Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - MELINTA THERAPEUTICS, INC. /NEW/ | d311250d8k.htm |
 Cempra
Corporate Presentation March 2012
Developing Well-Differentiated
Antibiotics to Meet Medical Needs
Exhibit 99.1 |
 Forward Looking
Statement 2
This
presentation
contains
forward-looking
statements
regarding
future
events.
These
statements
are
just
predictions
and
are
subject
to
risks
and
uncertainties
that
could
cause
the
actual
events
or
results
to
differ
materially.
These
risks
and
uncertainties
include,
among
others:
risks
related
to
the
costs,
timing,
regulatory
review
and
results
of
our
studies
and
clinical
trials;
our
ability
to
obtain
FDA
approval
of
our
product
candidates;
our
dependence
on
the
success
of
Solithromycin
and
Taksta;
our
need
to
obtain
additional
funding
and
our
ability
to
obtain
future
funding
on
acceptable
terms;
our
anticipated
capital
expenditures
and
our
estimates
regarding
our
capital
requirements;
the
possible
impairment
of,
or
inability
to
obtain,
intellectual
property
rights
and
the
costs
of
obtaining
such
rights
from
third
parties;
the
unpredictability
of
the
size
of
the
markets
for,
and
market
acceptance
of,
any
of
our
products,
including
Solithromycin
and
Taksta;
our
ability
to
produce
and
sell
any
approved
products
and
the
price
we
are
able
realize
for
those
products;
our
ability
to
retain
and
hire
necessary
employees
and
to
staff
our
operations
appropriately;
our
ability
to
compete
in
our
industry;
innovation
by
our
competitors;
and
our
ability
to
stay
abreast
of
and
comply
with
new
or
modified
laws
and
regulations
that
currently
apply
or
become
applicable
to
our
business.
Please
refer
to
the
documents
that
we
file
from
time
to
time
with
the
Securities
and
Exchange
Commission. |
 Lead program,
Solithromycin, has the potential to be the first Oral and IV macrolide approved since
Zithromax/Z-PAK and is ready to begin Phase 3 trials in CABP Second program, Taksta,
is an oral drug that is effective against MRSA, which we are developing for
long-term treatment of Prosthetic Joint Infection (PJI), a potential orphan
indication
Large market opportunity with global antibiotic sales in the multibillions
Significant need for new antibiotics driven by resistance and tolerability issues
New FDA guidance provides clear regulatory pathway
Strong management team with extensive successful experience in antibiotic drug
development and approval -
Azactam, Biaxin, Fidaxomicin, Synercid, Viread
Highlights
3 |
 Significant need
for new treatment driven by:
Resistance
Adverse events/lack of tolerability
Inappropriate spectrum
Lack of IV-oral
Lack of pediatric dosing formulation
Acceptability for long term use
Developing Differentiated Antibiotics
In a Large Market to Meet Significant Needs
4
Global antibiotics sales in 2009
Total $42B
The growing need:
At
least
30%
of
pneumococci
in
the
U.S.
are
resistant
to
azithromycin
(Z-Pak)
–
the
leading
macrolide
Growing
need
for
oral
therapies
to
address
chronic
Staph.
