Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Pernix Sleep, Inc. | c17447e8vk.htm |
Exhibit 99.1
Improvement in Sleep Maintenance and Early Morning Awakenings in Adult and Elderly Patients with
Insomnia Treated with Doxepin 3 and 6 mg
Andrew Krystal,1 Alan Lankford,2 Brian Dorsey,3 Roberta Rogowski,3 Elizabeth Ludington,3 H. Heith Durrence,
3 Thomas Roth 4
1Duke University Medical Center, 2Sleep Disorders Center of Georgia, 3Somaxon Pharmaceuticals, 4Henry Ford Sleep Disorders Center
INTRODUCTION
Insomnia is characterized by difficulty with sleep onset, sleep maintenance, waking too early,
and/or non restorative sleep despite adequate opportunity to sleep that is associated with daytime
impairment or distress. Sleep maintenance insomnia—defined as the inability to stay asleep
throughout the night or the propensity for early morning awakenings—is the most commonly reported
type of insomnia, comprising over 70% of those with insomnia.1,2
Early morning awakenings, a core symptom of DSM-IV insomnia, is seldom addressed in sleep trials.
Additionally, early morning awakenings are often assumed to be a problem that occurs predominantly
in the elderly. Recent evidence, however, indicates that difficulty with early morning awakenings
is a pervasive problem across all age groups.3 Unfortunately, early morning awakenings
are difficult to treat with medications without causing daytime residual effects.
Doxepin (DXP), a
potent and selective H1 antagonist at the sleep-specific doses
of 3 mg and 6 mg,4
has demonstrated significant efficacy in treating sleep maintenance.5-8 The present
poster reviews the efficacy of DXP 3 mg and 6 mg on parameters associated with sleep maintenance
and early morning awakenings across four Phase 3 trials.
METHODS AND STATISTICS
Objective and Study Design
Sleep maintenance, sleep duration, and early morning awakening endpoints from four double-blind
placebo-controlled trials are reported. In three trials, patients meeting DSM-IV-TR criteria for
primary insomnia were randomized for up to 12 weeks (WK) of treatment. Study A was a 12-WK trial of
elderly patients [N=240; DXP 3 mg vs placebo (PBO)]. Study B was a 5-WK trial of adult patients
(N=221; DXP 3 mg and 6 mg vs PBO). Study C was a 4-WK outpatient trial (n=255; DXP 6 mg vs PBO)
that utilized an interactive-voice-response system (IVRS) to assess patient-reported efficacy data.
Study D was a single-night trial that used a combination of the 1st night effect and a
4-hour phase advance to simulate transient insomnia in healthy adults (N=565; DXP 6 mg vs PBO). The
primary method of evaluating efficacy was polysomnography (PSG) in Studies A, B, and D, and
patient-reports in Study C. Studies A and B included both PSG and patient-reported data. Only the
data from the primary method are presented here. Sleep maintenance endpoints included
polysomnographic wake after sleep onset (WASO) and subjective WASO (sWASO). Sleep duration
endpoints included total sleep time (TST) and subjective TST (sTST). Early morning awakening was
assessed with PSG Sleep Efficiency % in the last quarter-of-the-night (SE-LQ). Data from the first
and final assessment point [Study A=Night (N) 85; Study B=N29; Study C=WK4] of the study are
reported. In terms of safety, standard safety measures, included adverse event reporting,
laboratory panels, ECGs, next-day residual effects, physical exams and sleep architecture were
included in each trial.
Statistics
Efficacy analyses were conducted using the Intent-to-treat analysis set for all trials. Analysis of
covariance (ANCOVA) methods were used to compare mean values for each variable between PBO, DXP 3
mg, or DXP 6 mg (as applicable), using a model that included main effects for treatment and study
center with the baseline value (except for Study D) as the covariate.
DEMOGRAPHICS AND DISPOSITION
In Study A, a total of 240 subjects met eligibility criteria and were randomized into the study (81
PBO, 77 DXP 1 mg, and 82 DXP 3 mg), with 214 subjects (89%) completing the study. In Study B, a
total of 221 subjects met eligibility criteria and were randomized into the study (73 PBO, 75 DXP 3
mg, 73 DXP 6 mg), with 203 subjects (92%) completing the study. In Study C, a total of 255 subjects
met eligibility criteria and were randomized into the study (125 PBO, 130 DXP 6 mg), with 237
subjects (93%) completing the study. In Study D, a total of 565 subjects met eligibility criteria
and were randomized into the study (282 PBO, 283 DXP 6 mg), with all subjects completing the study.
