Attached files
| file | filename |
|---|---|
| 10-K/A - Apyx Medical Corp | v167911_10ka.htm |
| EX-31.1 - Apyx Medical Corp | v167911_ex31-1.htm |
| EX-32.2 - Apyx Medical Corp | v167911_ex32-2.htm |
| EX-10.2 - Apyx Medical Corp | v167911_ex10-2.htm |
| EX-32.1 - Apyx Medical Corp | v167911_ex32-1.htm |
| EX-31.2 - Apyx Medical Corp | v167911_ex31-2.htm |
| EX-10.13 - Apyx Medical Corp | v167911_ex10-13.htm |
| EX-10.12 - Apyx Medical Corp | v167911_ex10-12.htm |
 |
|
|
Corporation
|
TERMINATION,
PURCHASE AND LICENSE AGREEMENT
|
This
Termination, Purchase and License Agreement ("Agreement") is made as of
____________________, 2008 ("Effective Date"), by and between Boston Scientific
Corporation, One Boston Scientific Place, Natick, MA 01760 ("Seller"), and Bovie
Medical Corporation, 7100 30th
Avenue-North, St. Petersburg, FL 33710 ("Buyer") for the purpose of
purchase and sale of certain rights and assets related to the Program (as
defined in Section 1.1) and use of the rights and assets in the development,
manufacture and sale of the Product (as defined in Section 1.2); assignments and
licenses for certain intellectual property of the Parties; and termination of
that certain Distribution Agreement between the Parties dated as of October 6,
2006, as amended on August 23, 2007 (the “Distribution Agreement”), all in
accordance with this Agreement. Buyer and Seller are herein referred
to collectively as “Parties” and individually as a “Party.”
MAILING
ADDRESSES AND FAX NUMBERS FOR NOTICES, ETC. UNDER
AGREEMENT
|
Buyer:
Bovie
Medical Corporation
7100
30th
Avenue North
St.
Petersburg, FL 33710
Attn: Moshe
Citronowicz, COO
Fax: (727)
344-3876
|
with copy
to:
Bovie
Medical Corporation
7100
30th
Avenue North
St.
Petersburg, FL 33710
Attn:
General Counsel
Fax: (727)
344-3876
|
|
|
Seller:
Boston
Scientific Corporation
100
Boston Scientific Way
Marlborough,
Massachusetts 01752
Attn: Michael
Phalen, President Endoscopy
Fax: (508)
683-5316
|
|
with copy
to:
Boston
Scientific Corporation
One
Boston Scientific Place
Natick,
Massachusetts 01760-1537
Attn: General
Counsel
Fax: (508)
650-8956
|
NOW, THEREFORE, for good and valuable
consideration, the receipt and sufficiency of which are hereby acknowledged, the
Parties hereto agree as follows:
1. Purchase and
Sale
1.1 Program. For
purposes of this Agreement, “Program” means the SEER
Sintered Tip Resection Device, BSC Project # M0380.
1.2 Product. For
purposes of this Agreement, “Product” means any medical
device having a sintered, conductive metal tip in accordance with the Product
Specifications attached hereto as Exhibit A (Revisions
A-1 & A-2) for use in the Field. Product specifically does not
include any generator or generator accessories. “Field” means liver, pancreatic
and kidney tumor, orthopedic, and blood vessel sealing, therapy by delivery of
RF current and sterile saline for resection, hemostatic sealing and coagulation
of soft tissue in open surgery and/or laparoscopic surgery.
1.3 Assets. At
the closing, and upon the terms and conditions of this Agreement, Seller hereby
sells, transfers, conveys, assigns and delivers to Buyer, and Buyer purchases
from Seller, free and clear of any and all liabilities, obligations, liens and
encumbrances, all right, title and interest in and to the following Program
assets of Seller (collectively, the “Purchased Assets”), wherever
located, :
(a) All
equipment, machinery, prototypes, tooling, supplies, and other personal property
of the Seller predominantly related to the Program and the design, development
and manufacture of the Product, including all records related thereto, as set
forth on Exhibit
X hereto;
|
BUYER:
________
|
Page 1 of
8
|
SELLER:
________
|
(b) all
of Seller’s rights and interest in and under the contracts, licenses, leases and
other agreements and instruments predominantly related to the Program and the
design, development and manufacture of the Product, as set forth on Exhibit X hereto,
together with true, complete and accurate copies of the same (the “Transferred
Contracts”);
(c) to
the extent assignable, approvals, clearances, authorizations, licenses and
registrations required by any governmental authority, predominantly related to
the Program and to permit the design, development, pre-clinical and clinical
testing, manufacturing, labeling, sale, distribution, and promotion of the
Product as set forth on Exhibit X hereto,
together with true, complete and accurate copies of the same (the “Transferred
Permits”);
(d) all
scientific, clinical, technical, marketing, pre-sales and other data,
predominantly related to the Program and the design, development and manufacture
of the Product, including, without limitation, files, formulas, compositions,
computer discs and tapes (and reasonable use of the means to access or convert
them to a form useable by Buyer, if necessary), laboratory notebooks, design
histories, operating manuals and procedures, instructions for use, device
history records, bills of materials, and manufacturing, inspection and quality
control records and procedures, as set forth on Exhibit X
hereto;
(e) the Generated Product IP (as defined in Section
2.1), together with true, complete and accurate copies of the documents
pertaining thereto including, without limitation, documents used by Seller (or
its counsel) in the preparation and prosecution of the patents comprising the
Generated Product IP, except as to the excluded document categories set forth on
Exhibit X
hereto;
(f) all guarantees,
warranties, indemnities and similar rights in favor of Seller with respect to
any Purchased Asset; and
(g) all rights to causes of
action, lawsuits, judgments, claims and demands of any nature available to, or
being pursued by, Seller, with respect to the Purchased Assets, whether arising
by way of counterclaim or otherwise.
1.4 Delivery of Purchased
Assets. No later than _____ (_)
business days after the closing, Seller shall package and ship (or cause to be packaged and shipped)
to Buyer all tangible personal property elements of the Purchased Assets not in
Buyer’s possession and custody as of the closing, at Seller’s
expense.
2. Assignment of Intellectual
Property; Cross-Licenses.
2.1 Assignment of Generated
Product IP. At the closing, and upon the terms and conditions
of this Agreement, Seller hereby sells, transfers, conveys and assigns to Buyer,
and Buyer purchases from Seller, free and clear of any and all liabilities,
obligations, liens and encumbrances, all right, title and interest in and to any
and all Intellectual Property Rights (as defined below) generated in the
performance of the Program and as a result of development work on the Product by
or on behalf of either Party (collectively, the “Generated Product IP”),
including but not limited to:
(a) ** **
(b) US2007/0156134,
filed December 29, 2005, ** **
(c) U.S.
