Attached files
 © 2009 EnteroMedics EMPOWER Study Summary of Ongoing Review November 2009 Exhibit 99.3 |
 © 2009 EnteroMedics EMPOWER Efficacy Outcomes |
 © 2009 EnteroMedics Optimal Hours of Usage Mean EWL from Implant (BMI Method) with Standard Error at 12 Months by Categories of Average Hours of Use per Day through 12 Months
|
 © 2009 EnteroMedics EWL Through 12 Months Mean EWL from Implant (BMI Method) by Average Hours of Use per Day with Standard Error for 12 Month Completer Cohort -30 -25 -20 -15 -10 -5 0 Study Visit < 9 hours/day >= 9 hours/day N=125 N=128 p<0.0001 at 6 and 12 Months N=118 N=126 Average Use = 6.9 hrs/day Average Use = 10.9 hrs/day |
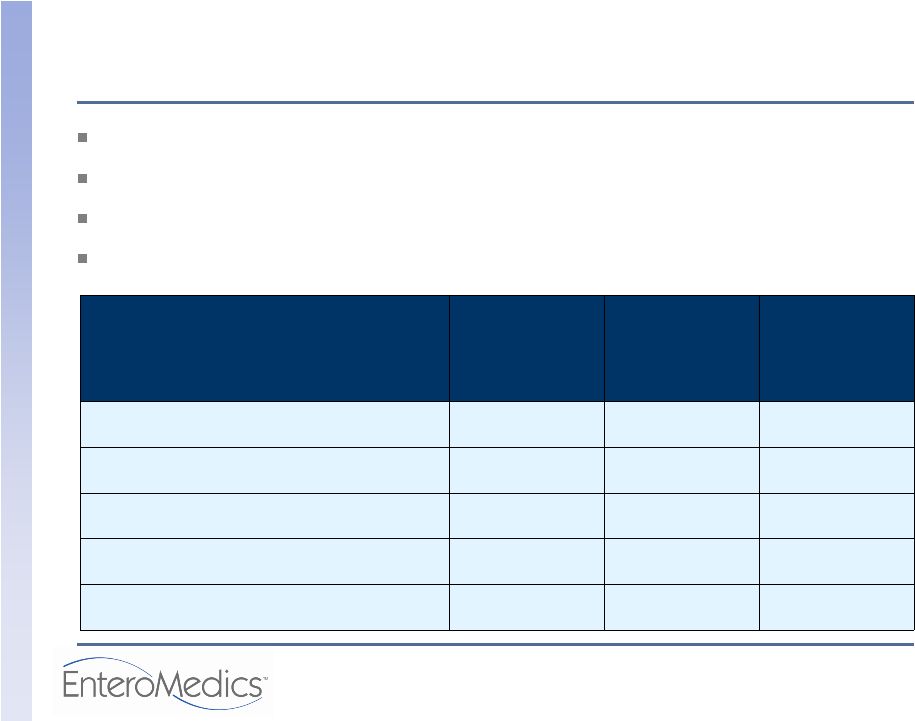 © 2009 EnteroMedics Safety Outcomes: Serious Adverse Events (SAEs) No deaths No Unanticipated Adverse Device Effects (UADEs) 30 SAEs (see table below) No therapy-related SAEs Clinical Events Committee- Adjudicated Relatedness of SAE (Event Origin) VBLOC Therapy N=192 Control N=102 Overall N=294 Device 3 (1.6%) 1 (1.0%) 4 (1.4%) General surgical procedure 4 (2.1%) 0 (0.0%) 4 (1.4%) Implant/revision procedure 3 (1.6%) 2 (2.0%) 5 (1.7%) Pre-existing condition 9 (4.7%) 8 (7.8%) 17 (5.8%) Therapy algorithm 0 (0.0%) 0 (0.0%) 0 (0.0%) |
 © 2009 EnteroMedics Safety Outcomes: Study Withdrawals 8.5% of patients withdrew in the first 12 Months |
 © 2009 EnteroMedics EMPOWER Study Summary of Ongoing Review November 2009 |
