Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - LEAP THERAPEUTICS, INC. | tm2127789d1_ex99-1.htm |
| 8-K - FORM 8-K - LEAP THERAPEUTICS, INC. | tm2127789d1_8k.htm |
Exhibit 99.2
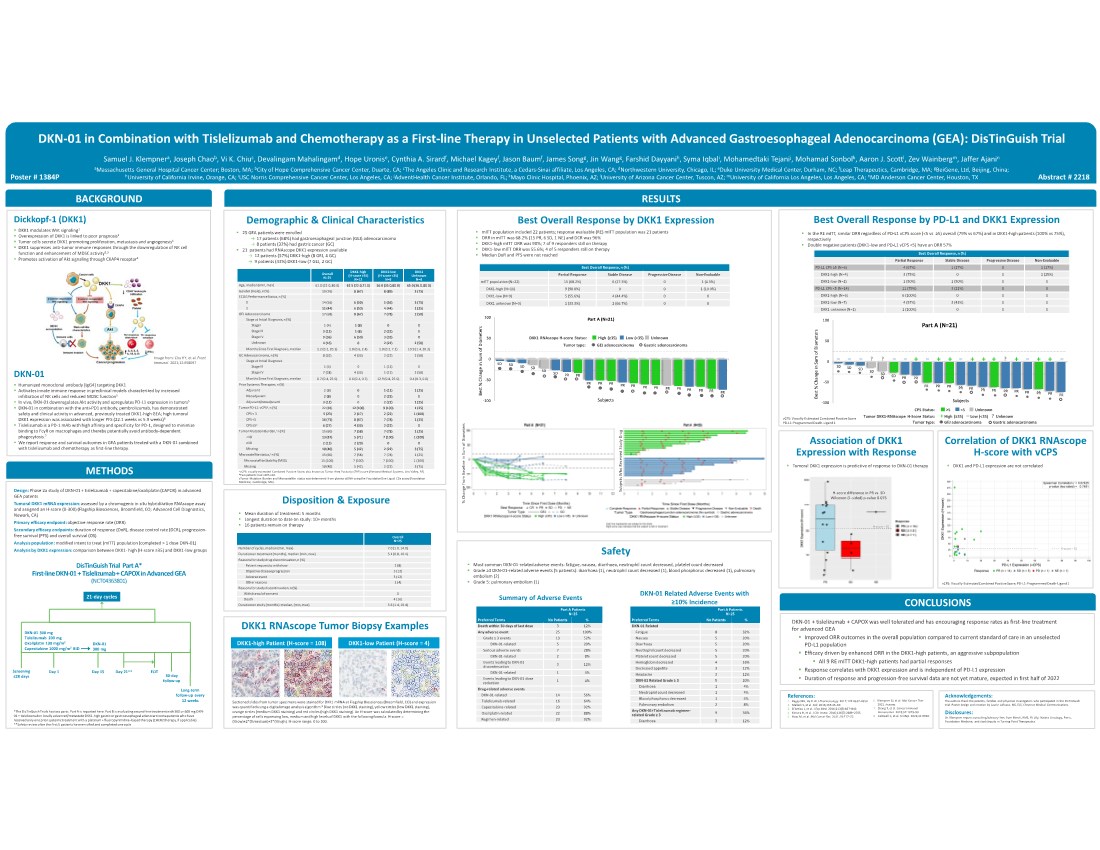
| DKN-01 in Combination with Tislelizumab and Chemotherapy as a First-line Therapy in Unselected Patients with Advanced Gastroesophageal Adenocarcinoma (GEA): DisTinGuish Trial Samuel J. Klempnera, Joseph Chaob, Vi K. Chiuc, Devalingam Mahalingamd, Hope Uronise, Cynthia A. Sirardf, Michael Kageyf, Jason Baumf, James Songg, Jin Wangg, Farshid Dayyanih, Syma Iqbali, Mohamedtaki Tejanij, Mohamad Sonbolk, Aaron J. Scottl, Zev Wainbergm, Jaffer Ajanin aMassachusetts General Hospital Cancer Center; Boston, MA; bCity of Hope Comprehensive Cancer Center, Duarte, CA; cThe Angeles Clinic and Research Institute, a Cedars-Sinai affiliate, Los Angeles, CA; dNorthwestern University, Chicago, IL; eDuke University Medical Center, Durham, NC; fLeap Therapeutics, Cambridge, MA; gBeiGene, Ltd, Beijing, China; hUniversity of California Irvine, Orange, CA; iUSC Norris Comprehensive Cancer Center, Los Angeles, CA; jAdventHealth Cancer Institute, Orlando, FL; kMayo Clinic Hospital, Phoenix, AZ; lUniversity of Arizona Cancer Center, Tuscon, AZ; mUniversity of California Los Angeles, Los Angeles, CA; nMD Anderson Cancer Center, Houston, TX Design: Phase 2a study of DKN-01 + tislelizumab + capecitabine/oxaliplatin (CAPOX) in advanced GEA patents Tumoral DKK1 mRNA expression: assessed by a chromogenic in situ hybridization RNAscope assay and assigned an H-score (0-300) (Flagship Biosciences, Broomfield, CO; Advanced Cell Diagnostics, Newark, CA) Primary efficacy endpoint: objective response rate (ORR) Secondary efficacy endpoints: duration of response (DoR), disease control rate (DCR), progression- free survival (PFS) and overall survival (OS) Analysis population: modified intent to treat (mITT) population (completed > 1 dose DKN-01) Analysis by DKK1 expression: comparison between DKK1- high (H-score ≥35) and DKK1-low groups . 25 GEA patients were enrolled → 17 patients (68%) had gastroesophageal junction (GEJ) adenocarcinoma → 8 patients (32%) had gastric cancer (GC) . 21 patients had RNAscope DKK1 expression available → 12 patients (57%) DKK1-high (8 GEJ, 4 GC) → 9 patients (43%) DKK1-low (7 GEJ, 2 GC) Abstract # 2218 RESULTS METHODS BACKGROUND Dickkopf-1 (DKK1) . DKK1 modulates Wnt signaling1 . Overexpression of DKK1 is linked to poor prognosis1 . Tumor cells secrete DKK1 promoting proliferation, metastasis and angiogenesis1 . DKK1 suppresses anti-tumor immune responses through the downregulation of NK cell function and enhancement of MDSC activity2,3 . Promotes activation of Akt signaling through CKAP4 receptor4 Demographic & Clinical Characteristics . mITT population included 22 patients; response evaluable (RE) mITT population was 21 patients . ORR in mITT was 68.2% (15 PR, 6 SD, 1 NE) and DCR was 96% . DKK1-high mITT ORR was 90%; 7 of 9 responders still on therapy . DKK1-low mITT ORR was 55.6%; 4 of 5 responders still on therapy . Median DoR and PFS were not reached Best Overall Response by DKK1 Expression Safety . In