aureus
(MRSA)
infections
–
chronic
therapy
of
prosthetic
joint
infections |
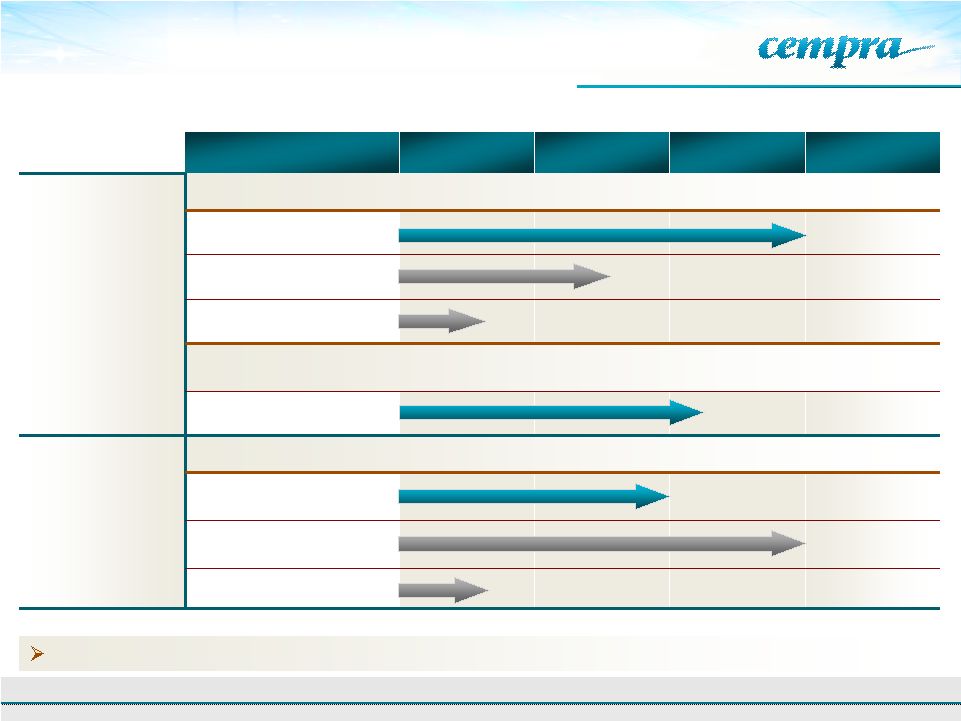 Cempra’s
Portfolio Addresses The Critical Needs
in The Antibiotic Market
Non-Antibiotic
Macrolide
Program:
GERD/Diabetic
gastroparesis
and
COPD
are
in
preclinical
stage
Product
Formulation
Preclinical
Phase 1
Phase 2
Phase 3
CEM-101
(Solithromycin)
Community Acquired Bacterial Pneumonia (CABP)
Oral
Intravenous (IV)
Oral Suspension/Pediatric
Future CEM-101 indications:
Urethritis,
Other respiratory tract infections (RTI’s), chronic obstructive
pulmonary disease (COPD), cystic fibrosis (CF), malaria, eye infections, etc.
Oral –
Urethritis
Taksta™
Fusidic Acid
Acute and Chronic Treatment of Staph (MRSA)
Oral –
Chronic Prosthetic
Joint Infections
Oral –
ABSSSI
Oral Suspension/Pediatric
5 |
 A next
generation macrolide for respiratory tract infections, including CABP, entering Phase
3 6
Solithromycin –
(CEM-101) |
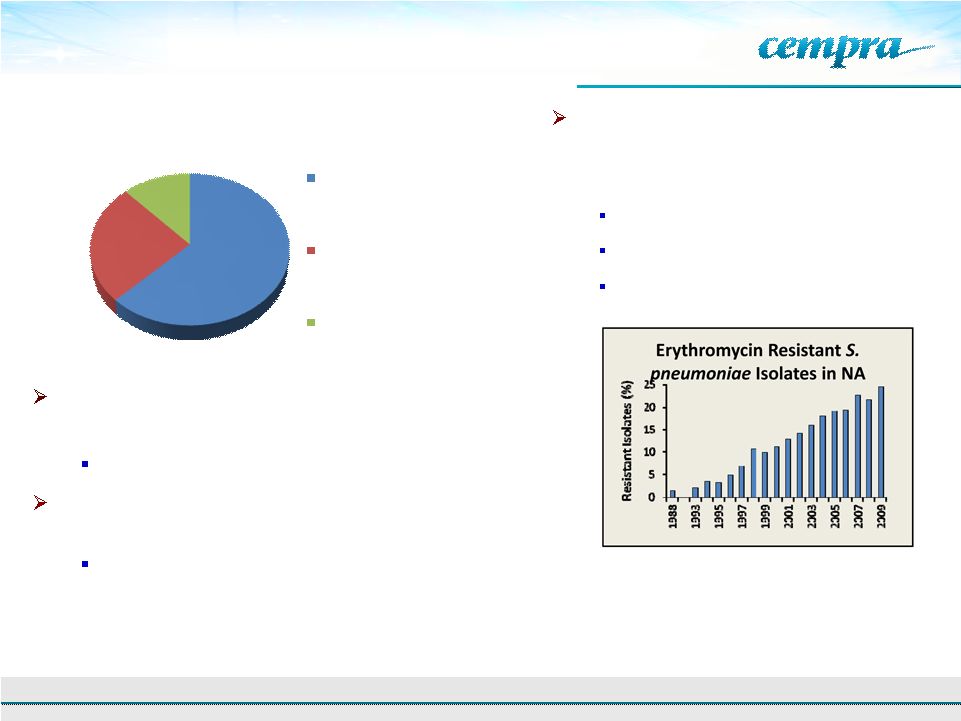 62.6%
25.5%
11.9%
Extended Spectrum
Macrolides
Quinolones
Other
Solithromycin Opportunity
7
Azithromycin (Zithromax/Z-Pak), the
leading macrolide, went generic in 2005
52 million prescriptions and $1.1 billion in sales in 2010
Widespread azithromycin use has led to resistance
issues
Lack of new macrolides with improved resistance profiles
has led physicians to turn to fluoroquinolones (Levaquin)
despite side effect concerns
Total 2009 Pneumonia Oral Prescriptions –
By
Class (Branded and Generic)
Source: IMS
Macrolides are the most widely
prescribed treatment for CABP and
other RTIs
Broad spectrum of activity
Good safety
Excellent tissue/intracellular distribution
and anti-inflammatory activity
Pneumococcal
resistance
rate
in
China
–
96.4%
Asian
Network
Surveillance:
AAC.
