Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HUMAN GENOME SCIENCES INC | c22238e8vk.htm |
Exhibit 99
| Benlysta(r) And beyond September 8, 2011 |
| Note Regarding Forward-Looking Statements 2 This presentation includes statements that are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include those regarding our expectations for BENLYSTA(r). These forward-looking statements are based on our current intentions, beliefs and expectations regarding future events. We cannot guarantee that any forward-looking statement will be accurate. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Investors are, therefore, cautioned not to place undue reliance on any forward- looking statement. Any forward-looking statement speaks only as of the date of this presentation, and, except as required by law, we do not undertake to update any forward-looking statement to reflect new information, events or circumstances. Some important factors that could cause our actual results to differ from our expectations in these forward-looking statements include: our lack of commercial experience and dependence on the sales growth of BENLYSTA; the occurrence of adverse safety events with our products; changes in the availability of reimbursement for BENLYSTA; the inherent uncertainty of the timing and success of, and expense associated with, research, development, regulatory approval and commercialization of our pipeline products; substantial competition in our industry, including from branded and generic products; the highly regulated nature of our business; uncertainty regarding our intellectual property rights and those of others; the ability to manufacture at appropriate scale, and in compliance with regulatory requirements, to meet market demand for our products; our substantial indebtedness and lease obligations; our dependence on collaborations over which we may not always have full control; the impact of our acquisitions and strategic transactions; changes in the health care industry in the U.S. and other countries, including government laws and regulations relating to sales and promotion, reimbursement and pricing generally; significant litigation adverse to the Company, including product liability and patent infringement claims; and increased scrutiny of the health care