Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HUMAN GENOME SCIENCES INC | c05842e8vk.htm |
Exhibit 99

| com com POISED for the MARKET: PREPARING FOR LAUNCH AND FUTURE GROWTH Company Update September, 2010 |

| Note Regarding Forward-Looking Statements This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. The forward- looking statements are based on Human Genome Sciences' current intent, belief and expectations. These statements are not guarantees of future performance and are subject to certain risks and uncertainties that are difficult to predict. Actual results may differ materially from these forward-looking statements because of Human Genome Sciences' unproven business model, its dependence on new technologies, the uncertainty and timing of clinical trials and regulatory approvals, Human Genome Sciences' ability to develop and commercialize products, its dependence on collaborators for services and revenue, its substantial indebtedness and lease obligations, its changing requirements and costs associated with facilities, intense competition, the uncertainty of patent and intellectual property protection, Human Genome Sciences' dependence on key management and key suppliers, the uncertainty of regulation of products, the impact of future alliances or transactions and other risks described in the Company's filings with the SEC. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of today's date. Human Genome Sciences undertakes no obligation to update or revise the information contained in this presentation whether as a result of new information, future events or circumstances or otherwise. HGS, Human Genome Sciences, and BENLYSTA are trademarks of Human Genome Sciences, Inc. Other trademarks referenced are the property of their respective owners. |
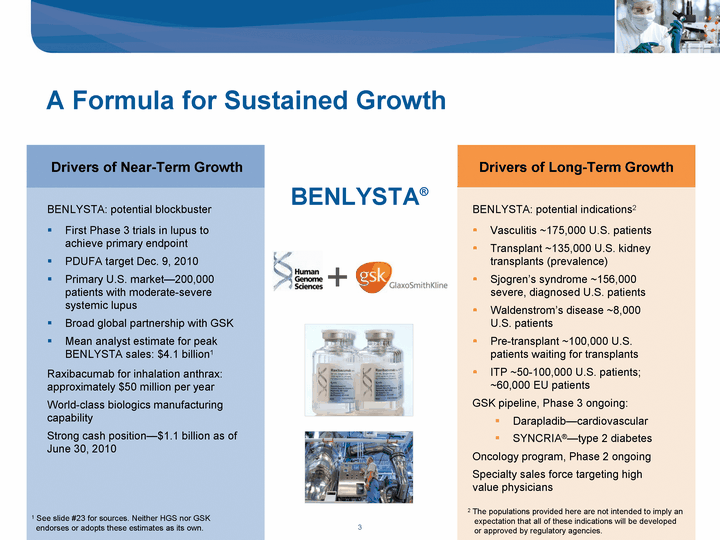
| A Formula for Sustained Growth BENLYSTA: potential blockbuster First Phase 3 trials in lupus to achieve primary endpoint PDUFA target Dec. 9, 2010 Primary U.S. market-200,000 patients with moderate-severe systemic lupus Broad global partnership with GSK Mean analyst estimate for peak BENLYSTA sales: $4.1 billion1 Oncology program, Phase 2 ongoing Specialty sales force targeting high value physicians Drivers of Long-Term Growth Drivers of Near-Term Growth + BENLYSTA(r) Raxibacumab for inhalation anthrax: approximately $50 million per year World-class biologics manufacturing capability Strong cash position-$1.1 billion as of June 30, 2010 