Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - aTYR PHARMA INC | life-ex991_19.htm |
| 8-K - 8-K - aTYR PHARMA INC | life-8k_20210913.htm |

A New Path to Medicine Results for Phase 1b/2a Study of ATYR1923 in Pulmonary Sarcoidosis SEPTEMBER 13, 2021 Exhibit 99.2

Forward Looking Statements The following slides and any accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” “opportunity,” or “continue,” and other similar expressions are intended to identify forward-looking statements. For example, all statements regarding: the potential therapeutic benefits of proteins derived from tRNA synthetase genes and our product candidates, including ATYR1923 and ATYR2810, and development programs, including our NRP2 antibody program and our tRNA synthetase program; the ability to successfully advance our product candidates and undertake certain development activities (such as the initiation of clinical trials, clinical trial enrollment, the conduct of clinical trials and announcement of clinical results) and accomplish certain development goals, and the timing of such events; the potential market opportunity for our product candidates; our ability to receive regulatory approvals for, and commercialize, our product candidates; our ability to identify and discover additional product candidates; potential activities and payments under collaboration agreements; and the ability of our intellectual property portfolio to provide protection are forward-looking statements. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These risks, uncertainties and other factors are more fully described in our filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q, and in our other filings. The forward-looking statements in this presentation speak only as of the date of this presentation and neither we nor any other person assume responsibility for the accuracy and completeness of any forward-looking statement. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. We own various U.S. federal trademark applications and unregistered trademarks, including our company name. All other trademarks or trade names referred to in this presentation are the property of their respective owners. Solely for convenience, the trademarks and trade names in this presentation are referred to without the symbols ® and ™, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. 2
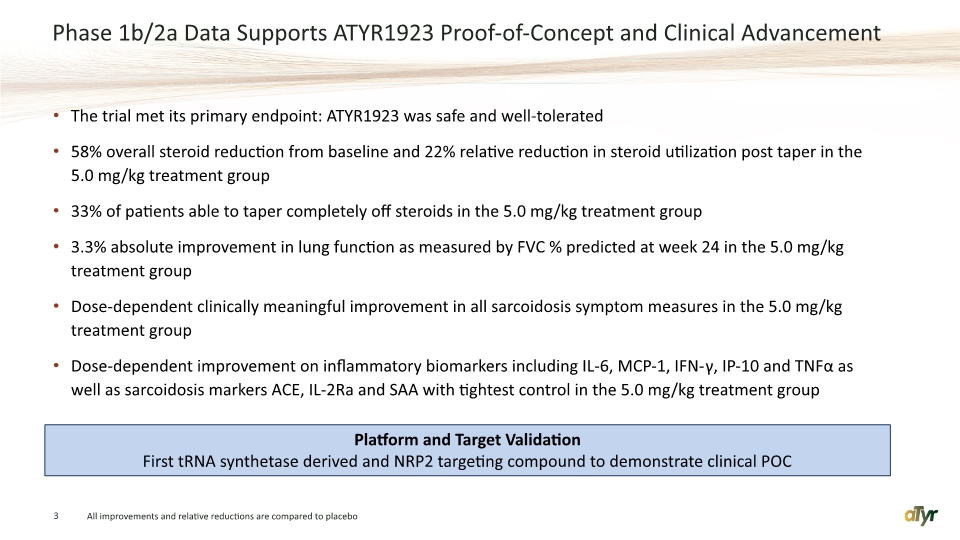
Phase 1b/2a Data Supports ATYR1923 Proof-of-Concept and Clinical Advancement The trial met its primary endpoint: ATYR1923 was safe and well-tolerated 58% overall steroid reduction from baseline and 22% relative reduction in steroid utilization post taper in the 5.0 mg/kg treatment group 33% of patients able to taper completely off steroids in the 5.0 