Attached files
| file | filename |
|---|---|
| 8-K - FORM 8K - WEST PHARMACEUTICAL SERVICES INC | form8k.htm |

WEST PHARMACEUTICAL SERVICES, INC.
Solutions for Injectable Drug Delivery NYSE:WST www.westpharma.com
© 2012 by West Pharmaceutical Services, Inc., Lionville, PA.
All rights reserved. This material is protected by copyright. No part of it may be reproduced, stored in a retrieval system, or transmitted in any
form or by any means, electronic, mechanical, photocopying or otherwise, without written permission of West Pharmaceutical Services, Inc.. All
trademarks and registered trademarks are property of West Pharmaceutical Services, Inc., unless noted otherwise.
form or by any means, electronic, mechanical, photocopying or otherwise, without written permission of West Pharmaceutical Services, Inc.. All
trademarks and registered trademarks are property of West Pharmaceutical Services, Inc., unless noted otherwise.
Jefferies 2012 Global Healthcare Conference
New York, NY
June 4, 2012

Safe Harbor Statement
Cautionary Statement Under the Private Securities Litigation Reform Act of 1995
This presentation and any accompanying management commentary contain “forward-looking statements”
as that term is defined in the Private Securities Litigation Reform Act of 1995. Such statements include,
but are not limited to statements about expected financial results for 2012 and future years.
as that term is defined in the Private Securities Litigation Reform Act of 1995. Such statements include,
but are not limited to statements about expected financial results for 2012 and future years.
Each of these estimates is based on preliminary information, and actual results could differ from these
preliminary estimates. We caution investors that the risk factors listed under “Cautionary Statement” in
our press releases, as well as those set forth under the caption "Risk Factors" in our most recent Annual
Report on Form 10-K as filed with the Securities and Exchange Commission and as revised or
supplemented by our quarterly reports on Form 10-Q, could cause our actual results to differ materially
from those estimated or predicted in the forward-looking statements. You should evaluate any statement
in light of these important factors. Except as required by law or regulation, we undertake no obligation to
publicly update any forward-looking statements, whether as a result of new information, future events, or
otherwise.
preliminary estimates. We caution investors that the risk factors listed under “Cautionary Statement” in
our press releases, as well as those set forth under the caption "Risk Factors" in our most recent Annual
Report on Form 10-K as filed with the Securities and Exchange Commission and as revised or
supplemented by our quarterly reports on Form 10-Q, could cause our actual results to differ materially
from those estimated or predicted in the forward-looking statements. You should evaluate any statement
in light of these important factors. Except as required by law or regulation, we undertake no obligation to
publicly update any forward-looking statements, whether as a result of new information, future events, or
otherwise.
Non-GAAP Financial Measures
Certain financial measures included in these presentation materials, and which may be referred to in
management’s discussion of the Company’s results and outlook, are Non-GAAP (Generally Accepted
Accounting Principles) financial measures. Please refer to the “Non-GAAP Financial Measures” and
“Notes to Non-GAAP Financial Measures” at the end of these materials for more information. Non-GAAP
financial measures should not be considered in isolation or as an alternative to such measures
determined in accordance with GAAP.
management’s discussion of the Company’s results and outlook, are Non-GAAP (Generally Accepted
Accounting Principles) financial measures. Please refer to the “Non-GAAP Financial Measures” and
“Notes to Non-GAAP Financial Measures” at the end of these materials for more information. Non-GAAP
financial measures should not be considered in isolation or as an alternative to such measures
determined in accordance with GAAP.
2

Pharmaceutical Packaging Systems
Pharmaceutical Delivery Systems
• A globally diverse manufacturer of
products used primarily in containing and
administering small-volume parenteral drugs
products used primarily in containing and
administering small-volume parenteral drugs
• Strong competitive position
– Substantial market shares
– Proprietary technology
– Diversified customer base
– Global footprint
– Preferred products for biologics
– Long-term customer relationships
• Stability with growth potential
• Proprietary Products
• Geographic Expansion
• Financial strength to invest
– Reliable operating cash flow
– Well capitalized
3