BENLYSTA: potential indications2 Vasculitis ~175,000 U.S. patients Transplant ~135,000 U.S. kidney transplants (prevalence) Sjogren's syndrome ~156,000 severe, diagnosed U.S. patients Waldenstrom's disease ~8,000 U.S. patients Pre-transplant ~100,000 U.S. patients waiting for transplants ITP ~50-100,000 U.S. patients; ~60,000 EU patients GSK pipeline, Phase 3 ongoing: Darapladib-cardiovascular SYNCRIA(r)-type 2 diabetes 1 See slide #23 for sources. Neither HGS nor GSK endorses or adopts these estimates as its own. 2 The populations provided here are not intended to imply an expectation that all of these indications will be developed or approved by regulatory agencies. |
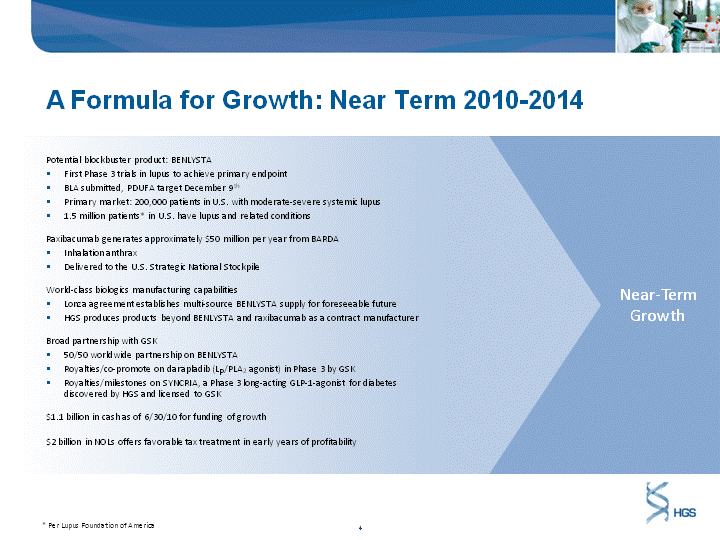
| A Formula for Growth: Near Term 2010-2014 Potential blockbuster product: BENLYSTA First Phase 3 trials in lupus to achieve primary endpoint BLA submitted, PDUFA target December 9th Primary market: 200,000 patients in U.S. with moderate-severe systemic lupus 1.5 million patients* in U.S. have lupus and related conditions Raxibacumab generates approximately $50 million per year from BARDA Inhalation anthrax Delivered to the U.S. Strategic National Stockpile World-class biologics manufacturing capabilities Lonza agreement establishes multi-source BENLYSTA supply for foreseeable future HGS produces products beyond BENLYSTA and raxibacumab as a contract manufacturer Broad partnership with GSK 50/50 worldwide partnership on BENLYSTA Royalties/co-promote on darapladib (LP/PLA2 agonist) in Phase 3 by GSK Royalties/milestones on SYNCRIA, a Phase 3 long-acting GLP-1-agonist for diabetes discovered by HGS and licensed to GSK $1.1 billion in cash as of 6/30/10 for funding of growth $2 billion in NOLs offers favorable tax treatment in early years of profitability * Per Lupus Foundation of America Near-Term Growth |

| A Formula for Growth Long Term: 2014 and Beyond BENLYSTA studies planned/underway for new indications and dosage form Sub Q formulation potentially increases dosing convenience for patients New studies planned in related B-cell diseases: Vasculitis, post-renal transplant, Sjogren's, Waldenstrom's, pre-transplant, and ITP (idiopathic thrombocytopenic purpura). Mean analyst estimate for peak BENLYSTA sales: $4.1 billion* (15 analysts reporting) Specialty sales force will address targeted, high-value physicians in U.S. and Europe Darapladib (Phase 3) offers potential new cardiovascular treatment, discovered and developed by GSK LP/PLA2 