Attached files
| file | filename |
|---|---|
| 8-K - 8-K - HELICOS BIOSCIENCES CORP | a09-34709_18k.htm |
| EX-99.1 - EX-99.1 - HELICOS BIOSCIENCES CORP | a09-34709_1ex99d1.htm |
| EX-99.2 - EX-99.2 - HELICOS BIOSCIENCES CORP | a09-34709_1ex99d2.htm |
Exhibit 99.3
|
|
Stan Lapidus Chairman © 2009 Helicos BioSciences Corporation Helicos Single Molecule Sequencing in Clinical Diagnostics |
|
|
Certain statements made in this presentation that are not based on historical information are forward-looking statements which are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation contains express or implied forward-looking statements relating to, among other things, the potential first mover advantages of Helicos’ single molecule sequencing in clinical diagnostics discussed in this presentation, and management's forecast of financial performance, estimates of expenses and future revenues and profitability, product development and marketing plans, and management's plans, objectives and strategies. These statements are neither promises nor guarantees, and are subject to a variety of risks and uncertainties, many of which are beyond Helicos' control, which could cause actual results to differ materially from those contemplated in these forward-looking statements. In particular, the risks and uncertainties include, among other things, our ability to successfully scale the manufacturing process and commercialize the HeliScope system; our history of operating losses and ability to achieve profitability; our ability to establish manufacturing capabilities; the research and development spending levels of academic, clinical and governmental research institutions and pharmaceutical, biotechnology and agriculture companies who may purchase our HeliScope system; our reliance on third-party suppliers; competition; changing technology and customer requirements; our ability to operate in an emerging market; market acceptance of our technology; the length of our sales and implementation cycles; our dependence on large contracts for the sale and implementation of our HeliScope system; failure of our technology and products; our ability to maintain customer relationships and contracts; ethical, legal and social concerns surrounding the use of genetic information; our ability to retain our personnel and hire additional skilled personnel; our ability to manage our growth with limited resources; our ability to obtain capital when desired on favorable terms; and the volatility of the market price of our common stock. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Helicos undertakes no obligation to update or revise the information contained in this presentation, whether as a result of new information, future events or circumstances or otherwise. For additional disclosure regarding these and other risks faced by Helicos, see the disclosure contained in Helicos' public filings with the Securities and Exchange Commission. PLEASE NOTE: The names HeliScope, tSMS, true Single Molecule Sequencing and Virtual Terminator and our logo are trademarks or service marks of Helicos BioSciences Corporation. Safe Harbor 1 |
