Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Keros Therapeutics, Inc. | exhibit992050updatepr.htm |
| EX-99.1 - EX-99.1 - Keros Therapeutics, Inc. | corporatepresentation.htm |
| 8-K - 8-K - Keros Therapeutics, Inc. | keros-form8xk050update.htm |

23 June 2021 KER-050 Update

Disclaimer Statements contained in this presentation regarding matters that are not historical facts are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as "anticipates," "believes," "expects," "intends," “plans,” “potential,” "projects,” “would” and "future" or similar expressions are intended to identify forward-looking statements. Examples of these forward-looking statements include statements concerning: Keros’ expectations regarding its growth, strategy, progress and the design, objectives and timing of its preclinical studies and clinical trials for KER-050, KER-047 and KER-012, including its regulatory plans; the potential impact of COVID-19 on Keros’ ongoing and planned preclinical studies, clinical trials, business and operations; and the potential of Keros’ proprietary discovery approach. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties include, among others: Keros’ limited operating history and historical losses; Keros’ ability to raise additional funding to complete the development and any commercialization of its product candidates; Keros’ dependence on the success of its lead product candidates, KER-050 and KER-047; that Keros may be delayed in initiating, enrolling or completing any clinical trials; competition from third parties that are developing products for similar uses; Keros’ ability to obtain, maintain and protect its intellectual property; Keros’ dependence on third parties in connection with manufacturing, clinical trials and pre-clinical studies; and risks relating to the impact on our business of the COVID-19 pandemic or similar public health crises. These and other risks are described more fully in Keros’ filings with the Securities and Exchange Commission (“SEC”), including the “Risk Factors” section of the Company’s Quarterly Report on Form 10-Q, filed with the SEC on May 6, 2021, and its other documents subsequently filed with or furnished to the SEC. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Except to the extent required by law, Keros undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and we make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. The trademarks included in this presentation are the property of the owners thereof and are used for reference purposes only. 2

Keros Attendees • Jasbir Seehra, Chief Executive Officer • Claudia Ordonez, Chief Medical Officer • Keith Regnante, Chief Financial Officer 3

Harnessing the Powerful Biology of the TGF-β Superfamily • Clinical-stage biopharmaceutical company developing novel therapeutics that target the TGF-β superfamily • Approach validated by marketed products, InfuseTM (BMP2) for spinal fusion and Reblozyl® (modified activin receptor IIB) for treatment of anemia in β-thalassemia and myelodysplastic syndromes • Leveraging our extensive experience in TGF-β superfamily protein structure, function and protein engineering to generate a clinical pipeline of differentiated therapeutics: Hematology KER-050: Modified activin receptor IIA (ActRIIA) ligand trap • Designed to address ineffective hematopoiesis by modulating TGF-β superfamily signaling • Potential to correct multiple cytopenias in patients with MDS and myelofibrosis (MF) KER-047: Activin receptor-like kinase-2 (ALK2) inhibitor • Designed to address anemias resulting from iron imbalance • Potential to treat iron-refractory iron deficiency anemia (IRIDA), iron deficiency anemia and other diseases Pulmonary and Musculoskeletal KER-012: Modified activin receptor IIB ligand trap • Designed to inhibit vascular remodeling and bone loss • Potential to treat pulmonary arterial hypertension (PAH) and bone loss in osteogenesis imperfecta and osteoporosis 4
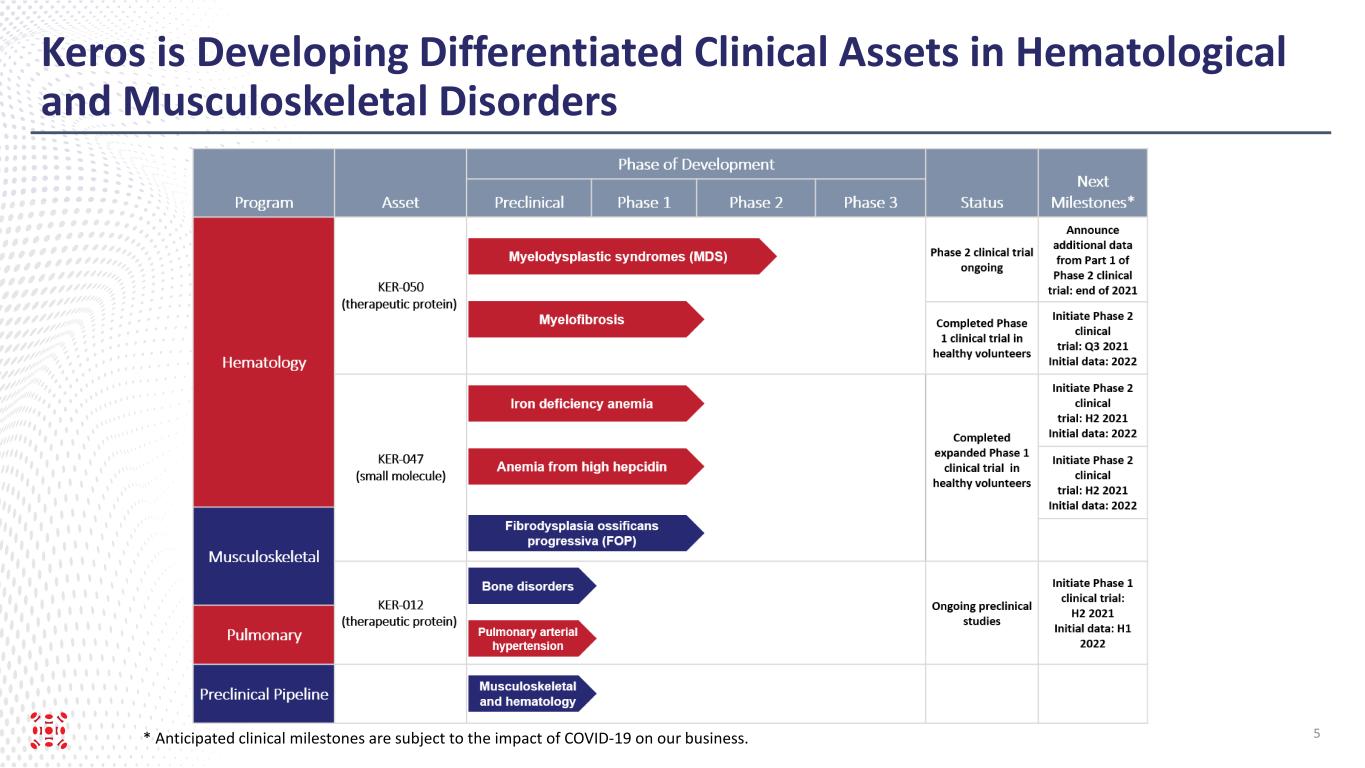
Keros is Developing Differentiated Clinical Assets in Hematological and Musculoskeletal Disorders 5* Anticipated clinical milestones are subject to the impact of COVID-19 on our business.

