Attached files
| file | filename |
|---|---|
| EX-3.2 - EXHIBIT 3.2 - UNIGENE LABORATORIES INC | ex3-2.htm |
| EX-23.1 - EXHIBIT 23.1 - UNIGENE LABORATORIES INC | ex23-1.htm |
| EX-32.2 - EXHIBIT 32.2 - UNIGENE LABORATORIES INC | ex32-2.htm |
| EX-23.2 - EXHIBIT 23.2 - UNIGENE LABORATORIES INC | ex23-2.htm |
| EX-10.8 - EXHIBIT 10.8 - UNIGENE LABORATORIES INC | ex10-8.htm |
| EX-31.2 - EXHIBIT 31.2 - UNIGENE LABORATORIES INC | ex31-2.htm |
| EX-32.1 - EXHIBIT 32.1 - UNIGENE LABORATORIES INC | ex32-1.htm |
| EX-31.1 - EXHIBIT 31.1 - UNIGENE LABORATORIES INC | ex31-1.htm |
| EX-10.67 - EXHIBIT 10.67 - UNIGENE LABORATORIES INC | ex10-67.htm |
| EX-10.66 - EXHIBIT 10.66 - UNIGENE LABORATORIES INC | ex10-66.htm |
| EX-10.38 - EXHIBIT 10.38 - UNIGENE LABORATORIES INC | ex10-38.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the Fiscal Year Ended December 31, 2010
OR
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
For the transition period from to .
Commission file number 0-16005
UNIGENE LABORATORIES, INC.
(Exact name of registrant as specified in its charter)
|
Delaware
|
22-2328609
|
|
|
(State or Other Jurisdiction of Incorporation or Organization)
|
(I.R.S. Employer Identification No.)
|
81 Fulton Street
Boonton, New Jersey 07005
(973) 265-1100
(Address and telephone number of principal executive offices)
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act:
Title of class:
Common Stock, $.01 Par Value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨.
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes £ No £
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer
|
o |
Accelerated filer
|
x |
|
Non-accelerated filer
|
o |
Smaller reporting company
|
o |
|
(Do not check if a smaller reporting company)
|
|||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x.
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked prices of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter. The aggregate market value as of June 30, 2010 was approximately $62,063,000.
APPLICABLE ONLY TO CORPORATE REGISTRANTS:
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date: Common Stock, $.01 Par Value, 92,540,982 shares as of March 2, 2011.
DOCUMENTS INCORPORATED BY REFERENCE
Information required by Part III (Items 10, 11, 12, 13 and 14) is incorporated by reference from portions of Unigene’s definitive proxy statement for its 2011 Annual Meeting of Stockholders, which will be filed with the Securities and Exchange Commission within 120 days of December 31, 2010.
1 of 59
Table of Contents
| Page Number | ||
|
PART I
|
||
|
ITEM 1.
|
BUSINESS
|
3 |
|
ITEM 1A.
|
RISK FACTORS
|
9 |
|
ITEM 1B.
|
UNRESOLVED STAFF COMMENTS
|
13 |
|
ITEM 2.
|
PROPERTIES
|
13 |
|
ITEM 3.
|
LEGAL PROCEEDINGS
|
14 |
|
ITEM 4.
|
(REMOVED AND RESERVED)
|
14 |
|
PART II
|
||
|
ITEM 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
|
14 |
|
ITEM 6.
|
SELECTED FINANCIAL DATA
|
15 |
|
ITEM 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
15 |
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
|
22 |
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
|
26 |
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
|
50 |
|
ITEM 9A.
|
CONTROLS AND PROCEDURES
|
50 |
|
ITEM 9B.
|
OTHER INFORMATION
|
52 |
| 2 | ||
|
PART III
|
||
|
ITEM 10.
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
|
52 |
|
ITEM 11.
|
EXECUTIVE COMPENSATION
|
52 |
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
|
52 |
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
|
52 |
|
ITEM 14.
|
PRINCIPAL ACCOUNTING FEES AND SERVICES
|
52 |
|
PART IV
|
||
|
ITEM 15.
|
EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
52 |
|
SIGNATURES
|
2 of 59
PART I
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Various statements in the items captioned “Business,” “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in this Form 10-K constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 (the “Reform Act”). These forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or activities of our business, or industry results, to be materially different from any future results, performance or activities expressed or implied by the forward-looking statements. These factors include: general economic and business conditions, our financial condition, product sales and royalties, competition, our dependence on other companies to commercialize, manufacture and sell products using our technologies, the uncertainty of results of animal and human testing, the risk of product liability and liability for human trials, our dependence on patents and other proprietary rights, dependence on key management officials, the availability and cost of capital, the availability of qualified personnel, changes in, or the failure to comply with, governmental regulations, the failure to obtain regulatory approvals for our products, litigation, and other factors discussed in this Form 10-K.
|
Item 1.
|
Business.
|
Overview
Unigene Laboratories, Inc., a biopharmaceutical company, was incorporated in the State of Delaware in 1980. Our single business segment focuses on the research, production and delivery of small proteins, referred to as peptides, for medical use. We have a patented manufacturing technology for producing many peptides cost-effectively. We also have patented oral and nasal delivery technologies that have been shown to deliver medically useful amounts of various peptides into the bloodstream. We have three operating locations: administrative and regulatory offices in Boonton, New Jersey; a laboratory research facility in Fairfield, New Jersey; and a pharmaceutical production facility in Boonton, New Jersey.
In March 2010, the Company entered into an amended and restated financing agreement for $33,000,000 three-year convertible senior secured term notes. Unigene issued $33,000,000 of new convertible senior secured notes due in 2013, in exchange for approximately $19,358,000 of existing non-convertible senior secured term notes which were due in 2011 and the payment to the Company of approximately $13,642,000 in cash at the closing, minus fees related to the restructuring. An entity managed by Victory Park Capital Advisors, LLC was the sole investor in the transaction. Richard P. Levy of Victory Park Capital was appointed Chairman of the Board in connection with the restructuring. Unigene also restructured notes held by founding family members and initiated a reorganization of the management team.
In June 2010, Ashleigh Palmer was appointed President and Chief Executive Officer. In the third quarter of 2010, the Company completed a comprehensive business audit and situational analysis resulting in a new strategy for the Company, an organizational realignment with the creation of two highly focused strategic business units, Unigene Biotechnologies and Unigene Therapeutics, and the appointment of a new executive leadership team.
Strategy
Our new three-step strategic intent is focused on increasing available cash for operations by prudent cash conservancy and incremental revenue generation; as resources permit, retiring debt and selectively investing in the advancement of existing core development programs; and maximizing shareholder value by exploiting the full potential of the Company's "Peptelligence(TM)" technology platform.
We are pursuing this strategic intent and, for marketing purposes only, we have focused our resources into two strategic business units.
Peptides are a rapidly growing therapeutic class with more than 130 programs currently in active development throughout the industry. To date, more than fifty peptide-based therapeutics have reached the market. Our goal is to see our Peptelligence(TM) platform, which represents a distinctive set of capabilities and assets, incorporated into as many peptide programs as possible as they advance through development towards commercialization.
Our Peptelligence(TM) encompasses extensive intellectual property covering drug delivery and manufacturing technologies, peptide research and development expertise, and proprietary know-how. Our core Peptelligence(TM) assets include proprietary oral and nasal peptide drug delivery technologies, and proprietary, high-yield, scalable and reproducible recombinant manufacturing technologies.
We are focusing our Unigene Therapeutics pipeline on metabolic and inflammatory diseases with significant unmet medical and socioeconomic needs. Programs which do not fit our core focus will be monetized, out-licensed or terminated. We also intend to focus our Unigene Biotechnologies business unit on generating near-term fee-for-service revenues and longer-term milestone payments and royalties by customizing delivery and manufacturing solutions for novel therapeutic peptides.
The Company's first product to market, Fortical(R), a nasal calcitonin product, received approval from the Food and Drug Administration (FDA) in 2005 and is marketed in the United States by Upsher-Smith Laboratories, Inc. (USL) for the treatment of postmenopausal osteoporosis. Other clinical programs include oral calcitonin licensed to Tarsa Therapeutics, Inc. (Tarsa), which recently completed Phase III testing for the treatment of osteoporosis, and oral parathyroid hormone (PTH), licensed to GlaxoSmithKline (GSK) and entering Phase II clinical studies for the treatment of osteoporosis. In addition, Unigene has a manufacturing license agreement with Novartis Pharma AG (Novartis), which is currently completing three Phase III studies of oral calcitonin for the treatment of osteoporosis and osteoarthritis.
Unigene Biotechnologies Business Unit
The Company is expanding its Peptelligence(TM) platform of peptide oral drug delivery and manufacturing assets and capabilities with the goal of establishing a portfolio of partnered opportunities. We plan to generate near-term revenue from feasibility studies and service fees and establish a solid foundation for potential high-value milestones and royalties. We hope to apply our Peptelligence platform to as many therapeutic peptide programs as possible and help our partners co-develop those peptides through advanced clinical testing and commercialization.
3 of 59
Unigene Therapeutics Business Unit
Unigene Therapeutics constitutes the Company's own pipeline of proprietary peptide development programs focused on metabolic disease and inflammation. Currently, we are concentrating our peptide development expertise on advancing the Company's PTH program for the treatment of osteoporosis and the development of our lead proprietary preclinical anorexigenic peptide UGP281 for obesity.
In 2009, Unigene licensed its late-stage oral calcitonin formulation to Tarsa, a venture-financed company founded exclusively to conduct Phase III clinical testing and prepare Unigene’s proprietary oral calcitonin formulation for commercialization. Unigene currently owns a 26% stake in Tarsa, subject to possible future dilution. During the third quarter of 2010, Tarsa announced it had completed patient enrollment for its multinational, randomized, double-blind, placebo-controlled Phase III ORACAL trial in postmenopausal women with osteoporosis and that an independent Data Monitoring Committee had conducted two separate safety reviews of patient data and recommended the trial proceed as planned. Tarsa has announced that it expects to report Phase III study top-line results in the second quarter of 2011 and submit registration filings with the FDA and European Medicines Agency (EMA) in the second half of 2011.
In December 2010, the Company entered into an amended and restated exclusive worldwide license agreement with GSK to develop and commercialize an oral formulation of a recombinantly produced parathyroid hormone analog for the treatment of osteoporosis in postmenopausal women. Under the terms of the amended and restated agreement, Unigene will conduct the Phase II study. In addition to the remaining milestone payments of up to $142,000,000 from the original agreement that we are still eligible to receive based upon the achievement of regulatory and commercialization milestones, in December 2010 we received an upfront payment of $4,000,000 to pay for Phase II costs. We are also eligible to receive an additional $4,000,000 milestone payment upon completion of Phase II patient enrollment. In addition, we could be eligible to receive royalties at a rate in the low-to-mid teens based on GSK's global sales of the product, subject to a reduction under certain circumstances as described in the agreement. Once the Phase II study has been completed, and based on a review of the data, GSK may elect to assume responsibility for all future development and commercialization of the product.
We plan to initiate the Phase II oral PTH study and commence patient enrollment in the first quarter of 2011. This multicenter, double blind, randomized, repeat dose placebo controlled study will include an open label comparator arm and will evaluate approximately 90 postmenopausal osteoporotic women for a period of 24 weeks. The primary endpoint will be an increase in bone mineral density in subjects at 24 weeks compared to baseline. Patient enrollment for this study is expected to be completed in the first half of 2011 in which case top-line results are expected by year end.
Our satiety program is in advanced pre-clinical development. In February 2011, we announced our plans to accelerate the development of our lead proprietary anorexigenic peptide, UGP281. An anorexigenic peptide is one that diminishes or controls appetite and offers potential therapeutic benefit to morbidly obese patients. We expect to file an Investigational New Drug (IND) application with the FDA and initiate Phase I clinical studies in the first half of 2012. Additionally, we are also currently exploring the opportunity to license a pharmacologically distinct sister analog to a veterinary partner for companion animal obesity to help subsidize a portion of the human proof of concept development costs of UGP281.
The Company's Annexin 1 analog and Site Directed Bone Growth (SDBG) programs have been put on hold to conserve cash in the near term. We are currently evaluating strategic opportunities with our SDBG program, including out-licensing or divesting this asset.
Scientific Accomplishments
Among our major accomplishments are:
|
•
|
Development, Licensing and Commercialization of Proprietary Technology for Nasal Delivery. We have developed and patented Fortical, our nasal calcitonin formulation that, in human studies, demonstrated similar blood levels to a competitor’s existing nasal calcitonin product and also demonstrated a comparable decrease in bone loss as measured by several industry-accepted blood markers and a comparable increase in bone mineral density. During 2002, we licensed U.S. rights to our nasal calcitonin product, Fortical, to USL. We filed a new drug application, or NDA, for Fortical in March 2003 and Fortical was approved by the FDA in August 2005. This was our first product approval in the United States. The launch of Fortical has resulted in sales to, and royalty payments from, USL.
|
|
•
|
Development and Licensing of a Proprietary Peptide Production Process. One of our principal scientific accomplishments is our success in developing a highly efficient biotechnology-based peptide production process. Several patents relating to this process have issued. We believe that this proprietary process is a key step in the more efficient and economical commercial production of medically-useful peptides. Many of these peptides cannot be produced at a reasonable cost in sufficient quantities for human testing or commercial use by other currently available production processes. We have constructed and are operating a manufacturing facility employing this process which is capable of producing PTH and calcitonin, and we have also produced laboratory-scale quantities of several other peptides. In 2004, we licensed worldwide rights to our patented manufacturing technology for the production of calcitonin to Novartis.
|
4 of 59
|
•
|
Development and Licensing of Proprietary Technology for Oral Delivery. We have licensed worldwide rights to our manufacturing and delivery technologies for PTH for the treatment of osteoporosis to GSK. We have developed and patented an oral formulation that has successfully delivered therapeutically relevant quantities of calcitonin and PTH into the bloodstream of human subjects in studies performed by Unigene and our collaborators. We believe that the components of our patented oral product also can enable the delivery of other peptides and we have initiated studies to investigate this possibility internally and in collaboration with others. During 2001, we reported successful oral delivery in animal studies of various peptides including PTH for the treatment of osteoporosis and insulin for the treatment of diabetes. During 2002, we licensed rights to our manufacturing and delivery technologies for oral PTH to GSK. During 2004, GSK successfully completed a Phase I study for oral PTH. During 2007, a small, short-term human study for oral calcitonin was initiated by us and successfully concluded. In February 2008, we initiated a Phase I/II clinical study for oral calcitonin. The tablets used in these studies utilized an improved solid dosage form of Unigene’s oral delivery technology for which a patent application has been filed A small, short-term Phase I human study for PTH was initiated in 2008 and successfully concluded in 2009 using our improved oral delivery technology. During 2009, we initiated a Phase III human study for our oral calcitonin technology and licensed this program to Tarsa. This study was fully enrolled in the first quarter of 2010 and completed in the first quarter of 2011.
|
Partnerships
|
•
|
Upsher-Smith Laboratories License Agreement. In November 2002, we signed an exclusive U.S. licensing agreement with USL for a value before royalties of $10,000,000 to market Fortical, our patented nasal formulation of calcitonin for the treatment of osteoporosis. Fortical was approved by the FDA and launched by USL in August 2005. We received the $10,000,000 from 2002 through 2005. We are responsible for manufacturing the product and USL packages the product and distributes it nationwide. Royalty revenue, computed in a range from the transfer price of product to USL to a royalty rate in the mid-thirties depending on the circumstances, is earned on net sales of Fortical by USL and is recognized in the period Fortical is sold by USL. Future sales and royalties are contingent upon many factors including competition, pricing, marketing and acceptance in the market place and, therefore, are difficult to predict. In July 2006, we and USL jointly filed a lawsuit against Apotex for infringement of our Fortical patent. We are seeking a ruling that Apotex’s ANDA and its nasal calcitonin product infringe our Fortical patent and its ANDA should not be approved before the expiration date of the Fortical patent. In the event that we do not prevail, then Apotex could be in a position to market its nasal calcitonin product if and when its pending ANDA receives FDA approval. In December 2008, Apotex and Sandoz launched nasal calcitonin products which are generic to Novartis’ nasal calcitonin product, but not to Fortical. In June 2009, Par also launched a product generic to Novartis’ nasal calcitonin product. Certain providers have substituted these products for Fortical, causing Fortical sales and royalties to decrease. Our agreement with USL is subject to certain termination provisions. We are currently dependent on USL to market and distribute Fortical and to provide a very significant portion of our revenue. The loss of USL as a licensee would have a material adverse effect on our business (see Note 19 to the audited financial statements and Item 3. Legal Proceedings).
|
|
•
|
Tarsa License Agreement. In October 2009, we licensed our Phase III oral calcitonin program to Tarsa, a new company formed by a syndicate of three venture capital funds specializing in the life sciences: MVM Life Science Partners; Quaker BioVentures; and Novo A/S. Simultaneously, Tarsa announced the closing of a $24 million Series A financing from the investor syndicate. In consideration for our sale to Tarsa of an exclusive license for the oral calcitonin program, we received from Tarsa approximately $8,993,000 in cash and 9,215,000 shares of common stock in Tarsa (which currently represents a 26% ownership, subject to possible future dilution). We are also eligible to receive milestone payments based on the achievement of certain sales benchmarks, as well as royalties, at rates in the single digits, on product sales. Tarsa will be responsible for the future costs of the global Phase III clinical program that was initiated in 2009. Tarsa shall pay us for any services, if requested and agreed upon by us. This agreement is subject to certain termination provisions. We may terminate this agreement in the event of Tarsa’s insolvency, material breach or in certain circumstances where Tarsa has ceased development and commercialization activities of the licensed product. Tarsa may terminate this agreement in the event of our insolvency, material breach or, in certain circumstances, without cause. (See Notes 10 and 19 to the audited financial statements).
|
5 of 59
|
•
|
GlaxoSmithKline License Agreement. In April 2002, we signed a licensing agreement with GSK for a value before royalties, PTH sales and reimbursement for development expenses, of up to $150,000,000 to develop an oral formulation of an analog of PTH currently in clinical development for the treatment of osteoporosis. In December 2010, we and GSK amended and restated this licensing agreement. Under the terms of the agreement, GSK received an exclusive worldwide license to develop and commercialize the product. GSK will reimburse us for certain development activities during the program, including our activities in the production of raw material for development and clinical supplies, and will pay us a royalty at a rate in the low to mid-teens based on its worldwide sales of the product, subject to a reduction under certain circumstances as described in the agreement. The royalty rate will be increased if certain sales milestones are achieved. A Phase I human trial, which commenced in 2004, demonstrated positive preliminary results. An aggregate of $12,000,000 in up-front and milestone payments has been received from inception through December 31, 2010 including the $4,000,000 payment described below. We have also received an additional $5,000,000 from GSK for PTH sales and in support of our PTH development activities from inception through December 31, 2010. Although there were no milestones achieved or PTH sales to GSK during 2010, we received $4,000,000 from GSK for Phase II development costs in December 2010. We are eligible to receive an additional $4,000,000 upon completion of Phase II patient enrollment, which is expected in the first half of 2011, in which case top-line Phase II results are expected before year-end. Bulk product sales to licensees, prior to product approval, are unpredictable and subject to the needs of the licensee. GSK could make additional milestone payments in the aggregate amount of up to $142,000,000 subject to the progress of the compound through clinical development and through to the market. As agreed with GSK, we conducted further development including a small Phase I study, which we initiated in 2008 and successfully completed in 2009, and we will conduct the Phase II study, which is expected to begin in the first quarter of 2011. This agreement is subject to certain termination provisions. Either party may terminate the license agreement if the other party (i) materially breaches the license agreement, which breach is not cured within 60 days (or 30 days for a payment default), (ii) voluntarily files, or has served against it involuntarily, a petition in bankruptcy or insolvency, which, in the case of involuntary proceedings, remains undismissed for 60 days, or (iii) makes an assignment for the benefit of creditors. Additionally, GSK may terminate the license agreement at any time for various reasons including safety or efficacy concerns of the PTH product, significant increases in development timelines or costs, or significant changes in the osteoporosis market or in government regulations (see Note 19 to the audited financial statements).
|
|
•
|
Novartis Agreement. In April 2004, we signed a worldwide licensing agreement with Novartis for a value before royalties of up to $18,700,000 to allow Novartis to manufacture calcitonin using our patented peptide production process. Through December 31, 2010, an aggregate of $13,700,000 has been received from Novartis under this agreement, including calcitonin purchases and a $5,500,000 milestone payment that we received in 2007 for the initiation of their oral calcitonin Phase III study for osteoporosis. There were no milestones achieved or additional calcitonin purchases in 2010. Sandoz, a Novartis affiliate, concluded a manufacturing campaign in 2005 based on our process and produced multiple kilograms of calcitonin at a scale that represents a ten-fold increase above our then current production capacity. Calcitonin produced by Sandoz is being used by Novartis in its ongoing Phase III clinical trials. Novartis will be conducting all future product development and clinical trials for its oral calcitonin product in conjunction with its partner, a competitor of ours. Therefore, the anticipated completion date is outside our control and unknown to us. We will receive royalties, at rates in the single digits, on net sales of any existing or future Novartis products that contain recombinant salmon calcitonin which in the future are approved for sale by health authorities, and are manufactured by Novartis using our technology. This agreement may be terminated by either party due to a material breach not cured within 60 days or due to insolvency or bankruptcy proceedings not dismissed within 60 days and for other customary events of default (see Note 19 to the audited financial statements).
|
Other License or Distribution Arrangements. To expand our product pipeline, we periodically perform feasibility studies for third parties. We are also working on the development of our anorexigenic peptide to diminish or control appetite, which would utilize our manufacturing and oral delivery technologies. In addition, we are actively seeking additional licensing and/or supply agreements with pharmaceutical companies for our products and technologies. However, we may not be successful in achieving milestones under our current agreements, obtaining regulatory approval for our products or in licensing any of our other products.
We operate as a single business segment and our revenue for the last three years, including product sales, royalties, licensing revenue and development services, has almost entirely been pursuant to our agreements with USL, Novartis and Tarsa, all primarily U.S. sources.
Revenue from Licensees:
|
2010
|
2009
|
2008
|
||||||||||
|
USL
|
73 | % | 87 | % | 87 | % | ||||||
|
Novartis
|
12 | % | 9 | % | 8 | % | ||||||
|
Tarsa
|
9 | % | - | - | ||||||||
|
GSK
|
2 | % | 1 | % | 1 | % | ||||||
|
Other revenue
|
4 | % | 3 | % | 4 | % | ||||||
For information regarding revenue, losses and total assets, please see our Selected Financial Data in Part II, Item 6 of this report.
Competition
Our primary business activity is biotechnology research and development. Biotechnology research is highly competitive, particularly in the field of human health care. We compete with specialized biotechnology and biopharmaceutical companies, major pharmaceutical and chemical companies, universities and other non-profit research organizations, many of which can devote considerably greater financial resources than we can to research activities. We believe that one of our main competitors in the field of oral delivery of peptides is Emisphere Technologies, Inc. Novartis’ nasal calcitonin product was the main competition for Fortical until December 2008, which was when Apotex and Sandoz launched nasal calcitonin products that are generic to Novartis’ nasal calcitonin product, but not to Fortical. In June 2009, Par also launched a product generic to Novartis’ nasal calcitonin product. Certain providers have substituted these products for Fortical, causing Fortical sales and royalties to decrease. There could also be future competition from products that are directly generic to our product. In July 2006, we and USL jointly filed a lawsuit against Apotex for infringement of our Fortical patent. We are seeking a ruling that Apotex’s abbreviated NDA, or ANDA, and its nasal calcitonin product infringe our Fortical patent and its ANDA should not be approved before the expiration date of the Fortical patent. In the event that we do not prevail, then Apotex could be in a position to market its nasal calcitonin product if and when its pending ANDA receives FDA approval (see Item 3. Legal Proceedings).
6 of 59
Our calcitonin and PTH products have potential applications in the treatment of osteoporosis. A third-party has conducted studies with an analog of PTH demonstrating the ability to build new bone and has launched an injectable PTH product in the U.S. Fortical currently faces competition from large pharmaceutical companies that produce other osteoporosis products and, if any of our other products receive regulatory approval, these other products would also face competition from large pharmaceutical companies that have substantially greater financial resources, research and development staffs and facilities, and regulatory experience than we do. Major companies in the field of osteoporosis treatment include Novartis, Merck, Eli Lilly and Sanofi-Aventis. We believe that we should be able to compete with these companies due both to our partnerships with GSK, USL and Tarsa and to our patented technologies. Our success will further depend on our ability to obtain adequate funding and on developing, testing, protecting, producing and marketing products and obtaining their timely regulatory approval. However, any of our competitors could, at any time, develop products or a manufacturing process that could render our technology or products noncompetitive or obsolete.
Product Manufacture
We have been producing calcitonin since 1992. We constructed a facility that complies with current good manufacturing practice guidelines, or cGMP, for the production of calcitonin at leased premises located in Boonton, New Jersey. The facility began producing calcitonin under cGMP guidelines in 1996. The facility is capable of producing our proprietary amidating enzyme for use in producing calcitonin as well as PTH. During 2008, we implemented refinements to our manufacturing process which improved our productivity and efficiency. We believe that our facility’s current capacity is sufficient to support expected Fortical sales. The facility also contains a filling line to fill and label Fortical. Since we currently maintain an adequate inventory of calcitonin and enzyme to support Fortical for the next several years, we have temporarily suspended manufacturing of those materials at our Boonton facility. However, we have been able to maintain the cGMP status of the facility and the ability to manufacture peptides at that location. Most of the raw materials we use in our peptide production process are readily available in sufficient quantities from a number of sources. However, certain raw materials have a single supplier. For example, for Fortical production, the pumps currently used are purchased by USL from a single source.
Although our facility currently is devoted primarily to calcitonin and Fortical production, it also is suitable for producing other peptide products. The facility can be modified to increase peptide production capacity. Because we manufacture peptides for human use, the FDA, European health authorities and our licensing partners have all inspected our facility at various times. However, there is always the risk that our operations might not remain in compliance or that approval by required regulatory agencies will not be obtained.
Government Regulation
Our laboratory research, development and production activities and those of our collaborators are subject to significant regulation by numerous federal, state, local and foreign governmental authorities. FDA approval, following the successful completion of various animal and human studies, is required for the sale of a pharmaceutical product in the U.S. Foreign sales require similar studies and approval by regulatory agencies.
The regulatory approval process for a pharmaceutical product requires substantial resources and can take many years. There is always a risk that any additional regulatory approvals required for our production facility or for any of our products will not be obtained in a timely manner. Our inability to obtain, or delays in obtaining, these approvals, would adversely affect our ability to continue to fund our programs, to produce marketable products, or to receive revenue from milestone payments, product sales or royalties. We also cannot predict the extent of any adverse governmental regulation that may arise from future legislative and administrative action such as healthcare reform.
The FDA or foreign regulatory agencies may audit our production facility at any time to ensure that it is operating in compliance with cGMP guidelines. These guidelines require that production operations be conducted in strict compliance with our established rules for manufacturing and quality control. These agencies are empowered to suspend our production operations and/or product sales if, in their opinion, significant or repeated deviations from these guidelines have occurred. A suspension by any of these agencies could have a material adverse impact on our operations.
In addition, we are subject to the U.S. Foreign Corrupt Practices Act, which prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity. Our present and future business is, and will continue to be, subject to various other laws, rules and/or regulations applicable to us as a result of our international business.
Patents and Proprietary Technology
We have filed a number of applications for U.S. patents relating to our proprietary peptide manufacturing process and our delivery technologies. Our most important U.S. manufacturing and delivery patents expire from 2017 to 2025. To date, the following fifteen U.S. patents are currently in force:
|
•
|
six patents covering improvements in our manufacturing technology;
|
|
•
|
three patents covering oral delivery of peptides;
|
|
•
|
two patents covering our nasal calcitonin formulation; and
|
|
•
|
four patents covering our SDBG technology;
|
We believe that our manufacturing patents give us a competitive advantage in producing peptides cost-effectively and in large quantities, because they cover a highly efficient bacterial fermentation process for producing peptides.
7 of 59
We also believe that our oral delivery patents give us a competitive advantage in enabling us to develop peptide products in oral forms, because they cover a process allowing delivery of significant quantities of peptides into the bloodstream. Peptides are small proteins that get broken down in the digestive system. Most commercial peptide products are currently available only in injectable or nasal spray formulations.
We also believe that our nasal formulation patent gives us a competitive advantage by enabling us to deliver the desired amount of calcitonin without requiring the presence of compounds that have been shown to cause irritation to the lining of the nasal cavity.
We also believe that our SDBG patent gives us a competitive advantage by enabling us to facilitate and accelerate bone growth at precisely targeted locations in the body using a simple surgical procedure that can be performed on an outpatient basis with minimal invasiveness. Animal studies have shown that, in combination with one or more therapeutic compounds, SDBG can grow significant amounts of high quality bone.
