Attached files
| file | filename |
|---|---|
| EX-32.2 - CERTIFICATION OF CFO PURSUANT TO SECTION 906 - MAKO Surgical Corp. | mako101092_ex32-2.htm |
| EX-99.1 - REGISTRATION RIGHTS AGREEMENT BY AND BETWEEN REGISTRANT AND Z-KAT, INC. - MAKO Surgical Corp. | mako101092_ex99-1.htm |
| EX-23 - CONSENT OF ERNST & YOUNG LLP - MAKO Surgical Corp. | mako101092_ex23.htm |
| EX-31.2 - CERTIFICATION OF CFO PURSUANT TO SECTION 302 - MAKO Surgical Corp. | mako101092_ex31-2.htm |
| EX-31.1 - CERTIFICATION OF CEO PURSUANT TO SECTION 302 - MAKO Surgical Corp. | mako101092_ex31-1.htm |
| EX-32.1 - CERTIFICATION OF CEO PURSUANT TO SECTION 906 - MAKO Surgical Corp. | mako101092_ex32-1.htm |
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
þ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF
THE SECURITIES
EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2009
Commission file number: 001-33966
MAKO SURGICAL CORP.
(Exact
Name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
|
20-1901148 |
|
(State or Other Jurisdiction of Incorporation or Organization) |
|
(I.R.S. Employer Identification No.) |
|
|
|
|
|
2555 Davie Road, Fort Lauderdale, FL |
|
33317 |
|
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
|
|
|
|
(954) 927-2044 |
||
|
(Registrant’s telephone number, including area code) |
||
|
|
|
|
|
Securities registered pursuant to Section 12(b) of the Act: |
||
|
|
|
|
|
Title of Class |
|
Name of Exchange on Which Registered |
|
|
|
|
|
Common stock, $0.001 par value per share |
|
The NASDAQ Global Market |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No þ
Indicate
by check mark whether the registrant (1) has filed all reports required to
be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934
(the “Exchange Act”) during the preceding 12 months (or for such shorter
period that the registrant was required to file such reports), and (2) has
been subject to such filing requirements for the past 90 days.
Yes þ No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes o No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
|
|
|
|
|
Large accelerated filer o |
Accelerated filer þ |
Non-accelerated filer o |
Smaller reporting company o |
|
|
(Do not check if a smaller reporting company) |
||
Indicate
by check mark whether the registrant is a shell company (as defined in
Rule 12b-2 of the Exchange Act)
Yes o No þ
The aggregate market value of the common stock held by non-affiliates of the registrant as of June 30, 2009 was approximately $134,589,080 (based on a closing price of $9.02 per share on The NASDAQ Global Market as of such date).
As of March 1, 2010, the registrant had outstanding 33,632,696 shares of common stock.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Company’s definitive proxy statement for the 2010 annual meeting of stockholders will be incorporated by reference into Part III of this Annual Report on Form 10-K when filed with the Securities and Exchange Commission.
MAKO Surgical Corp.
INDEX TO FORM 10-K
We have received or applied for trademark registration of and/or claim trademark rights, including in the following marks that appear in this report: “MAKOplasty®,” “RIO®,” “RESTORIS®,” “Tactile Guidance System” and “TGS,” as well as in the MAKO Surgical Corp. “MAKO” logo, whether standing alone or in connection with the words “MAKO Surgical Corp.” All other trademarks, trade names and service marks appearing in this report are the property of their respective owners. Unless the context requires otherwise, the terms “registrant,” “company,” “we,” “us” and “our” refer to MAKO Surgical Corp.
2
This report contains forward-looking statements within the meaning of the U.S. federal securities laws. Statements that are not historical facts, including statements about our beliefs and expectations, are forward-looking statements. Forward-looking statements include statements generally preceded by, followed by or that include the words “believe,” “could,” “expect,” “intend,” “may,” “anticipate,” “plan,” “predict,” “potential,” “estimate” or similar expressions. These statements include, but are not limited to, statements related to:
|
|
|
|
|
|
• |
the nature, timing and number of planned new product introductions; |
|
|
|
|
|
|
• |
market acceptance of the MAKOplasty solution; |
|
|
|
|
|
|
• |
the effect of anticipated changes in the size, health and activities of population on the demand for our products; |
|
|
|
|
|
|
• |
assumptions and estimates regarding the size and growth of certain market segments; |
|
|
|
|
|
|
• |
our ability and intent to expand into international markets; |
|
|
|
|
|
|
• |
the timing and anticipated outcome of clinical studies; |
|
|
|
|
|
|
• |
assumptions concerning anticipated product developments and emerging technologies; |
|
|
|
|
|
|
• |
the future availability from third-party suppliers, including single source suppliers, of implants for and components of our RIO® Robotic Arm Interactive Orthopedic system; |
|
|
|
|
|
|
• |
the viability of maintaining our licensed intellectual property or our ability to obtain additional licenses necessary to our growth; |
|
|
|
|
|
|
• |
the anticipated adequacy of our capital resources to meet the needs of our business; |
|
|
|
|
|
|
• |
our continued investment in new products and technologies; |
|
|
|
|
|
|
• |
the ultimate marketability of newly launched products and products currently being developed; |
|
|
|
|
|
|
• |
the ability to implement new technologies successfully; |
|
|
|
|
|
|
• |
our goals for sales and earnings growth; |
|
|
|
|
|
|
• |
our ability to sustain sales and earnings growth; |
|
|
|
|
|
|
• |
our success in achieving timely approval or clearance of products with domestic and foreign regulatory entities; |
|
|
|
|
|
|
• |
the stability of certain domestic and foreign economic markets; |
|
|
|
|
|
|
• |
the impact of anticipated changes in the U.S. healthcare industry and the medical device industry and our ability to react to and capitalize on those changes; |
|
|
|
|
|
|
• |
future declarations of cash dividends; and |
|
|
|
|
|
|
• |
the impact of any managerial changes. |
Forward-looking statements reflect our current expectations and are not guarantees of performance. These statements are based on management’s beliefs and assumptions, which in turn are based on currently available information. Important assumptions relating to these forward-looking statements include, among others, assumptions regarding demand for our products, expected pricing levels, raw material costs, the timing and cost of planned capital expenditures, competitive conditions and general regulatory and economic conditions. You are cautioned that reliance on any forward-looking statement involves risks and uncertainties. Although we believe that the assumptions on which the forward-looking statements contained herein are based are reasonable, any of those assumptions could prove to be inaccurate given the inherent uncertainties as to the occurrence or nonoccurrence of future events. There can be no assurance that the forward-looking statements contained in this
3
report will prove to be accurate. The inclusion of a forward-looking statement in this report should not be regarded as a representation by us that our objectives will be achieved.
Forward-looking statements also involve risks and uncertainties, which could cause actual results to differ materially from those contained in any forward-looking statement. Many of these factors are beyond our ability to control or predict and could, among other things, cause actual results to differ from those contained in forward-looking statements made in this report. Such factors, among others, may have a material adverse effect on our business, financial condition and results of operations and may include, but are not limited to, factors discussed under Item 1A, “Risk Factors,” and the following:
|
|
|
|
|
|
• |
a continued economic downturn or delayed economic recovery that may have a significant adverse effect on the ability of our customers to secure adequate funding, including access to credit, for the purchase of our products or that may cause our customers to delay a purchasing decision; |
|
|
|
|
|
|
• |
changes in general economic conditions and interest rates; |
|
|
|
|
|
|
• |
changes in the availability of capital and financing sources, for our company and our customers; |
|
|
|
|
|
|
• |
changes in competitive conditions and prices in our markets; |
|
|
|
|
|
|
• |
changes in the relationship between supply of and demand for our products; |
|
|
|
|
|
|
• |
fluctuations in costs of raw materials and labor; |
|
|
|
|
|
|
• |
changes in other significant operating expenses; |
|
|
|
|
|
|
• |
unanticipated issues related to intended product launches; |
|
|
|
|
|
|
• |
decreases in sales of our principal product lines; |
|
|
|
|
|
|
• |
slow downs or inefficiencies in our product research and development efforts; |
|
|
|
|
|
|
• |
increases in expenditures related to increased or changing government regulation or taxation of our business; |
|
|
|
|
|
|
• |
unanticipated issues associated with any healthcare reform legislation that may be enacted; |
|
|
|
|
|
|
• |
unanticipated changes in reimbursement to our customers for our products; |
|
|
|
|
|
|
• |
unanticipated issues in securing regulatory clearance or approvals for new products or upgrades or changes to our products; |
|
|
|
|
|
|
• |
developments adversely affecting our potential sales activities outside the United States; |
|
|
|
|
|
|
• |
increases in cost containment efforts by group purchasing organizations; |
|
|
|
|
|
|
• |
loss of key management and other personnel or inability to attract such management and other personnel; |
|
|
|
|
|
|
• |
increases in costs of retaining a direct sales force and building a distributor network; |
|
|
|
|
|
|
• |
unanticipated issues related to, or unanticipated changes in or difficulties associated with, the recruitment of agents and distributors of our products; |
|
|
|
|
|
|
• |
unanticipated expenditures related to any future litigation; and |
|
|
|
|
|
|
• |
unanticipated intellectual property expenditures required to develop, market and defend our products. |
We caution you not to place undue reliance on these forward-looking statements, as they speak only as of the date they were made. We do not undertake any obligation to release any revisions to these forward-looking statements publicly to reflect events or circumstances after the date of this report or to reflect the occurrence of unanticipated events.
4
|
|
|
|
BUSINESS |
Overview
We are an emerging medical device company that markets our advanced robotic arm solution and orthopedic implants for minimally invasive orthopedic knee procedures. We offer MAKOplasty, an innovative, restorative surgical solution that enables orthopedic surgeons to consistently, reproducibly and precisely treat patient specific, early to mid-stage osteoarthritic knee disease.
We were incorporated in Delaware in November 2004. In February 2008, our common stock began trading on The NASDAQ Global Market under the ticker symbol “MAKO” in connection with the closing of our initial public offering, or IPO.
MAKOplasty is performed using our proprietary RIO® Robotic Arm Interactive Orthopedic system, or RIO, and proprietary RESTORIS® unicompartmental and RESTORIS® MCK multicompartmental knee implant systems. The RIO is a technology platform that we believe has potential future application for other orthopedic procedures beyond the knee. The RIO utilizes tactile guided robotic arm technology and patient specific visualization to prepare the knee joint for the insertion and alignment of our resurfacing implants through a small incision in a minimally invasive, bone preserving and tissue sparing procedure. Our RESTORIS family of knee implants is designed to enable minimally invasive restoration of one or two of the diseased compartments of the knee joint. We believe MAKOplasty will empower physicians to address the needs of the large and growing, yet underserved population of patients with early to mid-stage osteoarthritic knee disease who desire a restoration of quality of life and reduction of pain, but for whom current surgical treatments are not appropriate or desirable due to the highly invasive nature of such procedures, the slow recovery and the substantial costs of rehabilitation, medication and hospitalization.
Unlike conventional knee replacement surgery, which requires extraction and replacement of the entire joint, MAKOplasty enables resurfacing of one or two specific diseased compartments of the joint, preserving significantly more soft tissue and healthy bone of the knee. We believe localized resurfacing can be optimized using our robotic arm technology, which offers consistently reproducible precision to surgeons to achieve optimal implant placement and alignment for smaller, more easily inserted modular implant components. We believe that the tissue sparing and bone conserving techniques enabled with MAKOplasty can offer substantial advantages to patients, surgeons and healthcare providers. Because of the minimally invasive nature of the procedure, smaller incisions are possible, which lead to less tissue damage and faster recoveries, thereby reducing the overall costs of rehabilitation, medication and hospitalization. In addition, because more of the patient’s natural anatomy is preserved and less trauma is inflicted on the knee, we believe that patients who undergo MAKOplasty have the potential to experience better functionality and more natural knee movements, thereby achieving an improved post-operative quality of life. Significantly, the expansion of our RESTORIS family of implants for use in single and bicompartmental knee resurfacing procedures provides the ability to address a broader range of the patient population suffering from early to mid-stage osteoarthritis. Finally, because our RIO system is easy to use, we believe that our MAKOplasty solution makes resurfacing procedures accessible to orthopedic surgeons with a broad range of training and skills and has the potential to lead to greater adoption of knee resurfacing solutions for early to mid-stage osteoarthritis of the knee.
In May 2005, we obtained 510(k) marketing clearance from the U.S. Food and Drug Administration, or FDA, for a haptic imageless surgical guidance system. In November 2005, we obtained 510(k) marketing clearance from the FDA for version 1.0 of our Tactical Guidance System, or TGS, a CT based patient specific visualization system with a robotic arm, which was the predecessor device to our RIO system and enabled MAKOplasty for a single compartment of the knee joint using a standard unicompartmental implant. In January 2008, we received 510(k) marketing clearance from the FDA for version 1.2 of our TGS, which allowed for MAKOplasty using an alternative standard unicompartmental knee implant and incorporated several software upgrades developed and introduced since the commercial introduction of version 1.0. We commercially launched
5
version 1.2 in the first quarter of 2008. In the third quarter of 2008, we commercially launched version 1.3 of our TGS (the modifications to which did not require 510(k) marketing clearance).
In the fourth quarter of 2008, we received 510(k) marketing clearance from the FDA for our RIO system, which is version 2.0 of our TGS, which we commercially launched in the first quarter of 2009. In the third quarter of 2009, we commercially launched version 2.1 of our RIO system (the modifications to which did not require 510(k) marketing clearance), which reflected further refinement of the RIO platform and knee application. In the fourth quarter of 2009, we commercially launched version 2.2 of our RIO system (the modifications to which did not require 510(k) marketing clearance), which enabled surgeons to treat lateral compartment knee arthritis.
In the first quarter of 2006, we received 510(k) marketing clearance for our tibial inlay knee implant system and in the fourth quarter of 2007 we received 510(k) marketing clearance for our tibial onlay knee implant system and for our combined inlay and onlay system, branded as RESTORIS. In the second quarter of 2008, we received 510(k) marketing clearance for our novel unicompartmental knee implant and 510(k) marketing clearance for our patellofemoral knee implant, predecessor components of our proprietary multicompartmental knee implant system. In the fourth quarter of 2008, we received 510(k) marketing clearance from the FDA for our multicompartmental knee implant system, branded as RESTORIS MCK. We commercially launched the RESTORIS MCK multicompartmental knee implant system in the second quarter of 2009.
As part of the original TGS sales contracts, TGS customers were entitled to receive an upgrade of their TGS unit to a RIO system at no additional charge, with the exception of one customer who had the right to receive it at a discounted price. During the first half of 2009, we upgraded all seventeen TGS units to RIO systems. In 2009, we commercially installed nineteen new RIO systems, bringing the total number of commercial MAKOplasty sites to 36 as of December 31, 2009. A total of 1,602 MAKOplasty procedures were performed in 2009 and as of December 31, 2009, 2,384 MAKOplasty procedures had been performed since commercial introduction in June 2006. As of March 1, 2010, we have 39 post-market studies of MAKOplasty, either recently completed or in progress, which are aimed at demonstrating the accuracy of the placement and alignment of our knee implants and the clinical value of the MAKOplasty procedure. To date, the results of these studies have been presented as peer-reviewed presentations at conferences in the United States and abroad. As of March 1, 2010, we have an intellectual property portfolio of more than 300 licensed or owned patents and patent applications relating to the areas of computer assisted surgery, robotics, haptics and implants.
We generate revenue from unit sales of our RIO system, sales of our implants and disposable products and the sale of extended warranty service contracts.
Industry Background
The Growing Osteoarthritis Problem
Osteoarthritis is a common medical condition that leads to the degeneration of joints from aging and repetitive stresses, resulting in a loss of the flexibility, elasticity and shock-absorbing properties of the joints. As osteoarthritis disease progresses, the cartilage and other soft tissues protecting the surfaces of key joints in the body, including knees, hips and shoulders, deteriorate, resulting in substantial and chronic joint pain, numbness and loss of motor function. This pain can be overwhelming for patients and can have significant physical, psychological, quality of life and financial implications. According to estimates by the National Institutes of Health, or NIH, 27 million people in the U.S., age 25 and older, suffer from osteoarthritis.
Compelling demographic trends, such as the growing, aging and more active population and rising obesity rates are expected to be key drivers in the continued growth of osteoarthritis. The NIH projects that by 2030, 20% of Americans, or approximately 72 million people, will be 65 years or older and will be at high risk of developing osteoarthritis. According to The Journal of the American Medical Association, it is estimated that of the U.S. population over the age of 20 approximately 68% was overweight and 34% was obese in 2007-2008. The Orthopaedic Industry Annual Report for the year ended June 15, 2009 reports that being overweight
6
significantly increases the risk of developing knee osteoarthritis and that obese women had nearly four times the risk of suffering from osteoarthritis of the knee as non-obese women, and obese men had nearly five times the risk of suffering from osteoarthritis of the knee as non-obese men.
For the most severe cases of osteoarthritis, in which patients suffer from extreme pain, reconstructive joint surgery may be required. Reconstructive joint surgery involves the removal of the bone area surrounding the affected joint and the insertion of one or more manufactured implants as a replacement for the affected bone. According to The Orthopaedic Industry Annual Report, global sales of joint replacement products in 2008, including knees, hips, elbows, wrists, digits and shoulders, were $12.7 billion, an increase in sales of nearly 9% over 2007, of which sales in the United States represented $6.7 billion. According to Frost & Sullivan, the joint implant market is expected to grow to approximately $9.7 billion by 2013, with knee and hip implant systems representing the two largest sectors.
Market for Osteoarthritis of the Knee
The knee joint consists of the medial, patellofemoral and lateral compartments. As depicted below by the shaded diseased areas of the knee joint, osteoarthritis of the knee usually begins with the deterioration of the soft tissue and cartilage in the medial (inner) compartment and progresses to either or both the patellofemoral (sub-kneecap) and lateral (outer) compartments. The progression of osteoarthritis of the knee can take many years, and even in the early stages, it can result in substantial pain for the patient and a reduction in the quality of life.
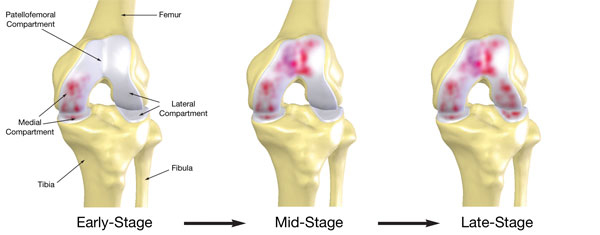
According to the Centers for Disease Control, there are currently more than 15 million people in the U.S. with osteoarthritis of the knee. According to a 2006 Duke University Survey of published literature, the growth of osteoarthritis of the knee among the U.S. population is expected to accelerate as the increasingly active population ages and obesity rates increase. As a result of this substantial clinical need, the market for orthopedic knee procedures in the U.S. has experienced tremendous growth over the past decade. According to data compiled by Orthopedic Network News, the U.S. knee implant market was greater than $3.7 billion in 2008, which represents growth of 9.2% from 2007 to 2008. In addition to the substantial costs of the procedure itself, total knee replacement and resurfacing procedures represent significant incremental costs to the healthcare system. These include costs associated with rehabilitation, medication, hospitalization and, over the long-term, costs incurred as a result of replacements or revisions that may be required due to wear and tear or improper placement.
Current Orthopedic Knee Arthroplasty Approaches
Arthroplasty options for treating osteoarthritis of the knee have historically been limited to either total knee replacement surgery or knee resurfacing procedures.
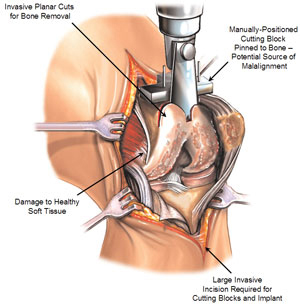
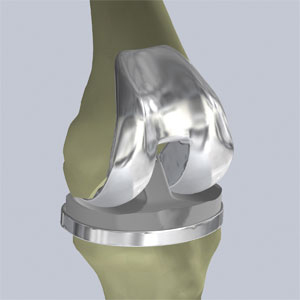
Total Knee Replacement. Currently, most people who choose to surgically address osteoarthritis of the knee elect to undergo total knee replacement surgery. Total knee replacement is a highly invasive surgical procedure in which a patient’s diseased knee joint is removed and replaced with a manufactured replacement knee joint comprised of several components that attempt to mimic the normal function of the knee joint. The procedure requires a large incision ranging from 4 to 12 inches to accommodate the complex scaffold of cutting blocks and jigs required to execute the blunt, planar cuts involved in total knee replacement surgery and to prepare the knee for insertion of the large implants. Both internal and external soft tissue damage is significant in this procedure as the entire knee joint is fully exposed and much of the bone and tissue surrounding it are removed. The bone cuts are also extensive, presenting a large surface area for bone bleeding. The implants are typically manufactured out of metal, ceramic or polymers and have an approximate useful life of between 15 and 20 years before they usually are revised or replaced.
7
The figures below illustrate a conventional total knee replacement surgery and implant:
|
|
|
|
|
|
|
|
|
Total Knee Replacement Surgery |
|
Total Knee Implant |
Despite its long history as an established and effective orthopedic procedure, total knee replacement surgery is not an ideal option for many patients suffering from early to mid-stage, unicompartmental or multicompartmental degeneration of the knee. Some of the principal limitations of total knee replacement surgeries include:
|
|
|
|
|
|
• |
highly invasive nature of the surgical procedure, which requires a large incision ranging from 4 to 12 inches to prepare and implant the large implants; |
|
|
|
|
|
|
• |
significant damage to the bone and tissue surrounding the joint; |
|
|
|
|
|
|
• |
substantial bone bleeding; |
|
|
|
|
|
|
• |
required removal of all three compartments of the knee, regardless of which compartments are actually diseased; |
|
|
|
|
|
|
• |
extended and often painful recovery time and rehabilitation; |
|
|
|
|
|
|
• |
reduced mobility and range of motion; and |
|
|
|
|
|
|
• |
likely implant replacement or revision in approximately 15 to 20 years when the implant reaches the end of its useful life. |
For these and other reasons, many people who are eligible for total knee replacement surgery elect not to undergo or postpone the procedure, choosing instead to suffer significant pain and limited mobility.
Unicompartmental and Bicompartmental Knee Resurfacing. Unicompartmental and bicompartmental knee resurfacing is a less invasive arthroplasty procedure in which only the arthritic region of the knee is removed and a small implant is inserted to resurface the diseased compartment of the knee. Unicompartmental and bicompartmental knee resurfacing procedures are ideal for patients with early to mid-stage osteoarthritis and are aimed at sparing the healthy bone, cartilage and other soft tissues typically removed in a conventional total knee replacement procedure. Today, these procedures are generally performed manually and require a level of training, expertise and precision that significantly exceeds what is required for the typical total knee replacement surgery. Orthopedic Network News has estimated that 49,400 unicompartmental knee resurfacing procedures were performed in 2008 in the United States, which represents a 6.9% increase from the estimated 46,200 such procedures performed in 2007 in the United States.
Unicompartmental and bicompartmental knee resurfacing are potentially more desirable procedures than total knee replacement surgery for patients suffering from early to mid-stage degeneration of the knee because they preserve more of the patient’s natural anatomy and result in less trauma to the patient. As a result, patients experience less tissue loss and faster recoveries. However, despite the potential clinical, quality of life and cost benefits of these procedures, they have achieved only limited adoption to date, in part, as a result of the following limitations that make performing these procedures very difficult:
|
|
|
|
|
|
• |
the restricted room to maneuver and impeded line of sight due to the smaller incision and minimally invasive nature of the procedures which make it difficult to insert, place and align the implant properly; and |
|
|
|
|
|
|
• |
the complex process of removing portions of the bone and resurfacing the knee joint in preparation for the implant. |
8
The difficulties in manually executing unicompartmental or bicompartmental knee resurfacing procedures can result in inaccurate implant alignment, which can lead to reduced range of motion and premature implant failure. In light of the difficulties, many physicians choose not to recommend these procedures and many patients choose either to live with the osteoarthritic pain or to undergo total knee replacement surgery. We currently believe that up to 20% of patients who underwent total knee replacement surgeries had osteoarthritis in only one or two compartments of the knee, which we believe may qualify them as appropriate candidates for a either a unicompartmental or bicompartmental implant.
Introduction of Minimally Invasive Surgery
Over the past thirty years, one of the most significant medical trends has been the development of minimally invasive methods of performing surgical procedures. Compared to traditional, open surgical techniques, minimally invasive techniques that employ image guided surgical systems offer potentially superior benefits for patients, surgeons and hospitals. For patients, these techniques result in reduced procedure related pain and less scarring at the incision site leading to faster recovery times and shorter post-operative hospital stays, as well as better aesthetic outcomes. For the surgeon, these techniques reduce procedure related complications and have the potential to reduce risks associated with more invasive procedures. For the hospital, these procedures can result in reduced hospital stays from faster recovery times and lower rates of complications.
Despite the many benefits of minimally invasive techniques, however, they also present several notable limitations due to the restricted surgical space, including:
|
|
|
|
|
|
• |
restricted vision at the anatomical site; |
|
|
|
|
|
|
• |
cumbersome handling of surgical instruments; |
|
|
|
|
|
|
• |
difficult hand eye coordination; and |
|
|
|
|
|
|
• |
limited tactile feedback. |
Minimally invasive approaches have seen substantial adoption in various surgical fields where procedures can be performed within existing anatomical cavities of the human body. However, because of the limitations of minimally invasive techniques, they have been less successful for complex surgical procedures requiring cutting and replacement of large anatomical parts that nevertheless require precision and control.
Introduction of Robotics into Other Surgical Fields
We believe that the application of robotics technologies in minimally invasive surgical procedures represents the next generation in the evolution of the surgical technique. These technologies are being developed to provide surgeons with a more precise, repeatable and controlled ability to perform complex procedures by offering increased visual acuity and greatly improved tactile feedback. These characteristics empower surgeons to better control their surgical technique and limit the margin of error.
With the assistance of robotics technology, an increasing number of surgeons have been able to perform procedures previously limited to a small subset of highly skilled surgeons. In addition, robotics technology has allowed these procedures to be performed in a more minimally invasive manner, requiring only small incisions, which result in reduced procedure related trauma, fewer infections and post-procedure complications and reduced recovery and hospitalization periods.
Robotics technology has been successfully applied in a variety of diverse fields including urology, gynecology, cardiothoracic surgery and catheter based interventional cardiology and radiology. The success of robotics technologies in these applications has led to the growing adoption and commercialization of these technologies in the medical world.
9
The Use of Robotics in Orthopedic Surgical Procedures
Despite the success of robotics technology in other medical fields, only limited applications have been commercialized in the field of orthopedics to date, although we are aware of current orthopedic robotic development by other companies. Some orthopedic companies have introduced instruments that are smaller than their predecessors, which are marketed as “minimally invasive,” but these instruments still require large incisions, trauma to the soft tissue and removal of large portions of the bone to perform the surgical procedure. Orthopedic companies have also introduced computer assisted surgical, or CAS, systems that are designed for use in open procedures. However, while these systems do provide a minimally invasive means of viewing the anatomical site, their benefits are marginal because they do not improve a surgeon’s ability to make consistently reproducible and precise surgical movements through a small incision.
We believe that the limitations of currently available surgical options for knee disease have created a sizeable market for treatment of a large, growing and underserved population of patients with early to mid-stage osteoarthritis of the knee. We believe that robotics technology is the key to enabling surgeons to perform the kind of minimally invasive knee surgery that results in restoration of function and improved post-operative outcomes for such patients.
The MAKO Solution
We have designed our MAKOplasty solution to provide the consistently reproducible precision, accuracy and dexterity necessary for a surgeon to successfully perform minimally invasive orthopedic arthroplasty procedures on the knee despite a limited field of vision in a confined anatomical space. Our MAKOplasty solution is composed of two critical components: the RIO system, which consists of the proprietary tactile robotic arm and our patient specific visualization system that provides both pre-operative and intra-operative guidance to the surgeon, and the RESTORIS family of knee implant systems that are designed for minimally invasive restoration of the diseased compartments of the joint. By integrating robotic arm and patient specific visualization technology with the touch and feel of the surgeon’s skilled hand, MAKOplasty is designed to enable a level of surgical precision and accuracy that is beyond the scope of the typical surgeon’s freehand capabilities, which we believe will result in broad adoption of our technologies by orthopedic surgeons and better outcomes for patients. We believe knee MAKOplasty offers the following key benefits to patients, surgeons and hospitals:
|
|
|
|
|
|
• |
Minimally Invasive Targeted Knee Arthroplasty. MAKOplasty enables surgeons to isolate and resurface just the diseased compartment or compartments of the knee joint through a minimally invasive incision, rather than replacing the entire joint. The precision of our RIO system robotic arm technology makes such minimally invasive targeted treatment possible by eliminating the complex scaffold of cutting blocks and jigs that would otherwise be required to execute the blunt, planar bone cuts and insert the large implants involved in conventional total knee replacement surgery or a manually executed resurfacing procedure. We believe that our solution will make minimally invasive orthopedic procedures, like unicompartmental and bicompartmental knee resurfacing, a viable option for a greatly expanded pool of patients and physicians. |
|
|
|
|
|
|
• |
Consistently Reproducible Precision. We believe that MAKOplasty reduces the variability of procedure outcomes and increases efficacy through the consistently reproducible precision provided by our computer assisted and tactile robotic arm technology. We believe that the precision of our cutting process and placement and alignment of implants leads to significantly improved and reliable results, compared to conventional, manually executed unicompartmental and bicompartmental resurfacing procedures. The surgeon retains control of the actual movements of the robotic arm within a pre-established volume of space, the tactile “safety zone,” which is tracked and bounded by the RIO system. We believe that the tactile safety zone enables improved placement and alignment of the resurfacing implant, while the visualization enables the procedure to be performed through a small incision without direct visualization. We believe that this consistently reproducible precision enables physicians to be trained in the use of |
10
|
|
|
|
|
|
|
MAKOplasty in a relatively short period of time and also will increase the number of physicians who are willing and able to perform unicompartmental and bicompartmental resurfacing procedures. |
|
|
|
|
|
|
• |
Proprietary Implants. We believe that our proprietary knee resurfacing implants allow surgeons to customize a knee resurfacing solution for individual patients facing early to mid-stage osteoarthritis in one or two compartments of the knee joint. Our original RESTORIS unicompartmental implant system allows for a choice of tibial implant based on patient bone quality. Our RESTORIS MCK implant system provides for this same choice for medial or lateral unicompartmental disease and allows for the resurfacing of the patellofemoral compartment as well, either independently or in combination with the medial compartment in a bicompartmental knee MAKOplasty. Because all implants are sized and planned for based on patient-specific anatomical indications, the potential for favorable clinical outcomes is enhanced. |
|
|
|
|
|
|
• |
Ease of Use. We believe that our RIO system leverages and complements the surgical skills and techniques already familiar to the surgeon, while providing substantial incremental control and precision that has not previously been possible. The customized, patient specific visualization system guides the surgeon through each step of the surgical procedure, while the tactile “safety zone” ensures that the surgeon does not apply the bone cutting instrument beyond the intended area of the knee joint. We believe that the RIO’s ease of use makes resurfacing procedures accessible to orthopedic surgeons with a broad range of training and skills and has the potential to lead to greater adoption of knee resurfacing solutions for early to mid-stage osteoarthritis of the knee. We also believe that the ease of use provided by the RIO can enable physicians to shorten operating room time and potentially increase the number of procedures performed. |
|
|
|
|
|
|
• |
Improved Restorative Post-Operative Outcomes. Due to the minimally invasive nature of the procedure, we believe that patients who undergo knee MAKOplasty are likely to experience less tissue loss, less visible scarring and a faster recovery, thereby reducing the cost of rehabilitation, physical therapy, medication and hospitalization. In addition, because more of the patient’s natural anatomy is preserved and less trauma is inflicted on the knee, patients who undergo MAKOplasty have the potential to experience better mobility, comfort, range of motion and more natural knee movements to achieve an improved post-operative quality of life. |
|
|
|
|
|
|
• |
Reduced Costs for Patients and Hospitals. The minimally invasive nature of the knee MAKOplasty solution aids hospitals and patients in reducing costs by shortening hospital stays and recovery periods and reducing the amount of rehabilitation and medication. |
The comprehensive nature of the MAKOplasty solution also provides hospitals with the implants and disposable products necessary to perform the procedures. We believe that our complete knee arthroplasty solution represents a substantial improvement over currently available approaches.
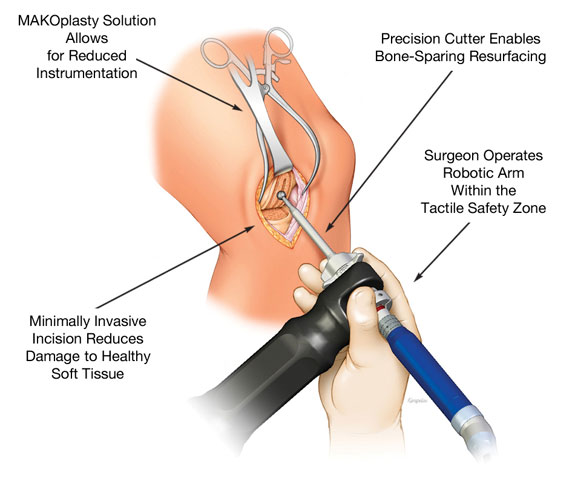
The figure below illustrates a MAKOplasty knee resurfacing procedure.
Our Strategy
Our goal is to continue to drive sales of the RIO system and generate recurring revenue through sales of implants, disposable products and service contracts by establishing MAKOplasty as the preferred surgical procedure for patients with early to mid-stage, unicompartmental and multicompartmental degeneration of the knee. We believe that we can achieve this objective by working with hospitals to demonstrate key benefits of MAKOplasty, such as consistently reproducible surgical precision, improved post-operative outcomes and reduced healthcare costs. Our strategy includes the following key elements:
11
|
|
|
|
|
|
• |
Focus on key physicians and thought leaders to encourage adoption of our MAKOplasty solution. We plan to continue to focus our marketing efforts on key orthopedic surgeons who currently perform the majority of unicompartmental and bicompartmental knee procedures or who are actively involved in the development of minimally invasive orthopedic approaches. We also plan to continue to focus our marketing efforts on the hospitals with which these key surgeons are affiliated and engage them to promote the benefits of MAKOplasty. Our strategy is to convince hospitals that through early adoption of MAKOplasty and acquisition of our RIO system, they can reinforce their reputations as leading institutions for the treatment of early to mid-stage osteoarthritis of the knee. |
|
|
|
|
|
|
• |
Drive volume sales of our RESTORIS family of knee implant systems and the disposable products for installed RIO systems. We intend to increase the number of orthopedic surgeons who use our RIO system and work with the hospitals and their surgeons to promote patient education about the benefits of knee MAKOplasty. Our goal is to increase usage per RIO system to drive higher volume sales of our RESTORIS family of knee implant systems and disposable products. |
|
|
|
|
|
|
• |
Expand the market for multicompartmental knee resurfacing. We plan to expand the market for multicompartmental knee resurfacing procedures by encouraging use of the MAKOplasty procedure for patients who, given only conventional surgical alternatives, would have opted for total knee replacement surgery or no surgery at all. Our current FDA cleared application of MAKOplasty is for unicompartmental and bicompartmental knee resurfacing procedures using the RESTORIS family of knee implant systems, allowing us the potential of accommodating varied patient profiles and surgeon preferences. We believe that the potential benefits of our MAKOplasty solution and the combination of these product offerings will facilitate our efforts to expand and capture the market for multicompartmental knee resurfacing. |
|
|
|
|
|
|
• |
Demonstrate the clinical and financial value proposition of MAKOplasty. We intend to continue to collaborate with leading surgeons and early adopting hospitals through such programs as our MAKOplasty Center of Excellence to build clinical and financial data that support the benefits of MAKOplasty. The MAKOplasty Center of Excellence is a program developed in conjunction with participating hospitals to educate surgeons and patients regarding the benefits of MAKOplasty. As part of the collaborative program, participating hospitals maintain and provide us with certain clinical and financial data that we use to support the business case for the MAKOplasty solution. Our goal is to obtain clinical data further supporting the value of MAKOplasty knee resurfacing procedures, as well as the accuracy and longevity of such implant placements, while demonstrating to hospitals the top and bottom line financial benefits of our MAKOplasty solution. Furthermore, if we are able to commercialize additional applications to our RIO system, we believe that we would be able to further demonstrate the financial value proposition of MAKOplasty to hospitals. |
Our Products
MAKOplasty knee resurfacing procedures are enabled through our proprietary technology consisting of two components: our RIO system and our RESTORIS family of knee implant systems.
The MAKO RIO Robotic Arm Interactive Orthopedic System
The centerpiece of MAKOplasty is the RIO system, our proprietary robotic arm, interactive, orthopedic system, that provides both pre-operative and intra-operative guidance to the orthopedic surgeon, enabling minimally invasive, tissue sparing bone removal and knee implant insertion. The RIO system consists of two elements: a tactile robotic arm utilizing an integrated bone cutting instrument and a patient specific visualization component.
12
The figures below identify the key components of the RIO system’s tactile robotic arm, stereo tracking system and instruments:
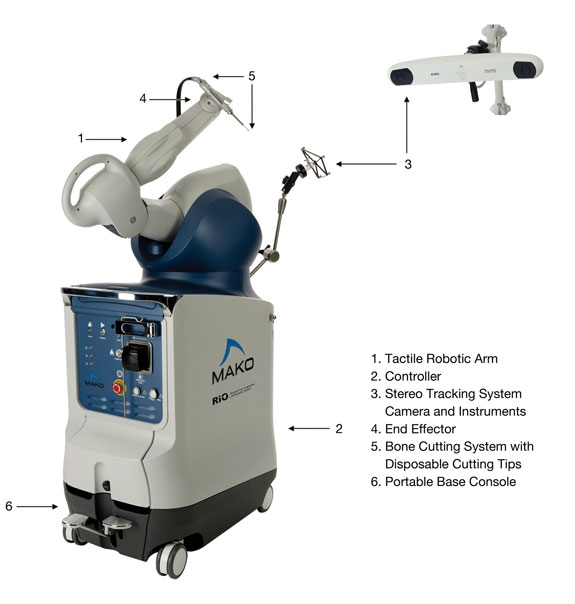
Tactile Robotic Arm System. The tactile robotic arm system consists of the key components identified in the figures above and incorporates the following specifications, features and benefits:
|
|
|
|
|
|
• |
Tactile Robotic Arm — The tactile robotic arm is designed to respond fluidly to movements initiated by the surgeon operating the bone cutting instrument. We have designed the robotic arm to achieve substantial dexterity and range of movement. The robotic arm helps enforce a tactile safety zone that is established by the patient specific visualization system by providing tactile resistance when the boundaries of the tactile safety zone are reached. This tactile resistance helps ensure that the surgeon does not apply the bone cutting instrument beyond the intended area of the knee joint. |
|
|
|
|
|
|
• |
Controller — The controller is the electronic hardware and firmware component of our computing system which interfaces with our proprietary surgical planning and execution software to allow the surgeon to safely guide the tactile robotic arm. The controller governs the basic, low-level functions of the tactile robotic arm, such as the tactile constraints and the safety circuit. |
|
|
|
|
|
|
• |
Stereo Tracking System Camera and Instruments — During a MAKOplasty procedure, the location of the tactile safety zone is updated continuously based on bone tracking data supplied to the computer system by an infrared stereo tracking system, which consists of a special camera that is directed toward a series of spheres and arrays attached to the patient’s anatomy by bone pins. The tracking system assists the robotic arm system in locating and physically tracking the patient’s anatomy and coordinating its real time position with the cutting instrument of the robotic arm. It has a sufficient refresh rate to provide the robotic arm system with an adequate flow of information regarding movements by both the patient and the robotic arm to ensure optimal cutting and placement. Using the system, the surgeon can freely move the robotic arm within the defined space, but encounters tactile resistance as the boundaries of such space are reached. |
|
|
|
|
|
|
• |
End Effector — The end effector is the mechanical component by which the bone cutting instrument is attached to the tactile robotic arm. It is designed to ensure the secure placement of the bone cutting instrument, while providing the flexibility necessary for the surgeon to manipulate the instrument. |
|
|
|
|
|
|
• |
Bone Cutting Instrument with Disposable Cutting Tip — The bone cutting instrument is integrated into the tactile robotic arm at the end effector. This instrument is composed of a high speed motor and a component that houses a variety of single use bone cutting tips. The design of the bone cutting instrument allows the surgeon to grip it in a manner similar to holding a pen-like cutting tool, making it easy to manipulate the instrument in the patient’s anatomy. The cutting tip is the disposable end tip of the bone cutting instrument that makes contact with the knee joint and actually removes the bone for placement of the implant in accordance with the pre-operative plan. In combination with our tactile robotic arm, the bone cutting instrument enables the smooth precision and accuracy necessary for resurfacing procedures. |
|
|
|
|
|
|
• |
Portable Base Console — The base component of our tactile robotic arm is a mobile unit that enables the portability of the tactile robotic arm from one operating room to another. The base console houses the controller and various electrical and mechanical components that help power the tactile robotic arm. Its design enables the console to be situated next to the patient during surgery and the tactile robotic arm to be conveniently positioned over the patient’s anatomy. |
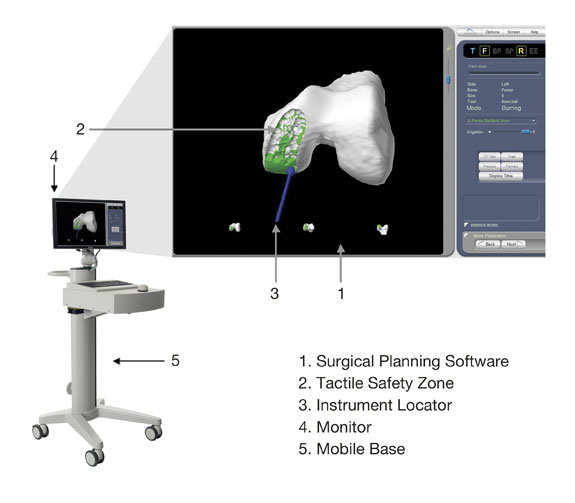
The figure below identifies the key components of the RIO system’s patient specific visualization system:
13
Patient Specific Visualization System. Our patient specific visualization system is a vital part of our ability to deliver minimally invasive surgical procedures for the knee. The surgical team uses our system pre-operatively to plan and intra-operatively to guide the surgical procedure. It consists of the key components identified in the figure above and incorporates the following specifications, features and benefits:
|
|
|
|
|
|
• |
Surgical Planning and Execution Software — Our surgical planning and execution software, which is integrated into our patient specific visualization system, is used during the pre-operative surgical planning process to visualize and map the exact portion of bone to be removed and resurfaced, define the anatomical boundaries of the tactile safety zone and plan the optimal placement and alignment of our implants. During the procedure, the visualization system guides the surgeon through each specific, well defined surgical technique and displays in real time each current and planned surgical activity. |
|
|
|
|
|
|
• |
Tactile Safety Zone — While the robotic arm enforces a tactile safety zone by providing tactile resistance when the boundaries of the tactile safety zone are reached, our patient specific visualization system provides a visual representation of the tactile safety zone and provides additional visual and auditory cues when the boundaries of such tactile safety zone are reached. The combination of this tactile resistance and patient specific visualization helps ensure that the surgeon does not apply the bone cutting instrument beyond the intended area of the knee joint. |
|
|
|
|
|
|
• |
Instrument Locator — The instrument locator provides visual guidance on the position of the bone cutting instrument and other surgical instruments in relation to the patient’s anatomy. |
|
|
|
|
|
|
• |
Monitors — Prior to surgery, patients undergo a conventional CT scan that captures an image of the diseased knee joint. This CT image is uploaded to the patient specific visualization system, where a MAKOplasty Specialist processes the image for display as a 3-D volume in space corresponding to the implant shape and placement overlaid onto the CT image of the patient’s knee joint. This patient specific visualization of our implant overlaid onto an image of the patient’s actual knee joint helps the surgeon to plan the procedure pre-operatively, by providing information which enables the surgeon to determine the optimal placement, alignment and sizing of the implant and establishing the boundaries of the tactile safety zone. During surgery, each monitor projects an active 3-D computer graphics visualization of the patient’s knee joint, showing the areas of the bone that are actually removed as the procedure progresses. The user can also change the viewpoint and zoom level of the visualization as the procedure progresses to focus on different portions of the anatomy. |
|
|
|
|
|
|
• |
Mobile Base — The base component of our patient specific visualization system is a mobile unit that enables the portability of the patient specific visualization system from one operating room to another. It houses our computer hardware and our surgical planning and execution software and various electrical and mechanical components that help power the visualization system. |
Early Versions of the Tactile Guidance System
In November 2005, we obtained 510(k) marketing clearance from the FDA for version 1.0 of our TGS for use with an inlay knee implant system, as described below. We subsequently developed and introduced several upgrades to our TGS, including improvements to our surgical planning software as well as changes to certain instrumentation to make the device easier to use. We determined that these modifications, embodied in version 1.1 of our TGS, did not require the submission of a new 510(k) application.
