Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ANI PHARMACEUTICALS INC | v355433_8k.htm |

A Specialty Pharmaceutical Company NASDAQ: ANIP HIGH POTENCY DRUGS – NARCOTIC DRUGS – RX LIQUIDS AND TABLETS – CONTRACT MANUFACTURING Corporate Presentation September 2013

2 Cautionary Statement Concerning Forward - Looking Statements This presentation and certain information incorporated herein by reference contain forward - looking statements under the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements about the Company’s plans, objectives, expectations and intentions with respect to future operations and products, the anticipated financial position, operating results and growth prospects of the Company and other statements that are not historical in nature, particularly those that utilize terminology such as “anticipates,” “will,” “expects,” “plans,” “potential,” “future,” “believes,” “intends,” “continue,” other words of similar meaning, derivations of such words and the use of future dates. Forward - looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause the Company’s actual results to be materially different than those expressed in or implied by such forward - looking statements. Uncertainties and risks include the risk that Company estimates regarding product development may not be realized; the Company may in the future fail to meet NASDAQ listing requirements; general business and economic conditions; the Company’s need for and ability to obtain additional financing; the difficulty of developing pharmaceutical products, obtaining regulatory and other approvals and achieving market acceptance; and the marketing success of the Company’s licensees or sublicensees . More detailed information on these and additional factors that could affect the Company’s actual results are described in the Company’s filings with the Securities and Exchange Commission, including its most recent annual report on Form 10 - K and quarterly report on Form 10 - Q, as well as its proxy statement/prospectus, filed with the Securities and Exchange Commission on May 8, 2013. All forward - looking statements in this quarterly report speak only as of the date made and are based on the Company’s current beliefs and expectations. The Company undertakes no obligation to update or revise any forward - looking statement, whether as a result of new information, future events or otherwise. 2

3 3 Mission Statement ANI Pharmaceuticals, Inc. (“ANI” or the “Company”) is an integrated specialty pharmaceutical company developing, manufacturing and marketing branded and generic prescription pharmaceuticals. ANI’s mission is to utilize its manufacturing assets to develop and market niche generic pharmaceuticals, focusing on opportunities in pain management (narcotics), anti - cancer ( oncolytics ), women’s health (hormones and steroids), as well as complex formulations including extended release and combination products.

4 Company Overview – Poised for Growth ANI Today ▪ Core competencies: marketing and manufacturing ▪ Experienced management team ▪ Existing business + potential future royalty stream – For the interim six - month period ended June 30, 2013 (1 ): □ $7.5 million Rx product revenues □ $4.2 million c ontract m anufacturing and services revenues □ Annual organic growth 17% year/year – Potential future royalty stream: Teva’s Bio - T - Gel™ – 12 products in development: total current market $850 million (2) ▪ Two manufacturing facilities: narcotics and potent compounds ▪ Well - capitalized balance sheet: +$12 million cash / no debt (1) Unaudited (2) Based on Company estimates, and recent IMS and NSP Audit data
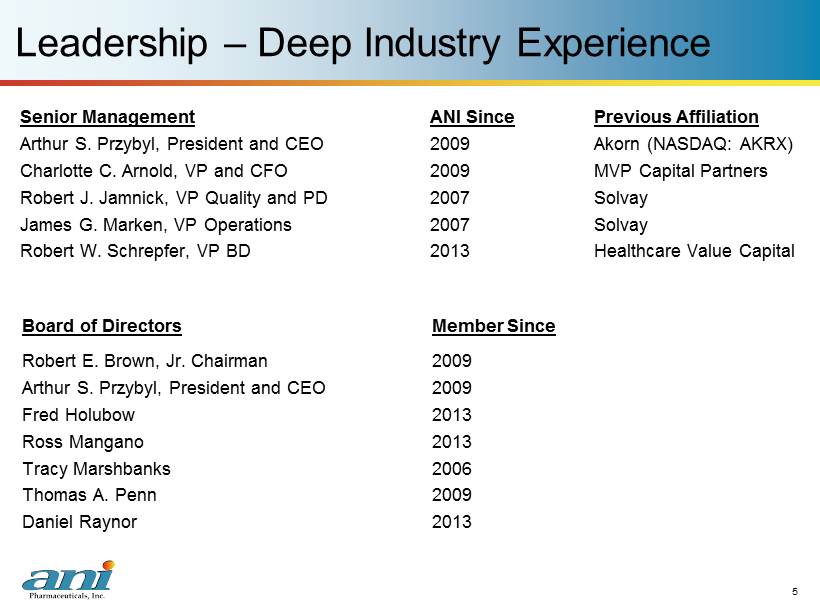
5 5 Leadership – Deep Industry Experience Senior Management ANI Since Previous Affiliation Arthur S. Przybyl, President and CEO 2009 Akorn (NASDAQ: AKRX) Charlotte C. Arnold, VP and CFO 2009 MVP Capital Partners Robert J. Jamnick, VP Quality and PD 2007 Solvay James G. Marken, VP Operations 2007 Solvay Robert W. Schrepfer, VP BD 2013 Healthcare Value Capital Board of Directors Member Since Robert E. Brown, Jr. Chairman 2009 Arthur S. Przybyl, President and CEO 2009 Fred Holubow 2013 Ross Mangano 2013 Tracy Marshbanks 2006 Thomas A. Penn 2009 Daniel Raynor 2013

