Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Chemomab Therapeutics Ltd. | exhibit_99-1.htm |
| 8-K - 8-K - Chemomab Therapeutics Ltd. | form8k.htm |
Exhibit 99.2

Pioneering Innovative Treatments for Fibrotic DiseasesCorporate Overview | Non-Confidential | September
2021

Forward Looking Statements This presentation contains "forward-looking statements" within the meaning of
the Private Securities Litigation Reform Act. These forward-looking statements include, among other things, statements regarding the clinical development pathway for CM-101; the future operations of Chemomab and its ability to successfully
initiate and complete clinical trials and achieve regulatory milestones; the nature, strategy and focus of Chemomab; the development and commercial potential and potential benefits of any product candidates of Chemomab; and that the product
candidates have the potential to address high unmet needs of patients with serious fibrosis-related diseases and conditions. Any statements contained in this communication that are not statements of historical fact may be deemed to be
forward-looking statements. These forward-looking statements are based upon Chemomab’s current expectations. Forward-looking statements involve risks and uncertainties.Because such statements deal with future events and are based on Chemomab’s
current expectations, they are subject to various risks and uncertainties and actual results, performance or achievements of Chemomab could differ materially from those described in or implied by the statements in this presentation, including:
the uncertain and time-consuming regulatory approval process; risks related to Chemomab’s ability to correctly manage its operating expenses and its expenses; Chemomab’s plans to develop and commercialize its product candidates, including
CM-101; the timing of initiation of Chemomab’s planned clinical trials; the timing of the availability of data from Chemomab’s clinical trials; the timing of any planned investigational new drug application or new drug application; Chemomab’s
plans to research, develop and commercialize its current and future product candidates; the clinical utility, potential benefits and market acceptance of Chemomab’s product candidates; Chemomab’s commercialization, marketing and manufacturing
capabilities and strategy; Chemomab’s ability to protect its intellectual property position; and the requirement for additional capital to continue to advance these product candidates, which may not be available on favorable terms or at all.
Additional risks and uncertainties relating to Chemomab’s and its business can be found under the caption “Risk Factors” and elsewhere in Chemomab’s filings and reports with the SEC. Chemomab expressly disclaims any obligation or undertaking to
release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Chemomab’s expectations with regard thereto or any change in events, conditions or circumstances on which any such statements
are based. This presentation (“Presentation”) is for informational purposes only and does not constitute an offer to sell, solicitation of an offer to buy, or a recommendation to purchase any equity, debt or other financial instruments of