Baseline characteristics and early termination rates were comparable in all studies, with lower
overall discontinuation rates for the DXP groups versus PBO.
The mean age in Study A was 71.4 years. In Study B, the mean age was 44.5 years. In Study C, the
mean age was 72.5 years, and finally, Study D had a mean age of 35.5 years. All four studies had a
higher percentage of women than men, with the distribution of women ranging from 65-73% in chronic
insomnia trials (Studies A, B, and C), with 55% in the transient study (Study D). Approximately 48%
to 50% of the adult patients/subjects and approximately 80% to 87% of the elderly patients were
Caucasian.
OVERALL RESULTS
DXP 3 mg and 6 mg significantly improved WASO on N1 of all three PSG trials
(p<0.0001). DXP 3 mg
(Study A and B; p≤0.0008)and 6 mg (Study B and D; p<0.0001) significantly improved SE-LQ on N1
of all three trials. DXP 6 mg (Study C; p<0.0001) significantly improved sWASO in WK1. The
significant improvements in sleep maintenance and early morning awakenings were maintained at the
final time point in all studies, excepting SE-LQ on N29 (Study B, 3mg p=0.07). There were no gender
differences in the sleep maintenance efficacy in any study. Table 1 summarizes the sleep
maintenance and duration results from these trials.
Supported by funding from Somaxon Pharmaceuticals, Inc.
SLEEP MAINTENANCE AND EARLY MORNING AWAKENING EFFICACY RESULTS
Table 1. Summary of Sleep Maintenance and Duration Efficacy for DXP 3 mg and 6 mg across all Phase 3 Trials
| Mean Improvement versus Placebo | ||||||||||||||||
| (minutes) | ||||||||||||||||
| Population | Duration of Effect | Variable | DXP 3 mg | DXP 6 mg | ||||||||||||
Study A |
Acute (Night 1) | WASO | 34 | ** | Dose not studied | |||||||||||
(Elderly Chronic Insomnia) |
TST | 43 | ** | Dose not studied | ||||||||||||
| Sustained (Night 85) | WASO | 34 | ** | Dose not studied | ||||||||||||
| TST | 30 | ** | Dose not studied | |||||||||||||
Study B |
Acute (Night 1) | WASO | 25 | ** | 30 | ** | ||||||||||
(Adult Chronic Insomnia) |
TST | 42 | ** | 47 | ** | |||||||||||
| Sustained (Night 29) | WASO | 15 | * | 21 | * | |||||||||||
| TST | 17 | * | 28 | * | ||||||||||||
Study C |
Acute (Week 1) | sWASO | Dose not studied | 22 | ** | |||||||||||
(Elderly Chronic Insomnia) |
sTST* | Dose not studied | 29 | ** | ||||||||||||
| Sustained (Week 4) | sWASO* | Dose not studied | 17 | * | ||||||||||||
| sTST* | Dose not studied | 21 | * | |||||||||||||
Study D |
Acute (Night 1) | WASO | Dose not studied | 39 | ** | |||||||||||
(Model of Transient Insomnia) |
TST | Dose not studied | 51 | ** | ||||||||||||
Note: Mean differences for sWASO and sTST represent the LS mean difference from placebo;
*p<0.05; **p ≤0.0007; the improvements demonstrated in Table 1 represent increases for TST and
decreases for WASO.
Individual Study Results
Study A. WASO (Figure 1) and TST (Figure 2) were significantly improved on N1 and N85 for DXP 3 mg.
In terms of early morning awakenings, SE-LQ was significantly improved on N1 (p<0.0001) and N85
(p=0.0014) for DXP 3 mg, with improvements versus PBO ranging from 11% to 15%.
