Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Emergent BioSolutions Inc. | ebs-20210729.htm |
| EX-99.3 - EX-99.3 - Emergent BioSolutions Inc. | emergentbiosolutionstore.htm |
| EX-99.1 - EX-99.1 - Emergent BioSolutions Inc. | ebs2021-06x30ex99earningsr.htm |

2Q21 Investor Update July 29, 2021

2Q 2021 Investor Update2 Introduction Robert G. Burrows Vice President, Investor Relations

Safe Harbor Statement INTRODUCTION This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact, including, without limitation, our financial guidance and related projections and statements regarding our ability to meet such projections in the anticipated timeframe, if at all; statements regarding continued procurement of AV7909 under our existing contract with BARDA; the full delivery in 2021 of vaccines procured under the July 2021 ACAM2000® option exercise; the potential award of a new procurement contract for raxibacumab; the strength of the naloxone market and the timing and number of naloxone competitor entrants expected by year end; the timing of the anticipated appellate decision on related pending patent litigation; progress across the vaccines, therapeutics, and devices portfolios and anticipated timing and number of regulatory submissions; the strength of our CDMO business unit and demand for biologics services; capacity expansion in our CDMO business portfolio; timing of CDMO revenues, our CDMO backlog and opportunity funnel; the stability of the U.S. government’s priority for procuring solutions to public health threats; the durability of our business and growth potential; remaining on track with respect to our 2024 strategy; capital expenditures and total contract value; and any other statements containing the words “will,” “believes,” “expects,” “anticipates,” “intends,” “plans,” “targets,” “forecasts,” “estimates” and similar expressions in conjunction with, among other things, discussions of the Company’s outlook, financial performance or financial condition, financial and operation goals, product sales, government development or procurement contracts or awards, government appropriations, manufacturing capabilities, and the timing of certain clinical trials and regulatory approvals are forward-looking statements. These forward-looking statements are based on our current intentions, beliefs and expectations regarding future events. We cannot guarantee that any forward-looking statement will be accurate. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Investors are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statements speak only as of the date of this presentation, and, except as required by law, we do not undertake to update any forward- looking statement to reflect new information, events or circumstances. There are a number of important factors that could cause our actual results to differ materially from those indicated by such forward-looking statements, including the continued exercise of discretion by BARDA to procure additional doses of AV7909 prior to approval by the FDA; our ability to negotiate follow-on procurement contracts for AV7909 and other follow-on procurement contracts for our public health threat products that have expired or will be expiring; the impact on our revenues from the hold of certain COVID-19 vaccine bulk drug substance lots; our ability to meet our commitments to continued quality and manufacturing compliance at our Baltimore Bayview facility identified by the FDA and the potential impact on our ability to continue production of bulk drug substance for Johnson & Johnson’s COVID-19 vaccine at the facility; the availability of U.S. government funding for procurement of our products and certain product candidates; our ability to perform under our contracts with the U.S. government including the timing of and specifications relating to deliveries; our ability to provide CDMO services for the development and/or manufacture of product candidates of our customers at required levels and on required timelines; our ability and the ability of our contractors and suppliers to maintain compliance with current good manufacturing practices and other regulatory obligations; our ability to obtain and maintain regulatory approvals for our product candidates and the timing of any such approvals; changes to U.S. government priorities for the SNS and the future exercise of all remaining options under our contract for the procurement of ACAM2000® and other government procurement contracts; the negotiation of further commitments or contracts related to the collaboration and deployment of capacity toward future commercial manufacturing under our CDMO contracts; the timing of our submission of an application for and our ability to secure licensure of AV7909 from the FDA within the anticipated timeframe, if at all; our ability to successfully appeal the patent litigation decision related to NARCAN® Nasal Spray 4mg/spray, and the impact of competition from potential generic and branded naloxone entrants on NARCAN® Nasal Spray; the results of pending shareholder litigation and the potential impact on our business; our ability to develop a safe and effective treatment for COVID-19 and obtain authorization for emergency use for or approval of such treatment from the FDA; our ability to identify and acquire companies, businesses, products or product candidates that satisfy our selection criteria; our ability to comply with the operating and financial covenants required by our senior secured credit facilities and our 3.875% Senior Unsecured Notes due 2028; the procurement of products by U.S. government entities under regulatory exemptions prior to approval by the FDA and corresponding procurement by government entities outside of the United States under regulatory exemptions prior to approval by the corresponding regulatory authorities in the applicable country; the full impact of COVID-19 disease on our markets, operations and employees as well as those of our customers and suppliers; the impact on our revenues from short-term declines in sales of our vaccine products that target travelers due to the reduction of international travel caused by the COVID-19 pandemic; the success of our commercialization, marketing and manufacturing capabilities and strategy; and the accuracy of our estimates regarding future revenues, expenses, capital requirements and needs for additional financing. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Investors should consider this cautionary statement as well as the risk factors identified in our periodic reports filed with the Securities and Exchange Commission when evaluating our forward-looking statements. 2Q 2021 Investor Update3

Non-GAAP Financial Measures / Trademarks 2Q 2021 Investor Update4 INTRODUCTION NON-GAAP FINANCIAL MEASURES This presentation contains financial measures (Adjusted Net Income, Adjusted Net Income Per Diluted Share, Adjusted EBITDA (Earnings Before Depreciation and Amortization, Interest and Taxes), Gross Margin, Adjusted Revenues and Net Research and Development expenses) that are considered “non-GAAP” financial measures under applicable Securities and Exchange Commission rules and regulations. These non-GAAP financial measures should be considered supplemental to and not a substitute for financial information prepared in accordance with generally accepted accounting principles. The Company’s definition of these non-GAAP measures may differ from similarly titled measures used by others. For its non-GAAP measures, the Company adjusts for specified items that can be highly variable or difficult to predict, or reflect the non-cash impact of charges or accounting changes. As needed, such adjustments are tax effected utilizing the federal statutory tax rate for the US, except for changes in the fair value of contingent consideration as the vast majority is non- deductible for tax purposes. The Company views these non-GAAP financial measures as a means to facilitate management’s financial and operational decision-making, including evaluation of the Company’s historical operating results and comparison to competitors’ operating results. These non-GAAP financial measures reflect an additional way of viewing aspects of the Company’s operations that, when viewed with GAAP results and the reconciliations to the corresponding GAAP financial measure may provide a more complete understanding of factors and trends affecting the Company’s business. For more information on these non-GAAP financial measures, please see the tables in the Appendix included at the end of this presentation. The determination of the amounts that are excluded from these non-GAAP financial measures are a matter of management judgment and depend upon, among other factors, the nature of the underlying expense or income amounts. Because non-GAAP financial measures exclude the effect of items that will increase or decrease the Company’s reported results of operations, management strongly encourages investors to review the Company’s consolidated financial statements and publicly filed reports in their entirety. TRADEMARKS BioThrax® (Anthrax Vaccine Adsorbed), RSDL® (Reactive Skin Decontamination Lotion Kit), BAT® (Botulism Antitoxin Heptavalent (A,B,C,D,E,F,G)-(Equine)), Anthrasil® (Anthrax Immune Globulin Intravenous (Human)), VIGIV (Vaccinia Immune Globulin Intravenous (Human)), Trobigard® (atropine sulfate, obidoxime chloride), ACAM2000® (Smallpox (Vaccinia) Vaccine, Live), Vivotif® (Typhoid Vaccine Live Oral Ty21a), Vaxchora® (Cholera Vaccine, Live, Oral), NARCAN® (naloxone HCI) Nasal Spray and any and all Emergent BioSolutions Inc. brands, products, services and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries. All other brands, products, services and feature names or trademarks are the property of their respective owners.

