Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Ra Medical Systems, Inc. | rmed-8k_20210824.htm |

Ra Medical Systems, Inc. NYSE American: RMED Corporate Presentation August 2021 1 Exhibit 99.1

Disclaimer Certain statements in this presentation and the accompanying oral commentary are forward-looking statements. These statements relate to future events or the future financial performance of Ra Medical Systems, Inc. (the “Company”) and involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. All statements other than statements of historical fact could be deemed forward-looking, including any expectations regarding investment returns; any projections of financial information; any statements about historical results that may suggest trends for our business; any statements of the plans, strategies, and objectives of management for future operations; any statements of expectation or belief regarding future events, potential markets, market size, market opportunities, or technology developments; any statements regarding sales and expansion strategies; any statements regarding our intention to seek additional indications for our products; and any statements of assumptions underlying any of the items mentioned. The Company has based these forward-looking statements on its current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company’s control. These and other important factors may cause actual results, performance or achievements to differ materially from those expressed or implied by these forward-looking statements. For a list and description of the risk and uncertainties inherent in the forward-looking statements, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2020, Quarterly Report on Form 10-Q for the period ended June 30, 2021, and in its other filings with the Securities and Exchange Commission. The forward-looking statements in this presentation are made only as of the date hereof. Except as required by law, the Company assumes no obligation and does not intend to update these forward-looking statements or to conform these statements to actual results or to changes in the Company's expectations. This presentation also contains estimates, projections and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry and our business. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. We have not independently verified the accuracy and completeness of the information obtained by third parties included in this presentation. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the solutions and services of the company.

Opportunity Focus on Engineering & Clinical Execution Engineering efforts focused on development of next-generation DABRA catheters with expected FDA submissions in 2022 U.S. clinical study underway to obtain an atherectomy indication with an expected FDA submission in 2022 Promising early research demonstrates the potential to perform intravascular lithotripsy in calcified arteries Targeting Large Peripheral Artery Disease (PAD) Market Opportunity An estimated 19-21 million people suffer from PAD in the US resulting in up to 200,000 lower limb amputations annually1,2 Targeting US peripheral atherectomy and CTO market segments with revenues projected to be greater than $900 million in 20213 Established, robust reimbursement for atherectomy procedures in US 2 The Sage Group 2016, Yost ML Critical limb ischemia volume 1, US Epidemiology, 2016 supplement 3 Millennium Research Group December 2020 Turnaround Company CEO transitioned to medtech veteran in 2020 with proven track record of success Strengthened management and technical teams including Engineering, Clinical, Operations, Compliance and Quality Completed extensive Quality Improvement Plan and delivered on 2020 communicated milestones Divested dermatology business in Q3 2021 in order to maintain focus on vascular business Significant opportunity to create value with the completion of clinical study and launch of next-generation catheters targeting US PAD market 1 The Sage Group 2021

DABRA Targets Large PAD Market 2 The Sage Group 2014 3 Millennium Research Group December 2020 4 FDA-approved atherectomy indication study enrolling in US 1 The Sage Group 2021

