Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - Protea Biosciences Group, Inc. | v365051_ex23-1.htm |
| As filed with the Securities and Exchange Commission on January 13, 2014 | Registration Statement No. 333- ______________ |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
Protea Biosciences Group, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 20-2903252 | ||
| (State or Other Jurisdiction of Incorporation or Organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
955 Hartman Run Road
Morgantown, West Virginia 26507
(304) 292-2226
(Address, including zip code, and telephone number, including area code,
of registrant’s principal executive offices)
Stephen Turner
Chief Executive Officer
Protea Biosciences Group, Inc.
955 Hartman Run Road
Morgantown, West Virginia 26507
(304) 292-2226
Copies to:
David N. Feldman, Esq.
RICHARDSON & PATEL LLP
405 Lexington Avenue, 49th Floor
New York, New York 10174
Tel: (212) 931-8700
Fax: (917) 677-8165
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. þ
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company.
| Large accelerated filer | ¨ | Accelerated filer | ¨ |
| Non-accelerated filer | ¨ | Smaller reporting company | þ |
CALCULATION OF REGISTRATION FEE
| Title of Each Class of
Securities to be Registered(1) | Amount to be Registered (2) | Proposed Maximum Offering Price Per Unit | Proposed Maximum Aggregate Offering Price | Amount of Registration Fee | ||||||||||||
| Common stock, $0.0001 par value per share | 17,511,197 | $ | 0.50 | $ | 8,755,599 | $ | 1,127.72 | (3) | ||||||||
| Common stock, $0.0001 par value per share, underlying $2.25 Warrants | 986,250 | $ | 2.25 | $ | 2,219,063 | $ | 285.82 | (4) | ||||||||
| Common stock, $0.0001 par value per share, underlying $1.10 Warrants | 2,898,375 | $ | 1.10 | $ | 3,188,213 | $ | 410.64 | (4) | ||||||||
| Common stock, $0.0001 par value per share, underlying A Warrants | 12,341,197 | $ | 0.50 | $ | 6,170,599 | $ | 794.77 | (4) | ||||||||
| Common stock, $0.0001 par value per share, underlying B Warrants | 6,170,598 | $ | 0.75 | $ | 4,627,949 | $ | 596.08 | (4) | ||||||||
| Common stock, $0.0001 par value per share, underlying Winter 2013 Placement Agent Warrants | 3,302,826 | $ | 0.75 | $ | 2,477,120 | $ | 319.05 | (4) | ||||||||
| TOTAL | 43,210,443 | $ | 27,438,543 | $ | 3,534.08 | (4) | ||||||||||
| (1) | This registration statement covers the resale (the “Resale”) of an aggregate of 17,511,197 shares of our common stock and 25,699,246 shares of common stock underlying warrants owned by two hundred nine (209) selling security holders identified in the prospectus below. The Company will not receive any proceeds from the Resale. |
| (2) | Pursuant to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), this registration statement also covers any additional securities that may be offered or issued in connection with any stock split, stock dividend or similar transaction. |
| (3) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under the Securities Act of 1933. |
| (4) | Calculated in accordance with Rule 457(g) of the Securities Act based upon the exercise price of the warrants held by the selling security holders named in this Registration Statement. |
THE REGISTRANT HEREBY AMENDS THIS REGISTRATION STATEMENT ON SUCH DATE OR DATES AS MAY BE NECESSARY TO DELAY ITS EFFECTIVE DATE UNTIL THE REGISTRANT SHALL FILE A FURTHER AMENDMENT WHICH SPECIFICALLY STATES THAT THIS REGISTRATION STATEMENT SHALL THEREAFTER BECOME EFFECTIVE IN ACCORDANCE WITH SECTION 8(A) OF THE SECURITIES ACT OR UNTIL THE REGISTRATION STATEMENT SHALL BECOME EFFECTIVE ON SUCH DATE AS THE SECURITIES AND EXCHANGE COMMISSION, ACTING PURSUANT TO SECTION 8(A), MAY DETERMINE.
The information in this prospectus is not complete and may be changed. The selling security holders may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
Subject to Completion, dated ________, 2014
PROSPECTUS
PROTEA BIOSCIENCES GROUP, INC.
43,210,443 Shares of Common Stock
This prospectus covers the resale by the selling security holders (the “Selling Security Holders”) named on page 65 of up to 43,210,443 shares of our common stock which include up to:
(i) 17,511,197 shares of common stock;
(ii) 986,250 shares of common stock issuable upon exercise of $2.25 Warrants;
(iii) 2,898,375 shares of common stock issuable upon exercise of $1.10 Warrants;
(iv) 12,341,197 shares of our Common Stock issuable upon exercise of A Warrants issued in connection with the Winter 2013 Offering (as defined below) at an exercise price of $0.50 per share;
(v) 6,170,598 shares of our Common Stock issuable upon exercise of B Warrants issued in connection with the Winter 2013 Offering at an exercise price of $0.75 per share; and
(vi) 3,302,826 shares of our common stock issuable upon exercise of Winter 2013 Placement Agent Warrants held by the Winter 2013 Placement Agent at an exercise price of $0.75 per share.
The shares being sold by the Selling Security Holders were issued to them in private placement transactions which were exempt from the registration and prospectus delivery requirements of the Securities Act. Our common stock and warrants are more fully described in “Description of Securities.”
There is not currently, and there has never been, any public market for our common stock. Our common stock is not currently eligible for trading on any national securities exchange, NASDAQ or any over-the-counter markets, including the OTC Bulletin Board or an OTC Market Group quotation system such as the OTCQB, and we cannot assure you that our common stock will become eligible for trading. We have arranged for a registered broker-dealer to apply to have our common stock quoted on the OTC Bulletin Board and/or an OTC Market Group quotation system such as the OTCQB. As a mandatory reporting company, we file annual, quarterly and current reports, proxy statements and other information about us with the SEC, as required.
We do not expect any secondary market in our common stock to develop for the foreseeable future. As a result, you should not expect to be able to resell your common stock regardless of how we perform and, if you are able to sell your common stock, you are likely to receive less than your purchase price. While we are currently in the process of seeking quotation of our shares on the OTC Bulletin Board and/or the OTCQB, there can be no assurance that we will be successful in listing our shares on the OTC Bulletin Board and/or the OTCQB. As a result of these factors, an investment in our common stock is not suitable for investors who require short or medium term liquidity.
We are an “emerging growth company” under the federal securities laws and will be subject to reduced public company reporting requirements. An investment in our common stock may be considered speculative and involves a high degree of risk, including the risk of a substantial loss of your investment. See “Risk Factors” beginning on page 8 to read about the risks you should consider before buying shares of our common stock. An investment in our common stock is not suitable for all investors. We intend to continue to issue common stock in this offering and, as a result, your ownership in us is subject to dilution. See “Risk Factors—Risks Relating to this Offering and Our Common Stock.”
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is _______, 2014
Table of Contents
| Prospectus Summary | 1 | |
| Risk Factors | 8 | |
| Special Note Regarding Forward-Looking Statements | 16 | |
| Business | 17 | |
| Management’s Discussion and Analysis or Plan of Operation | 42 | |
| Directors, Executive Officers, Promoters and Control Persons | 50 | |
| Executive Compensation | 54 | |
| Certain Relationships and Related Transactions | 56 | |
| Shares Eligible for Future Sale | 62 | |
| Market for Common Equity and Related Stockholder Matters | 63 | |
| Selling Security Holders | 64 | |
| Plan of Distribution | 71 | |
| Security Ownership of Certain Beneficial Owners and Management | 74 | |
| Description of Securities | 77 | |
| Disclosure of Commission Position on Indemnification for Securities Act Liabilities | 82 | |
| Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 83 | |
| Where You Can Find More Information | 83 | |
| Experts | 83 | |
| Legal Matters and Interests of Named Experts | 83 | |
| Financial Information | F-1 |
You should rely only on the information contained in this prospectus. We have not authorized any other person to provide you with information different from that contained in this prospectus. This prospectus is not an offer to sell, nor is it seeking an offer to buy, these securities in any state where the offer or sale is not permitted. The information in this prospectus speaks only as of the date of this prospectus unless the information specifically indicates that another date applies, regardless of the time of delivery of this prospectus or of any sale of our common stock.
We obtained industry and market data used throughout this prospectus through our research, surveys and studies conducted by third parties and industry and general publications. We have not independently verified market and industry data from third-party sources.
Prospectus Summary
This summary provides a brief overview of the key aspects of our business and our securities. The reader should read the entire prospectus carefully, especially the risks of investing in our common stock discussed under “Risk Factors.” Some of the statements contained in this prospectus, including statements under “Summary” and “Risk Factors” as well as those noted in the documents incorporated herein by reference, are forward-looking statements and may involve a number of risks and uncertainties. We note that our actual results and future events may differ significantly based upon a number of factors. The reader should not put undue reliance on the forward-looking statements in this document, which speak only as of the date on the cover of this prospectus. References to “we,” “our,” “us,” the “Company,” the “registrant,” or “Protea” refer to Protea Biosciences Group, Inc., a Delaware corporation.
About Our Business
Overview of Our Business
Protea operates in the field of bioanalytics. Bioanalytics is the identification and characterization of proteins, metabolites and other biomolecules, which are the products of all living cells and life forms. The Company is applying its technology to the development of a new generation of products and services that enable more rapid and comprehensive analysis of living cells and biofluids, thereby providing data that helps to define normal and disease processes. We believe that our proprietary technology is useful to support medical research and pharmaceutical development. Protea’s technologies enable the discovery and analysis of the proteins, metabolites and other biomolecules that regulate the biological functions of the human body and all other forms of life. Management believes this is a critical area of research, as pharmaceutical research is in need of more comprehensive biomolecular datasets that can be made available rapidly, in order to improve and accelerate the development of new therapeutics and diagnostic tests.
Risks Related to Our Business
Our business is subject to a number of risks. You should be aware of these risks before making an investment decision. These risks are discussed more fully in the section of this prospectus titled “Risk Factors”, which begins on page 8 of this prospectus.
Information Regarding our Capitalization
As of January 10, 2014, we had 65,442,735 shares of common stock issued and outstanding. This amount excludes the following securities that were outstanding as of January 10, 2014:
(i) $100,000 in convertible debt issued in April 2012 to the West Virginia Jobs Investment Trust Board as part of an original 3-month note in the amount of $400,000. The note bears interest at 10% providing for monthly interest-only payments starting May 2012 through June 2012, then final interest and principal payments due July 2012. The note includes a stock warrant for 88,889 shares. On June 18, 2012, the West Virginia Jobs Investment Trust Board signed an addendum to the note agreeing to extend the maturity date by 90 days to October 15, 2012. On March 11, 2013, the West Virginia Jobs Investment Trust Board approved an additional deferral of principal and interest until May 15, 2013 and reduced the price of converting into common shares to $0.50 per share. Subsequently the West Virginia Jobs Investment Trust Board further extended the maturity date until October 15, 2013. In November 2013 the West Virginia Jobs Investment Trust Board was paid $100,000 and the final $100,000 of the principal amount had its maturity date extended to January 15, 2014. An executive member of the West Virginia Jobs Investment Trust Board is a board member of the Company. Pursuant to the terms of the note, $200,000 of the principal amount is to be repaid in cash only.
| 1 |
(ii) $95,177 in convertible debt issued on May 24, 2012 to the West Virginia High Technology Consortium Foundation (“WVHTCF”) issued as a 30-month note in the amount of $200,000. The note bears interest at 8% providing for 25 monthly principal and interest payments of $9,001 starting December 2012 through November 2014, then final interest and principal payments due December 2014. The note is secured by a security interest in certain equipment and machinery owned by the Company pursuant to a security agreement dated May 24, 2012 in favor of WVHTCF, as amended by that certain Intercreditor Agreement dated June 11, 2012. In its sole discretion, at any time during the term of the WVHTCF note, elect to convert all or any portion of the unpaid principal and accrued interest due and payable under the WVHTCF note, into shares of Common Stock at an initial conversion price of $2.00 per share (the “Conversion Price”) subject to certain adjustments. In addition, if the Company issues any shares of Common Stock for a per share purchase price that is less than the Conversion Price then in effect (the “Original Conversion Price”), then the Original Conversion Price shall be reduced concurrently with such issue to a price determined by multiplying such Original Conversion Price by a fraction (x) the numerator of which shall be the number of shares of common stock outstanding immediately prior to such issue plus the number of shares of common stock which the aggregate consideration received by the Company for the total number of additional shares of common stock so issued would purchase at such Original Conversion Price, and (y) the denominator of which shall be the number of shares of common stock outstanding immediately prior to such issue plus the number of such additional shares of common stock so issued; provided, that, all shares of common stock issuable upon conversion of convertible securities shall be deemed to be outstanding. The WVHTCF Note is secured by a first lien interest, shared with the West Virginia Economic Development Authority (“WVEDA”), in all of the Company’s right, title and interest in and to the Collateral pursuant to the terms and conditions of a Security Agreement, dated May 24, 2012 by and between the Company and WVHTCF.
Unless otherwise specifically stated, information throughout this prospectus does not assume the exercise of outstanding options or warrants to purchase shares of our common stock or the conversion of convertible promissory notes.
| 2 |
Securities to be Registered
This prospectus relates to the resale of up to 43,210,443 shares of common stock and shares of common stock underlying warrants issued in connection with recent financings completed by the Company and described below.
December 2011 Offering
As of March 1, 2012, in connection with the terms of a private placement offering (the “December 2011 Offering”) of up to 100 units (the “December 2011Units”), each December 2011 Unit consisting of 50,000 shares of common stock and warrants to purchase 25,000 shares of common stock (the “December 2011 Warrants”), the Company issued an aggregate of 1,370,000 shares of common stock and December 2011 Warrants to purchase an aggregate of 685,000 shares of common stock at a per December 2011 Unit purchase price equal to $100,000, for aggregate gross proceeds equal to $2,740,000. The December 2011 Warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance (the “December 2011 Issue Date”) until the earlier of (i) a Qualified Public Offering (as defined below), or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the December 2011 Issue Date of the December 2011 Warrant. The term “Qualified Public Offering” means the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts, commissions and fees) are at least $15,000,000. The placement agent (the " December 2011 Placement Agent") in the December 2011 Offering received total cash commissions equal to $140,000, which amount represents 8% of the total gross proceeds received from investors that participated in the Offering and were introduced to the Company by the December 2011 Placement Agent. In addition, the December 2011 Placement Agent also received warrants to purchase an aggregate of 70,000 shares of common stock at an exercise price of $2.20 per share (the "December 2011 Placement Agent Warrants"). The December 2011 Placement Agent Warrants are exercisable any time after the December 2011 Issue Date until the earlier of (1) a Qualified Public Offering or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the December 2011 Issue Date.
The securities underlying the December 2011 Units and December 2011 Placement Agent Warrants were issued to accredited investors in accordance with Rule 506 of Regulation D under the Securities Act. The Company did not engage in any general advertisement or general solicitation in connection with the offering of the December 2011Units.
A total of 945,000 shares of common stock and 472,500 shares of common stock underlying December 2011 Warrants are being offered for resale pursuant to this prospectus.
May 2012 Offering
As of June 29, 2012, in connection with the terms of a private placement offering (the “May 2012 Offering”) of up to $6,000,000 of units (the “May 2012 Units”), each May 2012 Unit consisting of 50,000 shares of common stock and warrants to purchase 25,000 shares of common stock (the “May 2012 Warrants”), the Company issued an aggregate of 525,000 shares of Common Stock and Warrants to purchase an aggregate of 262,500 shares of common stock at a per May 2012 Unit purchase price of $100,000 for aggregate gross proceeds equal to $1,050,000. The May 2012 Warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance (the “May 2012 Issue Date”) until the earlier of (i) a Qualified Public Offering, or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the May 2012 Issue Date of the May 2012 Warrant. The term “Qualified Public Offering” means the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which (i) the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 and (ii) the offering price of the common stock in the Qualified Public Offering is at least 200% of the exercise price set forth in the May 2012 Warrants, as may be adjusted in accordance with the terms of the May 2012 Warrants.
| 3 |
All of the May 2012 Units, and securities underlying the May 2012 Units issued in the May 2012 Offering were issued to accredited investors in accordance with Rule 506 of Regulation D under the Securities Act. The placement agent in the May 2012 Offering has received total cash compensation equal to $103,862. The Company did not engage in any general advertisement or general solicitation in connection with the offering of the May 2012 Units.
A total of 437,500 shares of common stock and 218,750 shares of common stock underlying May 2012 Warrants are being offered for resale pursuant to this prospectus.
Summer 2012 Direct Issuances
As of September 21, 2012, in connection with a private placement offering (the “Summer 2012 Direct Issuances”) the Company issued an aggregate of 375,000 shares of common stock and warrants to purchase 187,500 shares of common stock (the “Summer 2012 Warrants”) to accredited investors at a per unit (the “Summer 2012 Units”) purchase price equal to $100,000 for an aggregate purchase price equal to $750,000. The Summer 2012 Warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the issue date thereof.
All of the Summer 2012 Units, and securities underlying the securities issued under the Summer 2012 Direct Issuances described above were issued to accredited investors in accordance with the exemption from registration provided by Section 4(2) of the Securities action in that the securities were offered and sold to accredited investors and the Company did not engage in any general advertisement or general solicitation in connection with the offering of the Summer 2012 Units.
A total of 375,000 shares of common stock and 187,500 shares of common stock underlying Summer 2012 Warrants are being offered for resale pursuant to this prospectus.
July 2012 Issuance
On July 27, 2012, the Company issued units (the “July 2012 Units”) consisting of an aggregate of 100,000 shares of common stock and warrants to purchase 50,000 shares of common stock (the “July 2012 Warrants”) to accredited investors at a per unit purchase price equal to $100,000 for an aggregate purchase price equal to $200,000. The July 2012 Warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof.
All of the July 2012 Units, and securities underlying the July 2012 Units were issued to accredited investors in accordance with the exemption from registration provided by Section 4(2) of the Securities Act in that the securities were offered and sold to accredited investors and the Company did not engage in any general advertisement or general solicitation in connection with the offering of the units.
A total of 75,000 shares of common stock and 37,500 shares of common stock underlying July 2012 Warrants are being offered for resale pursuant to this prospectus.
| 4 |
Fall 2012 Offering
As of October 17, 2012, in connection with the terms of a private placement offering (the “Fall 2012 Offering”) of up to $6,000,000 of units (the “Fall 2012 Units”), each Fall 2012 Unit consisting of 50,000 shares of common stock and warrants to purchase 25,000 shares of Common Stock (the “Fall 2012 Warrants”), the Company issued an aggregate of 452,500 shares of common stock and Fall 2012 Warrants to purchase an aggregate of 226,250 shares of common stock at a per Fall 2012 Unit purchase price of $100,000 for aggregate gross proceeds equal to $905,000. The Fall 2012 Warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of Common Stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof. The shares of common stock and Fall 2012 Warrants issued in connection with the Fall 2012 Offering Documents were subject to anti-dilution protection in the event the Company subsequently sold shares of common stock at a price per share that is less than $2.00 per share. The Company has since issued shares at a price equal to $0.50 per share, and therefore, on March 22, 2013, the Company’s board of directors (the “Board”) approved the issuance of an additional 1,057,500 shares of common stock and reduced the exercise price of the warrants to $1.12 per share.
All of the Fall 2012 Units, and securities underlying the Fall 2012 Units issued in the Fall 2012 Offering were issued to accredited investors in accordance with Rule 506 of Regulation D under the Securities Act. The Company did not engage in any general advertisement or general solicitation in connection with the offering of the Fall 2012 Units. The placement agent in the Fall 2012 Offering received total cash compensation equal to $56,400.
A total of 140,000 shares of common stock and 70,000 shares of common stock underlying Fall 2012 Warrants are being offered for resale pursuant to this prospectus.
Spring 2013 Direct Issuances
From April 23, 2013 to September 11, 2013, in connection with Company direct issuances (the “Spring 2013 Company Issuances”), the Company issued an aggregate of 3,197,500 shares of the Company’s common stock and warrants to purchase an aggregate of 2,898,375 shares of common stock to certain accredited investors for aggregate gross proceeds equal to $1,530,000 pursuant to the terms and conditions of a Securities Purchase Agreement and a warrant (the “Spring 2013 Warrants”). The Spring 2013 Warrants are exercisable for a term of five years from the issue date, and each purchaser has the right to purchase the number of shares of common stock equal to 75% of the number of shares purchased by such investor, at an exercise price of $1.10 per share. The shares and Spring 2013 Warrants are subject to certain anti-dilution rights in accordance with the terms of the Securities Purchase Agreement, if at any time after the issue date of the securities through December 31, 2013, the Company issues or sells any additional shares of common stock at a purchase price less than $0.50 per share.
The shares and Spring 2013 Warrants were issued pursuant to the exemption from registration provided by Rule 506 of Regulation D under the Securities Act in that they were issued to accredited investors, the Company did not engage in any general advertisement or general solicitation in connection with the offering of the securities, and the Company was available to answer any questions by and purchaser. Cash commissions were not paid in connection with the sale of the shares and Spring 2013 warrants.
A total of 3,197,500 shares of common stock and 2,898,375 shares of common stock underlying Spring 2013 Warrants are being offered for resale pursuant to this prospectus.
| 5 |
Winter 2013 Offering
As of December 31, 2013, the “Company had received $5,448,973 in aggregate gross cash proceeds from 131 accredited investors in connection with the sale of approximately 55 units (each a “Winter 2013 Unit” and collectively, the “Winter 2013 Units”) in an offering (the “Winter 2013 Offering”) of a minimum of $2,000,000 and up to a maximum of $6,000,000 (the “Maximum Offering Amount”), in Units of securities of the Company pursuant to the terms and conditions of a Subscription Agreement and Unit Purchase Agreement by and among the Company and each of the purchasers thereto. The Maximum Offering Amount excludes up to an additional $2,000,000 in Winter 2013 Units that may be sold to any existing stockholders, officers, and directors of the Company, including any of their affiliates (the “Company Investors”). Each Winter 2013 Unit consists of (i) 200,000 shares of common stock, par value $.0001 per share, (ii) and two warrants, including (a) a 1 year warrant to purchase 200,000 shares of common stock at an exercise price of $0.50 per share (the “A Warrants”) and (b) a 5 year warrant to purchase 100,000 shares of common stock at an exercise price of $0.75 per share (the “B Warrants” and together with the A Warrants the “Investor Warrants”). In addition, the Company issued an aggregate of approximately 21.5 in additional Units to the holders of bridge notes, in connection with the conversion of $1,994,500 (the “Conversion Amount”) in outstanding principal and accrued unpaid interest in bridge notes.
The Company also paid to the placement agent (the “Winter 2013 Placement Agent”) an aggregate of $733,410.67 in cash commissions, representing (1) 10% of the gross proceeds raised in the Winter 2013 Offering from purchasers introduced to the Company by the Winter 2013 Placement Agent; (2) 2% of the aggregate gross proceeds raised in the Winter 2013 Offering from Company Investors, including the Conversion Amount and (3) 2% of the aggregate gross proceeds raised in the Winter 2013 Offering, including the Conversion Amount, in connection with a non-accountable expense allowance. In addition, the Company agreed to issue warrants (the “Winter 2013 Placement Agent Warrants”) to purchase an aggregate of 3,302,826 of common stock underlying the Winter 2013 Units sold in the Winter 2013 Offering, including the shares of common stock underlying the Investor Warrants and (ii) 5% of the number of shares of common stock underlying the Winter 2013 Units issued in connection with the conversion of the bridge notes, including the shares of common stock underlying the Investor Warrants issued to bridge note holders.
In accordance with the terms of the Unit Purchase Agreement, until the one year anniversary of the closing date, purchasers shall have the right to participate in any subsequent offering by the Company of common stock or Common Stock Equivalents (as such terms is defined in the Unit Purchase Agreement) in which the Company raises gross proceeds of at least $1,000,000 (each a “Subsequent Offering”), on the same terms, conditions and price as provided for in the Subsequent Offering, in up to an amount of the Subsequent Offering equal to each purchaser’s proportionate share of the Subsequent Offering based on such purchaser’s participation in this Winter 2013 Offering. In addition, from the earlier of (1) five years from the closing Date or (2) the date on which any purchaser no longer owns shares of common stock sold in the Winter 2013 Offering, if the Company issues or sells any shares of common stock or any Common Stock Equivalent pursuant to which shares may be acquired at a price less than $0.50 per share (the “Base Price”) (subject to appropriate adjustments for any stock dividend, stock split, stock combination, reclassification or similar transaction) (each a “Dilutive Issuance”), the Company shall be required to issue additional shares of common stock to each purchaser, for no additional consideration, in an amount sufficient such that the pro rata portion of the Purchase Price paid by such purchaser for the shares of common stock then held, when divided by the total number of shares of common stock then held by such purchaser plus those shares of common stock issued as a result of the Dilutive Issuance will equal the Base Price.
The Winter 2013 Units, including the shares of common stock underlying the Winter 2013 Units, the Investor Warrants and the Winter 2013 Placement Agent Warrants were issued in connection with the exemption from registration provided by Rule 506(b) of Regulation D as promulgated under the Securities Act in that all purchasers and the Placement Agent were accredited investors and the Company did not engage in any general advertisement or general solicitation in connection with the purchase and sale of the Winter 2013 Units.
A total of 12,341,197 shares of common stock, 12,341,197 shares of common stock underlying A Warrants and 6,170,598 shares of common stock underlying B Warrants are being offered for resale pursuant to this prospectus.
| 6 |
Additional information regarding our issued and outstanding securities may be found in the section of this prospectus titled “Description of Securities.”
Corporate Information
Our principal executive office is located 955 Hartman Run Road, Morgantown, West Virginia 26507. Our telephone number is (304) 292-2226. Our web address is www.proteabio.com. Information included on our website is not part of this prospectus.
| 7 |
RISK FACTORS
You should carefully consider the risks described below, together with all other information included in this prospectus including our financial statements and related notes, before making an investment decision. The statements contained in this prospectus that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following risks actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and investors in our securities may lose all or part of their investment.
Risks Related to Our Business
We are a development stage company with a limited operating history and limited sales to date.
The Company is subject to all of the risks inherent in the establishment of a development stage company, including the absence of an operating history, and the risk that we may be unable to successfully develop, manufacture and sell our products. There can be no assurance that the Company will be able to execute its business plan, including without limitation the Company’s plans to develop, then manufacture, market and sell, its technologies, products and services. The Company has engaged in limited manufacturing operations to date, and although the Company believes that its plans to conduct manufacturing of its products internally will work, there is no assurance that this will be the case. The Company began to sell products and services in the fourth quarter of 2007, and sales to date are limited. There can be no assurance that the Company’s sales projections and marketing plans will be achieved as anticipated and planned. It is likely that losses will be incurred during the early stages of operations. The Company believes that its future success will depend on its ability to develop and introduce its instruments and services for mass spec molecular imaging, to meet a wide range of customer needs and achieve market acceptance. The Company cannot assure prospective investors that it will be able to successfully develop and market its products or that it will recover the initial investment that must be made to develop and market such products.
We have incurred net losses since inception.
The incurred a net loss of $9,531,259 and $11,497,332 for the fiscal years ended December 31, 2012 and 2011, respectively, a net loss of $7,916,882 for the nine months ended September 30, 2013 and net losses of $54,891,560 since inception. The opinion of our independent registered public accountants on our audited financial statements as of and for the year ended December 31, 2012 contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. Our ability to continue as a going concern is dependent upon raising capital from financing transactions. To stay in business, we will need to raise additional capital through public or private sales of our securities, debt financing or short-term bank loans, or a combination of the foregoing. The Company can provide no assurance as to whether our capital raising efforts will be successful or as to when, or if, we will be profitable in the future. Even if the Company achieves profitability, it may not be able to sustain such profitability.
Issuance of Common Stock to fund our operations or upon the exercise of outstanding warrants and options may dilute your investment.
We have been operating at a loss since inception and our working capital requirements continue to be significant. We have been supporting our business through the sale of debt and equity since inception. We will need additional funding for developing products and services, increasing our sales and marketing capabilities, technologies and assets, as well as for working capital requirements and other operating and general corporate purposes. Our working capital requirements depend and will continue to depend on numerous factors, including the timing of revenues, the expense involved in development of our products, and capital improvements. Accordingly, the proceeds from the Offering may not be sufficient to fund our future operations. If we are unable to generate sufficient revenue and cash flow from operations, we will need to seek additional equity or debt financing to provide the capital required to maintain or expand our operations, which may have the effect of diluting our existing stockholders or restricting our ability to run our business.
| 8 |
There can be no assurance that we will be able to raise sufficient additional capital on acceptable terms, or at all. If such financing is not available on satisfactory terms, or is not available at all, we may be required to delay, scale back or eliminate the development of business opportunities and our operations and financial condition may be materially adversely affected. Debt financing, if obtained, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, could increase our expenses and require that our assets be provided as a security for such debt. Debt financing would also be required to be repaid regardless of our operating results. Equity financing, if obtained, could result in dilution to our then existing stockholders. As of the date of this filing, we have warrants to purchase an aggregate of 54,752,952 shares of Common Stock issued and outstanding. The Company also has reserved an aggregate of 4,150,000 shares of Common Stock for issuance under its 2002 Equity Incentive Plan (the “2002 Plan”) and 5,000,000 shares of Common Stock have been reserved for issuance under the Company’s 2013 Equity Incentive Plan (the “2013 Plan”). As of the date of this filing, options to purchase an aggregate of 5,082,750 shares of Common Stock have been granted and are outstanding under the 2002 Plan and the 2013 Plan, collectively. There are also an additional 249,697 shares of Common underlying outstanding convertible promissory notes. The conversion of the notes and the exercise of the outstanding warrants and options could result in substantial dilution to stockholders. See “Description of Securities”.
We license a significant portion of our Intellectual Property from third parties; if the Company fails to remain in compliance with these agreements the Company’s business may be adversely affected.
The Company has entered into a number of technology license agreements with various universities for the exclusive use of a significant portion of the patent based intellectual property that the Company uses. While the Company is currently in compliance with the respective terms of these agreements, if there be one or more breaches thereunder, such as the failure to pay the applicable royalties, and one or more of these agreements are terminated, the Company will not be able to use such technology and the Company’s business may be adversely affected.
We may be unable to obtain or maintain patent or other intellectual property protection for any products or processes that we may develop.
The Company faces risks and uncertainties related to intellectual property rights. The Company may be unable to obtain or maintain its patents or other intellectual property protection for any products or processes that it may develop; third parties may obtain patents covering the manufacture, use or sale of these products or processes which may prevent the Company from commercializing its technology; or any patents that the Company may obtain may not prevent other companies from competing with it by designing their products or conducting their activities so as to avoid the coverage of the Company’s patents.
Since patent applications in the U.S. are maintained in secrecy for at least portions of their pendency periods (published on U.S. patent issuance or, if earlier, 18 months from earliest filing date for most applications) and since other publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we are the first to make the inventions to be covered by our patent applications. The patent position of biopharmaceutical firms generally is highly uncertain and involves complex legal and factual questions. The U.S. Patent and Trademark Office has not established a consistent policy regarding the breadth of claims that it will allow in biotechnology patents.
Proceedings to obtain, enforce or defend patents and to defend against charges of infringement are time consuming and expensive activities, and it is possible that the Company could become involved in such proceedings. Unfavorable outcomes in these proceedings could limit the Company’s activities and any patent rights that the Company may obtain, which could adversely affect its business or financial condition. Even if such proceedings ultimately are determined to be without merit, they can be expensive and distracting for the Company’s operations and personnel.
In addition, the Company’s success will depend in part on the ability of the Company to preserve its trade secrets. The Company cannot ensure investors that the obligations to maintain the confidentiality of trade secrets or proprietary information will not wrongfully be breached by employees, consultants, advisors or others or that the Company’s trade secrets or proprietary know how will not otherwise become known or be independently developed by competitors in such a manner that the Company has no legal recourse.
| 9 |
We are in a highly competitive market.
The Company is engaged in the highly competitive field of biotechnology. Competition from numerous existing biotechnology companies and others entering the proteomics field is intense and expected to increase. Many of these companies are larger, more established and recognized in the marketplace, and/or have substantially greater financial and business resources than the Company. Moreover, competitors who are able to develop and to commence commercial sales of their products before the Company could do so enjoy a significant competitive advantage. Likewise, innovations by competitors could cause the Company products or services to become obsolete or less attractive in the marketplace, adversely affecting sales and/or sales projections. The Company cannot assure investors that its technology will enable it to compete successfully in the future.
We are dependent on certain key personnel.
The success of the Company is dependent to a significant degree upon the skill and experience of its founders and other key personnel including Stephen Turner, Alessandro Baldi, Stanley Hostler, Daniel Dupret, Matthew Powell, Edward Hughes, and others. The loss of the services of any of these individuals would adversely affect the Company’s business. Although the Company has obtained a key man life insurance policy on Mr. Turner, its President and CEO, there is no assurance that policy proceeds would cover all potential costs or operational challenges that would result from the loss of services from Mr. Turner and in any event such policy would not cover the lives or loss of these other individuals. The Company cannot assure prospective investors that it would be able to find adequate replacements for these key individuals. In addition, the Company believes that its future success will depend in large part upon its continued ability to attract and retain highly skilled employees, who are in great demand. While the Company believes that its location in Morgantown, West Virginia, the home of West Virginia University, is helpful in attracting and retaining highly skilled employees, the Company cannot assure investors that it will be able to do so.
We are developing products in a rapidly evolving field and there are no assurances that the results of our research and development efforts will not be rendered obsolete by the research efforts and technological activities of others.
The bioanalytics field in which the Company is developing products is rapidly evolving. The Company cannot assure prospective investors that any results of the Company’s research and development efforts will not be rendered obsolete by the research efforts and technological activities of others, including the efforts and activities of governments, major research facilities, and large multinational corporations. While the Company believes that its initial efforts to develop its bioanalytics technology platform have been successful thus far, there can be no assurance that the Company will be able to successfully expand its operations in the future, to commercialize, market and sell products and services at projected levels, or to fully develop the technology in a timely and successful manner.
All of our pharmaceutical product candidates are in an early stage of development and we may never succeed in developing and/or commercializing them.
All of our pharmaceutical product candidates are in an early stage of development and we may never succeed in developing and/or commercializing them. If the Company is unable to commercialize them, or experiences significant delays in doing so, the Company’s business may be adversely affected. Even if the Company’s most advanced pharmaceutical candidate progresses through clinical trials as currently anticipated, it is not expected that it will be commercially available for at least several years. The Company will need to devote significant additional research and development, financial resources and personnel to develop commercially viable products and obtain regulatory approvals. The Company is likely to encounter hurdles and unexpected issues as it proceeds in the development of its pharmaceutical product candidates. There are many reasons that the Company may not succeed in its efforts to develop its product candidates, including the possibility that its product candidates will be deemed ineffective, unsafe or will not receive regulatory approvals; its product candidates will be too expensive to manufacture or market or will not achieve broad market acceptance; others will hold proprietary rights that will prevent the Company from marketing its pharmaceutical product candidates; or the Company’s competitors will market pharmaceutical products that are perceived as equivalent or superior.
| 10 |
There is no assurance that the Company’s manufacturing plans will be successful.
The Company employs internal and contract manufacturing. There is no assurance that the Company’s manufacturing plans will be successful. While the Company has a quality assurance program for its products, there nonetheless is inherent in any manufacturing process the risk of product defects or manufacturing problems that could result in potential liability for product liability risks.
Risks Relating to Ownership of Our Securities
There is no public trading market for our common stock and we cannot assure you that an active trading market may develop in the near future.
There is no public trading market for our Common Stock. Our Common Stock is not quoted in the over-the-counter markets or listed on any stock exchange. Therefore, outstanding shares of Common Stock cannot be offered, sold, pledged or otherwise transferred unless registered pursuant to, or exempt from registration under, the Securities Act and any other applicable federal or state securities laws or regulations. Stockholders may rely on the exemption from registration provided by Rule 144 of the Securities Act (“Rule 144”), subject to certain restrictions, only if the Company has been current in all of its periodic SEC filings for the 12 months preceding the contemplated sale of stock. Compliance with the criteria for securing exemptions under federal securities laws and the securities laws of the various states is extremely complex, especially in respect of those exemptions affording flexibility and the elimination of trading restrictions in respect of securities received in exempt transactions and subsequently disposed of without registration under the Securities Act or state securities laws. In addition, even if the Securities underlying the Units are registered pursuant to an effective registration statement filed in accordance with the Securities Act of 1933, as amended, if requested by the Company or an underwriter, the Securities and the Warrant Shares are subject to a market standoff agreement restricting their sale for up to 180 days following the date such securities are registered.
We intend to have a market maker file with the Financial Industry Regulatory Authority (FINRA) a Form 211 application to have our common stock quoted on the over-the-counter market, either through OTC Markets Group Inc. or the OTC Bulletin Board, or both. If we determine to have a market maker file the Form 211, we cannot predict when and whether FINRA will approve our application. Even if we are able to have our Common Stock become quoted in the over-the-counter market, an active trading market for our common stock may not develop in the future due to a number of other factors, including the fact that we are a small company that is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. Once our Common Stock is listed or quoted on an active trading market, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales. We cannot give you any assurance that an active public trading market for our Common Stock will develop or be sustained. You may not be able to liquidate your shares quickly or at the market price if trading in our Common Stock is not active.
Investors may face significant restrictions on the resale of our common stock due to federal regulation of penny stocks.
If our common stock be approved for quotation on the over-the-counter market, it may be subject to the requirements of Rule 15(g)-9, promulgated under the Securities Exchange Act so long as the price of our common stock is below $5.00 per share. Under such rule, broker-dealers who recommend low-priced securities to persons other than established customers and accredited investors must satisfy special sales practice requirements, including a requirement that they make an individualized written suitability determination for the purchaser and receive the purchaser's consent prior to the transaction. The Securities Enforcement Remedies and Penny Stock Reform Act of 1990, also requires additional disclosure in connection with any trades involving a stock defined as a penny stock. Generally, the Commission defines a penny stock as any equity security not traded on a national exchange or quoted on NASDAQ that has a market price of less than $5.00 per share.
| 11 |
The required penny stock disclosures include the delivery, prior to any transaction, of a disclosure schedule explaining the penny stock market and the risks associated with it. Such requirements could severely limit the market liquidity of the securities and the ability of purchasers to sell their securities in the secondary market.
We have established preferred stock which can be designated by the Company’s board of directors without shareholder approval.
The Company has authorized 10,000,000 shares of preferred stock. The shares of preferred stock of the Company may be issued from time to time in one or more series, each of which shall have a distinctive designation or title as shall be determined by the board of directors of the Company prior to the issuance of any shares thereof. The preferred stock shall have such voting powers, full or limited, or no voting powers, and such preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof as adopted by the board of directors. In each such case, we will not need any further action or vote by our stockholders. One of the effects of undesignated preferred stock may be to enable the board of directors to render more difficult or to discourage an attempt to obtain control of us by means of a tender offer, proxy contest, merger or otherwise, and thereby to protect the continuity of our management. The issuance of shares of preferred stock pursuant to the board of director's authority described above may adversely affect the rights of holders of common stock. For example, preferred stock issued by us may rank prior to the common stock as to dividend rights, liquidation preference or both, may have full or limited voting rights and may be convertible into shares of common stock. Accordingly, the issuance of shares of preferred stock may discourage bids for the common stock at a premium or may otherwise adversely affect the market price of the common stock.
Most of our total outstanding shares are subject to a market standoff agreement which provides that the purchaser will not, sell, assign or otherwise transfer or dispose of any common stock, warrants or other securities of the Company if so requested by an underwriter for a period not to exceed 180 days. If our securities are locked up at the request of any underwriter, and there are substantial sales of shares of our common stock, the price of our common stock could decline.
Most of our total outstanding shares are subject to a market standoff agreement which provides that the purchaser will not, sell, assign or otherwise transfer or dispose of any common stock, warrants or other securities of the Company if so requested by an underwriter for a period not to exceed 180 days. If our securities are locked up at the request of any underwriter, the price of our common stock could decline if there are substantial sales of our common stock following the “lock-up” period, particularly sales by our directors, executive officers and significant stockholders, or if there is a large number of shares of our common stock available for sale.
Our Certificate of Incorporation provides our directors with limited liability.
Article Seven of our Certificate of Incorporation states that our directors shall not be personally liable to us or any stockholder for monetary damages for breach of fiduciary duty as a director, except for any matter in respect of which such director shall be liable under Section 174 of the Delaware General Corporation Law or shall be liable because the director (1) shall have breached his duty of loyalty to us or our stockholders, (2) shall have acted not in good faith or in a manner involving intentional misconduct or a knowing violation of law or, in failing to act, shall have acted not in good faith or in a manner involving intentional misconduct or a knowing violation of law, or (3) shall have derived an improper personal benefit.
Article Seven further states that the liability of our directors shall be eliminated or limited to the fullest extent permitted by the Delaware General Corporation Law, as amended. These provisions may discourage stockholders from bringing suit against a director for breach of fiduciary duty and may reduce the likelihood of derivative litigation brought by stockholders on our behalf against a director.
Certain provisions of our Certificate of Incorporation and Delaware law make it more difficult for a third party to acquire us and make a takeover more difficult to complete, even if such a transaction were in the stockholders’ interest.
Our Certificate of Incorporation and the Delaware General Corporation Law contain provisions that may have the effect of making it more difficult or delaying attempts by others to obtain control of the Company, even when these attempts may be in the best interests of our stockholders.
| 12 |
We also are subject to the anti-takeover provisions of the Delaware General Corporation Law, which prohibit us from engaging in a “business combination” with an “interested stockholder” unless the business combination is approved in a prescribed manner and prohibit the voting of shares held by persons acquiring certain numbers of shares without obtaining requisite approval. The statutes have the effect of making it more difficult to effect a change in control of a Delaware company.
We are subject to the “seasoning” requirements imposed by the NYSE Euronext, NYSE MKT, and the NASDAQ Stock Market which will make us ineligible to list and trade on a national exchange until after trading for at least one year in the over-the-counter markets (or some other national or foreign regulated exchange), unless we are able to complete a firm commitment underwritten public offering with gross proceeds of at least $40 million.
Because of our status as a former SEC-reporting shell company, we are subject to SEC rules which require such companies to (1) trade in the over-the-counter markets (or some other national or foreign regulated exchange) for at least one year following the filing with the SEC of all required information about the reverse merger, including audited financial statements for the combined entity and (2) to timely file all required periodic reports with the SEC, including one annual report that includes audited financial statements for a full fiscal year commencing after filing the required information about the reverse merger, before seeking to “uplist” to a national securities exchange like NASDAQ, NYSE MKT or NYSE Euronext. We completed a reverse merger in September of 2011 and our Form 10-K filed for the fiscal year ended December 31, 2012 includes audited financial statements for a full fiscal year commencing after filing our Current Report on Form 8-K containing Form 10 information following the reverse merger. This means we will not be eligible to apply for listing on such exchanges until a market maker has filed a Form 211 to initiate trading and our securities begin to trade in the over-the-counter markets for at least one year. We may only bypass the requirements set forth above if we can complete a firm commitment underwritten public offering with gross proceeds of at least $40 million. As a result, our stockholders may find it more difficult to dispose of shares or obtain accurate quotations as to the market value of our Common Stock. In addition, we would be subject to an SEC rule that, if we failed to meet the criteria set forth in such rule, imposes various practice requirements on broker-dealers who sell securities governed by the rule to persons other than established customers and accredited investors. Consequently, such rule may deter broker-dealers from recommending or selling our Common Stock, which may further affect its liquidity. This would also make it more difficult for us to raise additional working capital through subsequent financings. In the event we do seek the listing of our Common Stock on a national securities exchange such as NASDAQ, NYSE MKT or NYSE Euronext, we cannot assure you that we will be able to meet the initial listing standards of either of those or any other stock exchange, or that we will be able to maintain a listing of our Common Stock on either of those or any other stock exchange.
Our financial controls and procedures may not be sufficient to ensure timely and reliable reporting of financial information, which, as a public company, could materially harm our stock price.
As a public reporting company, we require significant financial resources to maintain our public reporting status. We cannot assure you we will be able to maintain adequate resources to ensure that we will not have any future material weakness in our system of internal controls. The effectiveness of our controls and procedures may in the future be limited by a variety of factors, including:
| • | faulty human judgment and simple errors, omissions or mistakes; |
| • | fraudulent action of an individual or collusion of two or more people; |
| • | inappropriate management override of procedures; and |
| • | the possibility that any enhancements to controls and procedures may still not be adequate to assure timely and accurate financial information. |
If we fail to have effective controls and procedures for financial reporting in place, we could be unable to provide timely and accurate financial information and be subject to SEC investigation and civil or criminal sanctions.
| 13 |
We must implement additional and expensive procedures and controls in order to grow our business and organization and to satisfy new reporting requirements, which will increase our costs and require additional management resources.
As a public reporting company, we are required to comply with the Sarbanes-Oxley Act of 2002 and the related rules and regulations of the SEC, including the requirements that we maintain disclosure controls and procedures and adequate internal control over financial reporting. In the future, if our securities are listed on a national exchange, we may also be required to comply with marketplace rules and the heightened corporate governance standards. Compliance with the Sarbanes-Oxley Act and other SEC and national exchange requirements will increase our costs and require additional management resources. We recently have begun upgrading our procedures and controls and will need to continue to implement additional procedures and controls as we grow our business and organization and to satisfy new reporting requirements. If we are unable to complete the required assessment as to the adequacy of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act or if we fail to maintain internal control over financial reporting, our ability to produce timely, accurate and reliable periodic financial statements could be impaired.
If we do not maintain adequate internal control over financial reporting, investors could lose confidence in the accuracy of our periodic reports filed under the Securities Exchange Act of 1934, as amended. Additionally, our ability to obtain additional financing could be impaired or a lack of investor confidence in the reliability and accuracy of our public reporting could cause our stock price to decline.
We are an "emerging growth company" under the JOBS Act of 2012 and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company”, as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our Common Stock less attractive because we may rely on these exemptions. If some investors find our Common Stock less attractive as a result, there may be a less active trading market for our Common Stock and our stock price may be more volatile.
In addition, Section 107 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are choosing to take advantage of the extended transition period for complying with new or revised accounting standards.
We will remain an “emerging growth company” for up to five years following our initial public offering, although we will lose that status sooner if our revenues exceed $1 billion, if we issue more than $1 billion in non-convertible debt in a three year period, or if the market value of our Common Stock that is held by non-affiliates exceeds $700 million as of the last day of our most recently completed second fiscal quarter.
Our status as an “emerging growth company” under the JOBS Act may make it more difficult to raise capital as and when we need it.
Because of the exemptions from various reporting requirements provided to us as an “emerging growth company” and because we will have an extended transition period for complying with new or revised financial accounting standards, we may be less attractive to investors and it may be difficult for us to raise additional capital as and when we need it. Investors may be unable to compare our business with other companies in our industry if they believe that our financial accounting is not as transparent as other companies in our industry.
| 14 |
If we are unable to raise additional capital as and when we need it, our financial condition and results of operations may be materially and adversely affected.
Our directors, officers and principal stockholders have significant voting power and may take actions that may not be in the best interests of our other stockholders.
Our officers, directors and principal stockholders collectively beneficially own approximately 56% of our outstanding common stock, without giving effect to the purchase of Units in this Offering. As a result, these stockholders, if they act together, will be able to control the management and affairs of our company and most matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control and might adversely affect the market price of our common stock. This concentration of ownership may not be in the best interests of our other stockholders.
We have not paid dividends in the past and do not expect to pay dividends in the future, and any return on investment may be limited to the value of our stock.
We have never paid cash dividends on our common stock and do not anticipate paying cash dividends on our common stock in the foreseeable future. We currently intend to retain any future earnings to support the development of our business and do not anticipate paying cash dividends in the foreseeable future. Our payment of any future dividends will be at the discretion of our board of directors after taking into account various factors, including but not limited to our financial condition, operating results, cash needs, growth plans and the terms of any credit agreements that we may be a party to at the time. In addition, our ability to pay dividends on our Common Stock may be limited by Delaware state law. Accordingly, investors must rely on sales of their Common Stock after price appreciation, which may never occur, as the only way to realize a return on their investment. Investors seeking cash dividends should not purchase our Common Stock.
You should consult your own independent tax advisor regarding any tax matters arising with respect to the Units.
Participation in the Offering could result in various tax-related consequences for investors. All prospective purchasers of the Units are advised to consult their own independent tax advisors regarding the U.S. federal, state, local and non-U.S. tax consequences relevant to the purchase, ownership and disposition of the securities underlying the Units in their particular situations.
IRS CIRCULAR 230 DISCLOSURE: TO ENSURE COMPLIANCE WITH REQUIREMENTS IMPOSED BY THE INTERNAL REVENUE SERVICE, WE INFORM YOU THAT ANY U.S. TAX ADVICE CONTAINED HEREIN (INCLUDING ANY ATTACHMENTS) IS NOT INTENDED OR WRITTEN TO BE USED, AND CANNOT BE USED, FOR THE PURPOSE OF AVOIDING PENALTIES UNDER THE INTERNAL REVENUE CODE. IN ADDITION, ANY U.S. TAX ADVICE CONTAINED HEREIN (INCLUDING ANY ATTACHMENTS) IS WRITTEN TO SUPPORT THE “PROMOTION OR MARKETING” OF THE MATTER(S) ADDRESSED HEREIN. YOU SHOULD SEEK ADVICE BASED ON YOUR PARTICULAR CIRCUMSTANCES FROM YOUR OWN INDEPENDENT TAX ADVISOR.
| 15 |
IN ADDITION TO THE ABOVE RISKS, BUSINESSES ARE OFTEN SUBJECT TO RISKS NOT FORESEEN OR FULLY APPRECIATED BY MANAGEMENT. IN REVIEWING THIS FILING, POTENTIAL INVESTORS SHOULD KEEP IN MIND THAT OTHER POSSIBLE RISKS MAY ADVERSELY IMPACT THE COMPANY’S BUSINESS OPERATIONS AND THE VALUE OF THE COMPANY’S SECURITIES.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains “forward-looking statements”. These forward-looking statements are based on our current expectations, assumptions, estimates and projections about our business and our industry. Words such as “believe,” “anticipate,” “expect,” “intend,” “plan,” “may,” “will,” “would,” and other similar expressions identify forward-looking statements. In addition, any statements that refer to expectations, projections or other characterizations of future events or circumstances are forward-looking statements. These forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from those reflected in the forward-looking statements. Factors that might cause such a difference include, but are not limited to, those risks set forth in this prospectus under the title “Risk Factors” as well as the following:
| • | The current economic situation in the United States, which may reduce the funds available to businesses and government entities to purchase our products; |
| • | whether we will be able to raise capital as and when we need it; |
| • | our overall ability to successfully compete in our market and our industry; |
| • | unanticipated increases in development, production or marketing expenses related to our product and our business activities; and |
| • | other factors, some of which will be outside our control. |
You are cautioned not to place undue reliance on these forward-looking statements, which relate only to events as of the date on which the statements are made. We undertake no obligation to publicly revise these forward-looking statements to reflect events or circumstances that arise after the date hereof. You should refer to and carefully review the information in future documents we file with the Securities and Exchange Commission (the “Commission”.
Consequently, all of the forward-looking statements made in this prospectus are qualified by these cautionary statements, and there can be no assurance that the actual results anticipated by management will be realized or, even if substantially realized, that they will have the expected consequences to or effects on our business operations.
| 16 |
BUSINESS
History
Protea Biosciences Group, Inc. was incorporated under the name SRKP 5, Inc. in the State of Delaware on May 24, 2005 and was originally organized as a “blank check” shell company to investigate and acquire a target company or business seeking the perceived advantages of being a publicly held corporation.