• State of the Company • Financial Results: 2Q21 • Financial Guidance: Full Year 2021 and 3Q21 • Question and Answer Session Agenda 2Q 2021 Investor Update5 INTRODUCTION

2Q 2021 Investor Update6 State of the Company Robert G. Kramer President and Chief Executive Officer

• We are pleased to be resuming production of Johnson & Johnson’s Covid-19 vaccine bulk drug substance. • We continue to work collaboratively with AstraZeneca to complete all documents related to their batches and enable them to work with the US government on the disposition of those lots. • We are in year two of our 2020-2024 strategic plan and continue to make progress against that plan. • Our work supporting the USG’s priorities to protect the American public against smallpox, anthrax and other Category A biological agents remains stable. • The CDMO business unit remains strong; we see strong interest from current/potential clients across all three service pillars; industry demand for biologics manufacturing services continues to grow. • We continue to focus on the opioid epidemic, supporting public awareness and ongoing affordability and availability of Narcan® Nasal Spray and our role as a provider of solutions against this persistent threat to health and safety. • We still expect this year to initiate a Phase 3 trial for CHIKV VLP, our Chikungunya virus vaccine candidate, and possibly other Phase 1 studies with candidates addressing other infectious diseases, and to file with FDA our BLA for AV7909, our next generation anthrax vaccine candidate. • Our business remains durable, resilient and poised for growth; we are on track with our 2024 strategy; and, we remain well- positioned to play a meaningful role in strengthening our national public health threat preparedness. Key Themes for 2Q21 Status of the Company 2Q 2021 Investor Update7 STATE OF THE COMPANY

Financial Results Richard S. Lindahl Executive Vice President, Chief Financial Officer and Treasurer 2Q 2021 Investor Update8

2Q21 Performance Summary Points 2Q 2021 Investor Update9 FINANCIAL RESULTS • Solid top-line performance, consistent with expectations. • Expenses impacted by financial implications stemming from the situation at the Bayview facility. • Financial condition remains sound with liquidity and financial flexibility to fund operations and pursue opportunistic investments. • Despite challenges, remain steadfast in commitment to supporting global preparedness and response to public health threats (PHTs).
