Attached files
| file | filename |
|---|---|
| 8-K - 8-K - UNITED THERAPEUTICS Corp | a13-5931_18k.htm |
Exhibit 99.1
For Immediate Release
Contact: Andrew Fisher
(202) 483-7000
Afisher@unither.com
UNITED THERAPEUTICS CORPORATION REPORTS
2012 FOURTH QUARTER AND ANNUAL FINANCIAL RESULTS
· Total Annual Revenues of $916.1 million
· Annual Earnings per Share of $5.84 per Basic Share or $5.71 per Diluted Share
· Annual Earnings Before Non-Cash Charges of $9.97 per Basic Share or $9.75 per Diluted Share
Silver Spring, MD, February 26, 2013: United Therapeutics Corporation (NASDAQ: UTHR) today announced its financial results for the fourth quarter and year ended December 31, 2012.
“Our excellent annual results reflect both our leadership in pulmonary hypertension medicines and our discipline in focusing company resources on transformative growth opportunities,” noted Martine Rothblatt, Ph.D., United Therapeutics’ Chairman and Chief Executive Officer. “It is especially noteworthy that our medicines are now being prescribed to more pulmonary hypertension patients in the United States than any other company’s medicines.”
Total revenues for the quarter ended December 31, 2012 were $243.8 million, up from $195.2 million for the quarter ended December 31, 2011. Net income for the quarter ended December 31, 2012 was $83.3 million or $1.65 per basic share, compared to $43.2 million or $0.79 per basic share for the quarter ended December 31, 2011. For the year ended December 31, 2012, we had net income of $304.4 million, or $5.84 per basic share and $5.71 per diluted share, compared to $217.9 million, or $3.81 per basic share and $3.67 per diluted share, for the year ended December 31, 2011. Earnings before non-cash charges(1) for the quarter ended December 31, 2012, were $126.3 million or $2.50 per basic share, compared to $87.5 million or $1.61 per basic share for the quarter ended December 31, 2011.
(1)See definition of earnings before non-cash charges, a non-GAAP financial measure, and a reconciliation of net income to earnings before non-cash charges below.
Operating Results
Revenues
The table below summarizes the components of revenues (in thousands):
|
|
|
Three Months Ended |
|
Year Ended |
| ||||||||
|
|
|
2012 |
|
2011 |
|
2012 |
|
2011 |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
Cardiopulmonary products: |
|
|
|
|
|
|
|
|
| ||||
|
Remodulin |
|
$ |
116,214 |
|
$ |
107,116 |
|
$ |
457,969 |
|
$ |
430,132 |
|
|
Tyvaso |
|
86,036 |
|
64,547 |
|
325,614 |
|
240,382 |
| ||||
|
Adcirca |
|
38,182 |
|
22,647 |
|
122,540 |
|
70,580 |
| ||||
|
Other |
|
3,385 |
|
868 |
|
9,953 |
|
2,089 |
| ||||
|
Total revenues |
|
$ |
243,817 |
|
$ |
195,178 |
|
$ |
916,076 |
|
$ |
743,183 |
|
Revenues for the quarter ended December 31, 2012 increased by $48.6 million compared to the quarter ended December 31, 2011. The growth in revenues corresponded primarily to the continued increase in the number of patients being prescribed our products. In addition, approximately $3.6 million of the increase in Adcirca revenue was the result of shipments toward the end of December 2012, as wholesalers ordered additional product to avert near-term shortages of Adcirca due to the timing of the holidays. Other revenues include approximately $3.1 million recognized under our contract with the National Institute of Allergy and Infectious Diseases of the United States National Institutes for Health to develop antiviral agents.
Gross margins from sales for the quarters ended December 31, 2012 and 2011 were $202.8 million and $169.0 million, or 84% and 87%, respectively, of total revenues. The decrease in gross margins as a percentage of sales for the fourth quarter of 2012 reflects an $8.9 million increase in our reserves for inventory obsolescence, which represents the cost of the Tyvaso inhalation devices expected to be rendered obsolete based on the pending release of our modified inhalation device, the TD-100, in 2013.