|
|
Unique opportunity to shape clinical applications, regulation, and economics Application development & optimization IP: Early priority dates Informatics development: important for sequencing with its massive data and nascent computational biology infrastructure Building reference base of happy customers and scientific publications First Mover Advantage in SMS Executive Summary |
|
|
Diagnostic Opportunity |
|
|
SMS Advances Are Changing Lab Strategies Diagnostics represents a multi-billion market which no other current next-gen sequencing platform has the capability to address Establish position within reference labs through well-defined value proposition of cost reduction and MDx platform consolidation Opportunity to create a durable lead by aggregating complementary IP and establishing the HeliScope as the only HTS application platform Recent advances in sequencing have led all of the major clinical labs to reevaluate its role in diagnostics Senior management now believes that it is a revolutionary technology and essential to the future of their businesses A small, but growing, number of labs are doing commercial sequencing today Mayo, Myriad Genetics, Correlagen, and others |
|
|
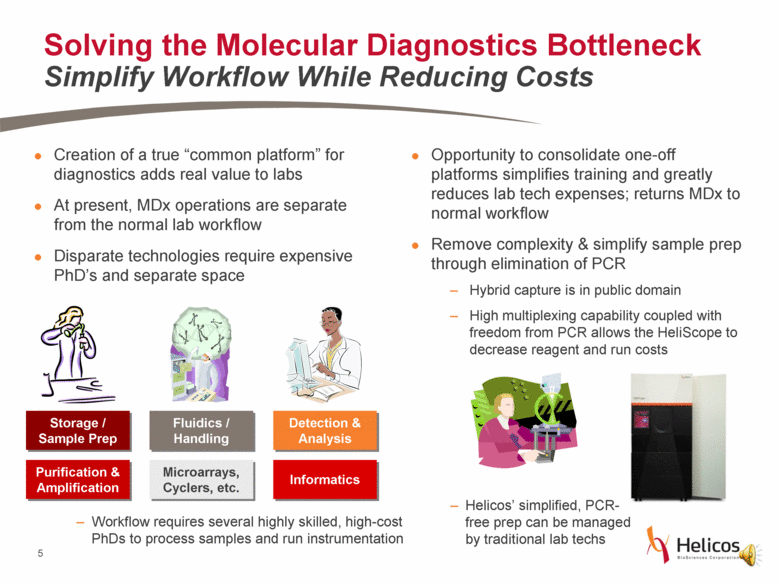
Creation of a true “common platform” for diagnostics adds real value to labs At present, MDx operations are separate from the normal lab workflow Disparate technologies require expensive PhD’s and separate space Solving the Molecular Diagnostics Bottleneck Simplify Workflow While Reducing Costs Opportunity to consolidate one-off platforms simplifies training and greatly reduces lab tech expenses; returns MDx to normal workflow Remove complexity & simplify sample prep through elimination of PCR Hybrid capture is in public domain High multiplexing capability coupled with freedom from PCR allows the HeliScope to decrease reagent and run costs Fluidics / Handling Storage / Sample Prep Informatics Purification & Amplification Microarrays, Cyclers, etc. Detection & Analysis Workflow requires several highly skilled, high-cost PhDs to process samples and run instrumentation Helicos’ simplified, PCR-free prep can be managed by traditional lab techs |
|
|