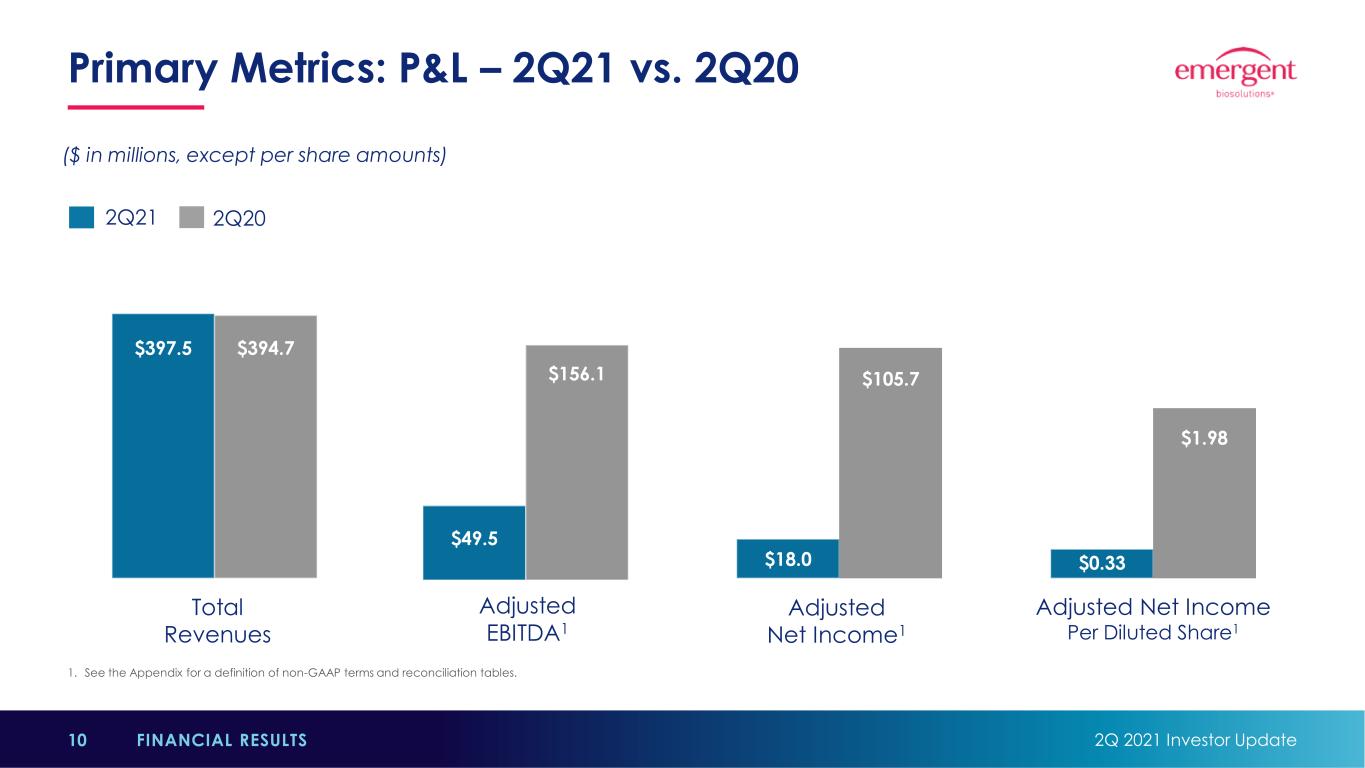
1. See the Appendix for a definition of non-GAAP terms and reconciliation tables. Primary Metrics: P&L – 2Q21 vs. 2Q20 2Q 2021 Investor Update10 FINANCIAL RESULTS $397.5 $394.7 $49.5 $156.1 $18.0 $105.7 $0.33 Total Revenues Adjusted EBITDA1 Adjusted Net Income1 Adjusted Net Income Per Diluted Share1 2Q21 2Q20 $1.98 ($ in millions, except per share amounts)
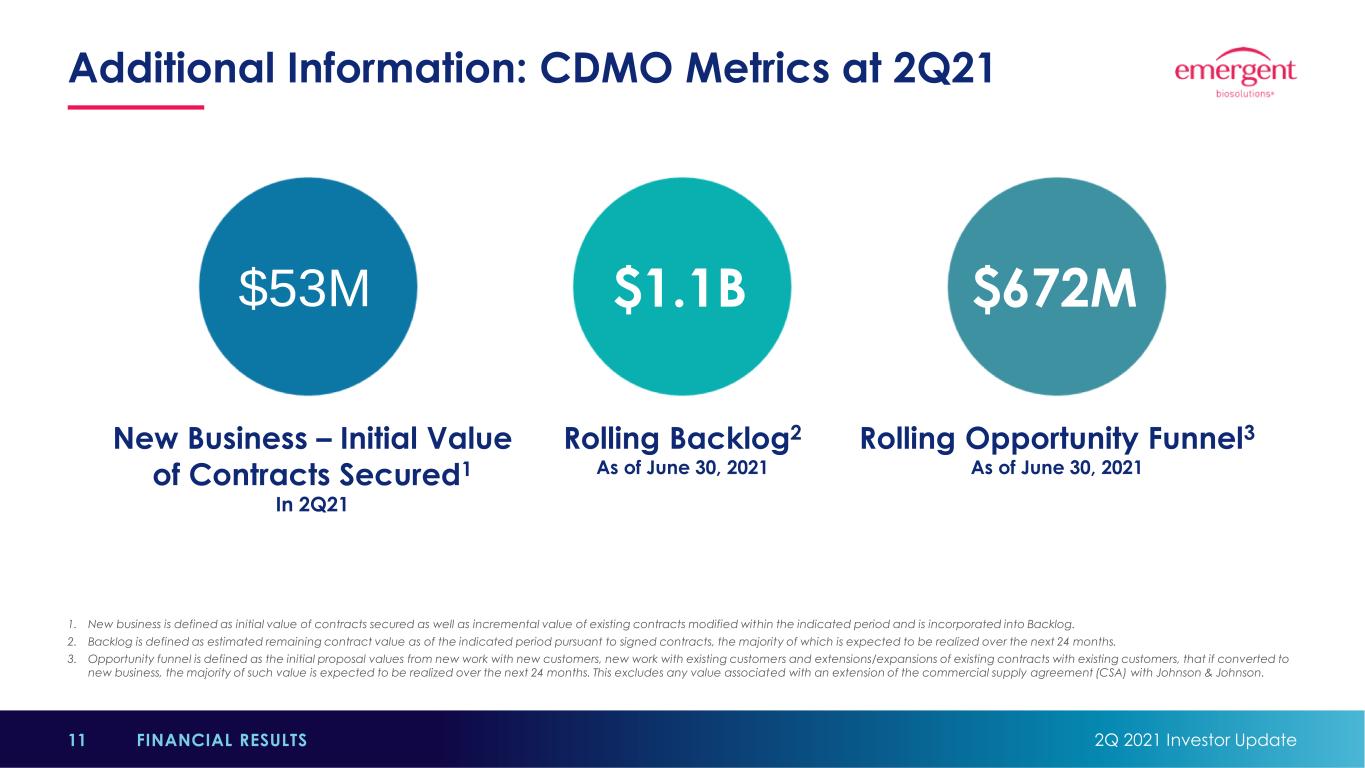
1. New business is defined as initial value of contracts secured as well as incremental value of existing contracts modified within the indicated period and is incorporated into Backlog. 2. Backlog is defined as estimated remaining contract value as of the indicated period pursuant to signed contracts, the majority of which is expected to be realized over the next 24 months. 3. Opportunity funnel is defined as the initial proposal values from new work with new customers, new work with existing customers and extensions/expansions of existing contracts with existing customers, that if converted to new business, the majority of such value is expected to be realized over the next 24 months. This excludes any value associated with an extension of the commercial supply agreement (CSA) with Johnson & Johnson. Additional Information: CDMO Metrics at 2Q21 2Q 2021 Investor Update11 FINANCIAL RESULTS $53M $1.1B $672M New Business – Initial Value of Contracts Secured1 In 2Q21 Rolling Backlog2 As of June 30, 2021 Rolling Opportunity Funnel3 As of June 30, 2021
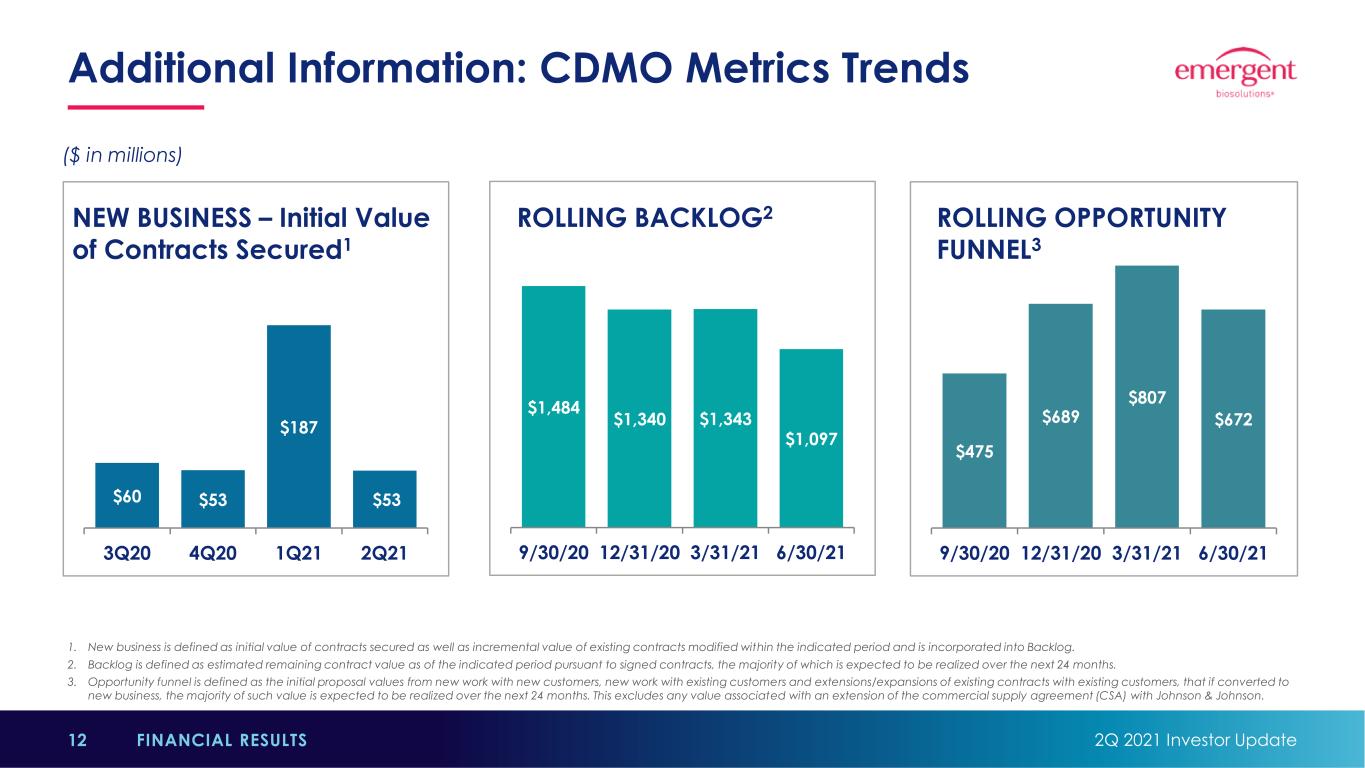
1. New business is defined as initial value of contracts secured as well as incremental value of existing contracts modified within the indicated period and is incorporated into Backlog. 2. Backlog is defined as estimated remaining contract value as of the indicated period pursuant to signed contracts, the majority of which is expected to be realized over the next 24 months. 