Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CADENCE PHARMACEUTICALS INC | d643222d8k.htm |
 Corporate
Presentation Improving the lives of hospitalized patients
December 16, 2013
EXHIBIT 99.1 |
 Caution on
forward-looking statements CADENCE
®
, OFIRMEV
®
and the OFIRMEV design logo are registered trademarks of Cadence Pharmaceuticals, Inc.
©
2013 Cadence Pharmaceuticals, Inc. All rights reserved.
2
This presentation includes forward-looking statements, which are based on our current
beliefs and expectations. Such statements include, without limitation, statements
regarding: the anticipated U.S. market opportunity for OFIRMEV; our financial estimates
and projections; our expectations regarding growth in customer base, frequency of
product use, order rates, share of surgical patients, utilization per patient, expectations for future use, and their
ability to drive revenue growth for OFIRMEV; the sustainability of our core business; the
sufficiency of our capital resources to fund our operations; and our ability to execute
our strategies for acquiring, in-licensing, developing and commercializing
proprietary products principally for use in the hospital setting.
You are cautioned not to place undue reliance on these forward-looking statements, which
speak only as of the date hereof. Our actual future results may differ materially from
our current expectations due to the risks and uncertainties inherent in our business.
In addition, past results and trends may not be indicative or a guarantee of future
results or trends. These risks include, but are not limited to: our dependence on the successful
commercialization of OFIRMEV, which is our only product; our ability to achieve broad market
acceptance and generate revenues from sales of OFIRMEV; our ability to successfully
enforce our marketing exclusivities and intellectual property rights and to defend the
patents covering OFIRMEV, including in our current and any future, additional
intellectual property litigation and U.S. patent office challenges; the potential introduction of generic
competition to OFIRMEV; our dependence on our contract manufacturers and our ability to ensure
an adequate and continued supply of OFIRMEV to meet market demand; potential product
liability exposure; the risk that we may not be able to raise sufficient capital when
needed, or at all; and other risks detailed under “Risk Factors” and
elsewhere in our most recent Quarterly Report on Form 10-Q, and our other filings made
with the Securities and Exchange Commission from time to time.
All forward-looking statements are qualified in their entirety by this cautionary
statement, which is made under the safe harbor provisions of Section 21E of the Private
Securities Litigation Reform Act of 1995 and we undertake no obligation to revise or
update this presentation to reflect events or circumstances after the date hereof.
|
 Investment
highlights •
Specialty biopharmaceutical company, focused on developing and
commercializing proprietary therapeutics utilized in the hospital setting
–
OFIRMEV®
(acetaminophen) injection –
Indicated for use in adults and
children >
2yrs for the:
•
Management of mild to moderate pain
•
Management of moderate to severe pain with adjunctive opioid analgesics
