Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Cardiff Oncology, Inc. | a12-26328_18k.htm |
Exhibit 99.1
|
|
Introduction to Trovagene, Inc. Antonius Schuh, PhD Chief Executive Officer November 2012 |
|
|
Forward-Looking Statements DISCLAIMER Statements in this presentation about the Company's expectations, applications of its technology, markets, launch of tests and other statements that are not historical facts are "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on management's current beliefs, assumptions, estimates and projections. Actual results may differ materially from those projected in the forward-looking statements for various reasons, including risks associated with product and test development, test transfer to contracting labs, government regulation, market acceptance, limited commercial experience, dependence on key personnel, obtaining financing and other factors discussed in the Company's periodic reports filed with the Securities and Exchange Commission. |
|
|
Company Overview Molecular diagnostics company Internal focus in oncology Collaboration opportunities in prenatal diagnostics, infectious diseases and transplant medicine IP protects detection of transrenal nucleic acids in urine (“DNA in urine”) Founded in 1999, $40M invested in R&D and IP Hired new management team (Q4 2011 – Q1 2012) Completed acquisition of CLIA lab in Feb 2012 NASDAQ listing in June 2012: TROV INTRODUCTION |
|
|
Experienced Management Team CEO - Antonius Schuh, PhD Sorrento Therapeutics, AviaraDx, Arcturus Biosciences, Sequenom CFO - Stephen Zaniboni, MBA, CPA Awarepoint, Xifin, AviaraDx, Sequenom, Arthur Anderson CTO - Charlie Rodi, PhD Sorrento Therapeutics, Icx BioSystems, Sequenom, Monsanto/G.D. Searle INTRODUCTION |