the RE mITT, similar ORR regardless of PD-L1 vCPS score (<5 vs ≥5) overall (79% vs 67%) and in DKK1-high patients (100% vs 75%), respectively . Double negative patients (DKK1-low and PD-L1 vCPS <5) have an ORR 57% DKK1 RNAscope Tumor Biopsy Examples DisTinGuish Trial Part A* First-line DKN-01 + Tislelizumab + CAPOX in Advanced GEA (NCT04363801) DKN-01 + tislelizumab + CAPOX was well tolerated and has encouraging response rates as first-line treatment for advanced GEA . Improved ORR outcomes in the overall population compared to current standard of care in an unselected PD-L1 population . Efficacy driven by enhanced ORR in the DKK1-high patients, an aggressive subpopulation . All 9 RE mITT DKK1-high patients had partial responses . Response correlates with DKK1 expression and is independent of PD-L1 expression . Duration of response and progression-free survival data are not yet mature, expected in first half of 2022 CONCLUSIONS References: 1. Kagey MH, He X. Br J Pharmacology. 2017;174:4637–4650. 2. Malladi S, et al. Cell. 2016;165:45–60. 3. D’Amico L, et al. J Exp Med. 2016;213(5):827–840. 4. Kimura H, et al. J Clin Invest. 2016;126(7):2689–2705. 5. Haas M, et al. Mol Cancer Res. 2021;19:717–25. DKK1-high Patient (H-score = 108) DKK1-high Patient (H-score = 108) DKK1-low Patient (H-score = 4) DKK1-low Patient (H-score = 4) DKN-01 . Humanized monoclonal antibody [IgG4] targeting DKK1 . Activates innate immune response in preclinical models characterized by increased infiltration of NK cells and reduced MDSC function5 . In vivo, DKN-01 downregulates Akt activity and upregulates PD-L1 expression in tumors5 . DKN-01 in combination with the anti-PD1 antibody, pembrolizumab, has demonstrated safety and clinical activity in advanced, previously treated DKK1-high GEA; high tumoral DKK1 expression was associated with longer PFS (22.1 weeks vs 5.9 weeks)6 . Tislelizumab is a PD-1 mAb with high affinity and specificity for PD-1, designed to minimize binding to FcγR on macrophages and thereby potentially avoid antibody-dependent phagocytosis.7 . We report response and survival outcomes in GEA patients treated with a DKN-01 combined with tislelizumab and chemotherapy as first-line therapy. Image from: Chu HY, et al. Front Immunol. 2021;12:658097 *The DisTinGuish Trials has two parts. Part A is reported here. Part B is evaluating second-line treatment with 300 or 600 mg DKN- 01 + tislelizumab in locally advanced/metastatic DKK1-high gastric or gastroesophageal adenocarcinoma patients who have received only one prior systemic treatment with a platinum + fluoropyrimidine–based therapy (±HER2 therapy, if applicable). **Safety review after the first 5 patients have enrolled and completed one cycle 21-day cycles Screening ≤28 days Day 1 Day 15 Day 21** EOT 30-day follow-up Long-term follow-up every 12 weeks DKN-01 300 mg DKN-01 300 mg Tislelizumab 200 mg Oxaliplatin 130 mg/m2 Capecitabine 1000 mg/m2 BID Disposition & Exposure . Mean duration of treatment: 5 months . Longest duration to date on study: 10+ months . 