mechanism favorably reviewed in December 2009 NEJM Two large scale patient trials underway, funded by GSK 10% royalty to HGS with 20% co-promote option in the U.S. and E.U. provides potentially substantial value SYNCRIA (Phase 3) offers long-acting GLP-1 agonist for diabetes, developed by HGS and licensed to GSK Eight Phase 3 studies underway, funded by GSK Royalties payable to HGS on global sales of product Raxibacumab potentially provides ongoing revenue source Oncology pipeline addresses promising mechanisms TRAIL, IAP inhibitor both target apoptotic pathway Long-Term Growth * See slide #23 for sources. Neither HGS nor GSK endorses or adopts these estimates as its own. |
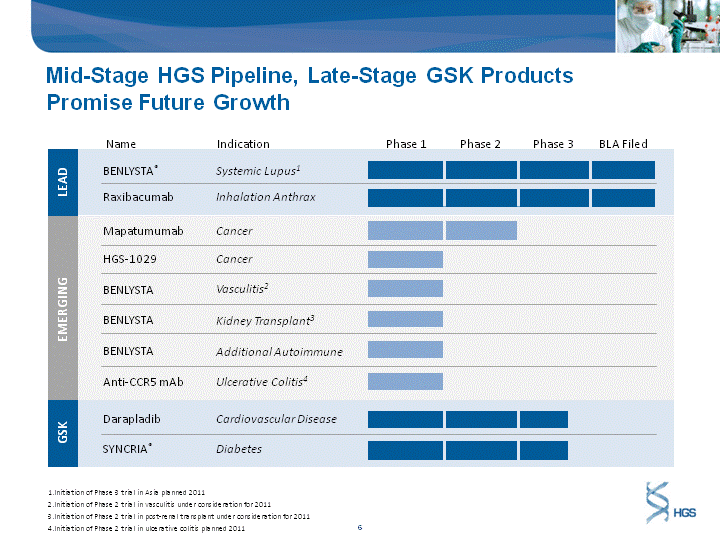
| EMERGING Mid-Stage HGS Pipeline, Late-Stage GSK Products Promise Future Growth BENLYSTA(r) Anti-CCR5 mAb HGS-1029 BENLYSTA BENLYSTA BENLYSTA Raxibacumab Mapatumumab LEAD GSK SYNCRIA(r) Darapladib Systemic Lupus1 Ulcerative Colitis4 Cancer Additional Autoimmune Vasculitis2 Kidney Transplant3 Inhalation Anthrax Cancer Diabetes Cardiovascular Disease Initiation of Phase 3 trial in Asia planned 2011 Initiation of Phase 2 trial in vasculitis under consideration for 2011 Initiation of Phase 2 trial in post-renal transplant under consideration for 2011 Initiation of Phase 2 trial in ulcerative colitis planned 2011 BLA Filed |
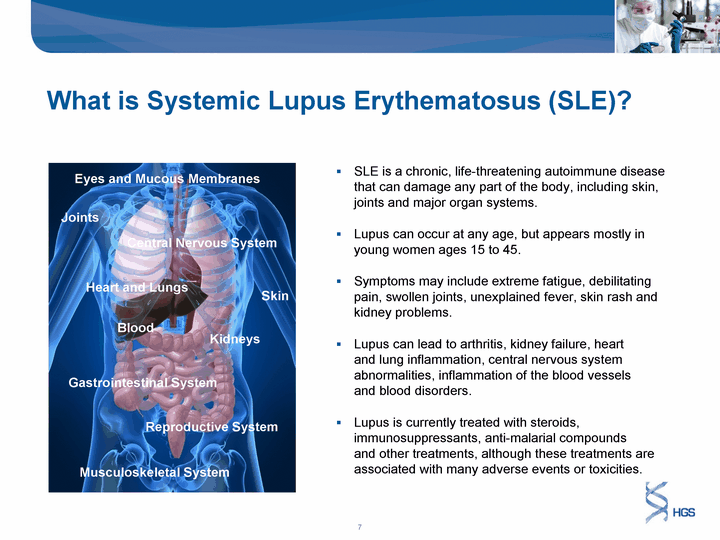
| SLE is a chronic, life-threatening autoimmune disease that can damage any part of the body, including skin, joints and major organ systems. Lupus can occur at any age, but appears mostly in young women ages 15 to 45. Symptoms may include extreme fatigue, debilitating pain, swollen joints, unexplained fever, skin rash and kidney problems. Lupus can lead to arthritis, kidney failure, heart and lung inflammation, central nervous system abnormalities, inflammation of the blood vessels and blood disorders. Lupus is currently treated with steroids, immunosuppressants, anti-malarial compounds and other treatments, although these treatments are associated with many adverse events or toxicities. What is Systemic Lupus Erythematosus (SLE)? What is Systemic Lupus Erythematosus (SLE)? Eyes and Mucous Membranes Heart and Lungs Skin Gastrointestinal System Reproductive System Musculoskeletal System Joints Kidneys Central Nervous System Blood |
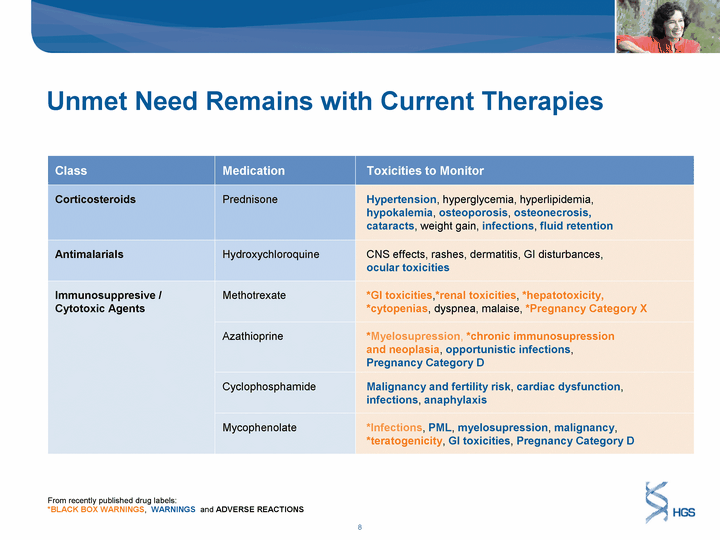