industry by government agencies and state attorneys general resulting in investigations and prosecutions. The foregoing list sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Investors should consider this cautionary statement, as well as the risk factors identified in our periodic reports filed with the SEC, when evaluating our forward-looking statements HGS, Human Genome Sciences, and BENLYSTA are trademarks of Human Genome Sciences, Inc. Other trademarks referenced are the property of their respective owners. |
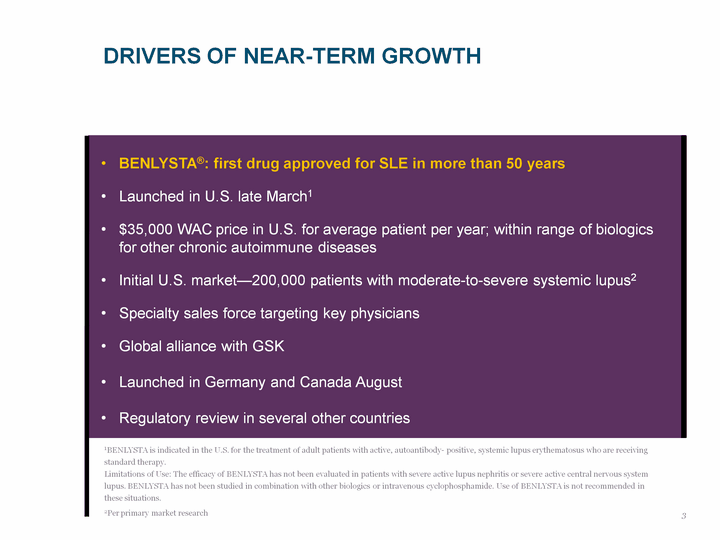
| DRIVERS OF NEAR-TERM GROWTH 1BENLYSTA is indicated in the U.S. for the treatment of adult patients with active, autoantibody- positive, systemic lupus erythematosus who are receiving standard therapy. Limitations of Use: The efficacy of BENLYSTA has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus. BENLYSTA has not been studied in combination with other biologics or intravenous cyclophosphamide. Use of BENLYSTA is not recommended in these situations. 2Per primary market research BENLYSTA(r): first drug approved for SLE in more than 50 years Launched in U.S. late March1 $35,000 WAC price in U.S. for average patient per year; within range of biologics for other chronic autoimmune diseases Initial U.S. market-200,000 patients with moderate-to-severe systemic lupus2 Specialty sales force targeting key physicians Global alliance with GSK Launched in Germany and Canada August Regulatory review in several other countries 3 |

| Drivers of Long-Term Growth BENLYSTA: expand market opportunity Expect to initiate Phase 3 subcutaneous formulation trial around end of 2011 Phase 2/3 vasculitis trial planning underway Proof of concept/exploratory studies in additional indications GSK pipeline, Phase 3s ongoing: Darapladib-cardiovascular Albiglutide-type 2 diabetes Oncology program World-class biologics manufacturing capability 4 |

| Redefining the market for SLE treatment 5 |

| Mild 33% Severe 22% Moderate 45% Initial Target 200,000 1,500,000 Patients with Lupus and Related Conditions* 325,000 SLE Patients Treated by Rheumatologists** * Per Lupus Foundation of America ** Per primary market research BENLYSTA INITIAL U.S. Market: 200,000 Patients 6 |
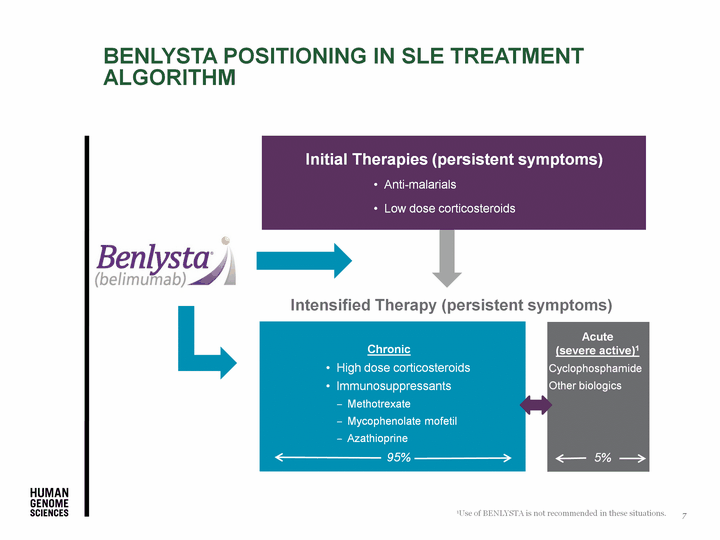
