Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Surrozen, Inc./DE | d148870dex991.htm |
| 8-K - 8-K - Surrozen, Inc./DE | d148870d8k.htm |

Exhibit 99.2 The Wnt Company – Targeted Regeneration © 2021 Surrozen, Inc.

Legal Disclaimers Forward–looking statements. Certain statements in this presentation may be considered forward-looking statements. Forward-looking statements generally relate to future events or Surrozen, Inc.’s future financial or operating performance. For example, statements concerning the following include forward-looking statements: Surrozen’s ability to identify, develop and commercialize drug candidates; the initiation, cost, timing, progress and results of research and development activities, preclinical or and clinical trials with respect to SZN-1326, SZN-043, and potential future drug candidates; estimates of Surrozen’s total addressable market, future revenue, expenses, and capital requirements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Surrozen and its management are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, factors associated with companies, such as Surrozen, that are engaged in preclinical studies and other research and development activities in the biopharma industry, including uncertainty in the timing or results of preclinical studies and clinical trials, product acceptance and/or receipt of regulatory approvals for product candidates, including any delays and other impacts from the COVID-19 pandemic, and other uncertainties and factors set forth in the section entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” in SEC filings by Surrozen (formerly known as Consonance-HFW Acquisition Corp.), including the registration statement on Form S-4 filed with the U.S. Securities and Exchange Commission. Nothing in this presentation should be regarded as a representation by any person that the forward- looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward- looking statements in this presentation, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. Surrozen does not undertake any duty to update these forward-looking statements. Certain Information. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and Surrozen’s own internal estimates and research. In addition, all of the market data included in this Presentation involve a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while Surrozen believes its internal research is reliable, such research has not been verified by any independent source. This presentation contains certain financial, including pro forma, information of Surrozen. The independent registered public accounting firm of Surrozen has not, audited, reviewed, compiled, or performed any procedures with respect to projections for the purpose of their inclusion in this presentation, and did not express an opinion or provide any other form of assurance with respect thereto for the purpose of this presentation. © 2021 Surrozen, Inc. 2Legal Disclaimers Forward–looking statements. Certain statements in this presentation may be considered forward-looking statements. Forward-looking statements generally relate to future events or Surrozen, Inc.’s future financial or operating performance. For example, statements concerning the following include forward-looking statements: Surrozen’s ability to identify, develop and commercialize drug candidates; the initiation, cost, timing, progress and results of research and development activities, preclinical or and clinical trials with respect to SZN-1326, SZN-043, and potential future drug candidates; estimates of Surrozen’s total addressable market, future revenue, expenses, and capital requirements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward-looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Surrozen and its management are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management’s control including general economic conditions and other risks, factors associated with companies, such as Surrozen, that are engaged in preclinical studies and other research and development activities in the biopharma industry, including uncertainty in the timing or results of preclinical studies and clinical trials, product acceptance and/or receipt of regulatory approvals for product candidates, including any delays and other impacts from the COVID-19 pandemic, and other uncertainties and factors set forth in the section entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” in SEC filings by Surrozen (formerly known as Consonance-HFW Acquisition Corp.), including the registration statement on Form S-4 filed with the U.S. Securities and Exchange Commission. Nothing in this presentation should be regarded as a representation by any person that the forward- looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward- looking statements in this presentation, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. Surrozen does not undertake any duty to update these forward-looking statements. Certain Information. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and Surrozen’s own internal estimates and research. In addition, all of the market data included in this Presentation involve a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while Surrozen believes its internal research is reliable, such research has not been verified by any independent source. This presentation contains certain financial, including pro forma, information of Surrozen. The independent registered public accounting firm of Surrozen has not, audited, reviewed, compiled, or performed any procedures with respect to projections for the purpose of their inclusion in this presentation, and did not express an opinion or provide any other form of assurance with respect thereto for the purpose of this presentation. © 2021 Surrozen, Inc. 2

Highlights - Compelling Breakthroughs in Harnessing Wnt Signaling Global leaders in developing antibodies targeting the Wnt pathway • World renowned scientific advisors, founders • Experienced management team to execute strategy Proprietary Wnt therapeutics platform designed to selectively stimulate tissue regeneration • Surrozen discoveries validate prominent Wnt biology role in normal & diseased tissues • Two technologies with broad library of receptor specific antibodies to confer selectivity Advancing two lead programs targeting billion+ dollar markets SZN-1326 | Ulcerative Colitis | FIH 2022 SZN-043 | Severe Alcoholic Hepatitis | FIH 2022 Scientifically driven strategy to build on leadership position in selective Wnt antibodies • Target high unmet needs to transform patient outcomes in broad spectrum of diseases • Leverage our platform to advance product candidates and to expand our patent portfolio (17 applications filed) • Potential to partner post value generating milestones Cash runway to advance lead programs through phase 1b and nominate additional IND candidates 3 © 2021 Surrozen, Inc. Highlights - Compelling Breakthroughs in Harnessing Wnt Signaling Global leaders in developing antibodies targeting the Wnt pathway • World renowned scientific advisors, founders • Experienced management team to execute strategy Proprietary Wnt therapeutics platform designed to selectively stimulate tissue regeneration • Surrozen discoveries validate prominent Wnt biology role in normal & diseased tissues • Two technologies with broad library of receptor specific antibodies to confer selectivity Advancing two lead programs targeting billion+ dollar markets SZN-1326 | Ulcerative Colitis | FIH 2022 SZN-043 | Severe Alcoholic Hepatitis | FIH 2022 Scientifically driven strategy to build on leadership position in selective Wnt antibodies • Target high unmet needs to transform patient outcomes in broad spectrum of diseases • Leverage our platform to advance product candidates and to expand our patent portfolio (17 applications filed) • Potential to partner post value generating milestones Cash runway to advance lead programs through phase 1b and nominate additional IND candidates 3 © 2021 Surrozen, Inc.
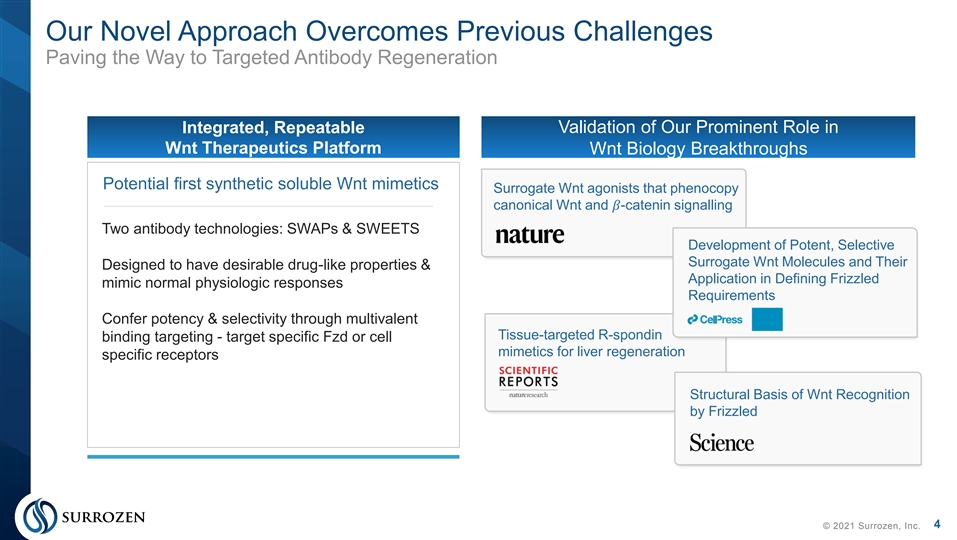
Our Novel Approach Overcomes Previous Challenges Paving the Way to Targeted Antibody Regeneration Integrated, Repeatable Validation of Our Prominent Role in Wnt Therapeutics Platform Wnt Biology Breakthroughs Potential first synthetic soluble Wnt mimetics Surrogate Wnt agonists that phenocopy canonical Wnt and �� -catenin signalling Two antibody technologies: SWAPs & SWEETS Development of Potent, Selective Surrogate Wnt Molecules and Their Designed to have desirable drug-like properties & Application in Defining Frizzled mimic normal physiologic responses Requirements Confer potency & selectivity through multivalent Tissue-targeted R-spondin binding targeting - target specific Fzd or cell mimetics for liver regeneration specific receptors Structural Basis of Wnt Recognition by Frizzled 4 © 2021 Surrozen, Inc.Our Novel Approach Overcomes Previous Challenges Paving the Way to Targeted Antibody Regeneration Integrated, Repeatable Validation of Our Prominent Role in Wnt Therapeutics Platform Wnt Biology Breakthroughs Potential first synthetic soluble Wnt mimetics Surrogate Wnt agonists that phenocopy canonical Wnt and �� -catenin signalling Two antibody technologies: SWAPs & SWEETS Development of Potent, Selective Surrogate Wnt Molecules and Their Designed to have desirable drug-like properties & Application in Defining Frizzled mimic normal physiologic responses Requirements Confer potency & selectivity through multivalent Tissue-targeted R-spondin binding targeting - target specific Fzd or cell mimetics for liver regeneration specific receptors Structural Basis of Wnt Recognition by Frizzled 4 © 2021 Surrozen, Inc.

