Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - MISONIX INC | c09237e8vk.htm |
Exhibit 10.1
Special Report
This report is published by Misonix, Inc. (“Misonix” or the “Company”) to provide the investor with
a general review of the Company’s technology and growth prospects for various products. This
report supplements some of the information that is available in the Company’s Form 10-K and related
documents. Except for historical information contained herein, the matters discussed in this report
contain forward-looking statements. The accuracy of these statements is subject to significant
risks and uncertainties. Actual results could differ materially from those contained in the
forward-looking statements.
December 1, 2010

Technology and Growth Status Report
Misonix develops, manufactures and markets ultrasonic medical devices for special surgery and
laboratory equipment. The Company’s medical systems are used for spine surgery, neurosurgery,
general surgery, maxillofacial surgery, cosmetic surgery and surgical wound debridement. The
company was founded in 1959 and has executive offices and production facilities in Farmingdale,
N.Y.
Symbol: MSON — Price: |
$ | 2.30 | ||
52-Week price range: |
$ | 1.61 - $3.84 | ||
Shares outstanding: |
7,000,000 | |||
Market capitalization: |
$16.1 million | |||
Shares held by insiders: |
Approx. 21 | %* | ||
% held by institutions: |
10.1 | % | ||
Daily trading vol. (avg): |
7,000 sh | |||
Cash/share (9/31/10) |
$ | 1.35 | ||
Book val./share (9/31/10) |
$ | 2.45 | ||
| FY | FY | 1Q | 1Q | |||||||||||||
| FY ends 6/30 | 2009 | 2010 | (9/09) | (9/10) | ||||||||||||
• Device sales (000) |
$ | 9,688 | $ | 10,737 | $ | 2,003 | $ | 2,692 | ||||||||
• Lab/other (000) |
3,025 | 2,634 | $ | 628 | $ | 566 | ||||||||||
Total sales |
$ | 12,713 | $ | 13,371 | $ | 2,631 | $ | 3,258 | ||||||||
Gross profit (000) |
$ | 5,218 | 6,526 | 1,009 | 1,637 | |||||||||||
Gross margin: |
41.0 | % | 48.8 | % | 38.4 | % | 50.3 | % | ||||||||
Net income (000)**: |
$ | (1,573 | ) | $ | (2,191 | ) | $ | (1,247 | ) | $ | (843 | ) | ||||
EPS** |
$ | (0.22 | ) | $ | (0.31 | ) | $ | (0.18 | ) | $ | (0.12 | ) | ||||
| * | Includes 1,164,410 shares which are issuable upon the exercise of presently vested options. |
|
| ** | Continuing operations |
Introduction
The worldwide market for ultrasonic tissue removal products is approximately $3.8 billion in
annual sales and is one of the fastest growing sectors of the medical device field. Misonix is a
technological leader in ultrasonic surgical devices and markets a wide range of products for
orthopedic surgery including spine and maxillofacial procedures, as well as neurosurgery, general
surgery, cosmetic surgery, wound debridement, etc.
We have recently undertaken a reorganization program that is now largely complete. A central
feature has been the divestiture of non-core assets over the past
18 months which — along with
other changes that have been put into place — has produced a more focused company, with over
$9 million in cash and a strong potential for growth and profit margin expansion. Other aspects
include expense belt-tightening, product rationalization, the launch of additional disposable
products, establishment of a U.S. sales force selling Misonix-labeled products direct to
hospitals, and expansion of international distribution including important new contracts in
Brazil, China, France, Germany and the Russian Federation.
Our share price is near the low end of the 3-5 year range. Price recovery depends on whether
recent revenue and profit margin trends continue in the coming quarters, and the extent to which
the Company begins to attract broader interest and attention. The current valuation is modest by
medical device standards as a multiple of annual sales. Our top three products — in bone
cutting, wound debridement and surgical aspiration systems — are strong entries in an
addressable world market of over $1 billion in annual sales. We expect significant market share
expansion in each of these areas in the years ahead.
| Senior Management | ||
Michael A. McManus, Jr. President, Chief Executive Officer |
CEO since 1999. Former President and CEO of New York Bancorp, Inc., prior to which he held senior positions with Jamcor Pharmaceutical, Pfizer and Revlon. Former Assistant to President Ronald Reagan. | |
Richard Zaremba Senior VP, CFO, Treasurer, Secretary |
With Company since 1999. Former VP and CFO of Comverse Information Systems, a manufacturer of digital voice recording systems, prior to which he was VP and CFO of Miltope Group, Inc. | |
Michael C. Ryan Senior VP, Medical Division |
Joined in 2007. Former Senior VP and General Manager for Nomos Radiation Oncology and Executive VP Business Development for Inter V, Inc., a medical device company marketing specialty products for interventional radiology, interventional cardiology and oncology. | |
Dan Voic VP, R&D and Engineering |
Approximately 15 years of senior scientific and product development experience at Misonix with demonstrated expertise in all phases of ultrasound technology. | |
Ronald Manna VP, Regulatory Affairs |
Present position since 2002. Former VP of R&D and Engineering, VP of Operations and Director of Engineering of the Company. | |
Frank Napoli VP, Operations |
Joined in 2004. Former VP of Manufacturing for Spellman High Voltage Electronics Corp. Prior Director of Manufacturing for Telephonics Corporation. |
| Board of Directors | ||
Michael A. McManus, Jr.
