Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Pernix Sleep, Inc. | c18737e8vk.htm |
Exhibit 99.1

| Patient-Reported Symptom Improvement in Sleep Maintenance Endpoints in Adult and Elderly Patients with Insomnia Treated with Doxepin 3 mg and 6 mg Alan Lankford, Andrew Krystal, Brian Dorsey, Roberta Rogowski, Elizabeth Ludington, H. Heith Durrence, Charles S. Davis, Thomas Roth Support for this study provided by Somaxon Pharmaceuticals, Inc. |

| Introduction The most frequent complaint of insomnia patients is sleep maintenance Defined as the inability to stay asleep throughout the night or the propensity for early morning awakenings Prevalence rates are over 70%1,2 Several well-established methods are available for assessing sleep Includes questionnaires, sleep diaries, polysomnography (PSG), and actigraphy Assessments broadly divided into two categories Objective (PSG is most often used) Subjective (patient-reported) 1Lichstein et al. Epidemiology of sleep...2004. Erlbaum 2Breslau et al. Sleep disturbance...Psychiatry 2004;39:411-8 |
| Mechanism of Action and Previous Results Sleep promoting effects of low dose doxepin (DXP) are likely mediated through histaminergic system DXP, at doses of 3 mg and 6 mg, is a highly potent and selective histamine receptor antagonist DXP at doses of 3 mg and 6 mg has demonstrated consistent efficacy in treating objective sleep maintenance1-4 1Krystal et al. Long-term Efficacy and Safety of DXP 1 mg and 3 mg...Sleep 2010;33:1553-1561 2Krystal et al. Efficacy and Safety of DXP 3 and 6 mg.... Sleep; In Press 3Segal et al. Efficacy and Safety of DXP 6 APA's 161st Meeting; 2008. Abstract NR6-091. 4Roth et al. Efficacy and Safety of DXP 6 mg... Sleep Medicine 2010;11:843-847 |

| Study Designs Subjective data from two double-blind placebo-controlled trials are reported Study A: 12-week trial of elderly patients with chronic primary insomnia N=240; DXP 1 mg and 3 mg versus placebo (PBO) Study B: 5-week trial of adult patients with chronic primary insomnia N=221; DXP 3 mg and 6 mg versus PBO Subjective endpoints included subjective wake after sleep onset (sWASO) and subjective total sleep time (sTST) Endpoints obtained from morning questionnaire completed the morning after a night spent in the sleep laboratory Interactive Voice Response System (IVRS) was also used to collect patient data at home |

| Study Design Schematics, Study A Note. Study A is Elderly 12 week trial |

| Study Design Schematics, Study B Note. Study B is Adult 5 week trial |
| Data Analyses These post-hoc efficacy analyses were performed on ITT data set Included all randomized patients who received at least one dose of double-blind study drug sTST and sWASO were analyzed with mixed-effect model repeated measures (MMRM) approach MMRM methods are used to analyze all available data across time Avoids need to impute missing data Accounts for within subject variability Model included fixed effects for treatment group, time, treatment-by-time interaction, and baseline value |
| Corresponding Objective Results DXP 3 mg and 6 mg significantly improved PSG sleep maintenance and sleep duration in both Phase 3 trials Significant improvements in WASO and TST on Night 1 (p<0.0001) that were maintained at final time points Improvements in PSG-defined TST ranged from 25-43 minutes better than PBO |

| Overall Results from MMRM Analyses No interaction tests were significant None approached significance (all p-values exceeded 0.5) Results indicate no evidence that the effect of DXP 3 mg or 6 mg varied substantially over time Estimated treatment differences were significant for both sWASO and sTST and at both doses Estimated overall treatment differences were significant |

| Summary of MMRM Results Population Measure Dose Difference vs PBO (minutes) p-value Study A sWASO DXP 3 mg -18.3 (6.49) 0.0052 Study A sTST DXP 3 mg 18.9 (7.41) 0.0114 Study B sWASO DXP 3 mg -10.2 (4.41) 0.0213 Study B sWASO DXP 6 mg -14.2 (4.41) 0.0014 Study B sTST DXP 3 mg 11.9 (5.97) 0.0469 Study B sTST DXP 6 mg 17.3 (5.96) 0.0042 Note. The overall treatment effect across time points is displayed; estimated differences reflect estimated least squares (LS) means (standard error). |