KER-050 A novel treatment designed to address diseases arising from ineffective hematopoiesis • Myelodysplastic syndromes • Myelofibrosis 6

KER-050 is a Modified ActRII Fusion Protein • Activin receptors are expressed on hematopoietic cells and modulate differentiation of precursor cells • KER-050 is a ligand trap composed of a modified extracellular domain of ActRIIA fused to the Fc region of human IgG • KER-050 is designed to increase red blood cell (RBC) and platelet production by inhibiting the signaling of ligands through activin receptors 7

KER-050 Increased RBC Parameters and Platelets following Single Doses in Phase 1 Clinical Trial in Healthy Volunteers • Single doses of KER-050 observed to lead to clinically meaningful changes in platelets • Maximum changes observed 7-15 days post-dosing 8 • Observed rapid and sustained increase in RBC parameters through day 29 are supportive of KER- 050 acting on multiple stages of erythropoiesis • Reticulocytes, RBCs and hemoglobin • Observed sustained increase from single dose supports monthly or less frequent dosing PLATELET (Mean Change from Baseline in Platelets at Each Dose) HEMOGLOBIN (Mean Change from Baseline)
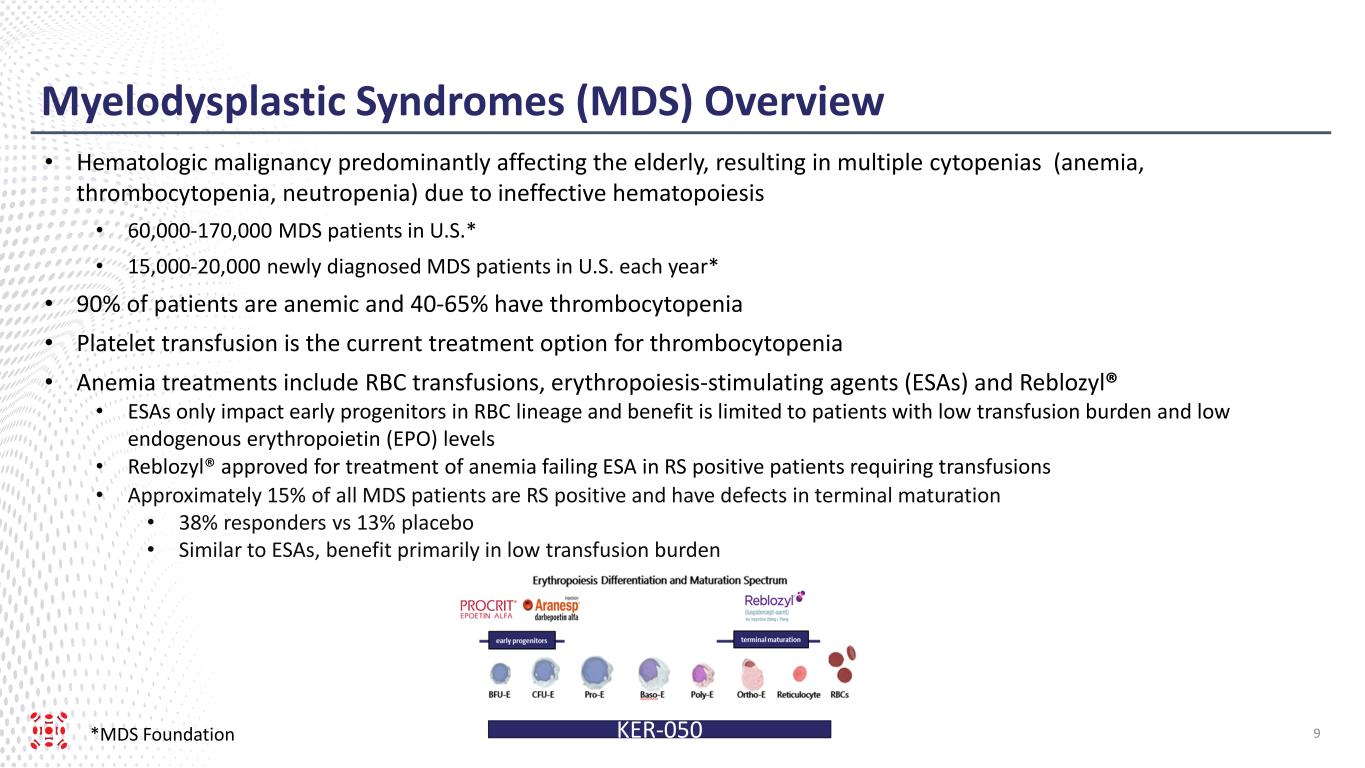
Myelodysplastic Syndromes (MDS) Overview *MDS Foundation • Hematologic malignancy predominantly affecting the elderly, resulting in multiple cytopenias (anemia, thrombocytopenia, neutropenia) due to ineffective hematopoiesis • 60,000-170,000 MDS patients in U.S.* • 15,000-20,000 newly diagnosed MDS patients in U.S. each year* • 90% of patients are anemic and 40-65% have thrombocytopenia • Platelet transfusion is the current treatment option for thrombocytopenia • Anemia treatments include RBC transfusions, erythropoiesis-stimulating agents (ESAs) and Reblozyl® • ESAs only impact early progenitors in RBC lineage and benefit is limited to patients with low transfusion burden and low endogenous