| Unmet Need Remains with Current Therapies Class Medication Toxicities to Monitor Corticosteroids Prednisone Hypertension, hyperglycemia, hyperlipidemia, hypokalemia, osteoporosis, osteonecrosis, cataracts, weight gain, infections, fluid retention Antimalarials Hydroxychloroquine CNS effects, rashes, dermatitis, GI disturbances, ocular toxicities Immunosuppresive / Cytotoxic Agents Methotrexate *GI toxicities,*renal toxicities, *hepatotoxicity, *cytopenias, dyspnea, malaise, *Pregnancy Category X Immunosuppresive / Cytotoxic Agents Azathioprine *Myelosupression, *chronic immunosupression and neoplasia, opportunistic infections, Pregnancy Category D Immunosuppresive / Cytotoxic Agents Cyclophosphamide Malignancy and fertility risk, cardiac dysfunction, infections, anaphylaxis Immunosuppresive / Cytotoxic Agents Mycophenolate *Infections, PML, myelosupression, malignancy, *teratogenicity, GI toxicities, Pregnancy Category D From recently published drug labels: *BLACK BOX WARNINGS, WARNINGS and ADVERSE REACTIONS |
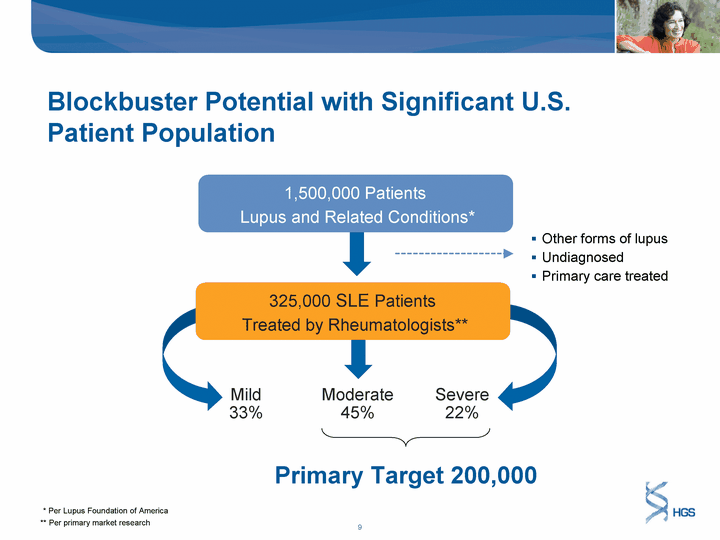
| Blockbuster Potential with Significant U.S. Patient Population 1,500,000 Patients Lupus and Related Conditions* Mild 33% Severe 22% 325,000 SLE Patients Treated by Rheumatologists** Moderate 45% Other forms of lupus Undiagnosed Primary care treated Primary Target 200,000 ** Per primary market research * Per Lupus Foundation of America |
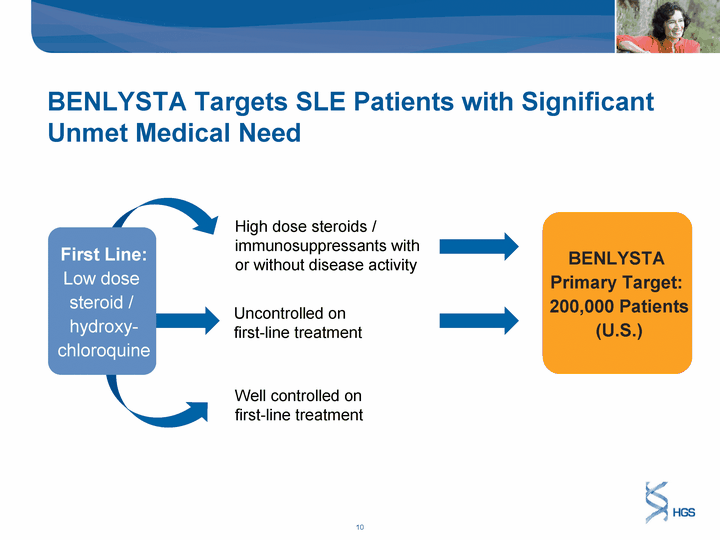
| BENLYSTA Targets SLE Patients with Significant Unmet Medical Need High dose steroids / immunosuppressants with or without disease activity Uncontrolled on first-line treatment Well controlled on first-line treatment BENLYSTA Primary Target: 200,000 Patients (U.S.) First Line: Low dose steroid / hydroxy- chloroquine |
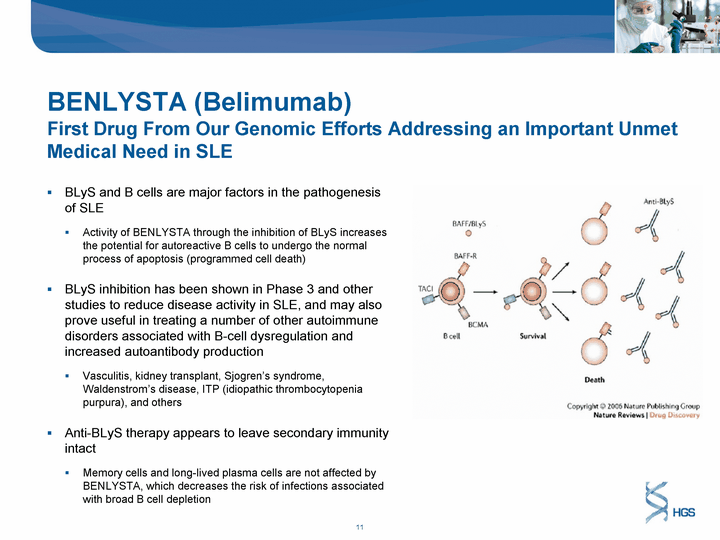
| BENLYSTA (Belimumab) First Drug From Our Genomic Efforts Addressing an Important Unmet Medical Need in SLE BLyS and B cells are major factors in the pathogenesis of SLE Activity of BENLYSTA through the inhibition of BLyS increases the potential for autoreactive B cells to undergo the normal process of apoptosis (programmed cell death) BLyS inhibition has been shown in Phase 3 and other studies to reduce disease activity in SLE, and may also prove useful in treating a number of other autoimmune disorders associated with B-cell dysregulation and increased autoantibody production Vasculitis, kidney transplant, Sjogren's syndrome, Waldenstrom's disease, ITP (idiopathic thrombocytopenia purpura), and others Anti-BLyS therapy appears to leave secondary immunity intact Memory cells and long-lived plasma cells are not affected by BENLYSTA, which decreases the risk of infections associated with broad B cell depletion |