mg/kg treatment group 3.3% absolute improvement in lung function as measured by FVC % predicted at week 24 in the 5.0 mg/kg treatment group Dose-dependent clinically meaningful improvement in all sarcoidosis symptom measures in the 5.0 mg/kg treatment group Dose-dependent improvement on inflammatory biomarkers including IL-6, MCP-1, IFN-γ, IP-10 and TNFα as well as sarcoidosis markers ACE, IL-2Ra and SAA with tightest control in the 5.0 mg/kg treatment group 3 All improvements and relative reductions are compared to placebo Platform and Target Validation First tRNA synthetase derived and NRP2 targeting compound to demonstrate clinical POC
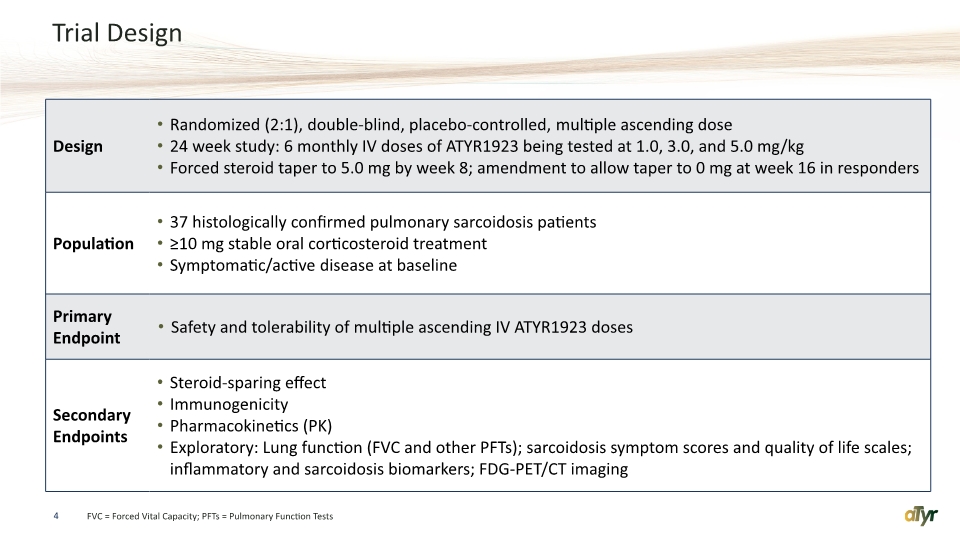
Trial Design 4 FVC = Forced Vital Capacity; PFTs = Pulmonary Function Tests
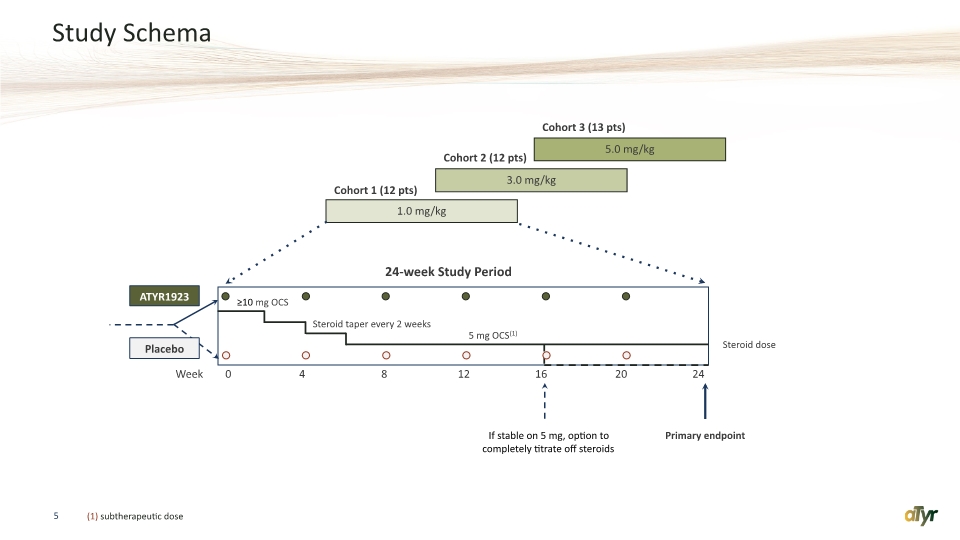
Study Schema 5 (1) subtherapeutic dose 0 4 8 12 16 20 24 24-week Study Period Week ATYR1923 Placebo 5 mg OCS(1) Steroid taper every 2 weeks Primary endpoint If stable on 5 mg, option to completely titrate off steroids ≥10 mg OCS Steroid dose
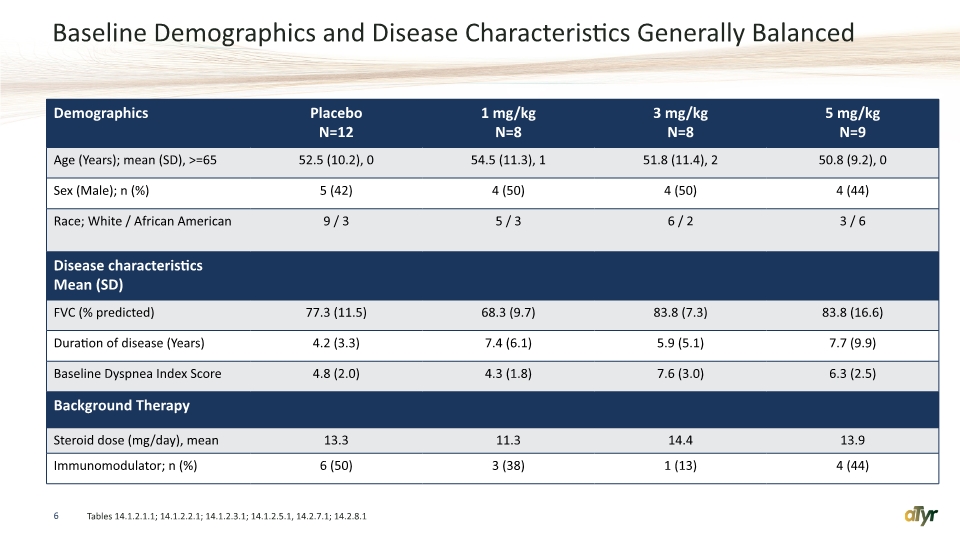
Baseline Demographics and Disease Characteristics Generally Balanced 6 Tables 14.1.2.1.1; 14.1.2.2.1; 14.1.2.3.1; 14.1.2.5.1, 14.2.7.1; 14.2.8.1
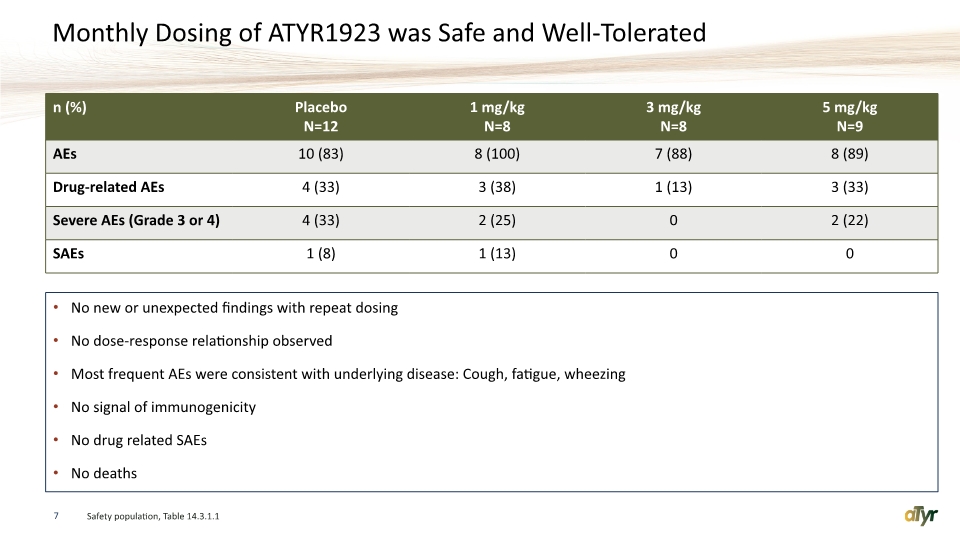