| Benlysta positioning in SLE treatment algorithm Initial Therapies (persistent symptoms) Anti-malarials Low dose corticosteroids Acute (severe active)1 Chronic Intensified Therapy (persistent symptoms) High dose corticosteroids Immunosuppressants Methotrexate Mycophenolate mofetil Azathioprine 1Use of BENLYSTA is not recommended in these situations. 7 95% 5% Acute (severe active)1 Cyclophosphamide Other biologics 5% |

| Launch update 90% of targeted accounts have indicated intent to use BENLYSTA Less than 10% of key accounts have ordered as of June 30 Over 20% have ordered as of mid-August Key uptake drivers Access Perceptions of reimbursement risk Hospital formularies Clinical experience Key focus of sales effort is driving initial trial in new accounts 8 |
| Payer reimbursement - relevant for community accounts ~70% of patients Some rheumatologists hesitant based upon reimbursement delays with previously launched biologics BENLYSTA experience to date positive Benefit verifications positive for >90% of patients, average turnaround 2-3 days BENLYSTA covered (via policy or exception) for nearly all covered lives in the U.S. Q-Code streamlines claims processing; J-Code expected early 2012 BENLYSTA reimbursement experience spreading Hospital formularies - ~30% of patients Acceptances in ~1/3 of hospitals as of end of Q2 Continued steady progress in additional institutions Access Is progressing well initial uptake is AMONG COMMUNITY-BASED PHYSICIANS 9 |
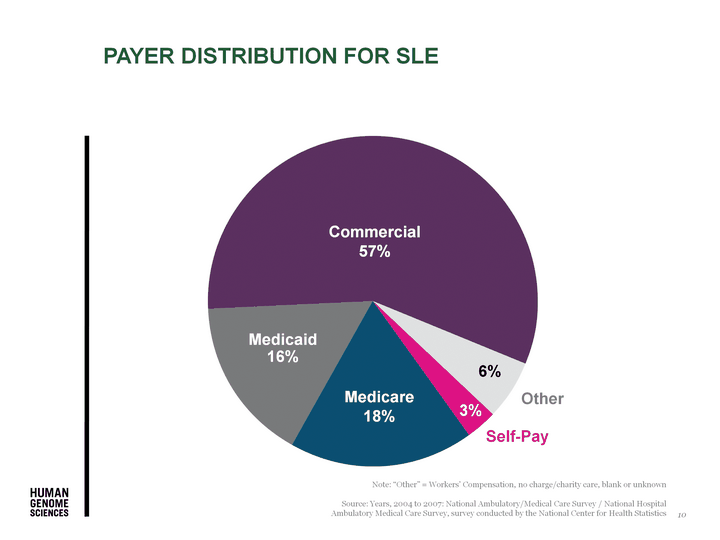
| Payer distribution for sle Note: "Other" = Workers' Compensation, no charge/charity care, blank or unknown Source: Years, 2004 to 2007: National Ambulatory/Medical Care Survey / National Hospital Ambulatory Medical Care Survey, survey conducted by the National Center for Health Statistics Self-Pay Commercial 57% Medicaid 16% Other Medicare 18% 3% 6% 10 |
| Patient identification Broad label covers majority of patients Community rheumatologists (initial driver) less experienced with BENLYSTA New promotional materials now available Initial trial and experience Clinical efficacy measurements vary by rheumatologist Physician adoption process is key Clinical driver of uptake 11 |
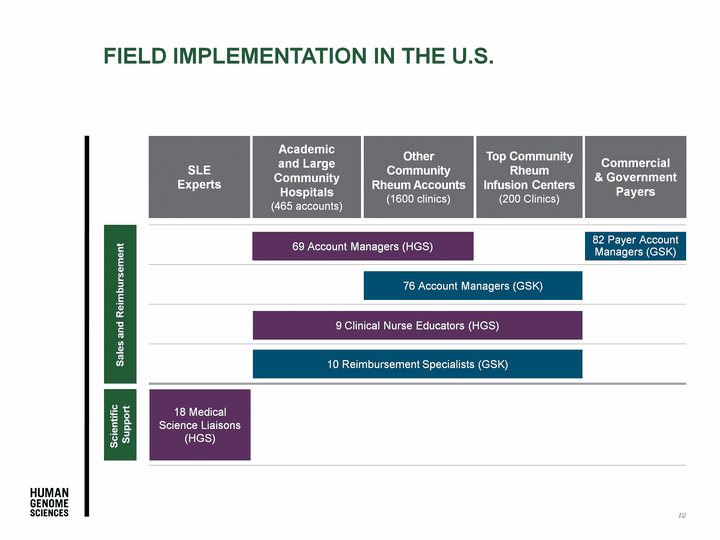
