Attached files
EXHIBIT 99.2

CORPORATE PRESENTATION (NASDAQ:AZRX) September 2021

Certain statements in this presentation constitute “forward-looking statements” within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. Any statements that refer to expectations or other characterizations of future events, circumstances or results are forward-looking statements. Such forward-looking statements include projections. Such projections were not prepared in accordance with public guidelines of the American Institute of Certified Public Accountants regarding projections and forecasts, nor have such projections been audited, examined or otherwise reviewed by independent auditors of the company. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of the company and its clinical trials to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. The views expressed are those of management and are based on currently available information. Estimates and projections contained herein have been prepared by management and involve significant elements of subjective judgment and analysis and are based on certain assumptions. No representation nor warranty, expressed or implied, is made as to the accuracy or completeness of the information contained in this document, and nothing contained herein is, or shall be relied upon, as a promise or representation, whether as to the past or the future. The projections are not intended to follow generally accepted accounting principles. Neither our accountants nor our legal counsel have compiled, audited, prepared, or contributed to the projections or the underlying assumptions. None of these parties express an opinion with respect to the projections. You are cautioned not to place undue reliance on these forward-looking statements. Except for ongoing obligations of the company to disclose material information under the federal securities laws, the company does not undertake any obligation to release any revisions to any forward-looking statements, to report events or to report the occurrence of unanticipated events. Company Disclaimer

Overview First Wave BioPharma is a clinical stage biotechnology company currently focused on the development of targeted, non-systemic therapies for gastrointestinal (GI) diseases, with two assets and six clinical indications spanning inflammatory bowel diseases (IBD), COVID viral infections, oncology therapy induced colitis, and pancreatic digestive disorders. Niclosamide: Re-purposed small molecule drug with potent anti-viral and anti-inflammatory properties, proprietary micronized formulation COVID-19 GI infections (Phase 2 launched 1H 2021) Immune Checkpoint Inhibitor-Associated Colitis (Phase 1b/2a in 2H 2021) IBD: Ulcerative Proctitis (Phase 2 in 2H 2021) IBD: Ulcerative Colitis (Phase 1b/2a) IBD: Crohn’s Disease (Phase 2a) Adrulipase: Recombinant lipase biologic for the treatment of Exocrine Pancreatic Insufficiency (EPI) 6. EPI in Cystic Fibrosis (CF); new enteric formulation (Phase 2b in 1H 2022) Assets leverage the Company’s core competencies and expertise in developing targeted, safe, non-systemic oral GI therapies Pipeline of gut-targeted GI therapies address significant unmet medical needs in billion-dollar markets

