Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Celldex Therapeutics, Inc. | a12-12945_1ex99d1.htm |
| 8-K - 8-K - Celldex Therapeutics, Inc. | a12-12945_18k.htm |
Exhibit 99.2
|
|
CDX-011 – EMERGE Topline Data May 23, 2012 |
|
|
Forward Looking Statement This communication contains "forward-looking" statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical fact are statements that could be forward-looking statements. You can identify these forward-looking statements through our use of words such as “may,” “will,” “can,” “anticipate,” “assume,” “should,” “indicate,” “would,” “believe,” “contemplate,” “expect,” “seek,” “estimate,” “continue,” “plan,” “point to,” “project,” “predict,” “could,” “intend,” “target,” “potential” and other similar words and expressions of the future. These forward-looking statements are subject to risks and uncertainties that may cause actual future experience and results to differ materially from those discussed in these forward-looking statements. Important factors that might cause such a difference include, but are not limited to, the timing, cost and uncertainty of obtaining regulatory approvals for product candidates; our ability to develop and commercialize products before competitors that are superior to the alternatives developed by such competitors; the validity of our patents and our ability to avoid intellectual property litigation, which can be costly and divert management time and attention; and the other factors listed under “Risk Factors” in our filings with the SEC, including Forms 10-K, 10-Q and 8-K. Celldex does not undertake any obligation to release publicly any revisions to such forward-looking statement to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events. 2 |
