Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CATALYST PHARMACEUTICALS, INC. | d482585d8k.htm |
| EX-99.2 - EX-99.2 - CATALYST PHARMACEUTICALS, INC. | d482585dex992.htm |
 Exhibit 99.1 |
 Page 2
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
This presentation contains forward-looking statements that are subject to a number
of risks and uncertainties, many
of
which
are
outside
our
control.
All
statements
regarding
our
strategy,
future
operations,
financial
position, estimated revenues or losses, projected costs, prospects, plans and
objectives, other than statements of historical fact included in our filings
with the U.S. Securities and Exchange Commission (the “SEC”), are
forward-looking statements. When used in this presentation or in answers given to questions
asked today, the words “may,”
“will,”
“could,”
“would,”
“expect,”
“intend,”
“plan,”
“anticipate,”
“believe,”
“estimate,”
“project,”
“potential,”
“continue,”
and similar expressions are intended to identify forward-looking
statements, although not all forward-looking statements contain these identifying
words. You should not place undue reliance on forward-looking
statements. While we believe that we have a reasonable basis for each
forward-looking statement that we make, we caution you that these statements are
based on a combination of facts and factors currently known by us and
projections of future events or conditions, about which we cannot be certain.
Forward-looking statements in this presentation should be evaluated together with the many
uncertainties
that
affect
our
business,
and
particularly
those
mentioned
in
the
“Risk
Factors”
section
of
our
Annual Report on Form 10-K filed with the SEC reporting our financial position and
results of operations as of and for the year ended December 31, 2011, in the
Registration Statement on Form S-1 that we filed with the SEC on April 6,
2012, as well as subsequent reports filed with the SEC during 2012. In addition, market and
industry statistics contained in this presentation are based on information available
to us that we believe is accurate. This information is generally based on
publications that are not produced for purposes of securities offerings or
economic analysis. All forward-looking statements speak only as of the date of this presentation.
Except as required by law, we assume no obligation to update these forward-looking
statements publicly or to update the factors that could cause actual results to
differ materially, even if new information becomes available in the future. Safe Harbor |
 Page 3
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Catalyst Pharmaceutical Partners is focused on the development and