Recent Developments: AzurRx BioPharma Acquires First Wave Bio First Wave BioPharma (“First Wave Bio” or “First Wave”) created through the merger of AzurRx BioPharma and First Wave Bio Total Consideration: Up to $229MM - $22MM Upfront Payment: $18MM Cash in Tranched Payments and $4MM Stock - Up to $207MM in Clinical, Regulatory and Commercial Milestone Payments Company acquires FirstWave’s robust Niclosamide IP portfolio and proprietary micronized formulations COVID-GI Infections Immune Checkpoint Inhibitor-Associated Colitis Inflammatory Bowel Diseases (IBD) Oral tablet and topical delivery mechanisms Pipeline Expansion into the Multi-Billion Dollar Mild-to-Moderate IBD Market - Ulcerative Proctitis - Ulcerative Colitis Crohn’s Disease NASDAQ “FWBI” Ticker to replace “AZRX” on Thursday, Sept. 23rd

First Wave BioPharma Management Team Combined Experience in Developing and Launching more than 25 Drugs Loreal McDonald General Counsel Image
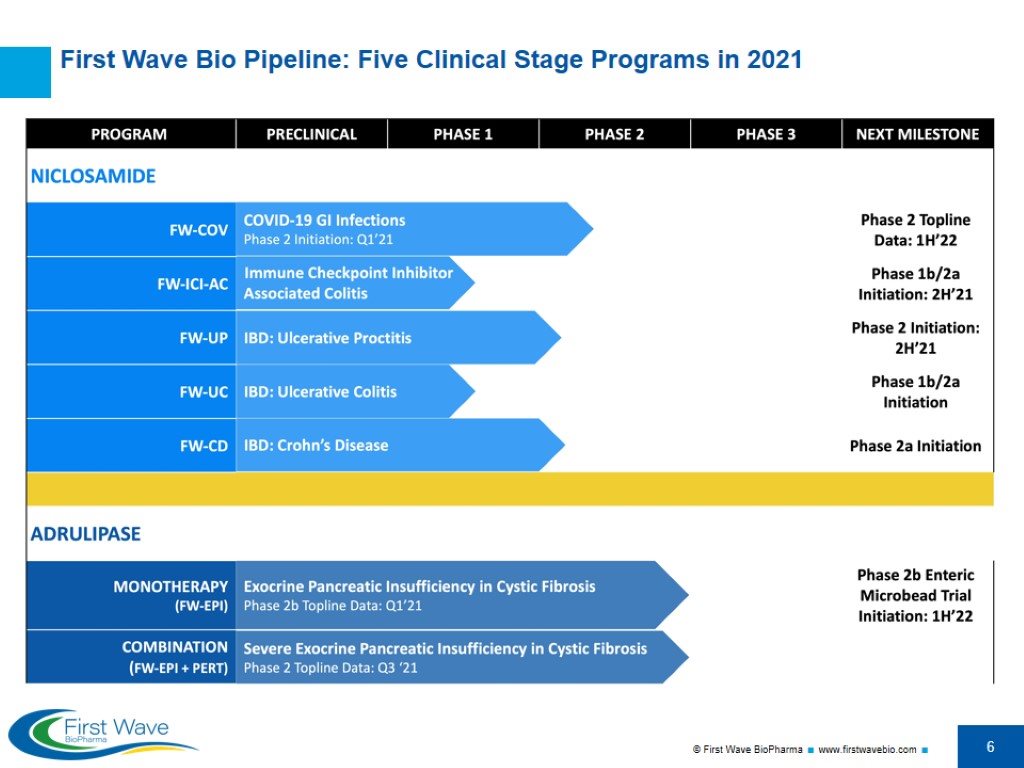
First Wave Bio Pipeline: Five Clinical Stage Programs in 2021
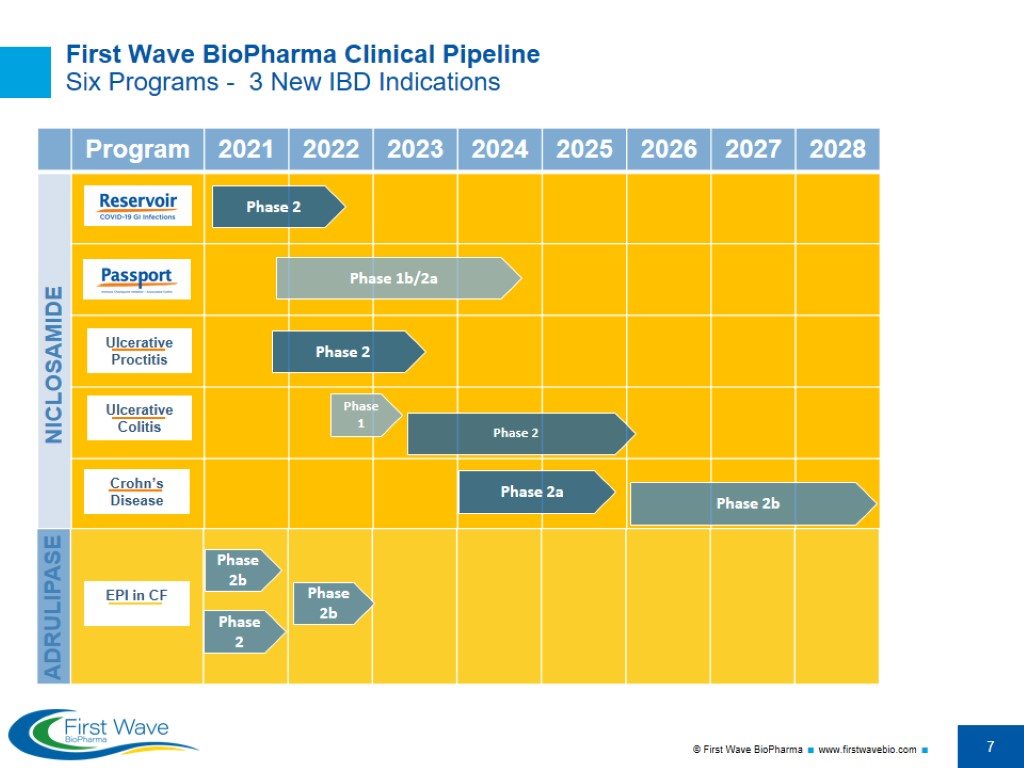
First Wave BioPharma Clinical Pipeline Six Programs - 3 New IBD Indications NICLOSAMIDE Phase 2 Phase 1b/2a Phase 2 Phase 1 Phase 2a Phase 2 Phase 2b ADRULIPASE Phase 2b Phase 2b Phase 2
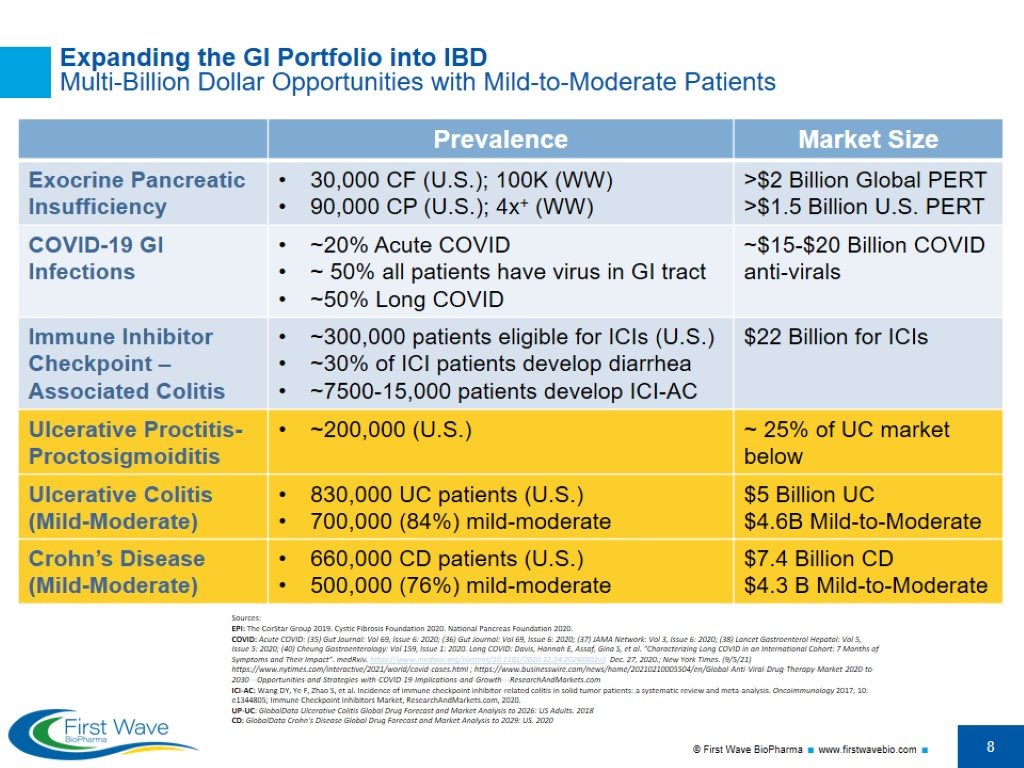
Expanding the GI Portfolio into IBD Multi-Billion Dollar Opportunities with Mild-to-Moderate Patients Sources: EPI: The CorStar Group 2019. Cystic Fibrosis Foundation 2020. National Pancreas Foundation 2020. COVID: Acute COVID: (35) Gut Journal: Vol 69, Issue 6: 2020; (36) Gut Journal: Vol 69, Issue 6: 2020; (37) JAMA Network: Vol 3, Issue 6: 2020; (38) Lancet Gastroenterol Hepatol: Vol 5, Issue 5: 2020; (40) Cheung Gastroenterology: Vol 159, Issue 1: 2020. Long COVID: Davis, Hannah E, Assaf, Gina S, et al. “Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact”. medRxiv. https://www.medrxiv.org/content/10.1101/2020.12.24.20248802v3 Dec. 27, 2020.; New York Times. (9/5/21) https://www.nytimes.com/interactive/2021/world/covid-cases.html ; https://www.businesswire.com/news/home/20210210005504/en/Global-Anti-Viral-Drug-Therapy-Market-2020-to-2030---Opportunities-and-Strategies-with-COVID-19-Implications-and-Growth---ResearchAndMarkets.com ICI-AC: Wang DY, Ye F, Zhao S, et al. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: a systematic review and meta-analysis. Oncoimmunology 2017; 10: e1344805; Immune Checkpoint Inhibitors Market, ResearchAndMarkets.com, 2020. UP-UC: GlobalData Ulcerative Colitis Global Drug Forecast and Market Analysis to 2026: US Adults. 2018 CD: GlobalData Crohn's Disease Global Drug Forecast and Market Analysis to 2029: US. 2020
NICLOSAMIDE: COVID-19 GI Infections Immune Checkpoint Inhibitor - Associated Colitis IBD: Ulcerative Proctitis IBD: Ulcerative Colitis IBD: Crohn’s Disease
History and Safety Profile of Niclosamide FDA approved (1982) small molecule anthelmintic drug used for intestinal tapeworm infections Clean safety history Ideal profile for GI-targeted agent Low oral bio-availability with minimal systemic exposure Niclosamide inhibits pro-inflammatory pathways Non-steroidal anti-inflammatory option Opportunities for combinations with standard of care for multiple indications without systemic immunosuppression