Patent #7,282,051, filed February 4, 2004, issued October 16, 2007, and
continuation 11/550,374 (US2007/0123848); and
(d) ** **
.
For
purposes of this Agreement, “Intellectual Property Rights”
means intellectual property or proprietary rights of any description worldwide
including without limitation (i) rights in any patent, patent registration,
patent application, copyrights, industrial designs, trademarks, (ii) trade
secrets, moral rights, shop rights and publicity rights, (iii) inventions,
discoveries, know-how, techniques, methodologies, designs or data, whether or
not patented, patentable or copyrightable, (iv) rights to sue for and remedies
against past, present and future infringements or misappropriations thereof, and
rights of priority and protection of interests therein under the laws of any
jurisdiction worldwide and all tangible embodiments thereof, and (v) goodwill
related to any of the foregoing.
|
BUYER:
________
|
Page 2 of
8
|
SELLER:
________
|
2.2 License to Generated Product
IP. At
the closing, and upon the terms and conditions of this Agreement, Buyer hereby
grants to Seller a non-exclusive, fully paid up, royalty-free, worldwide,
perpetual, irrevocable license to the Generated Product IP solely to make, have
made, use, offer for sale, sell, have sold, import and export any Seller
Products. For purposes of this Agreement, “Seller Products” means any
product or device of Seller, provided however, such product or device shall be
for use in applications outside of a Product for the Field. As of
January 1, 2016, the license granted to Seller under this Section 2.2 shall
cease to include the limitation in the previous sentence, i.e., “for use in
applications outside of a Product for the Field.” The license granted
to Seller hereunder is not sublicensable, but is transferable by Seller to a
buyer only in connection with the sale of all or substantially all of the assets
relating to any Seller Product.
2.3 License to Existing Product
Patents. At the closing, and upon the terms and conditions of
this Agreement, Seller hereby grants to Buyer a non-exclusive, fully paid up,
royalty-free, worldwide, perpetual, irrevocable license to Existing Product
Patents solely to make, have made, use, offer for sale, sell, have sold, import
and export the Product for use in the Field. For purposes of this
Agreement, “Existing Product
Patents” shall include any of Seller’s invention disclosures, trade
secrets and patent applications (but not to the extent of disclosing non-public
details of such disclosures, trade secrets, or patent applications), and issued
and issuing patents existing as of the Effective Date, including continuations
and foreign counterparts thereof obtained thereafter, necessary for the
non-infringing manufacture, use and sale of the Product. The
foregoing license is not sublicensable but is transferable by Buyer to a buyer
only in connection with the sale of all or substantially all of the assets
relating to the Product.
3. Termination of Distribution
Agreement and Releases.
3.1 Termination. The
Distribution Agreement is hereby terminated as of the Effective
Date.
3.2 Releases. Each
Party, along with its employees, officers, directors, affiliates,
subcontractors, agents, successors or assigns, hereby releases and discharges
the other Party, along with its employees, officers, directors, affiliates,
subcontractors, agents, successors or assigns, of any and all obligations,
liabilities and claims, whether known or unknown, accrued or not accrued,
contingent or otherwise, arising prior to the Effective Date, directly or
indirectly, out of or related to, the Distribution Agreement, the performance of
the Distribution Agreement or the termination of the Distribution
Agreement.
4. Confidential
Information
4.1 Confidential
Information.
(a) "Confidential Information"
means all proprietary information disclosed by or on behalf of either Party
hereto (a “Disclosing Party”) to the other Party hereto (a “Receiving Party”),
or any of the Disclosing Party’s or Receiving Party’s employees, officers,
directors, affiliates, subcontractors, agents, successors or assigns
(collectively “Representatives” and together
with the Disclosing Party, the “Disclosing Group” or together
with the Receiving Party, the “Receiving Group”), including
information relating to the matters that are the subject of this Agreement,
including the terms, existence and nature of this Agreement, or relating to the
Disclosing Party’s other past, present or future research, technology, know-how,
ideas, concepts, designs, products, markets, customer information, computer
programs, prototypes, processes, machines, articles of manufacture, compositions
of matter, business plans and operations, technical information, drawings, or
specifications; except information which is: (i) at the time of
disclosure, or thereafter becomes lawfully part of the public domain through no
act or omission by the Receiving Party; (ii) lawfully in the possession of
Receiving Party prior to disclosure by or on behalf of Disclosing Party, as
shown by written records; (iii) lawfully disclosed to the Receiving Party by a
third party which did not acquire the same under an obligation of
confidentiality from or through the Disclosing Group; or (iv) independently
developed by the Receiving Party without use of Confidential Information, as
shown by written records. If a Receiving Party believes in good faith
that it is required by law to disclose any Confidential Information, it shall
provide notice to the Disclosing Party, prior to making such disclosure so as to
allow Disclosing Party time to undertake legal or other action, to prevent such
disclosure or otherwise obtain confidential treatment of such
disclosure.
|
BUYER:
________
|
Page 3 of
8
|
SELLER:
________
|
(b) A
Receiving Party shall not, without the prior consent of the Disclosing Party,
disclose any Confidential Information to anyone for any reason at any time or
use any Confidential Information for any purpose except as requested by the
Disclosing Party. The Receiving Party shall limit dissemination of
Confidential Information to only those of the Receiving Group having a "need to
know." A Receiving Party shall not, except as permitted under this
Agreement: (i) appropriate or use a Disclosing Party’s Confidential
Information in Receiving Party’s own manufacture of products for itself or for
any third party or for any other purpose; or (ii) obtain any title to, or any
interest or license in, any Confidential Information of a Disclosing
Party.
(c) Neither
Party shall issue a press release or other public announcement concerning this
Agreement (or any term sheet, bids, negotiations or other related information),
the transactions contemplated herein, or the relationship between the Parties
without the prior written approval of an authorized representative of the other
Party, which such approval the other Party shall not unreasonably withhold or
delay, provided however, a Party may issue a press release, public announcement
or disclosure of this Agreement if required by the regulations of a securities
exchange on which such Party’s securities are listed if (i) it gives the other
Party prompt notice of such requirement and (ii) it cooperates with the other
Party with respect to reasonable requests for revisions to any announcement or
reasonable requests for confidential treatment of certain sections of this
Agreement.
(d) Neither
Party shall: (i) disclose to the other Party any confidential or
proprietary information belonging to any third party without the consent of such
third party; nor (ii) represent as being unrestricted any designs, plans,
models, samples, or other writings or products that the Disclosing Party knows
or has reason to know are covered by Intellectual Property Rights of a third
party.