Study B. WASO (Figure 3) and TST (Figure 4) were significantly improved on N1 and N29 for DXP 3 mg
and 6 mg. In terms of early morning awakenings, SE-LQ was significantly improved on N1 for DXP 3 mg
(p=0.0008) and 6 mg (p<0.0001), and on N29 for DXP 6 mg (p=0.0029), with improvements versus PBO
ranging from 7% to 10% on N29. Although not statistically significant, the improvement in SE-LQ on
N29 in the DXP 3 mg dose (p=0.0691) was comparable to the improvement observed on N1.
Study C. sWASO (Figure 5) and sTST (Figure 6) were significantly improved during Week 1 and Week 4
for DXP 6 mg. Early morning awakenings were not assessed.
Study D. WASO and TST were significantly improved on N1 (only night of trial; p-value<0.0001 for
both) for DXP 6 mg, with improvements versus PBO of 39 minutes for WASO and 51 minutes for TST. In
terms of early morning awakenings, SE-LQ was significantly improved on N1 (p<0.0001; DXP 6 mg
was 10.4% better than PBO).
Figure 1. Effects of DXP 3 mg on WASO in Study A
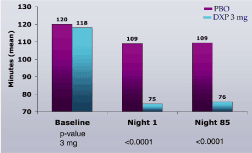
Figure 2. Effects of DXP 3 mg on TST in Study A
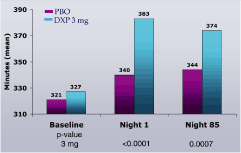
Figure 3. Effects of DXP 3 mg and 6 mg on WASO in Study B
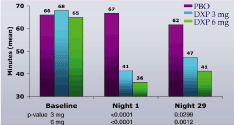
Figure 4. Effects of DXP 3 mg and 6 mg on TST in Study B
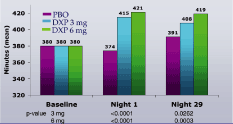
Figure 5. Effects of DXP 6 mg versus PBO on sWASO in Study C
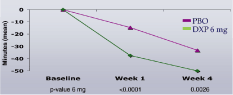
Note. Data reflect mean change from baseline and are in minutes (mean).
Figure 6. Effects of DXP 6 mg versus PBO on sTST in Study C
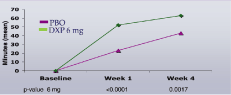
Note. Data reflect mean change from baseline and are in minutes (mean).
RESULTS SUMMARY
In these four Phase 3 trials:
| • | Compared with placebo, DXP 3 mg and 6 mg appeared to: |
| • | Significantly improve wake after sleep onset (WASO) and total sleep time (TST) on both Night 1 and Night 29 in adult patients |
| • | Data indicated that both doses provided an immediate and sustained effect on sleep maintenance |
| • | Prevent early morning awakenings as measured by SE in the last quarter-of-the-night |
| • | Improvements were sustained for most time points |
| • | The efficacy in preventing early morning awakenings was not accompanied by clinically significant residual effects the following morning (data not shown) 5,6,8 |
| • | DXP 3 mg and 6 mg were well-tolerated (data not shown) 5-8 |
| • | Rates of somnolence/sedation, although higher in the DXP groups in some studies, were reported by <10% in all groups |
| • | Incidence of treatment-emergent adverse reactions in long-term placebo-controlled clinical trials were |
| • | Somnolence/sedation (4% PBO, 6% 3 mg, 9% 6 mg), upper respiratory tract infection/nasopharyngitis (2% PBO, 4% 3 mg, 2% 6 mg), gastroenteritis (0% PBO, 2% 3 mg, 0% 6 mg), nausea (1% PBO, 2% 3 mg, 2% 6 mg), and hypertension (0% PBO, 3% 3 mg, <1% 6 mg) |
CONCLUSIONS
In these four Phase 3 trials, DXP 3 mg and 6 mg administered nightly demonstrated significant
improvements in WASO, TST, sWASO, sTST, and SE-LQ that were consistent across all Phase 3 trials
and that were maintained at the final time point for all but one assessment (N29 SE-LQ).
Importantly, these significant difference were robust compared with placebo. Additionally, these
significant improvements did not come at the expense of safety; there was no clinically significant
evidence of residual sedation the following morning nor was there evidence of rebound insomnia in
the trial assessing discontinuation effects. In summary, these data suggest DXP is effective at
treating both sleep maintenance (WASO and
sWASO) and early morning awakenings (SE-LQ) in both transient and chronic insomnia populations, and
in adult and elderly populations, with an excellent risk/benefit ratio.
