Attached files
| file | filename |
|---|---|
| 8-K - POLYMEDIX INC 8-K 4-23-2012 - POLYMEDIX, INC | form8k.htm |
| EX-99.1 - EXHIBIT 99.1 - POLYMEDIX, INC | ex99_1.htm |
Exhibit 99.2

OTCBB: PYMX
www.polymedix.com
Revolutionizing the Treatment of Infectious
Diseases with Defensin-Mimetics
Diseases with Defensin-Mimetics
PMX-30063 Phase 2 ABSSSI Clinical Trial
Results
Results
April 23, 2012

Disclaimer and Safe Harbor
Forward-looking statements
This presentation contains forward-looking statements made pursuant to the safe harbor provisions of
the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that
could cause PolyMedix’s actual results and experience to differ materially from anticipated results and
expectations expressed in these forward looking statements. PolyMedix has in some cases identified
forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,”
“expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other
factors that could cause actual results to differ materially from those expressed in forward-looking
statements are PolyMedix’s need for, and the availability of, substantial capital in the future to fund its
operations and research and development, and the fact that PolyMedix’s compounds may not successfully
complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the
United States or elsewhere. A more complete description of these risk factors is included in PolyMedix’s
filings with the Securities and Exchange Commission. You should not place undue reliance on any forward
-looking statements. PolyMedix undertakes no obligation to release publicly the results of any revisions to
any such forward-looking statements that may be made to reflect events or circumstances after the date of
this press release or to reflect the occurrence of unanticipated events, except as required by applicable
law or regulation.
the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that
could cause PolyMedix’s actual results and experience to differ materially from anticipated results and
expectations expressed in these forward looking statements. PolyMedix has in some cases identified
forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,”
“expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other
factors that could cause actual results to differ materially from those expressed in forward-looking
statements are PolyMedix’s need for, and the availability of, substantial capital in the future to fund its
operations and research and development, and the fact that PolyMedix’s compounds may not successfully
complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the
United States or elsewhere. A more complete description of these risk factors is included in PolyMedix’s
filings with the Securities and Exchange Commission. You should not place undue reliance on any forward
-looking statements. PolyMedix undertakes no obligation to release publicly the results of any revisions to
any such forward-looking statements that may be made to reflect events or circumstances after the date of
this press release or to reflect the occurrence of unanticipated events, except as required by applicable
law or regulation.
1

PMX-30063 Defensin-Mimetic --- New Class of Antibiotic
Positive Phase 2 study results
• Showed consistently high clinical response rates with PMX-30063
• Safe and generally well-tolerated
• First efficacy study in patients with this new class of antibiotic
PMX-30063 is unique ---- our goal is to dominate the multi-
billion $ hospital antibiotic market
billion $ hospital antibiotic market
• Novel mechanism of action and reduced susceptibility to resistance
is key differentiator
is key differentiator
2

The Urgent Need for New Antibiotics
“The world is on the brink of losing
these miracle cures.”
these miracle cures.”
Dr. Margarete Chang, Director-General World Health Organization
Many infections caused by highly resistant bacteria such as MRSA - 100’s of kinds
70% of infections resistant4
Demand exists for truly novel antibiotics with differentiated properties
In US antibiotic-resistance causes:
$20 billion in excess healthcare costs1
$35 billion in societal costs1
8 million additional hospital days1
7 million MRSA infections/year U.S. 2
Up to 300,000 deaths3
1 http://www.cdc.gov/media/releases/2011/p0407_antimicrobialresistance.html
2 Archives of Internal Medicine, 2008, A.L. Hersh et.al.
3 B. Spellberg, Rising Plague, 2009
4 Association for Professionals in Infection Control & Epidemiology; Feb 2008
3

Learning from evolution to solve the problem:
Killing bacteria the same way as the Host Defense Proteins
Killing bacteria the same way as the Host Defense Proteins
Target and directly disrupt
microbial membranes
Biophysical Approach
Host defense proteins
+ PMX-30063
Imitates natural immune
defense system
defense system
Rapidly bactericidal
Specific for bacteria
Evolutionarily conserved
+ Immunomodulatory activities
DNA
Ribosomes
Cell wall synthesis
Beta-lactams
Glycopeptides
Aminoglycosides
Macrolides
Tetracyclines
Fluoroquinolones
Sulfonamides
Biochemical Approach
Cell membrane depolarization
Lipopeptides
4