Expenses
The table below summarizes research and development expense by major project and non-project component (in thousands):
|
|
|
Three Months Ended |
|
Year Ended |
| ||||||||
|
|
|
2012 |
|
2011 |
|
2012 |
|
2011 |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
Project and non-project: |
|
|
|
|
|
|
|
|
| ||||
|
Cardiopulmonary |
|
$ |
30,892 |
|
$ |
28,142 |
|
$ |
122,350 |
|
$ |
150,501 |
|
|
Share-based compensation (benefit) expense |
|
(3,722 |
) |
9,687 |
|
11,237 |
|
(7,994 |
) | ||||
|
Other |
|
10,306 |
|
10,807 |
|
39,800 |
|
37,508 |
| ||||
|
Total research and development expense |
|
$ |
37,476 |
|
$ |
48,636 |
|
$ |
173,387 |
|
$ |
180,015 |
|
Cardiopulmonary. The increase in cardiopulmonary project expenses of $2.8 million for the quarter ended December 31, 2012 over the same quarter in 2011 resulted from $1.9 million and $1.3 million in expenses associated with on the development of self-injectable prostacyclin analogues and our Remodulin implantable pump program, respectively.
Share-based compensation. The $13.4 million decrease in share-based compensation expense for the quarter ended December 31, 2012 over the same quarter in 2011 reflects the 4 percent decline in the price of our common stock during the quarter ended December 31, 2012 compared to the 26 percent appreciation in the price of our common stock for the same quarter in 2011.
The table below summarizes selling, general and administrative expense by major category (in thousands):
|
|
|
Three Months Ended |
|
Year Ended |
| ||||||||
|
|
|
2012 |
|
2011 |
|
2012 |
|
2011 |
| ||||
|
|
|
|
|
|
|
|
|
|
| ||||
|
Category: |
|
|
|
|
|
|
|
|
| ||||
|
General and administrative |
|
$ |
31,118 |
|
$ |
26,608 |
|
$ |
116,899 |
|
$ |
97,785 |
|
|
Sales and marketing |
|
15,788 |
|
19,091 |
|
67,220 |
|
66,405 |
| ||||
|
Share-based compensation (benefit) expense |
|
(6,833 |
) |
12,008 |
|
17,627 |
|
(7,708 |
) | ||||
|
Total selling, general and administrative expense |
|
$ |
40,073 |
|
$ |
57,707 |
|
$ |
201,746 |
|
$ |
156,482 |
|
General and administrative. The $4.5 million increase in general and administrative expenses for the quarter ended December 31, 2012 compared to the same quarter in 2011 resulted from increases of: (1) $2.2 million in depreciation and amortization expense as a result of the expansion of our facilities in Maryland and North Carolina; and (2) $1.3 million in compensation expense due to an increase in both headcount and incentive-based compensation.
Sales and marketing. The $3.3 million decrease in sales and marketing expenses for the quarter ended December 31, 2012 compared to the same quarter in 2011 was attributable to a decrease of $2.8 million in professional fees and advertising expenses incurred in connection with the timing of our marketing-related activities.
Share-based compensation. The $18.8 million decrease in share-based compensation expense for the quarter ended December 31, 2012 over the same quarter in 2011 reflects the 4 percent decline in the price of our common stock during the quarter ended December 31, 2012 compared to the 26 percent appreciation in the price of our common stock for the same quarter in 2011.
Income Taxes
The provision for income taxes was $41.7 million for the quarter ended December 31, 2012, compared to $16.8 million for the quarter ended December 31, 2011. The increase in income tax expense was attributable to the increase in pre-tax earnings.
2013 Revenue Guidance
We reaffirm our 2013 full-year revenue guidance, as we continue to expect revenues to fall within a range of 5% above or below $1 billion for 2013.
This forward-looking guidance is inherently subject to variability; consequently, we anticipate reaffirming or updating our expectation for 2013 when we present our quarterly results during 2013.
