Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Vanda Pharmaceuticals Inc. | w77636e8vk.htm |
Exhibit 99.1

| Vanda Pharmaceuticals Inc. Cowen Healthcare Conference March 9, 2010 Mihael H. Polymeropoulos MD CEO |

| Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 with respect to our financial condition, results from operations and business, and our expectations and beliefs about future events. Actual results may vary materially from our expectations and beliefs. Meaningful factors which could cause actual results to differ from expectations include, but are not limited to, uncertainty of the Company's future profitability, uncertainty of market acceptance for the Company's products, delay in or failure to obtain regulatory approvals for the Company's product candidates, uncertainty regarding patents and proprietary rights, risks inherent in international transactions, limited sales and marketing experience, dependence on third party reimbursement, competition, uncertainty of clinical trial results, extent of government regulations, and inability to obtain requisite additional financing, as well as other factors discussed in the Company's Securities and Exchange Commission filings. All forward-looking statements in this presentation are expressly qualified by the above paragraph in their entirety. We have no obligation to update any forward- looking statements which are made in this presentation. |

| Company Summary Vanda Pharmaceuticals (Nasdaq: VNDA) Rockville, MD 22 Employees Established in 2003 IPO 2006 |

| Vanda Historical Stock Performance |

| Pipeline Phase I PhaseII PhaseIII NDA Market Fanapt(tm) (schizophrenia) Iloperidone depot Tasimelteon (CRSD) |

| Fanapt(tm) (iloperidone) (Schizophrenia) |

| Fanapt(tm) Overview Fanapt(tm) (iloperidone) tablets - oral formulation FDA approved in May 2009 for adults with Schizophrenia Iloperidone - depot formulation Phase II Oral formulation launched January 11, 2010 by Novartis in the U.S. |

| Deal Summary FDA approved for acute treatment of adult patients with schizophrenia Partnered with Novartis in the U.S. and Canada Vanda received $200 million upfront Eligible to receive up to an additional $265 million in payments tied to commercial and development milestones Low double-digit sales royalties Vanda retains exclusive rights outside the U.S. and Canada |

| Schizophrenia: the disease Severe, chronic, and debilitating brain disorder Prevalence: 1% worldwide ( ~2.4 million in the U.S.) Age of onset: 15-35 years Symptoms Positive Negative General Debilitating outcomes Near-universal disability Aggressive behavior High risk of suicide Sources: NAMI, NMA, DSM-IV |

| Antipsychotic Market: Summary Significant unmet needs remain CATIE study showed 74% discontinue in 18 months, primarily due to safety concerns Protected compound class on federal and private formularies due to disease severity Highly concentrated prescriber base $15+ billion market |
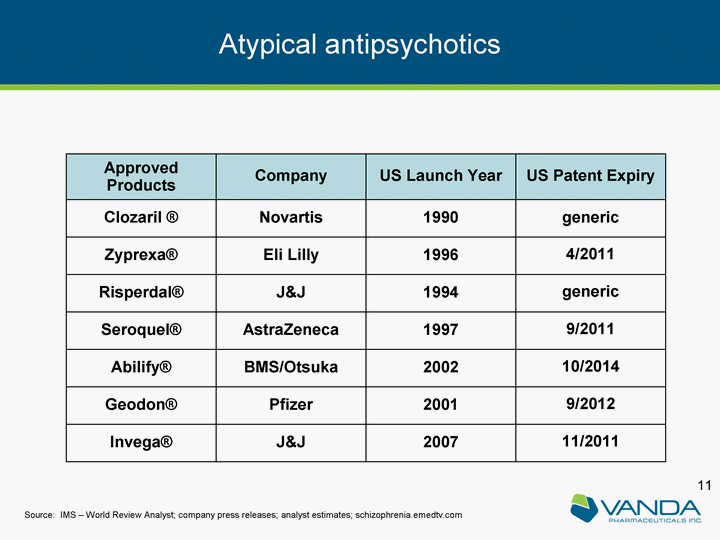
| Approved Products Company US Launch Year US Patent Expiry Clozaril (r) Novartis 1990 generic Zyprexa(r) Eli Lilly 1996 4/2011 Risperdal(r) J&J 1994 generic Seroquel(r) AstraZeneca 1997 9/2011 Abilify(r) BMS/Otsuka 2002 10/2014 Geodon(r) Pfizer 2001 9/2012 Invega(r) J&J 2007 11/2011 Source: IMS - World Review Analyst; company press releases; analyst estimates; schizophrenia.emedtv.com Atypical antipsychotics |