Proprietary Micronized Formulations: Potentially Transformative Efficacy Micronized niclosamide – a transformative treatment for multiple GI indications: Reduced particle size (~7 ) compared to regular non-micronized (~60 ) niclosamide Smaller particles have greater surface to solvent (GI fluids) ratio Improved dissolution: broader distribution and higher local GI concentrations Not systemically absorbed Preclinical studies confirm higher GI concentrations (~200x) with micronized niclosamide Micronized formulation, similar to non-micronized niclosamide, is not systemically absorbed (animal studies) Historically clean safety profile Avoids steroid-related complications; non-immunosuppressant
FW-COV: Treating COVID-19 GI Infections
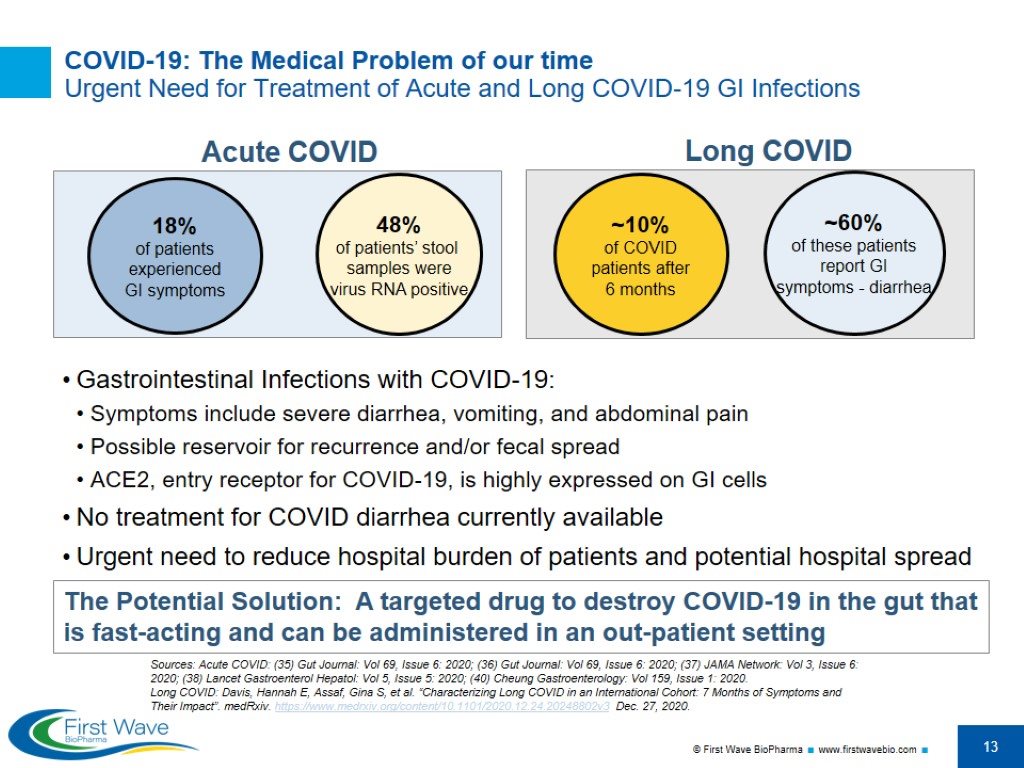
COVID-19: The Medical Problem of our time Urgent Need for Treatment of Acute and Long COVID-19 GI Infections Gastrointestinal Infections with COVID-19: Symptoms include severe diarrhea, vomiting, and abdominal pain Possible reservoir for recurrence and/or fecal spread ACE2, entry receptor for COVID-19, is highly expressed on GI cells No treatment for COVID diarrhea currently available Urgent need to reduce hospital burden of patients and potential hospital spread The Potential Solution: A targeted drug to destroy COVID-19 in the gut that is fast-acting and can be administered in an out-patient setting 18% of patients experienced GI symptoms 48% of patients’ stool samples were virus RNA positive Sources: Acute COVID: (35) Gut Journal: Vol 69, Issue 6: 2020; (36) Gut Journal: Vol 69, Issue 6: 2020; (37) JAMA Network: Vol 3, Issue 6: 2020; (38) Lancet Gastroenterol Hepatol: Vol 5, Issue 5: 2020; (40) Cheung Gastroenterology: Vol 159, Issue 1: 2020. Long COVID: Davis, Hannah E, Assaf, Gina S, et al. “Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact”. medRxiv. https://www.medrxiv.org/content/10.1101/2020.12.24.20248802v3 Dec. 27, 2020. ~10% of COVID patients after 6 months Acute COVID Long COVID ~60% of these patients report GI symptoms - diarrhea
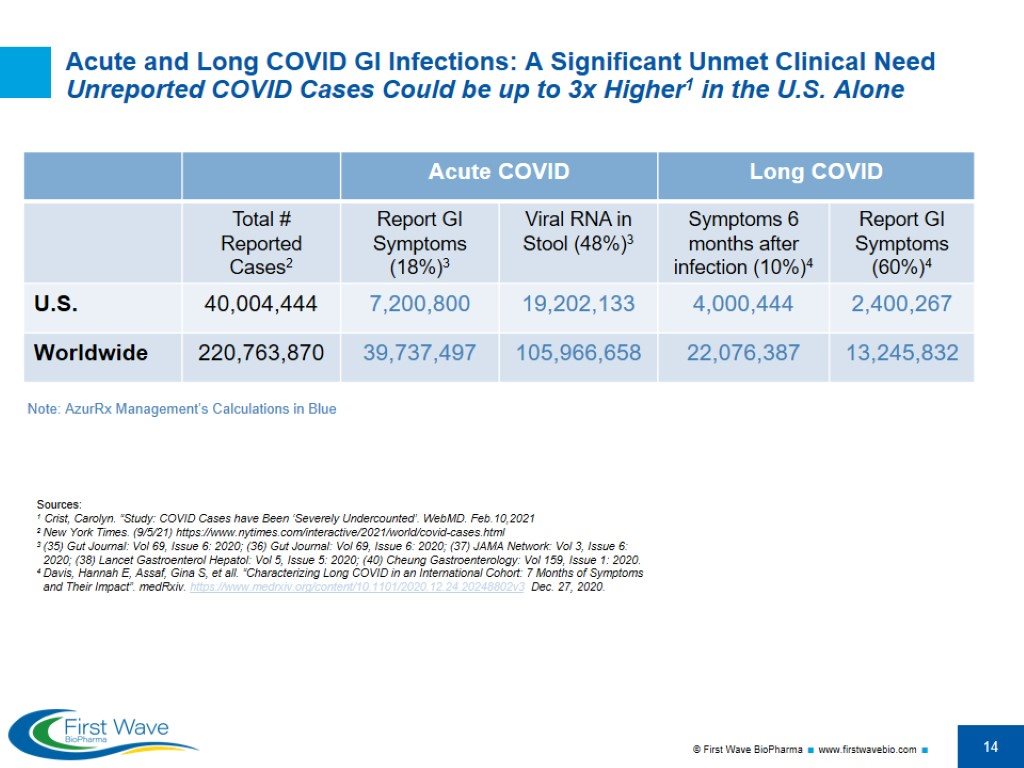
Acute and Long COVID GI Infections: A Significant Unmet Clinical Need Unreported COVID Cases Could be up to 3x Higher1 in the U.S. Alone Sources: 1 Crist, Carolyn. “Study: COVID Cases have Been ‘Severely Undercounted’. WebMD. Feb.10,2021 2 New York Times. (9/5/21) https://www.nytimes.com/interactive/2021/world/covid-cases.html 3 (35) Gut Journal: Vol 69, Issue 6: 2020; (36) Gut Journal: Vol 69, Issue 6: 2020; (37) JAMA Network: Vol 3, Issue 6: 2020; (38) Lancet Gastroenterol Hepatol: Vol 5, Issue 5: 2020; (40) Cheung Gastroenterology: Vol 159, Issue 1: 2020. 4 Davis, Hannah E, Assaf, Gina S, et all. “Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact”. medRxiv. https://www.medrxiv.org/content/10.1101/2020.12.24.20248802v3 Dec. 27, 2020. Note: AzurRx Management’s Calculations in Blue
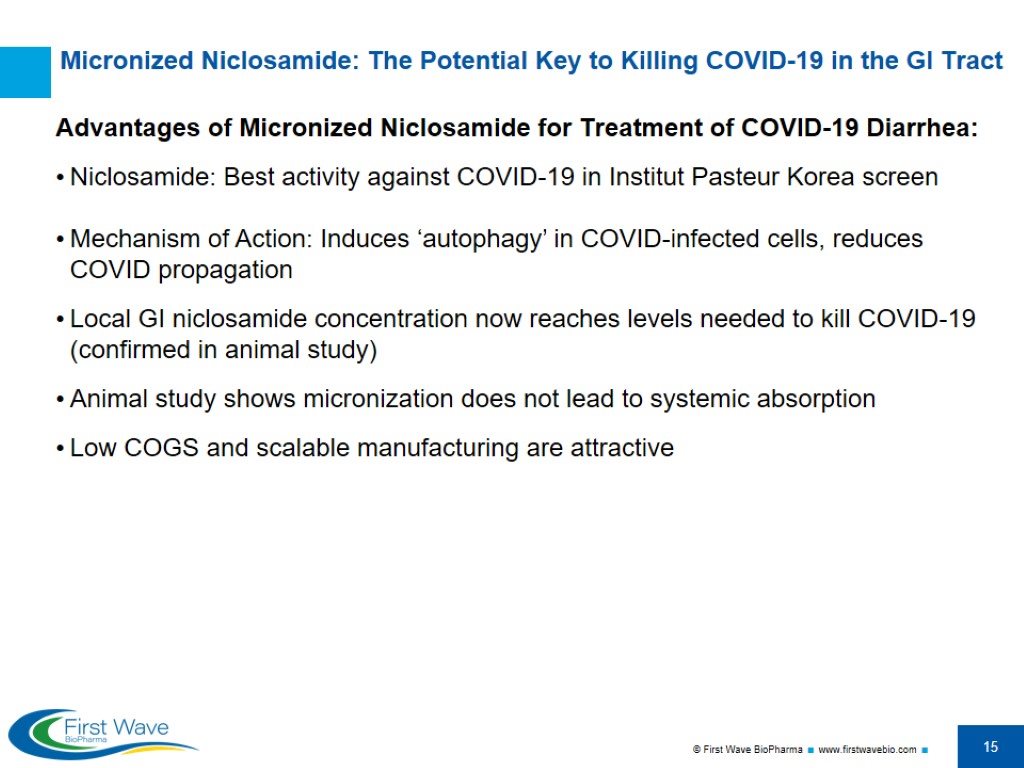
Micronized Niclosamide: The Potential Key to Killing COVID-19 in the GI Tract Advantages of Micronized Niclosamide for Treatment of COVID-19 Diarrhea: Niclosamide: Best activity against COVID-19 in Institut Pasteur Korea screen Mechanism of Action: Induces ‘autophagy’ in COVID-infected cells, reduces COVID propagation Local GI niclosamide concentration now reaches levels needed to kill COVID-19 (confirmed in animal study) Animal study shows micronization does not lead to systemic absorption Low COGS and scalable manufacturing are attractive

