Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ISTA PHARMACEUTICALS INC | d274572d8k.htm |
 Investor
Presentation December 2011
Exhibit 99.1 |
 ISTA PHARMACEUTICALS
2
Certain statements contained herein are “forward-looking”
statements (as such term is defined in the Private Securities
Litigation Reform Act of 1995). Because such statements include
risks and uncertainties, actual results may differ materially from
those expressed or implied by such forward-looking statements.
Factors that could cause actual results to differ materially from those
expressed or implied by such forward-looking statements include,
but are not limited to, failure to initiate clinical studies, failure to
achieve positive results in clinical trials, failure to receive market
clearance from regulatory agencies, and those risks and
uncertainties discussed in filings made by ISTA Pharmaceuticals,
Inc.,
with the Securities and Exchange Commission.
Safe
Harbor
Statement |
 ISTA PHARMACEUTICALS
3
3
1992
2000
2002
2004
2005
2009
2010
Founded as Advanced
Corneal Systems
Acquired ISTALOL & XIBROM
Name change
to
ISTA Pharmaceuticals, Inc.
IPO on NASDAQ
ISTALOL®
VITRASE®
XIBROM™
BEPREVE®
Revenue
>$100 million
BROMDAY™
ISTA
Profitable*
* On an adjusted cash net income basis
Company Timeline |
 ISTA PHARMACEUTICALS
4
•
Thriving core Rx eye business, emerging Rx allergy franchise
–
Third largest branded Rx eye care company in U.S.
•
Obtained 5 Rx eye and allergy product approvals in 6 years
–
ISTALOL, VITRASE, XIBROM, BEPREVE, BROMDAY
–
Products are #1 or #2 in their markets with market share growth potential
•
Deep R&D pipeline of new product candidates
–
Anticipate 5 new products < 5 years
•
Assembled experienced management team, formidable specialty sales
force:
~ 340 employees –
half are field based
•
Five-year (2006 –
2011)* compounded growth rate of 38%
•
Achieved profitability in 2010**
* Based on estimated 2011 revenues
Rx = Prescription
** On an
adjusted cash net income basis
ISTA’s Track Record
of Success |
 ISTA PHARMACEUTICALS
•
Highly successful switch from XIBROM to BROMDAY
•
BEPREVE projected to increase significantly over 2010
•
Strong pipeline progress, de-risking of programs
–
PROLENSA™
Phase 3 successful
–
BEPOMAX™
Phase 2 successful, BEPOSONE™
to enter Phase 2 shortly
–
OTC tear product formulated and successfully tested
–
T-PRED™
path forward clarified
5
2011 –
Solid Commercial and
Pipeline Progress |
 ISTA PHARMACEUTICALS
6
BEPREVE®
Fast growing, twice-daily
prescription eye drop for
ocular itching associated
with allergic conjunctivitis
ISTALOL®
Leading once-daily beta-
blocker eye drop for the
treatment of glaucoma
VITRASE®
Leading spreading agent
used to enhance absorption
and dispersion of other
injected drugs