erythropoietin (EPO) levels • Reblozyl® approved for treatment of anemia failing ESA in RS positive patients requiring transfusions • Approximately 15% of all MDS patients are RS positive and have defects in terminal maturation • 38% responders vs 13% placebo • Similar to ESAs, benefit primarily in low transfusion burden 9KER-050

KER050-MD-201 A Phase 2 Clinical Trial Of KER-050 For The Treatment Of Anemia In Patients With Very Low, Low Or Intermediate Risk Myelodysplastic Syndromes (MDS)

Phase 2 Clinical Trial of KER-050 in MDS • Phase 2, multicenter, open-label clinical trial in very low-, low- and intermediate-risk MDS patients • KER-050 administered once every four weeks (Q4W) for 12 weeks • Trial objectives: • Safety, tolerability and pharmacokinetics • Evaluate pharmacodynamic effects and efficacy of KER-050 • Trial designed to evaluate KER-050 effects on hematopoiesis in: • Ring sideroblast (RS) positive and non-RS patients • ESA naïve and experienced • In high and low transfusion burden and non- transfused patients 11

Phase 2 Clinical Trial of KER-050 in MDS Key Eligibility Criteria: • MDS with very low-, low-, or intermediate-risk disease, as classified by the International Prognostic Scoring System- Revised, including both RS positive and non-RS • ESA naïve and experienced patients are eligible • No prior treatment with azacitidine, decitabine, lenalidomide, luspatercept or sotatercept • Anemia, categorized in one of the following three groups: • Non-transfused (NT): hemoglobin (Hgb) <10 g/dL • Low transfusion burden (LTB): 1-3 units of RBC/8 weeks, Hgb <10 g/dL • High transfusion burden (HTB): ≥4 units of RBC/8 weeks Select Efficacy Endpoints: • Hemoglobin increase of ≥1.5 g/dL for 8 weeks (in NT and LTB patients) • Reduction of ≥4 units or ≥50% units transfused over 8 weeks compared to baseline (in HTB patients) • Transfusion independence for at least 8 weeks (in LTB and HTB patients) 12