Discontinued Operations
Results for the quarter and year ended December 31, 2011 do not include the results of Medicomp, Inc. (Medicomp), our former telemedicine subsidiary, which we sold during the first quarter of 2011. The results of Medicomp have been reported within discontinued operations on our consolidated statements of operations presented below.
Earnings Before Non-Cash Charges
Earnings before non-cash charges is defined as net income, adjusted for the following non-cash charges, as applicable: (1) interest; (2) income taxes; (3) license fees; (4) depreciation and amortization; (5) impairment charges; and (6) share-based compensation (stock option, share tracking award and employee stock purchase plan expense).
A reconciliation of net income to earnings before non-cash charges is presented below (in thousands, except per share data):
|
|
|
Year Ended December 31, |
|
Three Months Ended |
| ||||||||||||||
|
|
|
2012 |
|
2011 |
|
2010 |
|
2009 |
|
2012 |
|
2011 |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Net income, as reported |
|
$ |
304,442 |
|
$ |
217,868 |
|
$ |
105,916 |
|
$ |
19,462 |
|
$ |
83,255 |
|
$ |
43,189 |
|
|
Add (subtract) non-cash charges: |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Interest expense |
|
16,639 |
|
21,372 |
|
19,714 |
|
12,875 |
|
4,490 |
|
5,112 |
| ||||||
|
Income tax expense (benefit) |
|
136,229 |
|
82,183 |
|
41,923 |
|
(695 |
) |
41,697 |
|
16,800 |
| ||||||
|
License fees |
|
— |
|
37,049 |
|
— |
|
— |
|
— |
|
(4,283 |
) | ||||||
|
Depreciation and amortization |
|
27,145 |
|
20,535 |
|
17,919 |
|
11,394 |
|
7,290 |
|
4,898 |
| ||||||
|
Impairment charges |
|
4,839 |
(1) |
— |
|
7,688 |
|
5,457 |
|
— |
|
(250 |
) | ||||||
|
Share-based compensation expense (benefit) |
|
30,115 |
|
(15,715 |
) |
113,942 |
|
100,810 |
|
(10,453 |
) |
22,075 |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
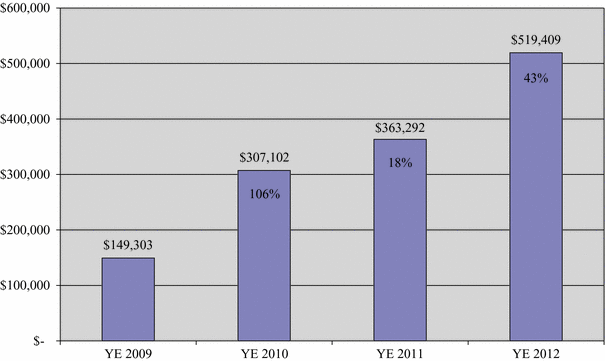
Earnings before non-cash charges |
|
$ |
519,409 |
|
$ |
363,292 |
|
$ |
307,102 |
|
$ |
149,303 |
|
$ |
126,279 |
|
$ |
87,541 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Earnings before non-cash charges per share: |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Basic |
|
$ |
9.97 |
|
$ |
6.36 |
|
$ |
5.47 |
|
$ |
2.80 |
|
$ |
2.50 |
|
$ |
1.61 |
|
|
Diluted |
|
$ |
9.75 |
|
$ |
6.12 |
|
$ |
5.16 |
|
$ |
2.66 |
|
$ |
2.42 |
|
$ |
1.56 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Weighted average number of common shares outstanding: |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Basic |
|
52,093 |
|
57,163 |
|
56,142 |
|
53,314 |
|
50,503 |
|
54,424 |
| ||||||
|
Diluted |
|
53,280 |
|
59,395 |
|
59,516 |
|
56,133 |
|
52,133 |
|
55,952 |
| ||||||
(1) Consists of a $6.8 million impairment loss relating to a contract-based intangible asset, upon the termination of the underlying license agreement during the year ending December 31, 2012, net of $2.0 million of deferred revenue we recognized as a result of the terminated license agreement and the termination of our obligation to perform future services thereunder.
