Attached files
| file | filename |
|---|---|
| EX-4.1 - SPECIMEN COMMON STOCK CERTIFICATE - NeurogesX Inc | d258817dex41.htm |
| EX-31.1 - CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER PURSUANT TO SECTION 302 - NeurogesX Inc | d258817dex311.htm |
| EX-10.4 - FORM OF INDEMNIFICATION AGREEMENT - NeurogesX Inc | d258817dex104.htm |
| EX-23.1 - CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM - NeurogesX Inc | d258817dex231.htm |
| EX-32.1 - CERTIFICATIONS OF THE PEO AND THE PFO PURSUANT TO SECTION 906 - NeurogesX Inc | d258817dex321.htm |
| EX-10.9 - AMENDED AND RESTATED EXECUTIVE EMPLOYMENT AGREEMENT - NeurogesX Inc | d258817dex109.htm |
| EX-31.2 - CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER PURSUANT TO SECTION 302 - NeurogesX Inc | d258817dex312.htm |
| EX-10.13 - EXECUTIVE EMPLOYMENT AGREEMENT - NeurogesX Inc | d258817dex1013.htm |
| EXCEL - IDEA: XBRL DOCUMENT - NeurogesX Inc | Financial_Report.xls |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011
Or
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-33438
NEUROGESX, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 94-3307935 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) |
2215 Bridgepointe Parkway, Suite 200
San Mateo, CA 94404
(650) 358-3300
(Address, including zip code, of registrant’s principal executive offices and telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Common Stock, $0.001 par value
Name of exchange on which registered: The Nasdaq Stock Market LLC (NASDAQ Global Market)
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ |
Accelerated filer ¨ | |
| Non-accelerated filer ¨ (Do not check if a smaller reporting company) |
Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates was $21.0 million computed by reference to the last sales price of $1.75 as reported by the NASDAQ Global Market, as of the last business day of the Registrant’s most recently completed second fiscal quarter, June 30, 2011. This calculation does not reflect a determination that certain persons are affiliates of the Registrant for any other purpose.
The number of shares outstanding of the Registrant’s common stock on February 29, 2012 was 32,896,871 shares.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s Proxy Statement for its 2012 Annual Meeting of Stockholders (the “Proxy Statement”), to be filed with the Securities and Exchange Commission, are incorporated by reference to Part III of this Annual Report on Form 10-K.
Table of Contents
NEUROGESX, INC.
FORM 10-K
Year Ended December 31, 2011
| Page | ||||||
| 1 | ||||||
| ITEM 1. |
2 | |||||
| ITEM 1A. |
29 | |||||
| ITEM 1B. |
47 | |||||
| ITEM 2. |
47 | |||||
| ITEM 3. |
47 | |||||
| ITEM 4. |
48 | |||||
| 49 | ||||||
| ITEM 5. |
49 | |||||
| ITEM 6. |
51 | |||||
| ITEM 7. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
52 | ||||
| ITEM 7A. |
70 | |||||
| ITEM 8. |
71 | |||||
| ITEM 9. |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
106 | ||||
| ITEM 9A. |
106 | |||||
| ITEM 9B. |
106 | |||||
| 107 | ||||||
| ITEM 10. |
107 | |||||
| ITEM 11. |
107 | |||||
| ITEM 12. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
107 | ||||
| ITEM 13. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
111 | ||||
| ITEM 14. |
111 | |||||
| 112 | ||||||
| ITEM 15. |
112 | |||||
-i-
Table of Contents
This report contains forward-looking statements that are based upon current expectations within the meaning of the Private Litigation Securities Reform Act of 1995. We intend that such statements be protected by the safe harbor created thereby. Forward-looking statements involve risks and uncertainties and our actual results and the timing of events may differ significantly from those results discussed in the forward-looking statements. Examples of such forward-looking statements include, but are not limited to, statements about or relating to:
| • | anticipated development of NGX-1998, including plans and timing with respect to an End of Phase 2 meeting with the U.S. Food and Drug Administration, or FDA, the potential entry of such product candidate into Phase 3 clinical trials and the timing of design of the applicator device for NGX-1998; |
| • | potential benefits of NGX-1998 as compared to Qutenza, including broader sites of application, timing and method of treatment necessary to obtain therapeutic benefits, treatment settings and potential additional indications for treatment; |
| • | potential benefits of cross application of our development experience with Qutenza for the development of NGX-1998; |
| • | our plans with regard to the commercialization of Qutenza® in the United States and the plans of Astellas Pharma Europe Ltd., or Astellas, for commercialization of Qutenza pursuant to the terms of the Distribution, Marketing Agreement and License Agreement, or the Astellas Agreement; |
| • | timing of completion of clinical trials for Qutenza being conducted by Astellas, and potential efforts for label expansion for Qutenza in the United States and the European Union that may be carried out if such trials are successful; |
| • | expectations with respect to potential entry into commercial strategic partnerships for Qutenza and potential entry into development and commercial strategic partnerships for NGX-1998; |
| • | maintenance of availability of Qutenza in the marketplace; |
| • | expectations with respect to revenues for Qutenza as a result of our restructuring of commercial operations; |
| • | sufficiency of existing resources to fund our operations through at least December 31, 2012; |
| • | capital requirements and our needs for additional financing including the potential forms such financing may take, including our needs for additional financing to fund development of NGX-1998; |
| • | anticipated development of other potential product candidates; |
| • | the potential benefits of, and markets for, Qutenza and our product candidates, as well as the potential target indications and size and scope of markets being explored for NGX-1998; |
| • | losses, costs, expenses, expenditures and cash flows; |
| • | potential competitors and competitive products; |
| • | future payments under lease obligations and equipment financing lines; |
| • | patents and our and others’ intellectual property; and |
| • | expected future sources of revenue and capital. |
We undertake no obligation to, and expressly disclaim any obligation to, revise or update the forward-looking statements made herein or the risk factors, whether as a result of new information, future events or otherwise. Forward-looking statements involve risks and uncertainties, which are more fully discussed in the “Risk Factors” section and elsewhere in this Annual Report, including, but not limited to, those risks and uncertainties relating to:
| • | difficulties or inability to meet our obligations and covenants under our debt arrangements, including our arrangements with Hercules Technology Growth Capital, Inc., or Hercules, and Cowen Royalty Partners, L.P., or Cowen Royalty; |
1
Table of Contents
| • | difficulties or delays in development, testing, obtaining regulatory approval for, and undertaking production and marketing of NGX-1998, and in potential label expansion development efforts for Qutenza; |
| • | unexpected adverse side effects or inadequate therapeutic efficacy of our product candidates, including NGX-1998 and Qutenza could slow or prevent product approval or approval for particular indications (including the risk that current and past results of clinical trials or preclinical studies are not indicative of future results of clinical trials, and the difficulties associated with clinical trials for pain indications); |
| • | positive results in clinical trials may not be sufficient to obtain FDA or European regulatory approval for NGX-1998 or for label expansion for Qutenza; |
| • | physician or patient reluctance to use Qutenza or payer coverage for Qutenza and for the procedure to administer it, which may impact physician utilization of Qutenza; |
| • | our inability to obtain additional financing if necessary; |
| • | changing standards of care and the introduction of products by competitors or alternative therapies for the treatment of indications we target; |
| • | the uncertainty of protection for our intellectual property, through patents, trade secrets or otherwise; and |
| • | potential infringement of the intellectual property rights or trade secrets of third parties. |
When used in this Annual Report, unless otherwise indicated, “NeurogesX,” “the Company,” “we,” “our” and “us” refers to NeurogesX, Inc. and its subsidiaries.
Qutenza® and NeurogesX® are registered trademarks in the United States. We have also applied for these trademarks in several other countries. Other service marks, trademarks and trade names referred to in this Annual Report on Form 10-K are the property of their respective owners.
| Item 1. | Business |
Overview
We are a specialty pharmaceutical company focused on developing and commercializing a portfolio of novel non-opioid, pain management therapies to address unmet medical needs and improve patients’ quality of life.
Our first commercial product, Qutenza, became commercially available in the United States and in certain European countries in the first half of 2010. Qutenza is a dermal delivery system designed to topically administer capsaicin to treat certain neuropathic pain conditions and was approved by the FDA in November 2009 for the management of neuropathic pain associated with postherpetic neuralgia, or PHN. Qutenza is also approved in the European Union for the treatment of peripheral neuropathic pain in non-diabetic adults. Qutenza is the first prescription strength capsaicin product approved in the United States.
Our most advanced drug candidate is NGX-1998. NGX-1998 is a topical liquid formulation of high concentration capsaicin that we are developing to treat neuropathic pain conditions. In November 2011, we announced positive top-line results from our Phase 2 clinical study of NGX-1998, in patients with PHN. The 12-week, multicenter, randomized, double-blinded, placebo-controlled clinical trial met its protocol-specified objectives, which include the primary endpoint of a percentage change from baseline as compared to placebo in a patient-reported numeric pain rating scale, or NPRS, score during weeks two through eight of the trial. We believe that the Phase 2 data support moving forward to a NGX-1998 Phase 3 clinical trial. We plan to have an End-of-Phase 2 meeting with the FDA, which we believe could occur in the second half of 2012. We are presently examining a range of potential indications for NGX-1998, including but not limited to PHN, HIV
2
Table of Contents
associated peripheral neuropathy, or HIV-PN, painful diabetic neuropathy, or PDN, chemotherapy-induced peripheral neuropathy, or CIPN, and post-traumatic (e.g. surgical) pain, or PTP. Our decision as to which indications to pursue in Phase 3 clinical trials is expected to be based on a number of criteria including, but not limited to, our assessments of probability of clinical development success, market size and potential regulatory pathways to possible approval.
We believe that NGX-1998 has the potential to represent a significant improvement over Qutenza. To date, the clinical data suggests that NGX-1998 does not require pre-treatment with a topical anesthetic and administration of NGX-1998 for five minutes may provide a similar response to that experienced with Qutenza after a 60-minute application, that is, that a patient may experience meaningful pain relief for up to 12 weeks from that single five minute application. As a result, we believe that NGX-1998 has the potential to be more readily accepted by physicians and may also demonstrate efficacy in a number of neuropathic pain conditions. Additionally, we believe that the liquid formulation represents a more versatile delivery model which is well suited for treating hard to reach places on the body. Further, we believe that our experience in conducting clinical trials with capsaicin and the approval of Qutenza may benefit our efforts to obtain clinical success for NGX-1998. Finally, in April, 2011, NGX-1998 was granted patent protection under a patent entitled “Methods and Compositions for Administration of TRPV1 Agonists.” The allowed claims include method of use, formulation and system claims. The patent, including the Patent Term Adjustment, expires in 2027. In February 2012, we received a notice of allowance from the Japanese Patent Office for a patent that covers NGX-1998 with a set of claims that are similar to those issued in the United States. A similar patent application is also under review in the European Union.
As a result of the opportunity we believe exists with NGX-1998, in March 2012, we decided to focus our resources on the preparation for advancing NGX-1998 into a Phase 3 clinical trial. To do so, we significantly restructured our operations, including eliminating most of sales and marketing expenditures in support of Qutenza, while continuing to maintain product availability of Qutenza for patients and physicians. While we believe a market opportunity exists for Qutenza, we believe that the resources required to attain that potential are better utilized to speed the development of NGX-1998. We are evaluating a number of alternative commercialization strategies which include the potential to license Qutenza to a commercialization partner in the United States and in other territories of the world outside of the territory addressed by Astellas.
In September 2011, we submitted a supplemental new drug application, or sNDA, to the FDA regarding potentially expanding the Qutenza label to include treatment of pain associated with HIV-PN. On February 9, 2012, the Anesthetic and Analgesic Drug Product Advisory Committee of the FDA Panel voted unanimously against approval of Qutenza in such indication as the committee determined that the data did not represent adequate evidence of efficacy. In March 2012, we received a complete response from the FDA regarding our sNDA. The complete response letter indicated that in order to gain approval for the use of Qutenza for the proposed indication, we would need to submit additional clinical data from at least one adequate and well-controlled Phase 3 clinical trial to support the proposed indication. However, we do not anticipate investing in further clinical trials for Qutenza at this time.
Although we are limiting our investment in Qutenza in the United States, Astellas continues to conduct studies with Qutenza for its Licensed Territory for commercialization of Qutenza which includes the 27 countries of the European Union, Iceland, Norway, and Liechtenstein as well as Switzerland, certain countries in Eastern Europe, the Middle East and Africa, which we refer to as the Licensed Territory. Astellas is currently conducting three clinical trials in response to follow up measures requested by the European Medicines Agency. These studies include a long-term safety study in on-label indications and two studies, a long-term safety study and an efficacy study, in patients with PDN. The results of these studies are not expected until sometime in 2013. However, if the PDN clinical trials are successful, we plan to explore the potential for such trials to support further label expansion opportunity in the United States.
3
Table of Contents
The Astellas Agreement, described below, provides Astellas an option to exclusively license NGX-1998 in the Licensed Territory. If Astellas was to exercise its option, they would collaborate with us on the Phase 3 clinical trials and share equally in certain of Phase 3 development costs that benefit the regulatory process in their territory. We are also exploring the possibility of a strategic alliance for development and commercialization of NGX-1998 in the United States.
In addition to Qutenza and NGX-1998, we have a series of compounds which represent an early stage pipeline of potential product candidates consisting of prodrugs of acetaminophen for potential use in acute pain, including traumatic pain, post-surgical pain and fever. These product candidates are in the pre-clinical stage of development and may or may not be the subject of further development in 2012 or beyond.
Astellas License Agreement
In May 2009, Qutenza received a marketing authorization, or MA, in the European Union for the treatment of peripheral neuropathic pain in non-diabetic adults, either alone or in combination with other medicinal products for pain. In June 2009, we entered into the Astellas Agreement, under which we granted Astellas an exclusive license to commercialize Qutenza in the Licensed Territory. Qutenza was made available on a country by country basis commencing in April 2010 and, by year-end 2011, Qutenza was available in 21 European countries and Astellas had filed for regulatory approval in numerous other countries in the non-EU geographies of their Licensed Territory. We believe that Astellas remains committed to Qutenza and its commercialization in Europe. Astellas is currently conducting three clinical trials in response to follow up measures requested by the European Medicines Agency. These studies include a long-term safety study in on-label indications and two studies, a long-term safety study and an efficacy study, as described above. We hold worldwide commercial rights to all of our products and product candidates, with the exception of rights granted to Astellas.
Financial Update
In July 2011, we completed a private placement under a Securities Purchase Agreement pursuant to which we issued units, or the Units, for an aggregate purchase price of approximately $20.2 million, at a per Unit price of $1.72. Each Unit was comprised of one share of common stock and a warrant to purchase 0.5 shares of common stock (representing 50% warrant coverage on the shares to be issued). The price of each Unit was based on the July 21, 2011 consolidated closing bid price of our common stock on the NASDAQ Global Market of $1.65 per share, and represented a purchase price of $1.65 for the one share of common stock issued for each Unit and a warrant purchase price of $0.07 for the 0.5 shares of common stock underlying the warrants issued for each Unit (resulting in a purchase price for the warrants of $0.14 per whole share of common stock underlying such warrants). The total number of Units issued in connection with the transaction was 11,749,552, representing an aggregate issuance of 11,749,552 shares of common stock and warrants to purchase an aggregate of 5,874,782 shares of common stock. The warrants have a term of five years, contain a net-exercise provision, and have an exercise price of $1.65 per share. The net proceeds to us, after deducting expenses of approximately $1.6 million, were $18.6 million. In February 2012, we completed a private placement of our common stock under a Securities Purchase Agreement pursuant to which we issued shares of common stock for an aggregate purchase price of approximately $3.0 million, at a per share price of $1.01. The price of each share of common stock is based on the January 31, 2012 consolidated closing bid price of our common stock on the NASDAQ Global Market of $1.01 per share. The total number of shares of common stock issued in connection with the transaction is 2,969,685. The net proceeds to us, after deducting expenses of less than $0.1 million, were approximately $3.0 million. The funding, initiated by an inbound inquiry, was led by a large, global institutional investor and included certain other existing investors.
In August 2011, we entered into a loan agreement with Hercules, or the Hercules Agreement, which includes both a $5.0 million accounts receivable line and a $15.0 million term loan, collectively. Under the terms of the loan agreement, the $15.0 million term loan is required to be repaid over the course of the 42-month maturity period, which includes a 12-month interest only period at the beginning of the term. In connection with our entry into the loan agreement, we issued to Hercules a warrant for 791,667 shares of our common stock (representing an aggregate
4
Table of Contents
exercise price equal to approximately 7.125% of the maximum aggregate funds potentially available to us under the loan agreement). The warrant has a term of five years, contains a net-exercise provision, and has an exercise price of $1.80 per share. The fair value of the warrants issued was approximately $1.1 million. The loan agreement also contains customary negative covenants and is subject to customary events of default, such as a failure to make a scheduled principal or interest payment, insolvency, and breaches of covenants under loan agreement and agreements and instruments entered into in connection with the loan agreement, including covenants in the Hercules warrant. In March 2012, we entered into an amendment to the Hercules warrant, in which we amended the covenant with respect to the requirement to maintain our listing on the NASDAQ Global Market to allow for the NASDAQ Capital Market and certain over the counter markets to be permissible alternative markets on which we can list our shares of common stock. Under the terms of the amendment, the number of shares underlying the warrant was increased to 1,950,000 and the exercise price per share of common stock was reduced to $0.50.
NASDAQ Listing
We received a notice of market capitalization insufficiency from the NASDAQ Global Market on October 18, 2011 as our market value of listed shares had remained below the $50 million minimum listing requirement for more than 30 consecutive business days. Additionally, on January 27, 2012, we received a notice of share price deficiency as the bid price of our shares of common stock had remained below the $1.00 minimum listing requirement for more than 30 consecutive business days. Each of these notices provide a 180 calendar day grace period during which time we may regain compliance with the minimum listing requirement by attaining the applicable minimum requirement for 10 consecutive business days. The grace period to regain compliance with the minimum market value of listed securities requirement expires on April 16, 2012 and the grace period to regain compliance with the minimum bid price requirement expires on July 28, 2012. If we have not regained compliance with the applicable minimum listing requirement on these dates, we will receive a de-listing determination letter with respect to the applicable requirement. Should that occur, we may seek a hearing with NASDAQ, wherein we would be required to provide a plan for regaining the applicable minimum listing requirement and through which we would seek an extension of the grace period during which we would seek to execute such plan. During the pendency of the hearing process, our delisting would be delayed, however, we cannot be assured that we would otherwise be granted any additional time to regain compliance as a result of the hearing process. If we are de-listed from the NASDAQ Global Market, we may have the opportunity to apply for and potentially obtain a listing of our common stock on the NASDAQ Capital Market, subject to our meeting the continued listing requirements of such market. However, we currently do not meet the continued listing requirements for that exchange. If we are ineligible for listing on the NASDAQ Capital Market, we may seek listing on the OTC Bulletin Board or other over-the-counter market. For a description of the risks associated with our potential de-listing from the NASDAQ Global Market, see the Risk Factors section of this Form 10-K.
Our Strategy
Our vision is to leverage our development and commercial expertise to become a sustainable specialty pain management company. Key elements of our strategy for achieving this goal include:
Advance the development of NGX-1998 rapidly and with a broad neuropathic pain focus
NGX-1998 is a topical liquid formulation of high concentration capsaicin. We have designed NGX-1998 to address what we believe to be the more significant limitations experienced with Qutenza, while attempting to preserve the safety profile of capsaicin and replicate the efficacy demonstrated with Qutenza. In November 2011, we announced positive top-line results from our Phase 2 clinical study of NGX-1998, a topical liquid formulation of high concentration capsaicin, in patients with PHN. We believe that the Phase 2 data support moving forward to a NGX-1998 Phase 3 clinical trial. We plan to have an End-of-Phase 2 meeting with the FDA and we believe this meeting could occur in the second half of 2012.
5
Table of Contents
We believe that NGX-1998 has the potential to represent an improvement over Qutenza for the following reasons:
| • | clinical data suggests that NGX-1998 does not require pre-treatment with a topical anesthetic which would reduce treatment time and make administering NGX-1998 more convenient for both patients and physicians; |
| • | administration of NGX-1998 for as little as five minutes may provide similar pain relief to that experienced with Qutenza, that is, a single five minute administration may provide meaningful pain relief for up to 12 weeks; |
| • | Clinical data suggests that NGX-1998 has a similar safety profile to Qutenza and that similar to Qutenza, does not result in significant systemic absorption; |
| • | NGX-1998 has the potential for broad market appeal to physicians due to its ease of use; |
| • | a liquid formulation represents a versatile delivery model that can be used on virtually any bodily surface including hard to reach places such as between the toes; and |
| • | NGX-1998 has patent protection in the United States through 2027 and a notice of allowance was issued by the Japanese Patent Office in early 2012. Additionally, patent applications are pending in the European Union and other countries. |
We believe that NGX-1998 has the potential to be efficacious in a number of neuropathic pain conditions and we are presently examining a range of indications for NGX-1998, including but not limited to PDN, CIPN, PTP, HIV–PN and PHN.
We are currently evaluating the potential to establish a development and commercial partnership to advance NGX-1998 on a broader scale than we would be able to accomplish on our own and also to assist in the commercialization of NGX-1998. We believe that NGX-1998 may be a product that is used by a variety of physician specialties and has the potential to be used first line in primary care, as well as a number of physician specialties. As a result, we believe there is the potential to enter into a strategic partnership to supplement our own commercial efforts.
Maintain market availability of Qutenza and seek potential opportunities for label expansion
Qutenza is a dermal delivery system designed to topically administer capsaicin to treat certain neuropathic pain conditions and was approved by the FDA in November 2009 for the management of neuropathic pain associated with PHN. Qutenza is also approved in the European Union for the treatment of peripheral neuropathic pain in non-diabetic adults.
We launched marketing of Qutenza in the United States in April 2010 with a specialty sales force. Although our sNDA for label expansion of Qutenza for the treatment of pain associated with HIV-PN was not approved, and although Qutenza revenues in the United States have been significantly less than our expectations since launch, we remain committed to maintaining availability of Qutenza in the United States.
In 2009, Qutenza was approved in the European Union for the treatment of neuropathic pain in non-diabetic adults. Astellas, our commercial partner in Europe, the Middle East and Africa initiated commercial availability of Qutenza in middle of 2010. Astellas is currently conducting additional clinical studies to support Qutenza including three clinical trials in response to follow up measures requested by the European Medicines Agency. These studies include a long term safety study in on-label indications and two studies, a long-term safety study and an efficacy study in patients with PDN. The results of these studies are not expected until sometime in 2013.
In March 2012, we decided to significantly reduce our investment in Qutenza commercialization including a decision to no longer promote Qutenza with our own sales force. We are currently evaluating promotional models for Qutenza that can better align near term revenue expectations with the cost to generate those revenues.
6
Table of Contents
These models may include out-licensing Qutenza for the U.S. market and promotional efforts that do not rely on our own sales force. We continue to believe that a significant commercial opportunity exists for Qutenza in the United States which may be further enhanced by clinical results from the PDN studies being conducted by Astellas.
Explore opportunities to augment our development and commercialization efforts through strategic alliances and collaborations.
We are currently evaluating the potential to establish a development and commercial partnership to advance NGX-1998 on a broader scale than we would be able to accomplish on our own and also to assist in the commercialization of NGX-1998. We believe that NGX-1998 may be a product that is used in a variety of care settings and has the potential to be used in the primary care setting, as well as a number of physician specialty settings. As a result, we believe there is the potential to enter into a strategic partnership to supplement our own commercial efforts.
In addition, we are evaluating a number of alternative commercialization strategies which include the potential to license Qutenza to a commercialization partner in the United States to supplement or replace our own Qutenza commercialization efforts.
Expand our product portfolio.
Our model is to develop innovative non-opioid therapies for the treatment of pain. We seek to balance market opportunity with a favorable clinical and regulatory pathway. We have begun to expand our product candidate pipeline through the pre-clinical evaluation of our acetaminophen pro-drug candidates. Further, we may expand our product candidate pipeline through in-license or acquisition of product candidates.
Neuropathic Pain
Pain results from sensory nerve stimulation often associated with actual or potential tissue damage. Pain is transmitted by specific nerve fibers that carry the pain signal across the nervous system to the brain, where it is recognized as pain. Pain can be acute or chronic. Acute pain is short in duration and tends to be reactive or protective against actual or potential tissue injury. Chronic pain lasts over an extended period of time and often serves no useful purpose. There are two broad categories of chronic pain, inflammatory and neuropathic. Inflammatory pain is associated with tissue damage, often occurring from injury or from inflammatory conditions, such as osteoarthritis or lower back pain. This class of pain is often treated with prescription drugs that act systemically, including opioids, and over-the-counter anti-inflammatory drugs.
Neuropathic pain is a type of chronic pain that results from injury to, or dysfunction of, nerves. The injury can be to the central nervous system, consisting of the brain and spinal cord, or to the peripheral nervous system, consisting of all other nerves. Neuropathic pain can occur in any part of the body and can significantly impair the affected individual’s quality of life. It can result from viruses, as is the case with PHN and HIV, or diseases, such as diabetes. Neuropathic pain can also result from the use of drugs that treat viruses or other diseases, such as drugs used to treat HIV or cancer. There are many different models of neuropathic pain. We are evaluating a subset of these models for potential future clinical study with NGX-1998, including without limitation PDN, CIPN, PTP, PHN and HIV-PN.
Capsaicin-Induced Effects on Peripheral Neuropathic Pain
Peripheral neuropathic pain results from injured or diseased nerve endings that can send aberrant pain signals to the brain in the absence of harmful stimuli, inappropriately causing the sensation of pain. We believe capsaicin can reduce the ability of these nerve fibers to initiate pain signals for a sustained period of time. Capsaicin is a naturally occurring substance that is responsible for making chili peppers pungent.
7
Table of Contents
Products containing low concentrations of capsaicin, including creams, lotions and patches, have long been sold over-the-counter for the treatment of minor arthritis, back and muscle pain, as well as for other conditions. Low-concentration capsaicin topical products have not been a viable treatment for chronic peripheral neuropathic pain conditions due in part to poor patient compliance resulting from the treatment of already painful skin with a compound that causes burning sensations, as well as the inconvenience of multiple daily applications. We believe that high-concentration capsaicin cream also does not appear to constitute a viable therapy because application causes significant patient discomfort, creams can allow the capsaicin to disperse beyond the treatment area, and existing creams have not been optimally formulated to allow capsaicin to penetrate the skin. To address the intrinsic limitations of existing, over the counter capsaicin therapies, we have developed Qutenza and are developing NGX-1998, utilizing prescription-strength capsaicin.
Existing Treatments and Their Limitations
While there are a number of products currently available for the management of neuropathic pain in general and PHN specifically, we believe that the market is still underserved due to the limitations of current therapies. The primary limitations of current therapies relate to their modest efficacy, unwanted systemic side effects, cumbersome treatment regimens, and the potential for abuse and drug-drug interaction.
Because patients react to pain and to pain therapies differently and because no one therapy offers complete pain relief to all patients without significant side effects, a single standard of care does not exist for the management of neuropathic pain. Initial systemic treatment typically involves one of a few anti-convulsants or anti-depressants. To the extent that the initial therapy does not provide adequate pain relief, the physician may try other anti-convulsants, anti-depressants or opioids alone or in combination, to treat the pain. These systemic treatments are often limited by side effects including dizziness, sedation, confusion, constipation and the potential for drug dependence. Due to these side effects, patient compliance is often poor and physicians often reduce dosing to less than optimal levels which limits the ability of these drugs to reduce pain. Additionally, topical analgesics have modest efficacy and require daily dosing. For these reasons, we believe there is an opportunity for localized, non-systemic analgesics to be used broadly either alone or in combination with other pain medications to reduce the patient’s pain.
Our Capsaicin Based Solutions to Neuropathic Pain
We have historically devoted significant development resources in two primary delivery models of high concentration capsaicin for the treatment of neuropathic pain: Qutenza (capsaicin 8%) dermal patch and NGX-1998, a product candidate which is a topical liquid formulation of high concentration capsaicin. We have treated over 2,000 patients in our various clinical trials with Qutenza and NGX-1998. Our experience with conducting these clinical trials, as well as our market experience in the commercialization of Qutenza, has convinced us that a convenient, versatile delivery model for capsaicin can be a very meaningful product opportunity which may address numerous neuropathic pain conditions. In March 2012, we focused our corporate resources on NGX-1998 with a goal of enabling the initiation of a Phase 3 clinical trial in late 2012. As described more fully below, we believe that NGX-1998 may have a broad market opportunity in a number of neuropathic pain conditions and that its delivery model and procedure may address certain major market impediments observed with Qutenza, including the application time. We believe that Qutenza has the opportunity to be a meaningful product for PHN patients and that the potential exists for further label expansion, depending on the outcome of clinical studies being conducted by Astellas in the PDN patient population. While we advance NGX-1998 through the clinic, we remain committed to maintaining Qutenza access for physicians and their PHN patients and we are evaluating various models for the continued promotion of Qutenza without requiring significant investments of our capital resources.
8
Table of Contents
NGX-1998—Topical Liquid High-Concentration Capsaicin
NGX-1998 is a topical liquid formulation containing a high concentration of capsaicin, the active ingredient in Qutenza. The formulation contains a 20% concentration of capsaicin in a semi viscous solution which includes a mixture of penetration enhancers. NGX-1998 is being developed to be applied using a sponge applicator that will provide for a predictable and reproducible delivery of the solution to the treatment area. In early development, we evaluated different concentration levels of capsaicin as well as combinations of penetration enhancers and other excipients. That evaluation led us to the current formulation which we intend to take into Phase 3 development in late 2012. As currently envisioned, NGX-1998 would be applied directly to the painful area by a healthcare professional and left on the skin for approximately five minutes, after which, the skin would be cleansed using a proprietary cleansing gel. We believe that NGX-1998 has the potential to treat a wide range of neuropathic pain conditions and we are currently evaluating our initial Phase 3 development plan which may include one or more of the following neuropathic pain indications: PDN, CIPN, PTP, HIV-PN and PHN. We intend to review our Phase 3 development plan with the FDA in an end of Phase 2 meeting later this year. We believe that our experience in conducting clinical trials with capsaicin positions us well to succeed in Phase 3 development. We also believe that the safety data accumulated to date with NGX-1998 is very similar to the safety profile that was developed during the significant clinical program for Qutenza, and that the nonsystemic nature of the product may position NGX-1998 for a more streamlined regulatory path.
Importantly, we believe that NGX-1998 may address many of the commercial issues that have challenged Qutenza in the market. Specifically, we believe that:
| • | clinical data suggests that NGX-1998 does not require pretreatment with a topical anesthetic thereby reducing the total treatment time making the treatment more convenient for both patient and physician; |
| • | administration of NGX-1998 for as little as five minutes may provide similar pain relief to that experienced with Qutenza, that is, a single five minute administration may provide meaningful pain relief for up to 12 weeks; |
| • | NGX-1998 has the potential for broad market appeal to physicians due to its ease of use; |
| • | a liquid formulation represents a versatile delivery model that can be used on virtually any bodily surface including hard to reach places such as between the toes; and |
| • | NGX-1998 has patent protection in the United States through 2027 and a notice of allowance was issued by the Japanese Patent Office in early 2012. Additionally, patent applications are pending in the European Union and other countries. |
We believe that capsaicin has the potential to be efficacious in a number of neuropathic pain conditions and represents a sizeable market opportunity. We are currently evaluating five potential indications for entering into Phase 3, including PDN, CIPN, PTP, HIV–PN and PHN.
Commercial Opportunity
We believe that NGX-1998 may have an opportunity to treat a broad range of neuropathic pain conditions. This belief is supported by both the nature of capsaicin and its effects on peripheral neuropathic pain as more fully described under the section entitled “Capsaicin induced effects on peripheral neuropathic pain” and is further supported by data that has been collected by our commercial partner, Astellas as well as discussions with physicians who have treated patients with capsaicin in wide range of conditions. We are currently evaluating five potential target indications for our initial Phase 3 development program. These include PDN, CIPN, PTP, HIV-PN and PHN, described below.
Painful Diabetic Neuropathy
Background on PDN: PDN is caused by injury to the sensory nerves, which arises from the toxic effects of glucose, some glucose metabolites and damage to blood vessels associated with nerves. The condition causes
9
Table of Contents
progressive pain or loss of feeling in the toes, feet, legs, hands and arms. Like HIV-PN, PDN is typically first felt as pain in the feet and hands. To fulfill a condition of approval of the MA, Astellas has submitted and received approval from the Committee for Medicinal Products for Human Use, or CHMP, of the European Medicines Agency, or EMA to conduct two protocols evaluating Qutenza in patients with PDN. One protocol is a long-term safety study and the other is a controlled efficacy study. These studies are planned to run concurrently beginning in 2011 with the safety study to be conducted in the European Union and the efficacy study to be conducted in North America. We plan to assess to what extent we will be able to use, if at all, the data from these studies to meet the requirements for a US regulatory submission.
Potential Market: The Centers for Disease Control, or CDC currently estimates that 25.8 million people in the United States have diabetes, of which it is estimated by Jain PharmaBiotech 2008, or Jain, that 6.0 million suffered from some form of neuropathy. The number of PDN sufferers in the United States is currently estimated by Jain to be 3.0 million. According to The Mattson Jack Group, there are approximately 2.85 million people with PDN in the United Kingdom, France, Germany, Italy and Spain, combined.
Chemotherapy Induced Peripheral Neuropathy
Background on CIPN: CIPN describes the damage to the peripheral nervous system incurred by a patient who has received a chemotherapeutic agent that is known to be neurotoxic. According to the National Comprehensive Cancer Network, neuropathic symptoms resulting from cancer therapies such as chemotherapy can have serious implications for cancer patients which can include treatment delay, dose reductions or even termination of therapy. Many chemotherapeutic agents are believed to significantly increase the risk of neuropathic pain including platinum-containing agents, vinca alkaloids, taxanes, bortezomib and thalidomide. While each class of chemotherapeutic agent has its own mechanism of action which can result in neuropathic pain, the CIPN that develops is thought to be a length dependent neuropathy that affects nerve endings furthest from the spinal cord first and as cumulative doses increase, the symptoms can increase in severity and also migrate closer to the spinal cord.
Potential Market: The American Cancer Society in its publication, Cancer Facts and Figures 2012, indicates that in the United States, there are an estimated 1.6 million new cancer cases each year. Data from the National Cancer Institute Surveillance Epidemiology and End Results (SEER) program show rising trends of chemotherapy treatment in cancers in which they have collected treatment data, such as colorectal, breast, and lung cancers. Ranges between 60% and 85% of patients were reported to be receiving chemotherapy treatment at the various stages within these indications. According to the National Cancer Institute, an estimated 30 to 40 percent of cancer patients treated with chemotherapy experience symptoms associated with CIPN.
Post Traumatic (e.g. Surgical) Pain
Background on PTP: A publication by the International Association for the Study of Pain defines persistent postsurgical pain as pain that develops after surgical intervention and lasts at least two months. Post Surgical neuropathic pain is believed to result from damage to nerves during a surgical procedure and the resulting pain can persist long after the wound has healed. As with most pain conditions, the effect of post surgical neuropathic pain can significantly impact a patients quality of life and can be debilitating. Certain surgical procedures appear to have greater incidence of PTP, such as mastectomy, coronary bypass surgery, for example.
Potential Market: Depending on how PTN is defined, the data on incidence varies widely across various indications. Overall, the incidence of chronic pain after major surgery is estimated to lie in a range between 10% and 50%. A publication in the ANZ Journal of Surgery, July 2008, reports the incidence of PTN in operations such as hernia repair or cesarean section can be 10% while the incidence of PTN in breast surgery can range from 20% to 30% and in both coronary artery bypass and amputation incidence can range from 30% to 50%. Based on this incidence data, we estimate that the foregoing surgical procedures may result in a potential annual incidence of PTP in the range of 500,000 to 650,000 in the United States.
10
Table of Contents
HIV Associated Peripheral Neuropathy
Background on HIV-PN: HIV-PN is caused primarily by three factors: direct activation of cells known as sensory neurons by the HIV virus, the immune system’s fight against the infection and certain drugs administered to treat HIV. Painful HIV-PN is characterized by significant pain in the feet and hands.
Potential Market: According to Frost & Sullivan, neuropathic pain is a common neurological complication of antiretroviral treatments of HIV and affects approximately 15% of the HIV-infected community. In July 2010, CDC estimated that there are more than one million people living with HIV in the United States. There are currently no specific treatments approved in the United States or Europe for HIV-PN. Estimates as to the number of people currently with HIV-PN in the United States vary from under 200,000 to nearly 300,000. The Mattson Jack Group estimated in 2007 that there were approximately 270,000 and 136,000 people with HIV-PN in the United States and in the United Kingdom, France, Germany, Italy and Spain, combined, respectively.
Post-Herpetic Neuralgia
Background on PHN: PHN is a painful condition affecting sensory nerve fibers. It is a complication of shingles, a second outbreak of the varicella-zoster virus, which initially causes chickenpox. Following an initial infection, some of the virus can remain dormant in nerve cells. Years later, age, illness, stress, medications or other factors that are not well understood can lead to reactivation of the virus. The rash and blisters associated with shingles usually heal within about six weeks, but some people continue to experience pain for years thereafter. This pain is known as postherpetic neuralgia, or PHN. PHN may occur in almost any area, but is especially common on the torso.
Potential Market: According to the CDC there are approximately 1.0 million cases of shingles in the United States each year, and approximately one in five shingles sufferers go on to develop PHN. The likelihood of developing PHN from shingles increases with age, with approximately 25% of people over 55, 50% of people over 60, and 75% of people over 70 estimated to eventually develop PHN after contracting shingles. Estimates of the number of people suffering from PHN in the United States range from under 200,000 to 500,000. We have been granted an orphan designation for capsaicin for the management of neuropathic pain in patients with postherpetic neuralgia by the FDA’s Office of Orphan Products Development.
To date, we have conducted four Phase 1 studies and one Phase 2 study in NGX-1998 which are summarized below:
| Indication |
Development Activity |
Trial Number |
Number of |
Status | ||||
| Healthy Volunteers | Phase 1 | C201 | 20 | Completed | ||||
| Healthy Volunteers |
Phase 1 | C202 | 30 | Completed | ||||
| Healthy Volunteers |
Phase 1 | C203 | 36 | Completed | ||||
| PHN |
Phase 2 | C204 | 183 | Completed | ||||
| Healthy volunteers |
Phase 1 | C205 | 24 | Completed—evaluation of data in process |
Phase 1 Studies
An investigational new drug application, or IND, was filed for NGX-1998 in June 2008. NGX-1998 (10% or 20% concentration) has been investigated for its safety and potential ability to affect the activity of cutaneous sensory nerve fibers in four Phase 1 clinical studies in healthy volunteers, studies C201, C202, C203 and C205. The activity of the cutaneous sensory nerve fibers in these studies was measured by punch biopsy seven days after treatment for the treated areas.
The first study, C201, investigated the effect of different formulations (all containing 10% capsaicin) and propylene glycol control applied for 15 minutes on epidermal nerve fiber density, or ENFD, thermal detection
11
Table of Contents
thresholds as measured by Quantitative Sensory Testing, or QST, mechanical (sharp pain) sensation and tactile thresholds, and the extent of pain and erythema produced at each application site. One formulation, NGX-1998 provided satisfactory ENFD reduction and acceptable tolerability. This formulation was tested in a second healthy volunteer trial, study C202. This study investigated the effects of three treatment times (5, 15 and 25 minutes) of NGX-1998, compared to a 1-hour application of NGX-4010 and a control (propylene glycol) on ENFD, thermal detection thresholds as measured by QST, and the extent of pain and erythema produced at each application site. This study demonstrated that NGX-1998 treatment for 5, 15 or 25 minutes reduced ENFD similar to Qutenza treatment for 60 minutes. A third study, study C203, was a randomized, single-blind study to investigate the tolerability and effects on EFND of 15-minute treatments of several low concentrations capsaicin topical liquid formulations (ranging from 0.001 to 1% concentration) as well as NGX-1998 (10%) and a control formulation. The goal was to identify a control formulation that did not reduce epidermal nerve fibers but still caused some erythema and pain. The results of the study showed that 15-minute treatments of NGX-1998 (10%) significantly reduced ENFD compared to vehicle control and indicated that the 1% capsaicin control formulation produced expected capsaicin related effects. Finally, we conducted and recently completed a Phase 1 study, study C205, in 24 healthy volunteers to evaluate alternative controls for possible usage in future clinical studies. In this study, we evaluated the effects of two formulations of low dose capsaicin (0% and 0.01%) and two formulations of high dose capsaicin (10% and 20% on epidermal nerve fiber density. The results of this study are currently being evaluated.
C204 Phase 2 Study in PHN
In November 2011, we completed a Phase 2 study in patients with PHN. The study met its protocol-specified objectives which included the primary endpoint of a percentage change from baseline compared to placebo in a patient reported NPRS during weeks 2 through 8 of the study after a single five minute application of either active or placebo. The 12-week, multicenter, randomized, double-blinded, placebo-controlled clinical trial included a total of 183 patients. Patients were randomized into one of three groups: NGX-1998 capsaicin 10% solution, NGX-1998 capsaicin 20% solution or placebo, according to an unequal allocation scheme of 2:2:1. NGX-1998 exhibited a dose response. The study utilized an adaptive design and was conducted in two stages. The first stage, which included a total of 39 patients, was designed to determine whether a topical anesthetic was required for patients to tolerate the treatment. The results of the first stage indicated that patients found the treatment procedure tolerable without topical anesthetic. Although no topical anesthetic was used during the second stage of the study the patients were able to tolerate the treatment procedure. No patients discontinued the study due to adverse events, and the incidence of adverse events and serious adverse events in patients treated with NGX-1998 were similar to the placebo-treated group.