On September 2, 2011, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Protea Biosciences, Inc., a Delaware corporation (“Protea Bio”) and SRKP 5 Acquisition Corp., a Delaware corporation and our wholly-owned subsidiary (“MergerCo”). Pursuant to the Merger Agreement, MergerCo merged with and into Protea Bio (the “Merger”). To effectuate the Merger and subject to the right of the Protea Bio stockholders to dissent, each share of Protea Bio common stock was automatically converted into one share of our common stock, all shares of Protea Bio common stock in treasury were canceled and we assumed all of Protea Bio’s rights and obligations for outstanding convertible securities and warrants. Following the Merger, we changed our name to Protea Biosciences Group, Inc.
Emerging Growth Company
The Company is an “emerging growth company”, as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”), and may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of section 404(b) of the Sarbanes-Oxley Act, and exemptions from the requirements of Sections 14A(a) and (b) of the Securities Exchange Act of 1934 to hold a nonbinding advisory vote of shareholders on executive compensation and any golden parachute payments not previously approved.
The Company has elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(1) of the JOBS Act. This election allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. As a result of this election, our financial statements may not be comparable to companies that comply with public company effective dates.
We will remain an “emerging growth company” for up to five years following our initial public offering, although we will lose that status sooner if our revenues exceed $1 billion, if we issue more than $1 billion in non-convertible debt in a three year period, or if the market value of our common stock that is held by non-affiliates exceeds $700 million as of any June 30.
To the extent that we continue to qualify as a “smaller reporting company”, as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, after we cease to qualify as an emerging growth company, certain of the exemptions available to us as an emerging growth company may continue to be available to us as a smaller reporting company, including: (1) not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes Oxley Act; (2) scaled executive compensation disclosures; and (3) the requirement to provide only two years of audited financial statements, instead of three years.
| 17 |
INDUSTRY AND MARKET OVERVIEW
The Mass Spectrometry Market
Mass spectrometry is an analytical technique that is used to determine the composition of a given sample based on the mass-to-charge ratio (m/z) of the biomolecules present in the sample. Mass spectrometer instruments are used extensively in the biopharmaceutical, industrial, chemical, materials and forensics industries as well as in academic research institutions. In its 12th Edition Global Assessment Report of The Laboratory Analytical & Life Science Instrumentation Industry, Strategic Directions International, Inc. (“SDI”) states, “The inherently strong identification and quantification capabilities of mass spectrometry are almost unrivaled in world of scientific instruments, and are why the technology is often associated with leading edge science in a variety of industries.” Protea is focused on developing and marketing instruments, software, products and services that enhance the capabilities and applications of mass spectrometer for end users in the biopharmaceutical, academic and chemical/industrial segments that are focused on pharmaceutical research and biomarker discovery.
According to the TechNavio Report, the global mass spec instrument market is expected to be valued at US$3.3 billion in 2013 and it is expected to reach US$3.92 billion by 2015, growing at a CAGR of 7.83 percent during the period 2011–2015. The United States accounts for 40% of the global mass spec instrument market representing an approximately US$1.3 billion market. Europe, Asia-Pacific and the rest of the world (ROW) account for the remaining US$2.0 billion and represent thirty percent (30%), twenty-three percent (23%) and seven percent (7%) of the global instrument market, respectively. The mass spectrometry instrument market can be segmented into three major sub segments; Biopharmaceuticals, Academia and Industrial which represented forty percent (40%), twenty-nine percent (29%) and thirty-one (31%) of the market, respectively.
Exhibit 1: Mass Spec Instrument Industry Size

Source: TechNavio Analysis, Global Mass Spectrometry Market 2011 – 2015.
Mass Spectrometry Instruments Types
The mass spectrometry instrument industry has witnessed numerous technological advancements leading to enhanced capabilities. As a result, a number of mass spec instrument types have emerged. According to SDI, the chart below indicates the market size for each respective instrument type for the years 2009 – 2011 and a forecast for 2014. It should be noted that SDI’s figures include instrument services and aftermarket products, which explains the discrepancy between SDI and the TechNavio Report.
| 18 |
Exhibit 2: Mass Spectrometry Instrument Classifications
| ($Million) | 2009 | 2010 | 2011 | 2014 | ||||
| Single Quadrupole LC/MS | 151 | 163 | 175 | 220 | ||||
| Time-of-Flight LC/MS | 77 | 82 | 91 | 118 | ||||
| Triple Quadrupole LC/MS | 571 | 628 | 680 | 879 | ||||
| Ion Trap LC/MS | 225 | 246 | 264 | 335 | ||||
| Q-TOF LC/MS | 292 | 331 | 366 | 510 | ||||
| FT-MS | 188 | 224 | 253 | 358 | ||||
| MALDI-TOF | 256 | 275 | 294 | 356 | ||||
| GC/MS | 446 | 474 | 500 | 580 | ||||
| Magnetic Sector MS | 94 | 98 | 101 | 110 | ||||
| ICP-MS | 298 | 323 | 344 | 408 | ||||
| Process MS | 33 | 36 | 38 | 43 | ||||
| Portable & In-Field MS | 55 | 61 | 68 | 94 | ||||
| SIMS | 81 | 86 | 96 | 106 | ||||
| Residual Gas Analyzers (RGAs) | 79 | 85 | 92 | 104 | ||||
| Leak Detectors | 121 | 127 | 136 | 152 | ||||
| Ion Mobility Spectroscopy (IMS) | 328 | 352 | 375 | 444 | ||||
| Overall | 3,293 | 3,591 | 3,874 | 4,816 |
Source: Strategic Directions International, Inc., Global Assessment Report 12th Edition, The Laboratory Analytical & Life Science Instrumentation Industry. Market Forecast 2012 – 2016. October 2012.
| 19 |
Exhibit 3: Mass Spectrometry Instrument Geographic Breakdown

Source: TechNavio Analysis, Global Mass Spectrometry Market 2011 – 2015.
Exhibit 4: Mass Spectrometer End-user Segmentation 2011–2015

Source: TechNavio Analysis, Global Mass Spectrometry Market 2011 – 2015.
The primary requirement for a mass spectrometer is to be able to identify and analyze specific components in a sample substance. Since the invention of the first mass spectrometer, there have been various upgrades that have allowed the mass spectrometer to be used across a wide variety of applications. Based on these applications, the Global Mass Spectrometry market can be classified into the following three main end-user segments: biopharmaceutical segment, academia segment and industrial segment
| 20 |
In 2011, the Biopharmaceutical Segment contributed the most to the revenue generated from sales of mass spectrometers. This is evident from the fact that this segment consists of a number of sub-segments, including Biotech Companies, Contract Research Organizations (“CROs”), Large Pharmaceutical Companies, and Generic Research Laboratories, which require high-cost mass spectrometers for a variety of applications. Moreover, with increasing focus on testing and research in the last decade, the demand for mass spectrometers had increased during this period. The key customers of mass spectrometers in this segment have a higher budget for research and testing purposes, as these purposes are essential for the final end-product of the respective customer.
The Academia Segment registered a moderate contribution to the revenue generated from sales of mass spectrometers in 2011. Educational institutions have been focusing on increasing their research capabilities in recent years, and hence the availability of the latest equipment for research is imperative. Furthermore, educational institutions are also rated based on the quantity and, more importantly, the quality of the equipment that is available for their students.
The Industrial Segment contributed the least revenue to the Global Spectrometry market in 2011. However, it is expected to be one of the fastest-growing segments for this market during the forecast period. Various regulations for clinical research are providing sufficient reasons for the purchase of mass spectrometers in this segment.
Mass Spectrometer Industry Structure
The vendor landscape in the Global Mass Spectrometry market is dominated by five key players: Agilent Technologies Inc., Bruker Corp., Danaher Corp., Thermo Fisher Scientific Inc. and Waters Corp. These companies are also involved in the sale of other research equipment. Overall, the top five vendors contributed close to 85 percent of the total revenue generated in the Global Mass Spectrometry market in 2011. Almost all these companies witnessed rapid growth in their respective revenue from mass spectrometers through the launch of several new products in 2010 and 2011.
Exhibit 5: Top 5 Vendor revenue and annual sales
| Company | Market Capitalization* | Annual Sales | ||||
| Agilent (NYSE:A) | $ | 16.01 B | $ | 580 M | ||
| Bruker (NASDAQ:BRKR) | $ | 3.35 B | $ | 290 M | ||
| Danaher (NYSE:DHR) | $ | 46.88 B | $ | 640 M | ||
| ThermoFisher (NYSE:TMO) | $ | 33 B | $ | 550 M | ||
| Waters Corp. (NYSE:WAT) | $ | 8.79 B | $ | 440 M | ||
Source: TechNavio Analysis, Global Mass Spectrometry Market 2011 – 2015.
Google Finance. Market Capitalization calculated on August 2, 2013
| 21 |
The following exhibit depicts the market shares of the respective
mass spectrometer vendors in the Global Mass Spectrometry market in 2011:
Exhibit 6: Market Shares by Vendor

Source: TechNavio Analysis, Global Mass Spectrometry Market 2011 – 2015.
The Company has done a thorough analysis of the installed mass spectrometers that the LAESI DP-1000 is currently compatible with. Based on information that the Company has compiled, Protea believes that the LAESI DP-1000 is currently compatible with 3,000 mass spectrometers worldwide from Waters Corp. and ThermoFisher Scientific. Protea intends to work towards making the LAESI DP-1000 compatible with a larger number of mass spectrometers through the integration with other mass spectrometry vendors such as AB Sciex (Danaher, (NYSE:DHR).
Pharmaceutical Research and Biomarker Discovery Market
The Company applies its core technologies and expertise to the development of instruments and services that seek to improve pharmaceutical research and biomarker discovery through the discovery and identification of biomolecules such as proteins, peptides, lipids and metabolites. Protea’s products and services are purchased and used primarily by pharmaceutical and academic/clinical research laboratories, including analytical chemists, translational researchers, biologists, oncologists and pathologists.
The Company’s commercial offering includes the LAESI DP-1000 instrument system and its ProteaWorks mass spec imaging services unit. It believes the market for these commercial offerings to be comprised of two main end user segments, the preclinical pharmaceutical research segment and the biomarker discovery segment, which believed to be the following size in totality.
Exhibit 7: Pharmaceutical Research & Biomarker Discovery Market
| Pharmaceutical Research* | Biomarker Discovery** | |
PhRma members spent ~ US $50 billion on R&D in 2012 NIH spent ~ US $30 billion on R&D in 2012 |
$13 Billion annual worldwide market | |
| Roughly one-third or ~ US $15 billion is dedicated to preclinical research including target validation and lead optimization. | $4.7 Billion annual worldwide market for molecular imaging |
Source: *PhRMA 2013 profile. Biopharmaceutical Research Industry
**BBC Research. Market Research Report. Biomarkers: Technologies and Global Markets. BIO061B. ISBN: 1-59623 721-X.
| 22 |
Preclinical pharmaceutical research refers to the discovery and testing of new therapeutic compound candidates prior to their use in human clinical trials. For every 5,000-10,000 preclinical drug candidates, only five enter human clinical trials. Thus, there is a great need to obtain better datasets faster to identify the most promising candidates.
“Biomarkers” are specific biomolecules that have been found to be associated with specific disease states. Biomarker discovery is useful for the development of new prognostic and diagnostic tests, as well as in the emerging field of personalized medicine, where biomarkers can identify those patients who will respond well to specific therapies. The human disease biomarker sector seeks to identify and validate biomolecules that are associated with the onset and progression of a specific disease, and thus can become new diagnostics, or biomarkers, to be used to better identify patient subgroups for more precise selection of their optimal treatment, as well as to guide new pharmaceutical development for specific patient subgroups. The largest area of biomarker research is oncology.
According to the Frost & Sullivan Analysis of the U.S. Cancer Biomarker Testing Market, June 2012, the U.S. cancer biomarker testing market is expected to grow from $7.86 billion in 2011 to $11.46 billion by 2017. The molecular-based cancer biomarker testing segment, including single-mutation companion diagnostics, multiplexed array, and other genomic analysis, were only 4% of total market revenues in 2011, but this will likely reach 9.4% by 2017 from 22.9% annual growth.
Exhibit 8: The U.S. Cancer Biomarker Testing Market - Annual Revenue (in USD Billion)
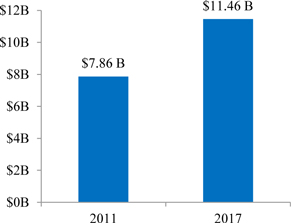
Source: Frost & Sullivan Analysis of the U.S. Cancer Biomarker Testing Market, June 2012
Exhibit 9: The molecular-based cancer biomarker testing segment - % of total market revenues
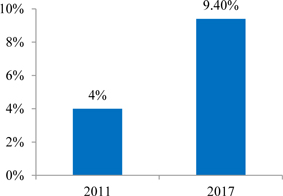
Source: Frost & Sullivan Analysis of the U.S. Cancer Biomarker Testing Market, June 2012
| 23 |
LIMITATIONS OF CURRENT MASS SPECTROMETRY PRODUCTS
Required Sample Preparation: Mass spectrometry is synonymous with sample preparation that is tedious, time consuming and results in operating complexity. Separation techniques such as gas chromatography (GC), liquid chromatography (GC) and others have been developed to facilitate sample preparation for mass spectrometry but current techniques require an experienced and highly skilled user. As a result, mass spectrometry has largely been limited to use by analytical chemists and has failed to be adopted by end users lacking this particular expertise.
Destruction of Sample: Mass spectrometry requires that a sample be homogenized, sometimes purified, or have additional chemicals introduced to it in order to be analyzed by a mass spectrometer. Homogenizing a sample means that molecules (ions) can be detected and quantified but their location in the original sample cannot be identified. Purification and chemicals result in the introduction of variation based on respective user techniques and protocols resulting in reproducibility issues. As a result of the sample being destroyed, further analysis after mass spectrometry analysis is not possible, which can cause users not to analyze samples that they perceive to have future value.
Limited Number of Compatible Samples: In order for a mass spectrometry analysis to occur, a sample must be in the gas phase and have a charged (charge results in molecules becoming ions). Therefore, the number of sample types that can be directly analyzed via a mass spectrometer is limited and can exclude biological samples such as tumor tissue biopsies, animal organ tissue and whole body animal tissue sections (mouse and rat). Also, mass spectrometry analysis occurs under vacuum conditions and therefore cannot analyze living cell samples like a bacterial colony or cell culture because the sample will be immediately desiccated (destroyed) from the vacuum conditions. These sample types are used extensively in pharmaceutical research and biomarker discovery.
No Imaging Capability: Mass spectrometers identify molecules based on their mass-to-charge ratio (m/z) and display each molecule identified in a sample on a mass spectra (mass spectrum). A mass spec is a displayed as an X/Y graph with the X-axis showing molecules based on their m/z and the Y-axis showing their relative intensity (amount of molecule present). Each molecule is represented as a bar or “peak” on the X-axis with the height of the peak being correlated to the abundance of that particular molecule. Mass spectra can be very complex data sets that are difficult to interpret and can have limited relevance to biology-centric applications. Molecular imaging techniques such as PET, SPECT and CT have become commonplace in pharmaceutical research and biomarker discovery due to their ability to display molecules in relation to biologic and anatomic features. Mass spec’s inability to perform imaging is considered a major limitation to these markets.
Exhibit 10: Representation of a Mass Spectrometer

Source: The Company
| 24 |
PROTEA SOLUTIONS
No Sample Preparation Required is required: Protea’s LAESI technology analyzes samples directly without the need for sample preparation. As a result, separation techniques and protocols are not required to obtain mass spectrometry data. Instead, virtually all sample types can be inserted directly into the LAESI DP-1000 instrument where they are ablated (put into gas phase), ionized (charged) and sent into the mass spectrometer, with this process taking fractions of a second. Also, the LAESI DP-1000 has a user-friendly interface that eliminates the operation complexity of traditional mass spectrometry a wide array of users beyond analytical chemists to generate mass spectrometry data on their samples.
No Sample Destruction: Protea’s LAESI technology does not require a sample to be homogenized, purified and does not require the introduction of any chemicals for mass spec analysis to occur. Because the sample is not destroyed, the identification of a molecule’s location in a sample is made possible via LAESI. Also, variability and reproducibility are eliminated as a result of LAESI.
Improvement in Analysis Speed: Due to the elimination of sample preparation and the rapid nature of LAESI, improvements in analysis speed as much as 10x are possible. This factor makes LAESI attractive to users that utilized mass spectrometry for high-throughput applications (i.e. a large number of samples must be analyzed in a limited number of time). Also, LAESI makes mass spectrometers more efficient, improve laboratory efficiencies and introduce time costs savings.
Maximum Number of Compatible Samples: LAESI is able to analyze virtually all sample types because of its novel design and the fact that it operates at ambient pressure (i.e. not a vacuum). As a result, tumor tissue biopsies, animal organ tissue and whole body animal tissue sections (mouse and rat) can all be analyzed. Also, living cell sample such as bacterial colonies and cell cultures can be directly analyzed. LAESI is able to analyze the largest number of sample types of any of the current mass spec technologies.
Imaging Ability: The LAESI DP-1000 system, through ProteaPlot, enables 2 dimensional and 3 dimensional imaging via mass spectrometry. LAESI’s “mass spec imaging” displays the distribution of molecules in a given sample. LAESI is the only technology that can facilitate this capability for mass spectrometry in tissue sections (animal and human tissue), living cell samples and other sample types.
Exhibit 11: Representation of a LAESI Mass Spec Image

Source: The Company
| 25 |
COMPANY STRATEGY
Protea seeks to become a leader in mass spectrometry enhancement for advanced pharmaceutical research and biomarker discovery applications. Centered around its LAESI technology platform the Company will seek to commercialize products and services that address the needs of and deliver solutions to customers focused on preclinical pharmaceutical research and biomarker discovery. To achieve this objective, the Company intends to employ the following strategy:
| · | Commercialize the LAESI DP-1000 instrument. Protea is building a direct sales force to market and sell the instrument to existing mass spectrometer users or those seeking to acquire compatible high-resolution mass spectrometers. In addition the Company will seek to execute co-marketing agreements with select mass spectrometry manufacturers and vendors. In March 2012 the Company executed a non-exclusive co-marketing agreement with Waters Corp. and may seek to execute additional agreements of similar nature. Also, Protea will execute agreements with select international dealers. |
| · | Commercialize mass spectrometry imaging services through the ProteaWorks business unit. Protea is building a direct sales force to market and sell the services offered through ProteaWorks to pharmaceutical companies, academic/clinical research laboratories and other industrial/chemical companies that are interested in Protea’s mass spectrometry imaging capabilities and expertise. In addition, the Company may seek to execute non-exclusive co-marketing agreements with select contract research organizations (CRO), however, to date it has no such agreements in place. |
| · | Develop consumable products to enhance the LAESI DP-1000 and mass spectrometry instruments. Specific mass spec applications exist that can benefit from consumable products that improve sensitivity, throughput and comprehensiveness of the mass spec analysis. Due to Protea’s expertise in the field, development efforts will be undertaken to develop LAESI DP-1000 specific consumable products that will bring a recurring revenue stream to the instrument platform. Consumable products for mass spectrometers will give Protea a deeper visibility into its intended sales channel and will bring a line of what is expected to be high-margin revenue. |
| · | Create new software products and introduce new software-driven data analytics capabilities. The LAESI DP-1000 generates very large data files that are not only valuable to researcher now but also data that will be valuable in the future. Protea is focused on creating software and analytical tools that enhance the value of the data generated by the LAESI DP-1000. The Company envisions creating disease, molecular and applications specific databases and software platforms that enhance the value of these databases. |
| · | Will partner and collaborate with academic and commercial based research laboratories to apply LAESI to leading edge research initiatives. The Company believes that LAESI will be a valuable tool for researchers pursuing new pharmaceutical and medical discoveries. As the trend of “personalized” or “molecular” medicine continues to emerge, comprehensive information on molecules that are associated with particular diseases (i.e. biomarkers) will see increased demand. Given LAESI’s ability to detect a wide range of molecules (e.g. proteins, peptides, lipids and metabolites) in a wide range of samples (tissue, biofluids and living cells), the Company believes it will be a sought after for its value in partnerships and collaborations. Protea believes it will be able to generate and secure intellectual property (“IP”) as a result of these partnerships and collaborations, which could potentially lead to new products, services, software or rights to royalty revenue. |
TECHNOLOGY
LAESI – Laser Ablation Electrospray Ionization – Mass Spectrometry
Believing that mass spectrometry represents a powerful analytical platform for biological applications, Protea licensed a technology known as Laser Ablation Electrospray Ionization (“LAESI”). Invented in the laboratory of Professor Akos Vertes, Ph.D., George Washington University in 2007 and exclusively-licensed to Protea in 2009, LAESI is a novel ion source that provides breakthrough capabilities that enable biological applications for mass spectrometry.
| 26 |
Exhibit 12: LAESI Illustration

As depicted above, LAESI utilizes a mid-range infrared (IR) laser pulse that rapidly boils the water in a given sample creating an ablation event that results in the biomolecules from that particular area being ejected vertically where they meet a stream of electrospray droplets and become charged biomolecules or ions. The ionized sample then moves through an inlet tube and into the mass spectrometer where the mass analyzer determines the mass-to-charge ratio of the representative biomolecules and their relative abundance is determined by the detector.
Exhibit 13: Key LAESI Components

LAESI Mass Spectrometry Advantages
Due to the manner in which LAESI ablates and ionizes samples, it eliminates two of the major drawbacks associated with mass spectrometry, sample preparation and sample destruction. LAESI can analyze a wide range of sample types with no sample preparation required. In addition, samples such as a tissue section or cells can be analyzed as is. With LAESI mass spectrometry, Protea has successfully analyzed tissue sections – human and animal (frozen or formalin-fixed), biofluids (blood, urine and serum), horticulture specimens, cell lines & pellets, bacterial colonies, hair fibers, contact lenses and hydrogels
| 27 |
Exhibit 14: Comparison between mass spectrometry with and without LAESI
| Technology | Mass Spectrometry With LAESI |
Mass Spectrometry without LAESI | ||
| No Sample Preparation | ü | û | ||
| Rapid Results (Up to 10x improvement) | ü | û | ||
| Sample in Native Form | ü | û | ||
|
Vacuum Incompatible Samples (i.e. bacterial colony) |
ü | û | ||
| Full Automation | ü | û | ||
| Imaging Capability | ü | û |
Source: The Company.
ProteaPlot™ Mass Spec Imaging Software
Protea also developed, internally, the software platform ProteaPlot to address two other limitations of mass spectrometry, the inability to determine the location of an ion in a sample and the difficulty a non-analytical chemist has in interpreting mass spectrometry data.
ProteaPlot was designed to create images known as ion maps from traditional mass spectrometry data (mass spectrum). Ion maps depict the distribution of an ion in a given sample and the relative intensity (abundance) of that ion.
The image below is a screen shot from the ProteaPlot software program. A traditional mass spectrum can be seen on the right side of the image. The image to the left is an ion map of the biomolecule with a mass-to-charge ratio (m/z) of 616 (circled in red), which is known to be a lipid (fat) molecule. The colorimetric scale represents the relative intensity of this ion with blue representing the regions with the lowest relative intensity, the areas in red representing the regions with the highest relative intensity and the areas in white representing regions where that molecule is not present at all.
Exhibit 15: ProteaPlot Software Screen Shot
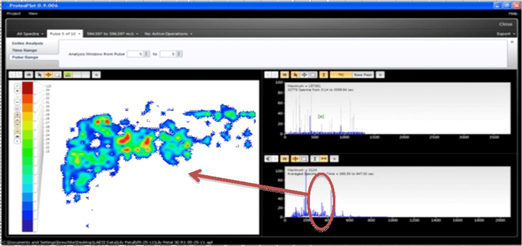
| 28 |
Exhibit 16: Ion Map of m/z 616 Displayed over its Originating Tissue Section
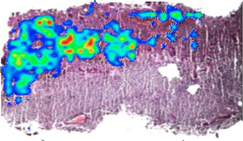
The same ion map for the lipid molecule m/z 616 is shown above overlaying the biopsied tissue section it was generated from. The patient that this biopsy was taken from was diagnosed with Renal Cell Carcinoma (a type of kidney cancer). Through pathologic analysis, the darker area on the top of the biopsy sample was diagnosed as being cancerous while the lighter area on the bottom was determined to be “normal”. As this ion map clearly shows, the lipid molecule m/z 616 is clearly localized in the cancerous part of the tissue while it is prominently absent from the normal area.
By transforming traditional mass spectrometry data like that shown in exhibit 10 into ion maps as shown in exhibits 15 and 16, the information obtained from LAESI-mass spectrometry and ProteaPlot becomes more relevant to biologists, who can analyze samples of choice with no sample preparation. Also, the sample is not destroyed so ion location information can be obtained and the ion maps can be visualized over the sample of origin for integration with more traditional biological analyses such histology.
PRODUCTS AND SERVICES
The Company applies its core technologies and expertise to the development of products, instruments and services that seek to improve the discovery and identification of proteins, metabolites and other biomolecules. Its products and services are purchased and used primarily by pharmaceutical and academic/clinical research laboratories, including analytical chemists, translational researchers, oncologists, pathologists and cell biologists.
Protea’s commercial offering is organized as follows: (a) LAESI DP-1000 Instrument Platform; and (b) ProteaWorks Imaging Service
LAESI DP-1000 Instrument Platform
In 2012 Protea completed the development of the LAESI DP-1000 and announced its commercial availability in April. The LAESI DP-1000 is a fully integrated system that incorporates the core LAESI technology licensed from George Washington University with the ProteaPlot software suite. The LAESI DP-1000 is compatible with high-resolution mass spectrometers manufactured by Waters Corp. (NYSE:WAT) and ThermoFisher Scientific (NYSE:TMO); engineering its compatibility with additional mass spec vendors is ongoing.
| 29 |
Exhibit 17: LAESI DP-1000 Instrument Images

LAESI is intended to meet the broad need of the biologist for the direct, unbiased identification and characterization of biomolecules in biological samples, which can remain untouched prior to their analysis. By virtue of LAESI’s improved speed and the comprehensive datasets it generates, the Company is pursuing its vision of what it believes will be a new era of human bioanalytics, where the molecular pathways of human disease will be more clearly elucidated, and datasets more rapidly available, thereby accelerating pharmaceutical and life science research.
The Company offers the LAESI DP-1000 instrument as well as software packages developed by the Company (LAESI desktop software and ProteaPlot™). Its software facilitates operating the instrument, and the storage and display of datasets, in a user friendly, intuitive software environment. The LAESI and ProteaPlot software include LAESI data mining and analytic tools to search for new, disease-specific biomarkers, and to elucidate disease-specific biomolecular pathways.
In addition, the Company provides an expanding line of LAESI consumables.
Instrument production has started. Protea has produced six units and six additional units are being produced. Five units have been shipped to date, to customers in the USA, the Netherlands and the United Kingdom.
LAESI DP-1000 Applications
Tissue Imaging
The LAESI DP-1000 is capable of rapidly analyzing and mapping the spatial distribution of biomolecules in tissue. At 200μ (micron) resolution, the LAESI DP-1000 can map a x cm2 piece of tissue in x minutes (x seconds per ablation). The LAESI DP-1000 can analyze and image both human and animal tissue. Below is a LAESI mass spec image of two molecules shown simultaneously in Z x Z mouse pup section. This analysis took approximately 180 minutes.
| 30 |
Exhibit 18: a LAESI 2 dimensional map image of two molecules shown simultaneously in Z x Z mouse pup section

Biofluid Analysis
The LAESI DP-1000 is capable of rapidly analyzing micro titer well plates filled with biofluids such as blood, urine or serum. The LAESI Desktop Software makes the automation of this type of analysis very easy for the user. A grid pattern can be quickly drawn for 96, 384 or 1515 well plates and the number of ablations per well can be set. Once the user’s settings have been entered the analysis will assess each well systematically with each ablation taking <10 seconds.
Exhibit 19: The LAESI DP1000 accepts 96, 384 and 1536 well plates for high throughput sample analysis

In Vivo Mass Spectrometry Imaging
Because LAESI operates at ambient pressure (non-vacuum) it is the only mass spectrometry imaging technology that can analyze vacuum incompatible samples such as living bacterial and cell colonies. Due to this unique capability, LAESI can be used to analyze a bacterial colony and then that colony can be re-incubated and analyzed at a later time, enabling biodynamics studies on living samples for the first time ever. Below is a LAESI mass spec image of bacterial colony.
Exhibit 20: LAESI 3 dimensional ion map image of a live bacterial colony showing the spatial distribution of an antibiotic molecule (green) and a biomolecule produced by the bacterial cells (orange)

3 Dimensional LAESI Mass Spectrometry Imaging
| 31 |
LAESI is the only mass spectrometry imaging technology that performs a volumetric sampling. All other mass spectrometry imaging technologies perform surface analysis or desorption whereby they are only extracting small amounts of molecular information. The nature of the LAESI DP-1000 mid-IR laser enables it to penetrate samples in the Z-plane enabling 3 dimensional analysis and imaging. Below is a plant sample that was analyzed in 3D by LAESI to assess the permeation of a pesticide that was applied to the surface of a leaf.
Exhibit 21: LASEI 3 dimensional ion map image of a leaf, displaying the presence of a chemical herbicide in the middle of the leaf
ProteaWorks Imaging Service
Protea offers proprietary ProteaWorks imaging services for the rapid identification of both small molecules (e.g. lipids and metabolites) and large molecules (e.g. proteins). The services unit is operated under GLP (Good Laboratory Practices), which are necessary for regulatory submissions and to meet the internal research and development standards of pharmaceutical research clients.
Exhibit 22: Service operated under Good Laboratory Practices

| 32 |
Protea’s services focus on the unique capability of LAESI technology to image and display the presence of specific biomolecules in cell and tissue samples. The Company believe that this field, called LAESI mass spectrometry imaging (“LAESI-MSI”), will become a major analytical method for biological investigation of tissues and living cell populations, such as cell cultures and colonies. LAESI-MSI provides new information based on molecule-specific images, without the need for labeling, staining or complex sample preparation protocols. Datasets are automatically generated, and are available in minutes, depending on the complexity of the analysis.
In LAESI-MSI, the chemical identity of biomarkers or other biomolecules present, for example in a tissue, is investigated as a function of spatial distribution. This paradigm allows accurate distribution profiling of chemical species that may help to understand pathologies or metabolic processes present in the specimen. LAESI-MSI has the potential to revolutionize biological investigation using objective chemical data by presenting the distribution of specific molecular structures in situ.
LAESI-MSI eliminates the need for sample preparation and allows the researcher to study the biochemical landscape of a sample as it exists in nature. LAESI-MSI services are available for 2D and 3D tissue biomolecular distribution profiling, high throughput biofluid analysis, biodynamic live cell colony monitoring, and biomaterial characterization.
Protea is applying LAESI-MSI to create cell-based biomolecular databases that will be specific to disease states and allow the analysis and integration of LAESI biomolecular datasets with the sample-related pathology, gene expression and demographic datasets. We are developing “high resolution” LAESI technology that will enable the analysis of single cells. We believe LAESI-MSI can generate important advances in bioinformatics to help provide time-based, biodynamic datasets for improved disease state assessment and management.
INTELLECTUAL PROPERTY AND MARKET EXCLUSIVITY
The Company is building a strong intellectual property position and currently owns four patents (with additional pending applications) and has an exclusive license to five additional patents and pending applications owned by George Washington University (GWU). The subjects of the patent applications include: Laser Ablation Electrospray Ionization (LAESI) for high throughput and imaging mass spectrometry, 2D and 3D biomolecular imaging; novel acid-cleavable chemical surfactants; and protein microscope.
| 33 |
Exhibit 23: IP Portfolio
| # | Claim | Expiration | Status | |||
| US 7,964,843 | 3D Molecular Imaging by Infrared Laser Ablation Electrospray Ionization Mass Spectrometry | 2026 | Issued | |||
| US 8,299,429 | To extend the intellectual property around this foundational patent | 2026 | Issued | |||
| A second CON patent has been allowed and is awaiting issuance by the USPTO | Pending | |||||
| US 8,067,730 | Laser Ablation Electrospray Ionization (LAESI) for Atmospheric Pressure, In Vivo, and Imaging Mass Spectrometry. | 2026 | Allowed | |||
| US 8,487,244 |
Laser Ablation Electrospray Ionization (LAESI) for Atmospheric Pressure, In Vivo, and Imaging Mass Spectrometry |
2026 | Issued | |||
| US 8,487,246 |
Three-Dimensional Molecular Imaging By Infrared Laser Ablation Electrospray Ionization Mass Spectrometry |
2026 | Issued |
PARTNERSHIPS
Agreement with George Washington University (GWU)
In December 2008, the Company entered into an Exclusive License Agreement, as amended on February 22, 2010 (the “GWU Effective Date”), and from time to time thereafter (“GWU Agreement”) with George Washington University (Washington D.C.) for technology developed in the laboratory of Akos Vertes Ph.D., Professor of Chemistry, Professor of Biochemistry & Molecular Biology, Founder and Co-Director of the W.M. Keck Institute for Proteomics Technology and Applications, the Department of Chemistry, who is a science advisor to the Company. The technology field is LAESI. Under the terms of the license agreement, the Company has the exclusive, worldwide, rights to commercialize the technology. The Company is obligated to pay expenses for the preparation, filing and prosecution of related patent applications, and license fees equal to $12,500 (which fees were paid as of August 18, 2008), annual royalties equal to 5% of the net sales of products and processes sold by the Company, or an affiliate that utilizes the GWU subject technology, after taking into account the annual minimum royalty fees described below, and 50% of payments received by the Company in connection with any sublicense of the technology under the GWU Agreement.
| 34 |
On the first anniversary of either the date on which the Company first sells a product or service utilizing the technology underlying the GWU Agreement or the date on which the Company enters into its first sublicense agreement, whichever occurs first (the “First Sale Date”), the Company is required to pay GWU a non-refundable minimum royalty payment equal to $5,000. GWU is entitled to the following non-refundable minimum royalty payments on each subsequent anniversary of the First Sale Date: 2nd Anniversary: $10,000; 3rd Anniversary: $15,000; 4th Anniversary and continuing annually through the expiration or termination of the GWU Agreement: $20,000.
The GWU Agreement expires upon the later of 20 years from the GWU Effective Date or the end of the term of the last underlying patent to expire. Currently, the LAESI patent (US 7,964,843) is the underlying patent and will expire on May 21, 2026. GWU may terminate the exclusivity of the license granted under the GWU Agreement or convert the license to a non-exclusive license in any national political jurisdiction if the Company, within 90 calendar days after receiving written notice from GWU of intended termination of exclusivity, fails to provide written evidence satisfactory to GWU that the Company or any of its sublicensees has commercialized or is actively attempting to commercialize a licensed product in such jurisdiction. Any time after 3 years from the GWU Effective Date, GWU may terminate the GWU Agreement in any national political jurisdiction if the Company, within 90 calendar days after receiving written notice from GWU of intended termination, fails to provide written evidence satisfactory to GWU that the Company or any of its sublicensees has commercialized or is actively attempting to commercialize a licensed invention in such jurisdiction. In addition, the GWU Agreement will terminate (a) automatically if the Company becomes bankrupt or insolvent and/or if the business of the Company is placed in the hands of a receiver, assignee, or trustee, whether by voluntary act of the Company or otherwise; or (b) upon 60 calendar days written notice from GWU if the Company breaches or defaults on its obligation to make payments or reports, unless before the end of the 60 day period the Company has cured the default or breach and so notifies GWU; or (c) upon 90 calendar days written notice if the Company breaches or defaults on any other obligation under the GWU Agreement, unless before the end of the 90 day period, the Company has, in GWU’s sole discretion, cured the default or breach and so notifies GWU; or (d) at any time by mutual written agreement between the Company and GWU, upon 180 calendar days written notice to all parties. The Company has made all the payments required under the GWU Agreement, and the Company is otherwise in full compliance with the terms of the GWU Agreement.
In November 2012, the Company entered into a Patent License Agreement with George Washington University (Washington D.C) for technology developed in the laboratory of Akos Vertes Ph.D. The technology field is Laser Desorption/Ionization and Peptide Sequencing on Laser-Induced Silicon Microcolumn Arrays (Matrix) and Nanophotonic Production, Modulation and Switching of Ions by Silicon Microcolumn Arrays.
Under the terms of the license agreement, the Company has an exclusive, worldwide license to make, have made, use, import, offer for sale and sell the licensed products. The Company is obligated to pay a license initiation fee of $25,000 and a minimum license diligence resources of $12,500 in year two and $20,000 each year thereafter to develop and commercialize the products, $30,000 milestone payment upon the first sale of a licensed product, and royalties will be 7% of the net sales of licensed products and 5% of the net sales of combination products for combined cumulative net sales up to $50 million. Thereafter, royalties reduce to 6% and 4%, respectively, after taking into account quarterly minimum royalties of $1,500 for the first four quarters after the first sale of a licensed product, $2,500 for the next four quarters, $3,000 for the next four quarters and $6,000 for each succeeding quarter. The Company has made all the payments required under the Patent License Agreement, and the Company is otherwise in full compliance with the terms of the Patent License Agreement.
| 35 |
Agreement with West Virginia University (WVU)
In September 2001, the Company entered into an Exclusive Option Agreement pursuant to which Protea was granted an exclusive option by West Virginia University Research Corporation, a nonprofit West Virginia corporation (“WVURC”) to license technology from the laboratories of certain West Virginia University (“WVU”) principal investigators in the field of protein discovery, for therapeutic, diagnostic and all other commercial fields worldwide. On December 21, 2005 (the “WVU Effective Date”), the Company entered into an Exclusive License Agreement (the “WVU Agreement”) with WVURC, acting for and on behalf of WVU. Under the terms of the WVU Agreement, the Company is required to pay (i) a license fee equal to $25,000 due within 90 days of the date on which the first notice of allowance is issued by the United States Patent and Trademark Office or a similar foreign country with respect to the technology underlying the WVU Agreement; (ii) annual royalties equal to 4% of the gross sales of products and services that utilize the WVU subject technology which are payable semi-annually within 30 days of June 30 and December 31 of each calendar year covering gross sales received during the preceding year; and (iii) expenses for the preparation, filing and prosecution of related patent applications. In the event the Company is required to license any intellectual property from third parties in order to practice or commercialize the technology underlying the WVU Agreement, the royalty payments will be reduced by the lesser of (a) 50% or (b) the royalty and licensing fees actually incurred by the Company to license the intellectual property rights from such third party. If such a reduction is applicable, WVURC is entitled to earned royalties of at least 2% on gross sales of products and services that utilize the WVU subject technology. For any sublicense granted to sub-licensees, WVURC is entitled to 10% of any license fee and other payments or fees received from the sublicensee, which is due and payable within ten days of receipt by the Company from the sublicensee.
The WVU Agreement automatically terminates upon the later of (1) the expiration of the last patent to expire issued in respect of the licensed technology, or (2) 20 years from the first commercial sale by the Company of the last licensed product included in the subject technology by amendment to the WVU Agreement. At present, there are no patent applications being pursued by WVU in respect of the technology licensed to the Company under the WVU Agreement. In addition, WVURC may cancel and terminate the WVU Agreement (1) 30 days following notice to the Company of an event of default or failure by the Company to perform, assuming such event of default has not been corrected during the 30 day period, or (2) immediately, in the event the Company (i) shall become involved in insolvency, dissolution, bankruptcy, or receivership proceedings affecting the operation of its business; (ii) make an assignment of all or substantially all of its assets for the benefit of creditors, or (iii) has a receiver or trustee appointed for it. The Company has made all the payments required under the WVU Agreement, and the Company is otherwise in full compliance with the terms of the WVU Agreement.
Agreement with Johns Hopkins University (JHU)
On June 9, 2009 (the “JHU Effective Date”), the Company entered into an Exclusive License Agreement with Johns Hopkins University (the “JHU Agreement”) for technology developed in the laboratory of Jennifer Van Eyk, Ph.D., Professor of Medicine, Division of Cardiology, Biological Chemistry and Biomedical Engineering. The technology field is albumin-bound protein complexes and their use in the diagnosis of cardiovascular disease. Under the terms of the JHU Agreement, the Company has the exclusive worldwide rights to commercialize this technology. The Company is obligated to reimburse JHU for all expenses for the preparation, filing and prosecution of related patent applications.
In addition, JHU is entitled to (i) a license fee of $75,000, which was paid on June 23, 2009, (ii) minimum annual royalties as follows: $5,000 for the first year; $10,000 for the second year; $20,000 for the third year; $30,000 for the fourth year; $40,000 for the fifth year; $50,000 for the sixth year and every year thereafter; (iii) quarterly royalties equal to 8% of the net sales and net service revenues for each licensed product or service and (iv) 50% of any payments received by the Company for sublicenses under the JHU Agreement. In addition, JHU is entitled to aggregate potential milestone payments of $325,000 as follows:
| · | $25,000 within 30 days of the issuance of the first U.S. patent underlying the subject technology; |
| 36 |
| · | $25,000 within 30 days of the first commercial sale of an albumin-bound protein/peptide complex-based research product; |
| · | $100,000 within 30 days of the submission of the first albumin-bound protein/peptide complex-based test for regulatory approval in any country; |
| · | $175,000 within 30 days of the first commercial sale of an albumin-bound protein/peptide complex-based approved diagnostic product. |
The JHU Agreement will expire on the expiration date of the last underlying patent to expire in any specific country or if no patents have been issued with respect to the technology licensed under the JHU Agreement, then for a term of 20 years from the JHU Effective Date. As of the date of this filing, no patent has been issued on the licensed technology. The JHU Agreement may also be terminated by either party, in the event that the other party (a) files or has filed against it a petition under the Bankruptcy Act, makes an assignment for the benefit of creditors, has a receiver approved for it or a substantial part of its assets, or otherwise takes advantage of any statute or law designed for relief of debtors or (b) fails to perform or otherwise breaches any of its obligation under the JHU Agreement, if, following the giving of notice by the terminating party of its intent to terminate and stating the grounds therefore, the party receiving such notice shall not have cured the failure or breach within 60 days. The Company may terminate the JHU Agreement for any reason upon giving JHU 90 days written notice.
Pharmaceutical Development Partnership Agreement with Laboratoires Mayoly Spindler
In May 2009, the Company, and its wholly-owned subsidiary ProteaBio Europe SAS, entered into a Joint Research and Development Agreement (the “Mayoly Agreement”) with Laboratoires Mayoly Spindler SAS (“Mayoly”), a pharmaceutical company with headquarters in Chatou, France, for the joint development of a recombinant lipase biopharmaceutical, MS1819. The Mayoly Agreement was subsequently amended and restated on March 22, 2010.
In December 2010, the Company and Mayoly successfully completed a first-in-man, Phase I/IIA clinical trial for the MS1819 recombinant lipase. The study was a randomized, double blind, placebo controlled, clinical trial with 12 patients who had been diagnosed with EPI resulting from chronic pancreatitis.
Each of the parties are responsible for using their best efforts to complete the Development Program which shall include, but not be limited to devoting sufficient, qualified personnel and resources. The Company is responsible for 40% of all required financing for the completion of the project. Under the terms of the Mayoly Agreement, the Company received the exclusive right to market, sell and distribute any human pharmaceutical medicine developed from lipase produced by the transformed yarrowia lipolytica strain (the “Product”) in the United States and Canada (the “Protea Assigned Territory”).
The Mayoly Agreement will continue in effect on a country-by-country and product-by-product basis until there is no remaining obligation for such product in such country.
RESEARCH AND DEVELOPMENT
The Company devotes most of its research and development (R&D) resources to advancing its core LAESI technology platform. As of the date of this filing, the initial research phase of the LAESI technology is complete and the Company has begun the marketing and distribution of the LAESI technology in 2013. New advances and applications for the LAESI technology platform are in development, including the integration of the LAESI-DP 1000 system with expanded mass spectrometer vendor coverage, utilization of high mass accuracy and ion mobility mass spectrometry, and capabilities in combination with LAESI-MSI work flows.
| 37 |
SALES AND MARKETING
The Company markets its products and services worldwide, utilizing a combination of field sales, distributors, in-house sales support and web-based marketing. The Company attends exhibitions in the U.S. and Europe to present its products, and in the last 12 months has exhibited at over 15 industry exhibitions. In Europe and Asia, the Company employs distributors who purchase the Company’s products and resell them to customers in their territory.
Two major business lines comprise the Company’s portfolio: instruments & consumables products and analytical services. The instruments & consumables products are serving the bioanalytical market and in particular laboratories that are using mass spectrometry as their core technology. The Company believes that its strong IP position allows offering of unique products aimed to improve the efficiency of the analytical laboratories in drug discovery and development. The Company believes that its flagship product, LAESI DP-1000 system, provides a chemical mapping of biological samples, greatly enhancing the research and development process of a drug.
A portfolio of services provided to the pharmaceutical and biomarker discovery markets composes the second business line. The Company leverages on its unique technologies to offer complete support and innovative analytical approaches to companies looking to study bio-dynamics of drugs, their metabolites, or specific molecules indicative of a diseased condition. Results of these services are used by pharmaceutical companies for the characterization of a drug, or by research institutes to advance their knowledge of a specific disease or condition.
Exhibit 24: Commercial Infrastructure

COMPETITIVE OVERVIEW
The Company believes that its technology differs substantially from what is currently on the market, bioanalytics is a major sector of the biotechnology industry, and competition is expected to be broad-based. However, the Company also believes that the industry affords opportunities for collaborative partnerships, and that industry participants or competitors can also be potential partners. The following list of competitors is not intended to be exhaustive, and there are other existing competitors and there likely will be new potential competitors in the future. Many competitors, including most of the companies identified below, are much larger than Protea in terms of capital, employees and other measures of size and have been in business longer than Protea and thus may have greater market acceptance or brand recognition.
These techniques enable mass spec imaging in their own particular manner resulting in each technique having unique characteristics including spot size resolution, mass range, limit of detection and dynamic range. As such, each technique has specific advantages, limitations and addressable applications. The chart below summarizes each of these points for each mass spec imaging technique.
| 38 |
Exhibit 25: Competitive Technology and Companies
| Technology | Companies | |||||||||||||
| LAESI | Protea | |||||||||||||
| MALDI | Agilent | Bruker | Danaher | JEOL | Shimadzu | ThermoFisher | Waters | |||||||
| DESI | Prosolia | |||||||||||||
| SIMS | Cameca | SAI | ||||||||||||
| DART | Ion Sense | |||||||||||||
| LESA | Advion | |||||||||||||
Source: The Company
MALDI – Matrix Assisted Laser Desorption Ionization
MALDI is an established mass spec imaging technology that is commercialized by a number of companies listed in Exhibit 25. MALDI was an integral technology development that leads to the integration of mass spec imaging for biological applications. According to the SDI 2012 Industry Report, it is estimated that the annual market for MALDI instrumentation is $US 300 million.
Exhibit 26: MALDI Annual Sales
| Technology | 2009 | 2010 | 2011 | 2014 | CAGR (2011-2014) | |||||
| MALDI | $256M | $275M | $294M | $356M | 6.8% |
Source: SDI 2012 Industry Report
Protea believes LAESI has significant competitive strengths even with respect to MALDI. Exhibit below gives the comparisons between MALDI and LAESI.
Exhibit 27: Comparison between LAESI and MALDI
| Technology | Mass Spectrometry With LAESI |
Mass Spectrometry with MALDI | ||
| Imaging Capability | ü | ü | ||
| No Sample Preparation | ü | û | ||
| Rapid Results | ü | û | ||
| Sample in Native Form | ü | û | ||
|
Vacuum Incompatible Samples (i.e. bacterial colony) |
ü | û | ||
| Ease of Use | ü | û |
Source: The Company
| 39 |
GOVERNMENT REGULATION
The Company’s products and instrument systems are sold for research use only and are not subject to FDA or other government agency approval.
SOURCES AND AVAILABILITY OF RAW MATERIALS
The Company does not believe that it has any critical issues of availability of raw materials or vendors where there are not multiple sources for the raw materials or vendor support that its business requires.
EQUIPMENT LEASE AGREEMENT
On March 12, 2007, Protea entered into equipment lease agreement, as amended on July 24, 2007 (the "Equipment Lease Agreement") with Monongalia County Development Authority ("Monongalia County") pursuant to which Monongalia County agreed to purchase certain equipment (the "Equipment") of Protea and to lease such equipment to Protea in accordance pursuant to the terms of the Equipment Lease Agreement. In accordance with the terms of the Equipment Lease Agreement, Protea agreed to lease the Equipment beginning on March 12, 2007 through April 1, 2015 at rate of 3% per month. In order to finance a portion of the purchase of the Equipment, Monongalia County entered into a loan agreement in an aggregate principal amount equal to $685,000 (the "Monongalia County Note"), with the West Virginia Infrastructure and Jobs Development Council (the "WVIJDC"), dated March 1, 2007. In connection with the Equipment Lease Agreement, Protea agreed to secure all amounts due and owing by Monongalia County to WVIJDC, pursuant to a Guaranty Agreement dated March 12, 2007. All amounts due under the Monongalia County Note have been paid to WVIJDC by Protea and have been recorded as a note payable on Protea's balance sheet. The Company is otherwise in compliance with the terms of Equipment Lease Agreement.
ENVIRONMENTAL MATTERS
We are subject to various laws and governmental regulations concerning environmental matters and employee safety and health in the United States and other countries. U.S. federal environmental legislation that affects us includes the Toxic Substances Control Act, the Resource Conservation and Recovery Act, the Clean Air Act, the Clean Water Act, the Safe Drinking Water Act, and the Comprehensive Environmental Response Compensation and Liability Act (CERCLA). We are also subject to regulation by the Occupational Safety and Health Administration (OSHA) concerning employee safety and health matters. The United States Environmental Protection Agency (EPA), OSHA, and other federal agencies have the authority to promulgate regulations that have an effect on Protea’s operations. As of the date of this filing, we did not have any accrued liabilities related to environmental matters.
EMPLOYEES
As of the date of this filing, the Company currently has 46 full-time employees, consisting of 27 technicians and scientists (8 PhD level), 8 management/administrative, 5 IT/software development, and 6 sales and marketing. There are no union employees.
FACILITIES
The Company currently leases laboratory and office space at 955 Hartman Run Road, Morgantown, West Virginia 26507. The space consists of approximately 11,000 square feet, leased from the Monongalia County Development Authority. The lease term expired on December 31, 2012 and is currently on a month-to-month status. For 2012, the base monthly rental amount was $14,407. The Company also paid a utility surcharge of $2,400 per month. In January 2013, the base monthly rental amount increased to $18,417.
| 40 |
The Company also leases an additional facility of approximately 10,412 square feet located at White Birch Towers II, 1311 Pineview Drive, Suite 501, Morgantown, WV 26505. The lease has an initial five-year term beginning on April 1, 2012, (the “Initial Term”). The rent during the Initial Term is equal to $16.10 per square foot per year or $13,969 per month. The Company has the exclusive option to renew the term of the lease for an additional five years following the expiration of the Initial Term, (the “Renewal Option”). The Renewal Option must be exercised at least 120 days prior to the end of the Initial Term. If the Renewal Option is exercised, the rent payable during the Renewal Period shall be equal to $17.75 per square foot per year. We have the option to terminate the lease after reaching the thirty-seventh month of the then existing term upon 90 days advance written notice to the lessor and the payment of an amount equal to two months’ rent.