We believe that the greatest competitive advantage of our manufacturing and oral delivery patent estates is realized through the combination of both technologies. To successfully commercialize an oral peptide product, an efficient manufacturing process is necessary because oral delivery systems are typically less efficient than injectable or nasal spray products. Reduced efficiency requires an increase in the amount of active pharmaceutical ingredient in each dose. Therefore, an efficient manufacturing process is needed to cost-effectively manufacture the increased quantities that are necessary to make a product that is commercially viable.
We believe that our manufacturing and oral delivery patent estates provide us with both a current and future advantage. Currently, the patent estates protect our intellectual property rights in the development of our manufacturing and oral delivery processes. In the future, we expect that the patent estates will give us a competitive advantage in commercializing our products.
Other patent applications are pending. We also have made filings in selected foreign countries, and fifty-eight foreign patents have issued. However, our pending applications may not issue as patents and our issued patents may not provide us with significant competitive advantages. Furthermore, our competitors may independently develop or obtain similar or superior technologies.
Although we believe our patents and patent applications are valid, the invalidity or unenforceability of one or more of our key patents could have a significant adverse effect upon our business. We are currently involved in ANDA litigation in which Apotex claims that our patent for Fortical is invalid. (See Item 3. Legal Proceedings.)
Detecting and proving infringement generally is more difficult with process patents than with product patents. In addition, a process patent’s value is diminished if others have patented the product that can be produced using the process. Under these circumstances, we would require the cooperation of, and likely be required to share royalties with, the product patent holder or its sublicensees in order to make and sell the product. In addition, the patent holder can refuse to license the product to us.
In some cases, we rely on trade secrets to protect our inventions. Our policy is to include confidentiality provisions in all research contracts, joint development agreements and consulting relationships that provide access to our trade secrets and other know-how. All of our employees, consultants and prospective partners sign confidentiality agreements. However, there is a risk that these secrecy obligations could be breached, causing us harm. To the extent licensees, consultants or other third parties apply technological information independently developed by them or by others to our projects, disputes may arise as to the ownership rights to information, which may not be resolved in our favor.
Employees
As of February 28, 2011, we had 68 full-time employees. Twenty-four were engaged in research, development and regulatory activities, 26 were engaged in production activities and 18 were engaged in general, administrative and support functions. Our employees are experts in molecular biology, including DNA cloning, synthesis, sequencing and expression; protein chemistry, including purification, amino acid analysis, synthesis and sequencing of proteins; immunology, including tissue culture, monoclonal and polyclonal antibody production and immunoassay development; chemical engineering; pharmaceutical production; quality assurance; and quality control. None of our employees is covered by a collective bargaining agreement.
Research and Development
We have established a multi-disciplinary research team to adapt proprietary amidation, biological production and oral and nasal delivery technologies to the development of proprietary products and processes. Approximately 74% of our employees are directly engaged in activities relating to production of, regulatory compliance for, and the research and development of pharmaceutical products. We spent $6.4 million on research and development activities in 2010, $12.4 million on research and development activities in 2009 ($3.3 million related to oral calcitonin), and $9.5 million in 2008.
Availability of Reports
Our Internet website address is www.unigene.com. The contents of our website are not part of this annual report on Form 10-K, and our Internet address is included in this document as an inactive textual reference only. Our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy and information statements and all amendments to those reports and statements filed by us with the Securities and Exchange Commission (the “SEC”) pursuant to Sections 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), are accessible free of charge through our website as soon as reasonably practicable after we electronically file those documents with, or otherwise furnish them to, the SEC. The SEC also maintains a website at www.sec.gov where such information is available.
8 of 59
|
Item 1A.
|
Risk Factors.
|
Our business is subject to the following risks:
We may require additional cash to sustain our operations and our ability to secure additional cash is uncertain.
We had cash flow deficits from operations of $1,669,000 for the year ended December 31, 2010, $11,291,000 for the year ended December 31, 2009 and $8,575,000 for the year ended December 31, 2008. We believe that, in the near-term, we will generate cash to apply toward funding a portion of our operations through sales of Fortical to USL and from royalties from USL sales of Fortical, as well as our anticipated receipt of a $4,000,000 milestone payment from GSK upon completion of Phase II patient enrollment. These funds may not be adequate to fully support our operations. Therefore, we may need additional sources of cash in order to maintain all of our future operations. Based upon our current level of revenue and expenses, we believe our current cash should be sufficient to support our current operations into the second half of 2012.
We may be unable to raise on acceptable terms, if at all, the substantial capital resources necessary to conduct our operations. If we are unable to raise the required capital, we may be forced to limit some or all of our research and development programs and related operations, curtail development of our product candidates and, ultimately, cease operations. Our future capital requirements will depend on many factors, including:
|
•
|
the level of Fortical sales, particularly in light of new and potential competition;
|
|
•
|
our ability to successfully defend our Fortical patent against Apotex’s ANDA;
|
|
•
|
the generation of revenue from our Tarsa, Novartis and GSK agreements;
|
|
•
|
continued scientific progress in our discovery and research programs;
|
|
•
|
progress with preclinical studies and clinical trials;
|
|
•
|
the magnitude and scope of our discovery, research and development programs;
|
|
•
|
our ability to maintain existing, and establish additional, corporate partnerships and licensing arrangements;
|
|
•
|
our partners’ ability to sell and market our products or their products utilizing our technologies;
|
|
•
|
the time and costs involved in obtaining regulatory approvals;
|
|
•
|
the time and costs involved in maintaining our production facility;
|
|
•
|
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims including our current litigation regarding Apotex’s ANDA; and
|
|
•
|
the pharmaceutical industry’s need to acquire or license new technologies and products.
|
There are numerous default provisions under our financing agreement with Victory Park.
Under our amended and restated March 2010 financing agreement with Victory Park, so long as our outstanding note balance is at least $5,000,000, we must maintain a minimum cash balance equal to at least $2,500,000 and our cash flow (as defined in the agreement) must be at least $2,000,000 in any fiscal quarter or $7,000,000 in any three consecutive quarters. These default provisions were temporarily waived under a forbearance agreement executed in December 2010. The forbearance period began December 10, 2010 and will end no later than June 30, 2012. The financing agreement specifies certain events of default including, without limitation: failure to pay principal or interest; filing for bankruptcy; breach of covenants, representations or warranties; the occurrence of a material adverse effect (as defined in the agreement); a change in control (as defined in the agreement); the failure of any registration statement required to be filed to be declared effective by the SEC, and maintained effective pursuant to the terms of our registration rights agreement with Victory Park; and any material decline or depreciation in the value or market price of the collateral. We are subject to certain cash damages, as set forth in the convertible notes, in the case of a failure to timely convert the notes and a failure to timely convert is also an event of default, subject to additional remedies. Upon any default, among other remedies, both principal and interest would be accelerated and additional charges would apply. There is no assurance that, after the forbearance period ends, we will be able to maintain a minimum cash balance of $2,500,000, or maintain an adequate cash flow, in order to avoid default. In addition, there is no assurance that the notes will be converted into common stock, in which case, we may not have sufficient cash from operations or from new financings to repay the Victory Park debt when it comes due in 2013, or that new financings will be available on favorable terms, if at all.
The conversion of the Victory Park notes will likely have a dilutive effect on our stock price and could lead to a change in control.
In March 2010, we issued to Victory Park $33,000,000 in convertible notes, which come due in 2013. As of March 17, 2011, these notes will be convertible into shares of Common Stock at the holder’s option. The initial conversion rate, which is subject to adjustment as set forth in the notes, is calculated by dividing the sum of the principal to be converted, plus all accrued and unpaid interest thereon, by $0.70 per share. If we subsequently make certain issuances of Common Stock or Common Stock equivalents at an effective purchase price less than the then-applicable conversion price, the conversion price of the notes will be reduced to such lower price. Assuming that the convertible notes are convertible in full on March 17, 2011 (with conversion of the original principal amount plus interest thereon into the conversion shares), then, together with the shares and other securities owned by them, Victory Park and its affiliates would beneficially own in the aggregate approximately 41% of our outstanding Common Stock (as diluted by outstanding options) as of that date.
9 of 59
We are required to make payments under the Levy loans, most of which are payable in June 2013.
We owe an aggregate original principal amount of $14,737,518 on notes payable to the Estate of Jean Levy and the Jaynjean Levy Family Limited Partnership. As of December 31, 2010, principal and interest on these notes aggregated $19,670,120. We may not be able to generate sufficient cash from operations or from other sources in order to make the payments when due which are required under these notes.
Our strategic realignment might not be successful.
In September 2010, we announced a realignment of our business and the creation of two new highly focused strategic business units. Our new strategic business units may not be successful or effectively implemented. Implementation of our new business strategy will require the intense involvement of our executive management team and may divert their efforts away from other important projects. Also, we may be unable to enter into mutually acceptable agreements with third party partners to use our peptide drug delivery platform and/or manufacturing capabilities. If we are unsuccessful, it could have a material adverse effect on our financial condition, results of operations and cash flow.
Our operations for future years are highly dependent on the successful marketing and sales of Fortical and could be further impacted by generic or other competition.
Fortical is our only product approved by the FDA. Any factors that adversely impact the marketing of Fortical including, but not limited to, competition, including generic competition and/or product substitutions, acceptance in the marketplace, or delays related to production and distribution or regulatory issues, will have a negative impact on our cash flow and operating results. For 2010, compared to 2009, Fortical sales decreased 12% while Fortical royalties decreased 40%. In December 2008, Apotex and Sandoz launched nasal calcitonin products which are generic to Novartis’ nasal calcitonin product, but not to Fortical. In June 2009, Par also launched a product generic to Novartis’ nasal calcitonin product. Certain providers have substituted these products for Fortical, causing Fortical sales and royalties to decrease. Further decreases in Fortical sales or royalties could have a material adverse effect on our business, financial condition and results of operations. In addition, Apotex has a pending ANDA for a nasal calcitonin product that we claim infringes our Fortical patent. We have sued Apotex for infringement of our Fortical Patent and Apotex has been enjoined from conducting any activities which would infringe the patent. Apotex has appealed that decision and if we do not prevail in this litigation, then Apotex could be in a position to market its nasal calcitonin product if and when its pending ANDA receives FDA approval.
We have significant historical losses and may continue to incur losses in the future.
We have incurred annual losses since our inception. As a result, at December 31, 2010, we had an accumulated deficit of approximately $171,000,000. Our gross revenues for the years ended December 31, 2010, 2009 and 2008 were $11,340,000, $12,792,000 and $19,229,000, respectively. Our revenues have not been sufficient to sustain our operations. Revenue for 2010, 2009 and 2008 consisted primarily of Fortical sales and royalties. As of December 31, 2010, we had four material revenue generating license agreements. We believe that to achieve profitability we will require at least the successful commercialization of one or more of our licensees’ oral or nasal calcitonin products, our oral PTH product, our obesity program or another peptide product or biotechnology in the U.S. and/or abroad. However, our products or technologies may never become commercially successful. For 2010, 2009 and 2008, we had losses from operations of $10,324,000, $12,380,000 and $4,950,000, respectively. Our net losses for the years ended December 31, 2010, 2009 and 2008 were $27,868,000, $13,380,000 and $6,078,000, respectively. We might never be profitable.
Most of our products are in early stages of development and we and our licensees may not be successful in efforts to develop a calcitonin, PTH or other peptide product that will produce revenues sufficient to sustain our operations.
Our success depends on our and our licensees’ ability to commercialize a calcitonin, PTH or other peptide product that will produce revenues sufficient to sustain our operations. Except for Fortical, most of our products are in early stages of development and we and our licensees may never develop a calcitonin, PTH or other peptide product that makes us profitable. Our ability to achieve profitability is dependent on a number of factors, including our and our licensees’ ability to complete development efforts, obtain regulatory approval for additional product candidates and successfully commercialize those product candidates or our technologies. We believe that the development of more desirable formulations is essential to expand consumer acceptance of peptide pharmaceutical products. However, we may not be successful in our development efforts, or other companies may develop such products, or superior products, before we do.
We may not be successful in our efforts to gain regulatory approval for our products other than Fortical and, if approved, the approval may not be on a timely basis.
Even if we or our licensees are successful in our development efforts, we may not be able to obtain the necessary regulatory approval for our products other than Fortical. The FDA must approve the commercial manufacture and sale of pharmaceutical products in the United States. Similar regulatory approvals are required for the sale of pharmaceutical products outside of the United States. None of our products other than Fortical has been approved for sale in the United States, and our other products may never receive the approvals necessary for commercialization. We must conduct further human testing on certain of our products before they can be approved for commercial sale and such testing requires the investment of significant resources. Any delay in receiving, or failure to receive, these approvals would adversely affect our ability to generate product revenues.
We may not be successful in efficiently developing, manufacturing or commercializing our products.
Because of our limited financial resources and our lack of a marketing organization, we are likely to rely on licensees or other parties to perform one or more tasks for the commercialization of our pharmaceutical products, such as USL to market and distribute Fortical in the U.S. If any of our products are approved for commercial sale, the product will need to be manufactured in commercial quantities at a reasonable cost in order for it to be a successful product that can generate profits. We may incur additional costs and delays while working with these parties, and these parties may ultimately be unsuccessful in the commercialization of a product.
10 of 59
Our success is dependent on our ability to establish and maintain commercial partnerships and currently we have only four significant license agreements.
We do not currently have, nor do we expect to have in the near future, sufficient financial resources and personnel to develop and market products on our own. Accordingly, we expect to continue to depend on pharmaceutical companies for revenues from sales of products, research sponsorship and distribution of our products.
The process of establishing partnerships is difficult and time-consuming. Our discussions with potential partners may not lead to the establishment of new partnerships on favorable terms, if at all. If we successfully establish new partnerships, the partnerships may never result in the successful development of our product candidates or the generation of significant revenue. Management of our relationships with these partners would require:
|
•
|
significant time and effort from our management team;
|
|
•
|
coordination of our research with the research priorities of our corporate partners;
|
|
•
|
effective allocation of our resources to multiple projects; and
|
|
•
|
an ability to attract and retain key management, scientific, production and other personnel.
|
We may not be able to manage these relationships successfully.
We currently have significant license agreements with Novartis worldwide for the production of calcitonin, with GSK worldwide for oral PTH, with USL in the United States for nasal calcitonin and with Tarsa worldwide (except for China) for oral calcitonin. We are pursuing additional opportunities to license or enter into distribution arrangements for products that utilize our oral and nasal delivery technologies and/or our manufacturing technology, as well as our other technologies. However, we may not be successful in any of these efforts.
Because we are a biopharmaceutical company, our operations are subject to extensive government regulation.
Our laboratory research, development and production activities, as well as those of our collaborators and licensees, are subject to significant regulation by federal, state, local and foreign governmental authorities. In addition to obtaining FDA approval and other regulatory approvals for our products, we must maintain approvals for our manufacturing facility to produce calcitonin, PTH and other peptides for human use. The regulatory approval process for a pharmaceutical product requires substantial resources and may take many years. Our inability to obtain approvals or delays in obtaining approvals would adversely affect our ability to continue our development program, to manufacture and sell our products, and to receive revenue from milestone payments, product sales or royalties.
The FDA and other regulatory agencies may audit our production facility at any time to ensure compliance with cGMP guidelines. These guidelines require that we conduct our production operation in strict compliance with our established rules for manufacturing and quality controls. Any of these agencies can suspend production operations and product sales if they find significant or repeated deviations from these guidelines. A suspension would likely cause us to incur additional costs or delays in product development. In addition, we are subject to the U.S. Foreign Corrupt Practices Act, which prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity. Our present and future business is, and will continue to be, subject to various other laws, rules and/or regulations applicable to us as a result of our international business.
Fortical currently faces competition from large pharmaceutical companies and, if our other products receive regulatory approval, these other products would also face competition from large pharmaceutical companies with superior resources.
We are engaged in developing pharmaceutical products, which is a rapidly changing and highly competitive field. To date, we have concentrated our efforts primarily on products to treat one indication — osteoporosis. Like the market for any pharmaceutical product, the market for treating osteoporosis has the potential for rapid, unpredictable and significant technological change. Competition is intense from specialized biotechnology and biopharmaceutical companies, major pharmaceutical and chemical companies and universities and research institutions. In December 2008, Apotex and Sandoz launched nasal calcitonin products which are generic to Novartis’ nasal calcitonin product, but not to Fortical. In June 2009, Par also launched a product generic to Novartis’ nasal calcitonin product. Certain providers have substituted these products for Fortical, causing Fortical sales and royalties to decrease. There could also be future competition from products that are generic to Fortical, including Apotex’s product that is the subject of our ANDA litigation. Fortical also faces competition from large pharmaceutical companies that produce other osteoporosis products and, if any of our other products receive regulatory approval, these other products likely would also face competition from large pharmaceutical companies with substantially greater financial resources, research and development staffs and facilities, and regulatory experience than we have. Major companies in the field of osteoporosis treatment include Novartis, Merck, Eli Lilly and Sanofi-Aventis. One or more of these potential competitors could, at any time, develop products or a manufacturing process that could render our technology or products noncompetitive or obsolete.
Our success depends upon our ability to protect our intellectual property rights.
We filed applications for U.S. patents relating to proprietary peptide manufacturing technology and oral and nasal formulations that we have invented in the course of our research. Our most important U.S. manufacturing and delivery patents expire from 2017 to 2025 and we have applications pending that could extend that protection. To date, fifteen U.S. patents have issued and other applications are pending. We have also made patent application filings in selected foreign countries and fifty-eight foreign patents have issued with other applications pending. We face the risk that any of our pending applications will not be issued as patents. In addition, our patents may be found to be invalid or unenforceable, including in the pending ANDA litigation with Apotex. Our business also is subject to the risk that our issued patents will not provide us with significant competitive advantages if, for example, a competitor were to independently develop or obtain similar or superior technologies. To the extent we are unable to protect our patents and patent applications, our investment in those technologies may not yield the benefits that we expect.
11 of 59
We also rely on trade secrets to protect our inventions. Our policy is to include confidentiality obligations in all research contracts, joint development agreements and consulting relationships that provide access to our trade secrets and other know-how. However, parties with confidentiality obligations could breach their agreements causing us harm. If a secrecy obligation were to be breached, we may not have the financial resources necessary for a legal challenge. If licensees, consultants or other third parties use technological information independently developed by them or by others in the development of our products, disputes may arise from the use of this information and as to the ownership rights to products developed using this information. These disputes may not be resolved in our favor and could materially impact our business.
Our technology or products could give rise to product liability claims.
Our business exposes us to the risk of product liability claims from human testing, manufacturing and sale of pharmaceutical products. The administration of drugs to humans, whether in clinical trials or commercially, can result in product liability claims even if our products are not actually at fault for causing an injury. Furthermore, our products may cause, or may appear to cause, adverse side effects or potentially dangerous drug interactions that we may not learn about or understand fully until the drug is actually manufactured and sold. Product liability claims can be expensive to defend and may result in large judgments against us. Even if a product liability claim is not successful, the adverse publicity, time and expense involved in defending such a claim may interfere with our business. We may not have sufficient resources to defend against or satisfy these claims. We currently maintain $10,000,000 in product liability insurance coverage. However, this amount may not be sufficient to protect us against losses or may be unavailable in the future on acceptable terms, if at all.
We may have financial obligations under the various agreements related to our joint venture in China.
Although we do not believe that we are required to make any further contributions to our joint venture in China, there exists a misunderstanding as to whether we owe an additional $1.2 million for discretionary loans or equity contributions to the China Joint Venture and we have recorded a $1.2 million liability on our balance sheet. To date, we have paid our cash capital requirement of $1,050,000 and also completed our required technology transfer, thereby satisfying in full our registered capital contribution. In addition, we believe that the China Joint Venture owes us the amount of $678,000 as reimbursement for monies paid, and engineering services provided to, or on behalf of, the China Joint Venture by us. The reimbursement amount is subject to confirmation by our China Joint Venture partner and therefore has not been recorded as a receivable since its collectability is uncertain. While there are no assurances that the parties will reach agreement with respect to these disputed amounts, we believe that we will reach an amicable resolution of the amounts due and owing each party. If we are unsuccessful in reaching an amicable resolution, it could have a material adverse effect on our financial condition, results of operations and cash flow.
The global financial crisis may adversely affect our business and financial results.
The global credit crisis that began in 2007 was further exacerbated by events occurring in the financial markets in the fall of 2008, which continued in 2009 and 2010. These events have negatively impacted the ability of corporations to raise capital through equity financings or borrowings. The credit crisis may continue for the foreseeable future. Based upon our current level of revenue and expenses, we believe our current cash should be sufficient to support our current operations into the second half of 2010. At that time, we may not be able to raise capital on reasonable terms, if at all. In addition, uncertainty about current and future global economic conditions may impact our ability to license our products and technologies to other companies and may cause consumers to defer purchases of prescription medicines, such as Fortical, in response to tighter credit, decreased cash availability and declining consumer confidence. Accordingly, future demand for our product could differ from our current expectations.
We have a new Chief Executive Officer and other new executives.
In June 2010 we hired a new Chief Executive Officer and recently hired certain other new members of our executive management team. Our new Chief Executive Officer or these other new executives may not be successfully integrated into our management team and may not effectively implement our business strategy. If the management team is unable to work together to successfully implement our business strategy, it could have a material adverse effect on our financial condition, results of operations and cash flow.
We may be unable to retain key employees or recruit additional qualified personnel.
Because of the specialized scientific nature of our business, we are highly dependent upon qualified scientific, technical, production and managerial personnel. There is intense competition for qualified personnel in our business. Therefore, we may not be able to attract and retain the qualified personnel necessary for the development of our business. The loss of the services of existing personnel, as well as the failure to recruit additional key scientific, technical, production and managerial personnel in a timely manner, could harm our programs and our business.
Compliance with regulations governing public company corporate governance and reporting is complex and expensive.
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 and related SEC regulations, have created uncertainty for public companies and significantly increased the costs and risks associated with accessing the public markets and public reporting. For example, on January 30, 2009, the SEC adopted rules requiring companies to provide their financial statements in interactive data format using the eXtensible Business Reporting Language, or XBRL. We will have to comply with these rules by June 15, 2011. More recently, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 was enacted into law. This legislation makes significant changes to corporate governance and executive compensation rules for public companies across all industries. Our management team will need to invest significant management time and financial resources to comply with both existing and evolving standards for public companies, which will lead to increased general and administrative expenses and a diversion of management time and attention from revenue generating activities to compliance activities.
12 of 59
The market price of our common stock is volatile.
The market price of our common stock has been, and we expect it to continue to be, highly unstable. Factors, including our announcement of technological improvements or announcements by other companies, regulatory matters, research and development activities, new or existing products or procedures, signing or termination of licensing agreements, concerns about competition, sales, our financial condition, operating results, litigation, government regulation, developments or disputes relating to agreements, patents or proprietary rights, and public concern over the safety of activities or products have had a significant impact on the market price of our stock. We expect such factors to continue to impact our market price for the foreseeable future. In addition, future sales of shares of Unigene common stock by us or our stockholders, and by the exercise and subsequent sale of Unigene common stock by the holders of outstanding and future warrants and options, could have an adverse effect on the price of our stock.
Our common stock is classified as a “penny stock” under SEC rules, which may make it more difficult for our stockholders to resell our common stock.
Our common stock is traded on the OTC Bulletin Board. As a result, the holders of our common stock may find it more difficult to obtain accurate quotations concerning the market value of the stock. Stockholders also may experience greater difficulties in attempting to sell the stock than if it was listed on a stock exchange. Because Unigene common stock is not traded on a stock exchange and the market price of the common stock is less than $5.00 per share, the common stock is classified as a “penny stock.” Rule 15g-9 of the Exchange Act imposes additional sales practice requirements on broker-dealers that recommend the purchase or sale of penny stocks to persons other than those who qualify as an “established customer” or an “accredited investor.” These include the requirement that a broker-dealer must make a determination that investments in penny stocks are suitable for the customer and must make special disclosures to the customer concerning the risks of penny stocks. Application of the penny stock rules to our common stock could adversely affect the market liquidity of the shares, which in turn may affect the ability of holders of our common stock to resell the stock.
In addition to the conversion of the Victory Park notes, the exercise of warrants and options, as well as other issuances of shares, will likely have a dilutive effect on our stock price.
As of December 31, 2010, there were outstanding warrants to purchase 60,000 shares of our common stock, all but 20,000 of which are currently exercisable, at an average exercise price of $2.13 per share. There were also outstanding stock options to purchase an aggregate of 9,208,200 shares of common stock, at an average exercise price of $1.03, of which 3,659,949 are currently exercisable. If our stock price should increase above the exercise price of these derivative securities, then such exercise at prices below the market price of our common stock could adversely affect the price of our common stock. Additional dilution may result from the issuance of shares of our common stock in connection with collaborations or licensing agreements or in connection with other financing efforts, including our issuance of stock upon the conversion of Victory Park's notes.
If provisions in our stockholder rights plan or Delaware law delay or prevent a change in control of Unigene, we may be unable to consummate a transaction that our stockholders consider favorable.
In December 2002, we adopted a stockholder rights plan. This stockholder rights plan increases the costs that would be incurred by an unwanted third party acquirer if such party owns or announces its intent to commence a tender offer for more than 15% of our outstanding common stock. The plan is designed to assure our board of directors a full opportunity to review any proposal to acquire us. However, the plan could prolong the take-over process and, arguably, deter a potential bidder. These provisions apply even if the offer may be considered beneficial by some stockholders, and a takeover bid otherwise favored by a majority of our stockholders might be rejected by our board of directors. In addition, specific sections of Delaware law may also discourage, delay or prevent someone from acquiring or merging with us.
Accounting for our revenues and costs involves significant estimates which, if actual results differ from estimates, could have a material adverse effect on our financial position and results of operations.
As further described in “Summary of Critical Accounting Policies” under “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations,” accounting for our contract related revenues and costs as well as other cost items requires management to make a variety of significant estimates and assumptions. Although we believe we have sufficient experience and processes in place to enable us to formulate appropriate assumptions and produce reliable estimates, these assumptions and estimates may change significantly in the future and these changes could have a material adverse effect on our financial position and the results of our operations.
|
Item 1B.
|
Unresolved Staff Comments.
|
Not applicable.
|
Item 2.
|
Properties.
|
We own a one-story office and laboratory facility consisting of approximately 12,500 square feet. The facility is located on a 2.2 acre site in Fairfield, New Jersey. At December 31, 2010, Victory Park and Jay Levy had first and second security interests, respectively, in this property. (See Notes 4 and 8 to the financial statements). In January 2011 we executed an agreement of sale on the property and expect to close on the sale of this property in the second quarter of 2011. One of the conditions of the sale is that we will lease the property from the new owner for an initial seven year term, which may be extended for one renewal term of five years. (See Note 25 to the financial statements.)
Our 32,000 square foot cGMP production facility, which has been used for the production of Fortical and calcitonin, and can be used for the production of other peptides, was constructed in a building located in Boonton, New Jersey. We lease the facility under a lease which began in February 1994. The current lease will expire in 2014. We have a 10-year renewal option under the lease, as well as an option to purchase the facility. We are currently leasing approximately 10,000 square feet of administrative and regulatory office space in Boonton, New Jersey. The initial term of the lease runs through May 2017. We have a 10-year renewal option under the lease, as well as an option to purchase the facility.
13 of 59
Fortical, our nasal calcitonin product for the treatment of postmenopausal osteoporosis, is covered by U.S. Patent No. 6,440,392 (the “Fortical Patent”). In June 2006, we received a “Paragraph IV certification letter” from Apotex Inc., a Canadian generic pharmaceutical manufacturer, alleging that this patent is invalid and therefore not infringed by Apotex’s nasal calcitonin product which is the subject of an Apotex pending ANDA. On July 24, 2006, we and USL jointly filed a lawsuit against Apotex Inc. and Apotex Corp., its U.S. subsidiary (together “Apotex”), in the U.S. District Court for the Southern District of New York for infringement of our Fortical Patent. Due to our filing of the above-mentioned lawsuit, the Hatch-Waxman Act provided for an automatic stay of FDA approval for Apotex’s ANDA of up to 30 months. In December 2008, this stay ended and we and Apotex entered a preliminary injunction enjoining Apotex from engaging in the commercial manufacture, use, marketing, distribution, selling, transportation or importation of any Fortical® generic equivalent product in the United States. The preliminary injunction will remain in effect until the court renders a final decision on the validity and infringement of Unigene’s U.S. Patent. In obtaining the preliminary injunction, Unigene and USL were required to post a bond and, if we do not prevail in the lawsuit, Unigene would be responsible for paying $1,662,500 to Apotex under the injunction agreement. In August 2009, the U.S. District Court, Southern District of New York, confirmed the validity of Unigene’s patent and issued an order permanently enjoining Apotex from engaging in any activity that infringes the patent. The motion of the plaintiffs, Unigene and its licensee USL, for summary judgment in the case was granted. The Court upheld the validity of the Fortical Patent and entered a permanent injunction against Apotex. Apotex appealed this decision. In October 2009, the District Court vacated its Order subject to reinstatement and asked the parties to identify any claims that remain in the case. As a result, Apotex’s appeal was deactivated. In July 2010, the Court ruled that no claims remain at issue and it reinstated its opinion and order of August 2009 in its entirety. Accordingly, the motion for summary judgment was entered in favor of Unigene and USL. Apotex appealed this decision to the United States Court of Appeals for the Federal Circuit and the parties are currently submitting briefs on appeal. There is the usual litigation risk that we will not be successful in the appeal and potentially subsequent litigation if it is remanded. In the event that we do not prevail, then Apotex could be in a position to market its nasal calcitonin product if and when its pending ANDA receives FDA approval. This could have a material adverse impact on our results and financial position.
