Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2009
Or
¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File No. 001-33635
CARDIUM THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 27-0075787 | |
| (State of incorporation) | (IRS Employer Identification No.) | |
| 12255 El Camino Real, Suite 250 San Diego, California 92130 |
(858) 436-1000 | |
| (Address of principal executive offices) | (Registrant’s telephone number) | |
Securities registered under Section 12(b) of the Exchange Act:
| Title of each class |
Name of exchange on which registered | |
| Common Stock, $0.0001 par value per share | NYSE Amex |
Securities registered under Section 12(g) of the Exchange Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ¨ Yes x No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. ¨ Yes x No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. x Yes ¨ No
Indicate by checkmark whether the registrant has submitted electronically and posted on it’s corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant for Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ¨ Yes ¨ No
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company.
| Large accelerated filer ¨ | Accelerated filer x | Non-accelerated filer ¨ | Smaller reporting company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ¨ Yes x No
The aggregate market value of voting and non-voting common equity held by non-affiliates computed as of the last business day of the registrant’s most recently completed second quarter was approximately $73,000,000 (based on the closing sale price of $1.85 reported by AMEX on June 30, 2009). For this purpose, all of Cardium’s officers and directors and each person who owned 10% or more of the outstanding common stock have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 15, 2010, 77,852,154 shares of Cardium’s common stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Part III (Items 10, 11, 12, 13 and 14) of this Form 10-K incorporates by reference portions of Cardium’s definitive proxy statement for its Annual Meeting of Stockholders to be held June 3, 2010 to be filed on or before April 30, 2010.
Table of Contents
| Page | ||||
| SPECIAL NOTE ABOUT FORWARD-LOOKING STATEMENTS | 1 | |||
| PART I | ||||
| Item 1. |
2 | |||
| Item 1A. |
9 | |||
| Item 1B. |
24 | |||
| Item 2. |
24 | |||
| Item 3. |
25 | |||
| Item 4. |
25 | |||
| PART II | ||||
| Item 5. |
Market for Our Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
26 | ||
| Item 6. |
27 | |||
| Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
29 | ||
| Item 7A. |
35 | |||
| Item 8. |
36 | |||
| Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
63 | ||
| Item 9A. |
63 | |||
| Item 9B. |
66 | |||
| PART III | ||||
| Item 10. |
67 | |||
| Item 11. |
67 | |||
| Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
67 | ||
| Item 13. |
Certain Relationships and Related Transactions and Director Independence |
67 | ||
| Item 14. |
67 | |||
| PART IV | ||||
| Item 15. |
Exhibits and Financial Statement Schedules | 68 | ||
| SIGNATURES | 75 | |||
Table of Contents
Unless the context requires otherwise, all references in this report to the “Company,” “Cardium,” “we,” “our,” and “us” refer to Cardium Therapeutics, Inc. and, as applicable, Post-Hypothermia Corporation (formerly, Innercool Therapies, Inc.) and Tissue Repair Company, each a wholly-owned subsidiary of Cardium.
Special Note about Forward-Looking Statements
Certain statements in this report, including information incorporated by reference, are “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, Section 21E of the Securities Exchange Act of 1934, and the Private Securities Litigation Reform Act of 1995. Forward-looking statements reflect current views about future events and financial performance based on certain assumptions. They include opinions, forecasts, intentions, plans, goals, projections, guidance, expectations, beliefs or other statements that are not statements of historical fact. Words such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “believes,” “anticipates,” “intends,” “estimates,” “approximates,” “predicts,” or “projects,” or the negative or other variation of such words, and similar expressions may identify a statement as a forward-looking statement. Any statements that refer to projections of our future financial performance, our anticipated growth and trends in our business, our goals, strategies, focus and plans, and other characterizations of future events or circumstances, including statements expressing general optimism about future operating results and the development of our products, are forward-looking statements. Forward-looking statements in this report may include statements about:
| • | future financial and operating results; |
| • | our ability to fund operations and business plans, and the timing of any funding or corporate development transactions we may pursue; |
| • | the timing, conduct and outcome of discussions with regulatory agencies, regulatory submissions and clinical trials, including the timing for completion of enrollment in clinical studies; |
| • | our beliefs and opinions about the safety and efficacy of our products and product candidates and the results of our clinical studies and trials; |
| • | our ability to enter into acceptable relationships with one or more contract manufacturers or other service providers on which we may depend and the ability of such contract manufacturers or other service providers to manufacture biologics, devices or key product components, or to provide other services, of an acceptable quality on a cost-effective basis; |
| • | our ability to enter into acceptable relationships with one or more development or commercialization partners to advance the commercialization of new products and product candidates and the timing of any product launches; |
| • | our growth, expansion and acquisition strategies, the success of such strategies, and the benefits we believe can be derived from such strategies; |
| • | our intellectual property rights and those of others, including actual or potential competitors; |
| • | the outcome of litigation matters; |
| • | our personnel, consultants and collaborators; |
| • | operations outside the United States; |
| • | current and future economic and political conditions; |
| • | overall industry and market performance; |
| • | the impact of accounting pronouncements; |
| • | management’s goals and plans for future operations; and |
| • | other assumptions described in this report underlying or relating to any forward-looking statements |
1
Table of Contents
The forward-looking statements in this report speak only as of the date of this report and caution should be taken not to place undue reliance on any such forward-looking statements. Forward-looking statements are subject to certain events, risks, and uncertainties that may be outside of our control. When considering forward-looking statements, you should carefully review the risks, uncertainties and other cautionary statements in this report as they identify certain important factors that could cause actual results to differ materially from those expressed in or implied by the forward-looking statements. These factors include, among others, the risks described under Item 1A and elsewhere in this report, as well as in other reports and documents we file with the United States Securities and Exchange Commission (“SEC”).
Overview
Cardium Therapeutics, Inc. was organized in Delaware in December 2003. Our business is focused on the acquisition and strategic development of product opportunities or businesses having the potential to address significant unmet medical needs, and definable pathways to commercialization, partnering or other monetization following the achievement of corresponding development objectives. As a development stage company, we have yet to generate positive cash flows from operations and are essentially dependent on debt and equity funding and partnering or other monetization transactions to finance our operations.
In October 2005, we acquired a portfolio of biologic growth factors and related delivery techniques from the Schering AG Group (now part of Bayer AG) for potential use in treating ischemic and other cardiovascular conditions, including GenerxTM, a product candidate being developed for patients with chronic myocardial ischemia (insufficient blood flow within the heart muscle) due to coronary heart disease.
In March 2006, we acquired the technologies and products of Innercool Therapies, Inc., a medical technology company in the emerging field of therapeutic hypothermia or patient temperature modulation, whose systems and products are designed to rapidly and controllably cool the body to reduce cell death and damage following acute ischemic events. On July 24, 2009, after completing a strategic turn-around and expansion of Innercool’s business and products, we sold the business to Royal Philips Electronics (NYSE: PHG) for $11.25 million (of which $1,125,000 is still in escrow as security for certain indemnification obligations), as well as the assumption by Philips of approximately $1.5 million in Innercool trade payables (the “Philips Transaction”). The operations of Innercool, which is now a part of Philips Healthcare, are shown as a discontinued operation in our consolidated statements of operations included with this report. After the closing, the name of our Innercool Therapies, Inc. subsidiary was changed to Post-Hypothermia Corporation.
In August 2006, we acquired rights to the assets and technologies of Tissue Repair Company, a company focused on the development of therapeutics for the potential treatment of chronic wounds and other tissue injuries. Tissue Repair Company’s ExcellagenTM and ExcellarateTM product candidates are initially being developed for the potential treatment of chronically non-healing diabetic foot ulcers. Tissue Repair Company is operated as a wholly-owned subsidiary of Cardium.
Our business model is designed to create multiple opportunities for success while avoiding reliance on any single technology platform or product type, and to leverage Cardium’s skills in late-stage product development in order to bridge the critical gap between promising new technologies and product opportunities that are ready for commercialization. Consistent with our long-term strategy, we intend to consider various corporate development transactions designed to place our product candidates into larger organizations or with partners having existing commercialization, sales and marketing resources, and a need for innovative products. Such transactions could involve the sale, partnering or other monetization of particular product opportunities or businesses. In parallel, as our businesses are advanced and corresponding valuations established, we plan to pursue new product opportunities and acquisitions with strong value enhancement potential.
2
Table of Contents
Cardium Biologics and Tissue Repair Company—Therapeutics and Devices for Ischemic Injuries and Other Indications
Cardium Biologics
The lead product candidate from our Cardium Biologics unit is Generx™ (alferminogene tadenovec, Ad5FGF-4), which is being developed as a potential treatment for myocardial ischemia (insufficient blood flow within the heart muscle) due to coronary heart disease. Generx represents a new therapeutic class of cardiovascular biologics designed to promote collateral angiogenesis, a natural process of blood vessel growth within the heart muscle, to increase blood supply to ischemic areas of the heart following a one-time intracoronary administration.
The FDA has cleared Generx for a Phase 3 clinical study in the U.S. for women with late stage coronary artery disease who are unresponsive to traditional drug therapy and are not appropriate candidates for mechanical revascularization (angioplasty/stents or by-pass surgery). However, in view of published results from an independent 10-year study among men and women with chronic coronary heart disease showing that improved collateral circulation was associated with substantially lower cardiac mortality (Circulation 116:975-983, 2007), and prior studies showing that a one-time infusion of Generx has the potential to achieve improved coronary collateral circulation in both men and women at levels approximately equivalent to bypass surgery as measured by SPECT imaging (Journal of American College of Cardiology 42(8):1339-1347, 2003), we believe that Generx could potentially be developed as a cost effective front-line therapy for patients with coronary artery disease in the large markets of newly-industrializing countries who often do not have access to costly procedures such as bypass surgery. We also believe that having such additional clinical evidence confirming the safety and effectiveness of Generx for improving coronary collateral circulation in men and women with severe coronary artery disease could potentially be used to optimize and broaden commercial development pathways in the U.S. and other industrialized countries.
Tissue Repair Company
Our Tissue Repair Company subsidiary is focused on the development of therapeutics and devices for the potential treatment of chronic wounds such as non-healing diabetic ulcers and other wounds, as well as the repair of other tissues, including both hard tissue injuries such as bone fracture, as well as soft tissue injuries affecting ligaments, tendons or cartilage.
On December 3, 2009 our Tissue Repair Company subsidiary filed a 510(k) premarket notification filing with the U.S. Food and Drug Administration (FDA) for its fibrillar collagen-based Excellagen™ topical gel for wound healing of diabetic foot ulcers and potentially other wounds. Our 510(k) filing covers ExcellagenXL™ and ExcellagenFXTM, advanced wound care management medical devices comprising customized protein-based gels designed for topical application by health care professionals for patients with dermal wounds, which can include diabetic ulcers, pressure ulcers, venous ulcers, tunneled/undermined wounds, surgical and trauma wounds, second degree burns, and other types of wounds. The 510(k) submission is based in part on positive findings from our Phase 2b Matrix clinical study, reported on October 14, 2009, demonstrating substantial improvements in wound healing responses in patients with non-healing diabetic foot ulcers following one or two applications of Excellagen, an enhanced, customized collagen-based gel matrix. ExcellagenXL is designed for use by health care professionals in a clinical setting and as an adjunct to standard of care topical wound therapy, which in the case of diabetic ulcers typically includes surgical debridement and off-loading. The ExcellagenFX kit is designed for use by health care providers in a clinical setting in the treatment of larger soft tissue or tunneling wounds that may occur with pressure, venous and diabetic ulcers, as well as surgical wounds. The ExcellagenFX flowable matrix product allows for deeper administration and direct intimate contact with the wound bed in these more complex, irregular and difficult to access wounds.
For Tissue Repair Company’s Excellarate™ product candidate, which comprises a mixture of our collagen-based gel with a biologic encoding a stimulatory growth factor (PDGF-B), we plan to introduce a combined
3
Table of Contents
formulation that allows for longer term stability without the need to maintain the biologic separately at -70 degrees centigrade, and to introduce the easier to use single-syringe product candidate into clinical studies designed to further evaluate the safety and effectiveness of Excellarate, and to allow for repeat dosing of Excellarate for wounds that are responding to treatment but have not yet achieved complete closure.
Incidence of Chronic Wounds
| • | An estimated 12.5 million patients worldwide suffer from chronic wounds with the industrialized countries making up 8 million, of which the U.S. totals approximately 3.7 million. |
| • | Over 800,000 patients in the U.S. develop diabetic foot ulcers annually. |
| • | Approximately 1.7 million patients suffer from pressure wounds, 1 million from diabetic foot ulcers and 1 million from venous status ulcers. |
| • | Diabetic ulcers cost the U.S. healthcare system approximately $5 billion per year with treatment and subsequent lower limb amputations adding an additional $1 billion per year. |
| • | Of the approximately 15 million diabetic patients, approximately 15 to 20 percent of this patient population will go on to suffer at least one chronic foot ulcer and of those six percent will be hospitalized due to infection or other ulcer-related complications. |
| • | Diabetes is the leading cause of non-traumatic lower extremity amputations and approximately 14 to 24 percent of patients with diabetes who develop foot ulcers eventually have an amputation. |
Current Treatment Approaches for Chronic Wounds
There are several treatment modalities currently used for severe chronic ulcers in diabetic patients, including topical dressings, off-loading, debridement and skin grafts. Regranex® Gel (becaplermin), which is marketed by Johnson & Johnson’s Ethicon Wound Management Division, is considered to be the only FDA-approved prescription medicine to treat such wounds. Regranex® Gel is a recombinant human platelet-derived growth factor (rrPDGF-BB) protein that is used as an adjunct with other current treatment modalities described above and is used to treat lower extremity diabetic neuropathic ulcers. Based on Regranex® Gel’s instructions for use, an estimated 70 administrations and 70 wound cleanings and redressings would be required over a 10-week treatment period (once daily administration followed by a subsequent wound cleaning and redressing without gel).
Gene Activated Matrix™ (GAM) Technology
We believe that patient compliance can be a major factor preventing or limiting improved medical outcomes, particularly when repeated administrations are required at a wound site. Tissue Repair Company’s Gene Activated Matrix technology is designed to provide a therapeutic level of protein synthesis at a particular site in the body and can be used in soft tissue such as skin, ligament, tendons and cartilage, as well as hard tissue such as bone. The technology is distinctive in that it is an immobilized form of local gene delivery that allows for control of gene uptake. GAM consists of a biocompatible matrix comprising a gene or DNA vector encoding a growth factor or other therapeutic protein.
For tissue repair, the application method involves placement of a GAM gel directly onto a wound site. TRC’s studies have shown that proliferative cells in the body can migrate into the GAM, take up the immobilized vector and gene and then transiently express the encoded therapeutic protein. Compared with topical applications of proteins, this in situ expression method significantly prolongs the availability of therapeutic protein to the cells involved in tissue repair. TRC’s GAM technology may have potential utility in several clinical indications where protein therapeutics have had limited success, including treatment of dermal wounds (such as diabetic foot ulcers), therapeutic angiogenesis (pharmacologically inducing new blood vessel growth), and orthopedic products for repair of various tissues, including hard tissue (bone) and soft tissue (ligament, tendon, cartilage).
4
Table of Contents
Other Biologics—Growth Factors for Regenerative Medicine
Biologics and Stem Cells for Regenerative Medicine
A complementary approach to the development of therapeutics for regenerative medicine involves the use of stem cells that can be incorporated into injured tissue like the heart as a means of preventing further damage and promoting healing of the affected organ. In that regard, filings by Cardium related to Ad5IGF-1 also described the potential use of stem cells, such as mesenchymal stem cells or MSCs transfected with an Ad5IGF-1 vector (such as that used in Corgentin™), for addressing coronary syndromes such as heart disease or heart attack.
In March 2009, Cardium reported that studies conducted by independent researchers at the University of Cincinnati showed in a preclinical model of heart attack that MSCs transfected with Ad5IGF-1 were effective at promoting angiogenesis within the heart and that the zone of heart attack related tissue damage (the infarct zone) was significantly reduced, and contractile function significantly improved, following administration of Ad5IGF-1 transfected MSCs—highlighting the potential value of these therapeutic approaches.
Business Strategy
Strategic Goals
Since December 31, 2008, we (i) completed the sale of Innercool Therapies to Royal Philips Electronics, (ii) completed Tissue Repair Company’s Matrix 2b clinical trial, (iii) submitted an FDA 510(k) application for the use of ExcellagenTM in the potential treatment of diabetic and other chronic wounds, and (iv) announced the Company’s new Orthobiologics initiative, designed to build on and extend the underlying technology developed by the Tissue Repair Company to hard tissue application such as bone.
Following the sale of our Innercool Therapies business, we do not currently have any products available for sale or use. Because of the limited nature of our revenues and the high costs we must incur to develop our product candidates, we have yet to generate positive cash flows or income from operations and do not anticipate doing so in the foreseeable future. As a result, we have been dependent on debt and equity funding to finance our operations. During September and October 2009, we raised net proceeds of approximately $9.7 million from the sale of common stock and warrants in two registered direct offerings. After year ended December 31, 2009, we raised additional net proceeds of approximately $10.4 from an additional registered direct offering of common stock and warrants.
Building on our core products and product candidates, our strategic goal is to develop a portfolio of medical products at various stages of development and secure additional financial resources to commercialize these products in a timely and effective manner.
The key elements of our strategy are to:
| • | complete an abbreviated FDA 510(k) registration process for ExcellagenTM and complete commercial development; candidate Excellagen; |
| • | advance the clinical development of ExcellarateTM; |
| • | evaluate the potential of GenerxTM to be a cost effective front-line therapy for patients with coronary artery disease in the large markets of newly-industrializing countries, and evaluate partnering opportunities designed to support the potential commercialization; |
| • | broaden and expand our product base and financial resources through other corporate development transactions in an attempt to enhance stockholder value, which could include acquiring other medical-related companies or product opportunities and/or securing additional capital; and |
| • | monetize the economic value of our product portfolio by establishing strategic collaborations and selling businesses and assets at appropriate valuation inflection points. |
5
Table of Contents
Government Regulation
New drugs, biologics and devices are subject to extensive regulation under the federal Food, Drug, and Cosmetic Act. In addition, biologics are also regulated under the Public Health Service Act. We believe that the pharmaceutical products we are attempting to develop will be regulated either as biological products or as new drugs. Both statutes and their corresponding regulations govern, among other things, the testing, manufacturing, distribution, safety, efficacy, labeling, storage, record keeping, advertising and other promotional practices involving biologics or new drugs. FDA approval or other clearances must be obtained before clinical testing, and before manufacturing and marketing, of biologics and drugs. Obtaining FDA approval has historically been a costly and time-consuming process. Different regulatory regimes are applicable in other major markets.
In addition, any gene therapy and other DNA-based products we develop will require regulatory approvals before human trials and additional regulatory approvals before marketing. New biologics are subject to extensive regulation by the FDA and the Center for Biological Evaluation and Research and comparable agencies in other countries. Currently, each human-study protocol is reviewed by the FDA and, in some instances, the NIH, on a case-by-case basis. The FDA and the NIH have published guidance documents with respect to the development and submission of gene therapy protocols.
To commercialize our product candidates, we must sponsor and file an investigational new drug (“IND”) application and be responsible for initiating and overseeing the human studies to demonstrate the safety and efficacy and, for a biologic product, the potency, which are necessary to obtain FDA approval of any such products. For our newly sponsored investigational new drug applications, we will be required to select qualified investigators (usually physicians within medical institutions) to supervise the administration of the products, and we will be required to ensure that the investigations are conducted and monitored in accordance with FDA regulations and the general investigational plan and protocols contained in the IND application.
The FDA receives reports on the progress of each phase of testing, and it may require the modification, suspension, or termination of trials if an unwarranted risk is present to patients. If the FDA imposes a clinical hold, trials may not recommence without FDA authorization and then only under terms authorized by the FDA. The IND application process can thus result in substantial delay and expense. Human gene therapy products, a primary area in which we are seeking to develop products, are a new category of therapeutics. Because this is a relatively new and expanding area of novel therapeutic interventions, there can be no assurance as to the length of the trial period, the number of patients the FDA will require to be enrolled in the trials to establish the safety, efficacy and potency of human gene therapy products, or that the data generated in these studies will be acceptable to the FDA to support marketing approval.
After the completion of trials of a new drug or biologic product, FDA marketing approval must be obtained. If the product is regulated as a biologic, the Center for Biological Evaluation and Research will require the submission and approval, depending on the type of biologic, of either a biologic license application or a product license application and a license application before commercial marketing of the biologic. If the product is classified as a new drug, we must file a new drug application with the Center for Drug Evaluation and Research and receive approval before commercial marketing of the drug. The new drug application or biologic license applications must include results of product development, laboratory, animal and human studies, and manufacturing information. The testing and approval processes require substantial time and effort and there can be no assurance that the FDA will accept the new drug application or biologic license applications for filing and, even if filed, that any approval will be granted on a timely basis, if at all. In the past, new drug applications and biologic license applications submitted to the FDA have taken, on average, one to two years to receive approval after submission of all test data. If questions arise during the FDA review process, approval can take more than two years.
Notwithstanding the submission of relevant data, the FDA may ultimately decide that the new drug application or biologic license application does not satisfy its regulatory criteria for approval and may require
6
Table of Contents
additional studies. In addition, the FDA may condition marketing approval on the conduct of specific post-marketing studies to further evaluate safety and effectiveness. Rigorous and extensive FDA regulation of pharmaceutical products continues after approval, particularly with respect to compliance with current good manufacturing practices (GMPs), reporting of adverse effects, advertising, promotion and marketing. Discovery of previously unknown problems or failure to comply with the applicable regulatory requirements may result in restrictions on the marketing of a product or withdrawal of the product from the market, as well as possible civil or criminal sanctions.
Ethical, social and legal concerns about gene therapy, genetic testing and genetic research could result in additional regulations restricting or prohibiting the processes we or our suppliers may use. Federal and state agencies, congressional committees and foreign governments have expressed interest in further regulating biotechnology. More restrictive regulations or claims that our products are unsafe or pose a hazard could prevent us from commercializing any such products.
The approval and/or clearance for marketing of medical devices, such as Excellagen and potentially other product candidates of our Tissue Repair Company subsidiary, are also subject to extensive controls by health regulatory and other authorities. Although some devices can be cleared for marketing pursuant to a procedure referred to as an FDA 501(k) clearance, other devices and/or indications may require additional clinical studies and may be subject to even more extensive regulatory and other controls.
In addition to the foregoing, state and federal laws regarding environmental protection and hazardous substances, including the Occupational Safety and Health Act, the Resource Conservancy and Recovery Act and the Toxic Substances Control Act, affect our business. These and other laws govern our use, handling and disposal of various biological, chemical and radioactive substances used in, and wastes generated by, our operations. If our operations result in contamination of the environment or expose individuals to hazardous substances, we could be liable for damages and governmental fines. We believe that we are in material compliance with applicable environmental laws and that continued compliance therewith will not have a material adverse effect on our business. We cannot predict, however, how changes in these laws may affect our future operations.
We are also subject to a variety of other regulations in the United States, including those relating to bioterrorism, taxes, labor and employment, import and export, and intellectual property.
To the extent we have operations outside the United States, any such operations would be similarly regulated by various agencies and entities in the countries in which we operate. The regulations of these countries may conflict with those in the United States and may vary from country to country. In markets outside the United States, we may be required to obtain approvals, licenses or certifications from a country’s ministry of health or comparable agency before we begin operations or the marketing of products in that country. Approvals or licenses may be conditioned or unavailable for certain products. These regulations may limit our ability to enter certain markets outside the United States.
Competition
The pharmaceutical, biotechnology and medical device industries are intensely competitive. Our products and any product candidates developed by us would compete with existing drugs, therapies, devices or procedures and with others under development. There are many pharmaceutical, biotechnology and medical device companies, public and private universities and research organizations actively engaged in research and development of products for the treatment of cardiovascular and related diseases, and/or products for the healing of chronic wounds. Many of these organizations have financial, technical, research, clinical, manufacturing and marketing resources that are greater than ours. If a competing company develops or acquires rights to a more efficient, more effective, or safer competitive therapy for treatment of the same or similar diseases we have targeted, or one that offers significantly lower costs of treatment, our business, financial condition and results of
7
Table of Contents
operations could be materially adversely affected. In view of the relatively early stage of the industry, we believe that the most significant competitive factor in the field of new therapeutics and devices is the effectiveness and safety of a product candidate, as well as its relative safety, efficacy and cost as compared to other products, product candidates or approaches that may be useful for treating a particular disease condition.
We believe that our product development programs will be subject to significant competition from companies using alternative technologies, some of which are described above, as well as to increasing competition from companies that develop and apply technologies similar to ours. Other companies may succeed in developing products earlier than we do, obtaining approvals for these products from the FDA more rapidly than we do or developing products that are safer, more effective or less expensive than those under development or proposed to be developed by us. We cannot assure you that research and development by others will not render our technology or product candidates obsolete or non-competitive or result in treatments superior to any product candidate developed by us, or that any product candidate developed by us will be preferred to any existing or newly developed technologies.
We are aware of products currently under development by competitors targeting the same or similar cardiovascular and vascular diseases as our Generx product development. These include biologic treatments using forms of genes and therapeutic proteins. For example, CardioVascular BioTherapeutics is developing injectable and topical forms of FGF-1 for the potential treatment of cardiovascular diseases. We will also face competition from entities using other traditional methods, including new drugs and mechanical therapies, to treat cardiovascular and vascular disease.
In the areas of tissue repair and wound healing, as being developed by our Tissue Repair subsidiary, there are a number of approaches being employed, including other collagen-based products, “living skin” equivalents, vacuum pumps and other devices, and biologics and small molecule drugs designed to promote repair and healing.
Manufacturing Strategy
To leverage our experience and available financial resources, we do not plan to develop company-owned and operated manufacturing facilities. We plan to outsource all product manufacturing to one or more contract manufacturers of clinical drug products that operate manufacturing facilities in compliance with current Good Manufacturing Practice regulations promulgated by the FDA. We may also seek to refine the current manufacturing process and final product formulation to achieve improvements in storage temperatures and the like.
The FDA has established guidelines and standards for the development and commercialization of molecular and gene-based drug products i.e.: Guidance for Industry—CMC for Human Gene Therapy INDs November 2004, Sterile Drug Products Produced by Aseptic Processing September 2004, Human Somatic Cell Therapy and Gene Therapy March 1998, PTC in the Characterization of Cell Lines Used to Produce Biologicals July 1993. These industry guidelines, among others, provide essential oversight with regard to process methodologies, product formulations and quality control standards to ensure the safety, efficacy and quality of these drug products.
