Attached files
| file | filename |
|---|---|
| EX-31.1 - SKYSTAR BIO-PHARMACEUTICAL CO | v218099_ex31-1.htm |
| EX-31.2 - SKYSTAR BIO-PHARMACEUTICAL CO | v218099_ex31-2.htm |
| EX-32.2 - SKYSTAR BIO-PHARMACEUTICAL CO | v218099_ex32-2.htm |
| EX-32.1 - SKYSTAR BIO-PHARMACEUTICAL CO | v218099_ex32-1.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
|
þ
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the fiscal year ended December 31, 2010
OR
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the transition period from ________________ to ________________
Commission file number: 001-15643

SKYSTAR BIO-PHARMACEUTICAL COMPANY
(Exact name of registrant as specified in its charter)
|
Nevada
|
33-0901534
|
|
|
(State or other jurisdiction of incorporation or
organization)
|
(I.R.S. Employer Identification No.)
|
|
|
4/F, Building B, Chuangye Square, No. 48 Keji Rd
Gaoxin District, Xi’an, Shaanxi Province, P.R.
China
|
N/A
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
Registrant’s telephone number: (8629) 8819-3188
Securities registered pursuant to Section 12(b) of the Act:
|
Common Stock $0.001 Par Value
|
NASDAQ Capital Market
|
|
|
(Title of Each Class)
|
(Name of Each Exchange on Which Registered)
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period than the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained herein, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ¨
|
Accelerated filer ¨
|
|
Non-accelerated filer ¨
|
Smaller reporting company þ
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No þ
As of March 21, 2011, the aggregate market value of the voting stock held by non-affiliates of the Registrant was approximately $32.0 million based on a closing price of $5.36 per share of common stock as reported on the NASDAQ Stock Market on such date.
On March 21, 2011, we had 7,161,919 shares of common stock issued and outstanding.
TO ANNUAL REPORT ON FORM 10-K
FOR YEAR ENDED DECEMBER 31, 2010
|
Page
|
||||
|
CAUTION REGARDING FORWARD-LOOKING INFORMATION
|
3 | |||
|
PART I
|
||||
|
Item 1.
|
Business
|
3
|
||
|
Item 2.
|
Properties
|
13
|
||
|
Item 3.
|
Legal Proceedings
|
14
|
||
|
PART II
|
16 | |||
|
Item 5.
|
Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
16
|
||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Conditions and Results of Operations
|
17
|
||
|
Item 8.
|
Financial Statements and Supplementary Data
|
25
|
||
|
Item 9.
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
|
26
|
||
|
Item 9A.
|
Controls and Procedures
|
26
|
||
|
PART III
|
28 | |||
|
Item 10.
|
Directors, Executive Officers and Corporate Governance
|
28
|
||
|
Item 11.
|
Executive Compensation
|
32
|
||
|
Item 12.
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
36
|
||
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
38
|
||
|
Item 14.
|
Principal Accounting Fees and Services
|
41
|
||
|
PART IV
|
||||
|
Item 15.
|
Exhibits, Financial Statement Schedules
|
41
|
||
|
SIGNATURES
|
45 | |||
2
This report contains forward-looking statements. All forward-looking statements are inherently uncertain as they are based on current expectations and assumptions concerning future events or future performance of the Company. Readers are cautioned not to place undue reliance on these forward-looking statements, which are only predictions and speak only as of the date hereof. Forward-looking statements usually contain the words “estimate,” “anticipate,” “believe,” “expect,” or similar expressions, and are subject to numerous known and unknown risks and uncertainties. In evaluating such statements, prospective investors should carefully review various risks and uncertainties identified in this annual report on Form 10-K, including the matters set forth under the captions “Risk Factors” and in our other SEC filings. These risks and uncertainties could cause our actual results to differ materially from those indicated in the forward-looking statements. We undertake no obligation to update or publicly announce revisions to any forward-looking statements to reflect future events or developments.
Although forward-looking statements in this annual report on Form 10-K reflect the good faith judgment of our management, such statements can only be based on facts and factors currently known by us. Consequently, forward-looking statements are inherently subject to risks and uncertainties, and actual results and outcomes may differ materially from the results and outcomes discussed in or anticipated by the forward-looking statements. Factors that could cause or contribute to such differences in results and outcomes include, without limitation, without limitation, the matters described in this report generally. Readers are urged not to place undue reliance on these forward-looking statements, which speak only as of the date of this report.
When used in this annual report, the terms the “Company,” “Skystar,” “we,” “us,” “our,” and similar terms refer to Skystar Bio-Pharmaceutical Company, a Nevada corporation, and our subsidiaries and variable interest entity.
PART I
ITEM 1. BUSINESS
Overview
We were incorporated in Nevada on September 24, 1998. We are a holding company that, through our wholly owned subsidiaries in China, Skystar Bio Technology Co.(Skystar Jingzhou) and variable interest entity (“VIE”), Xi’an Tianxing Bio-Pharmaceutical Co., Ltd. (“Xi’an Tianxing”), researches, develops, manufactures, and distributes veterinary health care and medical care products in the People’s Republic of China (“PRC”).
All of our operations are carried out by our subsidiaries in China and Xi’an Tianxing, which the Company controls through contractual arrangements between Xi’an Tianxing and Sida Biotechnology (Xi’an) Co., Ltd. (“Sida”), the wholly owned subsidiary of Fortunate Time International Limited, the wholly-owned subsidiary of Skystar Bio-Pharmaceutical (Cayman) Holdings Co., Ltd. (“Skystar Cayman”), which became our wholly owned subsidiary in 2005.
Such contractual arrangements are necessary to comply with PRC laws limiting foreign ownership of certain companies. Through these contractual arrangements, we have the ability to substantially influence Xi’an Tianxing’s daily operations and financial affairs, appoint its senior executives, and approve all matters requiring shareholder approval. As a result of these contractual arrangements, which enable us to control Xi’an Tianxing, we are considered the primary beneficiary of Xi’an Tianxing.
On August 21, 2007, Xi’an Tianxing invested $68,550 (RMB 500,000) to establish Shanghai Siqiang Biotechnological Company Limited (‘Shanghai Siqiang’). Xi’an Tianxing is the 100% shareholder. Shanghai Siqiang serves as a research and development center for Xi’an Tianxing to engage in research, development, production and sales of feed additives and veterinary disease diagnosis equipments.
In addition to Xi’an Tianxing, Skystar Jingzhou also manufactures and distributes veterinary medicines including aquaculture medicines in China.
3
Our current organizational structure is as follows (the percentages depict the current equity interests):
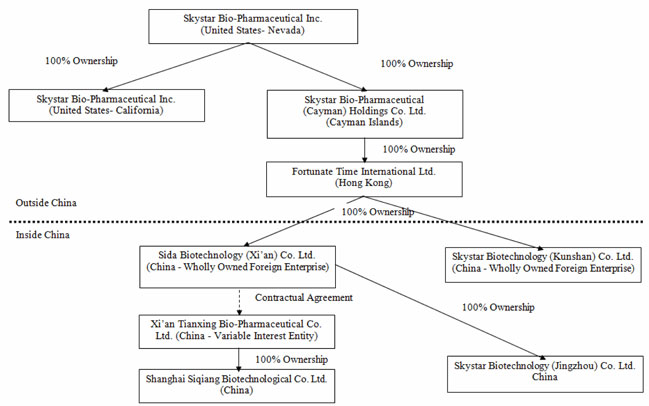
Current Developments
On September 18, 2009, Skystar Bio-Pharmaceutical Inc. (“Skystar California”) was incorporated in California and became a wholly-owned subsidiary of Skystar. On December 20, 2010, the California subsidiary was closed.
On May 7, 2010, Fortunate Time formed Skystar Biotechnology (Kunshan) Co., Limited (“Skystar Kunshan”) in Kunshan, Jiangsu province, China with registered capital of $15,000,000, of which $2,250,000 has been paid by Fortunate Time in cash, and the remaining $12,750,000 is due by May 7, 2012. Kunshan was formed in connection with a potential acquisition of assets. The Company intends to use the asset acquired to meet part of the registered capital requirements. Skystar Kunshan will be a micro-organism manufacturing facility for the Company once the acquisition is completed.
On August 11, 2010, Sida became the parent company of Skystar Biotechnology (Jingzhou) Co., Limited (“Skystar Jingzhou”), a company established in Jingzhou, Hubei Province, China on February 5, 2010, with registered capital of approximately $3.8 million, of which $3.5 million has been paid. The remaining $0.3 million is required to be invested by April 6, 2012. Skystar Jingzhou started production in the third quarter of 2010.
Hereinafter, Skystar, Skystar California, Skystar Cayman, Fortunate Time, Sida, Xi’an Tianxing, Shanghai Siqiang, Skystar Kunshan, and Skystar Jingzhou are collectively referred to as the “Company.”
4
1-for-10 Reverse Stock Split
On May 12, 2009, we effected a 1-for-10 reverse stock split of our issued and outstanding shares of common stock and concurrently reduced the number of authorized shares of common stock from 200,000,000 to 20,000,000. Under Section 78.2055 of the Nevada Revised Statues (“NRS”), to decrease the numbers of issued and outstanding shares of a class or series of a corporation’s capital stock requires the approval of stockholders holding a majority of the voting power of the affected class or series, or such greater proportion as may be provided in the articles of incorporation, regardless of limitations or restrictions on the voting power of the affected class or series. Under NRS Section 78.207, however, a corporation may change the number of shares of a class of its authorized stock by increasing or decreasing the number of authorized shares of the class and correspondingly increasing or decreasing the number of issued and outstanding shares of the same class held by each stockholder of record by a resolution adopted by the board of directors without obtaining the approval of the stockholders. Accordingly, we effected the 1-for-10 reverse stock split without the approval of our stockholders by concurrently effecting a corresponding reduction in the number of shares of our authorized common stock pursuant to NRS Section 78.207.
2-for-1 Forward Stock Split
On November 16, 2009, we effected a 2-for-1 forward stock split of our issued and outstanding shares of common stock and a proportional increase of our authorized shares of common stock from 20,000,000 to 40,000,000, pursuant to NRS Section 78.209.
Our Products
We have four major product lines:
|
|
1.
|
Our bio-pharmaceutical veterinary vaccine line includes over 10 products and accounted for 4% of our revenue in 2010.
|
|
|
2.
|
Our veterinary medicine line for poultry and livestock includes over 220 products and accounted for 67% of our revenue in 2010.
|
|
|
3.
|
Our feed additives line includes over 10 products and accounted for 4% of our revenue in 2010.
|
|
|
4.
|
Our micro-organism products line includes over 16 products and accounted for 25% of our revenue in 2010.
|
Industry and Market Overview
Based on analysis of sample statistics from the Chinese Ministry of Agriculture, the veterinary medicine market was valued at around $6.3 billion in 2010, of which, powder/solid-based medicines accounted for approximately $2.2 billion, injectables approximately $2.2 billion, biotech products approximately $700 million, Chinese herb-based products and feed additives approximately $2.2 billion, and aquaculture medicines accounted for about $300 million.
Distribution Methods of Our Products and Our Customers
As of December 31, 2010, we had over 2,703 customers in 29 provinces in China, including 1,959 distributors and 744 direct customers. Of the 1,959 distributors, 360 are physical stores that have outer signage with our logo and sell products from our four product lines exclusively that are known as “franchise distributors.”
We recognize the importance of branding as well as packaging. All of our products have uniform branding while being specifically designed to also differentiate our four product lines.
We conduct promotional marketing activities within the provinces we operate to publicize and enhance our image as well as to reinforce the recognition of our brand name, including:
|
·
|
publishing advertisements and articles in national as well as specialized and provincial newspapers, magazines, and in other media, including the Internet;
|
5
|
|
·
|
participating in national meetings, seminars, symposiums, exhibitions for bio-pharmaceutical and other related industries;
|
|
|
·
|
organizing cooperative promotional activities with distributors; and
|
|
|
·
|
sending direct mail to major farms.
|
None of our customers accounted for 10% or more of our total revenues in 2010.
Competition
We have three major competitors in China – Jielin Bio-Tech Production Co., Ltd., Qilu Animal Health Production Co., Ltd., and Zhongmu Industrial Joint Stock Co., Ltd. These companies have more assets and have a larger market share. Nevertheless, we believe we are able to compete with these competitors because of our quick response to market demand, a full range of product offerings, quality customer service, and lower prices. Other than these three competitors, most of our other competitors are privately held and vary greatly in scale of operations.
Sources and Availability of Raw Materials and Our Principal Suppliers
Xi’an Yanghua Chemical Co., Ltd., Xi’an Nanchen Trading Co., Ltd., and Xi’an Fandike Chemical Technology Co., Ltd., Xi’an Zhongsen Pharmaceuticals., Ltd, Xi’an Chenyue Trading Co., collectively supplied over 52% of the raw materials we used to manufacture our products in 2010. We design, create prototypes for, and manufacture our products at our facilities located at Xi’an city, Shaanxi Province, China. Our principal raw materials include various chemical compounds including dexamethasone sodium phosphate (a glucocorticoid with anti-inflammatory property), stachyose (a tetrasaccharide found naturally in many vegetables), and thiamphenicol (an antibiotic). We also use Chinese herbs such as Huoxiang (Patchouly), Huanglian (Chinese Goldthread), and Zhang Red Flowers as raw materials, which are supplied to us by Wan Shou Bei Lu Zhong Kui Cao Yao Xing. None of our products requires any raw materials that are scarce, and our raw materials generally are readily available from a wide range of sources. Accordingly, we do not have any continuing or long-term supply agreements with any of these suppliers, and purchase our raw materials from them on a per purchase order basis. The prices for these raw materials are nevertheless subject to market forces largely beyond our control, including energy costs, organic chemical feedstock, market demand, and freight costs. The prices for these raw materials have varied significantly in the past and may vary significantly in the future.
As a result of our research and development efforts in 2007 in cooperation with research institutes including Shaanxi Microbial Research Institute, Jiangsu Microbial Research Institute, China Northwestern University, and China Northwest A&F University, we now also internally produce microbial strains, which are key components of our micro-organism products. Our ability to produce microbial strains has translated into a significant cost reduction for these raw materials.
Intellectual Properties and Licenses
We rely on a combination of trademark, copyright and trade secret protection laws in China and other jurisdictions, as well as confidentiality procedures and contractual provisions to protect our intellectual property and our brand.
We intend to seek other licenses or apply for exclusivity as necessary in order to protect our rights, and we also enter into confidentiality, non-compete and invention assignment agreements with our employees and consultants and nondisclosure agreements with third parties. The Chinese characters that transliterate as “Jia Teng Jun,” “Liao Xiao Wang,” “An Jian,” “Hao Shou Yi,” and “Xing Ge” are our registered trademarks in the PRC.
Bio-pharmaceutical companies are at times involved in litigation based on allegations of infringement or other violations of intellectual property rights. Furthermore, the application of laws governing intellectual property rights in China and abroad is uncertain and evolving and could involve substantial risks to us.
6
Approved Drugs and Veterinary Products – Xi’an Tianxing
Additionally, Xi’an Tianxing is approved by the Chinese Ministry of Agriculture for the manufacture and distribution of 103 types of veterinary drugs. Such approvals certify Xi’an Tianxing’s products as conforming to government-mandated standards. The approvals are issued for a period of 5 years and may be renewed 6 months prior to their expiration date. The 103 veterinary drugs and their approval numbers are listed below:
|
Veterinary Drug Products
|
Approval Number
|
|
|
Metamizole Sodium Injection
|
Veterinary Drug (2007) 270261152
|
|
|
Antondine Injection
|
Veterinary Drug (2007) 270261160
|
|
|
Dexamethasone Sodium Phosphate Injection
|
Veterinary Drug (2007) 270262530
|
|
|
Enrofloxacin Injection
|
Veterinary Drug (2007) 270262518
|
|
|
Compound Vitamin B Injection
|
Veterinary Drug (2007) 270264572
|
|
|
Sulfamonomethoxine Sodium Injection
|
Veterinary Drug (2007) 270261616
|
|
|
Sulfadiazine Sodium Injection
|
Veterinary Drug (2007) 270261634
|
|
|
Kanamycin Sulfate Injection
|
Veterinary Drug (2007) 270261211
|
|
|
Gentamycin Sulfate Injection
|
Veterinary Drug (2007) 270261507
|
|
|
Gentamycin Micronomicin Sulfate Injection (10 ml:100,000 parts)
|
Veterinary Drug (2007) 270262751
|
|
|
Gentamycin Micronomicin Sulfate Injection (10ml: 200,000 parts)
|
Veterinary Drug (2007) 270262752
|
|
|
Mequindox Injection (10ml:0.5g)
|
Veterinary Drug (2007) 270261174
|
|
|
Mequindox Injection (10ml:0.2g)
|
Veterinary Drug (2007) 270264644
|
|
|
Vitamin C Injection
|
Veterinary Drug (2007) 270262795
|
|
|
Vitamin B1 Injection
|
Veterinary Drug (2007) 270261389
|
|
|
Lincomycin Hydrochloride Injection (10ml:0.3g)
|
Veterinary Drug (2007) 270262614
|
|
|
Lincomycin Hydrochloride Injection (10ml:1.5g)
|
Veterinary Drug (2007) 270262616
|
|
|
Danofloxacin Mesylate Powder
|
Veterinary Drug (2008) 270262036
|
|
|
Ofloxacin Injection
|
Veterinary Drug (2007) 270262126
|
|
|
Norfloxacin Nicotinate Injection
|
Veterinary Drug (2007) 270262593
|
|
|
Ciprofloxacin Hydrochloride Injection
|
Veterinary Drug (2007) 270262160
|
|
|
Pefloxacin Mesylate Granules
|
Veterinary Drug (2007) 270262042
|
|
|
Praziquantel Tablets
|
Veterinary Drug (2007) 270261174
|
|
|
Compound Sulfamethoxazole Tablets
|
Veterinary Drug (2007) 270261612
|
|
|
Ofloxacin Tablets
|
Veterinary Drug (2007) 270262123
|
|
|
Amoxicillin Soluble Powder
|
Veterinary Drug (2007) 270261199
|
|
|
Avermectin Powder
|
Veterinary Drug (2007) 270262066
|
|
|
Diclazuril Premix (0.2%)
|
Veterinary Drug (2007) 270261140
|
|
|
Diclazuril Premix (5%)
|
Veterinary Drug (2007) 270262528
|
|
|
Florfenicol Powder
|
Veterinary Drug (2007) 270262110
|
|
|
Compound Amoxicillin Powder
|
Veterinary Drug (2007) 270262092
|
|
|
Thiamphenicol Powder
|
Veterinary Drug (2007) 270262722
|
|
|
Erythromycin Thiocyanate Soluble Powder
|
Veterinary Drug (2007) 270261492
|
|
|
Apramycin Sulfate Soluble Powder
|
Veterinary Drug (2007) 270262745
|
|
|
Neomycin Sulfate Soluble Powder
|
Veterinary Drug (2007) 270262755
|
|
|
Colistin Sulfate Soluble Powder
|
Veterinary Drug (2007) 270262758
|
|
|
Salinomycin Sodium Premix
|
Veterinary Drug (2007) 270261379
|
|
|
Ciprofloxacin Hydrochloride Soluble Powder
|
Veterinary Drug (2007) 270262159
|
|
|
Spectinomycin Hydrochloride and Lincomycin Hydrochloride Soluble Powder
|
Veterinary Drug (2007) 270262602
|
|
|
Ofloxacin Soluble Powder
|
Veterinary Drug (2007) 270262124
|
|
|
Baitouweng San
|
Veterinary Drug (2007) 270265053
|
|
|
Baotai Wuyou San
|
Veterinary Drug (2007) 270265111
|
|
|
Chulijing
|
Veterinary Drug (2007) 270265192
|
|
|
Danjibao
|
Veterinary Drug (2007) 270265171
|
|
|
Feizhucai
|
Veterinary Drug (2007) 270265100
|
7
|
Fuzheng Jiedu San
|
Veterinary Drug (2007) 270265076
|
|
|
Gongying San
|
Veterinary Drug (2007) 270265028
|
|
|
Houyanjing San
|
Veterinary Drug (2007) 270265179
|
|
|
Huanglian Jiedu San
|
Veterinary Drug (2007) 270265178
|
|
|
Jianji San
|
Veterinary Drug (2007) 270265133
|
|
|
Jianwei San
|
Veterinary Drug (2007) 270265134
|
|
|
Jingfang Baidu San
|
Veterinary Drug (2007) 270265127
|
|
|
Mubin Xiaohuang San
|
Veterinary Drug (2007) 270265035
|
|
|
Qingfei Zhike San
|
Veterinary Drug (2007) 270265157
|
|
|
Qingshu San
|
Veterinary Drug (2007) 270265162
|
|
|
Qingwen Baidu San
|
Veterinary Drug (2007) 270265165
|
|
|
Quchong San
|
Veterinary Drug (2007) 270265089
|
|
|
Tongru San
|
Veterinary Drug (2007) 270265156
|
|
|
Xiaoji San
|
Veterinary Drug (2007) 270265146
|
|
|
Yimu Shenghua San
|
Veterinary Drug (2007) 270265148
|
|
|
Yujin San
|
Veterinary Drug (2007) 270265102
|
|
|
Zhili San
|
Veterinary Drug (2007) 270265037
|
|
|
Compound Sulfamethoxydiazine Sodium Injection
|
Veterinary Drug (2007) 270261608
|
|
|
Lomefloxacin Hydrochloride Soluble Powder
|
Veterinary Drug (2008) 270262166
|
|
|
Danofloxacin Mesylate Injection
|
Veterinary Drug (2008) 270262033
|
|
|
Sulfathiazole Sodium Injection
|
Veterinary Drug (2008) 270261645
|
|
|
Buzhong Yiqi San
|
Veterinary Drug (2008) 270265082
|
|
|
Fangji San
|
Veterinary Drug (2008) 270265072
|
|
|
Shenling Baishu San
|
Veterinary Drug (2008) 270265093
|
|
|
Qibu San
|
Veterinary Drug (2008) 270265220
|
|
|
Sulfaquinoxaline Sodium Soluble Powder (10%)
|
Veterinary Drug (2008) 270261624
|
|
|
Sulfaquinoxaline Sodium Soluble Powder (5%)
|
Veterinary Drug (2008) 270262580
|
|
|
Fenbendazole Powder
|
Veterinary Drug (2008) 270261189
|
|
|
Sulfachloropyrazin Sodium Soluble Powder
|
Veterinary Drug (2008) 270262703
|
|
|
Huoxiang Zhengqi San
|
Veterinary Drug (2008) 270265200
|
|
|
Cuiqing San
|
Veterinary Drug (2008) 270265188
|
|
|
Longdan Xiegan San
|
Veterinary Drug (2008) 270265057
|
|
|
Maxing Shigan San
|
Veterinary Drug (2008) 270265174
|
|
|
Qumai San
|
Veterinary Drug (2008) 270265067
|
|
|
Shengru San
|
Veterinary Drug (2008) 270265051
|
|
|
Xiaoshi Pingwei San
|
Veterinary Drug (2008) 270265145
|
|
|
Xiaochaihu San
|
Veterinary Drug (2008) 270265018
|
|
|
Yinqiao San
|
Veterinary Drug (2008) 270265172
|
|
|
Pefloxacin Mesylate Injection
|
Veterinary Drug (2008) 270262665
|
|
|
Enrofloxacin Injection (10ml:250mg)
|
Veterinary Drug (2008) 270261295
|
|
|
Florfenicol Injection
|
Veterinary Drug (2008) 270262546
|
|
|
Lomefloxacin Hydrochloride Injection
|
Veterinary Drug (2008) 270262169
|
|
|
Berberine Sulfate Injection
|
Veterinary Drug (2008) 270264595
|
|
|
Gentamycin Sulfate Injection (10ml:0.2g)
|
Veterinary Drug (2008) 270261506
|
|
|
Promethazine Hydrochloride Injection
|
Veterinary Drug (2008) 270262126
|
|
|
Bailong San
|
Veterinary Drug (2008) 270265055
|
|
|
Feizhu San
|
Veterinary Drug (2009) 270265101
|
|
|
Ivermectin Premix
|
Veterinary Drug (2009) 270263059
|
|
|
Kitasamycin Premix
|
Veterinary Drug (2008) 270262043
|
|
|
Pefloxacin Mesylate Soluble Powder
|
Veterinary Drug (2008) 270262040
|
|
|
Ciprofloxacin Lactate Soluble Powder
|
Veterinary Drug (2008) 270262073
|
|
|
Norfloxacin Nicotinic Soluble Powder
|
Veterinary Drug (2008) 270262178
|
|
|
Tylosin Tartrate Soluble Powder
|
Veterinary Drug (2008) 270262731
|
|
|
Lincomycin Hydrochloride Soluble Powder
|
Veterinary Drug (2008) 270262620
|
|
|
Tilmicosin Premix
|
Veterinary Drug (2010) 270262263
|
|
|
Ciprofloxacin Hydrochloride Soluble Powder (5%)
|
Veterinary Drug (2010) 270262605
|
8
|
Pefloxacin Mesylate Soluble Powder (10%)
|
Veterinary Drug (2010) 270262727
|
|
|
Ivermectin Injection (10ml:0.1g)
|
|
Veterinary Drug (2010) 270262646
|
Approved Drugs and Veterinary Products - Skystar Jingzhou
Skystar Jingzhou is approved by the Ministry of Agriculture to produce 103 veterinary products. The product names and their approval numbers are listed below. The approval licenses for these 103 products were acquired in conjunction with our acquisition of the Jingzhou facility.
