Attached files
Use these links to rapidly review the document
TABLE OF CONTENTS
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark one) | ||
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended December 31, 2009 |
||
or |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(D) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
Commission File Number 0-15006
CELLDEX THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 13-3191702 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
119 Fourth Avenue, Needham, Massachusetts 02494
(Address of principal executive offices) (Zip Code)
Registrant's telephone number, including area code: (781) 433-0771
Securities registered pursuant to Section 12(b) of the Act:
| Title of Class: | Name of Each Exchange on Which Registered: | |
|---|---|---|
| Common Stock, par value $.001 | NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this Chapter) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer ý | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller Reporting Company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes o No ý
The aggregate market value of the registrant's common stock held by non-affiliates as of June 30, 2009 was $100.8 million. Exclusion of shares held by any person should not be construed to indicate that such person possesses the power, direct or indirect, to direct or cause the actions of the management or policies of the registrant, or that such person is controlled by or under common control with the registrant.
The number of shares of common stock outstanding at February 25, 2010 was 31,711,124 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the definitive Proxy Statement for our 2010 Annual Meeting of Stockholders are incorporated by reference into Part III of this Report.
CELLDEX THERAPEUTICS, INC.
ANNUAL REPORT ON FORM 10-K
YEAR ENDED DECEMBER 31, 2009
i
Safe Harbor Statement under the Private Securities Litigation Reform Act of 1995: This report on Form 10-K contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 under Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements include statements with respect to our beliefs, plans, objectives, goals, expectations, anticipations, assumptions, estimates, intentions and future performance, and involve known and unknown risks, uncertainties and other factors, which may be beyond our control, and which may cause our actual results, performance or achievements to be materially different from future results, performance or achievements expressed or implied by such forward-looking statements. All statements other than statements of historical fact are statements that could be forward-looking statements. You can identify these forward-looking statements through our use of words such as "may," "will," "can," "anticipate," "assume," "should," "indicate," "would," "believe," "contemplate," "expect," "seek," "estimate," "continue," "plan," "point to," "project," "predict," "could," "intend," "target," "potential" and other similar words and expressions of the future.
There are a number of important factors that could cause the actual results to differ materially from those expressed in any forward-looking statement made by us. These factors include, but are not limited to:
- •
- our ability to successfully complete product research and further development, including animal, preclinical and clinical
studies, and commercialization of CDX-110, CDX-011, CDX-1307, CDX-1401, CDX-1135, and other products and the growth of the markets for those
product candidates;
- •
- our ability to raise sufficient capital on terms acceptable to us, or at all;
- •
- the cost, timing, scope and results of ongoing safety and efficacy trials of CDX-110, CDX-011,
CDX-1307, CDX-1401, CDX-1135, and other preclinical and clinical testing;
- •
- our ability to adapt our APC Targeting Technology™ to develop new, safe and effective vaccines against
oncology and infectious disease indications;
- •
- the ability to negotiate strategic partnerships or other disposition transactions for our non-core programs;
- •
- our ability to manage multiple clinical trials for a variety of product candidates at different stages of development;
- •
- the strategies and business plans of our partners, such as Pfizer's plans for CDX-110, GlaxoSmithKline's plans
with respect to Rotarix® and Vaccine Technologies' plans concerning the CholeraGarde® (Peru-15) and ETEC E. coli vaccines, which are not within our control, and our
ability to maintain strong, mutually beneficial relationships with those partners;
- •
- our ability to successfully integrate our and CuraGen's business without causing delays in the research and development
necessary to select drug development candidates and/or delays in clinical trials, and to operate the combined business efficiently;
- •
- our ability to develop technological capabilities and expand our focus to broader markets for vaccines;
- •
- the availability, cost, delivery and quality of clinical and commercial grade materials produced by our own manufacturing
facility or supplied by contract manufacturers and partners;
- •
- the timing, cost and uncertainty of obtaining regulatory approvals for product candidates;
- •
- our ability to develop and commercialize products before competitors that are superior to the alternatives developed by such competitors;
ii
- •
- the validity of our patents and our ability to avoid intellectual property litigation, which can be costly and divert
management time and attention; and
- •
- the factors listed under "Risk Factors" in this annual report on Form 10-K.
All forward-looking statements are expressly qualified in their entirety by this cautionary notice. You are cautioned not to place undue reliance on any forward-looking statements, which speak only as of the date of this report or the date of the document incorporated by reference into this report. We have no obligation, and expressly disclaim any obligation, to update, revise or correct any of the forward-looking statements, whether as a result of new information, future events or otherwise. We have expressed our expectations, beliefs and projections in good faith and we believe they have a reasonable basis. However, we cannot assure you that our expectations, beliefs or projections will result or be achieved or accomplished.
iii
General
As used herein, the terms "we," "us," "our," the "Company", or "Celldex" refer to Celldex Therapeutics, Inc. and its direct and indirect subsidiaries: Celldex Research Corporation ("Celldex Research") and Celldex Therapeutics, Ltd. ("Celldex Ltd."). Our principal activity since our inception has been research and product development conducted on our own behalf, as well as through joint development programs with several pharmaceutical companies and other collaborators.
We are an integrated biopharmaceutical company that applies our comprehensive Precision Targeted Immunotherapy Platform to generate a pipeline of candidates to treat cancer and other difficult-to-treat diseases. Our immunotherapy platform includes a complementary portfolio of monoclonal antibodies, antibody-targeted vaccines, antibody-drug conjugates and immunomodulators to create novel disease-specific drug candidates.
Our strategy is to develop and demonstrate proof-of-concept for our product candidates before leveraging their value through partnerships or, in appropriate situations, continuing late stage development through commercialization ourselves. Demonstrating proof-of-concept for a product candidate generally involves bringing it through Phase 1 clinical trials and one or more Phase 2 clinical trials so that we are able to demonstrate, based on human trials, good safety data for the product candidate and some data indicating its effectiveness. We thus leverage the value of our technology portfolio through corporate, governmental and non-governmental partnerships. This approach allows us to maximize the overall value of our technology and product portfolio while best ensuring the expeditious development of each individual product.
Our current collaborations include the commercialization of an oral human rotavirus vaccine and the development of oncology and infectious disease vaccines. Our product candidates address large market opportunities for which we believe current therapies are inadequate or non-existent.
AVANT Merger
On March 7, 2008, AVANT Immunotherapeutics, Inc. ("AVANT") merged with Celldex Research (formerly known as Celldex Therapeutics, Inc.), a privately-held company, (the "AVANT Merger"). Effective October 1, 2008, we changed our name from AVANT Immunotherapeutics, Inc. to Celldex Therapeutics, Inc.
The AVANT Merger was accounted for using the purchase method of accounting and was treated as an acquisition by Celldex Research of AVANT even though AVANT was the issuer of common stock and the surviving legal entity in the transaction. Because Celldex Research was determined to be the acquirer for accounting purposes, the historical financial statements of Celldex Research became our historical financial as of the closing of the AVANT Merger. Accordingly, our financial statements prior to the AVANT Merger reflect the financial position, results of operations and cash flows of Celldex Research, which during the historical periods presented in the accompanying consolidated financial statements, was then majority-owned by Medarex, Inc. ("Medarex"). Following the AVANT Merger, the financial statements reflect the financial position, results of operation and cash flows of the combined companies. The results of operations of AVANT are included in our results of operations beginning March 8, 2008.
Acquisition of CuraGen Corporation ("CuraGen")
On October 1, 2009, CuraGen, then a publicly-traded company, merged with a wholly-owned subsidiary of Celldex (the "CuraGen Merger"). In connection with the CuraGen Merger, effective
1
October 1, 2009, we (i) issued 15,722,713 shares of our common stock, or 0.2739 shares, in exchange for each share of outstanding CuraGen common stock, plus cash in lieu of fractional shares (the "CuraGen Exchange Ratio"), (ii) assumed all of the CuraGen stock options outstanding under the CuraGen 2007 Stock Plan (the "CuraGen 2007 Options"), and (iii) assumed the obligations of the $12.5 million in CuraGen 4% convertible subordinated debt due in February 2011 (the "CuraGen Debt"). The CuraGen 2007 Options are exercisable into 931,315 shares of our common stock after applying the CuraGen Exchange Ratio.
In connection with the consummation of the CuraGen Merger, effective October 1, 2009, Celldex, CuraGen, and The Bank of New York Mellon (formerly the Bank of New York) (the "Trustee") amended the CuraGen Debt to provide that the CuraGen Debt shall be convertible into 353,563 shares of Celldex common stock at the rate of 28.27823 shares of Celldex common stock per $1,000 principal amount of notes, or $35.36 per share.
Based on the closing price of our common stock on October 1, 2009 of $5.43, the fair value of the shares issued in the CuraGen Merger was $85.4 million. We have applied acquisition accounting as of October 1, 2009. Accordingly, the results of operations of CuraGen have been included in our results of operations beginning October 1, 2009.
On December 31, 2009, we completed the merger of our CuraGen subsidiary with and into Celldex pursuant to a short-form merger effected under Delaware law. As a result, the separate corporate existence of CuraGen has ceased and we have succeeded to all rights, privileges, powers and franchises of CuraGen.
We are a Delaware corporation organized in 1983. Our web site is located at http://www.celldextherapeutics.com. On our web site, investors can obtain a copy of our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and other reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act of 1934, as amended, as soon as reasonably practicable after we file such material electronically with, or furnishes it to, the Securities and Exchange Commission ("SEC"). None of the information posted on our website is incorporated by reference into this Annual Report.
Research and Development Activities
Our goal is to become a leading developer of innovative products that we call Precision Targeted Immunotherapeutics which are designed to address major unmet health care needs. Most of our products are derived from a set of complementary technologies (collectively known as our Precision Targeted Immunotherapy Platform). This platform includes monoclonal antibodies, antibody-targeted vaccines, antibody-drug conjugates and immunomodulators to create novel disease-specific drugs. We are using our Precision Targeted Immunotherapy Platform to develop targeted immunotherapies that prevent or treat specific forms of cancer, autoimmune disorders and disease caused by infectious organisms.
2
The following table includes the programs that we currently believe are material to our business:
Product (generic)
|
Indication/Field | Partner | Status | ||||
|---|---|---|---|---|---|---|---|
CLINICAL |
|||||||
CDX-110 |
Glioblastoma multiforme | Pfizer (PF-4948568) | Phase 2b | ||||
CDX-011 |
Metastatic melanoma and breast cancer | — | Phase 2 | ||||
CDX-1307 |
Colorectal, bladder, pancreas, ovarian and breast tumors | — | Phase 1 | ||||
CDX-1401 |
Multiple solid tumors | — | Phase 1/2 | ||||
CDX-1135 |
Renal disease | — | Phase 1/2 | ||||
PRECLINICAL |
|||||||
CDX-301 |
Cancer, autoimmune disease and transplant | — | Preclinical | ||||
CDX-1127 |
Immuno-modulation, multiple tumors | — | Preclinical | ||||
CDX-014 |
Renal and ovarian cancer | — | Preclinical | ||||
CDX-1189 |
Renal disease | — | Preclinical | ||||
MARKETED PRODUCTS |
|||||||
Rotarix® |
Rotavirus infection | GlaxoSmithKline | Marketed | ||||
Using our expertise in immunology, we are building business franchises in major disease areas: oncology, inflammatory and infectious diseases. Each of our business franchises addresses large market opportunities for which current therapies are inadequate or non-existent. We have pursued some of these opportunities independently in a highly focused manner. In other cases, we have leveraged the financial support and development capabilities of corporate and public sector partners to bring our development projects to fruition. The research we have pursued over the past several years has matured into what we believe is an exciting portfolio of product candidates.
Our success has depended and will continue to depend upon many factors, including our ability, and that of our licensees and collaborators, to successfully develop, obtain regulatory approval for and commercialize our product candidates. Commercial sales are currently only being generated from Rotarix®. We have had no commercial revenues from sales of our human therapeutic or other human vaccine products and we have had a history of operating losses. It is possible that we may not be able to successfully develop, obtain regulatory approval for or commercialize our product candidates, and we are subject to a number of risks that you should be aware of before investing in us. These risks are described more fully in "Item 1A. Risk Factors."
3
Development Strategy
Precision Targeted Immunotherapy Platform:
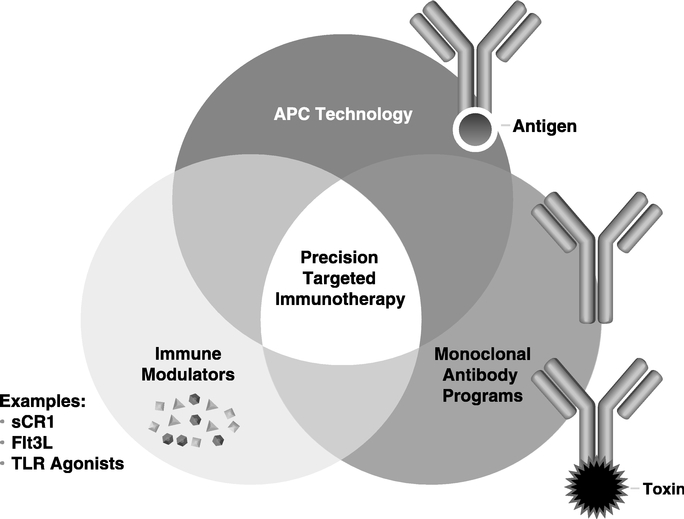
We believe there is tremendous untapped potential in immunotherapy that can be exploited through the right combination of therapeutic agents. Our industry has traditionally taken biologics that mediate effective cancer regression in mice and expected similar results in humans. There are many explanations why this strategy often does not succeed, but the most important is that immunotherapy has difficulties when following standard drug development. The mechanism of action is complex, activity is generally not dependent on highest tolerated dose, and patient response is highly variable. Our new understanding of the immune system, cancer's effect on immune mediated mechanisms, and the impact of conventional therapies on the immune system provides a new rationale for combining therapies that may lead to significant clinical responses. The concept of Precision Targeted Immunotherapy is to exploit this knowledge and the availability of good products that may not be sufficiently effective to be commercialized as a monotherapy, but which we believe may be very effective in combination approaches. Our goal is to develop products that maximize the efficacy of immunotherapy regimens through combinations of therapeutic agents. This includes:
Therapeutic Antibody Programs: These programs are based on the well validated approach to using antibodies that target to cancer and other diseases directly, or through interfering with critical interactions between the patient and the disease. Our antibody programs include antibody-drug conjugates (ADCs) that are designed to deliver potent cytotoxic molecules to cancer cells, and
4
traditional unmodified antibody approaches. Our current programs are based on fully human sequence antibodies to minimize patient reactivity against the drug. In addition, we have access through a Research and Commercialization Agreement with Medarex (now a subsidiary of Bristol-Myers Squibb) to the UltiMAb® Technology for generating fully human monoclonal antibodies. Under this agreement, we can exercise up to ten separate licenses to develop and commercialize therapeutic antibody products, either alone or through collaboration with our licensing partners.
Our APC Targeting Technology™: This is a new class of vaccines based on our proprietary antibody-targeted vaccine technology that is used to generate an immune response against cancer or other diseases. Our APC Targeting Technology™ uses human monoclonal antibodies linked to disease associated antigens to efficiently deliver the attached antigens to immune cells known as antigen presenting cells, or APCs. This technology has been designed to allow us to take advantage of many important characteristics of human monoclonal antibodies, including their long circulating half-life, well known safety profile, and standardized manufacturing procedures. We believe that our APC Targeting Technology™ provides significant manufacturing, regulatory and other practical advantages over patient specific and other immune-based treatments and can substantially reduce the dosage and cost currently required in conventional immunotherapies. Preclinical studies have demonstrated that APC Targeting Technology™ is more effective than conventional non-targeted vaccines. We have developed several proprietary monoclonal antibodies that can independently be developed to generate new product opportunities. Our CDX-1307 and CDX-1401 programs are in clinical development with the APC technology.
Immune System Modulators: Immune system modulators include drugs that activate or suppress specific parts of the immune system. Currently we are combining our APC technology product candidates with molecules known as Toll-Like Receptor (TLR) agonists that can activate patients' innate and adaptive immunity. We are also developing an immune cell growth factor called FMS-like tyrosine kinase 3 ligand (FLT3-L or CDX-301) designed to expand immune cells and stem cells. In addition, we are investigating the activity of a complement inhibitor (CDX-1135) that suppresses inflammatory reactions. These agents further support our Precision Targeted Immunotherapy Platform.
Our strategy is to utilize our expertise to design and develop targeted immunotherapeutics that have significant and growing market potential; to establish governmental and corporate alliances to fund development; and to commercialize our products either through corporate partners or, in appropriate circumstances, through our own direct selling efforts. Our goal is to demonstrate clinical proof-of-concept for each product, and then seek partners to help see those products which we cannot develop ourselves through to commercialization. This approach allows us to maximize the overall value of our technology and product portfolios while best ensuring the expeditious development of each individual product. Implementation of this strategy is exemplified by our lead programs which are discussed in the following sections.
Factors that may significantly harm our commercial success, and ultimately the market price of our common stock, include but are not limited to, announcements of technological innovations or new commercial products by our competitors, disclosure of unsuccessful results of clinical testing or regulatory proceedings and governmental approvals, adverse developments in patent or other proprietary rights, public concern about the safety of products developed by us and general economic and market conditions. See "Item 1A. Risk Factors."
Clinical Development Programs
CDX-110
Our lead clinical development program, CDX-110, is a peptide-based immunotherapy that targets the tumor specific molecule called EGFRvIII, a functional variant of the naturally expressed epidermal growth factor receptor ("EGFR"), a protein which has been well validated as a target for cancer
5
therapy. Unlike EGFR, EGFRvIII is not present in normal tissues, and has been shown to be a transforming oncogene that can directly contribute to the cancer cell growth. EGFRvIII is commonly present in glioblastoma multiforme, or GBM, the most common and aggressive form of brain cancer, and has also been observed in various other cancers such as breast, ovarian, prostate, colorectal, and head & neck cancer.
In April 2008, we and Pfizer Inc. ("Pfizer") entered into a License and Development Agreement (the "Pfizer Agreement") under which Pfizer was granted an exclusive worldwide license to CDX-110. The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Pfizer funds all development costs for these programs. We and Pfizer are currently pursuing the development of CDX-110 for GBM therapy and plan to expand the clinical development into other cancers through additional clinical studies. The Food and Drug Administration ("FDA") has granted orphan drug designation for CDX-110 for the treatment of EGFRvIII expressing GBM as well as fast track designation.
Initial clinical development of EGFRvIII immunotherapy was led by collaborating investigators at the Brain Center at Duke Comprehensive Cancer Center in Durham, North Carolina and at M.D. Anderson Cancer Center in Houston, Texas. The results from the Phase 1 (VICTORI) and Phase 2a (ACTIVATE) studies, which enrolled 16 and 21 patients, respectively, have demonstrated a significant increase in the time to disease progression (greater than 113%) in the patients who were vaccinated, and also in overall survival rates (greater than 100%), both relative to appropriately matched historical controls. An extension of the Phase 2a program (ACT II) at the same two institutions has enrolled 23 additional GBM patients treated in combination with temozolomide (the current standard of care). Preliminary results from this study (ACT II) currently estimates median overall survival to be 23.6 months, although the median has not yet been reached, while the survival of a matched historical control group was 15.0 months with a p value = 0.0237. Overall time to progression in the ACT II study was 15.2 months compared with 6.3 months for the historical control group.
We initiated a Phase 2b/3 randomized study (ACT III) of CDX-110 combined with standard of care, temozolomide, versus standard of care alone in patients with GBM in over 30 sites throughout the United States. In December 2008, we announced an amendment to convert the ACT III study to a single-arm Phase 2 clinical trial in which all patients will receive CDX-110 in combination with temozolomide. The decision, which followed the recommendation of the Independent Data Monitoring Committee, was based on the observation that the majority of patients randomized to the control (standard of care) arm withdrew from this open-label study after being randomized to the control arm. Patients participating on the control arm of the study were offered the option to receive treatment with CDX-110. Under this amendment, the ACT III study provided for a multi-center, non-randomized dataset for CDX-110 in patients with newly diagnosed GBM. These data will provide important additional information that can be used to better design the future development of CDX-110. Enrollment in ACT III is complete with a total of over 60 patients enrolled and we expect to present updated results during 2010.
CDX-011
CDX-011 (formerly CR011-vcMMAE) is an antibody-drug conjugate (ADC) that consists of a fully-human monoclonal antibody, CR011, linked to a potent cell-killing drug, monomethyl-auristatin E (MMAE). The CR011 antibody specifically targets glycoprotein NMB or (GPNMB) that is expressed in a variety of human cancers including breast cancer and melanoma. The ADC technology, comprised of MMAE and a stable linker system for attaching it to CR011, was licensed from Seattle Genetics, Inc. The ADC is designed to be stable in the bloodstream. Following intravenous administration, CDX-011 targets and binds to GPNMB, and upon internalization into the targeted cell, CDX-011 is designed to release MMAE from CR011 to produce a cell-killing effect. We acquired the rights to CDX-011 in connection with the CuraGen Merger.
6
Treatment of Breast Cancer: In June 2008, an open-label, multi-center Phase 1/2 study was initiated of CDX-011 administered intravenously once every three weeks to patients with locally advanced or metastatic breast cancer who have received prior therapy (median of seven prior regimens). The study began with a bridging phase to confirm the maximum tolerated dose ("MTD") and has expanded into a Phase 2 open-label, multi-center study.
The study confirmed the safety of CDX-011 at the pre-defined maximum dose level (1.88 mg/kg) in 6 patients. An additional 28 patients were enrolled as an expanded Phase 2 cohort (for a total of 34 treated patients at 1.88 mg/kg, the Phase 2 dose) to evaluate the progression-free survival ("PFS") rate at 12 weeks. As previously seen in melanoma patients, the 1.88 mg/kg dose was well tolerated in this patient population with the most common adverse events of rash, alopecia, and fatigue. The primary activity endpoint, which called for at least 5 of 25 (20%) patients in the Phase 2 study portion to be progression-free at twelve weeks, has been met. To date, 9 of 26 (35%) evaluable patients are without progression of disease at twelve weeks.
In addition, at the Phase 2 dose level, 4 of 32 (13%) evaluable patients achieved confirmed or unconfirmed Partial Responses ("PR") while 15 of 25 (60%) evaluable patients with measurable disease experienced some reduction in tumor size. GPNMB expression was identified in 10 of 14 (71%) of analyzed tumor samples and treatment with CDX-011 was associated with improved outcomes in all activity parameters in patients whose tumors expressed GPNMB. Notably, in patients who received the Phase 2 dose and whose tumors expressed GPNMB, 2 of 7 (29%) had confirmed PR, 5 of 7 (71%) had decreases in tumor size, and all 7 achieved at least stable disease with duration from 17.3 to 26.9 weeks. The median PFS in all patients was 9.1 weeks, but in patients whose tumors expressed GPNMB, median PFS was 18.3 weeks, compared to median PFS of 5.9 weeks for patients whose tumors did not express GPNMB. In patients with triple negative disease, 5 of 7 (71%) analyzed samples expressed GPNMB, 7 of 9 (78%) evaluable patients had tumor shrinkage, and the median PFS for these patients was 17.9 weeks.
We expect to initiate a randomized Phase 2b controlled study in patients with advanced breast cancer that express GPNMB in the second half of 2010.
Treatment of Metastatic Melanoma Cancer: In June 2006, a Phase 1/2 open-label, multi-center, dose escalation study was initiated to evaluate the safety, tolerability and pharmacokinetics of CDX-011 for patients with un-resectable Stage III or Stage IV melanoma who have failed no more than one prior line of cytotoxic therapy. During the Phase 1 portion of the study, doses of CDX-011 between 0.03 mg/kg to 2.63 mg/kg were evaluated and generally well tolerated, with rash and neutropenia emerging at higher doses. The MTD was determined to be 1.88 mg/kg administered intravenously (IV) once every three weeks.
In June 2009, CuraGen announced results for the 36 patients who were treated in the Phase 2 portion of the study. Of the patients enrolled, 94% had Stage IV disease of which two-thirds were classified as M1c, the poorest risk group. The study successfully met its primary activity endpoint, with 5 objective responses (1 unconfirmed) observed in 34 evaluable patients, and median duration of response of 5.3 months. The median overall PFS was 4.4 months. Tumor shrinkage was observed in 58% of patients, and 20 patients had best response of stable disease. Dermatologic adverse events consisting of rash, alopecia, and pruritus were the most common toxicities in this study. Other adverse events included fatigue, diarrhea, musculoskeletal pain, anorexia and nausea. Grade 3 or 4 neutropenia was observed in 5 patients. The absence of rash in the first cycle of treatment predicted a worse PFS. Additionally, in a subset of patients with tumor biopsies, high levels of tumor expression of GPNMB appeared to correlate with favorable outcome.
Enrollment has been completed in the Phase 1 portion of the melanoma trial to evaluate more frequent dosing schedules of CDX-011, including a weekly and a two out of every three-week regimen, to explore if more frequent administration can provide additional activity in patients with metastatic
7
melanoma. A dose of 1.0 mg/kg given once every week has been identified as the MTD in a weekly schedule, and a dose of 1.5mg/kg was being explored in the two out of three week schedule. Although median duration of follow-up was only 6 weeks, objective responses have thus far been observed in 3 of 11 evaluable patients treated with weekly CDX011 (1 confirmed) and 1 confirmed response in 8 evaluable patients treated with CDX-011 two out of every three weeks. We expect to present updated results during the first half of 2010.
CDX-1307
Our lead APC Targeting Technology™ product candidate, CDX-1307, is in development for the treatment of epithelial tumors such as colorectal, pancreatic, bladder, ovarian and breast cancers. CDX-1307 targets the beta chain of human chorionic gonadotropin, known as hCG-Beta, which is an antigen often found in epithelial tumors. The presence of hCG-Beta in these cancers correlates with a poor clinical outcome, suggesting that this molecule may contribute to tumor growth. Normal adult tissues have minimal expression of hCG-Beta; therefore, targeted immune responses are not expected to generate significant side effects.
Enrollment is complete in our two Phase 1 studies at multiple centers designed to explore safety and dose/effect relationships via two administration routes—intradermal (ID), a traditional vaccine route that allows efficient access to local dermal dendritic cells and intravenous (IV), a novel systemic approach to vaccination that might target a much larger population of dendritic cells. The Phase 1 studies investigated the safety and immunogenicity of CDX-1307 alone and in combination with adjuvants, including GM-CSF (known to increase mannose receptor expression on dendritic cells) and Toll-Like Receptor ("TLR") agonists (poly-ICLC or Hiltonol™ and R848 or resiquimod). Patients with an assortment of different tumor types that are known to express hCG-Beta were enrolled with retrospective analysis for hCG-Beta expression. An escalating four dose regimen was utilized with the possibility of retreatment if patients demonstrate tumor regression or stable disease.
The Phase 1 studies enrolled over 80 patients with heavily pretreated, advanced-stage breast, colon, bladder and pancreatic cancer, with an average of 4.6 prior therapies across the treatment population. All patient cohorts demonstrated a favorable safety profile with no dose limiting toxicity to date. The combination of CDX-1307 with TLR agonists significantly enhanced immune responses against hCG-Beta, providing humoral responses in 88% of patients and cellular immune responses in 57% of patients analyzed to date. Immune responses occurred even in the presence of high circulating levels of hCG-Beta, suggesting that the CDX-1307 can overcome antigen tolerance in advanced and heavily pretreated cancers. Nine patients in the studies experienced disease stabilization from 2.3 months to 11.4 months following the initiation of CDX-1307 vaccination. Two of these patients have received multiple courses of CDX-1307 and continue treatment with stable disease at 6.4 and 11.4 months. These data provide the basis for advancing CDX-1307 into a front-line patient population selected for hCG-Beta expressing cancers.
We expect to initiate a randomized Phase 2b controlled study in patients with newly diagnosed invasive bladder cancer in the second quarter of 2010. Patient's whose bladder cancer expresses hCG-Beta are predicted to have more aggressive disease and shorter survival. In this study we plan to select only patients with confirmed hCG-Beta expression using a specific diagnostic assay.
CDX-1401
CDX-1401 is a fusion protein consisting of a fully human monoclonal antibody with specificity for the dendritic cell receptor, DEC-205, linked to the NY-ESO-1 tumor antigen. In humans, NY-ESO-1 has been detected in 20 - 30% of cancers, thus representing a broad opportunity. This product is intended to selectively deliver the NY-ESO-1 antigen to APCs for generating robust immune responses against cancer cells expressing NY-ESO-1. Unlike CDX-1307, which targets the mannose receptor
8
expressing dendritic cells, CDX-1401 is the first APC product targeting DEC-205 expressing dendritic cells. We are developing CDX-1401 for the treatment of malignant melanoma and a variety of solid tumors which express the proprietary cancer antigen NY-ESO-1, which we licensed from the Ludwig Institute for Cancer Research in 2006. We believe that preclinical studies have shown that CDX-1401 is effective for activation of human T-cell responses against NY-ESO-1.
In September 2009, we initiated enrollment in a dose-escalating Phase 1/2 clinical trial aimed at determining the optimal dose for further development based on the safety, tolerability, and immunogenicity of the CDX-1401 vaccine. The trial will evaluate three different doses of the vaccine in combination with resiquimod, an activator of TLR 7 and 8. We expect to enroll approximately 36 patients with solid tumor cancers at multiple clinical sites in the United States.
CDX-1135
CDX-1135 is a molecule that inhibits a part of the immune system called the complement system. The complement system is a series of proteins that are important initiators of the body's acute inflammatory response against disease, infection and injury. Excessive complement activation also plays a role in some persistent inflammatory conditions. CDX-1135 is a soluble form of naturally occurring Complement Receptor 1 that inhibits the activation of the complement cascade in animal models and in human clinical trials. We believe that regulating the complement system could have therapeutic and prophylactic applications in several acute and chronic conditions, including organ transplantation, multiple sclerosis, rheumatoid arthritis, age-related macular degeneration ("AMD"), atypical Hemolytic Uremic Syndrome ("aHUS"), Paroxysmal Nocturnal Hemaglobinuria ("PNH"), Dense Deposit Disease ("DDD") in kidneys, and myasthenia gravis. We are currently defining the most appropriate clinical development path for CDX-1135 and are focusing on rare disease conditions of unregulated complement activation as the fastest route to FDA approval.
Preclinical Development Programs
CDX-301
CDX-301 is a FMS-like tyrosine kinase 3 ligand (Flt3L) that we licensed from Amgen in March 2009. CDX-301 is a growth factor for stem cells and immune cells called dendritic cells. Based on previous experience with this molecule, we believe that CDX-301 has considerable opportunity in various transplant settings as a stem cell mobilizing agent. In addition, CDX-301 is an immune modulating molecule that increases the numbers and activity of specific types of immune cells. We believe CDX-301 has significant opportunity for synergistic development in combination with proprietary molecules in our portfolio. We expect to file an Investigational New Drug ("IND") application for CDX-301 before the end of 2010.
CDX-1127
We have entered into a License Agreement with the University of Southampton, UK, to develop human antibodies to CD27, a potentially important target for immunotherapy of various cancers. In preclinical models, antibodies to CD27 alone have been shown to mediate anti-tumor effects, and may be particularly effective in combination with other immunotherapies. CD27 is a critical molecule in the activation pathway of lymphocytes. It is downstream from CD40, and may provide a novel way to regulate the immune responses. Engaging CD27 with the appropriate monoclonal antibody has proven highly effective at promoting anti-cancer immunity in mouse models. We are evaluating new human monoclonal antibodies in preclinical models.
9
CDX-014
CDX-014 (formerly CR014-vcMMAE) is a fully-human monoclonal ADC that targets TIM-1, an immunomudulatory protein that appears to down regulate immune response to tumors. The antibody, CDX-014, is linked to a potent chemotherapeutic, monomethyl auristatin E (MMAE), using Seattle Genetics' proprietary technology. The ADC is designed to be stable in the bloodstream, but to release MMAE upon internalization into TIM-1-expressing tumor cells, resulting in a targeted cell-killing effect. CDX-014 has shown potent activity in preclinical models of ovarian and renal cancer. We acquired the rights to CDX-014 in connection with the CuraGen Merger.
CDX-1189
We are developing therapeutic human antibodies to a signaling molecule known as CD89 or Fca receptor type I (FcaRI). CD89 is expressed by some white blood cells and leukemic cell lines, and has been shown to be important in controlling inflammation and tumor growth in animal models. We have proprietary, fully human antibodies to CD89 in preclinical development. Depending upon the specific antibody used, anti-CD89 antibodies can either be activating and thus stimulate immune responses, or down-regulating and act as an anti-inflammatory agent.
Partnerships
We have entered into collaborative partnership agreements with pharmaceutical and other companies and organizations that provide financial and other resources, including capabilities in research, development, manufacturing, and sales and marketing, to support our research and development programs. We depend on these relationships and may enter into more of them in the future. Some of our partners have substantial responsibility to commercialize a product and to make decisions about the amount and timing of resources that are devoted to developing and commercializing a product. As a result, we do not have complete control over how resources are used toward some of our products.
Some of our partnership agreements relate to products in the early stages of research and development. Others require us and our collaborators to jointly decide on the feasibility of developing a particular product using our technologies. In either case, these agreements may terminate without benefit to us if the underlying products are not fully developed. If we fail to meet our obligations under these agreements, they could terminate and we might need to enter into relationships with other collaborators and to spend additional time, money, and other valuable resources in the process.
We cannot predict whether our collaborators will continue their development efforts or, if they do, whether their efforts will achieve success. Many of our collaborators face the same kinds of risks and uncertainties in their business that we face. A delay or setback to a collaborator will, at a minimum, delay the commercialization of any affected products, and may ultimately prevent it. Moreover, any collaborator could breach its agreement with us or otherwise not use best efforts to promote our products. A collaborator may choose to pursue alternative technologies or products that compete with our technologies or products. In either case, if a collaborator failed to successfully develop one of our products, we would need to find another collaborator. Our ability to do so would depend upon our legal right to do so at the time and whether the product remained commercially viable.
GlaxoSmithKline plc ("Glaxo") and Paul Royalty Fund II, L.P. ("PRF")
Rotavirus is a major cause of diarrhea and vomiting in infants and children. In 1997, we licensed our oral rotavirus strain to Glaxo and Glaxo assumed responsibility for all subsequent clinical trials and all other development activities. Glaxo gained approval for its rotavirus vaccine, Rotarix®, in Mexico in July 2004, which represented the first in a series of worldwide approvals and commercial launches for the product leading up to the approval in Europe in 2006 and in the U.S. in 2008. We licensed-in our
10
rotavirus strain in 1995 and owe a license fee of 30% to Cincinnati Children's Hospital Medical Center ("CCH") on net royalties received from Glaxo. We are obligated to maintain a license with CCH with respect to the Glaxo agreement. The term of the Glaxo agreement is through the expiration of the last of the relevant patents covered by the agreement, although Glaxo may terminate the agreement upon 90 days prior written notice.
In May 2005, we entered into an agreement whereby an affiliate of PRF purchased an interest in the milestone payments and net royalties that we will receive on the development and worldwide sales of Rotarix®. We have received a total of $60 million in milestone payments under the PRF agreement. No additional milestone payments are due from PRF under the agreement.
Royalty rates on Rotarix® escalate from 7% to 10% based on net product sales in countries that have valid patent protection. These royalty rates are discounted by 30% for "non-patent" countries (primarily international markets). In September 2006, we received notice from Glaxo that Glaxo would begin paying royalties on sales of Rotarix® vaccine at the lower of the two royalty rates under their 1997 license agreement. Glaxo's decision to pay the lower royalty rate (which is 70% of the full rate) is based upon Glaxo's assertion that Rotarix® is not covered by the patents Glaxo licensed from us in Australia and certain European countries. We are currently evaluating the basis for Glaxo's action and our potential remedies. If Glaxo's position stands, the royalties to which PRF is entitled will no longer be limited by a $27.5 million annual threshold, which we projected may have been reached in later years as sales of Rotarix® increased. Irrespective of Glaxo's position, we will still retain approximately 65% of the royalties on worldwide sales of Rotarix® once PRF receives 2.45 times the aggregate cash payments of $60 million it made to us, though the potential amount of such residual royalties will be lower if Glaxo's position stands.
Pfizer Inc.
Pfizer License and Development Agreement: In April 2008, we and Pfizer entered into the Pfizer Agreement under which Pfizer was granted an exclusive worldwide license to a therapeutic cancer vaccine candidate, CDX-110, in Phase 2 development for the treatment of glioblastoma multiforme. The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Under the Pfizer Agreement, Pfizer made an upfront payment to us of $40 million and made a $10 million equity investment in us. Pfizer will fund all development costs for these programs. We are also eligible to receive potential milestone payments exceeding $390 million for the successful development and commercialization of CDX-110 and additional EGFRvIII vaccine products, as well as royalties on any product sales. The Pfizer Agreement became effective after clearance under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (as amended) on May 19, 2008. In connection with the Pfizer Agreement, we paid a total of $6.9 million in sublicense fees to Duke University and Thomas Jefferson University.