3. Opportunity funnel is defined as the initial proposal values from new work with new customers, new work with existing customers and extensions/expansions of existing contracts with existing customers, that if converted to new business, the majority of such value is expected to be realized over the next 24 months. This excludes any value associated with an extension of the commercial supply agreement (CSA) with Johnson & Johnson. Additional Information: CDMO Metrics Trends 2Q 2021 Investor Update12 FINANCIAL RESULTS ($ in millions) $60 $53 $187 $53 3Q20 4Q20 1Q21 2Q21 $475 $689 $807 $672 9/30/20 12/31/20 3/31/21 6/30/21 $1,484 $1,340 $1,343 $1,097 9/30/20 12/31/20 3/31/21 6/30/21 NEW BUSINESS – Initial Value of Contracts Secured1 ROLLING OPPORTUNITY FUNNEL3 ROLLING BACKLOG2
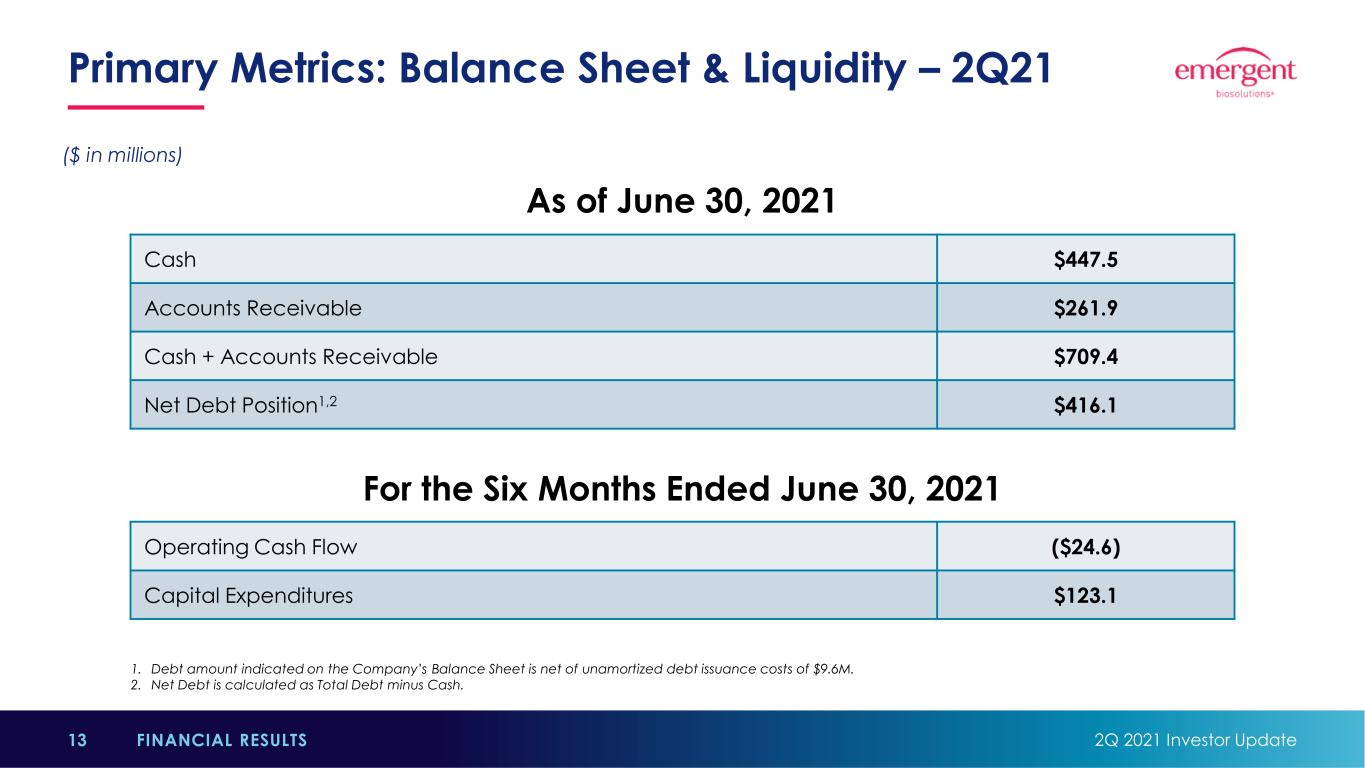
Primary Metrics: Balance Sheet & Liquidity – 2Q21 2Q 2021 Investor Update13 FINANCIAL RESULTS Cash $447.5 Accounts Receivable $261.9 Cash + Accounts Receivable $709.4 Net Debt Position1,2 $416.1 As of June 30, 2021 1. Debt amount indicated on the Company’s Balance Sheet is net of unamortized debt issuance costs of $9.6M. 2. Net Debt is calculated as Total Debt minus Cash. ($ in millions) Operating Cash Flow ($24.6) Capital Expenditures $123.1 For the Six Months Ended June 30, 2021
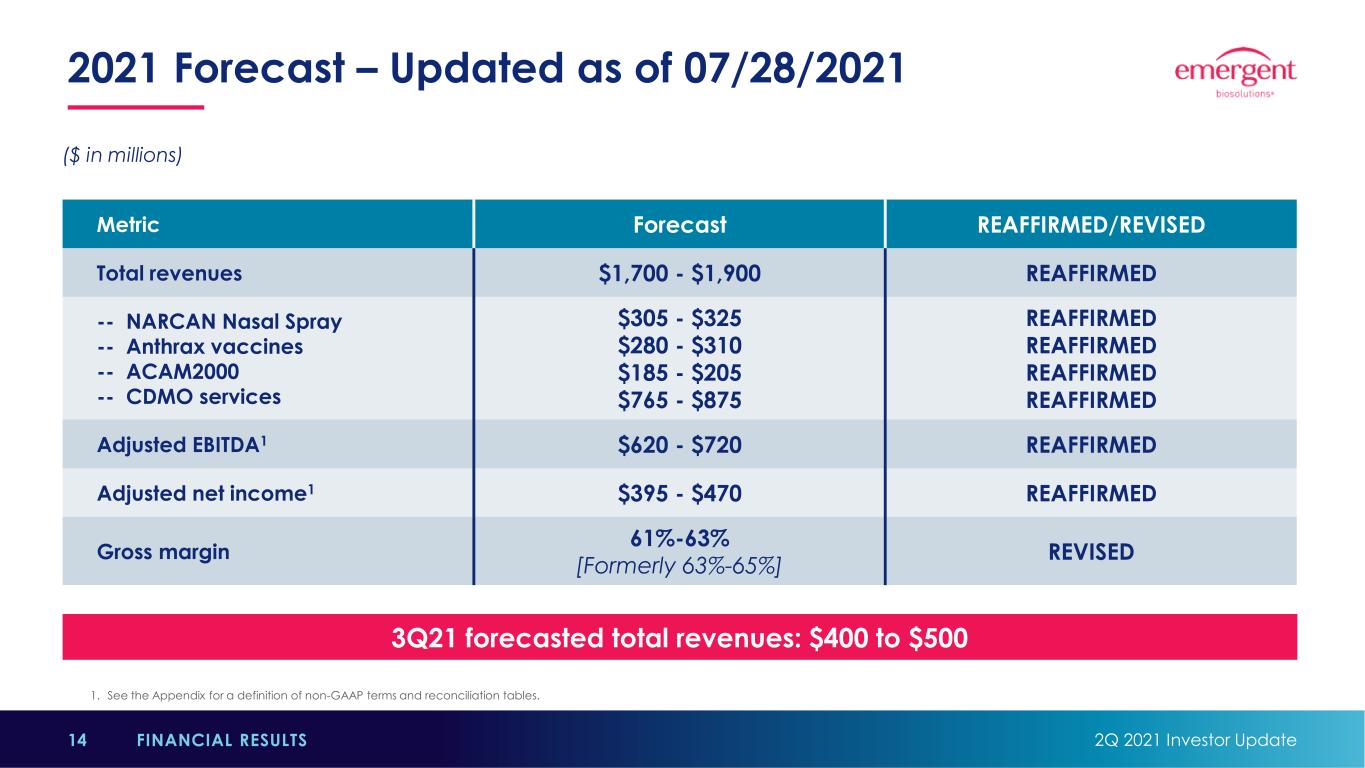
2021 Forecast – Updated as of 07/28/2021 2Q 2021 Investor Update14 FINANCIAL RESULTS 3Q21 forecasted total revenues: $400 to $500 Metric Forecast REAFFIRMED/REVISED Total revenues $1,700 - $1,900 REAFFIRMED -- NARCAN Nasal Spray -- Anthrax vaccines -- ACAM2000 -- CDMO services $305 - $325 $280 - $310 $185 - $205 $765 - $875 REAFFIRMED REAFFIRMED REAFFIRMED REAFFIRMED Adjusted EBITDA1 $620 - $720 REAFFIRMED Adjusted net income1 $395 - $470 REAFFIRMED Gross margin 61%-63% [Formerly 63%-65%] REVISED 1. See the Appendix for a definition of non-GAAP terms and reconciliation tables. ($ in millions)

2021 Forecast: Key Considerations 2Q 2021 Investor Update15 FINANCIAL RESULTS • Most assumptions remain consistent with those provided with April 30 guidance update, including: • No Raxibacumab revenue until 2022 • At least one new entrant to Naloxone market this year, but no generic competitor prior to resolution of patent litigation • CDMO revenue range reflects successful manufacturing of J&J’s COVID-19 vaccine at Bayview • Gross Margin revision reflects impact to FY21 from 2Q21 performance and expectations for the rest of the year. • Anticipate lower gross margin will be offset by R&D and SG&A cost savings.

