Attached files
Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
Form 10-K
(MARK ONE)
| þ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2011
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM TO
COMMISSION FILE NUMBER 001-31533
DUSA PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| NEW JERSEY | 22-3103129 | |
| (State or other jurisdiction of Incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 25 Upton Drive, Wilmington, MA | 01887 | |
| (Address of principal executive offices) | (Zip Code) | |
REGISTRANT’S TELEPHONE NUMBER, INCLUDING AREA CODE:
(978) 657-7500
SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT:
(TITLE OF CLASS)
NONE
SECURITIES REGISTERED PURSUANT TO SECTION 12(g) OF THE ACT:
(TITLE OF CLASS)
COMMON STOCK, NO PAR VALUE
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ¨ Accelerated filer þ Non-accelerated filer ¨ Smaller reporting company ¨
(Do not check if a smaller reporting company)
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No þ
As of March 2, 2012, the Registrant had 24,649,614 shares of Common Stock, no par value, outstanding.
Based on the last reported sale price of the Company’s common stock on the NASDAQ Global Market on June 30, 2011 ($6.22) (the last business day of the Registrant’s most recently completed second fiscal quarter), the aggregate market value of the voting stock held by non-affiliates of the Registrant was approximately $149,749,056.
DOCUMENTS INCORPORATED BY REFERENCE
| Document Description |
10-K Part III | |
| Portions of the Registrant’s proxy statement to be filed pursuant to Regulation 14A within 120 days after Registrant’s fiscal year end of December 31, 2011 are incorporated by reference into Part III of this report. | Items 10, 11, 12, 13 and 14 | |
Table of Contents
TABLE OF CONTENTS TO FORM 10-K
| PART I | ||||||
| ITEM 1. |
BUSINESS | 1 | ||||
| ITEM 1A. |
RISK FACTORS | 17 | ||||
| ITEM 1B. |
UNRESOLVED STAFF COMMENTS | 28 | ||||
| ITEM 2. |
PROPERTIES | 28 | ||||
| ITEM 3. |
LEGAL PROCEEDINGS | 28 | ||||
| ITEM 4. |
MINE SAFETY DISCLOSURES | 28 | ||||
| PART II | ||||||
| ITEM 5. |
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | 28 | ||||
| ITEM 6. |
SELECTED FINANCIAL DATA | 30 | ||||
| ITEM 7. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 31 | ||||
| ITEM 7A. |
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | 46 | ||||
| ITEM 8. |
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | 49 | ||||
| ITEM 9. |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 49 | ||||
| ITEM 9A. |
CONTROLS AND PROCEDURES | 49 | ||||
| ITEM 9B. |
OTHER INFORMATION | 51 | ||||
Table of Contents
| PART III | ||||||
| ITEM 10. |
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE | 51 | ||||
| ITEM 11. |
EXECUTIVE COMPENSATION | 51 | ||||
| ITEM 12. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | 51 | ||||
| ITEM 13. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | 51 | ||||
| ITEM 14. |
PRINCIPAL ACCOUNTING FEES AND SERVICES | 51 | ||||
| ITEM 15. |
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES | 51 | ||||
| EX 3.B |
||||||
| EX 10.H |
||||||
| EX-21.A |
||||||
| EX-23.A |
||||||
| EX-31.A |
||||||
| EX-31.B |
||||||
| EX-32.A |
||||||
| EX-32.B |
||||||
| EX-99.1 |
||||||
| EX-101 |
||||||
| Exhibit Index |
||||||
| EX 3.B |
AMENDED AND RESTATED BY-LAWS OF THE REGISTRANT | |||||
| EX 10.H: |
2011 AMENDED AND RESTATED PURCHASE AND SUPPLY AGREEMENT WITH NATIONAL BIOLOGICAL CORPORATION | |||||
| EX-21.A: |
SUBSIDIARIES OF THE REGISTRANT | |||||
| EX-23.A: |
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM | |||||
| EX-31.A: |
CERTIFICATION | |||||
| EX-31.B: |
CERTIFICATION | |||||
| EX-32.A: |
CERTIFICATION | |||||
| EX-32.B: |
CERTIFICATION | |||||
| EX-99.1: |
PRESS RELEASE | |||||
| EX-101 |
INSTANCE DOCUMENT | |||||
| EX-101 |
SCHEMA DOCUMENT | |||||
| EX-101 |
CALCULATION LINKBASE DOCUMENT | |||||
| EX-101 |
LABELS LINKBASE DOCUMENT | |||||
| EX-101 |
PRESENTATION LINKBASE DOCUMENT | |||||
| EX-101 |
DEFINITION LINKBASE DOCUMENT | |||||
Table of Contents
PART I
This Annual Report on Form 10-K and certain written and oral statements incorporated herein by reference of DUSA Pharmaceuticals, Inc. and subsidiaries (referred to as “DUSA,” “we,” and “us”) contain forward-looking statements that have been made pursuant to the provisions of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements are based on current expectations, estimates and projections about DUSA’s industry, management’s beliefs and certain assumptions made by our management. Words such as “anticipates,” “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” or variations of such words and similar expressions, are intended to identify such forward-looking statements. These statements are not guarantees of future performance and are subject to certain risks, uncertainties and assumptions that are difficult to predict particularly in the highly regulated pharmaceutical industry in which we operate. Therefore, actual results may differ materially from those expressed or forecasted in any such forward-looking statements. Such risks and uncertainties include those set forth herein under “Risk Factors” on pages 17 through 28, as well as those noted in the documents incorporated herein by reference. Unless required by law, we undertake no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise. However, readers should carefully review the statements set forth in other reports or documents we file from time to time with the Securities and Exchange Commission, particularly the Quarterly Reports on Form 10-Q and any Current Reports on Form 8-K.
General
DUSA is a vertically integrated dermatology company that is developing and marketing Levulan® photodynamic therapy, or Levulan® PDT. Our marketed products include Levulan® Kerastick® 20% Topical Solution with PDT and the BLU-U® brand light source.
We devote most of our resources to advancing the development and marketing of our Levulan® PDT technology platform. In addition to our marketed products, our drug, Levulan® brand of aminolevulinic acid HCl, or ALA, in combination with light, has been studied in a broad range of medical conditions. When Levulan® is used and followed with exposure to light to treat a medical condition, it is known as Levulan® PDT. The Kerastick® is our proprietary applicator that delivers Levulan®. The BLU-U® is our patented light device.
The Levulan® Kerastick® 20% Topical Solution with PDT and the BLU-U® were launched in the United States, or U.S., in September 2000 for the treatment of non-hyperkeratotic actinic keratoses, or AKs, of the face or scalp. AKs are precancerous skin lesions caused by chronic sun exposure that can develop over time into a form of skin cancer called squamous cell carcinoma. In addition, in September 2003 we received clearance from the United States Food and Drug Administration, or FDA, to market the BLU-U® without Levulan® PDT for the treatment of moderate inflammatory acne vulgaris and general dermatological conditions.
We manufacture our Levulan® Kerastick® in our Wilmington, Massachusetts facility. We are also responsible for the regulatory, sales, marketing, customer service and other related activities for all of our products, including our Levulan® Kerastick®. We began marketing Levulan® PDT under an exclusive worldwide license of patents, many of which have expired, and technology from PARTEQ Research and Development Innovations, the licensing arm of Queen’s University, Kingston, Ontario, Canada. We also own or license certain other patents relating to our BLU-U® device and methods for using pharmaceutical formulations which contain our drug and related processes and improvements. In the United States, DUSA®, DUSA Pharmaceuticals, Inc.®, Levulan®, Kerastick®, BLU-U®, ClindaReach®, Meted®, and Psoriacap® are registered trademarks which we own. Several of these trademarks are also registered in Europe, Australia, Canada, and in other parts of the world. Numerous other trademark applications are pending.
We achieved net income for 2011 of $7,320,000; however, as of December 31, 2011, we had an accumulated deficit of approximately $134,337,000.
1
Table of Contents
We were incorporated on February 21, 1991, under the laws of the State of New Jersey. Unless the context otherwise requires, the terms “we,” “our,” “us,” “the Company” and “DUSA” refer to DUSA Pharmaceuticals, Inc., a New Jersey corporation. Our principal executive office is located at 25 Upton Drive, Wilmington, Massachusetts 01887 (telephone: (978) 657-7500) (web address: www.dusapharma.com). On February 29, 1994, we formed DUSA Pharmaceuticals New York, Inc., a wholly owned subsidiary, to coordinate our research and development efforts. DUSA Acquisition Corp., now known as Sirius Laboratories, Inc., also a wholly-owned subsidiary of DUSA, was formed on January 26, 2006, in connection with our merger with Sirius Laboratories, Inc., an Illinois corporation. We have financed our operations to date, primarily from sales of our products, sales of securities in public offerings, private and offshore transactions that are exempt from registration under the Securities Act of 1933, as amended, or the Act, including private placements under Regulation D of the Act, and from payments received from marketing collaborators. See the sections entitled “Management’s Discussion and Analysis of Financial Condition — Overview; — Results of Operations; and — Liquidity and Capital Resources.”
Business Strategy
The key elements of our strategy include the following:
| • | Expand the Marketing and Sales of Our Products. Continue to drive PDT growth domestically through a focused effort to increase sales of our PDT products to both new and existing medical dermatology customers. |
| • | Conduct Selected Research Programs. During the fourth quarter of 2011 we initiated an exploratory DUSA-sponsored clinical trial designed to study the broad area application and/or short drug incubation, or BASDI, method of using the Levulan® Kerastick®, which is being carried out at 10 clinical trial sites. We expect that approximately 220 study subjects will be enrolled in this trial. The protocol objectives are to compare the safety and efficacy of various incubation times (1, 2 or 3 hours) of Levulan® plus BLU-U® PDT versus vehicle plus BLU-U® for the treatment of multiple actinic keratoses of the face or scalp and to investigate the potential for reduction in AK occurrence in the treatment areas. In addition to the BASDI clinical trial for the treatment of AKs of the face and scalp, a pilot DUSA-sponsored clinical trial designed to study the BASDI method of using the Levulan® Kerastick® for the treatment of AKs on extremities was initiated during the fourth quarter of 2011 at 3 clinical trial sites. We expect that approximately 64 study subjects will be enrolled in this study. The objective of the study is to determine and compare the safety and efficacy of ALA PDT versus vehicle PDT on AKs of the upper extremities, and to evaluate the effect of occlusion on the safety and efficacy of ALA PDT, using blue light after a three hour incubation period. |
| • | Enter into Strategic Alliances. If we determine that the development program for a given indication on our PDT technology may be beyond our own resources or may be advanced to market more rapidly by collaborating with a corporate partner, we may seek opportunities to license, market or co-promote our product opportunities. We also actively seek and explore potential opportunities to in-license or acquire products or technologies that are synergistic with our sales and marketing capabilities. |
| • | Improve Third-party Reimbursement for Our Products. We plan to continue to support activities to improve and/or pursue third-party reimbursement for our products. |
| • | Use the Results of Independent Researchers to Identify New Applications. We continue to support research by independent investigators so that we have the benefit of the resulting anecdotal human data for use in evaluating potential Levulan® clinical indications for corporate development. |
PDT Overview
In general, photodynamic therapy, or PDT, is a two-step process:
| • | the first step is the application of a drug known as a “photosensitizer,” or a pre-cursor of this type of drug, which tends to collect in specific cells; and |
2
Table of Contents
| • | the second step is activation of the photosensitizer by controlled exposure to a selective light source in the presence of oxygen. |
During this process, energy from the light activates the photosensitizer. In PDT, the activated photosensitizer transfers energy to oxygen molecules found in cells, converting the oxygen into a highly energized form known as “singlet oxygen,” which destroys or alters the sensitized cells.
The longer the wavelength of visible light, the deeper into tissue it penetrates. Different wavelengths, or colors of light, including red and blue light, may be used to activate photosensitizers. The selection of the appropriate color of light for a given indication is primarily based on two criteria:
| • | the desired depth of penetration of the light into the target tissue, and |
| • | the efficiency of the light in activating the photosensitizer. |
Blue light does not penetrate deeply into tissues, so it is generally better suited for treating superficial lesions. However, it is also a potent activator of some photosensitizers, including ours. Red light penetrates more deeply into tissues, and is therefore generally better suited for treating cancers and deeper tissues. However, it is generally not as strong an activator of photosensitizers, including ours. Different photosensitizers do not absorb all wavelengths (colors) of visible light in the same manner. For any given photosensitizer, some colors are more strongly absorbed than others.
Another consideration in selecting a light source is the location of the target tissue. Lesions on the skin which are easily accessible can be treated with either laser or non-laser light sources. Internal indications, which are often more difficult to access, usually require lasers in order to focus light into small fiber optic delivery systems that can be passed through an endoscope or into hollow organs.
PDT can be a highly selective treatment that targets specific tissues while minimizing damage to normal surrounding tissues. It also can allow for multiple courses of therapy. The most common side effect of photosensitizers that are applied topically or taken systemically is temporary skin sensitivity to bright light. Patients undergoing PDT treatments are usually advised to avoid direct sunlight and/or to wear protective clothing during this period. Patients’ indoor activities are generally unrestricted except that they are told to avoid bright lights. The degree of selectivity and period of skin photosensitivity varies among different photosensitizers and is also related to the drug dose given. Unless activated by light, photosensitizers have no direct PDT effects.
Our Levulan® PDT Platform
Our Levulan® Brand of ALA
We have a unique approach to PDT using the human cell’s own natural processes. Levulan® PDT takes advantage of the fact that ALA is the first product in a natural biosynthetic pathway present in virtually all living human cells. In normal cells, the production of ALA is tightly regulated through a feedback inhibition process. In our PDT system, excess ALA (as Levulan®) is added from outside the cell, bypassing this normal feedback inhibition. The ALA is then converted through a number of steps into a potent natural photosensitizer named protoporphyrin IX, or PpIX. This is the compound that is activated by light during Levulan® PDT, especially in fast growing cells. Any PpIX that remains after treatment is eliminated naturally by the same biosynthetic pathway.
We believe that Levulan® is unique among PDT agents. It has the following features:
| • | Naturally Occurring. ALA is a naturally occurring substance found in virtually all living human cells. |
| • | Small Molecule. Levulan® is a small molecule that is easily absorbed whether delivered topically, orally, or intravenously. |
3
Table of Contents
| • | Highly Selective. Levulan® is not itself a photosensitizer, but is a pro-drug that is converted through a cell-based process into the photosensitizer PpIX. The combination of topical application, tissue specific uptake, conversion into PpIX and targeted light delivery make this a highly selective process. Therefore, under appropriate conditions, we can achieve selective clinical effects in targeted tissues with minimal effects in normal surrounding and underlying tissues. |
| • | Controlled Activation. Levulan® has no PDT effect without exposure to light at specific wavelengths, so the therapy is easily controlled. |
Scientists believe that the accumulation of PpIX following the application of Levulan® is more pronounced in:
| • | rapidly growing diseased tissues, such as precancerous and cancerous lesions, |
| • | conditions characterized by rapidly proliferating cells such as those found in psoriasis and certain microbes, and |
| • | in certain normally fast-growing tissues, such as hair follicles, sebaceous glands, esophageal mucosa and the lining of the uterus. |
Our Kerastick® Brand Applicator
We designed our proprietary Kerastick® specifically for use with Levulan® and refer to it as the Levulan® Kerastick®. It is a single-use, disposable applicator, which allows for uniform application of Levulan® topical solution in standardized doses. The Kerastick® has two separate glass ampoules, one containing Levulan® powder and one containing a liquid vehicle, both enclosed within a single plastic tube and an outer cardboard sleeve. The dose is metered and filtered at the tip. Prior to application, the physician, nurse or other qualified healthcare practitioner crushes the ampoules and shakes the Kerastick® according to directions to mix the contents into a solution. The Kerastick® tip is then dabbed onto the individual AK lesions, releasing a predetermined amount of Levulan® 20% topical solution.
Our BLU-U® Light Source
Customized light sources are critical to successful Levulan® PDT because the effectiveness of Levulan® therapy depends on delivering light at an appropriate wavelength and intensity. We intend to continue to develop combination drug and light device systems, in which the light sources:
| • | are compact and tailored to fit specific medical needs, |
| • | are pre-programmed and easy to use, and |
| • | provide cost-effective therapy. |
Our proprietary BLU-U® is a continuous-wave (non-pulsed) fluorescent light source that can treat the entire face or scalp at one time. The light source is reasonably sized and can be moved from treatment room to treatment room if necessary. It can be used in a physician’s office, requires only a moderate amount of floor space, and plugs into a standard electrical outlet. The BLU-U® also incorporates a proprietary regulator that controls the optical power of the light source to within specified limits. It has a simple control panel consisting of an on-off key switch and digital timer which turns off the light automatically at the end of the treatment. The BLU-U® is also compliant with CE marking requirements.
We believe non-laser, non-pulsed light sources in comparison to lasers and high-intensity pulsed light sources, are:
| • | safer, |
| • | simpler to use, |
4
Table of Contents
| • | more reliable, and |
| • | less expensive. |
For treatment of AKs, our BLU-U® uses blue light which is a potent activator of PpIX and does not penetrate deeply into the skin. Longer red wavelengths penetrate more deeply into tissue but are not as potent activators of PpIX. Therefore, for treatment of superficial lesions of the skin, such as AKs, our therapy uses relatively low intensity, non-laser, non-pulsed BLU-U®, which is designed to treat areas such as the face or scalp. For treatment of diseases that may extend several millimeters into the skin or other tissues, including many forms of cancer; high-powered red light is usually preferable. We have also received clearance from the FDA to market the BLU-U® without Levulan® for the treatment of moderate inflammatory acne vulgaris and general dermatological conditions.
Our Products
The following table outlines the development status of our key products and planned product candidate. Our research and development expenses for the last three years were $5,275,000 in 2011, $4,930,000 in 2010 and $4,313,000 in 2009.
| Indication/Product |
Regulatory Status | |
|
Levulan® Kerastick® and BLU-U® for PDT of AKs |
Approved | |
|
BLU-U® Treatment of Moderate Inflammatory Acne Vulgaris and general dermatological conditions without Levulan® |
Market Clearance | |
| Broad area application, short drug incubation (BASDI) clinical trial for AKs of the face and scalp |
Clinical Trial(1) | |
| Broad area application, short drug incubation (BASDI) clinical trial for AKs of the upper extremities |
Clinical Trial(2) |
| (1) | A Phase 2 clinical trial was initiated during the fourth quarter of 2011. |
| (2) | A pilot clinical study was initiated during the fourth quarter of 2011. |
Dermatology Indications
Actinic Keratoses
AKs are superficial precancerous skin lesions usually appearing in sun-exposed areas as rough, scaly patches of skin with some underlying redness. The traditional methods of treating AKs are cryotherapy, or the deep freezing of skin, using liquid nitrogen; topical prescription products such as 5-fluorouracil cream, or 5-FU, diclofenac sodium and imiquimod; and surgery, for especially thick or suspicious lesions. Although any of these methods can be effective, each has limitations and can result in significant side effects. Cryotherapy is non-selective, can be painful at the site of freezing and can cause blistering and loss of skin pigmentation, leaving temporary or permanent white spots. In addition, because there is no standardized treatment protocol, results are not uniform. 5-FU can be highly irritating and requires twice-a-day application by the patient for approximately 2 to 4 weeks, resulting in inflammation, redness and erosion or rawness of the skin. Following the treatment, an additional 1 to 2 weeks of healing is required. Surgery is generally most useful for one or a few individual lesions, but not large numbers of lesions, and leaves permanent scars. Imiquimod or diclofenac require extended applications of cream, lasting up to 3 or 4 months, during which the skin is often very red and inflamed. Our approved treatment method involves applying Levulan® 20% topical solution using the Kerastick® to individual AK lesions, followed 14 to 18 hours later with exposure to our BLU-U® for approximately 17 minutes. In our Phase 3 trials, using this overnight drug application, our treatment produced varying degrees of pain during light treatment, but the therapy was generally well tolerated. The resulting redness and/or inflammation generally resolved within days without any change in pigmentation.
5
Table of Contents
Acne
Acne is a common skin condition caused in part by the blockage and/or inflammation of sebaceous (oil) glands. Traditional treatments for mild to moderate facial inflammatory acne include over-the-counter topical medications for mild cases, and prescription topical medications or oral antibiotics for mild to moderate cases. For nodulo-cystic acne, an oral retinoid drug called Accutane®1 is the most commonly prescribed treatment. It is also commonly used for moderate to severe inflammatory acne.
Over-the-counter treatments are not effective for many patients and can result in side effects including drying, flaking and redness of the skin. Prescription antibiotics lead to improvement in many cases, but patients must often take them on a long-term basis, with the associated risks including increased antibiotic resistance. Blue light alone has been shown to improve mild to moderate inflammatory acne, in part, by targeting the bacterium Propionibacterium acnes (P. acnes), which accumulates its own photosensitizer much like that produced by Levulan® in the skin, and possibly by other anti-inflammatory actions.
DUSA has clearance from the FDA to market the BLU-U® without Levulan® PDT for the treatment of moderate inflammatory acne vulgaris and general dermatological conditions.
Other Potential Levulan® Indications
We believe that there may be numerous other potential uses for Levulan® PDT in dermatology, and we intend to continue to support research in several of these areas, with corporate-sponsored trials, pilot trials, and/or investigator-sponsored studies, based on pre-clinical, clinical, regulatory and marketing criteria we have established through our strategic planning processes. Some of the additional potential uses for Levulan® in dermatology may include treatment of skin conditions such as psoriasis, onychomycosis, warts, molluscum contagiosum, oily skin, acne rosacea, cystic acne, inflamed or infected sweat glands (hidradenitis suppurativa), non-melanoma skin cancers, and cutaneous T-cell lymphomas as well as the prevention of non-melanoma skin cancers. Of these potential indications, we have supported or are currently supporting internal or investigator-sponsored studies for use of Levulan® PDT to treat hidradenitis suppurativa, acne vulgaris, non-melanoma skin cancer, warts, actinic keratoses of the lips (actinic cheilitis) and of the extremities, and inflammatory acne.
Supply Partners
National Biological Corporation
On November 29, 2011, we entered into the 2011 Amended and Restated Purchase and Supply Agreement, or the 2011 NBC Agreement, with National Biological Corporation, or NBC, the primary manufacturer of our BLU-U® light source. The 2011 NBC Agreement includes similar terms and conditions to our Amended and Restated Purchase and Supply Agreement dated as of June 21, 2004, as amended, or the 2004 Agreement, which was due to expire on December 31, 2011. The 2011 NBC Agreement replaces the 2004 Agreement and has a term of 2 years through December 31, 2013. We have an option to further extend the term of the 2011 NBC Agreement for an additional 2 years if we purchase a certain number of units.
Sochinaz SA
Under an agreement dated December 24, 1993, Sochinaz SA manufactures and supplies our requirements of Levulan® from its FDA approved facility in Switzerland. In 2009, the parties renewed the agreement until December 31, 2015 on substantially the same terms, albeit with a revised pricing schedule to cover the new term. Sochinaz is our sole source for Levulan® and while we can obtain alternative supply sources in certain circumstances, any new supplier would have to be inspected and qualified by the FDA.
| 1 | Accutane® is a registered trademark of Hoffmann-La Roche, Inc. |
6
Table of Contents
Licenses
PARTEQ
We license (or, in the case of the patents in Australia, were assigned) the patents underlying our Levulan® PDT system under a license agreement with PARTEQ Research and Development Innovations, or PARTEQ, the licensing arm of Queen’s University, Kingston, Ontario. Under the agreement, which became effective August 27, 1991, we have been granted an exclusive worldwide license, with a right to sublicense, under PARTEQ’s patent rights, to make, have made, use and sell products which are precursors of PpIX, including ALA. The agreement also covers any improvements discovered, developed or acquired by or for PARTEQ, or Queen’s University, to which PARTEQ has the right to grant a license. A non-exclusive right is reserved to Queen’s University to use the subject matter of the agreement for non-commercial educational and research purposes. A right is also reserved to the Department of National Defense Canada to use the licensed rights for defense purposes including defense procurement but excluding sales to third parties.
When we are selling our products directly, we have agreed to pay to PARTEQ royalties of 6% and 4% on 66% of the net selling price in countries where patent rights do and do not exist, respectively. In cases where we have a sublicensee, we will pay 6% and 4% when patent rights do and do not exist, respectively, on our net selling price less the cost of goods for products sold to the sublicensee, and 6% of royalty payments we receive on sales of products by the sublicensee. We are also obligated to pay 5% of any lump sum sublicense fees paid to us, such as milestone payments, excluding amounts designated by the sublicensee for future research and development efforts. The agreement is effective for the life of the latest United States patents and becomes perpetual and royalty-free when no United States patent subsists. Our AK product is covered by patents that start to expire in 2013 with certain new claims continuing through 2019. Annual minimum royalties to PARTEQ must total at least CDN $100,000 (U.S. $98,000 as of December 31, 2011) in order to retain the license. For 2011, royalties exceeded this minimum. We have the right to terminate the PARTEQ agreement with or without cause upon 90 days notice.
Together with PARTEQ and Draxis Health, Inc., our former parent, we entered into an agreement, known as the ALA Assignment Agreement, effective October 7, 1991. According to the terms of this agreement, we assigned to Draxis our rights and obligations under the PARTEQ license agreement to the extent they relate to Canada. On February 24, 2004, we reacquired these rights and agreed to pay an upfront fee and a 10% royalty on sales of the Levulan® Kerastick® in Canada over a 5-year term following the first commercial sale in Canada, which ended in the second quarter of 2010. Draxis also agreed to assign to us the Canadian regulatory approvals for the Levulan® Kerastick® with PDT for AKs. We also hold Canadian regulatory approval for the BLU-U®. In 2004, we appointed a Canadian distributor who launched our Levulan® Kerastick® and BLU-U® in Canada. See the section entitled “Distribution.”
PhotoCure ASA
On May 30, 2006, we entered into a patent license agreement with PhotoCure ASA whereby we granted a non-exclusive license to PhotoCure under the patents we license from PARTEQ for esters of ALA. Furthermore, we granted a non-exclusive license to PhotoCure for its existing formulations of its Hexvix® and Metvix® (known in the United States as Metvixia®) products for any DUSA patents that may issue or be licensed by us in the future.
PhotoCure received FDA approval to market Metvixia® for treatment of AKs in July 2004, and this product is directly competitive with our Levulan® Kerastick®. In 2009, PhotoCure transferred its license to Metvix®/Metvixia® to Galderma, S.A., a large dermatology company. While we are entitled to royalties on net sales of Metvixia®, Galderma has considerably more resources than we have, which could significantly hamper our ability to maintain or increase our market share. Metvixia® is commercially available in the U.S.; however, to our knowledge product revenues have not been significant to date.
7
Table of Contents
Patents and Trademarks
We actively seek, when appropriate, to protect our products and proprietary information through United States and foreign patents, trademarks and contractual arrangements. In addition, we rely on trade secrets and contractual arrangements to protect certain aspects of our proprietary information and products.
Our ability to compete successfully depends, in part, on our ability to defend our patents that have been issued, obtain new patents, protect trade secrets and operate without infringing the proprietary rights of others. Even where we have patent protection, there is no guarantee that we will be able to enforce our patents. Patent litigation is expensive, and we cannot assure you that we will defend or successfully defend our patents.
We have no product patent protection for the compound ALA itself, as our basic patents are for methods of detecting and treating various diseased tissues using ALA or related compounds called precursors, in combination with light. We own or exclusively license patents and patent applications related to the following:
| • | methods of using ALA and its unique physical forms in combination with light to treat conditions such as AKs and acne, |
| • | compositions and apparatus for those methods, and |
| • | unique physical forms of ALA. |
The reexamination by the USPTO of one of the patents we license from PARTEQ, the licensor of our ALA patents, that covers our approved product until 2013, has successfully concluded with the confirmation of the validity of all of the patent’s original claims and the addition of eight new claims. With claims which were issued on May 25, 2010, we now have additional claims that relate to our AK product, and these will not expire until 2019.
Under the license agreement with PARTEQ, we hold an exclusive worldwide license to certain patent rights in the United States and a limited number of foreign countries. See the section entitled “Business — Licenses.” The United States patents and patent applications licensed from PARTEQ relating to ALA are method of treatment patents. The patents relating to methods of using ALA for detecting or treating disease, other than for acne and our approved indication for AKs of the face or scalp, started to expire in July 2009. Method of treatment patents limit direct infringement to users of the methods of treatment covered by the patents. We have patents and/or pending patent applications in the United States and in a number of foreign countries covering unique physical forms of ALA, compositions containing ALA, as well as ALA applicators, light sources for use with ALA, including our BLU-U® brand light device, and other technology. We cannot guarantee that any pending patent applications will mature into issued patents.
We have limited patent protection outside the United States, which may make it easier for third parties to compete there. Our basic ALA method of treatment patents and applications have counterparts in only four foreign countries and under the European Patent Convention. See the section entitled “Risk Factors — Risks Related to DUSA.”
We can provide no assurance that a third-party or parties will not claim, with or without merit, that we have infringed or misappropriated their proprietary rights. A number of entities have obtained, and are attempting to obtain patent protection for various uses of ALA. We can provide no assurance as to whether any issued patents, or patents that may later issue to third parties, may affect the uses on which we are working or whether such patents can be avoided, invalidated or licensed if they cannot be avoided or invalidated. If any third-party were to assert a claim for infringement, we can provide no assurance that we would be successful in the litigation or that such litigation would not have a material adverse effect on our business, financial condition and results of operation. Furthermore, we may not be able to afford the expense of defending against any such additional claim.
In addition, we know that our patents, whether owned or licensed, or any future patents that may issue, have not prevented other companies from developing similar or functionally equivalent products. Further, we cannot
8
Table of Contents
guarantee that we will continue to develop our own patentable technologies or that our products or methods will not infringe upon the patents of third parties. In addition, we cannot guarantee that any of the patents that may be issued to us will effectively protect our technology or provide a competitive advantage for our products or will not be challenged, invalidated, or circumvented in the future.
We also attempt to protect our proprietary information as trade secrets. Generally, agreements with employees, licensing partners, consultants, universities, pharmaceutical companies and agents contain provisions designed to protect the confidentiality of our proprietary information. However, we can provide no assurance that these agreements will provide effective protection for our proprietary information in the event of unauthorized use or disclosure of such information. Furthermore, we can provide no assurance that our competitors will not independently develop substantially equivalent proprietary information or otherwise gain access to our proprietary information, or that we can meaningfully protect our rights in unpatentable proprietary information.
Even in the absence of composition of matter patent protection for ALA, we may receive financial benefits from: (i) patents relating to the use of such products (like PARTEQ’s patents); (ii) patents relating to special compositions and formulations; (iii) limited marketing exclusivity that may be available under the Hatch-Waxman Act and any counterpart protection available in foreign countries and (iv) patent term extension under the Hatch-Waxman Act. See the section entitled “Business — Government Regulation.” Effective patent protection also depends on many other factors such as the nature of the market and the position of the product in it, the growth of the market, the complexities and economics of the process for manufacture of the active ingredient of the product and the requirements of the new drug provisions of the Food, Drug and Cosmetic Act, or similar laws and regulations in other countries.
