Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Pernix Sleep, Inc. | c04013e8vk.htm |

| Somaxon Pharmaceuticals, Inc. August 5, 2010 BMO Capital Markets 10th Annual Focus on Healthcare Conference Nasdaq: SOMX Exhibit 99.1 |

| 2 Forward Looking Statements This presentation contains forward-looking statements about our business, including our commercialization timeline and plans, potential partnerships or other strategic collaborations, future financial results and events that have not yet occurred. Operating in the pharmaceutical industry inherently involves significant risks and uncertainties, including the risks outlined under "Risk Factors" and elsewhere in our Annual Report on Form 10-K and our other filings made with the SEC from time to time. Our actual results may differ materially from our expectations due to these risks and uncertainties, including our near-term dependence on the success of our lead product candidate, Silenor(r), and factors relating to ability to raise sufficient capital, ability to attract a strategic partner, competition, intellectual property protection, industry environment, and other matters. Somaxon undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. |
| 3 Somaxon Business Strategy Develop and Commercialize Highly Differentiated ProductsTarget late-stage or currently marketed assets Address unmet medical need and patient dissatisfaction Maximize value of SilenorDeploy a U.S. Commercial Organization Focus on the CNS therapeutic areaCover high-prescribing physicians with a targeted sales forceCreate efficiencies through strategic outsourcing Establish Commercial CollaborationsEffectively leverage partner resourcesReach broader prescriber universeConsider licensing, co-promotion or M&A to accelerate growth |
| 4 Highlights FDA approved Silenor for the treatment of insomnia characterized by sleep maintenance difficulty, March 17, 2010Raised $53MM in secondary offering in March 2010U.S. commercial collaboration discussions ongoingSomaxon’s commercial build and Silenor launch preparation nearing completionSilenor launch scheduled for late September |
| 5 Silenor for Insomnia Differentiated Mechanism of ActionH1 Antagonist vs. GABA or MelatoninTreat sleep maintenance insomniaThe most significant insomnia complaint7-8 hours of sleep with SilenorPrevention of early morning awakenings - a full night's sleepUnique potential positioning for SilenorAttractive safety profileNO clinically meaningful evidence of next-day residual effectsNOT DEA scheduledNO abuse potential NO risk of dependencyNO withdrawal symptomsNO tolerance with long term useNO complex sleep behaviors observedNO amnesia, hallucinations or other GABA-related side effectsNO disruption of sleep stages |