Pfizer Animal Health Agreement: We entered into a licensing agreement in December 2000 with Pfizer's Animal Health Division whereby Pfizer has licensed our technology for the development of animal health and food safety vaccines. Under the agreement, we may receive additional milestone payments of up to $3 million based upon attainment of specified milestones. We may receive royalty payments on eventual product sales. The term of this agreement is through the expiration of the last of the patents covered by the agreement. We have no obligation to incur any research and development costs in connection with this agreement.
Rockefeller University ("Rockefeller")
We are providing research and development support to Rockefeller on the development of their vaccine, DCVax-001, which we refer to as CDX-2401, aimed at providing protection from infection with HIV, the virus known to cause AIDS. Rockefeller's program is in a Bill & Melinda Gates Foundation
11
funded partnership called the Grand Challenges initiative. Preclinical studies and manufacturing development are in progress and our collaborators plan to file an IND for Phase 1 clinical studies in the first half of 2010. Rockefeller pays us on a time and materials basis.
Vaccine Technologies, Inc. ("VTI")
In January 2009, we entered into a license agreement with VTI under which we granted a worldwide exclusive license to VTI to develop and commercialize our CholeraGarde® and ETEC vaccine programs. We may receive milestones payments and royalties with respect to development and commercialization of the technology licensed to VTI.
TopoTarget A/S ("TopoTarget")
In connection with the CuraGen Merger, we assumed the rights under the April 2008 agreement ("TopoTarget Agreement") between CuraGen and TopoTarget whereby we could receive up to $6 million in either potential commercial milestone payments related to future net sales of Belinostat or 10% of any sublicense income received by TopoTarget ("TopoTarget Payments"). Under the TopoTarget Agreement, CuraGen sold back its Belinostat rights to TopoTarget and received $25 million in cash, 5 million shares of TopoTarget common stock (sold by CuraGen in 2008 for net proceeds of $12 million) and the right to receive the TopoTarget Payments. In addition, TopoTarget assumed all financial and operational responsibility for the clinical development of Belinostat under the TopoTarget Agreement. In February 2010, TopoTarget entered into a co-development and commercialization agreement for Belinostat with Spectrum Pharmaceuticals, Inc. resulting in our receipt of $3 million of the TopoTarget Payments.
Research Collaboration and Licensing Agreements
We have entered into licensing agreements with several universities and research organizations. Under the terms of these agreements, we have received licenses or options to license technology, specified patents or patent applications. Our licensing and development collaboration agreements generally provide for royalty payments equal to specified percentages of product sales, annual license maintenance fees and continuing patent prosecution costs. In addition, we have committed to make potential future milestone payments to third parties of up to approximately $116 million as part of our various collaborations including licensing and development programs. Payments under these agreements generally become due and payable only upon achievement of certain developmental, regulatory and/or commercial milestones.
Medarex, Inc., a subsidiary of Bristol-Myers Squibb ("Medarex")
We and Medarex, a former related party, have entered into the following agreements, each of which was approved by a majority of its independent directors who did not have an interest in the transaction. These agreements include:
- •
- An Assignment and License Agreement, as amended, ("Assignment and License Agreement") that provides for the assignment of
certain patent and other intellectual property rights and a license to certain Medarex technology; and
- •
- A Research and Commercialization Agreement, as amended, ("Research and Commercialization Agreement") that provides us with certain rights to obtain exclusive commercial licenses to proprietary monoclonal antibodies raised against certain antigens.
Under the terms of the Assignment and License Agreement and Research and Commercialization Agreement, we may be required to pay milestone and royalty payments to Medarex with respect to the development of any products containing such licensed antibodies.
12
In October 2007, we and Medarex entered into a settlement and mutual release agreement which settled disputed amounts we owed Medarex. We issued to Medarex 351,692 shares of our common stock equal in value to $3.0 million, based on the per share price of $8.64 set on the second trading day prior to the closing date of the AVANT Merger and exchanged releases. At December 31, 2008, we owed Medarex an additional $3.0 million related to a Master Services Agreement, which we paid Medarex in October 2009.
Rockefeller University ("Rockefeller")
In November 2005, we and Rockefeller entered into a license agreement for the exclusive worldwide rights to human DEC-205 receptor, with the right to sublicense the technology. The license grant is exclusive except that Rockefeller may use and permit other nonprofit organizations to use the human DEC-205 receptor patent rights for educational and research purposes. We may be required to pay milestone and royalty payments to Rockefeller with respect to development and commercialization of the human DEC-205 receptor. We may also be required to pay royalties on any product sales.
Duke University Brain Tumor Cancer Center ("Duke")
In September 2006, we and Duke entered into a license agreement that gave us access and reference to the clinical data generated by Duke and its collaborators in order for us to generate our own filing with the FDA relating to the CDX-110 product. We may be required to pay milestone and royalty payments to Duke with respect to development and commercialization of the CDX-110 product. In connection with the Pfizer Agreement, we determined that $2.4 million was payable to Duke as a sublicense fee. As provided for under the Duke license, we paid 50% of this amount to Duke in the form of 81,512 shares of our common stock in October 2008.
Ludwig Institute for Cancer Research ("Ludwig")
In October 2006, we and Ludwig entered into an agreement for the nonexclusive rights to six cancer tumor targets for use in combination with our APC Targeting Technology. The term of the agreement is for ten years. We may be required to pay milestone and royalty payments to Ludwig with respect to development and commercialization of the technology licensed from Ludwig.
Alteris Therapeutics, Inc. ("Alteris")
In October 2005, we completed the acquisition of the assets of Alteris, including the EGFRvIII molecule that we licensed to Pfizer under the Pfizer Agreement. We may be required to pay Alteris up to $5.0 million upon obtaining the first approval for commercial sale of a product containing EGFRvIII, including CDX-110.
Thomas Jefferson University ("TJU")
In February 2003, we entered into three exclusive license agreements with TJU. Under these licenses, we may be required to pay milestone and royalty payments to TJU with respect to development and commercialization of the technology licensed from TJU. In connection with the Pfizer Agreement, we amended our licenses with TJU to add additional sublicensing rights and paid $4.5 million in sublicense fees to TJU in 2008.
3M Company
In June 2008, we and 3M Company entered into a license agreement for the exclusive worldwide rights to access 3M Company's proprietary Immune Response Modifier, Resiquimod™, (and additional Toll-Like Receptor 7/8 agonists ("TLR")) for clinical study with our proprietary APC Targeting Technology, for use as vaccine adjuvants, with the right to sublicense the technology. We may be
13
required to pay milestone and royalty payments to 3M Company with respect to development and commercialization of the technology licensed from 3M Company.
University of Southampton, UK ("Southampton")
In November 2008, we entered into a license agreement with Southampton to develop human antibodies towards CD27, a potentially important target for immunotherapy of various cancers. CD27 is a critical molecule in the activation pathway of lymphocytes, is downstream from CD40, and may provide a novel way to regulate the immune responses. In preclinical models, antibodies to CD27 have been shown to mediate anti-tumor effects alone, and may be particularly effective in combination with our other immunotherapies. We may be required to pay milestone and royalty payments to Southampton with respect to development and commercialization of the technology licensed from Southampton.
Amgen Inc. ("Amgen")
In March 2009, we entered into a license agreement with Amgen to expand our Precision Targeted Immunotherapy Platform by acquiring exclusive rights to CDX-301 and CD40 ligand (CD40L). CDX-301 and CD40L are immune modulating molecules that increase the numbers and activity of immune cells that control immune responses. We may be required to pay milestone and royalty payments to Amgen with respect to development and commercialization of this technology licensed from Amgen.
Amgen Fremont (formerly Abgenix)
In connection with the CuraGen Merger, we assumed the license agreement between CuraGen and Amgen Fremont (successor in-interest to Abgenix) to develop fully-human monoclonal antibody therapeutics. In May 2009, an amendment to the license agreement ("Amgen Amendment") was entered into related to CuraGen's exclusive rights to develop and commercialize CDX-011 and 11 other licensed antigens. Under the Amgen Amendment, CuraGen and Amgen Fremont agreed to modify the terms of their existing cross-license of antigens whereby the amended license would be fully paid-up and royalty-free (except for any potentially required payments by CuraGen to the original licensor of CDX-011).
Seattle Genetics, Inc. ("Seattle Genetics")
In connection with the CuraGen Merger, we assumed the license agreement between CuraGen and Seattle Genetics whereby CuraGen acquired the rights to proprietary antibody-drug conjugate ("ADC") technology for use with its their proprietary antibodies for the potential treatment of cancer. We may be required to pay milestone and royalty payments to Seattle Genetics with respect to development and commercialization of the ADC technology.
Competition
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. Many of the products that we are attempting to develop and commercialize will be competing with existing therapies. In addition, a number of companies are pursuing the development of pharmaceuticals that target the same diseases and conditions that we are targeting. We face competition from pharmaceutical and biotechnology companies both in the United States and abroad. Our competitors may utilize discovery technologies and techniques or partner with collaborators in order to develop products more rapidly or successfully than us or our collaborators are able to do. Many of our competitors, particularly large pharmaceutical companies, have substantially greater financial, technical and human resources than we do. In addition, academic institutions,
14
government agencies and other public and private organizations conducting research may seek patent protection with respect to potentially competitive products or technologies and may establish exclusive collaborative or licensing relationships with our competitors.
We face intense competition in our development activities. We face competition from many companies in the United States and abroad, including a number of large pharmaceutical companies, firms specialized in the development and production of vaccines, adjuvants and vaccine and immunotherapeutic delivery systems and major universities and research institutions. These competitors include Alexion, Anadys, Antigenics, Baxter, BioSante, Crucell, Dendreon, Eli Lilly, Emergent, Genitope, GlaxoSmithKline, Idera, Intercell, Immunogen, Maxygen, Merck, NeoPharm, Northwest Biotherapeutics, Novavax, Pfizer, Roche, Sanofi-Aventis, Seattle Genetics, and Vical. We are aware that Dendreon is in late stage clinical trials for therapeutic vaccines for the treatment of prostate cancer which may compete with CDX-1307 and CDX-1401. In addition, companies such as Eli Lilly with its approved product Erbitux™ for the treatment of colorectal cancer, and Roche with its product Herceptin® for the treatment of metastatic breast cancer, have already commercialized antibody-based products that may compete with CDX-1307, CDX-1401 and CDX-110. Various other companies are developing or commercializing products in areas that we have targeted for product development. Some of these products use therapeutic approaches that may compete directly with our product candidates. Many of these companies and institutions, either alone or together with their partners, have substantially greater financial resources and larger research and development staffs than we do. These companies may succeed in obtaining approvals from the FDA and foreign regulatory authorities for their products sooner than we do for our products.
We are aware of a number of competitive products currently available in the marketplace or under development that are used for the prevention and treatment of the diseases that we have targeted for product development. Various companies are currently marketing or developing biopharmaceutical products that may compete with our product candidates that target colorectal cancer. Product candidates we may develop are also subject to competition in the treatment of colorectal cancer from a number of products already approved and on the market, including the following chemotherapy products: AstraZeneca PLC's Tomudex®, Hoffman-LaRoche's Xeloda® (capecitabine), Immunex Corporation's Leucovorin® calcium, ImClone Systems' Erbitux™, Pfizer, Inc.'s Camptosar® (irinotecan) and Aduracil® (5-FU), Sanofi-Synthelabo Group's Eloxatin™ (oxaliplatin), Genentech's anti-VEGF antibody, Avastin™, GlaxoSmithKline's Eniluracil™, and Titan Pharmaceuticals' CeaVac™, in the treatment of patients with advanced-stage colorectal cancer. In addition, we are aware that other companies such as Cell Genesys and Dendreon may be developing additional cancer vaccines that could potentially compete with other of our product candidates. We may also face competition from Medarex and Bristol-Myers Squibb, which are developing a therapeutic vaccine for the treatment of melanoma using Medarex's MDX-010 product candidate. We also face competition from a number of companies working in the fields of anti-angiogenesis and specific active immunotherapy for the treatment of solid tumor cancers. We expect that competition among specific active immunotherapy and anti-angiogenesis products approved for sale will be based on various factors, including product efficacy, safety, reliability, availability, price and patent position.
We are aware of specific companies that are developing antibody drug conjugates (ADCs) for use in the treatment of cancer. Trastuzumab-DM1 (T-DM1) is a first-in-class HER2 antibody drug conjugate comprised of Genentech's (a Member of the Roche Group) trastuzumab antibody linked to ImmunoGen's cell-killing agent, DM1. T-DM1 combines anti-HER2 activity and targeted intracellular delivery of the potent anti-microtubule agent, DM1 (a maytansine derivative). A Phase 3 clinical trial evaluating T-DM1 for second-line HER2-positive metastatic breast cancer is planned and may be competitive with our developmental program in the breast cancer indication. Other ADCs are in development by our collaborator of the MMAE technology, Seattle Genetics, using monomethylaurastatin derivatives as the cell-killing agent in hematologic cancers and other cancers.
15
Marketed products that are used in the treatment of melanoma include dacarbazine, temozolamide, and interleukin-2. In addition, several other pharmaceutical and biotechnology companies are engaged in research and development for the treatment of melanoma. Many more products are on the market or in development for the treatment of metastatic breast cancer. How CDX-011 will compete with these other commercial and development stage products in metastatic melanoma and breast cancer is not clear at this time.
We also face competition from pharmaceutical and biotechnology companies, academic institutions, government agencies and private research organizations in recruiting and retaining highly qualified scientific personnel and consultants and in the development and acquisition of technologies. Moreover, technology controlled by third parties that may be advantageous to our business may be acquired or licensed by our competitors, thereby preventing us from obtaining technology on commercially reasonable terms, if at all. We will also compete for the services of third parties that may have already developed or acquired internal biotechnology capabilities or made commercial arrangements with other biopharmaceutical companies to target the diseases on which we have focused both in the U.S. and outside of the U.S.
Our competitive position will also depend upon our ability to attract and retain qualified personnel, obtain patent protection or otherwise develop proprietary products or processes and secure sufficient capital resources for the often lengthy period between technological conception and commercial sales. We will require substantial capital resources to complete development of some or all of our products, obtain the necessary regulatory approvals and successfully manufacture and market our products. In order to secure capital resources, we anticipate having to sell additional capital stock, which would dilute existing stockholders. We may also attempt to obtain funds through research grants and agreements with commercial collaborators. However, these types of fundings are uncertain because they are at the discretion of the organizations and companies that control the funds. As a result, we may not receive any funds from grants or collaborations. Alternatively, we may borrow funds from commercial lenders, likely at high interest rates, which would increase the risk of any investment in us.
Manufacturing
We have no experience in large scale manufacturing and we have relied upon collaborators or contractors to manufacture some of our proposed products for both clinical and commercial purposes to date. We have established our own manufacturing facility in Fall River, Massachusetts, to produce antibodies, vaccines and other products that we may develop at scale for clinical trials. In order for us to establish a commercial manufacturing facility, we will require substantial additional funds and will be required to hire and retain significant additional personnel and comply with the extensive cGMP regulations of the FDA applicable to such facility. The commercial manufacturing facility would also need to be licensed for the production of antibodies, vaccines and other products by the FDA. We intend to establish manufacturing arrangements with manufacturers that comply with the FDA's requirements and other regulatory standards, although there can be no assurance that we will be able to do so.
While we believe that there is currently sufficient capacity worldwide for the production of our potential products by our collaborators or through contract manufacturers, establishing long-term relationships with contract manufacturers and securing multiple sources for the necessary quantities of clinical and commercial materials required can be a challenge. Qualifying the initial source of clinical and ultimately commercial material is a time consuming and expensive process due to the highly regulated nature of the pharmaceutical/biotech industry. These costs are hopefully mitigated in the economies of scale realized in commercial manufacture and product sale. The key difficulty in qualifying more than one source for each product is the duplicated time and expense in doing so without the potential to mitigate these costs if the secondary source is never utilized.
16
In connection with the Pfizer Agreement, the manufacture of CDX-110 is the responsibility of Pfizer. To date, we have utilized contract manufacturers for the manufacture of clinical trial supplies of CDX-011 and CDX-1135. Manufacture of the rotavirus vaccine is the responsibility of Glaxo, which has received from us a world-wide exclusive license to commercialize this vaccine. The two clinical lots of CDX-1307 used in our completed Phase 1 clinical trials of CDX-1307 were manufactured by contract manufacturers. In 2009, we completed the manufacture of additional quantities of CDX-1307 in our Fall River facility to meet planned Phase 2 clinical material requirements. We have also manufactured in our Fall River facility CDX-1401 clinical materials for our Phase 1/2 clinical trial currently enrolling and CDX-2401 clinical materials for a Rockefeller-sponsored Phase 1 clinical trial expected to begin in the first half of 2010.
The manufacturing processes for our other vaccine and immunotherapeutic delivery systems and vaccines utilize known technologies. We believe that the products we currently have under development can be scaled up to permit manufacture in commercial quantities. However, there can be no assurance that we will not encounter difficulties in scaling up the manufacturing processes.
Use of third party manufacturers limits our control over and ability to monitor the manufacturing process. As a result, we may not be able to detect a variety of problems that may arise and may face additional costs in the process of interfacing with and monitoring the progress of our contract manufacturers. If third party manufacturers fail to meet our manufacturing needs in an acceptable manner, we would face delays and additional costs while we develop internal manufacturing capabilities or find alternative third party manufacturers. It may not be possible to have multiple third party manufacturers ready to supply us with needed material at all or without incurring significant costs.
Marketing
Under the terms of existing and future partnership agreements, we rely and expect to continue to rely on the efforts of our collaborators, including Glaxo, Pfizer, VTI, and TopoTarget/Spectrum for the sale and marketing of our products. There can be no assurance that our collaborators will develop and market vaccine products incorporating our technologies, or, if marketed, that such efforts will be successful. The failure of our collaborators to successfully market products would harm our business.
We have retained, and in the future intend to retain, marketing rights to some of our product candidates, including vaccine and immunotherapeutic delivery systems and vaccine candidates, in selected geographic areas and for specified indications. We intend to seek marketing and distribution agreements and/or co-promotion agreements for the distribution of our products in these geographic areas and for these indications. We believe that these arrangements could enable us to generate greater financial return than might be obtained from early stage licensing and collaboration agreements. We have no marketing and sales staff and limited experience relating to marketing and distribution of commercial products, including vaccines. If we determine in the future to engage in direct marketing of our products, we will be required to recruit an experienced marketing group, develop a supporting distribution capability and incur significant additional expenditures. There can be no assurance that we will be able to establish a successful marketing force. We may choose or find it necessary to enter into strategic partnerships on uncertain, but potentially unfavorable, terms to sell, market and distribute our products. Any delay in the marketing or distribution of our products, whether it results from problems with internal capabilities or with a collaborative relationship, could harm the value of an investment in us.
17
Patents, Licenses and Proprietary Rights
In general, our intellectual property strategy is to protect our technology by filing patent applications and obtaining patent rights covering our own technology, both in the United States and in foreign countries that we consider important to our business. In addition, we have acquired and will seek to acquire as needed or desired, exclusive rights of others through assignment or license to complement our portfolio of patent rights. We also rely on trade secrets, unpatented know-how and technological expertise and innovation to develop and maintain our competitive position.
Patents
The successful development and marketing of products by us will depend in part on our ability to create and maintain intellectual property, including patent rights. We are the owner or exclusive licensee to proprietary patent positions in the areas of vaccine technologies, antibody technologies and complement inhibitor technology. Although we continue to pursue patent protection for our products, no assurance can be given that any pending application will issue as a patent, that any issued patent will have a scope that will be of commercial benefit, or that we will be able to successfully enforce our patent position against infringers. We routinely review our patent portfolio and adjusts its strategies for prosecution and maintenance of individual cases according to a number of factors including program priorities, stage of development, and patent term.
We own or license rights under more than 400 granted patents and national and regional patent applications around the world covering inventions relating to our business. The key patents owned by us or licensed to us that we consider important to our business include the following (the indicated and estimated patent expiry dates do not include any possible Patent Term Extensions or Supplementary Protection Certificates, if these may be secured in due course):
- •
- Patents for the technology used in CDX-110 have expiration dates through 2014 in the United States and from
2010 to 2015 in the United Kingdom, Germany and France. A pending patent application in Japan is currently under appeal. We also have rights under patent applications around the world relating to uses
of CDX-110 which are currently pending. If issued and maintained to full term in a form which covers commercial use of CDX-110, the latter filings could potentially provide
additional patent protection for the relevant use in the relevant territories to 2026.
- •
- Our patent portfolio for CDX-011 includes pending patent applications in the US, Europe and Japan. If issued
and maintained to full term in due course, these would have estimated patent expiry dates in 2025. In addition, patent rights relating to the toxin and conjugation technology used in
CDX-011 have been licensed from Seattle Genetics.
- •
- US patents and worldwide pending patent applications for the technology used in CDX-1307 have current or
estimated expiration dates (subject to issue in the case of pending applications) that range from 2021 to 2024.
- •
- We have a pending international patent application relating to the technology used in CDX-1401 which, if
issued in the main designated territories and maintained to full term in due course, would have estimated patent expiry dates in 2028.
- •
- Patents for the technology used in CDX-301 have current expiration dates that range from 2016 in the major
European territories to 2020 in the US.
- •
- We have licensed pending patent applications in the US, Europe and Japan relating to the technology used in CDX-1127. If issued and maintained to full term in due course, these would have estimated patent expiry dates in 2027. Further filings are also under preparation.
18
- •
- Our patent portfolio for CDX-014 includes pending patent applications in the US, Europe and Japan. If issued
and maintained to full term in due course, these would have estimated patent expiry dates in 2024.
- •
- Patents for the technology used in CDX-1135 have expiration dates that range from 2013 to 2016.
- •
- Our US patent and worldwide pending patent applications for the technology used in CDX-1189 have current or
estimated expiration dates (subject to issue in the case of pending applications) in 2022.
- •
- Patents for the technology used in the cholera and typhoid vaccines expire between 2013 and 2016. Our patent portfolio for
ETEC includes pending patent applications around the world which, if issued and maintained to full term in due course, would have estimated patent expiry dates in 2028.
- •
- Licensed patents for our rotavirus strain that we licensed to Glaxo have expiration dates in 2011 and 2012.
There can be no assurance that patent applications owned by or licensed to us will result in granted patents or that, if granted, the resultant patents will afford protection against competitors with similar technology. It is also possible that third parties may obtain patents or other proprietary rights that may be necessary or useful to us. In cases where third parties are first to invent a particular product or technology, it is possible that those parties will obtain patents that will be sufficiently broad to prevent us from using important technology or from further developing or commercializing important vaccine and immunotherapeutic systems and vaccine candidates. If licenses from third parties are necessary but cannot be obtained, commercialization of the covered products might be delayed or prevented. Even if these licenses can be obtained, they would probably require us to pay ongoing royalties and other costs, which could be substantial.
Although a patent has a statutory presumption of validity in the United States, the issuance of a patent is not conclusive as to validity or as to the enforceable scope of the patent claims. The validity or enforceability of a patent after its issuance by the Patent and Trademark Office can be challenged in litigation. As a business that uses a substantial amount of intellectual property, we face a heightened risk of intellectual property litigation. If the outcome of the litigation is adverse to the owner of the patent, third parties may then be able to use the invention covered by the patent without authorization or payment. There can be no assurance that our issued patents or any patents subsequently issued to or licensed by us will not be successfully challenged in the future. In addition, there can be no assurance that our patents will not be infringed or that the coverage of our patents will not be successfully avoided by competitors through design innovation.
We are aware that others, including universities and companies, have filed patent applications and have been granted patents in the United States and other countries which claim subject matter potentially useful or necessary to the commercialization of our products. The ultimate scope and validity of existing or future patents which have been or may be granted to third parties, and the availability and cost of acquiring rights in those patents necessary to the manufacture, use or sale of our products presently cannot be determined by us.
Third parties may have or may obtain valid and enforceable patents or proprietary rights that could block us from developing products using our technology, including:
- •
- certain patents and applications in the United States and Europe owned by Sanofi-Aventis, which relate to antibody-antigen conjugates and methods of their use for eliciting an immune response against the antigen;
19
- •
- certain patents and applications in the United States and foreign countries covering particular antigens and antigenic
fragments targeted by our current vaccine product candidates, including CDX-1307 and CDX-1401;
- •
- certain patents and pending applications related to particular receptors and other molecules on dendritic cells and
macrophages that may be useful for generating monoclonal antibodies and can be employed in our APC Targeting Technology;
- •
- two United States patents and related foreign patents and applications covering methods of diagnosing gliomas by detecting
the presence of the EGFRvIII (tumor specific splice variant) protein;
- •
- a United States patent relating to certain uses of GM-CSF;
- •
- a European patent relating to certain tumor antigen splice variants;
- •
- a United States patent owned by Genentech, Inc., relating to the production of recombinant antibodies in host
cells;
- •
- a United States patent owned by GlaxoSmithKline plc related to methods of culturing cells under certain conditions;
and
- •
- certain patents held by third parties relating to antibody expression in particular types of host cells.
The CholeraGarde® vaccine candidate and our VibrioVec® vaccine delivery system utilize mutated Vibrio cholerae strains. We are aware of an issued U.S. patent which claims a culture of mutated Vibrio cholerae. We believe that only one claim (the "Claim") of the patent may be pertinent to our CholeraGarde® and VibrioVec® products. The remaining claims of the patent cover other cultures, which we believe are not pertinent to the CholeraGarde® or VibrioVec® products. We have received an opinion of counsel from Fish & Richardson, P.C. that, based on the analysis set forth in their opinion and the facts known to them, the Claim is invalid. While a party challenging the validity of a patent has the burden of proving invalidity, the outcome of any litigation cannot be predicted with certainty. Accordingly, there can be no assurance that, if litigated, a court would conclude that the Claim is invalid.
In addition to the patents referred to in the previous paragraphs, there may be other patent applications and issued patents belonging to competitors that may require us to alter our vaccine candidates and vaccine and immunotherapeutic delivery systems, pay licensing fees or cease some of our activities. If our product candidates conflict with patents that have been or may be granted to competitors, universities or others, the patent owners could bring legal action against us claiming damages and seeking to enjoin manufacturing and marketing of the patented products. If any of these actions is successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to manufacture or market the affected products. There can be no assurance that we would prevail in any such action or that any license required under any such third party patent would be made available on acceptable terms or at all. We believe that there may be significant litigation in the biotechnology and vaccine industries regarding patent and other intellectual property rights. If we become involved in that litigation, we could consume substantial resources.
Licenses
We have entered into several significant license agreements relating to technology that is being developed by us and/or our collaborators. In general, these institutions have granted us an exclusive worldwide license (with right to sublicense) to make, use and sell products embodying the licensed technology, subject to the reservation by the licensor of a non-exclusive right to use the technologies for non-commercial research purposes. Generally, the term of each license is through the expiration of
20
the last of the patents issued with respect to the technologies covered by the license. We have generally agreed to use reasonable efforts to develop and commercialize licensed products and to achieve specified milestones and pay license fees, milestone payments and royalties based on the net sales of the licensed products or to pay a percentage of sublicense income. If we breach our obligations, the licensor has the right to terminate the license, and, in some cases, convert the license to a non-exclusive license. Generally, we control and are responsible for the cost of defending the patent rights of the technologies that we license.
Proprietary Rights
We also rely on unpatented technology, trade secrets and confidential information, and no assurance can be given that others will not independently develop substantially equivalent information and techniques or otherwise gain access to our know-how and information, or that we can meaningfully protect our rights in such unpatented technology, trade secrets and information. We require each of our employees, consultants and advisors to execute a confidentiality agreement at the commencement of an employment or consulting relationship with us. The agreements generally provide that all inventions conceived by the individual in the course of employment or in providing services to us and all confidential information developed by, or made known to, the individual during the term of the relationship shall be the exclusive property of us and shall be kept confidential and not disclosed to third parties except in limited specified circumstances. There can be no assurance, however, that these agreements will provide meaningful protection for our information in the event of unauthorized use or disclosure of such confidential information.
Government Regulation
Our activities and products are significantly regulated by a number of governmental entities, including the FDA in the United States and by comparable authorities in other countries. These entities regulate, among other things, the manufacture, testing, safety, effectiveness, labeling, documentation, advertising and sale of our products. We must obtain regulatory approval for a product in all of these areas before we can commercialize the product. Product development within this regulatory framework takes a number of years and involves the expenditure of substantial resources. Many products that initially appear promising ultimately do not reach the market because they are found to be unsafe or ineffective when tested. Our inability to commercialize a product would impair our ability to earn future revenues.
FDA Approval Process
In the United States, vaccines and immunotherapeutics for human use are subject to FDA approval as "biologics" under the Public Health Service Act and "drugs" under the Federal Food, Drug and Cosmetic Act. The steps required before a new product can be commercialized include: preclinical studies in animals, clinical trials in humans to determine safety and efficacy and FDA approval of the product for commercial sale.
Data obtained at any stage of testing is susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. Moreover, during the regulatory process, new or changed drug approval policies may cause unanticipated delays or rejection of our product. We may not obtain necessary regulatory approvals within a reasonable period of time, if at all, or avoid delays or other problems in testing our products. Moreover, even if we received regulatory approval for a product, the approval may require limitations on use, which could restrict the size of the potential market for the product.
The FDA provides that human clinical trials may begin thirty (30) days after receipt and review of an IND application, unless the FDA requests additional information or changes to the study protocol
21
within that period. An IND must be sponsored and filed by us for each of our proposed products. Authorization to conduct a clinical trial in no way assures that the FDA will ultimately approve the product. Clinical trials are usually conducted in three sequential phases. In a Phase 1 trial, the product is given to a small number of healthy volunteers to test for safety (adverse effects). Phase 2 trials are conducted on a limited group of the target patient population; safety, optimal dosage and efficacy are studied. A Phase 3 trial is performed in a large patient population over a wide geographic area to provide evidence for the safety of the product and to prove and confirm efficacy. The FDA has ongoing oversight over all these trials and can order a temporary or permanent discontinuation if warranted. Such an action could materially harm us. Clinical tests are critical to the success of our products but are subject to unforeseen and uncontrollable delay, including delay in enrollment of patients. Any delay in clinical trials could delay our commercialization of a product.
A product's safety and effectiveness in one test is not necessarily indicative of its safety and effectiveness in another test. Moreover, we may not discover all potential problems with a product even after completing testing on it. Some of our products and technologies have undergone only preclinical testing. As a result, we do not know whether they are safe or effective for humans. Also, regulatory authorities may decide, contrary to our findings, that a product is unsafe or not as effective in actual use as its test results indicated. This could prevent the product's widespread use, require its withdrawal from the market or expose us to liability.
The results of the clinical trials and all supporting data are submitted to the FDA for approval. A Biologics License Application ("BLA") is submitted for a biologic product; a New Drug Application ("NDA") for a drug product. The interval between IND filing and BLA/NDA filing is usually at least several years due to the length of the clinical trials, and the BLA/NDA review process can take over a year. During this time the FDA may request further testing or additional trials or may turn down the application. Even with approval, the FDA frequently requires post-marketing safety studies (known as Phase 4 trials) to be performed.
The FDA requires that the manufacturing facility that produces a licensed product meet specified standards, undergo an inspection and obtain an establishment license prior to commercial marketing. Subsequent discovery of previously unknown problems with a product or its manufacturing process may result in restrictions on the product or the manufacturer, including withdrawal of the product from the market. Failure to comply with the applicable regulatory requirements can result in fines, suspensions of regulatory approvals, product recalls, operating restrictions and criminal prosecution.
Expedited Review and Approval
The FDA has various programs, including fast track, priority review, and accelerated approval, that are intended to expedite or simplify the process for reviewing drugs, and/or provide for approval on the basis of surrogate endpoints. Generally, drugs that may be eligible for these programs are those for serious or life-threatening conditions, those with the potential to address unmet medical needs, and those that offer meaningful benefits over existing treatments, however, these programs do not affect the standards for approval. Fast track designation applies to the combination of the product and the specific indication for which it is being studied. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform post-marketing clinical trials.
Orphan Drug
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United
22
States for that drug. If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for seven years.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval. In addition, the FDA may require testing and surveillance programs to monitor the effect of approved products that have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval of a product by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for FDA approval. Approval by the FDA does not ensure approval by the regulatory bodies of other countries.
Under European Union regulatory systems, we may submit marketing authorization applications either under a centralized or decentralized procedure. The centralized procedure, which is compulsory for medicines produced by biotechnology and optional for those which are highly innovative, provides for the grant of a single marketing authorization that is valid for all European Union member states. The decentralized procedure provides for mutual recognition of national approval decisions. Under the decentralized procedure, the holder of a national marketing authorization may submit an application to the remaining member states. Within 90 days of receiving the applications and assessments report each member state must decide whether to recognize approval. If a member state does not recognize the marketing authorization, the disputed points are eventually referred to the European Commission, whose decision is binding on all member states. As in the United States, we may apply for designation of our products as orphan drug for the treatment of a specific indication in the European Union before the application for marketing authorization is made. Orphan drugs in Europe enjoy economic and marketing benefits, including a 10-year market exclusivity period for the approved indication, but not for the same drug, unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan-designated product.
Our collaborators are also subject to all of the above-described regulations in connection with the commercialization of products utilizing our technology.
Other Regulatory Processes
We are subject to a variety of financial disclosure and securities trading regulations as a public company in the U.S., including laws relating to the oversight activities of the SEC and the regulations of the NASDAQ Global Market, on which our shares are traded. We are also subject to regulation under other federal laws and regulation under state and local laws, including laws relating to occupational safety, laboratory practices, environmental regulations, and hazardous substance control.
23
Product Liability
The risk of product liability claims, product recalls and associated adverse publicity is inherent in the testing, manufacturing, marketing and sale of medical products. If and when we manufacture vaccines that are recommended for routine administration to children, we will be required to participate in the National Vaccine Injury Compensation Program. This program compensates children having adverse reactions to certain routine childhood immunizations with funds collected through an excise tax from the manufacturers of these vaccines.
Under our license agreements, we are required to maintain clinical trial liability insurance coverage up to $14 million. However, there can be no assurance that such insurance coverage is or will continue to be adequate or available. We may choose or find it necessary under our collaborative agreements to increase our insurance coverage in the future. We may not be able to secure greater or broader product liability insurance coverage on acceptable terms or at reasonable costs when needed. Any liability for mandatory damages could exceed the amount of our coverage. A successful product liability claim against us could require us to pay a substantial monetary award. Moreover, a product recall could generate substantial negative publicity about our products and business and inhibit or prevent commercialization of other product candidates.
Employees
As of December 31, 2009, we employed 90 full time persons and 3 part time or temporary persons, 14 of whom have doctoral degrees. Of these employees, 77 were engaged in or directly support research and development activities. We believe that our employee relations are good. We believe that our future success will depend in large part on our ability to attract and retain experienced and skilled employees.
You should consider carefully these risk factors together with all of the information included or incorporated by reference in this Annual Report in addition to our financial statements and the notes to our financial statements. This section includes forward-looking statements.
The following is a discussion of the risk factors that we believe are material to us at this time. These risks and uncertainties are not the only ones facing us and there may be additional matters that we are unaware of or that we currently consider immaterial. All of these could adversely affect our business, results of operations, financial condition and cash flows.
Risks Related to Our Business
Our products and product candidates are subject to extensive regulatory scrutiny.
All of our products and product candidates are at various stages of development and commercialization and our activities, products and product candidates are significantly regulated by a number of governmental entities, including the FDA in the United States and by comparable authorities in other countries. These entities regulate, among other things, the manufacture, testing, safety, effectiveness, labeling, documentation, advertising and sale of our products and product candidates. We or our partners must obtain regulatory approval for a product candidate in all of these areas before we can commercialize the product candidate. Product development within this regulatory framework takes a number of years and involves the expenditure of substantial resources. This process typically requires extensive preclinical and clinical testing, which may take longer or cost more than we anticipate, and may prove unsuccessful due to numerous factors. Many product candidates that initially appear promising ultimately do not reach the market because they are found to be unsafe or ineffective when tested. Companies in the pharmaceutical, biotechnology and vaccines industries have suffered
24
significant setbacks in advanced clinical trials, even after obtaining promising results in earlier trials. Our inability to commercialize a product or product candidate would impair our ability to earn future revenues.
If our products do not pass required tests for safety and effectiveness, we will not be able to derive commercial revenue from them.