In January 2008, we obtained 510(k) marketing clearance from the FDA for version 1.2 of our TGS, which became commercially available in the first quarter of 2008. Version 1.2 reflected further refinement of the basic instrumentation set and featured a customized bone cutting instrument and new surgical planning software applications necessary to support unicompartmental resurfacing procedures using a tibial onlay knee implant system. In the third quarter of 2008, we launched version 1.3 of our TGS, which enabled integration of components of both our tibial inlay and tibial onlay knee implant systems into a single MAKO-branded
14
RESTORIS unicompartmental knee implant system, for use with the TGS. These modifications did not require the submission of a new 510(k) application.
The RIO System
The RIO system, version 2.0 of the TGS, represents an important expansion from our first generation TGS, enabling and expanding application of MAKOplasty to multicompartmental resurfacing procedures, allowing orthopedic surgeons to treat degenerative osteoarthritis from early-stage, unicompartmental degeneration through mid-stage, multicompartmental degeneration with a modular knee implant system. In addition, the RIO system incorporates the following improvements, which we believe allow us to offer the benefits of knee MAKOplasty to more patients:
|
|
|
|
|
|
• |
improved dexterity and range of motion in the robotic arm to allow additional degrees of freedom in the movement of the robotic arm; |
|
|
|
|
|
|
• |
more efficient physical configuration of the patient specific visualization system, robotic arm, customized bone cutting instruments and electronic components; |
|
|
|
|
|
|
• |
support for the RESTORIS MCK knee implant system to enable bicompartmental knee procedures; |
|
|
|
|
|
|
• |
intelligent implant planning features to aid surgeon efforts to achieve optimal patient specific alignments; |
|
|
|
|
|
|
• |
redesign of system components to reduce operating room set up times; |
|
|
|
|
|
|
• |
modular design of certain components for ease of manufacturability and assembly and to make them more accessible for service repairs; and |
|
|
|
|
|
|
• |
sophisticated industrial design and state-of-the art user interface. |
In the fourth quarter of 2008, we received 510(k) marketing clearance for the RIO system, which we commercially launched in the first quarter of 2009. In the third quarter of 2009, we launched version 2.1 of our RIO system, which reflected further refinement of the RIO platform and knee application. In the fourth quarter of 2009, we launched version 2.2 of our RIO system, which enabled surgeons to treat lateral compartment knee arthritis. The modifications present in versions 2.1 and 2.2 did not require the submission of new 510(k) applications. The RIO System was approved for CE Marking in December 2009.
The RESTORIS Family of Knee Implant Systems
The second component of MAKOplasty is the knee implant system that is designed for insertion and cementation in a minimally invasive manner. Prior to the development and commercialization of the MAKO-branded, RESTORIS family of knee implant systems in late 2008, we purchased off-the-shelf unicompartmental tibial inlay and tibial onlay implants from third-party suppliers. Currently, we offer the RESTORIS unicompartmental knee implant system and the RESTORIS MCK multicompartmental knee implant system, for use with the RIO system.
The RESTORIS family of knee implant systems allows an orthopedic surgeon to treat early through mid-stage degenerative osteoarthritis of the knee with a modular implant system. We believe that modular components are key to the successful execution of minimally invasive knee surgeries because they can be more easily inserted into the knee joint through smaller incisions than a single, complete device. They can also be positioned independently to better accommodate the specific contours of the patient’s anatomy. Because of the technical design and programming, only the RESTORIS family of knee implant systems may be used effectively with our RIO systems. In addition, users of the RIO system are contractually required to purchase all implants and disposable products used in MAKOplasty procedures from us.
15
The RESTORIS Unicompartmental Knee Implant System
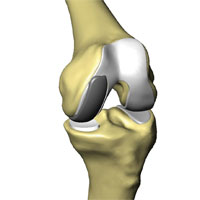
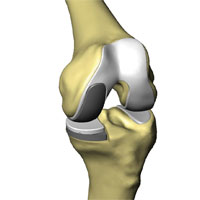
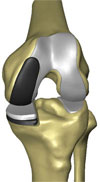
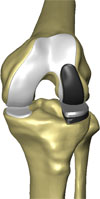
The classic RESTORIS unicompartmental knee implant system is designed to be used in MAKOplasty procedures for preserving critical tissue and bone to achieve improved outcomes. This RESTORIS system is composed of a rounded, anatomically shaped femoral component that attaches to the sculpted surface of the femur and either a flat tibial inlay polymer component that fits into a “pocket” that has been sculpted in the tibial bone or a tibial onlay insert and baseplate consisting of a flat polymer component that is backed by a metal support. Both the femoral and tibial components are offered in multiple sizes to best accommodate the size and shape of the patient’s knee.
Patients with relatively good tibial bone quality, including a sufficiently thick and appropriately located bed of hardened sclerotic tibial bone, are generally candidates for our RESTORIS inlay implant system. The RESTORIS onlay implant system is designed to accommodate patients who lack tibia sclerotic bone beds of sufficient quality. The metal support is placed horizontally on a planar surface prepared on the tibia using the RIO system, supported by the tibial cortical rim, rather than fitted into a pocket of the tibia. Some surgeons also prefer to utilize the tibial cortical rim support in all cases. In the first quarter of 2006, we received 510(k) marketing clearance for our tibial inlay knee implant system, and in the fourth quarter of 2007, we received 510(k) marketing clearance for our tibial onlay knee implant system and on our combined inlay and onlay system, branded as RESTORIS. Our classic RESTORIS onlay unicompartmental knee implant system was approved for CE marking in January 2010.
|
|
|
|
|
|
|
|
|
Inlay Implant |
|
Onlay Implant |
|
|
|
|
|
|
|
|
|
Post-operative |
|
Post-operative |
|
Inlay Implant |
|
Onlay Implant |
|
Placement |
|
Placement |
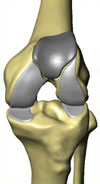
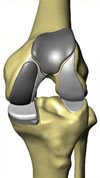
The RESTORIS MCK Multicompartmental Knee Implant System
The RESTORIS MCK multicompartmental knee implant system offers an implant geometry to support the tissue and bone sparing goals of MAKOplasty. Free from the limitations of manual instrumentation, RESTORIS MCK is designed to accurately mimic human anatomy, providing better coverage of diseased compartments while requiring less bone removal and tissue trauma than with traditional treatments.
The RESTORIS MCK system enables surgeons to treat patients suffering from osteoarthritis in any single compartment of the knee joint: the medial (inner), lateral (outer), or patellofemoral (sub-kneecap). The RESTORIS MCK system also enables bicompartmental treatment of the patellofemoral compartment in combination with the medial compartment. Utilizing a modular, bicompartmental system, a surgeon can use the pre- and intra-operative planning software in the RIO system to produce a patient specific implant plan, the result of which is the retention of a greater portion of the knee anatomy than patients treated with a total knee arthroplasty procedure. Surgeons are able to offer inlay and onlay implants medial configurations for both their unicompartmental and bicompartmental procedures. The product offering of the RESTORIS MCK multicompartmental knee implant system also features a patellofemoral component (under the kneecap), which is inset into the knee compartment between the medial and lateral (outer) compartments. Additionally, the surgeon has an array of patella “buttons” to affix to the back of the kneecap to replace the worn surface. In the second quarter of 2008, we received a 510(k) marketing clearance for our novel unicompartmental knee implant and a 510(k) marketing clearance for our patellofemoral knee implant, predecessor components of our proprietary RESTORIS MCK multicompartmental knee implant system. In the fourth quarter of 2008, we received 510(k) marketing clearance from the FDA for our RESTORIS MCK system. The CE marking certification process for the RESTORIS MCK onlay unicompartmental knee implant system is currently ongoing, with approval anticipated in 2010.
16
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Disposable Products
The RIO system utilizes disposable products such as bone pins and the spheres on the arrays used in our tracking system, irrigation clips and tubes that cool the cutting instruments, drapes to cover the robotic arm and other items that require disposal after each use. Disposables are not only a potential source of recurring revenue, but also an opportunity to differentiate our product platform from those of less comprehensive solutions offered by competitors.
Potential Future Applications
Hip MAKOplasty
We believe that the RIO system has the potential to add clinical and economic value in arthoplasty procedures in the hip joint, and we have directed resources to research and development activities in this area.
Market for Osteoarthritis of the Hip. Similar to the knee joint, osteoarthritis of the hip typically begins with degeneration of the hip joint caused by a local tear in the soft tissue surrounding the acetabulum (hip socket) of the hip or an excessive load on the cartilage caused by impingement conditions between the femur and the acetabulum. The progression of osteoarthritis of the hip can take years, but even in the early stages, it can result in substantial pain for the patient and a reduction in the quality of life. According to iData Data Research, Inc., the total hip replacement market in the U.S. in 2010 is estimated to be greater than 360,000 procedures.
Current Treatment Options for Osteoarthritis of the Hip. The current treatments available for osteoarthritis in the hip joint consist of early intervention, sports medicine procedures to treat soft tissue damage as well as more invasive procedures to treat impingement conditions. Conventional treatment for the replacement of the joint consists of a partial hip replacement where only one side of the joint is replaced (normally utilized for hip fractures), hip resurfacing, which is primarily indicated for male patients under the age of 65, and in late stage conditions, total hip arthroplasty. Total hip arthroplasty is a highly invasive surgical procedure in which a patient’s diseased hip joint is removed and replaced with a manufactured replacement hip joint comprised of several components that attempt to mimic the normal function of the hip joint. The procedure requires a large incision ranging from 6 to 12 inches, which often results in significant pain and an extended recovery time. Unlike the knee joint, the hip joint is covered with significant muscle and fat tissue, preventing direct access to the joint without a large incision.
The traditional total hip replacement implant consists of three main components:
|
|
|
|
|
|
• |
a metal cup and a plastic liner that together replace the acetabulum; |
|
|
|
|
|
|
• |
a metal stem inserted into the femoral canal that replaces the femoral neck; and |
17
|
|
|
|
|
|
• |
a highly polished metal ball that attaches onto the metal stem and replaces the femoral head. |
Despite its history as an established and effective orthopedic procedure, we believe that are several limitations of traditional total hip arthroplasty including malalignment of implant components, dislocation, differences in leg length, implant loosening, femoro-acetabular impingement and the eventual need for implant replacement or revision.
Dislocation, where the ball comes completely out of the replacement cup, is the most common reason for revision total hip surgery. According to The Journal of Bone and Joint Surgery, the national average for dislocation of the hip joint within six months of the primary hip replacement procedure is approximately four percent, or approximately 14,000 procedures, annually.
The current methodologies for measuring a patient’s leg length can result in a discrepancy in the length of a patient’s legs. Clinical studies show that leg length discrepancies may result in back pain, increased risk of nerve injury, dislocation, poor patient satisfaction, and the need for revision surgery. According to a study published in Physiotherapy, a leg length discrepancy of ten millimeters or more is associated with a significantly poorer outcome in terms of the clinical benefits of surgery as well as more than twice the incidence of limping than patients with a leg length discrepancy of ten millimeters or less. One methodology currently used by surgeons to measure leg length involves using a ruler to measure anatomical landmarks on the hip before surgery to assess the patient’s preoperative leg length and then measuring the leg length with the implants in place following surgery to assess the change in the patient’s leg length. Another popular methodology used by surgeons requires the surgeon to line the patient’s legs up on the operating room table to assess the leg length before surgery and then to do the same thing with the hip implants in place after surgery. This method requires the legs to be in the exact same position at the time of both measurements and uses only the surgeon’s eyes and sense of touch to assess the change in leg length. We believe that limitations exist with respect to both of these methodologies.
The MAKOplasty Solution. In February 2010, we received 510(k) marketing clearance from the FDA for an application that assists a surgeon in performing all components of a total hip arthroplasty using the RIO system. We believe a hip MAKOplasty application has the potential to provide a surgeon with the same consistently reproducible precision, accuracy, and dexterity as our knee MAKOplasty solution. The hip application would allow the surgeon to preoperatively plan the placement of the hip implants on a three dimensional image CT scan. The RIO system’s robotic arm would then assist the surgeon in preparing the bone accurately to the surgical plan as well as in the positioning of the hip implant, as opposed to the current method of implant placement which involves the use of mechanical jigs and visual alignment by the surgeon, which we believe may potentially decrease the likelihood and severity of leg length discrepancy.
In the same way that the cutting system of the RIO robotic arm allows for the precise resection of bone in the knee joint, we believe that surgeons will use the robotic arm to accurately prepare the patient’s acetabulum for the metal cup. During the insertion of the final implant, the robotic arm will help the surgeon position the cup at the orientation that was planned by the surgeon on the preoperative CT scan. A study at Massachusetts General Hospital, which was presented at the 39th Annual Course, Advances in Arthoplasty in October 2009, found that sixty percent of the acetabular cups placed by the surgeons in the study using commonly used mechanical jigs were placed outside of the optimal zone for avoiding post-operative dislocation. We believe that a hip MAKOplasty application would provide the surgeon with an accurate preoperative surgical plan, knowledge of the patient’s position on the operating room table and accurate implant sizing. This information would allow the surgeon to seat the implants to a final placement following the plan at a level of accuracy that we believe is extremely difficult to accomplish manually, resulting in placement of the implants in the best position to resist dislocation.
We are currently researching our hip MAKOplasty application and we anticipate that our pre-commercialization research and development efforts will continue through at least 2010.
18
Other Potential Applications
We believe that with further research and development, our robotic arm technology has the potential to serve as a platform technology with applications in other areas of the body, such as the shoulder and spine, and we are currently conducting initial research and development to test the viability of MAKOplasty outside of the knee and hip. Should we elect to commercialize additional potential applications of MAKOplasty outside of the knee and hip, we would seek the appropriate marketing clearance from the FDA and any other required regulatory approvals for such applications.
Sales and Marketing
We continue to expand the size of our sales and marketing organization, which is comprised of a direct sales force to sell RIO systems and to commercialize and market MAKOplasty in the U.S. and independent orthopedic product agents and distributors, who primarily generate RIO system sales leads for us. As of March 1, 2010, our sales and marketing group had a total of 79 employees, including 49 direct sales representatives, who are responsible for sales and marketing activity throughout the U.S and 2 global market and sales development employees, who are responsible for defining and executing our global commercialization strategy. We intend to continue to increase the number of sales and marketing personnel as we expand our business. We are currently developing a global market strategy and we believe that our entry into global markets may include the use of independent distributors to market, sell, and support our products.
A portion of our customers acquire our RIO system through a leasing arrangement with a third-party leasing company. In these instances, we typically sell the RIO system to the leasing company, and our customer enters into an independent leasing arrangement with the leasing company. We treat these leasing transactions the same as sales transactions for purposes of recognizing revenue for the sale.
Our sales and marketing goals are to continue to drive capital equipment sales of the RIO system and generate recurring revenue through sales of implants, disposable products and service contracts. To achieve these goals, we must continue to promote adoption of knee MAKOplasty by leading surgeons and hospitals and build demand for the procedure among patients through the following sales and marketing strategy:
|
|
|
|
|
|
• |
Target High Volume Orthopedic Facilities. Our sales representatives actively target hospitals with strong orthopedic reputations and significant knee replacement and resurfacing practices. We believe that adoption by such leading hospitals helps us to seed the market for MAKOplasty and provides the validation and visibility necessary for more widespread adoption. |
|
|
|
|
|
|
• |
Target Facilities with a Strong Strategic Commitment to Grow an Orthopedic Surgery Service Line. Our sales representatives actively target hospitals that have demonstrated a commitment to expand their orthopedic surgery service line. We believe that these hospitals will benefit from the growth in service associated with treating a large number of patients who, given only conventional surgical alternatives, would have delayed surgery or opted for no surgery at all. |
|
|
|
|
|
|
• |
Establish and Promote MAKOplasty Centers of Excellence. The MAKOplasty Center of Excellence is a joint marketing program that we promote in collaboration with participating hospitals to educate surgeons and patients regarding the benefits of MAKOplasty and to coordinate our public relations strategy. As part of the program, hospitals agree to maintain and provide us with certain clinical and financial data that we use in support of our business case for the MAKOplasty solution. As of December 31, 2009, we entered into 32 co-marketing agreements with hospitals to establish MAKOplasty Centers of Excellence. |
|
|
|
|
|
|
• |
Drive Patient Demand for MAKOplasty. During 2009, we continued our marketing efforts to directly educate patients on the benefits of MAKOplasty. We believe that patients are becoming increasingly more involved in the healthcare decision making process and have the potential to influence the adoption |
19
|
|
|
|
|
|
|
of new procedures such as MAKOplasty. Currently, our representatives primarily support hospitals participating in the MAKOplasty Center of Excellence program in their efforts to publicize the benefits of MAKOplasty and educate patients. |
The generation of recurring revenue through sales of our implants, disposable products and service contracts is an important part of the MAKOplasty business model. We anticipate that recurring revenue will constitute an increasing percentage of our total revenue as we leverage each new installation of the RIO system to generate recurring sales of implants and disposable products. We have designed our products so that our RIO system only works with our implant products and we contractually require purchasers of the RIO system to use only our implants in connection with providing MAKOplasty. We also offer a four-year supplemental service contract that provides enhanced levels of maintenance and support services related to the RIO system beyond the basic warranty period.
We provide training to surgeons and hospital staff on the use of the RIO system. Each of our customers also receives pre-operative and intra-operative support from our on-site MAKOplasty Specialist, or MPS, who provides clinical and technical support in connection with each MAKOplasty procedure. The MPS helps set up the equipment, participates in the pre-operative planning process and is present in the operating room with the surgeon, facilitating the surgeon’s use of the RIO system. By increasing familiarity with the system and helping to provide safe and proper usage of our equipment and products by surgeons and hospitals, we hope to promote seamless adoption of MAKOplasty. The presence of an MPS in the surgical theater also provides us with immediate feedback and understanding of our customers’ product preferences and requirements in clinical conditions.
Research and Development
Continued innovation through research and development is critical to our future success. Most of our research and development activity is performed internally. As of March 1, 2010, our research and development team, which is based at our headquarters in Fort Lauderdale, Florida, consisted of 63 employees. We have assembled an experienced team with recognized expertise in advanced robotics, software, instrumentation and orthopedic implants. Although we do not currently have plans to increase the size of our research and development team significantly, we may do so in the future, depending on the progress of our ongoing research and development efforts.
Our research and development efforts are currently focused on improvements to our current knee MAKOplasty solution, including customized bone cutting instruments, alternative registration and tracking systems, and enhanced application workflow, improvements to the RIO system based on customer feedback, and our hip MAKOplasty application. By continually researching improvements to our knee MAKOplasty solution and RIO system, we hope to refine the operating room experience for the surgeon and staff and identify new areas to enhance patient clinical outcomes. We are also conducting advanced research on new robotic platforms as well as early stage development on additional applications for the RIO system and corresponding implant systems for the knee, hip, and other major joints.
In June 2009, we entered into a Research and Development License and Supply Agreement, or the R&D Agreement, associated with the potential future product for RIO enabled hip MAKOplasty procedures. The R&D Agreement required an up-front payment of $450,000, and requires future milestone payments based on development progress. The aggregate milestone payments we are obligated to pay under the R&D Agreement are $1.6 million assuming the achievement of all development milestones. Through December 31, 2009, we had paid the $450,000 up-front payment and we had paid $550,000 of milestone payments which became due upon the achievement of the related milestones. The aggregate up-front payment and milestone payments of $2.0 million we are required to pay under the R&D Agreement will be recognized as research and development expense on a straight-line basis over the period development services are performed based on our current expectation that all development milestones will be achieved.
20
We have historically spent a significant portion of our capital resources on research and development. Our research and development expenses were $13.1 million in 2009, $12.5 million in 2008, and $8.3 million in 2007.
Manufacturing and Assembly
The MAKOplasty solution includes both off-the-shelf and custom made components produced to our specifications by various third parties. We purchase major components of the RIO system, including the computer hardware, the camera used in connection with our tracking system, robotic controller components, high speed bone cutting instrumentation, the molded plastic and machined metal parts, and the various electro-mechanical components that support the RIO system from a number of third-party suppliers. We internally develop the software components and license certain software components that are generally available for commercial use as open source software. We then assemble and integrate these various hardware components with our proprietary software to complete each RIO system. By assembling the final product at our facility, we are able to perform stringent quality assurance inspection and testing on each RIO system to best control the quality of the final product prior to shipment. A portion of our Fort Lauderdale facility is presently dedicated to these warehousing, assembly, testing and inspection activities.
Other than our proprietary software, single source suppliers currently provide us with many of the major components of the RIO system, including the bone cutting instrument and stereo tracking camera. Our RESTORIS family of knee implant systems consists of implants that are custom made to our specifications by several outside manufacturers.
We generally purchase our components through purchase orders and do not have long-term contracts with most of our suppliers. We have, however, entered into long-term contractual arrangements with some of our suppliers, including several single source suppliers, and we are currently negotiating long-term contractual arrangements with many of our remaining suppliers. We also have provided blanket purchase orders and have entered into long-term pricing arrangements with certain suppliers who are not under a long-term contractual arrangement such as key suppliers of the critical components of our products and suppliers to whom we make significant payments for products and services.
By providing blanket purchase orders and entering into long-term contracts and pricing arrangements, we intend to develop and maintain buffer inventory levels at various points in our supply chain to minimize risk. In addition, we intend to achieve improvements in our manufacturing operations and in our cost of revenue by continuing to improve our procurement and third-party manufacturing processes. We have also continued to upgrade our management information systems and to implement enhanced quality assurance, inventory and cost controls to improve the efficiency of our manufacturing operations, maintain product quality, reduce our cost of sales and increase our profitability.
Our operations and those of the third-party suppliers and manufacturers we use are subject to extensive regulation by the FDA under its Quality System Regulations, or QSR, as well as numerous post-market requirements. Our operations and those of third-party suppliers and manufacturers will also be subject to international regulatory requirements as we expand our operations or business overseas. Our facility is FDA registered and we believe is compliant with the FDA’s QSR. We have instituted a quality management system to evaluate and monitor compliance internally and by our third-party suppliers and manufacturers. Our facility and the facilities of the third-party suppliers and manufacturers we use are subject to periodic, announced and unannounced inspections by regulatory authorities, including the FDA and other governmental agencies. We were audited by the FDA in January 2009, during which we received certain inspectional observations. We have addressed the observations and submitted responses to the FDA on a voluntary basis. We received the establishment inspection report (EIR) from the FDA in August 2009. To date, our facilities have not been inspected by any other regulatory authorities. In November 2008, BSi, an independent global certification body, conducted an annual assessment of our quality management system, which concluded that our quality management system complied with the requirements of ISO13485:2003 in all material respects. In December 2009, we received a major nonconformance in our annual assessment by BSi. We implemented corrective actions
21
and in March 2010 the finding was officially closed by BSi. We expect that BSi will continue to conduct annual audits to assess our compliance with BSi certification standards.
Intellectual Property
We must develop, maintain and protect the proprietary aspects of our products and technologies to remain competitive in the marketplace. Our intellectual property portfolio includes rights to patents, patent applications and other intellectual property that we wholly own or license from others. We seek patent and other intellectual property protection in the U.S. and internationally for our products and technologies where available and when appropriate.
We also rely on other forms of intellectual property rights, including copyright, trademark, trade secrets and know-how, to develop, maintain and protect the proprietary aspects of our products and technologies. We require our employees and consultants to execute confidentiality agreements in connection with their employment or consulting relationships with us. We also require our employees and consultants to disclose and assign to us all inventions conceived during the term of their employment or engagement while using our property or which relate to our business.
Despite measures taken to protect our intellectual property, unauthorized parties may attempt to copy aspects of our products or to obtain and use information that we regard as proprietary. In addition, our competitors may independently develop similar technologies. Although patents may provide some degree of protection for our intellectual property, patent protection involves complex legal and factual determinations and is therefore uncertain.
Wholly Owned Patents and Patent Applications
Prior to February 2010, our largest licensing arrangement was with Z-Kat, Inc., or Z-Kat, which was our predecessor company, and from whom we licensed or sublicensed core technologies in CAS, haptics and robotics. In connection with our formation in November 2004, we were granted an exclusive, irrevocable, non-terminable license or sublicense to all intellectual property owned or licensed by Z-Kat in the field of medical orthopedic surgery to the extent Z-Kat’s licenses from third parties were exclusive. Our license from Z-Kat included a limited license to Z-Kat’s CAS and haptic robotic intellectual property portfolio for exclusive use in the field of orthopedics, subject to a prior license to Biomet Manufacturing Corp. to use Z-Kat’s CAS intellectual property, but not its haptic robotic intellectual property, in the field of orthopedics. Because of the prior license to Biomet and pursuant to our license with Z-Kat, we could not use the CAS intellectual property on a stand alone basis; we could only use the CAS intellectual property in combination with robotics technology. Z-Kat’s license also granted to us the sole right to prosecute and maintain all Z-Kat patents and patent applications that are licensed to us. In 2006, we obtained the right to take enforcement action against all third parties with respect to any intellectual property rights held by Z-Kat in the field of orthopedics. We had granted back to Z-Kat a fully paid, royalty-free, nonexclusive sublicense to our intellectual property portfolio in all fields other than orthopedic surgery. Through these and other arrangements, we had rights to Z-Kat’s wholly owned and third-party licensed intellectual property portfolio, which included a wide suite of intellectual property in the areas of haptic robotics and patient specific visualization.
In February 2010, we completed the acquisition of substantially all of the intellectual property portfolio of Z-Kat. The terms of the asset purchase agreement between us and Z-Kat terminated our prior licenses with Z-Kat, including Z-Kat’s nonexclusive sublicense to our intellectual property portfolio, and transferred to us ownership rights to certain intellectual property assets for core technologies in CAS, haptics and robotics, including U.S. and foreign patents and patent applications, proprietary software and documentation, trade secrets and trademarks owned by Z-Kat, and certain contractual and other rights to licensed patents, patent applications and other intellectual property licensed to Z-Kat under licenses. We paid the purchase price in full at the time of closing by issuing 230,458 unregistered shares of our common stock to Z-Kat. In connection with the acquisition, we also entered into a new license agreement with Z-Kat pursuant to which we obtained an exclusive worldwide, fully transferable, perpetual, royalty-free and fully paid-up sublicense to certain intellectual property for
22
technologies in CAS licensed by Z-Kat. This new license agreement expands our rights in this intellectual property from the field of orthopedics to the medical field generally. Certain of our rights under the asset purchase agreement and new license agreement remain subject to any prior license granted by Z-Kat, including the license from Z-Kat to Biomet Manufacturing Corp.
As of March 1, 2010, we held 6 wholly owned granted U.S. patents and 38 wholly owned pending U.S. patent applications. All of these patents and patent applications are either used in our current products or relate to core technologies used in our products, such as CAS, robotics, haptics and implants. The first of our currently pending patent applications was filed in March 2003 and should expire in March 2023, exclusive of any statutory extensions or reductions. As of March 1, 2010, we also held 13 wholly owned granted foreign patents and 80 wholly owned foreign patent applications. We are also continuing to pursue additional U.S. and foreign patent applications on key inventions to enhance our intellectual property portfolio.
Patents and Patent Applications Licensed from Third Parties
As of March 1, 2010, we had licensed rights to 114 U.S. and 40 foreign third-party granted patents, and we had licensed rights to 9 U.S. and 10 foreign third-party pending patent applications. The majority of these patents and applications are either used in our current products or relate to core technologies used in our products, such as CAS, robotics, haptics and implants. We also have rights to additional third-party patents and intellectual property that relate to our core technologies, but are not currently used in our products. Five of the licensed U.S. patents will expire by the end of 2010. One of these patents is considered material to our intellectual property portfolio because it deals with a core technology and potentially enables us to exclude others from practicing the claimed technology. The last licensed patent will expire in 2024.
License Arrangements with Third Parties
In September 2005, we entered into a license agreement pursuant to which we obtained an exclusive, worldwide license to patented technology relating to bone registration and tracking for use in the field of human interactive robotics in orthopedics and a nonexclusive license in the field of orthopedics generally. We paid a one time licensing fee that provides a fully paid, worldwide license for the life of the licensed patents.
In March 2006, we entered into a license agreement that covers a number of technologies related to the application of computers and robotics to surgery. Under the terms of this agreement, we have a nonexclusive, worldwide license to any of the licensor’s patents and patent applications with effective filing dates prior to March 31, 2011 in the field of robotic devices primarily designed for surgery in the medical field of orthopedics and/or primarily designed for spinal surgery in the medical field of neurology. We are obligated to make royalty payments based on the sale of each robotic product covered by the licensor’s patents. This license agreement will terminate upon the expiration of the last licensed patent.
In May 2006, we entered into a sublicense agreement which grants us nonexclusive rights in the field of CAS to a patent directed to core haptic technology. The sublicense also included an option to license or sublicense five additional patents, which we exercised in May 2007. We paid a one time sublicensing fee (and a one time option fee) that provides a fully paid, worldwide license for the life of the licensed patents.
In May 2009, we entered into a license agreement for patents relating to our RIO system, which we refer to as the robotic arm license. The robotic arm license requires minimum running royalties on sales of our RIO systems. The minimum running royalties are estimated to be approximately $600,000 for the year ended December 31, 2010, and increase annually thereafter through 2013. The minimum running royalties for the year ended December 31, 2013 and for each subsequent year through the term of the agreement are estimated to be approximately $1.3 million annually.
As noted above, in February 2010, we entered into a new license agreement with Z-Kat pursuant to which we obtained an exclusive worldwide, fully transferable, perpetual, royalty-free and fully paid-up sublicense to intellectual property for technologies in CAS in the medical field. This new license agreement expands our rights
23
in this intellectual property from the field of orthopedics to the medical field generally but remains subject to any prior license, including the license to Biomet Manufacturing Corp., an affiliate of Biomet, Inc.
Competition
Our success depends on convincing hospitals, surgeons and patients to utilize our robotic arm technology to perform unicompartmental and bicompartmental resurfacing of the knee. We face competition from large, well-known companies, principally Biomet, Inc., DePuy Orthopedics, Inc., a Johnson & Johnson company, Stryker Corporation, and Zimmer Holdings, Inc., that dominate the market for orthopedic products. Each of these companies, as well as other companies like Smith & Nephew, Inc., which introduced the non-modular Journey Deuce Bi-Compartmental Knee System in July 2007, offers conventional instruments and implants for use in conventional total and partial knee replacement surgeries as well as unicompartmental resurfacings procedures, which may compete with our MAKOplasty solution and negatively impact sales of our RIO system. A number of these and other companies also offer CAS systems for use in arthroplasty procedures that provide a minimally invasive means of viewing the anatomical site. In addition, Biomet has a license from Z-Kat and us to intellectual property rights in computer assisted surgery, or CAS intellectual property, for use in the field of orthopedics. The license is non-exclusive with respect to use of CAS intellectual property in combination with robotics technology and exclusive with respect to all other uses within the field of orthopedics, which could enable them to compete with us.
Currently, we are not aware of any well-known orthopedic companies that broadly offer robotics technology in combination with CAS. These large, well-known orthopedic companies, however, have the ability to acquire and develop robotics technology that may compete with our products. We are aware of certain early stage companies developing robotic applications in orthopedics and others commercializing customized implants and instruments for early and mid-stage arthroplasty solutions. For example, CUREXO Technology Corporation has engaged in marketing in the United States of its ROBODOC® Surgical System, which received 510(k) marketing clearance from the FDA in August 2008 for total hip arthroplasty procedures.
We also face competition from other medical device companies that may seek to extend robotics technology and minimally invasive approaches and products that they have developed for use in other parts of the human anatomy to minimally invasive arthroplasty of the knee. Even if these companies currently do not have an established presence in the field of minimally invasive surgery for the knee, they may attempt to apply their robotics technology to the field of knee replacement and resurfacing procedures to compete directly with us.
Even if our RIO system is commercially successful and becomes a market leader in the field of orthopedics with robotic arm technology, our implant products may face substantial competition from implants offered by the well-known companies currently in the market for orthopedic products. We have designed our products so that our RIO system only works with our implant products. We also contractually require purchasers of our RIO system to use only our implants in connection with MAKOplasty procedures. We cannot guarantee, however, that these measures will be effective or that our customers will agree to such contracts in the future. Accordingly, if use of the RIO system becomes more prevalent, competitors may attempt to market their implant products for use with the RIO system and compete directly with our implant products.
We believe that the principal competitive factors in our market include:
|
|
|
|
|
|
• |
the safety and efficacy of the procedure and product offerings, as documented through published studies and other clinical reports; |
|
|
|
|
|
|
• |
product benefits, including the ability to offer orthopedic surgeons a complete solution for minimally invasive orthopedic knee procedures; |
|
|
|
|
|
|
• |
the strength of acceptance and adoption by orthopedic surgeons and hospitals; |
24
|
|
|
|
|
|
• |
the ability to deliver new product offerings and enhanced technology to expand or improve upon existing applications through continued research and development; |
|
|
|
|
|
|
• |
the quality of training, services and clinical support provided to surgeons and hospitals; |
|
|
|
|
|
|
• |
the cost of product offerings and the availability of product coverage and reimbursement from third-party payors, insurance companies and others parties; |
|
|
|
|
|
|
• |
the ability to provide proprietary products protected by strong intellectual property rights; and |
|
|
|
|
|
|
• |
the ability to offer products that are intuitive and easy to learn and use. |
Many of our competitors have significantly greater financial, human and other resources than we do, and have established relationships with healthcare professionals, customers and third-party payors. In addition, many of our competitors have established sales networks, greater resources for product development, additional lines of products and the ability to offer financial incentives such as rebates, bundled products or discounts on other product lines that we cannot provide. Our products could also be rendered obsolete or uneconomical by technological advances developed by one or more of our competitors. These competitive factors may negatively affect our ability to convince individuals to utilize our RIO system and implant products and result in our inability to acquire technology, products and businesses from third parties to develop our current and planned versions of the RIO system and related products.
Regulatory Requirements of the U.S. Food and Drug Administration
Our research, development and clinical programs, as well as our manufacturing and marketing operations, are subject to extensive regulation in the U.S. and other countries. Most notably, all of our products sold in the U.S. are subject to regulation as medical devices under the Federal Food, Drug, and Cosmetic Act, or the FDCA, as implemented and enforced by the FDA. The FDA governs the following activities that we perform or that are performed on our behalf, to ensure that medical products we manufacture, promote and distribute domestically or export internationally are safe and effective for their intended uses:
|
|
|
|
|
|
• |
product design, preclinical and clinical development and manufacture; |
|
|
|
|
|
|
• |
product premarket clearance and approval; |
|
|
|
|
|
|
• |
product safety, testing, labeling and storage; |
|
|
|
|
|
|
• |
record keeping procedures; |
|
|
|
|
|
|
• |
product marketing, sales and distribution; and |
|
|
|
|
|
|
• |
post-marketing surveillance, complaint handling, medical device reporting, reporting of deaths, serious injuries or device malfunctions and repair or recall of products. |
FDA Premarket Clearance and Approval Requirements
Unless an exemption applies, each medical device we wish to commercially distribute in the U.S. will require either premarket notification, or 510(k), clearance or approval of a premarket approval application, or PMA, from the FDA. The FDA classifies medical devices into one of three classes. Class I devices, considered to have the lowest risk, are those for which safety and effectiveness can be assured by adherence to the FDA’s general regulatory controls for medical devices, which include compliance with the applicable portions of the FDA’s QSR, facility registration and product listing, reporting of adverse medical events, and appropriate, truthful and non-misleading labeling, advertising, and promotional materials (General Controls). Class II devices
25
are subject to the FDA’s General Controls, and any other special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device (Special Controls). Manufacturers of most class II and some class I devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA requesting permission to commercially distribute the device. This process is generally known as 510(k) marketing clearance. Devices deemed by the FDA to pose the greatest risks, such as life sustaining, life supporting or implantable devices, or devices that have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed device, are placed in class III, requiring approval of a PMA.
510(k) Marketing Clearance Pathway
To obtain 510(k) marketing clearance, we must submit a premarket notification demonstrating that the proposed device is “substantially equivalent” to a legally marketed “predicate device” that is either in class I or class II, or to a class III device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of a PMA. A Special 510(k) is an abbreviated 510(k) application which can be used to obtain clearance for certain types of device modification such as modifications that do not affect the intended use of the device or alter the device’s fundamental scientific technology. A Special 510(k) generally requires less information and data than a complete, or Traditional 510(k). In addition, a Special 510(k) application often takes a shorter period of time, which could be as short as 30 days, than a Traditional 510(k) marketing clearance application, which can be used for any type of 510(k) device. FDA’s 510(k) marketing clearance pathway usually takes from three to twelve months, but may take significantly longer. The FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence. There is no guarantee that the FDA will grant 510(k) marketing clearance for our future products and failure to obtain necessary clearances for our future products would adversely affect our ability to grow our business.
Medical devices can be marketed only for the indications for which they are cleared or approved. After a device receives 510(k) marketing clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) marketing clearance or, depending on the modification, PMA approval. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k) or a PMA, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) marketing clearance or PMA approval is obtained. Also, in these circumstances, we may be subject to significant regulatory fines or penalties. We have made and plan to continue to make additional product enhancements to the RIO system and other products that we believe do not require new 510(k) marketing clearances. We cannot assure you that the FDA would agree with any of our decisions not to seek 510(k) marketing clearance or PMA approval.