|
Chairman, President and Chief Executive Officer of Misonix (see above). | |
Howard Alliger
|
Chairman of the Board and CEO of Frontier Pharmaceutical, Inc. Founded the predecessor company to Misonix in 1955. Former president of the Ultrasonic Industry Association | |
T. Guy Minetti
|
CEO of Twig Tek, LLC, which is engaged in the recirculation and recycling of used electronics; prior to which he founded and was Managing Director of Senior Resource Advisors LLC, a management consulting firm; prior to which Mr. Minetti served as the Vice Chairman of the Board of Directors of 1-800- Flowers.Com, Inc.; prior to which he was the Managing Director of Bayberry Advisors, an investment banking firm he founded in 1989. From 1981 through 1989, Mr. Minetti was a Managing Director of Kidder, Peabody & Company where he worked in the investment banking and high yield bond departments. | |
Thomas F. O’Neill
|
Founding principal of Sandler O’Neill & Partners, LP, an investment banking firm. Serves on the Board of Archer-Daniels-Midland Company and The Nasdaq Stock Market, Inc. | |
John Gildea
|
Founding principal of Gildea Management Company, a management company focusing on special situations in the U.S. and Central Europe. Previously managed the Corporate Series Group at Donaldson Lufkin Jenrette. | |
Dr. Charles Miner III
|
Currently practices internal medicine in Darien, Connecticut. Serves on staff at Stamford and Norwalk Hospitals; Instructor in clinical medicine at Columbia University College of Physicians and Surgeons. | |
Executive
Offices
|
1938 New Highway, Farmingdale, NY 11735 Tel: (631) 694-9555 |
|
Auditors
|
Grant Thornton LLP Melville, NY |
|
Corporate Counsel
|
Joel I. Frank, Esq. Wilk Auslander LLP — New York, NY |
Table of Contents
| Page | ||||
Executive Summary |
4 | |||
Company Overview and Growth Strategy |
6 | |||
Leading Products |
9 | |||
• Misonix BoneScalpel™ |
9 | |||
• SonicOne® |
11 | |||
• SonaStar® |
14 | |||
Testimonials |
16 | |||
Other Products |
17 | |||
• AutoSonix™ |
17 | |||
• LySonix™ |
17 | |||
• Laboratory Filtration |
17 | |||
• High Intensity Focused Ultrasound (HIFU) |
18 | |||
Stock Analysis and Valuation |
20 | |||
Financial Statements |
21 | |||
Issued U.S. Patents |
23 | |||
Risk Factors |
25 | |||
Executive Summary
| • | Misonix is believed to be one of a limited number of companies in the world that has
the ability to apply ultrasound technology to a broad range of specialty surgical
devices. We develop, produce, and sell proprietary and patented products. All R&D,
engineering, product development and manufacturing is done at the Misonix headquarters
facility in Farmingdale, Long Island, New York. |
| • | The Misonix advantage is comprised of our large patent portfolio along with the skills
and know-how of our scientists accumulated over the last 20 years. Misonix is a leader
in ultrasonic surgical specialty systems with a wide range of products for general
surgery, neurosurgery, spine surgery, surgical wound debridement and liposuction. We
believe there are very few companies in the ultrasound field that know as much about the
design and manufacture of high-quality therapeutic medical devices than does Misonix. |
| • | We sell our surgical products on a private label or OEM basis through large partners
like Johnson & Johnson, Aesculap and Covidien and most recently,
through our own network of sales agencies and specialty distributors selling direct to
hospitals. International distribution has expanded throughout Europe, the Middle East,
Latin America and selected markets in Asia. Important contracts in Brazil, China,
France, Germany and Russia have recently been executed. |
| • | Each Misonix surgical product includes an ultrasonic generator and a disposable
component, which allows for continued sales after the placement of a unit at consistently
high profit margins. |
| • | Through our recently completed program of selling off non-core business units, we have
become a more focused surgical device company. Misonix has amassed over $9 million in
cash. With no debt on the balance sheet, the Company is in a strong position to acquire
additional products, develop new products or acquire distribution rights for products to
be sold to its present customer base. |
|
| • | Misonix has a focused strategy to: |
| • | Build a profitable high margin surgical device business. |
| • | Expand its channels of distribution throughout the world. |
| • | Build brand awareness for the Misonix name. |
| • | Focus on cost control, manufacturing efficiencies and expense reduction. |
| • | Increase margins and generate respectable net income. |
The following illustrates many of the expected drivers of our revised growth strategy.
Misonix Growth Model
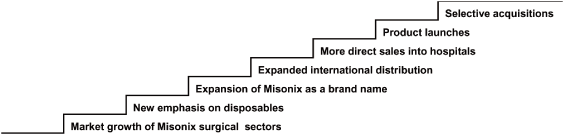
- 4 -
| • | Fiscal year 2010 was a transitional year owing to new initiatives and the phasing out
of former businesses, which in some cases distorted year-to-year quarterly comparisons.
Encouraging patterns have begun to emerge as seen by strong sales growth for medical
devices in the last three quarters. |
| Fiscal 2011 | ||||||||||||||||||||
| Fiscal 2010 | 1Q (Sep.) | 2Q (Dec.) | 3Q (Mar.) | 4Q (Jun.) | 1Q (Sep.) | |||||||||||||||
Medical Device Sales (000) |
$ | 2,003 | 2,494 | 2,635 | 3,605 | $ | 2,692 | |||||||||||||
% Gain |
(12 | %) | (31 | %) | 73 | % | 60 | % | 34.0 | % | ||||||||||
Gross Margin – Company |
38.4 | % | 47.8 | % | 51.8 | % | 53.6 | % | 50.3 | % | ||||||||||
Prior year |
37.0 | % | 40.0 | % | 42.8 | % | 45.0 | % | 38.4 | % | ||||||||||
| • | Profit margin improvement is partly the result of tighter control of corporate
overhead costs. On a more fundamental level, it reflects a series of initiatives that
are starting to take hold: |
| • | The Company is transitioning from our historic reliance on private label and OEM
customers to promotion of our own products under the Misonix brand name. Brand
name products now account for 46% of our sales, up from 33% over the course of the
past 12 months. |
| • | We are expanding direct to hospital marketing and sales through smaller,
specialty agencies and distributor organizations, which typically earn lower
commissions or discounts than is the case with exclusive contracts with large
distribution houses. |
| • | We are starting to shift from reusable products to reusable products with
disposable components. This should be beneficial not only for margins but building
customer loyalty and repeat business. The following table illustrates approximate
average hospital list prices for disposable components for some of the major
applications. |
| Surgical | Bone Cutting | |||||
| Aspiration | and Sculpting | Wound Debridement | ||||
| (in the OR) | ||||||
Avg. Sales Price: |
$500 | $450 | $400 | |||
Expected use
frequency: |
5x/month | 8x/month | 5x/month |
| • | The Company’s technology base consists of over 45 issued patents covering a wide
spectrum of products, methods and procedures, as well as extensive know-how and product
design skills that have been accumulating over the past 20 years. These skills range from
knowing how to work with the complexities of acoustical waves in order to optimize
performance, to in-house control of all phases of electronic design, which is a rare
capability in medical device manufacturing. |
| • | Misonix shares continue to trade near the bottom of the 3-5 year price range. Price
recovery depends on whether recent revenue and profit trends continue over the coming
quarters and the extent to which the Company begins to attract broader interest and
attention. The current valuation modestly exceeds trailing revenues and book value. |
- 5 -
| • | Our top three products — BoneScalpelÔ,
SonicOne® and
SonaStar® — are strong entries in an addressable world market with over $1
billion in annual sales. M&A activity in the ultrasound device field has been strong and
has left relatively few independent companies in the sector. |
| • | Another potential valuation driver for MSON is the development of promising HIFU
technology described in this report. Recently, the French company EDAP, which is the
current market leader in HIFU, reported favorable clinical outcomes for over 800 patients
with localized prostate cancer that were treated with its Ablatherm®-HIFU system. It was
reported that for a representative number of patients, the cancer specific survival rate
and the freedom from metastatic disease rate were 99% and 97%, respectively, at eight
years. We believe this further validation of HIFU as a cancer tumor ablation technology
may help to explain the recent rise in investor interest in EDAP shares. |
52- Week Shock Chart
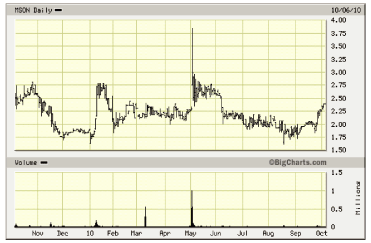
Source: Big Charts
Company Overview and Growth Strategy
The total market for ultrasonic tissue removal encompasses $3.8 billion on a worldwide basis.
Misonix participates in both hard and soft tissue sectors with a broad range of products, as
summarized in the following diagram. The use of ultrasound for tissue ablation is still in early
stage market development and potential for new uses abound.
| Hard Tissue | Soft Tissue | |||||
| Misonix Products | Misonix Products | |||||
| BoneScalpel | SonicOne, SonaStar, LySonix, AutoSonix | |||||
| Addressable Market: $1.45 billion | Addressable Market: $2.3 billion | |||||
| Current Markets | Potential Markets | Current Markets | Potential Markets | |||
• Spine
|
• ENT | • Wound debridement | • Tumor ablation | |||
• Maxillofacial
|
• Neurosurgery | • Neurosurgery | • Vascular | |||
| • Cartilage | • General surgery | • HIFU | ||||
| • Arm and hand | • Liposuction | |||||
| • Foot and ankle | ||||||
- 6 -
Revised Misonix Growth Strategy
Misonix is rapidly moving from its historical diversified products orientation towards becoming
a focused medical device company. We are also moving from a historical reliance on reusable
products by adding high margin disposables, from private label and OEM customers to greater
emphasis on Misonix as a brand name, from sales to large distribution houses with relatively low
margins to more direct to hospital sales in the U.S. and significant expansion in international
distribution agreements. These are classic growth strategies in the medical device sector and
we can now pursue them without the encumbrances of the past.