In order to succeed, we will need to derive commercial revenue from the products we have under development. The FDA has not approved our CDX-110 or CDX-011 product candidates or any of our other lead products for sale to date. Products in our vaccine programs are in various stages of preclinical and clinical testing. Preclinical tests are performed at an early stage of a product's development and provide information about a product's safety and effectiveness on laboratory animals. Preclinical tests can last years. If a product passes its preclinical tests satisfactorily, and we determine that further development is warranted, we would file an IND application for the product with the FDA, and if the FDA gives its approval we would begin Phase 1 clinical tests. Phase 1 testing generally lasts between 6 and 24 months. If Phase 1 test results are satisfactory and the FDA gives its approval, we can begin Phase 2 clinical tests. Phase 2 testing generally lasts between 6 and 36 months. If Phase 2 test results are satisfactory and the FDA gives its approval, we can begin Phase 3 pivotal studies. Phase 3 studies generally last between 12 and 48 months. Once clinical testing is completed and a new drug application is filed with the FDA, it may take more than a year to receive FDA approval.
In all cases we must show that a pharmaceutical product is both safe and effective before the FDA, or drug approval agencies of other countries where we intend to sell the product, will approve it for sale. Our research and testing programs must comply with drug approval requirements both in the United States and in other countries, since we are developing our lead products with companies, including Glaxo and Pfizer, which intend to or could later decide to commercialize them both in the U.S. and abroad. A product may fail for safety or effectiveness at any stage of the testing process. A major risk we face is the possibility that none of our products under development will come through the testing process to final approval for sale, with the result that we cannot derive any commercial revenue from them after investing significant amounts of capital in multiple stages of preclinical and clinical testing.
Product testing is critical to the success of our products but subject to delay or cancellation if we have difficulty enrolling patients.
As our portfolio of potential products moves from preclinical testing to clinical testing, and then through progressively larger and more complex clinical trials, we will need to enroll an increasing number of patients with the appropriate characteristics. At times we have experienced difficulty enrolling patients and we may experience more difficulty as the scale of our clinical testing program increases. The factors that affect our ability to enroll patients are largely uncontrollable and include principally the following:
- •
- the nature of the clinical test;
- •
- the size of the patient population;
- •
- patients' willingness to receive a placebo or less effective treatment on the control arm of a clinical study;
- •
- the distance between patients and clinical test sites; and
- •
- the eligibility criteria for the trial.
If we cannot enroll patients as needed, our costs may increase or it could force us to delay or terminate testing for a product.
25
Any delay in obtaining regulatory approval would have an adverse impact on our ability to earn future revenues.
It is possible that none of the products or product candidates that we develop will obtain the regulatory approvals necessary for us to begin commercializing them. The time required to obtain FDA and other approvals is unpredictable but often can take years following the commencement of clinical trials, depending upon the nature of the product candidate. Any analysis we perform of data from clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. Any delay or failure in obtaining required approvals could have a material adverse effect on our ability to generate revenues from the particular product candidate. Furthermore, if we, or our partners, do not reach the market with our products before our competitors offer products for the same or similar uses, or if we, or our partners, are not effective in marketing our products, our revenues from product sales, if any, will be reduced.
We face intense competition in our development activities. We face competition from many companies in the United States and abroad, including a number of large pharmaceutical companies, firms specialized in the development and production of vaccines, adjuvants and vaccine and immunotherapeutic delivery systems and major universities and research institutions. These competitors include Alexion, Anadys, Antigenics, Baxter, BioSante, Crucell, Dendreon, Eli Lilly, Emergent, Genitope, GlaxoSmithKline, Idera, Intercell, Immunogen, Maxygen, Merck, NeoPharm, Northwest Biotherapeutics, Novavax, Pfizer, Roche, Sanofi-Aventis, Seattle Genetics, and Vical. Most of our competitors have substantially greater resources, more extensive experience in conducting preclinical studies and clinical testing and obtaining regulatory approvals for their products, greater operating experience, greater research and development and marketing capabilities and greater production capabilities than those of ours. These companies might succeed in obtaining regulatory approval for competitive products more rapidly than we can for our products, especially if we experience any delay in obtaining required regulatory approvals.
Failure to comply with applicable regulatory requirements would adversely impact our operations.
Even after receiving regulatory approval, our products would be subject to extensive regulatory requirements, and our failure to comply with applicable regulatory requirements will adversely impact our operations. In the United States, the FDA requires that the manufacturing facility that produces a product meet specified standards, undergo an inspection and obtain an establishment license prior to commercial marketing. Subsequent discovery of previously unknown problems with a product or its manufacturing process may result in restrictions on the product or the manufacturer, including withdrawal of the product from the market. Failure to comply with the applicable regulatory requirements can result in fines, suspensions of regulatory approvals, product recalls, operating restrictions and criminal prosecution.
We depend greatly on the intellectual capabilities and experience of our key executives and scientists and the loss of any of them could affect our ability to develop our products.
The loss of Anthony S. Marucci, our President and Chief Executive Officer, or other key members of our staff, including Avery W. Catlin, our Chief Financial Officer, Dr. Thomas Davis, our Chief Medical Officer, or Dr. Tibor Keler, our Chief Scientific Officer, could harm us. We entered into employment agreements with Messrs. Marucci, Catlin, Davis and Keler. We also depend on our scientific and clinical collaborators and advisors, all of whom have outside commitments that may limit their availability to us. In addition, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled scientific, managerial and marketing personnel, particularly as we expand our activities in clinical trials, the regulatory approval process and sales and manufacturing. We routinely enter into consulting agreements with our scientific and clinical collaborators and advisors, opinion leaders and heads of academic departments in the ordinary course
26
of our business. We also enter into contractual agreements with physicians and institutions who recruit patients into our clinical trials on our behalf in the ordinary course of our business. Notwithstanding these arrangements, we face significant competition for this type of personnel from other companies, research and academic institutions, government entities and other organizations. We cannot predict our success in hiring or retaining the personnel we require for continued growth.
We rely on contract manufacturers. Should the cost, delivery and quality of clinical and commercial grade materials supplied by contract manufacturers vary to our disadvantage, our business operations could suffer significant harm.
Although we have small-lot manufacturing capability at our Fall River facility, we rely on sourcing from third-party manufacturers for suitable quantities of some of our clinical and commercial grade materials essential to preclinical and clinical studies currently underway and to planned clinical trials in addition to those currently being conducted by third parties or us. The inability to have suitable quality and quantities of these essential materials produced in a timely manner would result in significant delays in the clinical development and commercialization of products, which could adversely affect our business, financial condition and results of operations. We also rely on collaborators and contract manufacturers to manufacture proposed products in both clinical and commercial quantities in the future. Our leading vaccine candidates require specialized manufacturing capabilities and processes.
We may face difficulty in securing commitments from U.S. and foreign contract manufacturers as these manufacturers could be unwilling or unable to accommodate our needs. Relying on foreign manufacturers involves peculiar and increased risks, including the risk relating to the difficulty foreign manufacturers may face in complying with the FDA's Good Manufacturing Practices ("GMP") as a result of language barriers, lack of familiarity with GMP or the FDA regulatory process or other causes, economic or political instability in or affecting the home countries of our foreign manufacturers, shipping delays, potential changes in foreign regulatory laws governing the sales of our product supplies, fluctuations in foreign currency exchange rates and the imposition or application of trade restrictions.
There can be no assurances that we will be able to enter into long-term arrangements with third party manufacturers on acceptable terms, or at all. Further, contract manufacturers must also be able to meet our timetable and requirements, and must operate in compliance with GMP; failure to do so could result in, among other things, the disruption of product supplies. As noted above, non-U.S. contract manufacturers may face special challenges in complying with the FDA's GMP requirements, and although we are not currently dependent on non-U.S. collaborators or contract manufacturers, we may choose or be required to rely on non-U.S. sources in the future as we seek to develop stable supplies of increasing quantities of materials for ongoing clinical trials of larger scale. Our dependence upon third parties for the manufacture of our products may adversely affect our profit margins and our ability to develop and deliver products on a timely and competitive basis.
The significant third-parties who we currently rely on for sourcing of suitable quantities of some of our clinical and commercial grade materials include:
- •
- Pfizer, Bayer, and Genzyme for the CDX-110 drug product;
- •
- Dalton for Hiltonol which is an integral part of several of our drug products;
- •
- 3M for Resiquimod which is an integral part of several of our drug products; and
- •
- Piramal for the CDX-011 drug product.
If we or our third-party manufacturers are unable to produce drug material in suitable quantities of appropriate quality, in a timely manner, and at a feasible cost, our clinical tests will face delays.
27
We rely on third parties to plan, conduct and monitor our clinical tests, and their failure to perform as required would interfere with our product development.
We rely on third parties to conduct a significant portion of our clinical development activities. These activities include clinical patient recruitment and observation, clinical trial monitoring, clinical data management and analysis, safety monitoring and project management. We conduct project management and medical and safety monitoring in-house for some of our programs and rely on third parties for the remainder of our clinical development activities. Our significant third-party clinical development providers include Pfizer for the development of our CDX-110 drug product.
If any of these third parties fails to perform as we expect or if their work fails to meet regulatory standards, our testing could be delayed, cancelled or rendered ineffective.
We depend greatly on third party collaborators to license, develop and commercialize some of our products, and they may not meet our expectations.
We have agreements with companies, including Glaxo, Pfizer and VTI for the licensing, development and ultimate commercialization of some of our products. Some of those agreements give substantial responsibility over the products to the collaborator. Some collaborators may be unable or unwilling to devote sufficient resources to develop our products as their agreements require. They often face business risks similar to ours, and this could interfere with their efforts. Also, collaborators may choose to devote their resources to products that compete with ours. If a collaborator does not successfully develop any one of our products, we will need to find another collaborator to do so. The success of our search for a new collaborator will depend on our legal right to do so at the time and whether the product remains commercially viable.
The success of our products depends in great part upon our and our collaborators' success in promoting them as superior to other treatment alternatives. We believe that our products can be proven to offer disease prevention and treatment with notable advantages over drugs in terms of patient compliance and effectiveness. However, there can be no assurance that we will be able to prove these advantages or that the advantages will be sufficient to support the successful commercialization of our products.
We may face delays, difficulties or unanticipated costs in establishing sales, distribution and manufacturing capabilities for our commercially ready products.
To date, we have chosen to retain, rather than license, all rights to some of our lead products, such as CDX-011 and our APC Targeting Technology programs. If we proceed with this strategy, we will have full responsibility for commercialization of these products if and when they are approved for sale. We currently lack the marketing, sales and distribution capabilities that we will need to carry out this strategy. To market any of our products directly, we must develop a substantial marketing and sales force with technical expertise and a supporting distribution capability. We have little expertise in this area, and we may not succeed. We may find it necessary to enter into strategic partnerships on uncertain but potentially unfavorable terms to sell, market and distribute our products when they are approved for sale.
Some of our products are difficult to manufacture, especially in large quantities, and we have not yet developed commercial scale manufacturing processes for any of our products. We do not currently plan to develop internal manufacturing capabilities to produce any of our products at commercial scale if they are approved for sale. To the extent that we choose to market and distribute these products ourselves, this strategy will make us dependent on other companies to produce our products in adequate quantities, in compliance with regulatory requirements, and at a competitive cost. We may not find third parties capable of meeting those manufacturing needs.
28
Certain factors could negatively affect the demand for and sales and profitability of Rotarix®, which would have a material adverse affect on our revenues.
Both the demand and ultimately the profitability of Rotarix® are components to our success. We have licensed a rotavirus strain to Glaxo for the purposes of Glaxo developing and commercializing their Rotarix® vaccine worldwide. Glaxo gained approval for Rotarix® in Mexico in July 2004, in the European Union in February 2006 and in the United States in April 2008. In May 2005, we entered into an agreement whereby an affiliate of PRF purchased an interest in the net royalties we will receive on worldwide sales of Rotarix®. In addition, we retain upside participation in the worldwide net royalties from Rotarix® once, and if, PRF receives an agreed upon return on capital invested (2.45 times PRF's aggregate cash payments to us of $60 million). The following are potential factors, among others, that may negatively affect the demand for Rotarix®:
- •
- Competitors in the pharmaceuticals, biotechnology and vaccines market have greater financial and management resources, and
significantly more experience in bringing products to market, and may develop, manufacture and market products that are more effective or less expensive than Rotarix®;
- •
- Rotarix® could be replaced by a novel product and may become obsolete;
- •
- Glaxo may be unable to prevent third parties from infringing upon their proprietary rights related to
Rotarix®;
- •
- Users may not accept such a recently approved product without years of proven history; and
- •
- We are dependent on Glaxo for the manufacturing, testing, acquisition of regulatory approvals, marketing, distribution and commercialization of Rotarix®.
Any of these factors could have a material adverse effect on the sales of Rotarix® and our results of operations.
Other factors could affect the demand for and sales and profitability of Rotarix® and any other of our current or future products.
In general, other factors that could affect the demand for and sales and profitability of our products include, but are not limited to:
- •
- The timing of regulatory approval, if any, of competitive products;
- •
- Our, Glaxo's, Pfizer's or any other of our partners' pricing decisions, as applicable, including a decision to increase or
decrease the price of a product, and the pricing decisions of our competitors;
- •
- Government and third-party payer reimbursement and coverage decisions that affect the utilization of our products and
competing products;
- •
- Negative safety or efficacy data from new clinical studies conducted either in the U.S. or internationally by any party
could cause the sales of our products to decrease or a product to be recalled;
- •
- The degree of patent protection afforded our products by patents granted to or licensed by us and by the outcome of
litigation involving our or any of our licensor's patents;
- •
- The outcome of litigation involving patents of other companies concerning our products or processes related to production
and formulation of those products or uses of those products;
- •
- The increasing use and development of alternate therapies;
- •
- The rate of market penetration by competing products; and
- •
- The termination of, or change in, existing arrangements with our partners.
29
Any of these factors could have a material adverse effect on Glaxo's sales of Rotarix® and on any other of our current or future products and results of operations.
We may be unable to manage multiple late stage clinical trials for a variety of product candidates simultaneously.
As our current clinical trials progress, we may need to manage multiple late stage clinical trials simultaneously in order to continue developing all of our current products. The management of late stage clinical trials is more complex and time consuming than early stage trials. Typically early stage trials involve several hundred patients in no more than 10-30 clinical sites. Late stage (Phase 3) trials may involve up to several thousand patients in up to several hundred clinical sites and may require facilities in several countries. Therefore, the project management required to supervise and control such an extensive program is substantially larger than early stage programs. As the need for these resources is not known until some months before the trials begin it is necessary to recruit large numbers of experienced and talented individuals very quickly. If the labor market does not allow this team to be recruited quickly the sponsor is faced with a decision to delay the program or to initiate it with inadequate management resources. This may result in recruitment of inappropriate patients, inadequate monitoring of clinical investigators and inappropriate handling of data or data analysis. Consequently it is possible that conclusions of efficacy or safety may not be acceptable to permit filing of a BLA or NDA for any one of the above reasons or a combination of several.
We face the risk of product liability claims, which could exceed our insurance coverage, and produce recalls, each of which could deplete our cash resources.
As a participant in the pharmaceutical, biotechnology and vaccines industries, we are exposed to the risk of product liability claims alleging that use of our products or product candidates caused an injury or harm. These claims can arise at any point in the development, testing, manufacture, marketing or sale of our products or product candidates and may be made directly by patients involved in clinical trials of our products, by consumers or healthcare providers or by individuals, organizations or companies selling our products. Product liability claims can be expensive to defend, even if the product or product candidate did not actually cause the alleged injury or harm.
Insurance covering product liability claims becomes increasingly expensive as a product candidate moves through the development pipeline to commercialization. Under our license agreements, we are required to maintain clinical trial liability insurance coverage up to $14 million. However, there can be no assurance that such insurance coverage is or will continue to be adequate or available to us at a cost acceptable to us or at all. We may choose or find it necessary under our collaborative agreements to increase our insurance coverage in the future. We may not be able to secure greater or broader product liability insurance coverage on acceptable terms or at reasonable costs when needed. Any liability for damages resulting from a product liability claim could exceed the amount of our coverage, require us to pay a substantial monetary award from our own cash resources and have a material adverse effect on our business, financial condition and results of operations. Moreover, a product recall, if required, could generate substantial negative publicity about our products and business and inhibit or prevent commercialization of other products and product candidates.
In addition, some of our licensing and other agreements with third parties require or might require us to maintain product liability insurance. If we cannot maintain acceptable amounts of coverage on commercially reasonable terms in accordance with the terms set forth in these agreements, the corresponding agreements would be subject to termination, which could have a material adverse impact on our operations.
30
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them.
Because we rely on third parties to develop our products, we must share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements will typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are typically controlled exclusively by us, although in some cases we may share these rights with other parties. We also conduct joint research and development programs which may require us to share trade secrets under the terms of research and development partnership or similar agreements. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication. A competitor's discovery of our trade secrets would impair our competitive position.
We may not be able to successfully integrate newly-acquired technology with our existing technology or to modify our technologies to create new vaccines.
As part of our acquisition of technology assets from entities such as 3M Company and Amgen, we have acquired access to Resiquimod™ (a TLR 7/8 agonist) and Flt3L, which may improve the immunogenicity of our vaccines. If we are able to integrate these licensed assets with our vaccine technologies, we believe these assets will give our vaccines a competitive advantage. However, if we are unable to successfully integrate licensed assets, or other technologies which we have acquired or may acquire in the future, with our existing technologies and potential products currently under development, we may be unable to realize any benefit from our acquisition of these assets, or other technologies which we have acquired or may acquire in the future and may face the loss of our investment of financial resources and time in the integration process.
We believe that our vaccine technology portfolio may offer opportunities to develop vaccines that treat a variety of oncology, inflammatory and infectious diseases by stimulating a patient's immune system against those disease organisms. If our vaccine technology portfolio cannot be used to create effective vaccines against a variety of disease organisms, we may lose all or portions of our investment in development efforts for new vaccine candidates.
We license technology from other companies to develop products, and those companies could influence research and development or restrict our use of it.
Companies that license technologies to us that we use in our research and development programs may require us to achieve milestones or devote minimum amounts of resources to develop products using those technologies. They may also require us to make significant royalty and milestone payments, including a percentage of any sublicensing income, as well as payments to reimburse them for patent costs. The number and variety of our research and development programs require us to establish priorities and to allocate available resources among competing programs. From time to time we may choose to slow down or cease our efforts on particular products. If in doing so we fail to fully perform our obligations under a license, the licensor can terminate the licenses or permit our competitors to use the technology. Moreover, we may lose our right to market and sell any products based on the licensed technology.
31
We have many competitors in our field and they may develop technologies that make ours obsolete.
Biotechnology, pharmaceuticals and therapeutics are rapidly evolving fields in which scientific and technological developments are expected to continue at a rapid pace. We have many competitors in the U.S. and abroad. These competitors include Alexion, Anadys, Antigenics, Baxter, BioSante, Crucell, Dendreon, Eli Lilly, Emergent, Genitope, GlaxoSmithKline, Idera, Intercell, Immunogen, Maxygen, Merck, NeoPharm, Northwest Biotherapeutics, Novavax, Pfizer, Roche, Sanofi-Aventis, Seattle Genetics, and Vical. Our success depends upon our ability to develop and maintain a competitive position in the product categories and technologies on which we focus. Many of our competitors have greater capabilities, experience and financial resources than we do. Competition is intense and is expected to increase as new products enter the market and new technologies become available. Our competitors may:
- •
- develop technologies and products that are more effective than ours, making ours obsolete or otherwise noncompetitive;
- •
- obtain regulatory approval for products more rapidly or effectively than us; and
- •
- obtain patent protection or other intellectual property rights that would block our ability to develop competitive products.
We rely on patents, patent applications and other intellectual property protections to protect our technology and trade secrets; which are expensive and may not provide sufficient protection.
Our success depends in part on our ability to obtain and maintain patent protection for technologies that we use. Biotechnology patents involve complex legal, scientific and factual questions and are highly uncertain. To date, there is no consistent policy regarding the breadth of claims allowed in biotechnology patents, particularly in regard to patents for technologies for human uses like those we use in our business. We cannot predict whether the patents we seek will issue. If they do issue, a competitor may challenge them and limit their scope. Moreover, our patents may not afford effective protection against competitors with similar technology. A successful challenge to any one of our patents could result in a third party's ability to use the technology covered by the patent. We also face the risk that others will infringe, avoid or circumvent our patents. Technology that we license from others is subject to similar risks and this could harm our ability to use that technology. If we, or a company that licenses technology to us, were not the first creator of an invention that we use, our use of the underlying product or technology will face restrictions, including elimination.
If we must defend against suits brought against us or prosecute suits against others involving intellectual property rights, we will incur substantial costs. In addition to any potential liability for significant monetary damages, a decision against us may require us to obtain licenses to patents or other intellectual property rights of others on potentially unfavorable terms. If those licenses from third parties are necessary but we cannot acquire them, we would attempt to design around the relevant technology, which would cause higher development costs and delays, and may ultimately prove impracticable.
Our business requires us to use hazardous materials, which increases our exposure to dangerous and costly accidents.
Our research and development activities involve the use of hazardous chemicals, biological materials and radioactive compounds. Although we believe that our safety procedures for handling and disposing of hazardous materials comply with the standards prescribed by applicable laws and regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. In the event of an accident, an injured party will likely sue us for any resulting damages with
32
potentially significant liability. The ongoing cost of complying with environmental laws and regulations is significant and may increase in the future.
Health care reform and restrictions on reimbursement may limit our returns on potential products.
Because our strategy ultimately depends on the commercial success of our products, we assume, among other things, that end users of our products will be able to pay for them. In the United States and other countries, in most cases, the volume of sales of products like those we are developing depends on the availability of reimbursement from third-party payors, including national health care agencies, private health insurance plans and health maintenance organizations. Third-party payors increasingly challenge the prices charged for medical products and services. Accordingly, if we succeed in bringing products to market, and reimbursement is not available or is insufficient, we could be prevented from successfully commercializing our potential products.
The health care industry in the United States and in Europe is undergoing fundamental changes as a result of political, economic and regulatory influences. Reforms proposed from time to time include mandated basic health care benefits, controls on health care spending, the establishment of governmental controls over the cost of therapies, creation of large medical services and products purchasing groups and fundamental changes to the health care delivery system. We anticipate ongoing review and assessment of health care delivery systems and methods of payment in the United States and other countries. We cannot predict whether any particular reform initiatives will result or, if adopted, what their impact on us will be. However, we expect that adoption of any reform proposed will impair our ability to market products at acceptable prices.
Changes in laws affecting the health care industry could adversely affect our business.
In the U.S., there have been numerous proposals considered at the federal and state levels for comprehensive reforms of health care and its cost, and it is likely that federal and state legislatures and health agencies will continue to focus on health care reform in the future. Congress has considered legislation to reform the U.S. health care system by expanding health insurance coverage, reducing health care costs and making other changes. While health care reform may increase the number of patients who have insurance coverage for our products, it may also include cost containment measures that adversely affect reimbursement for our products. Congress has also considered legislation to change the Medicare reimbursement system for outpatient drugs, increase the amount of rebates that manufacturers pay for coverage of their drugs by Medicaid programs and facilitate the importation of lower-cost prescription drugs that are marketed outside the U.S. Some states are also considering legislation that would control the prices of drugs, and state Medicaid programs are increasingly requesting manufacturers to pay supplemental rebates and requiring prior authorization by the state program for use of any drug for which supplemental rebates are not being paid. Managed care organizations continue to seek price discounts and, in some cases, to impose restrictions on the coverage of particular drugs. Government efforts to reduce Medicaid expenses may lead to increased use of managed care organizations by Medicaid programs. This may result in managed care organizations influencing prescription decisions for a larger segment of the population and a corresponding constraint on prices and reimbursement for our products.
We and our collaborators and partners operate in a highly regulated industry. As a result, governmental actions may adversely affect our business, operations or financial condition, including:
- •
- new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations or decisions, related to
health care availability, method of delivery and payment for health care products and services;
- •
- changes in the FDA and foreign regulatory approval processes that may delay or prevent the approval of new products and result in lost market opportunity;
33
- •
- changes in FDA and foreign regulations that may require additional safety monitoring, labeling changes, restrictions on
product distribution or use, or other measures after the introduction of our products to market, which could increase our costs of doing business, adversely affect the future permitted uses of
approved products, or otherwise adversely affect the market for our products;
- •
- new laws, regulations and judicial decisions affecting pricing or marketing practices; and
- •
- changes in the tax laws relating to our operations.
The enactment in the U.S. of health care reform, possible legislation which could ease the entry of competing follow-on biologics in the marketplace, new legislation or implementation of existing statutory provisions on importation of lower-cost competing drugs from other jurisdictions, and legislation on comparative effectiveness research are examples of previously enacted and possible future changes in laws that could adversely affect our business. In addition, the Food and Drug Administration Amendments Act of 2007 included new authorization for the FDA to require post-market safety monitoring, along with an expanded clinical trials registry and clinical trials results database, and expanded authority for the FDA to impose civil monetary penalties on companies that fail to meet certain commitments.
If physicians, patients and third-party payors do not accept any future drugs that we may develop, we may be unable to generate significant revenue, if any.
Even if our drug candidates as well as any drug candidates that we may develop or acquire in the future obtain regulatory approval, they may not gain market acceptance among physicians, patients and health care payors. Physicians may elect not to recommend these drugs for a variety of reasons including:
- •
- timing of market introduction of competitive drugs;
- •
- lower demonstrated clinical safety and efficacy compared to other drugs;
- •
- lack of cost-effectiveness;
- •
- lack of availability of reimbursement from third-party payors;
- •
- convenience and ease of administration;
- •
- prevalence and severity of adverse side effects;
- •
- other potential advantages of alternative treatment methods; and
- •
- ineffective marketing and distribution support.
If our approved drugs fail to achieve market acceptance, we would not be able to generate sufficient revenue from product sales to maintain or grow our business.
If we are not successful in integrating CuraGen's organization, we may not be able to operate efficiently after the CuraGen Merger, which may harm the value of our common stock.
Achieving the benefits of the CuraGen Merger will depend in part on the successful integration of CuraGen's clinical and preclinical programs and personnel in a timely and efficient manner. The integration process requires coordination of different development, regulatory, and manufacturing teams, and involves the integration of systems, applications, policies, procedures, business processes and operations. This may be difficult and unpredictable because of possible cultural conflicts and different opinions on scientific and regulatory matters. If we cannot successfully integrate CuraGen's programs and personnel, we may not realize the expected benefits of the CuraGen Merger.
34
Integrating CuraGen's programs may divert management's attention away from our operations.
The successful integration of CuraGen's programs and personnel may place a significant burden on our management and internal resources, including time that will be spent on winding down CuraGen's facility in Connecticut and transitioning certain CuraGen employees to our facilities. The diversion of management's attention and any difficulties encountered in the transition and integration process could result in delays in our clinical trial programs and could otherwise harm our business, financial condition and operating results.
Risks Related to Our Capital Stock
Our history of losses and uncertainty of future profitability make our common stock a highly speculative investment.
We have had no commercial revenues to date from sales of our human therapeutic or vaccine products and cannot predict when we will. We have an accumulated deficit of $157.7 million as of December 31, 2009. We expect to spend substantial funds to continue the research and development testing of our products that we have in the preclinical and clinical testing stages of development that have not been partnered.
In anticipation of FDA approval of these products, we will need to make substantial investments to establish sales, marketing, quality control, and regulatory compliance capabilities. These investments will increase if and when any of these products receive FDA approval. We cannot predict how quickly our lead products will progress through the regulatory approval process. As a result, we may continue to lose money for several years.
We cannot be certain that we will achieve or sustain profitability in the future. Failure to achieve profitability could diminish our ability to sustain operations, pay dividends on our common stock, obtain additional required funds and make required payments on our present or future indebtedness.
If we cannot sell capital stock to raise necessary funds, we may be forced to limit our research, development and testing programs.
We will need to raise more capital from investors to advance our clinical and preclinical products and to fund our operations until we receive final FDA approval and our products begin to generate revenues for us. However, based on our history of losses and the on-going uncertainty of the U.S. capital markets, we may have difficulty raising sufficient capital on terms that are acceptable to us, or at all. As of December 31, 2009, we had cash, cash equivalents and marketable securities of $82.5 million, which, at that time, we believed would support expected operations for more than 12 months.
We continue to seek partnerships with pharmaceutical and biotech companies and with other organizations to support the clinical development of our programs, in addition to funded research grants. This kind of funding is at the discretion of other organizations and companies which have limited funds and many companies compete with us for those funds. As a result, we may not receive any research grants or funds from collaborators. If we are unable to raise the necessary funds, we may have to delay or discontinue the clinical development of programs, license out programs earlier than expected, raise funds at significant discount or on other unfavorable terms, if at all, or evaluate a sale of all or part of our business.
Until we begin generating revenue, we may seek funding through the sale of equity, or securities convertible into equity, and further dilution to the then existing stockholders may result. If we raise additional capital through the incurrence of debt, our business may be affected by the amount of leverage it incurs, and its borrowings may subject it to restrictive covenants.
35
Our share price has been and could remain volatile.
The market price of our common stock has historically experienced and may continue to experience significant volatility. From January 2009 through December 2009, the market price of our common stock has fluctuated from a high of $14.19 per share in the second quarter of 2009, to a low of $4.16 per share in the fourth quarter of 2009. Our progress in developing and commercializing our products, the impact of government regulations on our products and industry, the potential sale of a large volume of our common stock by stockholders, our quarterly operating results, changes in general conditions in the economy or the financial markets and other developments affecting us or our competitors could cause the market price of our common stock to fluctuate substantially with significant market losses. If our stockholders sell a substantial number of shares of common stock, especially if those sales are made during a short period of time, those sales could adversely affect the market price of our common stock and could impair our ability to raise capital. In addition, in recent years, the stock market has experienced significant price and volume fluctuations. This volatility has affected the market prices of securities issued by many companies for reasons unrelated to their operating performance and may adversely affect the price of our common stock. In addition, we could be subject to a securities class action litigation as a result of volatility in the price of our stock, which could result in substantial costs and diversion of management's attention and resources and could harm our stock price, business, prospects, results of operations and financial condition.
Our ability to use our net operating loss carryforwards will be subject to limitation and, under certain circumstances, may be eliminated.
Utilization of the net operating loss ("NOL") and R&D credit carryforwards may be subject to substantial annual limitation due to ownership change limitations that have occurred previously or that could occur in the future provided by Section 382 of the Internal Revenue Code of 1986 ("Section 382"), as well as similar state provisions. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50 percentage points over a three-year period.
In October 2007, June 2009 and in December 2009, Celldex Research experienced a change in ownership as defined by Section 382 of the Internal Revenue Code. Celldex Research, since its formation, has raised capital through the issuance of capital stock on several occasions which, combined with shareholders' subsequent disposition of those shares, has resulted in three changes of control, as defined by Section 382. As a result of the ownership change in October 2007, utilization of its Federal NOLs is subject to an annual limitation. Any unused annual limitation may be carried over to later years, and the amount of the limitation may, under certain circumstances, be subject to adjustment if the fair value of the our net assets are determined to be below or in excess of the tax basis of such assets at the time of the ownership change, and such unrealized loss or gain is recognized during the five-year period after the ownership change. Subsequent ownership changes, as defined in Section 382, could further limit the amount of net operating loss carryforwards and research and development credits that can be utilized annually to offset future taxable income.
We have not undertaken a study to assess whether an ownership change or multiple ownership changes has occurred for (i) AVANT, (ii) CuraGen, (iii) Celldex Research on the state level, or (iv) R&D credits. If there has been an ownership change at any time since its formation, utilization of NOL or tax credit carryforwards would be subject to an annual limitation under Section 382.
Refer to Note 9, "Income Taxes," in the accompanying notes to the consolidated financial statements for additional discussion on income taxes.
Item 1B. UNRESOLVED STAFF COMMENTS
None.
36
We lease approximately 81,600 square feet of office, manufacturing and laboratory space. Our major leased properties are described below:
Property Location
|
Approximate Square Feet | Use | Lease Expiration Date | ||||
|---|---|---|---|---|---|---|---|
| Needham, Massachusetts | 35,200 | Office Headquarters and Laboratory | April 2017 | ||||
| Fall River, Massachusetts | 23,400 | Manufacturing Facility | December 2010(1) | ||||
| Phillipsburg, New Jersey | 20,000 | Office and Laboratory | August 2011 | ||||
| New Haven, Connecticut | 3,000 | Office | January 2013 | ||||
- (1)
- Lease includes two renewal options of five years each.
Following the announcement of the proposed acquisition by Celldex of CuraGen, a putative class action complaint, Margaret Capps v. Timothy Shannon, et al., was filed in the Connecticut Superior Court, Judicial District of New Haven, on June 9, 2009. A second putative class action complaint, Cheryl Smith v. CuraGen Corporation, et al., was filed in the Court of Chancery of the State of Delaware on June 15, 2009. Both lawsuits purported to have been brought on behalf of all public stockholders of CuraGen, and named CuraGen, all of its former directors, Celldex, and Celldex's merger subsidiary as defendants. On July 21, 2009, the attorneys for the parties in the two actions executed a memorandum of understanding (the "MOU") pursuant to which such actions were subsequently dismissed with prejudice. CuraGen agreed to make certain revisions to the joint proxy statement/prospectus (which was prepared in connection with the approval by CuraGen's stockholders of the merger and by Celldex's stockholders of the stock issued to the former stockholders of CuraGen in the merger) as part of the agreement among the parties to settle the actions and agreed to pay attorneys' fees and expenses as awarded by the court, which had been expected to be $0.3 million but ultimately were reduced to $0.2 million. On August 27, 2009, a stipulation of settlement was submitted to the court for the action captioned Cheryl Smith v. CuraGen Corporation, et al, pending in the Court of Chancery of the State of Delaware, and thereafter a fairness hearing was held on November 9, 2009, at which the court approved the settlement of the Delaware action and thereafter both the Delaware and Connecticut actions were dismissed.
CuraGen Corporation's former landlord filed a complaint in the Connecticut Superior Court, Judicial District of New Haven, on September 9, 2009, in case captioned T.K.J. Associates LLC v. CuraGen Corporation. The plaintiff alleges that CuraGen failed to pay rent and additional charges, and, upon vacating previously leased space, failed to restore the vacated space to the condition of initial occupancy, failed to pay utility charges, failed to remove personal property, and failed to comply with applicable environmental laws and regulations. In January 2010, CuraGen and its former landlord entered into a settlement and release agreement pursuant to which the complaint was dismissed with prejudice. In connection with the settlement, CuraGen paid disputed rent and utility charges totaling $0.1 million, restored previously vacated space to the condition of initial occupancy and made certain notices to comply with local environmental law and regulations.
37
Item 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our common stock currently trades on The Nasdaq Global Market (the "NASDAQ") under the symbol "CLDX". Effective October 1, 2008, we changed our name from AVANT Immunotherapeutics, Inc. to Celldex Therapeutics, Inc. Prior to October 1, 2008, our common stock traded on NASDAQ under the symbol "AVAN". The following table sets forth for the periods indicated the high and low sale prices per share for our common stock, as reported by NASDAQ. The numbers below reflect the 1-for-12 reverse stock split effected on March 7, 2008.
Fiscal Period
|
High | Low | ||||||
|---|---|---|---|---|---|---|---|---|
Year Ended December 31, 2009 |
||||||||
First Quarter |
$ | 11.75 | $ | 5.13 | ||||
Second Quarter |
14.19 | 6.28 | ||||||
Third Quarter |
8.10 | 4.80 | ||||||
Fourth Quarter |
5.75 | 4.16 | ||||||
Year Ended December 31, 2008 |
||||||||
First Quarter |
$ | 9.91 | $ | 5.64 | ||||
Second Quarter |
19.79 | 9.55 | ||||||
Third Quarter |
16.98 | 9.67 | ||||||
Fourth Quarter |
12.69 | 4.24 | ||||||
As of February 25, 2010, there were approximately 792 shareholders of record of our common stock. On February 25, 2010 the closing price of our common stock, as reported by NASDAQ, was $5.13 per share. We have not paid any dividends on our common stock since our inception and do not intend to pay any dividends in the foreseeable future.
38
CELLDEX THEAPEUTICS, INC., NASDAQ MARKET INDEX—U.S. AND
PEER GROUP INDICES
The graph below compares the cumulative total stockholder return on the common stock for the period from December 31, 2004 through December 31, 2009, with the cumulative return on (i) NASDAQ Market Index—U.S. Companies and (ii) NASDAQ Pharmaceutical Index. The comparison assumes investment of $100 on December 31, 2004 in our common stock and in each of the indices and, in each case, assumes reinvestment of all dividends. The points on the graph are as of December 31 of the year indicated.
| |
2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Celldex Therapeutics, Inc. |
$ | 100 | $ | 94 | $ | 67 | $ | 25 | $ | 33 | $ | 19 | |||||||
NASDAQ Stock Market (U.S.) Index |
$ | 100 | $ | 102 | $ | 112 | $ | 122 | $ | 59 | $ | 84 | |||||||
NASDAQ Pharmaceutical Stock Index |
$ | 100 | $ | 110 | $ | 108 | $ | 113 | $ | 105 | $ | 119 | |||||||
Item 6. SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated financial data are derived from our financial statements. The consolidated statement of operations data for the years ended December 31, 2009, 2008 and 2007 and the consolidated balance sheet data as of December 31, 2009 and 2008 have been derived from our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K. This data should be read in conjunction with our audited consolidated financial statements and related notes which are included elsewhere in this Annual Report on Form 10-K, and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included in Item 7 below.