COVID GI Infection: Reservoir Trial Phase 2 Reservoir trial initiated in April 2021 Data Monitoring Committee (DMC) approved initiation of Part 2 150 patient efficacy study in U.S., Ukraine and India in Sept. 2021 Topline data anticipated in Q1 2022 Potential Emergency Use Authorization (EUA)
FW-ICI-AC: Immune Checkpoint Inhibitor-Associated Colitis
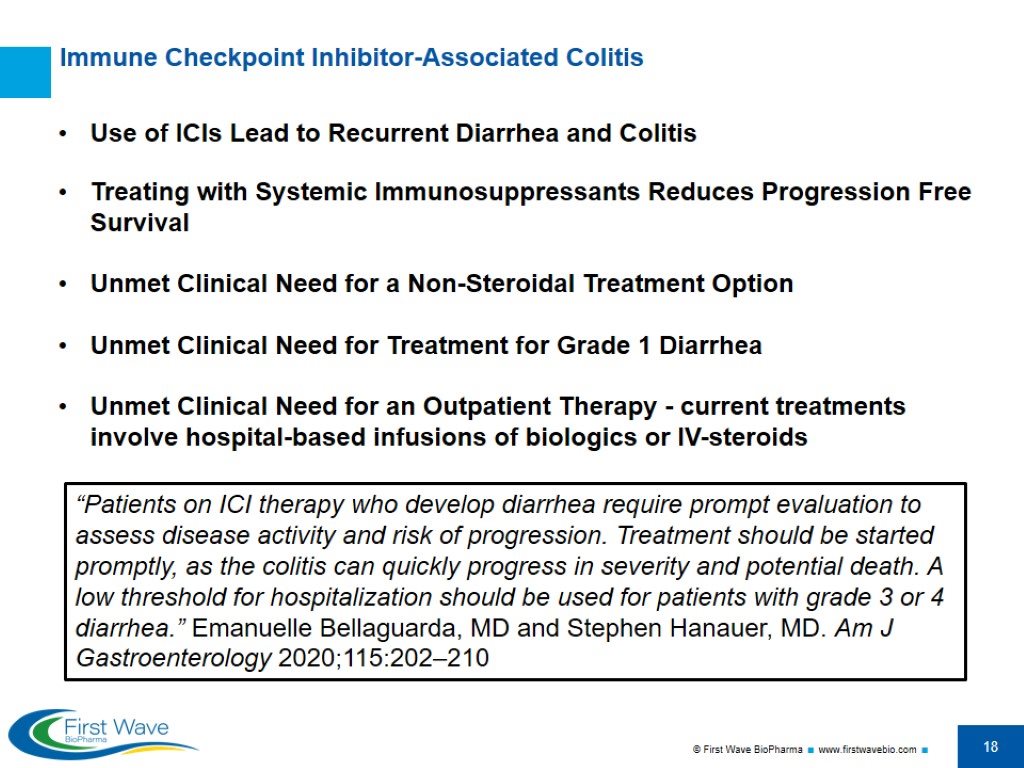
Use of ICIs Lead to Recurrent Diarrhea and Colitis Treating with Systemic Immunosuppressants Reduces Progression Free Survival Unmet Clinical Need for a Non-Steroidal Treatment Option Unmet Clinical Need for Treatment for Grade 1 Diarrhea Unmet Clinical Need for an Outpatient Therapy - current treatments involve hospital-based infusions of biologics or IV-steroids Immune Checkpoint Inhibitor-Associated Colitis “Patients on ICI therapy who develop diarrhea require prompt evaluation to assess disease activity and risk of progression. Treatment should be started promptly, as the colitis can quickly progress in severity and potential death. A low threshold for hospitalization should be used for patients with grade 3 or 4 diarrhea.” Emanuelle Bellaguarda, MD and Stephen Hanauer, MD. Am J Gastroenterology 2020;115:202–210
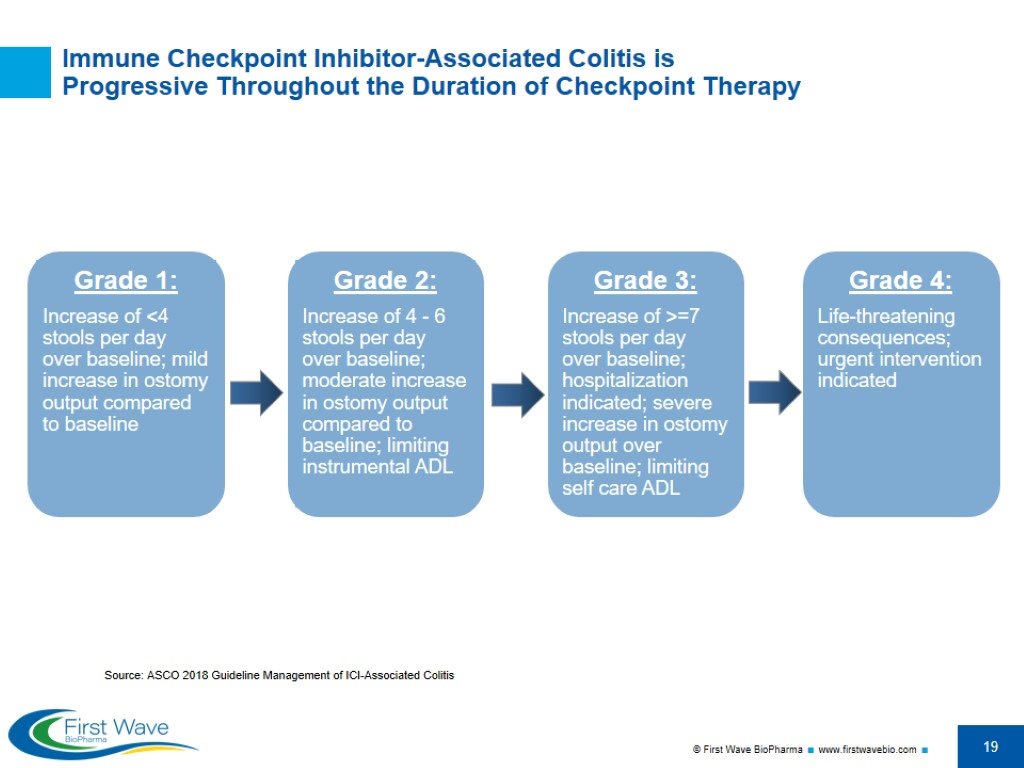
Immune Checkpoint Inhibitor-Associated Colitis is Progressive Throughout the Duration of Checkpoint Therapy Source: ASCO 2018 Guideline Management of ICI-Associated Colitis
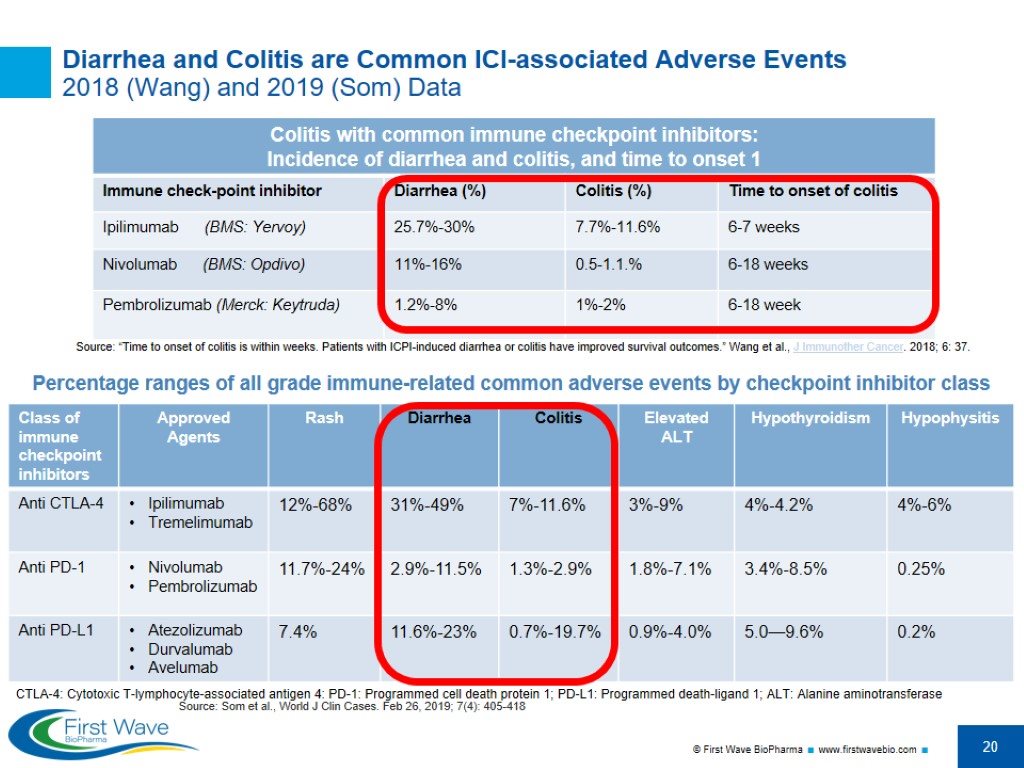
Diarrhea and Colitis are Common ICI-associated Adverse Events 2018 (Wang) and 2019 (Som) Data Source: Som et al., World J Clin Cases. Feb 26, 2019; 7(4): 405-418 Percentage ranges of all grade immune-related common adverse events by checkpoint inhibitor class CTLA-4: Cytotoxic T-lymphocyte-associated antigen 4: PD-1: Programmed cell death protein 1; PD-L1: Programmed death-ligand 1; ALT: Alanine aminotransferase Source: “Time to onset of colitis is within weeks. Patients with ICPI-induced diarrhea or colitis have improved survival outcomes.” Wang et al., J Immunother Cancer. 2018; 6: 37.

The Potential Solution: A gut targeted drug to treat the colitis with little or no systemic bioavailability that could counteract the activity of the ICI Niclosamide has the potential to prevent GI disease damage and stop disease progression to colitis in patients on ICIs by attacking the cell populations in the colon that cause this problem Agent can induce (with fast onset of action) and maintain long term remission GI-targeted Reduces systemic immune suppression Reduces off-target adverse effects Functions through clinically validated mechanisms Mitigates pathogenic lamina propria T cells Decreases the production of pro-inflammatory cytokines Enables Outpatient Treatment - may reduce hospital admissions for ICI-induced diarrhea Breakthrough Designation Potential - increases the therapeutic window for checkpoint inhibitors and the population of patients who benefit from checkpoint inhibitors.