5. Indemnification.
(a) Buyer
shall indemnify, defend and hold harmless Seller and its affiliates and their respective
directors, officers, employees and agents from and against any claim,
action, suit, demand, damage, expense or losses (including reasonable attorneys’
fees) by a third party (collectively, "Claims") resulting from or to
the extent relating to: (i) Buyer’s manufacture, use, sale and marketing of the
Product, including but not limited to Claims relating to personal injury; or
(ii) infringement or alleged infringement of any third party Intellectual
Property Rights with respect to the sale of the Product.
(b) Seller shall
indemnify, defend and hold harmless Buyer and its affiliates and their respective
directors, officers, employees and agents from and against any Claims
resulting from or to the extent relating to Seller’s material breach of this
Agreement including, without limitation, any material inaccuracy of Seller’s
representations or warranties, or material breach of Seller’s covenants, under
Section 7 hereof.
(c) Either
Party’s (the “indemnifying
Party”) obligations to the other Party (the “indemnified Party”) under this
Section 5 are conditioned upon the indemnified Party: (i) providing
written notice to the indemnifying Party of any Claims promptly, but not later
than fifteen (15) calendar days after the indemnified Party knows of such Claim;
(ii) permitting the indemnifying Party to assume full responsibility for the
defense of such Claim; (iii) assisting the indemnifying Party in defense of such
Claim at the indemnifying Party’s expense; and (iv) not compromising or settling
any such Claim without the indemnifying Party’s prior written
consent. Indemnifying Party may not settle a Claim without the
indemnified Party’s prior written consent, which consent shall not be
unreasonably withheld or delayed, unless such settlement includes a full release
of the indemnified Party from all liability and without any condition of future
consideration. Notwithstanding the foregoing, the indemnified Party’s
failure to give the notice specified in this Section or delay in giving such
notice, shall not affect the indemnified Party’s right to indemnification under
this Section except to the extent that indemnifying Party has been prejudiced by
such failure or delay.
6. Remedies. Termination
of this Agreement, or the exercise of any other remedy, shall not be deemed to
be an exclusive remedy hereunder, and shall be in addition to any other remedies
available at law or in equity (including a Party’s right to obtain specific
performance and other equitable relief for other Party’s material breach
hereof).
7. Representations and
Warranties; Covenants. Each Party hereby represents and
warrants to the other that: (a) the execution and delivery of
and performance under this Agreement by such Party does not, and will not,
conflict with or violate any other agreement or obligations with third parties
or any restrictions of any kind or any law to which it is bound or subject; and
(b) it has the unrestricted right to disclose any information it submits to the
other Party, free of all claims of third parties, and that such disclosures do
not breach or conflict with any confidentiality provisions of any agreement to
which it is a party.
|
BUYER:
________
|
Page 4 of
8
|
SELLER:
________
|
7.1 Purchased
Assets. Seller hereby represents and warrants to Buyer that
Seller has good title to the Purchased Assets, and that the Purchased Assets
transferred or otherwise conveyed hereunder to Buyer are and shall be free and clear of any and all
liabilities, obligations, liens and encumbrances.
7.2 Further
Assurances. Following the closing,
Seller shall, from time to time, execute and deliver such additional
instruments, documents, conveyances or assurances, and take such other actions
as shall be reasonably necessary, or otherwise reasonably requested by the
Buyer, to confirm and assure the rights and obligations provided for in this
Agreement and to render effective the consummation of the transactions
contemplated hereby.
7.3 Authorization. Each Party has the corporate power and
authority to execute and deliver this Agreement, to perform fully its
obligations hereunder, and to consummate the transactions contemplated thereby.
The execution and delivery by each Party of this Agreement, and the consummation
of the transactions contemplated hereby, have been duly authorized by all
requisite corporate action of each Party. This Agreement constitutes
legal, valid and binding obligations of each Party, enforceable against it in
accordance with the terms and conditions hereof.
7.4 Litigation. There is no action, claim, demand,
suit, proceeding, arbitration, grievance, citation, summons, subpoena, inquiry
or investigation of any nature, civil, criminal, regulatory or otherwise, in law
or in equity, pending or threatened against Seller in connection with the
Purchased Assets, and Seller does not know or have reason to be aware of any
basis for the same.
7.5 Brokers,
Finders. All negotiations
relating to this Agreement have been carried on without the participation of any
person acting on behalf of Seller or its affiliates in such manner as to give
rise to any valid claim against the Buyer for any brokerage or finder's
commission, fee or similar compensation, or for any bonus payable to any
officer, director, employee, agent or sales representative of or consultant to
Seller or their respective affiliates upon consummation of the transactions
contemplated hereby.
7.6 Liability
for Transfer Taxes. In the
event any sales (including, without limitation, bulk sales), use, value-added,
documentary, stamp, registration, transfer, conveyance, excise, recording,
license and other similar taxes and fees (collectively, "Transfer Taxes"),
arising out of or in connection with or attributable to the transactions
effected pursuant to this Agreement are due, the Parties shall agree as to which
Party shall prepare any required returns or notices and the Parties shall evenly
split the cost of preparing such returns and the amount of any Transfer Taxes
due.
8.
Miscellaneous
8.1 Except
as specifically set forth in Sections 2.2 and 2.3 neither Party shall assign
this Agreement or its obligations hereunder, whether voluntarily or
involuntarily, without the express prior written consent of the other
Party.
8.2 This
Agreement is fully binding upon the Parties’ successors and permitted
assigns.
8.3 All
requests, approvals, consents and notices must be in writing and will be
effective as of the date actually received and, unless otherwise specified in
this Agreement, shall be sent as follows: (i) certified mail - return receipt
requested; (ii) a nationally recognized overnight delivery service that
guarantees overnight delivery and requires the signature of recipient; or (iii)
facsimile, transmission confirmed; to the addresses and fax numbers indicated on
the first page of this Agreement; provided, however, that in the
case of a facsimile transmission a copy is also sent one of the foregoing
methods of subsection (i) or (ii), above.
8.4 This
Agreement: (i) is governed by the laws of The State of New York,
without reference to its internal principles of conflicts of laws, any disputes
shall be brought exclusively in a federal or state court residing in New York,
and the parties agree without objection to the jurisdiction and venue of such
court; (ii) together with all Exhibits thereto (which are hereby incorporated
into this Agreement) is the entire and exclusive set of terms and conditions and
supersedes all prior agreements and understandings, both written and oral,
between the Parties with respect to the subject matter of this Agreement and
termination of the Distribution Agreement; and (iii) may only be modified by a
writing signed by both Parties.
8.5 Headings
of the articles, sections and subsections of this Agreement, and the name of
this Agreement, are for reference purposes only and shall not limit or affect
the meaning or construction of the terms and conditions
hereof. Whenever the words “include”, “includes” or “including” are
used in this Agreement, they shall be deemed in each instance to be followed by
the words “without limitation.”
|
BUYER:
________
|
Page 5 of
8
|
SELLER:
________
|
8.6 No
failure of either Party to enforce any right under this Agreement shall be
deemed a waiver thereof.