A Diverse, Stable Customer Base
(representative healthcare customers)
(representative healthcare customers)
PHARMACEUTICAL / BIOTECHNOLOGY
GENERIC
MEDICAL DEVICE
4
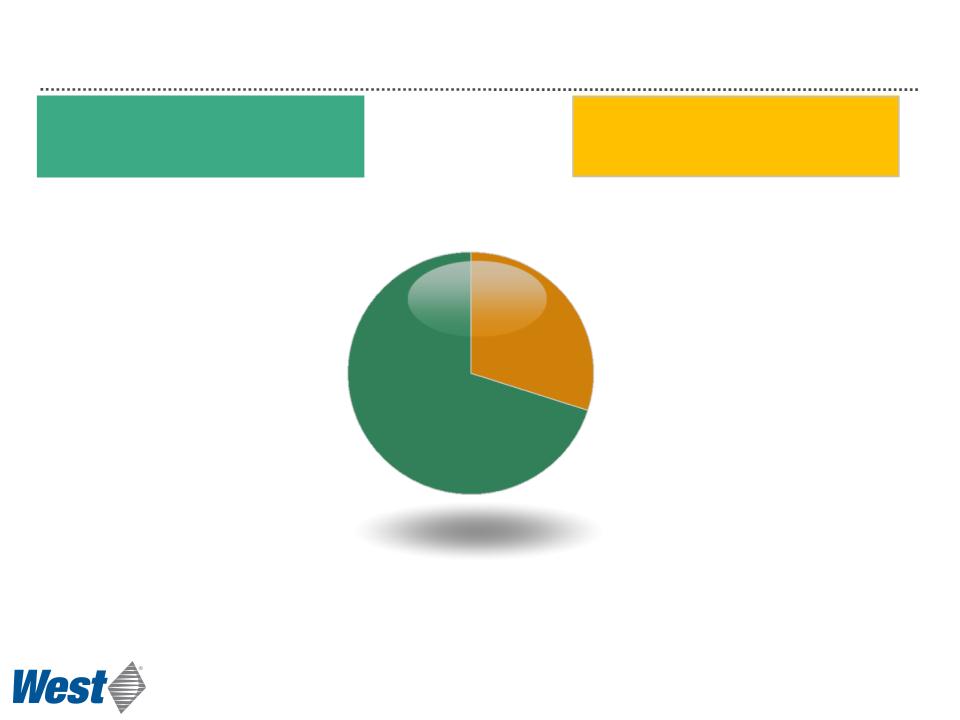
Business Segments
$857
$337
2011 Revenues
($ millions)
Delivery Systems
• Contract manufacturing base
• Multi-material
• Project management
• Automated assembly
• Regulated products
• Capabilities + IP = proprietary
delivery devices
delivery devices
• Proprietary devices are
expected to drive growth
expected to drive growth
Packaging Systems
• Established leadership
• Designed-in revenue base
• Diverse global capabilities
• High market shares
• Steady growth in base
• Increasing unit value of products
and geographic expansion are
expected to enhance growth
and geographic expansion are
expected to enhance growth
5

Summary Results
$ millions, except per-share data
$ millions, except per-share data
|
|
Three Months
Ended March 31,
|
|
|
2012
|
2011
|
|
|
|
|
|
|
Net Sales
|
$ 316.3
|
$ 295.4
|
|
Gross Profit
|
101.1
|
88.0
|
|
Reported Operating Profit
|
41.7
|
28.8
|
|
Adjusted Operating Profit (1)
|
42.3
|
30.7
|
|
Reported Diluted EPS
|
$ 0.81
|
$ 0.56
|
|
Adjusted Diluted EPS(1)
|
$ 0.83
|
$ 0.60
|
(1) These are Non-GAAP measurements. For an explanation and reconciliation of these items, see “Cautionary Statement”
(Slide 2) and “Non-GAAP Financial Measures” and “Notes to Non-GAAP Financial Measures” (Slides 25 -27).
(Slide 2) and “Non-GAAP Financial Measures” and “Notes to Non-GAAP Financial Measures” (Slides 25 -27).
6

2012 Outlook and Challenges
First-quarter results:
•Sales Grew 7.1% (9.1% at constant currency)
– Customer inventory building contributes to sales, backlog
•Operating leverage yielded sharply higher Adjusted Diluted EPS
Full-year / longer-term issues:
•Pharmaceutical Packaging and Device markets:
– Customer’s new product pipelines showing signs of strength
– Patent cliff front and center
– Shift to large molecule products continues
– Global shift in product sourcing (e.g., India generic growth)
– More demanding regulatory environment
•Continuing Fx and commodity price volatility
– Euro, European growth and sovereign debt
– Oil and regional political, civil and military turmoil
– Less predictable demand
7
Selected Factors That Impact Margin % in 2012(2)
(2) See “Cautionary Statement” on slide 2. This is not an exclusive list of risks associated with forward
looking statements made in connection with these slides.
looking statements made in connection with these slides.
8

Convertible Debenture Tender Offer
• Announced on May 8, 2012
– Offer to purchase any and all of $161.5 million of outstanding convertible
debentures
debentures
– All-cash offer, fixed plus variable component, based on interim share price
– Pricing on June 1, 2012; Tender period closes on June 5, 2012
– Information concerning the offer is contained in press release announcing the
offer, form 8-k, the offer to purchase, Schedule TO, and letter of transmittal,
all filed with the SEC on May 8, 2012
offer, form 8-k, the offer to purchase, Schedule TO, and letter of transmittal,
all filed with the SEC on May 8, 2012
• Plan to finance purchases pursuant to the tender offer
– Information concerning the financing plan is in a separate press release
dated May 8, 2012
dated May 8, 2012
• A successful tender offer is expected to be accretive to 2012
and subsequent years
and subsequent years
– Impact will be a function primarily of the number of Debentures purchased,
the final purchase price and related financing costs.
the final purchase price and related financing costs.
9
What will drive growth?
|
– Growth in emerging markets
– Escalating regulatory and
quality demands – Adding plants: China, India
– Expanding Westar® and
Envision® capacity, introducing NovaPure® components |
– Growth in combination products:
• safety, dosing accuracy, ease of
use, • deliver cost savings
• product differentiation
– Daikyo Crystal Zenith® products:
• Increasing awareness of glass
quality issues – Delivery technology platforms:
• SmartDose® electronic patch
injector • ConfiDose® auto-injector
– Reconstitution products
– Safety syringes
|
Daikyo Crystal Zenith® is a registered trademark of Daikyo Seiko, Ltd.
Packaging Systems
Delivery Systems
10