16 patients remain on therapy Poster # 1384P Overall N=25 Number of cycles, median (min, max) 7.0 (1.0, 14.0) Duration on treatment (months), median (min, max) 5.1 (0.8, 10.1) Reasons for study drug discontinuation, n (%) Patient request to withdraw 2 (8) Objective disease progression 3 (12) Adverse event 3 (12) Other reasons 1 (4) Reasons for study discontinuation, n (%) Withdrawal of consent 0 Death 4 (16) Duration on study (months): median, (min, max) 5.6 (1.4, 10.4) Best Overall Response, n (%) Partial Response Stable Disease Progressive Disease Non-Evaluable mITT population (N=22) 15 (68.2%) 6 (27.3%) 0 1 (4.5%) DKK1-high (N=10) 9 (90.0%) 0 0 1 (10.0%) DKK1-low (N=9) 5 (55.6%) 4 (44.4%) 0 0 DKK1 unknown (N=3) 1 (33.3%) 2 (66.7%) 0 0 Overall N=25 DKK1-high (H-score ≥35) N=12 DKK1-low (H-score <35) N=9 DKK1 Unknown N=4 Age, median (min, max) 61.0 (22.0, 80.0) 62.5 (22.0,71.0) 56.0 (35.0,80.0) 65.0 (36.0, 80.0) Gender (male), n (%) 19 (76) 8 (67) 8 (89) 3 (75) ECOG Performance Status, n (%) 0 14 (56) 6 (50) 5 (56) 3 (75) 1 11 (44) 6 (50) 4 (44) 1 (25) GEJ Adenocarcinoma 17 (68) 8 (67) 7 (78) 2 (50) Stage at Initial Diagnosis, n (%) Stage I 1 (4) 1 (8) 0 0 Stage III 3 (12) 1 (8) 2 (22) 0 Stage IV 9 (36) 6 (50) 3 (33) 0 Unknown 4 (16) 0 2 (22) 2 (50) Months Since First Diagnosis, median 1.2 (0.2, 20.3) 1.0 (0.6, 2.4) 1.0 (0.2, 7.1) 10.9 (1.4, 20.3) GC Adenocarcinoma, n (%) 8 (32) 4 (33) 2 (22) 2 (50) Stage at Initial Diagnosis Stage III 1 (4) 0 1 (11) 0 Stage IV 7 (28) 4 (33) 1 (11) 2 (50) Months Since First Diagnosis, median 0.7 (0.4, 25.0) 0.6 (0.4, 0.7) 12.9 (0.8, 25.0) 0.4 (0.3, 0.6) Prior Systemic Therapies, n (%) Adjuvant 2 (8) 0 1 (11) 1 (25) Neoadjuvant 2 (8) 0 2 (22) 0 Adjuvant/neoadjuvant 3 (12) 0 2 (22) 1 (25) Tumor PD-L1: vCPSa, n (%) 22 (88) 12 (100) 9 (100) 1 (25) CPS < 1 5 (23) 2 (17) 2 (22) 1 (100) CPS <5 16 (73) 8 (67) 7 (78) 1 (25) CPS ≥5b 6 (27) 4 (33) 2 (22) 0 Tumor Mutation Burden,c n (%) 15 (60) 7 (58) 7 (78) 1 (25) <10 13 (87) 5 (71) 7 (100) 1 (100) ≥10 2 (13) 2 (29) 0 0 Missing 10 (40) 5 (42) 2 (22) 3 (75) Microsatellite status,c n (%) 15 (60) 7 (58) 7 (78) 1 (25) Microsatellite Stability (MSS) 15 (100) 7 (100) 7 (100) 1 (100) Missing 10 (40) 5 (42) 2 (22) 3 (75) avCPS: visually-estimated Combined Positive Score, also known as Tumor Area Positivity (TAP) score (Ventana Medical Systems, Oro Valley, AZ). bTwo patients had vCPS ≥10. cTumor Mutation Burden and Microsatellite status was determined from plasma ctDNA using the FoundationOne Liquid CDx assay (Foundation Medicine, Cambridge, MA). Best Overall Response by PD-L1 and DKK1 Expression vCPS: Visually-Estimated Combined Positive Score PD-L1: Programmed Death-Ligand 1 Correlation of DKK1 RNAscope H-score with vCPS vCPS: Visually-Estimated Combined Positive Score; PD-L1: Programmed Death-Ligand 1 . Tumoral DKK1 expression is predictive of response to DKN-01 therapy -100 -50 0 50 100 Best % Change in Sum of Diameters Subjects Part A (N=21) SD SD SD SD SD SD PR PR PR PR PR PR PR PR PR PR PR PR PR PR PR + ++ +++ + ++ ???