Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ISTA PHARMACEUTICALS INC | d8k.htm |
 Lauren
Silvernail CFO
and
VP
Corporate
Development
Exhibit 99.1 |
 ISTA PHARMACEUTICALS
2
Safe Harbor Statement
Safe Harbor Statement
Certain statements contained herein are “forward-looking”
statements (as such term is defined in the Private Securities
Litigation Reform Act of 1995). Because such statements include
risks and uncertainties, actual results may differ materially from
those expressed or implied by such forward-looking statements.
Factors that could cause actual results to differ materially from those
expressed or implied by such forward-looking statements include,
but are not limited to, failure to initiate clinical studies, failure to
achieve positive results in clinical trials, failure to receive market
clearance from regulatory agencies, and those risks and
uncertainties discussed in filings made by ISTA Pharmaceuticals,
Inc.,
with the Securities and Exchange Commission. |
 ISTA PHARMACEUTICALS
3
3
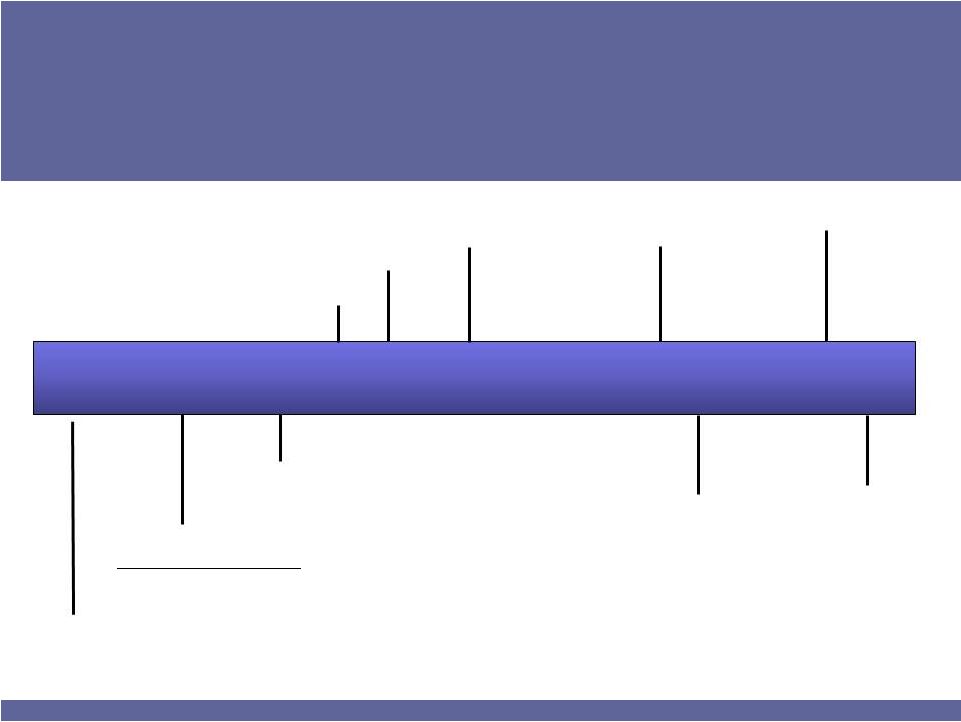
Company Timeline
Company Timeline
1992
2000
2002
2004
2005
2009
2010
Founded as Advanced
Corneal Systems
Acquired ISTALOL & XIBROM
Name change
to
ISTA Pharmaceuticals, Inc.
