Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Reneo Pharmaceuticals, Inc. | rphm-20210811x8k.htm |
Exhibit 99.1
| Nasdaq: RPHM August 2021 Focused on Improving the Lives of Patients with Rare Genetic Mitochondrial Diseases |
| 2 Forward-Looking Statements This presentation may not be reproduced, retransmitted or further distributed to any other person or published, in whole or in part, for any other purpose. This presentation regarding Reneo Pharmaceuticals, Inc.(the “Company”) is for you to familiarize yourself with the Company. This presentation is for information and education purposes only. No reliance may be placed for any purpose whatsoever on the information contained in this document or on assumptions made as to its completeness. This presentation contains forward-looking statements that are based on the beliefs and assumptions of the Company’s management team, and on information currently available to such management team. These forward-looking statements are subject to numerous risks and uncertainties, many of which are beyond the Company’s control. All statements, other than statements of historical fact, contained in this presentation, including statements regarding future events, future financial performance, business strategy and plans, and objectives of the Company for future operations, are forward-looking statements. Although the Company does not make forward-looking statements unless it believes it has a reasonable basis for doing so, the Company cannot guarantee their accuracy. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, which may cause the actual results, levels of activity, performance or achievements of the Company and the Company’s industry to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. You should not place undue reliance on any forward-looking statement. The Company undertakes no obligation to update or revise publicly any of the forward- looking statements after the date hereof to conform the statements to actual results or changed expectations except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. This presentation discusses a product candidate that is under clinical study and which has not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of this product candidate for the use for which it is being studied. |