Monthly Dosing of ATYR1923 was Safe and Well-Tolerated No new or unexpected findings with repeat dosing No dose-response relationship observed Most frequent AEs were consistent with underlying disease: Cough, fatigue, wheezing No signal of immunogenicity No drug related SAEs No deaths 7 Safety population, Table 14.3.1.1
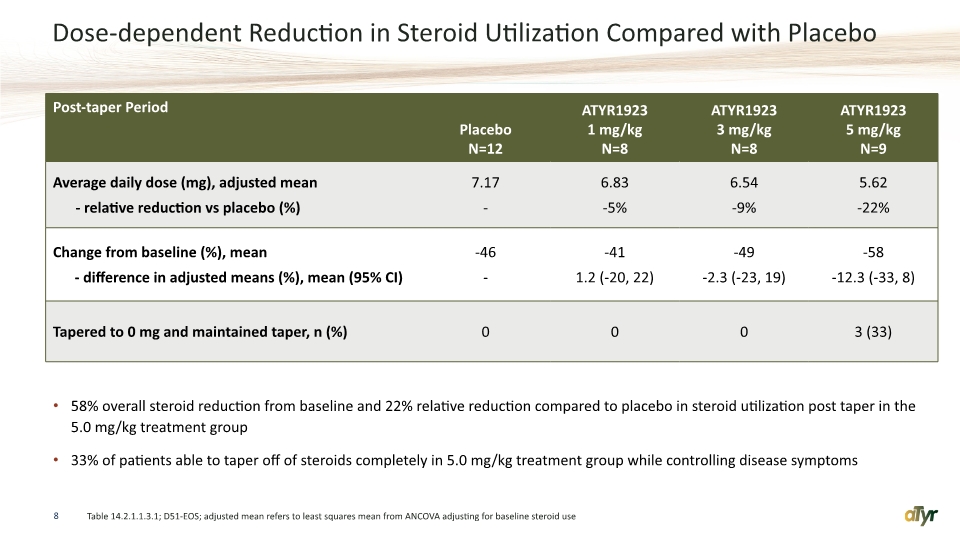
Dose-dependent Reduction in Steroid Utilization Compared with Placebo 58% overall steroid reduction from baseline and 22% relative reduction compared to placebo in steroid utilization post taper in the 5.0 mg/kg treatment group 33% of patients able to taper off of steroids completely in 5.0 mg/kg treatment group while controlling disease symptoms 8 Table 14.2.1.1.3.1; D51-EOS; adjusted mean refers to least squares mean from ANCOVA adjusting for baseline steroid use
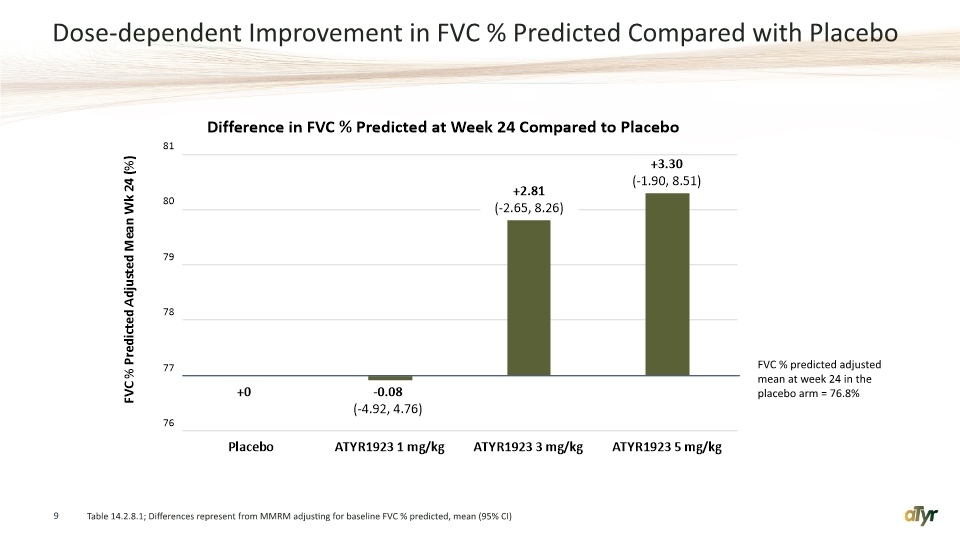
Dose-dependent Improvement in FVC % Predicted Compared with Placebo 9 Table 14.2.8.1; Differences represent from MMRM adjusting for baseline FVC % predicted, mean (95% CI) FVC % predicted adjusted mean at week 24 in the placebo arm = 76.8%
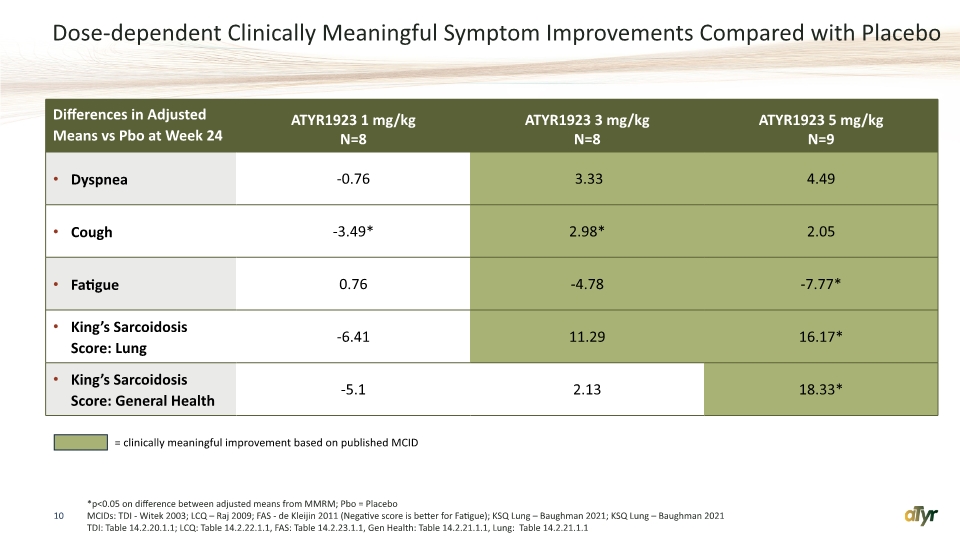
Dose-dependent Clinically Meaningful Symptom Improvements Compared with Placebo 10 *p<0.05 on difference between adjusted means from MMRM; Pbo = Placebo MCIDs: TDI - Witek 2003; LCQ – Raj 2009; FAS - de Kleijin 2011 (Negative score is better for Fatigue); KSQ Lung – Baughman 2021; KSQ Lung – Baughman 2021 TDI: Table 14.2.20.1.1; LCQ: Table 14.2.22.1.1, FAS: Table 14.2.23.1.1, Gen Health: Table 14.2.21.1.1, Lung: Table 14.2.21.1.1 = clinically meaningful improvement based on published MCID