|
|
New Website – www.trovagene.com INTRODUCTION Corporate Board Thomas Adams, PhD - Chairman John P. Brancaccio, CPA Gabriele M. Cerrone, MBA Gary S. Jacob, PhD Antonius Schuh, PhD Stanley Tennant, MD Scientific Advisory Board Carlo M. Croce, MD Riccardo Dalla-Favera, MD Mark Erlander, PhD Brunangelo Falini, MD Sinuhe Hahn, MD |
|
|
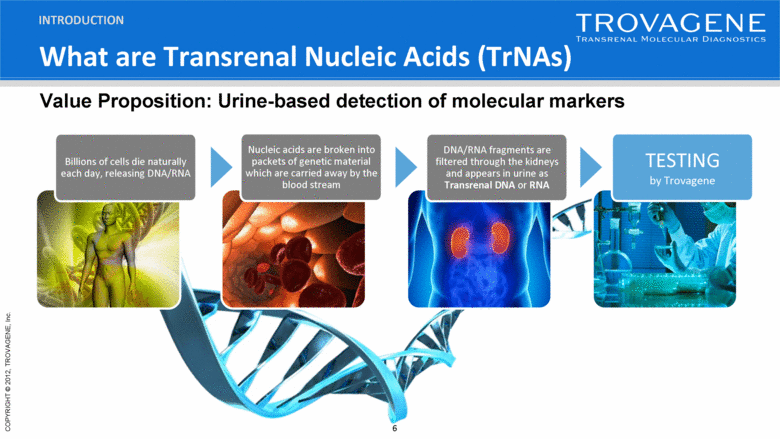
What are Transrenal Nucleic Acids (TrNAs) INTRODUCTION Billions of cells die naturally each day, releasing DNA/RNA Nucleic acids are broken into packets of genetic material which are carried away by the blood stream DNA/RNA fragments are filtered through the kidneys and appears in urine as Transrenal DNA or RNA TESTING by Trovagene Value Proposition: Urine-based detection of molecular markers |
|
|
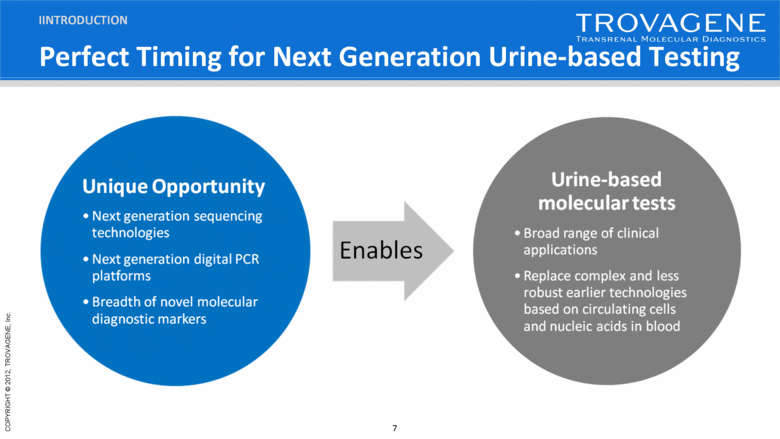
Perfect Timing for Next Generation Urine-based Testing IINTRODUCTION |
|
|
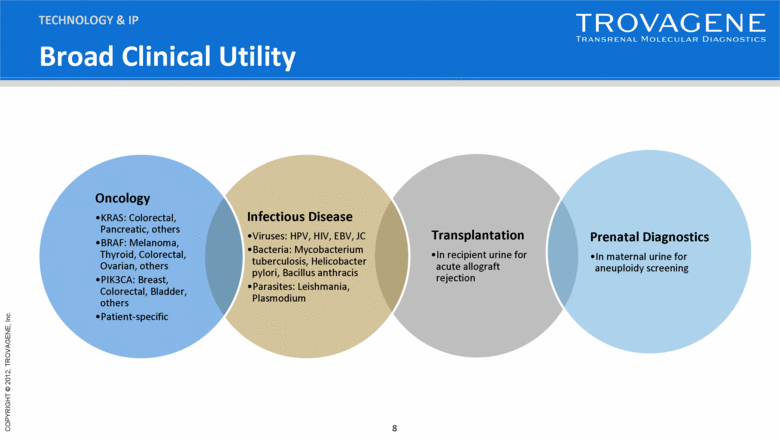
Transplantation In recipient urine for acute allograft rejection Prenatal Diagnostics In maternal urine for aneuploidy screening Infectious Disease Viruses: HPV, HIV, EBV, JC Bacteria: Mycobacterium tuberculosis, Helicobacter pylori, Bacillus anthracis Parasites: Leishmania, Plasmodium Oncology KRAS: Colorectal, Pancreatic, others BRAF: Melanoma, Thyroid, Colorectal, Ovarian, others PIK3CA: Breast, Colorectal, Bladder, others Patient-specific TECHNOLOGY & IP Broad Clinical Utility |
|
|
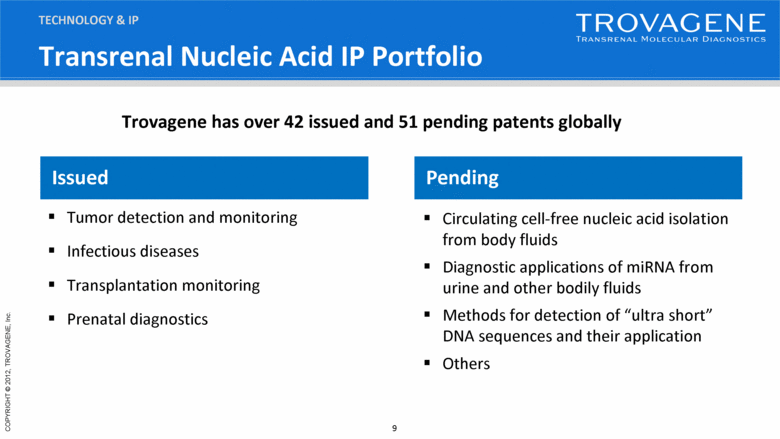
Transrenal Nucleic Acid IP Portfolio Issued Tumor detection and monitoring Infectious diseases Transplantation monitoring Prenatal diagnostics Pending Circulating cell-free nucleic acid isolation from body fluids Diagnostic applications of miRNA from urine and other bodily fluids Methods for detection of “ultra short” DNA sequences and their application Others TECHNOLOGY & IP Trovagene has over 42 issued and 51 pending patents globally |
