Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - TCR2 THERAPEUTICS INC. | d212739dex991.htm |
| 8-K - 8-K - TCR2 THERAPEUTICS INC. | d212739d8k.htm |

gavo-cel Phase 1/2 Clinical Update September 2021 Exhibit 99.2

Forward Looking Statements This presentation has been prepared by TCR2 Therapeutics Inc. (“we,” “us,” or “our”) and contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our clinical results and other future conditions. All statements, other than statements of historical facts, contained in this presentation, including express or implied statements regarding our expectations for the Phase 1/2 clinical trials of gavo-cel and TC-110, our expectations for the safety and efficacy of our product candidates and enhancements, including gavo-cel and TC-110, compared to current T-cell therapy approaches, our expectations regarding the timing of determining an RP2D for gavo-cel, our expectations regarding the estimated patient populations and related market opportunities in gavo-cel’s and TC-110’s targeted indications, and our expectations regarding manufacturing of our product candidates are forward-looking statements. These statements are based on management’s current expectations and beliefs and are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others: uncertainties inherent in clinical studies and in the availability and timing of data from ongoing clinical studies; whether interim results from a clinical trial will be predictive of the final results of a trial; the possibility that positive results from preclinical studies and correlative studies may not necessarily be predictive of the results of our planned clinical trials, including the Phase 1/2 clinical trials of gavo-cel and TC-110; the risk that the results from the Phase 1/2 clinical trials of gavo-cel and TC-110 will not support further development and marketing approval; the risk that we may be unable to gain approval of gavo-cel, TC-110 and our other product candidates on a timely basis, if at all; the risk that we have over-estimated the potential patient population for our product candidates, if approved; the risk that the current COVID-19 pandemic will impact our clinical trials and other operations; and the other risks set forth under the caption “Risk Factors” in our most recent Annual Report on Form 10-K for the year ended December 31, 2020, as filed with the SEC on March 16, 2021, and in our future filings with the SEC available at the SEC’s website at www.sec.gov. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. You should not place undue reliance on any forward‐looking statements, which speak only as of the date they are made. While we may elect to update these forward-looking statements at some point in the future, we assume no obligation to update or revise any forward-looking statements except to the extent required by applicable law. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements.

Introduction Garry Menzel, PhD Chief Executive Officer
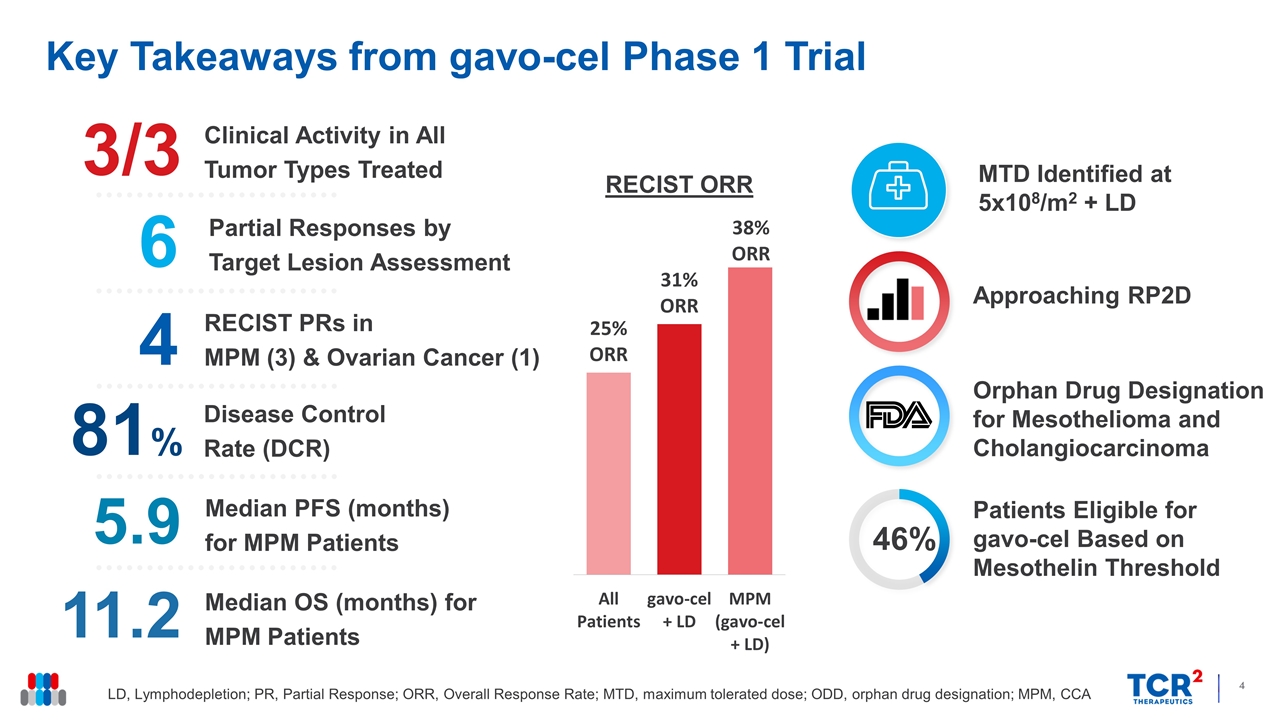
