Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Hoth Therapeutics, Inc. | ea146814-8k_hoththera.htm |
Exhibit 99.1

Clinical - Stage Biopharmaceutical Company Focused on Next Generation Therapeutics Meeting Unmet Patient Needs SEPTEMBER 2021

Safe Harbor Sta t ement This presentation contains "forward - looking statements" within the meaning of the “safe - harbor” provisions of the Private Securities Litigation Reform Act of 1995 . These statements are identified by the use of words “could,” “believe,” “anticipate,” “intend,” “estimate,” “expect,” “may,” “continue,” “predict,” “potential” and similar expressions that are intended to identify forward - looking statements . Such statements involve known and unknown risks, uncertainties and other factors that could cause the actual results of Hoth Therapeutics, Inc . (“Hoth” or the “Company”) to differ materially from the results expressed or implied by such statements . These forward - looking statements are made on the basis of the current beliefs, expectations and assumptions of management, are not guarantees of performance and are subject to significant risks and uncertainty . These forward - looking statements should, therefore, be considered in light of various important factors, including those set forth in Hoth’s reports that it files from time to time with the Securities and Exchange Commission (the “Commission”) and which you should review, including those statements under “Item 1 A – Risk Factors” in Hoth’s Annual Report on Form 10 - K, as amended by its Quarterly Reports on Form 10 - Q and other reports that Hoth files with the Commission . Important factors that could cause actual results to differ materially from those described in forward - looking statements contained in this presentation include, but are not limited to : the adverse impact on economies around the world of the current COVID - 10 pandemic ; changes to our anticipated sources of revenues ; competitive conditions ; difficulties in obtaining regulatory approvals for the Company’s product candidates ; changes in economic and political conditions ; the success of our research and development initiates ; and other factors . These forward - looking statements should not be relied upon as predictions of future events and Hoth cannot assure you that the events or circumstances discussed or reflected in these statements will be achieved or will occur . If such forward - looking statements prove to be inaccurate, the inaccuracy may be material . You should not regard these statements as representation or warranty by Hoth or any other person that we will achieve our objectives and plans in any specified timeframe, or at all . You are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date of this presentation . The Company disclaims any obligations to publicly update or release any revisions to the forward - looking information contained in this presentation, whether as a result of new information, future events or otherwise, after the date of this presentation or to reflect the occurrence of unanticipated events, except as required by law .

OUR MISSION Hoth Therapeutics is a biopharmaceutical visionary dedicated to finding, investigating and developing early - stage therapeutics that will change the way diseases are managed an d trea t ed. Guide d b y the belief that treatment should not make you sicker than the diseas e , Hoth The r a p eutics see k s out overlooked drugs that hold the promise to improve treatments and address significant unmet needs for patients. Hoth Therapeutics fol l o ws the science to find the ide a l indications for each asset and partners with leading com p an i es in the space to bring therapies to market. At Hoth Therapeutics, we understand how devastating it can be to live with a chronic illness, be diagnosed with a rare disease, or the only option is to take a medicine that causes unrelenting side effects. We believe that e v e r y p at i ent d e se r v es t o g et t h e therapies they need to address their disease and improve their quality of life.

Key Investment Highlights 2 Clinical Programs Robust Pre - Clinical Development Programs Targeting Unmet Medical Needs to Address Broad Market Experienced Mana g eme n t and Advisory Board

www.hoththerapeutics.com N A S D A Q: HOTH Pipeline: Multiple Shots on Goal 5

www.hoththerapeutics.com N A S D A Q: HOTH CLINICAL P R OGRAMS BioLexa Lotion Phase 1 b Clinical Trial in Humans for Treatment of Mild to Moderate Atopic Dermatitis in 2021 HT - 001 IND - Enabling Studies in 2021 & Phase 2a Clinical Trial in Patients Targeted for Q1 2022 6

www.hoththerapeutics.com 7 N A S D A Q: HOTH BioLexa Lotion : Value Proposition Market Growth: Atopic dermatitis market predicted to grow from $6.4B in 2017 to $18.3B by end of 2027* Mechanism of Action : Novel mixture of two previously approved compounds targeting the underlying Staphylococcus aureus infection hypothesize to potentiate Atopic Dermatitis (AD) or eczema flares - First compound prevents biofilm formation, which protects the underlying infection, allowing the second, an antibiotic, to more effectively treat the underlying infection . Addresses Unmet Need: Non - corticosteroid treatment targeted for treatment of both pediatric and adult mild to moderate AD populations *Atopic Dermatitis Market – Global Industry Analysis, Size and Forecast,2017 - 2027