|
|
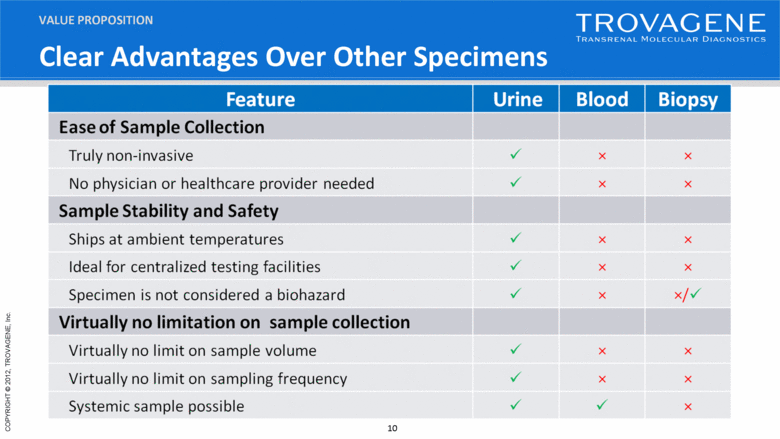
Clear Advantages Over Other Specimens VALUE PROPOSITION |
|
|
Oncogene Mutation Detection |
|
|
Market Entry: Oncology Very large market opportunity Demonstration of clinical utility is straight-forward Urine as a sample has enabling features for this market Role of oncogene mutation detection is already established Patients are concentrated at large oncology centers ADDRESSABLE MARKET |
|
|
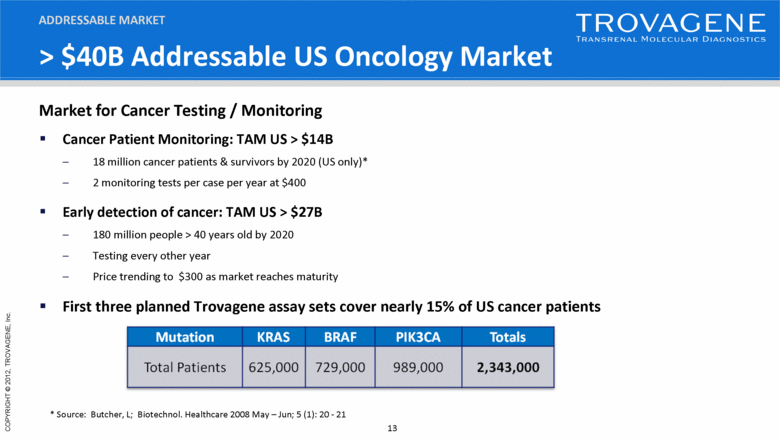
> $40B Addressable US Oncology Market Cancer Patient Monitoring: TAM US > $14B 18 million cancer patients & survivors by 2020 (US only)* 2 monitoring tests per case per year at $400 Early detection of cancer: TAM US > $27B 180 million people > 40 years old by 2020 Testing every other year Price trending to $300 as market reaches maturity First three planned Trovagene assay sets cover nearly 15% of US cancer patients ADDRESSABLE MARKET * Source: Butcher, L; Biotechnol. Healthcare 2008 May – Jun; 5 (1): 20 - 21 Market for Cancer Testing / Monitoring |
|
|
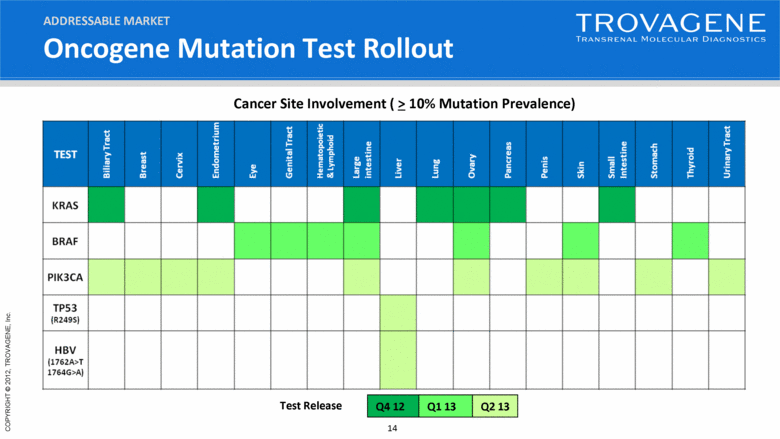
Oncogene Mutation Test Rollout Q4 12 Q1 13 Q2 13 Test Release Cancer Site Involvement ( > 10% Mutation Prevalence) ADDRESSABLE MARKET |
|
|
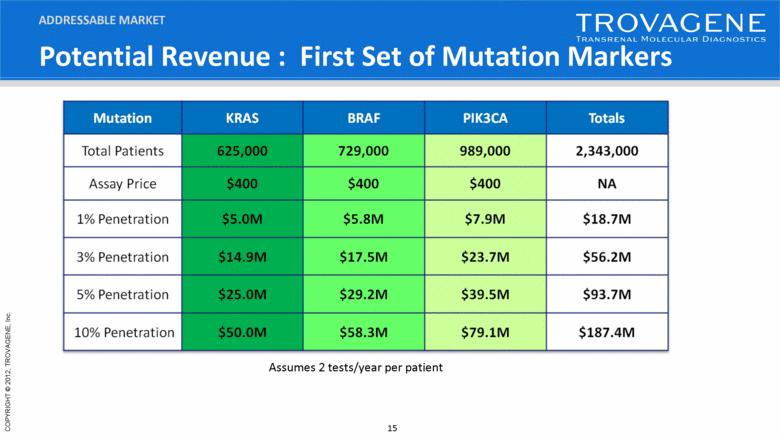
Potential Revenue : First Set of Mutation Markers ADDRESSABLE MARKET Assumes 2 tests/year per patient Mutation KRAS BRAF PIK3CA Totals Total Patients 625,000 729,000 989,000 2,343,000 Assay Price $400 $400 $400 NA 1% Penetration $5.0M $5.8M $7.9M $18.7M 3% Penetration $14.9M $17.5M $23.7M $56.2M 5% Penetration $25.0M $29.2M $39.5M $93.7M 10% Penetration $50.0M $58.3M $79.1M $187.4M |