REFERENCES
| 1. | Lichstein KL, Durrence HH, Taylor DJ, et al. Epidemiology of sleep: Age, gender, and ethnicity. 2004. Mahwah, NJ: Erlbaum | |
| 2. | Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders...Biol Psychiatry 2004;39:411-8 | |
| 3. | National Sleep Foundation. 2005 Sleep in America Poll | |
| 4. | Mansbach R. In Vitro Pharmacological Profile of DXP...Program and abstracts of the ECNP Annual Meeting; 2008; Barcelona, Spain. | |
| 5. | Krystal A, Durrence H, Scharf M, et al. Long-term Efficacy and Safety of DXP 1 mg and 3 mg...Sleep 2010;33:1553-1561 | |
| 6. | Krystal A, Lankford A, Durrence H, et al. Efficacy and Safety of DXP 3 and 6 mg... Sleep; In Press | |
| 7. | Segal S, Lankford A, Borders J, et al. Efficacy and Safety of DXP 6 mg...Program and abstracts of APA’s 161st Meeting; 2008. Abstract NR6-091. | |
| 8. | Roth T, Durrence H, Jochelson P, et al. Efficacy and Safety of DXP 6 mg in a Model of Transient Insomnia. Sleep Medicine 2010;11:843-847 |
Patient-Reported Symptom Improvement in Sleep Maintenance Endpoints in Adult and Elderly Patients with
Insomnia Treated with Doxepin 3 and 6 mg
Insomnia Treated with Doxepin 3 and 6 mg
Alan Lankford,1 Andrew Krystal,2 Brian Dorsey,3 Roberta Rogowski,3 Elizabeth Ludington,3 H. Heith Durrence,
3 , Charles S. Davis, 4 Thomas Roth5
1Sleep Disorders Center of Georgia, 2SDuke University Medical Center, 3Somaxon Pharmaceuticals, 4CSD Biostatistics, 5Henry Ford Sleep Disorders Center
INTRODUCTION
Insomnia is characterized by difficulty with sleep onset, sleep maintenance, waking too early,
and/or non restorative sleep despite adequate opportunity to sleep that is associated with daytime
impairment or distress. Sleep maintenance insomnia—defined as the inability to stay asleep
throughout the night or the propensity for early morning awakenings—is the most commonly reported
type of insomnia, with prevalence rates over 70%.1,2
Several well-established methods are available for assessing sleep and insomnia symptoms, including
questionnaires, sleep diaries, polysomnography, and actigraphy. These assessments are broadly
divided into two categories; objective and subjective. The most commonly used objective assessment
method is PSG. PSG sleep measures are typically used as dependent measures to quantify group
differences or intervention effects. Subjective assessment of insomnia parameters typically
involves some form of recall the following day of the previous night’s sleep, and is often referred
to as patient-reported data.
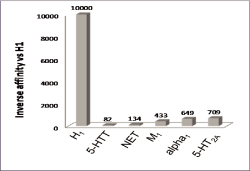
Doxepin (DXP), a
potent and selective H1 antagonist at doses of 3 mg and 6 mg (Figure 1),
4, has
demonstrated significant efficacy in treating
sleep maintenance.5-8 The
present poster reviews the efficacy of DXP 3 mg and 6 mg on patient-reported parameters associated
with sleep maintenance and sleep duration across two long-term Phase 3 trials.
Figure 1. Affinity of doxepin
for H1 Receptor versus Other Receptors
METHODS AND STATISTICS
Objective and Study Design
In two double-blind placebo-controlled trials, patients meeting DSM-IV-TR criteria for primary
insomnia were randomized for treatment. Study A was a 12-week trial in elderly [N=240; DXP 3 mg vs
placebo (PBO)]. Study B was a 5-week trial in adults (N=221; DXP 3 mg and 6 mg vs PBO).
Patient-reported endpoints included subjective wake after sleep onset (sWASO) and subjective total
sleep time (sTST). These sleep maintenance (sWASO) and sleep duration (sTST) endpoints were
obtained from the morning questionnaire that patients completed after a night in the sleep
laboratory. In terms of safety, standard safety measures, included adverse event reporting,
laboratory panels, ECGs, next-day residual effects, physical exams and sleep architecture were
included in each trial.