Recent Milestones: BioLexa Lotion • HREC Approval on December 9, 2020 to conduct phase 1b clinical trial in Australia • Site - Recruitment Completed • Dosing of Cohort 1 Complete • The interim safety review indicates that BioLexa was well tolerated with no serious adverse events and no drug - related treatment - emergent adverse events observed. • Cohort 2 submission and screening expected to start in Autumn of 2021

www.hoththerapeutics.com N A S D A Q: HOTH BioLexa Phase 1b Clinical Study Design A Randomised, Double - Blind, Vehicle Controlled, Sequential Group Study to Determine the Safety, Tolerability, Pharmacokinetics and Efficacy of Twice Daily Application of Topical BioLexa Πin Adult Healthy Subjects and Patients with Mild to Moderate Atopic Dermatitis 9

www.hoththerapeutics.com N A S D A Q: HOTH BioLexa: Proof - of - Concept Results Either Alone Not Adequate Combination Works Best DTPA alone G e n ta m i c i n alone Miller School of Medicine, of the University of Miami and University of Cincinnati - Determination of the effects of a novel antimicrobial agent used in conjunction with Gentamicin on Staphylococcus aureus using a porcine model: preliminary evaluations Jose Valdes, Joel Gil, Andrew Herr, Andrew Harding and Stephen Davis This study concluded that the combination of gentamicin and Ca - DTPA is more effective to reduce bacteria growth and inhibit the formation of biofilms than each compound individually. Combination reduced bacteria below LOQ Miller School of Medicine, of the University of Miami and University of Cincinnati - Determination of the effects of a novel antimicrobial agent used in conjunction with Gentamicin on Staphylococcus aureus using a porcine model: preliminary evaluations Jose Valdes, Joel Gil, Andrew Herr, Andrew Harding and Stephen Davis 10

www.hoththerapeutics.com 11 N A S D A Q: HOTH HT - 001 : Value Proposition Market Growth: EGFR Inhibitor Skin Toxicity market predicted to grow from $52M in 2018 to $391M by end of 2030* Mechanism of Action : 12 - week study conducted at GW suggests the topical application of HT - 001 significantly reduces erlotinib - induced cutaneous toxicities ( 71 % reduction compared to control) . It supports that HT - 001 may be used as a topical intervention to treat EGFR - inhibitor - induced cutaneous toxicity . ** Addresses Unmet Need: No current approved product on the market that specifically treats EGFR inhibitor cutaneous toxicities, which occur in up to 90% of patients*** undergoing EFGR inhibitor therapy *EGFR Inhibitors - Induced Skin Disorders - Market Insights, Epidemiology, and Market Forecast - 2030 **https://ir.hoththerapeutics.com/ht - 001 ***https://jamanetwork.com/journals/jamadermatology/article - abstract/2767656

Recent Milestones: HT - 001

www.hoththerapeutics.com N A S D A Q: HOTH Proposed HT - 001 Phase 2a Clinical Trial Design A Randomized, Placebo - Controlled, Parallel Phase 2a Dose Ranging Study to Investigate the Efficacy, Safety, and Tolerability of Topical HT - 001 for the Treatment of Skin Toxicities Associated with EGFR Inhibitors 13

www.hoththerapeutics.com N A S D A Q: HOTH • HT - 001 administered either via oral or topical application is effective to significantly reduce EGFR inhibitor - induced skin toxicities in rats HT - 001 : Topical HT - 001 Proof - of - Concept Results HT - 001 (O) alone – 12 weeks 14 Erlotonib (O) + HT - 001 (O) – week 12 Erlotonib (O) + HT - 001 (T) – week 12 HT - 001 (O) Erl (O) + HT - 001 (O) Erl (O) + HT - 001 (T )