LEGAL PROCEEDINGS
We are not currently a party to any legal proceedings.
| 41 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements and the notes to those statements included elsewhere in this prospectus. In addition to the historical financial information, the following discussion and analysis contains forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
We were originally incorporated under the name SRKP 5, Inc. in the State of Delaware on May 24, 2005 and were originally organized as a “blank check” shell company to investigate and acquire a target company or business seeking the perceived advantages of being a publicly-held corporation.
On September 2, 2011, we completed a merger in accordance with the terms and conditions of an Agreement and Plan of Merger by and among the Company, Protea Biosciences, Inc., a Delaware corporation ("PBI") and SRKP 5 Acquisition Corp., a Delaware corporation and our wholly-owned subsidiary ("Merger Co"), pursuant to which Merger Co merged with and into PBI (the "Merger"). As a result of the Merger, we (i) became the 100% parent of PBI, (ii) assumed the operations of PBI and (iii) changed our name to "Protea Biosciences Group, Inc."
Protea operates in the field of bioanalytics. Bioanalytics is the identification and characterization of proteins and other biomolecules, which are the products of all living cells and life forms. The Company is applying its technology to the development of a new generation of products and services that enable comprehensive, real-time analysis of living cells, thereby providing data that helps to define normal and disease processes. We believe this will, in turn, support the next generation of medical research and pharmaceutical development. Protea’s technologies seek to enable the discovery and analysis of the proteins and other biomolecules that regulate the biological functions of the human body and all other forms of life. This is a critical area of research, because most biological functions are carried out by proteins, and the discovery of proteins that are associated with specific diseases can lead to the development of new pharmaceuticals, designed to block the proteins’ aberrant biological activities. In 2012, the Company opened a dedicated Mass Spectrometry Imaging Center (MSIC) at their facility in Morgantown, WV. This MSIC offers access to advanced biomolecular imaging capabilities, including ultra-high resolution mass spectrometry coupled with Protea’s revolutionary LAESI platform.
Our Business Strategy and Products
The Company applies its core technologies and expertise to the development of products, instruments and services that seek to improve the discovery and identification of proteins, metabolites and other biomolecules. Our products and services are purchased and used primarily by pharmaceutical and academic/clinical research laboratories, including analytical chemists, translational researchers, oncologists, pathologists and cell biologists.
LAESI DP 1000 Instrument platform, software and consumables
In 2011, Protea completed the development of a novel bioanalytical instrument platform known as “LAESI” (laser ablation electrospray ionization). This technology enables the direct identification of proteins, lipids and metabolites in tissue, cells and biofluids, such as serum and urine, without any sample preparation prior to analysis. By eliminating sample preparation, the biological sample can be analyzed without the possible contamination, bias or sample loss that occurs with the current techniques, which require the introduction of chemicals, or the destruction of the sample itself, in order to enable analysis by mass spectrometry. LAESI then can display the data obtained by mass spectrometry analysis combined with actual images of the tissue and cell samples. Thus, mass spectrometry data can be evaluated in the context of the biology of the sample; this allows the integration of mass spectrometry data with current pathology and microscopic imaging techniques. Data is available in seconds to minutes, allowing rapid time to results and the capacity to analyze thousands of samples in a single work period.
| 42 |
As an example, a researcher testing a new drug’s effects on living cells can analyze changes in cells’ metabolism across a specific time course, thereby almost immediately obtaining data as to the activity of the new drug. The Company believes that LAESI technology has the potential to revolutionize bioanalytics in pharmaceutical research, as well as many other fields, including agriculture, pathology, biomarker discovery, biodefense and forensics.
LAESI is intended to meet the broad need of the biologist for the direct, unbiased identification and characterization of biomolecules in biological samples, which can remain untouched prior to their analysis. By virtue of LAESI’s improved speed and the comprehensive datasets it generates, the Company is pursuing its vision of what it believes will be a new era of human bioanalytics, where the molecular pathways of human disease will be more clearly elucidated, and datasets more rapidly available, thereby accelerating pharmaceutical and life science research.
The Company offers the LAESI DP-1000 instrument as well as software packages developed by the Company (LAESI desktop software and ProteaPlot™). Our software facilitates operating the instrument, and the storage and display of datasets, in a user friendly, intuitive software environment. The LAESI and ProteaPlot software include LAESI datamining and analytic tools to search for new, disease-specific biomarkers, and to elucidate disease-specific biomolecular pathways. In addition, the Company provides an expanding line of LAESI consumables.
Bioanalytical Consumable Products
This product group encompasses consumable products used in bioanalytical mass spectrometry, including a family of proprietary reagents, known as Progenta™ surfactants, that can rapidly remove proteins out of biological samples and into liquid phase, preparing them for analysis by mass spectrometry; single use products, including pipette tips and 96 well plates, that employ a novel chemistry technique, known as monolith chemistry, which enables recovery of proteins from solutions, as well as removal of salts, thereby improving the quality of analysis of the proteins by mass spectrometry; and, an extensive line of protein mass spectrometry standards, which provide defined and characterized proteins representing different protein structures and classes in order to improve the reproducibility of a researcher’s bioanalytical mass spectrometry results.
LAESI Services & Bioinformatics
Protea offers proprietary LAESI bioanalytical services for the rapid identification of both small molecules (e.g. lipids and metabolites) and large molecules (e.g. proteins). The services unit is operated under GLP (Good Laboratory Practices), which are necessary for regulatory submissions and to meet the internal research and development standards of pharmaceutical research clients.
We focus on the unique capability of LAESI technology to image and display the presence of specific biomolecules in cell and tissue samples. We believe that this field, called LAESI mass spectrometry imaging (“LAESI-MSI”), will become a major analytical method for biological investigation of tissues and living cell populations, such as cell cultures and colonies. LAESI-MSI provides new information based on molecule-specific images, without the need for labeling, staining or complex sample preparation protocols. Datasets are automatically generated, and are available in minutes, depending on the complexity of the analysis.
In LAESI-MSI, the chemical identity of biomarkers or other biomolecules present, for example in a tissue, is investigated as a function of spatial distribution. This paradigm allows accurate distribution profiling of chemical species that may help to understand pathologies or metabolic processes present in the specimen. LAESI-MSI has the potential to revolutionize biological investigation using objective chemical data by presenting the distribution of specific molecular structure in situ.
LAESI-MSI eliminates the need for sample preparation and allows the researcher to study the biochemical landscape of a sample as it exists in nature. LAESI-MSI services are available for 2D and 3D tissue biomolecular distribution profiling, high throughput biofluid analysis, biodynamic live cell colony monitoring, and biomaterial characterization.
| 43 |
We are applying LAESI-MSI to create cell-based biomolecular databases that will be specific to disease states and allow the analysis and integration of LAESI biomolecular datasets with the sample-related pathology, gene expression and demographic datasets. We are developing “high resolution” LAESI technology that will enable the analysis of single cells. We believe LAESI-MSI can generate important advances in bioinformatics to help provide time-based, biodynamic datasets for improved disease state assessment and management.
Emerging Growth Company
We are an “emerging growth company” under the federal securities laws and will be subject to reduced public company reporting requirements. In addition, Section 107 of the JumpStart Our Business Startups Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are choosing to take advantage of the extended transition period for complying with new or revised accounting standards.
Results of Operations
Fiscal Year Ended December 31, 2012 Compared to the Fiscal Year Ended December 31, 2011
We earned revenue of $834,250 for the fiscal year ended December 31, 2012 as compared to revenue of $713,028 for the fiscal year ended December 31, 2011, an increase of $121,222, or approximately 17%. The increase resulted mostly from growth in the Services area.
Selling, general and administrative expenses totaled $5,218,760 for the fiscal year ended December 31, 2012, compared to $5,245,985 for the fiscal year ended December 31, 2011, a decrease of $27,225 or approximately 1%. The decrease in selling, general and administrative resulted from management’s efforts to control costs.
Research and development expense totaled $4,595,830 for the fiscal year ended December 31, 2012, compared to research and development expense of $6,242,855 for the fiscal year ended December 31, 2011, a decrease of $1,647,025 or approximately 26%. Research and development expenses decreased as a result of a transition from development to production of the LAESI technology, reduced expenditures related to the recombinant lipase project, and other lesser items.
As a result of the decrease in both our selling, general and administrative expenses and our research and development expense, our loss from operations for the fiscal year ended December 31, 2012 was $8,980,340, compared to a loss from operations of $10,775,812 for the fiscal year ended December 31, 2011.
During the fiscal year ended December 31, 2012, we had other expense of $550,919 as compared to other expense for the fiscal year ended December 31, 2011 of $721,520. The decrease of $170,601 in other expenses largely resulted from the conversion of $8,455,000 of convertible debentures during 2011.
After foreign currency translation adjustments of $(21,222) and $18,617, respectively, we had a total comprehensive loss of $9,552,481, or $0.33 per share, for the fiscal year ended December 31, 2012 as compared to a total comprehensive loss of $11,478,715, or $0.55 per share, for the fiscal year ended December 31, 2011.
Three and Nine months Ended September 30, 2013 Compared to Three and Nine months Ended September 30, 2012
For the three months ended September 30, 2013 and September 30, 2012, we earned revenue of $178,475 and $165,538, respectively. This represents an increase of $12,937 or 8% from the prior period. We earned revenue of $938,080 and $568,994 for the nine month period ended September 30, 2013 and September 30, 2012, respectively, an increase of $369,086, or approximately 65%. During the first nine months of 2013, the Company sold four LAESI DP-1000 instruments. These sales represent the largest portion of the increase in revenue.
| 44 |
For the three months ended September 30, 2013 and September 30, 2012, selling, general and administrative expenses totaled $1,943,814 and $1,508,068, respectively. This represents an increase of $435,746 or 29% from prior year. Selling, general and administrative expenses totaled $6,015,077 for the nine months ended September 30, 2013 compared to $5,205,388 for the nine months ended September 30, 2012, an increase of $809,689 or approximately 16%. The increase in selling, general and administrative expenses relates largely to an increase in cost of goods and services sold, consulting fees and outside service expense and a reclassification to the quarter related to LAESI unit costs previously classified as research and development expense, which is partially off-set by a reduction in personnel costs. Cost of goods sold related to LAESI units include the cost to produce the unit, licensing costs, and accrued warranty expense.
For the three months ended September 30, 2013 and September 30, 2012, research and development expense totaled $658,880 and $722,352, respectively. This represents a decrease of $63,472 or 9% from prior year. Research and development expense totaled $2,367,014 for the nine months ended September 30, 2013, compared to research and development expense of $2,723,129 for the nine months ended September 30, 2012, a decrease of $356,115 or approximately 13%. Research and development expenses primarily include costs associated with the development of the LAESI technology. Research and development expenses have decreased as the Company has transitioned from development to production of the LAESI technology.
For the three months ended September 30, 2013 and September 30, 2012, loss from operations totaled $2,424,219 and $2,064,882, respectively. This represents an increase of $359,337 or 17% from prior period. Loss from operations totaled $7,444,011 for the nine months ended September 30, 2013, compared to a loss from operations of $7,359,523 for the nine months ended September 30, 2012, an increase of $84,488 or approximately 1%.
For the three months ended September 30, 2013 and September 30, 2012, other expense was $130,704 and $166,093, respectively. This is a decrease of $35,389 or 21% from the prior period. Other expense was $472,871 for the nine months ended September 30, 2013 compared to $398,581 for the nine months ended September 30, 2012. During 2012, the Company relied on related party debt to fund operations. As a result, other expense increased $74,290 or 19% for the nine months ended September 30, 2013 as compared to prior year due to the rise in related party debt.
After foreign currency translation adjustments of $5,797 and $2,136, respectively, total comprehensive loss was $2,549,126, or ($0.06) per share, for the three months ended September 30, 2013 as compared to a total comprehensive loss of $2,228,839, or ($0.08) per share, for the three months ended September 30, 2012. After foreign currency translation adjustments of $6,487 and ($19,944), respectively, total comprehensive loss was $7,910,395, or ($0.19) per share, for the nine months ended September 30, 2013 as compared to a total comprehensive loss of $7,778,048, or ($0.27) per share, for the nine months ended September 30, 2012.
Liquidity and Capital Resources
We have experienced negative cash flows from operations since inception. To date, our operations since inception have been funded primarily through proceeds received from the issuance of debt and sale of equity securities in private placement offerings.
Fiscal Year Ended December 31, 2012 Compared to the Fiscal Year Ended December 31, 2011
As of December 31, 2012, we had total current assets equal to $1,399,012 comprised of $27,604 in cash and cash equivalents, $436,655 in trade accounts receivable and other receivables, $905,186 in inventory and $29,567 in prepaid expenses. This compares with total current assets equal to $1,312,222 as of December 31, 2011 comprised of $505,277 in cash and cash equivalents, $49,979 in restricted cash, $392,205 in trade accounts receivable and other receivables, $223,336 in inventory and $141,425 in prepaid expenses. The Company's total current liabilities as of December 31, 2012 were equal to $10,032,836, comprised of $1,374,489 in current maturities on long term debt, $2,270,671 in trade accounts payable, $2,725,000 in connection with the United Bank line of credit, $2,898,216 in loans payable to shareholders and $764,460 in other payables and accrued expenses.
| 45 |
This compares with total current liabilities of $6,963,401 as of December 31, 2011 comprised of $398,383 in current maturities on long term debt, $2,504,094 in trade accounts payable, $3,000,000 in connection with the United Bank line of credit, $750,000 in loans payable to shareholders and $310,924 in other payables and accrued expenses.
Operating Activities
Net cash used in operating activities for the fiscal year ended December 31, 2012 increased $554,811, or 7%, to $8,521,997 as compared to $7,967,186 used during the fiscal year ended December 31, 2011. This consisted primarily of a net loss of approximately $9,531,259. Net loss was adjusted for non-cash items such as depreciation and amortization of $1,009,140, non-cash compensation of $345,662, accretion of convertible debenture discount of $53,294, bad debt expense of $30,032 and loss on disposal of fixed assets of $418. We also had an increase in trade accounts receivable of $81,103, an increase of inventory of $681,850, and an increase in other payables and accrued expenses of $453,536. Prepaid expenses decreased by $111,858 as well as a decrease in other receivables of $1,698 and a decrease in trade accounts payable of $233,423.
Our working capital deficit was $(8,633,824) at December 31, 2012 as compared to a working capital deficit of $(5,651,179) at December 31, 2011. The change in working capital of approximately $2,982,645 from December 31, 2011 to December 31, 2012 was primarily attributable to increased investment and activity into the LAESI project.
Investing Activities
Net cash used in investing activities during the fiscal year ended December 31, 2012 was $358,190, which was primarily used for deposits on equipment or equipment purchases. Net cash used in investing activities during the fiscal year ended December 31, 2011 was $1,241,966, which was primarily used for deposits on equipment or equipment purchases. We expect to continue to purchase property and equipment in the normal course of our business. The amount and timing of these purchases and the related cash outflows in future periods is difficult to predict and is dependent on a number of factors, including but not limited to any increase in the number of our employees and changes related to our development programs.
Financing Activities
Cash provided by financing activities during the year ended December 31, 2012 was $8,423,736, which was the result of the net proceeds of $5,738,521 from the sale of our common stock, proceeds of $1,090,000 from short and long-term debt, and proceeds from shareholder debt of $2,148,216. This was offset by $278,001 used in the repayment of long term debt and $275,000 used in the reduction on the bank line of credit. Cash provided by financing activities during the fiscal year ended December 31, 2011 was $9,285,116, which was the result of the net proceeds of $2,282,100 from the sale of our common stock and proceeds of $7,405,000 from long-term debt. This was offset by $401,984 used in the repayment of long-term debt.
Effect of Exchange Rate Changes on Cash
Exchange rate changes impact on cash during the year ended December 31, 2012 was $(21,222). For the year ended December 31, 2011, the impact was $18,617.
Nine months Ended September 30, 2013 Compared to Three and Nine months Ended September 30, 2012
We have a credit facility of $3,000,000 with United Bank, Inc. with a balance of $2,725,000 outstanding as of September 30, 2013. Interest is payable monthly and the loan is due on demand. In 2009 and 2010, we borrowed a total of $2,285,261 for the purchase of equipment from the West Virginia Economic Development Authority (WVEDA) and the West Virginia Water Development Authority. These loans have 10 year terms and interest rates ranging from 3.25% to 4%. In 2012, we borrowed a total of $400,000 for the purchase of equipment from the WVEDA and the West Virginia High Technology Consortium Foundation (WVHTC Foundation). The WVEDA loan has a 10 year term and an interest rate of 2%. The WVHTC Foundation loan has a 30-month term and an interest rate of 8%. Between 2011 and 2013, we borrowed $3,003,216 from various board members and spouses, of which all but $20,000 was converted into equity in July 2013.
| 46 |
The remaining note matures on December 31, 2013 and accrues simple interest at a rate of 10% per annum. In June and August 2013, we borrowed $600,000 from Summit Resources, Inc., an affiliate of one board member, under the Note and Warrant Purchase Agreement. The note matures one year from the anniversary date and accrues simple interest at a rate of 10% per annum. During the 3rd quarter of 2013, we borrowed $1,769,500 from various accredited investors. The convertible 1-year promissory notes bear simple interest at 10% per annum and are payable on the anniversary.
We will continue to require substantial funds to advance the research and development of our core technologies, to develop new products and services based upon our proprietary protein recovery and identification technologies and to continue development of our recombinant pharmaceutical compound. We intend to meet our operating cash flow requirements by raising additional funds from the sale of equity or issuance of debt and, possibly, pursuing partnerships to advance our drug and technology development activities. We may also consider the sale of certain assets, or entering into a transaction such as a merger with a business complimentary to ours. While we have been successful in raising funds to fund our operations since inception and we believe that we will be successful in obtaining the necessary financing to fund our operations going forward, we do not have any committed sources of funding and there are no assurances that we will be able to secure additional funding.
As of September 30, 2013, we had total current assets equal to $830,613, comprised of $41,039 in cash and cash equivalents, $66,596 in trade accounts receivable, $27,679 in other receivables, $591,404 in inventory and $103,895 in prepaid expenses. This compares with total current assets at December 31, 2012 equal to $1,399,012 comprised of $27,604 in cash and cash equivalents, $127,721 in trade accounts receivable, $308,934 in other receivables, $905,186 in inventory and $29,567 in prepaid expenses. The Company's total current liabilities as of September 30, 2013 were equal to $8,833,701, comprised of $3,037,991 in current maturities on long term debt, $1,662,314 in trade accounts payable, $2,725,000 in connection with the United Bank, Inc. line of credit, $620,000 in loans payable to stockholders $377,695 in the obligation related to the Letter of Credit and $410,701 in other payables and accrued expenses. The Company's total current liabilities as of December 31, 2012 were equal to $10,032,836, comprised of $1,374,489 in current maturities on long term debt, $2,270,671 in trade accounts payable, $2,725,000 in connection with United Bank, Inc. line of credit, $2,898,216 in loans payable to stockholders and $764,460 in other payables and accrued expenses.
Our working capital deficit at September 30, 2013 was $8,003,088 as compared to a working capital deficit of $8,633,824 at December 31, 2012. The decrease in working capital deficit was $630,736 from December 31, 2012 to September 30, 2013.
Operating Activities
Net cash used in operating activities for the nine months ended September 30, 2013 increased $581,238, or 9% from $6,688,148 for the nine months ended September 30, 2012 to $7,269,386. Net cash used in operating activities during the nine months ended September 30, 2013 was primarily the result of a net loss of $7,916,882. Net loss was adjusted for non-cash items such as depreciation and amortization of $633,535, non-cash compensation of $238,495, issuance of stock for services of $100,000, issuance of common stock for accrued interest of $228,384, accretion of convertible debenture discount of $17,208, bad debt expense of $5,000 and loss on disposal of fixed assets of $15,921. We also had a decrease in trade accounts receivable of $56,125, trade inventory of $313,782, in other receivables of $280,871, trade accounts payable of $813,738 and other payables and accrued expenses of $353,759. We also had an increase in prepaid assets of $74,328.
Investing Activities
Net cash used in investing activities during the nine months ended September 30, 2013 was $42,715, which was for equipment purchases. We expect to continue to purchase property and equipment in the normal course of our business. The amount and timing of these purchases and the related cash outflows in future periods is difficult to predict and is dependent on a number of factors including, but not limited to, any increase in the number of our employees and changes related to our development programs. Net cash used in investing activities during the nine month period ended September 30, 2012 was $340,645 and was also used for equipment purchases and partially offset by a movement in restricted cash.
| 47 |
Financing Activities
Cash provided by financing activities during the nine month period ended September 30, 2013 was $7,319,049, which was the result of net proceeds of $4,830,775 from the sale of our common stock, proceeds of $825,000 from the issuance of related party debt, proceeds of short term debt of $1,769,500, and proceeds of $600,000 from the Obligation related to the Letter of Credit. This was offset by $483,921 used in the repayment of long-term debt and $222,305 used in repayment of the Obligation related to Letter of Credit. Cash provided by financing activities during the nine month period ended September 30, 2012 was $6,599,805, which was the result of the net proceeds of $4,406,851 from the sale of our common stock, proceeds of $1,090,000 from the issuance of short and long-term debt and proceeds of $1,358,216 from the issuance of stockholder debt. This was offset by $255,262 used in the repayment of long-term debt.
Other than as discussed above, we know of no trends, events or uncertainties that are reasonably likely to impact our future liquidity.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet guarantees, interest rate swap transactions or foreign currency contracts. We do not engage in trading activities involving non-exchange traded contracts.
Critical Accounting Policies
The discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the U.S. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses for each period. The following represents a summary of our critical accounting policies, defined as those policies that we believe are the most important to the portrayal of our financial condition and results of operations and that require management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effects of matters that are inherently uncertain.
Revenue Recognition
We derive our revenue from the sale of products and services. Revenue is recognized when all four of the following criteria are met: (1) we have persuasive evidence that an arrangement exists, (2) the price is fixed and determinable, (3) title has passed, and (4) collection is reasonably assured. Product revenue is recognized when title passes, which is typically upon shipment of the product. Service revenue is recognized as the service is performed, generally through short-term contracts. However, where applicable, contract service revenue is earned and recognized according to the provisions of each agreement. As of September 30, 2013, we had deferred revenues of $2,500 compared to no deferred revenue as of September 30, 2012.
Shipping and handling costs are included in selling, general and administrative expenses. Shipping and handling costs charged to customers are recorded as revenue in the period the related product sales revenue is recognized.
Comprehensive Loss
The financial statements of our international subsidiary are translated into U.S. dollars using the exchange rate at each balance sheet date for assets and liabilities, the historical exchange rate for stockholders’ equity and an average exchange rate for each period of revenues, expenses, and gains and losses. The functional currency of our non-U.S. subsidiary is the local currency. Adjustments resulting from the translation of financial statements are reflected in accumulated other comprehensive income. Transactional gains and losses are recorded within operating results.
| 48 |
Research and Development
We follow the policy of charging the costs of research and development to expense as incurred. Research and development expense is $2,367,014 and $2,723,129, respectively, for the nine months ended September 30, 2013 and 2012.
Net Loss per Share
Basic and diluted loss per common share is computed based on the weighted average number of common shares outstanding. Common share equivalents (which may consist of options, warrants and convertible debt) are excluded from the computation of diluted loss per share since the effect would be anti-dilutive. Common share equivalents which could potentially dilute basic earnings per share in the future, and which were excluded from the computation of diluted loss per share, totaled approximately 34,958,000 and 18,697,000 for the nine months ended September 30, 2013 and year ended December 31, 2012, respectively.
Stock Based Compensation
We follow the provisions of FASB ASC 718, “Stock Based Compensation.” Stock-based compensation expense is estimated as of the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which generally represents the vesting period. Fair value of stock options issued by the Company is estimated using the Black-Scholes option-pricing model. The associated compensation expense is recognized on a straight-line basis over the requisite service period, net of estimated forfeitures.
Estimating the fair value for stock options for each grant requires judgment, including estimating stock-price volatility, expected term, expected dividends and risk-free interest rates. The expected volatility rates are estimated based on the volatility of similar entities whose share information is publicly available. The expected term represents the average time that options are expected to be outstanding and is estimated based on the average of the contractual term and the vesting period of the options as provided in SEC Staff Accounting Bulletin 110 as the “simplified” method. The risk-free interest rate for periods approximating the expected term of the options is based on the U.S. Treasury yield curve in effect at the time of grant. Expected dividends are zero as we have no plans to issue dividends.
| 49 |
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
The following table identifies our executive officers and directors, their ages, their respective offices and positions, and their respective dates of election or appointment.
| Name | Age | Position | Officer/Director
Since | |||||
| Stephen Turner | 68 | Chief Executive Officer and Chairman of the Board | 2001 | * | ||||
| Stanley Hostler | 86 | Vice President, Secretary and Director | 2006 | * | ||||
| Alessandro Baldi, Ph.D. | 57 | Vice President and General Manager | 2011 | |||||
| Edward Hughes | 60 | Chief Financial Officer | 2010 | * | ||||
| Matthew Powell | 39 | Chief Science Officer | 2006 | * | ||||
| Steven Antoline | 57 | Director | 2010 | * | ||||
| Leonard Harris | 77 | Director | 2003 | * | ||||
| Ed Roberson | 68 | Director | 2009 | * | ||||
| Scott Segal | 58 | Director | 2008 | * | ||||
| Roderick Jackson | 73 | Director | 2011 | * | ||||
| C. Andrew Zulauf | 50 | Director | 2012 | |||||
| Thijs Spoor | 41 | Director | 2013 | |||||
| Josiah T. Austin | 65 | Director | 2013 |
* - Represents the date on which such person was appointed to the referenced office of Protea Biosciences, Inc. Each such person was appointed to the identical position of the Company, effective September 2, 2011 upon the closing of the reverse merger.
There are no family relationships between any of our directors or executive officers. Our directors serve until the next annual meeting of stockholders and until their successors are elected by our stockholders, or until the earlier of their death, retirement, resignation or removal. Our executive officers are appointed by our board of directors and serve at the board’s discretion. Except as described below under “Certain Relationships and Related Transactions”, there is no arrangement or understanding between any of our directors or executive officers and any other person pursuant to which any director or officer was or is to be selected as a director or officer.
To the best of our knowledge, none of our directors or executive officers has, during the past ten years, been involved in any legal proceedings described in subparagraph (f) of Item 401 of Regulation S-K promulgated under the Securities Act of 1933, as amended.
| 50 |
Business Experience
Stephen Turner is Chief Executive Officer and Chairman of the Board, positions he has held since founding the company in July, 2001. From 1999 to 2001 he served as President and CEO of Quorum Sciences, Inc. From 1984 to 1997 he was President and CEO of Oncor, Inc. He founded Bethesda Research Laboratories, Inc. in 1975 and served as its Chairman and CEO from 1975 to 1983, at which time BRL became the molecular biology division of Life Technologies, Inc. Prior to commencing his career in biotechnology, Mr. Turner held the position of Director of Marketing for the Clinical Microbiology Division of Becton, Dickinson & Co. He received his B.A. from Stanford University in 1967. In 1994 he received the Ernst & Young Entrepreneur of the Year Award in Life Sciences for the Washington D.C. Region. Mr. Turner was appointed to serve as a director of the Company because he is the founder of Protea and his deep knowledge of our products and market opportunity led the board to determine that he should serve as a director.
Edward Hughes is Chief Financial Officer, and has served in this position since April 2010. Prior to this position he was CFO of Microbac Laboratories, Inc., an environmental and food testing company based in Pittsburgh, Pennsylvania from February, 2003 through March 2009. Prior to that, he was CFO of Silliker Group Corporation, a food testing company based in Greater Chicago. He is currently a Board member of the Pittsburgh Chapter of Financial Executives International. From 1987 to 1998 he was employed by Rhone Poulenc Rorer, where he was Manager, Financial Planning and Analysis (1987-88), Assistant Controller – Research and Development (1988-1991), Finance Director Asia/Pacific (1991-1996) and Corporate Finance Director (1997-1998).
Stanley Hostler is Vice President, Secretary and Director. He has been a Director of the Company since January 2006 and Vice President and Secretary since June 2006. Mr. Hostler is an attorney with a career practice in the field of labor and employment law. From 2000 to 2010 he served as Special Assistant to the Governor of the State of West Virginia. From 2002 to 2004 he served as Counsel to the Prim Law Firm. From 2000 to 2010 he served on the West Virginia University Foundation Board of Directors, and from 1995 to 2010 on the Advisory Committee of the WVU School of Medicine. He is a Graduate of the West Virginia University School of Law (1965). Mr. Hostler’s legal experience and business contacts and relationships with West Virginia University and the State of West Virginia have been an asset to the Company and led the board to determine that he should serve as a director of the Company.
Alessandro Baldi, Ph.D., is Vice President and General Manager and has served in this position since October 2011. Dr. Baldi has over 25 years of experience in separation sciences and mass spectrometry. Prior to joining Protea, Dr. Baldi served in various capacities for PerkinElmer, Inc., beginning in January 2001, as technology manager for the overall chromatography portfolio driving technology pipeline and leading a team of global support managers, application and technology scientists. In January, 2004, Dr. Baldi moved to a business manager position in liquid chromatography and then in January, 2006 to a business and marketing manager position for the entire chromatography and data systems portfolio responsible for providing strategic directions and program executions. He led a team of product managers and application scientists and coordinated communication and business activities on a global basis and drove a few acquisitions to expand the technology portfolio and create new business opportunities. As a result of these activities, in October of 2009, he then became Senior Business Director of Mass Spectrometry, leading the ramp up phase of the business within the organization providing strategic guidelines and organizing the business team on a global basis. Before joining PerkinElmer in 2001, Dr. Baldi was a part-time Professor at the University of Florence, Dept. of Pharmacy. He received his Ph.D. in Analytical Chemistry in 1995 from the University of Florence, Italy. Dr. Baldi has been active in the analytical industry for more than 20 years, as a researcher, consultant, teacher and manager, with operating experience in more than thirty countries.
Matthew Powell, Ph.D. is Protea’s Director, Research & Development and Chief Science Officer since 2006. He received his Ph.D. in Analytical Chemistry from West Virginia University in 2005. Dr. Powell is considered by the Company to be an expert in the field of biological mass spectrometry and is an inventor of several proprietary bioanalytical technologies in development at the Company.
| 51 |
Steven Antoline joined the Board of Directors in April 2010. He is a successful owner, developer and manager of coal and natural resource properties and inventor of new equipment for coal mining. From 1996 to 2006, he was President and owner of Superior Highwall Mining, Inc., which was sold to a partnership comprised of Lehman Bros. (60%) and Tennessee Valley Ventures (40%). From 2006-2010 Mr. Antoline was self-employed. Mr. Antoline was appointed to serve as a director of the Company because of his prior experience in the development and sale of companies, and in working with investment bankers.
Leonard Harris has been a member of the Board of Directors since April 2003. Since 1977 he is the founder and Chief Executive Officer of Southern Computer Consultants, Inc. located in Frederick, Maryland, a company which provides products and services to the United States government and Fortune 500 corporations. Mr. Harris' extensive experience in technology-based corporate development, which provides support and guidance to the Company’s LAESI instrument platform, led the board to determine that he should serve as a director of the Company.
Ed Roberson joined the Board of Directors in September 2009. From July 2006 to June 2010 he served as Chairman of the Board of the Methodist Healthcare System. He received his MBA in accounting in 1972 from the University of Georgia. From 2006-2011 he was President of Beacon Financial in Memphis, Tennessee, and from 2006-2007 President of Conwood LLC. He has been a Director of the Paragon National Bank from 2004 to present. From 1972 to 1992, Mr. Roberson was employed by KPMG, most recently as partner. Mr. Roberson’s experience, both as a Partner with KPMG and subsequently as a CEO, led the Board to determine that he should serve as a director of the Company.
Scott Segal joined the Board of Directors in February 2008. He is a practicing attorney, specializing in the fields of personal injury, product liability and related matters, and is the President of the Segal Law Firm, Charleston, West Virginia. He received his JD from the West Virginia University School of Law in 1981, and has been a member of the American Bar Association from 1981 to present. Mr. Segal has extensive relationships within the State of West Virginia and is considered by the Company to be an expert in several areas which may have use for the Company’s technology, including forensics and occupational health, which led the Board to determine that he should serve as a director of the Company.
Roderick Jackson joined the Board of Directors in January 2011. From 2005 to 2009 he was the founder, Chairman and Chief Executive Officer of Cobalt Laboratories, and from 2005 to 2009 a member of the Board of Directors of The Arrow Group, based in the United Kingdom. In June 2009 Cobalt Laboratories was sold, along with the Arrow Group, to Watson Pharmaceuticals. From 1986 to 2002 he was employed by Mylan Laboratories, Inc., first as Vice President Marketing and Sales (1986-1992) then as Senior Vice President and Member of the Office of the President (1992-2002). He received his B.B.A. from Texas A&M University. Mr. Jackson's experience in the development of marketing agreements both in the U.S. and internationally, which is beneficial to the Company as it seeks to market its products, led the board to determine that he should serve as a director of the Company.
C. Andrew Zulauf joined the Board of Directors in June 2012. He is Executive Director of the West Virginia Jobs Investment Trust (WVJIT) and has served in such capacity since March, 2009. WVJIT is a Charleston, West Virginia-based public venture capital firm created by the West Virginia Legislature in 1992 to promote new businesses in West Virginia. Previously, from 2006 to 2009, Mr. Zulauf was Vice President and Upper Middle Market Commercial Relationship Officer for Fifth Third Bank, headquartered in Cincinnati, Ohio. Mr. Zulauf was Partner and Managing Director of West Virginia Operations for Adena Ventures based in Athens, OH from 2002 to 2006, and Executive Director and Senior Loan Officer at the West Virginia Capital Corporation in Charleston, West Virginia from 1994 to 2002. Mr. Zulauf’s experience promoting new businesses in the area and his relationship with WVJIT, a stockholder and lender to the Company, led the Board to believe that Mr. Zulauf should serve as a director on the Board. Mr. Zulauf is a 1985 graduate of Marshall University and in 1994 received his MBA from the University of Charleston’s Executive MBA program.
| 52 |
Thijs Spoor joined the Board of Directors on January 28, 2013. Mr. Spoor has been the President, Chief Executive Officer and a member of the board of directors of Fluoropharma Medical, Inc. since February 14, 2011, and has been the Chairman of the board of directors of Fluoropharma Medical, Inc. since June 14, 2012. Mr. Spoor was the CFO for Sunstone BioSciences from February 2010 through September 2010. Prior to joining Sunstone BioSciences, he worked as a consultant at Oliver Wyman from December 2008 through February 2010, focusing on helping pharmaceutical and medical device companies evaluate their global revenue potential given the complex interplay of regulatory approvals, the reimbursement environment, as well as the impact of physician preference within constantly evolving standards of care. He further specialized on the implications of healthcare reform on new product approval and health insurance reform. Mr. Spoor was an equity research analyst at J.P. Morgan from July 2007 through October 2008 and Credit Suisse from November 2005 through July 2007, covering the biotechnology and medical device industries. Prior to his career on Wall Street, Mr. Spoor worked in the pharmaceutical industry spending 11 years with Amersham / GE Healthcare where he worked in 7 countries in a variety of roles including setting up GMP facilities, accountability for the nuclear cardiology portfolio and most recently as the Director of New Product Opportunities leading the PET strategic plan. Mr. Spoor holds a Nuclear Pharmacy degree from the University of Toronto as well as an M.B.A. from Columbia University with concentrations in finance and accounting. Mr. Spoor has been a guest lecturer at Columbia Business School, Kings College in London and the University of Newcastle in Australia. Mr. Spoor’s extensive experience and expertise in the bioscience industry led the Board to believe that Mr. Spoor should serve as a director on the Board.
Josiah T. Austin joined the Board of Directors on January 28, 2013 and has served as the managing member of El Coronado Holdings, L.L.C., a privately owned investment holding company which invests in public and private companies. He and his family own and operate agricultural properties in the states of Arizona, Montana, and northern Sonora, Mexico through El Coronado Ranch & Cattle Company, L.L.C. and other entities. Mr. Austin previously served on the Board of Directors of Monterey Bay Bancorp of Watsonville, California, and is a prior board member of New York Bancorp, Inc., and North Fork Bancorporation. He has served as a director of Goodrich Petroleum, Inc. since April 2002 and was named to the Board of Directors of Novogen Limited in September 2010. Mr. Austin also serves as a trustee of the Cuenca Los Ojos Foundation Trust, a non-profit organization working to preserve and restore the biodiversity of the borderland region between the United States and Mexico through land protection, habitat restoration and wildlife reintroduction. Mr. Austin graduated from the University of Denver with a Bachelor of Science in Finance in 1971.
| 53 |
EXECUTIVE COMPENSATION
Tabular Disclosure Regarding Executive Compensation
The following table illustrates the compensation paid by Protea to its Chief Executive Officer, its three most highly compensated executive officers other than the Chief Executive Officer who were serving as executive officers at the end of the last completed fiscal year and who earned in excess of $100,000, and up to one additional individual for whom disclosure would have been provided but for the fact that the individual was not serving as an executive officer of the Company at the end of the last completed fiscal year. We refer to these individuals as the “Named Executive Officers”. The disclosure is provided for the years ended December 31, 2012 and 2011.
SUMMARY COMPENSATION TABLE
| Name and Principal Position | Year | Salary ($) | Option Award (1) | Total ($) | ||||||||||
| Stephen Turner, Chief Executive Officer, President | 2012 | 240,000 | - | 240,000 | ||||||||||
| 2011 | 240,000 | - | 240,000 | |||||||||||
| Alessandro Baldi, Ph.D. Vice President, General Manger | 2012 | 288,551 | - | 288,551 | ||||||||||
| 2011 | 62,855 | 75,600 | 138,455 | |||||||||||
| Edward J. Hughes, Chief Financial Officer | 2012 | 145,600 | - | 145,600 | ||||||||||
| 2011 | 141,700 | - | 141,700 | |||||||||||
| Matthew Powell, Ph.D., Chief Science Officer | 2012 | 144,273 | - | 144,273 | ||||||||||
| 2011 | 138,640 | - | 138,640 | |||||||||||
(1) The amount included in the “Option Awards” column does not reflect compensation actually received by the Named Executive Officer but represents the compensation cost that we recognized in each year presented, determined in accordance with FASB ASC 718. The valuation assumptions used in determining such amounts are described in Note 8 of our financial statements for the fiscal year ended December 31, 2012.
We do not currently have employment agreements with our Named Executive Officers, although we may enter into such agreements in the future.
The following table provides information about equity awards granted to our Named Executive Officers that were outstanding on December 31, 2012.
| Number of Securities Underlying Unexercised Options | ||||||||||||||
| Name | Exercisable | Not Exercisable | Options Exercise Price ($) | Options Expiration Date | ||||||||||
| Stephen Turner | 100,000 | - | $ | 0.80 | 6/07/2016 | |||||||||
| 162,500 | (1) | 87,500 | $ | 1.50 | 4/23/2020 | |||||||||
| Alessandro Baldi, Ph.D. | 56,250 | (1) | 123,750 | $ | 1.50 | 10/01/2021 | ||||||||
| Edward J. Hughes | 49,500 | (1) | 40,500 | $ | 1.50 | 10/05/2020 | ||||||||
| Matthew Powell, Ph.D. | 80,000 | - | $ | 0.50 | 12/15/2015 | |||||||||
| 100,000 | - | $ | 1.25 | 1/19/2017 | ||||||||||
| 30,000 | (1) | 10,000 | $ | 1.50 | 12/31/2019 | |||||||||
| Stanley Hostler | 16,000 | - | $ | 1.50 | 12/31/2017 | |||||||||
| 48,000 | - | $ | 1.50 | 12/31/2018 | ||||||||||
| 48,000 | - | $ | 1.50 | 12/31/2019 | ||||||||||
| 48,000 | - | $ | 1.50 | 12/31/2020 | ||||||||||
| 36,000 | - | $ | 1.50 | 12/31/2021 | ||||||||||
| 12,000 | - | $ | 1.50 | 12/31/2021 | ||||||||||
| 44,000 | - | $ | 2.00 | 11/30/2022 | ||||||||||
| 4,000 | - | $ | 0.50 | 12/31/2022 | ||||||||||
| (1) | Options vest in 25% increments over a four-year vesting schedule. Grant dates are ten years prior to expiration date. |
| 54 |
Board Compensation
During the fiscal year ended December 31, 2012, our Board of Directors did not receive any compensation for their services as directors.
The following table provides information about equity awards granted to members of our Board of Directors that were outstanding on December 31, 2012, all of which were issued for services rendered prior to fiscal year 2012.
| Number of Securities Underlying Unexercised Options | ||||||||||||||
| Name | Exercisable | Not Exercisable | Options Exercise Price ($) | Options Expiration Date | ||||||||||
| Stanley Hostler | 100,000 | - | $ | 0.80 | 6/07/2016 | |||||||||
| 76,000 | (1) | 24,000 | $ | 1.50 | 9/17/2020 | |||||||||
| Steven Antoline | 88,000 | (1) | 12,000 | $ | 1.50 | 4/23/2020 | ||||||||
| Leo Harris | 100,000 | - | $ | 0.80 | 6/07/2016 | |||||||||
| 76,000 | (1) | 24,000 | $ | 1.50 | 9/17/2020 | |||||||||
| Ed Roberson | 88,000 | (1) | 12,000 | $ | 1.50 | 4/23/2020 | ||||||||
| Scott Segal | 100,000 | - | $ | 1.50 | 5/30/2018 | |||||||||
| Roderick Jackson | 71,000 | (1) | 29,000 | $ | 1.50 | 11/1/2020 | ||||||||
| C. Andrew Zulauf | - | - | - | - | ||||||||||
| Thijs Spoor | - | - | - | - | ||||||||||
| Josiah T. Austin | - | - | - | - | ||||||||||
| (1) | Options vest in 25% increments over a four-year vesting schedule. Grant dates are ten years prior to expiration date. |
Insider Participation in Meetings of Directors
Other than Mr. Turner, our Chief Executive Officer and President and Mr. Hostler, our Vice-President, all of our remaining directors are independent directors. All matters to be acted upon where potential conflicts exist between our executive officers and the Company (for example, the terms of employment agreements, option grants, bonus awards, etc.) are discussed and approved by the non-interested directors. During the year ended December 31, 2012, Mr. Turner did not participate in deliberations of the Company's Board of Directors concerning his compensation.
| 55 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Director Independence
Our determination of the independence of directors is made using the definition of “independent” contained in Rule 5605(a)(2) of NASDAQ. On the basis of information solicited from each director, the Board has determined that each of our directors with the exception of Mr. Turner and Mr. Hostler is independent within the meaning of such rule.
Described below are transactions or series of transactions that occurred from January 1, 2011 through the date of this report (the “Period Reported”) between us and our executive officers, directors or the beneficial owners of 5% or more of our common stock, and certain persons affiliated with or related to these persons, including family members, in which they had or will have a direct or indirect material interest in an amount that exceeds the lesser of $120,000 or 1% of the average of our total assets as of year-end for the last two completed fiscal years, other than compensation arrangements that are otherwise required to be described under “Executive Compensation.”
In March 2011, Summit Resources, Inc., an entity controlled by Steven Antoline, a director, paid the Company $250,000 in connection with a subscription for certain convertible debentures issued by the Company. The outstanding principal and interest on the debentures was converted into shares of Common Stock of the Company on September 2, 2011.
On December 20, 2011, we issued convertible promissory notes to Stanley Hostler, a director, and Summit Resources, Inc., an affiliate of Steven Antoline, a director, in an aggregate principal amount equal to $750,000. The notes accrue simple interest at a rate of 10% per annum and are due and payable on the earlier to occur of (i) the date that is 180 days from the issue date, or (ii) when declared due and payable by the holder upon the occurrence of an event of default. At the option of the holder, each $2.00 of outstanding principal amount and accrued unpaid interest is convertible into one share of common stock of the Company, at any time after the issue date.
In connection with the December 2011 Offering , during the period beginning on the date of the declaration of the effectiveness of a registration statement relating to a firm commitment underwritten offering covering the offer and sale of common stock for the account of the Company through the six month anniversary thereof, each of the officers and directors of the Company agreed not to, subject to certain exemptions, directly or indirectly, offer, sell, hedge, or pledge any option or contract to purchase, sell, grant any option, right or warrant to purchase or sell, or otherwise transfer or dispose of (or enter into any transaction or device that is designed to, or could be expected to, result in the disposition by any person at any time in the future), any securities of the Company beneficially owned by such person, pursuant to the terms and conditions of a lock-up agreement (the “Lock-up Agreement”) by and between the Company and each such officer and director.
On January 31, 2012, the Company issued 50,000 shares of common stock at $2.00 per share and warrants to purchase 25,000 additional shares of common stock for an aggregate purchase price of $100,000 to the West Virginia Investment Trust Board. The warrants are exercisable at an exercise price of $2.25 per share.
On April 16, 2012, the Company issued convertible promissory notes in an aggregate principal amount equal to $640,000 to Stanley Hostler, Scott Segal and Leonard Harris, each a director of the Company, Summit Resources, Inc., an affiliate of Steve Antoline, a director of the Company, Nancy Turner, the spouse of Stephen Turner, our Chief Executive Officer and a director of the Company and Virginia Child, the wife of Stanley Hostler. The notes accrue simple interest at a rate of 10% per annum and are due and payable on the earlier to occur of (i) the date that is 90 days from the issue date, or (ii) when declared due and payable by the holder upon the occurrence of an event of default. At the option of the holder, each $2.00 of outstanding principal amount and accrued unpaid interest is convertible into one share of common stock of the Company, at any time after the issue date. On March 22, 2013, the Company’s board approved the adjustment of the conversion price of these notes from $2.00 to $0.50 per share. In accordance with the terms and conditions of certain Conversion Agreements, as of June 30, 2013 (the “Conversion Agreements”) the $640,000 in principal and accrued unpaid interest were converted into shares of Common Stock of the Company issued at a rate of $0.50 per share. In addition, pursuant to the terms of the Conversion Agreements the Company issued a 5-year warrant to each of the note holders to purchase 75% of the number of shares into which the notes were convertible at an exercise price of $1.10 per share.
| 56 |
On July 27, 2012, the Company issued 75,000 shares of common stock at $2.00 per share and warrants of 37,500 to purchase additional shares of common stock for an aggregate purchase price of $150,000 to Stanley Hostler and Virginia Child, the wife of Stanley Hostler. The warrants are exercisable at an exercise price of $2.25 per share.
On September 25, 2012, the Company issued convertible promissory notes in an aggregate principal amount equal to $593,216 to Stanley Hostler, Scott Segal, Leonard Harris, Ed Roberson, each a director of the Company, Summit Resources, Inc., an affiliate of Steve Antoline, a director of the Company, Steven Turner, the Chief Executive Officer and a director of the Company, and his wife Nancy Turner and Virginia Child, the wife of Stanley Hostler in consideration for certain advances to the Company equal to the principal amount. The notes accrue simple interest at a rate of 10% per annum and are due and payable on the earlier to occur of (i) the date that is 60 days from the issue date, or (ii) when declared due and payable by the holder upon the occurrence of an event of default.
At the option of the holder, each $2.00 of outstanding principal amount and accrued unpaid interest is convertible into one share of common stock of the Company, at any time after the issue date. On March 22, 2013, the Company’s board approved the adjustment of the conversion price of these notes from $2.00 to $0.50 per share. In accordance with the terms and conditions of the Conversion Agreements, as of June 30, 2013, the $593,216 in principal and accrued unpaid interest were converted into shares of Common Stock of the Company issued at a rate of $0.50 per share. In addition, pursuant to the terms of the Conversion Agreements the Company issued a 5- year warrant to each the note holder to purchase 75% of the number of shares into which the notes were convertible at an exercise price of $1.10 per share.
On October 19, 2012, the Company issued 100,000 shares of common stock to the Milan Puskar Revocable Trust Restated 9/28/11 in connection with the exercise of outstanding options at an exercise price $1.25 per share.
On October 19, 2012, the Company issued 100,000 shares of common stock to the Milan Puskar Revocable Trust Restated 9/28/11 in connection with the exercise of outstanding options at an exercise price $1.50 per share.
On November 20, 2012, the Company entered into a Securities Purchase Agreement (the “El Coronado Purchase Agreement”) with El Coronado Holdings, LLC (“El Coronado”) an entity of which Josiah Austin, who subsequently became a director, is managing member, pursuant to which El Coronado was granted an option to purchase through December 31, 2012, up to 6,000,000 shares of common stock and warrants to purchase up to 4,500,000 shares of common stock (the “2012 El Coronado Warrants”) (at a purchase price of $0.50 per share) for an aggregate purchase price equal to $3,000,000. On November 20, 2012, the Company issued 600,000 shares of common stock and warrants to purchase 450,000 shares of common stock for aggregate gross proceeds of $300,000 to El Coronado, pursuant to the terms and conditions of the El Coronado Purchase Agreement. The 2012 El Coronado Warrants are exercisable at an exercise price of $1.10 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof. On November 29, 2012, pursuant to the terms of the El Coronado Purchase Agreement, the Company issued an aggregate of 400,000 shares of common stock and 2012 El Coronado Warrants to purchase 300,000 shares of common stock for aggregate gross proceeds of $200,000 to El Coronado. On December 10, 2012, pursuant to the terms of the El Coronado Purchase Agreement, the Company issued an aggregate of 1,000,000 shares of common stock and 2012 El Coronado Warrants to purchase 750,000 shares of common stock for aggregate gross proceeds of $500,000 to El Coronado.
| 57 |
On November 30, 2012, the Company issued convertible promissory notes in an aggregate principal amount equal to $915,000 to Stanley Hostler and Leonard Harris, each a director of the Company; Summit Resources, Inc., an affiliate of Steve Antoline, a director of the Company; Virginia Child, the wife of Stanley Hostler; Carl Hostler; and Brian Prim, in consideration for certain advances to the Company equal to the principal amount. The notes accrue simple interest at a rate of 10% per annum and are due and payable on the earlier to occur of (i) January 20, 2013, or (ii) when declared due and payable by the holder upon the occurrence of an event of default. The occurrence of any one of the following events will be deemed an event of default: (i) the failure of the Company to pay the principal balance or accrued interest on the Note when due; (ii) the consent or institution by or against the Company of bankruptcy or insolvency proceedings or the filing, or consent by the Company for the filing of a petition or answer or consent seeking reorganization or release under the federal Bankruptcy Act, or any other applicable federal or state law, or the appointment of a receiver, liquidator, assignee, trustee or other similar official of the Company, or of any substantial part of its property, or the making by it of an assignment for the benefit of creditors, or the taking of corporate action by the Company in furtherance of any such action; or (iii) if the commencement of an action against the Company seeking any bankruptcy, insolvency, reorganization, liquidation, dissolution or similar relief has not been resolved in favor of the Company or the consent or acquiescence of the Company of any trustee, receiver or liquidator of the Company or of all or any substantial part of the properties of the Company, shall not have been vacated within 60 days. At the option of the holder, each $2.00 of outstanding principal and accrued unpaid interest is convertible into one share of common stock of the Company at any time after the issue date. On March 22, 2013, the Company’s board approved the adjustment of the conversion price of these notes from $2.00 to $0.50 per share. In accordance with the terms and conditions of the Conversion Agreements, as of June 30, 2013, the $915,000 in principal and accrued unpaid interest were converted into shares of Common Stock of the Company issued at a rate of $0.50 per share. In addition, pursuant to the terms of the Conversion Agreements the Company issued a warrant to each of the note holders to purchase 75% of the number of shares into which the notes were convertible.