Proprietary Technologies Enable Potent, Selective Wnt Signaling SWAPs & SWEETS SWAP Technology SWEETS Technology Mutated RSPO2 anti-Lrp VHH anti-Fzd IgG Cell Specific Receptor Effector-less hIgG1 Effector-less hIgG1 Antibody Based Bi-Specific Antibody-based fusion protein Mimics natural Wnt in activating Wnt signaling Mimics natural R-Spondin in enhancing Wnt signaling Applied in disease states with deficient Wnt ligand Applied in diseases with adequate ligand, but deficient Wnt signaling Engineered to be tissue selective targeting with Engineered to be cell selective with cell specific receptors individual Fzd receptor selectivity 5 © 2021 Surrozen, Inc.Proprietary Technologies Enable Potent, Selective Wnt Signaling SWAPs & SWEETS SWAP Technology SWEETS Technology Mutated RSPO2 anti-Lrp VHH anti-Fzd IgG Cell Specific Receptor Effector-less hIgG1 Effector-less hIgG1 Antibody Based Bi-Specific Antibody-based fusion protein Mimics natural Wnt in activating Wnt signaling Mimics natural R-Spondin in enhancing Wnt signaling Applied in disease states with deficient Wnt ligand Applied in diseases with adequate ligand, but deficient Wnt signaling Engineered to be tissue selective targeting with Engineered to be cell selective with cell specific receptors individual Fzd receptor selectivity 5 © 2021 Surrozen, Inc.

Deep Wnt Signaling Expertise Supports Productive R&D Pipeline IND Enabling Studies Ongoing for SZN-1326 and SZN-043; Planned Phase 1 Clinical Trials 2022 Lead LEAD NEXT INDICATION/S RESEARCH PRECLINICAL PHASE 1 PHASE 2 PHASE 3 REGULATORY PROGRAMS MILESTONE Programs Moderate to First in Human SZN-1326 Severe IBD 2022 Severe Alcoholic First in Human SZN-043 Hepatitis 2022 Resear RESEARC ch H Tissue Indications Discovery Proof of Concept Lead Candidate PROGRAMS Programs Diabetic Retinopathy, Wet AMD Retinal Vasculature Fuch’s Dystrophy, Limbal Cell Def Cornea Dry AMD RPE Dry Eye, Sjögren’s Lacrimal Gland Short Bowel Syndrome Intestine Hearing Loss Cochlea IPF, COPD Lung Polycystic Kidney Disease, FSGS Renal 6 © 2021 Surrozen, Inc.Deep Wnt Signaling Expertise Supports Productive R&D Pipeline IND Enabling Studies Ongoing for SZN-1326 and SZN-043; Planned Phase 1 Clinical Trials 2022 Lead LEAD NEXT INDICATION/S RESEARCH PRECLINICAL PHASE 1 PHASE 2 PHASE 3 REGULATORY PROGRAMS MILESTONE Programs Moderate to First in Human SZN-1326 Severe IBD 2022 Severe Alcoholic First in Human SZN-043 Hepatitis 2022 Resear RESEARC ch H Tissue Indications Discovery Proof of Concept Lead Candidate PROGRAMS Programs Diabetic Retinopathy, Wet AMD Retinal Vasculature Fuch’s Dystrophy, Limbal Cell Def Cornea Dry AMD RPE Dry Eye, Sjögren’s Lacrimal Gland Short Bowel Syndrome Intestine Hearing Loss Cochlea IPF, COPD Lung Polycystic Kidney Disease, FSGS Renal 6 © 2021 Surrozen, Inc.

SZN-1326 & SZN-043 Represent Significant Market Opportunities • 2nd line biologics in UC represent a $4B market in US • Estimated 100,000 U.S. hospitalizations due to severe AH • Moderate to severe Crohn’s 2nd line market of > $7B in the US • ~50% of patients covered by commercial insurance • Opportunity for combination of SZN-1326 with all • Potential for expansion to other severe liver biological treatments diseases 6,000,000 1,600,000 1,400,000 4,800,000 1,200,000 1,000,000 3,600,000 800,000 500,000 2,400,000 600,000 400,000 1,200,000 250,000 200,000 0 0 IBD Moderate to IBD Moderate to Liver Dx Liver Severe UC Crohn’s Prevalence Severe UC Prevalence Severe Crohn’s Cirrhosis Disease AAH 7 © 2021 Surrozen, Inc.SZN-1326 & SZN-043 Represent Significant Market Opportunities • 2nd line biologics in UC represent a $4B market in US • Estimated 100,000 U.S. hospitalizations due to severe AH • Moderate to severe Crohn’s 2nd line market of > $7B in the US • ~50% of patients covered by commercial insurance • Opportunity for combination of SZN-1326 with all • Potential for expansion to other severe liver biological treatments diseases 6,000,000 1,600,000 1,400,000 4,800,000 1,200,000 1,000,000 3,600,000 800,000 500,000 2,400,000 600,000 400,000 1,200,000 250,000 200,000 0 0 IBD Moderate to IBD Moderate to Liver Dx Liver Severe UC Crohn’s Prevalence Severe UC Prevalence Severe Crohn’s Cirrhosis Disease AAH 7 © 2021 Surrozen, Inc.

