Attached files
| file | filename |
|---|---|
| EX-31.1 - Benda Pharmaceutical, Inc. | v186380_ex31-1.htm |
| EX-32.1 - Benda Pharmaceutical, Inc. | v186380_ex32-1.htm |
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
WASHINGTON,
D.C. 20549
FORM
10-Q
|
x
|
QUARTERLY
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For
the quarterly period ended March 31, 2010
|
o
|
TRANSITION
REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF
1934
|
For the transition period from
______to______.
BENDA
PHARMACEUTICAL, INC.
(Exact
name of registrant as specified in Charter
|
Delaware
|
000-16397
|
41-2185030
|
||
|
(State
or other jurisdiction of
incorporation
or organization)
|
(Commission
File No.)
|
(IRS
Employee Identification No.)
|
Taibei
Mingju, 4th
Floor,
6
Taibei Road, Wuhan, Hubei Province, 430015, PRC
(Address
of Principal Executive Offices)
+86
(27) 85494916
(Issuer
Telephone number)
(Former
Name or Former Address if Changed Since Last Report)
Check
whether the issuer (1) has filed all reports required to be filed by Section 13
or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter
period that the issuer was required to file such reports), and (2)has been
subject to such filing requirements for the past 90 days. Yes x No o
Indicate
by check mark whether the registrant has submitted electronically and posted on
its corporate Web site, if any, every Interactive Data File required to be
submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this
chapter) during the preceding 12 months (or for such shorter period that the
registrant was required to submit and post such files).
Yes o No o
Indicate
by check mark whether the registrant is a large accelerated filer, an
accelerated filer, a non-accelerated filer or a smaller reporting company filer.
See definition of “accelerated filer” and “large accelerated filer” in
Rule 12b-2 of the Exchange Act (Check one):
Large
Accelerated Filer o Accelerated
Filer o Non-Accelerated
Filer o Smaller
Reporting Company x
Indicate
by check mark whether the registrant is a shell company as defined in Rule 12b-2
of the Exchange Act.
Yes o No x
State the
number of shares outstanding of each of the issuer’s classes of common equity,
as of May 24, 2010: 105,155,355 shares of common stock.
BENDA
PHARMACEUTICAL, INC.
FORM
10-Q
March
31, 2010
INDEX
|
PART
I-- FINANCIAL INFORMATION
|
|||||
|
Item
1.
|
Financial
Statements
|
3 | |||
|
Item
2.
|
Management’s
Discussion and Analysis of Financial Condition
|
16 | |||
|
Item
3.
|
Quantitative
and Qualitative Disclosures About Market Risk
|
21 | |||
|
Item
4T.
|
Control
and Procedures
|
21 | |||
|
PART
II-- OTHER INFORMATION
|
|||||
|
Item
1
|
Legal
Proceedings
|
22 | |||
|
Item
2.
|
Unregistered
Sales of Equity Securities and Use of Proceeds
|
24 | |||
|
Item
3.
|
Defaults
Upon Senior Securities
|
24 | |||
|
Item
4.
|
(Removed
and Reserved)
|
25 | |||
|
Item
5.
|
Other
Information
|
25 | |||
|
Item
6.
|
Exhibits
|
25 | |||
|
SIGNATURE
|
26 | ||||
2
PART
I—FINANCIAL
INFORMATION
Item
1. Financial Information
Benda
Pharmaceutical, Inc.
Consolidated
Balance Sheets
(unaudited)
|
March
31
2010
|
December
31
2009
|
|||||||
|
Assets
|
||||||||
|
Current
Assets
|
||||||||
|
Cash
and cash equivalents
|
$ | 937,735 | $ | 191,095 | ||||
|
Trade
receivables, net
|
12,694,639 | 12,405,018 | ||||||
|
Advance
for inventory purchase
|
1,612,098 | 2,110,857 | ||||||
|
Note
receivables
|
- | 81,426 | ||||||
|
Inventories
|
3,657,029 | 2,038,987 | ||||||
|
Due
from related parties, short term
|
31,447 | 30,861 | ||||||
|
Prepaid
expenses and other current assets
|
1,300,024 | 1,720,237 | ||||||
|
Total
current assets
|
20,232,972 | 18,578,481 | ||||||
|
Due
from related parties, long term
|
3,054,840 | 3,032,726 | ||||||
|
Property
and equipments, net
|
28,478,714 | 28,658,131 | ||||||
|
Investment
|
117,024 | - | ||||||
|
Intangible
assets, net
|
6,465,608 | 6,629,501 | ||||||
|
Restricted
cash
|
4,471,257 | 4,409,334 | ||||||
|
Other
assets
|
2,285,268 | 2,285,581 | ||||||
|
Total
Assets
|
$ | 65,105,683 | $ | 63,593,754 | ||||
|
Liabilities
& Shareholders' Equity
|
||||||||
|
Current
Liabilities
|
||||||||
|
Accounts
payable
|
$ | 2,026,953 | $ | 902,079 | ||||
|
Customer
deposit
|
1,423,847 | 1,507,147 | ||||||
|
Other
payable
|
4,771,662 | 4,547,558 | ||||||
|
Accrued
liabilities
|
5,784,247 | 6,175,538 | ||||||
|
Convertible
notes
|
7,260,000 | 7,260,000 | ||||||
|
Short-term
debt
|
13,166,927 | 11,598,066 | ||||||
|
Accrued
VAT and other taxes
|
630,201 | 795,013 | ||||||
|
Acquisition
price payable
|
1,422,548 | 1,422,743 | ||||||
|
Wages
payable
|
1,214,685 | 1,187,075 | ||||||
|
Due
to related parties, short term
|
2,801,000 | 2,791,447 | ||||||
|
Redeemable
common stock, 2,049,560 shares at $3.6 per share
|
7,376,366 | 7,376,366 | ||||||
|
Total
current liabilities
|
47,878,436 | 45,563,032 | ||||||
|
Government
grant payable
|
1,789,195 | 1,789,439 | ||||||
|
Due
to related parties, long-term
|
118,186 | 118,202 | ||||||
|
Deferred
tax liability
|
764,362 | 778,026 | ||||||
|
Total
liabilities
|
50,550,179 | 48,248,699 | ||||||
|
Shareholders'
Equity
|
||||||||
|
Preferred
stock, $0.001 par value; 5,000,000 shares authorized;
|
||||||||
|
None
issued and outstanding
|
- | - | ||||||
|
Common
stock, $0.001 par value; 150,000,000 shares authorized; 105,155,355 shares
issued and outstanding
|
105,155 | 105,155 | ||||||
|
Additional
paid in capital
|
22,108,427 | 22,108,427 | ||||||
|
Accumulated
deficit
|
(18,248,187 | ) | (17,481,559 | ) | ||||
|
Statutory
surplus reserve fund
|
2,642,775 | 2,642,775 | ||||||
|
Accumulative
other comprehensive income
|
6,423,829 | 6,268,111 | ||||||
|
Shares
issuable for services
|
503,860 | 503,860 | ||||||
|
Total
Benda Pharmaceutical, Inc.'s Shareholers' Equity
|
13,535,859 | 14,146,769 | ||||||
|
Noncontrolling
Interest
|
1,019,645 | 1,198,286 | ||||||
|
Total
Shareholders' Equity
|
14,555,504 | 15,345,055 | ||||||
|
Total
Liabilities & Shareholders' Equity
|
$ | 65,105,683 | $ | 63,593,754 | ||||
The
accompany notes are an integral part of these consolidated financial
statements.
3
Benda
Pharmaceutical, Inc.
Consolidated
Statements of Operations
(unaudited)
|
THREEMONTHS
ENDED
MARCH
31,
|
||||||||
|
(Restated)
|
||||||||
|
2010
|
2009
|
|||||||
|
Revenue
|
$ | 4,140,615 | $ | 5,515,803 | ||||
|
Cost
of goods sold
|
(2,341,445 | ) | (3,142,791 | ) | ||||
|
Gross
profit
|
1,799,170 | 2,373,012 | ||||||
|
Selling
expenses
|
639,966 | 394,193 | ||||||
|
General
and administrative expenses
|
724,467 | 1,120,807 | ||||||
|
Depreciation
and Amortization Expense
|
424,106 | 222,829 | ||||||
|
Research
and development expenses
|
390,930 | 301,684 | ||||||
|
Total
operating expenses
|
2,179,469 | 2,039,513 | ||||||
|
Operating
income / (loss)
|
(380,299 | ) | 333,499 | |||||
|
Interest
Expense
|
(369,967 | ) | (1,270,381 | ) | ||||
|
Other
Income (expense)
|
70,277 | (17,189 | ) | |||||
|
Government
subsidies
|
5,103 | 26,372 | ||||||
|
Loss
before income taxes
|
(674,886 | ) | (927,699 | ) | ||||
|
Income
taxes
|
73,253 | 288,799 | ||||||
|
Net
Loss
|
(748,139 | ) | (1,216,498 | ) | ||||
|
Less:
Net gain (loss) attributable to the noncontrolling
Interests
|
18,489 | (210,154 | ) | |||||
|
Net
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (766,628 | ) | $ | (1,006,344 | ) | ||
|
Other
Comprehensive Loss
|
||||||||
|
Foreign
currency translation gain (loss)
|
(41,412 | ) | (428,439 | ) | ||||
|
Comprehensive
Loss
|
(789,551 | ) | (1,644,937 | ) | ||||
|
Comprehensive
gain (loss) attributable to the noncontrolling interest
|
(178,641 | ) | (200,286 | ) | ||||
|
Comprehensive
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (610,910 | ) | $ | (1,444,651 | ) | ||
|
Net
loss per share - basic and diluted
|
||||||||
|
Net
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (0.01 | ) | $ | (0.01 | ) | ||
|
Weighted
average shares outstanding - basic and diluted
|
105,155,355 | 105,155,355 | ||||||
The
accompany notes are an integral part of these consolidated financial
statements.
4
Benda
Pharmaceutical, Inc.