PART II
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
|
We have not declared or paid any cash dividends since inception, and do not anticipate paying any in the near future.
We became a public company in 1987. As of March 2, 2011, there were 537 holders of record of our common stock. Our common stock is traded on the OTC Bulletin Board under the symbol UGNE. The prices below represent high and low sale prices per share of our common stock.
|
2010
|
2009
|
|||||||
|
High-Low
|
High-Low
|
|||||||
|
1st Quarter:
|
$ | 1.01-0.56 | $ | 0.68-0.37 | ||||
|
2nd Quarter:
|
0.95-0.61 | 1.55-0.51 | ||||||
|
3rd Quarter:
|
0.78-0.52 | 1.54-0.91 | ||||||
|
4th Quarter:
|
0.75-0.40 | 1.96-0.61 | ||||||
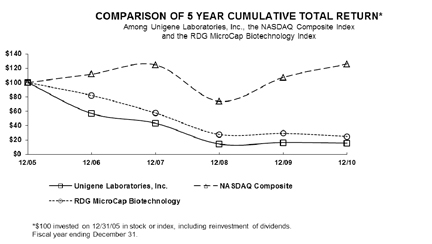
This stock performance information is “furnished” and shall not be deemed to be “soliciting material” or subject to Rule 14A of the Exchange Act, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, and shall not be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date of this Annual Report on Form 10-K and irrespective of any general incorporation by reference language in any such filing, except to the extent that we specifically incorporate this information by reference.
14 of 59
|
Item 6.
|
Selected Financial Data.
|
STATEMENT OF OPERATIONS DATA
(In thousands, except per share data)
|
Year Ended December 31,
|
||||||||||||||||||||
|
2010
|
2009
|
2008
|
2007
|
2006
|
||||||||||||||||
|
Revenue:
|
||||||||||||||||||||
|
Product sales, royalties, licensing and other revenue
|
$ | 11,340 | $ | 12,792 | $ | 19,229 | $ | 20,423 | 6,059 | |||||||||||
|
Costs and expenses:
|
||||||||||||||||||||
| Costs of goods sold and inventory reserve | 3,325 | 2,171 | 5,845 | 7,222 | 2,049 | |||||||||||||||
|
Research and development expenses and unallocated facility expenses
|
9,534 | 14,062 | 10,445 | 8,485 | 8,564 | |||||||||||||||
|
General and administrative and severance expenses
|
8,805 | 8,938 | 7,889 | 7,812 | 6,423 | |||||||||||||||
|
Net loss
|
(27,868 | ) | (13,380 | ) | (6,078 | ) | (3,448 | ) | (11,784 | ) | ||||||||||
|
Net loss per share, basic and diluted
|
(.30 | ) | (.15 | ) | (.07 | ) | (.04 | ) | (.14 | ) | ||||||||||
|
Weighted average number of shares outstanding
|
91,962 | 90,662 | 88,751 | 87,742 | 86,813 | |||||||||||||||
BALANCE SHEET DATA
(In thousands)
|
December 31,
|
||||||||||||||||||||
|
2010
|
2009
|
2008
|
2007
|
2006
|
||||||||||||||||
|
Cash and cash equivalents
|
$ | 12,201 | $ | 4,894 | $ | 8,583 | $ | 3,678 | $ | 3,357 | ||||||||||
|
Working capital (deficiency)
|
8,758 | (1,251 | ) | 14,914 | 6,390 | (9,527 | ) | |||||||||||||
|
Total assets
|
28,471 | 23,955 | 28,641 | 16,874 | 14,051 | |||||||||||||||
|
Total debt, including accrued interest-short-term
|
1,200 | 5,904 | -- | -- | 16,186 | |||||||||||||||
|
Total debt, including accrued interest-long-term
|
49,813 | 33,746 | 32,131 | 16,521 | -- | |||||||||||||||
|
Other long-term obligations, primarily deferred revenue, including current portion
|
13,915 | 11,217 | 12,354 | 13,712 | 9,361 | |||||||||||||||
|
Total liabilities
|
68,900 | 54,397 | 47,613 | 33,545 | 28,239 | |||||||||||||||
|
Total stockholders’ deficit
|
(40,429 | ) | (30,442 | ) | (18,972 | ) | (16,671 | ) | (14,188 | ) | ||||||||||
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations.
|
You should read the following discussion together with our financial statements and the notes appearing elsewhere in this Form 10-K. The following discussion contains forward-looking statements. Our actual results may differ materially from those projected in the forward-looking statements. Factors that might cause future results to differ materially from those projected in the forward-looking statements include those discussed in “Risk Factors” and elsewhere in this Form 10-K.
RESULTS OF OPERATIONS - Years ended December 31, 2010, 2009 and 2008
Introduction
We are a biopharmaceutical company engaged in the research, production and delivery of small proteins, referred to as peptides, for medical use. We have a patented manufacturing technology for producing many peptides cost-effectively. We also have patented oral and nasal delivery technologies that have been shown to deliver medically useful amounts of various peptides into the bloodstream. We currently have three operating locations: administrative and regulatory offices in Boonton, New Jersey, a laboratory research facility in Fairfield, New Jersey and a pharmaceutical production facility in Boonton, New Jersey.
In June 2010, we appointed Ashleigh Palmer as our Chief Executive Officer and since then, we have hired a number of new executive officers, and a new director has joined our Board of Directors. In addition, in September 2010, we announced a strategic realignment of our business into two strategic business units. One strategic business unit will concentrate on our pipeline of proprietary peptide development programs focused on metabolic disease and inflammation. The other strategic business unit will focus on opportunities where we can apply our platform of peptide drug delivery and manufacturing assets, expertise and capabilities to partners’ proprietary development programs. As part of this strategic realignment, we also determined that certain programs that do not fit our core focus, such as our China Joint Venture and our SDBG program will be monetized, out-licensed or terminated.
Historically, our primary focus has been on the development of calcitonin and other peptide products for the treatment of osteoporosis and other indications. Our revenue for the past three years has primarily been derived from domestic sources. We have licensed in the U.S. our nasal calcitonin product for the treatment of osteoporosis, which we have trademarked as Fortical, to USL. Fortical was approved by the FDA in 2005. This is our first product approval in the United States. We have licensed worldwide rights to our patented manufacturing technology for the production of calcitonin to Novartis. We have licensed worldwide rights to our manufacturing and delivery technologies for oral PTH for the treatment of osteoporosis to GSK. In October 2009, we licensed on a worldwide basis (except for China) our Phase III oral calcitonin program to Tarsa.
We have expanded our product pipeline with: (i) a novel peptide treatment for obesity; (ii) potential therapies for inflammation and cardiovascular disease based on technology we have in-licensed from Queen Mary, University of London; and (iii) SDBG technology, which we developed in conjunction with Yale University pursuant to a research agreement that terminated in the second quarter of 2010. In September 2010, we determined that SDBG does not fit our core focus and we will seek to monetize the investment or out-license or partner it to another company. All of these programs are in a preclinical, early stage of development. We also periodically perform feasibility studies for third parties. Our products, other than Fortical in the United States, will require clinical trials and/or approvals from regulatory agencies and all of our products will require acceptance in the marketplace. There are risks that these clinical trials will not be successful and that we will not receive regulatory approval or significant revenue for these products.
15 of 59
We compete with specialized biotechnology and biopharmaceutical companies, major pharmaceutical and chemical companies and universities and research institutions. Most of these competitors have substantially greater resources than we do. In December 2008, Apotex and Sandoz launched nasal calcitonin products which are generic to Novartis’ nasal calcitonin product, but not to Fortical. In June 2009, Par also launched a product generic to Novartis’ nasal calcitonin product. Certain providers have substituted these products for Fortical, causing Fortical sales and royalties to decrease. For 2010, compared to 2009, our Fortical sales decreased 12% while Fortical royalties decreased 40%. We cannot forecast whether, or to what extent, we will continue to experience further declines in sales or royalties. In addition, Apotex has a pending ANDA for a nasal calcitonin product that we claim infringes on our Fortical patent. If we do not prevail in that litigation, then Apotex could be in a position to market its nasal calcitonin product if and when its pending ANDA receives FDA approval.
The current need of major pharmaceutical companies to add products to their pipeline is a favorable sign for us and for other small biopharmaceutical companies. However, this need is subject to rapid change and it is uncertain whether any additional pharmaceutical companies will have interest in licensing our products or technologies.
REVENUE
Revenue is summarized as follows for the years ended December 31, 2010, 2009 and 2008:
|
2010
|
2009
|
2008
|
||||||||||
|
Product Sales
|
$ | 5,246,159 | $ | 5,941,254 | $ | 10,057,938 | ||||||
|
Royalty Revenue
|
2,974,365 | 4,991,266 | 6,519,942 | |||||||||
|
Licensing Revenue
|
1,300,763 | 1,253,260 | 1,256,760 | |||||||||
|
Development Fees and Other Revenue
|
1,818,927 | 606,058 | 1,394,793 | |||||||||
| $ | 11,340,214 | $ | 12,791,838 | $ | 19,229,433 | |||||||
Revenue for the year ended December 31, 2010 decreased $1,452,000 or 11% to $11,340,000 from $12,792,000 in 2009. Revenue for the year ended December 31, 2009 decreased $6,437,000, or 33%, to $12,792,000 from $19,229,000 in 2008. These decreases in revenue were due to a decline in Fortical sales and royalties as a result of increased competition in the nasal calcitonin market since the launch of competitive products in December 2008. In 2010, our Fortical sales declined $695,000, or 12%, and Fortical royalties declined $2,017,000, or 40%, from 2009. In 2009, Fortical sales to USL declined $4,117,000, or 41%, and Fortical royalties from USL declined $1,529,000, or 23%, from 2008. Fortical royalties fluctuate each quarter based upon the timing and pricing of USL’s shipment to its customers. Royalty revenue is earned on sales of Fortical by USL and is recognized in the period Fortical is sold by USL. Licensing revenue represents the partial recognition of milestones and up-front payments received in prior years. Licensing revenue remained relatively constant in 2010 from 2009 and in 2009 from 2008.
Development fees and other revenue increased 200% to $1,819,000 in 2010 from $606,000 in 2009 primarily due to an increase in development work and feasibility studies we did for various pharmaceutical and biotechnology companies, including Tarsa. Development fees and other revenue decreased 57% to $606,000 in 2009 from $1,395,000 in 2008 primarily due to the timing and amount of development work and feasibility studies we did. This revenue is dependent upon the needs of our partners.
Future sales and royalties are contingent upon many factors including competition, pricing, marketing and acceptance in the marketplace and, therefore, are difficult to predict. Milestone revenue is based upon one-time events and is generally correlated with the development strategy of our licensees. It is therefore subject to uncertain timing and not predictive of future revenue. Bulk peptide sales to our partners under license or supply agreements prior to product approval are typically of limited quantity and duration and also not necessarily predictive of future revenue. Additional peptide sales are dependent upon the future needs of our partners, which we cannot currently estimate. Sales revenue from Fortical in the future will depend on Fortical’s continued acceptance in the marketplace, as well as competition and other factors. In December 2008, Apotex and Sandoz launched nasal calcitonin products which are generic to Novartis’ nasal calcitonin product, but not to Fortical. In June 2009, Par also launched a product generic to Novartis’ nasal calcitonin product. Certain providers have substituted these products for Fortical, causing Fortical sales and royalties to decrease. Therefore, it is highly unlikely that Fortical alone will ever generate sufficient revenue for us to achieve profitability. In addition, Apotex has a pending ANDA for a nasal calcitonin product that we claim infringes on our Fortical patent. If we do not ultimately prevail in defending our patent, then Apotex could be in a position to market its nasal calcitonin product if and when its pending ANDA receives FDA approval.
EXPENSES
In December 2009, we announced a restructuring plan that included a reduction in workforce and expenses to improve operational efficiencies and to better match resources with market demand. Our restructuring expenses for 2009 were $353,000, of which $242,000 represented severance pay. These expenses are included in research and development expenses and in general and administrative expenses. There were no restructuring expenses in 2010. Under the comprehensive plan, we continued Fortical production and maintained all of our core programs and partnered activities while decreasing cash expenditures. We reached our target of decreasing cash expenditures by over $9 million for 2010. Since we currently maintain an adequate multi-year inventory of calcitonin and enzyme to support Fortical, we have temporarily suspended manufacturing of those materials at our Boonton facility. However, we are maintaining the cGMP status of the facility and the ability to manufacture peptides at that location.
Implementation of the plan resulted in an immediate company-wide workforce reduction of approximately 30%. The plan further provided for salary reductions in 2010 at all levels, including senior management, and resulted in other cost savings. From time to time, we will evaluate the feasibility of additional expenditures in areas such as preclinical and/or clinical studies in order to add value to certain of our current research projects.
16 of 59
Cost of Goods Sold
Cost of goods sold varies by product and consists primarily of material costs, personnel costs, manufacturing supplies and overhead costs such as depreciation and maintenance. Cost of goods sold increased 20% in 2010 to $2,608,000 from $2,171,000 in 2009. This increase was due to the utilization in 2010 of older calcitonin from inventory which was manufactured by a less efficient production process. The less efficient process produced batches with a lower yield per batch, therefore cost per gram of calcitonin was higher. This older calcitonin was fully utilized in 2010. Cost of goods sold decreased 61% in 2009 to $2,171,000 from $5,622,000 in 2008, due to a 41% reduction in Fortical sales to USL, as well as improved margins which resulted from improved batch yields for calcitonin production. Cost of goods sold represented our costs associated with Fortical production. Cost of goods sold as a percentage of sales was 50%, 37% and 56% respectively, for 2010, 2009 and 2008. The improvement in margins in 2009 was due to the utilization of calcitonin produced by our more efficient manufacturing process. Future production related expenses will be dependent upon the level of future Fortical sales, as well as possible peptide production to meet our partners’ needs.
Research and Development Expenses
Research and development expenses primarily consist of personnel costs, pre-clinical and clinical trials, supplies, outside testing and consultants primarily related to our research and development efforts or activities related to our license agreements, as well as depreciation and amortization expense.
Research and development expenses decreased $5,923,000, or 48%, in 2010 to $6,428,000 from $12,351,000 in 2009. This decrease was largely due to a reduction of $3,283,000 in oral calcitonin expenditures following the licensing of our oral calcitonin program to Tarsa in October 2009, as well as a reduction of $2,964,000 in personnel expenditures allocated to research and development due to staff and salary reductions. Research and development expense increased 30% in 2009 to $12,351,000 from $9,517,000 in 2008. The 2009 increase was due to an increase of $1,467,000 for our Phase III oral calcitonin program which was licensed to Tarsa in October 2009. In addition, production salaries and supplies allocated to research and development expenses increased $1,333,000 primarily due to the production of PTH for research purposes, as well as certain peptide batch failures during 2009.
Research and development expenses will fluctuate in future years depending on the timing of expenses, including preclinical and clinical trials. Expenditures for the sponsorship of collaborative research programs were $354,000, $712,000 and $702,000 in 2010, 2009 and 2008, respectively, which are included as research and development expenses. The 2009 and 2008 increases were due to expenses related to our collaboration with Queen Mary, University of London.
General and Administrative Expenses
General and administrative expenses decreased $1,253,000, or 14%, to $7,685,000 in 2010 from $8,938,000 in 2009. The decrease was due to a reduction of $1,267,000 in professional fees, primarily due to legal expenses in 2009 related to the Apotex litigation, as well as Tarsa licensing activities.
General and administrative expenses increased 13% to $8,938,000 in 2009 from $7,889,000 in 2008. The 2009 increase was primarily attributable to an increase of $1,216,000 in professional fees, including legal expenses related to the Tarsa transaction and to the Apotex litigation.
Unallocated Facility Cost
Unallocated facility cost represents underutilization of our Boonton manufacturing facility. These costs primarily consist of salaries, as well as overhead expenses, which increased $1,394,000 to $3,106,000 for 2010 as compared to $1,712,000 in 2009, and increased $784,000 in 2009 as compared to $928,000 in 2008. The expenses in 2010 primarily relate to our current cessation of calcitonin and enzyme production, as well as our decreased production of Fortical. The expenses in 2009 and 2008 primarily relate to manufacturing calcitonin, enzyme and Fortical at below normal production capacity.
Inventory Reserve
Inventory reserve charges were $717,000 for 2010. This expense is primarily due to our decision in the first quarter of 2010 not to pursue an additional improvement for our calcitonin manufacturing process. Therefore, calcitonin batches manufactured by this process have been fully reserved. There were no inventory reserve charges in 2009.
Inventory reserve charges in 2008 were $223,000, representing potential second source material components for Fortical which may not be usable.
Severance Expense
Severance expense of $1,120,000 in 2010 represents an accrual of salary and health insurance costs associated with the resignations of Warren Levy and Ronald Levy. These payments are expected to be paid out in March 2011 and are included in current accrued expenses.
Other Income/Expenses
Gain/Loss on Change in Fair Value of Embedded Conversion Feature
The Victory Park note is convertible into shares of our Common Stock and, until June 15, 2010, we did not have sufficient authorized shares to effectuate a full conversion of the notes. Therefore, the conversion feature was treated as a liability based on its fair value. The liability for the conversion feature was marked to the market at March 31, 2010 and at June 15, 2010, the date of our annual meeting of stockholders, when additional shares were authorized. We therefore recognized a non-cash expense of $10,034,000 in the first quarter of 2010 which represented the change in the fair value of the conversion feature of the note from the closing date of March 17, 2010 to the balance sheet date of March 31, 2010 and we recognized a non-cash gain of $1,909,000 which represented the change in the fair value of the conversion feature of the note from March 31, 2010 to June 15, 2010.
17 of 59
Qualifying Therapeutic Discovery Grant
In November 2010, we received notification that we were awarded an aggregate of $978,000 in grants under the Qualifying Therapeutic Discovery Project (QTDP) program of the Internal Revenue Service (IRS). The program, which targets therapeutic discovery projects that show potential to result in new therapies to treat areas of unmet medical need or treat chronic or acute diseases and conditions potentially resulting in reduced health care costs, was awarded to us to help fund the development of four of our programs.
Net Gain on License to Tarsa
In October 2009, we licensed our Phase III oral calcitonin program to Tarsa Therapeutics, Inc. a new company formed by a syndicate of three venture capital funds. In consideration for our sale to Tarsa of an exclusive license for the oral calcitonin program, we received from Tarsa approximately $8,993,000 in cash and 9,215,000 shares of common stock in Tarsa (which represents a 26% ownership). We valued the common stock at $0.23 per share or a total of $2,119,000 with the assistance of an outside valuation firm. In October 2009 we recognized $4,854,000 in expenses for our oral calcitonin program as well as an additional $573,000 in expenses related to the warrant exchange with Victory Park related to the Tarsa transaction. We therefore recognized a net gain of $5,685,000 on the licensing of our oral calcitonin program to Tarsa.
Interest Expense
Interest expense increased 91% in 2010 to $9,292,000 from $4,862,000 in 2009. The increase was primarily due to the debt restructuring with Victory Park in March 2010. The new notes for an aggregate of $33,000,000 bear interest at the prime rate plus 5%, with a floor of 15%. The prior Victory Park notes bore interest at the prime rate plus 7%, subject to a floor of 14% per annum and a cap of 18% per annum. During 2010 and 2009 we recognized $7,765,000 and $3,356,000, respectively, in cash and non-cash interest expense under these notes. In addition, interest expense on the Levy loans increased to $1,446,000 in 2010 from $1,403,000 in 2009 due to the increase in interest rate from 9% to 12% as a result of the Levy loan restructuring in March 2010. Due to the deferred payout schedules of both of the Levy notes , the effective interest rate of the restructured notes increased from 7.6% to 8.2%.
Interest expense increased 131% in 2009 to $4,862,000 from $2,102,000 in 2008. The increase was primarily due to the $15,000,000 and $5,000,000 notes issued to Victory Park on September 30, 2008 and May 22, 2009, respectively. These notes bore interest at the prime rate plus 7%, subject to a floor of 14% per annum and a cap of 18% per annum. During 2009 and 2008, we recognized $3,356,000 and $700,000, respectively, in cash and non-cash interest expense under these notes.
Gain/Loss On Equity Method Investments
China Joint Venture
This loss represents our 45% ownership percentage of our China joint venture’s profits and losses. Our share of the 2010 loss of the China joint venture was $149,000, a decrease of $20,000 from 2009.
Our technology and know-how contribution to our China joint venture gives rise to a basis difference between our investment in the joint venture and our 45% equity interest in the underlying assets of the joint venture. This basis difference is being recognized over 17 years, the estimated life of the transferred assets, at approximately $265,000 per year beginning in the fourth quarter of 2008 and is included under gain (loss) from investment in Tarsa and China joint venture on our statement of operations.
Tarsa
We currently have a 26% ownership interest in Tarsa, subject to possible future dilution. Our 2009 operations include a loss of $2,119,000 which represents our proportionate share of loss in Tarsa, limited to our total investment in Tarsa which was $2,119,000, as a result of our valuation of Tarsa’s common stock at $2,119,000, with the assistance of an outside appraiser. Our share of Tarsa’s loss would have been $3,795,000 so that the unrecognized loss of $1,676,000 is to be carried over to future periods. The loss reduced our investment in Tarsa to zero and, as a consequence, our 2010 and future financial results will not be negatively affected by Tarsa’s ongoing operations. We have no obligation to fund future operating losses of Tarsa as well as no further obligations of any kind.
Income Tax Benefit
The income tax benefits of $701,000 in 2010 and of $926,000 in 2008 consist primarily of proceeds received for sales of a portion of our state tax net operating loss carry forwards and research credits under a New Jersey Economic Development Authority program, which allows certain New Jersey taxpayers to sell their state tax benefits to third parties. The purpose of the New Jersey program is to provide financial assistance to high-technology and biotechnology companies in order to facilitate future growth and job creation. The income tax benefit of $67,000 in 2009 consists primarily of a partial refund of federal research and development tax credits. The New Jersey tax benefit sale for 2009 was concluded and recognized in February 2010.
Net Loss
Net loss for 2010 increased approximately $14,488,000, or 108%, to $27,868,000 from $13,380,000 for the corresponding period in 2009. This was primarily due to loss on change in fair value of embedded conversion feature of $8,125,000, debt issuance cost of $2,008,000, a reduction in revenue of $1,452,000, severance expense of $1,120,000, an inventory reserve of $717,000 and an increase in interest expense of $4,429,000. These were partially offset by a decrease in other operating expenses of $5,344,000. In addition, in 2009, we recognized a one-time, net gain in the amount of $5,685,000 on our license to Tarsa.
Net loss for 2009 increased 120% to $13,380,000 from $6,078,000 in 2008. This was due to increased operating expenses of $992,000, primarily due to expenditures for our oral calcitonin program partially offset by decreased cost of goods sold. Interest expenses increased $2,760,000, primarily due to our borrowings from Victory Park. In addition, Fortical revenue decreased $5,645,000 due to increased competition. These increased expenses and reduced Fortical revenue were partially offset by the Tarsa gain of $5,685,000.
18 of 59
Net losses will continue unless we achieve sufficient non-deferred revenue under our USL, GSK, Tarsa or Novartis agreements or sign new revenue generating research, licensing or distribution agreements.
Summary of Critical Accounting Policies
The SEC defines “critical accounting policies” as those that are both important to the portrayal of a company’s financial condition and results, and require management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain.
The following discussion of critical accounting policies represents our attempt to bring to the attention of the readers of this report those accounting policies which we believe are critical to our financial statements and other financial disclosure. It is not intended to be a comprehensive list of all of our significant accounting policies, which are more fully described in Note 3 of the Notes to the Financial Statements included in our Annual Report on Form 10-K for the year ended December 31, 2010. In many cases, the accounting treatment of a particular transaction is specifically dictated by generally accepted accounting principles (“GAAP”), with no need for management’s judgment in the application of GAAP.
Revenue Recognition: We recognize revenue from the sale of products and from royalties, licensing agreements, research services and grants. Revenue from the sale of product is recognized when title to product and risk of loss are transferred, collection is reasonably assured and we have no further obligations. If there are elements of the revenue recognition requirements that are not met at the time of shipment, the revenue is deferred and the corresponding cost of the product is included on our balance sheet as a deferred asset. Revenue from research services is recognized when services are rendered. We occasionally apply to various government agencies for research grants. Revenue from such research grants is recognized when work is conducted under the grant. Sales and grant revenues generally do not involve difficult, subjective or complex judgments. We recognize royalty revenue on an accrual basis in accordance with the terms of individual agreements. If the receipt of royalty revenue is contingent on a future event, revenue would be recognized when that event has occurred. Typically, royalties are recognized when third party results can be reliably measured and collectability is reasonably assured. In the case of USL, we recognize royalty revenue based upon the quarterly USL royalty report. This enables a reliable measure, as well as reasonable assurances of collectability. Royalty revenue is earned on sales of Fortical by USL and is recognized in the period Fortical is sold by USL.
We follow the accounting guidance for licensing agreements which typically include several elements of revenue, such as up-front payments, milestones, royalties upon sales of product and the delivery of product and/or research services to the licensee. Non-refundable license fees received upon execution of license agreements where we have continuing involvement are deferred and recognized as revenue over the estimated performance period of the agreement. This requires management to estimate the expected term of the agreement or, if applicable, the estimated life of its licensed patents. For our USL and GSK agreements, the following describes our revenue recognition accounting policy. Non-refundable milestone payments that represent the completion of a separate earnings process and a substantive step in the research and development process are recognized as revenue when earned at the lesser of 1) the non-refundable cash received or 2) the proportionate level of effort expended to date during the development stage multiplied by the development stage revenue. This sometimes requires management to judge whether or not a milestone has been met and when it should be recognized in the financial statements. Typically, this would involve discussions between management and our licensing partner. Payments for milestones for which the completion of the earnings process is not yet complete (for example, payment received for the commencement of an activity that represents a separate earnings process) are recognized over the estimated performance period of such activity. This is to comply with accounting guidance which provides for revenue to be recognized once delivery has occurred or services have been rendered. Royalties and/or milestones relating to sales of products by our licensees, and the delivery of products and research services, will be recognized as such events occur as they represent the completion of a separate earnings process.
In addition, accounting guidance requires a company to evaluate its arrangements under which it will perform multiple revenue-generating activities. For example, a license agreement with a pharmaceutical company may involve a license, research and development activities and/or contract manufacturing. Management is required to determine if the separate components of the agreement have value on a standalone basis and qualify as separate units of accounting, whereby consideration is allocated based upon their relative “fair values.” A delivered item or item(s) that do not qualify as a separate unit of accounting within the arrangement shall be combined with the other applicable undelivered item(s) within the arrangement. The allocation of arrangement consideration and the recognition of revenue then shall be determined for those combined deliverables as a single unit of accounting.
Accounting for Stock Options: For stock options granted to employees and directors, we recognize compensation expense based on the grant-date fair value estimated in accordance with accounting guidance. We estimate the fair value of each option award on the date of grant using the Black-Scholes option pricing model. Stock options and warrants issued to consultants are accounted for in accordance with accounting guidance. Compensation expense is calculated each quarter for consultants using the Black-Scholes option pricing model until the option is fully vested and is included in research and development expenses.
Inventory: Production inventories, at our Boonton, NJ location, are stated at the lower of cost or market, valued at specifically identified cost which approximates FIFO. Research inventories, at our Fairfield, NJ location, are stated at the lower of cost (using the FIFO method) or market. Typically, finished goods and work in process inventory are fully reserved except for the amounts deemed saleable by management based upon current or anticipated orders, primarily under contractual arrangements.
Use of Estimates: The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Our most significant estimates involve estimating the lives of our license agreements, which typically are determined by the estimated useful lives of our licensed patents. This would determine the period over which certain revenue would be recognized. Other significant estimates relate to valuation of inventory and intangible assets, both of which involve management estimates of the lives of the assets and the recoverability of costs.
19 of 59
Equity Method Investments: We account for our investment in the China joint venture and Tarsa under the equity method and therefore recognize our proportionate share of their earnings and losses. We own 45% of the Chinese joint venture and recognize 45% of the entity’s profits and losses. As part of the consideration for our sale to Tarsa of an exclusive license for our oral calcitonin program, we received 9,215,000 shares of common stock in Tarsa. This represents an approximate 26% ownership. In 2009, we therefore recognized 26% of the entity’s profits and losses, up to our investment in Tarsa. In 2009 our proportionate share of Tarsa’s loss was $3,795,000. However, our investment in Tarsa was $2,119,000 so the excess loss of $1,676,000 will be recognized in future years to offset our share of future profits, if any.
