Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Zentalis Pharmaceuticals, Inc. | zntl-midyearupdatedraftv11.htm |
| 8-K - 8-K - Zentalis Pharmaceuticals, Inc. | zntl-20210628.htm |

Mid-Year Update June 2021 Creating Differentiated Therapies to Improve the Lives of Cancer Patients

Forward-Looking Statements and Disclaimer 2 Zentalis Pharmaceuticals, Inc. (“we,” “us,” “our,” “Zentalis” or the “Company”) cautions that this presentation (including oral commentary that accompanies this presentation) contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation statements regarding our future financial or business performance, plans, prospects, trends or strategies, objectives of management, competition and other financial and business matters, the potential, safety, efficacy, and regulatory and clinical progress of our current and prospective product candidates, plans and timing for the initiation of and release of data from our clinical trials and our ability to meet other key milestones, planned preclinical activities, our current and prospective collaborations, the estimated size of the market for our product candidates, and the timing and success of our development and commercialization of our anticipated product candidates and the market acceptance thereof are forward-looking statements, as well as statements that include the words “expect,” “intend,” “plan,” “believe,” “project,” “forecast,” “estimate,” “may,” “should,” “anticipate” and similar statements of a future or forward-looking nature. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the outbreak of the novel coronavirus disease, COVID-19, has adversely impacted and may continue to adversely impact our business, including our preclinical studies and clinical trials; our limited operating history, which may make it difficult to evaluate our current business and predict our future success and viability; we have and expect to continue to incur significant losses; our need for additional funding, which may not be available; our substantial dependence on the success of our lead product candidate; failure to identify additional product candidates and develop or commercialize marketable products; the early stage of our development efforts; potential unforeseen events during clinical trials could cause delays or other adverse consequences; risks relating to the regulatory approval process or ongoing regulatory obligations; failure to obtain U.S. or international marketing approval; our product candidates may cause serious adverse side effects; interim, initial, “topline”, and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data; inability to maintain our collaborations, or the failure of these collaborations; our reliance on third parties; effects of significant competition; the possibility of system failures or security breaches; risks relating to intellectual property; our ability to attract, retain and motivate qualified personnel; and significant costs as a result of operating as a public company. These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2021 filed with the U.S. Securities and Exchange Commission (SEC) and our other filings with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of this presentation. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. While we may elect to update these forward-looking statements at some point in the future, we assume no obligation to update or revise any forward-looking statements except to the extent required by applicable law. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data and estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Neither we nor our affiliates, advisors or representatives makes any representation as to the accuracy or completeness of that data or undertake to update such data after the date of this presentation. Data of Fulvestrant, RAD1901, Abemaciclib, Alpelisib, AZD1775, Venetoclax and Osimertinib presented in this presentation is based on evaluation of comparable proxy chemical compounds purchased from commercial sources rather than the pharmaceutical company commercializing or developing, as applicable, the compound.

Mid-Year Update: Executive Summary • Since AACR 2021, ZN-c3 has generated new clinical responses, further depth of responses, increased durability and improved hematological tolerability; representing one of the most promising clinical advances in DNA Damage Response (DDR) and synthetic lethality to date • With broad utility across multiple large indications in both monotherapy and in combination, ZN-c3 is potentially both a first-in-class and best-in-class WEE1 inhibitor • Following a recent EOP1 meeting with FDA, Zentalis initiated a registrational trial for ZN-c3 in USC, and will also start a novel biomarker-enabled trial by EOY - both trials have potential accelerated approval pathways • ZN-c5’s favorable tolerability data suggests potential for superiority amongst the oral SERDs, rivaling leading competition • Clinical development plans for ZN-d5 (BCL-2) and ZN-e4 (EGFR) on track, expanding clinical and commercial opportunities with potential combinations 3 Zentalis is accelerating shareholder value accretion with the start of a registrational study with intent of an additional such trial by year end; both have potential for accelerated approvals

4 ZN-c3
ZN-c3 WEE1 Inhibitor - Executive Summary Since AACR 2021, ZN-c3 has generated new clinical responses, further depth of responses, increased durability and improved hematological tolerability; representing one of the most promising clinical advances in DNA Damage Response (DDR) and synthetic lethality to date With broad utility across multiple large indications in both monotherapy and in combination, ZN-c3 is potentially both a first-in- class and best-in-class WEE1 inhibitor Following a recent EOP1 meeting with FDA, Zentalis initiated a registrational trial for ZN-c3 in USC, and will also start a novel biomarker-enabled trial by EOY - both trials have potential accelerated approval pathways • Data presented at AACR 2021 continues to mature with both USC uPRs now confirmed PRs, and an additional uPR in a newly reported USC patient • Further depth of tumor response and extended durability (8+ months) from exceptional responder observed • Predictive biomarker may enable ZN-c3 to address tumor-agnostic indications • Even lower overall severe hematological adverse event rates, with more patients enrolled on ZN-c3 since AACR 2021 • Two key designations (orphan drug and rare pediatric disease) received from FDA for ZN-c3 in combination with chemotherapy for osteosarcoma • Two investigator-initiated trials (IIT) in GBM and TNBC with immunotherapy planned, in two very high unmet need indications 5

ZN-c3: Clinical Development Plan OverviewOngoing and Planned Clinical Programs • Initial Phase 1 monotherapy dose escalation and expansion data (1) – ZN-c3 was well-tolerated as a single agent – RP2D for ZN-c3 determined – ZN-c3 showed Exceptional Responses in heavily pre- treated subjects with advanced solid tumors • Corresponding studies with Zentera in Greater China • Two key designations now received from FDA for osteosarcoma for ZN-c3 combo with chemotherapy: – Orphan designation – Rare pediatric disease designation Solid Tumors: Monotherapy Dose Escalation and Expansion Initial data presented at AACR 2021 Phase 1 Phase 2 Ovarian Cancer Chemo Combination Ph 1b Study Initiated 4Q 2020 Osteosarcoma ZN-c3 + gemcitabine Ph 1/2 Study Expected Initiation 3Q 2021 Uterine Serous Carcinoma Monotherapy Ph 2 Study Initiated Ovarian Cancer ZN-c3 + niraparib Ph 1/2 Study Expected Initiation 4Q 2021 Monotherapy Study Combination Study Additional Clinical Studies 6 (1) Reported at AACR 2021 Predictive Biomarker Ph 2 Study Expected Initiation 4Q 2021 Registrational trial with potential accelerated approval
ZN-c3: Expanding Indications through Investigator Initiated Studies • New IITs to start: ‒ Glioblastoma Multiforme: Preclinical study completed. Clinical study to commence in 2021 planned ‒ Triple Negative Breast Cancer: Combination with anti-PDL1 and chemotherapy. Clinical study to commence in 2022. 7 Confidential

ZN-c3 has the Potential to be Both First-in-Class and Best-in-Class • Key attributes to ZN-c3’s Proof of Concept (POC) success: 1. Target biology known and validated 2. Clinical results shown with monotherapy • ZN-c3’s POC includes: A. Determination of RP2D B. Evidence of relevant clinical activity in target populations C. Data on tolerability D. Strategy based on potential accelerated approvals in US • ZN-c3’s POC has potential to lead to: I. Multiple, large commercial opportunities as monotherapy and in combination 8 ZN-c3 has potential to be both first-in-class and best-in-class WEE1 inhibitor with broad market indications and path(s) to potential accelerated approval in US

1. Target Biology Known and Validated 9 • Publication in Lancet, Jan 23, 2021 • The inhibition of WEE1 has already been shown in a randomized double- blind, placebo-controlled study to exhibit a statistically significant overall survival advantage • Rare for oncology drugs to show a survival advantage prior to approval • Highly likely for a drug target to be approved if it shows a meaningful survival advantage (the “gold” standard)

1. Target Biology Known and Validated 10 • Publication in JCO March 12, 2021 • A third party WEE1 inhibitor exhibited a 29% ORR in a Phase 2 study in very sick endometrial cancer patients with USC subtype (most of these women die within 5 years upon diagnosis) • AZ has just started a registrational P2 study in USC with monotherapy dosing