Key Takeaways from gavo-cel Phase 1 Trial LD, Lymphodepletion; PR, Partial Response; ORR, Overall Response Rate; MTD, maximum tolerated dose; ODD, orphan drug designation; MPM, CCA Approaching RP2D Orphan Drug Designation for Mesothelioma and Cholangiocarcinoma Median OS (months) for MPM Patients 11.2 Disease Control Rate (DCR) 81% Clinical Activity in All Tumor Types Treated 3/3 RECIST PRs in MPM (3) & Ovarian Cancer (1) 6 Partial Responses by Target Lesion Assessment 4 Median PFS (months) for MPM Patients 5.9 MTD Identified at 5x108/m2 + LD 46% Patients Eligible for gavo-cel Based on Mesothelin Threshold RECIST ORR

gavo-cel Clinical Trial Review Alfonso Quintás-Cardama, MD Chief Medical Officer
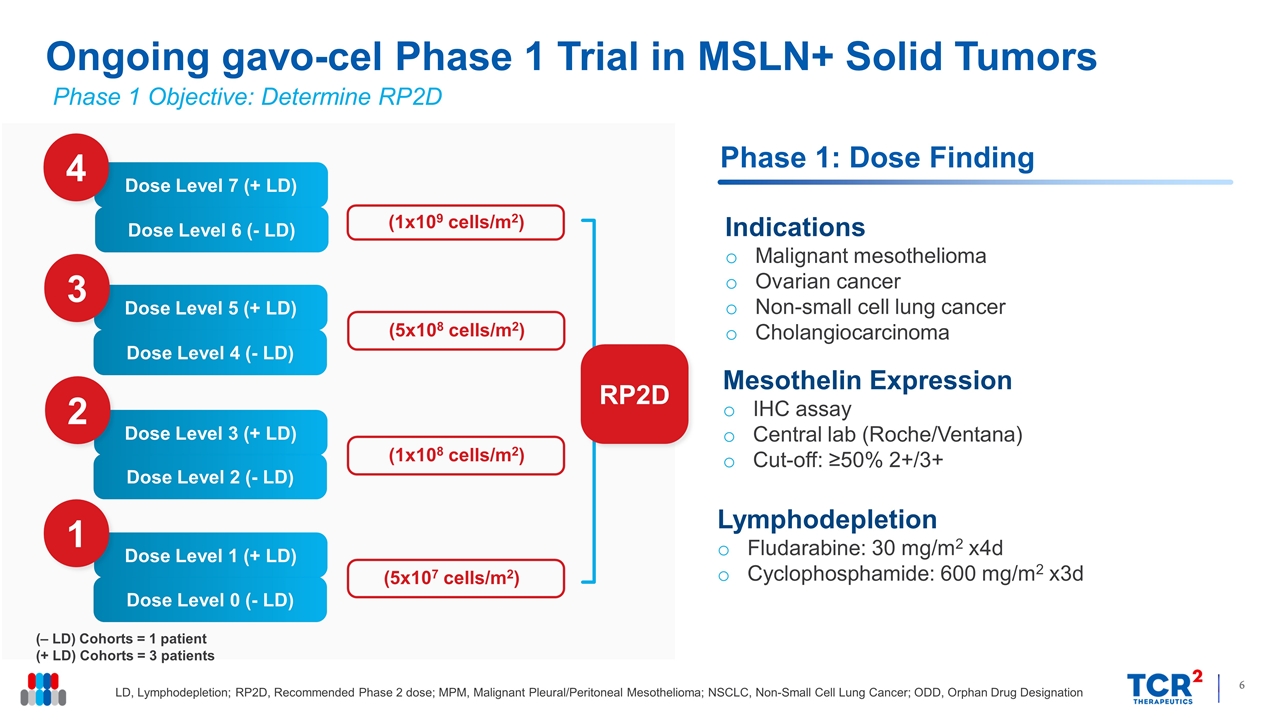
Ongoing gavo-cel Phase 1 Trial in MSLN+ Solid Tumors LD, Lymphodepletion; RP2D, Recommended Phase 2 dose; MPM, Malignant Pleural/Peritoneal Mesothelioma; NSCLC, Non-Small Cell Lung Cancer; ODD, Orphan Drug Designation RP2D Phase 1: Dose Finding (‒ LD) Cohorts = 1 patient (+ LD) Cohorts = 3 patients Dose Level 1 (+ LD) Dose Level 0 (- LD) 1 Dose Level 3 (+ LD) Dose Level 2 (- LD) 2 Dose Level 5 (+ LD) Dose Level 4 (- LD) 3 Dose Level 7 (+ LD) Dose Level 6 (- LD) 4 (5x107 cells/m2) (1x108 cells/m2) (5x108 cells/m2) (1x109 cells/m2) Lymphodepletion Fludarabine: 30 mg/m2 x4d Cyclophosphamide: 600 mg/m2 x3d Mesothelin Expression IHC assay Central lab (Roche/Ventana) Cut-off: ≥50% 2+/3+ Indications Malignant mesothelioma Ovarian cancer Non-small cell lung cancer Cholangiocarcinoma Phase 1 Objective: Determine RP2D
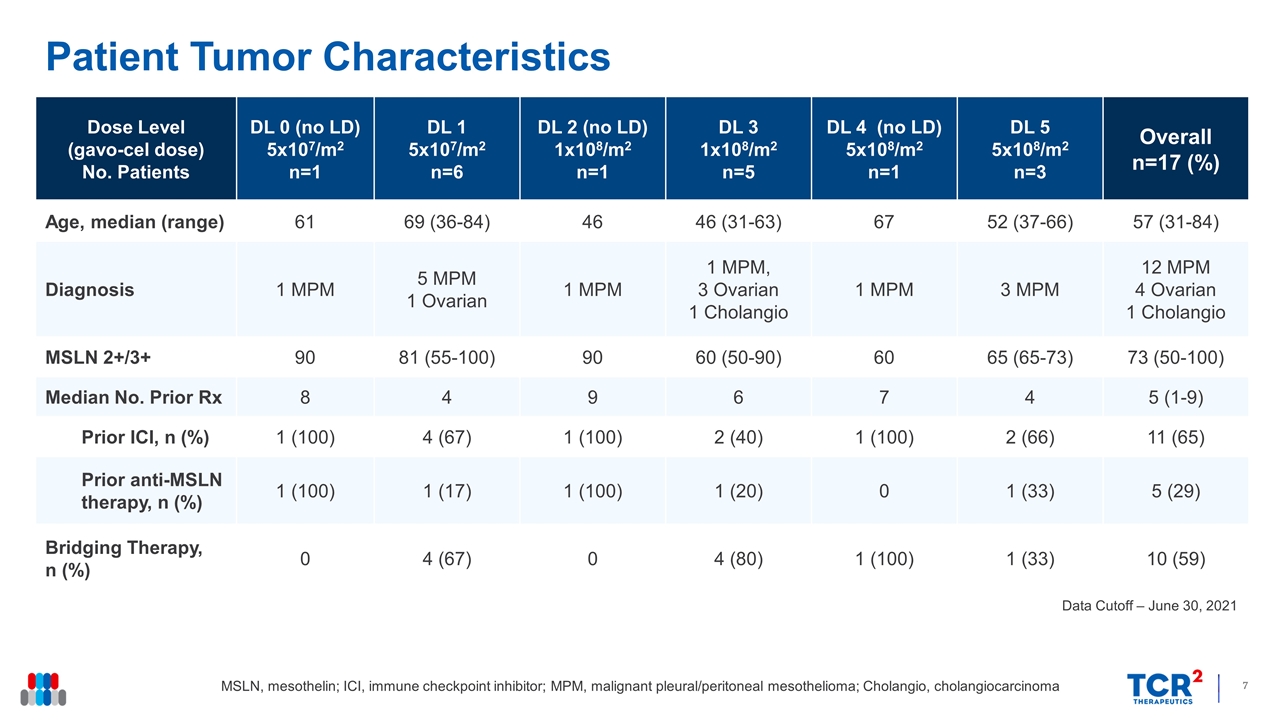