|
Veterinary Drug Products
|
Approval Number
|
|
|
Florfenicol Injection(10ml:1g)
|
Veterinary Drug (2006) 170142546
|
|
|
Sulfamonomethoxine Sodium Injection(10ml:1g)
|
Veterinary Drug (2006) 170141616
|
|
|
Enrofloxacin Injection(10ml:0.5g)
|
Veterinary Drug (2006) 170142519
|
|
|
Gentamycin Micronomicim Sulfate Injection(5ml:100mg)
|
Veterinary Drug (2006) 170142750
|
|
|
Ofloxacin Injection(10ml:0.4g)
|
Veterinary Drug (2006) 170142637
|
|
|
Tylosin Injection(10ml:0.5g)
|
Veterinary Drug (2006) 170143046
|
|
|
Mequindox Injection(10ml:0.5g)
|
Veterinary Drug (2006) 170144655
|
|
|
Mequindox Injection(10ml:0.2g)
|
Veterinary Drug (2006) 170144644
|
|
|
Naproxen Injection(10ml:0.5g)
|
Veterinary Drug (2006) 170142763
|
|
|
Injectio Bupleuri(10ml:10g)
|
Veterinary Drug (2006) 170145137
|
|
|
Injectio Houttuyniae(10ml:20g)
|
Veterinary Drug (2006) 170145109
|
|
|
Injectio Andrographini(10ml:10g)
|
Veterinary Drug (2006) 170145122
|
|
|
Metamizole Sodium Injection(10ml : 3g)
|
Veterinary Drug (2006) 170141152
|
|
|
Gentamycin Sulfate Injection(5ml:0.2g)
|
Veterinary Drug (2006) 170141505
|
|
|
Compound Aminophenzone Injection
|
Veterinary Drug (2006) 170141316
|
|
|
Kanamycin Sulfate Injection(10ml : 1g)
|
Veterinary Drug (2006) 170141211
|
|
|
Levamisole Hydrochloride Injection(5ml : 0.25g)
|
Veterinary Drug (2006) 170141351
|
|
|
Antondine Injection(10ml)
|
Veterinary Drug (2006) 170141160
|
|
|
Atropine Sulfate Injection(5ml : 25mg)
|
Veterinary Drug (2006) 170142742
|
|
|
Sulfadiazine Sodium Injection(10ml : 1g)
|
Veterinary Drug (2006) 170141634
|
|
|
Norfloxacin Nicotinic Injection(10ml:2g)
|
Veterinary Drug (2006) 170142180
|
|
|
Norfloxacin Nicotinic Injection(10ml:0.2g)
|
Veterinary Drug (2006) 170142179
|
|
|
Vitamin C Injection(10ml : 1g)
|
Veterinary Drug (2006) 170142795
|
|
|
Dexamethasone Sodium Phosphate Injection(1ml : 2mg)
|
Veterinary Drug (2006) 170141146
|
|
|
Dexamethasone Sodium Phosphate Injection(5ml : 2mg)
|
Veterinary Drug (2006) 170142529
|
|
|
Gentamycin Sulfate Injection(2ml : 0.08g)
|
Veterinary Drug (2006) 170141504
|
|
|
Lincomycin Hydrochloride Injection(10ml : 1.5g)
|
Veterinary Drug (2006) 170142616
|
|
|
Ciprofloxacin Lactate Injection(5ml : 0.25g)
|
Veterinary Drug (2006) 170142767
|
|
|
Duowei Jianwei San
|
Veterinary Drug (2006) 170145063
|
|
|
Baitouweng San
|
Veterinary Drug (2006) 170145053
|
|
|
Wumei San
|
Veterinary Drug (2006) 170145023
|
|
|
Cuiqing San
|
Veterinary Drug (2006) 170145188
|
|
|
Longdan Xiegan San
|
Veterinary Drug (2006) 170145057
|
|
|
Qingwen Baidu San
|
Veterinary Drug (2006) 170145057
|
|
|
Chulijing
|
Veterinary Drug (2006) 170145192
|
|
|
Qingfei Zhike San
|
Veterinary Drug (2006) 170145157
|
|
|
Jingfang Baidu San
|
Veterinary Drug (2006) 170145127
|
9
|
Florfenicol Powder(50g : 5g)
|
Veterinary Drug (2006) 170142110
|
|
|
Lincomycin Hydrochloride and Spectinomycin Sulfate Soluble Powder
|
Veterinary Drug (2006) 170143055
|
|
|
Artificial Carlsbad Salt
|
Veterinary Drug (2006) 170144548
|
|
|
Ofloxacin Soluble Powder(50g : 1g)
|
Veterinary Drug (2006) 170142124
|
|
|
Sulfachlorpyrazine Sodium Soluble Powder(100g : 30g)
|
Veterinary Drug (2006) 170141628
|
|
|
Neomycin Sulfate Soluble Powder(100g : 6.5g)
|
Veterinary Drug (2006) 170141523
|
|
|
Ciprofloxacin Lactate Soluble Powder(100g : 2g)
|
Veterinary Drug (2006) 170142159
|
|
|
Sulfaquinoxaline Sodium Soluble Powder(100g : 10g)
|
Veterinary Drug (2006) 170141624
|
|
|
Diclazuril Premix(100g : 0.5g)
|
Veterinary Drug (2006) 170141141
|
|
|
Colistin Sulfate Soluble Powder(100g : 2g)
|
Veterinary Drug (2006) 170143015
|
|
|
Oxytetracycline Tablets(0.25g)
|
Veterinary Drug (2006) 170141021
|
|
|
Amoxicillin Soluble Powder(50g : 5g)
|
Veterinary Drug (2006) 170141199
|
|
|
Enrofloxacin Solution(100ml : 5g)
|
Veterinary Drug (2006) 170141297
|
|
|
Sulfamidine Tablets(0.5g)
|
Veterinary Drug (2006) 170141620
|
|
|
Diclazuril Solution(100ml : 0.5g)
|
Veterinary Drug (2006) 170142045
|
|
|
Enrofloxacin Soluble Powder(100g : 2.5g)
|
Veterinary Drug (2006) 170142118
|
|
|
Norfloxacin Nicotinic Soluble Powder (100g : 5g)
|
Veterinary Drug (2006) 170142178
|
|
|
Norfloxacin Nicotinate Solution(100ml : 2g)
|
Veterinary Drug (2006) 170142180
|
|
|
Compound Vitamin B Solution
|
Veterinary Drug (2006) 170144573
|
|
|
Shuanghuanglian Koufuye
|
Veterinary Drug (2006) 170145030
|
|
|
Gandan Likang San(For Aquatic)
|
Veterinary Drug (2006) 170149200
|
|
|
Enrofloxacin Soluble Powder(100g : 5g)
|
Veterinary Drug (2006) 170142119
|
|
|
Sulfamonomethoxine Sodium Powder(For Aquatic) (10%)
|
Veterinary Drug (2006) 170149033
|
|
|
Sodium Thiosulfate Powder(For Aquatic) (90%)
|
Veterinary Drug (2006) 170149046
|
|
|
Sarafloxacin Hydrochloride Soluble Powder (For Aquatic) (100g : 5g)
|
Veterinary Drug (2006) 170149101
|
|
|
Enrofloxacin Powder(For Aquatic) (100g:5g)
|
Veterinary Drug (2006) 170149106
|
|
|
Banlangen Dahuang San(For Aquatic)
|
Veterinary Drug (2006) 170149203
|
|
|
Sanhuang San(For Aquatic)
|
Veterinary Drug (2006) 170149213
|
|
|
Qinghao Mo(For Aquatic)
|
Veterinary Drug (2006) 170149225
|
|
|
Lansailing(For Aquatic)
|
Veterinary Drug (2006) 170149227
|
|
|
Norfloxacin Powder(For Aquatic) (2.5%)
|
Veterinary Drug (2006) 170149094
|
|
|
Compound Sulfamethoxazole Powder(For Aquatic)
|
Veterinary Drug (2006) 170149021
|
|
|
Compound Sulfadiazine Powder (For Aquatic)
|
Veterinary Drug (2006) 170149022
|
|
|
Sodium Vitamin C Powder(For Aquatic) (10%)
|
Veterinary Drug (2006) 170149068
|
|
|
Zinc Sulfate Powder(For Aquatic) (60%)
|
Veterinary Drug (2006) 170149051
|
|
|
Niclosamide Powder(For Aquatic) (25%)
|
Veterinary Drug (2006) 170149093
|
|
|
Compound Sulfadimidine Powder(II)(For Aquatic)
|
Veterinary Drug (2006) 170149019
|
|
|
Sodium Percarborate(For Aquatic)
|
Veterinary Drug (2006) 170149029
|
|
|
Sodiumhumate Solution(For Aquatic) (1%)
|
Veterinary Drug (2006) 170149016
|
|
|
Florfenicol Powder(For Aquatic) (10%)
|
Veterinary Drug (2006) 170149014
|
|
|
Nortloxacin Lactate Soluble Powder(100g : 5g)
|
Veterinary Drug (2006) 170142181
|
|
|
Leiwan Binglang San(For Aquatic)
|
Veterinary Drug (2006) 170149230
|
10
|
Avermectin Powder(50g : 0.5g)
|
Veterinary Drug (2006) 170142066
|
|
|
Thiamphenicol Powder(100g : 5g)
|
Veterinary Drug (2006) 170141089
|
|
|
Ivermectin Injection(5ml : 0.05g)
|
Veterinary Drug (2006) 170141126
|
|
|
Levamisole Hydrochloride Injection(10ml : 0.5g)
|
Veterinary Drug (2006) 170141352
|
|
|
Albendazol Tablets(25mg)
|
Veterinary Drug (2006) 170141194
|
|
|
Levamisole Hydrochloride Tablets(50mg)
|
Veterinary Drug (2006) 170141349
|
|
|
Avermectin Injection(5ml : 50mg)
|
Veterinary Drug (2006) 170142065
|
|
|
Compound Albendazole Powder(1000g : 15g+600g+385g)
|
Veterinary Drug (2006) 170149018
|
|
|
Albendazole Powder(6%)
|
Veterinary Drug (2006) 170149001
|
|
|
Purified Trichlorphon Powder(For Aquatic) (20%)
|
Veterinary Drug (2006) 170149035
|
|
|
Beta-cypermethrin Solution(For Aquatic) (4.5%)
|
Veterinary Drug (2006) 170149055
|
|
|
Povidone Iodine Solution(100ml : 1g)
|
Veterinary Drug (2006) 170141574
|
|
|
Povidone Iodine Solution(100ml : 5g)
|
Veterinary Drug (2006) 170141575
|
|
|
Chlorinated Lime(For Aquatic)
|
Veterinary Drug (2006) 170149032
|
|
|
Hydrogen Peroxide Solution (25%)
|
Veterinary Drug (2006) 170149031
|
|
|
Trichloroisocyanuric Acid Powder(For Aquatic) (30%)
|
Veterinary Drug (2006) 170149062
|
|
|
Povidone Iodine Solution(2%)
|
Veterinary Drug (2006) 170149043
|
|
|
Purified Malathion Solution(For Aquatic) (20%)
|
Veterinary Drug (2006) 170149103
|
|
|
Bromochlorodimethylhydantion Powder(For Aquatic) (24%)
|
Veterinary Drug (2006) 170149075
|
|
|
Bromochlorodimethylhydantion Powder(For Aquatic) (8%)
|
Veterinary Drug (2006) 170149074
|
|
|
Sodium Periodate Solution(For Aquatic) (5%)
|
Veterinary Drug (2006) 170149250
|
|
|
Benzalkonium Bromide Solution(For Aquatic) (5%)
|
Veterinary Drug (2006) 170149002
|
|
|
Glutaral Solution(For Aquatic) (20%(g/g))
|
Veterinary Drug (2006) 170149073
|
|
|
Sodium Hypochlorite Solution(For Aquatic)
|
|
Veterinary Drug (2006) 170149056
|
Government Approval and Regulation of our Principal Products or Services
Government approval is required for the production of bio-pharmaceutical products. The Chinese Ministry of Agriculture has granted Xi’an Tianxing three government permits to produce the following products: Forage Additive Products, Additive and Mixed Forage Products, and Veterinary Medicine Products. For the production of the veterinary medicine, there is a national standard known as the Good Manufacturing Practice (“GMP”) standard. A company must establish its facility according to GMP standards, including both the facility and the production process. After establishing such facility, the company files an application to operate the facility with the PRC Ministry of Agriculture, which then sends a team of specialists to conduct an on-site inspection of the facility. A company cannot start production at the facility until it receives approval from the Ministry of Agriculture to begin operations. Xi’an Tianxing has the requisite approval and licenses from the Ministry of Agriculture in order to operate our production facilities.
Research and Development
We place great emphasis on product research and development, and we work closely with two research institutes in the veterinary science field.
With Shanghai Poultry Verminosis Institution, which is a part of the Chinese Academy of Agricultural Sciences, we jointly established the Skystar Research and Development Center in Shanghai. We are working on the following projects at this research center:
11
|
|
·
|
Development of new products for animal immunization by employing new technologies in micro-organism and bacterium. We expect to be placing greater resources into our research and development with the Institution of toxoid, multivalent inactivated vaccines, and attenuated live vaccine, which we believe will gradually replace traditional chemical drugs and greatly impact the animal vaccination industry.
|
|
|
·
|
Development of veterinary medicines for pets. We believe that markets for pet-related products, including vaccines, have been experiencing growth at a rate reflective of the growth rate for the general economy in China. We believe that this niche market is being overlooked by local manufacturers. To take advantage of this opportunity, we have over 20 products of veterinary medicines for pets that are in the course of development. These products are not currently in the market yet.
|
We also established an R&D center located on our premises in Huxian with the Shaanxi Microbial Institute, the only microbial research institute in northwest China. We provide for the running and operation of the research center, including research equipment and materials. In exchange, we have exclusive rights to any technology derived from any research project that we solely fund. We and the Shaanxi Microbial Institute mutually staff research personnel at, and joint-appointment of the director for, the research center. The Institute, however, is not obligated to us with respect to a specific amount of time or a specific project. We are working on the following projects at this research center:
|
|
·
|
Development of protein technology and enzyme mechanism. Introducing the technology in polypeptides, we are working to develop new products to cure piglet diarrhea. The products are expected to stimulate the release of growth hormones in piglets, improve their ability to produce antibody and excrete stomach acidity, enhance the activity of albumen enzyme, and adjust the activity of T.B. cells, thereby improving their all-around disease-resistance ability. We expect these new products will greatly reduce the use of traditional chemical drugs and lead to more environmentally-friendly livestock raising. These products are now in the interim stage of development. We are also developing complex enzyme preparations as new feed additives and aim to use anti-inflammatory enzyme, polyase, and cellulose to form the best combination to effectively dissolve and cause the additive to be absorbed in the feed. Our goal is to drastically improve the absorption rate of the feed, thereby reducing the ratio of usage of feed versus meat, while concurrently reducing the incidence of disease in livestock and poultry. We are looking to outsource certain aspects of these research projects to Shaanxi Jiuzhou Biotechnology Co., Ltd., a member of Shaanxi Jiuzhou Biomedicine Park; we have not, however, entered into any definitive agreement.
|
|
|
·
|
Development of non-pathogenic micro-organisms. We are also developing, in cooperation with the Institute, non-pathogenic micro-organisms and, based upon current products of microbe preparations, lactobacillus, bacillus, bifid bacterium baceroid, and combined with the most appropriate oligosaccharide preparations to produce living bacterium that will be applied to cure gastrointestinal tract diseases resulting from the maladjustment of flora. If successful, micro-organism preparations can be an effective cure and prevention for livestock disease, and can greatly reduce the use of antibiotic and other drugs.
|
During the first quarter of 2008, Xi’an Tianxing contracted with Northwestern Agricultural Technology University to jointly work on an R&D project concerning the application of nano-technology in the prevention of major milk cow disease. The project reached trial stage in March 2009. The project is still on-going. We expect to obtain veterinary permit for the new product from government sometime in 2011.
On September 23, 2009, we purchased an exclusive aquaculture vaccine technology from and signed a collaborative research and development agreement with China’s Fourth Military Medical University (“FMMU”) for RMB 8 million (approximately $1.2 million), granting the Company exclusive rights to sell and market the patented aquaculture vaccine through 2020. Under this patented technology, and in collaboration with FMMU, we will produce the first vaccine in China designed to safely prevent and treat certain bacterial infection and diseases in marine life without causing harmful side effects. Based on its first-to-market status, the Chinese Ministry of Agriculture has issued a Grade I Veterinary Certificate for our vaccine.
12
We also worked with Northwest A&F University in Shaanxi Province and Jiangsu Institute of Microbiology in the past, and we will continue to look for opportunities to collaborate with these and other research institutes in the future.
In 2010, we spent $684,778, or approximately 1.4%, of our revenue on R&D. In 2009, we spent $1,167,937, or approximately 3.5%, of our revenue on R&D.
Employees
As of March 14, 2011, we have approximately 213 employees, of which 172 are full-time employees.
ITEM 2. PROPERTIES
Our administrative headquarters moved to a new location whose address is: 4/F, Building B, Chuangye Square, No. 48 Keji Road, Gaoxin District, Xi’an, Shaanxi Province, China. This new office space was purchased by the Company’s Sida subsidiary, and has a total office space of 17,174 square feet. The original 3,700 square feet office space located at Rm. 10601, Jiezuo Plaza, No.4, Fenghui Road South, Gaoxin District, Xi’an, Shaanxi Province, China is now used by our sales department. This property is owned by our Chairman of the Board and Chief Executive Officer, Weibing Lu, and we are under a 5-year lease with Mr. Lu at rent of RMB 165,600 (approximately $24,000) per year.
The Company had a 3-year lease for an office in Sacramento, California with rent of $1,100 per month. We had intended to use this office to facilitate the exploration of business opportunities in the region. The California subsidiary related to this office was closed on December 20, 2010 as it no longer served our corporate strategy. The lease has since terminated, and no penalty accompanied the termination of the lease.
Shanghai Siqiang, Xi’an Tianxing’s wholly owned subsidiary, leases its office and facility space in Shanghai from Mr. Lu pursuant to a 10-year lease agreement at rent of approximately $21,000 per year.
Skystar Jingzhou, acquired in August 2010, owns the land use right and buildings located at No. 10 Yingxing Road, Songzi Economic Development Zone, East City Industrial Park, Jingzhou, Hubei Province, PRC. Total land area is 301,688 square feet. The total building space including office and plant facilities is 81,800 square feet.
Skystar Kunshan is in the process of completing the acquisition of a micro-organism manufacturing facility in Kunshan, Jiangsu Province. The Company received land use rights in October 2010. The Company is in the process of transferring the building ownership for all structures on the property and expects to finish the process in the near future.
Production Facilities
Xi’an Tianxing has two manufacturing sites located in Xi’an city, Shaanxi Province, China. One site is located in the town of Sanqiao and the other site is in the town of Huxian. Skystar Jingzhou has one manufacturing site located in Songzi, Jingzhou City, Hubei Province, China.
The Sanqiao Plant
Xi’an Tianxing entered into a ten-year lease agreement for the factory premises underlying its Sanqiao plant from October 1, 2004 to September 30, 2014. The annual rent has been adjusted to about $17,000 (RMB 116,000) and subject to a 10% increase every two years starting October 1, 2009. Following are descriptions of the production facilities:
|
|
·
|
Micro-Organism Plant. This production plant is run in cooperation with experts from Japan Kato Microbiology Institute, Microbiology Institute of Shaanxi Province, and Northwest Agro-Forestry Sci-tech University. This facility is approximately 21, 500 square feet and has a production permit and certain product approval numbers issued by the Chinese Ministry of Agriculture.
|
13
|
|
·
|
Feed Additive Plant. This production facility occupies approximately 10,700 square feet.
|
The Huxian Plant
In 2003, Xi’an Tianxing received approval from the State Council of China to expand its production facilities and construct a new GMP standard plant. In connection with the approval, Xi’an Tianxing acquired a long-term land use right for the land now underlying its Huxian plant. Our total investment in this project to date is estimated at $11,916,308. Because Xi’an Tianxing was accredited as a high-tech enterprise, its Huxian plant has the full support of both the Shaanxi provincial government and the Xi’an municipal government.
Construction of the Huxian plant commenced in late 2004 and portions of the plant were fully operational since the end of the second quarter of 2007. The vaccine facility finished construction in June 2010 and completed equipment installation, tooling and testing in the third quarter of 2010. Currently, the Company is awaiting response from the Ministry of Agriculture of China to set the appointment date for GMP certification. When the vaccine facility becomes operational, the Huxian plant will occupy approximately 7.7 acres and have a total plant and office area of approximately 151,700 square feet. The table below lists the primary facilities at the plant and the status of each facility:
|
Description
|
Approx. Size
|
Status
|
||
|
GMP standard veterinary medicine facility
|
|
45,200 sq. ft.
|
|
Complete
|
|
Quality control, research and development, and administration building
|
|
36,600 sq. ft.
|
|
Complete
|
|
GMP standard bio-pharmaceutical facility with the production lines for active bacteria, inactivated vaccines, coccidiosis vaccines and aquaculture vaccines.
|
|
59,201 sq. ft.
|
|
Awaiting GMP inspection & certification
|
|
Animal laboratory complying with Animal Bio-safety Level 2 (ABSL-2) requirements
|
|
10,700 sq. ft.
|
|
Completion expected in the second quarter of 2011
|
The Jingzhou Plant
The Company acquired the Jingzhou plant and related assets through bankruptcy court for approximately $3.5 million and completed the acquisition in August of 2010. The Jingzhou plant is GMP certified to produce veterinarian medicines including aqua culture medicines. The table below lists the primary facilities at the plant and their statuses.
|
Description
|
Approx. Size
|
Status
|
||
|
GMP standard veterinary medicine facility and warehouse
|
|
39,095 sq. ft.
|
|
Complete
|
|
Administration building, dorm and other
|
|
24,122 sq. ft.
|
|
Complete
|
ITEM 3. LEGAL PROCEEDINGS
Other than the proceedings described below, we are not involved in any material legal proceedings, and we are not aware of any material legal proceedings pending or threatened against us. We are also not aware of any material legal proceedings involving any of our directors, officers, or affiliates or any owner of record or beneficially of more than 5% of any class of our voting securities.
14
Andrew Chien v. Skystar Bio-Pharmaceutical Company, et. al. (US. District Court, District of Connecticut, Case No. 3:2007cv00781). On or around May 2007, Andrew Chien (“Chien” or "Plaintiff") filed suit against the Company, R. Scott Cramer, Steve Lowe, David Wassung and Weibing Lu (“Defendants”) in United States District Court for the District of Connecticut, alleging causes of action for violation of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934. In or around November 2007, the Defendants filed motions to dismiss the complaint for failure to state a claim and for lack of personal jurisdiction. The Plaintiff agreed to voluntarily amend the complaint after the motions were filed, and an amended complaint was subsequently filed on or around January 4, 2008. The amended complaint dropped Weibing Lu (who is a resident of China and had never been served) as a defendant. The remaining defendants contended that the amended complaint failed to correct the deficiencies of the original, and filed a renewed motion to dismiss for failure to state a claim, also preserving their challenge to personal jurisdiction. The defendants denied all claims and moved the Court to dismiss the complaint in its entirety in their motion to dismiss. The motion to dismiss also requested that the Court award sanctions against Mr. Chien under Federal Rule of Civil Procedure Rule 11 (“Rule 11”) and the Private Securities Litigation Reform Act (“PSLRA”). On July 17, 2008, in a decision that is now published, the Court granted defendants’ motion and subsequently dismissed the lawsuit, entering judgment on behalf of the defendants. Chien filed a Notice of Appeal of the Court’s dismissal of his lawsuit, opposed by the defendants. Defendants were invited to bring a post-judgment motion for sanctions pursuant to Rule 11 and the PSLRA, which they did. On February 5, 2009, Judge Kravitz issued a ruling on defendants’ Motion for Sanctions. He found the action filed by Chien to have been frivolous, and to have constituted a “substantial” violation of Rule 11, and imposed significant monetary sanctions on both Chien and his former attorney. As part of the basis for imposing sanctions on Mr. Chien personally, the Court specifically found that Chien had knowledge of facts directly contradicting the allegations of his complaint, as evident in internet postings he made on online message boards. Chien subsequently filed motions to “re-open” this case and to recuse Judge Kravitz, but both motions were denied. A Notice of Appeal concerning the ruling awarding sanctions against him was also filed by Chien. All appeals, including the one referenced below concerning Chien’s second lawsuit, were subsequently consolidated. The Court of Appeals issued a Mandate upholding the decision granting defendant’s motion to dismiss and found that the District Court did not “abuse its discretion” in issuing moderate sanctions against Chien in light of the circumstances and facts on record. This Mandate was entered on or about November 8, 2010.
Andrew Chien v. Skystar Bio-Pharmaceutical Company, et. al. (formerly Superior Court, State of Connecticut, Case No. NNH-CV-09-5025938-S, now U.S. District Court, District of Connecticut, Case No. 3:09-CV-00149 (MRK)). Andrew Chien, proceeding pro se (i.e., he represented himself without an attorney), filed another lawsuit against the Company, R. Scott Cramer, Steve Lowe, David Wassung and Weibing Lu (“Defendants”) in Connecticut Superior Court, alleging causes of action similar to those alleged in his federal complaint described above as well as state law causes of action. The Company argued in response that the new complaint was just as frivolous as Mr. Chien’s earlier federal action, which the new complaint substantially duplicated. The earlier federal action, described above, was found to be completely frivolous and dismissed in its entirety, with substantial monetary sanctions awarded against both Chien and his former attorney. A Notice of Removal to the U.S. District Court, District of Connecticut was filed in the state case on January 27, 2009, and the case was assigned to Judge Kravitz, the federal judge in the related federal case previously dismissed. The Company filed a Motion to Dismiss Chien’s new action. In their motion to dismiss, defendants argued that all the claims asserted by Chien were frivolous, including among other grounds that they were time-barred and otherwise substantively meritless, and that sanctions against Mr. Chien under Rule 11 and the PSLRA were again warranted. Rather than file an opposition to Defendants’ motion to dismiss, Chien filed a motion seeking to amend his complaint along with a proposed First Amended Complaint (“FAC”), which the Court ultimately granted. The FAC purported to drop all eleven claims for securities fraud asserted by Chien, all of which defendants had contended were frivolous and meritless. The Court ruled that these claims, abandoned in the wake of Defendant’s motion to dismiss, were all deemed dismissed with prejudice, and that no further briefing on defendant’s pending Motion to Dismiss the action was required. Subsequently, the Court granted defendant’s Motion to Dismiss, dismissing the action and all claims asserted in their entirety. In the ruling, the Court held that all claims asserted against the defendants were barred and failed to state a claim on a multiplicity of grounds, including on the basis of res judicata as well as other substantive defects. Defendants filed a second Motion for Sanctions under Rule 11 and the PSLRA. The Motion was subsequently granted by Judge Kravitz, and Chien was again ordered to pay additional monetary sanctions to the Company. Chien filed a Notice of Appeal concerning the ruling dismissing his second lawsuit. In its filings with the Court of Appeal, the Company argued that the appeals were groundless and the earlier rulings by Judge Kravitz should be upheld, including the two awards of sanctions against Mr. Chien. The Court of Appeals for the Second Circuit consolidated all of Chien’s appeals from both of his lawsuits. The Court of Appeals issued a Mandate upholding the decision granting defendant’s motion to dismiss and found that the District Court did not “abuse its discretion” in issuing moderate sanctions against Chien in light of the circumstances and facts on record. This Mandate was entered on November 8, 2010. On January 22, 2011, Chien filed a petition with the Supreme Court of the United States, appealing the lower court’s ruling. Appeals to the U.S. Supreme Court far exceed the number of cases it actually hears, and a hearing is not mandatory. As of this date we have not received notice of further action by the Supreme Court.
15
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASE OF EQUITY SECURITIES
Market Information
Our common stock trades on the Nasdaq Global Select Market under the symbol “SKBI.” Our common stock was previously quoted on the Over-The-Counter Bulletin Board, or OTCBB, under the symbol “SKBO.OB” until June 25, 2009.
The following table sets forth the high and low bid prices for our common stock on the OTCBB prior to June 26, 2009 and our common stock on the Nasdaq Capital Market since June 26, 2009 for the periods indicated. The high and low bid prices reflect inter-dealer prices, without retail markups, markdowns, or commissions, and may not represent actual transactions.
|
The OTCBB
Bid Price per Share (1)
|
The Nasdaq Capital Market
Sales Price per Share (2)
|
|||||||||||||||
|
Quarter ended
|
High
|
|
Low
|
|
High
|
|
Low
|
|
||||||||
|
December 31, 2010
|
$
|
N/A
|
$
|
N/A
|
$
|
9.73
|
$
|
6.31
|
||||||||
|
September 30, 2010
|
$
|
N/A
|
$
|
N/A
|
$
|
7.74
|
$
|
6.00
|
||||||||
|
June 30, 2010
|
$
|
N/A
|
$
|
N/A
|
$
|
11.63
|
$
|
6.21
|
||||||||
|
March 31, 2010
|
$
|
N/A
|
|
$
|
N/A
|
|
$
|
11.74
|
|
$
|
8.22
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
December 31, 2009
|
$
|
N/A
|
$
|
N/A
|
$
|
12.34
|
$
|
7.16
|
*
|
|||||||
|
September 30, 2009
|
$
|
N/A
|
|
$
|
N/A
|
|
$
|
8.14
|
*
|
|
$
|
5.85
|
*
|
|||
|
June 30, 2009
|
$
|
8.25
|
*
|
$
|
3.90
|
**
|
$
|
10.00
|
*
|
|
$
|
8.97
|
*
|
|||
|
March 31, 2009
|
$
|
5.05
|
**
|
$
|
1.50
|
**
|
$
|
N/A
|
|
$
|
N/A
|
|
||||
(1) Through June 25, 2009.
(2) From June 26, 2009 forward.
* Actual price adjusted to take into account 2-for-1 forward stock split effected on November 16, 2009.
**Actual price adjusted to take into account 10-for-1 reverse stock split effected on May 12, 2009 and 2 for 1 forward stock split effected on November 16, 2009.
Holders
As of March 21, 2010, we had approximately 393 registered stockholders of our common stock on record. This number does not include shares held by brokerage clearing houses, depositories, or otherwise in unregistered form.
16
Dividends
While there are no restrictions that limit our ability to pay dividends, we have not paid, and do not currently intend to pay, cash dividends on our common stock in the foreseeable future. Our policy is to retain all earnings, if any, to provide funds for operation and expansion of our business. The declaration of dividends, if any, will be subject to the discretion of our board of directors, which may consider such factors as our results of operations, financial condition, capital needs, and acquisition strategy, among others.