We are currently evaluating potential partnership opportunities with NGX-1998 for the United States as well as other world markets other than the Astellas Licensed Territory. The Astellas Agreement provides Astellas an option to exclusively license NGX-1998 in the Licensed Territory. If Astellas exercises this option, it is obligated to partner with us on the Phase 3 clinical trials and share equally in the cost of the trials that are pertinent to their market.
Manufacturing and NGX-1998 Applicator
We are currently working to establish the manufacturing process and establish the supply chain for NGX-1998 which includes an applicator. The liquid formulation is comprised of capsaicin, the active pharmaceutical ingredient, or API, and a certain penetration enhancers all of which belong to a class of pharmaceutical ingredients that are generally regarded as safe. The API is manufactured for us by Formosa Laboratories, Inc, or Formosa, in Taiwan, and is the same API used in Qutenza. We are currently working to establish manufacturing partners to address the conversion of the API into the liquid formulation at production scale levels as well as provide the filling of the liquid formulation into the applicator. We are also developing a container closure device which will be used as an applicator. The applicator device is currently in prototype development and our goal is complete the design and initial manufacturing of the applicator device by middle of 2012 to enable its use in our Phase 3 program by the end of 2012.
12
Table of Contents
Qutenza
Qutenza is a non-narcotic analgesic formulated in a localized treatment patch containing an 8% concentration of synthetic capsaicin. Capsaicin is released from the patch and, with the aid of penetration enhancers, penetrates into the skin during application without significant absorption of capsaicin into the bloodstream. Accordingly, we believe that users of Qutenza are unlikely to experience the common central nervous system side effects of systemically administered drugs used to treat neuropathic pain such as anti-convulsants, anti-depressants and opioids and the potential for abuse and addiction associated with some of these drugs. FDA labeling in the United States requires Qutenza to be administered by a physician or by a health care professional under the direct supervision of a physician in a non-invasive process that involves pre-treating the painful area with a topical anesthetic, followed by the application of Qutenza. Patches are cut to conform to the area to be treated. After the specified application period (60 minutes for PHN), the patch is removed and residual capsaicin is removed from the skin with a proprietary cleansing gel. Qutenza has been shown to provide a clinically meaningful reduction in peripheral neuropathic pain for up to 12 weeks in certain of our clinical studies. As noted on its label, the most common adverse reactions to Qutenza were application site redness, pain, itching and papules. The majority of these reactions were transient and self- limited. Serious adverse reactions included application site pain and increased blood pressure. Application associated pain can be treated with local cooling either alone or in conjunction with appropriate analgesic medication such as opioids. Blood pressure increases during or shortly after Qutenza treatment were on average less than 10 mm Hg, although some patients had greater increases and these changes lasted for approximately two hours after patch removal.
Qutenza can be used alone or in conjunction with other pain medications and as such may have place as first line or additive therapy.
Qutenza has been approved by the FDA for the management of neuropathic pain associated with PHN and is the first prescription strength product containing capsaicin, Qutenza’s active ingredient. Qutenza has also been approved in the European Union for the treatment of peripheral neuropathic pain in non-diabetic adults, either alone or in combination with other medicinal products for pain.
Commercialization of Qutenza
United States
We launched Qutenza in the United States in April 2010 with a focused approach to build the required infrastructure to support the long-term commercialization of the product. This approach consisted of educating and training healthcare providers, mostly pain specialists, who treat PHN with Qutenza, establishing a national network of Qutenza treatment centers, gaining access for Qutenza on formulary at key hospitals in major metropolitan areas, establishing a distribution network to allow customers to access Qutenza and establishing an efficient reimbursement process.
When we launched Qutenza in the United States, our own sales force was comprised of approximately 40 sales representatives and sales management personnel in three regions. However, as a result of lower than anticipated sales of Qutenza, and in an effort to preserve cash and focus our sales and marketing resources on the markets that we believed had the highest potential for continued sales growth, we reduced our sales force to 25 sales representatives in November 2011. In March 2012, we restructured our operations further, including significantly reducing our sales and marketing efforts on Qutenza to focus our resources on the development of NGX-1998. Our objective is to better align our commercial support costs with near term revenue expectations, while maintaining Qutenza availability for PHN patients and physicians. We are currently evaluating additional alternative strategies for commercializing Qutenza in the United States pursuant to a commercialization partnership and potentially other models. Additionally, our commercial partner in Europe, Astellas, is currently conducting several clinical trials, including two trials in patients with painful diabetic neuropathy. Should those studies return results that support the potential for a label expansion, we may consider further commercial investment in the U.S. either ourselves or through partnership.
13
Table of Contents
Reimbursement
Qutenza and its related treatment procedure are currently reimbursed under Medicare Part B. In January 2011, CMS granted a unique, Level II Health Care Common Procedural Coding System, or J-code, for Qutenza. Reimbursement for the treatment procedure is generally reimbursed using existing evaluation and management codes or miscellaneous procedure codes at amounts which are set by the regional Medicare carriers and commercial payers. Qutenza currently has broad coverage for PHN as demonstrated by the low benefit investigation denials for PHN, which are less than 3%.
Manufacturing
We manufacture Qutenza through the use of contract manufacturers. There are multiple primary raw material components of our active ingredient, synthetic capsaicin, all but one is generally available from more than one supplier. We currently obtain our supplies of synthetic capsaicin from Formosa, who obtains the raw materials from qualified suppliers. While Formosa is our sole supplier currently, other potential suppliers exist and we may consider qualifying a second source of supply in the future.
The Qutenza patch is manufactured exclusively by LTS Lohmann Therapie-Systeme AG, or LTS, in Germany. LTS manufactures the patch using a proprietary process which includes combining the active ingredient with adhesives and penetration enhancers and applying that mixture to a backing to create a dermal delivery system. Our commercial supply and license agreement with LTS establishes standard commercial terms on which product will be supplied, as well as continuing the licenses initially granted in our clinical supply agreement. The term of the commercial supply and license agreement extends until January 2020 and automatically renews for additional two year terms, unless terminated by either party with two years prior written notice. LTS has an alternative manufacturing site in the United States which is not currently an approved site for the manufacture of Qutenza. We may decide in the future to seek approval for that site as an alternative source of supply of Qutenza.
We have engaged Contract Pharmaceuticals Limited Canada, or Contract Pharmaceuticals, as the manufacturer of our cleansing gel, a component of the Qutenza treatment, which is used to cleanse residual capsaicin from the skin after a Qutenza application. While we currently have only one supplier for the cleansing gel, we believe there are a number of potential suppliers which we may evaluate as alternative sources of supply.
Distribution
We distribute Qutenza through a network of specialty distributors and specialty pharmacies. Healthcare professionals access Qutenza primarily through one of two distribution models. In one model, the physician practice, clinic or hospital, will take ownership of Qutenza, after having acquired it through a specialty distributor. Under this model, known as “buy and bill”, the physician is able to bill payers for Qutenza, as well as for their professional services related to its administration. In the second model, known as “assignment of benefit”, the physician would prescribe the product for a specific patient and a specialty pharmacy would dispense Qutenza for that patient, bill the patient’s health plan for Qutenza, and ship it to the treating physician for administration. The treating physician would administer Qutenza and bill the patient’s health plan for his or her professional services related to its administration.
Our distribution strategy does not include making Qutenza available in retail pharmacies, as is usually the case for self-administered products. As a result, we expect that product shipments will generally be limited and that our specialty distributors and specialty pharmacies will stock relatively small quantities of Qutenza. In December 2009, we signed a three-year agreement with Integrated Commercialization Solutions, Inc., or ICS, to outsource our finished goods warehousing, distribution, and customer service activities related to Qutenza. Our finished goods are held by ICS in their warehouse and ICS ships from that location to our specialty distributors and specialty pharmacies based on orders received.
14
Table of Contents
Territories outside of the United States
Astellas License Agreement and Supply Agreement
In June 2009, we entered into the Astellas Agreement, under which Astellas was granted an exclusive license to promote, distribute and market Qutenza in the Licensed Territory. At December 31, 2011, Qutenza was commercially available in 21 European countries in the Astellas Territory. The Astellas Agreement provides for Astellas to be responsible for seeking regulatory approvals in the remainder of the Licensed Territory and at December 31, 2011, Astellas had submitted for such approvals in additional countries.
The Astellas Agreement provided for an upfront payment for past development and the commercialization rights granted to Astellas, of 30.0 million Euro, or $41.8 million. In addition, the Astellas Agreement provided for an upfront payment of 5.0 million Euro, or $7.0 million, for future development expenses and an option to license NGX-1998. Other elements of the Astellas Agreement include future milestone payments of up to 65.0 million Euro if certain predefined sales thresholds of the products licensed under the agreement are met and royalty payments, as a percentage of net sales of products under the agreement, with royalty rates starting in the high teens and escalating into the mid twenties as revenues increase. These royalty payments are subject to a reduction upon entry of generic competition in the Licensed Territory. The Astellas Agreement requires us to participate on a joint steering committee with Astellas to oversee the development and commercialization activities related to Qutenza and NGX-1998 during the term of the agreement, but not to exceed ten years. On the seventh anniversary of the Astellas Agreement, we have the unilateral right to opt-out of participation on the joint steering committee. Additionally, the agreement provides for Astellas’ commitment to perform certain studies to support the marketing and promotion of Qutenza in the Licensed Territory, and Astellas is responsible for conducting certain post European Commission approval marketing commitments. To address these commitments, at December 31, 2011, Astellas had begun enrolling patients in a long-term safety study in on-label indications and had begun enrolling patients in a safety and efficacy study and a long-term safety study in patients with painful diabetic neuropathy. Astellas also has commenced an efficacy study in PDN patients in the first quarter of 2012.
The terms of the Astellas Agreement also provide for the development and potential commercial marketing and distribution of NGX-1998. Astellas’ development work with, and the right to distribute and market NGX-1998 in the Licensed Territory, are tied to affirmative opt-in provisions of the Astellas Agreement. Such provisions allow for Astellas to carry out its election by making (in addition to the initial upfront payment of 5.0 million Euro received by us as described above) two additional NGX-1998 option payments totaling 5.0 million Euro, each made at the election of Astellas after receipt from us of certain clinical development deliverables relating to the development of NGX-1998. If Astellas exercises such option by making all such payments, the Astellas Agreement provides that certain post-election development expenses for NGX-1998 will be shared equally by us and Astellas, and Astellas shall have the exclusive right to commercialize NGX-1998 in the Licensed Territory. If Astellas does not exercise its option to commercialize NGX-1998, the Astellas Agreement terms preclude us from marketing NGX-1998 in the Licensed Territory until the contract expires or terminates. The Astellas Agreement will remain effective on a country-by-country and product-by-product basis until the later of ten years after the first commercial sale, expiration or abandonment of the last valid patent claim or expiration of all applicable periods of regulatory data exclusivity. The Astellas Agreement may be terminated in the event of material breach or insolvency by either party, or Astellas may terminate the agreement for any reason without penalty, consequence or compensation on a country-by-country and product-by-product basis upon written notice to us.
Pursuant to a Supply Agreement that we entered into with Astellas in connection with our entry into the Astellas Agreement, we have agreed to provide Astellas with a sufficient supply of components of Qutenza to support its commercialization efforts, subject to certain limitations, until it can establish a direct supply relationship with product manufacturers. The Supply Agreement requires us to provide Qutenza to Astellas at a contractually agreed upon cost for materials and our related labor and overhead. We anticipate that Astellas may establish direct supply relationships with one or more of the three principal manufacturers of components of
15
Table of Contents
Qutenza and that the Supply Agreement will be terminated with respect to any manufacturer for which a direct supply relationship has been established. The three principal manufacturers identified in the Supply Agreement are LTS, Formosa, and Contract Pharmaceuticals.
In April 2010, we borrowed $40.0 million from Cowen Royalty. Such debt is secured by and will be repaid from future royalty, milestone, option and other payments (excluding development funding or supply purchase payments) we receive pursuant to the Astellas Agreement, referred to as the Revenue Interest payments. Under the arrangement, we are obligated to pay Cowen Royalty all such Revenue Interest payments until we have paid them $90.0 million. Additionally, we are obligated to pay Cowen Royalty five percent of amounts we receive from Astellas for royalty, milestone, option and other payments between $90.0 million and $106.0 million. These amounts due to Cowen Royalty may be reduced in accordance with certain prepayment provisions of the agreement. If Cowen Royalty has not received Revenue Interest payments totaling at least $40 million by the maturity date, we will be obligated to pay to Cowen Royalty the difference between such amount and the Revenue Interest payments paid to Cowen Royalty under the Financing Agreement up to such date. This represents our minimum obligation to Cowen.
Rest of World
Qutenza is not currently approved in countries other than the United States and the European Economic Area and certain additional countries in the Licensed Territory. We retain rights to all markets other than the Licensed Territory and we are currently working to identify potential commercialization partners in other world markets.
Competition
Qutenza competes against, and may be used in combination with, well-established products currently used both on and off-label in our target indications. The most directly competitive currently marketed products in the United States are Lidoderm, an FDA-approved 5% lidocaine topical patch for the treatment of PHN marketed by Endo Pharmaceuticals, and Lyrica, an oral anti-convulsant, marketed by Pfizer for use in the treatment of PHN. Additionally, in 2011, Depomed Inc. began commercializing Gralise, a formulation of gabapentin that is a once daily tablet for the treatment of PHN. In addition to these branded drugs, the FDA has approved gabapentin (Neurontin) for use in the treatment of PHN. Pfizer has also received FDA approval of Lyrica for the treatment of PDN, fibromyalgia and epilepsy indications. The FDA has approved Cymbalta from Eli Lilly & Co, or Eli Lilly, for use in the treatment of PDN, general anxiety disorder and depression; however we believe Cymbalta is used in PHN patients.
In addition to existing approved therapies for PHN, there are many other companies working to develop new drugs and other therapies to treat pain in general and neuropathic pain in particular, including Eli Lilly, Pfizer, UCB Pharma SA, GlaxSmithKline plc, Merz, GW Pharmaceuticals, Novartis AG, Novo Nordisk A/S, Dainippon Pharmaceutical Co Ltd., AVANIR Pharmaceuticals, Xenoport, EpiCept, Newron Pharmaceuticals SpA, WEX Pharmaceuticals, Arcion Therapeutics Inc., Endo Pharmaceuticals, AstraZeneca plc, Bristol-Myers Squibb Co., Bio3 Research Srl, Relevare Pharmaceuticals Ltd., and Ortho-McNeil-Janssen Pharmaceuticals. Many of the compounds in development by such companies are already marketed for other indications, such as anti-depressants, anti-convulsants or opioids. In addition, physicians employ other interventional procedures, such as nerve stimulation or nerve blocks, to treat patients with difficult to treat neuropathic pain conditions. Furthermore, new developments, including the development of other drug technologies and methods of preventing the incidence of disease, such as vaccines including Zostavax, occur in the biopharmaceutical industry at a rapid pace. Any of these developments may render our products or product candidates obsolete or noncompetitive.
We expect to compete on, among other things, the safety, efficacy and duration of action of Qutenza.
16
Table of Contents
Our ability to compete with Qutenza will be limited by our recent commercial restructuring and decision to no longer make significant investments in marketing and other activities in support of Qutenza in the market. Further, our ability to compete may be adversely affected because insurers and other third-party payers in some cases seek to encourage the use of generic products making branded products less attractive, from a cost perspective, to buyers.
Qutenza in other Neuropathic Pain Indications
In addition to PHN, we have studied Qutenza in two other neuropathic pain indications—HIV-PN and PDN as set forth in the following table.
| Indication |
Development Activity |
Trial |
Number of |
Status | ||||
| HIV-PN | Phase 3 | C119 | 494 | Completed; primary endpoint not met | ||||
| Phase 3 |
C107 | 307 | Completed; primary endpoint met (p = 0.0026) | |||||
| Open-label safety study |
C118 | 106* | Completed | |||||
| Phase 2 |
C109 | 12 | Completed | |||||
| PDN, PHN, HIV-PN |
Open-label tolerability study |
C111 | 117** | Completed | ||||
| * | C118 evaluated the safety of applications of Qutenza over a 12 month period. Of the 106 patients enrolled, 52 patients had HIV-PN and 54 patients had PHN. |
| ** | C111 evaluated the effect of topical anesthetic alternatives on tolerability of Qutenza and included 91 PDN patients, 25 PHN patients and 1 HIV-PN patient. |
In addition to the above studies, Astellas is currently conducting three studies in Qutenza. One study is a long-term safety study in on-label indications, that is, neuropathic pain in non-diabetic adults. The other two studies are in the patients with PDN. One study is a long-term, one year safety study and the other is an efficacy study. All of these studies were initiated near the end of 2011 or early 2012.
HIV-PN sNDA
In September 2011, we submitted a supplemental NDA to the FDA seeking an approval for Qutenza in the management of pain associated with HIV-PN. Our application was granted an expedited review with a PDUFA date of March 7, 2012. On February 9, 2012, the Analgesic and Anesthic Drug Product Advisory Committee of the FDA convened an advisory panel to evaluate the HIV-PN data to determine if the data supported a recommendation of approval. The panel voted unanimously against approval of Qutenza on the grounds that the data did not support efficacy of Qutenza in this patient population. The complete response letter indicated that in order to gain approval for the use of Qutenza for the proposed indication, the Company would need to submit additional clinical data from at least one adequate and well-controlled trial to support the proposed indication. We have advised the FDA that we do not intend to conduct further clinical studies with Qutenza in this patient population. A description of the HIV-PN studies that were included in this submission is set forth below.
General Objectives. The primary objective, or endpoint, of each of our Phase 3 clinical trials has been to assess the percent change in “average pain” from baseline to weeks 2-8, in the case of PHN, or to weeks 2-12, in the case of HIV-PN. If the primary endpoint is not met, the trial is generally considered to have been unsuccessful. Determining if the primary endpoint has been achieved is based on statistical analysis that has been defined in the protocol, the measurement of which is known as the “p value.” A successful trial is generally based on meeting a p value of less than 0.05, which means that there is a greater than 95% likelihood that the drug was responsible for the difference in effect observed between the treated patients and those receiving a placebo (or in our case, a control patch). The primary method of assessing baseline pain and pain over the course of the study is a Numeric Pain Rating Scale, or NPRS. Eligible subjects had moderate to severe neuropathic pain with a baseline
17
Table of Contents
average NPRS score, as measured over a period of one to two weeks prior to treatment, typically of 3 to 9 (with 0 = no pain and 10 = worst possible pain). Secondary efficacy measures included methods of assessing pain other than with NPRS, such as the Patient and Clinical Global Impression of Change, Gracely Pain Scale, Short-Form McGill Pain Questionnaire, and Brief Pain Inventory, as well as the proportion of “responders,” which are defined as patients who experience a certain minimal threshold of pain relief such as, the percentage of patients who achieve at least a 30% reduction in pain as measured by the NPRS. Each of our studies also assessed safety and tolerability.
General Safety Findings. Our clinical trials have consistently demonstrated that Qutenza is well tolerated. In our Phase 3 trials, over 98% of subjects completed the prescribed duration of patch application, both during the double-blind phase and during the open-label phase of our trials. Treatment-related adverse events have primarily consisted of application-site issues, such as redness, pain, burning, itching, dryness or swelling. Most of these events have been mild to moderate, however, severe application site events have been observed. The application site reactions have been generally short term and managed with the application of cool compresses, ice or the use of short-acting opioids to relieve the treatment-related discomfort. Transient changes in blood pressure have been observed during the treatment and appear to follow treatment-related changes in pain. We have not seen evidence of increased side effects with repeated treatment. In our clinical trials there have been three serious adverse events (totaling less than 1%) related to Qutenza, two related to pain and one case of hypertension.
Clinical Trial Results
Painful HIV-Associated Peripheral Neuropathy (HIV-PN)
We have conducted two controlled studies, an open-label extension study and an open-label long-term safety study evaluating the effect of Qutenza in subjects with HIV-AN. Overall, 632 subjects have received Qutenza in these studies with over 1,000 Qutenza treatments being administered.
Clinical Trial Results
C119 Phase 3 Clinical Trial. In February 2008, we completed a randomized, double-blind, controlled trial performed at 77 sites in the United States, Canada, Australia and the United Kingdom. Inclusion criteria included pain due to HIV-PN or neurotoxic antiretroviral drug exposure for at least two months and average NPRS scores during the screening period of three to nine, inclusive. The primary objective of this study was to assess efficacy, safety, and tolerability of two doses of Qutenza, 30- and 60-minute applications, over the 12-week study period. The primary efficacy assessment was the change in “average pain” from baseline in weeks 2-12. We enrolled 494 subjects and randomly assigned them to receive either a 30-minute or 60-minute application of Qutenza or control patches according to a 2:1 allocation scheme for each dose.
Study C119 did not meet its primary endpoint. Overall there was a 29.5% reduction in pain in the Qutenza treatment group, a result that was consistent with what we have observed in other Qutenza studies. The control group reported a 24.5% reduction in pain from baseline, a control group response greater than we observed in our prior HIV-PN Phase 3 study. The p-value for this comparison was p=0.1. For the individual dose groups, the 30-minute Qutenza group achieved a 26.2% reduction in pain from baseline compared to the 30-minute control group, which reported a 19.1% reduction in pain. The p-value for this comparison was p=0.1. The 60-minute dose group reported a 32.8% reduction in pain from baseline, however, the 60-minute control group reported a 30.0% reduction in pain (p=0.5).
C107 Phase 3 Clinical Trial. In 2005, we completed a randomized, double-blind, controlled, dose finding study of Qutenza for the treatment of HIV-PN performed at 32 sites in the United States. The primary objective of this study was to assess efficacy, safety, and tolerability of Qutenza. Efficacy was measured in terms of change in “average pain” from baseline to weeks 2-12. The study consisted of a 12-week randomized, double-blind, controlled phase and a 40-week open-label extension. Three different dose levels (30-, 60- and 90-minute applications) were evaluated together, and then each dose level was evaluated individually. The study also provided information about the efficacy, safety, and tolerability of repeated treatment with Qutenza over one
18
Table of Contents
year. A total of 307 subjects were enrolled at 32 clinical sites in the United States, divided approximately equally among the 30-, 60- and 90-minute dose levels, with three patients treated with Qutenza for every one subject treated with the control.
The results of the study demonstrated that in the aggregate, across all active treatment groups, Qutenza significantly reduced pain in subjects with HIV-PN compared to the control group. Subjects treated with Qutenza demonstrated a mean reduction in pain score from baseline of 22.8% that was statistically greater than the 10.7% decrease in the control group (p = 0.0026).
Among the individual dose groups, the 90-minute Qutenza group demonstrated a mean reduction in pain of 24.7% that was significantly greater than the decrease of 10.7% reported by the control group (p = 0.005). The 60-minute Qutenza group also demonstrated a greater reduction in pain scores of 15.8%, but the difference from control was not statistically significant. The 30-minute Qutenza group had a mean percent decrease from baseline of 27.7%, which was similar to the pain reduction reported for the 90-minute Qutenza group (p=0.0007). The effect of treatment was maintained for up to 12 weeks, with the Qutenza group demonstrating significantly greater pain reduction compared to the control group during the second week and at each subsequent week through week 12. Among several secondary measures of pain relief, all three doses showed meaningful improvement compared to control. This study demonstrated that treatment with Qutenza was generally well tolerated. A single Qutenza treatment provided a stable reduction in pain over a 12-week period. Although the pre-specified statistical testing of the primary analysis stopped after the 60-minute dose was found not to have reached statistical significance, the data from all the active dosing groups, including evaluation of secondary endpoints, support the conclusion that all the Qutenza doses tested (30-, 60-, and 90-minute) provided pain relief in subjects with HIV-PN. Repeated treatments for up to one year in an open-label extension study appeared to have been equally efficacious, generally well tolerated and without cumulative toxicity.
C118 Phase 2 Clinical Trial—Safety. This study was a multicenter, open-label trial of Qutenza for the treatment of peripheral neuropathic pain in patients with HIV-PN or PHN. The primary objective of this study was to assess the safety of up to four repeated applications of Qutenza for the treatment of HIV-PN and PHN. The study enrolled a total of 106 patients (52 HIV-PN and 54 PHN). Qutenza treatments were generally well tolerated with greater than 98% of subjects completing the prescribed duration of treatment. We believe the study demonstrated that Qutenza was not associated with increasing toxicity following multiple treatment cycles. Further, clinical assessments suggested no impairment of protective nerve function (such as the ability to feel touch or heat) over the one year study period.
Prior Clinical Experience. In 2003, we completed C109, an open-label pilot study of prescription strength capsaicin patches in the treatment of HIV-PN. The primary objective of this study was to obtain preliminary information on the efficacy, safety, and tolerability of Qutenza in subjects with HIV-PN. This Phase 2, multicenter, open-label trial enrolled 12 subjects at three clinical sites in the United States. Subjects received a single 60-minute treatment with Qutenza and were followed for 12 weeks. This study demonstrated that treatment with Qutenza was feasible and was generally well-tolerated. The study also provided preliminary evidence of efficacy indicating that Qutenza could reduce pain associated with HIV-PN for 12 weeks after treatment.
Painful Diabetic Neuropathy
Clinical Trial Results
Phase 2a Clinical Trial Description. In 2004, we completed C111, a randomized, open-label multicenter evaluation of the tolerability of treatment with Qutenza in conjunction with pre-patch topical application of one of three lidocaine 4%-based local anesthetic products. The study enrolled 25 subjects with PHN, 91 subjects with PDN and 1 subject with HIV-PN. Tolerability of the procedure was similar among all topical anesthetics tested. Preliminary efficacy data were obtained for the PHN group and the PDN group. PHN subjects experienced a 27.7% reduction in pain over weeks 2 through 12; pain was reduced by 31.4% in PDN subjects. This data suggests that PDN may respond similarly to treatment with Qutenza.
19
Table of Contents
As noted above, Astellas is currently conducting two studies in the PDN patient population in response to follow up measures agreed upon with the EMA at the time Qutenza was approved in the EU. These studies include a long-term safety study and an efficacy study. Depending on the outcome of these studies, we will evaluate the potential to utilize these studies for possible label expansion of Qutenza in the United States to include the PDN patient population.
Other Product Development Programs
Our current product development programs are focused on candidates in the field of pain with a primary focus on peripheral neuropathic pain. We retain worldwide rights to these product candidates with the exception of NGX-1998 for which we have granted Astellas an option to license in the Licensed Territory. Our portfolio consists of the following product candidates:
| Product Candidate |
Indication |
Phase of Development | ||
| Acetaminophen Prodrugs |
Traumatic pain, post-surgical pain and fever | Preclinical development—seeking out-license partner |
Acetaminophen Prodrugs—Pre-clinical Program
Acetaminophen, first approved by the FDA for marketing in the United States in 1955, is the most widely used drug for pain relief and the reduction of fever in the United States and is currently available in numerous pharmaceutical products. Acetaminophen is often perceived to be safer than other non-steroidal anti-inflammatory drugs, or NSAIDs. Acetaminophen in intravenous formulations has been available in the European Union since 2002, marketed by Bristol-Myers Squib under the trade name Perfalgan®. The same formulation was launched in January 2011 in the US by Cadence Pharmaceuticals under the trade name OFIRMEV®. These parenteral formulations require a 15-minute, 100 mL infusion, as these formulations provide only 10 mg of acetaminophen per mL due to acetaminophen’s modest water solubility. Despite this inconvenient dosing requirement, Perfalgan has become the market-leading injectable analgesic in the European Union.
Two major limitations exist regarding full utilization of acetaminophen as an injectable pain medicine. First, is the requirement of an intravenous drip bag and a 15-minute infusion. Second, is acetaminophen-induced liver toxicity, which is the most common cause of acute liver failure in the United States. In 2009, the FDA convened an Advisory Committee Meeting to address the public health problem of liver injury related to the use of acetaminophen, with the result that liver hepatotoxicity warnings on acetaminophen-containing products were expanded and restrictions were placed on the amount of acetaminophen in prescription pain medicines.
We believe that we have the potential to overcome these two limitations to expanded utilization of parenteral acetaminophen through the discovery of novel prodrugs To address the modest water solubility of acetaminophen, we have designed prodrugs with greatly improved water solubility (up to 30 times more water soluble). To address the potential for liver damage, we have developed prodrugs which couple acetaminophen to molecules known to prevent acetaminophen induced-liver toxicity. We have evaluated several of these prodrugs in preclinical studies, including in animal models. One such study in mice indicated that certain dose levels of one prodrug produced less liver-toxicity than equivalent doses of acetaminophen, with parallel pharmacokinetic data showing equivalent acetaminophen blood levels between prodrug and acetaminophen- treated mice.
These product candidates are in the pre-clinical stage of development and may or may not be the subject of further development in 2012 or beyond.
Patents and Proprietary Rights
Our success will depend in part on our ability to protect NGX-1998, Qutenza and other future products and product candidates by obtaining and maintaining a strong proprietary position both in the United States and in other countries. To develop and maintain our proprietary position, we will rely on patent protection, regulatory protection, trade secrets, know-how, continuing technological innovations and licensing opportunities.
20
Table of Contents
In 2011, we were granted patent protection under a patent entitled “Methods and Compositions for Administration of TRPV1 Agonists.” The issued claims include method of use, formulation and system claims, which we believe will provide protection for NGX-1998, should it be approved in the United States. The patent expiration date, including Patent Term Adjustment, is 2027. In early 2012, we received a notice of allowance by the Japanese Patent Office for a patent application covering a set of claims similar to the NGX-1998 patent issued in the United States. A similar patent application is currently under review in Europe.
Our ability to protect Qutenza depends, in part, on a device patent granted in the United States, and corresponding patents granted in certain European countries, Canada and Hong Kong. The device patent covers the use of a prescription-strength capsaicin dermal delivery system for the treatment of neuropathic pain. The United States device patent expires in November 2016 (although we have applied for patent term extension due to certain regulatory delay in the application process, which, if granted would extend the expiration date), with the corresponding patents outside the United States expiring in 2018. We exclusively license these patents on a worldwide basis from the University of California. We do not currently own, and do not have rights under this license agreement, to any issued patents that cover Qutenza other than in the United States, certain European countries, Canada and Hong Kong.
In addition, we licensed a method patent granted in the United States from the University of California concerning the use of prescription strength capsaicin delivery for the treatment of neuropathic pain. This patent expires in November 2016. Although our rights under the license agreement are worldwide, there are no corresponding granted patents or patent applications relating to this patent outside the United States. Two of the three inventors named in the method patent did not assign their patent rights to the University of California. These non-assigning inventors have made various claims related to inventorship and other matters. In late 2010, we entered into negotiations with these non-assigning inventors to settle any and all claims and to have them assign their rights related to the method patent and any other related patent rights that may exist at the time we enter into a final binding settlement agreement. We anticipate that any settlement will include us making cash payments and us issuing stock or stock options to them. We estimate the current cash and estimated fair value of the stock or stock options to be issued to approximate $0.6 million. We further anticipate that up to 50% of the cash settlement amount may be recovered over time, through a reduction in amounts due to the University of California under our license agreement with them. Our estimate of the value of the potential settlement has been expensed in 2010 with adjustments to reflect changes in the estimated value of the settlement recorded in 2011. Under the terms of our license agreement with the University of California, we are required to pay royalties on net sales of the licensed product up to a maximum of $1.0 million per annum as well as a percentage of upfront and milestone payments resulting from the sublicense of our rights under the agreement. As a result of the Astellas Agreement, in February 2010, we paid a sublicense fee totaling $1.2 million to the University of California. In addition, we were also required to make three annual cash payments in an aggregate amount of $12,000. The last of these payments was made in February 2010. Such agreement terminates on a country by country basis as to products covered by it on the date that such products are not covered by a claim of either an unexpired patent or a patent application that has been outstanding less than 10 years from its filing date.
We currently license the rights from LTS to three pending U.S. patent applications, patents granted in certain European countries and pending patent applications in certain European countries, Canada and more than ten other foreign countries, each filed and prosecuted by LTS. These patents and patent applications contain claims covering the composition and manufacture of microreservoir patches, including the type of patch comprised by Qutenza. We license the rights to these patents and patent applications on a worldwide basis under a January 2007 exclusive commercial supply and license agreement with LTS, which is subject to certain purchase and other obligations. The first of the issued European patents licensed under the commercial supply and license agreement expires in June 2020, with the last expiration date among such patents being September 2025. During 2009, as a result of achieving marketing approval for Qutenza, we made a one-time milestone payment to LTS of approximately $0.1 million. The term of the commercial supply and license agreement extends until January 2020 and automatically renews for additional two year terms, unless terminated by either party with two years prior written notice.
21
Table of Contents
We have also filed several patent applications in the United States, Europe, and certain other countries relating to kits, methods and formulations to remove residual capsaicin left on the skin after a topical application of capsaicin, as well as a number of patent applications that relate to differing formulations and delivery models of capsaicin at varying concentrations. We have also filed patent applications related to our acetaminophen prodrugs that contain both composition and method claims. The patent positions of pharmaceutical companies like us are generally uncertain and involve complex legal, scientific and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued. Consequently, we do not know whether any of the products or product candidates we acquire or license will result in the issuance of patents or, if any patents are issued, whether they will provide significant proprietary protection or will be circumvented or challenged and found to be unenforceable or invalid. Other parties may own patent rights that might be infringed by our products or other activities. In limited instances, patent applications in the United States and certain other jurisdictions are maintained in secrecy until patents issue, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications. Moreover, we may have to participate in interference proceedings declared by the U.S. Patent and Trademark Office to determine priority of invention or in opposition proceedings in a foreign patent office, any of which could result in substantial cost to us, even if the eventual outcome is favorable to us. There can be no assurance that a court of competent jurisdiction would hold the patents, if issued, valid. An adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease using such technology. To the extent prudent, we intend to bring litigation against third parties that we believe are infringing our patents. We also rely on trade secret protection for our confidential and proprietary information. No assurance can be given that others will not independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technology or that we can meaningfully protect our trade secrets. However, we believe that the substantial costs and resources required to develop technological innovations will help us protect our products. Our competitors or other intellectual property holders may assert that our products or trademarks and the methods we employ are covered by their intellectual property rights. For example, we have received a letter indicating a possible trademark conflict for Qutenza ® from a company claiming that our trademark was similar to theirs. While we believe that these assertions do not have merit and that we have defenses to them, there can be no assurance that we will be successful in defending our trademark. We intend to continue to use the Qutenza trademark and to vigorously defend our use of such trademark against such assertions.
It is our policy to require our employees, consultants, contractors, or scientific and other advisors, to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. These agreements provide that all inventions related to our business that are conceived by the individual during the course of our relationship, shall be our exclusive property. There can be no assurance, however, that these agreements will provide meaningful protection or adequate remedies for our trade secrets in the event of unauthorized use or disclosure of such information.
Government Regulation
United States
Regulation by governmental authorities in the United States and other countries is a significant factor in the development, manufacture and marketing of pharmaceuticals. Our product and product candidates require regulatory approval by governmental agencies prior to commercialization. Specifically, Qutenza is subject to significant ongoing review and evaluation by regulatory authorities which could result in discovery of previously unknown problems, side effects or other factors that may affect the marketing approval or labeling for the product, including causing a withdrawal of such approval or may require significant additional investment by the company to maintain the marketing approval.
22
Table of Contents
Our product candidates are subject to rigorous preclinical testing and clinical trials and other premarketing approval requirements of the FDA and regulatory authorities in other countries. Various federal, state and foreign statutes and regulations govern or affect the manufacturing, safety, labeling, storage, distribution, record-keeping and marketing of pharmaceutical products. The lengthy process of seeking required approvals and the continuing need for compliance with applicable statutes and regulations require the expenditure of substantial resources. When and if regulatory approval is obtained for any of our product candidates, the approval may be limited in scope, which may significantly limit the indicated uses for which our product candidates may be marketed, promoted and advertised. Additionally, approval may be conditioned upon our agreement to conduct further studies, which could either delay our planned product launch and/or significantly increase our costs in order to comply with these commitments. Following is a discussion of the regulatory environment that is typically applicable to pharmaceutical product candidates.
Preclinical Studies
Before testing any compounds with potential therapeutic value in human subjects in the United States, stringent governmental requirements for preclinical data must be satisfied. Preclinical testing includes both in vitro and in vivo laboratory evaluation and characterization of the safety and efficacy of a drug and its formulation. Preclinical testing results obtained from these studies, including tests in several animal species, are submitted to the FDA as part of an IND, and are reviewed by the FDA prior to the commencement of human clinical trials. These preclinical data must provide an adequate basis for evaluating both the safety and the scientific rationale for the initial trials in human volunteers.
Clinical Trials
If a company wants to conduct clinical trials in the United States to test a new drug in humans, an IND must be prepared and submitted with the FDA. The IND becomes effective, if not rejected or put on clinical hold by the FDA, within 30 days of filing the application. In addition, an Institutional Review Board or ethics committee must review and approve the trial protocol and monitor the trial on an ongoing basis. The FDA may, at any time during the 30-day review period or at any time thereafter, impose a clinical hold on proposed or ongoing clinical trials. If the FDA imposes a clinical hold, clinical trials cannot commence or recommence without FDA authorization and then only under terms authorized by the FDA. The IND application process can result in substantial delay and expense.
Clinical Trial Phases
Clinical trials typically are conducted in three sequential phases, Phases 1, 2 and 3, with Phase 4 trials potentially conducted after marketing approval. These phases may be compressed, may overlap or may be omitted in some circumstances.
| • | Phase 1 clinical trials. After an IND becomes effective, Phase 1 human clinical trials can begin. These trials typically evaluate a drug’s safety profile and the range of safe dosages that can be administered to healthy volunteers or patients, including the maximum tolerated dose that can be given to a trial subject. Phase 1 trials also examine how a drug is absorbed, distributed, metabolized and excreted by the body, and duration of its action. |
| • | Phase 2 clinical trials. Phase 2 clinical trials are generally designed to establish the optimal dose, to evaluate the potential effectiveness of the drug in patients who have the target disease or condition and to further ascertain the safety of the drug at the dosage and duration given in a larger patient population. |
| • | Phase 3 clinical trials. In Phase 3 clinical trials, the drug is usually tested in a controlled, randomized trial comparing the investigational new drug to a control (which may be an approved form of therapy) in an expanded and well-defined patient population and at multiple clinical sites. The goal of these |
23
Table of Contents
| trials is to obtain definitive statistical and substantial evidence of safety and effectiveness of the investigational new drug regime as compared to control in defined patient populations with a given disease and stage of illness. |
Additionally, the Food and Drug Administration Amendments Act of 2007, or FDAAA, requires that all controlled clinical trials conducted for our drug candidates and the results for those trials, be included in a clinical trials registry database that is available and accessible to the public through the internet. If we fail to properly participate in the clinical trial database registry we would be subject to significant civil monetary penalties.
Manufacturing Process Development
In order to gain marketing approval, a product candidate’s manufacturing process must be evaluated through a lengthy and detailed review process to ensure that it can be consistently manufactured to meet predetermined specifications. A robust manufacturing process must be developed and validated for both the active ingredient and the formulated product candidate which ensures that the product can be reproducibly manufactured at the intended commercial scale. Appropriate specifications must be developed and approved to ensure that the quality of the product can be assured. Analytical methods used for quality control testing must be developed and validated to ensure each lot of product meets the approved specifications. Stability testing of active ingredient and drug product must be performed to provide evidence that the product remains stable over time and that a shelf life for the product can be established. The FDA reviews the adequacy of the manufacturing process, specifications, quality control testing and stability during the New Drug Application, or NDA, review process. In addition, an inspection of the manufacturing site is performed to ensure the adequacy of the manufacturing facility to meet both the technical manufacturing requirements and compliance to current good manufacturing practices, or cGMP, regulations.
New Drug Application
After completion of clinical trials, if there is substantial evidence that the drug is both safe and effective, an NDA is prepared and submitted for the FDA to review. The NDA must contain all of the essential information on the drug gathered to that date, including data from preclinical studies and clinical trials, and the content and format of an NDA must conform with all FDA regulations and guidelines. In addition, the FDA generally requires successful completion of at least two adequate and well-controlled Phase 3 clinical trials to gain marketing approval for an indication. Accordingly, the preparation and submission of an NDA is an expensive and time consuming undertaking.
The FDA reviews all NDAs submitted before it accepts them for filing and may request additional information from the sponsor rather than accepting an NDA for filing. In such an event, the NDA must be submitted with the additional information and, again, is subject to review before filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. By law, the FDA has 180 days in which to review the NDA and respond to the applicant. Under the goals and policies agreed to by the FDA under the Prescription Drug User Fee Act IV, or PDUFA, the FDA has committed to reviewing an application within ten months from the filing date for a standard NDA and six months from the filing date for a priority NDA. The FDA may meet its PDUFA requirements by completing its initial review within the specified time-frames 90% of the time. Additionally, the FDA does not always meet the PDUFA goal dates for standard or priority NDAs. The review process is often significantly extended as a result of FDA requests for additional information and clarification. The FDA may refer the application to an appropriate advisory committee, typically a panel of clinicians with expertise in the disease to be treated by the product candidate, for review, evaluation and a recommendation as to whether the application should be approved and the scope of any approval. The FDA is not bound by the recommendation, but gives great weight to it. Upon completion of the FDA’s review of the NDA, it issues either an approval letter or a complete response letter. A complete response letter may describe any activities which may be required to gain approval or may indicate that a product candidate cannot be approved.