We seek registration of trademarks in the United States, and other countries where we may market our products. To date, we have been issued more than 135 trademark registrations, including trademarks for DUSA®, DUSA Pharmaceuticals, Inc.®, Levulan®, Kerastick®, BLU-U®, ClindaReach®, Meted®, Psoriacap®, and other applications are pending.
Manufacturing
We manufacture our Levulan® Kerastick® at our Wilmington, Massachusetts facility and we maintain a reasonable level of Kerastick® inventory based on our internal sales projections. In 2005, we received FDA approval to manufacture our BLU-U® brand light source in our Wilmington, Massachusetts facility. However, at this time, we expect to utilize our own facility only as a back-up to our current third-party manufacturer, or for repairs. Our drug, Levulan®, and the main component of the BLU-U® brand light source are each manufactured by single source suppliers.
Distribution
We have been a direct distributor of the BLU-U® since its launch. Effective January 1, 2006, we increased our own distribution capacity and have become the sole distributor for our Levulan® Kerastick® in the United States. In March 2004, we signed an exclusive Canadian marketing and distribution agreement for the Levulan® Kerastick® and BLU-U® with Clarion Medical Technologies, Inc., or Clarion (formerly known as Coherent-AMT), a leading Canadian medical device and laser distribution company. Clarion began marketing the BLU-U® in April 2004 and the Kerastick® in June 2004, following receipt of the applicable regulatory approval from Health Protection Branch — Canada. The agreement is automatically renewed for one-year terms, unless either party notifies the other party prior to a term expiration that it does not intend to renew the agreement. Clarion has the right for a period of time following termination of its agreement to return inventory of product.
9
Table of Contents
Marketing and Sales
DUSA markets its products in the United States. We have appointed Clarion as our marketing partner for our PDT products in Canada. See the section entitled “Business — Distribution.”
As of December 31, 2011 and 2010, respectively, we had 46 and 39 sales representatives and management personnel deployed nationally.
Competition
There are many pharmaceutical companies that compete with us in the field of dermatology, particularly in the acne market. The pharmaceutical industry is highly competitive, and many of our competitors have substantially greater financial, technical and marketing resources than we have. In addition, several of these companies have significantly greater experience than we do in developing products, conducting preclinical and clinical testing and obtaining regulatory approvals to market products for health care. Our competitors may succeed in developing products that are safer or more effective than ours and in obtaining regulatory marketing approval of future products before we do. Our competitiveness may also be affected by our ability to manufacture and market our products and by the level of reimbursement for the cost of our drug and treatment by third-party payors, such as insurance companies, health maintenance organizations and government agencies.
A number of companies are pursuing commercial development of PDT agents other than Levulan®. These include: Galderma S.A. (Switzerland); QLT Inc. (Canada); Miravant, Inc. (United States); and Pharmacyclics, Inc. (United States). We believe that several companies are also commercializing and/or conducting research with ALA or ALA-related compounds. These include: medac GmbH and photonamic GmbH & Co. KG (Germany); Biofrontera PhotoTherapeutics, Inc. (U.K.), and PhotoCure ASA (Norway).
PhotoCure has received marketing approval of its ALA precursor (ALA methyl-ester) compound for PDT treatment of AK and basal cell carcinoma, called BCC, in the European Union, New Zealand, Australia, and countries in Scandinavia. PhotoCure received FDA approval to market Metvixia® for treatment of AKs in July 2004, and this product is directly competitive with our Levulan® Kerastick® product. On October 1, 2009, PhotoCure announced that it had sold Metvixia® to Galderma, S.A., a large dermatology company.
On May 30, 2006, we entered into a patent license agreement under which we granted PhotoCure ASA a non-exclusive license under the patents we license from PARTEQ for ALA esters. In addition, we granted a non-exclusive license to PhotoCure for its existing formulations of Hexvix® and Metvix® (known in the U.S. as Metvixia®) for any patent we own now or in the future. PhotoCure is obligated to pay us royalties on sales of its ester products to the extent they are covered by our patents in the U.S. and certain other territories.
There are also non-PDT products for the treatment of AKs, including cryotherapy with liquid nitrogen, 5-fluorouracil (Efudex®)2, diclofenac sodium (Solaraze®)3, and imiquimod (ALDARA® and Zyclara®)4. On December 2, 2011, Medicis Pharmaceuticals Corporation, or Medicis, announced that it completed its acquisition of the assets of Graceway Pharmaceuticals, LLC, or Graceway. Medicis was the successful bidder at bankruptcy auction for Graceway’s U.S. and Canadian pharmaceutical assets. Medicis has the opportunity to launch two recently approved line extensions for the Zyclara® franchise. On January 25, 2012, the FDA approved Leo Pharma’s Picato® Gel (ingenol mebutate), a new treatment for AKs of both the face and scalp (0.015% formulation) and trunk and extremities (0.05% formulation).
Other AK therapies are also known to be under development by companies such as Medigene GmbH and others.
| 2 | Efudex® is a registered trademark of Valeant Pharmaceuticals International. |
| 3 | Solaraze® is a registered trademark of Fougera Pharmaceuticals Inc. |
| 4 | ALDARA® and Zyclara® are registered trademarks of Medicis Pharmaceuticals Corp. |
10
Table of Contents
We believe that comparisons of the properties of various photosensitizing PDT drugs will also highlight important competitive issues. We expect that our principal methods of competition with other PDT companies will be based upon such factors as the ease of administration of our photodynamic therapy; the degree of generalized skin sensitivity to light; the number of required doses; the selectivity of our drug for the target lesion or tissue of interest; and the type and cost of our light systems. New drugs or future developments in PDT, laser products or other drug technologies may provide therapeutic or cost advantages for competitive products. We believe that with increased reimbursement for our PDT-related procedure fee, including an increase effective January 2012, our treatment is increasingly more viable from a financial perspective for practitioners, and more competitive with alternative AK therapies from a practice management perspective. However, no assurance can be given that developments by other parties will not render our products uncompetitive or obsolete.
DUSA also markets the BLU-U® without Levulan® for the treatment of moderate inflammatory acne vulgaris and general dermatological conditions. Our competition for the BLU-U® without Levulan® for moderate inflammatory acne vulgaris is primarily oral antibiotics, topical antibiotics and other topical prescription drugs, as well as various laser and non-laser light sources. Blue light alone for acne is still a relatively new therapy and reimbursement has not been established by private insurance companies, which affects our competitive position as compared to traditional therapies which are reimbursed.
Government Regulation
The manufacture and sale of pharmaceuticals and medical devices in the United States and other countries are governed by a variety of statutes and regulations. These laws require, among other things:
| • | registration and inspection of manufacturing facilities, including adherence to current good manufacturing practice regulations, or cGMPs, quality system regulations, or QSRs, and good laboratory and good clinical practices or GLPs and GCPs; |
| • | adequate and well-controlled clinical studies and preclinical testing of products; |
| • | the submission of applications for marketing approval containing manufacturing, preclinical and clinical data and information to establish the safety and efficacy of the product for its intended use; |
| • | post-approval recordkeeping and reporting, including safety surveillance and reporting of adverse events to regulatory authorities; and |
| • | compliance with requirements and restrictions for marketing activities, including advertising and labeling. |
The process of obtaining marketing approval for a new drug or medical device normally takes several years and often involves significant costs. The steps required before a new drug or medical device can be produced and marketed for human use in the United States include:
| • | preclinical testing; |
| • | the filing of an Investigational New Drug application, or IND, for new drugs, and an Investigational Device Exemption application, or IDE, for medical devices; |
| • | human clinical trials, including the analysis of data collected from those trials; and |
| • | the preparation, submission, and approval of a New Drug Application, or NDA, for new drugs, or a Premarket Approval Application, or PMA, for medical devices. |
Preclinical testing is conducted in the laboratory and on animals to obtain preliminary information primarily on the safety of a new drug or device, although preclinical data may provide some information relevant to the potential efficacy of the product. The time required for conducting preclinical testing varies greatly depending on the nature of the product and the nature and design of the testing. The collection and analysis of preclinical data can take many years to complete. Such data are submitted to the FDA as part of the IND or IDE. Human studies can begin if the FDA does not object to the IND or IDE application.
11
Table of Contents
The human clinical testing program involves three phases, Phase 1, Phase 2, and Phase 3. Each clinical study is typically conducted under the auspices of an Institutional Review Board, or IRB, at the institution where the study will be conducted. An IRB will consider among other things, ethical factors, the potential risks to study subjects, and the possible liability of the institution. A clinical plan, or “protocol,” must be submitted to the FDA prior to commencement of each clinical trial. All subjects involved in the clinical trial must provide informed consent prior to their participation. The FDA may order the temporary or permanent discontinuance of a clinical trial at any time before or after a study begins for a variety of reasons, particularly if safety concerns exist. These clinical studies must be conducted in conformance with the FDA’s human subject protection and IND or IDE regulations.
In Phase 1 studies are usually conducted on a small number of healthy human volunteers to determine the maximum tolerated dose and any product-related side effects of a product. Phase 1 studies generally require several months to complete, but can take longer, depending on the drug and the nature of the study. Phase 2 studies are conducted on a small number of patients having a specific disease to determine the most effective doses and schedules of administration. Phase 2 studies generally require from several months to two years to complete, but can take longer, depending on the drug and the nature of the study. Phase 3 studies involve significant numbers of patients with the targeted disease or condition to provide comparisons against placebo or, in some cases, currently available therapies. Phase 3 studies generally require from six months to four years to complete, but can take longer, depending on the drug and the nature of the study.
Data from Phase 1, 2 and 3 trials are submitted to the FDA with the NDA. The NDA involves considerable data collection from preclinical testing and clinical studies, verification and analysis of data, as well as the preparation of summaries of the chemistry, manufacturing, and control processes. Submission of an NDA does not assure FDA approval for marketing. The application review process may take 180 days but more often it takes one to four years to complete, although reviews of treatments for AIDS, cancer and other serious and life-threatening diseases and conditions may be accelerated, expedited or subject to fast track treatment. The process may take substantially longer if, among other things, the FDA has questions or concerns about the safety and/or efficacy of a product. In general, the FDA requires properly conducted, adequate and well-controlled clinical studies demonstrating safety and efficacy for the product’s intended use with sufficient levels of statistical significance. However, additional information or data may be required. For example, the FDA may also request long-term toxicity studies, one or more additional pivotal or Phase 3 studies, or other studies relating to product safety or efficacy. Even with the submission of such data, the FDA may decide that the application does not satisfy its regulatory criteria for approval and may disapprove the NDA. Finally, the FDA may require additional clinical studies following NDA approval, often referred to as Phase 4 clinical trials, to confirm safety and efficacy for the intended use.
Upon approval, a prescription drug may only be marketed for the approved indications in the approved dosage forms and at the approved dosage with the approved labeling. Adverse experiences with the product must be reported to the FDA. In addition, the FDA may impose restrictions on the use of the drug that may be difficult and expensive to administer. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems occur or are discovered after the product reaches the market. After a product is approved for a given indication, the addition of new indications, any changes to the dosage form, or dosage levels, and certain other labeling changes for the same product may only be made after a supplemental NDA, or sNDA, is submitted to FDA support the safety and efficacy of the proposed changes and approved by the FDA.
The FDA regulations also require extensive recordkeeping and reporting of certain manufacturing deviations and certain safety and other information, often referred to as “adverse events” that become known to the manufacturer of an approved drug. Safety information collected through this process can result in changes to a product’s labeling or withdrawal of a product from the market. Usually the preparation and review of a sNDA takes less time than the original NDA because the sNDA may rely upon the data and information from the NDA to support the safety and efficacy of the proposed change.
12
Table of Contents
On December 3, 1999, the FDA approved the NDA we submitted for Levulan® Kerastick® 20% Topical Solution with PDT for treatment of non-hyperkeratotic actinic keratoses, or AKs, of the face or scalp. The commercial version of our BLU-U® medical device, used together with the Kerastick® to provide PDT for the treatment of non-hyperkeratotic AKs of the face or scalp, was approved on September 26, 2000. In September 2003, we received approval from the FDA to market the BLU-U® without Levulan® PDT for the treatment of moderate inflammatory acne vulgaris and general dermatological conditions.
We may develop other potential PDT products for other indications, which would require significant development, including approval and completion of preclinical and/or clinical testing and PMA approval prior to commercialization. The process of obtaining PMA approvals can be costly and time consuming and there can be no guarantee that the use of Levulan® with any future medical devices or for any other indication will be successfully developed, prove to be safe and effective in clinical trials, or receive applicable regulatory marketing approvals.
Medical devices, such as our light source device, are also subject to FDA-enforced laws and regulations. These products are required to be tested, developed, manufactured and distributed in accordance with FDA regulations, including cGMP, set forth under QSRs, and good laboratory and clinical practices. Under the Food, Drug and Cosmetic Act, all medical devices are classified as Class I, II or III devices. The classification of a device reflects the level of risk to a patient, with Class III devices having the highest risk and subject to the most stringent requirements and FDA review. Class I devices are generally the lowest risk devices and are subject only to general controls (for example, labeling and adherence to QSRs). Class II devices are generally moderate risk devices and are subject to general controls and special controls (for example, performance standards, postmarket surveillance, FDA guidelines specific to the type of product). Class III devices, which typically are life-sustaining or life-supporting and implantable devices, or new devices that have been found not to be substantially equivalent to a legally marketed Class I or Class II “predicate device,” are subject to general controls and also require clinical testing to assure safety and effectiveness before FDA approval is obtained. The FDA also has the authority to require clinical testing of Class I and II devices. The BLU-U® medical device is part of a combination product as defined by FDA and therefore has been classified as a Class III device. Approval of Class III devices require the filing of a PMA with extensive data, including preclinical and clinical trial data, to demonstrate the safety and effectiveness of the device for its intended use. If human clinical trials of a device are required and the device presents a “significant risk,” the manufacturer of the device must file an IDE and receive FDA approval prior to commencing human clinical trials.
Following receipt of the PMA, the FDA will determine whether the application is sufficiently complete to permit a substantive review, and if so, the agency will accept the PMA for filing and further review. Once the PMA is filed, the FDA begins a review of the PMA application. Under the Medical Device User Fee and Modernization Act, the FDA has 180 days to review a PMA and respond to the applicant. The review of PMAs more often occurs over a significantly protracted time period, and the FDA may take up to two years or more from the date of filing to complete its review. In addition, a PMA for a device which forms part of a combination product with a new drug will not be approved unless and until the NDA for the drug in the combination is also approved.
The PMA process can be expensive, uncertain and lengthy. A number of other companies have sought premarket approval for devices that have never been approved for marketing. The review time is often significantly extended by the FDA, which may require more information or clarification of information already provided in the submission. During the review period, an advisory committee may be convened to review and evaluate the data in a PMA and provide recommendations to the FDA as to whether the device should be approved for marketing. In addition, the FDA will inspect the medical device manufacturing facility to ensure compliance with QSR requirements prior to approval of the PMA. If granted, the premarket approval may include significant limitations on the indicated uses for which the product may be marketed, and the agency may require post-marketing studies of the device. The Medical Device Reporting regulations require that we provide information to the FDA whenever there is evidence to reasonably suggest that one of our devices may have
13
Table of Contents
caused or contributed to a death or serious injury or, if a malfunction were to recur, could cause or contribute to a death or serious injury. Under FDA regulations, we are required to submit reports of certain voluntary recalls and corrections to the FDA. If the FDA believes that a company is not in compliance with applicable regulations, the Agency may issue a notice of noncompliance in the form of inspectional observations on Form FDA-483, general correspondence, or a Warning Letter. If the noncompliance is not corrected to the satisfaction of the FDA, the agency may take any or all of the following legal actions: detain or seize the products, order a recall, impose operating restrictions, temporarily stop the manufacture of the product, seek injunctive relief to enjoin future violations, assess civil penalties against that company, its officers or its employees, and recommend criminal prosecution to the Department of Justice.
When a drug must be used with a specific medical device to be safe and effective for a specific indication, the drug and the device may be regulated as “combination products.” A combination product generally is defined as a product comprised of components from two or more regulatory categories (drug/device, device/biologic, drug/biologic, etc.). In December 2002, the FDA established the Office of Combination Products, or OCP, whose responsibilities, according to the FDA, will cover the entire regulatory life cycle of combination products, including jurisdictional decisions as well as the timeliness and effectiveness of pre-market review, and the consistency and appropriateness of post-market regulation.
In connection with our NDA for the Levulan® Kerastick® with PDT for AKs, a combination filing (including a PMA for the BLU-U® light source device and the NDA for the Levulan® Kerastick® drug) was submitted to the Center for Drug Evaluation and Research. The PMA was then separated from the NDA submission by the FDA and reviewed by the FDA’s Center for Devices and Radiological Health. Based upon this experience, we anticipate that any future NDAs for Levulan® PDT will be for combination products and will be accompanied by PMAs for the medical devices used in the combination. There is no guarantee that PDT products will continue to be regulated as combination products.
The United States Drug Price Competition and Patent Term Restoration Act of 1984 known as the Hatch-Waxman Act establishes a 5-year period of marketing exclusivity from the date of NDA approval for new chemical entities approved after September 24, 1984. Levulan® is a new chemical entity and we received 5 years of market exclusivity, which expired on December 3, 2004. After the expiration of the Hatch-Waxman exclusivity period, any third-party who submits an application for approval for a drug product containing ALA must provide a certification for each patent that claims the drug and is listed in the FDA’s Orange Book for Approved Drug Products with Therapeutic Equivalence Evaluations that: (i) no such patent information has been listed (ii) the patent has expired; (iii) marketing will not commence until the patent has expired; or (iv) the patent is invalid or will not be infringed by the manufacture, use, or sale of the product that is the subject of the new application.
If any person submits an abbreviated NDA, or ANDA, or an NDA that intends to rely on some or all of the data in our Levulan® NDA (also referred to as a “505(b)(2)” application), the applicant also must notify us as the NDA holder and the owner of the patent that claims the drug. Such notice will enable us to determine whether to bring a patent infringement lawsuit to protect our patent rights.
Generally, we try to design our protocols for clinical studies so that the results can be used in all the countries where we hope to market the product. However, countries sometimes require additional studies to be conducted on patients located in their country. Prior to marketing a product in other countries, approval by that nation’s regulatory authorities must be obtained. For Levulan® PDT, we have received such approval in Canada, Argentina, Brazil, Chile, Colombia and Mexico. Also, together with Daewoong, we have received approval in Korea. We expect to apply for approvals in additional territories.
Medical device regulations also are in effect in many of the countries outside the United States in which we do business. These laws range from comprehensive device approval and quality system requirements for some or all of our medical device products to simpler requests for product data or certifications. The number and scope of these requirements are increasing. Under the European Union Medical Device Directive, all medical devices
14
Table of Contents
must meet the Medical Device Directive standards and receive CE Mark certification. CE Mark certification requires a comprehensive quality system program and submission of data on a product to a “Notified Body” in Europe. The Medical Device Directive, ISO 9000 series and ISO 13485 are recognized international quality standards that are designed to ensure that we develop and manufacture quality medical devices. A recognized Notified Body (an organization designated by the national governments of the European Union member states to make independent judgments about whether or not a product complies with the protection requirements established by each CE marking directive) audits our facilities annually to verify our compliance with these standards. We will be required to meet these standards should we decide to sell our devices outside of the United States.
We are subject to laws and regulations that regulate the means by which companies in the health care industry may market their products to hospitals and health care professionals and may compete by discounting the prices of their products. This requires that we exercise care in structuring our sales and marketing practices and customer discount arrangements.
Our international operations are subject to laws and regulations regarding customs, import-export business transactions, and other local laws specific to each country. Among other things, laws in certain countries restrict, and in some cases prohibit, United States companies from directly or indirectly selling goods, technology or services to people or entities in those countries.
Our research, development and manufacturing processes involve the controlled use of certain hazardous materials. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these materials and certain waste products. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards prescribed by the controlling laws and regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. In the event of this type of an accident, we could be held liable for any damages that result and any liability could exceed our resources. Although we believe that we are in compliance in all material respects with applicable environmental laws and regulations, we could incur significant costs to comply with environmental laws and regulations in the future, and our operations, business or assets could be materially adversely affected by current or future environmental laws or regulations. During the design, construction and validation phases of our Kerastick® manufacturing facility, we have taken steps to ensure that appropriate environmental controls associated with the facility comply with environmental laws and standards. Furthermore, we cannot assure that current or future environmental laws or regulations will not materially adversely affect our operations, business or assets. Although we believe that our safety procedures for the handling and disposal of such hazardous materials comply with the standards prescribed by current environmental laws and regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of such an event, we could be held liable for any damages that result, and any such liability could exceed our resources.
In addition to the above regulations, we are and may be subject to regulation under federal and state laws, including, but not limited to, requirements regarding occupational health and safety, laboratory practices, state pharmacy and wholesale drug distribution laws, and the maintenance of personal health information. As a public company, we are subject to securities laws and regulations, including the Sarbanes-Oxley Act of 2002 and the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010. We may also be subject to other present and possible future local, state, federal and foreign regulations.
With the enactment of the Drug Export Amendments Act of the United States in 1986, products not yet approved by the FDA may be exported to certain foreign markets if the product is approved by the importing nation and meets applicable FDA criteria for export. We can provide no assurance that we will be able to get additional approvals for any of our products from any importing nations’ regulatory authorities or be able to participate in additional foreign pharmaceutical markets.
15
Table of Contents
Product Liability and Insurance
We are subject to the inherent business risk of product liability claims in the event that the use of our technology or any prospective product is alleged to have resulted in adverse effects during testing or following marketing approval of any such product for commercial sale. We maintain product liability insurance for coverage of our clinical trial activities and for our commercial supplies. There can be no assurance that such insurance will continue to be available on commercially reasonable terms or that it will provide adequate coverage against all potential claims.
Segment Reporting
We operate in one segment, Photodynamic Therapy, or PDT, Drug and Device Products. Our Levulan® Kerastick® and BLU-U® products comprise our commercial product offerings.
Following our acquisition of Sirius Laboratories, Inc., which occurred in 2006, we had operated in two segments, PDT Drug and Device Products and Non-Photodynamic Therapy, or Non-PDT, Drug Products. Our Non-PDT segment was comprised of the products acquired in the acquisition of Sirius Laboratories, Inc. At December 31, 2011, we ceased marketing and selling our remaining Non-PDT products, which were ClindaReach® and Meted®. The former Non-PDT Drugs Products segment is now reflected as discontinued operations in the accompanying financial statements for all periods presented.
Information About Geographic Sources of Revenue
For information about the geographic sources of our revenue, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Results of Operations.”
Employees
At the end of 2011, we had 96 employees, including 1 part-time employee. We also retain numerous independent consultants and temporary employees to support our business needs. We have employment agreements with all of our key executive officers.
Internet Information
Our Internet site is located at www.dusapharma.com. Copies of our reports filed pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, including Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, may be accessed from our website, free of charge, as soon as reasonably practicable after we electronically file such reports with, or furnish such reports to the SEC. Please note that our Internet address is being provided for reference only and no information contained therein is incorporated by reference into our Exchange Act filings. The public may read or copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Room 1580, Washington DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers, including DUSA, that file electronically with the SEC. The address of that site is www.sec.gov.
16
Table of Contents
| ITEM 1A. | RISK FACTORS |
Investing in our common stock is very speculative and involves a high degree of risk. You should carefully consider and evaluate all of the information in, or incorporated by reference in, this report. The following are among the risks we face related to our business, assets and operations. They are not the only ones we face. Any of these risks could materially and adversely affect our business, results of operations and financial condition, which in turn could materially and adversely affect the trading price of our common stock and you might lose all or part of your investment.
This report contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties, such as statements of our plans, objectives, expectations and intentions. We use words such as “anticipate”, “believe”, “expect”, “future” and “intend” and similar expressions to identify forward-looking statements. Our actual business, financial condition and results of operations could differ materially from those anticipated in these forward-looking statements for many reasons, including the factors described below and elsewhere in this report. You should not place undue reliance on these forward-looking statements, which apply only as of the date of this report.
Risks Related To DUSA
Any Failure To Comply With Ongoing Governmental Regulations In The United States And Elsewhere Will Limit Our Ability To Market Our Products And Achieve Profitability On A Quarterly Basis.
The manufacture and marketing of our products are subject to continuing FDA review as well as comprehensive regulation by the FDA and by state and local regulatory authorities. These laws require, among other things:
| • | approval of manufacturing facilities, including adherence to good manufacturing and laboratory practices during production and storage, |
| • | controlled research and testing of some of these products even after approval, |
| • | control of marketing activities, including sales promotions, advertising and labeling, and |
| • | state permits for the sale and distribution of products manufactured in and out-of-state. |
If we, or any of our contract manufacturers, fail to comply with these requirements, we may be limited in the jurisdictions in which we are permitted to sell our products. Additionally, if we or our manufacturers fail to comply with applicable regulatory approval requirements, a regulatory agency may:
| • | send warning letters, |
| • | impose fines and other civil penalties on us, |
| • | seize our products, |
| • | suspend our regulatory approvals, |
| • | cease the manufacture of our products, |
| • | refuse to approve pending applications or supplements to approved applications filed by us, |
| • | refuse to permit exports of our products from the United States, |
| • | require us to recall products, |
| • | require us to notify physicians of labeling changes and/or product related problems, |
| • | impose restrictions on our operations, and/or |
| • | criminally prosecute us. |
17
Table of Contents
We and our manufacturers must continue to comply with current Good Manufacturing Practice regulations, or cGMP, and Quality System Regulations, or QSR, and equivalent foreign regulatory requirements. The cGMP and QSR requirements govern quality control and documentation policies and procedures. In complying with cGMP, QSR and foreign regulatory requirements, we and our third-party manufacturers will be obligated to expend time, money and effort in production, record keeping and quality control to assure that our products meet applicable specifications and other requirements.
Manufacturing facilities are subject to ongoing periodic inspection by the FDA, including unannounced inspections. We cannot guarantee that our third-party supply sources, including our sole source supplier for the active ingredient in Levulan® and the component parts in the BLU-U®, or our own Kerastick® facility, will continue to meet all applicable FDA regulations. If we, or any of our manufacturers, fail to maintain compliance with FDA regulatory requirements, it would be time-consuming and costly to remedy the problem(s) or to qualify other sources. These consequences could have a significant adverse effect on our financial condition and operations. Additionally, if previously unknown problems with the product, a manufacturer or its facility are discovered in the future, changes in product labeling restrictions or withdrawal of the product from the market may occur. Any such problems could affect our ability to become profitable on an ongoing basis.
Any significant interruption in our operation caused by FDA could have a negative effect on our revenues.
We May Not Maintain Profitability On A Quarterly Basis Unless We Can Successfully Market And Sell Higher Quantities Of Our Products.
If A Competitive Product Is Successful Our Revenues Could Decline, And Our Ability To Maintain Profitability On A Quarterly Basis Could Be Delayed.
Galderma, S.A., a large dermatology company, holds a non-exclusive license from us to Metvixia®, which was transferred to Galderma by Photocure ASA, our original licensee. This product received FDA approval for treatment of AKs in July 2004 and is directly competitive with our Levulan® Kerastick® product. While we are entitled to royalties on net sales of Metvixia®, Galderma has considerably more resources than we have, which could adversely affect our ability to maintain or increase our market share and make it more difficult for us to achieve profitability on an ongoing basis. Metvixia’s U.S. product revenues have not been significant to date. Also, Photocure launched an ALA ester-based product, Allumera™, as a cosmetic, during the second quarter of 2011, which could cause disruption in the marketplace. On December 2, 2011, Medicis acquired Aldara®, a topical AK product, and Zyclara™, used to treat precancerous skin growths related to sun overexposure. In addition, in January, 2012, Leo Pharma, a Danish corporation, received FDA approval for Picato® Gel, a topical product, to treat AKs on the face and scalp and on the extremities. These products could negatively impact the market penetration of our PDT products.
Our ability to be profitable on a quarterly basis may also be affected by fluctuations in the demand for our products caused by both seasonal changes, such as when patient visits slow during the summer months, and the timing of pricing changes, which may impact the purchasing patterns of our customers.
If We Do Not Continue To Generate Positive Cash Flow, We May Need More Capital.
We have approximately $28,216,000 in cash, cash equivalents and marketable securities as of December 31, 2011. Our cash, cash equivalents and marketable securities should be sufficient for current operations for at least the next 12 months. Although we expect continued growth in our PDT segment revenues, we are susceptible to the uncertain economic conditions, particularly with increased competition from Metvixia®, Picato® Gel and Allumera™, and with Medicis’ acquisition of Aldara® and Zyclara®. If we are unable to continue to be profitable on an ongoing basis, we may have to reduce our headcount, curtail certain variable expenses, or raise funds through financing transactions.
18
Table of Contents
We Have Had Significant Cumulative Losses And May Have Losses In The Future.
Prior to 2010, we had a history of annual operating losses and we may incur losses in the future. We reported net income (loss) of $7,320,000, $2,703,000, and ($2,508,000) for the years ended December 31, 2011, 2010 and 2009, respectively. As of December 31, 2011, our accumulated deficit was approximately $134,337,000. We expect to incur significant additional research and development and other costs including costs related to preclinical studies and clinical trials. Our costs, including research and development costs for our product candidates and sales, marketing and promotion expenses for any of our existing or future products to be marketed by us or our distributors may exceed revenues in the future, which may result in future losses from operations.
If We Cannot Maintain Or Improve Physician Reimbursement And/Or Convince More Private Insurance Carriers To Adequately Reimburse Physicians For Our Product, Sales May Suffer.
Without adequate levels of reimbursement by government health care programs and private health insurers, the market for our Levulan® Kerastick® for AK therapy will be limited. While we continue to support efforts to improve reimbursement levels to physicians and are working with the major private insurance carriers to improve coverage for our therapy, if our efforts are not successful, broader adoption of our therapy and sales of our products could be negatively impacted. Although positive reimbursement changes related to AK were made over the last 5 years, some physicians still believe that reimbursement levels do not fully reflect the required efforts to routinely execute our therapy in their practices.
If insurance companies do not cover our products, or government payors reduce the amounts of coverage or stop covering our products which are covered, our sales could be dramatically reduced.
If Product Sales Do Not Continue to Increase, We May Not Be Able To Advance Development Of Other Potential Products As Quickly As We Would Like To, Which Would Delay The Approval Process And Marketing Of New Potential Products, If Approved.
If we do not generate sufficient revenues from our approved products, we may be forced to delay or abandon our development program for programs we may wish to initiate. The pharmaceutical development and commercialization process is time consuming and costly, and any delays might result in higher costs which could adversely affect our financial condition and results of operations. Without sufficient product sales, we would need alternative sources of funding. There is no guarantee that adequate funding sources could be found to continue the development of our technology.
If We Are Unable To Obtain The Necessary Capital To Fund Our Operations, We Will Have To Delay Our Development Program And May Not Be Able To Complete Our Clinical Trials.
We may need substantial additional funds to fully develop, manufacture, market and sell other potential products. We may obtain funds through other public or private financings, including equity financing, and/or through collaborative arrangements. We may also choose to license rights to third parties to commercialize products or technologies that we would otherwise have attempted to develop and commercialize on our own which could reduce our potential revenues.