anti-Lrp6 VHH anti-Fzd IgG (Fzd5) SZN-1326 Moderate to Severe IBD effector-less hIgG1 8anti-Lrp6 VHH anti-Fzd IgG (Fzd5) SZN-1326 Moderate to Severe IBD effector-less hIgG1 8
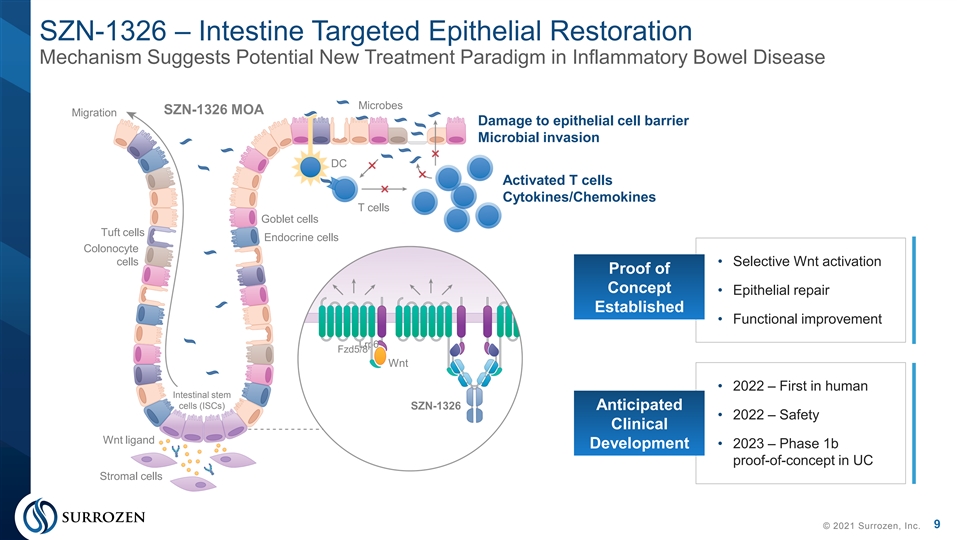
SZN-1326 – Intestine Targeted Epithelial Restoration Mechanism Suggests Potential New Treatment Paradigm in Inflammatory Bowel Disease Microbes SZN-1326 MOA Migration Damage to epithelial cell barrier Microbial invasion DC Activated T cells Cytokines/Chemokines T cells Goblet cells Tuft cells Endocrine cells Colonocyte cells • Selective Wnt activation Proof of Concept • Epithelial repair Established • Functional improvement Lrp6 Fzd5/8 Wnt • 2022 – First in human Intestinal stem cells (ISCs) SZN-1326 Anticipated • 2022 – Safety Clinical Wnt ligand • 2023 – Phase 1b Development proof-of-concept in UC Stromal cells 9 © 2021 Surrozen, Inc.SZN-1326 – Intestine Targeted Epithelial Restoration Mechanism Suggests Potential New Treatment Paradigm in Inflammatory Bowel Disease Microbes SZN-1326 MOA Migration Damage to epithelial cell barrier Microbial invasion DC Activated T cells Cytokines/Chemokines T cells Goblet cells Tuft cells Endocrine cells Colonocyte cells • Selective Wnt activation Proof of Concept • Epithelial repair Established • Functional improvement Lrp6 Fzd5/8 Wnt • 2022 – First in human Intestinal stem cells (ISCs) SZN-1326 Anticipated • 2022 – Safety Clinical Wnt ligand • 2023 – Phase 1b Development proof-of-concept in UC Stromal cells 9 © 2021 Surrozen, Inc.

SZN-1326 – Potential to Transform Treatment Paradigm in IBD Normal (No DSS Damage) High Unmet Need Need for rapid induction: SOC takes months to induce remission Better efficacy, especially mucosal healing: SOC achieve remission in <50% and low rates of mucosal healing (< 20%) Need for additional MOAs: Patients fail first-line anti-inflammatory Damaged (DSS Damage) nd rd biologics and subsequently fail 2 and 3 line therapies Differentiated Preclinical Data Repairs damaged colon epithelium Restores colon tissue structure, epithelial tight junctions and improves Restored (DSS Damage + SZN-1326) mucosal healing Reduces inflammation and improves disease activity index Superior to cyclosporin and anti-TNF’s © 2021 Surrozen, Inc. 10SZN-1326 – Potential to Transform Treatment Paradigm in IBD Normal (No DSS Damage) High Unmet Need Need for rapid induction: SOC takes months to induce remission Better efficacy, especially mucosal healing: SOC achieve remission in <50% and low rates of mucosal healing (< 20%) Need for additional MOAs: Patients fail first-line anti-inflammatory Damaged (DSS Damage) nd rd biologics and subsequently fail 2 and 3 line therapies Differentiated Preclinical Data Repairs damaged colon epithelium Restores colon tissue structure, epithelial tight junctions and improves Restored (DSS Damage + SZN-1326) mucosal healing Reduces inflammation and improves disease activity index Superior to cyclosporin and anti-TNF’s © 2021 Surrozen, Inc. 10

Initial Clinical Development Focus on Ulcerative Colitis Potential to Expand Into Additional IBD Indications • Phase 1a in healthy volunteers dosed for up to 12 weeks IV and SQ either weekly or biweekly • Phase 1b placebo controlled in UC patients provides potential to generate clinical proof of concept; endoscopies and biopsies enable blinded central reads of • Clinical remission (symptom scores) • Histologic remission/mucosal healing (histopathology) PHASE 1a SAD/MAD PHASE 1b MAD PHASE 2 Population UC Patients UC Patients Healthy Dose Escalation: Up to 24 N Expansion (Mono and Combo): Up to 60 120-150 Up to 24 Key Objectives Early Efficacy Inform Dose Proof of Mechanism Safety / PK/ ADA Additional UC-100, clinical remission and response, CRP, FC, cytokines, histology, PD markers endoscopic remission, endoscopy subscore, End-Points stool frequency, rectal bleeding, histology, histological remission, QOL, PD markers endoscopy subscore, PD markers 11 © 2021 Surrozen, Inc.Initial Clinical Development Focus on Ulcerative Colitis Potential to Expand Into Additional IBD Indications • Phase 1a in healthy volunteers dosed for up to 12 weeks IV and SQ either weekly or biweekly • Phase 1b placebo controlled in UC patients provides potential to generate clinical proof of concept; endoscopies and biopsies enable blinded central reads of • Clinical remission (symptom scores) • Histologic remission/mucosal healing (histopathology) PHASE 1a SAD/MAD PHASE 1b MAD PHASE 2 Population UC Patients UC Patients Healthy Dose Escalation: Up to 24 N Expansion (Mono and Combo): Up to 60 120-150 Up to 24 Key Objectives Early Efficacy Inform Dose Proof of Mechanism Safety / PK/ ADA Additional UC-100, clinical remission and response, CRP, FC, cytokines, histology, PD markers endoscopic remission, endoscopy subscore, End-Points stool frequency, rectal bleeding, histology, histological remission, QOL, PD markers endoscopy subscore, PD markers 11 © 2021 Surrozen, Inc.