|
Category
|
Key Customers
|
Projected
Growth |
|
Diabetes
|
|
> 10 %
|
|
Oncology
|
|
> 10 %
|
|
Vaccines
|
|
> 10 %
|
|
Autoimmune
|
|
> 8%
|
|
Generics
|
|
>10%
|
IMS April 2010 Report; Business Insights 2009; GBI Research 2009
Therapeutic Category Growth Drivers
11
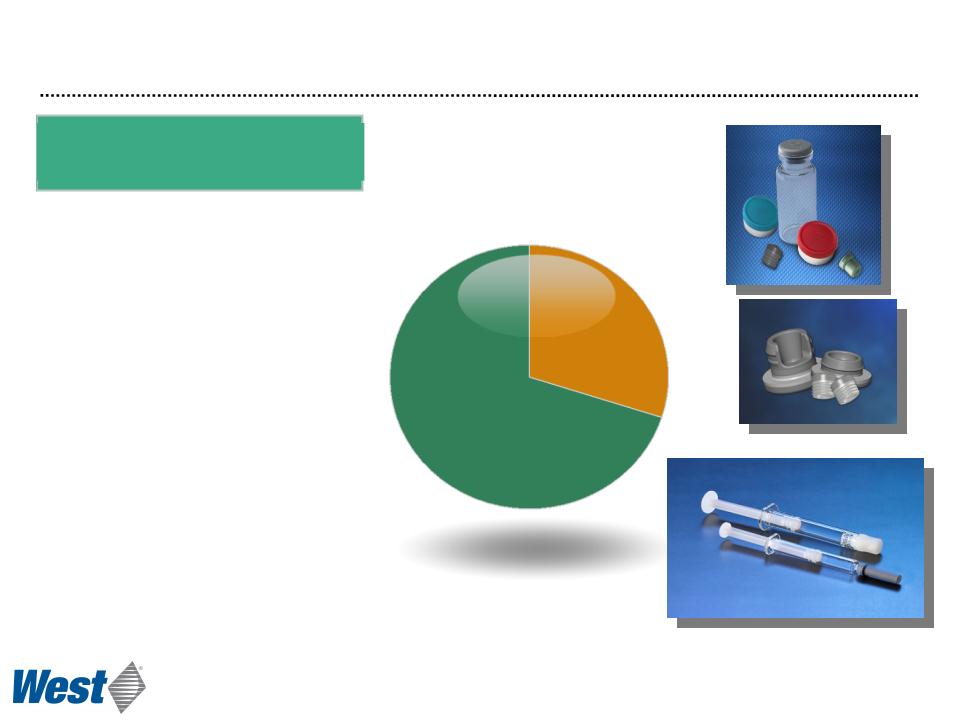
Packaging Segment Overview
2011 Revenue
($ millions)
Packaging Systems
• Market leader
• Strong recurring revenue base
• Global manufacturing
• Steady growth in base
• Future growth will be driven by:
added value per unit sold;
geographic expansion; and
growth in key therapeutic
segments
added value per unit sold;
geographic expansion; and
growth in key therapeutic
segments
$857
12
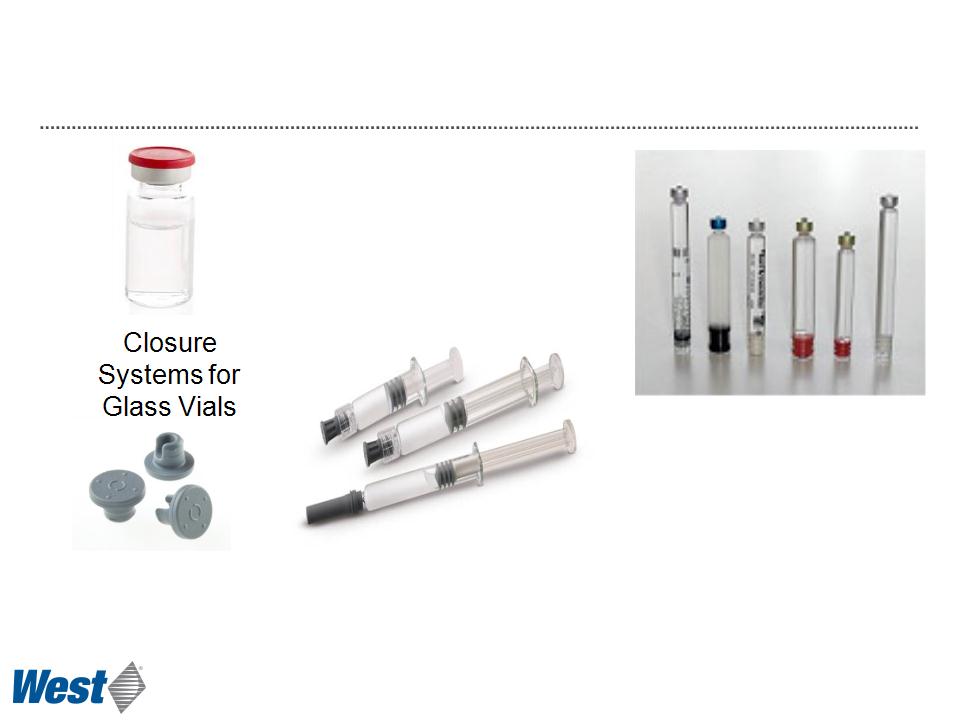
Pharmaceutical Packaging Systems
Packaging Components for Small Volume Parenterals
Packaging Components for Small Volume Parenterals
Plungers, Tip caps,
Needle shields for Glass
Syringes
Needle shields for Glass
Syringes
Plungers, lined seals
