Attached files
| file | filename |
|---|---|
| EX-23.2 - EX-23.2 - Ortho Clinical Diagnostics Holdings plc | d194092dex232.htm |
| EX-5.1 - EX-5.1 - Ortho Clinical Diagnostics Holdings plc | d194092dex51.htm |
| EX-1.1 - EX-1.1 - Ortho Clinical Diagnostics Holdings plc | d194092dex11.htm |
Table of Contents
As filed with the Securities and Exchange Commission on September 7, 2021.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
Ortho Clinical Diagnostics Holdings plc
(Exact name of registrant as specified in its charter)
| England and Wales | 2835 | 98-1574150 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
1001 Route 202
Raritan, New Jersey 08869
908-218-8000
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Michael A. Schlesinger
Executive Vice President, General Counsel and Secretary
Ortho Clinical Diagnostics Holdings plc
1001 Route 202
Raritan, New Jersey 08869
908-218-8000
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to:
| Patrick H. Shannon Jason M. Licht Latham & Watkins LLP 555 Eleventh Street, NW—Suite 1000 Washington, D.C. 20004 Tel: (202) 637-2200 Fax: (202) 637-2201 |
William J. Miller Noah B. Newitz Cahill Gordon & Reindel LLP 32 Old Slip New York, New York 10005 Tel: (212) 701-3000 Fax: (212) 269-5420 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☐ | |||
| Emerging growth company | ☐ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
|
| ||||||||
| Title of each class of securities to be registered |
Amount to be Registered(1) |
Proposed maximum offering price per ordinary share(2) |
Proposed maximum aggregate offering price (1)(2) |
Amount of registration fee | ||||
| Ordinary shares, $0.00001 par value per ordinary share |
25,300,000 | $20.38 | $515,614,000 | $56,253.49 | ||||
|
| ||||||||
| (1) | Includes 3,300,000 shares that the underwriters have the option to purchase. See “Underwriting.” |
| (2) | Estimated solely for the purpose of computing the amount of the registration fee. In accordance with Rule 457(c) under the Securities Act of 1933, as amended, the maximum offering price per share and maximum aggregate offering price are based on the average of the high and low sales price of the Registrant’s ordinary shares as reported by the NASDAQ Global Select Market on August 31, 2021, which date is within five business days prior to filing this Registration Statement. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. The selling shareholder may not sell these securities until the Registration Statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities, and the selling shareholder is not soliciting offers to buy the securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion
Preliminary Prospectus dated September 7, 2021
Prospectus
22,000,000 Shares
Ortho Clinical Diagnostics Holdings plc
Ordinary shares
This is a public offering of ordinary shares of Ortho Clinical Diagnostics Holdings plc.
The selling shareholder identified in this prospectus is offering 22,000,000 of our ordinary shares. We are not selling any ordinary shares under this prospectus and will not receive any proceeds from the sale of the shares by the selling shareholder.
Our ordinary shares are listed and traded on the NASDAQ Global Select Market (“Nasdaq”) under the symbol “OCDX.” On September 3, 2021, the last reported sale price of our ordinary shares on Nasdaq was $20.40 per ordinary share.
Investing in our ordinary shares involves risks that are described in the “Risk Factors” section beginning on page 17 of this prospectus and the risk factors in the documents incorporated by reference in this prospectus.
| Per share | Total | |||||||
| Public offering price |
$ | $ | ||||||
| Underwriting discount(1) |
$ | $ | ||||||
|
|
|
|
|
|||||
| Proceeds, before expenses, to selling shareholder |
$ | $ | ||||||
|
|
|
|
|
|||||
| (1) | See “Underwriting” for a description of the compensation payable to the underwriters. |
The underwriters may also exercise their option to purchase up to an additional 3,300,000 ordinary shares from the selling shareholder, at the public offering price, less the underwriting discount, for 30 days after the date of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The ordinary shares will be ready for delivery on or about , 2021.
| Goldman Sachs & Co. LLC | J. P. Morgan |
The date of this prospectus is , 2021.
Table of Contents
| Page | ||||
| 1 | ||||
| 17 | ||||
| 24 | ||||
| 27 | ||||
| 28 | ||||
| 29 | ||||
| 30 | ||||
| 33 | ||||
| 51 | ||||
| 54 | ||||
| 62 | ||||
| 71 | ||||
| 71 | ||||
| 72 | ||||
We, the selling shareholder and the underwriters have not authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses that we have prepared. We, the selling shareholder and the underwriters take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the ordinary shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is current only as of the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or any sale of the ordinary shares. Our business, financial condition, results of operations and prospects may have changed since the date on the front cover of this prospectus.
For investors outside the United States: We, the selling shareholder and the underwriters have not done anything that would permit a public offering of our ordinary shares or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the ordinary shares and the distribution of this prospectus outside of the United States.
Basis of Presentation
Unless otherwise indicated or the context otherwise requires, references in this prospectus to:
| • | the term “2025 Notes” refers to the 7.375% Senior Notes due 2025 issued by the Lux Co-Issuer and the U.S. Co-Issuer; |
| • | the term “2025 Notes Indenture” refers to the indenture governing the 2025 Notes, as amended, supplemented or otherwise modified from time to time; |
| • | the term “2028 Notes” refers to the 7.250% Senior Notes due 2028 issued by the Lux Co-Issuer and the U.S. Co-Issuer; |
| • | the term “2028 Notes Indenture” refers to the indenture governing the 2028 Notes, as amended, supplemented or otherwise modified from time to time; |
| • | the term “Acquisition” refers to the acquisition by Ortho-Clinical Diagnostics Bermuda Co. Ltd., a Bermuda exempted limited liability company and the Company’s predecessor (“Bermuda |
i
Table of Contents
| Holdco”), pursuant to a stock and asset purchase agreement, dated January 16, 2014 (the “Acquisition Agreement”), of (i) certain assets and liabilities and (ii) all of the equity interests and substantially all of the assets and liabilities of certain entities which, together with their subsidiaries, comprised the Ortho Clinical Diagnostics business from Johnson & Johnson; |
| • | the term “assay” refers to an investigative (analytic) procedure in laboratory medicine, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence or amount or the functional activity of a target entity (the analyte), which can be a drug or biochemical substance or a cell in an organism or organic sample; |
| • | the term “consumables” refers to miscellaneous items such as calibrators, pipettes and controls, which are run on our instruments in conjunction with and in support of assays and reagents; |
| • | the term “Credit Agreement” refers to that certain credit agreement governing our Senior Secured Credit Facilities; |
| • | the term “emerging markets” refers to all countries where we operate other than Australia, Austria, Belgium, Canada, Denmark, Finland, France, Germany, Italy, Japan, Luxembourg, the Netherlands, New Zealand, Norway, Portugal, Spain, Sweden, Switzerland, the United Kingdom, the United States and certain other developed countries; |
| • | the term “GAAP” refers to the generally accepted accounting principles in the United States of America; |
| • | the term “Holdings” refers to Ortho-Clinical Diagnostics Holdings Luxembourg S.à r.l., a société à responsabilité limitée organized under the laws of the Grand Duchy of Luxembourg, having its registered office at 89C, rue Pafebruch, L-8308 Capellen, Grand Duchy of Luxembourg and registered with the Luxembourg trade and companies register (Registre de Commerce et des Sociétés de Luxembourg) under number B185679 and a direct, wholly owned subsidiary of UK Holdco; |
| • | the term “Joint Business” refers to a long-term collaboration agreement with Grifols Diagnostic Solutions, Inc. that encompasses certain Clinical Laboratories and Donor Screening products; |
| • | the term “laboratories,” when we refer to our customers, refers to testing sites that process and provide results for in vitro diagnostic tests within hospitals, independent reference labs, physician office labs or physician clinics; |
| • | the term “Lux Co-Issuer” refers to Ortho-Clinical Diagnostics S.A., a société anonyme organized under the laws of the Grand Duchy of Luxembourg, having its registered office at 89C, rue Pafebruch, L-8308 Capellen Luxembourg and registered with the Luxembourg trade and companies register (Registre de Commerce et des Sociétés de Luxembourg) under number B 185693 and an indirect, wholly owned subsidiary of the Company; |
| • | the terms “Principal Shareholder” and “Carlyle” refer to The Carlyle Group Inc. and its affiliates; |
| • | the term “reagent” refers to a substance that is added during a test in order to bring about a reaction. The resulting reaction is used to confirm the presence of another substance. Our reagents are used to identify different properties of blood; |
| • | the term “Senior Notes” refers, collectively, to the 2025 Notes and the 2028 Notes; |
| • | the term “Senior Secured Credit Facilities” refers to (a) the senior secured term loan facility in an original amount of $2,175.0 million, as increased by the incremental term loan (the “Incremental Term Loan”) of $200 million (collectively, the “Dollar Term Loan Facility”), (b) the euro-denominated senior secured term loan facility in an amount equal to €337.4 million (the |
ii
Table of Contents
| “Euro Term Loan Facility” and, together with the Dollar Term Loan Facility, the “Term Loan Facilities”), and (c) the multi-currency senior secured revolving facility with commitments of $500.0 million (the “Revolving Credit Facility”); |
| • | the term “throughput” refers to the number of tests performed during a certain time period; |
| • | the term “U.K. TopCo” refers to Ortho Clinical Diagnostics Holdings plc and not any of its subsidiaries; |
| • | the term “U.S. Co-Issuer” refers to Ortho-Clinical Diagnostics, Inc., a corporation incorporated under the laws of the State of New York and an indirect, wholly owned subsidiary of the Company; |
| • | the terms “we,” “us,” “our,” “its,” our “Company” and “Ortho” refer to Ortho Clinical Diagnostics Holdings plc, a public limited company incorporated under the laws of England and Wales, and its consolidated subsidiaries. |
References to a year refer to our fiscal years ended on the Sunday nearest December 31 of the specified year and consist of 52 weeks (for example, the terms “2020” and “fiscal 2020” refer to our fiscal year ended January 3, 2021, the terms “2019” and “fiscal 2019” refer to our fiscal year ended December 29, 2019 and the terms “2018” and “fiscal 2018” refer to our fiscal year ended December 30, 2018).
References to our customers mean those customers we directly sell our products and services to, such as hospitals, clinics and our distributors. References to our end-customers mean customers who use our products, which include hospitals and clinics.
Certain monetary amounts, percentages and other figures included in this prospectus have been subject to rounding adjustments. Accordingly, figures shown as totals in certain tables and charts may not be the arithmetic aggregation of the figures that precede them, and figures expressed as percentages in the text may not total 100% or, as applicable, when aggregated may not be the arithmetic aggregation of the percentages that precede them.
Trademarks and Service Marks
We own or have rights to trademarks, service marks or trade names that we use in connection with the operation of our business. In addition, our names, logos and website names and addresses are our service marks or trademarks. Other trademarks, service marks and trade names appearing in this prospectus are the property of their respective owners. The trademarks we own or have the right to use include, among others, ORTHO®, ORTHO CLINICAL DIAGNOSTICS®, ORTHO VISION™, BIOVUE® and VITROS®. Solely for convenience, in some cases, the trademarks, service marks and trade names referred to in this prospectus are listed without the applicable ® and ™ symbols, but we will assert, to the fullest extent under applicable law, our rights to these trademarks, service marks and trade names.
Market, Industry and Other Data
Throughout this prospectus, we refer to our market position or market share in various markets or regions. These references represent our best estimates of our market share at the time of this prospectus and are based on management’s knowledge of the industry and the market data and other statistical information obtained from independent industry publications, reports by market research firms or other published independent sources. We confirm that such information has been accurately reproduced and that, so far as we are aware, and are able to ascertain from information published by
iii
Table of Contents
publicly available sources and other publications, no facts have been omitted which would render the reproduced information inaccurate or misleading. Industry publications, reports and other published data generally state that the information contained therein has been obtained from sources believed to be reliable. Certain other market and industry data included in this prospectus, including the size of certain markets, are based on estimates of our management. These estimates have been derived from our management’s knowledge and experience in the markets in which we operate, as well as information obtained from surveys, reports by market research firms, our customers, distributors, suppliers, trade and business organizations and other contacts in the markets in which we operate.
Unless specifically noted otherwise, information presented in this prospectus with respect to our position relative to other industry participants is based on information or sources for the year ended December 31, 2020. Market size and market growth information presented in this prospectus reflects estimates of market sizes and growth in such markets for the year ended December 31, 2020 and for the years ended December 31, 2020 through 2025, respectively. In each such case, management believes such information presented represents the most recent data available to the Company.
Projections, assumptions and estimates of our future performance and the future performance of the industry in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors” in this prospectus and Ortho Clinical Diagnostics Holdings plc’s Annual Report on Form 10-K for the fiscal year ended January 3, 2021 (“Ortho’s 2020 Form 10-K”). These and other factors could cause results to differ materially from those expressed in our estimates and beliefs and in the estimates prepared by independent parties.
Use of Non-GAAP Financial Measures
Adjusted EBITDA, Management EBITDA, Adjusted Net Income and Core revenue constant currency growth rate are our key non-GAAP financial measures. For more information about how we use these non-GAAP financial measures in our business, the limitations of these measures and a reconciliation of these measures to the most directly comparable GAAP measures, see “Prospectus Summary—Summary Consolidated Financial Data.” Adjusted EBITDA consists of net loss before interest expense, net, provision for (benefit from) income taxes and depreciation and amortization and eliminates (i) non-operating income or expense and (ii) impacts of certain non-cash, unusual or other items that are included in net loss that we do not consider indicative of our ongoing operating performance. Management EBITDA consists of Adjusted EBITDA plus certain other management adjustments. Adjusted Net Income consists of net loss before amortization and the elimination of (i) non-operating income or expense and (ii) impacts of certain non-cash, unusual or other items that are included in net loss that we do not consider indicative of our ongoing operating performance. Core revenue constant currency growth rate refers to the growth rate of revenue generated in our Clinical Laboratories and our Transfusion Medicine lines of business, which historically make up more than 95% of our net revenue, with any local currency revenue for all reporting periods translated into U.S. dollars using internally-derived foreign currency exchange rates held constant for each year. As needed, we translate prior periods based on our internally-derived currency exchange rates used for the current period in order to remove the impact of foreign currency exchange fluctuation.
We use these financial measures in the analysis of our financial and operating performance because they assist in the evaluation of underlying trends in our business. Additionally, Management EBITDA was the basis we used for assessing the profitability of our geographic-based reportable segments and was also utilized as a basis for calculating certain management incentive compensation programs through January 3, 2021. During the fiscal first quarter ended April 4, 2021, we changed the basis by which we measure segment profit or loss and calculate certain management incentive compensation programs from Management EBITDA to Adjusted EBITDA. In the case of Adjusted
iv
Table of Contents
EBITDA and Adjusted Net Income, we believe that making such adjustments provides management and investors meaningful information to understand our operating performance and ability to analyze financial and business trends on a period-to-period basis. We believe that the presentation of these financial measures enhances an investor’s understanding of our financial performance. We use certain of these financial measures for business planning purposes and measuring our performance relative to that of our competitors.
Other companies in our industry may calculate Adjusted EBITDA, Management EBITDA, Adjusted Net Income and Core revenue constant currency growth rate differently than we do. As a result, these financial measures have limitations as analytical and comparative tools and you should not consider these items in isolation, or as a substitute for analysis of our results as reported under GAAP. Adjusted EBITDA, Management EBITDA and Adjusted Net Income should not be considered as measures of discretionary cash available to us to invest in the growth of our business. In calculating these financial measures, we make certain adjustments that are based on assumptions and estimates that may prove to have been inaccurate. In addition, in evaluating these financial measures, you should be aware that in the future we may incur expenses similar to those eliminated in the presentation of these metrics included in this prospectus. Our presentation of Adjusted EBITDA, Management EBITDA and Adjusted Net Income should not be construed as an inference that our future results will be unaffected by unusual or non-recurring items.
Adjusted EBITDA, Management EBITDA, Adjusted Net Income and Core revenue constant currency growth rate have important limitations as analytical tools and you should not consider them in isolation or as substitutes for analysis of our results as reported under GAAP. Some of these limitations include the fact that:
| • | Adjusted EBITDA and Management EBITDA: |
| • | do not reflect the significant interest expense on our debt, including the Senior Secured Credit Facilities, the 2025 Notes and the 2028 Notes; |
| • | eliminate the impact of income taxes on our results of operations; and |
| • | although depreciation and amortization are non-cash charges, the assets being depreciated and amortized will often have to be replaced in the future, and Adjusted EBITDA and Management EBITDA do not reflect any cash requirements for such replacements; |
| • | Adjusted Net Income eliminates the effects of amortization related to purchased intangibles; and |
| • | Core revenue constant currency growth rate eliminates the effects of foreign currency exchange rate fluctuations. |
We compensate for these limitations by relying primarily on our GAAP results and using these financial measures only as a supplement to our GAAP results. For an explanation of the components of Core revenue constant currency growth rate, see footnotes (2) and (3) in “Prospectus summary—Summary consolidated financial data,” and for an explanation of the components of Adjusted EBITDA, Management EBITDA and Adjusted Net Income, see footnotes (4) and (5) in “Prospectus summary—Summary consolidated financial data.”
In calculating these financial measures, we make certain adjustments that are based on assumptions and estimates that may prove to have been inaccurate. In addition, in evaluating these financial measures, you should be aware that in the future we may incur expenses similar to those eliminated in this presentation. Our presentation of these metrics should not be construed as an inference that our future results will not be affected by unusual or non-recurring items or changes in our customer base. Additionally, our presentation of Adjusted EBITDA may differ from that included in the
v
Table of Contents
Credit Agreement, the 2025 Notes Indenture and the 2028 Notes Indenture for purposes of covenant calculation.
Where You Can Find Additional Information
We have filed with the Securities Exchange Commission (the “SEC”) a registration statement on Form S-1 under the Securities Act (the “Registration Statement”) with respect to the ordinary shares offered by this prospectus. This prospectus is a part of the Registration Statement and does not contain all of the information set forth in the Registration Statement and its exhibits and schedules, portions of which have been omitted as permitted by the rules and regulations of the SEC. For further information about us and our ordinary shares, you should refer to the Registration Statement and its exhibits and schedules.
We file annual, quarterly and current reports and other information with the SEC. Our filings with the SEC are available to the public on the SEC’s website at http://www.sec.gov. Those filings are also available to the public on, or accessible through, our website under the heading “Investor Relations” at www.orthoclinicaldiagnostics.com. The information we file with the SEC (except as described below in “Incorporation by reference”) or contained on or accessible through our corporate website or any other website that we may maintain is not part of this prospectus or the Registration Statement of which this prospectus is a part.
Incorporation by Reference
The rules of the SEC allow us to incorporate by reference information we file with the SEC. This means that we are disclosing important information to you by referring to other documents. The information incorporated by reference is considered to be part of this prospectus. To the extent there are inconsistencies between the information contained in this prospectus and the information contained in the documents filed with the SEC prior to the date of this prospectus and incorporated by reference, the information in this prospectus shall be deemed to supersede the information in such incorporated documents. We incorporate by reference the documents listed below (other than any portions thereof, which under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and applicable SEC rules, are not deemed “filed” under the Exchange Act):
| • | Ortho’s 2020 Form 10-K; |
| • | our Quarterly Reports on Form 10-Q for the quarterly period ended April 4, 2021 and for the quarterly period ended July 4, 2021 (such Quarterly Report on Form 10-Q for the quarterly period ended July 4, 2021, “Ortho’s 2021 Q2 Form 10-Q”); and |
| • | our Current Reports on Form 8-K filed with the SEC on February 4, 2021, February 9, 2021, February 23, 2021, May 3, 2021, May 11, 2021, May 25, 2021 and June 15, 2021. |
If we have incorporated by reference any statement or information in this prospectus and we subsequently modify that statement or information with information contained in this prospectus, the statement or information previously incorporated in this prospectus is also modified or superseded in the same manner. We will provide without charge to each person to whom a copy of this prospectus has been delivered, a copy of any and all of these filings. You may request a copy of these filings by writing to us at:
Investor Relations
1001 Route 202
Raritan, New Jersey 08869
e-mail: InvestorRelations@OrthoClinialDiagnostics.com
Exhibits to any documents incorporated by reference in this prospectus will not be sent, however, unless those exhibits have been specifically referenced in this prospectus.
vi
Table of Contents
This prospectus summary highlights selected information contained or incorporated by reference in this prospectus and may not contain all of the information that you should consider in making your investment decision. For a more complete understanding of our company and this offering and before making any investment decision regarding our ordinary shares, you should read this entire prospectus and the information incorporated by reference, including “Risk Factors.”
Our Company
We are a pure-play in vitro diagnostics (“IVD”) business driven by our credo, “Because Every Test is A Life.” This guiding principle reflects the crucial role diagnostics play in global health and guides our priorities as an organization. As a leader in IVD, we impact approximately 800,000 patients every day. We are dedicated to improving outcomes for these patients and saving lives through providing innovative and reliable diagnostic testing solutions to the clinical laboratory and transfusion medicine communities. Our global infrastructure and commercial reach allow us to serve these markets with significant scale. We have an intense focus on the customer. We support our customers with high quality diagnostic instrumentation, a broad test portfolio and market leading service. Our products deliver consistently fast, accurate and reliable results that allow clinicians to make better-informed treatment decisions. Our business model generates significant recurring revenues and strong cash flow streams from ongoing sales of high margin consumables. In 2020, these consumables contributed approximately 93% of our total revenue. We maintain close connectivity with our customers through our global presence, with approximately 4,700 employees, including approximately 2,200 commercial sales, service and marketing teammates. This global organization allows us to support our customers across more than 130 countries and territories.
We have been pioneering life-impacting advances in diagnostics for over 80 years, from our earliest work in blood typing, to our innovation in infectious diseases and our latest developments in laboratory solutions. In 2014, we were acquired by The Carlyle Group from Johnson & Johnson and became an independent organization, solely focused on delivering high quality IVD products and service to our diagnostic customer base. At the time of the separation, our business had global scale, a reputation for high quality products, a strong quality management system and a research and development team with extensive scientific expertise. Over the past six years, we have significantly invested in our business with the objective of creating a highly efficient, innovative and lean organization capable of scaling to meet our customers’ needs. These investments included the following focus areas:
| • | Infrastructure: We invested over $500 million in IT, finance, supply chain and other support functions to build out our infrastructure and capabilities as a standalone business and drive long-term, profitable growth. |
| • | Research and development: We increased our focus on innovation by investing over $650 million in research and development to enhance our existing capabilities and develop new instruments and assays to supplement our portfolio. |
| • | Commercial: We redesigned our go-to-market strategy across all key regions, expanded our sales force, implemented new customer information systems and launched ORTHOCARE to enhance our service capabilities. |
| • | Operations: We consolidated and streamlined our manufacturing capabilities and other global functions to improve profitability and cash flow, achieving more than $200 million in savings since our acquisition by Carlyle. |
1
Table of Contents
| • | Leadership: To capitalize on these investments, we recruited a highly qualified management team of experienced diagnostic and healthcare leaders focused on our customers and accelerated growth. |
With these investments, we have reinvigorated our portfolio, transformed our commercial model and emerged as a focused leader in the IVD market, which we believe positions our business for future growth.
IVD testing is a critical tool in evaluating health in many different settings around the world. IVD is a core component of routine health care check-ups for those who are presenting with symptoms or require procedures, and it influences up to 70% of critical healthcare clinical decision-making. Consequently, our IVD solutions have a profound impact on the assessment of health and the delivery of care. IVD is also critical in monitoring the transmission and spread of infectious disease outbreaks, where Ortho’s longstanding excellence in infectious disease testing is particularly relevant. Our solutions are central to the operations of hospitals, clinics, blood banks and donor centers around the world, where they are used to help diagnose certain conditions, such as cancer or heart attacks, and infectious diseases, such as hepatitis, HIV, and most recently, COVID-19, where we have launched two antibody tests and an antigen test, and we are actively expanding our menu of tests to address the global pandemic.