| A Restless Market |
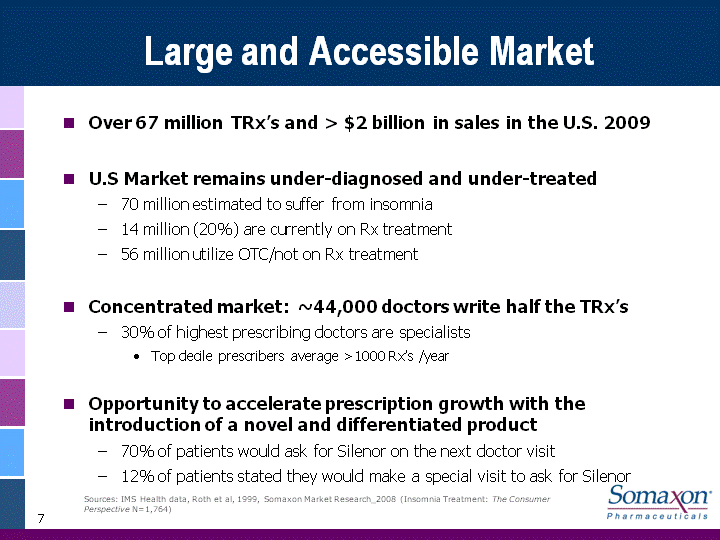
| 7 Large and Accessible Market Over 67 million TRx's and > $2 billion in sales in the U.S. 2009U.S Market remains under-diagnosed and under-treated70 million estimated to suffer from insomnia 14 million (20%) are currently on Rx treatment56 million utilize OTC/not on Rx treatmentConcentrated market: ~44,000 doctors write half the TRx's30% of highest prescribing doctors are specialistsTop decile prescribers average >1000 Rx's /yearOpportunity to accelerate prescription growth with the introduction of a novel and differentiated product70% of patients would ask for Silenor on the next doctor visit12% of patients stated they would make a special visit to ask for Silenor Sources: IMS Health data, Roth et al, 1999, Somaxon Market Research_2008 (Insomnia Treatment: The Consumer Perspective N=1,764) |
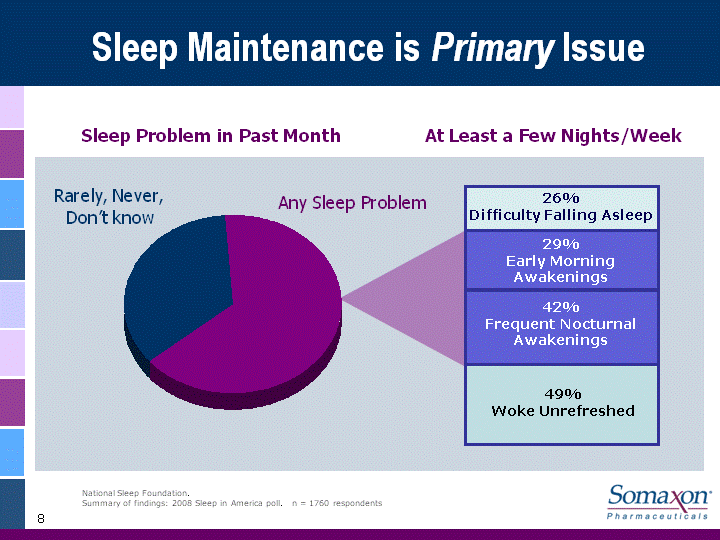
| 8 Sleep Maintenance is Primary Issue 65% 35% Sleep Problem in Past Month At Least a Few Nights/Week 49%Woke Unrefreshed 42%Frequent Nocturnal Awakenings 29%Early Morning Awakenings 26%Difficulty Falling Asleep National Sleep Foundation.Summary of findings: 2008 Sleep in America poll. n = 1760 respondents Any Sleep Problem 0.35 0.65 Any Sleep Problem Rarely, Never, Don't know |
| 9 Changing Market Dynamics Create Opportunities Solid "window of opportunity" for new treatmentsA void of new and differentiated insomnia treatments since 2006No "novel" compounds expected to launch in near term Clinical and regulatory setbacks for several drugs in developmentSomaxon estimates several years before new competition comes to marketNumber of primary details is decreasing Recent sales force reductionsCompetitive brands lack differentiated messageCompetitive promotional spending is decreasingDTC spending down dramatically year-over-yearConsistent trend across brands |

| Silenor Commercialization Silenor |

| Silenor Launch Preparation Marketing Campaign CompletedCore promotional material submitted to FDA for reviewPricing and Reimbursement Plan CompletedPremium pricing strategy Managed care dialogue underwaySales Management Team HiredVP of Sales10 Regional Managers10+ years of CNS experience on averageRecent sleep drug management experienceSales Force Recruitment Underway110 Specialty Sales RepresentativesTargeting 18,000 prescribers responsible for writing ~ 20MM insomnia drug prescriptions annually Dedicated to SilenorDeployed in September 11 |

| Silenor Launch Preparation Internal Staffing CompletedBrand TeamComplianceMedical AffairsContinue to outsource wherever possibleLaunch quantities of trade and sample packs nearing completionThird Party Logistics (3PL) Partner on-boardWholesaler/Retail Distribution Plan ready for implementationPublication PlanPhase 3 Elderly Trial (503 Study )Accepted by SLEEPPhase 3 Transient Insomnia Trial (502 Study) Accepted by Sleep Medicine Phase 3 Adult Trial (501 Study)Submitted/Pending 12 |

| 13 "Plan to Win" Capitalize on the Changing Market DynamicsCompetitor "turbulence"Lack of differentiated insomnia treatmentsShift from expensive DTC strategyPre-launch - Raise AwarenessWeb based initiativesPublicationsProfessional meetings (e.g. APSS)Launch with a Competitive Sales ForceTarget high value physiciansUtilize non-personal promotional efforts to reach Exploit Silenor sampling advantageIntegrated Professional Campaign DevelopedStrong branding elementsPatient Education ProgramsTechnology driven physician educationTargeted Consumer Campaign Developed"Direct to Insomniac" communication via WebPoint of Care Programs/Public Relations |