IPO on NASDAQ –
“ISTA”
ISTALOL®
VITRASE®
XIBROM™
BEPREVE®
Revenue
>$100 million
BROMDAY™
ISTA
Profitable*
* On an adjusted cash net income basis |
 ISTA PHARMACEUTICALS
4
ISTA’s Track Record
of Success
ISTA’s Track Record
of Success
•
Obtained 5 Rx product approvals in 6 years
–
ISTALOL®, VITRASE®, XIBROM™, BEPREVE®, BROMDAY™
•
Attained top market share positions
•
Built deep new product R&D pipeline
•
Assembled experienced management team, formidable
specialty sales force: ~ 330 employees
•
Converted business from 2/3 revenues from XIBROM
(Q3 ‘10) to 2/3 from BROMDAY and BEPREVE ( June ‘11)
|
 ISTA PHARMACEUTICALS
First Half Revenue Results
First Half Revenue Results
5
1H ’11
1H ’10
%
BROMDAY/XIBROM
$36.6
$41.4
(12%)
BEPREVE
$16.4
$ 6.7
145%
ISTALOL
$14.2
$ 9.7
46%
VITRASE
$ 6.7
$ 5.6
20%
NET REVENUES
$73.9
$63.4
17%
Tracking
to
our
guidance:
40%
1
st
half
/
60%
2
nd
half
of
year |
 ISTA PHARMACEUTICALS
ISTA
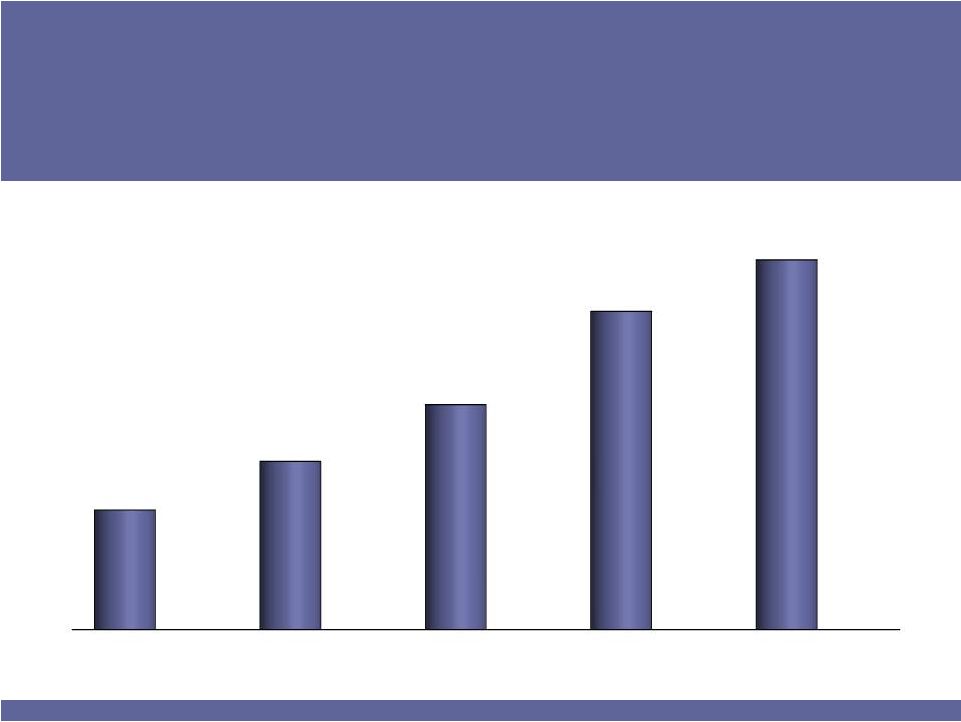
Fast-Growing, Niche Pharmaceutical Company
ISTA
Fast-Growing, Niche Pharmaceutical Company
Outlook
$175–
$190
2007
2008
2009
2010
2011
(F)
$59
$83
$111
$157
Net Revenues
(in $millions) |
 ISTA PHARMACEUTICALS
7
ISTA’s 2011 Financial Guidance
ISTA’s 2011 Financial Guidance
2011 Net Revenues
$175 -
$190 million
Gross Margin*
75% -
77%
R&D*
18% -
22%
SG&A*
44% -
48%
Operating Income
$13 -
$16 million
Adjusted Cash Net Income**
$13 -
$16 million
Adjusted Cash EPS** (50 mm shares)
$0.26 –
$0.32 per share
Cash at 12/31/11***
$80 -
$90 million
* % of Net Sales
** Adjusted Cash Net Income and EPS excludes non-cash warrant expense, non-cash
interest expense and non-cash stock-based compensation expense *** After debt
payments of $21m and including reserves for XIBROM/BROMDAY royalties and bank line
Growing revenues and improving SG&A ratio |
 ISTA PHARMACEUTICALS
8
Key Financial Notes for 2011
Key Financial Notes for 2011
•
Impact of healthcare reform: revenues $3m-$5m lower
•
Tax Rate: 2%-5% based on utilization of net loss carry
forwards
•
Warrants reduced by 8.1 million of 15 million shares in
first half
•
Debt: First of three annual ~$21 million principal
repayments made August 2011 from cash on-hand |
 ISTA PHARMACEUTICALS
9
BROMDAY
™
Once-Daily
(bromfenac ophthalmic solution) 0.09%
BROMDAY
™
Once-Daily
(bromfenac ophthalmic solution) 0.09%
•
First and only approved once-daily
Rx NSAID
–
For postoperative inflammation and reduction of ocular pain in patients
who have undergone cataract extraction
•
Rapid, successful switch from twice-daily XIBROM™
•
Fast-growing, market-leading $100+ million franchise
–
8
largest U.S. branded prescription eye product in 2010
•
$350 million U.S. market
•
No generic to BROMDAY until Oct 2013
–
Low-concentration BROMDAY in Phase 3 studies, patent pending 2024
th |
 ISTA PHARMACEUTICALS
BROMDAY
First Half Results
BROMDAY
First Half Results
•
Prescriptions (TRx) up 6.5%; revenues down 12% over first half ‘10
•
Why?