The following table summarizes the various non-core products and businesses that have been
divested over the past 15 months. The proceeds from these divestitures have helped to raise
cash to over $9 million and enabled us to not have to renew a former $8 million bank line of
credit. We expect net cash to climb towards $14 million if all of the proceeds from the
divestiture program are realized, which cannot be guaranteed.
| Proceeds | ||||||||||||||||||||||
| Total | Proceeds at | Proceeds | Released | |||||||||||||||||||
| Closing | Proceeds | Closing | Pending | Post Closing | ||||||||||||||||||
| (mil) | (mil) | (mil) | ||||||||||||||||||||
Labcaire Systems, Ltd.* |
Hospital products | 8/5/09 | $ | 5.6 | $ | 3.6 | $ | 2.0 | .250 | |||||||||||||
Laboratory Ultrasonics |
Lab products | 4/7/09 | 3.5 | 3.5 | — | |||||||||||||||||
Sonora Medical Systems |
Hospital Product refurbishment | 10/2/09 | 8.0 | 6.4 | 0.4 | .400 | ||||||||||||||||
Equity stake in Focus Surgery** |
HIFU | 5/28/10 | 5.8 | — | 5.8 | |||||||||||||||||
| Total | $ | 21.7 | $ | 13.5 | $ | 8.2 | .650 | |||||||||||||||
| * | $1.0m note payable in equal $250,000 installments over 4 years and up to $1.0m in
commissions based on product sales |
|
| ** | Paid by USHIFU, LLC on basis of earn-out mostly as a percentage of gross revenues |
We expect savings from the sale of these businesses and the reduction of associated overhead to
generate over $1 million in cost savings over the next 12 months. This includes a $750,000
reduction in personnel costs, $200,000 annual savings due to lower insurance premiums, and
$150,000 in savings from no longer having bank credit line carrying costs.
Shift to Misonix as a brand name. Misonix has historically had a heavy component of
private label and OEM business or has licensed our technology to others. While this has usually
resulted in less expensive product launches and quick launch times, it has often been to the
detriment of business predictability, quarter-by-quarter growth continuity and profit margins.
The progress that Misonix has recently made in promoting Misonix as a brand name is seen in the
accelerating sales growth of the BoneScalpel, SonicOne and SonaStar products. Brand name
products now account for 46% of Company sales, up from 33% roughly 12 months ago.
- 7 -
Shift from reuseables to disposables. The Company’s gross profit margin for
continuing operations, which has reached 49%, should significantly benefit to the extent that
disposable products as a percent of total sales continue to climb. This is partly a function
of manufacturing efficiencies that are lowering unit costs, thus making disposables more price
competitive, but also reflects entry into market sectors where the use of disposables is more
commonplace — such as in hospital operating rooms. The following table illustrates
approximate average list prices for disposable components of some lead products:
| SonaStar | BoneScalpel | SonicOne | ||||
Avg. List Price: |
$500 | $450 | $400 | |||
Expected use frequency: |
5x/month | 8x/month | 5x/month |
More direct sales and more profitable contracts. There is a positive trend currently
underway with respect to distribution channels. More growth is being generated through direct to
hospital and specialty distributor sales than is taking place through contracts with large
OEM/private label distribution partners like J&J, Aesculap and Covidien. The fact that
distributor pricing and profits are improving adds another dimension to the Company’s growth
potential — not unlike an effective price increase that could continue over the course of the
next several years.
Future Development and Growth
Misonix will continue its internal development of unique, ultrasonic medical devices using its
proprietary IP and knowhow, while adding to its worldwide distribution organization. It is
anticipated that growth may come from a number of diverse sources such as:
| • | Misonix expects to build on its present platform by adding new disposable probes
aimed at increasing product utilization in present markets. |
||
| • | Misonix will look for opportunities to use the present products in new markets or
for different procedures. Examples would be taking the BoneScalpel™ into the small
bone hand and foot markets. |
||
| • | Misonix expects to develop new ultrasound product applications for both soft and
hard tissue. |
||
| • | Misonix expects to negotiate business opportunities with companies that produce
products used in the same procedures as current Misonix products or products that can
be sold to the same Misonix customers. Misonix wants to be in a position where it is
selling multiple products to the same customers. These opportunities for growth would
come in the form of distribution agreements or product line acquisitions. |
||
| • | Misonix expects to meet with investment bankers and business brokers to find
synergistic product acquisition opportunities. |
||
| • | In some cases, Misonix may look at a new distribution capability for acquisition.
This could be in the form of a unique domestic or international sales force. |
||
| • | As noted previously, Misonix expects to meet with companies and investors interested
in working with Misonix to continue the development of its own unique HIFU technology. |
- 8 -
Leading Products
Misonix BoneScalpelÔ
Ultrasonic Bone Cutting System
Ultrasonic Bone Cutting System
The BoneScalpel has consistently generated strong growth since its U.S. introduction more than
two years ago. This product gives Misonix a unique niche in the dynamic field of spinal surgery,
and an opportunity to enter other areas of small bone orthopedic and maxillofacial surgery.
The BoneScalpel is a novel ultrasonic bone cutting
tool capable of making a 0.5mm clean cut with
minimal necrosis or burn artifacts, little
inflammation, and minimal effect on soft tissue.
The device can make linear and curved cuts, on any
plane, with precision previously unavailable.
The BoneScalpel is so precise that it can cut a
window in an eggshell while leaving the membrane
intact. The product is ideally suited for surgical
small bone applications involving the spine, the
maxillofacial area (facial, nasal and jawbones),
the skull, and the hand and foot. Minimal damage
to surrounding soft tissue is an important
feature, especially in spine surgery, and in other
areas of the body where critical nerve tissue and
vasculature may be in close proximity to the
surgical site.
BoneScalpel

Traditional powered cutting instruments like high-speed burrs or oscillating saws are far more
aggressive, do not distinguish between hard and soft tissue and are often less precise.
Additional opportunities may exist in selected indications for large bone surgery, i.e. the knee.
The BoneScalpel will compete mainly against pneumatic and electronic bone cutters, where the
largest companies in the sector include Stryker, Synthes and Medtronic.
The product addresses an estimated $600 million global market for spine, maxillofacial, ENT, foot
and ankle, and plastic surgery/reconstructive procedures.
