Attached files
Exhibit 10.32
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
AMENDED AND RESTATED
DEVELOPMENT AND LICENSE AGREEMENT
BY AND BETWEEN
BIOCRYST PHARMACEUTICALS, INC.
AND
MUNDIPHARMA INTERNATIONAL CORPORATION LIMITED
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
TABLE OF CONTENTS
| PAGE | ||||
| ARTICLE 1 - DEFINITIONS |
1 | |||
| ARTICLE 2 - GRANT |
7 | |||
| 2.1 License Grants |
7 | |||
| 2.2 BioCryst Retained Rights |
7 | |||
| 2.3 Mundipharma Exclusive Option |
7 | |||
| 2.4 BCX-4208 |
8 | |||
| 2.5 BCX-5235 |
8 | |||
| 2.6 Mundipharma Research Outside the Field |
8 | |||
| ARTICLE 3 - REPORTS |
8 | |||
| 3.1 Mundipharma Sole Control |
8 | |||
| 3.2 Reports |
8 | |||
| 3.3 Safety |
8 | |||
| ARTICLE 4 - DEVELOPMENT, COMMERCIALIZATION AND COOPERATION |
8 | |||
| 4.1 Transition Assistance |
8 | |||
| 4.2 Mundipharma Development Obligations |
9 | |||
| 4.3 Development Costs |
9 | |||
| 4.4 Commercialization |
9 | |||
| 4.5 Costs of Commercialization |
10 | |||
| 4.6 Report of Results, Data and Information |
10 | |||
| 4.7 Interactions with Government Agencies |
10 | |||
| ARTICLE 5 - UNDERTAKINGS OF BIOCRYST AND MUNDIPHARMA |
10 | |||
| 5.1 Non-Use and Non-Disclosure |
10 | |||
| 5.2 Authorized Disclosure |
10 | |||
| 5.3 Manufacturing |
10 | |||
| 5.4 Maintenance of License |
10 | |||
| ARTICLE 6 - PAYMENTS - ROYALTIES |
11 | |||
| 6.1 Payments Under Original Agreement |
11 | |||
| 6.2 Payments |
11 | |||
| 6.3 Royalties Payable by Mundipharma |
11 | |||
| 6.4 Royalty Reports |
12 | |||
| 6.5 Records |
12 | |||
| 6.6 Audit |
12 | |||
| 6.7 Foreign Currency Conversion |
12 | |||
| 6.8 Nature of Payments |
12 | |||
| 6.9 Payments to Third Parties |
12 | |||
| ARTICLE 7 - TRADEMARKS AND DOMAIN NAMES |
12 | |||
| 7.1 Right to Use Trademarks |
12 | |||
| 7.2 Secondary Marks |
12 | |||
i
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
TABLE OF CONTENTS
(continued)
| PAGE | ||||
| 7.3 Property in Trademarks and Payment of Fees |
13 | |||
| 7.4 Domain Names |
13 | |||
| ARTICLE 8 - LITIGATION, PATENT PROSECUTION AND ROYALTY OFFSET |
13 | |||
| 8.1 Litigation |
13 | |||
| 8.2 Patent Prosecution |
14 | |||
| 8.3 Registration of Patent License |
14 | |||
| 8.4 Royalty Offset |
14 | |||
| ARTICLE 9 - REPRESENTATIONS AND WARRANTIES |
15 | |||
| 9.1 BioCryst’s Representations and Warranties |
15 | |||
| 9.2 Mundipharma’s Representations and Warranties |
17 | |||
| 9.3 No other Representations |
17 | |||
| 9.4 Disclaimers |
18 | |||
| ARTICLE 10 - INDEMNITY AND PRODUCT LIABILITY |
18 | |||
| 10.1 Indemnification and Defense by Mundipharma |
18 | |||
| 10.2 Indemnification and Defense by BioCryst |
18 | |||
| 10.3 Defense Procedures |
18 | |||
| 10.4 Insurance |
18 | |||
| 10.5 Survival |
19 | |||
| 10.6 Disclaimer of Liability for Consequential Damages |
19 | |||
| ARTICLE 11 - TERM AND TERMINATION |
19 | |||
| 11.1 Term |
19 | |||
| 11.2 Termination by Parties |
19 | |||
| 11.3 Rights and Obligations of Parties upon Term Expiration or Termination |
20 | |||
| ARTICLE 12 - DISPUTE RESOLUTION AND GOVERNING LAW |
21 | |||
| 12.1 Disputes |
21 | |||
| 12.2 Dispute Resolution |
22 | |||
| 12.3 Governing Law |
22 | |||
| ARTICLE 13 - MISCELLANEOUS |
22 | |||
| 13.1 Mutual Release |
22 | |||
| 13.2 Covenants |
22 | |||
| 13.3 Non-Compete |
22 | |||
| 13.4 Delay of payment |
23 | |||
| 13.5 Assignment |
23 | |||
| 13.6 Pre-Existing Third Party License |
23 | |||
| 13.7 Press Releases and External Communications |
23 | |||
| 13.8 Use of Name |
23 | |||
| 13.9 Notices |
23 | |||
| 13.10 Effect of Waiver |
24 | |||
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
TABLE OF CONTENTS
(continued)
| PAGE | ||||
| 13.11 Effect of Partial Invalidity |
24 | |||
| 13.12 Force Majeure |
24 | |||
| 13.13 Entire Agreement; Amendment |
24 | |||
| 13.14 Status of Parties |
25 | |||
| 13.15 Further Assurances |
25 | |||
| 13.16 Performance by Associates |
25 | |||
| 13.17 Intellectual Property |
25 | |||
| 13.18 Counterparts; Facsimile Signature |
25 | |||
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
AMENDED AND RESTATED LICENSE AND DEVELOPMENT AGREEMENT
This Amended and Restated License and Development Agreement is made as of November 11, 2011 (the “Effective Date”) by and between BioCryst Pharmaceuticals, Inc., a company organized and existing under the laws of Delaware having offices at 4505 Emperor Blvd, Suite 200 Durham, NC 27703 (“BioCryst”) and Mundipharma International Corporation Limited, a Bermudan company, having offices at Mundipharma House, 14 Par-la-Ville Road, Hamilton, Bermuda HMJX (“Mundipharma”) (hereinafter, each of BioCryst and Mundipharma a “Party” and, collectively, the “Parties”).
W I T N E S S E T H :
WHEREAS, BioCryst owns or controls patents and know-how related to a series of proprietary compounds which act as PNP Inhibitors (as defined below), including the compound known as BCX-1777.
WHEREAS, Mundipharma has expertise in the discovery, development, manufacture and sale of pharmaceutical products.
WHEREAS, Mundipharma and BioCryst entered into a License and Development Agreement dated as of February 1, 2006 (the “Original Agreement”) pursuant to which BioCryst granted to Mundipharma, in certain countries, rights and licenses relating to BCX-1777 under certain patents, know-how and trademarks owned or controlled by BioCryst.
WHEREAS, certain disagreements developed between the Parties under the Original Agreement.
WHEREAS, the Parties desire to settle such disagreements by releasing each other from all claims relating thereto, and desire to re-affirm their license relationship by entering into this Agreement in place of the Original Agreement.
NOW, THEREFORE, in consideration of the mutual promises and covenants contained in this Agreement, the parties agree as follows:
ARTICLE 1 - DEFINITIONS
As used in this Agreement, the following terms shall have the following meanings:.
1.1 “Associate” of a Party means any person, firm, trust, corporation or other entity or combination thereof which directly or indirectly (a) controls said Party, (b) is controlled by said Party, or (c) is under common control with said Party; the terms “control” and “controlled” meaning direct or indirect ownership (including pursuant to any option, warrant or other arrangement or understanding) of fifty percent (50%) or more, including ownership by trusts with substantially the same beneficial interests, of the voting rights, shares or other equity interests of such person, firm, trust, corporation or other entity or combination thereof or the power to direct the management of such person, firm, trust, corporation or other entity or combination thereof.
1.2 “ATL” means adult T-cell leukaemia/lymphoma.
1.3 “Autoimmune Indications” means all indications that involve pathogenic consequences, including tissue injury, produced by autoantibodies or autoreactive T lymphocytes interacting with self epitopes, i.e. autoantigens. Autoimmune Indications shall include, without limitation, asthma, psoriasis, rheumatoid arthritis, systemic lupus erythematosus, scleroderma, juvenile rheumatoid arthritis, polymyositis, ankylosing spondylitis, Type I diabetes, sarcoidosis, Sjogrens syndrome, chronic active non-pathogenic hepatitis, non-infectious uveitis (Behcet’s), aplastic anemia, hemolytic anemia, idiopathic thrombocytopenia purpura, vasculitis, Hashimoto’s thyroiditis, atopic dermatitis, regional non-pathogenic enteritis (including ulcerative colitis, Crohn’s disease and inflammatory bowel disease), Kawasaki’s disease, post-infectious encephalitis, myasthenia gravis, multiple sclerosis, alopecia and tropic spastic paraparesis.
Page 1
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
1.4 “BCX-4208” means the PNP Inhibitor known as BCX-4208 having the following chemical structure * * *.
1.5 “BCX-5235” means * * *.
1.6 “BCX-5235 License Agreement” is defined in Section 2.5.1.
1.7 “BioCryst Indemnitees” is defined in Section 10.1.
1.8 “BioCryst Know-How” means all knowledge and proprietary information, including know-how, trade secrets, data, technology and scientific and technical information now or hereafter during the Term owned, developed or controlled by BioCryst or any of its Associates, which relate to the Compound or the Licensed Products, including but not limited to: (a) medical, clinical, toxicological or other scientific data, and (b) processes and analytical methodology useful in respect of the Initial Mundipharma Trials Plan, Mundipharma Trials Plan and/or the Development, testing, analysis, manufacture or packaging of the Compound or the Licensed Products.
1.9 “BioCryst Patents” means those patents and patent applications set forth on Schedule 1.9, and all patents and patent applications that claim priority to any of the foregoing or which claim the manufacture, use or sale of the Compound or Licensed Products in the Territory, in each case which patent applications and patents are owned or controlled by BioCryst or its Associates, or as to which BioCryst or any of its Associates have a license with rights to sublicense, during the Term, and any extensions, supplementary protection certificates, continuations, continuations-in-part, divisions, reissues, re-examinations, additions, substitutions, confirmations, registrations, or re-validations of or to any of the foregoing.
1.10 “Business Day” means a day that is not a Saturday, Sunday or a day on which banking institutions in New York, New York or London, England, are authorized by Legal Requirements to remain closed.
1.11 “Cancerous State” means a state in which cells exhibit aberrant and uncontrolled proliferation that are believed to be malignant.
1.12 “Claims” means any and all losses, liabilities, costs and expenses (including attorneys’ fees and expenses), debts and other obligations arising out of or resulting from claims, judgments, damages of any kind whatsoever (including but not limited to compensatory, exemplary and punitive damages), arbitral awards, and amounts paid in settlement of claims, judgments, legal (including but not limited to judicial, arbitral and administrative) proceedings and the like, which claims, judgments, damages, awards, settlements, legal proceedings and the like which arise out of or are connected or related in any way whatsoever to the design or clinical investigation or research or testing or labeling or manufacturing or packaging or marketing or sale or distribution of the Compound or Licensed Products, including (but not limited to) physical injury, death or product liability and similar Third Party claims.
1.13 “CLL” means chronic lymphocytic leukaemia.
1.14 “Commercialization” means, with respect to the Licensed Products, any and all processes and activities conducted to permit, establish, promote and maintain sales for the Licensed Products, including negotiating and obtaining Pricing Approvals, manufacturing, offering for sale, detailing, commercializing (including launch), promoting, storing, transporting, supporting, distributing, and importing the Licensed Products, and all Phase IV and post-marketing studies and tests of such Licensed Products, but in all cases excluding the Initial Mundipharma Trials Plan and any Mundipharma Trials Plan and Development. “Commercialize” and “Commercializing” shall have their correlative meanings.