Patient Tumor Characteristics Data Cutoff – June 30, 2021 Dose Level (gavo-cel dose) No. Patients DL 0 (no LD) 5x107/m2 n=1 DL 1 5x107/m2 n=6 DL 2 (no LD) 1x108/m2 n=1 DL 3 1x108/m2 n=5 DL 4 (no LD) 5x108/m2 n=1 DL 5 5x108/m2 n=3 Overall n=17 (%) Age, median (range) 61 69 (36-84) 46 46 (31-63) 67 52 (37-66) 57 (31-84) Diagnosis 1 MPM 5 MPM 1 Ovarian 1 MPM 1 MPM, 3 Ovarian 1 Cholangio 1 MPM 3 MPM 12 MPM 4 Ovarian 1 Cholangio MSLN 2+/3+ 90 81 (55-100) 90 60 (50-90) 60 65 (65-73) 73 (50-100) Median No. Prior Rx 8 4 9 6 7 4 5 (1-9) Prior ICI, n (%) 1 (100) 4 (67) 1 (100) 2 (40) 1 (100) 2 (66) 11 (65) Prior anti-MSLN therapy, n (%) 1 (100) 1 (17) 1 (100) 1 (20) 0 1 (33) 5 (29) Bridging Therapy, n (%) 0 4 (67) 0 4 (80) 1 (100) 1 (33) 10 (59) MSLN, mesothelin; ICI, immune checkpoint inhibitor; MPM, malignant pleural/peritoneal mesothelioma; Cholangio, cholangiocarcinoma
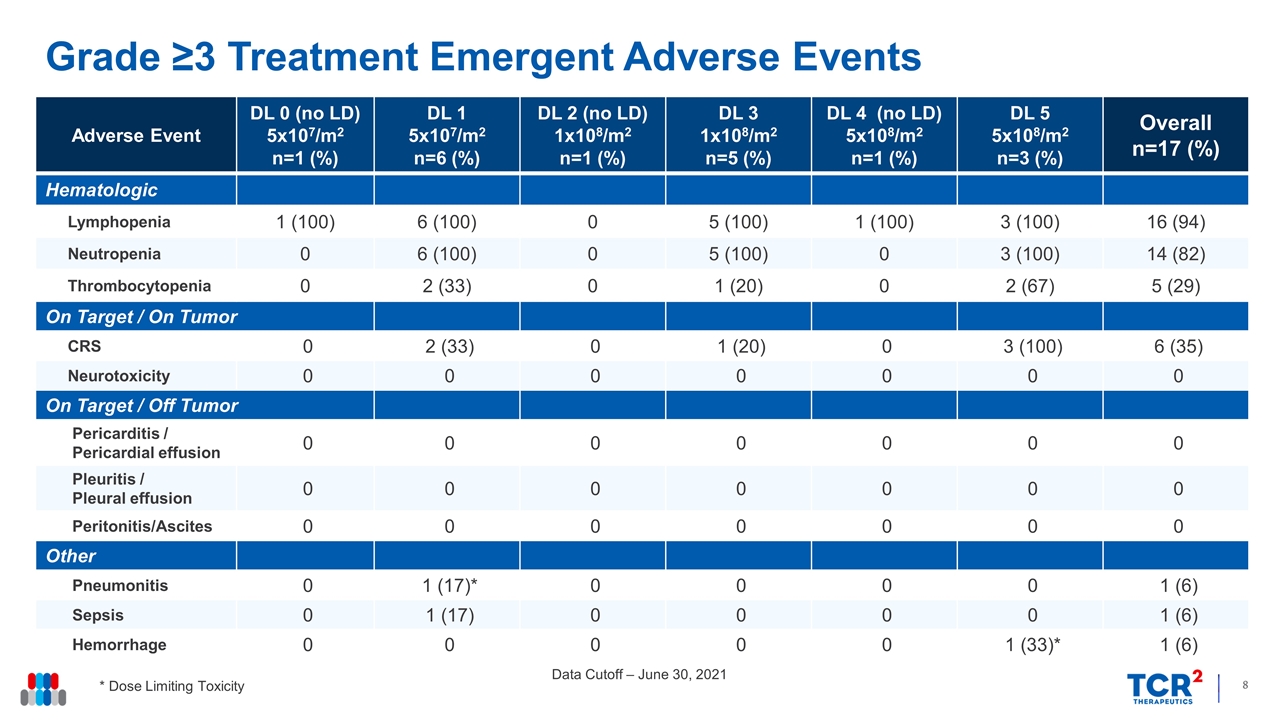
Grade ≥3 Treatment Emergent Adverse Events Data Cutoff – June 30, 2021 * Dose Limiting Toxicity Adverse Event DL 0 (no LD) 5x107/m2 n=1 (%) DL 1 5x107/m2 n=6 (%) DL 2 (no LD) 1x108/m2 n=1 (%) DL 3 1x108/m2 n=5 (%) DL 4 (no LD) 5x108/m2 n=1 (%) DL 5 5x108/m2 n=3 (%) Overall n=17 (%) Hematologic Lymphopenia 1 (100) 6 (100) 0 5 (100) 1 (100) 3 (100) 16 (94) Neutropenia 0 6 (100) 0 5 (100) 0 3 (100) 14 (82) Thrombocytopenia 0 2 (33) 0 1 (20) 0 2 (67) 5 (29) On Target / On Tumor CRS 0 2 (33) 0 1 (20) 0 3 (100) 6 (35) Neurotoxicity 0 0 0 0 0 0 0 On Target / Off Tumor Pericarditis / Pericardial effusion 0 0 0 0 0 0 0 Pleuritis / Pleural effusion 0 0 0 0 0 0 0 Peritonitis/Ascites 0 0 0 0 0 0 0 Other Pneumonitis 0 1 (17)* 0 0 0 0 1 (6) Sepsis 0 1 (17) 0 0 0 0 1 (6) Hemorrhage 0 0 0 0 0 1 (33)* 1 (6)