|
|
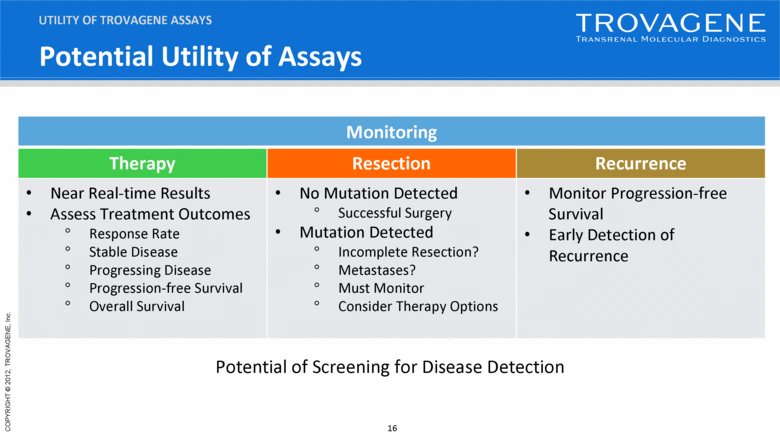
Potential Utility of Assays UTILITY OF TROVAGENE ASSAYS Monitoring Therapy Resection Recurrence Near Real-time Results Assess Treatment Outcomes Response Rate Stable Disease Progressing Disease Progression-free Survival Overall Survival No Mutation Detected Successful Surgery Mutation Detected Incomplete Resection? Metastases? Must Monitor Consider Therapy Options Monitor Progression-free Survival Early Detection of Recurrence Potential of Screening for Disease Detection |
|
|
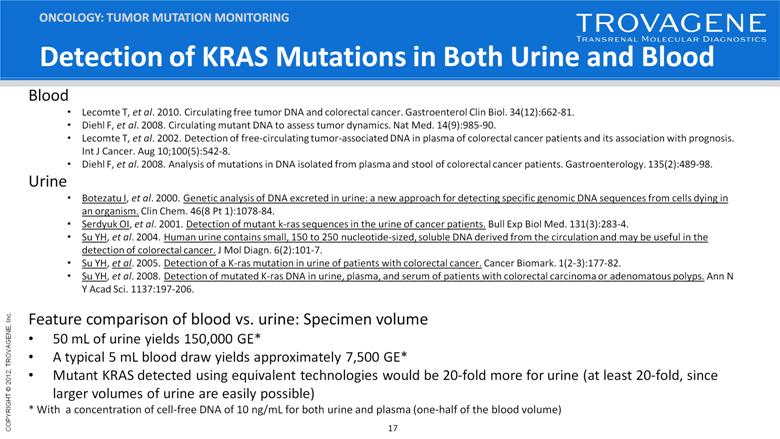
Detection of KRAS Mutations in Both Urine and Blood ONCOLOGY: TUMOR MUTATION MONITORING Blood Lecomte T, et al. 2010. Circulating free tumor DNA and colorectal cancer. Gastroenterol Clin Biol. 34(12):662-81. Diehl F, et al. 2008. Circulating mutant DNA to assess tumor dynamics. Nat Med. 14(9):985-90. Lecomte T, et al. 2002. Detection of free-circulating tumor-associated DNA in plasma of colorectal cancer patients and its association with prognosis. Int J Cancer. Aug 10;100(5):542-8. Diehl F, et al. 2008. Analysis of mutations in DNA isolated from plasma and stool of colorectal cancer patients. Gastroenterology. 135(2):489-98. Urine Botezatu I, et al. 2000. Genetic analysis of DNA excreted in urine: a new approach for detecting specific genomic DNA sequences from cells dying in an organism. Clin Chem. 46(8 Pt 1):1078-84. Serdyuk OI, et al. 2001. Detection of mutant k-ras sequences in the urine of cancer patients. Bull Exp Biol Med. 131(3):283-4. Su YH, et al. 2004. Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer. J Mol Diagn. 