We operate in an approximately $27 billion addressable market, which is expected to grow at a compound annual growth rate (“CAGR”) of approximately 5% from 2020 to 2025. We compete in the two largest IVD markets, Immunoassay and Clinical Chemistry, which together comprise our Clinical Laboratories business and represent approximately $25 billion of our current addressable market. We expect our Clinical Laboratories business will be favorably impacted by an aging population, an increased need for testing of chronic conditions, the expansion of access to healthcare services, the emergence of new disease states and other macro trends. We are also the global leader in Transfusion Medicine, which includes hospital-based Immunohematology and Donor Screening for blood and plasma at hospitals and other donation centers. Transfusion Medicine represents approximately $2 billion of our current addressable market. We expect our Transfusion Medicine business will be favorably impacted by increases in the number and type of surgical procedures, an aging population and other macro trends. There is significant overlap in our customer base given we currently sell to about 70% of the hospitals in the United States, and we are often able to leverage our leadership within Transfusion Medicine to cross-sell our Clinical Laboratories solutions. Because we focus primarily on acute or critical care diagnostics that are core to therapeutic decisions in hospitals, our markets are relatively resilient across business cycles. We offer our products and services globally, with distinct offerings targeted to the needs of customers in developed and emerging markets.
Today, we operate two lines of business, Clinical Laboratories and Transfusion Medicine, which together generate our core revenue:
| • | Clinical Laboratories: Comprised of Clinical Chemistry, which is the measurement of target chemicals in bodily fluids for the evaluation of health and the clinical management of patients, and Immunoassay, which is the measurement of proteins as they act as antigens in the spread of disease, antibodies in the immune response spurred by disease, or markers of proper organ function and health. |
| • | Transfusion Medicine: Comprised of Donor Screening, where blood and plasma is screened at donation for blood type and target diseases, and Immunohematology, where blood is typed and screened at the hospital blood bank before being transfused into the patient. |
2
Table of Contents
Our broad offerings allow us to support our customer base and maximize the opportunity to provide each of our customers with a comprehensive array of products and services over time. We refer to this mutually beneficial approach as focusing on Lifetime Customer Value (“LCV”). Our focus on LCV underpins everything we do, from the design and execution of our commercial and service model, to our instrument and assay innovation, to the composition of our global footprint. Our approach has informed our choice to focus on medium- to high-volume laboratories, which in turn has helped us become a focused leader in our selected markets and transformed our financial profile. We intend to continue to invest in and evolve our LCV framework to best support our customers and maximize our financial results.
As an example, we may begin a Clinical Laboratories customer relationship by providing a standalone instrument (often a clinical chemistry analyzer) and a set of assays that are relevant to that customer’s specific testing needs. As the customer and its testing needs grow, we look to migrate the customer, where appropriate, to an integrated analyzer that performs both clinical chemistry and immunoassay testing. This migration helps us increase our customers’ testing capabilities as well as the revenue we generate from customers. For our larger customers, we ultimately may expand their testing throughput by installing automation tracks that connect multiple analyzers along the automation track, while providing the full suite of our ever-expanding assay menu. As we have significantly expanded our test menu offering, our integrated analyzers are now more often where our Clinical Laboratories relationship starts. In Transfusion Medicine, the life cycle is similar, as customers graduate from manual testing processes to semi-automated capabilities to fully-automated blood and plasma screening instrumentation as their testing volumes and technical competencies grow over time. We focus on building long-term customer relationships and continuing to enhance both our offering and the customer’s ability to care for their patients—ultimately deepening our commercial relationship and driving our financial model.
We believe that the strong clinical performance of our assays, our instruments’ ease-of-use and reliability, our best-in-class customer service and low total cost of ownership contribute to our high revenue retention rate of approximately 98% in 2020. We have longstanding relationships with customers, with an average Clinical Laboratories customer relationship of almost 13 years and an average Transfusion Medicine customer relationship of almost 15 years. Our customer relationships are particularly strong in the medium- to high-volume laboratories (our “Focus Markets”), which compares to the broader market that includes low- and ultra high-volume laboratories. In addition, our dry slide technology has an environmentally friendly profile as it doesn’t require water system plumbing, it reduces hazardous waste and it requires less space for liquid storage. All of these attributes resonate particularly well with customers who are pursuing environmental goals or customers who operate in regions with scarce water supply.
Our revenue is driven by a “razor-razor blade” business model. Through this model, we generally sell or place instruments under long-term contracts, which support the ongoing sale of our assays, reagents and consumables. Our instruments are closed systems, requiring customers to purchase the assays, reagents and consumables from us. These sales generate a high proportion of recurring revenues. As of July 4, 2021, we had an installed base of approximately 20,300 instruments, which increased approximately 4% since June 28, 2020. We also generate non-core revenue, including through our contract manufacturing business and certain business collaborations, which accounted for approximately 3% of our net revenue during the fiscal year ended January 3, 2021. During the fiscal year ended January 3, 2021, we recorded net revenue of $1,766.2 million, net loss of $211.9 million and Adjusted EBITDA of $456.0 million. During the fiscal six months ended July 4, 2021, we recorded net revenue of $999.3 million, net loss of $59.1 million and Adjusted EBITDA of $280.6 million. As of July 4, 2021, we had total indebtedness of $2,295.0 million, and for the fiscal six months ended July 4,
3
Table of Contents
2021, our interest expense was $76.4 million. We note that our net revenue for the fiscal six months ended July 4, 2021 was 25.2% higher as compared to the prior year period, mainly driven by our core lines of business, as we recorded higher revenues in all geographic segments of our Clinical Laboratories business, and in certain geographic segments of our Transfusion Medicine business as well as a positive impact from foreign currency fluctuations, which was primarily driven by the weakening of the U.S. dollar against a variety of currencies, primarily the Euro, British Pound and Chinese Yuan, partially offset by the strengthening of the Brazilian Real. We note that our net revenue for the 2020 fiscal year was impacted by the global COVID-19 pandemic primarily due to decreased shipments to customers, most notably during the fiscal second quarter ended June 28, 2020. However, during the fiscal third quarter ended September 27, 2020, we began to experience a rebound towards the positive quarterly trajectory we saw prior to the beginning of the pandemic. As an example of such quarterly trajectory, during the four quarters prior to the fiscal second quarter ended June 28, 2020, our quarterly core revenue (excluding local HCV) was growing in the range of approximately 3.8% to 5.8% on a constant currency basis as compared to the relevant prior year period. Beginning in the fiscal third quarter ended September 27, 2020, and continuing through the fiscal second quarter ended July 4, 2021, we have returned to growth in our core revenue (excluding local HCV) on a constant currency basis, most recently increasing 25.0% during the fiscal second quarter ended July 4, 2021 as compared to the prior year period.
Our Competitive Strengths
We believe we are well-positioned to drive sustained and profitable growth through our relentless focus on LCV. This customer-centric approach informs the execution of our commercial and service model and underpins our go-to-market strategy. Our customer focus allows us to retain and grow our customers by providing a superior customer experience driven by unparalleled quality of service, continuous innovation and access to a diverse product portfolio. We are able to leverage our global footprint to gain differentiated and leading positions across our Focus Markets. This intense focus on our customers has resulted in an attractive business model with high recurring revenues that allows us to continue to invest to reinforce our competitive strengths, which include:
| • | Intense customer focus enabled by our broad portfolio and market leading positions; |
| • | Superior customer experience and brand loyalty resulting in high customer retention and win rates; |
| • | Highly compelling solutions supported by our leading and innovative research and development capabilities; |
| • | Extensive and balanced global commercial footprint with reinvigorated focus on growth; |
| • | Disciplined approach to streamline operations and optimize our cost structure; and |
| • | Deeply committed leadership team with broad experience in healthcare and diagnostics. |
Our Growth Strategy
Our heritage and strength lie in our long-term customer relationships and trusted brand, which create a steady base of recurring revenue and a foundation for future growth. By focusing on LCV, we identify ways in which we can utilize product innovation and impactful customer service and solutions to deepen these partnerships and add value over time. We plan to grow our business by broadening our existing relationships, winning new customers and targeting high-growth end markets and adjacencies. We are focused on sustainable long-term growth through commercial excellence that
4
Table of Contents
maximizes LCV, strengthens existing relationships through superior service and delivers innovative products for our customers and their patients. The key elements of our strategy to accelerate and sustain revenue growth and operating leverage include:
| • | Focusing relentlessly on maximizing LCV, which is designed to produce and maintain a growing and recurring, high margin, durable financial profile and contributes to growth; |
| • | Providing an unparalleled customer experience to retain and attract existing and new customers; |
| • | Leveraging our global footprint to deliver innovative solutions to meet our customers’ needs in both developed and emerging markets; |
| • | Creating meaningful product innovation through menu expansion, development of novel instruments and enhancement of automation and informatics; |
| • | Continuing to identify operating efficiencies to allow for reinvestment in growth and improve margins; and |
| • | Pursuing business development opportunities, partnerships and strategic acquisitions to enter adjacencies or expand our current business units. |
Our Products and Services
Clinical Laboratories
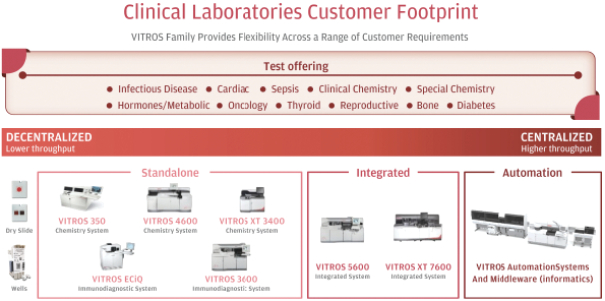
Our Clinical Laboratories business focuses on clinical chemistry and immunoassay instruments and tests to detect and monitor disease progression across a broad spectrum of therapeutic areas. Our flagship Clinical Laboratories platform is the VITROS family of instruments, which includes a series of clinical chemistry, immunoassay, integrated (combined chemistry and immunoassay) systems and automation and middleware solutions. VITROS instruments are placed in centralized, higher-throughput testing sites (hospitals and laboratories) and decentralized, lower-throughput sites (physician offices, clinics and specialty settings).
5
Table of Contents
Transfusion Medicine
Immunohematology
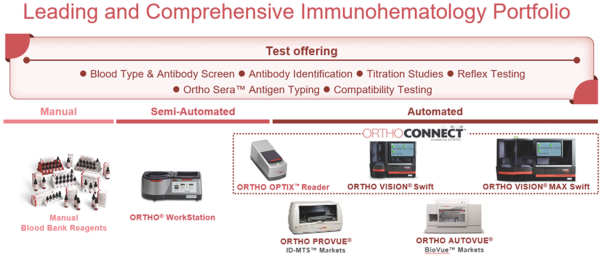
Within Transfusion Medicine, our Immunohematology business is focused on immunohematology instruments and tests used for blood typing to ensure patient-donor compatibility in blood transfusions. Our newly launched flagship Immunohematology analyzers are the ORTHO VISION Swift and ORTHO VISION Max Swift systems that automate blood typing and serology disease screening for blood banks. In addition, we sell the semi-automated ORTHO Workstation for blood bank customers that have lower volumes or need for test automation.
Donor Screening
Our Donor Screening business is focused on instruments and tests used for blood and plasma screening for infectious diseases for customers, which include some of the largest donor testing institutions, primarily in the United States. In Donor Screening, our core instrument offering is the ORTHO VERSEIA Integrated Processor—an automated pipetting and processing system that brings together the ORTHO VERSEIA pipettor and ORTHO Summit Processor to enable end-to-end pipetting and processing.
Our Services
In addition to the products we provide, ORTHOCARE Services are a critical element of how we deliver value to our customers. As of March 2021, we have approximately 900 service teammates globally. We employ highly trained service professionals including over 350 laboratory specialists with advanced qualifications. For example, more than 95% of our U.S. laboratory specialists have medical technician degrees.
Risks Related to our Business
Investing in our ordinary shares involves a high degree of risk. You should carefully consider these risks before investing in our ordinary shares, including the risks related to our business and
6
Table of Contents
industry described under “Risk Factors” in Ortho’s 2020 Form 10-K, which are incorporated by reference herein. In particular, the following considerations, among others, may offset our competitive strengths or have a negative effect on our business strategy, which could cause a decline in the price of our ordinary shares and result in a loss of all or a portion of your investment:
| • | the ongoing global coronavirus (COVID-19) pandemic; |
| • | increased competition; |
| • | manufacturing problems or delays or failure to develop and market new or enhanced products or services; |
| • | adverse developments in global market, economic and political conditions; |
| • | our ability to obtain additional capital on commercially reasonable terms may be limited or non-existent; |
| • | our inability to implement our strategies for improving growth or to realize the anticipated benefits of any acquisitions and divestitures, including as a result of difficulties integrating acquired businesses with, or disposing of divested businesses from, our current operations; |
| • | a need to recognize impairment charges related to goodwill, identified intangible assets and fixed assets; |
| • | our ability to operate according to our business strategy should our collaboration partners fail to fulfill their obligations; |
| • | risk that the insurance we maintain may not fully cover all potential exposures; |
| • | product recalls or negative publicity may harm our reputation or market acceptance of our products; |
| • | decreases in the number of surgical procedures performed, and the resulting decrease in blood demand; |
| • | fluctuations in our cash flows as a result of our reagent rental model; |
| • | terrorist acts, conflicts, wars and natural disasters that may materially adversely affect our business, financial condition and results of operations; |
| • | the outcome of legal proceedings instituted against us and/or others; |
| • | risks associated with our non-U.S. operations, including currency translation risks, the impact of possible new tariffs and compliance with applicable trade embargoes; |
| • | the effect of the United Kingdom’s withdrawal from the European Union; |
| • | our inability to deliver products and services that meet customers’ needs and expectations; |
| • | failure to maintain a high level of confidence in our products; |
| • | significant changes in the healthcare industry and related industries that we serve, in an effort to reduce costs; |
| • | reductions in government funding and reimbursement to our customers; |
| • | price increases or interruptions in the supply of raw materials, components for our products, and products and services provided to us by certain key suppliers and manufacturers; |
| • | our ability to recruit and retain the experienced and skilled personnel we need to compete; |
7
Table of Contents
| • | work stoppages, union negotiations, labor disputes and other matters associated with our labor force; |
| • | consolidation of our customer base and the formation of group purchasing organizations; |
| • | unexpected payments to any pension plans applicable to our employees; |
| • | our inability to obtain required clearances or approvals for our products; |
| • | failure to comply with applicable regulations, which may result in significant costs or the suspension or withdrawal of previously obtained clearances or approvals; |
| • | the inability of government agencies to hire, retain or deploy personnel or otherwise prevent new or modified products from being developed, cleared or approved or commercialized in a timely manner; |
| • | disruptions resulting from President Biden’s invocation of the Defense Production Act; |
| • | our inability to maintain our data management and information technology systems; |
| • | data corruption, cyber-based attacks, security breaches and privacy violations; |
| • | our inability to protect and enforce our intellectual property rights or defend against intellectual property infringement suits against us by third parties; |
| • | risks related to changes in income tax laws and regulations; |
| • | risks related to our substantial indebtedness, which includes $1,292.8 million outstanding under our Dollar Term Loan Facility, $380.9 million outstanding under our Euro Term Loan Facility, $240.0 million aggregate principal amount of 2025 Notes outstanding and $405.0 million aggregate principal amount of 2028 Notes outstanding, in each case, as of July 4, 2021; |
| • | our ability to generate cash flow to service our substantial debt obligations; |
| • | risks related to this offering and ownership of our ordinary shares; and |
| • | other factors set forth under “Risk Factors” in this prospectus or in the documents incorporated by reference herein. |
Our Principal Shareholder
Our Principal Shareholder is an investment fund affiliated with Carlyle. Carlyle acquired us from Johnson & Johnson in 2014 for aggregate consideration of $3,893.1 million, including debt financing consisting of $2,175.0 million of term loans pursuant to the Credit Agreement and $1,300.0 million of senior unsecured notes, all of which has since been refinanced. Affiliates of Carlyle currently hold approximately $61.0 million of indebtedness under the Dollar Term Loan Facility and approximately €56.4 million of indebtedness under the Euro Term Loan Facility. Pursuant to our articles of association, Carlyle has the right to appoint a number of the members of our board of directors. In addition, we are party to a shareholders agreement with Carlyle, pursuant to which Carlyle has the right to cause us to file registration statements under the Securities Act covering resales of our ordinary shares held by Carlyle, subject to certain exceptions.
Founded in 1987, Carlyle is a global investment firm and one of the world’s largest global private equity firms with approximately $276 billion of assets under management across 415 investment vehicles as of June 30, 2021. Carlyle invests across three segments—Global Private Equity, Global Credit, and Investment Solutions—in North America, South America, Europe, the Middle East, Africa
8
Table of Contents
and Asia. Carlyle has expertise in various industries, including aerospace, defense & government services, consumer & retail, energy, financial services, healthcare, industrials & transportation, technology & business services and telecommunications & media. Carlyle employs approximately 1,800 employees, including more than 675 investment professionals, in 27 offices across five continents.
Carlyle is one of the leading private equity investors in the healthcare sector, having completed 80 total healthcare transactions representing approximately $16 billion in equity invested since inception. Recent transactions include Grand Rounds (tech-enabled expert medical opinion and healthcare quality and clinical navigation provider), TriNetX (a global health research network optimizing clinical research), Sedgwick (a global multiline claims management firm), One Medical (technology-enabled primary care provider), CorroHealth (a business service provider for healthcare companies), Millicent (a pharmaceutical company), MedRisk (a physical therapy-focused workers’ compensation solutions company), Albany Molecular Research (a pharmaceutical contract development and manufacturing organization), WellDyneRx (an independent pharmacy benefit manager), Rede D’Or São Luiz S.A. (a leading hospital provider in Brazil) and Pharmaceutical Product Development (a global contract research organization).
Corporate Information
U.K. TopCo is a public limited company incorporated under the laws of England and Wales with the legal name Ortho Clinical Diagnostics Holdings plc and with the company number 13084624. Our principal executive offices are located at 1001 Route 202, Raritan, New Jersey 08869. Our telephone number at this address is 908-218-8000. Ortho’s registered address is Felindre Meadows, Pencoed, Bridgend, Mid Glamorgan, Wales, CF35 5PZ. Our website address is www.orthoclinicaldiagnostics.com. Information contained on, or that can be accessed through, our website does not constitute part of this prospectus, and inclusion of our website address in this prospectus is an inactive textual reference only. You should rely only on the information contained in this prospectus and in the documents incorporated by reference herein when making a decision as to whether to invest in the ordinary shares.
9
Table of Contents
THE OFFERING
| Ordinary shares offered by the selling shareholder |
22,000,000 shares. |
| Ordinary shares to be outstanding immediately after this offering |
234,921,002 shares. |
| Option to purchase additional ordinary shares |
The selling shareholder has granted to the underwriters an option to purchase up to 3,300,000 additional ordinary shares from us at any time within 30 days from the date of this prospectus. |
| Use of proceeds |
The selling shareholder will receive all of the net proceeds from the sale of the ordinary shares in this offering. We will not receive any proceeds from the sale of the ordinary shares by the selling shareholder or if the underwriters exercise their option to purchase additional ordinary shares. See “Use of Proceeds.” |
| Risk factors |
See “Risk Factors” and the other information included in this prospectus and incorporated by reference herein for a discussion of the factors you should consider carefully before deciding to invest in our ordinary shares. |
| Dividend policy |
We currently do not intend to declare any dividends on our ordinary shares in the foreseeable future. Our ability to pay dividends on our ordinary shares may be limited by the covenants contained in the agreements governing the Senior Notes and the Senior Secured Credit Facilities and applicable law. See “Dividend Policy.” |
| Nasdaq symbol |
“OCDX.” |
Except as otherwise indicated, all information in this prospectus regarding the number of ordinary shares that will be outstanding immediately after this offering is based on 234,921,002 ordinary shares outstanding as of July 4, 2021 and:
| • | does not reflect 15,857,759 ordinary shares issuable upon the exercise of options outstanding as of July 4, 2021 with a weighted average exercise price of $9.76 per ordinary share; and |
| • | does not reflect 7,864,810 ordinary shares available for future issuance under our Incentive Award Plan (the “2021 Incentive Award Plan”) (including shares subject to existing equity awards). |
10
Table of Contents
SUMMARY CONSOLIDATED FINANCIAL DATA
The following tables summarize the consolidated financial data of Ortho Clinical Diagnostics Holdings plc for the periods and dates indicated. The summary historical consolidated financial data as of and for each of the fiscal years ended January 3, 2021, December 29, 2019, December 30, 2018, December 31, 2017 and January 1, 2017 have been prepared in accordance with GAAP. The balance sheet data as of July 4, 2021 and the statement of operations and cash flow data for the fiscal six months ended July 4, 2021 and June 28, 2020 have been derived from our unaudited consolidated financial statements incorporated by reference from Ortho’s 2021 Q2 Form 10-Q. The statement of operations and cash flow data for the years ended January 3, 2021, December 29, 2019 and December 30, 2018 has been derived from our audited consolidated financial statements incorporated by reference from Ortho’s 2020 Form 10-K. The statement of operations and cash flow data for the years ended December 31, 2017 and January 1, 2017 have been derived from our audited consolidated financial statements not included or incorporated by reference in this prospectus. In the opinion of management, the unaudited financial statements include all adjustments, consisting of only normal and recurring adjustments, necessary for a fair statement of such financial data.