|
|
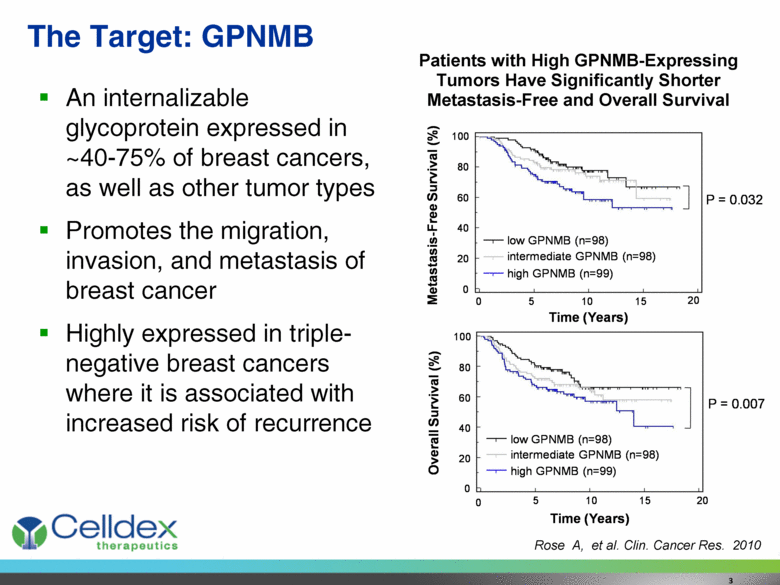
The Target: GPNMB An internalizable glycoprotein expressed in ~40-75% of breast cancers, as well as other tumor types Promotes the migration, invasion, and metastasis of breast cancer Highly expressed in triple-negative breast cancers where it is associated with increased risk of recurrence Patients with High GPNMB-Expressing Tumors Have Significantly Shorter Metastasis-Free and Overall Survival Rose A, et al. Clin. Cancer Res. 2010 3 Time (Years) 100 80 60 40 20 0 Metastasis - Free Survival (%) Time (Years) 0 5 10 15 20 intermediate GPNMB (n=98) low GPNMB (n=98) high GPNMB (n=99) 100 80 60 40 20 0 Overall Survival (%) 0 5 10 15 20 low GPNMB (n=98) intermediate GPNMB (n=98) high GPNMB (n=99) P = 0.032 P = 0.007 |
|
|
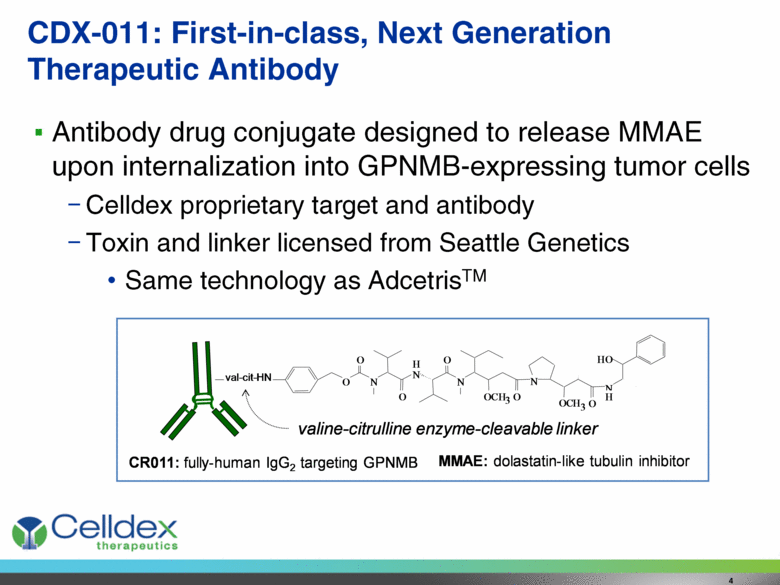
CDX-011: First-in-class, Next Generation Therapeutic Antibody Antibody drug conjugate designed to release MMAE upon internalization into GPNMB-expressing tumor cells Celldex proprietary target and antibody Toxin and linker licensed from Seattle Genetics Same technology as AdcetrisTM 4 MMAE: dolastatin-like tubulin inhibitor N H N N O O O O C H 3 O N O C H 3 N H H O O O val-cit-HN valine - citrulline enzyme - cleavable linker CR011: fully - human IgG 2 targeting GPNMB |
|
|
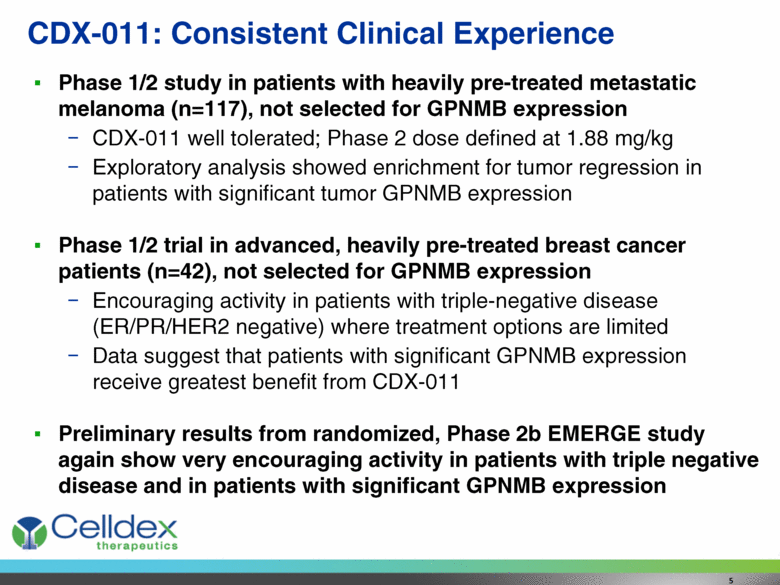