Oncology Testing Will Drive Initial Transition Other Large Market Applications Also Promising By 2010/2011, testing volumes for neuropsychiatric disorders, endocrinology, and cardiology are expected to increase markedly These diagnostics have emerged almost entirely from pathway-specific research and are currently being done commercially on Sanger platforms at great expense and therefore at smaller volumes Near-term, great value in sequencing will come from labs testing for inherited cancer risk & specific tumor characteristics BrCa, mismatch repair cancers, etc. EGFR mutations, resistance mutations, Gleevec susceptibility resistance, k-ras, PTEN, and others Pre-natal and neo-natal testing for a broad range of diseases in amniocentesis samples and perhaps maternal circulation The largest potential application by far will be for cancer screening in blood, stool, and urine (market size is tens of millions of tests per year) |
|
|
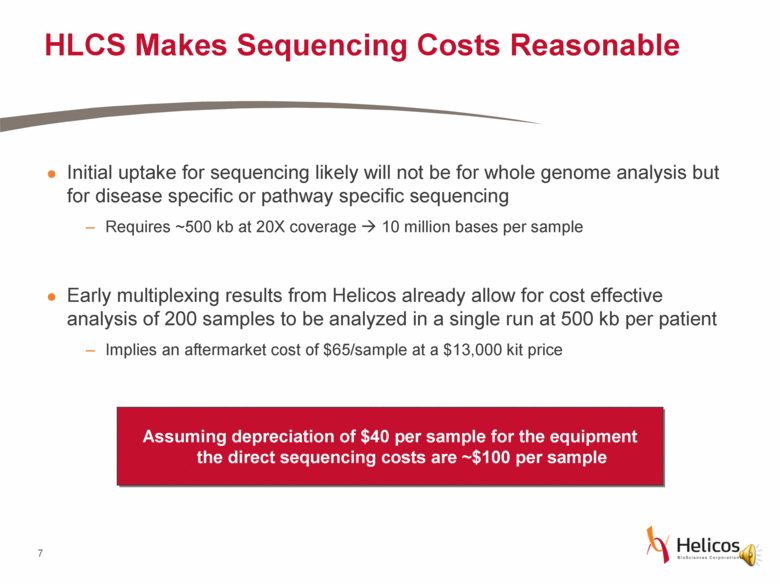
HLCS Makes Sequencing Costs Reasonable Initial uptake for sequencing likely will not be for whole genome analysis but for disease specific or pathway specific sequencing Requires ~500 kb at 20X coverage 10 million bases per sample Early multiplexing results from Helicos already allow for cost effective analysis of 200 samples to be analyzed in a single run at 500 kb per patient Implies an aftermarket cost of $65/sample at a $13,000 kit price Assuming depreciation of $40 per sample for the equipment the direct sequencing costs are ~$100 per sample |
|
|
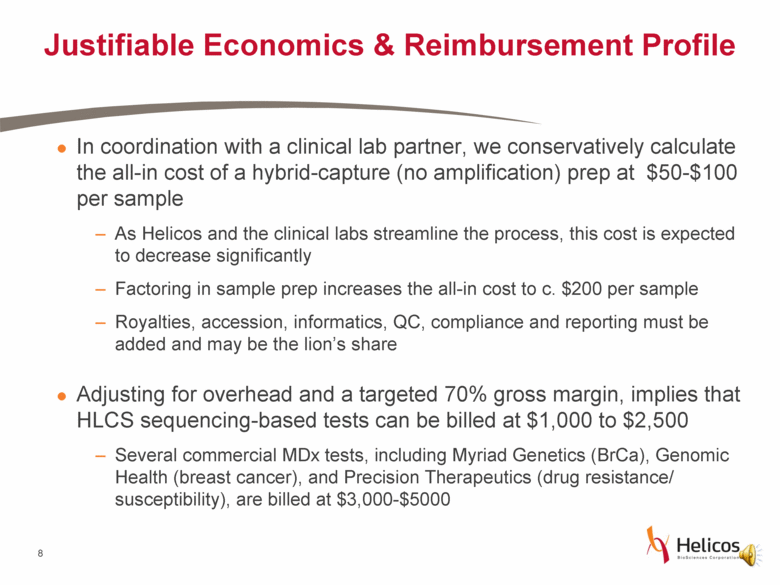
Justifiable Economics & Reimbursement Profile In coordination with a clinical lab partner, we conservatively calculate the all-in cost of a hybrid-capture (no amplification) prep at $50-$100 per sample As Helicos and the clinical labs streamline the process, this cost is expected to decrease significantly Factoring in sample prep increases the all-in cost to c. $200 per sample Royalties, accession, informatics, QC, compliance and reporting must be added and may be the lion’s share Adjusting for overhead and a targeted 70% gross margin, implies that HLCS sequencing-based tests can be billed at $1,000 to $2,500 Several commercial MDx tests, including Myriad Genetics (BrCa), Genomic Health (breast cancer), and Precision Therapeutics (drug resistance/ susceptibility), are billed at $3,000-$5000 |
|
|
New Markets Will Drive Dx Adoption HLCS Protocol Uniquely Addresses Lab Needs Early adopters in the lab business will be aggregators of IP Aggregation effort is often based on an initial single university license Many companies have been allocating material resources towards planning their sequencing based programs Primary challenge to adoption for low complex mixtures is to sequence a few thousand bases at high-coverage (1000x) with low-error rates Sequencing is not utilized because error rates of other systems are too high, in-part because of PCR errors For Helicos these error rates, even at current performance levels, can be brought in-line with market needs by deploying proprietary and patent-pending "melt-and-resequence" or "chewback-and-resequence" methods |