www.hoththerapeutics.com N A S D A Q: HOTH Recent Milestones and Upcoming Catalysts: Clinical Programs Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 15 BioLexa Lotion (Atopic Dermatitis) Initiate Phase 1 Cohort 1 dosing Preliminary Cohort 1 results Official Cohort 1 Results Initiate Phase 1b Cohort 2 Patient Dosing Initiate Phase 1b Cohort 2 Patient Dosing Official Cohort 1 Results Preliminary Results Phase 1b Cohort 2 Results HT - 001 (Cutaneous toxicity of EGFR Inhibitors) GMP Development No n - GL P to xi c o l ogy studies Clinical Protocol Development GLP Toxicology Studies Clinical Site Selection US IND submission Clinical Site Activation Initiate Phase 2a Clinical Trial Preliminary Results

www.hoththerapeutics.com N A S D A Q: HOTH 16 LATE STAGE PRE - CLINICAL P R OGRAMS H T - 003 Acne, Psoriasis HT - 004 Asthma, Allergic Inflammation HT - 005 Cutaneous Lupus Erythematosus (CLE) Vaxcelerate COVID - 19 Vaccine

www.hoththerapeutics.co m 17 N A S D A Q: HOTH Recent Preclinical Development Milestones • HT - 003 : Preclinical results show the ability of HT - 003 to inhibit TLR2 signaling pathway suggesting that HT - 003 is a potentially effective therapeutic for acne.* Additional animal model studies are currently underway to explore other inflammatory - driven dermatological indications. • HT - 004 : Mouse asthma model study demonstrates that HT - 004 delivered by inhalation is effective to reduce inflammatory cell recruitment around bronchioles, supporting a robust therapeutic response with no signs of tissue irritation. Development of a humanized mouse model is currently in progress to finalize the lead actives for further development. • HT - 005 Z - Pods : Rat model of Lupus with cutaneous lesions showed HT - 005 Z - Pods provided long term therapeutic response over 10 weeks to prevent development of lesions. • Vaxcelerate : A near GLP preclinical mouse study demonstrated that the self - assembling vaccine construct significantly increased both helper and cytotoxic T cell responses to the vaccine targeted antigens compared to controls.**** IND - enabling studies are planned with a Pre - IND meeting request targeted for Q4 2021. *See Appendix for HT - 003 preclinical results **See Appendix for HT - 005 Z - Pod preclinical results ***See Appendix for HT - 004 preclinical results ****Results reported from Voltron