6 6 History and Highlights 2004 Company founded with acquisition of over - the - counter pharmaceutical manufacturing plant in Gulfport, Mississippi 2007 ANI acquires two manufacturing plants located in Baudette , Minnesota from Solvay Pharmaceuticals 2009 New management team brought in by investors; Art Przybyl, CEO and Charlotte Arnold, CFO 2010 New strategy: ANI to focus on Rx products and contract manufacturing (Gulfport operation divested) 2011 ANI expands marketed Rx portfolio to seven products 2013 ANI completes reverse merger with BioSante Pharmaceuticals and obtains NASDAQ Global Market listing (NASDAQ: ANIP)

7 7 Sales and Marketing Overview
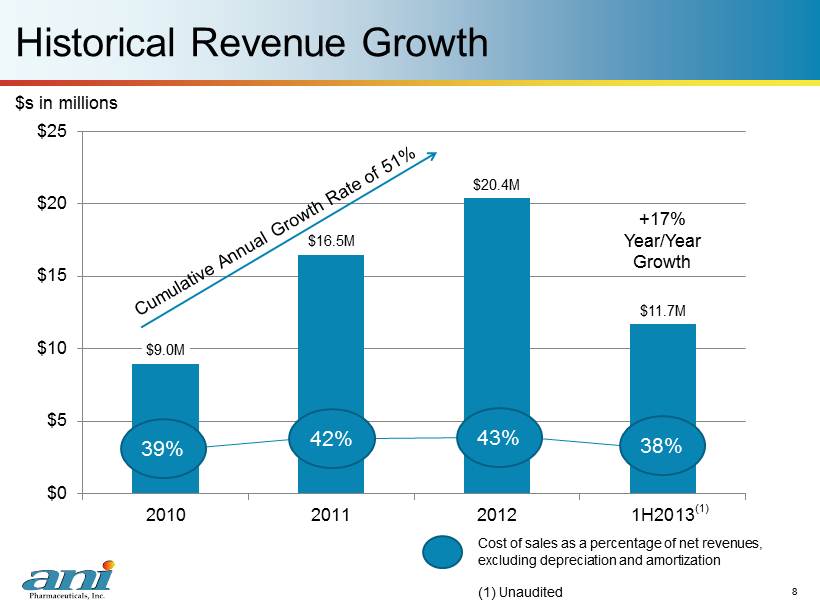
8 Historical Revenue Growth 8 $9.0M $16.5M $20.4M $11.7M $0 $5 $10 $15 $20 $25 2010 2011 2012 1H2013 $s in millions +17% Year/Year G rowth 39% 42% 43% 38% C ost of sales as a percentage of net revenues, excluding depreciation and amortization (1) Unaudited (1)
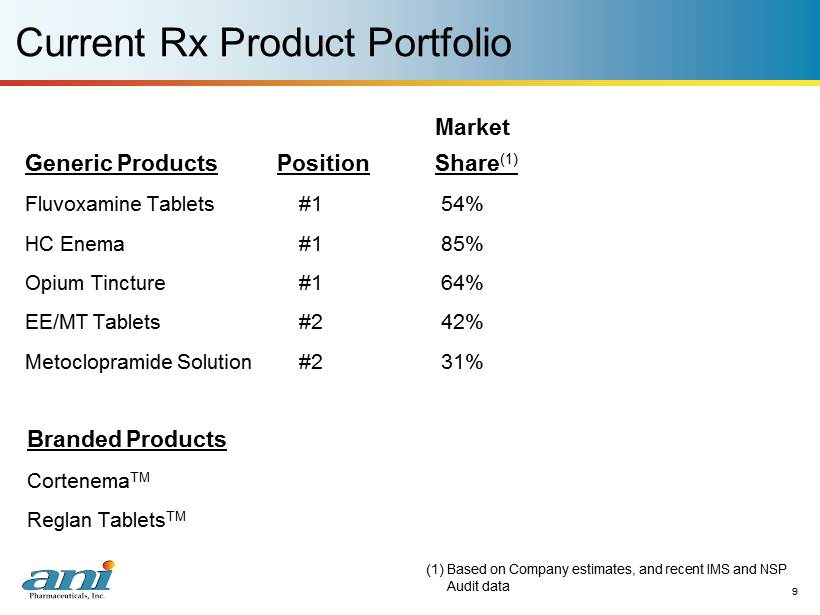