•
Reduction of fever
•
A differentiated, new class of IV pain medication
–
Non-opioid, non-NSAID analgesic
–
Foundation for multimodal approach to pain management
•
Widespread hospital formulary adoption and positive physician feedback
•
Solid revenue growth driven by a growing customer base, increasing re-order
rates and penetration in a variety of surgical settings
•
Sustainable OFIRMEV core business brings opportunities to diversify product
portfolio and leverage existing sales infrastructure
3 |
 OFIRMEV®
(acetaminophen) injection
•
Proprietary IV acetaminophen
formulation
•
First and only IV formulation of
acetaminophen approved in the United
States
•
New class of IV medication
–
non-narcotic / opioid
–
non-NSAID
•
Same formulation of IV acetaminophen
marketed by BMY in Europe since 2002 as
Perfalgan™
4
PERFALGAN™
is
a
trademark
of
Bristol-Myers
Squibb
Company. |
 Strong
foundation for commercial success Effective Pain
Control
•
$13.18/ vial**
•
Diagnosis-related group payment range for common procedures:
$12,000 -
$31,000
(3)
•
OFIRMEV may help reduce post surgical ambulation time
(4)
, time
to extubation in the ICU
(5)
, hospital length of stay
(6)
•
Significant pain relief
(1)
•
Reduced opioid consumption
(1)*
•
Improved patient satisfaction
(1),(2)
•
Sales force average >10 years hospital selling experience
•
Extensive relationships, significant overlap with prior territory
•
Substantial hospital commercial experience throughout
management
–
CEO > 25 years, CCO > 15 years, VP of Sales > 25 years
Experienced
Hospital Sales Force
Economic
Value
References: (1) Sinatra, et al., 2005, (2) Wininger, et al., 2010, (3) Birkmeyer,et al., 2010,
(4) Ohnesorge, et al., 2009, (5) Memis, et al., 2010, (6) Zafar, et al., 2010, Arici,
et al., 2009 * Clinical benefit of opioid reduction not evaluated or demonstrated in this
study ** WAC, effective July 2, 2013 5 |
 Label supports
the message Broad Label
Mild to moderate pain
Moderate to severe pain with adjunctive opioids
Treatment of fever
Adults and children 2 and older
Message
Significant pain relief
Reduced opioid consumption
Improved patient satisfaction
Established safety profile
6 |
 Limitations of
other IV pain therapies 7
Sedation
Nausea
Vomiting
Constipation
Headache
Cognitive impairment
Respiratory depression
Bleeding
GI complications
Kidney complications
Cardiovascular risks
Prolonged recovery
Increased length of stay
Higher costs to the institution
Limited use
Opioids
NSAIDs |
 Pain
Intensity Historical
US Approach
Emerging
US Approach
Severe
Opioids
IV acetaminophen
+ opioids
Moderate
Opioids
IV acetaminophen
+ opioids, if necessary
Mild
Opioids
IV acetaminophen
Multimodal analgesia is becoming standard
8 |
 Strong
commercial acceptance •
Rapid formulary adoption
–
On formulary in over 2,350 hospitals*
–
Minimal restrictions
•
Strong physician support and early experience positive
–
OFIRMEV physician market research:**
•
97% of physicians surveyed reported that OFIRMEV’s efficacy met or exceeded
their expectations
•
3 of 4 indicate they are very likely to recommend to colleagues
•
Rapid and sustained sales growth
–
Approximately 13.7 million vials purchased by hospitals***
–
Estimated 5.5 –
6.9 million patients treated through Oct. 31, 2013****
9
*
