Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Pernix Sleep, Inc. | a55050e8vk.htm |
Exhibit 99.1

| Somaxon Pharmaceuticals, Inc. February 8, 2010 BIO CEO & Investor Conference Nasdaq: SOMX |

| Forward Looking Statements This presentation contains forward-looking statements about our business, including results of our clinical trials, potential regulatory approval for our product candidate, development timelines, future financial results and events that have not yet occurred. Pharmaceutical development inherently involves significant risks and uncertainties, including the risks outlined under "Risk Factors" and elsewhere in our Annual Report on Form 10-K and our other filings made with the SEC from time to time. Our actual results may differ materially from our expectations due to these risks and uncertainties, including our near-term dependence on the success of our lead product candidate, Silenor(r), and factors relating to regulatory approval, research and development, intellectual property protection, ability to raise sufficient capital, ability to attract a strategic partner, competition, industry environment, and other matters. Somaxon undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. |
| Somaxon: A Focus on CNS Specialty pharmaceutical company Core asset: Silenor for insomnia NDA under FDA review - Class 1 Resubmission FDA Action date: March 21, 2010 Partnering discussions ongoing NASDAQ: SOMX |

| Focused Strategy Reduces Risk Develop and Commercialize Highly Differentiated Products Target late-stage or currently marketed assets Address unmet medical need and patient dissatisfaction Leverage development, regulatory and commercial expertise Deploy a U.S. Commercial Organization Focus on the CNS therapeutic area Cover high-prescribing physicians with a targeted sales force Create efficiencies through strategic outsourcing Establish Commercial Collaborations to Drive Growth and Profitability Effectively leverage partner resources Reach broader prescriber universe Attract additional product opportunities |

| Silenor for Insomnia Near-term Opportunity "Launch Ready" Marketing Campaign: 2H 2010 possible launch with partner Product profile supports attractive revenue estimates Changing insomnia market dynamics ? commercial upside Differentiated Mechanism of Action H1 specificity vs. GABA or melatonin Strong Clinical Efficacy Profile Primary efficacy endpoint achieved statistical significance in all four Phase 3 studies Sleep maintenance into the 8th hour of the night Favorable Safety and Tolerability Profile Not scheduled Adverse events comparable to placebo No GABA-ergic related side effects |

| A Restless Market |

| U.S. Insomnia Market: Large and Dissatisfied 70 million people in the U.S. suffer from insomnia Market remains under-diagnosed, under-treated, and dissatisfied 14 million (20%) are currently on Rx treatment 9 million (67%) are not satisfied 56 million utilize OTC and other remedies Current options force patients and physicians to compromise GABA-ergic drugs provide varying degrees of efficacy but involve compromise on tolerability and risk of addiction OTCs address addiction concerns but involve compromise on efficacy and tolerability Melatonin-receptor agonists address addiction and tolerability concerns but involve compromise on efficacy |

| Poorly/Not at All Controlled Completely/Well Controlled Somewhat Controlled 4th Qtr East 39 12 49 20.4 West 30.6 38.6 34.6 31.6 North 45.9 46.9 45 43.9 Market Dissatisfied with Current Options Somaxon Market Research, 2006; n = 451 "How well would you say your insomnia/sleep problem is controlled?" 12% 39% 49% Completely/ Well Controlled Somewhat Controlled Poorly/Not at All Controlled |

| Room for Improvement Patients Not Satisfied With Rx GABA Products Less Time to Get to Sleep Woke Less During Night Slept Longer Felt Rested on Waking Felt Better Overall East 76.2 60.8 48.6 47 48.1 % of Patients Taking "Z Drugs"* <50% report sleeping longer, feeling rested, or feeling better overall *Zolpidem, zopiclone, zaleplon. Siriwardena AN, et al. Br J Gen Pract. 2008;58(551):417-422. |

| Sleep Maintenance is Primary Issue 65% 35% Sleep Problem in Past Month At Least a Few Nights/Week 49% Woke Unrefreshed 42% Frequent Nocturnal Awakenings 29% Early Morning Awakenings 26% Difficulty Falling Asleep National Sleep Foundation. Summary of findings: 2008 Sleep in America poll. n = 1760 respondents Any Sleep Problem 0.35 0.65 Any Sleep Problem Rarely, Never, Don't know |
| Large and Accessible Market Over 70 million TRx's in the U.S. 2009 Demographics favorable for continued year over year growth Prescription growth accelerates with the introduction of novel, differentiated products Patients want new options 90% would be willing to try Silenor 70% would ask for it on the next doctor visit 12% stated they would make a special visit to ask for Silenor Concentrated market: ~44,000 doctors write half the TRx's Non-scheduled status results in significant sampling advantage for Silenor 80% of doctors willing to try Silenor |