Marketing and Sales
Our product candidates must generally undergo testing and development in clinical trials and pre-clinical studies. We do not currently have any products approved for marketing nor any present capacity to market and sell products that could be commercially developed based on our technology. If we should obtain any such marketing approvals, we expect that we would elect to engage in marketing or sales through or in collaboration with a commercialization partner, although we are not currently involved with such a partner.
8
Table of Contents
Intellectual Property
As part of our acquisition of Schering’s portfolio of cardiovascular growth factor therapeutic assets, pursuant to a Technology Transfer Agreement entered into between Cardium and Schering, we acquired from Schering a portfolio of methods and compositions directed at the treatment of cardiovascular diseases. In August 2006, we acquired the rights to various technologies and products now part of our Tissue Repair Company subsidiary, including Excellarate. There can be no assurance that our intellectual property assets will be sufficient to protect our commercialization opportunities, nor that our planned commercialization activities will not infringe any intellectual property rights held or developed by third parties.
Employees
As of December 31, 2009 we employed 13 full-time employees and one part-time employee. We do not expect to hire additional employees during the next 12 months. Our employees are not represented by a collective bargaining agreement and we have not experienced any work stoppages as a result of labor disputes. We believe our relationship with our employees is good. We also rely on various consultants and advisors to provide services to us.
Available Information
Our website address is www.cardiumthx.com. We make available, free of charge, through our website our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after we electronically file or furnish such reports to the SEC.
For additional financial information, including financial information about our business, please see the consolidated financial statements and accompanying notes to the consolidated financial statements included under Item 8 of this report.
You should carefully review and consider the risks described below, as well as the other information in this report and in other reports and documents we file with the SEC when evaluating our business and future prospects. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties, not presently known to us, or that we currently see as immaterial, may also occur. If any of the following risks or any additional risks and uncertainties actually occur, our business could be materially harmed, and our financial condition, results of operations and future growth prospects could be materially and adversely affected. In that event, the market price of our common stock could decline and you could lose all or a portion of the value of your investment in our stock. You should not draw any inference as to the magnitude of any particular risk from its position in the following discussion.
Risks Related to Our Business and Industry
Our product candidates require regulatory approvals, and in some cases additional prior development or testing, before marketing. We may be unable to develop, obtain regulatory approval or market any of our product candidates or expand the market of our existing products and technology. If our product candidates are delayed or fail, we will not be able to generate revenues and cash flows from operations, and we may have to curtail or cease our operations.
Our Excellagen™ collagen-based product candidates are expected to be regulated under an FDA 510(k) premarketing notification procedure allowing us or a commercialization partner to market and sell once 510(k) marketing clearance is obtained. There can be no assurance that marketing clearance will be achieved in a timely manner or at all, or that the FDA will not require additional studies or information to be provided for the 510(k) filing to be considered both complete and acceptable.
9
Table of Contents
Our other product candidates require additional research and development, clinical testing and regulatory clearances before we can market them. To our knowledge, the FDA has not yet approved any gene therapy or similar product and there can be no assurance that it will. There are many reasons that our products and product candidates may fail or not advance beyond clinical testing, including the possibility that:
| • | our products and product candidates may be ineffective, unsafe or associated with unacceptable side effects; |
| • | our product candidates may fail to receive necessary regulatory approvals or otherwise fail to meet applicable regulatory standards; |
| • | our product candidates may be too expensive to develop, manufacture or market; |
| • | physicians, patients, third-party payers or the medical community in general may not accept or use our products; |
| • | our potential collaborators may withdraw support for or otherwise impair the development and commercialization of our products or product candidates; |
| • | other parties may hold or acquire proprietary rights that could prevent us or our potential collaborators from developing or marketing our products or product candidates; or |
| • | others may develop equivalent, superior or less expensive products. |
In addition, our product candidates are subject to the risks of failure inherent in the development of biologics, gene therapy and other products based on innovative technologies. As a result, we are not able to predict whether our research, development and testing activities will result in any commercially viable products or applications. If our product candidates are delayed or we fail to successfully develop and commercialize our product candidates, or if we are unable to expand the market of our existing products or related technology, our business, financial condition or results of operations will be negatively affected, and we may have to curtail or cease our operations.
We rely on third party clinical research organizations to manage our clinical trials. Under this business model, we have less control over the clinical trials and may experience delays or errors in our clinical trials that could adversely affect our business, financial results and commercial prospects.
To obtain regulatory approvals for new products, we must, among other things, initiate and successfully complete multiple clinical trials demonstrating to the satisfaction of the FDA that our product candidates are sufficiently safe and effective for a particular indication. We currently rely on third party clinical research organizations to assist us in designing, administering and assessing the results of those trials. In relying on those third parties, we are dependent upon them to timely and accurately perform their services. We have experienced, and in the future may experience, delays in our clinical trials. Any such delay will result in additional costs, and defer any prospective opportunities to monetize the product candidate. Product development costs to us and our potential collaborators will increase, and our business may be negatively impacted, if we experience delays in testing or approvals or if we need to perform more or larger clinical trials than planned, for reasons such as the following:
| • | the FDA or other health regulatory authorities, or institutional review boards, do not approve a clinical study protocol or place a clinical study on hold; |
| • | suitable patients do not enroll in a clinical study in sufficient numbers or at the expected rate, or data is adversely affected by trial conduct or patient drop out; |
| • | patients experience serious adverse events, including adverse side effects of our drug candidate or device; |
| • | patients die during a clinical study for a variety of reasons that may or may not be related to our products, including the advanced stage of their disease and medical problems; |
10
Table of Contents
| • | patients in the placebo or untreated control group exhibit greater than expected improvements or fewer than expected adverse events; |
| • | third-party clinical investigators do not perform the clinical studies on the anticipated schedule or consistent with the clinical study protocol and good clinical practices, or other third-party organizations do not perform data collection and analysis in a timely or accurate manner; |
| • | service providers, collaborators or co-sponsors do not adequately perform their obligations in relation to the clinical study or cause the study to be delayed or terminated; |
| • | regulatory inspections of manufacturing facilities, which may, among other things, require us or a co-sponsor to undertake corrective action or suspend the clinical studies; |
| • | the interim results of the clinical study are inconclusive or negative; |
| • | the clinical study, although approved and completed, generates data that is not considered by the FDA or others to be sufficient to demonstrate safety and efficacy; and |
| • | changes in governmental regulations or administrative actions affect the conduct of the clinical trial or the interpretation of its results. |
Significant delays may adversely affect our financial results and the commercial prospects for our product candidates and delay our ability to become profitable. If third party organizations do not accurately collect and assess the trial data we may discontinue development of viable product candidates or continue allocating resources to the development and marketing of product candidates that are not efficacious. Either outcome could result in significant financial harm to our company and damage to our reputation.
If we are unable to enter into successful sales, marketing and distribution agreements with third parties, we may not be able to successfully commercialize our products.
In order to commercialize any products successfully, we expect to principally rely on collaborations or other arrangements with third parties to sell, market and distribute our products. To the extent that we enter into licensing, distributorship, co-promotion, co-marketing or other collaborative arrangements, our product revenues are likely to be lower than if we directly marketed and sold our products, and any revenues we receive will depend upon the efforts of third parties, whose efforts may not meet our expectations or be successful.
For example, we expect to depend upon the efforts of third parties to promote and sell our Excellagen™ products if and when they achieve FDA 510(k) marketing clearance, as well our Generx™ product if it should achieve regulatory approval, but there can be no assurance that the efforts of such third parties will meet our expectations or result in any significant product sales. While third parties would be largely responsible for the timing and extent of sales and marketing efforts, they may not dedicate sufficient resources to our product opportunities, and our ability to cause them to devote additional resources or to otherwise promote sales of our products may be limited. In addition, commercialization efforts could be negatively impacted by the delay or failure to obtain additional supportive data for our products. In some cases, third party partners could be responsible for conducting additional clinical trials to obtain such data and our ability to increase the efforts and resources allocated to these trials may be limited.
We sold all of the assets and business of our InnerCool Therapies, Inc. in July 2009 and may face claims for damages from the buyer if representations and warranties that we have made in connection with that sale have provided the buyer of those assets with certain indemnification rights.
On July 10, 2009 we entered into an Asset Purchase Agreement with Phillips Electronics North America Corporation (“Phillips”), pursuant to which we sold to Phillips certain assets and liabilities of our Innercool Therapies, Inc. subsidiary. The sales closed on July 24, 2009. In connection with the transaction, Phillips assumed only certain specified liabilities relating to the business. We retained responsibility for all other
11
Table of Contents
liabilities of the business, including contingent and unknown claims that may have existed at the time of sale. Also, in connection with the sale we made certain representations and warranties to Phillips that are standard for such transactions, including representations regarding the condition of the Innercool Therapies assets and liabilities. We have agreed to indemnify Phillips for any damages arising from the breach of any of those representations and warranties, as well as any breach of any covenant under the Asset Purchase Agreement. Our liability for breach of certain representations is contractually capped at $3.5 million; however, claims for damages arising out of certain “Excepted Representations” or any covenant under the Asset Purchase Agreement are not subject to the contractual limitation. Under the terms of the Asset Purchase Agreement, we deposited $1,125,000 of the proceeds from the sale into an escrow account to act as security for our indemnification obligations. We may, however, receive claims in excess of the funds in escrow. Any substantial indemnification claim under the Asset Purchase Agreement, or the defense of any such claim, could result in substantial costs, which would impair our financial condition and disrupt our ability to operate our remaining business.
We are a development stage company. We have incurred losses since our inception in December 2003 and expect to incur significant net losses in the foreseeable future and may never become profitable.
We have sustained operating losses to date and will likely continue to sustain losses as we seek to develop our products and product candidates. We expect these losses to be substantial because our product development and other costs, including significant amounts we expect to spend on development activities and clinical trials for our product candidates, cannot be offset by our limited revenues during our development stage. As of December 31, 2009, our accumulated deficit was approximately $76.2 million, and our cash and cash equivalents were approximately $3.4 million. To date, we have generated very limited revenues (mostly associated with our Innercool operations and a Tissue Repair Company grant, both of which have ended), and a large portion of our expenses are fixed, including expenses related to facilities, equipment, contractual commitments and personnel. As a result, we expect our net losses from operations to continue for at least the next five years. Our ability to generate additional revenues and potential to become profitable will depend largely on our ability, alone or with potential collaborators, to efficiently and successfully complete the development of our product candidates, successfully complete pre-clinical and clinical tests, obtain necessary regulatory approvals, and manufacture and market our products. There can be no assurance that any such events will occur or that we will ever become profitable. Even if we do achieve profitability, we cannot predict the level of such profitability. If we sustain losses over an extended period of time, we may be unable to continue our business.
Our business prospects are difficult to evaluate because we are a development stage company and are developing complex and novel medical products.
Since we have a relatively short operating history and our product candidates rely on complex technologies, it may be difficult for you to assess our growth, monetization and earnings potential. We have faced and it is likely we will continue to face many of the difficulties new technology companies often face. These include, among others: limited financial resources; developing, testing and marketing new products for which a market is not yet established and may never become established; challenges related to the development, approval and acceptance of a new product; delays in reaching our goals; lack of substantial revenues and cash flow; high product development costs; competition from larger, more established companies; and difficulty recruiting qualified employees for management and other positions. We will likely face these and other difficulties in the future, some of which may be beyond our control. If we are unable to successfully address these difficulties as they arise, our future growth and earnings will be negatively affected. We cannot be certain that our business strategies will be successful or that we will successfully address any problems that may arise.
We will need substantial additional capital to develop our products and for our future operations in the near term. If we are unable to obtain such funds when needed, we may have to delay, scale back or terminate our product development or our business.
Conducting the costly and time consuming research, pre-clinical and clinical testing necessary to obtain regulatory approvals and bring our products to market will require a commitment of substantial funds in excess
12
Table of Contents
of our current capital. Our future capital requirements will depend on many factors, including, among others: the progress of our current and new product development programs; the progress, scope and results of our pre-clinical and clinical testing; the time and cost involved in obtaining regulatory approvals; the cost of manufacturing our products and product candidates; the cost of prosecuting, enforcing and defending against patent claims and other intellectual property rights; competing technological and market developments; and our ability and costs to establish and maintain collaborative and other arrangements with third parties to assist in potentially bringing our products to market and/or to monetize the economic value of our product portfolio. The audit opinion accompanying our consolidated financial statements for the year ended December 31, 2009, included under Item 8 of this report, includes an explanatory paragraph indicating substantial doubt about our ability to continue as a going concern.
We will need to raise substantial additional capital to fund our future operations. We cannot be certain that additional financing will be available on acceptable terms, or at all. In recent years, it has been difficult for companies to raise capital due to a variety of factors. To the extent we raise additional capital through the sale of equity securities or we issue securities in connection with another transaction, the ownership position of existing stockholders could be substantially diluted. Anti-dilution adjustments to our securities currently outstanding would cause further dilution. If additional funds are raised through the issuance of preferred stock or debt securities, these securities are likely to have rights, preferences and privileges senior to our common stock and may involve significant fees, interest expense, restrictive covenants and the granting of security interests in our assets. Fluctuating interest rates could also increase the costs of any debt financing we may obtain. Raising capital through a licensing or other transaction involving our intellectual property could require us to relinquish valuable intellectual property rights and thereby sacrifice long term value for short-term liquidity.
Our failure to successfully address ongoing liquidity requirements would have a substantially negative impact on our business. If we are unable to obtain additional capital on acceptable terms when needed, we may need to take actions that adversely affect our business, our stock price and our ability to achieve cash flow in the future, including possibly surrendering our rights to some technologies or product opportunities, delaying our clinical trials or curtailing or ceasing operations.
We acquired the rights to develop the Excellarate product candidate of the Tissue Repair Company in August 2006 and may, in the future, pursue acquisitions of other companies or product rights that, if not successful, could adversely affect our business, financial condition and results of operations.
On August 11, 2006, we acquired rights to develop the Excellarate product candidate of the Tissue Repair Company, a medical technology company focused on the development of growth factor therapeutics for the potential treatment of chronic wounds such as dermal ulcers. These businesses are subject to all of the operational risks that can affect medical technology companies, including those related to regulatory approvals and clinical studies, acceptance of technology, competing technology, intellectual property rights, profitability, suppliers and third party collaborators, adverse publicity, litigation, and retention of key personnel.
In the future, we may pursue additional acquisitions of other companies, technologies or products. Acquisitions of businesses or product rights, including Tissue Repair Company transactions, involve numerous risks, including:
| • | our limited experience in evaluating businesses and product opportunities and completing acquisitions; |
| • | the use of any existing cash reserves or the need to obtain additional financing to pay for all or a portion of the purchase price of such acquisitions and to support the ongoing operations of the businesses acquired; |
| • | the potential need to issue convertible debt, equity securities, stock options and stock purchase warrants to complete an acquisition, which would dilute our stockholders and could adversely affect the market price of our common stock; |
13
Table of Contents
| • | potential difficulties related to integrating the technology, products, personnel and operations of the acquired company; |
| • | requirements of significant capital infusions in circumstances under which the acquired business, its products and/or technologies may not generate sufficient revenue or any revenue to offset acquisition costs or ongoing expenses; |
| • | entering markets in which we have no or limited prior direct experience and where competitors have stronger market or intellectual property positions; |
| • | disruptions to our ongoing business, diversion of resources, increases in our expenses and distraction of management’s attention from the normal daily operations of our business; |
| • | the potential to negatively impact our results of operations because an acquisition may require us to incur large one-time charges to earnings, amortize or write down amounts related to goodwill and other intangible assets, or incur or assume substantial debt or liabilities, or cause adverse tax consequences, substantial depreciation or deferred compensation charges; |
| • | an uncertain sales and earnings stream, or greater than expected liabilities and expenses, associated with the acquired company, product or product rights; |
| • | failure to operate effectively and efficiently as a combined organization utilizing common information and communication systems, operating procedures, financial controls and human resources practices; |
| • | potential loss of key employees of the acquired company; and |
| • | disruptions to our relationships with existing collaborators who could be competitive with the acquired business. |
There can be no assurance that our Tissue Repair transaction, or other transactions that we may pursue, will ultimately prove successful. If we pursue an acquisition but are not successful in completing it, or if we complete an acquisition but are not successful in integrating the acquired company’s employees, products or operations successfully, our business, financial condition or results of operations could be harmed.
Our technologies and product candidates may have unacceptable side effects that could delay or prevent product approval.
Possible side effects of therapeutic technologies may be serious and life threatening. The occurrence of any unacceptable side effects during or after pre-clinical and clinical testing of our product candidates, or the perception or possibility that our products cause or could cause such side effects, could delay or prevent approval of our products and negatively impact our business. For example, possible serious side effects of viral vector-based gene transfer could potentially include viral or gene product toxicity resulting in inflammation or other injury to the heart or other parts of the body. In addition, the development or worsening of cancer in a patient could potentially be a perceived or actual side effect of gene therapy technologies such as our own. Furthermore, there is a possibility of side effects or decreased effectiveness associated with an immune response toward any viral vector or gene used in gene therapy. The possibility of such response may increase if there is a need to deliver the viral vector more than once.
Even if approved for marketing, our technologies and product candidates are relatively novel and unproven and they may fail to gain market acceptance.
Our ongoing business and future depends on the success of our technologies and product candidates. Gene-based therapy is a new and rapidly evolving medical approach that has any not been shown to be effective on a widespread basis. Biotechnology and pharmaceutical companies have successfully developed and commercialized only a limited number of biologic-based products to date and no gene therapy has yet been successfully commercialized. Our product candidates, and the technology underlying them, are new and
14
Table of Contents
unproven and there is no guarantee that health care providers or patients will be interested in our products even if they are approved for use. Our success will depend in part on our ability to demonstrate sufficient clinical benefits, reliability, safety and cost effectiveness of our product candidates and technology relative to other approaches, as well as on our ability to continue to develop our product candidates to respond to competitive and technological changes. If the market does not accept our products or product candidates, when and if we are able to commercialize them, then we may never become profitable. It is difficult to predict the future growth of our business, if any, and the size of the market for our product candidates because the market and technology are continually evolving. There can be no assurance that our technologies and product candidates will prove superior to technologies and products that may currently be available or may become available in the future or that our technologies or research and development activities will result in any commercially profitable products.
We may not successfully establish and maintain collaborative and licensing arrangements, which could adversely affect our ability to develop and commercialize our product candidates.
Our strategy for the development, testing, manufacturing and commercialization of our product candidates generally relies on establishing and maintaining collaborations with licensors and other third parties. For example, we have various licenses from third parties relating to the use and delivery of our Generx product candidates. We may not be able to maintain or expand these or other licenses and collaborations or establish additional licensing and collaboration arrangements necessary to develop and commercialize our product candidates. Even if we are able to maintain or establish licensing or collaboration arrangements, these arrangements may not be on favorable terms and may contain provisions that will restrict our ability to develop, test and market our product candidates. Any failure to maintain or establish licensing or collaboration arrangements on favorable terms could adversely affect our business prospects, financial condition or ability to develop and commercialize our product candidates.
We expect to rely at least in part on third party service providers and collaborators to perform a number of activities relating to the development and commercialization of our product candidates, including the manufacture of product materials, the design and conduct of clinical trials, and potentially the obtaining of regulatory approvals and the marketing and distribution of any successfully developed products. Our collaborators also may have or acquire rights to control aspects of our product development and clinical programs. As a result, we may not be able to conduct these programs in the manner or on the time schedule we currently contemplate. In addition, if any of these collaborators withdraw support for our programs or product candidates or otherwise impair their development, our business could be negatively affected. To the extent we undertake any of these activities internally, our expenses may increase.
Our success hinges on the proper and effective performance of our service providers and collaborators of their responsibilities under their arrangements with us. Our existing or potential collaborators may not perform their obligations in a timely fashion or in a manner satisfactory to us. We and our present and future collaborators may fail to develop or effectively commercialize products covered by our present and future collaborations if, among other things:
| • | we do not achieve our objectives under our collaboration agreements; |
| • | we or our collaborators are unable to obtain patent protection for the products or proprietary technologies we develop in our collaborations; |
| • | we are unable to manage multiple simultaneous product discovery and development collaborations; |
| • | our collaborators become competitors of ours or enter into agreements with our competitors; |
| • | we or our collaborators encounter regulatory hurdles that prevent commercialization of our products; or |
| • | we develop products and processes or enter into additional collaborations that conflict with the business objectives of our other collaborators. |
15
Table of Contents
In addition, conflicts may arise with our collaborators, such as conflicts concerning the interpretation of clinical data, the achievement of milestones, the interpretation of financial provisions or the ownership of intellectual property developed during the collaboration. If any conflicts arise with our existing or future collaborators, they may act in their self-interest, which may be adverse to our best interest. If we or our collaborators are unable to develop or commercialize products, or if conflicts arise with our collaborators, we will be delayed or prevented from developing and commercializing products, which will harm our business and financial results.
We will rely on third parties to manufacture our product candidates. There can be no guarantee that we can obtain sufficient and acceptable quantities of our product candidates on acceptable terms, which may delay or impair our ability to develop, test and market such products.
Our business strategy relies on third parties to manufacture and produce our products and product candidates and the catheters used to deliver the products in accordance with good manufacturing practices established by the FDA and other regulators. These third party manufacturers are subject to extensive government regulation and must receive FDA approval before they can produce clinical material or commercial product.
Our products and product candidates may be in competition with other products for access to these facilities and may be subject to delays in manufacture if third parties give other products greater priority than our products. These third parties also may not deliver sufficient quantities of our products, manufacture our products in accordance with specifications, or comply with applicable government regulations. Successful large-scale manufacturing of gene-based therapy products has been accomplished by very few companies, and it is anticipated that significant process development changes will be necessary before commercializing and manufacturing any of our biologic product candidates. Additionally, if the manufactured products fail to perform as specified, our business and reputation could be severely impacted.
If any manufacturing agreement is terminated or any third party service provider or collaborator experiences a significant problem that could result in a delay or interruption in the supply of product materials to us, there are very few contract manufacturers who currently have the capability to produce our product candidates. There can be no assurance that manufacturers on whom we depend will be able to successfully produce our products or product candidates on acceptable terms, or on a timely or cost-effective basis, or in accordance with our product specifications and applicable FDA or other governmental regulations. We must have sufficient and acceptable quantities of our product materials to conduct our clinical trials and to market our product candidates, if and when such products have been approved by the FDA for marketing. If we are unable to obtain sufficient and acceptable quantities of our product material, we may be required to delay the clinical testing and marketing of our products, which would negatively impact our business.
If we do not comply with applicable regulatory requirements in the manufacture and distribution of our products and product candidates, we may incur penalties that may inhibit our ability to commercialize our products and adversely affect our financial condition and ability to become profitable.
Our failure or the failure of our potential collaborators or third party manufacturers to comply with applicable FDA or other product-related regulatory requirements including manufacturing, quality control, labeling, safety surveillance, promoting and reporting may result in criminal prosecution, civil penalties, recall or seizure of our products, total or partial suspension of production or an injunction, as well as other regulatory action against our products, product candidates or us. Discovery of previously unknown problems with a product, supplier, manufacturer or facility may result in restrictions on the sale of our products, including a withdrawal of such products from the market. The occurrence of any of these events would negatively impact our business and results of operations.
16
Table of Contents
If we are unable to create and maintain sales, marketing and distribution capabilities or enter into agreements with third parties to perform those functions, we will not be able to commercialize our product candidates or market our products.
We currently have limited sales, marketing and distribution capabilities. To commercialize our product candidates, if and when such products have been approved and are ready for marketing, we expect either to collaborate with third parties to perform these functions or develop them internally.
We have little experience in developing, training or managing a sales force and will incur substantial additional expenses for any products that we market directly. Developing a marketing and sales force is also time consuming and could delay the launch of new products or expansion of existing product sales. We expect that we or a partner will need to develop additional marketing and sales personnel, and/or work with outside providers, to achieve increased sales of our products. In addition, we or our partner will compete with many companies that currently have extensive and well-funded marketing and sales operations. Our or our partner’s marketing and sales efforts may be unable to compete successfully against these companies, in which event our business prospects may suffer.
We face intense and increasing competition and must cope with rapid technological change, which may adversely affect our financial condition and/or our ability to successfully commercialize and/or market our products and product candidates.
Our competitors and potential competitors include large pharmaceutical and medical device companies and more established biotechnology companies. These companies have significantly greater financial and other resources and greater expertise than us in research and development, manufacturing, pre-clinical and clinical testing, obtaining regulatory approvals and marketing. This may make it easier for them to respond more quickly than us to new or changing opportunities, technologies or market needs. Small companies may also prove to be significant competitors, particularly through collaborative arrangements with large pharmaceutical companies or through acquisition or development of intellectual property rights. Our larger competitors may be able to devote greater resources to research and development, marketing, distribution and other activities that could provide them with a competitive advantage. Many of these competitors operate large, well-funded research and development programs and have significant products approved or in development. Our potential competitors also include academic institutions, governmental agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for product and clinical development and marketing.
Our industry is characterized by extensive research and development, rapid technological change, frequent innovations and new product introductions, and evolving industry standards. Existing products and therapies to treat vascular and cardiovascular disease, including drugs and surgical procedures, as well as competitive approaches to wound healing and tissue repair, will compete directly or indirectly with the products that we are seeking to develop and market. In addition, our competitors may develop more effective or more affordable products, or achieve earlier patent protection or product commercialization and market penetration than us. As these competitors develop their technologies, they may develop proprietary positions that prevent us from successfully commercializing our future products. To be successful, we must be able to adapt to rapidly changing technologies by continually enhancing our products and introducing new products. If we are unable to adapt, products and technologies developed by our competitors may render our products and product candidates uneconomical or obsolete, and we may not be successful in marketing our products and product candidates against competitors. We may never be able to capture and maintain the market share necessary for growth and profitability and there is no guarantee we will be able to compete successfully against current or future competitors.
17
Table of Contents
Changes and reforms in the health care system or reimbursement policies may adversely affect the sale of our products and future products or our ability to obtain an adequate level of reimbursement or acceptable prices for our products or future products.
We currently have no products approved for marketing. Our ability to earn sufficient returns on our products and future products, if and when such products are approved and ready for marketing, will depend in part on the extent to which reimbursement for our products and related treatments will be available from government health administration authorities, private health coverage insurers, managed care organizations and other third-party payers. If we fail to obtain appropriate reimbursement, it could prevent us from successfully commercializing and marketing our products and future products.