Phase 1b-2a ICI-AC: Passport Trial Number of subjects/sites: Stage 1: 12 patients (safety) Stage 2: 90 patients (safety and efficacy) Timeline: IND submitted August 31 ~18 Months Enrollment

IBD Opportunity
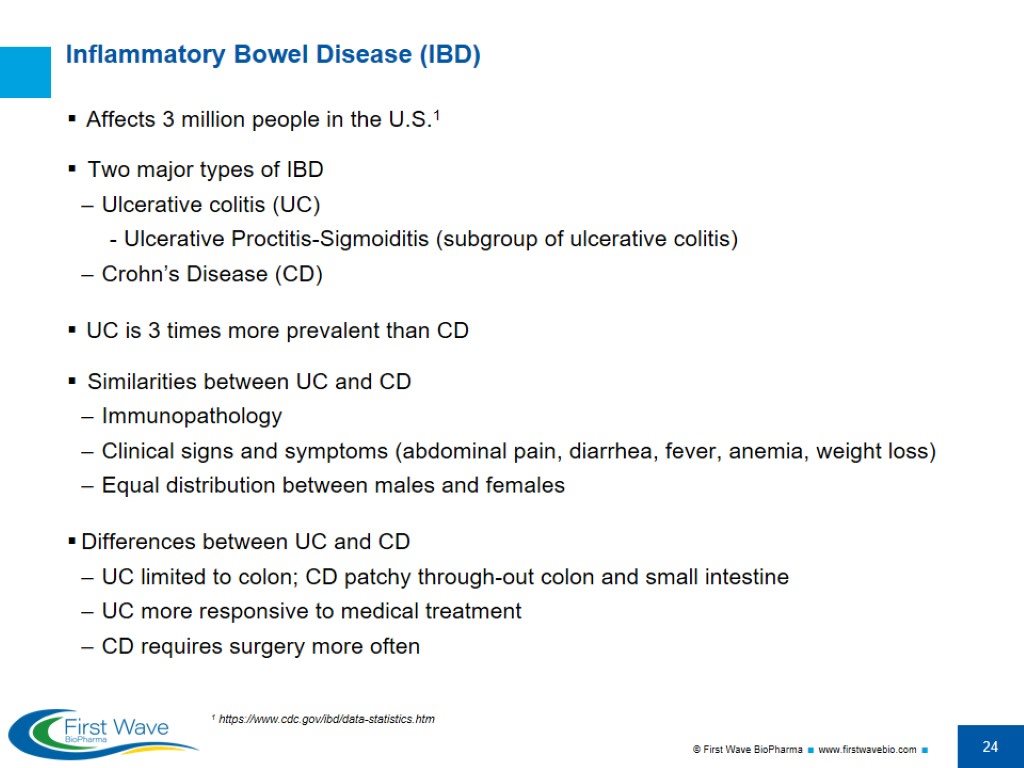
Inflammatory Bowel Disease (IBD) Affects 3 million people in the U.S.1 Two major types of IBD Ulcerative colitis (UC) Ulcerative Proctitis-Sigmoiditis (subgroup of ulcerative colitis) Crohn’s Disease (CD) UC is 3 times more prevalent than CD Similarities between UC and CD Immunopathology Clinical signs and symptoms (abdominal pain, diarrhea, fever, anemia, weight loss) Equal distribution between males and females Differences between UC and CD UC limited to colon; CD patchy through-out colon and small intestine UC more responsive to medical treatment CD requires surgery more often 1 https://www.cdc.gov/ibd/data-statistics.htm
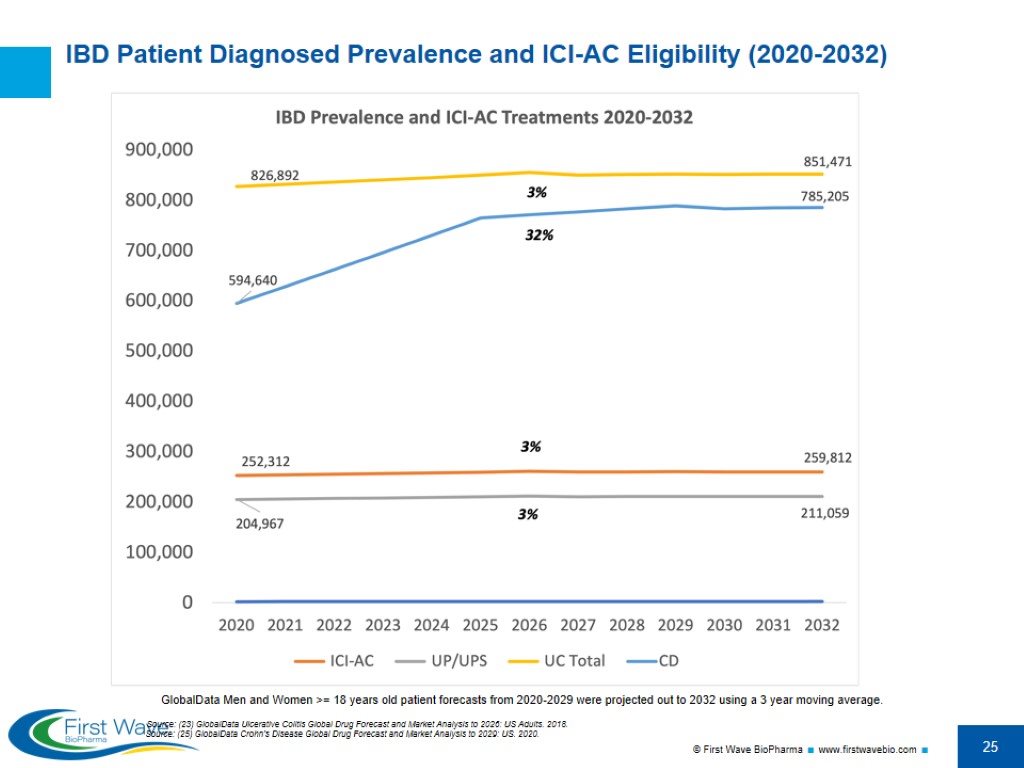
IBD Patient Diagnosed Prevalence and ICI-AC Eligibility (2020-2032) Source: (23) GlobalData Ulcerative Colitis Global Drug Forecast and Market Analysis to 2026: US Adults. 2018. GlobalData Men and Women >= 18 years old patient forecasts from 2020-2029 were projected out to 2032 using a 3 year moving average. Source: (25) GlobalData Crohn's Disease Global Drug Forecast and Market Analysis to 2029: US. 2020.

Treatments for IBD Ulcerative Colitis Mild disease: sulfasalazines; 5-ASAs (mesalamine) Moderate: steroids (budesonide; prednisone); azathioprine; 6-mercaptopurine; methotrexate Severe: Anti-TNF; Entyvio; Xeljanz; Stelara Crohn’s Disease Mild/Moderate: steroids Moderate/Severe: Anti-TNF; Entyvio; Stelara; Tysabri
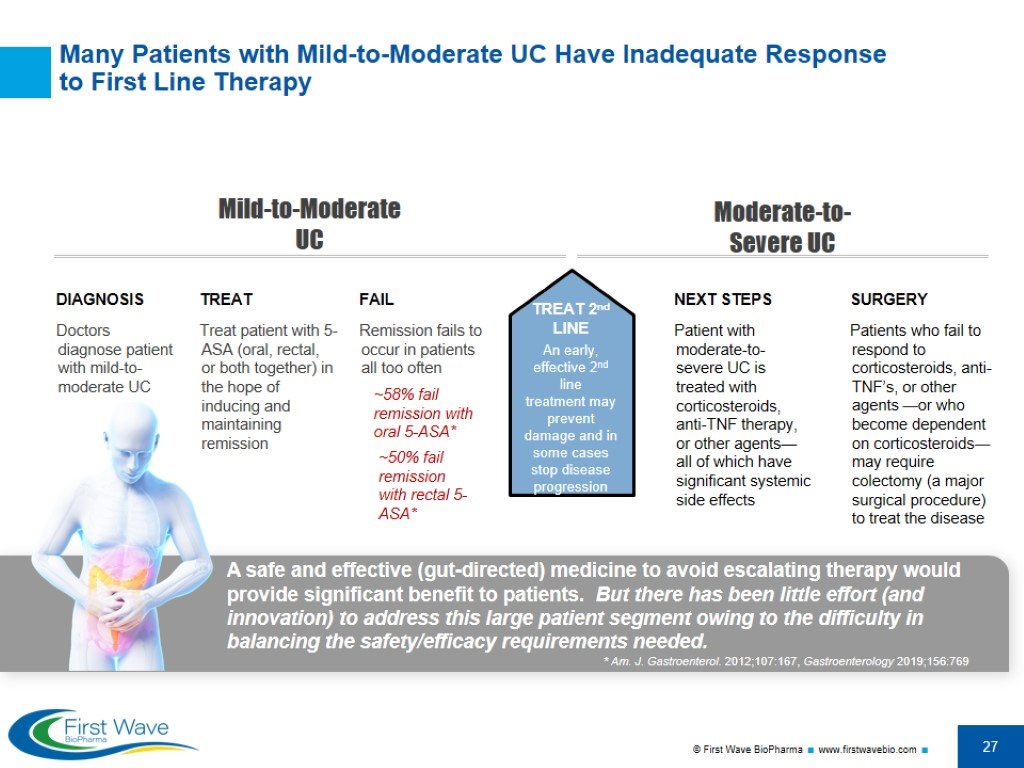
Many Patients with Mild-to-Moderate UC Have Inadequate Response to First Line Therapy * Am. J. Gastroenterol. 2012;107:167, Gastroenterology 2019;156:769 DIAGNOSIS Doctors diagnose patient with mild-to-moderate UC TREAT Treat patient with 5-ASA (oral, rectal, or both together) in the hope of inducing and maintaining remission FAIL Remission fails to occur in patients all too often NEXT STEPS Patient with moderate-to-severe UC is treated with corticosteroids, anti-TNF therapy, or other agents—all of which have significant systemic side effects SURGERY Patients who fail to respond to corticosteroids, anti-TNF’s, or other agents —or who become dependent on corticosteroids—may require colectomy (a major surgical procedure) to treat the disease ~58% fail remission with oral 5-ASA* ~50% fail remission with rectal 5-ASA* Mild-to-Moderate UC Moderate-to-Severe UC
Role for Niclosamide in IBD Pharmacology ideal for local bowel disease; not absorbed from GI tract Mechanism of action is to impair oxidative phosphorylation; i.e. how cells make energy. Pathogenic Th17 cells have overly active oxidative phosphorylation; niclosamide down regulates this over active cell. In vitro (cells from bowel wall of IBD patients); and in vivo (animal models of IBD) experiments demonstrate niclosamide provides beneficial effects. Preliminary data from human study of niclosamide in ulcerative proctitis show promising results Initial clinical trials of niclosamide in IBD will target mild or moderate non-responders to conventional therapy. Goal is to prevent advanced disease requiring more toxic immunosuppressive agents.