Chemomab. The data contained herein is derived from various internal and external sources. No representation is made as to the reasonableness of the assumptions made within or the accuracy or completeness of any other information contained
herein. All levels, prices and spreads are historical and do not represent current market levels, prices or spreads, some or all of which may have changed since the issuance of this document. Any data on past performance, modeling contained
herein is not an indication as to future performance. Chemomab assume no obligation to update the information in this Presentation. Chemomab does not accept any liability whatsoever for any losses arising from the use of this Presentation or
reliance on the information contained herein. Nothing herein shall be deemed to constitute investment, legal, tax, financial, accounting or other advice. This Presentation is being provided for use only by the intended recipient.
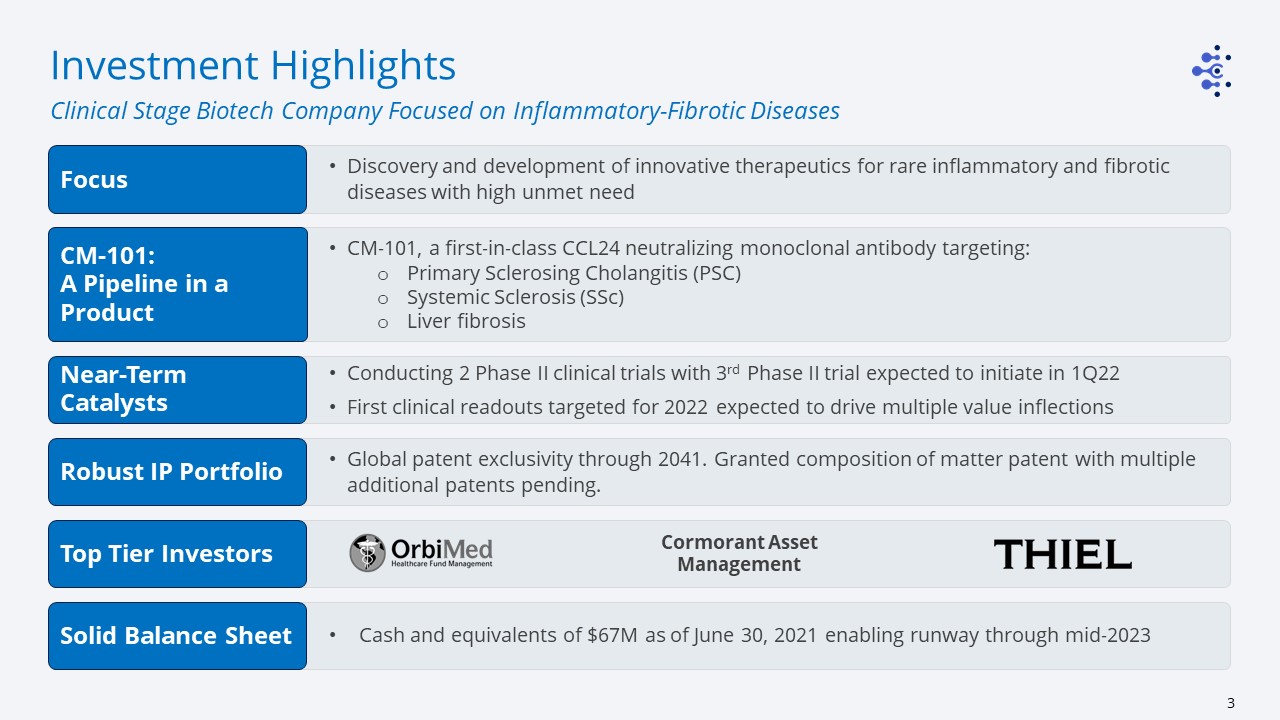
Discovery and development of innovative therapeutics for rare inflammatory and fibrotic diseases with
high unmet need CM-101, a first-in-class CCL24 neutralizing monoclonal antibody targeting:Primary Sclerosing Cholangitis (PSC)Systemic Sclerosis (SSc)Liver fibrosis Conducting 2 Phase II clinical trials with 3rd Phase II trial expected to
initiate in 1Q22First clinical readouts targeted for 2022 expected to drive multiple value inflections Global patent exclusivity through 2041. Granted composition of matter patent with multiple additional patents pending. Investment
Highlights Clinical Stage Biotech Company Focused on Inflammatory-Fibrotic Diseases Cormorant Asset Management Cash and equivalents of $67M as of June 30, 2021 enabling runway through mid-2023 Focus CM-101:A Pipeline in a
Product Near-Term Catalysts Robust IP Portfolio Top Tier Investors Solid Balance Sheet

Experienced Management ADI MOR, PhDFounding Chief Executive Officer &
Chief Scientific Officer ARNON AHARON, MDChief Medical Officer SIGAL FATTAL, CPA, MBAChief Financial Officer DALE PFOST, PhD* Incoming Chief Executive Officer SHARON ELKOBI, MSc, MBAVP Business Development MICHAL SEGAL-SALTO, PhDVP
Research and Development *Post shareholder vote

Stephen Squinto, PhD Chairman of the Board Adi Mor, PhDFounding Chief Executive Officer & Chief
Scientific Officer Dale Pfost, PhD* Incoming Chief Executive Officer Neil Cohen, MADirector Nissim Darvish, MD, PhDDirector Joel Maryles, CFA, MBADirector Alan Moses, MD Director Claude Nicaise, MDDirector Board of Directors Scientific
Advisory Board Prof. Marco Matucci-Cerinic, MD, PhDDirector of the Division of Rheumatology, University of Florence, Italy Scott L. Friedman, MDThe Dean for Therapeutic Discovery and Chief, Division of Liver Diseases, Mount Sinai, NY,
USA Prof. Dinesh Khanna MD, MBBS, MScDirector of the Scleroderma Program, University of Michigan, Ann Arbor, Michigan, USA Massimo Pinzani, MD, PhD, FRCP Sheila Sherlock Chair of Hepatology, Director UCLInstitute for Liver and Digestive
Health, RFH, London, UK Gideon Hirschfield, MA MB PhDLily and Terry Horner Chair in Autoimmune Liver Disease, University of Toronto, Toronto General Hospital, Canada Prof. Francesco Del Galdo, MD, PhDHead of the Scleroderma Program at NIHR,
