Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Celldex Therapeutics, Inc. | a13-2215_18k.htm |
Exhibit 99.1
|
|
Corporate Presentation January 2013 |
|
|
Forward Looking Statement This communication contains "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical fact are statements that could be forward-looking statements. You can identify these forward-looking statements through our use of words such as “may,” “will,” “can,” “anticipate,” “assume,” “should,” “indicate,” “would,” “believe,” “contemplate,” “expect,” “seek,” “estimate,” “continue,” “plan,” “point to,” “project,” “predict,” “could,” “intend,” “target,” “potential” and other similar words and expressions of the future. These forward-looking statements are subject to risks and uncertainties that may cause actual future experience and results to differ materially from those discussed in these forward-looking statements. Important factors that might cause such a difference include, but are not limited to, the timing, cost and uncertainty of obtaining regulatory approvals for product candidates; our ability to develop and commercialize products before competitors that are superior to the alternatives developed by such competitors; the validity of our patents and our ability to avoid intellectual property litigation, which can be costly and divert management time and attention; and the other factors listed under “Risk Factors” in our filings with the SEC, including Forms 10-K, 10-Q and 8-K. Celldex does not undertake any obligation to release publicly any revisions to such forward-looking statement to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. |
|
|
Multiple late-stage products with near-term inflection points Rindopepimut (ACT IV) Phase 3 trial in front-line GB to complete enrollment by YE 2013 Rindopepimut (ReACT) Phase 2 trial in recurrent GB (both Avastin naïve and refractory) data in 2H 2013 Initiate CDX-011 late-stage study in metastatic breast by 2H 2013 Late-stage programs supported by deep pipeline of clinical and preclinical programs CDX-1135 pilot study in DDD to initiate Q1 2013 CDX-1127 expanded Phase 1 studies in solid tumors/hematologic cancers; data in 2H 