Securities Authorized for Issuance under Equity Compensation Plans
Please see the discussion in Item 12 titled “Equity Compensation Plan Information” below.
Recent Sales of Unregistered Securities
None.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our results of operations and financial condition for the fiscal years ended December 31, 2010 and 2009 should be read in conjunction with our financial statements and the notes thereto that are included elsewhere in this report. Our discussion includes forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations, and intentions. Please see the section in this report titled “Caution Regarding Forward-Looking Information.”
Overview
Our financial statements are prepared in U.S. Dollars and in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). See “Exchange Rates” below for information regarding the exchanges rates at which Renminbi (“RMB”) were translated into U.S. Dollars (“USD”) at various pertinent dates and for pertinent periods.
In preparing the consolidated financial statements in accordance with U.S. GAAP, we make estimates and assumptions about the effect of matters that are inherently uncertain and may change in subsequent periods. The resulting accounting estimates will, by definition, vary from the related actual results. We consider the following to be the most critical accounting policies:
Principles of consolidation
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America. The consolidated financial statements include the financial statements of the Company, its wholly-owned subsidiaries, and its VIEs. All significant inter-company transactions and balances between the Company, its subsidiaries, and its VIEs have been eliminated in consolidation.
Revenue recognition
Revenue of the Company is primarily from the sales of veterinary healthcare and medical care products in China. Sales are recognized when the following four revenue criteria are met: persuasive evidence of an arrangement exists, delivery has occurred, the selling price is fixed or determinable, and collectability is reasonably assured. Sales are presented net of value added tax (“VAT”). No estimated allowance for sales returns is reflected on these consolidated financial statements as sales returns are de minimal based on historical experience.
17
There are two types of sales upon which revenue is recognized:
|
a.
|
Credit sales: revenue is recognized when the products have been delivered to the customers.
|
|
b.
|
Full payment before delivering: revenue is recognized when the products have been delivered to the customers.
|
Accounts receivable and other receivables
Accounts receivable are recorded at net realizable value consisting of the carrying amount less an allowance for uncollectible accounts, as needed. The Company uses the aging method to estimate the allowance for anticipated uncollectible receivable balances. Under the aging method, bad debt percentages determined by management, based on historical experience and current economic climate, are applied to customers’ balances categorized by the number of months the underlying invoices have remained outstanding. At each reporting period, the allowance balance is adjusted to reflect the amount computed as a result of the aging method. When facts subsequently become available to indicate that the allowance provided requires an adjustment, a corresponding adjustment is made to the allowance account as a change in estimate. The ultimate collection of the Company’s accounts receivable may take one year. Delinquent account balances are reserved after management determines that the likelihood of collection is not probable, and known bad debts are written-off against allowance for doubtful accounts when identified.
Intangible assets
Land Use Rights — Land use rights represent the amounts paid to acquire a long-term interest to utilize the land underlying the Company’s facilities. This type of arrangement is common for the use of land in the PRC. Land use rights are amortized on a straight-line basis over the term granted by the government.
Technological Know-How — Purchased technological know-how includes confidential formulas, manufacturing processes, and technical and procedural manuals, and is amortized using the straight-line method over the weighted average useful life of nine years, which reflects the period over which such confidential formulas, manufacturing processes, and technical and procedural manuals are kept confidential by the Company as agreed between the Company and the selling parties.
Impairment of Intangible Assets — The Company evaluates the carrying value of intangible assets annually or more often when factors indicating impairment are present. The Company determines the existence of such impairment by measuring the estimated future cash flows (undiscounted) and comparing such amount to the net asset carrying value. If the undiscounted cash flow estimated to be generated by any such intangible asset is less than its carrying amount, a loss is recognized based on the amount by which the carrying amount exceeds the intangible asset’s fair market value. Loss on intangible assets to be disposed of is determined in a similar manner, except that fair market values are reduced by the cost of disposal. Based on its review, the Company believes that, as of December 31, 2010, there was no impairment of its intangible assets.
Earnings per share
The Company reports earnings per share and present both basic and diluted earnings per share in conjunction with the disclosure of the methodology used in computing such earnings per share. Basic earnings per share is based upon the weighted-average number of common shares outstanding. Diluted earnings per share is based on the assumption that all dilutive convertible shares, including convertible preferred shares, warrants and stock options were converted or exercised. Further, the method requires that stock dividends or stock splits be accounted for retroactively if the stock dividends or stock splits occur during the period, or retroactively if the stock dividends or stock splits occur after the end of the period but before the release of the financial statements, by considering it outstanding of the entirety of each period presented. Dilution is computed by applying the treasury stock method. Under this method, options and warrants are assumed to be exercised at the beginning of the period (or at the time of issuance, if later), and as if funds obtained thereby were used to purchase common stock at the average market price during the period.
18
All share and per share amounts used in the Company's consolidated financial statements and notes thereto have been retroactively restated to reflect the 1-for-10 reverse stock split effectuated on May 12, 2009 and the 2-for-1 forward stock split effectuated on November 16, 2009.
Business combinations
Effective January 1, 2009, the Company adopted the accounting standard regarding business combinations. This standard retains the fundamental requirements that the acquisition method of accounting (which this standard called the purchase method) be used for all business combinations and for an acquirer to be identified for each business combination. This standard requires an acquirer to recognize the assets acquired, the liabilities assumed, and any non-controlling interest in the acquiree at the acquisition date, measured at their fair values as of that date, with limited exceptions. This replaces the old accounting standard’s cost-allocation process, which required the cost of an acquisition to be allocated to the individual assets acquired and liabilities assumed based on their estimated fair values.
Recently Issued Accounting Pronouncements
In December 2009, FASB issued ASU No. 2009-17, Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities. This Accounting Standards Update amends the FASB Accounting Standards Codification for the issuance of FASB Statement No. 167, Amendments to FASB Interpretation No. 46(R). The amendments in this Accounting Standards Update replace the quantitative-based risks and rewards calculation for determining which reporting entity, if any, has a controlling financial interest in a variable interest entity with an approach focused on identifying which reporting entity has the power to direct the activities of a variable interest entity that most significantly impact the entity’s economic performance and (1) the obligation to absorb losses of the entity or (2) the right to receive benefits from the entity. An approach that is expected to be primarily qualitative will be more effective for identifying which reporting entity has a controlling financial interest in a variable interest entity. The amendments in this Update also require additional disclosures about a reporting entity’s involvement in variable interest entities, which will enhance the information provided to users of financial statements. The Company adopted this standard, and the standard did not have a material effect on the Company’s consolidated financial statements.
In January 2010, FASB issued ASU No. 2010-01, Accounting for Distributions to Shareholders with Components of Stock and Cash. The amendments in this Update clarify that the stock portion of a distribution to shareholders that allows them to elect to receive cash or stock with a potential limitation on the total amount of cash that all shareholders can elect to receive in the aggregate is considered a share issuance that is reflected in EPS prospectively and is not a stock dividend for purposes of applying Topics 505 and 260 (Equity and Earnings Per Share). The amendments in this update are effective for interim and annual periods ending on or after December 15, 2009, and should be applied on a retrospective basis. The Company adopted this standard, and the standard did not have material effect on the Company’s consolidated financial statements.
In January 2010, the FASB issued ASU No. 2010-02 regarding accounting and reporting for decreases in ownership of a subsidiary. Under this guidance, an entity is required to deconsolidate a subsidiary when the entity ceases to have a controlling financial interest in the subsidiary. Upon deconsolidation of a subsidiary, an entity recognizes a gain or loss on the transaction and measures any retained investment in the subsidiary at fair value. In contrast, an entity is required to account for a decrease in its ownership interest of a subsidiary that does not result in a change of control of the subsidiary as an equity transaction. This ASU clarifies the scope of the decrease in ownership provisions, and expands the disclosures about the deconsolidation of a subsidiary or de-recognition of a group of assets. This ASU is effective beginning in the first interim or annual reporting period ending on or after December 31, 2009. The adoption of this ASU did not have a material impact the Company’s consolidated financial statements.
19
In January 2010, FASB issued ASU No. 2010-06 – Improving Disclosures about Fair Value Measurements. This update provides amendments to Subtopic 820-10 that requires new disclosure to include transfers in and out of Levels 1 and 2 and activity in Level 3 fair value measurements. Further, this update clarifies existing disclosures on level of disaggregation and disclosures about inputs and valuation techniques. A reporting entity should provide fair value measurement disclosures for each class of assets and liabilities and should provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements. Those disclosures are required for fair value measurements that fall in either Level 2 or Level 3. The new disclosures and clarifications of existing disclosures are effective for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements. Those disclosures are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. The Company is currently evaluating the impact of this ASU; however, the Company does not expect the adoption of this ASU to have a material impact on its consolidated financial statements.
In February 2010, the FASB issued ASU 2010-09, “Subsequent Events (Topic 855): Amendments to Certain Recognition and Disclosure Requirements”. ASU 2010-09 primarily rescinds the requirement that, for listed companies, financial statements clearly disclose the date through which subsequent events have been evaluated. Subsequent events must still be evaluated through the date of financial statement issuance; however, the disclosure requirement has been removed to avoid conflicts with other SEC guidelines. ASU 2010-09 was effective immediately upon issuance and was adopted in February 2010.
In April 2010, the FASB issued Accounting Standards Update 2010-13, “Compensation—Stock Compensation (Topic 718): Effect of Denominating the Exercise Price of a Share-Based Payment Award in the Currency of the Market in Which the Underlying Equity Security Trades,” or ASU 2010-13. ASU 2010-13 provides amendments to Topic 718 to clarify that an employee share-based payment award with an exercise price denominated in currency of a market in which a substantial porting of the entity’s equity securities trades should not be considered to contain a condition that is not a market, performance, or service condition. Therefore, an entity would not classify such an award as a liability if it otherwise qualifies as equity. The amendments in this Update are effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2010. The Company is currently evaluating the impact of this ASU; however, the Company does not expect the adoption of this ASU to have a material impact on its consolidated financial statements.
In April 2010, the FASB issued Accounting Standard Update 2010-17, “Revenue Recognition—Milestone Method (Topic 605): Milestone Method of Revenue Recognition” or ASU 2010-17. This Update provides guidance on the recognition of revenue under the milestone method, which allows a vendor to adopt an accounting policy to recognize all of the arrangement consideration that is contingent on the achievement of a substantive milestone (milestone consideration) in the period the milestone is achieved. The pronouncement is effective on a prospective basis for milestones achieved in fiscal years and interim periods within those years, beginning on or after June 15, 2010. The adoption of this ASU did not have a material impact the Company’s consolidated financial statements.
20
Results of Operations – Comparison of Two Years Ended December 31, 2010 and 2009
The following table summarizes our results of operations for the years ended December 31, 2010 and 2009.
|
|
Years Ended December 31,
|
|
||||||||||||||
|
|
|
2010
|
|
|
2009
|
|
||||||||||
|
|
|
Amount
|
|
|
Percentage of
total revenue
|
|
|
Amount
|
|
|
Percentage of
total revenue
|
|
||||
|
Revenues
|
|
$
|
47,556,383
|
|
|
|
100.0
|
%
|
|
$
|
33,778,305
|
|
|
|
100.0
|
%
|
|
Gross Profit
|
|
$
|
25,544,795
|
|
|
|
53.7
|
%
|
|
$
|
17,257,316
|
|
|
|
51.1
|
%
|
|
Operating Expense
|
|
$
|
7,434,822
|
|
|
|
15.6
|
%
|
|
$
|
5,562,848
|
|
|
|
16.5
|
%
|
|
Income from Operations
|
|
$
|
18,109,973
|
|
|
|
38.1
|
%
|
|
$
|
11,694,468
|
|
|
|
34.6
|
%
|
|
Other Expenses
|
|
$
|
720,931
|
|
|
|
1.5
|
%
|
|
$
|
813,162
|
|
|
|
2.4
|
%
|
|
Income Tax Expenses
|
|
$
|
3,297,758
|
|
|
|
6.9
|
%
|
|
$
|
2,029,374
|
|
|
|
6.0
|
%
|
|
Net Income
|
|
$
|
14,091,284
|
|
|
|
29.6
|
%
|
|
$
|
8,851,932
|
|
|
26.2
|
%
|
|
Revenues. All of our revenues are derived from the sale of veterinary healthcare and medical care products in the PRC. For the year ended December 31, 2010, we had revenues of $47,556,383 as compared to revenues of $33,778,305 for the year ended December 31, 2009, an increase of approximately 40.8%. Our customer count increased 36% in fiscal year 2010 compared to 2009. The selling prices of our products have not changed meaningfully in fiscal 2010 compared to those in 2009.
Revenues — Veterinary Medications. Revenues from sales of our veterinary medications product line increased from $22,920,479 for the year ended December 31, 2009 to $32,076,000 for the year ended December 31, 2010 for an increase of $9,155,521 or 39.9%. The increase in veterinary medication sales was primarily due to our increased utilization of our veterinary medicine facility expansion and increased sales efforts resulting in a larger customer base. We were able to utilize this capacity expansion as our customers increased the use of products for the treatment of livestock and poultry diseases during the year ended December 31, 2010. In addition, the newly acquired Skystar Jingzhou facility contributed $1,296,292 in sales revenue, nearly all of which occurred in the fourth quarter. Of total revenues from veterinary medications during the year ended December 31, 2010, approximately $12,198,522 or 25.7% of total revenue resulted from the sale of Praziquantel tablets which treats schistosomiasis.
Revenues — Micro-Organism. Revenues from sales of our micro-organism product line increased from $8,021,139 for the year ended December 31, 2009 to $11,543,988 for the year ended December 31, 2010 for an increase of $3,522,849 or 43.9%. The increase was the benefit of added capacity from the capacity expansion in our micro-organism facility that was completed in June 2010, as well as increased sales efforts of our probiotics micro-organism products during the year ended December 31, 2010.
Revenues — Feed Additives. Revenues from sales of our feed additives product line increased from $1,411,222 for the year ended December 31, 2009 to $1,953,416 for the year ended December 31, 2010 for an increase of $542,194 or 38.4%. This increase was the result of increasing usage of our multi-enzyme feed additive products by farmers.
Revenues — Vaccines. Revenues from sales of our vaccines product line increased from $1,425,465 for the year ended December 31, 2009 to $1,982,979 for the year ended December 31, 2010 or an increase of $557,514 or 39.1%. This increase was a result of increased demand for our vaccine products during the year ended December 31, 2010. We are presently operating at full production capacity for our vaccine product line and therefore cannot significantly increase sales until we expand our production capabilities which we presently have underway. The new vaccine facility in the Huxian plant is awaiting appointment date from the Ministry of Agriculture of China for the GMP certification inspection. While it is difficult to predict the date of the approval, the Company is confident in its qualifications and estimates the final approval date may be somewhere near the end of the second quarter this year.
Cost of Sales. For the year ended December 31, 2010, we had cost of sales, of $22,011,588 as compared to cost of sales of $16,520,989 for the year ended December 31, 2009, an increase of approximately 33.2%. Our cost of sales consists of four product lines – veterinary medications, micro-organism, feed additives, and vaccines. The increase was due to our overall sales increase of 39.9%. The Company’s cost of raw materials in 2010 remained relatively flat compared to that of 2009. Even though the market prices for many of the raw materials we used increased in 2010, the Company was able to minimize cost increases due to the prepayments that the Company made in 2009 locking down the prices at lower levels. The Company also did not see any meaningful increases in labor and overhead costs in fiscal 2010 compared to those in fiscal 2009.
21
Cost of Sales — Veterinary Medications. Cost of sales of our veterinary medications product line increased from $13,672,332 for the year ended December 31, 2009 to $17,870,668 for the year ended December 31, 2010, for an increase of $4,198,336 or approximately 30.7%. This increase was mainly due to the corresponding increase in veterinary medication sales.
Cost of Sales — Micro-Organism. Cost of sales of our micro-organism product line increased from $2,129,945 for the year ended December 31, 2009 to $3,113,667 for the year ended December 31, 2010 for an increase of $983,722 or approximately 46.2%. This increase was mainly due to a corresponding increase in micro-organism sales.
Cost of Sales — Feed Additives. Cost of sales of our feed additives product line increased from $568,007 for the year ended December 31, 2009 to $811,323 for the year ended December 31, 2010 for an increase of $243,316 or 42.8%. This increase was mainly due to a corresponding increase in feed additive sales.
Cost of Sales — Vaccines. Cost of sales of our vaccines product line increased from $150,705 for the year ended December 31, 2009 to $215,930 for the year ended December 31, 2010, for an increase of $65,225 or 43.3%. This increase was the result of an increase of vaccine product sales during the year ended December 31, 2010.
Operating Expenses
|
|
Years Ended December 31,
|
|||||||||||||||
|
|
2010
|
2009
|
||||||||||||||
|
|
Amount
|
Percentage
of
total revenue
|
Amount
|
Percentage of
total revenue
|
||||||||||||
|
Selling Expenses
|
|
$
|
2,124,952
|
|
|
|
4.5
|
%
|
|
$
|
1,928,441
|
|
|
|
5.7
|
%
|
|
General and Administrative Expenses
|
|
4,625,092
|
|
|
|
9.7
|
|
2,466,470
|
|
|
|
7.3
|
||||
|
Research and Development Costs
|
|
684,778
|
|
|
|
1.4
|
|
1,167,937
|
|
|
|
3.5
|
||||
|
Total Operating Expenses
|
|
$
|
7,434,822
|
|
|
|
15.6
|
%
|
|
$
|
5,562,848
|
|
|
|
16.5
|
%
|
Selling Expenses. Selling expenses, consisting of commissions, advertising and promotion expenses, freight charges, and salaries, totaled $2,124,952 for the year ended December 31, 2010 as compared to $1,928,441 for the year ended December 31, 2009, an increase of $196,511 or 10.2%. The percentage increase in selling expenses was less than that of the revenue increase during the year ended December 31, 2010 compared to fiscal year 2009. During 2010, the Company focused efforts on localizing the sales force and thus reducing sales related travel expenses compared to fiscal year 2009. The Company also reduced transportation and delivery costs by consolidating its logistics services in fiscal year 2010 compared to fiscal year 2009.
General and Administrative Expenses. General and administrative expenses totaled $4,254,549 for the year ended December 31, 2010 as compared to $2,466,470 for the year ended December 31, 2009, an increase of $1,788,079 or 72.5%. General and administrative expenses for our Chinese operating entities increased due to expanded operations and asset acquisitions related to our Kunshan and Jingzhou subsidiaries, with the Jingzhou-related acquisition closing in August 2010. Approximately $0.6 million was charged to the general and administrative expenses for expenses related to the Jingzhou acquisition. Approximately $0.4 million was charged to general and administrative expenses for expenses related to the Kunshan acquisition.
Research and Development Costs. Research and development costs, consisting of salaries, professional fees, and technical support fees, totaled $684,778 for the year ended December 31, 2010 as compared to $1,167,937 for the year ended December 31, 2009, a decrease of approximately 41.4%. During the year ended December 31, 2010, a significant portion of our research projects that year were in stages that were less costly compared to the research and development projects last year where we had costly testing and clinical trials. One notable project we have been working on is a joint research project with Northwestern Agricultural University to use nano-technology in the prevention of major diseases affecting dairy cows. As of December 31, 2010, the project was in the trial stage with a targeted completion date sometime in 2011.
22
Liquidity
During the 2010, cash provided by operating activities was $7,719,676 as compared to $1,265,727 in 2009. For the year ended December 31, 2010, the increase in net cash provided by operating activities was primarily due to net income of $14,091,284. Other activities contributing to the increases in cash from operations include higher levels of depreciation and amortization, valuation change of derivative liabilities, as well as higher accrued expenses and other payables. The total increases in depreciation were $847,662. The total increases in amortization were $423,195. The total increases in valuation of derivatives liability and non-cash equity transactions were $1,085,408.
Cash used in operating activities was mainly due to increases in prepayments for raw materials and prepaid expenses, higher inventory balances as a result of a larger scale of operations, and higher accounts receivable and other receivable balances as compared to 2009. The total increases in prepayments for raw materials and prepaid expenses were $4,654,016. There total increases in inventory balances were $2,914,627. The total increases in other receivable were $434,233. As of December 31, 2010, we have approximately fifty-two suppliers to which we have made advances to in order to secure our raw materials and obtain favorable pricing.
We used $16,419,318 in investing activities during 2010 as compared to $7,853,439 used in investing activities during 2009. Net cash used in investing activities was a result of significant investment the Company made in plant and equipment during 2010, short term loans issued to two of the Company’s suppliers, and purchases of intangible assets. During 2010, the total increases in plant and equipment, net of transfer from CIP, were $4,451,544. The increases in plant and equipments were mainly due to the completion of the micro-organism facility and the vaccine facility which resulted in the transfer of construction in progress to plant and equipment. The increases in plant and equipment also include assets acquired in the Skystar Jingzhou facilities in August, 2010. The prepayment for potential acquisitions decreased due to the completion of the Jingzhou acquisition, and the partial transfer to intangible assets of the balance related to the Kunshan acquisition for the land use right received in October, 2010. Cash used in investing activities also included loans to third parties totaling $7,840,820. This was mainly due to the short term loans the Company made to two of its suppliers in December 2010 totaling $7,487,000. The two short term loans were subsequently fully repaid with interest on March 15, 2011. Purchases of intangible asset used cash of $4,441,437 primarily due to the 103 licenses acquired in the Jingzhou acquisition, land used rights acquired in the Jingzhou acquisition, and land use rights acquired in the Kunshan acquisition. The Company valued these intangible assets based appraisal reports from certified appraisers in China.
Cash provided by financing activities was $2,649,547 during 2010 as compared to $17,709,238 during the 2009. Cash generated by financing activities for the year ended December 31, 2010 was the result of bank loans the Company secured from two banks in China totaling $2,728,975. The cash generated by financing for the 2009 fiscal year was mainly due to the financing we completed in July 2009.
As of December 31, 2010, we had cash totaling $5,887,831. Our total current assets were $44,740,779, and our total current liabilities were $7,353,613, which resulted in a net working capital of $37,387,166
Capital Resources
In July 2009, we completed a public offering of 3,220,000 shares of our common stock at a price of $6.49 per share resulting in net proceeds of $18,411,496.
Over the next 12 months, we plan to continue to market and sell our current products and to develop new products.
23
In 2003, we received approval from the State Council of China to expand our production facilities and construct a new GMP standard plant. We invested $10,501,000 (RMB 82,000,000) into this project, which includes our Huxian plant, split approximately $9,700,000 for the facilities and $800,000 for working capital respectively. Construction began in 2005, and we completed the veterinary medicine facility and the building that houses the quality control, research and development, and administration departments during 2007, both of which are fully operational. The vaccine facility of the Huxian plant has been completed and is currently waiting for GMP inspection and certification before it can become operational. We anticipate that the new factory will generate sufficient cash flows; thus, management has concluded that there is no impairment loss on the construction in progress.
Skystar Jingzhou plant started producing veterinarian medicines in the third quarter of 2010, and we expect
to start producing aquaculture medicines in that plant in the first half of this year.
Skystar intends to continue developing new products, including animal immunization products, non-pathogenic micro-organisms for the cure and prevention of livestock disease, complex enzyme preparations as animal feed additives, and several new veterinary medicine products within the next 12 months.
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
|
Payments Due by Period
|
||||||||||||||||||||
|
Contractual Obligations
|
Total
|
Less than
1 year
|
1 – 3 years
|
3 – 5 years
|
More than
5 years
|
|||||||||||||||
|
R&D Project Obligation
|
$
|
908,396
|
$
|
908,396
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||||||
|
Operating Lease Obligations
|
235,956
|
66,596
|
113,927
|
43,690
|
12,743
|
|||||||||||||||
|
Total
|
$
|
1,145,352
|
$
|
974,992
|
$
|
113,927
|
$
|
43,690
|
$
|
12,743
|
||||||||||
Bank Loans
|
Bank
|
Amt RMB
|
Amt USD
|
Due Date
|
Interest
Rate
|
||||||||||||
|
Yanta Credit Union
|
5,000,000
|
$
|
758,500
|
8/23/2011
|
8.658
|
% | ||||||||||
|
Bank of East Asia
|
14,946,500
|
2,267,384
|
9/6/2012
|
6.372
|
% | |||||||||||
|
Total
|
19,946,500
|
$
|
3,025,884
|
|||||||||||||
Off-Balance Sheet Arrangements
We do not have any outstanding financial guarantees or commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as stockholders’ equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity, or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk, or credit support to us or engages in leasing, hedging, or research and development services with us.
Exchange Rates
Xi’an Tianxing maintains its books and records in RMB, the currency of China. In general, for consolidation purposes, we translate Xi’an Tianxing’s assets and liabilities into USD using the applicable exchange rates prevailing at the balance sheet date, and the statement of income is translated at average exchange rates during the reporting period. Adjustments resulting from the translation of Xi’an Tianxing’s financial statements are recorded as accumulated other comprehensive income.
The exchange rates used to translate amounts in RMB into USD for the purposes of preparing the consolidated financial statements or otherwise stated in this MD&A were as follows:
24
|
December 31, 2010
|
December 31, 2009
|
|||||
|
Assets and liabilities
|
1 RMB = 0.1517 USD
|
1 RMB = 0.1467 USD
|
||||
|
Statements of operations and cash flows
|
1 RMB = 0.14794 USD
|
1 RMB = 0.14661 USD
|
No representation is made that RMB amounts have been, or would be, converted into USD at the above rates.
Inflation
We are seeing significant inflation pressures building in China. However, the Company was able to secure favorable pricing by prepaying for major components to certain suppliers to lock in prices ahead of time. As a result, we did not experience the same cost pressures as evidenced in the spot market prices of the raw materials. The raw materials cost pressures for the Company were relatively muted in 2010. The Company anticipates the inflationary pressures continuing in 2011. As a result, the Company is continuing the practice of prepayments to suppliers in order to have better control of costs for raw materials.
ITEM 8. FINANCIAL STATEMENTS
Our consolidated financial statements begin on page F-1 after the signature page to this report.
25
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURES
On December 17, 2010, the Company filed an 8K with the SEC disclosing the termination of Frazer Frost, LLP (“Frazer Frost”) as our independent auditors effective as of December 13, 2010. This action was approved by the Audit Committee of our Board of Directors and ratified by our Board. On December 14, 2010, we engaged Crowe Horwath LLP (“Crowe Horwath”) as our independent registered public accounting firm.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized, and reported within the time periods specified in the Commission’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer (Chief Executive Officer) and principal financial officer (Chief Financial Officer), as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management necessarily is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As of December 31, 2010, the end of the fiscal year covered by this report, our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act).
Based on the evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2010, our disclosure controls and procedures were ineffective at the reasonable assurance level.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rule 13a-15(f) and 15d-15(f) promulgated under the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. Our internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles in the United States of America, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of our assets that could have a material effect on the financial statements.
Any system of internal control, no matter how well designed, has inherent limitations, including the possibility that a control can be circumvented or overridden and misstatements due to error or fraud may occur and not be detected in a timely manner. Also, because of changes in conditions, internal control effectiveness may vary over time. Accordingly, even an effective system of internal control will provide only reasonable assurance with respect to financial statement preparation.
Our management assessed the effectiveness of our internal control over financial reporting based on criteria in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
26
Based on that evaluation, our management concluded that as of December 31, 2010, our internal control over financial reporting was not effective due to the following material weaknesses:
|
1.
|
Accounting and Finance Personnel Weaknesses – The current accounting staff is relatively inexperienced, and requires substantial training so as to meet with the higher demands necessary to fulfill the requirements of U.S. GAAP-based reporting and SEC rules and regulations.
|
As indicated in Item 9A (T) of our annual report on Form 10-K for the year ended December 31, 2009 (the “2009 Form 10-K”), we concluded that the Company had accounting and finance personnel weaknesses as of December 31, 2009. Management’s assessment of the control deficiency over accounting and finance personnel as of December 31, 2010 and 2009 considered the same factors, which included:
|
a.
|
the number of adjustments proposed by our independent auditors during our quarterly review and annual audit processes;
|
|
b.
|
the significance of the audit adjustments impact on the overall financial statements;
|
|
c.
|
how appropriately we complied with U.S. GAAP on transactions; and
|
|
d.
|
how accurately we prepared supporting information to provide to our independent auditors on a quarterly and annual basis.
|
Based on the above factors, management concluded that the control deficiency over accounting and finance personnel should be a material weakness as of December 31, 2010 and 2009 respectively because the situation in regard to our insufficient number of qualified resources in our U.S. reporting team remained the same in these two years.
|
2.
|
Lack of effective Internal Audit Function – We did not maintain effective controls over internal audit function due to the lack of qualified internal auditors who are familiar with internal audit and U.S. GAAP, and we did not implement adequate and proper supervisory review to ensure that significant internal control deficiencies can be detected or prevented.
|
As indicated in Item 9A(T) of the 2009 Form 10-K, we concluded that the Company lacked of effective internal audit function as of December 31, 2009 because we lacked an internal audit department, which rendered the Company ineffective in preventing and detecting control lapses and errors in the accounting of certain key areas. We are in the process of remediating this material weakness in year 2010 through setting up an internal audit department and appointing one person to carry out internal audit function. However, the material weakness had not been removed due to our insufficient number of qualified resources and lacking of substantial internal audit work.