University of Leeds, UK Experienced Leadership *Post shareholder vote
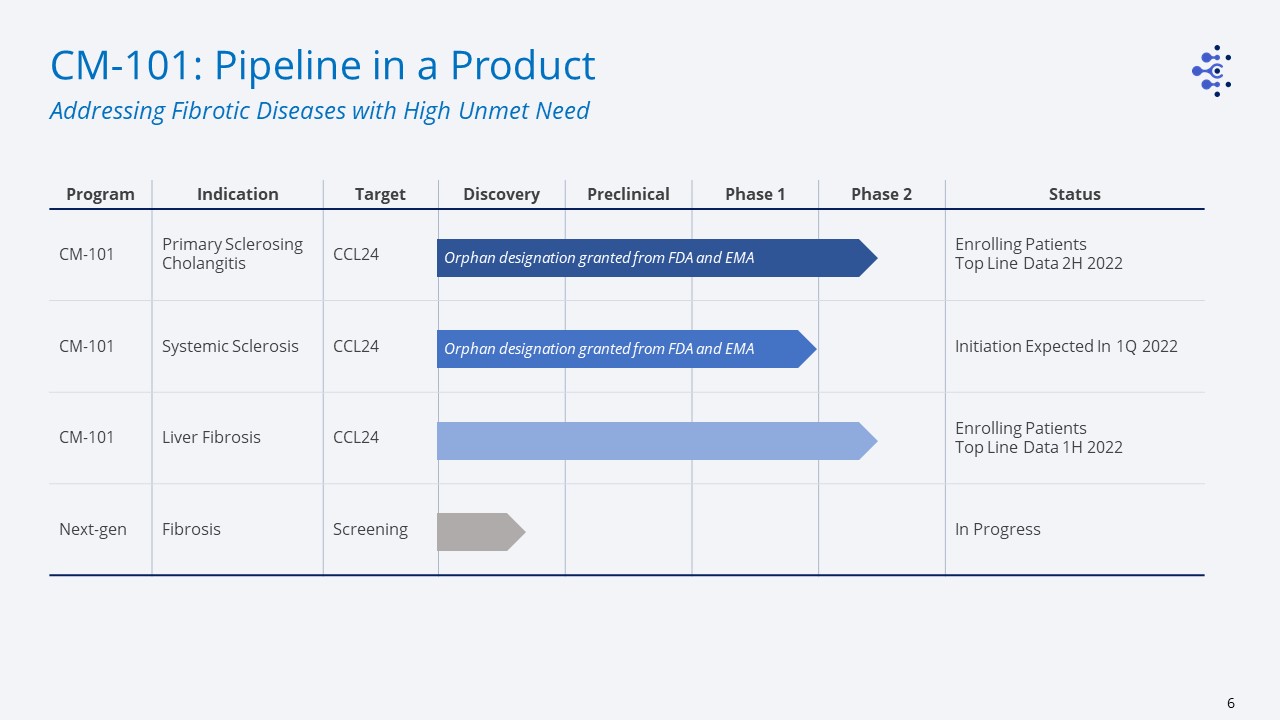
Program Indication Target Discovery Preclinical Phase 1 Phase 2 Status CM-101 Primary Sclerosing
Cholangitis CCL24 Enrolling PatientsTop Line Data 2H 2022 CM-101 Systemic Sclerosis CCL24 Initiation Expected In 1Q 2022 CM-101 Liver Fibrosis CCL24 Enrolling PatientsTop Line Data 1H
2022 Next-gen Fibrosis Screening In Progress CM-101: Pipeline in a Product Addressing Fibrotic Diseases with High Unmet Need Orphan designation granted from FDA and EMA Orphan designation granted from FDA and EMA
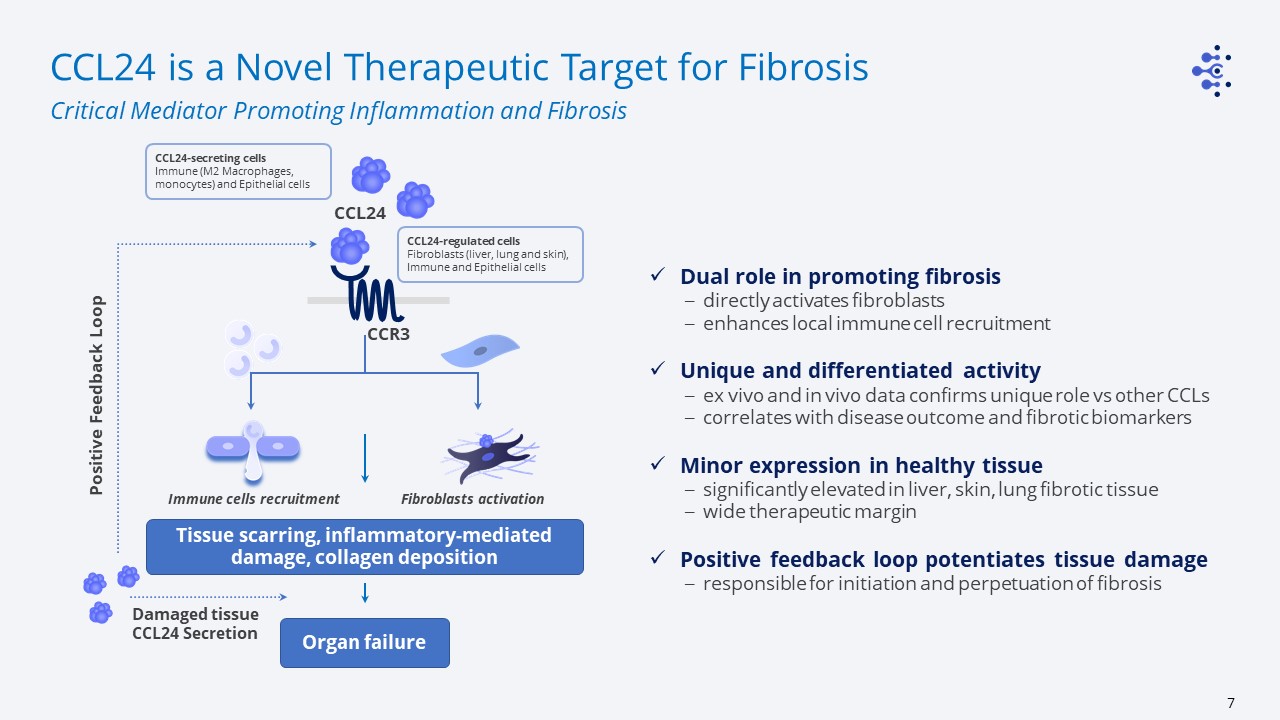
CCL24 is a Novel Therapeutic Target for Fibrosis Critical Mediator Promoting Inflammation and
Fibrosis CCL24 CCR3 CCL24-regulated cellsFibroblasts (liver, lung and skin), Immune and Epithelial cells Immune cells recruitment Fibroblasts activation Tissue scarring, inflammatory-mediated damage, collagen deposition Positive
Feedback Loop Organ failure Damaged tissue CCL24 Secretion CCL24-secreting cellsImmune (M2 Macrophages, monocytes) and Epithelial cells Dual role in promoting fibrosisdirectly activates fibroblastsenhances local immune cell
recruitmentUnique and differentiated activityex vivo and in vivo data confirms unique role vs other CCLscorrelates with disease outcome and fibrotic biomarkers Minor expression in healthy tissuesignificantly elevated in liver, skin, lung
fibrotic tissue wide therapeutic marginPositive feedback loop potentiates tissue damageresponsible for initiation and perpetuation of fibrosis

CCR3 Immune cells recruitment Fibroblasts activation &
proliferation CCL24 CM-101 Fibrosis Inflammation CM-101: A First-in-Class mAb Blocking CCL24 Dual Mechanism of Action Interfering with the Core Fibrotic Pathways CM-101 attenuates inflammation and fibrosis by inhibiting
fibroblasts activation and immune cells recruitment CM-101 Inhibits Primary Hepatic Fibroblasts Activation CM-101 Reduces In-vivo Monocyte Recruitment
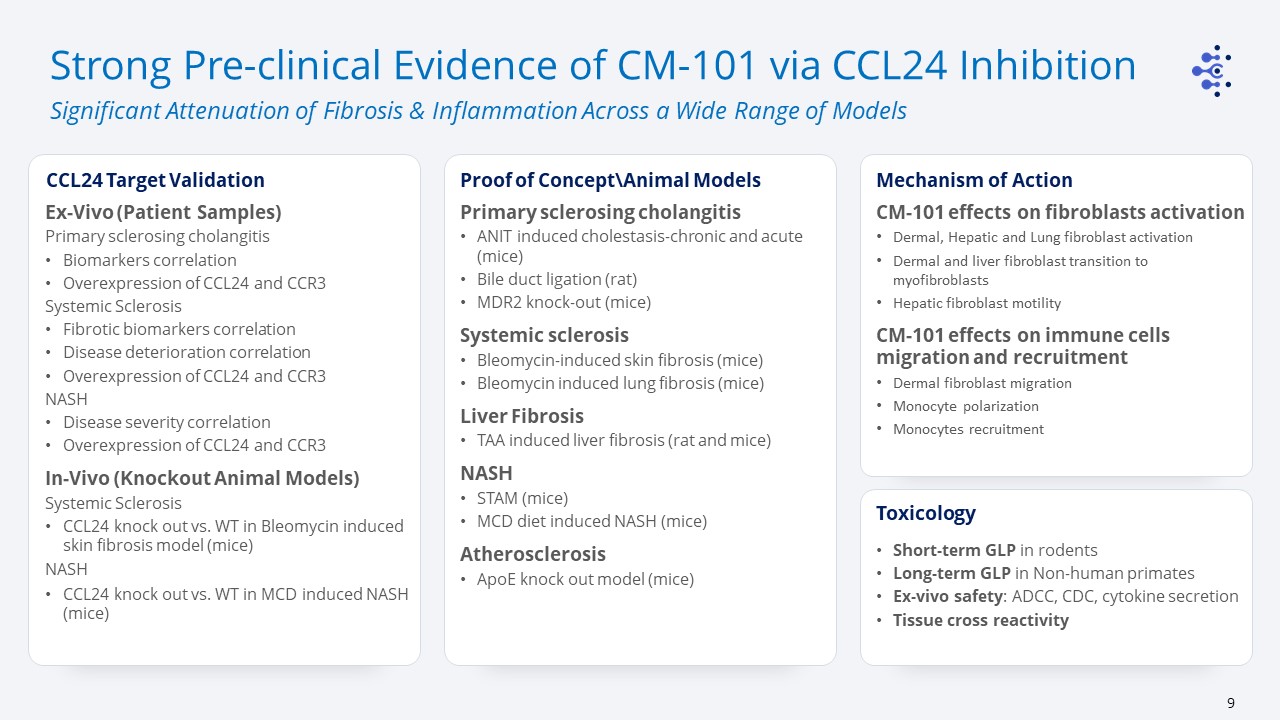
Strong Pre-clinical Evidence of CM-101 via CCL24 Inhibition Significant Attenuation of Fibrosis
& Inflammation Across a Wide Range of Models Primary sclerosing cholangitisANIT induced cholestasis-chronic and acute (mice)Bile duct ligation (rat)MDR2 knock-out (mice)Systemic sclerosisBleomycin-induced skin fibrosis (mice)Bleomycin
induced lung fibrosis (mice)Liver FibrosisTAA induced liver fibrosis (rat and mice)NASHSTAM (mice)MCD diet induced NASH (mice)AtherosclerosisApoE knock out model (mice) Proof of Concept\Animal Models Mechanism of Action Ex-Vivo (Patient
Samples)Primary sclerosing cholangitisBiomarkers correlationOverexpression of CCL24 and CCR3Systemic SclerosisFibrotic biomarkers correlationDisease deterioration correlationOverexpression of CCL24 and CCR3 NASHDisease severity
correlationOverexpression of CCL24 and CCR3In-Vivo (Knockout Animal Models)Systemic SclerosisCCL24 knock out vs. WT in Bleomycin induced skin fibrosis model (mice)NASHCCL24 knock out vs. WT in MCD induced NASH (mice) CCL24 Target
Validation Short-term GLP in rodentsLong-term GLP in Non-human primatesEx-vivo safety: ADCC, CDC, cytokine secretionTissue cross reactivity Toxicology CM-101 effects on fibroblasts activationDermal, Hepatic and Lung fibroblast