6(2):101-7. Su YH, et al. 2005. Detection of a K-ras mutation in urine of patients with colorectal cancer. Cancer Biomark. 1(2-3):177-82. Su YH, et al. 2008. Detection of mutated K-ras DNA in urine, plasma, and serum of patients with colorectal carcinoma or adenomatous polyps. Ann N Y Acad Sci. 1137:197-206. Feature comparison of blood vs. urine: Specimen volume 50 mL of urine yields 150,000 GE* A typical 5 mL blood draw yields approximately 7,500 GE* Mutant KRAS detected using equivalent technologies would be 20-fold more for urine (at least 20-fold, since larger volumes of urine are easily possible) * With a concentration of cell-free DNA of 10 ng/mL for both urine and plasma (one-half of the blood volume) |
|
|
Technology Leadership Isolation of short nucleic acid fragments from urine Yields are significantly higher than from competitors kits Higher yields mean more molecules to detect Development of ultra-short amplicon assays Optimizes detection of the short nucleic acids that pass through the kidney Shorter amplicon assays mean more molecules detected, better signals Application of droplet digital PCR ONCOLOGY: TUMOR MUTATION MONITORING |
|
|
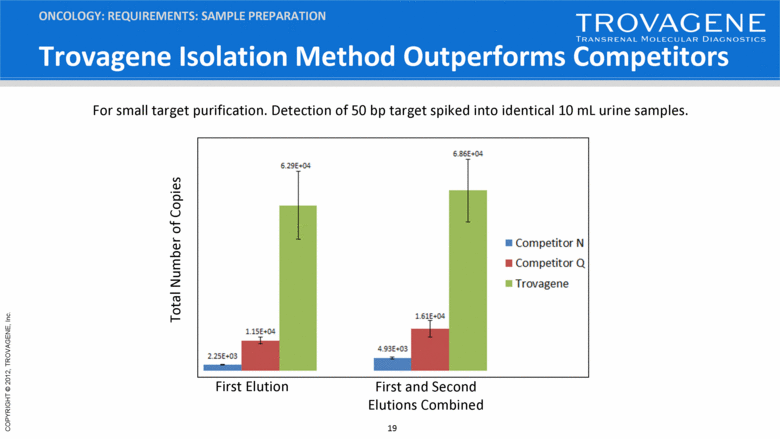
Trovagene Isolation Method Outperforms Competitors ONCOLOGY: REQUIREMENTS: SAMPLE PREPARATION For small target purification. Detection of 50 bp target spiked into identical 10 mL urine samples. First Elution First and Second Elutions Combined Total Number of Copies |
|
|
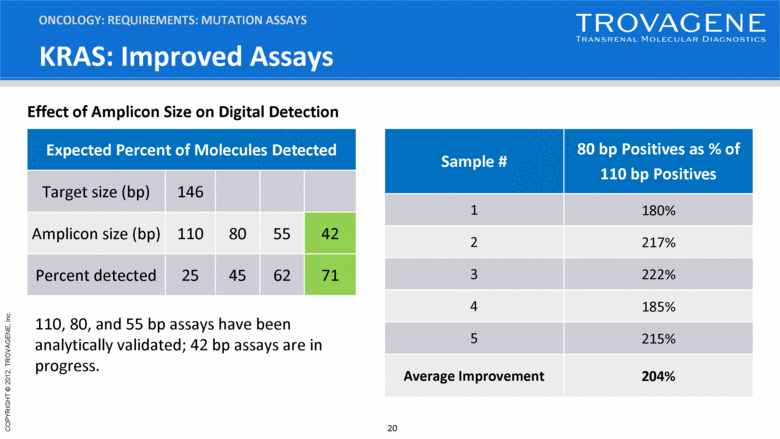