Launch through October 31, 2013
**
December 2012 attitude, trial, and usage (“ATU”) market research of surgeons and
anesthesiologists (n>180) conducted by GfK Healthcare (commissioned by
Cadence) ***
Cadence internal data through October 31, 2013
****
Based on our estimate of 2-2.5 doses per patient |
 Source:
Source Healthcare Analytics, Source® PHAST Institution, November 20, 2013 and
Cadence internal data. Strong OFIRMEV®
sales growth
•
WAC sales reported to be $11.8M for October 2013
•
Monthly vial sales of 895K in October 2013
10
-
100
200
300
400
500
600
0.0
2.0
4.0
6.0
8.0
10.0
12.0
14.0
OFIRMEV Monthly Sales
Monthly Sales ($M)
Sales/Day ($ 000's) |
 Historical
account growth metrics •
Growth in customer base
–
Number of unique customer accounts
increased to over 4,600 in Q3 2013
–
Number of repeat customers grew to over
3,900 in Q3 2013 and represents
approximately 85% of all customers
•
Increase in frequency of product use
–
Hospital reorder rates averaged
approximately 4.8 orders per account for
Q3 2013, an 8% increase over Q3 2012
•
Increase in average quantity of
product ordered per customer
–
Average order size in Q3 2013 increased
22% over Q3 2012 to over 105 vials/order
–
Anticipate increasing number of vials per
patient as adoption by surgeons broadens
11
Customer Base
Purchase Volume
Re-order rates
Product
Revenue |
 •
As of end of Q3 2013:
–
Over 2,350 hospital formulary approvals
–
Over 4,600 accounts have ordered OFIRMEV
12
Broad adoption |
 Significant
growth in new and repeat customers each quarter •
Approximately 33% growth in unique accounts ordering OFIRMEV in Q3 2013 vs. Q3 2012
•
39% increase in accounts that placed multiple orders in Q3 2013 vs. Q3 2012
Significant growth in new customers
13
0
500
1,000
1,500
2,000
2,500
3,000
3,500
4,000
4,500
5,000
Q1 11
Q2 11
Q3 11
Q4 11
Q1 12
Q2 12
Q3 12
Q4 12
Q1 13
Q2 13
Q3 13
Cumulative Accounts Ordering OFIRMEV®
Multiple Order
Single Order |
 Growing
average order size 14
Average Order Size/Quarter
(vials)
40
50
57
65
75
79
85
92
96
100
105
Q1 11
Q2 11
Q3 11
Q4 11
Q1 12
Q2 12
Q3 12
Q4 12
Q1 13
Q2 13
Q3 13 |
 Growth
drivers •
Expanding physician user base*
–
Market research indicates growing user base in targeted accounts
–
Proportion of surgeons using OFIRMEV in targeted accounts has tripled in
less than 2 years
•
Increasing share of surgical patients
–
Data from Premier healthcare alliance indicates consistent growth through
Q2 2013**
•
Growing utilization per patient
–
Number of vials used per patient has grown steadily since 2011**
–
Anticipate
increasing
number
of
vials
per
patient
as
adoption
by
surgeons
broadens
15
*
ATU surveys conducted from Sept ‘11 through July ‘13 indicate (general and
orthopedic) surgeon utilization has increased from 23% to 76% during this time
period. **
Patient discharge data from the hospital research database maintained by the Premier
healthcare alliance (October 10, 2013) Sample includes over 400 hospitals representing
approximately 4.5M surgical patient discharges/year |
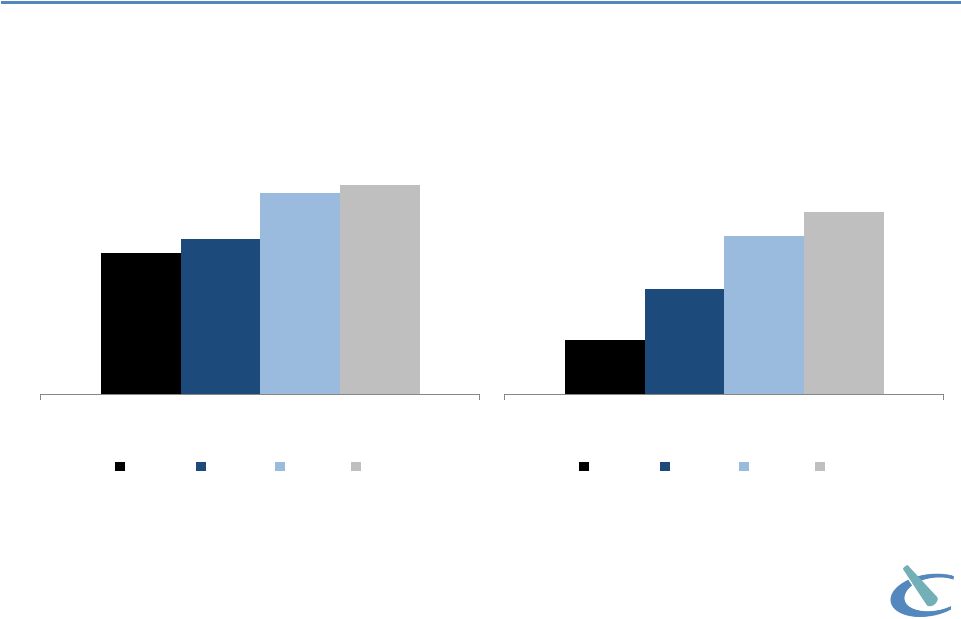 Market
research: increasing prescriber base More physicians in targeted accounts are using
OFIRMEV® Question:
Please
indicate
your
experience/familiarity
with
each
of
the
following
product
brands.