On January 3, 2013, the Company issued to El Coronado for an aggregate purchase price equal to $125,000 (1) a convertible promissory note in an aggregate principal amount equal to $125,000 and (2) a warrant to purchase 187,500 shares of common stock of the Company. The note accrues simple interest at a rate of 10% per annum and is due and payable on the earlier to occur of (i) April 1, 2013, or (ii) when declared due and payable by the holder upon the occurrence of an event of default. The warrant is exercisable at an exercise price of $1.10 per share any time after the Issue Date until the earlier of (i) a Qualified Public Offering (as such term is defined in the warrant) or (ii) 5:00 p.m. EST on the fifth anniversary of the issue date. In accordance with the terms and conditions of the Conversion Agreement, as of June 30, 2013, the $125,000 in principal and accrued unpaid interest was converted into shares of Common Stock of the Company issued at a rate of $0.50 per share. In addition, pursuant to the terms of the Conversion Agreement the Company issued a 5 year warrant to the note holder to purchase 75% of the number of shares into which the notes were convertible at an exercise price of $1.10 per share.
On January 11, 2013, the Company received an advance equal to an aggregate of $100,000 from Summit Resources, Inc. (“Summit”), an affiliate of Steve Antoline, a director of the Company. No terms of repayment have been specified on the aforementioned advance as of the filing date. This amount was subsequently converted into equity on July 23, 2013.
On March 6, 2013, the Company entered into that certain Warrant Purchase and Reimbursement Agreement, dated March 6, 2013 with Steve Antoline, a director of the Company, pursuant to which Mr. Antoline agreed to issue a letter of credit for the benefit of MPR Associates, Inc. for the purpose of guaranteeing an amount equal to $600,000 due by the Company to MPR in connection with the production of certain LAESI instruments in exchange for (i) the agreement by the Company to reimburse Mr. Antoline for any amounts paid by him pursuant to the Letter of Credit, up to the Purchase Order Amount and (ii) a warrant to purchase up to 1,100,000 shares of the Company’s common stock at an exercise price of $1.10 per share. The warrant was issued to Summit.
| 58 |
On March 21, 2013, the Company entered into a Securities Purchase Agreement (the “March Purchase Agreement”) with El Coronado pursuant to which El Coronado was granted an option to purchase through May 31, 2013, up to 6,000,000 shares of common stock and warrants to purchase up to 4,500,000 shares of common stock (the at a purchase price of $0.50 per share for an aggregate purchase price equal to $3,000,000. On March 21, 2013, the Company issued 1,360,000 shares of common stock and warrants to purchase 1,020,000 shares of common stock for aggregate gross proceeds of $685,000 to El Coronado, pursuant to the terms and conditions of the March Purchase Agreement. The warrants are exercisable at an exercise price of $1.10 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof.
On December 20, 2011, the Company issued convertible promissory notes (the “December 2011 Notes”) to Stanley Hostler and Summit (collectively, the “December 2011 Noteholders”), in an aggregate principal amount equal to $750,000. On April 16, 2012, the Company issued convertible promissory notes (the “April 2012 Notes”) to Hostler, Summit, Scott Segal, Virginia Child, Nancy Turner and Leo Harris (collectively, the “April 2012 Noteholders”), in an aggregate principal amount equal to $640,000. On September 25, 2012, the Company issued convertible promissory notes (the “September 2012 Notes”) to Hostler, Segal, Harris, Ed Roberson, Summit, Steven and Nancy Turner, and Child (collectively, the “September 2012 Noteholders”), in an aggregate principal amount equal to $593,216. On November 30, 2012, the Company issued convertible promissory notes (the “November 2012 Notes,” together with the “December 2011 Notes,” the “April 2012 Notes,” and the “September 2012 Notes” collectively, the “Related Party Notes”) to Hostler, Harris, Summit, Child, Carl Hostler and Brian Prim (the “November 2012 Noteholders” together with the “December 2011 Noteholders,” the “April 2012 Noteholders,” and the “September 2012 Noteholders” collectively the “Related Party Noteholders”) in an aggregate principal amount equal to $915,000. Each Related Party Note was previously amended to extend the original maturity date set forth in such Related Party Note. On March 22, 2013, the Company and each of the Related Party Noteholders amended each of the Related Party Notes to further extend the maturity dates to May 31, 2013. In exchange for the agreement by the Related Party Noteholders to extend the maturity date, the Company agreed to reduce the conversion price set forth in such Related Party Notes from $2.00 to $0.50 per share.
On April 5, 2013, the Company, issued and sold an aggregate of 280,000 shares (the "El Coronado Shares") of the Company’s common stock and a warrant (as defined below) to purchase 210,000 shares of common stock for aggregate gross proceeds of $140,000 to El Coronado in accordance with the terms and conditions of that certain Securities Purchase Agreement, dated April 5, 2013 (the "El Coronado SPA"). The warrant (the “El Coronado Warrant”) is exercisable for a term of five years from the issue date of the El Coronado Warrant, at an exercise price of $1.10 per share. The Company paid cash commissions equal to $11,200 in connection with the sale of the El Coronado Shares and the El Coronado Warrant.
On May 1, 2013, the Company and El Coronado extended the maturity date of the January 3, 2013 convertible promissory note an additional 60 days to May 31, 2013.
As of June 30, 2013, the Company entered into conversion agreements (the "Conversion Agreements") with the Related Party Noteholders who held an aggregate principal amount of $3,003,216 in Related Party Notes (the "Existing Notes") pursuant to which the Company agreed to issue 5 year warrants (the “Conversion Warrants”) to purchase up to 75% of the number of shares of common stock into which the Existing Notes were convertible, at an exercise price of $1.10 per share, provided that the conversion of the Existing Notes was exercised on or prior to June 30, 2013. In accordance with the terms and conditions of the Conversion Agreements, on June 30, 2013 the Related Party Noteholders notified the Company of their desire to convert the Existing Notes into an aggregate of 6,663,199 shares (the “Conversion Shares”). On July 23, 2013 and July 29, 2013, the Company issued the Conversion Shares and Conversion Warrants to purchase up to an aggregate of 4,997,400 shares of common stock.
On July 29, 2013, the Company issued a promissory note for $500,000 and a warrant to purchase up to 375,000 shares of the Company’s common stock to El Coronado and entered into a Note and Warrant Purchase Agreement by and between the Company and El Coronado.
| 59 |
On August 6, 2013, the Company and Steven and Nancy Turner extended the maturity date of the September 25, 2012 convertible promissory note to December 31, 2013.
On August 6, 2013, pursuant to the terms and conditions of a Note and Warrant Purchase Agreement, with Summit Resources the Company issued a promissory note in an aggregate principal amount of $600,000 to Summit. As of the issue date of the note, an aggregate of $600,000 was advanced to the Company against the note. In addition to the note, the Company agreed to issue warrants to purchase up to 250,000 shares of Common Stock of the Company at an exercise price of $1.10 per share, for each $150,000 borrowed against the note. As of the issue date of the note the Company issued warrants to purchase 1,000,000 shares of Common Stock to Summit.
On September 11, 2013, the Company issued a promissory note for $315,000 and a warrant to purchase up to 236,250 shares of the Company’s common stock to El Coronado and, entered into a Note and Warrant Purchase Agreement by and between the Company and El Coronado.
On September 20, 2013, the Company issued a promissory note for $300,000 and a warrant to purchase up to 225,000 shares of the Company’s common stock to El Coronado and, entered into a Note and Warrant Purchase Agreement by and between the Company and El Coronado.
On October 7, 2013, the Company issued a promissory note for $125,000 and a warrant to purchase up to 93,750 shares of the Company’s common stock to Summit and, entered into a Note and Warrant Purchase Agreement by and between the Company and Summit.
On October 25, 2013, the Company issued a promissory note for $125,000 to El Coronado and, entered into a Note and Warrant Purchase Agreement by and between the Company and El Coronado.
On October 25, 2013, the Company issued a promissory note for $150,000 to Summit. and, entered into a Note and Warrant Purchase Agreement by and between the Company and Summit Resources, Inc.
As of December 31, 2013, the “Company had received $5,448,973 in aggregate gross cash proceeds from 131 accredited investors in connection with the sale of approximately 55 units (each a “Winter 2013 Unit” and collectively, the “Winter 2013 Units”) in an offering (the “Winter 2013 Offering”) of a minimum of $2,000,000 and up to a maximum of $6,000,000 (the “Maximum Offering Amount”), in Units of securities of the Company pursuant to the terms and conditions of a Subscription Agreement and Unit Purchase Agreement by and among the Company and each of the purchasers thereto. The Maximum Offering Amount excludes up to an additional $2,000,000 in Winter 2013 Units that may be sold to any existing stockholders, officers, and directors of the Company, including any of their affiliates (the “Company Investors”). Each Winter 2013 Unit consists of (i) 200,000 shares of common stock, par value $.0001 per share, (ii) and two warrants, including (a) a 1 year warrant to purchase 200,000 shares of common stock at an exercise price of $0.50 per share (the “A Warrants”) and (b) a 5 year warrant to purchase 100,000 shares of common stock at an exercise price of $0.75 per share (the “B Warrants” and together with the A Warrants the “Investor Warrants”). In addition, the Company issued an aggregate of approximately 24.5 in additional Units to the holders of bridge notes, in connection with the conversion of $2,269,500 (the “Conversion Amount”) in outstanding principal and accrued unpaid interest in bridge notes.
The Company also paid to the placement agent (the “Winter 2013 Placement Agent”) an aggregate of $733,410.67 in cash commissions, representing (1) 10% of the gross proceeds raised in the Winter 2013Offering from purchasers introduced to the Company by the Winter 2013 Placement Agent; (2) 2% of the aggregate gross proceeds raised in the Winter 2013 Offering from Company Investors, including the Conversion Amount and (3) 2% of the aggregate gross proceeds raised in the Winter 2013 Offering, including the Conversion Amount, in connection with a non-accountable expense allowance. In addition, the Company agreed to issue warrants (the “Winter 2013 Placement Agent Warrants”) to purchase an aggregate of 3,302,826 shares of common stock underlying the Winter 2013 Units sold in the Winter 2013 Offering, including the shares of common stock underlying the Investor Warrants and (ii) 5% of the number of shares of common stock underlying the Winter 2013 Units issued in connection with the conversion of the bridge notes, including the shares of common stock underlying the Investor Warrants issued to bridge note holders.
| 60 |
In accordance with the terms of the Unit Purchase Agreement, until the one year anniversary of the closing date, purchasers shall have the right to participate in any subsequent offering by the Company of common stock or Common Stock Equivalents (as such terms is defined in the Unit Purchase Agreement) in which the Company raises gross proceeds of at least $1,000,000 (each a “Subsequent Offering”), on the same terms, conditions and price as provided for in the Subsequent Offering, in up to an amount of the Subsequent Offering equal to each purchaser’s proportionate share of the Subsequent Offering based on such purchaser’s participation in this Winter 2013 Offering. In addition, from the earlier of (1) five years from the closing Date or (2) the date on which any purchaser no longer owns shares of common stock sold in the Winter 2013 Offering, if the Company issues or sells any shares of common stock or any Common Stock Equivalent pursuant to which shares may be acquired at a price less than $0.50 per share (the “Base Price”) (subject to appropriate adjustments for any stock dividend, stock split, stock combination, reclassification or similar transaction) (each a “Dilutive Issuance”), the Company shall be required to issue additional shares of common stock to each purchaser, for no additional consideration, in an amount sufficient such that the pro rata portion of the Purchase Price paid by such purchaser for the shares of common stock then held, when divided by the total number of shares of common stock then held by such purchaser plus those shares of common stock issued as a result of the Dilutive Issuance will equal the Base Price.
| 61 |
SHARES ELIGIBLE FOR FUTURE SALE
There is not currently any market for our common stock. We cannot predict the effect, if any, that market sales of shares of our common stock or the availability of shares of our common stock for sale will have on the market price of our common stock prevailing from time to time. Sales of substantial amounts of our common stock, including shares issued upon exercise of outstanding warrants, in the public market after this offering, could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through the sale of our equity securities.
Resale Shares
All of the Resale Shares will be freely tradable, except that any shares acquired by our affiliates, as that term is defined in Rule 144 under the Securities Act, may only be sold in compliance with the limitations described below. As of January 10, 2014, our directors and executive officers held a total of approximately 26,050,151 shares or approximately 36% of the common stock outstanding as of January 10, 2014. The 47,931,538 shares of our outstanding common stock that are not registered and covered by this prospectus will be deemed restricted securities as defined under Rule 144. Sale limitations under Rule 144 for affiliates include the requirement for current public information about the Company; selling the shares pursuant to broker transactions; and limitations on the number of shares sold within a three-month period. Restricted shares may be sold in the public market only if registered or if they qualify for an exemption from registration promulgated under the Securities Act. Subject to the provisions of Rule 144, all of the outstanding shares of common stock that are currently restricted will be available for sale in the public market over the next six months under Rule 144.
In general, under Rule 144 as currently in effect, a person, or group of persons whose shares are required to be aggregated, who is deemed to have been an affiliate at any time during the three months preceding a sale, who has beneficially owned shares that are restricted securities as defined in Rule 144 for at least six months is entitled to sell, within any three-month period commencing 90 days after the date of this prospectus, a number of shares that does not exceed 1% of the then outstanding shares of our common stock, which will be approximately 654,428 shares.
In addition, a person who is not deemed to have been one of our affiliates at any time during the 90 days preceding a sale and who has beneficially owned shares of our common stock for at least six months, including the holding period of any prior owner, except if the prior owner was one of our affiliates, would be entitled to sell all of their shares, provided the availability of current public information about our company. To the extent that shares were acquired from one of our affiliates, a person's holding period for the purpose of effecting a sale under Rule 144 would commence on the date the shares were acquired from the affiliate.
After the date of this prospectus an aggregate of 43,210,443 shares (including shares underlying warrants) will have been registered and will be freely distributable and tradable by the Selling Security Holders. These shares consist of (i) 17,511,197 shares of common stock and (b) 25,699,246 shares of common stock underlying warrants. We will not receive any proceeds in connection with the sales, if any, of the Resale Shares.
| 62 |
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
The Company’s common stock is not quoted or listed on any quotation system or stock exchange. As of the date of this filing, there are 54,752,952 shares of our common stock subject to outstanding warrants.
Holders of Common Stock
As of January 10, 2014, we had 65,442,735 shares of common stock issued and outstanding and approximately 454 stockholders of record.
Dividends
We have not declared any cash dividends since inception and we do not anticipate paying any dividends in the foreseeable future. The payment of dividends is within the discretion of the board of directors and will depend on our earnings, capital requirements, financial condition, and other relevant factors. There are no restrictions that currently limit our ability to pay dividends on our common stock other than those generally imposed by applicable state law.
Securities Authorized for Issuance under Equity Compensation Plans
In 2002, the Board of Directors of PBI adopted the 2002 Equity Incentive Plan (the "Plan") that governs equity awards to employees, directors and consultants of the Company. There were 4,150,000 shares of common stock reserved for issuance under the Plan. Following the Reverse Merger, and in accordance with the Plan, the Company's Board of Directors approved the substitution of the shares of PBI's common stock underlying the options granted under the Plan with shares of Common Stock of the Company, subject to any further approvals or actions as may be required to ensure that the implementation of the substitution is in accordance with all state and federal rules and regulations that may be applicable.
The Plan had a term of ten years and expired in July 2012. The types of awards permitted under the Plan include qualified incentive stock options and non-qualified stock options, and restricted stock. Each option shall be exercisable at such times and subject to such terms and conditions as the Board may specify. Stock options generally vest over four years and expire no later than ten years from the date of grant. As of the date of the period covered by this report, options to purchase up to 3,864,750 shares of common stock have been granted of which 2,992,417 are vested.
On February 8, 2013, the Board of Directors of the Company adopted the 2013 Equity Incentive Plan, (the “2013 Plan”), which governs equity awards to employees, directors and consultants of the Company. The 2013 Plan has a term of ten years and permits the grant of qualified incentive stock options, non-qualified stock options, restricted stock awards as well as performance based cash compensation awards. Under the 2013 Equity Incentive Plan, an additional 5,000,000 shares of common stock are reserved for issuance.
The following table summarizes information about stock options at December 31, 2012:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Exercise Price | Outstanding | Weighted Average Remaining contractual life (in years) | Weighted Average Exercise Price | Exercisable | Weighted Average Exercise Price | |||||||||||||||
| $0.50 | 84,000 | 84,000 | ||||||||||||||||||
| $0.80 | 320,000 | 320,000 | ||||||||||||||||||
| $1.25 | 410,000 | 410,000 | ||||||||||||||||||
| $1.50 | 2,836,000 | 2,033,250 | ||||||||||||||||||
| $2.00 | 214,750 | 145,167 | ||||||||||||||||||
| $0.50 - $2.00 | 3,864,750 | 4.00 | $ | 1.42 | 2,992,417 | $ | 1.39 | |||||||||||||
| 63 |
SELLING SECURITY HOLDERS
The following table sets forth the names of the Selling Security Holders who may sell their Resale Shares under this prospectus from time to time. No Selling Security Holder has, or within the past three years has had, any position, office or other material relationship with us or any of our predecessors or affiliates other than as a result of the ownership of our securities.
The following table also provides certain information with respect to the Selling Security Holders’ ownership of our securities as of January 10, 2014, the total number of Resale Shares they may sell under this prospectus from time to time, and the number of securities they will own thereafter assuming no other acquisitions or dispositions of our securities. The Selling Security Holders can offer all, some or none of their Resale Shares, thus we have no way of determining the number they will hold after this offering. Therefore, we have prepared the table below on the assumption that the Selling Security Holders will sell all of the Resale Shares covered by this prospectus.
Some of the Selling Security Holders may distribute their shares, from time to time, to their limited and/or general partners or managers, who may sell Resale Shares pursuant to this prospectus. Each Selling Security Holder may also transfer shares owned by him or her by gift, and upon any such transfer the donee would have the same right of sale as the Selling Security Holder.
The Resale Shares described in the following table consist of shares of common stock and shares of common stock underlying common stock purchase warrants that were issued or are issuable in connection with the issuances described herein. A discussion of the material terms of the offerings is included in the section of this prospectus titled “Prospectus Summary”. We may amend or supplement this prospectus from time to time to update the disclosure set forth herein, however, if a Selling Security Holder transfers his or her interest in the common stock purchase warrants prior to the effective date of the registration statement of which this prospectus is a part, we will be required to file a post-effective amendment to the registration statement to provide the information concerning the transferee. Alternatively, if a Selling Security Holder transfers his or her interest in the common stock purchase warrants after the effective date of the registration statement of which this prospectus is a part, we may use a supplement to update this prospectus. None of the Selling Security Holders are or were affiliated with registered broker-dealers. See our discussion titled “Plan of Distribution” for further information regarding the Selling Security Holders’ method of distribution of the Resale Shares.
| 64 |
Selling Security Holder Table
| Name of Selling Security Holder | Securities Beneficially Owned Before Offering(1) | Percentage Beneficially Owned Before Offering(2) | Securities Offered Hereby(3) | Securities Beneficially Owned After Offering | Percentage Beneficially Owned Before Offering(4) | |||||||||||||||
| A.Y. Berman Consulting and Investments LTD(9) | 131,250 | * | 131,250 | 0 | ||||||||||||||||
| Aaron Lehmann(10) | 100,000 | * | 100,000 | 0 | ||||||||||||||||
| Abraham Barzelay and Hana Arad(9)(10) | 352,833 | * | 352,833 | 0 | ||||||||||||||||
| Alan and Angela Greenhalgh JTWROS(10) | 2,500,000 | 3.82 | % | 2,500,000 | 0 | |||||||||||||||
| Alok K. Agrawal(10) | 62,500 | * | 62,500 | 0 | ||||||||||||||||
| Andrew M. Klein(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Andrew S. Good(5)(6) | 56,250 | * | 56,250 | 0 | ||||||||||||||||
| Angelo De Rosa(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Anne P. Wells(8) | 37,500 | * | 22,500 | 15,000 | * | |||||||||||||||
| Anthony N. Parisi III(10) | 62,500 | * | 62,500 | 0 | ||||||||||||||||
| Arthur Smolensky(10) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Barbara B. Highland(5) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| Barry G. Pallay(6)(10) | 497,734 | * | 138,890 | 358,844 | * | |||||||||||||||
| Barry R. Shaw(10) | 24,900 | * | 24,900 | 0 | ||||||||||||||||
| Bejarano Ventures LTD(9) | 350,000 | * | 350,000 | 0 | ||||||||||||||||
| Bert K. Waits(5) | 106,250 | * | 18,750 | 87,500 | * | |||||||||||||||
| Billy Atkins(6)(7) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Birmingham Hematology/Oncology Assoc. PC 401(k) FBO Dr. Jimmie H. Harvey Jr.(5)(9) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Bradford E. Warden(7) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| Bradley T. Lehman(5) | 18,750 | * | 18,750 | 0 | ||||||||||||||||
| Brenda M. Hackney(5)(10) | 157,640 | * | 157,640 | 0 | ||||||||||||||||
| Brendan Sullivan(10) | 100,000 | * | 100,000 | 0 | ||||||||||||||||
| Brian Haughan(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Brian Kuhar(10) | 50,000 | * | 50,000 | 0 | ||||||||||||||||
| Brooks D. McCartney(5) | 22,500 | * | 22,500 | 0 | ||||||||||||||||
| Brown Communications, John Brown(6) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Bruce F. Levy(10) | 62,500 | * | 62,500 | 0 | ||||||||||||||||
| Burton Mark Paull(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Carlos J. and Zulma E Jurado JTIC(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Carmel Resources LLC(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Chad Elms(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Charles J. Magolske(10) | 50,000 | * | 50,000 | 0 | ||||||||||||||||
| Charles Morse(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Charlotte M. King(8) | 18,750 | * | 18,750 | 0 | ||||||||||||||||
| 65 |
| Craig H. Unger(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Daniel and Bettyann Kevles(9)(10) | 87,659 | * | 58,195 | 29,464 | * | |||||||||||||||
| Daniel Evan Etheredge(10) | 100,000 | * | 100,000 | 0 | ||||||||||||||||
| Daniel P. Wikel(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| David A. Kuhar(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| David A. Taylor(10) | 87,500 | * | 87,500 | 0 | ||||||||||||||||
| David G. Saoud(10) | 50,000 | * | 50,000 | 0 | ||||||||||||||||
| David L. Hawkins(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| David M. Laurenson(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| David M. Rosenfelt(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Dean L. Haas and Karen L. Haas, JTWROS(10) | 100,000 | * | 100,000 | 0 | ||||||||||||||||
| Del N. Wisler(6) | 18,750 | * | 18,750 | 0 | ||||||||||||||||
| Dianne M. Scheck(10) | 139,000 | * | 139,000 | 0 | ||||||||||||||||
| Donald K. Coffey(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Donald P. Sabetta(5) | 18,750 | * | 18,750 | 0 | ||||||||||||||||
| Emily Barnhart Trust, Todd Barnhart(7) | 30,000 | * | 30,000 | 0 | ||||||||||||||||
| Enterprise Inv. Partners, John D. Glass(5) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| Erich W. Wouters(5)(10) | 182,985 | * | 182,985 | 0 | ||||||||||||||||
| Eugene E. Eubank(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Foster T. Jordan and Carmela E. Jordan, JTWROS(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Frank Jernejcic(6)(10) | 141,945 | * | 69,445 | 72,500 | * | |||||||||||||||
| Frank T. Litton, Jr.(7) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Gary B. Rackliffe(10) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| George A. Meyers(10) | 62,500 | * | 62,500 | 0 | ||||||||||||||||
| George and Karin Alexa Elefther JTWROS(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| George Henshaw(7) | 18,750 | * | 18,750 | 0 | ||||||||||||||||
| George M. Zelinski(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Georges Zanellato(10) | 175,000 | * | 175,000 | 0 | ||||||||||||||||
| Gurpreet Ahluwalia(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Hackney One Investments, Morris Hackney(5) | 112,500 | * | 112,500 | 0 | ||||||||||||||||
| Harry A. Theochari(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Howard Wool(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Inbalim Solutions, LTD(9)(10) | 204,965 | * | 204,965 | 0 | ||||||||||||||||
| J. Finley McRae (Trust)(5) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Jack Lahav(9) | 350,000 | * | 350,000 | 0 | ||||||||||||||||
| James and Isolde Andrews(5) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| James G. Markey and Carolyn L. Markey, JTWROS(10) | 50,000 | * | 50,000 | 0 | ||||||||||||||||
| James T. Dietz and Barbara J. Dietz, JTWROS(10) | 117,500 | * | 117,500 | 0 | ||||||||||||||||
| Jan Backvall(10) | 62,500 | * | 62,500 | 0 | ||||||||||||||||
| Jeffrey Gropper(6) | 18,750 | * | 18,750 | 0 |
| 66 |
| Jeremy Evan Creson(10) | 50,000 | * | 50,000 | 0 | ||||||||||||||||
| Johanna Puskar(7) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| John Bennet Williamson(10) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| John C. Pryse, Jr. DDS(7) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| John E. Cook III(5) | 7,500 | * | 7,500 | 0 | ||||||||||||||||
| John Kellenyi(5)(10) | 810,033 | 1.24 | % | 633,000 | 177,033 | * | ||||||||||||||
| John Malfer and Toni Malfer, JTWROS(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| John R. B. Gould(10) | 499,650 | * | 499,650 | 0 | ||||||||||||||||
| John R. Harrison and Linda L. Harrison, JTWROS(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| John W. Broadbent(6) | 22,500 | * | 22,500 | 0 | ||||||||||||||||
| John W. Pyles(8) | 18,750 | * | 18,750 | 0 | ||||||||||||||||
| Jon H. Lytle and Carrie M. Lytle, JTWROS(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Joshua J. Gooden and Janeen Gooden, JTWROS(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Judith S. Raese(7) | 18,750 | * | 18,750 | 0 | ||||||||||||||||
| Julius E. Talton(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Kathryn H. Eckert (Trust)(5) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| Ken R. Klimitchek(10) | 150,000 | * | 150,000 | 0 | ||||||||||||||||
| Kenneth J. Broadbent(6) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Kerston Coombs(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Kevin T. MCDonough(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| KMR Agency Inc.(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Kyle M. Pratt(7) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| Lantis Consultants, Inc.(9)(10) | 469,860 | * | 469,860 | 0 | ||||||||||||||||
| Larry W. Schwartz(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Lawrence Solomon Revocable Living Trust, Lawrence Solomon Trustee(10) | 275,000 | * | 275,000 | 0 | ||||||||||||||||
| Lee Cleveland (10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Lee S. Good (Trust)(5)(6)(7) | 56,250 | * | 56,250 | 0 | ||||||||||||||||
| Lee Westwood(10) | 124,815 | * | 124,815 | 0 | ||||||||||||||||
| Leon Recanati(9)(10) | 1,484,330 | 2.27 | % | 1,484,330 | 0 | |||||||||||||||
| Liliana Jimenez and Patricia Jimenez, JTIC(10) | 62,500 | * | 62,500 | 0 | ||||||||||||||||
| Lindsay A. Rosenwald(8) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| M. Shane Harvey(7) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Mario Ayub(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Mark C. Jasek(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Mark S. King(5)(10) | 95,333 | * | 95,333 | 0 | ||||||||||||||||
| Martha C. Davis (Callie)(8) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Marvin Dale Martin(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Me And My Friends Ventures LLC(10) | 400,000 | * | 400,000 | 0 | ||||||||||||||||
| Memphis Acquisitions, William Ridgway(5) | 18,750 | * | 18,750 | 0 |
| 67 |
| Michael Bundschuh(10) | 25,000 | * | 25,000 | 0 | ||||||||||||||||
| Michael C. Karsonovich(10) | 166,500 | * | 166,500 | 0 | ||||||||||||||||
| Michael J. Pilbeam(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Michael K. Barber and Julia K. Barber, JTWROS(10) | 300,000 | * | 300,000 | 0 | ||||||||||||||||
| Michael S. Mullins(6) | 18,750 | * | 18,750 | 0 | ||||||||||||||||
| Moore Trading LLC(10) | 62,500 | * | 62,500 | 0 | ||||||||||||||||
| Moran Raz(9) | 175,000 | * | 175,000 | 0 | ||||||||||||||||
| Moshe Avramov(9)(10) | 738,667 | * | 738,667 | 0 | ||||||||||||||||
| N.C.L. Investments and Consulting, LTD(9)(10) | 176,917 | * | 176,917 | 0 | ||||||||||||||||
| Nathan Milikowsky(9) | 700,000 | 1.07 | % | 700,000 | 0 | |||||||||||||||
| Nelson H. and Dorothy M. Murphy JTWROS(10) | 62,500 | * | 62,500 | 0 | ||||||||||||||||
| Nicholas Femia and Christina A. Femia, JTWROS(10) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| Nicholas M. Finegold (UK)(5) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| Nikhilanand J. Hiremath(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Nissim Zarfati(9) | 262,500 | * | 262,500 | 0 | ||||||||||||||||
| Ori Bogomilsky(9)(10) | 147,222 | * | 147,222 | 0 | ||||||||||||||||
| Patrick S. Thomas(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Paul A. Wildberger(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Perry Family Trust, Bill Perry(6) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| Peter H. Colettis(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Phillip C. Altimari and Catherine M. Altimari, JTWROS(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Randall W. Miller(10) | 50,000 | * | 50,000 | 0 | ||||||||||||||||
| Reynold Duclas, Jr. and Janice Kannikal JTIC(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Richard Evans(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Richard Levine(10) | 1,500,000 | 2.29 | % | 1,500,000 | 0 | |||||||||||||||
| Robert B. Koplowitz(10) | 50,000 | * | 50,000 | 0 | ||||||||||||||||
| Robert John Kline-Schoder Revocable Living Trust DTD 1-27-95, Robert and Barbara Kline-Schoder Trustees(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Robert Masters(8)(10) | 471,328 | * | 353,472 | 117,856 | * | |||||||||||||||
| Robert N. Preite(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Robert P. Dickinson(5) | 56,250 | * | 56,250 | 0 | ||||||||||||||||
| Ron Mileikowsky(9) | 350,000 | * | 350,000 | 0 | ||||||||||||||||
| Ron Wilkinson(6) | 18,750 | * | 18,750 | 0 | ||||||||||||||||
| Ross Rodgers(10) | 25,000 | * | 25,000 | 0 | ||||||||||||||||
| RYLAKYAL Trust, David Weimer(6) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Sam J. Lewis(5) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Sandra F. Tomlinson(10) | 100,000 | * | 100,000 | 0 | ||||||||||||||||
| Savinis, D'Amico, and Kane LLC(6) | 100,000 | * | 75,000 | 25,000 | * | |||||||||||||||
| Sean Moore(10) | 110,000 | * | 110,000 | 0 |
| 68 |
| Sharon A. Hayes(6) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| Shawn and Jennifer Gilliam(7) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Shmuel Cabilly(9) | 350,000 | * | 350,000 | 0 | ||||||||||||||||
| Shoup Revocable Trust U/A/D 4/29/03(5)(8)(10) | 108,070 | * | 108,070 | 0 | ||||||||||||||||
| Shraga Bodin(9) | 525,000 | * | 525,000 | 0 | ||||||||||||||||
| Stanley Mansfield(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Starlight Inv. Holdings, Sarah Manning (UK)(8) | 93,750 | * | 93,750 | 0 | ||||||||||||||||
| Sten-Anders Fellman(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Stephen E. Haid(6)(10) | 100,000 | * | 100,000 | 0 | ||||||||||||||||
| Stephen Hart(10) | 25,000 | * | 25,000 | 0 | ||||||||||||||||
| Stephen J. Farley(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Stern Agee & Leach Inc. C/F Dennis Winson SEP IRA(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Sterne Agee & Leach Inc. C/F Brian Mark Miller Roth IRA(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Sterne Agee & Leach Inc. C/F John H. Welsh Roth IRA(10) | 150,000 | * | 150,000 | 0 | ||||||||||||||||
| Sterne Agee & Leach Inc. C/F John L. Sommer IRA(10) | 375,000 | * | 375,000 | 0 | ||||||||||||||||
| Sterne Agee & Leach Inc. C/F Pat Schneider IRA(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Sterne Agee & Leach Inc. C/F Ralph Wallis Kettell II SEP IRA(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Sterne Agee & Leach Inc. C/F Randy Payne IRA(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Sterne Agee & Leach Inc. C/F Robert S. Goldberg Roth IRA(10) | 62,500 | * | 62,500 | 0 | ||||||||||||||||
| Sterne Agee & Leach Inc. C/F Thomas Turley SEP IRA(10) | 100,000 | * | 100,000 | 0 | ||||||||||||||||
| Sterne Agee & Leach Inc. C/F Walter J. Lachewitz Jr. IRA(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Sterne Agee & Leach Inc. C/F William Bellinger IRA(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Sterne Agee & Leech Inc. C/F Peter J. Zaborowski IRA R/O(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Steve Octaviano(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Steven A. Hobbs(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Steven K. Nelson(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| Steven R. Eberly(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Steven Shaw(10) | 25,000 | * | 25,000 | 0 | ||||||||||||||||
| Stuart F. Bloch(6) | 15,000 | * | 15,000 | 0 | ||||||||||||||||
| Susan H. Lu(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Suzanne S. Barnhart (Trust)(5)(7) | 31,250 | * | 26,250 | 5,000 | * | |||||||||||||||
| T. Calvin Wells(5) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| Taylor Cardall(10) | 25,000 | * | 25,000 | 0 | ||||||||||||||||
| The Roger Conan Pension Fund(10) | 375,000 | * | 375,000 | 0 | ||||||||||||||||
| Thelma W. Andy(6) | 18,750 | * | 18,750 | 0 | ||||||||||||||||
| Thomas A. Lambert(5)(10) | 95,570 | * | 95,570 | 0 | ||||||||||||||||
| Thomas Cook(7) | 37,500 | * | 37,500 | 0 |
| 69 |
| Thomas G. Hoffman(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Thomas N. Metz(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Tim D. Lea(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Tim Wells(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Timothy A. Kippenhan(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Timothy Wieghaus(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| Timothy Williams(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| W. Charles Mayer III(5) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| W. Garner McNett(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| Walter W. & Karin H. Gloyer Trust, Walter & Karin Gloyer TTEES(10) | 125,000 | * | 125,000 | 0 | ||||||||||||||||
| West Virginia Jobs Investment Trust(5) | 75,000 | * | 75,000 | 0 | ||||||||||||||||
| William A. Legg, Jr.(5) | 56,250 | * | 56,250 | 0 | ||||||||||||||||
| William Allan Lucier(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| William and Leann Lane JTWROS(10) | 250,000 | * | 250,000 | 0 | ||||||||||||||||
| William F. Sneed(5) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| William J. Pulice II(6) | 37,500 | * | 37,500 | 0 | ||||||||||||||||
| William J. Solloway(10) | 500,000 | * | 500,000 | 0 | ||||||||||||||||
| William M. and Dorothy R. Wardell JTWROS(10) | 62,500 | * | 62,500 | 0 | ||||||||||||||||
| Yaron Sheinman(9)(10) | 1,160,750 | 1.77 | % | 1,160,750 | 0 | |||||||||||||||
| Yunsen LTD(9)(10) | 369,608 | * | 369,608 | 0 | ||||||||||||||||
| [Placement Agent Warrants](11) | [3,302,826 | ] | 5.05 | % | [3,302,826 | ] | 0 | |||||||||||||
| Total: | 44,098,640 | 67.39 | % | 43,210,443 | 888,197 | 1.36 | % |
* Less than 1%
(1) Represents (i) an aggregate of 17,511,197 shares of common stock and (ii) an aggregate of 25,699,246 shares of common stock underlying outstanding warrants.
(2) Based on 65,442,735 shares of common stock outstanding on January 10, 2014.
(3) The percentages set forth are not determinative of the Selling Shareholder's beneficial ownership of our common stock pursuant to Rule 13d-3 or any other provision under the Securities Exchange Act of 1934, as amended.
(4) There is no assurance that the Selling Security Holders will sell any or all of the shares of common stock offered hereby.
(5) Issued in connection with the December 2011 Offering.
(6) Issued in connection with the May 2012 Offering.
(7) Issued in connection with the Summer 2012 Direct Issuances.
(8) Issued in connection with the August 2012 Offering.
(9) Issued in connection with the Spring 2013 Direct Issuances.
(10) Issued in connection with the Winter 2013 Offering.
(11) To be issued in connection with the Winter 2013 Offering prior to the effective date of the registration statement of which this prospectus is a part.
| 70 |
PLAN OF DISTRIBUTION
Each Selling Security Holder of the common stock and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed or negotiated prices. A Selling Security Holder may use any one or more of the following methods when selling shares:
| · | ordinary brokerage transactions and transactions in which the broker dealer solicits purchasers; |
| · | block trades in which the broker dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| · | purchases by a broker dealer as principal and resale by the broker dealer for its account; |
| · | an exchange distribution in accordance with the rules of the applicable exchange; |
| · | privately negotiated transactions; |
| · | settlement of short sales entered into after the effective date of the registration statement of which this prospectus is a part; |
| · | broker dealers may agree with the Selling Security Holders |
| · | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| · | a combination of any such methods of sale; or |
| · | any other method permitted pursuant to applicable law. |
The Selling Security Holders may also Resale Shares under Rule 144 under the Securities Act, as amended, if available, rather than under this prospectus.
The Selling Security Holders will bear all brokerage or underwriting discounts or commissions paid to broker-dealers in connection with the sale of their Resale Shares. Broker-dealers engaged by the Selling Security Holders may arrange for other broker dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Security Holders (or, if any broker dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated by them.
In connection with the sale of the common stock or interests therein, the Selling Security Holders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the common stock in the course of hedging the positions they assume. The Selling Security Holders may also sell shares of the common stock short and deliver these securities to close out their short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The Selling Security Holders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities that require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
| 71 |
The Selling Security Holders and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act, in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. We know of no existing arrangements between the Selling Security Holders and any other security holder, broker, dealer, underwriter or agent relating to the sale or distribution of our common stock.
We are required to pay certain fees and expenses incurred by us incident to the registration of the Resale Shares. We have agreed to indemnify the Selling Security Holders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
If Selling Security Holders are deemed to be “underwriters” within the meaning of the Securities Act, they will be subject to the prospectus delivery requirements of the Securities Act, including Rule 172 thereunder. In addition, any securities covered by this prospectus that qualify for sale pursuant to Rule 144 under the Securities Act, may be sold under Rule 144 rather than under this prospectus. There is no underwriter or coordinating broker acting in connection with the proposed sale of the Resale Shares by the Selling Security Holders.
We have provided registration rights to various investors. These rights require us to keep the registration statement, of which this prospectus is a part, effective for periods ranging from the earlier of (i) the date on which the shares may be resold by the Selling Security Holders without registration and without regard to any volume limitations by reason of Rule 144 under the Securities Act, or any other rule of similar effect (ii) all of the shares have been sold pursuant to this prospectus or Rule 144 under the Securities Act, or any other rule of similar effect or (iii) two years from the effective date. We intend to keep the registration statement effective for the longest period required by the registration rights agreements we have signed. The Resale Shares will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the Resale Shares may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the Resale Shares may not simultaneously engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Security Holders will be subject to applicable provisions of the Exchange Act, and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of shares of the common stock by the Selling Security Holders or any other person. We will make copies of this prospectus available to the Selling Security Holders who are required to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
Our transfer agent is Island Stock Transfer located at 15500 Roosevelt Blvd., Suite 301, Clearwater, FL 33760. Their telephone number is (727) 289-0010.
Determination of Offering Price
There is no established public market for the shares of common stock that we are registering. The offering price has been arbitrarily determined and does not bear any relationship to our assets, results of operations, or book value, or to any other generally accepted criteria of valuation. The fixed price of $0.50 at which our common stock is being offered pursuant to this prospectus was determined as based on, without limitation, the estimates of the business potential and earnings prospects of the Company and the consideration of such potential earnings in relation to market valuations of comparable companies.
| 72 |
Penny Stock Rules
The Commission has also adopted rules that regulate broker-dealer practices in connection with transactions in “penny stocks” as such term is defined by Rule 15g-9. Penny stocks are generally equity securities with a price of less than $5.00 (other than securities registered on certain national securities exchanges or quoted on the NASDAQ system provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system).
The shares offered by this prospectus constitute penny stock under the Exchange Act. The shares will remain penny stock for the foreseeable future. The classification of penny stock makes it more difficult for a broker-dealer to sell the stock into a secondary market, which makes it more difficult for a purchaser to liquidate his or her investment. Any broker-dealer engaged by the purchaser for the purpose of selling his or her shares in our Company will be subject to the penny stock rules.
The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from those rules, deliver a standardized risk disclosure document prepared by the Commission, which: (i) contains a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; (ii) contains a description of the broker’s or dealer’s duties to the customer and of the rights and remedies available to the customer with respect to a violation to such duties or other requirements of Securities’ laws; (iii) contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks and significance of the spread between the bid and ask price; (iv) contains a toll-free telephone number for inquiries on disciplinary actions; (v) defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and (vi) contains such other information and is in such form as the Commission shall require by rule or regulation. The broker-dealer also must provide to the customer, prior to effecting any transaction in a penny stock, (i) bid and offer quotations for the penny stock; (ii) the compensation of the broker-dealer and its salesperson in the transaction; (iii) the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and (iv) monthly account statements showing the market value of each penny stock held in the customer’s account.
In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules; the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written acknowledgment of the receipt of a risk disclosure statement, a written agreement to transactions involving penny stocks, and a signed and dated copy of a written suitability statement. These disclosure requirements will have the effect of reducing the trading activity in the secondary market for our stock because it will be subject to these penny stock rules. Therefore, stockholders may have difficulty selling those securities.
| 73 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information, as of December 31, 2013, with respect to the holdings of (1) each person who is the beneficial owner of more than 5% of our common stock, (2) each of our directors, (3) each executive officer, and (4) all of our current directors and executive officers as a group.
Beneficial ownership of the common stock is determined in accordance with the rules of Commission and includes any shares of common stock over which a person exercises sole or shared voting or investment power, or of which a person has a right to acquire ownership at any time within 60 days of December 31, 2013. Except as otherwise indicated, we believe that the persons named in this table have sole voting and investment power with respect to all shares of common stock held by them. Applicable percentage ownership in the following table is based on 65,442,735 shares of common stock outstanding as of December 31, 2013 plus, for each individual, any securities that individual has the right to acquire within 60 days of December 31, 2013.
| Name and Address of Beneficial Owner Officers and Directors | Title | Beneficially Owned | Percent of Class | |||||||
| Stephen Turner | Chief Executive Officer and Chairman of the Board | 2,763,193 | (1) | 4.18 | % | |||||
| Stanley Hostler | Vice President, Secretary and Director | 10,502,971 | (2) | 15.03 | % | |||||
| Edward Hughes | Chief Financial Officer | 72,000 | (3) | * | ||||||
| Alessandro Baldi, Ph.D. | Vice President and General Manager | 101,250 | (4) | * | ||||||
| Matthew Powell | Director of Research & Development and Chief Science Officer | 220,000 | (5) | * | ||||||
| Steve Antoline | Director | 10,476,079 | (6) | 14.68 | % | |||||
| Leonard Harris | Director | 3,631,909 | (7) | 5.43 | % | |||||
| Ed Roberson | Director | 502,716 | (8) | * | ||||||
| Scott Segal | Director | 3,500,274 | (9) | 5.23 | % | |||||
| Roderick Jackson | Director | 336,044 | (10) | * | ||||||
| Andrew Zulauf | Director | 3,429,198 | (11) | 5.13 | % | |||||
| Thijs Spoor | Director | 67,639 | (12) | * | ||||||
| Josiah T. Austin | Director | 14,530,277 | (13) | 19.82 | % | |||||
| Officers and Directors as a Group (total of 13 persons) | 50,133,550 | 56.00 | % | |||||||
| 5% Stockholders | ||||||||||
| El Coronado Holdings, LLC | 14,484,695 | (14) | 19.77 | % | ||||||
| West Virginia Jobs Investment Trust Board | — | 3,429,198 | (15) | 5.13 | % | |||||
| Milan Puskar (Estate) | — | 3,373,057 | (16) | 5.07 | % | |||||
| Virginia Child | — | 3,954,627 | (17) | 5.92 | % | |||||
| Summit Resources, Inc. | — | 7,875,364 | (18) | 11.21 | % | |||||
| Steve A. Antoline 2006 Irrevocable Trust | — | 2,500,715 | (19) | 3.76 | % | |||||
| Hartwell Davis | — | 4,333,033 | (20) | 6.46 | % | |||||
| 74 |
| * | Represents ownership under 1%. |
| (1) | Includes 2,153,372 shares of common stock, 284,821 shares of common stock to be acquired upon the exercise of warrants and 325,000 shares of common stock to be acquired upon the exercise of stock options. |
| (2) | Includes 3,454,390 shares of common stock, 2,375,204 shares of common stock to be acquired upon the exercise of warrants and 718,750 shares of common stock to be acquired upon the exercise of stock options. Also includes 2,481,659 shares of common stock held by Mr. Hostler’s wife, Virginia Child and 1,213,422 shares of common stock to be acquired upon the exercise of warrants held by Mr. Hostler’s wife, Virginia Child. Also includes 148,312 shares of common stock and 111,234 shares of common stock to be acquired upon the exercise of warrants jointly held by Stanley Hostler and Virginia Child. |
| (3) | Includes 72,000 shares of common stock to be acquired upon the exercise of stock options. |
| (4) | Includes 101,250 shares of common stock to be acquired upon the exercise of stock options. |
| (5) | Includes 220,000 shares of common stock to be acquired upon the exercise of stock options. |
| (6) | Includes 1,514,048 shares of common stock and 986,667 shares of common stock to be acquired upon the exercise of warrants owned of record by the Steve A. Antoline 2006 Irrevocable Trust (the "Antoline Trust"). Also includes 3,051,184 shares of common stock and 4,824,180 shares of common stock to be acquired upon the exercise of warrants owned of record by Summit Resources, Inc. As the trustee of the Antoline Trust and president of Summit Resources, Inc., Mr. Antoline has voting and dispositive control over any securities owned of record by the Antoline Trust and Summit Resources, Inc. Therefore, he may be deemed to beneficially own the shares of common stock and the shares of common stock to be acquired upon the exercise of warrants held of record by the Antoline Trust and Summit Resources, Inc. Includes 100,000 shares of common stock to be acquired upon the exercise of stock options. |
| (7) | Includes 2,160,991 shares of common stock, 1,052,168 shares of common stock to be acquired upon the exercise of warrants and 418,750 shares of common stock to be acquired upon the exercise of stock options. |
| (8) | Includes 167,600 shares of common stock, 117,260 shares of common stock to be acquired upon the exercise of warrants and 100,000 shares of common stock to be acquired upon the exercise of stock options. Also includes 67,856 shares of common stock and warrants to purchase 50,000 shares of common stock owned of record by Morgan Keegan & Co, Inc., an IRA account of Ed Roberson. |
| (9) | Includes 1,957,161 shares of common stock, 1,224,363 shares of common stock to be acquired upon the exercise of warrants and 318,750 shares of common stock to be acquired upon the exercise of stock options. |
| (10) | Includes 136,044 shares of common stock, 100,000 shares of common stock to be acquired upon the exercise of warrants and 100,000 shares of common stock to be acquired upon the exercise of stock options. |
| (11) | Includes 2,082,809 shares of common stock, 946,389 shares of common stock to be acquired upon the exercise of warrants, and 400,000 shares of common stock to be acquired upon the conversion of debt. The holder’s address is 1012 Kanawha Boulevard East, 5th Floor, Charleston, West Virginia 25301. Andrew Zulauf, the Executive Director of WVJIT and a director of the Company, may be deemed to have voting and investment control over these securities. |
| (12) | Includes 67,639 shares of common stock to be acquired upon the exercise of stock options. |
| (13) | Includes 6,674,725 shares of common stock and 7,809,970 shares of common stock to be acquired upon the exercise of warrants. Also includes 45,583 shares of common stock to be acquired upon the exercise of stock options held by Josiah T. Austin. Josiah T. Austin is a managing member of El Coronado Holdings and a director of the Company and may be deemed to have voting and investment control over these securities. |
| (14) | Includes 6,674,725 shares of common stock and 7,809,970 shares of common stock to be acquired upon the exercise of warrants. Josiah T. Austin is a managing member of El Coronado Holdings and a director of the Company and may be deemed to have voting and investment control over these securities. |
| 75 |
| (15) | Includes 2,082,809 shares of common stock, 946,389 shares of common stock to be acquired upon the exercise of warrants, and 400,000 shares of common stock to be acquired upon the conversion of debt. The holder’s address is 1012 Kanawha Boulevard East, 5th Floor, Charleston, West Virginia 25301. Andrew Zulauf, the Executive Director of WVJIT and a director of the Company, may be deemed to have voting and investment control over these securities. |
| (16) | Includes 2,294,723 shares of common stock and 1,078,334 shares of common stock to be acquired upon the exercise of warrants. |
| (17) | Includes 2,481,659 shares of common stock and 1,213,422 shares of common stock to be acquired upon the exercise of warrants held by Virginia Child, the wife of Stanley Hostler. Also includes 148,312 shares of common stock and 111,234 shares of common stock to be acquired upon the exercise of warrants jointly held by Stanley Hostler and Virginia Child. |
| (18) | Includes 3,051,184 shares of common stock, 4,824,180 shares of common stock to be acquired upon the exercise of warrants. As president of Summit Resources, Inc., Mr. Antoline has voting and dispositive control over any securities owned of record by Summit Resources, Inc. Therefore, he may be deemed to beneficially own the shares of common stock and the shares of common stock to be acquired upon the exercise of warrants held of record by Summit Resources, Inc. |
| (19) | Includes 1,514,048 shares of common stock and 986,667 shares of common stock to be acquired upon the exercise of warrants owned of record by the Steve A. Antoline 2006 Irrevocable Trust (the "Antoline Trust"). As the trustee of the Antoline Trust, Mr. Antoline has voting and dispositive control over any securities owned of record by the Antoline Trust. Therefore, he may be deemed to beneficially own the shares of common stock and the shares of common stock to be acquired upon the exercise of warrants held of record by the Antoline Trust. |
| (20) | Includes 2,726,783 shares of common stock and 1,606,250 shares of common stock to be acquired upon the exercise of warrants. |
| 76 |
DESCRIPTION OF SECURITIES
General
We are presently authorized under our Certificate of Incorporation to issue 200,000,000 shares of Common Stock, $.0001 par value per share and 10,000,000 shares of preferred stock, $0.0001 par value per share. The following description of our Common Stock is only a summary and is subject to and qualified by our Certificate of Incorporation and By-Laws, copies of which will be provided by us upon request, and by the provisions of applicable corporate laws of the State of Delaware.
Common Stock
As of January 10, 2014, we had 65,442,735 shares of common stock issued and outstanding that were held of record by approximately 454 stockholders.
Voting Rights. Holders of our Common Stock are entitled to one vote per share on all matters to be voted upon by the stockholders. Holders of our Common Stock are not entitled to cumulative voting rights with respect to the election of directors.
Dividends. Subject to limitations under Delaware law and preferences that may apply to any shares of preferred stock that we may decide to issue in the future, holders of our Common Stock are entitled to receive ratably such dividends or other distributions, if any, as may be declared by our board of directors out of funds legally available therefor.
Liquidation. In the event of the liquidation, dissolution or winding up of our business, holders of our Common Stock are entitled to share ratably in all assets remaining after payment of liabilities, subject to the liquidation preference of any outstanding preferred stock we may decide to issue in the future.
