Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Panbela Therapeutics, Inc. | snbp20210620_8k.htm |
Exhibit 99.1


Certain statements in this presentation are forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and are provided under the protection of the safe harbor for forward-looking statements provided by that Act. Forward-looking statements are based on current expectations of future events and often can be identified by words such as “anticipate,” “believe,” “continue,” “estimate,” “expect,” “future,” “intend,” “may,” “plan,” “potential,” “target,” or other words of similar meaning or the use of future dates. Examples of forward-looking statements include future determinations of the characteristics of SBP-101 and its effectiveness, removal of the partial clinical hold, publication of results, other trial activities and the timing of the same, and expected financial or operating results. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our beliefs, expectations, and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially and adversely from the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: (i) our ability to obtain additional funding to complete a randomized clinical trial; (ii) progress and success of our Phase 1 clinical trial; (iii) the impact of the current COVID-19 pandemic on our ability to complete monitoring and reporting in our current clinical trial and procure the active ingredient; (iv) our ability to demonstrate the safety and effectiveness of our SBP-101 product candidate (v) our ability to obtain regulatory approvals for our SBP-101 product candidate in the United States, the European Union or other international markets; (vi) the market acceptance and level of future sales of our SBP-101 product candidate; (vii) the cost and delays in product development that may result from changes in regulatory oversight applicable to our SBP-101 product candidate; (viii) the rate of progress in establishing reimbursement arrangements with third-party payors; (ix) the effect of competing technological and market developments; (x) the costs involved in filing and prosecuting patent applications and enforcing or defending patent claims; and (xi) such other factors as discussed in Part I, Item 1A under the caption “Risk Factors” in our most recent Annual Report on Form 10-K, any additional risks presented in our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K. Any forward-looking statement made by us in this presentation is based