Phase 1/2 study in patients with heavily pre-treated metastatic melanoma (n=117), not selected for GPNMB expression CDX-011 well tolerated; Phase 2 dose defined at 1.88 mg/kg Exploratory analysis showed enrichment for tumor regression in patients with significant tumor GPNMB expression Phase 1/2 trial in advanced, heavily pre-treated breast cancer patients (n=42), not selected for GPNMB expression Encouraging activity in patients with triple-negative disease (ER/PR/HER2 negative) where treatment options are limited Data suggest that patients with significant GPNMB expression receive greatest benefit from CDX-011 Preliminary results from randomized, Phase 2b EMERGE study again show very encouraging activity in patients with triple negative disease and in patients with significant GPNMB expression CDX-011: Consistent Clinical Experience 5 |
|
|
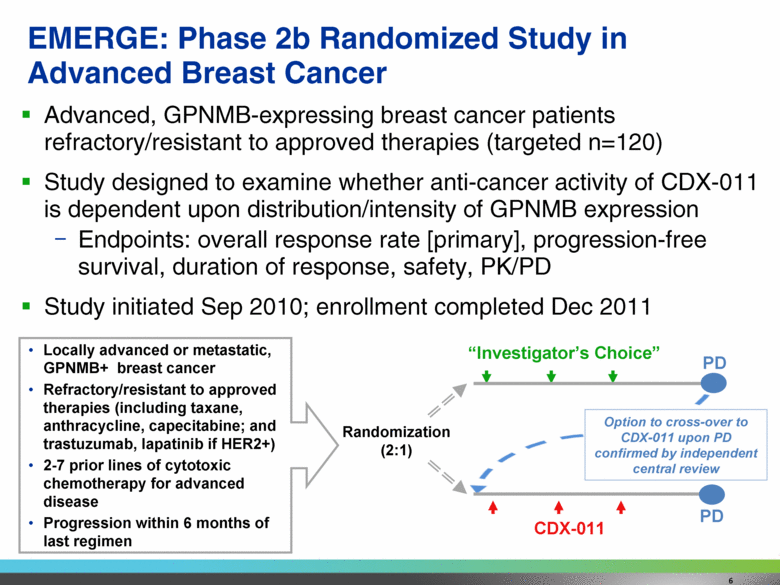
Advanced, GPNMB-expressing breast cancer patients refractory/resistant to approved therapies (targeted n=120) Study designed to examine whether anti-cancer activity of CDX-011 is dependent upon distribution/intensity of GPNMB expression Endpoints: overall response rate [primary], progression-free survival, duration of response, safety, PK/PD Study initiated Sep 2010; enrollment completed Dec 2011 EMERGE: Phase 2b Randomized Study in Advanced Breast Cancer “Investigator’s Choice” CDX-011 Option to cross-over to CDX-011 upon PD confirmed by independent central review Randomization (2:1) PD Locally advanced or metastatic, GPNMB+ breast cancer Refractory/resistant to approved therapies (including taxane, anthracycline, capecitabine; and trastuzumab, lapatinib if HER2+) 2-7 prior lines of cytotoxic chemotherapy for advanced disease Progression within 6 months of last regimen PD 6 |