for Glass Cartridges
for Pens
for Glass Cartridges
for Pens
Primary packaging components (those that touch the drug) are typically
proprietary to West and are “designed into” customers’ drug products
proprietary to West and are “designed into” customers’ drug products
13
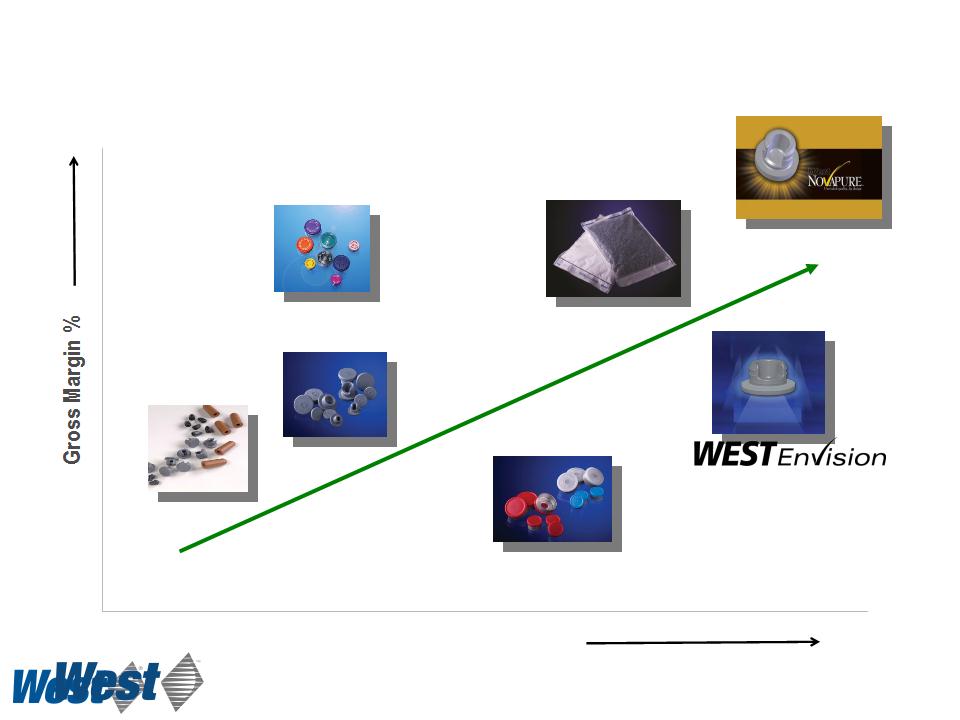
Standard High-Value
Products Products
Revenue Opportunity ($ per unit)
Plungers and
sleeve stoppers
sleeve stoppers
Stoppers
Seals
RU seals
Westar® RU
14
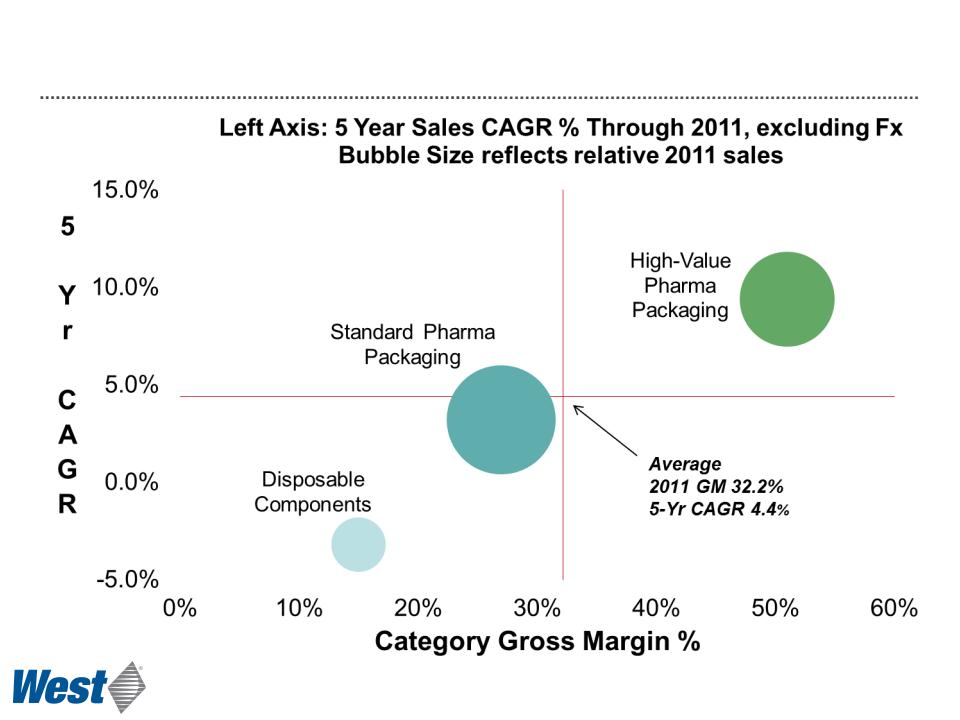
Faster Growth of High-Value Products
Pharmaceutical Packaging Systems
Pharmaceutical Packaging Systems
15
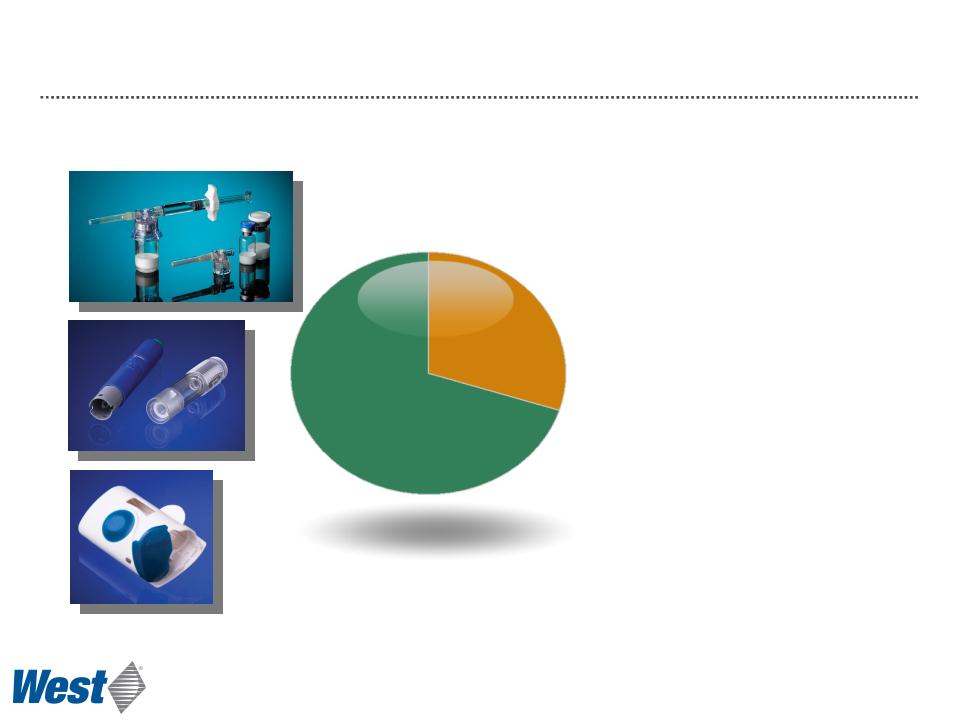
Delivery Systems Segment Overview
$337
• Contract manufacturing base
• Customer owned IP
• Project management
• Automated assembly
• Regulated products
• Proprietary Product
Development
Development
• West owned IP
• Multiple use platforms
• Focus on unmet needs
for biologics
for biologics
2011 Revenue
($ millions)
16