2013 CDX-1401 collaboration with NCI on Phase 2 combination study with CDX-301 CDX-301 final results by Q1 2013; initiation of pilot study in transplant setting by YE 2013 Balanced business strategy & risk management Experienced team involved in the development of ipilimumab (Yervoy) Fiscal discipline $77.6 mil in cash/investment as of Q3’12; financial runway into 2014 Celldex – Targeted Therapeutics for Patients with Devastating Diseases |
|
|
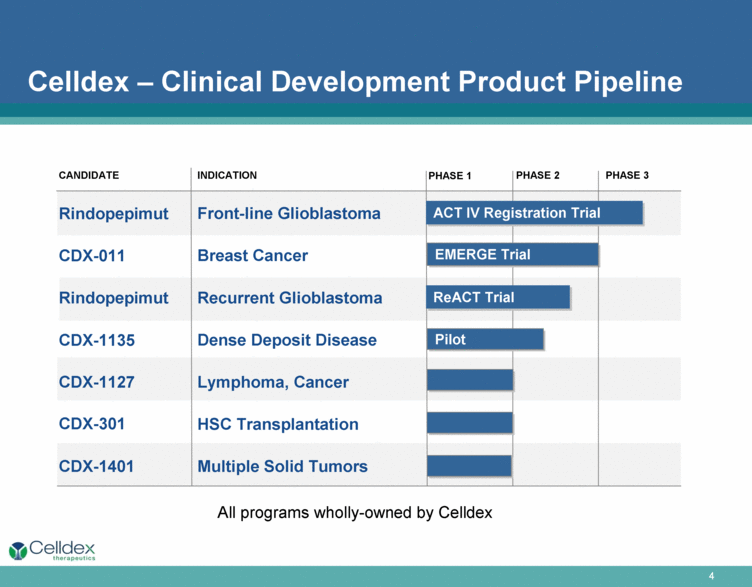
Celldex – Clinical Development Product Pipeline CANDIDATE PHASE 3 Rindopepimut CDX-011 Rindopepimut CDX-1135 CDX-1127 CDX-301 CDX-1401 INDICATION Front-line Glioblastoma Breast Cancer Recurrent Glioblastoma Dense Deposit Disease Lymphoma, Cancer HSC Transplantation Multiple Solid Tumors PHASE 2 PHASE 1 EMERGE Trial ReACT Trial ACT IV Registration Trial Pilot All programs wholly-owned by Celldex |
|
|
Celldex: Realizing the Potential of Immunotherapy Therapies that activate the patient’s immune system are changing the approach to cancer treatment Validation: antibodies that block negative signals to immune cells (anti-CTLA-4, anti-PD1) have shown profound clinical effects in various cancers These antibodies release a naturally developed immune response against the cancer Our targeted therapeutics (both antibody and protein based) specifically target and function through the immune system Rindopepimut activates a specific immune response against tumor cells and cancer stem cells that harbor the oncogene EGFRvIII and instructs the immune system to eliminate EGFRvIII expressing tumor cells |
|
|
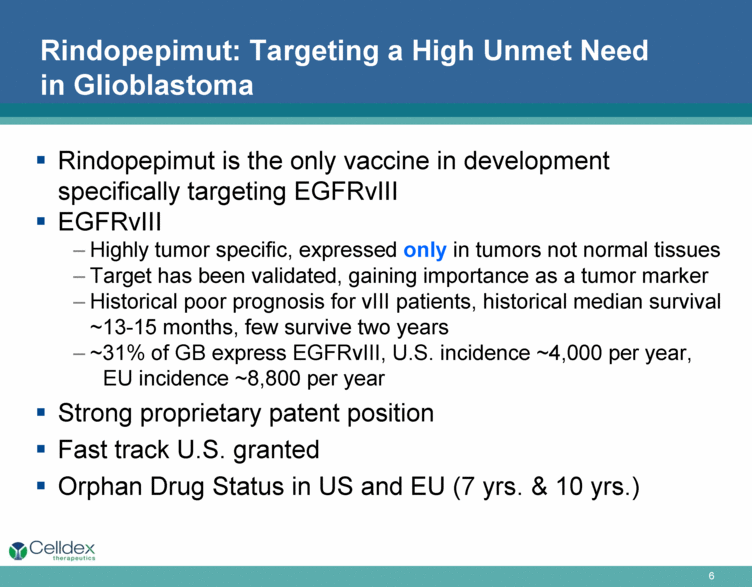
Rindopepimut: Targeting a High Unmet Need in Glioblastoma Rindopepimut is the only vaccine in development specifically targeting EGFRvIII EGFRvIII Highly tumor specific, expressed only in tumors not normal tissues Target has been validated, gaining importance as a tumor marker Historical poor prognosis for vIII patients, historical median survival ~13-15 months, few survive two years ~31% of GB express EGFRvIII, U.S. incidence ~4,000 per year, EU incidence ~8,800 per year Strong proprietary patent position Fast track U.S. granted Orphan Drug Status in US and EU (7 yrs. & 10 yrs.) |