Our historical financial data is not necessarily indicative of our future performance. The summary consolidated financial data set forth below should be read in conjunction with the sections herein entitled “Risk Factors” and “Capitalization” as well as Ortho’s 2020 Form 10-K and Ortho’s 2021 Q2 Form 10-Q, including our unaudited consolidated financial statements and audited consolidated financial statements, incorporated by reference in this prospectus.
| Fiscal six months ended |
Fiscal year ended | |||||||||||||||||||||||||||
| ($ in millions, except per share data) |
July 4, 2021 |
June 28, 2020 |
January 3, 2021 |
December 29, 2019 |
December 30, 2018 |
December 31, 2017 |
January 1, 2017 |
|||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||||||
| Statement of operations data: |
||||||||||||||||||||||||||||
| Net revenue |
$ | 999.3 | $ | 798.5 | $ | 1,766.2 | $ | 1,801.5 | $ | 1,787.3 | $ | 1,781.7 | $ | 1,695.6 | ||||||||||||||
| Cost of revenue, excluding amortization of intangible assets |
496.3 | 416.1 | 908.2 | 922.4 | 930.5 | 897.7 | 906.3 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Gross profit |
503.0 | 382.4 | 858.0 | 879.1 | 856.8 | 884.0 | 789.3 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Operating expenses: |
||||||||||||||||||||||||||||
| Selling, marketing and administrative expenses |
270.2 | 226.9 | 489.6 | 515.1 | 491.6 | 499.8 | 531.5 | |||||||||||||||||||||
| Research and development expense |
59.3 | 49.4 | 112.9 | 98.0 | 98.7 | 96.4 | 99.9 | |||||||||||||||||||||
| Amortization of intangible assets |
66.9 | 65.6 | 131.9 | 131.7 | 128.8 | 162.0 | 159.6 | |||||||||||||||||||||
| Intangible asset impairment charge |
— | — | — | — | — | 11.0 | — | |||||||||||||||||||||
| Other operating expense, net |
17.9 | 13.3 | 35.3 | 48.8 | 71.2 | 79.5 | 54.3 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total operating expenses |
414.2 | 355.2 | 769.7 | 793.6 | 790.3 | 848.7 | 845.3 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Income (loss) from operations |
88.8 | 27.2 | 88.3 | 85.5 | 66.5 | 35.3 | (56.0 | ) | ||||||||||||||||||||
| Other expense (income): |
||||||||||||||||||||||||||||
| Interest expense, net |
76.4 | 99.7 | 198.2 | 231.4 | 235.6 | 239.8 | 216.9 | |||||||||||||||||||||
| Tax indemnification (income) expense |
(0.4 | ) | (4.9 | ) | 31.2 | 29.2 | (13.1 | ) | (124.1 | ) | 5.5 | |||||||||||||||||
| Other expense (income), net |
53.4 | 67.0 | 84.2 | 5.7 | 61.6 | (66.1 | ) | 109.3 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total other expense, net |
129.4 | 161.8 | 313.6 | 266.3 | 284.1 | 49.6 | 331.7 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
11
Table of Contents
| Fiscal six months ended |
Fiscal year ended | |||||||||||||||||||||||||||
| ($ in millions, except per share data) |
July 4, 2021 |
June 28, 2020 |
January 3, 2021 |
December 29, 2019 |
December 30, 2018 |
December 31, 2017 |
January 1, 2017 |
|||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||||||
| Loss before provision for (benefit from) income taxes |
(40.7 | ) | (134.6 | ) | (225.3 | ) | (180.8 | ) | (217.6 | ) | (14.3 | ) | (387.7 | ) | ||||||||||||||
| Provision for (benefit from) income taxes |
18.4 | 7.9 | (13.4 | ) | (23.9 | ) | 31.2 | 102.0 | 3.0 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Net loss |
$ | (59.1 | ) | $ | (142.5 | ) | $ | (211.9 | ) | $ | (156.9 | ) | $ | (248.8 | ) | $ | (116.3 | ) | $ | (390.7 | ) | |||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Per share data: |
||||||||||||||||||||||||||||
| Basic and diluted net loss per share attributable to ordinary shareholders |
$ | (0.27 | ) | $ | (0.97 | ) | $ | (1.45 | ) | $ | (1.08 | ) | $ | (1.72 | ) | $ | (0.80 | ) | $ | (2.71 | ) | |||||||
| Basic and diluted weighted-average ordinary shares outstanding |
220.4 | 146.3 | 146.3 | 145.5 | 145.1 | 144.8 | 144.4 | |||||||||||||||||||||
| Cash flow data: |
||||||||||||||||||||||||||||
| Net cash provided by (used in): |
||||||||||||||||||||||||||||
| Operating activities |
$ | 122.8 | $ | (63.5 | ) | $ | 46.1 | $ | 143.0 | $ | 69.6 | $ | 68.0 | $ | (59.9 | ) | ||||||||||||
| Investing activities |
(4.3 | ) | (25.5 | ) | (45.4 | ) | (68.5 | ) | (87.1 | ) | (118.0 | ) | (187.4 | ) | ||||||||||||||
| Financing activities |
(50.2 | ) | 88.0 | 55.8 | (64.4 | ) | (8.2 | ) | 84.9 | 159.0 | ||||||||||||||||||
| Cash paid for interest |
83.6 | 105.6 | 191.8 | 189.7 | 218.8 | 222.3 | 201.8 | |||||||||||||||||||||
| Cash paid for income taxes, net |
8.3 | 10.8 | 16.8 | 8.4 | 13.4 | 9.1 | 20.6 | |||||||||||||||||||||
| As of | ||||||||||||||||||||||||
| ($ in millions) | July 4, 2021 |
January 3, 2021 |
December 29, 2019 |
December 30, 2018 |
December 31, 2017 |
January 1, 2017 |
||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||
| Consolidated balance sheet data: |
||||||||||||||||||||||||
| Cash and cash equivalents |
$ | 200.9 | $ | 132.8 | $ | 72.0 | $ | 56.4 | $ | 93.3 | $ | 60.8 | ||||||||||||
| Total assets |
3,304.2 | 3,401.5 | 3,589.2 | 3,687.4 | 3,936.8 | 3,865.7 | ||||||||||||||||||
| Working capital(1) |
389.8 | 230.8 | 138.7 | 188.8 | 234.0 | 79.0 | ||||||||||||||||||
| Total liabilities |
2,928.7 | 4,412.3 | 4,402.0 | 4,342.1 | 4,353.5 | 4,195.4 | ||||||||||||||||||
| Accumulated deficit |
(1,976.6 | ) | (1,917.5 | ) | (1,705.6 | ) | (1,548.9 | ) | (1,312.0 | ) | (1,195.7 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total shareholders’ equity (deficit) |
375.6 | (1,010.8 | ) | (812.8 | ) | (654.7 | ) | (416.7 | ) | (329.7 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Fiscal six months ended |
Fiscal year ended | |||||||||||||||||||||||
| ($ in millions) | July 4, 2021 |
June 28, 2020 |
January 3, 2021 |
December 29, 2019 |
December 30, 2018 |
December 31, 2017 |
||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||
| Other financial and operating data |
||||||||||||||||||||||||
| Net revenue—Clinical Laboratories |
$ | 663.1 | $ | 516.6 | $ | 1,154.2 | $ | 1,142.3 | $ | 1,102.5 | $ | 1,097.9 | ||||||||||||
| Net revenue—Transfusion Medicine |
323.8 | 273.9 | 580.6 | 598.0 | 616.1 | 598.7 | ||||||||||||||||||
| Core revenue(2) |
986.9 | 790.5 | 1,734.8 | 1,740.3 | 1,718.6 | 1,696.6 | ||||||||||||||||||
| Core revenue growth rate(2) |
24.8 | % | (6.9 | )% | (0.3 | %) | 1.3 | % | 1.3 | % | 4.8 | % | ||||||||||||
| Core revenue constant currency growth rate(2)(3) |
21.8 | % | (5.2 | )% | 0.4 | % | 2.9 | % | 1.0 | % | 4.8 | % | ||||||||||||
| Adjusted EBITDA(4)(5) |
280.6 | 202.9 | 456.0 | 477.5 | 467.8 | 477.6 | ||||||||||||||||||
| Adjusted EBITDA margin(4)(5)(6) |
28.1 | % | 25.4 | % | 25.8 | % | 26.5 | % | 26.2 | % | 26.8 | % | ||||||||||||
| Adjusted Net Income(4)(5) |
94.0 | 1.1 | 51.2 | 32.7 | 6.7 | 39.9 | ||||||||||||||||||
| (1) | We define working capital as current assets less current liabilities. Refer to our consolidated financial statements incorporated by reference in this prospectus for further details regarding our current assets and current liabilities. |
| (2) | Core revenue refers collectively to revenue generated in our Clinical Laboratories and our Transfusion Medicine lines of business which historically make up more than 95% of our net revenue. |
12
Table of Contents
| (3) | When we use the term “constant currency,” it means that we have translated current period and prior period amounts from local currency to U.S. dollars using internally-derived currency exchange rates held constant for each year. As needed, we translate prior periods based on our internally-derived currency exchange rates used in the current period in order to remove the impact of foreign currency exchange fluctuation. This additional non-GAAP financial information is not meant to be considered in isolation from or as substitute for financial information prepared in accordance with GAAP. The following table reconciles core revenue to core revenue constant currency growth rate for the periods presented: |
| Fiscal six months ended |
Fiscal year ended | |||||||||||||||||||
| ($ in millions) | July 4, 2021(a) |
June 28, 2020(a) |
January 3, 2021(a) |
December 29, 2019(a) |
December 30, 2018 |
|||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Core revenue |
$ | 986.9 | $ | 790.5 | $ | 1,734.8 | $ | 1,740.3 | $ | 1,718.6 | ||||||||||
| Foreign currency translation |
(13.6 | ) | 8.7 | 5.3 | (6.6 | ) | (31.6 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Core revenue constant currency |
973.3 | 799.2 | 1,740.1 | 1,733.7 | 1,687.0 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Core revenue constant currency growth rate |
21.8 | % | (5.2 | )% | 0.4 | % | 2.9 | % | 1.0 | % | ||||||||||
| (a) | Core revenue constant currency by quarter for fiscal 2019 and 2020 and year to date 2021 is as follows: |
| Fiscal quarter ended | ||||||||||||||||||||||||||||||||||||||||
| ($ in millions) | July 4, 2021 |
April 4, 2021 |
January 3, 2021 |
September 27, 2020 |
June 28, 2020 |
March 29, 2020 |
December 29, 2019 |
September 29, 2019 |
June 30 2019 |
March 31, 2019 |
||||||||||||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||||||||||||||||||
| Core revenue constant currency |
$ | 480.5 | $ | 492.8 | $ | 496.2 | $ | 444.7 | $ | 392.2 | $ | 407.0 | $ | 454.8 | $ | 435.9 | $ | 438.9 | $ | 404.0 | ||||||||||||||||||||
| Less: Local HCV(i) |
8.6 | 3.8 | 3.8 | 3.3 | 14.6 | 2.7 | 6.0 | 9.9 | 9.5 | 18.0 | ||||||||||||||||||||||||||||||
| Less: CoV-2 |
17.1 | 28.8 | 25.9 | 20.9 | 27.4 | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Core revenue constant currency (excl. local HCV and CoV-2) |
454.8 | 460.2 | 466.5 | 420.5 | 350.3 | 404.3 | 448.9 | 426.0 | 429.4 | 386.0 | ||||||||||||||||||||||||||||||
| Core revenue constant currency annual growth rate |
22.5 | % | 21.1 | % | 9.1 | % | 2.1 | % | (10.8 | )% | 0.9 | % | 2.9 | % | 5.5 | % | 3.7 | % | (0.5 | )% | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Core revenue constant currency annual growth rate (excl. local HCV) |
25.0 | % | 20.9 | % | 9.7 | % | 3.7 | % | (12.2 | )% | 4.8 | % | 4.3 | % | 5.8 | % | 3.8 | % | (3.0 | )% | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Core revenue constant currency annual growth rate (excl. local HCV and CoV-2) |
29.9 | % | 13.8 | % | 3.9 | % | (1.3 | )% | (18.4 | )% | 4.8 | % | 4.3 | % | 5.8 | % | 3.8 | % | (3.0 | )% | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| (i) | During the fiscal three months ended March 31, 2019, we signed a supply agreement in Japan related to our HCV business. Revenue recognition is based on the timing of periodic shipments which may create unusual year-over-year variances in certain quarters. |
13
Table of Contents
| (4) | Adjusted EBITDA consists of net loss before interest expense, net, provision for (benefit from) income taxes and depreciation and amortization and eliminates (i) non-operating income or expense and (ii) impacts of certain non-cash, unusual or other items that are included in net loss that we do not consider indicative of our ongoing operating performance. Management EBITDA consists of Adjusted EBITDA plus certain other management adjustments and was the basis we used for assessing the profitability of our geographic-based reportable segments. Additionally, Management EBITDA was utilized as a basis for calculating certain management incentive compensation programs. Beginning in the fiscal first quarter ended April 4, 2021, we began using Adjusted EBITDA as the basis for assessing the profitability of our geographic-based reportable segments and as a basis for calculating certain management incentive compensation programs. Adjusted Net Income consists of net loss before amortization and the elimination of (i) non-operating income or expense and (ii) impacts of certain non-cash, unusual or other items that are included in net loss that we do not consider indicative of our ongoing operating performance. In the case of Adjusted EBITDA and Adjusted Net Income, we believe that making such adjustments provides management and investors meaningful information to understand our operating performance and ability to analyze financial and business trends on a period-to-period basis. |
| (5) | Adjusted EBITDA, Management EBITDA, Adjusted Net Income and Core revenue constant currency growth rate are not calculated or presented in accordance with GAAP and other companies in our industry may calculate Adjusted EBITDA, Management EBITDA, Adjusted Net Income and Core revenue constant currency growth rate differently than we do. As a result, these financial measures have limitations as analytical and comparative tools and you should not consider these items in isolation, or as a substitute for analysis of our results as reported under GAAP. Adjusted EBITDA, Management EBITDA and Adjusted Net Income should not be considered as measures of discretionary cash available to us to invest in the growth of our business. In calculating these financial measures, we make certain adjustments that are based on assumptions and estimates that may prove to have been inaccurate. In addition, in evaluating these financial measures, you should be aware that in the future we may incur expenses similar to those eliminated in this presentation. Our presentation of Adjusted EBITDA, Management EBITDA and Adjusted Net Income should not be construed as an inference that our future results will be unaffected by unusual or non-recurring items. For additional information regarding Adjusted EBITDA, Management EBITDA, Adjusted Net Income and Core revenue constant currency growth rate and our use and presentation of those measures and the related risks, see “Use of Non-GAAP Financial Measures.” |
The following tables reconcile net loss to Adjusted EBITDA, Management EBITDA and Adjusted Net Income for the periods presented:
| Fiscal six months ended |
Fiscal year ended | |||||||||||||||||||||||
| ($ in millions) | July 4, 2021 |
June 28, 2020 |
January 3, 2021 |
December 29, 2019 |
December 30, 2018 |
December 31, 2017 |
||||||||||||||||||
| Net loss |
$ | (59.1 | ) | $ | (142.5 | ) | $ | (211.9 | ) | $ | (156.9 | ) | $ | (248.8 | ) | $ | (116.3 | ) | ||||||
| Interest expense, net |
76.4 | 99.7 | 198.2 | 231.4 | 235.6 | 239.8 | ||||||||||||||||||
| Provision for (benefit from) income taxes |
18.4 | 7.9 | (13.4 | ) | (23.9 | ) | 31.2 | 102.0 | ||||||||||||||||
| Depreciation and amortization |
165.8 | 159.7 | 325.9 | 327.5 | 332.2 | 333.3 | ||||||||||||||||||
| Unrealized foreign currency exchange losses (gains)(a) |
— | 52.3 | 63.0 | (19.6 | ) | 46.5 | (58.8 | ) | ||||||||||||||||
| Restructuring and severance-related costs(b) |
3.0 | 4.6 | 11.7 | 36.0 | 38.3 | 51.5 | ||||||||||||||||||
| Debt refinancing costs and loss on extinguishment of debt |
50.3 | 12.6 | 12.6 | — | 20.6 | 2.0 | ||||||||||||||||||
| Stock-based compensation(c) |
14.5 | 3.8 | 8.6 | 18.6 | 5.9 | 4.4 | ||||||||||||||||||
| Tax indemnification (income) expense, net(d) |
(0.4 | ) | (4.9 | ) | 31.2 | 29.2 | (13.1 | ) | (124.1 | ) | ||||||||||||||
| Quotient upfront payment(e) |
— | — | 7.5 | — | — | — | ||||||||||||||||||
| Impairment of equity investment |
— | — | — | — | — | 20.2 | ||||||||||||||||||
| Intangible asset impairment charge |
— | — | — | — | — | 11.0 | ||||||||||||||||||
| Impact of acquisition accounting and acquisition-related costs |
— | — | — | — | — | 2.4 | ||||||||||||||||||
| Other adjustments(f) |
11.6 | 9.7 | 22.6 | 35.2 | 19.4 | 10.2 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Adjusted EBITDA |
$ | 280.6 | $ | 202.9 | $ | 456.0 | $ | 477.5 | $ | 467.8 | $ | 477.6 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Management adjustments and realized foreign exchange losses (gains)(g) |
70.7 | 25.1 | 17.7 | 9.4 | ||||||||||||||||||||
| Management EBITDA |
$ | 526.7 | $ | 502.6 | $ | 485.5 | $ | 487.0 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
14
Table of Contents
| Fiscal six months ended |
Fiscal year ended | |||||||||||||||||||||||
| ($ in millions) | July 4, 2021 |
June 28, 2020 |
January 3, 2021 |
December 29, 2019 |
December 30, 2018 |
December 31, 2017 |
||||||||||||||||||
| Net loss |
$ | (59.1 | ) | $ | (142.5 | ) | $ | (211.9 | ) | $ | (156.9 | ) | $ | (248.8 | ) | $ | (116.3 | ) | ||||||
| Amortization of intangible assets |
66.9 | 65.6 | 131.9 | 131.7 | 128.8 | 162.0 | ||||||||||||||||||
| Unrealized foreign currency exchange losses (gains), net(a) |
— | 52.3 | 63.0 | (19.6 | ) | 46.5 | (58.8 | ) | ||||||||||||||||
| Restructuring and severance-related costs(b) |
3.0 | 4.6 | 11.7 | 36.0 | 38.3 | 51.5 | ||||||||||||||||||
| Debt refinancing costs and loss on extinguishment of debt |
50.3 | 12.6 | 12.6 | — | 20.6 | 2.0 | ||||||||||||||||||
| Stock-based compensation(c) |
14.5 | 3.8 | 8.6 | 18.6 | 5.9 | 4.4 | ||||||||||||||||||
| Impairment of equity investment |
— | — | — | — | — | 20.2 | ||||||||||||||||||
| Intangible asset impairment charge |
— | — | — | — | — | 11.0 | ||||||||||||||||||
| Quotient upfront payment(e) |
— | — | 7.5 | — | — | — | ||||||||||||||||||
| Impact of acquisition accounting and acquisition-related costs |
— | — | — | — | — | 2.4 | ||||||||||||||||||
| Other adjustments(f) |
11.6 | 9.7 | 22.6 | 35.2 | 19.4 | 10.2 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total adjustments |
146.3 | 148.6 | 257.9 | 201.9 | 259.5 | 204.9 | ||||||||||||||||||
| Tax effect of reconciling items(h) |
(3.9) | (5.0) | (6.3 | ) | (10.2 | ) | (9.0 | ) | (14.2 | ) | ||||||||||||||
| Discrete tax items(i) |
10.6 | — | 11.5 | (2.1 | ) | 5.0 | (34.5 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Adjusted Net Income |
$ | 94.0 | $1.1 | $ | 51.2 | $ | 32.7 | $ | 6.7 | $ | 39.9 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| (a) | Represents noncash unrealized gains and losses resulting from the remeasurement of transactions denominated in foreign currencies primarily related to intercompany loans. In the fiscal first quarter ended April 4, 2021, we initiated programs to mitigate the impact of foreign currencies related to intercompany loans in our results, and such non-cash net unrealized gains were approximately $13.8 million for the fiscal six months ended July 4, 2021. We expect these programs to continue to mitigate the impact of foreign currencies related to intercompany loans in our results of operations in future periods, and thus we did not exclude non-cash unrealized gains and losses resulting from the remeasurement of transactions denominated in foreign currencies from Adjusted EBITDA and Adjusted Net Income during the fiscal six months ended July 4, 2021 and onwards. |
| (b) | Represents restructuring and severance costs related to several discrete initiatives intended to strengthen operational performance and to support building our commercial capabilities including a project announced in fiscal year ended January 3, 2016 to outsource equipment manufacturing operations in Rochester, New York and a project announced in fiscal year ended December 30, 2018 to transfer certain production lines among facilities. |
| (c) | Represents stock-based compensation expense, including the impact of modifications to our stock option agreement of $6.0 million during the fiscal six months ended July 4, 2021 and $14.7 million during the fiscal year ended December 29, 2019. |
| (d) | Represents the reversal of the impact of tax indemnification income with Johnson & Johnson, primarily related to certain state tax matters, for which we recorded a tax reserve and indemnification expense. These state tax matters primarily include the taxability of the sale of our assets on the Acquisition date from Johnson & Johnson. Additionally, during the fiscal second quarter ended June 30, 2019, we recorded indemnification expense related to the release of certain tax reserves upon the settlement of certain state tax matters that were settled for an amount lower than what we had estimated. |
| (e) | Represents an initial, non-refundable upfront payment made to Quotient Ltd. (“Quotient”), one of our partners and suppliers. See note 9 to our unaudited consolidated financial statements and note 13 to our audited consolidated financial statements incorporated by reference in this prospectus for further discussion of the Quotient relationship. |
15
Table of Contents
| (f) | Represents miscellaneous other adjustments related to unusual items impacting our results including the elimination of management fees, non-cash derivative mark-to-market losses, net, and certain asset write-downs. See information below: |
| Fiscal six months ended |
Fiscal year ended | |||||||||||||||||||||||
| ($ in millions) | July 4, 2021 |
June 28, 2020 |
January 3, 2021 |
December 29, 2019 |
December 30, 2018 |
December 31, 2017 |
||||||||||||||||||
| Principal Shareholder management fee |
$ | 1.5 | $ | 1.5 | $ | 3.0 | $ | 3.1 | $ | 3.0 | $ | 3.1 | ||||||||||||
| Noncash losses on property, plant and equipment disposals |
— | 0.2 | 0.6 | 2.5 | 2.9 | 2.1 | ||||||||||||||||||
| EU Medical device regulation transition costs |
1.8 | 2.3 | 4.3 | 3.2 | 0.6 | — | ||||||||||||||||||
| Derivative mark-to-market loss (gain), net |
1.6 | (0.2 | ) | 1.5 | 16.0 | 2.7 | — | |||||||||||||||||
| Other |
6.7 | 5.9 | 13.2 | 10.4 | 10.2 | 5.0 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total Other Adjustments |
$ | 11.6 | $ | 9.7 | $ | 22.6 | $ | 35.2 | $ | 19.4 | $ | 10.2 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| (g) | Represents realized foreign currency losses (gains), impact from adoption of accounting standards, costs in connection with COVID-19 and other immaterial management adjustments. During the fiscal first quarter ended April 4, 2021, we changed our measure of the profitability of our geographic-based reportable segments and basis for calculating certain management incentive compensation programs from Management EBITDA to Adjusted EBITDA. Accordingly, Management EBITDA for the fiscal six months ended July 4, 2021 and June 28, 2020 has not been separately presented. |
| (h) | Non-GAAP adjustments were tax effected based on the nature of the expense and related jurisdiction, many of which are impacted by valuation allowances resulting in little to no tax impact. |
| (i) | We exclude deferred tax resulting from changes in tax law and expiration of statutes, adjustments for uncertain tax positions, and other unusual items not related to current operating results. |
| (6) | Adjusted EBITDA margin is defined as Adjusted EBITDA as a percentage of net revenue. |
16
Table of Contents
Investing in our ordinary shares involves a high degree of risk. You should carefully consider the following risk factors together with all of the other information included or incorporated by reference in this prospectus, including our consolidated financial statements and related notes incorporated by reference herein, before deciding whether to invest in our ordinary shares. The occurrence of any of the events described below could harm our business, financial condition, results of operations and growth prospects. In such an event, the trading price of our ordinary shares may decline and you may lose all or part of your investment.
Risks Related to this Offering and Ownership of Our Ordinary Shares
The market price of our ordinary shares has been volatile and may continue to fluctuate substantially, which could result in substantial losses for purchasers of our ordinary shares.
The trading price of our ordinary shares has been and is likely to continue to be volatile. The stock market has experienced extreme volatility. This volatility often has been unrelated or disproportionate to the operating performance of particular companies. The market price of our ordinary shares has been highly volatile and may continue to fluctuate substantially due to a number of factors such as those listed in “Risk Factors” in this prospectus and in the documents incorporated by reference herein and the following:
| • | results of operations that vary from the expectations of securities analysts and investors; |
| • | results of operations that vary from those of our competitors; |
| • | changes in expectations as to our future financial performance, including financial estimates and investment recommendations by securities analysts and investors; |
| • | declines in the market prices of stocks generally; |
| • | strategic actions by us or our competitors; |
| • | announcements by us or our competitors of significant contracts, new products, acquisitions, joint marketing relationships, joint ventures, other strategic relationships or capital commitments; |
| • | changes in general economic or market conditions or trends in our industry or markets; |
| • | changes in business or regulatory conditions; |
| • | additions or departures of key management personnel; |
| • | future sales of our ordinary shares or other securities by us or our existing shareholders, or the perception of such future sales; |
| • | investor perceptions of the investment opportunity associated with our ordinary shares relative to other investment alternatives; |
| • | the public’s response to press releases or other public announcements by us or third parties, including our filings with the SEC; |
| • | announcements relating to litigation; |
| • | guidance, if any, that we provide to the public, any changes in this guidance or our failure to meet this guidance; |
| • | the development and sustainability of an active trading market for our ordinary shares; |
| • | changes in accounting principles; and |
17
Table of Contents
| • | other events or factors, including those resulting from natural disasters, war, acts of terrorism or responses to these events. |
These broad market and industry fluctuations may materially adversely affect the market price of our ordinary shares, regardless of our actual operating performance. In addition, price volatility may be greater if the public float and trading volume of our ordinary shares are low.