On October 1, 2009, the CuraGen Merger became effective. The CuraGen Merger was accounted for using the acquisition method of accounting and was treated as our acquisition of CuraGen. Accordingly, the financial information presented below for periods prior to October 1, 2009 reflects the financial position and the results of operations of us alone, and for periods from October 1, 2009 forward the combined financial position and combined results of operations of us and CuraGen.
On March 7, 2008, the AVANT Merger became effective. The AVANT Merger was accounted for using the purchase method of accounting and was treated as our acquisition of AVANT. Accordingly, the financial information presented below for periods prior to March 8, 2008 reflects the financial position and the results of operations of us alone, and for periods from March 8, 2008 forward the
39
combined financial position and combined results of operations of us and AVANT. All amounts are in thousands except per share data.
CONSOLIDATED STATEMENTS OF OPERATIONS DATA
| |
2009 | 2008 | 2007 | 2006(2) | 2005 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
REVENUE: |
|||||||||||||||||
Product Development and Licensing Agreements |
$ | 5,662 | $ | 3,716 | $ | 466 | $ | 466 | $ | 14 | |||||||
Contracts and Grants |
1,802 | 533 | 940 | 433 | 57 | ||||||||||||
Product Royalties |
7,716 | 3,207 | — | — | — | ||||||||||||
Total Revenue |
15,180 | 7,456 | 1,406 | 899 | 71 | ||||||||||||
OPERATING EXPENSE: |
|||||||||||||||||
Research and Development |
26,169 | 22,636 | 9,892 | 10,013 | 4,826 | ||||||||||||
Royalty Expense |
8,397 | 3,711 | — | — | — | ||||||||||||
Charge for In-Process Research and Development(3) |
— | 14,756 | — | — | 8,447 | ||||||||||||
Other Operating Expense |
17,464 | 15,109 | 7,022 | 9,681 | 4,167 | ||||||||||||
Total Operating Expense |
52,030 | 56,212 | 16,914 | 19,694 | 17,440 | ||||||||||||
Operating Loss |
(36,850 | ) | (48,756 | ) | (15,508 | ) | (18,795 | ) | (17,369 | ) | |||||||
Investment and Other Income, Net |
248 | 1,411 | 435 | 960 | 290 | ||||||||||||
Interest Expense |
(452 | ) | (156 | ) | — | — | — | ||||||||||
Net Loss Before Income Taxes |
(37,054 | ) | (47,501 | ) | (15,073 | ) | (17,835 | ) | (17,079 | ) | |||||||
Income Tax Benefit |
529 | — | — | — | — | ||||||||||||
Net Loss |
$ | (36,525 | ) | $ | (47,501 | ) | $ | (15,073 | ) | $ | (17,835 | ) | $ | (17,079 | ) | ||
Basic and Diluted Net Loss Per Common Share |
$ | (1.84 | ) | $ | (3.34 | ) | $ | (1.81 | ) | $ | (2.15 | ) | $ | (3.00 | ) | ||
Shares Used in Calculating Basic and Diluted Net Loss Per Common Share(1) |
19,823 | 14,217 | 8,309 | 8,279 | 5,699 | ||||||||||||
- (1)
- Weighted
average common shares outstanding for the years 2005 to 2007 have been adjusted to reflect the AVANT Merger and a reverse stock split of
1-for-12 effective March 7, 2008.
- (2)
- In
2006, we changed the manner in which we account for stock-based compensation.
- (3)
- The 2008 amount arose as a result of the merger between AVANT and Celldex Research. The 2005 amount arose from the acquisition of Lorantis Limited.
CONSOLIDATED BALANCE SHEET DATA
| |
2009 | 2008 | 2007 | 2006 | 2005 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Working Capital |
$ | 69,569 | $ | 32,975 | $ | (4,438 | ) | $ | 12,178 | $ | 24,852 | |||||
Total Assets |
140,364 | 69,793 | 9,375 | 22,163 | 33,133 | |||||||||||
Long Term Liabilities |
52,190 | 37,558 | 370 | 914 | 1,152 | |||||||||||
Accumulated Deficit |
(157,674 | ) | (121,149 | ) | (73,648 | ) | (58,575 | ) | (40,739 | ) | ||||||
Total Stockholders' Equity (Deficit) |
73,767 | 18,134 | (1,132 | ) | 15,144 | 28,007 | ||||||||||
40
Item 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
OVERVIEW
We are an integrated biopharmaceutical company that applies our comprehensive Precision Targeted Immunotherapy Platform to generate a pipeline of candidates to treat cancer and other difficult-to-treat diseases. Our immunotherapy platform includes a complementary portfolio of monoclonal antibodies, antibody-targeted vaccines, antibody-drug conjugates and immunomodulators to create novel disease-specific drug candidates.
Our strategy is to develop and demonstrate proof-of-concept for our product candidates before leveraging their value through partnerships or, in appropriate situations, continuing late stage development through commercialization ourselves. Demonstrating proof-of-concept for a product candidate generally involves bringing it through Phase 1 clinical trials and one or more Phase 2 clinical trials so that we are able to demonstrate, based on human trials, good safety data for the product candidate and some data indicating its effectiveness. We thus leverage the value of our technology portfolio through corporate, governmental and non-governmental partnerships. This approach allows us to maximize the overall value of our technology and product portfolio while best ensuring the expeditious development of each individual product.
Our current collaborations include the commercialization of an oral human rotavirus vaccine and the development of oncology and infectious disease vaccines. Our product candidates address large market opportunities for which we believe current therapies are inadequate or non-existent.
Acquisition of CuraGen
In connection with the CuraGen Merger, effective October 1, 2009, we (i) issued 15,722,713 shares of our common stock, or 0.2739 shares, in exchange for each share of outstanding CuraGen common stock, plus cash in lieu of fractional shares (the "CuraGen Exchange Ratio"), (ii) assumed all of the CuraGen Stock Options and (iii) assumed the CuraGen Debt. We acquired CuraGen to gain access to a pipeline of oncology-focused antibodies, including CDX-011 (formerly CR011) currently in Phase 2 clinical development for the treatment of breast and melanoma cancer, and cash, cash equivalents and marketable securities of $70.3 million.
The transaction is being accounted for under the acquisition method of accounting. All of the assets acquired and liabilities assumed in the transaction are recognized at their acquisition-date fair values, while transaction costs associated with the transaction are expensed as incurred.
Purchase Price
The purchase price for CuraGen is based on the acquisition-date fair value of the consideration transferred, which was calculated based on the closing price of our common stock of $5.43 per share on October 1, 2009. The acquisition-date fair value of the consideration transferred consisted of the following (in thousands):
Fair value of common stock issued |
$ | 85,374 | ||
Fair value of CuraGen Stock Options |
2,868 | |||
Total consideration transferred |
$ | 88,242 | ||
U.S. GAAP requires that the fair value of replacement awards attributable to precombination service be included in the consideration transferred. Of the CuraGen Stock Options assumed, all but 1%, were immediately vested upon closing in accordance with the terms of the stock option agreements
41
and employment agreements. The fair value of the CuraGen Stock Options that has been attributed to precombination service is included in the consideration transferred.
Allocations of Assets and Liabilities
We have allocated the consideration transferred for CuraGen to net tangible assets, intangible assets, goodwill and a severance obligation. The difference between the aggregate consideration transferred and the fair value of assets acquired and liabilities assumed was allocated to goodwill. This goodwill relates to the potential synergies from the CuraGen Merger and a deferred tax liability related to acquired IPR&D intangible assets. None of the goodwill is expected to be deductible for income tax purposes. The following table summarizes the fair values of the assets acquired and liabilities assumed at the acquisition date (in thousands):
Cash and cash equivalents |
$ | 51,654 | |||
Marketable securities |
18,638 | ||||
Identifiable intangible assets: |
|||||
IPR&D |
11,800 | ||||
Amgen Amendment |
14,500 | ||||
TopoTarget Agreement |
2,400 | ||||
Other current and long-term assets |
756 | ||||
Goodwill |
8,965 | ||||
CuraGen Debt |
(11,503 | ) | |||
Deferred tax liabilities, net |
(5,190 | ) | |||
Other assumed liabilities |
(3,778 | ) | |||
Total |
$ | 88,242 | |||
The purchase price allocation has been prepared on a preliminary basis and is subject to change as additional information becomes available concerning the fair value and tax basis of the acquired assets and liabilities. Any adjustments to the purchase price allocation will be made as soon as practicable but no later than one year from the acquisition date.
The estimated fair value attributed to IPR&D intangible assets represents an estimate of the fair value of purchased in-process technology for CuraGen's research programs that, as of October 1, 2009, had not reached technological feasibility and have no alternative future use. Only those research programs that had advanced to a stage of development where we believed reasonable net future cash flow forecasts could be prepared and a reasonable likelihood of technical success existed were included in the estimated fair value. Accordingly, the IPR&D programs primarily represent the estimated fair value of CDX-011. The estimated fair value of the IPR&D programs was determined based on estimates of expected future net cash flows. These expected future net cash flows included estimates for revenue and associated costs for the IPR&D programs based on (i) relevant industry factors, (ii) current and expected trends in the product development life cycle, (iii) the ability to engage a strategic partner, (iv) the ability to obtain regulatory approval, and (v) the ability to manufacture and commercialize the products. The probability-adjusted future net cash flows which reflect the different stages of development of each program are then present valued utilizing an estimate of the appropriate discount rate which is consistent with the uncertainties of the cash flows utilized. Finally, the expected future net cash flows were calculated assuming the Amgen Amendment (defined below) was not entered into because the fair value attributable to the Amgen Amendment is separated from the fair value of the IPR&D programs.
The expected future net cash flows for CDX-011 were based on the expectation that a BLA for CDX-011 will be filed with the FDA by the end of 2015. We expect the commercial launch as promptly as commercially practicable after necessary regulatory approvals are received. Assuming a traditional
42
timeline for the regulatory review process, we expect CDX-011 will be commercially launched in 2016. These assumptions require various levels of in-house and external testing, clinical trials and approvals from the FDA or comparable foreign regulatory authorities before CDX-011 could be commercialized in the U.S. or other territories. Drug development involves a high degree of risk and most products that make it into clinical development do not receive marketing approval. Numerous risks and uncertainties can delay or stop clinical development of a pharmaceutical product prior to the receipt of marketing approval, including, but not limited to, results from clinical trials that do not support continuing development, issues related to manufacturing or intellectual property protection, and other events or circumstances that cause unanticipated delays, technical problems or other difficulties. Given these risks and uncertainties, there can be no assurance that the development of CDX-011 will be successfully completed. If the development of CDX-011 is not successful, in whole or in part, or completed in a timely manner, we may not realize the expected financial benefits from the development of CDX-011 or the transaction as a whole.
The estimated fair value attributed to the May 2009 amendment to the CuraGen and Amgen Fremont (successor in-interest to Abgenix) license agreement relates to CuraGen's exclusive rights to develop and commercialize CDX-011 and 11 other licensed antigens ("Amgen Amendment"). Under the Amgen Amendment, CuraGen and Amgen Fremont agreed to modify the terms of their existing cross-license of antigens whereby the amended license would be fully paid-up and royalty-free (except for any potentially required payments by CuraGen to the original licensor of CDX-011). The estimated fair value of the Amgen Amendment was based on the increase in expected future net cash flows for the IPR&D programs related to CDX-011 after the Amgen Amendment was entered into as compared to the expected future net cash flows if the Amgen Amendment was not entered into. The estimated fair value attributed to the Amgen Amendment is being amortized through the date of the last expiring patent covering CDX-011.
The estimated fair value attributed to TopoTarget Agreement between CuraGen and TopoTarget relates to CuraGen's rights under the TopoTarget Agreement to receive up to $6 million in either potential commercial milestone payments related to future net sales of Belinostat or 10% of any sublicense income received by TopoTarget ("TopoTarget Payments"). The estimated fair value of the TopoTarget Agreement was based on estimates of the probability-adjusted expected future net cash flows of the TopoTarget Payments. The estimated fair value attributed to the TopoTarget Agrement is being amortized through the date of the estimated receipt of the last payment under the TopoTarget Payments. In February 2010, TopoTarget entered into a co-development and commercialization agreement for Belinostat with Spectrum Pharmaceuticals, Inc. resulting in our receipt of $3 million of the TopoTarget Payments.
The deferred tax liability, net of $5.2 million primarily relates to the temporary differences associated with the IPR&D intangible assets, which are not deductible for tax purposes.
CURRENT PROGRAMS AND PARTNERSHIPS
Our products are derived from a broad set of complementary technologies which have the ability to utilize the human immune system and enable the creation of preventative and therapeutic agents. We are using these technologies to develop vaccines and targeted immunotherapeutics that prevent or treat cancer and disease caused by infectious organisms, and treatment vaccines that modify undesirable activity by the body's own proteins or cells. A number of our immunotherapeutic and vaccine product candidates are in various stages of clinical trials. We expect that a large percentage of our research and development expenses will be incurred in support of our current and future clinical trial programs.
43
The following table includes the programs that we currently believe are material to our business:
Product (generic)
|
Indication/Field | Partner | Status | ||||
|---|---|---|---|---|---|---|---|
CLINICAL |
|||||||
CDX-110 |
Glioblastoma multiforme | Pfizer (PF-4948568) | Phase 2b | ||||
CDX-011 |
Metastatic melanoma and breast cancer | — | Phase 2 | ||||
CDX-1307 |
Colorectal, bladder, pancreas, ovarian and breast tumors | — | Phase 1 | ||||
CDX-1401 |
Multiple solid tumors | — | Phase 1/2 | ||||
CDX-1135 |
Renal disease | — | Phase 1/2 | ||||
PRECLINICAL |
|||||||
CDX-301 |
Cancer, autoimmune disease and transplant | — | Preclinical | ||||
CDX-1127 |
Immuno-modulation, multiple tumors | — | Preclinical | ||||
CDX-014 |
Renal and ovarian cancer | — | Preclinical | ||||
CDX-1189 |
Renal disease | — | Preclinical | ||||
MARKETED PRODUCTS |
|||||||
Rotarix® |
Rotavirus infection | GlaxoSmithKline | Marketed | ||||
The expenditures that will be necessary to execute our business plan are subject to numerous uncertainties. Completion of clinical trials may take several years or more, and the length of time generally varies substantially according to the type, complexity, novelty and intended use of a product candidate. It is not unusual for the clinical development of these types of product candidates to each take five years or more, and for total development costs to exceed $100 million for each product candidate. Our estimates that clinical trials of the type we generally conduct are typically completed over the following timelines:
Clinical Phase
|
Estimated Completion Period |
|
|---|---|---|
Phase 1 |
1 - 2 Years | |
Phase 2 |
1 - 5 Years | |
Phase 3 |
1 - 5 Years |
The duration and the cost of clinical trials may vary significantly over the life of a project as a result of differences arising during the clinical trial protocol, including, among others, the following:
- •
- the number of patients that ultimately participate in the trial;
- •
- the duration of patient follow-up that seems appropriate in view of results;
- •
- the number of clinical sites included in the trials;
- •
- the length of time required to enroll suitable patient subjects; and
- •
- the efficacy and safety profile of the product candidate.
We test potential product candidates in numerous preclinical studies for safety, toxicology and immunogenicity. We may then conduct multiple clinical trials for each product candidate. As we obtain results from trials, we may elect to discontinue or delay clinical trials for certain product candidates in order to focus our resources on more promising product candidates.
44
An element of our business strategy is to pursue the research and development of a broad portfolio of product candidates. This is intended to allow us to diversify the risks associated with our research and development expenditures. As a result, we believe our future capital requirements and our future financial success are not substantially dependent on any one product candidate. To the extent we are unable to maintain a broad range of product candidates, our dependence on the success of one or a few product candidates increases.
Regulatory approval is required before we can market our product candidates as therapeutic or vaccine products. In order to proceed to subsequent clinical trial stages and to ultimately achieve regulatory approval, the regulatory agency must conclude that our clinical data is safe and effective. Historically, the results from preclinical testing and early clinical trials (through Phase 2) have often not been predictive of results obtained in later clinical trials. A number of new drugs, biologics and vaccines have shown promising results in early clinical trials, but subsequently failed to establish sufficient safety and efficacy data to obtain necessary regulatory approvals.
Furthermore, our business strategy includes the option of entering into collaborative arrangements with third parties to complete the development and commercialization of our product candidates. In the event that third parties take over the clinical trial process for one of our product candidates, the estimated completion date would largely be under control of that third party rather than us. We cannot forecast with any degree of certainty which proprietary products, if any, will be subject to future collaborative arrangements, in whole or in part, and how such arrangements would affect our development plan or capital requirements. Our programs may also benefit from subsidies, grants, contracts or government or agency-sponsored studies that could reduce our development costs.
As a result of the uncertainties discussed above, among others, we are unable to estimate the duration and completion costs of our research and development projects or when, if ever, and to what extent it will receive cash inflows from the commercialization and sale of a product. Our inability to complete our research and development projects in a timely manner or our failure to enter into collaborative agreements, when appropriate, could significantly increase our capital requirements and could adversely impact our liquidity. These uncertainties could force us to seek additional, external sources of financing from time to time in order to continue with our business strategy. Our inability to raise additional capital, or to do so on terms reasonably acceptable to us, would jeopardize the future success of our business.
During the past five years through December 31, 2009, we incurred an aggregate of $73.5 million in research and development expenses. The following table indicates the amount incurred for each of our significant research programs and for other identified research and development activities during the years ended December 31, 2009 and 2008. The amounts disclosed in the following table reflect direct research and development costs, license fees associated with the underlying technology and an allocation of indirect research and development costs to each program. Prior to 2008, the privately-held
45
Celldex Research did not maintain records that allowed for quantification of research and development expenses by project.
| |
Year Ended December 31, 2009 |
Year Ended December 31, 2008 |
||||||
|---|---|---|---|---|---|---|---|---|
| |
(In thousands) |
|||||||
CLINICAL |
||||||||
CDX-110 |
$ | 3,249 | $ | 7,621 | ||||
CDX-011 |
1,098 | — | ||||||
CDX-1307 |
6,510 | 3,446 | ||||||
CDX-1401 |
4,293 | 5,562 | ||||||
CDX-1135 |
473 | 159 | ||||||
PRECLINICAL |
||||||||
CDX-301 |
2,424 | — | ||||||
CDX-1127 |
1,308 | 1,040 | ||||||
CDX-014 |
8 | — | ||||||
CDX-1189 |
386 | 104 | ||||||
OTHER |
||||||||
Bacterial Vaccines (CholeraGarde®/ETEC/Ty800) |
124 | 1,481 | ||||||
CDX-2401 |
3,574 | 830 | ||||||
Other Programs |
2,722 | 2,393 | ||||||
Total R&D Expense |
$ | 26,169 | $ | 22,636 | ||||
Clinical Development Programs
CDX-110
Our lead clinical development program, CDX-110, is a peptide-based immunotherapy that targets the tumor specific molecule called EGFRvIII, a functional variant of the naturally expressed epidermal growth factor receptor ("EGFR"), a protein which has been well validated as a target for cancer therapy. Unlike EGFR, EGFRvIII is not present in normal tissues, and has been shown to be a transforming oncogene that can directly contribute to the cancer cell growth. EGFRvIII is commonly present in glioblastoma multiforme, or GBM, the most common and aggressive form of brain cancer, and has also been observed in various other cancers such as breast, ovarian, prostate, colorectal, and head & neck cancer.
In April 2008, we and Pfizer Inc. ("Pfizer") entered into a License and Development Agreement (the "Pfizer Agreement") under which Pfizer was granted an exclusive worldwide license to CDX-110. The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Pfizer funds all development costs for these programs. We and Pfizer are currently pursuing the development of CDX-110 for GBM therapy and plan to expand the clinical development into other cancers through additional clinical studies. The FDA has granted orphan drug designation for CDX-110 for the treatment of EGFRvIII expressing GBM as well as fast track designation.
Initial clinical development of EGFRvIII immunotherapy was led by collaborating investigators at the Brain Center at Duke Comprehensive Cancer Center in Durham, North Carolina and at M.D. Anderson Cancer Center in Houston, Texas. The results from the Phase 1 (VICTORI) and Phase 2a (ACTIVATE) studies, which enrolled 16 and 21 patients, respectively, have demonstrated a significant increase in the time to disease progression (greater than 113%) in the patients who were vaccinated, and also in overall survival rates (greater than 100%), both relative to appropriately matched historical controls. An extension of the Phase 2a program (ACT II) at the same two institutions has enrolled 23
46
additional GBM patients treated in combination with temozolomide (the current standard of care). Preliminary results from this study (ACT II) currently estimates median overall survival to be 23.6 months, although the median has not yet been reached, while the survival of a matched historical control group was 15.0 months with a p value = 0.0237. Overall time to progression in the ACT II study was 15.2 months compared with 6.3 months for the historical control group.
We initiated a Phase 2b/3 randomized study (ACT III) of CDX-110 combined with standard of care, temozolomide, versus standard of care alone in patients with GBM in over 30 sites throughout the United States. In December 2008, we announced an amendment to convert the ACT III study to a single-arm Phase 2 clinical trial in which all patients will receive CDX-110 in combination with temozolomide. The decision, which followed the recommendation of the Independent Data Monitoring Committee, was based on the observation that the majority of patients randomized to the control (standard of care) arm withdrew from this open-label study after being randomized to the control arm. Patients participating on the control arm of the study were offered the option to receive treatment with CDX-110. Under this amendment, the ACT III study provided for a multi-center, non-randomized dataset for CDX-110 in patients with newly diagnosed GBM. These data will provide important additional information that can be used to better design the future development of CDX-110. Enrollment in ACT III is complete with a total of over 60 patients enrolled and we expect to present updated results during 2010.
CDX-011
CDX-011 (formerly CR011-vcMMAE) is an antibody-drug conjugate (ADC) that consists of a fully-human monoclonal antibody, CR011, linked to a potent cell-killing drug, monomethyl-auristatin E (MMAE). The CR011 antibody specifically targets glycoprotein NMB or (GPNMB) that is expressed in a variety of human cancers including breast cancer and melanoma. The ADC technology, comprised of MMAE and a stable linker system for attaching it to CR011, was licensed from Seattle Genetics, Inc. The ADC is designed to be stable in the bloodstream. Following intravenous administration, CDX-011 targets and binds to GPNMB and upon internalization into the targeted cell, CDX-011 is designed to release MMAE from CR011 to produce a cell-killing effect. We acquired the rights to CDX-011 in connection with the CuraGen Merger.
Treatment of Breast Cancer: In June 2008, an open-label, multi-center Phase 1/2 study was initiated of CDX-011 administered intravenously once every three weeks to patients with locally advanced or metastatic breast cancer who have received prior therapy (median of seven prior regimens). The study began with a bridging phase to confirm the maximum tolerated dose ("MTD") and has expanded into a Phase 2 open-label, multi-center study.
The study confirmed the safety of CDX-011 at the pre-defined maximum dose level (1.88 mg/kg) in 6 patients. An additional 28 patients were enrolled as an expanded Phase 2 cohort (for a total of 34 treated patients at 1.88 mg/kg, the Phase 2 dose) to evaluate the progression-free survival ("PFS") rate at 12 weeks. As previously seen in melanoma patients, the 1.88 mg/kg dose was well tolerated in this patient population with the most common adverse events of rash, alopecia, and fatigue. The primary activity endpoint, which called for at least 5 of 25 (20%) patients in the Phase 2 study portion to be progression-free at twelve weeks, has been met. To date, 9 of 26 (35%) evaluable patients are without progression of disease at twelve weeks.
In addition, at the Phase 2 dose level, 4 of 32 (13%) evaluable patients achieved confirmed or unconfirmed Partial Responses ("PR") while 15 of 25 (60%) evaluable patients with measurable disease experienced some reduction in tumor size. GPNMB expression was identified in 10 of 14 (71%) of analyzed tumor samples and treatment with CDX-011 was associated with improved outcomes in all activity parameters in patients whose tumors expressed GPNMB. Notably, in patients who received the Phase 2 dose and whose tumors expressed GPNMB, 2 of 7 (29%) had confirmed PR, 5 of 7 (71%) had
47
decreases in tumor size, and all 7 achieved at least stable disease with duration from 17.3 to 26.9 weeks. The median PFS in all patients was 9.1 weeks, but in patients whose tumors expressed GPNMB, median PFS was 18.3 weeks, compared to median PFS of 5.9 weeks for patients whose tumors did not express GPNMB. In patients with triple negative disease, 5 of 7 (71%) analyzed samples expressed GPNMB, 7 of 9 (78%) evaluable patients had tumor shrinkage, and the median PFS for these patients was 17.9 weeks.
We expect to initiate a randomized Phase 2b controlled study in patients with advanced breast cancer that express GPNMB in the second half of 2010.
Treatment of Metastatic Melanoma Cancer: In June 2006, a Phase 1/2 open-label, multi-center, dose escalation study was initiated to evaluate the safety, tolerability and pharmacokinetics of CDX-011 for patients with un-resectable Stage III or Stage IV melanoma who have failed no more than one prior line of cytotoxic therapy. During the Phase 1 portion of the study, doses of CDX-011 between 0.03 mg/kg to 2.63 mg/kg were evaluated and generally well tolerated, with rash and neutropenia emerging at higher doses. The MTD was determined to be 1.88 mg/kg administered intravenously (IV) once every three weeks.
In June 2009, results were announced for the 36 patients who were treated in the Phase 2 portion of the study. Of the patients enrolled, 94% had Stage IV disease of which two-thirds were classified as M1c, the poorest risk group. The study successfully met its primary activity endpoint, with 5 objective responses (1 unconfirmed) observed in 34 evaluable patients, and median duration of response of 5.3 months. The median overall PFS was 4.4 months. Tumor shrinkage was observed in 58% of patients, and 20 patients had best response of stable disease. Dermatologic adverse events consisting of rash, alopecia, and pruritus were the most common toxicities in this study. Other adverse events included fatigue, diarrhea, musculoskeletal pain, anorexia and nausea. Grade 3 or 4 neutropenia was observed in 5 patients. The absence of rash in the first cycle of treatment predicted a worse PFS. Additionally, in a subset of patients with tumor biopsies, high levels of tumor expression of GPNMB appeared to correlate with favorable outcome.
Enrollment has been completed in the Phase 1 portion of the melanoma trial to evaluate more frequent dosing schedules of CDX-011, including a weekly and a two out of every three-week regimen, to explore if more frequent administration can provide additional activity in patients with metastatic melanoma. A dose of 1.0 mg/kg given once every week has been identified as the MTD in a weekly schedule, and a dose of 1.5mg/kg was being explored in the two out of three week schedule. Although median duration of follow-up was only 6 weeks, objective responses have thus far been observed in 3 of 11 evaluable patients treated with weekly CDX011 (1 confirmed) and 1 confirmed response in 8 evaluable patients treated with CDX-011 two out of every three weeks. We expect to present updated results during the first half of 2010.
CDX-1307
Our lead APC Targeting Technology™ product candidate, CDX-1307, is in development for the treatment of epithelial tumors such as colorectal, pancreatic, bladder, ovarian and breast cancers. CDX-1307 targets the beta chain of human chorionic gonadotropin, known as hCG-Beta, which is an antigen often found in epithelial tumors. The presence of hCG-Beta in these cancers correlates with a poor clinical outcome, suggesting that this molecule may contribute to tumor growth. Normal adult tissues have minimal expression of hCG-Beta; therefore, targeted immune responses are not expected to generate significant side effects.
Enrollment is complete in our two Phase 1 studies at multiple centers designed to explore safety and dose/effect relationships via two administration routes—intradermal (ID), a traditional vaccine route that allows efficient access to local dermal dendritic cells and intravenous (IV), a novel systemic approach to vaccination that might target a much larger population of dendritic cells. The Phase 1
48
studies investigated the safety and immunogenicity of CDX-1307 alone and in combination with adjuvants, including GM-CSF (known to increase mannose receptor expression on dendritic cells) and Toll-Like Receptor ("TLR") agonists (poly-ICLC or Hiltonol™ and R848 or resiquimod). Patients with an assortment of different tumor types that are known to express hCG-Beta were enrolled with retrospective analysis for hCG-Beta expression. An escalating four dose regimen was utilized with the possibility of retreatment if patients demonstrate tumor regression or stable disease.
The Phase 1 studies enrolled over 80 patients with heavily pretreated, advanced-stage breast, colon, bladder and pancreatic cancer, with an average of 4.6 prior therapies across the treatment population. All patient cohorts demonstrated a favorable safety profile with no dose limiting toxicity to date. The combination of CDX-1307 with TLR agonists significantly enhanced immune responses against hCG-Beta, providing strong humoral responses in 88% of patients and cellular immune responses in 57% of patients analyzed to date. Immune responses occurred even in the presence of high circulating levels of hCG-Beta, suggesting that the CDX-1307 can overcome antigen tolerance in advanced and heavily pretreated cancers. Nine patients in the studies experienced disease stabilization from 2.3 months to 11.4 months following the initiation of CDX-1307 vaccination. Two of these patients have received multiple courses of CDX-1307 and continue treatment with stable disease at 6.4 and 11.4 months. These data provide the basis for advancing CDX-1307 into a front-line patient population selected for hCG-Beta expressing cancers.
We expect to initiate a randomized Phase 2b controlled study in patients with newly diagnosed invasive bladder cancer in the second quarter of 2010. Patient's whose bladder cancer expresses hCG-Beta are predicted to have more aggressive disease and shorter survival. In this study we plan to select only patients with confirmed hCG-Beta expression using a specific diagnostic assay.
CDX-1401
CDX-1401 is a fusion protein consisting of a fully human monoclonal antibody with specificity for the dendritic cell receptor, DEC-205, linked to the NY-ESO-1 tumor antigen. In humans, NY-ESO-1 is one of the most immunogenic tumor antigens and has been detected in 20 - 30% of cancers, thus representing a broad opportunity. This product is intended to selectively deliver the NY-ESO-1 antigen to APCs for generating robust immune responses against cancer cells expressing NY-ESO-1. Unlike CDX-1307, which targets the mannose receptor expressing dendritic cells, CDX-1401 is the first APC product targeting DEC-205 expressing dendritic cells. We are developing CDX-1401 for the treatment of malignant melanoma and a variety of solid tumors which express the proprietary cancer antigen NY-ESO-1, which we licensed from the Ludwig Institute for Cancer Research in 2006. We believe that preclinical studies have shown that CDX-1401 is effective for activation of human T-cell responses against NY-ESO-1.
In September 2009, we initiated enrollment in a dose-escalating Phase 1/2 clinical trial aimed at determining the optimal dose for further development based on the safety, tolerability, and immunogenicity of the CDX-1401 vaccine. The trial will evaluate three different doses of the vaccine in combination with resiquimod, an activator of TLR 7 and 8. We expect to enroll approximately 36 patients with solid tumor cancers at multiple clinical sites in the United States.
CDX-1135
CDX-1135 is a molecule that inhibits a part of the immune system called the complement system. The complement system is a series of proteins that are important initiators of the body's acute inflammatory response against disease, infection and injury. Excessive complement activation also plays a role in some persistent inflammatory conditions. CDX-1135 is a soluble form of naturally occurring Complement Receptor 1 that inhibits the activation of the complement cascade in animal models and in human clinical trials. We believe that regulating the complement system could have therapeutic and
49
prophylactic applications in several acute and chronic conditions, including organ transplantation, multiple sclerosis, rheumatoid arthritis, age-related macular degeneration ("AMD"), atypical Hemolytic Uremic Syndrome ("aHUS"), Paroxysmal Nocturnal Hemaglobinuria ("PNH"), Dense Deposit Disease ("DDD") in kidneys, and myasthenia gravis. We are currently defining the most appropriate clinical development path for CDX-1135 and are focusing on rare disease conditions of unregulated complement activation as the fastest route to FDA approval.
Preclinical Development Programs
CDX-301
CDX-301 is a FMS-like tyrosine kinase 3 ligand (Flt3L) that we licensed from Amgen in March 2009. CDX-301 is a growth factor for stem cells and immune cells called dendritic cells. Based on previous experience with this molecule, we believe that CDX-301 has considerable opportunity in various transplant settings as a stem cell mobilizing agent. In addition, CDX-301 is an immune modulating molecule that increases the numbers and activity of specific types of immune cells. We believe CDX-301 has significant opportunity for synergistic development in combination with proprietary molecules in our portfolio. We expect to file an IND application for CDX-301 before the end of 2010.
CDX-1127
We have entered into a License Agreement with the University of Southampton, UK, to develop human antibodies to CD27, a potentially important target for immunotherapy of various cancers. In preclinical models, antibodies to CD27 alone have been shown to mediate anti-tumor effects, and may be particularly effective in combination with other immunotherapies. CD27 is a critical molecule in the activation pathway of lymphocytes. It is downstream from CD40, and may provide a novel way to regulate the immune responses. Engaging CD27 with the appropriate monoclonal antibody has proven highly effective at promoting anti-cancer immunity in mouse models. We are evaluating new human monoclonal antibodies in preclinical models.
CDX-014
CDX-014 (formerly CR014-vcMMAE) is a fully-human monoclonal ADC that targets TIM-1, an immunomudulatory protein that appears to down regulate immune response to tumors. The antibody, CDX-014, is linked to a potent chemotherapeutic, monomethyl auristatin E (MMAE), using Seattle Genetics' proprietary technology. The ADC is designed to be stable in the bloodstream, but to release MMAE upon internalization into TIM-1-expressing tumor cells, resulting in a targeted cell-killing effect. CDX-014 has shown potent activity in preclinical models of ovarian and renal cancer. We acquired the rights to CDX-014 in connection with the CuraGen Merger.
CDX-1189
We are developing therapeutic human antibodies to a signaling molecule known as CD89 or Fca receptor type I (FcaRI). CD89 is expressed by some white blood cells and leukemic cell lines, and has been shown to be important in controlling inflammation and tumor growth in animal models. We have proprietary, fully human antibodies to CD89 in preclinical development. Depending upon the specific antibody used, anti-CD89 antibodies can either be activating and thus stimulate immune responses, or down-regulating and act as an anti-inflammatory agent.
50
Marketed Products
Rotavirus Vaccine
Rotavirus is a major cause of diarrhea and vomiting in infants and children. In 1997, we licensed our oral rotavirus strain to Glaxo and Glaxo assumed responsibility for all subsequent clinical trials and all other development activities. Glaxo gained approval for its rotavirus vaccine, Rotarix®, in Mexico in July 2004, which represented the first in a series of worldwide approvals and commercial launches for the product leading up to the approval in Europe in 2006 and in the U.S. in 2008. We licensed-in our rotavirus strain in 1995 and owe a license fee of 30% to Cincinnati Children's Hospital Medical Center ("CCH") on net royalties received from Glaxo. We are obligated to maintain a license with CCH with respect to the Glaxo agreement. The term of the Glaxo agreement is through the expiration of the last of the relevant patents covered by the agreement, although Glaxo may terminate the agreement upon 90 days prior written notice.
In May 2005, we entered into an agreement whereby an affiliate of PRF purchased an interest in the milestone payments and net royalties that we will receive on the development and worldwide sales of Rotarix®. We have received a total of $60 million in milestone payments under the PRF agreement. No additional milestone payments are due from PRF under the agreement.
Royalty rates on Rotarix® escalate from 7% to 10% based on net product sales in countries that have valid patent protection. These royalty rates are discounted by 30% for "non-patent" countries (primarily international markets). In September 2006, we received notice from Glaxo that Glaxo would begin paying royalties on sales of Rotarix® vaccine at the lower of the two royalty rates under their 1997 license agreement. Glaxo's decision to pay the lower royalty rate (which is 70% of the full rate) is based upon Glaxo's assertion that Rotarix® is not covered by the patents Glaxo licensed from us in Australia and certain European countries. We are currently evaluating the basis for Glaxo's action and our potential remedies. If Glaxo's position stands, the royalties to which PRF is entitled will no longer be limited by a $27.5 million annual threshold, which we projected may have been reached in later years as sales of Rotarix® increased. Irrespective of Glaxo's position, we will still retain approximately 65% of the royalties on worldwide sales of Rotarix® once PRF receives 2.45 times the aggregate cash payments of $60 million it made to us, though the potential amount of such residual royalties will be lower if Glaxo's position stands.
CRITICAL ACCOUNTING POLICIES
Our significant accounting policies are described in Note 2 to the consolidated financial statements included in Item 8 of this Form 10-K. We believe our most critical accounting policies include accounting for business combinations, revenue recognition, property and equipment, impairment of long-lived assets, marketable securities, research and development expenses and stock-based compensation expense.