2012,
56:
1418-1426
2
Canadian Bacterial Surveillance Network.
http://microbiology.mtsinai.on.ca/research/cbsn/.
Accessed March 2011 |
 Community-Acquired Bacterial
Pneumonia (CABP)
No. 1 cause of death due to infection
Pneumococci is the most common
cause of fatal CABP
Most common cause of chronic bronchitis,
sinusitis, meningitis and middle ear
infection
5-6 million cases/year
~1 million hospital admissions/year
~1.6 million fatal cases of
pneumococcal disease worldwide annually
Pneumococcal diseases cause
more deaths per year in U.S. than
breast or prostate cancer
Centers for Disease Control and Prevention. 2010. Active Bacterial Core
Surveillance Report, Emerging Infections Program Network, Streptococcus
pneumoniae, 2009. http://www.cdc.gov/abcs/reports-
findings/survreports/spneu09.pdf. Accessed February 3, 2011.
Xu. et al. Deaths: Final Data for 2007. Natl Vital
Stat Rep. 2010; 58: 1-51
8
IDSA and KOLs recommend a
-lactam plus
a
macrolide for CABP
Several reports show that addition of a
macrolide results in better patient outcome
Addition of macrolide decreases mortality by
>50% in patients with highest PORT scores |
 Community-Acquired Bacterial
Pneumonia (CABP) –
Standard-of-Care
9
Macrolides have kept a large segment of the global antibiotic market in spite of
fluoroquinolones
Macrolide
segment
not
crowded
Mostly
occupied
by
azithromycin
and
clarithromycin
–
rising
incidence
of
resistance
No
new
macrolides
except
solithromycin
Solithromycin
is
being
developed
for
monotherapy
for
CABP
-
a
cephalosporin
would
not
be
needed, eliminating side effects and costs of two drugs
Solithromycin
has
the
spectrum
of
activity
that
provides
coverage
for
CABP
pathogens,
including
azithromycin-resistant
bacteria
Step
down
IV
to
oral
therapy
could
give
a
pharmacoeconomic
advantage
over
current
treatment
options |
 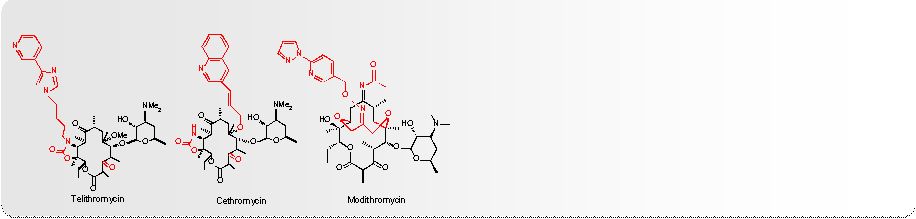   History of
Macrolide Development First Generation Macrolide
Second Generation Macrolide
In vitro activity is similar.