Comparison of Bone-Cutting Technology
| BoneScalpel | High-Speed Drill | Micro Saw | ||||
Cutting Frequency
|
23 kHz | 80,000 rpm | 20,000 cpm | |||
Tip Characteristic
|
Blunt blade | Abrasive surface | Sharp teeth | |||
Cutting mode
|
Longitudinal | Rotational | Traverse | |||
Minimum kerf size
|
0.54 mm | 2 mm | 0.4 mm | |||
Tip start/stop
|
Near instant | Delayed | Minor delay | |||
Tip cooling
|
Direct to vibrating edge | Indirect/ancillary | Indirect/ancillary | |||
Effect on soft tissue
|
Minimal | Very aggressive (wrapping, tearing) |
Very Aggressive (tearing) |
- 9 -
Additional Product Features
The BoneScalpel offers the speed and convenience of a powered instrument without the dangers
associated with conventional rotary instruments. With the BoneScalpel, bone yields to recurring
impacts resulting in a high-precision compression cut while the blade is being irrigated by a
patented jet nozzle that directs irrigation fluid over the blade to prevent bone necrosis. The
effect on soft tissue is substantially muted because the elastic and flexible structure of this
tissue tends to absorb the impact energy like a spring. This is a big advantage in anatomical
regions like the spinal dura, where accidental perforation of the spinal cord is not an uncommon
mishap, especially in revision procedures. Another feature of the BoneScalpel is that the
linear motion of the blunt tip avoids accidental trapping of soft tissue while eliminating the
spinning and tearing associated with rotary power instruments. Surgeons are able to improve on
existing techniques and design new approaches to performing osteotomies and removing bone, which
can lead to substantial time savings and increased efficiency in the operating room.
The BoneScalpel enables the reduction of bone resection times in laminectomies by up to 60
minutes, and more extensive spinal procedures by as much as 2 hours. According to the American
Association of Neurological Surgeons, there are 250,000 laminectomies performed in the U.S. each
year and the number of procedures continues to grow due mainly to expansion in the population of
elderly Americans and technological advances. It is possible that the BoneScalpel will become a
universal tool in spine surgery for nerve decompression, implant site preparation and the
correction of deformities like scoliosis. Spine surgery is only one aspect of the potential
market for the BoneScalpel. Current commercial opportunities exist in craniofacial surgery for
the correction of facial and jaw deformities and the treatment of sleep apnea, as well as for
pediatric and small bone surgery.
BoneScalpel Product Summary |
||
Current addressable market
|
$600 million | |
Potential future applications |
||
1) Cartilage
|
$400 million – spine | |
2) Large bone
|
$400 million – hip, knee | |
Target medical practitioners:
|
Orthopedic surgeons, neurosurgeons, plastic surgeons | |
Primary user sites:
|
Specialty/ General/ VA hospitals, Day Surgery Centers | |
Marketed by:
|
Domestic: Aesculap (Spine), Specialty Agencies International: Specialty Distributors |
|
Average unit price
|
$30,000 per system and $450 for disposables | |
Reimbursement status
|
Reimbursement varies by country and procedure | |
Primary competitors |
||
• High-speed burrs
|
Medtronic, Anspach, Stryker, Aesculap | |
Spine, neuro and ENT |
||
• Small bone power saws
|
Synthes, Stryker, MicroAire | |
Maxillofacial, hand, foot, ankle |
- 10 -
SonicOne®
Ultrasonic Wound Care System
Ultrasonic Wound Care System
The SonicOne is an innovative system that offers tissue specific debridement and cleansing of
wounds for effective removal of devitalized tissue and fibrin deposits while sparing viable
cells. This tissue specific capability is in part due to the fact that healthy and viable tissue
structures have a higher elasticity and flexibility than necrotic cells and are more resistant to
destruction from ultrasound effects. This ultrasound debridement process separates infected from
viable layers for a more defined treatment and reduced pain sensation. Clinical applications
include, but are not limited to, the debridement of diabetic foot ulcers, venous ulcers, pressure
sores, burns and bone. Ease of use and portability make the SonicOne suitable for a broad variety
of wound care settings: acute care, operating rooms, extended care facilities, outpatient
facilities and wound care clinics. The SonicOne is a new approach to advanced wound care and
progression towards patient healing.
The SonicOne facilitates an extremely thorough
debridement, by successfully removing necrotic materials
and devitalized tissue with minimal pain sensation and
excellent preservation of healthy, soft tissue.
The Debridement Probes are designed to address the needs
of different wound types. The unique shape of each probe
allows the clinician to choose the most appropriate probe
based upon the wound characteristics. The ultrasonic probe
allows for deep tissue penetration and causes cell
destruction within the wound bed. Continuous irrigation
(via the built in pump) provides a medium for cavitation
and flushes the wound of fibrin deposits and bacterial
growth while preserving most healthy tissue.
SonicOne

All of the ultrasonic probes are designed for direct contact with the wound surface, providing
maximum wound debridement efficacy.
Clinical experience with the SonicOne suggests exceptional wound bed granulation, accompanied by
significantly reduced bleeding and tissue trauma.
The standard of care for wound debridement procedures (the use of the traditional scalpel or
curette) has not changed in decades. Currently, in the USA, the use of the SonicOne can be billed
under certain existing codes for wound debridement, but no additional reimbursement is given for
use of ultrasound energy. Reimbursement codes can be expanded with published clinical evidence that
supports improved clinical efficacy and cost-effectiveness of advanced wound debridement options
like the SonicOne. With technology specific reimbursement as the ultimate goal, a randomized trial
involving a statistically significant number of patients will be submitted for publication in a
leading medical journal in the near future, comparing ultrasonic wound debridement with standard
manual approaches, focusing on reduction of wound area and incidence of complete healing.
Ultimately, this study will be the basis for the Company’s petition to the applicable agencies that
control healthcare reimbursements.
- 11 -
Market data indicates that chronic wounds afflict more than 8 million Americans. A large segment
of the chronic wound market is comprised of diabetics with foot ulcers (3-5 million in the U.S.).
Healthcare costs for the treatment of diabetic ulcers ranges from $7,400 — $20,600 per episode.
Close to 20% of diabetic patients with ulcers will require some type of lower extremity
amputation during their lifetime, accounting for more than 80,000 amputations per year in the
U.S. One of the main factors in this is poor wound care management. Another large segment is
the chronic leg ulcer. 2.5 million Americans have venous leg ulcers and the prevalence level is
3.5% in people over age 65. Cost of care per episode can exceed $40,000 and recurrence rates can
be as high as 70%. Total U.S. healthcare spending approximates $3 billion. The global market for
advanced wound management is valued at $3.7-4.0 billion and continues to expand primarily due to
the growth of the elderly population, sedentary lifestyles and the rising incidence of diabetes.
Secondary wound care market priorities include hospital-acquired pressure ulcers, which afflict
up to 28% of patients in long-term care facilities.
The SonicOne has been marketed to wound care clinics and outpatient facilities for quite some time,
but recently the emphasis has expanded to include long-term acute care hospitals and government
funded facilities (military, VA Hospitals and Indian reservations), where patient populations are
large and reimbursement is generally favorable.
| • | Long-term acute care hospitals. There are approximately 400 long-term acute
care hospitals in the U.S. with many owned by corporate entities such as Vibra
Healthcare and Triumph Healthcare. These institutions specialize in the
treatment and rehabilitation of medically complex patients who require an extended stay in
a hospital setting (25-30 days or longer). Reimbursement in these facilities is based on a
flat-fee, so using technologies that may reduce the length of stay, such as SonicOne, are
of great interest. Much of the Company’s business in this sector has been generated
through monthly rental programs where customers can pay as little as $2,000 per month for
the use of a SonicOne System. It is expected that most rentals will ultimately
convert to capital purchases. |
|
| • | Acute care general hospitals and Government Funded Facilities. Acute care
general, Veterans Administration (VA) and military hospitals present prime opportunities
for Misonix. The patient population of chronic and traumatic wounds is large. Misonix has
developed a line of disposable procedural trays for surgical debridement that are expected
to launch in early calendar 2011. The primary competitive product in the surgical wound
debridement space is the VersaJet™ (Smith & Nephew). This product uses a high-pressure
stream of saline, which, as compared to the SonicOne, is relatively dated technology. |
SonicOne will be available for purchase on a GSA contract for all government facilities in the near
future. Misonix has entered into an agreement with a strategic partner to place its products onto
a GSA contract. The strategic partner will generate sales leads, process bids and take orders for
Misonix through its own client base. This is an efficient way for Misonix to sell into the
government segment because it allows government accounts to easily access our products and reduces
the time to purchase.