Remediation of Material Weaknesses in Internal Control over Financial Reporting
During year 2010, we have carried out certain measures described below to address and remediate our previously identified material weaknesses:
|
1.
|
Streamlined main process cycles, including financial reporting, revenue, procurement, treasury and so on, to guide the routine work and improve the ability of accounting and financial reporting;
|
|
2.
|
Involved both internal accounting and operations personnel and outside contractors with technical accounting expertise, as needed, early in the evaluation of a complex, non-routine transaction to obtain additional guidance as to the applying of U.S. GAAP to such a proposed transaction; and
|
|
3.
|
Set up an internal audit department and assigned more resources to enhance the internal audit function, especially in the supervision of complex, non-routine transactions.
|
Although we implemented the remediation measures described above, we were unable to remediate these two material weaknesses due to the reasons stated in the previous paragraphs.
27
|
1.
|
Recruit sufficient qualified accounting and internal audit personnel and continue to engage outside contractor with technical accounting expertise, as needed.
|
|
2.
|
Reorganize the accounting and finance department to ensure that accounting personnel with adequate experience, skills and knowledge are directly involved in the review and accounting evaluation of our complex, non-routine transactions.
|
|
3.
|
Continue to evaluate our existing staffs and make adjustments as necessary, in addition to providing additional training on accounting principles and internal control procedures for our existing staffs.
|
|
4.
|
Improve the interaction among our management, audit committee, independent auditors and other external advisors.
|
While the Company has taken steps to remediate its material weaknesses, the Company believes that, such steps were not yet implemented to the extent required to fully remediate those material weaknesses and additional measures will be required. The effectiveness of these remediation efforts will not be known until the Company performs a test of these controls in connection with management’s tests of internal controls over financial reporting that the Company will undertake as of December 31, 2011.
Changes in Internal Controls over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act) during the year ended December 31, 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART III
Our directors and executive officers, their ages, their respective offices and positions, and their respective dates of election or appointment are as follows:
|
Name
|
Age
|
Position
|
Date of
Appointment
|
|||
|
Weibing Lu
|
48
|
Chief Executive Officer and Chairman of the Board of Directors
|
November 2005
|
|||
|
Michael H. Lan
|
43
|
Chief Financial Officer
|
April 16, 2010
|
|||
|
Wei Wen
|
45
|
Secretary and Director
|
November 2005
|
|||
|
R. Scott Cramer
|
47
|
Director
|
October 2001
|
|||
|
Qiang Fan
|
56
|
Director
|
July 2008
|
|||
|
Chengtun Qu
|
46
|
Director
|
July 2008
|
|||
|
Shouguo Zhao
|
48
|
Director
|
July 2008
|
|||
|
|
||||||
|
Mark D. Chen
|
43
|
Director
|
May 26, 2009
|
28
Business Experience Descriptions
Set forth below is a summary of our executive officers’ and directors’ business experience for the past 5 years. The experience and background of each of the directors, as summarized below, were significant factors in their previously being nominated as directors of the Company.
Weibing Lu, Chief Executive Officer and Chairman of the Board of Directors
Mr. Weibing Lu received his Bachelor’s degree in science from Wuhan University of Mapping Science and Technology (now known as Wuhan University) in 1985. In 1986, he was a teacher of College of Xi’an Geology. Mr. Lu attended Xi’an Jiao Tong University in 1999 where he received a Master’s degree in Business Administration in 2002. Mr. Lu has vast experience in the biotechnology field and in enterprise management. In 1992, he founded the Xi’an Xingji Electronic Engineering Company and served as its Chairman and President until 1997. In 2002, he was awarded as the title of “Outstanding Enterpriser of Xi’an Feed Industry” and appointed as a director of Xi’an Institute of Feed Industry. In July 1997, he founded Xi’an Tianxing Science and Technology Development Co., Ltd. In December 2003, Xi’an Tianxing Science and Technology Development Co., Ltd., was reorganized and became Xi’an Tianxing Bio-Pharmaceutical Co., Ltd. In addition to his role as our CEO and Chairman, since December 2003, Mr. Lu has served as Chairman of the Board and General Manager of Xi’an Tianxing Bio-Pharmaceutical Co., Ltd.
Michael H. Lan, Chief Financial Officer
Prior to joining the Company, Mr. Lan was an independent consultant providing consulting services to companies in the United States from February 2009 through March 2010. From December 2007 to January 2009, Mr. Lan served as the practice director of Innowave Technology, an information architecture consultancy specializing in complex integrations of service oriented architecture-based approaches. In 2007, Mr. Lan served as the senior manager of Gilead Science, Inc., a NASDAQ-listed company that discovers, develops, and commercializes innovative therapeutics in areas of unmet medical need. From 2004 to 2005, he was a project manager at Axion Solutions. From 1999 to 2004, Mr. Lan served as the senior consultant at Systems Management Inc. Mr. Lan received a B.A. in English Literature from Xiamen University and a Masters of Accounting from the University of Southern California and passed the Certified Public Accountant test in 1996.
Wei Wen, Secretary and Director
Mr. Wei Wen graduated from Xi’an University of Science and Industry (also known as Xi’an University of Technology) in 1989. From 1990 to 1994, Mr. Wen was the manager of Sales Department of Xi’an Zhongtian Science and Technology Development Co., Ltd. From 1994 to 1997, Mr. Wen served as Vice General Manager and Manager of Sales Department of Xi’an Xingji Electronic Engineering Company. Mr. Wen served as the Vice General Manager of Xi’an Tianxing Science and Technology Development Co., Ltd. from 1997 through December 2003. Concurrent to his services as our Secretary and a member of our Board, Mr. Wen serves as Vice General Manager and a Director of Xi’an Tianxing Bio-Pharmaceutical Co., Ltd. (including as secretary of its Board of Directors) since December 2003.
R. Scott Cramer, Director
Mr. R. Scott Cramer was previously our Chairman of the Board from November 2001 to November 2005, Chief Executive Officer from March 2002 to November 2005, and Chief Financial Officer from April 2003 to November 2005. He is currently a member of our Board of Directors. Mr. Cramer is the founder and serves as the President of Cramer & Rauchegger, Inc., a firm specializing in retirement management, estate planning and wealth management. He has been a Registered Investment Advisor since August 2001, a Securities Selling Representative since May 1999, and a General Securities Representative (Registered Representative) since July 2002. Mr. Cramer is a graduate of Seminole State College. H e received certification as a Chartered Retirement Planning Counselor from the College of Financial Planning in 2001, as a Certified Estate Planning Professional from the Abts Institute for Estate Preservation in 2001, and as a Certified Senior Advisor from the Society of Senior Advisors in 2002.
29
Qiang Fan, Director
Mr. Qiang Fan is the President and Founder of MIC Consulting Group, U.S.A., which he established in 1992 to provide operational and financial related problem solving services to privately owned companies. Since 2007, Mr. Fan is the exclusive representative of North America operation for China Venture Capital Research Institute, and the head analyst at Power Partner Institute focusing on IT trends since 2001. From 2006 to 2007, Mr. Fan was a Vice-president of Operation at Kantan Inc., a privately held boutique technology company focused on wireless solutions for device manufacturers. From 2005 to 2006, he was a Vice President at Third Wave Ventures, which provides corporate venture-related advisory, consulting, and management services. From 1998 to 2000, Mr. Fan was the exclusive representative in China for PowerQuest, a Utah-based international software company that focused on computer data storage management, as well as for ChipCoolers, a U.S. CPU cooler manufacturer. Mr. Fan received his B.A. degree from the Business School of California State University at San Francisco.
Chengtun Qu, Director
Dr. Chengtun Qu is the Vice Dean of the College of Chemistry and Chemical Engineering at Xi’an Shi You University, where he also teaches and heads the environmental engineering department. Dr. Qu is a board member of both the Shaanxi Province Environmental Protection Association and the Shaanxi Province Chemical Engineering Association. As a principal researcher, Dr. Qu has participated in various projects at both national and provincial levels, including ones sponsored by the Chinese Ministry of Science and Technology, and he is the recipient of numerous accolades from the Shaanxi provincial government in recognition of his contributions. Dr. Qu has three patents issued by the Chinese State Intellectual Property Office. He has also been extensively published in various scientific journals both in China and abroad. Dr. Qu has a B.S. degree in chemistry from Northwest University in Xi’an, a master’s degree from Southwest Petroleum University, and a doctorate degree from Xi’an Jiaotong University.
Shouguo Zhao, Director
Dr. Shouguo Zhao is an independent director of Shaanxi International Trust & Investment Corp., Ltd., a listed company on the Shenzhen Stock Exchange (SZSE: SZ000563), chairing its Remuneration and Assessment Committee and serving on its Strategy Committee. Dr. Zhao is also an independent non-executive director of Sungreen International Holdings Limited, a listed company on the Hong Kong Exchange (HKEX: HK8306), serving as a member of its audit committee. He is additionally an independent director of Tian Di Yuan Co., Ltd., a listed company on the Shenzhen Stock Exchange (SZSE: SH600665), chairing its Nominating Committee and serving on its Strategy Committee. From June 2005 to June 2008, Dr. Zhao was an independent director of IRICO Group Corporation, a listed company on the Shenzhen Stock Exchange (SZSE: SH600707), chairing its Remuneration and Assessment Committee and serving on its Strategy Committee. Dr. Zhao is the Vice Dean of the School of Economics and Management at Northwest University, where he also serves as a guide professor to doctorate candidates in finance and national economics. He has led and participated in 18 research programs sponsored by governments and the private sectors in areas of financial investment, modern enterprise systems and development strategies, and regional economic development strategies, and has more than 30 publications in various academic journals. Dr. Zhao is a member of Shaanxi Provincial Decision-making Consultative Committee, a member of the Executive Committee of the Tenth Session of Shaanxi Provincial Industrial and Commercial Association, the chairman of the Negotiable Securities Research Society of Shaanxi Province, and a consultant with the Listed Companies Association of Shaanxi Province. Dr. Zhao received his doctorate degree in economics from Northwest University.
Mark D. Chen, Director
Mr. Mark D. Chen is the Chairman of the Board and Chief Executive Officer of Pantheon China Acquisition Corp., a U.S. publicly traded acquisition company he founded in 2006 focusing on pre-IPO Chinese companies. Since 1998, Mr. Chen has been a founding general partner and has served in various positions, including managing director and currently a venture partner, with Easton Capital Investment Group and its various affiliated funds, a New York-based private equity investment firm. Mr. Chen has also worked extensively in China and was a founder and senior executive of SureData Inc., a marketing and distribution company in China in 1997. Mr. Chen received a B.S. from the Shanghai Jiao Tong University in Shanghai, China, an M.S. from Pennsylvania State University, and an M.B.A. from the Columbia Business School at Columbia University.
30
Family Relationships
There are no family relationships among our directors and executive officers.
Involvement in Certain Legal Proceedings
None of our directors and executive officers has, during the past ten years:
|
·
|
Had any petition under the federal bankruptcy laws or any state insolvency law filed by or against, or had a receiver, fiscal agent, or similar officer appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing;
|
|
|
·
|
Been convicted in a criminal proceeding or a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses);
|
|
|
·
|
Been the subject of any order, judgment, or decree, not subsequently reversed, suspended, or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities:
|
|
|
(i)
|
Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
|
|
|
(ii)
|
Engaging in any type of business practice; or
|
|
|
(iii)
|
Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of federal or state securities laws or federal commodities laws;
|
|
|
·
|
Been the subject of any order, judgment, or decree, not subsequently reversed, suspended, or vacated, of any federal or state authority barring, suspending, or otherwise limiting for more than 60 days the right of such person to engage in any activity described in (i) above, or to be associated with persons engaged in any such activity;
|
|
|
·
|
Been found by a court of competent jurisdiction in a civil action or by the SEC to have violated any federal or state securities law, where the judgment in such civil action or finding by the SEC has not been subsequently reversed, suspended, or vacated;
|
|
|
·
|
Been found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any federal commodities law, where the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended, or vacated;
|
|
|
·
|
Been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of:
|
|
|
(i)
|
Any federal or state securities or commodities law or regulation; or
|
|
|
(ii)
|
Any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or
|
31
|
|
(iii)
|
Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
|
|
|
·
|
Been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Securities Exchange Act of 1934), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
|
Compliance with Section 16(a) of the Exchange Act
Based solely on review of the copies of such forms furnished to the Company, or written representations that no reports were required, the Company believes that for the fiscal year ended December 31, 2010, our directors and executive officers complied with Section 16(a) filing requirements applicable to them.
Code of Ethics
We have adopted a code of ethics that applies to our officers, directors, and employees, including our principal executive officer and principal financial and accounting officer. Our code of ethics is available on our website at www.skystarbio-pharmaceutical.com. A copy of our code of ethics will also be provided to any person without charge, upon written request sent to us at our offices located at Rm. 10601, Jiezuo Plaza, No.4, Fenghui Road South, Gaoxin District, Xi’an, Shaanxi Province, P.R. China.
Material Changes to the Procedures by which Security Holders May Recommend Nominees to the Board of Directors
There have been no material changes to the procedures by which security holders may recommend nominees to the Board of Directors.
Our audit committee consists of three independent directors – Mr. Fan, Dr. Zhao, and Mr. Chen. Our board of directors has determined, based on information furnished by Mr. Chen and other available information, that he meets the requirements of an “audit committee financial expert” as such term is defined in the rules promulgated under the Securities Act and the Exchange Act. On May 26, 2009, Mr. Chen was appointed to serve as chairman of the audit committee.
ITEM 11. EXECUTIVE COMPENSATION
Summary Compensation
The following summary compensation table indicates the cash and non-cash compensation earned during the fiscal years ended December 31, 2010 and 2009 by (i) our Chief Executive Officer (principal executive officer), (ii) our Chief Financial Officer (principal financial officer), (iii) the three most highly compensated executive officers other than our CEO and CFO who were serving as executive officers at the end of our last completed fiscal year, whose total compensation exceeded $100,000 during such fiscal year ends, and (iv) up to two additional individuals for whom disclosure would have been provided but for the fact that the individual was not serving as an executive officer at the end of our last completed fiscal year, whose total compensation exceeded $100,000 during such fiscal year ends.
32
Summary Compensation Table
|
Name and
Principal Position
|
Year
|
|
Salary
($)
|
|
|
Bonus
($)
|
|
|
Stock
Awards
( $)(1)
|
|
|
Option
Awards
($)
|
|
|
Non-
Equity
Incentive
Plan
Comp-
ensation
($)
|
|
|
Non-
qualified
Deferred
Comp-
ensation
Earnings
($)
|
|
|
All Other
Comp-
ensation
( $)
|
|
|
Total
($)
|
||||||||||
|
Weibing Lu,
|
2010
|
$
|
105,000
|
$
|
$
|
$
|
$
|
$
|
$
|
$
|
105,000
|
|||||||||||||||||||||||
|
CEO (1)
|
2009
|
$
|
100,000
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
100,000
|
|||||||||||||||||
|
Michael H. Lan
|
2010
|
$
|
66,667
|
$
|
$
|
$
|
$
|
$
|
$
|
$
|
66,667
|
|||||||||||||||||||||||
|
CFO (2)
|
2009
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
|||||||||||||||||
|
Bennet P. Tchaikovsky
|
2010
|
$
|
42,625
|
$
|
$
|
38,738
|
$
|
$
|
$
|
$
|
$
|
81,363
|
||||||||||||||||||||||
|
former CFO (3)
|
2009
|
$
|
75,000
|
$
|
—
|
$
|
63,280
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
—
|
$
|
138,280
|
|||||||||||||||||
|
(1)
|
On May 5, 2008, we entered into an employment agreement with Mr. Lu pursuant to which he is entitled to an initial annual compensation of $100,000 as our Chief Executive Officer. Mr. Lu received no other form of compensation in the years shown, other than the salary set forth in this table
|
|
(2)
|
Mr. Lan was appointed as our CFO on April 16, 2010. On the same day, the Company entered into employment agreement with Mr. Lan that he is entitled to an annual pay of $100,000.
|
|
(3)
|
Mr. Tchaikovsky was our CFO from May 5, 2008 until April 16, 2010.
|
For the year ended December 31, 2010, there were no grants of an award made to a named executive officer.
Outstanding Equity Awards at Fiscal Year-End
For the year ended December 31, 2010, there were no grants of an award made to a named executive officer.
There were no outstanding equity awards, options, unvested stock awards or equity incentive plan awards held for any of our named executive officers. There were no options exercised and no stock that vested for Mr. Lu, Mr. Wei, or Mr. Lan.
Pension Benefits, Nonqualified Defined Contribution, and Other Nonqualified Deferred Compensation Plans
For the year ended December 31, 2010, the Company did not agree to any retirement payments and benefits, or payments and benefits that will be provided primarily following retirement, or other pension plans for executives or directors. For the year ended December 31, 2010, there were no Nonqualified Defined Contribution or Other Nonqualified Deferred Compensation plans.
Employment Agreements and Potential Payments Upon Termination or Change-in-Control
Except as described below, we have no employment agreements with any of the named executive officers, nor any compensatory plans or arrangements resulting from the resignation, retirement, or any other termination of any of the named executive officers, from a change-in-control, or from a change in such person’s responsibilities following a change-in-control.
Employment Agreement with Weibing Lu
We have a five-year Employment Agreement with Mr. Lu dated May 5, 2008. Under the agreement, Mr. Lu’s annual salary in 2009 was $100,000 and in 2010 was $105,000. His agreement provides that the company intends to increase his annual salary by 5% each year. Additionally, at the discretion of the Board’s Compensation Committee, Mr. Lu may be eligible for an annual bonus, which amount, if any, and payment will be determined by the Compensation Committee. Mr. Lu is entitled to medical, disability, and life insurance, as well as 4 weeks of vacation annually and reimbursement of all reasonable or authorized business expenses.
33
During its term, the Employment Agreement terminates upon Mr. Lu’s death, in which event we are obligated to pay Mr. Lu’s estate his base salary amount through the first anniversary of his death (or the expiration of the agreement if earlier than the anniversary date), as well as pro rata allocation of any bonus based on the days of service during the year of death, and all amounts owing to Mr. Lu at the time of termination, including for previously accrued but unpaid bonuses, expense reimbursements, and accrued but unused vacation pay.
If Mr. Lu is unable to perform his obligations under the agreement for over 180 consecutive days during any consecutive 12 month period, we may terminate the agreement by written notice to Mr. Lu delivered prior to the date that he resumes his duties. Upon receipt of such written notice, Mr. Lu may request a medical examination under which if he is certified to be incapable of performing his obligations for over 2 additional months, the agreement is terminated. We are obligated to pay Mr. Lu his base salary through the second anniversary of our notice to him of his termination, less any amount Mr. Lu may receive for such period from any Company-sponsored or Company-paid for source of insurance, disability compensation, or governmental program. We will also pay Mr. Lu pro rata allocation of any bonus based on the days of service during the year our notice is issued, and all amounts owing to Mr. Lu at the time of termination, including for previously accrued but unpaid bonuses, expense reimbursements, and accrued but unused vacation pay.
We may also terminate the agreement for cause upon notice if at any time Mr. Lu: (a) refuses in bad faith to carry out specific written directions of our board of directors; (b) intentionally takes fraudulent or dishonest action in his relations with us; (c) is convicted of a crime involving an act of significant moral turpitude; or (d) knowingly commits an act or omits to act in violation of our written policies, the agreement or any agreements that we may have with third parties and that is materially damaging to our business or reputation. Termination, however, for cause described in (a), (b), or (d) is predicated first on Mr. Lu receiving a 5-day written notice and a reasonable opportunity to present his position, then a subsequent written notice of the termination, with the termination to take effect 20 business days thereafter if Mr. Lu does not dispute the cause for the termination or fails to take corrective actions in good faith. Thereafter, if Mr. Lu takes corrective actions, he may be terminated for the same misconduct upon a 5-day written notice.
On the other hand, Mr. Lu may terminate the agreement upon written notice if: (w) there is a material adverse change in the nature of his title, duties, or obligations; (x) we materially breach the agreement; (y) we fail to make any payment to Mr. Lu (excepting any payment that is not material and that we are contesting in good faith); or (z) there is a change of control of the Company. Termination, however, for cause described in (w), (x), or (y) is predicated on our receiving a written notice from Mr. Lu specifying the cause, with the termination to take effect if we fail to take corrective action within 20 business days thereafter. If Mr. Lu terminates the agreement for any one of these reasons, or if we terminate the agreement without cause, we are obligated to pay to Mr. Lu (or in the case of his death, his estate), his base salary and any bonus, without any offset, as well as all amounts owing to Mr. Lu at the time of termination, including for previously accrued but unpaid bonuses, expense reimbursements, and accrued but unused vacation pay.
The agreement also contains restrictive covenants: (i) preventing the use and/or disclosure of confidential information during or at any time after termination; (ii) preventing competition with the Company during his employment and for a period of 3 years after termination (including contact with or solicitation of our customers, employees, or suppliers), provided that Mr. Lu may make investments of up to 2% in the publicly-traded equity securities of any competitor of the Company; (iii) requiring Mr. Lu to refer any business opportunities to the Company during his employment and for a period of 1 year after termination. Mr. Lu, however, shall have no further obligations with respect to competition and business opportunities if his employment is terminated without cause or if he terminates his employment for cause.
Lastly, we are obligated under the agreement to indemnify Mr. Lu for any claims made against him in his capacity as our Chief Executive Officer and, in connection with such obligation, we have included him under a directors’ and officers’ insurance policy that is in effect during his employment as our officer, director, or consultant.
34
Employment Agreement with Michael H. Lan
On April 16, 2010, we entered into an employment agreement with Mr. Lan pursuant to which we have engaged his service as our Chief Financial Officer for a period of one year for annual compensation of $100,000 as well as reimbursement for reasonable expenses incurred in connection with the performance of his duties, including travel expenses. We have also included Mr. Lan under a directors’ and officers’ insurance policy.
Loan-out Agreement for the Services of Bennet P. Tchaikovsky
On May 5, 2008, we entered into a Loan-out Agreement with Worldwide Officers, Inc. (“WOI”) pursuant to which we engaged the services of Mr. Tchaikovsky as our Chief Financial Officer for a period of one year. When the agreement expired on May 4, 2009, he continued to act as our Chief Financial Officer at our request. On May 26, 2009, we entered into an amendment to the agreement to continue the Mr. Tchaikovsky’s engagement as our Chief Financial Officer, subject to certain modification of terms. The amendment renewed Mr. Tchaikovsky’s term for an additional 1-year period, beginning on May 5, 2009. Under this amendment, we paid Mr. Tchaikovsky an annual fee of $75,000. Mr. Tchaikovsky was entitled to receive 14,440 restricted shares of our common stock vesting in four equal installments of 3,610 shares every three calendar months, with the first installment to vest on August 5, 2009.
Compensation of Directors
The following table provides compensation information for our directors during the fiscal year ended December 31, 2010:
|
|
Fees
Earned or
Paid in Cash
($)
|
Stock
Awards
($)(1)
|
Option
Awards ($)
|
Non-Equity
Incentive Plan
Compensation ($)
|
Non-Qualified
Deferred
Comp-
ensation
Earnings ($)
|
All Other
Comp-
ensation
($)
|
Total
($)
|
|
||||||||||||||||||||
|
Weibing Lu (2)
|
$
|
105,000
|
$
|
―
|
$
|
―
|
$
|
―
|
$
|
―
|
$
|
―
|
$
|
105,000
|
||||||||||||||
|
Wei Wen
|
$
|
$
|
$
|
$
|
$
|
$
|
$
|
|||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
|
R. Scott Cramer
|
$
|
30,000
|
$
|
106,100
|
$
|
$
|
$
|
$
|
$
|
136,100
|
||||||||||||||||||
|
|
||||||||||||||||||||||||||||
|
Qiang Fan
|
$
|
30,000
|
$
|
$
|
$
|
$
|
$
|
$
|
30,000
|
|||||||||||||||||||
|
Chengtun Qu
|
$
|
2,900
|
$
|
$
|
$
|
$
|
$
|
$
|
2,900
|
|||||||||||||||||||
|
Shouguo Zhao
|
$
|
7,400
|
$
|
$
|
$
|
$
|
$
|
$
|
7,400
|
|||||||||||||||||||
|
Mark D. Chen
|
$
|
14,000
|
$
|
$
|
$
|
$
|
$
|
$
|
14,000
|
|||||||||||||||||||
|
(1)
|
Please see stock-based compensation subsection under Note 15, Capital Transactions.
|
|
(2)
|
This individual’s compensation as a director is reflected in the Summary Compensation Table above.
|
Agreements with Directors
On March 30, 2010, we entered into an agreement with R. Scott Cramer to memorialize the terms under which he has been acting as our United States representative since November 2006. Under the terms of the agreement, we agreed to compensate Mr. Cramer from January 1, 2010 through March 31, 2010 in the amount of $7,500 and a stock grant of 2,500 shares of our restricted common stock. On April 16, 2010, we entered into a services agreement for Mr. Cramer to continue to act in this capacity. The services agreement renewed Mr. Cramer’s term for an additional 1-year period beginning on April 1, 2010, for an annual fee of $30,000 payable in four quarterly installments of $7,500 at the end of each quarter. Additionally, Mr. Cramer is entitled to receive 10,000 restricted shares of common stock under our 2010 Incentive Stock Plan. The shares will vest in four installments of 2,500 shares at the end of each quarter with the first vesting date on June 30, 2010. The number and the original value of the shares will be proportionately adjusted for any increase or decrease in the number of issued shares resulting from a recapitalization, subdivision, or consolidation of shares or the payment of a stock dividend, or any other increase or decrease in the number of such shares effected without receipt of consideration by the Company. We have the right to repurchase all or any portion of the shares at a price equal to the original amount paid for the shares by Mr. Cramer upon the termination of the services agreement or any attempted transfer of shares in violation of the services agreement. We have also included Mr. Cramer under a directors’ and officers’ insurance policy.
35
Under our agreement with Qiang Fan, Mr. Fan receives annual compensation of $30,000 for his services as a member of the Board, Chairman of the Compensation Committee, and a member of the Audit Committee. Mr. Fan’s annual compensation will be paid in cash, but, at the discretion of the Board, up to $8,000 of his annual compensation may be paid in the form of shares of our common stock under our Stock Incentive Plan #2. During his term as a director, we have included Mr. Fan under a directors’ and officers’ insurance policy. In addition, we agreed to reimburse Mr. Fan for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses.
Under our agreement with Chengtun Qu, Mr. Qu receives annual compensation of RMB 20,000 or about $2,900 for his services as a member of the Board. In addition, we agreed to reimburse Mr. Qu for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses. We have also included Mr. Qu under a directors’ and officers’ insurance policy.
Under our agreement with Shouguo Zhao, Mr. Zhao receives annual compensation of RMB 50,000 or about $7,400 for his services as a member of the Board, Audit Committee, and Compensation Committee. In addition, we agreed to reimburse Mr. Zhao for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses. We have also included Mr. Zhao under a directors’ and officers’ insurance policy.
