Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ANTARES PHARMA, INC. | atrs-20210818.htm |

1August 2021 NASDAQ: ATRS | August 2021 Investor Presentation EX99.1

2August 2021 Safe Harbor Statement This presentation contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: the Company’s ability to achieve the 2021 full-year revenue guidance; the uncertainty regarding the ongoing COVID-19 pandemic, including new strains of the virus, and the mitigation measures and other restrictions implemented in response to the same and the impact on demand for our products, new patients and prescriptions, future revenue, product supply, clinical trials and our overall business, operating results and financial condition; commercial success of XYOSTED® and future revenue from the same; market acceptance of Teva’s generic epinephrine auto-injector product and future revenue from the same; future prescriptions and sales of OTREXUP®; successful commercialization of NOCDURNA® in the U.S. and market acceptance and future revenue from the same; whether the FDA will withdraw marketing approval for AMAG Pharmaceuticals’ Makena® subcutaneous auto injector following the FDA letter seeking withdrawal, whether AMAG will be granted an appeal hearing and if granted, whether Makena® will be successful and future prescriptions, market acceptance and revenue from the same; Teva’s ability to successfully commercialize VIBEX® Sumatriptan Injection USP and the amount of revenue from the same; Teva’s ability to successfully commercialize generic teriparatide in Europe, Canada and Israel and future revenue from the same, successful development including the timing and results of the Phase 3 trial of the drug device combination product for selatogrel with Idorsia Pharmaceuticals and FDA and global regulatory approvals and future revenue from the same; the timing and results of the clinical development program for ATRS-1902 adrenal crisis rescue auto- injector, future NDA submission and FDA approval of the same, and if approved, future market acceptance and revenue for the same; FDA approval of Teva’s ANDAs for both generic Forteo® and generic Byetta® and future revenue from the same; the timing and results of the Company’s or its partners’ research projects or clinical trials of product candidates in development including the Company’s urology asset in development as well as Pfizer’s undisclosed development product; actions by the FDA or other regulatory agencies with respect to the Company’s products or product candidates of its partners; continued growth in product, development, licensing and royalty revenue; the Company’s ability to meet loan extension and interest only payment milestones and the ability to repay the debt obligation to Hercules Capital; the Company’s ability to obtain financial and other resources for its research, development, clinical, and commercial activities and other statements regarding matters that are not historical facts, and involve predictions. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results, performance, achievements or prospects expressed in or implied by such forward-looking statements. In some cases you can identify forward-looking statements by terminology such as ''may'', ''will'', ''should'', ''would'', ''expect'', ''intend'', ''plan'', ''anticipate'', ''believe'', ''estimate'', ''predict'', ''potential'', ''seem'', ''seek'', ''future'', ''continue'', or ''appear'' or the negative of these terms or similar expressions, although not all forward-looking statements contain these identifying words. Additional information concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward-looking statements is contained in the "Risk Factors" section of the Company's Annual Report on Form 10-K, and in the Company's other periodic reports and filings with the Securities and Exchange Commission. The Company cautions investors not to place undue reliance on the forward-looking statements contained in this presentation. All forward-looking statements are based on information currently available to the Company on the date hereof, and the Company undertakes no obligation to revise or update these forward-looking statements to reflect events or circumstances after the date of this presentation, except as required by law. ©2021 Copyright Antares Pharma, Inc. All Rights Reserved.

3August 2021 Leverage pharmaceutical and medical device expertise to develop innovative products that address needs in underserved therapeutic areas Investment Highlights Diversified revenue provides opportunities for continued growth Commercial XYOSTED®, OTREXUP® and NOCDURNA® Commercial Generic EpiPen®, Generic Forsteo® (ROW), Sumatriptan and Makena® Development Teva (Generic Forteo® (US) and Generic Byetta®), Idorsia Pharmaceuticals (selatogrel) and Pfizer (undisclosed) 2020 revenue of $149.6M (+21% vs. 2019) 2021 revenue guidance of $175-200M (+17-34% vs. 2020) Gross margin at 63% in 2Q 2021 as proprietary products represent 42% of total revenue Strong balance sheet with $45.1 million in cash and cash equivalents as of June 30, 2021 Generated $20.4M cash from operations for twelve-months ended June 30, 2021 Multiple opportunities for future value creation Partner Business Development ATRS-1901 & ATRS-1902 Proprietary Products