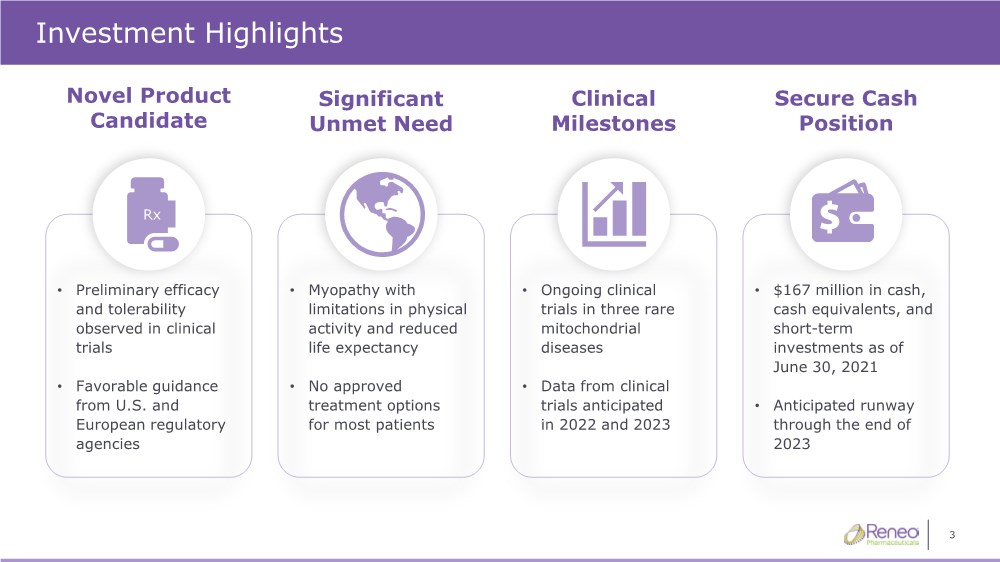
| 3 Investment Highlights Secure Cash Position Significant Unmet Need Clinical Milestones Novel Product Candidate • Preliminary efficacy and tolerability observed in clinical trials • Favorable guidance from U.S. and European regulatory agencies • Myopathy with limitations in physical activity and reduced life expectancy • No approved treatment options for most patients • Ongoing clinical trials in three rare mitochondrial diseases • Data from clinical trials anticipated in 2022 and 2023 • $167 million in cash, cash equivalents, and short-term investments as of June 30, 2021 • Anticipated runway through the end of 2023 Rx |
| REN001 Overview 11 4 |
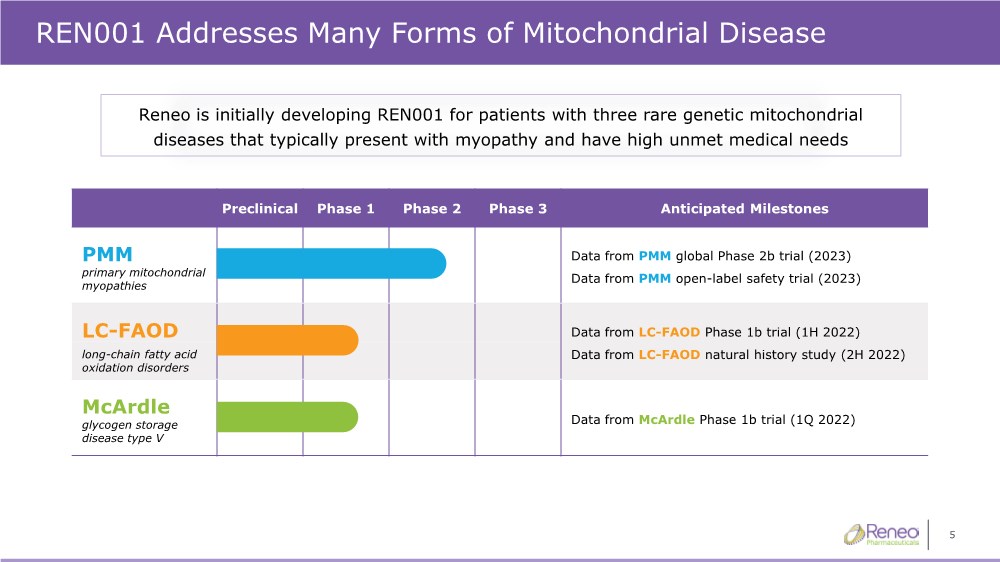
| 5 REN001 Addresses Many Forms of Mitochondrial Disease Preclinical Phase 1 Phase 2 Phase 3 Anticipated Milestones PMM Data from PMM global Phase 2b trial (2023) Data from PMM open-label safety trial (2023) primary mitochondrial myopathies LC-FAOD Data from LC-FAOD Phase 1b trial (1H 2022) Data from LC-FAOD natural history study (2H 2022) long-chain fatty acid oxidation disorders McArdle Data from McArdle Phase 1b trial (1Q 2022) glycogen storage disease type V Reneo is initially developing REN001 for patients with three rare genetic mitochondrial diseases that typically present with myopathy and have high unmet medical needs |
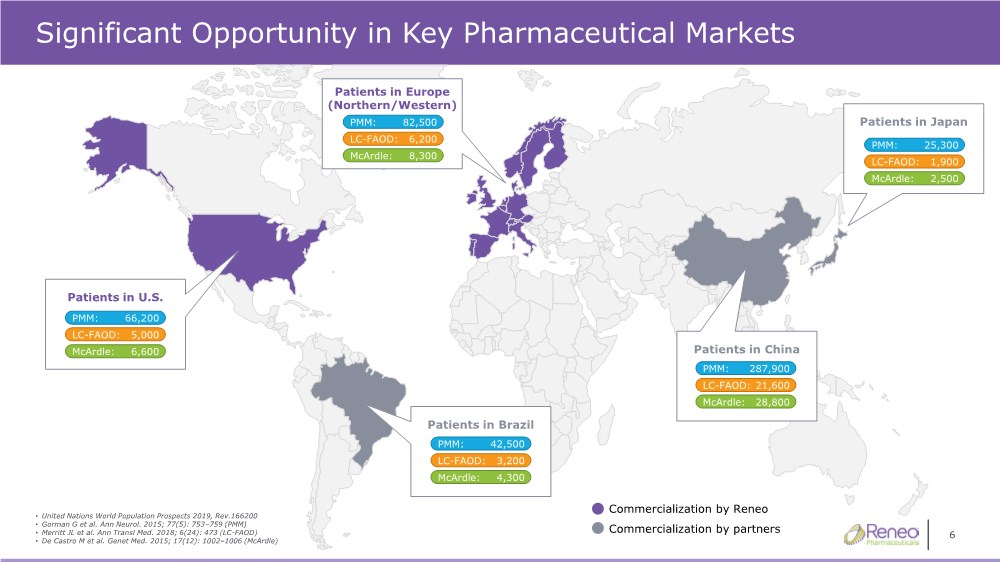
| 6 Significant Opportunity in Key Pharmaceutical Markets Patients in Europe (Northern/Western) • United Nations World Population Prospects 2019, Rev.166200 • Gorman G et al. Ann Neurol. 