- 12 -
The revenue prospects for SonicOne should substantially improve as the focus expands into the
operating room (“OR”) for wound debridement procedures. This will be accompanied by the launch of a
full line of disposable procedure trays to address the needs of the OR as compared to the
outpatient setting.
Domestically, the SonicOne is marketed through a network of specialty sales agencies. As marketing
and product developments expand into the OR many of the clinicians that are part of the current
call pattern in the wound care clinics and long-term acute care hospitals are the same people that
we will focus on with the OR based debridement initiative.
SonicOne Product Summary |
||
Addressable U.S. market:
|
$250 million in the U.S. | |
1) Outpatient/ wound care clinics
|
Approximately. 2,000 facilities in U.S. | |
2) Long Term acute care hospitals
|
Approximately 400 facilities in U.S. | |
3) Hospital (OR)
|
Over 7,000 facilities in U.S. | |
4) VA Locations
|
Over 500 facilities in the U.S. | |
Average unit selling price
|
$40,000 per system and $400 for operating procedure disposables | |
Marketed by:
|
Domestic: Specialty Agencies International: Specialty Distributors |
|
CMS reimbursement for outpatient
|
Reimbursed using established CPT codes for wound debridement | |
CMS reimbursement for inpatient/OR use |
Reimbursed using established codes for wound debridement. | |
Principal competitors
|
Ultrasonic Debridement in Clinic: Söring Sonoca (Germany), Arobella Qoustic (U.S.) | |
| Surgical Debridement in OR: Smith & Nephew VersaJet™ |
- 13 -
SonaStar®
Ultrasonic Surgical Aspiration System
Ultrasonic Surgical Aspiration System
SonaStar is primarily marketed for use in neurosurgery. A secondary market, liver resection,
presents a segment where growth prospects are believed to be particularly attractive for this
product. In the U.S., Misonix sells this product directly to hospitals using a network of
specialty sales agencies that employ more than 75 salespeople. In other countries, the SonaStar is
represented by a specialty distributor organization selling direct to hospitals. Direct to
hospital sales in the U.S. translates to higher profit margins with better account control and
steadily improving brand equity.
SonaStar is engineered to provide powerful and precise
ultrasonic soft tissue aspiration as well as fragmentation of
hard and soft tissues. It is indicated for open to minimally
invasive surgery; and can be used for neuro, general, thoracic
and gynecologic surgical procedures. SonaStar is also capable of
precise shaving of bony structures to gain access to the softer
tissue behind the bones.
The SonaStar operates at a powerful 23kHz frequency and allows
for quick and efficient removal of hard and calcified tumors.
The system brings surgical aspiration technology to a high
level. Active process control maintains all performance
parameters such as ultrasound, irrigation and aspiration at
optimum settings. Hand instruments are well-balanced and
ergonomically designed.
SonaStar

Dynamic tissue management enables intuitive control over loading characteristics from delicate
tumor removal to de-bulking of calcified tumors. Load adjustments are as intuitive as altering
automotive speed with a gas pedal. A wireless footswitch, the only wireless footswitch available
for an ultrasonic surgical aspirator, controls up to four functions: ultrasound, ultrasound + COAG,
COAG and fluid infiltration while offering the convenience of a cordless device. The system
includes a wide variety of single-use sterile products, including aspiration probes and associated
tubing.
Management believes that the SonaStar is highly competitive with, and in some respects superior to,
comparable products sold by Integra Life Sciences, which is the largest supplier of ultrasonic
surgical tissue ablation systems and has a market share in excess of 50%. The market for
ultrasound instruments continues to grow at the expense of older manual approaches to tumor
removal. Misonix is focusing its marketing on two surgical procedures that are believed to be
prime targets for expanded use of the SonaStar—the removal of brain tumors and liver resections.
- 14 -
Brain tumors. Approximately 20,000 primary brain tumors are diagnosed in the U.S. each
year. Secondary brain cancer occurs in 20–30% of patients with metastatic disease. In the United
States, about 100,000 cases of secondary brain cancer are diagnosed each year. Many tumor or cancer
types can spread to the brain, the most common being cancer of the lung, breast, kidney, bladder
and melanoma. Surgery is the most widely used treatment modality and is often done concurrently
with radiation and or chemotherapy. Surgery may be used for metastatic brain tumors when there is
a single lesion and when there is no cancer elsewhere in the body. Some may be completely removed.
Tumors that are deep or that infiltrate brain tissue may be de-bulked in order to reduce pressure
and relieve symptoms in cases when the tumor cannot be removed.
Liver Cancer. Liver cancer is the third most common cause of cancer-related death and the
liver is a common site for metastatic disease from cancer arising in other organs. In the U.S.,
liver metastases are much more common than primary liver cancer. The liver’s large size, high
volume of blood flow, and dual blood supply (the hepatic artery and portal vein) make it
particularly vulnerable to invasion by cancerous cells, especially those originating in the colon,
rectum, lung and breast. Surgical removal of the tumor is standard treatment for liver cancer.
Marketing. SonaStar marketing is focused on neurosurgery and liver surgery, supported by
specialty sales agents in the U.S. and specialty distributors worldwide.
SonaStar Product
Summary |
||
Addressable market:
|
$120 million in the U.S. | |
Procedure size of
addressable market:
|
5,000 brain tumor surgeries per year / 7,000 liver resections | |
Marketed by:
|
Domestic: Specialty Agencies International: Specialty Distributors |
|
Average unit price
|
$115,000 per system and $500 per procedure disposables | |
Primary competitors
|
Integra Life Sciences, Stryker, Soering |
- 15 -
Testimonials
BoneScalpel
• “I have performed over 100 laminectomies for single and multilevel cases and
including revisions. It saves me 30-60 min per case when performing decompressions. The
BoneScalpel gives me a high level of control in cutting bone structures with ease and
precision while being able to stop right on the ligamentum flavum. It offers a
considerable improvement in safety. The learning curve has been minimal and it is very
intuitive to use.”
Arnold M. Schwartz, MD, Orthopedic Spine Surgeon, Huntington Hospital, Huntington, NY
• “The device cuts through bone quickly and precisely. I observed no damage to
soft tissue once it comes in contact. The ultrasonic energy is dispersed. The system
qualities are essential for spinal bone incisions in close proximity to spinal nerve
roots and the dura such as laminectomies. With growing experience with the device. I
have observed shorter duration of surgery and a decrease in bleeding.”