activationDermal and liver fibroblast transition to myofibroblastsHepatic fibroblast motilityCM-101 effects on immune cells migration and recruitmentDermal fibroblast migrationMonocyte polarizationMonocytes recruitment
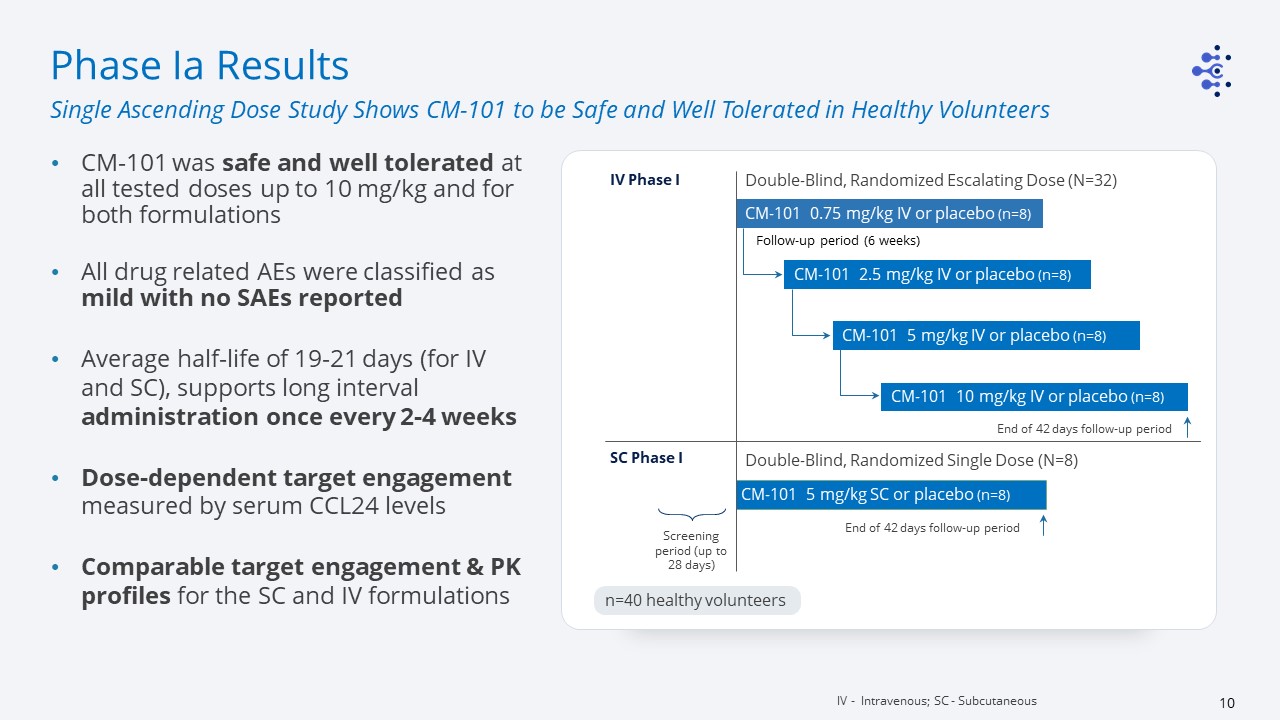
Double-Blind, Randomized Escalating Dose (N=32) CM-101 0.75 mg/kg IV or placebo (n=8) CM-101 2.5
mg/kg IV or placebo (n=8) CM-101 5 mg/kg IV or placebo (n=8) CM-101 10 mg/kg IV or placebo (n=8) Double-Blind, Randomized Single Dose (N=8) CM-101 5 mg/kg SC or placebo (n=8) IV Phase I End of 42 days follow-up period SC Phase I End
of 42 days follow-up period Screening period (up to 28 days) Follow-up period (6 weeks) n=40 healthy volunteers Phase Ia Results CM-101 was safe and well tolerated at all tested doses up to 10 mg/kg and for both formulations All drug
related AEs were classified as mild with no SAEs reportedAverage half-life of 19-21 days (for IV and SC), supports long interval administration once every 2-4 weeksDose-dependent target engagement measured by serum CCL24 levelsComparable target
engagement & PK profiles for the SC and IV formulations IV - Intravenous; SC - Subcutaneous Single Ascending Dose Study Shows CM-101 to be Safe and Well Tolerated in Healthy Volunteers

Wk 15End of treatment Wk 18End of study Double-Blind, Randomized Escalating Dose (N=16) CM-101
2.5 mg/kg IV or placebo (n=8) CM-101 5 mg/kg SC or placebo (n=8) Phase Ib Day 0Randomization Study population: NAFLD patients with normal liver functionMultiple CM-101 administrations were safe and well tolerated using both IV and SC
formulationsMost frequently reported AEs were mild with no drug-related SAEs reportedFavorable t1/2, supports long dosing interval (Q2W - Q4W) Phase Ib Results Multiple Administration Study Showed CM-101 to be Safe & Well Tolerated in
NAFLD Patients IV - Intravenous; SC – Subcutaneous; Wk - week Study Design Tested doses - 2.5 mg/kg IV infusion and 5 mg/kg SC injection 5 repeated administrations per patient; Q3WPrimary endpoint - safety and tolerability
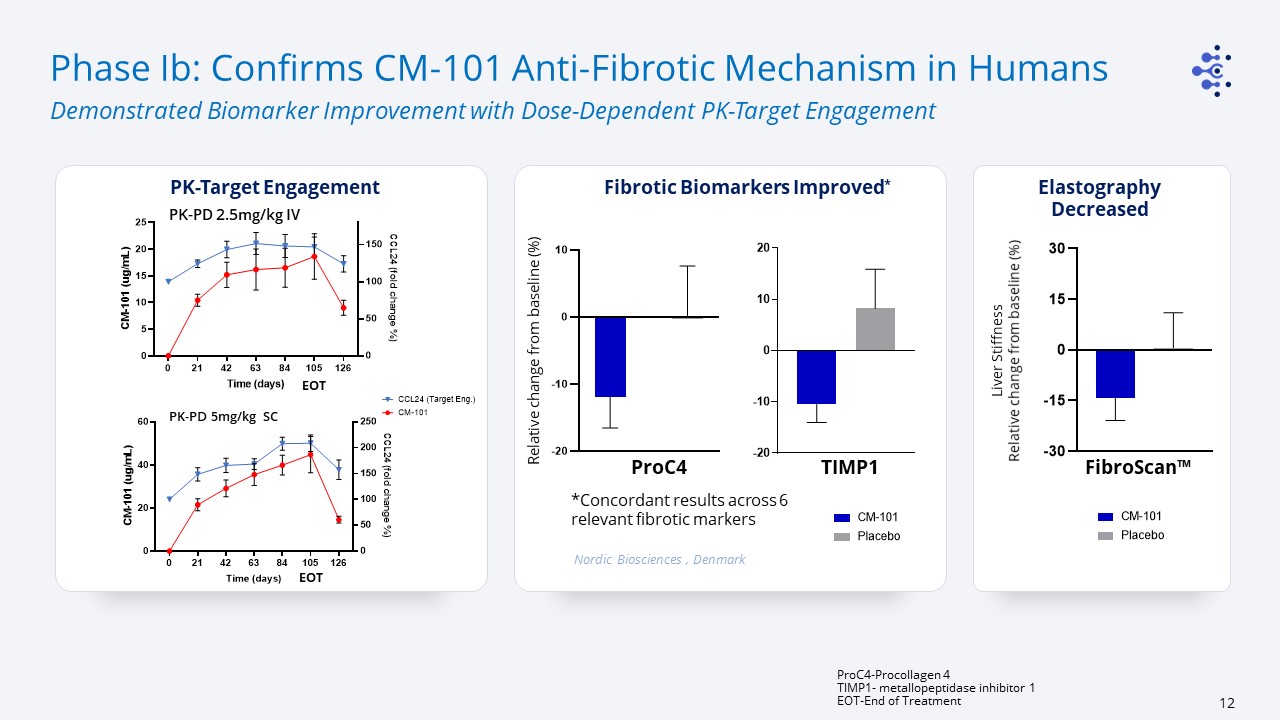
Phase Ib: Confirms CM-101 Anti-Fibrotic Mechanism in Humans Demonstrated Biomarker Improvement
with Dose-Dependent PK-Target Engagement ProC4-Procollagen 4TIMP1- metallopeptidase inhibitor 1EOT-End of Treatment PK-Target Engagement PK-PD 2.5mg/kg IV PK-PD 5mg/kg SC Liver Stiffness Relative change from baseline (%) Nordic
Biosciences , Denmark Fibrotic Biomarkers Improved* FibroScan™ ProC4 *Concordant results across 6 relevant fibrotic markers TIMP1 ElastographyDecreased Relative change from baseline (%) EOT EOT

CM-101 for the Treatment of Primary Sclerosing Cholangitis
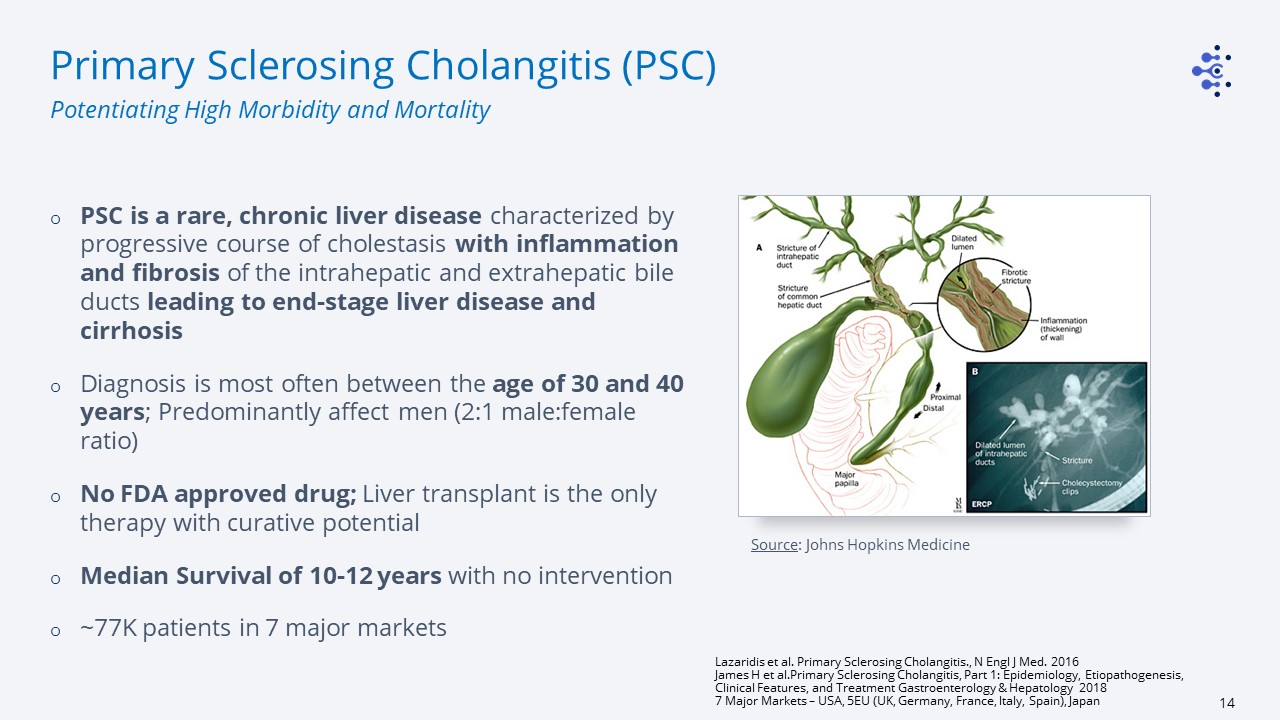
Source: Johns Hopkins Medicine Primary Sclerosing Cholangitis (PSC) PSC is a rare, chronic liver
disease characterized by progressive course of cholestasis with inflammation and fibrosis of the intrahepatic and extrahepatic bile ducts leading to end-stage liver disease and cirrhosisDiagnosis is most often between the age of 30 and 40
years; Predominantly affect men (2:1 male:female ratio)No FDA approved drug; Liver transplant is the only therapy with curative potentialMedian Survival of 10-12 years with no intervention~77K patients in 7 major markets Lazaridis et al.