Rights and Preferences. Our Common Stock has no preemptive, conversion or other rights to subscribe for additional securities. The rights, preferences and privileges of holders of Common Stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Fully Paid and Non-Assessable. All outstanding shares of our Common Stock are validly issued, fully paid and non-assessable.
Preferred Stock
Our authorized preferred stock consists of 10,000,000 shares of preferred stock, par value $0.0001 per share. As of the date of this Memorandum, no shares of preferred stock have been issued. Our board of directors has the authority, without any vote or action by the stockholders, to create one or more series of preferred stock up to the limit of our authorized but unissued shares of preferred stock and to fix the number of shares constituting such series and the designation of such series, the voting powers (if any) of the shares of such series and the relative participating, option or other special rights (if any), and any qualifications, preferences, limitations or restrictions pertaining to such series which may be fixed by the board of directors pursuant to a resolution or resolutions providing for the issuance of such series adopted by the board of directors.
| 77 |
Options
The Company has reserved 4,150,000 shares of common stock for issuance under the 2002 Equity Incentive Plan and 5,000,000 shares of Common Stock under the 2013 Equity Incentive Plan (collectively, the “Plans”). As of December 31, 2013 the Company had granted options to purchase a total of 5,082,750 shares of common stock under the Plans, of which 4,005,583 shares have vested as of the date hereof, at the exercise prices set forth below:
| (i) | 159,000 shares of common stock at an exercise price of $0.50 per share. |
| (ii) | 1,693,000 shares of common stock at an exercise price of $0.55 per share. |
| (iii) | 320,000 shares of common stock at an exercise price of $0.80 per share. |
| (iv) | 310,000 shares of common stock at an exercise price of $1.25 per share. |
| (v) | 2,386,000 shares of common stock at an exercise price of $1.50 per share. |
| (vi) | 214,750 shares of common stock at an exercise price of $2.00 per share. |
Warrants
There are warrants to purchase a total of 54,752,952 shares of Common Stock outstanding at the exercise prices set forth below:
| (i) | 15,524,642 shares of common stock at an exercise price of $0.50 per share. |
| (ii) | 7,762,321 shares of common stock at an exercise price of $0.75 per share. |
| (iii) | 18,304,350 shares of common stock at an exercise price of $1.10 per share. |
| (iv) | 11,503,180 shares of common stock at an exercise price of $2.00 per share. |
| (v) | 98,320 shares of common stock at an exercise price of $2.20 per share. |
| (vi) | 1,560,139 shares of common stock at an exercise price of $2.25 per share. |
$2.25 Warrants
Each $2.25 warrant is exercisable at an exercise price of $2.25 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of Common Stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof.
$1.10 Warrants
Each $1.10 warrant is exercisable for a term of five years from the issue date issuance at an exercise price of $1.10 per share.
| 78 |
Investor Warrants and Winter 2013 Placement Agent Warrants
A Warrants
Each A Warrant entitles the holder (the “A Warrant Holder”) to purchase from time to time the number of shares of common stock (the “A Warrant Shares”) equal to 100% of the number of shares purchased by the A Warrant Holder in the Winter 2013 Offering, at an exercise price of $0.50 per share (the “A Warrant Exercise Price”) in whole or in part from time to time after the date of issuance of the A Warrant until 5:00 p.m. Eastern time on the first anniversary of the termination of the Winter 2013 Offering (the “A Warrant Exercise Period”). Commencing at any time after the date on which after the common stock closing bid price reported by Bloomberg LP remains at an amount over One Dollar and Twenty-five Cents ($1.25) per share (as adjusted for forward or reverse stock splits, stock dividends or other similar proportionately-applied change) for at least thirty (30) consecutive trading days (the “Call Condition”), the Company shall have the right, upon 30 days’ notice to the A Warrant Holder given not later than ten (10) trading days after the date on which the Call Condition is satisfied (the “Redemption Notice”) to redeem the number of A Warrant Shares specified in the Redemption Notice, less any amount previously exercised, at a price of $0.01 per A Warrant Share (the “Redemption Price”), on the date set forth in the Redemption Notice, but in no event earlier than 30 days following the date of the receipt by the A Warrant Holder of the Redemption Notice (the “Redemption Date”). The A Warrant Holder may exercise the A Warrant at any time (in whole or in part) prior to the Redemption Date. Any portion of the A Warrant that is subject to the Call Condition which is not exercised by 5:30 p.m. (Eastern time) on the Redemption Date shall no longer be exercisable and shall be returned to the Company (and, if not so returned, shall automatically be deemed canceled), and the Company, upon its receipt of the unexercised portion of the A Warrant, shall issue therefore in full and complete satisfaction of its obligations under such called but unexercised portion of the A Warrant to the A Warrant Holder an amount equal to the number of shares of common stock called but remaining unexercised multiplied by the Redemption Price. The Redemption Price shall be mailed to such A Warrant Holder at its address of record, and the A Warrant shall be canceled. In the event that prior to the expiration of the purchase rights under the A Warrant by exercise or by the terms of the A Warrant, the Company effects a Fundamental Transaction, then, upon any subsequent exercise of the A Warrant, the A Warrant Holder shall have the right to receive, for each A Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, the number of shares of common stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and the Alternate Consideration receivable as a result of such merger, consolidation or disposition of assets by a holder of the number of shares of common stock for which the A Warrant is exercisable immediately prior to such event. For purposes of any such exercise, the determination of the A Warrant Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of common stock in such Fundamental Transaction, and the Company shall apportion the A Warrant Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration.
B Warrants
Each B Warrant entitles the holder (the “B Warrant Holder”) to purchase from time to time the number of shares of common stock (the “B Warrant Shares”) equal to 50% of the number of shares purchased by the B Warrant Holder in the Winter 2013 Offering, at an exercise price of $0.75 per share (the “B Warrant Exercise Price”) in whole or in part from time to time after the date of issuance of the B Warrant until 5:00 p.m. Eastern time on the fifth anniversary of the termination of the Winter 2013 Offering (the “B Warrant Exercise Period”). The B Warrant contains a cashless exercise provision.
| 79 |
In the event that prior to the expiration of such purchase rights by exercise or by the terms of the B Warrant, (i) the Company effects any merger or consolidation of the Company with or into another person, (ii) the Company effects any sale of all or substantially all of its assets in one or a series of related transactions, (iii) any tender offer or exchange offer (whether by the Company or another person) is completed pursuant to which holders of common stock are permitted to tender or exchange their shares for other securities, cash or property or (iv) the Company effects any reclassification of the common stock or any compulsory share exchange pursuant to which the common stock is effectively converted into or exchanged for other securities, cash or property (each a “Fundamental Transaction”), then, upon any subsequent exercise of the B Warrant, the B Warrant Holder shall have the right to receive, for each B Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, the number of shares of common stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such merger, consolidation or disposition of assets by a holder of the number of shares of common stock for which the B Warrant is exercisable immediately prior to such event. For purposes of any such exercise, the determination of the B Warrant Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of common stock in such Fundamental Transaction, and the Company shall apportion the B Warrant Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration.
Winter 2013 Placement Agent Warrants
At each closing of the Winter 2013 Offering, the Company shall issue to the Placement Agent and or its designee(s) warrants (the “Winter 2013 Placement Agent Warrants”) equal to 10% (ten percent) of the shares of common stock and shares underlying the Investor Warrants sold in the Winter 2013 Offering. The Winter 2013 Placement Agent Warrants shall have an exercise price equal to the lowest price per share of the shares or Investor Warrants. The Winter 2013 Placement Agent Warrants will otherwise contain the same terms as the Investor Warrants including that the Winter 2013 Placement Agent Warrants shall not be callable for five years, shall contain a cashless exercise provision and registration rights in respect of the shares of common stock underlying the Winter 2013 Placement Agent Warrants, and shall have a strike price of $0.75 per share.
Convertible Debt
As of December 31, 2013, the Company has a total of $198,560 in outstanding convertible debt consisting of $195,177 principal and $3,383 in accrued interest as of the date hereof, convertible into a total of 249,697 shares of Common Stock and comprised of the following:
| (i) | $100,000 in convertible debt issued in April 2012 to the West Virginia Jobs Investment Trust Board as part of an original 3-month note in the amount of $400,000. The note bears interest at 10% providing for monthly interest-only payments starting May 2012 through June 2012, then final interest and principal payments due July 2012. The note includes a stock warrant for 88,889 shares. On June 18, 2012, the West Virginia Jobs Investment Trust Board signed an addendum to the note agreeing to extend the maturity date by 90 days to October 15, 2012. On March 11, 2013, the West Virginia Jobs Investment Trust Board approved an additional deferral of principal and interest until May 15, 2013 and reduced the price of converting into common shares to $0.50 per share. Subsequently the West Virginia Jobs Investment Trust Board further extended the maturity date until October 15, 2013. In November 2013 the West Virginia Jobs Investment Trust Board was paid $100,000 and the final $100,000 of the principal amount had its maturity date extended to January 15, 2014. An executive member of the West Virginia Jobs Investment Trust Board is a board member of the Company. Pursuant to the terms of the note, $200,000 of the principal amount is to be repaid in cash only. |
| 80 |
| (ii) | $95,178 in convertible debt issued on May 24, 2012 to the West Virginia High Technology Consortium Foundation (“WVHTCF”) issued as a 30-month note in the amount of $200,000. The note bears interest at 8% providing for 25 monthly principal and interest payments of $9,001 starting December 2012 through November 2014, then final interest and principal payments due December 2014. The note is secured by a security interest in certain equipment and machinery owned by the Company pursuant to a security agreement dated May 24, 2012 in favor of WVHTCF, as amended by that certain Intercreditor Agreement dated June 11, 2012. In its sole discretion, at any time during the term of the WVHTCF note, elect to convert all or any portion of the unpaid principal and accrued interest due and payable under the WVHTCF note, into shares of common stock at an initial conversion price of $2.00 per share (the “WVHTCF Conversion Price”) subject to certain adjustments. |
| (iii) | In addition, if the Company issues any shares of common stock for a per share purchase price that is less than the WVHTCF Conversion Price then in effect (the “Original Conversion Price”), then the Original Conversion Price shall be reduced concurrently with such issue to a price determined by multiplying such Original Conversion Price by a fraction (x) the numerator of which shall be the number of shares of common stock outstanding immediately prior to such issue plus the number of shares of common stock which the aggregate consideration received by the Company for the total number of additional shares of common stock so issued would purchase at such Original Conversion Price, and (y) the denominator of which shall be the number of shares of common stock outstanding immediately prior to such issue plus the number of such additional shares of common stock so issued; provided, that, all shares of common stock issuable upon conversion of convertible securities shall be deemed to be outstanding. The WVHTCF Note is secured by a first lien interest, shared with the West Virginia Economic Development Authority (“WVEDA”), in all of the Company’s right, title and interest in and to the Collateral pursuant to the terms and conditions of a Security Agreement, dated May 24, 2012 by and between the Company and WVHTCF. |
Pre-Emptive Rights
| (i) | An aggregate of 527,500 shares of the Company’s common stock are subject to full ratchet anti-dilution rights in the event the Company issues any shares of common stock less than $2.00 per share until October, 2015 at the latest requiring the Company to issue an additional number of shares to such holders as may be required so that the purchase price paid for the shares shall be equal to the subsequent lower purchase price at which shares were issued by the Company; provided however, that shares shall not be issued at a purchase price that is less than $1.00 per share. As of the date hereof, the Company has issued 527,500 additional shares of common stock to such holders in accordance with this provision at the $1.00 floor price and is therefore not obligated to issue any additional shares of common stock to such holders. |
An additional 263,750 shares of common stock underlying outstanding warrants are also subject to full ratchet price protection, expiring October, 2015 which provide that if the Company issues or sells additional shares of common stock, for consideration that is less than $2.00, on the date of and immediately prior to the issuance or sale, the exercise price, then in effect, will be reduced concurrently with the issuance or sale to a price equal to the lowest price per share at which any additional share of common stock is issued or sold; provided, however, that in no event shall the exercise price be reduced to a price that is less than $1.12 per share.
| (ii) | An aggregate of 9,276,175 shares of common stock outstanding underlying warrants are subject to weighted average price protection. |
| 81 |
DISCLOSURE OF COMMISSION POSITION OF INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
We are subject to the laws of Delaware on corporate matters, including its indemnification provisions. Section 145 of the General Corporation Law of Delaware (the “GCL”) provides that Delaware corporations are empowered, subject to certain procedures and limitations, to indemnify any person against expenses (including attorney’s fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred by him in connection with any threatened, pending, or completed action, suit, or proceeding (including a derivative action) in which such person is made a party by reason of his being or having been a director, officer, employee, or agent of the company (each, an “Indemnitee”); provided that the right of an Indemnitee to receive indemnification is subject to the following limitations: (i) an Indemnitee is not entitled to indemnification unless he acted in good faith and in a manner that he reasonably believed to be in, or not opposed to, the best interests of the company, and, with respect to any criminal action or proceeding, had no reasonable cause to believe such conduct was unlawful, and (ii) in the case of a derivative action, an Indemnitee is not entitled to indemnification in the event that he is judged to be liable to the company (unless and only to the extent that the court determines that the Indemnitee is fairly and reasonably entitled to indemnification for such expenses as the court deems proper). The statute provides that indemnification pursuant to our provisions is not exclusive of other rights of indemnification to which a person may be entitled under any bylaw, agreement, vote of stockholders, or disinterested directors, or otherwise.
Pursuant to Article Seven of our Certificate of Incorporation (“Article Seven”), we are authorized to provide indemnification of (and advancement of expenses to) directors, officers, employees and agents (and any other persons to which Delaware law permits us to provide indemnification) through bylaw provisions, agreements with such agents or other persons, vote of stockholders or disinterested directors or otherwise, in excess of the indemnification and advancement otherwise permitted by Section 145 of the GCL, subject only to limits created by applicable Delaware law (statutory or non-statutory), with respect to action for breach of duty to us, our stockholders and others.
Article Seven also states that our directors shall not be personally liable to us or any stockholder for monetary damages for breach of fiduciary duty as a director, except of any matter in respect of which such director shall be liable under Section 174 of the GCL or shall be liable because the director (1) shall have breached his duty of loyalty to us or our stockholders, (2) shall have acted in a manner involving intentional misconduct or a knowing violation of law or, in failing to act, shall have acted in a manner involving intentional misconduct or a knowing violation of law, or (3) shall have derived an improper personal benefit. Article Seven further states that the liability of our directors shall be eliminated or limited to the fullest extent permitted by the GCL, as amended.
Under Article Eight of our Certificate of Incorporation, any person who was or is made a party or is threatened to be made a party to or is in any way involved in any threatened, pending or completed action suit or proceeding, whether civil, criminal, administrative or investigative, including any appeal therefrom, by reason of the fact that he is or was a director or officer of ours or was serving at our request as a director or officer of another entity or enterprise (including any subsidiary), shall be indemnified and held harmless by us and we are required to advance all expenses incurred by such person in defense of any such proceeding prior to its final determination, to the fullest extent authorized by the GCL. These rights are not exclusive of any other rights to which those seeking indemnification may otherwise be entitled.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. No pending material litigation or proceeding involving our directors, executive officers, employees or other agents as to which indemnification is being sought exists, and we are not aware of any pending or threatened material litigation that may result in claims for indemnification by any of our directors or executive officers.
| 82 |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
On December 17, 2013, Malin, Bergquist & Co., LLP (“Malin”) resigned from Protea Biosciences Group, Inc. (the “Company”) as its independent registered public accounting firm as a result of a merger, which the Company’s board of directors (the “Board”) accepted. On December 17, 2013 the Board also approved the engagement of Schneider Downs & Co., Inc. (“Schneider”), a top 60 accounting firm based in Pittsburgh, PA, as the Company’s new independent registered public accounting firm.
Neither the report of Malin for the years ended December 31, 2011 and 2012, nor subsequent interim periods contained an adverse opinion or disclaimer of opinion, or was qualified or modified as to uncertainty, audit scope or accounting principles, except that the Company's audited financial statements in its Form 10-K for the years ended December 31, 2011 and 2012 contained a going concern qualification. We have had no disagreements with Malin, whether or not resolved, on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which, if not resolved to Malin’s satisfaction would have caused them to make reference to the subject matter of the disagreement in connection with their reports on our financial statements.
There were no disagreements or other “reportable events” as that term is described in Item 304(a)(1)(iv) and Item 304(a)(1)(v), respectively, of Regulation S-K, occurring within the Company’s two most recent fiscal years and the subsequent interim periods through the date of dismissal.
On December 17, 2013 the Registrant engaged Schneider as its independent accountant. During the most recent fiscal year, since inception, and the interim periods preceding the engagement, the Company has not consulted Schneider regarding any of the matters set forth in Item 304(a)(2)(i) or (ii) of Regulation S-K.
WHERE YOU CAN FIND MORE INFORMATION
We are subject to the informational requirements of the Securities Exchange Act of 1934 and file annual, quarterly and current reports and other information with the Commission. You can read our filings over the Internet at the Commission’s website at www.sec.gov. You may also read and copy any document we file with the Commission at its public reference facility at 100 F Street, N.E., Washington, D.C., 20549, on official business days during the hours of 10 a.m. to 3 p.m. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the Securities and Exchange Commission at 100 F Street, N.E., Washington, D.C., 20549. Please call the Securities and Exchange Commission at 1-800-SEC-0330 for further information on the operation of the public reference facility. In addition you can find more information about us on our website at https://proteabio.com.
EXPERTS
Malin Bergquist & Co., LLP (“Malin”), an independent registered public accounting firm, audited our financial statements for the years ended December 31, 2012 and 2011, as set forth in their report. We have included our financial statements in this prospectus and elsewhere in the registration statement in reliance on the reports of Malin, given on their authority as experts in accounting and auditing.
LEGAL MATTERS AND INTERESTS OF NAMED EXPERTS
Richardson & Patel LLP and its principals have accepted our common stock in exchange for services rendered to us in the past and, although the law firm and its principals are under no obligation to do so, they may continue to accept our common stock for services rendered by them. As of the date of this prospectus, Richardson & Patel LLP and its principals collectively hold 50,000 shares of our common stock.
| 83 |
FINANCIAL STATEMENTS
PROTEA BIOSCIENCES GROUP, INC.
Table of Contents
| Page | |
| ANNUAL FINANCIAL INFORMATION | |
| Report of Independent Registered Public Accounting Firm | F-2 |
| Consolidated Balance Sheets – December 31, 2012 and 2011 | F-3 |
| Consolidated Statements of Operations - For the Years Ended December 31, 2012 and 2011 | F-4 |
| Consolidated Statements of Stockholders’ Equity (Deficit) - For the Years Ended December 31, 2012 and 2011 | F-5 |
| Consolidated Statements of Cash Flows - For the Years Ended December 31, 2012 and 2011 | F-9 |
| Notes to Consolidated Financial Statements | F-10 |
| INTERIM FINANCIAL INFORMATION | |
| Consolidated Balance Sheets at September 30, 2013 (Unaudited) and December 31, 2012 | F-27 |
| Consolidated Statements of Operations and Total Comprehensive Loss - For the Three and Nine Months Ended September 30, 2013; September 30, 2012; and for the period from July 13, 2001(inception) (Unaudited) | F-28 |
| Consolidated Statements of Stockholders’ Equity (Deficit) – As of September 30, 2013 (Unaudited) | F-29 |
| Consolidated Statements of Cash Flows - For the Nine Months Ended September 30, 2013; September 30, 2012; and for the period from July 13, 2001(inception) (Unaudited) | F-30 |
| Notes to Unaudited Consolidated Financial Statements | F-31 |
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Protea Biosciences Group, Inc.
Morgantown, West Virginia
We have audited the accompanying consolidated balance sheets of Protea Biosciences Group, Inc. and subsidiaries (a development stage enterprise) (the “Company”) as of December 31, 2012 and 2011, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity (deficit), and cash flows for the years then ended, and for the period from inception on July 13, 2001 to December 31, 2012. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2012 and 2011, and the consolidated results of their operations and their cash flows for the years then ended, and for the period from inception on July 13, 2001 to December 31, 2012, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company’s products are being developed and have not generated significant revenues. As a result, the Company has suffered recurring losses and its liabilities exceed its assets. This raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ Malin, Bergquist & Company, LLP | |
| Malin, Bergquist & Company, LLP | |
| Pittsburgh, PA | |
| March 27, 2013 |
| F-2 |
PROTEA BIOSCIENCES GROUP, INC.
(A DEVELOPMENT STAGE ENTERPRISE)
Consolidated Balance Sheets
See Accompanying Notes to Financial Statements
| December 31, 2012 | December 31, 2011 | |||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
| Cash and cash equivalents | $ | 27,604 | $ | 505,277 | ||||
| Restricted cash | - | 49,979 | ||||||
| Trade accounts receivable | 127,721 | 76,650 | ||||||
| Other receivables | 308,934 | 315,555 | ||||||
| Inventory | 905,186 | 223,336 | ||||||
| Prepaid expenses | 29,567 | 141,425 | ||||||
| Total current assets | 1,399,012 | 1,312,222 | ||||||
| Property and equipment, net | 2,790,464 | 3,319,219 | ||||||
| Other noncurrent assets | 22,555 | 17,632 | ||||||
| Total Assets | $ | 4,212,031 | $ | 4,649,073 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current Liabilities: | ||||||||
| Current maturities on short and long-term debt | $ | 1,374,489 | $ | 398,383 | ||||
| Accounts payable | 2,270,671 | 2,504,094 | ||||||
| Bank line of credit | 2,725,000 | 3,000,000 | ||||||
| Loans payable to stockholders | 2,898,216 | 750,000 | ||||||
| Other payables and accrued expenses | 764,460 | 310,924 | ||||||
| Total current liabilities | 10,032,836 | 6,963,401 | ||||||
| Long-term debt - net of current portion | 2,083,026 | 2,189,454 | ||||||
| Commitments and contingencies (see Notes) | - | - | ||||||
| Stockholders' Equity: | ||||||||
| Preferred stock ($.0001 par value; 10,000,000 shares authorized; none issued or outstanding) | - | - | ||||||
| Common stock ($.0001 par value; 100,000,000 shares authorized; 31,879,247 and 27,061,498 shares issued and outstanding at December 31, 2012 and December 31, 2011) | 3,188 | 2,706 | ||||||
| Additional paid in capital | 39,074,062 | 32,922,112 | ||||||
| Deficit accumulated during development stage | (46,974,678 | ) | (37,443,419 | ) | ||||
| Accumulated other comprehensive income (loss) | (6,403 | ) | 14,819 | |||||
| Total Stockholders' Equity (deficit) | (7,903,831 | ) | (4,503,782 | ) | ||||
| Total Liabilities and Stockholders' Equity | $ | 4,212,031 | $ | 4,649,073 | ||||
| F-3 |
PROTEA BIOSCIENCES GROUP, INC.
(A DEVELOPMENT STAGE ENTERPRISE)
Consolidated Statements of Operations and Total Comprehensive Loss
See Accompanying Notes to Financial Statements
| For the Year Ended December 31, | For the Year Ended December 31, | Period from July 13, 2001 (date of inception) to | ||||||||||
| 2012 | 2011 | December 31, 2012 | ||||||||||
| Revenue | $ | 834,250 | $ | 713,028 | $ | 3,492,855 | ||||||
| Selling, general, administrative expenses | (5,218,760 | ) | (5,245,985 | ) | (25,872,120 | ) | ||||||
| Research and development expense | (4,595,830 | ) | (6,242,855 | ) | (22,597,640 | ) | ||||||
| Loss from operations | (8,980,340 | ) | (10,775,812 | ) | (44,976,905 | ) | ||||||
| Other income (expense): | ||||||||||||
| Interest and exchange income (expense) | (8,657 | ) | 8,117 | 42,918 | ||||||||
| Interest expense | (541,844 | ) | (729,637 | ) | (2,030,454 | ) | ||||||
| Gain on debt settlement | - | - | 13,834 | |||||||||
| Loss on asset disposal | (418 | ) | - | (24,071 | ) | |||||||
| Total other income (expense) | (550,919 | ) | (721,520 | ) | (1,997,773 | ) | ||||||
| Loss before income taxes | (9,531,259 | ) | (11,497,332 | ) | (46,974,678 | ) | ||||||
| Income taxes | - | - | - | |||||||||
| Net loss | (9,531,259 | ) | (11,497,332 | ) | (46,974,678 | ) | ||||||
| Foreign currency translation adjustment | (21,222 | ) | 18,617 | (6,403 | ) | |||||||
| Total comprehensive loss | $ | (9,552,481 | ) | $ | (11,478,715 | ) | $ | (46,981,081 | ) | |||
| Net loss per share - basic and diluted | $ | (0.33 | ) | $ | (0.55 | ) | $ | (4.34 | ) | |||
| Weighted average number of shares outstanding - basic and diluted | 28,953,072 | 20,925,458 | 10,821,728 | |||||||||
| F-4 |
PROTEA BIOSCIENCES GROUP, INC.
(A DEVELOPMENT STAGE ENTERPRISE)
Consolidated Statements of Stockholders' Equity (Deficit)
See Accompanying Notes to Financial Statements
| Common Stock Par Value $0.0001 (1) | Additional Paid in | Stock Subscription | Deficit Accumulated During Development | Accumulated Other Comprehensive | Total Stockholders’ Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Receivable | Stage | Income (Loss) | (Deficit) | ||||||||||||||||||||||
| July 13, 2001 (date of inception) | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||
| Issuance of stock for cash | 320,000 | 320 | 59,680 | - | - | - | 60,000 | |||||||||||||||||||||
| Issuance of stock for services | 2,010,000 | 2,010 | 374,865 | - | - | - | 376,875 | |||||||||||||||||||||
| Net loss | - | - | - | - | (474,399 | ) | - | (474,399 | ) | |||||||||||||||||||
| December 31, 2001 | 2,330,000 | $ | 2,330 | $ | 434,545 | $ | - | $ | (474,399 | ) | $ | - | $ | (37,524 | ) | |||||||||||||
| Issuance of stock for cash | 1,050,000 | 1,050 | 373,950 | - | - | - | 375,000 | |||||||||||||||||||||
| Net loss | - | - | - | - | (416,491 | ) | - | (416,491 | ) | |||||||||||||||||||
| December 31, 2002 | 3,380,000 | $ | 3,380 | $ | 808,495 | $ | - | $ | (890,890 | ) | $ | - | $ | (79,015 | ) | |||||||||||||
| Issuance of stock for cash | 550,000 | 550 | 274,450 | - | - | - | 275,000 | |||||||||||||||||||||
| Stock-based compensation expense | - | - | 2,590 | - | - | - | 2,590 | |||||||||||||||||||||
| Issuance of stock for services | 40,000 | 40 | 19,960 | - | - | - | 20,000 | |||||||||||||||||||||
| Net loss | - | - | - | - | (420,431 | ) | - | (420,431 | ) | |||||||||||||||||||
| December 31, 2003 | 3,970,000 | $ | 3,970 | $ | 1,105,495 | $ | - | $ | (1,311,321 | ) | $ | - | $ | (201,856 | ) | |||||||||||||
| Issuance of stock for cash | 550,000 | 550 | 274,450 | - | - | - | 275,000 | |||||||||||||||||||||
| Stock-based compensation expense | - | - | 5,181 | - | - | - | 5,181 | |||||||||||||||||||||
| Net loss | - | - | - | - | (459,474 | ) | - | (459,474 | ) | |||||||||||||||||||
| December 31, 2004 | 4,520,000 | $ | 4,520 | $ | 1,385,126 | $ | - | $ | (1,770,795 | ) | $ | - | $ | (381,149 | ) | |||||||||||||
| Issuance of stock for cash | 1,034,000 | 1,034 | 515,966 | - | - | - | 517,000 | |||||||||||||||||||||
| Stock-based compensation expense | - | - | 44,711 | - | - | - | 44,711 | |||||||||||||||||||||
| Issuance of stock for services | 82,500 | 83 | 65,917 | - | - | - | 66,000 | |||||||||||||||||||||
| Net loss | - | - | - | - | (1,034,429 | ) | - | (1,034,429 | ) | |||||||||||||||||||
| December 31, 2005 | 5,636,500 | $ | 5,637 | $ | 2,011,720 | $ | - | $ | (2,805,224 | ) | $ | - | $ | (787,867 | ) | |||||||||||||
| Issuance of stock for cash | 3,191,000 | 3,191 | 2,929,809 | - | - | - | 2,933,000 | |||||||||||||||||||||
| Subscribed stock | - | - | - | (25,000 | ) | - | - | (25,000 | ||||||||||||||||||||
| Stock-based compensation expense | - | - | 26,851 | - | - | - | 26,851 | |||||||||||||||||||||
| Net loss | - | - | - | - | (1,336,317 | ) | - | (1,336,317 | ) | |||||||||||||||||||
| December 31, 2006 | 8,827,500 | $ | 8,828 | $ | 4,968,380 | $ | (25,000 | ) | $ | (4,141,541 | ) | $ | - | $ | 810,667 | |||||||||||||
| F-5 |
PROTEA BIOSCIENCES GROUP, INC.
(A DEVELOPMENT STAGE ENTERPRISE)
Consolidated Statements of Stockholders' Equity (Deficit) (continued)
See Accompanying Notes to Financial Statements
| Common Stock Par Value $0.0001 (1) | Additional Paid in | Stock Subscription | Deficit Accumulated During Development | Accumulated Other Comprehensive | Total Stockholders’ Equity | |||||||||||||||||||||||
| Shares | Amount | Capital | Receivable | Stage | Income (Loss) | (Deficit) | ||||||||||||||||||||||
| December 31, 2006 | 8,827,500 | $ | 8,828 | $ | 4,968,380 | $ | (25,000 | ) | $ | (4,141,541 | ) | $ | - | $ | 810,667 | |||||||||||||
| Issuance of stock for cash | 2,027,990 | 2,028 | 2,518,909 | - | - | - | 2,520,937 | |||||||||||||||||||||
| Issuance of stock for accrued interest on convertible debt | 169,332 | 169 | 135,289 | - | - | - | 135,458 | |||||||||||||||||||||
| Stock-based compensation expense | - | - | 91,446 | - | - | - | 91,446 | |||||||||||||||||||||
| Stock warrants exercised | 25,000 | 25 | 225 | - | - | - | 250 | |||||||||||||||||||||
| Net loss | - | - | - | - | (2,854,072 | ) | - | (2,854,072 | ) | |||||||||||||||||||
| December 31, 2007 | 11,049,822 | $ | 11,050 | $ | 7,714,249 | $ | (25,000 | ) | $ | (6,995,613 | ) | $ | - | $ | 704,686 | |||||||||||||
| Issuance of stock for cash | 2,136,671 | 2,137 | 3,196,614 | - | - | - | 3,198,751 | |||||||||||||||||||||
| Subscribed stock | - | - | - | (20,000 | ) | - | - | (20,000 | ) | |||||||||||||||||||
| Stock-based compensation expense | - | - | 113,262 | - | - | - | 113,262 | |||||||||||||||||||||
| Issuance of stock for services | 4,167 | 4 | 6,247 | - | - | - | 6,251 | |||||||||||||||||||||
| Net loss | - | - | - | - | (4,037,075 | ) | - | (4,037,075 | ) | |||||||||||||||||||
| December 31, 2008 | 13,190,660 | $ | 13,191 | $ | 11,030,372 | $ | (45,000 | ) | $ | (11,032,688 | ) | $ | - | $ | (34,125 | ) | ||||||||||||
| Issuance of stock for cash | 1,823,338 | 1,823 | 2,715,627 | - | - | - | 2,717,450 | |||||||||||||||||||||
| Issuance of stock for accrued interest on convertible debt | 45,042 | 45 | 67,517 | - | - | - | 67,562 | |||||||||||||||||||||
| Subscribed stock | - | - | - | 20,000 | - | - | 20,000 | |||||||||||||||||||||
| Stock-based compensation expense | - | - | 332,941 | - | - | - | 332,941 | |||||||||||||||||||||
| Issuance of stock for services | 20,000 | 20 | 29,986 | - | - | - | 30,006 | |||||||||||||||||||||
| Net loss | - | - | - | - | (5,329,737 | ) | - | (5,329,737 | ) | |||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | (1,450 | ) | (1,450 | ) | |||||||||||||||||||
| December 31, 2009 | 15,079,040 | $ | 15,079 | $ | 14,176,443 | $ | (25,000 | ) | $ | (16,362,425 | ) | $ | (1,450 | ) | $ | (2,197,353 | ) | |||||||||||
| F-6 |
PROTEA BIOSCIENCES GROUP, INC.
(A DEVELOPMENT STAGE ENTERPRISE)
Consolidated Statements of Stockholders' Equity (Deficit) (continued)
See Accompanying Notes to Financial Statements
| Common Stock Par Value $0.0001 (1) | Additional Paid | Stock Subscription | Deficit Accumulated During Development | Accumulated Other Comprehensive | Total Stockholders’ Equity | |||||||||||||||||||||||
| Shares | Amount | in Capital | Receivable | Stage | Income (Loss) | (Deficit) | ||||||||||||||||||||||
| December 31, 2009 | 15,079,040 | $ | 15,079 | $ | 14,176,443 | $ | (25,000 | ) | $ | (16,362,425 | ) | $ | (1,450 | ) | $ | (2,197,353 | ) | |||||||||||
| Issuance of stock for cash | 3,553,334 | 3,553 | 5,322,061 | - | - | - | 5,325,614 | |||||||||||||||||||||
| Subscribed stock | - | - | (25,000 | ) | (405,000 | ) | - | - | (430,000 | ) | ||||||||||||||||||
| Stock-based compensation expense | - | - | 295,573 | - | - | - | 295,573 | |||||||||||||||||||||
| Issuance of stock for services (net of $4,387 of financing expenses) | 20,004 | 20 | 29,986 | - | - | - | 30,006 | |||||||||||||||||||||
| Stock warrants issued as part of convertible debentures | - | - | 124,227 | - | - | - | 124,227 | |||||||||||||||||||||
| Net loss | - | - | - | - | (9,583,662 | ) | - | (9,583,662 | ) | |||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | (2,348 | ) | (2,348 | ) | |||||||||||||||||||
| December 31, 2010 | 18,652,378 | $ | 18,652 | $ | 19,923,290 | $ | (430,000 | ) | $ | (25,946,087 | ) | $ | (3,798 | ) | $ | (6,437,943 | ) | |||||||||||
| Issuance of stock for cash prior to Reverse Merger | 545,667 | 546 | 817,954 | - | - | - | 818,500 | |||||||||||||||||||||
| Subscribed stock prior to Reverse Merger | - | - | - | 430,000 | - | - | 430,000 | |||||||||||||||||||||
| Stock warrants issued in connection with convertible debentures | - | - | 852,995 | - | - | - | 852,995 | |||||||||||||||||||||
| Net carrying value of convertible debentures converted into shares of stock | 5,808,787 | 5,809 | 7,681,582 | - | - | - | 7,687,391 | |||||||||||||||||||||
| Change in Par Value upon completion of Reverse Merger | - | (22,506 | ) | 22,506 | - | - | - | - | ||||||||||||||||||||
| Stock issued upon conversion of convertible debentures | 1,033,333 | 103 | 1,549,897 | - | - | - | 1,550,000 | |||||||||||||||||||||
| Issuance of stock for cash | 1,021,333 | 102 | 1,721,898 | - | - | - | 1,722,000 | |||||||||||||||||||||
| Stock-based compensation expense | - | - | 351,990 | - | - | - | 351,990 | |||||||||||||||||||||
| Net loss | - | - | - | - | (11,497,332 | ) | - | (11,497,332 | ) | |||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | 18,617 | 18,617 | |||||||||||||||||||||
| December 31, 2011 | 27,061,498 | $ | 2,706 | $ | 32,922,112 | $ | - | $ | (37,443,419 | ) | $ | 14,819 | $ | (4,503,782 | ) | |||||||||||||
| F-7 |
PROTEA BIOSCIENCES GROUP, INC.
(A DEVELOPMENT STAGE ENTERPRISE)
Consolidated Statements of Stockholders' Equity (Deficit) (continued)
See Accompanying Notes to Financial Statements
| Stock Par Value Common $0.0001 (1) | Additional Paid in | Stock Subscription | Deficit Accumulated During Development | Accumulated Other Comprehensive | Total Stockholders’ | |||||||||||||||||||||||
| Shares | Amount | Capital | Receivable | Stage | Income (Loss) | Equity (Deficit) | ||||||||||||||||||||||
| December 31, 2011 | 27,061,498 | $ | 2,706 | $ | 32,922,112 | $ | - | $ | (37,443,419 | ) | $ | 14,819 | $ | (4,503,782 | ) | |||||||||||||
| Issuance of stock for cash (net of issuance cost of $502,235) | 4,417,500 | 442 | 5,332,323 | - | - | - | 5,332,765 | |||||||||||||||||||||
| Issuance of stock for placement agent | 124,871 | 12 | (12 | ) | - | - | - | - | ||||||||||||||||||||
| Stock-based compensation expense | - | - | 345,662 | - | - | - | 345,662 | |||||||||||||||||||||
| Stock warrants issued as part of convertible debentures | - | - | 68,250 | - | - | - | 68,250 | |||||||||||||||||||||
| Stock options and warrants exercised | 275,378 | 28 | 405,727 | - | - | - | 405,755 | |||||||||||||||||||||
| Net loss | - | - | - | - | (9,531,259 | ) | - | (9,531,259 | ) | |||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | - | (21,222 | ) | (21,222 | ) | |||||||||||||||||||
| December 31, 2012 | 31,879,247 | $ | 3,188 | $ | 39,074,062 | $ | - | $ | (46,974,678 | ) | $ | (6,403 | ) | $ | (7,903,831 | ) | ||||||||||||
Footnote:
(1) Prior to September 2, 2011, the Company's Par Value was $.001.
| F-8 |
PROTEA BIOSCIENCES GROUP, INC.
(A DEVELOPMENT STAGE ENTERPRISE)
Consolidated Statements of Cash Flows (Unaudited)
See Accompanying Notes to Financial Statements
| For the Year Ended December 31, | For the Year Ended December 31, | Period from July 13, 2001 (date of inception) to | ||||||||||
| 2012 | 2011 | December 31, 2012 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net loss | $ | (9,531,259 | ) | $ | (11,497,332 | ) | $ | (46,974,678 | ) | |||
| Adjustments to reconcile net loss to net cash from operating activities: | ||||||||||||
| Depreciation and amortization | 1,009,140 | 1,279,590 | 4,398,494 | |||||||||
| Non-cash compensation | 345,662 | 351,990 | 1,610,210 | |||||||||
| Issuance of common stock and warrants for services | - | - | 529,138 | |||||||||
| Issuance of common stock for accrued interest | - | 258,181 | 461,200 | |||||||||
| Accretion of convertible debenture discount | 53,294 | 132,689 | 193,128 | |||||||||
| Loss on disposal of fixed assets | 418 | - | 24,071 | |||||||||
| Bad debt expense | 30,032 | - | 30,032 | |||||||||
| Net change in assets and liabilities: | ||||||||||||
| Decrease (increase) | ||||||||||||
| Trade accounts receivable | (81,103 | ) | (25,999 | ) | (157,753 | ) | ||||||
| Prepaid expenses | 111,858 | (75,455 | ) | (29,667 | ) | |||||||
| Other receivables | 1,698 | 727,327 | 70,904 | |||||||||
| Inventory | (681,850 | ) | 43,729 | (905,186 | ) | |||||||
| Increase (decrease) | ||||||||||||
| Trade accounts payable | (233,423 | ) | 780,834 | 2,390,179 | ||||||||
| Other payables and accrued expenses | 453,536 | 57,260 | 764,459 | |||||||||
| Net cash used in operating activities | (8,521,997 | ) | (7,967,186 | ) | (37,595,469 | ) | ||||||
| Cash flows from investing activities: | ||||||||||||
| Movement in restricted cash | 49,979 | (86 | ) | - | ||||||||
| Purchase of and deposits on equipment | (408,169 | ) | (1,241,880 | ) | (4,491,474 | ) | ||||||
| Proceeds from sale of equipment | - | - | 47,450 | |||||||||
| Net cash used in investing activities | (358,190 | ) | (1,241,966 | ) | (4,444,024 | ) | ||||||
| Cash flows from financing activities: | ||||||||||||
| Net advances on bank line of credit | (275,000 | ) | - | 2,725,000 | ||||||||
| Proceeds from sale of common stock | 5,738,521 | 2,282,100 | 25,768,010 | |||||||||
| Proceeds from short and long-term debt | 1,090,000 | 7,405,000 | 11,630,000 | |||||||||
| Proceeds from shareholder debt | 2,148,216 | - | 3,398,216 | |||||||||
| Repayment of long-term debt | (278,001 | ) | (401,984 | ) | (1,443,339 | ) | ||||||
| Financing costs | - | - | (4,387 | ) | ||||||||
| Net cash provided by financing activities | 8,423,736 | 9,285,116 | 42,073,500 | |||||||||
| Effect of exchange rate changes on cash | (21,222 | ) | 18,617 | (6,403 | ) | |||||||
| Net increase (decrease) in cash | (477,673 | ) | 94,581 | 27,604 | ||||||||
| Cash, beginning of period | 505,277 | 410,696 | - | |||||||||
| Cash, end of period | $ | 27,604 | $ | 505,277 | $ | 27,604 | ||||||
| Supplemental disclosure of cash flow information: | ||||||||||||
| Cash paid during the period for interest | $ | 372,754 | $ | 338,767 | $ | 1,243,113 | ||||||
| Supplemental disclosure of non-cash investing and financing activities: | ||||||||||||
| Equipment financed through finance company debt | $ | 72,635 | $ | 283,125 | $ | 2,790,907 | ||||||
| Debt converted to company stock | $ | - | $ | 9,425,791 | $ | 9,628,710 | ||||||
| Stock subscription | $ | - | $ | - | $ | - | ||||||
| F-9 |
PROTEA BIOSCIENCES GROUP, INC. (A DEVELOPMENT STAGE ENTERPRISE)
Notes to Consolidated Financial Statements
1. Description of Company and Nature of Business
Protea Biosciences Group, Inc. (“Protea” or the “Company”) was incorporated in the state of Delaware on May 24, 2005. Pursuant to an Agreement and Plan of Merger, dated September 2, 2011 (the “Merger Agreement”), by and among the Company, Protea Biosciences, Inc. (“PBI”), and SRKP 5 Acquisition Corp., a wholly-owned subsidiary of the Company (“MergerCo”), PBI was merged with and into MergerCo, with PBI continuing as the surviving entity (the “Merger”). Upon the closing of the Merger, the Company changed its name from “SRKP 5, Inc.” to “Protea Biosciences Group, Inc.” and became the parent company of PBI.
PBI is an emerging biotechnology company incorporated in Delaware in July 2001, and based in Morgantown, West Virginia. The Company is engaged in developing and commercializing proprietary life science technologies, products and services that are used to recover and identify proteins in biological samples. Through its wholly-owned French subsidiary, it has a joint development arrangement with Laboratoires Mayoly Spindler SAS to develop and market a recombinant biopharmaceutical product.
The accompanying financial statements have been prepared on a going-concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has experienced negative cash flows from operations since inception and had an accumulated deficit at December 31, 2012 of approximately $47 million and at December 31, 2011 of approximately $37 million. The Company has funded its activities to date almost exclusively from debt and equity financings. The Company will continue to require substantial funds to advance the research and development of its core technologies, to continue to develop new products and services based upon its proprietary protein recovery and identification technologies, and to continue clinical trials of its recombinant pharmaceutical compound.
Management intends to meet its operating cash flow requirements from the sale of equity or issuance of debt. The Company seeks additional capital through sales of equity securities or convertible debt and, if appropriate, to pursue partnerships to advance its drug and technology development activities. The Company may also consider the sale of certain assets, or entering into a transaction such as a merger with a business complimentary to theirs.
While the Company believes that it will be successful in obtaining the necessary financing to fund its operations, they have no committed sources of funding and are not assured that additional funding will be available to them.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Protea Biosciences Group, Inc. and all of its wholly-owned subsidiaries. All material accounts and transactions have been eliminated in consolidation.
Development Stage Enterprise
The Company is a development-stage enterprise devoting substantial efforts to establishing new business, financial planning, raising capital, research and development activities and developing markets. All losses accumulated since inception have been considered as part of the Company’s development stage activities.
Estimates and Assumptions
The presentation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid debt instruments purchased with a maturity date of three months or less to be cash equivalents. The Company has cash on deposit at banks that may exceed the federally-insured limits at times.
| F-10 |
2. Summary of Significant Accounting Policies (continued)
Restricted Cash
Restricted cash represents a debt reserve account for the note payable to the West Virginia Development Office. The Company previously was required to maintain a minimum deposit balance, but was granted permission in 2012 to relinquish the minimum balance requirement. The amounts on deposit total $0 and $49,979 at December 31, 2012 and 2011, respectively. At no time during the period was the Company out of compliance.
Trade Accounts Receivable
Trade accounts receivable are reported at the amount management expects to collect from outstanding balances. Differences between the amount due and the amount management expects to collect are reported in the statement of operations of the year in which those differences are determined, with an offsetting entry to a valuation allowance for trade accounts receivable. Balances that are still outstanding after management has used reasonable collection efforts are written off through a charge to the valuation allowance and a credit to trade accounts receivable. The Company performs ongoing credit evaluations of its customers and generally does not require collateral.
The Company maintains allowances for doubtful accounts based on management’s analysis of historical losses from uncollectible accounts and risks identified for specific customers who may not be able to make required payments. If the financial condition of customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required. An allowance of $30,032 was deemed necessary on one receivable as of December 31, 2012. No allowance was deemed necessary at December 31, 2011.
Other Receivables
Other receivables, which reflect amounts due from non-trade activity, consist of the following at:
| December 31, 2012 | December 31, 2011 | |||||||
| French government R&D credit | $ | 282,036 | $ | 281,996 | ||||
| Employee loan | 4,000 | - | ||||||
| Stock subscription and Other | 22,898 | 33,559 | ||||||
| Other receivables - current | $ | 308,934 | $ | 315,555 | ||||
| Employee loan - noncurrent | $ | - | $ | 17,632 | ||||
| Deposits | 22,555 | - | ||||||
| Other receivables - noncurrent | $ | 22,555 | $ | 17,632 | ||||
Inventory
Inventory represents finished goods and work in progress. Finished goods and work in progress consist primarily of specifically identifiable items that are valued at the lower of cost or fair market value using the first-in, first-out (FIFO) method. Inventory consists of the following at:
| Inventory: | December 31, 2012 | December 31, 2011 | ||||||
| Finished goods | $ | 104,153 | $ | 155,368 | ||||
| Work in progress | 801,033 | 67,968 | ||||||
| Total Inventory | $ | 905,186 | $ | 223,336 | ||||
Property and Equipment
Expenditures for maintenance and repairs are charged to expense and the costs of significant improvements that extend the life of underlying assets are capitalized.
Property and equipment and leasehold improvements are capitalized at cost and depreciated using the straight-line method (the Company used the double-declining balance method for property and equipment placed in service prior to January 2011) over estimated lives as follows:
| F-11 |
2. Summary of Significant Accounting Policies (continued)
| Equipment | 5 - 10 years | |
| Vehicle | 5 years | |
| Leasehold improvements | 3 years | |
| Software | 3 years |
Property and equipment consists of the following at:
| December 31, 2012 | December 31, 2011 | |||||||
| Lab equipment | $ | 5,898,489 | $ | 5,569,965 | ||||
| Computer equipment | 389,720 | 362,826 | ||||||
| Office equipment and vehicle | 277,216 | 196,320 | ||||||
| Leasehold improvements | 465,790 | 245,684 | ||||||
| Construction in progress | - | 193,804 | ||||||
| 7,031,215 | 6,568,599 | |||||||
| Accumulated depreciation | (4,240,751 | ) | (3,249,380 | ) | ||||
| Property and equipment, net | $ | 2,790,464 | $ | 3,319,219 | ||||
Depreciation expense is charged to either research and development or administration expenses and totals $1,009,140 in 2012 and $1,279,590 in 2011. Depreciation expense for the period July 13, 2001 (date of inception) through 2012 totals $4,398,494.
The Company evaluates the potential impairment of property and equipment whenever events or changes in circumstances indicate that the carrying value of a group of assets may not be recoverable. An impairment loss would be recognized when the carrying amount of the asset group exceeds the estimated undiscounted future cash flows expected to be generated from the use of the asset group and its eventual disposition. The amount of impairment loss to be recorded is measured as the excess of the carrying value of the asset group over its fair value. Fair value is generally determined using a discounted cash flow analysis or market prices for similar assets.
Revenue Recognition
The Company derives its revenue from the sale of products and services. Product revenue is recognized upon shipment of the product. Service revenue is recognized upon completion of service contracts that are normally short-term in duration. However, where applicable, service revenue is recorded under proportional performance for projects in process to approximate the amount of revenue earned based on the percentage of effort completed.
Shipping and handling costs are included in cost of sales. Shipping and handling costs charged to customers are recorded as revenue in the period the related product sales revenue is recognized.
Foreign Currency
The financial statements of the Company’s international subsidiary are translated into U.S. dollars using the exchange rate at each balance sheet date for assets and liabilities, the historical exchange rate for stockholders’ equity and an average exchange rate for each period of revenues, expenses, and gains and losses. The functional currency of the Company’s non-U.S. subsidiary is the local currency. Adjustments resulting from the translation of financial statements are reflected in accumulated other comprehensive income/loss. Transactional gains and losses are recorded within operating results.
Advertising
The Company follows the policy of charging the costs of advertising to expense as incurred.
Research and Development
The Company follows the policy of charging the costs of research and development to expense as incurred. Research and Development expense is net of $279,688 in 2012 and $303,233 in 2011, which reflects the French Government Research Credit.
| F-12 |
2. Summary of Significant Accounting Policies (continued)
Net Loss per Share
Basic and diluted loss per common share are computed based on the weighted average number of common shares outstanding. Common share equivalents (which may consist of options, warrants and convertible debt) are excluded from the computation of diluted loss per share since the effect would be anti-dilutive. Common share equivalents which could potentially dilute basic earnings per share in the future, and which were excluded from the computation of diluted loss per share, totaled approximately 18,697,000 and 15,133,000 at December 31, 2012 and 2011, respectively.
Income Taxes
Deferred income taxes are reported using the liability method. Deferred tax assets are recognized for deductible temporary differences and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax bases. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates on the date of enactment.
Stock-based Compensation
The Company follows the provisions of FASB ASC 718, “Stock-Based Compensation”. Stock-based compensation expense is estimated as of the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which generally represents the vesting period. Fair value of Company stock options is estimated using the Black-Scholes option-pricing model. The associated compensation expense is recognized on a straight-line basis over the requisite service period, net of estimated forfeitures.
Estimating the fair value for stock options for each grant requires judgment, including estimating stock-price volatility, expected term, expected dividends and risk-free interest rates. The expected volatility rates are estimated based on the volatility of similar entities whose share information is publicly available. The expected term represents the average time that options are expected to be outstanding and is estimated based on the average of the contractual term and the vesting period of the options as provided in SEC Staff Accounting Bulletin #110 as the “simplified” method. The risk-free interest rate for periods approximating the expected term of the options is based on the U.S. Treasury yield curve in effect at the time of grant. Expected dividends are zero as the Company has no plans to issue dividends.
RECENTLY ISSUED ACCOUNTING PRONOUNCEMENTS:
Other Comprehensive Income
In February 2013, the FASB issued new guidance which requires disclosure of information about significant reclassification adjustments from accumulated other comprehensive income in a single note or on the face of the financial statements. This guidance will be effective for the Company in 2013. Adoption of this standard, which is related to disclosure only, will not have an impact on the Company’s consolidated financial position, results of operations or cash flows.