|
|
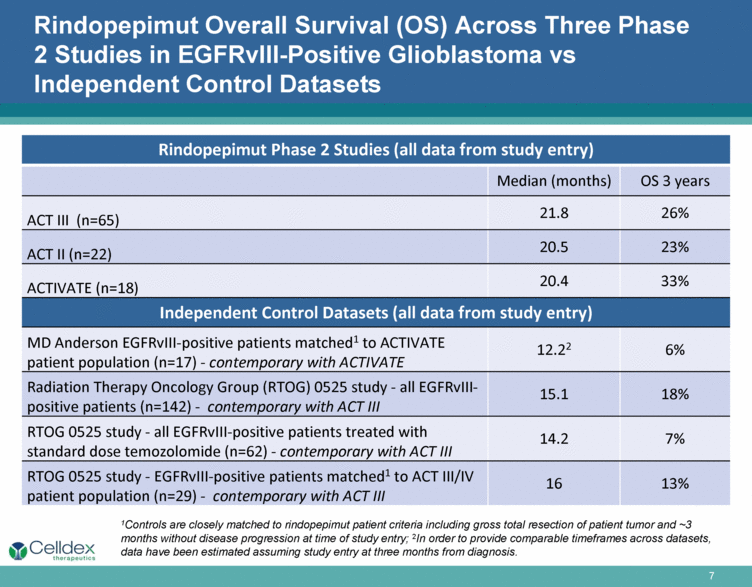
Rindopepimut Overall Survival (OS) Across Three Phase 2 Studies in EGFRvIII-Positive Glioblastoma vs Independent Control Datasets Rindopepimut Phase 2 Studies (all data from study entry) Median (months) OS 3 years ACT III (n=65) 21.8 26% ACT II (n=22) 20.5 23% ACTIVATE (n=18) 20.4 33% Independent Control Datasets (all data from study entry) MD Anderson EGFRvIII-positive patients matched1 to ACTIVATE patient population (n=17) - contemporary with ACTIVATE 12.22 6% Radiation Therapy Oncology Group (RTOG) 0525 study - all EGFRvIII-positive patients (n=142) - contemporary with ACT III 15.1 18% RTOG 0525 study - all EGFRvIII-positive patients treated with standard dose temozolomide (n=62) - contemporary with ACT III 14.2 7% RTOG 0525 study - EGFRvIII-positive patients matched1 to ACT III/IV patient population (n=29) - contemporary with ACT III 16 13% 1Controls are closely matched to rindopepimut patient criteria including gross total resection of patient tumor and ~3 months without disease progression at time of study entry; 2In order to provide comparable timeframes across datasets, data have been estimated assuming study entry at three months from diagnosis. |
|
|
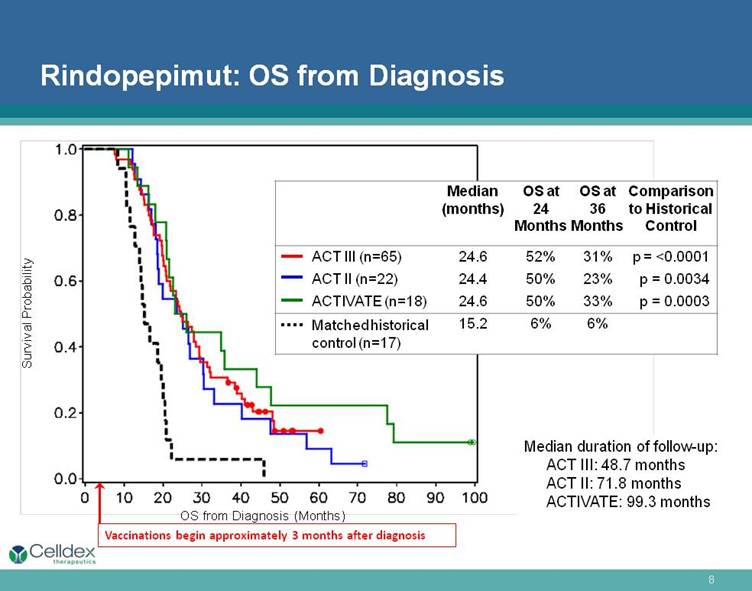
Rindopepimut: OS from Diagnosis Survival Probability Median (months) OS at 24 Months OS at 36 Months Comparison to Historical Control ACT III (n=65) 24.6 52% 31% p = <0.0001 ACT II (n=22) 24.4 50% 23% p = 0.0034 ACTIVATE (n=18) 24.6 50% 33% p = 0.0003 Matched historical control (n=17) 15.2 6% 6% Vaccinations begin approximately 3 months after diagnosis OS from Diagnosis (Months) Median duration of follow-up: ACT III: 48.7 months ACT II: 71.8 months ACTIVATE: 99.3 months |
|
|
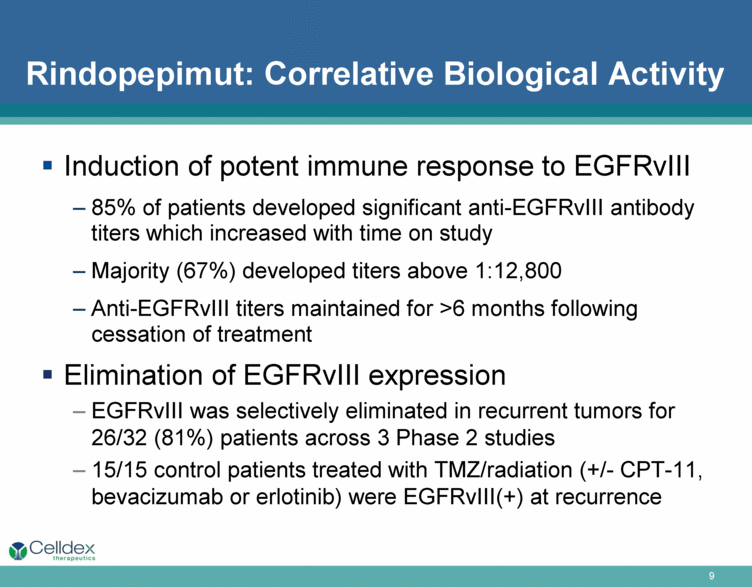
Rindopepimut: Correlative Biological Activity Induction of potent immune response to EGFRvIII 85% of patients developed significant anti-EGFRvIII antibody titers which increased with time on study Majority (67%) developed titers above 1:12,800 Anti-EGFRvIII titers maintained for >6 months following cessation of treatment Elimination of EGFRvIII expression EGFRvIII was selectively eliminated in recurrent tumors for 26/32 (81%) patients across 3 Phase 2 studies 15/15 control patients treated with TMZ/radiation (+/- CPT-11, bevacizumab or erlotinib) were EGFRvIII(+) at recurrence |