Under our agreement with Mark D. Chen, Mr. Chen receives annual compensation of $14,000 for his services as a member of the Board, Chairman of the Audit Committee, and a member of the Compensation Committee. Additionally, Mr. Chen has the right to receive 5,556 shares of our restricted common stock at the beginning of each term of his directorship. We have included Mr. Chen under a directors’ and officers’ insurance policy, and will reimburse him for reasonable expenses incurred in connection with the performance of duties as a director of the Company, including travel expenses.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
Plan Category
|
Number of securities to
be issued upon exercise
of outstanding options,
warrants and rights
|
Weighted-average
exercise price of
outstanding options,
warrants and rights
|
Number of securities
remaining available for future
issuance under equity
compensation plans
|
|||||||||
|
Equity compensation plans
approved by security holders (1)
|
0 | 0 | 690,000 | |||||||||
|
Equity compensation plans
not approved by security holders (2)(3)
|
0 | 0 | 538,620 | |||||||||
|
TOTAL
|
0 | 0 | 1,228,620 | |||||||||
|
On December 8, 2009, our Board approved a stock incentive plan for officers, directors, employees, and consultants entitled the “Skystar Bio-Pharmaceutical Company 2010 Stock Incentive Plan” (the “2010 Plan”). The maximum number of shares that may be issued under the 2010 Plan is 700,000 shares of our common stock. The 2010 Plan was approved by our stockholders on December 31, 2009, and awards may be granted under the 2010 Plan until December 7, 2019. Under the 2010 Plan, we may issue common stock and/or options to purchase common stock to certain officers, directors, and employees and consultants of the Company and our subsidiaries. The 2010 Plan is administered either by the Board or a committee appointed by the Board, which is comprised of two or more independent directors. The Board has full and complete authority, in its discretion, but subject to the express provisions of the 2010 Plan, to approve the eligible persons nominated by the management of the Company to be granted awards of common stock or stock options and to determine specific terms of awards or option grants.
|
36
|
(2)
|
On February 22, 2006, we adopted a stock incentive plan for consultants entitled the “2006 Consultant Stock Plan” (the “2006 Plan”). The maximum number of shares that may be issued under the 2006 Plan is 1,199,648 shares of our common stock. The 2006 Plan has not previously been approved by security holders and awards may be granted under this Plan until February 21, 2016. Under the 2006 Plan, we may issue common stock to certain consultants of the Company who are crucial to the future growth and success of the Company and our subsidiaries and affiliates. The 2006 Plan is administered by either a committee appointed by the Board, which is comprised of one or more members of the Board who is not serving on another plan committee, or the Board. The Board has full and complete authority, in its discretion, but subject to the express provisions of the Plan, to designate the persons or classes of persons eligible to receive awards of common stock awards and to determine specific terms of awards or option grants. As of December 31, 2010, there are 119,930 shares of our common stock remaining available for future issuance under the 2006 Plan.
|
|
(3)
|
On October 16, 2002, we adopted a stock incentive plan for officers, directors, employees, and consultants entitled the “Cyber Group Network Corporation Stock Incentive Plan # 2” (hereinafter the “2002 Plan”). The maximum number of shares that may be issued under the 2002 Plan is 40,000,000 shares of our common stock. The 2002 Plan has not previously been approved by security holders and awards may be granted under this Plan until October 15, 2012. Under the 2002 Plan, we may issue common stock and/or options to purchase common stock to certain officers, directors, and employees and consultants of the Company and our subsidiaries. The 2002 Plan is administered either by the compensation committee or a committee appointed by the Board, which is comprised of a combination of two or more officers and/or members of the Board. The board has full and complete authority, in its discretion, but subject to the express provisions of the Plan to approve the eligible persons nominated by the management of the Company to be granted awards of common stock awards or stock options and to determine specific terms of awards or option grants. As of December 31, 2010, there are 418,690 shares of our common stock remaining available for future issuance under the 2002 Plan.
|
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth information regarding the beneficial ownership of our common stock as of March 21, 2011, for each of the following persons:
|
|
·
|
each of our directors and each of the named executive officers;
|
|
|
·
|
all directors and named executive officers as a group; and
|
|
|
·
|
each person who is known by us to own beneficially 5% or more of our common stock.
|
Beneficial ownership is determined in accordance with the rules of the SEC. Unless otherwise indicated in the table, the persons and entities named in the table have sole voting and sole investment power with respect to the shares set forth opposite the stockholder’s name. Unless otherwise indicated, the address of each beneficial owner listed below is Rm. 10601, Jiezuo Plaza, No.4, Fenghui Road South, Gaoxin District, Xi’an, Shaanxi Province, People’s Republic of China. The percentage of class beneficially owned set forth below is based on 7,161,919 shares of common stock outstanding on March 21, 2011.
37
|
Title of Class
|
Name and Address of Beneficial Owners
|
Amount
of Beneficial
Ownership
|
Percent
of Class (1)
|
|||||||
|
Common Stock
|
Weibing Lu, Director and Chief Executive Officer (2)
|
939,126 | 13.1 | % | ||||||
|
Common Stock
|
Wei Wen, Director (3)
|
41,544 | * | |||||||
|
Common Stock
|
Michael H. Lan, Chief Financial Officer (4)
|
0 | * | |||||||
|
Common Stock
|
R. Scott Cramer, Director (5)
|
208,786 | 2.9 | % | ||||||
|
Common Stock
|
Qiang Fan, Director (6)
|
0 | 0 | % | ||||||
|
Common Stock
|
Chengtun Qu, Director (7)
|
0 | 0 | % | ||||||
|
Common Stock
|
Mark D. Chen, Director (8)
|
5,556 | * | |||||||
|
Common Stock
|
Shouguo Zhao, Director (9)
|
0 | * | |||||||
|
Common Stock
|
Bennet P. Tchaikovsky, former Chief Financial Officer (10)
|
2,880 | * | |||||||
|
Common Stock
|
Upform Group Limited (2)
|
939,126 | 13.1 | % | ||||||
|
Common Stock
|
All officers and directors as a group (8 total)
|
1,197,892 | 16.7 | % | ||||||
* Less than 1%.
|
(1)
|
Unless otherwise noted, the number and percentage of outstanding shares of our common stock is based upon 7,161,919 shares outstanding as of March 21, 2011.
|
|
(2)
|
Weibing Lu and Xinya Zhang are directors of Upform Group Limited. Mr. Lu is the majority stockholder and Chairman of the Board of Directors of Upform Group, and indirectly owns the shares held by Upform Group through his majority ownership. Thus, the number of shares reported herein as beneficially owned by Mr. Lu includes the shares held by Upform Group. Similarly, because Xinya Zhang is a director of Upform Group, he may be deemed to have or share investment control over Upform Group’s portfolio, and the number of shares reported herein as beneficially owned by Mr. Zhang also include the shares held by Upform Group. Upform Group’s address is Sea Meadow House, Blackburne Highway, P.O. Box 116, Road Town, Tortola, British Virgin Islands.
|
|
(3)
|
The number of shares reported herein as beneficially owned by Wei Wen are held by Clever Mind International Limited, which address is Sea Meadow House, Blackburne Highway, P.O. Box 116, Road Town, Tortola, British Virgin Islands. Mr. Wen is Chairman of the Board of Directors of Clever Mind and owns approximately 2.3% of the issued and outstanding shares of Clever Mind. As Mr. Wen is a director of Clever Mind, he may be deemed to have or share investment control over Clever Mind’s portfolio.
|
|
(4)
|
This shareholder’s address is 15333 Culver Drive, Ste 340-121, Irvine, California 92604.
|
|
(5)
|
This number includes 154,284 shares held by the Cramer Family Trust, of which Mr. Cramer is the sole trustee and sole primary beneficiary. This shareholder’s address is 1012 Lewis Dr., Winter Park, FL 32789.
|
|
(6)
|
This shareholder’s address is 9176 West Laguna Way, Elk Grove, CA 95758.
|
|
(7)
|
This shareholder’s address is No. 18 Dian Zi 2nd Road, School of Chemistry & Chemical Engineering, Xi'an Shiyou University, Xi'an, Shaanxi Province, People’s Republic of China.
|
|
(8)
|
This shareholder’s address is 10-64 #9 Jianguomenwai Avenue, Beijing, China 100600.
|
|
(9)
|
This shareholder’s address is No. 229 North Tai Bai Road, School of Economics and Management, Northwest University, Xi'an, Shaanxi Province, People’s Republic of China.
|
|
(10)
|
This shareholder’s address is 6571 Morningside Drive, Huntington Beach, CA 92648.
|
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Transactions with Related Persons
For the year ended December 31, 2010, there were no transactions whose amount involved exceeded $ 120,000 for which any related person had or will have a direct or indirect material interest, nor any currently proposed transactions, in which the Company was or is to be a participant.
38
Related Party Receivables and Payables
Set forth below are the related party receivables and payables between us and our officers and/or directors, and between Xi’an Tianxing and its stockholders, officers and/or directors, as of the date set forth on the table.
|
December 31,
2010
|
December 31,
2009
|
|||||||
|
|
||||||||
|
Short term loans from shareholders: (1) (2)
|
||||||||
|
Mr. Weibing Lu
|
$
|
-
|
$
|
36,675
|
||||
|
Mr. Wei Wen
|
-
|
36,675
|
||||||
|
Ms. Aixia Wang
|
-
|
36,675
|
||||||
|
Total
|
$
|
-
|
$
|
110,025
|
||||
|
Shares to be issued to a related party: (3)
|
||||||||
|
Mr. Mark Chen
|
$
|
—
|
$
|
25,002
|
||||
|
Mr. Scott Cramer
|
53,050
|
302,372
|
||||||
|
Total
|
$
|
53,050
|
$
|
327,374
|
||||
|
Amount due to(from) related parties: (4)
|
||||||||
|
Mr. Bennet P. Tchaikovsky – former CFO
|
-
|
$
|
-
|
|||||
|
Mr. Scott Cramer
|
170,937
|
143,556
|
||||||
|
Officer and shareholder
|
46,975
|
41,468
|
||||||
|
Total
|
$
|
217,912
|
$
|
185,024
|
||||
|
|
(1)
|
In 2008, Weibing Lu obtained an unsecured personal loan in the amount of $176,040 (RMB 1,500,000) from Huaxia Bank with annual interest rate of 7.47% and advanced it to Xi’an Tianxing to facilitate operations. Xi’an Tianxing guaranteed the loan. The loan principal and related interest was due on December 30, 2008. On January 4, 2009, Xi’an Tianxing paid the full principal amount to the bank, with related interest of $15,741.
|
|
|
(2)
|
On May 29, 2008, Weibing Lu, Wei Wen and Aixia Wang obtained personal loans from Yanta Credit Union and advanced cash to Xi’an Tianxing in the total amount of $132,030 to facilitate operations. These loans, which were due on May 29, 2009 with 8.436% interest per annum and guaranteed by Xi’an Tianxing, were paid in full on May 29, 2009. On June 2, 2009, Mr. Lu, Mr. Wen and Ms. Wang again obtained loans from the same bank and advanced cash to Xi’an Tianxing in the total amount of $110,025. These loans are due on June 1, 2010, with 10.11% interest per annum and are also guaranteed by Xi’an Tianxing. For the year ended December 31, 2010 and 2009, Xi’an Tianxing paid interest of $ 6,070 and $0, respectively, for these loans.
|
|
|
(3)
|
As of December 31, 2010 and December 31, 2009, the Company had $53,050 (representing 5,000 shares), and $302,372 (representing 47,334 common shares), respectively, under agreement to issue shares to Scott Cramer, respectively, as compensation for being a representative of the Company in the United States for the periods from May 2008 to June 30, 2009, respectively. As of December 31, 2009, the Company had $25,002 balance (representing 5,556 common shares) under agreement to issue shares to Mr. Mark D Chen as compensation at the beginning of each term of his directorship. Those shares were issued in February 2010.
|
|
|
(4)
|
Shaanxi Xinji Electronics Co., Ltd. is owned by the wife of Weibing Lu. The amounts due to Shaanxi Xinji Electronics as of December 31, 2010 and December 31, 2009 were short-term cash transfers for business operations, non-interest bearing, unsecured, and payable upon demand. As of December 31, 2010, the Company also had $4,301 payable to officers and shareholders for advance for short-term financing purposes. As of December 31, 2010 and December 31, 2009, the Company also had amounts due to Scott Cramer for bonus and the expenses paid by them on behalf of the Company.
|
39
Our Officers and Directors’ Relationship with Us, Our Subsidiaries and VIE
Mr. Weibing Lu, our Chairman and Chief Executive Officer, is a Director of Upform Group Limited, a British Virgin Islands company which owns approximately 13.1% of Skystar’s issued and outstanding common stock. Mr. Bennet P. Tchaikovsky, our former Chief Financial Officer, owns less than 0.1% of Skystar’s issued and outstanding common stock. Mr. Wei Wen, who is one of our directors, is Director of Clever Mind International Limited, a British Virgin Islands company which owns approximately 0.6% of Skystar’s issued and outstanding common stock. Mr. Scott Cramer, who is also one of our directors, owns and/or controls approximately 2.9% of Skystar’s issued and outstanding common stock. Mr. Mark D. Chen, who is also one of our directors, owns 0.1% of Skystar’s issued and outstanding common stock. Mr. Lu and Mr. Wen are both Directors of Skystar Cayman, our wholly owned subsidiary. Mr. Lan, Mr. Fan, Dr. Zhao and Dr. Qu do not own any shares of Skystar’s common stock as of the date of this prospectus.
Mr. Cramer is Director of Fortunate Time, wholly owned subsidiary of Skystar Cayman.
The management of Sida, the wholly owned subsidiary of Fortunate Time, includes Mr. Wen as General Manager
The management of Xi’an Tianxing, which we control through contractual arrangements between Sida and Xi’an Tianxing, includes Mr. Lu as Chairman and Chief Executive Officer and Mr. Wen as Vice-General Manager and Director. As of the date of this prospectus, Mr. Lu also owns approximately 41%, and Mr. Wen approximately 5%, of the issued and outstanding stock of Xi’an Tianxing.
Mr. Wei Shen is the General Manager of Shanghai Siqiang, wholly owned subsidiary of Xi’an Tianxing.
Skystar California has been closed.
Other Related Party Transactions
On January 1, 2007, we entered into a 5-year lease agreement with Mr. Weibing Lu, our chief executive officer, to lease the premises at Rm. 10601, Jiezuo Plaza, No.4, Fenghui Road South, Gaoxin District, Xi’an, Shaanxi Province, China, which belongs to Mr. Lu and which has been serving as our headquarters. The annual rent under the lease agreement is RMB 165,600 (approximately $24,000). The rent amount was deemed at fair value. Mr. Lu previously provided the premises rent-free, in 2005 and 2006, for the use of our administrative division.
On June 17, 2007, Shanghai Siqiang, wholly owned subsidiary of Xi’an Tianxing, entered into a 10-year lease agreement with Mr. Lu to lease the premises at 1715 Zhongchu Road, Building F, Unit 1001, Shanghai, China, which belongs to Mr. Lu. The annual rent under the lease agreement is RMB 144,000 (approximately $21,000). The rent amount was deemed at fair value.
Conflicts of interests between the duties of our officers and directors who are also management members of Xi’an Tianxing to our company and Xi’an Tianxing may arise. As our directors and/or executive officer (in the case of Mr. Lu), they have a duty of loyalty and care to us under U.S. and Cayman Islands law when there are any potential conflicts of interests between our company and Xi’an Tianxing. We cannot assure you, however, that when conflicts of interest arise, these individuals will act completely in our interests or that conflicts of interests will be resolved in our favor. In addition, they could violate their legal duties by diverting business opportunities from us to others. If we cannot resolve any conflicts of interest between us and them, we would have to rely on legal proceedings, which could result in the disruption of our business.
Director Independence
Our board of directors has determined that it has 4 members who qualify as “independent” as the term is defined under the listing standards of the NASDAQ Capital Market. The independent directors are Mr. Fan, Dr. Qu, Dr. Zhao, and Mr. Chen.
40
Audit Fees
The aggregate fees billed by our independent auditors, Frazer Frost LLP (“Frazer Frost”) was $75,000 for the three quarterly reviews of the nine months ended on September 30, 2010, and $195,000 for the year ended December 31, 2009 for professional services rendered for the audit of our annual financial statements and review of our quarterly financial statements. We have engaged Crowe & Horwath, LLP as our new auditor on December 14, 2011. The total fee for the audit of annual financial statements ending on December 31, 2010 was $180,000.
Audited-Related Fees
For the years ended December 31, 2010, and 2009, there were no fees billed by our independent auditors for services reasonably related to the performance of the audit or review of the financial statements outside of those fees disclosed above under “Audit Fees.”
Tax Fees
The aggregate fees billed by Frazer Frost for the year ended December 31, 2009, for services rendered for tax compliance, tax advice, and tax planning work to the Company was $12,000. For fiscal year 2010, we have retained a third party tax firm that is not our current or past auditors to work on the Company’s tax return.
All Other Fees
For the years ended December 31, 2010 and 2009, there were no other fees billed by our independent auditors for products and services outside of those fees disclosed above under “Audit Fees,” “Audit-Related Fees,” and “Tax Fees.”
ITEM 15. EXHIBITS
Financial Statements
The following consolidated financial statements are included in Part II, Item 8 of this Report:
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets at December 31, 2010 and 2009
Consolidated Statements of Operations and Comprehensive Income for the Years Ended December 31, 2010 and 2009
Consolidated Statements of Shareholders’ Equity for the Years Ended December 31, 2010 and 2009
Consolidated Statements of Cash Flows for the Years Ended December 31, 2010 and 2009
Notes to Consolidated Financial Statements
Financial Statement Schedules
Schedules are omitted because the required information is not present or is not present in amounts sufficient to require submission of the schedule or because the information required is given in the consolidated financial statements or the notes thereto.
41
Exhibits
|
Exhibit
Number
|
Description
|
|
|
3.1
|
Certificate of Incorporation, as amended (15)
|
|
|
3.2
|
Amended and Restated Bylaws (9)
|
|
|
4.1
|
Certificate of Designation of Series B Convertible Preferred Stock (1)
|
|
|
4.2
|
Form of Class A Convertible Debenture (2)
|
|
|
4.3
|
Form of Class B Convertible Debenture (2)
|
|
|
4.4
|
Form of Class A Warrant (2)
|
|
|
4.5
|
Form of Class B Warrant (2)
|
|
|
4.6
|
Form of Common Stock Certificate (12)
|
|
|
|
||
|
4.7
|
Form of Common Stock Purchase Option to be granted to the representative of the underwriters (12)
|
|
|
|
||
|
10.1
|
Form of Securities Purchase Agreement, dated as of February 26, 2007 by and among the Company and eight accredited investors (2)
|
|
|
10.2
|
Form of Registration Rights Agreement, dated as of February 26, 2007 by and among the Company and eight accredited investors (2)
|
|
|
10.3
|
Form of Company Principal Lockup Agreement in connection with the Securities Purchase Agreement dated as of February 26, 2007 (2)
|
|
|
10.4
|
Form of the Amendment, Exchange and Waiver Agreement between the Company and certain accredited investors dated November 9, 2007 (3)
|
|
|
10.5
|
Form of the Amendment and Waiver Agreement between Skystar Bio-Pharmaceutical Company and two institutional and accredited investors dated March 31, 2008 (6)
|
|
|
10.6
|
Amendment to Consulting Services Agreement among Skystar Cayman, Xi’an Tianxing and Sida Biotechnology (Xi’an) Co., Ltd. (“Sida”) dated March 10, 2008 (4)
|
|
|
10.7
|
Agreement to Transfer of Operating Agreement among Skystar Cayman, Xi’an Tianxing, Xi’an Tianxing’s Majority Stockholders, Weibing Lu and Sida dated March 10, 2008 (4)
|
|
|
10.8
|
Amendment to Equity Pledge Agreement among Skystar Cayman, Xi’an Tianxing, Xi’an Tianxing’s Majority Stockholders, and Sida dated March 10, 2008 (4)
|
|
|
10.9
|
Designation Agreement among Skystar Cayman, Xi’an Tianxing, Xi’an Tianxing’s Majority Stockholders, Weibing Lu and Sida dated March 10, 2008 (4)
|
|
|
10.10
|
Agreement to Transfer of Option Agreement among Skystar Cayman, Xi’an Tianxing, Xi’an Tianxing Majority Stockholders, Weibing Lu and Sida dated March 10, 2008 (4)
|
|
|
10.11
|
Employment Agreement with Weibing Lu dated May 5, 2008 (7)
|
|
|
10.13
|
Form of Director Offer Letter with Mr. Qiang Fan and Mr. Winston Yen (9)
|
|
|
10.14
|
Form of Director Offer Letter with Chengtun Qu and Shouguo Zhao (9)
|
|
|
10.16
|
Form of Director Offer Letter with Mr. Bennet P. Tchaikovsky (11)
|
42
|
10.17
|
Agreement with R. Scott Cramer dated March 30, 2010 (13)
|
|
|
10.18
|
Services Agreement with R. Scott Cramer dated April 16, 2010 (14)
|
|
|
10.19
|
Employment Agreement with Michael Lan dated April 16, 2010 (14)
|
|
|
14.1
|
Code of Ethics (10)
|
|
|
16.1
|
Letter from Frazer Frost, LLP to the U.S. Securities and Exchange Commission, dated December 17, 2010 (16)
|
|
|
21.1
|
List of Subsidiaries (10)
|
|
|
23.1
|
Consent of Independent Registered Public Accounting Firm *
|
|
|
|
||
|
23.2
|
Consent of prior auditor Frazer Frost LLP *
|
|
|
31.1
|
Certifications of Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 *
|
|
|
31.2
|
Certifications of Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 *
|
|
|
32.1
|
Certifications of Chief Executive Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 *
|
|
|
32.2
|
Certifications of Chief Financial Officer Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 *
|
|
|
99.1
|
Legal Opinion from Allbright Law Offices regarding the transfer of the contractual arrangements from Skystar Cayman to Sida, dated April 29, 2008 (8)
|
|
|
99.2
|
Lease Agreement between Xi’an Tianxing and Weibing Lu dated June 1, 2007 (5)
|
|
|
99.3
|
Lease Agreement between Shanghai Siqiang Biotechnological Co., Ltd. and Weibing Lu dated June 17, 2007 (8)
|
|
|
99.4
|
Summary of Research Arrangement between Shanghai Poultry Verminosis Institute and Xi’an Tianxing (8)
|
|
|
99.5
|
Cooperation Agreement between Shaanxi Microbial Institute and Xi’an Tianxing (8)
|
|
|
99.6
|
Technology Cooperation Agreement with Fourth Military Medical University (13)
|
* Filed herewith.
|
(1)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on November 14, 2005.
|
|
(2)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on March 5, 2007.
|
|
(3)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on December 11, 2007.
|
|
(4)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K on March 11, 2008.
|
|
(5)
|
Incorporated by reference from the Registrant’s Annual Report on Form 10-K on April 2, 2008.
|
|
(6)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K on April 23, 2008.
|
43
|
(8)
|
Incorporated by reference from the Registrant’s Registration Statement on Form S-1/A filed on June 26, 2008.
|
|
(9)
|
Incorporated by reference from the Registrant’s Current Report on Form 8-K on July 15, 2008.
|
|
(10)
|
Incorporated by reference from the Registration’s Annual Report on Form 10-K on April 15, 2009.
|
|
(11)
|
Incorporated by reference from the Registration’s Current Report on Form 8-K on May 27, 2009.
|
|
(12)
|
Incorporated by reference from the Registration’s Amendment to Registration Statement on Form S-1/A on June 26, 2009.
|
|
(13)
|
Incorporated by reference from the Registration’s Annual Report on Form 10-K on March 31, 2010.
|
|
(14)
|
Incorporated by reference from the Registration’s Current Report on Form 8-K on April 19, 2010.
|
|
(15)
|
Incorporated by reference from the Registration’s Quarterly Report on Form 10-Q on November 15, 2010.
|
|
(16)
|
Incorporated by reference from the Registration’s Current Report on Form 8-K on December 17, 2010.
|
44
SIGNATURES
Pursuant to the requirements of section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
SKYSTAR BIO-PHARMACEUTICAL COMPANY
(Registrant)
|
||||
|
Date: April 11, 2011
|
By:
|
/s/ Weibing Lu
|
||
|
Weibing Lu
Chief Executive Officer
|
||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Name
|
Title
|
Date
|
||
|
/s/ Weibing Lu
|
Chief Executive Officer (principal executive officer), Director
|
April 11, 2011
|
||
|
Weibing Lu
|
||||
|
/s/ Michael H. Lan
|
Chief Financial Officer (principal financial and accounting officer)
|
April 11, 2011
|
||
|
Michael Hongjie Lan
|
||||
|
/s/ Wei Wen
|
Secretary / Director
|
April 11, 2011
|
||
|
Wei Wen
|
||||
|
/s/ R. Scott Cramer
|
Director
|
April 11, 2011
|
||
|
R. Scott Cramer
|
||||
|
/s/ Qiang Fan
|
Director
|
April 11, 2011
|
||
|
Qiang Fan
|
||||
|
/s/ Chengtun Qu
|
Director
|
April 11, 2011
|
||
|
Chengtun Qu
|
||||
|
/s/ Mark D. Chen
|
Director
|
April 11, 2011
|
||
|
Mark D. Chen
|
||||
|
/s/ Shouguo Zhao
|
Director
|
April 11, 2011
|
||
|
Shouguo Zhao
|
45
Report of Independent Registered Public Accounting Firm
Board of Directors
Skystar Bio-Pharmaceutical Company and Subsidiaries
Gaoxin District, Xian Province, P.R. China
We have audited the accompanying consolidated balance sheet of Skystar Bio-Pharmaceutical Company and Subsidiaries as of December 31, 2010, and the related consolidated statements of income and comprehensive income, shareholders' equity, and cash flows for the year then ended. In connection with our audit of the 2010 consolidated financial statements, we have also audited the 2010 financial statements included in Schedule I listed in item 15(a) of this form 10-K. These consolidated financial statements and financial statement schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements and 2010 financial statement schedule referred to above present fairly, in all material respects, the financial position of Skystar Bio-Pharmaceutical Company and Subsidiaries as of December 31, 2010, and the results of their operations and their cash flows for the year then ended, in conformity with U.S. generally accepted accounting principles.
CROWE HORWATH LLP
/S/ CROWE HORWATH LLP
Sherman Oaks, California
April 11, 2010
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholder of
Skystar Bio-Pharmaceutical Company and Subsidiaries
We have audited the accompanying consolidated balance sheets of Skystar Bio-Pharmaceutical Company and Subsidiaries (the “Company”) as of December 31, 2009 and 2008, and the related consolidated statements of income and other comprehensive income, changes in equity, and cash flows for each of the years in the two-year period ended December 31, 2009. Our audits also included the financial statement schedules for the years ended December 31, 2009 and 2008. The Company’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Skystar Bio-Pharmaceutical Company and Subsidiaries as of December 31, 2009 and 2008, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America.