Statistics
In these supplemental post-hoc analyses, both sTST and sWASO were analyzed with a mixed-effect
model repeated measures (MMRM) approach that included fixed effects for treatment group, time (as a
discrete factor), the treatment-by-time interaction, and the baseline value of the endpoint. In
order to avoid the potential sensitivity of tests and estimators to the choice of an arbitrary
covariance structure, each analysis used an unstructured covariance matrix for the repeated
observations within each subject. Parameter estimates were estimated using the REML method.
DEMOGRAPHICS AND DISPOSITION
In Study A, a total of 240 subjects met eligibility criteria and were randomized into the study (81
PBO, 77 DXP 1 mg, and 82 DXP 3 mg), with 214 subjects (92%) completing the study. In Study B, a
total of 221 subjects met eligibility criteria and were randomized into the study (73 PBO, 75 DXP 3
mg, 73 DXP 6 mg), with 203 subjects (89%) completing the study. Baseline characteristics and early
termination rates were comparable in all studies, with lower overall discontinuation rates for the
DXP groups versus PBO.
The mean age in Study A was 71.4 years and the study included more women (65%) than men (35%). In
Study B, the mean age was 44.5 years and the study included more women (73%) than men (27%).
Subjects across all trials were predominantly Caucasian, with African Americans, and Hispanics
representing a noteworthy minority of the remaining subjects. Approximately 48% of the adult
patients and 80% of the elderly patients were Caucasian.
Supported by funding from Somaxon Pharmaceuticals, Inc.
EFFICACY RESULTS
Overall Results from Corresponding Objective Endpoints.
Although not the focus of this presentation, each study also examined the effects of DXP on PSG
parameters. Improvements in objective parameters are considered key indicators of treatment
efficacy. DXP 3 mg (Study A and B) and 6 mg (Study B) significantly improved PSG-defined WASO and
TST on N1 of both trials (p<0.0001). The significant improvements in WASO and TST were
maintained at the final time point in both studies (p<0.05). There were no apparent gender
differences in the sleep maintenance and duration endpoint efficacy in either trial.
Overall Results from MMRM Analyses
For the primary MMRM analyses none of the tests of interaction were statistically significant;
indeed, none of the tests of interaction by dose approached significance, with all p-values
exceeding 0.5. With regards to comparisons between each doxepin dose and placebo, the estimated
treatment differences were statistically significant for both sWASO and sTST. These results
indicate that there was no evidence that the effect of DXP 3 mg and 6 mg varies substantially over
time, with estimated overall treatment differences that were statistically significant. Table 1
summarizes these results.
Table 1. Summary of Results from MMRM Analyses for DXP 3 mg and 6 mg
| Estimated Difference from Placebo (Overall) | ||||||||||||||||
| LS Mean Difference | ||||||||||||||||
| Population | Measure | Dose group | (Standard Error) | p-value | ||||||||||||
Study A |
sWASO | DXP3 mg | -18.3 (6.49 | ) | 0.0052 | |||||||||||
(Elderly Chronic Insomnia) |
sTST | DXP3 mg | 18.9 (7.41 | ) | 0.0114 | |||||||||||
Study B |
sWASO | DXP3 mg | -10.2 (4.41 | ) | 0.0213 | |||||||||||
(Adult Chronic Insomnia) |
DXP6 mg | -14.2 (4.41 | ) | 0.0014 | ||||||||||||
| sTST | DXP3 mg | 11.9 (5.97 | ) | 0.0469 | ||||||||||||
| DXP6 mg | 17.3 (5.96 | ) | 0.0042 | |||||||||||||
Note: The overall treatment effect across time points is displayed.
Results from MMRM Analyses at Different Timepoints
Study A. For both sWASO and sTST, treatment effects were significantly
different from PBO (Table 1). MMRM analyses showed that the treatment
effects did not differ significantly across time for either sWASO and sTST
(Figure 2).
Study B. For both sWASO and sTST, treatment effects were significantly
different from PBO (Table 1). MMRM analyses showed that the treatment
effects did not differ significantly across time for either sWASO and sTST
(Figure 3).