|
|
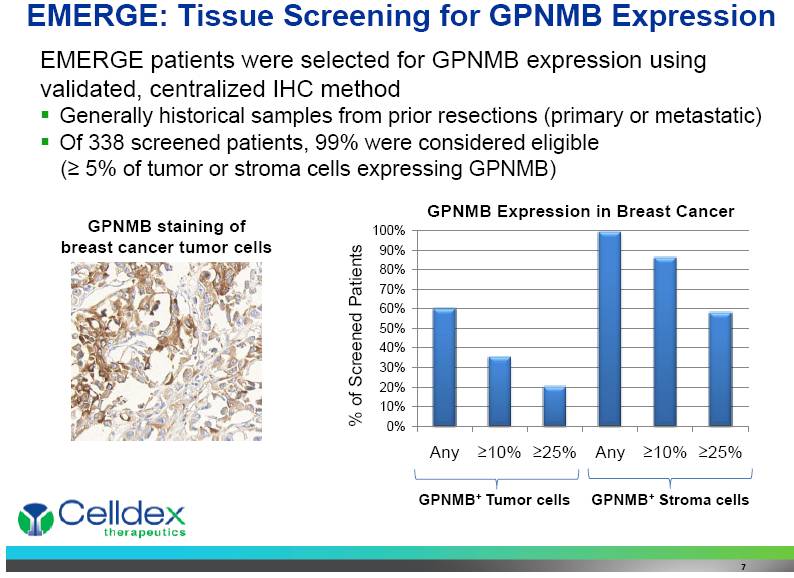
EMERGE patients were selected for GPNMB expression using validated, centralized IHC method Generally historical samples from prior resections (primary or metastatic) Of 338 screened patients, 99% were considered eligible (> 5% of tumor or stroma cells expressing GPNMB) EMERGE: Tissue Screening for GPNMB Expression % of Screened Patients GPNMB+ Tumor cells GPNMB+ Stroma cells GPNMB staining of breast cancer tumor cells GPNMB Expression in Breast Cancer 7 |
|
|
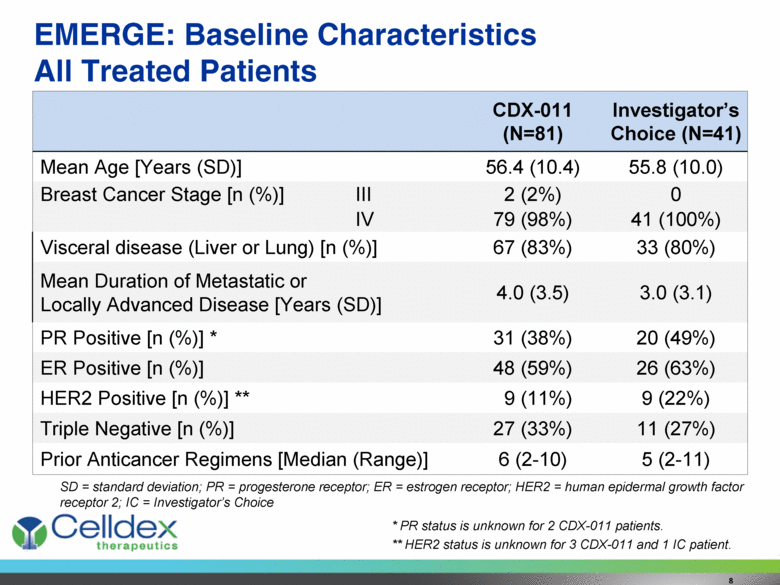
EMERGE: Baseline Characteristics All Treated Patients CDX-011 (N=81) Investigator’s Choice (N=41) Mean Age [Years (SD)] 56.4 (10.4) 55.8 (10.0) Breast Cancer Stage [n (%)] III 2 (2%) 0 IV 79 (98%) 41 (100%) Visceral disease (Liver or Lung) [n (%)] 67 (83%) 33 (80%) Mean Duration of Metastatic or Locally Advanced Disease [Years (SD)] 4.0 (3.5) 3.0 (3.1) PR Positive [n (%)] * 31 (38%) 20 (49%) ER Positive [n (%)] 48 (59%) 26 (63%) HER2 Positive [n (%)] ** 9 (11%) 9 (22%) Triple Negative [n (%)] 27 (33%) 11 (27%) Prior Anticancer Regimens [Median (Range)] 6 (2-10) 5 (2-11) 8 SD = standard deviation; PR = progesterone receptor; ER = estrogen receptor; HER2 = human epidermal growth factor receptor 2; IC = Investigator’s Choice * PR status is unknown for 2 CDX-011 patients. ** HER2 status is unknown for 3 CDX-011 and 1 IC patient. |
|
|