commercialization of prescription drugs targeting rare (orphan) neurological
diseases and disorders, including Lambert-Eaton Myasthenic Syndrome (LEMS),
infantile spasms, and Tourette’s disorder
Headquarters:
Coral Gables, FL
NASDAQ Capital Market:
CPRX
Shares outstanding:
41,420,687
Share price (2/7/13):
$0.54
Market capitalization:
$22.4M
Cash and investments:
$17M*
Lead institutional investors:
Federated, Millenium, Sophrosyne
Strategic investment:
BioMarin (16% and joint development
agreement)
*As of 9/30/12, pro-forma for BioMarin $5MM investment
Catalyst Overview |
 Page 4
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
CPP-109 for Cocaine Addiction
–
Pivotal Phase II(b) trial showed
no statistical difference for
primary and secondary end points
–
Disappointing for all Catalyst
stakeholders
–
Full
data
set
available
Q2
–
2013
–
Meet with NIDA to discuss
complete findings
–
Expect to present data at conference later this year
–
Trials
for
cocaine
addicts
-
difficult
patient
population
Catalyst will not continue
development of addiction drugs
A Glance In The Rear View Mirror |
 Page 5
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Announced strategic alliance with BioMarin (in October) for
the
development
of
Firdapse
TM
North
American
license
for
Firdapse
TM
to
treat
neuromuscular diseases including LEMS
Strategic fit with our other orphan drug programs
–
CPP-115 for Infantile Spasms and Tourette’s disorder
BioMarin invested $5 million for a 16.6% equity stake
Joint development agreement for several remaining
studies-
sharing costs 50/50
Several milestones to be paid later and a mid-teen royalty
payment
Firdapse
TM
/BioMarin
Alliance |
 Page 6
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
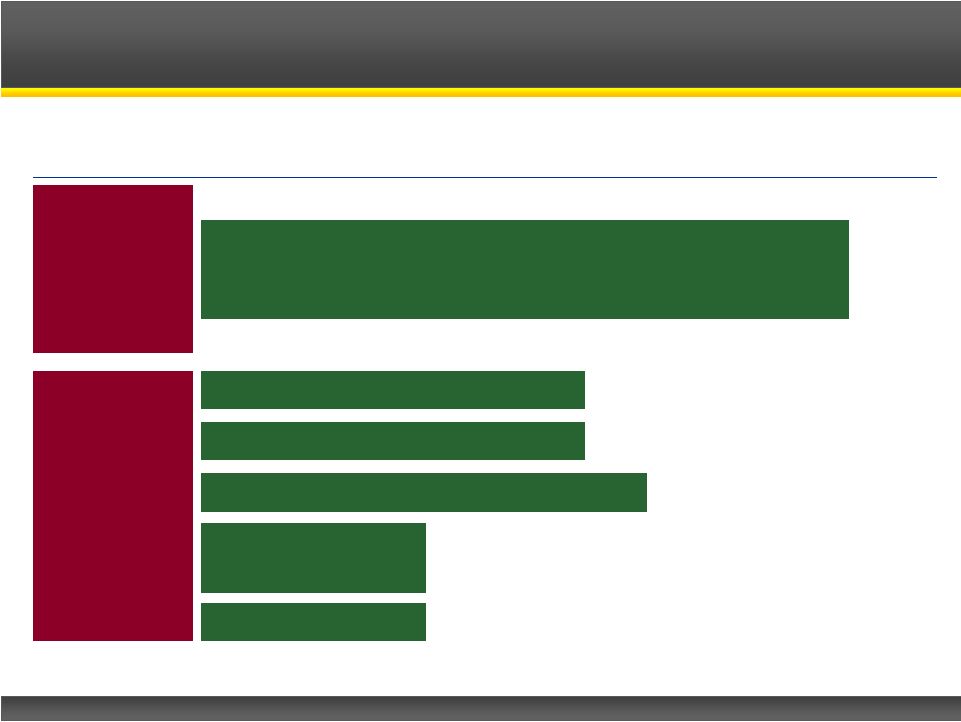
Phase I
Preclinical
Phase II
Phase III
Note: *Investigator sponsored study, CPP-109 is a model for CPP-115 to
treat this disorder Firdapse
TM
: Lambert-Eaton Myasthenic Syndrome (LEMS)
CPP-115: Complex Partial Seizures
CPP-109: Tourette’s Disorder*
CPP-115: Infantile Spasms
Potassium
Channel
Blocker
GABA-AT
Inhibitors
CPP-115: Dyskinesia
in Parkinson’s
CPP-115: MS
Product Pipeline |
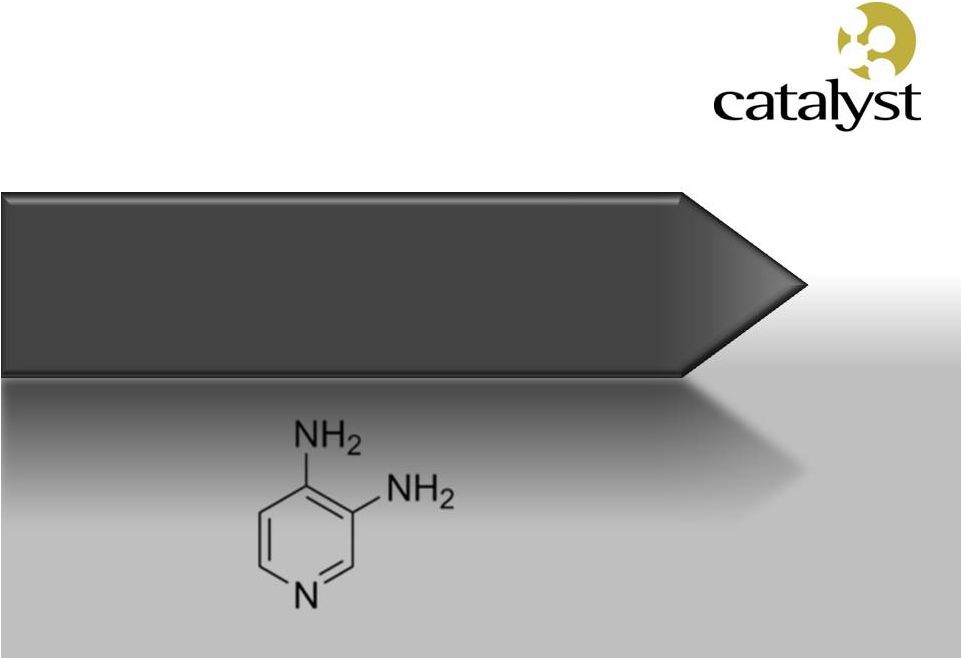 Firdapse
TM
Potassium Channel Blocker
Amifampridine
Phosphate
(3,4-Diaminopyridine) |
 Page 8
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Lambert-Eaton Myasthenic Syndrome (LEMS) is a rare autoimmune disease
caused by auto-antibodies that inhibit acetylcholine release from nerve
terminals
–
Chronic, often severly disabling and progressive
–
Usually managed by neurologists
–
~3,000 patients in the U.S. (10 per 1M prevalence)
Continuing pivotal Phase III trial designed and initiated by BioMarin with FDA
input
Orphan drug designation in the U.S.