Mutated RSPO2 Anti-ASGR1 IgG SZN-043 Severe Liver Disease Effector-less hIgG1 12Mutated RSPO2 Anti-ASGR1 IgG SZN-043 Severe Liver Disease Effector-less hIgG1 12

Potential for First Approved Treatment for Severe Alcoholic Hepatitis Liver Specific Wnt Activation and Regeneration SZN-043 MOA • Selective Wnt activation Proof of Concept • Specific hepatocyte proliferation Established • Functional improvement • 2022 - First in human • 2023 – Phase 1b in severe AH Anticipated • Potential for fast-track designation and fast Clinical path to approval Development • Potential for expansion to other severe liver diseases 13 © 2021 Surrozen, Inc.

SZN-043 – Potential to Significantly Improve Patient Outcomes in Severe Alcoholic Hepatitis AST:ALT ratio AST/ALT Ratio 20 D0 D3 D7 High Unmet Need 15 No approved drugs: SOC: steroids 10 **** 5 High mortality: 90-day mortality of 30% 0 Liver transplant denied: Limited availability, costly, denied due to Ammonia Plasma Ammonia alcoholism 300 D0 D3 D7 200 Differentiated Preclinical Data *** 100 • >25 preclinical studies conducted 0 • SZN-043 addresses underlying pathophysiology Liver IL6 Liver IL6 • Activates Wnt Signalling 8 D0 D3 D7 Pair Fed EtOH • Induces mature hepatocyte proliferation and improves clotting time 6 ** Anti-GFP SZN-043 • Reduces markers of liver injury & inflammation 4 ** 2 0 14 © 2021 Surrozen, Inc. AMMN (mmol/L) AST/ALT IL6/Actb mRNA (Fold)SZN-043 – Potential to Significantly Improve Patient Outcomes in Severe Alcoholic Hepatitis AST:ALT ratio AST/ALT Ratio 20 D0 D3 D7 High Unmet Need 15 No approved drugs: SOC: steroids 10 **** 5 High mortality: 90-day mortality of 30% 0 Liver transplant denied: Limited availability, costly, denied due to Ammonia Plasma Ammonia alcoholism 300 D0 D3 D7 200 Differentiated Preclinical Data *** 100 • >25 preclinical studies conducted 0 • SZN-043 addresses underlying pathophysiology Liver IL6 Liver IL6 • Activates Wnt Signalling 8 D0 D3 D7 Pair Fed EtOH • Induces mature hepatocyte proliferation and improves clotting time 6 ** Anti-GFP SZN-043 • Reduces markers of liver injury & inflammation 4 ** 2 0 14 © 2021 Surrozen, Inc. AMMN (mmol/L) AST/ALT IL6/Actb mRNA (Fold)

Clinical Development Plan Provides Fast Path to POC and Approval • Phase 1a: Potential to demonstrate clinical activity – methacetin breath test marker for hepatocyte proliferation • Phase 1b: Endpoints Lille and MELD scores highly correlated with survival; potentially lead to Fast Track Designation • Phase 2/3: Adaptive design may accelerate development timeline, primary endpoint readout at 90 days PHASE 1a SAD PHASE 1b MAD PHASE 2/3 HV/Early cirrhosis Severe Alcoholic Hepatitis Severe Alcoholic Hepatitis Pop 30-45 Up to 30 300 (placebo controlled) N Key Objectives Early Activity/Clinical Efficacy Inform Dose Proof of Mechanism Safety / PK PD markers (angiogenin, 7day Lille score, MELD score Additional Lect2, Methacetin) PD markers 90-day mortality End-Points 15 © 2021 Surrozen, Inc.Clinical Development Plan Provides Fast Path to POC and Approval • Phase 1a: Potential to demonstrate clinical activity – methacetin breath test marker for hepatocyte proliferation • Phase 1b: Endpoints Lille and MELD scores highly correlated with survival; potentially lead to Fast Track Designation • Phase 2/3: Adaptive design may accelerate development timeline, primary endpoint readout at 90 days PHASE 1a SAD PHASE 1b MAD PHASE 2/3 HV/Early cirrhosis Severe Alcoholic Hepatitis Severe Alcoholic Hepatitis Pop 30-45 Up to 30 300 (placebo controlled) N Key Objectives Early Activity/Clinical Efficacy Inform Dose Proof of Mechanism Safety / PK PD markers (angiogenin, 7day Lille score, MELD score Additional Lect2, Methacetin) PD markers 90-day mortality End-Points 15 © 2021 Surrozen, Inc.

Beyond Intestine and Liver… 16Beyond Intestine and Liver… 16
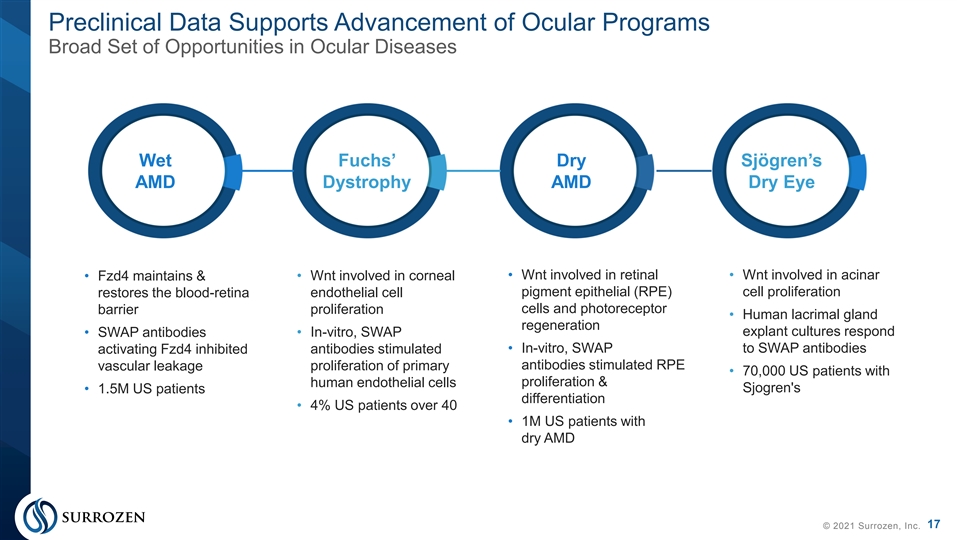
Preclinical Data Supports Advancement of Ocular Programs Broad Set of Opportunities in Ocular Diseases Wet Fuchs’ Dry Sjögren’s AMD Dystrophy AMD Dry Eye • Wnt involved in corneal • Wnt involved in retinal • Wnt involved in acinar • Fzd4 maintains & pigment epithelial (RPE) cell proliferation restores the blood-retina endothelial cell cells and photoreceptor barrier proliferation • Human lacrimal gland regeneration explant cultures respond • SWAP antibodies • In-vitro, SWAP • In-vitro, SWAP to SWAP antibodies activating Fzd4 inhibited antibodies stimulated proliferation of primary antibodies stimulated RPE vascular leakage • 70,000 US patients with proliferation & human endothelial cells Sjogren's • 1.5M US patients differentiation • 4% US patients over 40 • 1M US patients with dry AMD 17 © 2021 Surrozen, Inc.Preclinical Data Supports Advancement of Ocular Programs Broad Set of Opportunities in Ocular Diseases Wet Fuchs’ Dry Sjögren’s AMD Dystrophy AMD Dry Eye • Wnt involved in corneal • Wnt involved in retinal • Wnt involved in acinar • Fzd4 maintains & pigment epithelial (RPE) cell proliferation restores the blood-retina endothelial cell cells and photoreceptor barrier proliferation • Human lacrimal gland regeneration explant cultures respond • SWAP antibodies • In-vitro, SWAP • In-vitro, SWAP to SWAP antibodies activating Fzd4 inhibited antibodies stimulated proliferation of primary antibodies stimulated RPE vascular leakage • 70,000 US patients with proliferation & human endothelial cells Sjogren's • 1.5M US patients differentiation • 4% US patients over 40 • 1M US patients with dry AMD 17 © 2021 Surrozen, Inc.