DABRA Strategy Engineering focus is programs to enhance DABRA catheter deliverability with next-gen catheter products Clinical focus is execution of an FDA-approved clinical study to obtain an atherectomy indication Early research demonstrates the potential to perform intravascular lithotripsy in calcified arteries
DABRA Catheter Engineering Programs Extend shelf life of DABRA catheters Root causes of shelf-life limitations identified in 2020 Mitigation plans are being implemented with in-house accelerated & real-time aging data supporting 6-month shelf life for future generation catheters Next-gen DABRA catheter with improved deliverability and robustness Developing a next-generation catheter with an enhanced outer-jacket to allow physicians to better access difficult anatomy Engineering work to be completed in the first quarter of 2022 with subsequent FDA filing Next-gen DABRA catheter that is compatible with standard interventional guidewires Leveraging engineering work to develop a next-generation DABRA catheter with improved deliverability and robustness Design to be finalized by the end of 2021
Intravascular Lithotripsy Research Project Purpose Demonstrate feasibility of performing lithotripsy with an excimer laser-based system Progress to date Pressure measurements demonstrate peak shockwave pressures that exceed 50 atmospheres of peak pressure Successful in shattering medial calcium models that we believe are representative Next Steps Complete prototype systems Validate early benchtop results in a pre-clinical study with prototype systems Market Opportunity1 500,000 PAD procedures (BTK/SFA) are performed globally each year with heavily calcified arteries Coronary market is estimated to be 1 million procedures globally involving calcified vessels. 1 Shockwave Medical Investor Presentation August 2021
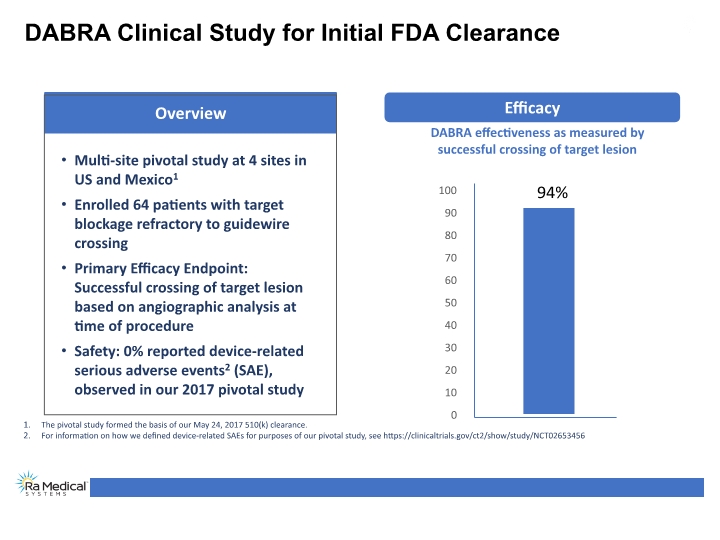
DABRA Clinical Study for Initial FDA Clearance DABRA effectiveness as measured by successful crossing of target lesion 94% The pivotal study formed the basis of our May 24, 2017 510(k) clearance. For information on how we defined device-related SAEs for purposes of our pivotal study, see https://clinicaltrials.gov/ct2/show/study/NCT02653456 Efficacy Overview Multi-site pivotal study at 4 sites in US and Mexico1 Enrolled 64 patients with target blockage refractory to guidewire crossing Primary Efficacy Endpoint: Successful crossing of target lesion based on angiographic analysis at time of procedure Safety: 0% reported device-related serious adverse events2 (SAE), observed in our 2017 pivotal study
FDA-Approved IDE Atherectomy Indication Study Primary safety endpoint: The incidence of 30-day Major Adverse Events (MAEs) as adjudicated by the Clinical Events Committee: All-cause mortality Unplanned major target limb amputation (at or above the ankle) and/or Clinically driven target limb revascularization. Status: 4 sites cleared to enroll1; 70 subjects treated as of August 16, 2021 Study size: Up to 10 sites, 100 patients Primary efficacy endpoint: Mean reduction in percent diameter stenosis in each subject’s primary lesion as measured by angiography following treatment with the DABRA and before any other treatment A 5th site, which was previously cleared, has moved locations and is in the process of being reactivated
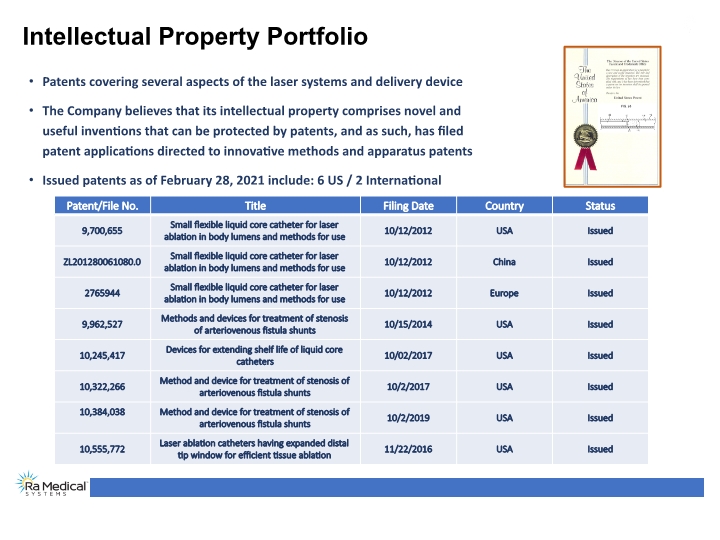
Intellectual Property Portfolio Patents covering several aspects of the laser systems and delivery device The Company believes that its intellectual property comprises novel and useful inventions that can be protected by patents, and as such, has filed patent applications directed to innovative methods and apparatus patents Issued patents as of February 28, 2021 include: 6 US / 2 International
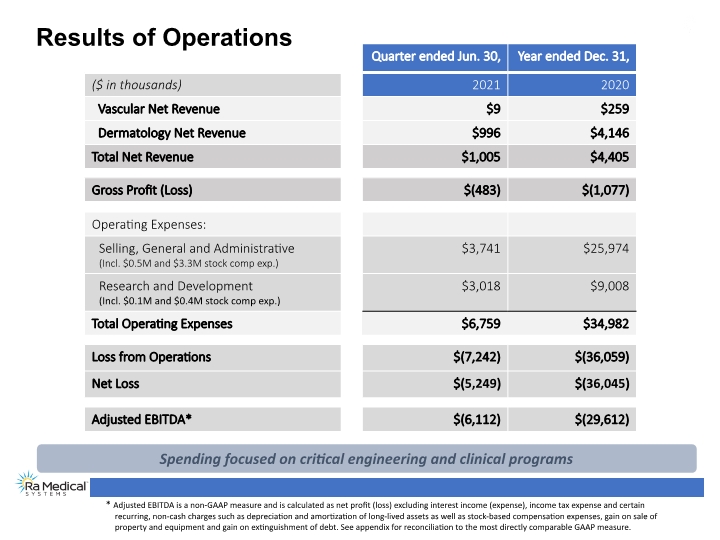
Results of Operations * Adjusted EBITDA is a non-GAAP measure and is calculated as net profit (loss) excluding interest income (expense), income tax expense and certain recurring, non-cash charges such as depreciation and amortization of long-lived assets as well as stock-based compensation expenses, gain on sale of property and equipment and gain on extinguishment of debt. See appendix for reconciliation to the most directly comparable GAAP measure. Spending focused on critical engineering and clinical programs
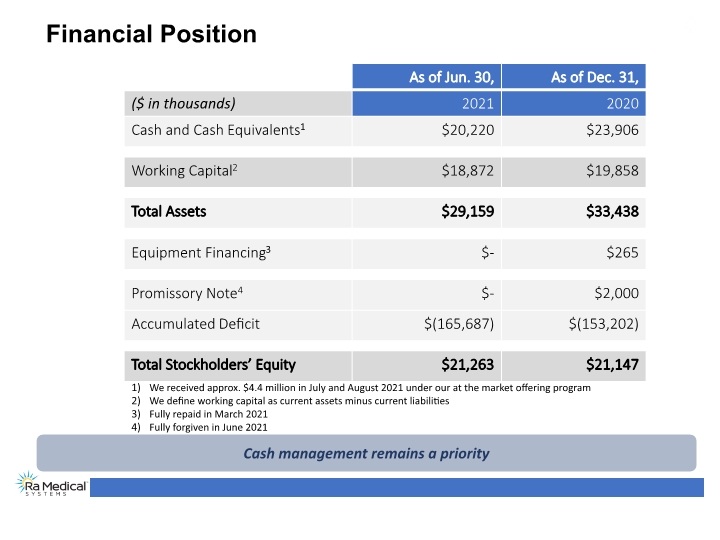
Financial Position We received approx. $4.4 million in July and August 2021 under our at the market offering program We define working capital as current assets minus current liabilities Fully repaid in March 2021 Fully forgiven in June 2021 Cash management remains a priority
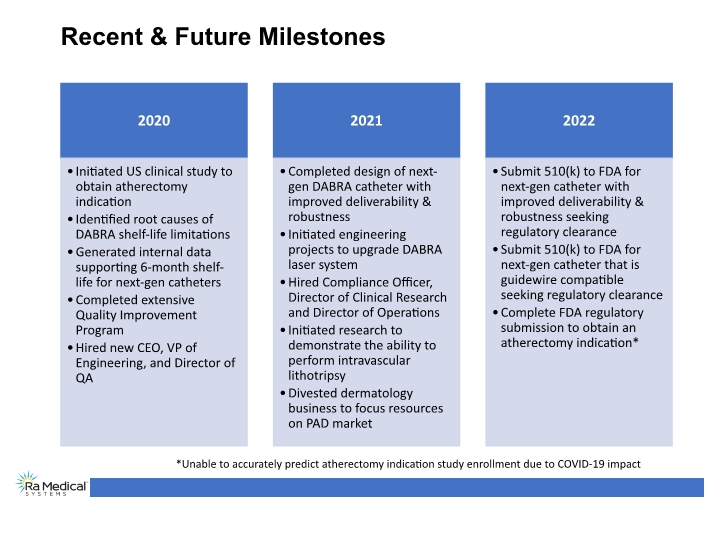
Recent & Future Milestones *Unable to accurately predict atherectomy indication study enrollment due to COVID-19 impact