Consolidated
Statements of Cash Flows
(unaudited)
|
THREE
MONTHS ENDED
MARCH
31
|
||||||||
|
2010
|
2009
|
|||||||
|
(Restated)
|
||||||||
|
Cash
Flows From Operating Activities
|
||||||||
|
Net
loss
|
$ | (748,139 | ) | $ | (1,216,498 | ) | ||
|
Adjustments
to reconcile net loss to net cash used in operating
activities:
|
||||||||
|
Loss
on disposals of fixed assets
|
4,551 | - | ||||||
|
Bad
debt provision
|
- | 256,338 | ||||||
|
Inventory
written down to net realizable value
|
- | 247,479 | ||||||
|
Depreciation,
including amounts in cost of sales
|
580,484 | 490,065 | ||||||
|
Amortization
of intangible assets
|
108,962 | 182,531 | ||||||
|
Amortization
of debt issuance costs
|
- | 55,485 | ||||||
|
Amortization
of convertible notes discount
|
- | 864,049 | ||||||
|
Changes
in operating assets and liabilities:
|
||||||||
|
Trade
receivables
|
(289,621 | ) | (832,110 | ) | ||||
|
Advance
for inventory purchase
|
498,759 | - | ||||||
|
Short-term
loan receivable
|
- | 44,130 | ||||||
|
Other
receivables
|
(127,848 | ) | (382,711 | ) | ||||
|
Prepaid
expenses and other current assets
|
548,061 | (338,363 | ) | |||||
|
Inventories
|
(1,618,042 | ) | (714,466 | ) | ||||
|
Accounts
payable
|
733,583 | 1,260,067 | ||||||
|
Customer
deposit
|
(83,300 | ) | - | |||||
|
Other
payable
|
202,159 | - | ||||||
|
Wages
payable
|
27,610 | - | ||||||
|
Government
grant payable
|
- | 1,283,960 | ||||||
|
Accrued
taxes
|
(178,476 | ) | (294,123 | ) | ||||
|
Net
cash provided by (used in) operating activities
|
(341,257 | ) | 905,833 | |||||
|
Cash
Flows From Investing Activities
|
||||||||
|
Investment
cost paid
|
(117,024 | ) | - | |||||
|
Purchases
of property and equipment and construction-in-progress
|
(623,627 | ) | (33,852 | ) | ||||
|
Collection
of notes receivable
|
81,426 | - | ||||||
|
Net
cash used in investing activities
|
(659,225 | ) | (33,852 | ) | ||||
|
Cash
Flows From Financing Actives
|
||||||||
|
Proceeds
and repayments of borrowings under related parties, net
|
(13,147 | ) | (29,716 | ) | ||||
|
Proceeds
and repayments of borrowings under short term debt
|
1,528,883 | (444,692 | ) | |||||
|
Net
cash provided by (used in) financing activities
|
1,515,736 | (474,408 | ) | |||||
|
Effect
of exchange rate changes on cash
|
231,386 | 32,573 | ||||||
|
Net
increase in cash and cash equivalents
|
746,640 | 430,146 | ||||||
|
Cash
and cash equivalents, beginning of period
|
191,095 | 584,266 | ||||||
|
Cash
and cash equivalents, end of period
|
$ | 937,735 | $ | 1,014,412 | ||||
|
Supplemental
Disclosure of Cash Flow Information
|
||||||||
|
Cash
paid for interest
|
$ | - | $ | 467,079 | ||||
|
Cash
paid for income taxes
|
$ | - | $ | 246,101 | ||||
The
accompany notes are an integral part of these consolidated financial
statements
5
Benda
Pharmaceutical, Inc.
Notes
to Consolidated Financial Statements
(Amounts
expressed in U.S. Dollars)
|
1.
|
Organization
|
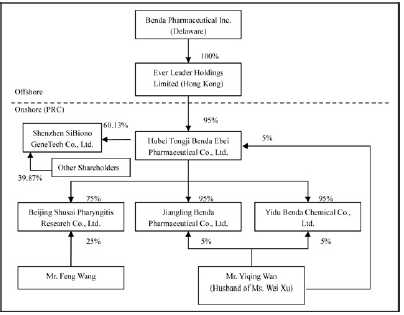
Benda
Pharmaceutical, Inc. (“Benda”) is a corporation organized under Delaware Law and
headquartered in Hubei Province, the People’s Republic of China
(“PRC”).
Ever
Leader Holdings Limited (“Ever Leader”), a wholly owned subsidiary of Benda, is
a company incorporated under the laws of Hong Kong SAR.
Ever
Leader owns 95% of the issued and outstanding capital of Hubei Tongji Benda Ebei
Pharmaceutical Co. Ltd. (“Benda Ebei”), a Sino-Foreign Equity Joint Venture
company incorporated under the laws of PRC. Mr. Yiqing Wan owns 5% of the issued
and outstanding capital stock of Benda Ebei. Benda Ebei owns: (i) 95% of the
issued and outstanding capital stock of Jiangling Benda Pharmaceutical Co. Ltd.,
(“Jiangling Benda”) a company formed under the laws of PRC; (ii) 95% of the
issued and outstanding capital stock of Yidu Benda Chemical Co. Ltd., (“Yidu
Benda”) a company incorporated under the laws of PRC; and (iii) 75% of the
issued and outstanding capital stock of Beijing Shusai Pharyngitis Research Co.
Ltd., (“Beijing Shusai”) a company incorporated under the laws of PRC. Mr.
Yiqing Wan owns: (i) 5% of the issued and outstanding capital stock of Jingling
Benda; and (ii) 5% of the issued and outstanding capital stock of Yidu Benda.
Mr. Feng Wang owns 25% of the issued and outstanding capital stock of Beijing
Shusai.
On April
5, 2007, Benda Ebei entered into an Equity Transfer Agreements with Shenzhen
Yuanzheng Investment Development Co., Ltd. and Shenzhen Yuanxing Gene City
Development Co., Ltd., the shareholders of Shenzhen SiBiono GeneTech Co., Ltd
(“SiBiono”), to purchase 27.57% and 30% respectively of the shares of SiBiono’s
common stock for total consideration of RMB 60 million due and payable on or
before April 30, 2007. On June 11, 2007, Benda Ebei entered into an Equity
Transfer Agreement with Huimin Zhang and Yaojin Wang, the individual
shareholders of SiBiono, to purchase 1.6% and 0.96% respectively of the shares
of SiBiono’s common stock for total consideration of RMB 2.56 million due and
payable on or before June 30, 2007. Altogether, the total consideration for
60.13% shares of SiBiono’s common stock was RMB 62.56 million or $8.58 million.
As of March 31, 2010 an accumulated amount, approximately RMB 52.83 million or
$7.16 million was paid leaving a balance of RMB 9.73 million or $1.42
million.
Benda,
Ever Leader, Benda Ebei, Jiangling Benda, Yidu Benda, Beijing Shusai and SiBiono
shall be referred to herein collectively as the “Company”. The Company is
engaged principally in the business of identifying, discovering, developing, and
manufacturing conventional medicines, active pharmaceuticals, bulk chemicals (or
pharmaceutical immediates), and Traditional Chinese Medicines (“TCM”) for the
treatment of some of the most widespread common ailments and diseases (e.g.
common cold, diabetes, and cancer).
As of March
31, 2010, the organization and ownership structure of the Company is as
follows:
Going
Concern
The
accompanying consolidated financial statements have been prepared assuming that
the Company will continue as a going concern. As reflected in the accompanying
consolidated financial statements, the Company has recurring losses and has a
working capital deficiency at March 31, 2010. These conditions raise substantial
doubt as to the Company’s ability to continue as a going concern.
6
While the
Company is attempting to produce sufficient revenues, the Company’s cash
position may not be enough to support the Company’s daily operations. Management
intends to raise additional funds by way of a public or private offering.
Management believes that the actions presently being taking to further implement
its business plan and generate sufficient revenues provide the opportunity for
the Company to continue as a going concern. While the Company believes in the
viability of its strategy to increase revenues and in its ability to raise
additional funds, there can be no assurance to that effect. The ability of the
Company to continue as a going concern is dependent upon the Company’s ability
to further implement its business plan and generate sufficient revenues. The
financial statements do not include any adjustments that might be necessary if
the Company is unable to continue as a going concern.
|
2.
|
Basis
of Preparation
|
The
accompanying unaudited consolidated financial statements of the Company have
been prepared in accordance with accounting principles generally accepted in the
United States of America and rules of the Securities and Exchange Commission,
and should be read in conjunction with the audited financial statements and
notes thereto contained in the Company’s annual report on Form 10-K for the
year ended December 31, 2009 filed with the SEC on May 18, 2010. In the opinion
of management, all adjustments, consisting of normal recurring adjustments,
necessary for a fair presentation of financial position and the results of
operations for the interim periods presented have been reflected herein. The
results of operations for interim periods are not necessarily indicative of the
results to be expected for the full ye;ar. Notes to the consolidated financial
statements which would substantially duplicate the disclosure contained in the
audited financial statements as reported in the 2009 annual report on Form 10-K
have been omitted.
These
consolidated financial statements include the accounts of Benda, Ever Leader,
Benda Ebei, Jiangling Benda, Yidu Benda, Beijing Shusai and Sibiono. All
significant inter-company balances and transactions have been eliminated in the
consolidation.
Certain
amounts in the consolidated financial statements for the prior year have been
reclassified to conform to the presentation of the current year.