There have been and will continue to be efforts by governmental and third-party payers to contain or reduce the costs of health care through various means, including limiting coverage and the level of reimbursement. We expect that there will continue to be a number of legislative proposals to implement government controls and other reforms to limit coverage and reimbursement. Additionally, third-party payers, including Medicare, are increasingly challenging the price of medical products and services and are limiting the reimbursement levels offered to consumers for these medical products and services. If purchasers or users of our products or future products are not able to obtain adequate reimbursement from third-party payers for the cost of using the products, they may forego or reduce their use. Significant uncertainty exists as to the reimbursement status of newly approved health care products, including gene therapy and other therapeutic products and devices, and whether adequate third-party coverage will be available. The announcement or considerations of these proposals or reforms could impair our ability to raise capital and negatively affect our business.
If we are unable to attract and retain key personnel and advisors, it may adversely affect our ability to obtain financing, pursue collaborations or develop or market our products or product candidates.
Our future success depends on our ability to attract, retain and motivate highly qualified management and scientific and regulatory personnel and advisors. The loss of any of our senior management team, in particular Christopher J. Reinhard, our Chairman of the Board, Chief Executive Officer, President and Treasurer, Tyler M. Dylan-Hyde, our director, Chief Business Officer, General Counsel, Executive Vice President and Secretary, and Dennis M. Mulroy, our Chief Financial Officer, or our vice presidents, or the operating officers of our subsidiaries, could harm our business. We do not maintain any key man life insurance on any of our executive officers.
To pursue our business strategy, we will need to hire or otherwise engage qualified scientific personnel and managers, including personnel with expertise in clinical trials, government regulation, manufacturing, marketing and other areas. Competition for qualified personnel is intense among companies, academic institutions and other organizations. If we are unable to attract and retain key personnel and advisors, it may negatively affect our ability to successfully develop, test, commercialize and market our products and product candidates.
Our facilities are located in or near seismic zones, and an earthquake or other natural disaster or resource shortage could delay our research and development efforts and adversely affect our business.
Our headquarters are in San Diego, California, and our third party manufacturing and storage facilities in Carlsbad, California, are both located in or near seismic zones, and there is a constant possibility that an earthquake or other natural disaster or resource shortage could be disruptive to our operations and result in delays in our research and development efforts. In the event of a natural or other disaster such as an earthquake, fire, flood or terrorist attack, if our facilities or the equipment in our facilities, or our clinical supplies, are significantly damaged or destroyed, we may not be able to rebuild or relocate our facility or replace any damaged equipment, records or clinical supplies in a timely manner and our business, financial condition and results of operations could be materially and adversely affected.
18
Table of Contents
We will use hazardous and biological materials in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our products and processes will involve the controlled storage, use and disposal of certain hazardous and biological materials and waste products. We and our suppliers and other collaborators are subject to federal, state and local regulations governing the use, manufacture, storage, handling and disposal of materials and waste products. Even if we and these suppliers and collaborators comply with the standards prescribed by law and regulation, the risk of accidental contamination or injury from hazardous materials cannot be completely eliminated. In the event of an accident, we could be held liable for any damages that result, and any liability could exceed the limits or fall outside the coverage of any insurance we may obtain and exceed our financial resources. We may not be able to maintain insurance on acceptable terms, or at all. We may incur significant costs to comply with current or future environmental laws and regulations.
To the extent we enter markets outside the United States, our business will be subject to political, economic, legal and social risks in those markets, which could adversely affect our business.
There are significant regulatory and legal barriers in markets outside the United States that we must overcome to the extent we enter or attempt to enter markets in countries other than the United States. We will be subject to the burden of complying with a wide variety of national and local laws, including multiple and possibly overlapping and conflicting laws. We also may experience difficulties adapting to new cultures, business customs and legal systems. Any sales and operations outside the United States would be subject to political, economic and social uncertainties including, among others:
| • | changes and limits in import and export controls; |
| • | increases in custom duties and tariffs; |
| • | changes in currency exchange rates; |
| • | economic and political instability; |
| • | changes in government regulations and laws; |
| • | absence in some jurisdictions of effective laws to protect our intellectual property rights; and |
| • | currency transfer and other restrictions and regulations that may limit our ability to sell certain products or repatriate profits to the United States. |
Any changes related to these and other factors could adversely affect our business to the extent we enter markets outside the United States.
Risks Related to Our Intellectual Property and Potential Litigation
If our products and product candidates are not effectively protected by valid, issued patents or if we are not otherwise able to protect our proprietary information, or if our right to use intellectual property that we license from third parties is terminated or adversely affected, our financial condition, operations or ability to develop and commercialize our product candidates may be harmed.
The success of our operations will depend in part on our ability and that of our licensors to: obtain patent protection for our therapeutics, devices and procedures, and other methods or components on which we rely, both in the United States and in other countries with substantial markets; defend patents once obtained; maintain trade secrets and operate without infringing upon the patents and proprietary rights of others; and obtain appropriate licenses upon reasonable terms to patents or proprietary rights held by others that are necessary or useful to us in commercializing our technology, both in the United States and in other countries with substantial markets.
19
Table of Contents
Our business substantially relies on our own or in-licensed intellectual property related to various technologies that are material to our products and processes. We depend on our and our licensors’ abilities to successfully prosecute and enforce the patents, file patent applications and prevent infringement of those patents and patent applications. The licenses and other intellectual property rights we acquire may or may not provide us with exclusive rights. To the extent we do not have exclusive rights, others may license the same technology and may develop the technology more successfully or may develop products similar to ours and that compete with our products. Even if we are provided with exclusive rights, the scope of our rights under our licenses may be subject to dispute and termination or reduction by our licensors or third parties. Our licenses also contain milestones that we must meet and/or minimum royalty or other payments that we must make to maintain the licenses. There is no assurance that we will be able to meet such milestones and/or make such payments. Our licenses may be terminated if we fail to meet applicable milestones or make applicable payments.
If we are not able to maintain adequate patent protection for our products and product candidates, we may be unable to prevent our competitors from using our technology or technology that we license.
The patent positions of the technologies being developed by us and our collaborators involve complex legal and factual uncertainties. As a result, we cannot be certain that we or our collaborators will be able to obtain adequate patent protection for our products or product candidates. There can be no assurance that (i) any patents will be issued from any pending or future patent applications of ours or our collaborators; (ii) the scope of any patent protection will be sufficient to provide us with competitive advantages; (iii) any patents obtained by us or our collaborators will be held valid if subsequently challenged; or (iv) others will not claim rights in or ownership of the patents and other proprietary rights we or our collaborators may hold. Unauthorized parties may try to copy aspects of our products and technologies or obtain and use information we consider proprietary. Policing the unauthorized use of our proprietary rights is difficult. We cannot guarantee that no harm or threat will be made to our or our collaborators’ intellectual property. In addition, changes in, or different interpretations of, patent laws in the United States and other countries may also adversely affect the scope of our patent protection and our competitive situation.
Due to the significant time lag between the filing of patent applications and the publication of such patents, we cannot be certain that our licensors were the first to file the patent applications we license or, even if they were the first to file, also were the first to invent, particularly with regards to patent rights in the United States. In addition, a number of pharmaceutical and biotechnology companies and research and academic institutions have developed technologies, filed patent applications or received patents on various technologies that may be related to our operations. Some of these technologies, applications or patents may conflict with our or our licensors’ technologies or patent applications. A conflict could limit the scope of the patents, if any, that we or our licensors may be able to obtain or result in denial of our or our licensors’ patent applications. If patents that cover our activities are issued to other companies, we may not be able to develop or obtain alternative technology.
Patents issued and patent applications filed internationally relating to gene therapy and biologics, collagen-based products, and other of our technologies are numerous, and we cannot assure you that current and potential competitors or other third parties have not filed or received, or will not file or receive applications in the future for patents or obtain additional proprietary rights relating to products or processes used or proposed to be used by us.
Additionally, there is certain subject matter that is patentable in the United States but not generally patentable outside of the United States. Differences in what constitutes patentable subject matter in various countries may limit the protection we can obtain outside of the United States. For example, methods of treating humans are not patentable in many countries outside of the United States. These and other issues may prevent us from obtaining patent protection outside of the United States, which would have a material adverse effect on our business, financial condition and results of operations.
20
Table of Contents
We may be subject to costly claims, and, if we are unsuccessful in resolving conflicts regarding patent rights, we may be prevented from developing, commercializing or marketing our products and/ or product candidates.
There has been, and will likely continue to be, substantial litigation regarding patent and other intellectual property rights in the biotechnology industry. As the biotechnology industry expands and more patents are issued, the risk increases that our processes, technology, products and product candidates may give rise to claims that they infringe on the patents of others. Others could bring legal actions against us claiming damages and seeking to stop clinical testing, manufacturing and marketing of the affected product or use of the affected process. Litigation may be necessary to enforce our or our licensors’ proprietary rights or to determine the enforceability, scope and validity of the proprietary rights of others. If we become involved in litigation, it could be costly and divert our efforts and resources. In addition, if any of our competitors file patent applications in the United States claiming technology also invented by us or our licensors, we may need to participate in interference proceedings held by the U.S. Patent and Trademark Office to determine priority of invention and the right to a patent for the technology. Like litigation, interference proceedings can be lengthy and often result in substantial costs and diversion of resources.
If we are unsuccessful in defending against any adverse claims, we could be compelled to seek licenses from one or more third parties who could be direct or indirect competitors and who might not make licenses available on terms that we find commercially reasonable or at all. In addition, such proceedings, even if decided in our favor, involve lengthy processes, are subject to appeals, and typically result in substantial costs and diversion of resources.
As more potentially competing patent applications are filed, and as more patents are actually issued, in the fields of gene therapy, biologics, collagen-based products, wound healing and tissue repair, adenoviral vectors or in other fields in which we may become involved and with respect to component methods or compositions that we may employ, the risk increases that we or our licensors may be subjected to litigation or other proceedings that claim damages or seek to stop our manufacturing, marketing, product development or commercialization efforts. Even if such patent applications or patents are ultimately proven to be invalid, unenforceable or non-infringed, such proceedings are generally expensive and time consuming and could consume a significant portion of our resources and substantially impair our marketing and product development efforts.
If there were an adverse outcome of any litigation or interference proceeding, we could have a potential liability for significant damages. In addition, we could be required to obtain a license to continue to make or market the affected product or use the affected process, or face an injunction to block our sale or marketing of affected products or use of the affected process. Costs of a license may be substantial and could include up-front payments as well as ongoing royalties. We may not be able to obtain such a license on acceptable terms, or at all, which could substantially impact our business.
We may not have adequate protection for our unpatented proprietary information, which could adversely affect our competitive position.
We also rely on trade secrets, know-how, continuing technological innovations and licensing opportunities to develop and maintain our competitive position. However, others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technology. To protect our trade secrets, we may enter into confidentiality agreements with employees, consultants and potential collaborators. However, these agreements may not provide meaningful protection of our trade secrets or adequate remedies in the event of unauthorized use or disclosure of such information. Likewise, our trade secrets or know-how may become known through other means or be independently discovered by our competitors. Any of these events could prevent us from developing or commercializing our product candidates.
21
Table of Contents
We face the risk of product liability claims, which could adversely affect our business and financial condition.
Our marketing and will expose us to product liability risks that are inherent in the testing, manufacturing and marketing of biotechnology and medical device products. Failure to obtain or maintain sufficient product liability insurance or otherwise protect against product liability claims could prevent or delay the commercialization or marketing of our products or product candidates or expose us to substantial liabilities and diversions of resources, all of which can negatively impact our business. Regardless of the merit or eventual outcome, product liability claims may result in withdrawal of product candidates from clinical trials, costs of litigation, damage to our reputation, substantial monetary awards to plaintiffs and decreased demand for products.
Product liability may result from harm to patients using our products, such as a complication that was either not communicated as a potential side effect or was more extreme than communicated. We will require all patients enrolled in our clinical trials to sign consents, which explain various risks involved with participating in the trial. However, patient consents provide only a limited level of protection, and it may be alleged that the consent did not address or did not adequately address a risk that the patient suffered from. Additionally, we will generally be required to indemnify the clinical product manufacturers, clinical trial centers, medical professionals and other parties conducting related activities in connection with losses they may incur through their involvement in the clinical trials. We may not be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities.
Risks Related to Our Common Stock
We will need substantial additional capital to develop our products and for our future operations in the near term, which can adversely affect our stock price and valuation
We will need to raise substantial additional capital to fund our future operations. To the extent we raise additional capital through the sale of equity securities or we issue securities in connection with another transaction, our stock price can be adversely affected and the ownership position of existing stockholders could be substantially diluted. Anti-dilution adjustments to our securities currently outstanding would cause further dilution. If additional funds are raised through the issuance of preferred stock or debt securities, these securities are likely to have rights, preferences and privileges senior to our common stock and may involve significant fees, interest expense, restrictive covenants and the granting of security interests in our assets. Fluctuating interest rates could also increase the costs of any debt financing we may obtain. Raising capital through a licensing or other transaction involving our intellectual property could require us to relinquish valuable intellectual property rights and thereby sacrifice long term value for short-term liquidity.
The exercise of our outstanding warrants will significantly dilute the ownership interest of existing stockholders.
We have a substantial number of warrants to purchase common stock outstanding. Some of these warrants contain antidilution purchase price protection such that the exercise price is reduced if we issue common stock or common stock equivalents to third parties at a purchase price that is less than the exercise price of the warrants. The exercise of some or all of our outstanding warrants would significantly dilute the ownership interests of existing stockholders. Any sales in the public market of the common stock issuable upon such exercise could adversely affect prevailing market prices of our common stock.
A delisting from the NYSE Amex could adversely affect the price of our common stock.
Our common stock is currently listed on the NYSE Amex (the “Exchange”). To maintain that listing, we must continue to comply with various listing standards of the Exchange, as set forth in Part 10 of the Exchange’s Company Guide. In December 2008, we received a notice from the staff of the Exchange noting that, based on their review of publicly available information, we did not meet certain of the Exchange’s continued listing standards related to the maintenance of a minimum level of stockholders’ equity and losses from ongoing
22
Table of Contents
operations. In January 2009, we submitted a plan of compliance (the “Plan”) advising the Exchange of the actions taken or to be taken to regain compliance with the Company Guide. In February 2009, our Plan was accepted and in July 2009, following completion of the Philips Transaction, we were informed that Cardium was considered to have regained compliance with the Exchange’s requirements, in advance of the June 23, 2010 deadline. On December 28, 2009, the Exchange noted that we were not considered to be in compliance with Section 1003(a)(iv) of the Company Guide because we reported stockholders’ equity of less than $4,000,000 and losses from continuing operations and net losses in three of our most recent fiscal years. With losses from continuing operations and net losses in five of our most recent fiscal years, the stockholders’ equity threshold would increase to $6,000,000. The Exchange asked us to supplement our previously-filed Plan advising the Exchange of the actions we have taken or will take to regain compliance with the Company Guide by June 23, 2010. Prior to the January 27, 2010 deadline, we provided a supplement to the Plan to the Exchange. That supplement was accepted; however, if we are not in compliance with the continued listing standards at the end of the Plan period, or we do not make progress consistent with the Plan during such period, then the Exchange could initiate delisting proceedings. If our common stock was ultimately delisted from the exchange, it would be expected to trade on the OTC Bulletin Board, a regulated quotation service that provides quotes, sale prices and volume information in over-the-counter equity securities which may reduce the liquidity of, and may adversely affect the price of, our common stock. Our common stock was traded on the OTC Bulletin Board until July 2007, when we elected to instead list our shares on the Exchange.
The price of our common stock is expected to be volatile and an investment in our common stock could decline substantially in value.
In light of our small size and limited resources, as well as the uncertainties and risks that can affect our business and industry, our stock price is expected to be highly volatile and can be subject to substantial drops, with or even in the absence of news affecting our business. The following factors, in addition to the other risk factors described in this report, and the potentially low volume of trades in our common stock, may have a significant impact on the market price of our common stock, some of which are beyond our control:
| • | changes in economic conditions in the United States and worldwide; |
| • | the availability to us or other companies of credit; |
| • | anticipated or unanticipated changes in financial condition, operating results or the perceived value of our business; |
| • | anticipated or unanticipated changes that affect our ability to maintain the listing of our common stock on a national exchange; |
| • | developments concerning any research and development, clinical trials, manufacturing, and marketing efforts or collaborations; |
| • | our announcement of significant acquisitions, strategic collaborations, joint ventures or capital commitments; |
| • | announcements of technological innovations; |
| • | new products or services that we or our competitors offer; |
| • | the initiation, conduct and/or outcome of intellectual property and/or litigation matters; |
| • | changes in financial or other estimates by securities analysts or other reviewers or evaluators of our business; |
| • | conditions or trends in bio-pharmaceutical or other healthcare industries; |
| • | regulatory developments in the United States and other countries; |
| • | changes in the economic performance and/or market valuations of other biotechnology and medical device companies; |
23
Table of Contents
| • | additions or departures of key personnel; |
| • | sales or other transactions involving our common stock; and |
| • | global unrest, terrorist activities, and economic and other external factors. |
The stock market in general has recently experienced relatively large price and volume fluctuations. In particular, the market prices of securities of smaller biotechnology and medical device companies have experienced dramatic fluctuations that often have been unrelated or disproportionate to the operating results of these companies. Continued market fluctuations could result in extreme volatility in the price of the common stock, which could cause a decline in the value of the common stock. You should also be aware that price volatility may be worse if the trading volume of the common stock remains limited or declines.
We could be difficult to acquire due to anti-takeover provisions in our charter, our stockholder rights plan and Delaware law.
Our board of directors has adopted a stockholder rights plan in which preferred stock purchase rights were distributed as a dividend. These provisions may make it more difficult for stockholders to take corporate actions and may have the effect of delaying or preventing a change in control. These provisions also could deter or prevent transactions that stockholders deem to be in their interests. In addition, we are subject to the anti- takeover provisions of Section 203 of the Delaware General Corporation Law. Subject to specified exceptions, this section provides that a corporation may not engage in any business combination with any interested stockholder during the three-year period following the time that such stockholder becomes an interested stockholder. This provision could have the effect of delaying or preventing a change of control of our company. The foregoing factors could reduce the price that investors or an acquirer might be willing to pay in the future for shares of our common stock.
We have never paid cash dividends on our capital stock and we do not anticipate paying dividends in the foreseeable future.
We have paid no cash dividends on any of our classes of capital stock to date, and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. In addition any future debt or credit facility we obtain also may preclude or limit our ability to pay any dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of potential gain for the foreseeable future.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
Our principal executive office is listed below and we believe our facility is adequate to meet our operating requirements for the foreseeable future.
| Location |
Nature of Use |
Square Feet |
How Held |
Monthly Base Rent |
Lease Expiration Date |
||||||||
| 12255 El Camino Real, Suite 250 San Diego, CA USA |
Principal executive office | 11,184 | Leased | $ | 48,650 | 1 | July 31 , 2013 | 2 | |||||
| 1 | The monthly base rent increases to $50,328 in April 2010 and is subject to an additional increase each year thereafter. In addition to base rent, we are also required to pay our proportionate share of any increase in operating expenses from 2008 levels for the office park in which our space is located. |
| 2 | The lease contains an option for one five-year lease renewal. |
24
Table of Contents
| ITEM | 3. LEGAL PROCEEDINGS |
From time to time, we may become involved in various investigations, claims and legal proceedings that arise in the ordinary course of our business. These matters may relate to intellectual property, employment, tax, regulation, contract or other matters. The resolution of these matters as they arise will be subject to various uncertainties and, even if such claims are without merit, could result in the expenditure of significant financial and managerial resources.
As of March 16, 2010, neither Cardium nor its subsidiaries were a party to any material pending legal proceeding nor was any of their property the subject of any material pending legal proceeding. In the course of our business, however, we could become engaged in various intellectual property, product-related and other matters in connection with the technology we develop or license and the products we develop or sell. To the extent we are not successful in defending against any adverse claims concerning our technology, we could be compelled to seek licenses from one or more third parties who could be direct or indirect competitors and who might not make licenses available on terms that we find commercially reasonable or at all. In addition, any such proceedings, even if decided in our favor, involve lengthy processes, are subject to appeals, and typically result in substantial costs and diversion of resources. In the course of our business, we are also routinely involved in proceedings such as disputes involving goods or services provided by various third parties to Cardium or its subsidiaries, which we do not consider likely to be material to Cardium, but which can nevertheless result in costs and diversions of resources to pursue.
ITEM 4. (REMOVED AND RESERVED)
25
Table of Contents
| ITEM 5. | MARKET FOR OUR COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock trades on NYSE Amex under the symbol “CXM.” Below are the high and low sale prices of our common stock as reported by the NYSE Amex for each quarter of the years ended December 31, 2009 and 2008:
| 2009 | 2008 | |||||||||||
| High | Low | High | Low | |||||||||
| First Quarter |
$ | 1.63 | $ | 0.40 | $ | 2.63 | $ | 2.10 | ||||
| Second Quarter |
$ | 2.90 | $ | 1.31 | $ | 2.50 | $ | 2.03 | ||||
| Third Quarter |
$ | 2.05 | $ | 1.61 | $ | 2.24 | $ | 1.65 | ||||
| Fourth Quarter |
$ | 1.96 | $ | 0.51 | $ | 1.70 | $ | 0.48 | ||||
Holders
As of March 11, 2010 there were approximately 119 stockholders of record of our common stock. Based on information we receive from brokerage firms in connection with proxy solicitations, we believe that there are approximately 2,800 beneficial owners of our common stock.
Dividends
We have not declared or paid any cash dividends on our common stock and we do not intend to declare or pay a dividend in the foreseeable future, as we are in our development stage and expect to sustain losses over the next several years. To the extent we do have earnings, we intend to retain any earnings to help provide funds for the development of our product candidates, the implementation of our business strategy and for our future growth.
Recent Sales of Unregistered Securities
During the years ended December 31, 2009, 2008 and 2007, we did not sell any unregistered securities.
Repurchases
During the year ended December 31, 2009, we did not repurchase any shares of our common stock, nor were any repurchases made on our behalf.
26
Table of Contents
Performance Graph
The graph below provides a comparison of cumulative total returns for our common stock, the Nasdaq Composite Index, the Nasdaq Biotechnology Index, and the RDG MicroCap Biotechnology Index for the five year period ended December 31, 2009. Please note that the information used to calculate the returns for periods before December 2005 is based on the share price of Aries Ventures Inc. On October 20, 2005, Cardium completed a reverse merger whereby it merged with a wholly-owned subsidiary of Aries. Thereafter, Aries was merged with and into Cardium with Cardium as the surviving entity and successor issuer to Aries. For the period from December 31, 2002 until October 20, 2005, Aries had no business operations. The graph below assumes an investment of $100 on December 31, 2004 in each of our common stock, and the stock comprising each of the indices shown. Each of the indices assumes that all dividends were reinvested. The graph lines merely connect the prices on the dates indicated and do not reflect fluctuations between those dates.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN
Among Cardium Therapeutics, The NASDAQ Composite Index,
The NASDAQ Biotechnology Index And The RDG MicroCap Biotechnology Index
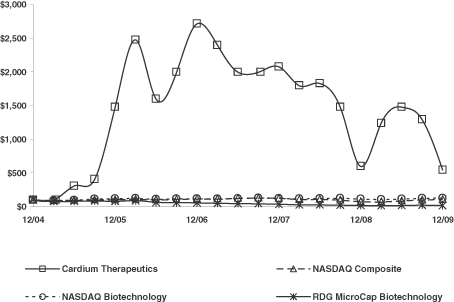
The stock performance shown above is not indicative of future performance.
The performance information above is not deemed to be filed with the SEC or subject to the liabilities of Section 18 of the Securities Exchange Act of 1934, as amended, and shall not be deemed incorporated by reference by any general statement incorporating by reference this report into any filing with the SEC, except to the extent we specifically incorporate this information by reference.
ITEM 6. SELECTED FINANCIAL DATA
The following tables contain certain financial information about the Company, including its subsidiaries. You should review this information together with our audited consolidated financial statements and the notes to the consolidated financial statements included under Item 8 in this report. Our future financial condition and results of operations will vary from our historical financial information below based on a variety of factors. You should carefully review the risks described under Items 1A and 7A and elsewhere in this report, which identify certain important factors that could cause our future financial condition and results of operations to vary from historical or anticipated results.