Phase 1b/2a Data Supports ATYR1923 Proof-of-Concept and Clinical Advancement ATYR1923 was safe and well-tolerated Strong suggestion of efficacy: Dose-response and consistent positive trends across key efficacy endpoints and multiple analysis populations Clinically meaningful symptom improvements in dyspnea, cough, fatigue and King’s Sarcoidosis scores Dose dependent control of inflammatory biomarkers confirms anti-inflammatory effect 11 Planned next steps: End of Phase 2 meeting with FDA Publish data Advance into registrational trial in pulmonary sarcoidosis
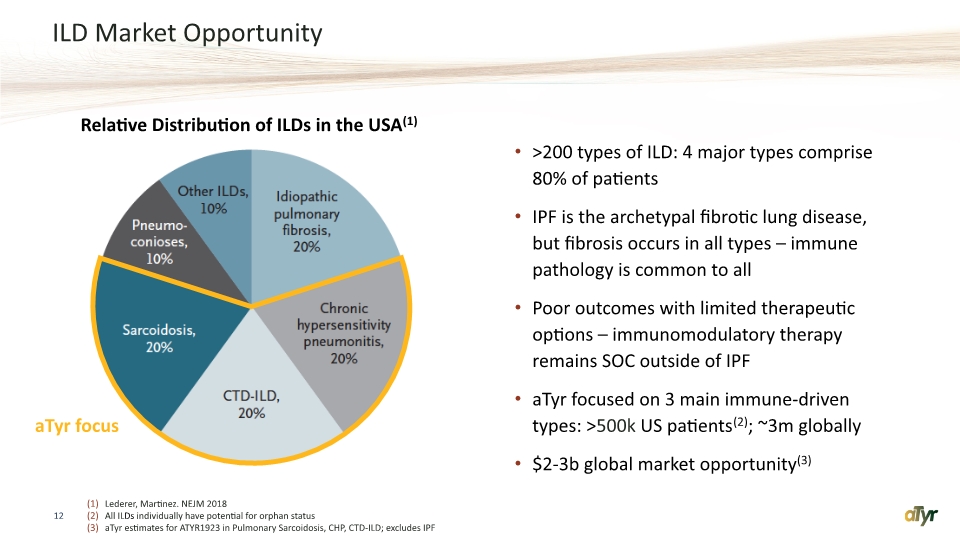
ILD Market Opportunity 12 Lederer, Martinez. NEJM 2018 All ILDs individually have potential for orphan status aTyr estimates for ATYR1923 in Pulmonary Sarcoidosis, CHP, CTD-ILD; excludes IPF >200 types of ILD: 4 major types comprise 80% of patients IPF is the archetypal fibrotic lung disease, but fibrosis occurs in all types – immune pathology is common to all Poor outcomes with limited therapeutic options – immunomodulatory therapy remains SOC outside of IPF aTyr focused on 3 main immune-driven types: >500k US patients(2); ~3m globally $2-3b global market opportunity(3) Relative Distribution of ILDs in the USA(1) aTyr focus

Q&A