Delivery Systems
Daikyo Crystal Zenith®
Life-cycle Containment Solutions
Life-cycle Containment Solutions
West MixJect® and
Vial2Bag®
Vial2Bag®
Custom Manufacturing of
Components and Devices
Components and Devices
Proprietary Components, Devices and Systems
West ConfiDose®
auto-injector platform
technology
auto-injector platform
technology
SmartDose® electronic
patch injector platform
technology
patch injector platform
technology
Daikyo Crystal Zenith® is a registered trademark of Daikyo Seiko, Ltd.
17
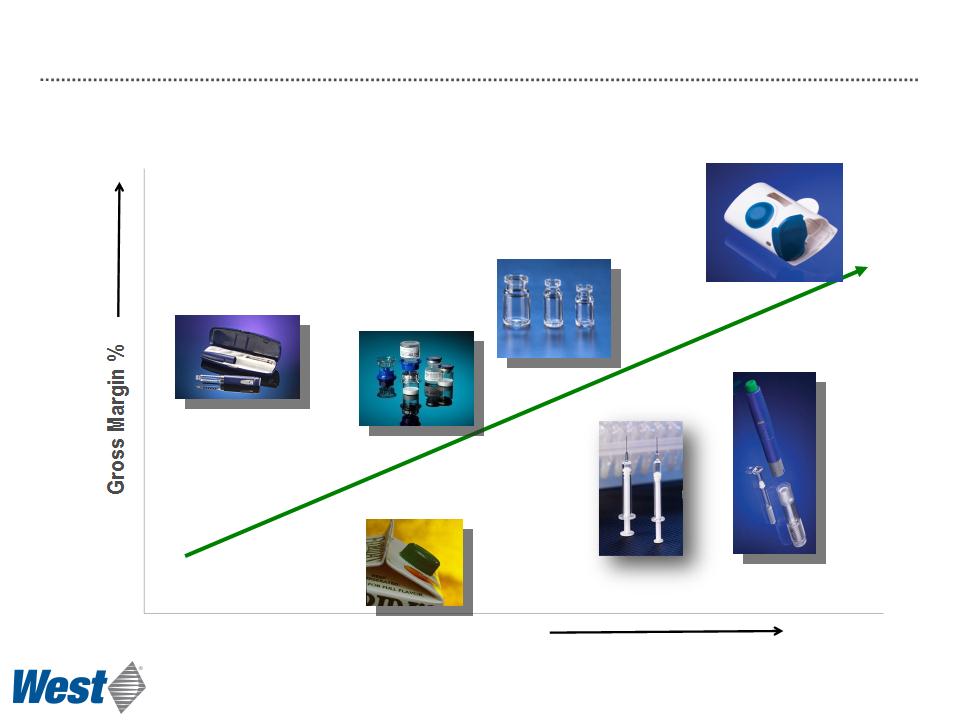
Revenue per-unit
Consumer product
manufacturing
manufacturing
Medical
device
manufacturing
device
manufacturing
Mix2Vial®
CZ vials
CZ Syringes
Effect of Increasing Proprietary Device Sales
Contract Manufacturing Proprietary Devices
Products
Auto-injector platform
technology
technology
Electronic patch
injector technology
injector technology
18