27
Table of Contents
Annual Financial Data
| Years Ended December 31, | ||||||||||||||||||||
| 2009(1) | 2008(1) | 2007(1) | 2006(1) | 2005 | ||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||
| Revenues |
$ | 444,946 | $ | 416,912 | $ | 445,958 | $ | 71,225 | $ | — | ||||||||||
| Loss from continuing operations |
$ | (16,158,417 | ) | $ | (17,601,990 | ) | $ | (16,885,355 | ) | $ | (13,395,064 | ) | $ | (5,588,288 | ) | |||||
| Loss from discontinued operation |
$ | (1,930,636 | ) | $ | (6,996,068 | ) | $ | (8,436,415 | ) | $ | (5,198,101 | ) | $ | — | ||||||
| Gain on sale of discontinued operation |
$ | (6,408,603 | ) | $ | — | $ | — | $ | — | $ | — | |||||||||
| Net loss |
$ | (11,680,450 | ) | $ | (24,598,058 | ) | $ | (25,321,770 | ) | $ | (18,593,165 | ) | $ | (5,441,694 | ) | |||||
| Net loss per common share—basic and diluted |
||||||||||||||||||||
| Net loss from continuing operations |
$ | (0.33 | ) | $ | (0.39 | ) | $ | (0.43 | ) | $ | (0.43 | ) | $ | (0.54 | ) | |||||
| Net income (loss) from discontinued operation |
$ | 0.09 | $ | (0.16 | ) | $ | (0.21 | ) | $ | (0.16 | ) | $ | — | |||||||
| Net loss per common share—basic and diluted |
$ | (0.24 | ) | $ | (0.55 | ) | $ | (0.64 | ) | $ | (0.59 | ) | $ | (0.54 | ) | |||||
| Weighted average shares outstanding—basic and diluted |
48,976,917 | 44,978,169 | 39,311,359 | 31,308,650 | 9,992,426 | |||||||||||||||
| (1) | On July 24, 2009 we sold our Innercool business unit. Results of the Innercool business unit are included as a discontinued operation for all periods presented. |
| Years Ended December 31, | |||||||||||||||||
| 2009 | 2008 | 2007 | 2006 | 2005 | |||||||||||||
| Consolidated Balance Sheets Data: |
|||||||||||||||||
| Current assets, net of current liabilities |
$ | (3,363,730 | ) | $ | (6,814,721 | ) | $ | 4,592,268 | $ | 4,754,127 | $ | 21,344,443 | |||||
| Total assets |
$ | 5,475,664 | $ | 10,296,921 | $ | 16,925,689 | $ | 14,117,423 | $ | 22,351,624 | |||||||
| Long-term liabilities, less current portion |
$ | 190,114 | $ | 195,315 | $ | 3,241,992 | $ | — | $ | — | |||||||
| Total stockholder’s (deficiency) equity |
$ | (2,154,575 | ) | $ | (754,756 | ) | $ | 8,428,305 | $ | 11,153,355 | $ | 21,738,116 | |||||
Quarterly Consolidated Financial Data—Unaudited
| Year Ended December 31, 2009(1) |
First Quarter | Second Quarter |
Third Quarter |
Fourth Quarter |
||||||||||||
| Revenues |
$ | 18,636 | $ | 6,996 | $ | 235,917 | $ | 183,397 | ||||||||
| (Loss) income from operations |
$ | (2,513,248 | ) | $ | (2,322,568 | ) | $ | (2,250,357 | ) | $ | (1,626,761 | ) | ||||
| (Loss) income from continuing operations |
$ | (13,743,176 | ) | $ | (10,110,163 | ) | $ | (1,621,205 | ) | $ | 9,316,127 | |||||
| (Loss) income from discontinued operation |
$ | (993,701 | ) | $ | (1,032,511 | ) | $ | 95,576 | $ | — | ||||||
| Gain on sale of discontinued operation |
$ | — | $ | — | $ | 6,408,603 | $ | — | ||||||||
| Net (loss) income |
$ | (14,736,877 | ) | $ | (11,142,674 | ) | $ | 4,882,974 | $ | 9,316,127 | ||||||
| Net (loss) income per common share—basic |
$ | (0.31 | ) | $ | (0.24 | ) | $ | 0.10 | $ | 0.17 | ||||||
| Net (loss) income per common share—diluted |
$ | (0.31 | ) | $ | (0.24 | ) | $ | 0.10 | $ | 0.17 | ||||||
| Weighted average shares outstanding—basic |
46,960,439 | 46,931,134 | 47,771,609 | 54,207,761 | ||||||||||||
| Weighted average shares outstanding—diluted |
46,960,439 | 46,931,134 | 49,620,079 | 54,374,522 | ||||||||||||
28
Table of Contents
| Year Ended December 31, 2008(1) |
First Quarter | Second Quarter |
Third Quarter | Fourth Quarter |
||||||||||||
| Revenues |
$ | 112,203 | $ | 262,430 | $ | — | $ | 42,279 | ||||||||
| Loss from operations |
$ | (4,666,040 | ) | $ | (4,639,798 | ) | $ | (4,290,914 | ) | $ | (3,557,612 | ) | ||||
| Loss from continuing operations |
$ | (4,593,851 | ) | $ | (4,624,351 | ) | $ | (4,282,886 | ) | $ | (4,100,902 | ) | ||||
| Loss from discontinued operation |
$ | (2,140,277 | ) | $ | (2,010,111 | ) | $ | (1,868,629 | ) | $ | (977,051 | ) | ||||
| Net loss |
$ | (6,734,128 | ) | $ | (6,634,462 | ) | $ | (6,151,515 | ) | $ | (5,077,953 | ) | ||||
| Net loss per common share—basic and diluted |
$ | (0.17 | ) | $ | (0.15 | ) | $ | (0.13 | ) | $ | (0.11 | ) | ||||
| Weighted average shares outstanding—basic and diluted |
40,709,247 | 43,629,975 | 46,603,700 | 46,930,439 | ||||||||||||
| (1) | On July 24, 2009 we sold our Innercool business unit. Results of the Innercool business unit are included as a discontinued operation for all periods presented. |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION |
The following discussion and analysis is intended to help you understand our financial condition and results of operations for the last three years ended December 31, 2009. You should read the following discussion and analysis together with our audited consolidated financial statements and the notes to the consolidated financial statements included under Item 8 in this report. Our future financial condition and results of operations will vary from our historical financial condition and results of operations described below based on a variety of factors. You should carefully review the risks described under Item 1A and 7A and elsewhere in this report, which identify certain important factors that could cause our future financial condition and results of operations to vary from historical or anticipated results.
Executive Overview
The following overview does not address all of the matters covered in the other sections of this Item 7 or other items in this report or contain all of the information that may be important to our stockholders or the investing public. This overview should be read in conjunction with the other sections of this Item 7 and this report.
We are a medical technology company primarily focused on the development and commercialization of novel therapeutics and medical devices for cardiovascular and ischemic disease, wound healing and tissue repair. Since we were initially funded in October 2005, we have made three strategic acquisitions and assembled a portfolio of innovative late-stage cardiovascular and regenerative medicine product candidates. We have established a pipeline of innovative products that are divided into two operating units, Cardium Biologics and the Tissue Repair Company. We report our operations in a single operating segment.
Our business is focused on the acquisition and strategic development of product opportunities or businesses having the potential to address significant unmet medical needs, and definable pathways to commercialization, partnering or other monetization following the achievement of corresponding development objectives. Consistent with our overall business strategy, as our product opportunities and businesses are advanced and corresponding valuations established, we intend to consider various corporate development transactions designed to place our product candidates into larger organizations or with partners having existing commercialization, sales and marketing resources, and a need for innovative products. Such transactions could involve the sale, partnering or other monetization of particular product opportunities or businesses.
Since December 31, 2008, we (i) completed the sale of Innercool Therapies to Royal Philips Electronics, (ii) completed Tissue Repair Company’s Matrix 2b clinical trial, (iii) submitted an FDA 510(k) application for the use of ExcellagenTM in the potential treatment of diabetic and other chronic wounds, and (iv) announced the Company’s new Orthobiologics initiative, designed to build on and extend the underlying technology developed by the Tissue Repair Company to hard tissue application such as bone.
29
Table of Contents
Following the sale of our Innercool Therapies business, we do not currently have any products available for sale or use. Because of the limited nature of our revenues and the high costs we must incur to develop our product candidates, we have yet to generate positive cash flows or income from operations and do not anticipate doing so in the foreseeable future. As a result, we are currently dependent on debt and equity funding to finance our operations. During the second half of 2009 we raised net proceeds of $9.7 million from the sale of common stock and warrants in two registered direct offerings. After year ended December 31, 2009, we raised additional net proceeds of approximately $10.4 from an additional registered direct offering of common stock and warrants.
More detailed information about our products, product candidates, our intended efforts to develop our products and our business strategy is included under Item 1 of this report.
Critical Accounting Policies and Estimates
Our consolidated financial statements included under Item 8 in this report have been prepared in accordance with accounting principles generally accepted in the United States of America (GAAP). The preparation of our financial statements in accordance with GAAP requires that we make estimates and assumptions that affect the amounts reported in our financial statements and their accompanying notes. We have identified certain policies such as derivative liabilities and stock option compensation expense that are calculated using the Black-Scholes Option Model that we believe are important to the portrayal of our financial condition and results of operations. For the year ended December 31, 2009 expenses related to derivative liabilities were calculated at $4,563,434 and expenses for stock based compensation was $909,142. These policies require the application of significant judgment by our management. We base our estimates on our historical experience, industry standards, and various other assumptions that we believe are reasonable under the circumstances. Actual results could differ from these estimates under different assumptions or conditions. An adverse effect on our financial condition, changes in financial condition, and results of operations could occur if circumstances change that alter the various assumptions or conditions used in such estimates or assumptions. If we were to undervalue of derivative liabilities or stock option compensation expense we would understate the expense recognized in our consolidated statements of operation. Conversely if we were to overvalue or derivative liabilities and stock option compensation expenses we would overstate the expense recognized in our consolidated statements of operations. Our significant accounting policies are described in the notes to our financial statements.
Results of Operations
Fiscal 2009 Compared to Fiscal 2008
Tissue Repair Company generated $444,946 in grant revenue during the year ended December 31, 2009, compared to $416,912, for the year ended December 31, 2008. The $28,034 increase was directly attributable to the level of activity expended on the grant until November 2009 when the grant ended.
Research and development expenses for the year ended December 31, 2009 were $4,302,298 compared to $11,041,930 for the year ended December 31, 2008. The decrease of $6,739,632 was primarily due to reductions in Generx (AWARE) Phase 3 clinical trial costs and related clinical support staff reductions as we chose to focus our resources on the MATRIX Phase 2b clinical trial, and costs related to our Excellarate (MATRIX) product candidate which substantially completed its Phase 2b clinical trial prior to fiscal year end.
For the year ended December 31, 2009, general and administrative expenses were $4,855,582 compared to $6,529,346 for the year ended December 31, 2008. The $1,673,764 decrease in general and administrative expenses was mainly due to decreases in payroll related costs, consulting fees, stock option compensation and legal fees.
We derive interest income from the investment of our available cash in various short-term obligations, such as certificates of deposit, commercial paper and money market funds. Interest income for the year ended December 31, 2009 was $12,160 compared to $102,201 for the same period last year. The $90,041 decrease in
30
Table of Contents
interest income for the year when compared to the same period last year was directly related to the decrease in cash available for investment as we used the proceeds from our equity financings and debt financing to fund operations.
Interest expense for the year ended December 31, 2009 was $6,339,523 compared to $549,827 for the year ended December 31, 2008. The $5,789,696 increase is as a result of the senior secured note financing from November 2008, senior subordinated secured note financing from March 2009 and unsecured note financing from June 2009. For the year ended December 31, 2009 interest expense consisted of $1,037,196 of interest paid or accrued, $738,893 of amortization of debt costs, and $4,563,434 of amortization of warrant value in connection with warrants issued with the debt financings.
Fiscal 2008 Compared to Fiscal 2007
Tissue Repair Company generated $416,912 in grant revenue during the year ended December 31, 2008, compared to $445,958, for the year ended December 31, 2007. The $29,046 decrease was directly attributable to the level of activity expended on the grant. During 2008 the primary focus was on the Excellarate clinical research study.
Research and development expenses for the year ended December 31, 2008 were $11,041,930 compared to $10,644,142 for the year ended December 31, 2007. The increase of $397,788 was primarily due to increased expenses in our efforts to advance our Excellarate product candidate in its Phase 2b clinical trial, offset by reductions in Generx (AWARE) Phase 3 clinical trial costs as spending in the year ended December 31, 2007 included product material and initial start-up costs not required in 2008. In addition we recorded a $500,000 reversal of research and development bonuses that were accrued at the end of 2007 and not paid. As of December 31, 2007, the Company recorded a liability for discretionary employee and officer bonuses for past performance. The payment of such bonuses was expected to take place sometime in 2008. Given the ongoing difficulties in the financial markets, which made it more challenging and expensive for companies to raise funds, the Compensation Committee of our Board of Directors felt it appropriate to forego the planned payment of these bonuses.
For the year ended December 31, 2008, selling, general and administrative expenses were $6,529,346 compared to $7,242,743 for the year ended December 31, 2007. The $713,397 decrease in selling, general and administrative expenses was mainly due to decreases in professional fees, payroll and payroll related cost with the reversal of $700,000 in administrative bonuses that were accrued at the end of 2007 and not paid, as noted above, and a significant reduction in our head count in the fourth quarter of 2008 as we moved towards strategic distribution relationships.
We derive interest income from the investment of our available cash in various short-term obligations, such as certificates of deposit, commercial paper and money market funds. Interest income for the year ended December 31, 2008 was $102,201 compared to $555,572 for the same period last year. The $453,371 decrease in interest income for the year when compared to the same period last year was directly related to the decrease in cash available for investment as we used the proceeds from our commercial credit facility, equity financings and debt financing to fund operations.
Interest expense for the year ended December 31, 2008 was $549,827. There was no interest expense for the year ended December 31, 2007. The increase was a result of our secured debt financing in November 2008.
Liquidity and Capital Resources
Liquidity
As of December 31, 2009 we had $3,363,665 in cash and cash equivalents. We did not have any accounts receivable, inventory or other assets on our balance sheet at December 31, 2009 that provided us with liquidity. Our working capital was a deficit of $3,358,458 at December 31, 2009, compared to a deficit of $1,838,048 at
31
Table of Contents
December 31, 2008. However, our working capital deficit at December 31, 2009 included $4,802,882 in derivative liabilities for warrants which we will not have to pay in cash. Excluding the effect of the derivative liabilities our working capital at December 31, 2009 would have been $1,444,444.
Net cash used in operating activities was $11,614,343 for the year ended December 31, 2009 compared to $16,613,779 for the same period last year. The decrease in net cash used in operating activities for the year ended December 31, 2009 was a result of the decrease in company wide spending as we focused on resources on the completion of near-term milestones including the MATRIX clinical study and advancement of Innercool’s portfolio of products, each of which provides partnering opportunities.
Our primary source of liquidity has been cash flows from financing activities and in particular proceeds from the sale of our common stock and our debt financing. Net cash provided by financing activities was $13,875,114 for the year ended December 31, 2009, and was primarily derived from proceeds we received from the sale of our common stock and debt financing, net of issuance costs and the sales of our Innercool business unit, offset by principal debt repayments in the amount of $10,750,000 during the year. On March 5, 2009 we completed a subordinated secured debt financing for which we received gross proceeds of approximately $3.5 million before placement agent fees and offering expenses of approximately $252,000. In June 2009 we completed an unsecured debt financing for which we received aggregate gross proceeds of approximately $750,000 before placement agent fees and offering expenses of approximately $50,000. During the third quarter of 2009 we closed the Philips transaction and received net proceeds of approximately $10.1 million (excluding $1,125,000 being held in an escrow account). In addition on September 16, 2009 we closed a securities purchase agreement for the sale of 3,000,000 shares of our common stock and 1,500,000 warrants to purchase our common stock in exchange for proceeds of approximately $4.1 million, net of issuance costs. On October 15, 2009, we sold an aggregate of 4,615,385 shares of our common stock and 3,000,000 warrants to purchase our common stock to certain institutional investors in exchange for proceeds of $5.6 million, net of issuance costs.
There was no net cash used in investing activities during the year ended December 31, 2009 compared to $1,681,604 for investing activities during the year ended December 31, 2008.
Since inception, our operations have consumed substantial amounts of cash and we have had only limited revenues. From inception (December 22, 2003) to December 31, 2009, net cash used in operating activities has been $69,994,499, net cash provided by financing activities was $77,617,899, and net cash used in investing activities has been $4,259,735.
Subsequent to the year ended December 31, 2009 we entered into a financing transaction. On March 12, 2010, we completed a registered direct offering of 2,266,998 units, which were sold to institutional and retail investors, at a price of $5.00 per unit. Each unit consisted of 10 shares of common stock and a warrant to purchase 5 shares of common stock. The common stock purchase warrants are exercisable at an exercise price of $0.64 per share, at any time after six months from the date of closing and have a term of exercise equal to five years from the initial exercise date. In the aggregate 22,669,980 shares of common stock and warrants to purchase an additional 11,334,990 shares were issued in the offering. The offering resulted in gross proceeds of $11.3 million, and net proceeds of approximately $10.4 million after deduction of offering fees and expenses.
Capital Resources
Our primary source of capital is the cash that we generate from the sale of debt or equity securities. We do not currently have any line of credit or other source of capital available to us.
We have generated significant losses from operation to date and anticipate that the negative cash flow from operations will continue for 2010. We raised in excess of $10 million in capital following the year ended December 31, 2010. We expect that capital will support our operations for at least the next twelve months, during
32
Table of Contents
which time we hope to complete a strategic licensing agreement or secure the approval and future sales of the Excellagen product family and/or other corporate transaction.
However, if we fail to enter into a significant licensing arrangement or generate sufficient product sales, we will not generate sufficient cash flows to cover our operating expenses. If needed, we intend to secure additional working capital through the sale of additional debt or equity securities. No arrangements or commitments for any such financing are in place at this time, and we cannot give any assurances about the availability or terms of any future financing.
Because of our historic net losses, and our working capital deficit, these conditions raise substantial doubt about our ability to continue as a going concern. The consolidated financial statements contained in this report do not include any adjustments related to the recoverability of assets or classifications of liabilities that might be necessary should we be unable to continue as a going concern.
Off-Balance Sheet Arrangements
As of December 31, 2009, we did not have any significant off-balance sheet debt nor did we have any transactions, arrangements, obligations (including contingent obligations) or other relationships with any unconsolidated entities or other persons that have or are reasonably likely to have a material current or future effect on financial condition, changes in financial condition, results of operations, liquidity, capital expenditures, capital resources, or significant components of revenue or expenses material to investors.
Contractual Obligations
The following table summarizes our known contractual obligations and commercial commitments at December 31, 2009:
| Payments Due By Period | |||||||||||||||
| Contractual Obligations |
Total | Less Than 1 Year |
1-3 Years | 3-5 Years | More than 5 Years | ||||||||||
| Long-Term Debt |
$ | — | $ | — | $ | — | $ | — | $ | — | |||||
| Operating Leases |
2,245,411 | 598,903 | 1,646,508 | — | — | ||||||||||
| Total Obligations |
$ | 2,245,411 | $ | 598,903 | $ | 1,646,508 | $ | — | $ | — | |||||
Recent Accounting Pronouncements
In September 2006, the FASB issued ASC topic 820 (formerly SFAS No. 157), “Fair Value Measurements,” which defines fair value, establishes a framework for measuring fair value and expands disclosure of fair value measurements. ASC topic 820 is applicable to other accounting pronouncements that require or permit fair value measurements, except those relating to lease accounting, and accordingly does not require any new fair value measurements. Our adoption of the provisions of ASC topic 820 on January 1, 2008, with respect to financial assets and liabilities measured at fair value, did not have an effect on our financial statements for the year ended December 31, 2008.
In December 2007, the FASB issued ASC topic 805 (formerly SFAS No. 141 (revised 2007), “Business Combinations.” ASC topic 805 retains the purchase method of accounting for acquisitions, but requires a number of changes, including changes in the way assets and liabilities are recognized in the purchase accounting. It also changes the recognition of assets acquired and liabilities assumed arising from contingencies, requires the capitalization of in-process research and development at fair value, and requires the expensing of acquisition-related costs as incurred. ASC topic 805 is effective for acquisitions occurring in fiscal periods beginning after December 15, 2008 and was required to be adopted by the Company in its first quarter of fiscal 2009. The adoption of ASC topic 805 could have an impact on the accounting for any future acquisition, if one were to occur.
33
Table of Contents
In December 2007, the FASB issued ASC topic 810 (formerly SFAS No. 160), “Noncontrolling Interests in Consolidated Financial Statements, an amendment of Accounting Research Bulletin No. 51, Consolidated Financial Statements” ASC topic 810 requires (i) that non-controlling (minority) interests be reported as a component of stockholders’ equity, (ii) that net income attributable to the parent and to the non-controlling interest be separately identified in the consolidated statement of operations, (iii) that changes in a parent’s ownership interest while the parent retains its controlling interest be accounted for as equity transactions, (iv) that any retained non-controlling equity investment upon the deconsolidation of a subsidiary be initially measured at fair value, and (v) that sufficient disclosures are provided that clearly identify and distinguish between the interests of the parent and the interests of the non-controlling owners. ASC topic 810 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. We adopted ASC topic 810 for our fiscal year beginning January 1, 2009, and the adoption did not have any impact on our consolidated financial position, results of operations and cash flows.
In March 2008, the FASB issued ASC topic 815, Statement of Financial Accounting Standards (formerly SFAS No. 161), “Disclosures about Derivative Instruments and Hedging Activities. ASC topic 818 changes the disclosure requirements for derivative instruments and hedging activities. Entities are required to provide enhanced disclosures about (a) how and why an entity uses derivative instruments, (b) how derivative instruments and related hedged items are accounted for under SFAS 133 and its related interpretations, and (c) how derivative instruments and related hedged items affect an entity’s financial position, financial performance and cash flows. . The adoption of this pronouncement on January 1, 3009 did not have a material impact on our consolidated financial statements.
In April 2008, the FASB issued ASC topic 815, “Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity’s Own Stock” and provided guidance on determining what types of instruments or embedded features in an instrument held by a reporting entity can be considered indexed to its own stock for the purpose of evaluating the first criteria of the scope exception and was effective for financial statements issued for fiscal years beginning after December 15, 2008. The adoption of this pronouncement had a material impact on our consolidated financial statements (See note 6).
Effective January 1, 2009 we adopted ASC topic 820 (formerly FSP FAS 157-3), “Determining the Fair Value of a Financial Asset When the Market for That Asset is Not Active” which clarifies the application in a market that is not active and provides an example to illustrate key considerations in determining the fair value of a financial asset when the market for that financial asset is not active. ASC topic 820 became effective immediately upon issuance, and its adoption did not have an effect on our financial statements. We currently determine the fair value of our property and equipment when assessing long-lived asset impairments and ASC topic 820 was effective for these fair value assessments as of January 1, 2009.
In April 2009, the FASB issued ASC topic 820 “Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly” which provides additional guidance for estimating fair value when the volume and level of activity for the asset or liability have significantly decreased. ASC topic 820 also includes guidance on identifying circumstances that indicate a transaction is not orderly and emphasizes that even if there has been a significant decrease in the volume and level of activity for the asset or liability and regardless of the valuation technique(s) used, the objective of a fair value measurement remains the same. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction (that is, not a forced liquidation or distressed sale) between market participants at the measurement date under current market conditions. ASC topic 820 was effective for interim and annual reporting periods ending after June 15, 2009, and is applied prospectively. The adoption of this pronouncement had a material impact on the Company’s consolidated financial statements.
In April 2009, the FASB issued ASC topic 825 (formerly SFAS 107), “Interim Disclosures about Fair Value of Financial Instruments,” which require disclosures about fair value of financial instruments for interim reporting periods of publicly traded companies, as well as in annual financial statements. The adoption of ASC
34
Table of Contents
topic 825 did not have a material impact on our consolidated financial position, results of operations and cash flows. The carrying value of our cash and cash equivalents approximates fair value because these instruments have original maturities of three months or less. The carrying value of our short-term debt approximates fair value because these instruments now have maturities of less than six months.
In May 2009, the FASB issued ASC topic 855 (formerly SFAS No. 165), “Subsequent Events”. The objective of ASC topic 855 is to establish general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. In particular, ASC topic 855 sets forth the period after the balance sheet date during which management should evaluate events or transactions that may occur for potential recognition or disclosure in the financial statements, the circumstances under which an entity should recognize events or transactions occurring after the balance sheet date in its financial statements, and the disclosures that an entity should make about events or transactions that occurred after the balance sheet date. The requirements of ASC topic 855 should be applied to interim or annual financial periods ending after June 15, 2009. Accordingly, we adopted ASC topic 855 in the second quarter of 2009. Management has evaluated subsequent events through the date of this report.
In June 2009, the FASB issued ASC topic 105 (formerly SFAS No. 168), “The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles establishes the FASB Accounting Standards Codification (the “Codification”) to become the source of authoritative accounting principles generally accepted in the United States of America (GAAP) recognized by the FASB to be applied by nongovernmental entities. Rules and interpretive releases of the SEC under authority of federal securities laws are also sources of authoritative GAAP for SEC registrants. On the effective date of ASC topic 105, the Codification superseded all then-existing non-SEC accounting and reporting standards. All other non-grandfathered, non-SEC accounting literature not included in the Codification will become non-authoritative. ASC topic 105 became effective for financial statements issued for interim and annual periods ending after September 15, 2009. The adoption of ASC topic 105 did not have a material impact on our consolidated financial statements and results of operations.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to a limited level of market risk, which is the potential loss arising from adverse changes in market rates and prices, such as interest rates, due to the investment of our available cash in various instruments.
The goal of our investment activities is to preserve principal while seeking to increase income received on our investments without significantly increasing risk. In the normal course of business, we employ established policies and procedures to manage our exposure to changes in the fair value of our investments. We generally do not, however, enter into derivatives or other financial instruments for trading or speculative purposes or to otherwise manage our exposure to interest rate changes. Generally, we seek to limit our exposure to risk by investing substantially in short-term, investment grade securities, such as commercial paper, certificates of deposit and money market funds. The amount of interest income we receive on our investments will vary with changes in the general level of interest rates in the United States, generally decreasing as interest rates decrease and increasing as interest rates increase.
While we cannot predict with any certainty our future exposure to fluctuations in interest rates or other market risks or the impact, if any, such fluctuations may have on our future business, consolidated financial condition, results of operations or cash flows, due to the short-term, investment grade nature of our investments, we do not believe our exposure to market risk from our investments is material.
35
Table of Contents
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee of
The Board of Directors and Stockholders of
Cardium Therapeutics, Inc. and Subsidiaries
We have audited the accompanying consolidated balance sheets of Cardium Therapeutics, Inc. and subsidiaries (the “Company”) (a development stage company) as of December 31, 2009 and 2008, and the related consolidated statements of operations, stockholders’ deficiency and cash flows for each of the years in the three-year period ended December 31, 2009 and for the period from December 22, 2003 (inception) through December 31, 2009. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Cardium Therapeutics, Inc. and subsidiaries (a development stage company) at December 31, 2009 and 2008, and the consolidated results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2009 and for the period from December 22, 2003 (date of inception) through December 31, 2009 in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 2 to the consolidated financial statements, the Company changed the manner in which it accounts for certain convertible debt and equity instruments (Note 6) effective January 1, 2009.
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As more fully described in Note 1, the Company has had recurring operating losses since its inception and must raise additional capital from external sources in order to sustain the business. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans with regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Cardium Therapeutics, Inc. and subsidiaries’ internal control over financial reporting as December 31, 2009, based on the criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report, dated March 16, 2010, expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
/s/ Marcum LLP
Marcum LLP
March 16, 2010
New York, New York
36
Table of Contents
CARDIUM THERAPEUTICS, INC. AND SUBSIDIARIES
(a development stage company)
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 3,363,665 | $ | 1,102,894 | ||||
| Accounts receivable |
115,138 | 42,279 | ||||||
| Deferred financing costs, net |
— | 432,966 | ||||||
| Prepaid expenses and other assets |
40,384 | 76,202 | ||||||
| Restricted cash |
562,500 | — | ||||||
| Current assets of business held for sale |
— | 7,363,973 | ||||||
| Total current assets |
4,081,687 | 9,018,314 | ||||||
| Restricted cash |
862,500 | 400,000 | ||||||
| Property and equipment, net |
351,539 | 746,169 | ||||||
| Deposits and other long term assets |
179,938 | 132,438 | ||||||
| Total assets |
$ | 5,475,664 | $ | 10,296,921 | ||||
| Liabilities and Stockholders’ Deficiency |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 2,300,786 | $ | 3,359,152 | ||||
| Accrued liabilities |
336,457 | 1,332,448 | ||||||
| Current liabilities of business held for sale |
— | 2,127,986 | ||||||
| Derivative liabilities—fair value of warrants |
4,802,882 | — | ||||||
| Short-term debt, net of debt discount of $1,963,224 at December 31, 2008 |
— | 4,036,776 | ||||||
| Current liabilities |
7,440,125 | 10,856,362 | ||||||
| Deferred rent |
190,114 | 195,315 | ||||||
| Total liabilities |
7,630,239 | 11,051,677 | ||||||
| Commitments and contingencies |
||||||||
| Stockholders’ deficiency: |
||||||||
| Common stock, $0.0001 par value; 200,000,000 shares authorized; issued and outstanding 55,182,174 at December 31, 2009 and 46,930,439 at December 31, 2008 |
5,518 | 4,693 | ||||||
| Additional paid-in capital |
74,065,539 | 73,199,199 | ||||||
| Deficit accumulated during development stage |
(76,225,632 | ) | (73,958,648 | ) | ||||
| Total stockholders’ deficiency |
(2,154,575 | ) | (754,756 | ) | ||||
| Total liabilities and stockholders’ deficiency |
$ | 5,475,664 | $ | 10,296,921 | ||||
See accompanying notes, which are an integral part of these consolidated financial statements.