Certain of our currently marketed products, such as our RIO system, are class II devices marketed pursuant to 510(k) marketing clearances. In January 2008, we obtained 510(k) marketing clearance from the FDA for version 1.2 of our TGS. We originally submitted a Special 510(k) application in September 2007, which the FDA subsequently indicated was converted to a Traditional 510(k) application. On November 1, 2007, the FDA provided us with a letter requesting additional information in which the FDA, among other things, asked us to justify our proposed use of the terms “haptic” and “robot” in the labeling of version 1.2 of our TGS. Through subsequent correspondence and communications, the FDA indicated that we needed to use the term “tactile” in lieu of “haptic” and the term “robotic arm” in lieu of “robotic,” as appropriate, when these terms are used to market our products and in order to obtain timely clearance of our 510(k) submission. The FDA granted 510(k) marketing clearance for version 1.2 of our TGS with those terms. See Item 1A, Risk Factors, “Risks Related to Our Business — We are currently required by the FDA to refrain from using certain terms to label and market our products, which could harm our ability to market and commercialize our current and future products.”
Version 1.3 of our TGS did not require submission of a 510(k) application. We received 510(k) marketing clearance from the FDA for the RIO system in the fourth quarter of 2008. Versions 2.1 and 2.2 of the RIO System did not require submission of a 510(k) application.
26
In June 2009, we received 510(k) marketing clearance from the FDA for an application that assists a surgeon in acetabular reaming during total hip arthroplasty using the TGS platform. In September 2009, we received 510(k) marketing clearance for the same application using the RIO system. In February 2010, we received 510(k) marketing clearance for an application that assists a surgeon in performing all components of a total hip arthroplasty using the RIO system.
PMA Approval Pathway
A PMA must be submitted to the FDA if the device cannot be cleared through the 510(k) process or is not otherwise exempt from the FDA’s premarket clearance and approval requirements. A PMA must generally be supported by extensive data, including, but not limited to, technical, preclinical, clinical trials, manufacturing and labeling, to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use. No device that we have marketed to date has required premarket approval. During the review period, the FDA will typically request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will generally conduct a pre-approval inspection of our or our third-party manufacturers’ or suppliers’ manufacturing facility or facilities to ensure compliance with the QSR.
New PMAs or PMA supplements are required for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel. None of our products is currently approved under a PMA approval. However, we may in the future develop devices which will require the approval of a PMA. There is no guarantee that the FDA will grant PMA approval of our future products and failure to obtain necessary approvals for our future products would adversely affect our ability to grow our business.
Clinical Trials
Clinical trials are generally required to support a PMA application and are sometimes required for 510(k) marketing clearance. Such trials generally require an investigational device exemption application, or IDE, approved in advance by the FDA for a specified number of patients and study sites, unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject. Clinical trials are subject to extensive monitoring, recordkeeping and reporting requirements. Clinical trials must be conducted under the oversight of an institutional review board, or IRB, for the relevant clinical trial sites and must comply with FDA regulations, including but not limited to those relating to good clinical practices. To conduct a clinical trial, we also are required to obtain the patient’s informed consent in form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. We, the FDA or the IRB could suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA clearance or approval to market the product in the U.S. Similarly, in Europe the clinical study must be approved by a local ethics committee and in some cases, including studies with high-risk devices, by the ministry of health in the applicable country.
Post-Market Studies
To date, none of our submissions to the FDA has required the submission of clinical data and all of our studies to date have been post-market studies. As of March 1, 2010, we have 39 studies either recently
27
completed or in progress. The studies, many of which are being conducted pursuant to IRB approval, range from cadaveric biomechanics studies to retrospective chart and radiographic reviews to prospective functional comparison studies to basic science histology studies. The results of these studies have been presented internationally as peer-reviewed presentations at conferences in the United States and abroad. One MAKOplasty surgical technique book chapter and 15 peer-reviewed manuscripts on the technology and early clinical results have been published as of March 1, 2010.
Pervasive and Continuing Regulation
After a device is placed on the market, numerous regulatory requirements continue to apply. In addition to the requirements below, the Medical Device Reporting, or MDR, regulations require that we report to the FDA any incident in which our products may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. See Item 1A, Risk Factors, “Risks Related to Regulatory Compliance,” for further information regarding our reporting obligations under MDR regulations. Additional regulatory requirements include:
|
|
|
|
|
|
• |
product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; |
|
|
|
|
|
|
• |
QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process; |
|
|
|
|
|
|
• |
labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication; |
|
|
|
|
|
|
• |
clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices; |
|
|
|
|
|
|
• |
approval of product modifications that affect the safety or effectiveness of one of our approved devices; |
|
|
|
|
|
|
• |
post-approval restrictions or conditions, including post-approval study commitments; |
|
|
|
|
|
|
• |
post-market surveillance regulations, which apply, when necessary, to protect the public health or to provide additional safety and effectiveness data for the device; |
|
|
|
|
|
|
• |
the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; and |
|
|
|
|
|
|
• |
notices of corrections or removals. |
We must also register with the FDA as a medical device manufacturer and must obtain all necessary state permits or licenses to operate our business. As a manufacturer, we are subject to announced and unannounced inspections by the FDA to determine our compliance with FDA’s QSR and other regulations. We were audited by the FDA in January 2009 and received the establishment inspection report (EIR) from the FDA in August 2009. Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
|
|
|
|
|
|
• |
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
|
|
|
|
|
|
• |
customer notifications or repair, replacement, refunds, recall, detention or seizure of our products; |
|
|
|
|
|
|
• |
operating restrictions or partial suspension or total shutdown of production; |
28
|
|
|
|
|
|
• |
refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products; |
|
|
|
|
|
|
• |
withdrawing 510(k) marketing clearances or PMA approvals that have already been granted; |
|
|
|
|
|
|
• |
refusal to grant export approval for our products; or |
|
|
|
|
|
|
• |
criminal prosecution. |
International Marketing Approvals
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for FDA clearance or approval, and the requirements may differ.
The European Union has adopted numerous directives and standards regulating the design, manufacture, clinical trials, labeling and adverse event reporting for medical devices. Each European Union member state has implemented legislation applying these directives and standards at the national level. Other countries, such as Switzerland, have voluntarily adopted laws and regulations that mirror those of the European Union with respect to medical devices. Devices that comply with the requirements of the laws of the relevant member state applying the applicable European Union directive are entitled to bear CE conformity marking and, accordingly, can be commercially distributed throughout the member states of the European Union and other countries that comply with or mirror these directives. The method of assessing conformity varies depending on the type and class of the product, but normally involves a combination of self-assessment by the manufacturer and a third-party assessment by a “Notified Body,” an independent and neutral institution appointed to conduct conformity assessment. This third-party assessment consists of an audit of the manufacturer’s quality system and clinical information, as well as technical review of the manufacturer’s product. An assessment by a Notified Body in one country within the European Union is required in order for a manufacturer to commercially distribute the product throughout the European Union. In addition, compliance with ISO 13845 on quality systems issued by the International Organization for Standards, among other standards, establishes the presumption of conformity with the essential requirements for a CE marking. In addition, many countries apply requirements in their reimbursement, pricing or health care systems that affect companies’ ability to market products. The RIO System and our classic RESTORIS onlay unicompartmental knee implant system were approved for CE marking in December 2009 and January 2010, respectively. The CE marking certification process for the RESTORIS MCK onlay unicompartmental knee implant system is currently ongoing, with approval anticipated in 2010.
Health Care Laws and Regulations
Third-Party Reimbursement
In the U.S. and elsewhere, health care providers that perform surgical procedures using medical devices such as ours generally rely on third-party payors, including governmental payors such as Medicare and Medicaid and private payors, to cover and reimburse all or part of the cost of the products. Consequently, sales of medical devices are dependent in part on the availability of reimbursement to the customer from third-party payors. The manner in which reimbursement is sought and obtained varies based upon the type of payor involved and the setting in which the product is furnished and utilized. In general, third-party payors will provide coverage and reimbursement for medically reasonable and necessary procedures and tests that utilize medical devices and may provide separate payments for the implanted or disposable devices themselves. Most payors, however, will not pay separately for capital equipment, such as the RIO system. Instead, payment for the cost of using the capital equipment is considered to be covered as part of payments received for performing the procedure. In determining payment rates, third-party payors are increasingly scrutinizing the prices charged for medical products and services in comparison to other therapies. Our products, and the procedures in which our products are used, may
29
not be reimbursed by these third-party payors at rates sufficient to allow us to sell our products on a competitive and profitable basis.
In addition, in many foreign markets, including the countries in the European Union, pricing of medical devices is subject to governmental control. In the U.S., there have been, and we expect that there will continue to be, a number of federal and state proposals to limit payments by governmental payors for medical devices, and the procedures in which medical devices are used. While we cannot predict whether such legislative or regulatory proposals will be adopted, the adoption of such proposals could have a material adverse effect on our business, financial condition and profitability.
Medicare and Medicaid
The Medicare program is a federal health benefit program administered by the Centers for Medicare and Medicaid Services, or CMS, that covers and pays for certain medical care items and services for eligible elderly, blind and disabled individuals, and individuals with end stage renal disease. The Medicaid program is a federal-state partnership under which states receive matching federal payments to fund healthcare services for the poor. Because we expect that a significant percentage of MAKOplasty patients will be Medicare beneficiaries, and because some private commercial health insurers and some state Medicaid programs may follow the coverage and payment policies for Medicare, Medicare’s coverage and payment policies are significant to our business.
Medicare coverage for procedures using our technology currently exists in the hospital inpatient setting, which falls under Part A of the Medicare program. Under Medicare Part A, Medicare reimburses acute care hospitals a flat prospectively determined payment amount for beneficiaries receiving covered inpatient services in an acute care hospital. This method of payment is known as the prospective payment system, or PPS. Under PPS, the prospective payment for a patient’s stay in an acute care hospital is determined by the patient’s condition and other patient data and procedures performed during the inpatient stay using a classification system known as diagnosis related groups, or DRGs. As of October 1, 2007, CMS implemented a revised version of the DRG system that uses 745 Medicare Severity DRGs, or MS-DRGs, instead of the approximately 540 DRGs Medicare previously used. The MS-DRGs are intended to account more accurately for the patient’s severity of illness when assigning each patient’s stay to a payment classification. Medicare pays a fixed amount to the hospital based on the MS-DRG into which the patient’s stay is classified, regardless of the actual cost to the hospital of furnishing the procedures, items and services that the patient’s condition requires. Accordingly, acute care hospitals generally do not receive direct Medicare reimbursement under PPS for the specific costs incurred in purchasing medical devices. Rather, reimbursement for these costs is deemed to be included within the MS-DRG based payments made to hospitals for the services furnished to Medicare eligible inpatients in which the devices are utilized. For cases involving unusually high costs, a hospital may receive additional “outlier” payments above the pre-determined amount. In addition, there is a mechanism by which new technology services can apply to Medicare for additional payments above the pre-determined amount, although such requests have not been granted frequently.
Because PPS payments are based on predetermined rates and may be less than a hospital’s actual costs in furnishing care, acute care hospitals have incentives to lower their inpatient operating costs by utilizing products, devices and supplies that will reduce the length of inpatient stays, decrease labor or otherwise lower their costs. For each MS-DRG, a relative weight is calculated representing the average resources required to care for cases grouped in that particular MS-DRG relative to the average resources used to treat cases in all MS-DRGs. MS-DRG relative weights are recalculated every year to reflect changes in technology and medical practice in a budget neutral manner. Under the MS-DRG payment system, there can be significant delays in obtaining adequate reimbursement amounts for hospitals for new technologies such that reimbursement may be insufficient to permit broad acceptance by hospitals.
We believe that there are existing reimbursement codes that can be used for MAKOplasty procedures performed in the hospital inpatient setting. Procedures for hospital inpatient billing are referenced by international classifications of diseases, clinical modification, or ICD-9-CM, volume 3 procedure codes. Knee arthroplasty is billed under ICD-9-CM code 81.54 (“Total Knee Replacement”), which is assigned to MS-DRG 469 (“Major
30
Joint Replacement or Reattachment of Lower Extremity with Complication or Comorbidity”) and MS-DRG 470 (“Major Joint Replacement or Reattachment of Lower Extremity without Major Complication or Comorbidity”). We anticipate that Medicare will continue to reimburse hospitals under MS-DRGs 469 and 470 for MAKOplasty procedures, but CMS can revise MS-DRG assignments from year to year.
In addition to payments to hospitals for procedures using our technology, Medicare makes separate payments to physicians for their professional services. The American Medical Association, or AMA, has developed a coding system known as the Current Procedural Terminology, or CPT, codes, which have been adopted by the Medicare program to describe and develop payment amounts for certain physician services. The Medicare physician fee schedule uses CPT codes (and other codes) as part of the determination of allowable payment amounts to physicians. In determining appropriate payment amounts for surgeons, CMS receives guidance from the AMA regarding the relative technical skill level, level of resources used, and complexity of a new surgical procedure. Generally, the FDA approval of a new product is necessary, but not necessarily sufficient, for the designation of a new procedure code for a new surgical procedure using that product. Codes are assigned by either the AMA (for CPT codes) or CMS (for Medicare specific codes) and new codes usually become effective on January 1st of each year. Physicians performing procedures using our technology submit bills under CPT codes 27446 (“Arthroplasty, knee, condyle and plateau; medial OR lateral compartment”), 27447 (“Arthoplasty, knee, condyle and plateau, medical and lateral with or without patella resurfacing”), and 27438 (“Arthoplasty, patella with prosthesis”). We cannot anticipate whether third-party payors will continue to reimburse physicians under these codes for services performed in connection with MAKOplasty procedures.
Commercial Insurers
In addition to the Medicare program, many private payors look to CMS policies as a guideline in setting their coverage policies and payment amounts. The current coverage policies of these private payors may differ from the Medicare program, and the payment rates they make may be higher, lower, or the same as the Medicare program. A decrease of, or limitation on, reimbursement payments for doctors and hospitals by CMS or other agencies may affect coverage and reimbursement determinations by many private payors. Additionally, some private payors do not follow the Medicare guidelines, and those payors may reimburse only a portion of the costs associated with the use of our products, or not at all.
Fraud and Abuse Laws
Because of the significant federal funding involved in Medicare and Medicaid, Congress and the states have enacted, and actively enforce, a number of laws whose purpose is to eliminate fraud and abuse in federal health care programs. Our business is subject to compliance with these laws.
Anti-Kickback Statutes and Federal False Claims Act
The federal healthcare programs’ Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as Medicare or Medicaid. The definition of “remuneration” has been broadly interpreted to include anything of value, including for example gifts, certain discounts, the furnishing of free supplies, equipment or services, credit arrangements, payments of cash and waivers of payments. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the statute has been violated. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. In addition, some kickback allegations have been claimed to violate the Federal False Claims Act, discussed in more detail below.
The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Recognizing that the Anti-Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress authorized the Office of Inspector
31
General of the U.S. Department of Health and Human Services, or OIG, to issue a series of regulations, known as “safe harbors.” These safe harbors, issued by the OIG beginning in July 1991, set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy each applicable safe harbor may result in increased scrutiny by government enforcement authorities such as the OIG.
Many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to referral of patients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
Government officials have focused their enforcement efforts on marketing of healthcare services and products, among other activities, and recently have brought cases against companies, and certain sales, marketing and executive personnel, for allegedly offering unlawful inducements to potential or existing customers in an attempt to procure their business.
Another development affecting the healthcare industry is the increased use of the federal Civil False Claims Act and, in particular, actions brought pursuant to the False Claims Act’s “whistleblower” or “qui tam” provisions. The False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The qui tam provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government alleging that the defendant has submitted a false claim to the federal government, and to share in any monetary recovery. In recent years, the number of suits brought against healthcare providers by private individuals has increased dramatically. In addition, various states have enacted false claim laws analogous to the Civil False Claims Act, although many of these state laws apply where a claim is submitted to any third-party payor and not merely a federal healthcare program.
When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between $5,500 to $11,000 for each separate false claim. There are many potential bases for liability under the False Claims Act. Liability arises, primarily, when an entity knowingly submits, or causes another to submit, a false claim for reimbursement to the federal government. The False Claims Act has been used to assert liability on the basis of inadequate care, kickbacks and other improper referrals, and improper use of Medicare numbers when detailing the provider of services, in addition to the more predictable allegations as to misrepresentations with respect to the services rendered. In addition, companies have been prosecuted under the False Claims Act in connection with alleged off-label promotion of products. Our future activities relating to the reporting of wholesale or estimated retail prices for our products, the reporting of discount and rebate information and other information affecting federal, state and third-party reimbursement of our products, and the sale and marketing of our products, may be subject to scrutiny under these laws. We believe our current consulting agreements with physicians represent legitimate compensation for needed documented services actually furnished to us. However, engagement of physician consultants by orthopedic medical device manufacturers has recently been subject to heightened scrutiny, and has resulted in four of the major orthopedic medical device implant manufacturers entering deferred prosecution agreements with the federal government and agreeing to pay substantial amounts to the federal government in settlement of Anti-Kickback Statute allegations, and all such companies submitting to supervision by a court appointed monitor throughout the term of the 18 month agreements. In this environment, our engagement of physician consultants in product development and product training and education could subject us to similar scrutiny. We are unable to predict whether we would be subject to actions under the False Claims Act or a similar state law, or the impact of such actions. However, the costs of defending such claims, as well as any sanctions imposed, could have a material adverse effect on our financial performance.
As part of our internal compliance program, we review our sales and marketing materials, contracts and programs with counsel, and require employees and marketing representatives to participate in regular training. We also have adopted and train our personnel on the Code of Ethics for Interactions with Health Care Professionals
32
promulgated by the Advanced Medical Technology Association, or AdvaMed, a leading trade association representing medical device manufacturers. However, we cannot rule out the possibility that the government or other third parties could interpret these laws differently and challenge one or more of our activities under these laws.
HIPAA and Other Fraud and Privacy Regulations
Among other things, the Health Insurance Portability and Accountability Act of 1996, or HIPAA, created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The HIPAA health care fraud statute prohibits, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment and/or exclusion from government sponsored programs. The HIPAA false statements statute prohibits, among other things, knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines and/or imprisonment.
In addition to creating the two new federal healthcare crimes, regulations implementing HIPAA also establish uniform standards governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of individually identifiable health information maintained or transmitted by healthcare providers, health plans and healthcare clearinghouses, which are referred to as “covered entities.” Three standards have been promulgated under HIPAA’s regulations: the Standards for Privacy of Individually Identifiable Health Information, which restrict the use and disclosure of certain individually identifiable health information, the Standards for Electronic Transactions, which establish standards for common healthcare transactions, such as claims information, plan eligibility, payment information and the use of electronic signatures, and the Security Standards, which require covered entities to implement and maintain certain security measures to safeguard certain electronic health information.
In 2009, Congress passed the American Recovery and Reinvestment Act of 2009, or ARRA, which included sweeping changes to HIPAA, including an expansion of HIPAA’s privacy and security standards. ARRA includes the Health Information Technology for Economic and Clinical Health Act, or HITECH, which, among other things, made HIPAA’s privacy and security standards directly applicable to “business associates” of covered entities effective February 17, 2010. A business associate is a person or entity that performs certain functions or activities on behalf of a covered entity that involve the use or disclosure of protected health information in connection with recognized health care operations activities. We believe that we are neither a covered entity nor, as of February 17, 2010, a business associate of our hospital customers. As such, we believe that we are not directly subject to these HIPAA standards; however, there is no guarantee that the government will agree with our determination. If the government determines that we are a business associate, we could be subject to enforcement measures, including civil and criminal penalties and fines. While the government intended this legislation to reduce administrative expenses and burdens for the healthcare industry, our compliance with certain provisions of these standards entails significant costs for us.
In addition to federal regulations issued under HIPAA, some states have enacted privacy and security statutes or regulations that, in some cases, are more stringent than those issued under HIPAA. In those cases, it may be necessary to modify our planned operations and procedures to comply with the more stringent state laws. If we fail to comply with applicable state laws and regulations, we could be subject to additional sanctions.
Employees
As of March 1, 2010, we had 240 employees, 73 of whom were engaged in sales and marketing, 63 in research and development, 53 in assembly, manufacturing and service, 27 in regulatory, clinical affairs and quality activities and 24 in general administrative and accounting activities. None of our employees is covered by a collective bargaining agreement, and we consider our relationship with our employees to be good.
33
Available Information
From our Internet website, http://www.makosurgical.com, you may obtain additional information about us including:
|
|
|
|
|
|
|
|
• |
Our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, including amendments to these reports, and other documents as soon as reasonably practicable after we file them with the Securities and Exchange Commission, or the SEC; |
||
|
|
|
|
||
|
|
• |
Beneficial ownership reports filed by officers, directors and principal security holders under Section 16(a) of the Securities Exchange Act of 1934, as amended, or the Exchange Act; and |
||
|
|
|
|
||
|
|
• |
Corporate governance information that includes our |
||
|
|
|
|
|
|
|
|
|
|
o |
Corporate Governance Guidelines |
|
|
|
|
|
|
|
|
|
|
o |
Audit Committee Charter |
|
|
|
|
|
|
|
|
|
|
o |
Compensation Committee Charter |
|
|
|
|
|
|
|
|
|
|
o |
Corporate Governance and Nominating Committee Charter |
|
|
|
|
|
|
|
|
|
|
o |
Code of Business Conduct and Ethics |
|
|
|
|
|
|
|
|
|
|
o |
Information on how to communicate directly with our board of directors |
We will also provide printed copies of any of these documents to any stockholder upon request. The contents of our Internet website are not intended to be incorporated by reference into this report or in any other report or document we file and any references to our website are intended to be inactive textual references only.
The following risk factors and other information included in this report should be carefully considered. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently treat as immaterial also may impair our business operations. If any of the following risks occur, our business, financial condition, operating results and cash flows could be materially adversely affected.
Risks Related to Our Business
Adverse changes in economic conditions and reduced spending on innovative medical technology may adversely impact our business.
The purchase of a RIO system is discretionary and requires our customers to make significant initial commitments of capital and other resources. In addition, purchase of a RIO system requires a commitment to purchase exclusively from us other products and services, including our proprietary RESTORIS family of knee implant systems. Continuing weak economic conditions, or a reduction in healthcare technology spending even if economic conditions improve, could adversely impact our business, operating results and financial condition in a number of ways, including longer sales cycles, lower prices for our products and services and reduced unit sales.
Current credit and financial market conditions could delay or prevent our customers from obtaining financing to purchase or lease a RIO system, which would adversely affect our business, financial condition and results of operations.
Due to the tightening of credit markets in the past year and concerns regarding the availability of credit, particularly in the United States, our customers may be delayed in obtaining, or may not be able to obtain, necessary financing for their purchases or leases of the RIO system. These delays may in some instances lead to our customers postponing the shipment and installation of previously ordered systems, cancelling their system orders, postponing their system installation or cancelling their agreements with us. An increase in delays and
34
order cancellations of this nature could adversely affect our product sales and revenues and, therefore, harm our business and results of operations.
Negative worldwide economic conditions and the long lead times required by certain suppliers could prevent us from accurately forecasting demand for our products, which could adversely affect our operating results.
The current negative worldwide economic conditions and market instability makes it increasingly difficult for us, our customers and our suppliers to accurately forecast future product demand trends, which could cause us to order and/or produce excess products that can increase our inventory carrying costs and result in obsolete inventory. Alternatively, this forecasting difficulty could cause a shortage of products, or materials used in our products, that could result in an inability to satisfy demand for our products and a resulting material loss of revenue.
In addition, certain of our suppliers may require extensive advance notice of our requirements in order to produce products in the quantities we desire. This long lead time may require us to place orders far in advance of the time when certain products will be offered for sale, thereby also making it difficult for us to accurately forecast demand for our products, exposing us to risks relating to shifts in consumer demand and trends and adversely affecting our operating results.
We may not have sufficient funding to complete the development and commercialization of our existing products and the weak worldwide economic conditions may hamper our efforts to raise additional capital to run our business.
To date, we have not achieved profitability. We anticipate that we will continue to incur substantial net losses for at least the next two or three years as we expand our sales and marketing capabilities in the orthopedic products market, continue our commercialization of the RIO system and the RESTORIS MCK multicompartmental knee implant system and continue to develop the corporate infrastructure required to sell and market our products and operate as a public company. We also expect to experience increased cash requirements for inventory and property and equipment in conjunction with the continued commercialization of our RESTORIS unicompartmental and RESTORIS MCK multicompartmental knee implant systems and our RIO system. Given the current weak economic conditions, we may be unable to obtain additional financing. As a result, we may be required to reduce the scope of, delay or eliminate some or all of our current and planned research, development and commercialization activities. We also may have to reduce marketing, customer support or other resources devoted to our products. Any of these factors could materially harm our business and results of operations.
We believe our existing cash, cash equivalents, short-term investment balances, and interest income we earn on these balances, if any, will be sufficient to meet our anticipated cash requirements through at least the next twelve months. To the extent our available cash, cash equivalents and short-term investment balances are insufficient to satisfy our operating requirements after that period, we will need to seek additional sources of funds, including selling additional equity or debt securities or entering into a credit facility, or modify our current business plan. The sale of additional equity and debt securities may result in dilution to our current stockholders or may require us to grant a security interest in our assets. If we raise additional funds through the issuance of debt securities, these securities may have rights senior to those of our common stock and could contain covenants that could restrict our operations. We may require additional capital beyond our currently forecasted amounts. Any such required additional capital may not be available on reasonable terms, or at all.
Because of the numerous risks and uncertainties associated with the development of medical devices, such as future versions of the RIO system and the RESTORIS family of knee systems, we are unable to estimate the exact amounts of capital outlays and operating expenditures necessary to complete the development of the products and successfully deliver commercial products to the market. Our future capital requirements will depend on many factors, including but not limited to the following:
|
|
|
|
|
|
• |
the revenue generated by sales of our current and future products; |
35
|
|
|
|
|
|
• |
the expenses we incur in selling and marketing our products; |
|
|
|
|
|
|
• |
the costs and timing of regulatory clearance or approvals for new products or upgrades or changes to our current products; |
|
|
|
|
|
|
• |
the rate of progress, cost, and success or failure of on-going development activities; |
|
|
|
|
|
|
• |
the emergence of competing or complementary technological developments; |
|
|
|
|
|
|
• |
the costs of filing, prosecuting, defending and enforcing any patent or license claims and other intellectual property rights, or participating in litigation related activities; |
|
|
|
|
|
|
• |
the terms and timing of any collaborative, licensing, or other arrangements that we may establish; |
|
|
|
|
|
|
• |
the acquisition of businesses, products and technologies, although we currently have no understandings, commitments or agreements relating to any material transaction of this type; and |
|
|
|
|
|
|
• |
general economic conditions and interest rates, including the current downturn. |
Our reliance on third-party suppliers, including single source suppliers, for our implants and nearly all components of our RIO system could harm our ability to meet demand for our products in a timely and cost effective manner.
We rely on third-party suppliers to manufacture and supply our implants and nearly all components used in our RIO system, other than software. We currently rely on a number of single source suppliers, such as The Anspach Effort, Inc., for our bone cutting instrument, Northern Digital Inc., or NDI, for the stereo tracking camera used in MAKOplasty and Millstone Medical Outsourcing, LLC, for sterile packaging of our RESTORIS family of knee implant systems. We currently do not have long-term contracts with all of our suppliers. As a result, some of our suppliers are not required to provide us with any guaranteed minimum production levels, and we cannot assure you that we will be able to obtain sufficient quantities of key components in the future. In addition, our reliance on third-party suppliers involves a number of risks, including, among other things:
|
|
|
|
|
|
• |
Our suppliers may encounter financial hardships as a result of unfavorable economic and market conditions unrelated to our demand for components, which could inhibit their ability to fulfill our orders and meet our requirements; |
|
|
|
|
|
|
• |
Suppliers may fail to comply with regulatory requirements, be subject to lengthy compliance, validation or qualification periods, or make errors in manufacturing components that could negatively affect the efficacy or safety of our products or cause delays in supplying of our products to our customers; |
|
|
|
|
|
|
• |
Newly identified suppliers may not qualify under the stringent regulatory standards to which our business is subject; |
|
|
|
|
|
|
• |
We or our suppliers may not be able to respond to unanticipated changes in customer orders, and if orders do not match forecasts, we or our suppliers may have excess or inadequate inventory of materials and components; |
|
|
|
|
|
|
• |
We may be subject to price fluctuations due to a lack of long-term supply arrangements for key components; |
|
|
|
|
|
|
• |
We may experience delays in delivery by our suppliers due to changes in demand from us or their other customers; |
|
|
|
|
|
|
• |
We or our suppliers may lose access to critical services and components, resulting in an interruption in the manufacture, assembly and shipment of our systems; |
|
|
|
|
|
|
• |
Our suppliers may be subject to allegations by third parties of misappropriation of proprietary information in connection with their supply of products to us, which could inhibit their ability to fulfill our orders and meet our requirements |
|
|
|
|
|
|
• |
Fluctuations in demand for products that our suppliers manufacture for others may affect their ability or willingness to deliver components to us in a timely manner; |
|
|
|
|
|
|
• |
Our suppliers may wish to discontinue supplying components or services to us for risk management reasons; and |
36
|
|
|
|
|
|
• |
We may not be able to find new or alternative components or reconfigure our system and manufacturing processes in a timely manner if the necessary components become unavailable. |
If any of these risks materialize, it could significantly increase our costs and impact our ability to meet demand for our products. If we are unable to satisfy commercial demand for the RIO system or the RESTORIS family of knee systems in a timely manner, our ability to generate revenue would be impaired, market acceptance of our products could be adversely affected, and customers may instead purchase or use our competitors’ products. In addition, we could be forced to secure new or alternative components through a replacement supplier. Securing a replacement supplier could be difficult, especially for complex components such as motors, encoders, brakes and certain RIO system components that are manufactured in accordance with our custom specifications. The introduction of new or alternative components may require design changes to our system that are subject to FDA and other regulatory clearances or approvals. We may also be required to assess the new manufacturer’s compliance with all applicable regulations and guidelines, which could further impede our ability to manufacture our products in a timely manner. As a result, we could incur increased production costs, experience delays in deliveries of our products, suffer damage to our reputation and experience an adverse effect on our business and financial results.
We are an early-stage medical device company with a limited operating history and our business may not become profitable.
We are an early-stage medical device company with a limited operating history. Our current products with 510(k) marketing clearance from the FDA are versions 1.0, 1.2 and 1.3 of our TGS, versions 2.0, 2.1, and 2.2 of our RIO system, and inlay and onlay implant systems for use in unicompartmental and bicompartmental knee resurfacing procedures. The future success of our business depends on our ability to continue to develop and obtain regulatory clearances or approvals for innovative and commercially successful products in our field, which we may be unable to do in a timely manner, or at all. Our success and ability to generate revenue or be profitable also depends on our ability to establish our sales and marketing force, generate product sales and control costs, all of which we may be unable to do. We have a limited history of operations upon which you can evaluate our business and our operating expenses are increasing. Our lack of any significant operating history also limits your ability to make a comparative evaluation of us, our products and our prospects.
We have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for at least the next two or three years.
We have sustained net losses in every fiscal year since our inception in 2004, including a net loss attributable to common stockholders of $34.0 million for the year ended December 31, 2009. As of December 31, 2009, we had total stockholders’ equity of $90.8 million. We expect to continue to incur significant operating losses as we increase our sales and marketing activities and otherwise continue to invest capital in the development of our products and our business generally. We also expect that our general and administrative expenses will increase due to additional operational and regulatory burdens associated with operating as a public company. Our losses have had and will continue to have an adverse effect on our stockholders’ equity and working capital. Any failure to achieve and maintain profitability would continue to have an adverse effect on our stockholders’ equity and working capital and could result in a decline in our stock price or cause us to cease operations.
We rely on intellectual property that we license from others, and if we are unable to maintain these licenses or obtain additional licenses that we may need, our ability to compete will be harmed.
We rely on intellectual property that we license or sublicense from others, including patented technology that is integral to our RIO system and RESTORIS family of knee systems. As of March 1, 2010, we had licensed rights to 114 U.S. and 40 foreign third-party granted patents, and we had licensed rights to 9 U.S. and 10 foreign third-party pending patent applications. The majority of these patents and applications are either used in our current products or relate to core technologies used in our products, such as computer assisted surgery, or CAS, robotics, haptics and implants. Five of the licensed U.S. patents will expire by the end of 2010. One of these
37
patents is considered material to our intellectual property portfolio because it deals with a core technology and potentially enables us to exclude others from practicing the claimed technology. Third parties may terminate a license in the event that we fail to make required payments or for other causes. In the event a third party terminates a license agreement, we cannot assure you that we could acquire another license to adequately replace the product, technology or method covered by the terminated license. If we fail to maintain our current licenses, our ability to compete in the orthopedic market will be harmed.
In addition, as we enhance our current product offerings and develop new ones, including the RIO system and the RESTORIS family of knee implant systems, we may find it advisable or necessary to seek additional licenses from third parties who hold patents covering technology or methods used in these products. If we cannot obtain these additional licenses, we could be forced to design around those patents at additional cost or abandon the product altogether. As a result, our ability to grow our business and compete in the knee implant market may be harmed.
We are currently required by the FDA to refrain from using certain terms to label and market our products, which could harm our ability to market and commercialize our current or future products.
On September 28, 2007, we submitted a Special 510(k) application to the FDA for version 1.2 of our TGS which the FDA converted to a Traditional 510(k) application. On November 1, 2007, the FDA provided us with a letter requesting additional information in which the FDA, among other things, asked us to justify our proposed use of the terms “haptic” and “robot” in the labeling of version 1.2 of our TGS. Through subsequent correspondence and communications, the FDA indicated that we needed to use the term “tactile” in lieu of “haptic” and the term “robotic arm” in lieu of “robotic,” as appropriate, when these terms are used to market our products. The FDA granted 510(k) marketing clearance in January 2008 for version 1.2 of our TGS with those terms. Because the FDA currently requires us to use the terms “tactile” or “robotic arm,” we revised the promotional and labeling materials for our existing products, including the RIO system, and may need to consider the use of modified language for our future products. As a result, our ability to market and commercialize our products and our growth may be harmed.
Modifications to our currently FDA cleared products or the introduction of new products may require new regulatory clearances or approvals or require us to recall or cease marketing our current products until clearances or approvals are obtained.
In November 2005, we obtained 510(k) marketing clearance from the FDA for version 1.0 of our TGS for use with our FDA cleared tibial knee inlay implant system. We were not required to obtain premarket approval, or PMA. We were also not required to conduct any clinical trials in support of our application for 510(k) marketing clearance. Modifications to our products, however, may require new regulatory approvals or clearances or require us to recall or cease marketing the modified products until these clearances or approvals are obtained. Any modification to one of our 510(k) cleared products that would constitute a major change in its intended use, or any change that could significantly affect the safety or effectiveness of the device would require us to obtain a new 510(k) marketing clearance and may even, in some circumstances, require the submission of a PMA, if the change raises complex or novel scientific issues or the product has a new intended use. The FDA requires every manufacturer to make the determination regarding the need for a new 510(k) submission in the first instance, but the FDA may review any manufacturer’s decision. Since obtaining 510(k) marketing clearance for version 1.0 of our TGS, we developed and commercially introduced several upgrades to our TGS that we believe did not require additional clearances or approvals. Our Special 510(k) application for version 1.2, which the FDA converted to a Traditional 510(k) application and cleared in January 2008, incorporated these upgrades. Since this 510(k) marketing clearance, we have made additional upgrades to the system (namely, version 1.3) that we believe were cleared under our most recent 510(k) marketing clearance and therefore did not require additional filings for clearance or approval. In the fourth quarter of 2008 we received 510(k) marketing clearance from the FDA for the RIO system and for the RESTORIS MCK multicompartmental knee implant system, which the RIO system is designed to support. Since obtaining 501(k) marketing clearance for the RIO system, we have made additional upgrades to the RIO system (namely, versions 2.1 and 2.2) that we believe were cleared under our 510(k) marketing clearance for the RIO system and therefore did not require additional filings for clearance or approval.
38
We may continue to make additional modifications in the future to the RIO system without seeking additional clearances or approvals if we believe such clearances or approvals are not necessary. If the FDA disagrees and requires new clearances or approvals for the modifications, we may be required to recall and stop marketing our products as modified, which could cause us to redesign our products, conduct clinical trials to support any modifications, and pay significant regulatory fines or penalties. Any of these actions would harm our operating results.
Obtaining clearances and approvals can be a difficult and time consuming process, and we may not be able to obtain any of these or other clearances or approvals in a timely manner, or at all. In addition, the FDA may not approve or clear our products for the indications that are necessary or desirable for successful commercialization or could require clinical trials to support any modifications. Any delay or failure in obtaining required clearances or approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
Moreover, clearances and approvals are subject to continual review, and the later discovery of previously unknown problems can result in product labeling restrictions or withdrawal of the product from the market. The loss of previously received approvals or clearances, or the failure to comply with existing or future regulatory requirements could reduce our sales, profitability and future growth prospects.
We depend on the success of a single line of products for our revenue, which could impair our ability to achieve profitability.
We expect to derive most of our revenue from capital sales of our RIO system, recurring sales of implants and disposable products required for each knee MAKOplasty procedure, and service plans that are sold with the RIO system. Currently, the only line of products that has been commercially introduced and received 510(k) marketing clearance is versions 1.0 and 1.2 of our TGS, the off-the-shelf inlay and onlay knee implant systems for use in unicompartmental knee resurfacing procedures, versions 2.0, 2.1, and 2.2 of the RIO system and our RESTORIS family of knee implant systems. Our future growth and success is dependent on the successful commercialization of the RIO system and the RESTORIS family of knee implant systems. If we are unable to achieve commercial acceptance of MAKOplasty for unicompartmental and bicompartmental knee resurfacing procedures or obtain regulatory clearances or approvals for future products, including products to treat the hip and other joints of the human body, our revenue would be adversely affected and we would not become profitable.
If our knee MAKOplasty solution does not gain market acceptance, we will not be able to generate the revenue necessary to develop a sustainable, profitable business.
Achieving patient, surgeon and hospital acceptance of MAKOplasty as the preferred method of treating early to mid-stage osteoarthritis of the knee is crucial to our success. We believe MAKOplasty represents a fundamentally new way of performing arthroplasty of the knee, employing computer assisted robotic arm technology and a patient specific visualization system to resurface only the diseased areas of the knee joint. The orthopedic market has been traditionally slow to adopt new products and treatment practices. We believe that if surgeons and hospitals do not broadly adopt the concept of computer assisted robotics enabled technology and do not perceive such technology as having significant advantages over conventional arthroplasty procedures, patients will be less likely to accept or be offered knee MAKOplasty and we will fail to meet our business objectives. Surgeons’ and hospitals’ perceptions of such technology having significant advantages are likely to be based on a determination that, among other factors, our products are safe, reliable, cost-effective and represent acceptable methods of treatment. Even if we can prove the clinical value of MAKOplasty through clinical use, surgeons may elect not to use our products for any number of other reasons. For example, surgeons may continue to recommend total knee replacement surgery simply because such surgery is already widely accepted. In addition, surgeons may be slow to adopt our products because of the perceived liability risks arising from the use of new products. Hospitals may not accept MAKOplasty because the RIO system is a piece of capital equipment, representing a significant portion of a hospital’s budget. The RIO system may not be cost-efficient if hospitals are not able to perform a significant volume of MAKOplasty procedures. If MAKOplasty fails to achieve market acceptance for
39
any of these or other reasons, we will not be able to generate the revenue necessary to develop a sustainable, profitable business.
We have only limited clinical data to support the value of knee MAKOplasty, which may make patients, surgeons and hospitals reluctant to purchase our products.
We believe that patients, surgeons and hospitals will only accept MAKOplasty or purchase our products if they believe that MAKOplasty is a safe and effective procedure with advantages over competing products and conventional arthroplasty procedures. To date, we have collected only limited, short-term clinical data with which to assess MAKOplasty’s clinical value. As of December 31, 2009, 2,384 MAKOplasty procedures had been performed since commercial introduction in 2006. We have not collected, and are not aware that others have collected, any long-term clinical data regarding the clinical value of knee MAKOplasty. The results of short-term studies, such as our post-market studies, do not necessarily predict long-term clinical results. As of March 1, 2010, we have one published MAKOplasty surgical technique book chapter, 15 peer-reviewed manuscripts, 40 peer-reviewed abstracts at conferences, and 11 completed whitepapers. We also have 39 studies either recently completed or in progress, which range from cadaveric biomechanics studies to retrospective chart and radiographic reviews to prospective functional comparison slides to basic science histology studies. If longer-term or more extensive clinical studies that may be performed by us or others indicate that MAKOplasty is a less safe or less effective procedure than our current data suggest, patients may choose not to undergo, and surgeons may choose not to perform, knee MAKOplasty. Furthermore, unsatisfactory patient outcomes or patient injury could cause negative publicity for our products, particularly in the early phases of product introduction. The FDA could also rescind our marketing clearances if future results and experience indicate that our products cause unexpected or serious complications or other unforeseen negative effects. See “Risks Related to Regulatory Compliance.” Surgeons may be slow to adopt our products if they perceive liability risks arising from the use of these new products. As a result, patients, surgeons and hospitals may not accept knee MAKOplasty or our products and we may fail to become profitable and may be subject to significant legal liability.