EMERGE: Safety Summary All Treated Patients 9 IC = Investigator’s Choice CDX-011 arm includes 15 patients who crossed over to receive CDX-011 treatment after progression on IC. Table presents treatment-related adverse events with incidence >15% overall, or >3% at Grade 3-4 severity, in either study arm. No Grade 5 treatment-related adverse events were reported. CDX-011 (n = 96) IC (n = 41) All Grades Grade 3 Grade 4 All Grades Grade 3 Grade 4 Hematologic Neutropenia/Neutrophil count decreased 25 (26%) 12 (13%) 6 (6%) 16 (39%) 7 (17%) 3 (7%) Leukopenia/White blood cell count decreased 9 (9%) 2 (2%) 1 (1%) 11 (27%) 6 (15%) 0 Thrombocytopenia/Platelet count decreased 4 (4%) 0 1 (1%) 6 (15%) 1 (2%) 0 Non-hematologic Rash (maculopapular, pruritic, erythematous, etc.) 36 (38%) 3 (3%) 0 1 (2%) 0 0 Fatigue 31 (32%) 6 (6%) 0 17 (41%) 2 (5%) 0 Nausea 29 (30%) 3 (3%) 0 14 (34%) 0 0 Alopecia 22 (23%) 0 0 6 (15%) 0 0 Decreased appetite 17 (18%) 1 (1%) 0 4 (10%) 1 (2%) 0 Peripheral neuropathy 17 (18%) 2 (2%) 0 3 (7%) 0 0 Vomiting 15 (16%) 1 (1%) 0 3 (7%) 0 0 Constipation 13 (14%) 0 0 7 (17%) 0 0 Stomatitis 9 (9%) 1 (1%) 0 6 (15%) 1 (2%) 0 Dehydration 7 (7%) 3 (3%) 0 2 (5%) 1 (2%) 0 |
|
|
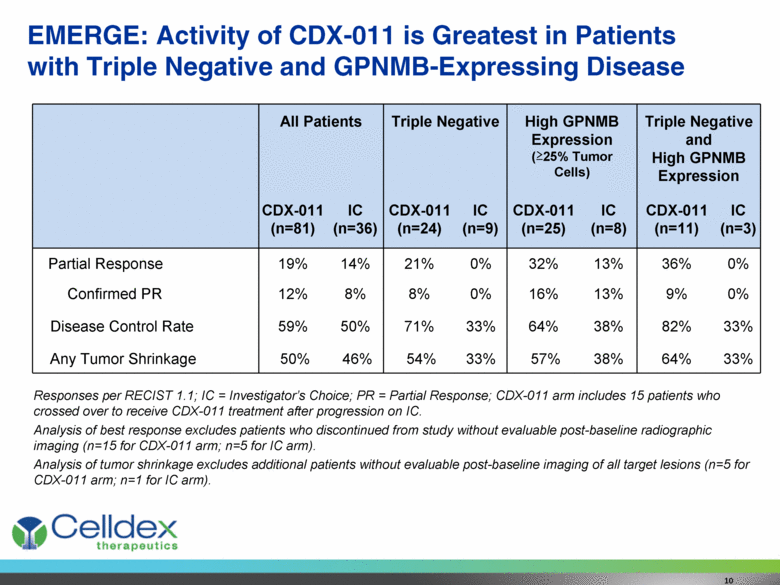
EMERGE: Activity of CDX-011 is Greatest in Patients with Triple Negative and GPNMB-Expressing Disease Responses per RECIST 1.1; IC = Investigator’s Choice; PR = Partial Response; CDX-011 arm includes 15 patients who crossed over to receive CDX-011 treatment after progression on IC. Analysis of best response excludes patients who discontinued from study without evaluable post-baseline radiographic imaging (n=15 for CDX-011 arm; n=5 for IC arm). Analysis of tumor shrinkage excludes additional patients without evaluable post-baseline imaging of all target lesions (n=5 for CDX-011 arm; n=1 for IC arm). All Patients Triple