1.15 “Commercially Reasonable Efforts” means with respect to the Initial Mundipharma Trials Plan and any Mundipharma Trials Plan a level of resources, efforts and urgency to develop the Licensed Products in accordance with the Initial Mundipharma Trials Plan and any subsequent Mundipharma Trials Plan that is consistent with Mundipharma (or its Associates’) practices in diligently and actively pursuing clinical trials in respect of its other pharmaceutical products at a similar stage of product life, and having similar safety, efficacy and commercial potential. Should Mundipharma, in its sole discretion, determine not to terminate this Agreement after
Page 2
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
the Initial Mundipharma Trial Plan or any Mundipharma Trials Plan has been completed or otherwise ended, then Commercially Reasonable Efforts means with respect to (i) Development a level of resources, efforts and urgency applied by Mundipharma to Develop the Licensed Products in accordance with Mundipharma’s current Development plan that is consistent with Mundipharma (or its Associates’) practices in diligently and actively pursuing such development activities in respect of its other pharmaceutical products at a similar stage of product life, and having similar safety, efficacy and commercial potential; and (ii) Commercialization a level of resources, efforts and urgency applied by Mundipharma in a country, following grant of both Regulatory Approval and Pricing Approval in a form satisfactory to Mundipharma, to Commercialize the Licensed Products in such country that is consistent with Mundipharma (or its Associates’) practices in diligently and actively pursuing commercialization of its other pharmaceutical products at a similar stage of product life, and having similar safety, efficacy and commercial potential. It is understood that any such resources, efforts and urgency may change from time to time during the Term based upon changing safety, efficacy, scientific, business and commercial considerations.
1.16 “Compound” means the PNP Inhibitor known as BCX-1777 as claimed in the BioCryst Patents having the following chemical structure
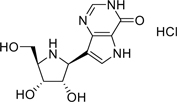
and including the salts, esters, metabolites, tautomers, isomers, conjugates and complexes thereof.
1.17 “Compound Transfer” is defined in Section 4.1.2.
1.18 “Confidential Information” means any and all information, data or know-how of a confidential nature (including BioCryst Know-How and Mundipharma Know-How), whether financial, business, legal, technical or non-technical, oral or written, related to the Compound or the Licensed Products or otherwise related to a Party or its licensors that is disclosed by one Party or its Associates (“Disclosing Party”) to the other Party or its Associates (“Receiving Party”).
Confidential Information shall not include any information which:
(i) either before or after disclosure to the Receiving Party, was or becomes published or generally known to the public through no fault or omission on the part of the Receiving Party; or
(ii) was known or used by the Receiving Party prior to its disclosure by the Disclosing Party to the Receiving Party; or
(iii) either before or after disclosure to the Receiving Party, is provided to the Receiving Party without restriction by a Third Party having the legal right to do so; or
(iv) is independently developed by the Receiving Party without access to or use of the Disclosing Party’s Information; or
(v) is required to be disclosed by the Receiving Party to comply with applicable laws, to defend or prosecute litigation or to comply with governmental regulations, provided that, the Receiving Party provides prior written notice of such disclosure to the Disclosing Party and, to the extent practicable, takes reasonable and lawful actions to minimize the degree of such disclosure.
1.19 “Decision Period” is defined in Section 8.1.4.
Page 3
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
1.20 “Develop” means with respect to Mundipharma for the Licensed Products, any and all processes and activities in accordance with a Mundipharma development plan subsequent to completion or termination of the Initial Mundipharma Trials Plan and any Mundipharma Trials Plan. “Develop”, “Development” and “Developing” shall have their correlative meanings.
1.21 “U.S. Dollars”, or “$” means dollars constituting legal tender for the payment of public and private debts in the United States of America.
1.22 “Effective Date” is defined in the preamble.
1.23 “EMEA” means the European Agency for the Evaluation of Medicinal Products.
1.24 “Field” means the treatment of all Cancerous and/or Pre-Cancerous States in humans.
1.25 “First Commercial Sale” means the first shipment for commercial sale of a Licensed Product in the Territory by Mundipharma or its Associates to a Third Party.
1.26 “Force Majeure Event” is defined in Section 13.12.
1.27 “Foreign Currency Sales” is defined in Section 6.3.2.
1.28 “Generic Compounds” means any pharmaceutical products, other than the Licensed Products, that (i) are marketed for sale by a Third Party, * * *.
1.29 “Governmental Authority” means any court, agency, department, authority or other instrumentality of any foreign, federal, state, county, city or other political subdivision.
1.30 “Gross Price” means, with respect to a Licensed Product, the unit price, without deduction, actually invoiced by Mundipharma, its sublicensees or its Associates for the sale of such Licensed Product.
1.31 “Hyperuricemea” means an excess of uric acid in the blood, whether or not symptoms of gout are present.
1.32 “IND” means an Investigational New Drug Application under the U.S. Federal Food, Drug and Cosmetic Act, as amended, and the regulations promulgated thereunder, or equivalent in another jurisdiction.
1.33 “Initial Mundipharma Trials Plan” means with respect to Mundipharma, for the Licensed Product the conduct until the first interim analysis of * * *.
1.34 “Initiating Party” is defined in Section 8.1.6.
1.35 “Legal Requirements” means all laws, statutes, rules, regulations, orders, decrees, judgments and/or ordinances of any Governmental Authority and any present and future supra-national, national, state and local laws (including rules and regulations having the force of law); requirements under permits; orders, decrees, judgments and directives; requirements of the Regulatory Authorities, including without limitation cGMPs, Council Regulation (EEC) No 2309/93, requirements imposed under the Federal Food, Drug and Cosmetic Act and the Public Health Service Act, each as amended from time to time, requirements in 21 C.F.R. Part 312, applicable requirements in 21 C.F.R. Parts 600-680 and similar requirements of Regulatory Authorities in jurisdictions outside the United States.
1.36 “Licensed Products” means all pharmaceutical preparations in all dosage strengths, formulations and methods of administration that contain the Compound as its active ingredient, alone or in combination with another active ingredient.
Page 4
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
1.37 “Licensed Products Sold” means sales of the Licensed Products by Mundipharma or its Associates or its permitted Third Party sublicensees to Third Parties (other than Third Party sublicensees) in the Territory.
1.38 “Marketing Authorization” means the product license or marketing authorization necessary as a prerequisite for marketing and selling the Licensed Products in each country of the Territory.
1.39 “Material Default” means, with respect to each Party:
(i) any default by any Party hereto of its covenants, representations, agreements or other performance obligations under this Agreement (other than a payment obligation) (a) that is a material breach or (b) that when aggregated with any other such uncured defaults by such Party, constitutes a material breach and, in the case of either clause (a) or (b), if such default is capable of being cured, shall have continued for * * * (* * *) days after written notice thereof was provided to the alleged defaulting Party by the non-defaulting Party (or, if such default cannot be cured within such * * *-day period, if the alleged defaulting Party does not promptly commence and diligently continue all reasonable actions to cure such defaults during such * * *-day period); or
(ii) any default by any Party hereto of its payment obligations hereunder that shall have continued for * * * (* * *) days after written notice thereof was provided to the alleged defaulting Party by the non-defaulting Party.
1.40 “Mundipharma Indemnitees” is defined in Section 10.2.
1.41 “Mundipharma Know-How” means all know-how, trade secrets, data, technology, scientific and technical information, improvements and inventions, now or hereafter during the Term owned, developed or acquired by Mundipharma or any of its Associates, or by any Third Party on behalf of Mundipharma or its Associates, which relate to the Compound or the Licensed Products, including but not limited to: (a) medical, clinical, toxicological or other scientific data, and (b) processes and analytical methodology useful in respect of the Mundipharma Trials Plan and/or the Development, testing, analysis, manufacture or packaging of the Compound or the Licensed Products.
1.42 “Mundipharma Notice” is defined in Section 2.5.1.
1.43 “Mundipharma Patents” means all patent applications and patents which claim improvements upon or modifications to the inventions and discoveries disclosed or claimed in any of the BioCryst Patents or which claim the manufacture, use or sale of Compound or Licensed Products, which patent applications and patents are owned or controlled by Mundipharma or its Associates, or as to which Mundipharma or any of its Associates have a license with rights to sublicense, during the Term, and any extensions, supplementary protection certificates, continuations, continuations-in-part, divisions, reissues, re-examinations, additions, substitutions, confirmations, registrations, or re-validations of or to any of the foregoing.
1.44 “Mundipharma Trials Plan” means with respect to Mundipharma, for the Licensed Product, any subsequent Mundipharma Trials Plan for the development or Regulatory Approval of the Licensed Products which is provided to BioCryst after completion or termination of the Initial Mundipharma Trials Plan or a prior Mundipharma Trials Plan.
1.45 “NDA” means a New Drug Application, including all supplements and amendments thereto, for the approval of a Licensed Product as a new drug under the U.S. Federal Food, Drug and Cosmetic Act, as amended, or equivalent in another jurisdiction, and the regulations promulgated thereunder filed with a Regulatory Authority.
1.46 “Net Sales” means (a) the Gross Price of Licensed Products multiplied by the quantity of Licensed Products Sold at such Gross Price LESS, only as specifically applicable to Licensed Products Sold, (b) the sum of * * *.
1.47 “New Indications” means any indication outside the Field (excluding hyperuricemia and gout).
1.48 “Original Agreement” is defined in the recitals.
Page 5
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
1.49 “Patent Activities” is defined in Section 8.2.1.
1.50 “Payments” is defined in Section 6.2.
1.51 “Phase II” means a human clinical trial performed to evaluate the efficacy of a Licensed Product for a particular indication or indications in patients with the disease or condition under study and/or to determine the common short-term side effects and risks associated with the drug, as described in 21 C.F.R. Part 312, as it may be amended.
1.52 “PNP Inhibitor” means * * *.
1.53 “Pre-Cancerous State” means any abnormal proliferation of cells exhibiting features characteristic of cancer that are of genetic or iatrogenic origin but are not in a Cancerous State, including without limitation actinic keratosis, Barrett’s oesophagus, cervical intraepithelial neoplasia, colonic polyposis, lymphomatoid papulosis, lymphomatoid granulomatosis, oral leukoplakia, other lymphoproliferative disorders, Putz-Jeghers Syndrome, Purtilo Syndrome and Xeroderma Pigmentosum.
1.54 “Pre-Existing Third Party License” means the agreement dated June 27, 2000 by and between, on the one hand, Albert Einstein College of Medicine of Yeshiva University, a division of Yeshiva University and Industrial Research Ltd., and on the other hand BioCryst, as amended on July 26, 2002 and on April 15, 2005, December 11, 2009, May 5, 2010 and as may be amended after the Effective Date.
1.55 “Pricing Approval” means, in a country of the Territory where a Governmental Authority or non-governmental body with relevant statutory authority approves or, thereafter, determines pricing for pharmaceutical products for reimbursement or otherwise, such approval or determination.
1.56 “PTCL” means peripheral T-cell lymphoma.
1.57 “Regulatory Approval” with respect to a particular jurisdiction in the Territory and Licensed Product means the receipt of all regulatory approvals necessary for sale of the Licensed Product in that jurisdiction, but excluding Pricing Approval.
1.58 “Regulatory Authority” means the FDA, or in the case of a jurisdiction outside of the United States, such corollary or other appropriate Regulatory Authority with similar responsibilities, including, without limitation, the EMEA.
1.59 “Secondary Marks” is defined in Section 7.2.