We have limited sales and marketing experience and capabilities, which could impair our ability to achieve profitability.
We have limited experience as a company in the sales and marketing of our products. We may not be successful in marketing and selling our products in the U.S. through our direct sales force with assistance from independent orthopedic product agents and distributors. Our sales and marketing organization is supported by clinical and technical representatives who provide training, clinical and technical support and other services to our customers before and during the surgery. As of March 1, 2010, we have 73 employees in our sales and marketing organization, which includes all clinical and technical representatives. To reach our revenue targets, we need to expand and strengthen our U.S. direct sales force. Developing a sales and marketing organization is expensive and time consuming and an inability to develop such an organization in a timely manner could delay the successful adoption of our products. Additionally, any sales and marketing organization that we develop may be competing against the experienced and well funded sales and marketing organizations of some of our competitors. We will face significant challenges and risks in developing our sales and marketing organization, including, among others:
|
|
|
|
|
|
• |
our ability to recruit, train and retain adequate numbers of qualified sales and marketing personnel; |
|
|
|
|
|
|
• |
the ability of sales personnel to obtain access to leading surgeons and persuade adequate numbers of hospitals to purchase our products; |
|
|
|
|
|
|
• |
costs associated with hiring, maintaining and expanding a sales and marketing organization; and |
|
|
|
|
|
|
• |
government scrutiny with respect to promotional activities in the healthcare industry. |
If we are unable to develop and maintain these sales and marketing capabilities, we may be unable to generate revenue and may not become profitable.
40
Surgeons, hospitals and orthopedic product agents and distributors may have existing relationships with other medical device companies that make it difficult for us to establish new relationships with them, and as a result, we may not be able to sell and market our products effectively.
We believe that to sell and market our products effectively, we must establish relationships with key surgeons and hospitals in the field of orthopedic surgery. Many of these key surgeons and hospitals already have long-standing relationships with large, better known companies that dominate the medical devices industry through collaborative research programs and other relationships. Because of these existing relationships, some of which may be contractually enforced, surgeons and hospitals may be reluctant to adopt MAKOplasty, particularly if MAKOplasty competes with or has the potential to compete with products supported through their own collaborative research program or by these existing relationships. Even if these surgeons and hospitals purchase our RIO system, they may be unwilling to enter into collaborative relationships with us to promote joint marketing programs such as the MAKOplasty Center of Excellence or to provide us with clinical and financial data.
In addition to our direct sales force, we work with distributors that primarily generate sales leads for us and we are pursuing relationships with potential foreign distributors. If these distributors believe that a relationship with us is less beneficial than other relationships they may have with more established or well known medical device companies, they may be unwilling to establish or continue their relationships with us, making it more difficult for us to sell and market our products effectively.
Because the markets for our products are highly competitive, customers may choose to purchase our competitors’ products, resulting in reduced revenue and harm to our financial results.
MAKOplasty requires the use of new robotics technology, and we face competition from large, well known companies, principally Biomet, Inc., DePuy Orthopedics, Inc., a Johnson & Johnson company, Stryker Corporation, and Zimmer Holdings, Inc., that dominate the market for orthopedic products. Each of these companies, as well as other companies like Smith & Nephew, Inc., which introduced the Journey Deuce Bi-Compartmental Knee System in July 2007, offers conventional instruments and implants for use in conventional total and partial knee replacement surgeries as well as unicompartmental resurfacing procedures, which may compete with our MAKOplasty solution and negatively impact sales of our robotic arm technology. A number of these and other companies also offer CAS systems for use in arthroplasty procedures that provide a minimally invasive means of viewing the anatomical site. In addition, Biomet has licenses from Z-Kat and us to intellectual property rights in CAS intellectual property for use in the field of orthopedics. The license is non-exclusive with respect to use of CAS intellectual property in combination with robotics technology and exclusive with respect to all other uses within the field of orthopedics, which could enable them to compete with us.
Currently, we are not aware of any well known orthopedic company that broadly offers robotics technology in combination with CAS. These large, well known orthopedics companies, however, have the ability to acquire and develop robotics technology that may compete with our products. We are aware of certain early stage companies developing robotic applications in orthopedics and others commercializing customized implants and instruments for early and mid-stage arthroplasty solutions. For example, CUREXO Technology Corporation has engaged in marketing in the United States of its ROBODOC® Surgical System, which received 510(k) marketing clearance from the FDA in August 2008 for total hip arthroplasty procedures.
We also may face competition from other medical device companies that may seek to extend robotics technology and minimally invasive approaches and products that they have developed for use in other parts of the human anatomy to minimally invasive arthroplasty of the knee. Even if these other companies currently do not have an established presence in the field of minimally invasive surgery for the knee, they may attempt to apply their robotics technology to the field of knee replacement and resurfacing procedures to compete directly with us.
Many of these medical device competitors enjoy competitive advantages over us, including:
|
|
|
|
|
|
• |
significantly greater name recognition; |
41
|
|
|
|
|
|
• |
longer operating histories; |
|
|
|
|
|
|
• |
established exclusive relations with healthcare professionals, customers and third-party payors; |
|
|
|
|
|
|
• |
established distribution networks; |
|
|
|
|
|
|
• |
additional lines of products and the ability to offer rebates or bundle products to offer higher discounts or incentives to gain a competitive advantage; |
|
|
|
|
|
|
• |
greater experience in conducting research and development, manufacturing, clinical trials, obtaining regulatory clearance for products and marketing approved products; and |
|
|
|
|
|
|
• |
greater financial and human resources for product development, sales and marketing and patent litigation. |
Moreover, our competitors in the medical device industry make significant investments in research and development, and innovation is rapid and continuous. If new products or technologies emerge that provide the same or superior benefits as our products at equal or lesser cost, they could render our products obsolete or unmarketable. Because our products can have long development and regulatory clearance or approval cycles, we must anticipate changes in the marketplace and the direction of technological innovation and customer demands. In addition, we face increasing competition from well financed orthopedic companies in our attempts to acquire such new technologies, products and businesses. As a result, we cannot be certain that surgeons will use our products to replace or supplement established surgical procedures or that our products will be competitive with current or future products and technologies resulting in reduced revenue and harm to our financial results.
If we do not timely achieve our development goals for new products, the commercialization of these products will be delayed and our business and financial results may be adversely affected.
The success of our business is dependent on our ability to develop new products, to introduce enhancements to our existing products and to develop these new products and enhancements within targeted time frames and budgets to meet customer expectations and requirements. For certain products we have in the past and may in the future utilize a surgeon preference evaluation process prior to full commercial release. The actual timing of these product releases can vary dramatically compared to our estimates for reasons that may or may not be within our control, including clearance or approval by the FDA to market future products and unfavorable clinical results or customer feedback prior to full commercial release through a surgeon preference evaluation process. Customers may forego purchases of our existing products and purchase our competitors’ products as a result of delays in the introduction of our new products and enhancements or failure by us to offer innovative products or enhancements at competitive prices and in a timely manner. Announcements of new products by us or by competitors may also result in a delay in or cancellation of purchasing decisions in anticipation of such new products. Any such losses of new customers would harm our business and financial results.
We have limited experience in assembling and testing our products and may encounter problems or delays in the assembly of our products or fail to meet certain regulatory requirements that could result in a material adverse effect on our business and financial results.
We have limited experience in assembling and testing our products, including the RIO system, and no experience in doing so on a large commercial scale. The current and intended future versions of our RIO system are complex and require the integration of a number of separate components and processes. To become profitable, we must assemble and test the RIO system in commercial quantities in compliance with regulatory requirements and at an acceptable cost. Increasing our capacity to assemble and test our products on a commercial scale will require us to improve internal efficiencies. We may encounter a number of difficulties in increasing our assembly and testing capacity, including:
|
|
|
|
|
|
• |
managing production yields; |
|
|
|
|
|
|
• |
maintaining quality control and assurance; |
|
|
|
|
|
|
• |
providing component and service availability; |
|
|
|
|
|
|
• |
maintaining adequate control policies and procedures; |
|
|
|
|
|
|
• |
hiring and retaining qualified personnel; and |
42
|
|
|
|
|
|
• |
complying with state, federal and foreign regulations. |
If we are unable to satisfy commercial demand for our RIO system due to our inability to assemble and test the system, our business and financial results, including our ability to generate revenue, would be impaired, market acceptance of our products could be materially adversely affected and customers may instead purchase or use, our competitors’ products.
Any failure in our efforts to train surgeons or hospital staff could result in lower than expected product sales and potential liabilities.
A critical component of our sales and marketing efforts is the training of a sufficient number of surgeons and hospital staff to properly perform knee MAKOplasty. As of December 31, 2009, we had trained 126 surgeons on the MAKOplasty system. We rely on surgeons and hospital staff to devote adequate time to learn to use our products. Convincing surgeons and hospital staff to dedicate the time and energy necessary for adequate training in the use of our system is challenging, and we cannot assure you we will be successful in these efforts. If surgeons or hospital staff are not properly trained, they may misuse or ineffectively use our products. If nurses or other members of the hospital staff are not adequately trained to assist in using our RIO system, surgeons may be unable to use our products. Insufficient training may result in reduced system use, unsatisfactory patient outcomes, patient injury and related liability or negative publicity, which could have an adverse effect on our product sales or create substantial potential liabilities.
We will likely continue to experience extended and variable sales cycles, which together with the unit price of the RIO system, could cause significant variability in our results of operations for any given quarter.
Our RIO system has a lengthy sales cycle because it is a major piece of capital equipment, the purchase of which will generally require the approval of senior management at hospitals, inclusion in the hospitals’ budget process for capital expenditures and, in some instances, a certificate of need from the state or other regulatory clearance. As a result, a relatively small number of units are installed each quarter. Based on our limited experience, we estimate that the sales cycle of the RIO system will continue to take between seven and eighteen months from the point of initial identification and contact with a qualified surgeon until closing of the purchase with the hospital. A limited number of sales of RIO systems may also be subject to a customer acceptance period, during which the customer may return the RIO system to us subject to a penalty. Although we believe that training can be accomplished in a relatively short period of time, there may be situations where training of physicians and staff may last an additional month or more after installation. In addition, the introduction of new products could adversely impact our sales cycle as customers take additional time to assess the capital products. Because of the lengthy sales cycle, the unit price of the RIO system and the relatively small number of systems installed each quarter, each installation of a RIO system can represent a significant component of our revenue for a particular quarter, particularly in the near term and during any other periods in which our sales volume is relatively low.
Certain factors that may contribute to variability in our operating results may include:
|
|
|
|
|
|
• |
timing and level of expenditures associated with new product development activities; |
|
|
|
|
|
|
• |
delays in shipment due, for example, to cancellations by customers, natural disasters or labor disturbances; |
|
|
|
|
|
|
• |
delays or unexpected difficulties in the manufacturing processes of our suppliers or in our assembly process; |
|
|
|
|
|
|
• |
timing of the announcement, introduction and delivery of new products or product upgrades by us and by our competitors; |
|
|
|
|
|
|
• |
timing and level of expenditures associated with expansion of sales and marketing activities and our overall operations; |
|
|
|
|
|
|
• |
disruptions in the supply or changes in the costs of raw materials, labor, product components or transportation services; and |
43
|
|
|
|
|
|
• |
changes in third-party coverage and reimbursement, changes in government regulation, or a change in a customer’s financial condition or ability to obtain financing. |
These factors are difficult to forecast and may contribute to substantial fluctuations in our quarterly revenue and substantial variation from our projections, particularly during the periods in which our sales volume is low. Moreover, many of our expenses, such as office leases and certain personnel costs, are relatively fixed. We may be unable to adjust spending quickly enough to offset any unexpected revenue shortfall. Accordingly, any shortfall in revenue may cause significant variation in operating results in any quarter. Based on the above factors, we believe that quarter-to-quarter comparisons of our operating results are not a good indication of our future performance. These and other potential fluctuations also mean that you will not be able to rely upon our operating results in any particular period as an indication of future performance.
If we receive a significant number of warranty claims or our RIO system units require significant amounts of service after sale, our costs will increase and our business and financial results will be adversely affected.
We currently warranty each RIO system against defects in materials and workmanship for a period of approximately twelve months from the first MAKOplasty procedure performed at a customer’s facility. We also provide technical and other services to customers beyond the warranty period pursuant to a supplemental service plan sold with each system. We have a limited history of commercial placements from which to judge our rate of warranty claims. If product returns or warranty claims are significant or exceed our expectations, we could incur unanticipated reductions in sales or additional expenditures for parts and service. In addition, our reputation could be damaged and our products may not achieve market acceptance. While we have established accruals for liability associated with product warranties, unforeseen warranty exposure in excess of those accruals could negatively impact our business and financial results.
We could become subject to product liability claims, product actions, including product recalls, and other field or regulatory actions that could be expensive, divert management’s attention and harm our business.
Our business exposes us to potential liability risks, product actions and other field or regulatory actions that are inherent in the manufacturing, marketing and sale of medical device products. We may be held liable if our RIO system or implants cause injury or death or is found otherwise unsuitable or defective during usage. The RIO system incorporates mechanical, electrical and optical parts, complex computer software and other sophisticated components, any of which can contain errors or failures. Complex computer software is particularly vulnerable to errors and failures, especially when first introduced. In addition, new products or enhancements to our existing products may contain undetected errors or performance problems that, despite testing, are discovered only after installation.
We may, from time to time, elect to initiate a product action concerning one or more of our products for the purpose of improving device performance. If any of our products are defective, whether due to design or manufacturing defects, improper use of the product or other reasons, we may voluntarily or involuntarily undertake a product action to remove, repair, or replace the product at our expense and, in some circumstances, to notify regulatory authorities of such product action pursuant to a product recall. During the year ended December 31, 2009, we initiated one voluntary product actions, which was a product recall reportable to the FDA pursuant to the correction / removal guidelines. We are required to submit an MDR report to the FDA for any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury.
We have experienced and anticipate in the future to experience events that may require reporting to the FDA pursuant to the MDR regulations. See “Risks Related to Regulatory Compliance.” A required notification to a regulatory authority could result in an investigation by regulatory authorities of our products, which could in turn result in product actions, restrictions on the sale of the products, civil or criminal penalties and other field corrective action. In addition, because our products are designed to be used to perform complex surgical procedures, defects could result in a number of complications, some of which could be serious and could harm or kill patients. The adverse publicity resulting from any of these events could cause surgeons or hospitals to review
44
and potentially terminate their relationships with us. Regulatory investigations or product actions could also result in our incurring substantial costs, losing revenue, and implementing a change in the design, manufacturing process or the indications for which our products may be used, each of which would harm our business.
It is also possible that defects in the design, manufacture or labeling of our products could result in a product liability claim. The medical device industry has historically been subject to extensive litigation over product liability claims. A product liability claim, regardless of its merit or eventual outcome, could result in significant legal defense costs. Although we maintain product liability insurance, the coverage is subject to deductibles and limitations, and may not be adequate to cover future claims. Additionally, we may be unable to maintain our existing product liability insurance in the future at satisfactory rates or adequate amounts. A product liability claim, regardless of its merit or eventual outcome could result in:
|
|
|
|
|
|
• |
decreased demand for our products; |
|
|
|
|
|
|
• |
injury to our reputation; |
|
|
|
|
|
|
• |
diversion of management’s attention; |
|
|
|
|
|
|
• |
significant costs of related litigation; |
|
|
|
|
|
|
• |
payment of substantial monetary awards by us; |
|
|
|
|
|
|
• |
product actions; |
|
|
|
|
|
|
• |
a change in the design, manufacturing process or the indications for which our products may be used; |
|
|
|
|
|
|
• |
loss of revenue; and |
|
|
|
|
|
|
• |
an inability to commercialize our products under development. |
If hospitals, surgeons and other healthcare providers are unable to obtain coverage or reimbursement from third-party payors for MAKOplasty procedures, hospitals may not purchase the RIO system and surgeons may not perform knee MAKOplasty, which would harm our business and financial results.
Our ability to successfully commercialize MAKOplasty depends significantly on the availability of coverage and reimbursement from third-party payors, including governmental programs such as Medicare and Medicaid as well as private insurance and private health plans. Reimbursement is a significant factor considered by hospitals in determining whether to acquire new capital equipment such as our technology. Although our customers have been successful in obtaining coverage and reimbursement, we cannot assure you that procedures using our technology will be covered or reimbursed by third-party payors in the future or that such reimbursements will not be reduced to the extent that they will adversely affect capital allocations for purchase of our RIO system.
We anticipate that in the U.S. our products will be purchased primarily by hospitals, which bill various third-party payors, including governmental healthcare programs, such as Medicare, and private insurance plans for procedures using our technology. Ensuring adequate Medicare reimbursement can be a lengthy and expensive endeavor and we cannot provide assurance that we will be successful. In addition, the U.S. Congress may pass legislation impacting coverage and reimbursement for healthcare services, including Medicare reimbursement to physicians and hospitals. Many private payors look to Medicare’s coverage and reimbursement policies in setting their coverage policies and reimbursement amounts. If the Centers for Medicare and Medicaid Services, or CMS, the federal agency that administers the Medicare program, or Medicare contractors limit payments to hospitals or surgeons for MAKOplasty procedures, private payors may similarly limit payments. In addition, state legislatures may enact laws limiting or otherwise affecting the level of Medicaid reimbursements. As a result, hospitals may not purchase the RIO system and surgeons may choose not to perform MAKOplasty, and, as a result, our business and financial results would be adversely affected.
Medicare pays acute care hospitals a prospectively determined amount for inpatient operating costs under the Medicare hospital inpatient prospective payment system, or PPS. Under the Medicare hospital inpatient PPS, the prospective payment for a patient’s stay in an acute care hospital is determined by the patient’s condition and other patient data and procedures performed during the inpatient stay using a classification system known as diagnosis related groups, or DRGs. As of October 1, 2007, CMS, implemented a revised version of the DRG
45
system that uses 745 Medicare Severity DRGs, or MS-DRGs, instead of the approximately 540 DRGs Medicare previously used. The MS-DRGs are intended to account more accurately for the patient’s severity of illness when assigning each patient’s stay to a payment classification. Medicare pays a fixed amount to the hospital based on the MS-DRG into which the patient’s stay is assigned, regardless of the actual cost to the hospital of furnishing the procedures, items and services provided. Accordingly, acute care hospitals generally do not receive direct Medicare reimbursement under PPS for the specific costs incurred in purchasing medical devices. Rather, reimbursement for these costs is deemed to be included within the MS-DRG based payments made to hospitals for the services furnished to Medicare eligible inpatients in which the devices are utilized. Accordingly, a hospital must absorb the cost of our products as part of the payment it receives for the procedure in which the device is used. In addition, physicians that perform procedures in hospitals are paid a set amount by Medicare for performing such services under the Medicare physician fee schedule. Medicare payment rates for both systems are established annually.
At this time, we do not know the extent to which hospitals and physicians would consider third-party reimbursement levels adequate to cover the cost of our products. Failure by hospitals and surgeons to receive an amount that they consider to be adequate reimbursement for procedures in which our products are used could deter them from purchasing or using our products and limit our sales growth. In addition, pre-determined MS-DRG payments or Medicare physician fee schedule payments may decline over time, which could deter hospitals from purchasing our products or physicians from using them. If hospitals are unable to justify the costs of our products or physicians are not adequately compensated for procedures in which our products are utilized, they may refuse to purchase or use them, which would significantly harm our business.
Notwithstanding current or future FDA clearances, if granted, third-party payors may deny reimbursement if the payor determines that a therapeutic medical device is unnecessary, inappropriate, not cost-effective or experimental, or is used for a non-approved indication. Although we are not aware of any potential customer that has declined to purchase our RIO system based upon third-party payors’ reimbursement policies, cost control measures adopted by third-party payors may have a significant effect on surgeries performed using MAKOplasty or as to the levels of reimbursement. All third-party payors, whether governmental or private, whether inside the U.S. or outside, are developing increasingly sophisticated methods of controlling healthcare costs. These cost control methods include prospective payment systems, capitated rates, benefit redesigns, pre-authorization or second opinion requirements prior to major surgery, an emphasis on wellness and healthier lifestyle interventions and an exploration of other cost-effective methods of delivering healthcare. These cost control methods also potentially limit the amount which healthcare providers may be willing to pay for medical technology which could, as a result, adversely affect our business and financial results. In addition, in the U.S., no uniform policy of coverage and reimbursement for medical technology exists among all these payors. Therefore, coverage and reimbursement for medical technology can differ significantly from payor to payor.
There also can be no assurance that current levels of reimbursement will not be decreased or eliminated in the future, or that future legislation, regulation, or reimbursement policies of third-party payors will not otherwise adversely affect the demand for our products or our ability to sell products on a profitable basis. Our customers are currently using existing reimbursement codes for knee arthroplasty. Knee arthroplasty performed in the hospital inpatient setting is currently assigned to MS-DRG 469 (“Major Joint Replacement or Reattachment of Lower Extremity with Major Complication or Comorbidity”) and MS-DRG 470 (“Major Joint Replacement or Reattachment of Lower Extremity without Major Complication of Comorbidity”), and surgeons currently bill Current Procedural Terminology, or CPT, code 27446 (“Arthroplasty, knee, condyle and plateau; medial OR lateral compartment”), code 27447 (“Arthroplasty, knee condyle and plateau, medial and lateral with or without patella resurfacing”), and code 27438 (“Arthoplasty, patella with prosthesis”) for services performed in connection with procedures using our technology. Our customers also have available Ambulatory Payment Classifications, or APC, code 0451 and CPT codes 27446 and 73700 (“CT lower extremity without contrast”) for out-patient procedures and procedures performed in an ambulatory surgery center. If unicompartmental and bicompartmental knee resurfacing procedures gain market acceptance and the number of such procedures increases, CMS and other payors may establish billing codes for unicompartmental and bicompartmental knee resurfacing procedures that provide for a smaller reimbursement amount than knee arthroplasty, which could adversely affect our financial results and business.
46
In international markets, market acceptance of our products will likely depend in large part on the availability of reimbursement within prevailing healthcare payment systems. Reimbursement and healthcare payment systems in international markets vary significantly by country, and by region in some countries, and include both government sponsored healthcare and private insurance. We may not obtain international reimbursement approvals in a timely manner, if at all. In addition, even if we do obtain international reimbursement approvals, the level of reimbursement may not be enough to commercially justify expansion of our business into the approving jurisdiction. To the extent we or our customers are unable to obtain coverage or reimbursement for procedures using our technology in major international markets in which we seek to market and sell our technology, our international revenue growth would be harmed, and our business and results of operations would be adversely affected.
Healthcare reforms, changes in healthcare policies and changes to third-party coverage and reimbursements may affect demand for our systems and products.
The U. S. government, state and local governments, and a number of foreign governments, are currently considering or may in the future consider healthcare policies and proposals intended to curb rising healthcare costs, including those that could significantly affect both coverage and reimbursement for healthcare services. Future significant changes in the healthcare systems in the United States or elsewhere, and current uncertainty about whether and how changes may be implemented, could have a negative impact on the demand for our products and services and our business. These changes may include basing coverage and reimbursement policies and rates on clinical outcomes, the comparative effectiveness and costs of different treatment technologies and modalities; imposing price controls on medical products and services providers; reducing reimbursement rates; imposing an excise tax on the medical device industry; and other measures. It is unclear which, if any, of the various U.S. healthcare reforms currently being discussed and/or proposed might be enacted by the U.S. Congress and signed into law by the President of the United States. We are unable to predict what healthcare reform legislation or regulations, if any, will be enacted in the United States or elsewhere; whether other healthcare legislation or regulations affecting our business may be proposed or enacted in the future; what effect any legislation or regulation would have on our business; or the effect ongoing uncertainty about these matters will have on the purchasing decisions of our customers.
We may attempt to acquire new products or technologies, and if we are unable to successfully complete these acquisitions or to integrate acquired businesses, products, technologies or employees, we may fail to realize expected benefits or harm our existing business.
Our success will depend, in part, on our ability to expand our product offerings and grow our business in response to changing technologies, customer demands and competitive pressures. In some circumstances, we may determine to do so through the acquisition of complementary businesses, products or technologies rather than through internal development. The identification of suitable acquisition candidates can be difficult, time consuming and costly, and we may not be able to successfully complete identified acquisitions. Furthermore, even if we successfully complete an acquisition, we may not be able to successfully integrate newly acquired organizations, products or technologies into our operations, and the process of integration could be expensive, time consuming and may strain our resources. Consequently, we may not achieve anticipated benefits of the acquisitions, which could harm our existing business. In addition, future acquisitions could result in potentially dilutive issuances of equity securities or the incurrence of debt, contingent liabilities or expenses, or other charges such as in-process research and development, any of which could harm our business and materially adversely affect our financial results or cause a reduction in the price of our common stock.
We depend on key employees, and if we fail to attract and retain employees with the expertise required for our business, we cannot grow or achieve profitability.
We are highly dependent on members of our senior management, in particular Maurice R. Ferré, M.D., our President, Chief Executive Officer and Chairman of the Board. Our future success will depend in part on our ability to retain these key employees and to identify, hire and retain additional qualified personnel with expertise
47
in research and development and sales and marketing. Competition for qualified personnel in the medical device industry is intense, and finding and retaining qualified personnel with experience in our industry is very difficult. We believe that there are only a limited number of individuals with the requisite skills to serve in many of our key positions, and we compete for key personnel with other medical equipment and software manufacturers and technology companies, as well as universities and research institutions. It is often difficult to hire and retain these persons, and we may be unable to replace key persons if they leave or fill new positions requiring key persons with appropriate experience. A significant portion of our compensation to our key employees is in the form of stock option grants. A prolonged depression in our stock price could make it difficult for us to retain our employees and recruit additional qualified personnel.
We do not maintain, and do not currently intend to obtain, key employee life insurance on any of our personnel other than Dr. Ferré. Although we have obtained key man insurance covering Dr. Ferré in the amount of $2,000,000, this would not fully compensate us for the loss of Dr. Ferré’s services. Dr Ferré may terminate his employment at will at any time with 30 days’ notice. Each of our other officers and key employees may terminate his or her employment at will at any time with 60 days’ notice. The loss of key employees, the failure of any key employee to perform or our inability to attract and retain skilled employees, as needed, could harm our business.
If we do not effectively manage our growth, we may be unable to successfully develop, market and sell our products.
Our future revenue and operating results will depend on our ability to manage the anticipated growth of our business. We have experienced significant growth in the scope of our operations and the number of our employees since our inception. This growth has placed significant demands on our management, as well as our financial and operations resources. In order to achieve our business objectives, we must continue to grow. However, continued growth presents numerous challenges, including:
|
|
|
|
|
|
• |
implementing appropriate operational and financial systems and controls; |
|
|
|
|
|
|
• |
expanding manufacturing and assembly capacity and increasing production; |
|
|
|
|
|
|
• |
developing our sales and marketing infrastructure and capabilities; |
|
|
|
|
|
|
• |
improving our information systems; |
|
|
|
|
|
|
• |
identifying, attracting and retaining qualified personnel in our areas of activity; and |
|
|
|
|
|
|
• |
hiring, training, managing and supervising our personnel. |
We cannot be certain that our systems, controls, infrastructure and personnel will be adequate to support our future operations. Any failure to effectively manage our growth could impede our ability to successfully develop, market and sell our products and our business will be harmed.
If we are successful in our efforts to market and sell MAKOplasty internationally, we will be subject to various risks relating to our international activities, which could adversely affect our business and financial results.
We have begun to pursue international markets for the sale of our products and we anticipate being exposed to risks separate and distinct from those we face in our U.S. operations. Our international business may be adversely affected by changing economic conditions in foreign countries. In addition, because international sales would most likely be denominated in the functional currency of the country where the product is being shipped, increases or decreases in the value of the U.S. dollar relative to foreign currencies could affect our results of operations. Engaging in international business inherently involves a number of other difficulties and risks, including:
|
|
|
|
|
|
• |
approval of product submissions with healthcare systems outside the United States; |
|
|
|
|
|
|
• |
gathering the clinical data that may be required for product submissions with healthcare systems outside the United States; |
|
|
|
|
|
|
• |
export restrictions and controls and other government regulation relating to technology; |
|
|
|
|
|
|
• |
the availability and level of reimbursement within prevailing foreign healthcare payment systems; |
48
|
|
|
|
|
|
• |
pricing pressures that we may experience internationally; |
|
|
|
|
|
|
• |
compliance with existing and changing foreign regulatory laws and requirements; |
|
|
|
|
|
|
• |
foreign laws and business practices favoring local companies; |
|
|
|
|
|
|
• |
longer payment cycles; |
|
|
|
|
|
|
• |
shipping delays; |
|
|
|
|
|
|
• |
difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; |
|
|
|
|
|
|
• |
political and economic instability; |
|
|
|
|
|
|
• |
potentially adverse tax consequences, tariffs and other trade barriers; |
|
|
|
|
|
|
• |
international terrorism and anti-American sentiment; |
|
|
|
|
|
|
• |
difficulties and costs of staffing and managing foreign operations; and |
|
|
|
|
|
|
• |
difficulties in enforcing intellectual property rights. |
Our exposure to each of these risks may increase our costs, impair our ability to market and sell our products and require significant management attention, resulting in harm to our business and financial results.
Our operations are currently conducted primarily at a single location in Florida, which may be at risk from hurricanes, storm, fire, terrorist attacks or other disasters.
We currently conduct all of our management activities, most of our research and development activities and assemble all of our products at a single location in Fort Lauderdale, Florida. We have taken various precautions to safeguard our facilities, such as obtaining insurance, installing hurricane shutters, establishing health and safety protocols and securing off-site storage of computer data. However, a casualty due to a hurricane, storm or other natural disasters, a fire, terrorist attack, or other unanticipated problems at this location could cause substantial delays in our operations, delay or prevent assembly of our RIO systems and shipment of our implants, damage or destroy our equipment and inventory, and cause us to incur substantial expenses. Our insurance does not cover losses caused by certain events such as floods or other activities and may not be adequate to cover our losses in any particular case. Any damage, loss or delay could seriously harm our business and have an adverse affect on our financial results.
Certain of our directors, executive officers and key employees have an interest in Z-Kat that could pose potential conflicts of interest, which could harm our business.
Prior to February 2010, we licensed or sublicensed intellectual property from Z-Kat and entered into various licensing and related arrangements with Z-Kat. In February 2010, we terminated our prior licenses with Z-Kat, including Z-Kat’s nonexclusive sublicense to our intellectual property portfolio, and entered into an asset purchase agreement with Z-Kat pursuant to which we obtained ownership rights to certain intellectual property assets owned by Z-Kat, and certain intellectual property licensed to Z-Kat. We also entered into a new license agreement with Z-Kat pursuant to which we obtained an exclusive sublicense to certain intellectual property for technologies in CAS and robotics in the medical field. We believe that certain of our directors, executive officers and key employees hold equity interests in Z-Kat. Accordingly, each of these individuals may face continuing potential conflicts of interest regarding these transactions as a result of their interests in Z-Kat. Dr. Ferré may face additional conflicts of interest regarding these transactions if he serves on the board of directors of Z-Kat. To address these potential conflicts of interest, we have adopted a Related Person Transaction Policy. In addition, the audit committee of our board of directors, under the terms of its charter, must review and approve all related person transactions. We cannot, however, assure you that any conflicts will be resolved in our favor, and as a result, our business could be harmed.
Risks Related to Our Intellectual Property
If we, or the third parties from whom we license intellectual property, are unable to secure and maintain patent or other intellectual property protection for the intellectual property contained in our products, our ability to compete will be harmed.
49
Our commercial success depends, in part, on obtaining patent and other intellectual property protection for the technologies contained in our products. The patent positions of medical device companies, including ours, can be highly uncertain and involve complex and evolving legal and factual questions. Our patent position is uncertain and complex, in part, because of our dependence on intellectual property that we license from others. If we, or the third parties from whom we license intellectual property, fail to obtain adequate patent or other intellectual property protection for intellectual property contained in our products, or if any protection is reduced or eliminated, others could use the intellectual property contained in our products, resulting in harm to our competitive business position. In addition, patent and other intellectual property protection may not provide us with a competitive advantage against competitors that devise ways of making competitive products without infringing any patents that we own or have rights to.
U.S. patents and patent applications may be subject to interference proceedings and U.S. patents may be subject to reexamination proceedings in the U.S. Patent and Trademark Office. Foreign patents may be subject to opposition or comparable proceedings in the corresponding foreign patent offices. Any of these proceedings could result in either loss of the patent or denial of the patent application, or loss or reduction in the scope of one or more of the claims of the patent or patent application. Changes in either patent laws or in interpretations of patent laws may also diminish the value of our intellectual property or narrow the scope of our protection. Interference, reexamination and opposition proceedings may be costly and time consuming, and we, or the third parties from whom we license intellectual property, may be unsuccessful in defending against such proceedings. Thus, any patents that we own or license may provide limited or no protection against competitors. In addition, our pending patent applications and those we may file in the future may have claims narrowed during prosecution or may not result in patents being issued. Even if any of our pending or future applications are issued, they may not provide us with adequate protection or any competitive advantages. Our ability to develop additional patentable technology is also uncertain.
Non-payment or delay in payment of patent fees or annuities, whether intentional or unintentional, may also result in the loss of patents or patent rights important to our business. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as do the laws of the U.S., particularly in the field of medical products and procedures.
If we are unable to prevent unauthorized use or disclosure of our proprietary trade secrets and unpatented know-how, our ability to compete will be harmed.
Proprietary trade secrets, copyrights, trademarks and unpatented know-how are also very important to our business. We rely on a combination of trade secrets, copyrights, trademarks, confidentiality agreements and other contractual provisions and technical security measures to protect certain aspects of our technology, especially where we do not believe that patent protection is appropriate or obtainable. We require our employees and consultants to execute confidentiality agreements in connection with their employment or consulting relationships with us. We also require our employees and consultants to disclose and assign to us all inventions conceived during the term of their employment or engagement while using our property or which relate to our business. We also have taken precautions to initiate reasonable safeguards to protect our information technology systems. However, these measures may not be adequate to safeguard our proprietary intellectual property and conflicts may, nonetheless, arise regarding ownership of inventions. Such conflicts may lead to the loss or impairment of our intellectual property or to expensive litigation to defend our rights against competitors who may be better funded and have superior resources. Our employees, consultants, contractors, outside clinical collaborators and other advisors may unintentionally or willfully disclose our confidential information to competitors. In addition, confidentiality agreements may be unenforceable or may not provide an adequate remedy in the event of unauthorized disclosure. Enforcing a claim that a third party illegally obtained and is using our trade secrets is expensive and time consuming, and the outcome is unpredictable. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. Unauthorized parties may also attempt to copy or reverse
50
engineer certain aspects of our products that we consider proprietary. As a result, third parties may be able to use our proprietary technology or information, and our ability to compete in the market would be harmed.
We could become subject to patent and other intellectual property litigation that could be costly, result in the diversion of management’s attention, require us to pay damages and force us to discontinue selling our products.
The medical device industry is characterized by competing intellectual property and a substantial amount of litigation over patent and other intellectual property rights. In particular, the fields of orthopedic implants, CAS, haptics and robotics are well established and crowded with the intellectual property of competitors and others. A number of companies in our market, as well as universities and research institutions, have issued patents and have filed patent applications which relate to the use of CAS.
Determining whether a product infringes a patent involves complex legal and factual issues, and the outcome of a patent litigation action is often uncertain. We have not conducted an extensive search of patents issued to third parties, and no assurance can be given that third-party patents containing claims covering our products, parts of our products, technology or methods do not exist, have not been filed or could not be filed or issued. Because of the number of patents issued and patent applications filed in our technical areas, our competitors or other third parties, including third parties from whom we license intellectual property, may assert that our products and the methods we employ in the use of our products are covered by U.S. or foreign patents held by them. In addition, because patent applications can take many years to issue and because publication schedules for pending applications vary by jurisdiction, there may be applications now pending of which we are unaware and which may result in issued patents which our current or future products infringe. Also, because the claims of published patent applications can change between publication and patent grant, there may be published patent applications that may ultimately issue with claims that we infringe. There could also be existing patents that one or more of our products or parts may infringe and of which we are unaware. As the number of competitors in the market for CAS and robotics assisted knee implant systems grows, and as the number of patents issued in this area grows, the possibility of patent infringement claims against us increases. In certain situations, we or third parties from whom we license intellectual property may determine that it is in our best interests or their best interests to voluntarily challenge a third party’s products or patents in litigation or other proceedings, including patent interferences or reexaminations. As a result, we may become involved in unwanted litigation that could be costly, result in diversion of management’s attention, require us to pay damages and force us to discontinue selling our products.
Infringement actions and other intellectual property claims and proceedings, whether with or without merit, may cause us to incur substantial costs and could place a significant strain on our financial resources, divert the attention of management from our business and harm our reputation. Some of our competitors may be able to sustain the costs of complex patent or intellectual property litigation more effectively than we can because they have substantially greater resources.
We cannot be certain that we will successfully defend against allegations of infringement of third-party patents and intellectual property rights. In the event that we become subject to a patent infringement or other intellectual property lawsuit and if the other party’s patents or other intellectual property were upheld as valid and enforceable and we were found to infringe the other party’s patents or violate the terms of a license to which we are a party, we could be required to pay damages. We could also be prevented from selling our products unless we could obtain a license to use technology or processes covered by such patents or were able to redesign the product to avoid infringement. A license may not be available at all or on commercially reasonable terms or we may not be able to redesign our products to avoid infringement. Modification of our products or development of new products could require us to conduct clinical trials and to revise our filings with the FDA and other regulatory bodies, which would be time consuming and expensive. In these circumstances, we may be unable to sell our products at competitive prices or at all, our business and operating results could be harmed and our stock price may decline. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
51
We may be subject to damages resulting from claims that our employees, our consultants or we have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees and consultants were previously employed at universities or other medical device companies, including our competitors or potential competitors. We could in the future be subject to claims that these employees or consultants, or we, have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending against such claims, a court could order us to pay substantial damages and prohibit us from using technologies or features that are essential to our products and processes, if such technologies or features are found to incorporate or be derived from the trade secrets or other proprietary information of the former employers. In addition, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize certain potential products, which could severely harm our business. Even if we are successful in defending against these claims, such litigation could result in substantial costs and be a distraction to management.
Risks Related to Regulatory Compliance
If we fail to comply with the extensive government regulations relating to our business, we may be subject to fines, injunctions and other penalties that could harm our business.
Our medical device products and operations are subject to extensive regulation by the FDA, pursuant to the Federal Food, Drug, and Cosmetic Act, or FDCA, and various other federal, state and foreign governmental authorities. Government regulations and foreign requirements specific to medical devices are wide ranging and govern, among other things:
|
|
|
|
|
|
• |
design, development and manufacturing; |
|
|
|
|
|
|
• |
testing, labeling and storage; |
|
|
|
|
|
|
• |
clinical trials; |
|
|
|
|
|
|
• |
product safety; |
|
|
|
|
|
|
• |
marketing, sales and distribution; |
|
|
|
|
|
|
• |
premarket clearance or approval; |
|
|
|
|
|
|
• |
record keeping procedures; |
|
|
|
|
|
|
• |
advertising and promotions; |
|
|
|
|
|
|
• |
recalls and field corrective actions; |
|
|
|
|
|
|
• |
post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; and |
|
|
|
|
|
|
• |
product export. |
In the U.S., before we can market a new medical device, or a new use of, or claim for, or significant modification to, an existing product, we must first receive either premarket clearance under Section 510(k) of the FDCA, or approval of a PMA from the FDA, unless an exemption applies. In the 510(k) marketing clearance process, the FDA must determine that a proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, with respect to intended use, technology and safety and effectiveness, in order to clear the proposed device for marketing. Clinical data is sometimes required to support substantial equivalence. The PMA approval pathway requires an applicant to demonstrate the safety and effectiveness of the device based, in part, on data obtained in clinical trials. Both of these processes can be expensive and lengthy and entail significant user fees, unless exempt. The FDA’s 510(k) marketing clearance process usually takes from three to 12 months, but it can last longer. The process of obtaining PMA approval is much more costly and uncertain than the 510(k) marketing clearance process. It generally takes from one to three years, or even longer, from the time the PMA application is submitted to the FDA until an approval is obtained. There is no assurance that we will be able to obtain FDA clearance or approval for any of our new products on a timely basis, or at all. We cannot predict whether the FDA will modify its 510(k) marketing clearance process in the future. Any such modification could have a material adverse effect on our ability to commercialize our products.
52
The FDA, state, foreign and other governmental authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in governmental agencies or a court taking action, including any of the following sanctions:
|
|
|
|
|
|
• |
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
|
|
|
|
|
|
• |
customer notifications or repair, replacement, refunds, detention or seizure of our products; |
|
|
|
|
|
|
• |
operating restrictions or partial suspension or total shutdown of production; |
|
|
|
|
|
|
• |
refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products; |
|
|
|
|
|
|
• |
withdrawing 510(k) marketing clearances or PMA approvals that have already been granted; |
|
|
|
|
|
|
• |
refusing to provide Certificates for Foreign Government (CFG); |
|
|
|
|
|
|
• |
refusing to grant export approval for our products; or |
|
|
|
|
|
|
• |
pursuing criminal prosecution. |
Failure to obtain regulatory approval in additional foreign jurisdictions will prevent us from expanding the commercialization of our products abroad.