The availability of additional capital to us is uncertain. There can be no assurance that additional funding will be available to us on favorable terms, if at all. Any equity financing, if needed, would likely result in dilution to our existing shareholders, and debt financing, if available, would likely involve significant cash payment obligations and could include restrictive covenants that would adversely affect the operation of our business. Failure to raise capital, if needed, could materially adversely affect our clinical program, our financial condition, results of operations and cash flows.
19
Table of Contents
Global Credit And Financial Market Conditions May Affect Our Business.
Sales of our products are dependent, in large part, on reimbursement from government health and administration authorities, private health insurers, distribution partners and other organizations. As a result of the global credit and financial market conditions, government authorities and private insurers may not satisfy their reimbursement obligations or may delay payment. In addition, federal and state health authorities may reduce Medicare and Medicaid reimbursements, and private insurers may increase their scrutiny of claims. A reduction in the availability or extent of reimbursement could negatively affect our product sales and revenues.
Due to the tightening of global credit, there may be disruption or delay in the performance by our third-party contractors, suppliers or collaborators. We rely on third parties for several important aspects of our business, including the active ingredient in Levulan® and key components of the BLU-U®, portions of our product manufacturing, conduct of clinical trials and the supply of raw materials. If such third parties are unable to satisfy their commitments to us, our business would be adversely affected.
If The Economic Slowdown Adversely Affects Our Customer’s Ability To Meet Our Payment Terms, Our Cash Flow Would Be Adversely Affected And Our Ability To Continue To Be Profitable Could Be Jeopardized.
If our customers were unable to pay us or pay us on a timely basis for their purchases of our products, we may not be able to maintain profitability on a sustainable on-going basis, and our financial position, results of operations and cash flows could be negatively affected.
We Have Only Three Therapies That Have Received Regulatory Approval Or Clearance, And We Cannot Predict Whether We Will Ever Develop Or Commercialize Any Other Levulan® Product Or Indications.
Potential Products Or PDT Indications Are In Early Stages Of Development And May Never Result In Any Additional Commercially Successful Products.
Except for Levulan® PDT for AKs, the BLU-U® for acne and the ClindaReach® pledget, which is reflected as discontinued operations in the accompanying financial statements, all of our other potential product candidates are being studied by independent investigators, or are at a very early stage of development, including our BASDI clinical studies. These candidates are subject to the risks of failure inherent in the development of new pharmaceutical products and products based on new technologies. These risks include:
| • | delays in product development, clinical testing or manufacturing, |
| • | unplanned expenditures in product development, clinical testing or manufacturing, |
| • | failure in clinical trials or failure to receive regulatory approvals, |
| • | emergence of superior or equivalent products, |
| • | inability to market products due to third-party proprietary rights, and |
| • | failure to achieve market acceptance. |
We cannot predict how long the development of our investigational stage products will take or whether they will be medically effective. We cannot be sure that a successful market will continue to develop for our Levulan® drug technology.
We Must Receive Separate Approval For Any Drug Or Medical Device Products Before We Can Sell Them Commercially In The United States Or Abroad.
Any potential Levulan® product will require the approval of the FDA before it can be marketed in the United States. Before an application to the FDA seeking approval to market a new drug, called an NDA, or a medical device, called either a PMA or 510(K) can be filed, a product must undergo, among other things,
20
Table of Contents
extensive testing and human clinical trials. The process of obtaining FDA approvals can be lengthy, costly, and time-consuming. Following the acceptance of an NDA, the time required for regulatory approval can vary and is usually one to three years or more. The FDA may require additional animal studies and/or human clinical trials before granting approval. Our Levulan® PDT products are based on relatively new technology. To our knowledge, the FDA has approved only 4 drugs for use in photodynamic therapy, including Levulan®. This factor may lengthen the approval process. We face much trial and error and we may fail at numerous stages along the way.
We cannot predict whether we will obtain any other regulatory approvals. Data obtained from preclinical testing and clinical trials can be susceptible to varying interpretations which could delay, limit or prevent regulatory approvals. Future clinical trials may not show that Levulan® PDT is safe and effective for any new use we may study. In addition, delays or disapprovals may be encountered based upon additional governmental regulation resulting from future legislation or administrative action or changes in FDA policy.
We Have Limited Patent Protection, And If We Are Unable To Protect Our Proprietary Rights, Competitors Might Be Able To Develop Similar Products To Compete With Our Products And Technology.
Our ability to compete successfully depends, in part, on our ability to defend patents that have issued, obtain new patents, protect trade secrets and operate without infringing the proprietary rights of others. We have no compound patent protection for our Levulan® brand of the compound ALA. Our basic ALA patents are for methods of detecting and treating various diseased tissues using ALA (or related compounds called precursors), in combination with light. We own or exclusively license ALA patents and patent applications related to the following:
| • | methods of using ALA and its unique physical forms in combination with light to treat conditions such as AKs and acne, |
| • | compositions and apparatus for those methods, and |
| • | unique physical forms of ALA. |
We also own patents covering our Kerastick® and BLU-U®, which also cover our AK therapy. However, other third parties may have blue light devices or drug delivery devices that do not infringe our patents.
The patents we license from PARTEQ, the licensor of our ALA patents, relating to methods of using ALA for detecting or treating disease, other than for acne and our approved indication for AKs of the face or scalp, started to expire in July 2009. The PARTEQ patent which covers our approved AK product expires in September 2013. Beyond September 2013 with the expiration of the PARTEQ patent, we may be more susceptible to certain types of competition, however, with the newly allowed claims which issued on May 25, 2010, relating to use of our BLU-U®, we now have additional claims that relate to our AK product, and these will not expire until June 2019.
We have limited ALA patent protection outside the United States, which may make it easier for third parties to compete there. Our basic methods of treatment patents and applications have counterparts in only 4 foreign countries, and certain countries under the European Patent Convention. Even where we have patent protection, there is no guarantee that we will be able to enforce our patents. Additionally, enforcement of a given patent may not be practicable or an economically viable alternative. Some of the indications for which we may develop PDT therapies may not be covered by the claims in any of our existing patents. Even with the issuance of additional patents to us, other parties are free to develop other uses of ALA, including medical uses, and to market ALA for such uses, assuming that they have obtained appropriate regulatory marketing approvals. ALA in the chemical form has been commercially supplied for decades, and is not itself subject to patent protection. There are reports of third parties conducting clinical studies with ALA in countries outside the United States where PARTEQ does not have patent protection. In addition, a number of third parties are seeking patents for uses of ALA not covered by our patents. These other uses, whether patented or not, and the commercial availability of ALA, could limit
21
Table of Contents
the scope of our future operations because ALA products could come on the market which would not infringe our patents, but would compete with our Levulan® product even though they are marketed for different uses.
Metvixia® was approved by the FDA as a treatment of AKs in July 2004, and this ALA-derived product is directly competitive with our Levulan® Kerastick® product. While we are entitled to royalties on net sales of Metvixia®, Galderma®, our licensee, has considerably more resources than we have, which could adversely affect our ability to maintain or increase our market share. Metvixia’s U.S. product revenues have not been significant to date. Physicians who use Allumera™, another ALA-derived product, for treatment of AKs, even though it is being marketed as a cosmetic product, may be infringing our patents.
While we attempt to protect our proprietary information as trade secrets through agreements with each employee, licensing partner, consultant, university, pharmaceutical company and agent, we cannot guarantee that these agreements will provide effective protection for our proprietary information. It is possible that all of the following issues could negatively impact our ability to be profitable:
| • | these persons or entities might breach the agreements, |
| • | we might not have adequate remedies for a breach, and/or, |
| • | our competitors will independently develop or otherwise discover our trade secrets. |
Since We Now Operate The Only FDA Approved Manufacturing Facility For The Kerastick® And Continue To Rely Heavily On Sole Suppliers For The Manufacture Of Levulan®, The BLU-U®, Any Supply Or Manufacturing Problems Could Negatively Impact Our Sales.
If we experience problems producing Levulan® Kerastick® units in our facility, or if any of our contract suppliers fail to supply our requirements for products or services, our business, financial condition and results of operations would suffer. Although we have received approval by the FDA to manufacture the BLU-U® and the Levulan® Kerastick® in our Wilmington, Massachusetts facility, at this time, with respect to the BLU-U®, we expect to utilize our own facility only as a back-up to our current third party manufacturers or for repairs.
Manufacturers and their subcontractors often encounter difficulties when commercial quantities of products are manufactured for the first time, or large quantities of products are manufactured, including problems involving:
| • | product yields, |
| • | quality control, |
| • | component and service availability, |
| • | compliance with FDA regulations, and |
| • | the need for further FDA approval if manufacturers make material changes to manufacturing processes and/or facilities. |
We cannot guarantee that problems will not arise with production yields, costs or quality as we and our suppliers manufacture our products. Any manufacturing problems could delay or limit our supplies which would hinder our marketing and sales efforts. If our facility, any facility of our contract manufacturers, or any equipment in those facilities is damaged or destroyed, we may not be able to quickly or inexpensively replace it. Likewise, if there is quality or supply problems with any components or materials needed to manufacture our products, we may not be able to quickly remedy the problem(s). Any of these problems could cause our sales to suffer and could increase costs.
22
Table of Contents
Our Ability To Use Net Operating Loss Carryforwards And Tax Credit Carryforwards To Offset Future Taxable Income May Be Further Limited As A Result Of Past Or Future Transactions Involving Our Common Stock.
Under Internal Revenue Code, or IRC, Section 382 the amount of our net operating loss carryforwards and other tax attributes that we may utilize to offset future taxable income, when earned, may be subject to certain limitations, based upon changes in the ownership of our common stock. In general, under IRC Section 382, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change net operating losses and certain other tax assets to offset future taxable income. An ownership change occurs if the aggregate stock ownership of certain shareholders increases by more than 50 percentage points over such shareholders’ lowest percentage ownership during the testing period, which is generally three years.
Based on an Internal Revenue Code (IRC) Section 382 study performed, we determined that we have experienced prior ownership changes, as defined under IRC Section 382, with the most recent change in ownership occurring in 2007 (the 2007 Ownership Change). Our pre-change NOL carryforwards are subject to an annual limitation of approximately $2.2 million per year. Further, additional rules provide for the enhancement of the aforementioned annual limitation for the first five years after the ownership change. A loss corporation may increase its IRC Section 382 limitation by the amount of the net unrealized built-in gain (NUBIG) recognized within five years of the ownership change. The calculated aggregate amount of NUBIG enhancement for us is approximately $4.3 million (i.e., approximately $868,000 per year for the first 5 years after the ownership change). This NUBIG enhancement will be utilized in conjunction with the approximately $2.2 million of IRC Section 382 base annual limitation, resulting in approximately $3.0 million per year for the first 5 years after the ownership change. Based on these additional factors, we estimate that we will be able to utilize approximately $49.9 million of its current net operating losses, provided that sufficient income is generated and no further ownership changes were to occur. However, it is reasonably possible that a future ownership change, which could be the result of transactions involving our common stock that are outside of its control (such as sales by existing shareholders), could occur during 2012 or thereafter. Future ownership changes could further restrict the utilization of our net operating losses and tax credits, reducing or eliminating the benefit of such net operating losses and tax credits. If such future ownership changes were to occur, it is a possibility that we could be required to pay federal income taxes in the near-term.
We Have Only A Limited Marketing And Sales Force Organization And As A Result, Our Revenues From Product Sales May Suffer.
If we are unable to successfully market and sell sufficient quantities of our products, revenues from product sales will be lower than anticipated and our financial condition may be adversely affected. We directly market our products in the United States. In Canada, we market Levulan® and the BLU-U® through a distributor. We have terminated our agreements with other foreign distributors. If our sales and marketing efforts fail, then sales of the Levulan® Kerastick®, the BLU-U®, and other products will be adversely affected, which would adversely affect our results of operations and financial condition.
The Commercial Success Of Any Product That We May Develop Will Depend Upon The Degree Of Market Acceptance Of Our Products Among Physicians, Patients, Health Care Payors, Private Health Insurers And The Medical Community.
Our ability to commercialize any product that we may develop will be highly dependent upon the extent to which the product gains market acceptance among physicians, patients, health care payors, such as Medicare and Medicaid, private health insurers, including managed care organizations and group purchasing organizations, and the medical community. If a product does not achieve an adequate level of acceptance, we may not generate material product revenues. The degree of market acceptance of our currently marketed products will depend on a number of factors, including:
| • | the effectiveness, or perceived effectiveness, of our product in comparison to competing products, |
23
Table of Contents
| • | the existence of any significant side effects, as well as their severity in comparison to any competing products, |
| • | potential advantages over alternative treatments, |
| • | the ability to offer our product for sale at competitive prices, |
| • | relative convenience and ease of administration, |
| • | the strength of marketing and distribution support, and |
| • | sufficient third-party coverage or reimbursement. |
Litigation Is Expensive And We May Not Be Able To Afford The Costs.
The costs of litigation or any proceeding relating to our intellectual property or contractual rights could be substantial even if resolved in our favor. Some of our competitors have far greater resources than we do and may be better able to afford the costs of complex litigation. Also, in a lawsuit against a third party for infringement of our patents in the United States, that third party may challenge the validity of our patent(s). We cannot guarantee that a third party will not claim, with or without merit, that our patents are not valid or that we have infringed their patent(s) or misappropriated their proprietary material. We could get drawn into or decide to join, litigation as the holder of the patent. Defending these types of legal actions involve considerable expense and could negatively affect our financial results.
Additionally, if a third-party were to file a United States patent application, or be issued a patent claiming technology also claimed by us in a pending United States application(s), we may be required to participate in interference proceedings in the USPTO to determine the priority of the invention. A third-party could also request the declaration of a patent interference between one of our issued United States patents and one of its patent applications. Any interference proceedings likely would require participation by us and/or PARTEQ, which could involve substantial legal fees and result in a loss or lessening of our patent protection.
Because Of The Nature Of Our Business, The Loss of Key Members Of Our Management Team Could Delay Achievement Of Our Goals.
We are a small company with only 96 employees, including 1 part-time employee, as of December 31, 2011. We are highly dependent on several key officer/employees with specialized scientific and technical skills without whom our business, financial condition and results of operations would suffer, especially in the photodynamic therapy portion of our business. The photodynamic therapy industry is still quite small and the number of experts is limited. The loss of these key employees could cause significant delays in achievement of our business and research goals since very few people with their expertise could be hired. Our growth and future success will depend, in large part, on the continued contributions of these key individuals as well as our ability to motivate and retain other qualified personnel in our specialty drug and light device areas.
Collaborations With Outside Scientists May Be Subject To Restriction And Change.
We work with scientific and clinical advisors and collaborators at academic and other institutions that assist us in our research and development efforts. These scientists and advisors are not our employees and may have other commitments that limit their availability to us. If a conflict of interest between their work for us and their work for another entity arises, we may lose their services. In addition, although our advisors and collaborators sign agreements not to disclose our confidential information, it is possible that valuable proprietary knowledge may become publicly known through them.
24
Table of Contents
Risks Related To Our Industry
Product Liability And Other Claims Against Us May Reduce Demand For Our Products Or Result In Damages.
We Are Subject To Risk From Potential Product Liability Lawsuits Which Could Negatively Affect Our Business.
The development, manufacture and sale of medical products expose us to product liability claims related to the use or misuse of our products. Product liability claims can be expensive to defend and may result in significant judgments against us. A successful claim could materially harm our business, financial condition and results of operations. Additionally, we cannot guarantee that continued product liability insurance coverage will be available in the future at acceptable costs. If we believe the cost of coverage is too high, we may self-insure.
Our Business Involves Environmental Risks And We May Incur Significant Costs Complying With Environmental Laws And Regulations.
We have used various hazardous materials, such as mercury in fluorescent tubes in our research and development activities. We are subject to federal, state and local laws and regulations which govern the use, manufacture, storage, handling and disposal of hazardous materials and specific waste products. We believe that we are in compliance in all material respects with currently applicable environmental laws and regulations. However, we cannot guarantee that we will not incur significant costs to comply with environmental laws and regulations in the future. We also cannot guarantee that current or future environmental laws or regulations will not materially adversely affect our operations, business or financial condition. In addition, although we believe our safety procedures for handling and disposing of these materials comply with federal, state and local laws and regulations, we cannot completely eliminate the risk of accidental contamination or injury from these materials. In the event of such an accident, we could be held liable for any resulting damages, and this liability could exceed our resources.
We May Not Be Able To Compete Against Traditional Treatment Methods Or Keep Up With Rapid Changes In The Biotechnology And Pharmaceutical Industries That Could Make Some Or All Of Our Products Non-Competitive Or Obsolete.
Competing Products And Technologies Based On Traditional Treatment Methods May Make Our Products Or Potential Products Noncompetitive Or Obsolete.
Well-known pharmaceutical, biotechnology and medical device companies are marketing well-established therapies for the treatment of AKs and acne. Doctors may prefer to use familiar methods, rather than trying our products. Reimbursement issues affect the economic competitiveness of our products as compared to other more traditional therapies.
Many companies are also seeking to develop new products and technologies, and receiving approval for treatment of AKs and acne. Our industry is subject to rapid, unpredictable and significant technological change. Competition is intense. Our competitors may succeed in developing products that are safer, more effective or more desirable than ours. Many of our competitors have substantially greater financial, technical and marketing resources than we have. In addition, several of these companies have significantly greater experience than we do in developing products, conducting preclinical and clinical testing and obtaining regulatory approvals to market products for health care.
We cannot guarantee that new drugs or future developments in drug technologies will not have a material adverse effect on our business. Increased competition could result in:
| • | price reductions, |
| • | lower levels of third-party reimbursements, |
25
Table of Contents
| • | failure to achieve market acceptance, and |
| • | loss of market share, |
any of which could adversely affect our business, results of operations and financial condition.
Further, we cannot give any assurance that developments by our competitors or future competitors will not render our technology obsolete or less advantageous.
Galderma, S.A., a large dermatology company, holds a non-exclusive license from us to Metvixia®, which was transferred to Galderma by Photocure ASA, our original licensee. This product received FDA approval for treatment of AKs in July 2004 and is directly competitive with our Levulan® Kerastick® product and its price is comparable to the price of Levulan®. While we are entitled to royalties on net sales of Metvixia®, Galderma has considerably more resources than we have, which could significantly hamper our ability to maintain or increase our market share. Metvixia’s U.S. product revenues have not been significant to date. Also, Leo Pharma’s Picato® Gel, could negatively impact the market penetration of our PDT products.
Our Competitors In The Biotechnology And Pharmaceutical Industries May Have Better Products, Manufacturing Capabilities Or Marketing Expertise.
We are aware of several companies commercializing and/or conducting research with ALA or ALA-related compounds, including: Galderma, medac GmbH and photonamic GmbH & Co. KG (Germany); Biofrontera, PhotoTherapeutics, Inc. (U.K.), and Photocure ASA (Norway). We also anticipate that we will face increased competition as the scientific development of PDT advances and new companies enter our markets. Several companies are developing PDT agents other than Levulan®. These include: QLT Inc. (Canada); Miravant, Inc. (U.S.); and Pharmacyclics, Inc. (U.S.). There are many pharmaceutical companies that compete with us in the field of dermatology, particularly in the acne market.
We expect that our principal methods of competition with other PDT products will be based upon such factors as:
| • | the ease of administration of our method of PDT, |
| • | the degree of generalized skin sensitivity to light, |
| • | the number of required doses, |
| • | the selectivity of our drug for the target lesion or tissue of interest, and |
| • | the type and cost of our light systems. |
Our primary competition in the acne market includes oral and topical antibiotics, other topical prescription and over-the-counter products, as well as various laser and non-laser light treatments. The market is highly competitive and other large and small companies have more experience than we do which could make it difficult for us to penetrate the market. The entry of new products from time to time would likely cause us to lose market share.
Risks Related To Our Stock
Our Stock Price Is Highly Volatile And Sudden Changes In The Market Value Of Our Stock Occur, Making An Investment Risky.
The price of our common stock has been highly volatile, which may create an increase in the risk of capital losses for our shareholders. From January 1, 2010 to December 31, 2011, the closing price of our stock has ranged from a low of $1.35 to a high of $6.77. The significant general market volatility in similar stage pharmaceutical and biotechnology companies also made the market price of our stock volatile.
26
Table of Contents
Significant Fluctuations In Orders For Our Products, On A Monthly And Quarterly Basis, Are Commonly Based On External Factors And Sales Promotion Activities. These Fluctuations Could Increase The Volatility Of Our Stock Price.
The price of our common stock may be affected by the amount of quarterly shipments of our products to end-users. Since our PDT products are still in relatively early stages of adoption, and sales volumes are still low, a number of factors could affect product sales levels and growth rates in any period. These could include the level of penetration in new markets outside of the United States, the timing of medical conferences, sales promotion activities, and large volume purchases by our higher usage customers. In addition, seasonal fluctuations in the number of patients seeking treatment at various times during the year could impact sales volumes. These factors could, in turn, affect the volatility of our stock price.
Future Sales Of Securities May Cause Our Stock Price To Decline.
As of December 31, 2011, there were outstanding options and warrants to purchase 3,956,000 shares of common stock, with exercise prices ranging from $1.10 to $15.90 per share for options, and $2.85 per share for warrants. In addition, there were approximately 901,000 shares of unvested common stock. The holders of the options and warrants have the opportunity to profit if the market price for the common stock exceeds the exercise price of their respective securities, without assuming the risk of ownership. Also, if some or all of such shares are sold into the public market over a short period of time, the value of all publicly traded shares could decline, as the market may not be able to absorb those shares at then-current market prices. Additionally, such sales may make it more difficult for us to sell equity securities or equity-related securities in the future at a time and price that our management deems acceptable, or at all. The holders may exercise their securities during a time when we would likely be able to raise capital from the public on terms more favorable than those provided in these securities.
Our Common Stock May Not Continue To Trade On The Nasdaq Global Market, Which Could Reduce The Value Of Your Investment And Make Your Shares More Difficult To Sell.
In order for our common stock to trade on the Nasdaq Global Market, we must continue to meet the listing standards of that market. Among other things, those standards require that our common stock maintain a minimum closing bid price of at least $1.00 per share. During 2010 our common stock traded at prices near and below $1.00. If we do not continue to meet Nasdaq’s applicable minimum listing standards, Nasdaq could delist us from the Nasdaq Global Market. If our common stock is delisted from the Nasdaq Global Market, we could seek to have our common stock listed on the Nasdaq Capital Market or other Nasdaq markets. However, delisting of our common stock from the Nasdaq Global Market could hinder your ability to sell, or obtain an accurate quotation for the price of, your shares of our common stock. Delisting could also adversely affect the perception among investors of DUSA and its prospects, which could lead to further declines in the market price of our common stock. Delisting may also make it more difficult and expensive for us to raise capital. In addition, delisting might subject us to a Securities and Exchange Commission rule that could adversely affect the ability of broker-dealers to sell or make a market in our common stock, thus hindering your ability to sell your shares.
Effecting A Change Of Control Of DUSA Would Be Difficult, Which May Discourage Offers For Shares Of Our Common Stock.
Our certificate of incorporation authorizes the board of directors to issue up to 100,000,000 shares of stock, 40,000,000 of which are common stock. The board of directors has the authority to determine the price, rights, preferences and privileges, including voting rights, of the remaining 60,000,000 shares without any further vote or action by the shareholders. The rights of the holders of our common stock will be subject to, and may be adversely affected by, the rights of the holders of any preferred stock that may be issued in the future.
On September 27, 2002, we adopted a shareholder rights plan at a special meeting of our board of directors. The rights plan could discourage, delay or prevent a person or group from acquiring 15% or more of our common stock, thereby limiting, perhaps, the ability of certain of our shareholders to benefit from such a transaction.
27
Table of Contents
The rights plan provides for the distribution of one right as a dividend for each outstanding share of our common stock to holders of record as of October 10, 2002. Each right entitles the registered holder to purchase one one-thousandths of a share of preferred stock at an exercise price of $37.00 per right. The rights will be exercisable subsequent to the date that a person or group either has acquired, obtained the right to acquire, or commences or discloses an intention to commence a tender offer to acquire, 15% or more of our outstanding common stock or if a person or group is declared an “Adverse Person”, as such term is defined in the rights plan. The rights may be redeemed by us at a redemption price of one one-hundredth of a cent per right until ten days following the date the person or group acquires, or discloses an intention to acquire, 15% or more, as the case may be, of DUSA, or until such later date as may be determined by our board of directors.
Under the rights plan, if a person or group acquires the threshold amount of common stock, all holders of rights (other than the acquiring person or group) may, upon payment of the purchase price then in effect, purchase shares of common stock of DUSA having a value of twice the purchase price. In the event that we are involved in a merger or other similar transaction where we are not the surviving corporation, all holders of rights (other than the acquiring person or group) shall be entitled, upon payment of the purchase price then in effect, to purchase common stock of the surviving corporation having a value of twice the purchase price. The rights will expire on October 10, 2012, unless previously redeemed. Our board of directors has also adopted certain amendments to our certificate of incorporation consistent with the terms of the rights plan.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
In May 1999 we entered into a 5-year lease for a portion of a building to be used for offices and manufacturing in Wilmington, Massachusetts. In December 2001 we entered into a 15 year lease covering the entire building through November 2016. Under the original lease terms, we had the ability to terminate the Wilmington lease after the 10th year (2011) of the lease by providing the landlord with notice at least seven and one-half months prior to the date on which the termination would be effective. In November 2010 we entered into a lease amendment, which reduces our lease payments and allows us to terminate the lease any time after November 2013 by notifying the landlord at least 9 months prior to the date on which the termination would be effective. Pursuant to the amendment, the end of the lease term remains November 2016; however, we have the right to extend the term of the lease for two periods of 5 years each.
| ITEM 3. | LEGAL PROCEEDINGS |
None.
| ITEM 4. | MINE SAFETY DISCLOSURES |
None.
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock is traded on the NASDAQ Global Market under the symbol “DUSA.” The following are the high and low prices for the common stock reported for the quarterly periods shown.
28
Table of Contents
Price range per common share by quarter, 2011:
| First | Second | Third | Fourth | |||||||||||||
| NASDAQ |
||||||||||||||||
| High |
$ | 5.30 | $ | 6.83 | $ | 6.60 | $ | 4.70 | ||||||||
| Low |
$ | 2.33 | $ | 4.40 | $ | 3.55 | $ | 3.25 | ||||||||
Price range per common share by quarter, 2010:
| First | Second | Third | Fourth | |||||||||||||
| NASDAQ |
||||||||||||||||
| High |
$ | 1.94 | $ | 2.75 | $ | 2.70 | $ | 2.75 | ||||||||
| Low |
$ | 1.32 | $ | 1.72 | $ | 2.07 | $ | 2.35 | ||||||||
On March 2, 2012, the closing price of our common stock was $4.70 per share on the NASDAQ Global Market. On March 2, 2012, there were 662 holders of record of our common stock.
We have never paid cash dividends on our common stock and have no present plans to do so in the foreseeable future.
RELATIVE STOCK PERFORMANCE
The graph below compares DUSA Pharmaceuticals, Inc.’s cumulative 5-year total shareholder return on common stock with the cumulative total returns of the NASDAQ Market index and Morningstar Group Index. The graph tracks the performance of a $100 investment in our common stock and in each of the indexes (with the reinvestment of all dividends) from December 31, 2006 to December 31, 2011. The comparisons in this graph are required by the SEC and are not intended to forecast or be indicative of possible future performance of our common stock. The list of companies in the Morningstar Group index is publicly available.
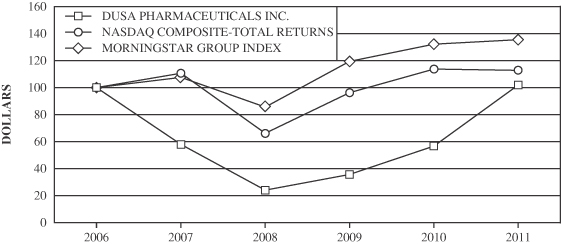
| COMPARISON OF CUMULATIVE TOTAL RETURN | ||||||||||||||||||||||||
| 12/31/06 | 12/31/07 | 12/31/08 | 12/31/09 | 12/31/10 | 12/31/11 | |||||||||||||||||||
| DUSA PHARMACEUTICALS, INC. |
$ | 100.00 | $ | 58.14 | $ | 24.42 | $ | 36.04 | $ | 56.97 | $ | 101.86 | ||||||||||||
| NASDAQ MARKET INDEX |
$ | 100.00 | $ | 110.55 | $ | 66.30 | $ | 96.34 | $ | 113.70 | $ | 112.80 | ||||||||||||
| MORNINGSTAR GROUP INDEX |
$ | 100.00 | $ | 107.46 | $ | 85.68 | $ | 119.29 | $ | 132.02 | $ | 135.26 | ||||||||||||
29
Table of Contents
| ITEM 6. | SELECTED FINANCIAL DATA |
The following information should be read in conjunction with our Consolidated Financial Statements and the Notes thereto and Management’s Discussion and Analysis of Financial Condition and Results of Operations included elsewhere in this report. The Statement of Operations data for the years ended December 31, 2008 and 2007 and the Balance Sheet data as of December 31, 2009, 2008, and 2007 are derived from audited consolidated financial statements not included herein. All periods presented have been reclassified to account for the presentation of our Non-PDT segment, which is now reflected as discontinued operations.
CONSOLIDATED STATEMENTS OF OPERATIONS DATA
| Year Ended December 31, | ||||||||||||||||||||
| 2011(1) | 2010(2) | 2009 | 2008 | 2007 | ||||||||||||||||
| Product revenues |
$ | 45,296,000 | $ | 36,423,000 | $ | 28,338,000 | $ | 23,931,000 | $ | 18,274,000 | ||||||||||
| Income (loss) from continuing operations |
6,793,000 | 2,563,000 | (2,759,000 | ) | (8,081,000 | ) | (15,116,000 | ) | ||||||||||||
| Income from discontinued operations |
527,000 | 140,000 | 251,000 | 1,831,000 | (3) | 402,000 | (4) | |||||||||||||
| Net income (loss) |
7,320,000 | 2,703,000 | (2,508,000 | ) | (6,250,000 | )(3) | (14,714,000 | )(4) | ||||||||||||
| Basic net income (loss) per share- Continuing operations |
$ | 0.28 | $ | 0.11 | $ | (0.11 | ) | $ | (0.34 | ) | $ | (0.74 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Discontinued operations |
$ | 0.02 | $ | 0.01 | $ | 0.01 | $ | 0.08 | $ | 0.02 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic net income (loss) per share |
$ | 0.30 | $ | 0.11 | $ | (0.10 | ) | $ | (0.26 | ) | $ | (0.73 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Diluted net income (loss) per share- Continuing operations |
$ | 0.26 | $ | 0.10 | $ | (0.11 | ) | $ | (0.34 | ) | $ | (0.74 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Discontinued operations |
$ | 0.02 | $ | 0.01 | $ | 0.01 | $ | 0.08 | $ | 0.02 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Diluted net income (loss) per share |
$ | 0.28 | $ | 0.11 | $ | (0.10 | ) | $ | (0.26 | ) | $ | (0.73 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
CONSOLIDATED BALANCE SHEET DATA
| Year Ended December 31, | ||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| Total assets -continuing operations |
$ | 37,983,000 | $ | 28,090,000 | $ | 24,693,000 | $ | 27,693,000 | $ | 31,296,000 | ||||||||||
| Total assets –discontinued operations |
39,000 | 203,000 | 240,000 | 517,000 | 1,596,000 | |||||||||||||||
| Long-term obligations(5) |
3,275,000 | 3,302,000 | 3,842,000 | 4,838,000 | 4,501,000 | |||||||||||||||
| Shareholders’ equity |
$ | 28,281,000 | $ | 19,523,000 | $ | 15,841,000 | $ | 17,712,000 | $ | 22,107,000 | ||||||||||
| (1) | 2011 includes the acceleration of deferred revenues of $1,379,000 and the acceleration of deferred cost of revenues of $71,000 related to our termination of our Asia Pacific distribution agreement Daewoong. |
| (2) | 2010 includes the acceleration of deferred revenues of $555,000 and the acceleration of deferred cost of revenues of $42,000 related to our termination of our Latin America distribution agreement with Stiefel. |
30
Table of Contents
| (3) | Includes an impairment charge of $1,500,000 resulting from our review of the carrying amount of our goodwill relating to our Non-PDT segment resulting from a contingent payout to the former shareholders of Sirius Laboratories, Inc. |
| (4) | Includes an impairment charge of $6,773,000 resulting from our review of the carrying amount of our goodwill relating to our Non-PDT segment. |
| (5) | Primarily comprised of deferred revenues related to milestone payments received under distribution agreements and the fair value of the warrants issued in connection with our October 29, 2007 private placement. |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
When you read this section of this report, it is important that you also read the financial statements and related notes included elsewhere in this report. This section contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those we anticipate in these forward-looking statements for many reasons, including the factors described below and in the section entitled “Risk Factors.”