1.60 “SLT” or “Second-Line Treatment” means the treatment of patients that have been previously treated for cancer, but have had a refraction or relapse of cancer.
1.61 “Stem-Cell Transplantation” means a procedure in which healthy stem cells are infused to help restore normal bone marrow function.
1.62 “Suit Notice” is defined in Section 8.1.4.
1.63 “Term” has the meaning defined in Section 11.1.1.
1.64 “Territory” means the world.
1.65 “Third Party(ies)” shall mean any party other than BioCryst, Mundipharma and their respective Associates from time to time.
1.66 “Trademarks” means the trademarks “Fodosine” and “Fodozan”.
1.67 “Transition Assistance Schedule” is defined in Section 4.1.
Page 6
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
1.68 “Transplantation Indications” means all indications that involve the suppression of rejection of transplanted organs, bone marrow or other tissue, including, without limitation, solid organ transplantation (including tolerance induction and xenotransplantation), bone marrow transplantation, graft versus host disease and cell transplantation.
1.69 “Valid Claim” means any claim in an issued and unexpired patent included within Schedule 1.9 which has not been revoked or held unenforceable or invalid by a final, non-appealable decision of a court of other Governmental Authority of competent jurisdiction.
1.70 Interpretations. The definitions of the terms herein apply equally to the singular and plural forms of the terms defined. Whenever the context may require, any pronoun will include the corresponding masculine, feminine and neuter forms. The words “include”, “includes” and “including” will be deemed to be followed by the phrase “without limitation.” Unless the context requires otherwise, (A) any definition of or reference to any agreement, instrument or other document herein will be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein or therein), (B) any reference to any laws herein will be construed as referring to such laws as from time to time enacted, repealed or amended, (C) any reference herein to any person will be construed to include the person’s permitted successors and assigns, (D) the words “herein”, “hereof” and “hereunder”, and words of similar import, will be construed to refer to this Agreement in its entirety and not to any particular provision hereof, (E) any reference herein to the words “mutually agree” or “mutual written agreement” will not impose any obligation on either Party to agree to any terms relating thereto, and (F) all references herein to Articles, Sections, Exhibits or Schedules will be construed to refer to Articles, Sections, Exhibits and Schedules of this Agreement. The table of contents, captions and Section headings appearing in this Agreement are inserted only as a matter of convenience and in no way define, limit, construe or describe the scope or intent of such Sections or of this Agreement, nor in any way affect this Agreement.
ARTICLE 2 - GRANT
2.1 License Grants. Subject to the rights retained by BioCryst and its licensors as set forth in Section 2.2, BioCryst grants to Mundipharma an exclusive, royalty-bearing, right and license in the Territory, with the right to sublicense without the consent of BioCryst (provided that such sublicensees agree in writing to be bound by all of the terms of this Agreement and no Third Party sublicensee shall have the right to further sublicense rights), to develop, register, make, have made, package and have packaged, use, promote, market, offer for sale, sell and import Licensed Products in the Field, in each case under the BioCryst Patents and BioCryst Know-How. Where Mundipharma has an obligation to the licensors of the Pre-Existing Third Party License to seek their consent for a sub-license, it shall give BioCryst the first opportunity to approach the said licensors on Mundipharma’s behalf to obtain such consent.
2.2 BioCryst Retained Rights. BioCryst retains all of the following rights:
2.2.1 subject to Section 2.3, all rights under the BioCryst Patents and the BioCryst Know-How outside of the Field;
2.2.2 the rights reserved to the licensors and the governments under the Pre-Existing Third Party License;
2.2.3 the right to conduct or continue further non-commercial research into the Licensed Products and/or the Compound, but not to conduct pre-clinical or clinical trials into the Licensed Products and/or the Compound without Mundipharma’s prior written consent; and
2.2.4 all rights with respect to BCX-5235 other than as explicitly set forth in Section 2.5.
2.3 Mundipharma Exclusive Option. In further consideration of the commitments made and payments to be made under this Agreement, BioCryst hereby grants to Mundipharma an exclusive option during the Term to negotiate a new license agreement to conduct research with, develop and commercialize the Compound and Licensed Products in New Indications, upon terms mutually agreeable to both parties, as
Page 7
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
determined by each party in its sole discretion. Such negotiations shall be conducted by both Parties in good faith. BioCryst further agrees, subject to Section 2.2.3, that during the Term it shall not, and shall not grant rights to an Associate or Third Party to, develop, register, make, package, use, promote, market, offer for sale, sell or commercialize the Compound or Licensed Products in an indication outside the Field.
2.4 BCX-4208. In further consideration of the commitments made and payments to be made under this Agreement, BioCryst agrees that during the Term it shall not, and shall not grant rights to an Associate or a Third Party to, develop, make, use, sell or commercialize pharmaceutical products containing BCX-4208 within the Field. Notwithstanding the foregoing and for the purposes of clarity, it is understood and agreed that (i) no rights related to BCX-4208 are granted to Mundipharma in this Agreement and (ii) the Field does not include Autoimmune Indications, Stem-Cell Transplantation, Transplantation Indications and Hyperuricemia.
2.5 BCX-5235. During the Term of this Agreement, BioCryst shall be obligated to negotiate with Mundipharma to grant it a license to BCX-5235 in accordance with this Section 2.5.
2.5.1 * * *.
2.5.2 * * *.
2.5.3 In the context of Sections 2.3, 2.5.1, and 2.5.2, ‘good faith’ shall include the use of commercially reasonable efforts to reach agreement of terms which are mutually acceptable to both Parties.
2.6 Mundipharma Research Outside the Field. Mundipharma shall not engage and shall not permit a Third Party to engage in any research outside the Field without BioCryst’s consent in accordance with this Section 2.6. In the event that Mundipharma desires to conduct certain in vitro studies or proof of concept in vivo studies with the Licensed Products in New Indications, or to supply Licensed Products to a Third Party investigator to permit him to do so, then Mundipharma shall first provide to BioCryst a detailed written description of the proposed studies along with any other information that BioCryst reasonably requests with respect to such studies. Upon receipt of sufficient information from Mundipharma, BioCryst shall determine, in its sole discretion, whether or not such studies could potentially have a negative impact on its PNP inhibitor compounds generally. In the event BioCryst determines that no potential for negative impact exists, then BioCryst’s consent for such studies shall not be unreasonably withheld.
ARTICLE 3 - REPORTS
3.1 Mundipharma Sole Control. It shall be agreed and understood that Mundipharma shall control all aspects of the development and Commercialization of the Compound and Licensed Products in the Territory.
3.2 Reports. During the Term of this Agreement, Mundipharma will provide semi-annual reports to BioCryst, with the first report due six (6) months after the Effective Date, regarding the Development and Commercialization of the Compound and Licensed Products which shall contain a summary of the progress of the Mundipharma Trials Plan, Development and Commercialization of the Compound and Licensed Products, copies of publications, copies of published Mundipharma Patents and safety information related to the Compound and the Licensed Product.
3.3 Safety. If either Party becomes aware of a safety signal, not previously known, which may materially impact PNP Inhibitors as a class, then such Party will promptly provide such information to the other Party in sufficient detail to allow a reasonable evaluation of the information.
ARTICLE 4 - DEVELOPMENT, COMMERCIALIZATION AND COOPERATION
4.1 Transition Assistance. Notwithstanding anything set forth in the Original Agreement to the contrary, as of the Effective Date, BioCryst shall have no further obligation to, and shall not in future, develop or commercialize the Licensed Products. As such, the Parties shall cooperate to facilitate and enable the transfer of certain activities and all data relating to the Compound and Licensed Products to Mundipharma in the Territory pursuant to the conditions and protocols set forth on Schedule 4.1 (the “Transition Assistance Schedule”). In addition to each Party’s obligations pursuant to the Transition Assistance Schedule, the Parties hereby agree that:
4.1.1 BioCryst Technical Assistance. During the transfer process of the data as specified on Schedule 4.1 and for a period of sixty (60) days thereafter, BioCryst shall render to Mundipharma technical assistance as may be reasonably required in transferring all aspects of the development program for the Licensed Product to Mundipharma and as otherwise agreed upon by the Parties in writing. Thereafter, BioCryst shall be reasonably compensated for any additional technical assistance provided to Mundipharma to further the development and Commercialization of the Licensed Products.
Page 8
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
4.1.2 Purchase of Supplies. No later than fifteen (15) business days after the Effective Date BioCryst shall deliver and Mundipharma shall take possession of and purchase (at * * *) lots * * * of the Compound, as more particularly described in Schedule 4.1.2 (“Compound Transfer”). Upon completion of the Compound Transfer, BioCryst shall have no further obligation to supply to Mundipharma any other materials or compounds relating to, or associated with, the Licensed Products. Any Compound delivered to Mundipharma hereunder shall be accompanied by a Certificate of Analysis from the manufacturer. Mundipharma will be entitled, at its cost and expense to test the Compound delivered to Mundipharma hereunder to determine whether such Compound complies with the specifications as set forth in Schedule 4.1.2. Mundipharma shall have until * * * or * * * (* * *) calendar days from the date of receipt of Compound, whichever is the later, to inspect and reject acceptance by written notice to BioCryst; provided, however, that any such notice shall set forth the reason for such rejection. If BioCryst does not receive Mundipharma’s written notice of rejection by * * *, or within * * * (* * *) calendar days from receipt of the Compound, whichever is the later, Mundipharma shall be deemed to have accepted the Compound. Mundipharma’s sole remedy for Compound which does not meet the specifications shall be a refund of amounts paid by Mundipharma for such Compound.
4.1.3 Future Data. BioCryst shall promptly during the Term provide all new BioCryst Know-How and any other data related to the Compound or the Licensed Products arising after the Effective Date to Mundipharma.
4.2 Mundipharma Development Obligations. Mundipharma will use Commercially Reasonable Efforts to undertake the Initial Mundipharma Trials Plan. Should Mundipharma, in its sole discretion, determine not to terminate this Agreement after the Initial Mundipharma Trials Plan has been completed or otherwise ended, Mundipharma shall submit to BioCryst a new Mundipharma Trials Plan for continued development of the Licensed Product. Upon completion or termination of any Mundipharma Trials Plan, Mundipharma shall, within a reasonable period of time after completion or termination of such Mundipharma Trials Plan, either terminate the Agreement or provide a new Mundipharma Trials Plan to BioCryst to continue development of the Licensed Product; provided, however, that upon completion or termination of the last Mundipharma Trials Plan, Mundipharma shall either terminate the Agreement or use Commercially Reasonable Efforts to Develop and Commercialize the Licensed Products in the Territory in compliance with Legal Requirements. The Parties acknowledge and agree that Mundipharma shall have no other obligation in respect of the development or Commercialization of the Compound or Licensed Products and shall be entitled to terminate this Agreement, at its sole discretion, in accordance with Section 11.2.3.3 at any time.
4.3 Development Costs. All Development costs incurred on or after the Effective Date shall be borne by Mundipharma.
4.4 Commercialization.
4.4.1 Mundipharma shall notify BioCryst within five (5) days of the date of the First Commercial Sale of a Licensed Product in each country within the Territory.
4.4.2 Mundipharma is entitled to engage contract sales organizations to supplement or complement Mundipharma’s sales force in the Territory, provided that Mundipharma shall at all times remain primarily responsible and liable for all such activities as if such activities had been undertaken by Mundipharma.
4.4.3 * * *.
Page 9
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
4.5 Costs of Commercialization. Mundipharma or its Associates shall be responsible for all costs associated with the Commercialization of Licensed Products within the Territory.