In the past, following periods of market volatility, shareholders have instituted securities class action litigation. If we were involved in securities litigation, it could have a substantial cost and divert resources and the attention of executive management from our business regardless of the outcome of such litigation.
Our quarterly operating results fluctuate and may fall short of prior periods, our projections or the expectations of securities analysts or investors, which could materially adversely affect the price of our ordinary shares.
Our operating results have fluctuated from quarter to quarter at points in the past, and they may do so in the future. Therefore, results of any one fiscal quarter are not a reliable indication of results to be expected for any other fiscal quarter or for any year. If we fail to increase our results over prior periods, to achieve our projected results or to meet the expectations of securities analysts or investors, the price of our ordinary shares may decline, and the decrease in the price of our ordinary shares may be disproportionate to the shortfall in our financial performance. Results may be affected by various factors, including those described in these risk factors. We maintain a forecasting process that seeks to align expenses to backlog conversion. If we do not control costs or appropriately adjust costs to actual results, or if actual results differ significantly from our forecast, our financial performance could be materially adversely affected.
We are a holding company with no operations and may not have access to sufficient cash to meet our financial obligations.
We are a holding company and have limited direct operations. Our most significant assets are the equity interests we directly and indirectly hold in our subsidiaries. As a result, we are dependent upon dividends and other payments from our subsidiaries to generate the funds necessary to meet our outstanding debt service and other obligations and such dividends may be restricted by law or the instruments governing our indebtedness, including the Credit Agreement, the 2028 Notes Indenture, the 2025 Notes Indenture or other agreements of our subsidiaries. Our subsidiaries may not generate sufficient cash from operations to enable us to meet our financial obligations. In addition, our subsidiaries are separate and distinct legal entities and may be legally or contractually prohibited or restricted from paying dividends or otherwise making funds available to us. In addition, payments to us by our subsidiaries will be contingent upon our subsidiaries’ earnings. Additionally, we may be limited in our ability to cause any future collaborative arrangements under which our subsidiaries distribute their earnings to us. Subject to certain qualifications, our subsidiaries are permitted, under the terms of our indebtedness, to incur additional indebtedness that may restrict payments from those subsidiaries to us. We cannot assure you that agreements governing the current and future indebtedness of our subsidiaries will permit those subsidiaries to provide us with sufficient cash to fund our financial obligations.
We currently do not intend to declare dividends on our ordinary shares in the foreseeable future and, as a result, your only opportunity to achieve a return on your investment is if the price of our ordinary shares appreciates.
We currently do not expect to declare any dividends on our ordinary shares in the foreseeable future. Instead, we anticipate that all of our earnings in the foreseeable future will be used to provide
18
Table of Contents
working capital, to support our operations and to finance the growth and development of our business. Any determination to declare or pay dividends in the future will be at the discretion of our board of directors, subject to applicable laws and dependent upon a number of factors, including our earnings, capital requirements and overall financial conditions. In addition, because we are a holding company, our ability to pay dividends on our ordinary shares may be limited by restrictions on our ability to obtain sufficient funds through dividends from subsidiaries, including restrictions under the covenants of the Credit Agreement, the 2025 Notes Indenture and the 2028 Notes Indenture, and may be further restricted by the terms of any future debt or preferred securities. Accordingly, your only opportunity to achieve a return on your investment in our company may be if the market price of our ordinary shares appreciates and you sell your ordinary shares at a profit. The market price for our ordinary shares may never exceed, and may fall below, the price that you pay for such ordinary shares.
If securities analysts do not publish research or reports about our business or if they downgrade our stock or our sector, our stock price and trading volume could decline.
The trading market for our ordinary shares relies in part on the research and reports that industry or financial analysts publish about us or our business or industry. We do not control these analysts. Furthermore, if one or more of the analysts who do cover us downgrade our stock or our industry, or the stock of any of our competitors, or publish inaccurate or unfavorable research about our business or industry, the price of our shares could decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, we could lose visibility in the market, which in turn could cause our share price or trading volume to decline.
Future sales, or the perception of future sales, by us or our existing shareholders in the public market following this offering could cause the market price for our ordinary shares to decline.
After this offering, the sale of our ordinary shares in the public market, or the perception that such sales could occur, could harm the prevailing market price of our ordinary shares. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
Our directors, our executive officers and certain significant equity holders have entered into lock-up agreements in connection with this offering, on substantially similar terms, which expire 90 days from the date of this prospectus. Upon completion of this offering, based on the number of ordinary shares outstanding on September 1, 2021, 122,176,446 of our ordinary shares (assuming no exercise of the underwriters’ option to purchase additional ordinary shares) will be restricted from sale as a result of lock-up agreements with the underwriters through the date that is 90 days from the date of this prospectus. Among other exceptions, the lock-up agreements entered into in connection with this offering permit our directors and executive officers to sell ordinary shares pursuant to existing trading plans pursuant to Rule 10b5-1 under the Exchange Act. Such sales could occur at any time, and could be substantial. See “Underwriting” for a description of the material terms of these lock-up agreements.
After this offering, the holders of an aggregate of 121,406,000 of our ordinary shares (assuming no exercise of the underwriters’ option to purchase additional ordinary shares), will have rights, subject to some conditions, to require us to file registration statements covering their ordinary shares or to include their ordinary shares in registration statements that we may file for ourselves or our shareholders. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares held by our affiliates as defined in Rule 144 under the Securities Act. See “Shares Eligible for Future Sale.”
As restrictions on resale end or if these shareholders exercise their registration rights, the market price of our ordinary shares could drop significantly if the holders of these shares sell them or are perceived by the market as intending to sell them. These factors could also make it more difficult for us to raise additional funds through future offerings of our ordinary shares or other securities.
19
Table of Contents
In addition, our ordinary shares reserved for future issuance under our 2021 Incentive Award Plan will become eligible for sale in the public market once those ordinary shares are issued, subject to provisions relating to various vesting agreements, lock-up agreements and Rule 144 under the Securities Act, as applicable. As of July 4, 2021, a total of 7,864,810 ordinary shares have been reserved for future issuance under the 2021 Incentive Award Plan (including shares subject to existing equity awards), which amount will increase on the first day of the fiscal year for up to 10 years by an amount equal to 3% of the ordinary shares outstanding on the last day of the prior fiscal year or such lesser amount as may be determined by our board of directors.
In the future, we may also issue our securities in connection with investments or acquisitions. The amount of our ordinary shares issued in connection with an investment or acquisition could constitute a material portion of our then-outstanding ordinary shares. Any issuance of additional securities in connection with investments or acquisitions may result in additional dilution to you.
Provisions in our organizational documents could delay or prevent a change of control.
Certain provisions of our articles of association and shareholders agreement may have the effect of delaying or preventing a merger, acquisition, tender offer, takeover attempt or other change of control transaction that a shareholder might consider to be in its best interest, including attempts that might result in a premium over the market price of our ordinary shares.
Subject to the provisions of the U.K. Companies Act 2006, these provisions provide for, among other things:
| • | the division of our board of directors into three classes, as nearly equal in size as possible, with directors in each class serving three-year terms and with terms of the directors of only one class expiring in any given year; |
| • | the reappointment of any member of our board of directors initially appointed by the Principal Shareholder in the event such director is removed in accordance with our articles of association and pursuant to the UK Companies Act 2006; |
| • | the ability of our board of directors to issue one or more series of preferred shares with voting or other rights or preferences that could have the effect of impeding the success of an attempt to acquire us or otherwise effect a change of control; |
| • | advance notice for nominations of directors by shareholders and for shareholders to include matters to be considered at shareholder meetings; |
| • | the right of the Principal Shareholder and certain of its respective affiliates to appoint the majority of the members of our board of directors for so long as the Principal Shareholder beneficially owns at least 25% of our ordinary shares, and such appointed members will serve as directors without standing for election by our shareholders; |
| • | certain limitations on convening special shareholder meetings; and |
| • | that certain provisions of our articles of association may be amended only with the consent of the Principal Shareholder for so long as the Principal Shareholder beneficially owns at least 5% of our ordinary shares. |
These provisions could make it more difficult for a third party to acquire us, even if the third-party’s offer may be considered beneficial by many of our shareholders. As a result, our shareholders may be limited in their ability to obtain a premium for their ordinary shares. See “Description of Share Capital and Articles of Association.”
20
Table of Contents
Private equity investment funds affiliated with the Principal Shareholder will continue to own a significant percentage of our equity after this offering and their interests may not be aligned with yours.
Upon the completion of this offering, the Principal Shareholder will own approximately 51.3% of our ordinary shares, or approximately 49.9% if the underwriters exercise in full their option to purchase additional ordinary shares, and will have the power to control or influence our affairs and policies. The Principal Shareholder will also control, to a large degree, the election of directors, the appointment of management, the entry into mergers, sales of substantially all of our assets and other extraordinary transactions. The directors so elected have authority to issue additional shares, implement stock repurchase programs, declare interim dividends and recommend final dividends, and make other decisions. The interests of the Principal Shareholder could conflict with your interests. For example, the Principal Shareholder may also have an interest in pursuing acquisitions, divestitures, financings or other transactions that, in its judgment, could enhance its equity investments. Additionally, the Principal Shareholder is in the business of making investments in companies, and may from time to time acquire interests in businesses that directly or indirectly compete with certain portions of our business or are suppliers or customers of ours.
We are a “controlled company” within the meaning of Nasdaq rules and the rules of the SEC. As a result, we qualify for, and rely on, exemptions from certain corporate governance requirements that provide protection to shareholders of other companies.
The Principal Shareholder owns, and, upon completion of this offering, will continue to own, a majority of our outstanding ordinary shares. As a result, we are a “controlled company” within the meaning of the corporate governance standards of Nasdaq. Under these rules, a company of which more than 50% of the voting power is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance requirements, including:
| • | the requirement that a majority of our board of directors consist of “independent directors” as defined under the rules of Nasdaq; |
| • | the requirement that we have a compensation committee that is composed entirely of directors who meet the Nasdaq independence standards for compensation committee members; and |
| • | the requirement that our director nominations be made, or recommended to our full board of directors, by our independent directors or by a nominations committee that consists entirely of independent directors. |
We currently rely on certain of the exemptions listed above. As a result, we may not have a majority of independent directors, our nominations committee and compensation committee will not consist entirely of independent directors and such committees may not be subject to annual performance evaluations. Accordingly, you will not have the same protections afforded to shareholders of companies that are subject to all of the corporate governance requirements of Nasdaq.
Failure to comply with requirements to design, implement and maintain effective internal controls could have a material adverse effect on our business and the price of our ordinary shares.
As a privately-held company, we were not required to evaluate our internal controls over financial reporting in a manner that meets the standards of publicly traded companies required by Section 404(a) of the Sarbanes-Oxley Act of 2002 (“Section 404” and the “Sarbanes-Oxley Act,” respectively). As a public company, we have significant requirements for enhanced financial reporting and internal controls. The process of designing and implementing effective internal controls that we
21
Table of Contents
manage is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company. If we are unable to establish or maintain appropriate internal financial reporting controls and procedures, it could cause us to fail to meet our reporting obligations on a timely basis, result in material misstatements in our consolidated financial statements and harm our results of operations. In addition, we are required, pursuant to Section 404, to furnish a report by management on, among other things, the effectiveness of our internal controls over financial reporting in our annual report on Form 10-K for the year ending January 2, 2022. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal controls over financial reporting. The rules governing the standards that must be met for our management to assess our internal controls over financial reporting are complex and require significant documentation, testing and possible remediation. Testing internal controls may divert our management’s attention from other matters that are important to our business.
In connection with the implementation of the necessary procedures and practices related to internal controls over financial reporting, we may identify deficiencies that we may not be able to remediate in time to meet the deadline imposed by the Sarbanes-Oxley Act for compliance with the requirements of Section 404. In addition, we may encounter problems or delays in completing the remediation of any deficiencies identified by our independent registered public accounting firm in connection with the issuance of their attestation report. While not required for our annual report on Form 10-K for the year ending January 2, 2022, our independent registered public accounting firm may be required to issue an attestation report on the effectiveness of internal controls over financial reporting in our annual report on Form 10-K for a fiscal year thereafter.
Our testing, or the subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses. A material weakness in internal controls could result in our failure to detect a material misstatement of our annual or quarterly consolidated financial statements or disclosures. We may not be able to conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404. If we are unable to conclude that we have effective internal controls over financial reporting, investors could lose confidence in our reported financial information, which could have a material adverse effect on the trading price of our ordinary shares.
Our board of directors is authorized to issue and designate preferred shares in additional series without shareholder approval.
Our articles of association authorize our board of directors, without the approval of our shareholders, to issue preferred shares, subject to limitations prescribed by applicable law, rules and regulations and the provisions of our articles of association, as preferred shares in series, to establish from time to time the number of preferred shares to be included in each such series and to fix the designation, powers, preferences and rights of the preferred shares of each such series and the qualifications, limitations or restrictions thereof. The powers, preferences and rights of these additional series of preferred shares may be senior to or on parity with our ordinary shares, which may reduce their value.
Our articles of association include exclusive jurisdiction and forum selection provisions, which may impact the ability of shareholders to bring actions against us or increase the costs of bringing such actions.
Our articles of association provide that the courts of England and Wales shall have exclusive jurisdiction to determine any and all disputes brought by a shareholder in their capacity as a shareholder against us, our officers or our board of directors arising out of or in connection with our
22
Table of Contents
articles of association or any non-contractual obligations arising out of or in connection with our articles of association, or otherwise. In addition, our articles of association provide that if a court were to find such provision invalid or unenforceable with respect to any complaint asserting a cause of action arising under the Securities Act, the federal courts of the United States will be the exclusive forum for resolving any such complaint. These limitations on the forum in which shareholders may initiate action against us may limit a shareholder’s ability to bring a claim in a judicial forum that it finds favorable and could increase the costs and inconvenience of pursuing claims or otherwise adversely affect a shareholder’s ability to seek monetary or other relief.
A court could decline to enforce these exclusive jurisdiction and forum provisions. If a court were to find these provisions to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions.
23
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements made herein or incorporated by reference in this prospectus contain forward-looking statements within the meaning of Section 27A of the Securities Act, and Section 21E of the Exchange Act. Such forward-looking statements reflect, among other things, our current expectations and anticipated results of operations, all of which are subject to known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements, market trends, or industry results to differ materially from those expressed or implied by such forward-looking statements. Therefore, any statements contained and incorporated by reference herein that are not statements of historical fact, including statements about our beliefs and expectations, may be forward-looking statements and should be evaluated as such. These statements often include words such as “anticipate,” “expect,” “suggest,” “plan,” “believe,” “intend,” “project,” “forecast,” “estimates,” “targets,” “projections,” “should,” “could,” “would,” “may,” “might,” “will,” and other similar expressions, including forward-looking statements about the impact from the COVID-19 pandemic. These forward-looking statements are contained or incorporated by reference in this prospectus.
We base these forward-looking statements or projections on our current expectations, plans and assumptions, which we have made in light of our experience in the industry, as well as our perceptions of historical trends, current conditions, expected future developments and other factors we believe are appropriate under the circumstances at this time, including the impact from the COVID-19 pandemic. As you read and consider this prospectus and the information incorporated by reference, you should understand that these statements are not guarantees of performance or results. The forward-looking statements and projections contained and incorporated by reference herein are subject to and involve risks, uncertainties and assumptions, and therefore you should not place undue reliance on these forward-looking statements or projections. Although we believe that these forward-looking statements and projections are based on reasonable assumptions at the time they are made, you should be aware that many factors could affect our actual financial results, and therefore actual results might differ materially from those expressed in the forward-looking statements and projections. Factors that might materially affect such forward-looking statements and projections include:
| • | the ongoing global COVID-19 pandemic; |
| • | increased competition; |
| • | failure to develop and market new or enhanced products or services; |
| • | adverse developments in global market, economic and political conditions; |
| • | our ability to obtain additional capital on commercially reasonable terms may be limited or non-existent; |
| • | our inability to realize the anticipated benefits of any acquisitions and divestitures, including as a result of difficulties integrating acquired businesses with, or disposing of divested businesses from, our current operations; |
| • | our ability to implement our strategies for improving growth; |
| • | a need to recognize impairment charges related to goodwill, identified intangible assets and fixed assets; |
| • | our inability to achieve some or all of the operational cost improvements and other benefits that we expect to realize; |
| • | our ability to operate according to our business strategy should our collaboration partners fail to fulfill their obligations; |
| • | risk that the insurance we maintain may not fully cover all potential exposures; |
24
Table of Contents
| • | product recalls or negative publicity may harm our reputation or market acceptance of our products; |
| • | decreases in the number of surgical procedures performed, and the resulting decrease in blood demand; |
| • | fluctuations in our cash flows as a result of our reagent rental model; |
| • | terrorist acts, conflicts, wars and natural disasters that may materially adversely affect our business, financial condition and results of operations; |
| • | the outcome of legal proceedings instituted against us and/or others; |
| • | risks associated with our non-U.S. operations that are not present in the United States; |
| • | currency translation risk and currency transaction risk; |
| • | the impact of possible new tariffs; |
| • | the effect of the United Kingdom’s withdrawal from the European Union; |
| • | our inability to deliver products and services that meet customers’ needs and expectations; |
| • | failure to maintain a high level of confidence in our products; |
| • | significant changes in the healthcare industry and related industries that we serve, in an effort to reduce costs; |
| • | reductions in government funding and reimbursement to our customers; |
| • | price increases or interruptions in the supply of raw materials, components for our products, and products and services provided to us by certain key suppliers and manufacturers; |
| • | our ability to recruit and retain the experienced and skilled personnel we need to compete; |
| • | work stoppages, union negotiations, labor disputes and other matters associated with our labor force; |
| • | consolidation of our customer base and the formation of group purchasing organizations; |
| • | manufacturing problems or delays; |
| • | unexpected payments to any pension plans applicable to our employees; |
| • | our inability to obtain required clearances or approvals for our products; |
| • | results of clinical studies, which may be delayed or fail to demonstrate the safety and effectiveness of our products; |
| • | failure to comply with applicable regulations, which may result in significant costs or the suspension or withdrawal of previously obtained clearances or approvals; |
| • | the inability of government agencies to hire, retain or deploy personnel or otherwise prevent new or modified products from being developed, cleared or approved or commercialized in a timely manner; |
| • | disruptions resulting from President Biden’s invocation of the Defense Production Act; |
| • | costs to comply with environmental and health and safety requirements, or costs related to liability for contamination or other potential environmental harm; |
| • | healthcare fraud and abuse regulations that could result in liability, require us to change our business practices and restrict our operations in the future; |
| • | failure to comply with the anti-corruption laws of the United States and various international jurisdictions; |
25
Table of Contents
| • | failure to comply with anti-terrorism laws and regulations and applicable trade embargoes; |
| • | failure to comply with the requirements of federal and state laws pertaining to the privacy and security of health information; |
| • | our inability to maintain our data management and information technology systems; |
| • | data corruption, cyber-based attacks, security breaches and privacy violations; |
| • | our inability to protect and enforce our intellectual property rights; |
| • | intellectual property infringement suits against us by third parties; |
| • | risks related to changes in income tax laws and regulations; |
| • | risks related to our substantial indebtedness; |
| • | risks related to this offering and ownership of our ordinary shares; |
| • | other factors disclosed or incorporated by reference in this prospectus; and |
| • | other factors beyond our control. |
These cautionary statements should not be construed by you to be exhaustive and speak only as of the date the statements are made. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. For further discussion of the risks relating to our business, see the section titled “Risk Factors” in the documents incorporated by reference herein.
26
Table of Contents
The selling shareholder is offering all of the ordinary shares in this offering, including any shares to be sold if the underwriters exercise their option to purchase additional shares, and will receive all of the net proceeds from the sale of ordinary shares in this offering. We are not offering any ordinary shares under this prospectus and will not receive any proceeds from the sale of ordinary shares by the selling shareholder.
27
Table of Contents
We currently do not expect to declare any dividends on our ordinary shares in the foreseeable future. Instead, we anticipate that all of our earnings in the foreseeable future will be used to provide working capital, support our operations, finance the growth and development of our business and reduce our net debt. Any determination to declare dividends in the future will be at the discretion of our board of directors, subject to applicable laws, and will be dependent on a number of factors, including our earnings, capital requirements and overall financial condition. We are controlled by the Principal Shareholder, who has the ability to appoint a majority of the members of our board of directors and therefore control the payment of dividends. In addition, because we are a holding company, our ability to pay dividends on our ordinary shares may be limited by restrictions on our ability to obtain sufficient funds through dividends from subsidiaries, including restrictions under the covenants of the Credit Agreement, the 2025 Notes Indenture and the 2028 Notes Indenture, and may be further restricted by the terms of any future debt or preferred securities. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and capital resources—Debt capitalization” in Ortho’s 2020 Form 10-K and Ortho’s 2021 Q2 Form 10-Q, which are incorporated by reference in this prospectus, for more information about our Senior Secured Credit Facilities and our Senior Notes.
28
Table of Contents
The following table sets forth our cash and cash equivalents and total capitalization as of July 4, 2021.
This table should be read in conjunction with the information presented under the caption “Prospectus Summary—Summary Consolidated Financial Data” in this prospectus, as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the unaudited interim financial statements and the related notes thereto included in Ortho’s 2021 Q2 Form 10-Q, which is incorporated by reference in this prospectus.
| As of July 4, 2021 | ||||
| ($ in millions) | ||||
| Cash and cash equivalents |
$ | 200.9 | ||
| Debt: |
||||
| Senior Secured Credit Facilities, consisting of the following(1): |
||||
| Dollar Term Loan Facility |
1,292.8 | |||
| Euro Term Loan Facility(2) |
380.9 | |||
| Revolving Credit Facility(3) |
— | |||
| 2028 Notes |
405.0 | |||
| 2025 Notes |
240.0 | |||
| Finance Leases and Other |
6.2 | |||
|
|
|
|||
| Total debt(4) |
$ | 2,324.9 | ||
|
|
|
|||
| Ortho Clinical Diagnostics Holdings plc shareholders’ equity: |
||||
| Ordinary shares, $0.00001 par value per ordinary share, 1,000,000,000 shares authorized, 234,921,002 shares issued and outstanding as of July 4, 2021 |
$ | — | ||
| Preferred redeemable shares, $1.39 nominal value per share, 50,000 shares issued and outstanding as of July 4, 2021 |
0.1 | |||
| Additional paid-in capital |
2,407.0 | |||
| Accumulated deficit |
(1,976.6 | ) | ||
| Accumulated other comprehensive loss |
(54.9 | ) | ||
|
|
|
|||
| Total shareholders’ equity |
$ | 375.6 | ||
|
|
|
|||
| Total capitalization |
$ | 2,700.5 | ||
|
|
|
|||
| (1) | The Senior Secured Credit Facilities consist of (a) the $500.0 million Revolving Credit Facility maturing on February 5, 2026, (b) the $2,375.0 million Dollar Term Loan Facility maturing on June 30, 2025 and (c) the €337.4 million Euro Term Loan Facility maturing on June 30, 2025. |
| (2) | Represents the aggregate dollar equivalent of our Euro Term Loan Facility as of July 4, 2021. |
| (3) | As of July 4, 2021, we had $466.8 million of capacity under the Revolving Credit Facility, net of $33.2 million of outstanding letters of credit. |
| (4) | Excludes debt discounts and debt issuance costs. |
29
Table of Contents
PRINCIPAL AND SELLING SHAREHOLDERS
The following table and accompanying footnotes set forth information with respect to the beneficial ownership of the ordinary shares of Ortho Clinical Diagnostics Holdings plc as of September 1, 2021 by:
| • | each person known by us to own beneficially 5% or more of our outstanding ordinary shares, including the selling shareholder; |
| • | each of our directors; |
| • | each of our named executive officers; and |
| • | our directors and executive officers as a group. |
The number of shares and percentages of beneficial ownership prior to this offering set forth below are based on the number of our ordinary shares to be issued and outstanding immediately prior to the consummation of this offering. The number of shares and percentages of beneficial ownership after this offering set forth below are based on the number of our ordinary shares to be issued and outstanding immediately after the consummation of this offering.