The
preparation of financial statements in conformity with US GAAP requires
management to make estimates and assumptions that affect the reported amounts of
assets and liabilities and disclosure of contingent assets and liabilities at
the date of the financial statements, and the reported amounts of revenue and
expenses during the reporting period. Actual results when ultimately realized
could differ from those estimates.
|
3.
|
Restatement
|
On May
12, 2010, the Company discovered that its financial statements for the year
ended December 31, 2008 and 2007, and the quarterly periods within the year 2007
to 2009 should not be relied upon due to multiple errors found in the accounting
treatment of a business combination transaction completed in March 2007
resulting in adjustment of assets and liabilities to fair market value. The
Company also adjusted certain other assets and intangible assets due to errors
in the accounting treatment of these assets resulting in additional expenses for
the prior periods. To correct the above noted errors, the Company has restated
the accompanying Consolidated Statements of Operations and Cash Flows for the
three months period ended March 31, 2009, and the notes to the consolidated
financial statements. The impact to the March 31, 2009 Balance Sheet is not
presented here. The following is a summary items affected by the corrections
described above:
7
|
THREE
MONTHS ENDED
MARCH
31, 2009
|
||||||||||||
|
As
Previously
Reported
|
Adjustments
|
As
Restated
|
||||||||||
|
Revenue
|
5,515,803 | - | 5,515,803 | |||||||||
|
Cost
of goods sold
|
(3,142,791 | ) | - | (3,142,791 | ) | |||||||
|
Gross
profit
|
2,373,012 | - | 2,373,012 | |||||||||
|
Selling
expenses
|
394,193 | - | 394,193 | |||||||||
|
General
and administrative expenses
|
1,065,321 | 55,486 | 1,120,807 | a | ||||||||
|
Depreciation
and Amortization Expense
|
284,801 | (61,972 | ) | 222,829 | b | |||||||
|
Research
and development expenses
|
301,684 | - | 301,684 | |||||||||
|
Total
operating expenses
|
2,045,999 | (6,486 | ) | 2,039,513 | ||||||||
|
Operating
income / (loss)
|
327,013 | 6,486 | 333,499 | |||||||||
|
Interest
Expense
|
(1,270,381 | ) | - | (1,270,381 | ) | |||||||
|
Other
Income
|
(17,189 | ) | - | (17,189 | ) | |||||||
|
Government
subsidies / grants
|
26,372 | - | 26,372 | |||||||||
|
Loss
before income taxes
|
(934,185 | ) | 6,486 | (927,699 | ) | |||||||
|
Income
taxes
|
302,378 | (13,579 | ) | 288,799 | b | |||||||
|
Net
Loss
|
(1,236,563 | ) | 20,065 | (1,216,498 | )c | |||||||
|
Less:
Net loss attributable to the noncontrolling Interests
|
(196,006 | ) | (14,148 | ) | (210,154 | )c | ||||||
|
Net
loss attributable to Benda Pharmaceutical, Inc.
|
(1,040,557 | ) | 34,213 | (1,006,344 | ) | |||||||
|
Other
Comprehensive Loss
|
||||||||||||
|
Foreign
currency translation loss
|
(52,320 | ) | (376,119 | ) | (428,439 | )c | ||||||
|
Comprehensive
Loss
|
(1,288,883 | ) | (356,054 | ) | (1,644,937 | ) | ||||||
|
Comprehensive
loss attributable to the noncontrolling interest
|
(196,006 | ) | (4,280 | ) | (200,286 | )c | ||||||
|
Comprehensive
loss attributable to Benda Pharmaceutical, Inc.
|
(1,092,877 | ) | (351,774 | ) | (1,444,651 | ) | ||||||
8
Benda
Pharmaceutical, Inc.
Consolidated
Statements of Cash Flows
(unaudited)
|
THREE
MONTHS ENDED
MARCH
31, 2009
|
||||||||||||
|
As
Previously
Reported
|
Adjustment
|
As
Restated
|
||||||||||
|
Cash
Flows From Operating Activities
|
||||||||||||
|
Net
loss
|
$ | (1,236,563 | ) | $ | 20,065 | $ | (1,216,498 | )c | ||||
|
Adjustments
to reconcile net loss to net cash used in operating
activities:
|
||||||||||||
|
Bad
debt provision
|
256,338 | - | 256,338 | |||||||||
|
Inventory
written down to net realizable value
|
247,479 | - | 247,479 | |||||||||
|
Depreciation,
including amounts in cost of sales
|
490,065 | - | 490,065 | |||||||||
|
Amortization
of intangible assets
|
182,531 | - | 182,531 | |||||||||
|
Amortization
of debt issuance costs
|
55,485 | - | 55,485 | |||||||||
|
Amortization
of convertible notes discount
|
864,049 | - | 864,049 | |||||||||
|
Changes
in operating assets and liabilities:
|
||||||||||||
|
Trade
receivables
|
(832,110 | ) | - | (832,110 | ) | |||||||
|
Short-term
loan receivable
|
44,130 | - | 44,130 | |||||||||
|
Other
receivables
|
92,668 | (475,379 | ) | (382,711 | )d | |||||||
|
Prepaid
expenses and other current assets
|
(130,932 | ) | (207,431 | ) | (338,363 | )d | ||||||
|
Inventories
|
(714,466 | ) | - | (714,466 | ) | |||||||
|
Accounts
payable
|
1,303,352 | (43,285 | ) | 1,260,067 | d | |||||||
|
Government
grant payable
|
- | 1,283,960 | 1,283,960 | d | ||||||||
|
Accrued
taxes
|
(197,167 | ) | (96,956 | ) | (294,123 | )d | ||||||
|
Net
cash provided by operating activities
|
424,859 | 480,974 | 905,833 | |||||||||
|
Cash
Flows From Investing Activities
|
||||||||||||
|
Purchases
of property and equipment and construction-in-progress
|
(33,852 | ) | - | (33,852 | ) | |||||||
|
Net
cash used in investing activities
|
(33,852 | ) | - | (33,852 | ) | |||||||
|
Cash
Flows From Financing Actives
|
||||||||||||
|
Proceeds
and repayments of borrowings under related parties,
net
|
(29,716 | ) | - | (29,716 | ) | |||||||
|
Proceeds
and repayments of borrowings under commercial bank notes,
net
|
94,824 | (539,516 | ) | (444,692 | )d | |||||||
|
Net
cash provided by (used in) financing activities
|
65,108 | (539,516 | ) | (474,408 | ) | |||||||
|
Effect
of exchange rate changes on cash
|
(10,722 | ) | 43,295 | 32,573 | c | |||||||
|
Net
increase in cash and cash equivalents
|
445,393 | (15,247 | ) | 430,146 | ||||||||
|
Cash
and cash equivalents, beginning of period
|
569,019 | 15,247 | 584,266 | d | ||||||||
|
Cash
and cash equivalents, end of period
|
$ | 1,014,412 | $ | - | $ | 1,014,412 | ||||||
a – These
are minor reclassifications between G&A expense items.
9
b - When
SiBiono was acquired at March 31, 2007, the assets and liabilities of SiBiono
were not fair valued at March 31, 2007. The differences in these items are due
to the difference between the fair value per valuation and book value at March
31, 2007.
c – The
combination of other adjustments above.
d – Due
to minor reclassifications between asset and liability items.
|
4.
|
Inventories
|
The
Company’s inventories were comprised as follows:
|
March
31,
2010
|
December
31,
2009
|
|||||||
|
Raw
materials
|
$ | 1,464,618 | $ | 489,348 | ||||
|
Packing
materials
|
442,602 | 290,601 | ||||||
|
Other
materials / supplies
|
76,942 | 83,247 | ||||||
|
Finished
goods
|
775,563 | 588,604 | ||||||
|
Work-in-process
|
906,565 | 596,449 | ||||||
|
Total
inventories at cost
|
3,666,290 | 2,048,249 | ||||||
|
Less:
Reserves on inventories
|
(9,261 | ) | (9,262 | ) | ||||
|
Total
inventories, net
|
$ | 3,657,029 | $ | 2,038,987 | ||||
|
5.
|
Short-term
debt
|
The
Company’s short term debt was comprised as follows:
|
March
31,
2010
|
December
31,
2009
|
|||||||
|
Ebei
- one year bank loan due in October 2010, bear interest at 9% per annum,
secured by Ebei Benda’s Machinery.
|
$ | 438,840 | $ | 438,900 | ||||
|
Ebei-
bank acceptance notes from SHPudong Development Bank with redemption dates
various from one to six months subsequent to year end, secured by
buildings, machinery and equipment of Benda Ebei and Jiangling
Benda.
|
8,942,058 | 8,160,503 | ||||||
|
Ebei
- Five-month loan from Shenzhen Shourenben Enterprise Consulting (SZ) Co.,
Ltd. due in May 2010, bear monthly interest at 1%.
|
531,786 | - | ||||||
|
Sibiono
- Three loans from Shourenben Enterprise Consulting (SZ) Co., Ltd. due in
May and June 2010, bear monthly interest at 1.5%.
|
277,932 | 21,945 | ||||||
|
Sibiono-
three-year bank loan due in April 2008 bearing annual interest at 6.366%.
Loan is currently in default.
|
2,976,311 | 2,976,718 | (a) | |||||
| $ | 13,166,927 | $ | 11,598,066 | |||||
(a)
SiBiono – Bank Loan in default
10
As of
March 31, 2010 and December 31, 2009, Sibiono, had an outstanding bank loan for
the amount of $2,976,311 and $2,976,718, respectively, which was used primarily
to fund construction in progress projects and for general working capital
purposes. The loan carries annual interest rate of 6.34% and matured in April
2008. The loan is personally guaranteed by Zhaohui Peng, the former Chairman and
a shareholder of SiBiono and is collateralized by Sibiono’s land use
right.
The loan
is in default since the maturity date. During 2008, SiBiono was sued for default
on the bank loan and judgment has been made requiring Sibiono to repay the
loan principle amount and related interest. The loan is collateralized by
Sibiono’s land use right, the judgment agreed that the lender bank can apply for
government permission to sell the land use right owned by Sibiono to repay the
debt. Sibiono’s management is actively seeking ways to refinance this loan,
currently the lender bank has not exercised its rights on the land use
right.
|
6.
|
Related
Party Transactions
|
Due from
related parties at March 31, 2010 and December 31, 2009 were comprised as
follows:
|
Relationship
|
March
31,
2010
|
December
31,
2009
|
||||||||
|
Current
|
||||||||||
|
Qin
Yu
|
Vice
president
|
|||||||||
|
Shenzhen
SiBiono
|
$ | 1,482 | $ | 2,024 | ||||||
|
Xiaoji
Zhang
|
Minority
shareholder
|
|||||||||
|
Shenzhen
SiBiono
|
5,423 | 5,423 | ||||||||
|
Hua
Xu
|
General
Manager's Sister
|
|||||||||
|
Shenzhen
SiBiono
|
22,723 | 22,726 | ||||||||
|
Rong
He
|
Manager
|
|||||||||
|
Shenzhen
SiBiono
|
1,819 | 688 | ||||||||
| $ | 31,447 | $ | 30,861 | |||||||
|
Non
current
|
||||||||||
|
Yiqing
Wan
|
CEO
& Director
|
|||||||||
|
Ever
Leader
|
$ | 649,536 | $ | 646,586 | ||||||
|
Benda
Ebei
|
539,048 | 520,712 | ||||||||
|
Hubei
Benda Science and Technology Co. Ltd
|
Controlled
by CEO
|
|||||||||
|
Yidu
Benda
|
1,602,731 | 1,602,950 | ||||||||
|
Ever
Leader
|
231,266 | 230,216 | ||||||||
|
Feng
Wang
|
Minority
shareholder
|
|||||||||
|
Beijing
Shusai
|
32,259 | 32,262 | ||||||||
| $ | 3,054,840 | $ | 3,032,726 | |||||||
The
balance owned by the Yiqing Wan, CEO & Director, and the Company under his
control, totaled $ 3,022,581 and $3,000,464 as of March 31, 2010 and December
31, 2009, respectively. This is a violation of Section 402 of the Sarbanes-Oxley
Act of 2002 which prohibits personal loans to executives.