8.7 All
obligations and rights which are by their nature continuing, including the
obligations contained in Sections 2.2, 2.3, 4, 5, 7, and 8 shall survive the
expiration or termination of this Agreement.
ACCEPTANCE
OF AGREEMENT
By
signing below the undersigned acknowledge and accept all terms and conditions of
this Agreement.
| BUYER: BOVIE MEDICAL CORPORATION |
SELLER: BOSTON
SCIENTIFIC CORPORATION
|
||||
|
By
|
/S/
Moshe Citronowicz
|
By:
|
/S/
Michael P. Phalen
|
||
|
(Signature)
|
Signature)
|
||||
|
Print
Name:
|
/S/ Moshe Citronowicz
|
|
Print
Name:
|
/S/ Michael P. Phalen
|
|
Title:
|
Vice President / COO
|
Title: Title:
|
Pres, Oncology
|
|
Date
signed:
|
4-29-08
|
Date
signed:
|
4-30-08
|
|
BUYER:
________
|
Page 6 of
8
|
SELLER:
________
|
Exhibit X: Purchased
Assets
Sect
1.3a)
Equipment, machinery, prototypes, tooling, supplies, and other personal property
predominantly related to the Program
Prototypes
|
12
assembled prototypes in trays
|
(BSC)
|
|
60
porous tips w/o powder coat
|
(BSC)
|
|
20
short samples – blue powder coat
|
(BSC)
|
|
20
laser and resistance welded samples
|
(BSC)
|
|
1
laparoscopic prototype
|
(BSC)
|
|
3
bipolar prototypes
|
(BSC)
|
|
Any
prototype from ** **
|
(Bovie)
|
|
Any
prototype with ** **
|
(Bovie)
|
|
Any
packaging prototypes
|
(Bovie)
|
PDM Project
Files
See 1.3d, below
Equipment
None
Tooling and
Molds
|
All
of BSC’s right, title and interest in component tooling with
Mott
|
||
|
Handle
Molds -9 tools
|
** **
|
|
|
Packaging
Molds
|
** **
|
|
Components and Test
Fixtures
|
Any
components with ** **
|
(Bovie)
|
|
Any
components with ** **
|
(Bovie)
|
Sect.
1.3b) Seller’s right and interest in
and under the contracts, licenses, leases and other agreements and instruments
predominantly related to the Program
Contracts
|
Mott
letter with terms and Conditions
|
(BSC)
|
|
PO’s
to Mott
|
(BSC)
|
|
Mott
quotes
|
(BSC)
|
Section
1.3c) Approvals, clearances, authorizations,
license and registrations required by any governmental authority, predominantly
related to the Program
None
Section
1.3d) Clinical, technical, marketing, pre-sales and
other data, predominantly related to the Program and the design, development and
manufacture of the Product
|
BUYER:
________
|
Page 7 of
8
|
SELLER:
________
|
Product and Marketing
Specifications
|
Product
Spec attached as part of contract
|
(Bovie)
|
|
Drawings
|
(Bovie)
|
|
Customer
Input
|
(BSC)
|
|
BSC
PDM Files
|
(BSC)
|
|
Design
History File
|
(Bovie)
|
Technical Documents,
Testing, Notebook Entries, Schedules and Activities
|
BSC
PDM Files
|
(BSC)
|
|
Personal
Files
|
(BSC)
|
|
Network
Drives
|
(BSC)
|
|
Mott
Documents
|
(BSC)
|
|
** **
Documents
|
(BSC)
|
|
** **Documents
|
(BSC)
|
Meeting Minutes,
Presentations, Trip Reports, Quality Documents, Design
Control
|
BSC
PDM Files
|
(BSC)
|
|
Personal
Files
|
(BSC)
|
|
Network
Drives
|
(BSC)
|
|
Mott
Documents
|
(BSC)
|
|
** **
Documents
|
(BSC)
|
Animal and Bench Testing,
Regulatory Documents, Physician Feedback
|
Video
and Photos
|
(BSC)
|
|
Includes
** ** demo
|
|
|
BSCPDMFiles
|
(BSC)
|
|
Personal
Files
|
(BSC)
|
|
Network
Drives
|
(BSC)
|
Marketing Data, Customer
Input
|
Personal
Files
|
(BSC)
|
|
Network
Drives
|
(BSC)
|
1.3e) Generated
Product IP
Excluded Document
Categories
Only documents or portions thereof, to
the extent they incorporate attorney-client privileged legal analysis, prepared
in response to BSC’s request to its internal or external counsel, related to the
General Product IP. Nothwithstanding the foregoing, patent search
results and drafts of unfiled patent applications shall not be
excluded.
|
BUYER:
________
|
Page 8 of
8
|
SELLER:
________
|
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
1-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
EXHIBIT A
Version A-1 to Exhibit 10.14 – Redacted

BOVIE
–
Resection
Device
Product
Specifications
BOVIE MEDICAL
CORPORATION
CONFIDENTIAL
FOR INTERNAL USE
ONLY
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
2-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
Table of
Contents
|
1 INTRODUCTION
|
4
|
|
1.1 Purpose
|
4
|
|
1.2 Scope
|
4
|
|
2 APPLICABLE
DOCUMENTS
|
4
|
|
2.1 Standards
|
4
|
|
3.0
GENERAL DESCRIPTION
|
4
|
|
3.1
Concept Drawing
|
5
|
|
3.2
Application
|
5
|
|
3.2.1
Intended Use
|
5
|
|
3.2.2
Intended Users
|
6
|
|
3.3
Quality System Requirements
|
6
|
|
3.3.1
US Requirements
|
6
|
|
3.3.2
Canadian Requirements
|
6
|
|
3.3.3
European Classifications
|
7
|
|
3.3.4
Quality System Requirements
|
7
|
|
4 PRODUCT
DOCUMENT STRUCTURE
|
7
|
|
4.1 Project
Documentation
|
7
|
|
4.2 Model Number
Format
|
7
|
|
5 PRODUCT
REQUIREMENTS
|
8
|
|
5.1 Electrical and
Mechanical
|
8
|
|
5.1.1 Insulated
Handle
|
8
|
|
5.1.2 Shaft
and Electrode Tip
|
9
|
|
5.1.3 Shipping
and Handling
|
10
|
|
5.1.4 Sterilization
|
10
|
|
6 Protection
Against Hazards
|
10
|
|
7 Packaging
|
10
|
|
7.1 Labeling
|
10
|
|
7.2 Packaging
Configuration
|
11
|
|
7.3 Manufacturing
|
11
|
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
3-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
List
of Tables
|
Table
One – Standards
|
Page
4
|
|
Table
Two – Device Classifications
|
Page
6
|
|
Table
Three – Model Number Format
|
Page
7
|
|
List
of Figures
|
|
|
Figure
One – Concept Drawing
|
Page
5
|
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
4-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
|
1
|
INTRODUCTION
|
|
1.1
|
Purpose
|
This
Product Specification defines preliminary product requirements and constitutes a
part of the Design Inputs for the Resection Device Project for Boston Scientific
Corporation (“BSC”).