Near Term Outlook and Potential Milestones Multiple Clinical Milestones with Potential for Early Proof of Concept 2021 2022 2023 Complete IND-enabling Phase 1a in Phase 1b in Ulcerative SZN-1326 Toxicology Studies Healthy Volunteers Colitis Patients INTESTINE SZN-043 LIVER 2021 2022 2023 Complete IND-enabling Phase 1a in Healthy Phase 1b in Severe Toxicology Studies Volunteers and Hepatic AH Patients Impairment Patients 2022+ Nominate Additional Lead Candidates RESEARCH PROGRAMS 2023+ File Additional INDs 18 © 2021 Surrozen, Inc. Near Term Outlook and Potential Milestones Multiple Clinical Milestones with Potential for Early Proof of Concept 2021 2022 2023 Complete IND-enabling Phase 1a in Phase 1b in Ulcerative SZN-1326 Toxicology Studies Healthy Volunteers Colitis Patients INTESTINE SZN-043 LIVER 2021 2022 2023 Complete IND-enabling Phase 1a in Healthy Phase 1b in Severe Toxicology Studies Volunteers and Hepatic AH Patients Impairment Patients 2022+ Nominate Additional Lead Candidates RESEARCH PROGRAMS 2023+ File Additional INDs 18 © 2021 Surrozen, Inc.

The Wnt Company - Targeted Regeneration © 2021 Surrozen, Inc.

Appendix © 2021 Surrozen, Inc.Appendix © 2021 Surrozen, Inc.

anti-Lrp6 VHH anti-Fzd IgG (Fzd5) SZN-1326 Preclinical Data effector-less hIgG1 21anti-Lrp6 VHH anti-Fzd IgG (Fzd5) SZN-1326 Preclinical Data effector-less hIgG1 21

SZN-1326 – Restores Wnt Signaling in Damaged Intestine Restores Wnt Signaling in Damaged Intestinal Selective Binding Profile Epithelium Selective Wnt Axin2 activation Normal Wnt No DSS Signaling Epithelial repair DSS Impaired Wnt anti-GFP Signaling Inflammation reduction DSS + Restored Wnt Functional SZN-1326 Signaling improvement Fzd10 Fzd9 Fzd8 Fzd7 Fzd6 Fzd5 Fzd4 Fzd3 Fzd2 Fzd1 Surrozen in vivo study (SRZ-279): Administered 4% DSS in mice for 7 days resulting in intestinal epithelial injury. SZN-1326 10mpk on days 4 and 7. 1% DSS on days 8-10. Readout on day 10 22 © 2021 Surrozen, Inc.SZN-1326 – Restores Wnt Signaling in Damaged Intestine Restores Wnt Signaling in Damaged Intestinal Selective Binding Profile Epithelium Selective Wnt Axin2 activation Normal Wnt No DSS Signaling Epithelial repair DSS Impaired Wnt anti-GFP Signaling Inflammation reduction DSS + Restored Wnt Functional SZN-1326 Signaling improvement Fzd10 Fzd9 Fzd8 Fzd7 Fzd6 Fzd5 Fzd4 Fzd3 Fzd2 Fzd1 Surrozen in vivo study (SRZ-279): Administered 4% DSS in mice for 7 days resulting in intestinal epithelial injury. SZN-1326 10mpk on days 4 and 7. 1% DSS on days 8-10. Readout on day 10 22 © 2021 Surrozen, Inc.
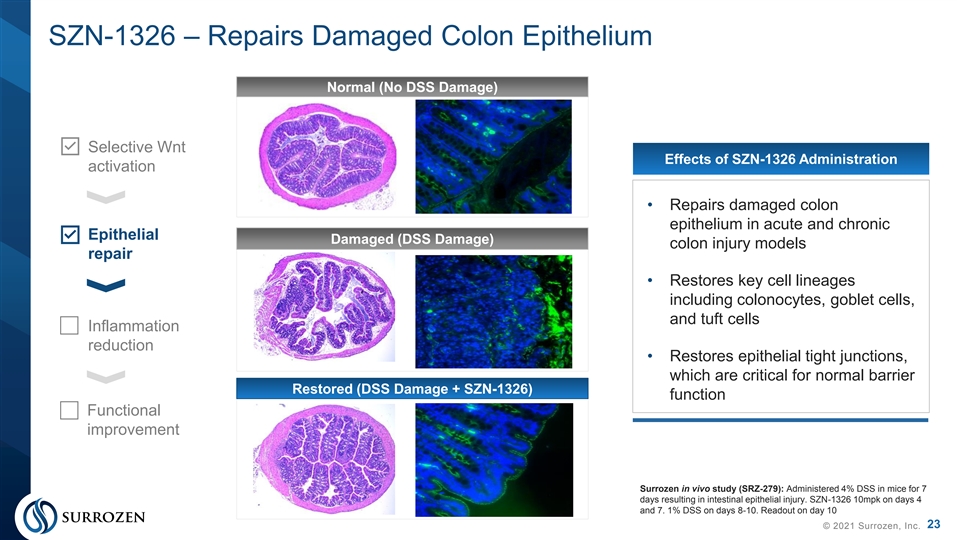
SZN-1326 – Repairs Damaged Colon Epithelium Normal (No DSS Damage) Selective Wnt Effects of SZN-1326 Administration activation • Repairs damaged colon epithelium in acute and chronic Epithelial Damaged (DSS Damage) colon injury models repair • Restores key cell lineages including colonocytes, goblet cells, and tuft cells Inflammation reduction • Restores epithelial tight junctions, which are critical for normal barrier Restored (DSS Damage + SZN-1326) function Functional improvement Surrozen in vivo study (SRZ-279): Administered 4% DSS in mice for 7 days resulting in intestinal epithelial injury. SZN-1326 10mpk on days 4 and 7. 1% DSS on days 8-10. Readout on day 10 23 © 2021 Surrozen, Inc.SZN-1326 – Repairs Damaged Colon Epithelium Normal (No DSS Damage) Selective Wnt Effects of SZN-1326 Administration activation • Repairs damaged colon epithelium in acute and chronic Epithelial Damaged (DSS Damage) colon injury models repair • Restores key cell lineages including colonocytes, goblet cells, and tuft cells Inflammation reduction • Restores epithelial tight junctions, which are critical for normal barrier Restored (DSS Damage + SZN-1326) function Functional improvement Surrozen in vivo study (SRZ-279): Administered 4% DSS in mice for 7 days resulting in intestinal epithelial injury. SZN-1326 10mpk on days 4 and 7. 1% DSS on days 8-10. Readout on day 10 23 © 2021 Surrozen, Inc.