11
Due to
related parties at March 31, 2010 and December 31, 2009 were comprised as
follows:
|
|
Relationship
|
March
31,
2010
|
December
31,
2009
|
|||||||
|
Current
|
||||||||||
|
Wei
Xu
|
VP,
CEO's Spouse & Director
|
|||||||||
|
Shenzhen
SiBiono
|
$ | 294,205 | $ | 234,569 | ||||||
|
Everleader
|
1,362,359 | 1,356,172 | ||||||||
|
BPMA
|
36,184 | 36,184 | ||||||||
|
Hubei
Benda Science and Technology Co. Ltd
|
Controlled
by CEO
|
|||||||||
|
Benda
Ebei
|
28,524 | 28,528 | ||||||||
|
Jiangliang
Benda
|
778,029 | 793,864 | ||||||||
|
Beijing
Shusai
|
14,109 | 14,111 | ||||||||
|
SiBiono
Zhongjia Gene Tech (SZ) Co., Ltd.
|
Associate
company
|
|||||||||
|
Shenzhen
SiBiono
|
59,896 | 103,948 | ||||||||
|
Yiqing,
Wan
|
CEO
& Director
|
|||||||||
|
Shenzhen
SiBiono
|
227,694 | 224,071 | ||||||||
| $ | 2,801,000 | $ | 2,791,447 | |||||||
|
Non
current
|
||||||||||
|
Wei
Xu
|
VP,
CEO's Spouse & Director
|
|||||||||
|
Benda
Ebei
|
$ | 23,891 | $ | 23,894 | ||||||
|
Beijing
Shusai
|
65,330 | 65,339 | ||||||||
|
Yiqing,
Wan
|
CEO
& Director
|
|||||||||
|
Yidu
Benda
|
559 | 559 | ||||||||
|
Hui
Xu
|
Manager
|
|||||||||
|
Benda
Ebei
|
28,406 | 28,410 | ||||||||
| $ | 118,186 | $ | 118,202 | |||||||
Except
for the loans from the shareholder Wei Xu by Everleader which bears interest
rate at 12% per annum, unsecure and matures within six months, the above
advances bear no interest and the above loans due to related parties are
unsecured, non-interest bearing and are not convertible into equity. Proceeds
from the above loans were used primarily for general working capital purposes,
among which the current portion does not have definitive terms and for
those portions which are long-term debts in nature, is expected to be repaid by
the Company in over 12-month period.
|
7.
|
Equity
Investment
|
Sibiono
and North American Gene Diagnostics and Therapeutics Ltd. (HK) entered into a
business agreement to set up Shenzhen Sibiono Zhongjia Gene Technology Ltd.
(Zhongjia) during June 2009. The business license of the new joint entity was
obtained in January 2010 and Sibiono made the capital contribution of RMB
800,000 in February 2010. The new entity's legal representative is Mr. Wan,
Yiqing. The registered capital is RMB 2 million. Sibiono's share of the
registered capital is 40% (RMB 800,000), the other party’s share is 60% (RMB 1.2
million).
Zhongjia
did not have significant operations during the quarter ended March 31,
2010.
|
8.
|
Segment
Information
|
The
Company states the segment information according to the requirement stated in
ASC 280-10-50. The Company produces five different categories of products and
each category of product is produced in different subsidiaries or operation
plants. The details are stated as follows:
|
1.
|
Benda
Ebei produces conventional medicines which including branded and generic
medicines;
|
|
2.
|
Jiangling
Benda produces active pharmaceutical ingredients,
APIs;
|
|
3.
|
Yidu
Benda produces bulk chemicals;
|
|
4.
|
Beijing
Shusai produces pharyngitis killer therapy;
and
|
|
5.
|
SiBiono
produces gene therapy medicines,
Gendicine.
|
Since
each subsidiary produces the corresponding products by using the production
facilities of each subsidiary, therefore according to the requirement stated ASC
280-10-50, the Company reports the segment information according to the
un-identical products that produced in each subsidiary.
12
Selected
financial information for each of these segments for the three months ended
March 31, 2010 and 2009 were as follows:
|
THREE
MONTHS ENDED
MARCH
31,
|
||||||||
|
Branded/Generic
medicine segment
|
2010
|
2009
|
||||||
|
Revenue
from external customers
|
$ | 2,815,568 | $ | 4,360,300 | ||||
|
Cost
of sales
|
(1,920,224 | ) | (2,659,782 | ) | ||||
|
Gross
profit
|
895,344 | 1,700,518 | ||||||
|
Gross
margin
|
32 | % | 39 | % | ||||
|
Research
and development
|
(340,961 | ) | (147 | ) | ||||
|
Selling
expense
|
(389,296 | ) | (202,469 | ) | ||||
|
General
and administrative expense
|
(163,522 | ) | (87,336 | ) | ||||
|
Segment
contribution
|
$ | 1,565 | $ | 1,410,566 | ||||
|
Contribution
margin
|
0 | % | 32 | % | ||||
|
Total
assets, segment
|
$ | 27,456,686 | $ | 22,399,392 | ||||
|
THREE
MONTHS ENDED
MARCH
31,
|
||||||||
|
Active
pharmaceutical ingredients segment
|
2010
|
2009
|
||||||
|
Revenue
from external customers
|
$ | 631,964 | $ | 422,262 | ||||
|
Cost
of sales
|
(345,910 | ) | (421,606 | ) | ||||
|
Gross
profit
|
286,054 | 656 | ||||||
|
Gross
margin
|
45 | % | 0 | % | ||||
|
Research
and development
|
- | - | ||||||
|
Selling
expense
|
(10,577 | ) | (3,914 | ) | ||||
|
General
and administrative expense
|
(118,842 | ) | (261,183 | ) | ||||
|
Segment
contribution
|
$ | 156,635 | $ | (264,441 | ) | |||
|
Contribution
margin
|
25 | % | -63 | % | ||||
|
Total
assets, segment
|
$ | 13,082,389 | $ | 13,011,848 | ||||
|
THREE
MONTHS ENDED
MARCH
31,
|
||||||||
|
Bulk
chemicals segment
|
2010
|
2009
|
||||||
|
Revenue
from external customers
|
$ | - | $ | - | ||||
|
Cost
of sales
|
- | - | ||||||
|
Gross
profit
|
- | - | ||||||
|
Gross
margin
|
- | - | ||||||
|
Research
and development
|
- | - | ||||||
|
Selling
expense
|
- | - | ||||||
|
General
and administrative expense
|
(147,142 | ) | (45,076 | ) | ||||
|
Segment
contribution
|
$ | (147,142 | ) | $ | (45,076 | ) | ||
|
Contribution
margin
|
0 | % | 0 | % | ||||
|
Total
assets, segment
|
$ | 8,367,685 | $ | 9,394,742 | ||||
|
THREE
MONTHS ENDED
MARCH
31,
|
||||||||
|
Pharynigitis
killer therapy segment
|
2010
|
2009
|
||||||
|
Revenue
from external customers
|
$ | - | $ | - | ||||
|
Cost
of sales
|
- | - | ||||||
|
Gross
profit
|
- | - | ||||||
|
Gross
margin
|
- | - | ||||||
|
Research
and development
|
- | - | ||||||
|
Selling
expense
|
- | - | ||||||
|
General
and administrative expense
|
(5,033 | ) | (7,246 | ) | ||||
|
Segment
contribution
|
$ | (5,033 | ) | $ | (7,246 | ) | ||
|
Contribution
margin
|
0 | % | 0 | % | ||||
|
Total
assets, segment
|
$ | 114,321 | $ | 103,421 | ||||
|
THREE MONTHS
ENDED
MARCH 31,
|
||||||||
|
Gendicine (Ad-p53)
segment
|
2010
|
2009
|
||||||
|
Revenue from external
customers
|
$ | 693,083 | $ | 733,083 | ||||
|
Cost of
sales
|
(75,311 | ) | (61,403 | ) | ||||
|
Gross
profit
|
617,772 | 671,680 | ||||||
|
Gross
margin
|
89 | % | 92 | % | ||||
|
Research and
development
|
(49,969 | ) | (301,537 | ) | ||||
|
Selling
expense
|
(240,093 | ) | (187,809 | ) | ||||
|
General and administrative
expense
|
(288,926 | ) | (683,093 | ) | ||||
|
Segment
contribution
|
$ | 38,784 | $ | (500,759 | ) | |||
|
Contribution
margin
|
6 | % | -68 | % | ||||
|
Total assets,
segment
|
$ | 14,950,202 | $ | 15,000,819 | ||||
|
THREE MONTHS
ENDED
MARCH 31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Total revenue from external
customers
|
$ | 4,140,615 | $ | 5,515,803 | ||||
|
Cost of
sales
|
(2,341,445 | ) | (3,142,791 | ) | ||||
|
Gross
profit
|
1,799,170 | 2,373,012 | ||||||
|
Gross
margin
|
43 | % | 43 | % | ||||
|
Research and
development
|
(390,930 | ) | (301,684 | ) | ||||
|
Selling
expense
|
(639,966 | ) | (394,192 | ) | ||||
|
General and administrative
expense
|
(723,465 | ) | (1,083,934 | ) | ||||
|
Segment
contribution
|
$ | 44,809 | $ | 593,202 | ||||
|
Contribution
margin
|
1 | % | 11 | % | ||||
|
Total assets,
segment
|
$ | 63,971,283 | $ | 59,910,222 | ||||
13
The
results of the total consolidated net profit before income taxes for the
reporting periods are as follows:
|
THREE
MONTHS ENDED
MARCH
31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Total
segment contribution
|
$ | 44,809 | $ | 593,202 | ||||
|
Unallocated
amounts:
|
||||||||
|
Government
subsidy
|
5,103 | 26,372 | ||||||
|
Other
income/(expenses)
|
(97,752 | ) | (17,189 | ) | ||||
|
Other
corporate expenses
|
(627,046 | ) | (1,530,084 | ) | ||||
|
Total
loss before noncontrolling interest and income taxes
|
$ | (674,886 | ) | $ | (927,699 | ) | ||
The other
corporate expenses per the above table for the three months ended March 31, 2010
and 2009 composed of the following events:
|
THREE
MONTHS ENDED
MARCH
31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Wages
and salaries
|
$ | 65,000 | $ | 98,333 | ||||
|
Audit
and accounting
|
- | 25,000 | ||||||
|
Amortization
of debt issue cost
|
- | 55,485 | ||||||
|
Consulting
fee
|
32,910 | 3,092 | ||||||
|
Investor
relation, transfer agent and filing fees
|
- | 2,720 | ||||||
|
Director
renumeration
|
22,500 | 22,500 | ||||||
|
Legal
fee
|
304,451 | 43,519 | ||||||
|
Travel
and transportation
|
- | 1,810 | ||||||
|
Interest
expense
|
201,938 | 1,270,381 | ||||||
|
Miscellaneous
|
247 | 7,244 | ||||||
|
Total
|
$ | 627,046 | $ | 1,530,084 | ||||
For the
details of information of this particular, it should be read in conjunction
with the management discussion and analysis section.
