Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Hepion Pharmaceuticals, Inc. | tm2127428d1_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Hepion Pharmaceuticals, Inc. | tm2127428d1_ex99-1.htm |
Exhibit 99.2

INVESTOR UPDATE September 13 , 2021 Nasdaq: HEPA

2 This presentation may contain forward - looking statements within the meaning of Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934 . Such forward - looking statements are characterized by future or conditional verbs such as “may,” “will,” “expect,” “intend,” “anticipate,” believe,” “estimate” and “continue” or similar words . You should read statements that contain these words carefully because they discuss future expectations and plans, which contain projections of future results of operations or financial condition or state other forward - looking information . Such statements are only predictions and our actual results may differ materially from those anticipated in these forward - looking statements . We believe that it is important to communicate future expectations to investors . However, there may be events in the future that we are not able to accurately predict or control . Factors that may cause such differences include, but are not limited to, those discussed under Risk Factors in our periodic reports filed with the Securities and Exchange Commission, including the uncertainties associated with product development, the risk that products that appeared promising in early clinical trials do not demonstrate safety and efficacy in larger - scale clinical trials, the risk that we will not obtain approval to market our products, risks associated with delays, increased costs and funding shortages caused by the COVID - 19 pandemic ; the risks associated with dependence upon key personnel and the need for additional financing . We do not assume any obligation to update forward - looking statements as circumstances change . This presentation does not constitute an offer or invitation for the sale or purchase of securities or to engage in any other transaction with Hepion Pharmaceuticals or its affiliates . The information in this presentation is not targeted at the residents of any particular country or jurisdiction and is not intended for distribution to, or use by, any person in any jurisdiction or country where such distribution or use would be contrary to local law or regulation . Statements Forward - Looking

3 Executive Management Introduction to • Dr. Robert Foster, Chief Executive Officer • Dr. Todd Hobbs, Chief Medical Officer • Dr. Daren Ure , Chief Scientific Officer • Dr. Patrick Mayo, Senior Vice President, Clinical Pharmacology & Analytics • Mr. John Cavan , Chief Financial Officer

4

5 NAFLD n on - a lcoholic f atty l iver d isease • “Fatty liver” disease associated with obesity, diabetes, hypertension, etc. • Approx. 25% of global population (up to 100 million in U.S.) NASH leads to cirrhosis, liver cancer (HCC), end stage liver disease, and death NASH n on - a lcoholic s teato h epatitis • A more severe form of NAFLD, with inflammation and liver scarring (fibrosis) • 1.5 – 6.5% globally (up to 17 million in U.S.) NASH is a Healthcare Crisis Large cost to healthcare system No drugs are approved for treating NASH Soon to be lead reason for liver transplantation NASH Drug Development The Need and Opportunity -

6 Through Cyclophilin Inhibition Multiple Therapeutic Actions Cyclophilin A (cytosol and secreted) Secreted from injured cells and acts as proinflammatory cytokine by binding to CD147 Cyclophilin B (endoplasmic reticulum) Promotes fibrotic scarring by controlling collagen production CRV431 Non - immunosuppressive analog of cyclosporine A Pan - Cyclophilin Inhibitor (Ki ≈ 1 nM ) Cyclophilin D (mitochondria) Regulates mitochondrial metabolism Promotes mitochondrial pore opening leading to mitochondrial and necrotic cell death INFLAMMATION x CD147 pro - inflammatory receptors x Procollagen x x FIBROTIC SCARRING Cyp A Cyp B Cyp D Mitochondrial pores (mPT) x NECROTIC CELL DEATH x

7

8 SAFETY TOLERABILITY PHARMACOKINETICS FIBROSCAN BIOMARKERS Day 1 Randomization * Day 28 End of treatment Day 42 End of trial CRV431 225 mg (n=17) Placebo (n=8) O ff - treatment O ff - treatment F2/F3 NASH Patients (N=43) Fasted Oral Dosing Day 29 Placebo (n=6) O ff - treatment CRV431 75 mg (n=12) O ff - treatment NASH Subjects - Safety, Tolerability, and Pharmacokinetics PHASE 2a AMBITION Study * Randomized assignment 2:1 CRV431. AMBITION: A Phase 2a, M ulti - center, Single - B l i nd, Placebo - Controlled, Proof of Concept Study to Evaluate the Safety & T olerab i lity of CRV431 Dosed O nce Daily in N ASH Induced F2 & F3 Subjects