|
1.2
|
Scope
|
This
Product Specification sets forth the requirements, provided by BSC, for the
Resection Device, (the “Device”).
|
2
|
APPLICABLE
DOCUMENTS
|
The
following is a list of all documents and other sources of information referenced
in this Product Specification.
|
2.1
|
Standards
|
|
Standard
|
|
Version/Date
|
|
Description
|
|
21
CFR Part 820
|
Medical
Devices, Current Good Manufacturing Practices, Final Rule, Quality System
Regulation.
|
|||
|
HF-18
|
2001
|
AAMI/ANSI
Electrosurgical Devices
|
||
|
10993-1
|
3;
Date 8-1-03
|
AAMI/ANSI/ISO
Biological evaluation of medical devices – Part 1: Evaluation and
Testing
|
||
|
EN
980
|
2003
04/16/2003
|
Graphical
symbols for use in the labeling of medical devices
|
||
|
EN
ISO 14971
|
2000
AMD 1 2003
03/01/2003
|
Medical
devices – Application of risk management to risk management to medical
devices (ISO 14971:2000)
|
||
|
ISTA
2A 2006
|
2006
|
Performance
Test for Packaged-Products Weighing 150 lbs (68 kg) or
Less
|
Table 1 -
Applicable Standards for Medical Devices (US)
|
3.0
|
GENERAL
DESCRIPTION
|
The
Device is a sterile, single use electrosurgical device intended to be used in
conjunction with an electrosurgical generator for the delivery of radiofrequency
(“RF”) current and sterile saline for hemostatic sealing and coagulation of soft
tissue in accordance with instructions and user procedures provided by
BSC. The device is not intended for any other unspecified uses.
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
5-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
|
3.1
|
Concept
Drawing
|
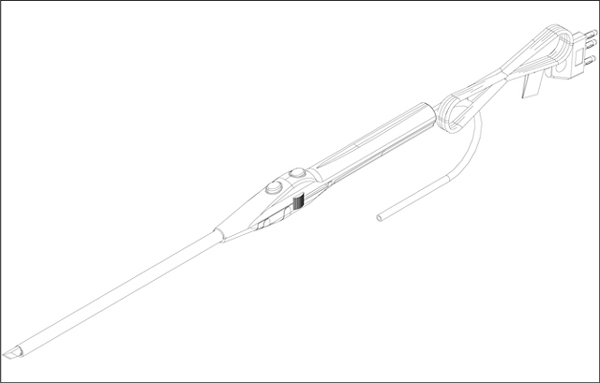
Figure 1
|
3.2
|
Application
|
|
3.2.1
|
Intended
Use
|
The
Device is a sterile, single use electrosurgical device intended to be used in
conjunction with an electrosurgical generator for the delivery of radiofrequency
current and sterile saline for hemostatic sealing and coagulation of the soft
tissue in accordance with instructions and user procedures provided by BSC. The
device is not intended for any other unspecified uses.
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
6-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
|
3.2.2
|
Intended
Users
|
Users of
this Device will be healthcare professionals such as medical doctors and nurses,
who are qualified and trained in electrosurgical procedures.
|
3.3
|
Quality
System Requirements
|
The
Device has the following classifications for FDA, CMDR, and MDD
classification.
|
Device
|
US Classification
|
Canada
Classification
|
MDD
Classification
|
|||
|
Resection
Device
|
II
|
III
|
IIb
|
Table 2 -
Device Classifications
|
3.3.1
|
US
Requirements
|
To market
medical products, the Food and Drug Administration (FDA) must determine that a
medical device is substantially equivalent to similar marketed medical
devices. The Bovie Regulatory department intends to use the
dissecting sealer device manufactured by TissueLink as a predicate
device.
|
3.3.1.1
|
Classification
|
The
Device’s classification is Class II.
|
3.3.1.2
|
Safety
and Effectiveness
requirements
|
The
Device will meet electrical safety requirements (ANSI/AAMI
HF-18:2001).
|
3.3.1.3
|
Quality
System Requirements
|
Quality
system requirements are specified as part of 21 CFR Part 820, Quality System
Regulation.
|
3.3.2
|
Canadian
Requirements
|
To market
medical products in Canada, a license application must be presented to Health
Canada. Once the license application is approved, the medical product can be
labeled and made available for sale in the Canadian provinces.
|
3.3.2.1
|
Classification
|
Class
III
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
7-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
|
3.3.2.2
|
Safety
and Effectiveness
requirements
|
Safety
and effectiveness requirements closely correspond to the essential requirements
of the Medical Device Directive. A list of Health Canada recognized
standards has been issued as of April 11, 2002 in Policy on Recognition and Use
of Standards under Medical Device Regulations.
|
3.3.2.3
|
Quality
System Requirements
|
Health
Canada requires manufacturers of Class II, III and IV device to demonstrate that
their devices are manufactured in accordance with internationally recognized
standards. Demonstration of compliance with the quality system
requirements will be required at the time an application is made for a medical
device license.
|
3.3.3
|
European
Classifications
|
|
3.3.3.1
|
Classification
|
The
Device is classified IIb (Rule 9 ) in accordance with the Medical Devices
Directive Annex IX.
(Reference:
Guidelines for the Classification of Medical Devices – MEDDEV 2.4/1 Rev. 8, July
2001)
|
3.3.3.2
|
Conformance
Assessment Route
|
The
Device assessment route will be via Annex II (full quality assurance
system).
|
3.3.4
|
Quality
System
Requirements
|
Bovie
Medical Corporation (“Bovie”) has been certified to ISO13485
|
4
|
PRODUCT
DOCUMENT STRUCTURE
|
|
4.1
|
Project Documentation
|
Documents
will be in standard Bovie document format.
|
4.2
|
Model Number Format
|
The
following table lists the Device’s model number and corresponding product /
catalog code.
|
Catalog #
|
Comments
|
|
|
TBD
|
Laparoscopic Monopolar
Device
|
|
|
TBD
|
Open
Abdominal Monopolar
Device
|
Table
Three
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
8-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
|
5
|
PRODUCT
REQUIREMENTS
|
The
following section will describe requirements for the insulated handle and shaft
with electrode tip.
|
5.1
|
Electrical
and Mechanical
|
The
Device hardware is comprised of two (2) major components: (i) an Insulated
Handle and (ii) a Shaft with Electrode Tip. RF energy is passed through the
Insulated Handle through the Shaft to the Electrode Tip by a powered lead from
an electrosurgical generator.
|
5.1.1
|
Insulated
Handle
|
The
Insulated Handle encases the controlling mechanism for the flow of saline, and
activation and selection of the RF current for the Device.