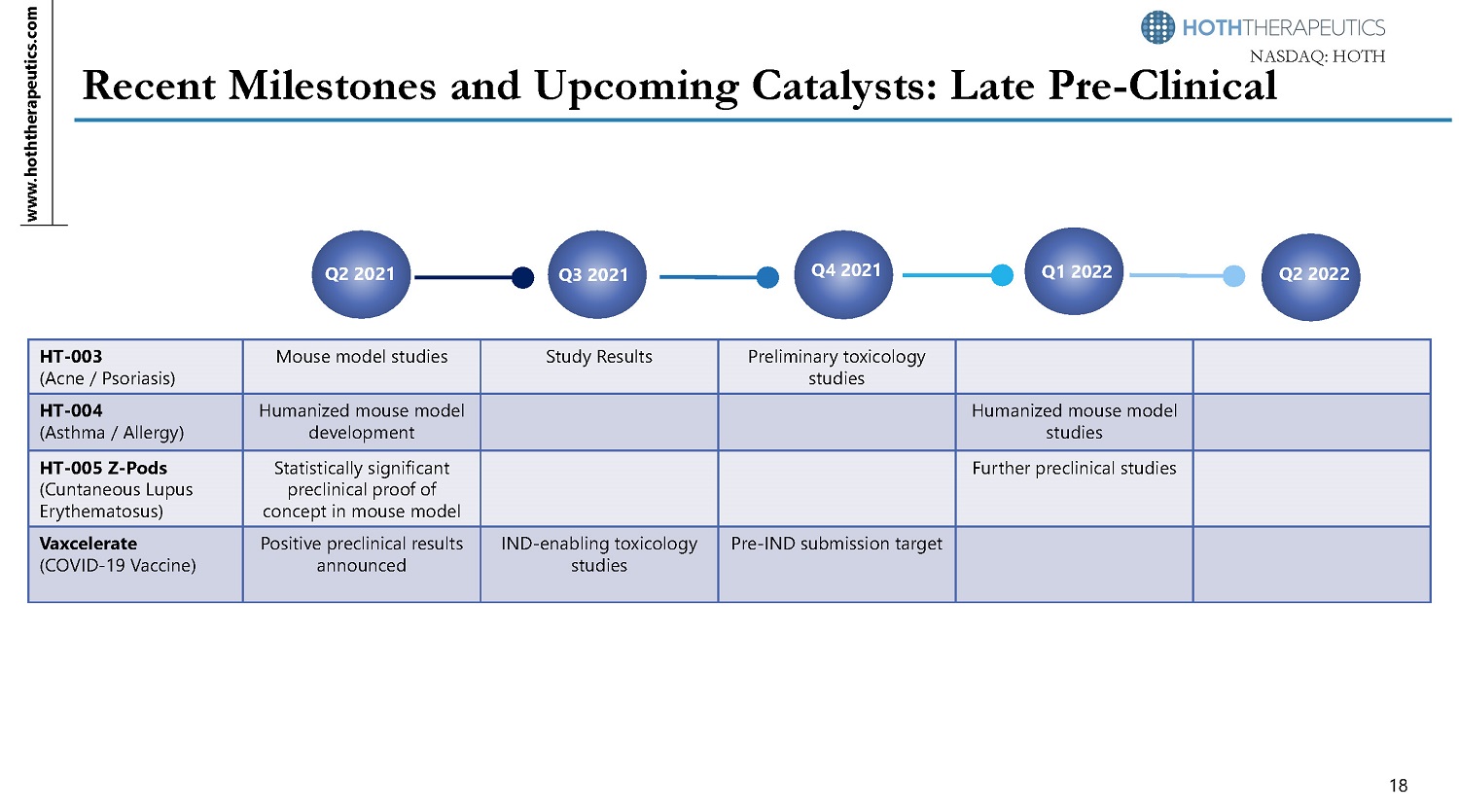
www.hoththerapeutics.com N A S D A Q: HOTH Recent Milestones and Upcoming Catalysts: Late Pre - Clinical Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 18 HT - 003 (Acne / Psoriasis) Mouse model studies Study Results P r eli mina r y to xi c o l ogy studies HT - 004 (Asthma / Allergy) Humanized mouse model development Humanized mouse model studies HT - 005 Z - Pods (Cuntaneou s Lupus Erythematosus) Statistically significant preclinical proof of concept in mouse model Further preclinical studies Vaxcelerate (COVID - 19 Vaccine) Positive preclinical results announced IND - enabling toxicology studies Pre - IND submission target

www.hoththerapeutics.com N A S D A Q: HOTH 19 EARLY PRE - CLINICAL P R OGRAMS HT - 003 Inflammatory Bowel Diseases HT - 006 Hospital - Acquired Pneumonia/ Ventilator - Acquired Pneumonia HT - ALZ Alzheimer’s Disease HT - KIT Mast Cell Neoplasms/ Anaphylaxis HT - 002 COVID - 19 Treatment/Prevention

www.hoththerapeutics.com 20 N A S D A Q: HOTH Recent Milestones and Upcoming Catalysts: Preclinical Candidates HT - 003 (IBD) Initiate proof of concept ex vivo studies Results from Proof of Concept Studies a Exclusive license granted P r eli mina r y to xi c o l ogy studies HT - 006 (Antibiotic for VAP/HAP) Antimicrobial characterization phase 1 Inhalation feasibility animal studies start Antimicrobial characterization phase 2 HT - ALZ (Alzheimer’s disease) Establish Research Plan with AD Research Leader Initiate proof of concept animal model studies at WashU Results from proof of concept studies HT - KIT (mast cell neoplasms/ anaphylaxis) Contract with CMO for drug development signed Initiate API synthesis Analytical method development Initiate drug product development P r eli mina r y to xi c o l ogy studies Pre - IND Request HT - 002 (COVID - 19 Therapy) New preclinical studies with SARS - CoV - 2 Variants Preliminary study results Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 a See slides 30 and 31 for preclinical results