In June 2011, the FASB issued new guidance pertaining to the presentation of comprehensive income. The new rule eliminates the previous option to report other comprehensive income and its components in the statement of changes in equity. The standard is intended to provide a more consistent method of presenting non-owner transactions that affect the Company’s equity. Under the new guidance, an entity can present items of net income and other comprehensive income in one continuous statement or in two separate, but consecutive, statements. The new guidance was effective for the Company on January 1, 2012 and did not have an impact on the Company’s consolidated financial position, results of operations or cash flows.
| F-13 |
3. Bank Line of Credit
The Company took out a line of credit that is authorized to $3,000,000 and payable on demand. The interest rate is variable and is .75% plus prime with a minimum rate of 5.87%. At December 31, 2012, the balance was $2,725,000 with interest payable at 5.87% and all covenants had been met. At December 31, 2011, the balance was $3,000,000 with interest payable at 5.87% and all covenants had been met. Borrowings under the line are secured by the personal guarantee of three board members and the estate of a former board member.
4. Loan Payable
In December of 2011, the Company borrowed $750,000 in an aggregate principal amount from two board members. The notes accrue simple interest at a rate of 10% per annum and were due and payable 180 days from the date of issue. At the option of the holders, the notes, including the principal and accrued interest, were convertible into common stock of the Company at $2.00 per share. On June 15, 2012, the board members signed addendums to the notes agreeing to extend the maturity date by 90 days to September 15, 2012. On September 25, 2012, the board members signed addendums to the notes agreeing to extend the maturity date by an additional 90 days to December 14, 2012. On March 22, 2013, the note holders signed addendums to the notes agreeing to extend the maturity date to May 31, 2013, and agreeing to reduce the price of converting into common shares to $0.50 per share.
In April of 2012, the Company borrowed $640,000 in an aggregate principal amount from various board members and their spouses. The notes accrue simple interest at a rate of 10% per annum and were due and payable 90 days from the date of issue. At the option of the holders, the notes, including the principal and accrued interest, were convertible into common stock of the Company at $2.00 per share. In June and July of 2012, the note holders signed addendums to the notes agreeing to extend the maturity date by 90 days to October 13, 2012. On October 31, 2012, the note holders signed addendums to the notes agreeing to extend the maturity date by an additional 90 days to January 11, 2012. On March 22, 2013, the note holders signed addendums to the notes agreeing to extend the maturity date to May 31, 2013, and agreeing to reduce the price of converting into common shares to $0.50 per share.
In September of 2012, the Company borrowed $593,216 in an aggregate principal amount from various board members and their spouses. The notes accrue simple interest at a rate of 10% per annum and were due and payable 60 days from the date of issue. At the option of the holders, the notes, including the principal and accrued interest, were convertible into common stock of the Company at $2.00 per share. On November 30, 2012, the board members signed addendums to the notes agreeing to extend the maturity date by an additional 90 days to February 22, 2013. On March 22, 2013, the note holders signed addendums to the notes agreeing to extend the maturity date to May 31, 2013, and agreeing to reduce the price of converting into common shares to $0.50 per share.
On November 30, 2012, the Company issued convertible promissory notes in an aggregate principal amount equal to $915,000 to various board members, their spouses and other related parties. The Notes accrue simple interest at a rate of 10% per annum and are due and payable 60 days from the date of issue. At the option of the holders, the notes, including the principal and accrued interest, were convertible into common stock of the Company at $2.00 per share. On March 22, 2013, the note holders signed addendums to the notes agreeing to extend the maturity date to May 31, 2013, and agreeing to reduce the price of converting into common shares to $0.50 per share.
5. Long-term Debt
1) Note Payable to the West Virginia Development Office
In March 2007, the Company obtained an 8-year loan in the amount of $685,000 from the West Virginia Development Office. The note bears interest at 3% providing for 96 monthly principal and interest payments of $8,035 through April 2015, at which time the note is due and payable. The note is secured by equipment. As of December 31, 2012, the Company was in arrears on the note. The repayment terms were modified, whereby the West Virginia Development Office approved a deferral of principal and interest until January 31, 2013. On March 11, 2013, the West Virginia Development Office approved an additional deferral of principal and interest until March 31, 2013.
2) Note Payable to Finance Company
In July 2007, the Company obtained a 5-year loan in the amount of $28,726 from a finance company. The note bears interest at 6.99% with monthly principal and interest payments of $569 through July 2012. The note was secured by a vehicle. The note was paid in full on July 11, 2012.
| F-14 |
5. Long-term Debt (continued)
3) Note Payable to the West Virginia Economic Development Authority
In August 2009, the Company obtained a 10-year loan in the amount of $242,631 from the West Virginia Economic Development Authority. The note bears interest at 4% providing for 120 monthly principal and interest payments of $2,457 through August 2019, at which time the note is due and payable. The note is secured by 50% of equipment costing $569,812. As of December 31, 2012, the Company was in arrears on the note. The repayment terms were modified, whereby the West Virginia Economic Development Authority approved a deferral of principal and interest until January 31, 2013. On March 8, 2013, the West Virginia Economic Development Authority approved an additional deferral of principal and interest until March 31, 2013.
4) Note Payable to the West Virginia Infrastructure and Jobs Development Council
In August 2009, the Company obtained a 10-year loan in the amount of $242,630 from the West Virginia Infrastructure and Jobs Development Council. The note bears interest at 3.25% providing for 120 monthly principal and interest payments of $2,371 through August 2019, at which time the note is due and payable. The note is secured by 50% of equipment costing $569,812. As of December 31, 2012, the Company was in arrears on the note. The repayment terms were modified, whereby the West Virginia Infrastructure and Jobs Development Council approved a deferral of principal and interest until January 31, 2013. On March 11, 2013, the West Virginia Infrastructure and Jobs Development Council approved an additional deferral of principal and interest until March 31, 2013.
5) Note Payable to the West Virginia Economic Development Authority
In October 2010, the Company obtained a 10-year note in the amount of $900,000 from the West Virginia Economic Development Authority. The note bears interest at 3.26% providing for 120 monthly principal and interest payments of $8,802 through October 2020, at which time the note is due and payable. The note is secured by equipment costing $997,248. As of December 31, 2012, the Company was in arrears on the note. The repayment terms were modified, whereby the West Virginia Economic Development Authority approved a deferral of principal and interest until January 31, 2013. On March 8, 2013, the West Virginia Economic Development Authority approved an additional deferral of principal and interest until March 31, 2013.
6) Note Payable to the West Virginia Infrastructure & Jobs Development Council
In December 2010, the Company obtained a 10-year note in the amount of $900,000 from the West Virginia Infrastructure & Jobs Development Council. The note bears interest at 3.25% providing for 120 monthly principal and interest payments of $8,781 through December 2020, at which time the note is due and payable. The note is secured by equipment costing $1,098,249. As of December 31, 2012, the Company was in arrears on the note. The repayment terms were modified, whereby the West Virginia Infrastructure and Jobs Development Council approved a deferral of principal and interest until January 31, 2013. On March 11, 2013, the West Virginia Infrastructure and Jobs Development Council approved an additional deferral of principal and interest until March 31, 2013.
7) Convertible Promissory Note Payable to the West Virginia Jobs Investment Trust Board
In March 2012, the Company issued an 18-month note in the amount of $290,000 from the West Virginia Jobs Investment Trust Board. The note bears interest at 6% providing for monthly interest-only payments starting April 2012 through August 2013, then final interest and principal payments due September 2013. The note includes a stock warrant for 72,500 shares (see Note 9, Stock Warrants). As of December 31, 2012, the Company was in arrears on the note. The repayment terms were modified, whereby the West Virginia Jobs Investment Trust Board approved an extension of the Company’s interest payments until January 15, 2013. On March 11, 2013, the West Virginia Jobs Investment Trust Board approved an additional deferral of principal and interest until May 15, 2013. An executive member of the West Virginia Jobs Investment Trust Board is a board member of the Company.
| F-15 |
5. Long-term Debt (continued)
8) Convertible Promissory Note Payable to the West Virginia Jobs Investment Trust Board
In April 2012, the Company issued a 3-month note in the amount of $400,000 from the West Virginia Jobs Investment Trust Board. The note bears interest at 10% providing for monthly interest-only payments starting May 2012 through June 2012, then final interest and principal payments due July 2012. The note includes a stock warrant for 88,889 shares (see Note 9, Stock Warrants). On June 18, 2012, the West Virginia Jobs Investment Trust Board signed an addendum to the note agreeing to extend the maturity date by 90 days to October 15, 2012. As of December 31, 2012, the Company was in arrears on the note. The repayment terms were modified, whereby the West Virginia Jobs Investment Trust Board approved an extension of the Company’s interest payments and principal repayment until January 15, 2013. On March 11, 2013, the West Virginia Jobs Investment Trust Board approved an additional deferral of principal and interest until May 15, 2013 and reduced the price of converting into common shares to $0.50 per share. An executive member of the West Virginia Jobs Investment Trust Board is a board member of the Company.
9) Convertible Promissory Note Payable to the West Virginia High Technology Consortium Foundation
In May 2012, the Company issued a 30-month note in the amount of $200,000 from the West Virginia High Technology Consortium Foundation. The note bears interest at 8% providing for 25 monthly principal and interest payments of $9,001 starting December 2012 through November 2014, then final interest and principal payments due December 2014. The note is secured by 50% of equipment costing $447,320.
10) Note Payable to the West Virginia Economic Development Authority
In June 2012, the Company issued a 10-year note in the amount of $200,000 from the West Virginia Economic Development Authority. The note bears interest at 2% providing for 120 monthly principal and interest payments of $1,840 through June 2022, at which time the note is due and payable. The note is secured by 50% of equipment costing $447,320. As of December 31, 2012, the Company was in arrears on the note. The repayment terms were modified, whereby the West Virginia Economic Development Authority approved a deferral of principal and interest until January 31, 2013. On March 8, 2013, the West Virginia Economic Development Authority approved an additional deferral of principal and interest until March 31, 2013.
11) Capital Leases
From time to time, in the normal course of business, the Company enters into capital leases to finance equipment. As of December 31, 2012, the Company had four capital lease obligations outstanding with imputed interest rates ranging from 2.9% to 8.0%. The leases require 36 monthly payments and begin to expire in March 2013 through May 2015. These leases are secured by equipment with an aggregate cost of $560,195. As of December 31, 2012, the Company was in arrears on all four capital leases.
Total debts outstanding are as follows:
| December 31, 2012 | December 31, 2011 | |||||||||
| 1) | Note Payable to the WV Development Office | $ | 258,497 | $ | 312,343 | |||||
| 2) | Note Payable to Finance Company | - | 3,343 | |||||||
| 3) | Note Payable to the WVEDA | 179,728 | 192,559 | |||||||
| 4) | Note Payable to the WVIJDC | 177,894 | 190,974 | |||||||
| 5) | Note Payable to the WVEDA | 756,217 | 802,916 | |||||||
| 6) | Note Payable to the WVIJDC | 768,259 | 814,652 | |||||||
| 7) | Note Payable to the WVJITB | 275,045 | - | |||||||
| 8) | Note Payable to the WVJITB | 400,000 | - | |||||||
| 9) | Note Payable to the WVHTCF | 191,373 | - | |||||||
| 10) | Note Payable to the WVEDA | 196,983 | - | |||||||
| 11) | Capital leases | 253,519 | 271,050 | |||||||
| Total | 3,457,515 | 2,587,837 | ||||||||
| Less: current portion | (1,374,489 | ) | (398,383 | ) | ||||||
| Long-term portion | $ | 2,083,026 | $ | 2,189,454 | ||||||
| F-16 |
5. Long-term Debt (continued)
Future required minimum principal repayments over the next five years are as follows:
| Year ending December 31: | Future required minimum principal repayments | |||
| 2012 (payment in arrears) | $ | 153,346 | ||
| 2013 | 1,221,143 | |||
| 2014 | 504,915 | |||
| 2015 | 287,768 | |||
| 2016 | 253,577 | |||
| 2017 | 262,002 | |||
| 2018 & Thereafter | 774,764 | |||
| Total | $ | 3,457,515 | ||
6. Common Stock
The Company is authorized to issue a total of 110,000,000 of shares of stock, of which 100,000,000 shares are designated Common Stock and 10,000,000 shares designated Preferred Stock.
Common Stock – par value of $.0001 per share with one vote in respect of each share held. Holders of Common Stock do not have cumulative voting rights. The members of the Board are elected by the affirmative vote of the holders of a majority of the Company’s outstanding Common Stock. Common Stock issues are as follows:
| # Shares Issued | Par Value | Price Per Share | Gross Proceeds | Value of Services Obtained | Par Value | Additional Paid in Capital (1) | ||||||||||||||||||||||
| July 13, 2001 (date of inception) | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||
| Stock issuance in 2001 | 2,010,000 | .001 | .188 | - | 376,875 | 2,010 | 374,865 | |||||||||||||||||||||
| Stock issuance in 2001 | 300,000 | .001 | .167 | 50,000 | - | 300 | 49,700 | |||||||||||||||||||||
| Stock issuance in 2001 | 20,000 | .001 | .500 | 10,000 | - | 20 | 9,980 | |||||||||||||||||||||
| Stock issuance in 2002 | 500,000 | .001 | .200 | 100,000 | - | 500 | 99,500 | |||||||||||||||||||||
| Stock issuance in 2002 | 550,000 | .001 | .500 | 275,000 | - | 550 | 274,450 | |||||||||||||||||||||
| Stock issuance in 2003 | 40,000 | .001 | .500 | - | 20,000 | 40 | 19,960 | |||||||||||||||||||||
| Stock issuance in 2003 | 550,000 | .001 | .500 | 275,000 | - | 550 | 274,450 | |||||||||||||||||||||
| Stock issuance in 2004 | 550,000 | .001 | .500 | 275,000 | - | 550 | 274,450 | |||||||||||||||||||||
| Stock issuance in 2005 | 1,034,000 | .001 | .500 | 517,000 | - | 1,034 | 515,966 | |||||||||||||||||||||
| Debt issuance cost in 2005 | 82,500 | .001 | .800 | - | 66,000 | 83 | 65,917 | |||||||||||||||||||||
| Stock issuance in 2006 | 66,000 | .001 | .500 | 33,000 | - | 66 | 32,934 | |||||||||||||||||||||
| Stock issuance in 2006 | 1,125,000 | .001 | .800 | 900,000 | - | 1,125 | 898,875 | |||||||||||||||||||||
| Stock issuance in 2006 | 2,000,000 | .001 | 1.00 | 2,000,000 | - | 2,000 | 1,998,000 | |||||||||||||||||||||
| Stock issuance in 2007 | 31,250 | .001 | .800 | 25,000 | - | 31 | 24,969 | |||||||||||||||||||||
| Stock warrants exercised in 2007 | 25,000 | .001 | .01 | 250 | - | 25 | 225 | |||||||||||||||||||||
| Debt converted for stock in 2007 | 169,322 | .001 | .800 | 135,458 | - | 169 | 135,288 | |||||||||||||||||||||
| Stock issuance in 2007 | 1,996,750 | .001 | 1.25 | 2,495,938 | - | 1,997 | 2,493,941 | |||||||||||||||||||||
| Stock issuance in 2008 | 25,000 | .001 | 1.25 | 31,250 | - | 25 | 31,225 | |||||||||||||||||||||
| Stock issuance in 2008 | 4,167 | .001 | 1.50 | - | 6,251 | 4 | 6,247 | |||||||||||||||||||||
| Stock warrants exercised in 2008 | 2,111,671 | .001 | 1.50 | 3,167,507 | - | 2,112 | 3,165,395 | |||||||||||||||||||||
| Stock issuance in 2009 | 20,004 | .001 | 1.50 | - | 30,006 | 20 | 29,986 | |||||||||||||||||||||
| Debt converted for stock in 2009 | 45,042 | .001 | 1.50 | 67,562 | - | 45 | 67,517 | |||||||||||||||||||||
| Stock warrants exercised in 2009 | 1,823,334 | .001 | 1.50 | 2,735,000 | - | 1,823 | 2,733,177 | |||||||||||||||||||||
| Stock issuance in 2010 | 20,004 | .001 | 1.50 | - | 30,006 | 20 | 29,986 | |||||||||||||||||||||
| Stock warrants exercised in 2010 | 3,553,334 | .001 | 1.50 | 5,330,000 | - | 3,553 | 5,326,448 | |||||||||||||||||||||
| Stock issuance in 2011 | 1,187,000 | .0001 | 1.50 | 1,780,500 | - | (16,668 | ) | 1,797,168 | ||||||||||||||||||||
| Debt converted for stock in 2011 | 6,842,120 | .0001 | 1.50 | 10,263,180 | - | 684 | 10,262,496 | |||||||||||||||||||||
| Stock issuance in 2011 | 380,000 | .0001 | 2.00 | 760,000 | - | 38 | 759,962 | |||||||||||||||||||||
| Total December 31, 2011 | 27,061,498 | $ | 31,226,645 | $ | 529,138 | $ | 2,706 | $ | 31,753,077 | |||||||||||||||||||
(1) Activity does not include issuance costs, deferred financing, warrants portion of convertible debentures and stock options.
| F-17 |
6. Common Stock (continued)
Common Stock issues during 2012 are as follows:
| # Shares Issued | Par Value | Price Per Share | Gross Proceeds | Value of Services Obtained | Par Value | Additional Paid in Capital (1) | ||||||||||||||||||||||
| December 31, 2011 Balance | 27,061,498 | $ | .0001 | Various | $ | 31,226,645 | $ | 529,138 | $ | 2,706 | $ | 31,753,077 | ||||||||||||||||
| Stock options exercised | 100,000 | .0001 | 1.25 | 125,000 | - | 10 | 124,990 | |||||||||||||||||||||
| Stock options exercised | 140,003 | .0001 | 1.50 | 210,005 | - | 14 | 209,991 | |||||||||||||||||||||
| Stock options exercised | 8,750 | .0001 | 2.00 | 17,500 | - | 1 | 17,499 | |||||||||||||||||||||
| Stock warrants exercised | 26,625 | .0001 | 2.00 | 53,250 | - | 3 | 53,247 | |||||||||||||||||||||
| Issuance of stock (2) | 2,000,000 | .0001 | 0.50 | 1,000,000 | - | 200 | 999,800 | |||||||||||||||||||||
| Issuance of stock (2) | 2,417,500 | .0001 | 2.00 | 4,835,000 | - | 242 | 4,834,758 | |||||||||||||||||||||
| Issuance of stock (3) | 124,871 | - | - | 12 | (12 | ) | ||||||||||||||||||||||
| Total December 31, 2012 | 31,879,247 | $ | 37,467,400 | $ | 529,138 | $ | 3,188 | $ | 37,993,350 | |||||||||||||||||||
(1) Activity does not include issuance costs, deferred financing, warrants portion of convertible debentures and stock options.
(2) Issuance includes stock warrants.
(3) Refer to Note 8 for details relating to shares issued as compensation to placement agent.
Pursuant to the terms and conditions in the Private Placement Memorandum dated August 6, 2012, executed purchasers of securities were granted anti-dilution rights in the event the Company subsequently sold shares of common stock at a price per share that is less than $2.00 per share. The Company issued and sold an aggregate of 352,500 shares of common stock at $2.00 per share and warrants to purchase up to 176,250 shares of common stock at an exercise price of $2.25 per share under the memorandum. Whereas the Company has since issued shares at $0.50 per share, on March 22, 2013, the Company’s board approved the issuance of an additional 1,057,500 shares of common stock for the anti-dilution provision and reduced the exercise price of the warrants to $1.12 per share.
7. Preferred Stock
The Company is authorized to issue Preferred Stock, in one or more series and containing such rights, privileges and limitations, including voting rights, dividend rates, conversion privileges, redemption rights and terms, redemption prices and liquidation preferences, as the Board may, from time to time, determine. No shares of the Preferred Stock have been issued.
8. Stock Options and Stock-based Compensation
In 2002, the Board of Directors of the Company adopted the 2002 Equity Incentive Plan that governed equity awards to employees, directors and consultants of the Company. Under the Plan, 450,000 shares of Common Stock were reserved for issuance. From 2006 through 2012, the Plan was amended several times and the Board of Directors authorized additional shares of Common Stock. The total shares reserved as of December 31, 2012 was 4,150,000. As of December 31, 2012, the Plan had expired and a new plan was adopted subsequent to the balance sheet date. (See Note 16, Subsequent Events)
The types of awards permitted under the Plan include qualified incentive stock options (ISO) and non-qualified stock options, and restricted stock. Each option shall be exercisable at such times and subject to such terms and conditions as the Board may specify. Stock options generally vest over four years and expire no later than ten years from the date of grant.
| F-18 |
8. Stock Options and Stock-based Compensation (continued)
A summary of stock option activity is as follows:
| Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (in years) | ||||||||||
| Outstanding at December 31, 2010 | 2,950,000 | $ | 1.39 | 4.88 | ||||||||
| Granted ($1.50) | 1,141,000 | $ | 1.50 | |||||||||
| Granted ($2.00) | 30,750 | $ | 2.00 | |||||||||
| Exercised | - | |||||||||||
| Cancelled or expired | 75,000 | $ | 1.50 | |||||||||
| Outstanding at December 31, 2011 | 4,046,750 | $ | 1.40 | 4.21 | ||||||||
| Granted | 188,000 | $ | 1.89 | |||||||||
| Exercised | 225,000 | $ | 1.42 | |||||||||
| Cancelled or expired | 145,000 | $ | 1.50 | |||||||||
| Outstanding at December 31, 2012 | 3,864,750 | $ | 1.42 | 4.00 | ||||||||
| Exercisable at December 31, 2011 | 2,518,854 | $ | 1.33 | 3.79 | ||||||||
| Exercisable at December 31, 2012 | 2,992,417 | $ | 1.39 | 3.59 | ||||||||
The following table summarizes information about stock options at December 31, 2012:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Exercise Price | Outstanding | Weighted Average Remaining contractual life (in years) | Weighted Average Exercise Price | Exercisable | Weighted Average Exercise Price | |||||||||||||||
| $0.50 | 84,000 | 84,000 | ||||||||||||||||||
| $0.80 | 320,000 | 320,000 | ||||||||||||||||||
| $1.25 | 410,000 | 410,000 | ||||||||||||||||||
| $1.50 | 2,836,000 | 2,033,250 | ||||||||||||||||||
| $2.00 | 214,750 | 145,167 | ||||||||||||||||||
| $0.50 - $2.00 | 3,864,750 | 4.00 | $ | 1.42 | 2,992,417 | $ | 1.39 | |||||||||||||
At December 31, 2012, the total aggregate intrinsic value for options currently exercisable and options outstanding was $0. These values represent the total pre-tax intrinsic value based on the estimated fair value of the Company’s stock price of $0.50 as of December 31, 2012. During the year ended December 31, 2012, 225,000 shares were exercised, whereas no options were exercised during December 31, 2011.
The following table summarizes the activity of the Company’s stock options that have not vested for the year ended December 31, 2012:
| Shares | Weighted Average Grant-date Fair Value | |||||||
| Nonvested at December 31, 2010 | 1,350,562 | $ | 0.466 | |||||
| Granted | 1,171,750 | $ | 0.426 | |||||
| Forfeited | 46,666 | $ | 0.388 | |||||
| Vested | 947,750 | $ | 0.471 | |||||
| Nonvested at December 31, 2011 | 1,527,896 | $ | 0.437 | |||||
| Granted | 69,583 | $ | 0.561 | |||||
| Forfeited | 145,000 | $ | 0.425 | |||||
| Vested | 580,146 | $ | 0.467 | |||||
| Nonvested at December 31, 2012 | 872,333 | $ | 0.441 | |||||
| F-19 |
8. Stock Options and Stock-based Compensation (continued)
The fair value of non-vested options to be recognized in future periods is $385,122, which is expected to be recognized over a weighted average period of 2 years. The total fair value of options vested during the twelve months ended December 31, 2012 was $345,662 and vested during the year ended December 31, 2011 was $351,990.
Stock-based compensation expense is as follows:
| Year ended | ||||||||
| December 31, 2012 | December 31, 2011 | |||||||
| Selling, general, and administrative expense | $ | 268,618 | $ | 282,836 | ||||
| Research and development expense | 77,044 | 69,154 | ||||||
| Total stock-based compensation expense | $ | 345,662 | $ | 351,990 | ||||
The weighted average grant-date fair value of options granted during the year ended December 31, 2012 was $0.554 and for the year ended December 31, 2011 was $0.426 per option.
The fair value of the option grants was estimated using the Black-Scholes option-pricing model with the following weighted average assumptions:
| Year ended | ||||||||
| December 31, 2012 | December 31, 2011 | |||||||
| Risk-free interest rate | 1.15 | % | 1.35 | % | ||||
| Volatility factor | 24.28 | % | 23.00 | % | ||||
| Weighted average expected life (in years) | 7 | 7 | ||||||
| Dividend rate | 0.0 | % | 0.0 | % | ||||
The Company based its estimate of expected stock price volatility on monthly price observations of the AMEX Biotech Index. Given the Company’s limited history with stock options, the Company’s expected term is based on average of the contractual term and the vesting period of the options (the SAB 110 “Simplified” method).
In January 2012, the Company issued 499,482 shares of common stock to a placement agent (the “Shares”) in connection with a private placement offering of units consisting of common stock and warrants, which were subject to forfeiture under certain conditions. As of December 31, 2012, an aggregate of 124,871 Shares are no longer subject to forfeiture, and the balance of 374,611 shares was forfeited.
9. Stock Warrants
In 2008, the Company issued stock warrants related to common stock issuances. The warrants are exercisable for five years from date of issuance. The warrant allows the holder the ability to purchase an additional share of common stock for every share purchased in connection with the warrant. The exercise price is $2.00 per share.
In November 2009, the Company issued stock warrants to four board members in exchange for personal guarantees on a bank line of credit (See Note 3, Bank Line of Credit). The warrants are exercisable for five years from date of issuance. The warrant allows the four holders the ability to purchase an additional 375,000 shares of common stock. The exercise price is $2.00 per share.
In March 2012, the Company issued stock warrants in connection with the issuance of a Convertible Debenture to the West Virginia Jobs Investment Trust Board (see Note 5, Long-term Debt). The warrant is exercisable for five years from date of issuance. The warrant allows the holder the ability to purchase 72,500 shares of common stock. The exercise price is $2.25 per share.
In April 2012, the Company issued stock warrants in connection with the issuance of a Convertible Debenture to the West Virginia Jobs Investment Trust Board (see Note 5, Long-term Debt). The warrant is exercisable for five years from date of issuance. The warrant allows the holder the ability to purchase 88,889 shares of common stock. The exercise price is $2.25 per share. As of December 31, 2012, warrants to purchase 14,633,319 shares of common stock were outstanding and exercisable.
| F-20 |
10. Treasury Stock
Treasury stock is accounted for using the par value method and is constructively cancelled when received.
11. Income Taxes
The provision for income taxes, if any, is comprised of current and deferred components. The current component, if any, presents the amount of federal and state income taxes that are currently reportable to the respective tax authorities and is measured by applying statutory rates to the Company’s taxable income as reported in its income tax returns. The Company has evaluated its income tax positions in accordance with FASB ASC 740. There were no changes to unrecognized tax benefits during 2012. The tax years 2008 through 2011 remain open to review by various taxing authorities.
ASC Topic 740, Income Taxes defines the confidence level that a tax position must meet in order to be recognized in the financial statements. Accordingly, we have assessed uncertain tax positions in each of the tax jurisdictions in which we have operations and account for the related financial statement implications. We recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities based on the technical merits of the position. The tax benefits recognized in the financial statements on a particular tax position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon settlement. The amount of unrecognized tax benefits is adjusted as appropriate for changes in facts and circumstances, such as significant amendments to existing tax law, new regulations or interpretations by the taxing authorities, new information obtained during a tax examination, or resolution of an examination. We recognize both accrued interest and penalties, where appropriate, related to unrecognized tax benefits in income tax expense.
Significant management judgment is required in determining the provision for income taxes, deferred tax assets and liabilities, and any valuation allowance recorded in connection with the deferred tax assets.
During the development stage of the Company when future benefit of the deferred tax asset is uncertain, the Company provides for a full valuation allowance against the deferred tax asset. Net operating loss carryforwards start to expire beginning 2022 for both federal and state purposes. The net operating tax loss carryforward totals approximately $41,500,000 and $32,700,000 at December 31, 2012 and December 31, 2011, respectively.
We have recorded a full valuation allowance of $16,765,500 and $13,228,300 as of December 31, 2012 and 2011, respectively, due to uncertainties related to our ability to utilize the Company’s deferred tax assets, primarily consisting of certain net operating losses carried forward. The valuation allowance is based on management’s current estimates of taxable income for the jurisdictions in which we operate and the period over which the deferred tax assets will be recoverable.
| December 31, 2012 | December 31, 2011 | |||||||
| Current deferred income tax asset: | ||||||||
| Tax net operating loss carry forward | $ | 16,170,277 | $ | 12,735,800 | ||||
| Tax-deferred stock option expense | 595,209 | 492,500 | ||||||
| Research and development expense | - | - | ||||||
| Total current deferred income tax asset | 16,765,486 | 13,228,300 | ||||||
| Valuation Allowance | (16,765,486 | ) | (13,228,300 | ) | ||||
| Net deferred income tax asset | $ | - | $ | - | ||||
In the event that actual results differ from these estimates, or these estimates are adjusted in future periods, realization of these deferred tax assets for which the valuation allowance has been provided occur, the provision for income taxes may decrease, raising income and positively impacting the Company’s financial position.
| F-21 |
12. Lease Commitments
The Company leases its USA facilities under operating leases beginning a) February 2005 and extended through December 2012 and b) April 2012 through March 2017. Additionally, the Company leases its Europe facility on a three-year lease beginning January 2012 through January 2015. The Company also has three equipment operating leases with terms of three to five years. Future minimum rental payments are as follows for the year ending December 31, 2012:
| Year ending | Future minimum rental payments | |||
| 2012 (payment in arrears) | $ | 61,552 | ||
| 2013 | $ | 283,570 | ||
| 2014 | $ | 258,771 | ||
| 2015 | $ | 173,395 | ||
| 2016 | $ | 167,628 | ||
| 2017 | $ | 41,907 | ||
| 2018 & Thereafter | $ | - | ||
Rent expense totals $413,401 for the twelve months ended December 2012 and $222,137 for 2011.
13. Retirement Plan
The Company provides a 401(k) Profit Sharing Plan for elective deferrals whereby participants can defer up to 100% of their wages not to exceed a maximum dollar amount determined by the Federal Government each year. The Company, at its discretion, can make matching contributions to the plan. The Company may also make qualified non-elective contributions to participants who are not highly compensated employees. All employees meeting age and hours of service requirements are eligible to participate in the Plan after completing one year of service. Participants become vested in employer contributions on a graduated scale with full vesting after five years. No Company contributions have yet been made.
14. Commitments and Contingencies
Legal Proceedings
The Company currently is not a party to any material legal proceeding and has no knowledge of any material legal proceeding contemplated by any governmental authority or third party. The Company may be subject to a number of claims and legal proceedings which, in the opinion of our management, are incidental to normal business operations. In managements’ opinion, although final settlement of these claims and suits may impact the financial statements in a particular period, they will not, in the aggregate, have a material adverse effect on the Company’s financial position, cash flows or results of operations.
Indemnity of Directors and Officers
As permitted under Delaware law and required by corporate by-laws, the Company indemnifies and holds harmless its directors and officers for certain events or occurrences while the director or officer is or was serving in such capacity. The maximum potential amount of future payments that could be required under these indemnification obligations is unlimited; however, the Company maintains a Directors and Officers liability insurance policy that enables it to recover a portion of any future amounts paid with a limit of liability of $5,000,000. The Company may incur an additional liability if indemnity for more than the limit of liability occurred, and such liability may have a material adverse effect on our financial position, cash flows and results of operations. As there were no known or pending claims, the Company has not accrued a liability for such claims as of December 31, 2012.
Warranty
In the ordinary course of business, the Company warrants to customers that its products will conform to published or agreed specifications. Generally, the applicable product warranty period is 90 days from the date of delivery of the goods. The Company provides for estimated warranty costs at the time of the product sale. As of December 31, 2012, management believes that no warranty allowance is necessary due to a limited sales history and that no goods have historically been returned.
| F-22 |
14. Commitments and Contingencies (continued)
Stock Options
The Company has an agreement with one board member for payment of services by stock options. The board member is to receive a stock option for 4,000 shares per month (awarded annually) with an exercise price equal to the current market price. The agreement is cancellable with 90 days’ notice.
University License Agreements
The Company has agreements with universities related to in-licensed technologies as follows:
AGREEMENT WITH WEST VIRGINIA UNIVERSITY (WVU)
The Company has entered into a License and Exclusive Option to License Agreement with the West Virginia University Research Corporation, a nonprofit West Virginia corporation (“WVURC”) acting for and on behalf of WVU. Under the terms of this Agreement, the WVURC has granted the Company an exclusive option to license technology from the laboratories of certain WVU principal investigators in the field of protein discovery, for therapeutic, diagnostic and all other commercial fields worldwide.
Under the terms of this Agreement, the Company pays expenses for the preparation, filing and prosecution of related patent applications, and the Company will pay royalties on the net revenue resulting from the sale of products and services that utilize the WVU subject technology.
AGREEMENT WITH JOHNS HOPKINS UNIVERSITY (JHU)
In June 2009, the Company entered into an Exclusive License Agreement with Johns Hopkins University for technology developed in the laboratory of Jennifer Van Eyk, Ph.D., Professor of Medicine, Division of Cardiology, Biological Chemistry and Biomedical Engineering. The technology field is albumin-bound protein complexes and their use in the diagnosis of cardiovascular disease. Under the terms of the license agreement, the Company has the exclusive, worldwide, rights to commercialize the technology. The Company is obligated to pay expenses for the preparation, filing and prosecution of related patent applications, and the Company will pay royalties on the net revenues resulting from the sale of products and services that utilize the JHU subject technology.
AGREEMENT WITH GEORGE WASHINGTON UNIVERSITY (GWU)
In March 2009, the Company entered into an Exclusive License Agreement with George Washington University (Washington D.C.) for technology developed in the laboratory of Dr. Akos Vertes Ph.D., Professor of Chemistry, Professor of Biochemistry & Molecular Biology, Founder and Co-Director of the W.M. Keck Institute for Proteomics Technology and Applications, the Department of Chemistry, who is a science advisor to the Company. The technology field is LAESI - laser ablation electrospray ionization, a new method of bioanalytical analysis that enables high throughput biomolecule characterization.
Under the terms of the license agreement, the Company has the exclusive, worldwide rights to commercialize the technology. The Company is obligated to pay expenses for the preparation, filing and prosecution of related patent applications, and the Company will pay royalties on the net revenues resulting from the sale of products and services that utilize the GWU subject technology.
In November 2012, the Company entered into a Patent License Agreement with George Washington University (Washington D.C) for technology developed in the laboratory of Akos Vertes Ph.D. The technology field is Laser Desorption/Ionization and Peptide Sequencing on Laser-Induced Silicon Microcolumn Arrays (Matrix) and Nanophotonic Production, Modulation and Switching of Ions by Silicon Microcolumn Arrays.
| F-23 |
14. Commitments and Contingencies (continued)
Under the terms of the license agreement, the Company has an exclusive, worldwide license to make, have made, use, import, offer for sale and sell the licensed products. The Company is obligated to pay a license initiation fee of $25,000 and a minimum license diligence resources of $12,500 in year two and $20,000 each year thereafter to develop and commercialize the products, $30,000 milestone payment upon the first sale of a licensed product, and royalties will be 7% of the net sales of licensed products and 5% of the net sales of combination products for combined cumulative net sales up to $50 million. Thereafter, royalties reduce to 6% and 4%, respectively, after taking into account quarterly minimum royalties of $1,500 for the first four quarters after the first sale of a licensed product, $2,500 for the next four quarters, $3,000 for the next four quarters and $6,000 for each succeeding quarter.
AGREEMENT WITH VIRGINIA TECH
In February 2010, the Company entered into an agreement with Virginia Tech (VT) for the exclusive option to license technology in development in the laboratory of Rafael Davalos, PhD, which is intended to enable the separation of specific cell populations, such as cancer cells, in blood and other fluids. It also has a co-exclusive license to the IRAK-1 compound as a regulator of diseases and disorders in the lab of Liwu Li, PhD. Per the licensing agreements, the Company may have been required to pay milestone payments of $12,500 in 2012.
Joint Pharmaceutical Development Agreement
AGREEMENT WITH LABORATOIRE MAYOLY SPINDLER
In May 2009, the Company, and its wholly-owned subsidiary, Proteabio Europe SAS, entered into a Joint Research and Development Agreement with Laboratories Mayoly Spindler SAS (Mayoly), a European Pharmaceutical company with headquarters in France, for the joint development of a recombinant lipase biopharmaceutical. Under terms of the agreement, Protea receives the exclusive marketing rights for the therapeutic in the territory of North America; will be responsible for paying 40% of the development expenses (with Mayoly funding the remaining 60%); will pay royalties on net sales of the biopharmaceutical, and a milestone payment of 1m Euros at the time the Company obtains the first FDA approval for the recombinant Lipase biotherapeutic. The Company also receives a sublicense to certain patents owned by Mayoly and licensed from the government of France. As of December 31, 2012 and December 31, 2011, the Company owed approximately $36,000 and $974,000 to Mayoly, which is reflected in Trade Accounts Payable on the Consolidated Balance Sheet. The two companies are in discussions for the purpose of making revisions to their agreement.
Engineering and Design Services
The Company has about $180,000 in outstanding commitments with MPR Associates, Inc. for engineering and design services related to their LAESI (Laser Ablation Electrospray Ionization) technology. These amounts relate to ongoing software development, completion of development and design, retrofit of beta units to production standards and production of eight additional instruments. At December 31, 2012, the Company had accrued expenses of approximately $105,000 owed to MPR.
| F-24 |
15. Unaudited Quarterly Information
| 2012 | ||||||||||||||||
| First Quarter | Second Quarter | Third Quarter | Fourth Quarter | |||||||||||||
| Revenue | $ | 179,494 | $ | 223,962 | $ | 165,538 | $ | 265,256 | ||||||||
| Loss from Operations | $ | (2,848,190 | ) | $ | (2,446,450 | ) | $ | (2,064,882 | ) | $ | (1,620,818 | ) | ||||
| Total Other Income (Expense) | $ | (83,064 | ) | $ | (149,424 | ) | $ | (166,093 | ) | $ | (152,338 | ) | ||||
| Net Loss | $ | (2,931,254 | ) | $ | (2,595,874 | ) | $ | (2,230,975 | ) | $ | (1,773,156 | ) | ||||
| Total Comprehensive Loss | $ | (2,965,600 | ) | $ | (2,583,608 | ) | $ | (2,228,839 | ) | $ | (1,774,435 | ) | ||||
| Earnings per share | $ | (0.11 | ) | $ | (0.09 | ) | $ | (0.08 | ) | $ | (0.05 | ) | ||||
| 2011 | ||||||||||||||||
| First Quarter | Second Quarter | Third Quarter | Fourth Quarter | |||||||||||||
| Revenue | $ | 147,386 | $ | 283,396 | $ | 215,379 | $ | 66,867 | ||||||||
| Loss from Operations | $ | (2,459,290 | ) | $ | (2,930,831 | ) | $ | (2,437,569 | ) | $ | (2,948,122 | ) | ||||
| Total Other Income (Expense) | $ | (114,195 | ) | $ | (128,447 | ) | $ | (413,513 | ) | $ | (65,365 | ) | ||||
| Net Loss | $ | (2,573,485 | ) | $ | (3,059,278 | ) | $ | (2,851,082 | ) | $ | (3,013,487 | ) | ||||
| Total Comprehensive Loss | $ | (2,592,565 | ) | $ | (3,076,738 | ) | $ | (2,838,732 | ) | $ | (2,970,680 | ) | ||||
| Earnings per share | $ | (0.14 | ) | $ | (0.16 | ) | $ | (0.13 | ) | $ | (0.12 | ) | ||||
16. Evaluation of Subsequent Events
New Equity Incentive Plan
Subsequent to the balance sheet date, the Board of Directors of the Company adopted a new plan, the 2013 Equity Incentive Plan, which governs equity awards to employees, directors and consultants of the Company. Under this Plan, an additional 5,000,000 shares of Common Stock are reserved for issuance.
Related Party Notes
On January 3, 2013, the Company issued a convertible promissory note in an aggregate principal amount equal to $125,000 to El Coronado Holdings, Inc., an affiliate of Josiah Austin, a director of the Company. The Note accrues simple interest at a rate of 10% per annum and is due and payable on the earlier to occur of (i) April 1, 2013, or (ii) when declared due and payable by the holder upon the occurrence of an event of default.
At the option of the holder, each $0.50 of outstanding Principal Amount and accrued unpaid interest is convertible into one share of common stock of the Company at any time after the Issue Date.
On March 22, 2013, the Company’s board approved the adjustment of the conversion price of all related party notes from $2.00 to $0.50 per share.
Advances from Stockholder
On January 11, 2013, the Company received an advance equal to an aggregate of $100,000 from Summit Resources, Inc., an affiliate of Steve Antoline, a director of the Company. No terms of repayment have been specified on the aforementioned advance as of the filing date.
On January 16, 2013, the Company received an advance equal to an aggregate of $50,000 from Stanley Hostler, a director of the Company. No terms of repayment have been specified on the aforementioned advance as of the filing date.
| F-25 |
16. Evaluation of Subsequent Events (continued)
Letters of Credit, Guarantees and Other Commitments
On February 25, 2013, the Company was the accommodation party in an irrevocable standby letter of credit (“letter of credit”). The letter of credit was issued in favor of MPR Associates, Inc., a major of supplier of engineering services to the Company. Steve Antoline, a director of the Company, applied for the letter of credit in an aggregate amount equal to $600,000. The letter of credit will expire on June 30, 2012. On February 26, 2013, MPR Associates, Inc. drew $300,000 on the letter of credit. On March 15, 2013, MPR Associates, Inc. drew an additional $300,000 on the letter of credit. Therefore, the Company owes $600,000 to Steve Antoline as required per the agreement.
Common Stock
Subsequent to the balance sheet date, in connection with a private placement offering, the Company sold and issued, at a price of $0.50 per share an aggregate of 3,373,900 shares of common stock and warrants to purchase 2,530,425 shares of common stock for gross proceeds of $1,686,950. The warrants are exercisable for five years from the date of issuance at an exercise price of $1.10 per share.
Subsequent to the balance sheet date, in connection with a private placement offering, the Company sold and issued at a price of $0.50 per share an aggregate of 1,370,000 shares of common stock and warrants of 1,027,500 to purchase shares of common stock for gross proceeds of $685,000 to El Coronado Holdings, LLC. The warrants are exercisable for five years from the date of issuance at an exercise price of $1.10 per share.
In connection with the above mentioned stock closings, the placement agent earned cash commissions of 8% and warrants to purchase 223,570 shares of common stock equal to 8% for each related closing.
| F-26 |
PROTEA BIOSCIENCES GROUP, INC.
(A DEVELOPMENT STAGE ENTERPRISE)
Consolidated Balance Sheets
See Accompanying Notes to Financial Statements
| September 30, 2013 (unaudited) | December 31, 2012 | |||||||
| ASSETS | ||||||||
| Current Assets: | ||||||||
| Cash and cash equivalents | $ | 41,039 | $ | 27,604 | ||||
| Trade accounts receivable | 66,596 | 127,721 | ||||||
| Other receivables | 27,679 | 308,934 | ||||||
| Inventory | 591,404 | 905,186 | ||||||
| Prepaid expenses | 103,895 | 29,567 | ||||||
| Total current assets | 830,613 | 1,399,012 | ||||||
| Property and equipment, net | 2,389,104 | 2,790,464 | ||||||
| Other noncurrent assets | 22,939 | 22,555 | ||||||
| Total Assets | $ | 3,242,656 | $ | 4,212,031 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current Liabilities: | ||||||||
| Current maturities on short and long-term debt | $ | 3,037,991 | $ | 1,374,489 | ||||
| Accounts payable | 1,662,314 | 2,270,671 | ||||||
| Bank line of credit | 2,725,000 | 2,725,000 | ||||||
| Loans payable to stockholders | 620,000 | 2,898,216 | ||||||
| Obligation related to the letter of credit | 377,695 | - | ||||||
| Other payables and accrued expenses | 410,701 | 764,460 | ||||||
| Total current liabilities | 8,833,701 | 10,032,836 | ||||||
| Long-term debt - net of current portion | 1,698,361 | 2,083,026 | ||||||
| Commitments and contingencies (see Notes) | - | - | ||||||
| Stockholders' Equity: | ||||||||
| Preferred stock ($.0001 par value; 10,000,000 shares authorized; none issued or outstanding) | - | - | ||||||
| Common stock ($.0001 par value; 200,000,000 shares authorized; 49,536,846 and 31,879,247 shares issued and outstanding at September 30, 2013 and December 31, 2012) | 4,954 | 3,188 | ||||||
| Additional paid in capital | 47,597,116 | 39,074,062 | ||||||
| Deficit accumulated during development stage | (54,891,560 | ) | (46,974,678 | ) | ||||
| Accumulated other comprehensive income (loss) | 84 | (6,403 | ) | |||||
| Total Stockholders' Equity (Deficit) | (7,289,406 | ) | (7,903,831 | ) | ||||
| Total Liabilities and Stockholders' Equity | $ | 3,242,656 | $ | 4,212,031 | ||||
| F-27 |
PROTEA BIOSCIENCES GROUP, INC.
(A DEVELOPMENT STAGE ENTERPRISE)
Consolidated Statements of Operations and Total Comprehensive Loss (Unaudited)
See Accompanying Notes to Financial Statements
| For the Three Months Ended September 30, | For the Three Months Ended September 30, | For the Nine Months Ended September 30, | For the Nine Months Ended September 30, | Period from July 13, 2001 (date of inception) to September 30, | ||||||||||||||||
| 2013 | 2012 | 2013 | 2012 | 2013 | ||||||||||||||||
| Gross revenue | $ | 178,475 | $ | 165,538 | $ | 938,080 | $ | 568,994 | $ | 4,430,935 | ||||||||||
| Selling, general, administrative expenses | (1,943,814 | ) | (1,508,068 | ) | (6,015,077 | ) | (5,205,388 | ) | (28,723,038 | ) | ||||||||||
| Research and development expense | (658,880 | ) | (722,352 | ) | (2,367,014 | ) | (2,723,129 | ) | (28,128,813 | ) | ||||||||||
| Loss from operations | (2,424,219 | ) | (2,064,882 | ) | (7,444,011 | ) | (7,359,523 | ) | (52,420,916 | ) | ||||||||||
| Other income (expense): | ||||||||||||||||||||
| Interest and exchange income (expense) | 573 | (2,369 | ) | 6,997 | (6,169 | ) | 49,915 | |||||||||||||
| Interest expense | (131,277 | ) | (138,621 | ) | (464,095 | ) | (367,309 | ) | (2,494,549 | ) | ||||||||||
| Gain on debt settlement | - | - | - | - | 13,834 | |||||||||||||||
| Loss on asset disposal | - | (25,103 | ) | (15,773 | ) | (25,103 | ) | (39,844 | ) | |||||||||||
| Total other income (expense) | (130,704 | ) | (166,093 | ) | (472,871 | ) | (398,581 | ) | (2,470,644 | ) | ||||||||||
| Loss before income taxes | (2,554,923 | ) | (2,230,975 | ) | (7,916,882 | ) | (7,758,104 | ) | (54,891,560 | ) | ||||||||||
| Income taxes | - | - | - | - | - | |||||||||||||||
| Net loss | (2,554,923 | ) | (2,230,975 | ) | (7,916,882 | ) | (7,758,104 | ) | (54,891,560 | ) | ||||||||||
| Foreign currency translation adjustment | 5,797 | 2,136 | 6,487 | (19,944 | ) | 84 | ||||||||||||||
| Total comprehensive loss | $ | (2,549,126 | ) | $ | (2,228,839 | ) | $ | (7,910,395 | ) | $ | (7,778,048 | ) | $ | (54,891,476 | ) | |||||
| Net loss per share - basic and diluted | $ | (0.06 | ) | $ | (0.08 | ) | $ | (0.19 | ) | $ | (0.27 | ) | $ | (4.33 | ) | |||||
| Weighted average number of shares outstanding - basic and diluted | 43,952,003 | 29,245,418 | 41,122,424 | 28,442,560 | 12,675,003 | |||||||||||||||
| F-28 |
PROTEA BIOSCIENCES GROUP, INC.
(A DEVELOPMENT STAGE ENTERPRISE)
Consolidated Statements of Stockholders' Equity (Deficit) (Unaudited)
See Accompanying Notes to Financial Statements
| Deficit | Accumulated | Total | ||||||||||||||||||||||
| Common Stock | Additional | Accumulated | Other | Stockholders' | ||||||||||||||||||||
| Par Value $.0001 | Paid in Capital | During | Comprehensive | Equity | ||||||||||||||||||||
| Shares | Amount | Common Stock | Development Stage | Income (Loss) | (Deficit) | |||||||||||||||||||
| December 31, 2012 | 31,879,247 | $ | 3,188 | $ | 39,074,062 | $ | (46,974,678 | ) | $ | (6,403 | ) | $ | (7,903,831 | ) | ||||||||||
| Issuance of stock for cash at $0.50 per share (net of issuance costs of $302,675) | 10,266,900 | 1,027 | 4,829,748 | - | - | 4,830,775 | ||||||||||||||||||
| Issuance of stock upon conversion of convertible debentures | 6,663,199 | 666 | 3,330,934 | - | - | 3,331,600 | ||||||||||||||||||
| Issuance of stock for services | 200,000 | 20 | 99,980 | - | - | 100,000 | ||||||||||||||||||
| Issuance of stock under anti-dilution provision | 527,500 | 53 | (53 | ) | - | - | - | |||||||||||||||||
| Stock-based compensation expense | - | - | 238,495 | - | - | 238,495 | ||||||||||||||||||
| Stock warrants issued as part of convertible debentures | - | - | 23,950 | - | - | 23,950 | ||||||||||||||||||
| Net loss | - | - | - | (7,916,882 | ) | - | (7,916,882 | ) | ||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | 6,487 | 6,487 | ||||||||||||||||||
| September 30, 2013 | 49,536,846 | $ | 4,954 | $ | 47,597,116 | $ | (54,891,560 | ) | $ | 84 | $ | (7,289,406 | ) | |||||||||||
| F-29 |
PROTEA BIOSCIENCES GROUP, INC.