5.1.1.1
Insulated Handle and Power Cord Insulation Resistance
The
insulation of the Insulated Handle and Power Cord shall meet Requirements of
Section 4.2.5.4 Dielectric withstands of accessories ANSI/AAMI
HF-18:2001
5.1.1.2
Insulated Handle Ergonomics
The
Insulated Handle shall be designed for comfortable and efficient hand
operation. It will incorporate an over-mold to give the body an
elegant feel and soft touch.
5.1.1.3
Insulated Handle Electrical Cord
The
Insulated Handle Electrical Cord shall be approximately ten (10) feet in length
and incorporate a 3-prong electrical plug.
5.1.1.4
Insulated Handle Flow Control Mechanism
The
Device will have a flow control mechanism, either on the tubing or in the handle
itself so the flow can be regulated by the user within the sterile
field.
5.1.1.5
Insulated Handle Tubing Length
The
tubing length should be approximately seven (7) feet in length and incorporate
an I.V. spike on the end to attach directly to a hanging IV bag.
5.1.1.6
Insulated Handle Tubing Material
The
tubing material should be a material that will resist kinking but should not
have so strong a memory as to pull the probe off of the sterile
field.
5.1.1.7
Insulated Handle Activation Buttons
The Device should have a Cut and Coagulation Mode. “Cut”/”Coag”
buttons must be easy to operate.
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
9-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
5.1.1.8
Insulated Handle Compatibility
|
|
·
|
The
Insulated Handle should have compatibility with standard RF generators
used for electrocautery and standard grounding
pad.
|
|
|
·
|
The
Insulated Handle should have compatibility with standard 0.9% saline I.V.
bag.
|
|
5.1.2
|
Shaft
and Electrode Tip
|
The Shaft
and Electrode Tip provide the actual working portion of this
system.
5.1.2.1
Saline Flow Rate of Device
The
Saline flow rate prior to use will be
** **
5.1.2.2
Device (Shaft) Length
The
Device should be straight and have two (2) lengths: one for laparoscopic
monopolar procedures (approximately thirty-two (32) cm) and one for open
monopolar procedures (approximately fourteen (14) cm).
5.1.2.3
Shaft Configuration
The Shaft
will be hollow to allow for fluid to flow to the sintered stainless steel tip.
The inside diameter of the Shaft should be equal to the inside diameter of the
tubing that feeds fluid to the Shaft to minimize any flow restriction in the
fluid circuit.
5.1.2.4
Tip Material
The Tip
shall be made of ** **
.
5.1.2.5
Tip Strength
The
porous blade tip to shaft tensile bond strength (axial mode) must be greater
than ** **
after
being subjected to a full-power simulated use duty cycle.
The
porous blade tip to shaft bond strength in an flexural mode must be greater than
** **
. after being subjected to a full-power simulated use duty
cycle.
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
10-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
5.1.2.6
Tip performance
|
|
·
|
Bovie
will exert its best efforts to meet the design goal that tip cutting
performance be superior to competitive devices with respect to cutting
speed. Tip will have the ability to coagulate soft tissue when performed
in a suitable simulated use
environment.
|
|
5.1.3
|
Shipping
and Handling
|
4.1.3.1
Shipping Temperatures
Tropical
(Wet and Dry) Conditions and Winter (Frozen) Conditions per ISTA
2A.
4.1.3.2
Transportation Testing
The Final
Product Packaging Configuration for the Device shall meet the finished device
requirements after being subjected to Simulated Transportation Conditioning per
ISTA 2A.
|
5.1.4
|
Sterilization
|
5.1.4.1
Withstand 2x ETO Sterilization.
5.1.4.2
EN 550: 1994 – Sterilization of Medical Devices – Validation and routine control
of ethylene oxide sterilization.
5.1.4.3
AAMI/ANSI/ISO 11135 -1994 Medical Devices – Validation and routine control of
ethylene oxide sterilization.
|
6
|
Protection
Against Hazards
|
A
Hazard/Risk Analysis will be performed throughout the design
process. The Hazard/Risk Analysis will be documented and filed as
part of the design history file.
|
7
|
Packaging
|
|
7.1
|
Labeling
|
7.1.1
Package must be labeled in accordance with both BSC and Bovie Labeling
Standards.
7.1.2 BSC and Bovie branding, including mutually approved trademarks, will
be on the Handle and/or the shaft.
7.1.3 The Device is to have a three (3) year shelf life under normal storage
conditions.
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
11-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
|
7.2
|
Packaging Configuration
|
|
|
·
|
The
Tip and Shaft protector shall be included into the
Design.
|
|
|
·
|
The
Scratch Pad shall be packaged with the
Device.
|
|
|
·
|
The
Device will be packaged in a Sterile
Pouch.
|
|
7.3
|
Manufacturing
|
Final
assembly and packaging will be performed by Bovie.
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-1
|
Page
12-12
|
|
|
Title: Product
Specification: Resection
Device
|
||||
APPROVALS
|
Author
|
|||
|
Thomas
Feldhaus
|
Date
|
||
|
Sales
& Marketing
|
|||
|
Rick
Pfahl
|
Date
|
||
|
Regulatory
|
|||
|
Rick
Kozloff
|
Date
|
||
|
Quality
|
|||
|
John
Woody
|
Date
|
||
|
Manufacturing
|
|||
|
Lillian
Eshem
|
Date
|
||
|
Engineering
|
|||
|
Fred
Baron
|
Date
|
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document Number
|
Rev
Level
A-2
|
Page
1-11
|
|
|
Title: Product
Specification: Resection Device
|
||||
EXHIBIT A
Version A-2 to Exhibit 10.14 – REDACTED

BOVIE
–
Resection
Device
Product
Specifications
BOVIE MEDICAL
CORPORATION
CONFIDENTIAL
FOR INTERNAL USE
ONLY
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document Number
|
Rev
Level
A-2
|
Page
2-11
|
|
|
Title: Product
Specification: Resection Device
|
||||
Table of
Contents
|
1 INTRODUCTION
|
4
|
|
1.1 Purpose
|
4
|
|
1.2 Scope
|
4
|
|
2 APPLICABLE
DOCUMENTS
|
4
|
|
2.1 Standards
|
4
|
|
3.0
GENERAL DESCRIPTION
|
4
|
|
3.1
Concept Drawing
|
5
|
|
3.2
Application
|
5
|
|
3.2.1
Intended Use
|
5
|
|
3.2.2
Intended Users
|
5
|
|
3.3
Quality System Requirements
|
6
|
|
3.3.1
US Requirements
|
6
|
|
3.3.2
Canadian Requirements
|
6
|
|
3.3.3
European Classifications
|
7
|
|
3.3.4
Quality System Requirements
|
7
|
|
4 PRODUCT
DOCUMENT STRUCTURE
|
7
|
|
4.1 Project
Documentation
|
7
|
|
4.2 Model
Number Format
|
7
|
|
5 PRODUCT
REQUIREMENTS
|
7
|
|
5.1
Electrical and
Mechanical
|
8
|
|
8
|
|
|
5.1.2
Shaft and Electrode Tip
|
9
|
|
5.1.3
Shipping and Handling
|
10
|
|
5.1.4
Sterilization
|
10
|
|
6 Protection
Against Hazards
|
10
|
|
7 Packaging
|
10
|
|
7.1 Labeling
|
10
|
|
10
|
|
|
7.3 Manufacturing
|
10
|
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document Number
|
Rev
Level
A-2
|
Page
3-11
|
|
|
Title: Product
Specification: Resection Device
|
||||
|
List
of Tables
|
|
|
Table
One – Standards
|
Page
4
|
|
Table
Two – Device Classifications
|
Page
6
|
|
Table
Three – Model Number Format
|
Page
7
|
|
List
of Figures
|
|
|
Figure
One – Concept Drawing
|
Page
5
|
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document Number
|
Rev
Level
A-2
|
Page
4-11
|
|
|
Title: Product
Specification: Resection Device
|
||||
|
1
|
INTRODUCTION
|
|
1.1
|
Purpose
|
This
Product Specification defines preliminary product requirements and constitutes a
part of the Design Inputs for the Resection Device Project for Boston Scientific
Corporation (“BSC”).