The
following table shows the reconciliation between the segments assets and the
total assets for the three months ended March 31, 2010 and 2009:
|
THREE
MONTHS ENDED
MARCH
31,
|
||||||||
|
2010
|
2009
|
|||||||
|
Total
assets, segment
|
$ | 63,971,283 | $ | 59,910,222 | ||||
|
Total
assets of corporate:
|
||||||||
|
Cash
and cash equivalent
|
11,828 | 31,070 | ||||||
|
Bank
indebtedness
|
- | 1,288,257 | ||||||
|
Prepaid
expenses
|
232 | 2,950 | ||||||
|
Due
from related parties
|
648,112 | 660,102 | ||||||
|
Construction-in-progress
capitalized interest
|
474,228 | - | ||||||
|
Debt
issuance cost
|
- | 55,485 | ||||||
|
Total
assets
|
$ | 65,105,683 | $ | 61,948,086 | ||||
14
The
following table shows how the noncontrolling interest for the three months
ended March 31, 2010 and 2009 was derived:
|
Three
Months Ended March 31, 2010
|
||||||||||||||||||||||||
|
Benda
Ebei
|
Jiangling
Benda
|
Yidu
Benda
|
Beijing
Shusai
|
SiBiono
|
Total
|
|||||||||||||||||||
|
Segment
operating profit / (loss)
|
$ | 1,565 | 156,635 | (147,142 | ) | (5,033 | ) | 38,784 | $ | 44,809 | ||||||||||||||
|
Interest
income/ (expenses)
|
(110,523 | ) | (1,388 | ) | - | - | (56,118 | ) | (168,029 | ) | ||||||||||||||
|
Other
income / (expenses)
|
(25,931 | ) | (609 | ) | 990 | - | 69,992 | 44,442 | ||||||||||||||||
|
Government
subsidy
|
- | 5,103 | - | - | - | 5,103 | ||||||||||||||||||
|
Income
taxes
|
(86,845 | ) | - | - | - | 13,592 | (73,253 | ) | ||||||||||||||||
|
Loss
before noncontrolling interest
|
$ | (221,734 | ) | 159,741 | (146,152 | ) | (5,033 | ) | 66,250 | $ | (146,928 | ) | ||||||||||||
|
Noncontrolling
interest percentage
|
5 | % | 5 | % | 5 | % | 25 | % | 39.87 | % | ||||||||||||||
|
Noncontrolling
interest
|
$ | (7,346 | ) | 7,987 | (7,308 | ) | (1,258 | ) | 26,414 | $ | 18,489 | |||||||||||||
|
Three
months Ended March 31, 2009
|
||||||||||||||||||||||||
|
Benda
Ebei
|
Jiangling
Benda
|
Yidu
Benda
|
Beijing
Shusai
|
SiBiono
|
Total
|
|||||||||||||||||||
|
Segment
operating profit / (loss)
|
$ | 1,410,724 | (264,441 | ) | (45,076 | ) | (7,246 | ) | (500,759 | ) | $ | 593,202 | ||||||||||||
|
Interest
income/ (expenses)
|
(60,541 | ) | 28 | 3 | - | (45,352 | ) | (105,862 | ) | |||||||||||||||
|
Other
income / (expenses)
|
(14,680 | ) | (1,574 | ) | 989 | - | 4,929 | (10,336 | ) | |||||||||||||||
|
Government
subsidy
|
26,372 | 26,372 | ||||||||||||||||||||||
|
Income
taxes
|
(302,537 | ) | - | - | - | - | (302,537 | ) | ||||||||||||||||
|
Income
(Loss) before noncontrolling interest
|
$ | 1,032,966 | (239,615 | ) | (44,084 | ) | (7,246 | ) | (541,182 | ) | $ | 200,839 | ||||||||||||
|
|
||||||||||||||||||||||||
|
Noncontrolling
interest percentage
|
5.00 | % | 9.75 | % | 9.75 | % | 28.75 | % | 42.88 | % | ||||||||||||||
|
Noncontrolling
interest
|
$ | 51,648 | (23,362 | ) | (4,298 | ) | (2,083 | ) | (232,059 | ) | $ | (210,154 | ) | |||||||||||
|
9.
|
Subsequent
Events
|
In April
2010, Jiangling Benda obtained a one year loan from Hubei Province Rural Credit
with principle amount of RMB 2 million ($292,556) that bears interest at 6% per
annum. The loan is secured by a third party commercial loan guarantee company
for a fee.
15
Item 2. Management’s Discussion and Analysis
or Plan of Operation
The
following discussion and analysis should be read in conjunction with the
information contained in the unaudited condensed consolidated financial
statements of the Company and the related notes thereto, appearing elsewhere
herein, and in conjunction with the Management’s Discussion and Analysis of
Financial Condition and Results of Operations set forth in the Company’s Annual
Report on Form 10-K for the year ended December 31, 2009, filed with the
Securities and Exchange Commission (“SEC”).
Forward
Looking Information
This Quarterly Report on Form 10-Q
(the “Report”) contains certain “forward-looking statements” within the meaning
of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the
Exchange Act of 1934, as amended, that are based on management’s exercise of
business judgment as well as assumptions made by, and information currently
available to, management. When used in this document, the words
“may”, "will”,
“anticipate”, “believe”, “estimate”, “expect”, “intend”, and words of similar
import, are intended to identify any forward-looking statements. You
should not place undue reliance on these forward-looking
statements. These statements reflect our current view of future
events and are subject to certain risks and uncertainties, as noted in the
Company’s Report on Form 10-K, filed with the SEC, and as noted
below. Should one or more of these risks or uncertainties
materialize, or should underlying assumptions prove incorrect, our actual
results could differ materially from those anticipated in these forward-looking
statements. We undertake no obligation, and do not intend, to update,
revise or otherwise publicly release any revisions to these forward-looking
statements to reflect events or circumstances after the date hereof, or to
reflect the occurrence of any unanticipated events. Although we
believe that our expectations are based on reasonable assumptions, we can give
no assurance that our expectations will materialize.
Critical Accounting
Policies
Accounting
policies discussed in this section are those that we consider to be most
critical to an understanding of our financial statements because they inherently
involve significant judgment and uncertainties. For all of these
estimates, we caution that future events rarely develop exactly as forecast, and
the best estimates routinely require adjustment.
Revenue
Recognition
Among the
most important accounting policies affecting the Group’s consolidated financial
statements is its policy of recognizing revenue. Under this policy, all of the
following criteria must be met in order for us to recognize
revenue:
1. Persuasive evidence of an arrangement
exists;
2. Delivery has occurred or services have
been rendered;
3. The seller's price to the buyer is fixed
or determinable; and
4. Collectibility is reasonably
assured.
The
majority of the Company's revenue results from sales contracts with distributors
and revenue is recorded upon the shipment of goods. Management conducts credit
background checks for new customers as a means to reduce the subjectivity of
assuring collectibility. Sales are presented net of value added tax (VAT). No
return allowance is made as products returns are insignificant based on
historical experience.
Operational
Results
The Three Months Ended March
31, 2010 and 2009
The
following table provides key components of our operational results for the three
months ended March 31, 2010 and 2009 for Benda Pharmaceutical, Inc.