Better PK, acid stable,
fewer GI effects
Third Generation Macrolides, “Ketolides”
More
potent
than
2
nd
generation
macrolides
Active against macrolide-resistant strains, 2 binding
sites, acid stable, good PK, better tissue
distribution
Telithromycin
–
major
adverse
events
Others
have
failed
–
no
other
new
macrolides
10
Resistance is now widespread
Fourth Generation Macrolide, Fluoroketolide
More
potent
than
3
rd
generation
macrolides
Active
against
1
st
,
2
nd
and
3
rd
generation
macrolide-resistant
strains,
3
binding
sites,
extended
spectrum,
good
PK
and
tissue
distribution,
intravenous
and
oral
dosing
Effective in Phase 2 oral and well-tolerated |
 Ketek, a third
generation macrolide, saw rapid uptake after approval Serious adverse events after
approval Visual disturbance
Myasthenia gravis
Hepatotoxicity
In 2006 FDA withdrew approval for all indications except for use
in CABP
No other macrolides have these issues
Cempra research published in peer-reviewed article in Antimicrobial Agents and
Chemotherapy Ketek, only macrolide to have a pyridine component
Pyridine component inhibits nACh receptors in the liver,
eye and muscle
CEM-101 has been administered to over 220 subjects/patients and has been well-tolerated
with none of the unusual side effects seen with Ketek
Market needs remain after Ketek failure
Ketek (Telithromycin) Failure Shows
the Opportunity for Our New Macrolide
11 |
 # of
Organisms Solithromycin
Azithromycin
MIC
90
(µg/ml)
Strep. pneumoniae
(150)
0.25
>16
Haemophilus influenzae
(100)
2
2
Strep. pyogenes
(100)
0.03
>16
Legionella pneumophila
(30)
0.015
2
Mycoplasma pneumoniae
(36)
0.000125
0.0005
Chlamydophila pneumoniae
(10)
0.25
0.125
Solithromycin: Spectrum of Activity That Addresses
CABP Pathogens
Solithromycin (CEM-101) is several fold more potent than azithromycin against Strep.
pneumoniae
and
Hemophilus
influenzae,
two
critical
pathogens
in
CABP
12
Solithromycin has
demonstrated class-leading
potency in vitro against
macrolide-resistant
pneumococcus
CEM-101’s
unique
chemical
structure
necessitates
mutations
at
three
distinct
sites
for
resistance
to
develop
–
no
other
macrolide
has
more
than
two |
 Population
Solithromycin
Levofloxacin
Success rate, %
Success rate, %
ITT*
72.3
71.6
MITT
77.8
71.4
Solithromycin: Comparable Efficacy to Standard of
Care (Levaquin) -
Oral Phase 2 Trial
13
CEM-101
performed
favorably
in
mITT
at
TOC
when
CABP
pathogens
were
isolated
-
key
criteria
used
by
the
FDA
Randomized 132 patients received either Solithromycin or Levofloxacin:
* Early Clinical Response
defined as:
Improvement at 72 hours in severity from baseline in at least two of the following
signs/symptoms: cough, dyspnea, chest pain, sputum production
Without
worsening
in
any
of
the
above
4
signs/symptoms
-
patient
is
clinically
stable
Proposed
by
FDA,
November
3
rd
2011
for
future