Potential Competitive Positioning:
PMX-30063 vs. Other Staph Antibiotics
PMX-30063 vs. Other Staph Antibiotics
|
|
Macrolide
class |
Vancomycin
(glycopeptides)
|
Linezolid
(oxazolidinones)
|
Daptomycin
(lipopeptides)
|
PMX-30063
(Defensin-
mimetic class) |
|
Short Dosing
Schedule |
ü
|
|
|
ü
|
ü
|
|
Bactericidal
Activity |
|
|
|
ü
|
ü
|
|
Gram+ and Gram-
Activity
|
|
|
|
|
ü
|
|
Long post-
antibiotic effect |
|
|
ü
|
ü
|
üü
|
|
Anti-inflammatory
activity |
ü
|
|
|
|
ü
|
|
Anti-biofilm
activity |
|
|
|
ü
|
ü
|
|
Resistance unlikely
|
|
|
|
|
ü
|
5

PMX-30063 Phase 2 ABSSSI Clinical Trial
First efficacy study in patients with this new class of antibiotic -
defensin-mimetic, PMX-30063
defensin-mimetic, PMX-30063
Efficacy
• High clinical success rates
• At early time points and sustained over time
• PMX-30063 similar to active control
Safety
• In this study PMX-30063 was safe and generally well-tolerated
Successfully achieved study objectives
6

Phase 2 Clinical Trial -- Overview
Acute Bacterial Skin and Skin Structure Infections (ABSSSI)
caused by Staph aureus:
caused by Staph aureus:
• One of the most common infections in U.S.; >14 million/year1
Rigorous study entry criteria, using FDA definitions for ABSSSI
1 Archives of Internal Medicine, 2008, A.L. Hersh et.al.
Multiple Objectives
• Assess safety and pharmacokinetics
• Demonstrate similar efficacy vs. active
comparator
comparator
• Guide dose selection for future clinical studies
7

Phase 2 Clinical Trial -- Design
Trial conducted in Canada & Europe (Russia, Ukraine)
Dosing: IV infusion 1x/day for 7 days (5 days on PMX-30063 +
2 days placebo; 7 days on daptomycin)
2 days placebo; 7 days on daptomycin)
200 patients, 4 arms, 50 patients per arm
Interim analysis after first 80 patients
Patient Screening
MSSA/MRSA
Randomization
Low (0.40 mg/kg load; 0.30 mg/kg qd x 4d)
Comparator (daptomycin)
Placebo
Placebo
Placebo
Visit Assessments:
Day 7
Day 3
Continuous safety assessments and analysis
Evaluation post treatment
Day 28
Day 10
Medium (0.75 mg/kg load; 0.35 mg/kg qd x 4d)
High (1.0 mg/kg load; 0.35 mg/kg qd x 4d)
8

Phase 2 Clinical Trial -- Study Populations
|
|
Low
dose
|
Medium
dose
|
High
dose
|
daptomycin
|
Total
patients |
|
Per Protocol Population1
|
40
|
35
|
39
|
47
|
161
|
|
mITT Population2
|
43
|
37
|
45
|
47
|
172
|
|
ITT Population3
|
54
|
54
|
54
|
53
|
215
|
1 - Per Protocol Population: All patients who received ≥ 80% of study drug, were culture confirmed for Staph aureus, and
were assessed
2 - mITT (modified Intent-to-Treat) Population: All patients with culture-confirmed Staph aureus
3 - ITT (Intent-to-Treat) Population: All patients who were randomized into the study
Rigorous Study Entry Criteria:
● All populations must meet FDA definition for ABSSSI
● PP and mITT require positive Staph aureus culture
● Photographic documentation at all time points
9

FDA Guidance - Baseline and Day 3 Assessment
Definitions per FDA Guidance for Industry, ABSSSI, August 2010
Lesion Size:
• ≥ 75 sq. cm2 (redness, edema, and/or induration)
Clinical Response:
• Cessation of spread or reduction in size of redness, edema, and/or
induration of lesion, and
induration of lesion, and
• Resolution of fever
Clinical Failure:
• Death, continued fever, increase in size of redness, edema and /or
induration of lesion, administration of rescue antibiotic therapy
induration of lesion, administration of rescue antibiotic therapy
10
Clinical Response - Day 3
Per FDA ABSSSI Guidance
Per FDA ABSSSI Guidance
|
Clinical
Response |
Low
dose
|
Medium
dose
|
High
dose
|
daptomycin
|
|
Per Protocol
|
85.0%
|
71.4%
|
89.7%
|
74.5%
|
|
mITT
|
81.4%
|
67.6%
|
77.8%
|
74.5%
|
|
ITT
|
79.6%
|
68.5%
|
75.9%
|
75.5%
|
Key Outcomes:
● Consistently high clinical response rates at all doses of PMX-30063
• Similar to daptomycin comparator
● Findings corroborate interim analysis
11