2. Evidence of Strong Clinical Activity Shown by ZN-c3 11 • Clinically relevant responses seen in a refractory solid tumor untargeted all-comer population as monotherapy. Responses seen across four different tumor types signaling potential for broad oncology applicability • We believe this is the first DDR drug (including ATM, ATR, CHK1/2, DNA-PK, or PARP) that has shown monotherapy responses in CRC and NSCLC pts (very difficult to treat) • Currently 43% ORR (3/7) in USC pts(3). AZD1775 observed a 29% ORR • Exceptional responses seen were dramatic, unexpected and durable (1) 3 subjects with no treatment scans (CRC, USC, Pancreas), and experienced clinical progressive disease (CPD). + denotes treatment ongoing (2) 3 confirmed Exceptional Responders PRs, including an additional confirmation of Exceptional Responder PR since AACR Presentation Press Release (3) Waterfall as of 05/15/2021; both uPRs reported at AACR 2021 in USC are confirmed PRs. Newly reported uPR in USC is included. ORR based on radiographic responses.
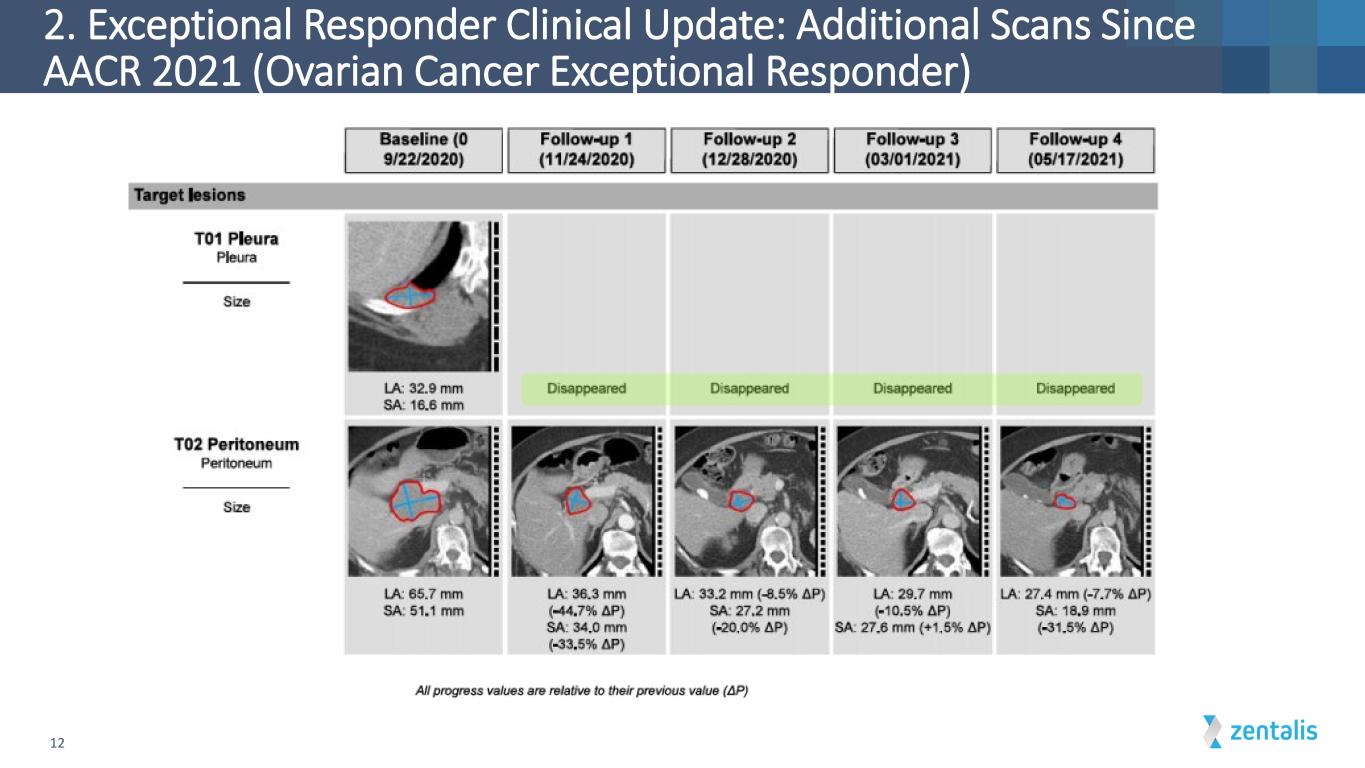
2. Exceptional Responder Clinical Update: Additional Scans Since AACR 2021 (Ovarian Cancer Exceptional Responder) 12

13 2. Exceptional Responder Clinical Update: Additional Scans Since AACR 2021 (Ovarian Cancer Exceptional Responder)
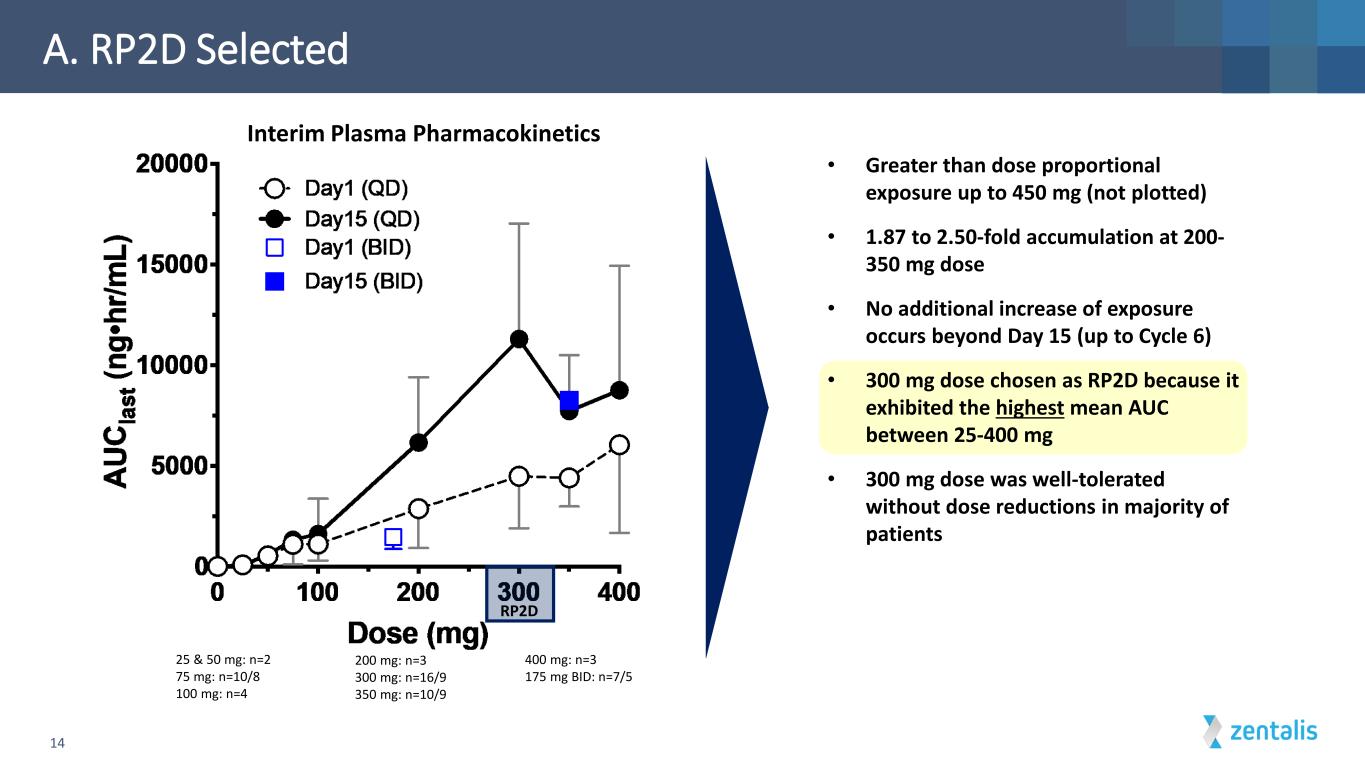
• Greater than dose proportional exposure up to 450 mg (not plotted) • 1.87 to 2.50-fold accumulation at 200- 350 mg dose • No additional increase of exposure occurs beyond Day 15 (up to Cycle 6) • 300 mg dose chosen as RP2D because it exhibited the highest mean AUC between 25-400 mg • 300 mg dose was well-tolerated without dose reductions in majority of patients A. RP2D Selected 25 & 50 mg: n=2 75 mg: n=10/8 100 mg: n=4 400 mg: n=3 175 mg BID: n=7/5 200 mg: n=3 300 mg: n=16/9 350 mg: n=10/9 14 Interim Plasma Pharmacokinetics RP2D