16
|
Benda
Pharmaceutical, Inc.
|
|
Consolidated
Statements of Operations
|
|
THREE
MONTHS ENDED MARCH 31,
|
||||||||
|
(Restated)
|
||||||||
|
2010
|
2009
|
|||||||
|
Revenue
|
$ | 4,140,615 | $ | 5,515,803 | ||||
|
Cost
of goods sold
|
(2,341,445 | ) | (3,142,791 | ) | ||||
|
Gross
profit
|
1,799,170 | 2,373,012 | ||||||
|
Selling
expenses
|
639,966 | 394,193 | ||||||
|
General
and administrative expenses
|
724,467 | 1,120,807 | ||||||
|
Depreciation
and Amortization Expense
|
424,106 | 222,829 | ||||||
|
Research
and development expenses
|
390,930 | 301,684 | ||||||
|
Total
operating expenses
|
2,179,469 | 2,039,513 | ||||||
|
Operating
income / (loss)
|
(380,299 | ) | 333,499 | |||||
|
Interest
Expense
|
(369,967 | ) | (1,270,381 | ) | ||||
|
Other
Income (expense)
|
70,277 | (17,189 | ) | |||||
|
Government
subsidies
|
5,103 | 26,372 | ||||||
|
Loss
before income taxes
|
(674,886 | ) | (927,699 | ) | ||||
|
Income
taxes
|
73,253 | 288,799 | ||||||
|
Net
Loss
|
(748,139 | ) | (1,216,498 | ) | ||||
|
Less:
Net gain (loss) attributable to the noncontrolling
Interests
|
18,489 | (210,154 | ) | |||||
|
Net
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (766,628 | ) | $ | (1,006,344 | ) | ||
|
Other
Comprehensive Loss
|
||||||||
|
Foreign
currency translation gain (loss)
|
(41,412 | ) | (428,439 | ) | ||||
|
Comprehensive
Loss
|
(789,551 | ) | (1,644,937 | ) | ||||
|
Comprehensive
gain (loss) attributable to the noncontrolling interest
|
(178,641 | ) | (200,286 | ) | ||||
|
Comprehensive
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (610,910 | ) | $ | (1,444,651 | ) | ||
|
Net
loss per share - basic and diluted
|
||||||||
|
Net
loss attributable to Benda Pharmaceutical, Inc.
|
$ | (0.01 | ) | $ | (0.01 | ) | ||
|
Weighted
average shares outstanding - basic and diluted
|
105,155,355 | 105,155,355 | ||||||
Net
Revenue:
The
Company has five core operating segments: Benda Ebei, Jiangling Benda, Yidu
Benda, Beijing Shusai and SiBiono. Benda Ebei manufactures branded/generic
medicines; Jiangling Benda manufactures active pharmaceutical ingredients (API);
Yidu Benda manufactures bulk chemicals; Beijing Shusai operates and distributes
Pharyngitis Killer Therapy; and SiBiono is a gene therapy company dedicated to
the development, manufacturing and commercialization of gene therapy product,
Gedicine.
Net
revenue decreased by $1.4 million (or 24.93%) to $4.1 million for the three
months ended March 31, 2010 from $5.5 million for the three months ended March
31, 2009. Decrease in revenue is principally attributed to the following
factors:
|
1.
|
One
of Benda’s subsidiaries, Benda Ebei’s net revenue decreased $1.6 million
(or 35.43%) to $2.8 million for the three months
ended March 31, 2010 from $4.4 million for the three months ended March
31, 2009. It was mainly due to maintenance activity during the three
months ended March 31, 2010, no such activity in the same period during
2009.
|
17
|
2.
|
One
of Benda’s subsidiaries, Jiangling Benda, achieved $0.63 million and $0.42
million for the three months ended March 31, 2010 and 2009,
respectively.
|
Jiangling
Benda plans to produce four types of active pharmaceutical ingredients and they
are Ribavirin, Asarin, Levofloxacin and Ribose whereas the production of Ribose
does not require the GMP certificate, but the production of the other three
products do require the GMP certificate.
On April 9, 2008, Jiangling Benda
received the approved GMP Certificate from the Chinese State Food and
Drug Administration ("SFDA") which authorized the production of
Ribavrin. The other two products, Asarin and Levolfozacin, are still
under the stage of GMP certificate approving process. Management could not
estimate the exact timing for obtaining those certificates.
|
3.
|
One
of Benda’s subsidiaries, Yidu Benda ceased operations due to the plant
closing in mid January 2007 to upgrade its waster water treatment system
to comply with new environmental standards enforced by PRC local
government.
|
Yidu
Benda completed its upgrading of the waster water system and passed the
government’s verification and testing of equipment in October 2007. It is now
permitted for the testing on actual production process. Once the actual products
are produced, then the environmental government bodies will re-test the
production results. Management cannot estimate the exact timing for obtaining
the final approval on the actual production process. Furthermore, management is
searching for new products to be produced in Yidu Benda with higher profit
margins.
|
4.
|
One
of Benda’s subsidiaries, Beijing Shusai was incorporated on July 15, 2006.
China’s State Food and Drug Administration (SFDA) recently experienced an
overhaul in its policies and regulatory systems in an effort to fight
against corruption in Chinese pharmaceutical industry. Beijing Shusai’s
operation has been adversely affected by this recent policy changes which
prohibits some state-owned hospitals from forming alliances with private
companies. Management cannot estimate that such situation could be
resolved in the coming future.
|
|
5.
|
One
of Benda’s subsidiaries, SiBiono, net revenue decreased $0.04 million (or
5.46%) to $0.69 million for the three months ended March 31, 2010 from
$0.73 million the three months ended March 31, 2009. The decrease in net
revenue is mainly due to the fact that SiBiono previously underwent a
process of re-engineering of the production department during the year of
2010.
|
SiBiono
GMP - On October 16, 2003, SiBiono successfully obtained a New Drug License from
the State Food & Drug Administration of China (SFDA), and then, in April 4,
2004, SiBiono obtained “Manufacture Certificate” and “Certificate of GMP for
Pharmaceutical Product”, so far being fully qualified for the market launch of
Recombinant Human Ad-p53 Injection, trademarked as Gendicine
® in China. Gendicine ® is the
commercialized gene therapy product approved in the PRC government agency. On
May 19, 2008, SiBiono received an official notice from the PRC State of SFDA in
which it mentioned that during the random inspection performed by the PRC State
of SFDA on April 8 to April 10, 2008, the PRC State of SFDA discovered there
were several production procedures that did not meet the requirement stated in
GMP, thus it required SiBiono to perform necessary improvements in order to
fulfill the GMP requirements and the PRC State of SFDA collected back the
distributed GMP certificate until the necessary improvements being carried out
and passed the examination that conducted by SFDA. On June 10, 2008,
SiBiono received another official notice from Guangdong Province SFDA and they
demanded the same requirements as stated in the official notice which issued by
the PRC State of SFDA dated on May 19, 2008. On November 24, 2008,
SiBiono received another official notice from Guangdong Province SFDA which
mentioned that after the examination conducted by Shenzhen City SFDA, the
Guangdong Province SFDA consent SiBiono to carry out production on a trial
basis. It further required SiBiono strictly to follow the
requirements of GMP to organize trial production and follow the procedures to
apply for GMP Certificate verification.
On July
14, 2009, SiBiono obtained the final approved GMP Certificate, in order words,
the SFDA allowed SiBiono to resume its production and sales.
18
Cost
of Goods Sold
Cost of
goods sold decreased $0.8 million (or 25.50%) to $2.34 million for the three
months ended March 31, 2010 from $3.14 million for the three months ended March
31, 2009 primarily due to the decrease in sales volume in Benda
Ebei.
Gross
Profit
Gross
profit decreased $0.57 million (or 24.2%) to $1.8 million for the three months
ended March 31, 2010 from $2.37 million for the three months ended
March 31, 2009, which was mainly due to the decrease in sales volume of
Gendicine which has a high gross profit margin.
Selling
Expenses:
Selling
expenses increased $0.25 million (or 62.35%) to $0.64 million for the three
months ended March 31, 2010 from $0.39 million the three months ended March 31,
2010, primarily due to the increased promotion efforts made by the
management.
General
and Administrative Expenses:
General
and administrative decreased $0.40 million (or 35.36%) to $0.72 million for the
three months ended March 31, 2010 from $1.12 million for the three months ended
March 31, 2009, primarily due to less bad debt expense incurred in
2010.
Operating
Income / (Loss):
The
Company had an operating loss of $0.38 million for the three months ended March
31, 2010, while the operating income from comparative period for 2009 was $0.33
million.
Interest
Expense:
Interest
expense was $0.37 million and $1.27 million for the three months ended March 31,
2010 and 2009 respectively. The decrease is mainly due to the amortization of
debt discount related to the convertible note during the first quarter of 2009
while there was no such expense in the same period of 2010.
Income
Taxes:
Benda is
subject to Delaware, United State of America tax, but no provision for income
taxes was made for the three months ended March 31, 2010 and 2009 as Benda did
not have reportable taxable income for the period.
Ever
Leader, a wholly owned subsidiary of Benda, is subject to Hong Kong tax, but no
provisions for income taxes was made for the three months ended
March 31, 2010 and 2009 as Ever Leader did not have reportable taxable income
for the periods.
Benda
Ebei was registered as a Sino-Foreign Equity Joint Venture on May 26, 2004 and
is subject to the tax laws applicable to Sino-Foreign Equity Joint Ventures in
the PRC. Benda Ebei, starting from 2005, is fully exempt from PRC
enterprise income tax for two years starting from the first profit-making year,
followed by a 50% reduction in the state income taxes, for the following three
years, commencing from the first profitable year.
Jiangling
Benda and Yidu Benda are cross-municipal investment entities and enjoy the same
tax treatment as Sino-Foreign Joint Ventures, starting from 2005, and were
therefore exempt from PRC enterprise income tax for two years starting from the
first profit-making year, followed by a 50% reduction in the state income taxes,
for the following three years, commencing from the first profitable year.
Cross-municipal investments entities refer to entities that are incorporated in
one municipal region but have investments in another municipal
region.
The
exemption periods for Benda Ebei, Jiangling Benda and Yidu Benda expired in the
year of 2006, after which they are subject to a 50% reduction in state income
taxes, at 18%; whereas the full income tax rate is 33%. The remaining tax
holidays will expire in 2010.
However,
starting and effective from January 1, 2008, the full income tax rate changed
from 33% to 25% according to the new PRC taxation regulations. Therefore these
subsidiaries are subject to the regular full income tax rate at 25% after the
tax holidays expire in 2010.
19
According
to the new taxation regulations starting and effective from January 1, 2008,
Beijing Shusai is subject to the full income tax rate of 25%.
According
to the new taxation regulations starting and effective from January 1, 2008,
SiBiono, which is located in Shenzhen, a Special Economic District of PRC, is
subject to the full income tax rate of 25% gradually in five years as
following:
|
Year
|
Tax
rate
|
|||
|
2008
|
18 | % | ||
|
2009
|
20 | % | ||
|
2010
|
22 | % | ||
|
2011
|
24 | % | ||
|
2012
and thereafter
|
25 | % | ||
Benda
Ebei recorded $86,845 income tax for the three months ended March 31,
2010.