4.6 Report of Results, Data and Information.
All preclinical and clinical data generated by or on behalf of Mundipharma beginning as of the Effective Date of this Agreement shall be owned by Mundipharma and shall constitute a part of Mundipharma’s Know-How. All preclinical and clinical data generated by or on behalf of Mundipharma prior to the Effective Date of this Agreement shall be governed by the terms of the Original Agreement as if the Original Agreement remained in force.
4.7 Interactions with Government Agencies.
4.7.1 Following the transfer of responsibility pursuant to Schedule 4.1, Mundipharma shall be solely responsible for the collection, review, assessment, tracking and filing with appropriate Regulatory Authorities of information related to adverse events associated with the Licensed Products in accordance with applicable FDA regulations, including without limitation 21 CFR §§ 312.32, 314.80, and with comparable Legal Requirements in relevant countries within the Territory. In accordance with Schedule 4.1, BioCryst shall transfer its global safety database for the Licensed Products to Mundipharma’s designee together with all other information and data in BioCryst’s possession or control which is relevant or useful in assisting Mundipharma to fulfill its responsibility pursuant to this Section 4.7. BioCryst shall in future forward any information related to adverse events associated with the Licensed Products to Mundipharma’s designee immediately upon receipt.
4.7.2 Governmental Authority Inquiries. Mundipharma shall notify BioCryst within two (2) Business Days after it receives information about the initiation of any investigation, review or inquiry by the FDA, EMEA or other Governmental Authority concerning (i) non-clinical or clinical research relating to the Compound or the Licensed Product; or (ii) the Commercialization of the Licensed Products.
ARTICLE 5 - UNDERTAKINGS OF BIOCRYST AND MUNDIPHARMA
5.1 Non-Use and Non-Disclosure. During the Term and thereafter, a Receiving Party shall (i) treat Confidential Information provided by Disclosing Party as it would treat its own information of a similar nature, (ii) take all reasonable precautions not to disclose such Confidential Information to Third Parties without the Disclosing Party’s prior written consent, and (iii) not use such Confidential Information other than in accordance with the terms of this Agreement. All information related to BioCryst’s licensors and the Pre-Existing Third Party License shall be deemed to be BioCryst Confidential Information.
5.2 Authorized Disclosure. Nothing in this Agreement shall prevent Mundipharma or its Associates from disclosing Confidential Information to (i) Governmental Authorities of any country to the extent required or desirable to secure government approval for the development, manufacture or Commercialization of Licensed Products, (ii) Third Parties acting on behalf of Mundipharma or its Associates, to the extent reasonably necessary for the development, manufacture or Commercialization of Licensed Products (and provided that Mundipharma has a written confidentiality agreement with such Third Party which is as protective of such Confidential Information as the terms of this Agreement), or (iii) Third Parties to the extent reasonably necessary to market Licensed Products (and provided that Mundipharma has a written confidentiality agreement with such Third Party which is as protective of such Confidential Information as the terms of this Agreement). Each Party shall be ultimately responsible for compliance with the terms of this Article 5 by its Associates, or any Third Party who receives Confidential Information as a result of a disclosure of such Confidential Information initially made by such Party.
5.3 Manufacturing. Mundipharma shall have sole responsibility (including complete decision making authority and discretion) to manufacture or have manufactured the Licensed Products for the Mundipharma Trials Plan and Development (including clinical trial material) and Commercialization by Mundipharma or its Associates in the Field. Manufacturing of the Licensed Product shall be conducted in accordance with cGMP.
5.4 Maintenance of License. * * *.
Page 10
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
ARTICLE 6 - PAYMENTS - ROYALTIES
6.1 Payments Under Original Agreement. The Parties acknowledge and agree that all payments made under the Original Agreement are non-refundable and non-creditable and that no further payments are due by either Party under the Original Agreement.
6.2 Payments. As partial consideration for the licenses granted by BioCryst to Mundipharma, Mundipharma shall pay to BioCryst the following one-time payment amounts (each a “Payment” and collectively the “Payments”) in U.S. Dollars listed in the table below.
| Event |
Payment Amount | |
| Regulatory Approval for * * * | $* * * | |
| Regulatory Approval for * * *. | $* * * |
6.2.1 Each such Payment shall be deemed earned as of the achievement of the corresponding event set forth above and shall be paid by Mundipharma within * * * (* * *) days after achievement of such event.
6.2.2 It is understood and agreed between the Parties that, subject to Section 5.4, the above Payments shall be non-refundable and non-creditable.
6.3 Royalties Payable by Mundipharma. In partial consideration for the licenses granted to Mundipharma, and subject to Sections 5.4, 8.1.3 and 8.4, Mundipharma shall pay to BioCryst:
6.3.1 a royalty equal to * * *.
6.3.2 Remittance of Royalties. Payments due under Section 6.3 shall be due quarterly on a calendar basis, in arrears, and shall be payable no later than * * * (* * *) days after the last Business Day of each such quarter. The payments due and payable under Section 6.3 shall be computed for each quarter with sales that occur in a currency other than U.S. Dollars (“Foreign Currency Sales”) to be converted in accordance with Section 6.7. All payments made by Mundipharma pursuant to this Section shall be made in immediately available funds by wire transfer to such bank and account of BioCryst as may be designated from time to time by BioCryst.
6.3.3 Deductions From Royalties. Mundipharma shall pay or procure the payment of the Royalties and other monies payable to BioCryst under this Agreement from Bermuda. As of the Effective Date, there is no Legal Requirement in Bermuda for Mundipharma to pay or withhold of any income or other taxes on behalf of BioCryst with respect to Royalties and any other monies payable to BioCryst under this Agreement. In the event that after the Effective Date, the payor of such Royalties and other monies payable to BioCryst under this Agreement shall change to an Associate of Mundipharma located in a jurisdiction with respect to which such payment or withholding is required by applicable Legal Requirements, then such income or other taxes shall be deducted from the amount of such payments, royalties and other monies due to BioCryst and paid to the relevant competent taxing authority; provided that (i) Mundipharma shall promptly notify BioCryst of such Legal Requirements in advance of the payment requiring the withholding; (ii) the sum payable shall be increased as necessary so that after making all required deductions, BioCryst receives an amount equal to the sum it would have received had no withholding been made; and (iii) Mundipharma shall furnish BioCryst with proof of such payments. Mundipharma shall promptly provide BioCryst with any available certificate or other documentary evidence that might enable BioCryst to support a claim for a refund or a foreign tax credit with respect to any such tax so withheld or deducted by Mundipharma, and BioCryst shall promptly (a) file a claim for refund with the relevant taxing authority and (b) pay to Mundipharma the actual amount of any refund received. Mundipharma and BioCryst will reasonably cooperate in completing and filing documents required under the provisions of any applicable tax treaty or under any other applicable law and to take any other reasonable actions, in order to enable Mundipharma to make such payments to BioCryst without any deduction or withholding, if possible consistent with Legal Requirements (including by maintaining or changing, as reasonably necessary, the payor of amounts under this Agreement).
Page 11
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
6.4 Royalty Reports. Each payment made to BioCryst under Section 6.3 shall be accompanied by a written report, showing Gross Price and Net Sales and the calculation of the same (including all deductions taken to arrive at Net Sales) together with the calculation of the royalties due for the quarter for which payment is being made. The report shall detail the amount of Licensed Products Sold, identified on a country-by-country basis.
6.5 Records. Mundipharma shall, and shall cause its Associates to, keep and maintain for * * * (* * *) years after payment of royalties pursuant to Section 6.3 complete and accurate books and records in sufficient detail so that Net Sales and royalties payable hereunder can be properly verified.
6.6 Audit. No more frequently than once during each calendar year during the Term and for * * * (* * *) years thereafter, Mundipharma shall permit independent auditors appointed by BioCryst, to whom Mundipharma has no reasonable objection and with reasonable notice at any time during normal business hours, to inspect, audit and copy relevant accounts and records of such Party for the purpose of verifying the accuracy of the calculation of royalty payments to BioCryst and the reports which accompanied them. The independent auditors shall not disclose to BioCryst any information other than information relating solely to the accuracy of the accounting and payments made by Mundipharma. If such audit determines that payments are due to BioCryst, Mundipharma shall pay to BioCryst any such additional amounts within * * * (* * *) days of the date on which such auditor’s written report is delivered to Mundipharma, unless such audit report is disputed, in which case the dispute shall be resolved in accordance with Article 12. If the auditor determines that Mundipharma’s payments are in excess of those required under this Agreement, BioCryst shall remit the difference to Mundipharma of such amount within * * * (* * *) days of the date on which such auditor’s report is delivered, unless such audit report is disputed, in which case the dispute shall be resolved in accordance with Article 12. Any such inspection of records shall be at BioCryst’s expense unless such audit discloses an underpayment of any payment of more than * * * percent (* * *%), in which case Mundipharma shall bear the cost of such audit. All payments due shall bear interest calculated as set forth in Section 13.4 below.
6.7 Foreign Currency Conversion. Payments made under this Agreement shall be payable in U.S. Dollars. The payments due and payable under Section 6.3 of this Agreement shall be computed for each calendar quarter with Foreign Currency Sales converted into U.S. Dollars using Mundipharma’s standard accounting procedures, consistently applied, which as of the Effective Date is calculated at the average of the daily foreign exchange mid-range rates, as quoted in the Bloomberg Financial Network (or another publication as notified by Mundipharma to BioCryst in writing), for such calendar quarter.
6.8 Nature of Payments. Subject to Sections 5.4, 8.1.3 and 8.4 all payments made pursuant to Section 6 shall be nonrefundable and noncreditable.
6.9 Payments to Third Parties. BioCryst shall maintain the Pre-Existing Third Party License at its own cost. In the event that a final court order or other binding order or ruling requires the payment of a royalty or other payment to a Third Party patent holder in respect of sales of the Licensed Products in the Territory (other than pursuant to the Pre-Existing Third Party License), Mundipharma shall pay such royalty or other payments in exchange for a grant of all licenses from such Third Party necessary to make, have made, use, offer for sale, sell or import Licensed Products in the Territory, with such royalty or other payment to be shared with BioCryst in accordance with Section 8.4.
ARTICLE 7 - TRADEMARKS AND DOMAIN NAMES
7.1 Right to Use Trademarks. Mundipharma shall have the exclusive right to use the Trademarks and BioCryst shall assign and transfer to Mundipharma or its designees the Trademarks, including all applications to register the Trademarks and all registrations thereof, and all goodwill relating to the Trademark to Mundipharma or its nominee. BioCryst shall execute in a timely fashion all documents reasonably required by Mundipharma to effect such assignments and transfers throughout the Territory.
7.2 Secondary Marks. At its discretion, Mundipharma may choose a new trademark (each such new trademark a “Secondary Mark”, and collectively the “Secondary Marks”) under which to develop and Commercialize the Licensed Products. Mundipharma will be entitled to file, maintain, and/or renew an application or registrations for such Secondary Marks at Mundipharma’s expense, in its own name in such country(ies) of the Territory.
Page 12
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
7.3 Property in Trademarks and Payment of Fees. As of the Effective Date, the Trademarks shall be the sole and exclusive property of Mundipharma or its nominees and Mundipharma or its Associates shall pay all fees falling due in future on the renewal of the Trademarks in the Territory. BioCryst shall cooperate with Mundipharma as Mundipharma may reasonably request in furtherance of Mundipharma’s application, prosecution, registration, or maintenance of any filings for the Trademarks and all Secondary Marks throughout the Territory.
7.4 Domain Names. Mundipharma shall have the right, at its expense, to apply for, acquire, register, maintain and use in the Territory any domain names specific to countries in the Territory that incorporate the Trademarks or are used primarily in connection with the Licensed Products.