–
Impact from XIBROM to BROMDAY conversion
•
Lower average price per script due to single, smaller bottle size
•
Lower refill rate for newly launched product
•
Wholesalers moderated BROMDAY inventories in Q2 after initial stocking for launch in
Q4/Q1. Meanwhile, no shipments of XIBROM in Q2
–
Generic twice-daily bromfenac launched in May garnered 13% of total RXs
of franchise
10 |
 ISTA PHARMACEUTICALS
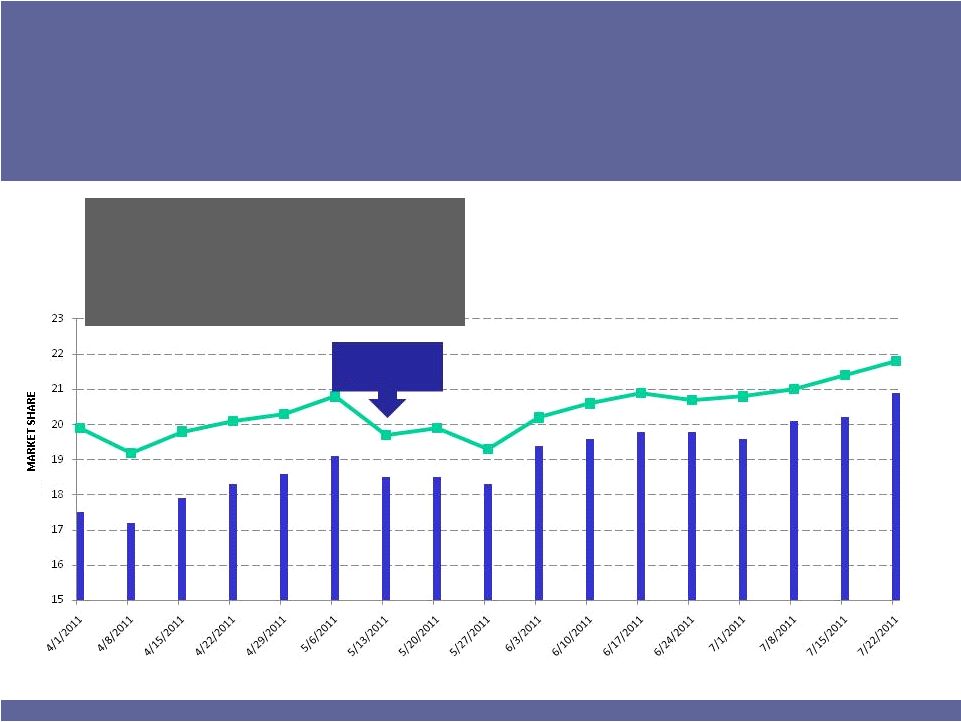
BROMDAY
Outlook Strong
Scripts Continue to Grow
BROMDAY
Outlook Strong
Scripts Continue to Grow
11
•
Continuing to build NRx and TRx share on a weekly basis
•
Sales force re-focused on BROMDAY post-allergy season
•
Generic to XIBROM stalled at ~3% market share
•
Major
new
managed
care
account
win
–
effective
July
1
•
Planning larger volume product for later this year
Generic to
XIBROM
launched
Source –
IMS Health Weekly NPA –
All MDs
BROMDAY
WEEKLY MARKET SHARE |
 ISTA PHARMACEUTICALS
12
BEPREVE
®
(bepotastine besilate ophthalmic solution) 1.5%
BEPREVE
®
(bepotastine besilate ophthalmic solution) 1.5%
•
For ocular itching associated
with allergic conjunctivitis
•
Launched Oct 2009
•
$16 million revenues in 2010
•
Estimated 2011 U.S. market
value: $740 million
•
Achieved #2 in NRx$ behind
Patanol/Pataday
•
Patent protection expected
through 2019, pending patent
expiring in 2023
*6/30/2011 NPA data.