Beneficial ownership for the purposes of the following table is determined in accordance with the rules and regulations of the SEC. A person is a “beneficial owner” of a security if that person has or shares “voting power,” which includes the power to vote or to direct the voting of the security, or “investment power,” which includes the power to dispose of or to direct the disposition of the security or has the right to acquire such powers within 60 days.
30
Table of Contents
Unless otherwise noted in the footnotes to the following table, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to their beneficially owned ordinary shares.
Except as otherwise indicated in the footnotes below, the address of each beneficial owner is c/o Ortho Clinical Diagnostics Holdings plc, 1001 Route 202, Raritan, New Jersey 08869.
| Shares beneficially owned prior to the offering |
Shares beneficially owned after the offering | |||||||||||||||||||||||
| Name of beneficial owner |
If underwriters’ option to purchase additional ordinary shares is not exercised |
If underwriters’ option to purchase additional shares is exercised in full |
||||||||||||||||||||||
| Shares | Percentage | Shares | Percentage | Shares | Percentage | |||||||||||||||||||
| 5% Shareholders: |
||||||||||||||||||||||||
| Carlyle Investor(1) |
143,406,000 | 60.6 | % | 121,406,000 | 51.3 | % | 118,106,000 | 49.9 | % | |||||||||||||||
| Directors and Named Executive Officers: |
||||||||||||||||||||||||
| Christopher M. Smith(2) |
1,057,833 | * | 1,057,833 | * | 1,057,833 | * | ||||||||||||||||||
| Joseph M. Busky(3) |
66,392 | * | 66,392 | * | 66,392 | * | ||||||||||||||||||
| Chad Dale(4) |
— | * | — | * | — | * | ||||||||||||||||||
| Michael S. Iskra(5) |
261,849 | * | 261,849 | * | 261,849 | * | ||||||||||||||||||
| Chockalingam Palaniappan(6) |
53,113 | * | 53,113 | * | 53,113 | * | ||||||||||||||||||
| Michael A. Schlesinger(7) |
426,291 | * | 426,291 | * | 426,291 | * | ||||||||||||||||||
| Karen H. Bechtel(8) |
1,300 | * | 1,300 | * | 1,300 | * | ||||||||||||||||||
| Evelyn Dilsaver(9) |
1,300 | * | 1,300 | * | 1,300 | * | ||||||||||||||||||
| Allan Holt |
— | — | — | — | — | — | ||||||||||||||||||
| Carl Hull(10) |
60,233 | * | 60,233 | * | 60,233 | * | ||||||||||||||||||
| Ron Labrum(11) |
108,035 | * | 108,035 | * | 108,035 | * | ||||||||||||||||||
| Thomas Mac Mahon(12) |
139,903 | * | 139,903 | * | 139,903 | * | ||||||||||||||||||
| David Perez(13) |
6,626 | * | 6,626 | * | 6,626 | * | ||||||||||||||||||
| Robert R. Schmidt |
— | — | — | — | — | — | ||||||||||||||||||
| Stephen H. Wise |
— | — | — | — | — | — | ||||||||||||||||||
| Robert Yates(14) |
1,026,525 | * | 1,026,525 | * | 1,026,525 | * | ||||||||||||||||||
| All directors and executive officers as a group (16 persons)(15) |
3,209,400 | 1.3 | % | 3,209,400 | 1.3 | % | 3,209,400 | 1.3 | % | |||||||||||||||
| * | Indicates beneficial ownership of less than 1%. |
| (1) | Reflects ordinary shares held of record by Carlyle Partners VI Cayman Holdings, L.P. (the “Carlyle Investor”). Carlyle Group Management L.L.C. holds an irrevocable proxy to vote a majority of the shares of The Carlyle Group Inc. (“Carlyle”), a publicly traded company listed on Nasdaq. Carlyle is the sole member of Carlyle Holdings II GP L.L.C., which is the managing member of Carlyle Holdings II L.L.C., which, with respect to the securities reported herein, is the managing member of CG Subsidiary Holdings L.L.C., which is the general partner of TC Group Cayman Investment Holdings, L.P., which is the general partner of TC Group Cayman Investment Holdings Sub L.P., which is the sole member of TC Group VI Cayman, L.L.C., which is the general partner of TC Group VI Cayman, L.P., which is the general partner of the Carlyle Investor. Voting and investment determinations with respect to the ordinary shares held of record by the Carlyle Investor are made by an investment committee of TC Group VI Cayman, L.P. |
31
Table of Contents
| Accordingly, each of the foregoing entities may be deemed to share beneficial ownership of the securities held of record by the Carlyle Investor. Each of them disclaims beneficial ownership of such securities. The address for each of TC Group Cayman Investment Holdings, L.P., TC Group Cayman Investment Holdings Sub L.P., TC Group VI Cayman, L.P. and the Carlyle Investor is c/o Walkers Corporate Limited, 190 Elgin Avenue, George Town, Grand Cayman KY1-9008, Cayman Islands. The address of each of the other entities named in this footnote is c/o The Carlyle Group, 1001 Pennsylvania Avenue, NW, Suite 220 South, Washington, D.C. 20004. |
| (2) | Includes 690,473 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (3) | Includes 66,392 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (4) | As disclosed in our Current Report on Form 8-K filed with the SEC on May 3, 2021, on April 27, 2021, Mr. Dale resigned as Chief Operating Officer of the Company, effective May 7, 2021. |
| (5) | Includes 261,848 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (6) | Includes 53,113 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (7) | Includes 338,653 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (8) | Includes 650 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (9) | Includes 650 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (10) | Includes 41,172 ordinary shares held by Mr. Hull in his capacity as a Trustee of the Hull Living Trust and 325 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (11) | Includes 325 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (12) | Includes 325 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (13) | Includes 325 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (14) | Includes 1,025,875 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
| (15) | Includes 2,438,954 ordinary shares issuable upon the exercise of options exercisable within 60 days following September 1, 2021. |
32
Table of Contents
DESCRIPTION OF SHARE CAPITAL AND ARTICLES OF ASSOCIATION
General
We were incorporated as a public limited company under the laws of England and Wales with the legal name Ortho Clinical Diagnostics Holdings plc on December 16, 2020, with company number 13084624. Our registered office is Felindre Meadows, Pencoed, Bridgend, Mid Glamorgan, Wales, CF35 5PZ. The principal legislation under which we operate and our ordinary shares are issued is the U.K. Companies Act 2006.
Share Capital
As of July 4, 2021, our issued share capital was $2,349.21. The nominal value of our ordinary shares is $0.00001 per ordinary share. Each issued ordinary share is fully paid.
Options
As of July 4, 2021, there were options to purchase 15,857,759 ordinary shares outstanding with a weighted average exercise price of $9.76 per ordinary share.
Ordinary Shares
The following summarizes the rights of holders of our ordinary shares:
| • | each holder of our ordinary shares is entitled to one vote per ordinary share at a meeting of shareholders; |
| • | the holders of the ordinary shares shall be entitled to receive notice of, attend, speak, and vote at our general meetings; and |
| • | holders of our ordinary shares are entitled to receive such dividends as are recommended by our directors and declared by our shareholders. |
Registered Shares
We are required by the U.K. Companies Act 2006 to keep a register of our shareholders. Under English law, the ordinary shares are deemed to be issued when the name of the shareholder is entered in our share register. The share register therefore is prima facie evidence of the identity of our shareholders and the ordinary shares that they hold. The share register generally provides limited, or no, information regarding the ultimate beneficial owners of our ordinary shares. Our share register is maintained by our registrar, Computershare Trust Company N.A.
Under the U.K. Companies Act 2006, we must enter an allotment of ordinary shares in our share register as soon as practicable and in any event within two months of the allotment. We will perform all procedures necessary to update the share register to reflect the ordinary shares being sold in an offering. We also are required by the U.K. Companies Act 2006 to register a transfer of ordinary shares (or give the transferee notice of and reasons for refusal) as soon as practicable and in any event within two months of receiving notice of the transfer.
We, any of our shareholders or any other affected person may apply to the court for rectification of the share register if:
| • | the name of any person, without sufficient cause, is wrongly entered in or omitted from our register of members; or |
33
Table of Contents
| • | there is a default or unnecessary delay in entering on the register the fact of any person having ceased to be a member or on which we have a lien, provided that such refusal does not prevent dealings in the ordinary shares taking place on an open and proper basis. |
Preemptive Rights
English law generally provides shareholders with preemptive rights when new shares are issued for cash; however, it is possible for the articles of association, or shareholders in a general meeting, to exclude preemptive rights. Such an exclusion of preemptive rights may be for a maximum period of up to five years from the date of adoption of the articles of association, if the exclusion is contained in the articles of association, or from the date of the shareholder resolution, if the exclusion is by shareholder resolution. In either case, this exclusion would need to be renewed by our shareholders upon its expiration (i.e., at least every five years). In connection with our initial public offering, our shareholders approved the exclusion of preemptive rights for a period of five years from the date of the approval in respect of the allotment of up to a maximum amount of one billion ordinary shares, which exclusion will need to be renewed upon expiration (i.e., at least every five years) to remain effective, but may be sought more frequently for additional five-year terms (or any shorter period).
Articles of Association
The following is a description of our articles of association.
Shares and Rights Attaching to Them
Objects
The objects of our company are unrestricted.
Share Rights
Subject to any special rights attaching to shares already in issue, our shares may be issued with or have attached to them any rights or restrictions as we may resolve by ordinary resolution of the shareholders or so far as the resolution does not make specific provision, as the board may determine.
Voting Rights
Without prejudice to any special rights, privileges or restrictions as to voting rights attached to any shares forming part of our share capital from time to time, all votes at a general meeting shall be taken on a poll and each holder of the shares of the class shall, on a poll, have one vote in respect of every share of the class held by them.
Restrictions on Voting
No shareholder shall be entitled to vote at any general meeting in respect of any share held by him unless all sums payable by him in respect of that share have been paid.
The board may from time to time make calls upon the shareholders in respect of any money unpaid on their shares and each shareholder shall (subject to at least 14 days’ notice specifying when and how the payment is to be made) pay at the time or times so specified the amount called on his shares.
34
Table of Contents
Dividends
We may by ordinary resolution of the shareholders declare dividends out of profits available for distribution in accordance with the respective rights of shareholders but no such dividend shall exceed the amount recommended by the directors. The board may from time to time pay shareholders such interim dividends as appear to the board to be justified by our financial position but, if at any time, our share capital is divided into different classes the board may not pay such interim dividends in respect of those shares which confer on the holders thereof deferred or non-preferential rights with regard to dividends if, at the time of payment, any preferential dividend is in arrears.
Subject to any special rights attaching to or the terms of issue of any share, all dividends shall be declared and paid according to the amounts paid up on the ordinary shares and shall be apportioned and paid pro rata according to the amounts paid up on the ordinary shares during any part or parts of the period in respect of which the dividend is paid.
No dividend or other moneys payable by us on or in respect of any share shall bear interest against us unless otherwise provided by the rights attached to the share or the provisions of another agreement between the shareholder and us. Any dividend unclaimed after a period of 12 years from the date such dividend became due for payment shall be forfeited and cease to remain owing.
Dividends may be declared or paid in any currency and the board may decide the rate of exchange for any currency conversions that may be required, and how any costs involved are to be met, in relation to the currency of any dividend.
Any general meeting declaring a dividend may by ordinary resolution of shareholders, upon the recommendation of the board, direct payment or satisfaction of such dividend wholly or in part by the distribution of non-cash assets of equivalent value, including shares or other securities in any company.
The directors may, if authorized by an ordinary resolution of shareholders, offer any holders of ordinary shares the right to elect to receive in lieu of a dividend, or part of a dividend, an allotment of ordinary shares credited as fully paid up.
Change of Control
Our articles of association contain provisions that are intended to enhance the likelihood of continuity and stability in the composition of our board of directors. These provisions are intended to avoid costly takeover battles, reduce our vulnerability to a hostile change of control and enhance the ability of our board of directors to maximize shareholder value in connection with any unsolicited offer to acquire us. However, these provisions may have the effect of delaying, deterring or preventing a merger or acquisition of our company by means of a tender offer, a proxy contest or other takeover attempt that a shareholder might consider in its best interest, including attempts that might result in a premium over the prevailing market price for the ordinary shares held by shareholders.
Distributions on Winding Up
If we are in liquidation, the liquidator may, if authorized by a special resolution of shareholders and any other authority required at law, divide among shareholders (excluding us to the extent we are a shareholder by virtue only of holding treasury shares) in specie or in kind the whole or any part of our assets (whether or not the assets consist of property of one kind or consist of properties of different kinds and the liquidator may for such purpose set such value as the liquidator deems fair upon any one or more class or classes of property and may determine how such division shall be carried out as
35
Table of Contents
between the shareholders or different classes of shareholders), or vest the whole or any part of such assets in trustees upon such trusts for the benefit of the shareholders as the liquidator determines (and the liquidation of the Company may be closed and the Company dissolved), but no shareholder shall be compelled to accept any shares or other assets upon which there is any liability or potential liability.
Variation of Rights
All or any of the rights and privileges attached to any class of shares issued may be varied or abrogated only with the consent in writing of the holders of not less than three-fourths in nominal value of the issued shares of that class (excluding any shares held as treasury shares) or by special resolution passed at a separate general meeting of the holders of such shares, subject to the other provisions of the U.K. Companies Act 2006 and the terms of their issue. The U.K. Companies Act 2006 also provides a right to object to the variation of the share capital by the shareholders who did not vote in favor of the variation. Should 15% or more of the shareholders of the issued shares in question apply to the court to have the variation cancelled, the variation shall have no effect unless and until it is confirmed by the court.
Alteration to Share Capital
We may, by ordinary resolution of shareholders, consolidate all or any of our share capital into shares of larger amount than our existing shares, or sub-divide our shares or any of them into shares of a smaller amount. We may, by special resolution of shareholders, confirmed by the court, reduce our share capital or any capital redemption reserve or any share premium account in any manner authorized by the U.K. Companies Act 2006. We may redeem or purchase all or any of our shares as described in “—Other U.K. Law Considerations—Purchase of own shares.”
Preemption Rights
In certain circumstances, our shareholders may have statutory preemption rights under the U.K. Companies Act 2006 in respect of the allotment of new shares as described in “—Preemptive Rights” and “—Differences in Corporate Law—Pre-emptive rights” in this prospectus.
Transfer of Shares
Any shareholder holding shares in certificated form may transfer all or any of his shares by an instrument of transfer in any usual form or any other form approved by the board. Any written instrument of transfer shall be signed by or on behalf of the transferor and (in the case of a partly paid share) the transferee.
In the case of uncertificated shares, the directors may take such action as they consider appropriate to achieve a transfer. The Uncertificated Securities Regulations 2001 permit shares to be issued and held in uncertificated form and transferred by means of a computer based system.
The board may decline to register any transfer of any share:
| • | which is not a fully paid share; |
| • | where the transfer is not lodged at our registered office or such other place as the directors have appointed; |
| • | where the transfer is not accompanied by the share certificate to which it relates, or such other evidence as the board may reasonably require to show the transferor’s right to make the transfer, or evidence of the right of someone other than the transferor to make the transfer on the transferor’s behalf; |
36
Table of Contents
| • | where the transfer is in respect of more than one class of share; and |
| • | where the number of joint holders to whom the share is to be transferred exceeds four. |
If the board declines to register a transfer, it must return to the transferee the instrument of transfer together with notice of the refusal, unless the board suspects that the proposed transfer may be fraudulent.
Shareholder Meetings
Annual General Meetings
In accordance with the U.K. Companies Act 2006, we are required in each year to hold an annual general meeting in addition to any other general meetings in that year and to specify the meeting as such in the notice convening it. The annual general meeting shall be convened whenever and wherever the board sees fit, subject to the requirements of the U.K. Companies Act 2006, as described in “—Differences in Corporate Law—Annual general meeting” and “—Differences in Corporate Law—Notice of general meetings” in this prospectus.
Notice of General Meetings
The arrangements for the calling of general meetings are described in “—Differences in Corporate Law—Notice of general meetings” in this prospectus.
Quorum of General Meetings
No business shall be transacted at any general meeting unless a quorum is present. At least two shareholders present in person or by proxy and entitled to vote shall be a quorum for all purposes.
Class Meetings
The provisions in our articles of association relating to general meetings apply to every separate general meeting of the holders of a class of shares.
Directors
Number of Directors
We may not have less than two directors on the board of directors and not more than fifteen.
Appointment of Directors
Subject to the provisions of our articles of association, we may, by ordinary resolution of the shareholders or a decision of the directors, elect any person to be a director, either to fill a casual vacancy or as an addition to the existing board, provided the total number of directors does not exceed the maximum number fixed by or in accordance with the articles of association, provided, further, that a vacancy caused by the departure of a Principal Shareholder appointee may only be filled by the Principal Shareholder. However, any person that is not a director retiring from the existing board must be recommended by the board or the person must have confirmed in writing to the Company their willingness to be elected as a director.
Our articles of association provide for a staggered board consisting of three classes of directors. Directors of each class are chosen for three-year terms, with only one class of directors being elected
37
Table of Contents
at each annual meeting of shareholders. At the first annual meeting of shareholders, the terms of the Class I directors (other than a Principal Shareholder appointee who is a Class I director) will expire and Class I directors (other than a Principal Shareholder appointee who is a Class I director) will be elected for a full term of three years. At the second annual meeting of shareholders, the terms of the Class II directors (other than a Principal Shareholder appointee who is a Class II director) will expire and Class II directors (other than a Principal Shareholder appointee who is a Class II director) will be elected for a full term of three years. At the third annual meeting of shareholders, the terms of the Class III directors (other than a Principal Shareholder appointee who is a Class III director) will expire and Class III directors (other than a Principal Shareholder appointee who is a Class III director) will be elected for a full term of three years. At each succeeding annual meeting, directors (other than Principal Shareholder appointees) will be elected for a full term of 3 years to succeed the directors of the class whose terms expire at such annual general meeting.
Directors’ Interests
If a situation arises in which a director has, or can have, a direct or indirect interest that conflicts, or possibly may conflict, with our interests (other than a situation that cannot reasonably be regarded as likely to give rise to a conflict of interest or a conflict of interest arising in relation to a transaction or arrangement with the Company), the board may authorize in accordance with the U.K. Companies Act 2006 the director’s interest and the continuing performance by the relevant director of his duties as a director on such terms as the board may determine.
A director shall not be accountable to us for any benefit which he derives from or in connection with a relationship involving a conflict of interest or possible conflict of interest which has been authorized by the directors or by the Company in a general meeting and any such transaction or arrangement shall not be liable to be avoided on the grounds of any such benefit.
Subject to the requirements under sections 175, 177 and 182 of the U.K. Companies Act 2006, a director shall declare the nature and extent of such conflicts.
A director may participate in the decision-making process and count in the quorum and vote on a proposed decision of the board which is concerned with such director’s interests (subject to any restrictions imposed by the other directors when providing such consent) if such director has declared the nature and extent of any interest of his and provided a majority of the other directors consent, or if one of the following situations applies:
| • | the director’s interest arises solely through an interest in shares, debentures or other securities of or otherwise in or through the Company; |
| • | an ordinary resolution of the Company permits the director to count in the quorum and vote on the proposed decision; |
| • | the conflict of interest arises from one of the following: |
| • | the giving of a guarantee, security or indemnity in respect of money lent or obligations incurred by them or any other person at the request of or for the benefit of, the Company or any of its subsidiary undertakings; |
| • | the giving of a guarantee, security or indemnity in respect of a debt or obligation of the Company or any of its subsidiary undertakings for which the director has assumed responsibility (in whole or part and whether alone or jointly with others) under a guarantee or indemnity or by the giving of security; |
| • | a contract, arrangement, transaction or proposal concerning an offer of shares, debentures or other securities of the Company or any of its subsidiary undertakings for subscription or |
38
Table of Contents
| purchase, in which offer they are or may be entitled to participate as a holder of securities or in the underwriting or sub-underwriting of which they are to participate; |
| • | proposals concerning another company in which the director is interested directly or indirectly (whether as officer, shareholder or otherwise), if the director and any other persons connected with him do not to his knowledge hold an interest in shares representing 1% or more of the issued shares of any class of the equity share capital of that company (or of any third company through which his or their interest is derived) or of the voting rights available to shareholders of the relevant company; |
| • | a contract, arrangement, transaction or proposal for the benefit of employees of the Company or of any of its subsidiary undertakings which does not award them any privilege or benefit not generally accorded to the employees to whom the arrangement relates; or |
| • | a contract, arrangement, transaction or proposal concerning any insurance which the Company is empowered to purchase or maintain for, or for the benefit of, and directors of the Company or for persons who include directors of the Company. |
A director shall not be counted in the quorum present at a meeting in relation to a resolution on which he is not entitled to vote.
If a question arises at a meeting of the board or of a committee of the board as to the right of a director to vote, the question may, before the conclusion of the meeting, be referred to the chair of the meeting and their ruling in relation to any director other than himself shall be final and conclusive, save where the nature of the interests of the direct have been fully disclosed.
Directors’ Fees and Remuneration
Each of the directors is entitled to remuneration as determined by the board for their service as directors and other services undertaken for the Company.
Each director may be paid his expenses in connection with such director’s attendance at meetings of the board or committees of the board or general meetings or separate meetings of the holders class of shares or of debentures, or otherwise in connection with the exercise of powers and the discharge of responsibilities in relation to the Company.
Indemnity
Every director, officer or former director or officer of our group may be indemnified against all costs, charges, losses, expenses and liabilities incurred by him in connection with any negligence, default, breach of duty, or breach of trust by him in relation to us or in connection with our activities as a trustee of an occupational pension scheme, in the actual or purported exercise of his powers or duties or otherwise as our officer, to the extent permitted under the U.K. Companies Act 2006.
Other U.K. Law Considerations
Mandatory Purchases and Acquisitions
Pursuant to Sections 979 to 991 of the U.K. Companies Act 2006, where a takeover offer has been made for us and the offeror has acquired or unconditionally contracted to acquire not less than 90% in value of the shares to which the offer relates and not less than 90% of the voting rights carried by those shares, the offeror may give notice to the holder of any shares to which the offer relates which the offeror has not acquired or unconditionally contracted to acquire that he wishes to acquire, and is
39
Table of Contents
entitled to so acquire, those shares on the same terms as the general offer. The offeror would do so by sending a notice to the outstanding minority shareholders telling them that it will compulsorily acquire their shares. Such notice must be sent within three months of the last day on which the offer can be accepted in the prescribed manner. The squeeze-out of the minority shareholders can be completed at the end of six weeks from the date the notice has been given, subject to the minority shareholders failing to successfully lodge an application to the court to prevent such squeeze-out any time prior to the end of those six weeks following which the offeror can execute a transfer of the outstanding shares in its favor and pay the consideration to us, which would hold the consideration on trust for the outstanding minority shareholders. The consideration offered to the outstanding minority shareholders whose shares are compulsorily acquired under the U.K. Companies Act 2006 must, in general, be the same as the consideration that was available under the takeover offer.
Sell Out
The U.K. Companies Act 2006 also gives our minority shareholders a right to be bought out in certain circumstances by an offeror who has made a takeover offer for all of our shares. The holder of shares to which the offer relates, and who has not otherwise accepted the offer, may require the offeror to acquire his shares if, prior to the expiry of the acceptance period for such offer, (i) the offeror has acquired or unconditionally agreed to acquire not less than 90% in value of the voting shares, and (ii) not less than 90% of the voting rights carried by those shares. The offeror may impose a time limit on the rights of minority shareholders to be bought out that is not less than three months after the end of the acceptance period. If a shareholder exercises his rights to be bought out, the offeror is required to acquire those shares on the terms of this offer or on such other terms as may be agreed.