Nachshon Knoller, MD, Sheba Medical Center, Tel-Hashomer, NA, Israel
• “I have utilized the device in over 90 patients in cervical, thoracic and lumbar
applications. Its major advantage is the ability to cut bone while preserving underlying
neural and non-bony structures. This greatly reduces the risk of dural laceration. The
ultrasonic cut reduces bleeding from the bone edges and efficiently eliminates soft tissue
bleeding.”
William C. Welch — MD, FACS, FICS, Chief of Neurosurgery, The Pennsylvania Hospital,
Philadelphia, PA
SonicOne
• “SonicOne is an extremely useful tool in our out-patient clinic to safely and
effectively debride wounds when other methods were totally ineffective. The SonicOne has now
become a standard of care in our wound care center for rapid wound healing. Bio-burden is a
significant contributor to non-healing chronic wounds. With routine use of SonicOne, I have
observed a significant improvement to overall healing when bio-burden was a factor or issue
to non-healing”.
Joseph P. Cavorsi, M.D., Medical Director,The Center for Advanced Wound Care
• “The SonicOne is indispensible in our clinic. There is a great sense of security to
be able to achieve reduction in necrosis, debris, and bacteria from our patient’s wounds
with minimal to no discomfort, as well as the satisfaction of promoting wound healing at the
same time.”
Dot Weir, RN, CWON, CWS, Program Director, Wound Healing Center, Osceola Regional Medical
Center
- 16 -
Other Products
AutoSonixTM
Soft Tissue Tran-section and Cauterization
Soft Tissue Tran-section and Cauterization
Misonix has been a pioneer in the development of ultrasonic instruments to transect and fragment
soft tissue, and to coagulate and seal blood vessels through a process that is generally regarded
as superior to electro-cauterization. This ultrasonically powered “cut and coagulate” approach has
grown sharply in parallel with the number of minimally invasive procedures, especially those done
laparoscopically. Misonix markets this product exclusively, worldwide, through Covidien. The
market is large, however, market leadership has gone to the Harmonic Scalpel® sold by
the Ethicon Division of Johnson & Johnson.
LySonix™
Ultrasound Assisted Liposuction
Ultrasound Assisted Liposuction
The LySonix 3000 Ultrasound Assisted Liposuction System (UAL) is distributed by Mentor
Corporation, now a division of Johnson & Johnson. The product will be emphasized by J&J and
growth in the large cosmetic and body sculpting markets should accelerate.
In UAL, ultrasonic waves emitted by a probe are used to break up the fat and liquefy it into an
emulsion. As the fat is emulsified, it can then be easily suctioned with the tumescent fluid.
The use of ultrasound reduces tissue damage and bleeding because no mechanical action is
necessary to break up the fat. Moreover, less suction is needed to pull out the fat when it is
in a liquid state. As stated by the American Society of Plastic Surgeons, “Ultrasonic Assisted
Lipoplasty has been shown to improve the ease and effectiveness of liposuction in fibrous areas
of the body and is commonly used in secondary procedures when enhanced precision is needed.”
UAL systems can be especially useful in removing large volumes of fat in a single operation with
up to 50% less bleeding. There have been reports that ultrasound methods actually contract the
skin as the procedure is underway. Ultrasound can also help break up fat in the face, neck,
abdomen, back, buttocks, and calf where tough and fibrous deposits cannot be removed with
traditional methods without significant damage to the surrounding tissue. The primary competitor
in UAL is Sound Surgical, Inc. which markets the Vaser SystemÔ.
Laboratory Air Filtration and Forensic Analysis
We manufacture and sell portable ductless fume hood systems to hospitals and laboratories, as well
as related forensic testing equipment, mainly to law enforcement agencies. Misonix has been in
this business since the mid-1990s and it is the only part of the industrial products side of the
Company that management has chosen to retain. This business generates roughly $3 million in annual
revenues and makes a solid contribution both to profits and the absorption of corporate overhead.
- 17 -
The Company’s portable ductless fume hoods are mostly self-contained carbon or HEPA filtered
enclosures that remove hazardous fumes, vapors and particles from virtually any laboratory
application. Ductless hoods require no installation and are deployed simply by placing a unit on an
existing countertop or cart in order to immediately improve the air quality. Misonix also designs
and manufactures related products for forensic analysis that are used for fingerprinting, evidence
drying or DNA testing and are used by law enforcement departments throughout the U.S.
High-Intensity Focused Ultrasound (“HIFU”)
After roughly a decade of involvement in the HIFU sector as a joint venture partner with others,
Misonix has recently changed tactics and, in so doing, has carved out its own franchise in the
sector and enhanced its long-term growth potential. In June of 2009 Misonix acquired three HIFU
patents from ProRhythm, Inc. for a relatively small investment. Approximately 10 months later,
Misonix exited its distribution agreement with USHIFU, LLC in the best interests of both companies’
growth strategies. Since the purchase, Misonix has filed for five additional patent claims. The
focused objective of the Company’s continuing product development effort is to market new HIFU
transducer technology either individually or in concert with a strategic partner.
One of the ProRhythm patents centers around the
use of a flat, novel HIFU transducer design
consisting of a flat shape transducer, and a
focusing Fresnel lens. The Fresnel lens was
developed in France more than a century ago, and
was first widely used in lighthouses to transmit
navigational lights further than was possible
before. Substantially thinner than a
conventional lens, the Fresnel lens is comprised
of concentric sections known as Fresnel zones.
The lens can effectively focus acoustical energy
many centimeters away from the surface to create
tissue ablation. This flat shape
transducer-Fresnel lens combination is highly
promising for use in HIFU applications in at
least the following respect:
Fresnel Lens (1)
Vs. Conventional Lens (2)
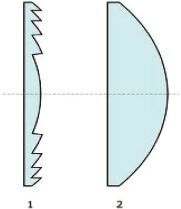
| • | It raises the possibility of developing much smaller, simpler, and less expensive HIFU
systems — especially the HIFU probe component containing the transducer and lens, which is
currently priced in the $5,000 — $8,000 range. By using less costly lens components and
transducers that are currently available from multiple suppliers, the HIFU probe could
conceivably open the door to a new generation of disposable products. |
- 18 -
Baseline evaluation tests of a new HIFU transducer design covered by our IP have recently been
completed successfully. Bench tests show that the transducer can create lesions up to 45mm in
depth and fully developed all the way back to the tissue surface. Reduced manufacturing costs
make the HIFU transducer suitable for use as a disposable component.
HIFU Background.
HIFU is a concentration of continuous beam ultrasound energy that raises the tissue in a
pre-determined focal zone to a high temperature sufficient to ensure coagulative necrosis without
blood loss or damage to the surrounding tissue. HIFU has significant clinical acceptance potential
due in part to its minimally invasive character, single-session treatment, minimal anesthesia, and
perceived short recovery period and quick return to daily activity.
The potential clinical efficacy for ablation of cancer tumors has not yet been thoroughly
investigated. The “piecemeal” nature of an ablation process in which the volume of lesion destroyed
at any given time is small (i.e. 1-3 mm wide / 5-20 mm high) makes it difficult to achieve complete
and homogeneous ablation of the entire gland. Another limiting factor is the relatively high cost
of current generation HIFU systems.