CABP
trial
design
and
defined
by
the
Foundation
for
the
NIH
(FNIH) |
 CEM-101
800/400 mg QD
(N=64)
Levofloxacin
750 mg QD
(N=68)
Any Treatment-Emergent Adverse Event (TEAE)
19 (29.7%)
31 (45.6%)
Any study drug related TEAE
7 (10.9)
13 (19.1)
Any Serious Adverse Event (SAE)
2* (3.1)
7** (10.3)
Premature Discontinuation of Study Drug from adverse events
0 (0.0)
6 (8.8)
Premature Discontinuation of Study Drug from study drug related
adverse events
0 (0.0)
2 (2.9)
Deaths
0 (0.0)
1 (1.5)
Solithromycin: Favorable Safety and
Tolerability in Phase 2
CEM-101 demonstrated a favorable safety and tolerability profile, with a lower incidence of
treatment emergent adverse events than levofloxacin
Fewer
treatment
emergent
AEs
(30%
vs
46%)
Fewer
study
subjects
with
SAEs
(2
vs
7
subjects)
Fewer
drug
discontinuations
due
to
AEs
(0
vs
6
subjects)
Fewer
GI
related
AEs
(14%
vs
24%)
No
liver
safety
or
QT
signals
of
concern,
no
bitter
after-taste
14
*Both were
unrelated to study drug **One was unrelated to study drug |
 Differentiation
from Fluoroquinolones 15
Fluoroquinolones
have
a
broad
spectrum
–
but
do
not
have
Anti-inflammatory
properties
-
lower
mortality
rates
are
noted
when
macrolides
are
added
for
CABP
treatment
Safety
record
of
macrolides
Use
in
pregnancy,
pediatrics
Resistance
to
fluoroquinolones
is
likely
to
increase
with
generic
levofloxacin
Fluoroquinolones
affect
bowel
flora
and
select
for
CDAD
Macrolides
have
less
broad
spectrum
effects
Fluoroquinolones
can
cause
tendonitis
and
other
side
effects
Avelox
has
QT
effects
Solithromycin
when
approved
Could
have
the
advantage
of
monotherapy
in
CABP
Could
be
priced
appropriately,
and
Could
be
noted
to
be
fluoroquinolone
sparing |
 Solithromycin:
Intravenous Development First Injectable Macrolide in 20 Years
16
Intravenous
macrolides
have
not
been
developed
because
of
safety
and
tolerability
issues
FDA
interest
in
intravenous
-
Allows
enrollment
of
PORT
III
–
IV
patients
Intravenous
and
oral
formulation
allow:
Flexibility
for
treating
severe
or
moderate
pneumonia
Severely
ill
patients
begin
treatment
in
the
hospital
and
then
go
home
earlier
on
oral
therapy
Pharmacoeconomic
advantage
IV
toxicology,
28-day
in
dog
and
monkey
was
well-tolerated
Phase
1
IV
clinical
trial
underway |
 Plan for Phase 3
CABP Studies 17
Phase
3
CABP
program
consistent
with
the
proposed
FDA
CABP
guidance
Nov.
2011:
One
oral
trial
Two
IV-to-oral
step-down
trials
Primary
endpoint:
Non-inferiority
of
early
response
(at
72
hours)
compared
to
a
fluoroquinolone
Secondary
endpoints:
Safety
and
pooled
mITT
at
early
response
Phase
3
oral
planned
to
begin
second
half
2012
and
complete
in
2014
Global
study
–
<50%
PORT
II,
50%
PORT
III
and
PORT
IV
Enroll
~800
patients
Comparator
moxifloxacin
–
a
fluoroquinolone
that
is
used
worldwide
at
same
dose
Enrollment
criteria
controlled
strictly
as
per