Key Takeaways 2Q 2021 Investor Update16 FINANCIAL RESULTS • Solid financial results in 2Q21 keep us on track with full year outlook. • YTD total revenues as a percentage of the midpoint of full year guidance in line with prior four years performance. • Remain confident in the strength of the business.

Q&A 2Q 2021 Investor Update17

APPENDIX 2Q 2021 Investor Update18

FY2021 Financial Forecast Considerations 2Q 2021 Investor Update19 APPENDIX Revised Considerations • Gross margin reflects the impact of the Q2 2021 performance as well as expectations for the remainder of the year. Reaffirmed Considerations • Narcan® (naloxone HCl) Nasal Spray revenues assume the naloxone market remains competitive and incorporates the impact of at least one new branded entrant into the market by year end, as well as that no generic entrant will enter the market prior to the anticipated appellate decision related to the pending patent litigation, which is expected in the second half of 2021. • Anthrax vaccines revenues are expected to continue to primarily reflect procurement of AV7909 under the terms of the Company’s existing contract with BARDA at a more normalized annual level. • ACAM2000®, (Smallpox (Vaccinia) Vaccine, Live) vaccine revenues incorporate the expected full delivery of product under the $182 million option exercise received in July 2021 as well as other international sales. • CDMO services revenue reflects the successful manufacturing of Johnson & Johnson’s COVID-19 vaccine bulk drug substance at the Company's Bayview facility. On July 29, the Company announced that it was informed by the FDA that it can resume production at its Bayview manufacturing facility. • Total revenues, specifically other product sales, are expected to be impacted due to the Company's assumption that a new raxibacumab contract will be awarded later than previously planned. • R&D expenses are expected to reflect continued pipeline progress across the vaccines, therapeutics, and devices portfolios, including the assumption of at least one Phase 3 launch and one Biologics License Application (BLA)/Emergency Use Authorization (EUA) filing. • Capital expenditures, net of reimbursement, are expected to be in a range of 8% to 9% of total revenues, reflecting ongoing investments in capacity and capability expansions in support of the Company's CDMO services business and product portfolio.