BioMarin
launched
Firdapse
TM
in
Europe
(2Q10)
for
the
treatment of LEMS
–
EFNS
recommends
Firdapse
TM
as
the
first-line
symptomatic treatment for LEMS
–
European annual cost of therapy ~$60,000 USD
Independent market research indicates
annual peak U.S. sales of ~$100 million
Opportunities for label expansion
Pending composition of matter patent
Firdapse
TM
Opportunity
Summary |
 Page 9
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
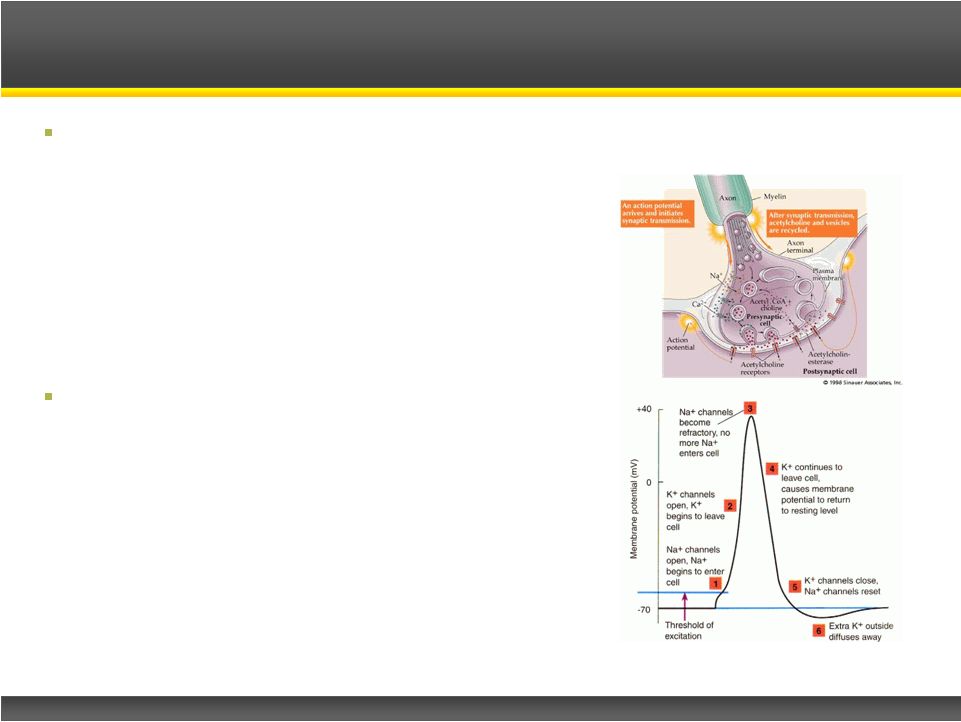
Insufficient ACh release due to
antibodies to the pre-synaptic P/Q type
voltage gated calcium channel
LEMS Disease:
–
Proximal muscle weakness
–
Straight forward differential diagnosis
–
Can be disabling
–
Often worsens after diagnosis
–
In some cases, may be life threatening
–
~50% of cases associated with SCLC, which has a
12-24 month life expectancy
Firdapse
TM
Treatment:
–
Potassium channel blocker
–
Delays neuron repolarization
–
Voltage gated calcium channels remain open
longer
–
Increased calcium influx causes more
acetylcholine to be released to the innervated
muscle cells
–
Restoration of lost muscle strength
LEMS
and
Firdapse
TM
Treatment |
 Page 10
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Unserved U.S. population due to no FDA approved therapy
–
Off-label therapies include pyridostigmine and
immunosuppressants
–
IVIg and plasmapheresis are also part of the standard of care
Amifampridine is currently available to patients via
investigator sponsored INDs and expanded access INDs
–
Difficult for patients to obtain drug
•
Even with legally allowed options, many physicians are
unwilling or unable to utilize them
–
Unclear if/how safety reporting is being done
–
Inadequate control of product manufacturing
•
Uniformity of potency
•
Stability
U.S. LEMS Product Need |
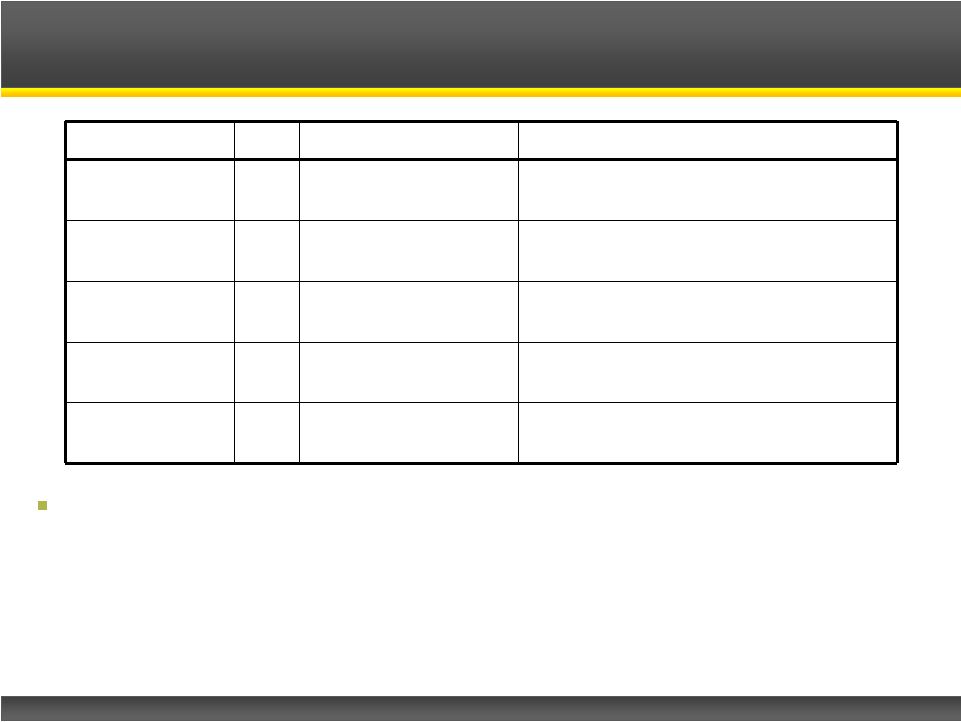 Page 11
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Study
N
Dose, duration
Efficacy Outcomes
McEvoy, 1989
12