|
1.2
|
Scope
|
This
Product Specification sets forth the requirements, provided by BSC, for the
Resection Device, (the “Device”).
|
2
|
APPLICABLE
DOCUMENTS
|
The
following is a list of all documents and other sources of information referenced
in this Product Specification.
|
2.1
|
Standards
|
|
Standard
|
Version/Date
|
Description
|
||
|
21
CFR Part 820
|
Medical
Devices, Current Good Manufacturing Practices, Final Rule, Quality System
Regulation.
|
|||
|
HF-18
|
2001
|
AAMI/ANSI
Electrosurgical Devices
|
||
|
10993-1
|
3;
Date 8-1-03
|
AAMI/ANSI/ISO
Biological evaluation of medical devices – Part 1: Evaluation and
Testing
|
||
|
EN
980
|
2003
04/16/2003
|
Graphical
symbols for use in the labeling of medical devices
|
||
|
EN
ISO 14971
|
2000
AMD 1 2003
03/01/2003
|
Medical
devices – Application of risk management to risk management to medical
devices (ISO 14971:2000)
|
||
|
ISTA
2A 2006
|
2006
|
Performance
Test for Packaged-Products Weighing 150 lbs (68 kg) or
Less
|
Table 1 -
Applicable Standards for Medical Devices (US)
3.0
GENERAL DESCRIPTION
The
Device is a sterile, single use electrosurgical device intended to be used in
conjunction with an electrosurgical generator for the delivery of radiofrequency
(“RF”) current and sterile saline for hemostatic sealing and coagulation of soft
tissue in accordance with instructions and user procedures provided by
BSC. The device is not intended for any other unspecified uses.
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document Number
|
Rev
Level
A-2
|
Page
5-11
|
|
|
Title: Product
Specification: Resection Device
|
||||
3.1
Concept Drawing

Figure
1
3.2
Application
3.2.1
Intended Use
The
Device is a sterile, single use electrosurgical device intended to be used in
conjunction with an electrosurgical generator for the delivery of radiofrequency
current and sterile saline for hemostatic sealing and coagulation of the soft
tissue in accordance with instructions and user procedures provided by BSC. The
device is not intended for any other unspecified uses.
3.2.2
Intended Users
Users of
this Device will be healthcare professionals such as medical doctors and nurses,
who are qualified and trained in electrosurgical procedures.
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document Number
|
Rev
Level
A-2
|
Page
6-11
|
|
|
Title: Product
Specification: Resection Device
|
||||
3.3
Quality System Requirements
The
Device has the following classifications for FDA, CMDR, and MDD
classification.
|
Device
|
US
Classification
|
Canada
Classification
|
|
MDD
Classification
|
||
|
Resection
Device
|
II
|
III
|
IIb
|
Table 2 -
Device Classifications
3.3.1
US Requirements
To market
medical products, the Food and Drug Administration (FDA) must determine that a
medical device is substantially equivalent to similar marketed medical
devices. The Bovie Regulatory department intends to use the
dissecting sealer device manufactured by TissueLink as a predicate
device.
3.3.1.1
Classification
The
Device’s classification is Class II.
3.3.1.2
Safety and Effectiveness requirements
The
Device will meet electrical safety requirements (ANSI/AAMI
HF-18:2001).
3.3.1.3
Quality System Requirements
Quality
system requirements are specified as part of 21 CFR Part 820, Quality System
Regulation.
3.3.2
Canadian Requirements
To market
medical products in Canada, a license application must be presented to Health
Canada. Once the license application is approved, the medical product can be
labeled and made available for sale in the Canadian provinces.
3.3.2.1
Classification
Class
III
3.3.2.2
Safety and Effectiveness requirements
Safety
and effectiveness requirements closely correspond to the essential requirements
of the Medical Device Directive. A list of Health Canada recognized
standards has been issued as of April 11, 2002 in Policy on Recognition and Use
of Standards under Medical Device Regulations.
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document Number
|
Rev
Level
A-2
|
Page
7-11
|
|
|
Title: Product
Specification: Resection Device
|
||||
3.3.2.3
Quality System Requirements
Health
Canada requires manufacturers of Class II, III and IV device to demonstrate that
their devices are manufactured in accordance with internationally recognized
standards. Demonstration of compliance with the quality system
requirements will be required at the time an application is made for a medical
device license.
3.3.3
European Classifications
3.3.3.1
Classification
The
Device is classified IIb (Rule 9 ) in accordance with the Medical Devices
Directive Annex IX.
(Reference:
Guidelines for the Classification of Medical Devices – MEDDEV 2.4/1 Rev. 8, July
2001)
3.3.3.2
Conformance Assessment Route
The
Device assessment route will be via Annex II (full quality assurance
system).
3.3.4
Quality System Requirements
Bovie
Medical Corporation (“Bovie”) has been certified to ISO13485
|
4
|
PRODUCT
DOCUMENT STRUCTURE
|
|
4.1
|
Project
Documentation
|
Documents
will be in standard Bovie document format.
|
4.2
|
Model
Number Format
|
The
following table lists the Device’s model number and corresponding product /
catalog code.
|
Catalog
#
|
Comments
|
|
|
TBD
|
Laparoscopic Monopolar
Device
|
|
|
TBD
|
Open
Abdominal Monopolar
Device
|
Table
Three
|
5
|
PRODUCT
REQUIREMENTS
|
The
following section will describe requirements for the insulated handle and shaft
with electrode tip.