4August 2021 Long-Term Growth Strategy Expand Partnership Opportunities A leader in self-administered injection technology Support life-cycle management solutions Disciplined Capital Allocation Corporate development In-licensing opportunities Enhance Proprietary Portfolio Support research and development Leverage salesforce Strong Financials Drive operational efficiency Increase margin profile and EPS

5August 2021 42% 34% 10% 14% FY 2020 32% 43% 6% 20% FY 2019 28% 48% 11% 14% FY 2018 Rapidly Growing and Diversified Revenue Mix Proprietary Products Partner Products Development Royalty Revenue $63.6M Revenue $123.9M Revenue $149.6M 43% 22% 14% 21% YTD 2021 Revenue $87.1M

6August 2021 Proprietary Products (desmopressin acetate) sublingual tablet

7August 2021 Patient-Centric Innovation Drives Strategy Targeting two therapeutic areas with significant market opportunities UROLOGY & ENDOCRINOLOGY Focus on patient populations with high unmet needs Target addressable physician audiences for efficient commercialization Identify and develop innovative, differentiated assets Leverage integrated capabilities $13.2 $15.1 $17.9 $17.5 $39.2 $62.9 $0 $10 $20 $30 $40 $50 $60 $70 $80 2015 2016 2017 2018 2019 2020 Proprietary Revenue (in millions)

8 Innovative self-delivery of testosterone (T) replacement therapy for at-home use • T levels maintained for as long as the patient remains on therapy* • Convenient, once-a-week dosing • Virtually painless subcutaneous injection ~75% of all commercial lives covered 16 Orange Book listed patents through year 2038 XYOSTED® (testosterone enanthate) for injection *Studied for 52 weeks when taken every week, as directed. Achieving desired blood levels may require dose adjustments at Week 7 based upon Week 6 blood levels. Some patients fell below minimum level of 300 ng/dL despite dose adjustments. Once Weekly Painless Steady PK Low Risk of Transfer Intramuscular Injection Topical Gels Please see Prescribing Information including important safety information and boxed warning.

9August 2021 Testosterone Market Once Weekly Painless Efficacious Steady PK No Risk of Transfer 0 500,000 1,000,000 1,500,000 2,000,000 2,500,000 3,000,000 3,500,000 4,000,000 4,500,000 2020 TRT Growth: 6.8% 2020 TRT INJECTABLE Growth: 7.3% 2020 TRT Topical Growth: 1.0% 2020 TRT TRx: 7.6M 2020 TRT Injectable TRx: 5.6M 2020 TRT Topical TRx: 1.8M IQVIA Data