1. A. RP2D Selected (Cont.) Hair bulb Basal epidermis WEE1 CDK1 Y15 ZN-c3 p-CDK1 = Brown Staining (subject with cPR) Baseline On Treatment Basal epidermis Hair bulb 40x -100% -99% 1. CDK1 phosphorylation by WEE1 2. p-CDK1 inhibition 3. Skin Biopsy 15 Source: Drawing adapted from Servier Medical Art 1. CDK1 phosphorylation (p-CDK1) is mediated by WEE1 2. Inhibition of WEE1 therefore will lead to inhibition of p-CDK1 3. Skin biopsies were performed at baseline (C1D1) and on- treatment (C1D15) to verify p-CDK1 levels, and hence level of target engagement of WEE1 Confirmation of WEE1i Target Engagement in Surrogate Tissue

A. RP2D Selected (Cont.) 16 M or e W EE 1 Ta rg et E ng ag em en t Higher Drug Exposure 0 5000 10000 15000 20000 -100 -50 0 50 100 Average drug exposure at RP2D of 300mg QD AUC0-24 hr (ng*h/mL) p- CD K1 (% in hi bi tio n) WEE1 Target Engagement • Inhibition of p-CDK1 demonstrated WEE1 target engagement • Increase in dose / drug exposure directly related to WEE1 target engagement • RP2D showed an AUC with excellent target engagement with p-CDK1 levels decreased at least by 50% • In short, RP2D showed highest AUC with excellent Pharmacodynamic data directly supportive of 300 mg QD `

17 B. Strong Comparative Efficacy Data of ZN-c3 vs AZD1775 • Early study published in JCO Oct 20, 2015, enabling comparison head-to-head with ZN- c3’s monotherapy refractory solid tumor study • Due to tolerability issues for AZD1775 as monotherapy, MTD was established as 225 mg BID for 5 days per 21-day cycle vs ZN-c3’s 300 mg QD continuous dosing • ZN-c3 delivered 6.3 grams vs 2.25 grams per 21 days cycle for AZD1775 with better tolerability (~3x more drug with ZN-c3 at its RP2D) • Zentalis has seen responses in four different tumor types including NSCLC, CRC, ovarian, and endometrial to date. AZ has not observed responses in CRC or NSCLC • AZD1775’s two PRs in USC were seen in BRCA mutant patients; ZN-c3 responses were seen in BRCA wildtype patients

Predictive Biomarker Driven Settings 18 Uterine Serous Carcinoma PARP Combo Chemo Combos Immunotherapy Combo Proprietary Combos Biomarker Identified “Exceptional Responders” Across Tumor Types Representative Indication(s) Orphan Setting ZN-c3 ORR 43% (3/7)1 Clinical Studies Initiated in PARP and Chemo combos Clinical Studies Planned in I/O Combos ORR 100% (3/3) AZD1775 ORR 29% (6/21)2 Conducted 49 Clinical Trials with WEE1 Positive ORRs in PARP Combos4 Positive ORRs in Chemo Combos4 AZ Does Not Possess Biomarker Drug Setting Single Agent Single Agent Combinations (1) Non-head-to-head comparison. Results of a head-to-head comparison may differ significantly from those set forth herein. In addition, because our Phase 1 clinical trial for ZN-c3 and the Adavosertib clinical trials were separate trials, differences between the results of the trials may not be statistically or clinically meaningful. Data as of 05/15/2021; both uPRs reported at AACR 2021 in USC are confirmed PRs. Newly reported uPR in USC is included in ORR. ORR based on radiographic responses. (2) Liu JF et al. J Clin Oncol. 2021 Mar 11:JCO2003167 (2) Lheureux S., Lancet (2021). Addition of a WEE1 to Gemcitabine shows a statistically significant improvement in mOS over Gemcitabine with placebo (HR=0.56, P=0.017) (3) J Clin Oncol. 2019;37:2643-2650; Clin Cancer Res 26:4767-4776, 2020; J Clin Oncol. 2016;34:4354-4361 B. Compelling Data for ZN-c3 vs AZD1775 (Cont.) Combination Therapy Settings Comparative Data for ZN-c3 vs AZD1775 Demonstrated Survival Benefit3 Expecting Initial Data 2022 Combinations with ZN-c3 Ovarian Plat Resistant Ovarian Plat Resist Additional uPR in USC newly reported June 3, 2021
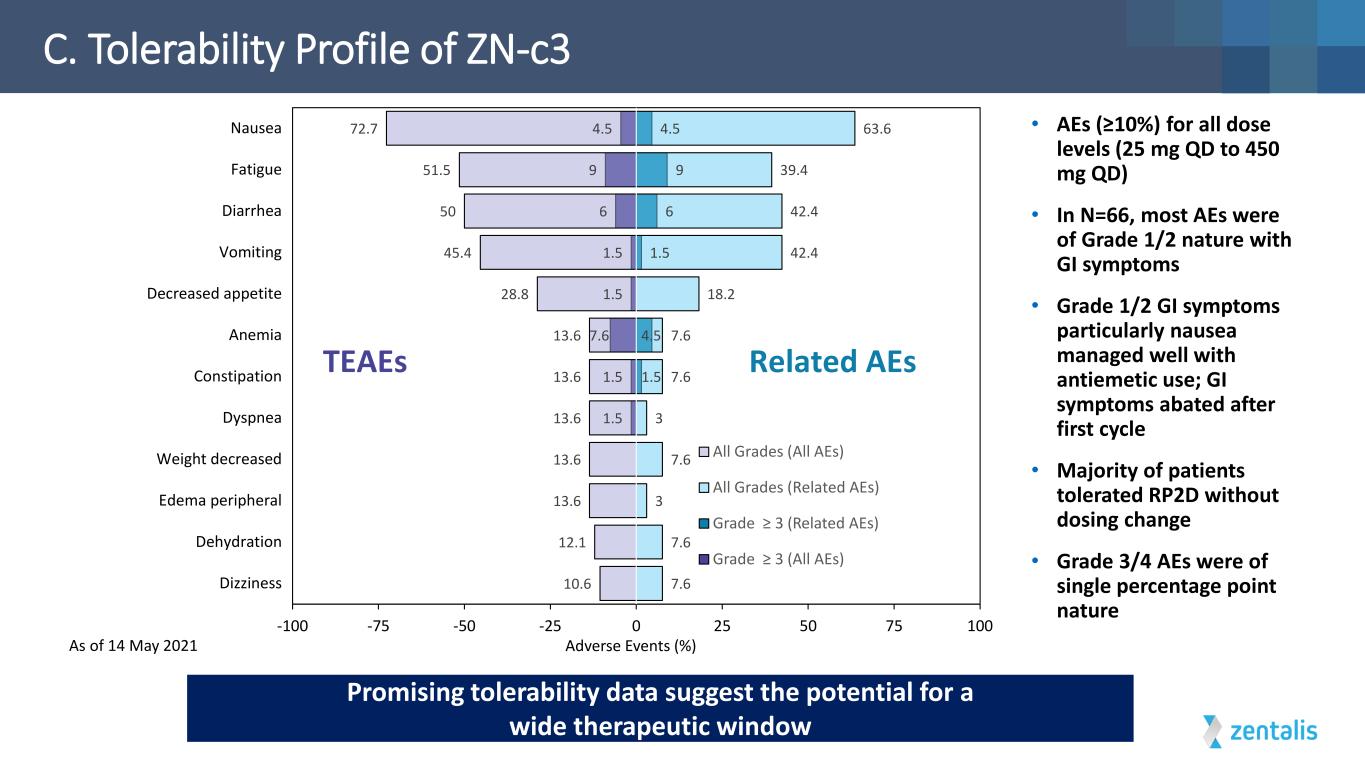
C. Tolerability Profile of ZN-c3 Adverse Events (%) 1.5 1.5 7.6 1.5 1.5 6 9 4.5 1.5 4.5 1.5 6 9 4.5 7.6 7.6 3 7.6 3 7.6 7.6 18.2 42.4 42.4 39.4 63.6 10.6 12.1 13.6 13.6 13.6 13.6 13.6 28.8 45.4 50 51.5 72.7 -100 -75 -50 -25 0 25 50 75 100 Dizziness Dehydration Edema peripheral Weight decreased Dyspnea Constipation Anemia Decreased appetite Vomiting Diarrhea Fatigue Nausea All Grades (All AEs) All Grades (Related AEs) Grade ≥ 3 (Related AEs) Grade ≥ 3 (All AEs) TEAEs Related AEs • AEs (≥10%) for all dose levels (25 mg QD to 450 mg QD) • In N=66, most AEs were of Grade 1/2 nature with GI symptoms • Grade 1/2 GI symptoms particularly nausea managed well with antiemetic use; GI symptoms abated after first cycle • Majority of patients tolerated RP2D without dosing change • Grade 3/4 AEs were of single percentage point nature Promising tolerability data suggest the potential for a wide therapeutic window As of 14 May 2021