Those
indicating
“currently
use”
shown
on
this
chart.
Source: September 2011-December 2012 ATU market research of surgeons and
anesthesiologists conducted by GfK Healthcare and July 2013 ATU conducted by
Life Science Strategy Group, LLC (commissioned by Cadence) 16
Physicians Currently Using OFIRMEV
n=120
n=122 n=61 n=54
n=279
n=344 n=236 n=137
59%
65%
84%
87%
Anesthesiologist
Sept '11
May '12
Dec '12
July '13
23%
44%
66%
76%
Surgeon (General & Orthopedic)
Sept '11
May '12
Dec '12
July '13 |
 Source:
Patient discharge data from the hospital research database maintained by the Premier
healthcare alliance (October 10, 2013) Sample includes over 400 hospitals representing
approximately 4.5M surgical patient discharges/year Increasing share of surgical
patients 17
0.0%
2.0%
4.0%
6.0%
8.0%
10.0%
12.0%
14.0%
16.0%
OFIRMEV
®
Share of US Hospital Surgical Procedures
(Premier Healthcare Alliance Database)
Inpatient
All Surgical
Outpatient |
 Source:
Patient discharge data from the hospital research database maintained by the Premier
healthcare alliance (October 10, 2013) Sample includes over 400 hospitals representing
approximately 4.5M surgical patient discharges/year Increasing utilization per
patient 18
2.00
2.25
2.50
2.75
3.00
3.25
3.50
OFIRMEV
®
Vial Utilization -
Surgical Inpatient Procedures
(Premier Healthcare Alliance Database) |
 Long term
opportunity Physicians indicate higher expectations for future use of OFIRMEV®
Question (September 2011): What percentage of your (surgical procedures) would you
expect to fill for OFIRMEV when you reach the peak usage? Question (December
2012): Thinking ahead to 3 years from now, in what proportion of your surgical procedures requiring IV analgesics do you expect to
include the use of OFIRMEV?
Source: December 2012 ATU market research of surgeons and anesthesiologists
(n>180) conducted by GfK Healthcare (commissioned by Cadence)
19
42%
43%
55%
60%
Anesthesiologists
Surgeons
Projected Future Use
(% of patients treated)
Sep 2011 Survey
Dec 2012 Survey |
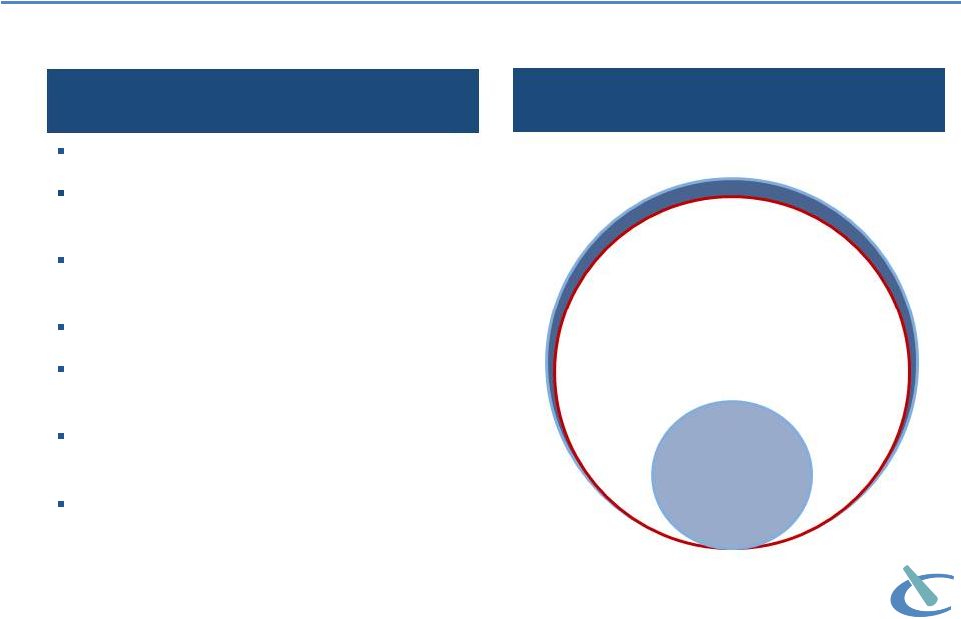 Long term
opportunity: ketorolac as a case study Limitations of NSAIDs
High Risk of Bleeding
Contraindicated as prophylactic analgesic
prior to any major surgery
Cardiovascular risks (CV thrombotic events,
MI and stroke)
Renal risks (advanced renal impairment)
Gastrointestinal risks (active or history of
peptic ulcers or GI bleeding)
Contraindicated in labor & delivery, and in
nursing mothers
Contraindicated in patients receiving aspirin
or other NSAIDs
Benchmarking potential future use of OFIRMEV®
against ketorolac utilization
OFIRMEV eligible
population
NSAID
treated
population
Perioperative Pain Population