ARTICLE 8 - LITIGATION, PATENT PROSECUTION AND ROYALTY OFFSET
8.1 Litigation.
8.1.1 Each Party shall promptly notify the other in writing (i) of any suspected or threatened infringement of a BioCryst Patent or a Mundipharma Patent by a Third Party in the Territory and in the Field, (ii) of any known or suspected unauthorized use or misappropriation by a Third Party of any BioCryst Know-How or any Mundipharma Know-How in the Territory and in the Field; and (iii) of any assertion or claim of alleged patent infringement by Mundipharma or its Associates with respect to the development, Commercialization, manufacture, use, sale, offer for sale or importation of the Compound or the Licensed Products in the Territory, and shall provide the other Party with all evidence in its possession that tends to prove the Third Party infringement or unauthorized use or misappropriation described in clauses (i) or (ii); or that tends to negate the alleged infringement described in clause (iii); in the case of each of clauses (i), (ii) and (iii), to the extent such Party becomes aware of it.
8.1.2 BioCryst shall promptly advise Mundipharma of any events of which BioCryst becomes aware that may have a material bearing on the validity or enforceability of the BioCryst Patents in the Field and in the Territory and shall inform Mundipharma of BioCryst’s plan, if any, to commence proceedings or to take other appropriate action in response to such events. BioCryst shall consider Mundipharma’s advice and comments in good faith.
8.1.3 If Mundipharma or any of its Associates becomes a party to a suit by a Third Party in any country of the Territory and it is alleged in the suit that Mundipharma’s or its Associate’s actions in the Territory with regards to Licensed Products infringe the Third Party’s intellectual property rights, then until such litigation is concluded, * * *% of the royalties from said country that may accrue after the institution of such suit shall be paid to BioCryst, and the other * * *% of such royalties shall be placed in a separate fund hereinafter referred to as a “Defense Fund”. Mundipharma may draw against such Defense Fund to satisfy therefrom all of the reasonable expenses of defending such suit as well as any damages that might be awarded or agreed upon. Any monies that accrue to the Defense Fund that are not required to satisfy such expenses and/or damages and/or agreed settlement in such litigation shall be paid to BioCryst within * * * (* * *) days after the non-appealable conclusion of such litigation.
8.1.4 Within a period of * * * (* * *) days after Mundipharma provides or receives written notice under 8.1.1 (“Decision Period”), Mundipharma, in its sole discretion, shall decide whether or not to initiate a suit or take other appropriate action in the Field and in the Territory and shall notify BioCryst in writing of its decision (“Suit Notice”). The Suit Notice shall provide a description of the suit or action contemplated by Mundipharma and shall provide details concerning the causes of action and grounds therefor.
8.1.5 * * *.
8.1.6 Upon written request, the Party bringing suit or taking action in the Territory and in the Field (“Initiating Party”) shall keep the other Party informed of the status of any such suit or action and shall provide the other Party with copies of all substantive documents and communications filed in such suit or action. The Initiating Party shall have the sole and exclusive right to select counsel for any such suit or action.
Page 13
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
8.1.7 The Initiating Party shall, except as provided below, pay all expenses of the suit or action, including, without limitation, the Initiating Party’s attorneys’ fees and court costs. Mundipharma shall be entitled to * * * per cent (* * *%) of any damages, settlement fees or other consideration received as a result of a suit or action initiated by either Party net of each Party’s actual counsel fees and out-of-pocket expenses, subject to payment of a royalty to BioCryst at the royalty rate then applicable pursuant to Section 6.3 on the net amount received by Mundipharma. The balance of * * * per cent of the damages, settlement fees or other consideration received as a result of a suit or action by either Party, net of each Party’s actual counsel fees and out-of-pocket expenses, shall be paid by BioCryst to the licensors under the Pre-Existing Third Party License Agreement for so long as BioCryst has an obligation to make such a payment under the Pre-Existing Third Party License Agreement. Once the obligation to make such a payment under the Pre-Existing Third Party License Agreement has expired, Mundipharma shall retain * * * per cent (* * *%) of such damages, settlement fees or other consideration and pay a royalty to BioCryst at the royalty rate then applicable pursuant to Section 6.3 on such amount net of each Party’s actual counsel fees and out-of-pocket expenses.
8.1.8 If the Initiating Party believes it reasonably necessary, upon written request the other Party shall join as a Party to the suit or action but shall be under no obligation to participate except to the extent that such participation is required as the result of its being named a Party to the suit or action. At the Initiating Party’s written request, the other Party shall offer reasonable assistance to the Initiating Party in connection therewith at no charge to the Initiating Party except for reimbursement of reasonable out-of-pocket expenses incurred by the other Party in rendering such assistance. The other Party shall have the right to participate and be represented in any such suit or action by its own counsel at its own expense.
8.1.9 When Mundipharma is the Initiating Party, Mundipharma shall not settle, consent to judgment or otherwise voluntarily dispose of the suit or action without the prior written consent of BioCryst, which consent shall not be unreasonably withheld. When BioCryst is the Initiating Party, BioCryst shall not settle, consent to judgment or otherwise voluntarily dispose of the suit or action without discussing such action with Mundipharma and considering any objection by Mundipharma in good faith.
8.2 Patent Prosecution.
8.2.1 BioCryst shall prepare, file, prosecute and maintain (hereinafter “Patent Activities”) the BioCryst Patents in the Territory, and Mundipharma shall reimburse BioCryst for its reasonable expenses incurred after the Effective Date in relation thereto (including, but not limited to, official patent office fees, attorney fees, and out-of-pocket expenses). BioCryst shall consult with Mundipharma as to the Patent Activities, and shall furnish to Mundipharma copies of all substantive documents relevant to the Patent Activities for the BioCryst Patents, all in sufficient time (at least one week) before any action by BioCryst is due, to allow Mundipharma to provide comments thereon. BioCryst shall consider Mundipharma’s comments in good faith. Mundipharma shall cooperate with BioCryst in all reasonable ways in connection with the Patent Activities for the BioCryst Patents.
8.2.2 Mundipharma shall conduct the Patent Activities in respect of the Mundipharma Patents at its own discretion and expense in the Territory.
8.3 Registration of Patent License. Upon Mundipharma’s request, the Parties shall enter into an appropriate memorandum of this license mutually agreed by the Parties which shall be recorded, as required or appropriate, in the patent or governmental office of any country or countries in the Territory in which BioCryst has a patent pending or granted.
8.4 Royalty Offset. If Mundipharma shall be subject to a final court or other binding order or ruling requiring the payment of a royalty or other payment to a Third Party holding patents to the Compound itself, but not to formulations of the Compound or methods of use or administration, or if the parties mutually agree in good faith that it is in the parties’ best commercial interests to settle a Third Party patent infringement proceeding initiated against Mundipharma or its Associates in the Territory on the basis of patents to the Compound itself, but not formulations of the Compound or methods of use or administration, by taking a license from a Third Party patent holder in any country in exchange for a royalty or other payment in respect of sales of the Licensed Products, then the amount of Mundipharma’s royalty payments to BioCryst under Section 6.3 with respect to Net Sales shall be reduced by the amount of the royalty or other payment made to such Third Party patent holder pursuant to such order, ruling or license, but in no event shall such reduction exceed * * * percent (* * *%) of such royalties payable to BioCryst.
Page 14
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
ARTICLE 9 - REPRESENTATIONS AND WARRANTIES
9.1 BioCryst’s Representations and Warranties. BioCryst hereby represents and warrants the following to Mundipharma as of the Effective Date :
9.1.1 BioCryst is a company duly organized, validly existing, and in good standing under the laws of Delaware, with its principal place of business as indicated in the preamble of this Agreement. BioCryst (i) is duly qualified as a corporation and in good standing under the laws of each jurisdiction where its ownership or lease of property or the conduct of its business requires such qualification, where the failure to be so qualified would have a material adverse effect on its financial condition or its ability to perform its obligations under this Agreement; (ii) has the requisite corporate power and authority and the legal right to conduct its business as now conducted and hereafter contemplated to be conducted; (iii) has all necessary licenses, permits, consents, or approvals from or by, and has made all necessary notices to, all governmental authorities having jurisdiction, to the extent required for such ownership and operation; and (iv) is in compliance with its instrument of corporate formation and by-laws or similar corporate governance rules.
9.1.2 The execution, delivery and performance of this Agreement by BioCryst and all instruments and documents to be delivered by BioCryst hereunder (i) are within its corporate power; (ii) are not in contravention of any provision of its instrument of corporate formation and by-laws or similar corporate governance rules; (iii) to BioCryst’s knowledge do not violate any law or regulation or any order or decree of any court of governmental instrumentality; (iv) do not violate any terms of any indenture, mortgage, deed of trust, lease, agreement, or other instrument to which it is a party or by which such entity or any of its property is bound, which violation would have a material adverse effect on its financial condition or on its ability to perform its obligations under this Agreement; and (v) do not require any filing or registration with or the consent or approval of any governmental body, agency, authority or any other Person, which has not been made or obtained previously, including any consent required under the Pre-Existing Third Party License (other than approvals required under the Regulatory Approvals required for the sale of Licensed Products and filings with regulatory authorities required in connection with Licensed Products).
9.1.3 This Agreement has been duly executed and delivered by BioCryst and constitutes a legal, valid and binding obligation of BioCryst, enforceable against it in accordance with its terms, except as such enforceability may be limited by the availability of equitable remedies.
9.1.4 To the knowledge of BioCryst, BioCryst has complied with all Legal Requirements in connection with the prosecution of the BioCryst Patents, including without limitation the duty of candor owed to any patent office under such laws, rules and regulations.
9.1.5 BioCryst has the right to grant Mundipharma the rights and licenses described in this Agreement.
9.1.6 BioCryst has not granted any rights with respect to (i) the Compound or the Licensed Products in the Territory, or (ii) the BioCryst Patents or the BioCryst Know-How in the Field in the Territory, in each case to any person or entity other than Mundipharma.
9.1.7 There are no claims or investigations pending or threatened against BioCryst or any of its Associates, at law or in equity, or before or by any governmental authority relating to the matters contemplated under this Agreement that would materially adversely affect BioCryst’s ability to perform its obligations hereunder or thereunder.
9.1.8 Neither BioCryst nor any of its Associates is under any obligation to any person, contractual or otherwise, that is in violation of the terms of this Agreement or that would impede the fulfillment of BioCryst’s obligations hereunder. Neither BioCryst nor any of its Associates will enter into any obligation to any person, contractual or otherwise, that is in violation of the terms of this Agreement or that would impede the fulfillment of BioCryst’s obligations hereunder.
Page 15
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
9.1.9 No employee of BioCryst has been debarred or is the subject of debarment proceedings by any Regulatory Authority. BioCryst shall not use in connection with its performance of its obligations or duties or its exercise of its rights under this Agreement any employee, consultant or investigator that has been debarred or the subject or debarment proceedings by any Regulatory Authority.
9.1.10 To the knowledge of BioCryst, in accordance with the terms hereof, BioCryst has not received written notice that the exercise of Mundipharma’s rights granted under this Agreement infringes any Third Party intellectual property rights, and to the knowledge of BioCryst, without inquiry or investigation, the exercise of Mundipharma’s rights granted under this Agreement, in accordance with the terms hereof, will not infringe or conflict with any Third Party intellectual property rights.
9.1.11 All material renewal and maintenance fees due as of the Effective Date with respect to the prosecution and maintenance of the BioCryst Patents and the Trademarks have been paid, except as would not have a material adverse effect on Mundipharma’s rights hereunder.
9.1.12 BioCryst has allowed, and will continue to allow, Mundipharma access to all material information in its possession or control (i) containing the results of all preclinical testing and human clinical testing of Licensed Product in its possession or control and (ii) concerning side effects, injury, toxicity or sensitivity reaction and incidents or severity thereof with respect to Licensed Product.