Patents and Other Intangibles: Amounts paid for patents and trademarks are capitalized. Patent and trademark costs are deferred pending the successful outcome of the relevant applications. These costs primarily consist of outside legal fees and typically relate to filings and prosecution of applications for improvements to existing patents or to the filings of foreign applications based upon domestic patents. Most of these applications relate to our patented peptide manufacturing and patented oral delivery technologies, as well as for our SDBG technology. Internal costs related to intangible assets are expensed as incurred. Successful patent costs are amortized using the straight-line method over the lives of the patents, typically five to fifteen years. Successful trademark costs are amortized using the straight-line method over their estimated useful lives of typically five to ten years. We assess the impairment of identifiable intangibles and other long-lived assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable, or we decide not to support the applications in certain jurisdictions. Some factors we consider important which could trigger an impairment review include (i) significant underperformance relative to expected historical or projected future operating results; (ii) significant changes in the manner of our use of the acquired assets or the strategy for our overall business; and (iii) significant negative industry or economic trends.
When we determine that the carrying value of intangible assets may not be recoverable based upon the existence of one or more of the above indicators of impairment, we first will perform an assessment of the asset’s recoverability based on expected undiscounted future net cash flow, and if the amount is less than the asset’s value, we will measure any impairment based on a projected discounted cash flow method using a discount rate determined by our management to be commensurate with the risk inherent in our current business model. Unsuccessful patent costs are expensed when determined worthless or when patent applications will no longer be pursued. Other intangibles are recorded at cost and are amortized over their estimated useful lives.
LIQUIDITY AND CAPITAL RESOURCES.
We have a number of future payment obligations under various agreements. They are summarized, at December 31, 2010, as follows:
|
Contractual Obligations
|
Total | 2011 | 2012 | 2013 | 2014 | 2015 | Thereafter | |||||||||||||||||||||
|
Notes payable – Victory Park
|
$ | 33,000,000 | $ | — | $ | — | $ | 33,000,000 | $ | — | $ | — | $ | — | ||||||||||||||
|
Interest – Victory Park
|
4,352,757 | — | — | 4,352,757 | — | — | — | |||||||||||||||||||||
|
China joint venture
|
1,200,000 | 1,200,000 | — | — | — | — | — | |||||||||||||||||||||
|
Notes payable-Levys
|
14,737,518 | — | 750,000 | 13,987,518 | — | — | — | |||||||||||||||||||||
|
Interest-Levys
|
4,932,602 | — | — | 4,932,602 | — | — | — | |||||||||||||||||||||
|
Operating leases
|
1,206,765 | 290,021 | 291,021 | 292,021 | 108,369 | 92,583 | 132,750 | |||||||||||||||||||||
|
Severance/vacation pay/deferred compensation-Levys
|
1,562,296 | 1,200,000 | 362,296 | — | — | — | — | |||||||||||||||||||||
|
Total Contractual Obligations
|
$ | 60,991,938 | $ | 2,690,021 | $ | 1,403,317 | $ | 56,564,898 | $ | 108,369 | $ | 92,583 | $ | 132,750 | ||||||||||||||
At December 31, 2010, we had cash and cash equivalents of $12,201,000, an increase of $7,307,000 from December 31, 2009. The increase was primarily due to funds received pursuant to a restructuring of our debt with Victory Park, Fortical revenue and a $4,000,000 payment received from GSK in December under our amended agreement. In March 2010, we entered into an amended and restated financing agreement with Victory Park. The restated financing agreement amends and restates in its entirety and replaces the financing agreement dated as of September 30, 2008.
Under the terms of the restated financing agreement, we issued to Victory Park $33,000,000 aggregate principal amount of three-year, senior secured convertible notes by way of surrender of the three-year, senior secured non-convertible notes previously issued pursuant to the original financing agreement, in the aggregate principal amount of approximately $19,358,000, and by way of cash payment of approximately $13,642,000 for the balance. After fees and expenses, we received net cash proceeds of approximately $11,635,000 on March 17, 2010. In addition, under certain circumstances, we may request that Victory Park purchase (which purchase shall be in Victory Park’s sole discretion) up to an additional $3 million aggregate principal amount of convertible notes at one subsequent closing. The maturity date of the convertible notes has been extended to March 17, 2013 from September 30, 2011 under the original notes. (See Note 8 to the audited financial statements.) Based upon our current level of revenue and expenses, we believe our current cash should be sufficient to support our current operations into the second half of 2012.
In December 2009, we announced a restructuring plan that included a reduction in workforce and expenses to improve operational efficiencies and to better match resources with market demand. Under the comprehensive plan, we are continuing Fortical production and maintaining all of our core programs and partnered activities while decreasing cash expenditures. We reached our target of decreasing cash expenditures by over $9 million for 2010. Since we currently maintain an adequate inventory of calcitonin and enzyme to support Fortical, we have temporarily suspended manufacturing of those materials at our Boonton facility. However, we will maintain the cGMP status of the facility and the ability to manufacture peptides at that location. Implementation of the plan resulted in an immediate company-wide workforce reduction of approximately 30%. The plan further provided for salary reductions in 2010 at all levels, including senior management, and other cost savings. From time to time we will evaluate the feasibility of additional expenditures in areas such as preclinical and/or clinical studies in order to add value to certain of our current research projects.
20 of 59
In addition to our debt restructuring in March 2010, other cash received during 2010 was primarily from Fortical sales and royalties received under our agreement with USL, as well as the $4,000,000 we received from GSK in December 2010. Our primary sources of cash have historically been (1) licensing fees for new agreements, (2) milestone payments under licensing or development agreements, (3) bulk peptide sales under licensing or supply agreements, (4) stockholder loans, (5) the sale of our common stock and (6) since 2005, Fortical sales and royalties. We cannot be certain that any of these cash sources will continue to be available to us in the future. Licensing fees from new collaborations are dependent upon the successful completion of complex and lengthy negotiations. Milestone payments are based upon progress achieved in collaborations, which cannot be guaranteed, and are often subject to factors that are controlled neither by our licensees nor us. Product sales to our partners under these agreements are based upon our licensees’ needs, which are sometimes difficult to predict. Sale of our common stock is dependent upon our ability to attract interested investors, our ability to negotiate favorable terms and the performance of the stock market in general and biotechnology investments in particular. Future Fortical sales and royalties will be affected by competition and continued acceptance in the marketplace and could be impacted by manufacturing, distribution or regulatory issues. We believe that in 2011 we will generate cash to apply toward funding a portion of our operations through sales of Fortical to USL and royalties on USL’s sales of Fortical, as well as our anticipated receipt of a $4,000,000 milestone payment from GSK upon completion of Phase II patient enrollment, and, in the long term, on sales and royalties from the sale of Fortical and oral calcitonin, the achievement of milestones under our existing license agreements and revenue on future licensed products and technologies. We are actively seeking additional licensing and/or supply agreements with pharmaceutical companies for various oral peptides, including our obesity peptide, and for our peptide manufacturing technology. However, we may not be successful in achieving milestones under our current agreements, in obtaining regulatory approval for our other products or in licensing any of our other products or technologies.
Due to our limited financial resources, any further decline in Fortical sales and/or royalties, or delay in achieving milestones under our existing license agreements, or in signing new license or distribution agreements for our products or technologies or loss of patent protection, could have a material adverse effect on our cash flow and operations (see Notes 10, 19 and 20 to the audited financial statements).
If we are unable to achieve significant milestones or sales under our existing agreements and/or enter into a new significant revenue generating license or other arrangement, at a certain point we would need to either secure another source of funding in order to satisfy our working capital needs and to remain in compliance with covenants in our financing agreement with Victory Park (see Note 8 to the audited financial statements) or significantly curtail our operations. Should the funding we require to sustain our working capital needs be unavailable or prohibitively expensive, the consequence would be a material adverse effect on our business, operating results, financial condition and prospects. We believe that the Victory Park financing has satisfied our current cash requirements, but satisfying our cash requirements over the long term will require the successful commercialization of one or more of our biotechnologies or our licensees’ oral or nasal calcitonin products, our oral PTH product, the obesity program or another peptide product in the U.S. and/or abroad. However, it is uncertain whether any of these products other than Fortical will be approved or will be commercially successful. The amount of future revenue we will derive from Fortical is also uncertain.
We have incurred annual operating losses since our inception and, as a result, at December 31, 2010, had an accumulated deficit of approximately $171,000,000. Our cash requirements in 2010 to operate our research and peptide manufacturing facilities and develop our products decreased from 2009 due to our December 2009 restructuring.
The global credit crisis that began in 2007 was further exacerbated by events occurring in the financial markets in the fall of 2008, which continued in 2009 and 2010. These events have negatively impacted the ability of corporations to raise capital through equity financings or borrowings. The credit crisis may continue for the foreseeable future. In addition, uncertainty about current and future global economic conditions may impact our ability to license our products and technologies to other companies and may cause consumers to defer purchases of prescription medicines, such as Fortical, in response to tighter credit, decreased cash availability and declining consumer confidence. Accordingly, future demand for our product could differ from our current expectations.
Net Cash Used in Operating Activities: Net cash used in operating activities was $1,669,000 in 2010, which was primarily due to our net loss adjusted for non-cash items of $14,302,000 and an increase in our operating assets and liabilities of $11,897,000, primarily from increased accrued interest and deferred revenue. Net cash used in operating activities was $11,291,000 in 2009, which was primarily due to our net loss adjusted for non-cash items of $2,836,000 and a decrease in our operating assets and liabilities of $4,925,000. The decrease in our operating assets and liabilities primarily resulted from a reduction in prepaid expenses and accounts receivable. Cash provided by operating activities has not been sufficient to meet our needs.
Net Cash Used in Investing Activities: Net cash used in investing activities was $678,000 in 2010, primarily resulting from costs related to our intellectual property. Net cash provided by investing activities was $3,030,000 in 2009, primarily due to our license to Tarsa.
Net Cash Provided by Financing Activities: Net cash provided by financing activities was $9,654,000 in 2010 and $4,572,000 in 2009 primarily resulting from our issuances of notes payable to Victory Park.
Off-Balance Sheet Arrangements.
None.
Recently Issued Accounting Pronouncements
In April 2010, an accounting standard update was issued to provide guidance on defining a milestone and determining when it is appropriate to apply the milestone method of revenue recognition for research and development transactions. Vendors can recognize consideration that is contingent upon achievement of a milestone in its entirety as revenue in the period the milestone is achieved if the milestone meets all the criteria stated in the guidance to be considered substantive and it must be considered substantive in its entirety. The amendments in this update became effective for our quarter ending June 30, 2010. The adoption of this update did not have a significant effect on our financial statements.
In January 2010, an accounting standards update was issued which includes new disclosure requirements related to fair value measurements, including transfers in and out of Levels 1 and 2 and information about purchases, sales, issuances and settlements for Level 3 fair value measurements. This update also clarifies existing disclosure requirements relating to levels of disaggregation and disclosures of inputs and valuation techniques. The adoption of this update did not have a significant impact on the Company's financial statements (see Note 21 to the audited financial statements).
21 of 59
In October 2009, an update to accounting guidance was issued to address the accounting for multiple-deliverable arrangements to enable vendors to account for products or services separately rather than as a combined unit. The amendments in this update will eliminate the residual method of allocation and require that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method. The amendments in this update will also require that a vendor determine its best estimate of selling price in a manner that is consistent with that used to determine the price to sell the deliverable on a standalone basis. The amendments in this update will become effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. We do not expect that the adoption of this update will have a material effect on our financial statements.
|
Item 7A.
|
Quantitative and Qualitative Disclosures About Market Risk.
|
On September 30, 2008, we entered into a financing agreement with Victory Park whereby Victory Park purchased $15,000,000 of three-year senior secured non-convertible term notes from us. In May 2009, we issued an additional $5,000,000 in notes to affiliates of Victory Park. In March 2010, we restructured our debt with Victory Park and increased our borrowing to $33,000,000. These new convertible notes bear interest at the prime rate plus 5%, subject to a floor of 15% per annum. Therefore, we are exposed to interest rate fluctuations in the near-term and long-term until these notes are converted or repaid in full. We do not employ specific strategies, such as the use of derivative instruments or hedging, to manage our interest rate exposure. We estimate that, due to the short-term nature of our cash and investments, a change of 100 basis points in interest rates would not have materially affected their fair value.
The information below summarizes our market risks associated with interest bearing debt obligations as of December 31, 2010. The table below presents principal cash flows and related interest rates by year of maturity based on the terms of the debt. Given our financial condition, described in “Liquidity and Capital Resources,” it is not practicable to estimate the fair value of our debt.
|
Debt Obligations
|
Carrying Amount
|
2011
|
2012
|
2013
|
Thereafter
|
|||||||||||||||
|
Note payable – Victory Park Variable interest rate: 15% (1)
|
$ | 33,000,000 | $ | -- | $ | -- | $ | 33,000,000 | $ | -- | ||||||||||
|
Notes payable – Levys – Fixed interest rate: 12%
|
14,737,518 | -- | 750,000 | 13,987,518 | -- | |||||||||||||||
|
Loan payable – CPG Fixed interest rate: 0%
|
1,200,000 | 1,200,000 | -- | -- | -- | |||||||||||||||
|
Total
|
$ | 48,937,518 | $ | 1,200,000 | $ | 750,000 | $ | 46,987,518 | $ | -- | ||||||||||
(1) Prime rate plus 5%, with a floor of 15%
22 of 59
INDEX TO FINANCIAL STATEMENTS
| Page Number | |
| Report of Independent Registered Public Accounting Firm | 24 |
| Report of Independent Registered Public Accounting Firm | 25 |
| BALANCE SHEETS – December 31, 2010 and 2009 | 26 |
| STATEMENTS OF OPERATIONS - Years Ended December 31, 2010, 2009 and 2008 | 27 |
| STATEMENTS OF STOCKHOLDERS’ DEFICIT - Years Ended December 31, 2010, 2009 and 2008 | 28 |
| STATEMENTS OF CASH FLOW - Years Ended December 31, 2010, 2009 and 2008 | 29 |
| NOTES TO FINANCIAL STATEMENTS | 30 |
23 of 59
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders
Unigene Laboratories, Inc.
We have audited the accompanying balance sheets of Unigene Laboratories, Inc. (the “Company) (a Delaware corporation) as of December 31, 2010 and 2009, and the related statements of operations, stockholders’ deficit, and cash flows for each of the three years in the period ended December 31, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. The 2009 financial statements of Tarsa Therapeutics, Inc. (an equity method investment) have been audited by other auditors whose report has been furnished to us, and our opinion on the 2009 financial statements, insofar as it related to the amounts included for Tarsa Therapeutics, Inc., is based solely on the report of other auditors. In the financial statements, the Company investment in Tarsa Therapeutics, Inc. is stated at $0 at December 31, 2009 and the Company’s equity in the net loss of Tarsa Therapeutics, Inc. is stated at $2,119,000 for the year ended December 31, 2009.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits and the report of the other auditors provide a reasonable basis for our opinion.
In our opinion, based on our audits and the report of the other auditors, the financial statements referred to above present fairly, in all material respects, the financial position of Unigene Laboratories, Inc. as of December 31, 2010 and 2009, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2010 in conformity with accounting principles generally accepted in the United States of America. The other auditor’s report on the 2009 financial statements of Tarsa Therapeutics, Inc., included an explanatory paragraph describing conditions that raised substantial doubt about its ability to continue as a going concern.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Unigene Laboratories, Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 16, 2011, expressed an unqualified opinion thereon.
/s/ GRANT THORNTON LLP
New York, New York
March 16, 2011
24 of 59
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
Tarsa Therapeutics, Inc.
We have audited the accompanying balance sheet of Tarsa Therapeutics, Inc. (a development-stage company) as of December 31, 2009, and the related statements of operations, shareholders’ deficit, and cash flows for the period from March 13, 2009 (date of inception) to December 31, 2009 (not presented separately herein). These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Tarsa Therapeutics, Inc. at December 31, 2009, and the results of its operations and its cash flows for the period from March 13, 2009 (date of inception) to December 31, 2009, in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming that Tarsa Therapeutics, Inc. will continue as a going concern. As more fully described in Note 2, the Company has incurred operating losses since inception and will need additional capital to continue its operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The 2009 financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
/s/ Ernst & Young LLP
Philadelphia, Pennsylvania
March 8, 2010
25 of 59
UNIGENE LABORATORIES, INC.
BALANCE SHEETS
DECEMBER 31, 2010 and 2009
|
|
2010
|
2009
|
||||||
|
ASSETS
|
|
|||||||
|
Current assets:
|
|
|||||||
|
Cash and cash equivalents
|
|
$
|
12,200,800
|
$
|
4,894,210
|
|||
|
Accounts receivable
|
|
1,553,091
|
2,201,505
|
|||||
|
Accounts receivable - Tarsa
|
186,263
|
19,593
|
||||||
|
Inventory, net
|
|
1,417,934
|
1,933,012
|
|||||
|
Prepaid expenses and other current assets
|
|
699,950
|
182,817
|
|||||
|
|
||||||||
|
Total current assets
|
|
16,058,038
|
9,231,137
|
|||||
|
Noncurrent inventory
|
2,881,980
|
4,989,668
|
||||||
|
Property, plant and equipment, net
|
|
3,190,117
|
3,679,561
|
|||||
|
Patents and other intangibles, net
|
|
2,670,044
|
2,467,111
|
|||||
|
Investment in China joint venture
|
|
3,175,925
|
3,060,151
|
|||||
|
Investment in Tarsa
|
--
|
--
|
||||||
|
Deferred financing costs, net
|
181,457
|
279,892
|
||||||
|
Other assets
|
|
313,382
|
247,421
|
|||||
|
Total assets
|
|
$
|
28,470,943
|
$
|
23,954,941
|
|||
|
|
||||||||
|
LIABILITIES AND STOCKHOLDERS’ DEFICIT
|
|
|||||||
|
Current liabilities:
|
|
|||||||
|
Accounts payable
|
|
$
|
974,998
|
$
|
1,144,396
|
|||
|
Accrued expenses
|
|
2,834,345
|
2,106,719
|
|||||
|
Current portion - deferred licensing fees
|
|
2,290,916
|
1,326,606
|
|||||
|
Notes payable – Levys
|
|
--
|
2,360,628
|
|||||
|
Accrued interest
|
--
|
1,533,360
|
||||||
|
Due to China joint venture partner, net of discount of $64,571 in 2009
|
1,200,000
|
2,010,429
|
||||||
|
Total current liabilities
|
|
7,300,259
|
10,482,138
|
|||||
|
Notes payable – Levys, excluding current portion
|
14,737,518
|
13,376,889
|
||||||
|
Note payable – Victory Park –net of discount of $7,209,700 in 2010 and $1,357,003 in 2009
|
25,790,300
|
18,180,203
|
||||||
|
Accrued interest –Victory Park and Levys, excluding current portion
|
9,285,359
|
2,189,242
|
||||||
|
Accrued expenses, excluding current portion
|
162,296
|
277,908
|
||||||
|
Deferred licensing fees, excluding current portion
|
|
11,152,037
|
9,452,809
|
|||||
|
Deferred compensation
|
471,890
|
437,413
|
||||||
|
|
||||||||
|
Total liabilities
|
|
68,899,659
|
54,396,602
|
|||||
|
Commitments and contingencies
|
|
|||||||
|
Stockholders’ deficit:
|
|
|||||||
|
Common Stock - par value $.01 per share, authorized 275,000,000 shares in 2010 and 135,000,000 shares in 2009;
issued and outstanding: 92,475,597 shares in 2010 and 91,730,117 shares in 2009
|
|
924,756
|
917,301
|
|||||
|
Additional paid-in capital
|
|
129,227,058
|
111,352,807
|
|||||
|
Accumulated deficit
|
|
(170,580,263
|
)
|
(142,711,769
|
)
|
|||
|
Treasury Stock – at cost (26,650 shares in 2010)
|
|
(267)
|
--
|
|||||
|
|
||||||||
|
Total stockholders’ deficit
|
|
(40,428,716
|
)
|
(30,441,661
|
)
|
|||
|
|
||||||||
|
Total liabilities and stockholders’ deficit
|
|
$
|
28,470,943
|
$
|
23,954,941
|
|||
The accompanying notes are an integral part of these financial statements.
26 of 59
STATEMENTS OF OPERATIONS
Years Ended December 31, 2010, 2009 and 2008
|
2010
|
2009
|
2008
|
||||||||||
|
Revenue:
|
||||||||||||
|
Product sales
|
$ | 5,246,159 | $ | 5,941,254 | $ | 10,057,938 | ||||||
|
Royalties
|
2,974,365 | 4,991,266 | 6,519,942 | |||||||||
|
Licensing revenue
|
1,300,763 | 1,253,260 | 1,256,760 | |||||||||
|
Development fees and other revenues
|
811,992 | 545,788 | 1,394,793 | |||||||||
|
Tarsa revenue
|
1,006,935 | 60,270 | -- | |||||||||
| 11,340,214 | 12,791,838 | 19,229,433 | ||||||||||
|
Operating expenses:
|
||||||||||||
|
Cost of goods sold
|
2,608,083 | 2,171,231 | 5,621,732 | |||||||||
|
Research and development
|
6,428,041 | 12,350,796 | 9,517,448 | |||||||||
|
General and administrative
|
7,685,039 | 8,937,683 | 7,889,260 | |||||||||
|
Unallocated facility expenses
|
3,105,989 | 1,711,642 | 927,553 | |||||||||
|
Inventory reserve
|
716,989 | -- | 223,413 | |||||||||
|
Severance expense - Levys
|
1,120,000 | -- | -- | |||||||||
| 21,664,141 | 25,171,352 | 24,179,406 | ||||||||||
|
Operating loss
|
(10,323,927 | ) | (12,379,514 | ) | (4,949,973 | ) | ||||||
|
Other income (expense):
|
||||||||||||
|
Loss on change in fair value of embedded conversion feature
|
(8,125,000 | ) | -- | -- | ||||||||
|
Debt issuance cost
|
(2,007,534 | ) | -- | -- | ||||||||
|
Qualifying therapeutic discovery grant
|
977,917 | -- | -- | |||||||||
|
Gain on technology license to Tarsa - net
|
-- | 5,685,530 | -- | |||||||||
|
Interest and other income
|
85,546 | 133,581 | 120,654 | |||||||||
|
Interest expense
|
(9,291,773 | ) | (4,862,319 | ) | (2,102,354 | ) | ||||||
|
Gain (loss) from investment in Tarsa and China joint venture
|
115,774 | (2,024,072 | ) | (72,337 | ) | |||||||
|
Loss before income taxes
|
(28,568,997 | ) | (13,446,794 | ) | (7,004,010 | ) | ||||||
|
Income tax benefit – principally from sale of New Jersey tax benefits in 2010 and 2008
|
700,503 | 67,115 | 925,588 | |||||||||
|
Net loss
|
$ | (27,868,494 | ) | $ | (13,379,679 | ) | $ | (6,078,422 | ) | |||
|
Loss per share – basic and diluted:
|
||||||||||||
|
Net loss per share
|
$ | (0.30 | ) | $ | (0.15 | ) | $ | (0.07 | ) | |||
|
Weighted average number of shares outstanding – basic and diluted
|
91,961,673 | 90,662,089 | 88,751,289 | |||||||||
The accompanying notes are an integral part of these financial statements.
27 of 59
STATEMENTS OF STOCKHOLDERS’ DEFICIT
Years Ended December 31, 2010, 2009 and 2008
| Common Stock |
Additional
|
|||||||||||||||||||||||
|
Number of
|
Paid-in
|
Accumulated |
Treasury
|
|||||||||||||||||||||
|
Shares
|
Par Value
|
Capital
|
Deficit
|
Stock
|
Total
|
|||||||||||||||||||
|
Balance, January 1, 2008
|
87,753,715 | $ | 877,537 | $ | 105,705,387 | $ | (123,253,668 | ) | $ | — | $ | (16,670,744 | ) | |||||||||||
|
Sale of common stock to CPG at $1.86 per share
(net of cash issuance costs of $74,000)
|
1,080,000 | 10,800 | 1,923,505 | — | — | 1,934,305 | ||||||||||||||||||
|
Issuance of stock to Victory Park
|
1,125,000 | 11,250 | 1,215,000 | — | — | 1,226,250 | ||||||||||||||||||
|
Recognition of stock option compensation expense – employees
and directors
|
— | — | 450,299 | — | — | 450,299 | ||||||||||||||||||
|
Recognition of stock option compensation benefit - consultants
|
— | — | (143,177 | ) | — | — | (143,177 | ) | ||||||||||||||||
|
Discount on note payable issued to joint venture partner
|
— | — | 160,064 | — | — | 160,064 | ||||||||||||||||||
|
Issuance of restricted stock
|
178,961 | 1,790 | (1,790 | ) | — | — | — | |||||||||||||||||
|
Recognition of restricted stock compensation expense
|
— | — | 144,567 | — | — | 144,567 | ||||||||||||||||||
|
Cashless warrant exercise
|
47,844 | 478 | (478 | ) | — | — | — | |||||||||||||||||
|
Exercise of stock options
|
10,000 | 100 | 4,300 | — | — | 4,400 | ||||||||||||||||||
|
Net loss
|
— | — | — | (6,078,422 | ) | — | (6,078,422 | ) | ||||||||||||||||
|
Balance, December 31, 2008
|
90,195,520 | 901,955 | 109,457,677 | (129,332,090 | ) | — | (18,972,458 | ) | ||||||||||||||||
|
Issuance of stock to Victory Park in connection with debt issuance and Tarsa transaction
|
675,000 | 6,750 | 930,000 | — | — | 936,750 | ||||||||||||||||||
|
Stock issued in lieu of director fees
|
91,886 | 919 | 72,581 | — | — | 73,500 | ||||||||||||||||||
|
Recognition of restricted stock compensation expense
|
— | — | 119,700 | — | — | 119,700 | ||||||||||||||||||
|
Issuance of restricted stock
|
60,000 | 600 | (600 | ) | — | — | — | |||||||||||||||||
|
Discount on note payable issued to joint venture partner
|
— | — | 27,978 | — | — | 27,978 | ||||||||||||||||||
|
Recognition of stock option compensation expense – employees and directors
|
— | — | 378,212 | — | — | 378,212 | ||||||||||||||||||
|
Recognition of stock option compensation expense - consultants
|
— | — | 43,393 | — | — | 43,393 | ||||||||||||||||||
|
Cashless warrant exercise
|
77,811 | 778 | (778 | ) | — | — | — | |||||||||||||||||
|
Exercise of stock options
|
629,900 | 6,299 | 324,644 | — | — | 330,943 | ||||||||||||||||||
|
Net loss
|
— | — | — | (13,379,679 | ) | — | (13,379,679 | ) | ||||||||||||||||
|
Balance December 31, 2009
|
91,730,117 | 917,301 | 111,352,807 | (142,711,769 | ) | — | (30,441,661 | ) | ||||||||||||||||
|
Stock issued in lieu of director fees
|
174,780 | 1,748 | 127,252 | — | — | 129,000 | ||||||||||||||||||
|
Issuance of restricted stock
|
372,100 | 3,721 | (3,721 | ) | — | — | — | |||||||||||||||||
|
Exercise of stock options
|
158,600 | 1,586 | 71,762 | — | — | 73,348 | ||||||||||||||||||
|
Embedded conversion feature
|
— | — | 16,724,000 | — | — | 16,724,000 | ||||||||||||||||||
|
Issuance of common stock to directors
|
40,000 | 400 | 30,400 | — | — | 30,800 | ||||||||||||||||||
|
Discount on note payable issued to joint venture partner
|
— | — | (1,918 | ) | — | — | (1,918 | ) | ||||||||||||||||
|
Forfeiture of restricted stock
|
— | — | 267 | — | (267 | ) | — | |||||||||||||||||
|
Recognition of restricted stock compensation
|
— | — | 243,240 | — | — | 243,240 | ||||||||||||||||||
|
Recognition of stock option compensation expense – employees and directors
|
— | — | 689,340 | — | — | 689,340 | ||||||||||||||||||
|
Recognition of stock option compensation expense - consultants
|
— | — | (6,371 | ) | — | — | (6,371 | ) | ||||||||||||||||
|
Net loss
|
— | — | — | (27,868,494 | ) | — | (27,868,494 | ) | ||||||||||||||||
|
Balance, December 31, 2010
|
92,475,597 | $ | 924,756 | $ | 129,227,058 | $ | (170,580,263 | ) | $ | (267 | ) | $ | (40,428,716 | ) | ||||||||||
The accompanying notes are an integral part of these financial statements.
28 of 59
UNIGENE LABORATORIES, INC.