24
Table of Contents
The Hatch-Waxman Act
Under the Drug Price Competition and Patent Term Restoration Act of 1984, known as the Hatch-Waxman Act, newly approved drugs may benefit from a statutory period of non-patent data exclusivity in the United States. The Hatch-Waxman Act provides 5 years of data exclusivity to the first applicant to gain approval of an NDA under Section 505(b) of the Food, Drug and Cosmetic Act for a new chemical entity. A drug qualifies as a new chemical entity if the FDA has not previously approved any other drug containing the same active ingredient. Hatch-Waxman provides data exclusivity by prohibiting abbreviated new drug applications, or ANDAs, and 505(b)(2) applications, which are marketing applications where the applicant does not own or have a legal right of reference to all the data required for approval, to be submitted by another company for another version of such drug during the exclusivity period. Protection under Hatch-Waxman will not prevent the filing or approval of a full NDA under Section 505(b)(1) for the same active ingredient, although the applicant would be required to conduct its own adequate and well-controlled clinical trials to demonstrate safety and effectiveness. Qutenza was granted new chemical entity status by the FDA and has therefore been granted five year data exclusivity under the Hatch-Waxman Act.
The Hatch-Waxman Act also provides three years of data exclusivity for the approval of supplemental NDAs for new indications, dosages or strengths of an existing drug if new clinical investigations are essential to the approval. This three-year exclusivity covers only the changes associated with the supplemental NDA and does not prohibit the FDA from approving ANDAs for drugs containing the original active ingredient.
The Hatch-Waxman Act also permits a patent extension term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent extension cannot extend the remaining term of a patent beyond a total of 14 years. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA, plus the time between the filing date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and it must be applied for prior to expiration of the patent. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for patent term extension. We have submitted an application for patent term extension for the device patent licensed from the University of California, and we believe that we may be eligible for an approximately four and a half year extension to the term for this patent.
Orphan Drug Designation
The FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition that affects fewer than 200,000 individuals in the United States. Orphan drug designation must be requested before submitting an NDA. If the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. If a product that has orphan drug designation subsequently receives FDA approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, which means the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for up to seven years after receiving FDA approval.
With respect to Qutenza, we were granted orphan status for the use of capsaicin for the management of neuropathic pain in patients with PHN and HIV-PN. When appropriate, we may seek orphan status for additional indications and products. We cannot predict the ultimate impact, if any, of orphan status on the timing or likelihood of FDA approval for use of Qutenza on additional, orphan indications or on any of our potential future products.
25
Table of Contents
Other Regulatory Requirements
Any products we manufacture or distribute under FDA approvals are subject to pervasive and continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the products. Drug manufacturers and their subcontractors are required to register with the FDA and, where appropriate, state agencies, and are subject to periodic unannounced inspections by the FDA and state agencies for compliance with cGMP regulations which impose procedural and documentation requirements upon us and each third party manufacturer we utilize.
The FDA closely regulates the marketing and promotion of drugs. The FDA expects that a company will make only those claims relating to safety and efficacy that are consistent with their products’ approved package insert. Failure to meet these expectations can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available drugs for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturers from communicating on the subject of off-label use.
The FDA’s policies may change and additional government regulations may be enacted which could affect Qutenza and our future product candidates or approval of new indications for our products. We cannot predict the likelihood, nature or extent of adverse governmental regulations that might arise from future legislative or administrative action, either in the United States or abroad.
European Union
Clinical Trials
In common with the United States, the various phases of preclinical and clinical research in the European Union are subject to significant regulatory controls. The regulatory controls on clinical research in the European Union are now largely harmonized following the implementation of the Clinical Trials Directive 2001/20/EC, or CTD. Compliance with the national implementations of the CTD has been mandatory from May 1, 2004. However, variations in the member state regimes continue to exist, particularly in the small number of member states that have yet to implement the CTD fully. Clinical trials must be separately authorized in each European Union member state where they are conducted.
All member states currently require regulatory and independent ethics committee approval of interventional clinical trials, as well as informed consent and other measures to protect the interest of human subjects. European regulators and ethics committees also require the submission of adverse event reports during a study and a copy of the final study report. Procedures exist to suspend studies if necessary to protect the safety of subjects.
Our MA for Qutenza has been transferred to Astellas pursuant to the Astellas Agreement and as a result, Astellas is responsible for ongoing maintenance and post approval requirements associated with Qutenza’s MA. In addition, Astellas is responsible for future regulatory applications in the Licensed Territory. Astellas also has an option to license our product candidate, NGX-1998. If they elect to exercise this option, Astellas will be responsible for regulatory matters associated with this product candidate in the Astellas Territory. If Astellas does not exercise this option, we have agreed to not seek regulatory approval for NGX-1998 in the Licensed Territory.
Approvals Outside of the United States and the Licensed Territory
We will also be subject to a wide variety of foreign regulations governing the development, manufacture and marketing of our products should we decide to seek regulatory approvals in other world markets. The
26
Table of Contents
approval process varies from country to country and the time needed to secure approval may be longer or shorter than that required for FDA approval. We offer no assurance that clinical trials conducted in one country will be accepted by other countries or that approval in one country will result in approval in any other country.
Third-Party Reimbursement and Pricing Controls
General. In the United States and elsewhere, patients’ access to pharmaceutical products depends in significant part on the coverage and reimbursement of a product or service by third party payers, such as government programs, private insurance plans and employers. Third-party payers increasingly are challenging the medical necessity of and prices charged for medical products and services. We may be unable to achieve reimbursement from some payers because they may not consider our products to be “reasonable and necessary” or cost-effective. Furthermore, it is possible that even if payers are willing to reimburse for our products, the reimbursement levels may not be sufficient to allow us to sell our products on a competitive and profitable basis.
In many foreign countries, particularly the countries in the European Union, the pricing of prescription drugs is subject to direct governmental control and is influenced by drug reimbursement programs that employ a variety of price control mechanisms. In the European Union, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national health care systems that fund a large part of the cost of such products to consumers. The approach taken varies from country to country. Some jurisdictions operate positive and/or negative list systems under which products may only be marketed once a reimbursement price has been agreed. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to restrict the entry of new products, as exemplified by the National Institute for Clinical Excellence in the United Kingdom which evaluates the data supporting new medicines and passes reimbursement recommendations to the government. In addition, in some countries cross-border imports from low-priced markets (parallel imports) exert a commercial pressure on pricing within a country. With regard to Qutenza, Astellas is responsible for seeking pricing approvals in the Licensed Territory.
In the United States, there have been, and we expect that there will continue to be, a number of federal and state proposals to implement means by which the government can negotiate lower drug prices for Medicare and Medicaid beneficiaries. While we cannot predict whether such legislative bills will become law, their enactment could have a material adverse effect on our business, financial condition and results of operations.
Medicare. We market Qutenza for use in the treatment of pain associated with PHN. We expect that in the United States a majority of the patients who are treated with Qutenza for this indication will be Medicare beneficiaries. CMS is the agency within the Department of Health and Human Services that administers both the Medicare and Medicaid programs. Because Qutenza is a dermal delivery system that in accordance with its FDA approved label must be applied by a physician or a health care professional working under the close supervision of a physician, two elements of Medicare reimbursement are relevant to Qutenza: the availability of reimbursement for services to administer Qutenza and the availability of reimbursement for Qutenza itself.
CMS has the authority not to cover particular products or services if it determines that they are not “reasonable and necessary” for the treatment of Medicare beneficiaries. CMS may make a national coverage determination, or NCD, for a product, which establishes on a nationwide basis the indications that will be covered, and any restrictions or limitations. However, for most new drugs that are eligible for payment, CMS does not create an NCD. For Qutenza, coverage under Medicare Part B has been confirmed in all Medicare regions and the amount of reimbursement for the Qutenza treatment procedure is being determined on a region by region basis. Although we have some local coverage determinations, CMS or a third party may request an NCD independent of us. If such request is made, we cannot assure you that such NCD will contain favorable coverage terms.
27
Table of Contents
In November 2010, CMS granted a unique, Level II Health Care Common Procedural Coding System, or J-code, for Qutenza which became effective on January 1, 2011. The issuance of the J7335 improves the efficiency of the reimbursement process for all customer segments, from large hospitals to individual physicians.
All eleven Medicare contractors are covering and reimbursing for medically necessary services related to the administration of Qutenza for PHN. Reimbursement for such services is being made to the physician, hospital or clinic, depending on where Qutenza is applied. Medicare payment for services related to the administration of Qutenza is determined according to prospectively set payment rates, linked to a procedure code established by the American Medical Association. These codes, called Current Procedural Terminology, or CPT codes, describe the procedure performed or services rendered. Currently, eight of eleven Medicare contractors are requiring the Qutenza application procedure be billed using an existing evaluation and management, or E&M, CPT code. The remaining three contractors have requested Qutenza administration be billed with miscellaneous codes with or without an E&M code.
Under Medicare Part B, reimbursement for Qutenza is currently limited to 106% of the manufacturer’s average sales price (as defined by statute and regulation). CMS has been considering other changes to Medicare reimbursement that could result in lower payments for physician-administered drugs, and Congress may also consider legislation that would mandate lower reimbursement levels. A reduction in reimbursement levels could materially and adversely affect our revenue.
Medicaid. Most State Medicaid programs have established preferred drug lists, or PDLs, and the process, criteria and timeframe for obtaining placement on the PDL varies from State to State. A federal law establishes a minimum rebate, currently 23.1%, that a manufacturer must pay for Medicaid utilization of a brand-name product, and many States have established supplemental rebate programs as a condition for including a drug product on a PDL. Submitting a PDL application to each State will be a time-consuming and expensive process, and it is not clear how many or which State programs will accept the applications. Review times for these applications can vary from weeks to 14 months or more.
Private Insurance Reimbursement. Commercial insurers usually offer two types of benefits: medical benefits and pharmacy benefits. In most private insurance plans, physician-administered drugs are provided under the medical benefit. If private insurers decide to cover Qutenza, they will likely reimburse for the drug and its administration in a variety of ways, depending on the insurance plan’s policies, employer and benefit manager input and contracts with their physician network. Like Medicare and Medicaid, commercial insurers have the authority to place coverage and utilization limits on prescription drugs. Private insurers often tend to adopt reimbursement methodologies for a product similar to those adopted by Medicare. Revenue for Qutenza may be materially and adversely affected if private payers make unfavorable reimbursement decisions or delay making favorable reimbursement decisions.
Employees
As of March 15, 2012, we had 35 employees, of which 14 are in research and development, 17 are in general and administrative and 4 are in commercial operations. None of our employees are represented by a labor union or are the subject of a collective bargaining agreement.
Facilities
We lease approximately 26,386 square feet of space in our headquarters in San Mateo, California under a lease that expires in July 2012. We are currently evaluating alternative office space for purposes of relocation before our lease expires later this year. We believe that our office space requirements may be substantially less than our currently leased facility. We have no laboratory, research or manufacturing facilities.
28
Table of Contents
Form of Organization
We were incorporated in California as Advanced Analgesics, Inc. on May 28, 1998 and changed our name to NeurogesX, Inc. in September 2000. In February 2007, we reincorporated into Delaware.
Available Information
We file electronically with the Securities and Exchange Commission, or SEC, our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act. The public may read or copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. The address of that site is http://www.sec.gov.
You may obtain a free copy of our annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K and amendments to those reports on the day of filing with the SEC on our website on the World Wide Web at http://www.neurogesx.com or by contacting our corporate offices by calling 650-358-3300. Information contained on our website is not part of this report or any other report filed with the SEC.
| Item 1A. | Risk Factors |
You should consider carefully the risk factors described below, and all other information contained in or incorporated by reference in this Annual Report on Form 10-K before making an investment decision. If any of the following risks actually occur, they may materially harm our business, financial condition, operating results or cash flows. As a result, the market price of our common stock could decline, and you could lose all or part of your investment. Additional risks and uncertainties that are not yet identified or that we think are immaterial may also materially harm our business, operating results or financial condition and could result in a complete loss of your investment.
Risks Related to our Business
Our success depends on our ability to obtain regulatory approval for our lead product candidate NGX-1998.
Our success depends substantially on the approval of our most advanced product candidate, NGX-1998, a topical liquid formulation of high concentration capsaicin, for use in certain neuropathic pain conditions. In order to obtain regulatory approval, we will need to demonstrate in Phase 3 clinical trials that NGX-1998 is safe and effective for use in each target indication. The FDA generally requires successful completion of at least two adequate and well-controlled Phase 3 clinical trials for each indication for which we seek regulatory approval. The successful outcome of Phase 3 clinical trials and the required capital expenditure for such trials will depend in part on our ability to successfully design, initiate and complete enrollment and conclude any such trials on a timely basis. We may not have adequate financial or other resources to pursue this product candidate for any indications through the clinical trial process. Additionally, such trials may not achieve positive results, and, even if the results are positive, may not adequately support approval by the FDA. If we fail to complete clinical trials for NGX-1998, or if such clinical trials fail to demonstrate with substantial evidence that NGX-1998 is both safe and effective, we will not be able to obtain regulatory approval to market the product candidate. To date, NGX-1998 has only been tested for efficacy in a single Phase 2 clinical trial in PHN patients. There can be no assurance that the results we obtained in such trial will be replicated in any subsequent trials or that the results obtained in the PHN population would be replicated in other neuropathic pain indications. Further, there can be no assurance that the clinical trial data for our approved Qutenza product will be relevant or provide any meaningful insight into the design of clinical trials for NGX-1998 or that we will be able to use the clinical trial data from Qutenza with respect to obtaining approval.
29
Table of Contents
Our clinical trials may fail to demonstrate adequately the safety and efficacy of our product candidates, which could prevent or delay regulatory approval and commercialization.
Before obtaining regulatory approvals for the commercial sale of our product candidates, we must demonstrate through lengthy, complex and expensive preclinical testing and clinical trials that the product is both safe and effective for use in each target indication. Clinical trial results from the study of neuropathic pain are inherently difficult to predict. The primary measure of pain is subjective patient feedback, which can be influenced by factors outside of our control, and can vary widely from day to day for a particular patient, and from patient to patient and site to site within a clinical study. The results we have obtained in completed clinical trials may not be predictive of results from our ongoing or future trials. Additionally, our product candidate may fail to show efficacy in additional clinical trials, even after promising results in earlier studies.
Some of our trial results have been negatively affected by factors that had not been fully anticipated prior to our examination of the trial results. For example, we have from time to time observed a significant “placebo effect” within our control groups—a phenomenon in which a sham treatment or, in the case of our studies, a low-dose capsaicin treatment that we believed would not be effective, results in a beneficial effect. Although we design our clinical study protocols to address known factors that may negatively affect our study results, there can be no assurance that our protocol designs will be adequate or that unknown factors will not have a negative effect on the results of our clinical trials, which could significantly disrupt our efforts to obtain regulatory approvals and commercialize our product candidates. In addition, clinical trials in new indications or in a larger patient population could reveal more frequent, more severe or additional side effects that were not seen or deemed unrelated in earlier studies, any of which could interrupt, delay or halt clinical trials of our product candidates and could result in the FDA or other regulatory authorities stopping further development of or denying approval of our product candidates. Based on results at any stage of clinical trials, we may decide to repeat or redesign a trial or, modify our regulatory strategy, which would likely delay the date of potential regulatory approval, or even discontinue development of one or more of our product candidates.
A number of companies in the biotechnology and pharmaceutical industries have suffered significant setbacks in advanced clinical trials, even after obtaining promising results in earlier trials. If our product candidates, including NGX-1998, are not shown to be both safe and effective in clinical trials, our business, financial condition and results of operations will be adversely affected.
Our success depends on our ability to secure adequate funding to complete the development program for NGX-1998.
Currently, we believe that we have sufficient cash reserves to fund operations into 2013. However, our current cash reserves are not be sufficient to fully fund Phase 3 development of NGX-1998, and as a result, we will need additional funding to complete the development of this product candidate and to fund operations generally. We are currently seeking to enter potential strategic partnerships related to both NGX-1998 and Qutenza to provide funding for continued development of NGX-1998, support of our commercialization operations with respect to Qutenza, potentially support commercialization for NGX-1998, if approved for marketing, and support funding of general operations. However, there can be no assurance that such a strategic partnership would be completed or on terms that are favorable to us. If we are unable to reach agreement with a potential strategic partner or if a strategic partnership does not provide adequate funding, we would need to obtain additional funding to complete the clinical development of NGX-1998. Raising additional equity capital may be difficult since our share price is near historical lows compared to its trading history, and due to our potential delisting from the NASDAQ Global Market, as a result of failure to meet certain minimum requirements. If we are unable to raise sufficient capital to fund our development programs and operations generally, we will be unable to proceed with the development of NGX-1998 and our business and financial condition will be significantly harmed.
30
Table of Contents
Additionally, Astellas has an option pursuant to the Astellas Agreement to obtain an exclusive license to commercialize NGX-1998 in the Licensed Territory. There can be no assurance that any data and other information we provide to Astellas regarding development of NGX-1998 and its prospects for potential approval and commercialization will be sufficient for Astellas to elect to exercise such option. If Astellas decides not exercise its option with respect to NGX-1998, we will not receive funding for Phase 3 development of NGX-1998 under the Astellas Agreement, and, additionally, we will not be permitted to commercialize NGX-1998 in the Licensed Territory. A failure of Astellas to exercise its co-development and commercialization option with respect to NGX-1998 may delay or prevent us from obtaining funding for the continued development of NGX-1998 and the operation of our business will be significantly harmed.
Raising additional funds by issuing securities, engaging in debt financings or through licensing arrangements may cause dilution to existing stockholders, restrict our operations or require us to relinquish proprietary rights.
To the extent that we raise additional capital by issuing equity securities as we did in July 2011 and February 2012, our existing stockholders’ ownership will be diluted. Royalty monetization structures may require us to give up or assign certain rights to certain current and future assets. Any debt financing we enter into may involve covenants that restrict our operations or our ability to enter into other funding arrangements. These restrictive covenants may include limitations on additional borrowing and specific restrictions on the use of our assets, as well as prohibitions on our ability to create liens, pay dividends, redeem our stock or make investments. In addition, if we raise additional funds through licensing arrangements, it may be necessary to relinquish potentially valuable rights to our potential products or proprietary technologies, or grant licenses on terms that are not favorable to us.
Failure to meet our loan obligations with Hercules could adversely affect our financial condition.
Under the terms of our agreement with Hercules, we are subject to certain negative covenants and events of default. If we were to default under Hercules Agreement, we would be obligated to pay to Hercules the amount we borrowed under such agreement together with any unpaid interest, a 5% interest penalty on any past due amount, and the end of loan charge of $0.6 million. If we were required to pay the amounts due in the event of default under the Hercules Agreement, we would be required to raise additional capital and there can be no assurances that we would be able to do so.
Failure to meet our loan obligations with Cowen could adversely affect our financial condition.
Under the terms of our Financing Agreement with Cowen, we are subject to certain negative covenants and events of default. Under the Financing Agreement, an event of default includes a covenant to maintain liquid assets equal to expected cash needs during the following three months. If we were to default under the Financing Agreement with Cowen we are obligated to immediately pay to Cowen the borrowed amount together with interest accrued thereon from the effective date of the Financing Agreement to the date of repayment at an internal rate of return equal to the lesser of the 19% rate of interest or the maximum rate permitted by law, less any payments received by Cowen. If we were required to pay the amounts due in the event of default under the Cowen Agreement, we would be required to raise additional capital and there can be no assurances that we would be able to do so.
If we fail to meet the requirements for continued listing on the NASDAQ Global Market and do not meet initial listing requirements to transfer to the NASDAQ Capital Market, our common stock could be delisted from trading, which would adversely affect the liquidity of our common stock and our ability to raise additional capital.
Our common stock is currently listed on the NASDAQ Global Market and to maintain our listing, we must meet certain financial requirements in accordance with the rules of Nasdaq. On October 18, 2011, we received a
31
Table of Contents
notification from Nasdaq that we failed to maintain the required minimum market value of listed securities level of $50.0 million for a 30 consecutive business day period. We have until April 16, 2012 in which to cure this non-compliance by meeting or exceeded such requirement for a minimum of ten consecutive business days. On January 27, 2012, we received a notification from Nasdaq that we failed to maintain the required $1.00 minimum bid price for a 30 consecutive business day period. We have until July 28, 2012 in which to cure this non-compliance by meeting or exceeded such requirement for a minimum of ten consecutive business days. There can be no assurance that we will be able to cure these violations by the end of their respective cure periods, be able to transfer to the NASDAQ Capital Market or maintain our listing on either market in the future. Any potential delisting of our common stock would adversely affect the liquidity of our common stock and our ability to raise additional capital.
If we do not raise additional capital, we may be forced to delay, reduce or eliminate our commercialization efforts or development programs.
Our future capital requirements will depend on, and could increase significantly as a result of many factors, including:
| • | the progress of our development programs including the number, size and scope of clinical trials and non-clinical development, including the initiation and conduct of Phase 3 clinical trials for NGX-1998; |
| • | our ability to meet our obligations under the loan agreement with Hercules and our Financing Agreement with Cowen; |
| • | the success of the commercialization of our products and the amount of revenue we generate; |
| • | the costs of maintaining sales and marketing infrastructure, distribution capabilities and other market support functions such as medical affairs and pharmacovigilance; |
| • | the conduct of manufacturing activities including the manufacture of commercial product supply and clinical product supply; |
| • | the costs of carrying out our obligations under our commercial arrangement with Astellas; |
| • | our ability to establish and maintain strategic collaborations, including licensing, distribution and other arrangements that we have or may establish; |
| • | our ability to meet our obligation under the Financing Agreement with Cowen including liquidity maintenance requirements; |
| • | the costs and timing of additional regulatory approvals; |
| • | the costs involved in enforcing or defending patent claims or other intellectual property rights; and |
| • | the extent to which we acquire or invest in other products, technologies and businesses. |
We intend to seek additional funding through strategic alliances and/or debt facilities or other financing vehicles which may include the public or private sales of our equity securities. There can be no assurance, however, that additional funding will be available on reasonable terms, if at all. If adequate funds are not available, we may be required to further delay, reduce the scope of, or eliminate one or more of our planned development, commercialization or expansion activities.
Our success depends in part on our ability to cost-effectively maintain Qutenza® (capsaicin) 8% patch on the market in the United States.
Our product Qutenza® (capsaicin) 8% patch, was approved by the FDA in November 2009 for the management of neuropathic pain associated with PHN. We initially launched Qutenza in April 2010 in the United States with our own commercial sales organization. Over the two years since launch, we invested
32
Table of Contents
significant time and cash resources into commercialization of Qutenza. In March 2012, we concluded that we could no longer invest significant resources in Qutenza. As a result, we ceased direct promotion of Qutenza by eliminating our sales force and by reducing our expenses in support of commercial operations. Despite our cost control efforts with respect to Qutenza, the costs of maintaining availability of Qutenza in the market could be greater, and the revenues deriving from sales of Qutenza could be less, than we currently anticipate. Further, there are certain ongoing activities that are required for maintaining a pharmaceutical product in the market, such as maintaining a safety database. If the costs of maintaining Qutenza in the market are significantly greater than we expect, or the revenues significantly lower, our business could be seriously harmed and our ability to continue development of NGX-1998 would be adversely affected.
Qutenza and our product candidates may never achieve market acceptance.
The commercial success of Qutenza or any other product candidates we develop including NGX-1998, if approved, will depend on, among other things, acceptance by healthcare professionals, patients and payers. Market acceptance of, and demand for any products that we develop and commercialize will depend on many factors, including:
| • | with respect to Qutenza, healthcare professionals and patients willingness and ability to accommodate the time necessary for each Qutenza treatment; |
| • | our ability to fund the size and scope of sales and marketing activities necessary to support a product such as Qutenza; |
| • | the effectiveness of our sales and marketing strategies; |
| • | the ability to obtain adequate pricing and sufficient insurance and other third party payer coverage and reimbursement; |
| • | patient acceptance of the treatment procedure, efficacy or safety of Qutenza; |
| • | availability, relative cost and relative efficacy and safety of alternative and competing treatments; |
| • | the effectiveness of our or our collaborators’ sales, marketing and distribution strategy; |
| • | publicity concerning our products or competing products and treatments; |
| • | potential product recalls; and |
| • | post marketing approval pharmacovigilance or other studies showing safety issues not seen in previous studies. |
If Qutenza, or our product candidates if approved, fail to gain market acceptance, we may be unable to earn sufficient revenue to continue our business.
We outsource the manufacturing of Qutenza and our other product candidates and accordingly depend on other parties for our manufacturing operations. If these manufacturers fail to meet our requirements and strict regulatory requirements, Qutenza commercialization efforts and further product development efforts may be negatively affected.
We currently depend on four contract manufacturers as single source suppliers for the components of Qutenza: an intermediate material used in the synthesis of capsaicin, synthetic capsaicin, the dermal delivery system and the associated cleansing gel. We have entered into long-term commercial supply agreements for these components. In addition we have entered into a long-term agreement for the assembly of the Qutenza treatment kits in the United States. If relationships with any of these manufacturers is terminated, or if any manufacturer is unable to produce required quantities on a timely basis or at all, our operations would be negatively impacted and our business harmed.
33
Table of Contents
We are currently in the process of identifying potential manufacturers for NGX-1998. Potential suppliers of NGX-1998 will need to meet technical and regulatory requirements related to safety manufacturing of the liquid formulation of capsaicin, filling the liquid formulation into its finished applicator and manufacturing of the applicator itself. The high concentration capsaicin such as that used in NGX-1998 is difficult to safely manufacture. If we are unable to identify one or more suitable suppliers in a timely manner, if those suppliers are unable to meet our technical or regulatory requirements then our development program may be delayed and the planned costs of the development program may increase significantly.
Any products we or our contract manufacturers manufacture or distribute under FDA approvals are subject to pervasive and continuing regulation by the FDA, including record-keeping requirements and reporting of adverse experiences with the products. Drug manufacturers and their subcontractors are required to register with the FDA and, where appropriate, state agencies, and are subject to periodic unannounced inspections by the FDA and state agencies for compliance with cGMP, regulations which impose procedural and documentation requirements upon us and each third party manufacturer we utilize. This ongoing regulation and evaluation results in an obligation on us to continually evaluate the manufacturing process, the applicability of our analytical methods and the specifications that are used to release product for sale and evaluate long term stability of our product to ensure they continue to meet their objective of ensuring that such processes and methods enable the consistent manufacture of product that meets regulatory approved specifications. This process often results in identification of matters that can result in changes to specifications, analytical methods and manufacturing processes as part of normal and ongoing process improvement. This process can also identify matters that can have significant implications including the potential for reduction in applicable product shelf life, suspension of product commercialization, or requirement of product recalls, all of which would have a material adverse effect on us. We are undertaking such ongoing process improvement processes with our contract manufacturers and we cannot assure you that the outcome of such initiatives will not have a material effect on our business.
Reliance on contract manufacturers exposes us to additional risks, including:
| • | failure of current and future manufacturers to comply with strictly-enforced regulatory requirements; |
| • | failure to manufacture products that meet our specifications throughout their shelf lives; |
| • | failure to deliver sufficient quantities in a timely manner; |
| • | the possibility that we may terminate a contract manufacturer and need to engage a replacement; |
| • | the possibility that current and future manufacturers may not be able to manufacture our product candidates and products without infringing the intellectual property rights of others; |
| • | the possibility that current and future manufacturers may not have adequate intellectual property rights to provide for exclusivity and prevent competition; and |
| • | insufficiency of intellectual property rights to any improvements in the manufacturing processes or new manufacturing processes for our products. |
Any of these factors could result in insufficient supply of Qutenza to meet demand which would have a significant impact on our financial results and adversely impact any revenues we may derive from Qutenza. With respect to our product candidates, such factors could result in significant delay or suspension of clinical trials, regulatory submissions, or receipt of required approvals, all of which could significantly harm our business.
Because our third party manufacturers operate outside of the United States, and many of the raw materials and the labor that are used to manufacture Qutenza and our product candidates are based in foreign countries, we may experience currency exchange rate risks, even though, in some instances our contracts are denominated in U.S. dollars. We do not currently engage in forward contracts to hedge this currency risk and as a result, may suffer adverse financial consequences as a result of this currency risk.
34
Table of Contents
Materials used by these entities to manufacture our product candidates originate outside the United States, including certain materials which may be sourced from China and India. The FDA has increased its diligence with regard to foreign-sourced materials and manufacturing processes. Increased FDA scrutiny of foreign manufacturers could result in delays in product availability, which could have a negative impact on our business.
Furthermore, because we are currently required to supply Qutenza to Astellas under the Supply Agreement, the foregoing risks to us related to supply of Qutenza and its components could also harm Astellas’ ability to commercialize Qutenza in the Licensed Territory. Whether we supply Qutenza to Astellas or Astellas enters into direct supply arrangements with our suppliers, Astellas’ ability to generate revenue and in turn our ability to generate royalty and sales-milestone revenue, would be impacted by these foregoing risks relating to manufacturing.
Our success will depend, in part, on the successful efforts of our collaboration partner in the European Union, certain countries in Eastern Europe, the Middle East and Africa.
The success of sales of Qutenza in the Licensed Territory will be dependent on Astellas’ ability to successfully launch and commercialize Qutenza pursuant to the Astellas Agreement. The manner in which Qutenza is being launched, including the timing of launch in each country of the European Union and the pricing that has or will be established in each such country, will have a significant impact on the ultimate success of Qutenza in the European Union, and ultimately the success of the overall commercial arrangement with Astellas. If commercial launch of Qutenza throughout the remainder of the Licensed Territory is delayed or prevented, our revenues will suffer and our stock price will decline. Further, if sales of Qutenza are less than anticipated, our stock price will decline. The outcome of Astellas’ commercialization efforts could also have an effect on investors’ perception of potential sales of Qutenza in the United States, which could cause a decline in our stock price if such efforts are unsuccessful.
The Astellas Agreement provides for Astellas to be responsible for conducting certain studies for Qutenza in the European Union, both to satisfy post-marketing commitments that were made in conjunction with the Qutenza MA in the European Union and to carry out in support of Astellas’ commercialization plans for Qutenza in the Licensed Territory. Astellas has now commenced three studies in this regard. The planning and execution of these studies is primarily the responsibility of Astellas, and may not be carried out in accordance with our indicated preferences, or may yield results that are detrimental to Astellas’ sales of Qutenza in the Licensed Territory or detrimental in our efforts to develop or commercialize Qutenza outside the Licensed Territory, including in the United States. Together with Astellas, we have attempted to design such studies with a view toward having the resulting data be supportive of regulatory and commercial efforts in both the European Union and the United States, such as potential label expansion studies that may be carried out with respect to use of Qutenza to treat pain associated with PDN. However, such studies may have limited or no utility to support such efforts and may negatively impact our revenues.
Within the Licensed Territory, regulatory approval for Qutenza has been obtained within the European Economic Area, Switzerland and Georgia. Other regulatory jurisdictions in the Licensed Territory may require further clinical and manufacturing activities to gain approval for sales of Qutenza in these countries. There can be no assurance that these activities will be successful and that approval in these countries will be obtained in a timely manner, if at all. Further, there can be no assurance that such clinical or manufacturing activities will not negatively impact the regulatory processes in the European Economic Area, United States or other markets where regulatory approval of Qutenza may have been obtained or may be in the process of being sought.
The Astellas Agreement requires us to expend resources in support of our commercial relationship with Astellas. Although we believe that our contractual arrangement with Astellas provides for reimbursement for many of these activities, the time and financial costs of managing such a relationship can be significant and may occur at a time when we are executing the commercialization of Qutenza in the United States.
35
Table of Contents
The Supply Agreement we entered into in connection with the Astellas Agreement requires us to supply components of Qutenza to Astellas at the same cost we obtain such components from our third-party manufacturers. Because we have never overseen commercial manufacturing processes and quantities of these components, we may incur non-reimbursable costs related to manufacturing scale up, quality assurance and release, which could negatively impact our financial results. Such Supply Agreement also provides for Astellas to enter into direct supply relationships with our current suppliers to replace the need for us to provide Astellas with pass-through supply of Qutenza components. There can be no assurance that the establishment of such separate supply relationships will not negatively impact our ability to obtain our own supply of Qutenza components in support of our commercialization of Qutenza in the United States or other markets outside the Licensed Territory that we seek to commercialize.
The Astellas Agreement grants to Astellas an option to exclusively license our second generation product, NGX-1998. In connection with this option, we are required to execute a development plan for NGX-1998 and achieve certain objectives. After completion of an initial set of objectives, Astellas may make an additional payment to us to retain its option to license NGX-1998 until further agreed upon development of NGX-1998 has been performed by us. Upon completion of these objectives, Astellas may elect to make a final payment to us in order to exercise its option. If Astellas determines not to make one of these payments, the option to license NGX-1998 will expire, Astellas will not be required to provide further funding for NGX-1998, and we would be precluded from marketing NGX-1998 in the Licensed Territory. If Astellas fails to exercise its options with respect to NGX-1998, we may not have adequate funding for development of NGX-1998, we will be unable to generate revenues from potential sales of NGX-1998 in the Licensed Territory, and our business will be harmed. Further, Astellas may be less inclined to exercise its co-development and commercialization option for NGX-1998 if commercial sales of Qutenza do not meet Astellas’ expectations.
Astellas’ commercialization of Qutenza in the Licensed Territory may result in lower levels of income to us than if we marketed Qutenza in the Licensed Territory on our own. Astellas may not fulfill its obligations or commercialize Qutenza as quickly as we would like. We could also become involved in disputes with Astellas, which could lead to delays in or termination of the Astellas Agreement and time-consuming and expensive litigation or arbitration. If Astellas terminates or breaches its agreement with us, or otherwise fails to complete its obligations in a timely manner, the chances of successfully developing or commercializing Qutenza in the Licensed Territory would be materially and adversely affected.
Qutenza will be subject to ongoing regulatory requirements and may face regulatory or enforcement action.
Any product candidate for which regulatory approval is obtained is subject to significant review and ongoing and changing regulation by the FDA, the EMA, and other worldwide regulatory agencies. These ongoing regulatory requirements may include, but are not limited to:
| • | obtaining additional post-approval clinical study data; |
| • | regulatory review of advertising, promotional and education activities for the product; |
| • | establishment and monitoring of pharmacovigilance programs and annual reports; and |
| • | periodic regulatory agency inspections and reviews of third-party manufacturing facilities and processes. |
Failure to comply with regulatory requirements may subject us or our collaboration partner to administrative and judicially-imposed sanctions. These may include warning letters, adverse publicity, civil and criminal penalties, injunctions, product seizures or detention, product recalls, total or partial suspension of production, and refusal to approve pending product marketing applications.
Even if regulatory approval to market a particular product candidate is obtained, the approval could be conditioned on conducting additional costly post-approval studies or could limit the indicated uses included in
36
Table of Contents
such product’s labeling. For example, the Qutenza MA owned by Astellas in the European Union requires the conduct of certain post-authorization follow up measurers including ongoing evaluations of safety of Qutenza’s use in the labeled indications, as well as clinical evaluation in patients with PDN. These studies, which are funded by Astellas pursuant to the Astellas Agreement, may prove difficult and expensive to complete and their results may identify safety, efficacy or other issues related to Qutenza’s use that could hinder or prevent commercialization efforts. Moreover, the product may later be found in the course of these studies or through our pharmacovigilance programs to cause adverse effects that limit its use, force us or our partner to withdraw it from the market or impede or delay the ability to obtain regulatory approvals in additional countries.
We are subject to various statutes and regulations pertaining to the marketing of our products.
We are subject to various federal and state laws pertaining to healthcare fraud and abuse, including inducing, facilitating or encouraging submission of false claims to government programs and prohibitions on the offer of payment or acceptance of kickbacks or other remuneration for the purchase of Qutenza. Specifically, these anti-kickback laws make it illegal for a prescription drug manufacturer to solicit, offer, or pay any remuneration in exchange for purchasing, leasing or ordering any service or items including the purchase or prescription of a particular drug for which payment may be made under a federal healthcare program. Because of the sweeping language of the federal anti-kickback statute, many potentially beneficial business arrangements would be prohibited if the statute were strictly applied. To avoid this outcome, the U.S. Department of Health and Human Services has published regulations – known as “safe harbors” – that identify exceptions or exemptions to the statute’s prohibitions. Arrangements that do not fit within the safe harbors are not automatically deemed to be illegal, but must be evaluated on a case-by-case basis for compliance with the statute. We seek to comply with anti-kickback statutes and if possible to fit within one of the defined “safe harbors”; we are unaware of any violations of these laws. However, due to the breadth of the statutory provisions and the absence of uniform guidance in the form of regulations or court decisions, there can be no assurance that our practices will not be challenged under anti-kickback or similar laws. Violations of such restrictions may be punishable by significant civil or criminal sanctions, including fines and civil monetary penalties, as well as the possibility of exclusion from U.S. federal healthcare programs (including Medicaid and Medicare). Any such violations could have a material adverse effect on our business, financial condition, results of operations and cash flows.
Several recently enacted state and federal laws in Massachusetts and Vermont, and the Federal Physician Payment Sunshine Act, now require, among other things extensive tracking and maintenance of data bases regarding disclosure of relationships and payments to physicians and healthcare providers. These laws require us to implement the necessary and costly infrastructure to track and report certainpayments to healthcare providers. Failure to comply with these new tracking and reporting laws could subject us to significant civil monetary penalties.
In addition, the FDA has the authority to regulate the claims we make in marketing our prescription drug products to ensure that such claims are true, not misleading, supported by scientific evidence and consistent with the labeled use of the drug. Failure to comply with FDA requirements in this regard could result in, among other things, warning letters, suspensions of approvals, seizures or recalls of products, injunctions against a product’s manufacturer, distribution, sales and marketing, operating restrictions, civil penalties and criminal prosecutions. Any of these FDA actions could negatively impact our product sales and profitability.
Delays in the commencement or completion of clinical testing could result in increased costs to us and delay our ability to generate additional revenues.
We do not know whether clinical trials will begin on time or be completed on schedule, if at all. The commencement and completion of clinical trials can be disrupted for a variety of reasons, including difficulties in:
| • | recruiting and enrolling patients to participate in a clinical trial; |
37
Table of Contents
| • | obtaining regulatory approval to commence a clinical trial; |
| • | reaching agreement on acceptable terms with prospective clinical research organizations and trial sites; |
| • | manufacturing sufficient quantities of a product candidate; and |
| • | obtaining institutional review board approval to conduct a clinical trial at a prospective site. |
A clinical trial may also be suspended or terminated by us, the FDA or other regulatory authorities due to a number of factors, including:
| • | failure to conduct the clinical trial in accordance with regulatory requirements or in accordance with our clinical protocols; |
| • | inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold; |
| • | unforeseen safety issues; or |
| • | inadequate patient enrollment or lack of adequate funding to continue the clinical trial. |
In addition, changes in regulatory requirements and guidance may occur and we may need to amend clinical trial protocols to reflect these changes, which could impact the cost, timing or successful completion of a clinical trial. If we experience delays in the commencement or completion of our clinical trials, the commercial prospects for our product candidates will be harmed, and our ability to generate product revenues will be delayed. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also lead to the denial of regulatory approval of a product candidate.
Finally, our discussions with potential or actual development and commercialization partners for NGX-1998 could result in significant delays in our planned timing of clinical trial initiation and may significantly change other elements of our current development plan. Any of these changes could result in delays, increased cost and changes to the potential probability of a successful outcome of our development program, any of which could negatively impact our business.
We rely on third parties to conduct our clinical trials. If these third parties do not perform as contractually required or otherwise expected, we may not be able to obtain regulatory approval for our product candidates.
We do not currently conduct clinical trials on our own, and instead rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to assist us with our clinical trials. We are also required to comply with regulations and standards, commonly referred to as good clinical practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the trial participants are adequately protected. If these third parties do not successfully carry out their duties to us or regulatory obligations or meet expected deadlines, if the third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our preclinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for our product candidates.
We face potential product liability exposure, and if successful claims are brought against us, we may incur substantial liability and may have to limit our commercialization efforts for Qutenza or our development efforts with respect to our product candidates.
The use of our product candidates in clinical trials and the sale of Qutenza expose us to the risk of product liability claims. Product liability claims might be brought against us by consumers, health care providers,
38
Table of Contents
pharmaceutical companies or others selling our products. If we cannot successfully defend ourselves against these claims, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for our product candidates; |
| • | impairment of our business reputation; |
| • | costs of related litigation; |
| • | substantial monetary awards to patients or other claimants; |
| • | loss of revenues; |
| • | the inability to commercialize our product candidates; and |
| • | withdrawal of clinical trial participants. |
Although we currently have product liability insurance coverage with limits that we believe are customary and adequate to provide us with coverage for foreseeable risks associated with our Qutenza commercialization and product candidate development efforts, our insurance coverage may not reimburse us or may be insufficient to reimburse us for the actual expenses or losses we may suffer. Moreover, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability.
Our products are expected to face substantial competition which may result in others discovering, developing or commercializing products before or more successfully than us or our collaborators.
Qutenza competes, and NGX-1998 will compete, if approved for marketing, against more established products marketed by large pharmaceutical companies with far greater name recognition and resources than we or Astellas have. The most directly-competitive currently-marketed products in the United States are Lidoderm, an FDA-approved 5% lidocaine topical patch for the treatment of PHN marketed by Endo Pharmaceuticals, and Lyrica, an oral anti-convulsant, marketed by Pfizer for use in the treatment of PHN. In January 2011, Depomed Inc. received FDA approval for Gralise, a formulation of gabapentin that is a once daily tablet for the treatment of PHN. In addition to these branded drugs, the FDA has approved gabapentin (Neurontin) for use in the treatment of PHN. Gabapentin is marketed by Pfizer and multiple generic manufacturers, and is the most widely-prescribed drug in the United States for treatment of neuropathic pain. Pfizer has also received FDA approval for Lyrica for the treatment of PDN, fibromyalgia, epilepsy and general anxiety disorder. Additionally, a number of products that are approved for other diseases are used by physicians to treat PHN.