C. Safety/Tolerability vs AZD1775 (1) Adverse Events (%)Source: Liu JF et al. J Clin Oncol. 2021 Mar 11:JCO2003167 (1) Non-head-to-head comparison. Results of a head-to-head comparison may differ significantly from those set forth herein. In addition, because our Phase 1 clinical trial for ZN-c3 and the Adavosertib clinical trials were separate trials, differences between the results of the trials may not be statistically or clinically meaningful • ≥300 mg QD continuously for 21 days of 21-day cycle • 6.3 grams dosed in cycle • Due to the tolerability issues of AZD1775 the drug was dosed intermittently while ZN- c3 was dosed continuously • Despite having a 2x higher dose intensity (6.3g vs 3g), ZN-c3 safety and tolerability were favorable 2.2 2.2 6.6 2.2 8.9 2.2 2.2 8.9 13.3 6.6 8.8 11.8 2.9 8.8 8.8 32.3 2.9 8.8 17.6 23.5 2.9 2.9 2.9 5.8 5.9 23.5 8.8 26.5 20.6 35.3 20.6 20.6 29.4 38.2 32.4 44.1 32.4 26.5 61.8 32.4 67.6 26.5 44.1 38.2 32.4 41.2 85.3 64.7 61.8 0 0 0 2.2 2.2 4.5 4.5 4.5 4.5 6.6 6.6 8.9 11.1 11.1 13.3 15.5 15.5 31.1 60 62.2 62.2 88.9 -100 -75 -50 -25 0 25 50 75 100 Insomnia Generalized muscle weakness Aspartate aminotransferase increased Cough Dysgeusia Hyponatremia Alanine aminotransferase increased Hypokalemia Neutrophil count decreased Hypomagnesemia Abdominal pain Platelet count decreased Dyspnea Anemia Back pain Edema peripheral Constipation Decreased appetite Vomiting Diarrhea Fatigue Nausea TEAEs AZD 1775 N = 34 ZN-c3 data as of 14 May 2021 TEAEs ZN-c3 N = 45 • 300 mg QD for days 1-5, then days 8-12 of 21-day cycle (published) • 3.0 grams dosed in cycle

C. Safety/Tolerability vs AZD1775 (Cont.) 21 Source: Liu JF et al. J Clin Oncol. 2021 Mar 11:JCO2003167 (1) Non-head-to-head comparison. Results of a head-to-head comparison may differ significantly from those set forth herein. In addition, because our Phase 1 clinical trial for ZN-c3 and the Adavosertib clinical trials were separate trials, differences between the results of the trials may not be statistically or clinically meaningful 23.5 32.3 14.7 6.6 2.2 6.6 0 5 10 15 20 25 30 35 Anemia Neutropenia Thrombocytopenia Chart Title Adavosertib 300mg Intermittent ZN-c3 300mg QD Continuous Interim Grade ≥3 Hematological TRAEs at ≥RP2D • Even lower overall severe hematological AE rate over AZD1775 even with 11 more ZN-c3 patients enrolled since AACR 2021 • Despite continuous dosing delivering twice the drug load, ZN-c3 induced markedly less hematological toxicity than AZD1775 did in its clinical trials • Better tolerability also unlocks the potential for wide ranging drug combinations providing potential for both increased efficacy and commercial potential % o f S ub je ct s 55% Less Thrombocytopenia For ZN-c3 93% Less Neutropenia For ZN-c372% Less Anemia for ZN-c3
• Conducted EOP1 Mtg with FDA re: study ZN-c3-004, a monotherapy trial planned with registrational intent in women with recurrent or persistent USC ‒ Proposed study for ZN-c3-004 designed with registrational intent for potential accelerated approval • In 2H 2021, Zentalis expects to approach FDA with a biomarker-driven, tumor- agnostic monotherapy clinical trial design with registrational intent • FDA has now granted two key designations for ZN-c3’s use in combination with chemotherapy in osteosarcoma ‒ Orphan Drug Designation ‒ Rare Pediatric Disease Designation D. Path to Potential Accelerated Approvals 22

Predictive Biomarker Driven Settings 23 Uterine Serous Carcinoma PARP Combo (e.g., Ovarian) Chemo Combo (e.g., CRC, NSCLC or GBM) Immunotherapy Combos (e.g., TNBC or CRC) Biomarker Identified “Exceptional Responders” Across Tumor Types Representative Indication(s) Orphan Setting Addressable Patient Populations1 ~11,500(2) Very Large~55,000(3) Drug Setting Single Agent ZN-c3 Single Agent ZN-c3 Combinations with ZN-c3 (1) North America, Western Europe and Japan (2) Gynecologic Oncology 115 (2009) 142-153, David Boruta et al.; National Cancer Institute, SEER 2020 Data (3) Observed predictive biomarker frequency data across solid tumor types; predictive biomarker not disclosed I. Commercial Opportunity Near-Term Monotherapy Registrational Paths Combination Therapy Settings Combinations with ZN-c3 Near-Term Combo Registrational Path Ovarian Plat Resist Ovarian Platinum Resistant ~13,000 Future Combo Registrational Path

24 ZN-c5

ZN-c5 Executive Summary • New interim clinical data from Phase 1/2 monotherapy studies suggest ZN- c5 has the potential to be best-in-class with favorable safety/tolerability data in mono and combo settings • New interim clinical data rival other oral SERD efficacy data; awaiting completion of study before final selection of RP2D (likely to be 50 mg QD) • Combination studies with palbociclib and abemaciclib continue on track 25

ZN-c5: Clinical Development Plan 26 Monotherapy Dose Escalation ZN-c5(1) Monotherapy Expansion ZN-c5 (2) Enrolled Combination Dose Escalation ZN-c5 + palbociclib (2) Enrolling Combination Phase 2 ZN-c5 + palbociclib Expected Initiation 2021 Monotherapy Phase 2 ZN-c5 Enrolling Combination Dose Escalation ZN-c5 + abemaciclib Enrolling Other studies • Window of Opportunity study initiated in 2020 to analyze tumor ER degradation (enrollment completed, 35 patients) • Food effect study (18 subjects) completed, CSR in preparation • Results showed ZN-c5 could be administered with or without food • Multiple dose cohorts may be chosen in monotherapy Phase 2 study Ongoing Clinical Programs Phase 1/2 Study (ZN-c5-001) Phase 1b Study Monotherapy Study Combination Study (1) As of May 11, 2021, n=24 were enrolled patients in the Phase 1, monotherapy dose escalation portion of this trial. Of these 24 patients, 3 were still on treatment and 21 discontinued due to disease progression (n = 20), and physician decision (n = 1). (2) As of May 11, 2021, 32 patients were enrolled in the Phase 1, monotherapy expansion portion of this trial. Of these 32 patients, 12 were still on treatment and 20 discontinued due to disease progression (n = 18), adverse event (n = 1, hypersensitivity) and physician decision (n = 1). As of May 11, 2021, we have enrolled 41 patients in the Phase 1, combination dose escalation portion of this trial. Of these 41 patients, 23 were still on treatment and 18 discontinued due to disease progression (n = 14), patient decision (n = 2), intercurrent illness (n = 1, endometrial cancer) and physician decision (n = 1).