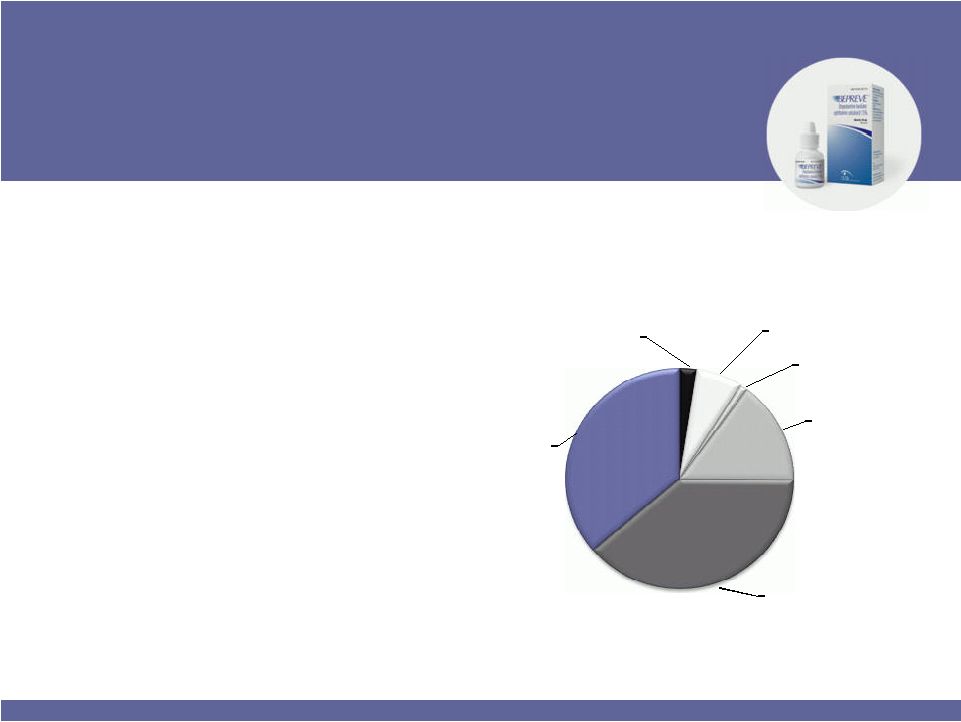
Elestat, Lastacaft, Patanol and Pataday
are
trademarks of their respective
owners. U.S. Prescription Allergy
Eye Competitors
NRx$$ 6/30/11
Lastacaft
2%
BEPREVE
7%
Elestat
1%
All Others
15%
Pataday
39%
Patanol
36% |
 ISTA PHARMACEUTICALS
BEPREVE
®
YTD Prescription Growth Strong
BEPREVE
®
YTD Prescription Growth Strong
Source –
IMS Health Monthly NPA –
All MDs
•
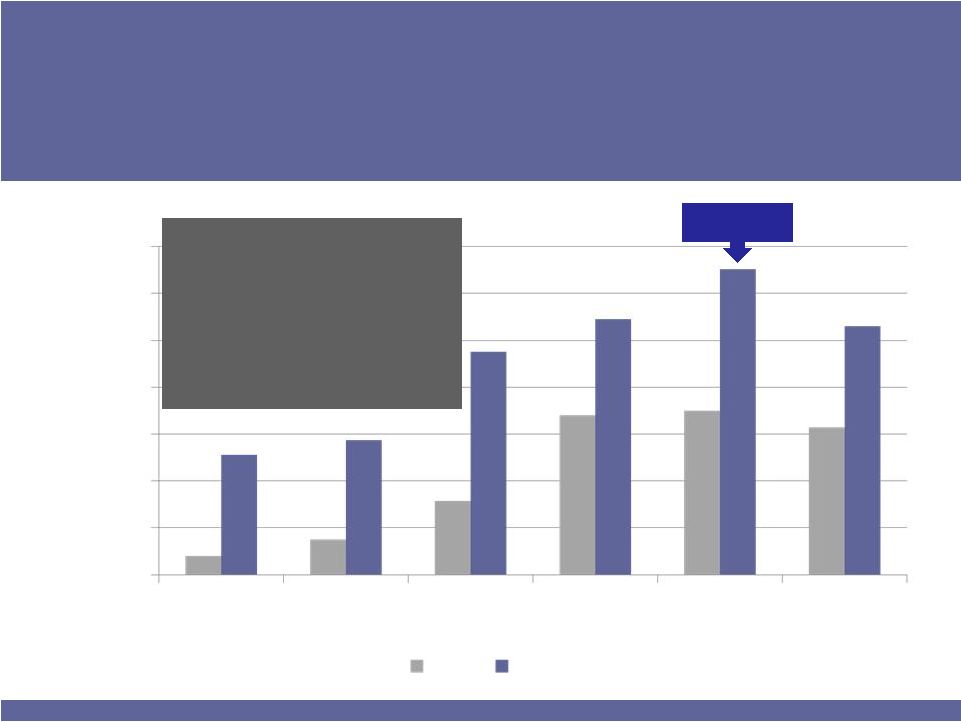
YTD June ‘11 TRx up 114%, TRx $$ up 195%.
Market share growth from 1.6% to 3.8%.
•
In its target audience, BEPREVE has 9.8% share.
•
An Rx of BEPREVE generates between 35-60%
more revenues than its competitors because of
the larger package size.
•
On track to at least double 2010 revenues
BEPREVE TRx
Peak Allergy
Season
0
5,000
10,000
15,000
20,000
25,000
30,000
35,000
Jan
Feb
March
April
May
June
2010
2011
13 |
 ISTA PHARMACEUTICALS
14
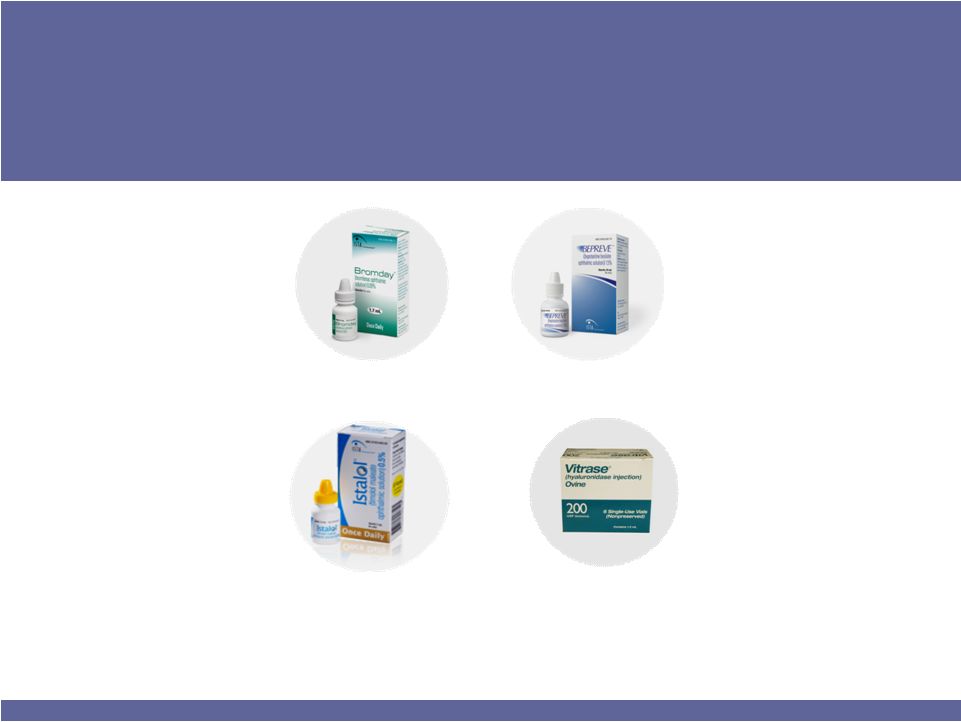
Currently Approved Products Can Drive ISTA
to >$300 Million in Annual Revenues
Currently Approved Products Can Drive ISTA
to >$300 Million in Annual Revenues
BEPREVE®
Twice-daily prescription
eye drop for ocular
itching associated with
allergic conjunctivitis
ISTALOL®
Once-daily eye drop for
the treatment of
glaucoma
VITRASE®
A spreading agent used
to enhance absorption
and dispersion of other
injected drugs
BROMDAY™
Once-daily prescription eye
drop for postoperative
inflammation and
reduction of ocular pain in
patients who have
undergone cataract
extraction |
 ISTA PHARMACEUTICALS
15
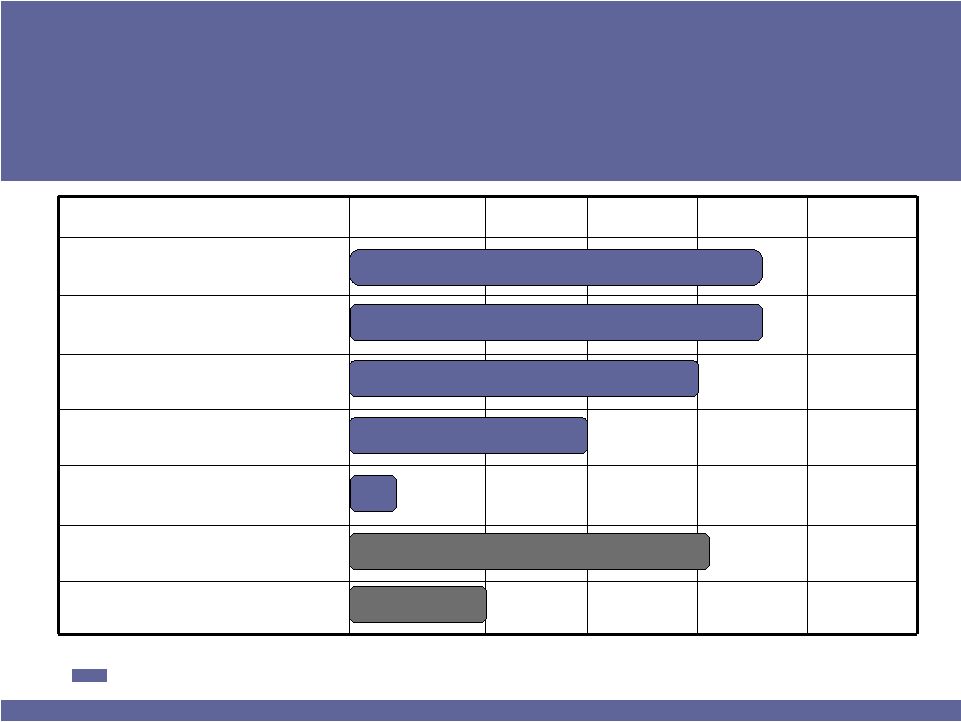