Competition may become stronger and more direct and products in development, including products that we are unaware of, may compete with Qutenza. There are many other companies working to develop new drugs and other therapies to treat pain in general and neuropathic pain in particular, including Eli Lilly & Co, Pfizer, UCB Pharma SA, GlaxSmithKline plc, Merz, GW Pharmaceuticals, Novartis AG, Novo Nordisk A/S, Dainippon Pharmaceutical Co Ltd., AVANIR Pharmaceuticals, Xenoport, EpiCept, Newron Pharmaceuticals SpA, Pharmagesic Holding Inc., Arcion Therapeutics Inc., Endo Pharmaceuticals, AstraZeneca plc, Allergan, Inc., Bristol-Myers Squibb Co., Bio3 Research Srl, Relevare Pharmaceuticals Ltd., Depomed, Inc., and Ortho-McNeil-Janssen Pharmaceuticals. Many of the compounds in development by such companies are already marketed for other indications, such as anti-depressants, anti-convulsants or opioids. In addition, physicians employ other interventional procedures, such as nerve stimulation or nerve blocks, to treat patients with difficult to treat neuropathic pain conditions.
Furthermore, new developments, including the development of other drug technologies and methods of preventing the incidence of disease, such as vaccines including Zostavax, occur in the biopharmaceutical industry at a rapid pace. Any of these developments may render our products or product candidates obsolete or noncompetitive.
39
Table of Contents
Many of our potential competitors, either alone or together with their partners, have substantially greater financial resources, research and development programs, clinical trial and regulatory experience, expertise in prosecution of intellectual property rights, and manufacturing, distribution and sales and marketing capabilities than we and Astellas do. As a result of these factors, our competitors may:
| • | initiate or withstand substantial price competition more successfully; |
| • | have greater success in recruiting skilled scientific workers from the limited pool of available talent; |
| • | more effectively negotiate third-party licenses and strategic alliances; |
| • | take advantage of acquisition or other opportunities more readily than we can; and |
| • | develop product candidates and market products that are less expensive, safer, more effective or involve more convenient treatment procedures than our current and future products. |
The life sciences industry is characterized by rapid technological change. If we fail to stay at the forefront of technological change our products may be unable to compete effectively.
The efforts of government entities and third-party payers to contain or reduce the costs of health care may adversely affect our sales and limit the commercial success of our products.
In certain foreign markets, pricing or profitability of pharmaceutical products is subject to various forms of direct and indirect governmental control, including the control over the amount of reimbursement provided to the patient who is prescribed specific pharmaceutical products.
In the United States, there have been, and we expect there will continue to be, various proposals to implement similar controls. For example, the health care reform act passed in March 2010, increased the rebate levels for pharmaceutical products sold to Medicaid patients by eight percent. The commercial success of our products could be limited if federal or state governments adopt any such proposals. In addition, in the United States and elsewhere, sales of pharmaceutical products depend in part on the availability of reimbursement to the consumer from third party payers, such as government and private insurance plans. These third-party payers are increasingly utilizing their significant purchasing power to challenge the prices charged for pharmaceutical products and seek to limit reimbursement levels offered to consumers for such products. We expect these third-party payers to focus their cost control efforts on our products, especially with respect to prices of and reimbursement levels for products prescribed outside their labeled indications. In these cases, their efforts may negatively impact our product sales and profitability.
The Hatch-Waxman Act data exclusivity and any equivalent regulatory data exclusivity protection in other jurisdictions for Qutenza, may not prevent new competition which may negatively impact our financial results.
The Hatch-Waxman Act provides five years of data exclusivity to the first applicant to gain approval of an NDA under Section 505(b) of the Food, Drug and Cosmetic Act for a new chemical entity. Qutenza has been recognized by the FDA as a new chemical entity and therefore has been granted five year data exclusivity under the Hatch-Waxman Act through November 2014. Hatch-Waxman provides data exclusivity by prohibiting abbreviated new drug applications, or ANDAs, and 505(b)(2) applications, which are marketing applications where the applicant does not own or have a legal right of reference to all the data required for approval, to be submitted by another company for another version of such drug during the exclusivity period. Protection under Hatch-Waxman will not prevent the filing or approval of a full NDA under Section 505(b)(1) for the same active ingredient, although the applicant would be required to conduct its own adequate and well-controlled clinical trials to demonstrate safety and effectiveness.
Even though Qutenza has been granted five year Hatch-Waxman data exclusivity in the United States and ten-year market exclusivity which may be extended to eleven-year market exclusivity upon meeting certain criteria
40
Table of Contents
and eight-year data exclusivity by the EMA, there can be no assurance that the exclusivity granted will effectively prevent competition, either generic or otherwise. Such competition could significantly harm our business.
Additional clinical trials of Qutenza may not support label expansion for Qutenza for such indication, which would adversely impact our long-term success.
We have not prepared for or conducted any Qutenza clinical trials for indications other than PHN, HIV-PN and PDN. Generally, two successful Phase 3 clinical trials are required to obtain regulatory approval in the U.S. We have conducted two Phase 3 clinical trials with Qutenza for patients with HIV-PN, one of which met its primary endpoint and one which did not meet its primary endpoint. Given that only one HIV-PN controlled study met its primary endpoint and that the pre-specified analysis plan in that study did not allow for examination of the proposed recommended dose, the FDA did not accept the data submitted as sufficient for approval of the HIV-PN indication under the sNDA we submitted with respect to such indication. We have also conducted one Phase 2 clinical trial that evaluated the use of Qutenza in patients with PDN. PDN represents a much larger market opportunity than either PHN or HIV-PN, and unless required clinical trials are successfully completed and regulatory approvals are obtained for the use of Qutenza for PDN patients, Qutenza will not be marketable for this indication in the United States and the European Union and possibly in other countries. If this occurs, our long-term ability to succeed may be significantly and negatively impacted. We believe that to market Qutenza in the United States for PDN, we may have to conduct two successful Phase 3 trials and we may be required to perform additional safety studies. In connection with the lower than expected sales of Qutenza for treatment of pain associated with PHN, we are currently not planning to pursue additional studies in PDN or HIV-PN. Although Astellas is pursuing clinical trials in PDN, there can be no assurance that the results of such trials would support label expansion of Qutenza in the European Union or support any efforts to seek label expansion in the United States.
We depend on our key personnel. If we are not able to retain them, our business will suffer.
We are highly dependent on the principal members of our management and commercial and scientific staff. The competition for skilled personnel among biopharmaceutical companies in the San Francisco Bay Area is intense and the employment services of our scientific, management and other executive officers are terminable at-will. We have encountered and may in the future experience turnover in our commercial organization. If we lose one or more of these key employees, our ability to implement and execute our business strategy successfully could be seriously harmed. Replacing key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to develop, gain regulatory approval of and commercialize products successfully. We do not carry key man life insurance on any of our key personnel.
We have incurred operating losses in each year since inception and expect to continue to incur substantial and increasing losses for the foreseeable future.
We have incurred operating and net losses each year since our inception in 1998. Our net loss for the year ended December 31, 2011 was approximately $49.5 million and as of December 31, 2011 we had an accumulated deficit of approximately $306.1 million. We had cash, cash equivalents and short-term investments totaling $34.3 million at December 31, 2011, and for the year ended December 31, 2011, we used cash of approximately $45.1 million in operating activities. We expect to continue to incur losses for several years, as we commercialize Qutenza and continue other research and development activities, including with respect to NGX-1998. If Qutenza or NGX-1998, if approved, do not achieve market acceptance in the United States or the European Union, we will not generate significant product related revenue. We cannot assure you that we will be profitable even if Qutenza is successfully commercialized or if NGX-1998 is successfully developed and commercialized. If we fail to achieve and maintain profitability, or if we are unable to fund our continuing losses, you could lose all or part of your investment.
41
Table of Contents
Risks Related to our Intellectual Property
The commercial success, if any, of Qutenza depends, in part, on the rights we have under certain patents.
The commercial success of NGX-1998 depends in part on a granted patent in the United States and a pending patent application in the European Union. The issued patent in the United States is entitled “Methods and Compositions for Administration of TRPV1 Agonists.” The commercial success, if any, of Qutenza depends, in part, on a patent granted in the United States and device patents granted in Canada, Hong Kong and certain countries of Europe concerning the use of a dermal delivery system for prescription strength capsaicin delivery for the treatment of neuropathic pain. We exclusively license these patents from the University of California. We do not currently own, and do not have rights under this license to any issued patents that cover Qutenza outside Europe, Hong Kong, Canada and the United States. Although we have filed for an extension of the term for one of the patents licensed from the University of California, there can be no assurance that such extension will be granted.
In addition to other patents and patent applications which have been licensed under our agreements with third party manufacturers, including the issued patents and pending applications licensed under our commercial supply agreement for Qutenza, we also license a method patent granted in the United States from the University of California concerning the delivery of prescription strength capsaicin for the treatment of neuropathic pain. Two of the three inventors named in the method patent did not assign their patent rights to the University of California. These two inventors asserted a claim of inventorship rights to the device patent. In late 2010, we entered into negotiations with these inventors to settle any and all claims and to have them assign their rights related to the method patent and any other related patent rights that may exist at the time we enter into a final binding settlement agreement. We anticipate that any settlement will include us making a cash payment and us issuing stock or stock options to them. The absence of exclusive rights to utilize such patent exposes us to a greater risk of direct competition and could materially harm our business.
If we are unable to maintain and enforce our proprietary rights, we may not be able to compete effectively or operate profitably.
Our commercial success will depend, in part, on obtaining and maintaining patent protection, trade secret protection and regulatory protection (such as Hatch-Waxman protection or orphan drug designation) of our proprietary technology and information as well as successfully defending against third-party challenges to our proprietary technology and information. We will be able to protect our proprietary technology and information from use by third parties only to the extent that valid and enforceable patents, trade secrets or regulatory protection cover them and we have exclusive rights to utilize them.
Our commercial success will continue to depend in part on the patent rights we own, the patent rights we have licensed, the patent rights of our collaborators and suppliers and the patent rights we may obtain related to future products we may market. Our success also depends on our and our licensors’, collaborators’ and suppliers’ ability to maintain these patent rights against third-party challenges to their validity, scope or enforceability. Further, we do not fully control the patent prosecution of our licensed patent applications. There is a risk that our licensors will not devote the same resources or attention to the prosecution of the licensed patent applications as we would if we controlled the prosecution of the patent applications, and the resulting patent protection, if any, may not be as strong or comprehensive as if we had prosecuted the applications ourselves.
The patent positions of biopharmaceutical companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies’ patents has emerged to date in the United States. The patent situation outside the United States is even more uncertain. Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property.
42
Table of Contents
Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. For example:
| • | we or our licensors might not have been the first to make the inventions covered by each of our pending patent applications and issued patents; |
| • | we or our licensors might not have been the first to file patent applications for these inventions; |
| • | others may independently develop similar or alternative technologies or duplicate any of our technologies; |
| • | it is possible that none of our pending patent applications or the pending patent applications of our licensors will result in issued patents; |
| • | our issued patents and the issued patents of our licensors may not provide a basis for commercially viable products, or may not provide us with any competitive advantages, or may be challenged and invalidated by third parties; |
| • | we may not develop additional proprietary technologies or product candidates that are patentable; or |
| • | the patents of others may have an adverse effect on our business. |
We also rely on trade secrets to protect our technology, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. While we seek to protect confidential information, in part, by confidentiality agreements with our employees, consultants, contractors, or scientific and other advisors, they may unintentionally or willfully disclose our information to competitors. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets.
If we are not able to defend the patent or trade secret protection position of our technologies and product candidates, then we will not be able to exclude competitors from developing or marketing competing products, and we may not generate enough revenue from product sales to justify the cost of development of our product candidates and to achieve or maintain profitability.
If we are sued for infringing intellectual property rights of other parties, such litigation will be costly and time consuming, and an unfavorable outcome would have a significant adverse effect on our business.
Although we believe that we would have valid defenses to allegations that our current product candidates, production methods and other activities infringe the valid and enforceable intellectual property rights of any third parties of which we are aware, we cannot be certain that a third party will not challenge our position in the future. Other parties may own patent or other intellectual property rights that might be infringed by our products, trademarks or activities. There has been, and we believe that there will continue to be, significant litigation and demands for licenses in our industry regarding patent and other intellectual property rights. Our competitors or other patent or intellectual property holders may assert that our products or trademarks and the methods we employ are covered by their intellectual property rights. For example, in 2009 we received a letter indicating a possible trademark conflict for Qutenza from a company claiming that our trademark was similar to theirs. While we believe that these assertions do not have merit and that we have defenses to them, there can be no assurance that we will be successful in defending our trademark. We intend to continue to use the Qutenza trademark and to vigorously defend our use of such trademark against such assertions. These parties could bring claims against us that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages or possibly prevent us from commercializing our product candidates using the Qutenza trademark which would have a significant negative effect on the Qutenza launch and global commercialization efforts. Further, if a patent infringement suit were brought against us, we could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit, or if a trademark infringement suit were brought against us or our collaboration partner Astellas, we or Astellas could be forced to cease using the name Qutenza in connection with our product.
43
Table of Contents
As a result of intellectual property infringement claims, or in order to avoid potential claims, we may choose to seek, or be required to seek, a license from the third party and would most likely be required to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if we were able to obtain a license, the rights may be non-exclusive, which would give our competitors access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations if, as a result of actual or threatened infringement claims, we or our collaborators are unable to enter into licenses on acceptable terms. This could harm our business significantly.
We may incur substantial costs as a result of litigation or other proceedings relating to patent and other intellectual property rights.
The cost to us of any litigation or other proceedings relating to intellectual property rights, even if resolved in our favor, could be substantial. Some of our potential competitors, other patent or intellectual property holders may be better able to sustain the costs of complex patent litigation because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to continue our operations. Should third parties file patent applications, or be issued patents claiming technology also claimed by us in pending applications, we may be required to participate in interference proceedings in the U.S. Patent and Trademark Office to determine priority of invention which could result in substantial costs to us or an adverse decision as to the priority of our inventions. An unfavorable outcome in an interference proceeding could require us to cease using the technology or to license rights from prevailing third parties. There is no guarantee that any prevailing party would offer us a license or that we could acquire any license made available to us on commercially acceptable terms.
If we fail to comply with our obligations in the agreements under which we license development or commercialization rights to products or technology from third parties, we could lose license rights that are important to our business.
We are a party to a number of technology licenses that are important to our business and expect to enter into additional licenses in the future. For example, we hold licenses from The University of California and LTS under patents and patent applications relating to Qutenza. These licenses impose various commercialization, milestone payment, royalty, insurance and other obligations on us. If we fail to comply with these obligations, the licensor may have the right to terminate the license, in which event we would not be able to market products covered by the license, including Qutenza.
We may be subject to damages resulting from claims that our employees or we have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize certain potential products, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
44
Table of Contents
Risks Related to an Investment in our Common Stock
We expect that our stock price will fluctuate significantly, and you may not be able to resell your shares at or above your investment price.
The stock market has experienced significant volatility, particularly with respect to pharmaceutical, biotechnology and other life sciences company stocks. The volatility of pharmaceutical, biotechnology and other life sciences company stocks often does not relate to the operating performance of the companies represented by the stock. Factors that could cause volatility in the market price of our common stock include, but are not limited to:
| • | failure to meet market expectations with respect to the launch of Qutenza by us in the United States and by Astellas in the European Union; |
| • | inability of us and Astellas to successfully commercialize Qutenza in the United States and the Licensed Territory, respectively; |
| • | results from and any delays related to the clinical trials for NGX-1998; |
| • | our ability to develop and market new and enhanced product candidates on a timely basis; |
| • | failure to maintain our listing on the NASDAQ Global Market or NASDAQ Capital Market; |
| • | potential inability to preserve or raise sufficient capital to maintain our operations; |
| • | potential negative stock market reaction in the event we raise or attempt to raise additional equity capital; |
| • | failure to meet revenue estimates and revenue growth rates; |
| • | actual or anticipated quarterly variations in our results of operations or those of our collaborators or competitors; |
| • | third-party healthcare reimbursement policies or determinations; |
| • | product recalls, reported incidents of unanticipated adverse effects with respected to Qutenza, or other negative publicity related to Qutenza; |
| • | general economic conditions and slow or negative growth of our expected markets; |
| • | delay in entering, or termination of, strategic partnership relationships; |
| • | our ability to meet our obligation under the Financing Agreement with Cowen including liquidity maintenance requirements; |
| • | our ability to meet our obligation under the loan from Hercules; |
| • | failure or delays in entering additional product candidates into clinical trials; |
| • | announcements by us or our collaborators or competitors of new commercial products, clinical progress or the lack thereof, significant contracts, commercial relationships or capital commitments; |
| • | issuance of new or changed securities analysts’ reports or recommendations for our stock or the discontinuation of one or more securities analysts’ research coverage for our stock; |
| • | inclusion in or removal from stock indices such as the Russell 3000; |
| • | developments or disputes concerning our intellectual property or other proprietary rights; |
| • | commencement of, or our involvement in, litigation; |
| • | changes in governmental statutes or regulations or in the status of our regulatory approvals; |
| • | market conditions in the life sciences sector; and |
| • | any major change in our board or management. |
45
Table of Contents
If we fail to meet the requirements for continued listing on the NASDAQ Global Market and do not meet initial listing requirements to transfer to the NASDAQ Capital Market, our common stock could be delisted from trading, which would adversely affect the liquidity of our common stock and our ability to raise additional capital.
Our common stock is currently listed on the NASDAQ Global Market and to maintain our listing, we must meet certain financial requirements in accordance with the rules of Nasdaq. On October 18, 2011, we received a notification from Nasdaq that we failed to maintain the required minimum market value of listed securities level of $50.0 million for a 30 consecutive business day period. We have until April 16, 2012 in which to cure this non-compliance by meeting such requirement for a minimum of ten consecutive business days. On January 27, 2012, we received a notification from Nasdaq that we failed to maintain the required $1.00 minimum bid price for a 30 consecutive business day period. We have until July 28, 2012 in which to cure this non-compliance by meeting such requirement for a minimum of ten consecutive business days. There can be no assurance that we will be able to cure these violations by the end of their respective cure periods, be able to transfer to the NASDAQ Capital Market or maintain our listing on either market in the future. Any potential delisting of our common stock would adversely affect the liquidity of our common stock and our ability to raise additional capital.
If the ownership of our common stock continues to be highly concentrated, it may prevent you and other stockholders from influencing significant corporate decisions and may result in conflicts of interest that could cause our stock price to decline.
Our executive officers, directors and their affiliates and 5% stockholders beneficially own or control approximately 79% of the outstanding shares of our common stock as of February 29, 2012 (after giving effect to the exercise of all of their outstanding vested options and warrants exercisable within 60 days of such date). Accordingly, these executive officers, directors and their affiliates and significant stockholders acting as a group, will have substantial influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets or any other significant corporate transactions. These stockholders may also delay or prevent a change of control of us, even if such a change of control would benefit our other stockholders. The significant concentration of stock ownership may adversely affect the trading price of our common stock due to investors’ perception that conflicts of interest may exist or arise.
Future sales of shares by existing stockholders could cause our stock price to decline.
The market price of our common stock could decline as a result of sales by our existing stockholders of shares of common stock in the market, or the perception that these sales could occur. In particular, the significant concentration of stock ownership may adversely affect the trading price of our common stock in the event that certain large stockholders elect to sell the shares of our common stock held by them. These sales might also make it more difficult for us to sell equity securities at a time and price that we deem appropriate.
We will continue to incur increased costs and demands upon management as a result of complying with the laws and regulations affecting public companies, which could affect our operating results.
As a public company, we will continue to incur significant legal, accounting and other expenses that we did not incur as a private company, including costs associated with public company reporting requirements. We also have incurred and will incur costs associated with corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002, the Dodd-Frank Act of 2010, as well as new rules implemented by the SEC and the NASDAQ Stock Market LLC. We expect these rules and regulations to increase our legal and financial compliance costs and to make some activities more time-consuming and costly.
46
Table of Contents
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our certificate of incorporation and bylaws may delay or prevent an acquisition of us or a change in our management. These provisions include a classified board of directors, a prohibition on actions by written consent of our stockholders and the ability of our board of directors to issue preferred stock without stockholder approval. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits stockholders owning in excess of 15% of our outstanding voting stock from merging or combining with us. Although we believe these provisions collectively provide for an opportunity to receive higher bids by requiring potential acquirers to negotiate with our board of directors, they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management.
Events in the credit markets have, and will continue to, impact our investment returns.
As a result of events in the credit markets, we maintain an investment portfolio of primarily U.S. Treasury securities. As a result of the credit crisis and our shift in investments, we anticipate that our investment returns will be below our historic rates of return. These lower returns will likely continue for some time and we cannot predict when market conditions will improve and when higher yielding investment options may be available to us.
We have never paid dividends on our capital stock, and we do not anticipate paying any cash dividends in the foreseeable future.
We have paid no cash dividends on any of our classes of capital stock to date, have contractual restrictions against paying cash dividends and currently intend to retain our future earnings to fund the development and growth of our business. As a result, capital appreciation, if any, of our common stock will be an investor’s sole source of gain for the foreseeable future.
| Item 1B. | Unresolved Staff Comments |
There are no unresolved staff comments regarding any of our periodic or current reports.
| Item 2. | Properties |
We lease approximately 26,386 square feet of space in our headquarters in San Mateo, California under a lease that expires in July 2012. We are currently evaluating alternative office space for purposes of relocation before our lease expires later this year. We believe that our office space requirements may be substantially less than our currently leased facility. We have no laboratory, research or manufacturing facilities.
| Item 3. | Legal Proceedings |
We are subject to various legal disputes that arise in the normal course of business. These include or may include employment matters, contract disputes, intellectual property matters, product liability actions, and other matters. We do not know whether we would prevail in these matters nor can it be assured that a remedy could be reached on commercially viable terms, if at all. Based on currently available information, we do not believe that any of these disputes will have a material adverse impact on our business, results of operations, liquidity or financial position.
47
Table of Contents
| Item 4. | Mine Safety Disclosures |
We are required, if applicable, to provide a statement that the information concerning mine safety violations or other regulatory matters required by Section 1503(a) of the Dodd-Frank Wall Street Reform and Consumer Protection Act and Item 104 of Regulation S-K (17 CFR 229.104) is included in exhibit 95 to the annual report. This item is not applicable.
S-K (17 CFR 229.104) is included in exhibit 95 to the annual report. This item is not applicable.
48
Table of Contents
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Our common stock is quoted on the NASDAQ Global Market under the symbol “NGSX.” The following table sets forth the high and low sales price per share of our common stock as reported on the NASDAQ Global Market for the periods indicated.
| Sale Price | ||||||||
| High | Low | |||||||
| Fiscal 2010: |
||||||||
| First Quarter |
$ | 9.99 | $ | 6.88 | ||||
| Second Quarter |
$ | 10.60 | $ | 6.52 | ||||
| Third Quarter |
$ | 7.55 | $ | 5.06 | ||||
| Fourth Quarter |
$ | 7.55 | $ | 5.32 | ||||
| Fiscal 2011: |
||||||||
| First Quarter |
$ | 6.41 | $ | 3.12 | ||||
| Second Quarter |
$ | 4.47 | $ | 1.55 | ||||
| Third Quarter |
$ | 2.85 | $ | 0.75 | ||||
| Fourth Quarter |
$ | 1.44 | $ | 0.66 | ||||
We did not declare or pay any cash dividends on our common stock during our fiscal years ended December 31, 2010 and 2011. We currently expect to retain future earnings, if any, for use in the operation and expansion of our business and do not in the foreseeable future anticipate paying any cash dividends. Any determination to pay dividends in the future will be at the discretion of our Board of Directors and will depend upon our results of operations, financial condition and other factors the Board of Directors, in its discretion, deems relevant. As of February 29, 2012 there were 48 holders of record of our common stock, one of which is Cede & Co., a nominee for Depository Trust Company, or DTC. All of the shares of our common stock held by brokerage firms, banks and other financial institutions as nominees for beneficial owners are deposited into participant accounts at DTC, and are therefore considered to be held of record by Cede & Co. as one stockholder.
The information regarding the securities authorized for issuance under our equity compensation plans is set forth in Item 12 of this Annual Report on Form 10-K.
49
Table of Contents
Comparison of Historical Cumulative Total Return (*) Among NeurogesX, Inc., the NASDAQ Composite Index and the NASDAQ Biotechnology Index
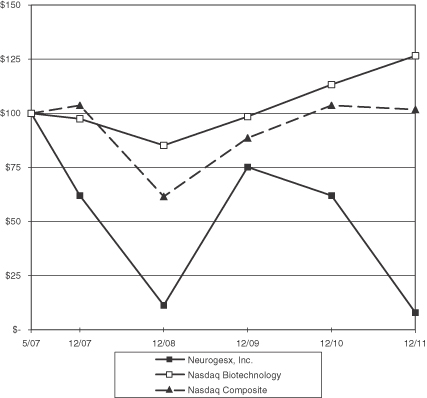
| Cumulative Total Return as of |
||||||||||||||||||||||||
| 5/2/2007 | 12/31/2007 | 12/31/2008 | 12/31/2009 | 12/31/2010 | 12/31/2011 | |||||||||||||||||||
| NeurogesX, Inc. |
100 | 62.05 | 11.41 | 75.22 | 62.05 | 8.00 | ||||||||||||||||||
| NASDAQ Composite Index |
100 | 103.69 | 61.65 | 88.71 | 103.72 | 101.85 | ||||||||||||||||||
| NASDAQ Biotechnology Index |
100 | 97.52 | 85.21 | 98.53 | 113.32 | 126.69 | ||||||||||||||||||
| (*) | The above graph shows the cumulative total stockholder return of an investment of $100 in cash on May 2, 2007, the date our stock began to trade on the NASDAQ Global Market, through December 31, 2011 for: (i) our common stock; (ii) the NASDAQ Composite Index; and (iii) the NASDAQ Biotechnology Index. All values assume reinvestment of the full amount of all dividends. Stockholder returns over the indicated period should not be considered indicative of future stockholder returns. |
The information contained under this caption “Comparison of Historical Cumulative Total Return(*) Among NeurogesX, Inc., the NASDAQ Composite Index and the NASDAQ Biotechnology Index” shall not be deemed to be soliciting material or to be filed with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate it by reference into such filing.
50
Table of Contents
| Item 6. | Selected Financial Data |
The following selected financial data should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and Item 8, “Financial Statements and Supplemental Data” of this Form 10-K.
| Years Ended December 31, | ||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| (in thousands except share and per share data) | ||||||||||||||||||||
| Consolidated Statement of Operations Data: |
||||||||||||||||||||
| Net product revenue |
$ | 2,625 | $ | 688 | $ | — | $ | — | $ | — | ||||||||||
| Collaboration revenue |
8,878 | 7,578 | 2,006 | — | — | |||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Cost of goods sold |
685 | 541 | — | — | — | |||||||||||||||
| Research and development |
13,566 | 11,151 | 11,235 | 16,104 | 25,321 | |||||||||||||||
| Selling, general and administrative |
36,408 | 36,383 | 12,420 | 10,182 | 7,455 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
50,659 | 48,075 | 23,655 | 26,286 | 32,776 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(39,156 | ) | (39,809 | ) | (21,649 | ) | (26,286 | ) | (32,776 | ) | ||||||||||
| Interest (expense) income, net |
(10,326 | ) | (5,315 | ) | (187 | ) | 314 | 499 | ||||||||||||
| Other income (expense), net |
(63 | ) | 608 | (31 | ) | (63 | ) | 321 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
(49,545 | ) | (44,516 | ) | (21,867 | ) | (26,035 | ) | (31,956 | ) | ||||||||||
| Accretion of redeemable convertible preferred stock |
— | — | — | — | (4,626 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss attributable to common stockholders |
$ | (49,545 | ) | $ | (44,516 | ) | $ | (21,867 | ) | $ | (26,035 | ) | $ | (36,582 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted net loss per share attributable to common stockholders(1) |
$ | (2.15 | ) | $ | (2.51 | ) | $ | (1.24 | ) | $ | (1.49 | ) | $ | (4.06 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average number of shares used to compute basic and diluted net loss per share attributable to common stockholders(1) |
23,011,015 | 17,768,881 | 17,610,796 | 17,519,415 | 9,017,627 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| As of December 31, | ||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||
| Cash and cash equivalents and short-term investments |
$ | 34,309 | $ | 46,830 | $ | 50,559 | $ | 24,506 | $ | 52,851 | ||||||||||
| Working capital |
19,058 | 32,615 | 39,286 | 19,109 | 44,139 | |||||||||||||||
| Restricted cash |
411 | 410 | 160 | 200 | 240 | |||||||||||||||
| Total assets |
39,227 | 50,850 | 52,905 | 25,590 | 54,185 | |||||||||||||||
| Long-term obligations—current portion |
4,829 | 2,222 | — | — | — | |||||||||||||||
| Notes payable—non-current portion |
— | — | — | 191 | 3,024 | |||||||||||||||
| Deferred revenue |
33,434 | 40,178 | 46,843 | — | — | |||||||||||||||
| Long-term obligations |
62,746 | 42,622 | — | — | — | |||||||||||||||
| Accumulated deficit |
(306,072 | ) | (256,527 | ) | (212,011 | ) | (190,144 | ) | (164,109 | ) | ||||||||||
| Total stockholders’ (deficit) equity |
(67,860 | ) | (41,291 | ) | 253 | 19,274 | 41,361 | |||||||||||||
| (1) | See Note 2 of the notes to the consolidated financial statements for an explanation of the method used to calculate the net loss per share attributable to common stockholders and the number of shares used in the computation of the per share amounts. |
51
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
This discussion and analysis should be read in conjunction with our financial statements and accompanying notes included elsewhere in this report. Operating results are not necessarily indicative of results that may occur in future periods. Readers are also urged to carefully review and consider the various disclosures made by us which attempt to advise interested parties of the factors which affect our business, including without limitation the disclosures made in Item 1A of Part I of this Annual Report under the caption “Risk Factors.”
Overview
We are a specialty pharmaceutical company focused on developing and commercializing a portfolio of novel non-opioid, pain management therapies to address unmet medical needs and improve patients’ quality of life.
Our first commercial product, Qutenza, became commercially available in the United States and in certain European countries in the first half of 2010. Qutenza is a dermal delivery system designed to topically administer capsaicin to treat certain neuropathic pain conditions and was approved by the FDA in November 2009 for the management of neuropathic pain associated with postherpetic neuralgia, or PHN. Qutenza is also approved in the European Union for the treatment of peripheral neuropathic pain in non-diabetic adults. Qutenza is the first prescription strength capsaicin product approved in the United States.
Our most advanced drug candidate is NGX-1998. NGX-1998 is a topical liquid formulation of high concentration capsaicin that we are developing to treat neuropathic pain conditions. In November 2011, we announced positive top-line results from our Phase 2 clinical study of NGX-1998, in patients with PHN. The 12-week, multicenter, randomized, double-blinded, placebo-controlled clinical trial met its protocol-specified objectives, which include the primary endpoint of a percentage change from baseline as compared to placebo in a patient-reported numeric pain rating scale, or NPRS, score during weeks two through eight of the trial. We believe that the Phase 2 data support moving forward to a NGX-1998 Phase 3 clinical trial. We plan to have an End-of-Phase 2 meeting with the FDA, which we believe could occur in the second half of 2012. We are presently examining a range of potential indications for NGX-1998, including but not limited to PHN, HIV associated peripheral neuropathy, or HIV-PN, painful diabetic neuropathy, or PDN, Chemotherapy-induced peripheral neuropathy, or CIPN, and post-traumatic (e.g. surgical) pain , or PTP. Our decision as to which indications to pursue in Phase 3 clinical trials is expected to be based on a number of criteria including, but not limited to, our assessments of probability of clinical development success, market size and potential regulatory pathways to possible approval.
We believe that NGX-1998 has the potential to represent a significant improvement over Qutenza. To date, the clinical data suggests that NGX-1998 does not require pre-treatment with a topical anesthetic and administration of NGX-1998 for five minutes may provide a similar response to that experienced with Qutenza after a 60-minute application, that is, that a patient may experience meaningful pain relief for up to 12 weeks from that single five minute application. As a result, we believe that NGX-1998 has the potential to be more readily accepted by physicians and may also demonstrate efficacy in a number of neuropathic pain conditions. Additionally, we believe that the liquid formulation represents a more versatile delivery model which is well suited for treating hard to reach places on the body. Further, we believe that our experience in conducting clinical trials with capsaicin and the approval of Qutenza may benefit our efforts to obtain clinical success for NGX-1998. Finally, in April, 2011, NGX-1998 was granted patent protection under a patent entitled “Methods and Compositions for Administration of TRPV1 Agonists.” The allowed claims include method of use, formulation and system claims. The patent, including the Patent Term Adjustment, expires in 2027. In February 2012, we received a notice of allowance from the Japanese Patent Office for a patent that covers NGX-1998 with a set of claims that are similar to those issued in the United States. A similar patent application is also under review in the European Union.
52
Table of Contents
As a result of the opportunity, we believe exists with NGX-1998, in March 2012, we decided to focus our resources on the preparation for advancing NGX-1998 into a Phase 3 clinical trial. To do so, we significantly restructured our operations, including eliminating most of sales and marketing expenditures in support of Qutenza, while continuing to maintain product availability of Qutenza for patients and physicians. While we believe a market opportunity exists for Qutenza, we believe that the resources required to attain that potential are better utilized to speed the development of NGX-1998. We are evaluating a number of alternative commercialization strategies which include the potential to license Qutenza to a commercialization partner in the United States and in other territories of the world outside of the territory addressed by Astellas.
In September 2011, we submitted a supplemental new drug application, or sNDA, to the FDA regarding potentially expanding the Qutenza label to include treatment of pain associated with HIV-PN. On February 9, 2012, the Anesthetic and Analgesic Drug Product Advisory Committee of the FDA Panel voted unanimously against approval of Qutenza in such indication as the committee determined that the data did not represent adequate evidence of efficacy. In March 2012, we received a complete response from the FDA regarding our sNDA. The complete response letter indicated that in order to gain approval for the use of Qutenza for the proposed indication, we would need to submit additional clinical data from at least one adequate and well-controlled Phase 3 clinical trial to support the proposed indication. However, we do not anticipate investing in further clinical trials for Qutenza at this time.
Although we are limiting our investment in Qutenza in the United States, Astellas continues to conduct studies with Qutenza for its Licensed Territory for commercialization of Qutenza which includes the 27 countries of the European Union, Iceland, Norway, and Liechtenstein as well as Switzerland, certain countries in Eastern Europe, the Middle East and Africa, which we refer to as the Licensed Territory. Astellas is currently conducting three clinical trials in response to follow up measures requested by the European Medicines Agency. These studies include a long-term safety study in on-label indications and two studies, a long-term safety study and an efficacy study, in patients with PDN. The results of these studies are not expected until sometime in 2013. However, if the PDN clinical trials are successful, we plan to explore the potential for such trials to support further label expansion opportunity in the United States.
The Astellas Agreement, described below, provides Astellas an option to exclusively license NGX-1998 in the Licensed Territory. If Astellas was to exercise its option, they would collaborate with us on the Phase 3 clinical trials and share equally in certain of Phase 3 development costs that benefit the regulatory process in their territory. We are also exploring the possibility of a strategic alliance for development and commercialization of NGX-1998 in the United States.
In addition to Qutenza and NGX-1998, we have a series of compounds which represent an early stage pipeline of potential product candidates consisting of prodrugs of acetaminophen for potential use in acute pain, including traumatic pain, post-surgical pain and fever. These product candidates are in the pre-clinical stage of development and may or may not be the subject of further development in 2012 or beyond.
To date, we have not generated any significant product revenue and have funded our operations primarily by selling equity securities, establishing debt facilities and through the Astellas Agreement. Our net loss for the fiscal years ended December 31, 2011, 2010 and 2009 was $49.5 million, $44.5 million and $21.9 million, respectively, and as of December 31, 2011 we had an accumulated deficit of $306.1 million. We had cash, cash equivalents and short-term investments totaling $34.3 million at December 31, 2011 and during the year ended December 31, 2011, we used cash of $45.0 million in operating activities. We expect to continue to incur annual operating losses for the foreseeable future as we support the continued availability of Qutenza in the United States and work to complete the NGX-1998 development program.
In April 2010, we borrowed $40.0 million from Cowen Healthcare Royalty Partners, L.P., or Cowen Royalty, and we agreed to repay Cowen Royalty up to $106 million by assigning Cowen Royalty one hundred percent of royalty, milestone, option and other payments that we may receive under the Astellas Agreement until our repayment obligation is satisfied in full.
53
Table of Contents
In August 2011, we entered into a loan agreement with Hercules Technology Growth Capital, Inc., or Hercules, which includes both a $5.0 million accounts receivable line and a $15.0 million term loan, collectively. Under the terms of the loan agreement, the $15.0 million term loan is required to be repaid over the course of the 42-month maturity period, which includes a 12-month interest only period at the beginning of the term. In connection with our entry into the loan agreement, we issued to Hercules a warrant for 791,667 shares of our common stock (representing an aggregate exercise price equal to approximately 7.125% of the maximum aggregate funds potentially available to us under the loan agreement). The warrant has a term of five years, contains a net-exercise provision, and has an exercise price of $1.80 per share. The fair value of the warrants issued was approximately $1.1 million. The loan agreement also contains customary negative covenants and is subject to customary events of default, such as a failure to make a scheduled principal or interest payment, insolvency, and breaches of covenants under loan agreement and agreements and instruments entered into in connection with the loan agreement, including covenants in the Hercules warrant. In March 2012, we entered into an amendment to the Hercules warrant, in which we amended the covenant with respect to the requirement to maintain our listing on the NASDAQ Global Market to allow for the NASDAQ Capital Market and certain over the counter markets to be permissible alternative markets on which we can list our shares of common stock. Under the terms of the amendment, the number of shares underlying the warrant was increased to 1,950,000 and the exercise price per share of common stock was reduced to $0.50.
We received a notice of market capitalization insufficiency from the NASDAQ Global Market on October 18, 2011 as our market value of listed shares had remained below the $50 million minimum listing requirement for more than 30 consecutive business days. Additionally, on January 27, 2012, we received a notice of share price deficiency as the bid price of our shares of common stock had remained below the $1.00 minimum listing requirement for more than 30 consecutive business days. Each of these notices provide a 180 calendar day grace period during which time we may regain compliance with the minimum listing requirement by attaining the applicable minimum requirement for 10 consecutive business days. The grace period to regain compliance with the minimum market value of listed securities requirement expires on April 16, 2012 and the grace period to regain compliance with the minimum bid price requirement expires on July 28, 2012. If we have not regained compliance with the applicable minimum listing requirement on these dates, we will receive a de-listing determination letter with respect to the applicable requirement. Should that occur, we may seek a hearing with NASDAQ, wherein we would be required to provide a plan for regaining the applicable minimum listing requirement and through which we would seek an extension of the grace period during which we would seek to execute such plan. During the pendency of the hearing process, our delisting would be delayed, however, we cannot be assured that we would otherwise be granted any additional time to regain compliance as a result of the hearing process. If we are de-listed from the NASDAQ Global Market, we may have the opportunity to apply for and potentially obtain a listing of our common stock on the NASDAQ Capital Market, subject to our meeting the continued listing requirements of such market. However, we currently do not meet the continued listing requirements for that exchange. If we are ineligible for listing on the NASDAQ Capital Market, we may seek listing on the OTC Bulletin Board or other over-the-counter market. For a description of the risks associated with our potential de-listing from the NASDAQ Global Market, see the Risk Factors section of this Form 10-K.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, expenses and related disclosures. Actual results could differ from those estimates.
While our significant accounting policies are described in more detail in Note 2 of the Notes to Consolidated Financial Statements included elsewhere in this report, we believe the following accounting policies to be critical to the judgments and estimates used in the preparation of our financial statements.
54
Table of Contents
Revenue Recognition
Effective January 1, 2011, we adopted the provisions of Accounting Standards Update, or ASU, 2009-13, Multiple-Deliverable Revenue Arrangements, or ASU 2009-13, which is included within the Codification as Revenue Recognition-Multiple Element Arrangements, on a prospective basis. Under the provisions of ASU 2009-13, we will no longer rely on objective and reliable evidence of the fair value of the elements in a revenue arrangement in order to separate a deliverable into a separate unit of accounting, and the use of the residual method has been eliminated. We will instead use a selling price hierarchy for determining the selling price of a deliverable, which will be used to determine the allocation of consideration to each unit of accounting under an arrangement. The selling price used for each deliverable will be based on vendor-specific objective evidence if available, third-party evidence if vendor-specific objective evidence is not available or estimated selling price if neither vendor-specific objective evidence nor third-party evidence is available. The provisions of ASU 2009-13 will be applied by us to revenue arrangements entered into, or materially modified, beginning January 1, 2011. As of December 31, 2011, we had not applied the provisions of ASU 2009-13 to any of our revenue arrangements as we had not entered into any new, or materially modified any of our existing, revenue arrangements in 2011. Therefore, there was no material impact on our financial position or results of operations from adopting ASU 2009-13. However, the provisions of ASU 2009-13 could have a material impact on the revenue recognized from any collaboration agreements that we enter into, or materially modifies, in future periods.
Product revenue
In April 2010, we made Qutenza commercially available in the United States to our specialty distributor and specialty pharmacy customers. Under the agreements with our customers, the customers take title to the product upon shipment and only have the right to return damaged product, product shipped in error and expired or short-dated product. As Qutenza is new to the marketplace, we are currently assessing the flow of product through our distribution channel, and currently cannot reasonably estimate returns. Until such time as an estimate of returns can be made, we are recognizing Qutenza product revenue, and related product costs, at the later of:
| • | the time the product is shipped by the customer to healthcare professionals; and |
| • | the date of cash collection. |
Healthcare professionals are generally ordering Qutenza in small quantities only after they have identified a patient for treatment. We believe that revenue recognition upon the later of customer shipment to the end user and the date of cash collection is appropriate until we have sufficient data on cash collection and return patterns of our customers. We are performing additional procedures to further analyze shipments made by our customers by utilizing shipping data provided by our customers to determine when product is shipped by the customers to healthcare professionals and are evaluating ending inventory levels at our customers each month to assess the risk of product returns.