Earnings Before Non-cash Charges
(in thousands)
Percentages note increase over prior year
Conference Call
We will host a half-hour teleconference on Tuesday, February 26, 2013, at 9:00 a.m. Eastern Time. The teleconference is accessible by dialing 1-877-351-5881, with international callers dialing 1-970-315-0533. A rebroadcast of the teleconference will be available for one week by dialing 1-855-859-2056, with international callers dialing 1-404-537-3406 and using access code 89593584.
This teleconference is also being webcast and can be accessed via our website at http://ir.unither.com/events.cfm.
About United Therapeutics
United Therapeutics Corporation is a biotechnology company focused on the development and commercialization of unique products to address the unmet medical needs of patients with chronic and life-threatening conditions.
Non-GAAP Financial Information
This press release contains a financial measure, earnings before non-cash charges, that does not comply with United States generally accepted accounting principles (GAAP). This measure supplements our financial results prepared in accordance with GAAP as reported below.
We use earnings before non-cash charges to assist us in: (1) planning, including the preparation of our annual operating budget; (2) allocating resources in an effort to enhance the financial performance of our business; (3) evaluating the effectiveness of our operational strategies; and (4) assessing our capacity to fund capital expenditures and expand our business. We believe this non-GAAP financial measure improves investors’ understanding of our financial results by excluding certain expenses that we do not consider when evaluating and comparing the performance of our core operations and making operating decisions. In addition, we have historically reported earnings before non-cash charges to investors, and believe the inclusion of this non-
GAAP financial measure provides investors with a consistent method of comparison to historical periods. However, there are limitations in the use of this non-GAAP financial measure in that it excludes certain operating expenses that are recurring in nature. In addition, our calculation of this non-GAAP financial measure may differ from the methodology used by other companies. The presentation of this non-GAAP financial measure should not be considered in isolation or as a substitute for our financial results prepared in accordance with GAAP. A reconciliation of net income, the most directly comparable GAAP financial measure, to earnings before non-cash charges can be found in the table above under the heading, Earnings Before Non-Cash Charges.
Forward-looking Statements
Statements included in this press release that are not historical in nature are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include, among others, our expectations about future operating results, including statements regarding our revenue guidance for 2013, our transformative growth opportunities and the pending release of the TD-100 in 2013. These forward-looking statements are subject to certain risks and uncertainties, such as those described in our periodic reports filed with the Securities and Exchange Commission, that could cause actual results to differ materially from anticipated results. Consequently, such forward-looking statements are qualified by the cautionary statements, cautionary language and risk factors set forth in our periodic reports and documents filed with the Securities and Exchange Commission, including our most recent Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K. We claim the protection of the safe harbor contained in the Private Securities Litigation Reform Act of 1995 for forward-looking statements. We are providing this information as of February 26, 2013, and assume no obligation to update or revise the information contained in this press release whether as a result of new information, future events or any other reason.
[uthr-g]
Remodulin and Tyvaso are registered trademarks of United Therapeutics Corporation.
Adcirca is a registered trademark of Eli Lilly and Company.