The methods, estimates and judgments we use in applying our most critical accounting policies have a significant impact on the results we report in our consolidated financial statements. We evaluate our estimates and judgments on an on-going basis. We base our estimates on historical experience and on assumptions that we believe to be reasonable under the circumstances. Our experience and assumptions form the basis for our judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may vary from what we anticipate and different assumptions or estimates about the future could materially change our reported results. We believe the following accounting policies are the most critical to us in that they are important to the portrayal of
51
our financial statements and they require our most difficult, subjective or complex judgments in the preparation of our consolidated financial statements:
Business Combinations
We account for business combinations that were completed after January 1, 2009 or will be completed in the future, including the CuraGen Merger, under the acquisition method of accounting. We assign the value of the consideration transferred to acquire a business to the tangible assets and identifiable intangible assets acquired and liabilities assumed on the basis of their fair values at the date of acquisition. We assess the fair value of assets, including intangible assets such as IPR&D, using a variety of methods including present-value models. Each asset is measured at fair value from the perspective of a market participant. The method used to estimate the fair values of IPR&D assets incorporates significant assumptions regarding the estimates a market participant would make in order to evaluate an asset, including a market participant's assumptions regarding the probability of completing IPR&D projects, which would require obtaining regulatory approval for marketing of the associated drug candidate; a market participant's estimates regarding the timing of and the expected costs to complete IPR&D projects; a market participant's estimates of future cash flows from potential product sales; and the appropriate discount rates for a market participant. Transaction costs and restructuring costs associated with the transaction are expensed as incurred.
IPR&D assets acquired in a business combination initially are recorded at fair value and accounted for as indefinite-lived intangible assets. These assets are maintained on our consolidated balance sheets until either the project underlying them is completed or the assets become impaired. If a project is completed, the carrying value of the related intangible asset is amortized over the remaining estimated life of the asset beginning in the period in which the project is completed. If a project becomes impaired or is abandoned, the carrying value of the related intangible asset is written down to its fair value and an impairment charge is taken in the period in which the impairment occurs. IPR&D assets will be tested for impairment on an annual basis during the third quarter, or earlier if impairment indicators are present.
Intangible assets acquired in a business combination with a finite life are recorded at fair value and amortized over the greater of economic consumption or on a straight-line basis over their estimated useful life.
The difference between the purchase price and the fair value of assets acquired and liabilities assumed in a business combination is allocated to goodwill. Goodwill will be evaluated for impairment on an annual basis during the third quarter, or earlier if impairment indicators are present.
For acquisitions completed prior to January 1, 2009, including the AVANT Merger, we expensed the fair value of IPR&D to research and development expense as of the acquisition date and included transaction costs associated with the business combination as part of the cost of the acquired company.
Revenue Recognition
We account for revenue arrangements that include multiple deliverables as separate units of accounting if (i) the delivered item has value to the customer on a standalone basis, (ii) there is objective and reliable evidence of the fair value of the undelivered items and (iii) if the right of return exists, delivery of the undelivered items is considered probable and substantially in the control of the vendor. If these criteria are not met, the revenue elements are considered a single unit of accounting for purposes of revenue recognition.
We have entered into various license and development agreements with pharmaceutical and biotechnology companies. The terms of the agreements typically include non-refundable license fees, funding of research and development, payments based upon achievement of certain milestones and
52
royalties on net product sales. Non-refundable license fees are recognized as revenue when we have a contractual right to receive such payments, provided that (i) a contractual arrangement exists, (ii) the contract price is fixed or determinable, (iii) the collection of the resulting receivable is reasonably assured and (iv) we have no further performance obligations under the license agreement. Upfront non-refundable fees associated with license and development agreements where we have continuing performance obligations under the terms of the agreement are recorded as deferred revenue and recognized as revenue over the estimated service period as we complete our obligations. Where our level of effort is relatively constant over the performance period or no other pattern is estimable, the revenue is recognized on a straight-line basis. Revenue is limited to the lesser of the cumulative amount of payments due or the cumulative amount of revenue earned, as determined using the straight-line basis, as of the period ending date. If the estimated service period is subsequently modified, the period over which the upfront fee is recognized is modified accordingly on a prospective basis. The determination of the performance period involves judgment on management's part. Funding of research and development is recognized as revenue over the term of the applicable contract as costs are incurred related to that contract.
Milestone payments are recognized as revenue upon the achievement of mutually agreed milestones, provided that there is no continuing performance obligations associated with the milestone payment. Revenues from milestone payments related to arrangements under which we have continuing performance obligations are recognized as revenue upon achievement of the milestone only if all of the following conditions are met: (i) the milestone payments are non-refundable; (ii) achievement of the milestone was not reasonably assured at the inception of the arrangement; (iii) substantive effort is involved in achieving the milestone; and, (iv) the amount of the milestone is reasonable in relation to the effort expended or the risk associated with achievement of the milestone. If any of these conditions are not met, the milestone payments are deferred and recognized as revenue over the term of the arrangement as we complete our performance obligations.
We have capitalized and deferred costs incurred in connection with the one-time signing and upfront payment (the initial deliverable) received with respect to a multiple deliverable arrangement. If there is deemed a single unit of accounting for such an arrangement, the capitalized deferred costs are amortized over the expected performance period of the arrangement.
Payments received to fund certain research activities are recognized as revenue in the period in which the research activities are performed. Revenue from contracts and grants is recognized as the services are performed and recorded as effort is expended on the contracted work and billed to the government or our contractual partner. Payments received in advance that are related to future performance are deferred and recognized as revenue when the research projects are performed.
Product royalty revenue consists of payments received from licensees for a portion of sales proceeds from products that utilize our licensed technologies and are recognized when the amount of and basis for such royalty payments are reported to us in accurate and appropriate form and in accordance with the related license agreement.
Property and Equipment
The treatment of costs to construct property and equipment depends on the nature of the costs and the stage of construction. Costs incurred in the project planning, design, construction and installation phases are capitalized as part of the cost of the asset. We stop capitalizing these costs when the asset is substantially complete and ready for its intended use. For manufacturing property and equipment, we also capitalizes the cost of validating these assets for the underlying manufacturing process. We complete the capitalization of validation costs when the asset is substantially complete and ready for its intended use. Costs capitalized include incremental labor and fringe benefits, and direct
53
consultancy services. We capitalize interest cost as part of the historical cost of acquiring certain assets during the period of time required to get the asset ready for its intended use.
Property and equipment is stated at cost and depreciated over the estimated useful lives of the related assets using the straight-line method. Laboratory equipment and office furniture and equipment are depreciated over five years and computer equipment is depreciated over three years. Manufacturing equipment is amortized over seven to ten years. Leasehold improvements are amortized over the shorter of the estimated useful life or the non-cancelable term of the related lease, including any renewals that are reasonably assured of occurring. Property and equipment under construction is classified as construction in progress and is depreciated or amortized only after the asset is placed in service. Expenditures for maintenance and repairs are charged to expense whereas the costs of significant improvements which extend the life of the underlying asset are capitalized. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation are eliminated and any resulting gain or loss is reflected in our consolidated statements of operations.
Impairment of Long-Lived Assets
We evaluate the recoverability of our long-lived assets, including property and equipment, and intangible assets when circumstances indicate that an event of impairment may have occurred. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written-down to their estimated fair values.
Marketable Securities
We invest our excess cash balances in marketable securities including municipal bond securities, U.S. government agency securities, and high-grade corporate bonds. We classify all of our marketable securities as current assets on the consolidated balance sheets because they are available-for-sale and available to fund current operations. Marketable securities are stated at fair value with their unrealized gains and losses included as a component of accumulated other comprehensive income (loss), which is a separate component of stockholders' equity, until such gains and losses are realized. The fair value of these securities is based on quoted prices for identical or similar assets. If a decline in the fair value is considered other-than-temporary, based on available evidence, the unrealized loss is transferred from other comprehensive income (loss) to the consolidated statements of operations. Realized gains and losses are determined on the specific identification method and are included in investment and other income, net.
Research and Development Expenses
Research and development costs, including internal and contract research costs, are expensed as incurred. Research and development expenses consist mainly of clinical trial costs, manufacturing of clinical material, toxicology and other studies, personnel costs, depreciation, license fees and funding of outside research.
Stock-Based Compensation Expense
We record stock-based compensation expense for all stock-based awards made to employees and directors based on the estimated fair values of the stock-based awards expected to vest at the grant date and is adjusted, if necessary, to reflect actual forfeitures. Compensation expense for all stock-based awards to employees and directors is recognized using the straight-line method over the term of vesting or performance.
54
We record stock-based compensation expense for stock options granted to non-employees based on the fair value of the stock options which is re-measured over the vesting term resulting in periodic adjustments to stock-based compensation expense.
RESULTS OF OPERATIONS
Our financial statements prior to the AVANT Merger reflect the financial position, results of operations and cash flows of Celldex Research. Following the AVANT Merger but prior to the CuraGen Merger, our financial statements reflect the financial position, results of operation and cash flows of the combined AVANT and Celldex Research. Following the CuraGen Merger, our financial statements reflect the financial position, results of operation and cash flows of the combined companies (AVANT, Celldex Research and CuraGen).
Year Ended December 31, 2009 compared with Year Ended December 31, 2008
| |
Year Ended December 31, |
|
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Increase/ (Decrease) $ |
Increase/ (Decrease) % |
||||||||||||
| |
2009 | 2008 | ||||||||||||
| |
(In thousands) |
|||||||||||||
Revenue: |
||||||||||||||
Product Development and Licensing Agreements |
$ | 5,662 | $ | 3,716 | $ | 1,946 | 52 | % | ||||||
Contracts and Grants |
1,802 | 533 | 1,269 | 238 | % | |||||||||
Product Royalties |
7,716 | 3,207 | 4,509 | 141 | % | |||||||||
Total Revenue |
$ | 15,180 | $ | 7,456 | $ | 7,724 | 104 | % | ||||||
Operating Expense: |
||||||||||||||
Research and Development |
26,169 | 22,636 | 3,533 | 16 | % | |||||||||
Royalty |
8,397 | 3,711 | 4,686 | 126 | % | |||||||||
Gain on Sale of Assets |
(604 | ) | — | (604 | ) | n/a | ||||||||
Charge for In-Process Research and Development |
— | 14,756 | (14,756 | ) | (100 | )% | ||||||||
General and Administrative |
17,119 | 14,748 | 2,371 | 16 | % | |||||||||
Amortization of Acquired Intangible Assets |
949 | 361 | 588 | 163 | % | |||||||||
Total Operating Expense |
52,030 | 56,212 | (4,182 | ) | (7 | )% | ||||||||
Operating Loss |
(36,850 | ) | (48,756 | ) | (11,906 | ) | (24 | )% | ||||||
Investment and Other Income, Net |
248 | 1,411 | (1,163 | ) | (82 | )% | ||||||||
Interest Expense |
(452 | ) | (156 | ) | 296 | 190 | % | |||||||
Net Loss Before Income Taxes |
(37,054 | ) | (47,501 | ) | (10,447 | ) | (22 | )% | ||||||
Income Tax Benefit |
529 | — | 529 | n/a | ||||||||||
Net Loss |
$ | (36,525 | ) | $ | (47,501 | ) | $ | (10,976 | ) | (23 | )% | |||
Net Loss
The $11.0 million decrease in net loss for the year ended December 31, 2009 compared to the year ended December 31, 2008 was primarily the result of a decrease in charges for acquired in-process research and development combined with increased revenues, partially offset by increased research and development, royalty and general and administrative expenses.
55
Revenue
The $1.9 million increase in product development and licensing agreement revenue for the year ended December 31, 2009 was primarily due to an increase of $2.3 million in Pfizer related revenue. The $1.3 million increase in contract and grant revenue for the year ended December 31, 2009 was primarily due to an increase of $1.4 million in revenue related to our vaccine development work on Rockefeller's CDX-2401 program. The $4.5 million increase in product royalty revenue for the year ended December 31, 2009 was primarily related to our retained interests in Rotarix® net royalties which were not sold to PRF and which is equal to the amount payable to CCH and recognized in royalty expense by us.
Research and Development Expense
Research and development expenses consist primarily of (i) personnel expenses, (ii) laboratory supply expenses relating to the development of our technology, (iii) facility expenses, and (iv) product development expenses associated with our product candidates as follows:
| |
Year Ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Increase/ (Decrease) $ |
Increase/ (Decrease) % |
|||||||||||
| |
2009 | 2008 | |||||||||||
| |
(In thousands) |
||||||||||||
Personnel |
$ | 11,108 | $ | 8,785 | $ | 2,323 | 26 | % | |||||
Laboratory Supplies |
2,517 | 2,179 | 338 | 16 | % | ||||||||
Facility |
4,782 | 4,180 | 602 | 14 | % | ||||||||
Product Development |
5,758 | 5,192 | 566 | 11 | % | ||||||||
Personnel expenses primarily include salary, benefits, stock-based compensation, payroll taxes and recruiting costs. The $2.3 million increase in personnel expenses for the year ended December 31, 2009 was primarily due to higher headcount and $0.9 million in severance expense related to the CuraGen Merger. We expect personnel expenses to remain relatively consistent over the next twelve months as the effect of our higher headcount will be offset by the reduction in severance expense.
Laboratory supply expenses include laboratory materials and supplies, services, and other related expenses incurred in the development of our technology. The $0.3 million increase in laboratory supply expenses for the year ended December 31, 2009 was primarily due to increased research, preclinical and manufacturing activities. We expect supply expenses to increase over the next twelve months as a result of increased research and development activities, although there may be fluctuations on a quarterly basis.
Facility expenses include depreciation, amortization, utilities, rent, maintenance, and other related expenses incurred at our facilities. The $0.6 million increase in facility expenses for the year ended December 31, 2009 was primarily due to higher depreciation and amortization expenses. We expect facility expenses to increase over the next twelve months as a result of continued capital expansion, although there may be fluctuations on a quarterly basis.
Product development expenses include clinical investigator site fees, external trial monitoring costs, data accumulation costs, contracted research and outside clinical drug product manufacturing. The $0.6 million increase in product development expenses for the year ended December 31, 2009 was primarily due to an increase in preclinical work related to the CDX-1401 and CDX-2401 programs. The decrease in clinical expenses for CDX-110 for the year ended December 31, 2009 due to the transfer of clinical management of our CDX-110 program to Pfizer was primarily offset by the increase in clinical expenses related to our CDX-011 program acquired from CuraGen. We expect product development expenses to increase over the next twelve months due to the increase in clinical trial expenses related
56
to our CDX-011, CDX-1307, and CDX-1401 programs, although there may be fluctuations on a quarterly basis.
Royalty Expense
Royalty expenses include product royalty and sublicense royalty fees on our out-licensed programs. The $4.7 million increase in royalty expenses for the year ended December 31, 2009 was primarily due to an increase in Rotarix® related royalty fees. Our retained interests in Rotarix® net royalties which were not sold to PRF are recorded as product royalty revenue and a corresponding amount that is payable to CCH is recorded as royalty expense. We expect royalty expenses to increase over the next twelve months, although there may be fluctuations on a quarterly basis.
General and Administrative Expense
The $2.4 million increase in general and administrative expenses for the year ended December 31, 2009 was primarily due to (i) $3.3 million in severance expense related to the CuraGen Merger, (ii) an increase in consultant and legal expense of $0.6 million during the year ended December 31, 2009 primarily related to the CuraGen Merger and (iii) $0.7 million in severance expense, including related non-cash stock-based compensation expense, incurred during the year ended December 31, 2009 related to our former SVP, Business Development. The effect of these increases was partially offset by $1.4 million in severance expense and $1.3 million in stock-based compensation expense incurred during the year ended December 31, 2008 related to our former President and Chief Executive Officer. We expect general and administrative expense to decrease over the next twelve months primarily due to the lack of CuraGen Merger related expenses.
Amortization Expense
The $0.6 million increase in amortization expenses for the year ended December 31, 2009 was primarily due to intangible assets acquired in connection with the CuraGen Merger. We expect amortization expense of acquired intangible assets to increase over the next twelve months primarily due to the CuraGen Merger.
Investment and Other Income, Net
The $1.2 million decrease in investment and other income, net for the year ended December 31, 2009 was primarily due to other income of $0.9 million recorded for the year ended December 31, 2008 related to the $10 million milestone payment we received from PRF in connection with the U.S. launch of Rotarix®. We anticipate investment income to increase over the next twelve months due to the CuraGen Merger.
Interest Expense
The $0.3 million increase in interest expense for the year ended December 31, 2009 was primarily due to the CuraGen Debt we assumed in connection with the CuraGen Merger. We anticipate interest expense to increase over the next twelve months due to the CuraGen Debt.
Income Tax Benefit
The $0.5 million increase in income tax benefit for the year ended December 31, 2009 was due to non-cash tax consequences as a result of the CuraGen Merger.
57
Year Ended December 31, 2008 compared with Year Ended December 31, 2007
| |
Year Ended December 31, |
|
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Increase/ (Decrease) $ |
Increase/ (Decrease) % |
||||||||||||
| |
2008 | 2007 | ||||||||||||
| |
(In thousands) |
|||||||||||||
Revenue: |
||||||||||||||
Product Development and Licensing Agreements |
$ | 3,716 | $ | 466 | $ | 3,250 | 697 | % | ||||||
Contracts and Grants |
533 | 940 | (407 | ) | (43 | )% | ||||||||
Product Royalties |
3,207 | — | 3,207 | n/a | ||||||||||
Total Revenue |
$ | 7,456 | $ | 1,406 | $ | 6,050 | 430 | % | ||||||
Operating Expense: |
||||||||||||||
Research and Development |
22,636 | 9,892 | 12,744 | 129 | % | |||||||||
Royalty |
3,711 | — | 3,711 | n/a | ||||||||||
Charge for In-Process Research and Development |
14,756 | — | 14,756 | n/a | ||||||||||
General and Administrative |
14,748 | 6,905 | 7,843 | 114 | % | |||||||||
Amortization of Acquired Intangible Assets |
361 | 117 | 244 | 209 | % | |||||||||
Total Operating Expense |
56,212 | 16,914 | 39,298 | 232 | % | |||||||||
Operating Loss |
(48,756 | ) | (15,508 | ) | 33,248 | 214 | % | |||||||
Investment and Other Income, Net |
1,411 | 435 | 976 | 224 | % | |||||||||
Interest Expense |
(156 | ) | — | (156 | ) | n/a | ||||||||
Net Loss |
$ | (47,501 | ) | $ | (15,073 | ) | $ | 32,428 | 215 | % | ||||
Net Loss
The $32.4 million increase in net loss for the year ended December 31, 2008 compared to the year ended December 31, 2007 was primarily the result of increased operating expenses as a result of the AVANT Merger including a one-time non-cash charge of $14.8 million for purchased in-process research and development. The effect of this increase was offset by higher revenue.
Revenue
The $3.3 million increase in product development and licensing agreement revenue for the year ended December 31, 2008 was primarily due to an increase of $2.9 million in Pfizer related revenue. The $0.4 million decrease in contract and grant revenue for the year ended December 31, 2008 was primarily due to lower levels of vaccine development work billable to Rockefeller and Harvard in 2008. The $3.2 million increase in product royalty revenue for the year ended December 31, 2008 was related to our retained interests in Rotarix® net royalties which were not sold to PRF and which is equal to the amount payable to CCH and recognized in research and development expense by us.
Research and Development Expense
| |
Year Ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Increase/ (Decrease) $ |
Increase/ (Decrease) % |
|||||||||||
| |
2008 | 2007 | |||||||||||
| |
(In thousands) |
||||||||||||
Personnel |
$ | 8,785 | $ | 3,250 | $ | 5,535 | 170 | % | |||||
Laboratory Supplies |
2,179 | 446 | 1,733 | 389 | % | ||||||||
Facility |
4,180 | 995 | 3,185 | 320 | % | ||||||||
Product Development |
5,192 | 2,455 | 2,737 | 111 | % | ||||||||
58
The $5.5 million increase in personnel expenses for the year ended December 31, 2008 was primarily due to higher headcount as a result of the AVANT Merger. The $1.7 million increase in laboratory supply expenses for the year ended December 31, 2008 was primarily due to the AVANT Merger. The $3.2 million increase in facility expenses for the year ended December 31, 2008 was primarily due to the combination of expenses for three facilities (Phillipsburg, NJ and Needham, MA and Fall River, MA) as a result of the AVANT Merger. The $2.7 million increase in product development expenses for the year ended December 31, 2008 was primarily due to the expansion of the clinical trials for the CDX-110 and CDX-1307 programs.
Royalty Expense
The $3.7 million increase in royalty expenses primarily related to Rotarix® for the year ended December 31, 2008 was due to the AVANT Merger.
General and Administrative Expense
The $7.8 million increase in our general and administrative expenses for the year ended December 31, 2008 was primarily due to (i) $1.4 million in severance expense and $1.3 million in stock-based compensation expense incurred during the year ended December 31, 2008 related to our former President and Chief Executive Officer and (ii) increases in legal and patent expense of $2.0 million, facility-related expenses of $1.1 million, insurance expenses of $0.6 million and professional services of $0.6 million, primarily due to the AVANT Merger.
Amortization Expense
The $0.2 million increase in amortization expenses for the year ended December 31, 2008 was primarily due to intangible assets acquired in connection with the AVANT Merger.
Investment and Other Income, Net
The $1.0 million increase in our investment and other income, net for the year ended December 31, 2008 was primarily due to other income of $0.9 million recorded for the year ended December 31, 2008 related to the $10 million milestone payment we received from PRF in connection with the U.S. launch of Rotarix®.
LIQUIDITY AND CAPITAL RESOURCES
At December 31, 2009, our principal sources of liquidity consisted of cash, cash equivalents and marketable securities of $82.5 million. Our working capital at December 31, 2009 was $69.6 million. At December 31, 2009, we had 4% convertible subordinated debt due in February 2011 of $12.5 million. We incurred a loss of $36.5 million for the year ended December 31, 2009. Net cash used in operations for the year ended December 31, 2009 was $29.9 million. We believe that the cash inflows from existing grants and collaborations, interest income on invested funds and our current cash, cash equivalents and marketable securities at December 31, 2009 are sufficient to meet estimated working capital requirements and fund planned operations for at least the next twelve months.
Our cash equivalents are highly liquid investments with a maturity of three months or less at the date of purchase and consist primarily of investments in money market mutual funds with commercial banks and financial institutions. We maintain cash balances with financial institutions in excess of insured limits. We do not anticipate any losses with respect to such cash balances. We invest our excess cash balances in marketable securities including municipal bond securities, U.S. government agency securities, and high-grade corporate bonds that meet high credit quality standards, as specified in our investment policy. Our investment policy seeks to manage these assets to achieve our goals of preserving principal and maintaining adequate liquidity.
59
The use of our cash flows for operations has primarily consisted of salaries and wages for our employees, facility and facility-related costs for our offices, laboratories and manufacturing facility, fees paid in connection with preclinical studies, clinical studies, contract manufacturing, laboratory supplies and services, consulting, legal and other professional fees. To date, and for the foreseeable future, the primary sources of cash flows from operations have been payments received from our collaborative partners and from government entities. The timing of any new collaboration agreements, government contracts or grants and any payments under these agreements, contracts or grants cannot be easily predicted and may vary significantly from quarter to quarter.
During the next twelve to twenty-four months, we may take further steps to raise additional capital to meet our long-term liquidity needs including, but not limited to, the licensing of technology programs with existing or new collaborative partners, possible business combinations, or the issuance of common stock or other securities via private placements or public offerings. While we may continue to seek capital through a number of means, there can be no assurance that additional financing will be available on acceptable terms, if at all, particularly in light of the recent disruptions in the financial markets and our negotiating position in capital-raising efforts may worsen as existing resources are used. There is also no assurance that we will be able to enter into further collaborative relationships. Additional equity financing may be dilutive to our stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict our ability to operate as a business; and licensing or strategic collaborations may result in royalties or other terms which reduce our economic potential from products under development. If we are unable to raise the funds necessary to meet our long-term liquidity needs, we may have to delay or discontinue the development of programs, license out programs earlier than expected, raise funds at significant discount or on other unfavorable terms, or sell all or part of us.
Operating Activities
Net cash used in operating activities was $29.9 million for the year ended December 31, 2009 compared to net cash provided by operating activities of $18.3 million for the year ended December 31, 2008. The increase in net cash used in operating activities was primarily due to Pfizer's up-front payment of $40 million received during the year ended December 31, 2008. We expect that cash used in operations will continue to increase in 2010 primarily related to the continued development of our therapeutic product pipeline, including bringing forward new product candidates into clinical development.
We have incurred and will continue to incur significant costs in the area of research and development, including preclinical and clinical trials, as our product candidates are developed. We plan to spend significant amounts to progress our current product candidates through the clinical trial and commercialization process as well as to develop additional product candidates. As our product candidates progress through the clinical trial process, we may be obligated to make significant milestone payments.
Investing Activities
Net cash provided by investing activities was $45.1 million for the year ended December 31, 2009 compared to $10.2 million for the year ended December 31, 2008. The increase in net cash provided by investing activities was primarily due to the $51.7 million in cash acquired in the CuraGen Merger in 2009 offset by higher purchases of marketable securities in 2009 and the cash acquired in the AVANT Merger in 2008. We expect to incur future facility cost as a result of continued capital expansion, renovation and replacements. Our investment in capital equipment is discretionary and there may be significant fluctuations on a quarterly basis.
60
Financing Activities
Net cash used in financing activities was $2.5 million for the year ended December 31, 2009 compared to net cash provided by financing activities of $10.9 million for the year ended December 31, 2008. The increase in net cash used in financing activities was primarily due the $3.0 million payment to Medarex in 2009 and Pfizer's one-time $10 million equity investment during the year ended December 31, 2008.
Other Liquidity Matters
Under the Pfizer Agreement, Pfizer made an upfront payment $40 million, an equity investment of $10.0 million and will fund all development costs for the licensed programs. We are also eligible to receive potential milestone payments exceeding $390.0 million for the successful development and commercialization of CDX-110 and additional EGFRvIII vaccine products, as well as royalties on any product sales.
AGGREGATE CONTRACTUAL OBLIGATIONS
We have entered into licensing agreements with several universities, research organizations and other corporations. Under the terms of these agreements, we have received licenses or options to license technology, specified patents or patent applications. Our licensing and development collaboration agreements generally provide for royalty payments equal to specified percentages of product sales, annual license maintenance fees and continuing patent prosecution costs. In addition, we have committed to make potential future milestone payments to third parties of up to approximately $116 million as part of our various collaborations including licensing and development programs. Payments under these agreements generally become due and payable only upon achievement of certain developmental, regulatory and/or commercial milestones. Because the achievement of these milestones had not occurred as of December 31, 2009, such contingencies have not been recorded in our financial statements. We expect to incur approximately $0.9 million of milestone payments in 2010.
The following table summarizes our contractual obligations (not including contingent royalty and milestone payments as described above) at December 31, 2009 and the effect such obligations and commercial commitments are expected to have on its liquidity and cash flow in future years. These obligations, commitments and supporting arrangements represent expected payments based on current operating forecasts, which are subject to change:
| |
Total | 2010 | 2011 - 2012 | 2013 - 2014 | Thereafter | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(In thousands) |
||||||||||||||||
Contractual obligations: |
|||||||||||||||||
Operating lease obligations(1) |
$ | 17,963 | $ | 2,465 | $ | 5,063 | $ | 5,047 | $ | 5,388 | |||||||
Other contractual obligations(2) |
2,607 | 2,607 | — | — | — | ||||||||||||
Other long-term liabilities(3) |
810 | 180 | 154 | 118 | 358 | ||||||||||||
Convertible subordinated debt(4) |
12,503 | — | 12,503 | — | — | ||||||||||||
CuraGen Severance obligations |
2,623 | 1,918 | 705 | — | — | ||||||||||||
Total contractual obligations |
$ | 36,506 | $ | 7,170 | $ | 18,425 | $ | 5,165 | $ | 5,746 | |||||||
- (1)
- Such
amounts primarily consist of payments for our facility leases and assumes the exercise of one five year renewal for our Fall River, MA facility.
- (2)
- Such amounts primarily consist of research and development commitments with third parties. Our obligation to pay certain of these amounts may increase or be reduced based on certain future events.
61
- (3)
- Such
amounts include the outstanding balance on a loan and note payable which accrue interest at 5.5% and is payable monthly.
- (4)
- Such amounts includes the outstanding balance on convertible subordinated debt due February 15, 2011 which accrues interest at 4% and is payable on February 15 and August 15.
RECENT ACCOUNTING PRONOUNCEMENTS
Refer to Note 2, "Summary of Significant Accounting Policies," in the accompanying notes to the consolidated financial statements for a discussion of recent accounting pronouncements.
OFF-BALANCE SHEET ARRANGEMENTS.
None.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We own financial instruments that are sensitive to market risk as part of our investment portfolio. Our investment portfolio is used to preserve our capital until it is used to fund operations, including our research and development activities. None of these market-risk sensitive instruments are held for trading purposes. We invest our cash primarily in money market mutual funds. These investments are evaluated quarterly to determine the fair value of the portfolio. From time to time, we invest our excess cash balances in marketable securities including municipal bond securities, U.S. government agency securities, and high-grade corporate bonds that meet high credit quality standards, as specified in our investment policy. Our investment policy seeks to manage these assets to achieve our goals of preserving principal and maintaining adequate liquidity. Because of the short-term nature of these investments, we do not believe we have material exposure due to market risk. The impact to our financial position and results of operations from likely changes in interest rates is not material.
We do not utilize derivative financial instruments. The carrying amounts reflected in the consolidated balance sheet of cash and cash equivalents, accounts receivables and accounts payable approximates fair value at December 31, 2009 due to the short-term maturities of these instruments.
62
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To
the Board of Directors and Stockholders of
Celldex Therapeutics, Inc.:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of stockholders' equity (deficit) and comprehensive loss, and of cash flows, present fairly, in all material respects, the financial position of Celldex Therapeutics, Inc. and its subsidiaries at December 31, 2009 and December 31, 2008, and the results of their operations and their cash flows for each of the two years in the period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Annual Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in which it accounts for business combinations in 2009.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Boston,
Massachusetts
March 12, 2010
63
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The
Board of Directors and Stockholders
Celldex Therapeutics, Inc.
We have audited the accompanying consolidated statement of operations, stockholders' equity (deficit) and comprehensive loss, and cash flow of Celldex Therapeutics, Inc. and subsidiary for the year ended December 31, 2007. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purposes of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated results of their operations and their cash flow for the year ended December 31, 2007 of Celldex Therapeutics, Inc. in conformity with U.S. generally accepted accounting principles.
/s/ Ernst & Young LLP
MetroPark,
New Jersey
May 7, 2008
64
CELLDEX THERAPEUTICS, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
| |
December 31, 2009 | December 31, 2008 | |||||||
|---|---|---|---|---|---|---|---|---|---|
ASSETS |
|||||||||
Current Assets: |
|||||||||
Cash and Cash Equivalents |
$ | 57,002 | $ | 44,257 | |||||
Marketable Securities |
25,451 | — | |||||||
Accounts and Other Receivables |
544 | 1,827 | |||||||
Prepaid and Other Current Assets |
979 | 992 | |||||||
Total Current Assets |
83,976 | 47,076 | |||||||
Property and Equipment, Net |
11,489 | 13,567 | |||||||
Intangible Assets, Net |
29,979 | 2,473 | |||||||
Goodwill |
8,965 | — | |||||||
Other Assets |
5,955 | 6,677 | |||||||
Total Assets |
$ | 140,364 | $ | 69,793 | |||||
LIABILITIES AND STOCKHOLDERS' EQUITY |
|||||||||
Current Liabilities: |
|||||||||
Accounts Payable |
$ | 1,445 | $ | 2,154 | |||||
Accrued Expenses |
5,615 | 3,841 | |||||||
Payable Due Medarex |
— | 2,957 | |||||||
Current Portion of Deferred Revenue |
5,191 | 4,931 | |||||||
Current Portion of Long-Term Liabilities |
2,156 | 218 | |||||||
Total Current Liabilities |
14,407 | 14,101 | |||||||
Deferred Revenue |
34,191 | 36,489 | |||||||
Convertible Subordinated Debt |
11,684 | — | |||||||
Other Long-Term Liabilities |
6,315 | 1,069 | |||||||
Total Liabilities |
66,597 | 51,659 | |||||||
Commitments and Contingent Liabilities (Notes 12 and 15) |
|||||||||
Stockholders' Equity: |
|||||||||
Convertible Preferred Stock, $.01 Par Value; 3,000,000 Shares Authorized; No Shares Issued and Outstanding at December 31, 2009 and 2008 |
— | — | |||||||
Common Stock, $.001 Par Value; 297,000,000 Shares Authorized; 31,685,061 and 15,789,756 Shares Issued and Outstanding at December 31, 2009 and 2008, respectively |
32 | 16 | |||||||
Additional Paid-In Capital |
228,863 | 136,661 | |||||||
Accumulated Other Comprehensive Income |
2,546 | 2,606 | |||||||
Accumulated Deficit |
(157,674 | ) | (121,149 | ) | |||||
Total Stockholders' Equity |
73,767 | 18,134 | |||||||
Total Liabilities and Stockholders' Equity |
$ | 140,364 | $ | 69,793 | |||||
The accompanying notes are an integral part of the consolidated financial statements.
65
CELLDEX THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| |
Year Ended December 31, 2009 |
Year Ended December 31, 2008 |
Year Ended December 31, 2007 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
REVENUE: |
|||||||||||
Product Development and Licensing Agreements |
$ | 5,662 | $ | 3,716 | $ | 466 | |||||
Contracts and Grants |
1,802 | 533 | 940 | ||||||||
Product Royalties |
7,716 | 3,207 | — | ||||||||
Total Revenue |
15,180 | 7,456 | 1,406 | ||||||||
OPERATING EXPENSE: |
|||||||||||
Research and Development |
26,169 | 22,636 | 9,892 | ||||||||
Royalty |
8,397 | 3,711 | — | ||||||||
Gain on Sale of Assets |
(604 | ) | — | — | |||||||
Charge for In-Process Research and Development |
— | 14,756 | — | ||||||||
General and Administrative |
17,119 | 14,748 | 6,905 | ||||||||
Amortization of Acquired Intangible Assets |
949 | 361 | 117 | ||||||||
Total Operating Expense |
52,030 | 56,212 | 16,914 | ||||||||
Operating Loss |
(36,850 | ) | (48,756 | ) | (15,508 | ) | |||||
Investment and Other Income, Net |
248 | 1,411 | 435 | ||||||||
Interest Expense |
(452 | ) | (156 | ) | — | ||||||
Net Loss Before Income Taxes |
(37,054 | ) | (47,501 | ) | (15,073 | ) | |||||
Income Tax Benefit |
529 | — | — | ||||||||
Net Loss |
$ | (36,525 | ) | $ | (47,501 | ) | $ | (15,073 | ) | ||
Basic and Diluted Net Loss Per Common Share (See Note 2) |
$ | (1.84 | ) | $ | (3.34 | ) | $ | (1.81 | ) | ||
Shares Used in Calculating Basic and Diluted Net Loss per Share (See Note 2) |
19,823 | 14,217 | 8,309 | ||||||||
The accompanying notes are an integral part of the consolidated financial statements.