on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement or reasons why actual results would differ from those anticipated in any such forward-looking statement, whether written or oral, whether as a result of new information, future developments or otherwise. This presentation includes information about investigational agents. The efficacy and safety of such investigational agents have not yet been established. Drug development is uncertain and investigative agents may be terminated along the development process. All trademarks, company and product names or logos are the property of their respective owners.
Developing small molecule polyamine metabolic inhibitors with tumor and organ-specific preferential uptake Multiple cancer types with known elevated polyamine levels represent potential targets Novel Trojan Horse polyamine metabolic inhibitor (PMI) mechanism and tolerability profile seen in early studies may enable use in combination with other agents Potential dual attack: growth inhibition + relieve polyamine-mediated immune suppressionPancreatic ductal adenocarcinoma (PDA) has the lowest survival rate among major cancers Fast track and orphan designation from FDA, SBP-101 is administered subcutaneously SBP-101 given first line with standard of care in Cohort 4 + Phase 1B study interim results:48% objective response rate; more than double historical standard of care 70% of patients with CA 19-9 biomarker reductions of greater than 60% Raised ~$37M in capital since inception to fund SBP-101 development Exclusive global license to SBP-101 from University of Florida Research Foundation Randomized Phase 2 ready, with improved, exclusive synthetic process, IP pending High quality management with proven oncology drug discovery, development and commercialization expertise

Collectively developed 10 FDA-approved therapies generating billions in sales Leadership Team Jennifer K. Simpson, PhD, MSN, CRNP President & CEO Susan Horvath, CPA (inactive), CMA VP of Finance & CFO Thomas X. Neenan, PhD Co-Founder, Chief Scientific Officer Suzanne Gagnon, MD, FACP Chief Medical Officer Board of Directors Michael T. Cullen, MD, MBA, ABIM Jennifer K. Simpson, PhD, MSN, CRNP Art Fratamico, MBA Suzanne Gagnon, MD, FACP Jeff Mathiesen, CPA Paul W. Schaffer, PharmD D. Robert Schemel
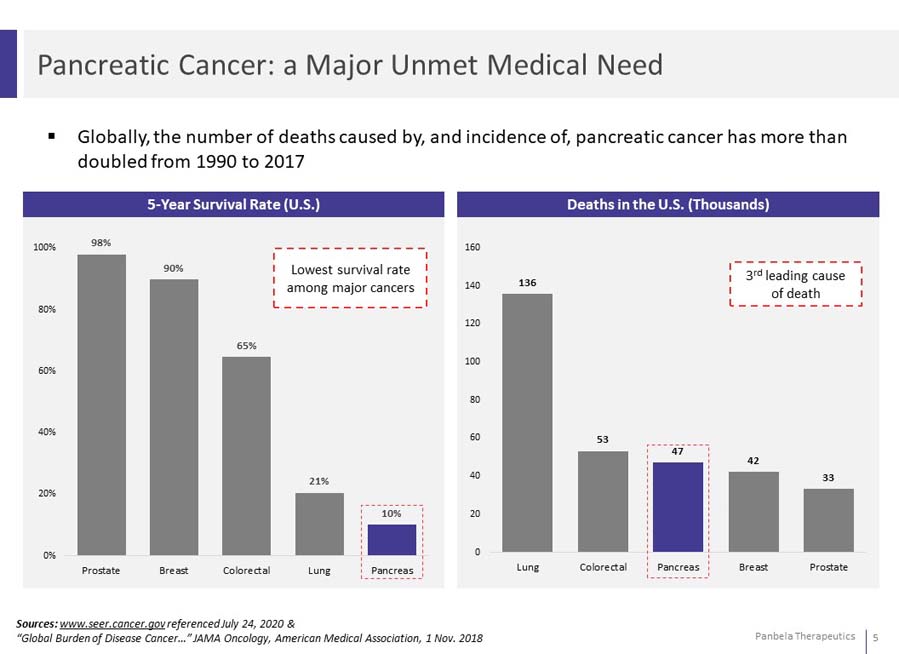
Globally, the number of deaths caused by, and incidence of, pancreatic cancer has more than doubled from 1990 to 2017
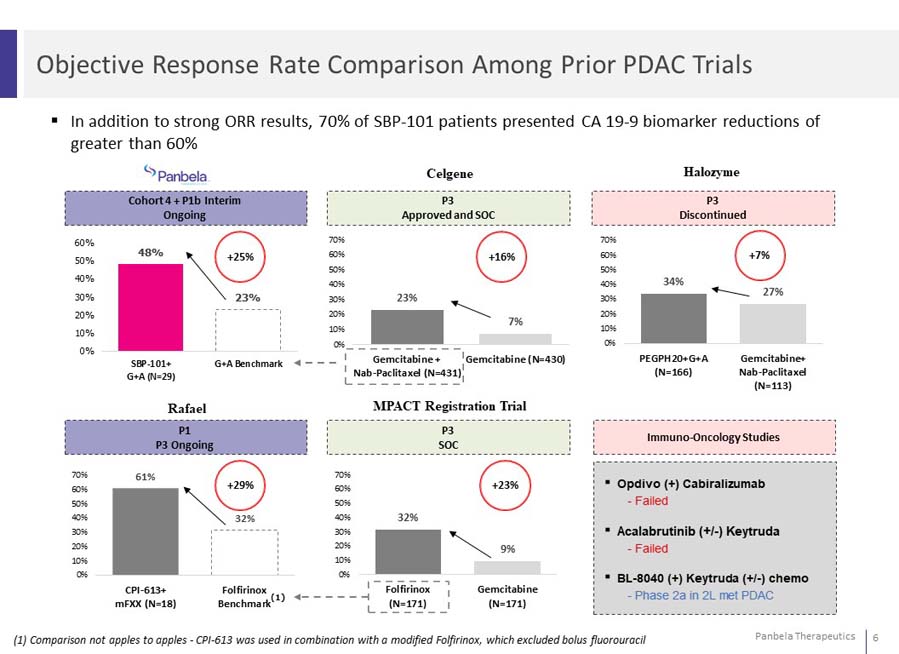