Figure 2. Mean Differences of DXP 3 mg from Placebo for sWASO and sTST in Study A
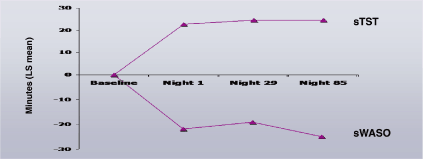
Figure 3. Mean Differences of DXP 3 mg from Placebo for sWASO and sTST in Study B
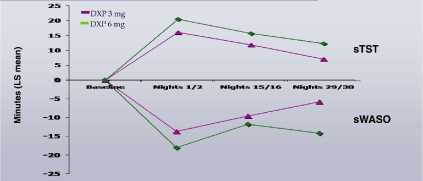
Note. Data reflect the average LS mean difference from placebo across two nights.
RESULTS SUMMARY
In these two Phase 3 trials, DXP 3 mg and 6 mg :
| • | Demonstrated an early and sustained (up to three months) benefit on patient-reported measures for adult and elderly patients with chronic insomnia, as evidenced by: |
| • | Estimated treatment differences from placebo that were statistically significant for both sWASO and sTST | ||
| • | Treatment effects that did not differ significantly across time, indicating that there was no evidence that the effect varied substantially over time |
| • | Demonstrated efficacy in sleep maintenance and sleep duration endpoints that was not accompanied by evidence of significant residual effects the following morning in either of these trials (data not shown) 4,5 | |
| • | Had consistent efficacy for sleep maintenance and sleep duration endpoints |
| • | Patient-reported data paralleled the significant improvements previously reported in PSG endpoints |
| • | Were well-tolerated (data not shown) 4-7 |
| • | Rates of somnolence/sedation, although higher in the DXP groups in Study B, were reported by <10% in both studies |
| • | Incidence of treatment-emergent adverse reactions in long-term placebo-controlled clinical trials were: |
| • | Somnolence/sedation (4% PBO, 6% 3 mg, 9% 6 mg), upper respiratory tract infection/nasopharyngitis (2% PBO, 4% 3 mg, 2% 6 mg), gastroenteritis (0% PBO, 2% 3 mg, 0% 6 mg), nausea (1% PBO, 2% 3 mg, 2% 6 mg), and hypertension (0% PBO, 3% 3 mg, <1% 6 mg) |
CONCLUSIONS
In both an adult (3 mg and 6 mg) and an elderly trial (3 mg), DXP 3 mg and 6 mg produced
significant improvements in subjective (patient-reported) sleep maintenance and duration endpoints.
Additionally, these data paralleled the significant improvements previously reported in objective
(PSG) endpoints, providing further evidence for the efficacy of DXP 3 mg and 6 mg for the treatment
of sleep maintenance insomnia. Finally, these significant improvements did not come at the expense
of safety; there was no evidence of residual sedation the following morning in either of these
trials nor was there evidence of rebound insomnia in the trial assessing discontinuation effects.
In summary, these data suggest DXP 3 mg and 6 mg are effective at treating sleep maintenance in
patients with chronic insomnia regardless of the population (adults and elderly) or whether the
endpoints reflect objective or subjective endpoints.
REFERENCES
| 1. | Lichstein KL, Durrence HH, Taylor DJ, et al. Epidemiology of sleep: Age, gender, and ethnicity. 2004. Mahwah, NJ: Erlbaum | |
| 2. | Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol Psychiatry 2004;39:411-8 | |
| 3. | Mansbach R. In Vitro Pharmacological Profile of DXP...Program and abstracts of the ECNP Annual Meeting; 2008; Barcelona, Spain. | |
| 4. | Krystal A, Durrence H, Scharf M, et al. Long-term Efficacy and Safety of DXP 1 mg and 3 mg...Sleep 2010;33:1553-1561 | |
| 5. | Krystal A, Lankford A, Durrence H, et al. Efficacy and Safety of DXP 3 and 6 mg... Sleep; In Press | |
| 6. | Segal S, Lankford A, Borders J, et al. Efficacy and Safety of DXP 6 mg...Program and abstracts of APA’s 161st Meeting; 2008. Abstract NR6-091. | |
| 7. | Roth T, Durrence H, Jochelson P, et al. Efficacy and Safety of DXP 6 mg in a Model of Transient Insomnia. Sleep Medicine 2010;11:843-847 |