We are a vertically integrated dermatology company that is developing and marketing Levulan® PDT. Our marketed products include Levulan® Kerastick® 20% Topical Solution with PDT and the BLU-U® brand light source.
We devote most of our resources to advancing the development and marketing of our Levulan® PDT technology platform. In addition to our marketed products, our drug, Levulan® brand of aminolevulinic acid HCl, or ALA, in combination with light, has been studied in a broad range of medical conditions. When Levulan® is used and followed with exposure to light to treat a medical condition, it is known as Levulan® PDT. The Kerastick® is our proprietary applicator that delivers Levulan®. The BLU-U® is our patented light device.
The Levulan® Kerastick® 20% Topical Solution with PDT and the BLU-U® were launched in the United States, or U.S., in September 2000 for the treatment of non-hyperkeratotic actinic keratoses, or AKs, of the face or scalp. AKs are precancerous skin lesions caused by chronic sun exposure that can develop over time into a form of skin cancer called squamous cell carcinoma. In addition, in September 2003 we received clearance from the United States Food and Drug Administration, or FDA, to market the BLU-U® without Levulan® PDT for the treatment of moderate inflammatory acne vulgaris and general dermatological conditions.
We are marketing Levulan® PDT under an exclusive worldwide license of patents, many of which have expired, and technology from PARTEQ Research and Development Innovations, the licensing arm of Queen’s University, Kingston, Ontario, Canada. We also own or license certain other patents relating to our BLU-U® device and methods for using pharmaceutical formulations which contain our drug and related processes and improvements. In the United States, DUSA®, DUSA Pharmaceuticals, Inc.®, Levulan®, Kerastick®, BLU-U®, ClindaReach®, Meted®, and Psoriacap® are registered trademarks. Several of these trademarks are also registered in Europe, Australia, Canada, and in other parts of the world. Numerous other trademark applications are pending.
We are responsible for manufacturing our Levulan® Kerastick® and for the regulatory, sales, marketing, and customer service and other related activities for all of our products, including our Levulan® Kerastick®.
31
Table of Contents
2011 TRANSACTIONS
The following significant transaction occurred during 2011:
Termination of Marketing, Distribution and Supply Agreement with Daewoong Pharmaceuticals
In the third quarter of 2011, we terminated our Marketing, Distribution and Supply Agreement, dated January 4, 2007, as amended, as of January 10, 2007, September 8, 2008 and June 20, 2011 with Daewoong Pharmaceutical, Co., LTD. and DNC Daewoong Derma & Plastic Surgery Network Company, or collectively Daewoong. We sent notice of termination of the agreement on August 31, 2011 based on our contractual right to unilaterally terminate the agreement as a result of certain regulatory milestones that were not achieved. As a result of the termination, we recorded $1,379,000 of revenues that were previously deferred, comprised of deferred drug shipments of $301,000 and the unamortized balance of milestone payments of $1,078,000, and recorded $71,000 of costs of revenues that were previously deferred. For 2011, the Daewoong termination resulted in the recognition of $1,308,000 of income from continuing operations.
Sale of Patent Covering Nicomide
In the second quarter of 2011, we entered into an Asset Purchase Agreement with Acella Pharmaceuticals, LLC, or Acella, pursuant to which we sold to Acella U.S. Patent No. 6,979,468 covering Nicomide® , together with the trademarks Nicomide® and Nicomide-T®, and related domain names, or the Divested Assets. The Divested Assets, which had a carrying value of $0, were sold for cash consideration of $750,000, all of which was paid at the closing. We ceased selling Nicomide® in June, 2008. The agreement included customary representations, warranties and covenants. The sale of the Divested Assets is recorded as a gain on assets, and is included in discontinued operations in the accompanying consolidated financial statements. See “Discontinued Operations” at Note 11 to the Notes to the Consolidated Financial Statements.
Discontinuance of Non-Photodynamic Therapy, or Non-PDT, Drug Products Segment
As of December 31, 2011 we ceased marketing and selling our remaining Non-PDT products, which were ClindaReach® and Meted®. Our former Non-PDT Drug Products segment has been presented as discontinued operations for all periods presented.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Critical accounting policies are those that require application of management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods and that can significantly affect our financial position and results of operations. Our accounting policies are disclosed in Note 2 to the Consolidated Financial Statements. We have discussed these policies and the underlying estimates used in applying these accounting policies with our Audit Committee. Since not all of these accounting policies require management to make difficult, subjective or complex judgments or estimates, they are not all considered critical accounting policies. We consider the following policies and estimates to be critical to our financial statements.
Revenue Recognition — We recognize revenues on Kerastick® and BLU-U® product sales in the U.S. and Canada when persuasive evidence of an arrangement exists, the price is fixed and determinable, delivery has occurred, and collection is reasonably assured. We offer programs that allow physicians and hospitals access to our BLU-U® device for a trial period. We do not recognize revenue on these units until the physician or hospital elects to purchase the equipment and all other revenue recognition criteria are met. Our terms with customers do not provide for the right of return for sales of Kerastick® and BLU-U®, unless the product does not comply with the technical specifications.
Inventory — Inventories are stated at the lower of cost or market value. Cost is determined using the first-in, first-out method. Inventories are continually reviewed for slow-moving, obsolete and excess items. Inventory items identified as slow-moving are evaluated to determine if an adjustment is required. Additionally, our industry is characterized by regular technological developments that could result in obsolete inventory.
32
Table of Contents
Although we make every effort to assure the reasonableness of our estimates, any significant unanticipated changes in demand, technological development, or significant changes to our business model could have a significant impact on the value of our inventory and our results of operations. We use sales projections to estimate the appropriate level of inventory reserves, if any, that are necessary at each balance sheet date.
Financial Instruments — Financial instruments recorded at fair value on the consolidated balance sheets include cash equivalents, marketable securities and the common stock purchase warrant liability.
Fair value is based on the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. We follow the fair value disclosure hierarchy that prioritizes observable and unobservable inputs used to measure fair value into three broad levels, which are described below:
| Level 1: | Quoted market prices in active markets for identical assets or liabilities. Level 1 primarily consists of financial instruments whose value is based on quoted market prices such as exchange-traded instruments and listed equities. | |||
| Level 2: | Observable market based inputs or unobservable inputs that are corroborated by market data. Level 2 includes financial instruments that are valued using models or other valuation methodologies. | |||
| Level 3: | Unobservable inputs that are not corroborated by market data. Level 3 is comprised of financial instruments whose fair value is estimated based on internally developed models or methodologies utilizing significant inputs that are generally less readily observable. | |||
Our cash equivalents are Level 1 instruments. The fair value is based on transacted prices in an active market. In determining the fair value of our marketable securities, we consider the level of market activity and the availability of prices for the specific securities that we hold. For our Level 2 financial instruments, comprising our corporate debt and United States government-backed securities, we use quoted market prices, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency in the determination of value. We also access publicly available market activity from third party databases and credit ratings of the issuers of the securities we hold to corroborate the data used in the fair value calculations obtained from our primary source. We take into account credit rating changes, if any, of the securities or recent marketplace activity. We do not have any Level 3 marketable securities.
The warrant liability is carried at fair value and fair value is measured using Level 3 inputs. We initially recorded the warrant liability at its fair value using the Black-Scholes option-pricing model and revalue it at each reporting date until the warrants are exercised or expire. Changes in the fair value of the warrants are reported in our Statements of Operations as non-operating income or expense under the caption “(Loss) gain on change in fair value of warrants.” The fair value of the warrants is subject to significant fluctuation based on changes in our stock price, expected volatility, remaining contractual life and the risk-free interest rate. The market price for our common stock has been and may continue to be volatile. Consequently, future fluctuations in the price of our common stock may cause significant increases or decreases in the fair value of the warrants.
Income Taxes — Prior to 2010, we had historically incurred domestic operating losses from both a financial reporting and tax return standpoint. We establish valuation allowances if it appears more likely than not that our deferred tax assets will not be realized. In concluding that it is not more-likely-than-not that our deferred tax assets will be realized, we have evaluated both positive and negative evidence regarding the future realization of these assets. This evaluation requires us to make difficult, subjective and complex judgments regarding our projections of taxable income and the amount and expiration dates of our net operating loss carryforwards and other deferred tax assets. In forming our conclusions as to whether the deferred tax assets are more likely than not to be realized we considered our historical cumulative profit and loss position, adjusted for non-recurring items, the sources of our future taxable income and the projected stability of those sources. We have established a
33
Table of Contents
full valuation allowance against our net deferred tax assets because of our history of generating operating losses and restrictions on the use of certain items after giving consideration to our profitability in the recent 2 fiscal years. If, in the future, we generate taxable income on a sustained basis, our conclusion regarding the need for full valuation allowances could change, resulting in the reversal of some or all of the valuation allowances. In the reporting period in which the valuation allowance is released, we will record a substantially large tax benefit related to the release, which will result in a large negative effective tax rate.
Results of Operations
Year Ended December 31, 2011 As Compared to the Year Ended December 31, 2010
Revenues — Total revenues for 2011 were $45,296,000, as compared to $36,423,000 in 2010 and were comprised of the following:
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | Increase/ (Decrease) |
||||||||||
| PDT DRUG AND DEVICE PRODUCT REVENUES |
||||||||||||
|
LEVULAN® KERASTICK® PRODUCT REVENUES |
||||||||||||
| United States |
$ | 40,940,000 | $ | 32,821,000 | $ | 8,119,000 | ||||||
| Canada |
485,000 | 491,000 | (6,000 | ) | ||||||||
| Korea |
1,720,000 | 424,000 | 1,296,000 | |||||||||
| Latin America |
— | 778,000 | (778,000 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Subtotal Levulan® Kerastick® product revenues |
43,145,000 | 34,514,000 | 8,631,000 | |||||||||
|
BLU-U® PRODUCT REVENUES |
||||||||||||
| United States |
2,151,000 | 1,892,000 | 259,000 | |||||||||
| Canada |
— | 17,000 | (17,000 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Subtotal BLU-U® product revenues |
2,151,000 | 1,909,000 | 242,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL PRODUCT REVENUES |
$ | 45,296,000 | $ | 36,423,000 | $ | 8,873,000 | ||||||
|
|
|
|
|
|
|
|||||||
For the year ended December 31, 2011, total PDT drug and device products revenues, comprised of revenues from our Kerastick® and BLU-U® products, were $45,296,000. This represents an increase of $8,873,000 or 24%, over the comparable 2010 total of $36,423,000. The incremental revenue was driven primarily by increased Kerastick® revenues in the United States and the acceleration of $1,379,000 of deferred revenues related to the termination of our Asia Pacific distribution agreement with Daewoong.
For the year ended December 31, 2011, Kerastick® revenues were $43,145,000, representing an increase of $8,631,000 or 25%, over the comparable 2010 totals of $34,514,000. Kerastick® unit sales to end-users for the year ended December 31, 2011 were 298,323 including 4,992 sold in Canada and 3,585 sold in Korea. This represents an increase from 262,046 Kerastick® units sold in the year ended December 31, 2010, including 5,208 sold in Canada, 4,170 sold in Korea and 1,772 sold in Latin America. Our average net selling price for the Kerastick® increased to $139.42 for the year ended December 31, 2011 from $128.04 in 2010. Our average net selling price for the Kerastick® includes sales made directly to our end-user customers, as well as sales made to our distributors. The increase in 2011 Kerastick® revenues were driven mainly by increased sales volumes in the United States along with an increase in our overall average unit selling price, and the acceleration of deferred revenues of $1,379,000, comprised of deferred drug shipments of $301,000 and the unamortized balance of milestone payments of $1,078,000, related to the termination of our Asia Pacific distribution agreement with Daewoong during 2011, while in 2010 we accelerated the recognition of $555,000 of deferred revenues related to the termination of our Latin American distribution agreement with Stiefel.
34
Table of Contents
For the year ended December 31, 2011, BLU-U® revenues were $2,151,000, an increase of $242,000, or 13%, from 2010 BLU-U® revenues of $1,909,000. The increase in 2011 BLU-U® revenues was the result of increases in both sales volumes and our average selling price. In the year ended December 31, 2011, there were 286 units sold, as compared to 270 units in 2010. The 2011 total were all sold in the United States. The 2010 total consists of 267 units sold in the United States and 3 sold in Canada. Our average net selling price for the BLU-U® increased to $7,377 for the year ended December 31, 2011 from $6,843 for 2010. The average net selling price of the BLU-U® increased, in part, due to incentive discounting in the prior year in advance of the introduction of our new upgraded unit, which was launched in April 2010. Our BLU-U® evaluation program allows customers to take delivery of BLU-U® units for a period of up to 4 months for private practitioners and up to 1 year for hospital clinics, before we require a purchase decision. At December 31, 2011, there were approximately 48 units in the field pursuant to this evaluation program, compared to 28 units in the field at December 31, 2010. The units are classified as inventory in the financial statements and are being amortized during the evaluation period to cost of goods sold using an estimated life for the equipment of 3 years.
We have to continue to demonstrate the clinical value of our unique therapy, and the related product benefits as compared to other well-established conventional therapies, in order for the medical community to accept our products on a large scale. We are aware that physicians are using Levulan® with the BLU-U® with short incubation times, and with light devices manufactured by other companies, and for uses other than our FDA-approved use. While we are not permitted to market our products for so-called “off-label” uses, we believe that these activities are positively affecting the sales of our products. Additionally, in 2011 we initiated 2 clinical trials to study broad area, short incubation methods, which, if successful, will allow us to enhance our product label and market our therapy under a treatment method being adopted by the medical community. The increase in our total product revenues for 2011 compared with 2010 results primarily from increased Kerastick® and BLU-U® revenues in the United States and the acceleration of deferred revenues of $1,379,000 mentioned above.
During 2011, our revenues in the United States grew as a result of increased demand for our product. With respect to Kerastick® prices, we announced a price increase in the fourth quarter of 2011 and intend to announce an annual price increase in the fourth quarter each year which will become effective on January 1 of the following year. This strategy is likely to have a positive impact on sales in the fourth quarter of each year. Although we expect continued growth in our revenues, we are susceptible to the uncertain economic conditions, particularly with our customer base where our product lacks reimbursement, and to increased competition particularly from Medicis, who in December 2011 acquired Aldara®, a topical AK product, and Zyclara®, used to treat precancerous skin growths related to sun overexposure, and Leo Pharma, who in January 2012 received FDA approval for Picato® Gel, a topical product, to treat AKs on the face and scalp and on the extremities. Also, Galderma, S.A., a large dermatology company, holds a non-exclusive license from us to Metvixia®, which was transferred to Galderma by PhotoCure ASA, our original licensee. This product received FDA approval for treatment of AKs in July 2004 and this product is directly competitive with our Levulan® Kerastick® product. While we are entitled to royalties on net sales of Metvixia®, Galderma has considerably more resources than we have, which could significantly hamper our ability to maintain or increase our market share. Metvixia® is commercially available in the U.S.; however, product revenues have not been significant to date. Also, in June 2011 PhotoCure announced the commercial launch of an ALA ester-based product, Allumera™, as a cosmetic, which could cause disruption in the marketplace.
Our ability to maintain profitability on a quarterly basis may be affected by fluctuations in the demand for our products caused by both seasonal changes, such as when patient visits slow during summer months, and the timing of pricing changes, which may impact the purchasing patterns of our customers.
Also see the section entitled “Risk Factors — We May Not Continue To Be Profitable Each Quarter Unless We Can Successfully Market And Sell Significantly Higher Quantities Of Our Products.”
35
Table of Contents
Cost Of Product Revenues and Royalties — Cost of product revenues and royalties for the year ended December 31, 2011 were $6,922,000 as compared to $6,419,000 for the year ended December 31, 2010. A summary of the components of cost of product revenues and royalties is provided below:
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | Increase/ (Decrease) |
||||||||||
|
Levulan® Kerastick ® Cost of Product Revenues and Royalties |
||||||||||||
| Direct and indirect Levulan® Kerastick® Product costs |
$ | 3,238,000 | $ | 3,231,000 | $ | 7,000 | ||||||
| Royalty and supply fees(1) |
1,653,000 | 1,331,000 | 322,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Subtotal Levulan® Kerastick® Cost of Product Revenues and Royalties |
4,891,000 | 4,562,000 | 329,000 | |||||||||
|
BLU-U® Cost of Product Revenues |
||||||||||||
| Direct BLU-U® Product Costs |
1,148,000 | 1,015,000 | 133,000 | |||||||||
| Other BLU-U® Product Costs including internal costs assigned to support products; as well as, costs incurred to ship, install and service the BLU-U® in physicians’ offices |
883,000 | 842,000 | 41,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Subtotal BLU-U® Cost of Product Revenues |
2,031,000 | 1,857,000 | 174,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL COST OF PRODUCT REVENUES AND ROYALTIES |
$ | 6,922,000 | $ | 6,419,000 | $ | 503,000 | ||||||
|
|
|
|
|
|
|
|||||||
| 1) | Royalty and supply fees reflect amounts paid to our licensor, PARTEQ, and amortization of an upfront fee and royalties paid to Draxis Health, Inc. on sales of the Levulan® Kerastick® in Canada. |
Margins — Total product margins for 2011 were $38,374,000, or 85%, as compared to $30,004,000, or 82% for 2010, as shown below:
| Year Ended December 31, | ||||||||||||||||||||
| 2011 | 2010 | Increase/ (Decrease) |
||||||||||||||||||
|
Levulan® Kerastick® Gross Margin |
$ | 38,254,000 | 89 | % | $ | 29,952,000 | 87 | % | $ | 8,302,000 | ||||||||||
|
BLU-U® Gross Margin |
120,000 | 6 | % | 52,000 | 3 | % | 68,000 | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| TOTAL GROSS MARGIN |
$ | 38,374,000 | 85 | % | $ | 30,004,000 | 82 | % | $ | 8,370,000 | ||||||||||
|
|
|
|
|
|
|
|||||||||||||||
Kerastick® gross margins for the year ended December 31, 2011 were 89%, as compared to 87% for the year ended December 31, 2010. The margin improvement for 2011 is attributable to increased U.S. sales volumes, an increase in our overall average selling price and the acceleration of deferred revenues related to the termination of our Asia-Pacific distribution agreement with Daewoong. The Daewoong termination resulted in the recognition of $1,308,000 of gross margin while in 2010 the Stiefel termination resulted in the recognition of $513,000 of gross margin.
Our long-term goal is to achieve higher gross margins on Kerastick® sales. We believe that we can achieve improved gross margins on our Kerastick® from further volume growth and price increases in the United States.
BLU-U® margins for the year ended December 31, 2011 were 6%, as compared to 3% for the year ended December 31, 2010. The increase in gross margin is a result of an increase in our average selling price. It is important for us to sell BLU-U® units in an effort to drive Kerastick® sales volumes and accordingly, we may sell BLU-U® units at low profit margins.
36
Table of Contents
Research and Development Costs — Research and development costs for 2011 were $5,275,000 as compared to $4,930,000 in 2010. The increase in 2011 compared to 2010 was due primarily to increased spending related to the addition of employees and the initiation of two clinical trials during 2011, as further described in the following paragraph.
An exploratory DUSA-sponsored Phase 2 clinical trial designed to study the broad area application and/or short drug incubation, or BASDI, method of using the Levulan® Kerastick® was initiated during the fourth quarter of 2011, and is being carried out at 10 clinical trial sites. DUSA expects that approximately 220 study subjects will be enrolled in this trial. The protocol objectives are to compare the safety and efficacy of various incubation times (1, 2 or 3 hours) of Levulan® plus BLU-U® PDT versus vehicle plus BLU-U® for the treatment of multiple actinic keratoses of the face or scalp and to investigate the potential for reduction in AK occurrence in the treatment areas. Assuming enrollment of subjects continues as planned, we expect that preliminary results of this trial will be available by the end of 2012. In addition to the BASDI clinical trial for the treatment of AKs of the face and scalp, a pilot DUSA-sponsored clinical trial designed to study the BASDI method of using the Levulan® Kerastick for the treatment of AKs on extremities was initiated during the fourth quarter of 2011 at 3 clinical trial sites. DUSA expects that approximately 64 study subjects will be enrolled in this study. The objective of the study is to determine and compare the safety and efficacy of ALA-PDT versus vehicle PDT on AKs of the upper extremities, and to evaluate the effect of occlusion on the safety and efficacy of ALA-PDT, using blue light after a 3 hour incubation period. Due to the initiation of these studies, we expect research and development costs for 2012 to be increased from 2011 levels. We expect that the total cost of these trials will be approximately $2.8 million over the course of the trials.
Marketing and Sales Costs — Marketing and sales costs for the year ended December 31, 2011 were $15,383,000 as compared to $13,241,000 for the year ended December 31, 2010. These costs consisted primarily of expenses such as salaries and benefits for the marketing and sales staff, commissions, and related support expenses such as travel, and telephone, totaling $11,609,000 in 2011, compared to $10,122,000 in 2010. The increase in spending in 2011 in this category is due primarily to increased headcount. The remaining expenses consisted of tradeshows, miscellaneous marketing and outside consultants totaling $3,774,000 in 2011, compared to $3,119,000 in 2010. The increase in this category is due primarily to an increase in expenditures related to sales training and promotional activities. We expect marketing and sales costs for 2012 to increase compared to 2011 levels, but to decrease as a percentage of revenues.
General and Administrative Costs — General and administrative costs for the year ended December 31, 2011 were $9,957,000 as compared to $9,106,000 for the year ended December 31, 2010. The increase is mainly attributable to compensation related charges and increased recruiting and hiring costs. General and administrative expenses are highly dependent on our legal and other professional fees, which can vary significantly from period to period. We expect general and administrative costs to increase in 2012 compared with 2011, but to decrease as a percentage of revenues.
Loss on Change in Fair Value of Warrants — The warrants issued to investors in connection with the October 29, 2007 private placement were recorded initially at fair value and are marked to market each reporting period. The increase in the liability during 2011 and 2010 was $1,013,000 and $391,000, respectively, which resulted in non-cash losses in the respective periods. The increases in fair value of the warrants are primarily due to increase in our stock price offset by a decreasing term to expiration.
Other Income — Other income for the year ended December 31, 2011 decreased to $46,000, as compared to $226,000 in 2010. This decrease reflects a general decrease in interest rates over that timeframe.
Income Taxes —There is no provision for income taxes due to the utilization of operating loss carryforwards for which a full valuation allowance had been provided. As of December 31, 2011, we had net operating loss carryforwards remaining of approximately $85,208,000 and tax credit carryforwards of approximately $1,641,000 for federal tax purposes. These amounts expire at various times through 2030. We have provided a full valuation allowance against the net deferred tax assets at December 31, 2011 and 2010.
37
Table of Contents
Based on an Internal Revenue Code (IRC) Section 382 study performed, we determined that we have experienced prior ownership changes, as defined under IRC Section 382, with the most recent change in ownership occurring in 2007 (the 2007 Ownership Change). Our pre-change NOL carryforwards are subject to an annual limitation of approximately $2.2 million per year. Further, additional rules provide for the enhancement of the aforementioned annual limitation for the first 5 years after the ownership change. A loss corporation may increase its IRC Section 382 limitation by the amount of the net unrealized built-in gain (NUBIG) recognized within 5 years of the ownership change. The calculated aggregate amount of NUBIG enhancement for us is approximately $4.3 million (i.e., approximately $868,000 per year for the first 5 years after the ownership change). This NUBIG enhancement will be utilized in conjunction with the approximately $2.2 million of IRC Section 382 base annual limitation, resulting in approximately $3.0 million per year for the first 5 years after the ownership change. Based on these additional factors, we estimate that we will be able to utilize approximately $49.9 million of our current net operating losses, provided that we generate sufficient income and no further ownership changes were to occur. However, it is reasonably possible that a future ownership change, which could be the result of transactions involving our common stock that are outside of our control (such as sales by existing shareholders), could occur during 2012 or thereafter. Future ownership changes could further restrict the utilization of our net operating losses and tax credits, reducing or eliminating the benefit of such net operating losses and tax credits. An ownership change occurs under IRC Section 382 if the aggregate stock ownership of certain shareholders increases by more than 50 percentage points over such shareholders’ lowest percentage ownership during the testing period, which is generally three years.
Income from Discontinued Operations, Net of Tax Provision — Income from discontinued operations, net of tax provision was $527,000 and $139,000 during 2011 and 2010, respectively. Discontinued operations reflect the results of our historically designated Non-PDT segment. See Note 11 in the Notes to the Consolidated Financial Statements for further discussion.
Net Income (Loss) — For 2011, we reported net income of $7,320,000, or $0.28 per diluted share, as compared to net income of 2,703,000, or $0.11 per diluted share, for 2010. The increase in our net income is attributable to the reasons discussed above.
38
Table of Contents
Year Ended December 31, 2010 As Compared to the Year Ended December 31, 2009
Revenues — Total revenues for 2010 were $36,423,000, as compared to $28,338,000 in 2009 and were comprised of the following:
| Year Ended December 31, | ||||||||||||
| 2010 | 2009 | Increase/ (Decrease) |
||||||||||
| PDT DRUG AND DEVICE PRODUCT REVENUES |
||||||||||||
|
LEVULAN® KERASTICK® PRODUCT REVENUES |
||||||||||||
| United States |
$ | 32,821,000 | $ | 24,756,000 | $ | 8,065,000 | ||||||
| Canada |
491,000 | 543,000 | (52,000 | ) | ||||||||
| Korea |
424,000 | 646,000 | (222,000 | ) | ||||||||
| Latin America |
778,000 | 434,000 | 344,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Subtotal Levulan® Kerastick® product revenues |
34,514,000 | 26,379,000 | 8,135,000 | |||||||||
|
BLU-U® PRODUCT REVENUES |
||||||||||||
| United States |
1,892,000 | 1,943,000 | (51,000 | ) | ||||||||
| Canada |
17,000 | 16,000 | 1,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Subtotal BLU-U® product revenues |
1,909,000 | 1,959,000 | (50,000 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL PRODUCT REVENUES |
$ | 36,423,000 | $ | 28,338,000 | $ | 8,085,000 | ||||||
|
|
|
|
|
|
|
|||||||
For the year ended December 31, 2010, total PDT drug and device products revenues, comprised of revenues from our Kerastick® and BLU-U® products, were $36,423,000. This represents an increase of $8,085,000 or 29%, over the comparable 2009 total of $28,338,000. The incremental revenue was driven primarily by increased Kerastick® revenues in the United States.
For the year ended December 31, 2010, Kerastick® revenues were $34,514,000, representing an increase of $8,135,000 or 31%, over the comparable 2009 totals of $26,379,000. Kerastick® unit sales to end-users for the year ended December 31, 2010 were 262,046, including 5,208 sold in Canada, 4,170 sold in Korea and 1,772 sold in Latin America. This represents an increase from 220,288 Kerastick® units sold in the year ended December 31, 2009, including 6,000 sold in Canada, 8,472 sold in Korea and 4,474 sold in Latin America. Our average net selling price for the Kerastick® increased to $128.04 for the year ended December 31, 2010 from $117.73 in 2009. Our average net selling price for the Kerastick® includes sales made directly to our end-user customers, as well as sales made to our distributors, in Canada, Korea and Latin America. The increase in 2010 Kerastick® revenues was driven mainly by increased sales volumes in the United States along with an increase in our overall average unit selling price, and the acceleration of the recognition of deferred revenues of $555,000 related to the termination of our Latin American distribution agreement with Stiefel.
For the year ended December 31, 2010, BLU-U® revenues were $1,909,000, a decrease of $50,000, or 3%, from 2009 BLU-U® revenues of $1,959,000. The slight decrease in 2010 BLU-U® revenues was the result of a decrease in our average selling price, partially offset by increased sales volumes. In the year ended December 31, 2010, there were 270 units sold, as compared to 252 units in 2009. The 2010 total consists of 267 sold in the United States and 3 sold in Canada. The 2009 total consists of 251 units sold in the United States and 1 sold in Canada. Our average net selling price for the BLU-U® decreased to $6,843 for the year ended December 31, 2010 from $7,418 for 2009. The decrease in our average selling price in 2010 compared with 2009 reflects lower pricing that we offered to customers in advance of the introduction of an upgraded BLU-U® design, which became available in April 2010. Our BLU-U® evaluation program allows customers to take delivery for a period of up to 4 months for private practitioners and up to 1 year for hospital clinics, before we require a purchase decision. At December 31, 2010, there were approximately 28 units in the field pursuant to this evaluation program, compared to 12 units in the field at December 31, 2009. The units are classified as inventory in the financial statements and are being amortized during the evaluation period to cost of goods sold using an estimated life for the equipment of 3 years.
39
Table of Contents
The increase in our total product revenues for 2010 compared with 2009 results primarily from increased Kerastick® revenues in the United States, along with an increase in our overall average unit selling price, and the acceleration of the recognition of deferred revenues of $555,000 related to the termination of our Latin American distribution agreement with Stiefel.