| Field Implementation in the U.S. SLE Experts Academic and Large Community Hospitals (465 accounts) Top Community Rheum Infusion Centers (200 Clinics) Other Community Rheum Accounts (1600 clinics) Commercial & Government Payers 18 Medical Science Liaisons (HGS) 69 Account Managers (HGS) 76 Account Managers (GSK) 9 Clinical Nurse Educators (HGS) 10 Reimbursement Specialists (GSK) 82 Payer Account Managers (GSK) Scientific Support Sales and Reimbursement 12 |
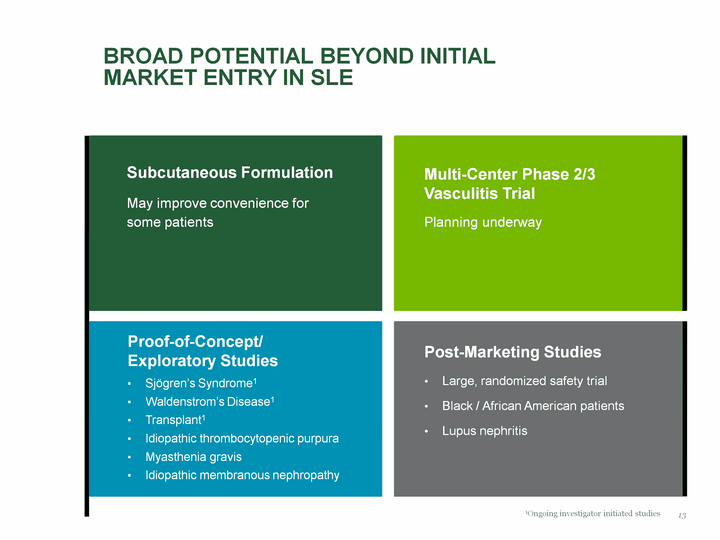
| Proof-of-Concept/ Exploratory Studies Sjogren's Syndrome1 Waldenstrom's Disease1 Transplant1 Idiopathic thrombocytopenic purpura Myasthenia gravis Idiopathic membranous nephropathy Broad Potential Beyond Initial Market Entry in SLE Subcutaneous Formulation May improve convenience for some patients Multi-Center Phase 2/3 Vasculitis Trial Planning underway Post-Marketing Studies Large, randomized safety trial Black / African American patients Lupus nephritis 1Ongoing investigator initiated studies 13 |

| BENLYSTA presents a significant opportunity in europe Launched in Germany August UK, Austria, Sweden, Denmark, Finland and Norway to follow Estimated 200-250K SLE patients1 Concentrated target group = lean organization Other major markets to follow France, Spain, and Italy 1Source: HGS and GSK market research 14 |
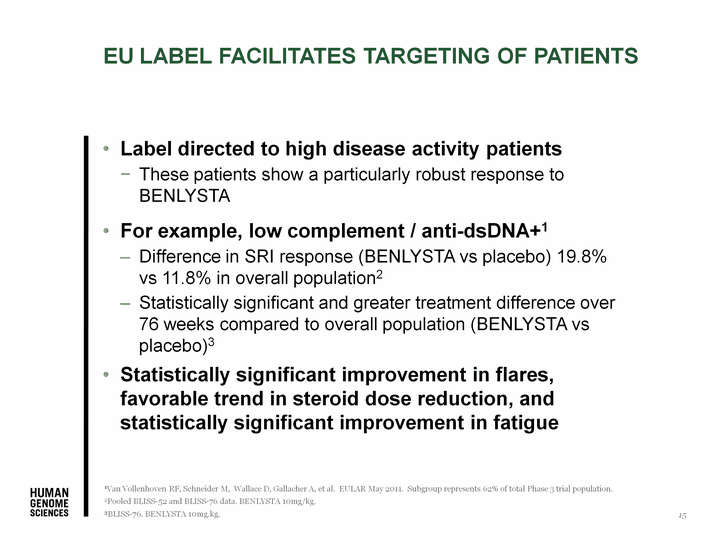
| EU label facilitates targeting of patients Label targets high disease activity patients These patients show a particularly robust response to BENLYSTA Helps physicians identify a group of patients in which they can first use BENLYSTA For example, low complement / anti-dsDNA+1 Difference in SRI response (BENLYSTA vs placebo) 19.8% vs 11.8% in overall population2 Statistically significant and greater treatment difference over 76 weeks compared to overall population3 Accounts for approximately 50% of patients in Phase 3 trials When other widely used definitions of high disease activity are also considered (e.g., high SELENA SLEDAI scores or steroid use), patients with high disease activity would account for approximately 70% of patients in Phase 3 trials Statistically significant improvement in flares, favorable trend in steroid dose reduction, and statistically significant improvement in fatigue 1Van Vollenhoven RF, Schneider M, Wallace D, Gallacher A, et al. EULAR May 2011. Subgroup represents 62% of total Phase 3 trial population. 2Pooled BLISS-52 and BLISS-76 data. BENLYSTA 10mg/kg. 3BLISS-76. BENLYSTA 10mg.kg. 15 |