Concerns With Glass Syringes
• Interaction with sensitive biologics
• Protein aggregation (silicone oil)
• Residual chemicals (tungsten, glue)
• Glass flakes
• Dimensional variation
• Variable silicone distribution
• High Cost of Quality
• Breakage
• In process/handling
• Within auto-injector systems
• Reduced Stability/Shelf-life for products
Siliconized Glass Syringe
Crystal Zenith Syringe
19

Daikyo CZ Solution
with Daikyo Flurotec® Barrier Film
with Daikyo Flurotec® Barrier Film
• Reduces:
– drug exposure to extractables
– risk of protein aggregation caused by silicone oil in the drug product
– returns and in-process clean-ups caused by broken glass
– risk of delamination and glass-particulate contamination
• Consistent piston release and travel forces without using silicone oil
Flurotec® is a registered trademark of Daikyo Seiko, Ltd.
20
Pharma Industry Drug Life-Cycle
Management
Management
Phase I
Phase II
Phase III
Post-Market Life Cycle Management
8 - 10 years
2 - 3 years
2 - 3 years
Regulatory
Approval
Discovery
21
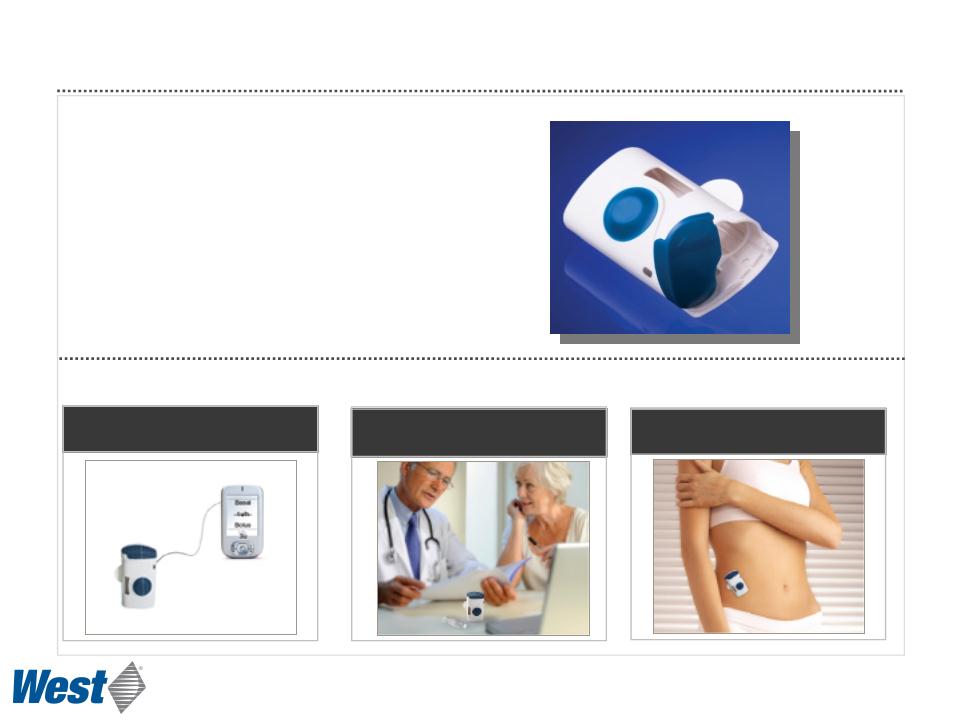
SmartDose®
Electronic Patch Injector Technology Platform
Programmed by PDA or PC
Dose may be customized
Applied and activated by patient
• Controlled, subcutaneous, micro-infusion delivery
of high volumes and high viscosity drugs
of high volumes and high viscosity drugs
• Prefilled cartridge, no need for user filling
• Based on Daikyo CZ cartridge
• Compact
• Hidden needle for safety
• Single push-button operation
Prototype Operation
22