ZN-c5-001: First-in-Human Study - Design & Endpoints • Monotherapy Dose escalation (3+3 design) → Maximum tolerated dose/Recommended Phase 2 Dose • Monotherapy Expansion → Safety and tolerability • Monotherapy Phase 2 → Clinical Benefit Rate (CBR) • Combination with Palbociclib Dose escalation (3+3) → Maximum tolerated dose/Recommended Phase 2 Dose • Combination with Palbociclib Phase 2 → Clinical Benefit Rate (CBR) • Safety and tolerability • Pharmacokinetics • Anti-tumor activity (RECIST): objective response rate, CBR, duration of response, progression-free survival, overall survival • Pharmacodynamic and prognostic biomarkers • Administered orally, doses from 25 to 300 mg/day, dose once (QD) or twice (BID) a day 27 Design Primary Endpoint Key Secondary Endpoints Dosing
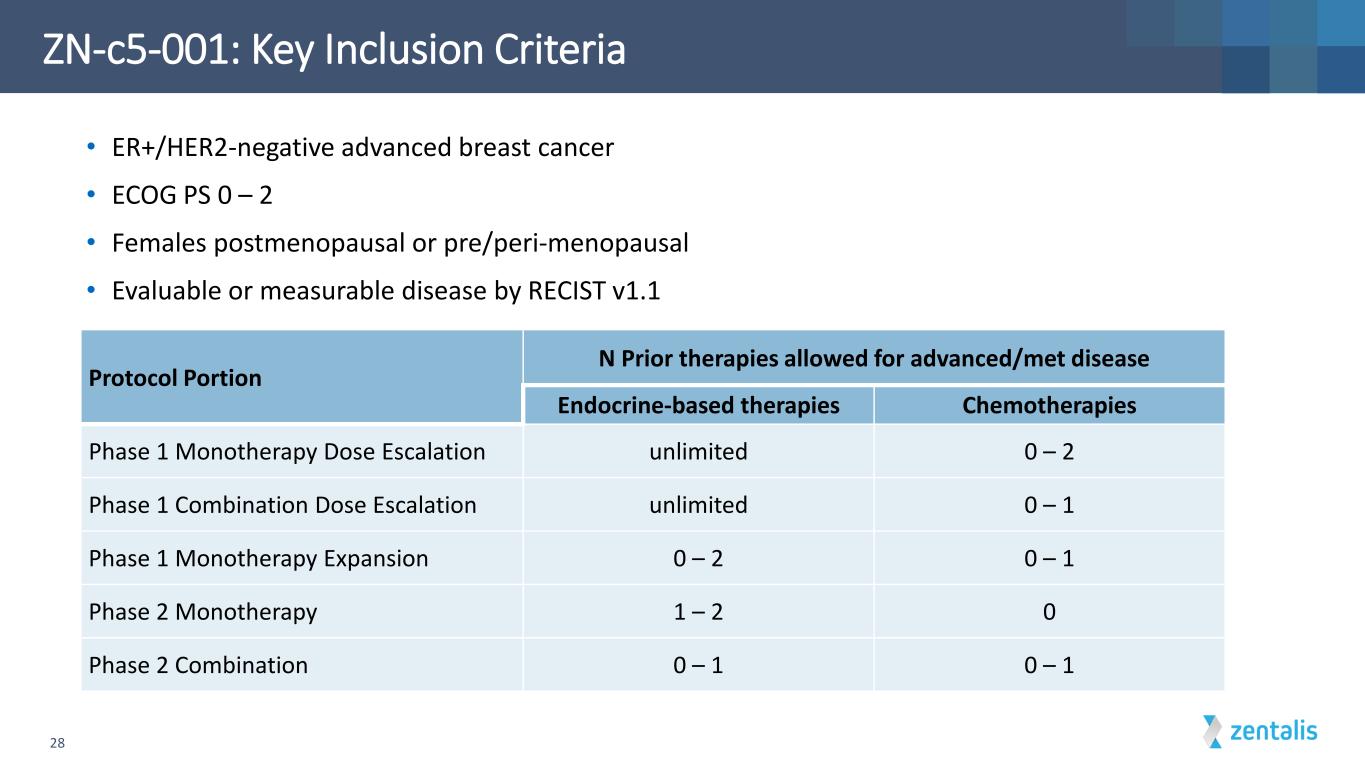
ZN-c5-001: Key Inclusion Criteria 28 Protocol Portion N Prior therapies allowed for advanced/met disease Endocrine-based therapies Chemotherapies Phase 1 Monotherapy Dose Escalation unlimited 0 – 2 Phase 1 Combination Dose Escalation unlimited 0 – 1 Phase 1 Monotherapy Expansion 0 – 2 0 – 1 Phase 2 Monotherapy 1 – 2 0 Phase 2 Combination 0 – 1 0 – 1 • ER+/HER2-negative advanced breast cancer • ECOG PS 0 – 2 • Females postmenopausal or pre/peri-menopausal • Evaluable or measurable disease by RECIST v1.1
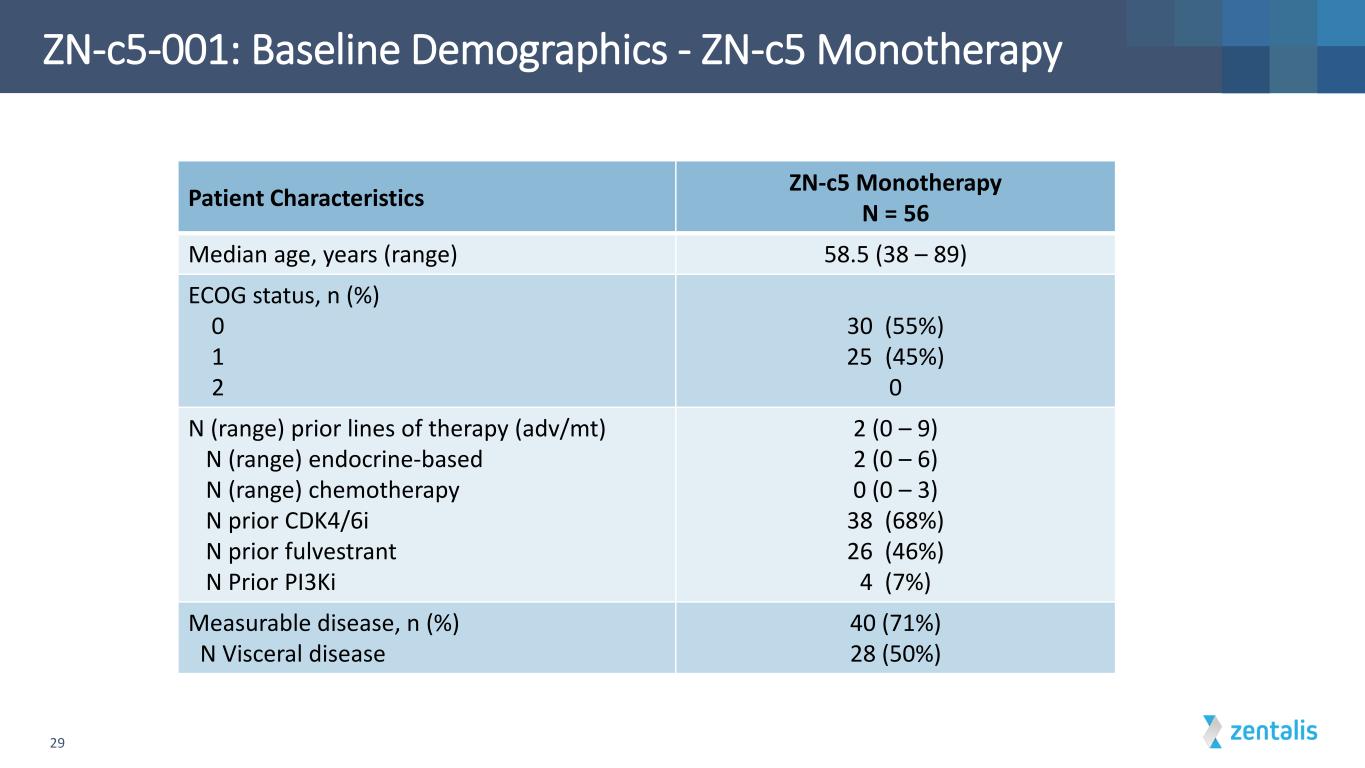
ZN-c5-001: Baseline Demographics - ZN-c5 Monotherapy 29 Patient Characteristics ZN-c5 Monotherapy N = 56 Median age, years (range) 58.5 (38 – 89) ECOG status, n (%) 0 1 2 30 (55%) 25 (45%) 0 N (range) prior lines of therapy (adv/mt) N (range) endocrine-based N (range) chemotherapy N prior CDK4/6i N prior fulvestrant N Prior PI3Ki 2 (0 – 9) 2 (0 – 6) 0 (0 – 3) 38 (68%) 26 (46%) 4 (7%) Measurable disease, n (%) N Visceral disease 40 (71%) 28 (50%)
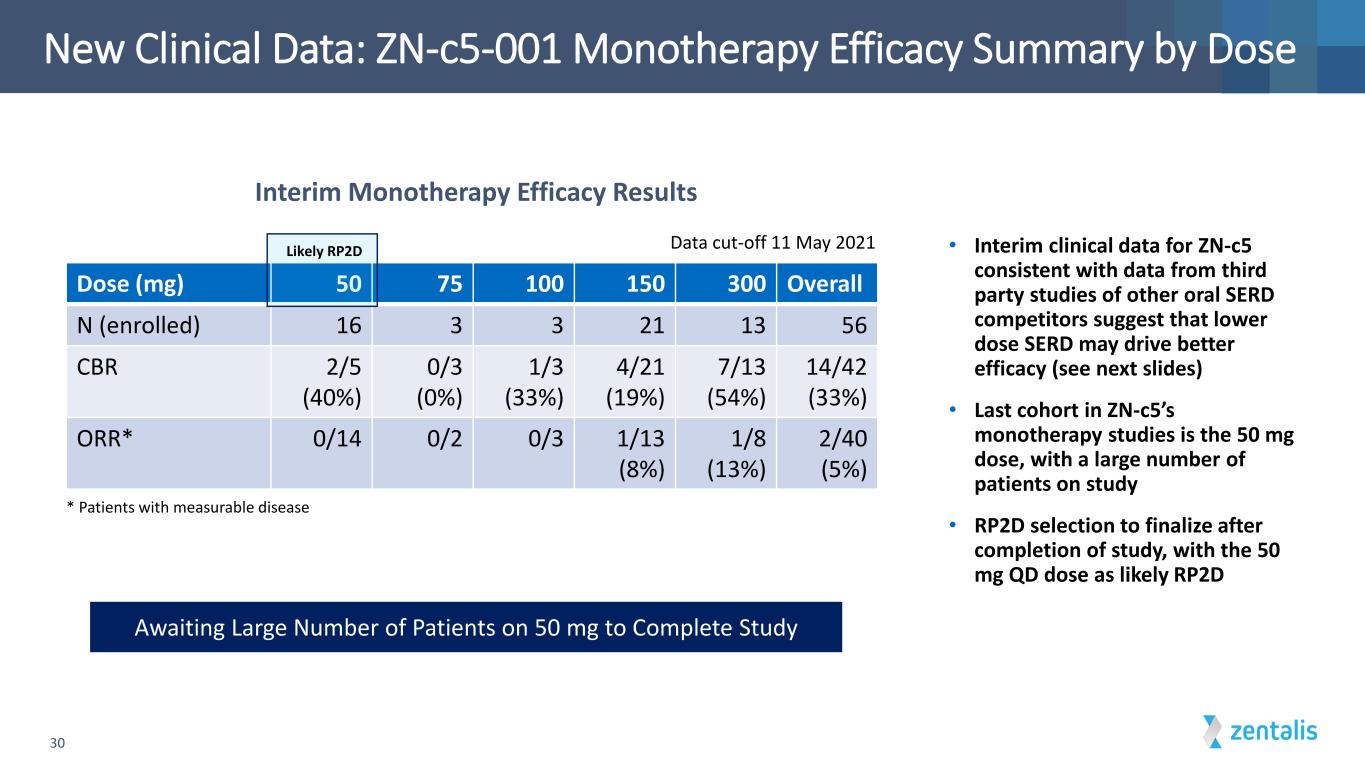
New Clinical Data: ZN-c5-001 Monotherapy Efficacy Summary by Dose 30 * Patients with measurable disease Dose (mg) 50 75 100 150 300 Overall N (enrolled) 16 3 3 21 13 56 CBR 2/5 (40%) 0/3 (0%) 1/3 (33%) 4/21 (19%) 7/13 (54%) 14/42 (33%) ORR* 0/14 0/2 0/3 1/13 (8%) 1/8 (13%) 2/40 (5%) Data cut-off 11 May 2021 Awaiting Large Number of Patients on 50 mg to Complete Study Interim Monotherapy Efficacy Results Likely RP2D • Interim clinical data for ZN-c5 consistent with data from third party studies of other oral SERD competitors suggest that lower dose SERD may drive better efficacy (see next slides) • Last cohort in ZN-c5’s monotherapy studies is the 50 mg dose, with a large number of patients on study • RP2D selection to finalize after completion of study, with the 50 mg QD dose as likely RP2D