| Depot Landscape Compliance remains a significant unmet need Limited competition Risperdal Consta: $1.3B revenue in 2009 Invega Sustenna: 2009 Launch Zyprexa Relprevv: 2009 Launch |

| Iloperidone Depot Formulation: Summary Differentiated profile Once every 4 weeks Well tolerated Weight gain/metabolics Movement disorders PK and Phase III studies needed for NDA |

| Iloperidone IP Summary Fanapt(tm) (oral) NCE (+ HW & pediatric) through mid 2017 Iloperidone depot microspheres US 2023 (allowed) EU 2022 (application) Iloperidone depot crystals US 2022 (application) EU 2022 (allowed) |

| Tasimelteon (VEC-162) (Circadian Rhythm Sleep Disorders or CRSD) |
| Tasimelteon Program Overview 2004: Acquired from BMS Mechanism: MT1, MT2 melatonin receptor agonist Vanda has completed 1 Phase II study and 2 Phase III studies Studies 2101 and 3101 establish tasimelteon as a circadian rhythm regulator |

| Vanda Clinical Trials to Date Design Outcome Assessments Outcome Assessments Outcome Assessments Design Phase Advance Sleep Onset Sleep Maintenance Phase II Phase II Phase II Phase II Phase II 2101 5-Hour Phase Advance R, DB, PC parallel-group study N=39 5 dose groups Treatment: 3 nights + + + Phase III Phase III Phase III Phase III Phase III 3101 5-Hour Phase Advance R, DB, PC parallel-group multi-center study N=412 4 dose groups Treatment: 1 night n.a. + + 3104 Sleep-Onset Insomnia R, DB, PC parallel-group multi-center study N=322 3 dose groups Treatment: 35 days n.a. + - |

| CRSD Population 80K 12.6 MM 35.7 MM N24HSWD SWSD/Early Risers DSPD 15.6 MM Jet-Lag Total Population: >65 MM |

| Non24Hour SWD Background Non-24-Hour Sleep Wake Disorder (N24HSWD), a type of Circadian Rhythm Sleep Disorder (CRSD) 65K-95K people in the U.S. Problems with sleep and wakefulness Endogenous circadian rhythm or "body clock" is unable to entrain to a normal 24- hour day. In sighted individuals, the strongest entrainer to the 24-hour clock is light. Other environmental factors can also help with entrainment but may not be sufficient in the totally blind. Person will "free run" slightly longer than 24-hours without environmental input, causing a phase delay in his/her body clock each day. Occurs almost entirely in subjects who are totally blind and lack the light sensitivity necessary to reset the circadian clock. |

| Orphan Indication Facts Orphan indication granted January 2010 Streamlined development Tax credit / User fees for NDA waived FDA protocol assistance 7 year market exclusivity |

| Non24Hour SWD Draft Clinical Development Plan Proposed Clinical Development Plan: |

| Tasimelteon IP Summary Tasimelteon NCE: December 2017 expiration (2023 including HW+pediatric) VXB-269 (back-up compound) NCE: June 2020 expiration |

| Finance Summary |

| Summary Financials ($ in millions) 12/31/09 Cash/cash equivalents/ST investments $205.3 ($ in millions) Year Ended 12/31/2009 Revenue Operating Expenses $4.5 COGS 2.9 R&D 13.9 G&A 23.7 Loss from Operations 40.5 Net Loss $36.0 Guidance: Vanda's fixed overhead costs are expected to be approximately $10.0 million to $12.0 million annually in 2010. (Inclusive of all FTE's but excludes additional R&D activities). |

| Tax Update Deferral for 2010 382 NOL Analysis On-going On-going discussion with IRS |

| Vanda Pharmaceuticals Inc. Cowen Healthcare Conference March 9, 2010 Mihael H. Polymeropoulos MD CEO |