2015; 77(5): 753–759 (PMM) •Merritt JL et al. Ann Transl Med. 2018; 6(24): 473 (LC-FAOD) • De Castro M et al. Genet Med. 2015; 17(12): 1002–1006 (McArdle) Patients in China Patients in Japan Commercialization by Reneo Commercialization by partners Patients in U.S. PMM: 66,200 LC-FAOD: 5,000 McArdle: 6,600 PMM: 82,500 LC-FAOD: 6,200 McArdle: 8,300 PMM: 287,900 LC-FAOD: 21,600 McArdle: 28,800 PMM: 25,300 LC-FAOD: 1,900 McArdle: 2,500 Patients in Brazil PMM: 42,500 LC-FAOD: 3,200 McArdle: 4,300 |
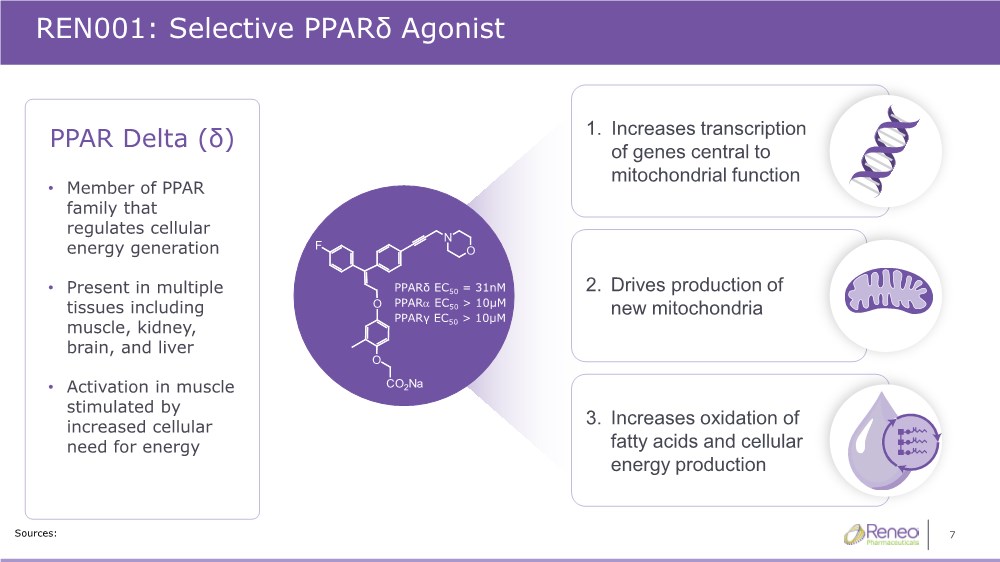
| 7 REN001: Selective PPARδ Agonist Sources: 1. Increases transcription of genes central to mitochondrial function 2. Drives production of new mitochondria 3. Increases oxidation of fatty acids and cellular energy production PPARδ EC50 = 31nM PPARa EC50 > 10μM PPARγ EC50 > 10μM PPAR Delta (δ) • Member of PPAR family that regulates cellular energy generation • Present in multiple tissues including muscle, kidney, brain, and liver • Activation in muscle stimulated by increased cellular need for energy |
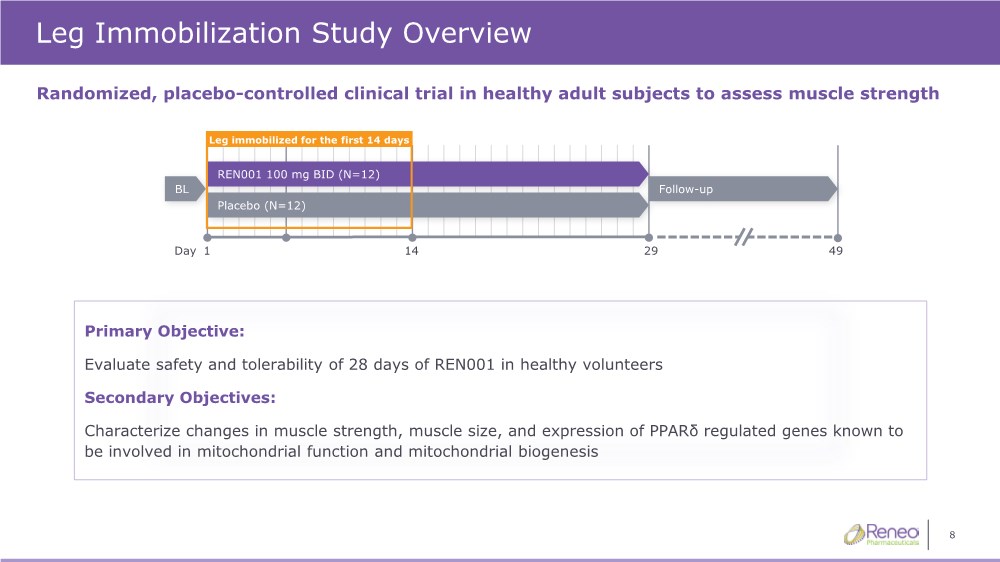
| 8 Leg Immobilization Study Overview Randomized, placebo-controlled clinical trial in healthy adult subjects to assess muscle strength Primary Objective: Evaluate safety and tolerability of 28 days of REN001 in healthy volunteers Secondary Objectives: Characterize changes in muscle strength, muscle size, and expression of PPARδ regulated genes known to be involved in mitochondrial function and mitochondrial biogenesis 29 1 14 Day REN001 100 mg BID (N=12) BL 49 Placebo (N=12) Follow-up Leg immobilized for the first 14 days |
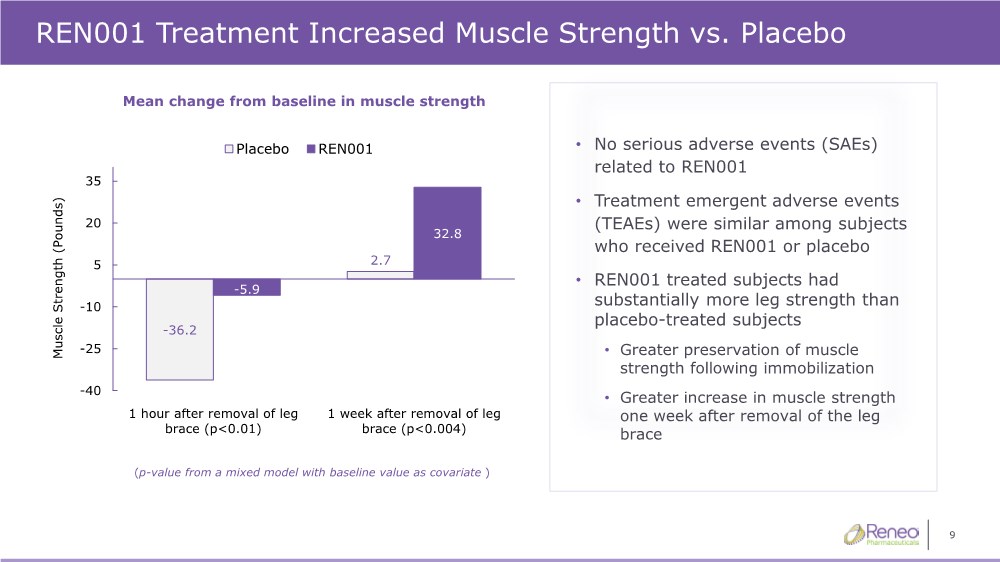
| 9 REN001 Treatment Increased Muscle Strength vs. Placebo -36.2 2.7 -5.9 32.8 -40 -25 -10 5 20 35 1 hour after removal of leg brace (p<0.01) 1 week after removal of leg brace (p<0.004) Muscle Strength (Pounds) Placebo REN001 (p-value from a mixed model with baseline value as covariate ) Mean change from baseline in muscle strength • No serious adverse events (SAEs) related to REN001 • Treatment emergent adverse events (TEAEs) were similar among subjects who received REN001 or placebo • REN001 treated subjects had substantially more leg strength than placebo-treated subjects • Greater preservation of muscle strength following immobilization • Greater increase in muscle strength one week after removal of the leg brace |