Our Long-Term Focus
• Pharmaceutical Packaging Systems
– Organic growth of 3-5% per year
– Margin expansion from efficiency, product mix
– Capital investments target enhanced quality and value
• Pharmaceutical Delivery Systems
– Deliver the potential of Daikyo CZ products
– Stronger mix of healthcare-consumable contract manufacturing
– Grow proprietary safety and delivery systems
• Financial discipline
– Operating cash flow supports R&D and capital spending
– Deliver returns (ROIC) that regularly exceed cost of capital (WACC)
– Maintain quarterly dividend
– Align incentives with financial performance and value creation
23

Pharmaceutical Packaging Systems
Pharmaceutical Delivery Systems
• Strong competitive position
• Stability with growth potential
• New products well positioned to
meet future market needs
meet future market needs
• The financial strength to invest
Summary
24

Non-GAAP Financial Measures(3)
Three Months Ended March 31, 2012 and 2011
Three Months Ended March 31, 2012 and 2011
(in millions, except per share data)
(3) See “Notes to Non-GAAP Financial Measures” (Slides 26-27), “Cautionary Statement” (Slide 2) for an explanation and
reconciliation of these items.
reconciliation of these items.
|
|
As Reported
March 31,
2012
|
Restructuring
and related charges |
Acquisition-
related contingencies |
Discrete
tax items |
Non-GAAP
March 31,
2012
|
|
Operating profit
|
$41.7
|
$0.4
|
$0.2
|
$-
|
$42.3
|
|
Interest expense, net
|
3.9
|
-
|
-
|
-
|
3.9
|
|
Income before income taxes
|
37.8
|
0.4
|
0.2
|
-
|
38.4
|
|
Income tax expense
|
9.8
|
0.1
|
0.1
|
(0.3)
|
9.7
|
|
Equity in net income of affiliated companies
|
1.2
|
-
|
-
|
-
|
1.2
|
|
Net income
|
$29.2
|
$0.3
|
$0.1
|
$0.3
|
$29.9
|
|
|
|
|
|
|
|
|
Net income per diluted share
|
$0.81
|
$0.01
|
$-
|
$0.01
|
$0.83
|
|
|
As Reported
March 31,
2011
|
Restructuring
and related charges |
Discrete
tax items |
Non-GAAP
March 31,
2011
|
|
Operating profit
|
$28.8
|
$1.9
|
$-
|
$30.7
|
|
Interest expense, net
|
4.5
|
-
|
-
|
4.5
|
|
Income before income taxes
|
24.3
|
1.9
|
-
|
26.2
|
|
Income tax expense
|
6.1
|
0.6
|
(0.2)
|
6.5
|
|
Equity in net income of affiliated companies
|
1.4
|
-
|
-
|
1.4
|
|
Net income
|
$19.6
|
$1.3
|
$0.2
|
$21.1
|
|
|
|
|
|
|
|
Net income per diluted share
|
$0.56
|
$0.04
|
$-
|
$0.60
|
25