10August 2021 XYOSTED® Quarterly TRx Growth Written by ~9,300 different physicians (since launch) 2Q 2021 TRx’s increased >50% year-over-year 2Q 2021 TRx’s increased ~15% sequentially 3,207 6,485 10,290 14,064 16,777 17,685 21,461 23,274 24,493 27,961 796 4,517 8,893 13,638 16,387 20,589 22,536 26,528 26,580 30,877 0 10,000 20,000 30,000 40,000 50,000 60,000 70,000 1Q 2019 2Q 2019 3Q 2019 4Q 2019 1Q 2020 2Q 2020 3Q 2020 4Q 2020 1Q 2021 2Q 2021 Quarterly TRx Growth NRx Refills 49,802 38,274 27,702 19,183 11,001 4,003 IQVIA Calculated Packaged Units 33,164 43,997 51,073 58,838
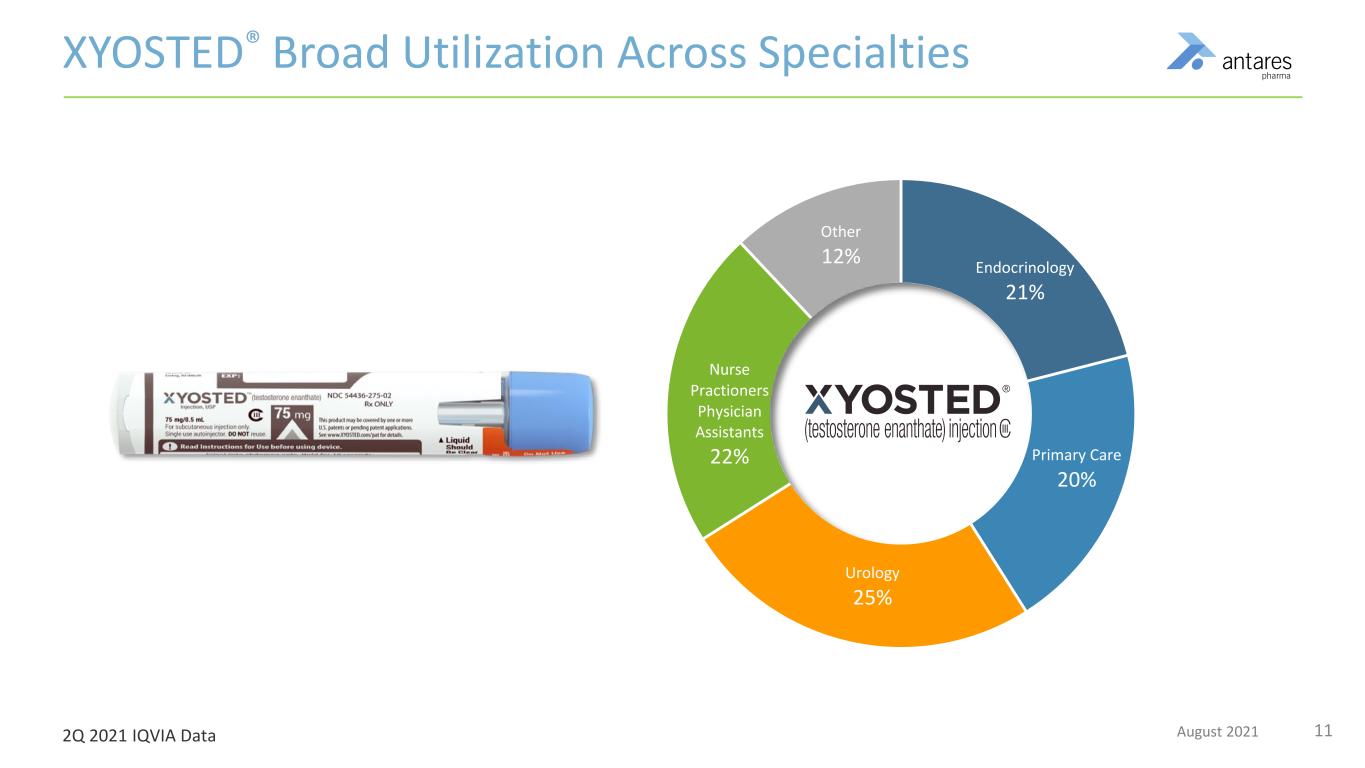
11August 2021 Endocrinology 21% Primary Care 20% Urology 25% Nurse Practioners Physician Assistants 22% Other 12% XYOSTED® Broad Utilization Across Specialties 2Q 2021 IQVIA Data