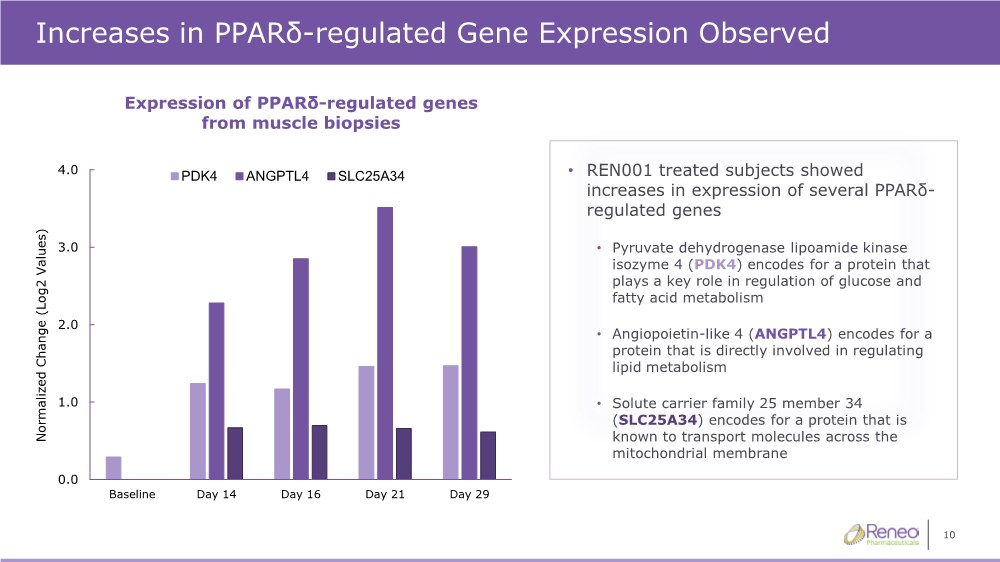
| 10 0.0 1.0 2.0 3.0 4.0 Baseline Day 14 Day 16 Day 21 Day 29 Normalized Change (Log2 Values) PDK4 ANGPTL4 SLC25A34 Increases in PPARδ-regulated Gene Expression Observed • REN001 treated subjects showed increases in expression of several PPARδ- regulated genes • Pyruvate dehydrogenase lipoamide kinase isozyme 4 (PDK4) encodes for a protein that plays a key role in regulation of glucose and fatty acid metabolism • Angiopoietin-like 4 (ANGPTL4) encodes for a protein that is directly involved in regulating lipid metabolism • Solute carrier family 25 member 34 (SLC25A34) encodes for a protein that is known to transport molecules across the mitochondrial membrane Expression of PPARδ-regulated genes from muscle biopsies |
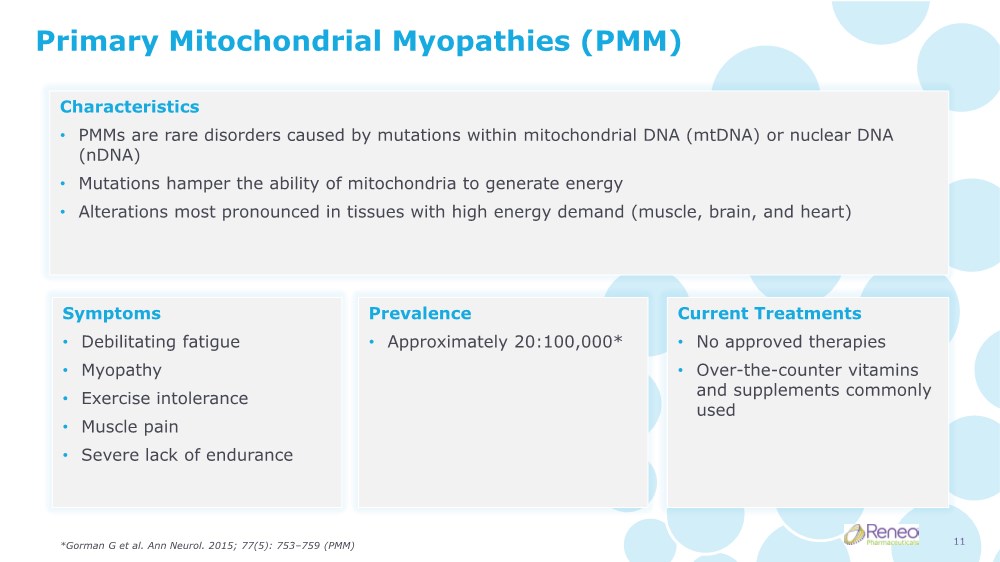
| Primary Mitochondrial Myopathies (PMM) Characteristics • PMMs are rare disorders caused by mutations within mitochondrial DNA (mtDNA) or nuclear DNA (nDNA) • Mutations hamper the ability of mitochondria to generate energy • Alterations most pronounced in tissues with high energy demand (muscle, brain, and heart) Symptoms • Debilitating fatigue • Myopathy • Exercise intolerance • Muscle pain • Severe lack of endurance Prevalence • Approximately 20:100,000* Current Treatments • No approved therapies • Over-the-counter vitamins and supplements commonly used *Gorman G et al. Ann Neurol. 2015; 77(5): 753–759 (PMM) 11 |
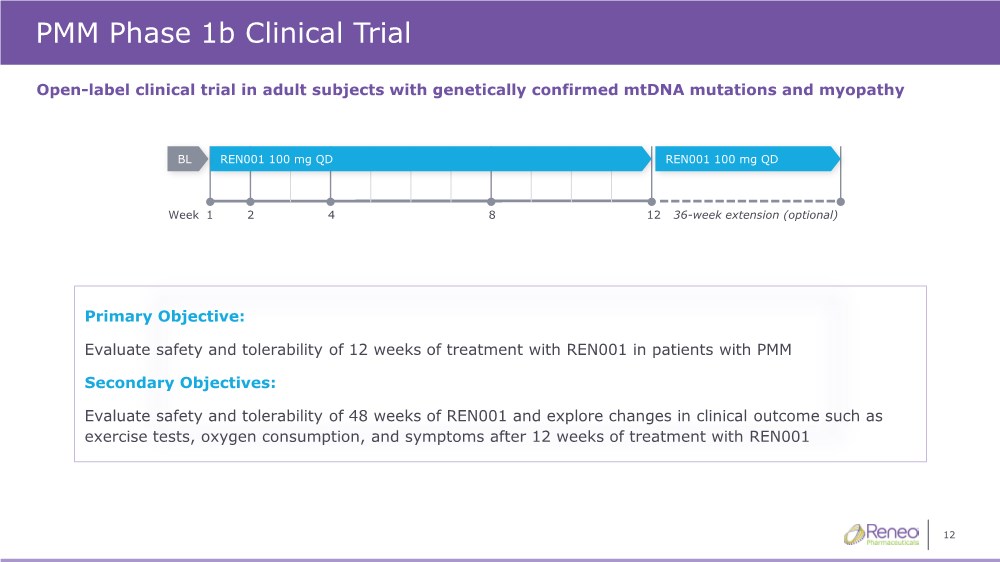
| 12 PMM Phase 1b Clinical Trial Open-label clinical trial in adult subjects with genetically confirmed mtDNA mutations and myopathy Primary Objective: Evaluate safety and tolerability of 12 weeks of treatment with REN001 in patients with PMM Secondary Objectives: Evaluate safety and tolerability of 48 weeks of REN001 and explore changes in clinical outcome such as exercise tests, oxygen consumption, and symptoms after 12 weeks of treatment with REN001 4 12 1 2 8 Week 36-week extension (optional) REN001 100 mg QD REN001 100 mg QD BL |