UP-UPS: Clinical remission efficacy with topical rectal niclosamide formulation superior to budesonide in Low Dose Phase 1b/2a Trial Clinical remission efficacy of 59% compares favorably to steroids as 2nd line therapy in mild-to-moderate Ulcerative Colitis (UC) Remission rate for budesonide in Ulcerative Proctitis (UP)/Ulcerative Proctosigmoiditis (UPS) is 38-44% Steroid use lowers patients’ ability to fight infections and leads to complications including bleeding, nausea, heartburn, and headaches Treatment-emergent adverse event (TEAE) reported in 35% (6/17 subjects) All but 1 TEAE was mild No serious or drug-related TEAEs First Ever Proof of Principle for Treatment of IBD with Niclosamide
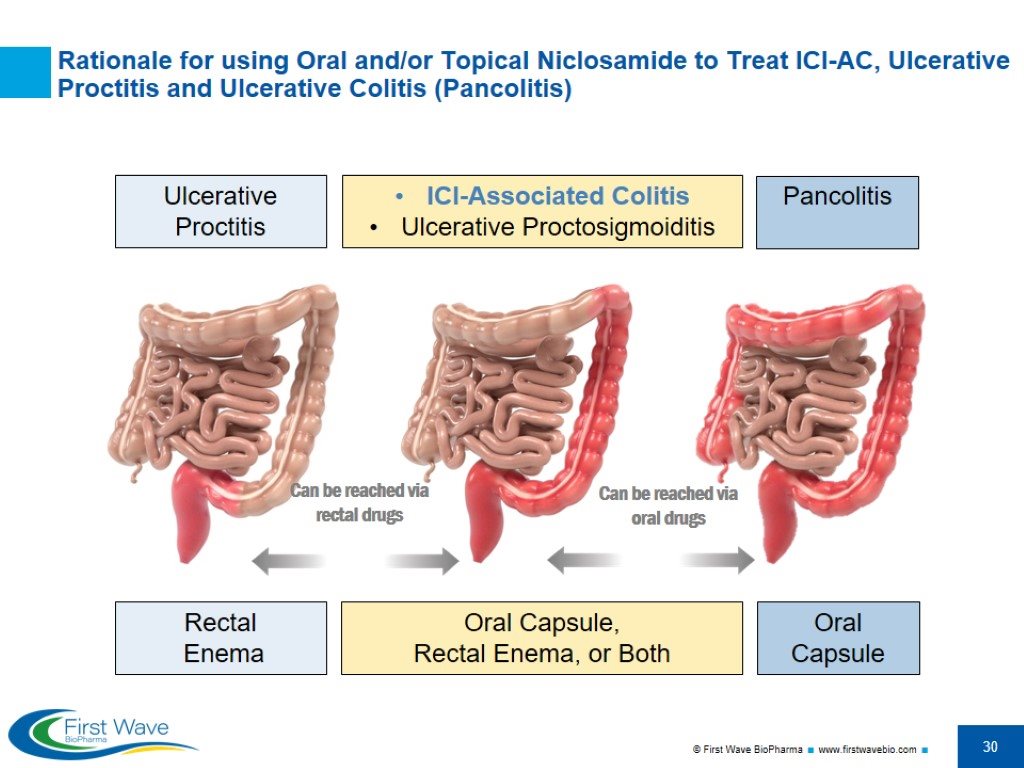
Ulcerative Proctitis ICI-Associated Colitis Ulcerative Proctosigmoiditis Pancolitis Rationale for using Oral and/or Topical Niclosamide to Treat ICI-AC, Ulcerative Proctitis and Ulcerative Colitis (Pancolitis) Rectal Enema Oral Capsule, Rectal Enema, or Both Oral Capsule
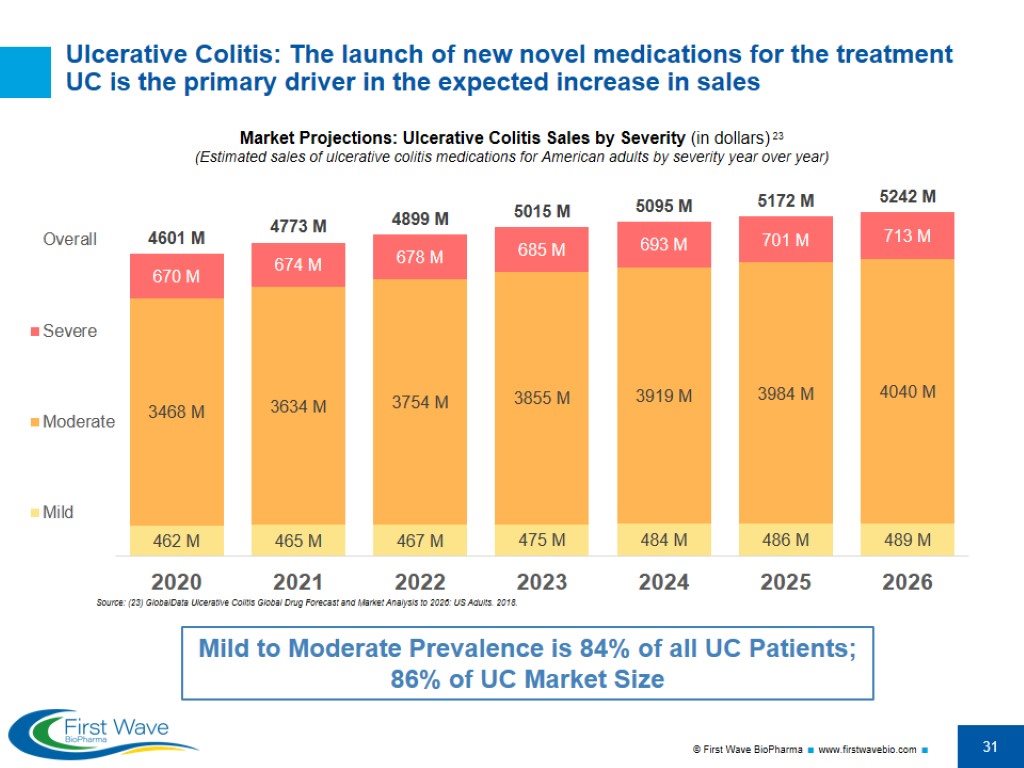
Ulcerative Colitis: The launch of new novel medications for the treatment UC is the primary driver in the expected increase in sales Market Projections: Ulcerative Colitis Sales by Severity (in dollars) 23 (Estimated sales of ulcerative colitis medications for American adults by severity year over year) Source: (23) GlobalData Ulcerative Colitis Global Drug Forecast and Market Analysis to 2026: US Adults. 2018. Mild to Moderate Prevalence is 84% of all UC Patients; 86% of UC Market Size
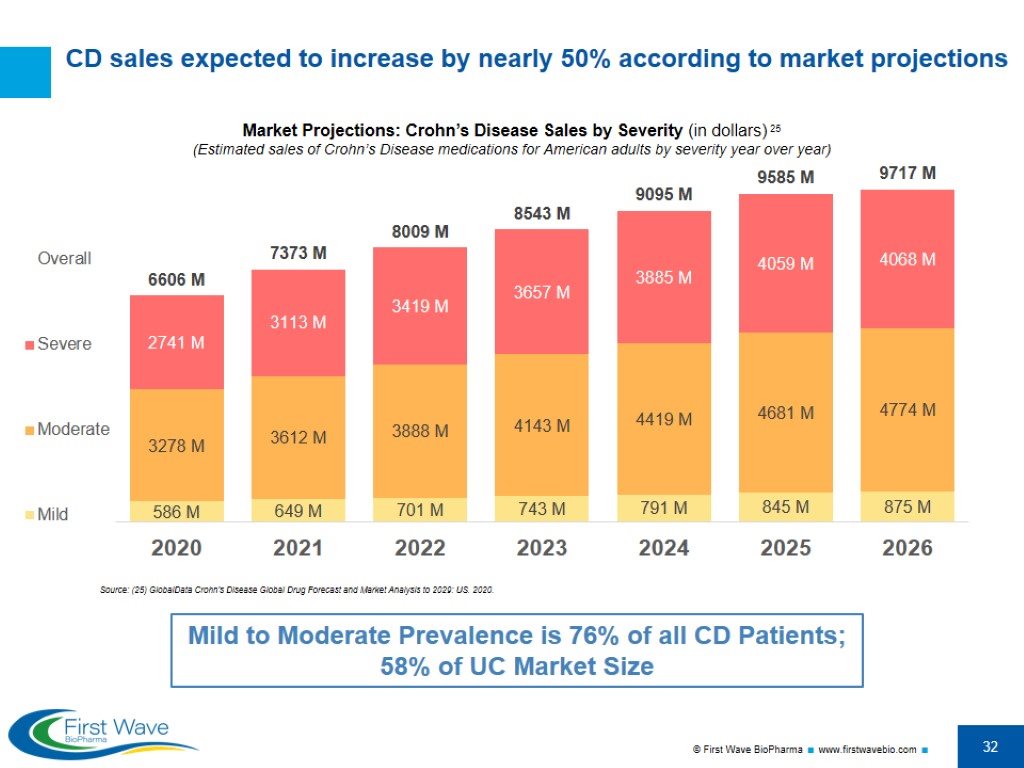
CD sales expected to increase by nearly 50% according to market projections Market Projections: Crohn’s Disease Sales by Severity (in dollars) 25 (Estimated sales of Crohn’s Disease medications for American adults by severity year over year) Source: (25) GlobalData Crohn's Disease Global Drug Forecast and Market Analysis to 2029: US. 2020. Mild to Moderate Prevalence is 76% of all CD Patients; 58% of UC Market Size