Up to 100 mg/day
3 day crossover
Significant on CMAP, disability score,
arm/leg strength
Sanders, 1993
18
Up to 100 mg/day
8 day crossover
Significant on QMG
Sanders, 2000
26
60 mg/day
6 day parallel arm
Significant
on
QMG,
CMAP
Wirtz, 2009
9
Single IV dose, 10
mg
Significant on isometric force, CMAP
Oh, 2009
8
75 –
80 mg/day
3-8 day crossover
Significant on symptom severity,
QMG, muscle strength and CMAP
Amifampridine Proven Efficacy and Safety in LEMS
Significant database of exposure in the literature
–
1174 patient exposures for all uses
–
169 in LEMS patients |
 Page 12
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Trial design
–
FDA concurred with design in June, 2010 meeting with BioMarin
–
Ethical design accepted by KOLs and FDA
FDA requires one randomized, placebo-controlled, treatment
discontinuation trial in LEMS patients
–
Compares amifampridine efficacy to placebo at the end of a 14-day
discontinuation period
–
Primary endpoint: Muscle strength (Quantitative Myasthenia Gravis score
[QMG])
–
Secondary endpoint: Walking speed (Timed 25-foot walking test)
–
Tertiary endpoint: Compound Muscle Action Potential (CMAP)
Approximately 1/3 enrolled
7 active sites (4 U.S./3 Europe) with up to 20 to be added to accelerate
the study
Data Monitoring Committee (DMC) review Q1 2013
Expect to complete double blind stage of trial around end of Q1 2014
Firdapse
TM
U.S. Phase III Clinical Trial |
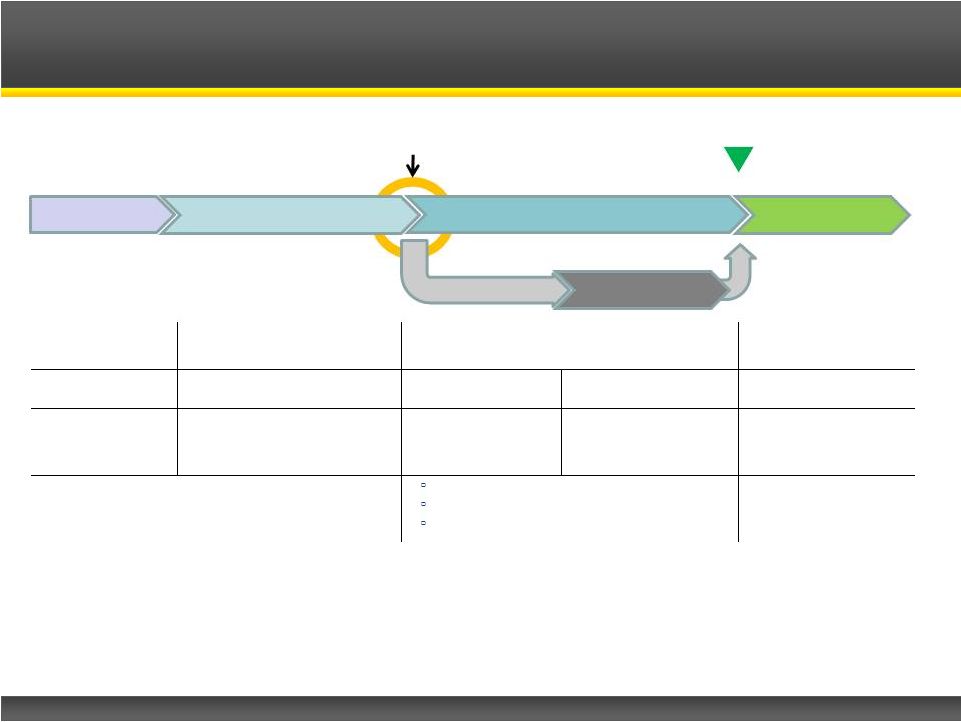 Page 13
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Randomization
N=30, 1:1
Dose Taper
Last Patient
~March 2014
Firdapse
TM
U.S. Phase III Clinical Trial
Firdapse™
Firdapse™
Firdapse™
Placebo
Screening
Open Label Run-In
Double-Blind Treatment Phase
Open-Label Safety
Extension
Days 1-4
Days 7-91
Day 1-7
Day 8-14
Up to 2 years
Efficacy/Baseline
Assessments
Screening
Efficacy/Eligibility
Assessments and Dose
Adjustments
Efficacy
Assessments
on Day 1
Efficacy Assessments
on Days 8 and 14