66
CELLDEX THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (DEFICIT) AND COMPREHENSIVE LOSS
(In thousands, except share amounts)
| |
Common Stock Shares |
Common Stock Par Value |
Class A Common Stock Shares |
Class A Common Stock Par Value |
Additional Paid-In Capital |
Accumulated Other Comprehensive Income |
Accumulated Deficit |
Total Stockholders' Equity (Deficit) |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance at December 31, 2006 |
5,498,273 | $ | 5 | 2,811,147 | $ | 3 | $ | 71,323 | $ | 2,388 | $ | (58,575 | ) | $ | 15,144 | |||||||||||
Share-Based Compensation |
— | — | — | — | 1,605 | — | — | 1,605 | ||||||||||||||||||
Medarex Return of Capital |
— | — | — | — | (3,039 | ) | — | — | (3,039 | ) | ||||||||||||||||
Comprehensive Income (Loss): |
||||||||||||||||||||||||||
Net Loss |
— | — | — | — | — | — | (15,073 | ) | (15,073 | ) | ||||||||||||||||
Translation Adjustments |
— | — | — | — | — | 231 | — | 231 | ||||||||||||||||||
Total Comprehensive Loss |
(14,842 | ) | ||||||||||||||||||||||||
Balance at December 31, 2007 |
5,498,273 | $ | 5 | 2,811,147 | $ | 3 | $ | 69,889 | $ | 2,619 | $ | (73,648 | ) | $ | (1,132 | ) | ||||||||||
Exchange of Class A for Common Stock |
2,811,147 | 3 | (2,811,147 | ) | (3 | ) | — | — | — | — | ||||||||||||||||
Shares Issued to Medarex in Settlement of a Payable |
351,692 | 1 | — | — | 3,038 | — | — | 3,039 | ||||||||||||||||||
Shares Received in Connection with the AVANT Merger |
6,265,882 | 6 | — | — | 46,869 | — | — | 46,875 | ||||||||||||||||||
Shares Issued to Pfizer in connection with the CDX-110 Licensing Agreement |
781,250 | 1 | 10,866 | 10,867 | ||||||||||||||||||||||
Shares Issued to Duke University in Settlement of a Payable |
81,512 | — | 1,183 | 1,183 | ||||||||||||||||||||||
Share-Based Compensation |
— | — | — | — | 4,816 | — | — | 4,816 | ||||||||||||||||||
Comprehensive Loss: |
||||||||||||||||||||||||||
Net Loss |
— | — | — | — | — | — | (47,501 | ) | (47,501 | ) | ||||||||||||||||
Translation Adjustments |
— | — | — | — | — | (13 | ) | — | (13 | ) | ||||||||||||||||
Total Comprehensive Loss |
(47,514 | ) | ||||||||||||||||||||||||
Balance at December 31, 2008 |
15,789,756 | $ | 16 | — | $ | — | $ | 136,661 | $ | 2,606 | $ | (121,149 | ) | $ | 18,134 | |||||||||||
Shares Issued in Connection with the CuraGen Merger |
15,722,713 | 16 | — | — | 88,227 | — | — | 88,243 | ||||||||||||||||||
Shares Issued under Stock Option and Employee Stock Purchase Plans |
172,592 | — | — | — | 917 | — | — | 917 | ||||||||||||||||||
Share-Based Compensation |
— | — | — | — | 3,058 | — | — | 3,058 | ||||||||||||||||||
Comprehensive Loss: |
||||||||||||||||||||||||||
Net Loss |
— | — | — | — | — | — | (36,525 | ) | (36,525 | ) | ||||||||||||||||
Translation Adjustments |
— | — | — | — | — | (12 | ) | — | (12 | ) | ||||||||||||||||
Net Change in Unrealized Loss on Marketable Securities |
— | — | — | — | — | (48 | ) | — | (48 | ) | ||||||||||||||||
Total Comprehensive Loss |
(36,585 | ) | ||||||||||||||||||||||||
Balance at December 31, 2009 |
31,685,061 | $ | 32 | — | $ | — | $ | 228,863 | $ | 2,546 | $ | (157,674 | ) | $ | 73,767 | |||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
67
CELLDEX THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
| |
Year Ended December 31, 2009 |
Year Ended December 31, 2008 |
Year Ended December 31, 2007 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Cash Flows From Operating Activities: |
||||||||||||
Net Loss |
$ | (36,525 | ) | $ | (47,501 | ) | $ | (15,073 | ) | |||
Adjustments to Reconcile Net Loss to Cash Provided by (Used in) Operating Activities: |
||||||||||||
Depreciation and Amortization |
2,583 | 2,176 | 710 | |||||||||
Amortization of Intangible Assets |
949 | 361 | 117 | |||||||||
Realized Loss on Sales and Maturities of Marketable Securities |
24 | — | — | |||||||||
(Gain) Loss on Sale or Disposal of Assets |
(556 | ) | 331 | — | ||||||||
Stock-Based Compensation Expense |
3,058 | 4,816 | 1,605 | |||||||||
Non-Cash Interest Expense |
181 | — | — | |||||||||
Non-Cash Tax Benefit |
(529 | ) | — | — | ||||||||
In-Process Research and Development |
— | 14,756 | — | |||||||||
Changes in Operating Assets and Liabilities, Net of Acquisitions |
||||||||||||
Accounts and Other Receivables |
1,283 | (1,656 | ) | 4,167 | ||||||||
Prepaid and Other Current Assets |
743 | 9,980 | (587 | ) | ||||||||
Other Assets |
722 | (6,414 | ) | — | ||||||||
Accounts Payable and Accrued Expenses |
(2,463 | ) | 1,221 | (28 | ) | |||||||
Deferred Revenue |
(2,038 | ) | 40,116 | 42 | ||||||||
Other Long-Term Liabilities |
2,699 | 94 | (79 | ) | ||||||||
Net Cash (Used in) Provided by Operating Activities |
(29,869 | ) | 18,280 | (9,126 | ) | |||||||
Cash Flows From Investing Activities: |
||||||||||||
Cash Acquired in AVANT Merger, Net of Transaction Costs |
— | 10,750 | — | |||||||||
Cash Acquired in CuraGen Merger |
51,654 | — | — | |||||||||
Sales and Maturities of Marketable Securities |
2,674 | — | — | |||||||||
Purchases of Marketable Securities |
(9,559 | ) | — | — | ||||||||
Other Non Current Assets |
— | — | (335 | ) | ||||||||
Restricted Cash Deposits |
— | (2 | ) | (3 | ) | |||||||
Acquisition of Property and Equipment |
(528 | ) | (1,305 | ) | (75 | ) | ||||||
Proceeds from Sale or Disposal of Assets |
850 | 712 | — | |||||||||
Net Cash Provided by (Used in) Investing Activities |
45,091 | 10,155 | (413 | ) | ||||||||
Cash Flows From Financing Activities: |
||||||||||||
Net Proceeds from Stock Issuances |
668 | 10,867 | — | |||||||||
Related Party Loan Due to Medarex |
(2,957 | ) | 160 | 265 | ||||||||
Payment of Other Long-Term Liabilities |
(176 | ) | (102 | ) | — | |||||||
Net Cash (Used in) Provided by Financing Activities |
(2,465 | ) | 10,925 | 265 | ||||||||
Effect of Exchange Rate Changes on Cash and Cash Equivalents |
(12 | ) | (13 | ) | 184 | |||||||
Net Increase (Decrease) in Cash and Cash Equivalents |
12,745 | 39,347 | (9,090 | ) | ||||||||
Cash and Cash Equivalents at Beginning of Period |
44,257 | 4,910 | 14,000 | |||||||||
Cash and Cash Equivalents at End of Period |
$ | 57,002 | $ | 44,257 | $ | 4,910 | ||||||
Supplemental Disclosure of Non-Cash Flow Information |
||||||||||||
Shares Received in Exchange in the Merger |
$ | — | $ | 46,252 | $ | — | ||||||
Shares Issued to Medarex in Settlement of a Payable |
$ | — | $ | 3,039 | $ | — | ||||||
Shares Issued to Duke University in Settlement of a Payable |
$ | — | $ | 1,183 | $ | — | ||||||
Shares Issued to Executive Officers |
$ | 250 | $ | — | $ | — | ||||||
Supplemental Disclosure of Cash Flow Information |
||||||||||||
Cash Paid for Interest |
$ | 157 | $ | 142 | $ | — | ||||||
The accompanying notes are an integral part of the consolidated financial statements.
68
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(1) NATURE OF BUSINESS AND OVERVIEW
Celldex Therapeutics, Inc. (the "Company" or "Celldex") is an integrated biopharmaceutical company that applies its comprehensive Precision Targeted Immunotherapy Platform to generate a pipeline of candidates to treat cancer and other difficult-to-treat diseases. The Company's immunotherapy platform includes a complementary portfolio of monoclonal antibodies, antibody-targeted vaccines and immunomodulators to create novel disease-specific drug candidates. The Company's collaboration with GlaxoSmithKline ("Glaxo") resulted in the commercialization of Rotarix®, an oral human rotavirus vaccine. In April 2008, the Company and Pfizer Inc. ("Pfizer") entered into a License and Development Agreement (the "Pfizer Agreement") under which Pfizer was granted an exclusive worldwide license to a therapeutic cancer vaccine candidate, CDX-110, in Phase 2 development for the treatment of glioblastoma multiforme ("GBM"). The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Under the Pfizer Agreement, Pfizer made an upfront payment to the Company of $40 million and made a $10 million equity investment in the Company. Pfizer will fund all development costs for these programs. The Company is also eligible to receive potential milestone payments exceeding $390 million for the successful development and commercialization of CDX-110 and additional EGFRvIII vaccine products, as well as royalties on any product sales. The Company's other clinical and preclinical product candidates address large market opportunities for which the Company believes current therapies are inadequate or non-existent.
AVANT Merger
On March 7, 2008, the Company (formerly known as AVANT Immunotherapeutics, Inc.) ("AVANT") merged with Celldex Research Corporation (formerly known as Celldex Therapeutics, Inc.) ("Celldex Research"), a privately-held company, (the "AVANT Merger"). Effective October 1, 2008, the Company changed its name from AVANT Immunotherapeutics, Inc. to Celldex Therapeutics, Inc.
The AVANT Merger was accounted for using the former purchase method of accounting and was treated as an acquisition by Celldex Research of AVANT, with Celldex Research being considered the accounting acquirer even though AVANT was the issuer of common stock and the surviving legal entity in the transaction. Under the former purchase method of accounting, the deemed purchase price was allocated to AVANT's underlying tangible and identifiable intangible assets acquired and liabilities assumed based upon the respective fair value of each with any excess deemed purchase price allocated to goodwill. The valuation analysis conducted by the Company determined that the fair value of assets acquired and the fair value of liabilities assumed by Celldex Research exceeded the purchase price for AVANT, resulting in negative goodwill of approximately $6.0 million. The negative goodwill was allocated to all of the acquired assets that were non-financial and non-current assets, including property and equipment, identifiable intangible assets, and in-process research and development ("IPR&D").
Because Celldex Research was determined to be the acquirer for accounting purposes, the historical financial statements of Celldex Research became the historical financial statements of the Company as of the closing of the AVANT Merger. Accordingly, the financial statements of the Company prior to the AVANT Merger reflect the financial position, results of operations and cash flows of Celldex Research, which during the historical periods presented in the accompanying consolidated financial statements, was then majority-owned by Medarex, Inc. ("Medarex"). Following the AVANT Merger, the financial statements reflect the financial position, results of operation and cash flows of the combined companies. Accordingly, this report reflects the financial condition, results of operations and liquidity of the combined companies at December 31, 2009 and 2008 and for the period from the AVANT Merger through December 31, 2009.
69
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(1) NATURE OF BUSINESS AND OVERVIEW (Continued)
Acquisition of CuraGen Corporation ("CuraGen")
As more fully discussed in Note 19, on October 1, 2009, CuraGen, a former publicly-traded company, merged with a wholly-owned subsidiary ("Merger Sub") of the Company (the "CuraGen Merger") in accordance with a definitive merger agreement dated May 28, 2009 (the "CuraGen Merger Agreement"). Following the CuraGen Merger, the financial statements reflect the financial position, results of operation and cash flows of the combined companies. Accordingly, this report reflects the financial condition, results of operations and liquidity of the combined companies at December 31, 2009 and for the period from the CuraGen Merger through December 31, 2009.
On December 31, 2009, the Company completed the merger of the Merger Sub with and into Celldex pursuant to a short-form merger effected under Delaware law. As a result, the separate corporate existence of CuraGen has ceased and the Company has succeeded to all rights, privileges, powers and franchises of CuraGen.
Capital Requirements
At December 31, 2009, the Company had cash, cash equivalents and marketable securities of $82.5 million; working capital of $69.6 million; and 4% convertible subordinated debt ("CuraGen Debt") due in February 2011 of $12.5 million. The Company incurred a loss of $36.5 million for the year ended December 31, 2009. Net cash used in operations for the year ended December 31, 2009 was $29.9 million. The Company believes that the cash, cash equivalents and marketable securities at December 31, 2009 in addition to the cash inflows from existing grants and collaborations and interest income on invested funds will be sufficient to meet estimated working capital requirements and fund planned operations for at least the next twelve months. The working capital requirements of the Company are dependent on several factors including, but not limited to, the costs associated with research and development programs, preclinical and clinical studies, manufacture of clinical materials, the scope of collaborative arrangements.
During the next twelve to twenty-four months, the Company may take steps to raise additional capital to meet its long-term liquidity needs including, but not limited to, the licensing of technology programs with existing or new collaborative partners, possible business combinations, or the issuance of common stock via private placements or public offerings. While the Company continues to seek capital through a number of means, there can be no assurance that additional financing will be available on acceptable terms, if at all, particularly in light of the recent disruptions in the financial markets. The Company's negotiating position in capital-raising efforts may worsen as existing resources are used. There is also no assurance that the Company will be able to enter into further collaborative relationships. Additional equity financing may be dilutive to the Company's stockholders; debt financing, if available, may involve significant cash payment obligations and covenants that restrict the Company's ability to operate as a business; and licensing or strategic collaborations may result in royalties or other terms that reduce the Company's economic potential from products under development. If the Company is unable to raise the funds necessary to meet its long-term liquidity needs, it may have to (i) delay or discontinue the development of programs, (ii) license out programs earlier than expected, (iii) raise funds at significant discount or on other unfavorable terms, or (iv) sell all or a part of the Company.
70
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The consolidated financial statements reflect the operations of the Company and its wholly-owned subsidiaries. All intercompany transactions have been eliminated. The Company operates in one segment, which is the business of development, manufacturing and commercialization of novel therapeutics for human health care.
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America ("U.S. GAAP") requires management to make estimates and use assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the dates of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with a maturity date of 90 days or less at the date of purchase to be cash equivalents. Cash equivalents consist principally of money market funds and debt securities.
Marketable Securities
The Company invests its excess cash balances in marketable securities including municipal bond securities, U.S. government agency securities, and high-grade corporate bonds. The Company classifies all of its marketable securities as current assets on the consolidated balance sheets because they are available-for-sale and available to fund current operations. Marketable securities are stated at fair value with their unrealized gains and losses included as a component of accumulated other comprehensive income (loss), which is a separate component of stockholders' equity, until such gains and losses are realized. The fair value of these securities is based on quoted prices for identical or similar assets. If a decline in the fair value is considered other-than-temporary, based on available evidence, the unrealized loss is transferred from other comprehensive income (loss) to the consolidated statements of operations. Realized gains and losses are determined on the specific identification method and are included in investment and other income, net.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk primarily consist of cash, cash equivalents, marketable securities and accounts receivable. The Company invests its cash, cash equivalents and marketable securities in debt instruments and interest bearing accounts at major financial institutions in excess of insured limits. The Company mitigates credit risk by limiting the investment type and maturity to securities that preserve capital, maintain liquidity and have a high credit quality.
The Company has not historically experienced credit losses from its accounts receivable and therefore has not established an allowance for doubtful accounts. Receivables of $0.2 million and $1.4 million were due from Pfizer at December 31, 2009 and 2008, respectively.
71
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Revenue from Glaxo and Pfizer represented 52% and 34% for the year ended December 31, 2009 and 50% and 38% for the year ended December 31, 2008, of total Company revenue, respectively. For the year ended December 31, 2007, certain other customers represented more than 10% of total Company revenue and these customers are not expected to represent 10% or more of total Company revenue in the future.
Fair Value of Financial Instruments
The Company enters into various types of financial instruments in the normal course of business. The carrying amounts of the Company's cash and cash equivalents, accounts receivable, accounts payable and accrued expenses approximate their fair values due to the short-term nature of these items. See Note 3 for additional information.
Property and Equipment
The treatment of costs to construct property and equipment depends on the nature of the costs and the stage of construction. Costs incurred in the project planning, design, construction and installation phases are capitalized as part of the cost of the asset. The Company stops capitalizing these costs when the asset is substantially complete and ready for its intended use. For manufacturing property and equipment, the Company also capitalizes the cost of validating these assets for the underlying manufacturing process. The Company completes the capitalization of validation costs when the asset is substantially complete and ready for its intended use. Costs capitalized include incremental labor and fringe benefits, and direct consultancy services. The Company capitalizes interest cost as part of the historical cost of acquiring certain assets during the period of time required to get the asset ready for its intended use.
Property and equipment is stated at cost and depreciated over the estimated useful lives of the related assets using the straight-line method. Laboratory equipment and office furniture and equipment are depreciated over five years and computer equipment is depreciated over three years. Manufacturing equipment is amortized over seven to ten years. Leasehold improvements are amortized over the shorter of the estimated useful life or the non-cancelable term of the related lease, including any renewals that are reasonably assured of occurring. Property and equipment under construction is classified as construction in progress and is depreciated or amortized only after the asset is placed in service. Expenditures for maintenance and repairs are charged to expense whereas the costs of significant improvements which extend the life of the underlying asset are capitalized. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation are eliminated and any resulting gain or loss is reflected in the Company's consolidated statements of operations.
Business Combinations
On January 1, 2009, the Company adopted the new acquisition method of accounting for business combinations that are completed after January 1, 2009, including the CuraGen Merger. The Company assigns the value of the consideration transferred to acquire a business to the tangible assets and identifiable intangible assets acquired and liabilities assumed on the basis of their fair values at the date of acquisition. The Company assesses the fair value of assets, including intangible assets such as IPR&D, using a variety of methods including present-value models. Each asset is measured at fair value from the perspective of a market participant. The method used to estimate the fair values of
72
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
IPR&D assets incorporates significant assumptions regarding the estimates a market participant would make in order to evaluate an asset, including a market participant's assumptions regarding the probability of completing IPR&D projects, which would require obtaining regulatory approval for marketing of the associated drug candidate; a market participant's estimates regarding the timing of and the expected costs to complete IPR&D projects; a market participant's estimates of future cash flows from potential product sales; and the appropriate discount rates for a market participant. Transaction costs and restructuring costs associated with the transaction are expensed as incurred.
For acquisitions completed prior to January 1, 2009, including the AVANT Merger, the Company expensed the fair value of IPR&D of $14.8 million to research and development expense as of the acquisition date and included transaction costs associated with the business combination as part of the cost of the acquired company. See Notes 18 and 19 for discussion of the AVANT and CuraGen Merger, respectively.
Intangible Assets
IPR&D assets acquired in a business combination initially are recorded at fair value and accounted for as indefinite-lived intangible assets. These assets are maintained on the Company's consolidated balance sheets until either the project underlying them is completed or the assets become impaired. If a project is completed, the carrying value of the related intangible asset is amortized over the remaining estimated life of the asset beginning in the period in which the project is completed. If a project becomes impaired or is abandoned, the carrying value of the related intangible asset is written down to its fair value and an impairment charge is taken in the period in which the impairment occurs. IPR&D assets will be tested for impairment on an annual basis during the third quarter, or earlier if impairment indicators are present.
Intangible assets acquired in a business combination with a finite life are recorded at fair value and amortized over the greater of economic consumption or on a straight-line basis over their estimated useful life.
Goodwill
The difference between the purchase price and the fair value of assets acquired and liabilities assumed in a business combination is allocated to goodwill. Goodwill will be evaluated for impairment on an annual basis during the third quarter, or earlier if impairment indicators are present.
Impairment of Long-Lived Assets
The Company evaluates the recoverability of its long-lived assets, including property and equipment, and intangible assets when circumstances indicate that an event of impairment may have occurred. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written-down to their estimated fair values.
73
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Revenue Recognition
The Company accounts for revenue arrangements that include multiple deliverables as separate units of accounting if (i) the delivered item has value to the customer on a standalone basis, (ii) there is objective and reliable evidence of the fair value of the undelivered items and (iii) if the right of return exists, delivery of the undelivered items is considered probable and substantially in the control of the vendor. If these criteria are not met, the revenue elements are considered a single unit of accounting for purposes of revenue recognition.
The Company has entered into various license and development agreements with pharmaceutical and biotechnology companies. The terms of the agreements typically include non-refundable license fees, funding of research and development, payments based upon achievement of certain milestones and royalties on net product sales. Non-refundable license fees are recognized as revenue when the Company has a contractual right to receive such payments, provided that (i) a contractual arrangement exists, (ii) the contract price is fixed or determinable, (iii) the collection of the resulting receivable is reasonably assured and (iv) the Company has no further performance obligations under the license agreement. Upfront non-refundable fees associated with license and development agreements where the Company has continuing performance obligations under the terms of the agreement are recorded as deferred revenue and recognized as revenue over the estimated service period as the Company completes its obligations. Where the Company's level of effort is relatively constant over the performance period or no other pattern is estimable, the revenue is recognized on a straight-line basis. Revenue is limited to the lesser of the cumulative amount of payments due or the cumulative amount of revenue earned, as determined using the straight-line basis, as of the period ending date. If the estimated service period is subsequently modified, the period over which the upfront fee is recognized is modified accordingly on a prospective basis. The determination of the performance period involves judgment on management's part. Funding of research and development is recognized as revenue over the term of the applicable contract as costs are incurred related to that contract.
Milestone payments are recognized as revenue upon the achievement of mutually agreed milestones, provided that there is no continuing performance obligations associated with the milestone payment. Revenues from milestone payments related to arrangements under which the Company has continuing performance obligations are recognized as revenue upon achievement of the milestone only if all of the following conditions are met: (i) the milestone payments are non-refundable; (ii) achievement of the milestone was not reasonably assured at the inception of the arrangement; (iii) substantive effort is involved in achieving the milestone; and, (iv) the amount of the milestone is reasonable in relation to the effort expended or the risk associated with achievement of the milestone. If any of these conditions are not met, the milestone payments are deferred and recognized as revenue over the term of the arrangement as the Company completes its performance obligations.
The Company has capitalized and deferred costs incurred in connection with the one-time signing and upfront payment (the initial deliverable) received with respect to a multiple deliverable arrangement. If there is deemed a single unit of accounting for such an arrangement, the capitalized deferred costs are amortized over the expected performance period of the arrangement.
Payments received to fund certain research activities are recognized as revenue in the period in which the research activities are performed. Revenue from contracts and grants is recognized as the services are performed and recorded as effort is expended on the contracted work and billed to the
74
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
government or the Company's contractual partner. Payments received in advance that are related to future performance are deferred and recognized as revenue when the research projects are performed.
Product royalty revenue consists of payments received from licensees for a portion of sales proceeds from products that utilize the Company's licensed technologies and are recognized when the amount of and basis for such royalty payments are reported to us in accurate and appropriate form and in accordance with the related license agreement.
Research and Development Expenses
Research and development costs, including internal and contract research costs, are expensed as incurred. Research and development expenses consist mainly of clinical trial costs, manufacturing of clinical material, toxicology and other studies, personnel costs, depreciation, license fees and funding of outside research.
Patent Costs
Patent costs are expensed as incurred. Certain patent costs are reimbursed by the Company's product development and licensing partners. Any reimbursed patent costs are recorded as product development and licensing agreement revenues in the Company's financial statements.
Stock-Based Compensation
The Company records stock-based compensation expense for all stock-based awards made to employees and directors based on the estimated fair values of the stock-based awards expected to vest at the grant date and is adjusted, if necessary, to reflect actual forfeitures. Compensation expense for all stock-based awards to employees and directors is recognized using the straight-line method over the term of vesting or performance.
The Company records stock-based compensation expense for stock options granted to non-employees based on the fair value of the stock options which is re-measured over the vesting term resulting in periodic adjustments to stock-based compensation expense.
Foreign Currency Translation
The functional currency of the Company's foreign subsidiary is the local currency. All assets and liabilities of the foreign subsidiary are re-measured into U.S. dollars at rates of exchange in effect at the end of the year. Revenue and expense amounts are re-measured using the average exchange rates for the period. Net unrealized gains and losses resulting from foreign currency translation are included in other comprehensive income (loss). At December 31, 2009 and December 31, 2008, accumulated other comprehensive income includes a net unrealized gain related to foreign currency translation of $2.6 million.
75
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Income Taxes
The Company uses the asset and liability method to account for income taxes, including the recognition of deferred tax assets and deferred tax liabilities for the anticipated future tax consequences attributable to differences between financial statement amounts and their respective tax basis. Quarterly, the Company reviews its deferred tax assets for recovery. A valuation allowance is established when the Company believes that it is more likely than not that its deferred tax assets will not be realized. Changes in valuation allowances from period to period are included in the Company's tax provision in the period of change.
The Company records uncertain tax positions in the financial statements only if it is more likely than not that the uncertain tax position will be sustained upon examination by the taxing authorities. The Company records interest and penalties related to uncertain tax positions in income tax expense.
Comprehensive Loss
Comprehensive loss is comprised of net loss and certain changes in stockholders' equity that are excluded from net loss. The Company includes foreign currency translation adjustments for subsidiaries in which the functional currency is not the U.S. dollar and unrealized gains and losses on marketable securities in other comprehensive loss. The consolidated statements of stockholders' equity (deficit) and comprehensive loss reflect total comprehensive loss for the years ended December 31, 2009, 2008 and 2007.
Net Loss Per Share
Basic net loss per common share is based upon the weighted-average number of common shares outstanding during the period, excluding restricted stock that has been issued but is not yet vested. Diluted net loss per common share is based upon the weighted-average number of common shares outstanding during the period plus additional weighted-average potentially dilutive common shares outstanding during the period when the effect is dilutive. The potentially dilutive common shares that have not been included in the net loss per common share calculations because the effect would have been anti-dilutive are as follows:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | |||||||
Stock options |
3,576,159 | 2,070,993 | 787,440 | |||||||
Convertible debt |
353,563 | — | — | |||||||
Restricted stock |
16,000 | — | — | |||||||
|
3,945,722 | 2,070,993 | 787,440 | |||||||
Recent Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board ("FASB") or other standard setting bodies that are adopted by the Company as of the specified effective date. Unless otherwise discussed, the Company believes that the impact of recently issued standards that are not yet effective will not have a material impact on the Company's financial position or results of operations upon adoption.
76
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
In October 2009, the FASB issued a new U.S. GAAP accounting standard which amends existing revenue recognition accounting guidance to provide accounting principles and application guidance on whether multiple deliverables exist, how the arrangement should be separated, and the consideration allocated. This guidance eliminates the requirement to establish objective evidence of fair value of undelivered products and services and instead provides for separate revenue recognition based upon management's estimate of the selling price for an undelivered item when there is no other means to determine the fair value of that undelivered item. The existing guidance previously required that the fair value of the undelivered item be the price of the item either sold in a separate transaction between unrelated third parties or the price charged for each item when the item is sold separately by the vendor. This was difficult to determine when the product was not individually sold because of its unique features. Under the existing guidance if the fair value of all of the elements in the arrangement was not determinable, then revenue was deferred until all of the items were delivered or fair value was determined. The Company expects to adopt the standard on January 1, 2010 on a prospective basis. While the Company does not expect the adoption of this standard to have a material impact on its financial position and results of operations, this standard may impact the Company in the event it completes future transactions or modifies existing collaborative relationships.
In January 2010, the FASB issued updated guidance to amend the disclosure requirements related to recurring and nonrecurring fair value measurements. This update requires new disclosures on significant transfers of assets and liabilities between Level 1 and Level 2 of the fair value hierarchy (including the reasons for these transfers) and the reasons for any transfers in or out of Level 3. This update also requires a reconciliation of recurring Level 3 measurements about purchases, sales, issuances and settlements on a gross basis. In addition to these new disclosure requirements, this update clarifies certain existing disclosure requirements. For example, this update clarifies that reporting entities are required to provide fair value measurement disclosures for each class of assets and liabilities rather than each major category of assets and liabilities. This update also clarifies the requirement for entities to disclose information about both the valuation techniques and inputs used in estimating Level 2 and Level 3 fair value measurements. This update will become effective for the Company with the interim and annual reporting period beginning January 1, 2010, except for the requirement to provide the Level 3 activity of purchases, sales, issuances, and settlements on a gross basis, which will become effective for the Company with the interim and annual reporting period beginning January 1, 2011. The Company will not be required to provide the amended disclosures for any previous periods presented for comparative purposes. Other than requiring additional disclosures, adoption of this update will not have a material effect on the Company's consolidated financial statements.
(3) FAIR VALUE MEASUREMENTS
The fair value of the Company's assets and liabilities reflects the Company's estimate of the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants on the measurement date. Market participants are buyers and sellers in the principal market that are (i) independent, (ii) knowledgeable, (iii) able to transact, and (iv) willing to transact.
In connection with measuring the fair value of its assets and liabilities, the Company seeks to maximize the use of observable inputs (market data obtained from sources independent from the Company) and to minimize the use of unobservable inputs (the Company's assumptions about how
77
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(3) FAIR VALUE MEASUREMENTS (Continued)
market participants would price assets and liabilities). The following fair value hierarchy is used to classify assets and liabilities based on the observable inputs and unobservable inputs used in order to value the assets and liabilities:
| Level 1: | Observable inputs such as quoted prices in active markets for identical assets or liabilities. An active market for an asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis. | |
Level 2: |
Observable inputs other than Level 1 prices, such as quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active. |
|
Level 3: |
Unobservable inputs based on the Company's assessment of the assumptions that market participants would use in pricing the asset or liability. |
The following tables set forth the Company's financial assets subject to fair value measurements:
| |
As of December 31, 2009 |
Level 1 | Level 2 | Level 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(In thousands) |
||||||||||||
Cash equivalents |
$ | 53,780 | $ | 53,780 | — | — | |||||||
Marketable securities |
$ | 25,451 | — | $ | 25,451 | — | |||||||
|
$ | 79,231 | $ | 53,780 | $ | 25,451 | — | ||||||
| |
As of December 31, 2008 |
Level 1 | Level 2 | Level 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(In thousands) |
||||||||||||
Cash equivalents |
$ | 43,457 | $ | 43,457 | — | — | |||||||
|
$ | 43,457 | $ | 43,457 | — | — | |||||||
Intangible assets acquired in connection with the CuraGen Merger were accounted for as described in Note 19. The estimated fair value of these nonfinancial assets was based on Level 3 inputs.
78
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(4) MARKETABLE SECURITIES
A summary of cash, cash equivalents and marketable securities as of December 31, 2009 is shown below:
| |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(In thousands) |
|||||||||||||||
Cash and cash equivalents |
||||||||||||||||
Cash and money market funds |
$ | 57,002 | $ | — | $ | — | $ | 57,002 | ||||||||
Total cash and cash equivalents |
$ | 57,002 | $ | — | $ | — | $ | 57,002 | ||||||||
Marketable securities |
||||||||||||||||
U.S. government obligations |
||||||||||||||||
Maturing in one year or less |
$ | 9,698 | $ | 5 | $ | — | $ | 9,703 | ||||||||
Maturing after one year through two years |
7,129 | 6 | 22 | 7,113 | ||||||||||||
Total U.S. government obligations |
$ | 16,827 | $ | 11 | $ | 22 | $ | 16,816 | ||||||||
Corporate debt securities |
||||||||||||||||
Maturing in one year or less |
$ | — | $ | — | $ | — | $ | — | ||||||||
Maturing after one year through two years |
8,672 | — | 37 | 8,635 | ||||||||||||
Total corporate debt securities |
$ | 8,672 | $ | — | $ | 37 | $ | 8,635 | ||||||||
Total marketable securities |
$ | 25,499 | $ | 11 | $ | 59 | $ | 25,451 | ||||||||
Total cash, cash equivalents and marketable securities |
$ | 82,501 | $ | 11 | $ | 59 | $ | 82,453 | ||||||||
As of December 31, 2009, unrealized losses in the portfolio were primarily due to increases in interest rates. The marketable securities held by the Company were high investment grade and there were no marketable securities that have been in a continuous unrealized loss position for more than 12 months at December 31, 2009. The Company did not consider these investments to be other-than-temporarily impaired as of December 31, 2009.
(5) STOCK-BASED COMPENSATION
At December 31, 2009, the Company had two stock-based compensation plans: the 2004 Employee Stock Purchase Plan (the "2004 ESPP Plan") and the 2008 Stock Option and Incentive Plan (the "2008 Plan").
Employee Stock Purchase Plan
At December 31, 2009, a total of 62,500 shares of common stock are reserved for issuance under the 2004 ESPP Plan. Under the 2004 ESPP Plan, each participating employee may purchase up to 250 shares of common stock per year, through payroll deductions, at a purchase price equal to 85% of the lower of the fair market value of the common stock at either the beginning of the offering period or the applicable exercise date. During the year ended December 31, 2009, the Company issued 2,979 shares under the 2004 ESPP Plan. During the year ended December 31, 2008, the Company issued no shares under the 2004 ESPP Plan. At December 31, 2009, 56,906 shares were available for issuance under the 2004 ESPP Plan.
79
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(5) STOCK-BASED COMPENSATION (Continued)
Employee Stock Option and Incentive Plan
The 2008 Plan permits the granting of incentive stock options (intended to qualify as such under Section 422A of the Internal Revenue Code of 1986, as amended), non-qualified stock options, stock appreciation rights, performance share units, restricted stock and other awards of restricted stock in lieu of cash bonuses to employees, consultants and outside directors.
At December 31, 2009, the 2008 Plan allowed for a maximum of 3,900,000 shares of common stock to be issued prior to October 19, 2017. The Company's board of directors determines the term of each option, option price, and number of shares for which each option is granted and the rate at which each option vests. Options generally vest over a period not to exceed four years. The term of each option cannot exceed ten years (five years for options granted to holders of more than 10% of the voting stock of the Company) and the exercise price of stock options cannot be less than the fair market value of the common stock at the date of grant (110% of fair market value for incentive stock options granted to holders of more than 10% of the voting stock of the Company). The 2008 Plan also provides for discretionary grants of non-qualified stock options to non-employee directors. Vesting of all employee and non-employee director stock option awards is accelerated upon a change in control as defined in the 2008 Plan.
In connection with the AVANT Merger, the Company assumed the obligations of Celldex Research under Celldex Research's 2005 Equity Incentive Plan (the "Celldex Research 2005 Plan") and each outstanding option to purchase Celldex Research common stock (a "Celldex Research Stock Option") granted under the Celldex Research 2005 Plan. Each Celldex Research Stock Option assumed by the Company is deemed to constitute an option to acquire, on the same terms and conditions as were applicable under the Celldex Research 2005 Plan, shares of the Company's common stock that have been adjusted consistent with the ratio at which the Company's common stock was issued in exchange for Celldex Research's common stock in the AVANT Merger. As of March 7, 2008, the Company assumed options to acquire 1,446,913 shares of its common stock at a weighted average exercise price of $8.35. The Celldex Research Stock Options generally vest over a two-to four-year period and the term of each option cannot exceed ten years from the date of grant. No additional awards will be issued under the Celldex Research 2005 Plan.
In connection with the CuraGen Merger, the Company assumed the obligations of CuraGen under CuraGen's 2007 Stock Plan (the "CuraGen 2007 Plan") and each outstanding option to purchase CuraGen common stock (a "CuraGen Stock Option") granted under the CuraGen 2007 Plan. Each CuraGen Stock Option assumed by the Company is deemed to constitute an option to acquire, on the same terms and conditions as were applicable under the CuraGen 2007 Plan, shares of the Company's common stock that have been adjusted consistent with the ratio at which the Company's common stock was issued in exchange for CuraGen's common stock in the CuraGen Merger. As of October 1, 2009, the Company assumed options to acquire 931,315 shares of its common stock with a weighted average exercise price of $3.17. As of October 1, 2009, all of the CuraGen Stock Options were fully vested except for 8,993 shares which generally vest over a two year period. No additional awards will be issued under the CuraGen 2007 Plan. The fair value of the CuraGen Stock Options that were attributed to precombination service was included in the CuraGen Merger consideration.
80
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(5) STOCK-BASED COMPENSATION (Continued)
A summary of stock option activity under the 2008 Plan, CuraGen 2007 Plan and Celldex Research 2005 Plan for the year ended December 31, 2009 is as follows:
| |
Shares | Weighted Average Exercise Price Per Share |
Weighted Average Remaining Contractual Term (In Years) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Options Outstanding at January 1, 2009 |
2,070,993 | $ | 8.39 | 8.7 | ||||||
Granted |
757,579 | 8.27 | ||||||||
CuraGen Stock Options assumed |
931,315 | 3.17 | ||||||||
Exercised |
(124,273 | ) | 5.21 | |||||||
Canceled/forfeited |
(34,519 | ) | 8.73 | |||||||
Expired |
(24,936 | ) | 9.79 | |||||||
Options Outstanding at December 31, 2009 |
3,576,159 | $ | 7.10 | 6.6 | ||||||
Options Vested and Expected to Vest at December 31, 2009 |
3,362,775 | $ | 7.03 | 6.5 | ||||||
Options Exercisable at December 31, 2009 |
2,506,684 | $ | 6.63 | 5.9 | ||||||
Shares Available for Grant under the 2008 Plan |
2,494,595 | |||||||||
The total intrinsic value of stock options exercised during the year ended December 31, 2009 was $0.3 million. No stock options were exercised during the year ended December 31, 2008. The weighted average grant-date fair value of stock options granted during the years ended December 31, 2009 and 2008 was $5.29 and $4.37, respectively. The total fair value of stock options vested during the year ended December 31, 2009 and 2008 was $2.6 million and $2.8 million, respectively.
The aggregate intrinsic value of stock options outstanding at December 31, 2009 was $1.5 million. The aggregate intrinsic value of stock options vested and expected to vest at December 31, 2009 was $1.5 million. As of December 31, 2009, total compensation cost related to non-vested employee and non-employee director stock options not yet recognized was approximately $3.5 million, net of estimated forfeitures, which is expected to be recognized as expense over a weighted average period of 2.6 years.