(A DEVELOPMENT STAGE ENTERPRISE)
Consolidated Statements of Cash Flows (Unaudited)
See Accompanying Notes to Financial Statements
| For the Nine Months Ended September 30, 2013 | For the Nine Months Ended September 30, 2012 | Period from July 13, 2001 (date of inception) to September 30, 2013 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net loss | $ | (7,916,882 | ) | $ | (7,758,104 | ) | $ | (54,891,560 | ) | |||
| Adjustments to reconcile net loss to net cash from operating activities: | ||||||||||||
| Depreciation and amortization | 633,535 | 749,155 | 5,032,029 | |||||||||
| Non-cash compensation | 238,495 | 238,484 | 1,848,705 | |||||||||
| Issuance of common stock and warrants for services | 100,000 | - | 629,138 | |||||||||
| Issuance of common stock for accrued interest | 228,384 | - | 689,584 | |||||||||
| Accretion of convertible debenture discount | 17,208 | 47,686 | 210,336 | |||||||||
| Loss on disposal of fixed assets | 15,921 | 25,103 | 39,992 | |||||||||
| Bad debt expense | 5,000 | - | 35,032 | |||||||||
| Net change in assets and liabilities: | ||||||||||||
| Decrease (increase) | ||||||||||||
| Trade accounts receivable | 56,125 | 15,315 | (101,628 | ) | ||||||||
| Prepaid expenses | (74,328 | ) | 18,804 | (103,995 | ) | |||||||
| Other receivables | 280,870 | 291,711 | 351,774 | |||||||||
| Inventory | 313,782 | (606,983 | ) | (591,404 | ) | |||||||
| Increase (decrease) | ||||||||||||
| Trade accounts payable | (813,738 | ) | 108,698 | 1,576,441 | ||||||||
| Other payables and accrued expenses | (353,758 | ) | 181,983 | 410,701 | ||||||||
| Net cash used in operating activities | (7,269,386 | ) | (6,688,148 | ) | (44,864,855 | ) | ||||||
| Cash flows from investing activities: | ||||||||||||
| Movement in restricted cash | - | 49,979 | - | |||||||||
| Purchase of and deposits on equipment | (42,715 | ) | (390,624 | ) | (4,534,189 | ) | ||||||
| Proceeds from sale of equipment | - | - | 47,450 | |||||||||
| Net cash used in investing activities | (42,715 | ) | (340,645 | ) | (4,486,739 | ) | ||||||
| Cash flows from financing activities: | ||||||||||||
| Net advances on bank line of credit | - | - | 2,725,000 | |||||||||
| Proceeds from sale of common stock | 4,830,775 | 4,406,851 | 30,598,785 | |||||||||
| Proceeds from short and long-term debt | 1,769,500 | 1,090,000 | 13,399,500 | |||||||||
| Proceeds from shareholder debt | 825,000 | 1,358,216 | 4,223,216 | |||||||||
| Repayment of long-term debt | (483,921 | ) | (255,262 | ) | (1,927,260 | ) | ||||||
| Proceeds from Obligation related to the Letter of Credit | 600,000 | - | 600,000 | |||||||||
| Repayment of Obligation related to the Letter of Credit | (222,305 | ) | - | (222,305 | ) | |||||||
| Financing costs | - | - | (4,387 | ) | ||||||||
| Net cash provided by financing activities | 7,319,049 | 6,599,805 | 49,392,549 | |||||||||
| Effect of exchange rate changes on cash | 6,487 | (19,944 | ) | 84 | ||||||||
| Net increase (decrease) in cash | 13,435 | (448,932 | ) | 41,039 | ||||||||
| Cash, beginning of period | 27,604 | 505,277 | - | |||||||||
| Cash, end of period | $ | 41,039 | $ | 56,345 | $ | 41,039 | ||||||
| Supplemental disclosure of cash flow information: | ||||||||||||
| Cash paid during the period for interest | $ | 403,140 | $ | 315,235 | $ | 1,646,253 | ||||||
| Supplemental disclosure of non-cash investing and financing activities: | ||||||||||||
| Financed equipment | $ | 205,381 | $ | 72,635 | $ | 2,996,288 | ||||||
| Debt converted to company stock | $ | 3,331,600 | $ | - | $ | 12,960,310 | ||||||
| Stock subscription | $ | - | $ | 23,605 | $ | - | ||||||
| F-30 |
PROTEA BIOSCIENCES GROUP, INC. (A DEVELOPMENT STAGE ENTERPRISE)
Notes to Consolidated Financial Statements – (Unaudited)
| 1. | Description of Company and Nature of Business |
Protea Biosciences Group, Inc. (“Protea” or the “Company”) was incorporated in the state of Delaware on May 24, 2005. Pursuant to an Agreement and Plan of Merger, dated September 2, 2011 (the “Merger Agreement”), by and among the Company, Protea Biosciences, Inc. (“PBI”), and SRKP 5 Acquisition Corp., a wholly-owned subsidiary of the Company (“MergerCo”), PBI was merged with and into MergerCo, with PBI continuing as the surviving entity (the “Merger”). Upon the closing of the Merger, the Company changed its name from “SRKP 5, Inc.” to “Protea Biosciences Group, Inc.” and became the parent company of PBI.
PBI is an emerging biotechnology company incorporated in Delaware in July 2001, and based in Morgantown, West Virginia. The Company is engaged in developing and commercializing proprietary life science technologies, products and services that are used to recover and identify proteins, metabolites and other biomolecules in tissue, cells and biological samples. The interim consolidated financial statements presented herein have been prepared by the Company, are unaudited and, in the opinion of management, reflect all adjustments of a normal recurring nature necessary for a fair statement of the financial position at September 30, 2013, the results of operations for the three and nine month periods ended September 30, 2013 and September 30, 2012, and the cash flows for the nine month periods ended September 30, 2013 and September 30, 2012. Interim results are not necessarily indicative of results for a full year.
The consolidated balance sheet presented as of December 31, 2012, has been derived from the audited consolidated financial statements as of that date. The consolidated financial statements and notes included in this report should be read in conjunction with the financial statements and notes included in the Company’s 2012 year-end audit.
The accompanying financial statements have been prepared on a going-concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The Company has experienced negative cash flows from operations since inception and had an accumulated deficit at September 30, 2013 of approximately $55 million and at December 31, 2012 of approximately $47 million. The Company has funded its activities to date almost exclusively from debt and equity financings. The Company will continue to require substantial funds to advance the research and development of its core technologies, to develop new products and services based upon its proprietary protein recovery and identification technologies, and to continue development of its recombinant pharmaceutical compound.
Management intends to meet its operating cash flow requirements primarily from the sale of equity and debt securities. The Company seeks additional capital through sales of equity securities or convertible debt and, if appropriate, to pursue partnerships to advance various research and development activities. The Company may also consider the sale of certain assets, or entering into a transaction such as a merger with a business complimentary to theirs.
While the Company believes that it will be successful in obtaining the necessary financing to fund its operations, they have no committed sources of funding and are not assured that additional funding will be available to them.
| F-31 |
| 2. | Summary of Significant Accounting Policies |
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Protea Biosciences Group, Inc. and all of its wholly-owned subsidiaries. All material intercompany accounts and transactions have been eliminated in consolidation.
Development Stage Enterprise
The Company is a development stage enterprise and devotes substantial efforts to establishing new business, raising capital, conducting research and development activities and developing markets. All losses accumulated, since inception, have been considered as part of the Company’s development stage activities.
Estimates and Assumptions
The presentation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Revenue Recognition
The Company derives its revenue from the sale of products and services. LAESI product revenue is recognized upon the transfer of title. Consumable product revenue is recognized upon shipment of the product. Service revenue is recognized upon completion of service contracts that are normally short-term in duration. However, where applicable, service revenue is recorded under proportional performance for projects in process to approximate the amount of revenue earned based on the percentage of effort completed.
Shipping and handling costs are included in selling, general and administrative expenses. Shipping and handling costs charged to customers are recorded as revenue in the period the related product sales revenue is recognized.
Warranty Costs
The Company provides for estimated warranty costs at the time product revenue is recognized. As the Company does not currently have historical data on warranty claims, the Company's estimates of anticipated rates of warranty claims and costs are primarily based on comparable industry metrics. The Company engages in extensive product quality programs and processes, including actively monitoring and evaluating the quality of its products. During the nine months ended September 30, 2013, the Company recorded accrued warranty expense of $65,000. The Company did not accrue warranty expense for the year ended December 31, 2012.
Reclassification
Certain amounts have been reclassified in the presentation of the Consolidated Financial Statements for the nine months ended September 30, 2012 to be consistent with the presentation in the Consolidated Financial Statements for the nine months ended September 30, 2013. This reclassification had no impact on previously reported net income, cash flow from operations or changes in stockholder equity.
| F-32 |
Total Comprehensive Loss
For the period from inception through September 30, 2013, the Company has a translation loss which represents other comprehensive loss and is included in the Statement of Operations and Total Comprehensive Loss in the financial statements.
Other Receivables
Other receivables, which reflect amounts due from non-trade activity, consist of the following at:
| September 30, 2013 | December 31, 2012 | |||||||
| French government R&D credit | $ | - | $ | 282,036 | ||||
| Employee loan – current | 2,400 | 4,000 | ||||||
| Stock subscription and other | 25,279 | 22,898 | ||||||
| Other receivables – current | $ | 27,679 | $ | 308,934 | ||||
| Deposits | $ | 22,939 | $ | 22,555 | ||||
| Other receivables – noncurrent | $ | 22,939 | $ | 22,555 | ||||
Net Loss per Share
Basic and diluted loss per common share is computed based on the weighted average number of common shares outstanding. Common share equivalents (which may consist of options, warrants and convertible debt) are excluded from the computation of diluted loss per share since the effect would be anti-dilutive. Common share equivalents which could potentially dilute basic earnings per share in the future, and which were excluded from the computation of diluted loss per share, totaled approximately 34,958,000 and 18,697,000 at September 30, 2013 and December 31, 2012, respectively.
RECENTLY ADOPTED ACCOUNTING PRONOUNCEMENTS:
Other Comprehensive Income
In February 2013, the FASB issued new guidance which requires disclosure of information about significant reclassification adjustments from accumulated other comprehensive income in a single note or on the face of the financial statements. This guidance is effective for the Company in 2013. Adoption of this standard, which is related to disclosure only, will not have an impact on the Company’s consolidated financial position, results of operations or cash flows.
In June 2011, the FASB issued new guidance pertaining to the presentation of comprehensive income. The new rule eliminates the previous option to report other comprehensive income and its components in the statement of changes in equity. The standard is intended to provide a more consistent method of presenting non-owner transactions that affect the Company’s equity. Under the new guidance, an entity can present items of net income and other comprehensive income in one continuous statement or in two separate, but consecutive, statements. The new guidance was effective for the Company on January 1, 2012 and did not have an impact on the Company’s consolidated financial position, results of operations or cash flows.
| 3. | Bank Line of Credit |
In August of 2009, the Company received a line of credit of up to $3,000,000 and is payable on demand. The interest rate is variable and is .75% plus prime with a minimum rate of 5.87%. The line of credit is subject to an annual review and certain covenants. At September 30, 2013 and December 31, 2012, the balance was $2,725,000 with interest payable at 5.87% and all covenants had been met. Borrowings under the line are secured by the personal guarantee of three board members and the estate of a former board member.
| F-33 |
| 4. | Loans Payable to Stockholders |
During 2011 and 2012, the Company issued convertible promissory notes in an aggregate principal amount equal to $2,898,216 to various board members, their spouses and other related parties. The notes accrue simple interest at a rate of 10% per annum and were due and payable 60 to 180 days from the date of issue. At the option of the holders, the notes, including the principal and accrued interest, were convertible into common stock of the Company at $2.00 per share. The noteholders signed multiple addendums to the notes agreeing to extend the maturity date. On March 22, 2013, the Company and the noteholders amended the notes to extend the maturity date to May 31, 2013 and reduce the conversion price to $0.50 per share. Prior to June 30, 2013, the Company entered into certain Conversion Agreements, which provided that if the noteholders agreed to convert the entire principal and interest balance due on these notes into common stock on or prior to June 30, 2013, the Company would issue additional five year warrants to purchase 75% of the number of shares of common stock into which the notes are convertible at an exercise price of $1.10 per share. The Company was notified of conversion of all but one note prior to June 30, 2013 and the related warrants and shares were issued in July 2013. On August 6, 2013, the Company along with one board member and his spouse agreed to further extend one note for $20,000 until December 31, 2013.
On January 3, 2013, the Company issued a convertible promissory note in an aggregate principal amount equal to $125,000 to El Coronado Holdings, Inc., an affiliate of Josiah Austin, a director of the Company. At the option of the holder, the note, including the principal and accrued interest, was convertible into common stock of the Company at $0.50 per share. On May 1, 2013, the Company and El Coronado Holdings, LLC extended the maturity date by 60 days to May 31, 2013. Prior to June 30, 2013, the Company entered into certain Conversion Agreements, which provided that if the noteholder agreed to convert the entire principal and interest balance due on these notes into common stock on or prior to June 30, 2013, the Company would issue additional five year warrants to purchase 75% of the number of shares of common stock into which the notes are convertible at an exercise price of $1.10 per share. The Company was notified of conversion prior to June 30, 2013 and the related warrants and shares were issued on July 23, 2013.
On January 11, 2013, the Company received an advance equal to an aggregate of $100,000 from Summit Resources, Inc., an affiliate of Steve Antoline, a director of the Company. On July 23, 2013, the Company issued 200,000 shares of common stock at $0.50 per share and a warrant to purchase up to 150,000 shares of common stock with an exercise price of $1.10 per share for the advanced funds.
On August 6, 2013, the Company entered into the Note and Warrant Purchase Agreement (the “Summit Agreement”) with Summit Resources, Inc. (“Summit”), an affiliate of Steve Antoline, a director of the Company, pursuant to which Summit acquired (a) a promissory note (the “Summit Note”) in an aggregate principal amount of $600,000 and (b) a five year warrant to purchase up to an aggregate of 250,000 shares of common stock for each $150,000 borrowed of common stock at an exercise price of $1.10 per share for up to a maximum of 1,000,000 shares of common stock as consideration for up to $600,000 in advances made or to be made, which was fully advanced to the Company as of September 30, 2013. In accordance with the terms of the Summit Agreement, up to $150,000 of the gross proceeds received by the Company from the sale of each LAESI instrument, beginning with the sale of the fourth LAESI instrument sold following the effective date, shall be used to repay the principal amounts due under the Summit Note. The Company issued a warrant to purchase up to 1,000,000 shares of common stock at an exercise price of $1.10 in connection with the Summit Agreement as of August 6, 2013.
| F-34 |
| 5. | Obligation Related to the Letter of Credit |
On March 6, 2013, the Company entered into that certain Warrant Purchase and Reimbursement Agreement, dated March 6, 2013 (the “Reimbursement Agreement”) with Steve Antoline, a director of the Company, pursuant to which Mr. Antoline agreed to issue a letter of credit, dated February 25, 2013 (the "Letter of Credit") for the benefit of MPR Associates, Inc. ("MPR") for the purpose of guaranteeing an amount equal to $600,000 (the “Purchase Order Amount”) due by the Company to MPR in connection with the production of certain LAESI instruments in exchange for (i) the agreement by the Company to reimburse Mr. Antoline for any amounts paid by him on behalf of the Company pursuant to the Letter of Credit, up to the Purchase Order Amount and (ii) a warrant to purchase up to 1,100,000 shares of the Company’s common stock, issued to an affiliate of Mr. Antoline. The Letter of Credit expired on September 30, 2013. On February 26, 2013, MPR drew $300,000 against the Letter of Credit and on March 15, 2013, it drew an additional $300,000. As of September 30, 2013, $377,695 was due to Steve Antoline in connection with the terms of the Reimbursement Agreement. On June 14, 2013, the Company received $147,860 as payment for one of the LAESI instruments. The Company and Steve Antoline entered into an Addendum to the Warrant Purchase and Reimbursement Agreement whereby the Company could use the funds for up to 80 days or until September 1, 2013. The Addendum was further amended whereby the Company and Mr. Antoline agreed to extend the maturity date until December 31, 2013. For the period the funds are used by the Company, Steve Antoline will receive interest on a 10% per annum basis. In addition, the Company will also issue a five-year warrant to purchase 250,000 shares of common stock at an exercise price of $1.10 per share upon the repayment of the funds. Subsequent to the balance sheet date, the Company remitted an additional $139,000 related to the proceeds from the sale of one LAESI unit to Mr. Antoline and reduced the balance due to $238,695.
| 6. | Long-term Debt |
1) Note Payable to the West Virginia Development Office
In March 2007, the Company obtained an 8-year loan in the amount of $685,000 from the West Virginia Development Office. The note bears interest at 3% providing for 96 monthly principal and interest payments of $8,035 through April 2015, at which time the note is due and payable. The note is secured by equipment costing $1,057,167.
2) Note Payable to the West Virginia Economic Development Authority
In August 2009, the Company obtained a 10-year loan in the amount of $242,631 from the West Virginia Economic Development Authority. The note bears interest at4% providing for 120 monthly principal and interest payments of $2,457 through August 2019, at which time the note is due and payable. The note is secured by 50% of equipment costing $569,812.
3) Note Payable to the West Virginia Infrastructure and Jobs Development Council
In August 2009, the Company obtained a 10-year loan in the amount of $242,630 from the West Virginia Infrastructure and Jobs Development Council. The note bears interest at 3.25% providing for 120 monthly principal and interest payments of $2,371 through August 2019, at which time the note is due and payable. The note is secured by 50% of equipment costing $569,812.
4) Note Payable to the West Virginia Economic Development Authority
In October 2010, the Company issued a 10-year note in the amount of $900,000 from the West Virginia Economic Development Authority. The note bears interest at3.26% providing for 120 monthly principal and interest payments of $8,802 through October 2020, at which time the note is due and payable. The note is secured by equipment costing $997,248.
5) Note Payable to the West Virginia Infrastructure and Jobs Development Council
In December 2010, the Company issued a 10-year note in the amount of $900,000 from the West Virginia Infrastructure and Jobs Development Council. The note bears interest at 3.25% providing for 120 monthly principal and interest payments of $8,781 through December 2020, at which time the note is due and payable. The note is secured by equipment costing $1,098,249.
6) Convertible Promissory Note Payable to the West Virginia Jobs Investment Trust Board
In March 2012, the Company issued an 18-month note in the amount of $290,000 from the West Virginia Jobs Investment Trust Board. The note bears interest at 6% providing for monthly interest-only payments starting April 2012 through August 2013, then final interest and principal payments due September 2013. The note includes a stock warrant for 72,500 shares (see Note 9, Stock Warrants). On September 3, 2013, the Company and the West Virginia Jobs Investment Trust Board entered into a Loan Modification Agreement whereby the maturity date changed from September 14, 2013 to $100,000 due on December 15, 2013, $100,000 due on March 15, 2014 and the remaining $90,000 due on June 15, 2014. An executive member of the West Virginia Jobs Investment Trust Board is a board member of the Company.
| F-35 |
7) Convertible Promissory Note Payable to the West Virginia Jobs Investment Trust Board
In April 2012, the Company issued a 3-month note in the amount of $400,000 from the West Virginia Jobs Investment Trust Board. The note bears interest at 10% providing for monthly interest-only payments starting May 2012 through June 2012, then final interest and principal payments due July 2012. The note includes a stock warrant for 88,889 shares (see Note 9, Stock Warrants). On June 18, 2012, the West Virginia Jobs Investment Trust Board signed an addendum to the note agreeing to extend the maturity date by 90 days to October 15, 2012. On March 11, 2013, the West Virginia Jobs Investment Trust Board approved an additional deferral of principal and interest until May 15, 2013 and reduced the price of converting into common shares to $0.50 per share. The West Virginia Jobs Investment Trust Board further extended the maturity date until October 15, 2013. Subsequent to the balance sheet date, the West Virginia Jobs Investment Trust Board further extended the maturity date until November 29, 2013, with a $100,000 principal payment due on or before November 15, 2013. An executive member of the West Virginia Jobs Investment Trust Board is a board member of the Company.
8) Convertible Promissory Note Payable to the West Virginia High Technology Consortium Foundation
In May 2012, the Company issued a 30-month note in the amount of $200,000 from the West Virginia High Technology Consortium Foundation. The note bears interest at 8% providing for 25 monthly principal and interest payments of $9,001 starting December 2012 through November 2014, then final interest and principal payments due December 2014. The note is secured by 50% of equipment costing $447,320.
9) Note Payable to the West Virginia Economic Development Authority
In June 2012, the Company issued a 10-year note in the amount of $200,000 from the West Virginia Economic Development Authority. The note bears interest at 2% providing for 120 monthly principal and interest payments of $1,840 through June 2022, at which time the note is due and payable. The note is secured by 50% of equipment costing $447,320.
10) Capital Leases
From time to time, in the normal course of business, the Company enters into capital leases to finance equipment. As of September 30, 2013, the Company had four capital lease obligations outstanding with imputed interest rates ranging from 2.9% to 8.0%. The leases require 36 monthly payments and begin to expire in March 2013 through May 2015. These leases are secured by equipment with an aggregate cost of $560,195. As of September 30, 2013, the Company was in arrears on all four capital leases.
11) Bridge Notes
In July and September 2013, the Company issued convertible debentures to eighteen (18) accredited investors. The convertible 1-year promissory notes bear simple interest at 10% and are due and payable on the anniversary date on an aggregate principal amount equal to $1,769,500 and include stock warrants in aggregate of1,327,125 shares (see Note 9, Stock Warrants.) The Bridge Notes and all accrued unpaid interest thereon shall automatically convert into a subsequent offering by the Company at a rate of two shares of the Company’s common stock and a) a 1-year warrant to purchase two shares of common stock at an exercise price of $0.50 per share for each $1 invested and b) a 5-year warrant to purchase one share of common stock exercisable at $0.75 per share for each $1.00 invested or on such other terms and conditions as may be determined by the Company; provided however that such terms shall be consistent with the terms of the subsequent offering. The Company issued three (3) notes to a director of the Company in aggregate of $1,115,000 and stock warrants in aggregate of 836,250 shares. Subsequent to the balance sheet date, the Bridge Notes converted into common stock (see Note 15, Subsequent Events.)
| F-36 |
Total debt outstanding as of September 30, 2013 and December 31, 2012 are as follows:
| September 30, 2013 | December 31, 2012 | |||||||||
| 1) | Note Payable to the WV Development Office | $ | 155,967 | $ | 258,497 | |||||
| 2) | Note Payable to the WVEDA | 155,099 | 179,728 | |||||||
| 3) | Note Payable to the WVIJDC | 152,935 | 177,894 | |||||||
| 4) | Note Payable to the WVEDA | 667,128 | 756,217 | |||||||
| 5) | Note Payable to the WVIJDC | 679,766 | 768,259 | |||||||
| 6) | Note Payable to the WVJITB | 290,000 | 275,045 | |||||||
| 7) | Note Payable to the WVJITB | 400,000 | 400,000 | |||||||
| 8) | Note Payable to the WVHTCF | 128,086 | 191,373 | |||||||
| 9) | Note Payable to the WVEDA | 177,129 | 196,983 | |||||||
| 10) | Capital Leases | 182,439 | 253,519 | |||||||
| 11) | Bridge Notes | 1,747,803 | - | |||||||
| Total | 4,736,352 | 3,457,515 | ||||||||
| Less: current portion | (3,037,991 | ) | (1,374,489 | ) | ||||||
| Long-term portion | $ | 1,698,361 | $ | 2,083,026 | ||||||
Future required minimum principal repayments over the next five years are as follows:
| Year ending December 31: | Future required minimum principal repayments | |||
| 2012 (payment in arrears) | $ | 17,296 | ||
| 2013 | $ | 698,226 | ||
| 2014 | $ | 2,440,570 | ||
| 2015 | $ | 289,917 | ||
| 2016 | $ | 253,577 | ||
| 2017 | $ | 262,002 | ||
| 2018 & Thereafter | $ | 774,764 | ||
| 7. | Common Stock |
The Company is authorized to issue a total of 210,000,000 shares of stock, of which 200,000,000 shares are designated common stock and 10,000,000 shares are designated preferred stock.
Common Stock – par value of $.0001 per share with one vote in respect of each share held. Holders of common stock do not have cumulative voting rights. The members of the Board of Directors are elected by the affirmative vote of the holders of a majority of the Company’s outstanding common stock.
Common stock issued during the first nine months of 2013 is as follows:
| # Shares Issued | Par Value | Price Per Share | Gross Proceeds | Value of Services Obtained | Par Value | Additional Paid in Capital (4) | ||||||||||||||||||||||
| December 31, 2012 Balance | 31,879,247 | $ | .0001 | Various | $ | 37,467,400 | $ | 529,138 | $ | 3,188 | $ | 37,993,350 | ||||||||||||||||
| Issuance of stock | 10,266,900 | .0001 | .50 | 5,133,450 | - | 1,027 | 5,132,423 | |||||||||||||||||||||
| Issuance of stock (1) | 200,000 | - | 100,000 | 20 | 99,980 | |||||||||||||||||||||||
| Issuance of stock (2) | 527,500 | - | - | 53 | (53 | ) | ||||||||||||||||||||||
| Issuance of stock (3) | 6,663,199 | 3,103,216 | - | 666 | 3,102,550 | |||||||||||||||||||||||
| September 30, 2013 Balance | 49,536,846 | $ | 45,704,066 | $ | 629,138 | $ | 4,954 | $ | 46,328,250 | |||||||||||||||||||
| (1) | Shares issued for services performed |
| (2) | Shares issued under anti-dilution provision |
| (3) | Shares issued upon conversion of convertible debentures |
| (4) | Activity does not include issuance costs, deferred financing, warrants portion of convertible debentures and stock options. |
| F-37 |
| 8. | Preferred Stock |
The Company is authorized to issue preferred stock in one or more series and containing such rights, privileges and limitations, including voting rights, dividend rates, conversion privileges, redemption rights and terms, redemption prices and liquidation preferences, as the Board may, from time to time, determine. No shares of the Preferred Stock have been issued.
| 9. | Stock Options and Stock-based Compensation |
In 2002, the Board adopted the 2002 Equity Incentive Plan (“the 2002 Plan”) that governed equity awards to employees, directors and consultants of the Company. Under the Plan, 450,000 shares of common stock were reserved for issuance. From 2006 through 2012, the 2002 Plan was amended several times to increase the total number of shares authorized under the 2002 Plan to 4,150,000 shares. During the first quarter 2013, the Board adopted the 2013 Equity Incentive Plan (the “2013 Plan”) and together with the 2002 Plan (the “Plans”) governs the equity awards to employees, directors and consultants of the Company. Under the 2013 Plan, an additional5,000,000 shares of common stock has been reserved for issuance. On June 18, 2013, the 2013 Plan was approved by holders of a majority of the issued and outstanding shares of common stock of the Company.
The types of awards permitted under the Plans include qualified incentive stock options (ISO), non-qualified stock options (NQO), and restricted stock. Each option shall be exercisable at such times and subject to such terms and conditions as the Board may specify. Stock options generally vest over four years and expire no later than ten years from the date of grant.
A summary of stock option activity is as follows:
| Shares | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (in years) | ||||||||||
| Outstanding at December 31, 2012 | 3,864,750 | $ | 1.42 | 4.00 | ||||||||
| Granted | 1,756,000 | $ | 0.55 | |||||||||
| Exercised | - | - | ||||||||||
| Cancelled or expired | 520,000 | $ | 1.45 | |||||||||
| Outstanding at September 30, 2013 | 5,100,750 | $ | 1.12 | 7.27 | ||||||||
| Exercisable at December 31, 2012 | 2,992,417 | $ | 1.39 | 3.59 | ||||||||
| Exercisable at September 30, 2013 | 3,660,959 | $ | 1.25 | 6.53 | ||||||||
The following table summarizes information about stock options at September 30, 2013:
| Options Outstanding | Options Exercisable | |||||||||||||||||||
| Exercise Price | Outstanding | Weighted Average Remaining Contractual Life (in years) | Weighted Average Exercise Price | Exercisable | Weighted Average Exercise Price | |||||||||||||||
| $0.50 | 159,000 | 102,688 | ||||||||||||||||||
| $0.55 | 1,681,000 | 645,833 | ||||||||||||||||||
| $0.80 | 320,000 | 320,000 | ||||||||||||||||||
| $1.25 | 310,000 | 310,000 | ||||||||||||||||||
| $1.50 | 2,416,000 | 2,095,813 | ||||||||||||||||||
| $2.00 | 214,750 | 186,625 | ||||||||||||||||||
| $ 0.50 - $2.00 | 5,100,750 | 7.27 | $ | 1.12 | 3,660,959 | $ | 1.25 | |||||||||||||
| F-38 |
At September 30, 2013 and December 31, 2012, the total aggregate intrinsic value for options currently exercisable and options outstanding was $0. These values represent the total pre-tax intrinsic value based on the estimated fair value of the Company’s stock price of $0.50 as of September 30, 2013 and December 31, 2012. During the year ended December 31, 2012, 225,000 options were exercised. During the first nine months of 2013, no additional options were exercised.
The following table summarizes the activity of the Company’s stock options that have not vested for the nine months ended September 30, 2013:
| Shares | Weighted Average Grant-date Fair Value | |||||||
| Nonvested at December 31, 2012 | 872,333 | $ | 0.441 | |||||
| Granted | 1,091,479 | $ | 0.110 | |||||
| Forfeited | 103,125 | $ | 0.448 | |||||
| Vested | 420,896 | $ | 0.328 | |||||
| Nonvested at September 30, 2013 | 1,439,791 | $ | 0.290 | |||||
The fair value of non-vested options to be recognized in future periods is $234,296, which is expected to be recognized over a weighted average period of 1.9 years. The total fair value of options vested during the nine months ended September 30, 2013 was $238,495 compared to $238,484 for the nine months ended September 30, 2012.
Stock-based compensation expense is as follows:
| Nine months Ended | ||||||||
| September 30, 2013 | September 30, 2012 | |||||||
| Selling, general, and administrative expense | $ | 220,840 | $ | 180,701 | ||||
| Research and development expense | 17,655 | 57,783 | ||||||
| Total stock-based compensation expense | $ | 238,495 | $ | 238,484 | ||||
The weighted average grant-date fair value of options granted during the nine months ended September 30, 2013 was $0.111 and for the nine months ended September 30, 2012 was $0.568 per option. The fair value of the option grants was estimated using the Black-Scholes option-pricing model with the following weighted average assumptions:
| Nine months Ended | ||||||||
| September 30, 2013 | September 30, 2012 | |||||||
| Risk-free interest rate | 0.35 | % | 1.04 | % | ||||
| Volatility factor | 24.48 | % | 24.04 | % | ||||
| Weighted average expected life (in years) | 7 | 7 | ||||||
| Dividend rate | 0.0 | % | 0.0 | % | ||||
The Company based its estimate of expected stock price volatility on monthly price observations of the AMEX Biotech Index. Given the Company’s limited history with stock options, the Company’s expected term is based on an average of the contractual term and the vesting period of the options (the SAB 110 “Simplified” method).
| 10. | Stock Warrants |
From 2008 through 2013, the Company has issued warrants mostly in connection with common stock issuances. The warrants are exercisable for five years from date of issuance. The warrant allows the holder the ability to purchase shares of common stock. The exercise price is $1.10 to $2.25 per share.
| F-39 |
In November 2009, the Company issued stock warrants to four board members in exchange for personal guarantees on a bank line of credit (See Note 3, Bank Line of Credit). The warrants are exercisable for five years from date of issuance. The warrant allows the four holders the ability to purchase 375,000 shares of common stock. The exercise price is $2.00 per share.
In March 2012, the Company issued stock warrants in connection with the issuance of a Convertible Debenture to the West Virginia Jobs Investment Trust Board (see Note 6, Long-term Debt). The warrant is exercisable for five years from date of issuance. The warrant allows the holder the ability to purchase 72,500 shares of common stock. The exercise price is $2.25 per share.
In April 2012, the Company issued stock warrants in connection with the issuance of a Convertible Debenture to the West Virginia Jobs Investment Trust Board (see Note 6, Long-term Debt). The warrant is exercisable for five years from date of issuance. The warrant allows the holder the ability to purchase 88,889 shares of common stock. The exercise price is $2.25 per share.
In March and April 2013, the Company issued stock warrants to a placement agent as commission in connection with the sale of common stock pursuant to two private placement offerings. The warrants are exercisable for five years from date of issuance. The warrant allows the holder the ability to purchase 387,720 shares of common stock. The exercise price is $1.10 to $2.20 per share.
In March 2013, the Company issued stock warrants in connection with the Reimbursement Agreement with Steve Antoline, a director of the Company (see Note 5, Obligation Related to the Letter of Credit.) The warrants are exercisable for five years from date of issuance. The warrant allows the holder the ability to purchase1,100,000 shares of common stock. The exercise price is $1.10 per share.
In August 2013, the Company also issued stock warrants in connection with the Note and Warrant Purchase Agreement with Summit Resources, Inc., which is an affiliate of Steve Antoline (see Note 4, Related Party Debt.) The warrants are exercisable for five years from date of issuance. The warrant allows the holder the ability to purchase 1,000,000 shares of common stock. The exercise price is $1.10 per share.
In July and September 2013, the Company issued stock warrants in connection with Bridge Notes (see Note 6, Bridge Notes.) The warrants are exercisable for five years from date of issuance. The warrants allow the holders the ability to purchase 1,327,125 shares of common stock. The exercise price is $1.10 per share.
In July 2013, the Company issued stock warrants in connection with the conversion of related party debt. The warrants are exercisable for five years from date of issuance. The warrants allow the holders the ability to purchase 4,997,399 shares of common stock. The exercise price is $1.10 per share.
As of September 30, 2013, warrants to purchase 31,297,239 shares of common stock were outstanding and exercisable.
| 11. | Income Taxes |
The provision for income taxes, if any, is comprised of current and deferred components. The current component, if any, presents the amount of federal and state income taxes that are currently reportable to the respective tax authorities and is measured by applying statutory rates to the Company’s taxable income as reported in its income tax returns.
The Company adopted ASC 740-10 Income Taxes effective January 1, 2007. The adoption of ASC 740-10 did not require an adjustment to the opening balance of retained earnings as of January 1, 2007. There were no changes to unrecognized tax benefits during 2012 or the first nine months of 2013. The tax years 2009 through 2012 remain open to review by various taxing authorities.
| F-40 |
Deferred income taxes are provided for the temporary differences between the carrying values of the Company’s assets and liabilities for financial reporting purposes and their corresponding income tax basis. These temporary differences are primarily attributable to a net operating loss, which, due to income tax laws and regulations, become taxable or deductible in different years than their corresponding treatment for financial reporting purposes. The temporary differences give rise to either a deferred tax asset or liability in the financial statements, which is computed by applying statutory tax rates to taxable or deductible temporary differences based upon the classification (i.e., current or noncurrent) of the asset or liability in the financial statements which relate to the particular temporary difference. Deferred taxes related to differences that are not attributable to a specific asset or liability are classified in accordance with the future period in which they are expected to reverse and be recognized for income tax purposes.
During the development stage of the Company when future benefit of the deferred tax asset is uncertain, the Company provides for a full valuation allowance against the deferred tax asset. Net operating loss carryforwards start to expire beginning 2021 for both federal and state purposes. The net operating tax loss carryforwards total approximately $48,100,000 and $41,500,000 at September 30, 2013 and December 31, 2012, respectively.
| 12. | Lease Commitments |
The Company leases its USA facilities under operating leases beginning a) January 2013 on a month to month basis and b) April 2012 through March 2017. Additionally, the Company leases its Europe facility on a three year lease beginning January 2012 through January 2015. The Company also has three equipment operating leases with terms of three to five years. Future required minimum principal repayments over the next five years are as follows:
| Year ending December 31: | Future required minimum lease payments | |||
| 2013 | $ | 208,057 | ||
| 2014 | $ | 260,376 | ||
| 2015 | $ | 173,500 | ||
| 2016 | $ | 167,628 | ||
| 2017 | $ | 41,907 | ||
Rent expense totals $108,446 for the three months ended and $325,980 for the nine months ended September 30, 2013, compared to $113,778 for the three months ended and $300,754 for the nine months ended September 30, 2012.
| 13. | Retirement Plan |
The Company provides a 401(k) Profit Sharing Plan (“401(k) Plan”) for elective deferrals whereby participants can defer up to 100% of their wages not to exceed a maximum dollar amount determined by the Federal Government each year. The Company, at its discretion, can make matching contributions to the 401(k) Plan. The Company may also make qualified non-elective contributions to participants who are not highly compensated employees. All employees meeting age and hours of service requirements are eligible to participate in the 401(k) Plan beginning their first full month of service. Participants become vested in employer contributions on a graduated scale with full vesting after five years. No company contributions have yet been made.
| 14. | Commitments and Contingencies |
Legal Proceedings
The Company currently is not a party to any material legal proceeding and has no knowledge of any material legal proceeding contemplated by any governmental authority or third party. The Company may be subject to a number of claims and legal proceedings which, in the opinion of our management, are incidental to normal business operations. In management’s opinion, although final settlement of these claims and suits may impact the financial statements in a particular period, they will not, in the aggregate, have a material adverse effect on the Company’s financial position, cash flows or results of operations.
| F-41 |
Warranty Obligations
In the ordinary course of business, the Company warrants to customers that its products will conform to published or agreed specifications. During the nine months ended September 30, 2013, the Company recorded accrued warranty expense of $65,000. The Company did not accrue warranty expense for the year ended December 31, 2012.
Indemnity of Directors and Officers
As permitted under Delaware law and required by corporate by-laws, the Company indemnifies and holds harmless its directors and officers for certain events or occurrences while the director or officer is or was serving in such capacity. The maximum potential amount of future payments that could be required under these indemnification obligations is unlimited; however, the Company maintains a Directors and Officers liability insurance policy that enables it to recover a portion of any future amounts paid with a limit of liability of $5,000,000. The Company may incur an additional liability if indemnity for more than the limit of liability occurred, and such liability may have a material adverse effect on its financial position, cash flows and results of operations. As there were no known or pending claims, the Company has not accrued a liability for such claims as of September 30, 2013.
University License Agreements
The Company has agreements with universities related to in-licensed technologies as follows:
AGREEMENT WITH WEST VIRGINIA UNIVERSITY (WVU)
The Company has entered into a License and Exclusive Option to License Agreement with the West Virginia University Research Corporation, a nonprofit West Virginia corporation (“WVURC”) acting for and on behalf of WVU. Under the terms of this Agreement, the WVURC has granted the Company an exclusive option to license technology from the laboratories of certain WVU principal investigators in the field of protein discovery, for therapeutic, diagnostic and all other commercial fields worldwide. Under the terms of this Agreement, the Company pays expenses for the preparation, filing and prosecution of related patent applications, and the Company will pay royalties on the net revenue resulting from the sale of products and services that utilize the WVU subject technology.
AGREEMENT WITH VIRGINIA TECH (VT)
In 2010, the Company entered into an agreement with VT for the exclusive option to license technology in development in the laboratory of Rafael Davalos, PhD., which is intended to enable the separation of specific cell populations, such as cancer cells, in blood and other fluids. It also has an exclusive license to the IRAK-1 compound as a regulator of diseases and disorders in the lab of Liwu Li, PhD. Per the licensing agreements, the Company may be required to pay milestone payments of $12,500 during 2013.
AGREEMENT WITH JOHNS HOPKINS UNIVERSITY (JHU)
In June 2009, the Company entered into an Exclusive License Agreement with Johns Hopkins University for technology developed in the laboratory of Jennifer Van Eyk, Ph.D., Professor of Medicine, Division of Cardiology, Biological Chemistry and Biomedical Engineering. The technology field is albumin-bound protein complexes and their use in the diagnosis of cardiovascular disease. Under the terms of the license agreement, the Company has the exclusive, worldwide rights to commercialize the technology. The Company is obligated to pay expenses for the preparation, filing and prosecution of related patent applications, and the Company will pay royalties on the net revenues resulting from the sale of products and services that utilize the JHU subject technology.
AGREEMENT WITH GEORGE WASHINGTON UNIVERSITY (GWU)
In March 2009, the Company entered into an Exclusive License Agreement with George Washington University (Washington D.C.) for technology developed in the laboratory of Dr. Akos Vertes Ph.D., Professor of Chemistry, Professor of Biochemistry & Molecular Biology, Founder and Co-Director of the W.M. Keck Institute for Proteomics Technology and Applications, the Department of Chemistry, who is a science advisor to the Company. The technology field is LAESI - laser ablation electrospray ionization, a new method of bioanalytical analysis that enables high throughput biomolecule characterization.
| F-42 |
Under the terms of the license agreement, the Company has the exclusive, worldwide rights to commercialize the technology. The Company is obligated to pay expenses for the preparation, filing and prosecution of related patent applications, and the Company will pay royalties of 5% on the net revenues resulting from the sale of products and services that utilize the GWU subject technology.
In November 2012, the Company entered into a Patent License Agreement with George Washington University (Washington D.C.) for technology developed in the laboratory of Akos Vertes Ph.D. The technology field is Laser Desorption/Ionization and Peptide Sequencing on Laser-Induced Silicon Microcolumn Arrays (Matrix) and Nanophotonic Production, Modulation and Switching of Ions by Silicon Microcolumn Arrays.
Under the terms of the license agreement, the Company has an exclusive, worldwide license to make, have made, use, import, offer for sale and sell the licensed products. The Company is obligated to pay a license initiation fee of $25,000 and a minimum license diligence resources of $12,500 in year two and $20,000 each year thereafter to develop and commercialize the products, $30,000 milestone payment upon the first sale of a licensed product, and royalties will be 7% of the net sales of licensed products and 5% of the net sales of combination products for combined cumulative net sales up to $50 million. Thereafter, royalties reduce to 6% and 4%, respectively, after taking into account quarterly minimum royalties of $1,500 for the first four quarters after the first sale of a licensed product, $2,500 for the next four quarters, $3,000 for the next four quarters and $6,000 for each succeeding quarter.
Joint Pharmaceutical Development Agreement
AGREEMENT WITH LABORATOIRE MAYOLY SPINDLER SAS (Mayoly)
In May 2009, the Company, and its wholly-owned subsidiary, Proteabio Europe SAS, entered into a Joint Research and Development Agreement with Mayoly, a European Pharmaceutical company with headquarters in France, for the joint development of a recombinant lipase biopharmaceutical. Under terms of the agreement, Protea receives the exclusive marketing rights for the therapeutic in the territory of North America; will be responsible for paying 40% of the development expenses (with Mayoly funding the remaining 60%); will pay royalties on net sales of the biopharmaceutical, and a milestone payment of 1 million Euros at the time the Company obtains the first FDA approval for the recombinant Lipase biotherapeutic. The Company also receives a sublicense to certain patents owned by Mayoly and licensed from the Government of France. As of September 30, 2013 and December 31, 2012, based on estimated expenditures by both companies and the anticipated sharing of expenditures, the Company estimates that it owed approximately $36,000 to Mayoly, which is reflected in Trade Accounts Payable on the Consolidated Balance Sheet.
Engineering and Design Services
The Company has approximately $254,000 in outstanding commitments with MPR Associates, Inc. for engineering and design services related to their LAESI (Laser Ablation Electrospray Ionization) technology. These amounts relate to further design enhancements to its instruments and the transfer of manufacturing knowledge to the Company's contract manufacturer, Dynamic Manufacturing, LLC. At September 30, 2013, the Company recorded approximately $7,800 in accrued expenses owed to MPR.
The Company has approximately $3,500 in outstanding commitments with Bridger Photonics for lasers related to the LAESI technology. At September 30, 2013, the Company did not have a related accrual for Bridger.
Contract Manufacturing Services
The Company has approximately $588,000 in outstanding commitments with Dynamic Manufacturing, LLC for contract manufacturing services related to their LAESI technology. These amounts relate to the production of instruments. At September 30, 2013, the Company did not have a related accrual for Dynamic.
| F-43 |
| 15. | Evaluation of Subsequent Events |
Issuance of Common Stock
On November 1, 2013 (the “Initial Closing”), the Company received $2,104,965 in aggregate gross cash proceeds from 36 accredited investors in connection with the sale of approximately 21 units (each a “Unit” and collectively, the “Units”) in an offering (the “Offering”) of a minimum of $2,000,000 (the “Minimum Offering Amount”) and up to a maximum of $6,000,000 (the “Maximum Offering Amount”), in Units of securities of the Company pursuant to the terms and conditions of a Subscription Agreement (the “Subscription Agreement”) and Unit Purchase Agreement (the “Purchase Agreement”) by and among the Company and each of the purchasers (the “Purchasers”) thereto. The Maximum Offering Amount excludes up to an additional $2,000,000 (the “Additional Offering Amount”) in Units that may be sold to any existing stockholders, officers, and directors of the Company, including any of their affiliates (the “Company Investors”). Each Unit consists of (i) 200,000 shares of common stock, par value $.0001 per share (the “Common Stock”), (ii) and two warrants, including (a) a 1 year warrant to purchase 200,000 shares of Common Stock at an exercise price of $0.50 per share (the “A Warrants”) and (b) a 5 year warrant to purchase 100,000 shares of Common Stock at an exercise price of $0.75 per share (the “B Warrants” and together with the A Warrants the “Investor Warrants”).
In connection with the Initial Closing of the Offering, the Company also paid to the placement agent (the “Placement Agent”) an aggregate of $314,669 in cash commissions, representing (1) 10% of the gross proceeds raised in the Offering from Purchasers introduced to the Company by the Placement Agent; (2) 2% of the aggregate gross proceeds raised in the offering from Company Investors, including the Conversion Amount and (3) 2% of the aggregate gross proceeds raised in the Offering, including the Conversion Amount, in connection with a non-accountable expense allowance. In addition, the Company agreed to issue warrants (the “Placement Agent Warrants”) to purchase an aggregate of 1,052,483 shares of Common Stock to the Placement Agent (or its designees), representing 10% of the number of shares of Common Stock underlying the Units sold in the Offering, including the shares of Common Stock underlying the Investor Warrants.
Conversion of Convertible Debentures
In addition, at the Initial Closing, the Company issued an aggregate of approximately 18.77 in additional Units to certain existing note holders, in connection with the conversion of $1,876,828 (the “Conversion Amount”) in outstanding principal and accrued unpaid interest in convertible promissory notes.
Issuance of Convertible Debentures
Subsequent to the balance sheet date (the “October Note Issue Date”), the Company has issued to three (3) accredited investors (each an “October Noteholder” and collectively, the “October Noteholders”) (1) 10% convertible 1-year promissory notes (each an “October Note” and collectively, the “October Notes”) in an aggregate principal amount equal to $225,000 and (2) warrants (each an “October Noteholder Warrant” and collectively, the “October Noteholder Warrants”) for each investor to purchase the number of shares of the Company’s common stock as is equal to 37.5% of the principal amount of the October Notes purchased by it, divided by $0.50, pursuant to the terms and conditions of those certain Note and Warrant Purchase Agreements (the “Purchase Agreements”).
The October Notes accrue simple interest at a rate of 10% per annum and are due and payable on the one-year anniversary of the October Note Issue Date. The October Notes and all accrued unpaid interest thereon shall automatically convert into units (the (“Units”) issued by the Company in a subsequent offering (the “Subsequent Offering”) at a rate that is equal to (a) two shares (the “Subsequent Offering Shares”) of the Company’s common stock and (b) two warrants including (1) a 1 year warrant (each, an “A Warrant” and collectively, the “A Warrants”) to purchase two shares of common stock at an exercise price of $0.50 per share and (2) a 5 year warrant (each, a “B Warrant” and collectively, the “B Warrants” and together with the A Warrants the “Subsequent Offering Warrants”) to purchase one share of common stock at an exercise price of $0.75 per share for each $1.00 invested in the Subsequent Offering or on such other terms and conditions as may be determined by the Company; provided however that such terms shall be consistent with the terms of the Subsequent Offering. At the Initial Closing, the Company issued an aggregate of approximately 1.36 Units to these note holders, in connection with the conversion of $135,900 (the “Conversion Amount”) in outstanding principal and accrued unpaid interest.
| F-44 |
Issuance of Related Party Debt
Subsequent to the balance sheet date, the Company issued Promissory Notes (the “Related Party Notes”) to two (2) board members’ affiliated companies in the aggregate amount of $275,000. The notes mature on October 25, 2014 and accrue simple interest of 10% per annum. The Related Party Notes and all accrued unpaid interest thereon shall automatically convert into a subsequent offering by the Company at a rate of two shares of the Company’s common stock and a) a 1-year warrant to purchase two shares of common stock at an exercise price of $0.50 per share for each $1 invested and b) a 5-year warrant to purchase one share of common stock exercisable at $0.75 per share for each $1.00 invested or on such other terms and conditions as may be determined by the Company; provided however that such terms shall be consistent with the terms of the subsequent offering.
| F-45 |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following is an itemized statement of all expenses, all of which we will pay, in connection with the registration of the common stock offered hereby:
| Amount | ||||
| SEC registration fee | $ | 3,534.08 | * | |
| Print fees | *(1) | |||
| Legal fees | *(1) | |||
| Accounting fees and expenses | *(1) | |||
| FINRA filing fee | *(1) | |||
| Miscellaneous | *(1) | |||
| Total | 3,534.08 | * | ||
* Estimated.
(1) To be filed via amendment.
INDEMNIFICATION OF DIRECTORS AND OFFICERS
We are subject to the laws of Delaware on corporate matters, including its indemnification provisions. Section 145 of the General Corporation Law of Delaware (the “GCL”) provides that Delaware corporations are empowered, subject to certain procedures and limitations, to indemnify any person against expenses (including attorney’s fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred by him in connection with any threatened, pending, or completed action, suit, or proceeding (including a derivative action) in which such person is made a party by reason of his being or having been a director, officer, employee, or agent of the company (each, an “Indemnitee”); provided that the right of an Indemnitee to receive indemnification is subject to the following limitations: (i) an Indemnitee is not entitled to indemnification unless he acted in good faith and in a manner that he reasonably believed to be in, or not opposed to, the best interests of the company, and, with respect to any criminal action or proceeding, had no reasonable cause to believe such conduct was unlawful, and (ii) in the case of a derivative action, an Indemnitee is not entitled to indemnification in the event that he is judged to be liable to the company (unless and only to the extent that the court determines that the Indemnitee is fairly and reasonably entitled to indemnification for such expenses as the court deems proper). The statute provides that indemnification pursuant to our provisions is not exclusive of other rights of indemnification to which a person may be entitled under any bylaw, agreement, vote of stockholders, or disinterested directors, or otherwise.
Pursuant to Article Seven of our Certificate of Incorporation (“Article Seven”), we are authorized to provide indemnification of (and advancement of expenses to) directors, officers, employees and agents (and any other persons to which Delaware law permits us to provide indemnification) through bylaw provisions, agreements with such agents or other persons, vote of stockholders or disinterested directors or otherwise, in excess of the indemnification and advancement otherwise permitted by Section 145 of the GCL, subject only to limits created by applicable Delaware law (statutory or non-statutory), with respect to action for breach of duty to us, our stockholders and others.
| 84 |
Article Seven also states that our directors shall not be personally liable to us or any stockholder for monetary damages for breach of fiduciary duty as a director, except of any matter in respect of which such director shall be liable under Section 174 of the GCL or shall be liable because the director (1) shall have breached his duty of loyalty to us or our stockholders, (2) shall have acted in a manner involving intentional misconduct or a knowing violation of law or, in failing to act, shall have acted in a manner involving intentional misconduct or a knowing violation of law, or (3) shall have derived an improper personal benefit. Article Seven further states that the liability of our directors shall be eliminated or limited to the fullest extent permitted by the GCL, as amended.
Under Article Eight of our Certificate of Incorporation, any person who was or is made a party or is threatened to be made a party to or is in any way involved in any threatened, pending or completed action suit or proceeding, whether civil, criminal, administrative or investigative, including any appeal therefrom, by reason of the fact that he is or was a director or officer of ours or was serving at our request as a director or officer of another entity or enterprise (including any subsidiary), shall be indemnified and held harmless by us and we are required to advance all expenses incurred by such person in defense of any such proceeding prior to its final determination, to the fullest extent authorized by the GCL. These rights are not exclusive of any other rights to which those seeking indemnification may otherwise be entitled.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. No pending material litigation or proceeding involving our directors, executive officers, employees or other agents as to which indemnification is being sought exists, and we are not aware of any pending or threatened material litigation that may result in claims for indemnification by any of our directors or executive officers.