Safety Assessments
and Dose
Adjustments
1 : Quantitative Myasthenia Gravis (QMG)
2 : Timed 25 Foot Walk
3 : Compound Muscle Action Potential
Not required for NDA
filing and approval |
 Page 14
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Congenital Myasthenic Syndrome (CMS)
–
Prevalence of ~1,500 patients in the U.S.
•
Eligible for orphan drug designation
•
Prevalence may be under reported due to complexities of diagnosis
–
No approved therapy
–
Differential diagnosis complex
–
Confirmation of diagnosis from genetic screening for one or more
of the 14 known genetic defects
Myasthenia Gravis (MG)
–
Prevalence of ~60,000 patients in the U.S.
•
Potential to treat a few thousand refractory patients with Firdapse
TM
–
First-line therapy is Mestinon (pyridostigmine), an ACh inhibitor
approved before 1982 that is not promoted
Firdapse
TM
Expansion Opportunities |
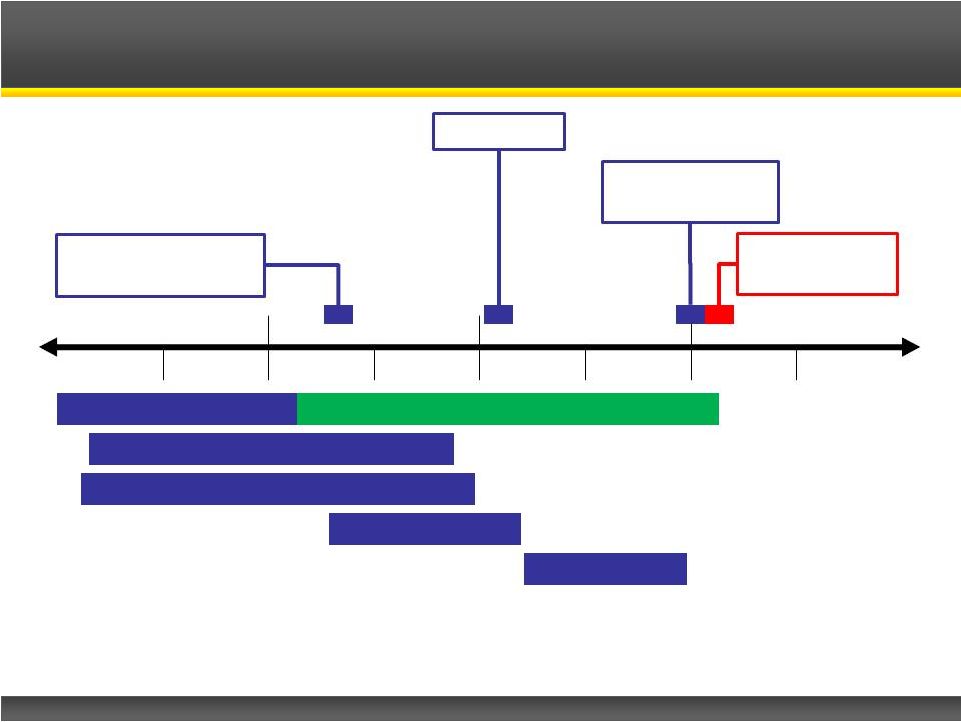 Page 15
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Phase III Clinical Trial
Phase III Safety Extension
Clinical Safety Studies
Pre-Clinical Studies
Prepare NDA
FDA Review
File NDA
Estimated FDA
Approval
Commercial
Launch
Phase III Top-Line
Results
Firdapse
TM
Regulatory
Pathway
2013
2014
2015
2016
H1
H2
H1
H2
H1
H2
H1
H2 |
 Page 16
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
First FDA approved drug for LEMS
Patient tracking and LEMS support groups to identify
patients
Market access through private and public payors
–
Market access research indicates drug will be widely
reimbursed
Specialty sales force
–
Initial sales force estimate of 20 sales representatives
Orphan drug pricing
Patient-assistance program
Registry support patients
Product education through KOLs
Expansion to new indications
Firdapse
TM
Commercialization Strategy |
 Beyond
Firdapse TM
: CPP-115
Next Generation GABA-AT Inhibitor |
 Page 18
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Invented by Richard Silverman, Ph.D.