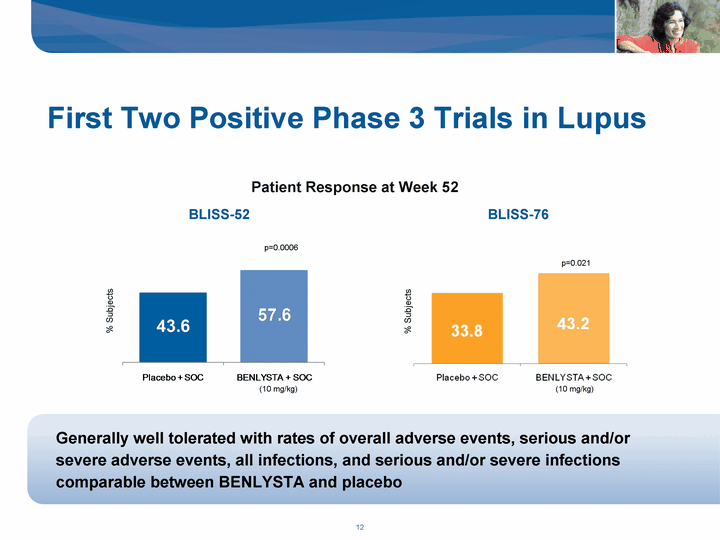
| First Two Positive Phase 3 Trials in Lupus Patient Response at Week 52 % Subjects Generally well tolerated with rates of overall adverse events, serious and/or severe adverse events, all infections, and serious and/or severe infections comparable between BENLYSTA and placebo p=0.0006 p=0.021 BLISS-52 BLISS-76 (10 mg/kg) (10 mg/kg) % Subjects |
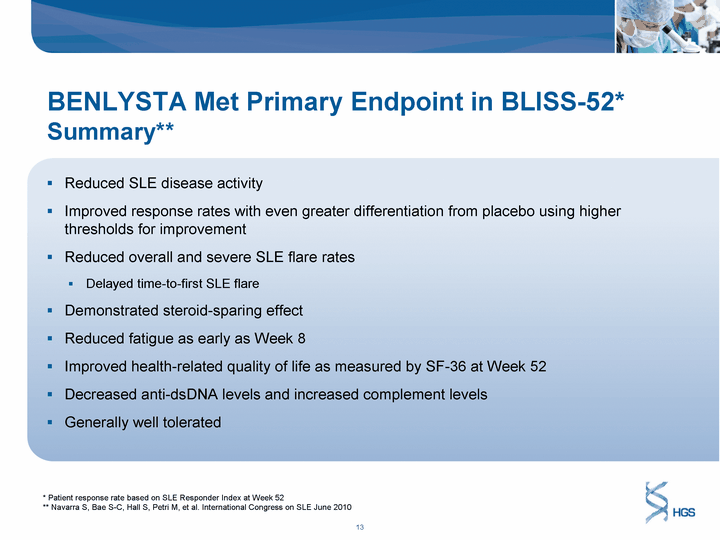
| BENLYSTA Met Primary Endpoint in BLISS-52* Summary** Reduced SLE disease activity Improved response rates with even greater differentiation from placebo using higher thresholds for improvement Reduced overall and severe SLE flare rates Delayed time-to-first SLE flare Demonstrated steroid-sparing effect Reduced fatigue as early as Week 8 Improved health-related quality of life as measured by SF-36 at Week 52 Decreased anti-dsDNA levels and increased complement levels Generally well tolerated * Patient response rate based on SLE Responder Index at Week 52 ** Navarra S, Bae S-C, Hall S, Petri M, et al. International Congress on SLE June 2010 |
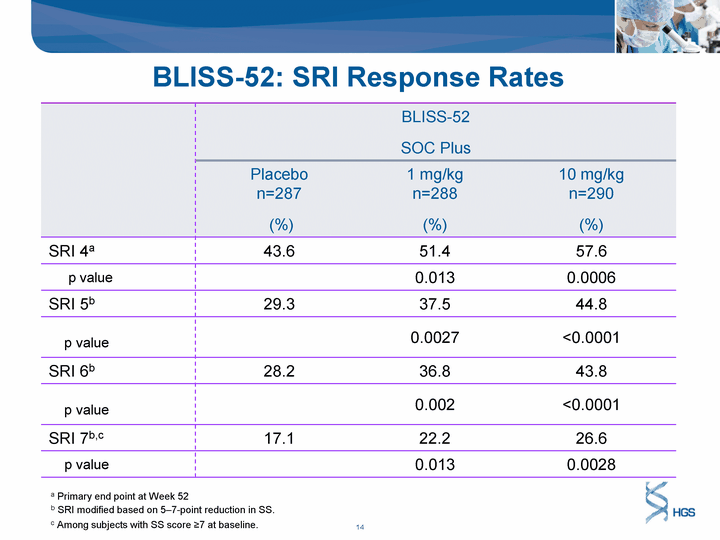
| BLISS-52: SRI Response Rates BLISS-52 SOC Plus BLISS-52 SOC Plus BLISS-52 SOC Plus Placebo n=287 (%) 1 mg/kg n=288 (%) 10 mg/kg n=290 (%) SRI 4a 43.6 51.4 57.6 p value 0.013 0.0006 SRI 5b 29.3 37.5 44.8 p value 0.0027 <0.0001 SRI 6b 28.2 36.8 43.8 p value 0.002 <0.0001 SRI 7b,c 17.1 22.2 26.6 p value 0.013 0.0028 a Primary end point at Week 52 b SRI modified based on 5-7-point reduction in SS. c Among subjects with SS score ^7 at baseline. |
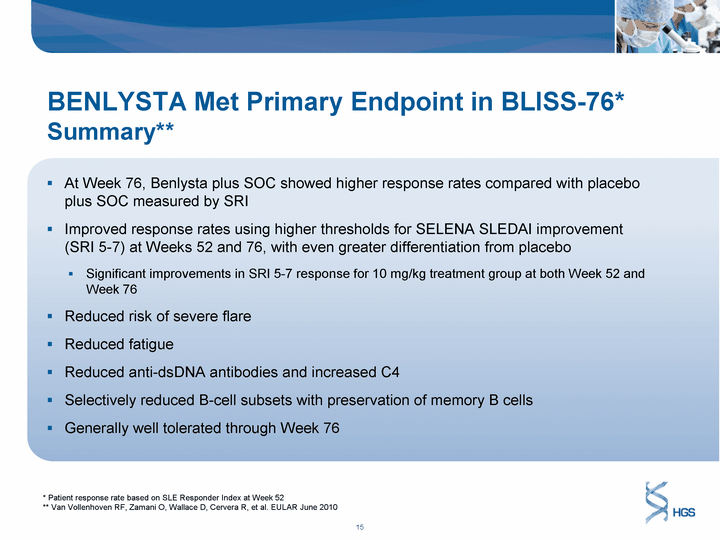
| BENLYSTA Met Primary Endpoint in BLISS-76* Summary** At Week 76, Benlysta plus SOC showed higher response rates compared with placebo plus SOC measured by SRI Improved response rates using higher thresholds for SELENA SLEDAI improvement (SRI 5-7) at Weeks 52 and 76, with even greater differentiation from placebo Significant improvements in SRI 5-7 response for 10 mg/kg treatment group at both Week 52 and Week 76 Reduced risk of severe flare Reduced fatigue Reduced anti-dsDNA antibodies and increased C4 Selectively reduced B-cell subsets with preservation of memory B cells Generally well tolerated through Week 76 * Patient response rate based on SLE Responder Index at Week 52 ** Van Vollenhoven RF, Zamani O, Wallace D, Cervera R, et al. EULAR June 2010 |