|
|
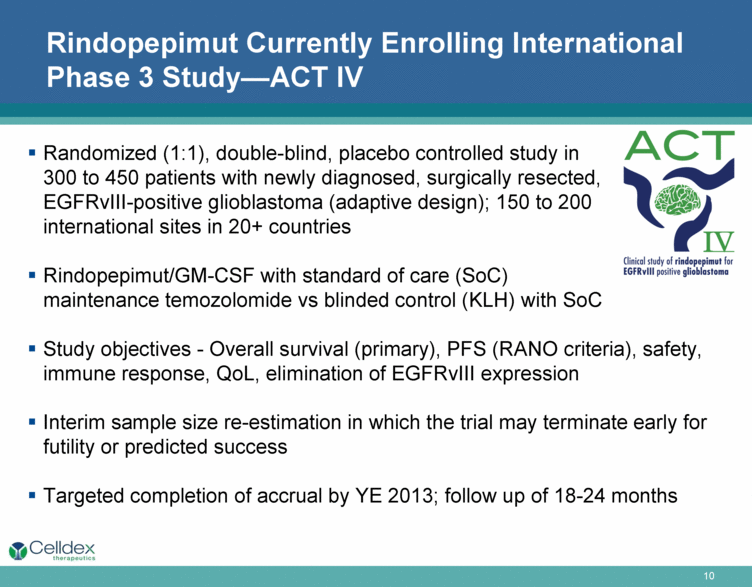
Rindopepimut Currently Enrolling International Phase 3 Study—ACT IV Randomized (1:1), double-blind, placebo controlled study in 300 to 450 patients with newly diagnosed, surgically resected, EGFRvIII-positive glioblastoma (adaptive design); 150 to 200 international sites in 20+ countries Rindopepimut/GM-CSF with standard of care (SoC) maintenance temozolomide vs blinded control (KLH) with SoC Study objectives - Overall survival (primary), PFS (RANO criteria), safety, immune response, QoL, elimination of EGFRvIII expression Interim sample size re-estimation in which the trial may terminate early for futility or predicted success Targeted completion of accrual by YE 2013; follow up of 18-24 months |
|
|
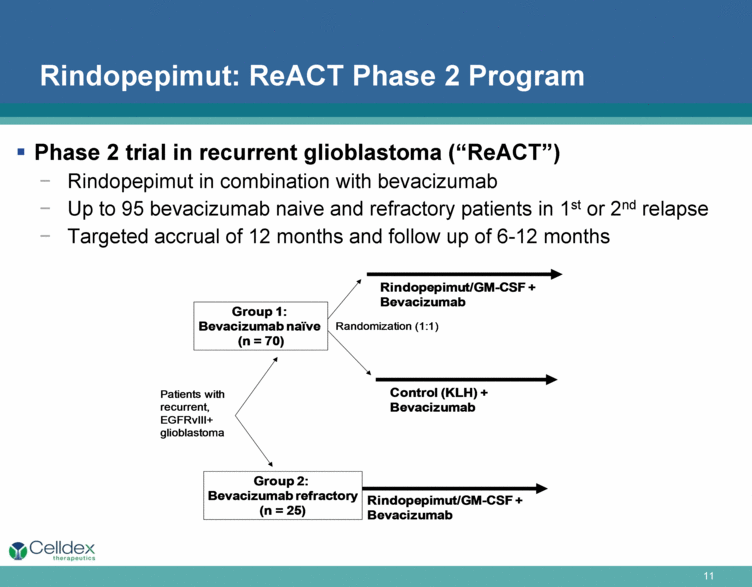
Rindopepimut: ReACT Phase 2 Program Phase 2 trial in recurrent glioblastoma (“ReACT”) Rindopepimut in combination with bevacizumab Up to 95 bevacizumab naive and refractory patients in 1st or 2nd relapse Targeted accrual of 12 months and follow up of 6-12 months Control (KLH) + Bevacizumab Rindopepimut/GM-CSF + Bevacizumab Randomization (1:1) Patients with recurrent, EGFRvIII+glioblastoma Rindopepimut/GM-CSF + Bevacizumab Group 1: Bevacizumab naïve (n = 70) Group 2: Bevacizumab refractory (n = 25) |
|
|
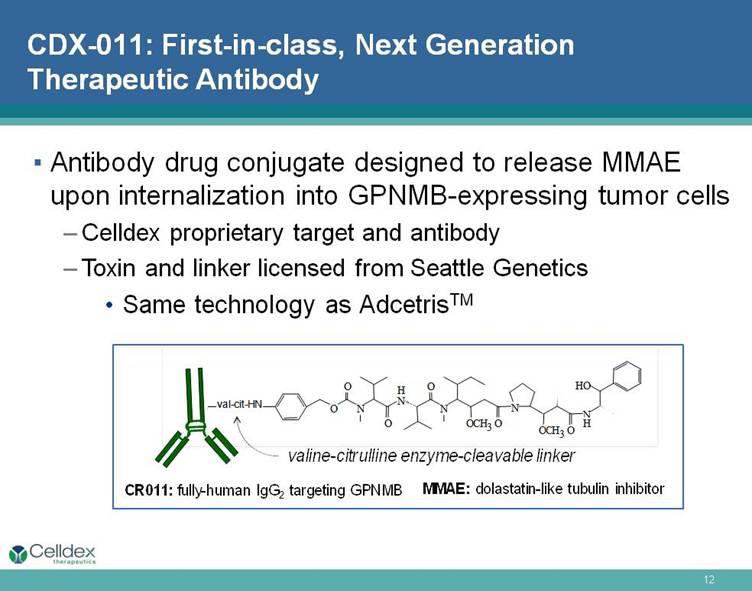
CDX-011: First-in-class, Next Generation Therapeutic Antibody Antibody drug conjugate designed to release MMAE upon internalization into GPNMB-expressing tumor cells Celldex proprietary target and antibody Toxin and linker licensed from Seattle Genetics Same technology as AdcetrisTM MMAE: dolastatin-like tubulin inhibitor N H N N O O O O C H 3 O N O C H 3 N H H O O O val-cit-HN valine-citrulline enzyme-cleavable linker CR011: fully-human IgG2 targeting GPNMB |
|
|