Shares Issued to Executive Officers
In January 2009, the Company granted 29,340 shares of common stock from the 2008 Plan to its executive officers. The value of these shares of $0.3 million was expensed during the year ended December 31, 2008.
81
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(5) STOCK-BASED COMPENSATION (Continued)
Restricted Stock
In December 2009, the Company granted 2,000 shares of restricted stock to each of its non-employee directors. A summary of restricted stock activity under the 2008 Plan for the year ended December 31, 2009 is as follows:
| |
Shares | Weighted Average Grant Date Fair Value (per share) |
|||||
|---|---|---|---|---|---|---|---|
Outstanding and unvested at December 31, 2008 |
— | — | |||||
Granted |
16,000 | $ | 4.48 | ||||
Vested |
— | — | |||||
Canceled |
— | — | |||||
Outstanding and unvested at December 31, 2009 |
16,000 | $ | 4.48 | ||||
Valuation and Expenses Information
Stock-based compensation expense related to employee and non-employee stock options, restricted stock and employee stock purchases for the years ended December 31, 2009, 2008 and 2007 was recorded as follows:
| |
2009 | 2008 | 2007 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
(In thousands) |
|||||||||
Research and development |
$ | 1,383 | $ | 2,035 | $ | 424 | ||||
General and administrative |
1,675 | 2,781 | 1,181 | |||||||
Total stock-based compensation expense |
$ | 3,058 | $ | 4,816 | $ | 1,605 | ||||
In connection with the AVANT Merger, the Company accounted for the exchange of Celldex Research Stock Options into options to acquire shares of the Company's common stock as a modification. The modification affected a total of 15 employees, including members of the Celldex Research board of directors. The total incremental compensation cost resulting from the modifications was $2.6 million, of which $0.9 million was related to vested awards and was recognized immediately as stock-based compensation during the three months ended March 31, 2008.
82
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(5) STOCK-BASED COMPENSATION (Continued)
The fair values of employee and non-employee director stock options and employee stock purchases granted during the years ended December 31, 2009, 2008 and 2007 were valued using the Black-Scholes option-pricing model with the following assumptions:
| |
Year Ended December 31, 2009 |
Year Ended December 31, 2008 |
Year Ended December 31, 2007(1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Expected stock price volatility (employees) |
65 - 68 | % | 55 - 67 | % | 80 | % | ||||
Expected stock price volatility (non-employee directors) |
67 | % | 57 - 67 | % | 80 | % | ||||
Expected stock price volatility (2004 ESPP) |
90 - 98 | % | 98 | % | n/a | |||||
Expected option term (employees) |
6.1 - 6.3 Years | 3 - 6.3 Years | 5 Years | |||||||
Expected option term (non-employee directors) |
5.5 Years | 4 - 6 Years | 5 Years | |||||||
Expected option term (2004 ESPP) |
.5 Years | .5 Years | n/a | |||||||
Risk-free interest rate (options) |
1.8 - 3.4 | % | 1.8 - 3.3 | % | 3.9 | % | ||||
Risk-free interest rate (2004 ESPP) |
0.3 | % | 0.3 | % | n/a | |||||
Expected dividend yield |
None | None | None | |||||||
- (1)
- The assumptions for 2007 were used by Celldex Research to calculate fair values of stock option grants. The expected volatility was based on the average volatility of a group of companies that Celldex Research believed would be considered a peer group had it been a publicly-held company.
U.S. GAAP allows companies to continue to utilize the simplified method in developing an estimate of the expected term of "plain vanilla" stock options when there have been structural changes in a Company's business such that historical exercise data does not provide a reasonable basis upon which to estimate expected term. Due to the AVANT Merger and the CuraGen Merger, historical exercise patterns do not provide a reasonable basis to estimate expected term of current option grants. Accordingly, the Company utilized the simplified method to estimate expected term of stock options granted during the years ended December 31, 2009 and 2008. The simplified method estimates the expected term as the mid-point between the vesting date and the expiration date. In 2008 and 2009, the Company used its daily historical stock price volatility consistent with the expected term of grant as the basis for its expected volatility assumption. The risk-free interest rate is based upon the yield of U.S. Treasury securities consistent with the expected term of the option. The dividend yield assumption is based on the Company's history of zero dividend payouts and expectation that no dividends will be paid in the foreseeable future. For the years ended December 31, 2009 and 2008, forfeitures were estimated based on historical experience by applying an eleven and zero percent forfeiture rate to employee and non-employee director stock option awards, respectively.
83
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(6) PROPERTY AND EQUIPMENT
Property and equipment include the following:
| |
December 31, 2009 |
December 31, 2008 |
||||||
|---|---|---|---|---|---|---|---|---|
| |
(In thousands) |
|||||||
Laboratory Equipment |
$ | 2,643 | $ | 2,449 | ||||
Manufacturing Equipment |
1,622 | 1,508 | ||||||
Office Furniture and Equipment |
1,165 | 1,085 | ||||||
Leasehold Improvements |
12,601 | 12,564 | ||||||
Construction in Progress |
180 | 71 | ||||||
Total Property and Equipment |
18,211 | 17,677 | ||||||
Less Accumulated Depreciation and Amortization |
(6,722 | ) | (4,110 | ) | ||||
|
$ | 11,489 | $ | 13,567 | ||||
Depreciation and amortization expense related to property and equipment was $2.6 million, $2.2 million and $0.7 million for the years ended December 31, 2009, 2008 and 2007, respectively.
(7) INTANGIBLE ASSETS AND GOODWILL
Intangible assets, net of accumulated amortization, and goodwill are as follows:
| |
December 31, 2009 | December 31, 2008 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Estimated Life |
Cost | Accumulated Amortization |
Net | Cost | Accumulated Amortization |
Net | |||||||||||||||
| |
|
(In thousands) |
||||||||||||||||||||
Intangible Assets: |
||||||||||||||||||||||
IPR&D |
Indefinite | $ | 11,800 | — | $ | 11,800 | — | — | — | |||||||||||||
Amgen Amendment |
16 years | 14,500 | $ | (224 | ) | 14,276 | — | — | — | |||||||||||||
TopoTarget Agreement |
1.75 years | 2,400 | (343 | ) | 2,057 | — | — | — | ||||||||||||||
Core Technology |
4.5 - 11 years | 2,193 | (832 | ) | 1,361 | $ | 2,193 | $ | (531 | ) | $ | 1,662 | ||||||||||
Strategic Partner Agreement |
8 years | 630 | (145 | ) | 485 | 630 | (65 | ) | 565 | |||||||||||||
Asset Held for Sale |
— | — | — | — | 274 | (28 | ) | 246 | ||||||||||||||
Total Intangible Assets |
$ | 31,523 | $ | (1,544 | ) | $ | 29,979 | $ | 3,097 | $ | (624 | ) | $ | 2,473 | ||||||||
Goodwill |
Indefinite |
$ |
8,965 |
— |
$ |
8,965 |
— |
— |
— |
|||||||||||||
84
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(7) INTANGIBLE ASSETS AND GOODWILL (Continued)
At December 31, 2008, the Company had recorded $0.2 million in intangible assets related to its poultry vaccines as a long-lived asset to be disposed of by sale due to the Company's negotiations with Lohmann Animal Health International ("LAHI"). In January 2009, the Company entered into a purchase agreement ("LAHI Agreement") to sell its poultry vaccines assets to LAHI. Under the LAHI Agreement, LAHI paid an upfront fee of $0.8 million and agreed to pay potential milestone payments. The Company recorded a gain of $0.6 million related to the LAHI Agreement based on the upfront fee less the net book value of the related asset.
Amortization expense for intangible assets was $0.9 million, $0.4 million and $0.1 million for the years ended December 31, 2009, 2008 and 2007, respectively.
The estimated future amortization expense of intangible assets for the next five years is as follows:
Year ending December 31,
|
Estimated Amortization Expense |
|||
|---|---|---|---|---|
| |
(In thousands) |
|||
2010 |
$ | 2,650 | ||
2011 |
1,964 | |||
2012 |
1,203 | |||
2013 |
1,127 | |||
2014 |
1,127 | |||
(8) ACCRUED EXPENSES
Accrued expenses include the following:
| |
December 31, 2009 |
December 31, 2008 |
|||||
|---|---|---|---|---|---|---|---|
| |
(In thousands) |
||||||
Accrued Royalty and License Fees |
$ | 1,323 | $ | 672 | |||
Accrued Payroll and Employee Benefits |
1,915 | 1,953 | |||||
Accrued Research and Development Contract Costs |
918 | 120 | |||||
Accrued Professional Fees |
512 | 432 | |||||
Other Accrued Expenses |
947 | 664 | |||||
|
$ | 5,615 | $ | 3,841 | |||
85
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(9) INCOME TAXES
The components of income tax expense attributable to continuing operations consist of the following:
| |
Year Ended December 31, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | 2007 | ||||||||
| |
(In thousands) |
||||||||||
Income tax benefit (provision): |
|||||||||||
Federal |
$ | 12,750 | $ | 10,198 | $ | 4,545 | |||||
State |
(1,757 | ) | 6,958 | 711 | |||||||
Foreign |
126 | 193 | 844 | ||||||||
Expiration of Net Operating Losses and Research & Development Tax Credits |
(3,992 | ) | (1,306 | ) | — | ||||||
|
7,127 | 16,043 | 6,100 | ||||||||
Deferred tax valuation allowance |
(6,598 | ) | (16,043 | ) | (6,100 | ) | |||||
|
$ | 529 | $ | — | $ | — | |||||
Included in the state tax provision above for the year ended December 31, 2009 is the effect of a rate decrease on the deferred tax asset and liabilities offset by a $0.5 million tax benefit due to non-cash tax consequences of the CuraGen Merger.
A reconciliation between the amount of reported income tax and the amount computed using the U.S. Statutory rate of 34% follows:
| |
2009 | 2008 | 2007 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
(In thousands) |
|||||||||
Pre-tax book income (loss) |
$ | (37,054 | ) | $ | (47,501 | ) | $ | (15,073 | ) | |
Loss at Statutory Rates |
(12,571 |
) |
(16,109 |
) |
(4,944 |
) |
||||
Research and Development Credits |
(1,456 | ) | (1,325 | ) | (306 | ) | ||||
State Taxes |
1,757 | (6,958 | ) | (711 | ) | |||||
Other |
1,151 | 85 | (139 | ) | ||||||
IPR&D |
— | 6,958 | — | |||||||
Expiration of Net Operating Losses and Research & Development Tax Credits |
3,992 | 1,306 | — | |||||||
Change in Valuation Allowance |
6,598 | 16,043 | 6,100 | |||||||
Income tax (benefit) provision |
$ | (529 | ) | $ | — | $ | — | |||
Deferred tax assets and liabilities are recognized based on temporary differences between the financial reporting and tax basis of assets and liabilities using future expected enacted rates. A valuation allowance is recorded against deferred tax assets if it is more likely than not that some or all of the deferred tax assets will not be realized.
86
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(9) INCOME TAXES (Continued)
The principal components of the deferred tax assets and liabilities at December 31, 2009 and 2008, respectively, are as follows:
| |
December 31, 2009 |
December 31, 2008 |
||||||
|---|---|---|---|---|---|---|---|---|
| |
(In thousands) |
|||||||
Gross Deferred Tax Assets |
||||||||
Net Operating Loss Carryforwards |
$ | 55,715 | $ | 94,406 | ||||
Tax Credit Carryforwards |
16,735 | 15,026 | ||||||
Deferred Expenses |
17,300 | 19,600 | ||||||
Stock-based Compensation |
3,795 | 2,049 | ||||||
Fixed Assets |
2,088 | 1,458 | ||||||
Accrued Expenses and Other |
1,122 | 474 | ||||||
Deferred Revenue |
15,535 | 2,094 | ||||||
|
112,290 | 135,107 | ||||||
Gross Deferred Tax Liabilities |
||||||||
Other Acquired Intangibles |
(6,500 | ) | (101 | ) | ||||
IPR&D Intangibles |
(4,661 | ) | — | |||||
Deferred License Costs and Other |
(2,651 | ) | (2,765 | ) | ||||
|
(13,812 | ) | (2,866 | ) | ||||
Total Deferred Tax Assets and Liabilities |
98,478 | 132,241 | ||||||
Deferred Tax Assets Valuation Allowance |
(103,139 | ) | (132,241 | ) | ||||
Net Deferred Tax Asset (Liability) |
$ | (4,661 | ) | $ | — | |||
As of December 31, 2009, the Company had the following federal net operating loss ("NOL") carryforwards:
- •
- Prior to the merger of Celldex and AVANT, $33.0 million was generated by Celldex Research which expire at various
dates starting in 2023 and going through 2028;
- •
- Prior to the merger of Celldex and AVANT, $145.2 million was generated by AVANT which expire at various dates
starting in 2010 and going through 2028;
- •
- Following the merger of Celldex and AVANT, $32.1 million was generated by the combined company which expire at
various dates starting in 2028 and going through 2029; and
- •
- Prior to its acquisition by Celldex, $521.3 million was generated by CuraGen.
In general, an ownership change, as defined by Section 382 of the Internal Revenue Code, results from transactions increasing the ownership of certain shareholders or public groups in the stock of a corporation by more than 50 percentage points over a three-year period. Such ownership changes can significantly limit the amount of NOL carryforwards that may be utilized in future periods. The Company currently expects that it is remote that the CuraGen loss carryforwards may be utilized and, as such, no related asset has been recorded for such losses. The Company has not completed an analysis of losses generated by AVANT, however, the Company believes it is remote that $105 million of the AVANT loss carryforwards may be utilized in future periods and there may be substantial limitations on the Company's ability to use the remaining losses of $40.2 million. Following the merger of Celldex and AVANT, the Company experienced changes in ownership as defined by Section 382 in
87
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(9) INCOME TAXES (Continued)
June 2009 and December 2009. Further, prior to the merger of AVANT and Celldex, Celldex Research as a stand alone company experienced a change in ownership in October 2007. As a result of the ownership change in October 2007, utilization of the Celldex Research Federal NOLs is subject to an annual limitation of $4.5 million on $28.3 million of NOLs generated before that date. As a result of the company ownership changes in June 2009 and December 2009, there is an annual limitation amount of $6.0 million on $65.1 million NOLs. Any unused annual limitation may be carried over to later years, and the amount of the limitation may, under certain circumstances, be subject to adjustment if the fair value of the Company's net assets are determined to be below or in excess of the tax basis of such assets at the time of the ownership change, and such unrealized loss or gain is recognized during the five-year period after the ownership change.
Similar to the AVANT and CuraGen NOL carryforwards above, the Company believes that it is remote that federal and state research and development ("R&D") credits of $20.6 million and $14.4 million, respectively, will be utilized in the future periods. Further, the Company's ability to use the state NOL carryforwards of approximately $76.2 million and the remaining federal and state R&D credit carryforwards of approximately $11.3 million and $7.7 million, respectively, may be substantially limited. These state NOLs and federal and state credits expire at various dates starting in 2010 going through 2029. The Company has not yet completed a study of these credits to substantiate the amounts. Until a study is completed, no amounts are being presented as an uncertain tax position.
Subsequent ownership changes, as defined in Section 382, could further limit the amount of net operating loss carryforwards and research and development credits that can be utilized annually to offset future taxable income.
As of December 31, 2009, the Company also has foreign NOL carryforwards of approximately $34.9 million in the UK which expire over various periods. These NOLs may be limited in the foreign jurisdiction and the Company has not yet undertaken any study to assess the usability of these NOLs.
Massachusetts, New Jersey and Connecticut are the three states in which the Company primarily operates or has operated and has income tax nexus. The Company is currently under examination for sales and use taxes by the State of Connecticut for the period from July 1, 2006 through June 30, 2009. The Company is not currently under examination by any other jurisdictions for any tax year.
The Company has evaluated the positive and negative evidence bearing upon the realizability of its net deferred tax assets, which are comprised principally of net operating loss carryforwards, capitalized R&D expenditures and R&D tax credit carryforwards. The Company has determined that it is more likely than not that it will not recognize the benefits of federal and state deferred tax assets and, as a result, a full valuation allowance was maintained at December 31, 2009 against the Company's net deferred tax assets.
(10) STOCKHOLDERS' EQUITY
Common Stock
The Company implemented a 1-for-12 reverse stock split of the Company's common stock on March 7, 2008, as approved by the Company's stockholders. The Company has retroactively applied the reverse stock split to all the share and per share amounts for all periods presented in these consolidated financial statements.
88
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(10) STOCKHOLDERS' EQUITY (Continued)
Convertible Preferred Stock
At December 31, 2009, the Company had authorized 3,000,000 shares of preferred stock all of which have been designated Class C Preferred Stock including 350,000 shares which have been designated Series C-1 Junior Participating Cumulative Preferred Stock (the "Series C-1 Preferred Stock")."
Shareholder Rights Plan
The Company's Board has adopted a Shareholder Rights Plan, as set forth in the Shareholder Rights Agreement, as amended, between the Company and Computershare Trust Company, N.A., as Rights Agent (the "Rights Agreement"). Pursuant to the terms of the Rights Agreement, the Board declared a dividend distribution of one Preferred Stock Purchase Right ("Right") for each outstanding share of the Company's common stock. Each Right, which expires in November 2014, entitles their holder to purchase from the Company one ten-thousandth of a share (a "Unit") of Series C-1 Preferred Stock at a cash exercise price of $35.00 per Unit, subject to adjustment. The Rights will trade separately from the common stock and will become exercisable only when a person or group has acquired 15% or more of the outstanding common stock or upon the commencement by a person or group of a tender offer that would result in such person or group acquiring 15% or more of the outstanding common stock other than as a result of repurchases of stock by the Company or certain inadvertent actions by a shareholder. In the event a person or group acquires 15% or more of the outstanding common stock each holder of a Right (except for any such person or group) would be entitled to receive upon exercise sufficient Units of Series C-1 Preferred Stock to equal a value of two times the exercise price of the Right. In the event the Company is acquired in a merger or other business combination transaction or if 50% or more of the Company's assets or earning power is sold, each holder of a Right (except for any such person or group described above) would receive upon exercise common stock of the acquiring company with a value equal to two times the exercise price of the Right.
(11) SIGNIFICANT REVENUE ARRANGEMENTS
A summary of the Company's significant revenue contracts and arrangements follows:
GlaxoSmithKline plc ("Glaxo") and Paul Royalty Fund II, L.P. ("PRF")
In 1997, the Company entered into an agreement with Glaxo to collaborate on the development and commercialization of the Company's oral rotavirus strain and Glaxo assumed responsibility for all subsequent clinical trials and all other development activities. The Company's licensed-in the rotavirus strain that was used to develop Glaxo's Rotarix® rotavirus vaccine in 1995 and owes a license fee of 30% to Cincinnati Children's Hospital Medical Center ("CCH") on net royalties received from Glaxo. The Company is obligated to maintain a license with CCH with respect to the Glaxo agreement. The term of the Glaxo agreement is through the expiration of the last of the relevant patents covered by the agreement, although Glaxo may terminate the agreement upon 90 days prior written notice.
In May 2005, the Company entered into an agreement whereby an affiliate of PRF purchased an interest in the net royalties the Company will receive on worldwide sales of Rotarix®. The Company's retained interests in Rotarix® net royalties which were not sold to PRF are recorded as product royalty revenue and a corresponding amount that is payable to CCH is recorded as royalty expense. Product royalty revenue and royalty expense related to the Company's retained interest in Rotarix® was
89
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(11) SIGNIFICANT REVENUE ARRANGEMENTS (Continued)
7.7 million and $3.0 million for the years ended December 31, 2009 and 2008, respectively. Under the PRF agreement, the Company also retained 50% of Glaxo milestone payments beginning on the effective date of the agreement with PRF, with 70% and 30% of the remaining balance payable to PRF and CCH, respectively. In April 2008, Rotarix® received Food and Drug Administration ("FDA") market approval for the prevention of rotavirus gastroenteritis in infants, which triggered a milestone from Glaxo. During the three months ended June 30, 2008, the Company recorded $0.2 million in product development and licensing agreement revenue and a corresponding amount payable to CCH as royalty expense related to the Glaxo milestone.
On October 1, 2008, the Company received a $10 million milestone payment from PRF related to the market launch of Rotarix® in the U.S. As of March 31, 2008, the Company recorded the estimated fair value of the $10 million milestone payment due from PRF of $9.1 million in connection with the purchase accounting for the AVANT Merger. During the three months ended September 30, 2008, the Company recognized the balance of $0.9 million as other income in the consolidated statement of operations. The Company has received $60 million in total milestone payments under the PRF agreement. No additional milestone payments are due from PRF under the agreement.
Royalty rates on Rotarix® escalate from 7% to 10% based on net product sales in countries that have valid patent protection. These royalty rates are discounted by 30% for "non-patent" countries (primarily international markets). In September 2006, the Company received notice from Glaxo that Glaxo would begin paying royalties on sales of Rotarix® at the lower of the two royalty rates under their 1997 license agreement. Glaxo's decision to pay the lower royalty rate (which is 70% of the full rate) is based upon Glaxo's assertion that Rotarix® is not covered by the patents Glaxo licensed from the Company in Australia and certain European countries. If Glaxo's position stands, the royalties to which PRF is entitled will no longer be limited by a $27.5 million annual threshold, which the Company projected may have been reached in later years as sales of Rotarix® increased. Irrespective of Glaxo's position, the Company will still retain approximately 65% of the royalties on worldwide sales of Rotarix® once PRF receives 2.45 times the aggregate cash payments of $60 million it made to the Company, though the potential amount of such residual royalties will be lower if Glaxo's position stands.
Glaxo and Corixa Corporation ("Corixa")
In December 2005, the Company and Corixa, a wholly-owned subsidiary of Glaxo, entered into a termination agreement of their collaboration of CDX-2101, or HepVax, for the development of a therapeutic vaccine for Hepatitis B (the "Termination Agreement"). Under the terms of the Termination Agreement, Glaxo paid the Company $1.6 million for which the Company recorded product development and licensing agreement revenue of $0.2 million, $0.5 million and $0.5 million during the years ended December 31, 2009, 2008 and 2007, respectively.
Pfizer Inc ("Pfizer")
On April 16, 2008, the Company and Pfizer entered into a License and Development Agreement (the "Pfizer Agreement") under which Pfizer was granted an exclusive worldwide license to a therapeutic cancer vaccine candidate, CDX-110, in Phase 2 development for the treatment of glioblastoma multiforme. The Pfizer Agreement also gives Pfizer exclusive rights to the use of EGFRvIII vaccines in other potential indications. Under the Pfizer Agreement, Pfizer made an upfront payment to the Company of $40 million and made a $10 million equity investment in the Company.
90
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(11) SIGNIFICANT REVENUE ARRANGEMENTS (Continued)
Pfizer will fund all development costs for these programs. The Company is also eligible to receive potential milestone payments exceeding $390 million for the successful development and commercialization of CDX-110 and additional EGFRvIII vaccine products, as well as royalties on any product sales. The Pfizer Agreement became effective after clearance under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (as amended) on May 19, 2008.
On May 27, 2008, the Company received $10 million from Pfizer in exchange for 781,250 shares of the Company's common stock having a fair value of $10.9 million, or $13.91 per share, on that date. The $0.9 million over the amount received from Pfizer was recorded as a reduction to deferred revenue of the $40 million upfront payment received from Pfizer on June 18, 2008.
The Company has determined that its performance obligations under this collaboration should be accounted for as a single unit of accounting. The Company's deliverables under this collaboration primarily include an exclusive license to its CDX-110 product candidate and its EGFRvIII technologies, research and development services as required under the collaboration and participation in the joint clinical development committee. The Company has estimated that its performance period under the collaboration will be 9.5 years based on an assessment of the period over which the Company will have met its performance obligations under the collaboration. Revenue, including research and development reimbursements, is being recognized on a straight-line basis over this period using the Contingency Adjusted Performance Model ("CAPM"). The $40 million up-front payment, less the $0.9 million in excess fair value for the Company's common stock discussed above, was recorded as deferred revenue and is being amortized over the 9.5-year performance period at a rate of $1.0 million per quarter.
The agreement also provides for reimbursement by Pfizer of all costs incurred by the Company in connection with the collaboration since the effective date. The Company invoices Pfizer monthly for its reimbursable costs and records the invoiced amount as deferred revenue. These deferred revenue amounts are amortized to revenue over the estimated 9.5-year performance period on a straight-line basis using the CAPM model. The Company incurred and invoiced Pfizer $3.2 million and $4.9 million in reimbursable costs related to the Pfizer collaboration for the years ended December 31, 2009 and 2008, respectively.
The Company recorded product development and licensing agreement revenue under the Pfizer Agreement as follows:
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | |||||
| |
(In thousands) |
||||||
Up-front portion |
$ | 4,119 | $ | 2,551 | |||
Reimbursable costs portion |
1,063 | 319 | |||||
|
$ | 5,182 | $ | 2,870 | |||
In connection with the Pfizer Agreement, the Company paid a total of $6.9 million in sublicense fees to Duke University and Thomas Jefferson University. The Company recorded these deferred sublicense fees to other assets in the consolidated balance sheets and is amortizing them to royalty expense over the 9.5-year performance period. The Company recorded $0.7 million and $0.5 million in royalty expense related to these deferred sublicense fees during the years ended December 31, 2009
91
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(11) SIGNIFICANT REVENUE ARRANGEMENTS (Continued)
and 2008, respectively. At December 31, 2009 and 2008, the unamortized balance of deferred costs was $5.7 million and $6.4 million, respectively.
Rockefeller University ("Rockefeller")
The Company is providing research and development support to Rockefeller on the development of their vaccine, DCVax-001, which the Company refers to as CDX-2401, aimed at providing protection from infection with HIV, the virus known to cause AIDS. This program is in a Bill & Melinda Gates Foundation funded partnership called the Grand Challenges initiative. Preclinical studies and manufacturing development are in progress and payments to the Company are made on a time and materials basis. The Company recorded grant revenue from Rockefeller of $1.8 million, $0.4 million and $0.8 million for the years ended December 31, 2009, 2008 and 2007, respectively.
(12) COLLABORATION AGREEMENTS
The Company has entered into licensing agreements with several universities and research organizations. Under the terms of these agreements, the Company has received licenses or options to license technology, specified patents or patent applications. The Company's licensing and development collaboration agreements generally provide for royalty payments equal to specified percentages of product sales, annual license maintenance fees and continuing patent prosecution costs. In addition, the Company has committed to make potential future milestone payments to third parties of up to approximately $116 million as part of the Company's various collaborations including licensing and development programs. Payments under these agreements generally become due and payable only upon achievement of certain developmental, regulatory and/or commercial milestones. Nonrefundable license fee expense was $0.7 million, $0.8 million and $0.2 million for the years ended December 31, 2009, 2008 and 2007, respectively.
Medarex, Inc., a subsidiary of Bristol-Myers Squibb ("Medarex")
Medarex, a former related party, and the Company have entered into the following agreements, each of which was approved by a majority of its independent directors who did not have an interest in the transaction. These agreements include:
- •
- An Assignment and License Agreement, as amended, ("Assignment and License Agreement") that provides for the assignment of
certain patent and other intellectual property rights and a license to certain Medarex technology; and
- •
- A Research and Commercialization Agreement, as amended, ("Research and Commercialization Agreement") that provides the Company with certain rights to obtain exclusive commercial licenses to proprietary monoclonal antibodies raised against certain antigens.
Under the terms of the Assignment and License Agreement and Research and Commercialization Agreement, the Company may be required to pay milestone and royalty payments to Medarex with respect to the development of any products containing such licensed antibodies.
In October 2007, the Company and Medarex entered into a settlement and mutual release agreement with settled disputed amounts the Company owed Medarex. The Company issued to Medarex 351,692 shares of the Company's common stock equal in value to $3.0 million, based on the per share price of $8.64 set on the second trading day prior to the closing date of the AVANT Merger and exchanged releases. At December 31, 2008, the Company owed Medarex an additional $3.0 million related to a Master Services Agreement which the Company paid Medarex in October 2009.
92
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(12) COLLABORATION AGREEMENTS (Continued)
Rockefeller University ("Rockefeller")
In November 2005, the Company and Rockefeller entered into a license agreement for the exclusive worldwide rights to human DEC-205 receptor, with the right to sublicense the technology. The license grant is exclusive except that Rockefeller may use and permit other nonprofit organizations to use the human DEC-205 receptor patent rights for educational and research purposes. The Company may be required to pay milestone and royalty payments to Rockefeller with respect to development and commercialization of the human DEC-205 receptor. The Company may also be required to pay royalties on any product sales.
Duke University Brain Tumor Cancer Center ("Duke")
In September 2006, the Company and Duke entered into a license agreement that gave the Company access and reference to the clinical data generated by Duke and its collaborators in order for the Company to generate its own filing with the FDA relating to its CDX-110 product. The Company may be required to pay milestone and royalty payments to Duke with respect to development and commercialization of the CDX-110 product. In connection with the Pfizer Agreement, the Company determined that $2.4 million was payable to Duke as a sublicense fee. As provided for under the Duke license, the Company paid 50% of this amount to Duke in the form of 81,512 shares of the Company's common stock in October 2008.
Ludwig Institute for Cancer Research ("Ludwig")
In October 2006, the Company and Ludwig entered into an agreement for the nonexclusive rights to six cancer tumor targets for use in combination with the Company's APC Targeting Technology. The term of the agreement is for ten years. The Company may be required to pay milestone and royalty payments to Ludwig with respect to development and commercialization of the technology licensed from Ludwig.
Alteris Therapeutics, Inc. ("Alteris")
In October 2005, the Company completed the acquisition of the assets of Alteris, including the EGFRvIII molecule that the Company licensed to Pfizer under the Pfizer Agreement. The Company may be required to pay Alteris up to $5.0 million upon obtaining the first approval for commercial sale of a product containing EGFRvIII, including CDX-110.
Thomas Jefferson University ("TJU")
In February 2003, the Company entered into three exclusive license agreements with TJU. Under these licenses, the Company may be required to pay milestone and royalty payments to TJU with respect to development and commercialization of the technology licensed from TJU. In connection with the Pfizer Agreement, the Company amended its licenses with TJU to add additional sublicensing rights and paid $4.5 million in sublicense fees to TJU in 2008.
3M Company
In June 2008, the Company and 3M Company entered into a license agreement for the exclusive worldwide rights to access 3M Company's proprietary Immune Response Modifier, Resiquimod™, (and
93
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(12) COLLABORATION AGREEMENTS (Continued)
additional Toll-Like Receptor 7/8 agonists ("TLR")) for clinical study with the Company's proprietary APC Targeting Technology, for use as vaccine adjuvants, with the right to sublicense the technology. The Company may be required to pay milestone and royalty payments to 3M Company with respect to development and commercialization of the technology licensed from 3M Company.
University of Southampton, UK ("Southampton")
In November 2008, the Company entered into a license agreement with Southampton to develop human antibodies towards CD27, a potentially important target for immunotherapy of various cancers. CD27 is a critical molecule in the activation pathway of lymphocytes, is downstream from CD40, and may provide a novel way to regulate the immune responses. In preclinical models, antibodies to CD27 have been shown to mediate anti-tumor effects alone, and may be particularly effective in combination with the Company's other immunotherapies. The Company may be required to pay milestone and royalty payments to Southampton with respect to development and commercialization of the technology licensed from Southampton.
Amgen Inc. ("Amgen")
In March 2009, the Company entered into a license agreement with Amgen to expand its Precision Targeted Immunotherapy Platform by acquiring exclusive rights to FMS-like tyrosine kinase 3 ligand (Flt3L) and CD40 ligand (CD40L). Flt3L and CD40L are immune modulating molecules that increase the numbers and activity of immune cells that control immune responses. The Company may be required to pay milestone and royalty payments to Amgen with respect to development and commercialization of technology licensed from Amgen.
Seattle Genetics, Inc. ("Seattle Genetics")
In connection with the CuraGen Merger, the Company assumed the license agreement between CuraGen and Seattle Genetics whereby CuraGen acquired the rights to proprietary antibody-drug conjugate ("ADC") technology for use with the Company's proprietary antibodies for the potential treatment of cancer. The Company may be required to pay milestone and royalty payments to Seattle Genetics with respect to development and commercialization of the ADC technology.
(13) 4% CONVERTIBLE DEBT DUE 2011
In connection with the CuraGen Merger, the Company assumed $12.5 million in 4% convertible subordinated debt due February 15, 2011 (the "CuraGen Debt"). In addition, effective October 1, 2009, Celldex, CuraGen, and The Bank of New York Mellon (the "Trustee") amended the CuraGen Debt to provide that the CuraGen Debt shall be convertible into 353,563 shares of Celldex common stock at the rate of 28.27823 shares of Celldex common stock per $1,000 principal amount of notes, or $35.36 per share. The Company has the right to redeem the notes at a redemption price equal to 100.571% of the principal amount of the notes plus accrued and unpaid interest, if any. The CuraGen Debt is convertible by the holders of the CuraGen Debt at any time prior to maturity.
As of October 1, 2009, the Company recorded the CuraGen Debt at its estimated fair value of $11.5 million as part of the acquisition accounting related to the CuraGen Merger. The initial carrying value of the CuraGen Debt is being accreted ratably, over the term of the CuraGen Debt, to $12.5 million due at maturity. Interest expense on the CuraGen Debt was $0.3 million for the three
94
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(13) 4% CONVERTIBLE DEBT DUE 2011 (Continued)
months ended December 31, 2009 and included $0.2 million in discount accretion. At December 31, 2009, the carrying value and the accrued interest on the CuraGen Debt were $11.7 million and $0.2 million, respectively. At December 31, 2009, the estimated fair value of the Company's outstanding $12.5 million in CuraGen Debt was approximately $11.9 million, based on quoted market prices.
(14) OTHER LONG-TERM LIABILITIES
Other long-term liabilities include the following:
| |
December 31, 2009 | December 31, 2008 | ||||||
|---|---|---|---|---|---|---|---|---|
| |
(In thousands) |
|||||||
Deferred Rent |
$ | 377 | $ | 301 | ||||
CuraGen Severance (See Note 16) |
2,623 | — | ||||||
Deferred Tax Liabilities (See Note 19) |
4,661 | — | ||||||
Loan Payable |
632 | 686 | ||||||
Note Payable |
178 | 300 | ||||||
Total |
8,471 | 1,287 | ||||||
Less Current Portion |
(2,156 | ) | (218 | ) | ||||
Long-Term Portion |
$ | 6,315 | $ | 1,069 | ||||
In December 2003, the Company entered into a Lease Agreement (the "Lease Agreement"), a Secured Promissory Note: Equipment Loan (the "Secured Promissory Note") and a Security Agreement with the Massachusetts Development Finance Agency ("MassDevelopment"), an economic development entity for the Commonwealth of Massachusetts, for the Company to occupy and build-out a manufacturing facility in Fall River, Massachusetts.
Under the Lease Agreement, the Company received a loan ("Loan Payable") that accrues interest at a rate of 5.5% per annum to finance the build-out of its Fall River facility. Principal and interest payments on the Loan Payable are due monthly using an amortization period of 15 years.
Under the Secured Promissory Note, the Company issued a note payable to MassDevelopment ("Note Payable") that accrues interest at a rate of 5.5% per annum to finance the purchases of manufacturing and laboratory equipment to be placed in its Fall River facility. The Note Payable has a term of 84 months and is collateralized by equipment with a net book value at December 31, 2009 of $0.3 million.
Based on current market interest rates available to the Company for long-term liabilities with similar terms and maturities, the Company believes the fair value of the Loan Payable and Note Payable approximates their carrying value at December 31, 2009.
95
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(14) OTHER LONG-TERM LIABILITIES (Continued)
The Company is obligated to repay the following principal amounts for the Loan Payable and Note Payable over the next five years and thereafter:
| |
Loan Payable | Note Payable | |||||
|---|---|---|---|---|---|---|---|
| |
(In thousands) |
||||||
2010 |
$ | 47 | $ | 133 | |||
2011 |
54 | 45 | |||||
2012 |
55 | — | |||||
2013 |
58 | — | |||||
2014 |
60 | — | |||||
Thereafter |
358 | — | |||||
Total |
$ | 632 | $ | 178 | |||
(15) COMMITMENTS AND CONTINGENCIES
In November 2005, the Company entered into a lease amendment that extended its lease of laboratory and office space in Needham, Massachusetts through April, 2017. Under this lease amendment, the Company is obligated to pay an escalating base annual rent and common area maintenance costs ("CAM") during the remaining lease term.
In December 2003, the Company entered into a lease, as amended, with MassDevelopment to occupy and build-out a manufacturing facility in Fall River, Massachusetts. The lease has an initial seven-year term that expires in December 2010 and two renewal options of five years each. Management has determined that it is reasonably assured that the Company will exercise one five-year renewal option.