–
Inventor of Lyrica®
(pregabalin); ~$4B in annual
sales for Pfizer
–
Rationally designed drug with enhanced potency,
specificity, and safety
Exclusive worldwide license to commercialize
new GABA-AT inhibitors
Includes composition of matter patents to a new
class of inhibitors
–
Protection through 2028 with patent extensions
allowed under Patent Term Restoration Act
Filed PCT application seeking to protect CPP-
115 in ex-U.S. markets
CPP-115 Innovation |
 Page 19
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
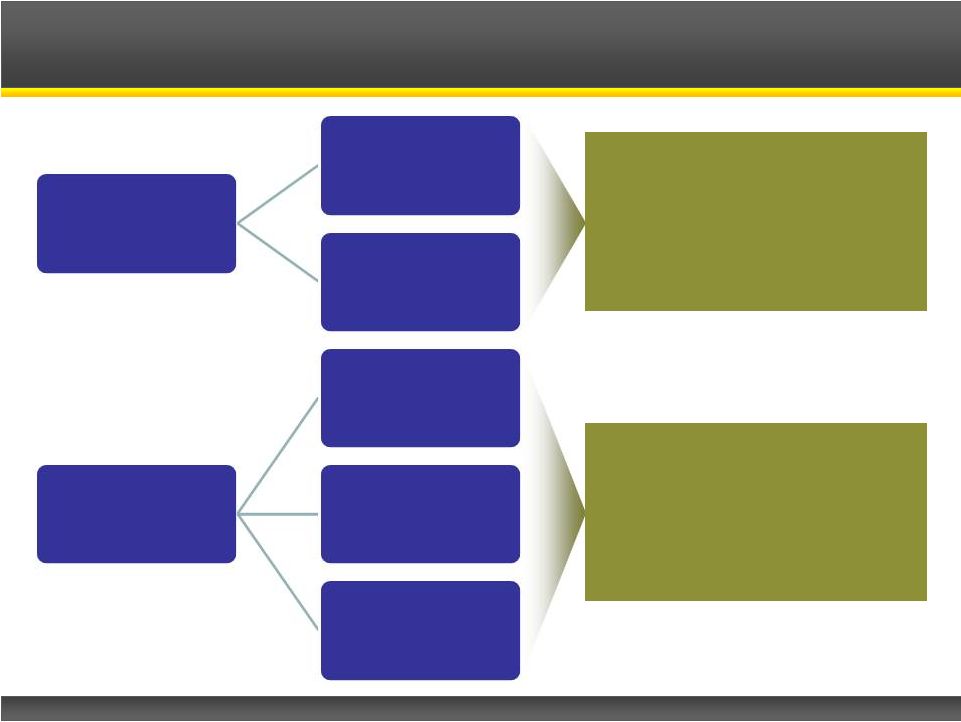
CPP-115 Targeted Indications
Proven
Indications
IS
CPS
Potential New
Indications
Tourette’s
PTSD
Movement
Disorders
Infantile spasms and complex
partial seizures are proven
indications for inhibition of
GABA-AT. CPP-115 could be a
safer and more effective
alternative.