| Mean Differences of DXP 3 mg from PBO for sWASO in Study A Minutes (LS mean) Note. sWASO data reflect improvement vs placebo, sWASO values are reduced vs placebo; error bars reflect standard error. |
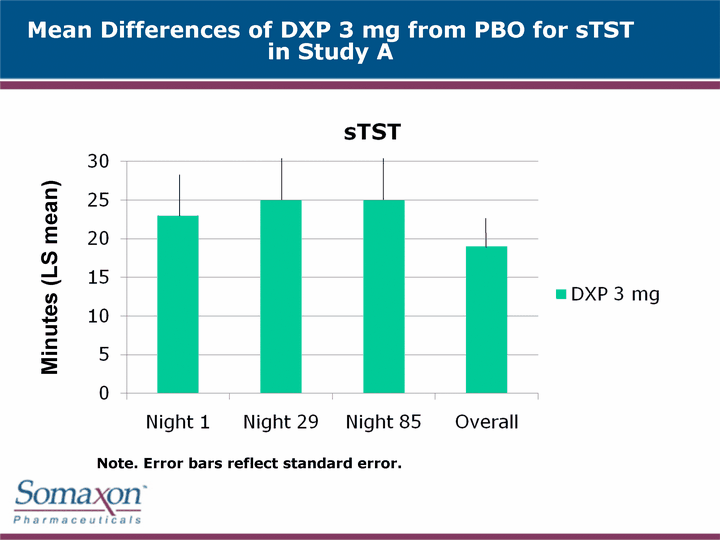
| Mean Differences of DXP 3 mg from PBO for sTST in Study A Minutes (LS mean) Note. Error bars reflect standard error. |
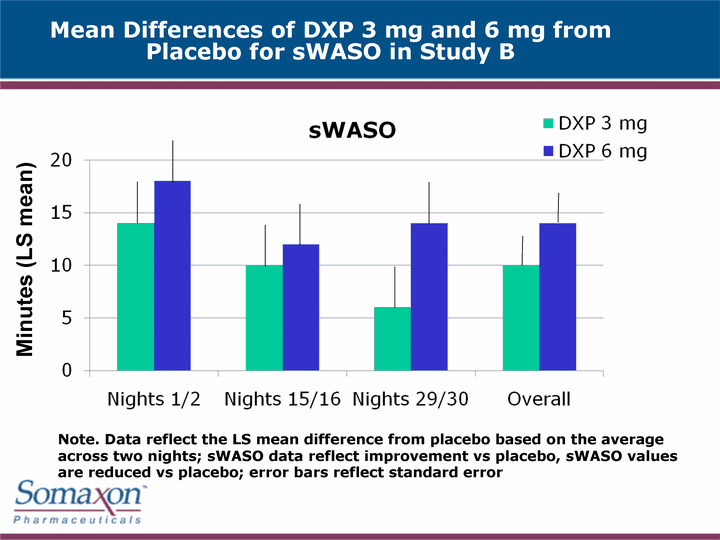
| Mean Differences of DXP 3 mg and 6 mg from Placebo for sWASO in Study B Minutes (LS mean) Note. Data reflect the LS mean difference from placebo based on the average across two nights; sWASO data reflect improvement vs placebo, sWASO values are reduced vs placebo; error bars reflect standard error |
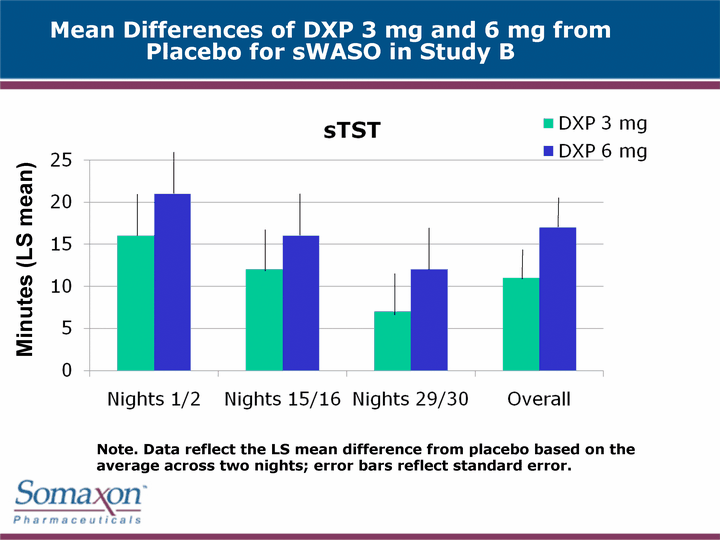
| Mean Differences of DXP 3 mg and 6 mg from Placebo for sWASO in Study B Minutes (LS mean) Note. Data reflect the LS mean difference from placebo based on the average across two nights; error bars reflect standard error. |
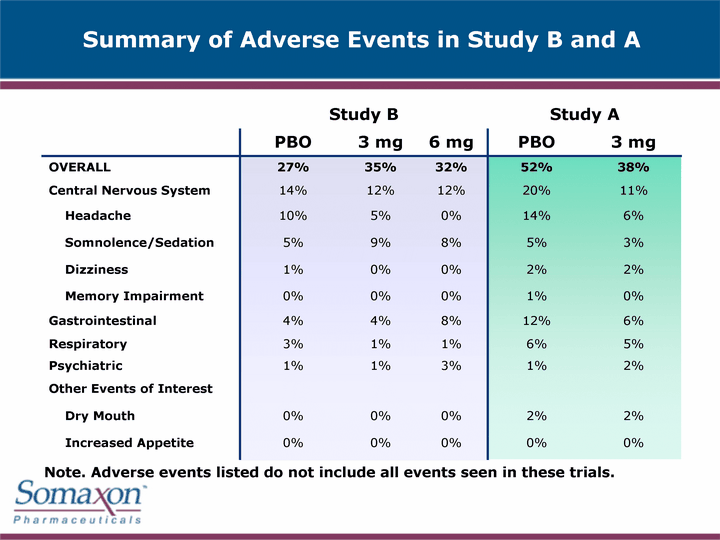
| Study B Study B Study B Study A Study A PBO 3 mg 6 mg PBO 3 mg OVERALL 27% 35% 32% 52% 38% Central Nervous System 14% 12% 12% 20% 11% Headache 10% 5% 0% 14% 6% Somnolence/Sedation 5% 9% 8% 5% 3% Dizziness 1% 0% 0% 2% 2% Memory Impairment 0% 0% 0% 1% 0% Gastrointestinal 4% 4% 8% 12% 6% Respiratory 3% 1% 1% 6% 5% Psychiatric 