Primary Sclerosing Cholangitis., N Engl J Med. 2016 James H et al.Primary Sclerosing Cholangitis, Part 1: Epidemiology, Etiopathogenesis, Clinical Features, and Treatment Gastroenterology & Hepatology 20187 Major Markets – USA, 5EU (UK,
Germany, France, Italy, Spain), Japan Potentiating High Morbidity and Mortality

CCL24 serum level in PSC patients Healthy Liver Collaboration with RFH, UK PSC Liver CCL24
STAINING CCL24 expression is significantly and selectively elevated in PSC livers CCL24 level in healthy vs PSC patients' livers tissues CCL24 correlates with ELF in PSC patients with elevated ALP CCL24 Plays a Key Role in PSC Clinical
Relevance of CCL24 in PSC Pathophysiology is Supported by Patient Samples ELF – Enhanced Liver Fibrosis; ALP – Alkaline Phosphatase CCL24 overexpressed in bile epithelial cells Enhanced Liver Fibrosis (ELF) test reflects fibrosis and
predicts clinical outcomes in PSC1 1. M. Vesterhus et al. Hepatology, 62 (1) (2015), pp. 188-197 CM-101 MoA guided the selection of ELF and ALP as Primary endpoints in Phase II
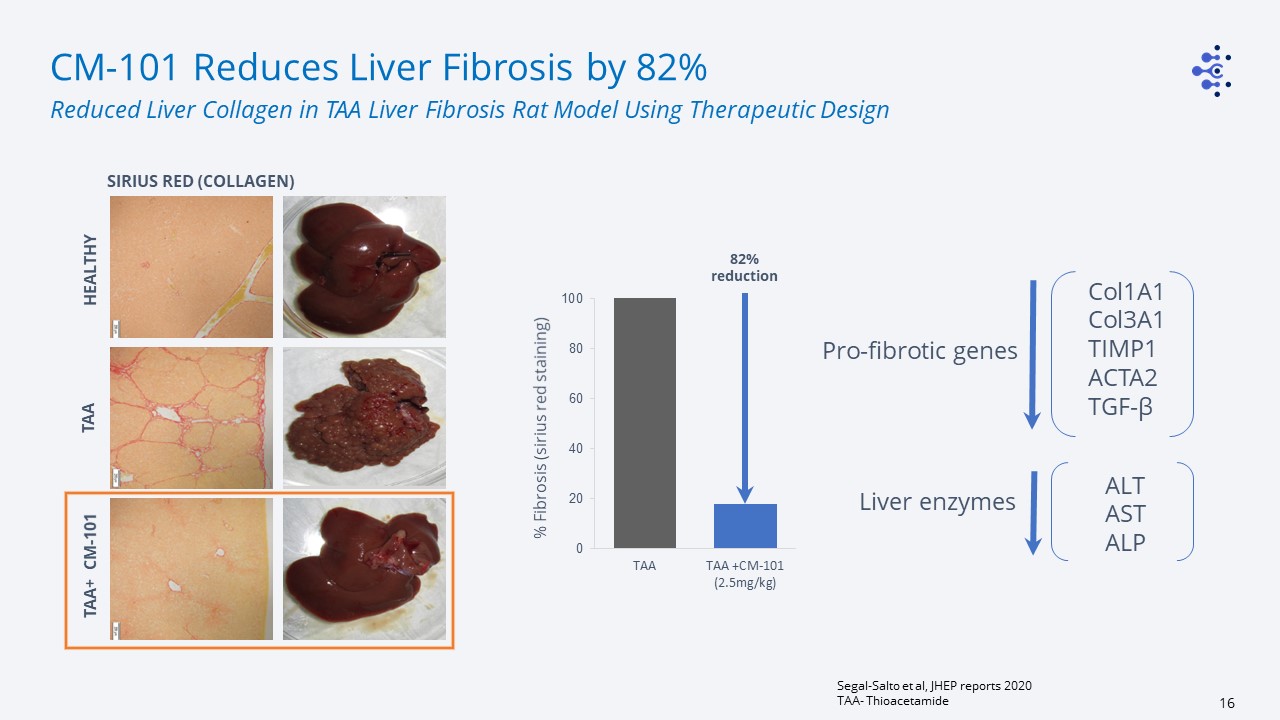
% Fibrosis (sirius red staining) 82% reduction Col1A1Col3A1TIMP1ACTA2TGF-β ALTASTALP Liver
enzymes Pro-fibrotic genes CM-101 Reduces Liver Fibrosis by 82% Segal-Salto et al, JHEP reports 2020TAA- Thioacetamide Reduced Liver Collagen in TAA Liver Fibrosis Rat Model Using Therapeutic Design HEALTHY TAA SIRIUS RED
(COLLAGEN) TAA+ CM-101
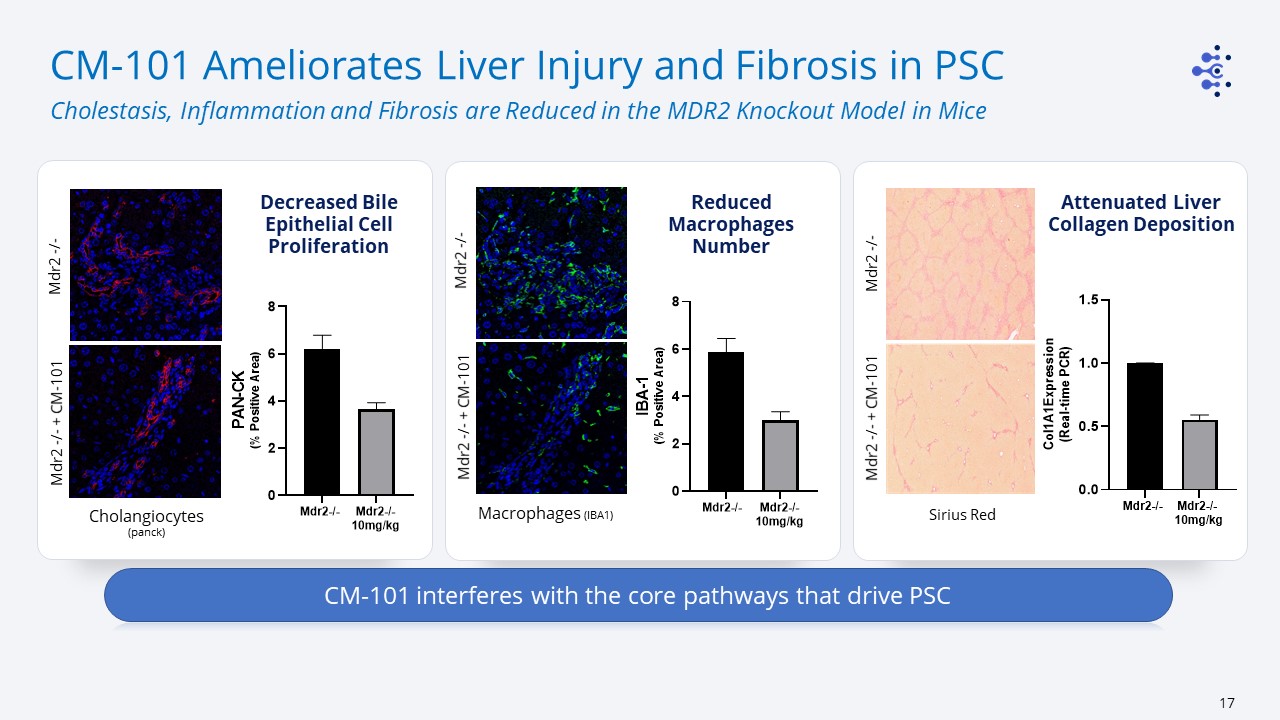
CM-101 Ameliorates Liver Injury and Fibrosis in PSC Cholestasis, Inflammation and Fibrosis are
Reduced in the MDR2 Knockout Model in Mice Macrophages (IBA1) Mdr2 -/- + CM-101 Cholangiocytes (panck) Sirius Red Mdr2 -/- Mdr2 -/- Reduced Macrophages Number Mdr2 -/- + CM-101 Mdr2 -/- Mdr2 -/- + CM-101 CM-101 interferes with