The Company leases office and laboratory space in Phillipsburg, New Jersey. The lease has an initial five-year term which expires in August 2011. As an incentive to enter into the lease agreement, the Company received four months of free rent which the Company is amortizing this over the original five-year term of the lease. In addition, the landlord provided the Company with an allowance on future rent payments towards tenant improvements that the Company made to the facility and that credit is included in deferred rent and is being amortized over the lease term.
The Company entered into a letter of credit facility with a national U.S. financial institution which is collateralized by a security deposit for the leased facility in Phillipsburg, New Jersey. The Company recorded restricted cash related to this security deposit of $0.2 million to other assets in the consolidated balance sheets at December 31, 2009 and December 31, 2008.
96
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(15) COMMITMENTS AND CONTINGENCIES (Continued)
Obligations for base rent and CAM costs under facility and other non-cancelable operating leases as of December 31, 2009, assuming the exercise of one five year renewal option for the Company's Fall River, MA facility, are approximately as follows:
Year ending December 31,
|
|
|||
|---|---|---|---|---|
| (In thousands) |
|
|||
2010 |
$ | 2,465 | ||
2011 |
2,613 | |||
2012 |
2,450 | |||
2013 |
2,495 | |||
2014 |
2,552 | |||
2015 and thereafter |
5,388 | |||
Total minimum lease payments |
$ | 17,963 | ||
The Company's total rent and CAM expense for all facility leases was $2.5 million, $2.2 million and $0.3 million for the years ended December 31, 2009, 2008 and 2007, respectively.
(16) SEVERANCE ARRANGEMENTS
Dr. Ronald C. Newbold, former Senior Vice President, Business Development, resigned from his position effective March 1, 2009 pursuant to the provision of his employment agreement that deems a resignation within the year following a change of control (in this case, the AVANT Merger) as a termination resulting from a change of control. In accordance with Dr. Newbold's employment agreement, the Company recorded severance expense during the three months ended March 31, 2009 of $0.7 million including non-cash stock-based compensation expense related to the acceleration of vesting of options to purchase 107,485 shares of Company common stock as provided for under Dr. Newbold's employment agreement.
The Company and Dr. Una S. Ryan, former President and Chief Executive Officer of the Company, executed a separation agreement effective July 16, 2008 (the "Separation Agreement") setting forth such terms regarding Dr. Ryan's separation from the Company. Pursuant to the Separation Agreement, the Company recorded severance expense during the three months ended June 30, 2008 of $1.4 million. The Separation Agreement also provided for the vesting of options to purchase 153,125 shares of Company common stock (of the options to purchase 612,500 shares of Company common stock which had been granted to Dr. Ryan on March 7, 2008). The remainder of Dr. Ryan's options terminated as of July 16, 2008. The Company recorded stock-based compensation expense of $1.3 million related to the acceleration of vesting of options in July 2008, when the criteria for establishing a grant date were met.
The Company and Dr. Robert F. Burns, former President and Chief Executive Officer of Celldex Research, entered into a separation and mutual release agreement dated as of October 19, 2007, under which (i) Dr. Burns' employment was terminated effective February 15, 2008, (ii) the Company agreed to nine months of severance payments and continuation of benefits through February 15, 2010, (iii) all of Dr. Burns' stock options became fully vested and exercisable through February 15, 2011, and (iv) Dr. Burns and the Company provided one another with mutual releases. The Company recorded
97
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(16) SEVERANCE ARRANGEMENTS (Continued)
$1.0 million in severance expense related to the separation and mutual release agreement during the three months ended December 31, 2007.
CuraGen employees who did not receive offers of employment were terminated upon the consummation of the CuraGen Merger. These employees were eligible for severance payments ("CuraGen Severance") upon termination of employment under certain circumstances, including following the CuraGen Merger. U.S. GAAP requires severance obligations that are incurred by the acquiree for the benefit of the acquirer to be recognized as an expense in the post-combination period. Because the offer of employment was at the option of the Company, the Company has deemed the CuraGen Severance to be at its benefit. On October 1, 2009, the Company recorded severance expense of $3.3 million and $0.9 million to general and administrative and research and development, respectively, related to the CuraGen Severance
The following table sets forth an analysis of the CuraGen Severance costs included in long-term liabilities at December 31, 2009:
| |
Balance at December 31, 2008 |
Charges | Paid Cash | Balance at December 31, 2009 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(In thousands) |
||||||||||||
CuraGen Severance |
— | $ | 4,246 | $ | (1,623 | ) | $ | 2,623 | |||||
(17) RETIREMENT SAVINGS PLAN
The Company's 401(k) Plan (the "401(k) Plan") is intended to be a tax-qualified plan covering substantially all employees. Under the terms of the 401(k) Plan, employees may elect to contribute up to 15% of their compensation, or the statutory prescribed limits. The Company may make 50% matching contributions on up to 4% of a participant's annual salary. Benefit expense for the 401(k) Plan was $0.1 million for the years ended December 31, 2009 and 2008.
(18) AVANT MERGER
In connection with the AVANT Merger, effective March 7, 2008, the Company issued 8,309,420 shares of its common stock in exchange for all of the outstanding capital stock of Celldex Research, such that Celldex Research shareholders owned 58% of the Company's common stock on a fully diluted basis and AVANT shareholders retained 42%. The purchase price of $47.6 million represents the shares attributable to former AVANT shareholders and consisted of (i) the 6,265,882 shares outstanding of AVANT common stock on the effective date of the Merger valued at $46.9 million and (ii) estimated transaction costs totaling $0.7 million.
98
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(18) AVANT MERGER (Continued)
The purchase price was allocated to the acquired tangible and identifiable intangible assets and assumed liabilities, based upon their fair value at the date of acquisition, as follows (in thousands):
Tangible assets acquired |
$ | 34,960 | |||
Less: Liabilities assumed |
(3,945 | ) | |||
Net tangible assets acquired |
31,015 | ||||
Intangible assets acquired: |
|||||
Core Technology |
897 | ||||
Developed Technology |
274 | ||||
Strategic Partner Agreement |
629 | ||||
In-Process Research and Development ("IPR&D") |
14,756 | ||||
Total |
$ | 47,571 | |||
The values assigned to the intangible assets acquired, including the IPR&D, were determined based on fair market value using a risk adjusted discounted cash flow approach. Fair values for long-term tangible and intangible assets and for IPR&D were then reduced by $6.0 million of negative goodwill.
The values assigned to IPR&D primarily related to the development of a typhoid-ETEC-cholera combination travelers vaccine and the CDX-1135 complement inhibitor in the amounts of $7.8 million and $6 million, respectively. The significant assumptions underlying the valuations included potential revenues, costs of completion, the timing of product approvals and the selection of appropriate probability of success and discount rates. None of the Company's IPR&D projects had reached technological feasibility nor did they have any alternative future use. Consequently, in accordance with the former purchase method of accounting, the fair value allocated to IPR&D was charged as an expense in the Company's consolidated financial statements as of the date of acquisition. The remaining acquired intangible assets arising from the acquisition are being amortized on a straight line basis over their estimated lives.
In January 2009, the Company entered into a license agreement with Vaccine Technologies, Inc. ("VTI") under which it granted a worldwide exclusive license to VTI to develop and commercialize our CholeraGarde® and ETEC vaccine programs. The Company may receive milestones payments and royalties with respect to development and commercialization of the technology licensed to VTI and no longer expects to incur significant costs on these projects. The Company is continuing the development of CDX-1135 and expects to incur approximately $6.3 million to move this project to the point of potentially out-licensing it to a third party. Estimated revenues from CDX-1135 are expected to be generated beginning in 2014.
(19) CURAGEN MERGER
In connection with the CuraGen Merger, effective October 1, 2009, the Company (i) issued 15,722,713 shares of common stock of the Company, or 0.2739 shares, in exchange for each share of outstanding CuraGen common stock, plus cash in lieu of fractional shares (the "CuraGen Exchange Ratio"), (ii) assumed all of the CuraGen Stock Options and (iii) assumed the CuraGen Debt. The Company acquired CuraGen to gain access to a pipeline of oncology-focused antibodies, including
99
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(19) CURAGEN MERGER (Continued)
CDX-011 (formerly CR011) currently in Phase 2 clinical development for the treatment of breast and melanoma cancer, and cash, cash equivalents and marketable securities of $70.3 million.
The transaction is being accounted for under the acquisition method of accounting. All of the assets acquired and liabilities assumed in the transaction are recognized at their acquisition-date fair values, while transaction costs associated with the transaction are expensed as incurred.
Purchase Price
The purchase price for CuraGen is based on the acquisition-date fair value of the consideration transferred, which was calculated based on the closing price of the Company's common stock of $5.43 per share on October 1, 2009. The acquisition-date fair value of the consideration transferred consisted of the following (in thousands):
Fair value of common stock issued |
$ | 85,374 | ||
Fair value of CuraGen Stock Options |
2,868 | |||
Total consideration transferred |
$ | 88,242 | ||
U.S. GAAP requires that the fair value of replacement awards attributable to precombination service be included in the consideration transferred. Of the CuraGen Stock Options assumed, all but 1%, were immediately vested upon closing in accordance with the terms of the stock option agreements and employment agreements. The fair value of the CuraGen Stock Options that has been attributed to precombination service is included in the consideration transferred.
Allocations of Assets and Liabilities
The Company has allocated the consideration transferred for CuraGen to net tangible assets, intangible assets, goodwill and a severance obligation. The difference between the aggregate consideration transferred and the fair value of assets acquired and liabilities assumed was allocated to goodwill. This goodwill relates to the potential synergies from the CuraGen Merger and a deferred tax liability related to acquired IPR&D intangible assets. None of the goodwill is expected to be deductible for income tax purposes. The following table summarizes the fair values of the assets acquired and liabilities assumed at the acquisition date (in thousands):
Cash and cash equivalents |
$ | 51,654 | |||
Marketable securities |
18,638 | ||||
Identifiable intangible assets: |
|||||
IPR&D |
11,800 | ||||
Amgen Amendment |
14,500 | ||||
TopoTarget Agreement |
2,400 | ||||
Other current and long-term assets |
756 | ||||
Goodwill |
8,965 | ||||
CuraGen Debt |
(11,503 | ) | |||
Deferred tax liabilities, net |
(5,190 | ) | |||
Other assumed liabilities |
(3,778 | ) | |||
Total |
$ | 88,242 | |||
100
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(19) CURAGEN MERGER (Continued)
The purchase price allocation has been prepared on a preliminary basis and is subject to change as additional information becomes available concerning the fair value and tax basis of the acquired assets and liabilities. Any adjustments to the purchase price allocation will be made as soon as practicable but no later than one year from the acquisition date.
The estimated fair value attributed to IPR&D intangible assets represents an estimate of the fair value of purchased in-process technology for CuraGen's research programs that, as of October 1, 2009, had not reached technological feasibility and have no alternative future use. Only those research programs that had advanced to a stage of development where the Company believed reasonable net future cash flow forecasts could be prepared and a reasonable likelihood of technical success existed were included in the estimated fair value. Accordingly, the IPR&D programs primarily represent the estimated fair value of CDX-011. The estimated fair value of the IPR&D programs was determined based on estimates of expected future net cash flows. These expected future net cash flows included estimates for revenue and associated costs for the IPR&D programs based on (i) relevant industry factors, (ii) current and expected trends in the product development life cycle, (iii) the ability to engage a strategic partner, (iv) the ability to obtain regulatory approval, and (v) the ability to manufacture and commercialize the products. The probability-adjusted future net cash flows which reflect the different stages of development of each program are then present valued utilizing an estimate of the appropriate discount rate which is consistent with the uncertainties of the cash flows utilized. Finally, the expected future net cash flows were calculated assuming the Amgen Amendment (defined below) was not entered into because the fair value attributable to the Amgen Amendment is separated from the fair value of the IPR&D programs.
The expected future net cash flows for CDX-011 were based on the expectation that a Biologics License Application ("BLA") for CDX-011 will be filed with the FDA by the end of 2015. The Company expects the commercial launch as promptly as commercially practicable after necessary regulatory approvals are received. Assuming a traditional timeline for the regulatory review process, the Company expects CDX-011 will be commercially launched in 2016. These assumptions require various levels of in-house and external testing, clinical trials and approvals from the FDA or comparable foreign regulatory authorities before CDX-011 could be commercialized in the U.S. or other territories. Drug development involves a high degree of risk and most products that make it into clinical development do not receive marketing approval. Numerous risks and uncertainties can delay or stop clinical development of a pharmaceutical product prior to the receipt of marketing approval, including, but not limited to, results from clinical trials that do not support continuing development, issues related to manufacturing or intellectual property protection, and other events or circumstances that cause unanticipated delays, technical problems or other difficulties. Given these risks and uncertainties, there can be no assurance that the development of CDX-011 will be successfully completed. If the development of CDX-011 is not successful, in whole or in part, or completed in a timely manner, the Company may not realize the expected financial benefits from the development of CDX-011 or the transaction as a whole.
The estimated fair value attributed to the May 2009 amendment to the CuraGen and Amgen Fremont (successor in-interest to Abgenix) license agreement relates to CuraGen's exclusive rights to develop and commercialize CDX-011 and 11 other licensed antigens ("Amgen Amendment"). Under the Amgen Amendment, CuraGen and Amgen Fremont agreed to modify the terms of their existing cross-license of antigens whereby the amended license would be fully paid-up and royalty-free (except for any potentially required payments by CuraGen to the original licensor of CDX-011). The estimated
101
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(19) CURAGEN MERGER (Continued)
fair value of the Amgen Amendment was based on the increase in expected future net cash flows for the IPR&D programs related to CDX-011 after the Amgen Amendment was entered into as compared to the expected future net cash flows if the Amgen Amendment was not entered into. The estimated fair value attributed to the Amgen Amendment is being amortized through the date of the last expiring patent covering CDX-011.
The estimated fair value attributed to the April 2008 agreement ("TopoTarget Agreement") between CuraGen and TopoTarget A/S ("TopoTarget") relates to CuraGen's rights under the TopoTarget Agreement to receive up to $6 million in either potential commercial milestone payments related to future net sales of Belinostat or 10% of any sublicense income received by TopoTarget ("TopoTarget Payments"). Under the TopoTarget Agreement, CuraGen sold back its Belinostat rights to TopoTarget and received $25 million in cash, 5 million shares of TopoTarget common stock (sold by CuraGen in 2008 for net proceeds of $12 million) and the right to receive the TopoTarget Payments. In addition, TopoTarget assumed all financial and operational responsibility for the clinical development of Belinostat under the TopoTarget Agreement. The estimated fair value of the TopoTarget Agreement was based on estimates of the probability-adjusted expected future net cash flows of the TopoTarget Payments. The estimated fair value attributed to the TopoTarget Agrement is being amortized through the date of the estimated receipt of the last payment under the TopoTarget Payments. In February 2010, TopoTarget entered into a co-development and commercialization agreement for Belinostat with Spectrum Pharmaceuticals, Inc. resulting in the Company's receipt of $3 million of the TopoTarget Payments.
The deferred tax liability, net of $5.2 million primarily relates to the temporary differences associated with the IPR&D intangible assets, which are not deductible for tax purposes.
Acquisition-Related Expenses, Including Severance
The Company incurred $2.9 million in acquisition-related expenses in the consolidated statements of operations for the year ended December 31, 2009. These costs include fees for investment banking services, legal, accounting, due diligence, tax, valuation, printing and other various services necessary to complete the transaction. In addition, the Company recorded $3.3 million and $0.9 million in CuraGen Severance expenses to general and administrative and research and development, respectively, in the consolidated statements of operations for the year ended December 31, 2009.
Pro Forma Financial Information
The operating results of CuraGen, which include approximately $2.3 million of research and development expense and $3.7 million in general and administrative expense, have been included in the accompanying consolidated financial statements from October 1, 2009, to December 31, 2009. CuraGen had no revenues from October 1, 2009 through December 31, 2009. The following unaudited pro forma financial summary is presented as if the operations of the Company and CuraGen were combined as of January 1, 2008. The unaudited pro forma combined results are not necessarily indicative of the actual
102
CELLDEX THERAPEUTICS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
(19) CURAGEN MERGER (Continued)
results that would have occurred had the acquisition been consummated at that date or of the future operations of the combined entities.
| |
Years Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2009 | 2008 | |||||
| |
(In thousands) |
||||||
Revenue |
$ | 15,180 | $ | 8,630 | |||
Net loss |
(40,262 | ) | (25,786 | ) | |||
(20) SELECTED QUARTERLY FINANCIAL DATA (Unaudited)
2009
|
Q1 2009 | Q2 2009 | Q3 2009 | Q4 2009 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(In thousands, except per share amounts) |
||||||||||||
Total revenue |
$ | 3,732 | $ | 2,685 | $ | 4,030 | $ | 4,733 | |||||
Net loss |
(7,703 | ) | (8,705 | ) | (7,174 | ) | (12,943 | ) | |||||
Basic and diluted net loss per common share |
(0.49 | ) | (0.55 | ) | (0.45 | ) | (0.41 | ) | |||||
2008
|
Q1 2008 | Q2 2008 | Q3 2008 | Q4 2008 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(In thousands, except per share amounts) |
||||||||||||
Total revenue |
$ | 147 | $ | 1,962 | $ | 2,358 | $ | 2,988 | |||||
Net loss |
(22,131 | ) | (10,260 | ) | (7,656 | ) | (7,454 | ) | |||||
Basic and diluted net loss per common share |
(2.19 | ) | (0.67 | ) | (0.49 | ) | (0.47 | ) | |||||
103
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
Item 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
As of December 31, 2009, we evaluated, with the participation of our Chief Executive Officer and Chief Financial Officer, the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the "Exchange Act")). Based on that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2009. Our disclosure controls and procedures are designed to provide reasonable assurance that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within time periods specified by the SEC's rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Management's Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act as the process designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of our financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that:
- •
- pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and
dispositions of assets;
- •
- provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in
accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with the authorizations of management and directors; and
- •
- provide reasonable assurance regarding the prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on our financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework provided in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management concluded that our internal control over financial reporting was effective as of December 31, 2009.
104
The effectiveness of our internal control over financial reporting as of December 31, 2009 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report, which is included herein.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting during the three months ended December 31, 2009 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Submission of Matters to a Vote of Security Holders
On December 16, 2009, we held our Annual Meeting of Stockholders at which the stockholders (i) elected nine directors to our Board of Directors; (ii) ratified the appointment of PricewaterhouseCoopers LLP as our independent registered public accounting firm for the year ending December 31, 2009; (iii) approved an amendment to our 2008 Stock Option and Incentive Plan to increase the shares reserved for issuance thereunder to 3,900,000 and (iv) approved an amendment to our 2004 Employee Stock Purchase Plan to increase the shares reserved for issuance thereunder to 62,500.
At the Annual Meeting of Stockholders, the following votes were tabulated for the proposals before our Stockholders:
Election of Directors:
| |
Number of Shares/Votes | ||||||
|---|---|---|---|---|---|---|---|
| |
For | Authority Withheld | |||||
Larry Ellberger |
22,320,214 | 1,334,572 | |||||
Anthony S. Marucci |
22,231,618 | 1,423,168 | |||||
Herbert J. Conrad |
22,314,720 | 1,340,066 | |||||
George O. Elston |
22,290,130 | 1,354,256 | |||||
Karen Shoos Lipton |
22,325,111 | 1,329,675 | |||||
Rajesh B. Parekh, Ph.D. |
19,731,867 | 3,922,919 | |||||
Harry H. Penner, Jr. |
22,211,760 | 1,443,026 | |||||
Charles R. Schaller |
22,221,826 | 1,432,960 | |||||
Timothy M. Shannon |
22,281,701 | 1,373,085 | |||||
Ratification of the appointment of PricewaterhouseCoopers LLP:
| FOR | AGAINST | ABSTAIN | BROKER NON-VOTES | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 22,824,672 | 703,037 | 127,077 | — | ||||||||
105
Approval of an amendment to our 2008 Stock Option and Incentive Plan to increase the shares reserved for issuance thereunder to 3,900,000:
| FOR | AGAINST | ABSTAIN | BROKER NON-VOTES | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 11,780,331 | 1,142,425 | 51,787 | 10,658,145 | ||||||||
Approval of an amendment to our 2004 Employee Stock Purchase Plan to increase the shares reserved for issuance thereunder to 62,500:
| FOR | AGAINST | ABSTAIN | BROKER NON-VOTES | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 11,936,847 | 989,380 | 48,316 | 10,658,145 | ||||||||
The number of shares issued, outstanding and eligible to vote as of the record date of November 2, 2009 was 31,602,188. A quorum was present with 23,654,786 shares represented by proxies or 74.9% of the eligible voting shares.
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item 10 will be included in the definitive Proxy Statement for our 2010 Annual Meeting of Stockholders, or the 2010 Proxy Statement, under "Information Regarding the Current Directors and Executive Officers of Celldex," "Section 16(a) Beneficial Ownership Reporting Compliance," "Code of Business Conduct and Ethics" and "The Board of Directors and Its Committees" and is incorporated herein by reference. If the 2010 Proxy Statement is not filed with the SEC within 120 days after the end of our most recent fiscal year, we will provide such information by means of an amendment to this Annual Report on Form 10-K.
Item 11. EXECUTIVE COMPENSATION
The information required by this Item 11 will be included in the 2010 Proxy Statement under "Executive Compensation," and "Compensation Committee Interlocks and Insider Participation," and is incorporated herein by reference. If the 2010 Proxy Statement is not filed with the SEC within 120 days after the end of our most recent fiscal year, we will provide such information by means of an amendment to this Annual Report on Form 10-K.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item 12 will be included in the 2010 Proxy Statement under "Security Ownership of Certain Beneficial Owners and Management" and "Equity Compensation Plan Information" and is incorporated herein by reference. If the 2010 Proxy Statement is not filed with the SEC within 120 days after the end of our most recent fiscal year, we will provide such information by means of an amendment to this Annual Report on Form 10-K.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item 13 will be included in the 2010 Proxy Statement under "Election of Directors" and "Approval of Related Person Transactions and Transactions with Related Persons" and is incorporated herein by reference. If the 2010 Proxy Statement is not filed with the SEC
106
within 120 days after the end of our most recent fiscal year, we will provide such information by means of an amendment to this Annual Report on Form 10-K.
Item 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this Item 14 will be included in the 2010 Proxy Statement under "Independent Registered Public Accounting Firm" and is incorporated herein by reference. If the 2010 Proxy Statement is not filed with the SEC within 120 days after the end of our most recent fiscal year, we will provide such information by means of an amendment to this Annual Report on Form 10-K.
107
Item 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
- (A)
- The
following documents are filed as part of this Form 10-K:
- (1)
- Financial Statements:
See "Index to Consolidated Financial Statements" at Item 8.
- (2)
- Financial Statement Schedules:
Schedules are omitted since the required information is not applicable or is not present in amounts sufficient to require submission of the schedule, or because the information required is included in the Consolidated Financial Statements or Notes thereto.
- (3)
- Exhibits:
| |
|
Incorporated by Reference to | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Description | Form and SEC File No. |
Exhibit No. |
SEC Filing Date |
|||||
| Plan of Acquisition, Reorganization, Arrangement, Liquidation or Succession | |||||||||
| 2.1 | Agreement and Plan of Merger, dated as of October 19, 2007, by and among AVANT, Celldex Merger Corporation, and Celldex Therapeutics, Inc. | 8-K (000-15006) |
2.1 | 10/22/07 | |||||
2.2 |
Agreement and Plan of Merger, dated as of May 28, 2009, by and among Celldex Therapeutics, Inc., CuraGen Corporation and Cottrell Merger Sub, Inc. |
8-K (000-15006) |
2.1 |
5/29/09 |
|||||
Articles of Incorporation and By-Laws |
|||||||||
| 3.1 | Third Restated Certificate of Incorporation | S-4 (333-59215) |
3.1 | 7/16/98 | |||||
3.2 |
Certificate of Amendment of Third Restated Certificate of Incorporation |
S-4 (333-59215) |
3.1 |
7/16/98 |
|||||
3.3 |
Second Certificate of Amendment of Third Restated Certificate of Incorporation |
S-4 (333-59215) |
3.2 |
7/16/98 |
|||||
3.4 |
Third Certificate of Amendment of Third Restated Certificate of Incorporation |
10-Q (000-15006) |
3.1 |
5/10/02 |
|||||
3.5 |
Fourth Certificate of Amendment of Third Restated Certificate of Incorporation |
8-K (000-15006) |
3.1 |
3/11/08 |
|||||
3.6 |
Fifth Certificate of Amendment of Third Restated Certificate of Incorporation |
8-K (000-15006) |
3.2 |
3/11/08 |
|||||
3.7 |
Amended and Restated By-Laws as of March 14, 2007 |
10-K (000-15006) |
3.5 |
3/18/08 |
|||||
Instruments Defining the Rights of Security Holders |
|||||||||
| 4.1 | Shareholder Rights Agreement dated November 5, 2004 | 8-A (000-15006) |
4.1 | 11/8/04 | |||||
4.2 |
Amendment No. 1 to Shareholder Rights Agreement dated October 19, 2007 |
8-A/A (000-15006) |
10.1 |
10/22/07 |
|||||
108
| |
|
Incorporated by Reference to | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Description | Form and SEC File No. |
Exhibit No. |
SEC Filing Date |
|||||
| 4.3 | Amendment No. 2 to Shareholder Rights Agreement dated March 7, 2008 | 8-A/A (000-15006) |
10.1 | 3/7/08 | |||||
4.4 |
Certificate of Designations, Preferences and Rights of a Series of Preferred Stock classifying and designating the Series C-1 Junior Participating Cumulative Preferred Stock |
8-A (000-15006) |
3.1 |
11/8/04 |
|||||
4.5 |
Indenture, dated February 17, 2004 between CuraGen and Trustee |
Filed herewith |
|||||||
4.6 |
Supplemental Indenture, dated September 30, 2009, by and among Celldex, CuraGen, Merger Sub, and Trustee. |
8-K (000-15006) |
4.1 |
10/2/09 |
|||||
4.7 |
Second Supplemental Indenture, dated December 31, 2009, by and among Celldex, CuraGen, and Trustee. |
8-K (000-15006) |
4.1 |
12/31/09 |
|||||
Material Contracts—Leases |
|||||||||
| 10.1 | Commercial Lease Agreement of May 1, 1996 between the Company and Fourth Avenue Ventures Limited Partnership | 10-Q/A (000-15006) |
10.11 | 8/23/96 | |||||
10.2 |
Extension of Lease Agreement of May 1, 1997 between the Company and DIV Needham 53 LLC (successor in interest to Fourth Avenue Ventures Limited Partnership) dated as of August 23, 2001 |
10-K (000-15006) |
10.9 |
3/27/02 |
|||||
10.3 |
First Amendment to Lease by and between the Company and DIV Needham 53 LLC dated November 29, 2005 |
10-K (000-15006) |
10.40 |
3/16/06 |
|||||
*10.4 |
Lease Agreement, by and between the Company and the Massachusetts Development Finance Agency, dated as of December 22, 2003 |
10-Q (000-15006) |
10.1 |
4/30/04 |
|||||
10.5 |
Second Amendment to Lease by and between the Company and the Massachusetts Development Finance Agency dated as of November 4, 2005 |
10-K (000-15006) |
10.41 |
3/16/06 |
|||||
10.6 |
Lease Agreement dated as of October 21, 2005 by and between Phillipsburg Associates, L.P. and the Company. |
S-4 (333-148291) |
10.10 |
1/18/08 |
|||||
Material Contracts—License, Collaboration, Supply and Distribution Agreements |
|||||||||
| *10.7 | License and Royalty Agreement by and between Pfizer Inc and the Company dated as of December 1, 2000 | 10-K (000-15006) |
10.13 | 3/27/01 | |||||
*10.8 |
Amendment to License and Royalty Agreement by and between Pfizer Inc and the Company dated as of December 1, 2000 |
10-K (000-15006) |
10.14 |
3/27/01 |
|||||
*10.9 |
Collaborative Research and Development Agreement by and between Pfizer Inc. and the Company dated as of December 1, 2000 |
10-K (000-15006) |
10.15 |
3/27/01 |
|||||
*10.10 |
License Agreement between the Company and SmithKline Beecham PLC dated as of December 1, 1997 |
10-K (000-15006) |
10.20 |
3/28/00 |
|||||
109
| |
|
Incorporated by Reference to | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Description | Form and SEC File No. |
Exhibit No. |
SEC Filing Date |
|||||
| 10.11 | Amendment Agreement, dated January 9, 2003, between the Company and SmithKline Beecham PLC | 10-K/A (000-15006) |
10.21 | 9/12/03 | |||||
10.12 |
License Agreement, dated as of January 31, 2003, by and between the Company and Elan Drug Delivery Limited |
10-K/A (000-15006) |
10.22 |
9/12/03 |
|||||
10.13 |
License and Clinical Trials Agreement, effective as of February 27, 1995, between the Company and the James N. Gamble Institute of Medical Research |
10-K/A (000-15006) |
10.23 |
9/12/03 |
|||||
10.14 |
License Agreement, dated as of November 25, 1988, by and among The Johns Hopkins University, Brigham and Women's Hospital and the Company |
10-K/A (000-15006) |
10.28 |
9/12/03 |
|||||
10.15 |
Purchase Agreement, dated as of May 16, 2005, by and between the Company and PRF Vaccine Holdings LLC |
8-K (000-15006) |
10.1 |
5/18/05 |
|||||
10.16 |
Amendment Agreement to Purchase Agreement between the Company and PRF Vaccine Holdings LLC, dated as of March 14, 2006 |
8-K (000-15006) |
10.1 |
3/15/06 |
|||||
*10.17 |
Exclusive License Agreement dated February 1, 2003 by and between Thomas Jefferson University ("TJU") and the Company |
S-4 (333-148291) |
10.1 |
1/18/08 |
|||||
*10.18 |
License Agreement dated as of November 1, 2005 by and between The Rockefeller University and the Company |
S-4 (333-148291) |
10.2 |
1/18/08 |
|||||
*10.19 |
License Agreement dated September 1, 2006 by and between Duke University and the Company |
S-4 (333-148291) |
10.3 |
1/18/08 |
|||||
*10.20 |
Assignment and License Agreement, as amended, dated April 6, 2004 by and among Medarex, Inc., GenPharm International, Inc. and the Company |
S-4 (333-148291) |
10.4 |
1/18/08 |
|||||
*10.21 |
Research and Commercialization Agreement, as amended, dated as of April 6, 2004 by and among Medarex, Inc., GenPharm International, Inc. and the Company |
S-4 (333-148291) |
10.5 |
1/18/08 |
|||||
*10.22 |
Supply Agreement dated August 18, 2006 by and between the Company and Biosyn |
S-4 (333-148291) |
10.9 |
1/18/08 |
|||||
10.23 |
License and Development Agreement dated as of April 16, 2008 between the Company and Pfizer Vaccines, LLC |
10-Q (000-15006) |
10.1 |
5/19/08 |
|||||
*10.24 |
Research Collaboration and Commercialization Agreement effective October 20, 2006 between the Company and the Ludwig Institute for Cancer Research |
10-K (000-15006) |
10.45 |
3/2/09 |
|||||
*10.25 |
Vaccine Adjuvant License and Collaboration Agreement dated on May 30, 2008 between the Company and 3M Innovation Properties Company |
10-K (000-15006) |
10.46 |
3/2/09 |
|||||
110
| |
|
Incorporated by Reference to | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Description | Form and SEC File No. |
Exhibit No. |
SEC Filing Date |
|||||
| *10.26 | Exclusive Patent and Know-How License Agreement dated as of November 5, 2008 between the Company and the University of Southampton | 10-K (000-15006) |
10.47 | 3/2/09 | |||||
*10.27 |
Collaboration Agreement dated June 18, 2004 between Seattle Genetics and CuraGen |
Filed herewith |
|||||||
*10.28 |
Second Restated Collaboration Agreement dated April 12, 2004 and amended October 19, 2004 between Abgenix Inc. and CuraGen |
Filed herewith |
|||||||
10.29 |
Amgen Letter Agreement, by and between CuraGen and Amgen Fremont, Inc. dated May 2, 2009 |
Filed herewith |
|||||||
*10.30 |
Transfer and Termination Agreement, dated as of April 21, 2008 by and between TopoTarget A/S and CuraGen |
Filed herewith |
|||||||
Material Contracts—Stock Purchase, Financing and Credit Agreements |
|||||||||
| *10.31 | Security Agreement, by and between the Company and the Massachusetts Development Finance Agency, dated as of December 22, 2003 | 10-Q (000-15006) |
10.2 | 4/30/04 | |||||
*10.32 |
Secured Promissory Note: Equipment Loan, by and between the Company and the Massachusetts Development Finance Agency, dated as of December 22, 2003 |
10-Q (000-15006) |
10.3 |
4/30/04 |
|||||
*10.33 |
Common Stock Purchase Agreement dated as of April 16, 2008 between the Company and Pfizer Vaccines, LLC |
10-Q (000-15006) |
10.2 |
8/11/08 |
|||||
Material Contracts—Management Contracts and Compensatory Plans |
|||||||||
| †10.34 | 2008 Stock Option and Incentive Plan, as amended and restated | Filed herewith | |||||||
†10.35 |
2004 Employee Stock Purchase Plan, as amended and restated |
Filed herewith |
|||||||
†10.36 |
Employment Agreement, dated January 6, 2009, by and between the Company and Avery W. Catlin |
8-K (000-15006) |
10.1 |
1/8/09 |
|||||
†10.37 |
Employment Agreement, dated January 6, 2009, by and between the Company and Thomas Davis, MD |
8-K (000-15006) |
10.2 |
1/8/09 |
|||||
†10.38 |
Employment Agreement, dated January 6, 2009, by and between the Company and Tibor Keler, Ph.D. |
8-K (000-15006) |
10.3 |
1/8/09 |
|||||
†10.39 |
Amended and Restated Employment Agreement, dated January 6, 2009, by and between the Company and Anthony S. Marucci. |
8-K (000-15006) |
10.4 |
1/8/09 |
|||||
†10.40 |
Form of Stock Option Agreement |
8-K (000-15006) |
10.1 |
1/25/10 |
|||||
111
| |
|
Incorporated by Reference to | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Description | Form and SEC File No. |
Exhibit No. |
SEC Filing Date |
|||||
| †10.41 | CuraGen 2007 Stock Incentive Plan, amended and restated | Filed herewith | |||||||
†10.42 |
Form of Restricted Stock Award |
Filed herewith |
|||||||
21.0 |
List of Subsidiaries |
Filed herewith |
|||||||
23.1 |
Consent of PricewaterhouseCoopers LLP Independent Registered Public Accounting Firm of Celldex Therapeutics, Inc. (formerly known as AVANT Immunotherapeutics, Inc.) |
Filed herewith |
|||||||
23.2 |
Consent of Ernst & Young LLP Independent Registered Public Accounting Firm of Celldex Research Corporation (formerly known as Celldex Therapeutics, Inc.) |
Filed herewith |
|||||||
31.1 |
Certification of President and Chief Executive Officer |
Filed herewith |
|||||||
31.2 |
Certification of Senior Vice President and Chief Financial Officer |
Filed herewith |
|||||||
32 |
Section 1350 Certifications |
Furnished herewith |
|||||||
- *
- Confidential
treatment has been requested for certain provisions of this Exhibit pursuant to Rule 24b-2 promulgated under the Securities
Exchange Act of 1934, as amended.
- †
- Indicates a management contract or compensation plan, contract or arrangement.
112
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| |
|
|
||
|---|---|---|---|---|
|
CELLDEX THERAPEUTICS, INC. | |||
|
By: |
/s/ ANTHONY S. MARUCCI |
||
Date |
Anthony S. Marucci | |||
March 12, 2010 |
President and Chief Executive Officer | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ ANTHONY S. MARUCCI Anthony S. Marucci |
President, Chief Executive Officer, and Director (Principal Executive Officer) |
March 12, 2010 | ||
/s/ AVERY W. CATLIN Avery W. Catlin |
Senior Vice President, Chief Financial Officer and Treasurer (Principal Financial and Accounting Officer) |
March 12, 2010 |
||
/s/ LARRY ELLBERGER Larry Ellberger |
Director, Chairman of the Board of Directors |
March 12, 2010 |
||
/s/ HERBERT J. CONRAD Herbert J. Conrad |
Director |
March 12, 2010 |
||
/s/ GEORGE O. ELSTON George O. Elston |
Director |
March 12, 2010 |
||
/s/ KAREN SHOOS LIPTON Karen Shoos Lipton |
Director |
March 12, 2010 |
||
/s/ DR. RAJESH B. PAREKH Dr. Rajesh B. Parekh |
Director |
March 12, 2010 |
||
/s/ HARRY H. PENNER, JR. Harry H. Penner, Jr. |
Director |
March 12, 2010 |
||
/s/ CHARLES R. SCHALLER Charles R. Schaller |
Director |
March 12, 2010 |
||
/s/ TIMOTHY M. SHANNON, M.D. Timothy M. Shannon, M.D. |
Director |
March 12, 2010 |
113