In addition to strong ORR results, 70% of SBP-101 patients presented CA 19-9 biomarker reductions of greater than 60%
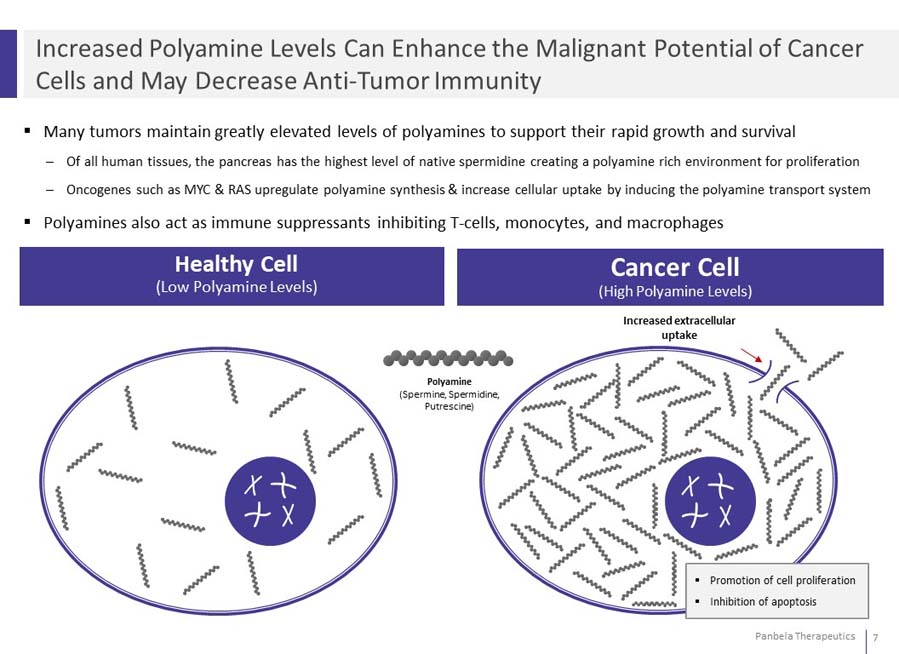
Many tumors maintain greatly elevated levels of polyamines to support their rapid growth and survival Of all human tissues, the pancreas has the highest level of native spermidine creating a polyamine rich environment for proliferation Oncogenes such as MYC & RAS upregulate polyamine synthesis & increase cellular uptake by inducing the polyamine transport system Polyamines also act as immune suppressants inhibiting T-cells, monocytes, and macrophages
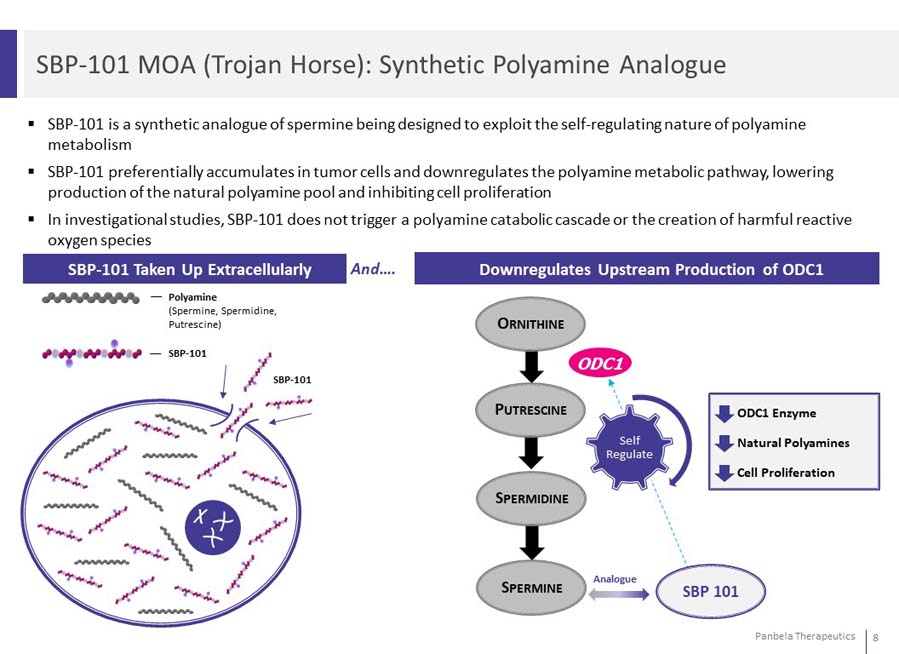
SBP-101 is a synthetic analogue of spermine being designed to exploit the self-regulating nature of polyamine metabolism SBP-101 preferentially accumulates in tumor cells and downregulates the polyamine metabolic pathway, lowering production of the natural polyamine pool and inhibiting cell proliferation In investigational studies, SBP-101 does not trigger a polyamine catabolic cascade or the creation of harmful reactive oxygen species
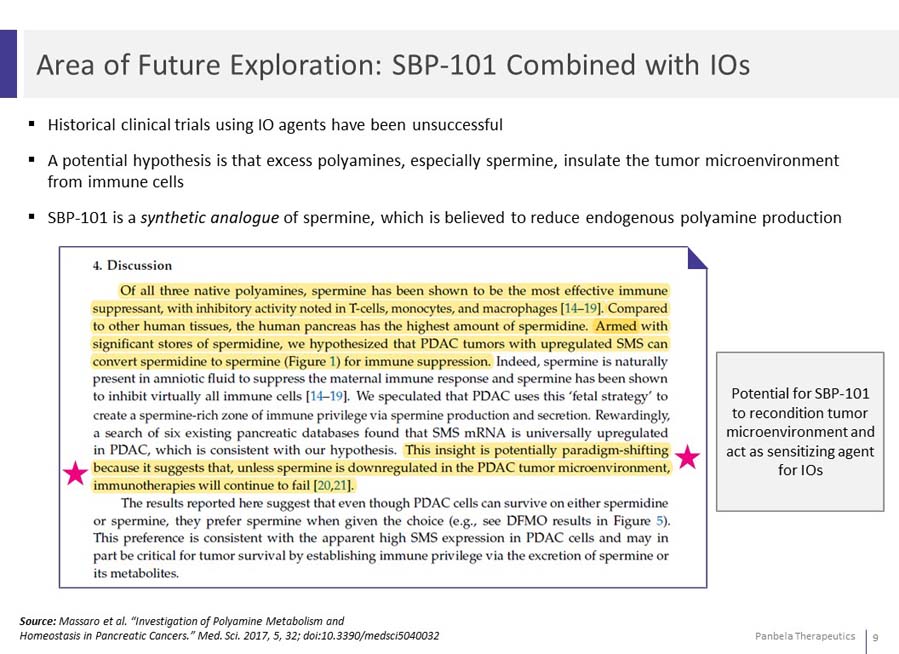