www.hoththerapeutics.com 21 N A S D A Q: HOTH Di a gnostic Device *U.S. Provisional Application No. 62/639,328
www.hoththerapeutics.com N A S D A Q: HOTH Direct Detect Breath Diagnostic Device System • Novel nanohole array (NHA) technology platform with direct sensing from breath sample type • Potential for home use by patients • Results in minutes • Technology licensed from George Washington University • Development of platform prototype in progress (6 - 9 months) • Future development will include selection of target analytes 22

www.hoththerapeutics.com N A S D A Q: HOTH Investment Highlights x Offers strong intellectual property portfolio, including exclusive licenses to patents and trademarks x Multiple shots on goal with diversified portfolio and market x Multiple assets have platform technology possibilities x 23.9 million shares outstanding (as of August 31, 2021) x Cash on hand is sufficient to take company through the clinical and pre - clinical programs in current pipeline Diverse and Robust Pipeline of Pre - Clinical Candidates x Addressing multi - billion dollar unmet market opportunities across indications x BioLexa Lotion - Novel mixture of two FDA - approved compounds – in clinical phase of development x HT - 001 – no approved product/competitor currently on the market , clinical trial projected for early 2022 Two Programs in Clinical Stage of Development Clean Financials Experienced M a n a gement , B o a r d a n d Scientific Advisors x Experienced management team, board of directors and scientific advisors with proven financial, capital markets and drug development experience 23

www.hoththerapeutics.com N A S D A Q: HOTH 24 Appendix

www.hoththerapeutics.com N A S D A Q: HOTH HT - 003 - D : Dermal Preclinical Study Results TLR2 is one of the most critical genes for acne pathophysiology Data shows that HT - 003 significantly downregulates TLR2 expression after challenge with PGN (TLR2 agonist) in an in vitro human keratinocyte model 25

www.hoththerapeutics.com N A S D A Q: HOTH HT - 004: Asthma & Allergic Inflammation • Peribronchiolar Inflammation was reduced by inhalation of HT - 004 that targets FcER1 - beta alternative exon splicing. • Ovalbumin inhalation induced airway - centric recruitment of inflammatory cells predominated by eosinophils admixed with lymphocytes, macrophages, and fewer mast cells. • Inflammatory cell recruitment was minimal in lungs of mice lacking the ovalbumin - induced allergic airway disease and administered only PBS vehicle control. • Inflammatory cell recruitment was moderate to marked resulting in expansion of peribronchiolar connective tissues by several cells thick in some areas for mice in control treatment groups with ovalbumin - induced allergic airway disease (vehicle control and oligo (non - target) control. • Despite ovalbumin - induced allergic airway induction, lungs from mice receiving inhalation of HT - 004 had reduced inflammatory cell recruitment around bronchioles. 0 1 2 3 4 5 Bronchiolar inflammation score n.s. **** **** 26

www.hoththerapeutics.com 27 N A S D A Q: HOTH HT - 005 Z - Pod Results Show Strong Therapeutic Potential HT - 005 in coconut oil (“neat”) provides a small therapeutic effect (although the lesions continue to progress), but this same active loaded in Z - pods Ρ provides an actual reduction in lesion score. Controlling Rapid Metabolism. Overcoming Poor Dermal Penetration. 11 11 11 11 11 10 9 9 7 10 10 10 7 6 6 7 6 10 10 4 10 10 8 6 6 4 0 10 3 - 0 .2 - 0 .1 0.1 0.2 0.3 0.4 0.5 0.6 1 2 3 4 5 6 7 7 8 9 10 Average score Time / weeks after 1st skin plaque observed Untreated AEA in coconut oil AEA - loaded Z - pods in coconut oil https://ir.hoththerapeutics.com/ht - 005 - z - pods HT - 005 in coconut oil HT - 005 - loaded Z - pods in coconut oil