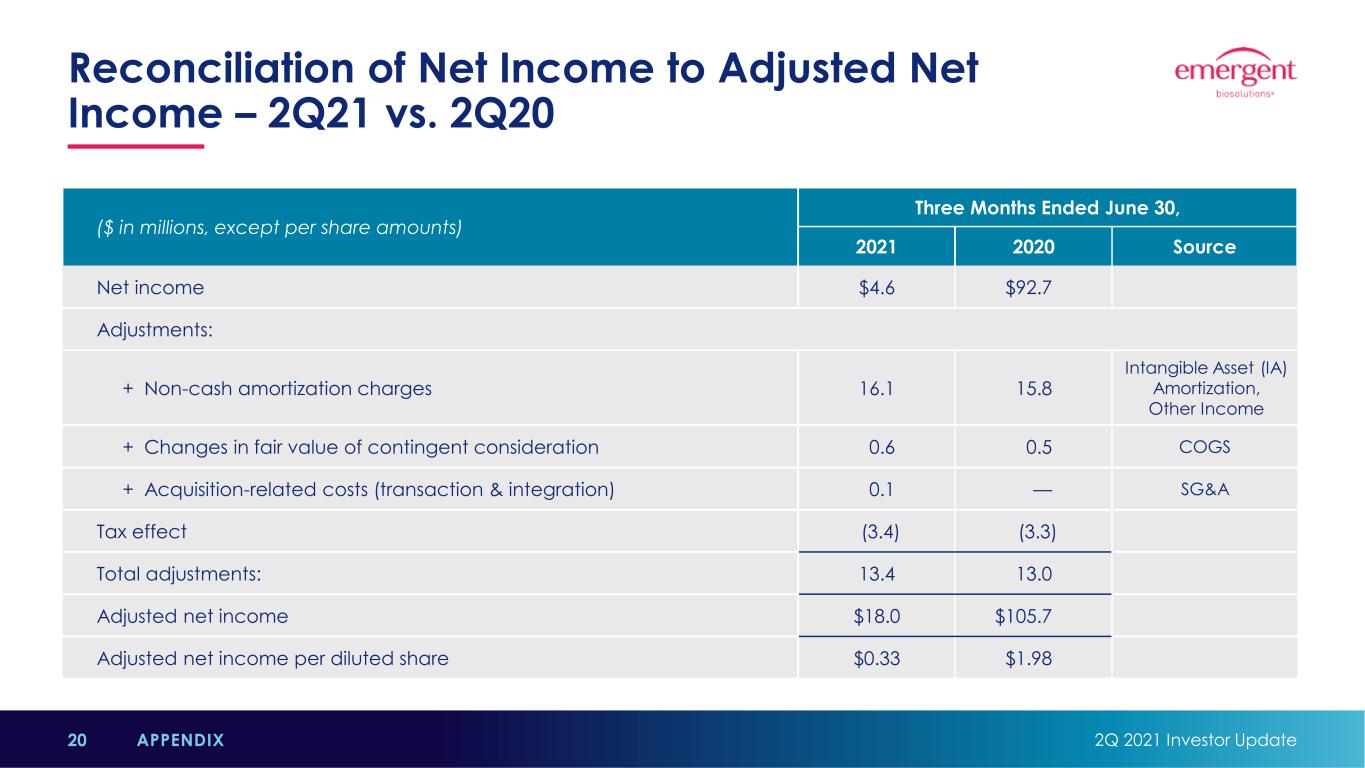
Reconciliation of Net Income to Adjusted Net Income – 2Q21 vs. 2Q20 2Q 2021 Investor Update20 APPENDIX ($ in millions, except per share amounts) Three Months Ended June 30, 2021 2020 Source Net income $4.6 $92.7 Adjustments: + Non-cash amortization charges 16.1 15.8 Intangible Asset (IA) Amortization, Other Income + Changes in fair value of contingent consideration 0.6 0.5 COGS + Acquisition-related costs (transaction & integration) 0.1 — SG&A Tax effect (3.4) (3.3) Total adjustments: 13.4 13.0 Adjusted net income $18.0 $105.7 Adjusted net income per diluted share $0.33 $1.98
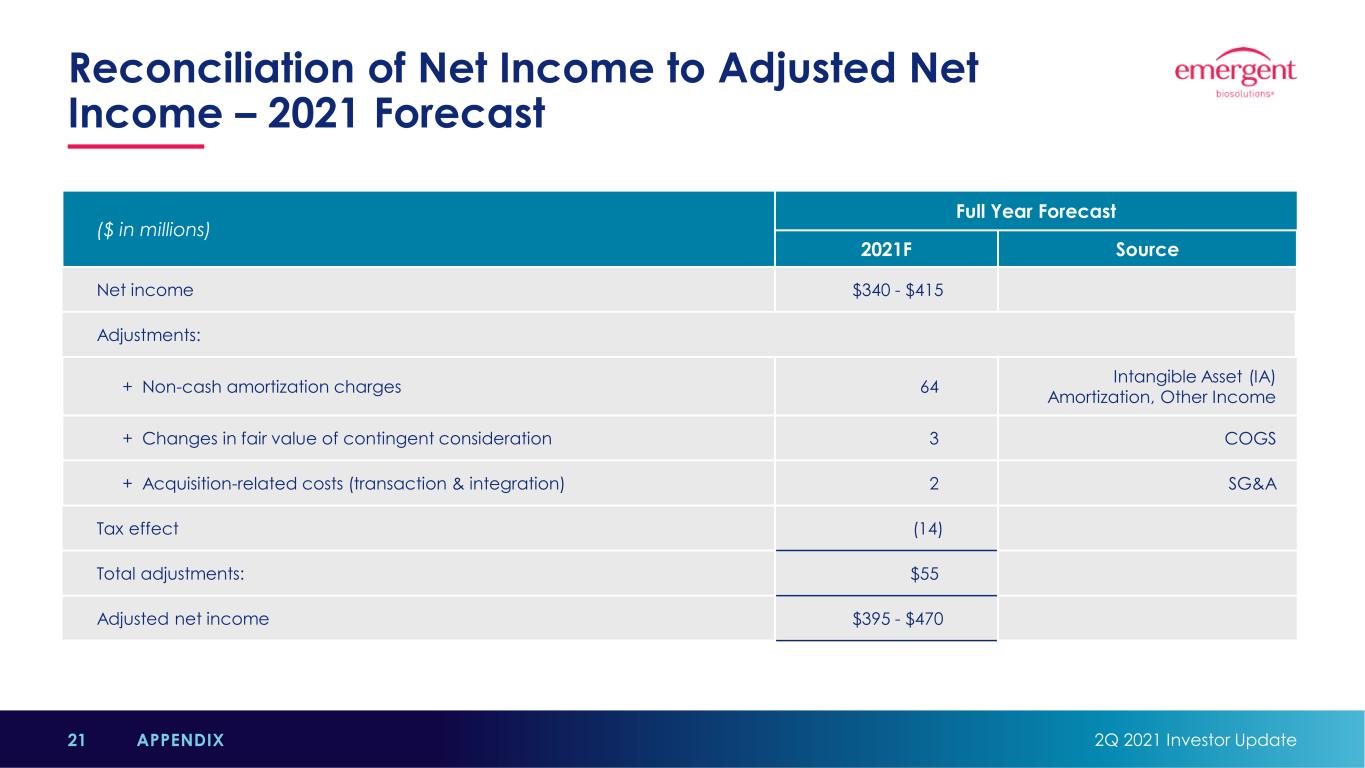
Reconciliation of Net Income to Adjusted Net Income – 2021 Forecast 2Q 2021 Investor Update21 APPENDIX ($ in millions) Full Year Forecast 2021F Source Net income $340 - $415 Adjustments: + Non-cash amortization