9 Current Rx Product Portfolio Market Generic Products Position Share (1) Fluvoxamine Tablets #1 54% HC Enema #1 85% Opium Tincture #1 64% EE/MT Tablets #2 42% Metoclopramide Solution #2 31% 9 Branded Products Cortenema TM Reglan Tablets TM (1) Based on Company estimates, and recent IMS and NSP Audit data

10 Contract Manufacturing and Royalties 10 Current Business ▪ $4.2 million in contract manufacturing and services revenues during the six - month period ended June 30, 2013 (1) ▪ Five customers – Eleven products and fourteen SKUs Future Opportunities ▪ Three customers in development – Three products and seven SKUs ▪ Potential future royalty: Teva’s Bio - T - Gel™ (1) Unaudited

11 11 Product Development / Business Development Overview
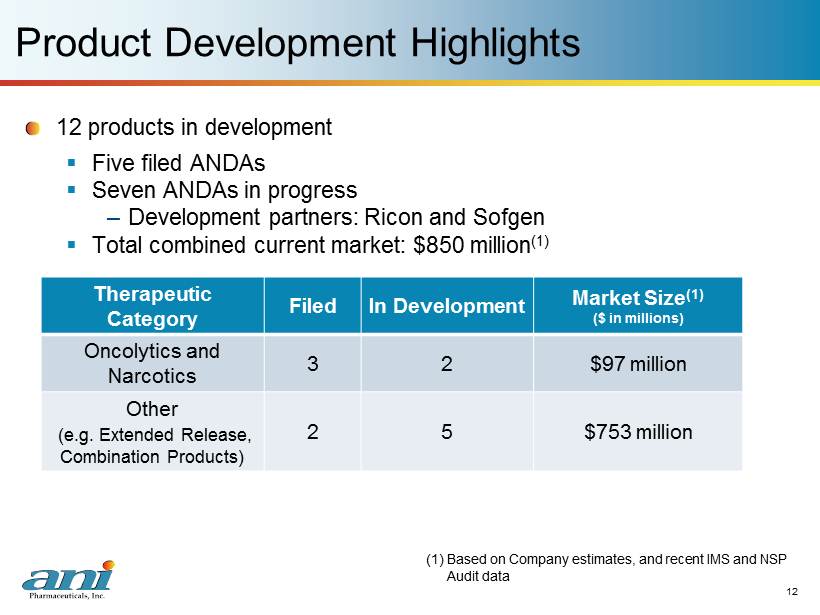
12 12 Product Development Highlights 12 p roducts in development ▪ Five filed ANDAs ▪ Seven ANDAs in progress – Development partners: Ricon and Sofgen ▪ Total combined current market: $850 million (1) Therapeutic Category Filed In Development Market Size (1) ($ in millions) Oncolytics and Narcotics 3 2 $97 million Other (e.g. Extended Release, Combination Products) 2 5 $753 million (1) Based on Company estimates, and recent IMS and NSP Audit data