Advanced breast cancer patients who are refractory/resistant to all approved therapies Patients selected for GPNMB expression 120 patients randomized (2:1) to receive CDX-011 or “Investigator’s Choice” single agent chemotherapy Endpoints included overall response rate, duration of response, PFS, O/S, and PK/PD Final data presented at SABC Symposium, Dec 2012 End of Phase 2 FDA meeting in Dec 2012; provide update on YE 2012 call in early March CDX-011: EMERGE Phase 2b Randomized Study in Advanced Breast Cancer |
|
|
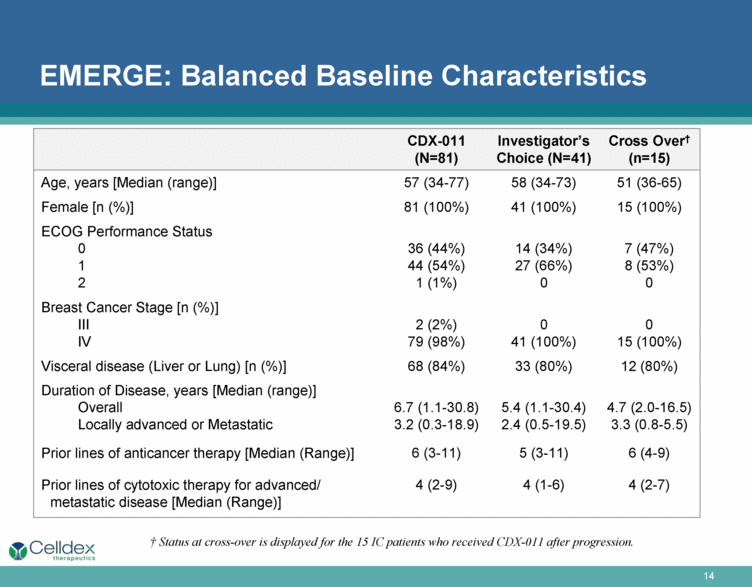
EMERGE: Balanced Baseline Characteristics † Status at cross-over is displayed for the 15 IC patients who received CDX-011 after progression. CDX-011 (N=81) Investigator’s Choice (N=41) Cross Over† (n=15) Age, years [Median (range)] 57 (34-77) 58 (34-73) 51 (36-65) Female [n (%)] 81 (100%) 41 (100%) 15 (100%) ECOG Performance Status 0 1 2 36 (44%) 44 (54%) 1 (1%) 14 (34%) 27 (66%) 0 7 (47%) 8 (53%) 0 Breast Cancer Stage [n (%)] III IV 2 (2%) 79 (98%) 0 41 (100%) 0 15 (100%) Visceral disease (Liver or Lung) [n (%)] 68 (84%) 33 (80%) 12 (80%) Duration of Disease, years [Median (range)] Overall Locally advanced or Metastatic 6.7 (1.1-30.8) 3.2 (0.3-18.9) 5.4 (1.1-30.4) 2.4 (0.5-19.5) 4.7 (2.0-16.5) 3.3 (0.8-5.5) Prior lines of anticancer therapy [Median (Range)] 6 (3-11) 5 (3-11) 6 (4-9) Prior lines of cytotoxic therapy for advanced/metastatic disease [Median (Range)] 4 (2-9) 4 (1-6) 4 (2-7) |
|
|
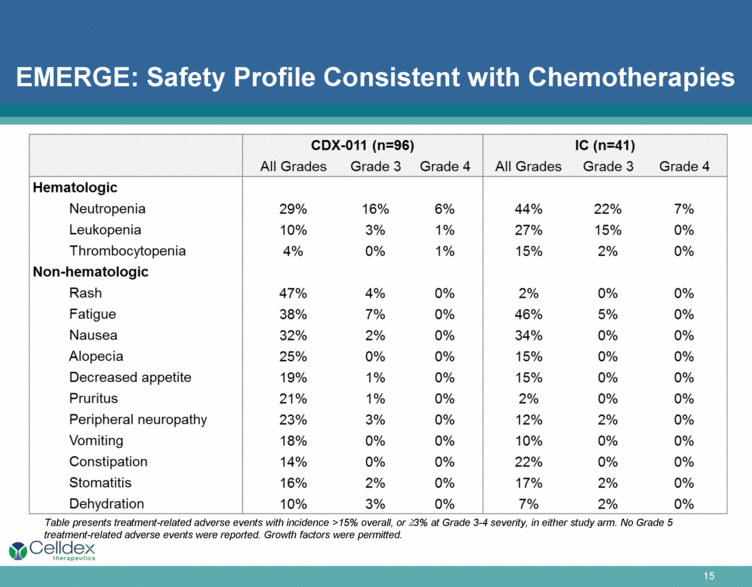
EMERGE: Safety Profile Consistent with Chemotherapies CDX-011(n=96) IC (n=41) All Grades Grade 3 Grade 4 All Grades Grade 3 Grade 4 Hematologic Neutropenia 29% 16% 6% 44% 22% 7% Leukopenia 10% 3% 1% 27% 15% 0% Thrombocytopenia 4% 0% 1% 15% 2% 0% Non-hematologic Rash 47% 4% 0% 2% 0% 0% Fatigue 38% 7% 0% 46% 5% 0% Nausea 32% 2% 0% 34% 0% 0% Alopecia 25% 0% 0% 15% 0% 0% Decreased appetite 19% 1% 0% 15% 0% 0% Pruritus 21% 1% 0% 2% 0% 0% Peripheral neuropathy 23% 3% 0% 12% 2% 0% Vomiting 18% 0% 0% 10% 0% 0% Constipation 14% 0% 0% 22% 0% 0% Stomatitis 16% 2% 0% 17% 2% 0% Dehydration 10% 3% 0% 7% 2% 0% Table presents treatment-related adverse events with incidence >15% overall, or >23% at Grade 3-4 severity, in either study arm. No Grade 5 treatment-related adverse events were reported. Growth factors were permitted. Celldex |
|