1% 1% 3% 1% 2% Other Events of Interest Dry Mouth 0% 0% 0% 2% 2% Increased Appetite 0% 0% 0% 0% 0% Summary of Adverse Events in Study B and A Note. Adverse events listed do not include all events seen in these trials. |
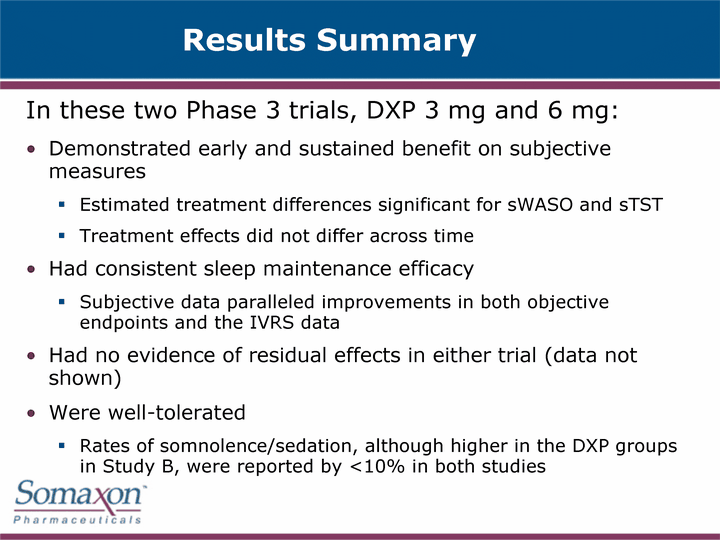
| Results Summary In these two Phase 3 trials, DXP 3 mg and 6 mg: Demonstrated early and sustained benefit on subjective measures Estimated treatment differences significant for sWASO and sTST Treatment effects did not differ across time Had consistent sleep maintenance efficacy Subjective data paralleled improvements in both objective endpoints and the IVRS data Had no evidence of residual effects in either trial (data not shown) Were well-tolerated Rates of somnolence/sedation, although higher in the DXP groups in Study B, were reported by <10% in both studies |

| Conclusion DXP 3 mg and 6 mg produced significant and sustained subjective improvements Improvements observed in both sleep maintenance and sleep duration endpoints Data paralleled improvements in objective endpoints Provides further evidence of DXP 3 mg and 6 mg efficacy for treating sleep maintenance insomnia Improvements did not come at expense of safety No evidence of residual sedation in either trial No evidence of rebound insomnia or other discontinuation effects (Study B; data not shown) |