www.hoththerapeutics.com 28 N A S D A Q: HOTH Animal Data U n t r e a t ed Control: Empty Z - pods Ρ HT - 005 Z - pods Ρ
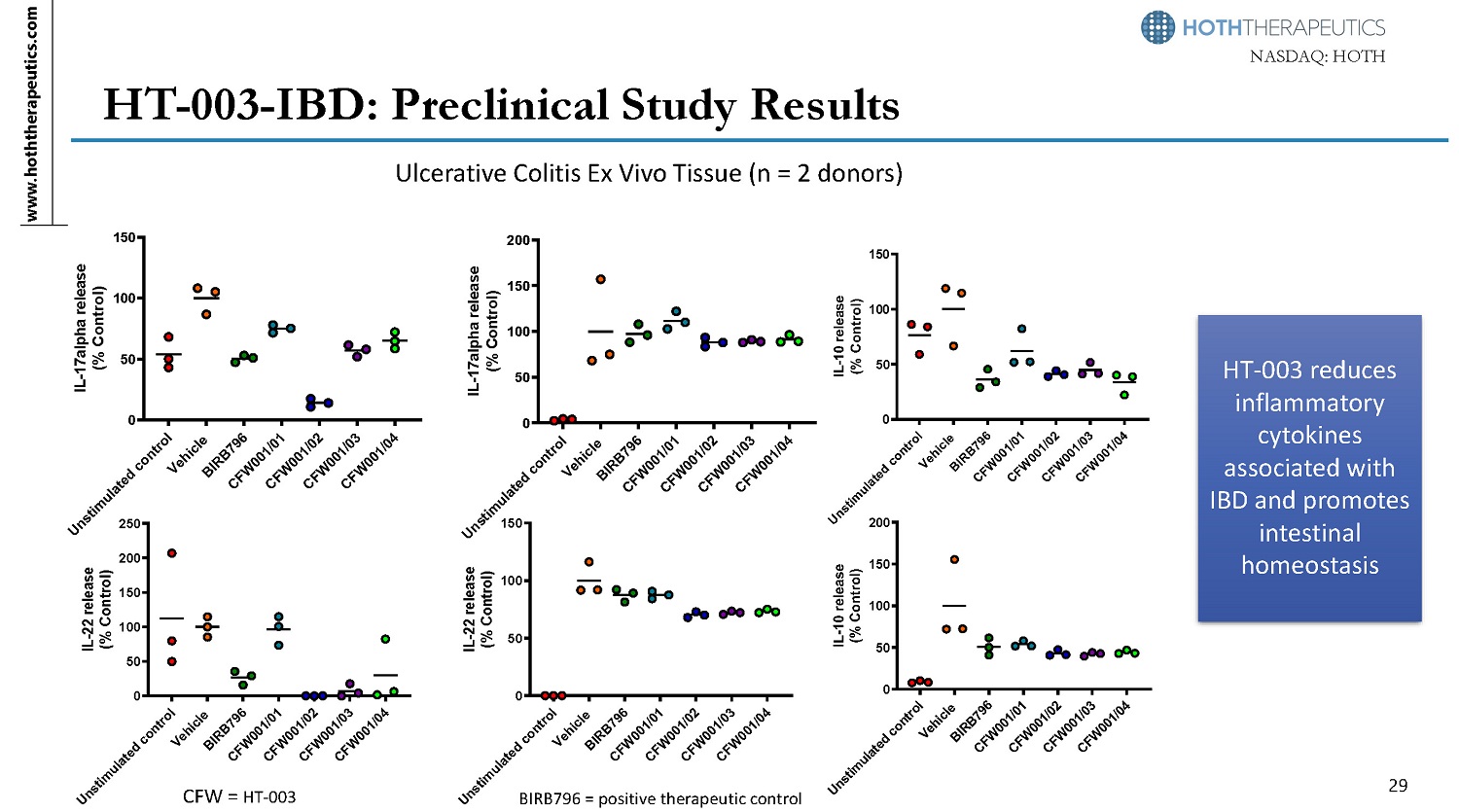
www.hoththerapeutics.com 29 N A S D A Q: HOTH HT - 003 - IBD: Preclinical Study Results 0 50 1 0 0 1 5 0 I L - 17 al ph a rel eas e (% Control) 0 50 1 0 0 1 5 0 2 0 0 2 5 0 I L - 2 2 rele as e (% Control) 0 50 1 0 0 1 5 0 I L - 2 2 r eleas e (% Control) 0 50 1 0 0 1 5 0 2 0 0 IL - 17alpha release (% Control) Ulcerative Colitis Ex Vivo Tissue (n = 2 donors) BIRB796 = positive therapeutic control CFW = HT - 003 0 50 1 0 0 1 5 0 I L - 1 0 rele as e (% Control) 0 50 1 0 0 1 5 0 2 0 0 I L - 1 0 r eleas e (% Control) HT - 003 reduces inflammatory cytokines associated with IBD and promotes intestinal homeostasis