|
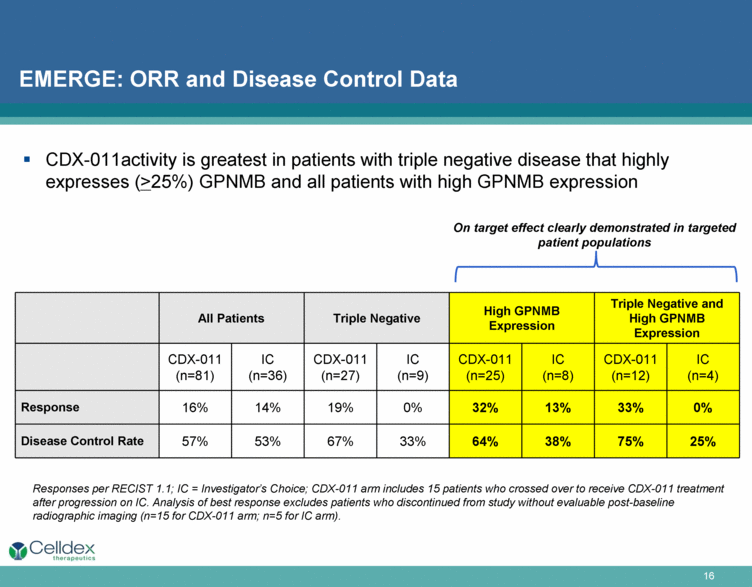
EMERGE: ORR and Disease Control Data Responses per RECIST 1.1; IC = Investigator’s Choice; CDX-011 arm includes 15 patients who crossed over to receive CDX-011 treatment after progression on IC. Analysis of best response excludes patients who discontinued from study without evaluable post-baseline radiographic imaging (n=15 for CDX-011 arm; n=5 for IC arm). All Patients Triple Negative High GPNMB Expression Triple Negative and High GPNMB Expression CDX-011 (n=81) IC (n=36) CDX-011 (n=27) IC (n=9) CDX-011 (n=25) IC (n=8) CDX-011 (n=12) IC (n=4) Response 16% 14% 19% 0% 32% 13% 33% 0% Disease Control Rate 57% 53% 67% 33% 64% 38% 75% 25% On target effect clearly demonstrated in targeted patient populations CDX-011activity is greatest in patients with triple negative disease that highly expresses (>25%) GPNMB and all patients with high GPNMB expression |
|
|
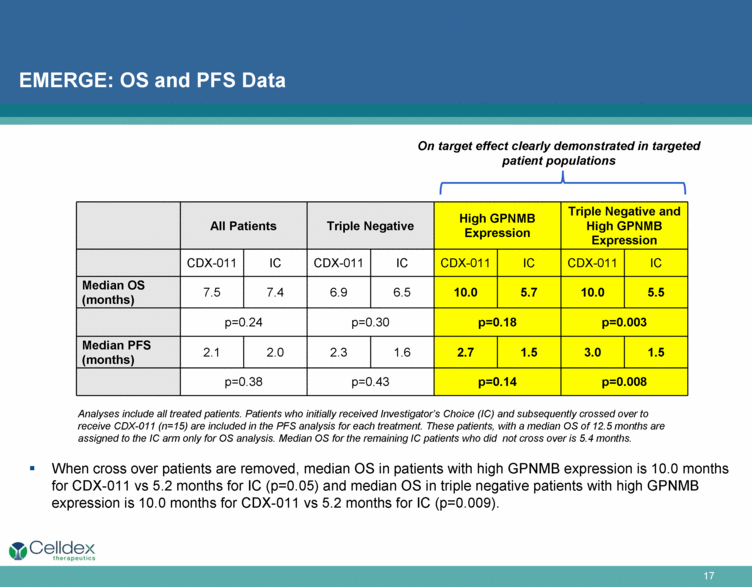
EMERGE: OS and PFS Data All Patients Triple Negative High GPNMB Expression Triple Negative and High GPNMB Expression CDX-011 IC CDX-011 IC CDX-011 IC CDX-011 IC Median OS (months) 7.5 7.4 6.9 6.5 10.0 5.7 10.0 5.5 p=0.24 p=0.30 p=0.18 p=0.003 Median PFS (months) 2.1 2.0 2.3 1.6 2.7 1.5 3.0 1.5 p=0.38 p=0.43 p=0.14 p=0.008 Analyses include all treated patients. Patients who initially received Investigator’s Choice (IC) and subsequently crossed over to receive CDX-011 (n=15) are included in the PFS analysis for each treatment. These patients, with a median OS of 12.5 months are assigned to the IC arm only for OS analysis. Median OS for the remaining IC patients who did not cross over is 5.4 months. On target effect clearly demonstrated in targeted patient populations When cross over patients are removed, median OS in patients with high GPNMB expression is 10.0 months for CDX-011 vs 5.2 months for IC (p=0.05) and median OS in triple negative patients with high GPNMB expression is 10.0 months for CDX-011 vs 5.2 months for IC (p=0.009). |
|
|
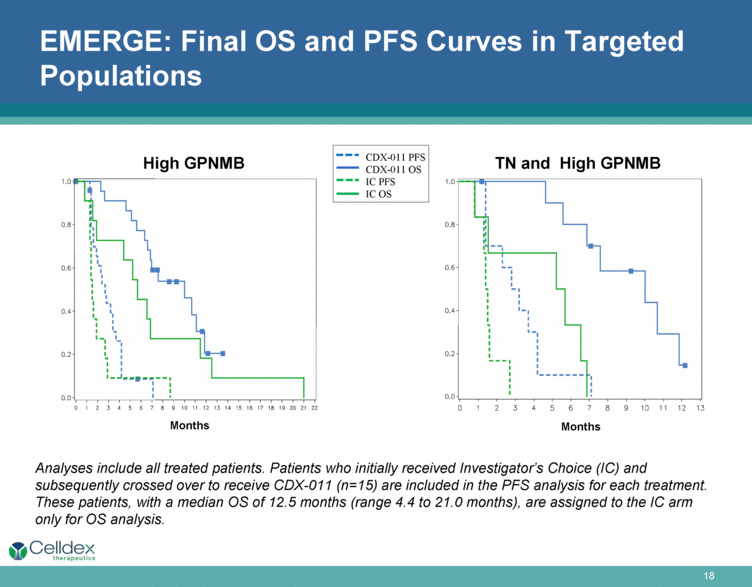
