Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Tonix Pharmaceuticals Holding Corp. | v315888_8k.htm |

Corporate Presentation June 12, 2012 OTC/QB: TNXP

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Forward - looking Statements The statements and discussions contained in this presentation that are not historical facts constitute forward - looking statement s. These can be identified by the use of forward - looking words, such as “believes”, “expects”, “may”, “intends”, “anticipates”, “plans”, “es timates”, or any other analogous or similar expressions intended to identify forward - looking statements. These forward - looking statements and es timates as to future performance, estimates as to future valuations, and other statements contained herein regarding matters that are no t h istorical facts, are only predictions and actual events or results may differ materially. We cannot assure or guarantee that any futur e r esults described in this presentation will be achieved, and actual results could vary materially from those reflected in such forward - looking sta tements. Information contained in this presentation has been complied from sources believed to be credible and reliable. However, we ca nnot guarantee such credibility and reliability. The forecasts and projections of events contained herein are based upon subjecti ve valuations, analyses, and personal opinions. Information Regarding Disclosures The Common Stock and Warrants have not and will not be registered under the Securities Act of 1933, as amended (the “Act”), o r u nder any state securities laws, nor has the Securities and Exchange Commission (the “Commission”) or any state regulatory authority en dor sed the Offering. Any representation to the contrary is a criminal offense. In making an investment decision, investors must rely upon their own examination of the company and the terms of the Offering , i ncluding the merits and risks involved. The acquisition of the Stock, if offered, should be considered only by persons who can bear t he economic risk of their investment of ran indefinite period of time and can afford a total loss of their investment. Each prospective inves tor in the Offering should, prior to purchasing any Stock, consult his own attorney and business advisor as to the legal, business, tax, and rel ate d matters concerning its investment and is urged to ask questions of, and receive answers from, the Company concerning the terms and co ndi tions of the Offering and request any additional information they may consider Necessary in making an informed investment decision. This presentation does not constitute an offer to sell or a solicitation of an offer to purchase any securities of any nature wh atsoever, nor do the contents of the presentation constitute legal, tax, or business advice. This presentation and the offering of the Company's Stock shall be kept confidential. The recipient agrees not to disclose t o a ny third party any information contained herein, or any terms, conditions, or other facts with respect to he Offering, including, without li mit ation, that the Company is or may be contemplating the Offering. Information included herewith has been obtained from the Company and other sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by the Company. Any representations and warranties will be contained only in a definitive agreement signed by the investor and the Company. 2 Disclosures

TONIX PHARMACEUTICALS • Specialty pharmaceutical company developing innovative non - addictive products for chronic pain syndromes - Fibromyalgia syndrome (FM ) - Post - traumatic stress disorder (PTSD) • Unmet medical needs and large commercial opportunities - Targeting sleep pathology - Central pain syndromes don’t respond to opiate pain drugs or benzodiazepine sleep drugs • Capital efficient, risk - mitigated development pathway - Near - term value - creating milestones • Experienced management and board Company Overview 3