Disclosure of Interest in Shares
Pursuant to Part 22 of the U.K. Companies Act 2006, we are empowered to give notice in writing to any person whom we know or have reasonable cause to believe to be interested in our shares, or to have been so interested at any time during the three years immediately preceding the date on which the notice is issued requiring such persons, within a reasonable time to disclose to us particulars of that person’s interest and (so far as is within his knowledge) particulars of any other interest that subsists or subsisted in those shares.
Under our articles of association, if a person defaults in supplying us with the required particulars in relation to the ordinary shares in question, or default shares, within the prescribed period, the directors may by notice direct that:
| • | in respect of the default shares, the relevant shareholder shall not be entitled to attend or vote (either in person or by proxy) at a general meeting or at a separate meeting of the holders of that class of shares or on a poll; |
| • | in respect of the default shares: |
| • | no payment shall be made by way of dividend and no share shall be allotted pursuant to scrip dividend; |
| • | no transfer of any default share shall be registered unless: |
(A) the shareholder is not himself in default as regards to supplying the information requested and the transfer when presented for registration is accompanied by a certificate by the shareholder in such form as the board may in its absolute discretion require to the effect that after due and careful enquiry the shareholder is satisfied that no person in default as regards supplying such information is interested in any of the shares the subject of the transfer; or
(B) the transfer is an approved transfer.
40
Table of Contents
Purchase of Own Shares
Under English law, a limited company may only purchase its own shares out of the distributable profits of the company or the proceeds of a fresh issue of shares made for the purpose of financing the purchase, provided that they are not restricted from doing so by their articles. A limited company may not purchase its own shares if, as a result of the purchase, there would no longer be any issued shares of the company other than redeemable shares or shares held as treasury shares. Shares must be fully paid in order to be repurchased.
We may purchase our own fully paid shares otherwise than on a recognized investment exchange pursuant to a purchase contract authorized by resolution of shareholders before the purchase takes place. Any authority will not be effective if any shareholder from whom we propose to purchase shares votes on the resolution and the resolution would not have been passed if he had not done so. The resolution authorizing the purchase must specify a date, not being later than five years after the passing of the resolution, on which the authority to purchase is to expire.
Distributions and Dividends
Under the U.K. Companies Act 2006, before a company can lawfully make a distribution or dividend, it must ensure that it has sufficient distributable reserves (on a non-consolidated basis). The basic rule is that a company’s profits available for the purpose of making a distribution are its accumulated, realized profits, so far as not previously utilized by distribution or capitalization, less its accumulated, realized losses, so far as not previously written off in a reduction or reorganization of capital duly made. The requirement to have sufficient distributable reserves before a distribution or dividend can be paid applies to us and to each of our subsidiaries that has been incorporated under English law.
It is not sufficient that we, as a public company, have made a distributable profit for the purpose of making a distribution. An additional capital maintenance requirement is imposed on us to ensure that the net worth of the company is at least equal to the amount of its capital. A public company can only make a distribution:
| • | if, at the time that the distribution is made, the amount of its net assets (that is, the total excess of assets over liabilities) is not less than the total of its called up share capital and distributable reserves; and |
| • | if, and to the extent that, the distribution itself, at the time that it is made, does not reduce the amount of the net assets to less than that total. |
On May 5, 2021, we capitalized $2,643,998,527 standing to the credit of our merger reserve by paying up the nominal value in full of 264,399,852,700 deferred shares (the “Capital Reduction Shares”), each with a nominal value of $0.01, and an aggregate nominal value of $2,643,998,527. On May 7, 2021, we applied to the High Court of Justice in England and Wales (the “Court”) seeking the Court’s approval for (i) the cancellation of the Capital Reduction Shares and the reduction of our share capital accordingly and (ii) the cancellation of $1,417,569,531, being the amount standing to credit in our share premium account as of June 14, 2021 (the “Capital Reduction”). The Capital Reduction was approved by the Court and took legal effect on June 15, 2021. The purpose of the Capital Reduction was to create distributable reserves in the amount of $4,061,568,058 in our books of account.
Compromises and Arrangements
Where we and our creditors or shareholders or a class of either of them propose a compromise or arrangement between us and our creditors or our shareholders or a class of either of them (as
41
Table of Contents
applicable), the High Court of Justice in England and Wales may order a meeting of the creditors or class of creditors or of our shareholders or class of shareholders (as applicable) to be called in such a manner as the court directs. Any compromise or arrangement approved by a majority in number present and voting at the meeting representing 75% or more in value of the creditors or 75% or more of the voting rights of shareholders or class of either of them (as applicable) if sanctioned by the court, is binding upon us and all the creditors, shareholders or members of the specific class of either of them (as applicable).
Whether the capital of the Company is to be treated as being divided into a single or multiple class(es) of shares is a matter to be determined by the court. The court may in its discretion treat a single class of shares as multiple classes, or multiple classes of shares as a single class, for the purposes of the shareholder approval referred to above taking into account all relevant circumstances, which may include circumstances other than the rights attaching to the shares themselves.
City Code on Takeovers and Mergers
The U.K. City Code on Takeovers and Mergers (the “Takeover Code”) applies, among other things, to an offer for a public company whose registered office is in the United Kingdom and which is considered by the Panel on Takeovers and Mergers (the “Takeover Panel”), to have its place of central management and control in the United Kingdom, the Channel Islands or the Isle of Man (in each case, a “Code Company”). This is known as the “residency test.” Under the Takeover Code, the Takeover Panel will determine whether we have our place of central management and control in the United Kingdom, the Channel Islands or the Isle of Man by looking at various factors, including the structure of our Board, the functions of the directors, and where they are resident.
If at the time of a takeover offer, the Takeover Panel determines that the residency test is satisfied and we have our place of central management and control in the United Kingdom, we would be subject to several rules and restrictions, including but not limited to the following: (i) our ability to enter into deal protection arrangements with a bidder would be extremely limited; (ii) we might not, without the approval of our shareholders, be able to perform certain actions that could have the effect of frustrating an offer, such as issuing shares or carrying out acquisitions or disposals; and (iii) we would be obliged to provide equality of information to all bona fide competing bidders. The Takeover Code also contains certain rules in respect of mandatory offers for Code Companies. Under Rule 9 of the Takeover Code, if a person:
| • | acquires an interest in shares of a Code Company that, when taken together with shares in which persons acting in concert with such person are interested, carry 30% or more of the voting rights of the Code Company; or |
| • | who, together with persons acting in concert with such person, is interested in shares that in the aggregate carry not less than 30% and not more than 50% of the voting rights in the Code Company, acquires additional interests in shares that increase the percentage of shares carrying voting rights in which that person is interested, the acquirer, and, depending on the circumstances, its concert parties, would be required (except with the consent of the Takeover Panel) to make a cash offer (or provide a cash alternative) for the Code Company’s outstanding shares at a price not less than the highest price paid for any interests in the shares by the acquirer or its concert parties during the previous 12 months. |
A majority of our Board currently resides outside of the United Kingdom, the Channel Islands and the Isle of Man. Based upon our current and intended plans for our directors and management, for the purposes of the Takeover Code, we anticipate that the residency test will not be met and that we will be considered to have our place of central management and control outside the United Kingdom, the Channel Islands or the Isle of Man. Therefore, the Takeover Code should not apply to us. It is possible
42
Table of Contents
that in the future changes in the Board’s composition, changes in the Takeover Panel’s interpretation of the Takeover Code, or other events may cause the Takeover Code to apply to us.
Exchange Controls
There are no governmental laws, decrees, regulations or other legislation in the United Kingdom that may affect the import or export of capital, including the availability of cash and cash equivalents for use by us, or that may affect the remittance of dividends, interest, or other payments by us to non-resident holders of our ordinary shares, other than withholding tax requirements. There is no limitation imposed by English law or in our articles of association on the right of non-residents to hold or vote shares.
Differences in Corporate Law
The applicable provisions of the U.K. Companies Act 2006 differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of certain differences between the provisions of the U.K. Companies Act 2006 applicable to us and the General Corporation Law of the State of Delaware relating to shareholders’ rights and protections.
| England and Wales |
Delaware | |||
| Number of directors |
Under the U.K. Companies Act 2006, a public limited company must have at least two directors and the number of directors may be fixed by or in the manner provided in a company’s articles of association. | Under Delaware law, a corporation must have at least one director and the number of directors shall be fixed by or in the manner provided in the bylaws. | ||
| Removal of directors |
Under the U.K. Companies Act 2006, shareholders may remove a director without cause by an ordinary resolution (which is passed by a simple majority of those voting in person or by proxy at a general meeting) irrespective of any provisions of any service contract the director has with the company, provided 28 clear days’ notice of the resolution has been given to the company and its shareholders. On receipt of notice of an intended resolution to remove a director, the company must forthwith send a copy of the notice to the director concerned. Certain other procedural requirements under the U.K. Companies Act 2006 must also be followed such as allowing the director to make representations against his or her removal either at the meeting or in writing. | Under Delaware law, any director or the entire board of directors may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors, except (a) unless the certificate of incorporation provides otherwise, in the case of a corporation whose board of directors is classified, shareholders may effect such removal only for cause, or (b) in the case of a corporation having cumulative voting, if less than the entire board of directors is to be removed, no director may be removed without cause if the votes cast against his removal would be sufficient to elect him if then cumulatively voted at an election of the entire board of directors, or, if there are classes of directors, at an election of the class of directors of which he is a part. | ||
43
Table of Contents
| England and Wales |
Delaware | |||
| Vacancies on the board of directors |
Under English law, the procedure by which directors, other than a company’s initial directors, are appointed is generally set out in a company’s articles of association, provided that where two or more persons are appointed as directors of a public limited company by resolution of the shareholders, resolutions appointing each director must be voted on individually. | Under Delaware law, vacancies and newly created directorships may be filled by a majority of the directors then in office (even though less than a quorum) or by a sole remaining director unless (a) otherwise provided in the certificate of incorporation or by-laws of the corporation or (b) the certificate of incorporation directs that a particular class of stock is to elect such director, in which case a majority of the other directors elected by such class, or a sole remaining director elected by such class, will fill such vacancy. | ||
| Annual general meeting |
Under the U.K. Companies Act 2006, a public limited company must hold an annual general meeting in each six-month period following the company’s annual accounting reference date. | Under Delaware law, the annual meeting of shareholders shall be held at such place, on such date and at such time as may be designated from time to time by the board of directors or as provided in the certificate of incorporation or by the bylaws. | ||
| General meeting |
Under the U.K. Companies Act 2006, a general meeting of the shareholders of a public limited company may be called by the directors.
Shareholders holding at least 5% of the paid-up capital of the company carrying voting rights at general meetings (excluding any paid up capital held as treasury shares) can require the directors to call a general meeting and, if the directors fail to do so within a certain period, may themselves convene a general meeting. |
Under Delaware law, special meetings of the shareholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws. | ||
| Notice of general meetings |
Under the U.K. Companies Act 2006, 21 clear days’ notice must be given for an annual general meeting and any resolutions to be proposed at the meeting.
Subject to a company’s articles of association providing for a longer period, at least 14 clear days’ |
Under Delaware law, unless otherwise provided in the certificate of incorporation or bylaws, written notice of any meeting of the shareholders must be given to each shareholder entitled to vote at the meeting not less than ten nor more than 60 days before the date of the meeting and shall specify the | ||
44
Table of Contents
| England and Wales |
Delaware | |||
| notice is required for any other general meeting. In addition, certain matters, such as the removal of directors or auditors, require special notice, which is 28 clear days’ notice. The shareholders of a company may in all cases consent to a shorter notice period, the proportion of shareholders’ consent required being 100% of those entitled to attend and vote in the case of an annual general meeting and, in the case of any other general meeting, a majority in number of the members having a right to attend and vote at the meeting, being a majority who together hold not less than 95% in nominal value of the shares giving a right to attend and vote at the meeting. | place, date, hour, and purpose or purposes of the meeting. | |||
| Pre-emptive rights |
Under the U.K. Companies Act 2006, “equity securities”, being (i) shares in the company other than shares that, with respect to dividends and capital, carry a right to participate only up to a specified amount in a distribution (“ordinary shares”) or (ii) rights to subscribe for, or to convert securities into, ordinary shares, proposed to be allotted for cash must be offered first to the existing equity shareholders in the company in proportion to the respective nominal value of their holdings, unless an exception applies or a special resolution to the contrary has been passed by shareholders in a general meeting or the articles of association provide otherwise in each case in accordance with the provisions of the U.K. Companies Act 2006. | Under Delaware law, shareholders have no preemptive rights to subscribe to additional issues of stock or to any security convertible into such stock unless, and except to the extent that, such rights are expressly provided for in the certificate of incorporation. | ||
| Authority to allot |
Under the U.K. Companies Act 2006, the directors of a company must not allot shares or grant of rights to subscribe for or to convert any security into shares unless an | Under Delaware law, if the corporation’s charter or certificate of incorporation so provides, the board of directors has the power to authorize the issuance of stock. It | ||
45
Table of Contents
| England and Wales |
Delaware | |||
| exception applies or an ordinary resolution to the contrary has been passed by shareholders in a general meeting or the articles of association provide otherwise in each case in accordance with the provisions of the U.K. Companies Act 2006. | may authorize capital stock to be issued for consideration consisting of cash, any tangible or intangible property or any benefit to the corporation or any combination thereof. It may determine the amount of such consideration by approving a formula. In the absence of actual fraud in the transaction, the judgment of the directors as to the value of such consideration is conclusive. | |||
| Liability of directors and officers |
Under the U.K. Companies Act 2006, any provision, whether contained in a company’s articles of association or any contract or otherwise, that purports to exempt a director of a company, to any extent, from any liability that would otherwise attach to him in connection with any negligence, default, breach of duty or breach of trust in relation to the company is void.
Any provision by which a company directly or indirectly provides an indemnity, to any extent, for a director of the company or of an associated company against any liability attaching to him in connection with any negligence, default, breach of duty or breach of trust in relation to the company of which he is a director is also void except as permitted by the U.K. Companies Act 2006, which provides exceptions for the company to (a) purchase and maintain insurance against such liability; (b) provide a “qualifying third party indemnity” (being an indemnity against liability incurred by the director to a person other than the company or an associated company or criminal proceedings in which he is convicted); and (c) provide a “qualifying pension scheme indemnity” (being an indemnity against liability incurred in connection with the company’s |
Under Delaware law, a corporation’s certificate of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation and its shareholders for damages arising from a breach of fiduciary duty as a director. However, no provision can limit the liability of a director for:
• any breach of the director’s duty of loyalty to the corporation or its shareholders;
• acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law;
• intentional or negligent payment of unlawful dividends or stock purchases or redemptions; or
• any transaction from which the director derives an improper personal benefit. | ||
46
Table of Contents
| England and Wales |
Delaware | |||
| activities as trustee of an occupational pension plan). | ||||
| Voting rights |
Under English law, unless a poll is demanded by the shareholders of a company or is required by the chairman of the meeting or the company’s articles of association, shareholders shall vote on all resolutions on a show of hands. Under the U.K. Companies Act 2006, a poll may be demanded by (a) not fewer than five shareholders having the right to vote on the resolution; (b) any shareholder(s) representing not less than 10% of the total voting rights of all the shareholders having the right to vote on the resolution (excluding any voting rights attaching to treasury shares); or (c) any shareholder(s) holding shares in the company conferring a right to vote on the resolution (excluding any voting rights attaching to treasury shares) being shares on which an aggregate sum has been paid up equal to not less than 10% of the total sum paid up on all the shares conferring that right. A company’s articles of association may provide more extensive rights for shareholders to call a poll.
Under English law, an ordinary resolution is passed on a show of hands if it is approved by a simple majority (more than 50%) of the votes cast by shareholders present (in person or by proxy) and entitled to vote. If a poll is demanded, an ordinary resolution is passed if it is approved by holders representing a simple majority of the total voting rights of shareholders present, in person or by proxy, who, being entitled to vote, vote on the resolution. Special resolutions require the affirmative vote of not less than 75% of the votes cast by shareholders present (in person or by proxy) at the meeting. If a poll is demanded, a special resolution is passed if it is approved by holders |
Delaware law provides that, unless otherwise provided in the certificate of incorporation, each shareholder is entitled to one vote for each share of capital stock held by such shareholder.
Generally, under Delaware law, unless the certificate of incorporation provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or substantially all of a corporation’s assets or dissolution requires:
• the approval of the board of directors; and
• approval by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per ordinary share, a majority of the votes of the outstanding stock of a corporation entitled to vote on the matter. | ||
47
Table of Contents
| England and Wales |
Delaware | |||
| representing not less than 75% of the total voting rights of shareholders present (in person or by proxy) who (being entitled to vote) vote on the resolutions. The U.K. Companies Act 2006 provides for schemes of arrangement, which are arrangements or compromises between a company and any class of shareholders or creditors and used in certain types of reconstructions, amalgamations, capital reorganizations, or takeovers. These arrangements require:
• the approval at a shareholders’ or creditors’ meeting convened by order of the court, of a majority in number of shareholders or creditors representing 75% in value of the capital held by, or debt owed to, the class of shareholders or creditors, or class thereof present and voting, either in person or by proxy; and
• the approval of the court. |
||||
| Standard of conduct for directors |
Under English law, a director owes various statutory and fiduciary duties to the company, including:
• to act in the way he considers, in good faith, would be most likely to promote the success of the company for the benefit of its members as a whole;
• to avoid a situation in which he has, or can have, a direct or indirect interest that conflicts, or possibly conflicts, with the interests of the company;
• to act in accordance with the company’s constitution and only exercise his powers for the purposes for which they are conferred; |
Delaware law does not contain specific provisions setting forth the standard of conduct of a director. The scope of the fiduciary duties of directors is generally determined by the courts of the State of Delaware. In general, directors have a duty to act without self-interest, on a well-informed basis and in a manner they reasonably believe to be in the best interest of the shareholders.
Directors of a Delaware corporation owe fiduciary duties of care and loyalty to the corporation and to its shareholders. The duty of care generally requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar | ||
48
Table of Contents
| England and Wales |
Delaware | |||
|
• to exercise independent judgment;
• to exercise reasonable care, skill, and diligence;
• not to accept benefits from a third party conferred by reason of his being a director or doing, or not doing, anything as a director; and
• a duty to declare any interest that he has, whether directly or indirectly, in a proposed or existing transaction or arrangement with the company. |
circumstances. Under this duty, a director must inform himself of all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he reasonably believes to be in the best interests of the corporation. He must not use his corporate position for personal gain or advantage. In general, but subject to certain exceptions, actions of a director are presumed to have been made on an informed basis, in good faith and in the honest belief that the action taken was in the best interests of the corporation. However, this presumption may be rebutted by evidence of a breach of one of the fiduciary duties. Delaware courts have also imposed a heightened standard of conduct upon directors of a Delaware corporation who take any action designed to defeat a threatened change in control of the corporation.
In addition, under Delaware law, when the board of directors of a Delaware corporation approves the sale or break-up of a corporation, the board of directors may, in certain circumstances, have a duty to obtain the highest value reasonably available to the shareholders. | |||
| Shareholder suits |
Under English law, generally, the company, rather than its shareholders, is the proper claimant in an action in respect of a wrong done to the company or where there is an irregularity in the company’s internal management. Notwithstanding this general position, the U.K. Companies Act 2006 provides that (i) a court may allow a shareholder to bring a derivative claim (that is, an action in respect of and on behalf of the | Under Delaware law, a shareholder may initiate a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must:
• state that the plaintiff was a shareholder at the time of the transaction of which the plaintiff complains or that the plaintiffs shares thereafter devolved on the plaintiff by operation of law; and | ||
49
Table of Contents
| England and Wales |
Delaware | |||
| company) in respect of a cause of action arising from a director’s negligence, default, breach of duty or breach of trust and (ii) a shareholder may bring a claim for a court order where the company’s affairs have been or are being conducted in a manner that is unfairly prejudicial to some of its shareholders. |
• allege with particularity the efforts made by the plaintiff to obtain the action the plaintiff desires from the directors and the reasons for the plaintiff’s failure to obtain the action; or
• state the reasons for not making the effort.
Additionally, the plaintiff must remain a shareholder through the duration of the derivative suit. The action will not be dismissed or compromised without the approval of the Delaware Court of Chancery. |
Transfer Agent and Registrar
The transfer agent and registrar for our ordinary shares is Computershare Trust Company N.A.
Listing
Our ordinary shares currently trade on Nasdaq under the symbol “OCDX.”
50
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
General
We cannot predict what effect, if any, market sales of ordinary shares or the availability of ordinary shares for sale will have on the market price of our ordinary shares prevailing from time to time. Nevertheless, sales of substantial amounts of ordinary shares, including shares issued upon the exercise of outstanding options, in the public market, or the perception that such sales could occur, could materially and adversely affect the market price of our ordinary shares and could impair our future ability to raise capital through the sale of our equity or equity-related securities at a time and price that we deem appropriate. See “Risk Factors—Risks Related to this Offering and Ownership of Our Ordinary Shares—Future sales, or the perception of future sales, by us or our existing shareholders in the public market following this offering could cause the market price for our ordinary shares to decline.”
As of July 4, 2021, we had 234,921,002 ordinary shares outstanding. All shares sold in this offering will be freely tradable without registration under the Securities Act and without restriction, except for shares held by our “affiliates” (as defined under Rule 144). The ordinary shares held by the Principal Shareholder and certain of our directors, officers, employees and other shareholders after this offering will be “restricted” securities under the meaning of Rule 144 and may not be sold in the absence of registration under the Securities Act, unless an exemption from registration is available, including the exemptions pursuant to Rule 144 under the Securities Act.
Pursuant to Rule 144, the restricted shares held by our affiliates and other shareholders will be available for sale in the public market at various times after the date of this prospectus following the expiration of the applicable lock-up period.
In addition, as of July 4, 2021, a total of 7,864,810 our ordinary shares have been reserved for issuance under the 2021 Incentive Award Plan (subject to adjustments for stock splits, stock dividends and similar events). The number of shares initially available for issuance will be increased by an annual increase on the first fiscal day of each calendar year beginning in 2022 and ending in and including 2031, equal to the least of (A) 3% of the ordinary shares outstanding on the final day of the immediately preceding calendar year and (B) a smaller number of shares determined by our board of directors. No more than 79,670,000 ordinary shares may be issued under the 2021 Plan upon the exercise of incentive stock options. Shares issued under the 2021 Plan may be authorized but unissued shares, shares purchased on the open market or treasury shares. We intend to file one or more registration statements on Form S-8 under the Securities Act to register ordinary shares issued or reserved for issuance under the 2021 Incentive Award Plan. Any such Form S-8 registration statement will automatically become effective upon filing. Accordingly, shares registered under such registration statement will be available for sale in the open market, unless such ordinary shares are subject to vesting or other contractual restrictions or the lock-up restrictions described below.
Rule 144
In general, under Rule 144, as currently in effect, a person (or persons whose shares are deemed aggregated) who is not deemed to be or have been one of our affiliates for purposes of the Securities Act at any time during 90 days preceding a sale and who has beneficially owned the ordinary shares proposed to be sold for at least six months, including the holding period of any prior owner other than an affiliate, is entitled to sell such ordinary shares without registration, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the ordinary shares proposed to be sold for at least one year, including the holding period of a prior owner other
51
Table of Contents
than an affiliate, then such person is entitled to sell such ordinary shares without complying with any of the requirements of Rule 144.
Under Rule 144, our affiliates or persons selling shares on behalf of our affiliates, who have met the six-month holding period for beneficial ownership of “restricted shares” of our ordinary shares, are entitled to sell within any three-month period, a number of shares that does not exceed the greater of:
| • | 1% of the number of our ordinary shares then outstanding, which will equal approximately 2,349,210 shares immediately after this offering; or |
| • | the average reported weekly trading volume of our ordinary shares on Nasdaq during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale. |
Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us. The sale of these shares, or the perception that sales will be made, could adversely affect the price of our ordinary shares after this offering because a great supply of shares would be, or would be perceived to be, available for sale in the public market.