Clinical Response - Day 3
Per FDA ABSSSI Guidance
Per FDA ABSSSI Guidance
12

Clinical Response - Days 3 and 7
Per Study Analysis Plan
Per Study Analysis Plan
|
Day 3
|
Low dose
|
Medium dose
|
High dose
|
daptomycin
|
|
Per Protocol
|
97.5%
|
91.4%
|
92.3%
|
91.5%
|
|
mITT
|
95.3%
|
89.2%
|
82.2%
|
91.5%
|
|
ITT
|
94.4%
|
90.7%
|
81.5%
|
90.6%
|
|
Day 7
|
Low dose
|
Medium dose
|
High dose
|
daptomycin
|
|
Per Protocol
|
92.5%
|
94.3%
|
97.4%
|
95.7%
|
|
mITT
|
86.0%
|
91.9%
|
84.4%
|
95.7%
|
|
ITT
|
87.0%
|
92.6%
|
83.3%
|
96.2%
|
Clinical response = cure + improvement
13

Clinical Response - Confidence Intervals
Per Study Analysis Plan - Per Protocol Population
Per Study Analysis Plan - Per Protocol Population
Day 3
Day 7
● Results similar across all treatment groups
• All confidence intervals overlap
● Results consistent in all populations (PP, mITT, ITT)
14

Clinical Response - Confidence Intervals
Per Study Analysis Plan - mITT Population
Per Study Analysis Plan - mITT Population
Day 3
Day 7
● Results similar across all treatment groups
• All confidence intervals overlap
● Results consistent in all populations (PP, mITT, ITT)
15

Clinical Response - Confidence Intervals
Per Study Analysis Plan - ITT Population
Per Study Analysis Plan - ITT Population
Day 3
Day 7
● Results similar across all treatment groups
• All confidence intervals overlap
● Results consistent in all populations (PP, mITT, ITT)
16

Sustained Clinical Response - Days 10 and 28
Per Study Analysis Plan
Per Study Analysis Plan
|
Day 10
|
Low dose
|
Medium dose
|
High dose
|
daptomycin
|
|
Per Protocol
|
92.3%
|
93.8%
|
100%
|
97.7%
|
|
mITT
|
87.8%
|
93.9%
|
97.3%
|
97.7%
|
|
ITT
|
90.2%
|
91.8%
|
95.5%
|
97.9%
|
|
Day 28
|
Low dose
|
Medium dose
|
High dose
|
daptomycin
|
|
Per Protocol
|
97.2%
|
87.5%
|
100%
|
97.8%
|
|
mITT
|
97.2%
|
87.9%
|
100%
|
97.8%
|
|
ITT
|
95.7%
|
89.6%
|
95.6%
|
98.0%
|
Sustained Response at Day 28 for Day 10 Clinical Successes
Sustained Response at Day 10 for Day 3 Clinical Successes
17