Historical clinical trials using IO agents have been unsuccessful A potential hypothesis is that excess polyamines, especially spermine, insulate the tumor microenvironment from immune cells SBP-101 is a synthetic analogue of spermine, which is believed to reduce endogenous polyamine production Potential for SBP-101 to recondition tumor microenvironment and act as sensitizing agent for IOs
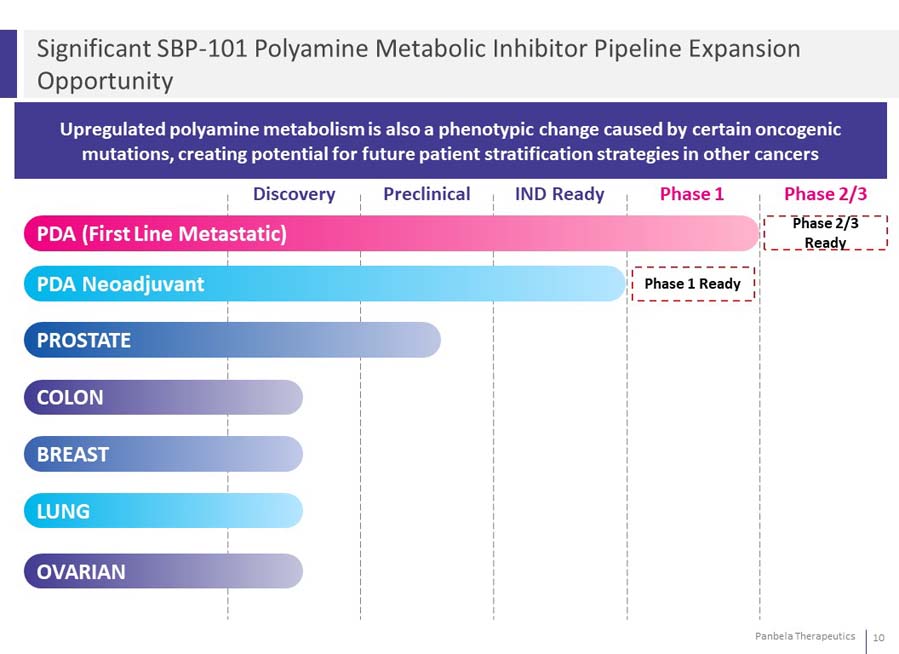
Upregulated polyamine metabolism is also a phenotypic change caused by certain oncogenic mutations, creating potential for future patient stratification strategies in other cancers
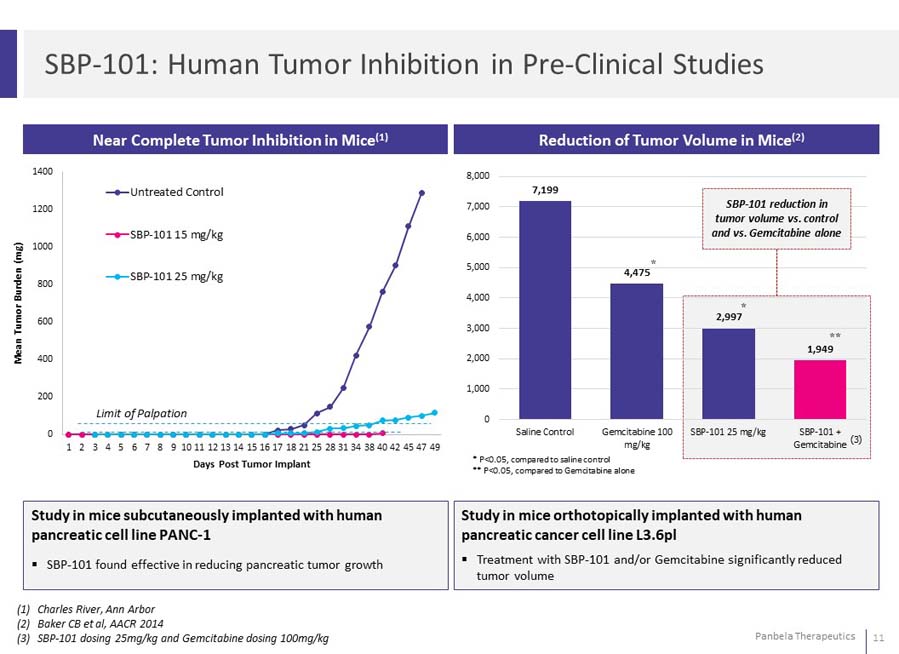
Study in mice subcutaneously implanted with human pancreatic cell line PANC-1 SBP-101 found effective in reducing pancreatic tumor growth Study in mice orthotopically implanted with human pancreatic cancer cell line L3.6pl Treatment with SBP-101 and/or Gemcitabine significantly reduced tumor volume (1)Charles River, Ann Arbor (2)Baker CB et al, AACR 2014 (3)SBP-101 dosing 25mg/kg and Gemcitabine dosing 100mg/kg
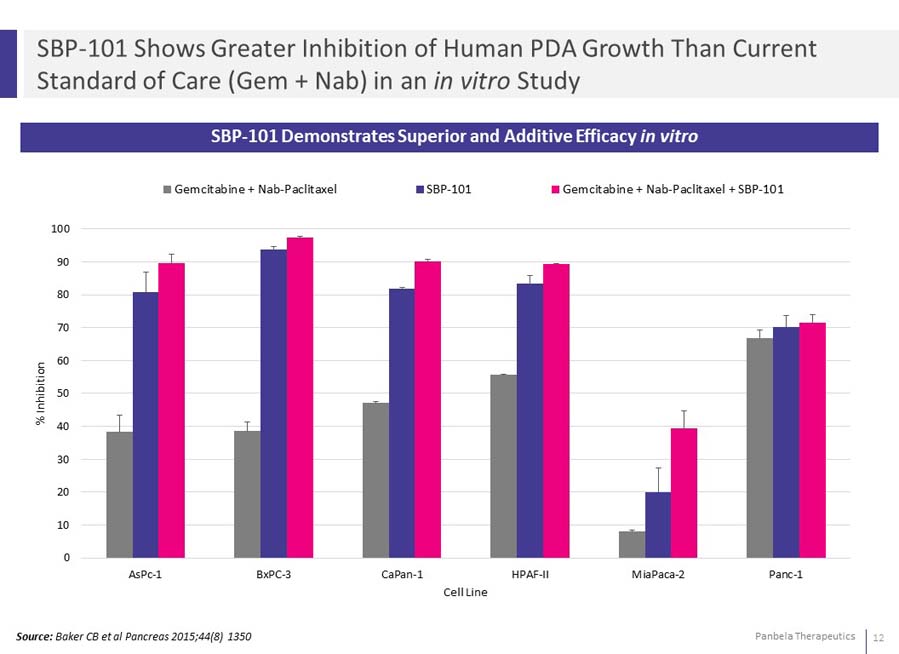
SBP-101 Demonstrates Superior and Additive Efficacy in vitro
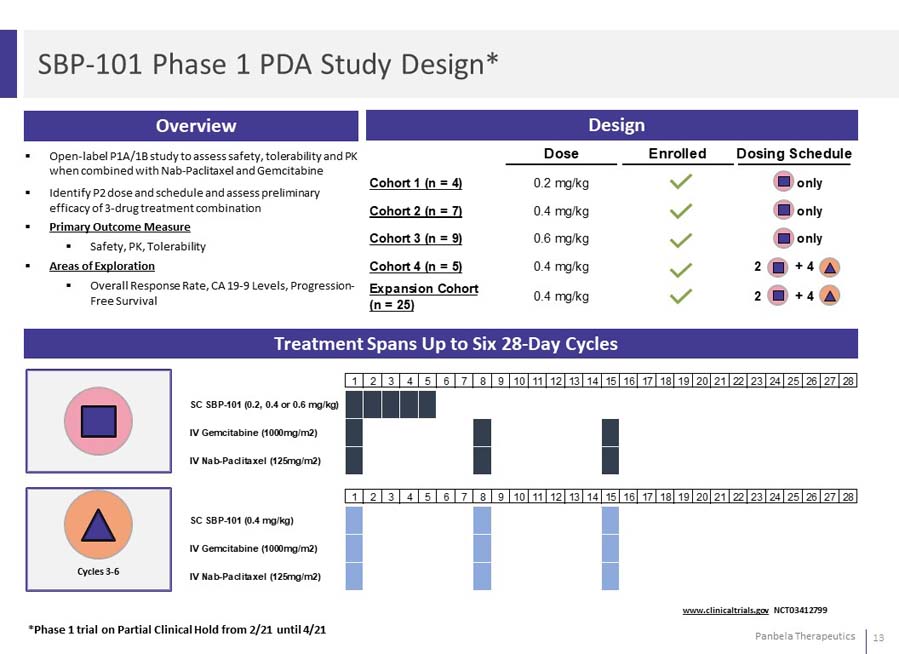
Open-label P1A/1B study to assess safety, tolerability and PK when combined with Nab-Paclitaxel and Gemcitabine Identify P2 dose and schedule and assess preliminary efficacy of 3-drug treatment combination Primary Outcome Measure Safety, PK, Tolerability Areas of Exploration Overall Response Rate, CA 19-9 Levels, Progression-Free Survival