20
Perioperative pain population |
 Long term
opportunity: share of patients Physicians project OFIRMEV®
to be much more broadly utilized than NSAIDs
** Question: Thinking ahead to 3 years from now, what proportion of your surgical
procedures do you expect to include the use of OFIRMEV? Source: ATU study conducted by
GfK Healthcare, May 2012 (n=180 surgeons and anesthesiologists) * Source: Patient discharge data from the hospital research database maintained by the Premier healthcare alliance (October 10, 2013)
Projected OFIRMEV Use
(ATU Market Research**)
21
56%
12%
25%
92%
MD Projection of OFIRMEV
Utilization In 3 yrs**
OFIRMEV Q2 2013
NSAIDs
OPIOIDS
IV Analgesic Use in Surgical Inpatients
(Premier Hospital Database*) |
 Long term
opportunity: doses per patient Physicians project significant increases in doses per
patient Source:
Awareness,
Trial,
Usage
Study
conducted
by
Life
Science
Strategy
Group,
LLC,
July
2013
Question 1: When you use OFIRMEV, how many vials are you using per one typical surgical
procedure? Question 2: How many vials of OFIRMEV do you expect to use per one typical
surgical procedure 3 years from now? Anesthesiologists
Surgeons
(n=43)
(n= 88)
Current Use
(July 2013)
Current Use
(July 2013)
Projected Use
in 3yrs
Projected Use
in 3yrs
22
(July 2013 Survey)
2.2
4.3
3.4
6.1
Doses Per Patient –
Inpatient Surgery |
 Delivering
sustainable growth •
Continue
to
broaden
and
deepen
use
of
OFIRMEV®
in
treating
post-op
pain
–
Current trends and primary market research indicate potential for sustained
growth in patient share and vials per patient
•
Support implementation of multi-modal pain management strategies to help
hospitals improve economics and quality outcomes
–
A recently-presented study analyzing a large-scale hospital billing database
examined the impact of IV acetaminophen on improving outcomes and cost
of care for treating acute pain associated with common joint replacement
procedures*
•
Launch OFIRMEV flexible bag presentation
–
sNDA expected to be filed Q4 13
–
Anticipated launch Q4 14/Q1 15
•
Extend use of OFIRMEV into non-operative acute pain management
–
Non-operative acute pain management estimated to represent 30-40% of IV
analgesic use
–
Non-operative applications currently account for only about 10% of OFIRMEV
use
•
Expand product portfolio through business development transactions
23
* Evaluation of Patient Outcomes, Length of Stay, and Average Hospital Costs with IV
Acetaminophen: A Case-Matched Analysis of a National Inpatient Hospital Database.
Christian Apfel, M.D., Ph.D., Adjunct Associate Professor, Department of Epidemiology and Biostatistics, UCSF, San Francisco, CA.