KRAS: Improved Assays ONCOLOGY: REQUIREMENTS: MUTATION ASSAYS Effect of Amplicon Size on Digital Detection Sample # 80 bp Positives as % of 110 bp Positives 1 180% 2 217% 3 222% 4 185% 5 215% Average Improvement 204% Expected Percent of Molecules Detected Target size (bp) 146 Amplicon size (bp) 110 80 55 42 Percent detected 25 45 62 71 110, 80, and 55 bp assays have been analytically validated; 42 bp assays are in progress. |
|
|
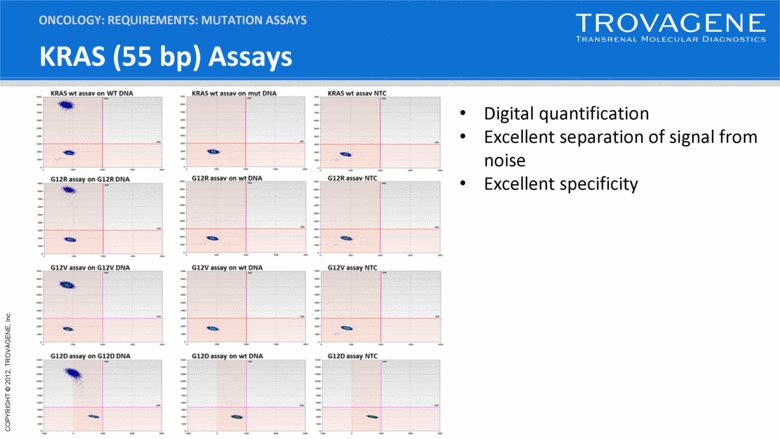
KRAS (55 bp) Assays ONCOLOGY: REQUIREMENTS: MUTATION ASSAYS Digital quantification Excellent separation of signal from noise Excellent specificity |
|
|
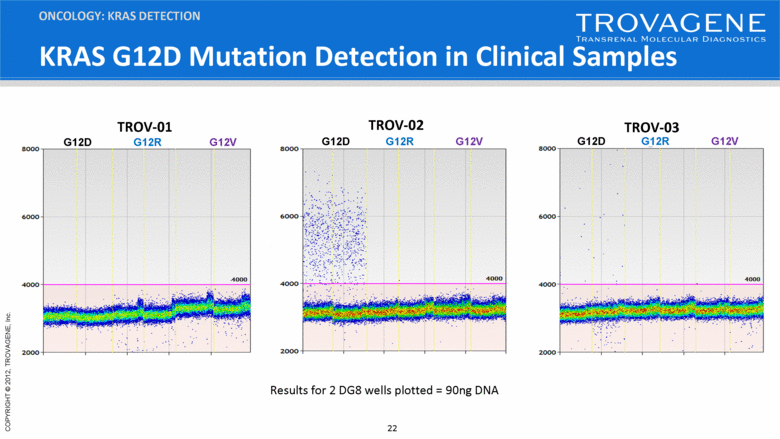
KRAS G12D Mutation Detection in Clinical Samples ONCOLOGY: KRAS DETECTION TROV-02 G12D G12R G12V TROV-01 G12D G12R G12V TROV-03 G12D G12R G12V Results for 2 DG8 wells plotted = 90ng DNA |
|
|
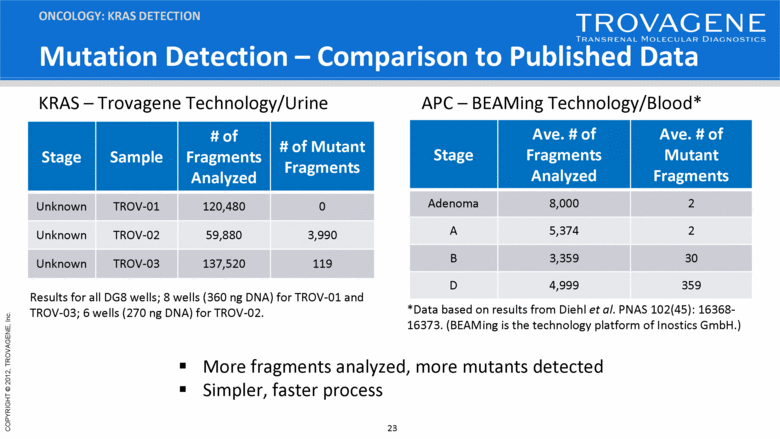
Mutation Detection – Comparison to Published Data ONCOLOGY: KRAS DETECTION Stage Ave. # of Fragments Analyzed Ave. # of Mutant Fragments Adenoma 8,000 2 A 5,374 2 B 3,359 30 D 4,999 359 Results for all DG8 wells; 8 wells (360 ng DNA) for TROV-01 and TROV-03; 6 wells (270 ng DNA) for TROV-02. Stage Sample # of Fragments Analyzed # of Mutant Fragments Unknown TROV-01 120,480 0 Unknown TROV-02 59,880 3,990 Unknown TROV-03 137,520 119 KRAS – Trovagene Technology/Urine APC – BEAMing Technology/Blood* *Data based on results from Diehl et al. PNAS 102(45): 16368-16373. (BEAMing is the technology platform of Inostics GmbH.) More fragments analyzed, more mutants detected Simpler, faster process |
|
|
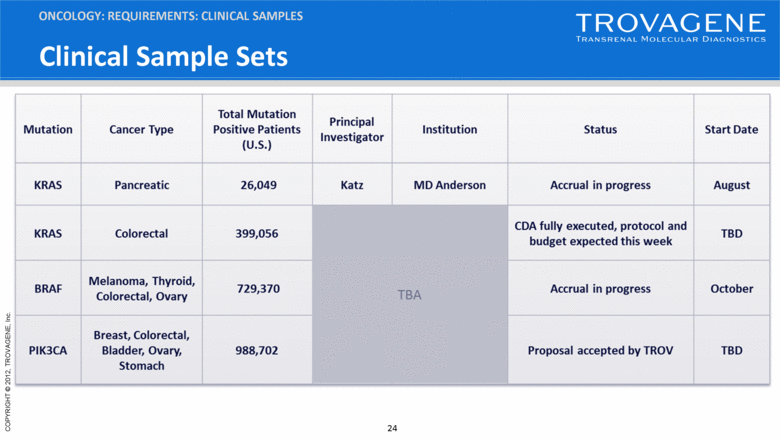
Clinical Sample Sets ONCOLOGY: REQUIREMENTS: CLINICAL SAMPLES |
|
|
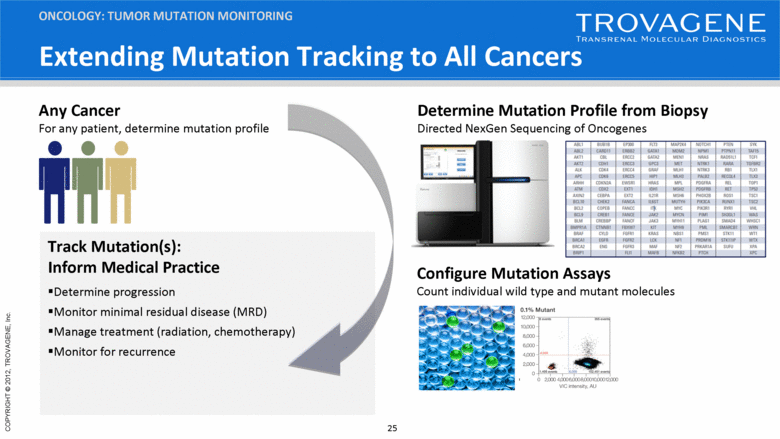