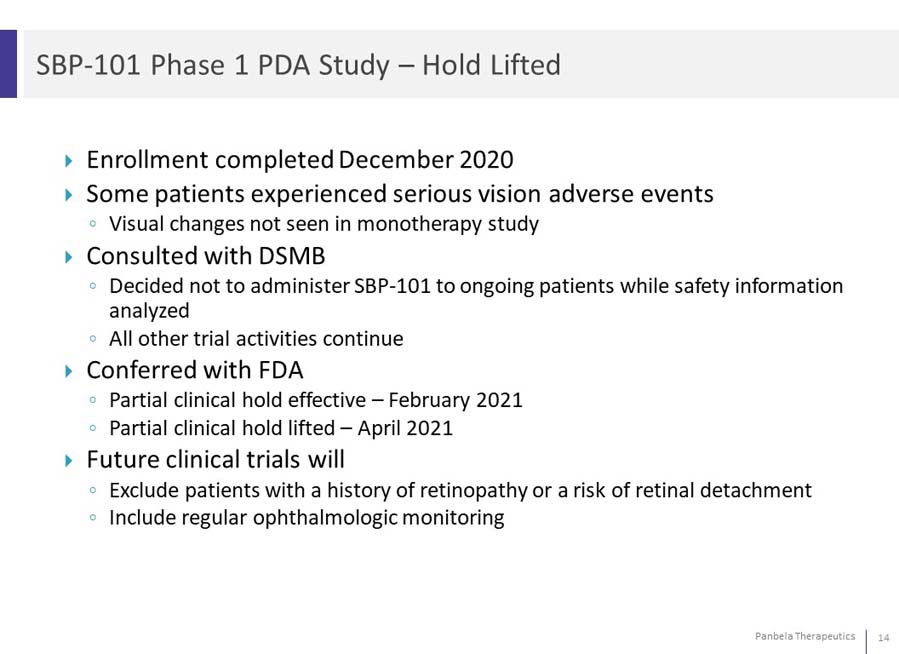
Enrollment completed December 2020 Some patients experienced serious vision adverse events Visual changes not seen in monotherapy study Consulted with DSMB Decided not to administer SBP-101 to ongoing patients while safety information analyzed All other trial activities continue Conferred with FDA Partial clinical hold effective – February 2021 Partial clinical hold lifted – April 2021 Future clinical trials will ◦Exclude patients with a history of retinopathy or a risk of retinal detachment Include regular ophthalmologic monitoring
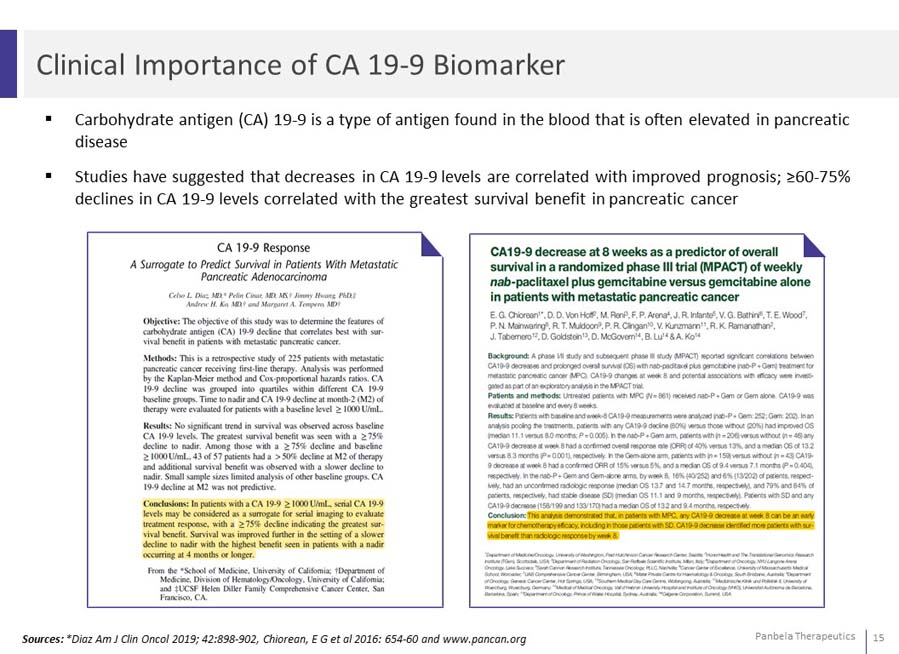
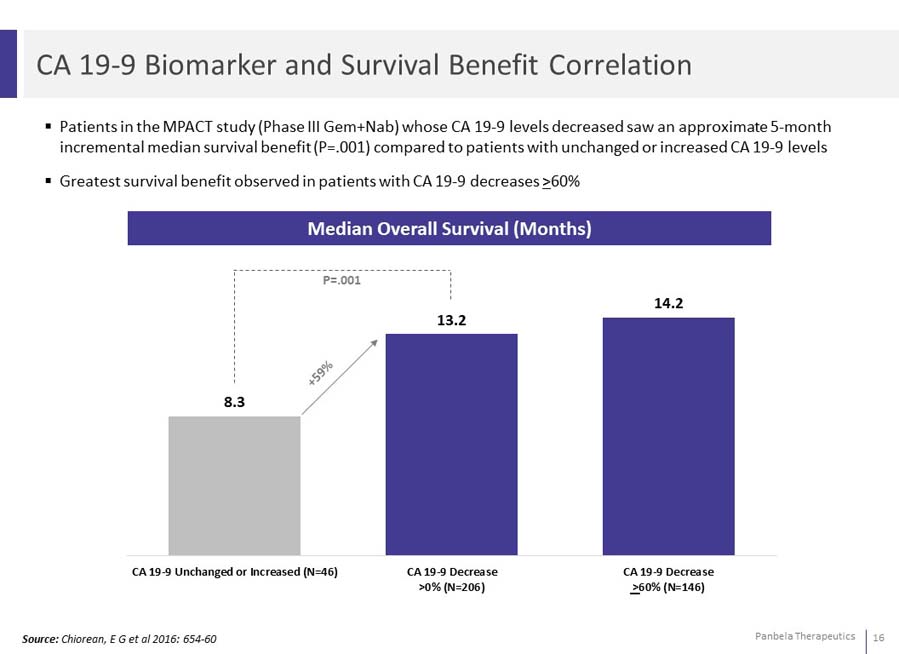
Patients in the MPACT study (Phase III Gem+Nab) whose CA 19-9 levels decreased saw an approximate 5-month incremental median survival benefit (P=.001) compared to patients with unchanged or increased CA 19-9 levels Greatest survival benefit observed in patients with CA 19-9 decreases >60%
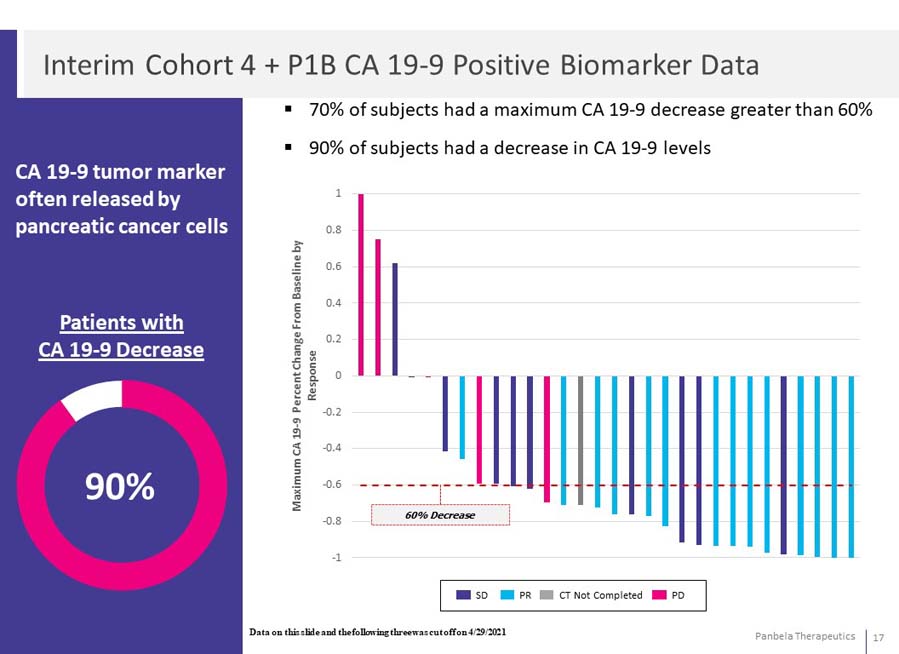
CA 19-9 tumor marker often released by pancreatic cancer cells 70% of subjects had a maximum CA 19-9 decrease greater than 60% 90% of subjects had a decrease in CA 19-9 levels