13 13 Business Development Highlights Acquired undisclosed A NDA, March 2010 Acquired Reglan TM tablets, June 2011 Product development partnership with Ricon , June 2011 Acquired Bio - T - Gel™ royalty arrangement with Teva , June 2013 Product development partnership with Sofgen , August 2013

14 14 Manufacturing Overview

15 15 Manufacturing – Main Street Facility Location: Baudette , Minnesota ▪ 52,000 square feet of manufacturing , packaging, and warehouse facilities ▪ Rx solutions , suspensions , topicals , tablets , and capsules ▪ DEA - licensed for Schedule II controlled substances ▪ 17,000 square feet of laboratory space for product development and analytical testing

16 16 Manufacturing – IDC Road Facility Location: Baudette, Minnesota ▪ Fully - contained h igh potency facility with capabilities to manufacture h ormone , steroid , and oncolytic products ▪ 47,000 square feet of manufacturing and packaging, and warehouse facilities ▪ 100 nano - gram per eight - hour weighted average maximum exposure limit to ensure employee safety ▪ DEA Schedule IIIN capability

17 17 Capitalization and Shareholders
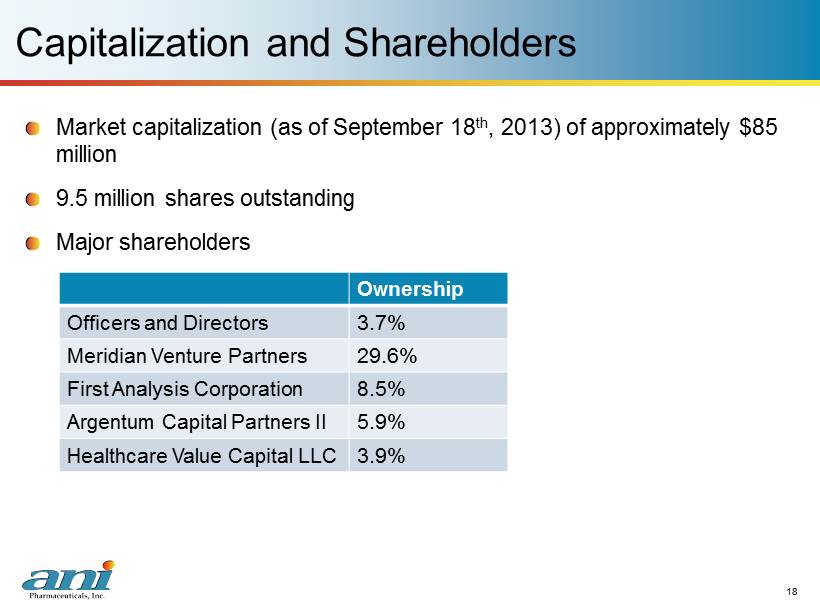
18 18 Capitalization and Shareholders Market capitalization (as of September 18 th , 2013) of approximately $85 million 9.5 million shares outstanding Major shareholders Ownership Officer s and Directors 3.7% Meridian Venture Partners 29.6% First Analysis Corporation 8.5% Argentum Capital Partners II 5.9% Healthcare Value Capital LLC 3.9%

19 ANI Pharmaceuticals Poised for Growth