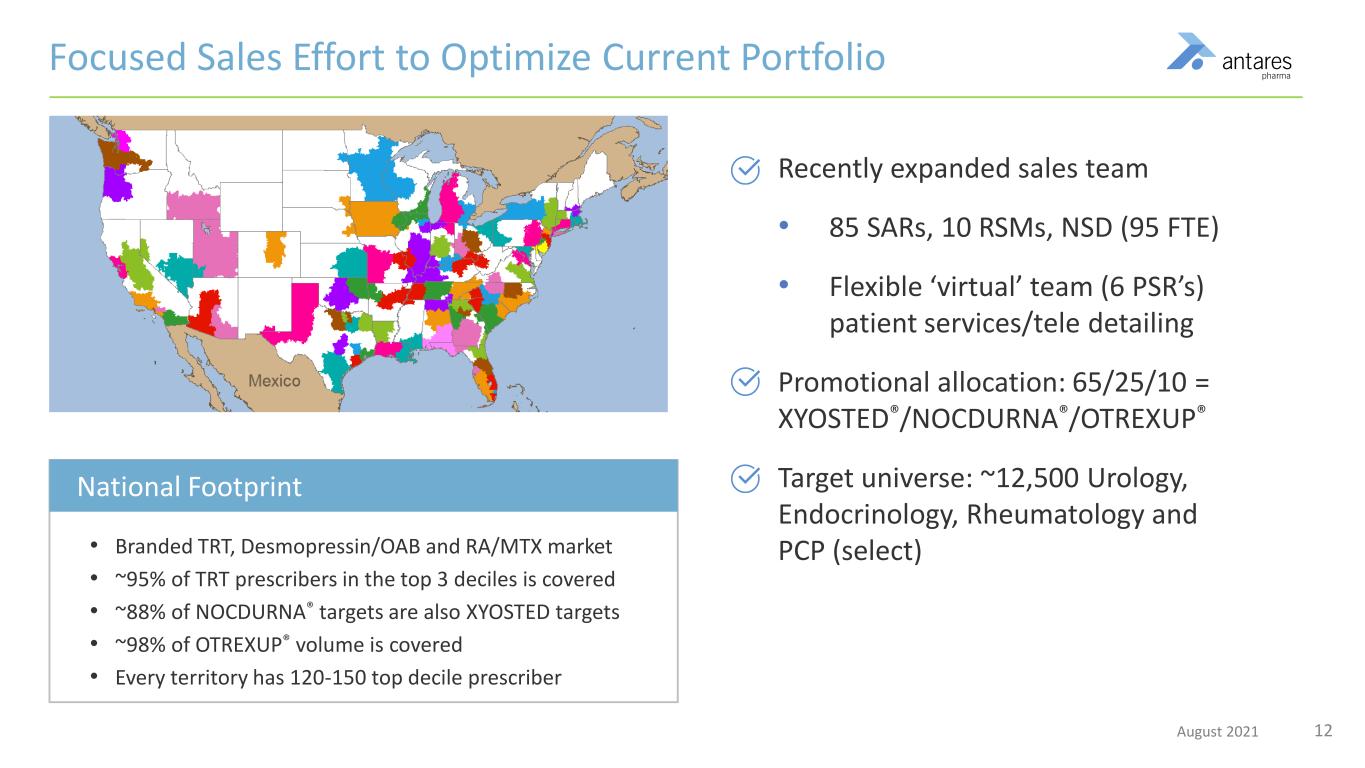
12August 2021 • Branded TRT, Desmopressin/OAB and RA/MTX market • ~95% of TRT prescribers in the top 3 deciles is covered • ~88% of NOCDURNA® targets are also XYOSTED targets • ~98% of OTREXUP® volume is covered • Every territory has 120-150 top decile prescriber Recently expanded sales team • 85 SARs, 10 RSMs, NSD (95 FTE) • Flexible ‘virtual’ team (6 PSR’s) patient services/tele detailing Promotional allocation: 65/25/10 = XYOSTED®/NOCDURNA®/OTREXUP® Target universe: ~12,500 Urology, Endocrinology, Rheumatology and PCP (select) Focused Sales Effort to Optimize Current Portfolio National Footprint