Turnaround Opportunity With Focus On Execution Focus on Engineering & Clinical Execution DABRA is an excimer laser and single-use catheter system used in the endovascular treatment of vascular blockages resulting from PAD Engineering efforts focused on development of next-generation DABRA catheters with expected FDA submissions in 2022 U.S. clinical study underway to obtain an atherectomy indication with an expected FDA submission in 2022 Targeting Large Peripheral Artery Disease (PAD) Market Opportunity An estimated 19-21 million people suffer from PAD in the US resulting in up to 200,000 lower limb amputations annually1,2 Targeting US peripheral atherectomy and CTO market segments with revenues projected to be greater than $900 million in 20213 Established, robust reimbursement for atherectomy procedures in US Turnaround Company CEO transitioned to medtech veteran in 2020 with proven track record of success Strengthened management and technical teams including Engineering, Clinical, Operations, Compliance and Quality Completed extensive Quality Improvement Plan and delivered on 2020 communicated milestones Divested dermatology business in Q3 2021 in order to maintain focus on vascular business Significant opportunity to create value with the completion of clinical study and launch of next-generation catheters targeting US PAD market 2 The Sage Group 2016, Yost ML Critical limb ischemia volume 1, US Epidemiology, 2016 supplement 3 Millennium Research Group December 2020 1 The Sage Group 2021