charges 64 Intangible Asset (IA) Amortization, Other Income + Changes in fair value of contingent consideration 3 COGS + Acquisition-related costs (transaction & integration) 2 SG&A Tax effect (14) Total adjustments: $55 Adjusted net income $395 - $470
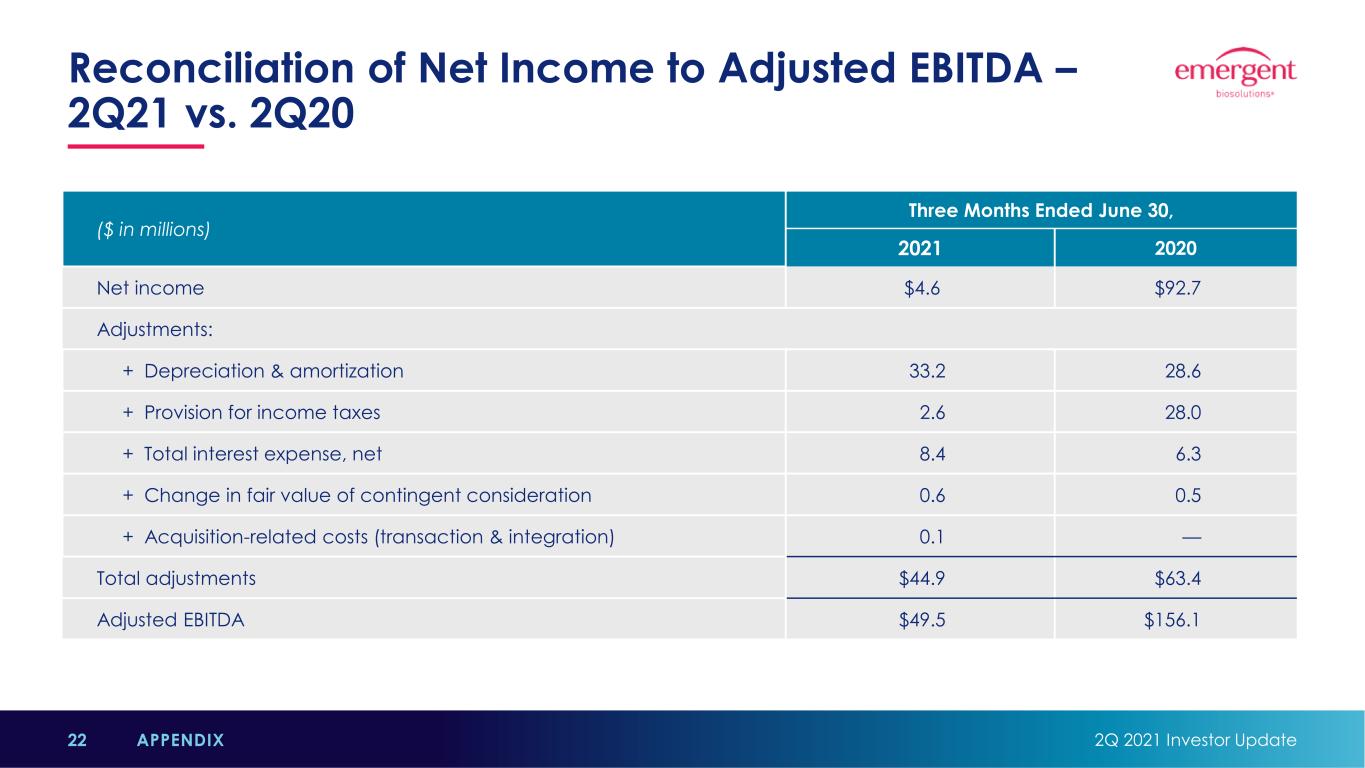
Reconciliation of Net Income to Adjusted EBITDA – 2Q21 vs. 2Q20 2Q 2021 Investor Update22 APPENDIX ($ in millions) Three Months Ended June 30, 2021 2020 Net income $4.6 $92.7 Adjustments: + Depreciation & amortization 33.2 28.6 + Provision for income taxes 2.6 28.0 + Total interest expense, net 8.4 6.3 + Change in fair value of contingent consideration 0.6 0.5 + Acquisition-related costs (transaction & integration) 0.1 — Total adjustments $44.9 $63.4 Adjusted EBITDA $49.5 $156.1
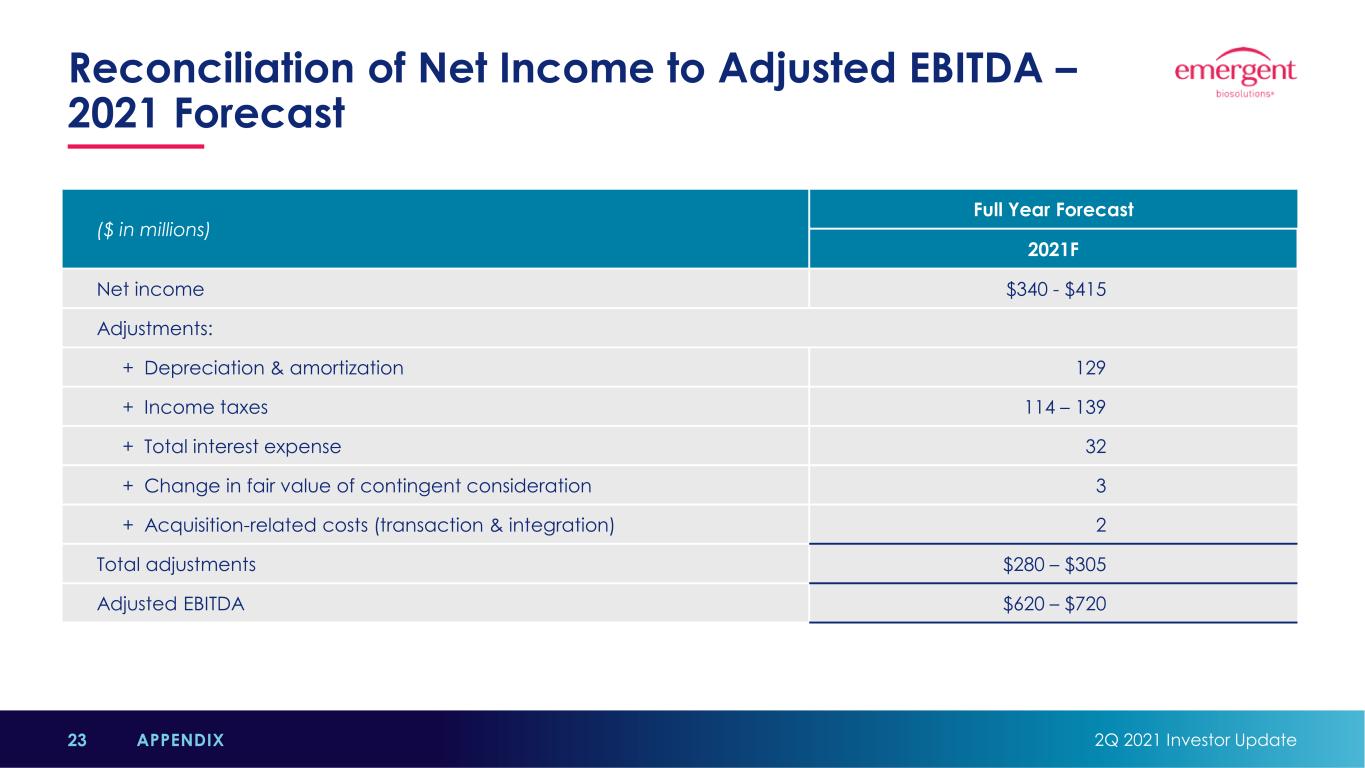