| 14 Silenor Branding |
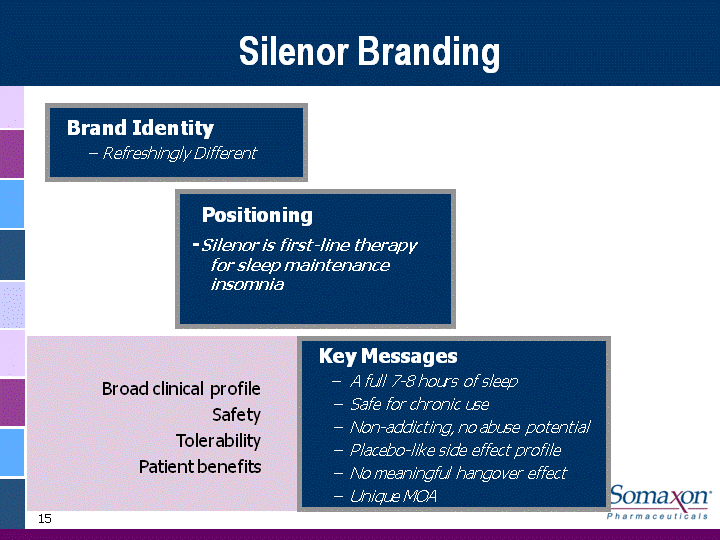
| 15 Silenor Branding Brand Identity - Refreshingly Different Key Messages - A full 7-8 hours of sleep - Safe for chronic use - Non-addicting, no abuse potential - Placebo-like side effect profile - No meaningful hangover effect - Unique MOA Positioning-Silenor is first-line therapy for sleep maintenance insomnia Broad clinical profileSafetyTolerabilityPatient benefits |

| 16 Professional Campaign Integrated marketing campaign to reach over 18,000 High Value Physicians (HVP) with both personal and non-personal promotional effortsUtilize high impact Brand Awareness programs Fully leverage web-based and media to reach prescribersSupportive sales aids and branded marketing materialsPackaging designed to reinforce Brand imagery and product featuresUnique dose pack to establish Silenor brand at the pharmacyDistinctive sample and trade packaging reinforces key branding elements for patientsSamples - a competitive advantageDrives physician access7 count Patient Starter Kit to top decile prescribers at launch4 count sample packs for sales rep delivery and direct distribution |
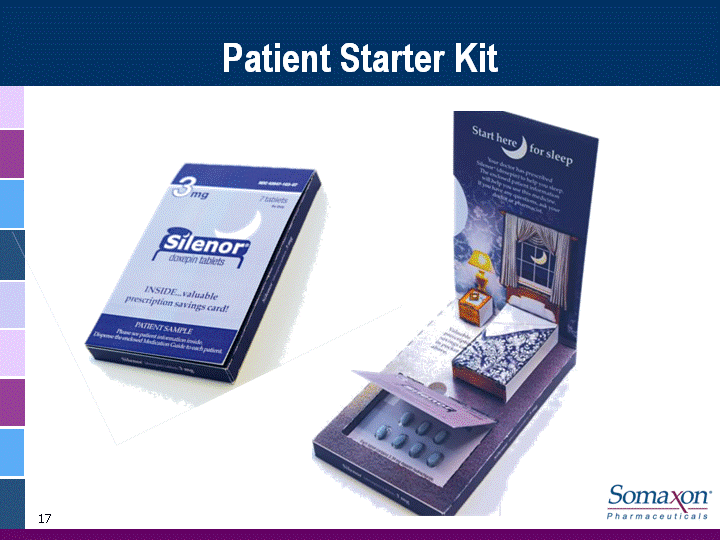
| 17 Patient Starter Kit |

| Patient Brochure and Co-Pay Card 18 |
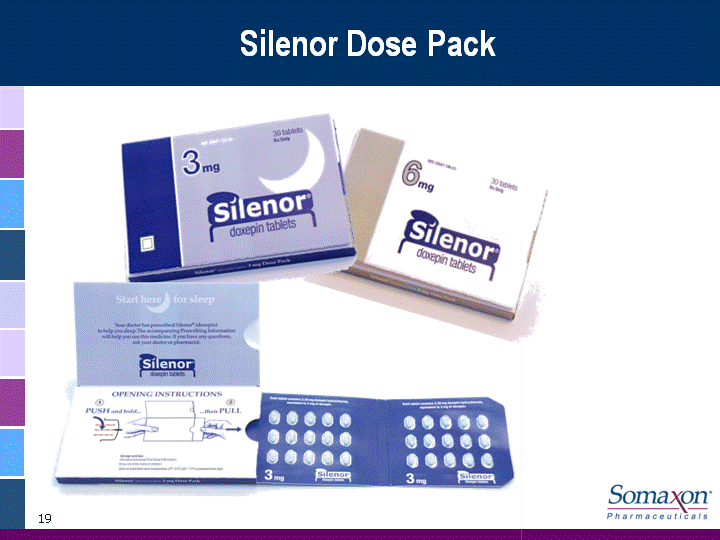
| 19 Silenor Dose Pack |
| Direct to Insomniac Campaign Internet rivals traditional DTC ads for Rx info by consumers before seeing MD1Consumers seek insomnia info in general before searching for specific medications1Physicians remain the most important source of Rx educationInternet a close second2 Physician internet use replacing traditional sources of medical info3 "Insomnia patients more likely than those with most other health conditions to use the internet for medical information"Trend report 2008 *Sources: 1) Somaxon market research, 2008; 2)Trend Report 2008; 3) Manhattan Research 20 |