/s/ Frazer Frost, LLP (Successor Entity of Moore Stephens Wurth Frazer and Torbet, LLP, see Form 8-K filed on January 7, 2010)
Brea, California
March 31, 2010
F-2
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
CURRENT ASSETS:
|
||||||||
|
Cash
|
$ | 5,887,831 | $ | 11,699,398 | ||||
|
Accounts receivable, net of allowance for doubtful accounts of $339,031 and $327,857, respectively
|
4,977,850 | 4,383,187 | ||||||
|
Inventories
|
7,202,223 | 4,074,645 | ||||||
|
Deposits and prepaid expenses
|
17,074,000 | 11,900,314 | ||||||
|
Loans receivable
|
8,040,100 | - | ||||||
|
Other receivables
|
1,558,775 | 490,712 | ||||||
|
Total current assets
|
44,740,779 | 32,548,256 | ||||||
|
PLANT AND EQUIPMENT, NET
|
22,613,113 | 8,829,058 | ||||||
|
CONSTRUCTION-IN-PROGRESS
|
1,590,720 | 9,389,120 | ||||||
|
OTHER ASSETS:
|
||||||||
|
Long-term prepayments
|
1,454,226 | 1,173,427 | ||||||
|
Long-term prepayments for asset acquisitions
|
4,806,352 | 6,806,880 | ||||||
|
Intangible assets, net
|
6,043,941 | 1,860,172 | ||||||
|
Total other assets
|
12,304,519 | 9,840,479 | ||||||
|
Total assets
|
$ | 81,249,131 | $ | 60,606,913 | ||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES:
|
||||||||
|
Accounts payable
|
$ | 201,850 | $ | 297,567 | ||||
|
Other payable and accrued expenses
|
1,845,051 | 917,284 | ||||||
|
Short-term loans
|
3,025,884 | 220,050 | ||||||
|
Short-term loans from shareholders
|
- | 110,025 | ||||||
|
Deposits from customers
|
1,260,030 | 1,275,958 | ||||||
|
Taxes payable
|
749,836 | 722,106 | ||||||
|
Shares to be issued to related parties
|
53,050 | 327,374 | ||||||
|
Due to related parties
|
217,912 | 185,024 | ||||||
|
Total current liabilities
|
7,353,613 | 4,055,388 | ||||||
|
OTHER LIABILITIES:
|
||||||||
|
Deferred government grant
|
986,050 | 1,100,250 | ||||||
|
Warrant liability
|
1,419,639 | 1,538,686 | ||||||
|
Total other liabilities
|
2,405,689 | 2,638,936 | ||||||
|
Total liabilities
|
9,759,302 | 6,694,324 | ||||||
|
COMMITMENTS AND CONTINGENCIES
|
||||||||
|
SHAREHOLDERS' EQUITY
|
||||||||
|
Preferred stock, $0.001 par value, 50,000,000 shares authorized, No Series "A" shares authorized, 48,000,000 Series "B" shares authorized, No Series "B" shares issued and outstanding
|
||||||||
|
Common stock, $0.001 par value, 40,000,000 shares authorized, 7,161,919 and 6,989,640 shares issued and outstanding, respectively
|
7,162 | 6,989 | ||||||
|
Paid-in capital
|
35,784,378 | 34,580,096 | ||||||
|
Statutory reserves
|
5,695,236 | 3,879,077 | ||||||
|
Retained earnings
|
24,847,290 | 12,574,906 | ||||||
|
Accumulated other comprehensive income
|
5,155,763 | 2,871,521 | ||||||
|
Total shareholders' equity
|
71,489,829 | 53,912,589 | ||||||
|
Total liabilities and shareholders' equity
|
$ | 81,249,131 | $ | 60,606,913 | ||||
THE ACCOMPANYING NOTES ARE AN INTEGRAL PART OF THESE CONSOLIDATED FINANCIAL STATEMENTS
F-3
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
|
Years ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
REVENUE, net
|
$ | 47,556,383 | $ | 33,778,305 | ||||
|
COST OF REVENUE
|
22,011,588 | 16,520,989 | ||||||
|
GROSS PROFIT
|
25,544,795 | 17,257,316 | ||||||
|
OPERATING EXPENSES:
|
||||||||
|
Research and development
|
684,778 | 1,167,937 | ||||||
|
Selling expenses
|
2,124,952 | 1,928,441 | ||||||
|
General and administrative
|
4,625,092 | 2,466,470 | ||||||
|
Total operating expenses
|
7,434,822 | 5,562,848 | ||||||
|
INCOME FROM OPERATIONS
|
18,109,973 | 11,694,468 | ||||||
|
OTHER INCOME (EXPENSE):
|
||||||||
|
Other income (expense), net
|
(49,202 | ) | 117,873 | |||||
|
Interest expense, net
|
(58,846 | ) | (62,590 | ) | ||||
|
Change in fair value of warrants
|
(612,883 | ) | (868,445 | ) | ||||
|
Total other expense, net
|
(720,931 | ) | (813,162 | ) | ||||
|
INCOME BEFORE PROVISION FOR INCOME TAXES
|
17,389,042 | 10,881,306 | ||||||
|
PROVISION FOR INCOME TAXES
|
3,297,758 | 2,029,374 | ||||||
|
NET INCOME
|
14,091,284 | 8,851,932 | ||||||
|
OTHER COMPREHENSIVE INCOME :
|
||||||||
|
Foreign currency translation adjustment
|
2,281,501 | 13,914 | ||||||
|
COMPREHENSIVE INCOME
|
$ | 16,372,785 | $ | 8,865,846 | ||||
|
EARNINGS PER SHARE:
|
||||||||
|
Basic
|
$ | 1.98 | $ | 1.65 | ||||
|
Diluted
|
$ | 1.97 | $ | 1.62 | ||||
|
WEIGHTED AVERAGE NUMBER OF COMMON SHARES:
|
||||||||
|
Basic
|
7,105,789 | 5,374,452 | ||||||
|
Diluted
|
7,138,279 | 5,459,528 | ||||||
THE ACCOMPANYING NOTES ARE AN INTEGRAL PART OF THESE CONSOLIDATED FINANCIAL STATEMENTS
F-4
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF SHAREHOLDERS' EQUITY
|
Accumulated
|
||||||||||||||||||||||||||||||||||||
|
Retained earnings
|
other
|
|||||||||||||||||||||||||||||||||||
|
Preferred stock
|
Common stock
|
Paid-in
|
Statutory
|
comprehensive
|
||||||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Shares
|
Amount
|
capital
|
reserves
|
Unrestricted
|
income
|
Total
|
||||||||||||||||||||||||||||
|
BALANCE, January 1, 2009, as adjusted
|
2,000,000 | $ | 2,000 | 3,733,038 | $ | 3,733 | $ | 15,237,267 | $ | 2,952,710 | $ | 4,649,341 | $ | 2,857,607 | $ | 25,702,658 | ||||||||||||||||||||
|
Shares issued for services
|
12,438 | 12 | 63,002 | 63,014 | ||||||||||||||||||||||||||||||||
|
Cancellation of preferred stock
|
(2,000,000 | ) | (2,000 | ) | 2,000 | - | ||||||||||||||||||||||||||||||
|
Fractional shares due to the one-for-ten reverse split
|
1,772 | 2 | (2 | ) | - | |||||||||||||||||||||||||||||||
|
Shares issued for cash
|
3,220,000 | 3,220 | 19,070,461 | 19,073,681 | ||||||||||||||||||||||||||||||||
|
Cashless exercise of warrants
|
22,392 | 22 | 207,368 | 207,390 | ||||||||||||||||||||||||||||||||
|
Foreign currency translation
|
13,914 | 13,914 | ||||||||||||||||||||||||||||||||||
|
Net income
|
8,851,932 | 8,851,932 | ||||||||||||||||||||||||||||||||||
|
Appropriation to statutory reserves
|
926,367 | (926,367 | ) | - | ||||||||||||||||||||||||||||||||
|
BALANCE, December 31, 2009
|
- | $ | - | 6,989,640 | $ | 6,989 | $ | 34,580,096 | $ | 3,879,077 | $ | 12,574,906 | $ | 2,871,521 | $ | 53,912,589 | ||||||||||||||||||||
|
Shares issued for services
|
64,380 | 66 | 472,459 | 472,525 | ||||||||||||||||||||||||||||||||
|
Cashless exercise of warrants
|
107,899 | 107 | 1,511,497 | 1,511,604 | ||||||||||||||||||||||||||||||||
|
Reclassification of stock purchase option to derivative liability
|
(779,674 | ) | (779,674 | ) | ||||||||||||||||||||||||||||||||
|
Foreign currency translation
|
2,281,501 | 2,281,501 | ||||||||||||||||||||||||||||||||||
|
Net income
|
14,091,284 | 14,091,284 | ||||||||||||||||||||||||||||||||||
|
Appropriation to statutory reserves
|
1,816,159 | (1,818,900 | ) | 2,741 | - | |||||||||||||||||||||||||||||||
|
BALANCE, December 31, 2010
|
- | $ | - | 7,161,919 | $ | 7,162 | $ | 35,784,378 | $ | 5,695,236 | $ | 24,847,290 | $ | 5,155,763 | $ | 71,489,829 | ||||||||||||||||||||
THE ACCOMPANYING NOTES ARE AN INTEGRAL PART OF THESE CONSOLIDATED FINANCIAL STATEMENTS
F-5
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
Years ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||
|
Net income
|
$ | 14,091,284 | $ | 8,851,932 | ||||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
||||||||
|
Depreciation
|
847,662 | 566,230 | ||||||
|
Amortization
|
423,195 | 212,826 | ||||||
|
Common stock issued for services
|
472,525 | 63,014 | ||||||
|
Common stock to be issued to related parties for compensation
|
- | 232,170 | ||||||
|
Change in fair value of warrant liability
|
612,883 | 868,445 | ||||||
|
Change in operating assets and liabilities
|
||||||||
|
Accounts receivable
|
(434,233 | ) | (1,957,883 | ) | ||||
|
Inventories
|
(2,914,627 | ) | (987,977 | ) | ||||
|
Deposits and prepaid expenses
|
(4,654,016 | ) | (7,310,495 | ) | ||||
|
Other receivables
|
(725,280 | ) | (705,365 | ) | ||||
|
Accounts payable
|
(103,235 | ) | (249,709 | ) | ||||
|
Accrued expenses
|
83,014 | 55,140 | ||||||
|
Deposits from customers
|
(57,945 | ) | 851,170 | |||||
|
Taxes payable
|
3,041 | 509,132 | ||||||
|
Other payables
|
75,408 | 267,097 | ||||||
|
Net cash provided by operating activities
|
7,719,676 | 1,265,727 | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||
|
Proceeds from short-term investment
|
- | 351,864 | ||||||
|
Refund of long-term prepayments
|
- | 2,713,950 | ||||||
|
Prepayment for asset acquisitions
|
(4,451,544 | ) | (6,802,704 | ) | ||||
|
Loans to third parties
|
(7,840,820 | ) | (2,580,336 | ) | ||||
|
Proceeds from loans receivable
|
- | 2,875,242 | ||||||
|
Purchases of intangible assets
|
- | (1,172,880 | ) | |||||
|
Purchases of plant and equipment
|
(4,126,954 | ) | (529,470 | ) | ||||
|
Payments on construction-in-progress
|
- | (2,709,105 | ) | |||||
|
Net cash used in investing activities
|
(16,419,318 | ) | (7,853,439 | ) | ||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||
|
Decrease in restricted cash
|
153 | 80,684 | ||||||
|
Proceeds from short-term loans
|
2,728,975 | 219,915 | ||||||
|
Repayment for short-term loans
|
(110,955 | ) | (747,711 | ) | ||||
|
Proceeds from equity offering
|
- | 18,411,496 | ||||||
|
Repayment to shareholders and directors
|
- | (307,881 | ) | |||||
|
Proceeds from shareholders and directors
|
- | 109,958 | ||||||
|
Due to (from) related parties
|
31,374 | (57,223 | ) | |||||
|
Net cash provided by financing activities
|
2,649,547 | 17,709,238 | ||||||
|
EFFECT OF EXCHANGE RATE CHANGES ON CASH
|
238,528 | 1,463 | ||||||
|
(DECREASE) INCREASE IN CASH
|
(5,811,567 | ) | 11,122,989 | |||||
|
CASH, beginning of year
|
11,699,398 | 576,409 | ||||||
|
CASH, end of year
|
$ | 5,887,831 | $ | 11,699,398 | ||||
| - | ||||||||
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION:
|
||||||||
|
Cash paid for interest
|
$ | 55,439 | $ | 73,085 | ||||
|
Cash paid for income taxes
|
$ | 3,130,855 | $ | 2,095,704 | ||||
|
Non-cash investing and financing activities
|
||||||||
|
Long-term prepayment transferred to construction-in-progress
|
$ | 915,130 | $ | 190,458 | ||||
|
Construction-in-progress transferred to property, plant and equipment
|
$ | 10,838,131 | $ | 1,818,403 | ||||
|
Cashless exercise of warrants
|
$ | 1,511,604 | $ | 207,390 | ||||
|
Expense paid thorugh contribution receivable
|
$ | - | $ | 662,185 | ||||
|
Reclassification from equity to warrant liability
|
$ | 779,674 | $ | - | ||||
|
Long term prepayment transferred to Land Use Rights
|
$ | 4,441,437 | $ | - | ||||
|
Long term prepayment transferred to plant and equipment
|
$ | 1,952,463 | $ | - | ||||
THE ACCOMPANYING NOTES ARE AN INTEGRAL PART OF THESE CONSOLIDATED FINANCIAL STATEMENTS
F-6
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Note 1 – ORGANIZATION
Organization and description of business
Skystar Bio-Pharmaceutical Company (“Skystar” or the “Company”), was incorporated in Nevada on September 24, 1998. Since its acquisition on November 7, 2005 of Skystar Bio-Pharmaceutical (Cayman) Holdings Co., Ltd. (“Skystar Cayman”), a Cayman Islands company, the Company has been engaged in research, development, production, marketing and sales of veterinary healthcare and medical care products. All current operations of the Company are in the People’s Republic of China (“China” or the “PRC”).
All of the Company’s operations are carried out by Xi’an Tianxing Bio-Pharmaceutical Co., Limited (“Xi’an Tianxing”), a PRC joint stock company that the Company controls through contractual arrangements originally between Skystar Cayman and Xi’an Tianxing. On March 10, 2008, the Company entered into a series of agreements transferring all of the rights and obligations of Skystar Cayman under the contractual arrangements to Sida Biotechnology (Xi’an) Co., Ltd. (“Sida”), a PRC company that is wholly-owned by Fortunate Time International Limited (“Fortunate Time”), a Hong Kong company and wholly-owned subsidiary of Skystar Cayman. Xi’an Tianxing also has a wholly-owned subsidiary, Shanghai Siqiang Biotechnological Co., Ltd. (“Shanghai Siqiang”), a PRC company.
As a result of these contractual arrangements, which obligates Sida to absorb all of the risk of loss from Xi’an Tianxing’s activities and enables Sida to receive all of its expected residual returns, the Company accounts for Xi’an Tianxing as a variable interest entity (“VIE”) under the Financial Accounting Standards Board’s (“FASB”) interpretation on consolidation of variable interest entities. Accordingly, the Company consolidates Xi’an Tianxing’s operating results, assets, and liabilities.
Current Developments
On September 18, 2009, Skystar Bio-Pharmaceutical Inc. (“Skystar California”) was incorporated in California and became a wholly-owned subsidiary of Skystar. In December 2010, the Company closed down the California subsidiary after determining that it no longer fit into the Company’s strategy.
On May 7, 2010, Fortunate Time formed Skystar Biotechnology (Kunshan) Co., Limited (“Skystar Kunshan”) in Kunshan, Jiangsu province, China. Skystar Kunshan was formed in connection with a potential acquisition of assets (see Note 10). As of December 31, 2010, the acquisition of assets has not been completed.
On August 11, 2010, Sida became the parent company of Skystar Biotechnology (Jingzhou) Co., Limited (“Skystar Jingzhou”), a company established in Jingzhou, Hubei Province, China on February 5, 2010.
Hereinafter, Skystar, Skystar California, Skystar Cayman, Fortunate Time, Sida, Xi’an Tianxing, Shanghai Siqiang, Skystar Kunshan, and Skystar Jingzhou are collectively referred to as the “Company.”
F-7
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Note 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of consolidation
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America. The consolidated financial statements include the financial statements of the Company, its wholly-owned subsidiaries, and its VIEs. All significant inter-company transactions and balances between the Company, its subsidiaries, and its VIEs have been eliminated in consolidation.
Use of estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. The level of uncertainty in estimates and assumptions increases with the length of time until the underlying transactions are completed. Actual results may differ from these estimates in amounts that may be material to the consolidated financial statements and accompanying notes. Significant estimates and assumptions made by the Company are used for, but not limited to the allowance for anticipated uncollectible receivable, obsolescence reserve against the inventory and the fair value for derivatives instruments.
Foreign currency translation
The Company uses the United States dollar (“U.S. dollar”) for financial reporting purposes and the Chinese Renminbi (“RMB”) as its functional currency. The Company’s subsidiaries and VIE maintain their books and records in their functional currency, being the primary currency of the economic environment in which their operations are conducted.
The Company translates the subsidiaries’ and VIEs’ assets and liabilities into U.S. dollars using the applicable exchange rates prevailing at the balance sheet dates, and the statements of operations and cash flows are translated at average exchange rates during the reporting period. As a result, amounts related to assets and liabilities reported on the consolidated statements of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheets. Equity accounts are translated at historical rates. Adjustments resulting from the translation of the subsidiaries’ and VIEs’ financial statements are recorded as accumulated other comprehensive income.
The quotation of the exchange rates does not imply free convertibility of RMB to other foreign currencies. All foreign exchange transactions continue to take place either through the People's Bank of China or other banks authorized to buy and sell foreign currencies at the exchange rate quoted by the People's Bank of China. The rates of exchange quoted by the People’s Bank of China on December 31, 2010 and December 31, 2009 were RMB 6.59 and RMB 6.82 to US $1.00, respectively. The average translation rates of RMB 6.76 and RMB 6.82 to US $1.00 was applied to the income statement accounts for the years ended December 31, 2010 and 2009, respectively.
Approval of foreign currency payments by the People’s Bank of China or other institutions requires submitting a payment application form together with invoices, shipping documents, and signed contracts. Transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
F-8
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Fair values of financial instruments
The accounting standards regarding fair value of financial instruments and related fair value measurements defines financial instruments and requires fair value disclosures of those financial instruments. This accounting standard defines fair value, establishes a three-level valuation hierarchy for disclosures of fair value measurement and enhances disclosures requirements for fair value measures. Certain current assets and current liabilities are financial instruments. Management believes their carrying amounts are a reasonable estimate of fair value because of the short period of time between the origination of such instruments and their expected realization and, if applicable, their current interest rates are equivalent to interest rates currently available. The three levels of valuation hierarchy are defined as follows:
|
•
|
Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets.
|
|
•
|
Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments.
|
|
•
|
Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement.
|
Effective January 1, 2009, the Company adopted the provisions of an accounting standard regarding whether an instrument (or embedded feature) is indexed to an entity’s own stock. This accounting standard specifies that a contract that would otherwise meet the definition of a derivative but is both (a) indexed to the Company’s own stock and (b) classified in stockholders’ equity in the statement of financial position would not be considered a derivative financial instrument. It provides a new two-step model to be applied in determining whether a financial instrument or an embedded feature is indexed to an issuer’s own stock and thus able to qualify for the scope exception within the standards.
As a result of the foregoing adoption, 309,100 common stock purchase warrants previously treated as equity instruments pursuant to the derivative liability treatment exemption are no longer afforded equity treatment because the exercise price of the warrants is denominated in U.S. dollars, a currency other than the Company’s functional currency, the RMB. As a result, the warrants are not considered indexed to the Company’s own stock, and as such, all future changes in the fair value of these warrants are recognized currently in earnings until such time as the warrants are exercised or expired.
As such, effective January 1, 2009, the Company reclassified the fair value of these warrants from equity to liability, as if these warrants were treated as a derivative liability since their issuance in February 2007. On January 1, 2009, the Company reclassified from additional paid-in capital, as a cumulative effect adjustment, $230,877 to beginning retained earnings and $877,631 to warrant liability to recognize the fair value of such warrants. On June 30, 2009, the Company and Rodman & Renshaw, LLC, as representative of underwriters (the "Underwriters") entered into an Underwriting Agreement in connection with a public offering of the Company’s common stock. In connection with this offering, the Company agreed to grant 140,000 common stock purchase options to five designees of the Underwriters. All those options were provided for services performed, and in accordance with the terms of the option agreement, the holders were entitled to exercise the options starting on June 30, 2010. Therefore, as of June 30, 2010, the purchase options were reclassified from equity to warrants liabilities, and the Company reclassified $779,674 from additional paid in capital to derivative liability.
As required by the FASB’s accounting standards, financial assets and liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The fair values of warrant liability were modeled using a series of techniques, including closed-form analytic formula, such as the Black-Scholes Model, which does not entail material subjectivity because the methodology employed does not necessitate significant judgment, and the pricing inputs are observed from actively quoted markets.
F-9
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Outstanding warrants and options do not trade in an active securities market, and, as such, the Company estimates the fair value of these instruments using the Black-Scholes Option Pricing Model (“Black-Scholes Model”) using the following assumptions:
|
Warrants (1)
|
Warrants (2)
|
Purchase options (3)
|
||||||||||||||
|
December
31, 2009
|
December
31, 2010
|
December
31, 2009
|
December 31, 2010
|
|||||||||||||
|
Stock price
|
$ | 10.10 | $ | 9.73 | $ | 10.10 | $ | 9.73 | ||||||||
|
Exercise price
|
$ | 6.00 | $ | 5.00 | $ | 5.00 | $ | 8.11 | ||||||||
|
Annual dividend yield
|
- | - | - | - | ||||||||||||
|
Expected term (years)
|
0.17 | 1.16 | 2.17 | 3.50 | ||||||||||||
|
Risk-free interest rate
|
0.04 | % | 0.30 | % | 1.14 | % | 1.50 | % | ||||||||
|
Expected volatility
|
34 | % | 51 | % | 178 | % | 180 | % | ||||||||
|
|
(1)
|
As of December 31, 2009, 145,000 warrants with an exercise price of $6.00 were outstanding. As of December 31, 2010, all of these warrants had been exercised on a “cashless” basis.
|
|
|
(2)
|
As of December, 31, 2009, 107,254 warrants with an exercise price of $5.00 were outstanding. As of December 31, 2010, 34,230 warrants were outstanding.
|
|
|
(3)
|
As of December 31, 2010, 140,000 options with an exercise price of $8.11 were outstanding.
|
Expected volatility is based on historical volatility. Historical volatility was computed using daily pricing observations for recent periods that correspond to the term of the warrants and options. The Company believes this method produces an estimate that is representative of future volatility over the expected term of these warrants and options. The Company has no reason to believe future volatility over the expected remaining life of these warrants and options is likely to differ materially from historical volatility. The expected life is based on the remaining term of the warrants and options. The risk-free interest rate is based on U.S. Treasury securities according to the remaining term of the warrants and options.
The fair value of the 34,230 warrants and options outstanding as of December 31, 2010 was determined using the Black-Scholes Model, defined in the FASB’s accounting standard of fair value measurement as level 3 inputs, and we recorded the change in earnings. As a result, the warrant liability is carried on the consolidated balance sheets at fair value.
The following table sets forth by level within the fair value hierarchy our financial assets and liabilities that were accounted for at fair value on a recurring basis as of December 31, 2010:
|
|
Carrying Value at
December 31, 2010
|
Fair Value Measurement at
December 31, 2010
|
||||||||||||||
|
Level 1
|
Level 2
|
Level 3 | ||||||||||||||
|
Derivative liability
|
$ | 1,419,639 | $ | — | $ | — | $ | 1,419,639 | ||||||||
F-10
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Below is the reconciliation for the derivatives liability changes from December 31, 2009 to December 31, 2010.
|
Beginning Balance (December 31, 2009)
|
$
|
1,538,686
|
||
|
Less cashless exercise of warrants
|
(1,511,604
|
) | ||
|
Add options vested on June 20, 2010 (reclassified from equity)
|
779,674
|
|||
|
Change in fair value
|
612,883
|
|||
|
Ending balance, December 31, 2010
|
$
|
1,419,639
|
The Company recognized losses of $612,883 and $868,445 from the change in fair value of derivative liability for the years ended December 31, 2010 and 2009, respectively.
In addition to assets and liabilities that are recorded at fair value on a recurring basis, the Company is required to record assets and liabilities at fair value on a non-recurring basis. Generally, assets are recorded at fair value on a non-recurring basis as a result of impairment charges. For the years ended December 31, 2010 and 2009, no impairment charges were incurred.
Revenue recognition
Revenue of the Company is primarily derived from the sales of veterinary healthcare and medical care products in China. Sales are recognized when the following four revenue criteria are met: persuasive evidence of an arrangement exists, delivery has occurred, the selling price is fixed or determinable, and collectability is reasonably assured. Sales are presented net of value added tax (“VAT”). No estimated allowance for sales returns is reflected on these consolidated financial statements as sales returns historically have been insignificant.
There are two types of sales upon which revenue is recognized:
|
a.
|
Credit sales: revenue is recognized when the products have been delivered to the customers.
|
|
b.
|
Full payment before delivering: Cash received is recorded as “deposits from customers,” and revenue is recognized when the products have been delivered to the customers.
|
Shipping and handling costs related to costs of goods sold are included in selling expenses, which totaled $937,478and $850,387 for the years ended December 31, 2010 and 2009, respectively.
The Company’s revenues and cost of revenues by product line were as follows:
|
|
Years Ended December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
Revenues
|
||||||||
|
Micro-organism
|
$
|
11,543,988
|
$
|
8,021,139
|
||||
|
Veterinary Medications
|
32,076,000
|
22,920,479
|
||||||
|
Feed Additives
|
1,953,416
|
1,411,222
|
||||||
|
Vaccines
|
1,982,979
|
1,425,465
|
||||||
|
Total Revenues
|
$
|
47,556,383
|
$
|
33,778,305
|
||||
|
Cost of Revenues
|
||||||||
|
Micro-organism
|
$
|
3,113,667
|
$
|
2,129,945
|
||||
|
Veterinary Medications
|
17,870,668
|
13,672,333
|
||||||
|
Feed Additives
|
811,323
|
568,007
|
||||||
|
Vaccines
|
215,930
|
150,705
|
||||||
|
Total Cost of Revenues
|
22,011,588
|
16,520,989
|
||||||
|
Gross Profit
|
$
|
25,544,795
|
$
|
17,257,316
|
||||
F-11
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Cash
Cash includes currency on hand, demand deposits with banks, and liquid investments with an original maturity of three months or less.
Restricted cash
Restricted cash, if and when received, is generally comprised of amounts received from the PRC government as subsidies and set aside for specific uses (see Note 14). Restricted cash is maintained as bank deposits and reflected as current or non-current assets based on the expected period when such funds will be put into their specific uses.
Accounts receivable and other receivables
Accounts receivable are recorded at net realizable value consisting of the carrying amount less an allowance for uncollectible accounts, as needed. The Company uses the aging method to estimate the allowance for anticipated uncollectible receivable balances. Under the aging method, a bad debt percentage is estimated by management based on historical experience and current economic climate. The resulting percentage is applied to customers’ balances categorized by the number of months the underlying invoices have remained outstanding. At each reporting period, the allowance balance is adjusted to reflect the amount computed as a result of the aging method. When facts subsequently become available to indicate that the allowance provided requires an adjustment, a corresponding adjustment is made to the allowance account as a change in estimate. The ultimate collection of the Company’s accounts receivable may take one year. Delinquent account balances are reserved after management determines that the likelihood of collection is not probable, and known bad debts are written-off against allowance for doubtful accounts when identified.
Inventories
Inventories are stated at the lower of cost or market, as determined on a moving weighted-average basis using the first-in, first-out (FIFO) cost method. Inventories include purchases and related costs incurred in bringing the inventories to their present location and condition. Management reviews inventories for obsolescence and cost in excess of net realizable value at least annually and records a reserve against the inventory and additional cost of goods sold when the carrying value exceeds net realizable value.
Plant and equipment
Plant and equipment are stated at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. Expenditures for maintenance and repairs that do not improve or extend the useful lives of the assets are charged to operations as incurred, while renewals and betterments are capitalized. Gains and losses on disposals are included in the results of operations. Estimated useful lives of the assets are as follows:
F-12
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
|
|
Estimated Useful Life
|
||
|
Buildings
|
20-40 years
|
||
|
Machinery and equipment
|
10 years
|
||
|
Computer, office equipment and furniture
|
5 years
|
||
|
Vehicles
|
5-10 years
|
||
Management assesses the carrying value of plant and equipment annually or more often when factors indicating impairment are present, and reduces the carrying value of such assets by the amount of the impairment. The Company determines the existence of such impairment by measuring the expected future cash flows (undiscounted) and comparing such amount to the net asset carrying value. An impairment loss, if it exists, is measured as the amount by which the carrying amount of the asset exceeds the fair value of the asset. Based on its review, management believes that, as of December 31, 2010 and 2009, there was no impairment for its plant and equipment.
Construction-in-progress
Construction-in-progress includes direct costs of construction of a factory building. Interest incurred during the period of construction, if significant, is capitalized. All other interest is expensed as incurred. Construction-in-progress is not depreciated until such time as the asset is completed and put into service.