(ASHP abstract 3-127) |
 Business
development: target profile Accelerate growth by leveraging Cadence’s existing
infrastructure through acquisitions of products or companies
Near Term
Long Term
Region
US
US / Global*
Channel
Hospital
Hospital**
Product Status &
Sales Potential
•
Modest sized marketed or ready-to-
launch products
•
Moderate-large marketed or ready-
to-launch products
•
Development-stage assets with
significant peak sales potential
Cost Synergies
•
Will target products that can leverage our hospital-focused commercial
infrastructure
Deal Structures
•
Will consider transaction structures,
incl. co-promotion, asset purchases,
and in-licenses
•
Will consider transaction structures
including acquisitions, asset
purchases, and in-licenses
*Anticipate partnering ex-US
rights **Anticipate partnering non-hospital component
24 |
 Financial
highlights 12 mos. ended
12/31/12
(in MM)
9 mos. ended
9/30/13
(in MM)
Net product revenue
$50.1
$77.2
Operating expenses
$103.6
$75.4
Cash, cash equivalents &
short-term investments
$62.1
$54.3
Shares outstanding
85.7
86.1
25 |
 Investment
highlights •
Specialty biopharmaceutical company, focused on developing and
commercializing proprietary therapeutics utilized in the hospital setting
–
OFIRMEV®
(acetaminophen) injection –
Indicated for use in adults and
children >
2yrs for the:
•
Management of mild to moderate pain
•
Management of moderate to severe pain with adjunctive opioid analgesics
•
Reduction of fever
•
A differentiated, new class of IV pain medication
–
Non-opioid, non-NSAID analgesic
–
Foundation for multimodal approach to pain management
•
Widespread hospital formulary adoption and positive physician feedback
•
Solid revenue growth driven by a growing customer base, increasing re-order
rates and penetration in a variety of surgical settings
•
Sustainable OFIRMEV core business brings opportunities to diversify product
portfolio and leverage existing sales infrastructure
26 |
 Take care when
prescribing, preparing, and administering OFIRMEV Injection to avoid dosing errors which could
result in accidental overdose and death.
OFIRMEV contains acetaminophen. Acetaminophen has been associated with cases of acute liver
failure, at times resulting in liver transplant and death. Most of the cases of liver
injury are associated with the use of acetaminophen at doses that exceed the recommended
maximum daily limits, and often involve more than one acetaminophen-containing product. OFIRMEV is contraindicated in patients with severe hepatic impairment, severe
active liver disease or with known hypersensitivity to acetaminophen or to any of the
excipients in the formulation. Acetaminophen should be used with caution in patients with
the following conditions: hepatic impairment or active hepatic disease, alcoholism, chronic
malnutrition, severe hypovolemia, or severe renal impairment. Rarely, acetaminophen may cause
serious skin reactions such as acute generalized exanthematous pustulosis (AGEP), Stevens-Johnson
Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal.
Discontinue OFIRMEV immediately if symptoms associated with allergy or hypersensitivity
occur, or at the first appearance of skin rash. Do not use in patients with acetaminophen
allergy. The most common adverse reactions in patients treated with
OFIRMEV were nausea, vomiting, headache, and insomnia in adult patients and nausea, vomiting,
constipation, pruritus, agitation, and atelectasis in pediatric patients.
The antipyretic effects of OFIRMEV may mask fever in patients treated with postsurgical
pain. Do not exceed the recommended maximum daily dose of OFIRMEV.
OFIRMEV should be administered only as a 15-minute infusion.
To report SUSPECTED ADVERSE REACTIONS, contact Cadence Pharmaceuticals, Inc. at
1-877-647-2239 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. OFIRMEV
is
approved
for
use
in
patients
2
years
of
age.
Important Safety Information about OFIRMEV®
(acetaminophen) injection
WARNING: RISK OF MEDICATION ERRORS AND HEPATOTOXICITY
Prescribing Information for OFIRMEV (acetaminophen) injection. San Diego, CA:
Cadence Pharmaceuticals, Inc., 2013. |