PD PD PD SD SD+ SD SD+ SD+ SD+ SD+ SD+ SD SD SD SD24 SD24 PD PD SD PD PD SD24 0.00 2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 1903-101 102-108 1002-102 102-107 102-109 104-002 (50 mg : M) 109-101 1003-105 1902-101 1902-102 1901-101 1003-103 109-103 1003-104 101-004 (50 mg : M) 104-005 (50 mg : NM) 106-007 (75 mg : M) 104-006 (75 mg : M) 106-008 (75 mg : NM) 104-010 (100 mg : M) 104-011 (100 mg : M)xxxxxxxxxxxxxxxx 106-009 (100 mg : M) xxx xxx xxx New Interim Clinical Data: ZN-c5-001 Monotherapy 50-100 mg Treatment Duration (months) 75 mg QD 100 mg QD 50 mg QD (1) Number of treatments reflect advanced or metastatic setting, not neo/adjuvant; also reflects combinations with targeted therapies CDK4/6, mTOR, PI3Ki (2) P-palbociclib, A- abemaciclib, R-ribociclib, E-experimental treatment, could be placebo ES R1 M ut at io n Pr io r Ho rm on al ba se d (1 ) Pr io r Ch em o (1 ) Pr io r CD K4 /6 (2 ) Pr io r Fu lv es tr an t + 3 1 P Y + 4 1 P Y - 3 1 P, R Y U 6 2 - Y + 3 0 P Y U 3 1 P Y U 2 2 P Y - 2 0 - - - 0 1 - - + 1 0 P - + 1 1 - - + 2 1 - - + 0 0 - - U 1 1 - - + 0 0 R - - 2 0 - - - 3 1 P, A Y Y 0 0 P - - 0 0 P - + 1 1 - - Y 0 0 P - + 0 0 - Y Treatment Duration (months) and Response by Dose as of 11 May 2021
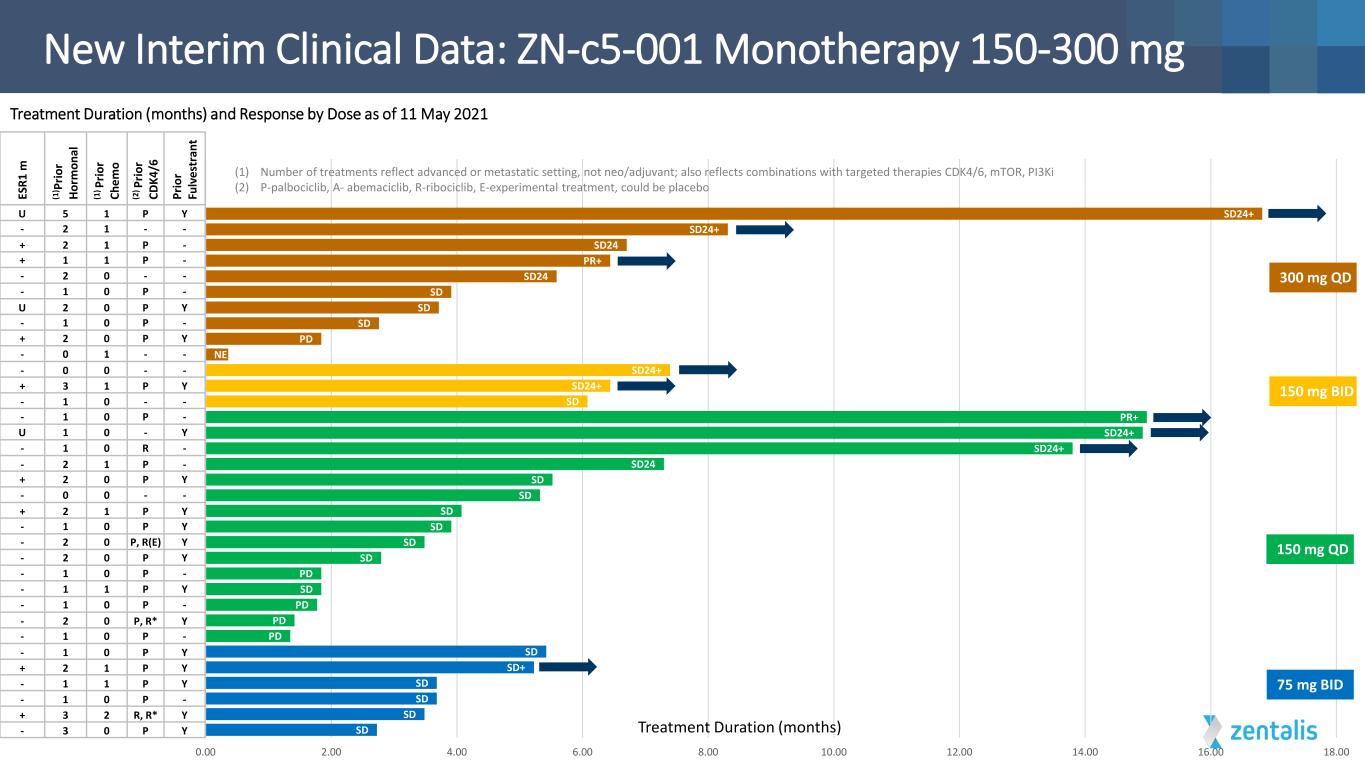
SD SD SD SD SD+ SD PD PD PD SD PD SD SD SD SD SD SD SD24 SD24+ SD24+ PR+ SD SD24+ SD24+ NE PD SD SD SD SD24 PR+ SD24 SD24+ SD24+ 0.00 2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00 18.00 105-003 (75 mg BID : NM) 107-001 (75 mg BID: M) 105-001 (75mg BID : M) 104-016 (75 mg BID : M) 106-018 (75 mg BID : NM) 105-002 (75 mg BID : NM) 107-103 104-015 (150 mg : M) 101-102 107-105 102-103 107-107 104-101xxxxxxxxxxxxxxxxxxxxxxxxxxxxxx 104-103 102-016 (150mg : NM) 102-101 104-102 106-014 (150 mg : NM) 102-102 107-101 107-102 109-002 (150 mg BID : M) 106-017 (150 mg BID : NM) 107-018 (150 mg BID : M) 1002-101 105-101 102-105 107-017 (300 mg : M) 102-104 105-102 107-108 106-016 (300 mg : M) 1003-102 106-015 (300 mg : NM) xxx xxx xxx (1) Number of treatments reflect advanced or metastatic setting, not neo/adjuvant; also reflects combinations with targeted therapies CDK4/6, mTOR, PI3Ki (2) P-palbociclib, A- abemaciclib, R-ribociclib, E-experimental treatment, could be placebo New Interim Clinical Data: ZN-c5-001 Monotherapy 150-300 mg Treatment Duration (months) 150 mg QD 300 mg QD 75 mg BID 150 mg BID ES R1 m (1 ) P rio r Ho rm on al (1 ) P rio r Ch em o (2 ) P rio r CD K4 /6 Pr io r Fu lv es tr an t U 5 1 P Y - 2 1 - - + 2 1 P - + 1 1 P - - 2 0 - - - 1 0 P - U 2 0 P Y - 1 0 P - + 2 0 P Y - 0 1 - - - 0 0 - - + 3 1 P Y - 1 - - - 1 0 P - U 1 0 - Y - 1 0 R - - 2 1 P - + 2 0 P Y - 0 0 - - + 2 1 P Y - 1 0 P Y - 2 0 P, R(Ε) Y - 2 0 P Y - 1 0 P - - 1 1 P Y - 1 0 P - - 2 0 P, R* Y - 1 0 P - - 1 0 P Y + 2 1 P Y - 1 1 P Y - 1 0 P - + 3 2 R, R* Y - 3 0 P Y Treatment Duration (months) and Response by Dose as of 11 May 2021