9.1.13 There is no action or proceeding related to, nor has BioCryst received any written notice of termination under, the Pre-Existing Third Party License, and to the knowledge of BioCryst, BioCryst is not in default of any material obligation under the Pre-Existing Third Party License.
9.1.14 BioCryst has not licensed or granted any rights in connection with BCX-4208 to any Third Party in the Field, and BioCryst is under no contractual or other obligation to develop BCX-4208 in the Field.
9.1.15 BioCryst has not licensed or granted any rights in connection with the Compound or the Licensed Products to any Third Party outside the Field, and BioCryst is under no contractual or other obligation to develop the Compound or the Licensed Products outside the Field.
9.1.16 To the knowledge of BioCryst, the inventory of the Compound to be purchased by Mundipharma pursuant to Section 4.1.2 is in good useable condition and has been manufactured, packaged, quality controlled and stored in accordance with all relevant United States Legal Requirements.
9.1.17 To the knowledge of BioCryst, all BioCryst Know-How and data provided to Mundipharma pursuant to the Original Agreement and to be provided to Mundipharma pursuant to this Agreement are materially accurate and complete and have been prepared materially in accordance with and materially comply with all relevant United States Legal Requirements; provided, however, that the data from clinical studies BCX-203 and BCX-210 which are provided to Mundipharma hereunder have been prepared materially in accordance with and materially comply with all relevant United States Legal Requirements only through the primary analysis of each completed study.
9.1.18 BioCryst will transfer the BioCryst Know-How and data existing at the Effective Date to Mundipharma in accordance with the Transition Assistance Schedule.
BioCryst acknowledges that Mundipharma is relying, and is entitled to rely, on the foregoing representations, warranties and covenants.
Page 16
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
9.2 Mundipharma’s Representations and Warranties. Mundipharma hereby represents and warrants the following to BioCryst as of the Effective Date:
9.2.1 Mundipharma (i) is a corporation duly organized, validly existing, and in good standing under the laws of Bermuda, with its principal place of business as indicated in the preamble of this Agreement; (ii) is duly qualified as a limited liability company and in good standing under the laws of each jurisdiction where ownership or lease of property or the conduct of its business requires such qualification, where the failure to be so qualified would have a material adverse effect on the financial condition of Mundipharma or the ability of Mundipharma to perform its obligations hereunder; (iii) has the requisite corporate power and authority and the legal right to conduct its business as now conducted and hereafter contemplated to be conducted; (iv) has all necessary licenses, permits, consents, or approvals from or by, and has made all necessary notices to, all governmental authorities having jurisdiction, to the extent required for such ownership and operation; and (v) is in compliance with its certificate of formation and limited liability company agreement.
9.2.2 The execution, delivery and performance of this Agreement by Mundipharma and all instruments and documents to be delivered by Mundipharma hereunder: (i) are within the corporate power of Mundipharma; (ii) are not in contravention of any provision of the certificate of formation or limited liability company agreement of Mundipharma; (iii) to the knowledge of Mundipharma will not violate any law or regulation or any order or decree of any court of governmental instrumentality; (iv) will not violate the terms of any indenture, mortgage, deed of trust, lease, agreement, or other instrument to which Mundipharma is a party or by which Mundipharma or any of its property is bound, which violation would have an adverse effect on the financial condition of Mundipharma or on the ability of Mundipharma to perform its obligations hereunder; and (v) do not require any filing or registration with, or the consent or approval of, any governmental body, agency, authority or any other Person, which has not been made or obtained previously (other than approvals required under the Regulatory Approvals required for the sale of Licensed Products and filings with regulatory authorities required in connection with Licensed Products).
9.2.3 This Agreement has been duly executed and delivered by Mundipharma and constitutes a legal, valid and binding obligation of Mundipharma, enforceable against Mundipharma in accordance with its terms, except as such enforceability may be limited by the availability of equitable remedies.
9.2.4 There are no claims or investigations pending or threatened against Mundipharma or any of its Associates, at law or in equity, or before or by any governmental authority relating to the matters contemplated under this Agreement that would materially adversely affect Mundipharma’s ability to perform its obligations hereunder or thereunder.
9.2.5 Neither Mundipharma nor any of its Associates is under any obligation to any person, contractual or otherwise, that is in violation of the terms of this Agreement or that would impede the fulfillment of Mundipharma’s obligations hereunder. Neither Mundipharma nor any of its Associates will enter into any obligation to any person, contractual or otherwise, that is in violation of the terms of this Agreement or that would impede the fulfillment of Mundipharma’s obligations hereunder.
9.2.6 No employee of Mundipharma has been debarred or is the subject of debarment proceedings by any Regulatory Authority. Mundipharma shall not use in connection with its performance of its obligations or duties or its exercise of its rights under this Agreement (including, without limitation, the Development of any Licensed Products) any employee, consultant or investigator that has been debarred or the subject or debarment proceedings by any Regulatory Authority.
9.2.7 Mundipharma (and its Associates) does not currently own or control rights underlying any PNP Inhibitor other than the Compound, and currently has no current plans to develop or acquire any PNP Inhibitor other than the Compound.
Mundipharma acknowledges that BioCryst is relying, and is entitled to rely, on the foregoing representations, warranties and covenants.
9.3 No other Representations. (i) No oral representations or warranties have been made by BioCryst to Mundipharma upon which Mundipharma is relying in connection with the transactions contemplated by this Agreement; (ii) no oral representations or warranties have been made by Mundipharma to BioCryst upon which BioCryst is relying in connection with the transactions contemplated by this Agreement; (iii) no written
Page 17
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
representations or warranties have been made by BioCryst to Mundipharma upon which Mundipharma is relying in connection with the transactions contemplated by this Agreement, other than as set forth in this Agreement; and (iv) no written representations or warranties have been made by Mundipharma or any of its Associates to BioCryst upon which BioCryst is relying in connection with the transactions contemplated by this Agreement.
9.4 Disclaimers. EXCEPT AS OTHERWISE PROVIDED IN THIS AGREEMENT, THE FOREGOING REPRESENTATIONS AND WARRANTIES ARE IN LIEU OF ALL OTHER REPRESENTATIONS AND WARRANTIES, EXPRESS OR IMPLIED, INCLUDING WITHOUT LIMITATION, WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OF COMPOUND AND LICENSED PRODUCTS. SPECIFICALLY, (i) BIOCRYST MAKES NO OTHER REPRESENTATIONS OR WARRANTIES IN RELATION TO THE BIOCRYST PATENTS, THE BIOCRYST KNOW-HOW, THE COMPOUND (INCLUDING COMPOUND AND LICENSED PRODUCTS SUPPLIED HEREUNDER), THE TRADEMARK OR THE LICENSED PRODUCTS, AND (ii) MUNDIPHARMA MAKES NO OTHER REPRESENTATIONS OR WARRANTIES IN RELATION TO THE MUNDIPHARMA PATENTS, THE MUNDIPHARMA KNOW-HOW, ANY SECONDARY TRADEMARKS, THE COMPOUND OR THE LICENSED PRODUCTS. EXCEPT AS OTHERWISE PROVIDED IN THIS AGREEMENT, BIOCRYST SHALL HAVE NO LIABILITY WHATSOEVER ARISING OUT OF OR RELATING TO COMPOUND OR LICENSED PRODUCTS SUPPLIED TO MUNDIPHARMA HEREUNDER.
ARTICLE 10 - INDEMNITY AND PRODUCT LIABILITY
10.1 Indemnification and Defense by Mundipharma. Mundipharma shall, at its sole expense, indemnify, defend and hold harmless BioCryst, its licensors under the Pre-Existing Third Party License, Associates and its or their respective officers, directors, agents and employees (the “BioCryst Indemnitees”) against any Third Party Claim arising out of or resulting from (i) gross negligence, willful misconduct or breach of Legal Requirements relevant to its activities under this Agreement or the Original Agreement (as applicable) by Mundipharma, its Associates or sublicensees, and/or (ii) Licensed Products manufactured, imported, marketed, distributed or sold by or on behalf of Mundipharma or its Associates, or sublicensees, and all activities related thereto except to the extent that such Third Party Claim is covered in Section 10.2.
10.2 Indemnification and Defense by BioCryst. BioCryst shall, at its sole expense, indemnify, defend and hold harmless Mundipharma, its Associates and its or their respective officers, directors, agents and employees (the “Mundipharma Indemnitees”) against any Third Party Claim arising out of or resulting from gross negligence, willful misconduct or breach of Legal Requirements relevant to its activities under this Agreement or the Original Agreement (as applicable) by BioCryst, its Associates or sublicensees (other than Mundipharma or its Associates).
10.3 Defense Procedures. BioCryst and Mundipharma shall notify each other promptly in writing upon learning of any Third Party Claim in respect of which indemnification may be sought under Section 10.1 or Section 10.2, as the case may be. The indemnifying Party shall actively defend against (or settle if appropriate) every Third Party Claim using counsel approved by the indemnified Party, such approval not to be unreasonably withheld or delayed, shall promptly inform the indemnified Party and its attorneys of all developments concerning the indemnified Party and shall generally consult with the indemnified Party regarding the strategy of the defense of any Third Party Claim. To the extent allowed by law, the BioCryst Indemnitees and the Mundipharma Indemnitees, as the case may be, shall reasonably cooperate with the indemnifying Party in defending or settling any such Third Party Claim. No settlement of any Third Party Claim for which indemnification is sought, shall be made without the prior written approval of the indemnifying Party. The indemnifying Party will have sole control over the defense and/or settlement, subject to the BioCryst Indemnitees’ and Mundipharma Indemnitees’, as the case may be, right to select and use their own counsel at their sole cost and expense.
10.4 Insurance. Each Party shall obtain and shall, as long as Mundipharma, directly or indirectly, is undertaking the Mundipharma Trials Plan or Developing, Commercializing, manufacturing, marketing, testing or distributing the Licensed Products and for at least * * * (* * *) years thereafter, maintain at such Party’s sole cost and expense product liability insurance, or shall set up, at its sole cost and expense, a self insurance arrangement, and such insurance shall meet the following requirements:
10.4.1 the insurance shall insure such Party against all liability related to the Licensed Products (whether such Party’s liability arises from its own conduct, that of its Associates, sublicensees or distributors or by virtue of its participation in this Agreement), including liability for bodily injury, property damage, wrongful death, and any contractual indemnity obligations imposed by this Agreement; and
Page 18
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
10.4.2 the insurance shall have a minimum limit of * * * U.S. Dollars ($* * *) per occurrence with an annual aggregate limit of not less than seven million U.S. Dollars ($* * *).
Each Party shall provide the other with a Certificate of Insurance evidencing such insurance coverage upon the request of the other Party. Each Party shall be entitled to substitute a program of self-insurance for all or a part of such Party’s Third Party insurance required hereunder at its sole option.
10.5 Survival. Neither the expiration nor termination of this Agreement shall in any way affect the provisions of this Article 10 or relieve or discharge any Party with respect thereto. The Parties understand and agree that the representations, warranties, covenants and agreements, including without limitation those set forth in this Article 10, shall survive without limitation.
10.6 Disclaimer of Liability for Consequential Damages. IN NO EVENT SHALL ANY PARTY OR ANY OF ITS RESPECTIVE AFFILIATES BE LIABLE UNDER THIS AGREEMENT FOR SPECIAL, INDIRECT, INCIDENTAL OR CONSEQUENTIAL DAMAGES, WHETHER IN CONTRACT, WARRANTY, TORT, NEGLIGENCE, STRICT LIABILITY OR OTHERWISE, INCLUDING, BUT NOT LIMITED TO, LOSS OF PROFITS OR REVENUE, SUFFERED BY BIOCRYST OR MUNDIPHARMA, RESPECTIVELY, UNDER THIS AGREEMENT, EXCEPT (A) TO THE EXTENT OF ANY SUCH DAMAGES PAID TO A THIRD PARTY AS PART OF A THIRD PARTY CLAIM WHICH IS INDEMNIFIABLE HEREUNDER, AND (B) IN THE EVENT OF AN INTENTIONAL AND WILLFUL BREACH IN BAD FAITH OF ANY REPRESENTATION, WARRANTY, COVENANT OR AGREEMENT BY BIOCRYST OR MUNDIPHARMA OR THEIR RESPECTIVE AFFILIATES (AS THE CASE MAY BE) OF THIS AGREEMENT.