SZN-1326 – Reduces Inflammatory Cytokines Colon TNF-a (pg/mg) Colon IL-6 (pg/mg) Colon IL-8 (pg/mg) Colon TNF-�� (pg/mg) Colon IL-6 (pg/mg) Colon IL-8 (pg/mg) 200 4 20 150 3 15 Selective Wnt activation 2 100 10 **** **** ** ** *** 50 1 5 Epithelial **** **** **** **** **** **** **** **** **** repair 0 0 0 SZN-1326 SZN-1326 SZN-1326 Inflammation reduction • Reduces key inflammatory cytokines induced by DSS and implicated in human IBD Functional • Results reproducible in both localized colon tissue and improvement systemic serum samples Statistical Analyses: One-way ANOVA, Holm-Sidak test (GraphPad Prism). All comparisons made with the anti-GFP group. Error bars: Mean with SD. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Surrozen in vivo study (SRZ-299): Administered 4% DSS in mice for 7 days resulting in intestinal epithelial injury. SZN-1326 24 treatment on days 4 and 7. 1% DSS on days 8-10. Readout on day 10. © 2021 Surrozen, Inc. No DSS Negative Control 0.3 mpk 1 mpk 3 mpk 10 mpk No DSS Negative Control 0.3 mpk 1 mpk 3 mpk 10 mpk No DSS Negative Control 0.3 mpk 1 mpk 3 mpk 10 mpk pg/mg pg/mg pg/mgSZN-1326 – Reduces Inflammatory Cytokines Colon TNF-a (pg/mg) Colon IL-6 (pg/mg) Colon IL-8 (pg/mg) Colon TNF-�� (pg/mg) Colon IL-6 (pg/mg) Colon IL-8 (pg/mg) 200 4 20 150 3 15 Selective Wnt activation 2 100 10 **** **** ** ** *** 50 1 5 Epithelial **** **** **** **** **** **** **** **** **** repair 0 0 0 SZN-1326 SZN-1326 SZN-1326 Inflammation reduction • Reduces key inflammatory cytokines induced by DSS and implicated in human IBD Functional • Results reproducible in both localized colon tissue and improvement systemic serum samples Statistical Analyses: One-way ANOVA, Holm-Sidak test (GraphPad Prism). All comparisons made with the anti-GFP group. Error bars: Mean with SD. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Surrozen in vivo study (SRZ-299): Administered 4% DSS in mice for 7 days resulting in intestinal epithelial injury. SZN-1326 24 treatment on days 4 and 7. 1% DSS on days 8-10. Readout on day 10. © 2021 Surrozen, Inc. No DSS Negative Control 0.3 mpk 1 mpk 3 mpk 10 mpk No DSS Negative Control 0.3 mpk 1 mpk 3 mpk 10 mpk No DSS Negative Control 0.3 mpk 1 mpk 3 mpk 10 mpk pg/mg pg/mg pg/mg

SZN-1326 – Reduces Disease Activity Disease Activity Index (DAI) at Body Weight Percent Change Fecal Score Day 10 (BW, fecal score) at Day 10 at Day 10 Selective Wnt activation Epithelial repair SZN-1326 SZN-1326 SZN-1326 Inflammation SZN-1326 decreases disease activity scores in acute and reduction chronic DSS mouse models: • Reverses DSS-induced weight loss Functional • Restores normal bowel function improvement Statistical Analyses: One-way ANOVA, Holm-Sidak test (GraphPad Prism). All comparisons made with the anti-GFP group. Error bars: Mean with SD. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Surrozen in vivo study (SRZ-299): Administered 4% DSS in mice for 7 days resulting in intestinal epithelial injury. SZN-1326 treatment on days 4 and 7. 1% DSS on days 8-10. Readout on day 10. 25 © 2021 Surrozen, Inc.SZN-1326 – Reduces Disease Activity Disease Activity Index (DAI) at Body Weight Percent Change Fecal Score Day 10 (BW, fecal score) at Day 10 at Day 10 Selective Wnt activation Epithelial repair SZN-1326 SZN-1326 SZN-1326 Inflammation SZN-1326 decreases disease activity scores in acute and reduction chronic DSS mouse models: • Reverses DSS-induced weight loss Functional • Restores normal bowel function improvement Statistical Analyses: One-way ANOVA, Holm-Sidak test (GraphPad Prism). All comparisons made with the anti-GFP group. Error bars: Mean with SD. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Surrozen in vivo study (SRZ-299): Administered 4% DSS in mice for 7 days resulting in intestinal epithelial injury. SZN-1326 treatment on days 4 and 7. 1% DSS on days 8-10. Readout on day 10. 25 © 2021 Surrozen, Inc.

SZN-1326 – Repairs Colon Epithelium In Vivo More Than Cyclosporine Cross Section of Transverse Colon: H&E Staining No DSS Anti-GFP Selective Wnt activation Epithelial repair Cyclosporine A SZN-1326 1 mpk BIW Inflammation reduction Functional improvement Surrozen in vivo study (SRZ-363): Administered 4% DSS in mice for 7 days followed by 1% DSS for 3 days resulting in intestinal epithelial injury. SZN-1326 treatment on days 4 and 7. Readout on day 10. 26 © 2020 Surrozen, Inc. CONFIDENTIALSZN-1326 – Repairs Colon Epithelium In Vivo More Than Cyclosporine Cross Section of Transverse Colon: H&E Staining No DSS Anti-GFP Selective Wnt activation Epithelial repair Cyclosporine A SZN-1326 1 mpk BIW Inflammation reduction Functional improvement Surrozen in vivo study (SRZ-363): Administered 4% DSS in mice for 7 days followed by 1% DSS for 3 days resulting in intestinal epithelial injury. SZN-1326 treatment on days 4 and 7. Readout on day 10. 26 © 2020 Surrozen, Inc. CONFIDENTIAL

SZN-1326 – Improves Colon Histology Score In Vivo More Than Cyclosporine Selective Wnt activation Epithelial repair Inflammation reduction Functional improvement Statistical Analyses: One-way ANOVA, Holm-Sidak test (GraphPad Prism). All comparisons made with the anti-GFP group. Error bars: Mean with SD. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Surrozen in vivo study (SRZ-363): Administered 4% DSS in mice for 7 days followed by 1% DSS for 3 days resulting in intestinal epithelial injury. SZN-1326 treatment on days 4 and 7. Readout on day 10. 27 © 2020 Surrozen, Inc. CONFIDENTIALSZN-1326 – Improves Colon Histology Score In Vivo More Than Cyclosporine Selective Wnt activation Epithelial repair Inflammation reduction Functional improvement Statistical Analyses: One-way ANOVA, Holm-Sidak test (GraphPad Prism). All comparisons made with the anti-GFP group. Error bars: Mean with SD. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Surrozen in vivo study (SRZ-363): Administered 4% DSS in mice for 7 days followed by 1% DSS for 3 days resulting in intestinal epithelial injury. SZN-1326 treatment on days 4 and 7. Readout on day 10. 27 © 2020 Surrozen, Inc. CONFIDENTIAL