37
Table of Contents
CARDIUM THERAPEUTICS, INC. AND SUBSIDIARIES
(a development stage company)
CONSOLIDATED STATEMENTS OF OPERATIONS
| Years Ended December 31, | Period from December 22, 2003 (Inception) to December 31, 2009 |
|||||||||||||||
| 2009 | 2008 | 2007 | ||||||||||||||
| Revenues |
||||||||||||||||
| Grant revenues |
$ | 444,946 | $ | 416,912 | $ | 445,958 | $ | 1,378,681 | ||||||||
| Operating expenses |
||||||||||||||||
| Research and development |
4,302,298 | 11,041,930 | 10,644,142 | 36,478,310 | ||||||||||||
| General and administrative |
4,855,582 | 6,529,346 | 7,242,743 | 27,911,549 | ||||||||||||
| Total operating expenses |
9,157,880 | 17,571,276 | 17,886,885 | 64,389,859 | ||||||||||||
| Loss from operations |
(8,712,934 | ) | (17,154,364 | ) | (17,440,927 | ) | (63,011,178 | ) | ||||||||
| Change in fair value of derivative liabilities |
(1,118,120 | ) | — | — | 8,519,497 | |||||||||||
| Interest income |
12,160 | 102,201 | 555,572 | 1,532,167 | ||||||||||||
| Interest expense |
(6,339,523 | ) | (549,827 | ) | — | (7,113,501 | ) | |||||||||
| Net loss from continuing operations, |
(16,158,417 | ) | (17,601,990 | ) | (16,885,355 | ) | (60,073,015 | ) | ||||||||
| Loss from discontinued operation |
(1,930,636 | ) | (6,996,068 | ) | (8,436,415 | ) | (22,561,220 | ) | ||||||||
| Gain on sale of discontinued operation |
6,408,603 | — | — | 6,408,603 | ||||||||||||
| Net Income (loss) from discontinued operation |
4,477,967 | (6,996,068 | ) | (8,436,415 | ) | (16,152,617 | ) | |||||||||
| Net loss |
$ | (11,680,450 | ) | $ | (24,598,058 | ) | $ | (25,321,770 | ) | $ | (76,225,632 | ) | ||||
| Net income (loss) per common share—basic and diluted |
||||||||||||||||
| Net loss from continuing operation |
$ | (0.33 | ) | $ | (0.39 | ) | $ | (0.43 | ) | |||||||
| Net income (loss) from discontinued operation |
0.09 | (0.16 | ) | (0.21 | ) | |||||||||||
| Net loss per common share—basic and diluted |
$ | (0.24 | ) | $ | (0.55 | ) | $ | (0.64 | ) | |||||||
| Weighted average common shares outstanding—basic and diluted |
48,976,917 | 44,978,169 | 39,311,359 | |||||||||||||
See accompanying notes, which are an integral part of these consolidated financial statements.
38
Table of Contents
CARDIUM THERAPEUTICS, INC. AND SUBSIDIARIES
(a development stage company)
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIENCY)
YEARS ENDED DECEMBER 31, 2009, 2008 AND 2007
| Common Stock | Additional Paid-In Capital |
Deficit Accumulated During Development Stage |
Total Stockholders’ Equity (Deficiency) |
||||||||||||||
| Shares | Amount | ||||||||||||||||
| Balance—December 31, 2006 |
32,190,804 | 3,218 | 35,188,957 | (24,038,820 | ) | 11,153,355 | |||||||||||
| Issuance of common stock for cash (March 9, 2007; $2.50 per share) |
8,636,000 | 875 | 20,092,489 | — | 20,093,364 | ||||||||||||
| Stock option compensation expense |
— | — | 2,329,440 | — | 2,329,440 | ||||||||||||
| Exercise of warrants ($1.50 per share) |
128,487 | 2 | 26,274 | 26,276 | |||||||||||||
| Warrants issued with debt (November 12, 2007) |
— | — | 147,640 | — | 147,640 | ||||||||||||
| Net Loss |
— | — | — | (25,321,770 | ) | (25,321,770 | ) | ||||||||||
| Balance—December 31, 2007 |
40,955,291 | 4,095 | 57,784,800 | (49,360,590 | ) | 8,428,305 | |||||||||||
| Issuance of common stock for cash (January 31, 2008; $2.00 per share) |
2,655,000 | 266 | 4,907,368 | — | 4,907,634 | ||||||||||||
| Issuance of common stock for cash (June 30, 2008; $2.00 per share) |
1,625,000 | 163 | 3,010,297 | — | 3,010,460 | ||||||||||||
| Issuance of common stock for cash (July 18, 2008; $2.00 per share) |
1,670,000 | 167 | 3,009,646 | — | 3,009,813 | ||||||||||||
| Stock option compensation expense |
— | — | 1,983,189 | — | 1,983,189 | ||||||||||||
| Exercise of warrants ($1.50 per share) |
25,148 | 2 | 22,499 | — | 22,501 | ||||||||||||
| Warrants issued in exchange for services and fees |
— | — | 162,382 | — | 162,382 | ||||||||||||
| Warrants issued with debt (November 5, 2008) |
— | — | 2,319,018 | — | 2,319,018 | ||||||||||||
| Net Loss |
— | — | — | (24,598,058 | ) | (24,598,058 | ) | ||||||||||
| Balance—December 31, 2008 |
46,930,439 | $ | 4,693 | $ | 73,199,199 | $ | (73,958,648 | ) | $ | (754,756 | ) | ||||||
| Cumulative effect of change in accounting principle (see note 6) |
— | — | (12,982,785 | ) | 9,413,466 | (3,569,319 | ) | ||||||||||
| Balance—January 1, as adjusted |
46,930,439 | $ | 4,693 | $ | 60,216,414 | $ | (64,545,182 | ) | $ | (4,324,075 | ) | ||||||
| Stock option compensation expense |
— | — | 909,142 | — | 909,142 | ||||||||||||
| Reclassification of derivative liabilities that no longer contain price protection provisions |
— | — | 2,538,793 | — | 2,538,793 | ||||||||||||
| Exercise of stock options and warrants |
636,350 | 64 | 711,009 | 711,073 | |||||||||||||
| Issuance of common stock for cash (September 16, 2009; $1.50 per share) |
3,000,000 | 300 | 4,129,971 | — | 4,130,271 | ||||||||||||
| Issuance of common stock for cash (October 15, 2009; $1.30 per share) |
4,615,385 | 461 | 5,560,210 | — | 5,560,671 | ||||||||||||
| Net Loss |
— | — | — | (11,680,450 | ) | (11,680,450 | ) | ||||||||||
| Balance—December 31, 2009 |
55,182,174 | $ | 5,518 | $ | 74,065,539 | $ | (76,225,632 | ) | $ | (2,154,575 | ) | ||||||
See accompanying notes, which are an integral part of these consolidated financial statements.
39
Table of Contents
CARDIUM THERAPEUTICS, INC. AND SUBSIDIARIES
(a development stage company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | Period from December 22, 2003 (Inception) To December 31, 2009 |
|||||||||||||||
| 2009 | 2008 | 2007 | ||||||||||||||
| Cash Flows From Operating Activities |
||||||||||||||||
| Net loss |
$ | (11,680,450 | ) | $ | (24,598,058 | ) | $ | (25,321,770 | ) | $ | (76,225,632 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||||||
| Gain on sale of discontinued operation |
(6,408,603 | ) | — | — | (6,408,603 | ) | ||||||||||
| Loss on abandonment of leaseholds |
135,344 | — | — | 135,344 | ||||||||||||
| Depreciation |
476,792 | 626,171 | 367,881 | 1,730,531 | ||||||||||||
| Amortization—intangibles |
443,651 | 789,656 | 789,656 | 2,696,193 | ||||||||||||
| Amortization—debt discount |
4,563,434 | 495,233 | 8,202 | 5,291,019 | ||||||||||||
| Amortization—deferred financing costs |
738,893 | 180,938 | 6,028 | 925,859 | ||||||||||||
| Provision for obsolete inventory |
— | (450,881 | ) | 50,402 | 200,000 | |||||||||||
| Provision for doubtful accounts |
— | (5,936 | ) | 5,936 | — | |||||||||||
| Change in fair value of warrants |
1,118,120 | — | — | (8,519,497 | ) | |||||||||||
| Common stock and warrants issued for services and reimbursement of expenses |
— | 162,382 | — | 203,882 | ||||||||||||
| Stock based compensation expense |
909,142 | 1,983,189 | 2,329,440 | 6,856,578 | ||||||||||||
| In-process purchased technology |
— | 1,000,000 | — | 2,027,529 | ||||||||||||
| Changes in operating assets and liabilities, excluding effects of acquisition: |
||||||||||||||||
| Accounts receivable |
83,372 | 275,433 | (295,959 | ) | (36,150 | ) | ||||||||||
| Inventories |
297,562 | (512,340 | ) | (230,532 | ) | (1,806,159 | ) | |||||||||
| Prepaid expenses and other assets |
37,759 | 276,620 | 168,547 | (152,974 | ) | |||||||||||
| Deposits |
(47,500 | ) | (77,780 | ) | (42,796 | ) | (193,380 | ) | ||||||||
| Accounts payable |
(1,259,383 | ) | 3,283,863 | 472,437 | 3,437,508 | |||||||||||
| Accrued liabilities |
(1,017,275 | ) | (237,584 | ) | 336,802 | (346,661 | ) | |||||||||
| Deferred rent |
(5,201 | ) | 195,315 | 190,114 | ||||||||||||
| Net cash used in operating activities |
(11,614,343 | ) | (16,613,779 | ) | (21,355,726 | ) | (69,994,499 | ) | ||||||||
| Cash Flows From Investing Activities |
||||||||||||||||
| In-process technology purchased from Tissue Repair Company |
— | (1,000,000 | ) | — | (1,500,000 | ) | ||||||||||
| Purchases of property and equipment |
— | (681,604 | ) | (1,227,236 | ) | (2,759,735 | ) | |||||||||
| Net cash used in investing activities |
— | (1,681,604 | ) | (1,227,236 | ) | (4,259,735 | ) | |||||||||
| Cash Flows From Financing Activities |
||||||||||||||||
| Proceeds from officer loan |
— | — | — | 62,882 | ||||||||||||
| Cash acquired in Aries merger and Innercool acquisition |
— | — | — | 1,551,800 | ||||||||||||
| Restricted cash – collateral for letter of credit |
100,000 | 100,000 | (500,000 | ) | (300,000 | ) | ||||||||||
| Restricted cash – proceeds placed in escrow from sale of discontinued operation |
(1,125,000 | ) | — | — | (1,125,000 | ) | ||||||||||
| Proceeds from the exercise of warrants, net |
711,073 | 22,501 | 26,276 | 1,258,448 | ||||||||||||
| Proceeds from debt financing agreement, net of deferred financing costs of $251,901 at December 31, 2009, $511,432 at December 31, 2008 and $108,500 at December 31, 2007 |
3,998,099 | 5,488,568 | 4,891,500 | 14,378,167 | ||||||||||||
| Proceeds from the sale business unit |
11,250,000 | — | — | 11,250,000 | ||||||||||||
| Repayment of debt |
(10,750,000 | ) | (4,863,515 | ) | (136,485 | ) | (15,750,000 | ) | ||||||||
| Proceeds from the sale of common stock, net of issuance cost |
9,690,942 | 10,927,907 | 20,093,364 | 66,291,602 | ||||||||||||
| Net cash provided by financing activities |
13,875,114 | 11,675,461 | 24,374,655 | 77,617,899 | ||||||||||||
| Net (decrease) increase in cash |
2,260,771 | (6,619,922 | ) | 1,791,693 | 3,363,665 | |||||||||||
| Cash and cash equivalents at beginning of period |
1,102,894 | 7,722,816 | 5,931,123 | — | ||||||||||||
| Cash and cash equivalents at end of period |
$ | 3,363,665 | $ | 1,102,894 | $ | 7,722,816 | $ | 3,363,665 | ||||||||
| Supplemental Disclosures of Cash Flow Information: |
||||||||||||||||
| Cash paid for interest |
$ | 1,023,990 | $ | 282,575 | $ | 73,731 | $ | 1,380,296 | ||||||||
| Cash paid for income taxes |
$ | 2,400 | $ | 2,400 | $ | 9,284 | $ | 22,162 | ||||||||
| Non-Cash Activity: |
||||||||||||||||
| Subscription receivable for common shares |
$ | — | $ | — | $ | — | $ | 17,000 | ||||||||
| Common stock issued for repayment of loans |
$ | — | $ | — | $ | — | $ | 62,882 | ||||||||
| Common stock and warrants issued for services and reimbursement of expenses |
$ | — | $ | 162,382 | $ | — | $ | 203,882 | ||||||||
| Net assets acquired for the issuance of common stock (exclusive of cash acquired) |
$ | — | $ | — | $ | — | $ | 5,824,000 | ||||||||
| Warrants issued with debt |
$ | 1,417,406 | $ | — | $ | — | $ | 15,861,172 | ||||||||
| Reclassification of derivative liabilities with expired price protection provisions |
$ | (2,538,793 | ) | $ | — | $ | — | $ | (2,538,793 | ) | ||||||
| Issuance of note for accrued milestone payment |
$ | 500,000 | $ | — | $ | — | $ | 500,000 | ||||||||
See accompanying notes, which are an integral part of these consolidated financial statements.
40
Table of Contents
CARDIUM THERAPEUTICS, INC. AND SUBSIDIAIRES
(a development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1—Organization and Liquidity
Organization
Cardium Therapeutics, Inc. (the “Company,” “Cardium,” “we,” “our” and “us”) was organized in Delaware in December 2003. Cardium’s business is focused on the acquisition and strategic development of product opportunities or businesses having the potential to address significant unmet medical needs, and definable pathways to commercialization, partnering or other monetization following the achievement of corresponding development objectives. In October 2005, we acquired a portfolio of biologic growth factors and related delivery techniques from the Schering AG Group (now part of Bayer AG) for potential use in treating ischemic and other cardiovascular conditions. In March 2006, we acquired the technologies and products of Innercool Therapies, Inc., a medical technology company in the emerging field of therapeutic hypothermia, or patient temperature modulation, whose systems and products are designed to rapidly and controllably cool the body to reduce cell death and damage following acute ischemic events such as cardiac arrest and stroke, and to potentially lessen or prevent associated injuries such as adverse neurologic outcomes. In August 2006, we acquired rights to assets and technologies of Tissue Repair Company, a company focused on the development of growth factor therapeutics for the potential treatment of tissue wounds such as chronic diabetic wounds, and whose product candidate, ExcellarateTM is initially being developed as a single administration for the treatment of non-healing, neuropathic diabetic foot ulcers. Innercool Therapies and Tissue Repair Company are each operated as a wholly-owned subsidiary of Cardium.
On July 24, 2009, we closed a transaction for the sale of Cardium’s InnerCool Therapies business to Philips Electronics North America Corporation for $11.25 million, of which $1,125,000 is held in escrow as security for certain indemnification obligations, as well as the transfer of approximately $1.5 million in trade payables (the “Philips Transaction”). The operations of Innercool are presented as a discontinued operation in our consolidated statements of operations. After the closing, the name of Innercool Therapies, Inc. was changed to Post-Hypothermia Corporation.
We are a development stage company. We have yet to generate positive cash flows from operations, and are essentially dependent on debt and equity funding to finance our operations. In October 2005, we closed a private placement of 19,325,651 shares of our common stock at a purchase price of $1.50 per share and received net proceeds of $25,542,389. In connection with the private placement, we completed a reverse merger, whereby Cardium merged with a wholly-owned subsidiary of Aries Ventures Inc. (“Aries”), a publicly-traded company. As a result of these transactions, the stockholders of Cardium became the controlling stockholders of Aries. Accordingly, the acquisition of Cardium by Aries was a reverse merger. The historical financial results before the reverse merger on October 20, 2005, are those of Cardium. Aries’ results of operations are included in Cardium’s financial results beginning October 20, 2005.
In January 2006, Aries was merged with and into Cardium, with Cardium as the surviving entity and the successor issuer to Aries. As a result, we are now in our present form a publicly-traded, Delaware corporation named Cardium Therapeutics, Inc.
Liquidity
As of December 31, 2009 we had $3,363,665 in cash and cash equivalents. Our working capital was a deficit of $3,358,458 at December 31, 2009, which included $4,802,882 in derivative liabilities for warrants.
Net cash used in operating activities was $11,614,343 for the year ended December 31, 2009 compared to $16,613,779 for the same period last year. The decrease in net cash used in operating activities for the year ended
41
Table of Contents
December 31, 2009 was a result of the decrease in company wide spending as we focused on resources on the completion of near-term milestones including the MATRIX clinical study and advancement of Innercool’s portfolio of products, each of which provides partnering opportunities.
Our primary source of liquidity has been cash flows from financing activities and in particular proceeds from the sale of our common stock and our debt financing. Net cash provided by financing activities was $13,875,114 for the year ended December 31, 2009, and was primarily derived from proceeds we received from the sale of our common stock and debt financing, net of issuance costs and the sales of our Innercool business unit, offset by principal debt repayments in the amount of $10,750,000 during the year. On March 5, 2009 we completed a subordinated secured debt financing for which we received gross proceeds of approximately $3.5 million before placement agent fees and offering expenses of approximately $252,000. In June 2009 we completed an unsecured debt financing for which we received aggregate gross proceeds of approximately $750,000 before placement agent fees and offering expenses of approximately $50,000. During the third quarter of 2009 we closed the Philips transaction and received net proceeds of approximately $10.1 million (excluding $1,125,000 being held in an escrow account). In addition on September 16, 2009 we closed a securities purchase agreement for the sale of 3,000,000 shares of our common stock and 1,500,000 warrants to purchase our common stock in exchange for proceeds of approximately $4.1 million, net of issuance costs. On October 15, 2009, we sold an aggregate of 4,615,385 shares of our common stock and 3,000,000 warrants to purchase our common stock to certain institutional investors in exchange for proceeds of $5.6 million, net of issuance costs.
There was no net cash used in investing activities during the year ended December 31, 2009 compared to $1,681,604 for investing activities during the year ended December 31, 2008.
Since inception, our operations have consumed substantial amounts of cash and we have had only limited revenues. From inception (December 22, 2003) to December 31, 2009, net cash used in operating activities has been $69,994,499, net cash provided by financing activities was $77,617,899, and net cash used in investing activities has been $4,259,735.
Subsequent to the year ended December 31, 2009 we entered into a financing transaction. On March 12, 2010, the Company completed a registered direct offering of 2,266,998 units, which were sold to institutional and retail investors, at a price of $5.00 per unit. Each unit consisted of 10 shares of common stock and a warrant to purchase 5 shares of common stock. The common stock purchase warrants are exercisable at an exercise price of $0.64 per share, at any time after six months from the date of closing and have a term of exercise equal to five years from the initial exercise date. In the aggregate 22,669,980 shares of common stock and warrants to purchase an additional 11,334,990 shares were issued in the offering. The offering resulted in gross proceeds to the Company of $11.3 million, and net proceeds of approximately $10.4 million after deduction of offering fees and expenses.
Our primary source of capital is the cash that we generate from the sale of debt or equity securities. We do not currently have any line of credit or other source of capital available to us.
We have generated significant losses from operation to date and anticipate that the negative cash flow from operations will continue for 2010. We raised in excess of $10 million in capital following the year ended December 31, 2010. We expect that capital will support our operations for at least the next twelve months, during which time we hope to complete a strategic licensing agreement or secure the approval and future sales of the Excellagen product family and/or other corporate transaction.
However, if we fail to enter into a significant licensing arrangement or generate sufficient product sales, we will not generate sufficient cash flows to cover our operating expenses. If needed, we intend to secure additional working capital through the sale of additional debt or equity securities. No arrangements or commitments for any such financing are in place at this time, and we cannot give any assurances about the availability or terms of any future financing.
42
Table of Contents
Because of our historic net losses, and our working capital deficit, these conditions raise substantial doubt about our ability to continue as a going concern. The consolidated financial statements do not include any adjustments related to the recoverability of assets or classifications of liabilities that might be necessary should we be unable to continue as a going concern.
NOTE 2—Summary of Significant Accounting Policies
Basis of Presentation
Our principal activities are expected to focus on the commercialization of our licensed technologies, other technologies and the expansion of our existing products. The accompanying financial statements have been prepared in accordance with ACS Topic 915 (formerly Statement of Financial Accounting Standard No. 7), “Development Stage Enterprises.”
FDIC Insured Limits
Financial instruments that subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents. The Company maintains all of its cash and cash equivalents in two financial institutions, although substantially all of the balance is within one institution. The Company performs periodic evaluations of the relative credit standing of the institutions. The cash balances are insured by either the Federal Deposit Insurance Corporation (FDIC), up to $250,000 per depositor currently set to expire on December 31, 2013, at which time the limit will be reduced to $100,000 per depositor. The Company’s bank, City National Bank is participating in the FDIC’s Transaction Guarantee Program. Through June 30, 2010 all non interest-bearing transaction accounts are fully guaranteed by the FDIC for the entire amount in the account. The Company’s cash balances on deposit with these two financial institutions at December 31, 2009 exceeded the FDIC limits by $167,000.
Fair Value of Financial Instruments
The carrying amounts of cash and cash equivalents, accounts receivable, accounts payable, and accrued liabilities approximate fair value due to the short term maturities of these instruments.
Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Principles of Consolidation
The accompanying consolidated financial statements include the financial statements of Cardium and its wholly-owned subsidiaries, Post-Hypothermia Corporation (a business that is presented as a discontinued operation as described in Note 3) and Tissue Repair Company. All inter-company balances and transactions have been eliminated in consolidation.
43
Table of Contents
Restricted Cash
We have a total of $400,000 invested in a certificate of deposit that serves as collateral for an outstanding letter of credit, and is therefore restricted. The letter of credit is a security deposit towards tenant improvements for the Company’s office space and is reduced by $100,000 every year on March 1. Therefore, $300,000 is classified as non-current restricted cash and $100,000 is classified as a cash equivalent. In addition under the terms of the Asset Purchase Agreement with Philips, we deposited $1,125,000 of the proceeds from the sale into an escrow account to act as security for our indemnification obligations that may arise. At December 31, 2009 half of the escrow amount is scheduled to be released in July 2010, therefore $562,500 is shown in current assets on our balance sheet. The remaining half is scheduled to be released in January 2011.
Accounts Receivable
Accounts receivable represent amounts due for the services provided under a grant from the National Institute of Health (“NIH”). Because of the nature of the grant the Company provides no allowances against this receivable. If circumstances were to change the Company would reevaluate the need for an allowance for doubtful accounts. There was no allowance for doubtful accounts at December 31, 2009 or 2008.
Deferred Financing Costs
The costs incurred in connection with the issuance of certain indebtedness were capitalized as deferred costs and were amortized over the term of the related indebtedness as a financing cost over the period of future benefit. The Company incurred related amortization expense of $738,893, $180,938 and $6,028 for the years ended December 31, 2009, 2008 and 2007, respectively. Amortization of the deferred costs are included as interest expense in the accompanying consolidated statements of operations for the period December 22, 2003 (inception) to December 31, 2009 amortization expense was $925,859.
Accounting for Debt/Proceeds Allocations
In accordance with ASC Topic 740 (formerly Accounting Principles Board Opinion No. 14) “Accounting for Convertible Debt and Debt Issued with Stock Purchase Warrants,” the Company recorded a $5,291,019 discount on notes payable based on the relative fair values of the notes and the common stock purchase warrants issued in connection with the notes and is amortizing the discount over the terms of the notes. The Company incurred related amortization expense of the discount on the notes of $4,563,434, $495,233 and $8,202 for the years ended December 31, 2009, 2008 and 2007, respectively. Amortization of the debt discount is included as interest expense in the accompanying consolidated statements of operations. For the period December 22, 2003 (inception) to December 31, 2009 amortization expense was $5,291,019.
Long-Lived Assets
The Company adopted ASC Topic 360 (formerly SFAS No. 144) “Accounting for the Impairment or Disposal of Long-Lived Assets.” Long-lived assets held for use are subject to an impairment assessment if the carrying value is no longer recoverable based upon the undiscounted cash flows of the assets. The amount of the impairment is the difference between the carrying amount and the fair value of the asset. Management does not believe there was any impairment of long-lived assets at December 31, 2009.
Property and Equipment
Property and equipment are stated at cost, net of accumulated depreciation. Property and equipment are depreciated on a straight-line basis over the estimated useful lives of the assets (three years for computer equipment and five years for furniture and fixtures). Leasehold improvements are being amortized on a straight-line basis over a period of six years.
44
Table of Contents
Common Stock Purchase Warrants
The Company accounts for the issuance of common stock purchase warrants issued in connection with capital financing transactions in accordance with the provisions of ASC Topic 815, (formerly Emerging Issues Task Force (“EITF”) Issue No. 07-5) “Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock.” Based upon the provisions of ASC Topic 815, the Company classifies as equity any contracts that (i) require physical settlement or net-share settlement or (ii) gives the Company a choice of net-cash settlement or settlement in its own shares (physical settlement or net-share settlement). The Company classifies as assets or liabilities any contracts that (i) require net-cash settlement (including a requirement to net-cash settle the contract if an event occurs and if that event is outside the control of the Company) or (ii) gives the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement).
As more fully described in Note 6, the Company classified certain common stock purchase warrants with contingent exercise features as derivative liabilities in the accompanying consolidated balance sheet as of December 31, 2009.
Revenue Recognition
Revenue is recognized on the grant when the services are provided and direct costs are incurred. Grant revenues are received by the Company to further advance its research and development activities associated with product development. As of December 31, 2009, the grant had concluded and all revenue had been recorded. The grant was awarded by the NIH specifically the National Heart, Lung & Blood Institute, a sponsored program by the federal government and provided 100% of our revenues for the years ended December 31, 2009, 2008 and 2007.
Reclassification
Certain prior year amounts have been reclassified to conform to the current year presentation. The reclassification did not have any effect on reported consolidated net losses for any periods presented.