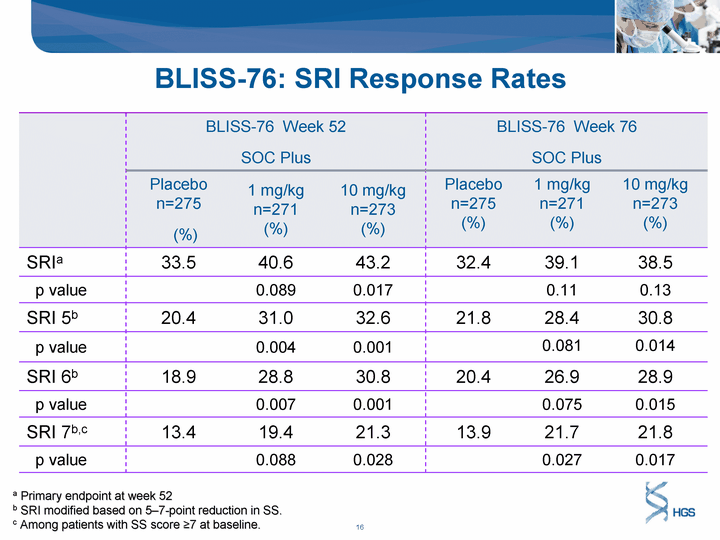
| BLISS-76 Week 52 SOC Plus BLISS-76 Week 52 SOC Plus BLISS-76 Week 52 SOC Plus BLISS-76 Week 76 SOC Plus BLISS-76 Week 76 SOC Plus BLISS-76 Week 76 SOC Plus Placebo n=275 (%) 1 mg/kg n=271 (%) 10 mg/kg n=273 (%) Placebo n=275 (%) 1 mg/kg n=271 (%) 10 mg/kg n=273 (%) SRIa 33.5 40.6 43.2 32.4 39.1 38.5 p value 0.089 0.017 0.11 0.13 SRI 5b 20.4 31.0 32.6 21.8 28.4 30.8 p value 0.004 0.001 0.081 0.014 SRI 6b 18.9 28.8 30.8 20.4 26.9 28.9 p value 0.007 0.001 0.075 0.015 SRI 7b,c 13.4 19.4 21.3 13.9 21.7 21.8 p value 0.088 0.028 0.027 0.017 BLISS-76: SRI Response Rates a Primary endpoint at week 52 b SRI modified based on 5-7-point reduction in SS. c Among patients with SS score ^7 at baseline. |
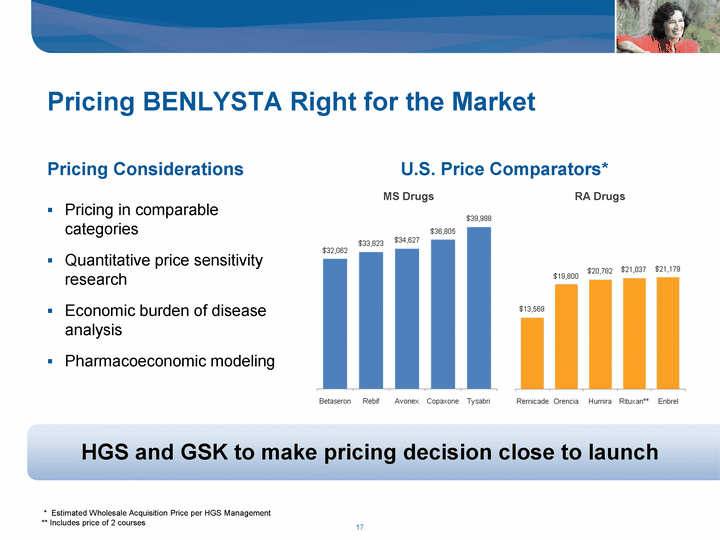
| Pricing BENLYSTA Right for the Market Pricing in comparable categories Quantitative price sensitivity research Economic burden of disease analysis Pharmacoeconomic modeling HGS and GSK to make pricing decision close to launch * Estimated Wholesale Acquisition Price per HGS Management ** Includes price of 2 courses Pricing Considerations U.S. Price Comparators* MS Drugs RA Drugs |

| Experienced Sales Organization Sales management team of 13 onboard 3 Area Managers and 10 District Managers Formerly with Abbott, Amgen, Biogen, BMS, Centocor, Genentech, Eisai Significant experience with rheumatology biologics, including Remicade(r), Rituxan(r), Orencia(r), Enbrel(r), Actemra(r) Full U.S. sales force in place by October 2010 Trained and ready to go prior to U.S. approval Building presence for BENLYSTA alongside GSK across Europe Recently added VP, HGS Europe-responsible for European sales, marketing and medical affairs Local operating teams in several key countries |
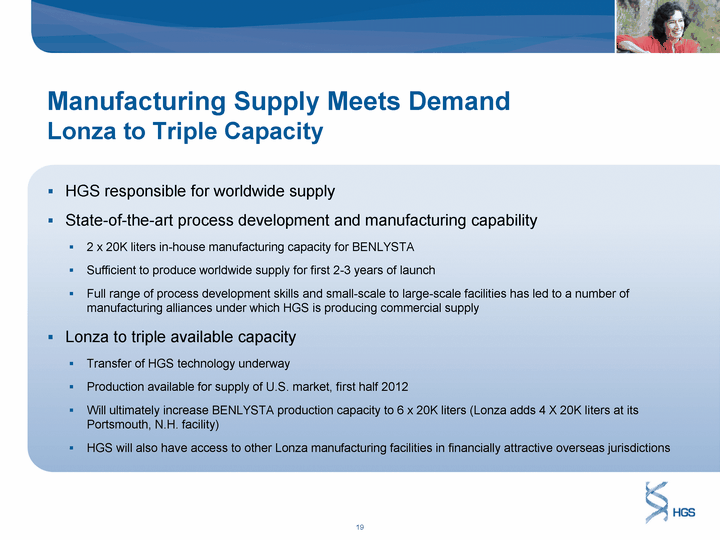
| Manufacturing Supply Meets Demand Lonza to Triple Capacity HGS responsible for worldwide supply State-of-the-art process development and manufacturing capability 2 x 20K liters in-house manufacturing capacity for BENLYSTA Sufficient to produce worldwide supply for first 2-3 years of launch Full range of process development skills and small-scale to large-scale facilities has led to a number of manufacturing alliances under which HGS is producing commercial supply Lonza to triple available capacity Transfer of HGS technology underway Production available for supply of U.S. market, first half 2012 Will ultimately increase BENLYSTA production capacity to 6 x 20K liters (Lonza adds 4 X 20K liters at its Portsmouth, N.H. facility) HGS will also have access to other Lonza manufacturing facilities in financially attractive overseas jurisdictions |