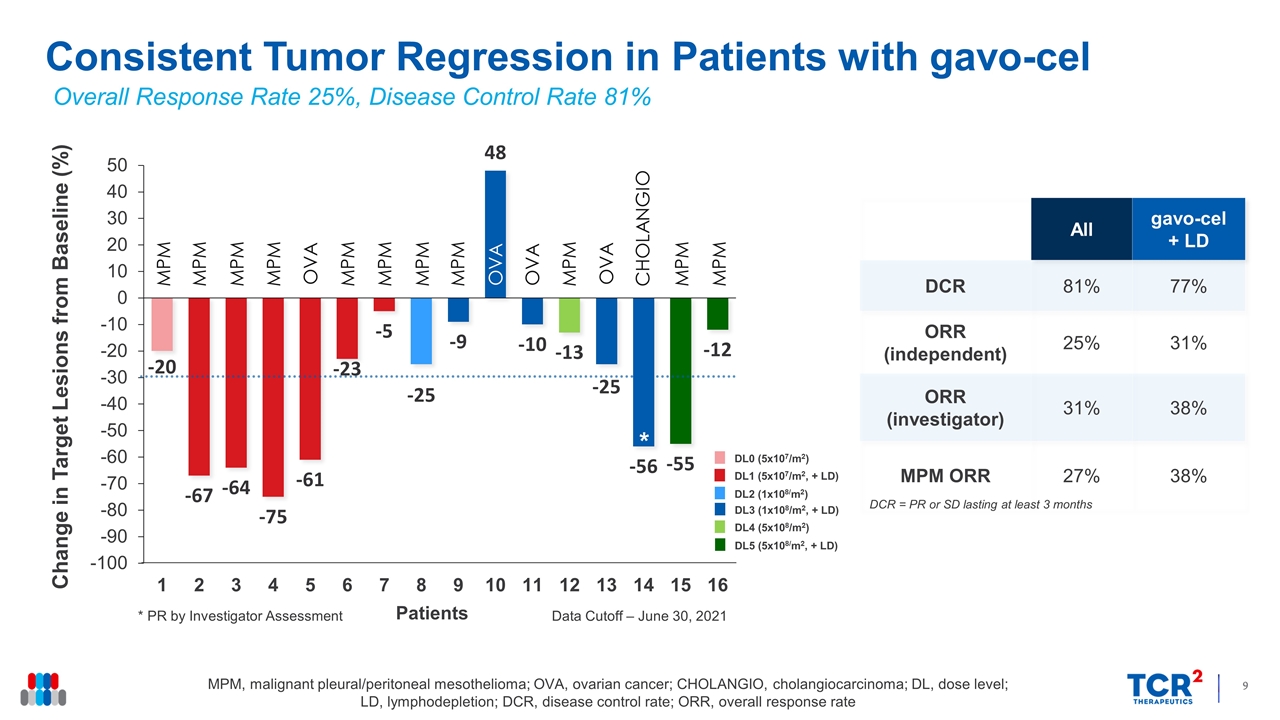
Consistent Tumor Regression in Patients with gavo-cel Change in Target Lesions from Baseline (%) MPM, malignant pleural/peritoneal mesothelioma; OVA, ovarian cancer; CHOLANGIO, cholangiocarcinoma; DL, dose level; LD, lymphodepletion; DCR, disease control rate; ORR, overall response rate All gavo-cel + LD DCR 81% 77% ORR (independent) 25% 31% ORR (investigator) 31% 38% MPM ORR 27% 38% Overall Response Rate 25%, Disease Control Rate 81% * * PR by Investigator Assessment Data Cutoff – June 30, 2021 DCR = PR or SD lasting at least 3 months DL0 (5x107/m2) DL1 (5x107/m2, + LD) DL2 (1x108/m2) DL3 (1x108/m2, + LD) DL4 (5x108/m2) DL5 (5x108/m2, + LD) * MPM MPM MPM MPM OVA MPM MPM MPM MPM OVA OVA MPM OVA CHOLANGIO MPM MPM Patients
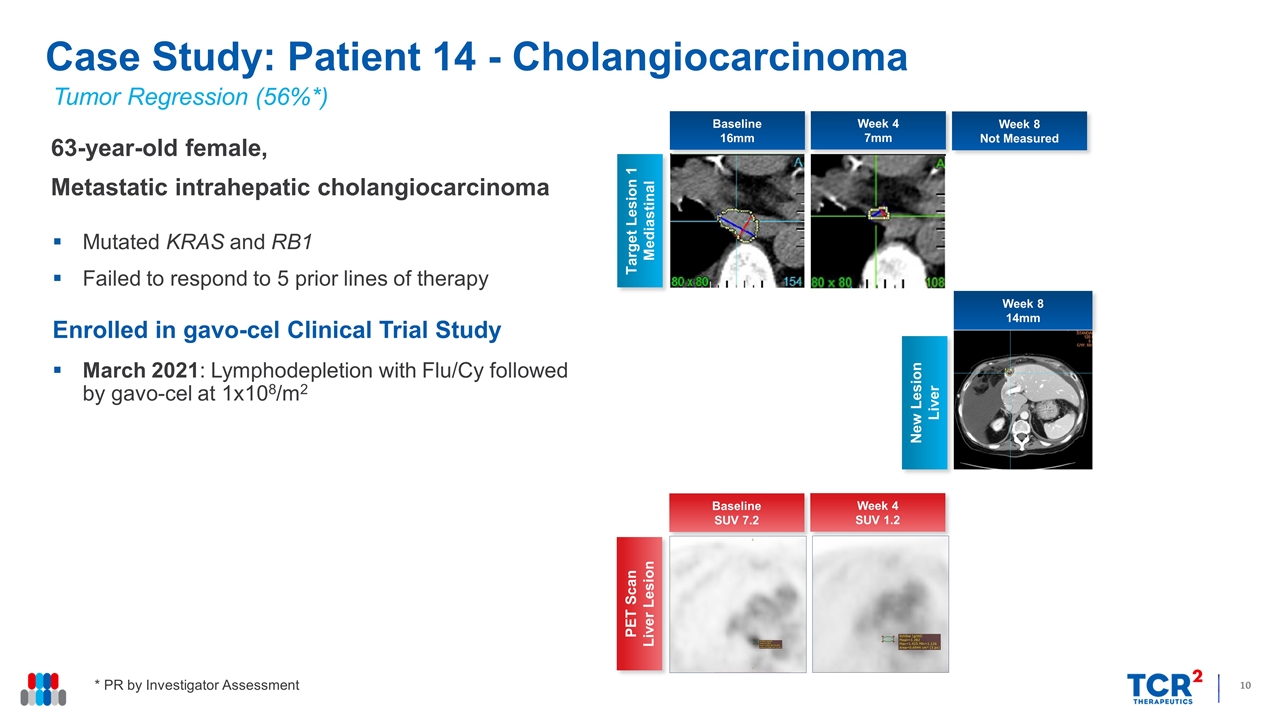
Tumor Regression (56%*) 63-year-old female, Metastatic intrahepatic cholangiocarcinoma Mutated KRAS and RB1 Failed to respond to 5 prior lines of therapy Enrolled in gavo-cel Clinical Trial Study March 2021: Lymphodepletion with Flu/Cy followed by gavo-cel at 1x108/m2 Case Study: Patient 14 - Cholangiocarcinoma Week 4 7mm Target Lesion 1 Mediastinal Week 8 Not Measured New Lesion Liver Baseline 16mm Week 8 14mm * PR by Investigator Assessment Week 4 SUV 1.2 PET Scan Liver Lesion Baseline SUV 7.2
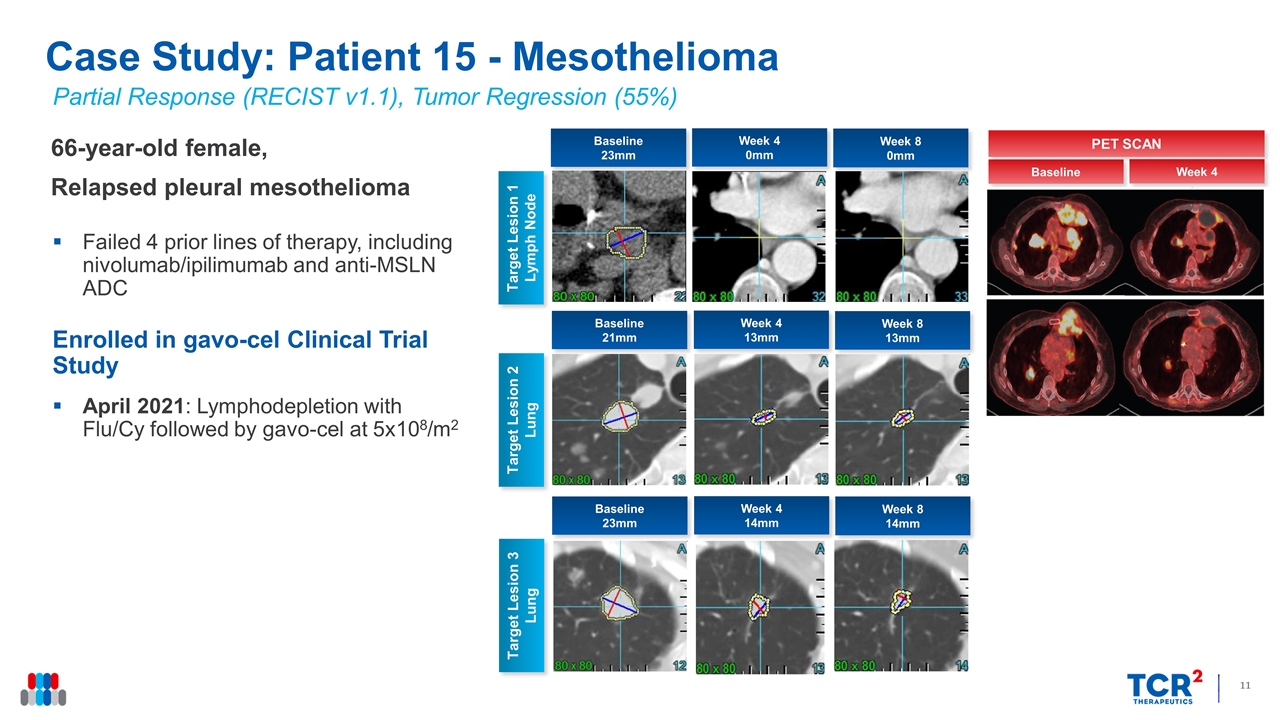