Research and Development
In accordance with ASC Topic 730 (formerly SFAS No. 2, “Research and Development Expenses”) research and development costs are expensed as incurred. Research and development expenses consist of purchased technology, purchased research and development rights and outside services for research and development activities associated with product development. In accordance with ASC Topic 730, the cost to purchase such technology and research and development rights are required to be charged to expense if there is currently no alternative future use for this technology and, therefore, no separate economic value.
Income Taxes
In accordance with ASC Topic 740 (formerly SFAS No. 109 “Accounting for Income Taxes”) we account for deferred income taxes under the liability method. Under this method, we recognize deferred tax assets and liabilities based on the tax effects of temporary differences between the financial statement and tax bases of assets and liabilities, as measured by current enacted tax rates. A valuation allowance is recorded to reduce deferred tax assets when necessary.
Loss Per Common Share
We compute loss per share, in accordance with ASC Topic 260 (formerly SFAS No. 128), “Earnings Per Share.” ASC 260 requires dual presentation of basic and diluted earnings per share.
45
Table of Contents
Basic income or loss per common share for continuing operations and discontinued operation is computed by dividing net income or loss by the weighted average number of common shares outstanding during the period. Diluted income or loss per common share is computed by dividing net income or loss by the weighted average number of common shares outstanding, plus the issuance of common shares, if dilutive, resulting from the exercise of outstanding stock options and warrants. These potentially dilutive securities were not included in the calculation of loss per common share for the years ended December 31, 2009, 2008 and 2007 because, due to the loss we incurred during such periods, their inclusion would have been anti-dilutive. Accordingly, basic and diluted loss per common share are the same for all periods presented. The common stock issued and outstanding with respect to the stockholders of Aries has been included since October 20, 2005, the effective date of the reverse merger.
Potentially dilutive securities consisted of outstanding stock options and warrants to acquire 27,586,356 shares as of December 31, 2009.
Stock-Based Compensation
In accordance with ASC 718 stock-based compensation costs are recognized on a straight-line basis over the requisite service period of the award, which is generally the vesting term of the award.
Total stock-based compensation expense included in the consolidated statements of operations was allocated as follows to research and development and general and administrative expenses as follows:
| December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Research and development |
$ | 240,095 | $ | 906,317 | $ | 1,064,554 | |||
| General and administrative |
669,047 | 1,076,872 | 1,264,886 | ||||||
| Total stock-based compensation |
$ | 909,142 | $ | 1,983,189 | $ | 2,329,440 | |||
Recently Issued and Adopted Accounting Pronouncements
In December 2007, the FASB issued ASC topic 805 (formerly SFAS No. 141 (revised 2007), “Business Combinations” ASC topic 805 retains the purchase method of accounting for acquisitions, but requires a number of changes, including changes in the way assets and liabilities are recognized in the purchase accounting. It also changes the recognition of assets acquired and liabilities assumed arising from contingencies, requires the capitalization of in-process research and development at fair value, and requires the expensing of acquisition-related costs as incurred. ASC topic 805 is effective for acquisitions occurring in fiscal periods beginning after December 15, 2008 and was required to be adopted by the Company in its first quarter of fiscal 2009. We adopted ASC Topic 805 on January 1, 2009 and it could have an impact on the accounting for any future acquisition, if one were to occur.
In December 2007, the FASB issued ASC topic 810 (formerly SFAS No. 160), “Noncontrolling Interests in Consolidated Financial Statements, an amendment of Accounting Research Bulletin No. 51, Consolidated Financial Statements” ASC topic 810 requires (i) that non-controlling (minority) interests be reported as a component of stockholders’ equity, (ii) that net income attributable to the parent and to the non-controlling interest be separately identified in the consolidated statement of operations, (iii) that changes in a parent’s ownership interest while the parent retains its controlling interest be accounted for as equity transactions, (iv) that any retained non-controlling equity investment upon the deconsolidation of a subsidiary be initially measured at fair value, and (v) that sufficient disclosures are provided that clearly identify and distinguish between the interests of the parent and the interests of the non-controlling owners. ASC topic 810 is effective for financial statements issued for fiscal years beginning after December 15, 2008, and interim periods within those fiscal years. We adopted ASC topic 810 for our fiscal year beginning January 1, 2009, and the adoption did not have any impact on our consolidated financial position, results of operations and cash flows.
46
Table of Contents
In March 2008, the FASB issued ASC topic 815, Statement of Financial Accounting Standards (formerly SFAS No. 161), “Disclosures about Derivative Instruments and Hedging Activities. ASC topic 818 changes the disclosure requirements for derivative instruments and hedging activities. Entities are required to provide enhanced disclosures about (a) how and why an entity uses derivative instruments, (b) how derivative instruments and related hedged items are accounted for under SFAS 133 and its related interpretations, and (c) how derivative instruments and related hedged items affect an entity’s financial position, financial performance and cash flows. The adoption of this pronouncement on January 1, 2009 did not have a material impact on the Company’s consolidated financial statements.
In April 2008, the FASB issued ASC topic 815, “Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity’s Own Stock” and provides guidance on determining what types of instruments or embedded features in an instrument held by a reporting entity can be considered indexed to its own stock for the purpose of evaluating the first criteria of the scope exception and was effective for financial statements issued for fiscal years beginning after December 15, 2008. The adoption of this pronouncement had a material impact on the Company’s consolidated financial statements (See note 7).
Effective January 1, 2009 we adopted ASC topic 820 (formerly FSP FAS 157-3), “Determining the Fair Value of a Financial Asset When the Market for That Asset is Not Active” which clarifies the application in a market that is not active and provides an example to illustrate key considerations in determining the fair value of a financial asset when the market for that financial asset is not active. ASC topic 820 became effective immediately upon issuance, and its adoption did not have an effect on our financial statements. We currently determine the fair value of our property and equipment when assessing long-lived asset impairments and ASC topic 820 was effective for these fair value assessments as of January 1, 2009.
In April 2009, (updated in January 2010) the FASB issued ASC topic 820 “Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly” which provides additional guidance for estimating fair value when the volume and level of activity for the asset or liability have significantly decreased. ASC topic 820 also includes guidance on identifying circumstances that indicate a transaction is not orderly and emphasizes that even if there has been a significant decrease in the volume and level of activity for the asset or liability and regardless of the valuation technique(s) used, the objective of a fair value measurement remains the same. Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction (that is, not a forced liquidation or distressed sale) between market participants at the measurement date under current market conditions. ASC topic 820 was effective for interim and annual reporting periods ending after June 15, 2009, and is applied prospectively. The adoption of this pronouncement did not have a material impact on the Company’s consolidated financial statements.
The fair value hierarchy distinguishes between assumptions based on market data (observable inputs) and an entity’s own assumptions (unobservable inputs). The hierarchy consists of three levels:
| • | Level one—Quoted market prices in active markets for identical assets or liabilities; |
| • | Level two—Inputs other than level one inputs that are either directly or indirectly observable; and |
| • | Level three—Unobservable inputs developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use. |
47
Table of Contents
Determining which category an asset or liability falls within the hierarchy requires significant judgment. We evaluate our hierarchy disclosures each quarter. Assets and liabilities measured at fair value on a recurring basis are summarized as follows:
| Assets | Level 1 | Level 2 | Level 3 | December 31, 2009 | ||||||||
| None |
$ | — | $ | — | $ | — | $ | — | ||||
| Liabilities | Level 1 | Level 2 | Level 3 | December 31, 2009 | ||||||||
| Fair value of common stock warrants (derivative liabilities—Note 6) |
$ | — | $ | — | $ | 4,802,882 | $ | 4,802,882 | ||||
| Total |
$ | — | $ | — | $ | 4,802,882 | $ | 4,802,882 | ||||
The following table provides the assets and liabilities carried at fair value measured on a recurring basis as of December 31, 2009:
| Fair Value Measurements at December 31, 2009 | ||||||||||||
| Total Carrying Value at December 31, 2009 |
Quoted prices in active markets (Level 1) |
Significant other observable inputs (Level 2) |
Significant unobservable inputs (Level 3) | |||||||||
| Derivative liabilities |
$ | 4,802,882 | $ | — | $ | — | $ | 4,802,882 | ||||
The following table sets forth a summary of the changes in the fair value of our Level 3 financial liabilities that are measured at fair value on a recurring basis:
| Years Ended December 31, | |||||||
| 2009 | 2008 | ||||||
| Beginning balance |
$ | 4,806,150 | $ | — | |||
| Net unrealized loss on derivative financial instruments |
1,118,120 | — | |||||
| Reclassification of derivative liabilities with expired price protection provisions |
(2,538,793 | ) | — | ||||
| Warrants issued with debt |
1,417,405 | ||||||
| Ending balance |
$ | 4,802,882 | $ | — | |||
NOTE 3—Disposal of Long-Lived Assets
In accordance with the provisions of ASC topic 360 (formerly SFAS No. 144), “Accounting for the Impairment or Disposal of Long-Lived Assets,” the disposal of our Innercool business segment is presented as assets and liabilities held for sale and as a discontinued operation in the accompanying consolidated financial statements.
48
Table of Contents
Assets and liabilities of business held for sale
The major categories of assets and liabilities held for sale included in the condensed consolidated balance sheet at December 31, 2008 were as follows:
| Assets held for sale: |
|||
| Accounts receivable |
$ | 253,837 | |
| Inventories, net |
2,000,385 | ||
| Prepaid expenses and other current assets |
133,078 | ||
| Property, plant and equipment, net |
959,896 | ||
| Acquired technology, net |
3,836,370 | ||
| Intangibles, net |
140,304 | ||
| Long term assets |
40,103 | ||
| Total assets held for sale |
$ | 7,363,973 | |
| Liabilities of business held for sale: |
|||
| Accounts payable |
$ | 1,386,169 | |
| Accrued liabilities |
741,817 | ||
| Total liabilities of business held for sale |
$ | 2,127,986 | |
The following results of operations of Innercool Therapies, Inc. are presented as a loss from a discontinued operation in the consolidated statements of operations:
| For the years ended December 31, | Period from December 22, 2003 (Inception) to December 31, 2009 |
|||||||||||||||
| 2009 | 2008 | 2007 | ||||||||||||||
| Revenues |
||||||||||||||||
| Product sales |
$ | 793,770 | $ | 2,000,473 | $ | 1,140,561 | $ | 4,620,076 | ||||||||
| Cost of goods sold |
579,895 | 1,431,574 | 1,348,335 | 4,313,998 | ||||||||||||
| Gross profit |
213,875 | 568,899 | (207,774 | ) | 306,078 | |||||||||||
| Operating expenses |
||||||||||||||||
| Research and development |
324,020 | 1,273,722 | 2,473,707 | 5,965,833 | ||||||||||||
| Selling, general and administrative |
1,357,249 | 5,070,769 | 4,891,814 | 13,681,733 | ||||||||||||
| Amortization—intangibles |
443,651 | 789,656 | 789,656 | 2,696,193 | ||||||||||||
| Total operating expenses |
2,124,920 | 7,134,147 | 8,155,177 | 22,343,759 | ||||||||||||
| Loss from operation |
(1,911,045 | ) | (6,565,248 | ) | (8,362,951 | ) | (22,037,681 | ) | ||||||||
| Interest, net |
(19,591 | ) | (430,820 | ) | (73,464 | ) | (523,539 | ) | ||||||||
| Net loss from discontinued operation |
$ | (1,930,636 | ) | $ | (6,996,068 | ) | $ | (8,436,415 | ) | $ | (22,561,220 | ) | ||||
On July 24, 2009, we closed the sale of our Innercool business unit to Philips. The purchase price was $11,250,000 net of a working capital adjustment of $7,148.
49
Table of Contents
The following is the calculation of the gain on the sale of Innercool.
| Selling price |
$ | 11,250,000 | ||
| Less working capital adjustment |
(7,148 | ) | ||
| Net cash received |
11,242,852 | |||
| Net assets sold: |
||||
| Accounts receivable |
97,606 | |||
| Prepaid expenses and other current assets |
131,137 | |||
| Inventory, net |
1,702,823 | |||
| Property and equipment, net |
742,390 | |||
| Intangible assets, net |
3,533,023 | |||
| Other long term assets |
40,103 | |||
| Accounts payable |
(1,185,152 | ) | ||
| Other liabilities |
(227,681 | ) | ||
| Subtotal |
4,834,249 | |||
| Gain on sale of discontinued operation |
$ | 6,408,603 | ||
NOTE 4—Property and Equipment
Property and equipment consisted of the following:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Computer and telecommunication equipment |
$ | 466,329 | $ | 466,329 | ||||
| Machinery and equipment |
31,779 | 31,779 | ||||||
| Office equipment |
53,050 | 53,050 | ||||||
| Instrumentation |
115,421 | 115,421 | ||||||
| Office furniture and equipment |
473,652 | 473,652 | ||||||
| Leasehold improvements |
152,774 | 343,844 | ||||||
| 1,293,005 | 1,484,075 | |||||||
| Accumulated depreciation and amortization |
(941,466 | ) | (737,906 | ) | ||||
| Property and equipment, net |
$ | 351,539 | $ | 746,169 | ||||
Depreciation and amortization of property and equipment from continuing operations totaled $259,286, $309,708, and $256,004 for the years ended December 31, 2009, 2008 and 2007, respectively. For the period from December 22, 2003 (date of inception) through December 31, 2009 depreciation and amortization of property and equipment from continuing operations totaled $997,192.
NOTE 5—Accrued Liabilities
Accrued Liabilities consisted of the following:
| December 31, | ||||||
| 2009 | 2008 | |||||
| Payroll and benefits |
$ | 311,849 | $ | 319,095 | ||
| In-process purchased technology |
— | 500,000 | ||||
| Clinical trial costs |
— | 358,891 | ||||
| Other |
24,608 | 154,462 | ||||
| Total |
$ | 336,457 | $ | 1,332,448 | ||
50
Table of Contents
NOTE 6—Derivative Liabilities
The adoption of ASC 815, as described under Note 2, can affect the accounting for warrants and convertible instruments with provisions that protect holders from a decline in the stock price (or “down-round” provisions). Down-round provisions reduce the exercise price or increase the number of shares underlying the common stock equivalents or issues new equity or equity linked securities at prices or with exercise prices that are more favorable than the security that features price protection. We evaluated whether warrants to acquire stock of the Company contain provisions that protect holders from declines in the stock price or otherwise could result in modification of the exercise price under the respective warrant agreements.
| Number of Warrants (in shares) |
Fair Value | ||||||
| Balance outstanding, January 1, 2009 |
15,854,279 | $ | 4,806,150 | ||||
| Warrants issued |
2,730,832 | 1,417,405 | |||||
| Warrants expired or cancelled |
(4,591,927 | ) | (2,538,793 | ) | |||
| Fair value adjustment |
— | 1,118,120 | |||||
| Balance outstanding, December 31, 2009 |
13,993,184 | $ | 4,802,882 | ||||
The Company had 15,854,279 warrants outstanding that contain anti-dilution or price protection on January 1, 2009, the date in which ASC 815 became effective. The aggregate fair value of all such warrants amounted to $14,443,766. The Company calculated the fair value of these warrants using the Black-Scholes Option Pricing Model with the following weighted average assumptions: exercise price $1.96, closing price of common stock $0.75, risk free interest rate of 1.16%, dividend yield of 0%, volatility of 82% and remaining contractual term of 4.17 years. The Company recorded an issuance date liability for these warrants by reducing additional paid in capital by $12,982,785, and increasing the discount of certain related indebtedness by an additional $1,460,981. The aggregate fair value of these warrants amounted to $4,806,150 as of January 1, 2009. Accordingly, the Company reduced the issuance date fair value of the warrant liability by $9,637,617 and increased it by $1,460,981 for the adjustment in the value of the debt discount. The Company then decreased the debt discount by $224,151 for amortization that would have been recorded from the dates of original issuance to the January 1, 2009. The effect of reducing the liability and the debt discount is reflected in the accompanying statement of stockholders’ deficiency as a $9,413,466 reduction of the accumulated deficit.
During the year ended December 31, 2009, the Company issued an additional 2,730,832 warrants that contain or price protection. The aggregate fair value of all such warrants issued during the year ended December 31, 2009 amounted to $1,417,405. The Company calculated the fair value of these warrants using the Black-Scholes Option Pricing Model with the following weighted average assumptions: exercise price $1.87, closing price of common stock $1.15, risk free interest rate of 1.9%, dividend yield of 0%, volatility of $92% and remaining contractual term of 4.69 years.
In addition to the above, 3,198,410 warrants expired or no longer contain price protection that requires them to be recorded as derivative liabilities. Accordingly, the Company reduced derivative liabilities $2,583,793 with respect to these warrants. Warrants to purchase 1,393,517 shares of common stock were exercised during the year ended December 31, 2009. As a result, the Company has 13,993,184 warrants outstanding that contain anti-dilution a price protection as of December 31, 2009. The aggregate fair value of all such warrants amounted to $4,802,882. The Company calculated the fair value of these warrants using the Black-Scholes Option Pricing Model with the following weighted average assumptions: exercise price $1.30, closing price of common stock $0.68, risk free interest rate of 1.27%, dividend yield of 0%, volatility of 98% and a remaining contractual term of 3.36 years. The Company recorded a $1,118,120 charge in the statement of operations during the year ended December 31, 2009 to increase the amount of the derivative liability to fair value as of December 31, 2009.
51
Table of Contents
NOTE 7—Debt Obligations
Short-term Debt
On November 10, 2008, we completed a secured debt financing pursuant to the terms of a Note and Warrant Purchase Agreement entered into with certain accredited investors. Under the terms of the purchase agreement we issued senior secured notes in the aggregate principal amount of $6 million to the investors, and five-year warrants to purchase an additional 9,386,625 shares of our common stock, in the aggregate, at an initial exercise price of $2.00 per share. The senior secured notes bear interest at a fixed rate of 12% per annum, payable monthly, have a one year term, are secured by all of our assets and intellectual property and are senior to, and have priority in right of payment over, any other indebtedness of our company. The senior secured notes may be prepaid, in whole or in part, at any time provided the investors receive an additional payment equal to the difference between the amount of interest they would have received through the maturity date of the notes and the amount of interest actually received as of the prepayment date. The warrants were fully exercisable when issued. We recorded deferred financing costs in the amount of $511,432 and debt discount in the amount of $3,780,000 in connection with this debt financing. As of November 5, 2009, all of the senior secured notes had been repaid.
On March 5, 2009 we completed a $3.5 million financing in the form of senior subordinated secured notes with accompanying warrants to purchase 1,505,000 shares of our common stock with an aggregate fair value of $675,960. The senior subordinated secured notes bear interest at a fixed rate of 12% per annum, payable upon maturity, are secured by all of the assets and intellectual property of Cardium, InnerCool and TRC, and are senior to, and have priority in right of payment over, any other indebtedness of Cardium with the exception of the senior secured notes. The warrants are fully exercisable, have a five year term and an exercise price of $2.00. We received gross proceeds of $3.5 million in this financing, less placement agent fees and offering expenses of $251,901. In addition, we issued warrants to purchase 90,300 shares of common stock to the placement agent on the same terms as the warrants issued to the lenders. The placement warrants at the time of issuance had an aggregate fair value of $37,926 and were included in deferred financing costs. The fair value of the warrants issued in this financing was $713,926. The Company calculated the fair value of these warrants using the Black-Scholes Option Pricing Model with the following weighted average assumptions: exercise price $2.00, closing price of common stock $0.80, risk free interest rate of 1.82%, dividend yield of 0%, volatility of 88% and remaining contractual term of 5 years. We also recorded deferred financing costs in the amount of $289,827 and debt discount in the amount of $675,960 in connection with this debt financing. As a result of the Phillips transaction, all of the senior subordinated secured notes have been repaid.
Under the terms of an Asset Purchase Agreement, dated as of August 11, 2006, Cardium, through its subsidiary, acquired substantially all of the assets and the business related to its wholly-owned subsidiary, Tissue Repair Company, from certain sellers now known as Tissue Repair Royalty Company, LLC (“TRC RC”). On February 23, 2009, Cardium, Tissue Repair and TRC RC agreed that a $500,000 milestone payment due in February 2009 in connection with Tissue Repair’s Phase 2 MATRIX clinical study for its Excellarate product candidate, for the potential treatment of non-healing diabetic ulcers, would be substituted by a convertible promissory note to TRC RC in the same amount (the “TRC Note”). The TRC Note bears interest at a rate of 0.6% per annum and provides for principal payments of $50,000 on each of March 1, 2009, April 1, 2009, May 1, 2009 and June 1, 2009 with the remaining principal balance and any interest due on June 11, 2009. If Cardium completes an equity financing of at least $2,000,000 or elects to sell its Innercool Therapies subsidiary, the maturity date would be accelerated and the remaining principal and unpaid interest would become due at that time. If Cardium did not repay the TRC Note when due, TRC RC would have the option to convert the remaining principal balance and any interest due into shares of Cardium’s common stock at a conversion price per share equal to the average of the closing or last sale price reported for the five trading days immediately preceding the maturity date of the Note, or such other price as specified in the Note if Cardium’s common stock is not then traded on the NYSE Amex (subject to certain adjustments). The note was repaid in two principal
52
Table of Contents
payments of $50,000 one on March 1, 2009 and the second one on April 1, 2009. The final principal payment of $400,000 was made on August 4, 2009.
On June 23, 2009 we completed a $750,000 unsecured debt financing involving the sale of unsecured notes with accompanying warrants to purchase 502,500 shares of our common stock with an aggregate value of $ 687,409. The unsecured notes bear interest at a fixed rate of 12% per annum, payable upon maturity. The maturity date of the unsecured notes was the earlier of June 27, 2009, or the closing of a qualified asset monetization qualified financing or qualified stock sale (see above). The warrants are fully exercisable, have a five-year term and an exercise price of $2.00. We received gross proceeds of approximately $750,000. In addition, we issued warrants to purchase 12,060 shares of common stock to the placement agent with and aggregate fair value of $16, 100 on the same terms as the warrants issued to the lenders. The Company calculated the fair value of these warrants using the Black-Scholes Option Pricing Model with the following weighted average assumptions: exercise price $2.00, closing price of common stock $1.90, risk free interest rate of 2.71%, dividend yield of 0%, volatility of 97% and remaining contractual term of 5 years. We recorded deferred financing costs in the amount of $16,100, the fair value of the placement agent warrants at the time of issuance, and debt discount in the amount of $687,419 in connection with this debt financing. Soon after the close of the Phillips transaction $539,000 of the unsecured notes were repaid. We repaid the remaining $211,000 of the unsecured notes which where due and payable on November 5, 2009.
During the years ended December 31, 2009 and 2008 contractual interest expense on the notes was $976,546 and $1,083,823, respectively.
Long-term Debt
On November 12, 2007, Cardium, InnerCool Therapies and Tissue Repair Company entered into a Loan and Security Agreement with Life Sciences Capital, LLC, whereby we obtained debt financing in the principal amount of $5 million to be used for general working capital purposes. In connection with the loan, we paid Life Sciences Capital, LLC a loan commitment fee and related legal expenses of $108,500, which was capitalized as deferred financing costs and amortized over the term of the loan. On July 1, 2008 we paid off the principal balance on our commercial credit facility out of the proceeds from the June 2008 offering, thereby retiring our remaining debt obligations to Life Sciences Capital.
NOTE 8—Commitments and Contingencies
Lease Commitments
Effective November 1, 2005, we entered into a two year lease for our principal executive offices. The lease contained two options, the first for an additional term of one year and the second for an additional term of two years. The second option was subject to a third party right of first refusal. During the first year of the lease, the monthly installment of base rent was approximately $21,500, which increased to approximately $22,335 in November 2006. In addition to base rent, we also were required to pay our proportionate share of operating and tax expenses for the office park in which our space was located. We chose not to exercise our option for an additional term and the lease expired in the fourth quarter of 2008.
On December 20, 2006, we entered into a six year lease for our technology center, which housed the operations of Innercool Therapies Inc. and Tissue Repair Company until Innercool was sold In July 2009. At the time of the sale the Philips assumed the lease for the technology center.
On November 19, 2007 we entered into a lease for approximately 11,184 square feet of office space in San Diego, California to be used as Cardium’s corporate headquarters. The lease commenced in April 2008 once substantial improvements were completed and has a term of 64 months from the commencement date with an option to renew for an additional five years. Monthly base rent was approximately $46,972 during the first year
53
Table of Contents
of the lease and increases to $48,650, $50,328, $52,117, $53, 907 and $55,808 each year thereafter. In addition to monthly base rent, we are also required to pay our proportionate share of any building operating expenses in excess of 2008 levels. In connection with entering into the lease, we paid a security deposit of $55,808 and delivered a $500,000 letter of credit to the landlord. The letter of credit is subject to annual reductions of $100,000 during the original term of the lease.
Future annual minimum rental payments under the leases are as follows:
| Year Ending December 31, |
Facilities (Operating Lease) | ||
| 2010 |
$ | 598,903 | |
| 2011 |
620,041 | ||
| 2012 |
641,514 | ||
| 2013 |
384,953 | ||
| Total |
$ | 2,245,411 | |
Rent expense included in continuing operations was $661,983, $815,275, and $369,983 for the years ended December 31, 2009, 2008 and 2007, respectively.
Our common stock is currently listed on the NYSE Amex (the “Exchange”). To maintain that listing, we must continue to comply with various listing standards of the Exchange, as set forth in Part 10 of the Exchange’s Company Guide. On December 23, 2008, we received a notice from the staff of the Exchange noting that, based on their review of publicly available information, we did not meet certain of the Exchange’s continued listing standards related to the maintenance of a minimum level of stockholders’ equity and losses from ongoing operations. On January 23, 2009, we submitted a plan of compliance (the “Plan”) advising the Exchange of the actions taken or to be taken to regain compliance with the Company Guide. In February 2009, our Plan was accepted and in July 2009, following completion of the Philips Transaction, we were informed that Cardium was considered to have regained compliance with the Exchange’s requirements, in advance of the June 23, 2010 deadline. On December 28, 2009, the Exchange noted that we were not considered to be in compliance with Section 1003(a)(iv) of the Company Guide because we reported stockholders’ equity of less than $4,000,000 and losses from continuing operations and net losses in three of our most recent fiscal years. With losses from continuing operations and net losses in five of our most recent fiscal years, the stockholders’ equity threshold would increase to $6,000,000. The Exchange asked us to supplement our previously-filed Plan advising the Exchange of the actions we have taken or will take to regain compliance with the Company Guide by June 23, 2010. Prior to the January 27, 2010 deadline, we provided a supplement to the Plan to the Exchange. The supplement was accepted; however, if we are not in compliance with the continued listing standards at the end of the Plan period, or we do not make progress consistent with the Plan during such period, then the Exchange could initiate delisting proceedings. If our common stock was ultimately delisted from the exchange, it would be expected to trade on the OTC Bulletin Board, a regulated quotation service that provides quotes, sale prices and volume information in over-the-counter equity securities which may reduce the liquidity of, and may adversely affect the price of, our common stock. Our common stock was traded on the OTC Bulletin Board until July 2007, when we elected to instead list its shares on the Exchange.