To be able to market and sell our products in other countries, we must obtain regulatory approvals and comply with the regulations of those countries. These regulations, including the requirements for approvals and the time required for regulatory review, vary from country to country. Obtaining and maintaining foreign regulatory approvals are expensive, and we cannot be certain that we will receive regulatory approvals in any foreign country in which we plan to market our products. If we fail to obtain or maintain regulatory approval in any foreign country in which we plan to market our products, our ability to generate revenue will be harmed.
As we modify existing products or develop new products in the future, including new instruments, we apply for permission to affix to such products a European Union CE mark, which is a legal requirement for medical devices intended for sale in Europe. In addition, we will be subject to annual regulatory audits in order to maintain those CE mark permissions. In November 2008, BSi, an independent global certification body, conducted an annual assessment of our quality management system, which concluded that our quality management system complied with the requirements of ISO13485:2003 in all material respects. We have updated our ISO certifications to include class III devices, such as orthopedic implants. In December 2009, we received a major nonconformance in our annual assessment by BSi. We implemented corrective actions and in March 2010 the finding was officially closed by BSi. BSi will continue to conduct annual audits to assess our compliance with BSi certification standards. In connection with achieving CE marking, which is a legal requirement for medical devices intended for sale in Europe, we have also submitted design dossiers to BSi for the purpose of review and approval. We do not know whether we will be able to obtain permission to affix the CE mark for new or modified products or that we will continue to meet the quality and safety standards required to maintain the permissions we have already received. If we are unable to maintain permission to affix the CE mark to our products, we will no longer be able to sell our products in member countries of the European Union or other areas of the world that require CE approval of medical devices.
If we or our third-party manufacturers or suppliers fail to comply with the FDA’s Quality System Regulation, our manufacturing operations could be interrupted and our product sales and operating results could suffer.
We and some of our third-party manufacturers and suppliers are required to comply with the FDA’s Quality System Regulation, or QSR, which covers the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our products. We and our manufacturers and suppliers are also subject to the regulations of foreign jurisdictions regarding the manufacturing process if we market our products overseas. The FDA enforces the QSR through periodic and unannounced inspections of manufacturing facilities. In January 2009, the FDA conducted its first audit of our facility, during which we received certain inspectional observations. We have addressed the observations and submitted a response to the FDA on a voluntary basis. We received a copy of the establishment inspection report
53
(EIR) and a cover letter from the FDA in August 2009. To date, our facilities have not been inspected by any other regulatory authorities. We anticipate that we and certain of our third-party manufacturers and suppliers will be subject to inspections by regulatory authorities in the future. If our facilities or those of our manufacturers or suppliers fail to take satisfactory corrective action in response to an adverse QSR inspection, the FDA could take enforcement action, including any of the following sanctions:
|
|
|
|
|
|
• |
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
|
|
|
|
|
|
• |
customer notifications or repair, replacement, refunds, detention or seizure of our products; |
|
|
|
|
|
|
• |
operating restrictions or partial suspension or total shutdown of production; |
|
|
|
|
|
|
• |
refusing or delaying requests for 510(k) marketing clearance or PMA approvals of new products or modified products; |
|
|
|
|
|
|
• |
withdrawing 510(k) marketing clearances or PMA approvals that have already been granted; |
|
|
|
|
|
|
• |
refusing to provide Certificates for Foreign Government (CFG); |
|
|
|
|
|
|
• |
refusing to grant export approval for our products; or |
|
|
|
|
|
|
• |
pursuing criminal prosecution. |
Any of these sanctions could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales and our ability to generate profits.
Our products may in the future be subject to product actions that could harm our reputation, business operations and financial results.
Manufacturers may, on their own initiative, initiate a product action, including a non-reportable market withdrawal or a reportable product recall, for the purpose of correcting a material deficiency, improving device performance, or other reasons. Additionally, the FDA and similar foreign governmental authorities have the authority to require an involuntary recall of commercialized products in the event of material deficiencies or defects in design, or manufacture or labeling. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious injury or death. In addition, foreign governmental bodies have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. Product actions involving any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. During the year ended December 31, 2009, we initiated one product action, which was reportable to the FDA pursuant to the correction / removal guidelines. This reportable recall was associated with a fiber optic cable used to connect the robotic arm to the camera stand and was communicated to the FDA in July 2009. We internally closed the action in November 2009 and are awaiting closure notification from the FDA. During the year ended December 31, 2009, we also closed a reportable recall which was initiated in 2008. This reportable recall was associated with two tracking array instruments for the patient specific visualization of the TGS and was communicated to the FDA in May 2008. We internally closed the action in January 2009 and received a closure notification from the FDA on August 6, 2009.
Companies are required to maintain certain records of product actions, even if they are not reportable to the FDA. If we determine that certain of those product actions do not require notification of the FDA, the FDA may disagree with our determinations and require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action, including any of the following sanctions for failing to report the recalls when they were conducted:
|
|
|
|
|
|
• |
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
|
|
|
|
|
|
• |
customer notifications or repair, replacement, refunds, recall, detention or seizure of our products; |
|
|
|
|
|
|
• |
operating restrictions or partial suspension or total shutdown of production; |
|
|
|
|
|
|
• |
refusing or delaying our requests for 510(k) marketing clearance or PMA approvals of new products or modified products; |
54
|
|
|
|
|
|
• |
withdrawing 510(k) marketing clearances or PMA approvals that have already been granted; |
|
|
|
|
|
|
• |
refusing to provide Certificates for Foreign Government (CFG); |
|
|
|
|
|
|
• |
refusing to grant export approval for our products; or |
|
|
|
|
|
|
• |
pursuing criminal prosecution. |
Any of these sanctions could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. We may also be required to bear other costs or take other actions that may have a negative impact on our future sales and our ability to generate profits.
If our products, or malfunction of our products, cause or contribute to a death or a serious injury, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA MDR regulations, we are required to report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. In addition, all manufacturers placing medical devices in European Union markets are legally bound to report any serious or potentially serious incidents involving devices they produce or sell to the relevant authority in whose jurisdiction the incident occurred. We have experienced and anticipate in the future to experience events that may require reporting to the FDA pursuant to the MDR regulations. Any adverse event involving our products could result in future voluntary corrective actions, such as product actions or customer notifications, or agency action, such as inspection, mandatory recall or other enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results. In addition, failure to report such adverse events to appropriate government authorities on a timely basis, or at all, could result in an enforcement action against us.
We may be subject to fines, penalties or injunctions if we are determined to be promoting the use of our products for unapproved or “off-label” uses, resulting in damage to our reputation and business.
Our promotional materials and training methods must comply with FDA and other applicable laws and regulations, including the prohibition of the promotion of a medical device for a use that has not been cleared or approved by FDA. Use of a device outside its cleared or approved indications is known as “off-label” use. We believe that the specific surgical procedures for which our products are marketed fall within the scope of the surgical applications that have been cleared by the FDA. However, physicians may use our products off-label, as the FDA does not restrict or regulate a physician’s choice of treatment within the practice of medicine. However, if the FDA determines that our promotional materials or training constitutes promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of the products would be impaired. Although our policy is to refrain from statements that could be considered off-label promotion of our products, the FDA or another regulatory agency could disagree and conclude that we have engaged in off-label promotion. In addition, the off-label use of our products may increase the risk of injury to patients, and, in turn, the risk of product liability claims. Product liability claims are expensive to defend and could divert our management’s attention and result in substantial damage awards against us.
We may be subject to fine, penalties, or licensure requirements if it is determined that our MAKOplasty Specialists are practicing medicine without a license.
55
State laws prohibit the practice of medicine without a license. Our MAKOplasty Specialists provide pre-operative and intra-operative clinical and technical support to our customers, including assistance setting up the equipment, participation in the pre-operative planning process, and facilitation of the surgeon’s use of the RIO system during surgery. We do not believe that our MAKOplasty Specialists are engaged in the practice of medicine, but rather are assisting our customers in the safe and proper usage of our equipment and products. Nevertheless, a state could consider the activities of our MAKOplasty Specialists to constitute the practice of medicine and may seek to have us discontinue the services provided by our MAKOplasty Specialists or subject us to fine, penalties or licensure requirements. Any determination that our MAKOplasty Specialists are practicing medicine without a license may result in significant liability to us.
The application of state certificate of need regulations could substantially limit our ability to sell our products and grow our business.
Some states require healthcare providers to obtain a certificate of need or similar regulatory approval prior to the acquisition of high-cost capital equipment such as our RIO system. In some states, the process required of our customers to obtain this certificate is lengthy and could result in a longer sales cycle for our RIO system. Further, in many cases, only a limited number of these certificates are available. As a result, our customers may be unable to obtain a certificate of need for the purchase of our RIO system which could cause our sales to decline.
Federal regulatory reforms may adversely affect our ability to sell our products profitably.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the clearance or approval, manufacture and marketing of a medical device. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be.
Without limiting the generality of the foregoing, Congress has enacted, and the President signed into law, the Food and Drug Administration Amendments Act of 2007, or the Amendments. This law requires, among other things, that the FDA propose, and ultimately implement, regulations that will require manufacturers to label medical devices with unique identifiers unless a waiver is received from the FDA. Once implemented, compliance with those regulations may require us to take additional steps in the manufacture of our products and labeling. These steps may require additional resources and could be costly. In addition, the Amendments will require us to, among other things, pay annual establishment registration fees to the FDA for each of our FDA registered facilities.
We may be subject, directly or indirectly, to federal and state healthcare regulations, including fraud and abuse laws, and could face substantial penalties if we are unable to fully comply with such regulations and laws.
While we do not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors, many healthcare laws and regulations apply to our business. For example, we could be subject to healthcare fraud and abuse and patient privacy regulation and enforcement by both the federal government and the states in which we conduct our business. The healthcare laws and regulations that may affect our ability to operate include:
|
|
|
|
|
|
• |
the federal healthcare programs’ Anti-Kickback Statute, which prohibits, among other things, persons or entities from soliciting, receiving, offering or providing remuneration, directly or indirectly, in return for or to induce either the referral of an individual for, or the purchase order or recommendation of, any item or service for which payment may be made under a federal healthcare program such as the Medicare and Medicaid programs; |
|
|
|
|
|
|
• |
federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third- |
56
|
|
|
|
|
|
|
party payors that are false or fraudulent, or are for items or services not provided as claimed, and which may apply to entities like us to the extent that our interactions with customers may affect their billing or coding practices; |
|
|
|
|
|
|
• |
the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which established new federal crimes for knowingly and willfully executing a scheme to defraud any healthcare benefit program or making false statements in connection with the delivery of or payment for healthcare benefits, items or services, as well as leading to regulations imposing certain requirements relating to the privacy, security and transmission of individually identifiable health information; |
|
|
|
|
|
|
• |
the Health Information Technology for Economic and Clinical Health Act, or HITECH, which made HIPAA’s privacy and security standards directly applicable to business associates of covered entities; and |
|
|
|
|
|
|
• |
state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Additionally, several bills have been passed or are pending, at both the state and federal levels, that expand the anti-kickback laws to require, among other things, extensive tracking and maintenance of databases regarding relationships to physicians and healthcare providers. The implementation of the infrastructure to comply with these bills could be costly.
While we do not believe that the provisions of HITECH which make HIPAA’s privacy and security standards directly applicable to business associates of covered entities apply to us since we do not believe that we are a business associate, there is no guarantee that the government will agree with our determination that we are not a business associate. If the government determines that we are a business associate, we could be subject to enforcement measures, including civil and criminal penalties and fines.
The orthopedic medical device industry is, and in recent years has been, under heightened scrutiny as the subject of government investigations and enforcement actions involving manufacturers who allegedly offered unlawful inducements to potential or existing customers in an attempt to procure their business, specifically including arrangements with physician consultants. We have arrangements with surgeons, hospitals and other entities which may be subject to scrutiny. For example, we have consulting agreements with orthopedic surgeons using or considering the use of our present and future RIO system and MAKOplasty implants and disposable products, for assistance in product development, and professional training and education, among other things. Payment for some of these consulting services has been in the form of stock options or royalties rather than per hour or per diem amounts that would require verification of time worked. We may continue in the future to make payment for these consulting services in the form of royalties or also possibly in the form of part time employment. In addition, we sometimes allow hospitals a period of evaluation of our products at no charge.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs and the curtailment or restructuring of our operations. Any penalties, damages, fines, exclusions, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that many of these laws are broad and their provisions are open to a variety of interpretations. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If the surgeons or other providers or entities with whom we do business are found to be non-compliant with applicable laws, they may be subject to sanctions, which could also have a negative impact on our business.
57
|
|
|
|
UNRESOLVED STAFF COMMENTS |
Not applicable.
|
|
|
|
PROPERTIES |
We lease approximately 36,000 square feet of office and warehouse space in Fort Lauderdale, Florida, which is used as our headquarters and for the assembly of our products. Our lease expires on July 31, 2011. Thereafter, we have the right to renew our lease for two three-year terms upon prior written notice and the fulfillment of certain conditions. We believe that this facility is adequate to meet our current needs and we are investigating leasing additional space to accommodate our anticipated growth.
|
|
|
|
LEGAL PROCEEDINGS |
None
|
|
|
|
RESERVED |
58
|
|
|
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock began trading on The NASDAQ Global Market under the symbol “MAKO” on February 14, 2008. Prior to that date, there was no identifiable public market for our common stock.
The following table sets forth the range of the high and low intraday prices for the period of April 1, 2008 through December 31, 2009 as reported by The NASDAQ Global Market.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
2008 |
|
||||||||
|
|
|
High |
|
Low |
|
High |
|
Low |
|
||||
|
First Quarter |
|
$ |
9.18 |
|
$ |
6.18 |
|
|
N/A |
|
|
N/A |
|
|
Second Quarter |
|
$ |
9.74 |
|
$ |
6.59 |
|
$ |
9.49 |
|
$ |
7.00 |
|
|
Third Quarter |
|
$ |
10.00 |
|
$ |
6.86 |
|
$ |
8.45 |
|
$ |
6.29 |
|
|
Fourth Quarter |
|
$ |
11.65 |
|
$ |
8.47 |
|
$ |
9.75 |
|
$ |
5.23 |
|
Our stock transfer records indicated that as of March 1, 2010, there were approximately 48 holders of record of our common stock.
Dividend Policy
We have never declared dividends or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our common stock for the foreseeable future. Any future determination related to dividend policy will be made at the discretion of our board of directors.
Equity Compensation Plan Information
The following table summarizes our equity compensation plans as of December 31, 2009.
|
|
|
|
|
|
|
|
|
|
|
|
|
Plan Category |
|
(a) |
|
(b) |
|
(c) |
|
|||
|
Equity compensation plans approved by our security holders |
|
3,478,000 |
(1) |
|
$ 6.71 |
|
|
727,000 |
(2) |
|
|
Equity compensation plans not approved by our security holders |
|
None |
|
|
None |
|
|
None |
|
|
|
TOTAL |
|
3,478,000 |
(1) |
|
$ 6.71 |
|
|
727,000 |
(2) |
|
(1) This number includes shares of our common stock to be issued upon exercise of outstanding options under our 2004 Stock Incentive Plan and our 2008 Omnibus Incentive Plan. No further awards will be made under the 2004 Stock Incentive Plan.
(2) This number includes 553,000 shares available for future issuance under our 2008 Employee Stock Purchase Plan. The 2008 Omnibus Incentive Plan contains an evergreen provision whereby the authorized shares increase on January 1 of each year in an amount equal to the least of (i) 2,500,000 shares, (ii) 4% of the total number of
59
shares of the Company’s common stock outstanding on December 31 of the preceding year, and (iii) a number of shares determined by the Company’s board of directors that is less than (i) and (ii).
Unregistered Sales of Equity Securities
On January 8, 2010, we entered into an asset purchase agreement with Z-Kat to acquire certain intellectual property assets from Z-Kat in consideration for $3,053,569, payable in shares of our common stock. We closed this transaction and issued 230,458 shares of our common stock to Z-Kat in a private placement on February 25, 2010. These shares were issued in a transaction exempt from registration pursuant to Section 4(2) of the Securities Act of 1933, as amended.
In connection with the closing, we entered into a registration rights agreement, dated as of February 25, 2010, with respect to the registration of the shares issued to Z-Kat at the closing. The registration rights agreement provides Z-Kat with “piggyback” registration rights for one year following the closing of the transaction and one demand registration right, which is exercisable beginning after the one year anniversary date of the closing but only to the extent that Z-Kat is not otherwise eligible to sell the shares pursuant to Rule 144 under the Securities Act of 1933, as amended.
Uses of Proceeds from Sale of Registered Securities
Not applicable.
Issuer Purchases of Equity Securities
The following table summarizes the surrenders of the Company’s common stock during the three month period ended December 31, 2009:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
Total
|
|
Average |
|
Total
Number |
|
Maximum
|
|
||
|
Period |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
October 1 to 31, 2009 |
|
— |
|
$ |
— |
|
— |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
November 1 to 30, 2009 |
|
15,834 |
|
|
8.97 |
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 1 to 31, 2009 |
|
— |
|
— |
|
— |
|
|
— |
|
|
|
|
|
15,834 |
|
$ |
8.97 |
|
— |
|
$ |
— |
|
|
|
|
|
(1) |
Represents the surrender of shares of common stock of the Company to satisfy the tax withholding obligations associated with the vesting of restricted stock. |
Performance Graph1
The following graph shows a comparison of cumulative total return for our common stock, the NASDAQ Composite Index, and the NASDAQ Medical Equipment Index. Such returns are based on historical results and are not intended to suggest future performance. The graph assumes $100 was invested in our common stock and in each of the indexes on February 14, 2008, the date our common stock commenced trading on The NASDAQ Global Market.
|
|
|
|
|
|
|
|
|
|
1 |
This section is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference into any of our filings of under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. |
60
Data for the NASDAQ Composite Index and the NASDAQ Medical Equipment Index assume reinvestment of dividends. The Company has never paid dividends on its common stock and has no present plans to do so.
The stockholder return shown on the graph below is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future stockholder returns.
COMPARISON OF 22-MONTH CUMULATIVE TOTAL
RETURN
Among
MAKO Surgical Corp.
The NASDAQ Composite Index
and
The NASDAQ Medical Equipment Index
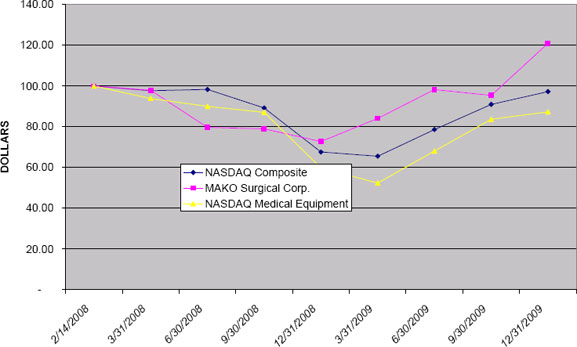
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2/14/2008 |
|
3/31/2008 |
|
6/30/2008 |
|
9/30/2008 |
|
12/31/2008 |
|
3/31/2009 |
|
6/30/2009 |
|
9/30/2009 |
|
12/31/2009 |
|
|
MAKO Surgical Corp. |
|
$100.00 |
|
$97.93 |
|
$79.74 |
|
$78.98 |
|
$72.77 |
|
$84.10 |
|
$98.26 |
|
$95.42 |
|
$120.92 |
|
|
NASDAQ Composite |
|
$100.00 |
|
$97.71 |
|
$98.30 |
|
$89.27 |
|
$67.61 |
|
$65.53 |
|
$78.67 |
|
$90.99 |
|
$ 97.28 |
|
|
NASDAQ Medical Equipment |
|
$100.00 |
|
$93.88 |
|
$90.03 |
|
$87.10 |
|
$59.87 |
|
$52.40 |
|
$68.08 |
|
$83.64 |
|
$ 87.31 |
|
61
|
|
|
|
SELECTED FINANCIAL DATA |
The following table sets forth certain financial data with respect to our business. The information set forth below is not necessarily indicative of results of future operations and should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Item 7 and the financial statements and related notes thereto in Item 8.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except per share data) |
|
Years Ended December 31, |
|
|||||||||||||
|
|
||||||||||||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
2006 |
|
2005 |
|
|||||
|
Statements of Operations Data |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
|
$ |
34,208 |
|
$ |
2,944 |
|
$ |
771 |
|
$ |
63 |
|
$ |
― |
|
|
Cost of revenue |
|
|
21,704 |
|
|
3,446 |
|
|
583 |
|
|
77 |
|
|
― |
|
|
Gross profit (loss) |
|
|
12,504 |
|
|
(502 |
) |
|
188 |
|
|
(14 |
) |
|
― |
|
|
Operating costs and expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
|
31,878 |
|
|
23,158 |
|
|
12,042 |
|
|
5,023 |
|
|
2,736 |
|
|
Research and development |
|
|
13,127 |
|
|
12,472 |
|
|
8,269 |
|
|
5,192 |
|
|
2,582 |
|
|
Depreciation and amortization |
|
|
1,951 |
|
|
1,828 |
|
|
1,297 |
|
|
644 |
|
|
98 |
|
|
Total operating costs and expenses |
|
|
46,956 |
|
|
37,458 |
|
|
21,608 |
|
|
10,859 |
|
|
5,416 |
|
|
Loss from operations |
|
|
(34,452 |
) |
|
(37,960 |
) |
|
(21,420 |
) |
|
(10,873 |
) |
|
(5,416 |
) |
|
Interest and other income |
|
|
432 |
|
|
988 |
|
|
1,073 |
|
|
476 |
|
|
269 |
|
|
Interest and other expenses |
|
|
(3 |
) |
|
(110 |
) |
|
(311 |
) |
|
(220 |
) |
|
― |
|
|
Net loss |
|
$ |
(34,023 |
) |
$ |
(37,082 |
) |
$ |
(20,658 |
) |
$ |
(10,617 |
) |
$ |
(5,147 |
) |
|
Net loss attributable to common stockholders |
|
$ |
(34,023 |
) |
$ |
(37,647 |
) |
$ |
(24,318 |
) |
$ |
(12,493 |
) |
$ |
(6,288 |
) |
|
Net loss per share: Basic and diluted attributable to common stockholders (1) |
|
$ |
(1.22 |
) |
$ |
(2.20 |
) |
$ |
(14.75 |
) |
$ |
(8.03 |
) |
$ |
(4.18 |
) |
|
Weighted average common shares outstanding: Basic and diluted (2) |
|
|
27,806 |
|
|
17,096 |
|
|
1,649 |
|
|
1,555 |
|
|
1,503 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
As of December 31, |
|
|||||||||||||
|
|
||||||||||||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
2006 |
|
2005 |
|
|||||
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
17,159 |
|
$ |
62,547 |
|
$ |
9,615 |
|
$ |
2,108 |
|
$ |
6,145 |
|
|
Short-term investments |
|
|
44,686 |
|
|
1,077 |
|
|
3,084 |
|
|
1,400 |
|
|
10,097 |
|
|
Long-term investments |
|
|
9,368 |
|
|
― |
|
|
― |
|
|
― |
|
|
― |
|
|
Total assets |
|
|
99,103 |
|
|
86,533 |
|
|
29,190 |
|
|
12,754 |
|
|
17,435 |
|
|
Long-term debt, net of current portion |
|
|
― |
|
|
― |
|
|
― |
|
|
― |
|
|
― |
|
|
Redeemable convertible preferred stock |
|
|
― |
|
|
― |
|
|
59,487 |
|
|
25,911 |
|
|
24,034 |
|
|
Accumulated deficit |
|
|
(114,195 |
) |
|
(80,172 |
) |
|
(42,843 |
) |
|
(19,366 |
) |
|
(6,820 |
) |
|
Total stockholders’ equity (deficit) |
|
|
90,794 |
|
|
66,514 |
|
|
(42,837 |
) |
|
(19,437 |
) |
|
(6,888 |
) |
|
|
|
|
(1) |
The basic and diluted net loss per share computation excludes potential common shares upon exercise of options and warrants to purchase common stock and unvested restricted stock as their effect would be anti-dilutive. See Item 8, Financial Statements and Supplementary Data, Note 2 to the Financial Statements, for a detailed explanation of the determination of shares used in computing basic and diluted loss per share. |
|
|
|
|
(2) |
Weighted average common shares outstanding and per share amounts have been retroactively adjusted to give effect to a one-for-3.03 reverse stock split of our common stock effected on February 8, 2008. |
62
|
|
|
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Overview
We are an emerging medical device company that markets our advanced robotic arm solution and orthopedic implants for minimally invasive orthopedic knee procedures. We offer MAKOplasty, an innovative, restorative surgical solution that enables orthopedic surgeons to consistently, reproducibly and precisely treat patient specific, early to mid-stage osteoarthritic knee disease. In February 2008, our common stock began trading on The NASDAQ Global Market under the ticker symbol “MAKO” in connection with the closing of our initial public offering, or IPO.
Through December 31, 2008, our recognized revenue was primarily generated from the sale of our implants and disposable products utilized in knee MAKOplasty procedures. In accordance with our revenue recognition policy, upon the sale of our first generation Tactile Guidance System, or TGS, we deferred recognition of the related revenue and direct cost of revenue until delivery of the RIO system, which is version 2.0 of the TGS. We commercially released the RIO system in the first quarter of 2009. Revenue for all previously deferred TGS sales was recognized in our statement of operations during the year ended December 31, 2009, upon delivery of the RIO system. We have incurred net losses in each year since our inception and, as of December 31, 2009, we had an accumulated deficit of $114.2 million. We expect to continue to incur significant operating losses as we increase our sales and marketing activities and otherwise continue to invest capital in the development and expansion of our products and our business generally. We also expect that our general and administrative expenses will increase due to additional operational and regulatory costs and burdens associated with the rapid expansion of our operations and operating as a public company.
Recent key milestones in the development of our business include the following:
|
|
|
|
|
• We commercially released our RIO system in the first quarter of 2009. Revenue for all previously deferred TGS sales was recognized in our statement of operations during the year ended December 31, 2009, upon delivery of the RIO system. In addition, we recognized the revenue and the direct cost of revenue from nineteen new unit sales of our RIO system during the year ended December 31, 2009, bringing the total number of commercial RIO systems to 36 as of December 31, 2009. |
|
|
|
|
|
• In the second quarter of 2009, we commercially released our RESTORIS MCK multicompartmental knee implant system, or RESTORIS MCK, which enables surgeons to perform bicompartmental knee MAKOplasty procedures. As of December 31, 2009, 133 RESTORIS MCK bicompartmental knee MAKOplasty procedures were performed since the release of RESTORIS MCK. |
|
|
|
|
|
• During the year ended December 31, 2009, a total of 1,602 knee MAKOplasty procedures were performed, including unicompartmental and bicompartmental procedures, representing a 167% increase over the year ended December 31, 2008. |
|
|
|
|
|
• In September 2009, we received 510(k) marketing clearance from the FDA for an application that assists a surgeon in acetabular reaming during total hip arthroplasty using the RIO system platform. In February 2010, we received 510(k) marketing clearance from the FDA for an application that assists a surgeon in performing all components of a total hip arthroplasty using the RIO system. We believe these represent achievement of necessary milestones towards what we anticipate will be our continuing development and future commercialization of a RIO-enabled hip application. |
We believe that the key to growing our near term business is expanding the acceptance and application of MAKOplasty to unicompartmental and multicompartmental knee resurfacing procedures by offering implants that address early to mid-stage, unicompartmental and multicompartmental knee degeneration. To successfully commercialize our products and grow our business, we must gain market acceptance for knee MAKOplasty.
63
Factors That May Influence Future Results of Operations
The following is a description of factors that may influence our future results of operations, including significant trends and challenges that we believe are important to an understanding of our business and results of operations.
Revenue
Revenue is generated from unit sales of our RIO systems, including installation services, training and upgrades and enhancements, from sales of implants and disposable products and sales of extended warranty service contracts. Through December 31, 2008, our recognized revenue was primarily generated from the sale of implants and disposable products utilized in knee MAKOplasty procedures. For the year ended December 31, 2009, we also recognized revenue from sales of our RIO systems in our statement of operations as described in the “Critical Accounting Policies and Significant Judgments and Estimates” section below.
Future revenue from sales of our products is difficult to predict and we expect that it will only modestly reduce our continuing and increasing losses resulting from selling, general and administrative expenses, research and development expenses and other activities for at least the next two or three years. Our future revenue may also be adversely affected by the current general economic downturn and the resulting tightening of the credit markets, which may cause purchasing decisions to be delayed or cause our customers to experience difficulties in securing adequate funding to buy our products.
The generation of recurring revenue through sales of our knee implants, disposable products and extended warranty service contracts is an important part of the MAKOplasty business model. We anticipate that recurring revenue will constitute an increasing percentage of our total revenue as we leverage each new installation of our RIO system to generate recurring sales of implants and disposable products and as we expand our implant product offering.
Cost of Revenue
Cost of revenue primarily consists of the direct costs associated with the manufacture of RIO systems, implants and disposable products for which revenue has been recognized or deferred in accordance with our revenue recognition policy. Costs associated with providing services are expensed as incurred. Cost of revenue also includes the allocation of manufacturing overhead costs, the cost associated with establishing at the time of installation an accrual for the RIO system standard one-year warranty liability, royalties related to the sale of products covered by licensing arrangements and write-offs of obsolete or impaired inventory.
The direct cost of revenue associated with the sale of TGS units was deferred until the recognition of the related revenue. The revenue and the direct cost of revenue for all previously deferred TGS sales was recognized in our statement of operations during the year ended December 31, 2009, upon delivery of the RIO system.
Selling, General and Administrative Expenses
Our selling, general and administrative expenses consist primarily of compensation, including stock-based compensation and benefits, for sales, marketing, clinical research, operations, regulatory, quality, executive, finance, legal and administrative personnel. Other significant expenses include costs associated with sales and marketing activities, marketing and advertising materials, insurance, professional fees for legal and accounting services, consulting fees, travel expenses, facility and related operating costs, and recruiting expenses. Our selling, general and administrative expenses are expected to continue to increase due to the planned increase in the number of employees necessary to support the sales and marketing efforts associated with the growing commercialization of MAKOplasty, an increased number of employees necessary to support our continued growth in operations, and the additional operational and regulatory burdens and costs associated with operating as a publicly traded company. In addition, we are currently in the process of securing European Union CE marking for the RESTORIS MCK onlay unicompartmental knee implant system, which is a legal requirement for medical
64
devices intended for sale in Europe, which may also increase our selling, general and administrative expenses, and we expect to incur additional costs associated with securing and protecting our intellectual property rights as necessary to support our future product offerings.
Research and Development Expenses
Costs related to research, design and development of products are charged to research and development expense as incurred. These costs include direct salary and benefit costs for research and development employees including stock-based compensation, cost for materials used in research and development activities and costs for outside services. We expect our research and development expense to increase as we continue to expand our research and development activities, including the support of existing products and the research of potential future products.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities as of the date of the financial statements as well as the reported expenses during the reporting periods. The accounting estimates that require our most significant, difficult and subjective judgments include revenue recognition, allowance for doubtful accounts, inventory valuation, valuation allowance for deferred income tax assets, impairment of long-lived assets and the determination of stock-based compensation. We evaluate our estimates and judgments on an ongoing basis. Actual results may differ materially from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Item 8, Financial Statements and Supplementary Data, Note 2 to the Financial Statements, we believe that the following accounting policies and estimates are most critical to a full understanding and evaluation of our reported financial results.
Revenue Recognition
Revenue is generated from unit sales of our RIO systems, including installation services, training and upgrades and enhancements, from sales of implants and disposable products and sales of extended warranty service contracts. Through December 31, 2008, our recognized revenue was primarily generated from the sale of implants and disposable products utilized in knee MAKOplasty procedures. For the year ended December 31, 2009, we also recognized revenue from sales of our RIO systems in our statement of operations as discussed below.
Since December 31, 2008, we no longer manufacture TGS units, to which associated TGS sales arrangements required us to provide upgrades and enhancements, through and including the delivery of the RIO system. We commercially released the RIO system in the first quarter of 2009. Sales arrangements for RIO systems do not require us to provide upgrades and enhancements. As a result, we anticipate that revenues related to RIO system sales will not be deferred and will be recognized upon installation of the system, delivery of associated instrumentation and training of at least one surgeon.
For sales of TGS units through December 31, 2008, the sales arrangements required us to provide upgrades and enhancements to the TGS unit through and including delivery of the RIO system. Prior to delivery of the RIO system, sales of TGS units were recorded as deferred revenue and the direct cost of revenue associated with the sale of TGS units was recorded as deferred cost of revenue. Upon satisfaction of the final deliverable of the RIO system, the revenue and direct cost of revenue associated with the sale of TGS units are recognized in our statement of operations. Revenue for all previously deferred TGS sales was recognized in our statement of operations during the year ended December 31, 2009, upon delivery of the RIO system. Our deferred revenue balance as of December 31, 2009 consists primarily of deferred service revenue for extended warranty services on the RIO system hardware.
65
A portion of our customers acquire our RIO system through a leasing arrangement with a third-party leasing company. In these instances, we typically sell the RIO system to the leasing company, and our customer enters into an independent leasing arrangement with the leasing company. We treat these leasing transactions the same as sales transactions for purposes of recognizing revenue for the sale.
Procedure revenue from the sale of implants and disposable products utilized in knee MAKOplasty procedures is recognized when persuasive evidence of an arrangement exists, delivery has occurred or service has been rendered, the price is fixed or determinable and collectability is reasonably assured. The implants and disposable products are a separate unit of accounting from the RIO systems as (1) they have value to the customer on a standalone basis, (2) objective and reliable evidence of the fair value of the item exists and (3) no right of return exists once the implants and disposable products are implanted or consumed. Accordingly, as our implants and disposable products are sold on a procedural basis, the revenue and costs associated with the sale of implants and disposable products are recognized at the time of sale (i.e., at the time of the related surgical procedure).
Service revenue consists of extended warranty services on the RIO system hardware, and is deferred and recognized ratably over the service period until no further obligation exists. Costs associated with providing extended warranty services are expensed as incurred.
Allowance for Doubtful Accounts
The allowance for doubtful accounts is based on our assessment of the collectability of customer accounts. We regularly review the allowance by considering factors such as historical experience, credit quality, the age of the accounts receivable balances and current economic conditions that may affect a customer’s ability to pay. We have not experienced any collectability issues to date and have no allowance, provision for doubtful accounts receivable or write-offs to date in the accompanying financial statements included in Item 8, Financial Statements and Supplementary Data, of this report.
Inventory Valuation
Inventory is stated at the lower of cost or market value on a first-in, first-out basis. Inventory costs include direct materials, direct labor and manufacturing overhead. We review our inventory periodically to determine net realizable value and consider product upgrades in our periodic review of realizability. We write down inventory, if required, based on forecasted demand and technological obsolescence. These factors are impacted by market and economic conditions, technology changes and new product introductions and require estimates that may include uncertain elements.
Beginning with the fourth quarter of 2008, manufacturing overhead costs have been capitalized and included in inventory. Previously, such overhead costs were fully expensed as selling, general and administrative expense as capitalizable amounts were not significant.
Valuation Allowance for Deferred Income Tax Assets
Deferred income taxes are determined based on the differences between financial reporting and income tax bases of assets and liabilities and are measured using the enacted income tax rates and laws that will be in effect when the differences are expected to reverse. Valuation allowances are established when necessary to reduce deferred income tax assets to the amounts expected to be realized. A full valuation allowance has been recorded in the accompanying financial statements relating to all our net deferred income tax assets.
Impairment of Long-Lived Assets
We evaluate our long-lived assets for indicators of impairment by comparison of the carrying amounts to future net undiscounted cash flows expected to be generated by such assets when events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Should an impairment exist, the impairment loss
66
would be measured based on the excess carrying value of the asset over the asset’s fair value or discounted estimate of future cash flows.
Determination of Stock-Based Compensation
We recognize compensation expense for our stock-based awards in accordance with ASC 718, Compensation-Stock Compensation. ASC 718 requires the recognition of compensation expense, using a fair value based method, for costs related to all share-based payments including stock options. ASC 718 requires companies to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model.
We account for stock-based compensation arrangements with non-employees in accordance with the ASC 505-50, Equity-Based Payments to Non-Employees. We record the expense of such services based on the estimated fair value of the equity instrument using the Black-Scholes-Merton pricing model. The value of the equity instrument is charged to expense over the term of the service agreement.
We selected the Black-Scholes-Merton pricing model to determine the fair value of stock options. The determination of the fair value of stock-based payment awards on the date of grant using an option-pricing model will be affected by our stock price as well as assumptions regarding a number of complex and subjective variables. These variables include our expected stock price volatility over the term of the awards, actual and projected employee stock option exercise behaviors, risk-free interest rates, forfeitures and expected dividends.
During the year ended December 31, 2009, we recognized $4.0 million of stock-based compensation expense including stock option grants, restricted stock grants, and compensation expense relating to shares issued under our employee share purchase plan, leaving $9.9 million to be recognized in future periods. Total unrecognized compensation cost will be adjusted for future changes in estimated forfeitures, and is expected to be recognized over a remaining weighted average period of 2.7 years as of December 31, 2009.
Results of Operations
Year Ended December 31, 2009 Compared to the Year Ended December 31, 2008
Revenue. Revenue was $34.2 million for the year ended December 31, 2009, compared to $2.9 million for the year ended December 31, 2008. The increase in revenue of $31.3 million was primarily due to $14.7 million of revenue from nineteen unit sales of our RIO system and the recognition of approximately $11.3 million of revenue from seventeen previously deferred unit sales of our TGS. In accordance with our revenue recognition policy, recognition of revenue on unit sales of our TGS was deferred until delivery of the RIO system, which we commercially released in the first quarter of 2009. Prior to 2009, recognized revenue was primarily generated from the sale of implants and disposable products utilized in knee MAKOplasty procedures. Total revenue was also positively impacted by a $5.1 million increase in procedure revenue attributable to an increase in knee MAKOplasty procedures performed during the year ended December 31, 2009 as compared with the year ended December 31, 2008. There were 1,602 knee MAKOplasty procedures performed during the year ended December 31, 2009 compared to 601 knee MAKOplasty procedures performed during year ended December 31, 2008. We expect our revenue to continue to increase as unit sales of our RIO system increase in future periods and the number of knee MAKOplasty procedures performed increases in future periods.
Cost of Revenue. Cost of revenue was $21.7 million for the year ended December 31, 2009, compared to $3.4 million for the year ended December 31, 2008. The increase in cost of revenue of $18.3 million was primarily due to the cost of revenue from nineteen unit sales of our RIO system, the recognition of the direct cost of revenue from seventeen previously deferred unit sales of our TGS, including the cost of providing the RIO system upgrades, as described in the “Critical Accounting Policies and Significant Judgments and Estimates” section above, and an increase in knee MAKOplasty procedures performed. We expect our cost of revenue to continue to increase as unit sales of our RIO system increase in future periods and the number of knee MAKOplasty procedures performed increases in future periods.
67
Selling, General and Administrative. Selling, general and administrative expense was $31.9 million for the year ended December 31, 2009, compared to $23.2 million for the year ended December 31, 2008. The increase of $8.7 million, or 38%, was primarily due to an increase in sales, marketing and operations costs associated with the production and commercialization of our products and an increase in general and administrative costs to support growth and costs associated with operating as a public company. Selling, general and administrative expense for the year ended December 31, 2009 also included $3.3 million of stock-based compensation expense compared to $1.9 million for the year ended December 31, 2008. The increase in stock-based compensation expense was primarily due to additional option and restricted stock grants made in 2009. We expect our selling, general and administrative expenses to continue to increase substantially due to our planned increase in the number of employees necessary to support the sales and marketing efforts associated with the growing commercialization of our products, continued growth in operations and the costs associated with operating as a public company.
Research and Development. Research and development expense was $13.1 million for the year ended December 31, 2009, compared to $12.5 million for the year ended December 31, 2008. The increase of $655,000, or 5%, was primarily due to an increase in research and development activities associated with on-going development of our RIO system, our MAKO implant systems and potential future products. This was partially offset by a nonrecurring charge of $949,000 incurred in the first quarter of 2008 associated with the vesting in full, upon completion of our IPO in February 2008, of restricted common stock issued pursuant to business consultation agreements entered into in December 2004. We expect our research and development expense to increase as we continue to expand our research and development activities, including the support of existing products and the research of potential future products.
Depreciation and Amortization. Depreciation and amortization expense was $2.0 million for the year ended December 31, 2009, compared to $1.8 million for the year ended December 31, 2008. The increase of $123,000, or 7%, was primarily due to an increase in depreciation of property and equipment as a result of purchases made during 2009 and 2008.
Interest and Other Income. Interest and other income was $432,000 for the year ended December 31, 2009, compared to $988,000 for the year ended December 31, 2008. The decrease of $556,000, or 56%, was primarily due to lower yields realized on our cash, cash equivalents and investments for the year ended December 31, 2009 compared with the same period of 2008.