BROMDAY™
Only once-daily
prescription eye drop for
postoperative
inflammation and
reduction of ocular pain in
patients who have
undergone cataract
extraction
Current Commercial
Products
$300 Million Mid-Term Potential Revenue |
 ISTA PHARMACEUTICALS
7
•
BROMDAY October 2011 prescriptions
equal to highest level achieved by
XIBROM
•
Major new managed care account wins
to drive increased share
•
Introduction of Twin Pack
–
5% of new prescriptions are Twin Pack as of
early December 2011
•
This added approximately $5 to the list price
•
No generic to BROMDAY until October
2013
–
Planning synchronized switch to PROLENSA™
in 2013
•
Patents pending 2024
The ONLY
approved once-daily
Rx non-steroidal
anti-inflammatory (NSAID) eye drop for pain and
inflammation post-cataract surgery
Estimated
NSAID
2011
Market
Size
TRx
$:
~$367MM
BRANDED
KETOROLAC
4%
GENERIC
KETOROLAC
27%
XIBROM+
BROMDAY
35%
NEVANAC
29%
GENERIC
BROMFENAC
2%
DICLOFENAC
3%
OTHER
0%
Based on Nov 2011 Twelve Month Rolling TRx$
BROMDAY
TM
Convenient Once-Daily Dosing |
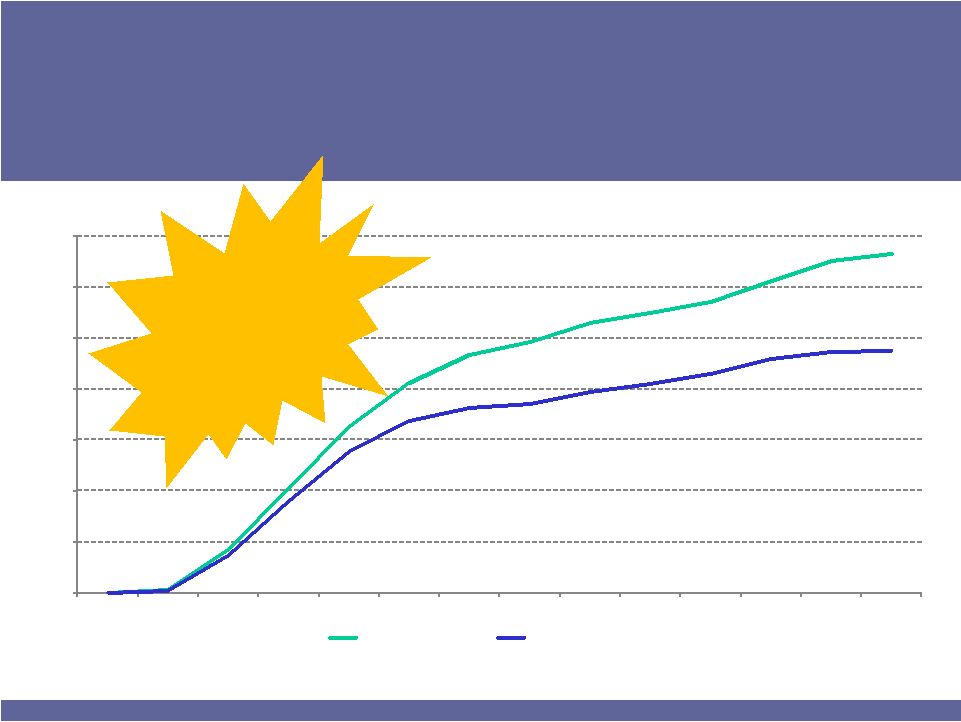 ISTA PHARMACEUTICALS
8
Source –
IMS Health Monthly NPA –
All MDs
0.0%
5.0%
10.0%
15.0%
20.0%
25.0%
30.0%
35.0%
Oct-10
Nov-10
Dec-10
Jan-11
Feb-11
Mar-11
Apr-11
May-11
Jun-11
Jul-11
Aug-11
Sep-11
Oct-11
Nov-11
BROMDAY TRX$ MS
BROMDAY TRX MS
33%
24%
BROMDAY Market Shares
#1 in Total
Prescription
Dollars (TRx$)
Achieved 33%
TRx$ share in
only 13 months
BROMDAY Outlook Strong
Prescription Dollars Reflect Increasing Average Price
|
 ISTA PHARMACEUTICALS
9
•
Twice-daily antihistamine eye drop to treat
itching associated with allergic conjunctivitis
•
Projected 2011 revenues to grow significantly
•
Major new managed care formulary wins to
drive market share in 2012
–
Currently #2 branded in NRx$ behind
Patanol/Pataday
•
Rx of BEPREVE generates 35-60% more
revenues than competitors
–
Larger package size
•
Patents to 2017 with extension expected to
2019
–
Additional patents pending through 2023
Patanol, Pataday and Lastacaft are trademarks of their respective owners
Opportunity for BEPREVE to gain additional market share
2011
Estimated
Allergy
Market
:
~$780
MM
PATADAY
40%
PATANOL
37%
BEPREVE
5%
LASTACAFT
2%
EPINASTINE
3%
AZELASTINE
5%
ALL
OTHER
8%
Nov 2011 Rolling 12 Month TRx
BEPREVE
®
Near-term tactical drivers in place |
 ISTA PHARMACEUTICALS
•
ISTALOL
–
Market-leading once daily beta-blocker eye drop for glaucoma
–
Growth through share gain and pricing
–
2010 market size: ~$ 180 million
–
Patent protection to 2018
•
VITRASE
–
Injectable biologic spreading agent, hyaluronidase, used to
enhance absorption and dispersion of other injected drugs
–
100% market share while competitors continue to deal with
manufacturing issues (likely through 2012)