Partial Response (RECIST v1.1), Tumor Regression (55%) 66-year-old female, Relapsed pleural mesothelioma Failed 4 prior lines of therapy, including nivolumab/ipilimumab and anti-MSLN ADC Enrolled in gavo-cel Clinical Trial Study April 2021: Lymphodepletion with Flu/Cy followed by gavo-cel at 5x108/m2 Case Study: Patient 15 - Mesothelioma Week 4 0mm Target Lesion 1 Lymph Node Week 8 0mm Target Lesion 2 Lung Baseline 23mm Week 4 13mm Week 8 13mm Baseline 21mm Target Lesion 3 Lung Week 4 14mm Week 8 14mm Baseline 23mm Week 4 Baseline PET SCAN
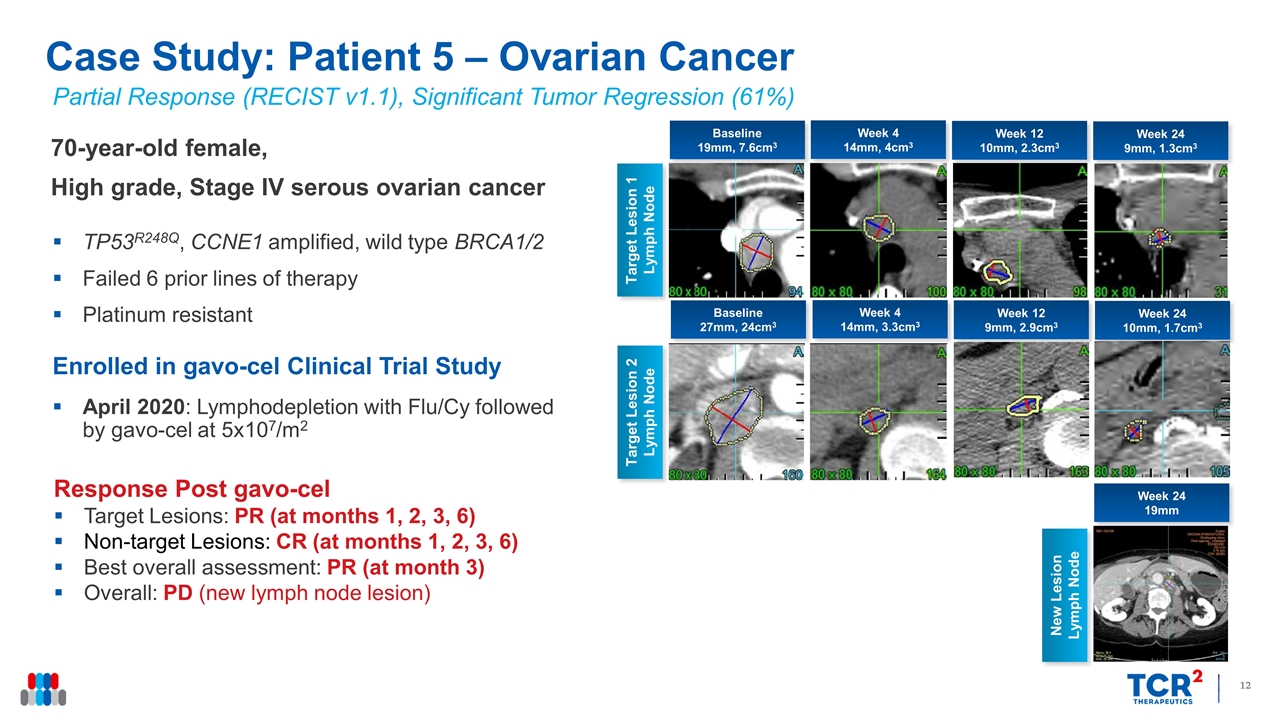
Partial Response (RECIST v1.1), Significant Tumor Regression (61%) 70-year-old female, High grade, Stage IV serous ovarian cancer TP53R248Q, CCNE1 amplified, wild type BRCA1/2 Failed 6 prior lines of therapy Platinum resistant Enrolled in gavo-cel Clinical Trial Study April 2020: Lymphodepletion with Flu/Cy followed by gavo-cel at 5x107/m2 Response Post gavo-cel Target Lesions: PR (at months 1, 2, 3, 6) Non-target Lesions: CR (at months 1, 2, 3, 6) Best overall assessment: PR (at month 3) Overall: PD (new lymph node lesion) Case Study: Patient 5 – Ovarian Cancer Week 4 14mm, 4cm3 Target Lesion 1 Lymph Node Week 12 10mm, 2.3cm3 Week 24 9mm, 1.3cm3 Target Lesion 2 Lymph Node Baseline 19mm, 7.6cm3 Week 4 14mm, 3.3cm3 Week 12 9mm, 2.9cm3 Week 24 10mm, 1.7cm3 Baseline 27mm, 24cm3 Week 24 19mm New Lesion Lymph Node
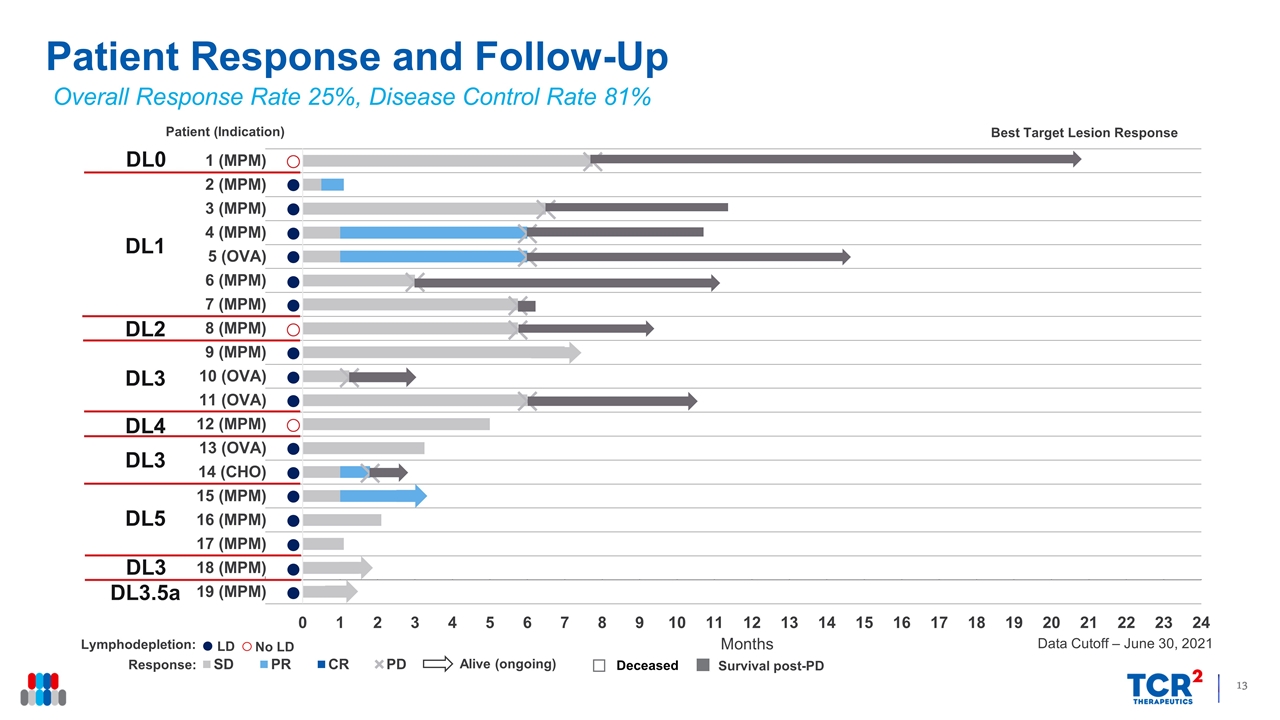
LD No LD Patient Response and Follow-Up Overall Response Rate 25%, Disease Control Rate 81% Data Cutoff – June 30, 2021 Alive (ongoing) Survival post-PD DL1 DL2 DL3 DL4 DL3 DL5 DL0 DL3 DL3.5a
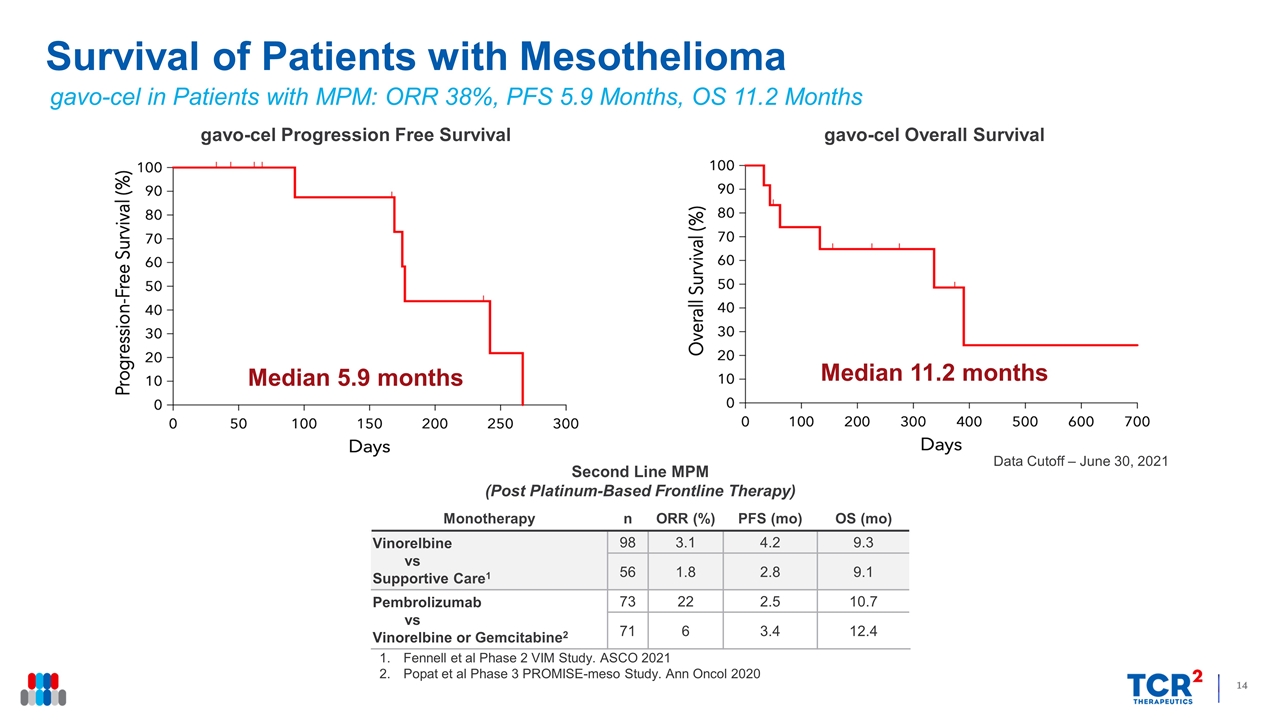