LIQUIDITY
AND CAPITAL RESOURCES
Net cash
provided by the operating activities was negative $0.34 million for the three
months ended March 31, 2010, while for the three months ended March 31, 2009 was
positive $0.9 million.
|
a)
|
Non-cash
operating activities, reconciliation items to net
loss
|
For the
three months ended March 31, 2009, an amount about $2.1 million non-cash
operating activities was reconciled back to the net loss and which mainly
included amortization of debt discount and debt issue cost, bad debt provision,
amortization of intangible assets, and depreciation.
However,
for the three months
ended March 31, 2010, about $0.69 million of non-cash operating activities was
reconciled back to the net loss and summarized as follows:
|
1.
|
Factors:
$0.58 million incurred on depreciation; and $0.11 million incurred on
amortization of intangible assets.
|
|
b)
|
Trade
receivables
|
The net
amount of trade receivable was increased by $0.29 million for the three months
ended March 31, 2010. Management acknowledges that the net balance of the trade
receivable, as of March 31, 2010, was a significant asset to the company.
However, management believes that the above situation is temporarily due to the
following reasons:
|
a)
|
Customers
whom have sales relationships with our company are all relatively big
business wholesale enterprises and they have all passed the examination of
GMP Certificate so that collectibility from those is out of
question;
|
|
b)
|
Management
realized that it did affect the cash flow situation of the company;
therefore the company will put more efforts to reduce the balance of trade
receivables.
|
For the
three months ended March 31, 2010 and 2009, the amount spent in investing
activities were $0.66 million and $0.03 million respectively. The investing
activities were relatively small for the reporting periods.
Financing
cash inflow was $1.52 million for the three months ended March 31, 2010, while
the financing cash outflow was $0.47 million for the three months ended March
31, 2009.
20
Item
3. Quantitative and Qualitative Disclosures About Market
Risk
The
Company is subject to certain market risks, including changes in interest rates
and currency exchange rates. The Company does not undertake any specific
actions to limit those exposures.
Item
4T. Evaluation of Disclosure Controls and Procedures
a)
Evaluation of Disclosure
Controls. Our Chief Executive Officer and Chief Accounting Officer
evaluated the effectiveness of our disclosure controls and procedures as of the
end of our first fiscal quarter 2010 pursuant to Rule 13a-15(b) of the
Securities and Exchange Act. Disclosure controls and procedures are
controls and other procedures that are designed to ensure that information
required to be disclosed by us in the reports that we file or submit under the
Exchange Act is recorded, processed, summarized and reported within the time
periods specified in the SEC’s rules and forms. Disclosure controls and
procedures include, without limitation, controls and procedures designed to
ensure that information required to be disclosed by us in the reports that we
file under the Exchange Act is accumulated and communicated to our management,
as appropriate to allow timely decisions regarding required disclosure. Based on
his evaluation, our Chief Executive Officer and Chief Accounting Officer
concluded that our disclosure controls and procedures were not effective as of
March 31, 2010 due the following factors:
1. There
is a risk of management override given that our officers have a high degree of
involvement in our day to day operations.
2.
Significant errors were found in our prior years accounting treatments that
require restatements of our prior years filed financial statements.
3. There
is personal loan to executives which is a violation of Section 402 of the
Sarbanes-Oxley Act of 2002
Management
is currently evaluating remediation plans for the above control
deficiencies.
It should
be noted that any system of controls, however well designed and operated, can
provide only reasonable, and not absolute, assurance that the objectives of the
system are met. In addition, the design of any control system is based in part
upon certain assumptions about the likelihood of future events. Because of these
and other inherent limitations of control systems, there can be no assurance
that any design will succeed in achieving its stated goals under all potential
future conditions
(b)
Changes in internal
control over financial reporting. There have been no changes in our
internal control over financial reporting that occurred during the first fiscal
quarter that has materially affected, or is reasonably likely to materially
affect, our internal control over financial reporting. Our management team will
continue to evaluate our internal control over financial reporting in 2010 as we
implement our Sarbanes Oxley Act testing.
21
PART
II - OTHER INFORMATION
Item
1. Legal Proceedings.
|
1.
|
On
November 23, 2006, Benda Ebei entered into an Equity Transfer Agreement
with Xiaozhi Zhang (“Zhang”), to purchase approximately 6.24% of SiBiono’s
common stock for a total consideration of Rmb12.48 million (Rmb6.24
million in cash and shares of our common stock equal to Rmb6.24 million)
(or $1.71 million) which was due and payable on or before March 31,
2007.
|
Due to
the fact that the signed agreement on November 23, 2006 was not practically
executable according to the PRC regulations, Benda Ebei asked Zhang to terminate
the signed agreement and sign a new agreement that was feasible under PRC
regulations with essentially the same terms.
However,
Zhang refused to sign the new agreement and applied to the Shenzhen Arbitration
Commission (the “Commission”) in April 2007 for enforcement of the original
agreement. Zhang requested the Commission to require Benda Ebei to pay for the
total consideration, penalty for late payment and the related legal and
arbitration expenses.
On
November 27, 2007, Shenzhen Arbitration Commission determined that:
|
1.
|
Benda
Ebei should pay for the consideration of Rmb 6.24 million, equal to 50% of
the total consideration set forth in the Equity Transfer Agreement. For
the other 50% of the total consideration which was supposed to be settled
in the form of issuing common stock, since Zhang did not make an
arbitration request on how to execute the arrangement, the Arbitration
Commission did not make an award on this particular
part.
|
|
|
2.
|
Benda
Ebei should pay for the penalty of Rmb
46,800;
|
|
3.
|
Benda
Eebi should pay for legal and arbitration expenses of Rmb
268,971.
|
On May
22, 2008, Benda Ebei applied to Shenzhen People Court to terminate above
mentioned arbitration. The termination is based on the ground that Xiaozhi Zhang
does not own all 6.24% of SiBiono’s common stock. In fact, he only owns 3.28% of
SiBiono’s stock. The application has been accepted by Shenzhen People Court
and is waiting for its further investigation.
|
2.
|
SiBiono
patents - on January 29, 2008, SiBiono entrusted Grandall Legal Group
Shenzhen Law Firm to issue a legal letter to Zhaohui Peng, one of the
shareholders of Sibiono and the inventor of Gendicine, demanding that he
transfer all the title of patents to
SiBiono.
|
On June
18, 1999, during the formation of SiBiono, Zhaohui Peng transferred the rights
to the patent “A new method for manufacturing recombinant adenovirus” and
related research results to SiBiono as a payment for the registered capital. In
return, Zhaohui Peng was granted 32.03% of the common stock of
SiBiono.
22
From 1999
to 2007, SiBiono successfully obtained various technology funds from various
government technology agencies to support the further research and development
activities of Gendicine. Due to this significant funding obtained by SiBiono,
Sibiono developed five additional patents which are summarized as
follows:
|
Countries
/
|
Application
|
Publication
|
Approved
Patent
|
Name
of Patent
|
Name
of
|
Patent
|
|||||||||||
|
Item
|
Patent
name
|
Date
|
Number
(1)
|
Number
(2)
|
Number
(3)
|
Inventor
(6)
|
Applicant
(6)
|
Assignees
|
|||||||||
|
1
|
A
new method for manufacturing recombinant adenovirus
|
||||||||||||||||
|
A
|
China
|
98123346.5
|
CN1228474A
|
ZL98123346.5
|
Peng
|
Peng
|
SiBiono
|
||||||||||
|
Date
|
1998/12/14
|
1999/9/15
|
2002/7/3
|
||||||||||||||
|
2
|
A
recombinant constructed
by a virus vector and a |
||||||||||||||||
|
human
tumor suppressor
gene
and its use
|
|||||||||||||||||
|
A
|
China
|
02115228.4
|
CN1401778A
|
ZL02115228.4
|
Peng
/ Zhang
|
Peng
/ Zhang
|
Peng
/ Zhang
|
||||||||||
|
Date
|
2002/5/8
|
2003/3/12
|
2004/11/24
|
||||||||||||||
|
B
|
PCT
|
(4)
|
5
|
WO2004/078987A1
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A
|
|||||||||
|
Date
|
2004/3/8
|
2004/9/16
|
N/A
|
||||||||||||||
|
3
|
Recombinant
gene medicine
of adenovirus vector and |
||||||||||||||||
|
and
gene p54 for treating
proloferative diseases |
|||||||||||||||||
|
A
|
China
|
03125129.3
|
CN1471977A
|
ZL03125129.3
|
Peng
/ Zhang
|
Peng
/ Zhang
|
Peng
/ Zhang
|
||||||||||
|
Date
|
2003/5/10
|
2004/2/4
|
2007/7/25
|
||||||||||||||
|
B
|
PCT
|
(4)
|
8
|
WO2004/104204A1
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A
|
|||||||||
|
Date
|
2004/5/9
|
2004/12/2
|
N/A
|
||||||||||||||
|
4
|
The
application of
recombinant adenoviral p53 |
||||||||||||||||
|
as
cancer vaccine (tentative title)
|
|||||||||||||||||
|
A
|
China
|
200510002779.1
|
CN1679641A
|
ZL200510002779.1
|
Peng
/ Zhang
|
Peng
/ Zhang
|
Peng
/ Zhang
|
||||||||||
|
Date
|
2005/1/26
|
2005/10/12
|
2007/8/29
|
||||||||||||||
|
B
|
PCT
|
(4)
|
1
|
WO2006/079244A1
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A
|
|||||||||
|
Date
|
2005/1/26
|
2006/8/3
|
N/A
|
||||||||||||||
|
C
|
US
|
(5)
|
11/075035
|
2005/0281785A1
|
Not
Approved
|
Peng
/ Zhang
|
Unidentified
Yet
|
N/A
|
|||||||||
|
Date
|
2005/3/7
|
2005/12/22
|
N/A
|
||||||||||||||
|
5
|
Human
Embryonic Kidney (HEK)
sub-clone cell line |
||||||||||||||||
|
A
|
China
|
03126889.7
|
CN1513985A
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A
|
||||||||||
|
Date
|
2003/6/13
|
2004/7/21
|
N/A
|
||||||||||||||
|
B
|
PCT
|
(4)
|
7
|
WO2004/111239
|
Not
Approved
|
Peng
/ Zhang
|
Peng
/ Zhang
|
N/A
|
|||||||||
|
Date
|
2004/5/9
|
2004/12/23
|
N/A
|
||||||||||||||
|
6
|
The
complex of polypeptide
liposome
and human VGEF
|
||||||||||||||||
|
gene,
and its use and human
VGEF
gene, and its use
|
|||||||||||||||||
|
A
|
China
|
02134321.7
|
CN1389269A
|
Not
Approved
|
Peng
/ Zhang / Zhu
|
Peng
/ Zhang / Zhu
|
N/A
|
||||||||||
|
Date
|
2002/7/4
|
2003/1/8
|
N/A
|
Note:
|
(1)
|
Application
number is obtained when application is submitted;
|
|
|
(2)
|
Publication
number is obtained after the first phase
examination;
|
|
(3)
|
Approved
patent number is obtained after the final examination;
|
|
|
(4)
|
PCT
is referred to an International Patent Organization in
Paris;
|
|
(5)
|
US
is referred to the application is made in United States of America
alone;
|
|
|
(6)
|
Peng
is referred to Zhaohui Peng; Zhang is referred to Xiaozhi Zhang; Zhu is
referred to Jinya Zhu.
|
As
indicated in the above table, Item 1, the patent “A new method for manufacturing
recombinant adenovirus” had been assigned to SiBiono; however, the other
approved patents (item 2 through item 4) in PRC still have not been
assigned to SiBiono. The Group believes that all the above mentioned patents
should be rightfully transferred to SiBiono, a subsidiary of the Group.