–
2010 market size: ~$13 million
10
ISTALOL and VITRASE
Established base products |
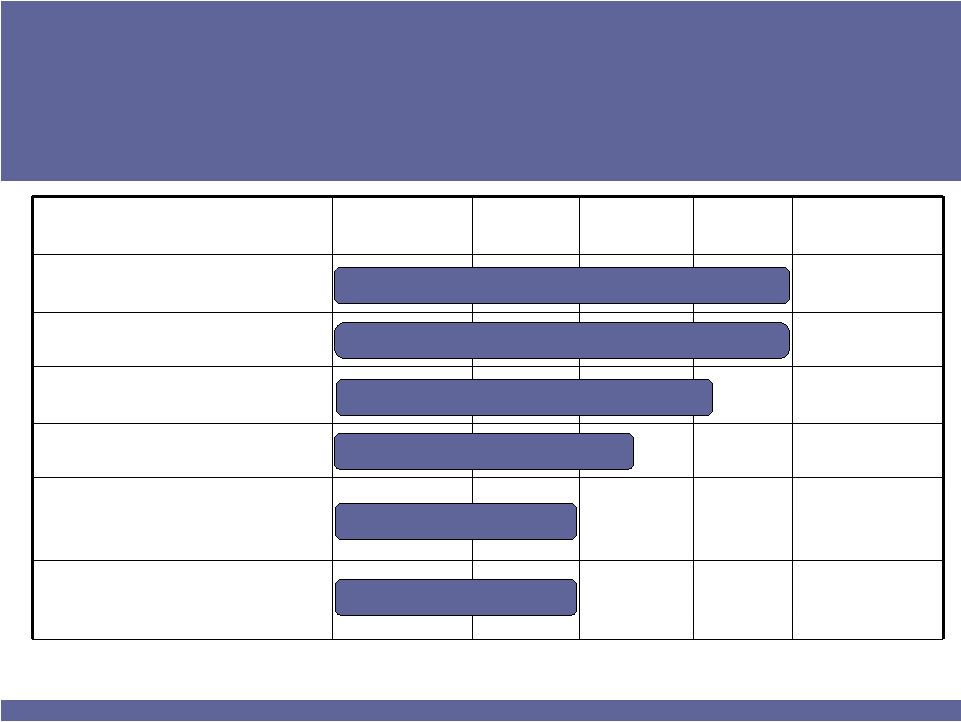 ISTA PHARMACEUTICALS
11
Candidates**
Formulation
Phase 1
Phase 2
Phase 3
2015 Est.
Market Size
OTC eye products
$300 million
PROLENSA
Low-concentration bromfenac
$500+ million
T-PRED
Antibiotic/steroid
$150 million
BEPOMAX
Bepotastine nasal spray
$400 million
BEPOSONE
Bepotastine/steroid
combo nasal spray
$2+ billion
Bromfenac Adjunct
for Age-related Macular
Degeneration
$400+ million
Allergic Rhinitis
Ocular Inflammation / Infection
* Includes BEPOMAX/BEPOSONE partnership
** Does not include all pre-clinical candidates
Ocular Inflammation & Pain
Dry eye
AMD
Allergic Rhinitis
Total Market Opportunity >$3.5 billion
ISTA’s Robust Near-Term Pipeline
Launches Drive Revenues to $500 by 2015* |
 ISTA PHARMACEUTICALS
•
Over-the-Counter eye drops for treatment of dry eye and other
ocular conditions
•
OTC developed and tested in 2011 during prescription dry eye
Phase 3 studies
–
Statistically significant improvements from patient baselines
•
Complete stability testing -
1H 2012
•
2015 market potential: ~$300
million
•
Looking to acquire OTC business to jump-start launch
12
Existing sales force to drive practitioner-recommended sales
OTC Eye Products
Expansion of Rx Business into High-
Operating Margin Consumer Cash Business |
 ISTA PHARMACEUTICALS
13
•
Bromfenac-based NSAID for ocular pain and
inflammation
associated
with
cataract
extraction
–
projected
to
replace
BROMDAY
•
Positive
Phase
3
Study
Results
Reported
Oct.
2011
–
Clinical
work
done
•
Plan to file NDA with U.S. FDA 1H 2012
•
Launch by early 2013
•
2015 market potential: $500+ million
•
Patents pending through 2024
Key extension of successful bromfenac business
PROLENSA
™
New Formula with Pending Patents through 2024
–
Lowest number of adverse events (greater than 2%) vs. prior bromfenac trials
–
Optimized, lower concentration formula enhances penetration into ocular tissues
–
Will implement switch strategy similar to XIBROM to BROMDAY
–
Complete switch before BROMDAY Hatch-Waxman exclusivity expires in October 2013 |
 ISTA PHARMACEUTICALS
14
•
Fixed combination prednisolone & tobramycin prescription eye
drop
•
For steroid-responsive inflammatory ocular conditions for which
a corticosteroid is indicated and where superficial bacterial