ARTICLE 11 - TERM AND TERMINATION
11.1 Term.
11.1.1 This Agreement shall commence on and as of the Effective Date and shall continue for the Commercial Life of the Licensed Products, unless terminated earlier as set forth below (the “Term”). As used in this Section 11.1.1, “Commercial Life” shall mean as long as there is any development or Commercialization of the Licensed Products in the Territory by Mundipharma, its Associates or its permitted Third Party sublicensees or distributors.
11.2 Termination by Parties.
11.2.1 By Either Party. Either Party may terminate this Agreement immediately on written notice to the other Party in the event that the Pre-Existing Third Party License expires, subject always to Mundipharma’s rights pursuant to Section 5.4.
11.2.2 Termination by BioCryst. BioCryst may terminate this Agreement as follows:
11.2.2.1 If Mundipharma is generally unable to meet its debts when due, or makes a general assignment for the benefit of creditors, or there shall have been appointed a receiver, trustee or other custodian for Mundipharma for all or a substantial part of its assets, or any case or proceeding shall have been commenced or other action taken by or against Mundipharma in bankruptcy or seeking the reorganization, liquidation, dissolution or winding-up of Mundipharma or any other relief under any bankruptcy, insolvency, reorganization or other similar act or Legal Requirements, and any such event (other than any such event which shall have been instituted by Mundipharma) shall have continued for sixty (60) days undismissed, unstayed, unbonded and undischarged, then BioCryst may, upon notice to Mundipharma, terminate this Agreement.
Page 19
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
11.2.2.2 BioCryst may notify Mundipharma that a Material Default by Mundipharma has occurred, in which case BioCryst may terminate this Agreement, without prejudice to Mundipharma’s right to dispute the notified Material Default in accordance with the dispute resolution procedures set out in Article 12 and Exhibit A. For purposes of example and not limitation, Mundipharma’s challenge or any challenge by a Third Party acting on behalf of Mundipharma to the validity or enforceability of or opposition by such persons to any BioCryst Patents shall be deemed to be a Material Default of this Agreement and shall give rise to BioCryst’s right to terminate this Agreement pursuant to this Section 11.2.2.2.
11.2.3 Termination by Mundipharma. Mundipharma may terminate this Agreement as follows:
11.2.3.1 If BioCryst is generally unable to meet its debts when due, or makes a general assignment for the benefit of creditors, or there shall have been appointed a receiver, trustee or other custodian for BioCryst for all or a substantial part of its assets, or any case or proceeding shall have been commenced or other action taken by or against BioCryst in bankruptcy or seeking the reorganization, liquidation, dissolution or winding-up of BioCryst or any other relief under any bankruptcy, insolvency, reorganization or other similar act or Legal Requirements, and any such event (other than any such event which shall have been instituted by BioCryst) shall have continued for sixty (60) days undismissed, unstayed, unbonded and undischarged, then Mundipharma may, upon notice to BioCryst, terminate this Agreement.
11.2.3.2 Mundipharma may notify BioCryst that a Material Default by BioCryst has occurred, in which case Mundipharma may terminate this Agreement, without prejudice to BioCryst’s right to dispute the notified Material Default in accordance with the dispute resolution procedures set out in Article 12 and Exhibit A.
11.2.3.3 Mundipharma may, in its sole discretion upon sixty (60) days’ prior written notice to BioCryst, terminate this Agreement; provided that Mundipharma shall pay to BioCryst the applicable Payments and Royalties accruing on or prior to the termination date.
11.2.3.4 Mundipharma may terminate this Agreement immediately on written notice to BioCryst in the event that a Regulatory Approval in the Territory is cancelled, withdrawn or suspended as a result of a serious safety issue of the Licensed Products.
11.2.3.5 Mundipharma may terminate this Agreement immediately on written notice to BioCryst in the event that the Pre-Existing Third Party License terminates. * * *.
11.3 Rights and Obligations of Parties upon Term Expiration or Termination.
11.3.1 Any termination (i) shall be without prejudice to a Party’s right to damage or legal redress that a Party hereto may be entitled to for any breach or Material Default of this Agreement, provided that neither Mundipharma nor BioCryst will incur any liability to the other Party by rightfully terminating this Agreement as provided in Section 11.2, whether for loss of goodwill, anticipated profits or otherwise, (ii) shall not release a Party hereto from any indebtedness, liability or other obligation incurred hereunder by such Party prior to the date of termination or expiration, (iii) shall allow both parties to immediately exercise their audit rights under Section 6.6 whether or not such Party had already exercised such rights in that calendar year, and (iv) shall be without prejudice to a Party’s right to dispute the existence of a Material Default notified by the other Party as the basis for termination, in which event the termination of this Agreement shall be held in abeyance pending the outcome of the dispute resolution procedures set out in Article 12.
11.3.2 In case of termination of this Agreement, each Party shall promptly pay to the other Party all amounts due. To the extent not otherwise required by Legal Requirements, in the event of termination under Sections 11.2.1, 11.2.2, 11.2.3.3, 11.2.3.4 and 11.2.3.5, (i) Mundipharma shall use all reasonable efforts to return to BioCryst all documents (including copies) of any kind concerning the Compound or the Licensed Products received from BioCryst and (ii) BioCryst shall be entitled to a non-exclusive license to use Mundipharma’s Know-how in connection with the Development and Commercialization of Licensed Products in the Field upon payment of one hundred and fifty percent (150%) of Mundipharma’s and its Associates’ costs of generating such Mundipharma Know-how; provided, however, that BioCryst’s obligation to pay for use of the data shall not include the use by BioCryst of safety information which may have an effect on PNP inhibitors generally.
Page 20
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
11.3.3 In case of termination of this Agreement under Sections 11.2.1, 11.2.2, 11.2.3.3, 11.2.3.4 and 11.2.3.5, the licenses set forth in Article 2 shall terminate.
11.3.4 In case of termination of this Agreement by BioCryst under Section 11.2.2 or termination by Mundipharma under Sections 11.2.3.3 or 11.2.3.4 Mundipharma shall promptly assign all of its Trademark applications or registrations in the Territory to BioCryst free of charge. In case of termination for any reason other than under Sections 11.2.2, 11.2.3.3 or 11.2.3.4, Mundipharma will assign to BioCryst all right, title, and interest in and to any such Trademark applications or registrations, including the goodwill symbolized thereby, promptly upon receipt of BioCryst’s payment of Mundipharma’s reasonable cost of acquiring, maintaining and transferring such Trademark applications or registrations.
11.3.5 Within * * * (* * *) Business Days from the date of notice of termination of this Agreement under Sections 11.2.1, 11.2.2, 11.2.3.3 and 11.2.3.4, Mundipharma shall commence all action necessary or advisable to transfer to BioCryst or such entity as BioCryst may designate, all Regulatory Approvals relating to the Licensed Products, which are then held by Mundipharma or its Associates in the Territory. Mundipharma’s obligation hereunder shall include, but not be limited to, the execution and delivery of all necessary documents in a form reasonably acceptable to BioCryst in order to complete and fully implement the definitive transfer and assignment thereof to BioCryst, and to register all such transfers to BioCryst with a Regulatory Authority, including but not limited to all IND and NDA applications and Marketing Authorizations. Such transfer shall be made free of charge to BioCryst, except as set forth in Section 11.3.8.
11.3.6 The Parties hereby agree that as soon as any transfer pursuant to Section 11.3.5 of a Regulatory Approval has been registered with a Regulatory Authority, BioCryst or its designee will be the sole owner of said Regulatory Approval.
11.3.7 Promptly after notice of any termination, Mundipharma shall provide BioCryst with copies of all relevant Third Party sublicenses, agreements with clinical research organizations and other Third Party agreements relating to Licensed Products hereunder, and allow BioCryst * * * (* * *) days from the date of such delivery to choose whether to assume any or all of such contracts to the extent allowed by the applicable contract or law. Mundipharma shall, subject to its ability to do so, assign to BioCryst those Third Party agreements BioCryst chooses to assume.
11.3.8 If this Agreement is terminated pursuant to 11.2.3.2 as a result of BioCryst’s uncured Material Breach, then without prejudice to Sections 11.3.3. 11.3.4 and 11.3.5, BioCryst shall reimburse Mundipharma for Mundipharma’s reasonable out of pocket expenses incurred in the transfer of rights and documents required hereunder, which expenses shall be pre-approved by BioCryst before Mundipharma makes the transfers incurring such expenses.
11.3.9 Upon termination of this Agreement, Sections 4.7, 5.1, 5.2, 6.2 (to the extent that the payment obligation accrues prior to termination), 6.3 (to the extent that the payment obligation accrues prior to termination), 6.4, 6.5, 6.6, 6.8, 11.2.3.3 (to the extent that the Royalties, and/or Payments accrue prior to termination) 11.3, 13.1, 13.7, 13.8, 13.9, and Articles 1, 10, and 12 (and the Exhibits attached thereto) shall survive without limitation.
ARTICLE 12 - DISPUTE RESOLUTION AND GOVERNING LAW
12.1 Disputes. Unless otherwise set forth in this Agreement, in the event of a dispute arising under this Agreement between the Parties and/or their Associates, such dispute shall be referred to the respective executive officers of the Parties designated below, or their successors, for good faith negotiations attempting to resolve the dispute. The designated executive officers are as follows:
| For Mundipharma: | Regional Director, Europe | |
| For BioCryst: | President and CEO |
Page 21
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
12.2 Dispute Resolution.
12.2.1 Any dispute or claim arising out of or relating to this Agreement (other than with respect to patent, copyright, trademark or trade secret rights), or to the breach, termination, or validity of this Agreement, will be resolved as follows: the officers of each Party referred to in Section 12.1 above will meet to attempt to resolve such dispute by good faith negotiations. If such officers cannot resolve the dispute within * * * days after a Party requests such a meeting, then each Party will attempt in good faith to settle the dispute by mediation pursuant to Section 12.2.2.
12.2.2 The mediation of any dispute is to be administered by JAMS or such other mediator as may be mutually agreed to by the Parties. If mediation is unsuccessful within * * * days after the Parties request mediation pursuant to this Section 12.2.2, the Parties may then resort to the alternative dispute resolution procedures set forth on Exhibit A.
12.2.3 Notwithstanding anything to the contrary in Sections 12.2.1 or 12.2.2, if either Party in its sole judgment believes that any such dispute could cause it irreparable harm, such Party (a) will be entitled to seek equitable relief in order to avoid such irreparable harm, and (b) will not be required to follow the procedures set forth in Sections 12.2.1.
12.3 Governing Law. This Agreement is made in accordance with and shall be governed and construed under the laws of New York, without regard to its choice of law principles. The parties hereby irrevocably submit to the jurisdiction of the courts located in the County and State of New York.
ARTICLE 13 - MISCELLANEOUS
13.1 Mutual Release.
13.1.1 Release of BioCryst. Upon execution of this Agreement, each of Mundipharma and any predecessors, successors and Associates, and each of their respective officers, directors, employees and agents, shall release and discharge, to the fullest extent of the law, each of BioCryst and any predecessors, successors and Associates, and each of their respective officers, directors, employees and agents, from any and all claims, debts, sums of money, contracts, agreements, obligations, damages, and liabilities of any kind or nature, including attorneys’ fees, costs, and expenses of every kind and however denominated, including interest thereon, arising out of or in connection with the Original Agreement; provided, however, that the foregoing release shall not apply to release or discharge the performance of any obligations under this Agreement.