9 Baseline Demographics Phase 2a: AMBITION Study CRV431 75 mg (n = 12) CRV431 225 mg (n = 17)* Pooled Placebo (n = 14) Age (years) Mean (SD) 61.9 (8.0) 54.0 (13.3) 61.1 (12.0) Range 48 - 72 27 - 71 27 - 72 Gender Male n (%) 7 (58.3) 7 (41.2) 9 (64.3) Female n (%) 5 (41.7) 10 (58.8) 5 (35.7) Race White n (%) 11 (91.7) 17 (100) 13 (92.9) Hispanic n (%) 1 (8.3) 1 (7.1) 2 (4.7) BMI ( kg/m 2 ) Mean (SD) 35.0 (8.0) 37.7 (6.4) 38.9 (8.8) Range 25 - 53 28 - 53 29 - 57 *1 - Covid Patient

10 Dose AE Term Severity Outcome Relation to Study Drug Placebo Body Aches Moderate Ongoing Possible 75 mg Sensation of Heart Beating Strongly Moderate Resolved Probable 75 mg Constipation Mild Resolved Probable 225 mg Diarrhea Mild Resolved Probable 225 mg Constipation Severe Resolved Probable 225 mg Fatigue Mild Resolved Probable 225 mg Lips Tingling Mild Resolved Probable 225 mg Constipation Mild Ongoing Probable 225 mg Increased Weight Mild Ongoing Probable 225 mg Headache Mild Ongoing Probable 225 mg Diarrhea Mild Ongoing Probable Well Tolerated Primary Endpoint: Safety – No Serious Adverse Events Note: all events we categorized as not serious.

11 Adequate Exposures Anticipated for Efficacy Primary Endpoint: Pharmacokinetics –
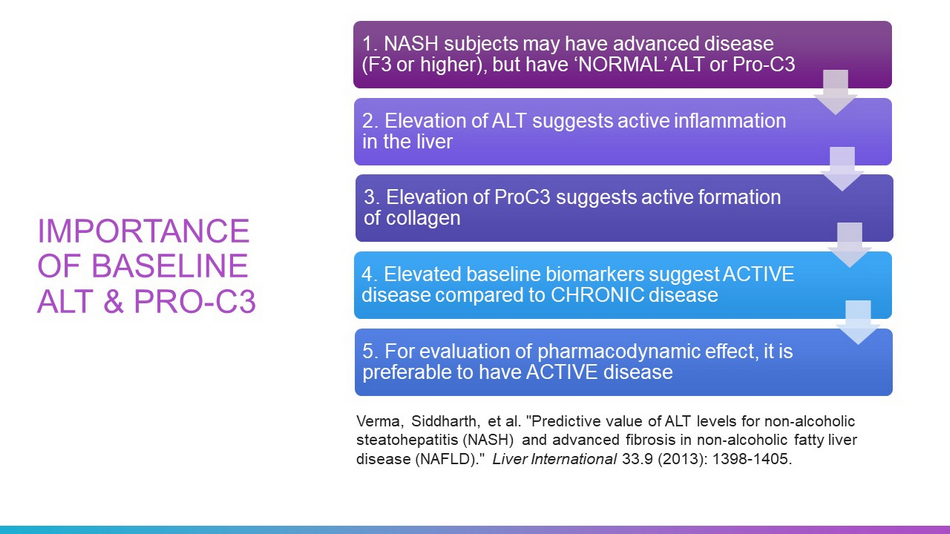
IMPORTANCE OF BASELINE ALT & PRO - C3 1. NASH subjects may have advanced disease (F3 or higher), but have ‘NORMAL’ ALT or Pro - C3 2. Elevation of ALT suggests active inflammation in the liver 3. Elevation of ProC3 suggests active formation of collagen 4. Elevated baseline biomarkers suggest ACTIVE disease compared to CHRONIC disease 5. For evaluation of pharmacodynamic effect, it is preferable to have ACTIVE disease Verma, Siddharth, et al. "Predictive value of ALT levels for non - alcoholic steatohepatitis (NASH) and advanced fibrosis in non - alcoholic fatty liver disease (NAFLD)." Liver International 33.9 (2013): 1398 - 1405.