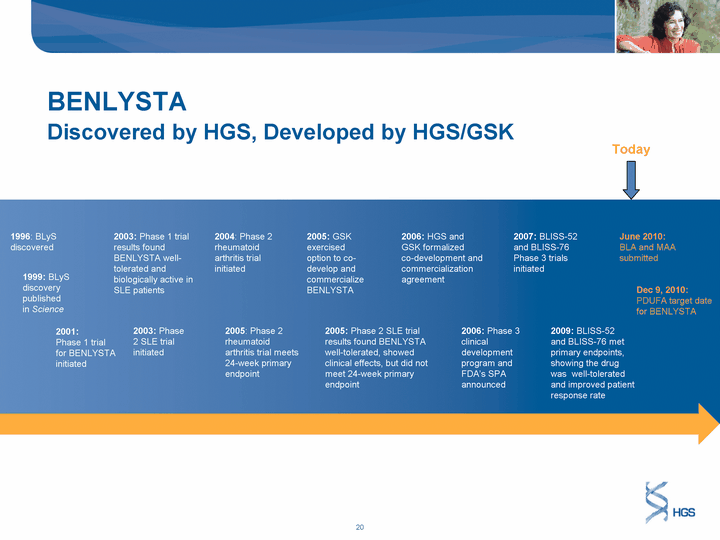
| BENLYSTA Discovered by HGS, Developed by HGS/GSK 1996: BLyS discovered 1999: BLyS discovery published in Science 2001: Phase 1 trial for BENLYSTA initiated 2003: Phase 1 trial results found BENLYSTA well- tolerated and biologically active in SLE patients 2003: Phase 2 SLE trial initiated 2004: Phase 2 rheumatoid arthritis trial initiated 2005: Phase 2 rheumatoid arthritis trial meets 24-week primary endpoint 2005: GSK exercised option to co- develop and commercialize BENLYSTA 2005: Phase 2 SLE trial results found BENLYSTA well-tolerated, showed clinical effects, but did not meet 24-week primary endpoint 2006: HGS and GSK formalized co-development and commercialization agreement 2006: Phase 3 clinical development program and FDA's SPA announced Dec 9, 2010: PDUFA target date for BENLYSTA 2007: BLISS-52 and BLISS-76 Phase 3 trials initiated 2009: BLISS-52 and BLISS-76 met primary endpoints, showing the drug was well-tolerated and improved patient response rate June 2010: BLA and MAA submitted Today |
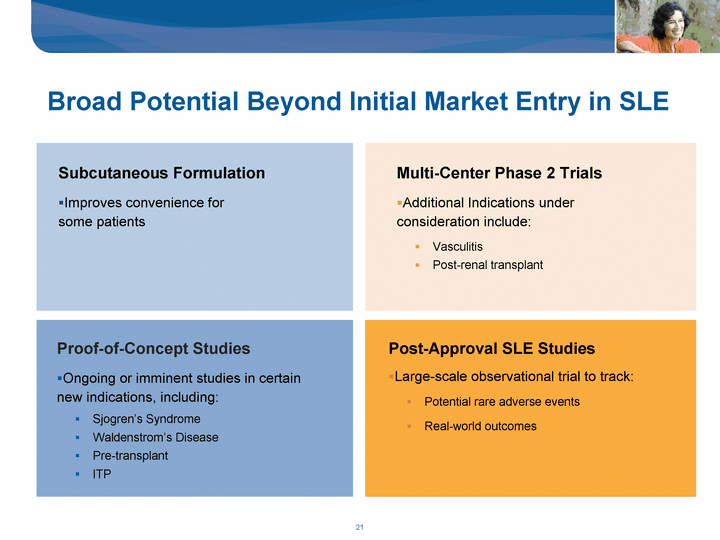
| Subcutaneous Formulation Improves convenience for some patients Multi-Center Phase 2 Trials Additional Indications under consideration include: Vasculitis Post-renal transplant Proof-of-Concept Studies Ongoing or imminent studies in certain new indications, including: Sjogren's Syndrome Waldenstrom's Disease Pre-transplant ITP Post-Approval SLE Studies Large-scale observational trial to track: Potential rare adverse events Real-world outcomes Broad Potential Beyond Initial Market Entry in SLE |
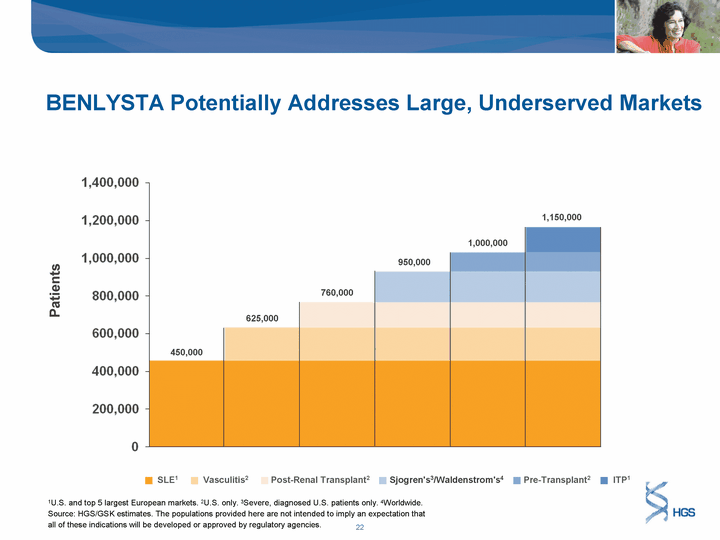
| BENLYSTA Potentially Addresses Large, Underserved Markets Patients SLE1 Vasculitis2 Post-Renal Transplant2 Sjogren's3/Waldenstrom's4 Pre-Transplant2 ITP1 1U.S. and top 5 largest European markets. 2U.S. only. 3Severe, diagnosed U.S. patients only. 4Worldwide. Source: HGS/GSK estimates. The populations provided here are not intended to imply an expectation that all of these indications will be developed or approved by regulatory agencies. 450,000 625,000 760,000 950,000 1,000,000 1,150,000 |
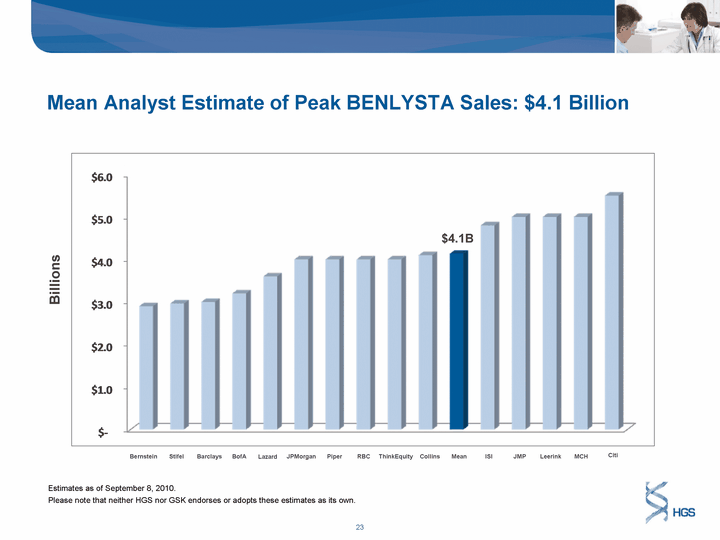