Rule 701
In general, under Rule 701 of the Securities Act as currently in effect, any of our employees, consultants or advisors who received shares from us in connection with a compensatory stock or option plan or other written agreement in a transaction in reliance on Rule 701 that was completed before the effective date of the registration statement on Form S-1 for our initial public offering are now eligible to resell such ordinary shares in reliance on Rule 144, but without compliance with certain restrictions, including the holding period, contained in Rule 144. Such sales may, however, remain subject to the restrictions related to the lock-up agreements discussed below.
Registration Rights
Our Principal Shareholder has certain registration rights with respect to our ordinary shares pursuant to the Principal Shareholders Agreement. See “Certain Relationships and Related Party Transactions—Shareholders agreements—Principal Shareholders Agreement” in Ortho’s 2020 Form 10-K which is incorporated by reference in this prospectus.
Lock-up Agreements
In connection with this offering, we, our directors and executive officers, and the selling shareholder have each agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any of our or their ordinary shares or securities convertible into or exchangeable for ordinary shares during the period from the date of this prospectus continuing through the date 90 days after the date of this prospectus, except with the prior written consent of certain representatives of the underwriters.
Immediately following the consummation of this offering, equity holders subject to lock-up agreements will hold 122,176,446 of our ordinary shares (assuming no exercise of the underwriters’ option to purchase additional ordinary shares), representing approximately 51.6% of our then outstanding ordinary shares, or 118,876,446 of our ordinary shares if the underwriters exercise in full their option to purchase additional ordinary shares representing approximately 50.2% of our then outstanding ordinary shares.
52
Table of Contents
We have agreed not to issue, sell or otherwise dispose of any our ordinary shares during the 90-day period following the date of this prospectus. We may, however, grant options to purchase ordinary shares, issue ordinary shares upon the exercise of outstanding options, issue ordinary shares in connection with certain acquisitions or business combinations or an employee stock purchase plan and in certain other circumstances.
53
Table of Contents
Material U.S. Federal Income Tax Considerations
The following summary describes certain material U.S. federal income tax considerations generally applicable to U.S. Holders (as defined below), and solely to the extent described below under the heading “U.S. Foreign Account Tax Compliance Act,” to persons other than U.S. Holders, of an investment in the ordinary shares pursuant to this offering. This summary applies only to U.S. Holders that hold the ordinary shares as capital assets within the meaning of Section 1221 of the Internal Revenue Code of 1986, as amended (the “Code”), that have acquired the ordinary shares in this offering and that have the U.S. dollar as their functional currency.
This discussion is based on the tax laws of the United States, including the Code, as in effect on the date hereof and on U.S. Treasury regulations as in effect or, in some cases, as proposed, on the date hereof, as well as judicial and administrative interpretations thereof available on or before such date. All of the foregoing authorities are subject to change or differing interpretations, which change or differing interpretation could apply retroactively and could affect the tax consequences described below. No ruling will be requested from the Internal Revenue Service (the “IRS”) regarding the tax consequences of this offering and there can be no assurance that the IRS will agree with the discussion set out below. This summary does not address any estate or gift tax consequences, the alternative minimum tax, the Medicare tax on net investment income or any state, local or non-U.S. tax consequences.
This summary also does not address the tax consequences that may be relevant to persons in special tax situations such as:
| • | banks; |
| • | certain financial institutions; |
| • | insurance companies; |
| • | regulated investment companies; |
| • | real estate investment trusts; |
| • | individual retirement accounts and other tax-deferred accounts; |
| • | broker-dealers; |
| • | traders that elect to mark to market their securities; |
| • | U.S. expatriates; |
| • | tax-exempt entities; |
| • | persons that own the ordinary shares as part of a “straddle,” “hedge,” “conversion transaction” or integrated transaction; |
| • | persons that actually or constructively own 10% or more of the Company’s share capital (by vote or value); |
| • | persons that are resident or ordinarily resident in or have a permanent establishment in a jurisdiction outside the United States; |
| • | persons who acquired the ordinary shares pursuant to the exercise of any employee share option or otherwise as compensation; |
| • | persons liable for alternative minimum tax of the Medicare contribution tax on net investment income; |
54
Table of Contents
| • | persons subject to special tax accounting rules as a result of any item of gross income with respect to the ordinary shares being taken into account in an applicable financial statement; or |
| • | pass-through entities or arrangements, or persons holding ordinary shares through pass-through entities or arrangements. |
THE SUMMARY OF U.S. FEDERAL INCOME TAX CONSIDERATIONS SET OUT BELOW IS FOR GENERAL INFORMATION ONLY. PROSPECTIVE PURCHASERS SHOULD CONSULT THEIR TAX ADVISORS AS TO THE APPLICATION OF THE U.S. FEDERAL TAX RULES TO THEIR PARTICULAR CIRCUMSTANCES AS WELL AS THE STATE, LOCAL, NON-U.S. AND OTHER TAX CONSEQUENCES TO THEM OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF THE ORDINARY SHARES.
As used herein, the term “U.S. Holder” means a beneficial owner of the ordinary shares that is, for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) created or organized under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
| • | a trust that (1) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons for all its substantial decisions or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person. |
If an entity or other arrangement treated as a partnership for U.S. federal income tax purposes holds ordinary shares, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. Partnerships considering an investment in ordinary shares and partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences of owning and disposing of ordinary shares.
Taxation of Dividends and Other Distributions on the Ordinary Shares
Subject to the passive foreign investment company rules discussed below, the gross amount of any distributions made by the Company with respect to our ordinary shares (including the amount of non-U.S. taxes withheld therefrom, if any) generally will be includible in a U.S. Holder’s gross income as dividend income on the date of receipt, but only to the extent the distribution is paid out of the Company’s current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Because the Company does not maintain calculations of its earnings and profits under U.S. federal income tax principles, a U.S. Holder should expect all cash distributions to be reported as dividends for U.S. federal income tax purposes. Such dividends will not be eligible for the dividends received deduction allowed to U.S. corporations with respect to dividends received from other U.S. corporations.
With respect to certain non-corporate U.S. Holders, including individual U.S. Holders, dividends may be taxed at the lower capital gain rates applicable to “qualified dividend income,” provided that (i) the ordinary shares are readily tradable on an established securities market in the United States, (ii) certain holding period and at-risk requirements are met and (iii) the Company is not a passive foreign investment company (as discussed below) with respect to the relevant U.S. Holder for either the taxable year in which the dividend was paid or the preceding taxable year. In this regard, the ordinary shares will generally be considered to be readily tradable on an established securities market in the United States if they are listed on Nasdaq.
55
Table of Contents
Dividends on the ordinary shares generally will constitute foreign source income for foreign tax credit limitation purposes. Subject to certain complex conditions and limitations, foreign taxes withheld on any distributions on ordinary shares, if any, may be eligible for credit against a U.S. Holder’s federal income tax liability. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by the Company with respect to ordinary shares will generally constitute “passive category income.” However, if the Company is a “United States-owned foreign corporation,” solely for foreign tax credit purposes, a portion of the dividends allocable the Company’s U.S.-source earnings and profits may be re-characterized as U.S. source. A “United States-owned foreign corporation” is any foreign corporation in which United States persons own, directly or indirectly, 50% or more (by vote or by value) of the stock. Under an exception, dividends will not be allocated to U.S.-source income if less than 10% of the earnings and profits of the United States-owned foreign corporations are attributable to sources within the United States. We cannot provide any assurances that we will not be characterized as a “United States-owned foreign corporation.” If we are a United States-owned foreign corporation, if 10% or more of our earnings and profits are attributable to sources within the United States, the portion of the dividends paid on the ordinary shares allocable to our United States source earnings and profits would be treated as U.S.-source, and a U.S. Holder would not be permitted to offset any foreign tax withheld as a credit against U.S. federal income tax imposed on that portion of the dividends. The rules relating to the determination of the U.S. foreign tax credit are complex. U.S. Holders should consult their tax advisors regarding the availability of a foreign tax credit in their particular circumstances and the possibility of claiming an itemized deduction (in lieu of the foreign tax credit) for any foreign taxes paid or withheld.
Taxation of Disposition of the Ordinary Shares
Subject to the passive foreign investment company rules discussed below, upon a sale or other taxable disposition of ordinary shares, a U.S. Holder will recognize capital gain or loss in an amount equal to the difference between the amount realized and the U.S. Holder’s adjusted tax basis in such ordinary shares. In general, a U.S. Holder’s adjusted tax basis in its ordinary shares will be equal to the cost of such ordinary shares to the U.S. Holder. Any such gain or loss generally will generally be treated as long-term capital gain or loss if the U.S. Holder’s holding period in the ordinary shares exceeds one year. Non-corporate U.S. Holders (including individuals) generally will be subject to U.S. federal income tax on long-term capital gain at preferential rates. The deductibility of capital losses is subject to significant limitations. Gain or loss, if any, recognized by a U.S. Holder on the sale or other disposition of ordinary shares generally will be treated as U.S. source gain or loss for U.S. foreign tax credit limitation purposes.
Passive Foreign Investment Company Rules
The Company will be classified as a passive foreign investment company (a “PFIC”) for any taxable year if either: (a) at least 75% of its gross income is “passive income” for purposes of the PFIC rules or (b) at least 50% of the value of its assets (determined on the basis of a quarterly average) is attributable to assets that produce or are held for the production of passive income. For this purpose, the Company will be treated as owning its proportionate share of the assets and earning its proportionate share of the income of any other corporation in which it owns, directly or indirectly, 25% or more (by value) of the stock.
Under the PFIC rules, if the Company were considered a PFIC at any time that a U.S. Holder holds ordinary shares, the Company would continue to be treated as a PFIC with respect to such investment unless (i) the Company ceases to be a PFIC and (ii) the U.S. Holder has made a “deemed sale” election under the PFIC rules.
56
Table of Contents
Based on the recent, current and anticipated composition of the income, assets and operations of the Company and its subsidiaries, the Company does not expect to be treated as a PFIC for the taxable year ended January 3, 2021 or the current taxable year. This is a factual determination, however, that depends on, among other things, the composition of the income and assets, and the market value of the shares and assets, of the Company and its subsidiaries from time to time, and thus the determination can be made only annually after the close of each taxable year. Therefore there can be no assurances that the Company will not be classified as a PFIC for the taxable year ended January 3, 2021, the current taxable year or for any future taxable year.
If the Company is considered a PFIC at any time that a U.S. Holder holds ordinary shares, any gain recognized by the U.S. Holder on a sale or other disposition of the ordinary shares, as well as the amount of any “excess distribution” (defined below) received by the U.S. Holder, would be allocated ratably over the U.S. Holder’s holding period for the ordinary shares. The amounts allocated to the taxable year of the sale or other disposition (or the taxable year of receipt, in the case of an excess distribution) and to any year before the Company became a PFIC would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for individuals or corporations, as appropriate, for that taxable year, and an interest charge would be imposed. For purposes of these rules, an excess distribution is the amount by which any distribution received by a U.S. Holder on ordinary shares exceeds 125% of the average of the annual distributions on the ordinary shares received during the preceding three years or the U.S. Holder’s holding period, whichever is shorter. Certain elections may be available that would result in alternative treatments (such as qualified electing fund treatment or mark-to-market treatment) of our ordinary shares if we are considered a PFIC. We do not intend to provide the information necessary for U.S. Holders of our ordinary shares to make qualified electing fund elections, which, if available, would result in tax treatment different from the general tax treatment for an investment in a PFIC described above. If we are treated as a PFIC with respect to a U.S. Holder for any taxable year, the U.S. Holder will be deemed to own shares in any of our subsidiaries that are also PFICs. However, an election for mark-to-market treatment would likely not be available with respect to any such subsidiaries.
If the Company is considered a PFIC, a U.S. Holder would also be subject to annual information reporting requirements. Failure to comply with such information reporting requirements may result in significant penalties and may suspend the running of the statute of limitations. U.S. Holders should consult their tax advisors about the potential application of the PFIC rules to an investment in ordinary shares.
Information Reporting and Backup Withholding
Dividend payments with respect to ordinary shares and proceeds from the sale, exchange or redemption of ordinary shares may be subject to information reporting to the IRS and U.S. backup withholding. A U.S. Holder may be eligible for an exemption from backup withholding if the U.S. Holder furnishes a correct taxpayer identification number and makes any other required certification or is otherwise exempt from backup withholding. U.S. Holders who are required to establish their exempt status may be required to provide such certification on IRS Form W-9. U.S. Holders should consult their tax advisors regarding the application of the U.S. information reporting and backup withholding rules.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. Holder’s U.S. federal income tax liability, and such U.S. Holder may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing an appropriate claim for refund with the IRS and furnishing any required information.
57
Table of Contents
Information with Respect to Foreign Financial Assets
Certain U.S. Holders who are individuals (and certain entities) that hold an interest in “specified foreign financial assets” (which may include the ordinary shares) are required to report information relating to such assets, subject to certain exceptions (including an exception for ordinary shares held in accounts maintained by certain financial institutions). U.S. Holders should consult their tax advisors regarding the effect, if any, of this requirement on their ownership and disposition of the ordinary shares.
U.S. Foreign Account Tax Compliance Act (FATCA)
Certain provisions of the Code and the Treasury regulations (commonly collectively referred to as “FATCA”) generally impose withholding at a rate of 30% on “foreign passthru payments” made by a “foreign financial institution” (as defined in the Code) (an “FFI”). If the Company were to be treated as an FFI, such withholding may be imposed on such payments to any other FFI (including an intermediary through which an investor may hold the ordinary shares) that is not a “participating FFI” (as defined under FATCA) or any other investor who does not provide information sufficient to establish that the investor is not subject to withholding under FATCA, unless such other FFI or investor is otherwise exempt from FATCA. In addition, under those circumstances, the Company may be required to report certain information regarding investors to the relevant tax authorities, which information may be shared with taxing authorities in the United States. Under current guidance, the term “foreign passthru payment” is not defined. Consequently, it is not clear whether or to what extent payments on the ordinary shares would be considered foreign passthru payments. Withholding on foreign passthru payments would not be required with respect to payments made before the date that is two years after the date of publication in the Federal Register of final regulations defining the term “foreign passthru payment.” Prospective investors should consult their tax advisors regarding the potential impact of FATCA, any applicable inter-governmental agreement relating to FATCA, and any non-U.S. legislation implementing FATCA on the investment in the ordinary shares.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL TAX MATTERS THAT MAY BE IMPORTANT TO YOU. EACH PROSPECTIVE PURCHASER SHOULD CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES OF AN INVESTMENT IN ORDINARY SHARES UNDER THE INVESTOR’S OWN CIRCUMSTANCES.
Material U.K. Tax Considerations
The following statements are of a general nature and do not purport to be a complete analysis of all potential United Kingdom tax consequences of acquiring, holding and disposing of the ordinary shares pursuant to this offering. They are based on current United Kingdom tax law and on the current published practice of HMRC (which may not be binding on HMRC), as of the date of this prospectus, all of which are subject to change, possibly with retrospective effect. They are intended to address only certain United Kingdom tax consequences for holders who are tax resident in (and only in) the United Kingdom, and in the case of individuals, domiciled in (and only in) the United Kingdom (except where expressly stated otherwise) who are the absolute beneficial owners of the ordinary shares and any dividends paid on them and who hold the ordinary shares as investments (other than in an individual savings account or a self-invested personal pension). They do not address the United Kingdom tax consequences which may be relevant to certain classes of holders such as traders, brokers, dealers, banks, financial institutions, insurance companies, investment companies, collective investment schemes, tax-exempt organisations, trustees, persons connected with the Company, persons holding its ordinary shares as part of hedging or conversion transactions, holders who have (or are deemed to have) acquired their ordinary shares by virtue of an office or employment, and holders who are or have been officers or employees of the Company. The statements do not apply to any holder who either
58
Table of Contents
directly or indirectly holds or controls 10% or more of the Company’s share capital (or class thereof), voting power or profits.
The following is intended only as a general guide and is not intended to be, nor should it be considered to be, legal or tax advice to any particular prospective subscriber for, or purchaser of, the ordinary shares. Accordingly, prospective subscribers for, or purchasers of, the ordinary shares who are in any doubt as to their tax position regarding the acquisition, ownership or disposition of the ordinary or who are subject to tax in a jurisdiction other than the United Kingdom should consult their own tax advisers.
(1) Taxation of Dividends
Withholding Tax
The Company will not be required to withhold United Kingdom tax at source when paying dividends. The amount of any liability to United Kingdom tax on dividends paid by the Company will depend on the individual circumstances of a holder.
Income Tax
An individual holder who is resident for tax purposes in the United Kingdom may, depending on his or her particular circumstances, be subject to United Kingdom tax on dividends received from the Company. An individual holder who is not resident for tax purposes in the United Kingdom should not be chargeable to United Kingdom income tax on dividends received from the Company unless he or she carries on (whether solely or in partnership) any trade, profession or vocation in the United Kingdom through a branch or agency to which the ordinary shares are attributable. There are certain exceptions for trading in the United Kingdom through independent agents, such as some brokers and investment managers.
All dividends received by a United Kingdom tax resident individual holder from the Company or from other sources will form part of the holder’s total income for income tax purposes and will constitute the top slice of that income. A nil rate of income tax will apply to the first £2,000 of taxable dividend income received by the holder in a tax year. Income within the nil rate band will be taken into account in determining whether income in excess of the nil rate band falls within the basic rate, higher rate or additional rate tax bands. Where the dividend income is above the £2,000 dividend allowance, the first £2,000 of the dividend income will be charged at the nil rate and any excess amount will be taxed at 7.5%. to the extent that the excess amount falls within the basic rate tax band, 32.5%. to the extent that the excess amount falls within the higher rate tax band and 38.1%. to the extent that the excess amount falls within the additional rate tax band.
Corporation Tax
Corporate holders which are resident for tax purposes in the United Kingdom should not be subject to United Kingdom corporation tax on any dividend received from the Company so long as the dividends qualify for exemption (as is likely) and certain conditions are met (including anti-avoidance conditions). If the conditions for exemption are not met or cease to be satisfied, or such a holder elects for an otherwise exempt dividend to be taxable, the holder will be subject to United Kingdom corporation tax on dividends received from the Company, at the rate of corporation tax applicable to that holder (the main rate of United Kingdom corporation tax is currently 19%).
Corporate holders who are not resident in the United Kingdom will not generally be subject to United Kingdom corporation tax on dividends unless they are carrying on a trade, profession or vocation in the United Kingdom through a permanent establishment in connection with which the ordinary shares are used, held, or acquired.
59
Table of Contents
A holder who is resident outside the United Kingdom may be subject to non-United Kingdom taxation on dividend income under local law.
(2) Taxation of Capital Gains
United Kingdom Resident Holders
A disposal or deemed disposal of ordinary shares by an individual or corporate holder who is tax resident in the United Kingdom may, depending on the holder’s circumstances and subject to any available exemptions or reliefs, give rise to a chargeable gain or allowable loss for the purposes of United Kingdom taxation of chargeable gains.
Any chargeable gain (or allowable loss) will generally be calculated by reference to the consideration received for the disposal of the ordinary shares less the allowable cost to the holder of acquiring such ordinary shares.
The applicable tax rates for individual holders realising a gain on the disposal of ordinary shares is, broadly, 10% for basic rate taxpayers and 20% for higher and additional rate taxpayers. For corporate holders, corporation tax is generally charged on chargeable gains at the rate applicable to the relevant corporate holder.
Non-United Kingdom Holders
Holders who are not resident in the United Kingdom and, in the case of an individual holder, not temporarily non-resident, should not be liable for United Kingdom tax on capital gains realised on a sale or other disposal of ordinary shares unless (i) such ordinary shares are used, held or acquired for the purposes of a trade, profession or vocation carried on in the United Kingdom through a branch or agency or, in the case of a corporate holder, through a permanent establishment or (ii) where certain conditions are met, the Company derives 75% or more of its gross value from United Kingdom land. Holders who are not resident in the United Kingdom may be subject to non-United Kingdom taxation on any gain under local law.
Generally, an individual holder who has ceased to be resident in the United Kingdom for United Kingdom tax purposes for a period of five years or less and who disposes of ordinary shares during that period may be liable on their return to the United Kingdom to United Kingdom taxation on any capital gain realised (subject to any available exemption or relief).
(3) United Kingdom Stamp Duty (“Stamp Duty”) and United Kingdom Stamp Duty Reserve Tax (“SDRT”)
The statements in this paragraph 3 are intended as a general guide to the current position relating to stamp duty and SDRT and apply to any holder irrespective of their place of tax residence. Certain categories of person, including intermediaries, brokers, dealers and persons connected with depositary receipt arrangements and clearance services, may not be liable to stamp duty or SDRT or may be liable at a higher rate or may, although not primarily liable for the tax, be required to notify and account for it under the Stamp Duty Reserve Tax Regulations 1986.
Transfers of ordinary shares within a clearance service or depositary receipt system should not give rise to a liability to stamp duty or SDRT, provided that no instrument of transfer is entered into and that no election that applies to the ordinary shares is, or has been, made by the clearance service under Section 97A of the United Kingdom Finance Act 1986.
60
Table of Contents
Transfers of ordinary shares within a clearance service where an election has been made by the clearance service under Section 97A of the Finance Act 1986 will generally be subject to SDRT (rather than stamp duty) at the rate of 0.5% of the amount or value of the consideration.
Transfers of ordinary shares that are held in certificated form will generally be subject to stamp duty at the rate of 0.5% of the consideration given (rounded up to the nearest £5). An exemption from stamp duty is available for a written instrument transferring an interest in ordinary shares where the amount or value of the consideration is £1,000 or less, and it is certified on the instrument that the transaction effected by the instrument does not form part of a larger transaction or series of transactions for which the aggregate consideration exceeds £1,000. SDRT may be payable on an agreement to transfer such ordinary shares, generally at the rate of 0.5% of the consideration given in money or money’s worth under the agreement to transfer the ordinary shares. This charge to SDRT would be discharged if an instrument of transfer is executed pursuant to the agreement which gave rise to SDRT and stamp duty is duly paid on the instrument transferring the ordinary shares within six years of the date on which the agreement was made or, if the agreement was conditional, the date on which the agreement became unconditional.
If ordinary shares (or interests therein) are subsequently transferred into a clearance service or depositary receipt system, stamp duty or SDRT will generally be payable at the rate of 1.5% of the amount or value of the consideration given or, in certain circumstances, the value of the shares (save to the extent that an election has been made under section 97A of the United Kingdom Finance Act 1986). This liability for stamp duty or SDRT will strictly be accountable by the clearance service or depositary receipt system, as the case may be, but will, in practice, generally be reimbursed by participants in the clearance service or depositary receipt system.
61
Table of Contents
The selling shareholder is offering the ordinary shares described in this prospectus through a number of underwriters. Goldman Sachs & Co. LLC and J.P. Morgan Securities LLC are acting as representatives of the underwriters. The selling shareholder has entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, the selling shareholder has agreed to sell to the underwriters, and each underwriter has severally agreed to purchase from the selling shareholder, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of ordinary shares listed next to its name in the following table:
| Name |
Number of shares |
|||
| Goldman Sachs & Co. LLC |
||||
| J.P. Morgan Securities LLC |
||||
|
|
|
|||
| Total |
22,000,000 | |||
|
|
|
|||
The underwriters are committed to purchase all the ordinary shares offered by the selling shareholder if they purchase any shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or the offering may be terminated.
The underwriters propose to offer the ordinary shares directly to the public at the public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per share. After the initial offering to the public, the offering price, concession or any other term of the offering may be changed. The offering of the shares by the underwriters is subject to receipt and acceptance and subject to the underwriters’ right to reject any order in whole or in part.
The underwriters have an option to buy up to 3,300,000 additional ordinary shares from the selling shareholder to cover sales of shares by the underwriters which exceed the number of shares specified in the table above. The underwriters have 30 days from the date of this prospectus to exercise this option to purchase additional shares. If any shares are purchased with this option to purchase additional shares, the underwriters will purchase shares in approximately the same proportion as shown in the table above. If any additional ordinary shares are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered.