Data Cutoff – June 30, 2021 Survival of Patients with Mesothelioma Monotherapy n ORR (%) PFS (mo) OS (mo) Vinorelbine vs Supportive Care1 98 3.1 4.2 9.3 56 1.8 2.8 9.1 Pembrolizumab vs Vinorelbine or Gemcitabine2 73 22 2.5 10.7 71 6 3.4 12.4 Second Line MPM (Post Platinum-Based Frontline Therapy) gavo-cel Overall Survival Median 11.2 months gavo-cel Progression Free Survival Median 5.9 months Fennell et al Phase 2 VIM Study. ASCO 2021 Popat et al Phase 3 PROMISE-meso Study. Ann Oncol 2020 gavo-cel in Patients with MPM: ORR 38%, PFS 5.9 Months, OS 11.2 Months
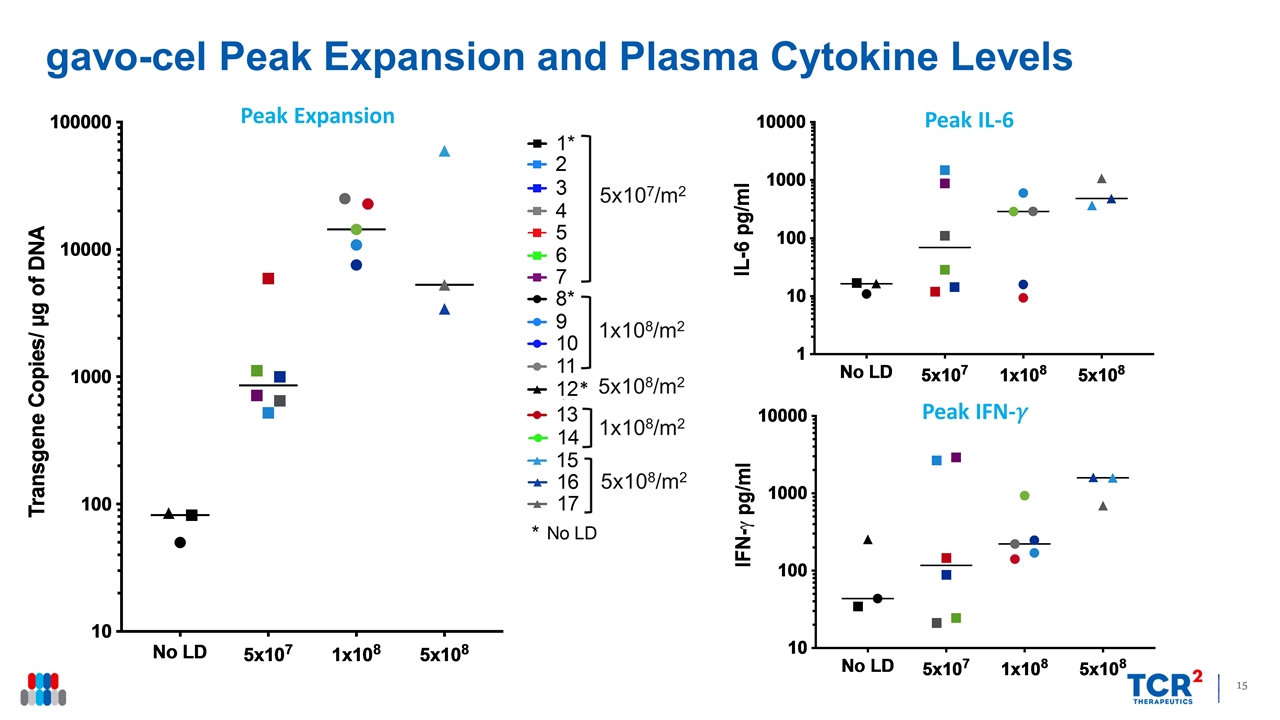
gavo-cel Peak Expansion and Plasma Cytokine Levels Peak Expansion Peak IFN-�� Peak IL-6 5x107/m2 1x108/m2 5x108/m2 1x108/m2 5x108/m2 *
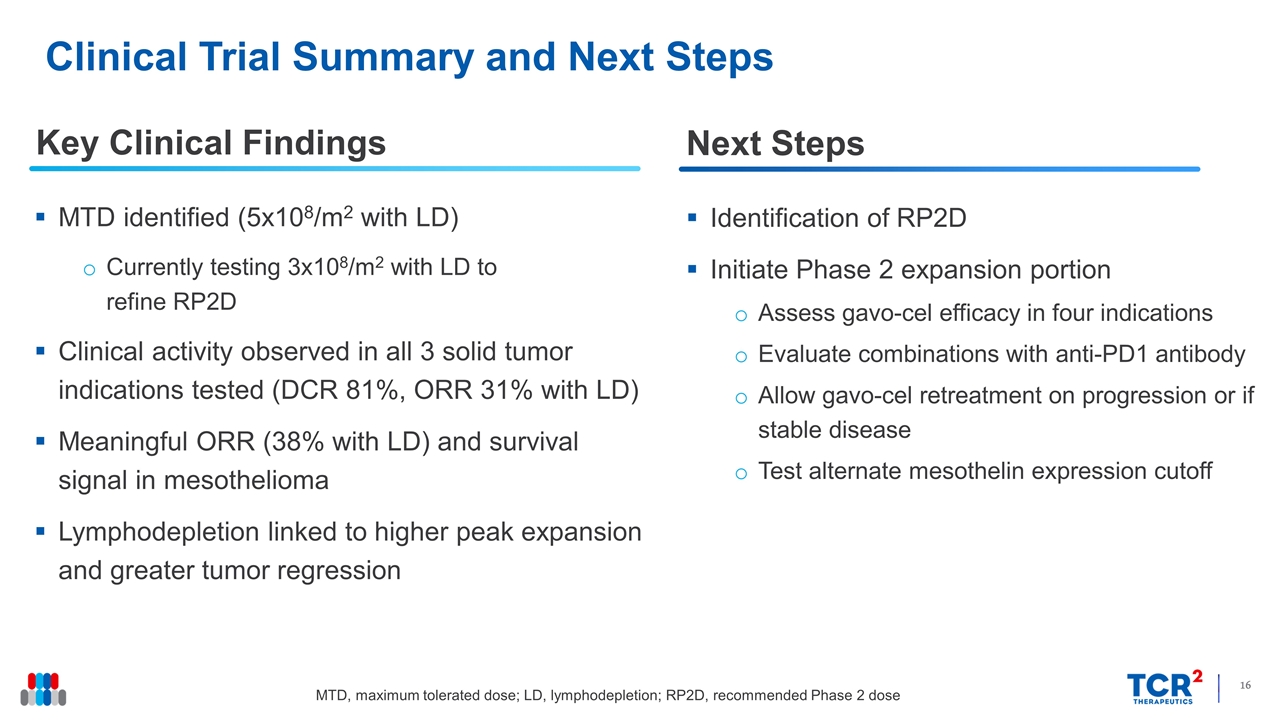
Clinical Trial Summary and Next Steps Key Clinical Findings MTD identified (5x108/m2 with LD) Currently testing 3x108/m2 with LD to refine RP2D Clinical activity observed in all 3 solid tumor indications tested (DCR 81%, ORR 31% with LD) Meaningful ORR (38% with LD) and survival signal in mesothelioma Lymphodepletion linked to higher peak expansion and greater tumor regression Identification of RP2D Initiate Phase 2 expansion portion Assess gavo-cel efficacy in four indications Evaluate combinations with anti-PD1 antibody Allow gavo-cel retreatment on progression or if stable disease Test alternate mesothelin expression cutoff Next Steps MTD, maximum tolerated dose; LD, lymphodepletion; RP2D, recommended Phase 2 dose
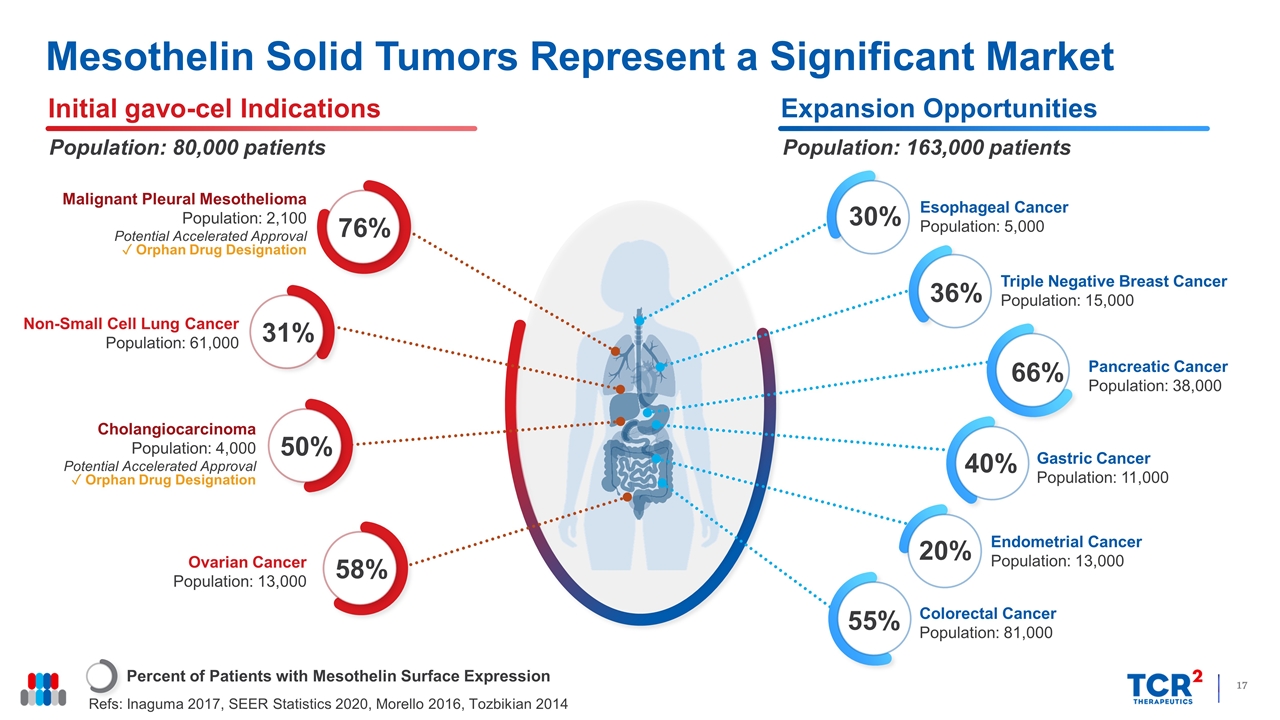