FDA
guidance
Success
criteria:
as
specified
by
newly
proposed
FDA
guidance
Safety
and
tolerability:
secondary
endpoints |
 18
Effective Macrolides Needed for CABP and
Other Therapies
A new macrolide is needed –
for CABP because of resistance and mortality associated with
treatment failures –
no second chance upon failure
Monotherapy
and
IV-Oral
could
allow
pricing
and
efficacy
advantage
over
combination
of
-lactam, such as a cephalosporin, plus
macrolide which is the standard of care
Secondary studies in pharyngitis planned to be run against azithromycin to show
superiority-
high resistance rates makes this possible
Solithromycin could have intravenous advantage, has good oral bioavailability and could
have better anti-inflammatory properties than any other macrolide
|
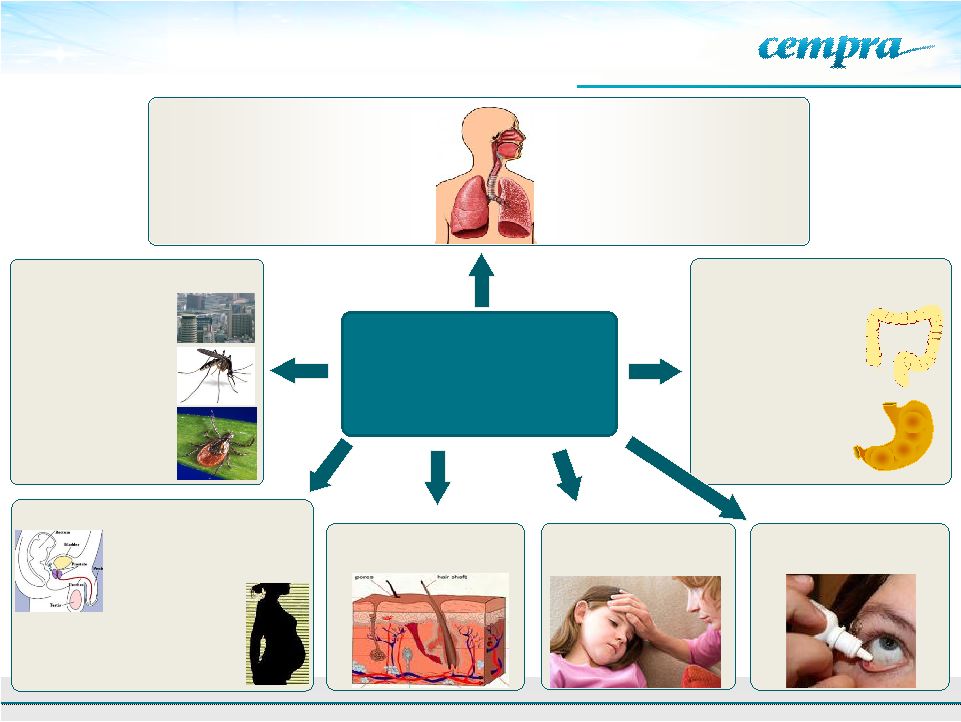 19
Solithromycin for CABP and Multiple
Indications –
Potential Broad Use
Solithromycin-
The Next Generation
Macrolide-
First Fluoroketolide
Campylobacter
diarrhea
Helicobacter
gastritis
Gonococcal and Non-
Gonococcal Urethritis
Simple skin infections
Group B Strep
infections in
pregnancy
Lyme disease and
other tick borne
diseases
Other Diseases:
Ophthalmic drops
Pediatric infections
Malaria
Prophylaxis
Biodefense
GI Tract Diseases:
Antibacterial and Anti-
inflammatory:
Primary Indication : CABP
Simple RTI’s, Pharyngitis, Sinusitis,
Bronchitis, Acute Exacerbation of Chronic
Bronchitis (AECB)
GU Tract Diseases:
Respiratory Tract Infections (RTI):
COPD
Cystic fibrosis
Panbronchiolitis |
 20
TAKSTA™
(Fusidic acid)
An oral antibiotic for MRSA infections
being developed for chronic use in the U.S. |
 21
What is Taksta
TM
?
Taksta is Cempra’s proprietary fusidic acid dosing regimen
Fusidic acid approved in Western Europe, Australia, and other countries
40
years
of
established
safety
and
efficacy
profile
in
acute
and
chronic
use
in
staph
infections ex-U.S.
NCE in the US, Hatch Waxman exclusivity obtained by Cempra, dosing regimen patent,
supply
agreement
of
drug
substance
-
a
fermentation
product
Unique structure, no-cross resistance with any other antibiotic
Orally bioavailable
Targeted
against
Gram-positive
pathogens,
including
MRSA
-
a
pathogen
of
great
concern |
   Antimicrobial
agents
MIC (µg/ml)
90%
% susceptible
Fusidic acid
0.12
99.6
Clindamycin
>2
69.7
Erythromycin
>4
11.2
Levofloxacin
>4
30.2
Linezolid
1
99.9
Tetracycline
1
95.8
TMP/SMX
0.5
97.9
Compared with other Oral Agents
Compared with other Oral Agents
Tetracyclines
and
Bactrim
(TMP/SMX)
have
significant
limitations
for
oral,
outpatient
use
Linezolid is the only oral antibiotic approved for MRSA but is not recommended for long term
use
TAKSTA has Excellent Activity Against
S.
aureus
in
the
U.S.
(1,710
U.S.
strains)
22
Taksta is highly effective against S. aureus strains found in the U.S.
Almost all staph
and MRSA
in the U.S.