13August 2021 FDA-approved vasopressin analog indicated for the treatment of nocturia due to nocturnal polyuria in adults who awaken at least two times a night to void • First and only sublingual tablet that targets the kidneys • Short-acting desmopressin is underutilized due to poor disease state and product awareness Nocturia affects ~40 million adults in U.S. ~50%+ prescriber alignment overlap between NOCDURNA® and XYOSTED® In-Licensed: NOCDURNA® (desmopressin acetate)

14August 2021 NOCDURNA® Works Quickly A sublingual tablet that dissolves rapidly1 Administered without water1 Onset action occurs within 30 minutes1 Therapeutic effect as early as the first night1 Elimination from the body starts quickly, within a half-life of 2.8 hours1 Antidiuretic effect lasts 6 hours1 Sublingual tablet formulation does not undergo first-pass hepatic metabolism1 NOCDURNA® reduced nighttime voids by nearly half1 WOMEN (N=118) MEN (N=102) 52% 43% References: 1. NOCDURNA prescribing information. Parsippany, NJ: Ferring Pharmaceuticals Inc. 2. Aditya S et al. Int J Appl Basic Med Res. 2012;2(2):77-83 Please see Prescribing Information including important safety information and boxed warning.

15August 2021 ATRS-1901 Urology Asset Antares Assets in Development ATRS-1902 for Adrenal Crisis Rescue Completed Pre-IND meeting with FDA Filed IND with FDA in June 2021 Expect to initiate BC study followed by BE study Expect to file 505(b)(2) NDA with FDA towards the end 2022 ATRS-1901: Urology Asset Completed Pre-IND meeting with FDA Expected IND filing in 2H 2021

16August 2021 ATRS-1902 for Adrenal Crisis Rescue + 62% 6% 6% 12% 14% ENDOCRINOLOGY PEDIATRICS NURSE PRACTITIONER PRIMARY CARE ALL OTHERS Solu-Cortef® Prescribers Simple (2-step), integrated device versus standard-of-care, Solu-Cortef® sterile powder that requires reconstitution and multiple steps Liquid stable formulation of hydrocortisone at room temperature ATRS-1902 seeking indication for acute adrenal insufficiency, or adrenal crisis, in adults and adolescents using a novel auto-injector platform to deliver hydrocortisone Estimated ~140K U.S. patient population with adrenal insufficiency (1)(2)(3) Endocrinology prescriber overlap with XYOSTED® (1) Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism. 2016;101(2):364–369. (2) Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383(9935):2152–2167.2 of 3 (3) Chabre O, Goichot B, Zenaty D, Bertherat J. Group 1. Epidemiology of primary and secondary adrenal insufficiency: prevalence and incidence, acute adrenal insufficiency, long-term morbidity and mortality. Annals of Endocrinology (Paris). 2017;78(6):490–494.

17August 2021 Partner Business

18August 2021 Deep Device Knowledge Drug/Device Combination Product Partner Extensive Regulatory Expertise and Success Tailored Durable Asset Development PHARMACEUTICAL TECHNOLOGY PARTNER $0.0 $20.0 $40.0 $60.0 $80.0 $100.0 2015 2016 2017 2018 2019 2020 Partner Product License and Development Royalties $32.4 $46.0 $36.6$37.1 $84.6 $86.7 Partner Business Revenue (in millions)

19August 2021 Commercial Experience Teva’s Generic EpiPen FDA-approved as therapeutically equivalent to Mylan’s EpiPen® and fully substitutable at the pharmacy Antares receives cost plus margin on all devices sold to Teva plus mid-to-high, single-digit royalties on net sales EpiPen, Jr. launched in August 2019 Teva garnered ~58% share of EpiPen market in 2Q 2021

20August 2021 Generic EpiPen® Quarterly TRx Prescription Trends Bloomberg/Symphony Health Solutions 28,750 / 6% 125,000 / 22% 237,000 / 27% 183,000 / 36% 206,000 / 43% 176,000 / 38% 293,000 / 43% 232,000 / 48% 305,000 / 53% 445,000 / 58%448,750 568,000 862,000 506,000 484,000 465,000 676,000 483,000 580,000 767,000 0 100,000 200,000 300,000 400,000 500,000 600,000 700,000 800,000 900,000 1,000,000 1Q 2019 2Q 2019 3Q 2019 4Q 2019 1Q 2020 2Q 2020 3Q 2020 4Q 2020 1Q 2021 2Q 2021 Teva Mylan Total Epi Market

21August 2021 Teva launched ROW 11 European countries Israel and Canada Forteo® 2020 revenue $510 million in U.S. $536 million in ROW by Lilly Attractive economics to ATRS Supply devices at reasonable margin Royalties escalating to mid-teens Potential FDA approval Expect fully substitutable at pharmacy : Generic Forteo® (teriparatide)