the core pathways that drive PSC Decreased Bile Epithelial Cell Proliferation Attenuated Liver Collagen Deposition
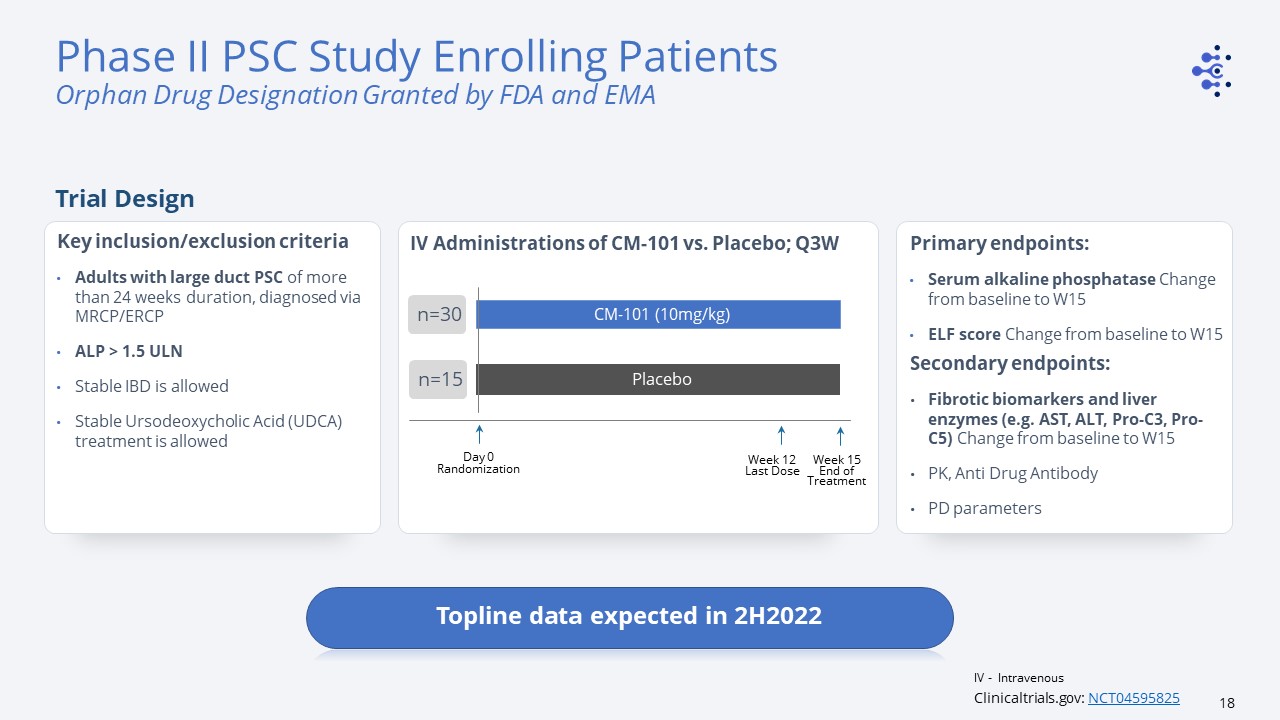
IV Administrations of CM-101 vs. Placebo; Q3W Week 12Last Dose CM-101 (10mg/kg) Placebo Day
0Randomization n=30 n=30 n=15 Primary endpoints: Serum alkaline phosphatase Change from baseline to W15 ELF score Change from baseline to W15 Secondary endpoints: Fibrotic biomarkers and liver enzymes (e.g. AST, ALT, Pro-C3, Pro-C5)
Change from baseline to W15 PK, Anti Drug AntibodyPD parameters Key inclusion/exclusion criteriaAdults with large duct PSC of more than 24 weeks duration, diagnosed via MRCP/ERCPALP > 1.5 ULNStable IBD is allowed Stable Ursodeoxycholic Acid
(UDCA) treatment is allowed Week 15End of Treatment Clinicaltrials.gov: NCT04595825 Phase II PSC Study Enrolling PatientsOrphan Drug Designation Granted by FDA and EMA Trial Design Topline data expected in 2H2022 IV - Intravenous

CM-101 for the Treatment of Systemic Sclerosis

Systemic Sclerosis (SSc) Rare autoimmune rheumatic disease characterized by inflammation and
fibrosis of the internal organs as well as vasculopathyAge of diagnosis 30-50 years, Predominantly affect women (3:1 female:male ratio)There is no approved disease modifying drug; Treatments focus on managing disease symptoms (Nintedanib,
Tocilizumab)Median Survival of 10 years - the highest mortality rate among the systemic rheumatic diseasesPulmonary involvement is the leading cause of death~170K patients in 7 major markets Global data; Bergamasco et al., Clin Epidemiol.
2019; 11: 257–2737 Major Markets – USA, 5EU (UK, Germany, France, Italy, Spain), Japan Most Lethal Among Systemic Rheumatic Diseases Source:
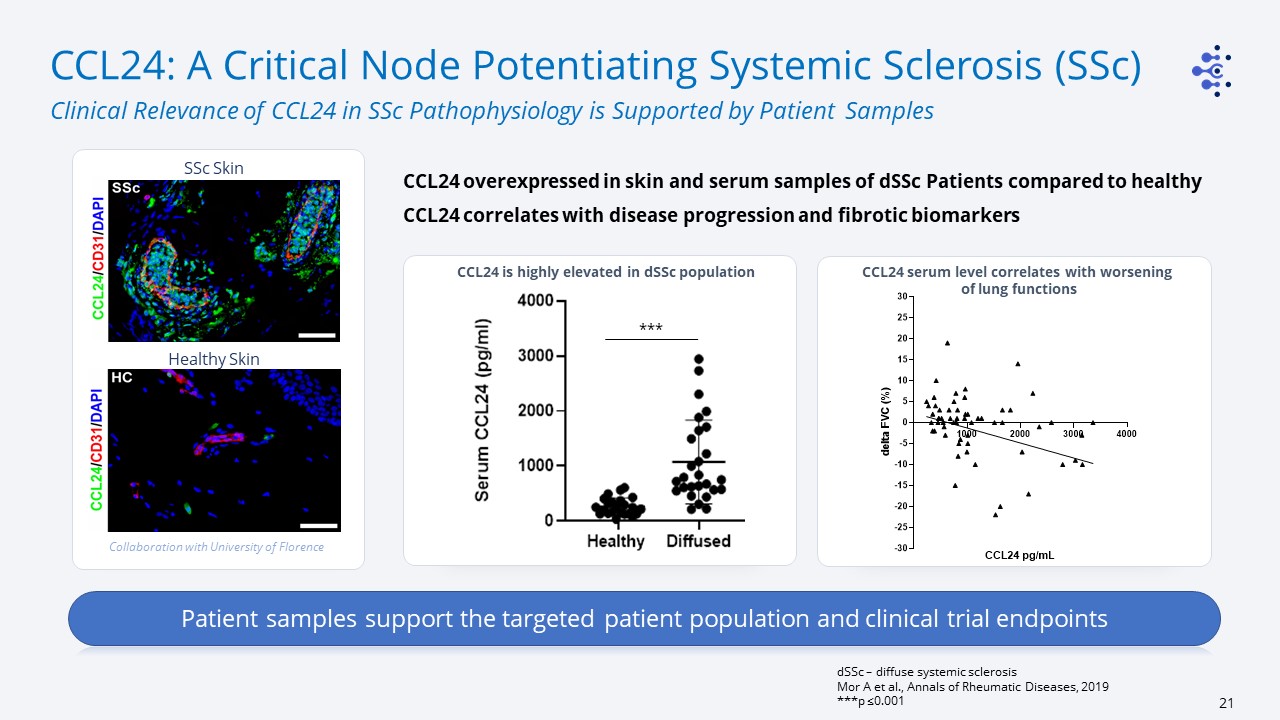
CCL24: A Critical Node Potentiating Systemic Sclerosis (SSc) Clinical Relevance of CCL24 in SSc