Sustained Clinical Response - Confidence Intervals
Per Study Analysis Plan - Per Protocol Population
Per Study Analysis Plan - Per Protocol Population
Day 28
Day 10
● Results similar across all treatment groups
• All confidence intervals overlap
● Results consistent in all populations (PP, mITT, ITT)
18
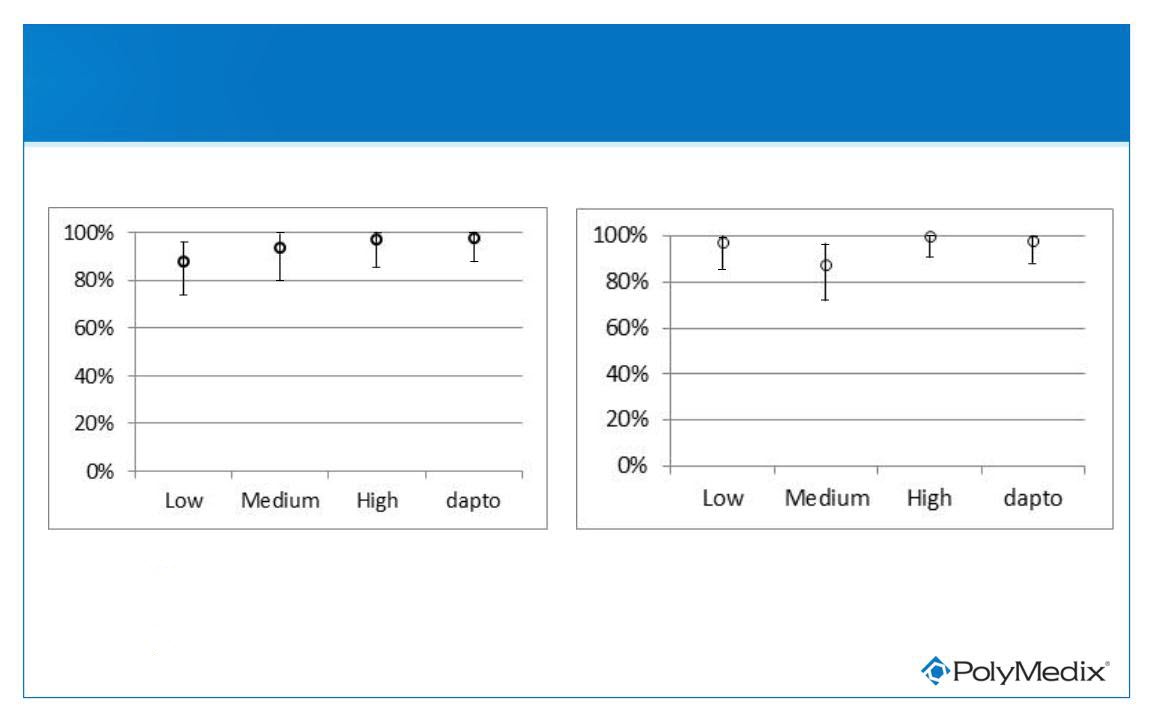
Sustained Clinical Response - Confidence Intervals
Per Study Analysis Plan - mITT Population
Per Study Analysis Plan - mITT Population
Day 28
Day 10
● Results similar across all treatment groups
• All confidence intervals overlap
● Results consistent in all populations (PP, mITT, ITT)
19

Sustained Clinical Response - Confidence Intervals
Per Study Analysis Plan - ITT Population
Per Study Analysis Plan - ITT Population
Day 28
Day 10
● Results similar across all treatment groups
• All confidence intervals overlap
● Results consistent in all populations (PP, mITT, ITT)
20

Time-to-Clinical Response Assessment
Per Protocol Population
Per Protocol Population
Subjects who demonstrate at least 80% decrease in lesion size from baseline, and had a 2-point reduction
from baseline in severity for all signs and symptoms or no presence of all signs and symptoms.
from baseline in severity for all signs and symptoms or no presence of all signs and symptoms.
21