-100 -80 -60 -40 -20 0 20 40 60 80 100 Baseline Week 8 Week 16 Week 24 Week 32 Week 40 Week 48 Target Lesion size - % Change from Baseline 101-004 (50 : SD24) 104-002 (50 : SD) 104-006 (75 : PD) 106-007 (75 : PD) 106-009 (100 : SD24) 104-010 (100 : SD) 107-001 (75 BID : SD) 105-001 (75 BID : SD) 104-016 (75 BID : SD) 104-015 (150 : PD) 107-018 (150 BID : SD24+) 109-002 (150 BID : uPR+) 106-016 (300 : SD24) 107-017 (300 : PD) 1002-102 (50 : PD) 1003-103 (50 : SD) 1003-104 (50 : SD) 1003-105 (50 : SD+) 102-107 (50 : SD) 102-108 (50 : PD) 109-101 (50 : SD+) 109-103 (50 : SD) 1901-101 (50 : SD+) 1902-101 (50 : SD+) 1902-102 (50 : SD+) 1903-101 (50 : PD) 104-101 (150 : SD) 104-102 (150 : SD) 102-101 (150 : SD) 101-102 (150 : PD) 107-102 (150 : PR+) 107-103 (150 : PD) 107-105 (150 : SD) 107-107 (150 : SD) 102-103 (150 : PD) 1003-102 (300 : SD+) 107-108 (300 : PR+) New Interim Clinical Data: ZN-c5-001 Monotherapy xxx-yyy [zz : rr] indicates: patient number [dose : best response, + if ongoing] PR PD Subjects with Measurable Disease as of 11 May 2021

New Interim Clinical Data: ZN-c5 Plasma PK Parameters in Combo Arms Confidential34 ZN-c5 Dose (mg) ZN-c5 on Day 1 ZN-c5 on Day 15 ZN-c5 Accum by AUC Cmax (ng/mL) Tmax (hr) AUC0-24hr (ng*h/mL) Cmax (ng/mL) Tmax (hr) AUC0-24hr (ng*h/mL 25 (n=3/2) Mean 2,230 2 26,800 2,360 2 26,500 1.2 SD 447 2-2 7,040 (2,230, 2,490) 2,2 28,000, 25,100) (1.2, 1.2) 50 (n=6) Mean 5,140 1.5 62,700 5,390 1.5 60,800 1.1 SD 1,080 1-24 27,100 1,380 1-4 18,400 0.36 100 (n=8) Mean 6,960 2 89,600 6,640 2 80,000 0.90 SD 1,290 1-8 18,500 1,050 2-8 22,000 0.19 150 (n=3) Mean 18,500 2 226,000 13,300 2 158,000 0.71 SD 4,370 1-8 22,700 3,370 1-2 33,800 0.23 25BID (n=3) Mean 3,280 2 AUC0-inf 38,200 4,730 1 69,500* 1.0** SD 121 1-2 5,930 2,300 1-2 37,100 50BID (n=2) Mean 5,030 (3990, 6070) 2 (2, 2) AUC0-inf 48,900 4,430 (3,900, 4,950) 1.5 (1, 2) 63,400* (48,400, 78,200) 0.94** ZN-c5 PK at 50 & 100 mg is consistent with mono, at 150 mg – higher than in mono *For BID, AUC0-24hr on Day 15 is calculated as 2 x AUC0-12hr **For BID, accumulation is calculated based in AUC0-12hr ZN-c5 exposure on Day 15 was approximately dose proportional between 25 and 150 mg QD (with somewhat lower exposure at 100 mg) 25 – 150 mg ZN-c5 + 125 mg Palbociclib (preliminary) as of 1 May 2021

35 New Interim Clinical Data: ZN-c5 Window of Opportunity Initial Biomarker Study N Mean STD All doses 21 -17% 22% 25 BID 3 -23% 21% 50 QD 9 -15% 26% 50 mg* 12 -17% 24% 150 mg* 6 -20% 16% 300 QD 3 -8% 29% *total daily dose Linear Regression %H-score change from baseline = intercept + slope*total daily dose R-squared=0.013 intercept= 20% 95% CI (-37%, -3%) p-value=0.022 slope= 0 95% CI (-.1, .1) p-value=0.623 Initial Impressions of Interim Data: • No dose correlation with ER degradation status • High variability with assay and difficulty in cross comparing with other studies • One pt at 300 mg dose with Grade 3/4 LFT increases resolved without incident after study end: patient with concomitant issues of fever, infection, acetaminophen use and steatosis • Study continues to enroll; full study results to be published in future as of 1 February 2021