Extending Mutation Tracking to All Cancers ONCOLOGY: TUMOR MUTATION MONITORING Track Mutation(s): Inform Medical Practice Determine progression Monitor minimal residual disease (MRD) Manage treatment (radiation, chemotherapy) Monitor for recurrence Configure Mutation Assays Count individual wild type and mutant molecules Any Cancer For any patient, determine mutation profile Determine Mutation Profile from Biopsy Directed NexGen Sequencing of Oncogenes |
|
|
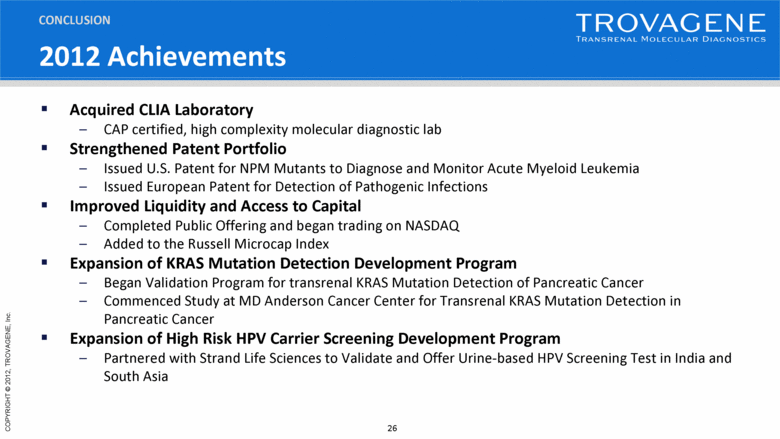
2012 Achievements CONCLUSION Acquired CLIA Laboratory CAP certified, high complexity molecular diagnostic lab Strengthened Patent Portfolio Issued U.S. Patent for NPM Mutants to Diagnose and Monitor Acute Myeloid Leukemia Issued European Patent for Detection of Pathogenic Infections Improved Liquidity and Access to Capital Completed Public Offering and began trading on NASDAQ Added to the Russell Microcap Index Expansion of KRAS Mutation Detection Development Program Began Validation Program for transrenal KRAS Mutation Detection of Pancreatic Cancer Commenced Study at MD Anderson Cancer Center for Transrenal KRAS Mutation Detection in Pancreatic Cancer Expansion of High Risk HPV Carrier Screening Development Program Partnered with Strand Life Sciences to Validate and Offer Urine-based HPV Screening Test in India and South Asia |
|
|
Summary and Financials Through June 30, 2012 |
|
|
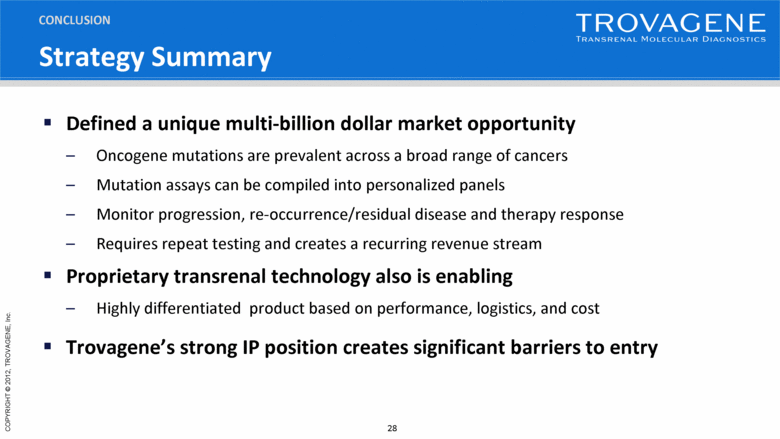
Strategy Summary Defined a unique multi-billion dollar market opportunity Oncogene mutations are prevalent across a broad range of cancers Mutation assays can be compiled into personalized panels Monitor progression, re-occurrence/residual disease and therapy response Requires repeat testing and creates a recurring revenue stream Proprietary transrenal technology also is enabling Highly differentiated product based on performance, logistics, and cost Trovagene’s strong IP position creates significant barriers to entry CONCLUSION |