SZN-1326 – Improves Disease Activity In Vivo More Than Cyclosporine Selective Wnt activation Epithelial repair Inflammation reduction Functional improvement Statistical Analyses: One-way ANOVA, Holm-Sidak test (GraphPad Prism). All comparisons made with the anti-GFP group. Error bars: Mean with SD. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Surrozen in vivo study (SRZ-363): Administered 4% DSS in mice for 7 days followed by 1% DSS for 3 days resulting in intestinal epithelial injury. SZN-1326 treatment on days 4 and 7. Readout on day 10. 28 © 2020 Surrozen, Inc. CONFIDENTIAL
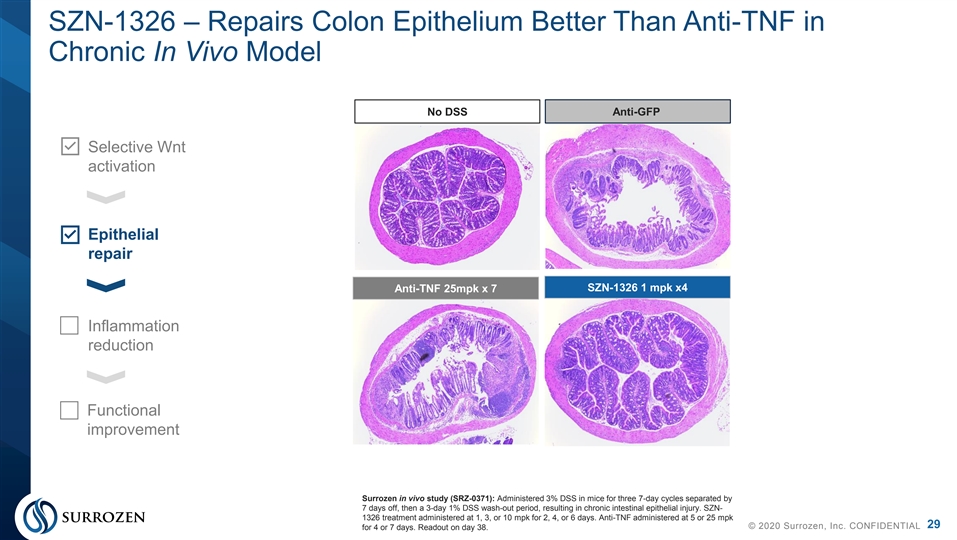
SZN-1326 – Repairs Colon Epithelium Better Than Anti-TNF in Chronic In Vivo Model No DSS Anti-GFP Selective Wnt activation Epithelial repair SZN-1326 1 mpk x4 Anti-TNF 25mpk x 7 Inflammation reduction Functional improvement Surrozen in vivo study (SRZ-0371): Administered 3% DSS in mice for three 7-day cycles separated by 7 days off, then a 3-day 1% DSS wash-out period, resulting in chronic intestinal epithelial injury. SZN- 1326 treatment administered at 1, 3, or 10 mpk for 2, 4, or 6 days. Anti-TNF administered at 5 or 25 mpk 29 © 2020 Surrozen, Inc. CONFIDENTIAL for 4 or 7 days. Readout on day 38. SZN-1326 – Repairs Colon Epithelium Better Than Anti-TNF in Chronic In Vivo Model No DSS Anti-GFP Selective Wnt activation Epithelial repair SZN-1326 1 mpk x4 Anti-TNF 25mpk x 7 Inflammation reduction Functional improvement Surrozen in vivo study (SRZ-0371): Administered 3% DSS in mice for three 7-day cycles separated by 7 days off, then a 3-day 1% DSS wash-out period, resulting in chronic intestinal epithelial injury. SZN- 1326 treatment administered at 1, 3, or 10 mpk for 2, 4, or 6 days. Anti-TNF administered at 5 or 25 mpk 29 © 2020 Surrozen, Inc. CONFIDENTIAL for 4 or 7 days. Readout on day 38.

SZN-1326 – Improves Colon Histology Score More Than Anti-TNF in Chronic In Vivo Model Selective Wnt activation Epithelial repair Inflammation reduction Functional improvement Statistical Analyses: One-way ANOVA, Holm-Sidak test (GraphPad Prism). All comparisons made with the anti-GFP group. Error bars: Mean with SD. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Surrozen in vivo study (SRZ-0371): Administered 3% DSS in mice for three 7-day cycles separated by 7 days off, then a 3-day 1% DSS wash-out period, resulting in chronic intestinal epithelial injury. SZN-1326 treatment administered at 1, 3, or 10 mpk for 2, 4, or 6 days. Anti-TNF administered at 5 or 25 mpk for 4 or 7 days. Readout on day 38. 30 © 2020 Surrozen, Inc. CONFIDENTIALSZN-1326 – Improves Colon Histology Score More Than Anti-TNF in Chronic In Vivo Model Selective Wnt activation Epithelial repair Inflammation reduction Functional improvement Statistical Analyses: One-way ANOVA, Holm-Sidak test (GraphPad Prism). All comparisons made with the anti-GFP group. Error bars: Mean with SD. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Surrozen in vivo study (SRZ-0371): Administered 3% DSS in mice for three 7-day cycles separated by 7 days off, then a 3-day 1% DSS wash-out period, resulting in chronic intestinal epithelial injury. SZN-1326 treatment administered at 1, 3, or 10 mpk for 2, 4, or 6 days. Anti-TNF administered at 5 or 25 mpk for 4 or 7 days. Readout on day 38. 30 © 2020 Surrozen, Inc. CONFIDENTIAL
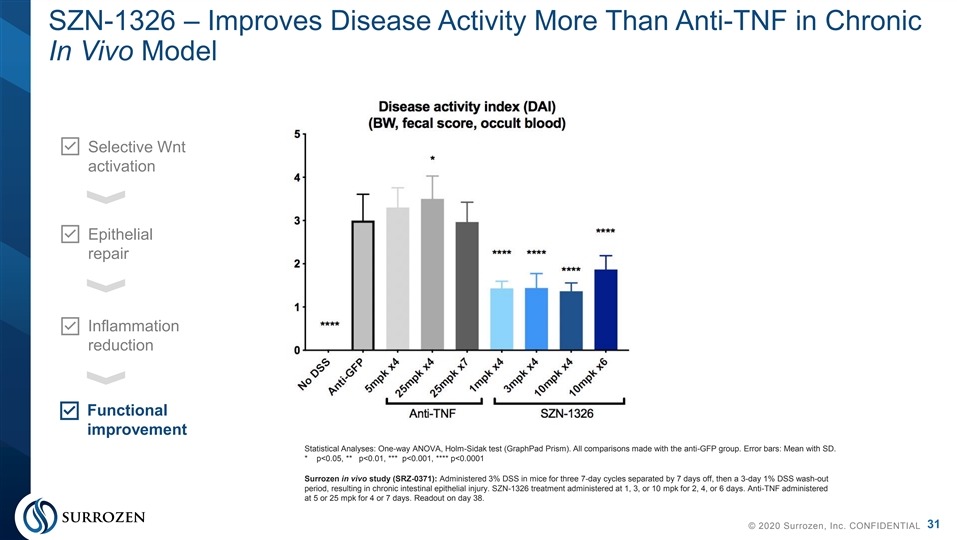
SZN-1326 – Improves Disease Activity More Than Anti-TNF in Chronic In Vivo Model Selective Wnt activation Epithelial repair Inflammation reduction Functional improvement Statistical Analyses: One-way ANOVA, Holm-Sidak test (GraphPad Prism). All comparisons made with the anti-GFP group. Error bars: Mean with SD. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Surrozen in vivo study (SRZ-0371): Administered 3% DSS in mice for three 7-day cycles separated by 7 days off, then a 3-day 1% DSS wash-out period, resulting in chronic intestinal epithelial injury. SZN-1326 treatment administered at 1, 3, or 10 mpk for 2, 4, or 6 days. Anti-TNF administered at 5 or 25 mpk for 4 or 7 days. Readout on day 38. 31 © 2020 Surrozen, Inc. CONFIDENTIALSZN-1326 – Improves Disease Activity More Than Anti-TNF in Chronic In Vivo Model Selective Wnt activation Epithelial repair Inflammation reduction Functional improvement Statistical Analyses: One-way ANOVA, Holm-Sidak test (GraphPad Prism). All comparisons made with the anti-GFP group. Error bars: Mean with SD. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 Surrozen in vivo study (SRZ-0371): Administered 3% DSS in mice for three 7-day cycles separated by 7 days off, then a 3-day 1% DSS wash-out period, resulting in chronic intestinal epithelial injury. SZN-1326 treatment administered at 1, 3, or 10 mpk for 2, 4, or 6 days. Anti-TNF administered at 5 or 25 mpk for 4 or 7 days. Readout on day 38. 31 © 2020 Surrozen, Inc. CONFIDENTIAL