Pathophysiology is Supported by Patient Samples dSSc – diffuse systemic sclerosisMor A et al., Annals of Rheumatic Diseases, 2019***p ≤0.001 CCL24 overexpressed in skin and serum samples of dSSc Patients compared to healthyCCL24 correlates
with disease progression and fibrotic biomarkers *** SSc Skin Collaboration with University of Florence Healthy Skin CCL24 serum level correlates with worsening of lung functions CCL24 is highly elevated in dSSc population Patient
samples support the targeted patient population and clinical trial endpoints
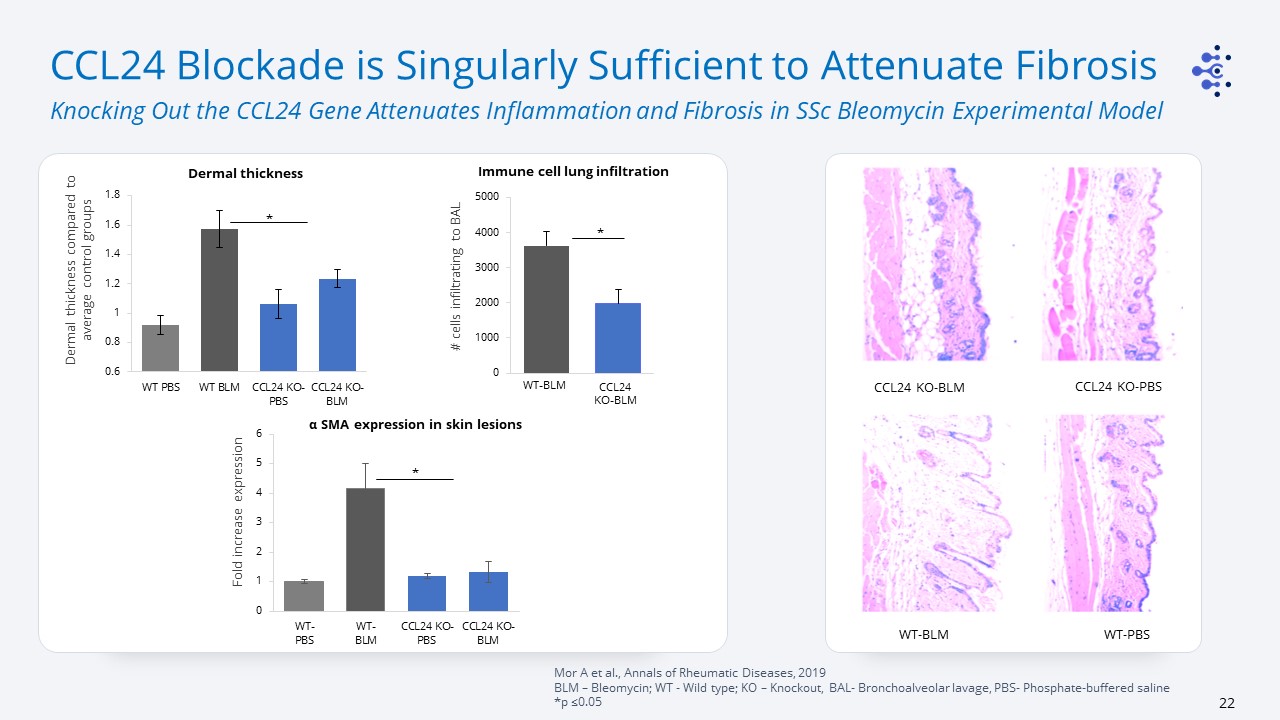
CCL24 Blockade is Singularly Sufficient to Attenuate Fibrosis Knocking Out the CCL24 Gene Attenuates
Inflammation and Fibrosis in SSc Bleomycin Experimental Model Mor A et al., Annals of Rheumatic Diseases, 2019BLM – Bleomycin; WT - Wild type; KO – Knockout, BAL- Bronchoalveolar lavage, PBS- Phosphate-buffered saline *p ≤0.05 WT-PBS CCL24
KO-PBS WT-BLM CCL24 KO-BLM Fold increase expression α SMA expression in skin lesions Dermal thickness Dermal thickness compared to average control groups * * # cells infiltrating to BAL Immune cell lung infiltration
* WT-BLM CCL24KO-BLM
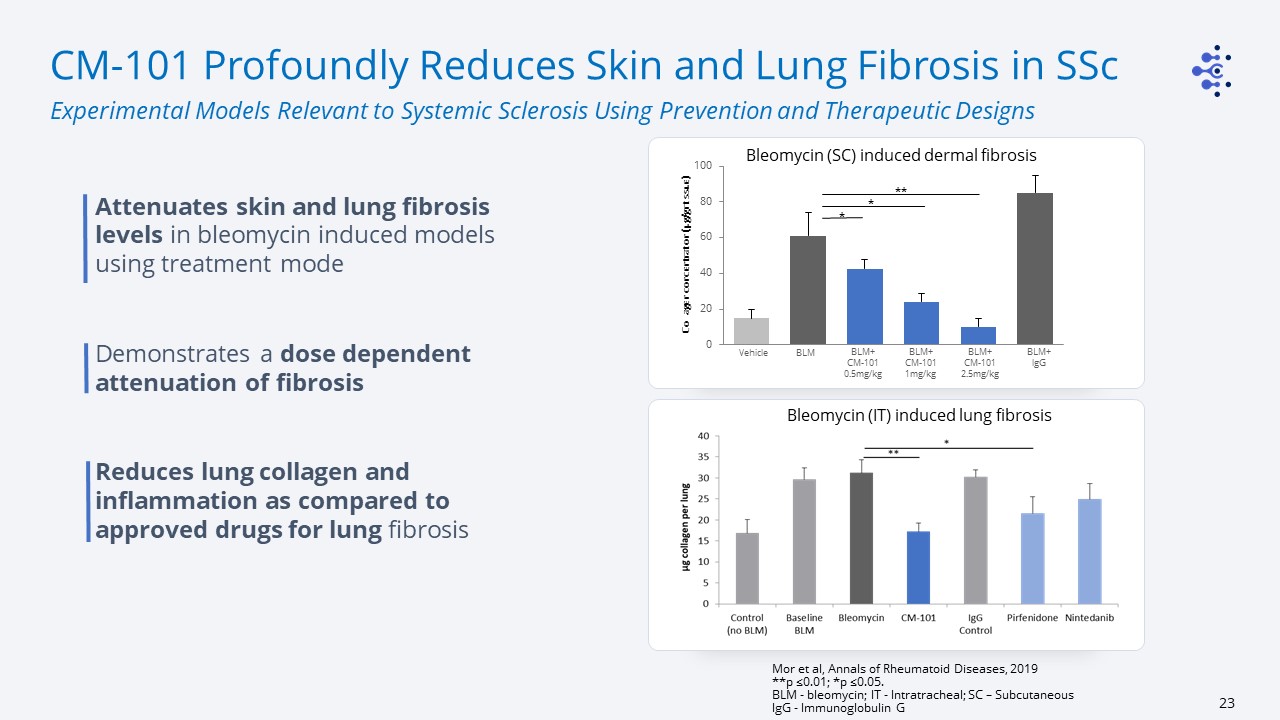
CM-101 Profoundly Reduces Skin and Lung Fibrosis in SSc Experimental Models Relevant to Systemic
Sclerosis Using Prevention and Therapeutic Designs Mor et al, Annals of Rheumatoid Diseases, 2019**p ≤0.01; *p ≤0.05. BLM - bleomycin; IT - Intratracheal; SC – SubcutaneousIgG - Immunoglobulin G Attenuates skin and lung fibrosis levels in
bleomycin induced models using treatment modeDemonstrates a dose dependent attenuation of fibrosisReduces lung collagen and inflammation as compared to approved drugs for lung fibrosis Collagen concentration (µg/gr