22August 2021 Global Development Agreement with Idorsia Pharmaceuticals for selatogrel, a New Chemical Entity, with the QuickShot® auto injector selatogrel Selatogrel is a potent and highly selective P2Y12 receptor antagonist intended for the treatment of suspected Acute Myocardial Infarction (AMI) Phase 2 data demonstrated that subcutaneous administration of selatogrel showed fast and reversible inhibition of platelet aggregation in patients Idorsia initiates global Phase 3 study in June 2021 “SOS-AMI” Selatogrel Outcome Study in Acute Myocardial Infarction Special Protocol Assessment Agreement Granted fast track designation

23August 2021 • ~800,000 occurrences of new or recurrent MI1 annually • 600,000 have a first MI + 200,000 have a recurrent MI Myocardial Infarction Market Opportunity Mozaffarian D et al. Circulation 2016 * American Heart Association ~8.4 million Americans* have survived a Myocardial Infarction (MI) Product Justification to potentially change the way AMI is treated Potent and highly selective antagonist of P2Y12 receptor “Fast” onset of action (within 15 min) - for emergency use - to quickly restore blood flow - to keep heart muscle alive - to stop heart attack process “Short” duration of action - limits bleeding risk - to allow safe catherization and/or angioplasty Easy to use and suitable for subcutaneous injection - no HCP required to begin treatment Safety demonstrated in Phase 2 results

24August 2021 Diversified Product Portfolio Testosterone Methotrexate Desmopressin Acetate Sumatriptan Epinephrine Hydroxyprogesterone Teriparatide Teriparatide Exenatide P2Y12 Receptor Antagonist Undisclosed Hydrocortisone Undisclosed XYOSTED® OXTREXUP® NOCDURNA® SUMATRIPTAN EPINEPHRINE MAKENA® TERIPARATIDE (ROW) TERIPARATIDE (US) EXENATIDE SELATOGREL UNDISCLOSED ATRS-1902 ATRS-1901 PRODUCT MOLECULE PRECLINICAL CLINICAL FILED APPROVED MARKETEDCOMPANY Targeted investments designed to fuel growth through 2025 and beyond

25August 2021 45% 50% 51% 59% 58% 62% 0% 10% 20% 30% 40% 50% 60% 70% 2016 2017 2018 2019 2020 YTD 2021 Gross Margin $52.2M $54.5M $63.6M $123.9M $149.6M $175-200M* 2016 2017 2018 2019 2020 2021 +4% vs. 2016 +21% vs. 2019 +17% vs. 2017 +95% vs. 2018 Revenue Growth and 2021 Projections Projected 5-Year CAGR of ~29%** * Revenue Guidance **Based on mid-point of 2021 revenue guidance +14% vs. 2015 ($24.3) ($16.7) ($6.5) ($2.0) $9.9 $9.9 (25.0) (20.0) (15.0) (10.0) (5.0) 0.0 5.0 10.0 2016 2017 2018 2019 2020 YTD 2021 Net Income / (Loss) Before Taxes (in millions) 91% 80% 77% 58% 49% 48% 0% 20% 40% 60% 80% 100% 2016 2017 2018 2019 2020 YTD 2021 R&D + SG&A Expenses as % of Revenue +17-34% vs. 2020

26August 2021 Income Statement Summary (in millions, except EPS) 2Q 2021 2Q 2020 Increase (Decrease) Total Revenue 45.0 32.4 39% Cost of Products Sales and Development Revenue 16.4 12.5 31% Total Gross Profit 28.6 19.9 44% Gross Margin 63% 61% --- R&D and SG&A Expenses 21.8 16.9 29% Net Income $4.4 $2.2 103% Basic and Diluted Earnings Per Share $0.03 $0.01 131%

27August 2021 Antares Pharma: Value Proposition Diverse portfolio of commercialized products Multiple growth drivers • Continued XYOSTED® prescription growth • Continued generic EpiPen® prescription growth • Relaunch of NOCDURNA® • Potential FDA approval and U.S. launch of Teva’s generic teriparatide and exenatide • Pfizer development program • Idorsia’s selatogrel rescue pen development program Proprietary R&D portfolio • ATRS-1902 for adrenal crisis rescue • ATRS-1901 for urology Disciplined capital allocation • Invest to diversify portfolio Expanding operational capabilities

28August 2021 Thank You!