| Changing Market Dynamics Create Opportunities Solid "window of opportunity" for new treatments A void of new and differentiated insomnia treatments since 2006 No "novel" compounds expected to launch in near term Clinical and Regulatory setbacks for several drugs in development Somaxon estimates several years before new competition comes to market Number of primary details is decreasing Recent sales force reductions Competitive brands lack differentiated message Competitive promotional spending is decreasing DTC spending down dramatically year-over-year Consistent trend across brands |
| A Novel Treatment for Insomnia Silenor |
| Starting With a Differentiated Mechanism of Action Histamine (H1) is one of several waking neuro-transmitters important in the arousal system Silenor has high affinity for H1 receptors Twenty-fold more potent than diphenhydramine, and significantly more selective Silenor has significant effects at night through the blockade of H1, which promotes and maintains sleep Specific H1 blockade does not prevent or diminish arousal due to other neurotransmitters in the arousal system |

| Finishing With a Strong Clinical Profile Six controlled clinical trials completed Adult and elderly populations Chronic and transient insomnia Primary efficacy endpoint achieved statistical significance in each of Phase 2 and 3 studies Comprehensive Phase 1/Non-clinical program completed Cardiac study Non-clinical toxicology Drug-drug interaction Next-day residual effects Clinically meaningful improvement in sleep maintenance, sleep efficiency and prevention of early morning awakenings |

| Silenor: Just What the Patient Ordered Treat sleep maintenance insomnia The most significant insomnia complaint 7-8 hours of sleep with Silenor Prevention of early morning awakenings - a full night's sleep Unique potential positioning for Silenor Sleep onset (<30 min) Clinically meaningful Proposed to be described in the clinical trials section Attractive safety profile NO clinically relevant evidence of next-day residual effects NOT DEA scheduled NO abuse potential NO risk of dependency NO withdrawal symptoms NO tolerance with long term use NO complex sleep behaviors observed NO amnesia, hallucinations or other GABA-related side effects NO disruption of sleep stages |

| Silenor Commercialization Silenor |

| A Refreshingly Different Sleep Solution Silenor Commercialization |

| Silenor Branding Platform Brand Identity - Refreshingly Different Key Messages - A full 7-8 hours of sleep - Safe for chronic use - Non-addicting, no abuse potential - Placebo-like side effect profile - No residual hangover effect - Unique MOA Positioning -Silenor is first-line therapy for sleep maintenance insomnia Broad clinical profile Safety Tolerability Patient benefits |

| "Plan to Win" Capitalize on the Changing Market Dynamics Competitor "turbulence" Lack of differentiated insomnia treatments Shift from expensive DTC strategy Launch with a Competitive Share of Voice with a Partner Target high value physicians Utilize non-personal promotional efforts to leverage into lower deciles Exploit Silenor sampling advantage Integrated Professional Campaign Developed Strong branding elements Patient Education Programs Technology driven physician education Competitive publication plan at launch Targeted Consumer Campaign Developed "Direct to Insomniac" communication via Web Point of Care Programs/Public Relations |

| Somaxon Pharmaceuticals, Inc. Corporate Summary |

| Strategic Collaboration for Silenor GOALS Successful Silenor launch in 2H 2010 Build a focused Somaxon commercial organization Maximize shareholder value Traditional licensing with co-promotion rights Co-promotion with shared revenues and costs M&A to gain access to sales infrastructure/ products |
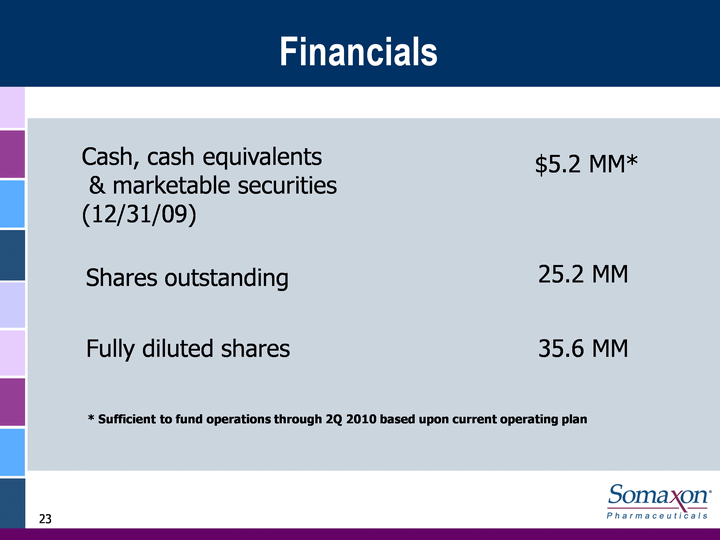
| Financials Cash, cash equivalents & marketable securities (12/31/09) Shares outstanding Fully diluted shares $5.2 MM* 23.1 MM 34.9 MM * Sufficient to fund operations through 2Q 2010 based upon current operating plan |

| Near-Term Priorities Obtain regulatory approval for Silenor NDA re-submission accepted March 21, 2010 FDA action date Partner Silenor Ongoing dialogue with multiple parties Build commercial infrastructure Execute post-approval Launch Silenor in U.S. in 2H 2010 |

| Somaxon Value Proposition Silenor Highly differentiated insomnia treatment Favorable market dynamics "Launch Ready" campaign following regulatory approval Attractive revenue potential Experienced management team Significant commercial launch experience Building a focused commercial organization Capable of driving product sales and profitability Able to leverage/support additional products |

| Somaxon Pharmaceuticals, Inc. Thank You! |