STATEMENTS OF CASH FLOWS
Years Ended December 31, 2010, 2009 and 2008
|
|
2010
|
2009
|
2008
|
|||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|
|||||||||||
|
Net loss
|
|
$
|
(27,868,494)
|
$
|
(13,379,679)
|
$
|
(6,078,422)
|
|||||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|||||||||||
|
Amortization of deferred revenue
|
|
(1,326,620)
|
(1,367,664)
|
(1,456,760)
|
||||||||
|
Amortization of discounts and deferred financing fees
|
2,844,736
|
845,803
|
174,760
|
|||||||||
|
Non-cash equity compensation
|
|
1,086,009
|
614,805
|
451,689
|
||||||||
|
Gain on technology license to Tarsa, net
|
—
|
(5,685,530)
|
—
|
|||||||||
|
Debt issuance cost
|
2,007,534
|
—
|
—
|
|||||||||
|
Loss on change in fair value of embedded conversion feature
|
8,125,000
|
—
|
—
|
|||||||||
|
Depreciation, amortization and impairment of long-lived assets
|
|
965,008
|
732,464
|
903,018
|
||||||||
|
Inventory reserve provision
|
716,989
|
—
|
223,413
|
|||||||||
|
(Gain)/loss on investment in Tarsa and China joint venture
|
(115,774)
|
2,024,072 | 72,337 | |||||||||
|
|
||||||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Decrease (increase) in accounts receivables
|
|
481,744
|
2,413,938
|
(1,492,840)
|
||||||||
|
Decrease (increase) in prepaid interest
|
—
|
525,000
|
(525,000)
|
|||||||||
|
Decrease (increase) in inventory
|
|
1,905,777
|
(2,092,971)
|
(1,098,813)
|
||||||||
|
Decrease (increase) in other assets
|
|
(583,095)
|
1,716,948
|
(1,037,396)
|
||||||||
|
Increase (decrease) in accounts payable and accrued expenses
|
|
505,271
|
503,892
|
(163,229)
|
||||||||
|
Increase in accrued interest - Vicotory Park and Levys
|
|
5,562,757
|
1,627,629
|
1,311,694
|
||||||||
|
Increase (decrease) in deferred compensation
|
34,477
|
66,267
|
(3,354)
|
|||||||||
|
Increase in deferred revenue
|
|
3,990,160
|
164,252
|
143,750
|
||||||||
|
|
||||||||||||
|
Net cash used in operating activities
|
|
(1,668,521)
|
(11,290,774)
|
(8,575,153)
|
||||||||
|
|
||||||||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|
|||||||||||
|
Net proceeds from technology license to Tarsa
|
—
|
4,139,080
|
—
|
|||||||||
|
Investment in China joint venture
|
—
|
(317,354)
|
(583,491)
|
|||||||||
|
Construction of leasehold and building improvements
|
|
(2,941)
|
(76,916)
|
(521,474)
|
||||||||
|
Purchase of equipment and furniture
|
|
(144,478)
|
(220,055)
|
(884,640)
|
||||||||
|
Increase in patents and other intangibles
|
|
(531,078)
|
(494,548)
|
(559,892)
|
||||||||
|
Decrease in other assets
|
|
—
|
—
|
4,335
|
||||||||
|
|
||||||||||||
|
Net cash (used in) provided by investing activities
|
|
(678,497)
|
3,030,207
|
(2,545,162)
|
||||||||
|
|
||||||||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|
|||||||||||
|
Issuance of note payable – Victory Park, net
|
13,642,473
|
4,803,402
|
14,129,142
|
|||||||||
|
Expenses related to issuance of note payable – Victory Park
|
(2,007,534)
|
—
|
—
|
|||||||||
|
Proceeds from sale of stock, net
|
|
—
|
—
|
1,934,305
|
||||||||
|
Repayment of notes payable – Levys
|
|
(1,000,000)
|
—
|
—
|
||||||||
|
Repayment on note payable – Victory Park
|
(179,679)
|
(462,794)
|
—
|
|||||||||
|
Repayment on note payable-China joint venture
|
(875,000)
|
(100,000)
|
—
|
|||||||||
|
Repayment of capital lease obligations
|
|
—
|
—
|
(41,943)
|
||||||||
|
Proceeds from exercise of stock options
|
|
73,348
|
330,943
|
4,400
|
||||||||
|
|
||||||||||||
|
Net cash provided by financing activities
|
|
9,653,608
|
4,571,551
|
16,025,904
|
||||||||
|
|
||||||||||||
|
Net increase (decrease) in cash and cash equivalents
|
|
7,306,590
|
(3,689,016)
|
4,905,589
|
||||||||
|
Cash and cash equivalents at beginning of year
|
|
4,894,210
|
8,583,226
|
3,677,637
|
||||||||
|
|
||||||||||||
|
Cash and cash equivalents at end of year
|
|
$
|
12,200,800
|
$
|
4,894,210
|
$
|
8,583,226
|
|||||
|
|
||||||||||||
|
SUPPLEMENTAL CASH FLOW INFORMATION:
|
|
|||||||||||
|
Non-cash investing and financing activities:
|
|
|||||||||||
|
Issuance of convertible note in exchange for non-convertible note
|
$
|
19,357,527
|
$
|
—
|
$
|
—
|
||||||
|
Common stock received for license to Tarsa
|
$
|
—
|
$
|
2,119,450
|
$
|
—
|
||||||
|
Contribution to joint venture financed through interest-free note payable to joint venture partner
|
|
$
|
—
|
$
|
1,200,000
|
$
|
975,000
|
|||||
|
Common stock issued with debt financing (see Note 8)
|
|
$
|
—
|
$
|
363,750
|
$
|
1,226,250
|
|||||
|
Common stock issued in exchange for warrant
|
$
|
—
|
$
|
573,000
|
$
|
—
|
||||||
|
Cash payments:
|
|
|||||||||||
|
Cash paid for interest
|
|
$
|
803,000
|
$
|
1,750,000
|
$
|
1,085,000
|
|||||
|
Cash paid for income taxes
|
|
$
|
—
|
$
|
—
|
$
|
—
|
|||||
The accompanying notes are an integral part of these financial statements.
29 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
1. Description of Business
We are a biopharmaceutical company engaged in the research, production and delivery of small proteins, referred to as peptides, for medical use. We have a patented manufacturing technology for producing many peptides cost-effectively. We also have patented oral and nasal delivery technologies that have been shown to deliver medically useful amounts of various peptides into the bloodstream. We have three operating locations: administrative and regulatory offices in Boonton, New Jersey; a laboratory research facility in Fairfield, New Jersey; and a pharmaceutical production facility in Boonton, New Jersey. Our primary focus has been on the development of calcitonin and other peptide products for the treatment of osteoporosis and other indications. We have licensed in the U.S. our nasal calcitonin product for the treatment of osteoporosis, which we have trademarked as Fortical, to USL. Fortical was approved by the FDA in 2005. We have licensed worldwide rights to our patented manufacturing technology for the production of calcitonin to Novartis. We have licensed worldwide rights to our manufacturing and delivery technologies for oral PTH for the treatment of osteoporosis to GSK. In 2009, we licensed on a worldwide basis (except for China) our Phase III oral calcitonin program to Tarsa. We have expanded our product pipeline with a novel peptide for obesity; our SDBG technology developed in conjunction with Yale University; in-licensed technology from Queen Mary, University of London relating to potential therapies for inflammation and cardiovascular disease; and we periodically perform feasibility studies for third parties. During the period of 2008 through 2010, most of our revenue was generated from one customer, USL (see Note 19 to the financial statements).
2. Liquidity
At December 31, 2010, we had cash and cash equivalents of $12,201,000, an increase of $7,307,000 from December 31, 2009. The increase was primarily due to funds received pursuant to a restructuring of our debt with Victory Park and Fortical revenue, as well as a $4,000,000 payment received from GSK in December under our amended agreement. (See Note 19.) In March 2010, we entered into an amended and restated financing agreement with Victory Park. The restated financing agreement amends and restates in its entirety and replaces the financing agreement dated as of September 30, 2008.
Under the terms of the restated financing agreement, we issued to Victory Park $33,000,000 aggregate principal amount of three-year, senior secured convertible notes by way of surrender of the three-year, senior secured non-convertible notes previously issued pursuant to the original financing agreement, in the aggregate principal amount of approximately $19,358,000, and by way of cash payment of approximately $13,642,000 for the balance. After fees and expenses, we received net cash proceeds of approximately $11,635,000 on March 17, 2010. In addition, under certain circumstances, we may request that Victory Park purchase (which purchase shall be in Victory Park’s sole discretion) up to an additional $3 million aggregate principal amount of convertible notes at one subsequent closing. The maturity date of the convertible notes has been extended to March 17, 2013 from September 30, 2011 under the original notes (See Note 8). Based upon our current level of revenue and expenses, we believe our current cash should be sufficient to support our current operations into the second half of 2012.
In December 2009, we announced a restructuring plan that included a reduction in workforce and expenses to improve operational efficiencies and to better match resources with market demand. Our restructuring expenses for 2009 were $353,000, of which $242,000 represented severance pay. These expenses are included in research and development expenses and in general and administrative expenses. There were no restructuring expenses in 2010. Under the comprehensive plan, we are continuing Fortical production and maintaining all of our core programs and partnered activities while decreasing cash expenditures. We decreased cash expenditures by approximately $9 million for 2010. Since we currently maintain an adequate inventory of calcitonin and enzyme to support Fortical, we have temporarily suspended manufacturing of those materials at our Boonton facility. However, we will maintain the cGMP status of the facility and the ability to manufacture peptides at that location. Implementation of the plan resulted in an immediate company-wide workforce reduction of approximately 30%. The plan further provided for salary reductions in 2010 at all levels, including senior management, and other cost savings. From time to time we will evaluate the feasibility of additional expenditures in areas such as preclinical and/or clinical studies in order to add value to certain of our current research projects.
In addition to our debt restructuring in March 2010, other cash received during 2010 was primarily from Fortical sales and royalties received under our agreement with USL, as well as the $4,000,000 we received from GSK in December 2010. Our primary sources of cash have historically been (1) licensing fees for new agreements, (2) milestone payments under licensing or development agreements, (3) bulk peptide sales under licensing or supply agreements, (4) stockholder loans, (5) the sale of our common stock and (6) since 2005, Fortical sales and royalties. We cannot be certain that any of these cash sources will continue to be available to us in the future. Licensing fees from new collaborations are dependent upon the successful completion of complex and lengthy negotiations. Milestone payments are based upon progress achieved in collaborations, which cannot be guaranteed, and are often subject to factors that are controlled neither by our licensees nor us. Product sales to our partners under these agreements are based upon our licensees’ needs, which are sometimes difficult to predict. Sale of our common stock is dependent upon our ability to attract interested investors, our ability to negotiate favorable terms and the performance of the stock market in general and biotechnology investments in particular. Future Fortical sales and royalties will be affected by competition and continued acceptance in the marketplace and could be impacted by manufacturing, distribution or regulatory issues.
We believe that in 2011 we will generate cash to apply toward funding a portion of our operations through sales of Fortical to USL and royalties on USL’s sales of Fortical, as well as our anticipated receipt of a $4,000,000 milestone payment from GSK upon completion of Phase II patient enrollment, and, in the long term, on sales and royalties from the sale of Fortical and oral calcitonin, the achievement of milestones under our existing license agreements and revenue on future licensed products and technologies. We are actively seeking additional licensing and/or supply agreements with pharmaceutical companies for various oral peptides, including our obesity peptide, and for our peptide manufacturing technology. However, we may not be successful in achieving milestones under our current agreements, in obtaining regulatory approval for our other products or in licensing any of our other products or technologies.
30 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
Due to our limited financial resources, any further decline in Fortical sales and/or royalties, or delay in achieving milestones under our existing license agreements, or in signing new license or distribution agreements for our products or technologies or loss of patent protection, could have a material adverse effect on our cash flow and operations (see Notes 10, 19 and 20).
If we are unable to achieve significant milestones or sales under our existing agreements and/or enter into a new significant revenue generating license or other arrangement, we would eventually need to either secure another source of funding in order to satisfy our working capital needs and to remain in compliance with covenants in our restated financing agreement with Victory Park (see Note 8) or significantly curtail our operations. Under the restated financing agreement we must maintain a cash balance equal to at least $2,500,000 and our cash flow (as defined in the restated financing agreement) must be at least $2,000,000 in any fiscal quarter or $7,000,000 in any three consecutive quarters. These default provisions were temporarily waived under a forbearance agreement executed in December 2010. The forbearance period began December 10, 2010 and will terminate upon the earliest to occur of (i) the termination of the amended GSK license agreement, (ii) June 30, 2012 (or such later date as the parties may agree in writing), (iii) GSK’s failure to pay the Company certain specific amounts pursuant to the amended license agreement, or (iv) the date when the Company (a) repudiates or asserts a defense to any obligation or liability under the forbearance agreement or any transaction document (as defined in the restated financing agreement or (b) makes or pursues a claim against Victory Park or any secured party named in the forbearance agreement. Should the funding we require to sustain our working capital needs be unavailable or prohibitively expensive, the consequence would be a material adverse effect on our business, operating results, financial condition and prospects. We believe that the Victory Park financing has satisfied our current cash requirements, but satisfying our cash requirements over the long term will require the successful commercialization of one or more of our biotechnologies or our licensees’ oral or nasal calcitonin products, our oral PTH product, the obesity program or another peptide product in the U.S. and/or abroad. However, it is uncertain whether any of these products other than Fortical will be approved or will be commercially successful. The amount of future revenue we will derive from Fortical is also uncertain.
We have incurred annual operating losses since our inception and, as a result, at December 31, 2010, had an accumulated deficit of approximately $171,000,000. Our cash requirements in 2010 to operate our research and peptide manufacturing facilities and develop our products decreased from 2009 due to our December 2009 restructuring.
The global credit crisis that began in 2007 was further exacerbated by events occurring in the financial markets in the fall of 2008, which continued in 2009 and 2010. These events have negatively impacted the ability of corporations to raise capital through equity financings or borrowings. The credit crisis may continue for the foreseeable future. In addition, uncertainty about current and future global economic conditions may impact our ability to license our products and technologies to other companies and may cause consumers to defer purchases of prescription medicines, such as Fortical, in response to tighter credit, decreased cash availability and declining consumer confidence. Accordingly, future demand for our product could differ from our current expectations.
3. Summary of Significant Accounting Policies & Practices
Revenue Recognition: We recognize revenue from the sale of products and from royalties, licensing agreements, research services and grants. Revenue from the sale of product is recognized when title to product and risk of loss are transferred, collection is reasonably assured and we have no further obligations. If there are elements of the revenue recognition requirements that are not met at the time of shipment, the revenue is deferred and the corresponding cost of the product is included on our balance sheet as a deferred asset. Revenue from research services, such as for Tarsa, is recognized when services are rendered. We occasionally apply to various government agencies for research grants. Revenue from such research grants is recognized when work is conducted under the grant. Sales and grant revenues generally do not involve difficult, subjective or complex judgments. We recognize royalty revenue on an accrual basis in accordance with the terms of individual agreements. If the receipt of royalty revenue is contingent on a future event, revenue would be recognized when that event has occurred. Typically, royalties are recognized when third party results can be reliably measured and collectability is reasonably assured. In the case of USL, we recognize royalty revenue based upon the quarterly USL royalty report. This enables a reliable measure, as well as reasonable assurances of collectability.
We follow the accounting guidance for licensing agreements which typically include several elements of revenue, such as up-front payments, milestones, royalties upon sales of product and the delivery of product and/or research services to the licensor. Non-refundable license fees received upon execution of license agreements where we have continuing involvement are deferred and recognized as revenue over the estimated performance period of the agreement. This requires management to estimate the expected term of the agreement or, if applicable, the estimated life of its licensed patents. For our USL and GSK agreements, the following describes our revenue recognition accounting policy. Non-refundable milestone payments that represent the completion of a separate earnings process and a substantive step in the research and development process are recognized as revenue when earned at the lesser of 1) the non-refundable cash received or 2) the proportionate level of effort expended to date during the development stage multiplied by the development stage revenue. This sometimes requires management to judge whether or not a milestone has been met and when it should be recognized in the financial statements. Typically, this would involve discussions between management and our licensing partner. Payments for milestones for which the completion of the earnings process is not yet complete (for example, payment received for the commencement of an activity that represents a separate earnings process) are recognized over the estimated performance period of such activity. This is to comply with accounting guidance, which provides for revenue to be recognized once delivery has occurred or services have been rendered. Management must determine if a milestone payment is substantive, i.e. whether or not it is reasonable relative to all of the deliverables and payment terms in the agreement; commensurate with our level of effort; and commensurate with the enhanced value of the deliverables. Royalties and/or milestones relating to sales of products by our licensees, and the delivery of products and research services, will be recognized as such events occur as they represent the completion of a separate earnings process.
In addition, accounting guidance requires a company to evaluate its arrangements under which it will perform multiple revenue-generating activities. For example, a license agreement with a pharmaceutical company may involve a license, research and development activities and/or contract manufacturing. Management is required to determine if the separate components of the agreement have value on a standalone basis and qualify as separate units of accounting, whereby consideration is allocated based upon their relative “fair values.” A delivered item(s) that does not qualify as a
31 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
separate unit of accounting within the arrangement shall be combined with the other applicable undelivered item(s) within the arrangement. The allocation of arrangement consideration and the recognition of revenue then shall be determined for those combined deliverables as a single unit of accounting.
Accounting for Stock Options - For stock options granted to employees and directors, we recognize compensation expense based on the grant-date fair value estimated in accordance with accounting guidance. We estimate the fair value of each option award on the date of grant using the Black-Scholes option pricing model. Stock options and warrants issued to consultants are accounted for in accordance with accounting guidance at their fair value on the measurement date using the Black-Scholes option pricing model. The measurement of share-based compensation is subject to periodic adjustment as the underlying awards vest. Compensation expense is calculated each quarter for consultants using the Black-Scholes option pricing model until the option is fully vested and is included in research and development expenses.
Inventory - Production inventories at our Boonton, NJ location are stated at the lower of cost or market, valued at specifically identified cost which approximates FIFO. Research inventories at our Fairfield, NJ location are stated at the lower of cost (using the FIFO method) or market. Typically, finished goods and work in process inventory are fully reserved except for the amounts deemed saleable by management based upon current or anticipated orders, primarily under contractual arrangements.
Use of Estimates - The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Our most significant estimates involve estimating the lives of our license agreements, which typically are determined by the estimated useful lives of our licensed patents. This would determine the period over which certain revenue would be recognized. Other significant estimates relate to valuation of inventory, fixed assets, deferred tax assets and intangible assets, which involve management estimates of the lives of the assets and the recoverability of costs.
Equity Method Investments - We account for our investment in the China joint venture and Tarsa under the equity method and therefore recognize our proportionate share of each joint venture’s earnings and losses based upon our ownership percentages. For our China Joint Venture, we recognize 45% of the entity’s profits or losses. For Tarsa, in 2009, we recognized approximately 26% of the entity’s profits or losses. Our share of Tarsa’s 2009 loss that we recognized was $2,119,000, the amount of our investment in Tarsa, as a result of our valuation of Tarsa’s common stock with the assistance of an outside appraiser. Our share of Tarsa’s loss would have been $3,795,000 so that the unrecognized loss of $1,676,000 is to be carried over to future periods. Since we have no future funding obligations, we discontinued the equity method of accounting for our investment in Tarsa.
Patents and Other Intangibles - Amounts paid for patents and trademarks are capitalized. Patent and trademark costs are deferred pending the successful outcome of the relevant applications. These costs primarily consist of outside legal fees and typically relate to filings and prosecution of applications for improvements to existing patents or to the filings of foreign applications based upon domestic patents. Most of these applications relate to our patented peptide manufacturing and patented oral delivery technologies, as well as for our SDBG technology. Internal costs related to intangible assets are expensed as incurred. Successful patent costs are amortized using the straight-line method over the lives of the patents, typically five to fifteen years. Successful trademark costs are amortized using the straight-line method over their estimated useful lives of typically five to ten years. We assess the impairment of identifiable intangibles and other long-lived assets whenever events or changes in circumstances indicate that the carrying value may not be recoverable. Some factors we consider important which could trigger an impairment review include (i) significant underperformance relative to expected historical or projected future operating results; (ii) significant changes in the manner of our use of the acquired assets or the strategy for our overall business; and (iii) significant negative industry or economic trends.
When we determine that the carrying value of intangible assets may not be recoverable based upon the existence of one or more of the above indicators of impairment, we first will perform an assessment of the asset’s recoverability based on expected undiscounted future net cash flow, and if the amount is less than the asset’s value, we will measure any impairment based on a projected discounted cash flow method using a discount rate determined by our management to be commensurate with the risk inherent in our current business model. Unsuccessful patent costs are expensed when determined worthless or when patent applications will no longer be pursued. Other intangibles are recorded at cost and are amortized over their estimated useful lives.
Segment Information - We are managed and operated as one business. Our entire business is managed by a single management team that reports to the chief executive officer. We do not operate separate lines of business or separate business entities with respect to any of our product candidates. Accordingly, we do not prepare discrete financial information with respect to separate product areas or by location and do not have separately reportable segments as defined by accounting guidance.
Deferred Financing Costs – Amounts paid related to debt financing activities are capitalized and amortized over the term of the loan. Our expenses incurred related to the Victory Park financing are being amortized over the three-year term of the notes to interest expense on a straight-line basis which approximates the effective interest rate method.
Cash Equivalents - We consider all highly liquid securities purchased with an original maturity of three months or less to be cash equivalents, which currently consist of money market and checking accounts.
32 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
Accounts Receivable - During 2010 and 2009, most of our accounts receivable involved transactions with established pharmaceutical companies, Novartis and USL. The terms of payments and collections in these transactions are governed by contractual agreements. Our policy is that an allowance for doubtful accounts is established for estimated losses resulting from the inability of our customers to make required payments. Such allowance is computed based upon a specific customer account review of larger customers and balances in excess of 90 days old. Our assessment of our customers’ ability to pay generally includes direct contact with the customer, investigation into our customers’ financial status, as well as consideration of our customers’ payment history with us. If the financial condition of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required. If we determine, based on our assessment, that it is more likely than not that our customers will be unable to pay, we will write-off the account receivables. At both December 31, 2010 and 2009, no allowances were recorded based upon the Company’s analysis that all receivables were deemed collectible.
Property, Plant and Equipment - Property, plant and equipment are carried at cost. Equipment under capital leases is stated at the present value of the minimum lease payments. Depreciation is computed using the straight-line method. Amortization of equipment under capital leases and leasehold improvements is computed over the shorter of the lease term or estimated useful life of the asset. Additions and improvements are capitalized, while repairs and maintenance are expensed as incurred.
Fair Value of Financial Instruments - The fair value of a financial instrument represents the amount at which the instrument could be exchanged in a current transaction between willing parties, other than in a forced sale or liquidation. Significant differences can arise between the fair value and carrying amounts of financial instruments that are recognized at historical cost amounts. Given our financial condition described in Note 2, it is not practicable to estimate the fair value of our notes payable at December 31, 2010 and 2009. We believe that the carrying amounts of cash and cash equivalents, our accounts receivable and accounts payable approximate fair value due to their short-term nature.
Research and Development - Research and development expenses include the costs associated with our internal research and development and research and development conducted for us by third parties. These costs primarily consist of salaries, pre-clinical and clinical trials, outside consultants, supplies, and indirect costs. Indirect costs such as depreciation, rent, utilities, insurance, taxes, and maintenance are allocated to research and development based on specific criteria such as square footage utilized. All research and development costs discussed above are expensed as incurred. Third-party expenses reimbursed under research and development contracts, which are not refundable, are recorded as a reduction to research and development expense in the statement of operations. These expenses have not been material.
Sales Concentrations – Our revenue for the last three years has almost entirely been from product sales, royalties and licensing revenue under our agreements with USL, Novartis and Tarsa.
Revenue from Licensees:
|
2010
|
2009
|
2008
|
||||||||||
|
USL
|
73 | % | 87 | % | 87 | % | ||||||
|
Novartis
|
12 | % | 9 | % | 8 | % | ||||||
|
Tarsa
|
9 | % | -- | -- | ||||||||
|
GSK
|
2 | % | 1 | % | 1 | % | ||||||
|
Other
|
4 | % | 3 | % | 4 | % | ||||||
Concentrations of Credit Risk – Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable. We maintain our holdings in cash in excess of federally insured amounts and in cash equivalents with high credit-quality financial institutions in order to minimize credit exposure. We have concentrations of credit risk with respect to accounts receivable, as most of our accounts receivable are concentrated among a very small number of customers. As of December 31, 2010 and December 31, 2009, USL accounted for approximately 84% and 98%, respectively, of our total accounts receivable. We perform credit evaluations of our customers, but generally do not require collateral to support accounts receivable.
Income Taxes - Income taxes are accounted for under the asset and liability method with deferred tax assets and liabilities recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis and loss carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be reversed or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment date. A valuation allowance is established to reduce deferred tax assets if it is more likely than not that all, or some portion, of such deferred tax assets will not be realized.
Reclassifications – Certain reclassifications to prior years’ financial statements have been made to conform to the current presentation.
Net Loss per Share - We compute and present both basic and diluted earnings per share (“EPS”) on the face of the statement of operations. Basic EPS is computed using the weighted average number of common shares outstanding during the period. Diluted EPS reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock at the beginning of the period (or as of date of issuance if issued in the current year) being reported on, and the effect was dilutive. Our weighted average shares outstanding used for computing diluted loss per share were the same as that used for computing basic loss per share for each of the years ended December 31, 2010, 2009 and 2008 because inclusion of our restricted stock, stock options and warrants (approximately 7,374,000, 7,025,000 and 6,876,000 shares of common stock, if vested or exercised, at December 31, 2010, 2009 and 2008, respectively) would be antidilutive.
33 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
At December 31, 2010, there were warrants outstanding to purchase an aggregate of 60,000 shares of common stock at exercise prices ranging from $2.00 to $2.20 per share, with expiration dates ranging from 2012 to 2015. At December 31, 2009, there were warrants outstanding to purchase an aggregate of 2,121,571 shares of common stock at exercise prices ranging from $1.77 to $4.25 per share, with expiration dates ranging from 2010 to 2015. The convertible notes to Victory Park have not been included above because they were not convertible as of December 31, 2010.
Reconciliation between the number of issued shares shown on the balance sheet and the number of shares used to determine EPS at December 31, 2010 and December 31, 2009:
|
2010
|
2009
|
|||||||
|
Issued shares per balance sheet
|
92,475,597 | 91,730,117 | ||||||
|
Unvested restricted stock
|
(164,000 | ) | (50,000 | ) | ||||
|
Treasury Stock
|
(26,650 | ) | -- | |||||
|
Shares used in determining EPS
|
92,284,947 | 91,680,117 | ||||||
New Accounting Pronouncements
In April 2010, an accounting standard update was issued to provide guidance on defining a milestone and determining when it is appropriate to apply the milestone method of revenue recognition for research and development transactions. Vendors can recognize consideration that is contingent upon achievement of a milestone in its entirety as revenue in the period the milestone is achieved if the milestone meets all the criteria stated in the guidance to be considered substantive and must be considered substantive in its entirety. The amendments in this update became effective for our quarter ending June 30, 2010. The adoption of this update did not have a significant effect on our financial statements.
In January 2010, an accounting standards update was issued which includes new disclosure requirements related to fair value measurements, including transfers in and out of Levels 1 and 2 and information about purchases, sales, issuances and settlements for Level 3 fair value measurements. This update also clarifies existing disclosure requirements relating to levels of disaggregation and disclosures of inputs and valuation techniques. The adoption of this update did not have a material impact on the Company's financial statements (see Note 21).
In October 2009, an update to accounting guidance was issued to address the accounting for multiple-deliverable arrangements to enable vendors to account for products or services separately rather than as a combined unit. The amendments in this update will eliminate the residual method of allocation and require that arrangement consideration be allocated at the inception of the arrangement to all deliverables using the relative selling price method. The amendments in this update will also require that a vendor determine its best estimate of selling price in a manner that is consistent with that used to determine the price to sell the deliverable on a standalone basis. The amendments in this update will become effective prospectively for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. We do not expect that the adoption will have a significant effect on our financial statements.
4. Notes Payable - Levys
To satisfy our short-term liquidity needs, Jay Levy, Jean Levy, Warren Levy and Ronald Levy (the “Levys”) from time to time (prior to 2003) made loans to us. Jay Levy, our former director, Chairman of the Board, Treasurer and Assistant Secretary, and Jean Levy are/were the parents of Warren Levy and Ronald Levy, former executive officers and directors. At May 10, 2007, the outstanding principal and interest on these loans were $7,095,000 and $8,642,517, respectively, with interest rates ranging from 8.5% to 14.2%. The total owed on May 10, 2007 aggregated $15,737,517, of which approximately $8,900,000 in principal and interest were in default, and was restructured as eight-year term notes, with a fixed simple interest rate of 9% per annum. Interest expense was calculated using an effective interest method, at a rate of 7.6%, over the life of the notes due to the deferred payment schedule contained in the notes. No gain or loss was recognized on the restructuring transaction. Required quarterly payments of principal and interest under these new notes were to begin in May 2010 and continue over a five-year period.