Negative High GPNMB Expression (>25% Tumor Cells) Triple Negative and High GPNMB Expression CDX-011 (n=81) IC (n=36) CDX-011 (n=24) IC (n=9) CDX-011 (n=25) IC (n=8) CDX-011 (n=11) IC (n=3) Partial Response 19% 14% 21% 0% 32% 13% 36% 0% Confirmed PR 12% 8% 8% 0% 16% 13% 9% 0% Disease Control Rate 59% 50% 71% 33% 64% 38% 82% 33% Any Tumor Shrinkage 50% 46% 54% 33% 57% 38% 64% 33% 10 |
|
|
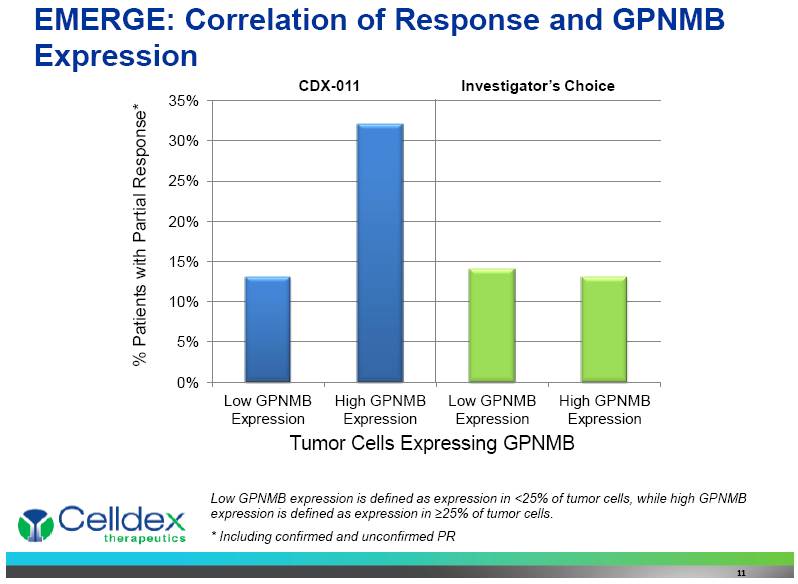
EMERGE: Correlation of Response and GPNMB Expression 11 Tumor Cells Expressing GPNMB % Patients with Partial Response* CDX-011 Investigator’s Choice Low GPNMB expression is defined as expression in <25% of tumor cells, while high GPNMB expression is defined as expression in >25% of tumor cells. * Including confirmed and unconfirmed PR 0% 5% 10% 15% 20% 25% 30% 35% Low GPNMB Expression High GPNMB Expression Low GPNMB Expression High GPNMB Expression |
|
|
EMERGE: Triple-Negative Patients Correlation of Response and GPNMB Expression 12 Tumor Cells Expressing GPNMB % Patients with Partial Response* CDX-011 Investigator’s Choice Low GPNMB expression is defined as expression in <25% of tumor cells, while high GPNMB expression is defined as expression in >25% of tumor cells. * Including confirmed and unconfirmed PR 0% 5% 10% 15% 20% 25% 30% 35% 40% Low GPNMB Expression High GPNMB Expression Low GPNMB Expression High GPNMB Expression |
|
|
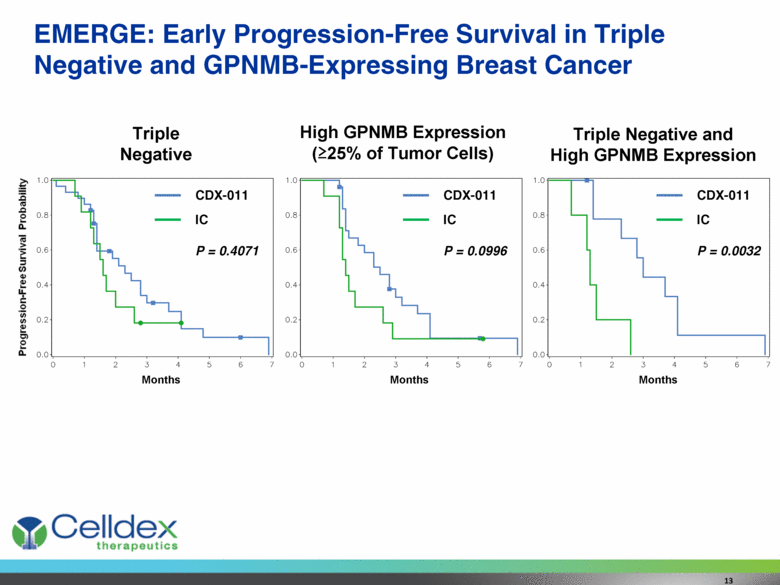
EMERGE: Early Progression-Free Survival in Triple Negative and GPNMB-Expressing Breast Cancer Triple Negative High GPNMB Expression (>25% of Tumor Cells) Triple Negative and High GPNMB Expression Months Months Months CDX-011 IC P = 0.0996 CDX-011 IC P = 0.0032 CDX-011 IC P = 0.4071 13 Progression-Free Survival Probability |