are susceptible |
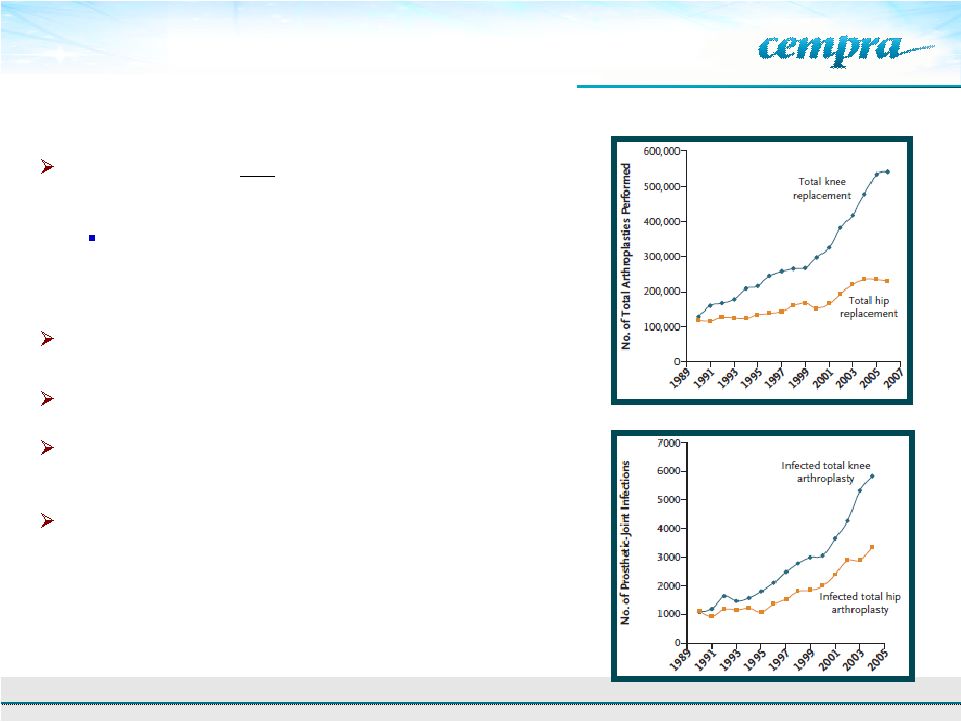 23
Osteomyelitis/Prosthetic Joint Infection is a
Significant Opportunity
Growing need for oral
therapies to address MRSA
infections
Physicians cite staph (MRSA) as top pathogen of
concern in prosthetic joint infections and
osteomyelitis
Prosthetic joint replacement is increasing
Risk of life-long bacterial infection of implant
Minimum duration of treatment is 4-6 weeks, with
many patients requiring life-long treatment
Leading drugs for prosthetic joint infections and
osteomyelitis are the same as those for ABSSSI:
vancomycin, daptomycin and linezolid
Total prosthetic-joint infections 1990-2004 (Kurtz et. al)
New England J Medicine, 2009
Total arthroplasties performed from 1990-2006 (CDC)
|
 European
dosing Cempra’s loading dose
1500 BID loading dose followed by 600 mg maintenance
dose –
minimizes resistance development
Loading
dose
regimen
validated
–
resistance
not
noted
in Phase 2 study
Patent pending
Cempra’s Proprietary TAKSTA Loading Dose
Validated -
Phase 2 Study With MRSA Infections
Cempra developed a proprietary loading dose regimen to prevent resistance to fusidic acid
that has occurred outside the U.S.
24
Data from 155-patient Phase 2 trial demonstrates
efficacy and safety in ABSSSI
Comparable efficacy
to Zyvox (linezolid)
Proprietary loading dose
regimen was well
tolerated and overcame
resistance
Comparable safety to linezolid, despite trial design excluding patients for whom linezolid is
contra- indicated (e.g., those on SSRIs)
End of Phase 2 meeting for ABSSSI with FDA completed |
 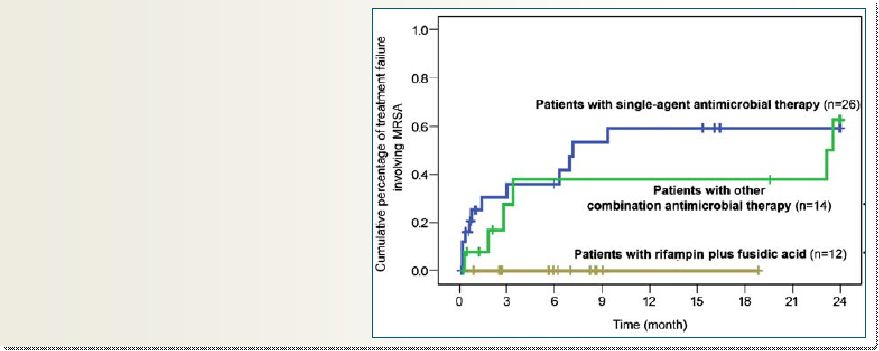 Use In Prosthetic
Joint Infections 25
Used outside the U.S. in Osteomyelitis/PJI
Significant physician interest for chronic use in the U.S.