–– –– – –– –– Association of DKK1 Expression with Response . DKK1 and PD-L1 expression are not correlated Best Overall Response, n (%) Partial Response Stable Disease Progressive Disease Non-Evaluable PD-L1 CPS ≥5 (N=6) 4 (67%) 1 (17%) 0 1 (17%) DKK1-high (N=4) 3 (75%) 0 0 1 (25%) DKK1-low (N=2) 1 (50%) 1 (50%) 0 0 PD-L1 CPS <5 (N=14) 11 (79%) 3 (21%) 0 0 DKK1-high (N=6) 6 (100%) 0 0 0 DKK1-low (N=7) 4 (57%) 3 (43%) 0 0 DKK1 unknown (N=1) 1 (100%) 0 0 0 Disclosures: Dr. Klempner reports consulting/advisory fees from Merck, BMS, Eli Lilly, Natera Oncology, Pieris, Foundation Medicine, and stock/equity in Turning Point Therapeutics. Acknowledgements: The authors thank the patients, families and physician investigators who participated in the DisTinGuish trial. Poster design and creation by Laurie LaRusso, MS, ELS, Chestnut Medical Communications. Sectioned slides from tumor specimens were stained for DKK1 mRNA at Flagship Biosciences (Broomfield, CO) and expression was quantified using a digital image analysis algorithm.8 Blue circles (no DKK1 staining), yellow circles (low DKK1 staining), orange circles (medium DKK1 staining) and red circles (high DKK1 staining). An H-score was calculated by determining the percentage of cells expressing low, medium and high levels of DKK1 with the following formula. H-score = (%low)+2*(%medium)+3*(%high). H-score range: 0 to 300. Part A Patients N=25 Preferred Terms No Patients % Death within 30 days of last dose 3 12% Any adverse event 25 100% Grade ≥ 3 events 13 52% DKN-01-related 5 20% Serious adverse events 7 28% DKN-01-related 2 8% Events leading to DKN-01 discontinuation 3 12% DKN-01-related 1 4% Events leading to DKN-01 dose reduction 1 4% Drug-related adverse events DKN-01-related 14 56% Tislelizumab-related 16 64% Capecitabine-related 23 92% Oxaliplatin-related 22 88% Regimen-related 23 92% Summary of Adverse Events Part A Patients N=25 Preferred Terms No Patients % DKN-01 Related Fatigue 8 32% Nausea 5 20% Diarrhoea 5 20% Neutrophil count decreased 5 20% Platelet count decreased 5 20% Hemoglobin decreased 4 16% Decreased appetite 3 12% Headache 3 12% DKN-01 Related Grade ≥ 3 5 20% Diarrhoea 1 4% Neutrophil count decreased 1 4% Blood phosphorus decreased 1 4% Pulmonary embolism 2 8% Any DKN-01+Tislelizumab regimen- related Grade ≥ 3 9 36% Diarrhoea 3 12% DKN-01 Related Adverse Events with ≥10% Incidence . Most common DKN-01-related adverse events: fatigue, nausea, diarrhoea, neutrophil count decreased, platelet count decreased . Grade ≥3 DKN-01-related adverse events (5 patients): diarrhoea (1), neutrophil count decreased (1), blood phosphorus decreased (1), pulmonary embolism (2) . Grade 5: pulmonary embolism (1) CPS Status: ≥5 <5 Unknown Tumor DKK1-RNAscope H-Score Status: + High (≥35) – Low (<35) ? Unknown H-score = 35 H-score difference in PR vs. SD Wilcoxon (1-sided) p-value 0.075 H-score = 35 Tumor type: GEJ adenocarcinoma Gastric adenocarcinoma -100 -50 0 50 100 Best % Change in Sum of Diameters Subjects Part A (N=21) SD SD SD SD SD SD PR PR PR PR PR PR PR PR PR PR PR PR PR PR PR DKK1 RNAscope H-score Status: High (≥35) Low (<35) Unknown Tumor type: GEJ adenocarcinoma Gastric adenocarcinoma % Change from Baseline in Sum of Diameters Subjects Who Received Study Drug 6. Klempner SJ, et al. Mol Cancer Ther. 2021. In press. 7. Zhang T, et al. Cancer Immunol Immunother. 2018;67:1079–90. 8. Caldwell C, et al. Sci Rep. 2021;11:9920. |