Trial Status and Baseline Characteristics • Data cut-off date: May 14, 2021 • Preliminary data presented from two lowest dose cohorts: • Cohort 1: 0.75 mg/kg Q4W for 12 weeks • Cohort 2: 1.5 mg/kg Q4W for 12 weeks • 12 patients received at least one dose of KER-050 as of the data cut-off date • 9 patients completed 8 weeks of treatment with KER-050 as of the data cut-off date • 2 patients withdrew from the trial prior to completing 8 weeks of treatment with KER-050 • 1 patient had not completed 8 weeks of treatment with KER-050 as of the data cut-off date • Baseline characteristics (n=12): • 50% RS positive and non-RS • 50% had erythropoietin >100 mIU/mL • 50% HTB (≥ 4 units/8 weeks) • 85% had multilineage dysplasia • Mean platelet count of 192.4 x 109/L 13

Safety Profile as of May 14, 2021 • Safety Review Committee has reviewed preliminary 0.75 mg/kg (Cohort 1) and 1.5 mg/kg (Cohort 2) data • Summary of safety profile* (Cohorts 1 and 2; n=12): • No drug related serious adverse events (SAEs) • 4 treatment-emergent SAEs deemed unrelated to study drug (anemia, febrile illness, pneumonia and death) • 1 treatment-related adverse event of maculopapular rash (Grade 2) • 2 withdrawals (death deemed unrelated to study drug; patient decision) • Cohort 3 (2.5 mg/kg Q4W) has been initiated following Safety Review Committee recommendation 14 *Data cut-off date: May 14, 2021

Preliminary Results Preliminary results*: • Consistent with the data from the Phase 1 clinical trial, increases in reticulocytes, Hgb and platelets were observed in MDS patients • 5 patients that completed 8 weeks of treatment with KER-050 as of the data cut-off date met at least one of the following three endpoints: • Increase in hemoglobin ≥ 1.5 g/dL for 8 weeks, or • 50% reduction in transfusion requirements over 8 weeks, or • Transfusion independence for at least 8 weeks • 3 patients achieved transfusion independence ≥ 8 weeks in duration • Reduction in transfusions observed in both RS positive and non-RS patients 15 *Data cut-off date: May 14, 2021
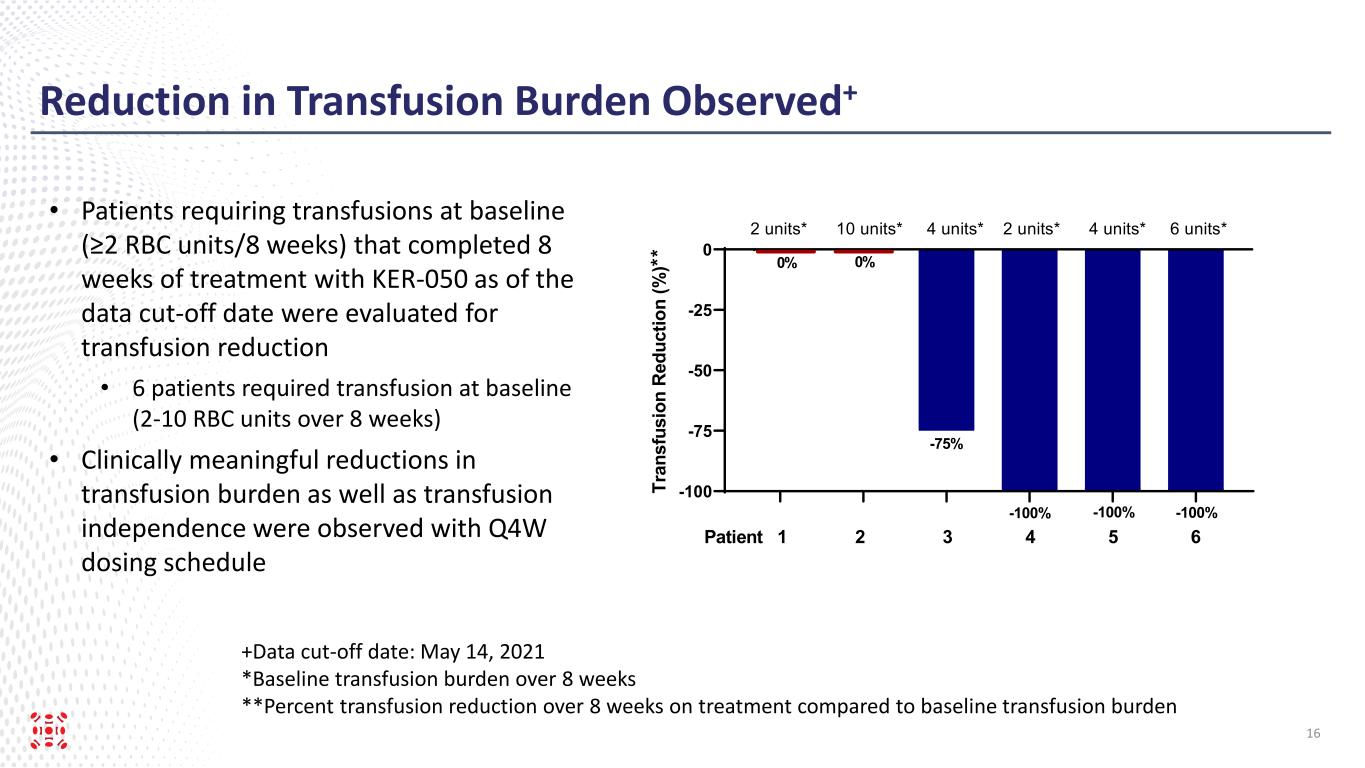
Reduction in Transfusion Burden Observed+ +Data cut-off date: May 14, 2021 *Baseline transfusion burden over 8 weeks **Percent transfusion reduction over 8 weeks on treatment compared to baseline transfusion burden • Patients requiring transfusions at baseline (≥2 RBC units/8 weeks) that completed 8 weeks of treatment with KER-050 as of the data cut-off date were evaluated for transfusion reduction • 6 patients required transfusion at baseline (2-10 RBC units over 8 weeks) • Clinically meaningful reductions in transfusion burden as well as transfusion independence were observed with Q4W dosing schedule 16 -100 -75 -50 -25 0 Tr an sf us io n R ed uc tio n (% ) * * Patient 1 2 3 4 5 6 -75% 0% 0% -100% -100% -100% 2 units* 10 units* 4 units* 2 units* 4 units* 6 units*