| $4.1B Billions Bernstein BofA JPMorgan Piper RBC ThinkEquity Collins Mean ISI JMP Leerink MCH Citi Stifel Barclays Lazard Mean Analyst Estimate of Peak BENLYSTA Sales: $4.1 Billion Estimates as of September 8, 2010. Please note that neither HGS nor GSK endorses or adopts these estimates as its own. |

| Raxibacumab: First Product Sales and Second Order Achieved First 20,000 doses delivered H1 2009 to the U.S. Strategic National Stockpile; $163 million in revenue recognized through Q2 2009 45,000 more doses to be delivered over a three-year period; $142 million award ~14,000 doses delivered and $44 million revenue recognized Q4 2009 and H1 2010 Submitted BLA in Q2 2009 $20 million in revenue upon FDA licensure Complete Response Letter received November 2009; working closely with FDA to obtain approval |

| GSK Products Offer Significant Therapeutic and Commercial Potential Darapladib |

| Eight Phase 3 studies ongoing to demonstrate durable efficacy and safety as mono- and add-on therapy in patients with type 2 diabetes Primary endpoint for all eight studies: change from baseline in HbA1c compared with placebo and/or active comparators Expected study duration of up to 3 years GSK Products Offer Significant Therapeutic and Commercial Potential SYNCRIA(r) Fees and milestone payments up to $183 million, up to $150 million still to be received Single-digit net royalties on worldwide sales if SYNCRIA is commercialized |
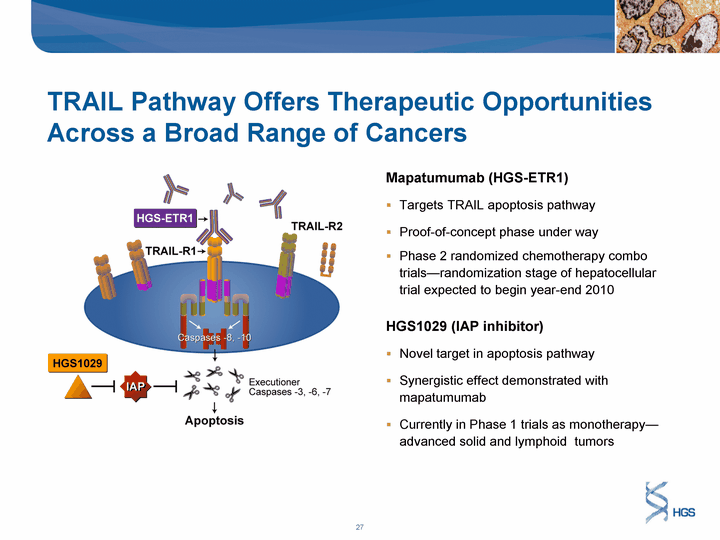
| TRAIL Pathway Offers Therapeutic Opportunities Across a Broad Range of Cancers Mapatumumab (HGS-ETR1) Targets TRAIL apoptosis pathway Proof-of-concept phase under way Phase 2 randomized chemotherapy combo trials-randomization stage of hepatocellular trial expected to begin year-end 2010 HGS1029 (IAP inhibitor) Novel target in apoptosis pathway Synergistic effect demonstrated with mapatumumab Currently in Phase 1 trials as monotherapy- advanced solid and lymphoid tumors |
| Strong Financial Position to Fuel Product Launches, Pipeline Advancement Cash and investments at June 30, 2010: ~ $1.1 billion Expected net cash burn 2010: $300-$350 million Expected cash and investments at year-end 2010: $840-890 million Products Nearing Commercialization Strong Cash Position High- Potential Pipeline |

| Preparing for Launch, Future Growth World-Class Partner Commercial Readiness Manufacturing Excellence Potential Blockbuster Product Seasoned Leadership Pipeline to Fuel Future Growth A Robust Formula for Commercial Success |

| com com THE END Company Update September, 2010 |