Accordingly, the above mentioned legal letter was issued on this
ground.
On August
27, 2008, the Group through its subsidiary, SiBiono filed an application to the
Guongdong Province Shenzhen City (Middle) Peoples’ Court and demand Zhaozhu Peng
to transfer back all the mentioned patents that mentioned in above to
SiBiono. The case has been
accepted by the Court and is waiting for its further
investigation.
|
3.
|
Excalibur
Limited Partnership and Excalibur Limited Partnership II (the
"Plaintiffs") filed a motion for summary judgment in lieu of a complaint
pursuant to CPLR § 3213 (the "Motion") with the Supreme Court of the State
of New York (the "Court"), alleging that the Company has been delinquent
on the payment of an aggregate sum of $600,000 and accrued interest and
costs arising from the Convertible Promissory Notes that were issued to
the Plaintiffs in April 2007 in connection with a $7,560,000 private
placement. Pursuant to the motion, the Plaintiffs requested that the Court
(1) enter summary judgment in favor of Excalibur Limited Partnership
(“Excalibur Limited”) in the amount of $390,000 plus all accrued interest
and costs, and, (2) enter summary judgment in favor of Excalibur Small Cap
Opportunities LP (“Excalibur Small Cap”) in the amount of $210,000 and
accrued interest and costs. On July 29, 2009, the Court entered a
judgment against the Company in favor of the Plaintiffs in the amount of
$674,251.65 in connection with the Convertible Promissory Notes issued to
the Plaintiffs in April 2007 in a private
placement.
|
23
On March
4, 2009, the Company received a Notice and Default and Payment Demand letter
(the "Default Letter") from Pope Investments LLC ("Pope") in connection with its
convertible promissory note in the amount of $5,520,000 (the "Note") purchased
in our April 2007 private placement offering. The Default Letter provided notice
of default based on the Company's failure to make a required interest payment on
the Note by February 20, 2009. The Default Letter further demanded full payment
of all interest, liquidated damages and accrued interest thereon in the amount
of $130,364.37 by March 14, 2009, or Pope will accelerate the maturity date of
the full principal amount of the Note. On April 7, 2009, the Company
received further Default Letters and Payment Demand from Pope, Excalibur Limited
and Excalibur Small Cap demanding payment in full of the balance of the Notes,
which matured on March 28, 2009. The Company was notified on June 15,
2009, that on May 11, 2009 Pope filed a motion for summary judgment in lieu of a
complaint against us pursuant to CPLR § 3213 (the “Motion”) with the Supreme
Court of the State of New York (the “Court”), alleging that the Company has been
delinquent on the payment of an aggregate sum of $5,520,000 and accrued interest
and costs arising from the Note that the Company issued to Pope in April
2007. Pursuant to the motion, the Plaintiffs requested that the Court
enter summary judgment in favor of Plaintiff in the amount of $5,994,617.53
constituting principal and interest, plus costs.
On June
23, 2009, the Company filed an Affidavit in Opposition to Motion for Summary
Judgment in Lieu of Complaint with the Court requesting that Plaintiff’s Motion
be denied. On October 14, 2009, this motion was denied, and the court entered a
judgment in favor of Pope in the amount of $5,520,000 plus
interest.
On July
30, 2009, the Company received a Notice of Default from three additional
investors in the April 2007 private placement offering holding Notes totaling
$90,000.
|
4.
|
On
December 30, 2009, the Company was served with a Summons and Complaint
filed by Pope Investments, LLC (“Pope”) in the Court of Chancery of the
State of Delaware (the “Court”) against the Company and our officers and
directors. Pope filed the Summons and Complaint as a judgment
creditor and as a shareholder of the Company. Pope alleges
that the assets and profits of our subsidiary company have been
wrongfully diverted by our officers and directors and requests the
appointment of a receiver to liquidate and wind down the business affairs
of the Company. In connection with the filing of the Summons and
Complaint, Pope has also filed a motion for a Preliminary Injunction
Motion seeking to enjoin the Company and our officers and directors from
taking any further actions to divert the corporate assets and profits of
our subsidiary company and for expedited discovery proceedings.
Pope further requests the imposition of a constructive trust, an
accounting, damages for an alleged breach of fiduciary duty by the
Company’s officers and directors, and attorney fees.
|
|
A
hearing on the application of Pope Investments, LLC ("Pope") seeking the
appointment of a receiver was held on March 29, 2010. The parties filed
post-trial briefs on April 7, 2010. As of this date, the Court has not yet
ruled on Pope's application. Since the hearing, the remaining defendants,
including the Benda directors and officers named as defendants, have filed
a motion to dismiss Pope's complaint against them. No date has yet been
set for Pope's response to the motion or the parties' arguments to the
Court on the motion.
|
Item
2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item
3. Defaults Upon Senior Securities.
Excalibur
Limited Partnership and Excalibur Limited Partnership II (the "Plaintiffs")
filed a motion for summary judgment in lieu of a complaint pursuant to CPLR §
3213 (the "Motion") with the Supreme Court of the State of New York (the
"Court"), alleging that the Company has been delinquent on the payment of an
aggregate sum of $600,000 and accrued interest and costs arising from the
Convertible Promissory Notes that were issued to the Plaintiffs in April 2007 in
connection with a $7,560,000 private placement. Pursuant to the motion, the
Plaintiffs requested that the Court (1) enter summary judgment in favor of
Excalibur Limited Partnership (“Excalibur Limited”) in the amount of $390,000
plus all accrued interest and costs, and, (2) enter summary judgment in favor of
Excalibur Small Cap Opportunities LP (“Excalibur Small Cap”) in the amount of
$210,000 and accrued interest and costs. On July 29, 2009, the Court
entered a judgment against the Company in favor of the Plaintiffs in the amount
of $674,251.65 in connection with the Convertible Promissory Notes issued to the
Plaintiffs in April 2007 in a private placement.
24
On March
4, 2009, the Company received a Notice and Default and Payment Demand letter
(the "Default Letter") from Pope Investments LLC ("Pope") in connection with its
convertible promissory note in the amount of $5,520,000 (the "Note") purchased
in our April 2007 private placement offering. The Default Letter provided notice
of default based on the Company's failure to make a required interest payment on
the Note by February 20, 2009. The Default Letter further demanded full payment
of all interest, liquidated damages and accrued interest thereon in the amount
of $130,364.37 by March 14, 2009, or Pope will accelerate the maturity date of
the full principal amount of the Note. On April 7, 2009, the Company
received further Default Letters and Payment Demand from Pope, Excalibur Limited
and Excalibur Small Cap demanding payment in full of the balance of the Notes,
which matured on March 28, 2009. We were notified on June 15, 2009,
that on May 11, 2009 Pope filed a motion for summary judgment in lieu of a
complaint against us pursuant to CPLR § 3213 (the “Motion”) with the Supreme
Court of the State of New York (the “Court”), alleging that we have been
delinquent on the payment of an aggregate sum of $5,520,000 and accrued interest
and costs arising from the Note that we issued to the Pope in April
2007. Pursuant to the motion, the Plaintiffs requested that the Court
enter summary judgment in favor of Plaintiff in the amount of $5,994,617.53
constituting principal and interest, plus costs.
On June
23, 2009, we filed an Affidavit in Opposition to Motion for Summary Judgment in
Lieu of Complaint with the Court requesting that Plaintiff’s Motion be
denied. On October 14, 2009, this motion was denied, and the court entered
a judgment in favor of Pope in the amount of $5,520,000 plus
interest.
On July
30, 2009, we received a Notice of Default from three additional investors in the
April 2007 private placement offering holding Notes totaling
$90,000.
Item
4. (Removed and Reserved)
Item
5. Other Information.
None
Item
6. Exhibits and Reports of Form 8-K.
|
(a)
|
Exhibits
|
|
31.1
|
Certifications
pursuant to Section 302 of Sarbanes Oxley Act of 2002
|
|
32.1
|
Certifications
pursuant to Section 906 of Sarbanes Oxley Act of 2002
|
|
(b)
|
Reports
on Form 8-K
|
|
On
January 12, 2010, the Company filed a Form 8-K disclosing a Complaint
filed against the Company.
|
|
|
On
March 3, 2010, the Company filed a Form 8-K disclosing a change in the
Company’s auditor.
|
25
SIGNATURES
In
accordance with Section 13 or 15(d) of the Exchange Act, the registrant caused
this report to be signed on its behalf by the undersigned, there unto duly
authorized.
|
BENDA
PHARMACEUTICAL, INC.
Registrant
|
|||
|
Date:
May 24, 2010
|
By:
|
/s/ Yiqing
Wan
|
|
|
Yiqing
Wan
|
|||
|
President,
Chief Executive Officer,
|
|||
|
Chief
Financial and Accounting Officer
|
|||
|
Chairman
of Board of Directors
|
|||
26