EMERGE: Final OS and PFS Curves in Targeted Populations Months Months TN and High GPNMB High GPNMB Analyses include all treated patients. Patients who initially received Investigator’s Choice (IC) and subsequently crossed over to receive CDX-011 (n=15) are included in the PFS analysis for each treatment. These patients, with a median OS of 12.5 months (range 4.4 to 21.0 months), are assigned to the IC arm only for OS analysis. CDX-011 PFS CDX-011 OS IC PFS IC OS |
|
|
CDX-011: Next Steps and Additional Therapeutic Indications Initiate late-stage study in metastatic breast cancer in 2H 2013 Positive end of Phase 2 meeting with the FDA in late December Finalize study design; provide update on YE 2012 call in early March Activity in melanoma Phase 2 open-label trial completed Promising PFS results in heavily pretreated patients and clinical responses with tumor shrinkage in 58% of patients (median duration of response of 5.3 months) Additional opportunities in squamous cell lung cancer, osteosarcoma and lymphoma/leukemia |
|
|
CDX-1135: A Potent Complement Inhibitor Complement inhibition in rare disease indications is a successful business model CDX-1135 is a clinically proven effective complement inhibitor with unique properties—blocks both alternative and classical complement activation pathways Clear path to clinical opportunity in Dense Deposit Disease (DDD) Potent activity in mouse model of DDD CDX-1135 inhibits C3 deposition in kidneys CDX-1135 normalizes serum C3 levels Inhibits complement activation in serum from all DDD patients tested to date CDX-1135 inhibition was not impacted by the presence of auto-antibodies (C3Nef) Data generated in the laboratory of Dr. Richard Smith at Iowa University, a leading expert in DDD |
|
|
CDX-1135: Next Steps Initiate open-label, multicenter pilot study in pediatric and adult patients with DDD in up to 5 patients in Q1 2013 Areas of investigation: Normalization of complement Improvement in kidney function Pathologic improvement in the kidneys With positive results, pursue study expansion suitable for approval |
|
|
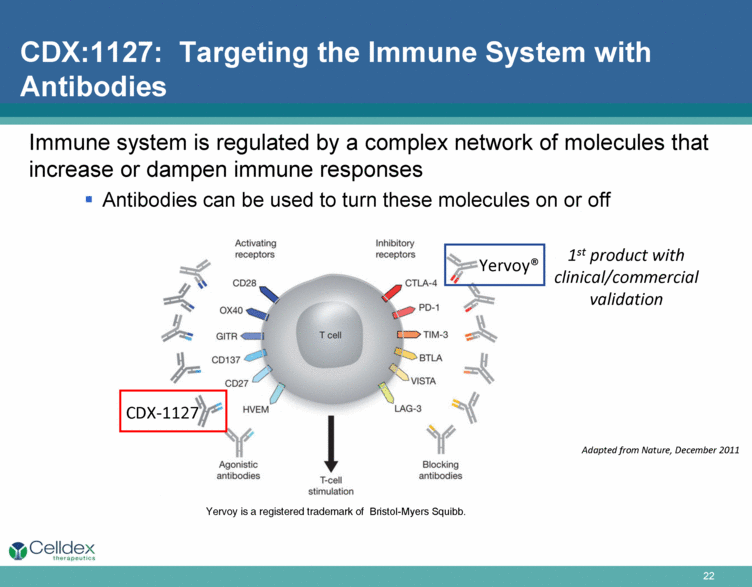
Immune system is regulated by a complex network of molecules that increase or dampen immune responses Antibodies can be used to turn these molecules on or off Adapted from Nature, December 2011 CDX:1127: Targeting the Immune System with Antibodies Yervoy is a registered trademark of Bristol-Myers Squibb. Yervoy® CDX-1127 1st product with clinical/commercial validation |