Time-to-Clinical Response Assessment
mITT Population
mITT Population
22

Time-to-Clinical Response Assessment
ITT Population
ITT Population
23

Safety Analysis
Safety Population; N=215
Safety Population; N=215
|
|
Low dose
(n=52)
|
Medium dose
(n=54)
|
High dose
(n=54)
|
daptomycin
(n=55)
|
|
Treatment Related Adverse Events
|
|
|
|
|
|
Excluding numbness & tingling
|
9.6% (5)
|
5.6% (3)
|
7.4% (4)
|
10.9% (6)
|
|
Numbness & tingling
|
65.4% (34)
|
64.8% (35)
|
87.0% (47)
|
1.8% (1)
|
|
Total TRAE
|
75.0% (39)
|
70.4% (38)
|
94.4% (51)
|
12.7% (7)
|
|
Discontinued due to TRAE
|
1.9% (1)
|
3.7% (2)
|
9.2% (5)
|
0
|
Numbness & tingling was most common TRAE
• No patient discontinued drug because of numbness/tingling
• Most cases were mild
• Completely transient, all resolved
• Mechanism thoroughly studied
24
Safety Analysis
Safety Population; N=215
Safety Population; N=215
|
|
Low dose
(n=52)
|
Medium dose
(n=54)
|
High dose
(n=54)
|
daptomycin
(n=55)
|
|
Serious Adverse Events
|
1.9% (1)
|
3.7% (2)
|
3.7% (2)
|
0
|
|
TRSAE
|
1.9% (1)
|
1.8% (1)
|
1.8% (1)
|
0
|
|
Discontinued due to TRSAE
|
0
|
1.8% (1)
|
1.8% (1)
|
0
|
3 patients experienced a TRSAE
Hypertension - 1 medium dose; 1 high dose (both discontinued)
Increased platelets - 1 low dose
25
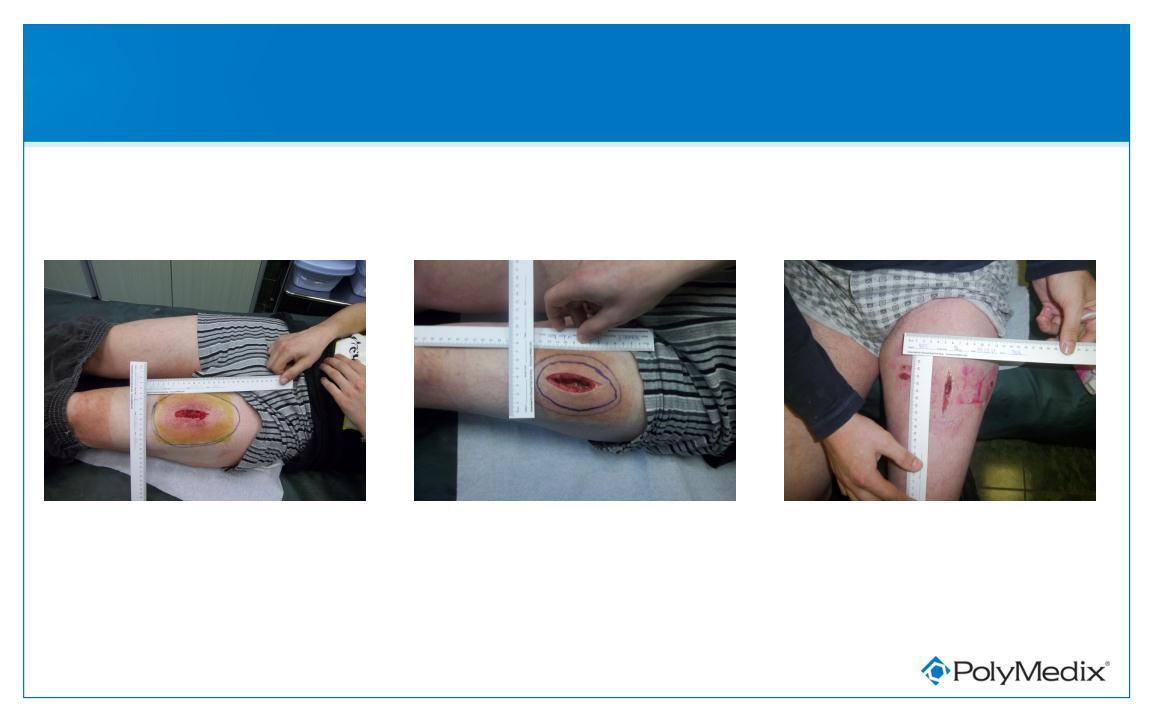
Patient Received Low Dose PMX-30063
Pre-treatment
Day 10
Day 3
26
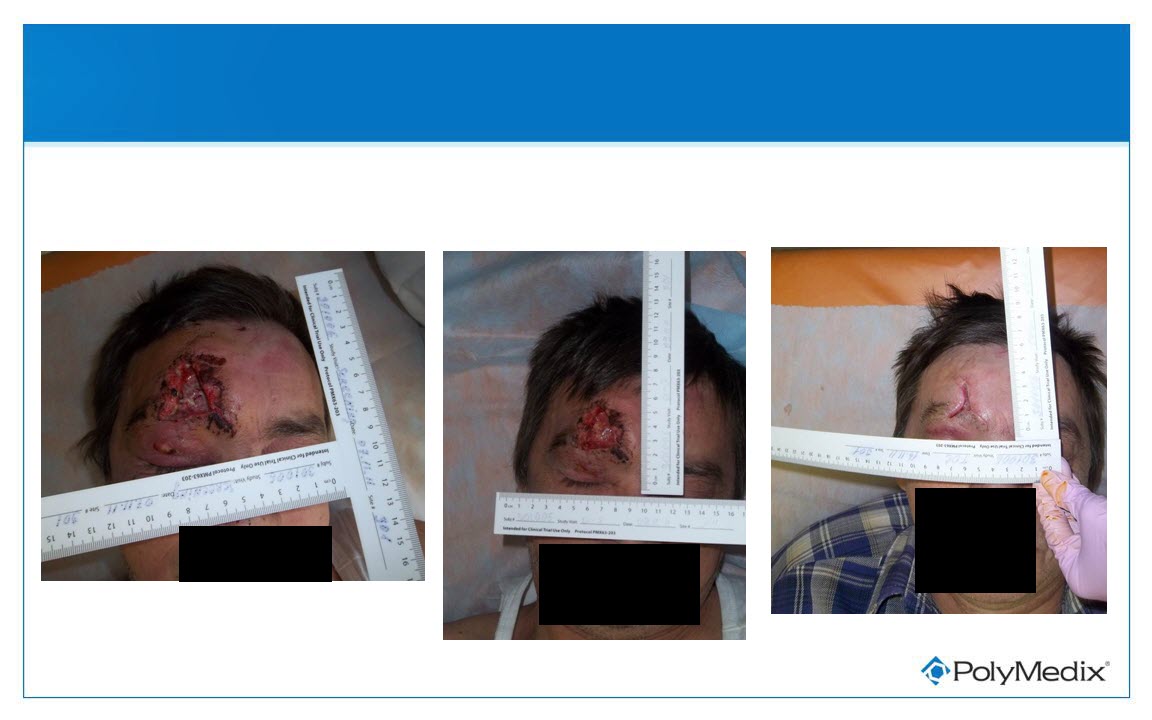
Patient Received Medium Dose PMX-30063
Pre-treatment
Day 10
Day 3
27