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS 4 Experienced Leadership Selected Previous Corporate Affiliations Selected Previous Product Affiliations Seth Lederman, MD CEO & Chairman • Vela • Targent • Validus • Fontus Benjamin Selzer COO • Reliant • Aton • Investment Banking Leland Gershell, MD, PhD CFO • Cowen • Apothecary • Favus • Madison Williams Bruce Daugherty, PhD, MBA Senior Director of Drug Development • Merck • Roche Institute
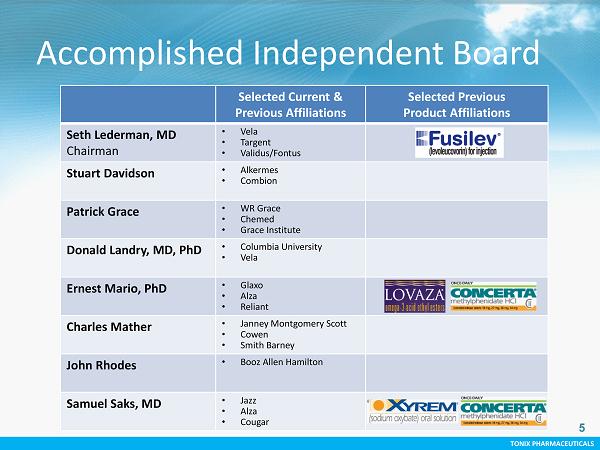
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS 5 Accomplished Independent Board Selected Current & Previous Affiliations Selected Previous Product Affiliations Seth Lederman, MD Chairman • Vela • Targent • Validus/Fontus Stuart Davidson • Alkermes • Combion Patrick Grace • WR Grace • Chemed • Grace Institute Donald Landry, MD, PhD • Columbia University • Vela Ernest Mario, PhD • Glaxo • Alza • Reliant Charles Mather • Janney Montgomery Scott • Cowen • Smith Barney John Rhodes • Booz Allen Hamilton Samuel Saks, MD • Jazz • Alza • Cougar
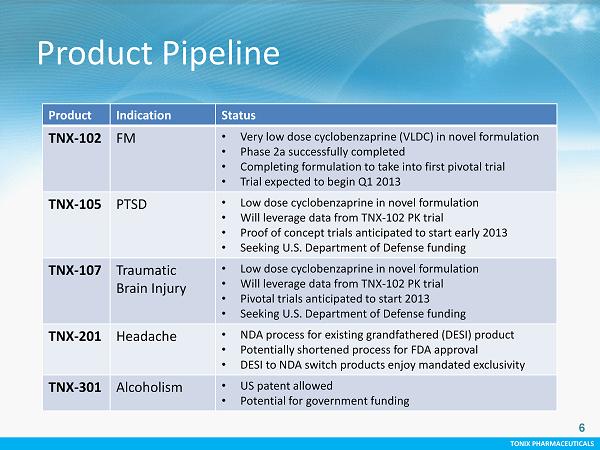
TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS Product Pipeline 6 Product Indication Status TNX - 102 FM • Very low dose cyclobenzaprine (VLDC) in novel formulation • Phase 2a successfully completed • Completing formulation to take into first pivotal trial • T rial expected to begin Q1 2013 TNX - 105 PTSD • Low dose cyclobenzaprine in novel formulation • Will leverage data from TNX - 102 PK trial • Proof of concept trials anticipated to start early 2013 • Seeking U.S. Department of Defense funding TNX - 107 Traumatic Brain Injury • Low dose cyclobenzaprine in novel formulation • Will leverage data from TNX - 102 PK trial • Pivotal trials anticipated to start 2013 • Seeking U.S. Department of Defense funding TNX - 201 Headache • NDA process for existing grandfathered (DESI) product • Potentially shortened process for FDA approval • DESI to NDA switch products enjoy mandated exclusivity TNX - 301 Alcoholism • US patent allowed • Potential for government funding
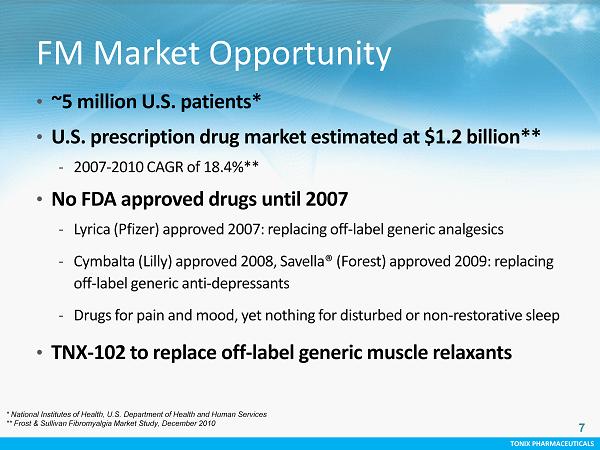
TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS FM Market Opportunity • ~5 million U.S. patients* • U.S. prescription drug market estimated at $1.2 billion** - 2007 - 2010 CAGR of 18.4 %** • No FDA approved drugs until 2007 - Lyrica (Pfizer) approved 2007: replacing off - label generic analgesics - Cymbalta (Lilly) approved 2008, Savella® (Forest) approved 2009: replacing off - label generic anti - depressants - Drugs for pain and mood, yet nothing for disturbed or non - restorative sleep • TNX - 102 to replace off - label generic muscle relaxants * National Institutes of Health, U.S. Department of Health and Human Services ** Frost & Sullivan Fibromyalgia Market Study, December 2010 7
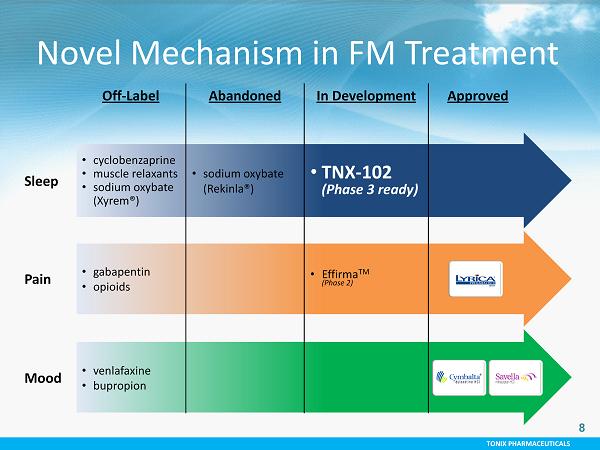
TONIX PHARMACEUTICALS 8 Off - Label Abandoned In Development Approved Sleep • cyclobenzaprine • muscle relaxants • sodium oxybate (Xyrem®) • sodium oxybate (Rekinla®) • TNX - 102 (Phase 3 ready) Pain • gabapentin • opioids • Effirma TM (Phase 2) Mood • venlafaxine • bupropion Novel Mechanism in FM Treatment