HypoGABAergic signaling
pathways are known to play a
role in these conditions. |
 Page 20
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
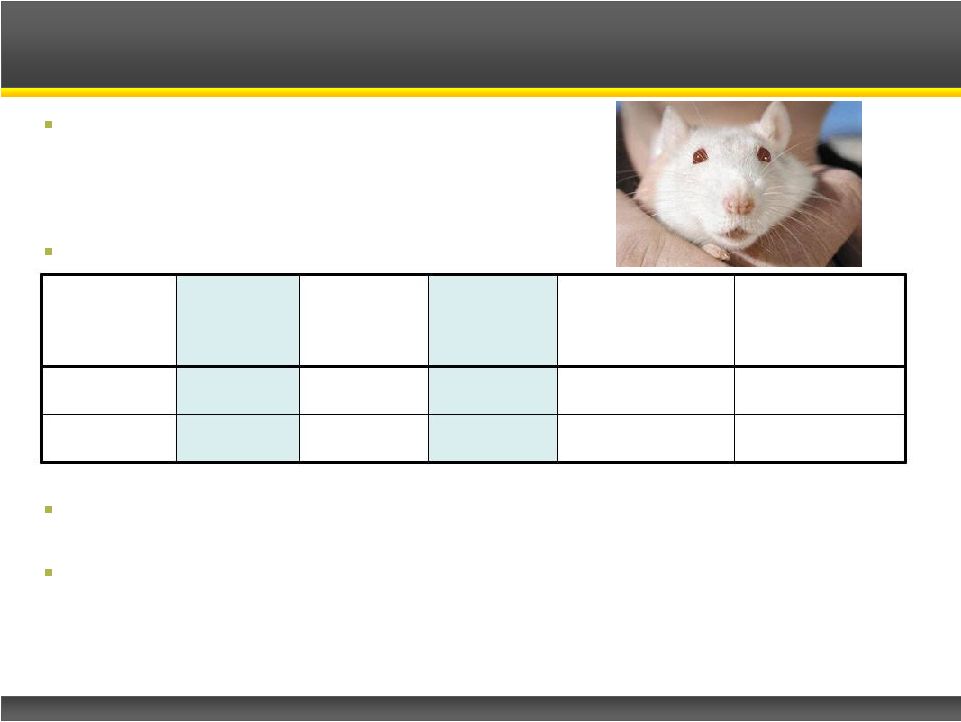
Risk of visual field deficits with vigabatrin use
–
Black box warning on label and REMS program
–
Occurs in 1/3 to 1/2 of patients
–
Permanent loss of some peripheral vision
Comparative vision safety study in rats
1
For infantile spasms in Multiple Hit Model, and dose shown to inhibit GABA-AT
in other studies CPP-115, at 20 times its effective dose, is safer than
vigabatrin at its effective
dose
CPP-115, at its effective dose, will likely be even safer
–
Potentially no Visual Field Defect (VFD) risk
CPP-115 Superior Visual Safety
Effective
Dose
(Rats)
Vision
Study
Dose
Vision
Safety
Margin
45 Day Retinal
Function Loss
(ERG)
90 Day Retinal
Function Loss
(ERG)
Vigabatrin
300 mg/kg
200 mg/kg
~1
~30-60%
~45-60%
CPP-115
<
1
mg/kg
1
20 mg/kg
> 20
~5-30%
~10-35% |
 Page 21
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
0
2,000
4,000
6,000
U.S.
Europe
Incidence
Prevalence
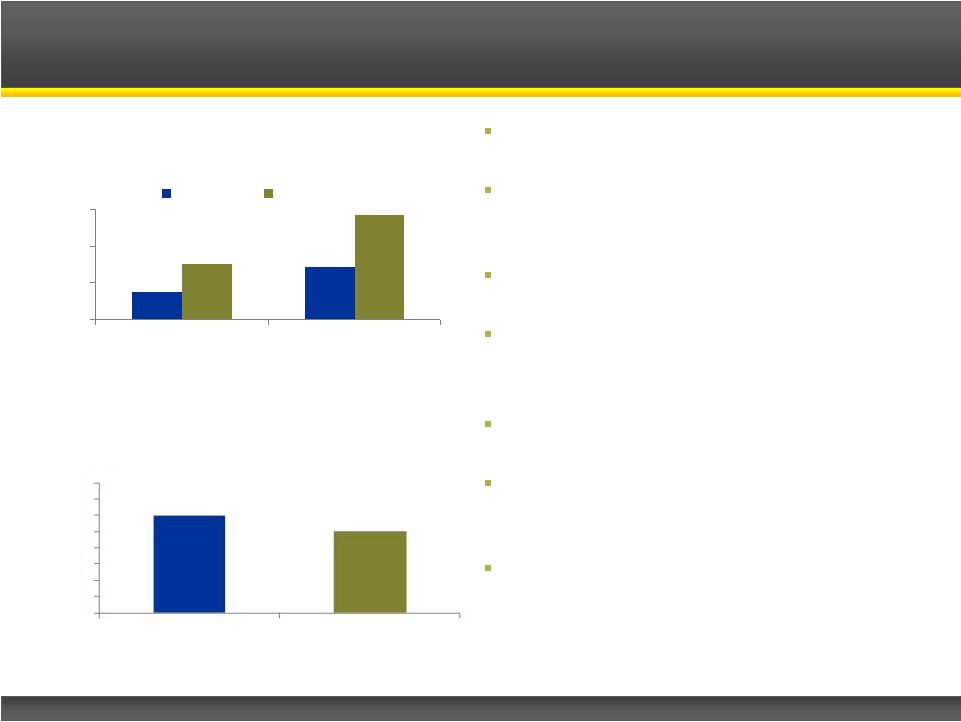
Infantile spasms, or West Syndrome, is a
catastrophic form of epilepsy for infants
Affects 10K –
20K infants worldwide, with
nearly one-half of them in the U.S. and
Europe
60-70% of patients have underlying
disorder
Leading
therapies
are
Acthar
®
Gel
and
Sabril
®
; generate ~$100M in U.S. sales
–
In spite of significant side effects
CPP-115 has U.S. and EU orphan drug
designations
CPP-115 will be a new first-line therapy,
as well as for non-responders to existing
therapies
Opportunity valuation
–
Lundbeck
paid
~$300M
for
Sabril
®
rights
–
Oppenheimer
values
Questcor’s
Acthar
®
Gel infantile spasm franchise at ~$300M
Infantile Spasms Opportunity
Infantile Spasms Epidemiology (2010)
310M
Source: Epilepsia 2010; Population Reference Bureau 2010;
Company Reports; Catalyst Estimates
U.S. Infantile Spasms Sales (2011E)
Sales ($M)
739M
Population:
0
10
20
30
40
50
60
$70
$80
Acthar Gel
Sabril |
 Page 22
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
“Multiple-Hit Model”
for ACTH-refractory infantile spasms
(Albert Einstein College of Medicine)
–
Widely Respected model of infantile spasms
–
CPP-115
(0.1-1
mg/kg/day
i.p.)