Purchase of Technology from Schering AG
In October 2005, we completed a transaction with Schering AG Group, Germany (now part of Bayer AG) and related licensors, including the University of California, New York University and Yale University, for the transfer or license of certain assets and technology for potential use in treating ischemic and other cardiovascular conditions. Under the terms of the transaction, we paid Schering a $4 million fee, and would be required to pay a $10 million milestone payment upon the first commercial sale of each resulting product. We also may be obligated to pay the following future royalties to Schering: (i) 5% on net sales of an FGF-4 based product such as Generx, or (ii) 4% on net sales of other products developed based on technology transferred to Cardium by
54
Table of Contents
Schering. As part of the Schering transaction, we acquired rights and corresponding obligations under the Regents of the University of California (Regents) September 1995 agreement, as amended. The agreement as amended may be canceled by us at any time on 60 days’ notice, following which we would continue to be responsible only for obligations and liabilities accrued before termination. Under the agreement, we are obligated to pay (1) an annual royalty fee of 2% based on net sales of products incorporating the technology licensed under the agreement, and (2) a minimum annual royalty fee (which may be offset against the net sales-based royalty fee) of $150,000 for 2009, $200,000 for 2010, $250,000 for 2011, $300,000 for 2012, $400,000 for 2013 and $500,000 for 2014 and thereafter.
Legal Proceedings
From time to time, we may become involved in various investigations, claims and legal proceedings that arise in the ordinary course of our business. These matters may relate to intellectual property, employment, tax, regulation, contract or other matters. The resolution of these matters as they arise will be subject to various uncertainties and, even if such claims are without merit, could result in the expenditure of significant financial and managerial resources. At December 31, 2009 we were not a party to any material pending legal proceeding.
NOTE 9—Income Taxes
The Company files income tax returns in the United States (federal) and in various state and local jurisdictions. In most instances, the Company is no longer subject to federal, state and local income tax examinations by tax authorities for years prior to 2005.
In accordance with ASC topic 740, interest costs related to unrecognized tax benefits are required to be calculated (if applicable) and would be classified as Interest expense, net. Penalties if incurred would be recognized as a component of general and administrative expenses.
The Company files corporate income tax returns in the United States (federal) and in various state and local jurisdictions. In most instances, the Company is no longer subject to federal, state and local income tax examinations by tax authorities for years prior to 2006.
As of December 31, 2009 and 2008, no liability for unrecognized tax benefits was required to be recorded. The Company does not expect its unrecognized tax benefit position to change during the next 12 months.
The Company recognized a deferred tax asset of approximately $28 million as of December 31, 2009 related to net operating loss carryforwards (which excludes net operating losses of $71 million that represent pre-merger losses for which the use is limited in accordance with Section 382 of the Internal Revenue Code of 1986, as amended), available to offset future taxable income through 2029. The net operating losses begin to expire in 2023 for federal tax purposes and in 2013 for state income tax purposes.
The ultimate realization of deferred tax assets depends on the generation of future taxable income during the periods in which those net operating losses are available. The Company considers projected future taxable income and tax planning strategies in making its assessment. At present, the Company does not have a sufficient history of income to conclude that it is more-likely-than-not that the Company will be able to realize all of its tax benefits in the near future and therefore a valuation allowance was established for the full value of the deferred tax asset.
A valuation allowance will be maintained until sufficient positive evidence exists to support the reversal of any portion or all of the valuation. Should the Company become profitable in future periods with supportable trends, the valuation allowance will be reversed accordingly.
55
Table of Contents
The Company’s net deferred tax asset consisted of the following at December 31, 2009 and 2008:
| December 31, | ||||||||
| 2009 | 2008 | |||||||
| Deferred tax asset: |
||||||||
| Net operating loss carryforwards |
$ | 28,430,918 | $ | 24,448,171 | ||||
| Deferred compensation—options |
656,270 | 569,467 | ||||||
| Deferred rent |
75,730 | 77,803 | ||||||
| Accrued expenses |
124,223 | 126,791 | ||||||
| Other |
25,627 | 55,502 | ||||||
| Total deferred tax assets |
29,312,768 | 25,277,734 | ||||||
| Less: Valuation allowance |
(29,312,768 | ) | (25,277,734 | ) | ||||
| Net deferred tax asset |
$ | — | $ | — | ||||
The Company’s recorded income tax benefit, net of change in the valuation allowance for each period presented is as follows:
| December 31, | ||||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Income tax benefit |
4,035,034 | 9,008,022 | 9,171,031 | |||||||||
| Change in valuation allowance |
(4,035,034 | ) | (9,008,022 | ) | (9,171,031 | ) | ||||||
| Net deferred tax asset |
$ | — | $ | — | $ | — | ||||||
As a result of the Company’s significant operating loss carryforwards and the corresponding valuation allowance, no income tax benefit was recorded at December 31, 2009, 2008 or 2007. The provision for income taxes using the statutory federal tax rate as compared to the Company’s effective tax rate is summarized as follows:
| December 31, | |||||||||
| 2009 | 2008 | 2007 | |||||||
| Expected federal statutory rate |
(34.00 | )% | (34.00 | )% | (34.00 | )% | |||
| State income taxes, net of federal benefits |
(5.83 | )% | (5.83 | )% | (5.83 | )% | |||
| Change in net operating loss due to subsidiary with discontinued operation |
11.04 | % | (15.04 | )% | (18.21 | )% | |||
| Effect of permanent differences |
3.82 | % | 3.69 | % | 3.73 | % | |||
| (24.97 | )% | (51.18 | )% | (54.31 | )% | ||||
| Change in valuation allowance |
24.97 | % | 51.18 | % | 54.31 | % | |||
| Totals |
— | — | — | ||||||
As described in Note 3, the Company sold substantially all of the net assets of its Innercool subsidiary in the Philips Transaction completed on July 23, 2009. The Company is reporting Innercool’s historical results as part of discontinued operations in the accompanying consolidated statements of operations; however, the Company still files consolidated income tax returns with Innercool as a wholly-owned subsidiary for which it is entitled to the benefit of the Innercool’s net operating loss carryforwards.
56
Table of Contents
NOTE 10—Stockholders’ Deficiency
Common Stock
On March 9, 2007, we closed a private placement of 8,636,000 shares of common stock at a purchase price of $2.50 per share and received net proceeds of approximately $20 million. Investors received five-year warrants to buy up to 35% of the number of shares of common stock purchased in the private placement, at an exercise price of $3.75 per share. Warrants to purchase approximately 3,022,600 shares of common stock, in the aggregate, were issued to such investors. The warrants are subject to price protection provisions pursuant to the purchase agreement. These warrants had a price protection provision which when triggered on September 9, 2009 were reduced to an exercise price of $1.50 and then on October 15, 2009 were further reduced to $1.30.
In connection with the private placement, we incurred selling commissions, and expenses payable to the placement agent, totaling approximately $1,480,300, and legal, accounting and other fees and expenses totaling approximately $100,000 In addition, a five-year warrant to purchase 518,160 shares of our common stock was issued to the placement agent at an exercise price of $3.78 per share.
In November 2007, we entered into a Loan and Security Agreement with Life Sciences Capital, LLC whereby we obtained debt financing in the principal amount of $5 million to be used for general working capital purposes. The proceeds were immediately made available to us under this credit agreement. In connection with this financing, we issued a warrant to Life Sciences Capital, LLC to purchase 93,333 shares of our common stock at an exercise price of $3.75. We also recorded deferred financing costs in the amount of $108,500 in connection with this debt financing.
On January 31, 2008, we completed a registered direct offering of our common stock that resulted in the sale of 2,655,000 shares, in the aggregate, of our common stock at a purchase price of $2.00 per share, and five year warrants to purchase an additional 929,250 shares of our common stock at an exercise price of $2.00. The warrants were fully exercisable when issued. We received gross proceeds of approximately $5,300,000, before placement agent fees and offering expenses of approximately $400,000. In addition, we issued warrants to purchase 159,300 shares of our common stock to the placement agent on terms substantially the same as the warrants issued to investors.
On June 27, 2008, we completed a follow-on registered direct offering of our common stock that resulted in the sale of 1,625,000 shares, in the aggregate, of our common stock at a purchase price of $2.00 per share, and five year warrants to purchase an additional 568,750 shares of our common stock at an exercise price of $2.00. The warrants were fully exercisable when issued. We received gross proceeds of approximately $3,250,000, before placement agent fees and offering expenses of approximately $224,000. In addition, we issued warrants to purchase 97,500 shares of our common stock to the placement agent. These warrants had an exercise price of $2.29 and otherwise have substantially the same terms as the warrants issued to investors. The investor warrants had a price protection provision which when triggered on September 9, 2009 were reduced to an exercise price of $1.50 and then on October 15, 2009 were further reduced to $1.30. As a result of the price reductions an additional 306,249 warrants were issued.
On July 18, 2008, we completed a second follow-on registered direct offering of our common stock that resulted in the sale of 1,670,000 shares, in the aggregate, of our common stock at a purchase price of $2.00 per share, and five year warrants to purchase an additional 584,500 shares of our common stock at an exercise price of $2.00. The warrants were fully exercisable when issued. We received gross proceeds of approximately $3,340,000, before placement agent fees, offering expenses and expense reimbursements of approximately $330,000. In addition, we issued warrants to purchase 100,200 shares of our common stock to the placement agent. The warrants had an exercise price of $2.20 and otherwise have substantially the same terms as the warrants issued to investors. The investor warrants had a price protection provision which when triggered on September 9, 2009 were reduced to an exercise price of $1.50 and then on October 15, 2009 were further reduced to $1.30. As a result of the price reductions an additional 314,723 warrants were issued.
57
Table of Contents
On November 5, 2008, we completed a secured debt financing pursuant to the terms of a Note and Warrant Purchase Agreement entered into with certain accredited investors. Under the terms of the purchase agreement we issued notes in the aggregate principal amount of $6 million to the investors, and five year warrants to purchase an additional 9,386,625 shares of our common stock, in the aggregate, at an exercise price of $2.00 per share. The notes bear interest at a fixed rate of 12% per annum, payable monthly, have a one year term, are secured by all of our assets and intellectual property and are senior to, and have priority in right of payment over, any other indebtedness of our company. The notes may be prepaid, in whole or in part, at any time provided the investors receive an additional payment equal to the difference between the amount of interest they would have received through the maturity date of the notes and the amount of interest actually received as of the prepayment date. The warrants were fully exercisable when issued. We also recorded deferred financing costs in the amount of $511,432 and debt discount in the amount of $2,319,018 in connection with this debt financing. The warrants are subject to price protection provisions pursuant to the purchase agreement. These warrants had a price protection provision which when triggered on September 9, 2009 were reduced to an exercise price of $1.50 and then on October 15, 2009 were further reduced to $1.30.
In September 16, 2009, we sold an aggregate of 3,000,000 at a price of $1.50 per share of our common stock and 2,250,000 warrants to common stock to certain institutional investors in exchange for proceeds of $4.1 million, net of issuance costs. Each investor received warrants to purchase a number of shares equal to 75% of the number of shares of common stock purchased by the investor in the offering. The exercise price of the warrants is $1.77. In addition, the placement agent for the September financing received 150,000 warrants to purchase common stock at an exercise price of $1.87 on substantially identical terms; provided, however that the warrants to the placement agent expire on December 19, 2012.
On October 15, 2009, we sold an aggregate of 4,615,385 shares of our common stock and 3,000,000 warrants to common stock to certain institutional investors in exchange for proceeds of $5.6 million, net of issuance costs. Each investor received warrants to purchase a number of shares equal to 75% of the number of shares of common stock purchased by the investor in the offering. The units were sold at a price of $1.30 per unit. The exercise price of the warrants is $1.40. In addition, the placement agent for the October financing received 230,769 warrants to purchase common stock at an exercise price of $1.63 on substantially identical terms; provided, however that the warrants to the placement agent expire on December 19, 2012.
During 2007, 128,487 shares of common stock were issued when warrants to purchase 222,815 shares of common stock were exercised in cashless transactions, whereby a portion of the respective warrants representing the right to purchase 111,849 shares of common stock, in the aggregate, was cancelled as the method of payment for the exercise of the warrants. Also during 2007, 17,521 shares of common stock were issued upon the exercise of warrants for which Cardium received $26,276 as payment of the exercise price.
During 2008, 10,148 shares of common stock were issued when warrants to purchase 25,000 shares of common stock were exercised in cashless transactions, whereby a portion of the respective warrants representing the right to purchase 14,852 shares of common stock, in the aggregate, was cancelled as the method of payment for the exercise of the warrants. Also during 2008, 15,000 shares of common stock were issued upon the exercise of a warrant for which Cardium received $22,500 as payment of the exercise price.
During 2009, 270,590 shares of common stock were issued when warrants to purchase 1,097,207 shares of common stock were exercised in cashless transactions, whereby a portion of the respective warrants representing the right to purchase 826,617 shares of common stock, in the aggregate, were cancelled as the method of payment for the exercise of the warrants. Cardium paid $15 to various warrant holders for fractional shares resulting from the cashless exercises. Also during 2009, 356,310 shares of common stock were issued upon the exercise of warrants for which Cardium received $707,620 as payment of the exercise price.
58
Table of Contents
Stockholder Rights Plan
On July 10, 2006, Cardium’s Board of Directors approved the adoption of a Stockholder Rights Plan (“Rights Plan”) with the intention to protect against potential takeover tactics that are not in the best interest of Cardium and its stockholders, such as acquisitions of control without paying all stockholders a fair premium, coercive tender offers and inadequate offers. The Rights Plan was not adopted in response to any specific effort to acquire control of Cardium and it is not intended to prevent an offer that the Board of Directors concludes is in the best interests of Cardium and its stockholders. Pursuant to the Rights Plan, Cardium issued a dividend of one right for each share of its common stock held by stockholders of record as of the close of business on July 21, 2006. The rights are not immediately exercisable and will become exercisable only upon the occurrence of certain events. In general, if a person or group acquires, or announces a tender or exchange offer that would result in the acquisition of, 15% or more of Cardium’s common stock while the Rights Plan remains in place, then, unless the rights are redeemed by Cardium for $0.001 per right, the rights will become exercisable by all rights holders except the acquiring person or group, for 0.001 of a share of newly created Series A Preferred Stock of the Company at an exercise price of $40.00. Until the rights become exercisable, the rights will be represented by, and will automatically trade with, the Company’s common stock certificates.
The Rights Plan will be reviewed and evaluated every three years by a committee of independent directors of Cardium’s Board of Directors to consider whether the plan continues to be in the best interests of Cardium and its stockholders. The Rights Plan may be amended or revoked by Cardium at any time and unless earlier terminated or amended, the rights will expire on July 10, 2016.
Stock Options and Other Equity Compensation Plans
We have an equity incentive plan that was established in 2005 under which 5,665,856 shares of our common stock have been reserved for issuance to employees, non-employee directors and consultants of the Company. In addition we have one other equity compensation plan, Warrants—Tissue Repair. The equity compensation plans, Warrants for Common Stock, Innercool Incentive Warrant Plan, terminated 90 days after the sale of Innercool.
At December 31, 2009 the following shares were outstanding and available for future issuance:
| Plan |
Shares Outstanding | Shares Available for Issuance | ||
| 2005 Equity Incentive Plan |
3,340,000 | 2,321,169 | ||
| Warrants—Tissue Repair |
685,000 | — | ||
| Total all plans |
4,025,000 | 2,321,169 | ||
A summary of stock options and warrants that we granted during the years December 31, 2007, 2008 and 2009 are as follows:
| Date |
Quantity Issued |
Expected Life (Years) |
Strike Price | Volatility | Dividend Yield |
Risk-Free Interest Rate |
Grant Date Fair Value Per Option |
Share Based Compensation Expense | ||||||||||||||
| 05/08/07 |
235,000 | 5.25 | $ | 2.95 | 76 | % | 0 | % | 4.75 | % | $ | 1.95 | $ | 458,250 | ||||||||
| 08/14/07 |
20,000 | 5.25 | $ | 2.60 | 76 | % | 0 | % | 4.75 | % | $ | 1.72 | $ | 34,000 | ||||||||
| 08/14/07 |
100,000 | 7.00 | $ | 2.60 | 76 | % | 0 | % | 4.75 | % | $ | 1.91 | $ | 191,000 | ||||||||
| 11/06/07 |
10,000 | 5.25 | $ | 2.85 | 76 | % | 0 | % | 4.15 | % | $ | 1.87 | $ | 18,700 | ||||||||
| 03/10/08 |
87,500 | 5.25 | $ | 2.19 | 76 | % | 0 | % | 2.48 | % | $ | 1.41 | $ | 123,375 | ||||||||
| 05/05/08 |
165,000 | 5.25 | $ | 2.19 | 76 | % | 0 | % | 3.50 | % | $ | 1.42 | $ | 234,300 | ||||||||
| 06/16/08 |
350,000 | 5.25 | $ | 2.33 | 76 | % | 0 | % | 3.73 | % | $ | 1.52 | $ | 532,000 | ||||||||
| 08/04/08 |
45,000 | 5.25 | $ | 1.92 | 75 | % | 0 | % | 3.30 | % | $ | 1.23 | $ | 55,350 | ||||||||
| 11/10/08 |
75,000 | 5.25 | $ | 0.99 | 75 | % | 0 | % | 2.29 | % | $ | 0.63 | $ | 47,250 | ||||||||
| 03/09/09 |
1,542,500 | 5.25 | $ | 0.74 | 88 | % | 0 | % | 1.82 | % | $ | 0.52 | $ | 802,100 | ||||||||
59
Table of Contents
As of December 31, 2009, the Company had $860,441 of unvested stock-based compensation at fair value remaining to be expensed ratably over the period January 2010 through May 2013. During the year ended December 31, 2009 the Company recognized $909,142 of stock option compensation expense.
During the year ended December 31, 2007 the options and warrants granted had contractual terms ranging from seven to ten years, and vest over approximately four years. During the year ended December 31, 2008 and 2009, the options and warrants granted had a contractual term of seven years, and vest over approximately four years.
We calculate the fair value of stock options using the Black-Scholes option-pricing model. In determining the expected term, we separate groups of employees that have historically exhibited similar behavior with regard to option exercises and post-vesting cancellations. The option-pricing model requires the input of subjective assumptions, such as those included in the table above. The volatility rates are based principally on our historical stock prices and expectations of the future volatility of our common stock. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant. The total expense to be recorded in future periods will depend on several variables, including the number of share-based awards and expected vesting.
The following is a summary of stock option and warrant activity under our equity incentive plan and warrants issued outside of the plan to employees and consultants, during the years ended December 31, 2009, 2008 and 2007:
| Number of Options or Warrants |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life (in years) | ||||||
| Balance outstanding, December 31, 2006 |
5,304,000 | $ | 2.27 | 8.4 | ||||
| Granted |
365,000 | 2.83 | 6.6 | |||||
| Exercised |
— | — | — | |||||
| Cancelled |
(126,844 | ) | 2.52 | 7.9 | ||||
| Expired |
(50,656 | ) | 2.52 | 7.9 | ||||
| Balance outstanding, December 31, 2007 |
5,491,500 | $ | 2.30 | 7.3 | ||||
| Granted |
722,500 | 2.12 | 6.5 | |||||
| Exercised |
— | — | — | |||||
| Cancelled |
(490,898 | ) | 2.32 | 6.9 | ||||
| Expired |
(362,696 | ) | 2.72 | 7.1 | ||||
| Balance outstanding, December 31, 2008 |
5,360,406 | $ | 2.22 | 6.2 | ||||
| Granted |
1,542,500 | 0.74 | 6.2 | |||||
| Exercised |
(4,687 | ) | 0.74 | 6.2 | ||||
| Cancelled |
(732,344 | ) | 2.17 | 5.2 | ||||
| Expired |
(2,140,875 | ) | 2.21 | 2.9 | ||||
| Balance outstanding, December 31, 2009 |
4,025,000 | $ | 1.67 | 3.4 | ||||
| Balance exercisable, December 31, 2009 |
2,560,200 | $ | 2.04 | 5.4 | ||||
At December 31, 2008, and 2007 the number of options exercisable were 3,915,478, and 2,758,302 respectively, and the weighted-average exercise prices of those options were $2.18, and $2.20, respectively.
60
Table of Contents
Warrants
The following table summarizes warrant activity for the years ended December 31, 2009, 2008 and 2007:
| Number of Warrants |
Exercise Price | Weighted Average Remaining Contractual Life (in years) | ||||||
| Balance outstanding, December 31, 2006 |
2,307,853 | $ | 1.50-$1.75 | 2-4 | ||||
| Warrants issued |
3,634,093 | 3.75-3.78 | 4 | |||||
| Warrants exercised |
(128,487 | ) | 1.50 | 3 | ||||
| Warrants expired |
— | — | — | |||||
| Warrants cancelled |
(111,849 | ) | — | — | ||||
| Balance outstanding, December 31, 2007 |
5,701,610 | $ | 1.50-3.78 | 1-4 | ||||
| Warrants issued |
11,826,125 | 2.00 | 4.5 | |||||
| Warrants exercised |
(25,148 | ) | 1.50 | 2 | ||||
| Warrants expired |
(824,263 | ) | 1.50 | — | ||||
| Warrants cancelled |
(14,852 | ) | 1.50 | 2 | ||||
| Balance outstanding, December 31, 2008 |
16,663,472 | $ | 2.03 | 4.2 | ||||
| Warrants issued |
8,361,601 | 1.62 | 5.1 | |||||
| Warrants exercised |
(626,900 | ) | 1.55 | 1.9 | ||||
| Warrants expired |
— | — | — | |||||
| Warrants cancelled |
(836,817 | ) | 1.51 | 1.4 | ||||
| Balance outstanding, December 31, 2009 |
23,561,356 | $ | 1.52 | 4.0 | ||||
| Warrants exercisable at December 31, 2009 |
17,930,587 | $ | 1.50 | 3.5 | ||||
The table above does not include warrants issued to employees and consultants as they are included under “Option Activity” above.
61
Table of Contents
NOTE 12—Supplemental Financial Data (unaudited)
The following table presents selected unaudited financial results for each of the eight quarters during the two-year period ended December 31, 2009. In the opinion of management, this unaudited information has been prepared on the same basis as the audited information and includes all adjustments (consisting of only normal recurring adjustments) necessary for the fair statement of the financial information for the periods presented,.
| Year Ended December 31, 2009(1) |
First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
||||||||||||
| Revenues |
$ | 18,636 | $ | 6,996 | $ | 235,917 | $ | 183,397 | ||||||||
| Loss from operations |
$ | (2,513,248 | ) | $ | (2,322,568 | ) | $ | (2,250,357 | ) | $ | (1,626,761 | ) | ||||
| (Loss) income from continuing operations |
$ | (13,743,176 | ) | $ | (10,110,163 | ) | $ | (1,621,205 | ) | $ | 9,316,127 | |||||
| (Loss) income from discontinued operation |
$ | (993,701 | ) | $ | (1,032,511 | ) | $ | 95,576 | $ | — | ||||||
| Gain on discontinued operation |
$ | — | $ | — | $ | 6,408,603 | $ | — | ||||||||
| Net (loss) income |
$ | (14,736,877 | ) | $ | (11,142,674 | ) | $ | 4,882,974 | $ | 9,316,127 | ||||||
| Net (loss) income per common share—basic |
$ | (0.31 | ) | $ | (0.24 | ) | $ | 0.10 | $ | 0.17 | ||||||
| Net (loss) income per common share—diluted |
$ | (0.31 | ) | $ | (0.24 | ) | $ | 0.10 | $ | 0.17 | ||||||
| Weighted average shares outstanding—basic |
46,960,439 | 46,931,134 | 47,771,609 | 54,207,761 | ||||||||||||
| Weighted average shares outstanding—diluted |
46,960,439 | 46,931,134 | 49,620,079 | 54,374,522 | ||||||||||||
| Year Ended December 31, 2008(1) |
First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
||||||||||||
| Revenues |
$ | 112,203 | $ | 262,430 | $ | — | $ | 42,279 | ||||||||
| Loss from operations |
$ | (4,666,040 | ) | $ | (4,639,798 | ) | $ | (4,290,914 | ) | $ | (3,557,612 | ) | ||||
| Loss from continuing operations |
$ | (4,593,851 | ) | $ | (4,624,351 | ) | $ | (4,282,886 | ) | $ | (4,100,902 | ) | ||||
| Loss from discontinued operation |
$ | (2,140,277 | ) | $ | (2,010,111 | ) | $ | (1,868,629 | ) | $ | (977,051 | ) | ||||
| Net loss |
$ | (6,734,128 | ) | $ | (6,634,462 | ) | $ | (6,151,515 | ) | $ | (5,077,953 | ) | ||||
| Net loss per common share—basic and diluted |
$ | (0.17 | ) | $ | (0.15 | ) | $ | (0.13 | ) | $ | (0.11 | ) | ||||
| Weighted average shares outstanding—basic and diluted |
40,709,247 | 43,629,975 | 46,603,700 | 46,930,439 | ||||||||||||
| (1) | On July 24, 2009 we sold our Innercool business unit. Results of the Innercool business unit are included as a discontinued operation for all periods presented. |
NOTE 13—Subsequent Events
On March 12, 2010, the Company completed a registered direct offering of 2,266,998 units, which were sold to institutional and retail investors, at a price of $5.00 per unit. The offering resulted in gross proceeds to the Company of $11.3 million and net proceeds of approximately $10.4 million after payment of offering fees and expenses. Each unit consisted of 10 shares of common stock and a warrant to purchase 5 shares of common stock. The common stock purchase warrants are exercisable at an exercise price of $0.64 per share, at any time after six months from the date of closing and have a term of exercise equal to five years from the initial exercise date. In the aggregate 22,669,980 shares of common stock and warrants to purchase an additional 11,334,990 shares were issued in the offering. Dawson James Securities, Inc. acted as exclusive placement agent for the offering. Dawson James received placement agent fees of $793,449 and a warrants to purchase an aggregate of 1,133,499 shares of common stock, exercisable at $0.64 per share. The placement agent warrants expire on December 19, 2012. The offering was made pursuant to a shelf registration statement that was filed by Cardium Therapeutics with the Securities and Exchange Commission (the “SEC”) and declared effective by the SEC on December 19, 2007. A prospectus supplement related to the public offering was filed with the SEC on March 9, 2010. Copies of the final prospectus and accompanying preliminary prospectus supplement relating to the offering may be obtained from the SEC’s website at http://www.sec.gov. At December 31, 2009 the Company had 13,706,202 warrants outstanding with an exercise price of $1.30. The exercise price of these warrants was reduced to $0.50 per share as a result of the offering. As a result of the price reductions an additional 2,838,718 warrants were issued.