| Beyond U.S. and EU Launched in Canada August Indication and recommended use closely aligned with U.S. Regulatory applications currently under review in Australia, Brazil, Colombia, Israel, The Philippines, Russia, Singapore, Switzerland, and Taiwan Regulatory submissions anticipated in additional countries during 2011 Phase 3 program for China, Japan and S. Korea recently initiated Regulatory submissions during or after 2013 16 |
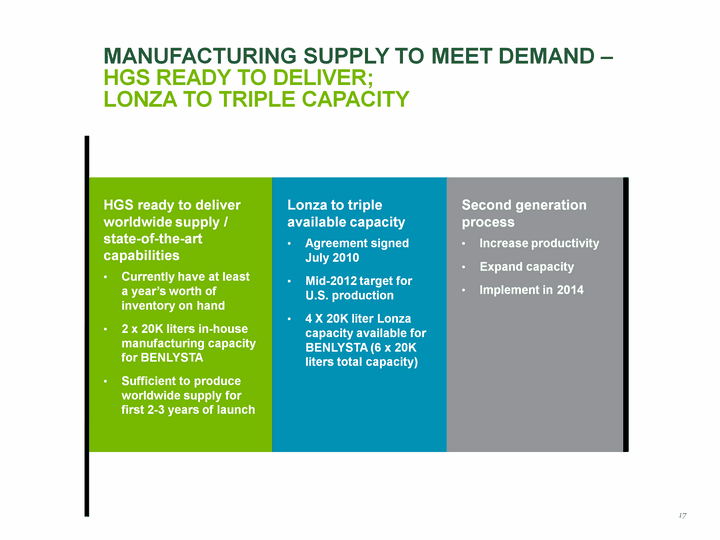
| Manufacturing Supply To Meet Demand - HGS Ready to deliver; Lonza to Triple Capacity HGS ready to deliver worldwide supply / state-of-the-art capabilities Currently have at least a year's worth of inventory on hand 2 x 20K liters in-house manufacturing capacity for BENLYSTA Sufficient to produce worldwide supply for first 2-3 years of launch Lonza to triple available capacity Agreement signed July 2010 Mid-2012 target for U.S. production 4 X 20K liter Lonza capacity available for BENLYSTA (6 x 20K liters total capacity) Second generation process Increase productivity Expand capacity Implement in 2014 17 |

| Beyond 18 |
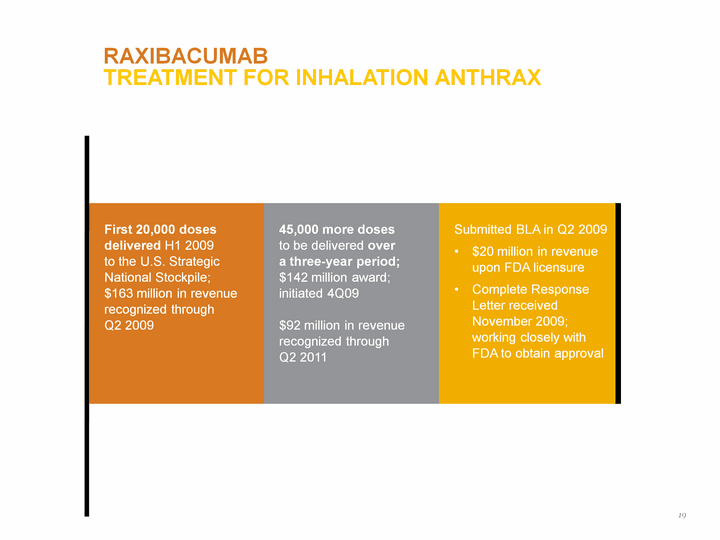
| RAXIBACUMAB Treatment for Inhalation Anthrax First 20,000 doses delivered H1 2009 to the U.S. Strategic National Stockpile; $163 million in revenue recognized through Q2 2009 45,000 more doses to be delivered over a three-year period; $142 million award; initiated 4Q09 $92 million in revenue recognized through Q2 2011 Submitted BLA in Q2 2009 $20 million in revenue upon FDA licensure Complete Response Letter received November 2009; working closely with FDA to obtain approval 19 |
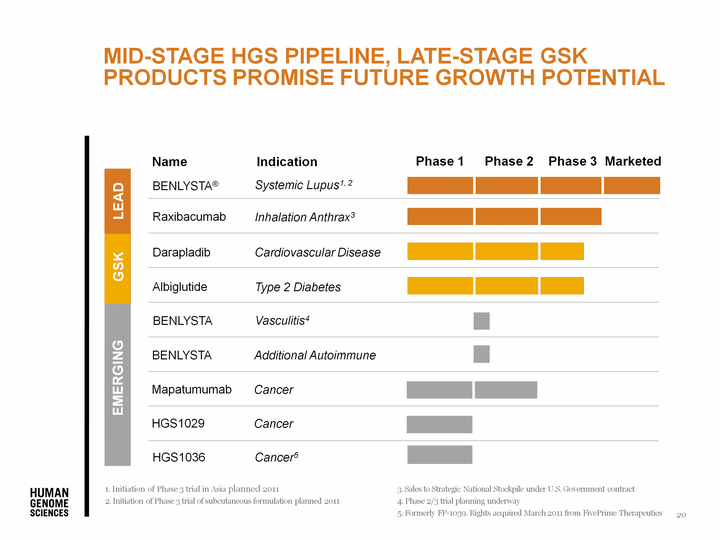
| Initiation of Phase 3 trial in Asia planned 2011 Initiation of Phase 3 trial of subcutaneous formulation planned 2011 Sales to Strategic National Stockpile under U.S. Government contract Phase 2/3 trial planning underway Formerly FP-1039. Rights acquired March 2011 from FivePrime Therapeutics Raxibacumab Inhalation Anthrax3 BENLYSTA(r) Systemic Lupus1, 2 Type 2 Diabetes Albiglutide Darapladib Cardiovascular Disease Marketed EMERGING GSK LEAD Mid-Stage HGS Pipeline, Late-Stage GSK Products Promise Future Growth POTENTIAL 20 |