We have established terms pursuant to distribution agreements with our specialty distributor customers providing for payment within 120 days of the date of shipment to our specialty distributor customers and 30 days of the date of shipment to our specialty pharmacy customers. Such distribution agreements generally have a term of three years.
The application of the above revenue recognition policy resulted in the recognition of net revenue of $2.6 million from gross shipments of $3.4 million for the year ended December 31, 2011. Our gross shipments are reduced for chargebacks, certain fees paid to distributors and product sales allowances including rebates to qualifying federal and state government programs. At December 31, 2011, net deferred product revenue totaled $1.1 million.
55
Table of Contents
Revenue from the Astellas Agreement
In June 2009, we entered into the Astellas Agreement which provides for an exclusive license by us to Astellas for the promotion, distribution and marketing of Qutenza in the Licensed Territory, an option to license NGX-1998, a product candidate that is a non-patch liquid formulation of capsaicin, in the Licensed Territory, participation on a joint steering committee and, through a related Supply Agreement entered into with Astellas supply of product until direct supply arrangements between Astellas and third-party manufacturers are established, some of which we anticipate to occur in 2012. Revenue under this arrangement includes upfront non-refundable fees and may also include additional option payments for the development of and license to NGX-1998, milestone payments upon achievement of certain product sales levels and royalties on product sales.
We analyze multiple-element arrangements, such as the Astellas Agreement, to determine whether the various performance obligations, or elements, can be separated or whether they must be accounted for as a single unit of accounting. The determination of whether an element can be separated or accounted for as a single unit of accounting is based on the availability of evidence with regard to standalone value of each delivered element. If the fair value of all undelivered performance obligations can be determined, then all obligations would then be accounted for separately as performed. If any delivered element is considered to either: 1) not have standalone value or 2) have standalone value but the fair value of any of the undelivered performance obligations are not determinable, then any delivered elements of the arrangement would be accounted for as a single unit of accounting and the multiple elements would be grouped and recognized as revenue over the longest estimated performance obligation period.
Significant management judgment is required in determining the level of effort required under an arrangement and the period over which the performance obligations are estimated to be completed. In addition, if we are involved in a joint steering committee as part of a multiple-element arrangement that is accounted for as a single unit of accounting, an assessment is made as to whether the involvement in the steering committee constitutes a performance obligation or a right to participate.
Revenue recognition of non-refundable upfront license fees commences when there is a contractual right to receive such payment, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and there are no further performance obligations under the license agreement.
We view the Astellas Agreement and related agreements, as a multiple-element arrangement with the key deliverables consisting of an exclusive license to Qutenza in the Licensed Territory, an obligation to conduct certain development activities associated with NGX-1998, participation on a joint steering committee and supply of Qutenza components to Astellas until such time that Astellas can establish a direct supply relationship with product vendors. Because not all of these elements have both standalone value and objective and reliable evidence of fair value, we are accounting for such elements as a single unit of accounting. Further, the agreement provides for our mandatory participation in a joint steering committee, which we believe represents a substantive performance obligation and also the estimated last delivered element under the Astellas Agreement and related agreements. Therefore, we are recognizing revenue associated with upfront payments and license fees ratably over the term of the joint steering committee performance obligation. We estimate this performance obligation will be delivered ratably through June 2016.
At the inception of the Astellas Agreement, the upfront license fees were subject to a refund right, which expired upon transfer of our MA for Qutenza in the European Union to Astellas. In September 2009, the transfer of the MA to Astellas was completed and, therefore, the upfront license fees became non-refundable and revenue recognition of the upfront license fees commenced.
In the accompanying financial statements, collaboration revenue represents revenue associated with the Astellas Agreement and related agreements. Deferred collaboration revenue arises from the excess of cash received or receivable over cumulative revenue recognized for the period.
56
Table of Contents
Under the terms of the Astellas Agreement, we earn royalties based on each sale of Qutenza into the Licensed Territory. The royalty rate is tiered based on the level of sales achieved. These royalties are considered contingent consideration as we have no remaining contract performance obligation and, therefore, the royalties are excluded from the single unit of accounting discussed above. We report royalty revenue based upon Astellas’ reported net sales of Qutenza in the Licensed Territory. We recognize royalty revenue from Astellas on a quarter lag basis due to the timing of Astellas reporting such royalties to us.
Effective January 1, 2011, we also adopted the provisions of ASU 2010-17, Milestone Method of Revenue Recognition, or ASU 2010-17, which is included within the Codification as Revenue Recognition-Milestone Method. The provisions of ASU 2010-17 are being applied on a prospective basis for milestones achieved starting in 2011. The adoption of ASU 2010-17 did not have a material impact on our financial position or results of operations in the year ended December 31, 2011, and the provisions of ASU 2010-17 are not expected to have a material impact on the revenue recognized from any existing collaboration agreements.
For events for which the occurrences are contingent solely upon the passage of time or are the result of the collaborative partner’s performance, the contingent payments will not be accounted for in accordance with the provisions of ASU 2010-17 and will be recognized as revenue when payments are earned, the amounts are fixed or determinable and collectability is reasonably assured.
Additionally, we can earn contingent event-based payments from Astellas if they achieve certain sales levels as outlined in the Astellas Agreement. As these payments are contingent on Astellas’ performance, they are excluded from the single unit of accounting discussed above. The contingent event-based payments will be recorded as revenue when earned as we have no remaining contract performance obligation.
We recognize revenue net of related costs for collaboration supplies sold to Astellas upon the product being delivered to Astellas. The revenue and related costs of the product supplied to Astellas are included in collaboration revenue. Prior to delivery to Astellas, the costs accumulated for the manufacturing of the collaboration supplies are included as Prepaid expenses and other current assets on our consolidated balance sheets.
Stock-Based Compensation
We account for our stock-based compensation in accordance with the fair value recognition provisions of current authoritative guidance. Under the authoritative guidance, stock-based awards, including stock options, are recorded at fair value as of the grant date and recognized to expense over the employee’s requisite service period (generally the vesting period), which we have elected to amortize on a straight-line basis. Because non-cash stock-based compensation expense is based on awards ultimately expected to vest, it has been reduced by an estimate for future forfeitures. We estimate forfeitures at the time of grant and revise our estimate, if necessary, in subsequent periods.
We estimate the fair value of options granted using the Black-Scholes option valuation model and the assumptions used shown in Note 11 to the Consolidated Financial Statements in Item 8 of this Annual Report on Form 10-K. Significant judgment is required on the part of management in determining the proper assumptions used in this model. The assumptions used in the Black-Scholes option valuation model include the risk free interest rate, expected term, expected volatility and expected dividend yield. We base our assumptions on historical data when available or when not available, on a peer group of companies. However, these assumptions consist of estimates of future market conditions, which are inherently uncertain, and therefore subject to management’s judgment. See Note 11 of Notes to Consolidated Financial Statements included elsewhere in this report for further detail.
57
Table of Contents
Inventories
Inventories are valued at the lower of cost or market with cost determined under the specific identification method. Inventories consist of raw materials, work-in-process and finished goods. Raw materials include certain starting materials used in the production of the active pharmaceutical ingredient of Qutenza. Work-in-process includes the bulk inventory of the active pharmaceutical ingredient, patches and cleansing gel of Qutenza that are in the process of being manufactured or have completed manufacture and are awaiting final packaging, including related internal labor and overhead costs. Finished goods include the final packaged kits that contain the Qutenza patch and cleansing gel and that are ready for commercial sale. Qutenza received FDA approval for sale in the United States during the fourth quarter of 2009; costs incurred prior to FDA approval were recorded as research and development expense in our consolidated statements of operations. On a quarterly basis, we analyze our inventory levels and write down inventory that has become obsolete, inventory that has a cost basis in excess of the expected net realizable value and inventory that is in excess of expected requirements based upon anticipated product revenues. During the year ended December 31, 2011, we recorded a charge to cost of goods sold of $0.4 million for Qutenza patches which we do not expect to sell prior to their expiration date. Additionally, as a result of a corporate restructuring announced on March 8, 2012, we expect to take an additional charge that we estimate will be in the range of approximately $0.5 million to $0.6 million during the first quarter of 2012. This represents our estimate of inventory on hand that is in excess of our expected requirements based upon revised estimates of anticipated product revenues, mainly resulting from changes to our planned commercialization strategy.
Clinical Trials
We accrue and expense costs for clinical trial activities performed by third parties, including clinical research organizations and clinical investigators, based upon estimates made, as of the reporting date, of the work completed through the reporting date as a percentage of the total work contracted for under our agreements with such third parties. We make our estimates at the end of each reporting period after discussion with internal personnel and outside service providers and thorough evaluation as to the progress or stage of completion of trials or services. On a periodic basis we adjust our estimated clinical trial costs to reflect actual expenses incurred to date. Due to the nature of the estimation of clinical trial costs, we may be required to make changes to our estimates in the future as we become aware of additional information about the status or conduct of clinical trials.
Long-term Obligations
On April 30, 2010, the Company entered into a Financing Agreement, or the Financing Agreement, with Cowen Royalty. Under the terms of the Financing Agreement, we borrowed $40 million from Cowen Royalty, or the Borrowed Amount, and we agreed to repay such Borrowed Amount together with a return to Cowen Royalty, out of royalty, milestone, option and certain other payments, collectively, the Revenue Interest, that we may receive under the Astellas Agreement.
The accounting for the Financing Arrangement requires us to make certain estimates and assumptions, including the timing and extent of royalty, milestone, option and other payments to be received from Astellas and an internal rate of return we are to pay to Cowen. Changes in the estimated Revenue Interest payments can result in changes in our classification of the current and long-term portions of the amounts payable pursuant to the Financing Agreement, as well as the internal rate of return paid to Cowen.
In August 2011, we entered into a loan agreement with Hercules which includes both a $5.0 million accounts receivable line and a $15.0 million term loan. Under the terms of the loan agreement, the $15.0 million term loan is required to be repaid over the course of the 42-month maturity period, which includes a 12-month interest only period at the beginning of the term. Principal payments are scheduled to commence in September, 2012. Interest on the term loan has a minimum rate of 9.5%, which can increase based on fluctuations in the prime rate. The $5.0 million accounts receivable line is a revolving line that is available until December 31, 2012
58
Table of Contents
and then is renewable by Hercules each year thereafter. Under the terms of the loan agreement with Hercules and the warrant as originally issued on August 5, 2011, if our shares of common stock were delisted from the NASDAQ Global Market, as may occur pursuant to the market value of listed shares and bid price delinquency notices we received from NASDAQ, such an event would trigger an event under the loan agreement. As a result, in March 2012, we entered into an amendment to the Hercules warrant, in which we amended the covenant with respect to the requirement to maintain our listing on the NASDAQ Global Market to allow for the NASDAQ Capital Market and certain over the counter markets to be permissible alternative markets on which we can list our shares of common stock. Under the terms of the amendment, the number of shares underlying the warrant was increased to 1,950,000 and the exercise price per share of common stock was reduced to $0.50.
We are also required to make assumptions for the calculation of the estimated fair market value of the Long-term obligation disclosed in the footnotes to our financial statements. The key assumptions required for the estimated fair market value calculation are estimates of the amounts and timing of the future royalty revenues and other payments to be received from Astellas, the amount and timing of payments to Cowen Royalty to repay the Borrowed Amount and an estimated cost of capital.
The estimate of the future royalty revenues and other payments to be received from Astellas is subject to significant variability due to the recent product launch and regulatory process still to be conducted for significant portions of the Astellas territory and thus are subject to significant uncertainty (see further discussion of this Long-term obligations under Liquidity and Capital Resources).
Results of Operations
In November 2009, we received marketing approval from the FDA for Qutenza for the management of neuropathic pain associated with PHN. In April, 2010, we launched Qutenza in the United States. During 2011, we were marketing and selling Qutenza in the United States through our own specialty sales force. We have established a distribution network to allow health care providers to access Qutenza through specialty distributors and specialty pharmacies. Our marketing efforts were focused on high volume prescribing physicians who treat PHN patients in private practices, hospitals, military treatment facilities and veterans associations. Our approach required considerable time and effort to educate and train physicians and their staff prior to their initial Qutenza treatments. In March 2012, we revised our commercial strategy to, in part, bring our investment in commercial operations, including sales and marketing, in line with our near-term expected revenue. As a result, we eliminated our sales force and are currently evaluating alternative promotional models and potential commercial partnership for Qutenza in the United States.
We commenced recording collaboration revenue for the upfront payments from Astellas in 2009, when the upfront payment of $48.8 million received from Astellas became nonrefundable as a result of our transfer of the Qutenza Marketing Authorization. We have $32.4 million remaining in deferred collaborative revenue which will be recognized on a straight-line basis over the remaining service period of the joint steering committee, which we estimate will expire in June 2016. Under our Supply Agreement with Astellas, we are obligated to supply product at a contractual price until such time as Astellas establishes its own supply relationship.
As of December 31, 2011, Astellas has made Qutenza commercially available in 21 European countries and Astellas has informed us that it is continuing its focus on intensive site training on a country by country basis. We believe that Astellas plans to expand Qutenza availability in the European Union and seek regulatory approvals in certain countries in the remainder of the Licensed Territory in 2012.
Cost of goods sold includes the cost of products sold, costs associated with services provided by our third-party logistics partner for managing the shipping, billing and other administrative functions associated with the distribution channel and also includes royalty obligations due to our intellectual property licensors, UC and LTS. We recognize costs associated with our third-party logistics provider in the period in which those costs are incurred, however, costs to manufacture product and royalty obligations due to third parties are deferred and recognized in the period in which the associated revenue is recognized. Costs associated with the manufacture of Qutenza prior to regulatory approval were previously expensed in the period in which those costs were incurred. We believe that the
59
Table of Contents
effect on cost of goods sold of previously expensed materials prior to approval is immaterial as compared to what cost of goods sold would have been if such costs were expensed in the period that the product was sold.
Our research and development expenses consist of internal and external costs. Our internal costs are primarily employee salaries and benefits, contract employee expense, non-cash stock-based compensation expense, allocated facility and other overhead costs. Our external costs are primarily expenses related to the development of product candidates including formulation development, manufacturing process development, non-clinical studies, clinical trial costs, such as the cost of clinical research organizations and clinical investigators, milestone payments to our licensors in connection with the use of certain intellectual property, and costs associated with preparation and filing of regulatory submissions. Additionally, external and internal costs related to manufacturing and quality assurance activities for production batches of our active pharmaceutical ingredient, patches and cleansing gel, which are destined for commercial sale in the United States, were recorded to research and development expense if purchase of such components occurred prior to FDA approval.
We commence tracking the separate, external costs of a project when we determine that a project has a reasonable chance of entering clinical development. Senior management discusses the possibility of entering clinical development, along with the possibility of meeting other regulatory milestones, throughout the development cycle. These discussions include consideration of the costs and time required to bring the project to market and potential market acceptance for a product from the project, if one receives marketing approval. We do not maintain and evaluate research and development costs by specific therapeutic indications within a program due to the difficulties in allocating such costs to specific indications. We use our internal research and development resources across several projects and some of their efforts may not be attributable to specific projects or indications. Beginning in 2011, we allocate internal resources to specific development programs.
In the years ended December 31, 2011, 2010 and 2009, our research and development costs totaled $13.6 million, $11.2 million and $11.2 million, respectively. The following table reflects the distribution of our research and development costs by development program (in thousands):
| Year ended December 31, |
Qutenza | NGX-1998 | Acetaminophen Prodrug and Opioid Prodrug |
Internal costs | Total | |||||||||||||||
| 2011 |
$ | 4,867 | $ | 7,437 | $ | 65 | $ | 1,197 | $ | 13,566 | ||||||||||
| 2010 |
1,159 | 2,757 | 199 | 7,036 | 11,151 | |||||||||||||||
| 2009 |
3,634 | 133 | 6 | 7,462 | 11,235 | |||||||||||||||
The process of conducting pre-clinical testing and clinical trials necessary to obtain marketing approvals in the United States and other regions is costly and time consuming. The probability of success for each product candidate and clinical trial may be affected by a variety of factors, including, among others, patient enrollment, manufacturing capabilities, successful clinical results, funding, and competitive and commercial viability. As a result of these and other factors, we are unable to determine the duration and completion costs of current or future clinical stages of our product candidates or when or to what extent we will generate revenues from commercialization and sale of any of our unapproved product candidates. In November 2011, we completed a Phase 2 clinical study of NGX-1998 in patients with PHN. NGX-1998 is our most advanced product candidate and we expect that advancing its development program will be the primary focus for us for at least the next year, as we prepare to initiate Phase 3 development as early as late 2012. Costs to complete development of NGX-1998 could be significant. Pursuant to the Astellas Agreement, we might receive future option payments related to this program at Astellas’ election; however, we are obligated to pay any such amounts to Cowen Royalty under the terms of the Financing Agreement. Further, if Astellas exercises its option to license NGX-1998, Astellas would be responsible for 50% of the costs incurred to support approval in both the European and US markets. Additionally, we are currently evaluating the potential for development and commercial partnership for NGX-1998 in the United States and other markets of the World. If we do not obtain regulatory approval for NGX-1998 or our other product candidates, or for other indications for Qutenza, our ability to achieve additional revenue, and therefore our operating results and financial position, will be adversely affected.
60
Table of Contents
Our overall research and development expenses, excluding non-cash stock-based compensation expense increased in 2011 as compared to 2010. We expect that it will continue to increase during 2012 as we expand development activities for NGX-1998, including the on-going Phase 2 clinical study and initiate activities with the intent of filing a sNDA for Qutenza’s label expansion to cover patients with HIV-AN.
Our selling, general and administrative expenses consist primarily of salaries and benefits, including those of our sales, marketing, commercial operations and administrative functions such as finance, human resources, legal, medical affairs and information technology. Additionally, selling, general and administrative expenses include marketing expenses, commercialization costs, pharmacovigilance costs and costs associated with our status as a public company and patent costs. In addition, selling, general and administrative expenses include allocated facility, basic operational and support costs and insurance costs. As a result of the organizational restructuring that was announced on March 8, 2012, we anticipate that our selling, general and administrative expenses will decrease significantly in 2012 from 2011 levels and will remain lower for the foreseeable future. The lower expenses are due to our decision to curtail our investment in the commercialization of Qutenza in order to focus our corporate resources on the advancement of NGX-1998 into its Phase 3 development program.
Comparison of Years Ended December 31, 2011 and 2010
| Year Ended December 31, | Increase (Decrease) |
% Increase (Decrease) |
||||||||||||||
| 2011 | 2010 | |||||||||||||||
| (in thousands, except percentages) | ||||||||||||||||
| Product revenue |
$ | 2,625 | $ | 688 | $ | 1,937 | 282 | % | ||||||||
| Collaboration revenue |
8,878 | 7,578 | 1,300 | 17 | % | |||||||||||
| Cost of goods sold |
(685 | ) | (541 | ) | 144 | 27 | % | |||||||||
| Research and development expenses |
(13,566 | ) | (11,151 | ) | 2,415 | 22 | % | |||||||||
| Selling, general and administrative expenses |
(36,408 | ) | (36,383 | ) | 25 | — | % | |||||||||
| Interest income |
79 | 98 | (19 | ) | (19 | %) | ||||||||||
| Interest expense |
(10,405 | ) | (5,413 | ) | 4,992 | 92 | % | |||||||||
| Other income (expense), net |
(63 | ) | 608 | (671 | ) | (110 | %) | |||||||||
Product Revenue. Due to the limited history of product returns and cash collections from our customers, (specialty distributors and specialty pharmacies), we recognize revenue for Qutenza at the later of the time our customers ship product to healthcare professionals and cash collections are received by us from our customers. We recorded gross shipments of Qutenza to our customers totaling $3.4 million in 2011. Based on our revenue recognition methodology, we recognized net revenue of $2.6 million as Qutenza revenue in 2011. We have deferred product revenue of $1.1 million at December 31, 2011, which is reported net of associated cost of goods sold of $0.1 million in our consolidated balance sheet at December 31, 2011. Our expectation of future U.S. Qutenza product revenue is that it may decline over the coming months as a result of our decision to curtail our investment in marketing and sales activity.
Collaboration Revenue. Collaboration revenue increased $1.3 million, or 17%, to $8.9 million for the year ended December 31, 2011, compared to $7.6 million for the year ended December 31, 2010. This increase was attributable to higher royalties earned from Astellas of $0.8 million and $0.5 million more in collaboration revenue under our Supply Agreement with Astellas.
Cost of goods sold. Cost of goods sold totaled $0.7 million for the year ended December 31, 2011. Cost of goods sold includes $0.3 million for the cost of the finished goods sold that were recognized as revenue in 2011 and a charge of $0.4 million for Qutenza patches which we do not expect to sell prior to their expiration date. The cost of the finished goods sold includes fixed costs of $0.2 million and variable costs of $0.1 million. Our expectation of future U.S. Qutenza product revenue is that it may decline over the coming months as a result of our decision to curtail our investment in marketing and sales activity. As a result, gross margins will likely
61
Table of Contents
decrease as the fixed costs component of cost of goods sold has a more significant impact on overall gross margins. Cost of goods sold for 2010 includes $0.1 million for the cost of the finished goods sold in that year and a charge of $0.4 million for excess Qutenza patches.
Research and Development expenses. Research and development expenses were $13.6 million for the year ended December 31, 2011, an increase of $2.4 million or 22% compared to $11.2 million in 2010. The increase was primarily related to a $1.3 million increase in costs for our Phase 2 clinical trial of NGX-1998. We completed enrollment and dosing for this clinical trial in the second quarter of 2011 and completed the study in November 2011, although residual costs from this study will continue to be expensed into the first quarter of 2012. Also contributing to the increased costs were $0.4 million of higher formulation and applicator development costs for NGX-1998. We incurred $0.6 million higher consulting and contractor costs in 2011 related to the sNDA for a possible label expansion for Qutenza for patients with HIV-PN. We submitted the sNDA for the HIV indication in September 2011. We also incurred higher employee-related costs totaling $0.3 million compared to 2010. We expect that research and development expenses will be lower in 2012, as most of our activities will be conducted by our internal personnel and will be focused on preparing for the NGX-1998 Phase 3 development program with a goal of potentially initiating that program by late 2012.
Selling, General and Administrative expenses. Selling, general and administrative expenses were $36.4 million for each of the years ended December 31, 2011 and 2010. Similar to 2010, we spent significant resources to promote Qutenza in 2011. However, our marketing related expenditures have transitioned as we progressed through our launch. In 2011, we focused on gathering and analyzing market data and designing and delivering programs to promote patient awareness, whereas, in 2010, a significant component of our costs related to initial launch activities, including marketing materials development and marketing events. The shift in focus has resulted in a cost reduction of $2.8 million from the prior year. In addition, our general corporate matters and patent legal costs have decreased by $1.0 million in 2011 compared to 2010. In 2010, we accrued $1.0 million as an estimated settlement cost. In 2011, we reduced that accrual by $0.5 million based on the final settlement terms and revised valuation of the non-cash stock compensation component of that settlement. Offsetting these decreases were higher employee-related costs in our field sales and marketing organization totaling $1.5 million. In October 2011, we enacted a restructuring plan and reduced our workforce, including reducing the covered sales territories from 40 to 25. Travel costs were $0.5 million higher in the 2011 than 2010 as the sales team traveled for a full calendar year compared to an abbreviated post-launch period in 2010. General and administrative employee costs increased $0.9 million compared to 2010 due to additional headcount. In 2011, we also incurred $0.6 million of cost related to the retirement of our former chief executive. In March 2012, we announced a corporate reorganization, which included a substantial reduction in our commercial investment in support of Qutenza to better align our investment in commercial operations to near-term revenue potential, while continuing to ensure Qutenza availability for PHN patients and their physicians. As a result, selling, general and administrative expenses are expected to be significantly lower in 2012 than 2011 levels.
Interest expense. Interest expense was $10.4 million for the year ended December 31, 2011 as compared to $5.4 million in 2010. This increase was primarily due to interest on the $40 million long-term obligation related to the Financing Agreement entered into with Cowen Royalty in April 2010 and the loan agreement entered into with Hercules in August 2011. Interest expense on the Financing Agreement, continues to accrue at 19%. If Cowen Royalty has not received Revenue Interest payments totaling at least $40 million by the maturity date, we will be obligated to pay to Cowen Royalty the difference between such amount and the Revenue Interests paid to Cowen Royalty under the Financing Agreement up to such date. This represents our minimum obligation to Cowen. However, the facility is repaid only through proceeds received from the Astellas Agreement and thus the effect of this increasing interest charge is expected to have no effect on near term net cash flows. In accordance with our agreement with Cowen, monies received from Astellas for such things as royalties and milestones are paid to Cowen in satisfaction of this debt obligation but, except for an event of default. At maturity of the Agreement in April 2020, if amounts received from Astellas have not, in total returned to Cowen $40 million, then we will be required to pay the difference between amounts already paid to Cowen and $40 million.
62
Table of Contents
Other income (expense), net. Other income (expense), net of $0.1 million for the year ended December 31, 2011 was primarily related to investment advisory fees. Other income (expense), net for the year ended December 31, 2010 primarily consisted of a grant earned under Section 48D of the Internal Revenue Code (as enacted on March 23, 2010 by the “Patient Protection and Affordable Care Act”). We applied for a grant based upon certain of our 2009 and 2010 research and development activities. The payment for the 2009 grant of $0.4 million was received in 2010, whereas the 2010 grant of $0.2 million was received in early 2011.
Comparison of Years Ended December 31, 2010 and 2009
| Year Ended December 31, |
Increase (Decrease) |
% Increase (Decrease) |
||||||||||||||
| 2010 | 2009 | |||||||||||||||
| (in thousands, except percentages) | ||||||||||||||||
| Product revenue |
$ | 688 | $ | — | $ | 688 | 100 | % | ||||||||
| Collaboration revenue |
7,578 | 2,006 | 5,572 | 278 | % | |||||||||||
| Cost of goods sold |
(541 | ) | — | 541 | 100 | % | ||||||||||
| Research and development expenses |
(11,151 | ) | (11,235 | ) | (84 | ) | (1 | %) | ||||||||
| Selling, general and administrative expenses |
(36,383 | ) | (12,420 | ) | 23,963 | 193 | % | |||||||||
| Interest income |
98 | 89 | 9 | 10 | % | |||||||||||
| Interest expense |
(5,413 | ) | (276 | ) | 5,112 | 1,852 | % | |||||||||
| Other income (expense), net |
608 | (31 | ) | 614 | 1,981 | % | ||||||||||
Product Revenue. Due to the limited history of product returns and cash collections from our customers, (specialty distributors and specialty pharmacies), we recognize revenue for Qutenza at the later of the time our customers ship product to healthcare professionals and cash collections are received by us from our customers. We recorded gross shipments of Qutenza to our customers totaling $1.4 million in 2010. Based on our revenue recognition methodology, we recognized net revenue of $0.7 million as Qutenza revenue for the year. We had deferred product revenue of $0.6 million at December 31, 2010, which is reported net of associated cost of goods sold of $0.1 million in our consolidated balance sheets at December 31, 2010.
Collaboration Revenue. Collaboration revenue increased $5.6 million, or 278%, to $7.6 million for the year ended December 31, 2010, compared to $2.0 million for the year ended December 31, 2009. This increase was primarily attributable to the amortization of upfront payments received pursuant to the Astellas Agreement over a full fiscal year and the recognition of $0.3 million of collaboration revenue under our Supply Agreement with Astellas.
Cost of goods sold. Cost of goods sold totaled $0.5 million for the year ended December 31, 2010. Cost of goods sold includes $0.1 million for the cost of the finished goods sold that were recognized as revenue in 2010 and a charge of $0.4 million for Qutenza patches which we do not expect to sell prior to their expiration date. The cost of the finished goods sold includes fixed costs of $0.1 million and variable costs of $41,000.
Research and Development expenses. Research and development expenses were $11.2 million for each of the years ended December 31, 2010 and 2009. During 2010, we incurred higher expenses of $1.6 million related to the preparation and commencement of our Phase 2 study of NGX-1998 and higher expenses of $0.2 million related to our pre-clinical program. However, these expenses were offset by a $1.2 million decrease in royalties paid to UC in 2009 relating to the Astellas Agreement, a $0.3 million decrease in clinical consulting fees related to our 2009 MA and NDA preparation costs and a $0.4 million decrease in stock-based compensation expense due to the achievement of performance based milestones and the granting of options which vested immediately in 2009.
Selling, General and Administrative expenses. Selling, general and administrative expenses increased $24.0 million, or 193%, to $36.4 million for the year ended December 31, 2010, from $12.4 million for the same period
63
Table of Contents
in 2009. This increase was due in large part to the commercial launch of Qutenza in the United States in April 2010. Specifically, the increases included $8.5 million in costs associated with our sales and commercial operation organization including compensation and benefit costs and travel expenses; $9.1 million increase in expenses for marketing materials development and other launch related marketing costs; $1.6 million in costs due to an increase in general and administrative salary and related expenses, temporary help and consultants, professional service fees and other public company costs; $1.6 million of costs associated with our medical affairs organization including salary and related expenses; $1.0 million in estimated settlement costs; $0.7 million increase in facilities and office expenses due to expansion of headcount to support commercialization; and a $0.2 million increase in stock-based compensation costs due to the granting of options to employees and officers.
Interest expense. Interest expense was $5.4 million for the year ended December 31, 2010 as compared to $0.3 million for the same period in 2009. This increase was primarily due to interest on the $40 million long-term obligation related to the Financing Agreement entered into with Cowen Royalty in April 2010.
Other income (expense), net. Other income for the year ended December 31, 2010 primarily consisted of a grant earned under Section 48D of the Internal Revenue Code (as enacted on March 23, 2010 by the “Patient Protection and Affordable Care Act”). We applied for a grant based upon certain of our 2009 and 2010 research and development activities. The payment for the 2009 grant of $0.4 million was received in 2010, whereas the 2010 grant of $0.2 million was received in early 2011.
Liquidity and Capital Resources
Since our inception through December 31, 2011, we have funded our operations primarily by selling equity securities, establishing debt facilities and through a collaboration agreement with Astellas. On May 7, 2007, we completed an initial public offering of our common stock which resulted in net cash proceeds, after deducting total expenses including underwriting discounts and commissions and other-offering related expenses, of $38.1 million. In December 2007, we completed the first closing of a private placement of our common stock and warrants resulting in net cash proceeds of $21.5 million and in January 2008, we completed the second and final closing of this private placement of our common stock and warrants resulting in net cash proceeds of $2.3 million. In April 2010, we borrowed $40.0 million under a Financing Agreement with Cowen Royalty. In July 2011, we completed a private placement of our common stock and warrants which resulted in net cash proceeds of $18.6 million, after deducting total expenses including placement agent fees and other offering-related expenses. In August 2011, we borrowed $15.0 million under a loan agreement with Hercules.
In February 2012, we completed a private placement of our common stock which resulted in net cash proceeds of approximately $3.0 million, after deducting offering-related expenses.
At December 31, 2011, we had approximately $34.3 million in cash, cash equivalents and short-term investments and working capital of $27.4 million, adjusted to exclude the current portion of non-cash deferred revenue of approximately $8.3 million. During 2011, our cash and investment balances were generally held in money market funds and obligations of U.S. government agencies. Cash in excess of immediate operational requirements is invested in accordance with our investment policy primarily with a view to capital preservation and liquidity. Further, to reduce portfolio risk, our investment policy specifies a concentration limit of 10% in any one issuer or group of issuers of corporate bonds or commercial paper at the time of purchase. Our investment holdings at December 31, 2011 consist of $6.5 million in U.S. Treasury securities, $23.9 million of government sponsored enterprise debt securities, and $3.9 million in cash and money market funds.
Operating Activities
Net cash used by operating activities was $45.0 million in 2011 as compared to $42.4 million for 2010. The increase in cash used in operating activities in 2011 as compared to 2010 resulted primarily from the timing of
64
Table of Contents
material supply transactions with our collaboration partner and the timing of payments of our expenses. We anticipate that cash used in operating activities will decrease in 2012 as a result of the organization restructuring which occurred on March 8, 2012.
The increase in cash used in operating activities in 2010 as compared to 2009, after adjusting for the effects of the Astellas payment, was due to the large increase in selling, general and administrative expense due to the launch of Qutenza in early 2010.
Net cash provided by operating activities in 2009 was $29.2 million that resulted primarily from the receipt of an upfront payment under the Astellas Agreement, partially offset by our net loss during the period. After adjusting for the effects of the Astellas payment, our net cash used in operating activities would have been $17.7 million in 2009.
Investing Activities
Net cash provided by investing activities was $12.0 million in 2011 and cash used in investing activities was $18.2 million and $7.4 million in 2010 and 2009, respectively. Investing activities consisted primarily of the purchase, sale and maturity of marketable securities and to a lesser extent, the purchase of capital equipment.
Fluctuations in our investing activities have primarily been driven by the timing and receipt of proceeds from financing activities and the upfront payments from Astellas. We expect that property and equipment expenditures in 2012 will remain low but may increase somewhat over 2011 levels in 2012, as a result of planned relocation of our corporate offices in mid 2012.
Financing Activities
Net cash provided by financing activities in 2011 was $33.4 million due to proceeds from the Hercules term loan and the July 2011 private placement of common stock. Net cash provided by financing activities in 2010 was $39.6 million, primarily due to the proceeds received from the Cowen Royalty financing. Net cash used in financing activities in 2009 was $2.5 million.
On April 30, 2010, we entered into a Financing Agreement with Cowen Royalty. Under the terms of the Financing Agreement, we borrowed $40 million from Cowen Royalty and we agreed to repay the borrowed amount together with a return to Cowen Royalty, as described below, out of royalty, milestone, option and certain other payments that we may receive under the Astellas Agreement including payments that Astellas may make to us to maintain its option to commercialize NGX-1998 in the Licensed Territory, (we refer to such payments under the Astellas Agreement as the Revenue Interest).
Under the Financing Agreement, we are obligated to pay to Cowen Royalty:
| • | All of the Revenue Interest payments due to us under the Astellas Agreement, until Cowen Royalty has received $90 million of Revenue Interest payments; and |
| • | 5% of the Revenue Interest payments due to us under the Astellas Agreement for Revenue Interests received by Cowen Royalty over $90 million and until Cowen Royalty has received $106 million of Revenue Interest payments. |
We also have the option to pay a prepayment amount to terminate the Financing Agreement at our election at any time or in connection with a change of control of NeurogesX. Such amount is set at a base amount of $76 million (or $68 million if it is being exercised in connection with a change of control), or, if higher, an amount that generates a specified internal rate of return on the borrowed amount as of the date of prepayment, in each case reduced by the Revenue Interest payments received by Cowen Royalty up to the date of prepayment.
65
Table of Contents
Our obligation to pay the Revenue Interest during the term of the Financing Agreement is secured by our rights to the Revenue Interest under the Astellas Agreement, and by intellectual property and other rights we have to the extent necessary or used to commercialize products covered by the Astellas Agreement in the Licensed Territory.
Unless terminated earlier pursuant to a prepayment, the Financing Agreement also terminates on the earlier of:
| • | The time when Cowen Royalty has received at least $106 million of Revenue Interest payments; or |
| • | The maturity date, which is the latest to occur of 10 years following the first commercial sale of Qutenza in the Licensed Territory or the last to expire of any patents or regulatory exclusivity covering the products commercialized under the Astellas Agreement in the Licensed Territory. |
If Cowen Royalty has not received Revenue Interest payments totaling at least $40 million by the maturity date, we will be obligated to pay to Cowen Royalty the difference between such amount and the Revenue Interests paid to Cowen Royalty under the Financing Agreement up to such date. This represents our minimum obligation to Cowen.
In addition, in the event we default under the Financing Agreement, we are obligated to pay to Cowen the borrowed amount together with interest accrued thereon from the effective date of the Financing Agreement, to the date of repayment at a rate equal to the lesser of the 19% rate of interest or the maximum rate permitted by law, less Revenue Interest payments received by Cowen Royalty.
In July 2011, we completed a private placement under a Securities Purchase Agreement pursuant to which we issued units, or the Units, for an aggregate purchase price of approximately $20.2 million, at a per Unit price of $1.72. Each Unit was comprised of one share of common stock and a warrant to purchase 0.5 shares of common stock (representing 50% warrant coverage on the shares to be issued). The price of each Unit was based on the July 21, 2011 consolidated closing bid price of our common stock on the NASDAQ Global Market of $1.65 per share, and represented a purchase price of $1.65 for the one share of common stock issued for each Unit and a warrant purchase price of $0.07 for the 0.5 shares of common stock underlying the warrants issued for each Unit (resulting in a purchase price for the warrants of $0.14 per whole share of common stock underlying such warrants). The total number of Units issued in connection with the transaction was 11,749,552, representing an aggregate issuance of 11,749,552 shares of common stock and warrants to purchase an aggregate of 5,874,782 shares of common stock. The warrants have a term of five years, contain a net-exercise provision, and have an exercise price of $1.65 per share. In addition, the warrants contain terms that prevent the warrants from being exercised to the extent that such exercise would cause a stockholder’s beneficial ownership (along with its affiliates and others with whom such stockholder’s holdings would be aggregated for purposes of Section 13(d) of the Securities Exchange Act of 1934, as amended) to exceed 19.999% of the total number of then issued and outstanding shares of our common stock.
The fair value of the warrants issued was approximately $5.9 million. The fair value was allocated from the net proceeds of the private placement and was recorded in additional paid-in capital. The net proceeds to us, after deducting expenses of approximately $1.6 million, was $18.6 million.
Pursuant to a Registration Rights Agreement entered into in connection with the July 2011 Securities Purchase Agreement, we filed a registration statement covering the resale of the common stock and the shares of common stock underlying the Warrants issued and issuable to the purchasers of Units on August 24, 2011. The registration statement was declared effective on September 8, 2011.
In August 2011, we entered into a loan agreement with Hercules which includes both a $5.0 million accounts receivable line and a $15.0 million term loan. Under the terms of the loan agreement, the $15.0 million term loan is required to be repaid over the course of the 42-month maturity period, which includes a 12-month
66
Table of Contents
interest only period at the beginning of the term. Principal repayment is scheduled to begin September, 2012. Interest on the term loan has a minimum rate of 9.5%, which can increase based on fluctuations in the prime rate. The $5.0 million accounts receivable line is a revolving line that is available until December 31, 2012 and then is renewable by Hercules each year thereafter. The accounts receivable line bears interest at a minimum interest rate of 6.75%, which can also increase based on fluctuations in the prime rate, and the maximum borrowing availability under the revolving line is based upon a percentage of eligible account receivables. We may prepay and terminate the term loan and the accounts receivable line at any time, subject to certain prepayment fees, and we are obligated to also pay a fee of $0.6 million when the term loan is repaid. Our obligations under the loan agreement are secured by certain of our personal property. The loan agreement also contains customary negative covenants and is subject to customary events of default, such as a failure to make a scheduled principal or interest payment, insolvency, and breaches of covenants under loan agreement and agreements and instruments entered into in connection with the loan agreement, including a covenant in the Hercules warrant requiring us to maintain our listing on the NASDAQ Global Market. Under the terms of the loan agreement with Hercules and the warrant as originally issued on August 5, 2011, if our shares of common stock were delisted from the NASDAQ Global Market, as may occur pursuant to the market value of listed shares and bid price delinquency notices we received from NASDAQ, such an event would trigger an event under the loan agreement. As a result we entered into an amendment to the Hercules warrant in March 2012 in which we amended the covenant with respect to the requirement to maintain our listing on the NASDAQ Global Market to allow for the NASDAQ Capital Market and certain over the counter markets to be permissible alternative markets on which we can list our shares of common stock. Under the terms of the amendment, the number of shares underlying the warrant was increased to 1,950,000 and the exercise price per share of common stock was reduced to $0.50.
In connection with our entry into the loan agreement, we issued to Hercules a warrant for 791,667 shares of our common stock (representing an aggregate exercise price equal to approximately 7.125% of the maximum aggregate funds potentially available to us under the loan agreement). The warrant has a term of five years, contains a net-exercise provision, and has an exercise price of $1.80 per share. The fair value of the warrants issued was approximately $1.1 million.
We had $0.8 million available for draw down on the accounts receivable line at December 31, 2011 based upon a percentage of the then eligible account receivables. We have not drawn any funds from the accounts receivable line.
In February 2012, we completed a private placement of our common stock under a Securities Purchase Agreement, pursuant to which we issued shares of common stock for an aggregate purchase price of $3.0 million, at a per share price of $1.01. The price of each share of common stock is based on the January 31, 2012 consolidated closing bid price of our common stock on the NASDAQ Global Market of $1.01 per share. The total number of shares of common stock issued in connection with the transaction is 2,969,685.
Pursuant to a Registration Rights Agreement entered into in connection with the February 2012 Securities Purchase Agreement, we are obligated to file a registration statement covering the resale of the common stock.