The Company evaluates events that have occurred after the balance sheet date but before the consolidated financial statements are issued.
62
Table of Contents
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We maintain certain disclosure controls and procedures. They are designed to ensure that material information is: (1) gathered and communicated to our management, including our principal executive and financial officers, as appropriate, to allow timely decisions regarding required disclosure; and (2) recorded, processed, summarized, reported and filed with the SEC as required under the Securities Exchange Act of 1934, as amended.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2009. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at the reasonable assurance level for their intended purpose described above.
Internal Control Over Financial Reporting
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting for the Company. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America. Internal control over financial reporting includes policies and procedures for maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for the preparation of our financial statements; providing reasonable assurance that receipts and expenditures of the Company are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use or disposition of Company assets that could have a material effect on our financial statements would be prevented or detected on a timely basis. Because of inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected.
Management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, management concluded that the Company’s internal control over financial reporting was effective as of December 31, 2009. Marcum, LLP, our independent registered public accounting firm, has issued an attestation report on our internal control over financial reporting as of December 31, 2009.
Our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls or internal controls over financial reporting will prevent all errors or all instances of fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Controls can also be circumvented by the individual acts of some
63
Table of Contents
persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and any design may not succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures. Because of the inherent limitation of a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
64
Table of Contents
Attestation Report of the Registered Public Accounting Firm
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Audit Committee of
The Board of Directors and Stockholders of
Cardium Therapeutics, Inc. and subsidiaries
We have audited Cardium Therapeutics, Inc.’s and subsidiaries’ (the “Company”) internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Management’s Annual Report on Internal Control Over Financial Reporting.” Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that degree of compliance with the policies or procedures may deteriorate.
In our opinion, Cardium Therapeutics, Inc. and subsidiaries maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Cardium Therapeutics, Inc. and subsidiaries as of December 31, 2009 and 2008, and the related consolidated statements of operations, stockholders’ deficiency and cash flows for each of the years in the three-year period ended December 31, 2009 and for the period from December 22, 2003 (date of inception) through December 31, 2009, and our report, dated March 16, 2010, expressed an unqualified opinion on those consolidated financial statements and included an explanatory paragraph as to the Company’s ability to continue as a going concern.
/s/ Marcum, LLP
Marcum, LLP
New York, New York
March 16, 2010
65
Table of Contents
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended December 31, 2009 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
None.
66
Table of Contents
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
The information for this item is incorporated by reference to the sections “Our Board of Directors,” “Our Executive Officers,” “Section 16(a) Beneficial Ownership Reporting Compliance,” and “Code of Ethics” in our definitive proxy statement for our Annual Meeting of Stockholders to be held on June 3, 2010, to be filed on or before April 30, 2010.
ITEM 11. EXECUTIVE COMPENSATION
The information for this item is incorporated by reference to the sections “Director Compensation” and “Executive Officer Compensation” in our definitive proxy statement for our Annual Meeting of Stockholders to be held on June 3, 2010, to be filed on or before April 30, 2010.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information for this item is incorporated by reference to the sections “Stock Holdings of Certain Owners and Management” and “Securities Authorized for Issuance Under Equity Compensation Plans” in our definitive proxy statement for our Annual Meeting of Stockholders to be held on June 3, 2010, to be filed on or before April 30, 2010.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE |
The information for this item is incorporated by reference to the sections “Certain Relationships and Related Transactions” and “Our Board of Directors” in our definitive proxy statement for our Annual Meeting of Stockholders to be held on June 3, 2010, to be filed on or before April 30, 2010.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information for this item is incorporated by reference to the sections “Audit Fees,” “Audit-Related Fees,” “Tax Fees,” “All Other Fees” and “Pre-Approval Policies and Procedures” in our definitive proxy statement for our Annual Meeting of Stockholders to be held on June 3, 2010, to be filed on or before April 30, 2010.
67
Table of Contents
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
The following documents are filed as part of this report:
(1) Financial Statements. The financial statements listed below are included under Item 8 of this report:
| • | Consolidated Balance Sheets as of December 31, 2009 and 2008; |
| • | Consolidated Statements of Operations for the years ended December 31, 2009, 2008 and 2007 and for the period from December 22, 2003 (inception) to December 31, 2009; |
| • | Consolidated Statements of Stockholders’ Equity (Deficiency) for the years ended December 31, 2009, 2008 and 2007; |
| • | Consolidated Statements of Cash Flows for the years ended December 31, 2009, 2008 and 2007 and for the period from December 22, 2003 (inception) to December 31, 2009; and |
| • | Notes to Consolidated Financial Statements. |
(2) Financial Statement Schedules. The following financial statement schedules are included under Item 8 of this report: None.
(3) Exhibits. The following exhibit index shows those exhibits filed with this report and those incorporated by reference:
EXHIBIT INDEX
| Exhibit |
Description |
Incorporated By Reference To | ||
| 3.1 | Second Amended and Restated Certificate of Incorporation of Cardium Therapeutics, Inc. as filed with the Delaware Secretary of State on January 13, 2006 | Exhibit 3(i) of our Registration Statement on Form SB-2 (File No. 333-131104), filed with the commission on January 18, 2006 | ||
| 3.2 | Amended and Restated Bylaws of Cardium Therapeutics, Inc. as adopted on January 12, 2006 | Exhibit 3(ii) of our Registration Statement on Form SB-2 (File No. 333-131104), filed with the Commission on January 18, 2006 | ||
| 3.3 | Certificate of Designation of Series A Junior Participating Preferred Stock | Exhibit 3.2 of our Registration Statement on Form 8-A, filed with the Commission on July 11, 2006 | ||
| 4.1 | Form of Warrant issued to employees and consultants of Innercool Therapies, Inc. | Exhibit 4.1 of our Current Report on Form 8-K dated March 8, 2006, filed with the Commission on March 14, 2006 | ||
| 4.2 | Form of Common Stock Certificate for Cardium Therapeutics, Inc. | Exhibit 4.5 of our Annual Report on Form 10-KSB for the fiscal year ended December 31, 2005, filed with the Commission on March 31, 2006 | ||
| 4.3 | Form of Rights Agreement dated as of July 10, 2006, between Cardium Therapeutics, Inc. and Computershare Trust Company, Inc., as Rights Agent | Exhibit 4.1 of our Registration Statement on Form 8-A, filed with the Commission on July 11, 2006 | ||
| 4.4 | Form of Rights Certificate | Exhibit 4.2 of our Registration Statement on Form 8-A, filed with the Commission on July 11, 2006 | ||
68
Table of Contents
| Exhibit |
Description |
Incorporated By Reference To | ||
| 4.5 | Form of Warrant issued to purchasers in 2007 private financing | Exhibit 4.1 of our Current Report on Form 8-K dated March 6, 2007, filed with the Commission on March 6, 2007 | ||
| 4.6 | Form of Warrant issued to Oppenheimer & Co. Inc. as Placement Agent in 2007 private financing | Exhibit 4.7 of our Annual Report on Form 10-KSB for the fiscal year ended December 31, 2006, filed with the Commission on March 15, 2007 | ||
| 4.7 | Form of Warrant issued to Life Sciences Capital LLC | Exhibit 10.42 of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2007, filed with the Commission on November 14, 2007 | ||
| 4.8 | Form of Warrant issued to purchasers in January 2008 registered direct offering | Exhibit 4.1 of our Current Report on Form 8-K dated January 30, 2008, filed with the Commission on January 31, 2008 | ||
| 4.9 | Form of Warrant issued to purchasers in June 2008 registered direct offering | Exhibit 4.1 of our Current Report on Form 8-K dated June 27, 2008, filed with the Commission on June 30, 2008 | ||
| 4.10 | Form of Warrant issued to Empire Asset Management Company in June 2008 registered direct offering | Exhibit 4.2 of our Current Report on Form 8-K dated June 27, 2008, filed with the Commission on June 30, 2008 | ||
| 4.11 | Form of Warrant issued to purchasers in July 2008 registered direct offering | Exhibit 4.1 of our Current Report on Form 8-K dated July 18, 2008, filed with the Commission on July 21, 2008 | ||
| 4.12 | Form of Warrant issued to Empire Asset Management Company in July 2008 registered direct offering | Exhibit 4.2 of our Current Report on Form 8-K dated July 18, 2008, filed with the Commission on July 21, 2008 | ||
| 4.13 | Form of Common Stock Purchase Warrant issued to investors and the placement agent in the November 2008 debt financing | Exhibit 4.2 of our Current Report on Form 8-K dated November 5, 2008, filed with the Commission on November 13, 2008 | ||
| 4.14 | Convertible Promissory Note issued to TRC Royalty Co. | Exhibit 4.1 of our Current Report on Form 8-K dated February 23, 2009, filed with the Commission on February 26, 2009 | ||
| 4.15 | Form of Senior Subordinated Secured Promissory Note issued to investors in the February 2009 debt financing | Exhibit 4.1 of our Current Report on Form 8-K dated February 27, 2009, filed with the Commission on March 5, 2009 | ||
| 4.16 | Form of Common Stock Purchase Warrant issued to investors and the placement agent in the February 2009 debt financing | Exhibit 4.2 of our Current Report on Form 8-K dated February 27, 2009, filed with the Commission on March 5, 2009 | ||
| 4.17 | Form of Promissory Note issued to investors in the June 2009 debt financing | Exhibit 4.1 of our Current Report on Form 8-K dated June 11, 2009, filed with the Commission on June 16, 2009 | ||
| 4.18 | Form of Common Stock Purchase Warrant issued to investors and the placement agent in the June 2009 debt financing | Exhibit 4.2 of our Current Report on Form 8-K dated June 11, 2009, filed with the Commission on June 16, 2009 | ||
69
Table of Contents
| Exhibit |
Description |
Incorporated By Reference To | ||
| 4.19 | Form of Amendment to Promissory Notes by and among Cardium Therapeutics, Inc. and the investors in the June 2009 debt financing | Exhibit 4.3 of our Current Report on Form 8-K dated 23, 2009, filed with the Commission on June 29, 2009 | ||
| 4.20 | Form of Amendment to Promissory Notes by and among Cardium Therapeutics, Inc. and the holders of senior secured subordinated promissory notes dated February 27, 2009 and March 5, 2009 | Exhibit 4.1 of our Current Report on Form 8-K dated July 10, 2009, filed with the Commission on July 13, 2009 | ||
| 4.21 | Form of Amendment to Promissory Notes by and among Cardium Therapeutics, Inc. and the holders of unsecured promissory notes dated June 11, 2009 and June 23, 2009 | Exhibit 4.2 of our Current Report on Form 8-K dated July 10, 2009, filed with the Commission on July 13, 2009 | ||
| 4.22 | Form of Note Amendment and Release of Collateral by and among Cardium Therapeutics, Inc. and the holders of senior subordinated secured promissory notes dated February 27, 2009 and March 5, 2009 | Exhibit 4.1 of our Current Report on Form 8-K dated July 20, 2009, filed with the Commission on July 21, 2009 | ||
| 4.23 | Form of Note Amendment by and among Cardium Therapeutics, Inc. and the holders of unsecured promissory notes dated June 11, 2009 and June 23, 2009 | Exhibit 4.2 of our Current Report on Form 8-K dated July 20, 2009, filed with the Commission on July 21, 2009 | ||
| 4.24 | Form of Letter Amendment between Cardium Therapeutics, Inc. and certain holders of the November Notes | Exhibit 4.3 of our Current Report on Form 8-K, filed with the Commission on July 21, 2009 | ||
| 4.25 | Form of Common Stock Purchase Warrant issued to investors in the September 2009 registered direct offering | Exhibit 4.1 of our Current Report on Form 8-K dated September 14, 2009, filed with the Commission on September 15, 2009 | ||
| 4.26 | Form of Common Stock Purchase Warrant issued to investors in the October 2009 registered direct offering | Exhibit 4.1 of our Current Report on Form 8-K dated October 15, 2009, filed with the Commission on October 15, 2009 | ||
| 4.27 | Form of Warrant Agreement between Cardium Therapeutics, Inc. and Computershare Trust Company, NA. | Exhibit 4.1 of our Current Report on Form 8-K, filed with the Commission on February 18, 2009 | ||
| 4.28 | Form of Warrant Agreement between Cardium Therapeutics, Inc. and Computershare Trust Company, NA. | Exhibit 4.1 of our Current Report on Form 8-K, filed with the Commission on March 15, 2010 | ||
| 10.1 | Transfer, Consent to Transfer, Amendment and Assignment of License Agreement effective as of August 31, 2005, by and among New York University, Collateral Therapeutics, Inc. and Cardium Therapeutics, Inc. | Exhibit 10.1 of our Current Report on Form 8-K dated October 20, 2005, filed with the Commission on October 26, 2005 | ||
| 10.2 | Transfer, Consent to Transfer, Amendment and Assignment of License Agreement effective as of August 31, 2005, by and among Yale University, Schering Aktiengesellschaft and Cardium Therapeutics, Inc. | Exhibit 10.2 of our Current Report on Form 8-K dated October 20, 2005, filed with the Commission on October 26, 2005 | ||
70
Table of Contents
| Exhibit |
Description |
Incorporated By Reference To | ||
| 10.3 | Transfer, Consent to Transfer, Amendment and Assignment of License Agreement effective as of July 31, 2005, by and among the Regents of the University of California, Collateral Therapeutics, Inc. and Cardium Therapeutics, Inc. | Exhibit 10.3 of our Current Report on Form 8-K dated October 20, 2005, filed with the Commission on October 26, 2005 | ||
| 10.4 | Transfer, Consent to Transfer, Amendment and Assignment of License Agreement effective as of July 31, 2005, by and among the Regents of the University of California, Collateral Therapeutics, Inc. and Cardium Therapeutics, Inc. | Exhibit 10.4 of our Current Report on Form 8-K dated October 20, 2005, filed with the Commission on October 26, 2005 | ||
| 10.5 | Technology Transfer Agreement effective as of October 13, 2005, by and among Schering AG, Berlex, Inc., Collateral Therapeutics, Inc. and Cardium Therapeutics, Inc. | Exhibit 10.5 of Aries’ Current Report on Form 8-K dated October 20, 2005, filed with the Commission on October 26, 2005 | ||
| 10.6 | Amendment to the Exclusive License Agreement for “Angiogenesis Gene Therapy” effective as of October 20, 2005, between the Regents of the University of California and Cardium Therapeutics, Inc. | Exhibit 10.6 of our Current Report on Form 8-K dated October 20, 2005, filed with the Commission on October 26, 2005 | ||
| 10.7 | Amendment to License Agreement effective as of October 20, 2005, by and between New York University and Cardium Therapeutics, Inc. | Exhibit 10.7 of our Current Report on Form 8-K dated October 20, 2005, filed with the Commission on October 26, 2005 | ||
| 10.8 | Second Amendment to Exclusive License Agreement effective as of October 20, 2005, by and between Yale University and Cardium Therapeutics, Inc. | Exhibit 10.8 of our Current Report on Form 8-K dated October 20, 2005, filed with the Commission on October 26, 2005 | ||
| 10.9 | 2005 Equity Incentive Plan as adopted effective as of October 20, 2005* | Exhibit 10.9 of our Current Report on Form 8-K dated October 20, 2005, filed with the Commission on October 26, 2005 | ||
| 10.10 | Employment Agreement dated as of October 20, 2005 by and between Aries Ventures Inc. and Christopher Reinhard* | Exhibit 10.10 of our Current Report on Form 8-K dated October 20, 2005, filed with the Commission on October 26, 2005 | ||
| 10.11 | Employment Agreement dated as of October 20, 2005 by and between Aries Ventures Inc. and Tyler Dylan* | Exhibit 10.11 of our Current Report on Form 8-K dated October 20, 2005, filed with the Commission on October 26, 2005 | ||
| 10.12 | Yale Exclusive License Agreement between Yale University and Schering Aktiengesellschaft dated September 8, 2000 | Exhibit 10.13 of our Annual Report on Form 10-KSB for the fiscal year ended September 30, 2005, filed with the Commission on December 22, 2005 | ||
| 10.13 | Research and License Agreement between New York University and Collateral Therapeutics, Inc. dated March 24, 1997 (with amendments dated April 28, 1998 and March 24, 2000) | Exhibit 10.14 of our Annual Report on Form 10-KSB for the fiscal year ended September 30, 2005, filed with the Commission on December 22, 2005 | ||
71
Table of Contents
| Exhibit |
Description |
Incorporated By Reference To | ||
| 10.14 | Exclusive License Agreement for “Angiogenesis Gene Therapy” between the Regents of the University of California and Collateral Therapeutics, Inc. dated as of September 27, 1995 (with amendments dated September 19, 1996, June 30, 1997, March 11, 1999 and February 8, 2000) | Exhibit 10.15 of our Annual Report on Form 10-KSB for the fiscal year ended September 30, 2005, filed with the Commission on December 22, 2005 | ||
| 10.15 | Michigan License Agreement between the Regents of the University of Michigan and Matrigen, Inc. dated July 13, 1995 | Exhibit 10.33 of our Annual Report on Form 10-KSB for the fiscal year ended December 31, 2006, filed with the Commission on March 15, 2007 | ||
| 10.16 | Amendment to License Agreement between the Regents of the University of Michigan and Matrigen, Inc. dated August 10, 1995 | Exhibit 10.34 of our Annual Report on Form 10-KSB for the fiscal year ended December 31, 2006, filed with the Commission on March 15, 2007 | ||
| 10.17 | Office Lease dated as of September 16, 2006 and commencing on January 20, 2007, by and between Cardium Therapeutics, Inc. and Jaguar Properties, L.L.C. | Exhibit 10.30 of our Annual Report on Form 10-KSB for the fiscal year ended December 31, 2006, filed with the Commission on March 15, 2007 | ||
| 10.18 | Second Amendment to the Michigan License agreement between the Regents of the University of Michigan and Selective Genetics, Inc. dated February 1, 2004 | Exhibit 10.35 of our Annual Report on Form 10-KSB for the fiscal year ended December 31, 2006, filed with the Commission on March 15, 2007 | ||
| 10.19 | Third Amendment to Michigan License Agreement between the Regents of the University of Michigan, and Tissue Repair Company, and Cardium Biologics Inc. dated August 10, 2006 | Exhibit 10.36 of our Annual Report on Form 10-KSB for the fiscal year ended December 31, 2006, filed with the Commission on March 15, 2007 | ||
| 10.20 | First Amendment dated March 16, 2007 to Employment Agreement dated as of October 20, 2005 by and between Aries Ventures Inc. and Christopher Reinhard* | Exhibit 10.38 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2007, filed with the Commission on May 15, 2007. | ||
| 10.21 | First Amendment dated March 16, 2007 to Employment Agreement dated as of October 20, 2005 by and between Aries Ventures Inc. and Tyler Dylan* | Exhibit 10.39 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2007, filed with the Commission on May 15, 2007. | ||
| 10.22 | Office Lease by and between Paseo Del Mar CA LLC and Cardium Therapeutics, Inc., effective as of November 19, 2007 | Exhibit 10.43 of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2007, filed with the Commission on November 14, 2007 | ||
| 10.23 | Form of Securities Purchase Agreement dated January 30, 2008, by and between Cardium Therapeutics, Inc. and each purchaser in the January 2008 registered direct offering (an agreement on substantially this form was signed by each purchaser in the offering) | Exhibit 10.1 of our Current Report on Form 8-K dated January 30, 2008, filed with the Commission on January 31, 2008 | ||
72
Table of Contents
| Exhibit |
Description |
Incorporated By Reference To | ||
| 10.24 | Form of Securities Purchase Agreement dated June 27, 2008, by and between Cardium Therapeutics, Inc. and each purchaser in the June 2008 registered direct offering (an agreement on substantially this form was signed by each purchaser in the offering) | Exhibit 10.1 of our Current Report on Form 8-K dated June 27, 2008, filed with the Commission on June 30, 2008 | ||
| 10.25 | Form of Securities Purchase Agreement dated July 18, 2008, by and between Cardium Therapeutics, Inc. and each purchaser in the July 2008 registered direct offering (an agreement on substantially this form was signed by each purchaser in the offering) | Exhibit 10.1 of our Current Report on Form 8-K dated July 18, 2008, filed with the Commission on July 21, 2008 | ||
| 10.26 | Form of Note and Warrant Purchase Agreement, dated as of November 5, 2008, by and among Cardium Therapeutics, Inc., Innercool Therapies, Inc., Tissue Repair Company and each investor in the November 2008 debt financing | Exhibit 10.1 of our Current Report on Form 8-K dated November 5, 2008, filed with the Commission on November 13, 2008 | ||
| 10.27 | Security Agreement dated as of November 5, 2008, by and among Cardium Therapeutics, Inc., Innercool Therapies, Inc., Tissue Repair Company and Robert Marvin as collateral agent | Exhibit 10.2 of our Current Report on Form 8-K dated November 5, 2008, filed with the Commission on November 13, 2008 | ||
| 10.28 | Form of Note and Warrant Purchase Agreement, dated as of February 27, 2009, by and among Cardium Therapeutics, Inc., Innercool Therapies, Inc., Tissue Repair Company and each investor in the February 2009 debt financing | Exhibit 10.1 of our Current Report on Form 8-K dated February 27, 2009, filed with the Commission on March 5, 2009 | ||
| 10.29 | Security Agreement dated as of February 27, 2009, by and among Cardium Therapeutics, Inc., Innercool Therapies, Inc., Tissue Repair Company and Dr. Robert Marshall as collateral agent | Exhibit 10.2 of our Current Report on Form 8-K dated February 27, 2009, filed with the Commission on March 5, 2009 | ||
| 10.30 | Placement Agency Agreement dated February 27, 2009, by and among Cardium Therapeutics, Inc., Innercool Therapies, Inc., Tissue Repair Company and Empire Asset Management Company | Exhibit 10.3 of our Current Report on Form 8-K dated February 27, 2009, filed with the Commission on March 5, 2009 | ||
| 10.31 | Form of Promissory Note and Warrant Purchase Agreement with investors in the June 2009 debt financing | Exhibit 10.1 of our Current Report on Form 8-K dated June 11, 2009, filed with the Commission on June 16, 2009 | ||
| 10.32 | Placement Agency Agreement dated June 5, 2009, by and among Cardium Therapeutics, Inc. and Empire Asset Management Company | Exhibit 10.2 of our Current Report on Form 8-K dated June 6, 2009, filed with the Commission on June 16, 2009 | ||
| 10.33 | Asset Purchase Agreement dated July 10, 2009, by and among Innercool Therapies, Inc., Cardium Therapeutics, Inc., and Philips Electronics North America Corporation | Exhibit 10.1 of our Current Report on Form 8-K dated July 10, 2009, filed with the Commission on July 15, 2009. | ||
73
Table of Contents
| Exhibit |
Description |
Incorporated By Reference To | ||
| 10.34 | Letter Agreement dated September 10, 2009, by and between Cardium Therapeutics, Inc. and Dawson James Securities Inc. | Exhibit 10.2 of our Current Report on Form 8-K dated September 14, 2009, filed with the Commission on September 15, 2009 | ||
| 10.35 | Securities Purchase Agreement dated September 14, 2009, by and among Cardium Therapeutics, Inc. and the investors named therein with respect to the September 2009 registered direct offering. | Exhibit 10.1 of our Current Report on Form 8-K dated September 14, 2009, filed with the Commission on September 15, 2009 | ||
| 10.36 | First Amendment to Letter Agreement dated October 15, 2009, by and between Cardium Therapeutics, Inc. and Dawson James Securities Inc. | Exhibit 10.3 of our Current Report on Form 8-K dated October 15, 2009, filed with the Commission on October 15, 2009 | ||
| 10.37 | Securities Purchase Agreement dated October 15, 2009, by and among Cardium Therapeutics, Inc. and the investors named therein with respect to the October 2009 registered direct offering. | Exhibit 10.1 of our Current Report on Form 8-K dated October 15, 2009, filed with the Commission on October 15, 2009 | ||
| 10.38 | Placement Agent Agreement dated February 16, 2010, by and between Cardium Therapeutics, Inc. and Dawson James Securities, Inc. | Exhibit 10.1 of our Current Report on Form 8-K dated February 16, 2010, filed with the Commission on February 18, 2010 | ||
| 21 | Subsidiaries of the registrant | Filed herewith | ||
| 23.1 | Consent of Marcum LLP | Filed herewith | ||
| 31.1 | Rule 13a-14(a)/15d-14(a) Certification of Chief Executive Officer | Filed herewith | ||
| 31.2 | Rule 13a-14(a)/15d-14(a) Certification of Chief Financial Officer | Filed herewith | ||
| 32 | Section 1350 Certification | Filed herewith | ||
| * | Indicates management contract or compensatory plan or arrangement. |
74
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, Cardium Therapeutics, Inc., the registrant, has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: March 16, 2010
| CARDIUM THERAPEUTICS, INC. | ||
| By: | /s/ CHRISTOPHER J. REINHARD | |
| Christopher J. Reinhard, Chief Executive Officer | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby severally constitutes and appoints Christopher J. Reinhard and Dennis M. Mulroy, and each of them, his true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution for him and in his name, place and stead, in any and all capacities to sign any and all amendments to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Commission, granting unto said attorney-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that each said attorneys-in-fact and agents or any of them or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of Cardium Therapeutics, Inc., in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ CHRISTOPHER J. REINHARD
(Christopher J. Reinhard) |
Chief Executive Officer and Chairman of the Board of Directors (principal executive officer) |
March 16, 2010 | ||
| /s/ DENNIS M. MULROY
(Dennis M. Mulroy) |
Chief Financial Officer (principal financial officer and principal accounting officer) |
March 16, 2010 | ||
| /s/ TYLER M. DYLAN-HYDE
(Tyler M. Dylan-Hyde) |
Director |
March 16, 2010 | ||
| /s/ EDWARD W. GABRIELSON
(Edward W. Gabrielson) |
Director |
March 16, 2010 | ||
| /s/ MURRAY H. HUTCHISON
(Murray H. Hutchison) |
Director |
March 16, 2010 | ||
| /s/ ANDREW M. LEITCH
(Andrew M. Leitch) |
Director |
March 16, 2010 | ||
| /s/ GERALD J. LEWIS
(Gerald J. Lewis) |
Director |
March 16, 2010 | ||
| /s/ LON E. OTREMBA
(Lon E. Otremba) |
Director |
March 16, 2010 | ||
75