ISTA’s Near-Term Pipeline
Key Approvals Can
Take
Revenue to $500 million by 2015
ISTA’s Near-Term Pipeline
Key Approvals Can
Take
Revenue to $500 million by 2015
Candidates*
Formulation
Phase 1
Phase 2
Phase 3
NDA
Low-concentration
BROMDAY™
REMURA™
(bromfenac for dry eye)
Bepotastine Nasal
Bepotastine Nasal/Combo
Antihistamine/steroid
Bromfenac Adjunct
for AMD
T-PRED™
Antibiotic/steroid
Strong Steroid
Dry Eye
Allergic Rhinitis
Ocular Inflammation / Infection
Drug Candidates with 2011 Milestones
* Does not include all pre-clinical candidates
Ocular Inflammation & Pain
Inflammation
AMD
Allergic Rhinitis |
 ISTA PHARMACEUTICALS
16
Drug Candidate
Active Ingredient
Rx Indication
Current
Stage/Status
2015 Market
Potential
Exclusivity
Low-concentration
BROMDAY™
Lower
concentration,
new formulation
bromfenac
Inflammation &
pain post cataract
surgery
Initiated Phase 3
study,
preliminary results
Q4 2011
$400 MM
3-year Hatch
Waxman. Pending
patents 2024
REMURA™
Lower
concentration,
new formulation
bromfenac
Dry eye
Phase 3 efficacy
studies. WEST
completed, EAST
results due Q4 2011
$1+ billion
3-year Hatch
Waxman. Pending
patents 2024
Bepotastine Nasal
Spray
Bepotastine
Nasal symptoms of
allergic rhinitis
Positive Phase 2
results
1H 2011
>$250 million
3-year Hatch
Waxman. Patented
thru 2017. Pending
patent 2023
Bepotastine/
Steroid Nasal
Spray
Combination
bepotastine +
steroid
Nasal symptoms of
allergic rhinitis
Initiate
Phase
2
2H 2011 or 1H 2012
~ $2+ Billion
3-year Hatch
Waxman. Patented
thru 2017. Pending
patent 2023
Bromfenac Adjunct
Bromfenac