Contractual Obligations
Contractual obligations represent future cash commitments and liabilities under agreements with third parties, and exclude contingent liabilities for which we cannot reasonably predict future payment. The following table represents our total contractual obligations, including principal and interest, at December 31, 2011, aggregated by type (in thousands):
| Payments due by period | ||||||||||||||||||||
| Contractual Obligations |
Total | 2012 | 2013 and 2014 | 2015 and 2016 | 2017 and thereafter |
|||||||||||||||
| Operating lease obligations(1) |
$ | 407 | $ | 391 | $ | 16 | $ | — | $ | — | ||||||||||
| Long-term obligations(2) |
57,467 | 3,661 | 13,529 | 1,196 | 39,081 | |||||||||||||||
| Purchase obligations(3) |
1,532 | 790 | 616 | 116 | 10 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 59,406 | $ | 4,842 | $ | 14,161 | $ | 1,312 | $ | 39,091 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Represent our future minimum rental commitments for our noncancelable operating lease obligations for our facilities and office equipment. |
67
Table of Contents
| (2) | The amounts shown relate to the Cowen Royalty Agreement and the Hercules Agreement. Under the terms of the Cowen Royalty agreement, payments are contingent upon cash received from Astellas under the Astellas Agreement (refer above for discussion). The amounts shown all relate to the loan agreement with Hercules. |
| (3) | Consists of commitments to third-party manufacturers of Qutenza and NGX-1998 for manufacturing, stability and related services including reimbursable amounts from Astellas for supply of Qutenza based on the Astellas Agreement. The $1.5 million includes $1.0 million for stability and related services, $0.2 million related to Qutenza product manufacturing costs for the U.S. market and NGX-1998 clinical supply development cost along with $0.3 million of commitments related to supply of Qutenza to Astellas. |
In addition to the above, we have commitments to pay royalties on certain patents we licensed from the University of California for prescription strength capsaicin for neuropathic pain. Under the terms of this license agreement we are required to pay royalties on net sales of the licensed product up to a maximum of $1.0 million per annum as well as a percentage of up-front and milestone payments or payments resulting from the sublicensing of our rights under the agreement.
We also have commitments to pay royalties under a Commercial Supply and License Agreement with LTS to manufacture commercial and clinical supply of Qutenza. Under the terms of the agreement, we are required to pay a transfer price for product purchased under the agreement as well as a royalty on net sales of product purchased under such agreement.
Our future capital uses and requirements depend on numerous forward-looking factors. These factors include but are not limited to the following:
| • | the progress of our development programs including the number, size and scope of clinical trials and non-clinical development; |
| • | the success of the commercialization of our products; |
| • | the scope and cost of commercial activities including, but not limited to, costs associated with pharmacovigilance and medical affairs activities; |
| • | the conduct of manufacturing activities; |
| • | the costs of carrying out our obligations under our commercial arrangement with Astellas; |
| • | our ability to establish and maintain strategic collaborations, including licensing and other arrangements that we have or may establish; |
| • | the costs and timing of seeking regulatory approvals; |
| • | the costs involved in enforcing or defending patent claims or other intellectual property rights; and |
| • | the extent to which we acquire or invest in other products, technologies and businesses. |
At December 31, 2011, we had $34.3 million in cash, cash equivalents and short-term investments. We anticipate that cash used in operating activities will decrease in 2012 as a result of the organization restructure that was announced on March 8, 2012. We anticipate that our existing cash and investments will be sufficient to meet our projected operating requirements through at least December 31, 2012.
To date, we have incurred recurring net losses and negative cash flows from operations, after excluding the upfront cash received from Astellas in 2009. Until we can generate significant cash from our operations, if ever, we expect to continue to fund our operations with existing cash resources generated from the proceeds of offerings of our equity securities and proceeds from one or more collaboration agreements as well as potentially through royalty monetization, debt financing or the sale of other equity securities. However, we may not be successful in obtaining additional collaboration agreements, or in receiving milestone or royalty payments under those agreements. In addition, we cannot be sure that our existing cash and investment resources will be adequate or that additional financing will be available when needed or that, if available, financing will be obtained on
68
Table of Contents
terms favorable to us or our stockholders. Having insufficient funds may require us to delay or potentially eliminate some or all of our development programs, relinquish some or even all rights to product candidates at an earlier stage of development or negotiate less favorable terms than we would otherwise choose. Failure to obtain adequate financing also may adversely affect the launch of our product candidates or our ability to continue in business. If we raise additional funds by issuing equity securities, substantial dilution to existing stockholders would likely result. If we raise additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict our ability to operate our business.
Off-balance Sheet Arrangements
Since inception, we have not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities.
Recently Issued Accounting Standards
Not Yet Adopted
In June 2011, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update, or ASU, No. 2011-05, “Presentation of Comprehensive Income” an update to Accounting Standards Codification, or ASC, Topic 220, “Comprehensive Income.” The amendments of this update require that comprehensive income be presented either in a single continuous statement of comprehensive income or in two separate but consecutive statements. This update is to be applied retroactively and is effective for financial statements issued for fiscal years, and interim periods within those years, beginning after December 15, 2011, and interim and annual periods thereafter. This update will be effective for the Company January 1, 2012. The Company does not expect the adoption of this guidance to have a material impact on its consolidated financial statements.
Adopted in 2011
In October 2009, FASB issued ASU No. 2009-13, Revenue Recognition (ASC Topic 605) – Multiple-Deliverable Revenue Arrangements. This guidance modifies previous guidance for multiple element arrangements by allowing the use of the “best estimate of selling price” in the absence of vendor-specific objective evidence, or VSOE, or third-party evidence, or TPE, of selling price for determining the selling price of a deliverable. A vendor is now required to use its best estimate of the selling price when VSOE or TPE of the selling price is not available. In addition, the residual method of allocating arrangement consideration is no longer permitted. This ASU is effective for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010 and early adoption was permitted. We adopted this ASU on January 1, 2011 and the adoption of the new guidance did not have a material impact on our consolidated financial statements. We currently only have one revenue agreement with multiple elements, the Astellas Agreement. If we were to enter into new multiple element arrangements or make a material modification to the Astellas Agreement after January 1, 2011, the contract would be accounted for under the provisions of ASU No. 2009-13.
In April 2010, the FASB issued ASU No. 2010-17, Revenue Recognition—Milestone Method (ASC Topic 605): Milestone Method of Revenue Recognition. This ASU codifies the consensus reached in Emerging Issues Task Force Issue No. 08-9, “Milestone Method of Revenue Recognition.” The amendments to the FASB Accounting Standards Codification provide guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research or development transactions. Consideration that is contingent on achievement of a milestone in its entirety may be recognized as revenue in the period in which the milestone is achieved only if the milestone is judged to meet certain criteria to be considered substantive. Milestones should be considered substantive in their entirety and may not be bifurcated. An arrangement may contain both substantive and non-substantive milestones, and each milestone should be evaluated individually to determine if it is substantive. ASU No. 2010-17 is effective for fiscal years, and interim
69
Table of Contents
periods within those years, beginning on or after June 15, 2010, and may be applied prospectively to milestones achieved after the adoption date or retrospectively for all periods presented. On January 1, 2011, we prospectively adopted and elected the Milestone Method of Revenue Recognition as our accounting policy for substantive milestones achieved after the adoption date. The adoption of the new guidance did not have a material impact on our consolidated financial statements.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risks |
Interest Rate and Market Risk
Our exposure to market risk is confined to our cash, cash equivalents and short term investments which have maturities of less than one year. The goal of our investment policy is primarily liquidity and capital preservation while attempting to maximize the income we receive without assuming significant risk. To achieve these objectives our investment policy allows us to maintain a portfolio of cash equivalents and short term investments in a variety of securities, including U.S. government agencies, corporate bonds, commercial paper and money market funds. Further, to reduce portfolio risk through diversification, our investment policy specifies a concentration limit of 10% in any one issuer or group of issuers of corporate bonds or commercial paper at the time of purchase. Our cash and investments as of December 31, 2011 consisted of U.S. Treasury securities and money market funds consisting of only U.S. Treasury securities.
If interest rates rise, the market value of our investments may decline, which could result in a realized loss if we are forced to sell an investment before its scheduled maturity. A hypothetical increase in interest rates by 25 basis points would not result in a material decrease in the fair value of our net investment position.
Foreign Currency Exchange Rate Risk
Our third-party manufacturers including the manufacturer of our active pharmaceutical ingredient, trans-capsaicin, the manufacturer of Qutenza and the manufacturer of our cleansing gel, are located in countries outside the United States. As a result, we may experience changes in product supply costs as a result of changes in exchange rates between the U.S. dollar and the local currency where the manufacturing activities occur. In addition, as we continue to scale up supply of our product components to Astellas for commercial sale in the European Union and to supply the U.S. market, our exposure to foreign currency exchange rates, primarily the Euro, will increase.
Amounts paid to us by Astellas under the Astellas Agreement may be based in Euro or other currencies which will then be converted to U.S. dollars before payment to us. To the extent that these potential receipts are greater than our net payable exposure in Euro to our suppliers mentioned above, and to the extent that the timing of these potential payments and receipts differ, we will have a net receivable exposure in Euro once Qutenza is launched in the European Union and certain other foreign territories.
We currently do not engage in foreign currency hedging activities as the short-term exposure to fluctuations in foreign currency is relatively small.
70
Table of Contents
| Item 8. | Financial Statements and Supplementary Data |
NEUROGESX, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||
| 72 | ||||
| 73 | ||||
| 74 | ||||
| 75 | ||||
| 76 | ||||
| 77 | ||||
71
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of NeurogesX, Inc.
We have audited the accompanying consolidated balance sheets of NeurogesX, Inc. as of December 31, 2011 and 2010, and the related consolidated statements of operations, stockholders’ (deficit) equity, and cash flows for each of the three years in the period ended December 31, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of NeurogesX, Inc. at December 31, 2011 and 2010, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2011, in conformity with U.S. generally accepted accounting principles.
/s/ ERNST & YOUNG LLP
Redwood City, California
March 28, 2012
72
Table of Contents
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 9,148 | $ | 8,705 | ||||
| Short-term investments |
25,161 | 38,125 | ||||||
| Trade receivables |
1,166 | 607 | ||||||
| Receivable from collaboration partner |
502 | 65 | ||||||
| Inventories |
654 | 786 | ||||||
| Prepaid expenses and other current assets |
1,364 | 1,082 | ||||||
| Restricted cash |
160 | 290 | ||||||
|
|
|
|
|
|||||
| Total current assets |
38,155 | 49,660 | ||||||
| Property and equipment, net |
547 | 809 | ||||||
| Restricted cash |
251 | 120 | ||||||
| Other assets |
274 | 261 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 39,227 | $ | 50,850 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Deficit |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 1,321 | $ | 1,544 | ||||
| Accrued compensation |
2,084 | 1,867 | ||||||
| Accrued research and development |
282 | 582 | ||||||
| Other accrued expenses |
2,245 | 3,011 | ||||||
| Deferred product revenue, net |
1,075 | 577 | ||||||
| Deferred collaboration revenue |
7,261 | 7,242 | ||||||
| Long-term obligations—current portion |
4,829 | 2,222 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
19,097 | 17,045 | ||||||
| Non-current liabilities: |
||||||||
| Deferred collaboration revenue |
25,098 | 32,359 | ||||||
| Other long-term liabilities |
146 | 115 | ||||||
| Long-term obligations |
62,746 | 42,622 | ||||||
|
|
|
|
|
|||||
| Total non-current liabilities |
87,990 | 75,096 | ||||||
| Commitments and contingencies |
||||||||
| Stockholders’ deficit: |
||||||||
| Common stock, $0.001 par value; |
30 | 18 | ||||||
| Additional paid-in capital |
238,181 | 215,220 | ||||||
| Accumulated other comprehensive income (loss) |
1 | (2 | ) | |||||
| Accumulated deficit |
(306,072 | ) | (256,527 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ deficit |
(67,860 | ) | (41,291 | ) | ||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ deficit |
$ | 39,227 | $ | 50,850 | ||||
|
|
|
|
|
|||||
See accompanying notes.
73
Table of Contents
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share data)
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Net product revenue |
$ | 2,625 | $ | 688 | $ | — | ||||||
| Collaboration revenue |
8,878 | 7,578 | 2,006 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
11,503 | 8,266 | 2,006 | |||||||||
| Operating expenses: |
||||||||||||
| Cost of goods sold |
685 | 541 | — | |||||||||
| Research and development |
13,566 | 11,151 | 11,235 | |||||||||
| Selling, general and administrative |
36,408 | 36,383 | 12,420 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
50,659 | 48,075 | 23,655 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(39,156 | ) | (39,809 | ) | (21,649 | ) | ||||||
| Interest income |
79 | 98 | 89 | |||||||||
| Interest expense |
(10,405 | ) | (5,413 | ) | (276 | ) | ||||||
| Other income (expense), net |
(63 | ) | 608 | (31 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (49,545 | ) | $ | (44,516 | ) | $ | (21,867 | ) | |||
|
|
|
|
|
|
|
|||||||
| Basic and diluted net loss per share |
$ | (2.15 | ) | $ | (2.51 | ) | $ | (1.24 | ) | |||
|
|
|
|
|
|
|
|||||||
| Shares used to compute basic and diluted net loss per share |
23,011,015 | 17,768,881 | 17,610,796 | |||||||||
|
|
|
|
|
|
|
|||||||
See accompanying notes.
74
Table of Contents
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ (DEFICIT) EQUITY
(in thousands, except share and per share data)
| Common Stock | Additional Paid-in Capital |
Deferred Stock-Based Compensation |
Accumulated Other Comprehensive Income (Loss) |
Accumulated Deficit |
Total Stockholders’ Equity (Deficit) |
|||||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||||||
| Balance at December 31, 2008 |
17,568,402 | $ | 18 | $ | 209,370 | $ | (2 | ) | $ | 32 | $ | (190,144 | ) | $ | 19,274 | |||||||||||||
| Issuance of common stock upon exercise of stock options for cash at $1.20 - $7.54 per share |
104,262 | 288 | 288 | |||||||||||||||||||||||||
| Issuance of common stock at $3.00 - $3.75 per share upon vesting of early exercised stock options |
785 | 2 | 2 | |||||||||||||||||||||||||
| Issuance of common stock under the Employee Stock Purchase Plan |
52,510 | 77 | 77 | |||||||||||||||||||||||||
| Stock-based compensation |
2,517 | 2,517 | ||||||||||||||||||||||||||
| Amortization of deferred stock-based compensation |
2 | 2 | ||||||||||||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||||||
| Change in unrealized gain/(loss) on investments |
(40 | ) | (40 | ) | ||||||||||||||||||||||||
| Net loss |
(21,867 | ) | (21,867 | ) | ||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Total comprehensive loss |
(21,907 | ) | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2009 |
17,725,959 | 18 | 212,254 | — | (8 | ) | (212,011 | ) | 253 | |||||||||||||||||||
| Issuance of common stock upon exercise of stock options for cash at $0.90 - $8.32 per share |
79,869 | 258 | 258 | |||||||||||||||||||||||||
| Issuance of common stock under the Employee Stock Purchase Plan |
63,280 | 354 | 354 | |||||||||||||||||||||||||
| Stock-based compensation |
2,354 | 2,354 | ||||||||||||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||||||
| Change in unrealized gain/(loss) on investments |
6 | 6 | ||||||||||||||||||||||||||
| Net loss |
(44,516 | ) | (44,516 | ) | ||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Total comprehensive loss |
(44,510 | ) | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balances at December 31, 2010 |
17,869,108 | 18 | 215,220 | — | (2 | ) | (256,527 | ) | (41,291 | ) | ||||||||||||||||||
| Issuance of common stock upon exercise of stock options for cash at $1.20 - $3.75 per share |
22,207 | 41 | 41 | |||||||||||||||||||||||||
| Issuance of common stock under the Employee Stock Purchase Plan |
97,319 | 162 | 162 | |||||||||||||||||||||||||
| Stock-based compensation |
3,065 | 3,065 | ||||||||||||||||||||||||||
| Issuance of common stock, net of offering costs |
11,749,552 | 12 | 11,854 | 11,866 | ||||||||||||||||||||||||
| Issuance of warrant to purchase stock to investors at $0.14 per share including fair value |
6,699 | 6,699 | ||||||||||||||||||||||||||
| Issuance of warrant to purchase stock to investor at fair value |
1,140 | 1,140 | ||||||||||||||||||||||||||
| Release of restricted stock units |
159,000 | |||||||||||||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||||||
| Change in unrealized gain/(loss) on investments |
3 | 3 | ||||||||||||||||||||||||||
| Net loss |
(49,545 | ) | (49,545 | ) | ||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Total comprehensive loss |
(49,542 | ) | ||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balances at December 31, 2011 |
29,897,186 | $ | 30 | $ | 238,181 | $ | — | $ | 1 | $ | (306,072 | ) | $ | (67,860 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
See accompanying notes.
75
Table of Contents
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Operating activities |
||||||||||||
| Net loss |
$ | (49,545 | ) | $ | (44,516 | ) | $ | (21,867 | ) | |||
| Adjustments to reconcile net loss to cash (used in) provided by operating activities: |
||||||||||||
| Depreciation |
376 | 343 | 184 | |||||||||
| Amortization of debt issuance costs and accretion of debt discount |
620 | 54 | 123 | |||||||||
| Amortization of investment premiums |
677 | 520 | 130 | |||||||||
| Stock-based compensation |
3,426 | 2,354 | 2,492 | |||||||||
| Gain on disposal of property and equipment |
— | 2 | — | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Restricted cash |
(2 | ) | (250 | ) | — | |||||||
| Trade receivables |
(559 | ) | (607 | ) | — | |||||||
| Receivable from collaboration partner |
(437 | ) | 613 | (678 | ) | |||||||
| Prepaid expenses and other current assets |
38 | (344 | ) | (310 | ) | |||||||
| Inventories |
132 | (729 | ) | (58 | ) | |||||||
| Accounts payable |
(223 | ) | 1,479 | (305 | ) | |||||||
| Accrued compensation |
(145 | ) | (289 | ) | 1,118 | |||||||
| Accrued research and development |
(300 | ) | (87 | ) | (397 | ) | ||||||
| Accrued license fees |
— | (1,213 | ) | 1,213 | ||||||||
| Accrued interest payable on long-term obligations |
8,457 | 5,306 | — | |||||||||
| Deferred product revenue, net |
498 | 577 | — | |||||||||
| Deferred collaboration revenue |
(7,242 | ) | (7,242 | ) | 46,843 | |||||||
| Other long-term liabilities |
(176 | ) | (123 | ) | 114 | |||||||
| Other accrued expenses |
(703 | ) | 1,735 | 585 | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash (used in) provided by operating activities |
(45,108 | ) | (42,417 | ) | 29,187 | |||||||
|
|
|
|
|
|
|
|||||||
| Investing activities |
||||||||||||
| Purchases of short-term investments |
(31,684 | ) | (90,029 | ) | (37,459 | ) | ||||||
| Proceeds from maturities of short-term investments |
36,870 | 72,254 | 30,496 | |||||||||
| Proceeds from sales of short-term investments |
7,104 | — | — | |||||||||
| Change in restricted cash |
— | — | 40 | |||||||||
| Purchases of property and equipment |
(114 | ) | (415 | ) | (456 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
12,176 | (18,190 | ) | (7,379 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Financing activities |
||||||||||||
| Repayment of notes payable |
— | (191 | ) | (2,913 | ) | |||||||
| Proceeds from issuance of long-term obligations |
14,820 | 39,505 | — | |||||||||
| Payment of debt issuance costs |
(213 | ) | (309 | ) | — | |||||||
| Proceeds from issuance of warrants |
822 | — | — | |||||||||
| Proceeds from issuance of common stock |
17,946 | 612 | 365 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) financing activities |
33,375 | 39,617 | (2,548 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
443 | (20,990 | ) | 19,260 | ||||||||
| Cash and cash equivalents, beginning of period |
8,705 | 29,695 | 10,435 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 9,148 | $ | 8,705 | $ | 29,695 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental cash flow information |
||||||||||||
| Cash paid for interest |
$ | 1,325 | $ | 53 | $ | 181 | ||||||
| Non-cash financing activities |
||||||||||||
| Contingent put option liability |
$ | 146 | $ | — | $ | — | ||||||
| Warrants issued in connection with private equity placement and debt financing |
$ | 7,016 | $ | — | $ | — | ||||||
See accompanying notes.
76
Table of Contents
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. The Company
Nature of Operations
NeurogesX, Inc. (the “Company”) is a specialty pharmaceutical company focused on developing and commercializing a portfolio of novel non-opioid, pain management therapies to address unmet medical needs and improve patients’ quality of life.
The Company’s first commercial product, Qutenza, became commercially available in the United States and in certain European countries in the first half of 2010. Qutenza is a dermal delivery system designed to topically administer capsaicin to treat certain neuropathic pain conditions and was approved by the Food and Drug Administration, or FDA, in November 2009 for the management of neuropathic pain associated with postherpetic neuralgia, or PHN. Qutenza is also approved in the European Union for the treatment of peripheral neuropathic pain in non-diabetic adults. Qutenza is the first prescription strength capsaicin product approved in the United States.
The Company’s most advanced drug candidate is NGX-1998. NGX-1998 is a topical liquid formulation of high concentration capsaicin that the Company is developing to treat neuropathic pain conditions. In November 2011, the Company announced positive top-line results from its Phase 2 clinical study of NGX-1998, in patients with PHN. The 12-week, multicenter, randomized, double-blinded, placebo-controlled clinical trial met its protocol-specified objectives, which include the primary endpoint of a percentage change from baseline, as compared to placebo in a patient-reported numeric pain rating scale, or NPRS, score during weeks two through eight of the trial. The Company believes that the Phase 2 data support moving forward to a NGX-1998 Phase 3 clinical trial. The Company plans to have an End-of-Phase 2 meeting with the FDA, which the Company believes could occur in the second half of 2012. The Company is presently examining a range of potential indications for NGX-1998, including but not limited to PHN, HIV associated peripheral neuropathy, or HIV-PN, painful diabetic neuropathy, or PDN, chemo-induced peripheral neuropathy, CIPN, and post-traumatic (e.g. surgical) pain, or PTP. The Company’s decision as to which indications to pursue in Phase 3 clinical trials is expected to be based on a number of criteria including, but not limited to, its assessments of probability of clinical development success, market size and potential regulatory pathways to possible approval.
As a result of the opportunity, the Company believes exists with NGX-1998, in March 2012, the Company decided to focus its resources on the preparation for advancing NGX-1998 into a Phase 3 clinical trial. To do so, the Company significantly restructured its operations, including eliminating most of sales and marketing expenditures in support of Qutenza, while continuing to maintain product availability of Qutenza for patients and physicians. While the Company believes a market opportunity exists for Qutenza, it believes that the resources required to attain that potential are better utilized to speed the development of NGX-1998. The Company is evaluating a number of alternative commercialization strategies which include the potential to license Qutenza to a commercialization partner in the United States and in other territories of the world outside of the Astellas Territory.
In May 2009, Qutenza received a marketing authorization (“MA”) in the European Union for the treatment of peripheral neuropathic pain in non-diabetic adults, either alone or in combination with other medicinal products for pain. In June 2009, the Company entered into a Distribution, Marketing and License Agreement (the “Astellas Agreement”) with Astellas Pharma Europe Ltd. (“Astellas” or “Collaboration Partner”), under which Astellas was granted an exclusive license to commercialize Qutenza in the European Economic Area, which includes the 27 countries of the European Union, Iceland, Norway, and Liechtenstein as well as Switzerland, certain countries in Eastern Europe, the Middle East and Africa (“Licensed Territory”). Qutenza was made available on a country by country basis commencing in April 2010 and by year end was available in 21 European countries.
77
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
At December 31, 2011, the Company had $34.3 million in cash, cash equivalents and short-term investments. The Company anticipate that cash used in operating activities will decrease in 2012 as a result of the organization restructure that was announced on March 8, 2012. The Company anticipate that our existing cash and investments will be sufficient to meet its projected operating requirements through at least December 31, 2012.
To date, the Company has incurred recurring net losses and negative cash flows from operations, after excluding the upfront cash received from Astellas in 2009. Until the Company can generate significant cash from its operations, if ever, the Company expects to continue to fund its operations with existing cash resources generated from the proceeds of offerings of its equity securities and proceeds from one or more collaboration agreements, as well as potentially through royalty monetization, debt financing or the sale of other equity securities. However, the Company may not be successful in obtaining additional collaboration agreements, or in receiving milestone or royalty payments under those agreements. In addition, the Company cannot be sure that its existing cash and investment resources will be adequate or that additional financing will be available when needed or that, if available, financing will be obtained on terms favorable to the Company or its stockholders. Having insufficient funds may require the Company to delay or potentially eliminate some or all of its development programs, relinquish some or even all rights to product candidates at an earlier stage of development or negotiate less favorable terms than it would otherwise choose. Failure to obtain adequate financing also may adversely affect the launch of the Company’s product candidates or its ability to continue in business. If the Company raises additional funds by issuing equity securities, substantial dilution to existing stockholders would likely result. If the Company raises additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations, as well as covenants and specific financial ratios that may restrict its ability to operate its business.
The Company was incorporated in California as Advanced Analgesics, Inc. on May 28, 1998 and changed its name to NeurogesX, Inc. in September 2000. In February 2007, the Company reincorporated into Delaware. The Company is located in San Mateo, California. All of the Company’s assets are located within the United States.
Certain amounts in the prior years’ consolidated financial statements have been reclassified to conform to the current-year presentation.
Recent Event
The Company’s common stock is currently listed on the NASDAQ Global Market and to maintain its listing, it must meet certain financial requirements in accordance with the rules of Nasdaq. On October 18, 2011, the Company received a notification from Nasdaq that it failed to maintain the required minimum market value of listed securities level of $50.0 million for a 30 consecutive business day period. The Company has until April 16, 2012 in which to cure this non-compliance by meeting such requirement for a minimum of ten consecutive business days. On January 27, 2012, the Company received a notification from Nasdaq that it failed to maintain the required $1.00 minimum bid price for a 30 consecutive business day period. The Company has until July 28, 2012 in which to cure this non-compliance by meeting such requirement for a minimum of ten consecutive business days. Any potential delisting of the Company’s common stock could adversely affect the liquidity of its common stock and its ability to raise additional capital. In addition, delisting from the NASDAQ Global Market would have triggered an event of default under the Company’s loan agreement with Hercules Technology Growth Capital, Inc. (“Hercules”), which would have provided Hercules the option to accelerate repayment of amounts due under such agreement. As a result, in March 2012, the Company entered into an amendment to the Hercules warrant, in which it amended the covenant with respect to the requirement to maintain its listing on the NASDAQ Global Market to allow for the NASDAQ Capital Market and certain over the counter markets to be permissible alternative markets on which the Company can list its shares of common
78
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
stock. Under the terms of the amendment, the number of shares underlying the warrant was increased to 1,950,000 and the exercise price per share of common stock was reduced to $0.50.
Principles of Consolidation
The accompanying financial statements include the accounts of the Company and its wholly-owned subsidiary, NeurogesX UK Limited, which was incorporated as of June 1, 2004. NeurogesX UK Limited was established for the purposes of conducting clinical trials in the UK and marketing approval submission. The subsidiary has no assets other than the initial formation capital totaling one Pound Sterling.
Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
2. Summary of Significant Accounting Policies
Cash and Cash Equivalents and Short-Term Investments
The Company considers all highly liquid investments with an original maturity of three months or less at the time of purchase to be cash equivalents. The Company invests its available cash balances in bank deposits, money market funds, U.S. government securities and other investment grade debt securities that have strong credit ratings.
Management determines the appropriate classification of securities at the time of purchase. To date, all marketable securities have been classified as available-for-sale, and are carried at fair value as determined based on quoted market prices or other observable market inputs with unrealized gains and losses reported as a component of accumulated other comprehensive income (loss) in stockholders’ deficit. The Company views its available-for-sale portfolio as available for use in current operations. Accordingly, the Company has classified all investments as short-term.
The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization is included in interest income. Realized gains and losses and declines in value judged to be other-than-temporary for available-for-sale securities, if any, are included in other income (expense), net and have not been significant to date. Realized gains and losses are computed on a specific identification basis. Interest and dividends are included in interest income.
Concentrations of Credit Risk and Financial Instruments
The Company invests cash that is not currently being used for operational purposes in accordance with its investment policy. The policy allows for the purchase of low risk debt securities issued by U.S. government agencies and highly rated corporations subject to concentration limits of 10% of any one issuer or group of issuers at the time of purchase, with the exception of debt securities issued by U.S. government agencies where the Company is not subject to any concentration limit. The maturities of these securities on a weighted-average basis may be no longer than 12 months. The Company believes that it has established guidelines for investment of its excess cash that maintains safety and liquidity through its policies on diversification and investment maturity.
79
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Financial instruments that potentially subject the Company to concentrations of credit risk consist of cash and cash equivalents and short-term investments, available for sale investment securities in high-credit quality corporate debt, and debt securities issued by the U.S. government and government-sponsored enterprises.
The Company utilizes a network of seven specialty distributors and specialty pharmacies to distribute Qutenza. For the year ended December 31, 2011, four of the distributors each accounted for 10% or more of the Company’s 2011 gross product shipments. As of December 31, 2011, the Company had accounts receivable balances due from four of the distributors in amounts in excess of 10% of the Company’s total accounts receivable balance and one such distributor’s accounts receivable balance represented 43% of the total. If any of these distributors were to discontinue distributing Qutenza, the Company’s product revenue could be negatively impacted, at least in the short term.
The Company currently depends on four contract manufacturers as single source suppliers for the components of Qutenza. The Company has entered into long-term commercial supply agreements for these components. In addition, the Company entered into a long-term agreement for the assembly of the Qutenza treatment kits in the United States. If relationships with any of these manufacturers is terminated, or if any manufacturer is unable to produce required quantities on a timely basis or at all, the Company’s operations would be negatively impacted and our business harmed.
Trade Receivables
Trade accounts receivable are recorded net of allowances for customer chargebacks related to government rebate programs, doubtful accounts and sales returns. Estimates for customer chargebacks for government rebates and sales returns are based on contractual terms, historical trends and the Company’s expectations regarding the utilization rates for these programs. The Company’s estimate for allowance for doubtful accounts is determined based on existing contractual payment terms, historical payment patterns of its customers and individual customer circumstances. To date, sales returns and rebates have been insignificant, therefore, the Company has determined that an allowance for uncollectible accounts receivable is not required. The Company does not require collateral in support of its trade receivables.
Inventories
Inventories are determined at the lower of cost or market value with cost determined under the specific identification method. Inventories consist of raw materials, work-in-process and finished goods. Raw materials include certain starting materials used in the production of the active pharmaceutical ingredient of Qutenza. Work-in-process includes the bulk inventory of the active pharmaceutical ingredient, patches and cleansing gel of Qutenza that are in the process of being manufactured or have completed manufacture and are awaiting final packaging, including related internal labor and overhead costs. Finished goods include the final packaged kits that contain the Qutenza patch and cleansing gel and that are ready for commercial sale. Qutenza received FDA approval for sale in the United States during the fourth quarter of 2009; costs incurred prior to FDA approval had been recorded as research and development expense on the Company’s consolidated statements of operations. On a quarterly basis, the Company analyzes its inventory levels and writes down inventory that has become obsolete, inventory that has a cost basis in excess of the expected net realizable value and inventory that is in excess of expected requirements based upon anticipated product revenues. For each of the years ended December 31, 2011 and 2010, the Company recorded a charge to Cost of goods sold of $0.4 million for Qutenza patches, which the Company does not expect to sell prior to their expiration date. As a result of the Company’s re-organization in March 2012 which has resulted in a significant reduction of its expected revenue for United States Qutenza sales, the Company anticipates additional write-downs of inventory in the first quarter of 2012 to reflect these lower anticipated revenue levels. Expected inventory requirements based on product revenue and the timing of
80
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
production runs are subject to significant judgment and management estimate. The Company expects that its sales forecast will change over time and thus it will continue to review the quantities of inventories on hand compared to the sales forecast.
Prepaid Collaboration Supplies
Costs to manufacture product under the supply agreement with Astellas, including internal labor costs and third-party manufacturing, packaging and transportation costs are classified as prepaid collaboration supplies, which are included in Prepaid expenses and other current assets on the Company’s consolidated balance sheets, until such time as those supplies are delivered to Astellas.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation. Depreciation is determined using the straight-line method and recorded over the estimated useful lives of the respective assets, generally three to five years. Leasehold improvements are amortized over the life of the lease or the useful economic life, whichever is shorter.
Product Revenue
In April 2010, the Company made Qutenza commercially available in the United States to its specialty distributor and specialty pharmacy customers. Under the Company’s agreements with its customers, the customers take title to the product upon shipment and only have the right to return damaged product, product shipped in error and expired or short-dated product. As Qutenza is new to the marketplace, the Company is currently assessing the flow of product through its distribution channel, and cannot reasonably estimate returns. Until such time as an estimate of returns can be made, the Company is recognizing Qutenza product revenue, and related product costs, at the later of:
| • | the time the product is shipped by the customer to healthcare professionals, and |
| • | the date of cash collection. |
Healthcare professionals are generally ordering Qutenza in small quantities only after they have identified a patient for treatment. The Company believes that revenue recognition upon the later of customer shipment to the end user and the date of cash collection is appropriate until the Company has sufficient data on the cash collection and return patterns of its customers. The Company is performing additional procedures to further analyze shipments made by its customers by utilizing shipping data provided by its customers to determine when product is shipped by customers to healthcare professionals and are evaluating ending inventories at the Company’s customers each month to assess the risk of product returns.
The Company has established terms pursuant to distribution agreements with its customers providing for payment within 120 days of the date of shipment to its specialty distributor customers and 30 days of the date of shipment to its specialty pharmacy customers. Such distribution agreements generally have a term of three years.
The application of the Company’s revenue recognition policy resulted in the recognition of net revenue of $2.6 million from gross shipments of $3.4 million for the year ended December 31, 2011. The Company’s gross shipments are reduced for chargebacks, certain fees paid to distributors and product sales allowances including rebates to qualifying federal and state government programs. At December 31, 2011, net deferred product revenue totaled $1.1 million.
81
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Revenue from the Astellas Agreement
In June 2009, NeurogesX entered into the Astellas Agreement which provides for an exclusive license by the Company to Astellas for the promotion, distribution and marketing of Qutenza in the Licensed Territory, an option to license NGX-1998, a product candidate that is a non-patch liquid formulation of capsaicin, in the Licensed Territory, participation on a joint steering committee and, through a related supply agreement entered into with Astellas (the “Supply Agreement”), supply of product until direct supply arrangements between Astellas and third-party manufacturers are established. The Company anticipates certain of the direct supply arrangements will be established in the first half of 2012. Revenue under this arrangement includes upfront non-refundable fees and may also include additional option payments for the development of and license to NGX-1998, contingent event-based payments upon achievement of certain product sales levels and royalties on product sales.
The Company analyzes multiple-element arrangements, such as the Astellas Agreement, to determine whether the various performance obligations, or elements, can be separated or whether they must be accounted for as a single unit of accounting. The determination of whether an element can be separated or accounted for as a single unit of accounting is based on the availability of evidence with regard to standalone value of each delivered element. If the fair value of all undelivered performance obligations can be determined, then all obligations would then be accounted for separately as performed. If any delivered element is considered to either: 1) not have standalone value or 2) have standalone value but the fair value of any of the undelivered performance obligations are not determinable, then any delivered elements of the arrangement would be accounted for as a single unit of accounting and the multiple elements would be grouped and recognized as revenue over the longest estimated performance obligation period.
Significant management judgment is required in determining the level of effort required under an arrangement and the period over which the performance obligations are estimated to be completed. In addition, if the Company is involved in a joint steering committee as part of a multiple-element arrangement that is accounted for as a single unit of accounting, an assessment is made as to whether the involvement in the steering committee constitutes a performance obligation or a right to participate.
Revenue recognition of non-refundable upfront license fees commences when there is a contractual right to receive such payment, the contract price is fixed or determinable, the collection of the resulting receivable is reasonably assured and there are no further performance obligations under the license agreement.
The Company views the Astellas Agreement and related agreements, as a multiple-element arrangement with the key deliverables consisting of an exclusive license to Qutenza in the Licensed Territory, an obligation to conduct certain development activities associated with NGX-1998, participation on a joint steering committee and supply of Qutenza components to Astellas until such time that Astellas can establish a direct supply relationship with product vendors. Because not all of these elements have both standalone value and objective and reliable evidence of fair value, the Company is accounting for such elements as a single unit of accounting. Further, the agreement provides for the Company’s mandatory participation in a joint steering committee, which the Company believes represents a substantive performance obligation and also the estimated last delivered element under the Astellas Agreement and related agreements. Therefore, the Company is recognizing revenue associated with upfront payments and license fees ratably over the term of the joint steering committee performance obligation. The Company estimates this performance obligation will be delivered ratably through June 2016.
At the inception of the Astellas Agreement, the upfront license fees were subject to a refund right, which expired upon transfer of the Company’s MA for Qutenza in the European Union to Astellas. In September 2009, the transfer of the MA to Astellas was completed and, therefore, the upfront license fees became non-refundable and revenue recognition of the upfront license fees commenced.
82
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
In the accompanying financial statements, Collaboration revenue represents revenue associated with the Astellas Agreement and related agreements. Deferred collaboration revenue arises from the excess of cash received or receivable over cumulative revenue recognized for the period.
Under the terms of the Astellas Agreement, the Company earns royalties based on each sale of Qutenza into the Licensed Territory. The royalty rate is tiered based on the level of sales achieved. These royalties are considered contingent consideration as there is no remaining contract performance obligation of the Company and, therefore, the royalties are excluded from the single unit of accounting treatment discussed above. The Company reports royalty revenue based upon Astellas’ reported net sales of Qutenza in the Licensed Territory. The Company recognizes royalty revenue from Astellas on a quarter lag basis due to the timing of Astellas reporting such royalties to the Company.
Additionally, the Company can earn contingent event-based payments from Astellas if Astellas achieves certain sales levels as outlined in the Astellas Agreement. As these payments are contingent on Astellas’ performance, they are excluded from the single unit of accounting treatment discussed above. The contingent event-based payments will be recorded as revenue when earned as there is no remaining contractual performance obligation of the Company.
The Company recognizes revenue net of related costs for collaboration supplies sold to Astellas upon product being delivered to Astellas. The revenue and related costs of the product supplied to Astellas are included in Collaboration revenue. Prior to delivery to Astellas, the costs accumulated for the manufacturing of the collaboration supplies are included as Prepaid expenses and other current assets on the Company’s consolidated balance sheets.
Cost of Goods Sold
Cost of goods sold includes costs to procure, manufacture and distribute Qutenza including certain shipping and handling costs incurred in the distribution channel, as well as royalties payable to The Regents of the University of California, or UC, and Lohmann Therapie-Systeme AG or LTS. Costs associated with the manufacture of Qutenza prior to FDA approval were previously expensed as research and development in the period incurred. Cost of goods sold associated with deferred product revenue will be deferred and recognized in the same period as the associated product revenue. At December 31, 2011, the Company had deferred Cost of goods sold of $0.1 million.
During the years ended December 31, 2011 and 2010, the Company recorded charges of $0.4 million and $0.4 million, respectively, for Qutenza patches which the Company does not expect to sell prior to their expiration date.
Research and Development
The Company expenses research and development costs as incurred. Research and development expenses include personnel and personnel related costs, costs associated with pre-clinical and clinical development activities, such as clinical trials, including amounts paid to clinical research organizations and clinical investigators; product and manufacturing costs, such as process development, clinical product supply costs and the cost of commercially saleable product that is manufactured prior to market approval for that product candidate; internal and external costs associated with the Company’s regulatory compliance and quality assurance functions including the costs of outside consultants and contractors that assist in the process of submitting and maintaining regulatory filings; and overhead costs including allocated facility and related expenses.
83
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Clinical Trials
The Company accrues and expenses costs for clinical trial activities performed by third parties, including clinical research organizations and clinical investigators, based upon estimates of the work completed over the life of the individual study in accordance with agreements established with contract research organizations and clinical trial sites. The Company determines these estimates through discussion with internal personnel and outside service providers as to progress or stage of completion of trials or services pursuant to contracts with numerous clinical trial centers and clinical research organizations and the agreed upon fee to be paid for such services.
Advertising Costs
The Company expenses both the production costs and the placement costs of advertising as incurred. Advertising expense which includes Qutenza’s website development, eLearning, eMarketing, media campaigns, patient testimonial videos and other items was $2.3 million, $5.8 million and $0.7 million for the years ended December 31, 2011, 2010 and 2009, respectively.
Interest Expense
Interest expense is comprised of interest relating to the Company’s long-term obligations, the accretion of the debt discount, and the amortization of debt issuance costs recorded as Prepaid expenses and other current assets and Other assets on the consolidated balance sheets. Interest expense includes both currently payable interest charges and interest charges that accrue but are not currently due as a result of the terms of the underlying debt agreement. See Note 7 for a discussion of the Cowen Healthcare Royalty debt facility.
Other Income (Expense), Net
Other income (expense), net, in 2011 was a net expense of $0.1 million primarily related to investment advisory fees. Other income (expense), net, was a net income of $0.6 million in 2010 that resulted primarily from income tax credits earned under Section 48D of the Internal Revenue Code (as enacted on March 23, 2010 by the “Patient Protection and Affordable Care Act”). The Company applied for a credit based upon certain of its 2009 and 2010 research and development activities. The payment for the 2009 credit of $0.4 million was received in 2010 whereas the payment for the 2010 credit of $0.2 million was received in 2011.
Income Taxes
The Company utilizes the liability method of accounting for income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax reporting bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. Uncertain tax positions are evaluated and if appropriate, the amount of unrecognized tax benefits are recorded within deferred tax assets, as further described in Note 12. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized. Currently, there is no provision for income taxes as the Company has incurred operating losses to date.
Comprehensive (Loss)/Income
Comprehensive (loss)/income includes net loss and other comprehensive (loss)/income. For each of the years ended December 31, 2011, 2010 and 2009, the difference between comprehensive loss and net loss represented net unrealized gains or losses on available-for-sale securities.
84
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Net Loss Per Share
Basic and diluted net loss per share is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period less the weighted-average unvested common shares subject to repurchase. For purposes of this calculation, warrants and options to purchase common stock are considered to be common stock equivalents but have been excluded from the calculation of diluted net loss per share as their effect is anti-dilutive.