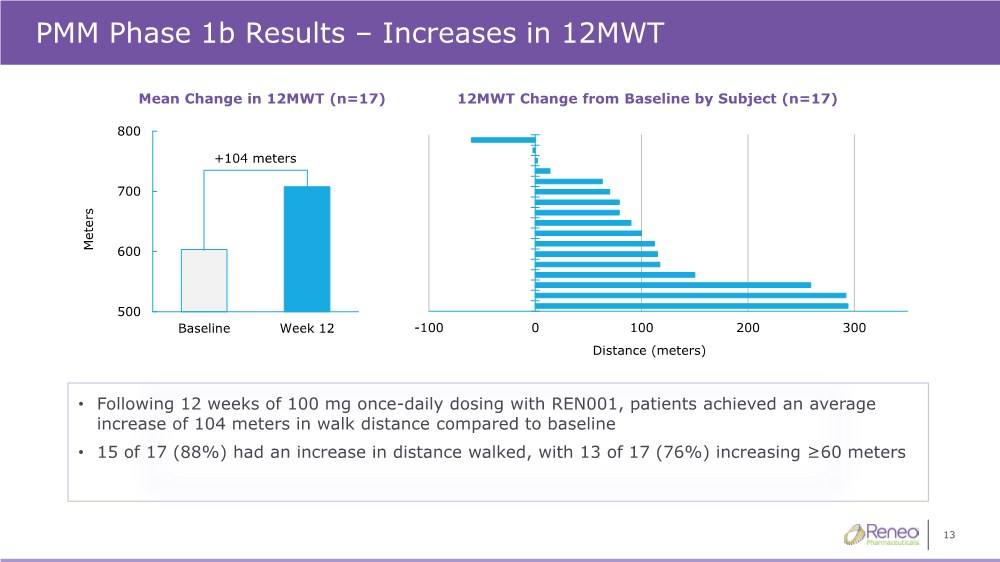
| 13 500 600 700 800 Baseline Week 12 Meters • Following 12 weeks of 100 mg once-daily dosing with REN001, patients achieved an average increase of 104 meters in walk distance compared to baseline • 15 of 17 (88%) had an increase in distance walked, with 13 of 17 (76%) increasing ≥60 meters PMM Phase 1b Results – Increases in 12MWT -100 0 100 200 300 Distance (meters) 12MWT Change from Baseline by Subject (n=17) Mean Change in 12MWT (n=17) +104 meters |
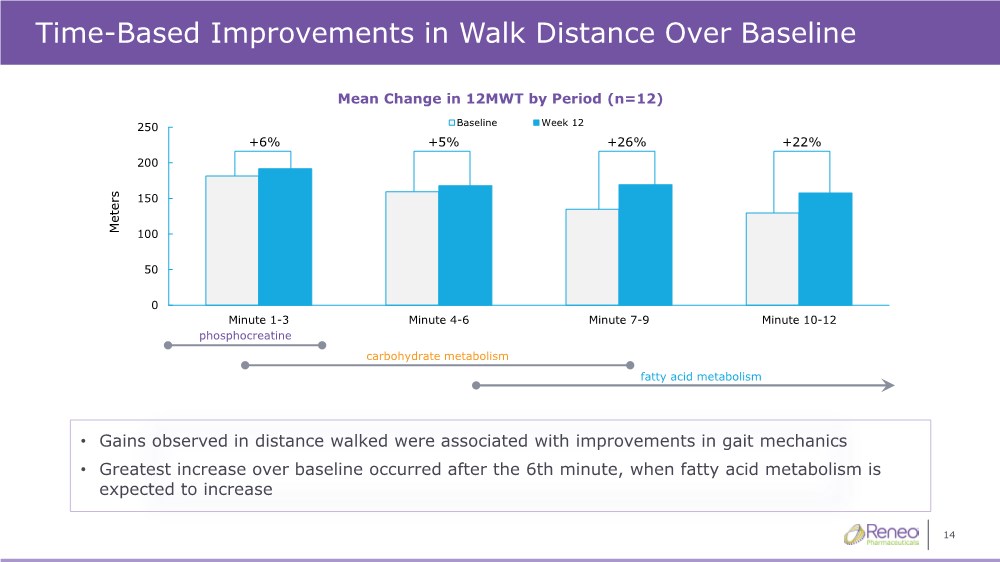
| 14 Time-Based Improvements in Walk Distance Over Baseline • Gains observed in distance walked were associated with improvements in gait mechanics • Greatest increase over baseline occurred after the 6th minute, when fatty acid metabolism is expected to increase phosphocreatine carbohydrate metabolism fatty acid metabolism +6% +5% +26% +22% 0 50 100 150 200 250 Minute 1-3 Minute 4-6 Minute 7-9 Minute 10-12 Meters Baseline Week 12 Mean Change in 12MWT by Period (n=12) |
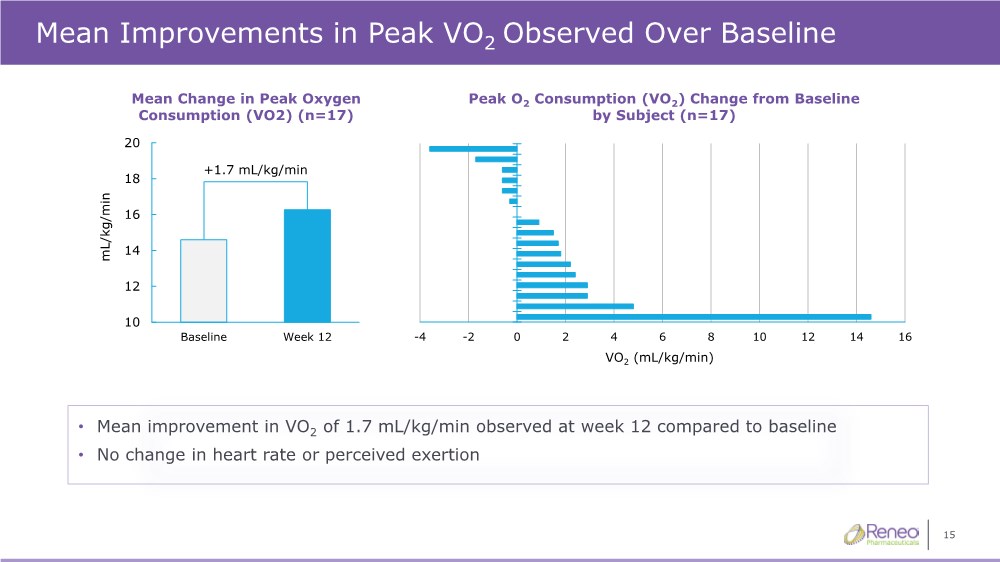
| 15 Mean Improvements in Peak VO2 Observed Over Baseline • Mean improvement in VO2 of 1.7 mL/kg/min observed at week 12 compared to baseline • No change in heart rate or perceived exertion Peak O2 Consumption (VO2) Change from Baseline by Subject (n=17) Mean Change in Peak Oxygen Consumption (VO2) (n=17) +1.7 mL/kg/min -4 -2 0 2 4 6 8 10 12 14 16 VO2 (mL/kg/min) 10 12 14 16 18 20 Baseline Week 12 mL/kg/min |
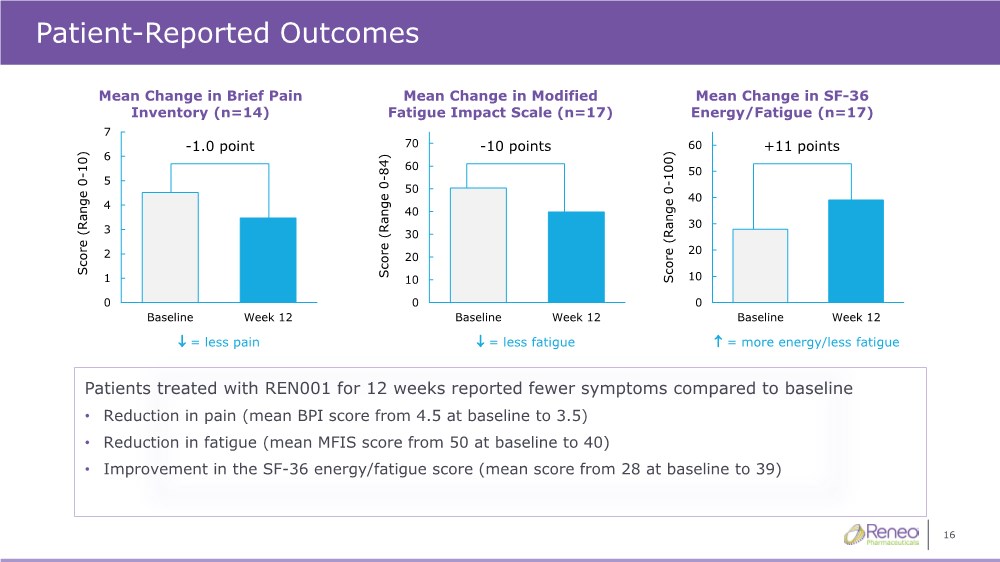
| 16 Patient-Reported Outcomes Patients treated with REN001 for 12 weeks reported fewer symptoms compared to baseline • Reduction in pain (mean BPI score from 4.5 at baseline to 3.5) • Reduction in fatigue (mean MFIS score from 50 at baseline to 40) • Improvement in the SF-36 energy/fatigue score (mean score from 28 at baseline