Intangible assets
Land Use Rights — Land use rights represent the amounts paid to acquire a long-term interest to utilize the land underlying the Company’s facilities. This type of arrangement is common for the use of land in the PRC. Land use rights are amortized on the straight-line method over the contractual lease terms. The land use right granted to the Company’s Huxian facility was for 50 years. The land use right granted to the Company’s Jingzhou facility was 30 years.
Technological Know-How — Purchased technological know-how includes confidential formulas, manufacturing processes, and technical and procedural manuals, and is amortized using the straight-line method over estimated useful life between five to ten years that reflects the period over which such confidential formulas, manufacturing processes, and technical and procedural manuals are kept confidential by the Company as agreed between the Company and the selling parties. Drug approval licenses are typically granted in five year terms by the Ministry of Agriculture.
Impairment of Intangible Assets — the Company evaluates the carrying value of intangible assets annually or more often when factors indicating impairment are present. The Company determines the existence of such impairment by measuring the estimated future cash flows (undiscounted) and comparing such amount to the net asset carrying value. If the undiscounted cash flow estimated to be generated by any such intangible asset is less than its carrying amount, a loss is recognized based on the amount by which the carrying amount exceeds the intangible asset’s fair market value. Loss on intangible assets to be disposed of is determined in a similar manner, except that fair market values are reduced by the cost of disposal. Based on its review, the Company believes that, as of December 31, 2010, there was no impairment of its intangible assets.
Comprehensive income
Comprehensive income and loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. Accumulated other comprehensive income is comprised of the changes in foreign currency exchange rates.
F-13
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Research and development costs
Research and development costs are charged to operations as incurred and include salaries, professional fees, and technical support fees related to such efforts.
Advertising costs
Advertising costs are charged to operations currently. Advertising costs for the years ended December 31, 2010 and 2009 were $46,385 and $253,006, respectively.
Income taxes
The Company accounts for income taxes using an asset and liability method. Under this method, deferred income taxes are recognized for the tax consequences of temporary differences by applying enacted statutory tax rates applicable to future years to differences between the financial statement carrying amounts and the tax bases of existing assets and liabilities. The effect on deferred income taxes of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is recognized if it is more likely than not that some portion or all of a deferred tax asset will not be realized.
A tax position is recognized as a benefit only if it is “more likely than not” that the tax position would be sustained in a tax examination, with a tax examination being presumed to occur. The amount recognized is the largest amount of tax benefit that is greater than 50% likely of being realized on examination. For tax positions not meeting the “more likely than not” test, no tax benefit is recorded. As of December 31, 2010, there are no unrecognized tax benefits, and the Company does not expect a significant change in tax benefits in the next 12 months. Penalties and interest levied by taxing authorities, if any, are classified as income tax expense in the year incurred. No significant penalties or interest relating to income taxes have been incurred during the years ended December 31, 2010 and 2009.
The Company’s operations are subject to income and transaction taxes in the United States and in the PRC jurisdictions. Significant estimates and judgments are required in determining the Company’s worldwide provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations. The ultimate amount of tax liability may be uncertain as a result.
According to the PRC Tax Administration and Collection Law, the statute of limitations is three years if the underpayment of taxes is due to computational or other errors made by the taxpayer or the withholding agent. The statute of limitations extends to five years under special circumstances. In the case of transfer pricing issues, the statute of limitations is ten years. There is no statute of limitations in the case of tax evasion. Accordingly, the income tax returns of the Company’s PRC operating subsidiaries for the years ended December 31, 2006 through 2010 are open to examination by the PRC state and local tax authorities.
The Company does not anticipate any events that could cause a change to these uncertainties.
Stock-based compensation
The Company records and reports stock-based compensation by measuring the cost of services received in exchange for an award of equity instruments based on the grant-date fair value of the award. That cost is recognized over the period during which services are received. Stock compensation for stock granted to non-employees is determined as the fair value of the consideration received or the fair value of equity instruments issued, whichever is more reliably measured.
F-14
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Earnings per share
Basic earnings per share is based upon the weighted-average number of common shares outstanding. Diluted earnings per share is based on the assumption that all dilutive convertible shares, including convertible preferred shares, warrants and stock options were converted or exercised. Further, the method requires that stock dividends or stock splits be accounted for retroactively if the stock dividends or stock splits occur during the period or after the end of the period but before the release of the financial statements, by considering it outstanding for the entirety of each period presented. Diluted earnings per share is computed by applying the treasury stock method. Under this method, options and warrants are assumed to be exercised at the beginning of the period (or at the time of issuance, if later), and as if funds obtained thereby were used to purchase common stock at the average market price during the period.
All share and per share amounts used in the Company's consolidated financial statements and notes thereto have been retroactively restated to reflect the 1-for-10 reverse stock split on May 12, 2009 and the 2-for-1 forward stock split on November 16, 2009.
Related parties
Parties are considered to be related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of such principal owners and management, and other parties with which the Company may deal if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests.
Reclassifications
Certain prior period amounts have been reclassified to conform to the current period presentation. These reclassifications had no impact on previously reported net income or cash flows.
Business combinations
Effective January 1, 2009, the Company adopted the accounting standard regarding business combinations. This standard retains the fundamental requirements that the acquisition method of accounting (which this standard refers to as the purchase method) be used for all business combinations and for an acquirer to be identified for each business combination. This standard requires an acquirer to recognize the assets acquired, the liabilities assumed, and any noncontrolling interest in the acquiree at the acquisition date, measured at their fair values as of that date, with limited exceptions, to the individual assets acquired and liabilities assumed based on their estimated fair values.
Recently issued accounting pronouncements
In January 2010, the FASB issued ASU No. 2010-01, Accounting for Distributions to Shareholders with Components of Stock and Cash. The amendments in this Update clarify that the stock portion of a distribution to shareholders that allows them to elect to receive cash or stock with a potential limitation on the total amount of cash that all shareholders can elect to receive in the aggregate is considered a share issuance that is reflected in EPS prospectively and is not a stock dividend for purposes of applying Topics 505 and 260 (Equity and Earnings Per Share). The amendments in this update are effective for interim and annual periods ending on or after December 15, 2009, and should be applied on a retrospective basis. The Company adopted this standard, and the standard did not have material effect on the Company’s consolidated financial statements.
F-15
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
In January 2010, the FASB issued ASU No. 2010-02 regarding accounting and reporting for decreases in ownership of a subsidiary. Under this guidance, an entity is required to deconsolidate a subsidiary when the entity ceases to have a controlling financial interest in the subsidiary. Upon deconsolidation of a subsidiary, an entity recognizes a gain or loss on the transaction and measures any retained investment in the subsidiary at fair value. In contrast, an entity is required to account for a decrease in its ownership interest of a subsidiary that does not result in a change of control of the subsidiary as an equity transaction. This ASU clarifies the scope of the decrease in ownership provisions, and expands the disclosures about the deconsolidation of a subsidiary or de-recognition of a group of assets. This ASU is effective beginning in the first interim or annual reporting period ending on or after December 31, 2009. The adoption of this ASU did not have a material impact the Company’s consolidated financial statements.
In January 2010, the FASB issued ASU No. 2010-06 – Improving Disclosures about Fair Value Measurements. This update provides amendments to Subtopic 820-10 that requires new disclosure to include transfers in and out of Levels 1 and 2 and activity in Level 3 fair value measurements. Further, this update clarifies existing disclosures on level of disaggregation and disclosures about inputs and valuation techniques. A reporting entity should provide fair value measurement disclosures for each class of assets and liabilities and should provide disclosures about the valuation techniques and inputs used to measure fair value for both recurring and nonrecurring fair value measurements. Those disclosures are required for fair value measurements that fall in either Level 2 or Level 3. The new disclosures and clarifications of existing disclosures are effective for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements. Those disclosures are effective for fiscal years beginning after December 15, 2010, and for interim periods within those fiscal years. The adoption of this ASU did not have a material impact on its consolidated financial statements.
In February 2010, the FASB issued ASU 2010-09, “Subsequent Events (Topic 855): Amendments to Certain Recognition and Disclosure Requirements”. ASU 2010-09 primarily rescinds the requirement that, for listed companies, financial statements clearly disclose the date through which subsequent events have been evaluated. Subsequent events must still be evaluated through the date of financial statement issuance; however, the disclosure requirement has been removed to avoid conflicts with other SEC guidelines. ASU 2010-09 was effective immediately upon issuance and was adopted in February 2010.
In April 2010, the FASB issued Accounting Standards Update 2010-13, “Compensation—Stock Compensation (Topic 718): Effect of Denominating the Exercise Price of a Share-Based Payment Award in the Currency of the Market in Which the Underlying Equity Security Trades,” or ASU 2010-13. ASU 2010-13 provides amendments to Topic 718 to clarify that an employee share-based payment award with an exercise price denominated in currency of a market in which a substantial portion of the entity’s equity securities trades should not be considered to contain a condition that is not a market, performance, or service condition. Therefore, an entity would not classify such an award as a liability if it otherwise qualifies as equity. The amendments in this Update are effective for fiscal years, and interim periods within those fiscal years, beginning on or after December 15, 2010. The Company is currently evaluating the impact of this ASU; however, the Company does not expect the adoption of this ASU to have a material impact on its consolidated financial statements.
F-16
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
In April 2010, the FASB issued Accounting Standard Update 2010-17, “Revenue Recognition—Milestone Method (Topic 605): Milestone Method of Revenue Recognition” or ASU 2010-17. This Update provides guidance on the recognition of revenue under the milestone method, which allows a vendor to adopt an accounting policy to recognize all of the arrangement consideration that is contingent on the achievement of a substantive milestone (milestone consideration) in the period the milestone is achieved. The pronouncement is effective on a prospective basis for milestones achieved in fiscal years and interim periods within those years, beginning on or after June 15, 2010. The adoption of this ASU did not have a material impact the Company’s consolidated financial statements.
In December 2010, the FASB issued Accounting Standards Update 2010-28, Intangibles—Goodwill and Other (Topic 350): When to Perform Step 2 of the Goodwill Impairment Test for Reporting Units with Zero or Negative Carrying Amounts (a consensus of the FASB Emerging Issues Task Force). ASU No. 2010-28 addresses questions about entities that have reporting units with zero or negative carrying amounts. The amendments modify Step 1 of the goodwill impairment test for reporting units with zero or negative carrying amounts. For those reporting units, an entity is required to perform Step 2 of the goodwill impairment test if it is more likely than not that a goodwill impairment exists. In determining whether it is more likely than not that goodwill impairment exists, an entity should consider whether there are any adverse qualitative factors indicating that impairment may exist. The qualitative factors are consistent with the existing guidance and examples in paragraph 350-20-35-30, which requires that goodwill of a reporting unit be tested for impairment between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. In addition, current GAAP will be improved by eliminating an entity’s ability to assert that a reporting unit is not required to perform Step 2 because the carrying amount of the reporting unit is zero or negative despite the existence of qualitative factors that indicate the goodwill is more likely than not impaired. As a result, goodwill impairments may be reported sooner than under current practice. The provisions of ASC No. 2010-28 are effective for fiscal years, and interim periods within those years, beginning after Dec. 15, 2010. Early adoption is not permitted. The Company does not expect the adoption of this ASU to have a material impact on its consolidated financial statements.
In December 2010, the FASB issued Accounting Standards Update 2010-29, Business Combinations (Topic 805) — Disclosure of Supplementary Pro Forma Information for Business Combinations. ASU No. 2010-29 clarifies that, when presenting comparative financial statements, SEC registrants should disclose revenue and earnings of the combined entity as though the current period business combinations had occurred as of the beginning of the comparable prior annual reporting period only. The amendments expand the supplemental pro forma disclosures to include a description of the nature and amount of material, nonrecurring pro forma adjustments directly attributable to the business combination included in the reported pro forma revenue and earnings. ASU 2010-29 is effective prospectively for material (either on an individual or aggregate basis) business combinations entered into in fiscal years beginning on or after December 15, 2010 with early adoption permitted.
Note 3 - CONCENTRATIONS AND CREDIT RISK
The Company’s operations are all carried out in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by the political, economic, and legal environments in the PRC, and by the general state of the PRC’s economy. The Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies in North America and Western Europe. These include risks associated with, among others, the political, economic, and legal environments and foreign currency exchange. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
F-17
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Financial instruments which subject the Company to concentration of credit risk consist of cash and accounts receivable. Balances at financial institutions or state-owned banks within the PRC are not covered by insurance. The Company has not experienced any losses in such accounts. The Company provides credit terms for sales to certain customers. As a result, there are credit risks with the accounts receivable balances. The Company constantly re-evaluates the credit worthiness of customers buying on credit and maintains an allowance for doubtful accounts.
For the year ended December 31, 2010 and 2009, all of the Company’s sales occurred in the PRC. No customer accounted for 10% or more of the Company’s total revenues. All accounts receivable at December 31, 2010 and 2009 are from customers located in the PRC.
The Company’s five largest vendors accounted for approximately 54% of the Company’s total purchases for the year ended December 31, 2010, while the Company’s five largest vendors accounted for 59% of the Company’s total purchases for the year ended December 31, 2009. As of December 31, 2010 and 2009, there were no amounts due to the five largest vendors.
The Company had one product that accounted for 26% and 20% of the Company’s total revenues for the years ended December 31, 2010 and 2009, respectively.
Note 4 - ACCOUNTS RECEIVABLE, NET
Accounts receivable consisted of the following:
|
|
December 31,
2010
|
December 31,
2009
|
||||||
|
Account receivable
|
$ | 5,316,881 | $ | 4,711,044 | ||||
|
Allowance for doubtful accounts
|
(339,031 | ) | (327,857 | ) | ||||
|
Account receivable, net
|
$ | 4,977,850 | $ | 4,383,187 | ||||
The following table presents the movement of allowance for doubtful accounts:
|
Allowance January 1, 2009
|
$
|
327,857
|
||
|
Addition
|
—
|
|||
|
Recovery
|
—
|
|||
|
Translation adjustment
|
—
|
|||
|
Allowance December 31, 2009
|
327,857
|
|||
|
Addition
|
||||
|
Recovery
|
||||
|
Translation adjustment
|
11,174
|
|||
|
Allowance December 31, 2010
|
$
|
339,031
|
F-18
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Note 5 – INVENTORIES
Inventories consisted of the following:
|
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Raw materials
|
$ | 5,466,902 | $ | 2,997,481 | |||||
|
Packing materials
|
170,843 | 159,620 | |||||||
|
Work-in-process
|
23,071 | 886 | |||||||
|
Finished goods
|
1,721,943 | 1,096,547 | |||||||
|
Other
|
25,723 | 19,571 | |||||||
|
Total
|
7,408,482 | 4,274,105 | |||||||
|
Less: Allowance for slow moving raw materials
|
(206,259 | ) | (199,460 | ) | |||||
|
Total
|
$ | 7,202,223 | $ | 4,074,645 | |||||
The Company periodically reviews its reserves for slow-moving and obsolete inventories.
Note 6 - DEPOSITS AND PREPAID EXPENSES
Deposits and prepaid expenses were comprised of the following:
|
|
December 31, 2010
|
December 31, 2009
|
||||||
|
Prepayment for raw materials purchases
|
$ | 15,967,236 | $ | 10,990,913 | ||||
|
Prepayment for packaging materials purchases
|
767,623 | 489,392 | ||||||
|
Other
|
339,141 | 420,009 | ||||||
|
Total
|
$ | 17,074,000 | $ | 11,900,314 | ||||
As part of the Company’s strategy to reduce inventory costs, the Company maintains a balance for prepayment to suppliers in order to secure favorable pricing for raw materials. As inventory is received throughout the year, this balance will fluctuate with the business operations.
Note 7 - PLANT AND EQUIPMENT, NET
Plant and equipment consisted of the following:
|
|
December 31, 2010
|
December 31, 2009
|
||||||
|
Building and improvements
|
$ | 20,757,554 | $ | 6,798,616 | ||||
|
Machinery and equipment
|
3,640,635 | 3,035,814 | ||||||
|
Office equipment and furniture
|
256,690 | 191,424 | ||||||
|
Vehicles
|
566,718 | 485,156 | ||||||
|
Total
|
25,221,597 | 10,511,010 | ||||||
|
Less: accumulated depreciation
|
(2,608,484 | ) | (1,681,952 | ) | ||||
|
Plant and equipment, net
|
$ | 22,613,113 | $ | 8,829,058 | ||||
Depreciation expense was $847,662 and $566,230 for the years ended December 31, 2010 and 2009, respectively.
F-19
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Note 8 - CONSTRUCTION-IN-PROGRESS
Construction-in-progress (“CIP”) relates to a plant being built in accordance with the PRC’s Good Manufacturing Practices (“GMP”) Standard. During the year ended December 31, 2010, the micro-organism facility and some general facility improvements were completed and placed in service, which resulted in a transfer from CIP to plant and equipment of $1,389,602. The Vaccine facility was completed and resulted in a transfer to plant and equipment of $9,448,505. No depreciation is provided for construction-in-progress until such time as the assets are completed and placed into service.
The construction projects the Company was in the progress of completing are as follows:
|
Total in CIP
as of
|
Estimate cost to
|
Estimated
|
Estimated
|
|||||||||||
|
Project
|
12/31/2010
|
Complete
|
Total Cost
|
Completion Date
|
||||||||||
|
Vaccine facility
|
$ | 1,501,332 | $ | 1,501,332 |
July 2011
|
|||||||||
|
Kunshan facility
|
89,388 | 89,388 |
December 2011
|
|||||||||||
|
TOTAL CIP Balance
|
$ | 1,590,720 | $ | - | $ | 1,590,720 | ||||||||
The construction of the vaccine facility was completed in June 2010. The Company has finished installing, tooling, and testing of equipment and submitted the application for GMP certification to the Ministry of Agriculture. The Company is waiting for occupancy and operation approval from the Ministry of Agriculture.
As of December 31, 2010 and 2009, the Company had CIP amounting to $1,590,720 and $9,389,120, respectively. Interest expense capitalized for construction for CIP. For the years ended December 31, 2010 and 2009 was $0 and $71,467, respectively.
Note 9 - LONG-TERM PREPAYMENTS
Long-term prepayments consisted of the following:
|
|
December 31,
2010
|
December 31, 2009
|
||||||
|
R&D project
|
$ | 303,400 | $ | 293,400 | ||||
|
Construction deposit
|
455,100 | 440,100 | ||||||
|
Deposit for building and equipment purchase
|
695,726 | 439,927 | ||||||
|
Total
|
$ | 1,454,226 | $ | 1,173,427 | ||||
In 2009, the Company prepaid $293,400 (RMB 2,000,000) to a vendor for equipment and raw materials related to a research and development project on nano-technology in the prevention of milk cow disease. As of December 31, 2010, the entire prepayment was used for expenses incurred for this project, and the project started its trial phase in the third quarter of 2010 (see Note 20).
As of December 31, 2009, deposits totaling $439,927 (RMB 2,998,820) were made for the purchase of a new office building. The purchase was completed and the deposit transferred to fixed asset in March 2010. Additional prepayments made in 2010 were transferred to fixed assets as of December 31, 2010.
Note 10 - LONG-TERM PREPAYMENTS FOR ACQUISITIONS
The Company paid $3,731,820 (RMB 24,600,000) to acquire the assets of the Hubei-based veterinary manufacturer through its bankruptcy proceedings. The purchase price was prepaid in 2009. On August 11, 2010, the Hubei purchase was completed. In connection with the purchase, an independent third party appraiser which is a certified public appraiser under the laws of PRC was engaged by the Company to perform an appraisal of the assets acquired. The purchase price was allocated to fixed assets of $1.5 million (RMB 10,160,000), land use rights of $1.3 million (RMB 8,324,000) and patents of $303,400 (RMB 2.0 million). Approximately $3.1 million (RMB 20,484,000) was transferred as investment on Skystar Jingzhou, and approximately $0.6 million (RMB 4,116,000) was expensed.
F-20
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Through December 31, 2010, the Company has spent $8,179,664 (RMB 53,920,000) in connection with the purchase of a Kunshan based micro-organism manufacturing facility located in Jiangsu Province, PRC, of which, approximately $0.38 million has been expensed. As of December 31, 2010 the purchase was not yet completed. The Company received the title of land use right in October 2010. Consequently, $2,994,062 was transferred to land use rights from long term prepayments. The value of the land use right was appraised by an independent, certified public appraiser. The Company is still waiting for the completion of the transfers of other property rights and certificates. When the purchase is complete, another appraisal must be performed for all the assets related to the purchase as required by government regulation.
Deposits made for potential purchases amounted to $4,806,352 and $6,806,880 at December 31, 2010 and December 31, 2009, respectively, all of which was held by an unrelated third party engaged to identify and facilitate acquisition targets for the Company. The Company is in the final stages of clearing all the necessary government approvals and expects to close the purchase early second quarter of 2011.
Note 11 – INTANGIBLE ASSETS
Intangible assets consisted of the following:
|
|
December 31, 2010
|
December 31, 2009
|
||||||
|
Land use rights
|
$
|
4,650,521
|
$
|
378,853
|
||||
|
Technological know-how
|
2,123,800
|
2,053,800
|
||||||
|
Patent
|
303,400
|
-
|
||||||
|
Total
|
7,077,721
|
2,432,653
|
||||||
|
Less: accumulated amortization
|
(1,033,780
|
)
|
(572,481
|
)
|
||||
|
Intangible assets, net
|
$
|
6,043,941
|
$
|
1,860,172
|
||||
In 2009, the Company paid $1,197,600 (RMB 8,000,000) for the right of a fish disease vaccine technology for an eleven-year term from September 2009 through September 2020. This amount was included in technological know-how at December 31, 2010. The technology was transferred to the Company on June 17, 2010, and the Company began using such technology since that date and recorded the amortization expense based on the remaining term. In 2010, land use rights increased by the Jingzhou acquisition totaling $1,264,693 and the Kunshan acquisition totaling $2,994,062.
For the year ended December 31, 2010 and 2009, the amortization expense for intangibles amounted to $423,195 and $212,826, respectively.
F-21
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Amortization expense for the next five years and thereafter is as follows:
|
Years ending December 31,
|
|
Amount
|
|
|
|
2011
|
$
|
524,214
|
||
|
2012
|
350,842
|
|||
|
2013
|
350,842
|
|||
|
2014
|
350,842
|
|||
|
2015
|
278,670
|
|||
|
Thereafter
|
2,191,594
|
|||
|
Total
|
$
|
4,047,004
|
||
Note 12 – Loan Receivable
In December 2010, the Company provided two of its largest suppliers with a short term loan totaling $7,585,000 at the prevailing interest rate of 5%. The two suppliers, Fandike and Chenyue, are among the largest suppliers to the Company. On March 15, 2011, the suppliers repaid the entire $7,585,000 loan plus interest of $83,989.
In August 2010, the Company issued an unsecured loan to an unrelated third party in the amount of $455,100 (RMB 3,000,000) for one year from August 10, 2010 through August 9, 2011, with an annual interest rate of 6%.
Note 13 – SHORT-TERM LOANS
On January 14, 2009, the Company obtained a one year loan with Shaanxi Agricultural Yanta Credit Union for $220,050 (RMB 1,500,000) at an annual interest rate of 8.66% and secured by the personal property of Weibing Lu, the Company’s Chief Executive Officer. This amount was repaid on January 15, 2010. On August 24, 2010, the Company obtained a one year loan with the same bank for $748,500 (RMB 5,000,000) at an annual interest rate of 8.66%. This loan was secured by the Company’s office buildings located in Xi’an City.
The Company entered into a line of credit agreement with Bank of East-Asia that allows the Company to borrow up to $3,000,000 (RMB 20,000,000). This line of credit agreement expires within two years starting with the first withdrawal. The Company withdrew $468,753 (RMB 3,090,000), $514,870 (RMB 3,394,000) and $1,283,761 (RMB 8,462,500) on September 7, 2010, September 9, 2010 and October 12, 2010, respectively, at an annual interest rate of 6.372% with interest due every three months starting on the date of the first withdrawal. This line of credit is secured by the Company’s land use right and manufacturing plant located in Huxian County.
Interest expense incurred and associated with the short-term loans amounted to $55,105(RMB 372,484.85) for the year ended December 31, 2010. Interest expense incurred and associated with the short term loan amounted to $71,467 for the year ended December 31, 2009, which has been capitalized as part of construction-in-progress.
Note 14 - DEFERRED GOVERNMENT GRANT
Deferred government grant represents subsidies for Good Manufacturing Practice projects granted by various levels of the PRC government. To date, the Company received government subsidies totaling $986,050 (RMB 6,500,000,) of which, RMB 5,000,000 were granted by the PRC Government, of which, RMB 1,000,000 was re-paid on December 7, 2010, with the remaining fund to be paid back in 2012. RMB 2,000,000 was granted by Shaanxi Provincial Government, and RMB 500,000 was granted by Xi’an Municipal Government. The Shaanxi Provincial Government grant and Xi’an Municipal Government Grant do not need to be re-paid.
F-22
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Note 15 - CAPITAL TRANSACTIONS
On May 12, 2009, the Company effected a 1-for-10 reverse stock split of its issued and outstanding shares of common stock and a proportional reduction of its authorized shares of common stock. On November 16, 2009, the Company effected a 2-for-1 forward split of its issued and outstanding common stock and a proportional increase of its authorized shares of common stock. The amounts and exercise prices of common stock, warrants, and options disclosed in the Company’s consolidated financial statements and the footnotes thereto that were issued prior to such stock splits, including the financial statements for the year ended December 31, 2010 and these footnotes, have been retroactively restated to reflect both the reverse stock split and the forward stock split.
Preferred stock
On June 25, 2009, the Company’s board of directors concluded that 2,000,000 shares of series “A” preferred stock issued in 2001 were not valid because no certificate of designation was filed prior to their issuance as required under Nevada corporate law. On December 21, 2009, the Company instructed its transfer agent to remove these preferred shares officially from its shareholder records. As of December 31, 2010 and December 31, 2009, no share of series “A” preferred stock was authorized or outstanding.
Stock-based compensation
On March 30, 2010, the Company agreed to issue 2,500 shares of common stock to a non-executive director in exchange for services unrelated to directorship at the fair market value of $11.74 per share based on the closing price of March 30, 2010. On April 16, 2010, the Company entered into another agreement to grant 10,000 shares of common stock to that director for his one-year service from April 1, 2010. The closing price per share on the grant date was $10.61. The common stock compensation vests in four equal quarterly installments of 2,500 shares. Shares owed were accrued at end of each quarter at the fair market value of the grant date at $10.61 per share. A total of $170,940 was charged to general and administrative expense for the year ended on December 31, 2010. On October 25, 2010, 5,000 shares were issued to the director for the shares vested in the first two quarters of 2010. As of December 31, 2010, 5,000 shares were accrued at the closing price of the grant date of $10.61 per share and pending to be issued.
The Company accrued $53,050 and $302,372 under shares to be issued as of December 31, 2010 and 2009, respectively, which represented 5,000 and 47,334 common shares to be issued to that non-executive director for his service provided for the period from April 2010 to December 31, 2010 and for the period from May 2008 to December 2009, respectively. Based on the 2010 service agreements, restricted stocks are treated as equity awards and un-issued shares as of December 31, 2010 were recorded as accrued compensation. The Company issued 52,334 common shares to the director on October 25, 2010 at the fair market value of $7.81. The issuance includes the 47,334 shares owed as of December 31, 2009, and 5,000 shares vested during the first two quarters of 2010.
On May 26, 2009, the Company renewed the one-year service agreement with the former CFO and agreed to issue 14,440 shares of common stock, which would vest in four equal installments of 3,610 shares every quarter starting August 5, 2009. Compensation expense is recognized on the straight-line method over the vesting period. On February 26, 2010, 10,830 shares were issued at the fair market value of $8.57. Effective April 16, 2010, the former CFO resigned from his position and the last installment of 3,610 shares was prorated to 2,880 shares. The Company issued the 2,880 common shares to the former CFO on October 25, 2010 at the fair market value of $7.81 per share. Total compensation expense of $25,781 and $32,490 were charged to general and administrative expenses for the years ended December 31, 2010 and 2009, respectively.