Adjunct with
Lucentis or Avastin
for AMD
Proof of concept
complete, dose-
ranging Phase 2/3
next step
$400-800 million
3-year Hatch
Waxman. Pending
patents expiring
2024 & 2028
Active Pipeline Summary
Active Pipeline Summary |
 ISTA PHARMACEUTICALS
17
Low-Concentration BROMDAY™
Phase 3 Efficacy Studies Initiated May 2011
Low-Concentration BROMDAY™
Phase 3 Efficacy Studies Initiated May 2011
•
Two Studies: East/West, 400 patients, 40 sites
–
1x daily for 16 days
–
1 dose prior to surgery, 1 dose day of surgery, 1 dose per day for 14 days
•
Primary outcome: cleared ocular inflammation
–
Defined as summed ocular inflammation score (SOIS) of grade 0 at
any visit prior to
Day 15
•
Secondary outcome: proportion of patients pain-free at Day 1
–
Grading of “None”
on Ocular comfort grading assessment
•
Anticipate reporting data Q4 2011
•
Expands value of bromfenac franchise
–
Launch expected 1H 2013 |
 ISTA PHARMACEUTICALS
REMURA™
for Dry Eye
Underserved, $700 million market in 2011
REMURA™
for Dry Eye
Underserved, $700 million market in 2011
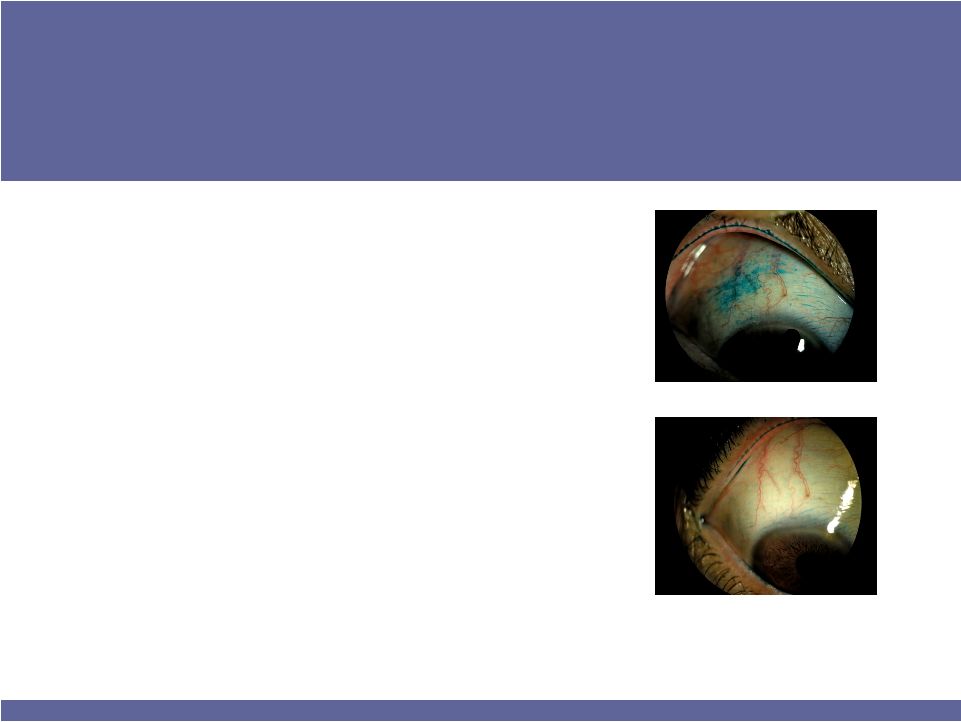
Baseline
After six weeks (BID)
•
Targets inflammatory component
•
Future market potential >$2 billion
•
Strong Phase 2 results (at right)
–
70% of patients responded
–
30% reduction in ocular surface disease index (OSDI)
•
Two Phase 3 efficacy studies
–
~1,000 patients, 2x daily, 42 days
–
Signs of conjunctival staining measured using Lissamine Green
–
Symptoms measured using Ocular Surface Disease Index (OSDI)
–
WEST study results: While REMURA was highly effective in