13 ALT, Area Under the Curve (AUC) p = 0.0493 ANOVA % Change From Baseline Dose Placebo 75 mg 225 mg Mean ± SD - 6.1 ± 13.3 - 18.4 ± 25.8 - 21.1 ± 21.0 Median (Range) - 5.2 ( - 23.8 – 13.8) - 15.9 ( - 58.3 – 17.2) - 20.0 ( - 81.5 – 11.1) N (%) Reduction ALT 7/14 (50%) 8/12 (67%) 13/15 (87%) AUC of ALT (IU*D/L) 1465.1 ± 810.9 1190.5 ± 712.1 859.9 ± 387.0* *p < 0.05 versus placebo

14 Volume (L) Absorption Ka (h - 1 ) Elimination (k h - 1 ) Inhibitory Imax Model - Baseline (ALT) IC50:Half - Maximal Inhibition • Assess patient characteristics (COVARIATES) that help refine prediction of both PK and PD • Final Covariate Model: ↑Cholesterol ~ ↓Absorption ↑Baseline AST ~ ↑Effect ↑Lean Body Weight ~ ↓ IC50 • Confirms Concentration - Effect for ALT • Allows simulations for Phase 2b and Phase 3 PK PD Observed vs Predicted: ALT PK - PD Model Successfully Predicts ALT

15 ALT Responder Analysis By Flexible Discriminate Analysis: Misclassification Error = 0.04255 ALT Non - Responder Responder Unknown Age 61.6 57.2 60.6 BMI 40.2 36.1 36.1 Sex > Male > Female 2.0 Day 1, 2h 284.2 596.5 264.8 Day 14, 0h 483.4 882.5 667.9 Day 28, 0h 557.9 1040.7 706.0 Day 28, 2h 724.0 1243.0 870.1 BASO 0.0677 0.0741 0.0540 CPK 128.8 100.0 114.4 CREAT 0.700 0.683 0.700 GLUCOSE 117.3 134.8 109.4 PLT 228.5 235.7 237.8 TRIGs 164.5 174.6 169.0 WBC 7.2 7.6 6.5 CHOL 160.5 176.5 188.2 AST 42.8 50.4 75.6 ALT 51.8 59.8 79.6 A1C 6.7 6.9 6.5 DX_Y = Day X, Y - Hour CRV431 Concentration
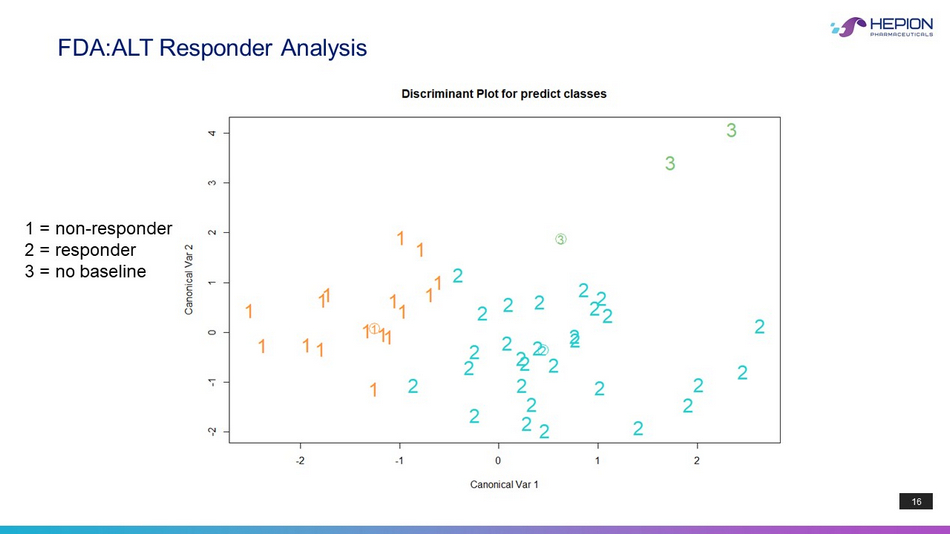
16 FDA:ALT Responder Analysis 1 = non - responder 2 = responder 3 = no baseline

17 Note stratification by Baseline Example: Resmetirom Harrison, Stephen A., et al . Lancet 2019; published online Nov 11. http:// dx.doi.org /10.1016/S0140 - 6736(19)32517 - 6. Note stratification by Baseline
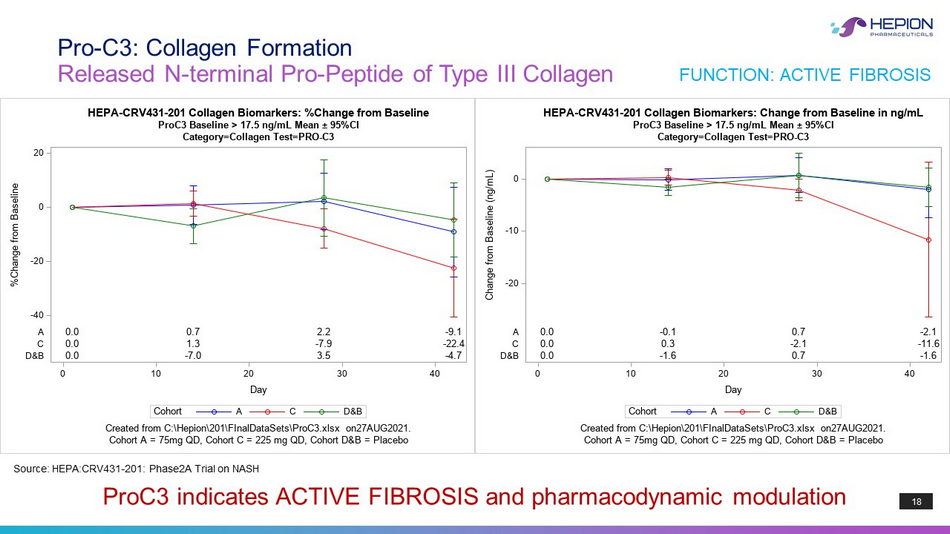
18 Released N - terminal Pro - Peptide of Type III Collagen Pro - C3: Collagen Formation ProC3 indicates ACTIVE FIBROSIS and pharmacodynamic modulation Source: HEPA:CRV431 - 201: Phase2A Trial on NASH FUNCTION: ACTIVE FIBROSIS