to 39) Mean Change in Brief Pain Inventory (n=14) Mean Change in Modified Fatigue Impact Scale (n=17) Mean Change in SF-36 Energy/Fatigue (n=17) 0 1 2 3 4 5 6 7 Baseline Week 12 Score (Range 0 - 10) -1.0 point 0 10 20 30 40 50 60 70 Baseline Week 12 Score (Range 0 - 84) -10 points 0 10 20 30 40 50 60 Baseline Week 12 Score (Range 0 - 100) +11 points = less pain = less fatigue = more energy/less fatigue |
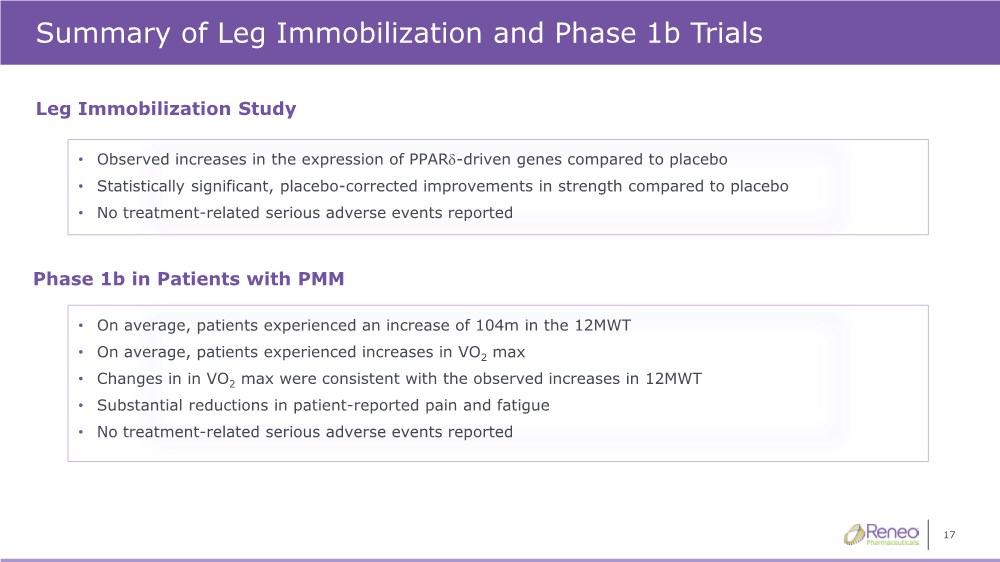
| 17 Summary of Leg Immobilization and Phase 1b Trials • On average, patients experienced an increase of 104m in the 12MWT • On average, patients experienced increases in VO2 max • Changes in in VO2 max were consistent with the observed increases in 12MWT • Substantial reductions in patient-reported pain and fatigue • No treatment-related serious adverse events reported Leg Immobilization Study Phase 1b in Patients with PMM • Observed increases in the expression of PPARd-driven genes compared to placebo • Statistically significant, placebo-corrected improvements in strength compared to placebo • No treatment-related serious adverse events reported |
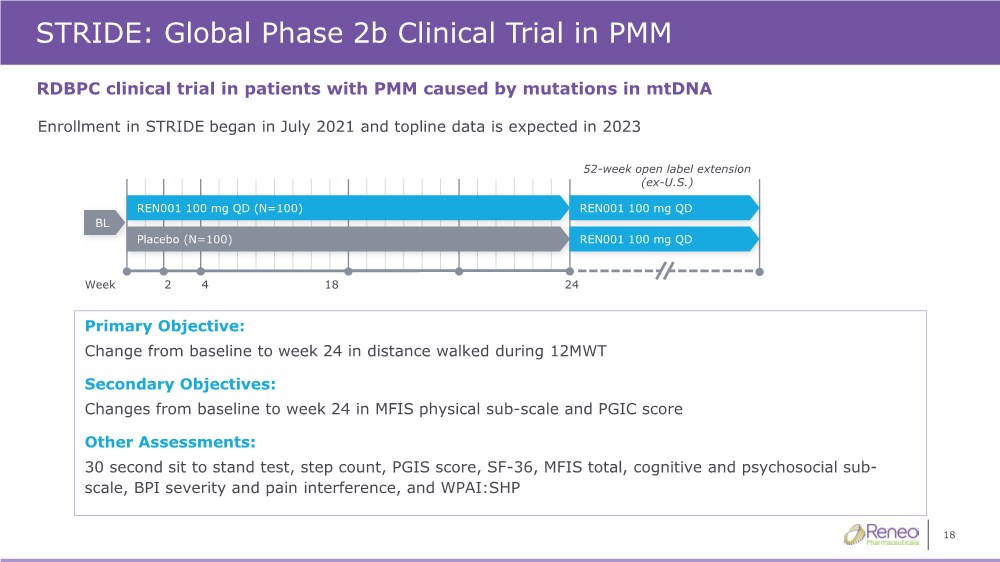
| 18 STRIDE: Global Phase 2b Clinical Trial in PMM RDBPC clinical trial in patients with PMM caused by mutations in mtDNA 52-week open label extension (ex-U.S.) Primary Objective: Change from baseline to week 24 in distance walked during 12MWT Secondary Objectives: Changes from baseline to week 24 in MFIS physical sub-scale and PGIC score Other Assessments: 30 second sit to stand test, step count, PGIS score, SF-36, MFIS total, cognitive and psychosocial sub- scale, BPI severity and pain interference, and WPAI:SHP 24 2 18 Week REN001 100 mg QD (N=100) BL Placebo (N=100) REN001 100 mg QD REN001 100 mg QD 4 Enrollment in STRIDE began in July 2021 and topline data is expected in 2023 |
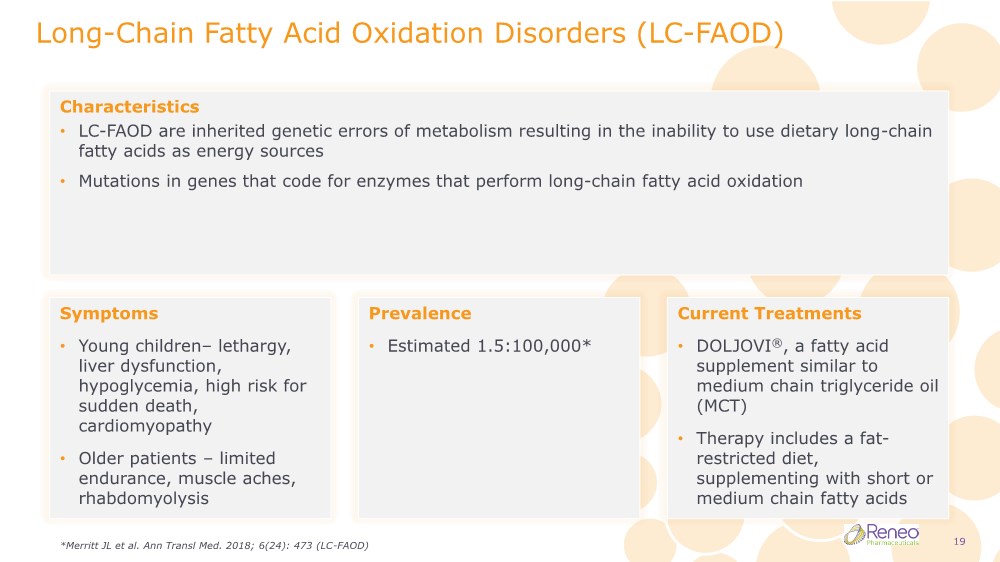