Cost Of Product Revenues and Royalties — Cost of product revenues and royalties for the year ended December 31, 2010 were $6,419,000 as compared to $6,007,000 for the year ended December 31, 2009. A summary of the components of cost of product revenues and royalties is provided below:
| Year Ended December 31, | ||||||||||||
| 2010 | 2009 | Increase/ (Decrease) |
||||||||||
|
Levulan® Kerastick ® Cost of Product Revenues and Royalties |
||||||||||||
| Direct and indirect Levulan® Kerastick® Product costs |
$ | 3,231,000 | $ | 3,339,000 | $ | (108,000 | ) | |||||
| Royalty and supply fees(1) |
1,331,000 | 1,042,000 | 289,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Subtotal Levulan® Kerastick® Cost of Product Revenues and Royalties |
4,562,000 | 4,381,000 | 181,000 | |||||||||
|
BLU-U® Cost of Product Revenues |
||||||||||||
| Direct BLU-U® Product Costs |
1,015,000 | 907,000 | 108,000 | |||||||||
| Other BLU-U® Product Costs including internal costs assigned to support products; as well as, costs incurred to ship, install and service the BLU-U® in physicians’ offices |
842,000 | 719,000 | 123,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Subtotal BLU-U® Cost of Product Revenues |
1,857,000 | 1,626,000 | 231,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL COST OF PRODUCT REVENUES AND ROYALTIES |
$ | 6,419,000 | $ | 6,007,000 | $ | 412,000 | ||||||
|
|
|
|
|
|
|
|||||||
| 1) | Royalty and supply fees reflect amounts paid to our licensor, PARTEQ, and amortization of an upfront fee and royalties paid to Draxis Health, Inc. on sales of the Levulan® Kerastick® in Canada. |
Margins — Total product margins for 2010 were $30,004,000, or 82%, as compared to $22,331,000, or 79% for 2009, as shown below:
| Year Ended December 31, | ||||||||||||||||||||
| 2010 | 2009 | Increase/ (Decrease) |
||||||||||||||||||
|
Levulan® Kerastick® Gross Margin |
$ | 29,952,000 | 87 | % | $ | 21,998,000 | 83 | % | $ | 7,954,000 | ||||||||||
|
BLU-U® Gross Margin |
52,000 | 3 | % | 333,000 | 17 | % | (281,000 | ) | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| TOTAL GROSS MARGIN |
$ | 30,004,000 | 82 | % | $ | 22,331,000 | 79 | % | $ | 7,673,000 | ||||||||||
|
|
|
|
|
|
|
|||||||||||||||
Kerastick® gross margins for the year ended December 31, 2010 were 87%, as compared to 83% for the year ended December 31, 2009. The margin improvement for 2010 is attributable to increased U.S. sales volumes, an increase in our overall average selling price and the acceleration of deferred revenues related to the termination of our Latin American distribution agreement with Stiefel. The Stiefel termination resulted in the recognition of $513,000 of gross margin.
BLU-U® margins for the year ended December 31, 2010 were 3%, as compared to 17% for the year ended December 31, 2009. The decrease in gross margin is a result of a decrease in our average selling price.
40
Table of Contents
Research and Development Costs — Research and development costs for 2010 were $4,930,000 as compared to $4,313,000 in 2009. The increase in 2010 compared to 2009 was due primarily to increased spending on development projects and investigator studies. In the third quarter of 2010, we announced that we would be closing out our solid organ transplant recipient, or SOTR, clinical trial program in response to the FDA’s denial of our application for Orphan Drug Designation for the use of Levulan® PDT for the prevention of squamous cell carcinomas, or SCC, in patients who have a proven history of multiple localized cutaneous SCCs, such as SOTRs.
Marketing and Sales Costs — Marketing and sales costs for the year ended December 31, 2010 were $13,241,000 as compared to $12,897,000 for the year ended December 31, 2009. These costs consisted primarily of expenses such as salaries and benefits for the marketing and sales staff, commissions, and related support expenses such as travel, and telephone, totaling $10,122,000 in 2010, compared to $9,389,000 in 2009. The increase in spending in 2010 in this category is due primarily to higher commissions expense in 2010. The remaining expenses consisted of tradeshows, miscellaneous marketing and outside consultants totaling $3,119,000 in 2010, compared to $3,508,000 in 2009. The decrease in this category is due primarily to a decrease in tradeshow and other promotional spending in 2010.
General and Administrative Costs — General and administrative costs for the year ended December 31, 2010 were $9,106,000 as compared to $7,956,000 for the year ended December 31, 2009. The increase is mainly attributable to an increase in compensation related expense, which partially resulted from a non-cash charge related to a modification of stock options for former members of the board of directors. General and administrative expenses are highly dependent on our legal and other professional fees, which can vary significantly from period to period.
Loss on Change in Fair Value of Warrants — The warrants issued to investors in connection with the October 29, 2007 private placement were recorded initially at fair value and are marked to market each reporting period. The increase in the liability during 2010 and 2009 was $391,000 and $376,000, respectively, which resulted in non-cash losses in the respective periods. The increases or decreases in fair value of the warrants are primarily due to increase in our stock price offset by a decreasing term to expiration.
Other Income — Other income for the year ended December 31, 2010 decreased to $226,000, as compared to $291,000 in 2009. This decrease reflects a general decrease in interest rates over that timeframe.
Income Tax Benefit — Overall, there is no provision for income taxes due to the full valuation allowance. In 2009, in accordance with intra-period tax allocation accounting rules, we recorded an income tax benefit to continuing operations, which is offset by a corresponding charge to income tax expense in discontinued operations.
Income from Discontinued Operations, Net of Tax Provision — Income from discontinued operations, net of tax provision was $139,000 and $251,000 during 2010 and 2009, respectively. See Note 11 to the Notes to Consolidated Financial Statements for further discussion.
Net Income (Loss) — For 2010, we reported net income of $2,703,000, or $0.11 per share, as compared to a net loss of $(2,508,000), or $(0.10) per share, for 2009. The increase in our net income, or decrease in our net loss, is attributable to the reasons discussed above.
41
Table of Contents
Quarterly Results of Operations
The following is a summary of the unaudited quarterly results of operations for the years ended December 31, 2011 and 2010, respectively:
| Quarterly Results for Year Ended December 31, 2011 | ||||||||||||||||
| March 31 | June 30 | Sept 30(1) | Dec 31 | |||||||||||||
| Product revenues |
$ | 10,982,000 | $ | 9,671,000 | $ | 9,374,000 | $ | 15,269,000 | ||||||||
| Gross margin |
9,351,000 | 8,200,000 | 7,962,000 | 12,861,000 | ||||||||||||
| Income (loss) from continuing operations |
(571,000 | ) | 396,000 | 3,562,000 | 3,406,000 | |||||||||||
| Income from discontinued operations |
(34,000 | ) | 714,000 | (3) | (55,000 | ) | (99,000 | ) | ||||||||
| Net income (loss) |
(605,000 | ) | 1,110,000 | 3,507,000 | 3,307,000 | |||||||||||
| Basic net income (loss) per share - Continuing operations |
$ | (0.02 | ) | $ | 0.02 | $ | 0.14 | $ | 0.14 | |||||||
| Discontinued operations |
$ | — | $ | 0.03 | $ | — | $ | — | ||||||||
| Basic net income (loss) per share |
$ | (0.02 | ) | $ | 0.05 | $ | 0.14 | $ | 0.13 | |||||||
| Diluted net income (loss) per share - |
||||||||||||||||
| Continuing operations |
$ | (0.02 | ) | $ | 0.01 | $ | 0.13 | $ | 0.13 | |||||||
| Discontinued operations |
$ | — | $ | 0.03 | $ | — | $ | — | ||||||||
| Diluted net income (loss) per share |
$ | (0.02 | ) | $ | 0.04 | $ | 0.13 | $ | 0.13 | |||||||
| Quarterly Results for Year Ended December 31, 2010 | ||||||||||||||||
| March 31 | June 30 | Sept 30(2) | Dec 31 | |||||||||||||
| Product revenues |
$ | 8,296,000 | $ | 8,411,000 | $ | 7,837,000 | $ | 11,879,000 | ||||||||
| Gross margin |
6,676,000 | 6,912,000 | 6,499,000 | 9,917,000 | ||||||||||||
| Income (loss) from continuing operations |
(640,000 | ) | 186,000 | 148,000 | 2,869,000 | |||||||||||
| Income from discontinued operations |
215,000 | 2,000 | (115,000 | ) | 37,000 | |||||||||||
| Net income (loss) |
(424,000 | ) | 188,000 | 33,000 | 2,906,000 | |||||||||||
| Basic net income (loss) per share - |
||||||||||||||||
| Continuing operations |
$ | (0.03 | ) | $ | 0.01 | $ | 0.01 | $ | 0.12 | |||||||
| Discontinued operations |
$ | 0.01 | $ | — | $ | — | $ | — | ||||||||
| Basic net income (loss) per share |
$ | (0.02 | ) | $ | 0.01 | $ | 0.00 | $ | 0.12 | |||||||
| Diluted net income (loss) per share - |
||||||||||||||||
| Continuing operations |
$ | (0.03 | ) | $ | 0.01 | $ | 0.01 | $ | 0.12 | |||||||
| Discontinued operations |
$ | 0.01 | $ | — | $ | — | $ | — | ||||||||
| Diluted net income (loss) per share |
$ | (0.02 | ) | $ | 0.01 | $ | 0.00 | $ | 0.12 | |||||||
| (1) | The third quarter of 2011 includes the acceleration of deferred revenues of $1,379,000 and the acceleration of deferred cost of revenues of $71,000 related to our termination of our Asia Pacific distribution agreement with Daewoong Pharmaceutical Co., LTD. and Daewoong Derma & Plastic Surgery Network Company, or Daewoong. |
| (2) | The third quarter of 2010 includes the acceleration of deferred revenues of $555,000 and the acceleration of deferred cost of revenues of $42,000 related to our termination of our Latin America distribution agreement with Stiefel Laboratories, Inc., or Stiefel. |
| (3) | Income from discontinued operations in the second quarter of 2011 includes a gain of $750,000 related to our sale of our patents covering Nicomide®, together with the trademarks Nicomide® and Nicomide-T®, and related domain names. |
42
Table of Contents
Liquidity and Capital Resources
At December 31, 2011, we had approximately $28,216,000 of total liquid assets, comprised of $24,424,000 of cash and cash equivalents and marketable securities available-for-sale totaling $3,792,000. We believe that our liquidity will be sufficient to meet our cash requirements for at least the next 12 months. As of December 31, 2011, our marketable securities had a weighted average yield to maturity of 1.39% and maturity dates ranging from June 2012 to January 2013. Our net cash generated by operations in 2011 was $8,039,000 versus $3,324,000 in 2010. The year-over-year increase in cash generated by operations is primarily attributable to an increase in our net income. Our net cash provided by (used in) investing activities was $7,218,000 in 2011 versus $(2,073,000) in 2010. Net cash provided by (used in) investing activities is primarily the result of our net maturities and purchases of marketable securities, and in 2011 also includes the proceeds from the sale of an asset, which is included in discontinued operations. Our net cash provided by (used in) financing activities is primarily the result of proceeds from stock option exercises, net of settlement of tax withholding obligations from restricted stock vesting. As of December 31, 2011 working capital, which is our total current assets minus our total current liabilities, was $29,721,000, as compared to $21,000,000 as of December 31, 2010. Total current assets increased by $9,720,000 during 2011, due primarily to an increase in our cash and cash equivalents, offset by a decrease in our marketable securities. Total current liabilities increased by $998,000 during 2011 due primarily to increases in accounts payable and accrued expenses, offset in part by a decrease in deferred revenues. In response to the instability in the financial markets, we regularly review our marketable securities holdings, and have invested primarily in securities of the U.S. government and its agencies.
We may expand or enhance our business in the future by using our resources to acquire by license, purchase or other arrangements, additional businesses, new technologies, or products in the field of dermatology. In 2011 and 2010, we focused primarily on increasing the sales of the Levulan® Kerastick® and the BLU-U®. If we cannot maintain profitability and positive cash flow, we may reduce our headcount or reduce spending in other areas. We may also seek to raise funds through financing transactions. We cannot predict whether financing will be available at all or on reasonable terms.
In April 2009, we paid to the former Sirius shareholders, on a pro rata basis, $100,000. In addition, in the event that the $1,000,000 milestone payment that would have been due under the merger agreement if cumulative net sales of the Sirius products reached $35,000,000 was not, in fact, triggered by December 31, 2011, then we agreed to pay $250,000 to the former Sirius shareholders on a pro rata basis on or before January 6, 2012. Cumulative net sales of the Sirius products did not reach $35,000,000 by December 31, 2011, and accordingly, the guaranteed $250,000 milestone payment, which is included as a liability in discontinued operations in the accompanying Consolidated Balance Sheets as of December 31, 2011, was paid in January 2012. No other payments are due pursuant to the merger agreement.
We have no off-balance sheet financing arrangements.
Contractual Obligations and Other Commercial Commitments
Perrigo Pharmaceuticals Company
On October 21, 2005, the former Sirius entered into a supply agreement with L. Perrigo Company, or Perrigo, for the exclusive manufacture and supply of a proprietary device/drug kit designed by Sirius pursuant to an approved abbreviated new drug application, or ANDA, owned by Perrigo. The agreement, which covers our ClindaReach® product, was assigned to us as part of the Sirius merger, and has been assigned by Perrigo to its affiliate, Perrigo Pharmaceuticals Company. During the fourth quarter of 2010, the parties executed an amendment to the agreement, which extended the initial term of the agreement through December 31, 2011, subject to certain rights to early termination. Perrigo’s affiliate was entitled to royalties on net sales of the product, including certain minimum royalties. For calendar year 2011, the minimum annual royalty of $250,000, which is included in Current Liabilities of Discontinued Operations in the accompanying Consolidated Balance Sheets as of December 31, 2011, was paid in January 2012. This agreement has terminated.
43
Table of Contents
Merger With Sirius Laboratories, Inc.
In March 2006, we closed our merger to acquire all of the common stock of Sirius Laboratories Inc. in exchange for cash and common stock worth up to $30,000,000. Of the up to $30,000,000, up to $5,000,000, ($1,500,000 of which would be paid in cash, and $3,500,000 of which would be paid in cash or common stock) may be paid based on a combination of new product approvals or launches, and achievement of certain pre-determined total cumulative sales milestones for Sirius products. With the launch of ClindaReach®, we were obligated to make a cash payment of $500,000 to the former shareholders of Sirius. Also, as a consequence of the decision not to launch the product under development with another third party and pursuant to the terms of the merger agreement with Sirius, we paid $250,000 on a pro rata basis to the former Sirius shareholders. Similarly, with our decision in early 2008 not to develop a third product from a list of product candidates acquired as part of the merger, another $250,000 was paid on a pro rata basis to the former Sirius shareholders. The payments for ClindaReach® and the other two product decisions satisfied our obligations for the $1,500,000 portion of the purchase price mentioned above. In the third quarter of 2008, the first of the pre-determined total cumulative sales milestones for Sirius products was achieved, and accordingly, we made a cash payment of $1,500,000 to the former Sirius shareholders in consideration of the milestone achievement.
Pursuant to the agreements we entered into in April 2009, we agreed to extend the milestone termination date from 50 months from the date of the closing of the merger until December 31, 2011 and to include in the definition of net sales in the merger agreement payments which we may receive from the divestiture of Sirius products by December 31, 2011. This amendment to the merger agreement also removed our obligation to market the Sirius products according to certain previously required standards and allows us to manage all business activities relating to the products acquired from Sirius without further approval from the former Sirius shareholders.
In April 2009, we paid to the former Sirius shareholders, on a pro rata basis, $100,000. In addition, in the event that the $1,000,000 milestone payment that would have been due under the merger agreement if cumulative net sales of the Sirius products reached $35,000,000 was not, in fact, triggered by December 31, 2011, then we agreed to pay $250,000 to the former Sirius shareholders on a pro rata basis on or before January 6, 2012. Cumulative net sales of the Sirius products did not reach $35,000,000 by December 31, 2011, and accordingly, the guaranteed $250,000 milestone payment, which is included in Current Liabilities of Discontinued Operations in the accompanying Consolidated Balance Sheets as of December 31, 2011, was paid in January 2012. No other payments are due pursuant to the merger agreement.
PARTEQ Agreement
We license certain patents underlying our Levulan® PDT systems under a license agreement with PARTEQ Research and Development Innovations, or PARTEQ. Under the agreement, we have been granted an exclusive worldwide license, with a right to sublicense, under PARTEQ patent rights, to make, have made, use and sell certain products, including ALA. The agreement covers certain use patent rights. When we sell our products directly, we have agreed to pay to PARTEQ royalties of 6% and 4% on 66% of the net selling price in countries where patent rights do and do not exist, respectively. In cases where we have a sublicensee, we will pay 6% and 4% when patent rights do and do not exist, respectively, on our net selling price less the cost of goods for products sold to the sublicensee, and 6% of payments we receive on sales of products by the sublicensee. We are also obligated to pay to PARTEQ 5% of any lump sum sublicense fees received, such as milestone payments, excluding amounts designated by the sublicensee for future research and development efforts.
For the years ended December 31, 2011, 2010 and 2009, actual royalties based on product sales were approximately $1,653,000, $1,331,000 and $1,019,000, respectively. Annual minimum royalties to PARTEQ must total at least CDN $100,000 (U.S. $98,000 as of December 31, 2011).
44
Table of Contents
National Biological Corporation Amended And Restated Purchase And Supply Agreement
On November 29, 2011, we entered into the 2011 Amended and Restated Purchase and Supply Agreement, or the 2011 NBC Agreement, with National Biological Corporation, or NBC, the primary manufacturer of our BLU-U® light source. The 2011 NBC Agreement includes similar terms and conditions to our Amended and Restated Purchase and Supply Agreement dated as of June 21, 2004, as amended, or the 2004 Agreement, which was due to expire on December 31, 2011. The 2011 NBC Agreement replaces the 2004 Agreement and has a term of two (2) years through December 31, 2013. We have an option to further extend the term of the 2011 NBC Agreement for an additional two years if we purchase a certain number of units.
Sochinaz SA
Under an agreement dated December 24, 1993, Sochinaz SA manufactures and supplies our requirements of Levulan® from its FDA approved facility in Switzerland. In 2009, our agreement was renewed until December 31, 2015 on substantially the same terms, albeit with a revised pricing schedule to cover the new term. While we can obtain alternative supply sources in certain circumstances, any new supplier would have to be GMP compliant and complete process development, validation and stability programs to become fully qualified by us and acceptable to FDA.
Lease Agreements
We have entered into a lease commitment for office space in Wilmington, Massachusetts. The minimum lease payments disclosed below include the non-cancelable terms of the leases.
Research Agreements
We have entered into various agreements for research projects and clinical studies. As of December 31, 2011, future payments to be made pursuant to these agreements, under certain terms and conditions, totaled approximately $3,093,000. Included in this future payment is a master service agreement, effective June 15, 2001, with Therapeutics, Inc. for management services in connection with the clinical development of our products in the field of dermatology. The agreement was renewed on June 15, 2011 for a one year period and is renewable annually. Therapeutics is entitled to receive a bonus valued at $50,000, in cash or stock at our discretion, upon each anniversary of the effective date.
Our contractual obligations and other commercial commitments to make future payments under contracts, including lease agreements, research and development contracts, manufacturing contracts, or other related agreements are as follows at December 31, 2011:
| Total | 1 Year or less | 2-3 Years | 4-5 Years | After 5 Years | ||||||||||||||||
| Operating lease obligations |
$ | 1,152,000 | $ | 389,000 | $ | 763,000 | $ | — | $ | — | ||||||||||
| Purchase obligations(1, 2) |
5,303,000 | 5,288,000 | 15,000 | — | — | |||||||||||||||
| Minimum royalty obligations(3) |
360,000 | 286,000 | 74,000 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total obligations |
$ | 6,815,000 | $ | 5,963,000 | $ | 852,000 | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| 1) | Research and development projects include various commitments including obligations for our study on a broad area application, short drug incubation method of using the Levulan® Kerastick®. |
| 2) | In addition to the obligations disclosed above, we have contracted with Therapeutics, Inc., a clinical research organization, to manage the clinical development of our products in the field of dermatology. This organization has the opportunity for additional stock grants, bonuses, and other incentives for each product indication ranging from $250,000 to $1,250,000, depending on the regulatory phase of development of products under Therapeutics’ management. |
| 3) | Minimum royalty obligations relate to our agreements with PARTEQ and Perrigo described above. |
45
Table of Contents
Rent expense incurred under these operating leases was approximately $357,000, $386,000, and $398,000 for the years ended December 31, 2011, 2010, and 2009, respectively.
Inflation
Although inflation rates have been comparatively low in recent years, inflation is expected to apply upward pressure on our operating costs. We have included an inflation factor in our cost estimates. However, the overall net effect of inflation on our operations is expected to be minimal.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Interest Rates
Our exposure to market risk for changes in interest rates relates primarily to our investment portfolio. We do not use derivative financial instruments in our investment portfolio. Our investment policy specifies credit quality standards for our investments and limits the amount of credit exposure to any non-U.S. government single issue, issuer or type of investment.
Our investments consist of U.S. government securities and high grade corporate bonds. All investments are carried at fair value.
As of December 31, 2011, the weighted average yield to maturity on our investments was 1.39%. If market interest rates were to increase immediately and uniformly by 100 basis points from levels as of December 31, 2011, the fair market value of the portfolio would decline by $28,000. Declines in interest rates could, over time, reduce our interest income.
Derivative Financial Instruments
The warrants that we issued on October 29, 2007 in connection with the private placement of our common stock, which expire in April 2013, were determined to be derivative financial instruments and accounted for as a liability. We revalue these warrants on a quarterly basis with any change in value reflected in our earnings. We value these warrants using various assumptions, including our common stock price as of the end of each reporting period, the historical volatility of our common stock price, and risk-free interest rates commensurate with the remaining contractual term of the warrants. Changes in our common stock price or in interest rates would result in a change in the value of the warrants and impact our consolidated statement of operations. A 10% increase in our common stock price would cause the fair value of the warrants and our warrant liability to increase by approximately $428,000.
Currency Exchange Rates
Exchange rates that we are subject to, primarily the Canadian dollar, are not material to our operations.
Forward-Looking Statements Safe Harbor
This report, including the Management’s Discussion and Analysis of Financial Condition and Results of Operations, contains various “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and 21E of the Securities Exchange Act of 1934 which represent our expectations or beliefs concerning future events, including, but not limited to our expectations regarding the potential for reduction of headcount, and our desire to raise funds through financing transactions, management’s beliefs regarding the unique nature of Levulan® and its use and potential uses, expectations regarding the enrollment of our BASDI clinical trial, beliefs regarding the future development of Levulan® and other potential indications, expectations concerning manufacture of the BLU-U® in our facility, intention to pursue licensing, marketing, co-promotion, other arrangements, additional business or new technologies, our
46
Table of Contents
beliefs regarding the safety, simplicity, reliability and cost-effectiveness of light sources, our expectations regarding product launches in other countries and territories, expectations regarding additional market expansion, the impact on our market share resulting from Galderma’s promotion of Metvixia®, the approval of Leo Pharma’s Picato® Gel, Medicis’ potential launch of two recently approved line extensions for the Zyclara® franchise and Photocure launch of AllumeraTM, expectations regarding the confidentiality of our proprietary information, beliefs regarding regulatory classifications, filings, timelines, off-label use, and environmental compliance, beliefs concerning patent disputes or patents issued to third parties, beliefs regarding the impact of litigation and ability to afford the costs, ability and intentions to obtain, secure, defend and enforce our patents, beliefs regarding the result of the reexamination process of our patents, beliefs regarding the impact of a third-party’s regulatory compliance status and fulfillment of contractual obligations, expectations of increases or decreases in the prices we charge for our products and their margins, our beliefs regarding the size of the market for our products and our product candidate, expected use of cash resources, beliefs regarding requirements of cash resources for our future liquidity, and research and development programs, beliefs regarding investments and economic conditions including the impact of our customer’s failure to meet our payment terms, expectations regarding outstanding options and warrants, anticipation of increases or decreases in personnel, beliefs regarding the effect of reimbursement policies on revenues and market acceptance of our therapies, expectations for future strategic opportunities and research and development programs and expenses, expectations for continuing operating losses and beliefs regarding competition, expectations regarding the adequacy and availability of insurance, expectations regarding general and administrative costs, expectations regarding sales and marketing costs and research and development costs, levels of interest income and our, beliefs regarding the impact of raising additional funds to meet capital requirements and the potential dilution to our existing shareholders, beliefs regarding the potential for additional inspection and testing of our manufacturing facilities or additional FDA actions, beliefs regarding our manufacturing capabilities, beliefs regarding interest rate risks to our investments and effects of inflation, beliefs regarding the impact of any current or future legal proceedings, beliefs regarding the dependence on key personnel, beliefs concerning product liability insurance, beliefs regarding the enforceability of our patents, beliefs regarding financial condition, results of operations and profitability, our beliefs regarding our sales and marketing efforts, beliefs regarding competition with other companies and effect of increased reimbursement, beliefs regarding the adoption of our products, our beliefs regarding our compliance with applicable laws, rules and regulations, our beliefs regarding available reimbursement for our products and plans to seek improvement, our beliefs regarding the current and future clinical development and testing of our potential products and technologies and the costs thereof, beliefs regarding the volatility of our stock price, beliefs regarding the impact of our rights plan, beliefs regarding the impact of future sales of securities, expectations related to the change in revenues of our PDT products, beliefs regarding market share, beliefs regarding profitability, beliefs regarding the change in growth in our PDT Drug and Device Products segment, expectations regarding our manufacturing facility or any facility of our contract manufacturers, beliefs regarding our Nasdaq Global Market listing, beliefs regarding Section 382 on our current and future NOLs, beliefs regarding evaluation of wholesaler inventory levels and our applicable revenue related reserves, beliefs regarding factors which could trigger an impairment review, beliefs regarding our ability to use net operating loss carryforwards and tax credit carryforwards to offset future taxable income, beliefs regarding our NUBIG enhancements, beliefs regarding a future ownership change or change of control, beliefs regarding the outcome if some or all of our shares are sold into the public market over a short period of time, beliefs regarding our ability to sell equity securities or equity-related securities in the future, beliefs regarding the impact that any manufacturing or supply problems could have on our sales, intentions to support research, anticipation of future NDAs for Levulan® PDT as combination products, beliefs concerning safety procedures for hazardous materials, our compliance and risks of liability, beliefs regarding competitive products, beliefs concerning revenues, beliefs regarding our capital resource needs, beliefs regarding the sufficiency of our cash, cash equivalents and marketable securities, beliefs regarding softness in the international markets, beliefs regarding economic recovery, beliefs regarding the failure to comply with FDA or other governmental regulatory requirements and the impact of such failure, beliefs regarding the global credit and financial market conditions on our business, beliefs regarding cash flows, beliefs regarding production yields, costs or quality of our products, beliefs
47
Table of Contents
regarding market acceptance of our products, beliefs regarding collaborations with outside scientists, beliefs regarding our products becoming non-competitive or obsolete, beliefs regarding our inventory becoming obsolete or our inventory significantly changing in value, expectation to hire additional sales representatives, expectations for losses from operations, intention to continue to develop combination drug and light device systems, beliefs regarding patent protection of our technology and from competition, beliefs regarding financial benefits from patent protection, beliefs regarding the FDA and preclinical and clinical testing process beliefs regarding the PMA process, beliefs regarding improved gross margins on Kerastick® from further volume growth and price increases in the United States, beliefs regarding the timing of pricing changes, beliefs regarding the purchasing patterns of our customers, our expectations regarding BASDI research and development costs, beliefs regarding our intention to announce a price increase on an annual basis in the fourth quarter of each year and our beliefs regarding our ability to be profitable each quarter and the affect of fluctuations in the demand for our products during the year. These forward-looking statements are further qualified by important factors that could cause actual results to differ materially from those in the forward-looking statements. These factors include, without limitation, changing market and regulatory conditions, actual clinical results of our trials, the impact of competitive products and pricing, the timely development, FDA and foreign regulatory approval, and market acceptance of our products, environmental risks relating to our products, reliance on third-parties for the production, manufacture, sales and marketing of our products, the availability of products for acquisition and/or license on terms agreeable to us, sufficient sources of funds, the securities regulatory process, the maintenance of our patent portfolio and ability to obtain competitive levels of reimbursement by third-party payors, none of which can be assured. Results actually achieved may differ materially from expected results included in these statements as a result of these or other factors.
48
Table of Contents
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The following financial statements required to be filed pursuant to this Item 8 are appended to this Annual Report on Form 10-K:
| F-1 | ||||
| F-2 | ||||
| F-3 | ||||
| Consolidated Statements of Shareholders’ Equity and Comprehensive Income (Loss) |
F-4 | |||
| F-5 | ||||
| F-6 |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures. We carried out an evaluation, under the direction of our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)). Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to the issuer’s management, including its principal executive and principal financial officers or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. Based upon that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this report.
Changes In Internal Control Over Financial Reporting. The Chief Executive Officer and Chief Financial Officer have concluded that there have been no changes in our internal control over financial reporting during the quarter ended December 31, 2011 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control — Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2011.
The effectiveness of our internal control over financial reporting as of December 31, 2011 has been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report which is included herein.
49
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of DUSA Pharmaceuticals, Inc.
Wilmington, Massachusetts
We have audited the internal control over financial reporting of DUSA Pharmaceuticals, Inc. and subsidiaries (the “Company”) as of December 31, 2011, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on the criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements as of and for the year ended December 31, 2011 of the Company and our report dated March 6, 2012 expressed an unqualified opinion on those financial statements.
/s/ Deloitte & Touche LLP
Boston, Massachusetts
March 6, 2012
50
Table of Contents
| ITEM 9B. OTHER | INFORMATION |
On March 6, 2012, DUSA Pharmaceuticals, Inc. issued a press release announcing summary financial results for the fiscal quarter and year ended December 31, 2011. The press release issued in connection with such announcement is attached hereto as Exhibit 99.1.
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by Item 10 is hereby incorporated by reference to the sections entitled “Nominees,” “Executive Officers who are not Directors,” “Compliance with Section 16(a) of the Exchange Act,” “Meetings and Committees of the Board,” and “Code of Ethics Applicable to Senior Officers” of the Registrant’s 2012 Proxy Statement.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by Item 11 is hereby incorporated by reference to the sections entitled “Director Compensation,” “Executive Compensation,” “Summary Compensation Table,” “Grants of Plan-Based Awards,” “Outstanding Equity Awards at Fiscal Year-End,” “Option Exercises and Stock Vested,” “NonQualified Deferred Compensation,” “Compensation Discussion and Analysis,” and “Board Compensation Committee Report” of Registrant’s 2012 Proxy Statement.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by Item 12 is hereby incorporated by reference to the section entitled “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information” of the Registrant’s 2012 Proxy Statement.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by Item 13 is hereby incorporated by reference to the section entitled “Certain Relationships and Related Transactions” and “Meetings and Committees of the Board” of the Registrant’s 2012 Proxy Statement.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by Item 14 is hereby incorporated by reference to the section entitled “Ratification of the Selection of Independent Registered Public Accounting Firm” of the Registrant’s 2012 Proxy Statement.