www.hoththerapeutics.com 30 N A S D A Q: HOTH HT - 003 - IBD: Preclinical Study Results 0 50 1 0 0 1 5 0 I L - 17 al ph a rel eas e (% Control) 0 50 1 0 0 1 5 0 I L - 2 2 rele as e (% Control) Crohn’s Ex Vivo Tissue (n = 1 donor) BIRB796 = positive therapeutic control CFW = HT - 003 HT - 003 reduces inflammatory cytokines associated with IBD and promotes intestinal homeostasis 0 50 1 0 0 1 5 0 I L - 1 0 rele as e (% Control)

www.hoththerapeutics.com N A S D A Q: HOTH Management Team Jonathan Zippin, M.D., Ph.D. Senior Scientific Advisor Hoth Therapeutics, Inc. Associate Attending Dermatologist Vice Chair of Research Associate Professor of Dermatology & Pharmacology Weill Cornell Medical College David Briones Chief Financial Officer 31 Stefanie Johns, Ph.D. Chief Scientific Officer Robb Knie Chief Executive Officer

www.hoththerapeutics.com N A S D A Q: HOTH Boar d of Di r ecto r s Graig Springer, J.D. Director Hoth Therapeutics, Inc. Vice President Legal & Regulatory Brookfield Asset Management 32 David Sarnoff, Esq. Director Hoth Therapeutics, Inc. Founder & Principal The Sarnoff Group, LLC Vadim Mats, C.P.A. Director Hoth Therapeutics, Inc. BeSpoke CFO Wayne Linsley Director Hoth Therapeutics, Inc. Vice President CFO Oncall, Inc.

www.hoththerapeutics.com N A S D A Q: HOTH Scientific Advisory Board Adam Friedman, M.D. F.A.A.D. Professor & Chair of Dermatology Residency Program Director Di r ecto r T r an slational Research Director of Supportive Oncodermatology The George Washington University Richard Granstein, M.D. Chair of Dermatology Weill Cornell Medical Center 33 Mario Lacouture, M.D. Attending Physician Director of Oncodermatology Memorial Sloan Kettering Cancer Center Professor in Department of Dermatology Weill Cornell Medicine Andrew Herr, Ph.D. Associate Professor Division of Immunobiology and Center for Systems Immunology Cincinnati Childrens Hospital Glenn Cruse, Ph.D. Assistant Professor of Immunology North Carolina State University

www.hoththerapeutics.com N A S D A Q: HOTH Scientific Advisory Board Michael Peters, Ph.D. Professor of Chemical and Life Science Engineering V irgi n ia Common w ealth University 34 Vincent Njar, Ph.D. Professor of Medicinal Chemistry and Pharmacology University of Maryland, Baltimore Head, Medicinal Chemistry Section Center for Biomolecular Therapeutics William Weglicki, M.D. Professor of Biochemistry and Molecular Medicine Professor of Medicine George Washington School of Medicine & Health Sciences

THAN K YOU Media Relations: Makovsky Contact Information Investor Relations: LR Advisors Hoth Therapeutics, Inc. (678) 570 - 6791 investorrelations@hoththerapeutics.com hoth - mak@makovsky.com :H O TH