| Long-Chain Fatty Acid Oxidation Disorders (LC-FAOD) Characteristics • LC-FAOD are inherited genetic errors of metabolism resulting in the inability to use dietary long-chain fatty acids as energy sources • Mutations in genes that code for enzymes that perform long-chain fatty acid oxidation Symptoms • Young children– lethargy, liver dysfunction, hypoglycemia, high risk for sudden death, cardiomyopathy • Older patients – limited endurance, muscle aches, rhabdomyolysis Prevalence • Estimated 1.5:100,000* Current Treatments • DOLJOVI®, a fatty acid supplement similar to medium chain triglyceride oil (MCT) • Therapy includes a fat- restricted diet, supplementing with short or medium chain fatty acids *Merritt JL et al. Ann Transl Med. 2018; 6(24): 473 (LC-FAOD) 19 |
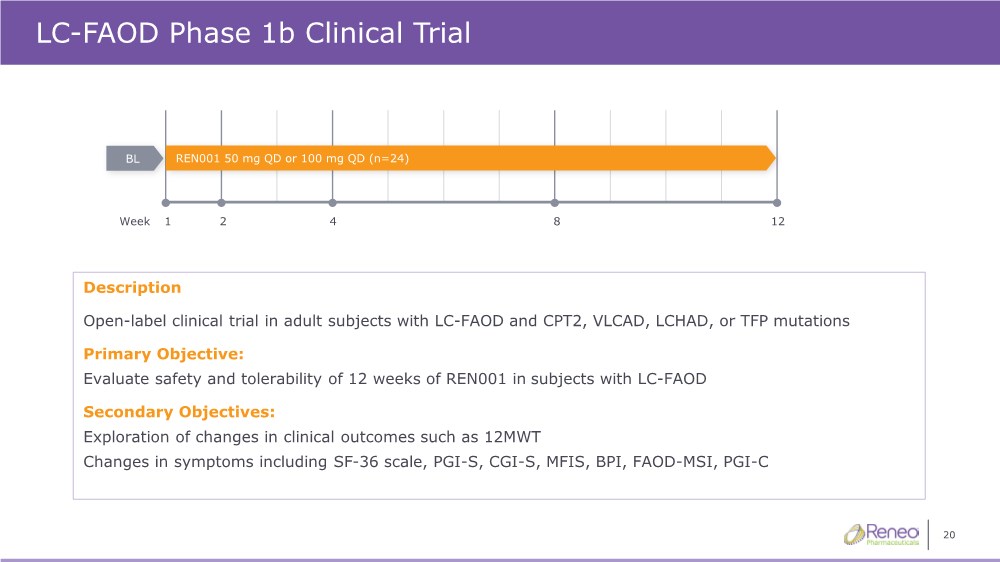
| 20 LC-FAOD Phase 1b Clinical Trial Description Open-label clinical trial in adult subjects with LC-FAOD and CPT2, VLCAD, LCHAD, or TFP mutations Primary Objective: Evaluate safety and tolerability of 12 weeks of REN001 in subjects with LC-FAOD Secondary Objectives: Exploration of changes in clinical outcomes such as 12MWT Changes in symptoms including SF-36 scale, PGI-S, CGI-S, MFIS, BPI, FAOD-MSI, PGI-C BL REN001 50 mg QD or 100 mg QD (n=24) 1 12 Week 2 4 8 |
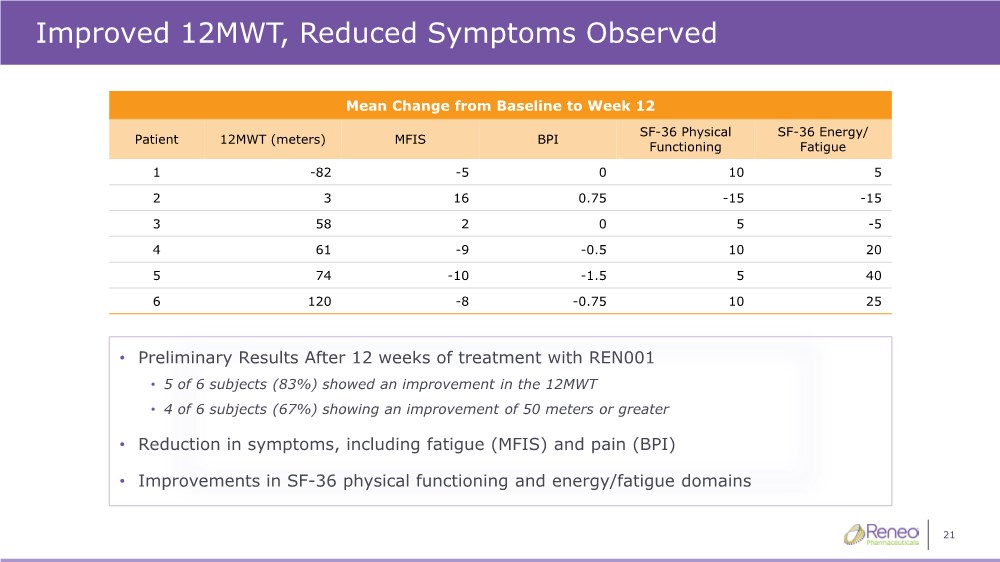
| 21 Improved 12MWT, Reduced Symptoms Observed • Preliminary Results After 12 weeks of treatment with REN001 • 5 of 6 subjects (83%) showed an improvement in the 12MWT • 4 of 6 subjects (67%) showing an improvement of 50 meters or greater • Reduction in symptoms, including fatigue (MFIS) and pain (BPI) • Improvements in SF-36 physical functioning and energy/fatigue domains Mean Change from Baseline to Week 12 Patient 12MWT (meters) MFIS BPI SF-36 Physical Functioning SF-36 Energy/ Fatigue 1 -82 -5 0 10 5 2 3 16 0.75 -15 -15 3 58 2 0 5 -5 4 61 -9 -0.5 10 20 5 74 -10 -1.5 5 40 6 120 -8 -0.75 10 25 |
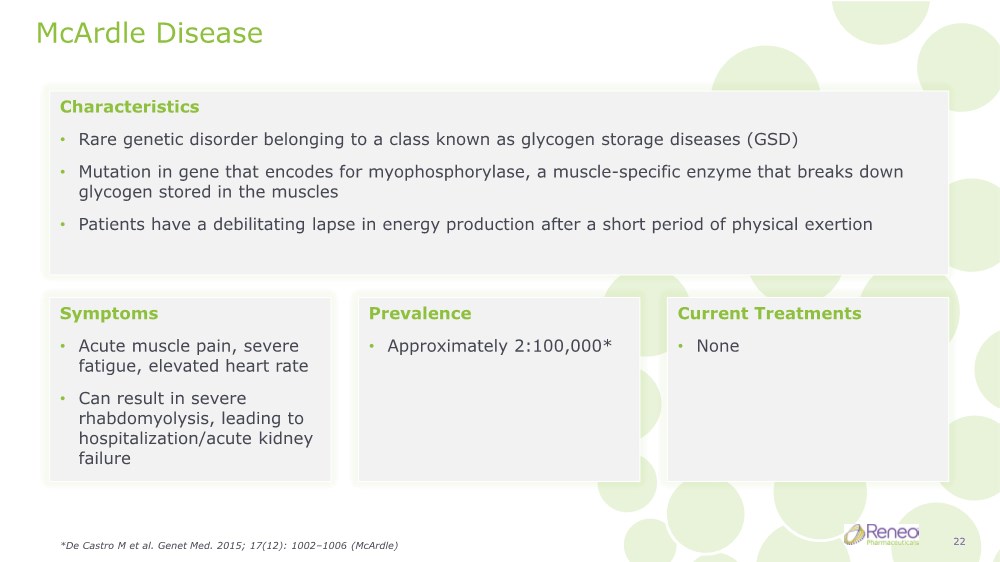