RECENT SALES OF UNREGISTERED SECURITIES
During the past three years, the registrant has issued and sold the following unregistered securities:
Common Stock and Warrants
On September 2, 2011, in connection with the closing of the reverse acquisition the Company issued a total of 25,006,831 shares of our common stock to the stockholders of PBI in exchange for all of the issued and outstanding shares of PBI's capital stock. In addition, upon the closing of the Merger, all outstanding warrants or other securities of PBI exercisable or convertible into shares of common stock of PBI prior to the Merger, thereafter, became exercisable or convertible, as applicable, for shares of common stock of the Company. We relied on Section 4(2) of the Securities Act of 1933 and Rule 506 of Regulation D, promulgated thereunder, to issue the securities. PBI had no more than 35 unaccredited stockholders, each PBI stockholder was provided with the information required by Rule 502 of Regulation D, at a reasonable time prior to the consummation of the Merger the PBI stockholders were given the opportunity to ask questions and receive answers concerning the terms and conditions of the Merger and we did not engage in any general solicitation or general advertising relating to the Merger.
On November 10, 2011 the Company issued an aggregate of 80,000 shares of common stock and warrants to purchase an aggregate of 40,000 shares of common stock to two accredited investors for an aggregate purchase price equal to $200,000 in connection with a private placement offering of units consisting of 40,000 shares of Common stock and warrants to purchase up to 20,000 shares of common stock. The warrants were exercisable at an exercise price of $3.25 per share after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts, commissions and fees) are at least $15,000,000, or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the issue date. On December 27, 2011, the Company issued an additional 10,000 shares of Common Stock and exchanged the warrants issued in the referenced offering, for a warrant to purchase up to 25,000 shares of Common Stock at an exercise price of $2.25 per share to each of the two investors that participated in this offering. The shares of common stock and warrants were issued to accredited investors in connection with the exemption from registration provided by Regulation D promulgated under the Securities Act. The Company did not engage in any general advertisement or general solicitation in connection with the sale of the shares and warrants.
| 85 |
As of March 1, 2012, in connection with the terms of a private placement offering (the “December 2011 Offering”) of up to 100 units (the “December 2011Units”), each December 2011 Unit consisting of 50,000 shares of common stock and warrants to purchase 25,000 shares of common stock (the “December 2011 Warrants”), the Company issued an aggregate of 1,370,000 shares of common stock and December 2011 Warrants to purchase an aggregate of 685,000 shares of common stock at a per December 2011 Unit purchase price equal to $100,000, for aggregate gross proceeds equal to $2,740,000. The December 2011 Warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance (the “December 2011 Issue Date”) until the earlier of (i) a Qualified Public Offering (as defined below), or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the December 2011 Issue Date of the December 2011 Warrant. The term “Qualified Public Offering” means the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts, commissions and fees) are at least $15,000,000. The placement agent (the " December 2011 Placement Agent") in the December 2011 Offering received total cash commissions equal to $140,000, which amount represents 8% of the total gross proceeds received from investors that participated in the Offering and were introduced to the Company by the December 2011 Placement Agent. In addition, the December 2011 Placement Agent also received warrants to purchase an aggregate of 70,000 shares of common stock at an exercise price of $2.20 per share (the "December 2011 Placement Agent Warrants"). The December 2011 Placement Agent Warrants are exercisable any time after the December 2011 Issue Date until the earlier of (1) a Qualified Public Offering or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the December 2011 Issue Date.
The securities underlying the December 2011 Units and December 2011 Placement Agent Warrants were issued to accredited investors in accordance with Rule 506 of Regulation D under the Securities Act. The Company did not engage in any general advertisement or general solicitation in connection with the offering of the December 2011Units.
On March 31, 2012, the Company issued 10,000 shares of common stock to Nathan Hostler and 20,000 shares of common stock to Melissa Hostler in connection with the exercise of a warrant and options issued to John A. Hostler at an aggregate purchase price equal to $47,500. The shares of common stock were issued in connection with the exemption from registration provided by Section 4(2) of the Securities Act.
As of June 29, 2012, in connection with the terms of a private placement offering (the “May 2012 Offering”) of up to $6,000,000 of units (the “May 2012 Units”), each May 2012 Unit consisting of 50,000 shares of common stock and warrants to purchase 25,000 shares of common stock (the “May 2012 Warrants”), the Company issued an aggregate of 525,000 shares of Common Stock and Warrants to purchase an aggregate of 262,500 shares of common stock at a per May 2012 Unit purchase price of $100,000 for aggregate gross proceeds equal to $1,050,000. The May 2012 Warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance (the “May 2012 Issue Date”) until the earlier of (i) a Qualified Public Offering, or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the May 2012 Issue Date of the May 2012 Warrant. The term “Qualified Public Offering” means the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which (i) the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 and (ii) the offering price of the common stock in the Qualified Public Offering is at least 200% of the exercise price set forth in the May 2012 Warrants, as may be adjusted in accordance with the terms of the May 2012 Warrants.
All of the May 2012 Units, and securities underlying the May 2012 Units issued in the May 2012 Offering were issued to accredited investors in accordance with Rule 506 of Regulation D under the Securities Act. The placement agent in the May 2012 Offering has received total cash compensation equal to $103,862. The Company did not engage in any general advertisement or general solicitation in connection with the offering of the May 2012 Units.
| 86 |
As of September 21, 2012, in connection with a private placement offering (the “Summer 2012 Direct Issuances”) the Company issued an aggregate of 375,000 shares of common stock and warrants to purchase 187,500 shares of common stock (the “Summer 2012 Warrants”) to accredited investors at a per unit (the “Summer 2012 Units”) purchase price equal to $100,000 for an aggregate purchase price equal to $750,000. The Summer 2012 Warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the issue date thereof.
All of the Summer 2012 Units, and securities underlying the securities issued under the Summer 2012 Direct Issuances described above were issued to accredited investors in accordance with the exemption from registration provided by Section 4(2) of the Securities action in that the securities were offered and sold to accredited investors and the Company did not engage in any general advertisement or general solicitation in connection with the offering of the Summer 2012 Units.
On July 27, 2012, the Company issued units (the “July 2012 Units”) consisting of an aggregate of 100,000 shares of common stock and warrants to purchase 50,000 shares of common stock (the “July 2012 Warrants”) to accredited investors at a per unit purchase price equal to $100,000 for an aggregate purchase price equal to $200,000. The July 2012 Warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof.
All of the July 2012 Units, and securities underlying the July 2012 Units were issued to accredited investors in accordance with the exemption from registration provided by Section 4(2) of the Securities Act in that the securities were offered and sold to accredited investors and the Company did not engage in any general advertisement or general solicitation in connection with the offering of the units.
On August 13, 2012, the Company issued units consisting of an aggregate of 12,500 shares of common stock and warrants to purchase 6,250 shares of common stock to accredited investors at a per unit purchase price equal to $100,000 for an aggregate purchase price equal to $25,000. The warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of Common Stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof.
On August 31, 2012, the Company issued units consisting of an aggregate of 62,500 shares of common stock and warrants to purchase 31,250 shares of common stock to accredited investors at a per unit purchase price equal to $100,000 for an aggregate purchase price equal to $125,000. The warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of Common Stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof.
All of the units, and securities underlying the units issued on July 3, 2012, July 27, 2012, August 13, 2012 and August 31, 2012 described above were issued to accredited investors in accordance with the exemption from registration provided by Section 4(2) of the Securities action in that the securities were offered and sold to accredited investors and the Company did not engage in any general advertisement or general solicitation in connection with the offering of the units.
| 87 |
As of October 17, 2012, in connection with the terms of a private placement offering (the “Fall 2012 Offering”) of up to $6,000,000 of units (the “Fall 2012 Units”), each Fall 2012 Unit consisting of 50,000 shares of common stock and warrants to purchase 25,000 shares of Common Stock (the “Fall 2012 Warrants”), the Company issued an aggregate of 452,500 shares of common stock and Fall 2012 Warrants to purchase an aggregate of 226,250 shares of common stock at a per Fall 2012 Unit purchase price of $100,000 for aggregate gross proceeds equal to $905,000. The Fall 2012 Warrants are exercisable at an exercise price of $2.25 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of Common Stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof. The shares of common stock and Fall 2012 Warrants issued in connection with the Fall 2012 Offering Documents were subject to anti-dilution protection in the event the Company subsequently sold shares of common stock at a price per share that is less than $2.00 per share. The Company has since issued shares at a price equal to $0.50 per share, and therefore, on March 22, 2013, the Company’s board of directors (the “Board”) approved the issuance of an additional 1,057,500 shares of common stock and reduced the exercise price of the warrants to $1.12 per share.
All of the Fall 2012 Units, and securities underlying the Fall 2012 Units issued in the Fall 2012 Offering were issued to accredited investors in accordance with Rule 506 of Regulation D under the Securities Act. The Company did not engage in any general advertisement or general solicitation in connection with the offering of the Fall 2012 Units. The placement agent in the Fall 2012 Offering received total cash compensation equal to $56,400.
On November 20, 2012, the Company entered into a Securities Purchase Agreement (the “El Coronado Purchase Agreement”) with El Coronado Holdings, LLC (“El Coronado Holdings”), an entity of which Josiah Austin, who subsequently became a director of the Company, is managing member, pursuant to which El Coronado Holdings was granted an option (the “El Coronado Option”) to purchase through December 31, 2012, up to 6,000,000 shares of common stock (the “El Coronado Shares”) and warrants to purchase up to 4,500,000 shares of common stock (the “El Coronado Warrants”) at a purchase price of $0.50 per share for an aggregate purchase price equal to $3,000,000. On November 20, 2012, the Company issued 600,000 shares of common stock and warrants to purchase 450,000 shares of Common Stock for aggregate gross proceeds of $300,000 to El Coronado Holdings, pursuant to the terms and conditions of the El Coronado Purchase Agreement. The El Coronado Warrants are exercisable at an exercise price of $1.10 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of Common Stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof. The Company paid cash commissions equal to $24,000 in connection with the sale of the El Coronado Shares and El Coronado Warrants issued on November 20, 2012.
On November 29, 2012, pursuant to the terms of the El Coronado Purchase Agreement, the Company issued an aggregate of 400,000 shares of common stock and warrants to purchase 300,000 shares of common stock for aggregate gross proceeds of $200,000 to El Coronado.
On December 10, 2012, pursuant to the terms of the El Coronado Purchase Agreement, the Company issued an aggregate of 1,000,000 shares of common stock and warrants to purchase 750,000 shares of common stock for aggregate gross proceeds of $500,000 to El Coronado. The Company paid cash commissions equal to $56,000 in connection with the sale of the El Coronado Shares and El Coronado Warrants issued on November 29, 2012 and December 10, 2012.
The El Coronado Shares and El Coronado Warrants were exempt from the registration requirements of the Securities Act pursuant to Section 4(2) thereof and the rules promulgated thereunder. The above-referenced investor represented to us that it was an accredited investor and was acquiring the shares for investment and not for distribution, that it could bear the risks of the investment and could hold the securities for an indefinite period of time. The investor received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration or an available exemption from such registration.
| 88 |
The sale of the foregoing securities was made without any form of general solicitation or advertising and all of the foregoing securities are deemed restricted securities for purposes of the Securities Act.
On March 6, 2013, the Company issued a warrant (the “Antoline Warrant”) to an affiliate of Steve Antoline, a director of the Company, to purchase up to 1,100,000 shares of the Company’s common stock at an exercise price of $1.10 per share pursuant to that certain Warrant Purchase and Reimbursement Agreement, dated March 6, 2013 by and between the Company and Steve Antoline (the “Reimbursement Agreement”), a director of the Company, as consideration for Mr. Antoline’s agreement to issue a letter of credit for the benefit of MPR Associates, Inc. for the purpose of guaranteeing an amount equal to $600,000 due by the Company to MPR in connection with the production of certain LAESI instruments. The Antoline Warrant was issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that it was issued to an accredited investor and the Company did not engage in any general solicitation, the purchaser was the sole offeree and had full access to all information concerning the Company that was requested. No commissions were paid in connection with the issuance of the Antoline Warrant.
On March 21, 2013, the Company entered into a Securities Purchase Agreement (the “March Purchase Agreement”) with El Coronado pursuant to which El Coronado was granted an option to purchase, through May 31, 2013, up to 6,000,000 shares of common stock and warrants to purchase up to 4,500,000 shares of common stock at a purchase price of $0.50 per share for an aggregate purchase price equal to $3,000,000 pursuant to which the Company issued 1,370,000 shares of common stock (the “2013 El Coronado Shares”) and warrants to purchase 1,027,500 shares of common stock (the “2013 El Coronado Warrants”) for aggregate gross proceeds of $685,000. The 2013 El Coronado Warrants are exercisable at an exercise price of $1.10 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts and commissions) are at least $15,000,000 or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the warrant issue date hereof. The 2013 El Coronado Shares and Warrants were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that they were issued to an accredited investor and the Company did not engage in any general solicitation. The Company paid cash commissions equal to $54,800 in connection with the issuance of the 2013 El Coronado Shares and 2013 El Coronado Warrants.
On April 5, 2013, the Company issued 150,000 shares of common stock at $0.50 per share and warrants to purchase 187,500 shares of common stock for $1.10 per share for services rendered pursuant to the terms of a Consulting Agreement by and between the Company and the consultant, dated March 25, 2013. The warrants are exercisable for a term of five years from the issue date of the warrants. The shares and warrants were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that the investor was the sole offeree, had access to all information concerning the Company and the Company did not engage in any general solicitation.
On April 17, 2013, as a result of the sale of shares of common stock of the Company at a per share price less than $2.00 per share, pursuant to the terms and conditions of triggering the anti-dilution provisions set forth in those certain subscription agreements and warrants (the “Offering Documents”) issued in connection with that certain Private Placement Memorandum dated August 6, 2012, the Company issued 150,000 shares of common stock for no additional consideration to certain investors that purchased securities pursuant to the Offering Documents.
From April 23, 2013 to June 5, 2013, in connection with Company direct issuances (the “Spring 2013 Company Issuances”), the Company issued an aggregate of 3,060,000 shares of the Company’s common stock and warrants to purchase an aggregate of 2,295,000 shares of common stock to certain accredited investors for aggregate gross proceeds equal to $1,530,000 pursuant to the terms and conditions of a Securities Purchase Agreement and a warrant (the “Spring 2013 Warrants”). The Spring 2013 Warrants are exercisable for a term of five years from the issue date, and each purchaser has the right to purchase the number of shares of common stock equal to 75% of the number of shares purchased by such investor, at an exercise price of $1.10 per share. The shares and Spring 2013 Warrants are subject to certain anti-dilution rights in accordance with the terms of the Securities Purchase Agreement, if at any time after the issue date of the securities through December 31, 2013, the Company issues or sells any additional shares of common stock at a purchase price less than $0.50 per share.
| 89 |
The shares and Spring 2013 Warrants were issued pursuant to the exemption from registration provided by Rule 506 of Regulation D under the Securities Act in that they were issued to accredited investors, the Company did not engage in any general advertisement or general solicitation in connection with the offering of the securities, and the Company was available to answer any questions by and purchaser. Cash commissions were not paid in connection with the sale of the shares and Spring 2013 warrants.
On July 17, 2013, the Company issued an aggregate of 377,500 shares of common stock for no additional consideration to certain stockholders in connection with the anti-dilution rights applicable to such investor’s investments. In addition, the exercise price of the warrants issued to such investors in connection with the anti-dilution rights were reduced from $2.25 to $1.12 per share.
On July 23, 2013, in connection with a private placement offering, the Company issued an aggregate of 300,000 shares of common stock at $0.50 per share and warrants to purchase up to 225,000 shares of common stock at a purchase price of $150,000. The warrants are exercisable for five years from date of issuance at an exercise price of $1.10 per share. The shares and warrants were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that the investors were the sole offerees, had access to all information concerning the Company and the Company did not engage in any general solicitation. Cash commissions were not paid in connection with the sale of the shares and warrants.
Promissory Notes
On December 20, 2011 (the "December 2011 Issue Date"), the Company issued convertible promissory notes (the "December 2011 Notes") to Stanley Hostler, a director of the Company and Summit Resources, Inc., an affiliate of Steve Antoline, a director of the Company, in an aggregate principal amount equal to $750,000 (the "December 2011 Principal Amount"). The December 2011 Notes accrue simple interest at a rate of 10% per annum and are due and payable on the earlier to occur of (i) the date that is 180 days from the December 2011 Issue Date, or (ii) when declared due and payable by the holder upon the occurrence of an event of default. At the option of the holder, each $2.00 of outstanding December 2011 Principal Amount and accrued unpaid interest is convertible into one share of common stock of the Company, at any time after the December 2011 Issue Date. The December 2011 Notes were issued in connection with the exemption from registration provided by Section 4(2), in that the December 2011 Notes were issued to two accredited investors and the Company did not engage in any general advertisement or general solicitation in connection with the sale of the shares and warrant.
On March 20, 2012, the Company issued a convertible debenture (the “March 2012 Debenture”), dated as of March 15, 2012, for an aggregate principal amount of $290,000 (the “March 2012 Principal Amount”) to the West Virginia Jobs Investment Trust Board (the “WVJITB”), evidencing a convertible loan in that amount from the WVJITB to finance the construction and leasehold improvements required in connection with the Company’s newly leased premises. The March 2012 Debenture accrues interest at a rate of 6% per annum and is subordinated to outstanding senior indebtedness of the Company. The entire March 2012 Principal Amount is due on September 14, 2013 (the “March 2012 Maturity Date”) and all accrued and unpaid interest is due and payable in cash monthly, beginning on April 14, 2012 through and including the March 2012 Maturity Date. At the option of the WVJITB, the March 2012 Debenture is convertible into shares of common stock of the Company, at an initial conversion rate of $2.00 per share, subject to certain adjustments.
In addition, the Company also issued a warrant to the WVJITB, dated as of March 15, 2012, to purchase 72,500 shares of common stock of the Company at an exercise price of $2.25 per share (the “March 2012 Warrant”). The March 2012 Debenture and the March 2012 Warrant were issued to an accredited investor in accordance with Rule 506 of Regulation D under the Securities Act of 1933, as amended. The Company did not engage in any general advertisement or general solicitation in connection with issuance and sale of the securities.
| 90 |
On April 16, 2012 (the “April 2012 Issue Date”), the Company issued convertible promissory notes ("April 2012 Notes") in an aggregate principal amount equal to $640,000 (the "April 2012 Principal Amount") to certain directors of the Company and their affiliates. The April 2012 Notes accrue simple interest at a rate of 10% per annum and were originally due and payable on the earlier to occur of (i) the date that is 90 days from the April 2012 Issue Date, or (ii) when declared due and payable by the holder upon the occurrence of an event of default (the "April 2012 Maturity Date").At any time after the April 2012 Issue Date, at the option of the holder, each $2.00 of outstanding principal amount and accrued unpaid interest is convertible into one share of common stock of the Company. On or around June 15, 2012, the April 2012 Maturity Date, with respect to each of the April 2012 Notes was extended to October 13, 2012. The April 2012 Notes were issued to accredited investors (as such term is defined by Rule 501 of Regulation D under the Securities Act), pursuant to the exemption from registration provided by Section 4(2) under the Securities Act. The Company did not engage in any general advertisement or general solicitation in connection with the offer and sale of the April 2012 Notes. The Company did not pay any commissions in connection with the sale of the April 2012 Notes.
On April 18, 2012, the Company issued a convertible debenture (the “April 2012 Debenture”), for an aggregate principal amount of $400,000 (the “April 2012 Principal Amount”) to the WVJITB. The April 2012 Debenture accrues interest at a rate of 10% per annum and is subordinated to outstanding senior indebtedness of the Company. The entire April 2012 Principal Amount was originally due on July 17, 2012 (the “April 2012 Maturity Date”). On June 18, 2012, the April 2012 Maturity Date was extended to October 15, 2012. At the option of the WVJITB the April 2012 Debenture is convertible into shares of (1) common stock of the Company, at an initial conversion rate of $2.00 per share, subject to certain adjustments or (2) preferred stock of the Company, at an initial conversion rate equal to the lowest price paid for such preferred stock by other purchasers, subject to certain adjustments. Upon the issuance of the April 2012 Debenture, the Company also issued a warrant to the WVJITB to purchase 88,889 shares of common stock of the Company at an exercise price of $2.25 per share (the “April 2012 Warrant”). The April 2012 Warrant is exercisable at an exercise price of $2.25 per share any time after the date of issuance until the earlier of (i) the closing of a firm commitment underwritten offering pursuant to an effective registration statement under the Securities Act covering the offer and sale of common stock for the account of the Company in which the net cash proceeds to the Company (after deduction of underwriting discounts, commissions and fees) are at least $15,000,000, or (ii) 5:00 p.m. Eastern time on the fifth anniversary of the issue date of the April 2012 Warrant. The April 2012 Debenture and the April 2012 Warrant were issued pursuant to the exemption from registration provided by Section 4(2) under the Securities Act in reliance upon the representation by WVJITB that it is an accredited investor as such term is defined by Rule 501 of Regulation D under the Securities Act and the Company did not engage in any general advertisement or general solicitation in connection with the offer and sale of the April 2012 Debenture and April 2012 Warrant. The Company did not pay any commission in connection with the sale of the April 2012 Debenture and April 2012 Warrant.
On May 24, 2012, the Company issued a Secured Convertible Note and Investment Agreement (the "WVHTCF Note") with a principal amount of $200,000 to West Virginia High Technology Consortium Foundation, a West Virginia non-profit corporation (“WVHTCF”). The WVHTCF Note accrues interest at a rate of eight percent (8%) per annum and has a maturity date of December 1, 2014. At any time during the term of the WVHTCF Note, WVHTCF may elect to convert all or any portion of the unpaid principal and accrued interest due and payable under the WVHTCF Note, into shares of common stock at a conversion price of $2.00 per share subject to certain adjustments in the event of stock splits or dividend issuances. The WVHTCF Note is secured by a first lien interest, shared with the West Virginia Economic Development Authority (“WVEDA”), in all of the Company’s right, title and interest in and to the equipment referred to as the Waters Synapt G2 Mass Spectrometer (Serial Number UEB072 01) (the “Mass Spectrometer”), including all attachments, accessories, tools, parts, manuals, software, parts, records and data, all cash and non-cash proceeds from the sale, destruction, loss or other disposition of the Mass Spectrometer (the “Collateral”) pursuant to the terms and conditions of a Security Agreement, dated May 24, 2012 by and between the Company and WVHTCF (the “Security Agreement”). The obligations of the Company under the WVHTCF Note are guaranteed by the Company’s wholly owned subsidiary, Protea Biosciences, Inc. (the “Subsidiary”) pursuant to the terms and conditions of a Guaranty of Payment, dated May 22, 2012.
On June 11, 2012, the Company issued a promissory note, in an aggregate principal amount of $200,000.00 (the “WVEDA Note”) to the West Virginia Economic Development Authority, in accordance with the terms and conditions of a Loan Agreement of even date. The WVEDA Note accrues interest at a rate of two percent (2%) per annum and has a ten year term. The obligations of the Company under the WVEDA Note are guaranteed by the Company’s wholly owned subsidiary, Protea Biosciences, Inc. pursuant to the terms and conditions of a Guaranty of Payment, dated June 6, 2012 and secured by the Collateral pursuant to the terms of the Security Agreement.
| 91 |
The WVHTCF Note and WVEDA Note were issued in connection with the exemption from registration provided by Section 4(2) under the Securities Act as a transaction by an issuer not involving a public offering, in which the investors are accredited and have acquired the securities for investment purposes only and not with a view to or for sale in connection with any distribution thereof.
On September 25, 2012 (the "September 2012 Issue Date"), the Company issued convertible promissory notes (the " September 2012 Notes") in an aggregate principal amount equal to $593,216 (the " September 2012 Principal Amount") to Stanley Hostler, Scott Segal, Leonard Harris, Ed Roberson, each a director of the Company, Summit Resources, Inc., an affiliate of Steve Antoline, a director of the Company, Steven Turner, the Chief Executive Officer and a director of the Company, and his wife Nancy Turner and Virginia Child, the wife of Stanley Hostler (each a " September 2012 Holder" and collectively, the " September 2012 Holders") in consideration for certain advances to the Company equal to the September 2012 Principal Amount. The September 2012 Notes accrue simple interest at a rate of 10% per annum and are due and payable on the earlier to occur of (i) the date that is 60 days from the September 2012 Issue Date, or (ii) when declared due and payable by the holder upon the occurrence of an event of default.
The occurrence of any one of the following events will be deemed an event of default: (i) the failure of the Company to pay the principal balance or accrued interest on the September 2012 Note when due; (ii) the consent or institution by or against the Company of bankruptcy or insolvency proceedings or the filing, or consent by the Company for the filing of a petition or answer or consent seeking reorganization or release under the federal Bankruptcy Act, or any other applicable federal or state law, or the appointment of a receiver, liquidator, assignee, trustee or other similar official of the Company, or of any substantial part of its property, or the making by it of an assignment for the benefit of creditors, or the taking of corporate action by the Company in furtherance of any such action; or (iii) if the commencement of an action against the Company seeking any bankruptcy, insolvency, reorganization, liquidation, dissolution or similar relief has not been resolved in favor of the Company or the consent or acquiescence of the Company of any trustee, receiver or liquidator of the Company or of all or any substantial part of the properties of the Company, shall not have been vacated within 60 days.
At the option of the September 2012 Holder, each $2.00 of outstanding principal and accrued unpaid interest is convertible into one share of common stock of the Company, at any time after the September 2012 Issue Date. The September 2012 Notes were issued to accredited investors (as such term is defined by Rule 501 of Regulation D under the Securities Act), pursuant to the exemption from registration provided by Section 4(2) under the Securities Act. The Company did not engage in any general advertisement or general solicitation in connection with the offer and sale of the September 2012 Notes. The Company did not pay any commissions in connection with the sale of the September 2012 Notes.
On October 25, 2012, the Company issued 1,000 shares of common stock to Thomas E. Taliaferro, Jr. in connection with the exercise of outstanding warrants at an exercise price $1.50 per share.
On November 30, 2012, the Company issued convertible promissory notes in an aggregate principal amount equal to $915,000 to Stanley Hostler and Leonard Harris, each a director of the Company; Summit Resources, Inc., an affiliate of Steve Antoline, a director of the Company; Virginia Child, the wife of Stanley Hostler; Carl Hostler; and Brian Prim, in consideration for certain advances to the Company equal to the principal amount. The notes accrue simple interest at a rate of 10% per annum and are due and payable on the earlier to occur of (i) January 20, 2013, or (ii) when declared due and payable by the holder upon the occurrence of an event of default. The occurrence of any one of the following events will be deemed an event of default: (i) the failure of the Company to pay the principal balance or accrued interest on the Note when due; (ii) the consent or institution by or against the Company of bankruptcy or insolvency proceedings or the filing, or consent by the Company for the filing of a petition or answer or consent seeking reorganization or release under the federal Bankruptcy Act, or any other applicable federal or state law, or the appointment of a receiver, liquidator, assignee, trustee or other similar official of the Company, or of any substantial part of its property, or the making by it of an assignment for the benefit of creditors, or the taking of corporate action by the Company in furtherance of any such action; or (iii) if the commencement of an action against the Company seeking any bankruptcy, insolvency, reorganization, liquidation, dissolution or similar relief has not been resolved in favor of the Company or the consent or acquiescence of the Company of any trustee, receiver or liquidator of the Company or of all or any substantial part of the properties of the Company, shall not have been vacated within 60 days. At the option of the holder, each $2.00 of outstanding principal and accrued unpaid interest is convertible into one share of common stock of the Company at any time after the issue date. On March 22, 2013, the Company’s board approved the adjustment of the conversion price of these notes from $2.00 to $0.50 per share.
| 92 |
On January 3, 2013, the Company issued to El Coronado for an aggregate purchase price equal to $125,000 (1) a convertible promissory note in an aggregate principal amount equal to $125,000 (the “El Coronado Note”) and (2) a warrant to purchase 187,500 shares of common stock of the Company (the “El Coronado Note Warrant”). The note accrues simple interest at a rate of 10% per annum and is due and payable on the earlier to occur of (i) April 1, 2013, or (ii) when declared due and payable by the holder upon the occurrence of an event of default. The warrant is exercisable at an exercise price of $1.10 per share any time after the issue date until the earlier of (i) a Qualified Public Offering (as such term is defined in the El Coronado Note Warrant) or (ii) 5:00 p.m. EST on the fifth anniversary of the issue date. As of the date hereof the entire principal amount remains outstanding and no amounts have been paid on the note. On May 1, 2013, the Company and El Coronado extended the maturity date by 60 days to May 31, 2013. The El Coronado Note and El Coronado Note Warrant were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that they were issued to an accredited investor and the Company did not engage in any general solicitation, the purchaser was the sole offeree and had full access to all information concerning the Company that was requested. The Company paid cash commissions equal to $10,000 in connection with the issuance of the El Coronado Note and El Coronado Note Warrant.
During the 3rd quarter of 2013 (the “July and September Note Issue Dates”), the Company issued to eighteen (18) accredited investors (each a “July and September Noteholder” and collectively, the “July and September Noteholders”) (1) 10% convertible 1-year promissory notes (each a “July and September Note” and collectively, the “July and September Notes”) in an aggregate principal amount equal to $1,769,500 and (2) warrants (each a “July and September Noteholder Warrant” and collectively, the “July and September Noteholder Warrants”) for each investor to purchase the number of shares of the Company’s common stock as is equal to 37.5% of the principal amount of the July and September Notes purchased at $1.10 per share, pursuant to the terms and conditions of those certain Note and Warrant Purchase Agreements (the “Purchase Agreements”).
During the 4th quarter of 2013 (the "October Note Issue Dates"), the Company issued to three (3) accredited investors (each an "October Noteholder" and collectively, the "October Noteholders") (1) 10% convertible I-year promissory notes (each an "October Note" and collectively, the "October Notes") in an aggregate principal amount equal to $225,000 and (2) warrants (each an "October Noteholder Warrant" and collectively, the "October Noteholder Warrants") for each investor to purchase the number of shares of the Company's common stock as is equal to 37.5% of the principal amount of the October Notes purchased by it, divided by $0.50, pursuant to the terms and conditions of those certain Note and Warrant Purchase Agreements (the "October Purchase Agreements").
During the 4th quarter of 2013 (the “Related Party Note Issue Dates”) the Company issued Promissory Notes (the “Related Party Notes” and together with the July and September Notes and the October Notes, the “2013 Notes”) to two (2) board members' affiliated companies in the aggregate amount of $275,000. The notes mature on October 25, 2014 and accrue simple interest of 10% per annum.
The 2013 Notes accrue simple interest at a rate of 10% per annum and are due and payable on the one-year anniversary of their respective Issue Dates. As of December 31, 2013, the 2013 Notes and all accrued unpaid interest thereon were converted into the Winter 2013 Offering at a rate of two shares of the Company's common stock and a) a 1-year warrant to purchase two shares of common stock at an exercise price of $0.50 per share for each $1 invested and b) a 5-year warrant to purchase one share of common stock exercisable at $0.75 per share for each $1.00 invested.
The Notes and the July and September Noteholder Warrants and the October Noteholder Warrants were issued to accredited investors in accordance with Rule 506 of Regulation D under the Securities Act in that the Company did not engage in any general advertisement or general solicitation in connection with the offering of the Notes, the July and September Noteholder Warrants and the October Noteholder Warrants, and the Company was available to answer any questions from any purchaser. Cash commissions were not paid in connection with the sale of the Notes, the July and September Noteholder Warrants and the October Noteholder Warrants.
| 93 |
On August 6, 2013, the Company entered into the Note and Warrant Purchase Agreement (the “Summit Agreement”) with Summit Resources, Inc. (“Summit”), an affiliate of Steve Antoline, a director of the Company pursuant to which Summit acquired (a) a promissory note (the “Summit Note”) in an aggregate principal amount of $600,000 and (b) a five year warrant to purchase up to an aggregate of 250,000 shares of common stock for each $150,000 borrowed of common stock at an exercise price of $1.10 per share for up to a maximum of 1,000,000 shares of common stock as consideration for up to $600,000 in advances made or to be made to the Company as consideration for advances of up to $600,000, of which $550,000 was advanced to the Company as of June 30, 2013. In accordance with the terms of the Summit Agreement, up to $150,000 of the gross proceeds received by the Company from the sale of each LAESI instrument beginning with the sale of the fourth LAESI instrument sold following the effective date shall be used to repay the principal amounts due under the Summit Note. The Summit Note and warrants were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that the investor was the sole offeree, had access to all information concerning the Company that was requested and the Company did not engage in any general solicitation.
Conversion of Promissory Notes
As of June 30, 2013, the Company entered into conversion agreements (the "Conversion Agreements") with certain related party holders (the “Existing Noteholders”) of its existing convertible promissory notes with an aggregate principal amount of $3,003,216 (the "Existing Notes") pursuant to which the Company agreed to issue 5 year warrants (the “Conversion Warrants”) to purchase up to 75% of the number of shares of common stock into which the Existing Notes were convertible, at an exercise price of $1.10 per share, provided that the conversion of the Existing Notes was exercised on or prior to June 30, 2013. In accordance with the terms and conditions of the Conversion Agreements, on June 30, 2013 the Existing Noteholders notified the Company of their desire to convert the Existing Notes into an aggregate of 6,663,199 shares (the “Conversion Shares”). On July 23 and 29, 2013, the Company issued the Conversion Shares and Conversion Warrants to purchase up to an aggregate of 4,997,399 shares of common stock. The Conversion Shares and Conversion Warrants were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that the investors were the sole offerees, had access to all information concerning the Company and the Company did not engage in any general solicitation. Cash commissions were not paid in connection with the sale of the Conversion Shares and Conversion Warrants.
Consultant Shares
On October 22, 2012, the Company issued 23,753 shares of common stock to a consultant as compensation for services performed with a value equal to $40,005. There were no commissions paid in connection with the sale of the consultant shares.
On June 5, 2013, the Company issued 48,000 shares of common stock at $0.50 per share for services rendered pursuant to the terms of a Consulting Termination Agreement by and between the Company and the consultant, dated April 15, 2013.
On December 10, 2013, the Company issued 375,000 shares of common stock at $0.50 per share for services rendered pursuant to the terms of a Consulting Agreement by and between the Company and the consultant dated March 25, 2013.
The shares issued to consultants as described above were issued in connection with the exemption from the registration requirements of the Securities Act pursuant to Section 4(2) thereof and the rules promulgated thereunder. The investor received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration or an available exemption from such registration. The sale of the foregoing securities was made without any form of general solicitation or advertising and all of the foregoing securities are deemed restricted securities for purposes of the Securities Act.
Options
On October 19, 2012, the Company issued 100,000 shares of common stock to the Milan Puskar Revocable Trust Restated 9/28/11 in connection with the exercise of outstanding options at an exercise price $1.25 per share.
| 94 |
On October 19, 2012, the Company issued 100,000 shares of common stock to the Milan Puskar Revocable Trust Restated 9/28/11 in connection with the exercise of outstanding options at an exercise price $1.50 per share.
During the quarter ended March 31, 2013, the Company granted options to purchase an aggregate of 87,000 shares of common stock in connection with the Company’s 2013 Equity Incentive Plan at an exercise price of $0.50 per share and 1,050,000 shares of common stock in accordance with the 2013 Equity Incentive Plan at an exercise price of $0.55 per share.
During the quarter ended June 30, 2013, the Company granted options to purchase an aggregate of 367,000 shares of common stock in connection with the Company’s 2013 Equity Incentive Plan at an exercise price of $0.55 per share.
During the quarter ended September 30, 2013, the Company granted options to purchase an aggregate of 252,000 shares of common stock in connection with the Company’s 2013 Equity Incentive Plan at an exercise price of $0.55 per share.
Consulting Agreement
On March 25, 2013, the Company entered into a consulting agreement (the “Consulting Agreement”) with an existing shareholder to provide business and management consulting services pursuant to which the Company agreed to issue to the consultant and its designees an aggregate of 300,000 shares of common stock and five year warrants to purchase up to 375,000 shares of common stock at an exercise price of $1.10 per share, of which 50% of the shares of common stock and warrants were payable upon execution of the Consulting Agreement and the balance is payable on the one year anniversary of the Consulting Agreement. On April 5, 2013, the Company issued an aggregate of 150,000 shares of common stock and a warrant to purchase up to 187,500 shares of common stock to the consultant. The shares of common stock and warrants issued pursuant to the Consulting Agreement were issued pursuant to the exemption from registration provided by Section 4(2) of the Securities Act in that they were issued to an accredited investor, the Company did not engage in any general solicitation, the purchaser was the sole offeree and had full access to all information concerning the Company that was requested. No cash commissions were paid in connection with the issuance of the securities pursuant to the Consulting Agreement.
Related Party Debt
On August 6, 2013, the Company and one board member and his spouse agreed to further extend the maturity date of the Convertible Promissory Note dated September 25, 2012 for $20,000 until December 31, 2013.
| 95 |
EXHIBITS
| No. | Description | |
| 2.1 | Merger Agreement by and among Protea Biosciences, Inc., SRKP 5, Inc., and SRKP 5 Acquisition Corp. Inc.(1) | |
| 3.1 | Certificate of Incorporation.(1) | |
| 3.2 | Bylaws.(1) | |
| 3.3 | Certificate of Ownership and Merger filed with the Office of Secretary of State of Delaware on September 2, 2011.(1) | |
| 3.4 | Certificate of Amendment to Certificate of Incorporation.(2) | |
| 4.1 | Form of Warrant issued by Protea Biosciences, Inc. in connection with sale of convertible debentures. (1) | |
| 4.2 | Form of Warrant issued by Protea Biosciences, Inc. in connection with sale of Class A Common Stock. (1) | |
| 4.3 | Promissory Note issued by Protea Biosciences, Inc. to West Virginia Economic Development Authority, dated August 3, 2009. (1) | |
| 4.4 | Promissory Note issued by Protea Biosciences, Inc. to West Virginia Water Development Authority, acting by and on behalf of the West Virginia Infrastructure and Jobs Development Council, dated August 3, 2009.(1) | |
| 4.5 | Promissory Note issued by Protea Biosciences, Inc. to West Virginia Economic Development Authority, dated October 21, 2010. (1) | |
| 4.6 | Promissory Note issued by Protea Biosciences, Inc. to West Virginia Water Development Authority, acting by and on behalf of the West Virginia Infrastructure and Jobs Development Council, dated December 10, 2010.(1) | |
| 4.7 | Commercial Promissory Note between Protea Biosciences, Inc. and Centra Bank, Inc., dated August 27, 2009. (1) | |
| 4.8 | Form of Warrant issued to El Coronado Holdings, LLC(3) | |
| 4.9 | Warrant to Purchase Common Stock of the Company issued to Summit Resources, Inc. in connection with Warrant and Reimbursement Agreement.(4) | |
| 4.10 | Form of Placement Agent Warrant issued in connection with the Fall 2012 Offering.(4) | |
| 4.11 | Form of Warrants to Purchase Common Stock issued in connection with the Spring 2013 Direct Issuances.(4) | |
| 5.1 | Legal Opinion of Richardson & Patel LLP** | |
| 10.1 | Share Cancellation Agreement dated as of September 2, 2011 by and between the registrant and the Persons signatory thereto.(1) | |
| 10.2 | Second Amendment to Exclusive License Agreement between George Washington University and Protea Biosciences, Inc., dated January 14, 2011. (1) | |
| 10.3 | Third Amendment to Exclusive License Agreement between George Washington University and Protea Biosciences, Inc., dated April 27, 2011. (1) | |
| 10.4 | Fourth Amendment to Exclusive License Agreement between George Washington University and Protea Biosciences, Inc., dated June 21, 2011. (1) | |
| 10.5 | Form of Convertible Promissory Notes, dated December 20, 2011(5) | |
| 10.6 | Share Cancellation Agreement dated as of September 2, 2011. (1) | |
| 10.7 | Lease Agreement, dated January 25, 2012, by and between Protea Biosciences, Inc. and White Birch Properties LLC.(6) | |
| 10.8 | Form of Convertible Promissory Notes, dated as of April 16, 2012, issued to directors and certain related parties.(7) | |
| 10.9 | 10% Convertible Debenture, dated as of April 18, 2012, issued to the West Virginia Jobs Investment Trust Board.(8) | |
| 10.10 | Secured Convertible Note and Investment Agreement, dated as of May 24, 2012, issued to the West Virginia High Technology Consortium Foundation.(9) | |
| 10.11 | Security Agreement, dated as of May 24, 2012, issued to the West Virginia High Technology Consortium Foundation.(9) | |
| 10.12 | Loan Agreement, dated as of June 11, 2012, issued to the West Virginia Economic Development Authority Foundation.(9) |
| 96 |
| 10.13 | Promissory Note, dated as of June 11, 2012, issued to the West Virginia Economic Development Authority Foundation.(9) | |
| 10.14 | Security Agreement, dated as of June 11, 2012, issued to the West Virginia Economic Development Authority Foundation.(9) | |
| 10.15 | Guaranty, dated as of May 22, 2012, issued to the West Virginia High Technology Consortium Foundation.(9) | |
| 10.16 | Guaranty, dated as of June 6, 2012, issued to the West Virginia Economic Development Authority Foundation.(9) | |
| 10.17 | Convertible Promissory Note Addendum, dated as of June 15, 2012, executed by Summit Resources, Inc. with respect to the December Note by and between the Company and Summit Resources, Inc.(10) | |
| 10.18 | Convertible Promissory Note Addendum, dated as of June 15, 2012, executed by Stanley Hostler with respect to the December Note by and between the Company and Stanley Hostler. (10) | |
| 10.19 | Convertible Promissory Note Addendum, dated as of June 15, 2012, executed by Summit Resources, Inc. with respect to the April Note by and between the Company and Summit Resources, Inc.(10) | |
| 10.20 | Convertible Promissory Note Addendum, dated as of June 15, 2012, executed by Scott Segal with respect to the April Note by and between the Company and Scott Segal.(10) | |
| 10.21 | Convertible Promissory Note Addendum, dated as of June 15, 2012, executed by Stanley Hostler with respect to the April Note by and between the Company and Stanley Hostler.(10) | |
| 10.22 | Convertible Promissory Note Addendum, dated as of June 18, 2012, executed by Virginia Child with respect to the April Note by and between the Company and Virginia Child.(10) | |
| 10.23 | Convertible Promissory Note Addendum, dated as of June 22, 2012, executed by Nancy Turner with respect to the April Note by and between the Company and Nancy Turner.(10) | |
| 10.24 | Convertible Promissory Note Addendum, dated as of July 2, 2012, executed by Leonard Harris with respect to the April Note by and between the Company and Leonard Harris.(10) | |
| 10.25 | Letter Agreement, dated June 18, 2012, by and between the Company and the WVJITB.(10) | |
| 10.26 | Convertible Promissory Note Addendum, dated as of September 25, 2012, executed by Stanley Hostler with respect to the December Note by and between the Company and Stanley Hostler.(11) | |
| 10.27 | Convertible Promissory Note Addendum, dated as of September 25, 2012, executed by Summit Resources, Inc. with respect to the December Note by and between the Company and Summit Resources, Inc.(11) | |
| 10.28 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Summit Resources, Inc. with respect to the April Note by and between the Company and Summit Resources, Inc. (12) | |
| 10.29 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Scott S. Segal with respect to the April Note by and between the Company and Scott S. Segal. (12) | |
| 10.30 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Stanley M. Hostler with respect to the April Note by and between the Company and Stanley M. Hostler.(12) | |
| 10.31 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Virginia E. Child with respect to the April Note by and between the Company and Virginia E. Child.(12) | |
| 10.32 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Nancy Turner with respect to the April Note by and between the Company and Nancy Turner.(12) | |
| 10.33 | Convertible Promissory Note Addendum, dated as of October 31, 2012, executed by Leonard P. Harris with respect to the April Note by and between the Company and Leonard P. Harris.(12) | |
| 10.34 | Letter Agreement, dated as of October 31, 2012, executed by the Company and WVJITB.(12) | |
| 10.35 | Form of Convertible Promissory Notes, dated as of November 30, 2012, issued to directors and certain related parties.(13) | |
| 10.36 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Stanley M. Hostler with respect to the September Note by and between the Company and Stanley M. Hostler. (13) | |
| 10.37 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Scott Segal with respect to the September Note by and between the Company and Scott Segal. (13) | |
| 10.38 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Leo Harris with respect to the September Note by and between the Company and Leo Harris. (13) | |
| 10.39 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Virginia Child with respect to the September Note by and between the Company and Virginia Child. (13) |
| 97 |
| 10.40 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Summit Resources, Inc. with respect to the September Note by and between the Company and Summit Resources, Inc. (13) | |
| 10.41 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Ed Roberson with respect to the September Note by and between the Company and Ed Roberson. (13) | |
| 10.42 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Stanley Hostler and Virginia Child with respect to the September Note by and between the Company, Stanley Hostler and Virginia Child. (13) | |
| 10.43 | Convertible Promissory Note Addendum, dated as of November 30, 2012, executed by Stephen Turner with respect to the September Note by and between the Company and Stephen Turner. (13) | |
| 10.44 | Convertible Promissory Note Due April 1, 2013, executed by Josiah T. Austin, Managing Member of El Coronado Holdings, LLC with respect to the note by and between the Company and El Coronado Holdings, LLC. (14) | |
| 10.45 | Warrant to Purchase Common Stock, executed by Josiah T. Austin, Managing Member of El Coronado Holdings, LLC with respect to the warrant by and between the Company and El Coronado Holdings, LLC.(14) | |
| 10.46 | Securities Purchase Agreement dated as of March 21, 2013 by and between the Company and El Coronado Holdings, LLC.(3) | |
| 10.47 | Warrant and Reimbursement Agreement, dated March 6, 2013 by and between the Company and Steve Antoline.(4) | |
| 10.48 | 2013 Equity Incentive Plan.(4) | |
| 10.49 | Form of Securities Purchase Agreement by and between the Company and investors in the Spring 2013 Direct Issuances.(4) | |
| 10.50 | Form of Conversion Agreement.(15) | |
| 10.51 | Form of Conversion Warrant.(15) | |
| 10.52 | Form of Bridge Note Purchase Agreement.(15) | |
| 10.53 | Form of Bridge Note.(15) | |
| 10.54 | Form of Bridge Noteholder Warrant.(15) | |
| 10.55 | Convertible Promissory Note Addendum, dated as of August 6, 2013, executed by Nancy Turner with respect to the September 2012 Note by and between the Company and Nancy Turner.(16) | |
| 10.56 | Form of Related Party Advance Note Purchase Agreement.(17) | |
| 10.57 | Form of Related Party Advance Note.(17) | |
| 14.1 | Code of Ethics(18) | |
| 16.1 | Letter Regarding Change in Certifying Accountant.(19) | |
| 21.1 | List of Subsidiaries.(1) | |
| 23.1 | Consent of Malin, Bergquist & Company, LLP* | |
| 23.2 | Consent of Richardson & Patel LLP (included in Exhibit 5.1)** |
*Filed herewith.
**To be filed by amendment.
(1) Form 8-K filed with the SEC on September 9, 2011 and amended on November 14, 2011 and incorporated herein by this reference.
(2) Form 8-K filed with the SEC on June 24, 2013 and incorporated herein by this reference.
(3) Form 8-K filed with the SEC on March 27, 2013 and incorporated herein by this reference.
(4) Form 10-Q filed with the SEC on May 10, 2013 and incorporated herein by this reference.
(5) Form 8-K filed with SEC on December 28, 2011 and amended on April 16, 2012 and incorporated herein by this reference.
(6) Form 8-K filed with the SEC on January 25, 2012 and incorporated herein by this reference.
(7) Form 8-K filed with the SEC on April 20, 2012 and amended on June 15, 2012 and incorporated herein by this reference.
(8) Form 8-K filed with the SEC on April 20, 2012 and amended on October 31, 2012 and incorporated herein by this reference.
(9) Form 8-K filed with the SEC on June 15, 2012 and incorporated herein by this reference.
| 98 |
(10) Form 8-K filed with the SEC on July 16, 2012 and amended on September 25, 2012 and incorporated herein by this reference.
(11) Form 8-K filed with the SEC on October 4, 2012 and incorporated herein by this reference.
(12) Form 8-K filed with the Securities and Exchange Commission on November 7, 2012 and incorporated herein by this reference.
(13) Form 8-K filed with the SEC on December 6, 2012 and incorporated herein by this reference.
(14) Form 8-K filed with the SEC on January 9, 2013 and incorporated herein by this reference.
(15) Form 8-K filed with the SEC on July 23, 2013 and incorporated herein by this reference.
(16) Form 8-K filed with the SEC on August 12, 2013 and incorporated herein by this reference.
(17) Form 8-K filed with the SEC on October 30, 2013 and incorporated herein by this reference.
(18) Form 10-KSB filed with the SEC on March 7, 2008 and incorporated herein by this reference.
(19) Form 8-K filed with the SEC on December 20, 2013 and incorporated herein by this reference.
| 99 |
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
(2) That, for purposes of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
If the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness.
Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| 100 |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
The undersigned registrant hereby undertakes to deliver or cause to be delivered with the prospectus, to each person to whom the prospectus is sent or given, the latest annual report, to security holders that is incorporated by reference in the prospectus and furnished pursuant to and meeting the requirements of Rule 14a-3 or Rule 14c-3 under the Securities Exchange Act of 1934; and, where interim financial information required to be presented by Article 3 of Regulation S-X is not set forth in the prospectus, to deliver, or cause to be delivered to each person to whom the prospectus is sent or given, the latest quarterly report that is specifically incorporated by reference in the prospectus to provide such interim financial information.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer of controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of the registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497 (b) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (2) | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| 101 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Morgantown, State of West Virginia, on January 13, 2014.
| PROTEA BIOSCIENCES GROUP, INC. | ||
| By: | /s/ Stephen Turner | |
| Stephen Turner | ||
| President and Director | ||
| Principal Executive Officer | ||
| By: | /s/ Edward Hughes | |
| Edward Hughes | ||
| Chief Financial Officer | ||
| Principal Financial Officer | ||
SIGNATURES AND POWER OF ATTORNEY
Each person whose signature appears below constitutes and appoints Mr. Stephen Turner and Mr. Edward Hughes his or her true and lawful attorneys-in-fact and agents, each with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments, including post-effective amendments, to this Registration Statement, and any registration statement relating to the offering covered by this Registration Statement and filed under the Securities Act of 1933, and to file the same, with exhibits thereto and other documents in connection therewith, with the Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that each of said attorneys-in-fact and agents or their substitute or substitutes may lawfully so or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on January 13, 2014.
| /s/ Stephen Turner | /s/ Stanley Hostler | /s/ C. Andrew Zulauf | ||
| Stephen Turner, Chief | Stanley Hostler | C. Andrew Zulauf | ||
| Executive Officer and | Director | Director | ||
| Director | ||||
| /s/ Edward Hughes | /s/ Roderick Jackson | /s/ Thijs Spoor | ||
| Edward Hughes, Chief | Roderick Jackson | Thijs Spoor | ||
| Financial Officer | Director | Director | ||
| /s/ Steven Antoline | /s/ Ed Roberson | /s/ Josiah T. Austin | ||
| Steven Antoline | Ed Roberson | Josiah T. Austin | ||
| Director | Director | Director | ||
| /s/ Leonard Harris | /s/ Scott Segal | |||
| Leonard Harris | Scott Segal | |||
| Director | Director |
| 102 |