In March 2010, in conjunction with the Victory Park refinancing, the Levy loans were amended and restated to modify the terms therein. The amended notes, which continue to be secured by a secondary lien on our equipment and certain of our United States patents and patent applications, as well as a secondary mortgage on certain of our real property, bore interest at a rate of 9% per annum from May 10, 2007 to March 17, 2010 and 12% per annum thereafter, each of which shall be non-compounding. As of March 17, 2010, interest expense is calculated using an effective interest method, at a rate of 8.2%, over the life of the notes due to the deferred payment schedule contained in the notes. Subject to the conditions set forth in our financing agreement with Victory Park, we are obligated to make the following payments as set forth in, and pursuant to, the amended notes: an aggregate principal payment of $1,000,000 on May 10, 2010, which has been paid; an aggregate principal payment of $500,000 on November 10, 2010 (unpaid as of December 31, 2010); an aggregate principal payment of $250,000 on May 10, 2011; and payment of all unpaid principal and accrued and unpaid interest on June 18, 2013. These loans remain subordinated to the Victory Park notes. In March 2011 we signed a settlement and release agreement with the Levys to defer and reschedule certain severance payments, vacation pay, deferred compensation and loan payments. Under the agreement, we will make a lump sum payment to the Levys in March 2011 in the amount of $1,200,000 for severance payments and a portion of accrued vacation pay. All loan payments are being temporarily deferred. In addition, we agreed to pay an aggregate of $1,112,296 in monthly payments from May 2012 through December 2012. Approximately $900,000 in payments is being deferred.
Total interest expense on all Levy loans was approximately $1,446,000 and $1,403,000 respectively, for 2010 and 2009. As of December 31, 2010, total accrued interest on all Levy loans was $4,932,602 and the outstanding loans by these persons to us totaled $14,737,518 (after a required $1,000,000 principal payment in May 2010), for an aggregate owed to them of $19,670,120.
34 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
Outstanding Levy loans consisted of the following at December 31, 2010 and December 31, 2009 (in thousands):
|
2010
|
2009
|
|||||||
|
Long-term loans, current portion
|
$ | -- | $ | 2,361 | ||||
|
Long-term loans
|
14,738 | 13,377 | ||||||
| 14,738 | 15,738 | |||||||
|
Accrued interest, short-term
|
-- | 1,298 | ||||||
|
Accrued interest, long-term
|
4,932 | 2,189 | ||||||
|
Total loans and interest
|
$ | 19,670 | $ | 19,225 | ||||
5. Property, Plant and Equipment
Property, plant and equipment consisted of the following at December 31, 2010 and 2009:
|
2010
|
2009
|
Estimated
Useful Lives
|
|||||||
|
Building and improvements
|
$ | 1,497,425 | $ | 1,494,483 |
25 years
|
||||
|
Leasehold improvements
|
10,470,946 | 10,470,946 |
Lesser of lease term or useful life
|
||||||
|
Manufacturing equipment
|
5,759,643 | 5,717,841 |
10 years
|
||||||
|
Laboratory equipment
|
3,603,600 | 3,576,178 |
5 years
|
||||||
|
Other equipment
|
724,580 | 671,489 |
10 years
|
||||||
|
Office equipment and furniture
|
1,395,019 | 1,372,857 |
5 years
|
||||||
|
Equipment formerly under capital leases
|
1,093,424 | 1,093,424 |
Lesser of lease term or useful life
|
||||||
| 24,544,637 | 24,397,218 | ||||||||
|
Less accumulated depreciation and amortization
|
(21,475,687 | ) | (20,838,824 | ) | |||||
| 3,068,950 | 3,558,394 | ||||||||
|
Land
|
121,167 | 121,167 | |||||||
|
Property, plant and equipment, net
|
$ | 3,190,117 | $ | 3,679,561 |
Depreciation and amortization expense on property, plant and equipment was approximately $637,000, $641,000 and $596,000 in 2010, 2009 and 2008, respectively. Our fully depreciated assets included above at December 31, 2010 and December 31, 2009, respectively, were approximately $19,000,000 and $18,400,000.
6. Intangible Assets
Details of intangible assets are summarized as follows:
|
December 31, 2010
|
December 31, 2009
|
|||||||||||||||||||||||
|
Cost
|
Accumulated
Amortization
|
Net
|
Cost
|
Accumulated
Amortization
|
Net
|
|||||||||||||||||||
|
Trademarks
|
$ | 144,000 | $ | 142,000 | $ | 2,000 | $ | 144,000 | $ | 141,000 | $ | 3,000 | ||||||||||||
|
Patents
|
1,440,000 | 571,000 | 869,000 | 1,235,000 | 410,000 | 825,000 | ||||||||||||||||||
|
Deferred Patents
|
1,784,000 | -- | 1,784,000 | 1,624,000 | -- | 1,624,000 | ||||||||||||||||||
|
Deferred Trademarks
|
15,000 | -- | 15,000 | 15,000 | -- | 15,000 | ||||||||||||||||||
| $ | 3,383,000 | $ | 713,000 | $ | 2,670,000 | $ | 3,018,000 | $ | 551,000 | $ | 2,467,000 | |||||||||||||
35 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
Trademarks and patents are all intangible assets with definite useful lives and therefore continue to be amortized in accordance with accounting guidance. Amortization expense amounted to $104,000, $92,000 and $90,000 for the years ended December 31, 2010, 2009 and 2008, respectively. The weighted-average amortization period for trademarks for 2010 and 2009 was approximately two years and three years, respectively. The weighted-average amortization period for patents for both 2010 and 2009 was approximately 11 years and 12 years, respectively. Future amortization expense on our amortizable intangible assets over the next five years is estimated as follows:
|
2011
|
$ | 90,000 | ||
|
2012
|
89,000 | |||
|
2013
|
89,000 | |||
|
2014
|
88,000 | |||
|
2015
|
87,000 | |||
| $ | 443,000 |
During 2010, 2009 and 2008, we wrote off certain foreign patents in countries that had limited commercial potential and/or wrote off certain foreign and domestic patent applications for technologies that were no longer being pursued or had limited commercial potential in their respective countries. Charges in 2010, 2009 and 2008 to general and administrative expense to write off certain abandoned intangible assets were $225,000, $3,000 and $217,000, respectively, representing their carrying value. As of December 31, 2010, fifteen of our patents had issued in the U.S. and fifty-eight had issued in various foreign countries. Various other domestic and foreign applications are pending.
7. China Joint Venture
In June 2000, we entered into a joint venture agreement with SPG, a pharmaceutical company in the People’s Republic of China. We currently own 45% of the joint venture (the “China Joint Venture”) and have a 45% interest in the entity’s profits and losses. We account for this investment under the equity method. Our share of its losses for 2010, 2009 and 2008 were $149,000, $169,000 and $139,000, respectively.
Although the Company does not believe that it is required to make any further contributions to the China Joint Venture, there exists a misunderstanding as to whether Unigene owes an additional $1.2 million for discretionary loans or equity contributions to the China Joint Venture and the $1.2 million has been recorded as a liability on our balance sheet. To date, Unigene has paid its cash capital requirement of $1,050,000 and also completed its required technology transfer, thereby satisfying in full its registered capital contribution. In addition, Unigene believes that the China Joint Venture owes Unigene the amount of $678,000 as reimbursement for monies paid, and engineering services provided to, or on behalf of, the China Joint Venture by Unigene. The reimbursement amount is subject to confirmation by our China Joint Venture partner and therefore has not been recorded as a receivable since its collectability is uncertain. While there are no assurances that the parties will reach agreement with respect to these disputed amounts, Unigene believes that it will reach an amicable resolution of the amounts due and owing each party.
In 2008 we initiated the transfer of technology and know-how to the joint venture. This technology and know-how contribution gives rise to a basis difference between our investment in the joint venture and our 45% equity interest in the underlying assets of the joint venture. This basis difference is being recognized over 17 years, the estimated life of the transferred assets, at approximately $265,000 per year beginning the fourth quarter of 2008.
As part of our strategic realignment announced in September 2010, we have determined that the China Joint Venture no longer fits our core focus and therefore we will look to monetize our investment therein to the greatest extent possible, and terminate our participation in the China Joint Venture.
8. Notes Payable – Victory Park
On September 30, 2008, we entered into a financing agreement with Victory Park pursuant to which we borrowed $15,000,000 from Victory Park and, in connection therewith, we issued to Victory Park a three-year senior secured non-convertible term note. Richard Levy (no relation to the Levys), our Chairman of the Board, is the Managing Principal and Founder of Victory Park. We received net proceeds of $14,372,000 after fees and closing expenses paid as of September 30, 2008. On May 22, 2009, we drew down the remaining $5,000,000 available under this agreement. These term notes were purchased by Victory Park affiliates at a 3% discount to the face amount and we received net proceeds of $4,803,000 after fees and closing expenses. Pursuant to the financing agreement, we issued an aggregate of 1,500,000 shares of Common Stock to Victory Park. Subsequently, pursuant to a Warrant Exchange Agreement, dated as of October 19, 2009, with Victory Park, we issued 300,000 shares of Common Stock to Victory Park in exchange for Victory Park’s surrender of a warrant to purchase 1,000,000 shares of Common Stock.
During January 2009 and February 2010, respectively, we repaid approximately $463,000 and $180,000 in principal in accordance with mandatory prepayment terms. For 2010 and 2009 we recognized approximately $7,765,000 and $3,356,000, respectively, in cash and non-cash interest expense on these notes.
36 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
These notes bore interest through March 16, 2010 at the prime rate plus 7%, subject to a floor of 14% per annum and a cap of 18% per annum. In conjunction with this financing, loans held by the Levys were subordinated to the Victory Park notes. We pledged all of our current and future assets, including intellectual property, as collateral under the Victory Park notes.
In March 2010 we entered into an amended and restated financing agreement with Victory Park. The restated financing agreement amends and restates in its entirety and replaces the financing agreement dated as of September 30, 2008.
Under the terms of the restated financing agreement, we issued to Victory Park $33,000,000 aggregate principal amount of three-year, senior secured convertible notes by way of surrender of the three-year, senior secured non-convertible notes previously issued pursuant to the original financing agreement, in the aggregate principal amount of approximately $19,358,000, and by way of cash payment of approximately $13,642,000 for the balance. These convertible notes were purchased at a 2% discount to the face amount. Total fees and expenses at closing were approximately $2,007,000 and these closing costs are shown on our Statement of Operations as debt issuance costs. The financing was considered a troubled debt restructuring due to our financial condition and due to a decrease in the effective rate of the note. Therefore, these costs were expensed in March 2010. We therefore received net cash proceeds of approximately $11,635,000. The balance of deferred financing costs ($181,457 at December 31, 2010) and note discount ($7,209,700 at December 31, 2010) from the 2008, 2009 and 2010 Victory Park financings are being amortized over the three-year term of the new convertible notes to interest expense on a straight-line basis which approximates the effective interest rate method. In addition, under certain circumstances, we may request that Victory Park purchase (which purchase shall be in Victory Park’s sole discretion) up to an additional $3 million aggregate principal amount of convertible notes at one subsequent closing. The maturity date of the convertible notes has been extended to March 17, 2013 from September 30, 2011 under the original notes. After the first anniversary of the first closing, under certain circumstances, we have the right to prepay up to $10,642,472 (this was reduced from $13,642,472 in conjunction with the forbearance agreement described below) of the convertible notes at a price equal to 110% of the convertible notes being repaid plus accrued and unpaid interest, subject to customary conditions. The convertible notes are secured by a first priority lien on all of our current and future assets. The convertible notes will accrue interest at a rate per annum equal to the greater of the prime rate plus 5% and 15%, which, in the absence of an event of default, shall be capitalized and added to the outstanding principal balance of the convertible notes on each anniversary of the date of issuance other than the maturity date. Interest expense is calculated using an effective interest method, at a rate of 15.9%, over the three-year life of the notes due to the deferred payment schedule contained in the notes. The notes are convertible into shares of Common Stock at the holder’s option as of March 17, 2011. The initial conversion rate, which is subject to adjustment as set forth in the notes, is calculated by dividing the sum of the principal to be converted, plus all accrued and unpaid interest thereon, by $0.70 per share. If we subsequently make certain issuances of Common Stock or Common Stock equivalents at an effective purchase price less than the then-applicable conversion price, the conversion price of the notes will be reduced to such lower price. We lacked sufficient shares of Common Stock to deliver all of the shares of Common Stock to be issued upon conversion of the notes and therefore, we were required to obtain stockholder approval to amend our certificate of incorporation to increase the number of authorized shares. We obtained this approval on June 15, 2010 at our annual meeting of stockholders.
As the Victory Park notes are convertible into shares of our Common Stock and prior to June 15, 2010 we did not have sufficient authorized shares to effectuate a full conversion of the notes, the conversion feature was being treated as a liability. As such we established a liability for the conversion feature based on its fair value. The liability for the conversion feature was marked to the market at the end of each quarter until such time as additional shares were authorized. We therefore recognized a non-cash expense of $10,034,000 in the first quarter of 2010 which represented the change in the fair value of the conversion feature of the notes from the closing date of March 16, 2010 to the balance sheet date of March 31, 2010. In the second quarter of 2010, we recognized a non-cash gain of $1,909,000 due to the change in the fair value of the conversion feature of the note from March 31, 2010 to June 15, 2010, the date of stockholder approval of the amendment to increase the number of authorized shares. In addition, at March 31, 2010, we had a liability on our balance sheet for the embedded conversion feature in the amount of $18,633,000, which was the value of the embedded conversion feature as of March 31, 2010. After reducing this liability by the $1,909,000 gain as described above, the balance of $16,724,000 was reclassified to additional paid-in capital since the embedded conversion feature no longer met the bifurcation criteria.
In addition, pursuant to the restated financing agreement, in March 2010, Richard Levy became a member of the Board, Chairman of the Board and a member of our Nominating and Corporate Governance Committee. Victory Park has the right, subject to certain conditions, to designate an individual to fill the current vacant seat on the Board. That individual (the “VPC Designee”) will become a member of the Board’s Compensation Committee and Audit Committee. Moreover, we agreed that until such time as (i) the aggregate principal amount outstanding under the senior secured convertible notes issued to Victory Park is less than $5 million and (ii) Victory Park beneficially owns less than twenty percent of the issued and outstanding shares of our Common Stock, our Nominating and Corporate Governance Committee shall take all actions reasonably necessary to recommend the nomination of, and the Board shall nominate for reelection to the Board, Richard Levy and the VPC Designee (or substitutes or replacements designated by Victory Park).
37 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
Under the restated financing agreement, we must maintain a cash balance equal to at least $2,500,000 and our cash flow (as defined in the restated financing agreement) must be at least $2,000,000 in any fiscal quarter or $7,000,000 in any three consecutive quarters. These default provisions were temporarily waived under a forbearance agreement executed in December 2010. The forbearance period began December 10, 2010 and will terminate upon the earliest to occur of (i) the termination of the amended GSK license agreement, (ii) June 30, 2012 (or such later date as the parties may agree in writing), (iii) GSK’s failure to pay the Company certain specific amounts pursuant to the amended license agreement, or (iv) the date when the Company (a) repudiates or asserts a defense to any obligation or liability under the forbearance agreement or any transaction document (as defined in the restated financing agreement or (b) makes or pursues a claim against Victory Park or any secured party named in the forbearance agreement. The restated financing agreement specifies certain events of default including, without limitation: failure to pay principal or interest; filing for bankruptcy; breach of covenants, representations or warranties; the occurrence of a material adverse effect (as defined in the restated financing agreement); a change in control (as defined in the restated financing agreement); lack of timely filing or effectiveness of a required registration statement; and any material decline or depreciation in the value or market price of the collateral. We are subject to certain cash damages, as set forth in the convertible notes, in the case of a failure to timely convert the notes, and a failure to timely convert is also an event of default, subject to additional remedies. Upon any default, among other remedies, both principal and interest would be accelerated and additional charges would apply. As of December 31, 2010, we were in compliance with all of these covenants.
Pursuant to the amended and restated registration rights agreement executed in connection with the March 2010 financing, we filed a registration statement with the SEC registering the resale of the shares currently or to be held by Victory Park including the conversion shares, which registration statement was declared effective on July 13, 2010. We agreed to keep the registration statement effective at all times until the earlier of (i) the date as of which all the registrable securities may be sold without restriction pursuant to Rule 144 and (ii) the date on which all of the registrable securities covered by such registration statement have been sold. If on any day after the effective date of the registration statement, sales of all of the registrable securities cannot be made (other than during an allowable grace period) pursuant to the registration statement (including because of a suspension or delisting of the Common Stock on its principal market or a failure to register a sufficient number of shares of Common Stock), then as partial relief for the damages to any holder of registrable securities we shall pay to each holder of registrable securities an amount in cash equal to two percent (2%) of the aggregate value of such holder’s registrable securities required to be included in such registration statement or the initial date of such failure and on every 30th day thereafter (pro rated for periods totaling less than 30 days) until March 17, 2012, excluding for days prior to the date that the convertible notes become convertible, the value of any conversion shares included in such registrable securities. These damages do not have a maximum limitation.
9. Debt
We have short-term and long-term debt outstanding to Victory Park, the Levys and our China joint venture partner.
Aggregate maturities of all outstanding debt at December 31, 2010 were as follows:
|
2011
|
$ | 1,200,000 | ||||||
|
2012
|
750,000 | |||||||
|
2013
|
46,987,518 | |||||||
|
2014
|
-- | |||||||
|
2015
|
-- | |||||||
|
Thereafter
|
-- | |||||||
| 48,937,518 | ||||||||
|
Discount – Victory Park (see Note 8)
|
(7,209,700 | ) | ||||||
| $ | 41,727,818 | |||||||
|
Presentation on Balance Sheet:
|
||||||||
| December 31, 2010 | December 31, 2009 | |||||||
|
Notes payable – Levys: long-term (see Note 4)
|
$ | 14,737,518 | $ | 13,376,889 | ||||
|
Notes payable – Levys: short-term (see Note 4)
|
-- | 2,360,628 | ||||||
|
Notes payable – Victory Park –net of discount of $7,209,700 in 2010 and $1,357,003 in 2009 (see Note 8)
|
25,790,300 | 18,180,203 | ||||||
|
Note payable-China Joint Venture Partner-net of discount of $64,571 in 2009 (see Note 7)
|
1,200,000 | 2,010,429 | ||||||
| $ | 41,727,818 | $ | 35,928,149 | |||||
38 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
10. Tarsa
In October 2009, we licensed our Phase III oral calcitonin program (“the Program”) to Tarsa Therapeutics, Inc. (“Tarsa”), a new company formed by a syndicate of three venture capital funds specializing in the life sciences: MVM Life Science Partners, Quaker BioVentures and Novo A/S. Simultaneously, Tarsa announced the closing of a $24 million Series A financing from the investor syndicate.
In consideration for our sale to Tarsa of an exclusive license for the Program, we received from Tarsa approximately $8,993,000 in cash and 9,215,000 shares of common stock in Tarsa (which represents a 26% ownership). The cash consideration was derived based upon the expenses we had incurred for the calcitonin program. We valued the common stock at $0.23 per share or a total of $2,119,000 with the assistance of an outside valuation firm. These shares are pledged as security under the Victory Park loans.
Tarsa will be solely responsible for the future costs of the global Phase III clinical trials of the Program that was initiated in 2009 and completed in the first quarter of 2011. We are eligible to receive up to $3,000,000 in milestone payments based on the achievement of certain sales benchmarks, as well as royalties, at rates in the single digits, on product sales. We have no further cash or non-cash obligations to Tarsa or the Program.
Additionally, prepaid Phase III expenses of $4.6 million deferred through September 30, 2009 ($1,507,000 as of December 31, 2008) were recognized and netted against the consideration received from Tarsa.
As of the effective date of the agreement, Tarsa is responsible for all expenses incurred related to the Program, thus no additional expenses will be incurred by us related to the clinical study, except as agreed upon in subsequent statement of works. Furthermore, we note there is no joint development or steering committee between us and Tarsa related to the manufacturing and development of Phase III and future product. Any additional involvement by Unigene requires a separate statement of works to be negotiated and executed.
As we have no commitment to fund the losses of Tarsa, our 2009 operations include a loss of $2,119,000, which represents our proportionate share of Tarsa’s loss of $3,795,000 up to the amount of our investment in Tarsa. The loss reduced our investment in Tarsa to zero and, as a consequence, our future financial results will not be negatively affected by Tarsa’s ongoing operations. We therefore discontinued the equity method of accounting for our investment in Tarsa.
We recognized $1,007,000 and $60,000, respectively, for development, testing and other services performed for Tarsa under statements of work in 2010 and 2009.
11. Inventory
Inventories consisted of the following as of December 31, 2010 and 2009:
|
2010
|
2009
|
|||||||
|
Current Inventory
|
||||||||
|
Finished goods – net of allowances of
$1,214,000 and $692,000, respectively
|
$ | 896,471 | $ | 1,385,782 | ||||
|
Raw materials – net of allowances of $63,000 and $25,000, respectively
|
521,462 | 547,230 | ||||||
|
Total
|
$ | 1,417,933 | $ | 1,933,012 | ||||
|
Noncurrent Inventory
|
||||||||
|
Raw Materials
|
$ | 1,439,460 | $ | 1,574,370 | ||||
|
Finished Goods
|
1,442,520 | 3,415,298 | ||||||
|
Total
|
$ | 2,881,980 | $ | 4,989,668 | ||||
Typically, finished goods and work in process inventory are fully reserved except for the amounts deemed saleable by management based upon current or anticipated orders, primarily under contractual arrangements. Our reserves for finished goods and raw materials were $1,277,000 at December 31, 2010, an increase of $560,000 from December 31, 2009. Based upon expected future orders, $1,443,000 of our finished goods inventory and $1,439,000 of our raw material inventory were classified as a noncurrent asset at December 31, 2010. Noncurrent inventory decreased in 2010 due to the cessation of production of calcitonin and enzyme in 2010 and the utilization of previously manufactured calcitonin. We expect this inventory to be fully recoverable, therefore no reserve was established.
39 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
12. Accrued expenses
Accrued expenses consisted of the following at December 31, 2010 and 2009:
|
Current Accrued Expenses
|
||||||||
|
2010
|
2009
|
|||||||
|
Severance payments to former executives
|
$ | 911,000 | $ | -- | ||||
|
Vacation, payroll taxes and other payroll-related expenses
|
734,970 | 903,049 | ||||||
|
Consultants, clinical trials
|
938,642 | 908,946 | ||||||
|
Professional fees and other
|
249,733 | 294,724 | ||||||
|
Total
|
$ | 2,834,345 | $ | 2,106,719 | ||||
|
Noncurrent Accrued Expenses
|
||||||||
|
Vacation Pay
|
$ | 162,296 | $ | 277,908 | ||||
13. Obligations Under Operating Leases
We are obligated under a net-lease for our manufacturing facility located in Boonton, New Jersey that expires in 2014. We also lease office space in a second location in Boonton, New Jersey that expires in 2017. We have 10-year renewal options on both Boonton locations, as well as options to purchase these facilities. Total future minimum rentals under these non-cancelable operating leases are as follows:
|
2011
|
$ | 290,021 | ||
|
2012
|
291,021 | |||
|
2013
|
292,021 | |||
|
2014
|
108,369 | |||
|
2015
|
92,583 | |||
|
2016 and thereafter
|
132,750 | |||
| $ | 1,206,765 |
Total rent expense was approximately $290,000 for each of 2010, 2009 and 2008. The rent on our Boonton, NJ office space increases by $1,000 per year.
14. Warrants
As of December 31, 2010, there were 60,000 warrants outstanding, all but 20,000 of which are currently exercisable, to purchase an aggregate of 60,000 shares of Common Stock at exercise prices ranging from $2.00 to $2.20 per share. The following summarizes warrant activity for the past three years:
|
Warrants
|
Warrants
Exercisable
At
End of
Year
|
Weighted
Average
Exercise Price
|
||||||||||
|
Outstanding January 1, 2008
|
2,371,571 | 2,351,571 | $ | 2.73 | ||||||||
|
Granted
|
-- | -- | ||||||||||
|
Cancelled
|
-- | -- | ||||||||||
|
Exercised
|
(250,000 | ) | 0.90 | |||||||||
|
Outstanding December 31, 2008
|
2,121,571 | 2,101,571 | $ | 2.95 | ||||||||
|
Granted
|
-- | -- | ||||||||||
|
Cancelled – Victory Park stock swap
|
(1,000,000 | ) | 4.25 | |||||||||
|
Exercised – Fusion Capital
|
(1,061,571 | ) | 1.77 | |||||||||
|
Outstanding December 31, 2009
|
60,000 | 40,000 | $ | 2.13 | ||||||||
|
Granted
|
-- | -- | ||||||||||
|
Cancelled
|
-- | -- | ||||||||||
|
Exercised
|
-- | -- | ||||||||||
|
Outstanding December 31, 2010
|
60,000 | 40,000 | $ | 2.13 | ||||||||
40 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
A summary of warrants outstanding as of December 31, 2010 follows:
|
Warrants Outstanding
|
||||||||||||
|
Exercise
Price
|
Number
Outstanding
|
Weighted Ave.
Remaining Life
(years)
|
Weighted Ave.
Exercise Price
|
|||||||||
|
$2.00
|
20,000 | 4.4 | $ | 2.00 | ||||||||
|
$2.20
|
40,000 | 1.9 | 2.20 | |||||||||
| 60,000 | $ | 2.13 | ||||||||||
15. Rights Plan
In December 2002, pursuant to a rights agreement, we distributed common stock purchase rights to stockholders of record as a dividend at the rate of one right for each share of common stock. Each right entitles the holder to purchase from us one ten-thousandth of a share of common stock at an exercise price of $4.00. Initially the rights are attached to the common stock and are not exercisable. There is one right outstanding for every share of common stock outstanding.
The rights become exercisable and will separate from the common stock ten calendar days after a person or group acquires beneficial ownership of fifteen percent or more of our common stock, or ten business days (or a later date following such announcement as determined by our Board of Directors) after the announcement of a tender offer or an exchange offer to acquire fifteen percent or more of our outstanding common stock.
The rights are redeemable for $.00001 per right at the option of the Board of Directors at any time prior to the close of business on the tenth calendar day after a person or group acquires beneficial ownership of fifteen percent or more of our common stock. If not redeemed, the rights will expire on December 30, 2012. Prior to the date upon which the rights would become exercisable under the rights agreement, our outstanding stock certificates will represent both the shares of common stock and the rights, and the rights will trade only with the shares of common stock.
Generally, if the rights become exercisable, then each stockholder, other than the stockholder whose acquisition has caused the rights to become exercisable, is entitled to purchase, for the exercise price, that number of shares of common stock that, at the time of the transaction, will have a market value of two times the exercise price of the rights. In addition, if, after the rights become exercisable, we are acquired in a merger or other business combination, or fifty percent or more of our assets, cash flow or earning power are sold, each right will entitle the holder to purchase, at the exercise price of the rights, that number of shares of common stock of the acquiring company that, at the time of the transaction, will have a market value of two times the exercise price of the rights.
The rights plan is intended to improve the ability of our Board of Directors to protect the interests of Unigene and our stockholders in the event of an unsolicited proposal to acquire a significant interest in Unigene.
16. Stock Option Plans
We have had various shareholder-approved stock option plans for employees and directors under which we have granted non-qualified and incentive stock options. Options granted under these plans must be at a price per share not less than the fair market value per share of common stock on the date the option is granted. The options generally vest over a one to four year period and typically expire ten years from the date of grant. Shares for option exercises are issued from authorized and unissued shares.
At our June 2006 Annual Meeting, the stockholders approved the adoption of the 2006 Stock Based Incentive Compensation Plan (as amended, the “2006 Plan”). All employees and directors, as well as certain consultants, are eligible to receive grants under the 2006 Plan. Allowable grants under the 2006 Plan include stock options, phantom stock, stock appreciation rights, restricted stock and deferred stock. Options granted under the 2006 Plan have a ten-year term and an exercise price equal to the market price of the common stock on the date of the grant. The 2006 Plan replaced our older stock option plans and, initially, had 3,426,000 shares authorized for issuance. At December 31, 2010, we had reserved approximately 3,012,000 shares for future grants under the 2006 Plan.
Compensation expense is calculated each quarter for consultants using the Black-Scholes option pricing model, until the option is fully vested. Based upon options issued to consultants, we recognized a compensation benefit of $6,000 in 2010, compensation expense of $43,000 in 2009 and a compensation benefit of $143,000 for 2008. These amounts are included in research and development expenses.
41 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
For 2010 we recognized share-based compensation cost of $689,000, which consisted of $601,000 in general and administrative expenses and $88,000 in research and development expenses. For 2009, we recognized share-based compensation costs of $378,000, which consisted of $270,000 in general and administrative expense and $108,000 in research and development expenses. For 2008, we recognized share-based compensation cost of $450,000, which consisted of $396,000 in general and administrative expenses and $54,000 in research and development expenses. We did not capitalize any share-based compensation cost.
As of December 31, 2010, there was $1,735,000 of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the stock option plans. That cost is expected to be recognized over a weighted-average period of approximately 1.4 years.
For 2010, 2009 and 2008, we estimated the fair value of each option award on the date of grant using the Black-Scholes model. We utilized the risk-free interest rate for periods equal to the expected term of the option based upon U.S. Treasury securities in effect at the time of the grant. We have no present intention of declaring any dividends. We based expected volatility on historical volatility. Option forfeiture rates are based on our historical forfeiture rates. We estimated the expected term of stock options using historical exercise experience.