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| (in thousands except share and per share data) | ||||||||||||
| Numerator: |
||||||||||||
| Net loss |
$ | (49,545 | ) | $ | (44,516 | ) | $ | (21,867 | ) | |||
|
|
|
|
|
|
|
|||||||
| Denominator: |
||||||||||||
| Weighted-average common shares outstanding |
23,011,015 | 17,768,881 | 17,611,189 | |||||||||
| Less: Weighted-average unvested common shares subject to repurchase |
— | — | (393 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Denominator for basic and diluted net loss per share |
23,011,015 | 17,768,881 | 17,610,796 | |||||||||
|
|
|
|
|
|
|
|||||||
| Basic and diluted net loss per share |
$ | (2.15 | ) | $ | (2.51 | ) | $ | (1.24 | ) | |||
|
|
|
|
|
|
|
|||||||
| Outstanding securities not included in diluted net loss per share: |
||||||||||||
| Options to purchase common stock |
3,037,039 | 3,002,493 | 2,264,535 | |||||||||
| Warrants outstanding |
7,932,295 | 1,265,846 | 1,265,846 | |||||||||
| Restricted stock units |
781,000 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| 11,750,334 | 4,268,339 | 3,530,381 | ||||||||||
|
|
|
|
|
|
|
|||||||
Recently Issued and Adoption of New Accounting Standards
Not Yet Adopted
In June 2011, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2011-05, “Presentation of Comprehensive Income” an update to Accounting Standards Codification (“ASC”) Topic 220, “Comprehensive Income”. The amendments of this update require that comprehensive income be presented either in a single continuous statement of comprehensive income or in two separate but consecutive statements. This update is to be applied retroactively and is effective for financial statements issued for fiscal years, and interim periods within those years, beginning after December 15, 2011, and interim and annual periods thereafter. This update will be effective for the Company January 1, 2012. The Company does not expect the adoption of this guidance to have a material impact on its consolidated financial statements.
Adopted in 2011
In October 2009, FASB issued ASU No. 2009-13, Revenue Recognition (ASC Topic 605)—Multiple-Deliverable Revenue Arrangements. This guidance modifies previous guidance for multiple element arrangements by allowing the use of the “best estimate of selling price” in the absence of vendor-specific objective evidence (“VSOE”) or third-party evidence (“TPE”) of selling price for determining the selling price of a deliverable. A vendor is now required to use its best estimate of the selling price when VSOE or TPE of the selling price is not available. In addition, the residual method of allocating arrangement consideration is no longer permitted. This ASU is effective for revenue arrangements entered into or materially modified in fiscal
85
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
years beginning on or after June 15, 2010 and early adoption was permitted. The Company adopted this ASU on January 1, 2011 on a prospective basis and the adoption of the new guidance did not have a material impact on the Company’s consolidated financial statements. The Company currently only has one revenue agreement with multiple elements, the Astellas Agreement. If the Company was to enter into new multiple element arrangements or make a material modification to the Astellas Agreement after January 1, 2011, the contract would be accounted for under the provisions of ASU No. 2009-13.
In April 2010, the FASB issued ASU No. 2010-17, Revenue Recognition—Milestone Method (ASC Topic 605): Milestone Method of Revenue Recognition (“ASU No. 2010-17”). This ASU codifies the consensus reached in EITF Issue No. 08-9, “Milestone Method of Revenue Recognition.” The amendments to the Codification provide guidance on defining a milestone and determining when it may be appropriate to apply the milestone method of revenue recognition for research or development transactions. Consideration that is contingent on the achievement of a milestone in its entirety may be recognized as revenue in the period in which the milestone is achieved only if the milestone is judged to meet certain criteria to be considered substantive. Milestones should be considered substantive in their entirety and may not be bifurcated. An arrangement may contain both substantive and non-substantive milestones, and each milestone should be evaluated individually to determine if it is substantive. ASU No. 2010-17 is effective for fiscal years, and interim periods within those years, beginning on or after June 15, 2010, and may be applied prospectively to milestones achieved after the adoption date or retrospectively for all periods presented. On January 1, 2011, the Company prospectively adopted and elected the Milestone Method of Revenue Recognition as its accounting policy for substantive milestones achieved after the adoption date. The adoption of the new guidance did not have a material impact on the Company’s consolidated financial statements.
3. Cash and Cash Equivalents and Short-Term Investments
The following are summaries of cash, cash equivalents and short-term investments (in thousands):
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
|||||||||||||
| As of December 31, 2011: |
||||||||||||||||
| Cash and money market funds |
$ | 3,908 | $ | — | $ | — | $ | 3,908 | ||||||||
| Government sponsored enterprise debt securities |
23,900 | 2 | (1 | ) | 23,901 | |||||||||||
| U.S. Treasury securities |
6,500 | — | — | 6,500 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 34,308 | $ | 2 | $ | (1 | ) | $ | 34,309 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Reported as: |
||||||||||||||||
| Cash and cash equivalents |
$ | 9,148 | ||||||||||||||
| Short-term investments |
25,161 | |||||||||||||||
|
|
|
|||||||||||||||
| $ | 34,309 | |||||||||||||||
|
|
|
|||||||||||||||
| As of December 31, 2010: |
||||||||||||||||
| Cash and money market funds |
$ | 6,705 | $ | — | $ | — | $ | 6,705 | ||||||||
| Commercial paper |
2,000 | — | — | 2,000 | ||||||||||||
| U.S. Treasury securities |
38,127 | 1 | (3 | ) | 38,125 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 46,832 | $ | 1 | $ | (3 | ) | $ | 46,830 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Reported as: |
||||||||||||||||
| Cash and cash equivalents |
$ | 8,705 | ||||||||||||||
| Short-term investments |
38,125 | |||||||||||||||
|
|
|
|||||||||||||||
| $ | 46,830 | |||||||||||||||
|
|
|
|||||||||||||||
86
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
At December 31, 2011 and 2010, the contractual maturities of investments held were one year or less. The Company sold $7.1 million of its investments prior to the maturity date in the twelve months ended December 31, 2011 and recorded an immaterial gain on the sale. The Company did not sell any of its investments prior to maturity during 2010. The Company’s short-term restricted cash of $0.2 million at December 31, 2011 is related to its obligation under the sublease for its corporate headquarters which is secured by a certificate of deposit. The Company’s long-term restricted cash of $0.3 million at December 31, 2011 is related to its corporate credit card program.
4. Fair Value Measurements
The Company discloses its cash equivalents and short-term investments under a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are the following:
Level 1—Quoted prices in active markets for identical assets or liabilities.
Level 2—Inputs other than quoted prices included within Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
87
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
In accordance with ASC 820, the following table represents the Company’s financial assets (cash equivalents and short-term investments) measured at fair value on a recurring basis and their level within the fair value hierarchy as of December 31, 2011 and December 31, 2010 (in thousands):
| Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
Total | |||||||||||||
| Fair Value Measurements at December 31, 2011: |
||||||||||||||||
| Components of cash equivalents and short-term investments measured at fair value: |
||||||||||||||||
| Money market funds |
$ | 3,855 | $ | — | $ | — | $ | 3,855 | ||||||||
| U.S. Treasury securities |
— | 6,500 | — | 6,500 | ||||||||||||
| Government sponsored enterprise debt securities |
— | 23,901 | — | 23,901 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total financial assets measured at fair value |
$ | 3,855 | $ | 30,401 | $ | — | 34,256 | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Components of cash and cash equivalents not measured at fair value: |
||||||||||||||||
| Operating cash |
53 | |||||||||||||||
|
|
|
|||||||||||||||
| Total cash and cash equivalents and short-term investments |
$ | 34,309 | ||||||||||||||
|
|
|
|||||||||||||||
| Fair Value Measurements at December 31, 2010: |
||||||||||||||||
| Components of cash equivalents and short-term investments measured at fair value: |
||||||||||||||||
| Money market funds |
$ | 6,370 | $ | — | $ | — | $ | 6,370 | ||||||||
| Commercial paper |
— | 2,000 | — | 2,000 | ||||||||||||
| U.S. Treasury securities |
— | 38,125 | — | 38,125 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total financial assets measured at fair value |
$ | 6,370 | $ | 40,125 | $ | — | 46,495 | |||||||||
|
|
|
|
|
|
|
|||||||||||
| Components of cash and cash equivalents not measured at fair value: |
||||||||||||||||
| Operating cash |
335 | |||||||||||||||
|
|
|
|||||||||||||||
| Total cash and cash equivalents and short-term investments |
$ | 46,830 | ||||||||||||||
|
|
|
|||||||||||||||
The Company revised its 2010 disclosure to indicate that commercial paper and U.S. treasury securities are valued using Level 2 inputs as opposed to Level 1 inputs as originally reported.
In addition to Level 1 and Level 2 assets held by the Company at December 31 2011, the Company measures its contingent put option liability related to the loan agreement with Hercules (see Note 7), utilizing Level 3 measurements. The fair value of the contingent put option of $0.1 million was determined by evaluating multiple potential outcomes of an event of default, including a change of control, using an income approach and discounting the values back to December 31, 2011.
88
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
5. Inventories
The components of inventories are as follows (in thousands):
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Raw materials |
$ | 10 | $ | 7 | ||||
| Work-in-process(1) |
591 | 581 | ||||||
| Finished goods |
53 | 198 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 654 | $ | 786 | ||||
|
|
|
|
|
|||||
| (1) | Included in work-in-process at December 31, 2011 and 2010 is $0.2 million and $0.2 million, respectively, of product awaiting final packaging. |
The above inventory balances relate to the manufacturing of Qutenza and are costs capitalized since the Company received FDA approval for the sale of Qutenza in the US market in the fourth quarter of 2009. Prior to FDA approval, all manufacturing and product related costs for Qutenza and for the Company’s other development programs were expensed to research and development. On a quarterly basis, the Company analyzes its inventory levels and writes down inventory that has become obsolete, inventory that has a cost basis in excess of the expected net realizable value and inventory that is in excess of expected requirements based upon anticipated product revenues. In each of the years ended December 31, 2011 and 2010, the Company recorded a charge to Cost of goods sold of $0.4 million for Qutenza patches which the Company does not expect to sell prior to their expiration date.
6. Property and Equipment
Property and equipment consists of the following (in thousands):
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Computer equipment |
$ | 828 | $ | 745 | ||||
| Leasehold improvements |
275 | 275 | ||||||
| Software |
520 | 373 | ||||||
| Office furniture and equipment |
327 | 327 | ||||||
| Marketing related equipment |
149 | 149 | ||||||
| Research equipment |
56 | 46 | ||||||
| Construction in progress |
20 | 146 | ||||||
|
|
|
|
|
|||||
| 2,175 | 2,061 | |||||||
| Less: accumulated depreciation and amortization |
(1,628 | ) | (1,252 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 547 | $ | 809 | ||||
|
|
|
|
|
|||||
Depreciation expense was $0.4 million, $0.3 million and $0.2 million for the years ended December 31, 2011, 2010 and 2009, respectively. Construction in progress primarily represents software that was not yet placed into service by yearend. As a result of the Company’s re-organization in March 2012, it expects to write-down certain of its property and equipment in the first quarter of 2012.
89
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
7. Long-Term Obligations
The following table summarizes the carrying amount of the Company’s borrowings under various long-term obligations (in thousands):
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Cowen Financing Agreement |
$ | 53,344 | $ | 44,844 | ||||
| Hercules Technology Growth Capital, Inc.—Term loan |
14,231 | — | ||||||
|
|
|
|
|
|||||
| Total |
67,575 | 44,844 | ||||||
| Less: Long-term obligations—current portion |
4,829 | 2,222 | ||||||
|
|
|
|
|
|||||
| Long-term obligations |
$ | 62,746 | $ | 42,622 | ||||
|
|
|
|
|
|||||
Cowen Financing Agreement
On April 30, 2010, the Company entered into a Financing Agreement (the “Financing Agreement”) with Cowen Healthcare Royalty Partners, L.P., a limited partnership organized under the laws of the State of Delaware (“Cowen”). Under the terms of the Financing Agreement, the Company borrowed $40.0 million from Cowen (the “Borrowed Amount”) and the Company agreed to repay such Borrowed Amount together with a return to Cowen, as described below, out of royalty, milestone, option and certain other payments (collectively, “Revenue Interest”) that the Company may receive under the Astellas Agreement or a replacement licensee, as a result of commercializing the Company’s product, Qutenza, in the Licensed Territory, including payments that Astellas may make to the Company to maintain its option to commercialize NGX-1998 in the Licensed Territory.
Under the Financing Agreement, the Company is obligated to pay to Cowen:
| • | All of the Revenue Interest payments due to the Company under the Astellas Agreement, until Cowen has received $90.0 million of Revenue Interest payments; and |
| • | 5% of the Revenue Interest payments due to the Company under the Astellas Agreement for Revenue Interest received by Cowen over $90.0 million and until Cowen has received $106.0 million of Revenue Interest payments. |
The Company also has the option to pay a prepayment amount to terminate the Financing Agreement at the Company’s election at any time or in connection with a change of control of the Company. Such amount is set at a base amount of $76.0 million (or $68.0 million if it is being exercised in connection with a change of control), or, if higher, an amount that generates a specified internal rate of return, as noted below, on the Borrowed Amount as of the date of prepayment, in each case reduced by the Revenue Interest payments received by Cowen up to the date of prepayment.
The obligation of the Company to pay Revenue Interest during the term of the Financing Agreement (which is defined below) is secured by rights that the Company has to Revenue Interest under the Astellas Agreement, and by intellectual property and other rights of the Company to the extent necessary or used to commercialize products covered by the Astellas Agreement in the Licensed Territory.
Unless terminated earlier pursuant to a prepayment, the Financing Agreement terminates on the earlier of:
| • | The time when Cowen has received at least $106.0 million of Revenue Interest payments; or |
| • | The maturity date, which is the latest to occur of 10 years following the first commercial sale of Qutenza in the Licensed Territory or the last to expire of any patents or regulatory exclusivity covering the products commercialized under the Astellas Agreement in the Licensed Territory. |
90
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
If Cowen has not received Revenue Interest payments totaling at least $40.0 million by the maturity date, the Company will be obligated to pay to Cowen the difference between such amount and the Revenue Interest paid to Cowen under the Financing Agreement up to such date. This represents the Company’s minimum financial obligation under the Financing Agreement.
In addition, in the event of the Company’s default under the Financing Agreement, the Company is obligated to pay to Cowen the borrowed amount together with interest accrued thereon from the effective date of the Financing Agreement, to the date of repayment at an internal rate of return equal to the lesser of a 19% rate of interest or the maximum rate permitted by law, less any Revenue Interest payments received by Cowen. Under the Financing Agreement, an event of default includes standard insolvency and bankruptcy terms, including a failure to maintain liquid assets equal to at least the Company’s liabilities due and payable during the following three months.
The upfront cash receipt of $40.0 million less a discount of $0.5 million in cost reimbursements paid to Cowen was recorded in Long-term obligations at issuance. The Company is accreting the discount to interest expense over the term of the related debt. The carrying value of the Long-term obligation at December 31, 2011, including accrued interest of $13.8 million, is $53.3 million. The carrying value of the Long-term obligation at December 31, 2010, including accrued interest of $5.4 million, was $44.8 million. The Company received $0.9 million of royalty payments from Astellas in the year ended December 31, 2011, and consistent with the provisions of the arrangement, the Company paid that amount to Cowen as a reduction of the obligation. The Company made payments to Cowen of less than $0.1 million in the year ended December 31, 2010.
The best estimate of future payments, the timing of which determines the short- and long-term classification of the obligation under the Financing Agreement, was based upon returning to Cowen an internal rate of return of 19% through Revenue Interest payments. Under this methodology, the Company recorded interest expense of $9.4 million and $5.4 million for the years ended December 31, 2011 and 2010, respectively. The accrued interest on the Long-term debt obligation is presented on the consolidated balance sheets as of December 31, 2011 in two components, the Long-term obligation—current portion, which totals $2.5 million and the remaining $11.3 million included in Long-term obligation. At December 31, 2010, the Long-term obligation—current portion was $2.2 million of accrued interest and the remaining $3.1 million of accrued interest was included in Long-term obligations.
The Company capitalized $0.3 million of debt issuance costs related to the Financing Agreement which are being amortized over the term. At both December 31, 2011 and December 31, 2010, the unamortized debt issuance cost was $0.3 million, and is included in “Prepaid expenses and other current assets” and “Other assets” on the Company’s consolidated balance sheets.
The estimated fair value of the Cowen Borrowed Amount at December 31, 2011 and December 31, 2010 was $43.6 million and $40.9 million, respectively, and the fair value was measured using Level 3 inputs. The estimated fair market value was calculated using the income method of valuation. The key assumptions required for the calculation were an estimate of the amount and timing of future royalty revenues to be received from Astellas and an estimated cost of capital. Management’s estimate of the future royalty revenues to be received from Astellas is subject to significant variability due to the recent product launch, regulatory process still to be conducted for significant portions of the Astellas territory and the extended time period associated with the Cowen Financing Agreement, and thus is subject to significant uncertainty.
Hercules Loan Agreement
In August 2011, the Company entered into a loan agreement with Hercules, which includes both a $5.0 million accounts receivable line and a $15.0 million term loan. Under the terms of the loan agreement, the $15.0
91
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
million term loan is required to be repaid over the course of the 42-month maturity period, which includes a 12-month interest only period at the beginning of the term. Interest on the term loan has a minimum rate of 9.5%, which can increase based on fluctuations in the prime rate. The $5.0 million accounts receivable line is a revolving line that is available until December 31, 2012 and then is renewable at the option of Hercules each year thereafter. The accounts receivable line bears interest at a minimum interest rate of 6.75%, which can also increase based on fluctuations in the prime rate, and the maximum borrowing availability under the revolving line is based upon a percentage of eligible accounts receivable of the Company. The Company may prepay and terminate the term loan at any time, subject to certain prepayment fees, and is obligated to also pay a fee of $0.6 million when the term loan is repaid and which is being accrued to interest expense over the term of the loan. The obligations of the Company under the loan agreement are secured by certain personal property of the Company.
The loan agreement also contains customary negative covenants and is subject to customary events of default, such as a failure to make a scheduled principal or interest payment, insolvency, and breaches of covenants under loan agreement and agreements and instruments entered into in connection with the loan agreement, including covenants in the Hercules warrant. Upon an event of default, including a change of control, Hercules has the option to accelerate repayment of the loan, including payment of any applicable prepayment charges, which range from 1%-3% of the outstanding loan balance and accrued interest, as well as a final payment fee of $0.6 million. This option is considered a contingent default provision liability as the holder of the loan may exercise the option in the event of default and, is a derivative which must be bifurcated and valued separately in the Company’s financial statements. As of December 31, 2011, the estimated fair value of the contingent put option upon an event of default liability was $0.1 million which was determined by evaluating multiple potential outcomes of an event of default, including a change of control, using an income approach and discounting the values back to December 31, 2011. As of December 31, 2011, the contingent put option liability is included in Other long-term liabilities on the consolidated balance sheets and the fair value of the warrants at the time of issuance resulted in a debt discount. The contingent put option liability will be revalued at the end of each reporting period and any change in the fair value will be recognized in the statements of operations.
In connection with the entry of the Company into the loan agreement, Hercules was issued a warrant for 791,667 shares of the Company’s common stock (representing an aggregate exercise price equal to approximately 7.125% of the maximum aggregate funds potentially available to the Company under the loan agreement) (the Hercules Warrant). The warrants have a term of five years, contains a net-exercise provision, and has an exercise price of $1.80 per share. The fair value of the warrants issued was approximately $1.1 million based on a Black-Scholes valuation model; $0.8 million of which was attributable to the warrants issued in connection with the term loan, and $0.3 million of which was attributable to warrants issued in connection with the accounts receivable line. The fair value of the warrants issued in connection with the term loan resulted in a debt discount, which is being amortized to interest expense over the life of the loan using the effective interest method. The fair value of the warrants issued in connection with the accounts receivable line was recorded in Prepaid and other current assets and Other assets, respectively. The Company will amortize these assets over the initial term of the accounts receivable line that ends December 31, 2012 on a straight-line basis. The Company also paid $0.4 million in issuance costs which were allocated to the term loan and accounts receivable line and accounted for consistent with the warrants as described above.
In March 2012, the Company entered into an amendment to the Hercules Warrant in which the Company amended the covenant with respect to the requirement to maintain its listing on the NASDAQ Global Market to allow for the NASDAQ Capital Market and certain over-the-counter markets to be permissible alternative markets on which the Company can list its shares of common stock. Under the terms of the amendment, the number of shares underlying the warrant was increased to 1,950,000 and the exercise price per share of common stock was reduced to $0.50.
92
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The recorded value of the term loan is $13.8 million after deducting expenses of $0.3 million paid to Hercules, $0.8 million for the value of the warrants allocated to the term loan and $0.1 million for the value ascribed to the default provision of the agreement. The estimated fair value of the Hercules term loan and the default provision was $15.0 million and $0.1 million, respectively, at December 31, 2011 and the liabilities were measured at fair value using Level 3 inputs.
The Company had $0.8 million available for draw-down on the accounts receivable line at December 31, 2011 based upon a percentage of the then eligible accounts receivable of the Company. The Company has not drawn any funds from the accounts receivable line.
8. Commitments and Contingencies
Operating Leases
The Company entered into an operating lease in September 2007 for its corporate headquarters, consisting of approximately 26,386 square feet of office space in San Mateo, California. The lease, which expires in July 2012, includes total base rent of $2.3 million, a period of free rent and rent escalation, all of which are accounted for on a straight-line basis over the lease term. Also included in the terms of the lease is a tenant improvement allowance of $106,000, which was received in 2009, was recorded to deferred rent and is being amortized on a straight-line basis over the remaining term of the lease. The Company’s obligation under the sublease is secured by a letter of credit in the original amount of $240,000, and in accordance with the provisions of the sublease agreement, the Company can reduce the letter of credit, if certain conditions are met, by $40,000 on each of the first three anniversaries of the lease commencement date. As a result of having met these conditions, the Company reduced the letter of credit to $160,000 at December 31, 2010, of which the full amount is secured by a certificate of deposit that is included in the Company’s consolidated balance sheet at December 31, 2011 as Restricted cash in current assets. The Company has a deferred rent liability of $115,000 as of December 31, 2011, of which the full amount is classified as a current liability.
Total rent expense under all operating leases, which also includes rent expense for certain office equipment, was $0.5 million for each of the years ended December 31, 2011, 2010 and 2009. Future minimum payments under all non-cancellable operating lease obligations are as follows as of December 31, 2011 (in thousands):
| Year End December 31, |
||||
| 2012 |
$ | 391 | ||
| 2013 |
16 | |||
| 2014 |
— | |||
| 2015 and beyond |
— | |||
|
|
|
|||
| Total minimum lease payments |
$ | 407 | ||
|
|
|
|||
Purchase Obligations
The Company has issued non-cancellable purchase orders to third-party manufacturers of Qutenza and NGX-1998 for manufacturing, stability and related services including reimbursable amounts from Astellas for the supply of Qutenza based on the Astellas Agreement. These purchase orders totaled $1.5 million as of December 31, 2011 and included $1.0 million for stability and related services, $0.2 million related to Qutenza product manufacturing costs for the U.S. market and NGX-1998 clinical supply development cost along with $0.3 million of commitments related to supply of Qutenza to Astellas. Actual payments for purchases from these third-party manufacturers, a portion of which were made to satisfy Astellas supply requests, were $2.9 million, $3.2 million and $1.7 million during the years ended December 31, 2011, 2010 and 2009, respectively.
93
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Indemnifications
In the normal course of business, the Company enters into contracts and agreements that contain a variety of representations and warranties and generally provide for various indemnifications including indemnification from claims resulting from clinical trial activities and intellectual property matters. The Company’s liability under these agreements is unknown because it involves the potential for future claims that may be made against the Company, but have not yet been made. To date, the Company has not received any claims under its various indemnification obligations. However, the Company may record charges in the future as a result of these indemnification obligations.
The Company, as permitted under Delaware law and in accordance with its bylaws, indemnifies its officers and directors for certain events or occurrences, subject to certain limits, while the officer or director is or was serving at the Company’s request in such capacity. The Company may terminate the indemnification agreements with its officers and directors upon 90 days written notice, but termination will not affect claims for indemnification relating to events occurring prior to the effective date of termination. The maximum amount of potential future indemnification is unlimited, however, the Company’s director and officer insurance policy reduces the Company’s exposure and may enable the Company to recover a portion of any future amounts paid. The Company believes that the fair value of these indemnification agreements is minimal. Accordingly, the Company has not recorded any liabilities for these agreements as of December 31, 2011.
Legal Matters
The Company is subject to various legal disputes that arise in the normal course of business which include or may include employment matters, contract disputes, intellectual property matters, product liability actions, and other matters. The Company does not know whether it would prevail in these matters nor can it be assured that any remedy could be reached on commercially viable terms, if at all. Based on currently available information, the Company does not believe that any of these disputes will have a material adverse impact on the Company’s results of operations, liquidity or financial position. The Company records a liability when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated. The Company recorded a $1.0 million charge in 2010 related to intellectual property matters as discussed in Note 10, and subsequently reversed $0.3 million of such accrual in 2011.
9. Restructuring
In October 2011, the Company’s board of directors approved a restructuring plan whereby the Company’s workforce was reduced by 26 individuals. The Company communicated the plan to the impacted employees on October 28, 2011 and the last day of employment for a majority of the affected employees was November 2, 2011. The restructuring decision resulted from the Company’s evaluation of the U.S. Qutenza launch. The restructuring plan reduced the Company’s sales force from 40 to 25 employees and eliminated an additional 11 positions across all functions. The Company recorded a charge of approximately $0.8 million in the fourth quarter of 2011 related to this restructuring, which is presented in research and development and selling, general and administrative expenses in the consolidated statements of operations.
In March 2012 the Company undertook an additional restructuring. See Note 13.
10. License Agreements
Collaboration Agreement—Astellas
In June 2009, NeurogesX entered into the Astellas Agreement granting Astellas an exclusive license to commercialize Qutenza in the Licensed Territory. The Astellas Agreement provided for an upfront payment for
94
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
past development and the commercialization rights granted, of 30 million Euro, or $41.8 million. In addition, the agreement provided for an upfront payment of 5 million Euro, or $7.0 million, for future development expenses and an option to license NGX-1998, a product candidate in Phase 1 development at that time. Other elements of the Astellas Agreement include future payments of up to 65 million Euro if certain pre-defined sales thresholds of the products licensed under the agreement are met and royalties, as a percentage of net sales made by Astellas of products under the agreement, with such royalties starting in the high teens and escalating into the mid twenties as revenues increase. The Company is participating on a joint steering committee with Astellas to oversee the development and commercialization activities related to Qutenza and NGX-1998 during the term of the agreement, not to exceed ten years. On the seventh anniversary of the Astellas Agreement, the Company has the unilateral right to opt-out of participation on the joint steering committee. The Astellas Agreement further provides that upon delivery of certain data related to NGX-1998, Astellas may pay two additional NGX-1998 option payments totaling 5 million Euro. Subsequent to Astellas’ exercise of the option to exclusively license NGX-1998 in the Territory, both companies would cooperate on Phase III clinical trials and will share such costs equally.
The Company commenced recognizing revenue related to the upfront and option payments upon transfer of the Marketing Authorization for Qutenza to Astellas, which occurred in September 2009. For the years ended December 31, 2011, 2010 and 2009, the Company recognized $7.2 million, $7.2 million and $1.9 million as Collaboration revenue related to the upfront and option payments, respectively. As of December 31, 2011, the Company had deferred revenue totaling $32.4 million, of which $7.3 million is reflected as current. The Astellas upfront license fee and option payments are accounted for as a single unit of accounting, and accordingly, such payments will be recognized ratably through June 2016, which is the Company’s estimate of its substantive performance obligation period related to the joint steering committee. Subsequent option exercise payments received, if any, are expected to be recognized ratably over the remaining estimated period of performance.
The agreement will remain effective on a country-by-country and product-by-product basis until the later of ten years after the first commercial sale, expiration or abandonment of the last valid patent claim or expiration of all applicable periods of regulatory exclusivity. Astellas may terminate the agreement for any reason without penalty, consequence or compensation on a country-by-country and product-by-product basis upon written notice to the Company.
Pursuant to the Supply Agreement, the Company has agreed to provide Astellas with a sufficient supply of Qutenza to support their commercialization efforts until Astellas can establish a direct supply relationship with product vendors. These products will be charged to Astellas at contractually agreed upon costs per unit which are intended to reflect the Company’s direct cost of goods and related internal labor and overhead costs without mark-up for profit. The Company reported amounts received from product transactions net of direct costs incurred as a component of Collaboration revenue.
For the years ended December 31, 2011, 2010 and 2009, the Company included in Collaboration revenue $0.8 million, $0.3 million and $0.1 million from product transactions, net of direct costs incurred as a component of Collaboration revenue, respectively.
For the years ended December 31, 2011 and 2010, the Company recognized $0.9 million and a nominal amount, respectively, in royalty revenue related to the Astellas Agreement as a component of Collaboration revenue. There was no royalty revenue in 2009.
95
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
University Of California
In October 2000 and as amended, the Company licensed certain patents, including a method patent, from the University of California (“UC”) for prescription strength capsaicin for neuropathic pain. Under the terms of the agreement, the Company is required to pay royalties on net sales of the licensed product up to a maximum of $1.0 million per annum, as well as a percentage of upfront and milestone payments received by the Company from sublicensing the Company’s rights under the agreement (“Sublicense Fee”). As a result of the Astellas license payment received in July 2009, the Company accrued a $1.2 million sublicense fee to research and development expense during the third quarter of 2009, which was paid in February 2010. The Company recognized $14,000 and $4,000, respectively, in royalty expense due to UC for the years ended December 31, 2011 and 2010 related to Qutenza product sales in the United States.
Two of the three inventors named in the method patent did not assign their patent rights to UC. These non-assigning inventors have made various claims related to inventorship and other matters. In 2010, the Company entered into negotiations with these non-assigning inventors to settle any and all claims and to have them assign their rights related to the method patent and any other related patent rights that may exist at the time we enter into a final binding settlement agreement. The Company anticipates that a final settlement may include the Company issuing stock or stock options to the two non-assigning inventors and making current and future cash payments. The Company anticipates that approximately 50% of the cash settlement amount will be recovered over time, through a reduction in amounts due to the University of California under the Company’s license agreement with them. During the year ended December 31, 2010, the Company recorded a $1.0 million charge within selling, general and administrative expense representing its estimate of the potential settlement. In 2011, the Company continued negotiations and has revised its estimate of the potential settlement down to $0.7 million, which is the current cash and estimated fair value of the stock or stock options to be issued as part of a potential settlement. The estimated amount to be paid is included in Other accrued expenses on the consolidated balance sheets.
LTS Lohmann Therapie-Systeme AG
In January 2007, the Company entered into a Commercial Supply and License Agreement (“LTS Agreement”) with LTS Lohmann Therapie-Systeme AG (“LTS”) to manufacture commercial and clinical supply of Qutenza. Under the terms of the agreement, the Company is required to pay a transfer price for product purchased, as well as a royalty on net sales of product purchased under the LTS Agreement. In addition, upon first market approval of Qutenza, the Company is required to make a one-time milestone payment of 100,000 Euros. During the second quarter of 2009, the Company received market approval of Qutenza in the European Union and as a result, paid LTS a one-time milestone payment of 100,000 Euros, or approximately $140,000, which was charged to research and development expense. The Company recognized $47,000 and $12,000, respectively, in royalty expense due to LTS Lohmann Therapie-Systeme AG for the years ended December 31, 2011 and 2010 related to Qutenza product sales in the United States.
The Company’s December 31, 2011 consolidated balance sheet includes a liability for royalties due UC and LTS totaling $21,000 related to gross product shipments in the year ended December 31, 2011.
11. Stockholders’ Equity
Common Stock
On July 26, 2011, the Company completed a private placement under a Securities Purchase Agreement pursuant to which the Company issued “Units” for an aggregate purchase price of approximately $20.2 million, at a per Unit price of $1.72. Each Unit was comprised of one share of common stock and a warrant to purchase
96
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
0.5 shares of common stock (representing 50% warrant coverage on the shares to be issued) (the “Warrants”). The price of each Unit was based on the July 21, 2011 consolidated closing bid price of the Company’s common stock on the NASDAQ Global Market of $1.65 per share, and represented a purchase price of $1.65 for the one share of common stock issued for each Unit and a Warrant purchase price of $0.07 for the 0.5 shares of common stock underlying the Warrants issued for each Unit (resulting in a purchase price for the Warrants of $0.14 per whole share of common stock underlying such Warrants). The total number of Units issued in connection with the transaction was 11,749,552, representing an aggregate issuance of 11,749,552 shares of common stock and Warrants to purchase an aggregate of 5,874,782 shares of common stock. The Warrants have a term of five years, contain a net-exercise provision, and have an exercise price of $1.65 per share. In addition, the Warrants contain terms that prevent the Warrants from being exercised to the extent that such exercise would cause a stockholder’s beneficial ownership (along with its affiliates and others with whom such stockholder’s holdings would be aggregated for purposes of Section 13(d) of the Securities Exchange Act of 1934, as amended) to exceed 19.999% of the total number of then issued and outstanding shares of common stock of the Company. The fair value of the warrants issued was approximately $5.9 million based on a Black-Scholes valuation model. The net proceeds to the Company in connection with the Securities Purchase Agreement were $18.6 million, after deducting expenses of approximately $1.6 million.
Pursuant to a Registration Rights Agreement entered into in connection with the Securities Purchase Agreement, the Company filed a registration statement covering the resale of the common stock and the shares of common stock underlying the Warrants issued and issuable to the purchasers of Units on August 24, 2011. The registration statement was declared effective on September 8, 2011.
Warrants to Purchase Common Stock
In May 2007, the Company completed its IPO as a result of which all of the existing shares of the Company’s preferred stock were converted to common stock. At the time of the completion of the IPO, the Company had outstanding warrants to purchase 33,600 shares of its Series A preferred stock, 20,000 shares of its Series B preferred stock, and 840,000 shares of its Series C2 preferred stock. Upon closing of the Company’s IPO, warrants to purchase 893,600 shares of its preferred stock converted to warrants to purchase 59,573 shares of the Company’s common stock. These warrants remain outstanding at December 31, 2011. Of these warrants to purchase 59,573 shares of the Company’s stock, warrants to purchase 56,000 shares of the Company’s common stock automatically exercise in the event of an acquisition of the Company. The Company performed a final re-measurement to determine the fair value of such financial instruments immediately prior to the conversion. The resulting fair value of $0.4 million was reclassified to additional paid-in capital during the year ended December 31, 2007.
In connection with private placements of common stock in December 2007 and January 2008, the Company issued to the investors warrants to purchase 1,206,273 shares of common stock at $8.034 per share. The warrants became exercisable immediately upon issuance and expire five years from issuance. The fair value of the warrants to purchase 1,091,622 shares of common stock issued in conjunction with the first closing of the private placement in December 2007 was approximately $3.4 million and was determined using the Black-Scholes method with the following assumptions: expected volatility of 67%, a dividend yield of 0%, a risk-free interest rate of 3.52%, and an expected life of five years. The fair value of the warrants to purchase 114,651 shares of common stock issued in conjunction with the second closing of the private placement in January 2008 was approximately $0.4 million and was determined using the Black-Scholes method with the following assumptions: expected volatility of 67%, a dividend yield of 0%, a risk-free interest rate of 3.26%, and an expected life of five years. The fair value of the warrants issued was allocated from the proceeds of the financing. All of the 1,206,273 warrants remain outstanding at December 31, 2011.
97
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
2000 Stock Incentive Plan
The Company’s 2000 Stock Incentive Plan (the “2000 Plan”) provides for the grant of incentive and nonstatutory stock options by the board of directors to employees, officers, directors, and consultants of the Company. Incentive stock options may be granted with exercise prices not less than estimated fair value, and nonstatutory stock options may be granted with an exercise price of not less than 85% of the estimated fair value of the common stock on the date of grant. Options granted under the 2000 Plan expire no later than 10 years from the date of grant. Options granted and shares underlying stock purchase rights issued under the 2000 Plan vest over periods determined by the board of directors, generally over four to six years. Unvested shares of common stock purchased under stock purchase rights are subject to a repurchase option by the Company upon termination of the purchaser’s employment or services. The repurchase right lapses over a period of time, as determined by the board of directors. At December 31, 2011 and 2010, there were no stock purchase rights outstanding that were subject to a repurchase right by the Company. For certain options, vesting accelerates upon the achievement of specified milestones.
The 2000 Plan automatically terminated on June 5, 2010 as the plan provisions included an automatic termination 10 years after its adoption by the board of directors. The termination does not impact unexercised stock options outstanding.
The 2000 Plan allowed for the early exercise of stock options prior to vesting. The Company issued an aggregate of 11,945 shares of common stock pursuant to the early exercise of stock options, which were not deemed to be issued until those shares vested. As of December 31, 2011 and 2010, all early exercised shares were fully vested.
2007 Stock Plan
The Company’s 2007 Stock Plan provides for the grant of incentive stock options by the Company’s board of directors to employees and for the grant of nonstatutory stock options, restricted stock, restricted stock units, stock appreciation rights, performance units and performance shares to its employees, directors and consultants.
The Company’s board of directors has the authority to determine the terms of the awards, including exercise price, the number of shares subject to each such award, the exercisability of the awards and consideration payable upon exercise. Incentive stock options may be granted with exercise prices at least equal to the fair market value of the Company’s common stock on the date of grant. The term of an incentive stock option granted under this plan may not exceed ten years, except that with respect to any participant who owns 10% of the voting power of all classes of the Company’s outstanding stock as of the grant date, the term must not exceed five years and the exercise price must equal at least 110% of the fair market value on the date of grant.
Upon adoption of the plan, the Company reserved 1,333,333 shares of its common stock for issuance under the 2007 Stock Plan. Any shares returned to the 2000 Plan as a result of termination of options or the repurchase of shares issued under the 2000 Plan are added to the 2007 Stock Plan. In addition, the 2007 Stock Plan provides for annual increases in the number of shares available for issuance thereunder on the first day of each fiscal year, beginning with the Company’s 2008 fiscal year, equal to the lesser of:
| • | 5% of the outstanding shares of the Company’s common stock on the last day of the immediately preceding fiscal year; |
| • | 1,333,333 shares; or |
| • | such other amount as the Company’s board of directors may determine. |
98
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The Company’s 2007 Stock Plan also provides for the automatic grant of non-statutory options to the Company’s non-employee directors. Each non-employee director that is newly appointed to the board of directors will receive an initial option to purchase 20,000 shares upon such appointment. Additionally, non-employee directors who have been directors for at least six months will receive a subsequent option to purchase 10,000 shares immediately following each annual meeting of the Company’s stockholders.
The following table summarizes stock option activity under both the 2000 Plan and 2007 Stock Plan:
| Options Outstanding | ||||||||||||
| Shares Available for Grant |
Number of Shares | Weighted-Average Exercise Price |
||||||||||
| Balance at December 31, 2008 |
1,578,202 | 1,393,545 | $ | 5.31 | ||||||||
| Additional shares authorized |
878,459 | — | — | |||||||||
| Options granted |
(1,061,642 | ) | 1,061,642 | $ | 3.85 | |||||||
| Options exercised |
— | (105,247 | ) | $ | 2.76 | |||||||
| Options canceled |
85,405 | (85,405 | ) | $ | 7.68 | |||||||
|
|
|
|
|
|||||||||
| Balance at December 31, 2009 |
1,480,424 | 2,264,535 | $ | 4.65 | ||||||||
| Additional shares authorized |
886,297 | — | — | |||||||||
| Options granted |
(1,109,539 | ) | 1,109,539 | $ | 7.39 | |||||||
| Options exercised |
— | (79,869 | ) | $ | 3.23 | |||||||
| Options canceled |
291,712 | (291,712 | ) | $ | 7.08 | |||||||
|
|
|
|
|
|||||||||
| Balance at December 31, 2010 |
1,548,894 | 3,002,493 | $ | 5.47 | ||||||||
| Additional shares authorized |
1,893,455 | — | — | |||||||||
| Options granted |
(3,017,468 | ) | 3,017,468 | $ | 2.70 | |||||||
| Options exercised |
— | (22,207 | ) | $ | 1.84 | |||||||
| Options canceled |
2,960,715 | (2,960,715 | ) | $ | 5.46 | |||||||
|
|
|
|
|
|||||||||
| Balance at December 31, 2011 |
3,385,596 | 3,037,039 | $ | 2.75 | ||||||||
|
|
|
|
|
|||||||||
The options granted in 2011 in the table above include options granted to certain employees and officers to purchase a total of 580,000 shares of the Company’s common stock in the form of market-based stock options with vesting tied to stock price targets. The grant date fair value of these options was $2.1 million as estimated using a Monte Carlo valuation methodology, using the following assumptions: expected volatility of 88.7%; expected risk-free interest rate of 1.45%; median range of expected life of 3.7 years to 4.3 years; and expected dividend yield of zero percent. The fair value of these options is expected to be amortized through 2015. Employees were granted 90,000 market-based stock options with a fair value of $0.3 million, and officers were granted 490,000 market-based stock options with a fair value of $1.8 million. For the year ended December 31, 2011, the Company recognized $0.5 million of stock-based compensation expense related to these market-based options.
The options granted and canceled in 2011 in the table above include options that were exchanged and replaced under the Company’s option exchange program executed in December 2011. Under the exchange offer, the Company accepted for cancellation options to purchase an aggregate of 2,110,821 shares of its common stock, which were cancelled as of December 12, 2011, and, in exchange, granted new options to purchase an aggregate of 1,225,468 shares of its common stock. The exercise price per share of the new options granted in the offer was $1.07, the closing price of the Company’s common stock as reported by the NASDAQ Global market on December 12, 2011. The unamortized value of the cancelled grants was added to the incremental grant date fair value of the replacement grants, and the combined value will be amortized over the vesting period of the new grant. The incremental grant date fair value of the replacement grants is approximately $0.4 million.