The world market for HIFU systems approximates $100 million in annual sales, and is fragmented both
regionally and by medical specialty — for example, Chinese surgeons and European urologists. The
two principal suppliers of ultrasound-guided transrectal HIFU devices for prostate cancer are
EDAP of France (Ablatherm®) and USHIFU, LLC (Sonablate® 500). Although
these devices are approved in Europe and the Far East, their use is currently limited in the U.S.
to the treatment of uterine fibroids. HIFU is still investigational in the U.S. for cancer tumor
ablation and Phase III trials are underway. China Medical Technologies (CMED) of Beijing,
is a dominant supplier in China, which presently accounts for roughly half of all worldwide HIFU
orders. Meanwhile, GE Healthcare has been a principal investor in Insightec, Ltd.
(Israel) which is selling HIFU systems, mainly for treating uterine fibroids, in many large markets
except Japan and Russia.
Future growth of HIFU will in large part be determined by FDA approval for ablation of cancer
tumors, and the development of new technologies that can lower costs and increase the therapeutic
range of HIFU as a treatment modality.
- 19 -
Stock Analysis and Valuation
Misonix has had a difficult time attracting investor or Wall Street interest while our story was
confused by a mix of laboratory and medical device businesses. The sale of the non-core businesses,
the focus on large market medical products, the international distribution, and the recurring
revenues from the sale of disposables are expected to attract more interest.
52- Week Shock Chart
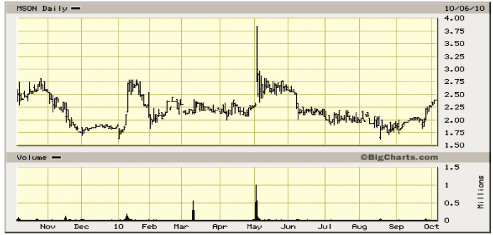
Source: Big Charts
The stock price remains at the lower end of its historic range. The market capitalization is only
modestly higher trailing 12-month revenues and the Company’s book value. Our three leading
medical devices — BoneScalpelÔ, SonicOneÔ and SonaStar®
— are believed to be strong entries in a $1 billion addressable market and each of these
products is believed by our management to have outstanding growth potential.
A potential valuation driver for the Company could be the development of promising HIFU technology
described in this report. Recently, the French company EDAP reported favorable clinical outcomes
for over 800 patients with localized prostate cancer that were treated with its HIFU system. It
was reported that for a representative number of patients, the cancer specific survival rate and
the freedom from metastatic disease rate were 99% and 97%, respectively, at 8 years.
Management and directors control 20.7% of the Company’s shares while most of the remainder is
held in the form of small institutional holdings or by individual investors.
| Shares Beneficially | ||||||||||||
| Name | Affiliation | Owned on 11/02/2010 | % of Class | |||||||||
Michael A. McManus, Jr. |
CEO and Chairman | 836,751 | 11.0 | |||||||||
Dimensional Fund Advisors LP |
511,508 | 6.8 | ||||||||||
Howard Alliger |
Director | 251,508 | 3.5 | |||||||||
Richard Zaremba |
Officer | 148,000 | 2.1 | |||||||||
Other Officers and Directors (8) |
292,750 | 4.1 | ||||||||||
All Officers and Directors (11) |
1,693,111 | 20.7 | %* | |||||||||
| * | Includes 1,164,410 shares which are issuable upon the exercise of presently vested options |
- 20 -
Financial Statements
Misonix, Inc. and Subsidiaries
Consolidated Balance Sheets
Consolidated Balance Sheets
| June 30, | September 30, | |||||||
| 2010 | 2010 | |||||||
| (Derived from | ||||||||
| audited | ||||||||
| statements) | (Unaudited) | |||||||
Assets |
||||||||
Current assets |
||||||||
Cash and cash equivalents |
$ | 9,900,605 | $ | 9,413,437 | ||||
Accounts receivable, less doubtful account allowance |
2,335,653 | 1,920,689 | ||||||
Investments, net |
2,699,717 | 2,919,243 | ||||||
Prepaid expenses and other current assets |
515,427 | 362,283 | ||||||
Note receivable |
1,075,105 | 920,145 | ||||||
Total current assets |
16,526,507 | 15,535,797 | ||||||
Property, plant and equipment, net |
500,215 | 526,084 | ||||||
Goodwill |
1,701,094 | 1,701,094 | ||||||
Other assets |
1,730,339 | 1,465,004 | ||||||
Total assets |
$ | 20,458,155 | $ | 19,227,979 | ||||
Liabilities and stockholders’ equity |
||||||||
Current liabilities |
||||||||
Notes payable |
177,679 | 84,491 | ||||||
Accounts payable |
888,654 | 832,940 | ||||||
Accrued expenses and current liabilities |
1,000,523 | 915,704 | ||||||
Total current liabilities |
2,066,856 | 1,833,135 | ||||||
Capital lease obligations |
14,274 | 10,474 | ||||||
Deferred lease liability |
— | 1,404 | ||||||
Deferred income |
250,739 | 214,420 | ||||||
Total liabilities |
2,331,869 | 2,059,433 | ||||||
Stockholders’ equity |
||||||||
Common stock ($0.01 par value shares, 20,000,00 authorized) |
70,792 | 70,792 | ||||||
Additional paid-in capital |
25,502,717 | 25,562,823 | ||||||
Accumulated deficit |
(7,034,799 | ) | (8,052.645 | ) | ||||
Treasury stock, at cost, 77,800 shares |
(412,424 | ) | (412,424 | ) | ||||
Total stockholders’ equity |
18,126,286 | 17,168,546 | ||||||
Total liabilities and stockholders’ equity |
$ | 20,458,155 | $ | 19,227,979 | ||||
- 21 -
Misonix, Inc. and Subsidiaries
Income Statement
Income Statement
| FY 2010 | FY 2009 | Fiscal 1Q | Fiscal 1Q | |||||||||||||
| (ended 6/30) | (ended 6/30) | (9/30/10) | (9/30/09) | |||||||||||||
Net sales — total |
$ | 13,371,275 | $ | 12,713,273 | $ | 3,257,988 | $ | 2,631,017 | ||||||||
Medical devices |
10,737,379 | 9,688,294 | 2,692,268 | 2,003,284 | ||||||||||||
Laboratory and other |
2,633,896 | 3,024,979 | 565,720 | 627,733 | ||||||||||||
Gross profit — total |
$ | 6,526,495 | $ | 5,218,271 | 1,637,285 | 1,009,124 | ||||||||||
Medical devices |
5,799,713 | 4,319,395 | 1,472,571 | 908,585 | ||||||||||||
Laboratory and other |
726,782 | 898,876 | 164,714 | 100,539 | ||||||||||||
Operating expenses |
||||||||||||||||
Selling |
$ | 3,625,072 | $ | 2,619,510 | 965,007 | 919,607 | ||||||||||
General & administrative |
5,055,848 | 5,018,143 | 1,217,805 | 1,312,680 | ||||||||||||
Research & development |
1,803,524 | 1,377,807 | 460,494 | 422,469 | ||||||||||||
Total operating expenses |
$ | 10,484,444 | $ | 9,015,460 | 2,643,306 | 2,654,756 | ||||||||||
Loss from operations |
(3,957,949 | ) | (3,797,189 | ) | (1,006,021 | ) | (1,645,632 | ) | ||||||||
Other income (expenses) |
||||||||||||||||
Interest income |
28,227 | 67,170 | 50 | 14,025 | ||||||||||||
Interest expense |
(53,194 | ) | (158,007 | ) | (3,641 | ) | (28,088 | ) | ||||||||
Royalty income and |
||||||||||||||||
licensing fees |
614,663 | 616,336 | 179,115 | 156,623 | ||||||||||||
Royalty expense |
(117,630 | ) | (24,822 | ) | (19,343 | ) | — | |||||||||
Recovery of Focus
Surgery, Inc.