Interest and Other Expense. Interest and other expense was $3,000 for the year ended December 31, 2009, compared to $110,000 for the year ended December 31, 2008. Through February 2008, interest and other expense consisted primarily of the amortization of a $590,000 discount associated with a deferred license fee payment of $4.0 million which had been fully amortized and paid upon the completion of our IPO in February 2008.
Income Taxes. No income taxes were recognized for the year ended December 31, 2009 and 2008, due to net operating losses in each period. In addition, no current or deferred income taxes were recorded for the year ended December 31, 2009 and 2008, as all income tax benefits were fully offset by a valuation allowance against our net deferred income tax assets.
Year Ended December 31, 2008 Compared to the Year Ended December 31, 2007
Revenue. Revenue was $2.9 million for the year ended December 31, 2008, compared to $771,000 for the year ended December 31, 2007, and was primarily generated from the sale of implants and disposable products utilized in knee MAKOplasty procedures. The increase in revenue of $2.2 million was primarily due to an increase in MAKOplasty procedures performed during the year ended December 31, 2008 as compared with the year ended December 31, 2007. There were 601 procedures performed during the year ended December 31, 2008 compared to 168 procedures performed during the year ended December 31, 2007. Total revenue was also positively impacted by a $434,000 increase in other revenue, which consists primarily of service revenue on extended warranty services and net royalty revenues.
68
Cost of Revenue. Cost of revenue was $3.4 million for the year ended December 31, 2008, compared to $583,000 for the year ended December 31, 2007. The increase in cost of revenue of $2.9 million was primarily due to an increase in knee MAKOplasty procedures performed, a $730,000 write-off of obsolete and discontinued inventory in 2008 primarily due to the launch of our RESTORIS implant system in 2008 and the anticipated launch of our RIO system in the first half 2009, the establishment of warranty accruals on sales of twelve TGS units and royalties incurred on sales of twelve TGS units during the year ended December 31, 2008.
Selling, General and Administrative. Selling, general and administrative expense was $23.2 million for the year ended December 31, 2008, compared to $12.0 million for the year ended December 31, 2007. The increase of $11.1 million, or 92%, was primarily due to an increase in sales, marketing and operations costs associated with the production and commercialization of our products, an increase in general and administrative costs to support growth and costs associated with operating as a public company. Selling, general and administrative expense for the year ended December 31, 2008 also included $1.9 million of stock-based compensation expense compared with $1.0 million for the year ended December 31, 2007. The increase in stock-based compensation expense was primarily due to additional option and restricted stock grants made in 2008 and 2007.
Research and Development. Research and development expense was $12.5 million for the year ended December 31, 2008, compared to $8.3 million for the year ended December 31, 2007. The increase of $4.2 million, or 51%, was primarily due to an increase in research and development activities associated with on-going development of our RIO system and the RESTORIS and RESTORIS MCK implant systems. Research and development expense for the year ended December 31, 2008 also included $1.4 million of stock-based compensation expense compared with $184,000 for the year ended December 31, 2007. The increase in stock-based compensation expense is due primarily to a nonrecurring charge of $949,000 associated with the vesting in full, upon completion of our IPO in February 2008, of restricted common stock issued pursuant to business consultation agreements entered into in December 2004.
Depreciation and Amortization. Depreciation and amortization expense was $1.8 million for the year ended December 31, 2008, compared to $1.3 million for the year ended December 31, 2007. The increase of $531,000, or 41%, was primarily due to an increase in depreciation of property and equipment as a result of purchases made during 2008 and 2007.
Interest and Other Income. Interest income was $988,000 for the year ended December 31, 2008, compared to $1.1 million for the year ended December 31, 2007. The decrease of $85,000, or 8%, was primarily due to lower yields realized on our cash, cash equivalents and investments for the year ended December 31, 2008 compared with the year ended December 31, 2007.
Interest and Other Expense. Interest and other expense was $110,000 for the year ended December 31, 2008, compared to $311,000 for the year ended December 31, 2007. Through February 2008, interest and other expense consisted primarily of the amortization of the $590,000 discount associated with a deferred license fee payment of $4.0 million which had been fully amortized and paid upon the completion of our IPO in February 2008. Interest and other expense also included a $63,000 write down of our variable auction rate securities in the first quarter of 2008.
Income Taxes. No income taxes were recognized for the years ended December 31, 2008 and 2007, due to net operating losses in each period. In addition, no current or deferred income taxes were recorded for the years ended December 31, 2008 and 2007, as all income tax benefits were fully offset by a valuation allowance against our net deferred income tax assets.
69
Liquidity and Capital Resources
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 |
|
Change |
|
2008 |
|
Change |
|
2007 |
|
|||||
|
Cash and cash equivalents |
|
$ |
17,159 |
|
$ |
(45,388 |
) |
|
62,547 |
|
$ |
52,932 |
|
$ |
9,615 |
|
|
Short-term investments |
|
|
44,686 |
|
|
43,609 |
|
|
1,077 |
|
|
(2,007 |
) |
|
3,084 |
|
|
Long-term investments |
|
|
9,368 |
|
|
9,368 |
|
|
― |
|
|
― |
|
|
― |
|
|
Total cash, cash equivalents, and investments |
|
$ |
71,213 |
|
$ |
7,589 |
|
$ |
63,624 |
|
$ |
50,925 |
|
$ |
12,699 |
|
|
Cash used in operating activities |
|
$ |
(45,572 |
) |
$ |
(15,752 |
) |
$ |
(29,820 |
) |
$ |
(13,745 |
) |
$ |
(16,075 |
) |
|
Cash used in investing activities |
|
|
(54,180 |
) |
|
(50,631 |
) |
|
(3,549 |
) |
|
(333 |
) |
|
(3,216 |
) |
|
Cash provided by financing activities |
|
|
54,364 |
|
|
(31,937 |
) |
|
86,301 |
|
|
59,503 |
|
|
26,798 |
|
|
Net increase (decrease) in cash and cash equivalents |
|
$ |
(45,388 |
) |
$ |
(98,320 |
) |
$ |
52,932 |
|
$ |
45,425 |
|
$ |
7,507 |
|
We have incurred net losses and negative cash flow from operating activities for each period since our inception in November 2004. As of December 31, 2009, we had an accumulated deficit of $114.2 million and have financed our operations principally through the sale of Series A, B and C redeemable convertible preferred stock, the sale of common stock in our IPO in February 2008, our equity financing in October 2008 and our sale of common stock in August 2009. In February 2008, we completed our IPO of common stock, issuing a total of 5.1 million shares at an offering price to the public of $10.00 per share, resulting in net proceeds to us, after underwriting discounts and commission and expenses, of approximately $43.8 million. In conjunction with the closing of the IPO in February 2008, all of our outstanding Series A, Series B and Series C redeemable convertible preferred stock was converted into 10,945,080 shares of common stock, as adjusted for a one-for-3.03 reverse stock split, which has been retroactively reflected in the accompanying financial statements.
In October 2008, we entered into a Securities Purchase Agreement for an equity financing of up to approximately $60 million, with initial gross proceeds of approximately $40.2 million, which we closed on October 31, 2008, and conditional access to an additional $20 million. The financing resulted in net proceeds of approximately $39.7 million, after expenses of approximately $525,000. In connection with the financing, we issued and sold to the participating investors 6,451,613 shares of our common stock at a purchase price of $6.20 per share and issued to participating investors, at the purchase price of $0.125 per warrant, warrants to purchase 1,290,323 shares of common stock at an exercise price of $7.44 per share. The warrants became exercisable on April 29, 2009 and have a seven-year term.
Subject to the satisfaction of certain business related milestones before December 31, 2009, we had the right, which we refer to as the call right, to require certain participants in the financing to purchase an additional $20 million of common stock and warrants to purchase common stock. We did not exercise our call right, which expired on December 31, 2009, to require these participants to purchase an additional $20 million of common stock. At the initial closing, the investors that agreed to provide the additional $20 million investment received warrants to purchase an additional 322,581 shares of our common stock at a purchase price of $0.125 per warrant and an exercise price of $6.20 per share. These warrants became exercisable on December 31, 2009.
In August 2009, we completed a public offering of our common stock, issuing 8,050,000 shares at an offering price to the public of $7.25 per share, resulting in net proceeds of approximately $54.3 million, after underwriting discounts and commissions and expenses.
As of December 31, 2009, we had approximately $71.2 million in cash, cash equivalents and investments. Our cash and investment balances are held in a variety of interest bearing instruments, including notes and bonds from U.S. government agencies, certificates of deposit and investment grade rated U.S. corporate debt.
Net Cash Used in Operating Activities
Net cash used in operating activities primarily reflects the net loss for those periods, which was reduced in part by depreciation and amortization, stock-based compensation and inventory write-downs. Net cash used in
70
operating activities was also affected by changes in operating assets and liabilities. Included in changes in operating assets and liabilities for the year ended December 31, 2009 are approximately $11.0 million and $3.6 million of decreases to the deferred revenue balance and deferred cost of revenue balance, respectively, due to the recognition of seventeen previously deferred unit sales of our TGS, $7.4 million of increases in inventory necessitated by the commercial release of the RIO system, the commercial release of the RESTORIS MCK implant system and increased sales of implants and disposable products and $3.8 million of increases in accounts receivable due to increased sales in the forth quarter of 2009 as compared to the forth quarter of 2008. Included in changes in operating assets for the year ended December 31, 2008 are approximately $8.2 million of increases to the deferred revenue balance, which was partially offset by approximately $2.7 million of increases to the deferred cost of revenue balance. The increases to the deferred revenue and deferred cost of revenue balances are primarily related to twelve unit sales of our TGS during the year ended December 31, 2008. In accordance with our revenue recognition policy, recognition of revenue and direct cost of revenue associated with the unit sales of our TGS was deferred until delivery of the RIO system, which we commercially released in the first quarter of 2009.
Net Cash Used in Investing Activities
Net cash used in investing activities for the year ended December 31, 2009 was primarily attributable to the purchase of investments of $60.0 million and purchases of property and equipment of $790,000, which was partially offset by proceeds of $6.7 million from sales and maturities of investments. Net cash used in investing activities for the year ended December 31, 2008 was primarily attributable to the payment of the $4.0 million deferred license fee due upon completion of our IPO, purchases of $1.6 million of property and equipment as we invested in the infrastructure to support the growth of our company and $2.0 million for the purchase of investments, which was partially offset by proceeds of $4.0 million from sales and maturities of investments.
Net Cash Provided by Financing Activities
Net cash provided by our financing activities for the year ended December 31, 2009 was primarily attributable to net proceeds received in connection with our equity financing in August 2009. Net cash provided by our financing activities for the year ended December 31, 2008 was primarily attributable to net proceeds received in connection with our IPO in February 2008 and to net proceeds received in connection with our equity financing in October 2008.
Operating Capital and Capital Expenditure Requirements
To date, we have not achieved profitability. We anticipate that we will continue to incur substantial net losses for at least the next two or three years as we expand our sales and marketing capabilities in the orthopedic products market, commercialize our RIO system and RESTORIS unicompartmental and RESTORIS MCK multicompartmental knee implant systems, continue research and development of existing and future products and continue development of the corporate infrastructure required to sell and market our products, support operations and operate as a public company. We also expect to experience increased cash requirements for inventory and property and equipment in conjunction with the continued commercialization of our RESTORIS unicompartmental and RESTOIS MCK multicompartmental knee implant systems and our RIO system.
In executing our current business plan, we believe our existing cash, cash equivalents and investment balances, and interest income we earn on these balances will be sufficient to meet our anticipated cash requirements for at least the next twelve months. To the extent our available cash, cash equivalents and investment balances are insufficient to satisfy our operating requirements after that period, we will need to seek additional sources of funds, including selling additional equity, debt or other securities or entering into a credit facility, or modify our current business plan. The sale of additional equity and convertible debt securities may result in dilution to our current stockholders. If we raise additional funds through the issuance of debt securities, these securities may have rights senior to those of our common stock and could contain covenants that could restrict our operations and issuance of dividends. We may also require additional capital beyond our currently forecasted amounts. Any required additional capital, whether forecasted or not, may not be available on reasonable terms, or at all. If we
71
are unable to obtain additional financing, we may be required to reduce the scope of, delay or eliminate some or all of our planned research, development and commercialization activities, which could materially harm our business and results of operations.
Because of the numerous risks and uncertainties associated with the development of medical devices and the current economic situation, we are unable to estimate the exact amounts of capital outlays and operating expenditures necessary to complete the development of our products and successfully deliver commercial products to the market. Our future capital requirements will depend on many factors, including but not limited to the following:
|
|
|
|
|
|
• |
the revenue generated by sales of our current and future products; |
|
|
|
|
|
|
• |
the expenses we incur in selling and marketing our products; |
|
|
|
|
|
|
• |
the costs and timing of regulatory clearance or approvals for upgrades or changes to our products; |
|
|
|
|
|
|
• |
the rate of progress, cost and success of on-going product development activities; |
|
|
|
|
|
|
• |
the emergence of competing or complementary technological developments; |
|
|
|
|
|
|
• |
the costs of filing, prosecuting, defending and enforcing any patent or license claims and other intellectual property rights, or participating in litigation related activities; |
|
|
|
|
|
|
• |
the acquisition of businesses, products and technologies, although we currently have no understandings, commitments or agreements relating to any material transaction of this type; and |
|
|
|
|
|
|
• |
the continued downturn in general economic conditions and interest rates. |
Contractual Obligations
The following table summarizes our outstanding contractual obligations as of December 31, 2009 and the effect those obligations are expected to have on our liquidity and cash flows in future periods:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
Payment Due by Period |
|
|||||||||||||
|
|
|
|
|
December 31, |
|
After |
|
|||||||||
|
|
|
Total |
|
2010 |
|
2011-2012 |
|
2013-2014 |
|
|
||||||
|
Contractual Obligations |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Minimum royalty payments – licenses |
|
$ |
11,724 |
|
$ |
1,258 |
|
$ |
3,358 |
|
$ |
3,085 |
|
$ |
4,023 |
|
|
Purchase commitments and obligations |
|
|
7,140 |
|
|
7,140 |
|
|
― |
|
|
― |
|
|
― |
|
|
Development agreement obligations |
|
|
1,000 |
|
|
1,000 |
|
|
― |
|
|
― |
|
|
― |
|
|
Operating lease – real estate |
|
|
639 |
|
|
400 |
|
|
239 |
|
|
― |
|
|
― |
|
|
Total |
|
$ |
20,503 |
|
$ |
9,798 |
|
$ |
3,597 |
|
$ |
3,085 |
|
$ |
4,023 |
|
Our commitments for minimum royalty payments relate to payments under various licenses and sublicenses as discussed in Item 8, Financial Statements and Supplementary Data, Note 7 to the Financial Statements. Our commitments for purchase commitments and obligations include an estimate of open purchase orders and contractual obligations in the ordinary course of business, including commitments with contract manufacturers and suppliers, for which we have not received the goods or services. Our commitments for development agreement obligations relate to payments under a research and development agreement as discussed in Item 8, Financial Statements and Supplementary Data, Note 7 to the Financial Statements. Our commitments for operating leases relate to the lease for our headquarters in Fort Lauderdale, Florida.
Recent Accounting Pronouncements
Adopted Accounting Pronouncements
72
In June 2008, the Financial Accounting Standards Board, or FASB, issued an accounting standard update. As codified in Accounting Standards Codification 815-40, or ASC 815-40, Derivatives and Hedging, this update provides guidance for determining whether an equity-linked financial instrument (or embedded feature) is indexed to an entity’s own stock. The update applies to any freestanding financial instrument or embedded feature that has all the characteristics of a derivative, for purposes of determining whether that instrument or embedded feature qualifies for the first part of the scope exception under ASC 815-10-15. The update also applies to any freestanding financial instrument that is potentially settled in an entity’s own stock, regardless of whether the instrument has all the characteristics of a derivative under previous derivative Generally Accepted Accounting Principals, or GAAP, for purposes of determining whether the instrument is within the scope of derivative accounting. ASC 815-40 was effective beginning first quarter of fiscal 2009. The adoption did not have a material impact on our results of operations and financial position.
Effective January 1, 2009, we adopted a new accounting standard update regarding business combinations. As codified under ASC 805, Business Combinations, this update requires an entity to recognize the assets acquired, liabilities assumed, contractual contingencies, and contingent consideration at their fair value on the acquisition date. It further requires that acquisition-related costs be recognized separately from the acquisition and expensed as incurred; that restructuring costs generally be expensed in periods subsequent to the acquisition date; and that changes in accounting for deferred tax asset valuation allowances and acquired income tax uncertainties after the measurement period be recognized as a component of provision for taxes. In addition, acquired in-process research and development is capitalized as an intangible asset and amortized over its estimated useful life. The adoption did not have a material impact on our results of operations and financial position.
In December 2007, the FASB issued accounting guidance regarding noncontrolling interests, as codified in ASC 810-10-65. ASC 810-10-65 establishes accounting and reporting standards for ownership interests in subsidiaries held by parties other than the parent, the amount of consolidated net income attributable to the parent and to the noncontrolling interest, changes in a parent’s ownership interest and the valuation of retained noncontrolling equity investments when a subsidiary is deconsolidated. ASC 810-10-65 also establishes reporting requirements that provide sufficient disclosures that clearly identify and distinguish between the interests of the parent and the interests of the noncontrolling owners. The adoption did not have a material impact on our results of operations and financial position.
Effective April 1, 2009, we adopted a new accounting standard, as codified in ASC 820-10-65, which provides additional guidance for estimating fair value when the volume and level of activity for the asset or liability have significantly decreased. ASC 820-10-65 also includes guidance on identifying circumstances that indicate a transaction is not orderly. The adoption did not have a material impact on our results of operations and financial position.
In April 2009, the FASB issued an accounting standard update, as codified in ASC 320-10-65, to amend the other-than-temporary impairment guidance in debt securities to be based on intent to sell instead of ability to hold the security and to improve the presentation and disclosure of other-than-temporary impairments on debt and equity securities in the financial statements. This pronouncement is effective for periods ending after June 15, 2009. The adoption did not have a material impact on our results of operations and financial position.
Effective April 1, 2009, we adopted a new accounting standard for subsequent events, as codified in ASC 855-10, which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date, but before the financial statements are issued or available to be issued (“subsequent events”). ASC 855-10 is effective for interim or annual periods ending after June 15, 2009. See Item 8, Financial Statements and Supplementary Data, Note 11 to the Financial Statements for discussion of subsequent event.
Effective July 1, 2009, we adopted The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles, or ASC 105. ASC 105 establishes the FASB Accounting Standards Codification as the source of authoritative accounting principles recognized by the FASB to be applied in the
73
preparation of financial statements in conformity with generally accepted accounting principles. ASC 105 explicitly recognizes rules and interpretive releases of the SEC under federal securities laws as authoritative GAAP for SEC registrants. As ASC 105 was not intended to change or alter existing GAAP, it did not have any impact on our financial statements.
Recent Accounting Pronouncements
In September 2009, the FASB issued Update No. 2009-13, Multiple-Deliverable Revenue Arrangements, a consensus of the FASB Emerging Issues Task Force, or ASU 2009-13. ASU 2009-13 updates the existing multiple-element revenue arrangements guidance currently included under ASC 605-25. ASU 2009-13 eliminates the need for objective and reliable evidence of the fair value for the undelivered element in order for a delivered item to be treated as a separate unit of accounting and eliminates the residual method to allocate the arrangement consideration. In addition, the guidance also expands the disclosure requirements for revenue recognition. ASU 2009-13 will be effective for the first annual reporting period beginning on or after June 15, 2010, with early adoption permitted provided that the revised guidance is retroactively applied to the beginning of the year of adoption. We are currently evaluating the future impact that ASU 2009-13 will have on our financial statements.
In September 2009, the FASB issued Update No. 2009-14, Certain Revenue Arrangements That Include Software Elements, a consensus of the FASB Emerging Issues Task Force, or ASU 2009-14. ASU 2009-14 modifies the scope of ASC 985-605 to exclude from its requirements (a) non-software components of tangible products and (b) software components of tangible products that are sold, licensed, or leased with tangible products when the software components and non-software components of the tangible product function together to deliver the tangible product’s essential functionality. ASU 2009-14 will be effective for the first annual reporting period beginning on or after June 15, 2010, with early adoption permitted provided that the revised guidance is retroactively applied to the beginning of the year of adoption. We are currently evaluating the future impact that ASU 2009-14 will have on our financial statements.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
|
|
|
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Our exposure to market risk is confined to our cash, cash equivalents and investments. The goals of our cash investment policy are the security of the principal invested and fulfillment of liquidity needs, with the need to maximize value being an important consideration. To achieve our goals, we maintain a portfolio of cash equivalents and investments in a variety of securities including notes and bonds from U.S. government agencies, certificates of deposit and investment grade rated U.S. corporate debt. The securities in our investment portfolio are not leveraged and are classified as available-for-sale. We currently do not hedge interest rate exposure. We do not believe that a variation in market rates of interest would significantly impact the value of our investment portfolio.
74
|
|
|
|
ITEM 8. |
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
MAKO SURGICAL CORP.
Index to the Financial Statements
|
|
|
|
|
|
76 |
|
|
|
78 |
|
|
Statements of Operations for the years ended December 31, 2009, 2008 and 2007 |
|
79 |
|
|
80 |
|
|
Statements of Cash Flows for the years ended December 31, 2009, 2008 and 2007 |
|
82 |
|
|
83 |
75
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
MAKO Surgical Corp.
We have audited the accompanying balance sheets of MAKO Surgical Corp. as of December 31, 2009 and 2008, and the related statements of operations, redeemable convertible preferred stock and stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of MAKO Surgical Corp. at December 31, 2009 and 2008, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2009, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), MAKO Surgical Corp.’s internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 10, 2010 expressed an unqualified opinion thereon.
|
|
|
|
|
/S/ Ernst & Young LLP |
|
|
Certified Public Accountants |
|
Fort Lauderdale, Florida |
|
|
March 10, 2010 |
|
76
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
MAKO Surgical Corp.
We have audited MAKO Surgical Corp.’s internal control over financial reporting as of December 31, 2009, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). MAKO Surgical Corp.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, MAKO Surgical Corp. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2009, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of MAKO Surgical Corp. as of December 31, 2009 and 2008, and the related statements of operations, redeemable convertible preferred stock and stockholders’ equity (deficit), and cash flows for each of the three years in the period ended December 31, 2009 of MAKO Surgical Corp. and our report dated March 10, 2010 expressed an unqualified opinion thereon.
|
|
|
|
|
/s/ Ernst & Young LLP |
|
|
Certified Public Accountants |
|
Fort Lauderdale, Florida |
|
|
March 10, 2010 |
|
77
MAKO SURGICAL CORP.
Balance Sheets
(in thousands, except share and per share data)
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
||||
|
|
|
2009 |
|
2008 |
|
||
|
ASSETS |
|
|
|
|
|
|
|
|
Current Assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
17,159 |
|
$ |
62,547 |
|
|
Short-term investments |
|
|
44,686 |
|
|
1,077 |
|
|
Accounts receivable |
|
|
6,536 |
|
|
2,727 |
|
|
Inventory |
|
|
10,190 |
|
|
7,673 |
|
|
Deferred cost of revenue |
|
|
― |
|
|
3,608 |
|
|
Prepaids and other assets |
|
|
532 |
|
|
483 |
|
|
Total current assets |
|
|
79,103 |
|
|
78,115 |
|
|
Long-term investments |
|
|
9,368 |
|
|
― |
|
|
Property and equipment, net |
|
|
6,205 |
|
|
3,424 |
|
|
Intangible assets, net |
|
|
4,234 |
|
|
4,817 |
|
|
Other assets |
|
|
193 |
|
|
177 |
|
|
Total assets |
|
$ |
99,103 |
|
$ |
86,533 |
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
Current Liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
1,159 |
|
$ |
1,809 |
|
|
Accrued compensation and employee benefits |
|
|
3,709 |
|
|
2,338 |
|
|
Other accrued liabilities |
|
|
2,872 |
|
|
4,283 |
|
|
Deferred revenue |
|
|
548 |
|
|
11,518 |
|
|
Total current liabilities |
|
|
8,288 |
|
|
19,948 |
|
|
|
|
|
|
|
|
|
|
|
Deferred revenue |
|
|
21 |
|
|
71 |
|
|
Total liabilities |
|
|
8,309 |
|
|
20,019 |
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
|
|
Preferred stock, $0.001 par value; 27,000,000 authorized; 0 shares issued and outstanding as of December 31, 2009 and 2008 |
|
|
― |
|
|
― |
|
|
Common stock, $0.001 par value; 135,000,000 authorized; 33,036,378 and 24,684,786 shares issued and outstanding as of December 31, 2009 and 2008, respectively |
|
|
33 |
|
|
25 |
|
|
Additional paid-in capital |
|
|
204,977 |
|
|
146,607 |
|
|
Accumulated deficit |
|
|
(114,195 |
) |
|
(80,172 |
) |
|
Accumulated other comprehensive income (loss) |
|
|
(21 |
) |
|
54 |
|
|
Total stockholders’ equity |
|
|
90,794 |
|
|
66,514 |
|
|
Total liabilities and stockholders’ equity |
|
$ |
99,103 |
|
$ |
86,533 |
|
See accompanying notes.
78
MAKO SURGICAL CORP.
Statements of Operations
(in thousands, except per share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31, |
|
|||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
Procedures |
|
$ |
7,550 |
|
$ |
2,457 |
|
$ |
718 |
|
|
Systems – RIO |
|
|
14,715 |
|
|
― |
|
|
― |
|
|
Systems – TGS, previously deferred |
|
|
11,297 |
|
|
― |
|
|
― |
|
|
Service and other |
|
|
646 |
|
|
487 |
|
|
53 |
|
|
Total revenue |
|
|
34,208 |
|
|
2,944 |
|
|
771 |
|
|
Cost of revenue: |
|
|
|
|
|
|
|
|
|
|
|
Procedures |
|
|
3,337 |
|
|
1,521 |
|
|
197 |
|
|
Systems – RIO |
|
|
9,032 |
|
|
1,692 |
|
|
361 |
|
|
Systems – RIO upgrades |
|
|
5,183 |
|
|
― |
|
|
― |
|
|
Systems – TGS, previously deferred |
|
|
3,606 |
|
|
― |
|
|
― |
|
|
Service and other |
|
|
546 |
|
|
233 |
|
|
25 |
|
|
Total cost of revenue |
|
|
21,704 |
|
|
3,446 |
|
|
583 |
|
|
Gross profit (loss) |
|
|
12,504 |
|
|
(502 |
) |
|
188 |
|
|
Operating costs and expenses: |
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative |
|
|
31,878 |
|
|
23,158 |
|
|
12,042 |
|
|
Research and development |
|
|
13,127 |
|
|
12,472 |
|
|
8,269 |
|
|
Depreciation and amortization |
|
|
1,951 |
|
|
1,828 |
|
|
1,297 |
|
|
Total operating costs and expenses |
|
|
46,956 |
|
|
37,458 |
|
|
21,608 |
|
|
Loss from operations |
|
|
(34,452 |
) |
|
(37,960 |
) |
|
(21,420 |
) |
|
Interest and other income |
|
|
432 |
|
|
988 |
|
|
1,073 |
|
|
Interest and other expenses |
|
|
(3 |
) |
|
(110 |
) |
|
(311 |
) |
|
Net loss |
|
|
(34,023 |
) |
|
(37,082 |
) |
|
(20,658 |
) |
|
Accretion of preferred stock |
|
|
― |
|
|
(44 |
) |
|
(301 |
) |
|
Dividends on preferred stock |
|
|
― |
|
|
(521 |
) |
|
(3,359 |
) |
|
Net loss attributable to common stockholders |
|
$ |
(34,023 |
) |
$ |
(37,647 |
) |
$ |
(24,318 |
) |
|
Net loss per share – Basic and diluted attributable to common stockholders |
|
$ |
(1.22 |
) |
$ |
(2.20 |
) |
$ |
(14.75 |
) |
|
Weighted average common shares outstanding – Basic and diluted |
|
|
27,806 |
|
|
17,096 |
|
|
1,649 |
|
See accompanying notes.
79
MAKO SURGICAL CORP.
Statements of
Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficit)
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Redeemable
|
|
Common |
|
Stock |
|
Additional |
|
Note |
|
Accumulated |
|
Other |
|
Total |
|
|||||||||
|
|
|
Shares |
|
Amount |
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2006 |
|
19,650 |
|
$ |
25,911 |
|
1,556 |
|
$ |
2 |
|
$ |
— |
|
$ |
(71 |
) |
$ |
(19,366 |
) |
$ |
(2 |
) |
$ |
(19,437 |
) |
|
Issuance of Series C redeemable convertible preferred stock, net of issuance costs of $84,000 |
|
13,514 |
|
|
29,916 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
Issuance of common stock upon exercise of options |
|
— |
|
|
— |
|
1 |
|
|
— |
|
|
2 |
|
|
— |
|
|
— |
|
|
— |
|
|
2 |
|
|
Employee share-based compensation expense |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
531 |
|
|
— |
|
|
— |
|
|
— |
|
|
531 |
|
|
Interest on note receivable from stockholder |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
4 |
|
|
(4 |
) |
|
— |
|
|
— |
|
|
— |
|
|
Modification of restricted stock |
|
— |
|
|
— |
|
300 |
|
|
— |
|
|
394 |
|
|
75 |
|
|
— |
|
|
— |
|
|
469 |
|
|
Return of 35,244 shares due to modification of restricted stock |
|
— |
|
|
— |
|
(35 |
) |
|
— |
|
|
(392 |
) |
|
— |
|
|
— |
|
|
— |
|
|
(392 |
) |
|
Restricted common stock compensation expense |
|
— |
|
|
— |
|
49 |
|
|
— |
|
|
302 |
|
|
— |
|
|
— |
|
|
— |
|
|
302 |
|
|
Accretion to redemption value of Series A, B and C redeemable convertible preferred stock |
|
— |
|
|
301 |
|
— |
|
|
— |
|
|
(301 |
) |
|
— |
|
|
— |
|
|
— |
|
|
(301 |
) |
|
Accrued dividends on Series A, B and C redeemable convertible preferred stock |
|
— |
|
|
3,359 |
|
— |
|
|
— |
|
|
(540 |
) |
|
— |
|
|
(2,819 |
) |
|
— |
|
|
(3,359 |
) |
|
Change in unrealized gain on available-for-sale securities |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
6 |
|
|
6 |
|
|
Net loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(20,658 |
) |
|
— |
|
|
(20,658 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(20,652 |
) |
|
Balance at December 31, 2007 |
|
33,164 |
|
|
59,487 |
|
1,871 |
|
|
2 |
|
|
— |
|
|
— |
|
|
(42,843 |
) |
|
4 |
|
|
(42,837 |
) |
|
Issuance of common stock in initial public offering |
|
— |
|
|
— |
|
5,100 |
|
|
5 |
|
|
43,789 |
|
|
— |
|
|
— |
|
|
— |
|
|
43,794 |
|
|
Issuance of common stock in equity financing |
|
— |
|
|
— |
|
6,451 |
|
|
7 |
|
|
39,726 |
|
|
— |
|
|
— |
|
|
— |
|
|
39,733 |
|
|
Issuance of common stock upon exercise of options |
|
— |
|
|
— |
|
62 |
|
|
— |
|
|
46 |
|
|
— |
|
|
— |
|
|
— |
|
|
46 |
|
|
Employee share-based compensation expense |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
1,467 |
|
|
— |
|
|
— |
|
|
— |
|
|
1,467 |
|
|
Restricted common stock compensation expense |
|
— |
|
|
— |
|
256 |
|
|
— |
|
|
1,856 |
|
|
— |
|
|
— |
|
|
— |
|
|
1,856 |
|
|
Accretion to redemption value of Series A, B and C redeemable convertible preferred stock |
|
— |
|
|
44 |
|
— |
|
|
— |
|
|
(44 |
) |
|
— |
|
|
— |
|
|
— |
|
|
(44 |
) |
|
Accrued dividends on Series A, B and C redeemable convertible preferred stock |
|
— |
|
|
521 |
|
— |
|
|
— |
|
|
(274 |
) |
|
— |
|
|
(247 |
) |
|
— |
|
|
(521 |
) |
|
Conversion of Series A, B and C redeemable convertible preferred shares into common shares |
|
(33,164 |
) |
|
(53,667 |
) |
10,945 |
|
|
11 |
|
|
53,656 |
|
|
— |
|
|
— |
|
|
— |
|
|
53,667 |
|
|
Reclassification of accrued dividends on redeemable convertible preferred stock to additional paid-in capital |
|
— |
|
|
(6,385 |
) |
— |
|
|
— |
|
|
6,385 |
|
|
— |
|
|
— |
|
|
— |
|
|
6,385 |
|
|
Change in unrealized gain on available-for-sale securities |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
50 |
|
|
50 |
|
|
Net loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(37,082 |
) |
|
— |
|
|
(37,082 |
) |
|
Total comprehensive loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(37,032 |
) |
|
Balance at December 31, 2008 |
|
— |
|
$ |
— |
24,685 |
|
$ |
25 |
|
$ |
146,607 |
|
$ |
— |
|
$ |
(80,172 |
) |
$ |
54 |
|
$ |
66,514 |
|
|
(continued)
80
MAKO SURGICAL CORP.
Statements of Redeemable Convertible
Preferred Stock and Stockholders’ Equity (Deficit)
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common |
|
Stock |
|
Additional |
|
Note |
|
Accumulated |
|
Other |
|
Total |
|
|||||||||
|
|
|
Shares |
|
Amount |
|
|
|
|
|
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008 |
|
— |
|
$ |
— |
|
24,685 |
|
$ |
25 |
|
$ |
146,607 |
|
$ |
— |
|
$ |
(80,172 |
) |
$ |
54 |
|
$ |
66,514 |
|
|
Issuance of common stock in equity financing |
|
— |
|
|
— |
|
8,050 |
|
|
8 |
|
|
54,300 |
|
|
— |
|
|
— |
|
|
— |
|
|
54,308 |
|
|
Issuance of common stock under employee stock purchase plan |
|
— |
|
|
— |
|
72 |
|
|
— |
|
|
455 |
|
|
— |
|
|
— |
|
|
— |
|
|
455 |
|
|
Issuance of common stock upon exercise of options and warrants |
|
— |
|
|
— |
|
140 |
|
|
— |
|
|
149 |
|
|
— |
|
|
— |
|
|
— |
|
|
149 |
|
|
Employee share-based compensation expense |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
3,032 |
|
|
— |
|
|
— |
|
|
— |
|
|
3,032 |
|
|
Restricted common stock compensation expense |
|
— |
|
|
— |
|
145 |
|
|
— |
|
|
982 |
|
|
— |
|
|
— |
|
|
— |
|
|
982 |
|
|
Receipt of 56,045 shares delivered in payment of payroll taxes |
|
— |
|
|
— |
|
(56 |
) |
|
— |
|
|
(492 |
) |
|
— |
|
|
— |
|
|
— |
|
|
(492 |
) |
|
Deferred equity financing costs |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
(56 |
) |
|
— |
|
|
— |
|
|
— |
|
|
(56 |
) |
|
Change in unrealized gain (loss) on available-for-sale securities |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(75 |
) |
|
(75 |
) |
|
Net loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(34,023 |
) |
|
— |
|
|
(34,023 |
) |
|
|
||||||||||||||||||||||||||
|
Total comprehensive loss |
|
— |
|
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(34,098 |
) |
|
Balance at December 31, 2009 |
|
— |
|
$ |
— |
|
33,036 |
|
$ |
33 |
|
$ |
204,977 |
|
$ |
— |
|
$ |
(114,195 |
) |
$ |
(21 |
) |
$ |
90,794 |
|
See accompanying notes.
81
MAKO SURGICAL CORP.
Statements of
Cash Flows
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31, |
|
|||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
Operating activities: |
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
$ |
(34,023 |
) |
$ |
(37,082 |
) |
$ |
(20,658 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
Depreciation |
|
|
1,769 |
|
|
1,502 |
|
|
678 |
|
|
Amortization of intangible assets |
|
|
682 |
|
|
660 |
|
|
645 |
|
|
Stock-based compensation |
|
|
4,014 |
|
|
3,323 |
|
|
1,227 |
|
|
Inventory write-down |
|
|
1,081 |
|
|
730 |
|
|
8 |
|
|
Amortization of premium on investment securities |
|
|
188 |
|
|
― |
|
|
― |
|
|
Loss on asset impairment |
|
|
51 |
|
|
― |
|
|
14 |
|
|
Accrued interest expense on deferred license fee |
|
|
― |
|
|
45 |
|
|
305 |
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
|
(3,809 |
) |
|
(514 |
) |
|
(1,634 |
) |
|
Inventory |
|
|
(7,358 |
) |
|
(7,056 |
) |
|
(2,123 |
) |
|
Prepaid and other assets |
|
|
(49 |
) |
|
(173 |
) |
|
97 |
|
|
Other assets |
|
|
(16 |
) |
|
(7 |
) |
|
156 |
|
|
Accounts payable |
|
|
(650 |
) |
|
298 |
|
|
1,078 |
|
|
Accrued compensation and employee benefits |
|
|
1,371 |
|
|
1,305 |
|
|
523 |
|
|
Other accrued liabilities |
|
|
(1,411 |
) |
|
1,603 |
|
|
1,664 |
|
|
Deferred cost of revenue |
|
|
3,608 |
|
|
(2,682 |
) |
|
(716 |
) |
|
Deferred revenue |
|
|
(11,020 |
) |
|
8,228 |
|
|
2,661 |
|
|
Net cash used in operating activities |
|
|
(45,572 |
) |
|
(29,820 |
) |
|
(16,075 |
) |
|
Investing activities: |
|
|
|
|
|
|
|
|
|
|
|
Purchase of investments |
|
|
(59,961 |
) |
|
(1,990 |
) |
|
(15,159 |
) |
|
Proceeds from sales and maturities of investments |
|
|
6,721 |
|
|
4,047 |
|
|
13,480 |
|
|
Acquisition of property and equipment |
|
|
(790 |
) |
|
(1,606 |
) |
|
(1,087 |
) |
|
Acquisition of intangible assets |
|
|
(150 |
) |
|
― |
|
|
(450 |
) |
|
Payment of deferred license fee |
|
|
― |
|
|
(4,000 |
) |
|
― |
|
|
Net cash used in investing activities |
|
|
(54,180 |
) |
|
(3,549 |
) |
|
(3,216 |
) |
|
Financing activities: |
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock in equity financing, net of underwriting fees of $3,502 and $0 for the years ended December 31, 2009 and 2008, respectively |
|
|
54,861 |
|
|
40,202 |
|
|
― |
|
|
Deferred equity financing costs |
|
|
(609 |
) |
|
(469 |
) |
|
― |
|
|
Proceeds from initial public offering of common stock, net of underwriting fees of $3,570 |
|
|
― |
|
|
47,430 |
|
|
― |
|
|
Deferred initial public offering costs |
|
|
― |
|
|
(908 |
) |
|
(2,728 |
) |
|
Proceeds from issuance of Series C redeemable convertible preferred stock, net of stock issuance costs |
|
|
― |
|
|
― |
|
|
29,916 |
|
|
Proceeds from employee stock purchase plan |
|
|
455 |
|
|
― |
|
|
― |
|
|
Exercise of common stock options for cash |
|
|
149 |
|
|
46 |
|
|
2 |
|
|
Payment of payroll taxes relating to vesting of restricted stock |
|
|
(492 |
) |
|
― |
|
|
― |
|
|
Payment of CEO payroll taxes relating to restricted stock modification |
|
|
― |
|
|
― |
|
|
(392 |
) |
|
Net cash provided by financing activities |
|
|
54,364 |
|
|
86,301 |
|
|
26,798 |
|
|
Net increase (decrease) in cash and cash equivalents |
|
|
(45,388 |
) |
|
52,932 |
|
|
7,507 |
|
|
Cash and cash equivalents at beginning of year |
|
|
62,547 |
|
|
9,615 |
|
|
2,108 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end of year |
|
$ |
17,159 |
|
$ |
62,547 |
|
$ |
9,615 |
|
|
Non-cash investing and financing activities: |
|
|
|
|
|
|
|
|
|
|
|
Receipt of 56,045 and 35,244 shares of common stock delivered in payment of payroll taxes for the years ended December 31, 2009 and 2007, respectively |
|
$ |
492 |
|
$ |
― |
|
$ |
392 |
|
|
Transfers of inventory to property and equipment |
|
|
3,760 |
|
|
999 |
|
|
695 |
|
|
Accretion of redeemable convertible preferred stock |
|
|
― |
|
|
44 |
|
|
301 |
|
|
Accrued dividends on redeemable convertible preferred stock |
|
|
― |
|
|
521 |
|
|
3,359 |
|
|
Conversion of redeemable convertible preferred stock into 10,945,080 common shares |
|
|
― |
|
|
53,667 |
|
|
― |
|
|
Reclassification of accrued dividends on redeemable convertible preferred stock to additional paid-in capital |
|
|
― |
|
|
6,385 |
|
|
― |
|
|
Reclassification of deferred initial public offering costs to additional paid-in capital |
|
|
― |
|
|
3,636 |
|
|
― |
|
|
Licensing of intellectual property |
|
|
― |
|
|
― |
|
|
30 |
|
|
Deferred license fee payable |
|
|
― |
|
|
― |
|
|
30 |
|
|
Interest on note receivable for common stock |
|
|
― |
|
|
― |
|
|
4 |
|
See accompanying notes.