|
|
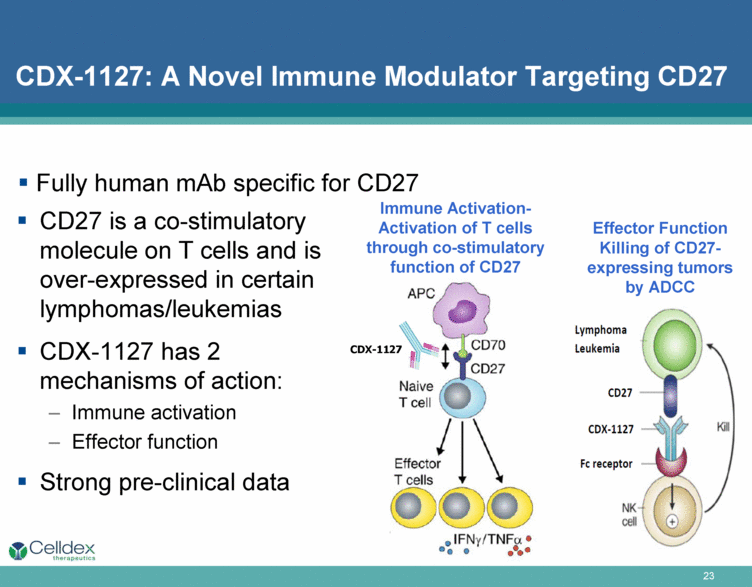
CDX-1127: A Novel Immune Modulator Targeting CD27 CD27 is a co-stimulatory molecule on T cells and is over-expressed in certain lymphomas/leukemias CDX-1127 has 2 mechanisms of action: Immune activation Effector function Strong pre-clinical data Immune Activation- Activation of T cells through co-stimulatory function of CD27 Fully human mAb specific for CD27 CDX-1127 Effector Function Killing of CD27-expressing tumors by ADCC |
|
|
CDX-1127: Phase 1 Study – 2 Arms Solid Tumors: Immune sensitive tumors Include melanoma, NSCLC, prostate, ovarian, RCC, colorectal Dose range 0.1mg/kg – 10 mg/kg 5 cohorts: single and multiple doses have completed accrual; well tolerated to date, including at the highest dose level Expansion cohort planned in 2013 Lymphoma/Leukemia (CD27+) Tumors express CD27 at high levels Dual mechanism of action (MoA): targeting tumor and immune system Dose range 0.1 mg/kg – 10 mg/kg 5 cohorts: single and multiple doses; expansion cohort planned in 2013 |
|
|
CDX-1127: Product Opportunity Monotherapy Melanoma, RCC (“naturally immunogenic” tumors) CD27+ cancers (various lymphoma and leukemia) Combination with chemotherapy Chemo or ADC that reduce tumor burden and provide source of tumor antigens Combination with vaccines/immunotherapy APC Targeting combinations Rindopepimut Other immune modulators - anti-CTLA-4, anti-PD-1 |
|
|
Anticipated Milestones for 2013 Rindopepimut Compete accrual of ACT IV registration study by YE 2013 Complete accrual of ReACT Phase 2 study— recurrent Q3 2013/refractory 1H 2013; data in 2H 2013 from both studies CDX-011 - Initiate late stage study in metastatic breast cancer in 2H 2013 CDX-1135 - Initiate pilot study in DDD in Q1 2013 CDX-1127 - Expansion cohorts planned; data in 2H 2013 CDX-301 - Initiate pilot study in transplant setting by YE 2013 CDX-1401 - Collaboration with NCI on Phase 2 combination study with CDX-301 |
|
|
For additional information, please visit: www.celldextherapeutics.com |