19 Volume (L) Absorption Ka (h - 1 ) • No Covariates were required to predict both PK and PD. • Final Covariate Model: PK 1 - Compartment PD: Inhibitory Imax Model with Baseline Pro - C3 • Quantitative Concentration - Effect for Pro - C3 • Allows simulation of Phase 2b and Phase 3 trials PK PD Observed vs Predicted: Pro - C3 PK - PD Model Successfully Predicts Pro - C3 Elimination (k h - 1 ) Inhibitory Imax Model - Baseline (Pro - C3) IC50:Half - Maximal Inhibition

20 Pro - C3 Responder Analysis By Mixture Discriminate Analysis: Misclassification Error = 0.02128 Pro - C3 Non - Responder Responder Sex >Male >Female Age 60.4 55.4 BMI 36.3 39.1 CHOL 177.9 163.5 TRIGs 181.0 150.1 AST 45.6 62.5 ALT 55.3 69.0 PLT 239.8 221.3 Day 1, 2h 449.8 528.2 Day 14, 0h 732.3 785.5 Day 28, 0h 828.1 964.2 Day 28, 2h 1012.8 1160.0 BASO 0.0697 0.0713 WBC 7.6 7.1 GLUCOSE 128.3 124.9 A1C 6.9 6.5 CPK 105.5 118.1 CREAT 0.666 0.740 DX_Y = Day X, Y - Hour CRV431 Concentration

21 PRO - C3 CRV431 Responder Analysis 1=Non - Responder 2=Responder 1 = non - responder 2 = responder
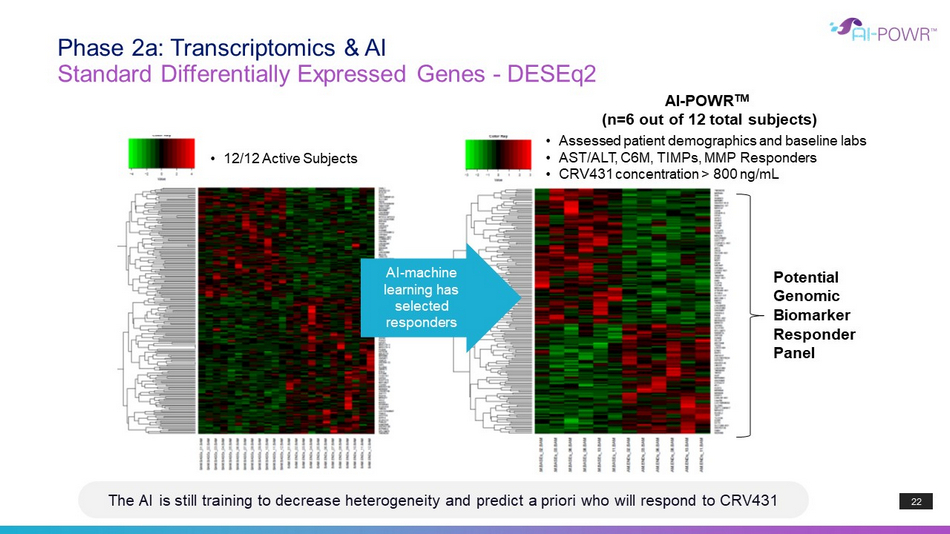
AI - POWR TM (n=6 out of 12 total subjects) • Assessed patient demographics and baseline labs • AST/ALT, C6M, TIMPs, MMP Responders • CRV431 concentration > 800 ng / mL Potential Genomic Biomarke r Responder Panel • 12/12 Active Subjects AI - machine learning has selected responders The AI is still training to decrease heterogeneity and predict a priori who will respond to CRV431 22 Standard Differentially Expressed Genes - DESEq2 Phase 2a: Transcriptomics & AI