treating a sign and symptom of dry eye, it was not statistically
significantly better than placebo (vehicle)
–
EAST study fully enrolled. Amending statistical plan. Anticipate
reporting data Q4 2011
•
Pending patents expiring 2024 |
 ISTA PHARMACEUTICALS
19
Bepotastine Nasal Spray
Positive Phase 2 Study Results, April 2011
Bepotastine Nasal Spray
Positive Phase 2 Study Results, April 2011
•
600 patients, multi-sites in Texas
–
Patients presenting allergic rhinitis caused by Mountain Cedar Pollen
•
3 concentrations dosed 2x day for 14 days
–
Patients graded both individual nasal and ocular symptoms on a daily basis
•
Primary outcome: Efficacy of any of the 3 doses of bepotastine nasal spray
in individuals with seasonal allergic rhinitis
–
Statistically significant results for all three concentrations
–
Statistically significant results seen from Day 1
•
Secondary outcome: Safety & tolerability of 3 doses of bepotastine nasal
spray
–
Safety similar to placebo & other antihistamine nasal sprays
•
Next Steps
–
Pursuing combination bepotastine/steroid nasal spray
–
Initiate Phase 2 study for bepotastine/steroid combination either before end of 2011 or
early 2012. |
 ISTA PHARMACEUTICALS
20
ISTA: Strategy for Long-Term Success ISTA:
Strategy for Long-Term Success
Marketed
Products
Product
Pipeline
Business
Development
•
Bepotastine
-
Nasal spray/combo
•
Acquire OTC
business platform
•
Add bolt-on deals
> $300 million annual revenues
> $500 million annual revenues
~$1 billion annual revenues
•
Low-concentration
BROMDAY™
•
REMURA™
for dry eye