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Impressive Safety, Widely Used • Off - label cyclobenzaprine is the third most widely prescribed medication for FM* • 1977: FDA approved Flexeril® (Merck) • 1990s: Extensive safety and efficacy studies (Merck) • 2007: FDA approved controlled - release formulations (15/30 mg) • 2010: >1 billion tablets prescribed • Not a controlled substance, no recognized addictive potential 9 * Frost & Sullivan Fibromyalgia Market Study, December 2010
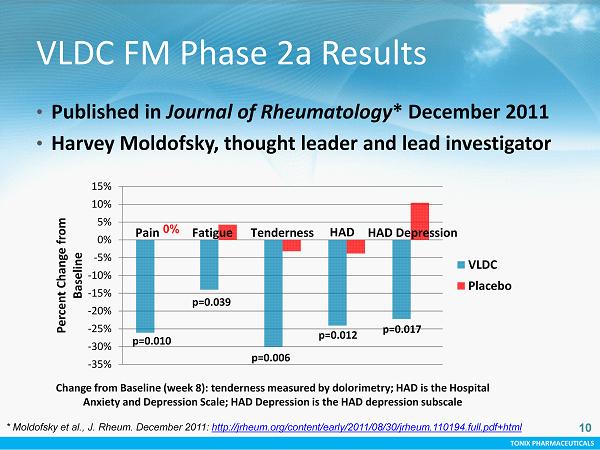
TONIX PHARMACEUTICALS Change from Baseline (week 8): tenderness measured by dolorimetry; HAD is the Hospital Anxiety and Depression Scale; HAD Depression is the HAD depression subscale -35% -30% -25% -20% -15% -10% -5% 0% 5% 10% 15% Percent Change from Baseline VLDC Placebo p=0.010 p=0.039 p=0.006 p=0.012 p=0.017 Fatigue HAD HAD Depression Pain Tenderness 0% 10 * Moldofsky et al., J. Rheum. December 2011: http://jrheum.org/content/early/2011/08/30/jrheum.110194.full.pdf+html • P ublished in Journal of Rheumatology * December 2011 • Harvey Moldofsky, thought leader and lead investigator VLDC FM Phase 2a Results
TONIX PHARMACEUTICALS TNX - 102: Addressing the Limitations of Cyclobenzaprine for Fibromyalgia 11 • Cyclobenzaprine currently widely used off label in the management of FM • Current dose and formulation not optimal for FM - Long half life contributes to somnolence and accumulation - Lowest approved daily dose is 15mg • TNX - 102 is designed specifically for FM - Fast absorption - Elevated blood levels during the night to improve sleep quality - Low next - day blood levels to minimize morning somnolence - Significantly lower dose

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS TNX - 102: Unique Market Position • Specifically designed for the treatment of FM • Differentiated from / not competitive with other FM therapies - First - in - class sleep quality treatment indicated for bedtime dosing - Restorative sleep shown to improve pain and fatigue - High patient dissatisfaction, physicians frequently switch drugs • With a unique formulation and new indication, reimbursement coverage is expected 12

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS TNX - 102: Development Plan • Formulation and PK trials - 30 - subject, 3 - week PK study on first version completed - Completion of PK study on second version expected in September 2012 - Pharmacodynamic study contemplated by year - end • Pivotal efficacy trial - 2 - arm, 12 - week study with 150 patients per arm - Study design and endpoints to mirror those used by Lyrica and Cymbalta • Pain and a composite endpoint of other FM symptoms - Final study results expected 1H 2014 • Partnership for second pivotal trial and commercialization 13