ocular infection or risk of infection exists
•
Initiate Phase 3 trials 2H 2012
•
2015 market potential: ~$150
million
•
Potential primary care market
•
Pending patent through 2025
Ideal supplemental product to leverage sales infrastructure
T-Pred
Promising Anti-Infective/Steroid |
 ISTA PHARMACEUTICALS
15
•
Single agent antihistamine nasal spray
•
Active ingredient, bepotastine, already approved in Rx eye drop BEPREVE
•
For treatment of seasonal allergic rhinitis
•
Positive Phase 2 Study results reported April 2011
•
Safety similar to placebo and other antihistamine nasal sprays
•
Patented through 2017
–
Pending patent through 2023
–
Additional patents filed through 2031
Large Market Opportunity Exists
2015 Potential: ~$400+ million
BEPOMAX
Major
Extension of Allergy Franchise |
 ISTA PHARMACEUTICALS
16
•
Combination patented antihistamine / steroid
•
Antihistamine, bepotastine, already approved in Rx eye drop BEPREVE
•
For treatment of seasonal allergic rhinitis
•
Filed Investigational New Drug (IND) Q4 2011
•
Plan to initiate 4-armed Phase 2 Mountain Cedar Pollen study in December
2011
•
To accelerate growth, seek marketing partner for access to primary care MDs
•
Launch expected 2015
•
Patented through 2017
–
Pending patent through 2023
–
Additional patents to be filed
Large Market Opportunity Exists
2015 Potential: $2+ Billion
BEPOSONE ™
"Holy-Grail" Rx Nasal Allergy Product |
 ISTA PHARMACEUTICALS
17
•
Higher-concentration, new formulation of bromfenac
•
For adjunctive treatment of age-related macular degeneration (AMD)
•
Paper published in Sept 2011 Issue of RETINA suggests bromfenac 0.09%
(XIBROM), administered twice daily may have an additive effect when used
with intravitreal ranibizumab (LUCENTIS®)
in reducing retinal thickness in
neovascular AMD
•
Determining path forward with FDA as adjunctive therapy with intravitreal
injections
•
2015 market potential: $400+ million
•
Pending patents through 2024 & 2028
Continued growth of successful bromfenac franchise
* Improvement in visual acuity not demonstrated in this study
LUCENTIS®
is a registered trademark of Genentech, a member of the Roche Group
Bromfenac Adjunct for AMD
Lower-Risk "Back of the Eye" Approach
|
 ISTA PHARMACEUTICALS
18
PRODUCT(S)
PATENT(S) AND EXPIRATION
KEY CLAIM(S)
BEPREVE
BEPOSONE
ISSUED
Exp: Dec 2017 , filed for extension to Sept 2019
Bepotastine API and methods of mfg.