82
MAKO SURGICAL CORP.
1. Description of the Business
MAKO Surgical Corp. (the “Company” or “MAKO”) is an emerging medical device company that markets its advanced robotic arm solution and orthopedic implants for minimally invasive orthopedic knee procedures. The Company was incorporated in the State of Delaware on November 12, 2004 and is headquartered in Fort Lauderdale, Florida.
In February 2008, the Company effected a one for 3.03 reverse split of its issued and outstanding common stock, which has been retroactively reflected in these financial statements and accompanying notes. Also, in February 2008, the Company completed its initial public offering (“IPO”) of common stock, issuing a total of 5.1 million shares at an offering price to the public of $10.00 per share, resulting in net proceeds to the Company, after underwriting discounts and commissions and expenses, of approximately $43.8 million.
In conjunction with the completion of the Company’s IPO in February 2008, all of the Company’s outstanding Series A, B and C redeemable convertible preferred stock was converted into 10,945,080 shares of common stock, adjusted for the February 2008 reverse stock split. In connection therewith, all remaining redeemable convertible preferred stock discounts and accrued dividends were reclassified to additional paid-in capital and were not paid.
In October 2008, the Company entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) for an equity financing of up to approximately $60 million, with initial gross proceeds of approximately $40.2 million, which the Company closed on October 31, 2008, and conditional access to an additional $20 million (which conditional access expired on December 31, 2009). The financing resulted in net proceeds to the Company of approximately $39.7 million, after expenses of approximately $525,000. See Note 5 for further discussion of the Securities Purchase Agreement.
In August 2009, the Company completed a public offering of its common stock, issuing 8,050,000 shares at an offering price to the public of $7.25 per share, resulting in net proceeds to the Company, after underwriting discounts and commissions and expenses, of approximately $54.3 million.
2. Summary of Significant Accounting Policies
Basis of Presentation and Use of Estimates
The financial statements have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. The accounting estimates that require management’s most significant, difficult and subjective judgments include revenue recognition, allowance for doubtful accounts, inventory valuation, valuation allowance for deferred income tax assets, impairment of long-lived assets and the determination of stock-based compensation. Actual results could differ significantly from these estimates.
Liquidity and Operations
In executing its current business plan, the Company believes its existing cash, cash equivalents and investment balances and interest income earned on these balances will be sufficient to meet its anticipated cash requirements for at least the next twelve months. To the extent the Company’s available cash, cash equivalents and investment balances are insufficient to satisfy its operating requirements after that period, the Company will need to seek additional sources of funds, including selling additional equity, debt or other securities or entering into a credit
83
facility, or modifying its current business plan. The sale of additional equity and convertible debt securities may result in dilution to the Company’s current stockholders. If the Company raises additional funds through the issuance of debt securities, these securities may have rights senior to those of its common stock and could contain covenants that could restrict its operations and issuance of dividends. The Company may also require additional capital beyond its currently forecasted amounts. Any required additional capital, whether forecasted or not, may not be available on reasonable terms, or at all. If the Company is unable to obtain additional financing, the Company may be required to reduce the scope of, delay or eliminate some or all of its planned research, development and commercialization activities, which could materially harm its business and results of operations.
Concentrations of Credit Risk and Other Risks and Uncertainties
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash and cash equivalents and investments. The Company’s cash and cash equivalents are held in demand and money market accounts at three large financial institutions. The Company’s investments are held in a variety of interest bearing instruments, including notes and bonds from U.S. government agencies, certificates of deposit and investment grade rated U.S. corporate debt at three large financial institutions. Such deposits are generally in excess of insured limits. The Company has not experienced any historical losses on its deposits of cash and cash equivalents.
The Company is subject to risks common to emerging companies in the medical device industry including, but not limited to: new technological innovations, dependence on key personnel, dependence on key suppliers, changes in general economic conditions and interest rates, protection of proprietary technology, compliance with changing government regulations and taxes, uncertainty of widespread market acceptance of products, access to credit for capital purchases by our customers, product liability and the need to obtain additional financing. The Company’s products include components subject to rapid technological change. Certain components used in manufacturing have relatively few alternative sources of supply and establishing additional or replacement suppliers for such components cannot be accomplished quickly. The inability of any of these suppliers to fulfill the Company’s supply requirements may negatively impact future operating results. While the Company has ongoing programs to minimize the adverse effect of such uncertainty and considers technological change in estimating the net realizable value of its inventory, uncertainty continues to exist.
The Company’s current versions of its RIO® Robotic Arm Interactive Orthopedic system (“RIO”), which is the version 2.0 of its Tactile Guidance System™ (“TGS™”), its RESTORIS® unicompartmental and RESTORIS MCK multicompartmental knee implant systems and its TGS have been cleared by the U.S. Food and Drug Administration (“FDA”). Certain products currently under development by the Company will require clearance or approval by the FDA or other international regulatory agencies prior to commercial sale. There can be no assurance that the Company’s products will receive the necessary clearances or approvals. If the Company were to be denied such clearance or approval or such clearance or approval were delayed, it could have a material adverse impact on the Company.
The Company may perform credit evaluations of its customers’ financial condition and, generally, requires no collateral from its customers. The Company will provide an allowance for doubtful accounts when collections become doubtful but has not experienced any credit losses to date.
Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 280, Segment Reporting, establishes standards for reporting information about operating segments. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker, or decision making group, in deciding how to allocate resources and in assessing performance. The Company’s chief operating decision maker is its CEO. The Company’s CEO reviews financial information presented on an aggregate basis for purposes of allocating resources and evaluating financial performance. The Company has one business activity and there are no segment managers who are held accountable for operations, operating results and plans for products or components below the aggregate Company level. Accordingly, the Company reports as a single operating segment. To date, all of the Company’s revenue is from companies located in the United States. No one customer accounted for more than 10% of the Company’s
84
total revenue for the year ended December 31, 2009. The following table presents information about the Company’s revenue by significant customer for the years ended December 31, 2008 and 2007:
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
Years Ended December 31, |
|
||||
|
|
|
2008 |
|
2007 |
|
||
|
Company A |
|
$ |
417 |
|
$ |
331 |
|
|
Company B |
|
|
277 |
|
|
161 |
|
|
Company C |
|
|
493 |
|
|
126 |
|
|
Company D |
|
|
274 |
|
|
18 |
|
|
Others |
|
|
996 |
|
|
82 |
|
|
Net Revenue |
|
$ |
2,457 |
|
$ |
718 |
|
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity at date of purchase of 90 days or less to be cash equivalents.
Fair Value of Financial Instruments
Carrying amounts of certain of the Company’s financial instruments, including cash and cash equivalents, investments, accounts receivable and other accrued liabilities approximate fair value due to their short maturities or market rates of interest.
Allowance for Doubtful Accounts
The allowance for doubtful accounts is based on the Company’s assessment of the collectability of customer accounts. The Company regularly reviews the allowance by considering factors such as historical experience, credit quality, the age of the accounts receivable balances and current economic conditions that may affect a customer’s ability to pay. The Company has not experienced any collectability issues to date and has no allowance, provision for doubtful accounts receivable or write-offs to date in the accompanying financial statements.
Accrual for Warranty Costs
Upon installation of a RIO system, the Company establishes an accrual for the estimated costs associated with providing a standard one-year warranty for defects in materials and workmanship.
Inventory
Inventory is stated at the lower of cost or market value on a first-in, first-out basis. Inventory costs include direct materials, direct labor and manufacturing overhead. The Company reviews its inventory periodically to determine net realizable value and considers product upgrades in its periodic review of realizability. The Company writes down inventory, if required, based on forecasted demand and technological obsolescence. These factors are impacted by market and economic conditions, technology changes and new product introductions and require estimates that may include uncertain elements.
Beginning with the fourth quarter of 2008, manufacturing overhead costs have been capitalized and included in inventory. As of December 31, 2009 and 2008, capitalized manufacturing overhead included in inventory was approximately $1.1 million and $282,000, respectively. Previously, such overhead costs were fully expensed as selling, general and administrative expense as capitalizable amounts were not significant.
85
Property and Equipment
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation of property and equipment is computed using the straight-line method over their estimated useful lives of two to seven years. Leasehold improvements are amortized on a straight-line basis over the lesser of their useful life or the term of the lease and are included in depreciation expense in the accompanying statements of operations. Upon retirement or sale, the cost and related accumulated depreciation are removed from the balance sheet and the resulting gain or loss is reflected in operations. Maintenance and repairs are charged to operations as incurred.
Intangible Assets
The Company’s intangible assets are comprised of licenses to intellectual property rights. These intangible assets are carried at cost, net of accumulated amortization. Amortization is recorded using the straight-line method, over their respective useful lives (generally the life of underlying patents), which range from approximately 5 to 13 years.
Impairment of Long-Lived Assets
The Company evaluates its long-lived assets for indicators of impairment by comparison of the carrying amounts to future net undiscounted cash flows expected to be generated by such assets when events or changes in circumstances indicate the carrying amount of an asset may not be recoverable. Should an impairment exist, the impairment loss would be measured based on the excess carrying value of the asset over the asset’s fair value or estimated discounted future cash flows.
Revenue Recognition
Revenue is generated from unit sales of the Company’s RIO system, including installation services, training, upgrades and enhancements, from sales of implants and disposable products, and by providing extended warranty services. The Company’s RIO system, as well as upgrades and enhancements to its RIO system, include software that is essential to the functionality of the product and, accordingly, the Company accounts for the sale of the RIO system pursuant to ASC 985-605, Software – Revenue Recognition (“ASC 985-605”).
The Company recognizes system revenue for sales of the RIO system when there is persuasive evidence of a sales arrangement, the fee is fixed or determinable, collection of the fee is probable and delivery has occurred as prescribed by ASC 985-605. For all sales, the Company uses either a signed agreement or a binding purchase order as evidence of an arrangement.
For arrangements with multiple elements, the Company allocates arrangement consideration to the RIO systems, upgrades, enhancements and services based upon vendor specific objective evidence (“VSOE”) of fair value of the respective elements. Revenue and direct cost of revenue associated with the sale of the RIO systems are recognized upon the earlier of (1) delivery of all elements or (2) establishment of VSOE of fair value for all undelivered elements.
Subsequent to December 31, 2008, the Company no longer manufactures TGS units, to which associated TGS sales arrangements required it to provide upgrades and enhancements, through and including the delivery of the RIO system. The Company commercially released the RIO system in the first quarter of 2009. Sales arrangements for RIO systems do not require the Company to provide upgrades and enhancements. As a result, revenues related to RIO system sales will be recognized upon installation of the system, delivery of associated instrumentation and training of at least one surgeon.
For sales of TGS units through December 31, 2008, VSOE of fair value was not established for upgrades and enhancements (through and including delivery of the RIO), which the TGS sales arrangements required the Company to provide. Accordingly, prior to delivery of the RIO system, sales of TGS units were recorded as deferred revenue and the direct cost of revenue associated with the sale of TGS units was recorded as deferred
86
cost of revenue. Revenue for all previously deferred TGS sales was recognized in our statement of operations during the year ended December 31, 2009, upon delivery of the RIO system. As of December 31, 2009, the deferred revenue balance consists primarily of deferred service revenue as discussed below.
A portion of the Company’s customers acquire the RIO system through a leasing arrangement with a third-party leasing company. In these instances, the Company typically sells the RIO system to the leasing company, and the customer enters into an independent leasing arrangement with the leasing company. The Company treats these leasing transactions the same as sales transactions for purposes of recognizing revenue for the sale. The Company sells implants and disposable products utilized in knee MAKOplasty procedures directly to the customers.
Procedure revenue from the sale of implants and disposable products utilized in knee MAKOplasty procedures is recognized when persuasive evidence of an arrangement exists, delivery has occurred or service has been rendered, the price is fixed or determinable and collectability is reasonably assured. The implants and disposable products are a separate unit of accounting from the RIO systems as (1) they have value to the customer on a standalone basis, (2) objective and reliable evidence of the fair value of the item exists and (3) no right of return exists once the implants and disposable products are implanted or consumed. Accordingly, as the Company’s implants and disposable products are sold on a procedural basis, the revenue and costs associated with the sale of implants and disposable products are recognized at the time of sale (i.e., at the time of the related surgical procedure).
Costs associated with establishing an accrual for the RIO system standard one-year warranty liability and royalties covered by licensing arrangements related to the sale of RIO systems are expensed upon installation and are included in cost of revenue - systems, in the statements of operations.
Service revenue, which is included in other revenue, consists of extended warranty services on the RIO system hardware, and is deferred and recognized ratably over the service period until no further obligation exists. Costs associated with providing extended warranty services are expensed as incurred.
Deferred Revenue and Deferred Cost of Revenue
Deferred revenue consists of deferred system revenue and deferred service revenue. Deferred system revenue arises from timing differences between the installation of RIO systems and satisfaction of all revenue recognition criteria consistent with the Company’s revenue recognition policy. Deferred service revenue also results from the advance payment for services to be delivered over a period of time, usually in one-year increments. Service revenue is recognized ratably over the service period. Deferred cost of revenue consists of the direct costs associated with the manufacture of RIO systems for which the revenue has been deferred in accordance with the Company’s revenue recognition policy. Deferred revenue and associated deferred cost of revenue expected to be realized within one year are classified as current liabilities and current assets, respectively. The deferred revenue balance as of December 31, 2009 consists primarily of deferred service revenue for extended warranty services on the RIO system hardware.
Research and Development Costs
Costs related to research, design and development of products are charged to research and development expense as incurred. These costs include direct salary costs for research and development personnel, costs for materials used in research and development activities and costs for outside services.
87
Shipping and Handling Costs
Costs incurred for shipping and handling are included in cost of revenue at the time the expense is incurred.
Software Development Costs
Software development costs are included in research and development and are expensed as incurred. After technological feasibility is established, material software development costs are capitalized. The capitalized cost is then amortized on a straight-line basis over the estimated product life, or on the ratio of current revenue to total projected product revenue, whichever is greater. To date, the period between achieving technological feasibility, which the Company has defined as the establishment of a working model which typically occurs when the verification and validation testing is complete, and the general availability of such software has been short and software development costs qualifying for capitalization have been insignificant. Accordingly, the Company has not capitalized any software development costs to date.
Stock-Based Compensation
The Company recognizes compensation expense for its stock-based awards in accordance with ASC 718, Compensation-Stock Compensation. ASC 718 requires the recognition of compensation expense, using a fair value based method, for costs related to all share-based payments including stock options. ASC 718 requires companies to estimate the fair value of share-based payment awards on the date of grant using an option-pricing model.
The Company accounts for stock-based compensation arrangements with non-employees in accordance with the ASC 505-50, Equity-Based Payments to Non-Employees. The Company records the expense of such services based on the estimated fair value of the equity instrument using the Black-Scholes-Merton pricing model. The value of the equity instrument is charged to expense over the term of the service agreement.
See Note 8 for a detailed discussion of the various stock option plans and related stock-based compensation.
Advertising Costs
Advertising costs are expensed as incurred. Advertising costs were approximately $1.3 million, $1.4 million and $431,000 for the years ended December 31, 2009, 2008 and 2007, respectively.
Income Taxes
The Company accounts for income taxes under ASC 740, Income Taxes. Deferred income taxes are determined based upon differences between financial reporting and income tax bases of assets and liabilities and are measured using the enacted income tax rates and laws that will be in effect when the differences are expected to reverse. Valuation allowances are established when necessary to reduce deferred income tax assets to the amounts expected to be realized. The Company recognizes any interest and penalties related to unrecognized tax benefits as a component of income tax expense.
Operating Leases
Rental payments and incentives, if any, are recognized on a straight-line basis over the life of a lease. See Note 7 for further discussion of operating leases.
Net Loss Per Share
The Company calculated net loss per share in accordance with ASC 260, Earnings per Share. Basic earnings per share (“EPS”) is calculated by dividing the net income or loss available to common stockholders adjusted for redeemable convertible preferred stock accretion and dividends by the weighted average number of common
88
shares outstanding for the period, without consideration for common stock equivalents. Diluted EPS is computed by dividing the net income or loss available to common stockholders by the weighted average number of common shares outstanding for the period and the weighted average number of dilutive common stock equivalents outstanding for the period determined using the treasury stock method. The following table sets forth potential shares of common stock that are not included in the calculation of diluted net loss per share because to do so would be anti-dilutive as of the end of each period presented:
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
December 31, |
|
|||||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
|||
|
Stock options outstanding |
|
|
3,478 |
|
|
2,193 |
|
|
1,917 |
|
|
Warrants to purchase common stock |
|
|
2,065 |
|
|
2,076 |
|
|
463 |
|
|
Unvested restricted stock |
|
|
222 |
|
|
267 |
|
|
428 |
|
|
Redeemable convertible preferred stock |
|
|
― |
|
|
― |
|
|
33,164 |
|
Comprehensive Loss
Comprehensive loss is defined as the change in equity from transactions and other events and circumstances other than those resulting from investments by owners and distributions to owners. For the years ended December 31, 2009, 2008 and 2007, the Company recorded comprehensive losses of approximately $34.1 million, $37.0 million and $20.7 million, respectively. The difference between comprehensive loss and net loss for the years ended December 31, 2009, 2008 and 2007 is due to changes in unrealized gains and losses on the Company’s available-for-sale securities.
Recent Accounting Pronouncements
Adopted Accounting Pronouncements
In June 2008, the Financial Accounting Standards Board (“FASB”) issued an accounting standard update. As codified in ASC 815-40, Derivatives and Hedging, this update provides guidance for determining whether an equity-linked financial instrument (or embedded feature) is indexed to an entity’s own stock. The update applies to any freestanding financial instrument or embedded feature that has all the characteristics of a derivative, for purposes of determining whether that instrument or embedded feature qualifies for the first part of the scope exception under ASC 815-10-15. The update also applies to any freestanding financial instrument that is potentially settled in an entity’s own stock, regardless of whether the instrument has all the characteristics of a derivative under previous derivative Generally Accepted Accounting Principals (“GAAP”), for purposes of determining whether the instrument is within the scope of derivative accounting. ASC 815-40 was effective beginning with the first quarter of fiscal 2009. The adoption did not have a material impact on the Company’s results of operations and financial position.
Effective January 1, 2009, the Company adopted a new accounting standard update regarding business combinations. As codified under ASC 805, Business Combinations, this update requires an entity to recognize the assets acquired, liabilities assumed, contractual contingencies, and contingent consideration at their fair value on the acquisition date. It further requires that acquisition-related costs be recognized separately from the acquisition and expensed as incurred; that restructuring costs generally be expensed in periods subsequent to the acquisition date; and that changes in accounting for deferred tax asset valuation allowances and acquired income tax uncertainties after the measurement period be recognized as a component of provision for taxes. In addition, acquired in-process research and development is capitalized as an intangible asset and amortized over its estimated useful life.
Effective April 1, 2009, the Company adopted a new accounting standard, as codified in ASC 820-10-65, which provides additional guidance for estimating fair value when the volume and level of activity for the asset or liability have significantly decreased. ASC 820-10-65 also includes guidance on identifying circumstances that
89
indicate a transaction is not orderly. The adoption did not have a material impact on the Company’s results of operations and financial position.
In April 2009, the FASB issued an accounting standard update, as codified in ASC 320-10-65, to amend the other-than-temporary impairment guidance in debt securities to be based on intent to sell instead of ability to hold the security and to improve the presentation and disclosure of other-than-temporary impairments on debt and equity securities in the financial statements. This pronouncement is effective for periods ending after June 15, 2009. The adoption did not have a material impact on the Company’s results of operations and financial position.
Effective April 1, 2009, the Company adopted a new accounting standard for subsequent events, as codified in ASC 855-10, which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date, but before the financial statements are issued or available to be issued (“subsequent events”). ASC 855-10 is effective for interim or annual periods ending after June 15, 2009. See Note 11 for discussion of subsequent event.
Effective July 1, 2009, the Company adopted The FASB Accounting Standards Codification and the Hierarchy of Generally Accepted Accounting Principles (“ASC 105”). ASC 105 establishes the FASB Accounting Standards Codification as the source of authoritative accounting principles recognized by the FASB to be applied in the preparation of financial statements in conformity with generally accepted accounting principles. ASC 105 explicitly recognizes rules and interpretive releases of the SEC under federal securities laws as authoritative GAAP for SEC registrants. As ASC 105 was not intended to change or alter existing GAAP, it did not have any impact on the Company’s financial statements.
New Accounting Pronouncements
In September 2009, the FASB issued Update No. 2009-13, Multiple-Deliverable Revenue Arrangements, a consensus of the FASB Emerging Issues Task Force (“ASU 2009-13”). ASU 2009-13 updates the existing multiple-element revenue arrangements guidance currently included under ASC 605-25. ASU 2009-13 eliminates the need for objective and reliable evidence of the fair value for the undelivered element in order for a delivered item to be treated as a separate unit of accounting and eliminates the residual method to allocate the arrangement consideration. In addition, the guidance also expands the disclosure requirements for revenue recognition. ASU 2009-13 will be effective for the first annual reporting period beginning on or after June 15, 2010, with early adoption permitted provided that the revised guidance is retroactively applied to the beginning of the year of adoption. The Company is currently evaluating the future impact that ASU 2009-13 will have on its financial statements.
In September 2009, the FASB issued Update No. 2009-14, Certain Revenue Arrangements That Include Software Elements, a consensus of the FASB Emerging Issues Task Force (“ASU 2009-14”). ASU 2009-14 modifies the scope of ASC 985-605 to exclude from its requirements (a) non-software components of tangible products and (b) software components of tangible products that are sold, licensed, or leased with tangible products when the software components and non-software components of the tangible product function together to deliver the tangible product’s essential functionality. ASU 2009-14 will be effective for the first annual reporting period beginning on or after June 15, 2010, with early adoption permitted provided that the revised guidance is retroactively applied to the beginning of the year of adoption. The Company is currently evaluating the future impact that ASU 2009-14 will have on its financial statements.
90
Reclassifications
Certain insignificant reclassifications have been made to the prior periods’ statements of cash flows to conform to the current period’s presentation.
3. Investments
The Company’s investments are classified as available-for-sale. Available-for-sale securities are carried at fair value, with the unrealized gains and losses included in other comprehensive income within stockholders’ equity (deficit). Realized gains and losses and declines in value determined to be other-than-temporary on available-for-sale securities are included in interest and other expenses. During the years ended December 31, 2009, 2008 and 2007, realized gains or losses recognized on the sale of investments were not significant. Interest and dividends on securities classified as available-for-sale are included in interest and other income. The cost of securities sold is based on the specific identification method.
The amortized cost and fair value of short and long-term investments, with gross unrealized gains and losses, were as follows:
As of December 31, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
Amortized |
|
Gross |
|
Gross |
|
Fair |
|
||||
|
Short-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. government agencies |
|
$ |
32,860 |
|
$ |
31 |
|
$ |
(24 |
) |
$ |
32,867 |
|
|
Certificates of deposit |
|
|
10,297 |
|
|
1 |
|
|
(25 |
) |
|
10,273 |
|
|
U.S. corporate debt |
|
|
1,532 |
|
|
14 |
|
|
― |
|
|
1,546 |
|
|
Long-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. government agencies |
|
|
5,418 |
|
|
― |
|
|
(18 |
) |
|
5,400 |
|
|
Certificates of deposit |
|
|
2,462 |
|
|
― |
|
|
(10 |
) |
|
2,452 |
|
|
U.S. corporate debt |
|
|
1,506 |
|
|
10 |
|
|
― |
|
|
1,516 |
|
|
Total investments |
|
$ |
54,075 |
|
$ |
56 |
|
$ |
(77 |
) |
$ |
54,054 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31, 2008 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
Amortized |
|
Gross |
|
Gross |
|
Fair |
|
||||
|
Short-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Certificates of deposit |
|
$ |
61 |
|
$ |
― |
|
$ |
― |
|
$ |
61 |
|
|
Variable auction rate securities |
|
|
962 |
|
|
54 |
|
|
― |
|
|
1,016 |
|
|
Total short-term investments |
|
$ |
1,023 |
|
$ |
54 |
|
$ |
― |
|
$ |
1,077 |
|
As of December 31, 2009 and December 31, 2008, all short-term investments had maturity dates or interest reset dates of less than one year. As of December 31, 2009, all long-term investments had maturity dates between one and two years.
91
The fair values of the Company’s investments based on the level of inputs are summarized below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
|
|
Fair Value Measurements at the Reporting Date Using |
|
||||||||
|
|
|
December |
|
Quoted |
|
Significant |
|
Significant |
|
||||
|
Short-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. government agencies |
|
$ |
32,867 |
|
$ |
32,867 |
|
$ |
― |
|
$ |
― |
|
|
Certificates of deposit |
|
|
10,273 |
|
|
10,273 |
|
|
― |
|
|
― |
|
|
U.S. corporate debt |
|
|
1,546 |
|
|
1,546 |
|
|
― |
|
|
― |
|
|
Long-term investments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
U.S. government agencies |
|
|
5,400 |
|
|
5,400 |
|
|
― |
|
|
― |
|
|
Certificates of deposit |
|
|
2,452 |
|
|
2,452 |
|
|
― |
|
|
― |
|
|
U.S. corporate debt |
|
|
1,516 |
|
|
1,516 |
|
|
― |
|
|
― |
|
|
Total investments |
|
$ |
54,054 |
|
$ |
54,054 |
|
$ |
― |
|
$ |
― |
|
The table below provides a reconciliation of auction rate securities assets measured at fair value on a recurring basis which use Level 3 or significant unobservable inputs for the year ended December 31, 2009.
|
|
|
|
|
|
|
(in thousands) |
|
Fair Value Measurements Using Significant Unobservable Inputs |
|
|
|
|
|
Year Ended |
|
|
|
Balance at beginning of year |
|
$ |
1,016 |
|
|
Transfers into Level 3 |
|
|
― |
|
|
Total gains realized included in earnings |
|
|
63 |
|
|
Total change in other comprehensive income |
|
|
(54 |
) |
|
Sales/Redemptions |
|
|
(1,025 |
) |
|
Balance at December 31, 2009 |
|
$ |
― |
|
|
|
|
|
|
|
|
The total amount of gains or losses for the period included in earnings attributable to the change in unrealized gains or losses relating to assets still held at the reporting date |
|
$ |
― |
|
In February 2008, the FASB issued an accounting standard update, as codified in ASC 820-10, that delayed the effective date of fair value measurements accounting for certain nonfinancial assets and certain nonfinancial liabilities, until the beginning of the first quarter of fiscal 2009. The Company adopted this accounting standard update effective January 1, 2009. The adoption of this update did not have a material impact on the Company’s financial position or its results of operations.
92
4. Selected Balance Sheet Components
The following table provides details of selected balance sheet items:
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
December 31, |
|
||||
|
|
|
2009 |
|
2008 |
|
||
|
Inventory: |
|
|
|
|
|
|
|
|
Raw materials |
|
$ |
2,770 |
|
$ |
3,809 |
|
|
Work-in-process |
|
|
932 |
|
|
748 |
|
|
Finished goods |
|
|
6,488 |
|
|
3,116 |
|
|
Total inventory |
|
$ |
10,190 |
|
$ |
7,673 |
|
The Company incurred write-offs totaling approximately $1.1 million and $730,000 during the years ended December 31, 2009 and 2008, respectively. Write-offs in 2009 primarily relate to technology changes associated with the launch of the RIO system in 2009 and disposal of spare TGS inventory associated with the launch of the RIO system. Write-offs in 2008 primarily relate to discontinued portions of the Company’s existing implant lines in connection with the launch of RESTORIS and to the discontinuation of the manufacturing of the Company’s TGS in anticipation of the launch of the RIO system.
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
December 31, |
|
Estimated |
|
|||||
|
|
|
2009 |
|
2008 |
|
|
||||
|
Property and equipment: |
|
|
|
|
|
|
|
|
|
|
|
Consigned RIO systems and instruments |
|
$ |
2,551 |
|
$ |
705 |
|
|
2-5 years |
|
|
Service and demo RIO systems and instruments |
|
|
2,354 |
|
|
549 |
|
|
2-5 years |
|
|
Computer equipment and software |
|
|
2,160 |
|
|
1,669 |
|
|
3-5 years |
|
|
Manufacturing and laboratory equipment |
|
|
1,654 |
|
|
1,261 |
|
|
5 years |
|
|
Office furniture and equipment |
|
|
882 |
|
|
804 |
|
|
7 years |
|
|
Leasehold improvements |
|
|
607 |
|
|
387 |
|
|
Lesser of useful life or lease term |
|
|
|
|
|
10,208 |
|
|
5,375 |
|
|
|
|
|
Less accumulated depreciation and amortization |
|
|
(4,003 |
) |
|
(1,951 |
) |
|
|
|
|
Total property and equipment, net |
|
$ |
6,205 |
|
$ |
3,424 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
December 31, |
|
||||
|
|
|
2009 |
|
2008 |
|
||
|
Other accrued liabilities: |
|
|
|
|
|
|
|
|
Accrued royalties |
|
$ |
413 |
|
$ |
429 |
|
|
Accrued legal fees |
|
|
172 |
|
|
586 |
|
|
Other |
|
|
2,287 |
|
|
3,268 |
|
|
|
|
$ |
2,872 |
|
$ |
4,283 |
|
5. Related Parties
Employee Loans
During 2006, the Company issued $225,000 in employee loans to certain officers of the Company (the “Employee Loans”). The Employee Loans accrued interest at a rate of 4.0% per annum, compounded annually. The interest was paid biweekly. The Employee Loans and accrued interest were due upon the earlier of one year from the date of the Employee Loan or a liquidation event, as defined. The Employee Loans were fully repaid in
93
April 2007. In May and June 2007, the Company issued $225,000 in employee loans to certain officers of the Company under terms that were substantially similar to the Employee Loans issued in 2006. In August and September 2007, the Company forgave the $225,000 of outstanding loans, including accrued interest, with a charge to the statement of operations. No Employee Loans were outstanding as of December 31, 2009 and 2008.
Restricted Stock and Note Receivable from Related Party
In July 2005 and May 2006, the Company issued a total of 446,287 shares of restricted common stock to its CEO and 49,504 shares of unrestricted common stock to an entity affiliated with the CEO in exchange for promissory notes from the CEO totaling approximately $631,000 (representing the fair value of the shares on the date of issuance) approximately 50% of which was nonrecourse. The promissory notes accrued interest at a rate of 8% per annum, with 25% of the restricted stock vesting immediately and the remainder vesting monthly over 48 months as service is provided. The restricted stock was pledged as collateral against the promissory notes. In March 2007, the Company issued 82,508 shares of restricted common stock to its CEO at a purchase price of $2.48 per share (the estimated fair value at the date of issuance) in exchange for a promissory note of $205,000, 50% of which was nonrecourse and a pledge agreement. The March 2007 restricted stock, pledge agreement and promissory note were issued under terms substantially similar to the July 2005 and May 2006 restricted stock issuances. Because it was unclear as to whether the recourse portion had substance as of the dates of issuance of the restricted stock and the promissory notes, the Company determined to treat the entire amount of the promissory notes related to the restricted stock as nonrecourse for accounting purposes. A nonrecourse note issued for restricted stock is in substance an option to acquire the stock. Accordingly, the Company recorded compensation expense for the restricted stock grants and the promissory notes and the restricted stock were not then recorded in the financial statements. The compensation expense was determined under the Black-Scholes-Merton model assuming a risk free interest rate of 0.0% (as the interest rate on the promissory notes was greater than the risk free interest rate and the excess was not significant to the Black-Scholes-Merton valuation — risk free interest rate ranging from 4.08% to 4.96% less the stated interest rate of 8% implicit in the promissory notes), a volatility factor ranging from 57.1% to 66.5% and a 6.25 year estimated life. The value of the common stock was initially determined by the Company’s board of directors and was validated as reasonable on a retrospective basis in a March 2007 valuation by an independent valuation firm.
On September 5, 2007, the Company forgave approximately $1,149,000 of outstanding loans, including accrued interest of $113,000, to its CEO, which represents all loans outstanding to the Company’s CEO. Of this amount $949,000 was associated with the issuances of the restricted and unrestricted stock and $200,000 was associated with the employee loans discussed above. In connection with the forgiveness of the loans, 35,244 shares of common stock were surrendered by the CEO to the Company to pay for the payroll taxes associated with the taxable income from the forgiveness of the loans. The forgiveness of the notes receivable resulted in a modification to the original award. Accordingly, the Company accounted for the modification by determining the amount of the incremental compensation charge to be recorded in accordance with ASC 718-20-35. The original award, which was accounted for as a stock option, was revalued on the date of modification using the Black-Scholes-Merton model with current inputs for risk-free rate, volatility and market value. This calculated amount was compared to the fair value of the restricted stock award on the date of modification resulting in the incremental charge. Due to the forgiveness of the note, the Company ceased to record the award as a stock option and commenced the recording of the award as a restricted stock award. Accordingly, on the date of modification, the Company recognized the incremental charge for the portion of the vested shares and is recording the additional portion related to the unvested shares over the remaining term. The forgiveness resulted in a modification to the original terms of the restricted stock-based awards with a charge of approximately $395,000 recorded in the financial statements in September 2007. The remaining unrecognized compensation expense of approximately $533,000 relating to the unvested restricted stock will be recorded in the financial statements over the remaining vesting period, along with the related vested common stock. The compensation expense associated with the modification of the terms of the restricted stock was determined under the Black-Scholes-Merton model assuming a risk free interest rate of 0.0% (as the interest rate on the promissory notes was greater than the risk free interest rate and the excess was not significant to the Black-Scholes-Merton valuation — risk free interest rate of 4.29% less the stated interest rate of 8% implicit in the promissory notes), a volatility factor of 54.07% and
94
an estimated life ranging from 4.10 to 5.80 years. The value of the common stock on the modification date was determined in an August 2007 valuation by an independent valuation firm.
See Note 8 for further discussion of restricted stock.
Securities Purchase Agreement
In October 2008, the Company entered into a Securities Purchase Agreement for an equity financing of up to approximately $60 million, with initial gross proceeds of approximately $40.2 million, which the Company closed on October 31, 2008, and conditional access to an additional $20 million. The financing resulted in net proceeds to the Company of approximately $39.7 million, after expenses of approximately $525,000. In connection with the financing, the Company issued and sold to the participating investors 6,451,613 shares of its common stock at a purchase price of $6.20 per share and issued to participating investors, at the purchase price of $0.125 per warrant, warrants to purchase 1,290,323 shares of common stock at an exercise price of $7.44 per share. The warrants became exercisable on April 29, 2009 and have a seven-year term.
Subject to the Company’s satisfaction of certain business related milestones before December 31, 2009, the Company had the right (the “Call Right”) to require certain participants in the financing to purchase an additional $20 million of common stock and warrants to purchase common stock. The Company did not exercise its Call Right, which expired on December 31, 2009, to require these participants to purchase an additional $20 million of common stock. At the initial closing, the investors that agreed to provide the additional $20 million investment received warrants to purchase an additional 322,581 shares of common stock at a purchase price of $0.125 per warrant and an exercise price of $6.20 per share. These warrants became exercisable on December 31, 2009 and have a seven-year term.
The participating investors consisted of eleven accredited investors, six of which were existing stockholders of the Company who were deemed to be affiliates of the Company by virtue of their being represented on the Company’s Board of Directors or by virtue of their Board membership.
6. Intangible Assets
The Company’s intangible assets are comprised of a purchased patent application and licenses to intellectual property rights (the “Licenses”). The Licenses are amortized on a straight line basis over their estimated useful lives which range from approximately 5 to 13 years. See Note 7 for additional discussion of Licenses.
The following tables present details of MAKO’s intangible assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
||||||||||
|
(in thousands) |
|
2009 |
|
2008 |
|
||||||||
|
|
|
Amount |
|
Weighted |
|
Amount |
|
Weighted |
|
||||
|
Licenses |
|
$ |
6,679 |
|
|
9.9 |
|
$ |
6,549 |
|
|
10.0 |
|
|
Patent |
|
|
― |
|
|
|
|
|
60 |
|
|
17.8 |
|
|
|
|
|
6,679 |
|
|
9.9 |
|
|
6,609 |
|
|
10.1 |
|
|
Less: accumulated amortization |
|
|
(2,445 |
) |
|
|
|
|
(1,792 |
) |
|
|
|
|
Intangible assets, net |
|
$ |
4,234 |
|
|
|
|
$ |
4,817 |
|
|
|
|
Amortization expense related to intangible assets was approximately $682,000, $660,000 and $645,000 for the years ended December 31, 2009, 2008 and 2007, respectively.
95
The estimated future amortization expense of intangible assets for the next five years as of December 31, 2009 is as follows:
|
|
|
|
|
|
|
(in thousands) |
|
|
|
|
|
2010 |
|
$ |
688 |
|
|
2011 |
|
|
688 |
|
|
2012 |
|
|
688 |
|
|
2013 |
|
|
683 |
|
|
2014 |
|
|
655 |
|
|
Total |
|
$ |
3,402 |
|
7. Commitments and Contingencies
Operating Leases
The Company leases its facility under an operating lease that expires in July 2011. The Company has the option to renew its facility lease for two consecutive three year periods. Rent expense on a straight-line basis was $613,000, $498,000 and $314,000 for the years ended December 31, 2009, 2008 and 2007, respectively. The rent expense for the years ended December 31, 2009, 2008 and 2007 included the Company’s monthly variable operating costs of the facility.
Future minimum lease commitments, excluding monthly variable operating costs, under the Company’s operating lease as of December 31, 2009 are approximately as follows:
|
|
|
|
|
|
|
(in thousands) |
|
|
|
|
|
2010 |
|
|
400 |
|
|
2011 |
|
|
239 |
|
|
|
|
$ |
639 |
|
Purchase Commitments
At December 31, 2009, the Company was committed to make future purchases for inventory related items under various purchase arrangements with fixed purchase provisions aggregating approximately $7.1 million.
License and Royalty Agreements
In December 2004, the Company was granted a limited license to Z-Kat, Inc.’s (“Z-Kat”) computer assisted surgery (“CAS”) and haptic robotic intellectual property portfolio for use in the field of orthopedics (the “Z-Kat License”). In December 2006, the Company entered into an addendum to the Z-Kat License (the “Addendum”). Under the Addendum, the Company obtained the right to take enforcement action against all third parties with respect to any intellectual property rights held by Z-Kat in the field of orthopedics; and MAKO assumed the obligation to pay the annual minimum royalty to a third-party CAS licensor due to the importance of maintaining the licensed rights. The Z-Kat License is fully paid up as to intellectual property owned by Z-Kat. The Z-Kat License includes sublicenses to third-party intellectual property rights for which the Company is obligated to make ongoing royalty payments of 2% on the sale of certain products or components thereof and minimum annual payments totaling $555,000. By their terms, the Z-Kat License and the component sublicenses generally continue until all of the licensed patents have expired, which, based on the licensed granted patents and presently pending patent applications is currently estimated to be December 2024.
In December 2008, a third-party CAS intellectual property licensee of Z-Kat from which MAKO had the right to receive royalty payments under the Addendum terminated its license. Accordingly, the Company no longer receives royalties under this license.
96
See Note 11 for further discussion of the Z-Kat License and Addendum.
In March 2006, the Company entered into a license agreement that covers a number of technologies related to the application of computers and robotics to surgery in exchange for a payment of $2 million upon execution of the agreement (the “Upfront License Fee”) and a deferred payment of $4 million payable upon a change of control, as defined (e.g., IPO, acquisition or change in voting ownership greater than 50.01%) (the “Deferred License Fee”). The license also requires royalty payments of 2% of the selling price of each RIO system. The Upfront License Fee and net present value of the Deferred License Fee were included in intangible assets in the accompanying balance sheets. The net present value of the Deferred License Fee obligation was approximately $3.4 million, net of a discount of $590,000 and was recorded as a long-term debt obligation as the Company believed it was probable at the inception of the agreement that the contingent obligation would become payable. The net present value of the Deferred License Fee was determined using an incremental borrowing rate of 8% and an expected payment date of approximately two years from the effective date of the license agreement. The discount on the debt obligation was being amortized over the estimated term of the Deferred License Fee obligation as interest expense which was approximately $45,000 and $305,000 for the years ended December 31, 2008 and 2007, respectively, in the accompanying statements of operations. In February 2008, the Company paid the $4 million Deferred License Fee due upon completion of the Company’s IPO.
In May 2009, the Company entered into a license agreement for patents relating to its RIO system (the “Robotic Arm License”). The Robotic Arm License requires minimum running royalties on sales of the Company’s RIO systems. The minimum running royalties are estimated to be approximately $600,000 for the year ended December 31, 2010, and increase annually thereafter through 2013. The minimum running royalties for the year ended December 31, 2013 and for each subsequent year through the term of the agreement are estimated to be approximately $1.3 million annually.