|
|
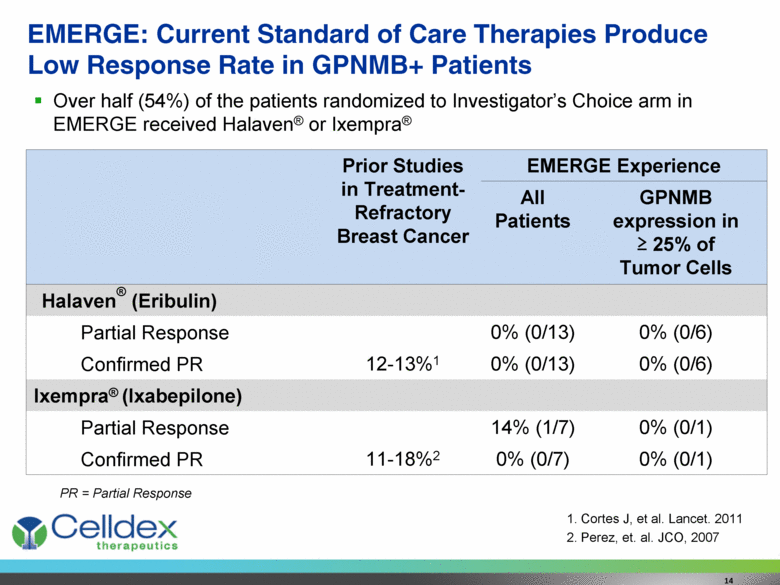
EMERGE: Current Standard of Care Therapies Produce Low Response Rate in GPNMB+ Patients 14 Prior Studies in Treatment-Refractory Breast Cancer EMERGE Experience All Patients GPNMB expression in > 25% of Tumor Cells Halaven® (Eribulin) Partial Response 0% (0/13) 0% (0/6) Confirmed PR 12-13%1 0% (0/13) 0% (0/6) Ixempra® (Ixabepilone) Partial Response 14% (1/7) 0% (0/1) Confirmed PR 11-18%2 0% (0/7) 0% (0/1) Over half (54%) of the patients randomized to Investigator’s Choice arm in EMERGE received Halaven® or Ixempra® Cortes J, et al. Lancet. 2011 Perez, et. al. JCO, 2007 PR = Partial Response |
|
|
Preliminary EMERGE Results Consistent with Previous Data in Similar Population Promising activity in triple-negative breast cancer (TNBC), where treatment options are limited CDX-011 response rate of 21% in TNBC compared to 0% for Investigator’s Choice (IC) therapy Significant tumor expression of GPNMB associated with greater activity CDX-011 response rate of 32% compared to 13% for IC High GPNMB expression seen in 39% of patients with TNBC; in these patients, CDX-011 response rate of 36% and progression-free survival advantage of 130% (p=0.0032) Celldex will explore possible registrational paths in these patients with significant unmet medical need Additional Phase 2 development possible in other GPNMB-expressing tumor types (melanoma, lymphoma, lung cancer) 15 |
|
|
For additional information, please visit: www.celldextherapeutics.com 16 |