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS TNX - 105: VLDC for PTSD • 3.5% of U.S. adult population has suffered from PTSD in past 12 months* - Any trauma can lead to PTSD • Unsatisfied market - Only Zoloft® and Paxil® have FDA approval • Widespread painkiller abuse and addiction • Leverage formulation and clinical work of TNX - 102 to advance TNX - 105 14 * National Institutes of Mental Health & National Institutes of Health

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS FM & PTSD are Related Conditions • Symptom overlap - PTSD is thought to be exacerbated by non - restorative sleep - Some are believed to suffer from both conditions simultaneously - Some patients with FM meet PTSD criteria, and vice versa • PTSD has both combat and civilian forms - Zoloft and Paxil are approved for PTSD - Brand prescriptions filled by generic sertraline and paroxetine - DOD has a strong interest in promoting research on therapeutics 15
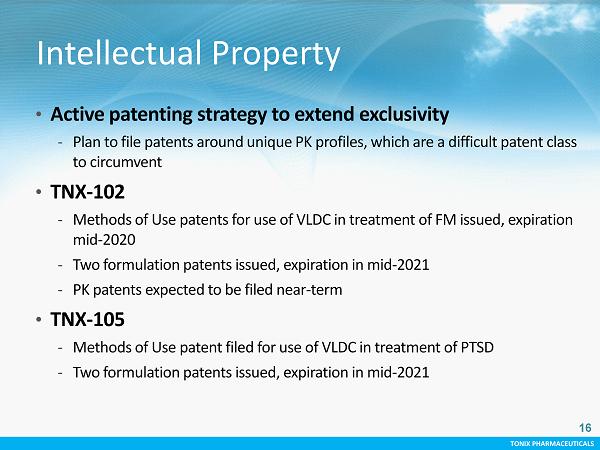
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Intellectual Property • Active patenting strategy to extend exclusivity - Plan to file patents around unique PK profiles, which are a difficult patent class to circumvent • TNX - 102 - Methods of Use patents for use of VLDC in treatment of FM issued, expiration mid - 2020 - Two formulation patents issued, expiration in mid - 2021 - PK patents expected to be filed near - term • TNX - 105 - Methods of Use patent filed for use of VLDC in treatment of PTSD - Two formulation patents issued, expiration in mid - 2021 16
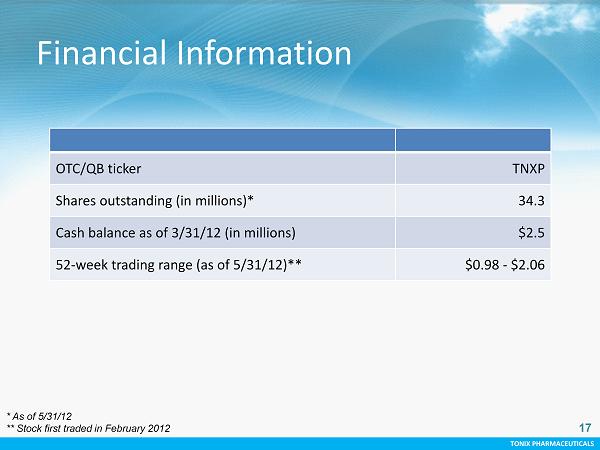
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS 17 OTC/QB ticker TNXP Shares outstanding (in millions)* 34.3 Cash balance as of 3/31/12 (in millions) $2.5 52 - week trading range (as of 5/31/12)** $0.98 - $2.06 * As of 5/31/12 ** Stock first traded in February 2012 Financial Information

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS 18 Timing Milestones Related to TNX - 102 Q2 2012 • PK study on second formulation Q3 2012 • PK/PD on “commercial” formulation and dose Q1 2013 • Commencement of initial pivotal trial Q3 2013 • Interim look at initial pivotal trial data H1 2014 • Final study results of initial pivotal trial • Partnering Timing Milestones Related to TNX - 105 H1 2013 • Commencement of proof of concept study in PTSD patients Upcoming Milestones

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Investment Summary • Significant unmet needs and large market opportunities • First - in - class products; not competitive with existing therapies • Capital efficient, low risk drug development strategy • Near - term value inflection points • Experienced management and board 19

OTC/QB: TNXP