|
|
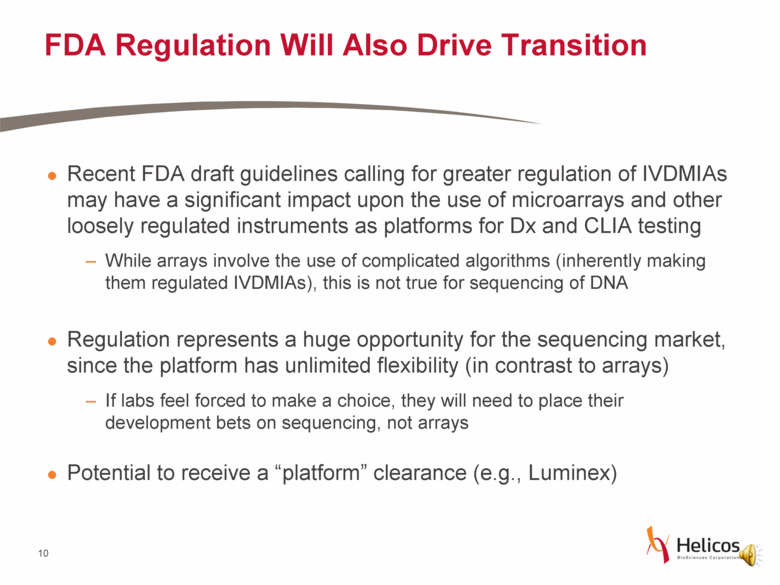
FDA Regulation Will Also Drive Transition Recent FDA draft guidelines calling for greater regulation of IVDMIAs may have a significant impact upon the use of microarrays and other loosely regulated instruments as platforms for Dx and CLIA testing While arrays involve the use of complicated algorithms (inherently making them regulated IVDMIAs), this is not true for sequencing of DNA Regulation represents a huge opportunity for the sequencing market, since the platform has unlimited flexibility (in contrast to arrays) If labs feel forced to make a choice, they will need to place their development bets on sequencing, not arrays Potential to receive a “platform” clearance (e.g., Luminex) |
|
|
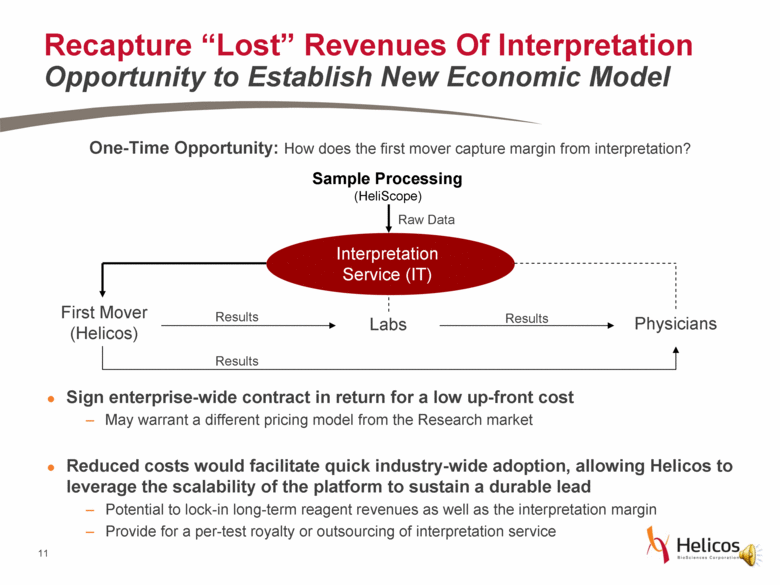
One-Time Opportunity: How does the first mover capture margin from interpretation? Sign enterprise-wide contract in return for a low up-front cost May warrant a different pricing model from the Research market Reduced costs would facilitate quick industry-wide adoption, allowing Helicos to leverage the scalability of the platform to sustain a durable lead Potential to lock-in long-term reagent revenues as well as the interpretation margin Provide for a per-test royalty or outsourcing of interpretation service (HeliScope) Raw Data Interpretation Service (IT) First Mover (Helicos) Physicians Labs Sample Processing Results Results Results Recapture “Lost” Revenues Of Interpretation Opportunity to Establish New Economic Model |
|
|
First Movers In Dx Establish Market Protocols The first instrument company to develop an FDA-approvable sequencing platform will have an unshakable first-mover advantage FDA approval implies that the machine can be used by IVD companies in their FDA submissions for 510(k) and PMA approvals The effort to implement a quality system, design controls, hazard analysis & first rate documentation is not trivial but the pay-off is large Once an IVD company, and for that matter a CLIA lab, settles on a platform, it will be very hard to dislodge The market dynamics are very different from the life-science tools business |
|
|
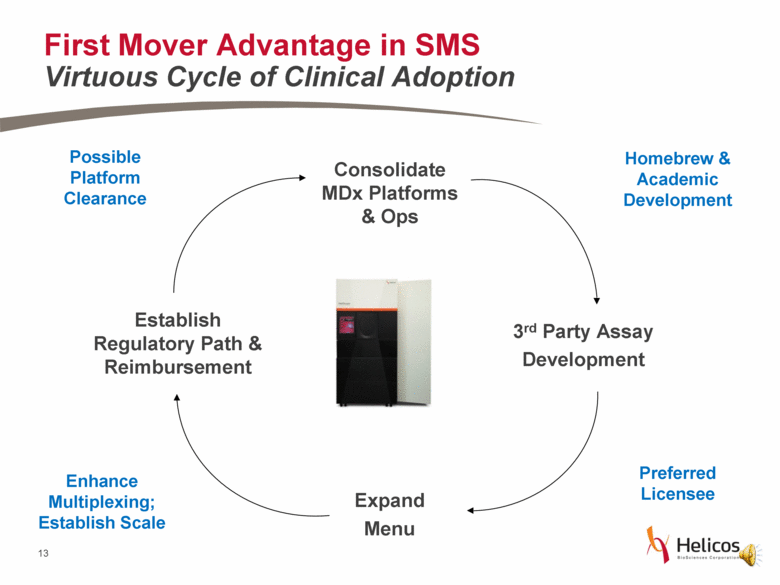
First Mover Advantage in SMS Virtuous Cycle of Clinical Adoption Consolidate MDx Platforms & Ops 3rd Party Assay Development Expand Menu Establish Regulatory Path & Reimbursement Homebrew & Academic Development Preferred Licensee Enhance Multiplexing; Establish Scale Possible Platform Clearance |
|
|
www.helicosbio.com |