23 A1BG SDC2 MMP 23B FBN2 EMID1 LOXL1 SERP INB8 ORM1 ICAM1 VCAN CLEC3B PXDN HTRA1 F13A1 COCH COL9A2 COL4A3 EMILIN2 MMRN1 LAMB1 COL6AE LAMA5 ADAM9 LTBP1 TIMP2 COL 26A1 HRNR COLQ HSPG2 C1QB CLU ADAM 19 BCAM MXRA7 PLSCR1 PCSK6 BCHE N1D1 F5 FN1 FBN1 HGF PKLR NID2 COL3A1 LAMB2 LAMC1 IL2RA EGFL7 PLSCR4 USH2A MMP8 CLUL1 TMEM256 - PLSCR3 PKM ACHE Clinical Collagen - Related Gene Regulatory Network collagen - containing extracellular matrix collagen binding collagen type IV trimer collagen type IX trimer collagen fibril organization collagen catabolic process Consistent antifibrotic effects observed in all preclinical and clinical models

24 Phase 2a Study Conclusions • Phase 2a study provided safety and exposure data in NASH subjects • Phase 2a study demonstrated signals of efficacy (inflammation and fibrosis) within 4 weeks • Phase 2a study provided post - hoc responder analysis data to further train AI - POWR for a priori responder analysis • Data from the Phase 2a was utilized to add a 150 mg dose cohort for the Phase 2b protocol and to adjust inclusion criteria (Pro - C3 > 14 ng/mL).

25

26 Primary Objective: Evaluate the efficacy and safety of once - daily 75mg, 150 mg, and 225 mg doses of CRV431 compared to placebo in subjects with biopsy proven NASH and stage 2 liver fibrosis (F2) / stage 3 liver fibrosis (F3) Phase 2b ASCEND - NASH F2/F3 NASH Patients (N=336) RANDOMIZED 1:1:1:1 Day 1 12 Months End of treatment CRV431 225 mg qd (n=84) Placebo (n=84) CRV431 150 mg qd (n=84) 13 Months End of trial Data to inform phase 3 trial patient selection and biomarkers for analysis. AI - POWR identification of biomarkers of CRV431 response Off - treatment follow - up CRV431 75 mg qd (n=84) Interim Analysis Interim Analysis • Targeted 12 – 14 - month recruitment period will include 86 US sites and 39 ex - US sites (Canada, France, UK, Spain, Germany, Italy, Belgium, Australia) • Up to 20 additional F4 subjects discovered during screening will be enrolled in an open label cohort dosed with 225mg CRV431 • Power = 90% to distinguish each dose level

27 Primary Efficacy Endpoint: Superiority of CRV431 (75mg, 150 mg, 225 mg) compared to placebo on liver histology at month 12 relative to the screening biopsy, by assessing the proportion of subjects with improvement in fibrosis by at least 1 stage (NASH CRN system) OR NASH resolution without worsening of fibrosis Secondary Efficacy Endpoints: Superiority of CRV431 (75mg, 150 mg, 225 mg) compared to placebo on histology at month 12 relative to screening by assessing: • Proportion of subjects with improvement in fibrosis by at least 1 stage (NASH CRN system), regardless of effect on NASH • Proportion of subjects with improvement in fibrosis by at least 2 stages (NASH CRN system), regardless of effect on NASH • Proportion of subjects with improvement in fibrosis by at least 2 stages (NASH CRN system) AND no worsening of NASH. By at least 1 stage regardless of the effect on NASH Endpoints

28

29 Financial Position as of June 30, 2021 Cash - $110.1 MM Working Capital - $106.6 MM No Debt Common Shares Outstanding - 76,225,245

30

31 • CRV431, once - daily oral, multi - modal • Cyclophilin inhibition allows for several benefits, including anti - fibrotic, anti - inflammatory, cytoprotective • Phase 2a NASH trial completed with success • Safe, well - tolerated, PK defined • Efficacy signals in only 4 weeks • Phase 2b activities initiated • Hepion’s Proprietary Artificial Intelligence Platform (AI - POWR Ρ ) • Core scientific team with >100 years collective cyclophilin expertise • Core scientific team discovered and developed voclosporin (currently marketed) • Robust IP $ 110.1 M as of 6/30/21 CASH TWO VALUE DRIVERS A Therapy for NASH with indications for several other conditions AI - Driven, Bioinformatic Platform Summary 31 76.2 M common shares outstanding