The Company has other license agreements related to current product offerings and research and development projects. Upfront license fees paid for these agreements total approximately $1.1 million. Royalty payments related to these agreements are anticipated to range between 1% and 5% of future sales of the Company’s RIO system and components thereof and/or products. These royalty payments are subject to certain minimum annual royalty payments as shown in the schedule below. The terms of these license agreements continue until the related licensed patents and intellectual property rights expire, which is expected to range between 7 and 17 years. The net expense related to the Company’s license and royalty agreements was approximately $1.5 million, $525,000 and $304,000 for the years ended December 31, 2009, 2008 and 2007, respectively.
As of December 31, 2009, future annual minimum royalty payments under the licenses and sublicenses are anticipated to be as follows:
|
|
|
|
|
|
|
(in thousands) |
|
|
|
|
|
2010 |
|
$ |
1,258 |
|
|
2011 |
|
|
1,499 |
|
|
2012 |
|
|
1,859 |
|
|
2013 |
|
|
1,689 |
|
|
2014 |
|
|
1,396 |
|
|
Thereafter |
|
|
4,023 |
|
|
|
|
$ |
11,724 |
|
Development Agreement
In June 2009, the Company entered into a Research and Development License and Supply Agreement, or the R&D Agreement, associated with a potential future product for RIO enabled hip MAKOplasty procedures. The R&D Agreement required an up-front payment of $450,000, and requires future milestone payments based on development progress. The aggregate milestone payments the Company is obligated to pay under the R&D Agreement are $1.6 million assuming the achievement of all development milestones. Through December 31,
97
2009, the Company paid the $450,000 up-front payment and the Company paid $550,000 of milestone payments which became due upon the achievement of the related milestones. The aggregate up-front payment and milestone payments of $2.0 million the Company is required to pay under the R&D Agreement will be recognized as research and development expense on a straight-line basis over the period development services are performed based on the current expectation that all development milestones will be achieved.
Contingencies
The Company is a party to legal contingencies or claims arising in the normal course of business, none of which the Company believes is material to its financial position, results of operations or cash flows.
8. Preferred Stock and Stockholders’ Equity
Preferred Stock
As of December 31, 2009 and 2008, the Company was authorized to issue 27,000,000 shares of $0.001 par value preferred stock. As of December 31, 2009 and 2008, there were no shares of preferred stock issued or outstanding. All shares of Series A, B and C redeemable convertible preferred stock that were issued and outstanding as of December 31, 2007 converted into 10,945,080 shares of common stock upon closing of the Company’s IPO in February 2008.
Common Stock
As of December 31, 2009 and 2008, the Company was authorized to issue 135,000,000 shares of $0.001 par value common stock. Common stockholders are entitled to dividends as and if declared by the Board of Directors, subject to the rights of holders of all classes of stock outstanding having priority rights as to dividends. There have been no dividends declared to date on the common stock. The holder of each share of common stock is entitled to one vote.
In December 2004, the Company issued 189,768 shares of restricted common stock to certain consultants (the “Consultant Restricted Stock”). The Consultant Restricted Stock vested in tranches upon the Company’s achievement of certain business milestones and any unvested restricted stock vested immediately upon completion of an initial public offering of common stock. Upon vesting, the Company recorded a consulting expense equal to the estimated fair value of the Company’s common stock on the date of vesting. As of January 1, 2008, 94,884 shares of the Consultant Restricted Stock were unvested. Upon closing of the IPO in February 2008, the vesting of the remaining 94,884 shares of Consultant Restricted Stock was accelerated and the Company recognized $949,000 of compensation expense associated with the accelerated vesting of the Consultant Restricted Stock during the year ended December 31, 2008 based on the IPO price of $10.00 per share.
401K Plan
The Company maintains a qualified deferred compensation plan under Section 401K of the Internal Revenue Code, covering substantially all full-time employees, which permits employees to contribute up to 84% of pre-tax annual compensation up to annual statutory limitations. The discretionary company match for employee contributions to the plan is 25% of up to the first 6% of the participant’s earnings contributed to the plan. The discretionary company match commenced in 2008 and to date has not been significant.
Employee Stock Purchase Plan
In January 2008, the Company’s Board of Directors and stockholders approved the MAKO Surgical Corp. 2008 Employee Stock Purchase Plan (the “2008 Employee Stock Purchase Plan”). The 2008 Employee Stock Purchase Plan became effective upon closing of the IPO. The 2008 Employee Stock Purchase Plan authorizes the issuance of 625,000 shares of the Company’s common stock for purchase by eligible employees of the Company or any of its participating affiliates. The shares of common stock issuable under the 2008 Employee Stock
98
Purchase Plan may be authorized but unissued shares, treasury shares or shares purchased on the open market. The purchase price for a purchase period may not be less than 85% of the fair market value of the Company’s common stock on the first trading day of the applicable purchase period or the last trading day of such purchase period, whichever is lower. The initial purchase period of the 2008 Employee Stock Purchase Plan began on October 1, 2008. During the year ended December 31, 2009, the Company issued approximately 72,000 shares under the 2008 Employee Stock Purchase Plan. As of December 31, 2009, there were approximately 553,000 shares reserved for future grant under the 2008 Employee Stock Purchase Plan.
Stock Option Plans and Stock-Based Compensation
The Company recognizes compensation expense for its stock-based awards in accordance with ASC 718, Compensation-Stock Compensation. ASC 718 requires the recognition of compensation expense, using a fair value based method, for costs related to all stock-based payments including stock options. ASC 718 requires companies to estimate the fair value of stock-based payment awards on the date of grant using an option-pricing model.
During the year ended December 31, 2009, 2008 and 2007, stock-based compensation expense was $4.0 million, $3.3 million and $1.2 million respectively. Included within stock-based compensation expense for the year ended December 31, 2009 were $2.9 million related to stock option grants, $982,000 related to the partial vesting of shares of restricted stock granted to the Company’s CEO at various dates from 2005 through 2009, and $164,000 related to employee stock purchases under the 2008 Employee Stock Purchase Plan.
In December 2004, the Company’s stockholders approved the Company’s 2004 Stock Incentive Plan (the “2004 Plan”). Under the 2004 Plan, the Board of Directors was authorized to grant restricted common stock and options to purchase shares of common stock to employees, directors and consultants. No further awards will be made under the 2004 Plan. In January 2008, the Company’s Board of Directors and stockholders approved the MAKO Surgical Corp. 2008 Omnibus Incentive Plan (the “2008 Plan,” and together with the 2004 Plan, the “Plans”). The 2008 Plan became effective upon the closing of the IPO and will expire January 9, 2018 unless earlier terminated by the Board of Directors. The aggregate number of shares of the Company’s common stock that may be issued initially pursuant to stock awards under the 2008 Plan is 1,084,703 shares, which includes approximately 85,000 shares previously reserved but unallocated under the 2004 Plan. Awards under the 2008 Plan may be made in the form of: stock options, which may be either incentive stock options or non-qualified stock options; stock appreciation rights; restricted stock; restricted stock units; dividend equivalent rights; performance shares; performance units; cash-based awards; other stock-based awards, including unrestricted shares; and any combination of the foregoing.
The 2008 Plan contains an evergreen provision whereby the authorized shares increase on January 1st of each year in an amount equal to the least of (1) four percent (4%) of the total number of shares of the Company’s common stock outstanding on December 31st of the preceding year, (2) 2.5 million shares and (3) a number of shares determined by the Company’s Board of Directors that is lesser than (1) and (2). The number of additional shares authorized under the 2008 Plan on January 1, 2009 and 2010 was approximately 998,000 and 1,330,000, respectively.
Generally, the Company’s outstanding stock options vest over four years. Stock options granted to certain non-employee directors generally vest over three years. Continued vesting typically terminates when the employment or consulting relationship ends. Vesting generally begins on the date of grant; however, certain stock options granted in 2007 began vesting upon the achievement of performance conditions.
Under the terms of the Plans, the maximum term of options intended to be incentive stock options granted to persons who own at least 10% of the voting power of all outstanding stock on the date of grant is 5 years. The maximum term of all other options is 10 years. Options issued under the 2008 Plan that are forfeited or expire will again be made available for issuing grants under the 2008 Plan. Options issued under the 2004 Plan that are forfeited or expire will not be made available for issuing grants under the 2008 Plan. All future awards will be made under the Company’s 2008 Plan.
99
As of December 31, 2009, the Company had reserved shares of common stock for the issuance of common stock under the 2008 Employee Stock Purchase Plan, the exercise of warrants and the issuance of options granted under the 2008 Plan as follows:
|
|
|
|
|
|
|
(in thousands) |
|
|
|
|
|
2008 Employee Stock Purchase Plan |
|
|
625 |
|
|
Warrants to purchase common stock |
|
|
2,076 |
|
|
2008 Plan |
|
|
2,083 |
|
|
|
|
|
4,784 |
|
Only employees are eligible to receive incentive stock options. Non-employees may be granted non-qualified options. The Board of Directors has the authority to set the exercise price of all options granted, subject to the exercise price of incentive stock options being no less than 100% of the estimated fair value, as determined by the Board of Directors, of a share of common stock on the date of grant; and no less than 85% of the estimated fair value for non-qualified stock options, except for an employee or non-employee with options who owns more than 10% of the voting power of all classes of stock of the Company, in which case the exercise price shall be no less than 110% of the fair market value per share on the grant date. Options become exercisable as determined by the Board of Directors.
Activity under the Plans is summarized as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except per share data) |
|
|
|
Outstanding Options |
|
|||||
|
|
|
Shares/Options |
|
Number of |
|
Weighted |
|
|||
|
Balance at December 31, 2008 |
|
|
733 |
|
|
2,193 |
|
|
5.56 |
|
|
Shares reserved |
|
|
998 |
|
|
― |
|
|
― |
|
|
Restricted stock issued |
|
|
(100 |
) |
|
― |
|
|
― |
|
|
Options granted |
|
|
(1,482 |
) |
|
1,482 |
|
|
8.03 |
|
|
Options exercised |
|
|
― |
|
|
(133 |
) |
|
1.13 |
|
|
Options forfeited under the 2004 Plan |
|
|
― |
|
|
(39 |
) |
|
9.25 |
|
|
Options forfeited under the 2008 Plan |
|
|
25 |
|
|
(25 |
) |
|
8.30 |
|
|
Balance at December 31, 2009 |
|
|
174 |
|
|
3,478 |
|
$ |
6.71 |
|
The options outstanding and exercisable, by exercise price, at December 31, 2009 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Options Outstanding |
|
Options Exercisable |
|
||||||||||||||||
|
(in thousands, |
|
Number |
|
Weighted |
|
Weighted |
|
Aggregate |
|
Number |
|
Weighted |
|
Weighted |
|
Aggregate |
|
||||
|
Range of Exercise Prices: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
$0.67 |
|
241 |
|
|
|
$ |
0.67 |
|
|
|
|
241 |
|
|
|
$ |
0.67 |
|
|
|
|
|
$1.27 – $2.48 |
|
798 |
|
|
|
$ |
1.72 |
|
|
|
|
680 |
|
|
|
$ |
1.63 |
|
|
|
|
|
$6.90 – $8.06 |
|
1,483 |
|
|
|
$ |
7.94 |
|
|
|
|
282 |
|
|
|
$ |
7.98 |
|
|
|
|
|
$8.27 – 11.39 |
|
956 |
|
|
|
$ |
10.49 |
|
|
|
|
383 |
|
|
|
$ |
10.57 |
|
|
|
|
|
|
|
3,478 |
|
7.82 |
|
$ |
6.71 |
|
$ |
15,271 |
|
1,586 |
|
6.80 |
|
$ |
4.77 |
|
$ |
10,041 |
|
100
|
|
|
|
(1) |
The aggregate intrinsic value represents the total pre-tax intrinsic value, based on the Company’s closing stock price of $11.10 on December 31, 2009, which would have been received by the option holders had all option holders exercised their options as of that date. |
As of December 31, 2009, approximately 3,390,000 options were vested and expected to vest at a weighted average exercise price of $6.67 per share, a weighted average contractual life of 7.8 years and aggregate intrinsic value of $15.0 million.
The weighted average fair values of options granted were $4.43, $5.08 and $4.54 for the years ended December 31, 2009, 2008 and 2007, respectively. The total fair value of shares vested was approximately $2.8 million, $1.3 million and $260,000 during the years ended December 31, 2009, 2008 and 2007, respectively. The total intrinsic value of options exercised was $1.0 million and $491,000 for the years ended December 31, 2009 and 2008. The total intrinsic value of options exercised was not significant for the year ended December 31, 2007.
The Company records stock-based compensation expense on a straight-line basis over the vesting period. As of December 31, 2009, there was total unrecognized compensation cost of approximately $7.9 million, net of estimated forfeitures, related to non-vested stock option grants to the Company’s employees and non-employee directors. The unrecognized compensation cost will be adjusted for future changes in estimated forfeitures, and is expected to be recognized over a remaining weighted average period of 2.8 years as of December 31, 2009.
On May 22, 2009, the Company issued 100,000 shares of restricted stock to its CEO at an estimated fair value of $8.70 per share on the date of issuance. The restricted stock will vest over a four-year period. For the year ended December 31, 2009, 56,045 shares of common stock were surrendered by the CEO to the Company to cover payroll taxes associated with the taxable income from the vesting of restricted stock previously granted to the Company’s CEO. As of December 31, 2009, 755,105 shares of restricted stock granted to the Company’s CEO were issued and outstanding.
Restricted stock activity for the year ended December 31, 2009 is as follows:
|
|
|
|
|
|
|
|
|
(in thousands, except per share data) |
|
Shares |
|
Weighted Average |
|
|
|
Unvested shares at December 31, 2008 |
|
267 |
|
$ |
7.78 |
|
|
Unvested shares at December 31, 2009 |
|
222 |
|
|
8.86 |
|
|
Shares granted in 2009 |
|
100 |
|
|
8.70 |
|
|
Shares vested in 2009 |
|
145 |
|
|
6.77 |
|
As of December 31, 2009, the remaining stock-based compensation expense for the restricted stock awards was approximately $2.0 million, which will be recognized on a straight line basis over a remaining weighted average period of 2.30 years.
The Company uses the Black-Scholes-Merton pricing model to determine the fair value of stock options. The determination of the fair value of stock-based payment awards on the date of grant using a pricing model is affected by our stock price as well as assumptions regarding a number of complex and subjective variables. These variables include our expected stock price volatility over the term of the awards, actual and projected employee stock option exercise behaviors, risk-free interest rates and expected dividends.
101
The estimated grant date fair values of the employee stock options were calculated using the Black-Scholes-Merton valuation model, based on the following assumptions:
|
|
|
|
|
|
|
|
|
Stock Option Plans |
|
Years Ended December 31, |
||||
|
|
|
2009 |
|
2008 |
|
2007 |
|
Risk-free interest rate |
|
1.99% - 3.53% |
|
1.59% - 3.62% |
|
4.50% - 5.14% |
|
Expected life |
|
6.25 years |
|
6.25 years |
|
6.25 years |
|
Expected dividends |
|
― |
|
― |
|
― |
|
Expected volatility |
|
54.43% - 57.71% |
|
56.36% - 58.31% |
|
56.24% - 60.00% |
The Company estimates the fair value of each share of stock which will be issued under the 2008 Employee Stock Purchase Plan based upon its stock prices at the beginning of each offering period using a Black-Scholes-Merton pricing model and amortizes that value to expense over the plan purchase period. The fair values determined for the years ended December 31, 2009 and 2008, as well as the assumptions used in calculating those values are as follows:
|
|
|
|
|
|
|
|
|
2008 Employee Stock Purchase Plan |
|
|
|
Year Ended |
|
Year Ended |
|
|
|
|
|
2009 |
|
2008 |
|
Fair Value |
|
|
|
$1.82 - $2.54 |
|
$1.82 - $1.89 |
|
Assumptions |
|
|
|
|
|
|
|
Risk-free interest rate |
|
|
|
0.60% - 3.20% |
|
1.87% - 3.29% |
|
Expected life |
|
|
|
0.25 years |
|
0.25 years |
|
Expected dividends |
|
|
|
― |
|
― |
|
Expected volatility |
|
|
|
34.50% - 60.68% |
|
57.05% - 60.68% |
Risk-Free Interest Rate. The risk-free rate is based on U.S. Treasury zero-coupon issues with remaining terms similar to the expected term on the options.
Weighted-Average Expected Term. The expected term of options granted is determined using the average period the stock options are expected to remain outstanding and is based on the options vesting term, contractual terms and historical exercise and vesting information used to develop reasonable expectations about future exercise patterns and post-vesting employment termination behavior. The expected term of the 2008 Employee Stock Purchase Plan is equal to the duration of the purchase period.
Dividend Yield. The Company has never declared or paid any cash dividends and does not plan to pay cash dividends in the foreseeable future, and, therefore, used an expected dividend yield of zero in the valuation model.
Volatility. Since the Company was a private entity until February 2008 with no historical data regarding the volatility of its common stock, the expected volatility used for the years ended December 31, 2009, 2008 and 2007, is based on volatility of similar entities, referred to as “guideline” companies. In evaluating similarity, the Company considered factors such as industry, stage of life cycle and size.
Forfeitures. ASC 718 requires the Company to estimate forfeitures at the time of grant, and revise those estimates in subsequent periods if actual forfeitures differ from those estimates. The Company uses historical data to estimate pre-vesting option forfeitures and records stock-based compensation expense only for those awards that are expected to vest. All stock-based payment awards are amortized on a straight-line basis over the requisite service periods of the awards, which are generally the vesting periods. If the Company’s actual forfeiture rate is materially different from its estimate, the stock-based compensation expense could be significantly different from what the Company has recorded in the accompanying periods.
102
Warrants
In December 2004, the Company issued at the purchase price of $0.03 per share warrants to purchase 462,716 shares of common stock. The warrants are immediately exercisable at an exercise price of $3.00 per share, with the exercise period expiring in December 2014. As of December 31, 2009 and 2008, 451,916 and 462,716 warrants were outstanding and exercisable, respectively.
As more fully described in Note 5, in October 2008, the Company entered into a Securities Purchase Agreement for an equity financing of up to $60 million, with initial gross proceeds of approximately $40.2 million, and conditional access to an additional $20 million. In connection with the financing, the Company issued warrants to the participating investors to purchase 1,290,323 shares of common stock at a purchase price of $0.125 per warrant and an exercise price of $7.44 per share. The warrants became exercisable on April 29, 2009 and have a seven-year term. In addition, as consideration for the Call Right, the Company issued warrants to purchase 322,581 shares of common stock at a purchase price of $0.125 per warrant and an exercise price of $6.20 per share to participating investors. These warrants became exercisable on December 31, 2009 and have a seven-year term.
9. Income Taxes
The provision for income taxes is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
Years Ended |
|
|||||||
|
|
|
December 31, |
|
December 31, |
|
December 31, |
|
|||
|
Current income taxes: |
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
― |
|
$ |
― |
|
$ |
― |
|
|
State |
|
|
― |
|
|
― |
|
|
― |
|
|
Total current income taxes |
|
|
― |
|
|
― |
|
|
― |
|
|
Deferred income taxes |
|
|
(12,593 |
) |
|
(13,031 |
) |
|
(7,835 |
) |
|
Change in valuation allowance |
|
|
12,593 |
|
|
13,031 |
|
|
7,835 |
|
|
Provision for income taxes |
|
$ |
― |
|
$ |
― |
|
$ |
― |
|
The Company accounts for income taxes under ASC 740, Income Taxes. Deferred income taxes are determined based upon differences between financial reporting and income tax bases of assets and liabilities and are measured using the enacted income tax rates and laws that will be in effect when the differences are expected to reverse. The Company recognizes any interest and penalties related to unrecognized tax benefits as a component of income tax expense.
No current or deferred income taxes were recorded for the years ended December 31, 2009, 2008 and 2007, as the Company’s income tax benefits were fully offset by a corresponding increase to the valuation allowance against its net deferred income tax assets.
At December 31, 2009, 2008 and 2007, the Company had federal and state net operating loss carryforwards of approximately $100.2 million, $60 million and $32.9 million, respectively, available to offset future taxable income. These net operating loss carryforwards will expire in varying amounts from 2024 through 2029.
The Tax Reform Act of 1986 limits the annual utilization of net operating loss and tax credit carryforwards, following an ownership change of the Company. Note that as a result of the Company’s equity financings in 2008, the Company underwent a change of ownership for purposes of the Tax Reform Act during the tax year ended December 31, 2008.
103
Deferred income taxes reflect the net tax effect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s net deferred income taxes are as follows:
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
December 31, |
|
||||
|
|
|
2009 |
|
2008 |
|
||
|
Current deferred income tax assets: |
|
|
|
|
|
|
|
|
Deferred revenue |
|
$ |
212 |
|
$ |
4,443 |
|
|
Total current deferred income tax assets |
|
|
212 |
|
|
4,443 |
|
|
|
|
|
|
|
|
|
|
|
Noncurrent deferred income tax assets: |
|
|
|
|
|
|
|
|
Net operating loss carryforwards |
|
|
38,650 |
|
|
23,144 |
|
|
Amortization |
|
|
404 |
|
|
322 |
|
|
Other |
|
|
481 |
|
|
656 |
|
|
Total noncurrent deferred income tax assets |
|
|
39,535 |
|
|
24,122 |
|
|
|
|
|
|
|
|
|
|
|
Current deferred income tax liabilities: |
|
|
|
|
|
|
|
|
Other deferred income tax liabilities |
|
|
(2 |
) |
|
― |
|
|
Deferred costs |
|
|
― |
|
|
(1,392 |
) |
|
Total current deferred income tax liabilities |
|
|
(2 |
) |
|
(1,392 |
) |
|
|
|
|
|
|
|
|
|
|
Noncurrent deferred income tax liabilities: |
|
|
|
|
|
|
|
|
Other deferred income tax liabilities |
|
|
― |
|
|
(21 |
) |
|
Total noncurrent deferred income tax liabilities |
|
|
― |
|
|
(21 |
) |
|
|
|
|
|
|
|
|
|
|
Less valuation allowance |
|
|
(39,745 |
) |
|
(27,152 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred income tax assets, net |
|
$ |
― |
|
$ |
― |
|
Due to uncertainty surrounding realization of the deferred income tax assets in future periods, the Company has recorded a 100% valuation allowance against its net deferred tax assets. If it is determined in the future that it is more likely than not that the deferred income tax assets are realizable, the valuation allowance will be reduced.
The reconciliation of the income tax provision computed at the U.S. federal statutory rate to income tax provision is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended |
|
||||
|
|
|
December 31, |
|
December 31, |
|
December 31, |
|
|
|
|
|
|
|
|
|
|
|
Tax at U.S. statutory rate |
|
(35.00 |
)% |
(35.00 |
)% |
(35.00 |
)% |
|
State taxes, net of federal impact |
|
(3.28 |
)% |
(3.26 |
)% |
(3.55 |
)% |
|
Non-deductible items |
|
2.92 |
% |
3.13 |
% |
0.28 |
% |
|
Change in valuation allowance |
|
35.13 |
% |
35.14 |
% |
37.93 |
% |
|
Other, net |
|
0.23 |
% |
(0.01 |
)% |
0.34 |
% |
|
Effective income tax rate |
|
0.00 |
% |
0.00 |
% |
0.00 |
% |
In accordance with ASC 740, the Company has decided to classify any interest and penalties as a component of income tax expense. To date, there have been no interest or penalties charged to the Company in relation to the
104
underpayment of income taxes. The Company’s primary tax jurisdictions are the United States, Florida, California, Rhode Island, Ohio, New York, Georgia, Texas, Illinois, North Carolina, Pennsylvania, Maryland, Colorado, Oklahoma, Washington, West Virginia, Wisconsin, Michigan, Mississippi and Tennessee. The tax years from 2005 through 2009 remain open and are subject to examination by the appropriate governmental agencies.
10. Selected Quarterly Data (Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except per share data) |
|
2009 |
|
||||||||||
|
|
|
Q1 |
|
Q2 |
|
Q3 |
|
Q4 |
|
||||
|
Revenue |
|
$ |
3,727 |
|
$ |
14,904 |
|
$ |
6,726 |
|
$ |
8,851 |
|
|
Gross profit (loss) |
|
|
655 |
|
|
4,622 |
|
|
2,765 |
|
|
4,462 |
|
|
Loss from operations |
|
|
(9,107 |
) |
|
(6,491 |
) |
|
(9,498 |
) |
|
(9,356 |
) |
|
Net loss |
|
|
(8,885 |
) |
|
(6,424 |
) |
|
(9,439 |
) |
|
(9,275 |
) |
|
Net loss attributable to common stockholders |
|
|
(8,885 |
) |
|
(6,424 |
) |
|
(9,439 |
) |
|
(9,275 |
) |
|
Net loss per share – basic and diluted attributable to common stockholders |
|
|
(0.36 |
) |
|
(0.26 |
) |
|
(0.33 |
) |
|
(0.28 |
) |
Revenue for the first and second quarter of 2009 includes approximately $2.5 million and $8.8 million, respectively, of revenue from previously deferred unit sales of our TGS. In accordance with our revenue recognition policy, recognition of revenue on unit sales of our TGS was deferred until delivery of the RIO system, which we commercially released in the first quarter of 2009.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except per share data) |
|
2008 |
|
||||||||||
|
|
|
Q1 |
|
Q2 |
|
Q3 |
|
Q4 |
|
||||
|
Revenue |
|
$ |
498 |
|
$ |
704 |
|
$ |
777 |
|
$ |
965 |
|
|
Gross profit (loss) |
|
|
128 |
|
|
224 |
|
|
(584 |
) |
|
(270 |
) |
|
Loss from operations |
|
|
(8,552 |
) |
|
(7,805 |
) |
|
(10,443 |
) |
|
(11,160 |
) |
|
Net loss |
|
|
(8,501 |
) |
|
(7,565 |
) |
|
(10,202 |
) |
|
(10,814 |
) |
|
Net loss attributable to common stockholders |
|
|
(9,066 |
) |
|
(7,565 |
) |
|
(10,202 |
) |
|
(10,814 |
) |
|
Net loss per share – basic and diluted attributable to common stockholders |
|
|
(0.95 |
) |
|
(0.42 |
) |
|
(0.56 |
) |
|
(0.48 |
) |
11. Subsequent Event
In February 2010, the Company completed the acquisition of substantially all of the intellectual property portfolio of Z-Kat. The terms of the Asset Purchase Agreement between the Company and Z-Kat (the “Asset Purchase Agreement”) terminated the Company’s prior licenses with Z-Kat, including Z-Kat’s nonexclusive sublicense to the Company’s intellectual property portfolio, and transferred to the Company ownership rights to certain intellectual property assets for core technologies in CAS, haptics and robotics, including U.S. and foreign patents and patent applications, proprietary software and documentation, trade secrets and trademarks owned by Z-Kat, and certain contractual and other rights to patents, patent applications and other intellectual property licensed to Z-Kat under licenses. In connection with the acquisition, the Company also entered into a new license agreement with Z-Kat (the “License Agreement”) pursuant to which the Company obtained an exclusive worldwide, fully transferable, perpetual, royalty-free and fully paid-up sublicense to certain intellectual property for technologies in CAS licensed by Z-Kat. This new License Agreement expands the Company’s rights in this intellectual property from the field of orthopedics to the medical field generally. Certain of the Company’s rights under the Asset Purchase Agreement and License Agreement remain subject to any prior license granted by Z-Kat, including the license to Biomet Manufacturing Corp. In consideration for consummation of the transactions
105
contemplated by the Asset Purchase Agreement and License Agreement, the Company issued 230,458 shares of its unregistered common stock to Z-Kat in a private placement. The Asset Purchase Agreement and License Agreement, the entry into which was deemed to be a related party transaction as certain directors and executive officers of the Company have a material interest in Z-Kat by virtue of their ownership of Z-Kat stock, were approved by the board of directors and audit committee of the Company.
|
|
|
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
|
|
|
|
CONTROLS AND PROCEDURES |
In accordance with Rule 13a-15(b) of the Securities Exchange Act of 1934, or the Exchange Act, our management evaluated, with the participation of our chief executive officer and chief financial officer, or the Certifying Officers, the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of December 31, 2009. Based upon their evaluation of these disclosure controls and procedures, our Certifying Officers concluded that the disclosure controls and procedures were effective as of December 31, 2009 to provide reasonable assurance that information required to be disclosed by us in the reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time period specified in the SEC rules and forms, and to provide reasonable assurance that information required to be disclosed by us in the reports we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive and principal financial officers, as appropriate, to allow timely decisions regarding required disclosure.
We believe that a controls system, no matter how well designed and operated, is based in part upon certain assumptions about the likelihood of future events, and therefore can only provide reasonable, not absolute, assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) and 15d-15(f) promulgated under the Securities Exchange Act of 1934, as amended, as a process designed by, or under the supervision of, a company’s principal executive and financial officers, or the certifying officers, and effected by a company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Internal control over financial reporting includes policies and procedures that pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; provide reasonable assurance that transactions are recorded as necessary to permit preparation of our financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with the authorization of our board of directors and management; and provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Under the supervision and with the participation of our management, including the certifying officers, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on this evaluation under the criteria established in Internal Control – Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2009. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject
106
to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The effectiveness of our internal control over financial reporting as of December 31, 2009 has been audited by our independent registered public account firm, as stated in their report, which is included herein.
During the most recently completed fiscal quarter, there was no change in our internal control over financial reporting that has materially affected or is reasonably likely to materially affect, our internal control over financial reporting.
|
|
|
|
OTHER INFORMATION |
None
107
|
|
|
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required by this item will be contained under the following headings in our definitive proxy statement to be filed in connection with our 2010 annual meeting of stockholders and, upon filing with the SEC, will be incorporated herein by reference:
|
|
|
|
|
|
• |
Section 16(a) Beneficial Ownership Reporting Compliance |
|
|
|
|
|
|
• |
Election of Directors |
|
|
|
|
|
|
• |
Board of Directors and Corporate Governance |
|
|
|
|
|
|
• |
Executive Officers |
|
|
|
|
EXECUTIVE COMPENSATION |
The information required by this item will be contained under the following headings in our definitive proxy statement to be filed in connection with our 2010 annual meeting of stockholders and, upon filing with the SEC, will be incorporated herein by reference:
|
|
|
|
|
|
• |
Director Compensation |
|
|
|
|
|
|
• |
Compensation Discussion and Analysis |
|
|
|
|
|
|
• |
Compensation Committee Report |
|
|
|
|
|
|
• |
Executive Compensation |
|
|
|
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this item will be contained under the following heading in our definitive proxy statement to be filed in connection with our 2010 annual meeting of stockholders and, upon filing with the SEC, will be incorporated herein by reference:
|
|
|
|
|
|
• |
Principal Stockholders |
The information under “Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities—Equity Compensation Plan Information” in this annual report on Form 10-K is also incorporated herein by reference.
|
|
|
|
CERTAIN RELATIONSHIPS, RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE |
The information required by this item will be contained under the following heading in our definitive proxy statement to be filed in connection with our 2010 annual meeting of stockholders and, upon filing with the SEC, will be incorporated herein by reference:
|
|
|
|
|
|
• |
Board of Directors and Corporate Governance – Independent Directors |
|
|
|
|
|
|
• |
Certain Relationships and Related Person Transactions |
108
|
|
|
|
PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this item will be contained under the following heading in our definitive proxy statement to be filed in connection with our 2010 annual meeting of stockholders and, upon filing with the SEC, will be incorporated herein by reference:
|
|
|
|
|
|
• |
Ratification of the Appointment of Ernst & Young LLP as Independent Registered Public Accounting Firm |
109
|
|
|
|
EXHIBITS, FINANCIAL STATEMENTS and FINANCIAL STATEMENT SCHEDULES |
|
|
|
|
|
(a) The following documents are filed as a part of this Annual Report on Form 10-K: |
|
|
|
|
|
1. Financial Statements |
|
|
|
|
|
See Item 8, Financial Statements and Supplementary Data, Index to Financial Statements. |
|
|
|
|
|
2. Financial Statement Schedules |
|
|
|
|
No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or the notes thereto. |
|
|
|
|
|
|
(b) Exhibits |
|
|
|
|
|
|
Exhibit |
|
|
|
|
|
|
|
|
|
3.1 |
|
Third Amended and Restated Certificate of Incorporation of the Registrant, dated February 20, 2008 (2) |
|
|
|
|
|
|
|
3.2 |
|
Fourth Amended and Restated Bylaws of the Registrant effective October 31, 2008 (6) |
|
|
|
|
|
|
|
4.1 |
|
Securities Purchase Agreement by and among the Registrant and Investors named therein, dated as of October 28, 2008 (6) |
|
|
|
|
|
|
|
4.2 |
|
Form of Warrant (6) |
|
|
|
|
|
|
|
4.3 |
|
Form of Call Warrant (6) |
|
|
|
|
|
|
|
4.4 |
|
Form of Call Exercise Warrant (6) |
|
|
|
|
|
|
|
10.1 |
|
Form of Indemnity Agreement for Directors and Executive Officers (3) |
|
|
|
|
|
|
|
10.2+ |
|
2004 Stock Incentive Plan and forms of agreements related thereto (3) |
|
|
|
|
|
|
|
10.3+ |
|
2008 Omnibus Incentive Plan (3) |
|
|
|
|
|
|
|
10.4+ |
|
2008 Employee Stock Purchase Plan (3) |
|
|
|
|
|
|
|
10.5+ |
|
Amended Employment Agreement, dated as of November 12, 2007, by and between Registrant and Maurice R. Ferré, M.D (3) |
|
|
|
|
|
|
|
10.6+ |
|
Employment Agreement, dated as of January 1, 2005, by and between Registrant and Rony Abovitz (3) |
|
|
|
|
|
|
|
10.7+ |
|
Amendment to Employment Agreement, dated as of February 5, 2007, by and between Registrant and Rony Abovitz (3) |
|
|
|
|
|
|
|
10.8 |
|
Multi-Tenant Lease, by and between Registrant and Westport Business Park Associates LLP, last dated January 31, 2006 (3) |
|
|
|
|
|
|
|
10.9+ |
|
Form of Incentive Stock Option Agreement related to the 2008 Omnibus Incentive Plan (4) |
|
|
|
|
|
|
|
10.10+ |
|
Employment Agreement between Registrant and Duncan Moffat, effective as of April 28, 2008 (5) |
|
|
|
|
|
|
|
10.11+ |
|
Form of Non-Qualified Stock Option Agreement related to the 2008 Omnibus |
|
110
|
|
|
|
|
|
|
Incentive Plan (5) |
|
|
|
|
|
10.12+ |
|
Form of Restricted Stock Unit Agreement related to the 2008 Omnibus Incentive Plan (5) |
|
|
|
|
|
10.13+ |
|
Form of Subscription Agreement related to the 2008 Employee Stock Purchase Plan (5) |
|
|
|
|
|
10.14+ |
|
2009 Leadership Cash Bonus Plan (7) |
|
|
|
|
|
10.15+ |
|
2009 Performance Bonus Plan for S. Nunes – SVP of Sales & Marketing (7) |
|
|
|
|
|
10.16+ |
|
Amendment to Amended Employment Agreement by and between Registrant and Maurice R. Ferré, M.D., effective February 13, 2009 (8) |
|
|
|
|
|
10.17+ |
|
Amended and Restated Employment Agreement by and between Registrant and Fritz L. LaPorte, effective February 13, 2009 (8) |
|
|
|
|
|
10.18+ |
|
Amended and Restated Employment Agreement by and between Registrant and Menashe R. Frank, effective February 13, 2009 (8) |
|
|
|
|
|
10.19+ |
|
Amended and Restated Employment Agreement by and between Registrant and Steven J. Nunes, effective February 13, 2009 (8) |
|
|
|
|
|
10.20+ |
|
Employment Agreement by and between Registrant and Ivan Delevic, effective April 27, 2009 (9) |
|
|
|
|
|
10.21+ |
|
2010 Leadership Cash Bonus Plan (10) |
|
|
|
|
|
10.22+ |
|
2010 Performance Bonus Plan for S. Nunes – SVP of Sales & Marketing (10) |
|
|
|
|
|
10.23+ |
|
Second Amendment to Amended Employment Agreement by and between Registrant and Maurice R. Ferré, M.D., effective February 17, 2010 (10) |
|
|
|
|
|
23 |
|
Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm (1) |
|
|
|
|
|
31.1 |
|
Certification of Chief Executive Officer pursuant to Rule 13a-14(a) of the Exchange Act (1) |
|
|
|
|
|
31.2 |
|
Certification of Chief Financial Officer pursuant to Rule 13a-14(a) of the Exchange Act (1) |
|
|
|
|
|
32.1 |
|
Certification of Chief Executive Officer pursuant to18 U.S.C. §1350 (1) |
|
|
|
|
|
32.2 |
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. §1350 (1) |
|
|
|
|
|
99.1 |
|
Registration Rights Agreement by and between Registrant and Z-Kat, Inc. dated February 25, 2010 (1) |
|
|
|
|
(1) |
Filed herewith |
|
|
|
|
(2) |
Incorporated by reference to Registrant’s Annual Report on Form 10-K for the period ended December 31, 2007 filed with the SEC on March 31, 2008 |
|
|
|
|
(3) |
Incorporated by reference to Registrant’s Registration Statement on Form S-1, as amended, filed with the SEC on September 19, 2007 (Registration No. 333-146162) |
|
|
|
|
(4) |
Incorporated by reference to Registrant’s Current Report on Form 8-K filed with the SEC on February 26, 2008 |
|
|
|
|
(5) |
Incorporated by reference to Registrant’s Current Report on Form 8-K filed with the SEC on April 29, 2008 |
|
|
|
|
(6) |
Incorporated by reference to Registrant’s Current Report on Form 8-K filed with the SEC on October 30, 2008 |
111
|
|
|
|
(7) |
Incorporated by reference to Registrant’s Current Report on Form 8-K filed with the SEC on February 13, 2009 |
|
|
|
|
(8) |
Incorporated by reference to Registrant’s Current Report on Form 8-K filed with the SEC on February 20, 2009 |
|
|
|
|
(9) |
Incorporated by reference to Registrant’s Current Report on Form 8-K filed with the SEC on April 28, 2009 |
|
|
|
|
(10) |
Incorporated by reference to Registrant’s Current Report on Form 8-K filed with the SEC on February 23, 2010 |
|
|
|
|
+ |
Indicates management contract or compensatory plan. |
112
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
|
By: |
/s/ Maurice R. Ferré, M.D. |
|
|
|
|
President, Chief Executive Officer |
|
|
|
|
and Chairman of the Board |
|
|
|
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
Dated: March 10, 2010 |
|
|
|
|
|
|
|
|
|
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated. |
|||
|
|
|
|
|
|
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Maurice R. Ferré, M.D. |
|
President, Chief Executive Officer and Chairman of the Board (Principal Executive Officer) |
|
March 10, 2010 |
|
Maurice R. Ferré, M.D. |
|
|
||
|
|
|
|
|
|
|
/s/ Fritz L. LaPorte |
|
Senior Vice President of Finance and Administration, Chief Financial Officer and Treasurer (Principal Accounting and Financial Officer) |
|
March 10, 2010 |
|
Fritz L. LaPorte |
|
|
||
|
|
|
|
|
|
|
/s/ S. Morry Blumenfeld, Ph.D. |
|
Director |
|
March 10, 2010 |
|
S. Morry Blumenfeld, Ph.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Marcelo G. Chao |
|
Director |
|
March 10, 2010 |
|
Marcelo G. Chao |
|
|
|
|
|
|
|
|
|
|
|
/s/ Christopher C. Dewey |
|
Director |
|
March 10, 2010 |
|
Christopher C. Dewey |
|
|
|
|
|
|
|
|
|
|
|
/s/ Charles W. Federico |
|
Director |
|
March 10, 2010 |
|
Charles W. Federico |
|
|
|
|
|
|
|
|
|
|
|
/s/ John G. Freund, M.D. |
|
Director |
|
March 10, 2010 |
|
John G. Freund, M.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ Frederic H. Moll, M.D. |
|
Director |
|
March 10, 2010 |
|
Frederic H. Moll, M.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ William D. Pruitt |
|
Director |
|
March 10, 2010 |
|
William D. Pruitt |
|
|
|
|
|
|
|
|
|
|
|
/s/ John J. Savarese, M.D. |
|
Director |
|
March 10, 2010 |
|
John J. Savarese, M.D. |
|
|
|
|
113
|
|
|
|
|
|
Exhibit |
|
|
|
|
23 |
|
Consent of Ernst & Young LLP, Independent Public Registered Accounting Firm |
|
|
|
|
|
|
|
31.1 |
|
Certification of Chief Executive Officer pursuant to Rule 13a-14(a) of the Exchange Act |
|
|
|
|
|
|
|
31.2 |
|
Certification of Chief Financial Officer pursuant to Rule 13a-14(a) of the Exchange Act |
|
|
|
|
|
|
|
32.1 |
|
Certification of Chief Executive Officer pursuant to18 U.S.C. §1350 |
|
|
|
|
|
|
|
32.2 |
|
Certification of Chief Financial Officer pursuant to18 U.S.C. §1350 |
|
|
|
|
|
|
|
99.1 |
|
Registration Rights Agreement by and between Registrant and Z-Kat, Inc. dated February 25, 2010 |
|
114