ADRULIPASE: Exocrine Pancreatic Insufficiency in Cystic Fibrosis & Chronic Pancreatitis

Recombinant lipase for treatment of Exocrine Pancreatic Insufficiency (EPI) Targeting patients with Cystic Fibrosis (CF) and Chronic Pancreatitis (CP) Addressing established global market (>$2 billion) (1) Recombinant alternative to porcine pancreatic enzyme replacement therapy (PERT) Clear unmet medical need Demonstrated safety and efficacy profile in two Phase 2 clinical trials in two indications Pursuing parallel monotherapy and combination therapy clinical pathways Topline Phase 2b CF monotherapy data announced in Q1 2021 Topline Interim Phase 2 CF combination (MS1819 + PERT) therapy data announced in Q3 2021; final Clinical Study Reports in Q4 2021 New enteric granule formulation being developed, Phase 2b trial in 1H 2022 (1) The CorStar Group 2019. Symphony Health 2019. ADRULIPASE: MS1819 Clinical Program
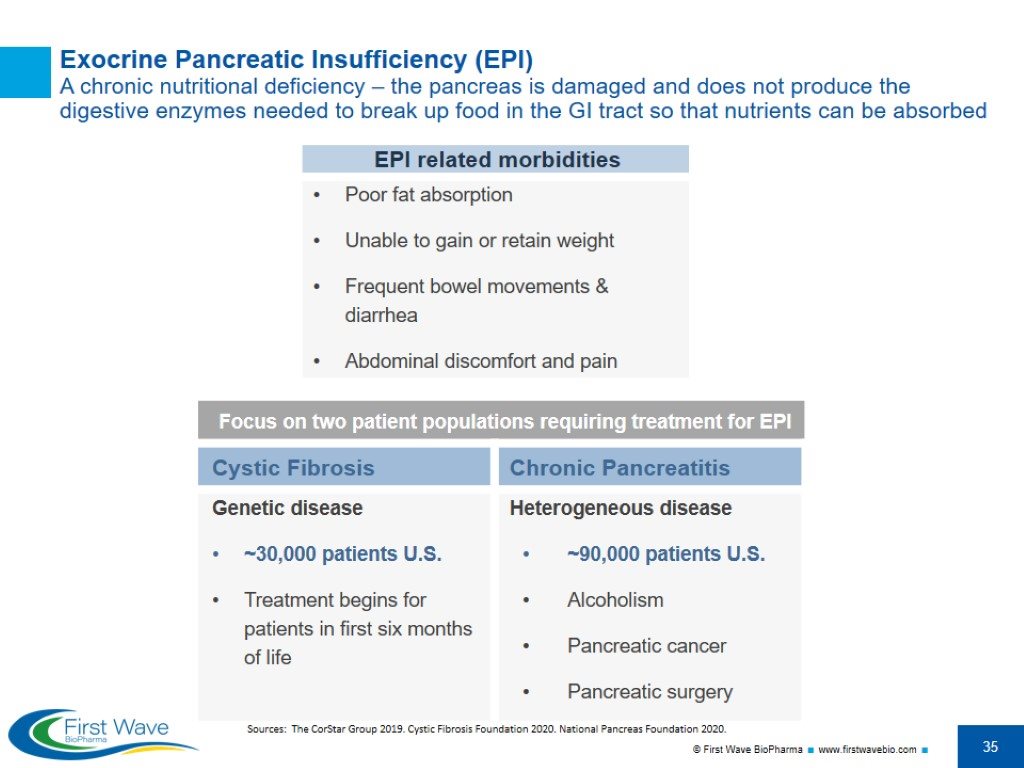
Sources: The CorStar Group 2019. Cystic Fibrosis Foundation 2020. National Pancreas Foundation 2020. Exocrine Pancreatic Insufficiency (EPI) A chronic nutritional deficiency – the pancreas is damaged and does not produce the digestive enzymes needed to break up food in the GI tract so that nutrients can be absorbed
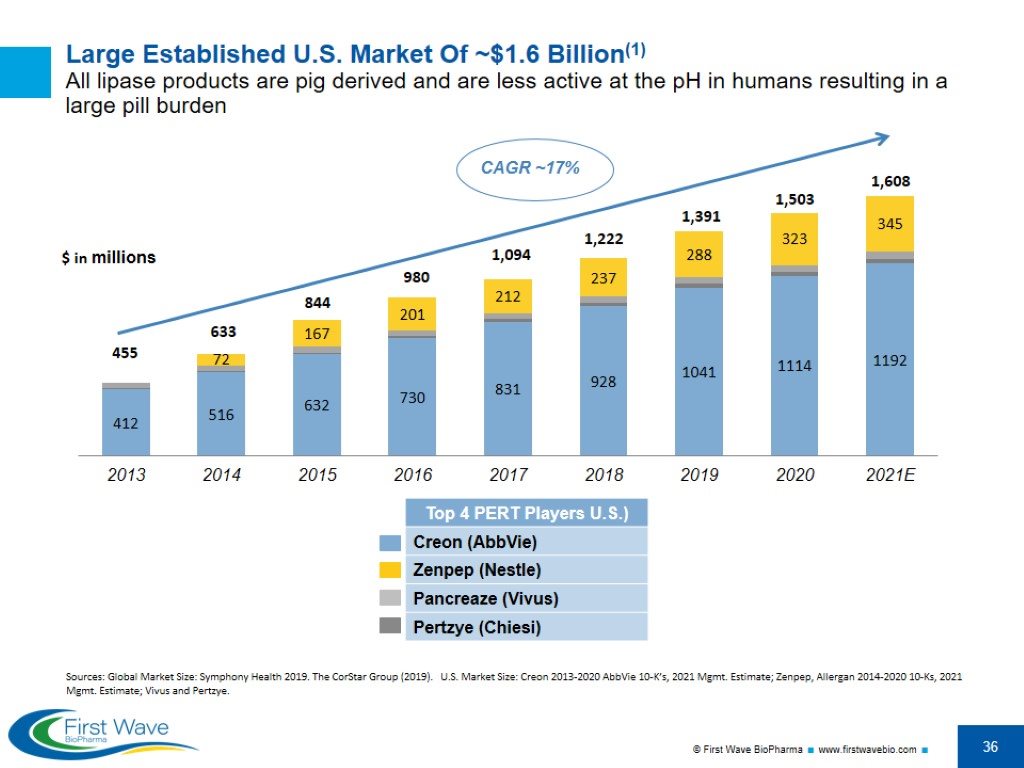
Large Established U.S. Market Of ~$1.6 Billion(1) All lipase products are pig derived and are less active at the pH in humans resulting in a large pill burden $ in millions 1,094 980 633 455 1,222 844 CAGR ~17% 1,391 1,503 1,608 Sources: Global Market Size: Symphony Health 2019. The CorStar Group (2019). U.S. Market Size: Creon 2013-2020 AbbVie 10-K’s, 2021 Mgmt. Estimate; Zenpep, Allergan 2014-2020 10-Ks, 2021 Mgmt. Estimate; Vivus and Pertzye.