| Discovered by GSK based on HGS technology 10% royalty on worldwide sales 20% co-promotion option in North America and Europe CV disease one of the largest markets in the pharmaceutical industry Darapladib Phase 3 for Cardiovascular Disease Lp-PLA2 inhibitor as a potential anti-atherosclerosis agent for cardiovascular disease Two Phase 3 trials to evaluate Major Adverse Cardiovascular Events (MACE) STABILITY: ~15,800 patients; 39 countries; initiated December 2008; fully enrolled SOLID-TIMI 52: ~11,500 patients in 40 countries; initiated December 2009 21 |
| Licensed to GSK Fees and milestone payments up to $183 million; up to $150 million still to be received1 Single-digit net royalties on worldwide sales Approximately 18.8 million diagnosed type 2 diabetes patients in the U.S.2 Albiglutide Phase 3 for Type 2 Diabetes Once-a-week GLP-1 agonist Eight Phase 3 studies ongoing to evaluate efficacy and safety as mono- and add-on therapy in patients with type 2 diabetes First Phase 3 clinical trial initiated 1Q09; expected study duration of up to 3 years 1Includes regulatory and sales milestones 2National Diabetes Fact Sheet, 2011, Centers for Disease Control and Prevention 22 |
| HGS ONCOLOGY PIPELINE Mapatumumab (monoclonal antibody to the TRAIL-R1; HGS-ETR1) Targets TRAIL apoptosis pathway Q1 2011 initiation of randomization stage-Phase 2 chemotherapy combo trial in hepatocellular carcinoma HGS1029 (small molecule IAP inhibitor) Novel intracellular target in apoptosis pathway Synergistic preclinical activity demonstrated with mapatumumab and other therapeutic agents Currently in Phase 1 trials as monotherapy-advanced solid and lymphoid tumors Planning underway for Phase 1b combination oncology studies HGS1036 (first-in-class ligand trap targeting FGF pathway) Potential in various cancer indications Ongoing phase 1 clinical study (nearing completion); safe and well tolerated to date Phase 1b chemotherapy combination studies planned for 2012 Patients currently being screened for phase 2 trial for a form of endometrial cancer 23 |
| Potential Blockbuster Product Pipeline to Fuel Future Growth Manufacturing Excellence Commercial Readiness World-Class Partner Seasoned Leadership A Formula for Sustainable Growth Building Shareholder Value 24 |

| Thank You 25 |