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
| A. | List of Financial Statements |
| F-1 | ||||
| F-2 | ||||
| F-3 | ||||
| Consolidated Statements of Shareholders’ Equity and Comprehensive Income (Loss) |
F-4 | |||
| F-5 | ||||
| F-6 |
51
Table of Contents
| B. | Exhibits filed as part of this Report |
| 2(a.1)* |
Merger Agreement by and among the Registrant, Sirius Laboratories, Inc., and the shareholders of Sirius dated as of December 30, 2005 filed as Exhibit 2(a.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference; | |
| 2(a.2) |
First Amendment to Merger Agreement by and among the Registrant, Sirius Laboratories, Inc. and the shareholders of Sirius, dated as of February 6, 2006 filed as Exhibit 2(a.2) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference; and | |
| 2(a.3) |
Third Amendment to Merger Agreement by and among the Registrant, Sirius Laboratories, Inc. and the shareholders of Sirius, dated as of April 21, 2009; filed as Exhibit 2(a.3) to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2009, and is incorporated herein by reference. | |
| 3(a.1) |
Certificate of Incorporation, as amended, filed as Exhibit 3(a) to the Registrant’s Form 10-K for the fiscal year ended December 31, 1998, and is incorporated herein by reference; | |
| 3(a.2) |
Certificate of Amendment to the Certificate of Incorporation, as amended, dated October 28, 2002 and filed as Exhibit 99.3 to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2002, filed November 12, 2002, and is incorporated herein by reference; and | |
| 3(b) |
Amended and Restated By-laws of the Registrant. | |
| 4(a) |
Common Stock specimen, filed as Exhibit 4(a) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2002, and is incorporated herein by reference; | |
| 4(b) |
Rights Agreement filed as Exhibit 4.0 to Registrant’s Current Report on Form 8-K filed October 11, 2002, and is incorporated herein by reference; | |
| 4(c) |
Rights Certificate relating to the rights granted to holders of common stock under the Rights Agreement filed as Exhibit 4.0 to Registrant’s Current Report on Form 8-K filed October 11, 2002, and is incorporated herein by reference; | |
| 4(d) |
Form of Common Stock Purchase Warrant, dated October 29, 2007 filed as Exhibit 4.2 to the Registrant’s Registration Statement on Form S-3, No. 333-147614, and is incorporated herein by reference; and | |
| 4(e) |
Registration Rights Agreement, dated October 29, 2007, by and between the Registrant and each of the respective selling shareholders named therein filed as Exhibit 4.3 to the Registrant’s Registration Statement on Form S-3, No. 333-147614, and is incorporated herein by reference. | |
| 10(a) |
License Agreement between the Registrant, PARTEQ and Draxis Health Inc. dated August 27, 1991, filed as Exhibit 10.1 to the Registrant’s Registration Statement on Form S-1, No. 33-43282, and is incorporated herein by reference; | |
| 10(b) |
Termination and Transfer Agreement between the Registrant and Draxis Health Inc. dated as of February 24, 2004, filed as Exhibit 10(b.2) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2003, portions of which have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
| 10(c) |
Consulting Agreement and General Release of D. Geoffrey Shulman, MD, FRCPC dated as of December 1, 2008, filed as Exhibit 10(d.3) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(d) |
Amended and Restated License Agreement between the Registrant and PARTEQ dated March 11, 1998, filed as Exhibit 10(e) to the Registrant’s Form 10-K/A filed on June 18, 1999, portions of Exhibit A have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
52
Table of Contents
| 10(e) |
Incentive Stock Option Plan, filed as Exhibit 10.11 of Registrant’s Registration Statement on Form S-1, No. 33-43282, and is incorporated herein by reference;+ | |
| 10(f) |
1994 Restricted Stock Option Plan, filed as Exhibit 1 to Registrant’s Schedule 14A definitive Proxy Statement dated April 26, 1995, and is incorporated herein by reference;+ | |
| 10(g) |
1996 Omnibus Plan, as amended, filed as Appendix A to Registrant’s Schedule 14A Definitive Proxy Statement dated April 26, 2001, and is incorporated herein by reference;+ | |
| 10(g.1) |
1996 Omnibus Plan, as amended on May 1, 2003, filed as Exhibit 10(h.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2003, and is incorporated herein by reference;+ | |
| 10(g.2) |
1996 Omnibus Plan, as amended April 23, 2004, filed as Appendix A to Registrant’s Schedule 14A definitive Proxy Statement dated April 28, 2004, and is incorporated herein by reference;+ | |
| 10(h) |
2011 Amended and Restated Purchase and Supply Agreement between the Registrant and National Biological Corporation dated as of November 29, 2011, portions of which have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended; | |
| 10(i) |
Supply Agreement between the Registrant and Sochinaz SA dated December 24, 1993, filed as Exhibit 10.1 to Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2011, and is incorporated herein by reference; | |
| 10(i.1) |
First Amendment to Supply Agreement between the Registrant and Sochinaz SA dated July 7, 1994, filed as Exhibit 10(q.1) to Registrant’s Annual Report on Form 10-K for the fiscal year ended December 31, 1999, and is incorporated herein by reference; | |
| 10(i.2) |
Second Amendment to Supply Agreement between the Registrant and Sochinaz SA dated as of June 20, 2000, filed as Exhibit 10.1 to Registrant’s Current Report on Form 8-K dated June 28, 2000, and is incorporated herein by reference; | |
| 10(i.3) |
Third Amendment to Supply Agreement between the Registrant and Sochinaz SA dated July 29, 2005, filed as Exhibit 10.1 to the Registrant’s Form 10-Q filed on August 3, 2005, portions of which have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
| 10(i.4) |
Fifth Amendment to Supply Agreement between the Registrant and Sochinaz SA dated September 10, 2009, filed as Exhibit 10 to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2009, portions of which have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
| 10(j) |
Master Service Agreement between the Registrant and Therapeutics, Inc. dated as of October 4, 2001, filed as Exhibit 10(b) to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2001, filed November 8, 2001, portions of which have been omitted pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
| 10(k) |
License and Development Agreement between the Registrant and photonamic GmbH & Co. KG dated as of December 30, 2002, filed as Exhibit 10(r) to Registrant’s Annual Report on Form 10-K for the fiscal year ended December 31, 2002, portions of which have been omitted pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
| 10(l) |
Supply Agreement between the Registrant and medac GmbH dated as of December 30, 2002, filed as Exhibit 10(r) to Registrant’s Annual Report on Form 10-K for the fiscal year ended December 31, 2002, portions of which have been omitted pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
53
Table of Contents
| 10(m) |
License and Supply Agreement dated August 7, 2007 among the Registrant, photonamic GmbH & Co. KG and medac, GmbH, portions of which have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, filed as Exhibit 10 to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2007 and is incorporated herein by reference; | |
| 10(n) |
License, Promotion, Distribution and Supply Agreement between the Registrant and Coherent-AMT dated as of March 31, 2004 filed as Exhibit 10(a) to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2004, filed May 4, 2004, and is incorporated herein by reference; | |
| 10(o) |
Employment Agreement of Scott L. Lundahl dated as of June 23, 1999 filed as Exhibit 10(u) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(o.1) |
Amendment No. 1 to Employment Agreement of Scott Lundahl dated as of April 10, 2008, filed as Exhibit 10(s.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(p) |
Amended Employment Agreement of Stuart L. Marcus, MD, PhD dated December 9, 1999 filed as Exhibit 10(v) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(p.1) |
Amendment No. 2 to Employment Agreement of Stuart L. Marcus, MD, PhD dated as of April 10, 2008, filed as Exhibit 10(t.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(q) |
Employment Agreement of Mark C. Carota dated as of February 14, 2000 filed as Exhibit 10(w.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(q.1) |
First Amendment to Employment Agreement of Mark C. Carota dated October 31, 2001 filed as Exhibit 10(w.2) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(q.2) |
Amendment No. 2 to Employment Agreement of Mark C. Carota dated as of April 10, 2008, filed as Exhibit 10(u.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(r) |
Employment Agreement of Richard Christopher dated as of January 1, 2004 filed as Exhibit 10(y) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(s) |
Amendment No. 1 to Employment Agreement of Richard Christopher dated as of October 18, 2006 filed as Exhibit 10.A to the Registrant’s Form 10-Q for the fiscal quarter ended September 30, 2004, and is incorporated herein by reference; | |
| 10(s.1) |
Amendment No. 2 to Employment Agreement of Richard Christopher dated as of April 10, 2008, filed as Exhibit 10(w.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(t) |
Employment Agreement of Robert F. Doman dated as of December 29, 2004 filed as Exhibit 10(z) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(u) |
First Amendment to Employment Agreement of Robert F. Doman dated November 26, 2008, filed as Exhibit 10(x.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
54
Table of Contents
| 10(v) |
Compensation Policy Applicable to the Registrant’s Non-Employee Directors filed as Exhibit 10(cc) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(w) |
Employment Agreement of William O’Dell dated as of April 4, 2006 filed as Exhibit 10(ii) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference; | |
| 10(w.1) |
Amendment No. 1 to Employment Agreement of William O’Dell dated as of April 10, 2008, filed as Exhibit 10(jj.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(x) |
Patent License Agreement between the Registrant and PhotoCure ASA, dated as of May 30, 2006, portions of which have been omitted pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended, filed as Exhibit 10.A to the Registrant’s Form 10-Q for the fiscal quarter ended June 30, 2006, and is incorporated herein by reference; | |
| 10(y) |
Employment Agreement of Michael Todisco dated as of September 20, 2006 filed as Exhibit 10(11) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference; | |
| 10(y.1) |
Amendment No. 1 to Employment Agreement of Michael Todisco dated as of April 10, 2008, filed as Exhibit 10(mm.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(z) |
DUSA Pharmaceuticals, Inc. 2006 Equity Compensation Plan, filed as Appendix A to Registrant’s Schedule 14A definitive Proxy Statement dated April 24, 2006, and is incorporated herein by reference;+ | |
| 10(aa) |
DUSA Pharmaceuticals, Inc. 2006 Equity Compensation Plan, as amended October 18, 2006 filed as Exhibit 10(pp) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference;+ | |
| 10(bb) |
DUSA Pharmaceuticals, Inc. 2006 Deferred Compensation Plan, October 18, 2006 filed as Exhibit 10(qq) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference;+ | |
| 10(cc) |
Asset Purchase Agreement between Registrant and Acella Pharmaceuticals LLC dated June 30, 2011 portions of which have been omitted pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Act of 1934, as amended, filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2011, and is incorporated herein by reference; and | |
| 10(dd) |
DUSA Pharmaceuticals, Inc. Amended and Restated 2011 Equity Compensation Plan, filed as Appendix A to Registrant’s Schedule 14A definitive Proxy Statement dated April 20, 2011, and is incorporated herein by reference.+ | |
| 14(a) |
Form of DUSA Pharmaceuticals, Inc. Code of Ethics Applicable to Senior Officers, filed as Exhibit 14(a) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference. | |
| 21(a) |
Subsidiaries of the Registrant. | |
| 23(a) |
Consent of Independent Registered Public Accounting Firm. | |
| 31(a) |
Rule 13a-14(a)/15d-14(a) Certification of the Chief Executive Officer; and | |
| 31(b) |
Rule 13a-14(a)/15d-14(a) Certification of the Chief Financial Officer. | |
55
Table of Contents
| 32(a) |
Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002; and | |
| 32(b) |
Certification of the Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 99.1 |
Press Release dated March 6, 2012. | |
| 101.INS |
XBRL Instance Document | |
| 101.SCH |
XBRL Taxonomy Extension Schema | |
| 101.CAL |
XBRL Taxonomy Extension Calculation Linkbase | |
| 101.LAB |
XBRL Taxonomy Extension Label Linkbase | |
| 101.PRE |
XBRL Taxonomy Extension Presentation Linkbase | |
| 101.DEF |
XBRL Taxonomy Extension Definition Document | |
| + | Management contract or compensatory plan or arrangement. |
| * | Schedules and exhibits omitted pursuant to Item 601(b)(2) of Regulation S-K. The Registrant agrees to furnish supplementally a copy of any omitted schedule or exhibit to the Commission upon request. |
56
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of DUSA Pharmaceuticals, Inc.
Wilmington, Massachusetts
We have audited the accompanying consolidated balance sheets of DUSA Pharmaceuticals, Inc. and subsidiaries (the “Company”) as of December 31, 2011 and 2010, and the related consolidated statements of operations, shareholders’ equity and comprehensive income (loss), and cash flows for each of the three years in the period ended December 31, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of DUSA Pharmaceuticals, Inc. and subsidiaries as of December 31, 2011 and 2010, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2011, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2011, based on the criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 6, 2012 expressed an unqualified opinion on the Company’s internal control over financial reporting.
/s/ Deloitte & Touche LLP
Boston, Massachusetts
March 6, 2012
F-1
Table of Contents
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS |
||||||||
| Cash and cash equivalents |
$ | 24,423,682 | $ | 8,884,402 | ||||
| Marketable securities, at fair value |
3,791,942 | 10,762,559 | ||||||
| Accounts receivable, net of allowance for doubtful accounts of $50,000 and $35,000 in 2011 and 2010, respectively |
3,729,303 | 3,225,296 | ||||||
| Inventory |
2,823,173 | 2,047,926 | ||||||
| Prepaid and other current assets |
1,380,763 | 1,344,062 | ||||||
| Current assets of discontinued operations |
38,671 | 203,465 | ||||||
|
|
|
|
|
|||||
| TOTAL CURRENT ASSETS |
36,187,534 | 26,467,710 | ||||||
| Restricted cash |
175,810 | 174,753 | ||||||
| Property, plant and equipment, net |
1,601,101 | 1,582,777 | ||||||
| Deferred charges and other assets |
57,833 | 68,099 | ||||||
|
|
|
|
|
|||||
| TOTAL ASSETS |
$ | 38,022,278 | $ | 28,293,339 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES |
||||||||
| Accounts payable |
$ | 803,639 | $ | 160,742 | ||||
| Accrued compensation |
2,351,342 | 2,243,997 | ||||||
| Other accrued expenses |
2,459,562 | 1,967,293 | ||||||
| Deferred revenues |
— | 712,338 | ||||||
| Current liabilities of discontinued operations |
851,775 | 383,545 | ||||||
|
|
|
|
|
|||||
| TOTAL CURRENT LIABILITIES |
6,466,318 | 5,467,915 | ||||||
| Deferred revenues |
900,769 | 1,917,237 | ||||||
| Warrant liability |
2,216,763 | 1,203,553 | ||||||
| Other liabilities |
157,238 | 181,153 | ||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES |
9,741,088 | 8,769,858 | ||||||
|
|
|
|
|
|||||
| COMMITMENTS AND CONTINGENCIES (NOTE 13) |
||||||||
| SHAREHOLDERS’ EQUITY |
||||||||
| Capital stock authorized: 100,000,000 shares; 40,000,000 shares designated as common stock, no par, and 60,000,000 shares issuable in series or classes; and 40,000 junior Series A preferred shares. Issued and outstanding: 24,649,614 and 24,239,365 shares of common stock, no par, at December 31, 2011 and December 31, 2010, respectively |
151,985,930 | 151,703,468 | ||||||
| Additional paid-in capital |
10,606,654 | 9,399,434 | ||||||
| Accumulated deficit |
(134,336,998 | ) | (141,656,600 | ) | ||||
| Accumulated other comprehensive income |
25,604 | 77,179 | ||||||
|
|
|
|
|
|||||
| TOTAL SHAREHOLDERS’ EQUITY |
28,281,190 | 19,523,481 | ||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY |
$ | 38,022,278 | $ | 28,293,339 | ||||
|
|
|
|
|
|||||
See the accompanying Notes to the Consolidated Financial Statements.
F-2
Table of Contents
CONSOLIDATED STATEMENTS OF OPERATIONS
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Product revenues |
$ | 45,296,065 | $ | 36,423,171 | $ | 28,338,168 | ||||||
| Cost of product revenues |
6,921,906 | 6,419,446 | 6,007,034 | |||||||||
|
|
|
|
|
|
|
|||||||
| GROSS MARGIN |
38,374,159 | 30,003,725 | 22,331,134 | |||||||||
| Operating costs: |
||||||||||||
| Research and development |
5,275,001 | 4,929,622 | 4,313,313 | |||||||||
| Marketing and sales |
15,382,721 | 13,240,543 | 12,897,286 | |||||||||
| General and administrative |
9,956,608 | 9,105,846 | 7,956,410 | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL OPERATING COSTS |
30,614,330 | 27,276,011 | 25,167,009 | |||||||||
|
|
|
|
|
|
|
|||||||
| INCOME (LOSS) FROM OPERATIONS |
7,759,829 | 2,727,714 | (2,835,875 | ) | ||||||||
| Other income |
46,446 | 226,055 | 290,681 | |||||||||
| Loss on change in fair value of warrants |
(1,013,210 | ) | (390,648 | ) | (376,447 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| INCOME (LOSS) FROM CONTINUING OPERATIONS, BEFORE INCOME TAX BENEFIT |
6,793,065 | 2,563,121 | (2,921,641 | ) | ||||||||
| Income tax benefit |
— | — | 162,661 | |||||||||
|
|
|
|
|
|
|
|||||||
| INCOME (LOSS) FROM CONTINUING OPERATIONS |
6,793,065 | 2,563,121 | (2,758,980 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| INCOME FROM DISCONTINUED OPERATIONS, NET OF TAX PROVISION |
526,537 | 139,496 | 250,688 | |||||||||
|
|
|
|
|
|
|
|||||||
| NET INCOME (LOSS) |
$ | 7,319,602 | $ | 2,702,617 | $ | (2,508,292 | ) | |||||
|
|
|
|
|
|
|
|||||||
| BASIC NET INCOME (LOSS) PER SHARE — CONTINUING OPERATIONS |
$ | 0.28 | $ | 0.11 | $ | (0.11 | ) | |||||
|
|
|
|
|
|
|
|||||||
| DISCONTINUED OPERATIONS |
0.02 | 0.01 | 0.01 | |||||||||
|
|
|
|
|
|
|
|||||||
| BASIC NET INCOME (LOSS) PER SHARE |
$ | 0.30 | $ | 0.11 | $ | (0.10 | ) | |||||
|
|
|
|
|
|
|
|||||||
| DILUTED NET INCOME (LOSS) PER SHARE — CONTINUING OPERATIONS |
$ | 0.26 | $ | 0.10 | $ | (0.11 | ) | |||||
|
|
|
|
|
|
|
|||||||
| DISCONTINUED OPERATIONS |
0.02 | 0.01 | 0.01 | |||||||||
|
|
|
|
|
|
|
|||||||
| DILUTED NET INCOME (LOSS) PER SHARE |
$ | 0.28 | $ | 0.11 | $ | (0.10 | ) | |||||
|
|
|
|
|
|
|
|||||||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING, BASIC |
24,531,798 | 24,188,163 | 24,102,085 | |||||||||
|
|
|
|
|
|
|
|||||||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING, DILUTED |
26,352,535 | 24,765,910 | 24,134,627 | |||||||||
|
|
|
|
|
|
|
|||||||
See the accompanying Notes to the Consolidated Financial Statements.
F-3
Table of Contents
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY AND COMPREHENSIVE INCOME (LOSS)
| Common Stock | Additional Paid-in Capital |
Accumulated Deficit |
Accum.
Other Comprehensive Income |
Total | ||||||||||||||||||||
| Number of Shares |
Amount | |||||||||||||||||||||||
| Balance, January 1, 2009 |
24,089,452 | 151,663,943 | 7,514,900 | (141,850,925 | ) | 384,281 | 17,712,199 | |||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||
| Net loss |
(2,508,292 | ) | (2,508,292 | ) | ||||||||||||||||||||
| Net unrealized gain on marketable securities available-for-sale |
(158,794 | ) | (158,794 | ) | ||||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total comprehensive loss |
$ | (2,667,086 | ) | |||||||||||||||||||||
| Share-based compensation expense |
800,774 | 800,774 | ||||||||||||||||||||||
| Vesting of common stock grants |
22,750 | 22,750 | (22,750 | ) | ||||||||||||||||||||
| Settlements of restricted stock for tax withholding obligations |
(3,294 | ) | (3,294 | ) | (1,119 | ) | (4,413 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2009 |
24,108,908 | 151,683,399 | 8,291,805 | (144,359,217 | ) | 225,487 | 15,841,474 | |||||||||||||||||
| Comprehensive loss: |
||||||||||||||||||||||||
| Net loss |
2,702,617 | 2,702,617 | ||||||||||||||||||||||
| Net unrealized loss on marketable securities available-for-sale |
(148,308 | ) | (148,308 | ) | ||||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total comprehensive loss |
$ | 2,554,309 | ||||||||||||||||||||||
| Share-based compensation expense |
1,107,629 | 1,107,629 | ||||||||||||||||||||||
| Exercises of options |
38,300 | 62,560 | 62,560 | |||||||||||||||||||||
| Vesting of common stock grants |
115,250 | |||||||||||||||||||||||
| Settlements of restricted stock for tax withholding obligations |
(23,093 | ) | (42,491 | ) | (42,491 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2010 |
24,239,365 | $ | 151,703,468 | $ | 9,399,434 | $ | (141,656,600 | ) | $ | 77,179 | $ | 19,523,481 | ||||||||||||
| Comprehensive income: |
||||||||||||||||||||||||
| Net income |
7,319,602 | 7,319,602 | ||||||||||||||||||||||
| Net unrealized loss on marketable securities available-for-sale |
(51,575 | ) | (51,575 | ) | ||||||||||||||||||||
|
|
|
|||||||||||||||||||||||
| Total comprehensive income |
$ | 7,268,027 | ||||||||||||||||||||||
| Share-based compensation expense |
1,207,220 | 1,207,220 | ||||||||||||||||||||||
| Exercises of options |
273,588 | 516,219 | 516,219 | |||||||||||||||||||||
| Vesting of common stock grants |
191,250 | |||||||||||||||||||||||
| Settlements of restricted stock for tax withholding obligations |
(54,589 | ) | (233,757 | ) | (233,757 | ) | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance, December 31, 2011 |
24,649,614 | $ | 151,985,930 | $ | 10,606,654 | $ | (134,336,998 | ) | $ | 25,604 | $ | 28,281,190 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
See the accompanying Notes to the Consolidated Financial Statements.
F-4
Table of Contents
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| CASH FLOWS PROVIDED BY (USED IN) OPERATING ACTIVITIES |
||||||||||||
| Net income (loss) |
$ | 7,319,602 | $ | 2,702,617 | $ | (2,508,292 | ) | |||||
| Less: Income from discontinued operations |
(526,537 | ) | (139,496 | ) | (250,688 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net income (loss) from continuing operations |
6,793,065 | 2,563,121 | (2,758,980 | ) | ||||||||
| Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities: |
||||||||||||
| Accretion of premiums and discounts on marketable securities |
(13,126 | ) | 2,388 | 46,145 | ||||||||
| Realized loss on sales of marketable securities |
— | — | 43,678 | |||||||||
| Share-based compensation |
1,207,220 | 1,107,629 | 800,774 | |||||||||
| Depreciation and amortization |
445,233 | 393,611 | 455,528 | |||||||||
| Loss on change in fair value of warrants |
1,013,210 | 390,648 | 376,447 | |||||||||
| Deferred revenues recognized |
(1,728,806 | ) | (1,179,042 | ) | (960,290 | ) | ||||||
| Changes in other assets and liabilities impacting cash flows from operations: |
||||||||||||
| Accounts receivable |
(504,007 | ) | (735,007 | ) | (327,416 | ) | ||||||
| Inventory |
(775,247 | ) | 20,999 | 432,322 | ||||||||
| Prepaids and other assets |
(26,435 | ) | 217,405 | 404,935 | ||||||||
| Accounts payable |
642,897 | (464,045 | ) | 335,193 | ||||||||
| Accrued compensation and other accrued expenses |
599,614 | 1,154,851 | (871,773 | ) | ||||||||
| Other liabilities |
(23,915 | ) | (41,863 | ) | (121,656 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| NET CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES FROM CONTINUING OPERATIONS |
7,629,703 | 3,430,695 | (2,145,093 | ) | ||||||||
| NET CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES FROM DISCONTINUED OPERATIONS |
409,561 | (106,313 | ) | 362,676 | ||||||||
|
|
|
|
|
|
|
|||||||
| NET CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES |
8,039,264 | 3,324,382 | (1,782,417 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| CASH FLOWS PROVIDED BY (USED IN) INVESTING ACTIVITIES |
||||||||||||
| Purchases of marketable securities |
(4,497,832 | ) | (12,742,296 | ) | (12,049,905 | ) | ||||||
| Proceeds from maturities and sales of marketable securities |
11,430,000 | 10,885,000 | 17,748,159 | |||||||||
| Restricted cash |
(1,057 | ) | (498 | ) | (411 | ) | ||||||
| Purchases of property, plant and equipment |
(463,557 | ) | (215,633 | ) | (178,308 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES FROM CONTINUING OPERATIONS |
6,467,554 | (2,073,427 | ) | 5,519,535 | ||||||||
| NET CASH PROVIDED BY INVESTING ACTIVITIES FROM DISCONTINUED OPERATIONS |
750,000 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES |
7,217,554 | (2,073,427 | ) | 5,519,535 | ||||||||
|
|
|
|
|
|
|
|||||||
| CASH FLOWS PROVIDED BY (USED IN) FINANCING ACTIVITIES |
||||||||||||
| Proceeds from exercise of options |
516,219 | 62,560 | — | |||||||||
| Settlements of restricted stock for tax withholding obligations |
(233,757 | ) | (42,491 | ) | (4,413 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| NET CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES |
282,462 | 20,069 | (4,413 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| NET INCREASE IN CASH AND CASH EQUIVALENTS |
15,539,280 | 1,271,024 | 3,732,705 | |||||||||
| Less (increase) decrease in cash and cash equivalents from discontinued operations |
(1,159,561 | ) | 106,313 | (362,676 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Increase (decrease) in cash and cash equivalents from continuing operations |
14,379,719 | 1,377,337 | 3,370,029 | |||||||||
| CASH AND CASH EQUIVALENTS AT BEGINNING OF YEAR |
8,884,402 | 7,613,378 | 3,880,673 | |||||||||
|
|
|
|
|
|
|
|||||||
| CASH AND CASH EQUIVALENTS AT END OF YEAR |
$ | 24,423,682 | $ | 8,884,402 | $ | 7,613,378 | ||||||
|
|
|
|
|
|
|
|||||||
See the accompanying Notes to the Consolidated Financial Statements.
F-5
Table of Contents
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
1) NATURE OF BUSINESS
DUSA Pharmaceuticals, Inc. (“DUSA” or the “Company”) is a vertically integrated dermatology company that is developing and marketing Levulan® photodynamic therapy (“PDT”) and other products for common skin conditions. The Company is based in Wilmington, Massachusetts. The Company’s marketed products include, among others, Levulan® Kerastick® 20% Topical Solution with PDT and the BLU-U® brand light source.
The Levulan® Kerastick® 20% Topical Solution with PDT and the BLU-U® brand light source were launched in the United States, or U.S., in September 2000 for the treatment of non-hyperkeratotic actinic keratoses, or AKs, of the face or scalp. AKs are precancerous skin lesions caused by chronic sun exposure that can develop over time into a form of skin cancer called squamous cell carcinoma. In addition, in September 2003, the Company received clearance from the U.S. Food and Drug Administration, or FDA, to market the BLU-U® without Levulan® PDT for the treatment of moderate inflammatory acne vulgaris and general dermatological conditions.
Following its acquisition of Sirius Laboratories, Inc., which occurred in 2006, the Company had operated in two segments, Photodynamic Therapy (“PDT”) Drug and Device Products and Non-Photodynamic Therapy (“Non-PDT”) Drug Products. The Company’s Levulan® Kerastick® and BLU-U® products comprised its PDT segment, while its Non-PDT segment was comprised of the products acquired in the acquisition of Sirius. At December 31, 2011, the Company ceased marketing and selling its remaining Non-PDT products, which were ClindaReach® and Meted®. The former Non-PDT Drug segment is now reflected as discontinued operations for all periods presented.
2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
a) Principles of Consolidation — The Company’s consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, DUSA Pharmaceuticals New York, Inc. and Sirius Laboratories, Inc. All intercompany balances and transactions have been eliminated in consolidation.
b) Basis of Presentation and Use of Estimates — These financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). Such principles require management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
c) Cash and Cash Equivalents — Cash equivalents include short-term highly liquid money market funds. All other investments are classified as marketable securities. The Company maintained cash of $176,000 and $175,000 at December 31, 2011 and 2010, respectively, in a separate bank account in support of a letter of credit of $172,000 that was issued in lieu of a security deposit on the lease for its manufacturing facility in Wilmington, Massachusetts. The cash is presented in restricted cash as a non-current asset in the Consolidated Balance Sheets.
d) Marketable Securities — The Company records marketable securities at fair value as available-for-sale with unrealized holding gains (losses) recorded in accumulated other comprehensive income. The Company amortizes or accretes the premiums and discounts paid for the securities into interest income over the period to maturity of the securities. As the Company’s marketable securities are available to fund operations and as management may sell a portion of its marketable securities in the next fiscal year in order to meet its working capital requirements, all marketable securities are classified as current assets. Realized gains and losses are determined on the specific identification method.
F-6
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
e) Inventory — Inventory is stated at the lower of cost (first-in, first-out method) or market. Inventory identified for research and development activities is expensed in the period in which such inventory is designated for such use. BLU-U® commercial light sources placed in physicians’ offices for an initial evaluation period are included in inventory in the accompanying Consolidated Balance Sheets and amortized over a three year period or until sold to the physician’s office evidenced by the fact that all revenue recognition criteria have been met. Inventories are continually reviewed for slow moving, obsolete and excess items. Sales projections are used to estimate the appropriate level of inventory reserves, if any, that are necessary at each balance sheet date.
f) Property, Plant and Equipment — Property, plant and equipment is carried at cost less accumulated depreciation and amortization. Depreciation is computed on a straight-line basis over the estimated lives of the related assets. Leasehold improvements are amortized over the lesser of their useful lives or the lease terms.
g) Valuation of Long-Lived Assets — The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of a long-lived asset may not be recoverable or that the useful lives of these assets are no longer appropriate. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. When it is determined that the carrying value of a long-lived asset is not recoverable, the asset is written down to its estimated fair value on a discounted cash flow basis. There have been no impairment charges recorded for long-lived assets in the Consolidated Statements of Operations in 2011, 2010, or 2009.
h) Goodwill and Other Intangible Assets — At December 31, 2011, the Company has no goodwill or intangible assets. In connection with the acquisition of Sirius Laboratories, Inc., (which became the Company’s Non-PDT segment), the Company recorded goodwill of approximately $8.3 million. In fiscal years prior to 2009, impairment charges for goodwill were recorded that totaled approximately $8.3 million.
In 2008 and 2007, we recorded impairment charges to goodwill of $6.8 million and $1.5 million, respectively. The impairment charges were related to our review of the carrying amounts of our goodwill related to our former Non-PDT segment, which is reflected in Discontinued Operations for all periods presented.
i) Revenue Recognition —Revenues on Kerastick® and BLU-U® product sales in the U.S. and Canada are recognized when persuasive evidence of an arrangement exists, the price is fixed and determinable, delivery has occurred, and collection is reasonably assured. DUSA offers programs that allow physicians access to our BLU-U® device for a trial period. No revenue is recognized on these units until the physician elects to purchase the equipment and all other revenue recognition criteria are met. DUSA’s terms with customers do not provide for the right of return for sales of Kerastick® and BLU-U®, unless the product does not comply with the technical specifications.
For revenues associated with contractual agreements with multiple elements, the Company applies the revenue recognition criteria outlined in Securities and Exchange Commission (“SEC”) Staff Accounting Bulletin Topic 13, Revenue Recognition (“SAB Topic 13”) and ASC 605-25, Multiple Element Arrangements. Each contract is analyzed in order to separate each deliverable into separate units of accounting, if applicable, and then recognize revenue for those separated units as earned. Significant judgment is required in determining the units of accounting and the attribution method for such arrangements.