The following table shows the weighted average assumptions we used to develop the fair value estimates for employee and director stock options:
|
2010
|
2009
|
2008
|
||||||||||
|
Expected volatility
|
59% | 52% | 58% | |||||||||
|
Expected dividends
|
0% | 0% | 0% | |||||||||
|
Expected term (in years)
|
6.0 | 6.0 | 5.3 | |||||||||
|
Risk-free rate
|
1.9% | 1.9% | 2.7% | |||||||||
|
Forfeiture rate - employees
|
20% | 20% | 20% | |||||||||
|
Forfeiture rate – officers and directors
|
0% | 0% | 0% |
The total fair value of shares vested during 2010, 2009 and 2008 was approximately $385,000, $378,000 and $860,000, respectively. The weighted average grant-date fair value of the 685,249 options that vested during 2010 was $.52 per share. The total intrinsic value, which represents the difference between the underlying stock’s market price and the option’s exercise price, of options exercised during 2010, 2009 and 2008 was $ 42,000, $498,000 and $2,000, respectively. The aggregate intrinsic value for all fully vested shares at December 31, 2010 was $335,000.
The weighted average grant-date fair value per share of non-vested options as of December 31, 2010 and 2009 was $.41 and $.55, respectively. Total non-vested options as of December 31, 2010 and 2009 were 5,548,251 and 1,501,000, respectively. Of the total non-vested options at December 31, 2010, approximately 5,407,000 are expected to vest.
Cash received from option exercises under all share-based payment arrangements for 2010, 2009 and 2008 was approximately $73,000, $331,000 and $4,000, respectively. No tax benefit was realized from option exercises of the share-based payment arrangements for 2010, 2009 and 2008. During 2010, certain options were modified for former officers and/or directors whereby the period of time to exercise was extended from 3 months to 12 months. Total incremental expense associated with these modifications was $67,000 of which $48,000 represented options held by Jay Levy.
The following summarizes activity for options granted to directors, employees and consultants:
|
Options
|
Weighted Average
Exercise Price
|
Options
Exercisable At
End of Year
|
Weighted Average Exercise
Price
|
Weighted Average
Grant-date
Fair
Value
|
||||||||||||||||
|
Outstanding January 1, 2008
|
3,962,215 | $ | 1.43 | 3,340,836 | $ | 1.21 | ||||||||||||||
|
Granted
|
1,229,000 | 1.32 | $ | 0.71 | ||||||||||||||||
|
Forfeited
|
(15,900 | ) | 1.34 | |||||||||||||||||
|
Exercised
|
(10,000 | ) | 0.44 | |||||||||||||||||
|
Expired
|
(301,000 | ) | 2.06 | |||||||||||||||||
|
Outstanding December 31, 2008
|
4,864,315 | $ | 1.37 | 3,475,285 | $ | 1.34 | ||||||||||||||
|
Granted
|
731,000 | 0.63 | $ | 0.31 | ||||||||||||||||
|
Forfeited
|
(116,500 | ) | 1.50 | |||||||||||||||||
|
Exercised
|
(629,900 | ) | 0.53 | |||||||||||||||||
|
Expired
|
(66,000 | ) | 0.81 | |||||||||||||||||
|
Outstanding December 31, 2009
|
4,782,915 | $ | 1.37 | 3,281,915 | $ | 1.53 | ||||||||||||||
|
Granted
|
4,747,000 | $ | 0.70 | $ | 0.38 | |||||||||||||||
|
Forfeited
|
(98,000 | ) | 1.85 | $ | $1.29 | |||||||||||||||
|
Exercised
|
(158,600 | ) | 0.46 | |||||||||||||||||
|
Expired
|
(65,115 | ) | 2.00 | |||||||||||||||||
|
Outstanding December 31, 2010
|
9,208,200 | $ | 1.03 | 3,659,949 | $ | 1.48 | ||||||||||||||
42 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
A summary of options outstanding and exercisable as of December 31, 2010, follows:
|
Options Outstanding
|
Options Exercisable
|
|||||||||||||||||||
|
Range of
Exercise Price
|
Number
Outstanding
|
Weighted Ave.
Remaining
Life (years)
|
Weighted
Ave.
Exercise
Price
|
Number
Exercisable
|
Weighted
Ave.
Exercise
Price
|
|||||||||||||||
|
$ 0.28-0.49
|
1,097,700 | 1.23 | $ | 0.40 | 1,097,700 | $ | 0.40 | |||||||||||||
|
$ 0.50-0.99
|
5,526,000 | 7.59 | 0.67 | 442,749 | 0.65 | |||||||||||||||
|
$ 1.00-1.99
|
1,419,500 | 7.05 | 1.48 | 977,500 | 1.50 | |||||||||||||||
|
$ 2.00-2.99
|
778,000 | 5.29 | 2.26 | 755,000 | 2.25 | |||||||||||||||
|
$ 3.00-3.99
|
87,000 | 5.15 | 3.42 | 87,000 | 3.42 | |||||||||||||||
|
$ 4.00-4.69
|
300,000 | 5.30 | 4.02 | 300,000 | 4.02 | |||||||||||||||
| 9,208,200 | 6.45 | $ | 1.03 | 3,659,949 | $ | 1.48 | ||||||||||||||
Restricted Stock Awards
During 2008, 2009 and 2010, we granted restricted stock awards to certain officers and other employees, as well as directors, that vest six months to one year from their grant date. We recognized $243,000 of compensation expense during the year ended December 31, 2010 related to restricted stock awards. Stock compensation expense is recognized over the vesting period of the restricted stock. At December 31, 2010, we had $12,000 of total unrecognized compensation cost related to non-vested restricted stock, all of which will be recognized in 2011.
The following table summarizes restricted stock activity:
|
Shares
|
Weighted Average
Grant-Date Fair Value
|
|||||||
|
Non-vested balance at January 1, 2008
|
-- | -- | ||||||
|
Shares granted
|
178,961 | $ | 1.28 | |||||
|
Shares vested
|
-- | -- | ||||||
|
Shares forfeited
|
-- | -- | ||||||
|
Non-vested balance at January 1, 2009
|
178,961 | $ | 1.28 | |||||
|
Shares granted
|
60,000 | $ | 0.80 | |||||
|
Shares vested
|
(188,961 | ) | 0.74 | |||||
|
Shares forfeited
|
-- | -- | ||||||
|
Non-vested balance at December 31, 2009
|
50,000 | $ | 0.80 | |||||
|
Shares granted
|
372,100 | $ | 0.70 | |||||
|
Shares vested
|
(231,450 | ) | 0.72 | |||||
|
Shares forfeited
|
(26,650 | ) | 0.70 | |||||
|
Non-vested balance at December 31, 2010
|
164,000 | $ | 0.70 | |||||
17. Income Taxes
As of December 31, 2010, we had available for federal income tax reporting purposes net operating loss carryforwards in the approximate amount of $98,000,000, expiring from 2011 through 2030, which are available to reduce future earnings which would otherwise be subject to federal income taxes. Our ability to use such net operating losses may be limited by change in control provisions under Internal Revenue Code Section 382. Approximately $3,200,000 of these net operating losses is related to deductions resulting from exercises of employee stock options. The tax benefit related to these stock options would be credited to additional paid-in capital when realized. In addition, as of December 31, 2010, we had federal research and development credits in the approximate amount of $3,420,000, which are available to reduce the amount of future federal income taxes. These credits expire from 2011 through 2030.
43 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
We have New Jersey operating loss carryforwards in the approximate amount of $32,000,000, expiring from 2013 through 2017, which are available to reduce future earnings, which would otherwise be subject to state income tax. As of December 31, 2010, approximately $11,000,000 of these New Jersey loss carryforwards had been approved for future sale under a program of the New Jersey Economic Development Authority, which we refer to as the NJEDA. In order to realize these benefits, we must apply to the NJEDA each year and must meet various requirements for continuing eligibility. In addition, the program must continue to be funded by the State of New Jersey, and there are limitations based on the level of participation by other companies. As a result, future tax benefits will be recognized in the financial statements as specific sales are approved. We sold tax benefits and received cash of $701,000 in 2010 (for 2009 and 2010) and $926,000 in 2008. In addition, as of December 31, 2010, we had New Jersey research and development credits in the approximate amount of $1,000,000, which are available to reduce the amount of future New Jersey income taxes. These credits expire from 2011 through 2017.
Income tax benefits recorded are summarized as follows:
|
Year ended December 31,
|
||||||||||||
|
2010
|
2009
|
2008
|
||||||||||
|
Proceeds from sale of New Jersey tax benefits
|
$ | (701,000 | ) | $ | 0 | $ | (926,000 | ) | ||||
|
Federal research and development credit benefit
|
0 | (69,000 | ) | 0 | ||||||||
|
Federal alternative minimum tax expense
|
0 | 0 | 0 | |||||||||
|
State tax expense
|
0 | 2,000 | 0 | |||||||||
|
Total income tax benefit
|
$ | (701,000 | ) | $ | (67,000 | )) | $ | (926,000 | ) | |||
|
The reconciliation for the benefit from income taxes and the amount computed by applying the federal statutory income tax rate (34%) is summarized as follows:
|
||||||||||||
|
Pre-tax book loss at statutory rate
|
$ | (9,720,000 | ) | $ | (4,572,000 | ) | $ | (2,381,000 | ) | |||
|
Change in valuation allowance
|
10,299,000 | 5,293,000 | 2,202,000 | |||||||||
|
Permanent tax differences
|
5,000 | 6,000 | 8,000 | |||||||||
|
State taxes
|
(1,797,000 | ) | (958,000 | ) | (617,000 | ) | ||||||
|
Other
|
512,000 | 164,000 | (138,000 | ) | ||||||||
|
Recorded income tax benefit
|
$ | (701,000 | ) | $ | (67,000 | ) | $ | (926,000 | ) | |||
Deferred tax assets are summarized as follows:
|
|
December 31,
|
|||||||
|
|
2010
|
2009
|
||||||
|
Federal net operating loss carryforwards
|
|
$
|
33,169,000
|
$
|
32,294,000
|
|||
|
State net operating loss carryforwards
|
|
1,906,000
|
1,599,000
|
|||||
|
Tax credits carryforward
|
|
4,224,000
|
4,637,000
|
|||||
|
Deferred revenue
|
|
5,389,000
|
4,331,000
|
|||||
|
Fixed asset
|
|
1,923,000
|
1,935,000
|
|||||
|
Stock options
|
|
1,152,000
|
780,000
|
|||||
|
Deferred compensation
|
552,000
|
176,000
|
||||||
|
Accrued vacation
|
239,000
|
233,000
|
||||||
|
Patent costs
|
896,000
|
968,000
|
||||||
|
Inventory Reserve
|
510,000
|
89,000
|
||||||
|
Deferred financing costs
|
601,000
|
0
|
||||||
|
Embedded conversion feature
|
(2,528,000)
|
0
|
||||||
|
Investment in China joint venture
|
1,682,000
|
1,799,000
|
||||||
|
Investment in Tarsa
|
846,000
|
851,000
|
||||||
|
|
|
50,561,000
|
|
49,692,000
|
|
|||
| Valuation allowance |
|
(50,561,000
|
) |
(49,692,000
|
) | |||
|
Net deferred tax assets
|
|
$
|
— 0 —
|
$
|
— 0 —
|
|||
44 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
On January 1, 2007, we adopted the provisions of ASC 740-10-25. ASC 740-10-25 provides recognition criteria and a related measurement model for uncertain tax positions taken or expected to be taken in income tax returns. ASC 740-10-25 requires that a position taken or expected to be taken in a tax return be recognized in the financial statements when it is more likely than not that the position would be sustained upon examination by tax authorities. Tax positions that meet the more likely than not threshold are then measured using a probability weighted approach recognizing the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate settlement. The Company records tax related interest and penalty in the tax provision. The Company has not recognized any tax related interest or penalty in the statement of operations due to its net operating loss carry forwards.
The following is a reconciliation of the beginning and ending amount of unrecognized tax benefit:
|
2010
|
2009
|
2008
|
||||||||||
|
Balance at January 1
|
0 | 0 | 0 | |||||||||
|
Additions based on tax positions related to the current year
|
20,000 | - | - | |||||||||
|
Additions based on tax positions related to the prior years
|
360,000 | - | - | |||||||||
|
Balance at December 31
|
380,000 | - | - | |||||||||
The Company’s 2007, 2008 and 2009 federal tax returns remain subject to examination by the IRS and the Company’s 2006, 2007, 2008 and 2009 New Jersey tax returns are also open to potential examination. In addition, net operating losses arising from prior years are also subject to examination at the time that they are utilized in future years. Neither the Company’s federal nor state tax returns are currently under examination.
18. Employee Benefit Plan
We maintain a deferred compensation plan covering all full-time employees. The plan allows participants to defer a portion of their compensation on a pre-tax basis pursuant to Section 401(k) of the Internal Revenue Code of 1986, as amended, up to an annual maximum for each employee set by the IRS. Our discretionary matching contribution expense for 2010, 2009 and 2008 was approximately $0, $117,000 and $125,000, respectively.
19. Research, Licensing and other Revenue
Research Agreement
GSK
In April 2002, we signed a licensing agreement with GSK for a value before royalties, PTH sales and reimbursement of development expenses, of up to $150,000,000 to develop an oral formulation of an analog of PTH currently in clinical development for the treatment of osteoporosis. Under the terms of the agreement, GSK received an exclusive worldwide license to develop and commercialize the product. In addition, GSK will reimburse us for certain development activities during the program, including our activities in the production of raw material for development and clinical supplies, and will pay us a royalty at a rate in the low to mid-teens based on its worldwide sales of the product, subject to a reduction under certain circumstances as described in the agreement. The royalty rate will be increased if certain sales milestones are achieved. A Phase I human trial, which commenced in 2004, demonstrated positive preliminary results. As agreed with GSK, we conducted further development including a small Phase I study, which we initiated in 2008 and successfully completed in 2009. An aggregate of $12,000,000 in up-front and milestone payments has been received from inception through December 31, 2010 including the $4,000,000 payment described below. We have also received an additional $5,000,000 from GSK for PTH sales and in support of our PTH development activities from inception through December 31, 2010. In December 2010, the Company entered into an amended and restated exclusive worldwide license agreement with GSK, with a Phase II trial to begin in the first quarter of 2011 and we received $4,000,000 for the Phase II development costs and this revenue is being recognized over four years, the estimated performance period under the amended agreement. This payment was considered substantive due to its reasonableness relative to all of the deliverables and payment terms in the agreement; commensurate with our level of effort; and commensurate with the enhanced value of the deliverables. We recognized $50,000 of revenue under this milestone in December 2010. We are eligible to receive an additional $4,000,000 upon completion of Phase II patient enrollment, which is expected in the first half of 2011. Phase II results are expected before year-end. Bulk product sales to licensees, prior to product approval, are unpredictable and subject to the needs of the licensee. GSK could make additional milestone payments in the aggregate amount of up to $142,000,000 subject to the progress of the compound through clinical development and through to the market. This agreement is subject to certain termination provisions. Either party may terminate the license agreement if the other party (i) materially breaches the license agreement, which breach is not cured within 60 days (or 30 days for a payment default), (ii) voluntarily files, or has served against it involuntarily, a petition in bankruptcy or insolvency, which, in the case of involuntary proceedings, remains undismissed for 60 days, or (iii) makes an assignment for the benefit of creditors. Additionally, GSK may terminate the license agreement at any time for various reasons including safety or efficacy concerns of the PTH product, significant increases in development timelines or costs, or significant changes in the osteoporosis market or in government regulations. During 2010, 2009 and 2008, and since inception, direct and
45 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
indirect costs associated with this project, included in research, development and facility expenses, were approximately $1,134,000, $1,747,000, $801,000 and $11,622,000, respectively.
License Agreements
Tarsa
In October 2009, we licensed our Phase III oral calcitonin program to Tarsa, a new company formed by a syndicate of three venture capital funds specializing in the life sciences: MVM Life Science Partners; Quaker BioVentures; and Novo A/S. Simultaneously, Tarsa announced the closing of a $24 million Series A financing from the investor syndicate. In consideration for our sale to Tarsa of an exclusive license for the oral calcitonin program, we received from Tarsa approximately $8,993,000 in cash and 9,215,000 shares of common stock in Tarsa (which represents a 26% ownership). We valued the common stock at $0.23 per share or a total of $2,119,000 with the assistance of an outside valuation firm. Tarsa will be responsible for the future costs of the global Phase III clinical program that was initiated in 2009. We are eligible to receive up to $3,000,000 in milestone payments based on the achievement of certain sales benchmarks, as well as royalties, at rates in the single digits, on product sales. This study was fully enrolled in the first quarter of 2010 and completed in the first quarter of 2011. In addition, in the first quarter of 2011, Tarsa initiated a Phase II clinical trial for the prevention of postmenopausal osteoporosis.
USL
In November 2002, we signed an exclusive U.S. licensing agreement with USL for a value before royalties of $10,000,000 to market our patented nasal formulation of calcitonin for the treatment of osteoporosis. We are responsible for manufacturing the product and USL packages the product and distributes it nationwide. Fortical was approved by the FDA and launched by USL in August 2005. During 2010, we recognized $5,246,000 in sales revenue and $2,974,000 in royalty revenue. During 2009, we recognized $5,941,000 in sales revenue and $4,991,000 in royalty revenue. Revenue for the year ended December 31, 2008 includes the $10,058,000 in sales to USL and $6,520,000 in royalties from USL. We recognize USL royalty revenue based upon the quarterly USL royalty report. This provides for a reliable measure as well as reasonable assurances of collectability. Royalty revenue, computed in a range from the transfer price of product to USL to a royalty rate in the mid-thirties depending on the circumstances, is earned on net sales of Fortical by USL and is recognized in the period Fortical is sold by USL. Future sales and royalties are contingent upon many factors including competition, pricing, marketing and acceptance in the market place and, therefore, are difficult to predict. In December 2008, Apotex and Sandoz launched nasal calcitonin products which are generic to Novartis’ nasal calcitonin product, but not to Fortical. In June 2009, Par also launched a product generic to Novartis’ nasal calcitonin product. Certain providers have substituted these products for Fortical, causing Fortical sales and royalties to decrease. This agreement may be terminated by either party by mutual agreement or due to breach of any material provision of the agreement not cured within 60 days. In addition, USL may terminate the agreement under certain circumstances where USL assigns the agreement and we do not approve the assignment. The term of the USL agreement shall continue through the expiration of USL’s obligation to pay royalties and continue thereafter.
Novartis
In April 2004, we signed a worldwide licensing agreement with Novartis for a value before royalties of up to $18,700,000 to allow Novartis to manufacture calcitonin using our patented peptide production process. We have received an aggregate of $13,700,000 from Novartis under this agreement and there are up to $5,000,000 in potential milestone payments remaining. We will receive royalties, at rates in the single digits, on net sales of any existing or future Novartis products that contain recombinant salmon calcitonin which in the future are approved for sale by health authorities, and are manufactured by Novartis using our technology. In each of 2010, 2009 and 2008, we recognized $893,000 in licensing revenue. During 2004, Novartis purchased calcitonin from us for use in their oral calcitonin development program and implemented our patented manufacturing process at Novartis facilities. Sandoz, a Novartis affiliate, concluded a manufacturing campaign in 2005 based on our process and produced multiple kilograms of calcitonin at a scale that represents a ten-fold increase above our current production capacity. Calcitonin produced by Sandoz is being used by Novartis in ongoing Phase III clinical trials. In July 2010, an independent Data Monitoring Committee (“DMC”) reviewed and conducted a “futility” analysis of one-year data for all patients enrolled in one of Novartis’s osteoarthritis studies, including both an assessment of safety and efficacy parameters. The DMC concluded there was no reason to stop the study because of safety findings. In addition, the DMC concluded there was no reason to continue the study because of efficacy findings, but also determined that the final decision whether to continue this study rests with the study sponsors. In October 2010, Novartis and its partner Nordic Bioscience announced that this study of patients with osteoarthritis of the knee is currently ongoing. In addition, in October 2010, Novartis released information on a different study on oral calcitonin for the treatment of osteoarthritis. The top-level results indicated that the study did not meet the first of three co-primary endpoints and results from the other two co-primary endpoints indicated clinical efficacy related to certain symptom modification. Novartis and its partner Nordic Bioscience announced their plan to continue to analyze and evaluate the results of this study and to determine appropriate next steps. Novartis and Nordic Bioscience also confirmed that a concurrent third study of oral calcitonin for the treatment of osteoporosis is proceeding as planned.
46 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
We will receive royalties on net sales of any existing or future Novartis products that contain calcitonin manufactured by Novartis using our technology. Since Novartis will be conducting all future product development and clinical trials for its own oral calcitonin product in conjunction with its partner, a competitor of ours, the anticipated completion date is outside our control. If Tarsa (see above) and Novartis each successfully develop oral calcitonin products, they would be competing in this area. This agreement may be terminated by either party due to a material breach not cured within 60 days or due to insolvency or bankruptcy proceedings not dismissed within 60 days and for other customary events of default. The Novartis license agreement shall continue in full force and effect until the earlier of (a) the date Novartis and its affiliates cease to manufacture calcitonin for use in Novartis drug products and in Unigene drug products and (b) the date Novartis and/or its affiliates decide not to manufacture calcitonin.
Deferred licensing fees
|
December 31, 2010
|
December 31, 2009
|
|||||||
|
Current portion
|
||||||||
|
GSK
|
$ | 1,200,000 | $ | 200,000 | ||||
|
USL
|
157,895 | 157,895 | ||||||
|
Novartis
|
892,861 | 892,857 | ||||||
|
Tarsa and other
|
40,160 | 75,854 | ||||||
| $ | 2,290,916 | $ | 1,326,606 | |||||
|
Long-term
|
||||||||
|
GSK
|
$ | 4,000,000 | $ | 1,250,000 | ||||
|
USL
|
1,565,789 | 1,723,643 | ||||||
|
Novartis
|
5,586,248 | 6,479,166 | ||||||
| $ | 11,152,037 | $ | 9,452,809 | |||||
20. Legal Matters
Fortical, our nasal calcitonin product for the treatment of postmenopausal osteoporosis is covered by the Fortical Patent. In June 2006, we received a Paragraph IV certification letter from Apotex Inc., a Canadian generic pharmaceutical manufacturer, alleging that this patent is invalid and therefore not infringed by Apotex’s nasal calcitonin product, which is the subject of an Apotex pending ANDA. On July 24, 2006, we and USL jointly filed a lawsuit against Apotex in the U.S. District Court for the Southern District of New York for infringement of our Fortical Patent. Due to our filing of the above-mentioned lawsuit, the Hatch-Waxman Act provided for an automatic stay of FDA approval for Apotex’s ANDA of up to 30 months. In December 2008 this stay ended and we and Apotex entered a preliminary injunction enjoining Apotex from engaging in the commercial manufacture, use, marketing, distribution, selling, transportation or importation of any Fortical generic equivalent product in the United States. The preliminary injunction will remain in effect until the court renders a final decision on the validity and infringement of Unigene’s U.S. Patent. In obtaining the preliminary injunction, Unigene and USL were required to post a bond and, if we do not prevail in the lawsuit, Unigene would be responsible for paying $1,662,500 to Apotex under the injunction agreement. In August 2009, the U.S. District Court, Southern District of New York, confirmed the validity of Unigene’s patent and issued an order permanently enjoining Apotex from engaging in any activity that infringes the patent. The motion of the plaintiffs, Unigene and its licensee USL, for summary judgment in the case was granted. The Court upheld the validity of the Fortical Patent and entered a permanent injunction against Apotex. Apotex appealed this decision. In October 2009, the District Court vacated its order subject to reinstatement and asked the parties to identify any claims that remain in the case. As a result, Apotex’s appeal was deactivated. In July 2010, the Court ruled that no claims remain at issue and it reinstated its opinion and order of August 2009 in its entirety. Accordingly, the motion for summary judgment was entered in favor of Unigene and USL. Apotex appealed this decision to the United States Court of Appeals for the Federal Circuit and the parties are currently submitting briefs on appeal. There is the usual litigation risk that we will not be successful in the appeal and potentially subsequent litigation if it is remanded. In the event that we do not prevail, then Apotex could be in a position to market its nasal calcitonin product if and when its pending ANDA receives FDA approval. This could have a material adverse impact on our results and financial position.
47 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
21. Fair Value Measurements
U.S. GAAP defines fair value as the price that would be received to sell an asset or paid to transfer a liability to a third party with the same credit standing (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. In many cases, the exit price and the transaction (or entry) price will be the same at initial recognition. However, in certain cases, the transaction price may not represent fair value. Given our financial condition described in Note 2, it is not practicable to estimate the fair value of our notes payable at December 31, 2010 and 2009. We believe that the carrying amounts of cash and cash equivalents, our accounts receivable and accounts payable approximate fair value due to their short-term nature. Fair value is a market-based measurement determined based on a hypothetical transaction at the measurement date, considered from the perspective of a market participant, not based solely upon the perspective of the reporting entity. When quoted prices are not used to determine fair value, consideration is given to three broad valuation techniques: (i) the market approach, (ii) the income approach, and (iii) the cost approach. Entities are required to determine the most appropriate valuation technique to use, given what is being measured and the availability of sufficient inputs. Inputs to fair valuation techniques are prioritized, allowing for the use of unobservable inputs to the extent that observable inputs are not available. The applicable guidance establishes a three-level hierarchy, based on the priority of the inputs to the respective valuation technique. The fair value hierarchy gives the highest priority to quoted prices in active markets for identical assets or liabilities (Level 1) and the lowest priority to unobservable inputs (Level 3). An asset or liability’s classification within the fair value hierarchy is based on the lowest level of significant input to its valuation. The input levels are defined as follows:
Level 2 Quoted prices in markets that are not active or inputs that are observable either directly or indirectly. Level 2 inputs include quoted prices for similar assets or liabilities other than quoted prices in Level 1, quoted prices in markets that are not active, or other inputs that are observable or can be derived principally from or corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 Unobservable inputs that are supported by little or no market activity and are significant to the fair value of the assets or liabilities. Unobservable inputs reflect the reporting entity’s own assumptions about the assumptions that market participants would use in pricing the asset or liability. Level 3 assets and liabilities include those whose values are determined using pricing models, discounted cash flow methodologies, or similar techniques, as well as those for which the determination of fair value requires significant management judgment or estimation.
The Company has categorized its liabilities measured at fair value into the three-level fair value hierarchy, as defined above, based upon the priority of inputs to respective valuation techniques. Liabilities included within Level 3 of the fair value hierarchy included an embedded conversion feature which requires fair value on a recurring basis. The valuation methodology uses a combination of observable and unobservable inputs in calculating fair value. The fair value of the conversion feature was determined using a lattice pricing model.
The changes in Level 3 liabilities measured at fair value on a recurring basis during the year ended December 31, 2010 are summarized as follows:
|
Balance
Beginning of
Period
|
Issuance
|
(Gain) or Loss Recognized in Earning from Change in Fair Value |
Reclassification to Additional Paid-in Capital
|
Balance End of Period
|
||||||||||||||||
|
For the year ended,
|
||||||||||||||||||||
|
December 31, 2010
|
||||||||||||||||||||
|
Embedded conversion feature
|
$ | -- | $ | 8,599,000 | $ | 8,125,000 | $ | (16,724,000 | ) | $ | -- | |||||||||
For the year ended December 31, 2010, total loss of approximately $8,125,000 is included in the Statements of Operations caption “Loss on change in fair value of embedded conversion feature.” The balance of $16,724,000 was reclassified to additional paid-in capital since the embedded conversion feature no longer met the bifurcation criteria after stockholder approval of the increase in authorized shares in June 2010.
48 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
22. Deferred Compensation Plan
In December 2005, our Board of Directors approved the adoption of a deferred compensation plan for Ronald Levy, former Executive Vice President and Director of the Company, and Warren Levy, former President, Chief Executive Officer and Director of the Company. The major features of the plan are as follows: The Company agrees to credit a book account with $25,000 per year on January 1st of such year beginning on January 1, 2006 and ending on January 1, 2014 for each participant; the credits to the accounts would be 100% vested; upon the death of a participant, any remaining contributions would be made to his account; and in the event of a “change in control” of Unigene, all remaining contributions shall be made to each participant’s account. The related contracts were finalized and executed in February 2006. Therefore, we recognized this liability in the first quarter of 2006 in the amount of $304,000, which represented the net present value of the future payments. As of December 31, 2010, this liability was approximately $472,000. As of December 31, 2010, both of these accounts had been funded to date in the aggregate amount of $303,000 ($250,000 plus $53,000 in interest and investment gains) and these accounts are included on the Balance Sheet in other assets.
The entire value of the account would be distributed as follows: upon attainment of age 75, 25% of balance, upon attainment of age 76, 33.33% of remaining balance, upon attainment of age 77, 50% of remaining balance, and the remainder of the balance upon attainment of age 78; in the event of a participant’s death or disability, 50% of the participant’s account balance shall be distributed following his death or disability and the remainder distributed on the first anniversary of his death or disability.
23. Unconsolidated Affiliates
We use the equity method to account for our 45% ownership of the China joint venture and our current 26% ownership of Tarsa. Our proportionate share of each joint venture’s net loss was included in our Statements of Operations. Below is summarized balance sheet information as of December 31, 2010 and 2009 and summarized statement of operations information for the years ended December 31, 2010 and 2009.