Mesothelin Solid Tumors Represent a Significant Market Percent of Patients with Mesothelin Surface Expression Refs: Inaguma 2017, SEER Statistics 2020, Morello 2016, Tozbikian 2014 Initial gavo-cel Indications Population: 80,000 patients Expansion Opportunities Population: 163,000 patients 76% Malignant Pleural Mesothelioma Population: 2,100 Potential Accelerated Approval ✓ Orphan Drug Designation 58% Ovarian Cancer Population: 13,000 Cholangiocarcinoma Population: 4,000 Potential Accelerated Approval ✓ Orphan Drug Designation 50% Non-Small Cell Lung Cancer Population: 61,000 31% Gastric Cancer Population: 11,000 40% Endometrial Cancer Population: 13,000 20% Colorectal Cancer Population: 81,000 55% Esophageal Cancer Population: 5,000 30% Triple Negative Breast Cancer Population: 15,000 36% Pancreatic Cancer Population: 38,000 66%
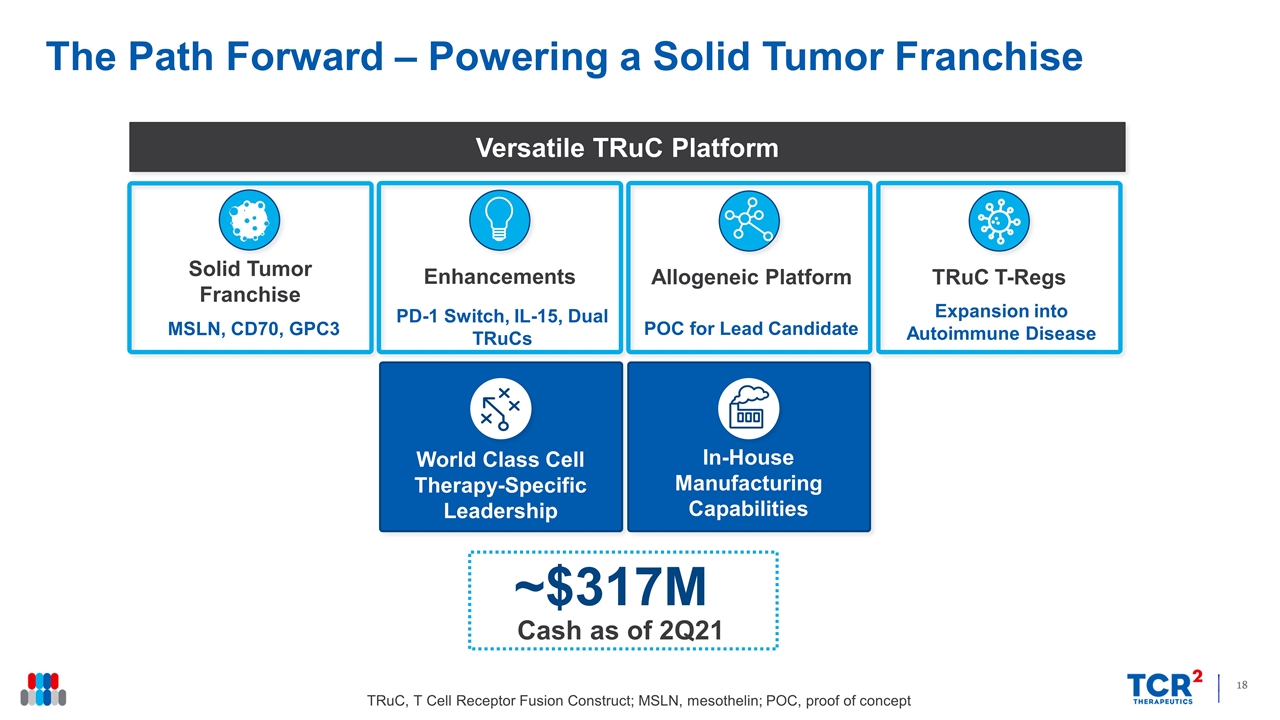
MSLN, CD70, GPC3 PD-1 Switch, IL-15, Dual TRuCs POC for Lead Candidate Solid Tumor Franchise Enhancements Allogeneic Platform Versatile TRuC Platform World Class Cell Therapy-Specific Leadership In-House Manufacturing Capabilities Cash as of 2Q21 ~$317M Expansion into Autoimmune Disease TRuC T-Regs The Path Forward – Powering a Solid Tumor Franchise TRuC, T Cell Receptor Fusion Construct; MSLN, mesothelin; POC, proof of concept

R&D Day – October 20, 2021 Further update on gavo-cel clinical trial and Phase 2 strategy Individual program reviews and pipeline strategy Allogeneic development candidate and strategy/timelines Development and data for TRuC T-reg T cells

Thank You