The underwriting fee is equal to the public offering price per share of ordinary shares less the amount paid by the underwriters to the selling shareholder per share of ordinary shares. The underwriting fee is $ per share. The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Without exercise of option to purchase additional shares |
With full exercise of option to purchase additional shares exercise |
|||||||
| Per share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
|
|
|
|
|
|||||
We estimate that the total expenses of this offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and commissions, will be approximately $1.1 million.
62
Table of Contents
A prospectus in electronic format may be made available on the web sites maintained by one or more underwriters, or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representatives to underwriters and selling group members that may make Internet distributions on the same basis as other allocations.
We have agreed that we will not (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any of our ordinary shares or securities convertible into or exercisable or exchangeable for any of our ordinary shares, or publicly disclose the intention to make any offer, sale, pledge, loan, disposition or filing, or (ii) enter into any swap or other arrangement that transfers all or a portion of the economic consequences associated with the ownership of any ordinary shares or any such other securities (regardless of whether any of these transactions are to be settled by the delivery of ordinary shares or such other securities, in cash or otherwise), in each case without the prior written consent of each of Goldman Sachs & Co. LLC and J.P. Morgan Securities LLC for a period of 90 days after the date of this prospectus, subject to certain exceptions, including any shares to be sold in this offering.
The restrictions on our actions, as described above, do not apply to certain transactions, including (i) the issuance of ordinary shares or securities convertible into or exercisable for our ordinary shares pursuant to the conversion or exchange of convertible or exchangeable securities or the exercise of warrants or options (including net exercise) or the settlement of RSUs (including net settlement), in each case outstanding on the date of the underwriting agreement and described in this prospectus; (ii) grants of stock options, stock awards, restricted stock, RSUs, or other equity awards and the issuance of our ordinary shares or securities convertible into or exercisable or exchangeable for our ordinary shares (whether upon the exercise of stock options or otherwise) to our employees, officers, directors, advisors, or consultants pursuant to the terms of an equity compensation plan in effect as of the closing of this offering and described in this prospectus; or (iii) our filing of any registration statement on Form S-8 relating to securities granted or to be granted pursuant to any plan described in this prospectus or any assumed benefit plan pursuant to an acquisition or similar strategic transaction.
Our directors and executive officers, and the selling shareholder (such persons, the “lock-up parties”) have entered into lock-up agreements with the underwriters prior to the commencement of this offering pursuant to which each lock-up party, with limited exceptions, for a period of days after the date of this prospectus (such period, the “restricted period”), may not (and may not cause any of their direct or indirect affiliates to), without the prior written consent of each of Goldman Sachs & Co. LLC and J.P. Morgan Securities LLC, (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any of our ordinary shares or any securities convertible into or exercisable or exchangeable for our ordinary shares (including, without limitation, ordinary shares or such other securities which may be deemed to be beneficially owned by such lock-up parties in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of a stock option or warrant (collectively with the ordinary shares, the “lock-up securities”)), (2) enter into any hedging, swap or other agreement or transaction that transfers, in whole or in part, any of the economic consequences of ownership of the lock-up securities, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of lock-up securities, in cash or otherwise or (3) publicly disclose the intention to do any of the foregoing. Such persons or entities have further acknowledged that these undertakings preclude them from engaging in any hedging or other transactions or arrangements (including, without limitation, any short sale or the purchase or sale of, or entry into, any put or call option, or combination thereof,
63
Table of Contents
forward, swap or any other derivative transaction or instrument, however described or defined) designed or intended, or which could reasonably be expected to lead to or result in, a sale or disposition or transfer (by any person or entity, whether or not a signatory to such agreement) of any economic consequences of ownership, in whole or in part, directly or indirectly, of any lock-up securities, whether any such transaction or arrangement (or instrument provided for thereunder) would be settled by delivery of lock-up securities, in cash or otherwise.
The restrictions described in the immediately preceding paragraph and contained in the lock-up agreements between the underwriters and the lock-up parties do not apply, subject in certain cases to various conditions, to certain transactions, including (a) transfers of lock-up securities: (i) as bona fide gifts, or for bona fide estate planning purposes, (ii) by will or intestacy, (iii) to any trust for the direct or indirect benefit of the lock-up party or any immediate family member, (iv) to a partnership, limited liability company or other entity of which the lock-up party and its immediate family members are the legal and beneficial owner of all of the outstanding equity securities or similar interests, (v) to a nominee or custodian of a person or entity to whom a disposition or transfer would be permissible under clauses (i) through (iv), (vi) in the case of a corporation, partnership, limited liability company, trust or other business entity, (A) to another corporation, partnership, limited liability company, trust or other business entity that is an affiliate of the lock-up party, or to any investment fund or other entity controlling, controlled by, managing or managed by or under common control with the lock-up party or its affiliates or (B) as part of a distribution to members or stockholders of the lock-up party, (vii) by operation of law, (viii) to us from an employee upon death, disability or termination of employment of such employee, (ix) as part of a sale of lock-up securities acquired in open market transactions after the completion of this offering, (x) to us in connection with the vesting, settlement or exercise of restricted stock units, options, warrants or other rights to purchase shares of our ordinary shares (including “net” or “cashless” exercise), including for the payment of exercise price and tax and remittance payments, (xi) pursuant to a bona fide third-party tender offer, merger, consolidation or other similar transaction approved by our board of directors and made to all shareholders involving a change in control, provided that if such transaction is not completed, all such lock-up securities would remain subject to the restrictions in the immediately preceding paragraph, or (xii) in connection with sales of ordinary shares made pursuant to a trading plan that complies with Rule 10b5-1 under the Exchange Act that has been entered into prior to the date of the lock-up agreement; (b) exercise of the options, settlement of RSUs or other equity awards, or the exercise of warrants granted pursuant to plans described in in this prospectus, provided that any lock-up securities received upon such exercise, vesting or settlement would be subject to restrictions similar to those in the immediately preceding paragraph; (c) the conversion of outstanding preferred stock, warrants to acquire preferred stock, or convertible securities into shares of our ordinary shares or warrants to acquire shares of our ordinary shares, provided that any ordinary shares or warrant received upon such conversion would be subject to restrictions similar to those in the immediately preceding paragraph; and (d) the establishment by lock-up parties of trading plans under Rule 10b5-1 under the Exchange Act, provided that such plan does not provide for the transfer of lock-up securities during the restricted period.
Goldman Sachs & Co. LLC and J.P. Morgan Securities LLC, in their sole discretion, may release the securities subject to any of the lock-up agreements with the underwriters described above, in whole or in part at any time.
The selling shareholder has agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act.
Our ordinary shares are currently traded on Nasdaq under the symbol “OCDX”.
In connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling ordinary shares in the open market for the purpose of
64
Table of Contents
preventing or retarding a decline in the market price of the ordinary shares while this offering is in progress. These stabilizing transactions may include making short sales of ordinary shares, which involves the sale by the underwriters of a greater number of ordinary shares than they are required to purchase in this offering, and purchasing ordinary shares on the open market to cover positions created by short sales. Short sales may be “covered” shorts, which are short positions in an amount not greater than the underwriters’ option to purchase additional shares referred to above, or may be “naked” shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their option to purchase additional shares, in whole or in part, or by purchasing shares in the open market. In making this determination, the underwriters will consider, among other things, the price of shares available for purchase in the open market compared to the price at which the underwriters may purchase shares through the option to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the ordinary shares in the open market that could adversely affect investors who purchase in this offering. To the extent that the underwriters create a naked short position, they will purchase shares in the open market to cover the position.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act of 1933, they may also engage in other activities that stabilize, maintain or otherwise affect the price of the ordinary shares, including the imposition of penalty bids. This means that if the representatives of the underwriters purchase ordinary shares in the open market in stabilizing transactions or to cover short sales, the representatives can require the underwriters that sold those shares as part of this offering to repay the underwriting discount received by them.
These activities may have the effect of raising or maintaining the market price of the ordinary shares or preventing or retarding a decline in the market price of the ordinary shares, and, as a result, the price of our ordinary shares may be higher than the price that otherwise might exist in the open market. The underwriters may carry out these transactions on Nasdaq, in the over-the-counter market or otherwise.
Neither we, the selling shareholder, nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our ordinary shares. In addition, neither we, the selling shareholder, nor any of the underwriters make any representation that the representatives will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Other than in the United States, no action has been taken by us, the selling shareholder or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Notice to Prospective Investors in Canada
The ordinary shares may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined
65
Table of Contents
in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the ordinary shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Notice to Prospective Investors in the European Economic Area
In relation to each Member State of the European Economic Area (each a “Member State”), no ordinary shares have been offered or will be offered pursuant to the offering to the public in that Member State prior to the publication of a prospectus in relation to the ordinary shares which has been approved by the competent authority in that Member State or, where appropriate, approved in another Member State and notified to the competent authority in that Member State, all in accordance with the Prospectus Regulation, except that offers of ordinary shares may be made to the public in that Member State at any time under the following exemptions under the Prospectus Regulation:
(a) to any legal entity which is a qualified investor as defined under the Prospectus Regulation;
(b) to fewer than 150 natural or legal persons (other than qualified investors as defined under the Prospectus Regulation), subject to obtaining the prior consent of the underwriters; or
(c) in any other circumstances falling within Article 1(4) of the Prospectus Regulation,
provided that no such offer of ordinary shares shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation and each person who initially acquires any ordinary shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the underwriters and the Company that it is a “qualified investor” within the meaning of Article 2(e) of the Prospectus Regulation. In the case of any ordinary shares being offered to a financial intermediary as that term is used in the Prospectus Regulation, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the ordinary shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any ordinary shares to the public other than their offer or resale in a Member State to qualified investors as so defined or in circumstances in which the prior consent of the underwriters have been obtained to each such proposed offer or resale.
For the purposes of this provision, the expression an “offer to the public” in relation to ordinary shares in any Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any ordinary shares to be offered so as to enable an investor to decide to purchase or subscribe for any ordinary shares, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
66
Table of Contents
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Regulation as it forms part of domestic law by virtue of the European Union (Withdrawal) Act 2018) (i) who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the “Order”) and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”) or otherwise in circumstances which have not resulted and will not result in an offer to the public of the ordinary shares in the United Kingdom within the meaning of the Financial Services and Markets Act 2000.
Any person in the United Kingdom that is not a relevant person should not act or rely on the information included in this document or use it as a basis for taking any action. In the United Kingdom, any investment or investment activity that this document relates to may be made or taken exclusively by relevant persons.
Notice to Prospective Investors in Switzerland
The ordinary shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document does not constitute a prospectus within the meaning of, and has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the ordinary shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company, the ordinary shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of ordinary shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (FINMA), and the offer of ordinary shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of ordinary shares.
Notice to Prospective Investors in Australia
This prospectus:
| • | does not constitute a disclosure document or a prospectus under Chapter 6D.2 of the Corporations Act 2001 (Cth) (the “Corporations Act”); |
| • | has not been, and will not be, lodged with the Australian Securities and Investments Commission (“ASIC”), as a disclosure document for the purposes of the Corporations Act and does not purport to include the information required of a disclosure document for the purposes of the Corporations Act; and |
| • | may only be provided in Australia to select investors who are able to demonstrate that they fall within one or more of the categories of investors, available under section 708 of the Corporations Act (“Exempt Investors”). |
67
Table of Contents
The ordinary shares may not be directly or indirectly offered for subscription or purchased or sold, and no invitations to subscribe for or buy the ordinary shares may be issued, and no draft or definitive offering memorandum, advertisement or other offering material relating to any ordinary shares may be distributed in Australia, except where disclosure to investors is not required under Chapter 6D of the Corporations Act or is otherwise in compliance with all applicable Australian laws and regulations. By submitting an application for the ordinary shares, you represent and warrant to us that you are an Exempt Investor.
As any offer of ordinary shares under this document will be made without disclosure in Australia under Chapter 6D.2 of the Corporations Act, the offer of those securities for resale in Australia within 12 months may, under section 707 of the Corporations Act, require disclosure to investors under Chapter 6D.2 if none of the exemptions in section 708 applies to that resale. By applying for the ordinary shares you undertake to us that you will not, for a period of 12 months from the date of issue of the ordinary shares, offer, transfer, assign or otherwise alienate those ordinary shares to investors in Australia except in circumstances where disclosure to investors is not required under Chapter 6D.2 of the Corporations Act or where a compliant disclosure document is prepared and lodged with ASIC.
Notice to Prospective Investors in Japan
The ordinary shares have not been and will not be registered pursuant to Article 4, Paragraph 1 of the Financial Instruments and Exchange Act. Accordingly, none of the ordinary shares nor any interest therein may be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any “resident” of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to or for the benefit of a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Act and any other applicable laws, regulations and ministerial guidelines of Japan in effect at the relevant time.
Notice to Prospective Investors in Hong Kong
The ordinary shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (the “SFO”) of Hong Kong and any rules made thereunder; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32) of Hong Kong) (the “CO”) or which do not constitute an offer to the public within the meaning of the CO. No advertisement, invitation or document relating to the ordinary shares has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to ordinary shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the SFO and any rules made thereunder.
Notice to Prospective Investors in Singapore
Each underwriter has acknowledged that this prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, each underwriter has represented and agreed that it has not offered or sold any ordinary shares or caused the ordinary shares to be made the
68
Table of Contents
subject of an invitation for subscription or purchase and will not offer or sell any ordinary shares or cause the ordinary shares to be made the subject of an invitation for subscription or purchase, and has not circulated or distributed, nor will it circulate or distribute, this prospectus or any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the ordinary shares, whether directly or indirectly, to any person in Singapore other than:
| (a) | to an institutional investor (as defined in Section 4A of the Securities and Futures Act (Chapter 289) of Singapore, as modified or amended from time to time (the “SFA”)) pursuant to Section 274 of the SFA; |
| (b) | to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA; or |
| (c) | otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA. |
Where the ordinary shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| (a) | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| (b) | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, |
securities or securities-based derivatives contracts (each term as defined in Section 2(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the ordinary shares pursuant to an offer made under Section 275 of the SFA except:
| (i) | to an institutional investor or to a relevant person, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA; |
| (ii) | where no consideration is or will be given for the transfer; |
| (iii) | where the transfer is by operation of law; |
| (iv) | as specified in Section 276(7) of the SFA; or |
| (v) | as specified in Regulation 37A of the Securities and Futures (Offers of Investments) (Securities and Securities-based Derivatives Contracts) Regulations 2018. |
Notice to Prospective Investors in the Dubai International Financial Centre (“DIFC”)
This prospectus relates to an Exempt Offer in accordance with the Markets Rules 2012 of the Dubai Financial Services Authority (“DFSA”). This prospectus is intended for distribution only to persons of a type specified in the Markets Rules 2012 of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this prospectus supplement nor taken steps to verify the information set forth herein and has no responsibility for this prospectus. The securities to which this prospectus relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the securities offered should conduct their own due diligence on the securities. If you do not understand the contents of this prospectus you should consult an authorized financial advisor.
69
Table of Contents
In relation to its use in the DIFC, this prospectus is strictly private and confidential and is being distributed to a limited number of investors and must not be provided to any person other than the original recipient, and may not be reproduced or used for any other purpose. The interests in the securities may not be offered or sold directly or indirectly to the public in the DIFC.
Other Relationships
Certain of the underwriters and their affiliates have provided in the past to us and our affiliates and may provide from time to time in the future certain commercial banking, financial advisory, investment banking and other services for us and such affiliates in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, from time to time, certain of the underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future. Furthermore, certain of the underwriters and/or affiliates of certain of the underwriters are lenders and/or agents under our Senior Secured Credit Facilities, including an affiliate of Barclays Capital Inc. that serves as administrative agent under our Senior Secured Credit Facilities, and accordingly, are entitled to fees and expenses in connection therewith.
70
Table of Contents
Certain legal matters in connection with this offering relating to United States federal securities law will be passed upon for us by Latham & Watkins LLP, Washington, District of Columbia. The validity under English law of the ordinary shares offered by this prospectus will be passed upon for us by Latham & Watkins LLP, London, United Kingdom. Certain legal matters relating to this offering will be passed upon for the underwriters by Cahill Gordon & Reindel LLP, New York, New York.
The financial statements incorporated in this Prospectus by reference to the Annual Report on Form 10-K for the year ended January 3, 2021 have been so incorporated in reliance on the report of PricewaterhouseCoopers LLP (“PwC”), an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
PwC completed an independence assessment to evaluate the services and relationships with the Company and its affiliates that may bear on PwC’s independence under the SEC and the PCAOB (United States) independence rules for an audit period commencing January 1, 2018, PwC informed us that certain of its member firms within PricewaterhouseCoopers International Limited, each member firm of which is a separate legal entity (“PwC member firm”), were engaged to perform non-audit services by and had relationships with entities that are under common control with Ortho Clinical Diagnostics Holdings plc. These non-audit services and relationships are not in accordance with the SEC and PCAOB auditor independence rules and are described below:
| • | From April 2019 to September 2020, a PwC member firm assisted in the preparation and submission of two statistical reports to a non-tax authority for an entity under common control with the Company. The fees for these services were approximately $1,600. |
| • | From April 2018 to March 2019, a PwC covered person, who was not involved in the audit of the Company, held an impermissible financial interest in an affiliate of the Company. |
| • | From August 2017 to January 2018, a PwC member firm had a business relationship with an entity under common control with the Company. The fees for these services were approximately $180,000. |
| • | From November 2018 to February 2019, a PwC member firm provided an expert service to an entity under common control with the Company. The fees for these services were approximately $360,000 (fees for the services were not invoiced or collected). |
| • | During March 2019, a PwC member firm held custody of documents and provided a registered office address for an entity under common control with the Company. There were no fees earned for this service. |
PwC provided an overview of the facts and circumstances surrounding the services and relationships to our audit committee and management, including the entities involved, the nature of the relationships, where applicable, an approximation of the fees earned related to the services and relationships and other relevant factors. Considering the facts presented, our audit committee and PwC have concluded that the relationships would not impair PwC’s application of objective and impartial judgment on any matters encompassed within the audit engagement performed by PwC for our consolidated financial statements for each of the three years in the period ended January 3, 2021, and that no reasonable investor would conclude otherwise.
71
Table of Contents
U.K. TopCo is a public limited company organized under the laws of England and Wales. As a result, the rights of holders of our ordinary shares is governed by U.K. law and our articles of association. The rights of shareholders under U.K. law may differ from the rights of shareholders of companies incorporated in other jurisdictions. A substantial amount of our assets are located outside the United States. As a result, it may be difficult for investors to enforce in the United States judgments obtained in U.S. courts against us based on the civil liability provisions of the U.S. securities laws. It is doubtful whether courts in England and Wales will enforce judgments obtained in other jurisdictions, including the United States, against us or our directors or officers under the securities laws of those jurisdictions or entertain actions in England and Wales against us or our directors or officers under the securities laws of other jurisdictions.
Our registered address in the United Kingdom is Felindre Meadows, Pencoed, Bridgend, Mid Glamorgan, Wales, CF35 5PZ.
72
Table of Contents
Ortho Clinical Diagnostics
Goldman Sachs & Co. LLC
J.P. Morgan
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, other than the underwriting discount, payable by the registrant in connection with the sale and distribution of the securities being registered. All amounts are estimated except the Securities and Exchange Commission (“SEC”) registration fee and the Financial Industry Regulatory Authority (“FINRA”) filing fee.
| Amount to be paid |
||||
| SEC Registration Fee |
$ | 57,000 | ||
| FINRA Filing Fee |
78,000 | |||
| Legal Fees and Expenses |
500,000 | |||
| Accounting Fees and Expenses |
250,000 | |||
| Printing Fees and Expenses |
100,000 | |||
| Transfer Agent and Registrar Fees |
25,000 | |||
| Miscellaneous Expenses |
50,000 | |||
|
|
|
|||
| Total |
$ | 1,062,000 | ||
|
|
|
|||
Item 14. Indemnification of Directors and Officers
Every director, officer or former director or officer of our group may be indemnified against all costs, charges, losses, expenses and liabilities incurred by him in connection with any negligence, default, breach of duty or breach of trust by him in relation to us or in connection with our activities as a trustee of an occupational pension scheme, in the actual or purported exercise of his powers or duties or otherwise as our officer, to the extent permitted under the U.K. Companies Act 2006 and the General Corporation Law of the State of Delaware.
The underwriting agreement the registrant will enter into in connection with the offering being registered hereby provides that the underwriters will indemnify, under certain conditions, the registrant’s board of directors and its officers against certain liabilities arising in connection with the offering.
Item 15. Recent Sales of Unregistered Securities
On May 5, 2021, we issued a separate class of 264,399,852,700 deferred shares, with nominal value of $0.01 per share (the “Capital Reduction Shares”), to Computershare Trustees (Jersey) Limited, as our nominee, to hold for, and on behalf of, the holders of our ordinary shares. The Capital Reduction Shares were issued for the purpose of capitalizing a portion of the available merger reserve created following completion of our initial public offering and related reorganization transactions. We did not receive any proceeds from the issuance of the Capital Reduction Shares. The issuance of the Capital Reduction Shares was made in reliance on the exemption from the registration requirements of the Securities Act of 1933, as amended (the “Securities Act”), provided by Section 4(a)(2) of the Securities Act and certain rules and regulations promulgated under that section and pursuant to exemptions under state securities laws.
II-1
Table of Contents
Item 16. Exhibits and Financial Statement Schedules
(a) Exhibits. See Exhibit Index immediately preceding the signature page hereto, which is incorporated by reference as if fully set forth herein.
(b) Financial Statement Schedules. All financial statement schedules are omitted because the information required to be set forth therein is not applicable or is shown in the consolidated financial statements or the notes thereto.
Item 17. Undertakings
(1) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(2) The undersigned registrant hereby undertakes that:
(a) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus as filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(b) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-2
Table of Contents
EXHIBIT INDEX
II-3
Table of Contents
II-4
Table of Contents
II-5
Table of Contents
II-6
Table of Contents
Pursuant to the requirements of the Securities Act, we have duly caused this registration statement on Form S-1 to be signed on our behalf by the undersigned, thereunto duly authorized, in the city of Raritan, New Jersey, on September 7, 2021.
| By: | /s/ Christopher M. Smith | |
| Name: | Christopher M. Smith | |
| Title: | Chairman and Chief Executive Officer |
The undersigned directors and officers of Ortho Clinical Diagnostics Holdings plc hereby constitute and appoint Christopher M. Smith the individual’s true and lawful attorney in fact and agent, with full power of substitution and resubstitution, for the person and in his name, place and stead, in any and all capacities, to sign this registration statement and any or all amendments, including post effective amendments to the Registration Statement, including a prospectus or an amended prospectus therein and any registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act, and all other documents in connection therewith to be filed with the SEC, granting unto said attorney in fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney in fact as agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereto.
Pursuant to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities indicated on September 7, 2021.
| Signature |
Title | |
| /s/ Christopher M. Smith Christopher M. Smith |
Chairman and Chief Executive Officer (Principal Executive Officer) | |
| /s/ Joseph M. Busky Joseph M. Busky |
Chief Financial Officer and Authorized Representative in the United States (Principal Financial Officer and Principal Accounting Officer) | |
| /s/ Karen H. Bechtel Karen H. Bechtel |
Director | |
| /s/ Evelyn Dilsaver Evelyn Dilsaver |
Director | |
| /s/ Allan Holt Allan Holt |
Director | |
| /s/ Carl Hull Carl Hull |
Director | |
| /s/ Ron Labrum Ron Labrum |
Director | |
II-7
Table of Contents
| Signature |
Title | |
| /s/ Thomas Mac Mahon Thomas Mac Mahon |
Director | |
| /s/ David Perez David Perez |
Director | |
| /s/ Robert R. Schmidt Robert R. Schmidt |
Director | |
| /s/ Stephen H. Wise Stephen H. Wise |
Director | |
| /s/ Robert Yates Robert Yates |
Director | |
II-8