| 21 Direct to Insomniac Campaign Web property deploymentwww.silenor.com Search engine optimizationUser databaseOutreach through Web MDDirected approach Most trusted and searched website for medical inquiriesPublic Relations Media outreach at launchPoint of Care - PharmacyDirect product messaging to OTC sleep aid and anti-depressant usersPoint of Care - MD OfficeIn-office video and print materialsRep and direct distributed |

| 22 Silenor.com |
| Managed Care Targeting Tier 3 at launch for majority of plans-Minimize step edits and quantity limitsNormalize the cost to the patient through co-pay assistanceFirst Rx and two refillsConfigured to be delivered as physical part of the sampleOut of pocket expense expected to be less than $1 per day to patientTarget largest/influential commercial plans for potential tier two coverageDossier and formulary kit finalizedPrioritize Government Programs Product profile supports use in Medicare populationActively pursuing opportunity in the VA/DOD system 23 |

| Somaxon Pharmaceuticals, Inc. Corporate Summary |

| 25 Commercial Collaboration for Silenor GOALSMaximize shareholder valueBuild a sustainable pharmaceutical business Traditionallicensingwithco-promotion rights Co-promotionwith sharedrevenues and costs M&A to gain access to sales infrastructure/products |
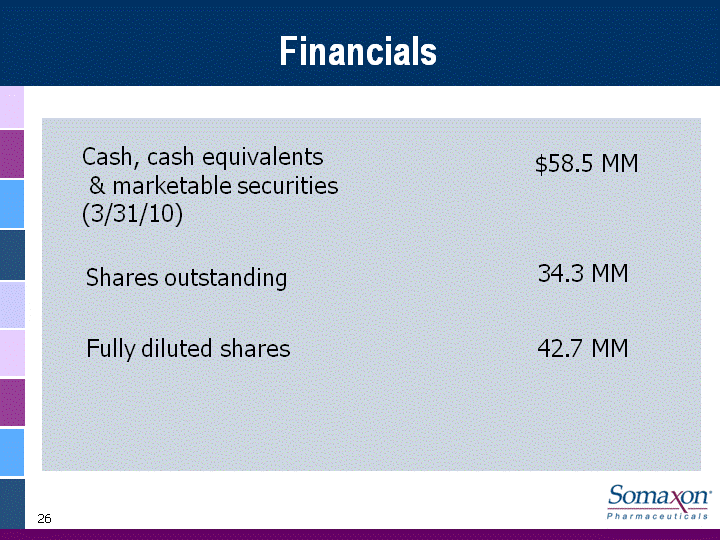
| 26 Financials Cash, cash equivalents & marketable securities (3/31/10) Shares outstanding Fully diluted shares $58.5 MM 34.3 MM 42.7 MM |
| 27 Intellectual Property Position Two issued US patents listed in Orange Book - protection until 2020Low-dose doxepin for treating chronic insomnia and transient insomniaApproved indication is "treatment of insomnia"No subset of this indication has ever been recognized by the FDAFormulation patents and exclusivityExclusive license to use key excipient, and to list related patents in Orange Book (expire in 2015)Somaxon formulation patent application pendingMultiple other patent applications pending that cover information in approved labelMethods of improving pharmacokineticsEfficacy into 8th hour, unaccompanied by next-day residual effects Method of treating insomnia without tolerance, rebound insomnia or weight gainMethod of treating insomnia in the elderly |
| 28 Somaxon Value Proposition Silenor Highly differentiated insomnia treatment Favorable market dynamics Attractive clinical and safety profile Significant revenue generating potential Experienced management team Significant commercial transaction and product launch experience 2010 milestonesPotential Silenor commercial collaboration(s) Deploying a specialty commercial operation September 2010 Silenor launch in US |

| Somaxon Pharmaceuticals, Inc. |