| McArdle Disease Characteristics • Rare genetic disorder belonging to a class known as glycogen storage diseases (GSD) • Mutation in gene that encodes for myophosphorylase, a muscle-specific enzyme that breaks down glycogen stored in the muscles • Patients have a debilitating lapse in energy production after a short period of physical exertion Symptoms • Acute muscle pain, severe fatigue, elevated heart rate • Can result in severe rhabdomyolysis, leading to hospitalization/acute kidney failure Prevalence • Approximately 2:100,000* Current Treatments • None *De Castro M et al. Genet Med. 2015; 17(12): 1002–1006 (McArdle) 22 |
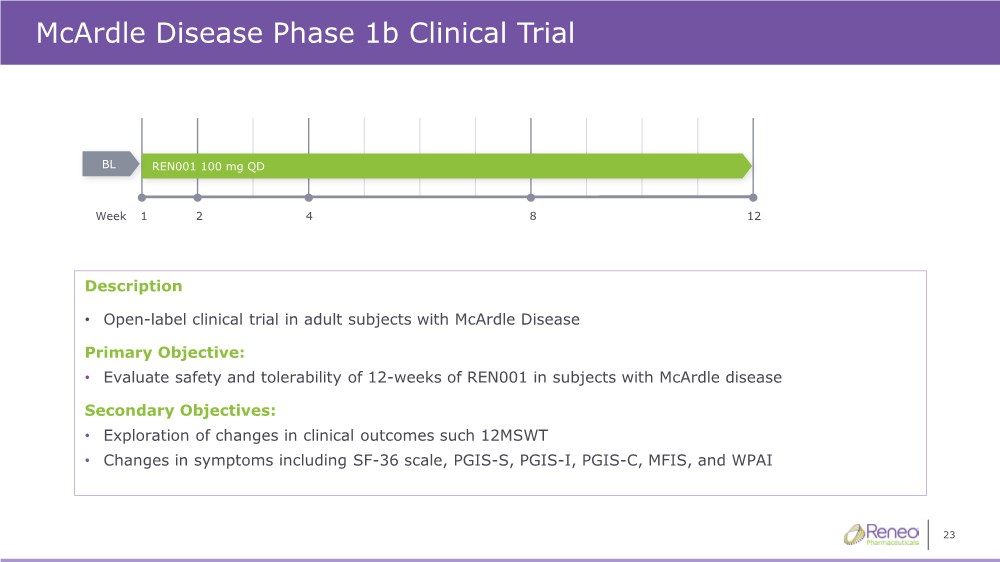
| 23 McArdle Disease Phase 1b Clinical Trial REN001 100 mg QD BL Description • Open-label clinical trial in adult subjects with McArdle Disease Primary Objective: • Evaluate safety and tolerability of 12-weeks of REN001 in subjects with McArdle disease Secondary Objectives: • Exploration of changes in clinical outcomes such 12MSWT • Changes in symptoms including SF-36 scale, PGIS-S, PGIS-I, PGIS-C, MFIS, and WPAI 1 12 Week 2 4 8 |
| Patents and Exclusivity |
| 25 Patents & Exclusivity REN001 patent portfolio • 6 U.S. and 19 foreign issued patents covering composition of matter of REN001 (expiry in 2026) • 3 U.S. and 5 foreign issued patents, plus 1 U.S. and 2 foreign pending applications covering methods of use (expires 2034) • Pending patent applications covering methods of use, methods of manufacturing, and crystalline forms (polymorphs) (expires 2040-2042, if issued) Anticipated market exclusivity • 7 years for new orphan drug in the U.S. (10 years in Europe and Japan) • 6 months additional exclusivity for pediatric populations |
| Clinical Milestones |
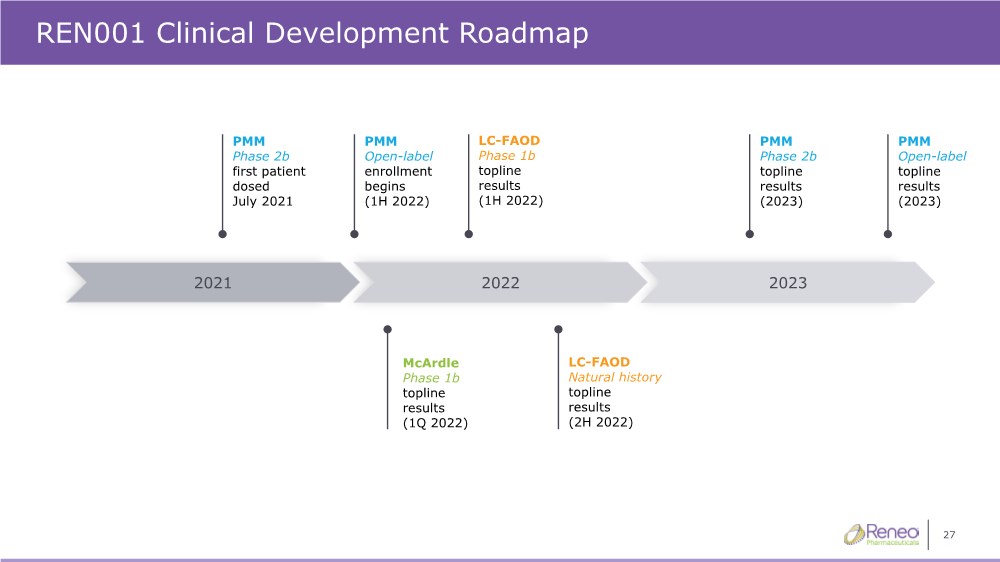
| 27 REN001 Clinical Development Roadmap 2023 2022 2021 PMM Open-label enrollment begins (1H 2022) PMM Phase 2b topline results (2023) PMM Phase 2b first patient dosed July 2021 PMM Open-label topline results (2023) McArdle Phase 1b topline results (1Q 2022) LC-FAOD Phase 1b topline results (1H 2022) LC-FAOD Natural history topline results (2H 2022) |
| To learn more, please contact: Vinny Jindal Chief Financial Officer vjindal@reneopharma.com |