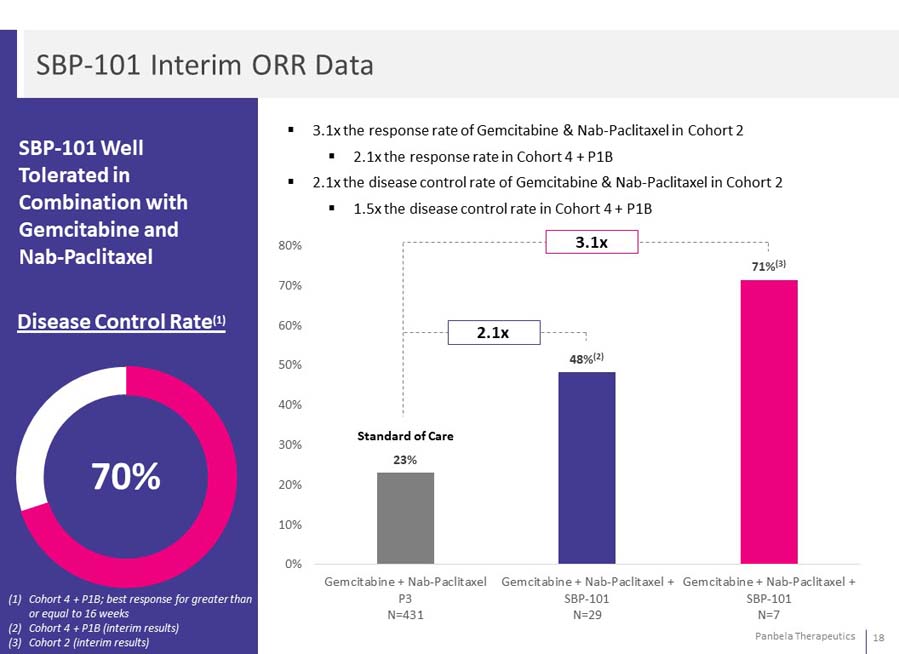
SBP-101 Well Tolerated in Combination with Gemcitabine and Nab-Paclitaxel 3.1x the response rate of Gemcitabine & Nab-Paclitaxel in Cohort 2 2.1x the response rate in Cohort 4 + P1B 2.1x the disease control rate of Gemcitabine & Nab-Paclitaxel in Cohort 2 1.5x the disease control rate in Cohort 4 + P1B
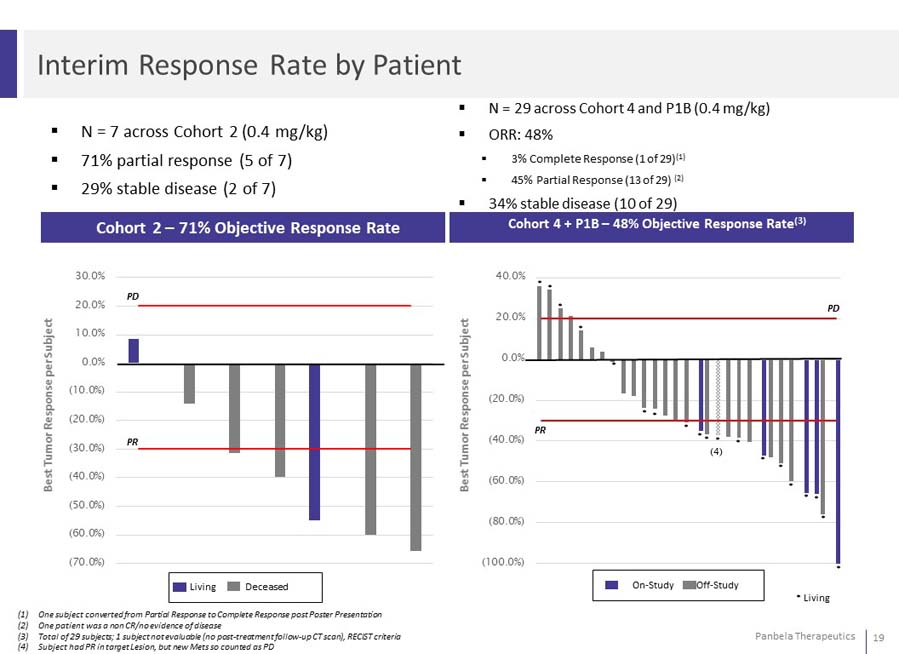
N = 7 across Cohort 2 (0.4 mg/kg) 71% partial response (5 of 7) 29% stable disease (2 of 7) N = 29 across Cohort 4 and P1B (0.4 mg/kg) ORR: 48% 3% Complete Response (1 of 29)(1) 45% Partial Response (13 of 29) (2) 34% stable disease (10 of 29)
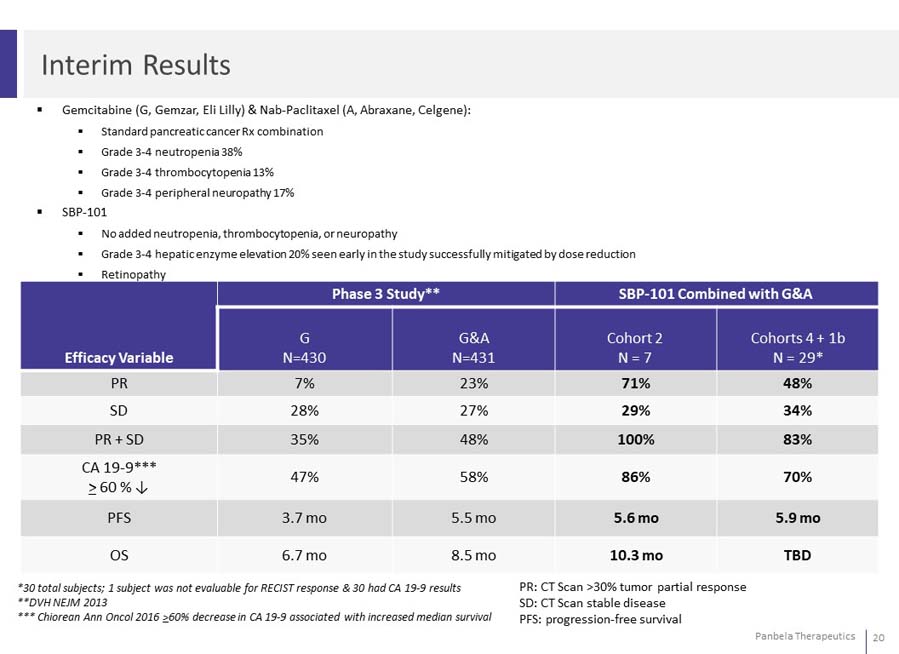
Gemcitabine (G, Gemzar, Eli Lilly) & Nab-Paclitaxel (A, Abraxane, Celgene): Standard pancreatic cancer Rx combination Grade 3-4 neutropenia 38% Grade 3-4 thrombocytopenia 13% Grade 3-4 peripheral neuropathy 17% SBP-101 No added neutropenia, thrombocytopenia, or neuropathy Grade 3-4 hepatic enzyme elevation 20% seen early in the study successfully mitigated by dose reduction Retinopathy
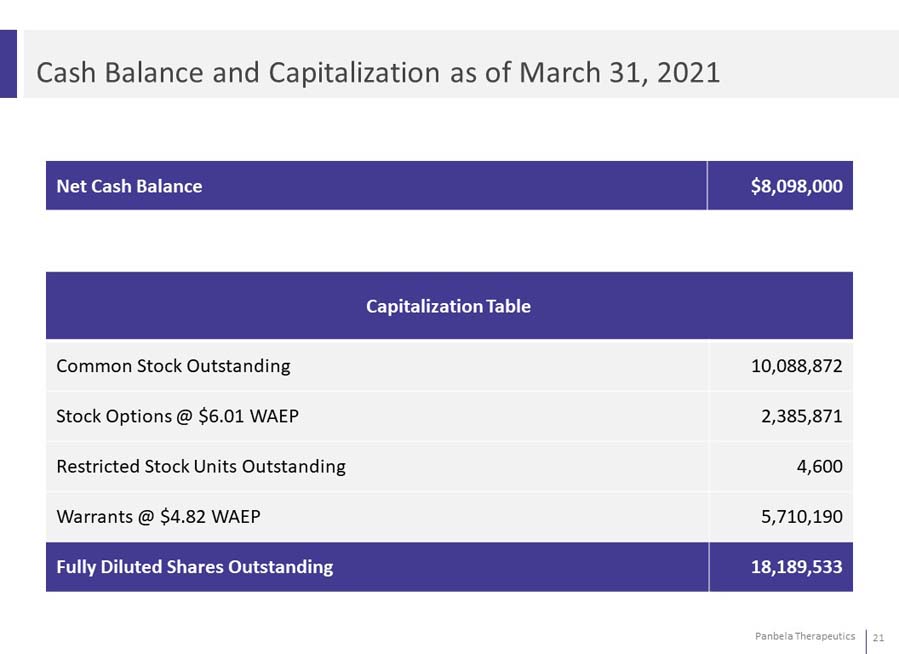
Cash Balance and Capitalization as of March 31, 2021
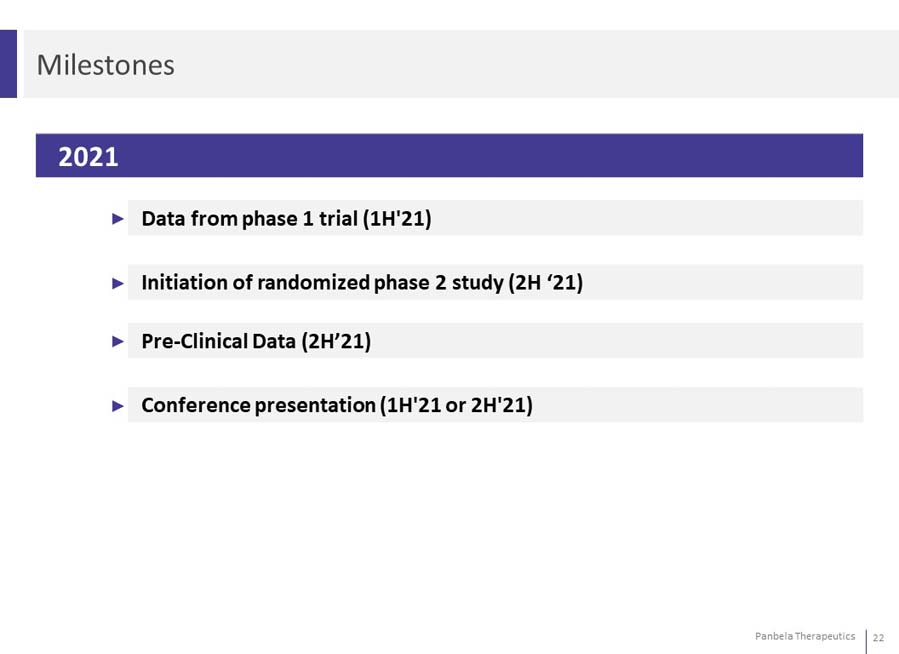
Milestones
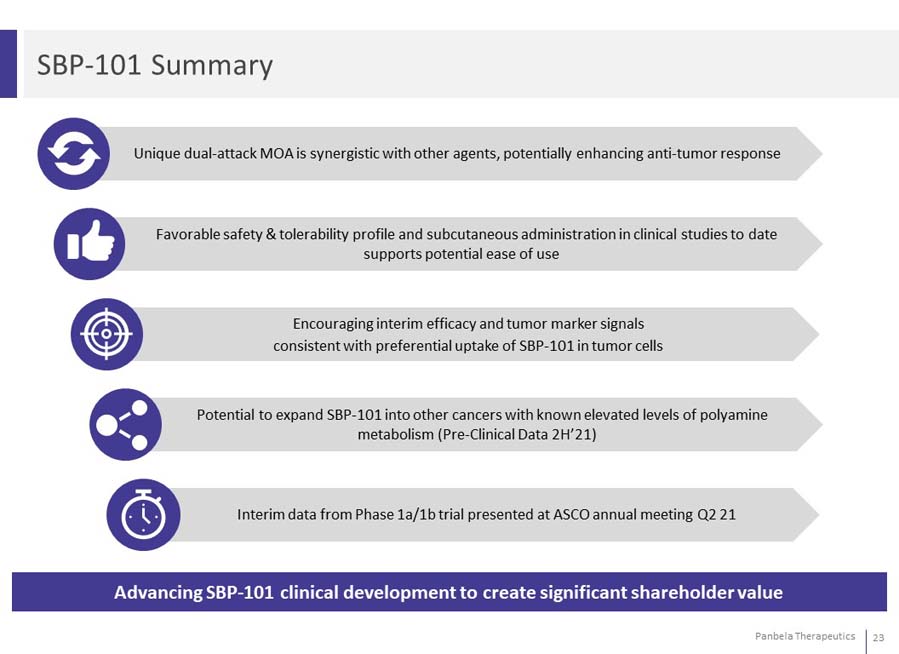
SBP-101 Summary