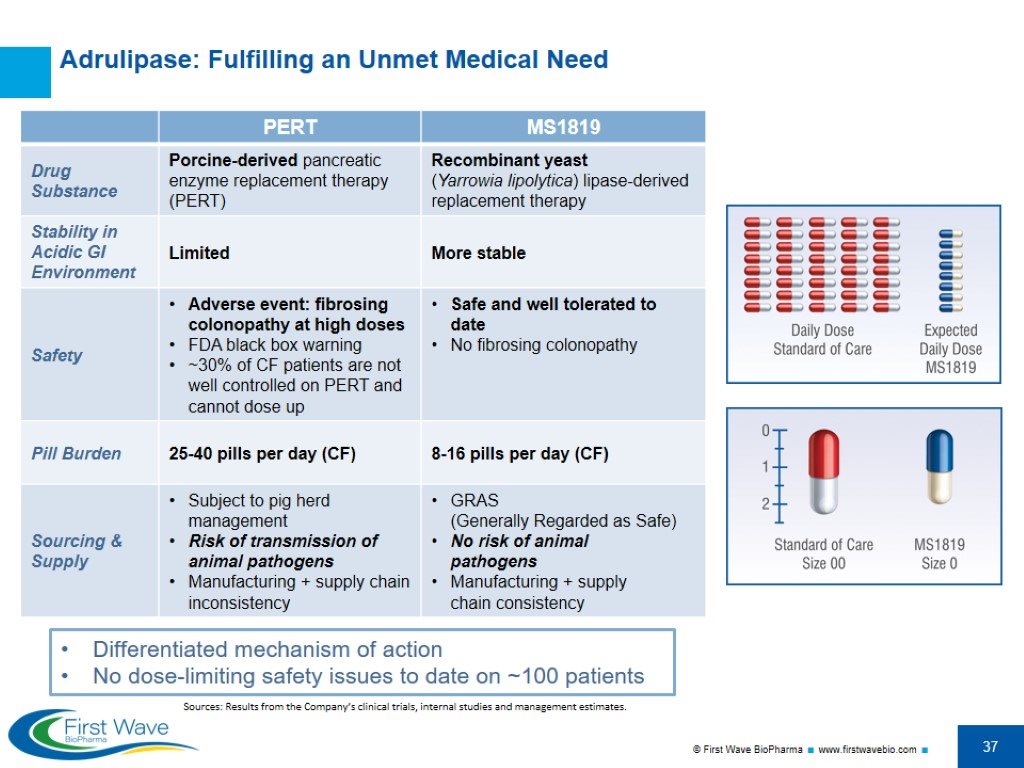
Sources: Results from the Company’s clinical trials, internal studies and management estimates. Adrulipase: Fulfilling an Unmet Medical Need Differentiated mechanism of action No dose-limiting safety issues to date on ~100 patients
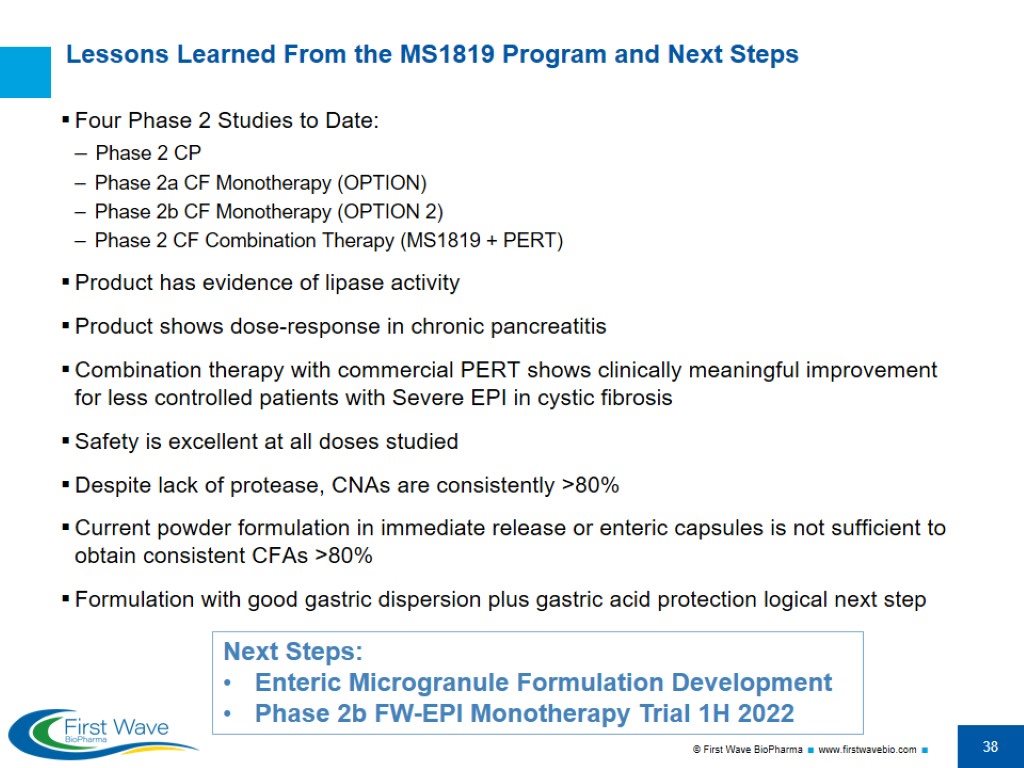
Lessons Learned From the MS1819 Program and Next Steps Four Phase 2 Studies to Date: Phase 2 CP Phase 2a CF Monotherapy (OPTION) Phase 2b CF Monotherapy (OPTION 2) Phase 2 CF Combination Therapy (MS1819 + PERT) Product has evidence of lipase activity Product shows dose-response in chronic pancreatitis Combination therapy with commercial PERT shows clinically meaningful improvement for less controlled patients with Severe EPI in cystic fibrosis Safety is excellent at all doses studied Despite lack of protease, CNAs are consistently >80% Current powder formulation in immediate release or enteric capsules is not sufficient to obtain consistent CFAs >80% Formulation with good gastric dispersion plus gastric acid protection logical next step Next Steps: Enteric Microgranule Formulation Development Phase 2b FW-EPI Monotherapy Trial 1H 2022

Intellectual Property Investment Highlights
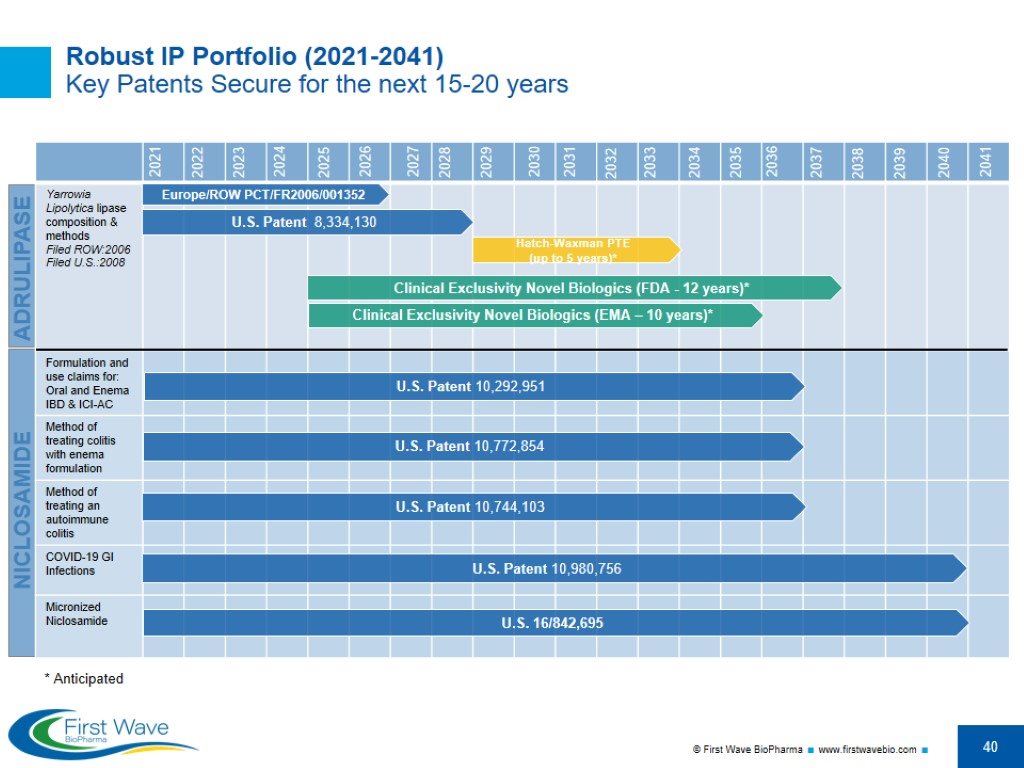
Robust IP Portfolio (2021-2041) Key Patents Secure for the next 15-20 years NICLOSAMIDE U.S. Patent 8,334,130 Clinical Exclusivity Novel Biologics (EMA – 10 years)* ADRULIPASE Hatch-Waxman PTE (up to 5 years)* U.S. Patent 10,292,951 U.S. Patent 10,980,756 U.S. 16/842,695 Europe/ROW PCT/FR2006/001352 Clinical Exclusivity Novel Biologics (FDA - 12 years)* U.S. Patent 10,772,854 U.S. Patent 10,744,103 2021 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 2033 2034 2035 2036 2037 2038 2039 2040 2041 * Anticipated

Overview First Wave BioPharma is a clinical stage biotechnology company currently focused on the development of targeted, non-systemic therapies for gastrointestinal (GI) diseases, with two assets and six clinical indications spanning inflammatory bowel diseases (IBD), COVID viral infections, oncology therapy induced colitis, and pancreatic digestive disorders. Niclosamide: Re-purposed small molecule drug with potent anti-viral and anti-inflammatory properties, proprietary micronized formulation COVID-19 GI infections (Phase 2 launched 1H 2021) Immune Checkpoint Inhibitor-Associated Colitis (Phase 1b/2a in 2H 2021) IBD: Ulcerative Proctitis (Phase 2 in 2H 2021) IBD: Ulcerative Colitis (Phase 1b/2a) IBD: Crohn’s Disease (Phase 2a) Adrulipase: Recombinant lipase biologic for the treatment of Exocrine Pancreatic Insufficiency (EPI) 6. EPI in Cystic Fibrosis; new enteric formulation (Phase 2b in 1H 2022) Assets leverage the Company’s core competencies and expertise in developing targeted, safe, non-systemic oral GI therapies Pipeline of gut-targeted GI therapies address significant unmet medical needs in billion-dollar markets