New Interim Clinical Data: ZN-c5 Monotherapy – Related AEs in ≥ 10% 36 AEs in N 50 mg QD N = 16 75 mg QD N = 3 100 mg QD N = 3 75 mg BID N = 6 150 mg QD N = 15 150 mg BID N = 3 300 mg QD N = 10 Total N = 56 Grade 1 2 3 1 2 3 1 2 3 1 2 3 1 2 3 1 2 3 1 2 3 1 2 3 All N (%) Any AE 6 2 0 1 0 0 0 0 0 2 2 0 5 4 0 1 1 1 5 2 1 20 11 2 33 (59%) Hot Flushes 2 3 1 2 6 2 0 8 (14%) Nausea 1 1 1 1 1 1 2 4 4 0 8 (14%) Fatigue 1 1 2 1 1 1 6 1 0 7 (13%) Data cut-off 11 MAY 2021 Diarrhea events: 2 out of 56 subjects (3.6%), only grade 1 or 2 events observed Grade 3 events: n=1 hypersensitivity (300 mg qd); n=1 γGT increase (150 mg bid) No observed bradycardia, no visual disturbances, no QTC, no dizziness TEAE’s Related to ZN-c5
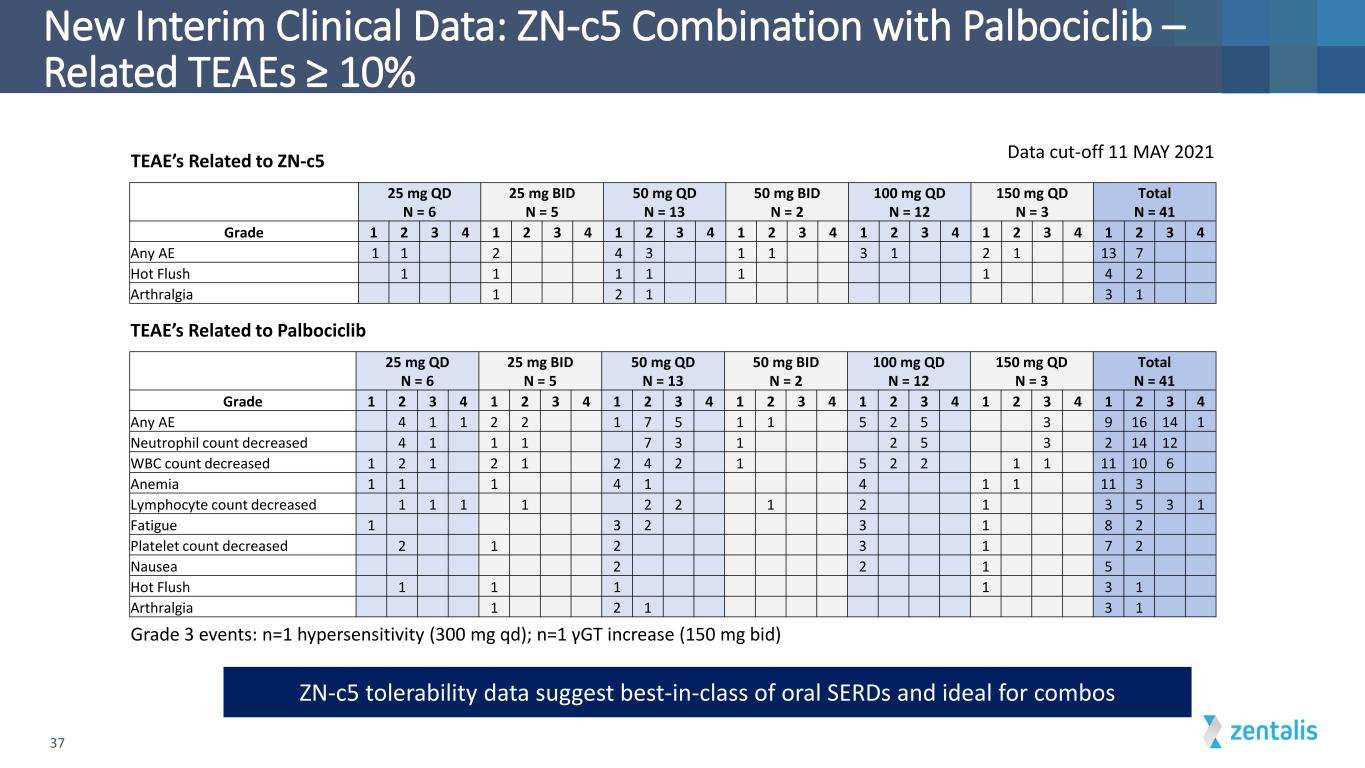
New Interim Clinical Data: ZN-c5 Combination with Palbociclib – Related TEAEs ≥ 10% 37 25 mg QD N = 6 25 mg BID N = 5 50 mg QD N = 13 50 mg BID N = 2 100 mg QD N = 12 150 mg QD N = 3 Total N = 41 Grade 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 Any AE 4 1 1 2 2 1 7 5 1 1 5 2 5 3 9 16 14 1 Neutrophil count decreased 4 1 1 1 7 3 1 2 5 3 2 14 12 WBC count decreased 1 2 1 2 1 2 4 2 1 5 2 2 1 1 11 10 6 Anemia 1 1 1 4 1 4 1 1 11 3 Lymphocyte count decreased 1 1 1 1 2 2 1 2 1 3 5 3 1 Fatigue 1 3 2 3 1 8 2 Platelet count decreased 2 1 2 3 1 7 2 Nausea 2 2 1 5 Hot Flush 1 1 1 1 3 1 Arthralgia 1 2 1 3 1 Grade 3 events: n=1 hypersensitivity (300 mg qd); n=1 γGT increase (150 mg bid) Data cut-off 11 MAY 2021 TEAE’s Related to Palbociclib TEAE’s Related to ZN-c5 ZN-c5 tolerability data suggest best-in-class of oral SERDs and ideal for combos 25 mg QD N = 6 25 mg BID N = 5 50 mg QD N = 13 50 mg BID N = 2 100 mg QD N = 12 150 mg QD N = 3 Total N = 41 Grade 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 1 2 3 4 Any AE 1 1 2 4 3 1 1 3 1 2 1 13 7 Hot Flush 1 1 1 1 1 1 4 2 Arthralgia 1 2 1 3 1

38 ZN-d5

ZN-d5: Clinical Development Plan 39 Ongoing and Planned Clinical Programs AML and Non-Hodgkin’s Lymphoma: Monotherapy Dose Escalation Initiated 4Q 2020 Phase 1 Phase 2 Undisclosed Indication: Phase 1b Combination Study Expected Initiation 1H 2022 Monotherapy Phase 2 Study Expected Initiation 1Q 2022 (1) Enrollment of trial ongoing (2) Trial designs will be based off data generated from Phase 1 trials (1) (2) Monotherapy Study Combination Study

ZN-d5 Update • ZN-d5 is a highly selective, oral BCL-2 inhibitor • In internal preclinical studies, ZN-d5 is 14x more selective for BCL-2 over BCL-xL than venetoclax, potentially yielding less thrombocytopenia • Entered the clinic in October 2020 in a dose-escalation study in relapsed/ refractory non-Hodgkin’s lymphoma (NHL) and acute myeloid leukemia (AML) • 14 subjects with NHL enrolled (diffuse large B cell, mantle cell, follicular and marginal zone lymphomas all represented) • Completed first 4 dosing cohorts without dose-limiting toxicities; 5th cohort ongoing • No unexpected safety findings; evidence of biological activity • Plan to open the study to AML this summer 40

41 ZN-e4
ZN-e4 Update • ZN-e4 is a potent, third generation EGFR inhibitor • Lack of active metabolite binding to wild type EGFR provides potential for better tolerability than osimertinib • First-in-human dose escalation study in EGFR-mutant non-small cell lung cancer is ongoing • Enrolled 26 subjects, both osimertinib-naïve and -experienced • Escalated from 20 mg through 480 mg, with clinical activity at doses >80 mg QD • Well-tolerated at all doses; rash AE observed in one subject and only grade 1 (1/26 subjects, 4%) as of March 25, 2021 data cutoff • Currently back-filling several dose cohorts to have robust PK and exposure-toxicity data to support Phase 2 dose selection 42

43 Milestones

44 Updated Key Milestones Event Expected Timing ZN-c3 (WEE1 Inhibitor) Initiate Phase 2 monotherapy in uterine serous carcinoma Initiate Phase 1/2 chemotherapy combo in osteosarcoma Initiate Phase 1/2 niraparib combo in ovarian cancer Initiate Phase 1/2 tumor agnostic, predictive biomarker study Initial readouts on Phase 1 USC expansion cohort and Phase 1b ovarian chemo combo Initial readouts on Phase 2 USC trial and Phase 1/2 chemotherapy combo in osteosarcoma Completed 3Q 2021 4Q 2021 4Q 2021 1H 2022 2H 2022 ZN-c5 (Oral SERD) Phase 1 interim results from monotherapy dose expansion and escalation studies, Window of Opportunity study, palbo combo safety Initiate Phase 2 monotherapy study Phase 1b combination study topline results with palbociclib; Phase 1b combination study topline results with abemaciclib Completed Completed 1H 2022 ZN-d5 (BCL-2 Inhibitor) Initiate monotherapy Phase 2 trial Phase 1 initial results from dose escalation study in AML and Non-Hodgkin’s Lymphoma Initiate combination Phase 1b trial in undisclosed indication 1Q 2022 1H 2022 1H 2022 ZN-e4 (EGFR Inhibitor) Phase 1 initial results from dose escalation study Evaluate potential for use in combinations for treatment of lung cancer 4Q 2021 2021+ Integrated Discovery Engine R&D Day 4Q 2021 Zentera Submit ZN-c5, ZN-c3, ZN-d5 CTAs in China Potential HK listing Completed 2022