The Company previously had exclusive marketing, distribution and supply agreements with distributors in Asia Pacific and Latin America. These agreements were terminated by the Company in 2011 and 2010 in Asia Pacific and Latin America, respectively. These agreements had contained multiple deliverables, which were treated as a single unit of accounting utilizing the attribution method for each of the separate payment streams.
F-7
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Under these agreements, the Company received non-refundable milestone payments, a fixed price per unit sold and royalties based on a percentage of net sales to end-users. Prior to termination, the milestones had been deferred and recognized as license revenues on a straight-line basis over the contractual terms of the agreements, and the fixed price and the royalty payments were recognized upon sell through to end users. The terminations resulted in the acceleration of the recognition of deferred revenues in 2011 and 2010 of $1,379,000 and $555,000, respectively. The terminations of these agreements, and the resulting accounting, are further described in Note 9 to the Consolidated Financial Statements “Significant Product Agreements”.
j) Warranty Costs — The Company accrues for estimated future warranty costs on its BLU-U® sales at the time of sale. The Company’s products are subject to rigorous regulation and quality standards. Warranty costs were immaterial in the periods presented.
k) Research and Development Costs — Costs related to the conceptual formulation and design of products and processes are expensed as research and development costs as incurred. Purchased technology, including the costs of licensed technology for a particular research project that do not have alternative future uses, is expensed as incurred.
l) Marketing and Sales Costs — Costs included in marketing and sales consist mainly of overhead expenses such as salaries and benefits for the marketing and sales staff, commissions, and related support expenses such as travel, and telephone, as well as costs related to trade shows costs, miscellaneous marketing and outside consultants. All such costs are expensed as incurred. Advertising costs were immaterial in the periods presented.
m) Income Taxes — The Company recognizes deferred income tax assets and liabilities for the expected future tax consequences for events that have been included in the Company’s financial statements or tax returns. Deferred tax assets and liabilities are based on the difference between the financial statement and tax bases of assets and liabilities using tax rates expected to be in effect in the years in which these differences are expected to reverse. A valuation allowance is provided to reduce the deferred tax assets to the amount that will more likely than not be realized.
The Company determines whether it is more likely than not that a tax position will be sustained upon examination. If it is not more likely than not that a position will be sustained, no amount of the benefit attributable to the position is recognized. The tax benefit to be recognized for any tax position that meets the more likely than not recognition threshold is calculated as the largest amount that is more than 50% likely of being realized upon resolution of the contingency.
The Company recognizes interest and penalties related to unrecognized tax benefits in operating expenses. Since a full valuation allowance was recorded against the Company’s net deferred tax assets and the unrecognized tax benefits would not result in a tax liability, the Company has not accrued for any interest and penalties relating to these unrecognized tax benefits.
n) Basic and Diluted Net Income (Loss) Per Common Share — Basic net income (loss) per common share is based on the weighted-average number of common shares outstanding during each period. Diluted net income (loss) is based on the weighted-average shares outstanding and any contingently issuable shares. The net outstanding shares are adjusted for the dilutive effect of shares issuable upon the assumed conversion of the Company’s common stock equivalents, which consist of outstanding stock options, warrants and unvested shares of common stock using the treasury stock method.
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Weighted average common shares outstanding-basic |
24,531,798 | 24,188,163 | 24,102,085 | |||||||||
| Stock options and unvested shares of common stock |
1,820,737 | 577,747 | 32,542 | |||||||||
|
|
|
|
|
|
|
|||||||
| Weighted average common shares outstanding-diluted |
26,352,535 | 24,765,910 | 24,134,627 | |||||||||
|
|
|
|
|
|
|
|||||||
F-8
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The following were not included in weighted average common shares outstanding because they are anti-dilutive:
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Stock options |
893,000 | 2,837,000 | 2,660,000 | |||||||||
| Warrants |
— | 1,395,000 | 1,395,000 | |||||||||
| Unvested shares of common stock |
21,000 | 236,000 | 364,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
914,000 | 4,468,000 | 4,419,000 | |||||||||
|
|
|
|
|
|
|
|||||||
o) Share-Based Compensation — The Company’s stock-based compensation cost is measured at the grant date based on the fair value of the award and is recognized as expense over the requisite service period, which generally represents the vesting period, and includes an estimate of the awards that will be forfeited. The Company uses the Black-Scholes valuation model for estimating the fair value on the date of grant of stock options.
p) Comprehensive Income (Loss) — The Company has reported accumulated comprehensive income (loss) and its components as part of its Consolidated Statements of Shareholders’ Equity and Comprehensive Income. Comprehensive income (loss), apart from net income (loss), relates to net unrealized gains and losses on marketable securities.
q) Segment Reporting — At December 31, 2011, the Company ceased the marketing and selling of its Non-PDT products, ClindaReach® and Meted®, and as a result, the former Non-PDT Drug Products segment has been classified as discontinued operations in the accompanying Consolidated Financial Statements for all periods presented. The Company is now managed as a single operating segment.
r) Concentrations — The Company invests cash in accordance with a policy objective that seeks to preserve both liquidity and safety of principal. The Company manages the credit risk associated with its investments in marketable securities by investing in U.S. government securities and investment grade corporate bonds. The Company’s exposure to credit risk relating to its accounts receivable is limited. To manage credit risk in accounts receivable, the Company performs regular credit evaluations of its customers and provides allowances for potential credit losses, when applicable. The Company is dependent upon sole-source suppliers for a number of its products. There can be no assurance that these suppliers will be able to meet the Company’s future requirements for such products or parts or that they will be available at favorable terms.
s) Derivative Financial Instruments — The Company has issued common stock warrants in connection with the October 2007 private placement (See Note 8). The warrants are accounted for as derivative liabilities at fair value. Changes in fair value of derivative liabilities are recorded in the Consolidated Statements of Operations under the caption “Loss on change in fair value of warrants.” The fair value of the warrant liability is determined using the Black-Scholes option-pricing model. The fair value of the warrants is subject to significant fluctuation based on changes in the Company’s stock price, expected volatility, remaining contractual life and the risk-free interest rate.
t) Discontinued Operations — At December 31, 2011, the Company ceased the marketing and selling of its Non-PDT products, ClindaReach® and Meted®, and as a result, the former Non-PDT Drug Products segment has been classified as discontinued operations in the accompanying financial statements for all periods presented.
F-9
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The remaining asset is primarily accounts receivable and the remaining liabilities are primarily for the returns of product from wholesalers, for the final milestone payment to the former Sirius shareholders, and for the final payment to a licensor. As of December 31, 2011, the carrying value of Non-PDT inventory was $0. All of the intangible asset value related to the Non-PDT segment, including goodwill, was previously deemed to be impaired and was written down to zero in prior years.
3) FINANCIAL INSTRUMENTS
Fair Value Measurements
The Company’s financial instruments at December 31, 2011 and 2010 consisted primarily of cash and cash equivalents, accounts receivable, marketable securities, accounts payable, and warrant liability. The Company believes the carrying value of accounts receivable and accounts payable approximates their fair values due to their short-term nature.
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. In order to increase consistency and comparability in fair value measurements, financial instruments are categorized based on a hierarchy that prioritizes observable and unobservable inputs used to measure fair value into three broad levels, which are described below:
| Level 1: | Quoted market prices in active markets for identical assets or liabilities. Level 1 primarily consists of financial instruments whose value is based on quoted market prices such as exchange-traded instruments and listed equities. | |
| Level 2: | Observable market based inputs or unobservable inputs that are corroborated by market data. Level 2 consists of financial instruments that are valued using quoted market prices, broker or dealer quotations, or alternative pricing sources with reasonable levels of price transparency in the determination of value. The Company accesses publicly available market activity from third party databases and credit ratings of the issuers of the securities it holds to corroborate the data used in the fair value calculations obtained from its primary pricing source. The Company also takes into account credit rating changes, if any, of the securities or recent marketplace activity. | |
| Level 3: | Unobservable inputs that are not corroborated by market data. Level 3 is comprised of financial instruments whose fair value is estimated based on internally developed models or methodologies utilizing significant inputs that are generally less readily observable. The warrant liability was recorded initially at its fair value using the Black-Scholes option-pricing model and is revalued at each reporting date until the warrants are exercised or expire. The fair value of the warrants is subject to significant fluctuation based on changes in our stock price, expected volatility, remaining contractual life and the risk-free interest rate. | |
The Company’s cash equivalents and investments are classified within Level 1 or Level 2 of the fair value hierarchy because they are valued using quoted market prices, or broker dealer quotations and matrix pricing compiled by third party pricing vendors, respectively, which are based on third party pricing sources with reasonable levels of price transparency. The Company’s investments are valued based on a market approach in which all significant inputs are observable or can be derived from or corroborated by observable market data such as interest rates, yield curves, and credit risk.
F-10
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The following table presents the Company’s financial instruments recorded at fair value in the Consolidated Balance Sheet, classified according to the three categories described above:
| Fair Value Measurements at December 31, 2011 | ||||||||||||||||
| Carrying Value | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
| Assets |
||||||||||||||||
| Cash and cash equivalents |
$ | 24,424,000 | $ | 24,424,000 | — | — | ||||||||||
| United States government-backed securities |
3,569,000 | — | $ | 3,569,000 | — | |||||||||||
| Certificate of Deposit – Restricted Cash |
176,000 | 176,000 | ||||||||||||||
| Corporate debt securities |
223,000 | 223,000 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets |
$ | 28,392,000 | $ | 24,600,000 | $ | 3,792,000 | — | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities |
||||||||||||||||
| Warrant liability |
2,217,000 | — | — | $ | 2,217,000 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
$ | 2,217,000 | $ | — | $ | — | $ | 2,217,000 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Fair Value Measurements at December 31, 2010 | ||||||||||||||||
| Carrying Value | (Level 1) | (Level 2) | (Level 3) | |||||||||||||
| Assets |
||||||||||||||||
| Cash and cash equivalents |
$ | 8,884,000 | $ | 8,884,000 | — | — | ||||||||||
| United States government-backed securities |
9,985,000 | — | $ | 9,985,000 | — | |||||||||||
| Certificate of Deposit – Restricted Cash |
175,000 | 175,000 | ||||||||||||||
| Corporate debt securities |
778,000 | 778,000 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets at fair value |
$ | 19,822,000 | $ | 9,059,000 | $ | 10,763,000 | — | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities |
||||||||||||||||
| Warrant liability |
1,204,000 | — | — | $ | 1,204,000 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities at fair value |
$ | 1,204,000 | $ | — | $ | — | $ | 1,204,000 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The Company reviewed the level classifications of its financial instruments at December 31, 2011 compared to December 31, 2010 and determined that there were no significant transfers between levels in the year ended December 31, 2011.
The table below includes a rollforward of the balance sheet amounts for the years ended December 31, 2011, 2010 and 2009 for the warrant liability, which is classified as Level 3.
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) Year Ended December 31, 2011 |
||||||||||||||||||||||||
| Fair Value at January 1, 2011 |
Total Unrealized Loss Recognized in Statement of Operations |
Purchases, Sales, Issuances, Settlements, Net |
Transfers In and/or Our Out of Level 3 |
Fair Value at December 31 2011 |
Change in Unrealized Loss in 2011 |
|||||||||||||||||||
| Warrant Liability |
$ | 1,204,000 | $ | 1,013,000 | $ | — | $ | — | $ | 2,217,000 | $ | (1,013,000 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
F-11
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) Year Ended December 31, 2010 |
||||||||||||||||||||||||
| Fair Value at January 1, 2010 |
Total Unrealized Loss Recognized in Statement of Operations |
Purchases, Sales, Issuances, Settlements, Net |
Transfers In and/or Our Out of Level 3 |
Fair Value at December 31 2010 |
Change in Unrealized Loss in 2010 |
|||||||||||||||||||
| Warrant Liability |
$ | 813,000 | $ | 391,000 | $ | — | $ | — | $ | 1,204,000 | $ | (391,000 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Fair Value Measurements Using Significant Unobservable Inputs (Level 3) Year Ended December 31, 2009 |
||||||||||||||||||||||||
| Fair Value at January 1, 2009 |
Total Unrealized Loss Recognized in Statement of Operations |
Purchases, Sales, Issuances, Settlements, Net |
Transfers In and/or Our Out of Level 3 |
Fair Value at December 31 2009 |
Change in Unrealized Loss in 2009 |
|||||||||||||||||||
| Warrant Liability |
$ | 436,000 | $ | 377,000 | $ | — | $ | — | $ | 813,000 | $ | (377,000 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Marketable Securities
The Company’s marketable securities consist of the following:
| December 31, 2011 | ||||||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value |
|||||||||||||
| United States government-backed securities |
$ | 3,552,000 | $ | 17,000 | $ | — | $ | 3,569,000 | ||||||||
| Corporate debt securities |
215,000 | 8,000 | — | 223,000 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total marketable securities |
$ | 3,767,000 | $ | 25,000 | $ | — | $ | 3,792,000 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| December 31, 2010 | ||||||||||||||||
| Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Fair Value |
|||||||||||||
| United States government-backed securities |
$ | 9,954,000 | $ | 34,000 | $ | (3,000 | ) | $ | 9,985,000 | |||||||
| Corporate debt securities |
732,000 | 46,000 | — | 778,000 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total marketable securities |
$ | 10,686,000 | $ | 80,000 | $ | (3,000 | ) | $ | 10,763,000 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
The decrease in net unrealized gains on such securities for the years ended December 31, 2011, 2010 and 2009 was $(52,000), $(148,000) and $(159,000), respectively, which has been recorded in accumulated other comprehensive income and is reported as part of shareholders’ equity in the Consolidated Balance Sheets. Realized losses on sales of marketable securities were $0, $0 and $44,000 in 2011, 2010 and 2009, respectively. As of December 31, 2011, current yields range from 0.25% to 4.52% and maturity dates range from June 2012 to January 2013.
F-12
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Common Stock Warrants.
Upon issuance of the warrants on October 29, 2007, the Company recorded the warrant liability at its initial fair value of $1,950,000. Warrants that are classified as a liability are revalued at each reporting date until the warrants are exercised or expire with changes in the fair value reported in the Company’s Consolidated Statements of Operations as gain or loss on fair value of warrants. Non-cash losses for 2011, 2010 and 2009, were $(1,013,000), $(391,000) and $(376,000), respectively. At December 31, 2011 and 2010, the aggregate fair value of these warrants was $2,217,000 and $1,204,000, respectively. Assumptions used for the Black-Scholes option-pricing models in determining the fair value as of December 31, 2011, 2010 and 2009 are as follows:
| December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Expected volatility |
61 | % | 81 | % | 88 | % | ||||||
| Remaining contractual term (years) |
1.3 | 2.3 | 3.3 | |||||||||
| Risk-free interest rate |
0.2 | % | 0.7 | % | 1.9 | % | ||||||
| Expected dividend yield |
0 | % | 0 | % | 0 | % | ||||||
| Common stock price |
$ | 4.38 | $ | 2.45 | $ | 1.6 | ||||||
4) INVENTORY
Inventory consisted of the following at December 31:
| 2011 | 2010 | |||||||
| Finished goods |
$ | 1,110,000 | $ | 790,000 | ||||
|
BLU-U® evaluation units |
225,000 | 129,000 | ||||||
| Work in process |
291,000 | 371,000 | ||||||
| Raw materials |
1,197,000 | 758,000 | ||||||
|
|
|
|
|
|||||
| $ | 2,823,000 | $ | 2,048,000 | |||||
|
|
|
|
|
|||||
BLU-U® commercial light sources placed in physicians’ offices for an initial evaluation period are included in inventory until all revenue recognition criteria are met. The Company amortizes the cost of the evaluation units during the evaluation period of three years to cost of product revenues to approximate its net realizable value.
5) PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment, at cost, consisted of the following at December 31:
| Useful Life | 2011 | 2010 | ||||||||
| (In years) | ||||||||||
| Computer equipment and software |
3 | $ | 3,307,000 | $ | 3,006,000 | |||||
| Furniture, fixtures and equipment |
5 | 1,243,000 | 1,183,000 | |||||||
| Manufacturing facility |
Term of lease | 2,204,000 | 2,204,000 | |||||||
| Manufacturing equipment |
5 | 2,562,000 | 2,490,000 | |||||||
| Leasehold improvements |
Lesser of useful life or term of lease |
992,000 | 962,000 | |||||||
|
|
|
|
|
|||||||
| 10,308,000 | 9,845,000 | |||||||||
| Accumulated depreciation and amortization |
(8,707,000 | ) | (8,262,000 | ) | ||||||
|
|
|
|
|
|||||||
| $ | 1,601,000 | $ | 1,583,000 | |||||||
|
|
|
|
|
|||||||
F-13
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Depreciation and amortization related to property, plant and equipment was $445,000, $394,000, and $456,000 for 2011, 2010 and 2009, respectively.
6) OTHER ACCRUED EXPENSES
Other accrued expenses consisted of the following at December 31:
| 2011 | 2010 | |||||||
| Research and development costs |
$ | 323,000 | $ | 231,000 | ||||
| Marketing and sales costs |
249,000 | 195,000 | ||||||
| Other product related costs |
918,000 | 798,000 | ||||||
| Legal and other professional fees |
363,000 | 308,000 | ||||||
| Employee benefits |
368,000 | 298,000 | ||||||
| Other expenses |
239,000 | 137,000 | ||||||
|
|
|
|
|
|||||
| $ | 2,460,000 | $ | 1,967,000 | |||||
|
|
|
|
|
|||||
7) INCOME TAXES
The tax effect of significant temporary differences representing deferred tax assets and liabilities at December 31, 2011 and 2010 are as follows:
| 2011 | 2010 | |||||||
| DEFERRED TAX ASSETS |
||||||||
| Current |
||||||||
| Reserves |
$ | 134,000 | $ | 64,000 | ||||
| Accrued Charges |
511,000 | 481,000 | ||||||
|
|
|
|
|
|||||
| Total current deferred tax assets |
645,000 | 545,000 | ||||||
| Non-current |
||||||||
| Operating loss carryforwards |
29,091,000 | 32,717,000 | ||||||
| Capitalized research and development |
4,117,000 | 5,431,000 | ||||||
| Research and development tax credit carryforwards |
1,728,000 | 1,844,000 | ||||||
| Deferred revenue |
335,000 | 1,024,000 | ||||||
| Intangible assets |
115,000 | 154,000 | ||||||
| Accrued charges |
318,000 | 225,000 | ||||||
| Stock-based compensation |
1,782,000 | 1,557,000 | ||||||
| Equipment |
684,000 | 782,000 | ||||||
|
|
|
|
|
|||||
| Total noncurrent deferred tax assets |
38,170,000 | 43,734,000 | ||||||
|
|
|
|
|
|||||
| Net deferred tax assets before allowance |
38,815,000 | 44,279,000 | ||||||
|
|
|
|
|
|||||
| Valuation allowance |
(38,815,000 | ) | (44,279,000 | ) | ||||
|
|
|
|
|
|||||
| Total |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
During the years ended December 31, 2011, 2010 and 2009, the change in the valuation allowance was approximately $(5,464,000), $(2,702,000), and $177,000, respectively. The change in the valuation allowance in 2011, 2010 and 2009 was principally due to the utilization of federal and state net operating loss (NOL) carryforwards, federal and state NOL carryforward expirations and a reduction in the temporary differences for
F-14
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
capitalized research and development. During 2011, the Company utilized federal and state net operating loss carryforwards of $4,211,000 and $2,686,000, respectively, to reduce the current tax provision. The Company has generated net operating loss carryforwards from stock compensation deductions and the amount of federal and state excess tax benefits totaling $2,493,000 will be credited to additional paid-in-capital when realized.
Management has concluded that it is not more-likely-than-not that the Company’s deferred tax assets will be realized and therefore the Company has provided a full valuation allowance against the deferred tax assets at December 31, 2011 and December 31, 2010. In concluding that it is not more-likely-than-not that the Company’s deferred tax assets will be realized, the Company evaluated both positive and negative evidence regarding the future realization of these assets. This evaluation requires the Company to make difficult, subjective and complex judgments regarding its projections of taxable income and the amount and expiration dates of its net operating loss carryforwards and other deferred tax assets. In forming its conclusion as to whether the deferred tax assets are more-likely-than-not to be realized, the Company weighed evidence including its historical cumulative profit and loss position, adjusted for non-recurring items, its sources of future taxable income and the projected stability of those sources.
As of December 31, 2011, the Company has federal net operating loss carryforwards of approximately $85,208,000 and research and development tax credits of approximately $1,641,000, both of which, if not utilized, will expire on various dates through 2031 as follows:
| Operating Loss Carryforwards |
Research and Development Tax Credits |
|||||||
| 2011 |
— | — | ||||||
| 2012 |
6,841,000 | — | ||||||
| 2013 |
— | |||||||
| 2014 |
— | — | ||||||
| 2015 |
— | — | ||||||
| 2016 |
— | — | ||||||
| 2017 |
— | — | ||||||
| 2018 |
5,738,000 | — | ||||||
| 2019 |
— | — | ||||||
| 2020 |
— | 110,000 | ||||||
| 2021 |
3,052,000 | 288,000 | ||||||
| 2022 |
16,018,000 | 309,000 | ||||||
| 2023 |
12,872,000 | 148,000 | ||||||
| 2024 |
10,498,000 | 196,000 | ||||||
| 2025 |
13,425,000 | 167,000 | ||||||
| 2026 |
5,923,000 | 114,000 | ||||||
| 2027 |
5,321,000 | 18,000 | ||||||
| 2028 |
1,024,000 | 51,000 | ||||||
| 2029 |
4,496,000 | 80,000 | ||||||
| 2030 |
— | 66,000 | ||||||
| 2031 |
— | 94,000 | ||||||
|
|
|
|
|
|||||
| $ | 85,208,000 | $ | 1,641,000 | |||||
|
|
|
|
|
|||||
As of December 31, 2011, the Company has state net operating loss carryforwards for tax purposes of approximately $20,992,000 which expire on various dates beginning in 2012 through 2030.
F-15
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
Based on an Internal Revenue Code (IRC) Section 382 study performed, the Company determined that it has experienced prior ownership changes, as defined under IRC Section 382, with the most recent change in ownership occurring in 2007 (the 2007 Ownership Change). The Company’s pre-change NOL carryforwards are subject to an annual limitation of approximately $2.2 million per year. Further, additional rules provide for the enhancement of the aforementioned annual limitation for the first five years after the ownership change. A loss corporation may increase its IRC Section 382 limitation by the amount of the net unrealized built-in gain (NUBIG) recognized within five years of the ownership change. The calculated aggregate amount of NUBIG enhancement for the Company is approximately $4.3 million (i.e., approximately $868,000 per year for the first five years after the ownership change). This NUBIG enhancement will be utilized in conjunction with the approximately $2.2 million of IRC Section 382 base annual limitation, resulting in approximately $3.0 million per year for the first five years after the ownership change. Based on these additional factors, the Company estimates that it will be able to utilize approximately $49.9 million of its current net operating losses, provided that sufficient income is generated and no further ownership changes were to occur. However, it is reasonably possible that a future ownership change, which could be the result of transactions involving the Company’s common stock that are outside of its control (such as sales by existing shareholders), could occur during 2012 or thereafter. Future ownership changes could further restrict the utilization of the Company’s net operating losses and tax credits, reducing or eliminating the benefit of such net operating losses and tax credits. An ownership change occurs under IRC Section 382 if the aggregate stock ownership of certain shareholders increases by more than 50 percentage points over such shareholders’ lowest percentage ownership during the testing period, which is generally three years.
Of the $49.9 million of net operating losses available to the Company, approximately $14.4 million, which is comprised of post-2007 Ownership Change net operating losses and accumulated Section 382 limited net operating losses, are available to the Company at December 31, 2011. The available net operating losses will increase annually based on the limitations presented above.
A reconciliation between the effective tax rate and the statutory federal rate is as follows:
| 2011 | % | 2010 | % | 2009 | % | |||||||||||||||||||
| Income tax provision (benefit) at statutory rate |
$ | 2,489,000 | 34.0 | $ | 919,000 | 34.0 | $ | (853,000 | ) | (34.0 | ) | |||||||||||||
| State taxes |
1,465,000 | 19.9 | 564,000 | 20.9 | (61,000 | ) | (2.4 | ) | ||||||||||||||||
| Tax credit carryforwards |
(134,000 | ) | (1.8 | ) | (149,000 | ) | (5.5 | ) | (61,000 | ) | (2.4 | ) | ||||||||||||
| Warrant valuation adjustment |
344,000 | 4.7 | 133,000 | 4.9 | 128,000 | 5.1 | ||||||||||||||||||
| Change in valuation allowance including revisions of prior year estimates |
(5,303,000 | ) | (72.4 | ) | (2,761,000 | ) | (102.2 | ) | 265,000 | 10.6 | ||||||||||||||
| Federal NOL expirations |
825,000 | 11.3 | 797,000 | 29.5 | — | — | ||||||||||||||||||
| Expirations and adjustments of vested, non-qualified stock options |
— | — | 363,000 | 13.4 | 303,000 | 12.1 | ||||||||||||||||||
| Other |
314,000 | 4.3 | 134,000 | 5.0 | 279,000 | 11.0 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Effective tax rate |
$ | — | — | $ | — | — | $ | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
F-16
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
As of December 31, 2011 and 2010, the Company’s total amount of unrecognized tax benefits was $1,442,000 and $1,355,000, respectively, which, if recognized, would affect the effective tax rate prior to the adjustment for the Company’s valuation allowance. The Company has not recognized a tax liability for the unrecognized tax benefits because the Company has recorded a tax net operating loss carryforward that would offset this liability.
The change in unrecognized tax benefits for each of the years ended December 31, 2011, 2010 and 2009 is as follows:
| 2011 | 2010 | 2009 | ||||||||||
| Balance at January 1, |
$ | 1,355,000 | $ | 1,399,000 | $ | 1,483,000 | ||||||
| Additions for current year tax positions |
182,000 | — | — | |||||||||
| Reductions for expiration of statute of limitations |
(95,000 | ) | (44,000 | ) | (84,000 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Balance at December 31, |
$ | 1,442,000 | $ | 1,355,000 | $ | 1,399,000 | ||||||
|
|
|
|
|
|
|
|||||||
The Company does not expect substantial changes in its unrecognized tax benefits or positions over the next twelve months.
Tax years ended December 31, 2008, 2009, 2010 and 2011 remain subject to examination by major tax jurisdictions, which are Federal and the Commonwealth of Massachusetts. However, since the Company has net operating loss and tax credit carryforwards which may be utilized in future years to offset taxable income, the years in which such losses originate may also be subject to review by relevant taxing authorities if utilized.
8) WARRANTS AND SHARE BASED AWARDS
Common Stock Warrants
On October 29, 2007, the Company sold, through a private placement, 4,581,043 shares of our common stock and warrants to purchase 1,145,259 shares of common stock with an exercise price of $2.85. The warrants have a 5.5 year term and became exercisable on April 30, 2008. The warrants are recorded as a derivative liability at fair value.
On October 18, 2006, the Company’s Board of Directors extended the term of 250,000 Class B warrants, originally issued to the Company’s Chairman of the Board of Directors and Chief Executive Officer at the time of the Company’s initial public offering, for an additional four years to January 29, 2011. These warrants had an exercise price of $6.00 per share. On January 29, 2011, all 250,000 of the Class B warrants expired.
Share-based Awards
Under the Company’s 2011 Amended and Restated Equity Compensation Plan, (the “2011 Plan”), the Company may grant stock-based awards in amounts not to exceed the lesser of: (i) 25% of the total number of shares of the Company’s common stock issued and outstanding at any given time less the number of shares issued and outstanding under any other equity compensation plan of the Company at such time; or (ii) 6,108,492 shares less the number of shares issued and outstanding under any other equity compensation plan of the Company from time to time. The maximum number of shares of common stock that may be granted to any individual during any calendar year is 500,000.
The 2011 Plan is administered by the Compensation Committee of the Board of Directors (the “Committee”). The 2011 Plan provides for the grant of incentive stock options (“ISO”), nonqualified stock
F-17
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
options (“NSO”), stock awards, and stock appreciation rights to (i) employees, consultants, and advisors; (ii) the employees, consultants, and advisors of the Company’s parents, subsidiaries, and affiliates; and (iii) and the Company’s non-employee directors.
Non-Qualified Stock Options — All the NSOs granted under the 2011 Plan have an expiration period not exceeding seven years and are issued at a price not less than the market value of the common stock on the grant date. The Committee may establish such vesting and other conditions with respect to options as it deems appropriate. In addition, the Company initially grants each individual who agrees to become a director 15,000 NSOs to purchase common stock of the Company. Thereafter, on June 30th of each year, each non-employee director will receive automatically an NSO to purchase 10,000 shares of the Company’s common stock. Each NSO will vest in full on the date of the grant and have a term not to exceed seven (7) years from the date of grant. The Compensation Committee has the discretion to award 8,000 shares of restricted stock annually in lieu of a grant of 10,000 options to each continuing non-employee director, subject to the vesting provisions of the 2011 Plan.
Incentive Stock Options — ISOs granted under the 2011 Plan have an expiration period not exceeding seven years (five years for ISOs granted to employees who are also ten percent shareholders) and are issued at a price not less than the market value of the common stock on the grant date. The Committee may establish such vesting and other conditions with respect to options as it deems appropriate.
The 2011 Plan replaced the Company’s 2006 Equity Compensation Plan, as amended (the “2006 Plan”). A summary of stock option activity in both the 2011 Plan and the 2006 Plan, for 2011 follows:
| Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term (Years) |
Aggregate Intrinsic Value |
||||||||||||||
| Outstanding, beginning of year |
3,064,050 | $ | 4.09 | |||||||||||||
| Options granted |
158,300 | $ | 4.32 | |||||||||||||
| Options forfeited |
(25,037 | ) | $ | 2.34 | ||||||||||||
| Options expired |
(112,500 | ) | $ | 13.02 | ||||||||||||
| Options exercised |
(273,588 | ) | $ | 1.89 | ||||||||||||
|
|
|
|||||||||||||||
| Outstanding, end of year |
2,811,225 | $ | 3.97 | 3.89 | $ | 4,822,000 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Exercisable, end of year |
1,828,064 | $ | 5.06 | 3.33 | $ | 2,413,000 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Options vested and expected to vest, end of year |
2,730,495 | $ | 4.02 | 3.84 | $ | 4,664,000 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
A summary of stock options outstanding at December 31, 2011 follows:
| Range of Exercise Prices |
Outstanding as of December 31, 2011 |
Weighted Average Remaining Contractual Life |
Weighted Average Exercise Price |
Exercisable as of December 31, 2011 |
Weighted Average Exercise Price |
|||||||||||||||
| $1.10-$1.22 |
592,050 | 4.23 | $ | 1.21 | 266,176 | $ | 1.20 | |||||||||||||
| $1.27-$1.60 |
78,625 | 2.27 | $ | 1.50 | 68,625 | $ | 1.52 | |||||||||||||
| $1.65 |
570,225 | 5.18 | $ | 1.65 | 135,488 | $ | 1.65 | |||||||||||||
| $1.74-$3.37 |
669,850 | 3.04 | $ | 2.70 | 608,150 | $ | 2.74 | |||||||||||||
| $3.40-$15.90 |
900,475 | 3.61 | $ | 8.43 | 749,625 | $ | 9.25 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| 2,811,225 | 3.89 | $ | 3.97 | 1,828,064 | $ | 5.06 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||
F-18
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The total intrinsic value for stock options exercised in 2011, 2010 and 2009 was approximately $982,000, $26,000 and $0, respectively. At December 31, 2011, total unrecognized estimated compensation cost related to unvested stock options was $830,000, which is expected to be recognized over a weighted average period of 1.92 years.