KER-050 MDS Program Update • Dosing has been initiated in Cohort 3 at 2.5 mg/kg Q4W following Safety Review Committee recommendation • Keros expects to report additional Part 1 data and initiate Part 2 of the trial by the end of 2021 • Based on the preliminary results as of the data cut-off date (May 14, 2021), Keros plans to: • Extend treatment duration from 12 weeks to up to 2 years to define response rate following 6 months of treatment and to confirm durability of response • Update protocol to increase size of Part 2 dose confirmation to confirm response rates and guide design of registration program • Keros plans to share the Part 2 trial design by the end of 2021 17

Summary • Keros believes the initial preliminary data from this 12-week treatment Phase 2 clinical trial demonstrate proof-of-concept of KER-050 in patients with very low-, low- or intermediate-risk MDS • Data consistent with observations from the Phase 1 clinical trial in healthy volunteers • Increases in hematological parameters were observed in RS positive and non-RS patients that received doses of KER-050 Q4W • Increases in reticulocytes, hemoglobin and platelets were observed • Clinically meaningful reductions in transfusion burden as well as transfusion independence were observed • Lowest two doses were well tolerated as of the data cut-off date • Cohort 3 dosing at 2.5 mg/kg Q4W has been initiated • Keros plans to: • Update protocol to increase size of Part 2 (dose confirmation) to confirm response rates and guide design of registration program • Extend treatment duration from 12 weeks to up to 2 years to define response rate following 6 months of treatment, confirm durability of response and guide design of registration program • Share additional Part 1 dose-escalation data and Part 2 trial design by the end of 2021 18

Anticipated Key Milestones* KER-050 • Announce additional data from Part 1 of Phase 2 trial in MDS End of 2021 • Initiate Phase 2 trial in myelofibrosis Q3 2021 (initial data 2022) KER-047 • Initiate Phase 2 trial in IDA H2 2021 (initial data 2022) • Initiate Phase 2 trial in IRIDA H2 2021 (initial data 2022) KER-012 • Present preclinical data on PAH at major conference 2021 • Initiate Phase 1 trial in healthy volunteers H2 2021 (initial data H1 2022) 19 *Anticipated preclinical and clinical milestones are subject to the impact of COVID-19 on our business.

Q+A Session 20