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document Number
|
Rev
Level
A-2
|
Page
8-11
|
|
|
Title: Product
Specification: Resection Device
|
||||
|
5.1
|
Electrical
and Mechanical
|
The
Device hardware is comprised of two (2) major components: (i) an Insulated
Handle and (ii) a Shaft with Electrode Tip. RF energy is passed through the
Insulated Handle through the Shaft to the Electrode Tip by a powered lead from
an electrosurgical generator.
|
5.1.1
|
Insulated
Handle
|
The Insulated Handle encases the controlling
mechanism for the flow of saline, and activation and selection of the RF current
for the Device.
5.1.1.1
Insulated Handle and Power Cord Insulation Resistance
The
insulation of the Insulated Handle and Power Cord shall meet Requirements of
Section 4.2.5.4 Dielectric withstands of accessories ANSI/AAMI
HF-18:2001
5.1.1.2
Insulated Handle Ergonomics
The
Insulated Handle shall be designed for comfortable and efficient hand
operation. It will incorporate an over-mold to give the body an
elegant feel and soft touch.
The
Insulated Handle Electrical Cord shall be approximately ten (10) feet in length
and incorporate a 3-prong electrical plug.
5.1.1.4
Insulated Handle Flow Control Mechanism
The
Device will have a flow control mechanism in the handle so the flow can be
regulated by the user within the sterile field.
The
tubing length should be approximately ten (10) feet in length and incorporate an
I.V. spike on the end to attach directly to a hanging IV bag.
5.1.1.6
Insulated Handle Tubing Material
The
tubing material should be a material that will resist kinking but should not
have so strong a memory as to pull the probe off of the sterile
field.
5.1.1.8
Insulated Handle Compatibility
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document Number
|
Rev
Level
A-2
|
Page
9-11
|
|
|
Title: Product
Specification: Resection Device
|
||||
|
|
·
|
The
Insulated Handle should have compatibility with standard RF generators
used for electrocautery and standard grounding
pad.
|
|
|
·
|
The
Insulated Handle should have compatibility with standard 0.9% saline I.V.
bag.
|
|
Shaft
and Electrode Tip
|
The Shaft
and Electrode Tip provide the actual working portion of this
system.
5.1.2.1
Saline Flow Rate of Device
The
Saline flow rate prior to use will be
** **.
5.1.2.2
Device (Shaft) Length
The
Device should be straight and have two (2) lengths: one for laparoscopic
monopolar procedures (approximately thirty-two (32) cm) and one for open
monopolar procedures (approximately fourteen (14) cm).
5.1.2.3
Shaft Configuration
The Shaft
will be hollow to allow for fluid to flow to the sintered stainless steel tip.
The inside diameter of the Shaft should be equal to the inside diameter of the
tubing that feeds fluid to the Shaft to minimize any flow restriction in the
fluid circuit.
5.1.2.4
Tip Material
The Tip
shall be made of ** **.
5.1.2.5
Tip Strength
The
porous blade tip to shaft tensile bond strength (axial mode) must be greater
than ** ** after being subjected to a
full-power simulated use duty cycle.
The
porous blade tip to shaft bond strength in an flexural mode must be greater than
** ** after being subjected to a
full-power simulated use duty cycle.
5.1.2.6
Tip performance
|
|
·
|
Bovie
will exert its best efforts to meet the design goal that tip cutting
performance be superior to competitive devices with respect to cutting
speed. Tip will have the ability to coagulate soft tissue when performed
in a suitable simulated use
environment.
|
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document Number
|
Rev
Level
A-2
|
Page
10-11
|
|
|
Title: Product
Specification: Resection Device
|
||||
|
Shipping
and Handling
|
4.1.3.1
Shipping Temperatures
Tropical
(Wet and Dry) Conditions and Winter (Frozen) Conditions per ISTA
2A.
4.1.3.2
Transportation Testing
The Final
Product Packaging Configuration for the Device shall meet the finished device
requirements after being subjected to Simulated Transportation Conditioning per
ISTA 2A.
|
5.1.4
|
Sterilization
|
5.1.4.1
Withstand 2x ETO Sterilization.
5.1.4.2
EN 550: 1994 – Sterilization of Medical Devices – Validation and routine control
of ethylene oxide sterilization.
5.1.4.3
AAMI/ANSI/ISO 11135 -1994 Medical Devices – Validation and routine control of
ethylene oxide sterilization.
|
Protection
Against Hazards
|
A
Hazard/Risk Analysis will be performed throughout the design
process. The Hazard/Risk Analysis will be documented and filed as
part of the design history file.
|
Packaging
|
|
7.1
|
Labeling
|
|
|
7.1.1
Package must be labeled in accordance with both BSC and Bovie
Labeling Standards.
|
|
|
7.1.2
BSC and Bovie branding, including mutually approved trademarks, will
be on the Handle and/or the shaft.
|
7.1.3
The Device is to have a three (3) year shelf life under normal storage
conditions.
|
Packaging
Configuration
|
|
|
·
|
The
Tip and Shaft protector shall be included into the
Design.
|
|
|
·
|
The
Scratch Pad shall be packaged with the
Device.
|
|
|
·
|
The
Device will be packaged in a Sterile
Pouch.
|
|
7.3
|
Manufacturing
|
Final
assembly and packaging will be performed by
Bovie.
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
|
Author
Thomas
Feldhaus
|
Document
Number
|
Rev
Level
A-2
|
Page
11-11
|
|
|
Title: Product
Specification: Resection Device
|
||||
APPROVALS
|
Author
|
|||
|
Thomas
Feldhaus
|
Date
|
||
|
Sales
& Marketing
|
|||
|
Rick
Pfahl
|
Date
|
||
|
Regulatory
|
|||
|
Rick
Kozloff
|
Date
|
||
|
Quality
|
|||
|
John
Woody
|
Date
|
||
|
Manufacturing
|
|||
|
Lillian
Eshem
|
Date
|
||
|
Engineering
|
|||
|
Fred
Baron
|
Date
|
BOVIE
MEDICAL CONFIDENTIAL
THIS
DOCUMENT IS THE PROPERTY OF BOVIE MEDICALCORPORATION AND MUST BE ACCOUNTED
FOR. INFORMATION HEREIN IS CONFIDENTIAL. DO NOT REPRODUCE,
USE, OR CONVEY ANY PORTION OF THIS DOCUMENT TO UNAUTHORIZED PERSONS, WITHOUT
PROPER AUTHORIZATION.