UNITED THERAPEUTICS CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
|
|
|
Three Months Ended December 31, |
|
Year Ended December 31, |
| ||||||||
|
|
|
2012 |
|
2011 |
|
2012 |
|
2011 |
| ||||
|
Revenues: |
|
|
|
|
|
|
|
|
| ||||
|
Net product sales |
|
$ |
240,431 |
|
$ |
194,310 |
|
$ |
906,123 |
|
$ |
741,094 |
|
|
Other |
|
3,386 |
|
868 |
|
9,953 |
|
2,089 |
| ||||
|
Total revenue |
|
243,817 |
|
195,178 |
|
916,076 |
|
743,183 |
| ||||
|
Operating expenses: |
|
|
|
|
|
|
|
|
| ||||
|
Research and development |
|
37,476 |
|
48,636 |
|
173,387 |
|
180,015 |
| ||||
|
Selling, general and administrative |
|
40,073 |
|
57,707 |
|
201,746 |
|
156,482 |
| ||||
|
Cost of product sales |
|
37,665 |
|
25,327 |
|
119,297 |
|
88,904 |
| ||||
|
Total operating expenses |
|
115,214 |
|
131,670 |
|
494,430 |
|
425,401 |
| ||||
|
Operating income |
|
128,603 |
|
63,508 |
|
421,646 |
|
317,782 |
| ||||
|
Other (expense) income: |
|
|
|
|
|
|
|
|
| ||||
|
Interest income |
|
716 |
|
929 |
|
3,941 |
|
3,450 |
| ||||
|
Interest expense |
|
(4,490 |
) |
(5,112 |
) |
(16,639 |
) |
(21,367 |
) | ||||
|
Equity loss in affiliate |
|
(33 |
) |
(9 |
) |
(143 |
) |
(119 |
) | ||||
|
Other, net |
|
156 |
|
673 |
|
31,866 |
|
(629 |
) | ||||
|
Total other (expense) income, net |
|
(3,651 |
) |
(3,519 |
) |
19,025 |
|
(18,665 |
) | ||||
|
Income from continuing operations before income taxes |
|
124,952 |
|
59,989 |
|
440,671 |
|
299,117 |
| ||||
|
Income tax expense |
|
(41,697 |
) |
(16,800 |
) |
(136,229 |
) |
(81,874 |
) | ||||
|
Income from continuing operations |
|
83,255 |
|
43,189 |
|
304,442 |
|
217,243 |
| ||||
|
Discontinued operations |
|
|
|
|
|
|
|
|
| ||||
|
Income from discontinued operations, net of tax |
|
— |
|
— |
|
— |
|
7 |
| ||||
|
Gain on disposal of discontinued operations, net of tax |
|
— |
|
— |
|
— |
|
618 |
| ||||
|
Income from discontinued operations |
|
— |
|
— |
|
— |
|
625 |
| ||||
|
Net income |
|
$ |
83,255 |
|
$ |
43,189 |
|
$ |
304,442 |
|
$ |
217,868 |
|
|
Net income per common share: |
|
|
|
|
|
|
|
|
| ||||
|
Basic |
|
|
|
|
|
|
|
|
| ||||
|
Continuing operations |
|
$ |
1.65 |
|
$ |
0.79 |
|
$ |
5.84 |
|
$ |
3.80 |
|
|
Discontinued operations |
|
— |
|
— |
|
— |
|
0.01 |
| ||||
|
Net income per basic common share |
|
$ |
1.65 |
|
$ |
0.79 |
|
$ |
5.84 |
|
$ |
3.81 |
|
|
Diluted |
|
|
|
|
|
|
|
|
| ||||
|
Continuing operations |
|
$ |
1.60 |
|
$ |
0.77 |
|
$ |
5.71 |
|
$ |
3.66 |
|
|
Discontinued operations |
|
— |
|
— |
|
— |
|
0.01 |
| ||||
|
Net income per diluted common share |
|
$ |
1.60 |
|
$ |
0.77 |
|
$ |
5.71 |
|
$ |
3.67 |
|
|
Weighted average number of common shares outstanding: |
|
|
|
|
|
|
|
|
| ||||
|
Basic |
|
50,503 |
|
54,424 |
|
52,093 |
|
57,163 |
| ||||
|
Diluted |
|
52,133 |
|
55,952 |
|
53,280 |
|
59,395 |
| ||||
SELECTED CONSOLIDATED BALANCE SHEET DATA
(In thousands)
|
|
|
December 31, |
| ||||
|
|
|
2012 |
|
2011 |
| ||
|
Cash, cash equivalents and marketable securities (excluding restricted amounts of $5,377 and $5,123, respectively) |
|
$ |
784,931 |
|
$ |
747,378 |
|
|
Total assets |
|
1,626,595 |
|
1,518,079 |
| ||
|
Total liabilities and common stock subject to repurchase |
|
542,614 |
|
569,591 |
| ||
|
Total stockholders’ equity |
|
1,083,981 |
|
948,488 |
| ||