Summarized balance sheet information:
|
Dec. 31, 2010
|
Dec. 31, 2009
|
|||||||
|
Current Assets
|
$ | 7,557,116 | $ | 1,329,892 | ||||
|
Fixed assets - net
|
12,183,164 | 8,978,003 | ||||||
|
Intangible and other assets
|
5,403,461 | 2,722,489 | ||||||
|
Current liabilities
|
19,862,510 | 7,998,140 | ||||||
| Non-current liabilities | 659,756 | - | ||||||
|
Stockholders’ equity
|
4,621,475 | 5,032,244 | ||||||
Summarized statement of operations information:
|
Year Ended
Dec. 31, 2010
|
Year Ended
Dec. 31, 2009
|
|||||||
|
Revenue
|
$ | -- | $ | - | ||||
|
Operating loss
|
(13,971,628 | ) | (14,993,982 | ) | ||||
|
Net loss
|
$ | (14,149,614 | ) | $ | (14,991,715 | ) | ||
|
2010
|
|
1st Quarter
|
2nd Quarter
|
3rd Quarter
|
4th Quarter
|
|||||||||||
|
Revenue
|
|
$
|
2,532,502
|
|
$
|
3,028,313
|
|
$
|
2,856,389
|
|
$
|
2,923,010
|
|
|||
|
Operating loss
|
|
(2,800,972
|
)
|
(3,001,274
|
)
|
(1,834,088
|
)
|
(2,687,593
|
)
|
|||||||
|
Net loss
|
|
(15,946,719
|
)
|
(3,643,788
|
)
|
(4,383,557
|
)
|
(3,894,430
|
)
|
|||||||
|
Net loss per share, basic and diluted
|
|
$
|
(0.17
|
)
|
$
|
(0.04
|
)
|
$
|
(0.05
|
)
|
$
|
(0.04
|
)
|
|||
|
2009
|
|
1st Quarter
|
2nd Quarter
|
3rd Quarter
|
4th Quarter
|
|||||||||||
|
Revenue
|
|
$
|
3,191,416
|
$
|
4,296,586
|
$
|
2,731,764
|
$
|
2,572,072
|
|||||||
|
Operating loss
|
|
(2,252,616
|
)
|
(2,348,113
|
)
|
(4,599,444
|
)
|
(3,179,341
|
)
|
|||||||
|
Net loss
|
|
(3,275,307
|
)
|
(3,460,115
|
)
|
(5,833,978
|
)
|
(810,279
|
)
|
|||||||
|
Net loss per share, basic and diluted
|
|
$
|
(0.04
|
)
|
$
|
(0.04
|
)
|
$
|
(0.06
|
)
|
$
|
(0.01
|
)
|
|||
49 of 59
UNIGENE LABORATORIES, INC.
NOTES TO FINANCIAL STATEMENTS—(Continued)
YEARS ENDED DECEMBER 31, 2010, 2009 AND 2008
25. Subsequent Events
On January 31, 2011, we entered into an agreement for sale of the Company’s real property located at 110 Little Falls Road, Fairfield, NJ, with the buildings and improvements thereon. The purchaser will pay the Company an aggregate purchase price of $1,200,000. The closing is expected to occur no later than April 29, 2011, is subject to the receipt of the release of existing mortgages on the property and to certain other conditions and contingencies. The Company will lease the property from the purchaser for an initial term of seven years, which may be extended for one renewal term of five years, for an annual base rent during the initial term ranging from $135,000 to $145,000, and up to approximately $156,000 in the renewal term. This Agreement is subject to certain termination provisions.
On March 16, 2011, we signed a settlement and release agreement with the Levys to defer and reschedule certain severance payments, vacation pay, deferred compensation and loan payments. Under the agreement, we will make a lump sum payment to the Levys on or about March 17, 2011 in the amount of $1,200,000 for severance payments and a portion of accrued vacation pay. All loan payments are being temporarily deferred. In addition, we agreed to make an aggregate of $1,112,296 in monthly payments from May 2012 through December 2012. Under this settlement and release agreement, the Levys agreed to release any liens on the Fairfield property in order to effectuate the sale/leaseback described above. The balances presented at December 31, 2010 reflect this settlement.
|
Item 9.
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
|
None.
Evaluation of Disclosure Controls and Procedures. We carried out an evaluation, under the supervision and with the participation of our management, including our principal executive officer and our principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Rules 13a-15(e) and 15d-15(e) promulgated under the Exchange Act, as of the end of the period covered by this report. Based upon this evaluation, the principal executive officer and principal financial officer concluded that, as of December 31, 2010, our disclosure controls and procedures were effective and provide reasonable assurance that the information required to be disclosed by the Company in reports filed or submitted under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC.
Management’s Annual Report on Internal Control Over Financial Reporting. The management of Unigene Laboratories, Inc. (the “Company”), is responsible for establishing and maintaining adequate internal control over financial reporting. The Company’s internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) promulgated under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of, the Company’s principal executive and principal financial officers and effected by the Company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the Company’s financial statements for external purposes in accordance with generally accepted accounting principles in the United States and includes those policies and procedures that:
1. Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of assets of the Company;
2. Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and
3. Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our internal control over financial reporting, using the criteria set forth in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission, for the period covered by this report. Based on our evaluation, our principal executive officer and principal financial officer concluded that the Company’s internal control over financial reporting was effective as of December 31, 2010.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company’s independent registered public accounting firm has audited, and reported on, the Company’s internal control over financial reporting as of December 31, 2010. This report appears on page 51.
Changes in Internal Control Over Financial Reporting. Our principal executive officer and principal financial officer determined that there were no changes in our internal control over financial reporting during the quarterly period ended December 31, 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
50 of 59
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders
Unigene Laboratories, Inc.
We have audited Unigene Laboratories, Inc.’s (a Delaware Corporation) internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Unigene Laboratories, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on Unigene Laboratories, Inc.’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Unigene Laboratories, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control - Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Unigene Laboratories, Inc. as of December 31, 2010 and 2009, and the related statements of operations, cash flows, and stockholders’ deficit, for each of the three years in the period ended December 31, 2010 and our report dated March 16, 2011, expressed an unqualified opinion thereon.
/s/ Grant Thornton LLP
New York, New York
March 16, 2011
51 of 59
Item 9B. Other Information.
None.
The information required by this item will be included in the sections entitled “Information Regarding Directors, Nominees and Executive Officers,” “Section 16(a) Beneficial Ownership Reporting Compliance” and “Corporate Governance” of the Registrant's definitive proxy statement for its Annual Meeting of Stockholders to be held on June 2, 2011 and is hereby incorporated by reference.
Item 11. Executive Compensation.
The information required by this item will be included in the sections entitled “Compensation Committee Interlocks and Insider Participation,” “Compensation Discussion and Analysis,” “Compensation Committee Report,” “Executive Compensation” and “Director Compensation” of the Registrant's definitive proxy statement for its Annual Meeting of Stockholders to be held on June 2, 2011 and is hereby incorporated by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item will be included in the sections entitled “Security Ownership of Certain Beneficial Owners,” “Security Ownership of Management” and “Equity Compensation Plan Information” of the Registrant's definitive proxy statement for its Annual Meeting of Stockholders to be held on June 2, 2011 and is hereby incorporated by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item will be included in the sections entitled “Certain Relationships and Related Transactions” and “Director Independence” of the Registrant's definitive proxy statement for its Annual Meeting of Stockholders to be held on June 2, 2011 and is hereby incorporated by reference.
Item 14. Principal Accounting Fees and Services.
The information required by this item will be included in the section entitled “Proposal 2: Ratification of the Appointment of Independent Auditors” of the Registrant's definitive proxy statement for its Annual Meeting of Stockholders to be held on June 2, 2011 and is hereby incorporated by reference.
PART IV
Item 15. Exhibits and Financial Statement Schedules.
(a) Financial Statements.
The Financial Statements and Supplementary Data are listed under Item 8 of this Report on Form 10-K.
(b) Exhibits.
See Index to Exhibits which appears on Pages 53-58.
(c) Financial Statement Schedules.
None.
52 of 59
INDEX TO EXHIBITS
|
Exhibit
Number
|
|
Description
|
|
3.1
|
Certificate of Incorporation of Unigene Laboratories, Inc., dated October 31, 1980, as filed with the Secretary of State in the State of Delaware on November 3, 1980, and all amendments thereto through June 15, 2006 (incorporated by reference from Exhibit 3.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2006).
|
|
|
3.2
|
Certificates of Amendment to Certificate of Incorporation of Unigene Laboratories, Inc., dated June 16, 2010, as filed with the Secretary of State in the State of Delaware on June 16, 2010.
|
|
|
3.3
|
Amended By-Laws of Unigene Laboratories, Inc. (incorporated by reference from Exhibit 3.2 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2004).
|
|
|
4.1
|
Rights Agreement between Unigene Laboratories, Inc. and Registrar and Transfer Company, dated December 20, 2002 (incorporated by reference from Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, dated December 23, 2002).
|
|
|
4.2
|
Amendment to Rights Agreement, dated March 16, 2010 (incorporated by reference from Exhibit 4.1 to the Registrant’s Current Report on Form 8-K, dated March 17, 2010 (the “March 2010 Form 8-K”)).
|
|
|
4.3
|
Specimen Certificate for Common Stock, par value $.01 per share (incorporated by reference from Exhibit 3.1.1 to the Registrant’s Registration Statement No. 33-6877 on Form S-1, filed July 1, 1986).
|
|
|
10.1
|
Lease agreement between Unigene Laboratories, Inc. and Fulton Street Associates, dated May 20, 1993 (incorporated by reference from Exhibit 10.1 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1992).
|
|
|
10.2
|
First Amendment to Lease Agreement between Unigene Laboratories, Inc. and Fulton Street Associates, dated May 20, 1993 (incorporated by reference from Exhibit 10.2 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2007).
|
|
|
10.3
|
Second Amendment to Lease between Fulton Street Associates and Unigene Laboratories, Inc., dated as of May 15, 2003 (incorporated by reference from Exhibit 10.48 to Post-Effective Amendment No. 1 to Registrant’s Registration Statement No. 333-75960, filed August 1, 2003).
|
|
|
10.4
|
Directors Stock Option Plan (incorporated by reference from Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 1999).**
|
|
|
10.5
|
Amendment to Directors Stock Option Plan (incorporated by reference from Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2009).**
|
|
|
10.6
|
2000 Stock Option Plan (incorporated by reference from Attachment A to the Registrant’s Schedule 14A, filed April 28, 2000, containing the Registrant’s Definitive Proxy Statement for its 2000 Annual Meeting of Stockholders). **
|
|
|
10.7
|
Unigene Laboratories, Inc. 2006 Stock-Based Incentive Compensation Plan, as amended by Amendment No. 1 (incorporated by reference from Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2009). **
|
|
|
10.8
|
Amendment No. 2 to the Unigene Laboratories, Inc. 2006 Stock-Based Incentive Compensation Plan. **
|
|
|
10.9
|
Form of Incentive Stock Option Agreement under 2006 Stock-Based Incentive Compensation Plan (incorporated by reference from Exhibit 10.8 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2007).**
|
|
|
10.10
|
Form of Non-qualified Stock Option Agreement under 2006 Stock-Based Incentive Compensation Plan (incorporated by reference from Exhibit 10.9 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2007).**
|
|
53 of 59
|
Exhibit
Number
|
Description
|
|
|
10.11
|
Form of Restricted Stock Agreement under 2006 Stock-Based Incentive Compensation Plan (incorporated by reference from Exhibit 10.11 to the Registrant's Annual Report of Form 10-K for the year ended December 31, 2009).**
|
|
|
10.12
|
Employment Agreement by and between Unigene Laboratories, Inc. and Ashleigh Palmer, dated June 9, 2010 (incorporated by reference from Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, dated June 9, 2010).**
|
|
|
10.13
|
Employment Agreement by and between the Company and Gregory T. Mayes, dated September 12, 2010 (incorporated by reference from Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2010).**
|
|
|
10.14
|
Employment Agreement between Unigene Laboratories, Inc. and Warren P. Levy, dated January 1, 2000 (incorporated by reference from Exhibit 10.6 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1999).**
|
|
|
10.15
|
First Amendment to Employment Agreement between Unigene Laboratories, Inc. and Warren P. Levy, dated December 22, 2008 (incorporated by reference from Exhibit 10.12 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2008).**
|
|
|
10.16
|
Second Amendment to Employment Agreement, dated March 17, 2010, by and between the Company and Warren P. Levy (incorporated by reference from Exhibit 10.11 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010).**
|
|
|
10.17
|
Employment Agreement between Unigene Laboratories, Inc. and Ronald S. Levy, dated January 1, 2000 (incorporated by reference from Exhibit 10.7 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1999).**
|
|
|
10.18
|
First Amendment to Employment Agreement between Unigene Laboratories, Inc. and Ronald S. Levy, dated December 22, 2008 (incorporated by reference from Exhibit 10.14 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2008).**
|
|
|
10.19
|
Second Amendment to Employment Agreement, dated March 17, 2010, by and between the Company and Ronald Levy (incorporated by reference from Exhibit 10.12 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010).**
|
|
|
10.20
|
Employment Agreement between Unigene Laboratories, Inc. and Jay Levy, dated January 1, 2000 (incorporated by reference from Exhibit 10.8 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 1999).**
|
|
|
10.21
|
First Amendment to Employment Agreement between Unigene Laboratories, Inc. and Jay Levy, dated December 22, 2008 (incorporated by reference from Exhibit 10.15.1 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2008).**
|
|
|
10.22
|
Separation Agreement, dated March 16, 2010, by and between the Company and Jay Levy (incorporated by reference from Exhibit 10.13 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010).**
|
|
|
10.23
|
Summary of Deferred Compensation Plan between Unigene Laboratories, Inc. and Dr. Warren P. Levy and Dr. Ronald S. Levy, dated December 14, 2005 (incorporated by reference from Exhibit 10.1 to Registrant’s Current Report on Form 8-K, dated December 14, 2005).**
|
|
|
10.24
|
Non-qualified Deferred Compensation Agreement between Unigene Laboratories, Inc. and Dr. Warren P. Levy, dated February 1, 2006 (incorporated by reference from Exhibit 10.17 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2008).**
|
|
|
10.25
|
Non-qualified Deferred Compensation Agreement between Unigene Laboratories, Inc. and Dr. Ronald S. Levy, dated February 1, 2006 (incorporated by reference from Exhibit 10.18 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2008).**
|
|
|
10.26
|
Amendment to Non-qualified Deferred Compensation Agreement between Unigene Laboratories, Inc. and Dr. Warren P. Levy, dated December 19, 2008 (incorporated by reference from Exhibit 10.19 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2008).**
|
|
|
10.27
|
Amendment to Non-qualified Deferred Compensation Agreement between Unigene Laboratories, Inc. and Dr. Ronald S. Levy, dated December 22, 2008 (incorporated by reference from Exhibit 10.20 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2008).**
|
|
54 of 59
|
Exhibit
Number
|
Description
|
|
|
10.28
|
Form of Amended and Restated Change in Control Agreement, by and between the Company and the vice president named therein (incorporated by reference from Exhibit 10.8 to the March 2010 Form 8-K).**
|
|
|
10.29
|
Amended and Restated Change in Control Agreement, dated April 5, 2010, by and between the Company and Dr. James P. Gilligan (incorporated by reference from Exhibit 10.15 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010).**
|
|
|
10.30
|
Director Stock Option Agreement between Allen Bloom and Unigene Laboratories, Inc., dated December 5, 2001 (incorporated by reference from Exhibit 10.45 to Registrant’s Annual Report on Form 10-K for the year ended December 31, 2002).**
|
|
|
10.31
|
Director Stock Option Agreement between J. Thomas August and Unigene Laboratories, Inc., dated December 5, 2001 (incorporated by reference from Exhibit 10.46 to Registrant’s Annual Report on Form 10-K for the year ended December 31, 2002).**
|
|
|
10.32
|
Joint Venture Contract between Shijiazhuang Pharmaceutical Group Company, Ltd., and Unigene Laboratories, Inc., dated June 15, 2000 (incorporated by reference from Exhibit 10.1 to the Registrant’s Amended Quarterly Report on Form 10-Q/A , filed April 2, 2008, for the quarter ended June 30, 2000).
|
|
|
10.33
|
Articles of Association of Shijiazhuang-Unigene Pharmaceutical Corporation Limited, dated June 15, 2000 (incorporated by reference from Exhibit 10.2 to the Registrant’s Amended Quarterly Report on Form 10-Q/A, , filed April 2, 2008 for the quarter ended June 30, 2000).
|
|
|
10.34
|
Agreement by and between Shijiazhuang Pharmaceutical Group Company, Ltd. and Unigene Laboratories, Inc., dated April 23, 2008 (incorporated by reference from Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008).*
|
|
|
10.35
|
Technology Transfer Agreement by and between Shijiazhuang Pharmaceutical Group Corporation and Unigene Laboratories, Inc., dated April 23, 2008 (incorporated by reference from Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008).*
|
|
|
10.36
|
Agreement of Assignment among Shijiazhuang Pharmaceutical Group Corporation, Unigene Laboratories Inc., and China Pharmaceutical Group Limited, dated April 23, 2008 (incorporated by reference from Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008).
|
|
|
10.37
|
Stock Purchase Agreement, dated as of May 10, 2008, between Unigene Laboratories, Inc. and Tin Lon Investment Limited (incorporated by reference from Exhibit 10.1 to the Registrant’s Current Report on Form 8-K dated May 10, 2008).
|
|
|
10.38
|
Amended and Restated License Agreement, dated as of December 10, 2010, by and between Unigene Laboratories, Inc. and GlaxoSmithKline LLC.*
|
|
55 of 59
|
Exhibit
Number
|
Description
|
|
|
10.39
|
License and development agreement between Upsher-Smith Laboratories, Inc. and Unigene Laboratories, Inc., dated November 26, 2002 (incorporated by reference from Exhibit 10.1 to Registrant’s Current Report on Form 8-K/A, dated October 16, 2007). *
|
|
|
10.40
|
License Agreement, dated April 7, 2004, between Unigene Laboratories, Inc. and Novartis Pharma AG (incorporated by reference from Exhibit 10.1 to Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2007).*
|
|
|
10.41
|
Supply Agreement, dated January 22, 2007, by and between Unigene Laboratories, Inc. and Novartis Pharma AG (incorporated by reference from Exhibit 10.1 to the Registrant’s Amended Quarterly Report on Form 10-Q/A filed March 20, 2008, for the quarter ended March 31, 2007). *
|
|
|
10.42
|
Amended and Restated Security Agreement, dated May 10, 2007, by and between Unigene Laboratories, Inc. and Jay Levy (incorporated by reference from Exhibit 10.3 to Registrant’s Current Report on Form 8-K, dated May 10, 2007). *
|
|
|
10.43
|
Patent Security Agreement, dated March 13, 2001, between Unigene Laboratories, Inc. and Jay Levy (incorporated by reference from Exhibit 10.38 to Amendment No. 2 to Registrant’s Registration Statement No. 333-04557 on Form S-1, filed December 12, 2001).
|
|
|
10.44
|
First Amendment to Patent Security Agreement, dated May 29, 2001, between Unigene Laboratories, Inc. and Jay Levy (incorporated by reference from Exhibit 10.39 to Amendment No. 2 to Registrant’s Registration Statement No. 333-04557 on Form S-1, filed December 12, 2001).
|
|
|
10.45
|
Second Amendment to Patent Security Agreement, dated November 26, 2002, by and between Unigene Laboratories, Inc. and Jay Levy (incorporated by reference from Exhibit 10.33 to the Registrant’s Annual Report on Form 10-K for the year ended December 31, 2007).
|
|
|
10.46
|
Third Amendment to Patent Security Agreement, dated May 10, 2007, by and between Unigene Laboratories, Inc. and the Jaynjean Family Limited Partnership (incorporated by reference from Exhibit 10.4 to Registrant’s Current Report on Form 8-K, dated May 10, 2007).*
|
|
56 of 59
|
Exhibit
Number
|
Description
|
|
|
10.47
|
Mortgage and Security Agreement, dated July 13, 1999, by and between Unigene Laboratories, Inc. and Jay Levy (incorporated by reference from Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 1999).
|
|
|
10.48
|
Modification of Mortgage and Security Agreement, dated August 5, 1999, by and between Unigene Laboratories, Inc. and Jay Levy (incorporated by reference from Exhibit 10.7 to the Registrant’s Quarterly Report on Form 10-Q for the quarter ended September 30, 1999).
|
|
|
10.49
|
Modification of Mortgage and Security Agreement, dated May 10, 2007, by and between Unigene Laboratories, Inc. and Jay Levy (incorporated by reference from Exhibit 10.5 to Registrant’s Current Report on Form 8-K, dated May 10, 2007).*
|
|
|
10.50
|
Third Modification of Mortgage and Security Agreement, dated September 30, 2008, by and between Unigene Laboratories, Inc. and Jay Levy (incorporated by reference from Exhibit 10.8 to the Company’s Current Report on Form 8-K filed on October 6, 2008 (the “October 2008 8-K Report”)).
|
|
|
10.51
|
Assignment of Mortgage by Jay Levy to Jean Levy, dated February 25, 2010 (incorporated by reference from Exhibit 10.56 to the Registrant's Annual Report on Form 10-K for the year ended December 31, 2009).
|
|
|
10.52
|
Senior Secured Term Note, dated May 22, 2009, issued by Unigene Laboratories, Inc. in favor of the Lenders signatory to the Financing Agreement, dated as of September 30, 2008 (the "VPC Financing Agreement") by and among Unigene Laboratories, Inc., the Lenders and Victory Park Management, LLC, as agent (incorporated by reference from Exhibit 10.1 to the Registrant’s Current Report on Form 8-K, dated May 29, 2009).
|
|
|
10.53
|
Pledge and Security Agreement, dated as of September 30, 2008, by and among Unigene Laboratories, Inc., Victory Park Management, LLC, as agent, and the Secured Parties (incorporated by reference from Exhibit 10.3 to the October 2008 8-K Report).*
|
|
|
10.54
|
Amended and Restated Financing Agreement (the “Restated Financing Agreement”), dated as of March 16, 2010, by and among the Company, the Lenders and Victory Park Management, LLC, as agent (incorporated by reference from Exhibit 10.1 to the March 2010 Form 8-K).
|
|
|
10.55
|
Master Reaffirmation and Amendment to Transaction Documents, dated as of March 17, 2010, by and among the Company and Victory Park Management, LLC, as agent (incorporated by reference from Exhibit 10.2 to the March 2010 Form 8-K).*
|
|
|
10.56
|
Senior Secured Convertible Term Note, dated March 17, 2010, issued by the Company in favor of the Lenders signatory to the Restated Financing Agreement (incorporated by reference from Exhibit 10.3 to the March 2010 Form 8-K).
|
|
|
10.57
|
Amended and Restated Registration Rights Agreement, dated as of March 17, 2010, by and among the Company and the Lenders (incorporated by reference from Exhibit 10.4 to the March 2010 Form 8-K).
|
|
|
10.58
|
Mortgage, Security Agreement, Assignment of Rents and Leases and Fixture Filing, dated as of September 30, 2008, by and between the Company and Victory Park Management, LLC, as agent (incorporated by reference from Exhibit 10.5 to the October 2008 Form 8-K).
|
|
|
10.59
|
First Amendment to Mortgage, Security Agreement, Assignment of Rents and Leases and Fixture Filing, dated as of March 17, 2010, by and between the Company and Victory Park Management, LLC, as agent (incorporated by reference from Exhibit 10.6 to the October 2008 Form 8-K).
|
|
|
10.60
|
|
Affiliate Subordination Agreement, dated as of September 30, 2008, by and among Unigene Laboratories, Inc., Jay Levy, Jaynjean Levy Family Limited Partnership and Victory Park Management, LLC, as agent for the Lenders signatory to the VPC Financing Agreement (incorporated by reference from Exhibit 10.5 to the October 2008 8-K Report).
|
|
10.61
|
Assignment and Assumption Agreement, dated January 11, 2010, by and between Jay Levy and Jean Levy (incorporated by reference from Exhibit 10.64 to the Company's Annual Report on Form 10-K for the year ended December 31, 2009).
|
|
57 of 59
|
10.62
|
Affiliate Subordination Reaffirmation, dated as of March 17, 2010, by and among the Company, Jean Levy, Jaynjean Levy Family Limited Partnership and Victory Park Management, LLC, as agent for the Lenders (incorporated by reference from Exhibit 10.7 to the March 2010 Form 8-K).
|
|
|
10.63
|
Amended and Restated Secured Promissory Note, dated March 17, 2010, issued by the Company in favor of Jean Levy (incorporated by reference from Exhibit 10.8 to the Registrant’s quarterly report on Form 10-Q for the quarter ended March 31, 2010).
|
|
|
10.64
|
Amended and Restated Secured Promissory Note, dated March 17, 2010, issued by the Company in favor of the Jaynjean Levy Family Limited Partnership (incorporated by reference from Exhibit 10.9 to the Registrant’s quarterly report on Form 10-Q for the quarter ended March 31, 2010).
|
|
|
10.65
|
Fourth Modification of Mortgage, in favor of Jean Levy, dated March 17, 2010 (incorporated by reference from Exhibit 10.10 to the Registrant’s quarterly report on Form 10-Q for the quarter ended March 31, 2010).
|
|
|
10.66
|
Forbearance Agreement, dated December 10, 2010, by and among Unigene Laboratories, Inc., Victory Park Management, LLC, as agent, and the Secured Parties named therein.
|
|
|
10.67
|
Letter Agreement, dated as of December 10, 2010, by and among Unigene Laboratories, Inc., Victory Park Management, LLC, as agent, and the Secured Partied named therein.
|
|
|
10.68
|
License Agreement, dated October 19, 2009, by and between Unigene Laboratories, Inc. and Tarsa Therapeutics, Inc. (incorporated by reference from Exhibit 10.67 to the Company's Annual Report on Form 10-K for the year ended December 31, 2009).*
|
|
|
10.69
|
Amendment to License Agreement, dated January 15, 2010, by and among Unigene Laboratories, Inc. and Tarsa Therapeutics, Inc. (incorporated by reference from Exhibit 10.68 to the Company's Annual Report on Form 10-K for the year ended December 31, 2009).*
|
|
|
10.70
|
Omnibus Amendment Agreement, dated October 19, 2009, by and among Unigene Laboratories, Inc., Victory Park Management, LLC, as agent, and the holders signatory thereto (incorporated by reference from Exhibit 10.69 to the Company's Annual Report on Form 10-K for the year ended December 31, 2009).
|
|
|
10.71
|
Collateral Assignment Agreement, dated October 19, 2009, by and between Unigene Laboratories, Inc. and Victory Park Management, LLC, as agent (incorporated by reference from Exhibit 10.70 to the Company's Annual Report on Form 10-K for the year ended December 31, 2009).
|
|
|
10.72
|
Warrant Exchange Agreement, dated October 19, 2009, by and between Unigene Laboratories, Inc. and Victory Park Special Situations Master Fund, Ltd. (incorporated by reference from Exhibit 10.71 to the Company's Annual Report on Form 10-K for the year ended December 31, 2009).
|
|
23.1
|
|
Consent of Grant Thornton LLP.
|
|
| 23.2 | Consent of Ernst & Young LLP. | ||
|
24.1
|
Power of Attorney (included on signature pages to this report).
|
||
|
31.1
|
Certification by Ashleigh Palmer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
||
|
31.2
|
Certification by William Steinhauer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.
|
||
|
32.1
|
Certification by Ashleigh Palmer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
||
|
32.2
|
Certification by William Steinhauer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
||
|
*
|
Portions of the exhibit have been omitted pursuant to a request for confidential treatment.
|
|
**
|
Management contract or compensatory plan.
|
58 of 59
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
UNIGENE LABORATORIES, INC.
|
|||
|
March 16, 2011
|
/s/ Ashleigh Palmer
|
||
|
Ashleigh Palmer, President
|
|||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
March 16, 2011
|
/s/ Ashleigh Palmer
|
|||
|
Ashleigh Palmer, President, Chief Executive Officer and Director (Principal Executive Officer)
|
||||
|
March 16, 2011
|
/s/ William Steinhauer
|
|||
|
William Steinhauer, Vice President of Finance (Principal Financial Officer and Principal Accounting Officer)
|
||||
|
March 16, 2011
|
/s/ Richard Levy
|
|||
|
Richard Levy, Chairman of the Board
|
||||
|
March 16, 2011
|
/s/ Allen Bloom
|
|||
|
Allen Bloom, Director
|
||||
|
March 16, 2011
|
/s/ Zvi Eiref
|
|||
|
Zvi Eiref, Director
|
||||
|
March 16, 2011
|
/s/ Marvin L. Miller
|
|||
|
Marvin L. Miller, Director
|
||||
|
March 16, 2011
|
/s/ Joel Tune
|
|||
|
Joel Tune, Director
|
||||
Each person whose signature appears above in so signing also makes, constitutes and appoints each of Ashleigh Palmer, President and Chief Executive Officer of the registrant, and William Steinhauer, Vice President of Finance of the registrant, his true and lawful attorney-in-fact, in his name, place and stead to execute and cause to be filed with the Securities and Exchange Commission any or all amendments to this Form 10-K.
Page 59 of 59