Appendix

R&D and Manufacturing Facility Carlsbad, CA 41,000 sq. ft. in Carlsbad, CA with three controlled environments Sizable capacity for laser and catheter production Fully capitalized with all equipment owned ISO13485 certified, FDA and CA state inspected Laser Assembly Controlled Environments

Management Team

Board of Directors
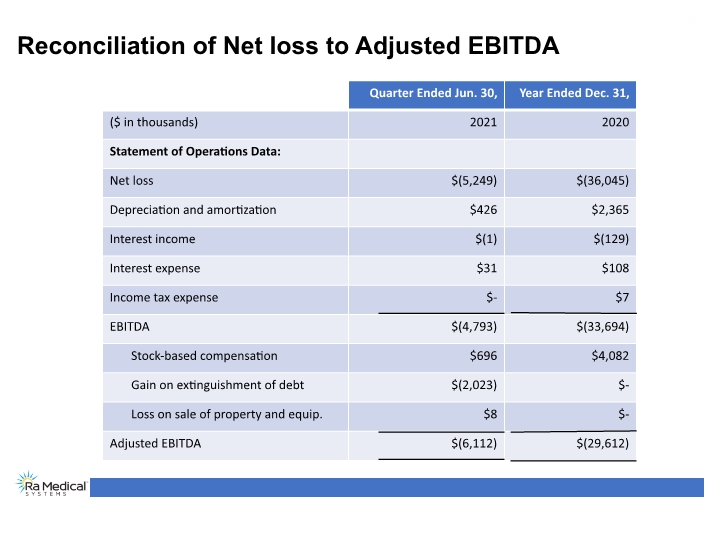
Reconciliation of Net loss to Adjusted EBITDA