Eur J. Clin Microbiol Infect Dis (2010) 29: 171-180
Several reports from ex-USA that PJI
can be successfully treated by
adding FA to the standard of care
Taksta
could
address
the
need
for
a
safe,
oral
product
for
acute
and
chronic
treatment in prosthetic joint infections |
    Safety
Tedizolid
Tedizolid
Linezolid
Linezolid
Taksta
Taksta
26
Two compassionate use cases of bone/prosthesis infections in North America
Efficacy is
comparable to linezolid but has
better safety –
useful for oral
chronic use in all
patient populations
PJI Facts:
200K Hip Replacements; 550K Knee Replacements in 2007
Surgeries increase by 3% per year
1% of hips and 2% of knees develop PJI's
Chronic daily therapy
Potential for Taksta for Chronic Use in
Prosthetic Joint Device Infections |
 Current
Treatment: Debride
and
treat
intravenously
and
orally
for
months
–
replace
prosthesis
if possible –
may not be possible in older patients
Study Design:
Success
Criteria:
The
primary
outcome
–
retention
of
a
functional
prosthesis
at the end of 3 months
Other outcome measures: longer term safety and tolerability, joint & mobility
function
Cempra
will
confirm
trial
design
with
the
FDA
–
Orphan
indication
potential
27
Prosthetic Joint Infection Phase 2 Trial Plan –
Chronic Oral Treatment of MRSA
50 patients with
Knee or Hip PJI
Patient
Population
IV vancomycin
+ Taksta
+ Rifampin
Discontinue
vancomycin at
7-28 days
Add Taksta to
Current Standad
of Care
Continue with
Taksta +
Rifampin
for 3 months
Continue for
3 Months
Taksta as oral
monotherapy
beyond 3
months
Long-Term
Therapy |
  2012
Milestones 2Q 12: CEM-101 End of Phase 2 review of Oral Phase 3 protocol
2H 12: CEM-101 Initiation of Phase 3 Oral Trial for CABP
2H 12: CEM-101 Completion of Phase 1 IV
4Q 12: Taksta Initiation of Phase 2 Prosthetic Joint Infection Trial
4Q 12 Solithromycin top line data of Phase 2 Gonococcal Urethritis Study
2013 Milestones
1H 13: CEM-101 Initiation of First Phase 3 IV-to-Oral CABP Trial (with partner
funding) 2H 13: CEM-101 Initiation of Second Phase 3 IV-to-Oral Trial (with
partner funding) 4Q 13: Taksta top line Prosthetic Joint Infection results
2014 Milestones
1H 14: Taksta Phase 2 Prosthetic Joint Study Results
1H 14: CEM-101 Phase 3 Oral CABP Top line data
28
Clinical Development Plan and
Milestones |
 Capitalization
29
Cash & Equivalents (at 9/30/11) *
$ 9M
Long-Term Debt (at 9/30/11)
$ 5M
Shares Outstanding
21.0M
Market Capitalization (at 2/28/12)
$160.2M
Cash Runway **
1H 2014
* Does not include proceeds of 12/11 $10M venture debt or 2/12 $57.9M
IPO ** Includes initiation of CEM-101 P3 oral trial and Taksta P2 PJI
trial; Does not include any potential partnerships. |
 Kenneth Touw,
PhD EVP Regulatory
Carl Foster
EVP Business Development
Mark Hahn, CPA
CFO
David Oldach, MD
SVP Clinical
GILEAD
Prabhavathi Fernandes, PhD
President & CEO
Proven Management Team
David Pereira, PhD
SVP Chemistry
•
Azactam
(aztreonam)
•
Biaxin
(clarithromycin)
•
Dificid
(fidaxomicin)
•
Plenaxis
(abarelix)
•
Zavesca
(miglustat)
•
OncoVax
•
Prilosec
(omeprazole)
•
IPO and M&A
•
Athenix-Bayer CropScience
•
Charles & Colvard (CTHR)
•
E&Y
•
Viread
•
GS-9190
•
Combinations against HCV
•
Synercid
(quinupristin/
dalfopristin)
•
Altace
(ramipril)
•
Embeda
(morphine
and
naltrexone)
•
Acurox
(oxycodone)
•
Injectable Penicillins
•
Dobutamine HCl Injection
•
Ranitidine Injection
30 |