13.1.2 Release of Mundipharma. Upon execution of this Agreement, each of BioCryst and any predecessors, successors and Associates, and each of their respective officers, directors, employees and agents, shall release and discharge, to the fullest extent of the law, each of Mundipharma and any predecessors, successors and Associates, and each of their respective officers, directors, employees and agents, from any and all claims, debts, sums of money, contracts, agreements, obligations, damages, and liabilities of any kind or nature, including attorneys’ fees, costs, and expenses of every kind and however denominated, including interest thereon, arising out of or in connection with the Original Agreement; provided, however, that the foregoing release shall not apply to release or discharge the performance of any obligations under this Agreement
13.2 Covenants. Mundipharma covenants that, during the term of this Agreement, it shall carry out the Development and Commercialization of the Licensed Products and its other obligations and activities hereunder in accordance with (i) the terms of this Agreement, (ii) accepted applicable pharmaceutical industry codes of practice and (iii) applicable Legal Requirements.
13.3 Non-Compete. * * *.
Page 22
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
13.4 Delay of payment. If Mundipharma fails to make a timely payment pursuant to the terms of this Agreement, interest shall accrue on the past due amount at a rate of interest equal to the 30-day U.S. Dollar London Inter-Bank Offering Rate (“LIBOR”) in the case of payments denominated in U.S. Dollars as published in The Financial Times, effective for the date on which the payment was due; provided, that if such failure to pay continues for more than * * * (* * *) days, the applicable rate of interest shall be the 30-day LIBOR rate effective for the date on which payment was due, plus * * * percent (* * *%) for the entire period of delinquency. All interest due pursuant to this Section shall be computed on an actual/360 basis.
13.5 Assignment. This Agreement shall not be assignable in part or in whole (by operation of law or otherwise) by any Party without the prior written consent of the other; provided, however, that BioCryst, without notice and at any time for any reason, may assign this Agreement in whole or in part to (i) any of its Associates who agree to be bound by the terms and conditions of this Agreement or (ii) any successor of BioCryst by merger or sale of all or substantially all of its business assets to which this Agreement relates, and provided further that Mundipharma, without the written consent of BioCryst, may assign this Agreement in whole or in part to any of its Associates with exactly the same or greater financial standing and resources as Mundipharma and who agree to be bound by the terms and conditions of this Agreement.
13.6 Pre-Existing Third Party License. Mundipharma acknowledges and agrees that the terms of this Agreement are subject in all respects to the terms of the Pre-Existing Third Party License, which has been previously provided to Mundipharma. Mundipharma further agrees that (i) the licensors under the Pre-Existing Third Party License retained certain rights, which are not granted to Mundipharma hereunder; (ii) such licensors shall be deemed to be Third Party beneficiaries of this Agreement, entitled to enforce BioCryst’s rights hereunder; (iii) all Confidential Information provided to BioCryst hereunder may be shared with such licensors; and (iv) Mundipharma shall assist BioCryst in complying with its obligations (including but not limited to recordkeeping and the provision of information to such licensors) under the Pre-Existing Third Party License. BioCryst agrees to update Mundipharma on the activities of BioCryst’s licensors under the Pre-Existing Third Party License, the U.S. government, the National Cancer Institute and the New Zealand Foundation for Research, Science and Technology in connection with the Compound to the extent that BioCryst is aware of such activities.
13.7 Press Releases and External Communications. Neither Party shall issue press releases or make public announcements relating to this Agreement without the other Party’s prior written approval, which approval shall not be unreasonably withheld or delayed; provided, however, that nothing in this Section shall impair either Party’s compliance with any requirements of the Securities and Exchange Commission or the national securities exchange or other stock market on which such Party’s securities are traded; and, provided further, that BioCryst may issue external media and investor communications related to the transactions contemplated by this agreement if such external media communications are previously approved by Mundipharma, which approval shall not be unreasonably withheld or delayed. In connection with any filing by either Party of a copy of this Agreement with the Securities and Exchange Commission (or the national securities exchange or other stock market on which such Party’s securities are traded), the filing Party shall endeavor to obtain confidential treatment of economic and trade secret information. Reasonably in advance of filing, the filing Party shall provide to the other Party a copy of the proposed filing and the Parties shall work cooperatively in good faith, taking into consideration the other Party’s suggestions, regarding the information for which the filing Party will seek to obtain confidential treatment. Notwithstanding the foregoing, BioCryst shall be entitled to make public disclosures regarding the status of Mundipharma’s Development work provided that no Confidential Information of Mundipharma is disclosed.
13.8 Use of Name. Neither Party shall use the other Party’s name or trademarks for publicity or advertising purposes, except with the prior written consent of the other Party.
13.9 Notices. Any notices, requests, reports, approvals, designations, responses, or other communications provided for in this Agreement to be made by either of the Parties to the other shall be in writing and shall be sufficiently given when made by prepaid registered or certified air mail or by an internationally reputable overnight courier addressed to the other at its address set forth below. Any such notice or communication may also be given by hand or telecommunicated. Either Party may by like notice specify an address to which notices and communications shall thereafter be sent. Any such notice, instruction or communication shall be deemed to have been delivered upon receipt if delivered by hand, three (3) Business Days after it is sent by registered or certified mail, return receipt requested, postage prepaid, one (1) Business Day after it is sent via an
Page 23
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
internationally reputable overnight courier service, or when transmitted with electronic confirmation of receipt, if transmitted by facsimile (if such transmission is on a Business Day; otherwise, on the next Business Day following such transmission).
| In the case of BioCryst: | With a required copy to: | |
| BioCryst Pharmaceuticals, Inc. 4505 Emperor Boulevard Durham, North Carolina 27703
Attention: President & CEO
Facsimile No.: 919-859-1314 |
Proskauer Rose LLP 11 Times Square New York, New York 10036
Attention: Daryn Grossman, Esq.
Facsimile No.: (212) 969-2900 | |
| In the case of Mundipharma: | With required copies to: | |
| 14 Par-la-Ville Road Hamilton HMJX Bermuda
Attention: General Manager
Facsimile No.: +1 441 292 1472 |
Mundipharma International Limited Cambridge Science Park Milton Road Cambridge CB4 0GW
Attention: (1) Regional Director, Europe, and
(2) European General Counsel
Facsimile No.: +44 1223 424442 | |
13.10 Effect of Waiver. The waiver from time to time by either of the Parties of any of their respective rights or privileges or their failure to exercise any remedy shall not operate or be construed as a continuing waiver of same or of any other of such Party’s rights, privileges or remedies provided in this Agreement.
13.11 Effect of Partial Invalidity. If any term, covenant or condition of this Agreement or the application thereof to any Party or circumstances shall, to any extent, be held to be invalid or unenforceable, then (i) the remainder of this Agreement, or the application of such term, covenant or condition to parties or circumstances other than those as to which it is held invalid or unenforceable, shall not be affected thereby and each term, covenant or condition of this Agreement shall be valid and be enforced to the fullest extent permitted by law; and (ii) the Parties hereto covenant and agree to renegotiate any such term, covenant or application thereof in good faith in order to provide a reasonably acceptable alternative to the term, covenant or condition of this Agreement or the application thereof that is invalid or unenforceable.
13.12 Force Majeure. Each Party will be excused from the performance of its obligations under this Agreement to the extent that such performance is prevented by a Force Majeure Event (as defined below) and the non-performing Party promptly provides written notice to the other Party of such inability and of the period for which such inability is expected to continue. Such excused performance will be continued so long as the condition constituting a Force Majeure Event continues and the non-performing Party takes reasonable efforts to remove or cure the condition. For purposes of this Agreement, a Force Majeure Event means conditions caused by occurrences beyond the control of the Party affected, including an act of God, an act, pronouncement, omission or delay in acting by any Governmental Authority or Regulatory Authority or the other Party, war, an act of war, terrorism, insurrection, riot, civil commotion, epidemic, failure or default of public utilities or common carriers, shortages of raw materials or other supplies necessary for Mundipharma’s Third Party manufacturer to manufacture the Licensed Products, sabotage, labor strike, lockout, labor disturbance, embargo, fire, explosion, earthquake, flood, storm or like catastrophe (each a “Force Majeure Event”).
13.13 Entire Agreement; Amendment. This Agreement (including the Schedules and Exhibits hereto) sets forth all the covenants, promises, agreements, warranties, representations, conditions and understandings
Page 24
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
between the Parties hereto and supersedes and terminates all prior agreements and understanding between the Parties (including but not limited to the Original Agreement). There are no covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between them concerning the subject matter hereof, other than as are herein set forth. No subsequent alteration, amendment, change or addition to this Agreement shall be binding upon the Parties hereto unless reduced to writing and signed by the respective authorized officers of the Parties.
13.14 Status of Parties. The status of each Party hereunder is that of an independent contractor. No provision of this Agreement shall be construed to place the Parties in the relationship of partners or joint venturers. Neither Party is, and neither will represent itself to be, an agent, representative or employee of the other Party, and neither Party has any right or authority to obligate the other in any manner or thing whatsoever. No third parties shall be entitled to rely upon the terms and conditions of this Agreement.
13.15 Further Assurances. After the Effective Date, the Parties shall and, to the extent this Agreement expressly imposes obligations on its Associates, each Party shall cause such Associates to, from time to time, execute and deliver such additional instruments, documents, conveyances or assurances and take such other action as shall be necessary or other reasonably requested by the other Party, to confirm and assume the rights and obligations provided for in this Agreement.
13.16 Performance by Associates. Each of Mundipharma and BioCryst acknowledges that certain obligations under this Agreement may be performed by Associates of Mundipharma and BioCryst. Each of Mundipharma and BioCryst guarantees the performance of this Agreement by any of its Associates, and shall remain responsible therefor. Any Associate of Mundipharma or BioCryst to which rights are extended or which performs any of the obligations required of the respective Party hereunder will be deemed to have accepted and be bound by the relevant terms and conditions of this Agreement, including the dispute resolution procedures set forth in Section 12.2.
13.17 Intellectual Property. The Parties acknowledge and agree that the BioCryst Patents and BioCryst Know-How licensed under this Agreement are “intellectual property” within the meaning of Section 101(35(A)) of Title 11 of the U.S. Code (the “Bankruptcy Code”), and that this Agreement is an executory contract governed by Section 365(n) of the Bankruptcy Code in the event that a bankruptcy proceeding is commenced involving BioCryst.
13.18 Counterparts; Facsimile Signature. This Agreement may be executed in counterparts, each of which counterparts, when so executed and delivered, will be deemed to be an original, and both of which counterparts, taken together, will constitute one and the same instrument even if both Parties have not executed the same counterpart. Signatures provided by facsimile transmission will be deemed to be original signatures.
Page 25
Pursuant to 17 CFR 240.24b-2, confidential information has been omitted in places marked “* * *” and has been filed separately with the Securities and Exchange Commission pursuant to a Confidential Treatment Application filed with the Commission
IN WITNESS WHEREOF the parties hereto have executed this Agreement by their proper officers on the date and year first above written.
| BIOCRYST PHARMACEUTICALS, INC. | ||
| By: | /s/ Alane Barnes | |
| Name: | Alane Barnes | |
| Title: | VP, General Counsel | |
| MUNDIPHARMA INTERNATIONAL CORPORATION LIMITED | ||
| By: | /s/ Douglas Docherty | |
| Name: | Douglas Docherty | |
| Title: | General Manager | |
Page 26