The amount of cash received from the exercise of stock options in 2011, 2010 and 2009 was approximately $516,000, $63,000, and $0, respectively. No tax benefits were realized during this period due to the existence of tax net operating loss carryforwards.
Unvested Shares of Common Stock — The Company has issued unvested shares of common stock, which vest over 4 years at a rate of 25% per year, or for members of the Board of Directors, 25% immediately and 25% per year thereafter. The changes in unvested common stock during 2011 are as follows:
| Outstanding unvested shares of common stock, beginning of year |
586,000 | |||
| Shares granted |
506,000 | |||
| Shares vested |
(191,250 | ) | ||
|
|
|
|||
| Outstanding unvested shares of common stock, end of year |
900,750 | |||
|
|
|
|||
| Weighted average grant date fair value of shares vested during year |
$ | 1.88 | ||
| Weighted average grant date fair value of shares granted during year |
$ | 4.42 | ||
| Weighted average grant date fair value of unvested shares, end of year |
$ | 3.08 | ||
| Weighted average remaining years to vest |
2.48 |
At December 31, 2011 total unrecognized estimated compensation cost related to unvested common shares was $2,041,000, which is expected to be recognized over a weighted average period of 2.48 years.
Share-based Compensation
Total share-based compensation expense, related to all of the Company’s share-based awards, recognized for the years ended December 31, 2011, 2010 and 2009 is included in the following line items:
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Cost of product revenues |
$ | 55,000 | $ | 49,000 | $ | 60,000 | ||||||
| Research and development |
146,000 | 123,000 | 140,000 | |||||||||
| Selling and marketing |
223,000 | 114,000 | 110,000 | |||||||||
| General and administrative |
783,000 | 822,000 | 491,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Share-based compensation expense |
$ | 1,207,000 | $ | 1,108,000 | $ | 801,000 | ||||||
|
|
|
|
|
|
|
|||||||
The weighted-average estimated fair value of employee stock options granted during the years ended December 31, 2011, 2010 and 2009 was $2.85, $1.18 and $0.81 per share, respectively, determined using the Black-Scholes option valuation model with the following weighted-average assumptions (annualized percentages):
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Volatility |
77.3 | % | 75.5 | % | 73.6 | % | ||||||
| Risk-free interest rate |
1.05 - 2.37 | % | 1.79 - 2.73 | % | 1.87 - 2.54 | % | ||||||
| Expected dividend yield |
0 | % | 0 | % | 0 | % | ||||||
| Expected life-directors and officers |
6.1 years | 5.9 years | 6.0 years | |||||||||
| Expected life-non-officer employees |
5.6 years | 5.6 years | 5.8 years | |||||||||
F-19
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The Company used historical volatility in the Company’s stock for the expected volatility assumption input to the Black-Scholes model measured over a look back period commensurate with the expected life of the options. The decision to use historical volatility data to estimate expected volatility was based upon the lack of actively traded options in the Company’s stock, and the Company’s assessment that historical volatility is the most representative measure of future stock price trends.
The risk-free interest rate assumption is based upon observed interest rates appropriate for the term of the Company’s employee stock options. The expected life is based on the Company’s historical option cancellation and employee exercise information. The expected life of employee stock options includes the weighted-average period the stock options are expected to remain outstanding post-vesting. In calculating the expected life of the options, the Company classified its grantee population into two groups, directors and officers and non-officer employees. As share-based compensation expense recognized in the Consolidated Statements of Operations is based on awards ultimately expected to vest, it is reduced for estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. In 2011 and 2010, forfeiture rates were estimated to be approximately 2.95% and 3.35%, respectively, for officers and directors and 10.75% and 9.67%, respectively, for non-officer employees.
9) SIGNIFICANT PRODUCT AGREEMENTS
Daewoong Agreement
In the third quarter of 2011, the Company terminated its Marketing, Distribution and Supply Agreement, dated January 4, 2007, as amended, as of January 10, 2007, September 8, 2008 and June 20, 2011 with Daewoong Pharmaceutical, Co., LTD. and DNC Daewoong Derma & Plastic Surgery Network Company (collectively “Daewoong”). The Company sent notice of termination of the agreement on August 31, 2011 based on DUSA’s contractual right to unilaterally terminate the agreement as a result of certain regulatory milestones that were not achieved. As a result of the termination, the Company recorded $1,379,000 of revenues that were previously deferred, comprised of deferred drug shipments of $301,000 and the unamortized balance of milestone payments of $1,078,000, and recorded $71,000 of costs of revenues that were previously deferred. For 2011 the Daewoong termination resulted in the recognition of $1,308,000 of income from continuing operations.
Stiefel Agreement
In the third quarter of 2010, the Company gave notice to Stiefel Laboratories, Inc. terminating the parties’ Marketing, Distribution and Supply Agreement, dated January 12, 2006, as amended, as of September 26, 2007. The termination of this Agreement, which had appointed Stiefel as the Company’s exclusive marketing and distribution partner for the Company’s product, the Levulan® Kerastick, in Latin America, resulted in the acceleration of the recognition of deferred revenues of $555,000, comprised of deferred drug shipments of $87,000 and the unamortized balance of milestone payments of $468,000, and the acceleration of deferred cost of revenues of $42,000. For 2010 the Stiefel termination resulted in the recognition of $513,000 of income from continuing operations.
PhotoCure Agreement
On May 30, 2006, the Company entered into a patent license agreement under which the Company granted PhotoCure ASA a non-exclusive license under the patents the Company licenses from PARTEQ for ALA esters. In addition, the Company granted a non-exclusive license to PhotoCure for its existing formulations of Hexvix® and Metvix® (known in the U.S. as Metvixia®) for any patent the Company owns now or in the future. On
F-20
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
October 1, 2009, Photocure announced that it had sold Metvix/Metvixia to Galderma, S.A., a large dermatology company. While we are entitled to royalties on net sales of Metvixia, Galderma has considerably more resources than we have, which could significantly hamper our ability to maintain or increase our market share.
PhotoCure is obligated to pay the Company royalties on sales of its ester products to the extent they are covered by its patents in the U.S. and certain other territories. As part of the agreement, PhotoCure paid the Company a prepaid royalty in the amount of $1,000,000 in 2006. Revenues recognized pursuant to the Photocure Agreement have not been material to date. The balance of the prepaid royalty under the Photocure Agreement is included in deferred revenues in the accompanying Consolidated Balance Sheets.
10) RETIREMENT PLAN
The Company has a tax-qualified employee savings and retirement 401(k) Profit Sharing Plan (the “401(k) Plan”), covering all qualified employees. Participants may elect a salary deferral of at least 1% as a contribution to the 401(k) Plan, up to the statutorily prescribed annual limit for tax-deferred contributions. The Company matches a participant’s contribution up to 1.25% of a participant’s salary (the “Match”), subject to certain limitations of the 401(k) Plan. Participants will vest in the Match at a rate of 25% for each year of service to the Company. The Company’s matching contributions in 2011, 2010 and 2009 were $72,000, $65,000 and $63,000, respectively.
11) DISCONTINUED OPERATIONS
At December 31, 2011, the Company ceased marketing and selling its remaining Non-PDT products, which were ClindaReach® and Meted®. The former Non-PDT Drug Products segment is now reflected as discontinued operations in the accompanying financial statements for all periods presented.
The following is a summary of income from discontinued operations, net of income tax benefit, for each of the years in the three-year period ended December 31, 2011:
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Revenues |
$ | 282,000 | $ | 1,010,000 | $ | 1,470,000 | ||||||
| Cost of revenues |
487,000 | 853,000 | 667,000 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross Margin(1) |
(205,000 | ) | 157,000 | 803,000 | ||||||||
| Operating Expenses |
||||||||||||
| Selling, general and administrative |
18,000 | 18,000 | 314,000 | |||||||||
| Settlements, net |
— | — | 75,000 | |||||||||
| Gain on sale of assets |
(750,000 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
(732,000 | ) | 18,000 | 389,000 | ||||||||
| Income from discontinued operations, before income tax provision |
527,000 | 139,000 | 414,000 | |||||||||
| Income tax provision |
— | — | (163,000 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Income from discontinued operations |
$ | 527,000 | $ | 139,000 | $ | 251,000 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Historical gross margin disclosures for the Non-PDT Drug Products segment included general corporate overhead allocations of $59,000, $149,000 and $272,000, for 2011, 2010, and 2009, respectively. These amounts have been allocated to continuing operations for purposes of discontinued operations. |
F-21
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
The Company includes only revenues and costs directly attributable to the discontinued operations, and not those attributable to the ongoing entity. Accordingly, no general corporate overhead costs have been allocated to the Non-PDT operations for purposes of discontinued operations reporting.
The following is a summary of assets and liabilities associated with discontinued operations as of December 31, 2011 and 2010:
| 2011 | 2010 | |||||||
| Assets from discontinued operations: |
||||||||
| Accounts receivable, net of allowance for doubtful accounts |
$ | 39,000 | $ | 86,000 | ||||
| Inventory |
— | 117,000 | ||||||
|
|
|
|
|
|||||
| Total assets from discontinued operations |
39,000 | 203,000 | ||||||
|
|
|
|
|
|||||
| Liabilities from discontinued operations: |
||||||||
| Accounts payable |
3,000 | 2,000 | ||||||
| Sales returns reserve |
252,000 | 125,000 | ||||||
| Deferred revenues |
78,000 | — | ||||||
| Payment due to former Sirius shareholders |
250,000 | 232,000 | ||||||
| Non-PDT license payable |
250,000 | — | ||||||
| Other |
19,000 | 25,000 | ||||||
|
|
|
|
|
|||||
| Total liabilities from discontinued operations |
$ | 852,000 | $ | 384,000 | ||||
|
|
|
|
|
|||||
The following is a summary of net cash provided by (used in) operating and investing activities from discontinued operations for each of the years in the three-year period ended December 31, 2011:
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Income from discontinued operations, net of tax provision |
$ | 527,000 | $ | 139,000 | $ | 251,000 | ||||||
| Decrease in assets |
164,000 | 37,000 | 276,000 | |||||||||
| Increase (decrease) in liabilities |
469,000 | (282,000 | ) | (164,000 | ) | |||||||
| Gain on sale of assets |
(750,000 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by(used in) operating activities from discontinued operations |
$ | 410,000 | $ | (106,000 | ) | $ | 363,000 | |||||
|
|
|
|
|
|
|
|||||||
| Proceeds from sale of assets |
750,000 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by investing activities from discontinued operations |
$ | 750,000 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
The Company establishes an accrual in an amount equal to its estimate of Non-PDT products expected to be returned. The Company determines the estimate of the sales return accrual primarily based on historical experience regarding sales and related returns and incorporating other factors that could impact sales returns in the future. These other factors include, for example, levels of inventory in the distribution channel, estimated shelf life and product discontinuances. The Company’s policy is to accept returns when product is within six months of expiration. The Company considers all of these factors and adjusts the accrual periodically to reflect actual experience.
F-22
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
A summary of activity in the Company’s sales returns reserve accounts is as follows:
| Balance at January 1, 2011 |
Provision | Actual Returns or Credits |
Balance at December 31, 2011 |
|||||||||||||
| Sales returns reserve |
$ | 125,000 | $ | 189,000 | $ | (62,000 | ) | $ | 252,000 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance at January 1, 2010 |
Provision | Actual Returns or Credits |
Balance at December 31, 2010 |
|||||||||||||
| Sales returns reserve |
$ | 225,000 | $ | 166,000 | $ | (266,000 | ) | $ | 125,000 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance at January 1, 2009 |
Provision | Actual Returns or Credits |
Balance at December 31, 2009 |
|||||||||||||
| Sales returns reserve |
$ | 500,000 | $ | 290,000 | $ | (565,000 | ) | $ | 225,000 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
12) SEGMENT REPORTING
Following its acquisition of Sirius Laboratories, Inc., which occurred in 2006, the Company had operated in two segments, Photodynamic Therapy (“PDT”) Drug and Device Products and Non-Photodynamic Therapy (“Non-PDT”) Drug Products. The Company’s Levulan® Kerastick® and BLU-U® products comprised its PDT segment, while its Non-PDT segment was comprised of the products acquired in the acquisition of Sirius. At December 31, 2011, the Company ceased marketing and selling its Non-PDT products, which were ClindaReach® and Meted®. The former Non-PDT Drugs Products segment is now reflected as discontinued operations in the accompanying financial statements for all periods presented.
During the years ended December 31, 2011, 2010 and 2009, the Company derived revenues from the following geographies based on the location of the customer (as a percentage of product revenues):
| Year Ended December 31, |
||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| United States |
95 | % | 96 | % | 94 | % | ||||||
| Canada |
1 | % | 1 | % | 2 | % | ||||||
| Korea |
4 | % | 1 | % | 2 | % | ||||||
| Latin America |
— | % | 2 | % | 2 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Total |
100 | % | 100 | % | 100 | % | ||||||
|
|
|
|
|
|
|
|||||||
F-23
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
13) COMMITMENTS AND CONTINGENCIES
Lease Arrangements
The Company leases its facilities under operating leases. The Company’s lease arrangements have terms which expire through 2014. Total rent expense under operating leases was approximately $357,000, $386,000 and $398,000 for the years ended December 31, 2011, 2010 and 2009, respectively. Future minimum payments under lease arrangements at December 31, 2011 are as follows:
| Years Ending December Years 31, |
Operating Lease Obligations |
|||
| 2012 |
$ | 389,000 | ||
| 2013 |
396,000 | |||
| 2014 |
367,000 | |||
|
|
|
|||
| Total |
$ | 1,152,000 | ||
|
|
|
|||
The Company has not accrued amounts for any potential contingencies as of December 31, 2011.
The Company is involved in legal matters arising in the ordinary course of business. Management does not expect that the resolution of these matters will have a material adverse effect on the Company’s financial position or its results of operations. There are no matters for which management believes it is reasonably possible that a material loss has been incurred as of December 31, 2011.
| 14) | SELECTED QUARTERLY FINANCIAL INFORMATION (UNAUDITED) |
| Quarterly Results for Year Ended December 31, 2011 | ||||||||||||||||
| March 31 | June 30 | Sept 30(1) | Dec 31 | |||||||||||||
| Product revenues |
$ | 10,982,000 | $ | 9,671,000 | $ | 9,374,000 | $ | 15,269,000 | ||||||||
| Gross margin |
9,351,000 | 8,200,000 | 7,962,000 | 12,861,000 | ||||||||||||
| (Income (loss) from continuing operations |
(571,000 | ) | 396,000 | 3,562,000 | 3,406,000 | |||||||||||
| (Loss) income from discontinued operations |
(34,000 | ) | 714,000 | (3) | (55,000 | ) | (99,000 | ) | ||||||||
| Net income (loss) |
$ | (605,000 | ) | $ | 1,110,000 | $ | 3,507,000 | $ | 3,307,000 | |||||||
| Basic net income (loss) per share - |
||||||||||||||||
| Continuing operations |
$ | (0.02 | ) | $ | 0.02 | $ | 0.14 | $ | 0.14 | |||||||
| Discontinued operations |
$ | — | $ | 0.03 | $ | — | $ | — | ||||||||
| Basic net income (loss) per share |
$ | (0.02 | ) | $ | 0.05 | $ | 0.14 | $ | 0.13 | |||||||
| Diluted net income (loss) per share - |
||||||||||||||||
| Continuing operations |
$ | (0.02 | ) | $ | 0.01 | $ | 0.13 | $ | 0.13 | |||||||
| Discontinued operations |
$ | — | $ | 0.03 | $ | — | $ | — | ||||||||
| Diluted net income (loss) per share |
$ | (0.02 | ) | $ | 0.04 | $ | 0.13 | $ | 0.13 | |||||||
F-24
Table of Contents
DUSA PHARMACEUTICALS, INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS — (Continued)
| Quarterly Results for Year Ended December 31, 2010 | ||||||||||||||||
| March 31 | June 30 | Sept 30(2) | Dec 31 | |||||||||||||
| Product revenues |
$ | 8,296,000 | $ | 8,411,000 | $ | 7,837,000 | $ | 11,879,000 | ||||||||
| Gross margin |
6,676,000 | 6,912,000 | 6,499,000 | 9,917,000 | ||||||||||||
| Income (loss) from continuing operations |
(640,000 | ) | 186,000 | 148,000 | 2,869,000 | |||||||||||
| Income (loss) from discontinued operations |
215,000 | 2,000 | (115,000 | ) | 37,000 | |||||||||||
| Net income (loss) |
(424,000 | ) | 188,000 | 33,000 | 2,906,000 | |||||||||||
| Basic net income (loss) per share - |
||||||||||||||||
| Continuing operations |
$ | (0.03 | ) | $ | 0.01 | $ | 0.01 | $ | 0.12 | |||||||
| Discontinued operations |
$ | 0.01 | $ | — | $ | — | $ | — | ||||||||
| Basic net income (loss) per share |
$ | (0.02 | ) | $ | 0.01 | $ | 0.00 | $ | 0.12 | |||||||
| Diluted net income (loss) per share - |
||||||||||||||||
| Continuing operations |
$ | (0.03 | ) | $ | 0.01 | $ | 0.01 | $ | 0.12 | |||||||
| Discontinued operations |
$ | 0.01 | $ | — | $ | — | $ | — | ||||||||
| Diluted net income (loss) per share |
$ | (0.02 | ) | $ | 0.01 | $ | 0.00 | $ | 0.12 | |||||||
| (1) | The third quarter of 2011 includes the acceleration of deferred revenues of $1,379,000 and the acceleration of deferred cost of revenues of $71,000 related to the Company’s termination of its Asia Pacific distribution agreement with Daewoong Pharmaceutical Co., LTD. and Daewoong Derma & Plastic Surgery Network Company, or Daewoong. |
| (2) | The third quarter of 2010 includes the acceleration of deferred revenues of $555,000 and the acceleration of deferred cost of revenues of $42,000 related to the Company’s termination of its Latin America distribution agreement with Stiefel Laboratories, Inc., or Stiefel. |
| (3) | Income from discontinued operations in the second quarter of 2011 includes a gain of $750,000 related to the Company’s sale of its patents covering Nicomide®, together with the trademarks Nicomide® and Nicomide-T®, and related domain names. |
F-25
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| (Registrant) DUSA Pharmaceuticals, Inc. | ||
| By | (Signature and Title) | |
| /s/ Robert F. Doman | ||
| President and Chief Executive Officer | ||
Date: March 6, 2012
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| /s/ Robert F. Doman Robert F. Doman |
Director, President and Chief Executive Officer |
March 6, 2012 Date | ||
| /s/ Richard C. Christopher Richard C. Christopher |
Vice President, Finance and |
March 6, 2012 Date | ||
| /s/ Alfred Altomari Alfred Altomari |
Director |
March 6, 2012 Date | ||
| /s/ David Bartash David Bartash |
Vice Chairman of the Board and Lead Director |
March 6, 2012 Date | ||
| /s/ Alexander W. Casdin Alexander W. Casdin |
Director |
March 6, 2012 Date | ||
| /s/ Jay M. Haft, Esq. Jay M. Haft, Esq. |
Chairman of the Board and Director |
March 6, 2012 Date | ||
| /s/ Paul J. Hondros Paul J. Hondros |
Director |
March 6, 2012 Date | ||
| /s/ Magnus Moliteus Magnus Moliteus |
Director |
March 6, 2012 Date | ||
| /s/ David M. Wurzer David M. Wurzer |
Director |
March 6, 2012 Date | ||
Table of Contents
EXHIBIT INDEX
| 2(a.1)* | Merger Agreement by and among the Registrant, Sirius Laboratories, Inc., and the shareholders of Sirius dated as of December 30, 2005 filed as Exhibit 2(a.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference; | |
| 2(a.2) | First Amendment to Merger Agreement by and among the Registrant, Sirius Laboratories, Inc. and the shareholders of Sirius, dated as of February 6, 2006 filed as Exhibit 2(a.2) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference; and | |
| 2(a.3) | Third Amendment to Merger Agreement by and among the Registrant, Sirius Laboratories, Inc. and the shareholders of Sirius, dated as of April 21, 2009; filed as Exhibit 2(a.3) to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2009, and is incorporated herein by reference. | |
| 3(a.1) | Certificate of Incorporation, as amended, filed as Exhibit 3(a) to the Registrant’s Form 10-K for the fiscal year ended December 31, 1998, and is incorporated herein by reference; | |
| 3(a.2) | Certificate of Amendment to the Certificate of Incorporation, as amended, dated October 28, 2002 and filed as Exhibit 99.3 to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2002, filed November 12, 2002, and is incorporated herein by reference; and | |
| 3(b) | Amended and Restated By-laws of the Registrant. | |
| 4(a) | Common Stock specimen, filed as Exhibit 4(a) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2002, and is incorporated herein by reference; | |
| 4(b) | Rights Agreement filed as Exhibit 4.0 to Registrant’s Current Report on Form 8-K filed October 11, 2002, and is incorporated herein by reference; | |
| 4(c) | Rights Certificate relating to the rights granted to holders of common stock under the Rights Agreement filed as Exhibit 4.0 to Registrant’s Current Report on Form 8-K filed October 11, 2002, and is incorporated herein by reference; | |
| 4(d) | Form of Common Stock Purchase Warrant, dated October 29, 2007 filed as Exhibit 4.2 to the Registrant’s Registration Statement on Form S-3, No. 333-147614, and is incorporated herein by reference; and | |
| 4(e) | Registration Rights Agreement, dated October 29, 2007, by and between the Registrant and each of the respective selling shareholders named therein filed as Exhibit 4.3 to the Registrant’s Registration Statement on Form S-3, No. 333-147614, and is incorporated herein by reference. | |
| 10(a) | License Agreement between the Registrant, PARTEQ and Draxis Health Inc. dated August 27, 1991, filed as Exhibit 10.1 to the Registrant’s Registration Statement on Form S-1, No. 33-43282, and is incorporated herein by reference; | |
| 10(b) | Termination and Transfer Agreement between the Registrant and Draxis Health Inc. dated as of February 24, 2004, filed as Exhibit 10(b.2) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2003, portions of which have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
| 10(c) | Consulting Agreement and General Release of D. Geoffrey Shulman, MD, FRCPC dated as of December 1, 2008, filed as Exhibit 10(d.3) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
Table of Contents
| 10(d) | Amended and Restated License Agreement between the Registrant and PARTEQ dated March 11, 1998, filed as Exhibit 10(e) to the Registrant’s Form 10-K/A filed on June 18, 1999, portions of Exhibit A have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
| 10(e) | Incentive Stock Option Plan, filed as Exhibit 10.11 of Registrant’s Registration Statement on Form S-1, No. 33-43282, and is incorporated herein by reference;+ | |
| 10(f) | 1994 Restricted Stock Option Plan, filed as Exhibit 1 to Registrant’s Schedule 14A definitive Proxy Statement dated April 26, 1995, and is incorporated herein by reference;+ | |
| 10(g) | 1996 Omnibus Plan, as amended, filed as Appendix A to Registrant’s Schedule 14A Definitive Proxy Statement dated April 26, 2001, and is incorporated herein by reference;+ | |
| 10(g.1) | 1996 Omnibus Plan, as amended on May 1, 2003, filed as Exhibit 10(h.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2003, and is incorporated herein by reference;+ | |
| 10(g.2) | 1996 Omnibus Plan, as amended April 23, 2004, filed as Appendix A to Registrant’s Schedule 14A definitive Proxy Statement dated April 28, 2004, and is incorporated herein by reference;+ | |
| 10(h) | 2011 Amended and Restated Purchase and Supply Agreement between the Registrant and National Biological Corporation dated as of November 29, 2011, portions of which have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended; | |
| 10(i) | Supply Agreement between the Registrant and Sochinaz SA dated December 24, 1993, filed as Exhibit 10.1 to Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2011, and is incorporated herein by reference; | |
| 10(i.1) | First Amendment to Supply Agreement between the Registrant and Sochinaz SA dated July 7, 1994, filed as Exhibit 10(q.1) to Registrant’s Annual Report on Form 10-K for the fiscal year ended December 31, 1999, and is incorporated herein by reference; | |
| 10(i.2) | Second Amendment to Supply Agreement between the Registrant and Sochinaz SA dated as of June 20, 2000, filed as Exhibit 10.1 to Registrant’s Current Report on Form 8-K dated June 28, 2000, and is incorporated herein by reference; | |
| 10(i.3) | Third Amendment to Supply Agreement between the Registrant and Sochinaz SA dated July 29, 2005, filed as Exhibit 10.1 to the Registrant’s Form 10-Q filed on August 3, 2005, portions of which have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
| 10(i.4) | Fifth Amendment to Supply Agreement between the Registrant and Sochinaz SA dated September 10, 2009, filed as Exhibit 10 to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2009, portions of which have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
| 10(j) | Master Service Agreement between the Registrant and Therapeutics, Inc. dated as of October 4, 2001, filed as Exhibit 10(b) to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2001, filed November 8, 2001, portions of which have been omitted pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
| 10(k) | License and Development Agreement between the Registrant and photonamic GmbH & Co. KG dated as of December 30, 2002, filed as Exhibit 10(r) to Registrant’s Annual Report on Form 10-K for the fiscal year ended December 31, 2002, portions of which have been omitted pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
Table of Contents
| 10(l) | Supply Agreement between the Registrant and medac GmbH dated as of December 30, 2002, filed as Exhibit 10(r) to Registrant’s Annual Report on Form 10-K for the fiscal year ended December 31, 2002, portions of which have been omitted pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended, and is incorporated herein by reference; | |
| 10(m) | License and Supply Agreement dated August 7, 2007 among the Registrant, photonamic GmbH & Co. KG and medac, GmbH, portions of which have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended, filed as Exhibit 10 to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2007 and is incorporated herein by reference; | |
| 10(n) | License, Promotion, Distribution and Supply Agreement between the Registrant and Coherent-AMT dated as of March 31, 2004 filed as Exhibit 10(a) to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2004, filed May 4, 2004, and is incorporated herein by reference; | |
| 10(o) | Employment Agreement of Scott L. Lundahl dated as of June 23, 1999 filed as Exhibit 10(u) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(o.1) | Amendment No. 1 to Employment Agreement of Scott Lundahl dated as of April 10, 2008, filed as Exhibit 10(s.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(p) | Amended Employment Agreement of Stuart L. Marcus, MD, PhD dated December 9, 1999 filed as Exhibit 10(v) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(p.1) | Amendment No. 2 to Employment Agreement of Stuart L. Marcus, MD, PhD dated as of April 10, 2008, filed as Exhibit 10(t.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(q) | Employment Agreement of Mark C. Carota dated as of February 14, 2000 filed as Exhibit 10(w.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(q.1) | First Amendment to Employment Agreement of Mark C. Carota dated October 31, 2001 filed as Exhibit 10(w.2) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(q.2) | Amendment No. 2 to Employment Agreement of Mark C. Carota dated as of April 10, 2008, filed as Exhibit 10(u.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(r) | Employment Agreement of Richard Christopher dated as of January 1, 2004 filed as Exhibit 10(y) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(s) | Amendment No. 1 to Employment Agreement of Richard Christopher dated as of October 18, 2006 filed as Exhibit 10.A to the Registrant’s Form 10-Q for the fiscal quarter ended September 30, 2004, and is incorporated herein by reference; | |
| 10(s.1) | Amendment No. 2 to Employment Agreement of Richard Christopher dated as of April 10, 2008, filed as Exhibit 10(w.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
Table of Contents
| 10(t) | Employment Agreement of Robert F. Doman dated as of December 29, 2004 filed as Exhibit 10(z) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(u) | First Amendment to Employment Agreement of Robert F. Doman dated November 26, 2008, filed as Exhibit 10(x.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(v) | Compensation Policy Applicable to the Registrant’s Non-Employee Directors filed as Exhibit 10(cc) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference;+ | |
| 10(w) | Employment Agreement of William O’Dell dated as of April 4, 2006 filed as Exhibit 10(ii) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference; | |
| 10(w.1) | Amendment No. 1 to Employment Agreement of William O’Dell dated as of April 10, 2008, filed as Exhibit 10(jj.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(x) | Patent License Agreement between the Registrant and PhotoCure ASA, dated as of May 30, 2006, portions of which have been omitted pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Exchange Act of 1934, as amended, filed as Exhibit 10.A to the Registrant’s Form 10-Q for the fiscal quarter ended June 30, 2006, and is incorporated herein by reference; | |
| 10(y) | Employment Agreement of Michael Todisco dated as of September 20, 2006 filed as Exhibit 10(11) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference; | |
| 10(y.1) | Amendment No. 1 to Employment Agreement of Michael Todisco dated as of April 10, 2008, filed as Exhibit 10(mm.1) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2008, and is incorporated herein by reference;+ | |
| 10(z) | DUSA Pharmaceuticals, Inc. 2006 Equity Compensation Plan, filed as Appendix A to Registrant’s Schedule 14A definitive Proxy Statement dated April 24, 2006, and is incorporated herein by reference;+ | |
| 10(aa) | DUSA Pharmaceuticals, Inc. 2006 Equity Compensation Plan, as amended October 18, 2006 filed as Exhibit 10(pp) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference;+ | |
| 10(bb) | DUSA Pharmaceuticals, Inc. 2006 Deferred Compensation Plan, October 18, 2006 filed as Exhibit 10(qq) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2006, and is incorporated herein by reference;+ | |
| 10(cc) | Asset Purchase Agreement between Registrant and Acella Pharmaceuticals LLC dated June 30, 2011 portions of which have been omitted pursuant to a request for confidential treatment under Rule 24b-2 of the Securities Act of 1934, as amended, filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2011, and is incorporated herein by reference; and | |
| 10(dd) | DUSA Pharmaceuticals, Inc. Amended and Restated 2011 Equity Compensation Plan, filed as Appendix A to Registrant’s Schedule 14A definitive Proxy Statement dated April 20, 2011, and is incorporated herein by reference.+ | |
| 14(a) | Form of DUSA Pharmaceuticals, Inc. Code of Ethics Applicable to Senior Officers, filed as Exhibit 14(a) to the Registrant’s Form 10-K for the fiscal year ended December 31, 2004, and is incorporated herein by reference. | |
Table of Contents
| 21(a) | Subsidiaries of the Registrant. | |
| 23(a) | Consent of Independent Registered Public Accounting Firm. | |
| 31(a) | Rule 13a-14(a)/15d-14(a) Certification of the Chief Executive Officer; and | |
| 31(b) | Rule 13a-14(a)/15d-14(a) Certification of the Chief Financial Officer. | |
| 32(a) | Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002; and | |
| 32(b) | Certification of the Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 99.1 | Press Release dated March 6, 2012. | |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Taxonomy Extension Schema | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase | |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase | |
| 101.DEF | XBRL Taxonomy Extension Definition Document | |
| + | Management contract or compensatory plan or arrangement. |
| * | Schedules and exhibits omitted pursuant to Item 601(b)(2) of Regulation S-K. The Registrant agrees to furnish supplementally a copy of any omitted schedule or exhibit to the Commission upon request. |