tissue) Vehicle BLM BLM+CM-101 0.5mg/kg BLM+CM-101 1mg/kg BLM+CM-101 2.5mg/kg BLM+lgG * * ** Bleomycin (SC) induced dermal fibrosis Bleomycin (IT) induced lung fibrosis
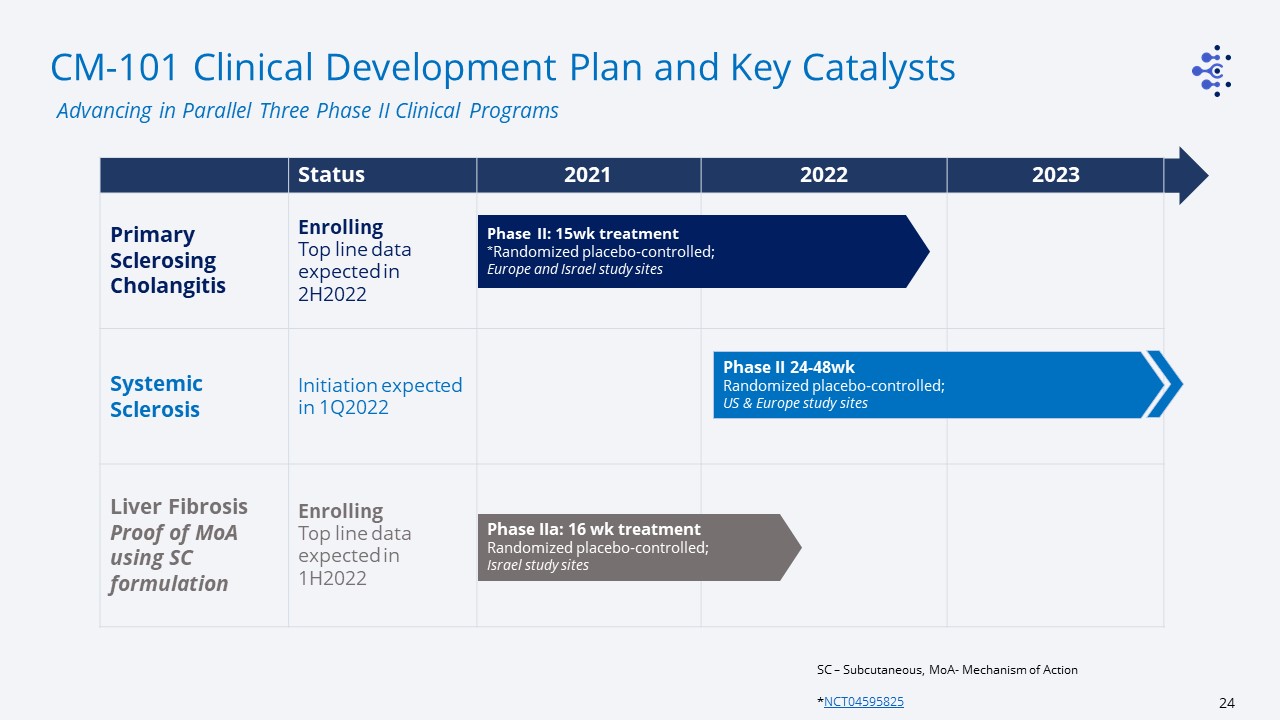
CM-101 Clinical Development Plan and Key Catalysts SC – Subcutaneous, MoA- Mechanism of Action
*NCT04595825 Advancing in Parallel Three Phase II Clinical Programs Status 2021 2022 2023 Primary Sclerosing Cholangitis EnrollingTop line data expected in 2H2022 Systemic Sclerosis Initiation expected in
1Q2022 Liver FibrosisProof of MoA using SC formulation EnrollingTop line data expected in 1H2022 Phase II: 15wk treatment*Randomized placebo-controlled;Europe and Israel study sites Phase II 24-48wkRandomized
placebo-controlled;US & Europe study sites Phase IIa: 16 wk treatmentRandomized placebo-controlled;Israel study sites
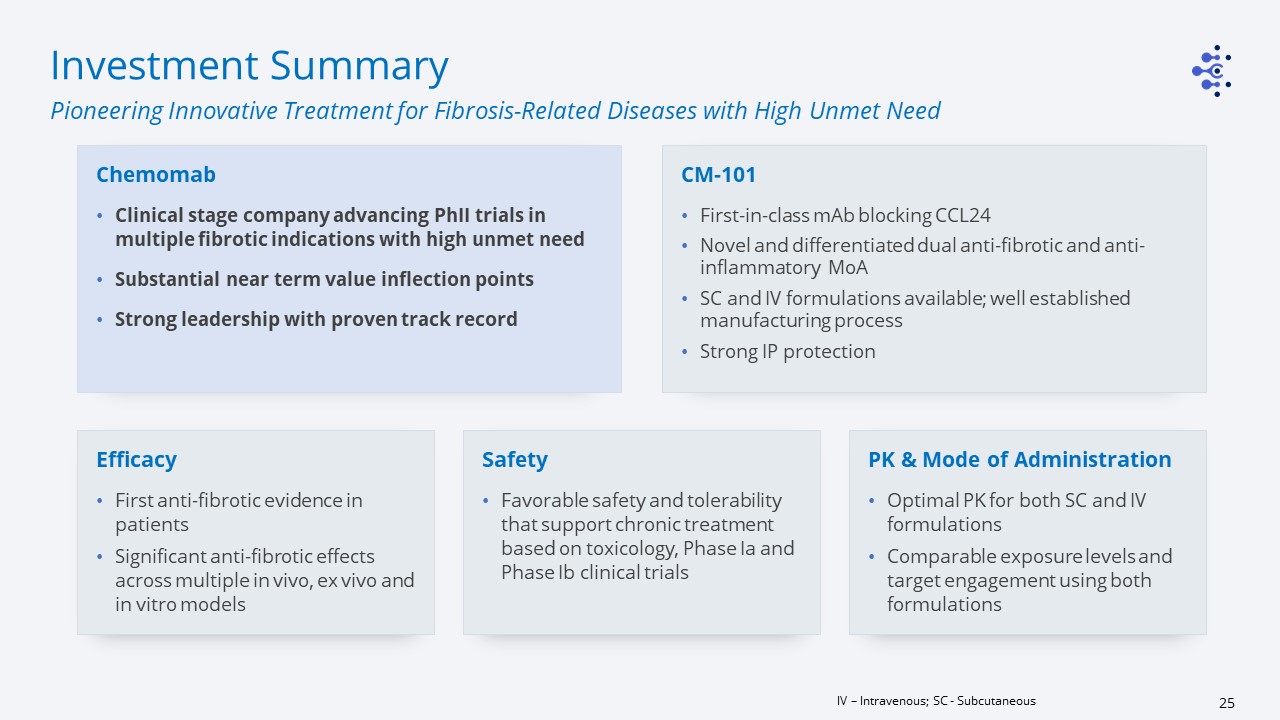
Investment Summary Pioneering Innovative Treatment for Fibrosis-Related Diseases with High Unmet
Need IV – Intravenous; SC - Subcutaneous Chemomab Clinical stage company advancing PhII trials in multiple fibrotic indications with high unmet need Substantial near term value inflection pointsStrong leadership with proven track record PK
& Mode of AdministrationOptimal PK for both SC and IV formulationsComparable exposure levels and target engagement using both formulations EfficacyFirst anti-fibrotic evidence in patientsSignificant anti-fibrotic effects across multiple in
vivo, ex vivo and in vitro models CM-101First-in-class mAb blocking CCL24Novel and differentiated dual anti-fibrotic and anti-inflammatory MoA SC and IV formulations available; well established manufacturing processStrong IP
protection SafetyFavorable safety and tolerability that support chronic treatment based on toxicology, Phase Ia and Phase Ib clinical trials

Presentation name Thank You