Reconciliation of Net Income to Adjusted EBITDA – 2021 Forecast 2Q 2021 Investor Update23 APPENDIX ($ in millions) Full Year Forecast 2021F Net income $340 - $415 Adjustments: + Depreciation & amortization 129 + Income taxes 114 – 139 + Total interest expense 32 + Change in fair value of contingent consideration 3 + Acquisition-related costs (transaction & integration) 2 Total adjustments $280 – $305 Adjusted EBITDA $620 – $720
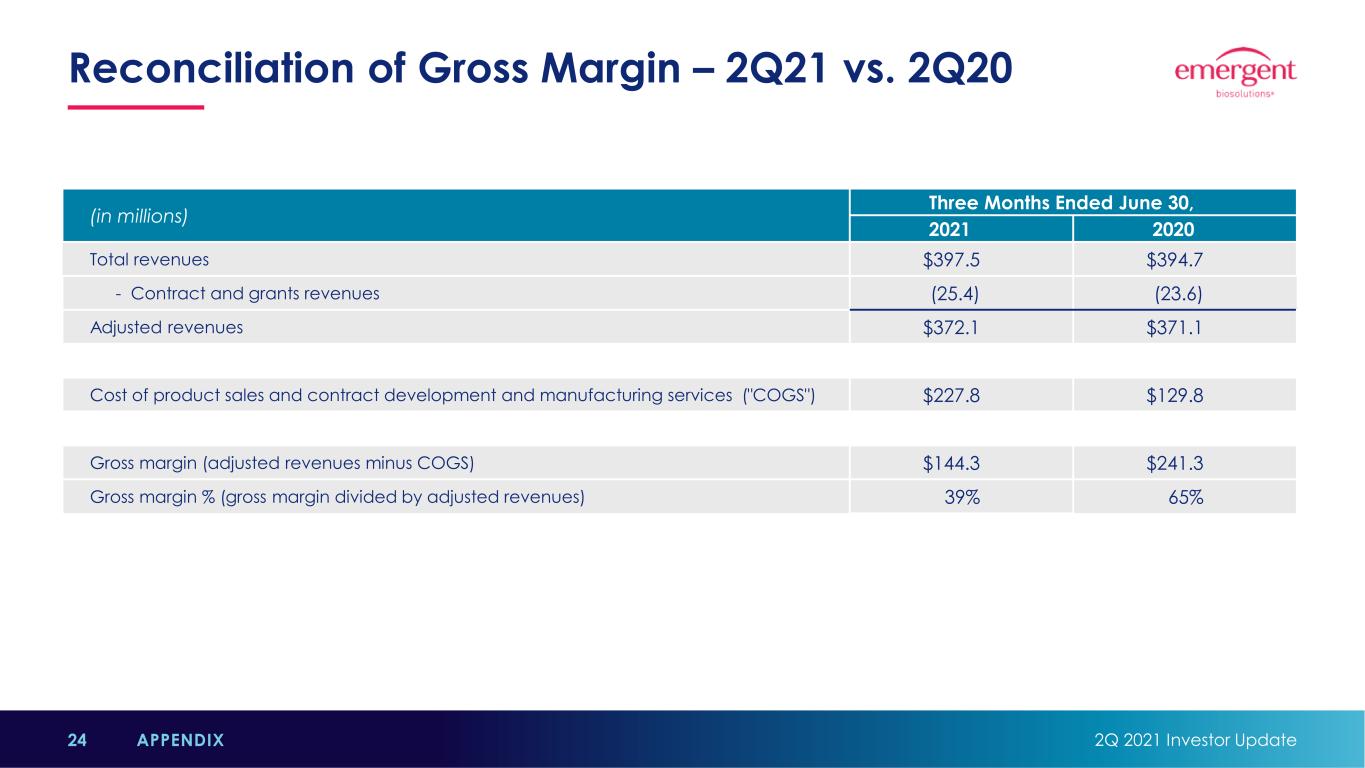
Reconciliation of Gross Margin – 2Q21 vs. 2Q20 2Q 2021 Investor Update24 APPENDIX (in millions) Three Months Ended June 30, 2021 2020 Total revenues $397.5 $394.7 - Contract and grants revenues (25.4) (23.6) Adjusted revenues $372.1 $371.1 Cost of product sales and contract development and manufacturing services ("COGS") $227.8 $129.8 Gross margin (adjusted revenues minus COGS) $144.3 $241.3 Gross margin % (gross margin divided by adjusted revenues) 39% 65%