Mutated RSPO2 Anti-ASGR1 IgG SZN-043 Preclinical Data Effector-less hIgG1 32Mutated RSPO2 Anti-ASGR1 IgG SZN-043 Preclinical Data Effector-less hIgG1 32

SZN-043 Selectively Stimulates Hepatocyte Proliferation Hepatocyte Proliferation Results in Rapid Improvement in Liver Function Ki-67 HNF4α Overlay Liver Mki67 Selective Wnt 20 24 h 48 h 72 h Anti-GFP activation SZN-043 **** 15 *** 10 Hepatocyte 5 Proliferation 0 Functional Improvement • SZN-043 induces Axin-2 expression selectively in the liver in normal mice • Induces mature hepatocyte proliferation in alcoholic hepatitis mouse model and TAA mouse model • SZN-043 treatment restores normal clotting function in TAA liver injury model by day 3 Surrozen in vivo study (SRZ-347): Mice were preconditioned with 5% EtOH liquid diet for 10 days followed by a 20% EtOH p.o. binge to establish alcoholic-induced liver injury. Treatment followed with 1 dose of SZN-043 at 30 mpk or 1 equivalent dose of anti-GFP as a negative control. All images from 72 h after SZN-043 treatment. 33 © 2021 Surrozen, Inc. Anti-GFP SZN-043 Mki67/Actb mRNA (Fold)SZN-043 Selectively Stimulates Hepatocyte Proliferation Hepatocyte Proliferation Results in Rapid Improvement in Liver Function Ki-67 HNF4α Overlay Liver Mki67 Selective Wnt 20 24 h 48 h 72 h Anti-GFP activation SZN-043 **** 15 *** 10 Hepatocyte 5 Proliferation 0 Functional Improvement • SZN-043 induces Axin-2 expression selectively in the liver in normal mice • Induces mature hepatocyte proliferation in alcoholic hepatitis mouse model and TAA mouse model • SZN-043 treatment restores normal clotting function in TAA liver injury model by day 3 Surrozen in vivo study (SRZ-347): Mice were preconditioned with 5% EtOH liquid diet for 10 days followed by a 20% EtOH p.o. binge to establish alcoholic-induced liver injury. Treatment followed with 1 dose of SZN-043 at 30 mpk or 1 equivalent dose of anti-GFP as a negative control. All images from 72 h after SZN-043 treatment. 33 © 2021 Surrozen, Inc. Anti-GFP SZN-043 Mki67/Actb mRNA (Fold)

SZN-043 Reduces Markers of Liver Injury and Inflammation Activity in Alcohol Injury Model Support Clinical Development Path Selective Wnt AST:ALT ratio Ammonia Liver IL6 activation AST/ALT Ratio Plasma Ammonia Liver IL6 20 300 8 D0 D3 D7 D0 D3 D7 D0 D3 D7 Pair Fed EtOH 15 6 ** Anti-GFP 200 SZN-043 10 4 *** Hepatocyte ** **** 100 5 2 Proliferation 0 0 0 • Surrozen established a rodent model of alcohol-induced liver injury Functional Improvement • Alcohol injury in the model leads to characteristics of severe alcoholic hepatitis in humans, e.g. hepatocyte injury, increased ammonia, elevated cytokines • SZN-043 treatment reduces ammonia • SZN-043 treatment reduces the AST:ALT ratio, IL1β, and IL6 34 © 2021 Surrozen, Inc. AST/ALT AMMN (mmol/L) IL6/Actb mRNA (Fold)SZN-043 Reduces Markers of Liver Injury and Inflammation Activity in Alcohol Injury Model Support Clinical Development Path Selective Wnt AST:ALT ratio Ammonia Liver IL6 activation AST/ALT Ratio Plasma Ammonia Liver IL6 20 300 8 D0 D3 D7 D0 D3 D7 D0 D3 D7 Pair Fed EtOH 15 6 ** Anti-GFP 200 SZN-043 10 4 *** Hepatocyte ** **** 100 5 2 Proliferation 0 0 0 • Surrozen established a rodent model of alcohol-induced liver injury Functional Improvement • Alcohol injury in the model leads to characteristics of severe alcoholic hepatitis in humans, e.g. hepatocyte injury, increased ammonia, elevated cytokines • SZN-043 treatment reduces ammonia • SZN-043 treatment reduces the AST:ALT ratio, IL1β, and IL6 34 © 2021 Surrozen, Inc. AST/ALT AMMN (mmol/L) IL6/Actb mRNA (Fold)

Additional Information © 2021 Surrozen, Inc.Additional Information © 2021 Surrozen, Inc.

Glossary AH – Alcoholic hepatitis IND – Investigational new Drug ALT – Alanine Aminotransferase Lille – Model for end-stage liver disease score AMD – Age-related macular degeneration Lrp – Lipoprotein receptor-related protein ASGR1 – Asiaglycoprotein receptor 1 MELD – Model for end-stage liver disease score AST – Aspartate aminotransferase MOA – Mechanism of action AT1/AT2 – Alveolar type epithelial cells PD – Pharmacodynamics BW – Body weight Pg – Picogram COPD – Chronic Obstructive Pulmonary Disease Mg – Milligrams DC – dendritic cell PIPE – Private investment in public equity DSS – Dextran sodium sulfate PK – Pharmacokinetic EtOH – Ehyl alcohol SAD – Single ascending dose FSGS – Focal segmental glomerulosclerosis MAD – Multiple ascending dose Fzd – Frizzled RPE – Retinal pigment epithelium GFP – Green fluorescence protein SOC – Standard of care GI – Gastrointestinal SWAP – Surrozen Wnt signal activating proteins HNF alpha - Hepatocyte nuclear factor 4 alpha SWEETS – Surrozen Wnt enhancer engineered for tissue specificity IBD – inflammatory Bowel Disease TAA – Thioacetamide IgG – Immunoglobulin G UC – Ulcerative colitis IPF – Idiopathic pulmonary fibrosis VHH – Single variable domain on a heavy chain (VHH) antibodies 36 © 2021 Surrozen, Inc.