(composition of matter for
Bepotastine besilate)
APPLICATION
Estimated Exp: July 2023
Aqueous formulation
BEPOMAX
APPLICATION
Estimated Exp: October 2031
Formulation and use
PROLENSA
APPLICATION
Estimated Exp: Jan 2024
Formulation containing tyloxapol
APPLICATION
Estimated Exp: Jan 2024
Formulation and method of use
Bromfenac adjunctive
therapy
APPLICATION
Estimated Exp: Feb 2028
Method of use in AMD (also covered
by PROLENSA patent apps)
ISTALOL
ISSUED
Exp: Nov 2018
Method of use in eye drops
T-PRED
APPLICATION
Estimated Exp: April 2025
Formulation and method of use
ISTA’s Key Patents and
Applications |
 ISTA PHARMACEUTICALS
19
~$1 billion annual revenues*
* Includes BEPOMAX/BEPOSONE partnership
Marketed
Products
Product
Pipeline
Business
Development
•
BEPOMAX nasal spray
•
BEPOSONE combo
nasal spray
•
Acquire OTC
business platform
•
Add bolt-on deals
> $300 million annual revenues
> $500 million annual revenues*
•
Acquire marketed products
•
License late stage assets
in 2013+
•
Acquire marketed products
•
Partner for access to
primary care physicians
and consumer branding
•
PROLENSA
(low-
concentration bromfenac)
•
T-PRED
•
OTC tear products
BROMDAY
ISTALOL
VITRASE BEPREVE
ISTA Strategy for Long-Term Success |
 ISTA PHARMACEUTICALS
•
Disciplined approach to evaluation of opportunities
•
Rx Eye and Rx Allergy for growth and commercial synergy
–
Acquire marketed or soon-to-be marketed products
–
License late-stage products
–
Partner for access to primary care physicians and consumer branding
•
OTC Eye and Allergy for growth without heavy R&D investment
–
Acquire OTC business platform
–
License and/or develop new OTC products
•
Artificial tears
•
Ocular vitamins
•
Other
Goal –
To Become $1 Billion Specialty Pharmaceutical Firm
20
Business Development
External Value Creation |
 ISTA PHARMACEUTICALS
Outlook
$160 –
$175
21
ISTA
Fast-Growing, Niche Pharmaceutical Company |
 ISTA PHARMACEUTICALS
•
Webcast
and
Conference
call
–
Jan.
4,
2012
4:30
pm
ET
–
Internet: http://www.istavision.com/investors.html
–
Domestic dial-in:
866-270-6057
International : 617-213-8891
–
Passcode: 58694464
22
2012
Guidance
and Pipeline Update |
 ISTA PHARMACEUTICALS
•
"5 in 5"
–
5 new product launches in next 5 years
•
Rx Eye, Rx Allergy, Over-the-Counter
–
$500 million in revenues by 2015
•
Forecasted compounded 5-year revenue growth rate ~ 40%
•
Organic growth from current products drives revenues majority of
growth
•
Revenues include partnering for primary care access –
BEPOMAX/BEPOSONE
•
Accelerate revenue growth through:
–
Establishment of OTC business, de-risks future sales
–
Licensing of late-stage assets
–
Acquisition of marketed drugs
23
Realizing Value -
Looking to
2015 and Beyond |
 |