99
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
On April 29, 2011, the Company announced the planned retirement of Anthony DiTonno, the Company’s President and Chief Executive Officer, by December 31, 2011. In connection with Mr. DiTonno’s retirement, the Company’s board of directors approved accelerating the vesting of options to purchase the Company’s common stock held by Mr. DiTonno by 12 months upon termination of his employment or consulting services, if any, and the extension of time to exercise such options up to 24 months. The Company recorded a non-cash charge of $0.1 million in the 2011 selling, general and administration expense related to the acceleration.
The Company’s board of directors granted 899,000 restricted common stock units (“RSUs”) to its officers and employees. The RSUs vest 50% on September 1, 2012 and 12.5% on each of December 1, 2012, March 10, 2013, June 1, 2013 and September 1, 2013. The fair market value of the Company’s common stock on the date of grant was $1.60. The grant date fair value of these RSUs was $1.4 million and it is being amortized over the vesting period. Employees were granted 809,000 RSUs with a fair value of $1.3 million, and officers were granted 90,000 RSUs with a fair value of $0.1 million. In addition, the Company’s board of directors granted 75,000 RSUs to an officer, in which the fair market value of the Company’s common stock on the date of grant was $1.07. These RSUs vest 25% per year on the anniversary grant date.
The following table summarizes restricted stock unit activity under the 2007 Stock plan:
| Number of RSUs | Weighted-Average Grant Date Fair Value |
|||||||
| Non-vested balance at December 31, 2010 |
— | — | ||||||
| Awards granted |
974,000 | $ | 1.56 | |||||
| Awards released |
(159,000 | ) | $ | 1.60 | ||||
| Awards canceled |
(34,000 | ) | $ | 1.60 | ||||
|
|
|
|
|
|||||
| Balance at December 31, 2011 |
781,000 | $ | 1.55 | |||||
|
|
|
|||||||
In October 2011, the Company’s board of directors approved a restructuring plan whereby it reduced its workforce by 26 individuals. The Company communicated the plan to the impacted employees on October 28, 2011 and their last day of employment was November 2, 2011. The impacted employees are eligible to receive severance contingent on their signing of a general release with the Company. Part of the severance given included a full acceleration of vesting on their RSUs and an extension of the exercise period for vested stock options. The Company recorded a non-cash charge of $0.2 million in 2011 related to these modifications. In March 2012, the Company undertook an additional restructuring. The severance associated with such restructuring included acceleration of RSU vesting for terminated employees in addition to other modifications of equity grants.
2011 Inducement Stock Plan
On September 20, 2011, the Company’s board of directors adopted the 2011 Inducement Stock Plan (the “Inducement Plan”). The purposes of the Inducement Plan are to provide a material inducement for the best available individuals to enter into the employment of the Company and thereby to promote the success of the Company’s business. The Inducement Plan provides for the grant of nonstatutory stock options, stock appreciation rights, restricted stock, restricted stock units, performance units and performance shares by the Company’s board of directors its employees. The term of the Inducement Plan is ten years from the date adopted by the board of directors.
The Company’s independent compensation committee has the authority to determine the terms of the awards, including exercise price, the number of shares subject to each such award, individuals to whom awards may be granted, the exercisability of the awards and consideration payable upon exercise.
100
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Upon adoption of the plan, the Company reserved 1,000,000 shares of its common stock for issuance under the Inducement Plan. At December 31, 2011, 1,000,000 shares were available for grant. In connection with the hiring of the Company’s new Chief Executive Officer on January 2, 2012, the Company has issued 600,000 shares under the Inducement Plan.
Stock-Based Compensation Expense
The Company recognizes stock-based compensation expense for all share-based payments granted subsequent to December 31, 2005. Stock-based compensation expense for awards made to employees and directors is measured at the date of grant, based on the fair value of the award, and is recognized as expense on a straight-line basis over the requisite service period. The following table summarizes stock-based compensation expense for the years ended December 31, 2011, 2010 and 2009:
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Research and development |
$ | 877 | $ | 679 | $ | 1,068 | ||||||
| Selling, general and administrative |
2,549 | 1,675 | 1,424 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 3,426 | $ | 2,354 | $ | 2,492 | ||||||
|
|
|
|
|
|
|
|||||||
The fair value for the Company’s employee stock options was estimated at the date of grant using the Black-Scholes valuation model with the following average assumptions:
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Expected volatility |
67 | % | 66 | % | 70 | % | ||||||
| Expected term (years) |
6.0 | 6.0 | 6.0 | |||||||||
| Risk-free interest rate |
1.8 | % | 2.8 | % | 2.2 | % | ||||||
| Dividend yield |
0 | % | 0 | % | 0 | % | ||||||
The Company’s computation of expected volatility for the years ended December 31, 2011, 2010 and 2009 is based on an average of the historical volatility of a peer-group of similar companies. The Company’s computation of expected term in the years ended December 31, 2011, 2010 and 2009 utilizes the simplified method. The risk-free interest rate for periods within the term of the option is based on the U.S. Treasury yield curve in effect at the time of grant. Stock compensation expense relating to options with acceleration of vesting dependent upon the achievement of milestones is recognized on a straight-line basis over a period which is the shorter of:
| • | the Company’s evaluation of the probability of achievement of each respective milestone and the related estimated date of achievement; or |
| • | the otherwise stated time-based vesting period. |
101
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The following table represents stock options activity for the year ended December 31, 2011:
| Number of Shares |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contract Life |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding options at beginning of period |
3,002,493 | $ | 5.47 | |||||||||||||
| Granted |
3,017,468 | 2.70 | ||||||||||||||
| Exercised |
(22,207 | ) | 1.84 | |||||||||||||
| Forfeited |
(1,396,840 | ) | 5.46 | |||||||||||||
| Expired |
(1,563,875 | ) | 5.45 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding options at end of period |
3,037,039 | $ | 2.75 | 6.9 | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Vested and expected to vest at December 31, 2011 |
2,858,590 | $ | 2.77 | 6.8 | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Exercisable options at end of period |
1,259,845 | $ | 3.41 | 5.2 | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The weighted-average grant date fair value of stock options granted during the years ended December 31, 2011, 2010 and 2009 was $1.75, $4.53 and $2.47 per share, respectively. Total intrinsic value of options exercised for the years ended December 31, 2011, 2010 and 2009 was less than $0.1 million, $0.3 million, and $0.5 million, respectively. Cash received from stock option exercises and employee stock purchases under the employee stock purchase plan for the years ended December 31, 2011, 2010 and 2009 was $0.2 million, $0.6 million and $0.4 million, respectively. Because of the Company’s net operating losses, it did not realize any tax benefits for the tax deductions from share-based payment arrangements during the years ended December 31, 2011, 2010 and 2009. As of December 31, 2011, the total unamortized compensation expense related to stock-based awards granted to employees and directors was $5.0 million and it is expected to be amortized over the next 4.3 years. As of December 31, 2011, the total unamortized compensation expense related to RSUs granted to employees and directors was $0.9 million and it is expected to be amortized over the next 1.08 years.
2007 Employee Stock Purchase Plan
Under the Company’s 2007 Employee Stock Purchase Plan (the “Purchase Plan”), eligible employees can participate and purchase common stock semi-annually through accumulated payroll deductions. The Purchase Plan is administered by the Company’s board of directors or a committee appointed by the Company’s board of directors. Under the Purchase Plan eligible employees may purchase stock at 85% of the lower of the fair market value of a share of Common Stock on the offering date or the exercise date. The Purchase Plan provides for consecutive, overlapping twelve-month offering periods generally starting on the first trading day on or after May 15 and November 15 of each year. There are two 6-month purchase periods in each offering period. Eligible employees may contribute up to 15% of their eligible compensation which includes a participant’s straight time gross earnings, commissions, overtime and shift premium, exclusive of payments for incentive compensation, bonuses and other compensation. A participant may purchase a maximum of 1,333 shares of common stock per purchase period. If the fair market value of the Company’s common stock at the end of a purchase period is less than the fair market value at the beginning of the offering period, participants will be withdrawn from the then current offering period following the purchase of shares on the purchase date and automatically will be re-enrolled in a new offering period.
102
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
The Purchase Plan was effective upon the completion of the Company’s IPO, at which time a total of 333,333 shares of the Company’s common stock were made available for sale. Annual increases in the number of shares available for issuance will be made on the first day of each fiscal year, beginning with the Company’s 2008 fiscal year. The annual increases will be equal to the lesser of:
| • | 2% of the outstanding shares of the Company’s common stock on the first day of the fiscal year; |
| • | 533,333 shares; or |
| • | such other amount as may be determined by the Company’s board of directors. During the years ended December 31, 2011, 2010 and 2009, 97,319, 63,280 and 52,510 shares were issued, respectively. |
The Company accounts for its Purchase Plan as a compensatory plan and recorded compensation expense of approximately $0.2 million, $0.2 million and $0.1 million for the years ended December 31, 2011, 2010 and 2009, respectively. The estimated fair value of shares granted under the Purchase Plan was determined at the date of grant using the Black-Scholes pricing model with the following assumptions:
| Year Ended December 31, | ||||||
| 2011 | 2010 | 2009 | ||||
| Expected dividend yield |
0% | 0% | 0% | |||
| Expected volatility |
55 – 58% | 50 – 89% | 56 – 89% | |||
| Expected life (in years) |
0.5 to 1.0 | 0.5 to 1.0 | 0.5 to 1.0 | |||
| Risk-free interest rate |
0.1 – 0.4% | 0.21 – 0.5% | 0.2 – 2.1% | |||
As of December 31, 2011, 1,458,906 shares were reserved for future issuance under the Purchase Plan.
12. Income Taxes
Due to the ongoing operating losses and the inability to recognize any income tax benefit, there is no provision for income taxes.
Deferred tax assets and liabilities reflect the net tax effects of net operating loss and tax credit carryovers and the temporary differences between the carrying amounts of assets and liabilities for financial reporting and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets are as follows (in thousands):
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Deferred revenue |
$ | 13,450 | $ | 16,392 | ||||
| Accrued expenses and other current assets |
1,714 | 1,103 | ||||||
| Net operating loss carryforward |
69,808 | 48,451 | ||||||
| Capitalized research |
3,167 | 4,944 | ||||||
| Research and development and other credits |
7,076 | 4,867 | ||||||
| Basis difference in fixed assets |
256 | 260 | ||||||
| Stock options |
2,341 | 1,641 | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
97,812 | 77,658 | ||||||
| Valuation allowance |
(97,812 | ) | (77,658 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
103
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Realization of deferred tax assets is dependent upon future earnings, if any, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by $20.2 million and $18.8 million during 2011 and 2010, respectively. The Company’s deferred tax assets reflect an estimate of the potential limitation of its utilization of its net operating loss carryforwards and research and development and other credits.
As of December 31, 2011, the Company had net operating loss carryforwards for federal income tax purposes of approximately $196.6 million which will begin to expire in the year 2020 and federal research and development tax credits of approximately $4.7 million which will begin to expire in the year 2020. The Company also had orphan drug credits of approximately $4.9 million which will begin to expire in the year 2029.
As of December 31, 2011, the Company had net operating loss carryforwards for state income tax purposes of approximately $183.4 million which will begin to expire in the year 2012 and state research and development tax credits of approximately $4.5 million which have no expiration date.
Utilization of the net operating losses may be subject to substantial annual limitation due to federal and state ownership limitations. The annual limitation could result in the expiration of the net operating losses before utilization.
The Company received payments of $46.8 million from Astellas related to the License Agreement in 2009 and deferred that amount to future years for book purposes. For tax purposes, the Company elected to use the deferral method to reflect the upfront payments from Astellas for tax purposes. In accordance with IRS Revenue Procedure 2004-34, the deferral method allowed the Company to defer this advance payment to 2010 for tax purposes.
The Company utilizes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The Company classifies interest and penalties as a component of tax expense.
The Company had unrecognized tax benefits of $2.3 million and $2.1 million as of December 31, 2011 and December 31, 2010, respectively, all of which are offset by a full valuation allowance. These unrecognized tax benefits, if recognized, would not affect the effective tax rate for the periods presented. No interest or penalties have been accrued. The Company does not believe it is reasonably possible that its unrecognized tax benefits will significantly change within the next twelve months.
The Company files income tax returns in the U.S. federal and various state tax jurisdictions. The tax years 2000 to 2011 remain open to examination by the U.S. and state tax authorities.
A reconciliation of the change in the unrecognized tax benefits balance from January 1, 2010 to December 31, 2011 is as follows:
| (In thousands) |
Federal and State Tax |
|||
| Balance at December 31, 2009 |
$ | 1,794 | ||
| Additions for tax positions related to current year |
255 | |||
| Additions for tax positions related to prior years |
24 | |||
|
|
|
|||
| Balance at December 31, 2010 |
2,073 | |||
| Additions for tax positions related to current year |
268 | |||
| Reductions for tax positions related to prior years |
(19 | ) | ||
|
|
|
|||
| Balance at December 31, 2011 |
$ | 2,322 | ||
|
|
|
|||
104
Table of Contents
NEUROGESX, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
13. Subsequent Events
On February 3, 2012, the Company completed a private placement of its common stock under a Securities Purchase Agreement, dated as of January 31, 2012, by and among the Company and the Purchasers (as defined therein), pursuant to which the Company issued shares of common stock for an aggregate purchase price of $3.0 million, at a per share price of $1.01. The price of each share of common stock is based on the January 31, 2012 consolidated closing bid price of the Company’s common stock on the NASDAQ Global Market of $1.01 per share. The net proceeds to the Company, after deducting expenses of less than $0.1 million, were $3.0 million. The total number of shares of common stock issued in connection with the transaction was 2,969,685.
On March 5, 2012, the Company’s board of directors committed to a restructuring plan whereby the Company proposed to reduce its workforce by 43 individuals. The restructuring plan was approved in connection with the Company’s plan to focus its resources on NGX-1998 development program. The Company communicated the plan to the impacted employees on March 7, 2012 with the expectation that the restructuring would be effective and completed on or about such date. The Company expects to record a cash charge for severance and other payroll related termination costs of approximately $1.6 million in the first quarter of 2012. The Company also expects that there will be an additional non-cash compensation charge related to modifications to the impacted employee’s equity awards under the 2000 Stock Incentive Plan and 2007 Stock Plan, as well as charges related to excess inventory, capital equipment write-downs and other miscellaneous restructure charges that in total will be in the range of $0.5 million to $0.8 million.
14. Unaudited Quarterly Information
Certain unaudited quarterly financial information for the years ended December 31, 2011 and 2010 is presented below:
| First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
|||||||||||||
| (In thousands, except per share amounts) | ||||||||||||||||
| 2011 |
||||||||||||||||
| Product revenue |
$ | 575 | $ | 486 | $ | 763 | $ | 801 | ||||||||
| Collaboration revenue |
$ | 2,595 | $ | 1,997 | $ | 2,128 | $ | 2,158 | ||||||||
| Net loss |
$ | (13,363 | ) | $ | (14,567 | ) | $ | (11,375 | ) | $ | (10,240 | ) | ||||
| Basic and diluted net loss per share |
$ | (0.75 | ) | $ | (0.81 | ) | $ | (0.43 | ) | $ | (0.34 | ) | ||||
| 2010 |
||||||||||||||||
| Product revenue |
$ | — | $ | 33 | $ | 197 | $ | 458 | ||||||||
| Collaboration revenue |
$ | 1,786 | $ | 2,051 | $ | 1,854 | $ | 1,887 | ||||||||
| Net loss |
$ | (9,163 | ) | $ | (10,233 | ) | $ | (12,760 | ) | $ | (12,360 | ) | ||||
| Basic and diluted net loss per share |
$ | (0.52 | ) | $ | (0.58 | ) | $ | (0.72 | ) | $ | (0.69 | ) | ||||
105
Table of Contents
| Item 9. | Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
| (a) | Evaluation of disclosure controls and procedures |
Our management evaluated, with the participation and under the supervision of our Chief Executive Officer and our Chief Financial Officer, the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K. Based on this evaluation, our Chief Executive Officer and our Chief Financial Officer have concluded, subject to the limitations described below, that our disclosure controls and procedures are effective to ensure that information we are required to disclose in reports that we file or submit under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in Securities and Exchange Commission, or SEC, rules and forms, and that such information is accumulated and communicated to management as appropriate to allow timely decisions regarding required disclosures.
| (b) | Internal control over financial reporting |
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. We maintain a system of internal controls that is designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Management assessed our internal control over financial reporting as of December 31, 2011, the end of our last fiscal year. Management based its assessment on criteria established in “Internal Control—Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission. Management’s assessment included evaluation of such elements as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies and our overall control environment.
Based on our assessment, management has concluded that our internal control over financial reporting was effective as of December 31, 2011 to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with generally accepted accounting principles.
There have not been any changes in our internal controls over financial reporting (as such item is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during our fiscal year ended December 31, 2011 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
The information contained under this caption “Internal control over financial reporting” shall not be deemed to be filed with the SEC, nor shall such information be incorporated by reference into any future filing under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate it by reference into such filing.
| (c) | Limitations on the Effectiveness of Controls |
A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the controls are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company have been detected. Accordingly, our controls and procedures are designed to provide reasonable, not absolute, assurance that the objectives of our control system are met.
| Item 9B. | Other Information |
106
Table of Contents
| Item 10. | Directors, Executive Officers and Corporate Governance |
The information required to be disclosed under this Item, other than as set forth below, is incorporated by reference from our Proxy Statement for our 2012 Annual Meeting of Stockholders where it appears under the heading “Directors and Executive Officers.”
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our officers and directors, and persons who beneficially own more than ten percent of a registered class of our equity securities, to file reports of ownership and changes in ownership with the SEC. Such officers, directors and ten-percent stockholders are also required by SEC rules to furnish us with copies of all forms that they file pursuant to Section 16(a). Based solely on our review of the copies of such forms received by us, or written representations from certain reporting persons, we believe that during fiscal 2011, our executive officers, directors and ten-percent stockholders complied with all applicable filing requirements.
Code of Ethics
We have adopted a Code of Ethics that applies to all directors, officers and employees of the Company. We publicize the Code of Ethics through posting the policy on our website, http://www.neurogesx.com. We will disclose on our website any waivers of, or amendments to, our Code of Ethics.
| Item 11. | Executive Compensation |
The information required by this Item is incorporated by reference from our definitive Proxy Statement referred to in Item 10 above where it appears under the heading “Executive Compensation and other Matters.”
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of February 29, 2012 for:
| • | each person known by us to beneficially own more than 5% of our outstanding shares of our common stock, |
| • | each of our directors and each nominee for director, |
| • | each of our named executive officers, and |
| • | all such directors, nominees for director and executive officers as a group. |
The percentage of ownership is based on 32,896,871 shares of Common stock outstanding on February 29, 2012, adjusted to include shares of common stock that could be issued upon the exercise of outstanding options and warrants held by that person that are currently exercisable or exercisable within 60 days of February 29, 2012. Shares underlying these options and warrants, however, are not considered outstanding when computing the percentage ownership of any other person. We do not know of any arrangements, including any pledge by any person of our securities, the operation of which may at a subsequent date result in our change of control.
107
Table of Contents
| Beneficial Ownership (1) | ||||||||
| Name and Address of Beneficial Owner |
Number of Shares | Percent of Common stock Outstanding |
||||||
| 5% Stockholders |
||||||||
| Wasatch Advisors, Inc. (2) 150 Social Hall Avenue, Suite 400 Salt Lake City, UT 84111 |
6,103,409 | 18.21 | % | |||||
| Entities affiliated with ARCH Venture Partners (3) 8725 West Higgins Road, Suite 290 Chicago, IL 60631 |
5,644,428 | 16.7 | % | |||||
| Entities affiliated with FMR LLC (4) 82 Devonshire Street Boston, MA 02109 |
2,969,276 | 9.03 | % | |||||
| Entities affiliated with Sphera Global Healthcare (5) Platinum House, 21 Ha’arba’ah Street Tel Aviv 64739, Israel |
2,648,107 | 8.1 | % | |||||
| Entities affiliated with Montreux Equity Partners (6) 3000 Sand Hill Road, Bldg. 1, Suite 260 Menlo Park, CA 94025 |
2,515,385 | 7.51 | % | |||||
| Entities affiliated with SV Life Sciences Fund (7) 60 State Street, Suite 3650 Boston, MA 02109 |
2,221,315 | 6.6 | % | |||||
| Named Executive Officers and Directors |
||||||||
| Jean-Jacques Bienaimé (8) |
78,895 | * | ||||||
| Anthony A. DiTonno (9) |
413,545 | 1.3 | % | |||||
| Stephen F. Ghiglieri (10) |
199,144 | * | ||||||
| Bradford S. Goodwin (11) |
145,000 | * | ||||||
| Neil M. Kurtz (12) |
40,666 | * | ||||||
| Gary Lyons (13) |
100,000 | * | ||||||
| Michael E. Markels (14) |
150,539 | * | ||||||
| Ronald A. Martell |
- | * | ||||||
| Robert T. Nelsen (3)(15) |
5,674,428 | 16.8 | % | |||||
| Steven H. Nelson (16) |
5,000 | * | ||||||
| John A. Orwin (17) |
20,000 | * | ||||||
| Stephen J. Peroutka |
- | * | ||||||
| All directors and executive officers as a group (12 persons ) (18) |
6,827,217 | 20.2 | % | |||||
| * | Beneficial ownership representing less than 1%. |
| (1) | This table is based upon information supplied by officers and directors and upon information gathered by us about principal stockholders known to us based on Schedules 13D, 13G, and Forms 3 and 4 filed with the SEC or otherwise provided to us. |
| (2) | Represents 5,486,576 shares of common stock held by Wasatch Advisors, Inc., a Utah corporation, and currently exercisable warrants to purchase 616,833 shares of common stock. |
| (3) | Represents (a) 3,960,777 shares of common stock held by ARCH Venture Fund V, L.P. and the right to acquire 986,744 shares of common stock pursuant to the exercise of warrants exercisable within 60 days of February 29, 2012, (b) 458,903 shares of common stock held by Healthcare Focus Fund, L.P., and (c) 11,004 shares of common stock held by ARCH V Entrepreneurs Fund, L.P. The people who have investment control of the ARCH Venture Fund V, L.P., the Healthcare Focus Fund, L.P., and ARCH V Entrepreneurs Fund, L.P., shares are Robert T. Nelsen, Steven Lazarus, Clinton Bybee and Keith Crandell, each of whom disclaims beneficial ownership except to the extent of their pecuniary interest therein. |
| (4) | Represents (a) 2,804,676 shares of common stock held by Fidelity Select Biotechnology Portfolio Fund and (b) 164,600 shares of common stock held by Fidelity Advisor Series VII: Fidelity Advisor Biotechnology |
108
Table of Contents
| Fund. Fidelity Management & Research Company, or Fidelity, 82 Devonshire Street, Boston, Massachusetts 02109, a wholly-owned subsidiary of FMR LLC and an investment adviser registered under Section 203 of the Investment Advisers Act of 1940, is the beneficial owner of all such shares as a result of acting as investment adviser to various investment companies registered under Section 8 of the Investment Company Act of 1940. Edward C. Johnson 3d and FMR LLC, through its control of Fidelity and the funds each has sole power to dispose of such shares. Members of the family of Edward C. Johnson 3d, Chairman of FMR LLC, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR LLC. Neither FMR LLC nor Edward C. Johnson 3d, Chairman of FMR LLC, has the sole power to vote or direct the voting of the shares owned directly by the Fidelity Funds, which power resides with the Funds’ Boards of Trustees. Fidelity carries out the voting of the shares under written guidelines established by the Funds’ Boards of Trustees. Fidelity, 82 Devonshire Street, Boston, Massachusetts 02109, a wholly-owned subsidiary of FMR LLC and an investment adviser registered under Section 203 of the Investment Advisers Act of 1940, is the beneficial owner of all such shares as a result of acting as investment adviser to various investment companies registered under Section 8 of the Investment Company Act of 1940. Edward C. Johnson 3d and FMR LLC, through its control of Fidelity and the funds each has sole power to dispose of such shares. Members of the family of Edward C. Johnson 3d, Chairman of FMR LLC, are the predominant owners, directly or through trusts, of Series B voting common shares of FMR LLC, representing 49% of the voting power of FMR LLC. The Johnson family group and all other Series B shareholders have entered into a shareholders’ voting agreement under which all Series B voting common shares will be voted in accordance with the majority vote of Series B voting common shares. Accordingly, through their ownership of voting common shares and the execution of the shareholders’ voting agreement, members of the Johnson family may be deemed, under the Investment Company Act of 1940, to form a controlling group with respect to FMR LLC. Neither FMR LLC nor Edward C. Johnson 3d, Chairman of FMR LLC, has the sole power to vote or direct the voting of the shares owned directly by the Fidelity Funds, which power resides with the Funds’ Boards of Trustees. Fidelity carries out the voting of the shares under written guidelines established by the Funds’ Boards of Trustees. |
| (5) | Represents 2,648,107 shares of common stock held by Moshe Arkin. |
| (6) | Represents (a) 946,494 shares of common stock held by Montreux Equity Partners II SBIC, L.P., a California limited partnership (“MEP II”) and currently exercisable warrants to purchase 290,698 shares of common stock; and (b) 987,495 shares of common stock held by Montreux Equity Partners III SBIC, L.P., a California limited partnership (“MEP III”), and currently exercisable warrants to purchase 290,698 shares of common stock. Montreux Equity Management II SBIC, LLC, a California limited liability company (“MEM II”), Montreux Equity Management III SBIC, LLC, a California limited liability company (“MEM III”), Howard D. Palefsky (“Palefsky”), and Daniel K. Turner III (“Turner”). MEM II serves as the sole general partner of MEP II and owns no securities of the Issuer directly. MEM III serves as the sole general partners of MEP III and owns no securities of the Issuer directly. Palefsky and Turner are directors and/or members of MEM III and share voting and dispositive power over the shares held by MEP III; however, they disclaim beneficial ownership of the shares held by MEP III except to extent of their pecuniary interests therein. |
| (7) | Represents (a) 1,456,207 shares of common stock held by SV Life Science Fund IV, L.P. and the right to acquire 703,784 shares of common stock pursuant to currently exercisable warrants and (b) 41,343 shares of common stock held by SV Life Science Fund IV Strategic Partners, L.P. and the right to acquire 19,981 shares of Common stock pursuant to currently exercisable warrants. SV Life Sciences Fund IV (GP), L.P. is a general partner of SV Life Science Fund IV, L.P. and SV Life Science Fund IV Strategic Partners, L.P. and has sole dispositive and voting power over the shares owned by of SV Life Science Fund IV, L.P. and SV Life Science Fund IV Strategic Partners, L.P. SVLSF IV, LLC is a general partner of SV Life Sciences Fund IV (GP), L.P. and has sole dispositive and voting power over the shares owned by of SV Life Science Fund IV, L.P. and SV Life Science Fund IV Strategic Partners, L.P. The people at SVLSF IV, LLC who |
109
Table of Contents
| have investment control of the SV Life Science Fund IV, L.P. and SV Life Science Fund IV Strategic Partners, L.P. shares are Kate Bingham, James Garvey, Lutz Giebel, Eugene Hill, David Milne, Michael Ross and Henry Simon, each of whom disclaims beneficial ownership except to the extent of their pecuniary interest therein. |
| (8) | Represents (a) 21,748 shares of common stock held by Mr. Bienaimé, and (b) the right to acquire 57,147 shares of common stock exercisable within 60 days of February 29, 2012. |
| (9) | Represents (a) 107,283 shares of common stock held by Mr. DiTonno (11,283 of those shares are directly held by Mr. DiTonno and 96,000 shares are held by the DiTonno Family Trust, dated 8/3/01), and (b) the right to acquire 306,262 shares of common stock exercisable within 60 days of February 29, 2012. |
| (10) | Represents (a) 66,210 shares of common stock held by Mr. Ghiglieri (34,115 of those shares are held directly by Mr. Ghiglieri and 32,095 shares are held by the Ghiglieri Family Trust, dated 5/15/08), and (b) the right to acquire 132,934 shares of common stock exercisable within 60 days of February 29, 2012. |
| (11) | Represents: (a) 95,000 shares of common stock held by the Goodwin Family Trust; (b) warrants to purchase 30,000 shares of common stock held by Spencer and Eliot 2, LLC; and (c) the right to acquire 20,000 shares of common stock pursuant to options held by Mr. Goodwin that are exercisable within 60 days of February 29, 2012. Mr. Goodwin is the trustee of the Goodwin Family Trust and the managing member of Stewart and Spencer 2, LLC. |
| (12) | Represents the right to acquire 40,666 shares of common stock, by Mr. Kurtz, exercisable within 60 days of February 29, 2012. |
| (13) | Represents the right to acquire 100,000 shares of common stock, by Mr. Lyons, exercisable within 60 days of February 29, 2012. |
| (14) | Represents (a) 54,873 shares of common stock held by Mr. Markels, and (b) the right to acquire 95,666 shares of Common stock exercisable within 60 days of February 29, 2012. |
| (15) | Represents the right to acquire 30,000 shares of common stock exercisable within 60 days of February 29, 2012 and Mr. Nelsen’s pecuniary interest in the ARCH Fund shares as defined in note 3 above. |
| (16) | Represents the right to acquire 5,000 shares of common stock, by Mr. Nelson, exercisable within 60 days of February 29, 2012. |
| (17) | Represents the right to acquire 20,000 shares of common stock, by Mr. Orwin, exercisable within 60 days of February 29, 2012. |
| (18) | See note 3 and notes 8 through 17. |
The following table summarizes the securities authorized for issuance under our equity compensation plans as of December 31, 2011:
| Plan Category |
Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
Weighted Average Exercise Price of Outstanding Options, Warrants and Rights |
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans(1) |
|||||||||
| Equity compensation plans approved by stockholders |
3,037,039 | $ | 2.75 | 3,904,569 | ||||||||
| Equity compensation plans not approved by stockholders |
7,932,295 | $ | 2.71 | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Total |
10,969,334 | $ | 2.72 | 3,904,569 | ||||||||
|
|
|
|
|
|
|
|||||||
| (1) | The number of authorized shares under the 2007 Stock Plan automatically increases on January 1 of each year by a number of shares equal to the lesser of (i) 1,333,333 shares, (ii) 5.0% of the outstanding shares on the last day of the immediately preceding fiscal year, or (iii) an amount determined by the Board of Directors. The number of authorized shares under the 2007 Employee Stock Purchase Plan automatically increases on January 1 of each year by a number of shares equal to the lesser of (i) 533,333 shares, (ii) 2.0% of the outstanding shares on such date, or (iii) an amount determined by the Board of Directors. |
110
Table of Contents
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this Item is incorporated by reference from our definitive Proxy Statement referred to in Item 10 above where it appears under the heading “Certain Business Relationships and Related Party Transactions.”
| Item 14. | Principal Accounting Fees and Services |
The information required by this Item is incorporated by reference from our definitive Proxy Statement referred to in Item 10 above where it appears under the heading “Principal Accountant Fees and Services.”
111
Table of Contents
| Item 15. | Exhibits and Financial Statement Schedules |
| (a) | The following documents are filed as part of this Form 10-K: |
| (1) | Financial Statements (included in Part II of this report): |
| • | Report of Independent Registered Public Accounting Firm |
| • | Consolidated Balance Sheets |
| • | Consolidated Statements of Operations |
| • | Consolidated Statements of Stockholders’ Equity (Deficit) |
| • | Consolidated Statements of Cash Flows |
| • | Notes to Consolidated Financial Statements |
| (2) | Financial Statement Schedules: |
None—All financial statement schedules are omitted because the information is inapplicable or presented in the notes to the financial statements.
| (3) | Exhibits: |
| Exhibit |
Exhibit Title | |
| 3.1(1) | Amended and Restated Certificate of Incorporation. | |
| 3.2(1) | Amended and Restated Bylaws. | |
| 4.1 | Specimen Common Stock Certificate. | |
| 4.2(1) | Third Amended and Restated Investors’ Rights Agreement by and between NeurogesX, Inc. and certain stockholders, dated as of November 14, 2005. | |
| 4.3(2) | Amendment No. 1 to the Third Amended and Restated Investors’ Rights Agreement by and between NeurogesX, Inc. and certain stockholders, dated as of December 28, 2007. | |
| 4.4(3) | Amendment No. 2 to the Third Amended and Restated Investors’ Rights Agreement by and between NeurogesX, Inc. and certain stockholders, dated as of August 5, 2010. | |
| 4.5(1) | Warrant to Purchase Series A Preferred Stock by and between NeurogesX, Inc. and Silicon Valley Bank, dated as of December 14, 2000. | |
| 4.6(1) | Warrant to Purchase Series B Preferred Stock by and between NeurogesX, Inc. and Silicon Valley Bank, dated as of May 1, 2002. | |
| 4.7(1) | Warrant to Purchase Shares of Series C2 Preferred Stock by and between NeurogesX, Inc. and Horizon Technology Funding Company II LLC, dated as of July 7, 2006. | |
| 4.8(1) | Warrant to Purchase Shares of Series C2 Preferred Stock by and between NeurogesX, Inc. and Horizon Technology Funding Company III LLC, dated as of July 7, 2006. | |
| 4.9(1) | Warrant to Purchase Shares of Series C2 Preferred Stock by and between NeurogesX, Inc. and Oxford Finance Corporation, dated as of July 7, 2006. | |
| 4.10(1) | Form of First Warrant to Purchase Series C2 Preferred Stock. | |
| 4.11(1) | Form of Second Warrant to Purchase Series C2 Preferred Stock. | |
| 4.12(2) | Registration Rights Agreement by and between NeurogesX, Inc. and certain investors, dated as of December 23, 2007. | |
112
Table of Contents
| Exhibit |
Exhibit Title | |
| 4.13(2) | Form of Warrant to Purchase Common Stock. | |
| 4.14(10) | Registration Rights Agreement dated July 21, 2011. | |
| 4.15(10) | Form of Warrant to Purchase Common Stock issued on July 21, 2011. | |
| 4.16(11) | Warrant Agreement between NeurogesX, Inc. and Hercules Technology Growth Capital, Inc. dated as of August 5, 2011. | |
| 10.1(4) | 2007 Stock Plan as amended. | |
| 10.2(1) | 2007 Employee Stock Purchase Plan. | |
| 10.3(12) | 2011 Inducement Stock Plan. | |
| 10.4 | Form of Indemnification Agreement entered into between NeurogesX, Inc. and each of its directors and officers. | |
| 10.5(5) | Exclusive License Agreement between NeurogesX, Inc. and The Regents of the University of California, executed as of October 6, 2008. | |
| 10.6(1)† | Clinical Supply, Development and License Agreement between NeurogesX, Inc. and LTS Lohmann Therapie- Systeme AG, dated as of January 15, 2004. | |
| 10.7(6)† | Manufacturing and Supply Agreement, effective as of August 19, 2008 between NeurogesX, Inc. and Formosa Laboratories, Inc. | |
| 10.8(5)† | Commercial Supply and License Agreement with Lohmann Therapie-Systeme AG and NeurogesX, Inc., effective as of January 2007. | |
| 10.9 | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Anthony DiTonno, effective as of November 9, 2011. | |
| 10.10(13) | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Stephen Ghiglieri, effective as of September 19, 2011. | |
| 10.11(13) | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Michael Markels, effective as of September 19, 2011. | |
| 10.12(13) | Amended and Restated Executive Employment Agreement by and between NeurogesX, Inc. and Jeffrey Tobias, effective as of September 19, 2011. | |
| 10.13 | Executive Employment Agreement by and between NeurogesX, Inc. and Stephen J. Peroutka, MD, PhD, effective as of November 9, 2011. | |
| 10.14(13) | Consulting Agreement by and between NeurogesX, Inc. and Jeffrey Tobias, effective as of September 27, 2011. | |
| 10.15(10) | Securities Purchase Agreement, dated as of July 21, 2011. | |
| 10.16(11) | Loan and Security agreement between NeurogesX, Inc. and Hercules Technology Growth Capital, Inc., dated as of August 5, 2011. | |
| 10.17(7) | Sublease between NeurogesX, Inc. and Oracle USA, Inc., dated September 6, 2007. | |
| 10.18(2) | Securities Purchase Agreement by and between NeurogesX, Inc. and certain investors, dated as of December 23, 2007. | |
| 10.19(8) | Distribution, Marketing and License Agreement between NeurogesX, Inc. and Astellas Pharma Europe Limited, effective as of June 19, 2009. | |
| 10.20(8) | Supply Agreement between NeurogesX, Inc. and Astellas Pharma Europe Limited, effective as of June 19, 2009. | |
113
Table of Contents
| Exhibit |
Exhibit Title | |
| 10.21(9)† | Financing Agreement between NeurogesX, Inc. and Cowen Healthcare Royalty Partners, L.P., dated as of April 30, 2010. | |
| 23.1 | Consent of Independent Registered Public Accounting Firm. | |
| 24.1 | Power of Attorney (see page 116). | |
| 31.1 | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 31.2 | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 32.1 | Certifications of the Principal Executive Officer and the Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (18 U.S.C. Section 1350). | |
| 101.INS(14) | XBRL Instance. | |
| 101.SCH(14) | XBRL Taxonomy Extension Schema. | |
| 101.CAL(14) | XBRL Taxonomy Extension Calculation. | |
| 101.DEF(14) | XBRL Taxonomy Extension Definition. | |
| 101.LAB(14) | XBRL Taxonomy Extension Labels. | |
| 101.PRE(14) | XBRL Taxonomy Extension Presentation. | |
| (1) | Incorporated by reference from our registration statement on Form S-1, registration number 333-140501, declared effective by the Securities and Exchange Commission on May 1, 2007. |
| (2) | Incorporated by reference from our Current Report on Form 8-K, filed with the Securities and Exchange Commission on December 28, 2007. |
| (3) | Incorporated by reference from our Current Report on Form 8-K, filed with the Securities and Exchange Commission on August 10, 2010. |
| (4) | Incorporated by reference from our Current Report on Form 8-K, filed with the Securities and Exchange Commission on June 7, 2010. |
| (5) | Incorporated by reference from our Annual Report on Form 10-K, filed with the Securities and Exchange Commission on March 26, 2009. |
| (6) | Incorporated by reference from our Current Report on Form 8-K, filed with the Securities and Exchange Commission on August 22, 2008. |
| (7) | Incorporated by reference from our Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission on November 14, 2007. |
| (8) | Incorporated by reference from our Current Report on Form 8-K/A, filed with the Securities and Exchange Commission on July 1, 2009. |
| (9) | Incorporated by reference from our Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission on August 11, 2010. |
| (10) | Incorporated by reference from our Current Report on Form 8-K, filed with the Securities and Exchange Commission on July 27, 2011. |
| (11) | Incorporated by reference from our Current Report on Form 8-K, filed with the Securities and Exchange Commission on August 9, 2011. |
| (12) | Incorporated by reference from our Current Report on Form 8-K, filed with the Securities and Exchange Commission on September 26, 2011. |
| (13) | Incorporated by reference from our Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission on November 14, 2011. |
| (14) | Pursuant to applicable securities laws and regulations, we have deemed to have complied with the reporting obligation relating to the submission of interactive data files in such exhibits and are not subject to liability under any anti-fraud provisions or other liability provisions of the federal securities laws as long, as we have made a good faith attempt to comply with the submission requirements and promptly amend the interactive |
114
Table of Contents
| data files after becoming aware that the interactive data files fail to comply with the submission requirements. In addition, users of this data are advised that, pursuant to Rule 406T of Regulation S-T, these interactive data files are deemed furnished and not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933 or Section 18 of the Securities Exchange Act of 1934 and otherwise are not subject to liability under these sections. |
| † | Confidential treatment has been granted for portions of this exhibit. These portions have been omitted from this filing and have been filed separately with the Securities and Exchange Commission. |
| (b) | Exhibits |
The exhibits listed under Item 14(a)(3) hereof are filed as part of this Form 10-K other than Exhibit 32.1 which shall be deemed furnished.
| (c) | Financial Statement Schedules |
All financial statement schedules are omitted because the information is inapplicable or presented in the notes to the financial statements.
115
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities and Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| NEUROGESX, INC. | ||
| By: |
/S/ RONALD A. MARTELL | |
| Ronald A. Martell President, Chief Executive Officer and Director | ||
Dated: March 28, 2012
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Ronald A. Martell and Stephen F. Ghiglieri, and each of them, his true and lawful attorneys-in-fact, each with full power of substitution, for him in any and all capacities, to sign any amendments to this Annual Report on Form 10-K and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact or their substitute or substitutes may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities and Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /S/ RONALD A. MARTELL Ronald A. Martell |
President, Chief Executive Officer and Director (Principal Executive Officer) | March 28, 2012 | ||
| /S/ STEPHEN F. GHIGLIERI Stephen F. Ghiglieri |
Executive Vice President, Chief Operating Officer and Chief Financial Officer (Principal Financial and Accounting Executive) | March 28, 2012 | ||
| /S/ JEAN-JACQUES BIENAIME Jean-Jacques Bienaimé |
Lead Independent Director | March 28, 2012 | ||
| /S/ NEIL M. KURTZ Neil M. Kurtz |
Director | March 28, 2012 | ||
| /S/ ROBERT T. NELSEN Robert T. Nelsen |
Director | March 28, 2012 | ||
| /S/ BRADFORD S. GOODWIN Bradford S. Goodwin |
Director | March 28, 2012 | ||
| /S/ JOHN ORWIN John Orwin |
Director | March 28, 2012 | ||
| /S/ STEVEN H. NELSON Steven H. Nelson |
Director | March 28, 2012 | ||
| /S/ ANTHONY A. DITONNO Anthony A. DiTonno |
Director | March 28, 2012 | ||
116