On May 26, 2009, the Company agreed to issue 5,556 shares of common stock to a director at the beginning of each term of his directorship. The trading value of the common stock on May 26, 2009 was $4.50 per share. The total value of $0 and $25,002 was charged to general and administrative expenses for the years ended December 31, 2010 and 2009, respectively. As of December 31, 2009, the shares were not issued and the balance was included in shares to be issued. The Company issued 5,556 shares on February 26, 2010. In accordance with the agreement between the Company and this director, this director must continue to serve as a member of the Board until his successor is duly elected and qualified in order to receive the shares. This director has continued in his position and his next calendar term started on May 26, 2010. The Company is in the process of finalizing its agreement with this director and expects to complete this process shortly.
A summary of changes in the Company’s non-vested shares for the year follows:
|
Non-vested Shares
|
Weighted-Average Grant
Date Fair Value
|
|||
|
Non-vested at January 1, 2010
|
- | |||
|
Granted
|
10,000 | |||
|
Vested
|
10,000 | |||
|
Forfeited
|
- | |||
|
Non-vested at December 31, 2010
|
- | |||
As of December 31, 2010, there was $0 of total unrecognized compensation cost related to non-vested shares granted. The total fair value of shares vested during the years ended December 31, 2010 and 2009 was $129,815 and $359,864 based on the closing price of the grant date, respectively.
F-23
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Warrants and purchase options
On February 28, 2007, in connection with a financing the Company issued 195,000 warrants to four investors with an exercise price of $6.00 per share for a term of three years (number of warrants and exercise price adjusted for 1-for-10 reverse stock split on May 12, 2009 and 2-for-1 forward stock split on November 16, 2009). On the same date, the Company also issued warrants to the placement agent, exercisable for 114,100 shares of the Company’s common stock at a price of $5.00 per share for a five-year term (number of warrants and exercise price adjusted for 1-for-10reverse stock split on May 12, 2009 and 2-for-1 forward stock split on November 16, 2009). For the year ended December 31, 2009, 56,846 warrants were exercised. For the year ended December 31, 2010, there was a cashless exercise of 218,024 warrants.
In connection with the 2009 equity offering discussed below, the Company granted 140,000 common stock purchase options to five designees of the Underwriters with a vesting date of June 30, 2010. The options are exercisable from June 30, 2010 to June 30, 2014, and each option is exercisable for one share of the Company’s common stock at an exercise price at $8.11 per share. All options were provided for services performed. On June 30, 2010, the purchase options were reclassified from equity to warrant liabilities, and the Company reclassified $779,674 from additional paid in capital to derivative liability.
The fair value of each warrant and purchase option is estimated on the date of grant using the Black-Scholes option-pricing model that uses the assumptions noted in the following table. Expected volatilities are based on historical volatility of the Company’s stock, and reflect the assumption that the historical volatilities are indicative of future trends, which may not necessarily be the actual outcome. Expected term of each warrant and purchase option award represents the period of time that options granted are expected to be outstanding and is estimated based on the historical exercise behavior of separate groups of employees or officers. The risk-free rate reflects the interest rate for United States Treasury Notes with similar time-to-maturity to that of the options.
|
2010
|
2009
|
|||
|
Expected term (year)
|
1.2 – 3.5
|
0.17- 2.17
|
||
|
Expected volatility
|
51% - 180%
|
34% - 178%
|
||
|
Weighted average volatility
|
51% - 180%
|
34% - 178%
|
||
|
Expected dividend yield
|
0%
|
0%
|
||
|
Risk-free rate
|
0.3% - 1.5%
|
.04% - 1.14%
|
A summary of the Company’s warrant and purchase option activity in 2009 and 2010 follows:
|
Number of warrants/purchase options
|
Weighted –
average
exercise price
|
Weighted-
average
remaining
contractual term
Years)
|
||||||||||
|
Outstanding at January 1, 2009
|
309,100 | $ | 5.63 | |||||||||
|
Granted
|
140,000 | $ | 8.11 | |||||||||
|
Forfeited
|
- | |||||||||||
|
Exercised
|
(56,846 | ) | ||||||||||
|
Outstanding at December 31, 2009
|
392,254 | $ | 5.57 | |||||||||
|
Granted
|
- | - | ||||||||||
|
Forfeited
|
- | - | ||||||||||
|
Exercised
|
(218,024 | ) | $ | 5.72 | ||||||||
|
Outstanding at December 31, 2010
|
174,320 | $ | 7.50 | 3.04 | ||||||||
|
Vested and expected to vest at December 31, 2010
|
174,320 | $ | 7.50 | 3.04 | ||||||||
|
Exercisable at December 31, 2010
|
174,320 | $ | 7.50 | 3.04 | ||||||||
2009 Equity offering
On June 30, 2009, the Company and Rodman & Renshaw, LLC, as representative of underwriters (the "Underwriters") entered into an Underwriting Agreement. Pursuant to the Underwriting Agreement, the Company agreed to issue and sell an aggregate 3,220,000 shares (including 420,000 over-allotment shares) of its common stock at a price of $6.49 per share in a public offering. The closing date of this offering was July 3, 2009.
F-24
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Costs incurred in this offering, including underwriting commission, legal fees, and printing costs, were $1,730,477, and were directly deducted from the proceeds. The gross proceeds of this offering were $20,897,800. The Company received cash proceeds of $19,167,323 on July 6, 2009.
Equity Compensation Plan
On December 8, 2009, the Company’s board of directors approved a stock incentive plan for officers, directors, employees and consultants entitled the “Skystar Bio-Pharmaceutical Company 2010 Stock Incentive Plan” (the “2010 Plan”). The maximum number of shares that may be issued under the 2010 Plan is 700,000 shares of common stock. The 2010 Plan was approved by the Company’s stockholders on December 31, 2009, and awards may be granted thereunder until December 7, 2019. As of December 31, 2010, there are 690,000 shares of the Company’s common stock remaining available for future issuance under the Plan.
Note 16 - STATUTORY RESERVES
Statutory reserves represent restricted retained earnings. Based on the legal formation of the entities, all PRC entities are required to set aside 10% of net income as reported in their statutory accounts on an annual basis to the statutory surplus reserve fund. Once the total statutory surplus reserve reaches 50% of the entity’s registered capital, further appropriations are discretionary. The statutory surplus reserve can be used to increase the entity’s registered capital (upon approval by relevant government authorities) and eliminate its future losses under PRC GAAP (upon a resolution by the board of directors). The statutory surplus reserve is not distributable to shareholders except in the event of liquidation. As of December 31, 2010, Xi’an Tianxing has met the statutory surplus reserve requirement, and approximately $10,418,157 still needs to be transferred to the statutory surplus reserve from other Chinese subsidiaries.
The reserve fund can be used to increase the registered capital upon approval by relevant government authorities and eliminate future losses of the respective companies upon a resolution by the board of directors.
Appropriations to the above statutory reserves are accounted for as a transfer from unrestricted earnings to statutory reserves. There are no legal requirements in the PRC to fund these statutory reserves by the transfer of cash to any restricted accounts, and as such, the Company has not transferred any cash to these accounts. These reserves are not distributable as cash dividends.
Note 17 – TAXES
Skystar is subject to United States federal income tax provisions. Skystar Cayman is a tax-exempt company incorporated in the Cayman Islands and conducts all of its business through its subsidiaries, Fortune Time, Sida, Fortune Time’s subsidiary Skystar Kunshan, Sida’s subsidiary Skystar Jingzhou, and Sida’s PRC VIEs, Xi’an Tianxing, and Shanghai Siqiang.
Sida, Skystar Jingzhou, Skystar Kunshan, Xi’an Tianxing, and Shanghai Siqiang are subject to the PRC’s Enterprise Income Tax. Pursuant to the PRC Income Tax Laws, Enterprise Income Tax is generally imposed at a statutory rate of 25%. Xi’an Tianxing has been approved as a new technology enterprise, and under PRC Income Tax Laws is entitled to a preferential tax rate of 15%.
F-25
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
The following table reconciles the U.S. statutory rates to the Company's effective tax rate for the year ended December 31, 2010 and 2009:
|
For the year ended
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
U.S. Statutory rate
|
34.0 | % | 34.0 | % | ||||
|
Foreign income not recognized in the U.S.
|
(34.0 | ) | (34.0 | ) | ||||
|
China income tax rate
|
25.0 | 25.0 | ||||||
|
China income tax exemption
|
(10.0 | ) | (10.0 | ) | ||||
|
Other item (1)
|
3.6 | 3.7 | ||||||
|
Total provision for income taxes
|
18.6 | % | 18.7 | % | ||||
|
(1)
|
Other item is for operating expenses incurred by Skystar that are not deductible in the PRC and expenses incurred by other subsidiaries that are not deductible on the consolidated level, which resulted in an increase in effective tax rate 3.6% and 3.7% for the years ended December 31, 2010 and 2009, respectively.
|
Taxes payable consisted of the following:
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Income taxes payable
|
$ | 432,722 | $ | 104,261 | ||||
|
Value added tax
|
203,684 | 561,646 | ||||||
|
Other taxes
|
113,430 | 56,199 | ||||||
|
Total
|
$ | 749,836 | $ | 722,106 | ||||
The estimated tax savings due to the reduced tax rate for the years ended December 31, 2010 and 2009 amounted to $2,089,862 and $202,934, respectively. If the statutory income tax had been applied, the Company would have decreased basic earnings per share from $1.98 to $1.68 and decreased diluted earnings per share from $1.97 to $1.67 for the year ended December 31, 2010. For the year ended December 31, 2009, the basic earnings per share would have decreased from $1.65 to $1.61, and the diluted earnings per share would have decreased from $1.62 to $1.58, if the statutory income tax had been applied.
Skystar was incorporated in the U.S. and has incurred a net operating loss for income tax purposes for the year ended December 31, 2010. As of December 31, 2010, the estimated net operating loss carry forwards for U.S. income tax purposes amounted to $5,241,042, which may be available to reduce future years’ taxable income. These carry forwards will expire, if not utilized, beginning in 2026 and through 2030. Management believes that the realization of the benefits arising from this loss appears to be uncertain due to the Company’s limited operating history and continuing losses for U.S. income tax purposes. Accordingly, the Company has provided a 100% valuation allowance at December 31, 2010 and December 31, 2009. The valuation allowance at December 31, 2010 and 2009 was $1,781,954 and $1,553,677, respectively. The Company’s management reviews this valuation allowance periodically and makes adjustments as necessary.
The Company has cumulative undistributed earnings of foreign subsidiaries of approximately $37 million as of December 31, 2010, which were included in consolidated retained earnings and will continue to be indefinitely reinvested in international operations. Accordingly, no provision has been made for U.S. deferred taxes related to future repatriation of these earnings, nor is it practicable to estimate the amount of income taxes that would have to be provided if we concluded that such earnings will be remitted in the future.
F-26
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Note 18 - EARNINGS PER SHARE
The following is the calculation of earnings per share:
|
For the years ended
December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Net income
|
$ | 14,091,284 | $ | 8,851,932 | ||||
|
Weighted average shares used in basic computation
|
7,105,789 | 5,374,452 | ||||||
|
Dilutive effect of stock warrants and purchase options
|
32,490 | 85,076 | ||||||
|
Weighted average shares used in diluted computation
|
7,138,279 | 5,459,528 | ||||||
|
Earnings per share:
|
||||||||
|
Basic
|
$ | 1.98 | $ | 1.65 | ||||
|
Diluted
|
$ | 1.97 | $ | 1.62 | ||||
For the years ended December 31, 2010 and 2009, the average stock price was greater than the exercise prices of warrants, which resulted in additional weighted-average common stock equivalents of 32,490 and 85,076, respectively.
Note 19 - RELATED PARTY TRANSACTIONS AND ARRANGEMENTS
Amounts receivable from and payable to related parties are summarized as follows:
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Short-term loans from shareholders
|
||||||||
|
Mr. Weibing Lu – officer and shareholder (1)
|
$ | - | $ | 36,675 | ||||
|
Mr. Wei Wen – officer and shareholder (1)
|
- | 36,675 | ||||||
|
Ms. Aixia Wang – shareholder (1)
|
- | 36,675 | ||||||
|
Total
|
$ | - | $ | 110,025 | ||||
|
Shares to be issued to related party
|
||||||||
|
Scott Cramer – non-executive director (2)
|
$ | 53,050 | $ | 302,372 | ||||
|
Mark D. Chen – non-executive director(2)
|
- | 25,002 | ||||||
|
Total
|
$ | 53,050 | $ | 327,374 | ||||
|
Amounts due to related parties
|
||||||||
|
Scott Cramer – non-executive director and shareholder (3)
|
170,937 | 143,556 | ||||||
|
Officer and shareholder (3)
|
46,975 | 41,468 | ||||||
|
Total
|
$ | 217,912 | $ | 185,024 | ||||
F-27
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
(1) On June 2, 2009, Weibing Lu, Wei Wen, and Aixia Wang obtained personal loans from Yanta Credit Union and advanced cash to Xi’an Tianxing in the total amount of $110,025. These loans were due on June 1, 2010 with 10.1% interest per annum, were guaranteed by Xi’an Tianxing, and were paid in full in June 2010. For the year ended December 31, 2010, Xi’an Tianxing paid interest of $6,122 for these loans directly. For the year ended December 31, 2009, Xi’an Tianxing paid interest of $0 for these loans.
(2) As of December 31, 2010 and 2009, the Company had the obligation under an agreement to issue 5,000 and 47,334 shares of common stock to Scott Cramer as compensation valued at $53,050 and $302,372 for being a representative of the Company in the United States for the periods from April 2010 to December 31, 2010, and May 2008 to December 31, 2009, respectively. A total of 52,334 shares, including 47,334 shares accrued in 2009 and 5,000 shares accrued in the first two quarters of 2010 were issued on October 25, 2010. A total of 5,000 shares were vested in the second half of 2010 and remain un-issued as of December 31, 2010 were recorded as deferred compensation.
In addition, as of December 31, 2009, the Company had the obligation under an agreement to issue 5,556 shares of common stock, respectively, to Mark D. Chen as compensation valued at $25,002 at the beginning of each term of his directorship. The Company issued 5,556 shares on February 26, 2010.
Effective April 16, 2010, Bennet Tchaikovsky resigned as our chief financial officer, and the last installment of 3,610 shares of common stock owed to him was prorated to 2,880 shares. These shares were issued on October 25, 2010.
(3) Shaanxi Xinji Electronics Co., Ltd. is owned by the wife of Weibing Lu. The amounts due to Shaanxi Xinji Electronics as of December 31, 2010 and December 31, 2009 were short-term cash transfers for business operations, non-interest bearing, unsecured, and payable upon demand. As of December 31, 2010 and 2009, the Company also had unpaid reimbursement and compensation due to Scott Cramer. In addition, Mr. Wen Wei advanced money from the company for travel expenses totaling $16,481 as of December 31, 2010.
Note 20 - COMMITMENTS AND CONTINGENCIES
(a) Lease commitments
The Company recognizes lease expense on the straight-line method over the term of the lease. The Company entered into a tenancy agreement for the lease of factory premises for a period of ten years from October 1, 2004 to December 31, 2014. The annual rent for the factory premises has been adjusted to about $17,000 (RMB 116,000) and subject to a 10% increase every two years starting October 1, 2009.
The Company leases office space from Weibing Lu, the Company’s chief executive officer, for a period of five years from January 1, 2007 to December 31, 2011, with annual rent of approximately $24,790 (RMB 165,600). The Company also entered into a tenancy agreement with Mr. Lu for the lease of Shanghai Siqiang’s office for a period of ten years from August 1, 2007 to August 1, 2017, with annual rent of approximately $21,560 (RMB 144,000).
The Company entered into a tenancy agreement for the lease of an office space in California for a period of three years from July 1, 2009 to July 1, 2012 with monthly rent of $1,100. The Company dissolved the California entity in December 2010. There are no future lease liabilities related to this office.
The Company entered into a one-year tenancy agreement for leasing Tianxing’s sales office space in Tianjin from April 21, 2010 to April 20, 2011 with annual rent of approximately $3,600 (RMB 24,000).
F-28
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
The minimum future lease payments for the next five years and thereafter are as follows:
|
Period
|
Amount
|
|||
|
Year ending December 31, 2011
|
$ | 66,596 | ||
|
Year ending December 31, 2012
|
39,442 | |||
|
Year ending December 31, 2013
|
39,442 | |||
|
Year ending December 31, 2014
|
35,043 | |||
|
Year ending December 31, 2015 and thereafter
|
56,432 | |||
|
Total
|
$ | 236,955 | ||
Rental expense for the years ended December 31, 2010 and 2009 amounted to $62,066 and $63,660, respectively.
(b) Legal proceedings
From time to time, the Company is involved in legal matters arising in the ordinary course of business. Management currently is not aware of any legal matters or pending litigation which would have a significant effect on the Company’s consolidated financial statements as of December 31, 2010.
In May 2007, Andrew Chien filed suit against the Company, Scott Cramer, Steve Lowe, David Wassung and Weibing Lu in United States District Court for the District of Connecticut, alleging causes of action for violation of Sections 10(b) and 20(a) of the Exchange Act. On July 17, 2008, in a decision that is now published, the Court granted defendants' motion to dismiss and subsequently dismissed the lawsuit, entering judgment on behalf of the defendants. Mr. Chien filed a notice of appeal of the Court's dismissal of his lawsuit, opposed by the defendants, which remains pending. Additionally, on February 5, 2009, the Court issued a ruling on defendants' motion for sanctions, finding the action filed by Mr. Chien to have been entirely frivolous, and to have constituted a "substantial" violation of Federal Rule of Civil Procedure Rule 11, and imposed significant monetary sanctions on both Mr. Chien and his former attorney. As part of the basis for imposing sanctions on Mr. Chien personally, the Court specifically found that Mr. Chien had knowledge of facts directly contradicting the allegations of his complaint, as evident in internet postings he made on online message boards. Mr. Chien subsequently filed motions seeking to "re-open" this case, and to recuse the judge, but both motions were denied. A Notice of Appeal concerning the ruling awarding sanctions against him was also filed by Mr. Chien. All appeals, including the one referenced below concerning Mr. Chien's second lawsuit, were subsequently consolidated. On May 26, 2010, the court of appeals for the Second Circuit upheld Judge Kravtiz’s ruling against Mr. Chien.
Subsequently, Mr. Chien, proceeding pro se (i.e., meaning he represented himself without an attorney), filed another lawsuit against the Company, Scott Cramer, Steve Lowe, David Wassung, and Weibing Lu in Connecticut Superior Court, alleging causes of action similar to those alleged in his federal complaint described above as well as state law causes of action. The case was removed to the U.S. District Court, District of Connecticut, and assigned to the same Judge who dismissed Mr. Chien’s related federal action. On June 8, 2009, the Court granted defendants’ motion to dismiss this action in its entirety, and denied Mr. Chien’s motion to further amend his complaint. Mr. Chien filed a Notice of Appeal concerning the ruling dismissing this lawsuit, which has been consolidated with Mr. Chien’s appeal of his other lawsuit. On May 26, 2010, the court of appeals for the Second Circuit upheld Judge Kravtiz’s ruling against Mr. Chien.
Subsequently, The Court of Appeals issued a Mandate upholding the decision granting defendant’s motion to dismiss and found that the District Court did not “abuse its discretion” in issuing moderate sanctions against Chien in light of the circumstances and facts on record. This Mandate was entered on November 8, 2010. On January 22, 2011, Chien filed a petition with the Supreme Court of the United States, appealing the lower court’s ruling. Appeals to the U.S. Supreme Court far exceed the number of cases it actually hears, and a hearing is not mandatory. As of this date we have not received notice of further action by the Supreme Court.
F-29
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
Other than the above described legal proceedings, the Company is not aware of any legal matters in which any director, officer, or any owner of record or beneficial owner of more than five percent of any class of voting securities of the Company, or any affiliate of purchaser, or of any such director, officer, affiliate of the Company, or security holder, is a party adverse to the Company or has a material adverse interest to the Company. No provision has been made in the consolidated financial statements for the above contingencies.
(c) Ownership of leasehold property
In 2005, a shareholder contributed a leasehold office building as additional capital of Xi’an Tianxing. However, as of December 31, 2010, title to this leasehold property has not passed to the Company. The Company does not believe there are any legal barriers for the shareholder to transfer the ownership to the Company. However, in the event that the Company fails to obtain the ownership certificate for the leasehold property, there is a risk that we may be required to vacate the building. Management believes that this possibility is remote, and, as such, no provision has been made in the consolidated financial statements for this potential occurrence.
(d) R&D project
During the first quarter of 2008, Xi’an Tianxing contracted with Northwestern Agricultural Technology University to jointly work on an R&D project concerning the application of nano-technology in the prevention of milk cow diseases. The total projected budget for this project is approximately $592,000 (RMB 4,000,000), of which, approximately $147,000 (RMB 1,000,000) was expensed when incurred in previous years, and approximately $293,000 (RMB 2,000,000) was prepaid in 2008. During year ended December 31, 2010, the Company incurred $369,850 (RMB 2,500,000) of expenses relating to this project. The project is still in the trial stage, and we expect to obtain a veterinary permit for the new product from the PRC government in June 2011.
During the year ended December 31, 2009, Xi’an Tianxing contracted with the Fourth Military Medical University to jointly work on an R&D project with a contracted amount of approximated $880,200 (RMB 6,000,000). The project is expected to complete in September 2014. The Company paid $411,880(RMB 2,800,000) in the fourth quarter of 2009. The remaining RMB 3,200,000 will be paid from 2012 to2014.
(e) Registered capital commitment
Skystar Kunshan’s remaining registered capital of $12,750,000 is required to be invested by May 7, 2012. The prepayments the Company has been making in relation to the Kunshan acquisition will be used to satisfy the registered capital requirement upon the completion of the Kunshan acquisition. Skystar Jingzhou has remaining registered capital of $377,000 (RMB 2,520,000).
Note 21 - RESTRICTED NET ASSETS
Schedule I of Article 5-04 of Regulation S-X requires the condensed financial information of registrant (Parent Company) shall be filed when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end of the most recently completed fiscal year. For purposes of this test, restricted net assets of consolidated subsidiaries shall mean that amount of the registrant’s proportionate share of net assets of consolidated subsidiaries (after intercompany eliminations) which as of the end of the most recent fiscal year may not be transferred to the parent company by subsidiaries in the form of loans, advances or cash dividends without the consent of a third party (i.e., lender, regulatory agency, foreign government, etc.).
F-30
SKYSTAR BIO-PHARMACEUTICAL COMPANY AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2010
The condensed parent company financial statements have been prepared in accordance with Rule 12-04, Schedule I of Regulation S-X as the restricted net assets of the subsidiaries of Skystar Bio-Pharmaceutical Company exceed 25% of the consolidated net assets of Skystar Bio-Pharmaceutical Company. The ability of our Chinese operating affiliates to pay dividends may be restricted due to the foreign exchange control policies and availability of cash balances of the Chinese operating subsidiaries. Because a significant portion of our operations and revenues are conducted and generated in China, all of our revenues being earned and currency received are denominated in Renminbi (RMB). RMB is subject to the exchange control regulation in China, and, as a result, we may be unable to distribute any dividends outside of China due to PRC exchange control regulations that restrict our ability to convert RMB into US Dollars.
F-31
SCHEDULE I – CONDENSED FINANCIAL INFORMATION OF REGISTRANT
SKYSTAR BIO-PHARMACEUTICAL COMPANY
PARENT COMPANY STATEMENTS OF OPERATIONS
|
Years ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
REVENUE, net
|
$ | - | $ | - | ||||
|
OPERATING EXPENSES:
|
||||||||
|
Salaries and Consulting Expenses
|
519,034 | 608,704 | ||||||
|
General and administrative
|
1,131,339 | 1,147,969 | ||||||
|
Total operating expenses
|
1,650,373 | 1,756,673 | ||||||
|
LOSS FROM OPERATIONS
|
(1,650,373 | ) | (1,756,673 | ) | ||||
|
OTHER INCOME (EXPENSE):
|
||||||||
|
Interest income
|
375 | 9,815 | ||||||
|
Change in fair value of warrants
|
(612,883 | ) | (868,445 | ) | ||||
|
Total other expense, net
|
(612,508 | ) | (858,630 | ) | ||||
|
LOSS ATTRIBUTABLE TO PARENT ONLY
|
(2,262,881 | ) | (2,615,303 | ) | ||||
|
EQUITY IN EARNINGS OF SUBSIDIARIES
|
16,354,165 | 11,467,235 | ||||||
|
NET INCOME AVAILABLE TO SHAREHOLDERS
|
$ | 14,091,284 | $ | 8,851,932 | ||||
F-32
SKYSTAR BIO-PHARMACEUTICAL COMPANY
PARENT COMPANY BALANCE SHEETS
|
December 31,
|
December 31,
|
|||||||
|
2010
|
2009
|
|||||||
|
ASSETS
|
||||||||
|
CURRENT ASSETS:
|
||||||||
|
Cash
|
$ | 224,372 | $ | 3,583,292 | ||||
|
Prepaid expenses
|
- | 193,177 | ||||||
|
Total current assets
|
224,372 | 3,776,469 | ||||||
|
Investment in and advances to subsidiaries
|
69,116,404 | 44,482,490 | ||||||
|
Total assets
|
$ | 69,340,776 | $ | 48,258,959 | ||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
||||||||
|
CURRENT LIABILITIES:
|
||||||||
|
Salary and consulting fees payable
|
$ | 162,858 | $ | - | ||||
|
Accrued expenses
|
158,677 | 99,217 | ||||||
|
Other payable
|
170,937 | 470,930 | ||||||
|
Total current liabilities
|
492,472 | 570,147 | ||||||
|
OTHER LIABILITIES:
|
||||||||
|
Warrant liability
|
1,419,639 | 1,538,686 | ||||||
|
Total liabilities
|
1,912,111 | 2,108,833 | ||||||
|
COMMITMENTS AND CONTINGENCIES
|
||||||||
|
SHAREHOLDERS' EQUITY
|
67,428,665 | 46,150,126 | ||||||
|
Total liabilities and shareholders' equity
|
$ | 69,340,776 | $ | 48,258,959 | ||||
F-33
SKYSTAR BIO-PHARMACEUTICAL COMPANY
PARENT COMPANY STATEMENTS OF CASH FLOWS
|
Years ended December 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||
|
Net income available to shareholders
|
$ | 14,091,284 | $ | 8,851,932 | ||||
|
Adjustments to reconcile net income to net cash used in operating activities:
|
||||||||
| Equity in earnings of subsidiaries | (16,354,165 | ) | (11,467,235 | ) | ||||
|
Common stock issued for services
|
475,525 | 63,014 | ||||||
|
Common stock to be issued to related parties for compensation
|
- | 232,170 | ||||||
|
Change in fair value of warrant liability & equity accounts
|
612,883 | 868,445 | ||||||
|
Change in operating assets and liabilities
|
||||||||
|
Prepaid expenses
|
193,177 | (193,177 | ) | |||||
|
Salary and consulting fee payable
|
162,858 | - | ||||||
|
Accrued expenses
|
59,460 | 787,551 | ||||||
|
Other payables
|
(299,993 | ) | (275,747 | ) | ||||
|
Net cash used by operating activities
|
(1,058,971 | ) | (1,133,047 | ) | ||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||
|
Investment in subsidiaries
|
(2,299,949 | ) | (13,560,773 | ) | ||||
|
Net cash used in investing activities
|
(2,299,949 | ) | (13,560,773 | ) | ||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||
|
Proceeds from equity offering
|
- | 18,411,496 | ||||||
|
Due to (from) related parties
|
- | (137,874 | ) | |||||
|
Net cash provided by financing activities
|
- | 18,273,622 | ||||||
|
INCREASE (DECREASE) IN CASH
|
(3,358,920 | ) | 3,579,802 | |||||
|
CASH, beginning of year
|
3,583,292 | 3,490 | ||||||
|
CASH, end of year
|
$ | 224,372 | 3,583,292 | |||||
The condensed parent company financial statements have been prepared using the equity method to account for its subsidiaries. Refer to the consolidated financial statements and notes presented above for additional information and disclosures with respect to these financial statements.
F-34