Patient Received High Dose PMX-30063
Pre-treatment
Day 10
Day 3
28

Phase 2 ABSSSI Clinical Trial - Conclusions
Excellent clinical efficacy results for a first phase 2 study
Efficacy:
• High clinical response rates
• Similar across all treatment groups (95% CIs overlap)
• Efficacy comparable to active control (daptomycin)
• Early & sustained clinical response
• Results corroborate Interim Analysis
• Rigorous study entry criteria, using FDA definitions for ABSSSI
Safety:
• PMX-30063 is safe and generally well-tolerated
• Other than expected numbness & tingling, consistently low TRAE’s
29

Anticipated Next Steps
Continued regulatory efforts
Presentation of data at scientific meeting
Initiate Second Phase 2 trial in 2012
• Optimize dosing
• Maximize efficacy
• Maximize safety
• Study single dose regimen(s)
Goal: initiate pivotal trials in 2013
30

Goals for PMX-30063
First-line therapy for Staph aureus including resistant strains like MRSA
Short course therapy would promote
• Patient compliance
• Pharmacoeconomic benefits
Novel Defensin-Mimetic mechanism of action
• Active in preclinical studies vs. bacteria that are resistant to current antibiotics
• Resistance should be unlikely to develop to PMX-30063
• Multi-functional mechanism
Expand development for other potential indications and modes of delivery
• i.v.: pneumonia, bacteremia
• Topical oral rinse: oral mucositis (cancer)
31

Q & A
32

Why use PMX-30063 Antibiotic?
New mechanism and class of drug - “Defensin-Mimetic”
• Unlikely to encounter a resistant strain
• Unlikely to develop resistance in the future
Short course of therapy possible
• Next clinical trial plan to evaluate single dose regimen(s)
• Goal to shorten hospital stays, reduce healthcare costs, potential out-
patient treatment
patient treatment
Broader spectrum than other drugs for ABSSSI
Multi-functional mechanism - anti-biofilm, anti-inflammatory
• Additional indications possible - bacteremia, pneumonia
• Additional applications possible - cancer oral mucositis
33

No resistance seen for PMX-30063 vs. daptomycin in pre-clinical
studies
studies
After 40 Passages, no resistance developed to PMX-30063
APIC, 2008, resistance
rates in U.S.
34

PMX-30063 - Successful Phase 2 Results
Efficacy demonstrated
Safety demonstrated
Entirely novel drug - goal to dominate the antibiotic market
Plan to advance with further clinical development
35

OTCBB: PYMX
www.polymedix.com
Revolutionizing the Treatment of Infectious
Diseases with Defensin-Mimetics
Diseases with Defensin-Mimetics