•
T-PRED™
•
Strong steroid
•
Acquire marketed products
•
License late stage (Phase 3)
•
Acquire marketed products
•
Partner for access to
primary care physicians
and consumer branding
Rx Eye
Rx Allergy &
Respiratory
OTC Eye
and Allergy |
 ISTA PHARMACEUTICALS
21
21
ISTA's 2011
Milestones
to Drive Shareholder Value
ISTA's 2011
Milestones
to Drive Shareholder Value
Product / Candidates
Milestone
Timing
BROMDAY once-daily
Complete switch from XIBROM
DONE Q1
Low-concentration BROMDAY
for inflammation and pain assoc
with cataract extraction
Initiate Phase 3
Prelim Phase 3 results
Done Q2
Q4
REMURA for dry eye
Prelim Phase 3 efficacy results:
WEST Study
EAST Study
DONE Q3
Q4
Bromfenac adjunct for AMD
Data publication
2H
Bepotastine spray for nasal allergies
Prelim Phase 2 results
DONE Q2
Bepotastine / steroid combination
spray for nasal allergies
Initiate Phase 2 study
2H ‘11 or
1H ‘12 |
 ISTA PHARMACEUTICALS
22
ISTA’s Strategic Vision
“5 in 5”
ISTA’s Strategic Vision
“5 in 5”
•
Become one of top 3 branded Rx eye care companies in the U.S.
•
Achieve #1 or #2 position in each product market
•
Deliver EPS* of $1.00 by 2013 (based on 50 million shares)
•
Generate $500 million in revenue in 5 years
–
Develop products for larger markets
•
Obtain 5 new product approvals in next 5 years
–
Rx Eye and Rx Allergy
•
Accelerate growth in sales via organic growth, partnering and
acquisitions
–
Target: $1 billion in revenues
* Adjusted Cash EPS excludes non-cash warrant expense, non-cash interest expense and
non-cash stock-based compensation expense |
 |