NOTES TO NON-GAAP FINANCIAL MEASURES
See also “Safe Harbor Statement” (slide 2)
See also “Safe Harbor Statement” (slide 2)
These slides use non-GAAP financial measures. West believes that these non-GAAP measures of financial results provide
useful information to management and investors regarding certain business trends relating to West’s financial condition, results
of operations and the Company’s overall performance. Our executive management team uses adjusted operating profit and
adjusted diluted EPS to evaluate the performance of the Company in terms of profitability and to compare operating results to
prior periods. Adjusted operating profit is also used to evaluate changes in the operating results of each segment and to allocate
resources to our segments. The Company believes that the use of these non-GAAP financial measures provides an additional
tool for investors to use in evaluating ongoing operating results and trends in comparing its financial measures with other
companies.
useful information to management and investors regarding certain business trends relating to West’s financial condition, results
of operations and the Company’s overall performance. Our executive management team uses adjusted operating profit and
adjusted diluted EPS to evaluate the performance of the Company in terms of profitability and to compare operating results to
prior periods. Adjusted operating profit is also used to evaluate changes in the operating results of each segment and to allocate
resources to our segments. The Company believes that the use of these non-GAAP financial measures provides an additional
tool for investors to use in evaluating ongoing operating results and trends in comparing its financial measures with other
companies.
Our executive management does not consider such non-GAAP measures in isolation or as an alternative to such measures
determined in accordance with GAAP. The principal limitation of such non-GAAP financial measures is that they exclude
significant expenses and income that are required by GAAP to be recorded. In addition, they are subject to inherent limitations
as they reflect the exercise of judgment by management about which items are excluded from the non-GAAP financial
measures. In order to compensate for these limitations, our executive management presents its non-GAAP financial measures
in connection with its GAAP results. We urge investors and potential investors to review the reconciliation of our non-GAAP
financial measures to the comparable GAAP financial measures, and not rely on any single financial measure to evaluate the
Company’s business.
determined in accordance with GAAP. The principal limitation of such non-GAAP financial measures is that they exclude
significant expenses and income that are required by GAAP to be recorded. In addition, they are subject to inherent limitations
as they reflect the exercise of judgment by management about which items are excluded from the non-GAAP financial
measures. In order to compensate for these limitations, our executive management presents its non-GAAP financial measures
in connection with its GAAP results. We urge investors and potential investors to review the reconciliation of our non-GAAP
financial measures to the comparable GAAP financial measures, and not rely on any single financial measure to evaluate the
Company’s business.
In calculating adjusted operating profit and adjusted diluted EPS, we exclude the impact of items that are not considered
representative of ongoing operations. Such items include restructuring and related costs, certain asset impairments, other
specifically identified gains or losses, and discrete income tax items. Reconciliations of these adjusted non-GAAP measures to
the comparable GAAP financial measures are included in the preceding (current and prior-year periods).
representative of ongoing operations. Such items include restructuring and related costs, certain asset impairments, other
specifically identified gains or losses, and discrete income tax items. Reconciliations of these adjusted non-GAAP measures to
the comparable GAAP financial measures are included in the preceding (current and prior-year periods).
The following is a description of the items excluded from adjusted operating profit and adjusted diluted EPS:
(continued on following slide)
26

NOTES TO NON-GAAP FINANCIAL MEASURES
(continued)
(continued)
Restructuring and related charges: During the three months ended March 31, 2012, we incurred restructuring and
related charges of $0.4 million associated with the restructuring plan announced in December 2010. Charges
associated with the plan for the three months ended March 31, 2012 were primarily facility closure costs associated
with the 2011 closure of a plant in the United States and a reduction of operations at a manufacturing facility in
England.
related charges of $0.4 million associated with the restructuring plan announced in December 2010. Charges
associated with the plan for the three months ended March 31, 2012 were primarily facility closure costs associated
with the 2011 closure of a plant in the United States and a reduction of operations at a manufacturing facility in
England.
During the three months ended March 31, 2011, we incurred restructuring and related charges of $1.9 million
associated with the restructuring plan announced in December 2010. Charges associated with the plan for the three
months ended March 31, 2011 were primarily for employee severance and benefits.
associated with the restructuring plan announced in December 2010. Charges associated with the plan for the three
months ended March 31, 2011 were primarily for employee severance and benefits.
Acquisition-related contingencies: During the three months ended March 31, 2012, we increased the liability for
contingent consideration related to our 2010 acquisition of technology used in our SmartDose™ electronic patch
injector system by $0.2 million.
contingent consideration related to our 2010 acquisition of technology used in our SmartDose™ electronic patch
injector system by $0.2 million.
Discrete tax items: During the three months ended March 31, 2012, we recorded a discrete tax charge of $0.3
million, primarily due to the reduction of deferred tax assets associated with the legal restructuring of the ownership of
our Puerto Rico operations.
million, primarily due to the reduction of deferred tax assets associated with the legal restructuring of the ownership of
our Puerto Rico operations.
During the three months ended March 31, 2011, we recorded a discrete tax charge of $0.2 million, resulting from the
impact of changes in tax laws in certain foreign tax jurisdictions on our deferred tax balances.
impact of changes in tax laws in certain foreign tax jurisdictions on our deferred tax balances.
27

WEST PHARMACEUTICAL SERVICES, INC.
Solutions for Injectable Drug Delivery NYSE:WST www.westpharma.com
© 2012 by West Pharmaceutical Services, Inc., Lionville, PA.
All rights reserved. This material is protected by copyright. No part of it may be reproduced, stored in a retrieval system, or transmitted in any
form or by any means, electronic, mechanical, photocopying or otherwise, without written permission of West Pharmaceutical Services, Inc.. All
trademarks and registered trademarks are property of West Pharmaceutical Services, Inc., unless noted otherwise.
form or by any means, electronic, mechanical, photocopying or otherwise, without written permission of West Pharmaceutical Services, Inc.. All
trademarks and registered trademarks are property of West Pharmaceutical Services, Inc., unless noted otherwise.