Investment |
693,044 | 1,516,866 | — | — | ||||||||||||
Other |
(92,799 | ) | 282,721 | 45,409 | 10,164 | |||||||||||
Total other income |
$ | 1,072,311 | $ | 2,300,264 | 201,590 | 152,724 | ||||||||||
Net loss from continuing
operations |
||||||||||||||||
Before income tax (benefit) |
(2,885,638 | ) | (1,496,925 | ) | (804,431 | ) | (1,492,908 | ) | ||||||||
Income tax (benefit) |
(694,796 | ) | 76,329 | 38,100 | (245,764 | ) | ||||||||||
Net loss from
continuing operations |
$ | (2,190,842 | ) | $ | (1,573,254 | ) | (842,531 | ) | (1,247,144 | ) | ||||||
Net loss from cont.
operations attributed to
Misonix
shareholders—Diluted |
$ | (0.31 | ) | $ | (0.22 | ) | $ | (0.12 | ) | $ | (0.18 | ) | ||||
Weighted average shares |
7,001,369 | 7,001,369 | 7,001,369 | 7,001,369 | ||||||||||||
- 22 -
Issued U.S. Patents
June 30, 2010
| Expiration | ||||||
| Number | Description | Date | ||||
| 5,248,296 | Wire with sheath — relating to the
Company’s Alliger System for
reducing transverse motion in its
catheters. |
12/24/10 | ||||
| 5,306,261 | Guidewire guides — relating to the
Company’s Alliger System for a
catheter with collapsible wire
guide. |
1/22/13 | ||||
| 5,443,456 | Guidewire guides — relating to the
Company’s Alliger System for a
catheter with collapsible wire
guide. |
2/10/14 | ||||
| 5,371,429 | Flow-thru transducer — relating to
the Company’s liposuction system and
its ultrasonic laboratory and
scientific products for an
electromechanical transducer device.
|
09/28/13 | ||||
| 5,397,293 | Catheter sheath — relating to the
Company’s Alliger System for an
ultrasonic device with sheath and
transverse motion damping. |
11/25/12 | ||||
| 5,419,761 | Liposuction — relating to the
Company’s liposuction apparatus and
associated method. |
8/03/13 | ||||
| D409 746 | Cannula for ultrasonic probe. |
5/11/13 | ||||
| D408 529 | Cannula for ultrasonic probe. |
4/20/13 | ||||
| D478165 | Cannula for ultrasonic probe. |
8/05/17 | ||||
| 5,465,468 | Flow-thru transducer — relating to
the method of making an
electro-mechanical transducer device
to be used in conjunction with the
Company’s soft tissue aspiration
system and ultrasonic laboratory and
scientific products. |
12/06/14 | ||||
| 5,527,273 | Ultrasonic probes — relating to an
ultrasonic lipectomy probe to be
used with the Company’s soft tissue
aspiration technology. |
10/6/14 | ||||
| 5,769,211 | Autoclavable switch — relating to a
medical handpiece with autoclavable
rotary switch to be used in medical
procedures. |
1/21/17 | ||||
| 5,562,609 | Ultrasonic surgical probe. |
10/07/14 | ||||
| 5,562,610 | Needle for ultrasonic surgical probe. |
10/07/14 | ||||
- 23 -
| Expiration | ||||||
| Number | Description | Date | ||||
| 6,033,375 | Ultrasonic probe with isolated and Teflon coated outer cannula. |
12/23/17 | ||||
| 6,270,471 | Ultrasonic probe with isolated outer cannula. |
12/23/17 | ||||
| 6,443,969 | Ultrasonic blade with cooling. |
8/15/20 | ||||
| 6,379,371 | Ultrasonic blade with cooling. |
11/15/19 | ||||
| 6,375,648 | Infiltration cannula with Teflon coated outer surface. |
10/02/18 | ||||
| 6,063,050 | Ultrasonic dissection and coagulation system. |
10/16/17 | ||||
| 6,036,667 | Ultrasonic dissection and coagulation system. |
08/14/17 | ||||
| 6,582,440 | Non-clogging catheter for lithotripsy. |
12/26/16 | ||||
| 6,454,730 | Thermal film ultrasonic dose indicator. |
04/02/19 | ||||
| 6,613,056 | Ultrasonic probe with low-friction bushings. |
02/17/19 | ||||
| 6,648,839 | Ultrasonic medical treatment device for RF cauterization and related
method. |
05/08/22 | ||||
| 6,660,054 | Fingerprint processing chamber with airborne contaminant containment
and adsorption. |
09/10/21 | ||||
| 6,736,814 | Ultrasonic medical treatment device for bipolar RF cauterization and
related method. |
02/28/22 | ||||
| 6,799,729 | Ultrasonic cleaning and atomizing probe. |
10/05/21 | ||||
| 6,869,439 | Ultrasonic dissector. |
03/22/22 | ||||
| 6,902,536 | RF cauterization and ultrasonic ablation. |
06/07/22 | ||||
| 6,377,693 | Tinnitus masking using ultrasonic signals. |
06/23/14 | ||||
| 6,173,062 | Frequency transpositional hearing aid with digital and single
sideband modulation. |
03/16/14 | ||||
| 6,169,813 | Frequency transpositional hearing aid with single sideband modulation. |
03/16/14 | ||||
| 5,663,727 | Frequency response analyzer and shaping apparatus and digital hearing
enhancement apparatus and method utilizing the same. |
06/23/15 | ||||
| 7,442,168 | High efficiency medical transducer with ergonomic shape and method
manufacture. |
04/01/23 | ||||
| 7,223,267 | Ultrasonic probe with detachable slidable cauterization forceps. |
02/06/24 | ||||
| 7,717,913 | Cauterization and ultrasonic ablation instrument with multi hole
collar and electrode MTG sleeve. |
11/04/24 | ||||
| 7,776,027 | Medical Handpiece with automatic power switching means |
7/11/22 | ||||
| 6,492,762 | Ultrasonic Transducer, Transducer Array, And Fabrication Method |
3/22/20 | ||||
| 6,787,974 | Ultrasound Transducer Unit And Planar Ultrasound Lens |
11/21/21 | ||||
| 6,461,314 | Intrabody HIFU Applicator |
2/2/20 | ||||
- 24 -
Risk Factors
An investment in Misonix involves risk. This report supplements information available in the
Company’s Annual Report on Form 10-K for the year ended June 30, 2010 (the “10-K”) and related
documents. Our business financial condition or results of operations could be materially adversely
affected by any of these risks. These risks are more specifically set forth in the Risk Factors
section of the 10-K. You should refer to the qualification and limitations of forward looking
statements set forth immediately prior to the beginning of Item 1 of the 10-K. An investor should
also refer to our subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. We
disclaim any obligation to update our forward- looking statements. Additional risks not presently
known to us, or that we currently deem immaterial, may also adversely affect our business,
financial condition or results of operations.
- 25 -
Published by:
Misonix, Inc.
1938 New Highway
Farmingdale, NY 11735
Phone: 631-694-9555
Fax: 631-694-9412
Farmingdale, NY 11735
Phone: 631-694-9555
Fax: 631-694-9412
Website:
http://www.misonix.com
http://www.misonix.com