|
|
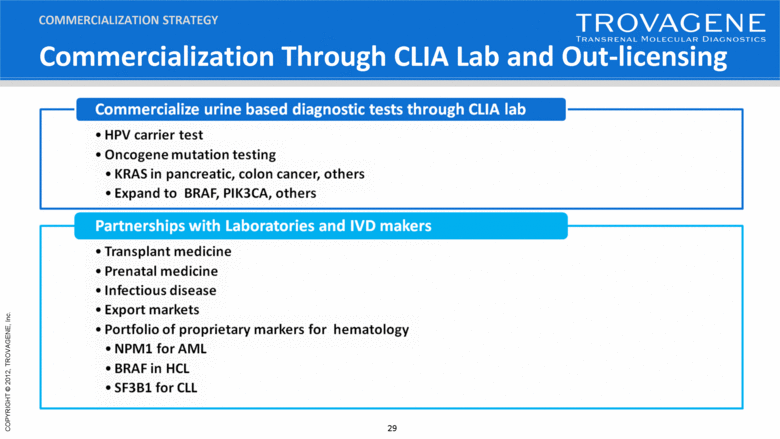
Commercialization Through CLIA Lab and Out-licensing COMMERCIALIZATION STRATEGY |
|
|
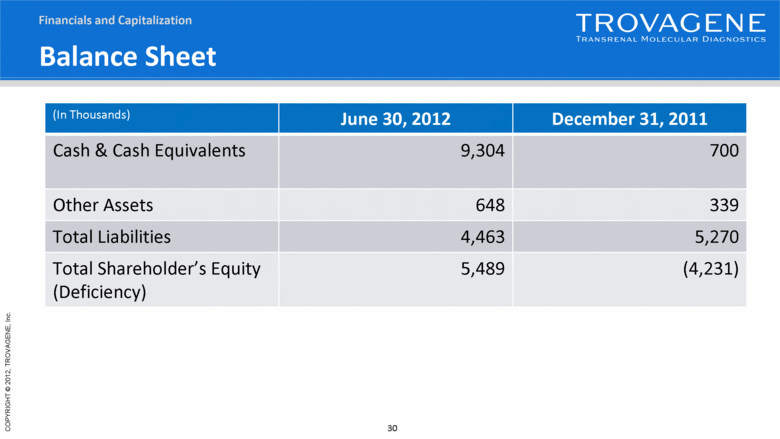
Balance Sheet (In Thousands) June 30, 2012 December 31, 2011 Cash & Cash Equivalents 9,304 700 Other Assets 648 339 Total Liabilities 4,463 5,270 Total Shareholder’s Equity (Deficiency) 5,489 (4,231) Financials and Capitalization |
|
|
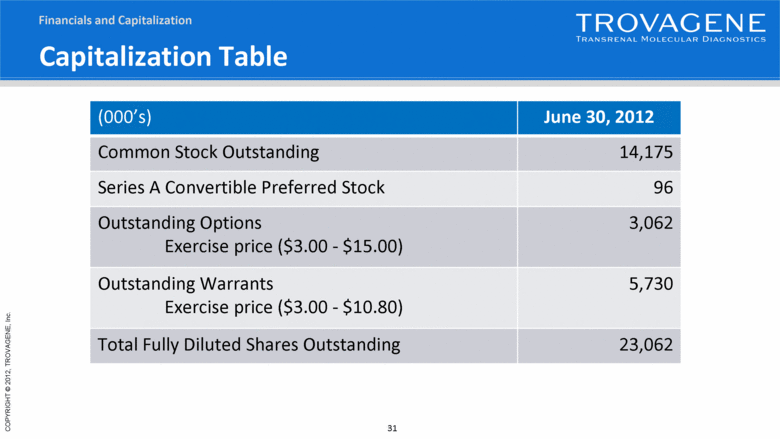
Capitalization Table (000’s) June 30, 2012 Common Stock Outstanding 14,175 Series A Convertible Preferred Stock 96 Outstanding Options Exercise price ($3.00 - $15.00) 3,062 Outstanding Warrants Exercise price ($3.00 - $10.80) 5,730 Total Fully Diluted Shares Outstanding 23,062 Financials and Capitalization |
|
|
For further information contact: Antonius Schuh, CEO aschuh@trovagene.com Stephen Zaniboni, CFO szaniboni@trovagene.com |
|
|
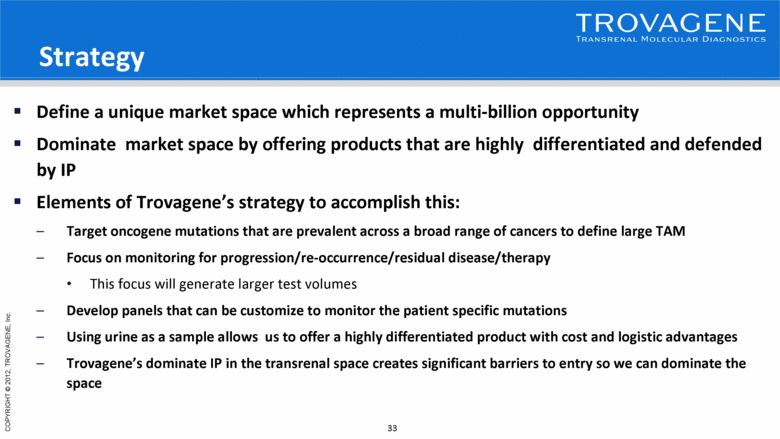
Strategy Define a unique market space which represents a multi-billion opportunity Dominate market space by offering products that are highly differentiated and defended by IP Elements of Trovagene’s strategy to accomplish this: Target oncogene mutations that are prevalent across a broad range of cancers to define large TAM Focus on monitoring for progression/re-occurrence/residual disease/therapy This focus will generate larger test volumes Develop panels that can be customize to monitor the patient specific mutations Using urine as a sample allows us to offer a highly differentiated product with cost and logistic advantages Trovagene’s dominate IP in the transrenal space creates significant barriers to entry so we can dominate the space |