suppressed
spasms
at
1/100th
the
dose
of
vigabatrin,
with
better
tolerance
than
vigabatrin
1
–
CPP-115 more effective than vigabatrin
•
Magnitude and duration of seizure reduction greater than
vigabatrin
•
CPP-115 causes no sedation in contrast to vigabatrin which
causes severe sedation at therapeutic doses
1
Briggs SW, Ono T, Moshé
SL, Galanopoulou AS (2011): presented at the American Epilepsy Society
Meeting CPP-115 Infantile Spasms Screening
(December 2011) |
 Page 23
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Met with FDA Oct 2011 to discuss IS development plans
–
Agreed on development strategy through phase II
–
Relatively standard development pathway to enter phase II
•
No unusual phase 1 or preclinical requirements, other than preclinical work in
juvenile animals for IS indication
–
Agreed on phase II design
•
Escalating dose study, N=25-30 infants
Utilizing experts Jack Pellock, MD and Don Shields, MD as consultants
–
Widely respected KOLs for infantile spasms
–
Accompanied Catalyst to FDA meeting
Phase 1 studies (supports any indication)
–
Phase 1 SAD study completed Q2 2012
–
Phase 1 MAD study designed (includes MRI efficacy biomarker)
Phase 2 enabling toxicology studies are needed
Will seek additional development funding
–
Potential partners
–
NIH
CPP-115 Development Strategy |
 Page 24
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Indications for which no predictive animal models exist
–
Tourette’s disorder
•
6-10 patient, open label, phase I/II study in progress
•
Top-line results Q4 2013
–
Post Traumatic Stress Disorder (PTSD)
–
CPP-109 (vigabatrin) used as a research “surrogate”
for CPP-115
•
CPP-115 and CPP-109 have same mechanism of action
•
Extended duration use in man for CPP-109 allowed with frequent vision
testing •
Will use CPP-109 until CPP-115 is developed sufficiently to support use in
phase 2 studies
Indications for which predictive animal models exist
–
Dyskinesia in Parkinson’s Disease
–
Multiple Sclerosis
CPP-115: Other Potential Indications |
 Page 25
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Acquired
Firdapse
TM
,
a
phase
III
asset,
for development and commercialization
Completed $5,000,000 strategic
investment by BioMarin
Initiated Phase I/II study for Tourette’s
Disorder
Met with FDA to define development plan
for CPP-115 to treat infantile spasms
Granted orphan medicinal product
designation in EU for CPP-115 for
treatment of West Syndrome (infantile
spasms)
Reported CPP-115 Phase I(a) study
results
Filed U.S. provisional patent application
for GABA-AT inhibitor use in treatment of
Tourette Syndrome
Completed common stock public offering
Q1 2013
–
Firdapse
TM
DMC
meeting
results
Q4 2013
–
Complete
enrollment
of
Firdapse
TM
phase III clinical trial
–
Top-line results from Tourette’s
Disorder study
Q2 2014
–
Top-line
results
from
Firdapse
TM
phase III clinical trial
Q1 2015
–
File
Firdapse
TM
NDA
Catalyst Milestones
Recent Milestones
Expected Milestones |
 Page 26
Copyright
©
2013 Catalyst Pharmaceutical Partners, Inc.
Contact Information
Catalyst Pharmaceutical Partners, Inc.
Investor Relations
355 Alhambra Circle, Suite 1500
Rx Communications
Coral Gables, FL 33134
Melody Carey
(305) 529-2522
(917) 322-2568
mcarey@rxir.com
Patrick J. McEnany
Chairman and Chief Executive Officer
pmcenany@catalystpharma.com |
 |
